Pharmaceutical composition of sitagliptin
A technology of sitagliptin phosphate and its composition, which is applied in the field of pharmaceutical preparations, can solve the problems of poor particle fluidity, poor stability, and low release rate, and achieve large-scale industrial production, good stability, and good dissolution rate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
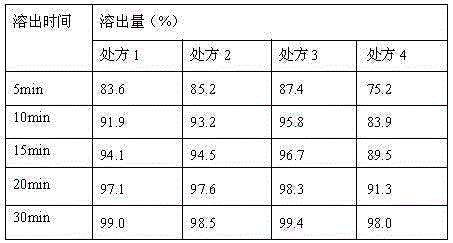
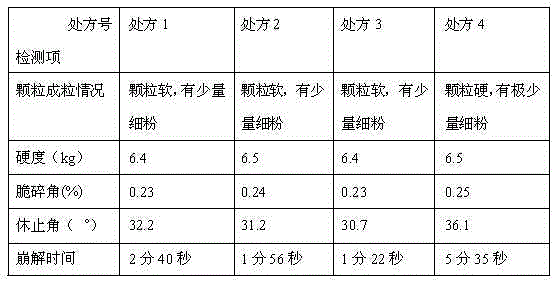
Embodiment 1
[0060] (1) Prescription
[0061] sitagliptin phosphate 128.5
[0062] Microcrystalline Cellulose 80g
[0063] Calcium hydrogen phosphate 80g
[0064] Croscarmellose Sodium 6.5g
[0065] Magnesium Stearate 12.5g
[0066] Coating agent 12.5g
[0067] 50% ethanol aqueous solution appropriate amount
[0068] Makes 1000 pieces
[0069] (2) Preparation method
[0070] 1) Preparation and processing of raw and auxiliary materials: pass sitagliptin phosphate and other auxiliary materials through an 80-mesh sieve for later use;
[0071] 2) Weighing and mixing: Weigh the above raw and auxiliary materials according to the prescription amount and calculate the feeding amount after checking by two people;
[0072] 3) Mixing: Mix the prescribed amount of sitagliptin phosphate with the prescribed amount of calcium hydrogen phosphate, and then mix it with the prescribed amount of microcrystalline cellulose to make it fully mixed;
[0073] 4) Granulation: Add 50% ethanol water solution t...
Embodiment 2
[0082] (1) Prescription
[0083] Sitagliptin Phosphate 128.5g
[0084] Microcrystalline Cellulose 128.5g
[0085] Calcium hydrogen phosphate 128.5g
[0086] Croscarmellose Sodium 6.5g
[0087] Magnesium Stearate 12.5g
[0088] Coating agent 12.5g
[0089] 50% ethanol aqueous solution appropriate amount
[0090] Makes 1000 pieces
[0091] (2) The preparation method is the same as in Example 1.
Embodiment 3
[0093] (1) Prescription
[0094] Sitagliptin Phosphate 128.5g
[0095] Microcrystalline Cellulose 96.5g
[0096] Calcium hydrogen phosphate 64.5g
[0097] Croscarmellose Sodium 6.5g
[0098] Magnesium Stearate 12.5g
[0099] Coating agent 12.5g
[0100] 50% ethanol aqueous solution appropriate amount
[0101] Makes 1000 pieces
[0102] (2) The preparation method is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com