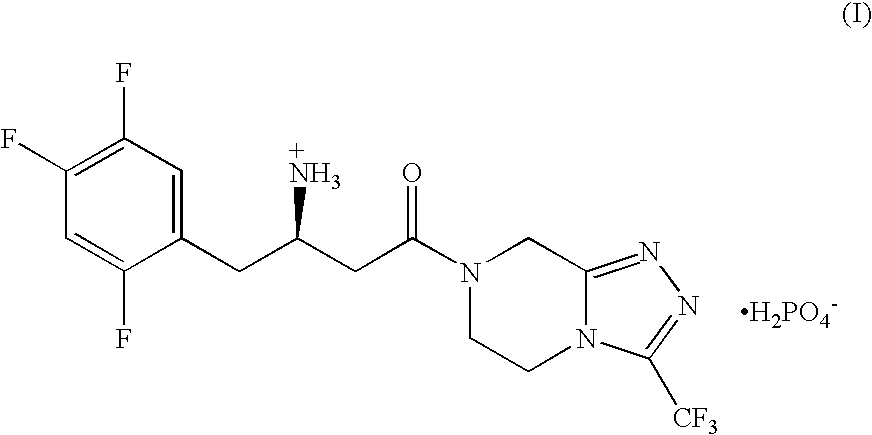
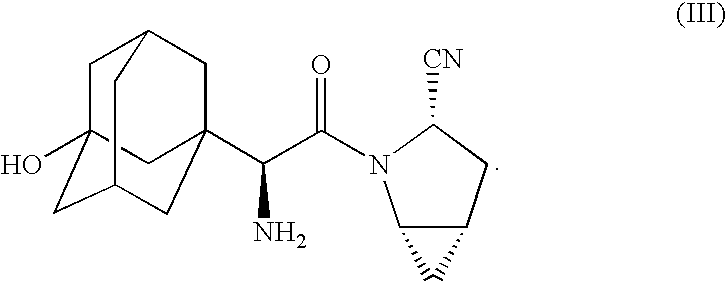
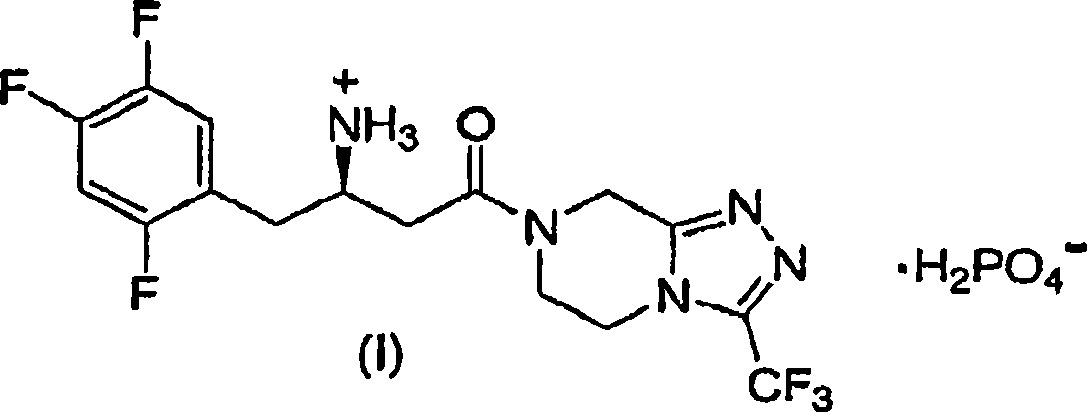
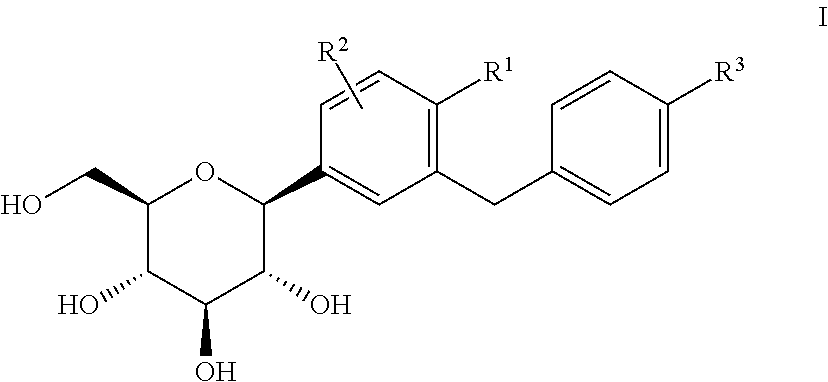
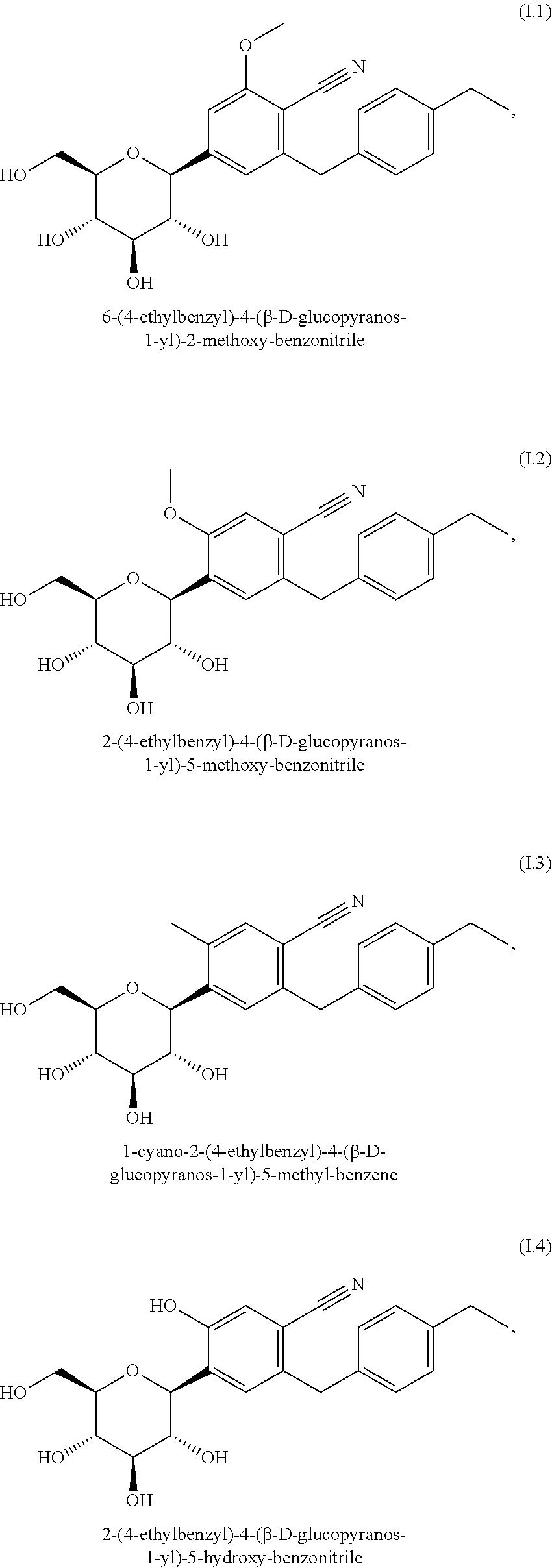
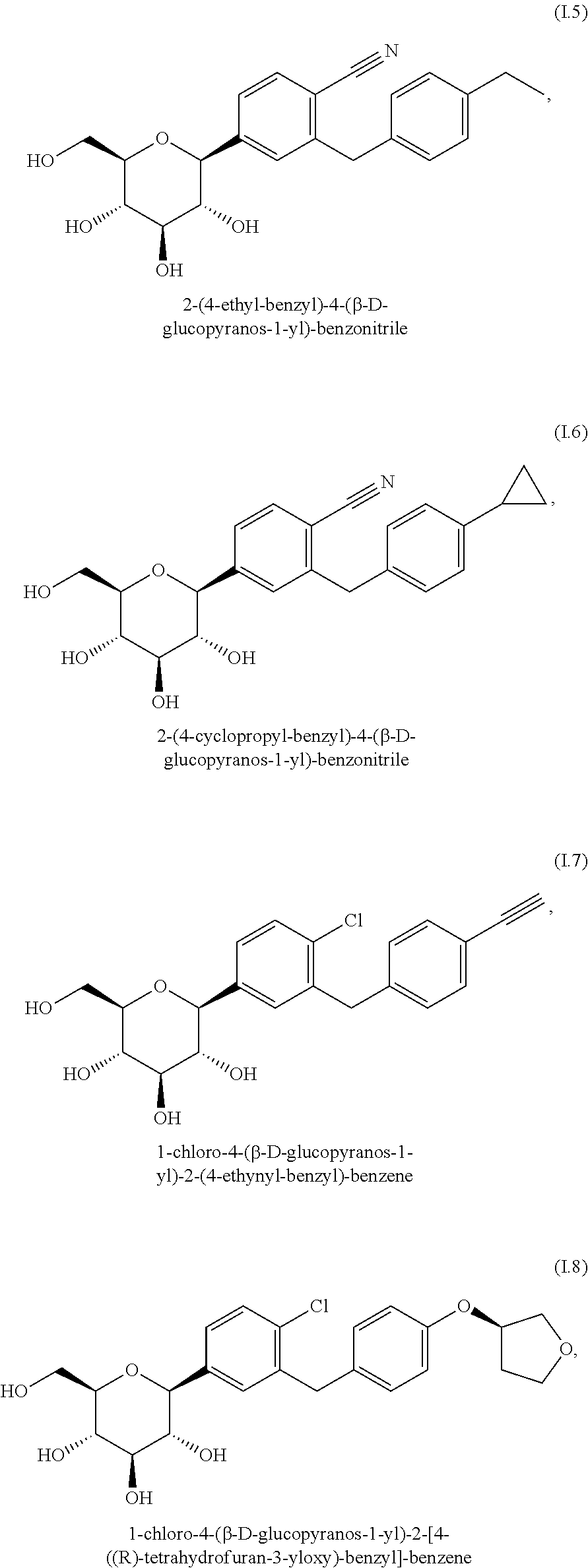
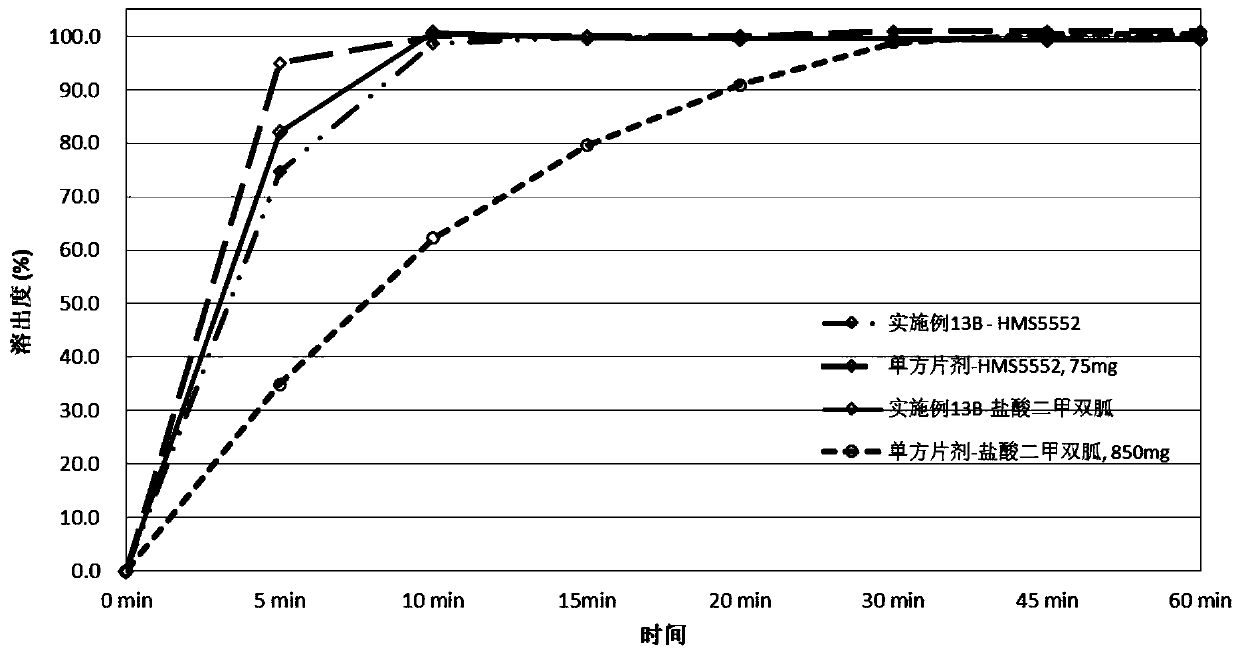
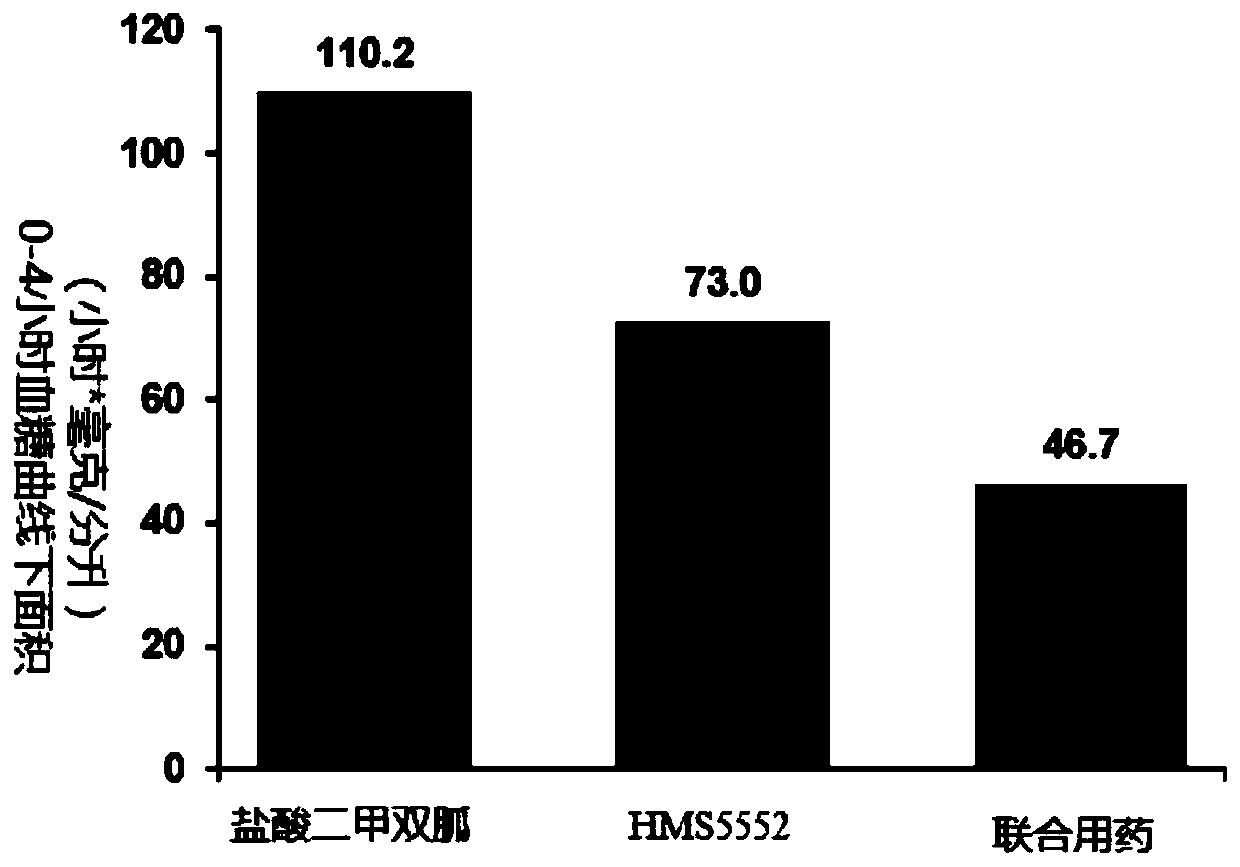
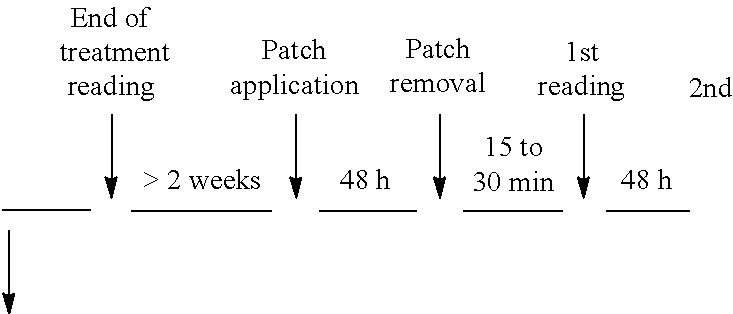
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
89 results about "Fixed-dose combination" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Fixed-dose combinations of antiretrovirals are multiple antiretroviral drugs combined into a single pill, which helps reduce pill burden. They may combine different classes of antiretrovirals (e.g., Atripla) or contain only a single class (e.g., Epzicom). Licensed fixed-dose combinations are shown in the table below. Some are complete single-tablet regimens for the management of HIV/AIDS (the drug is one pill taken once daily); the others must be combined with one or more additional pills to complete a regimen.
Oral dosage combination pharmaceutical packaging
InactiveUS20090087483A1Overall design flexibilityIncreased riskAntibacterial agentsBiocideMedicinePharmaceutical packaging
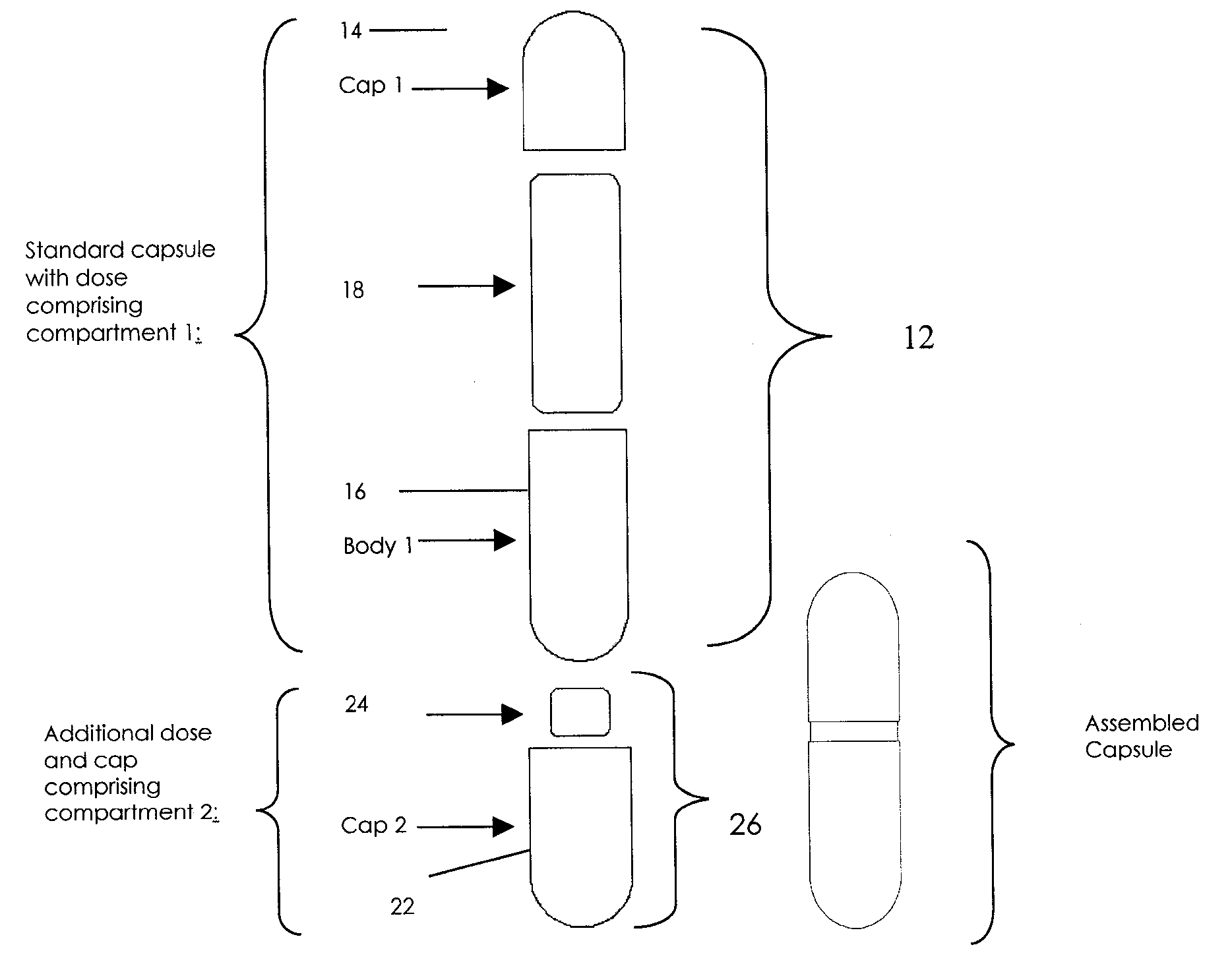
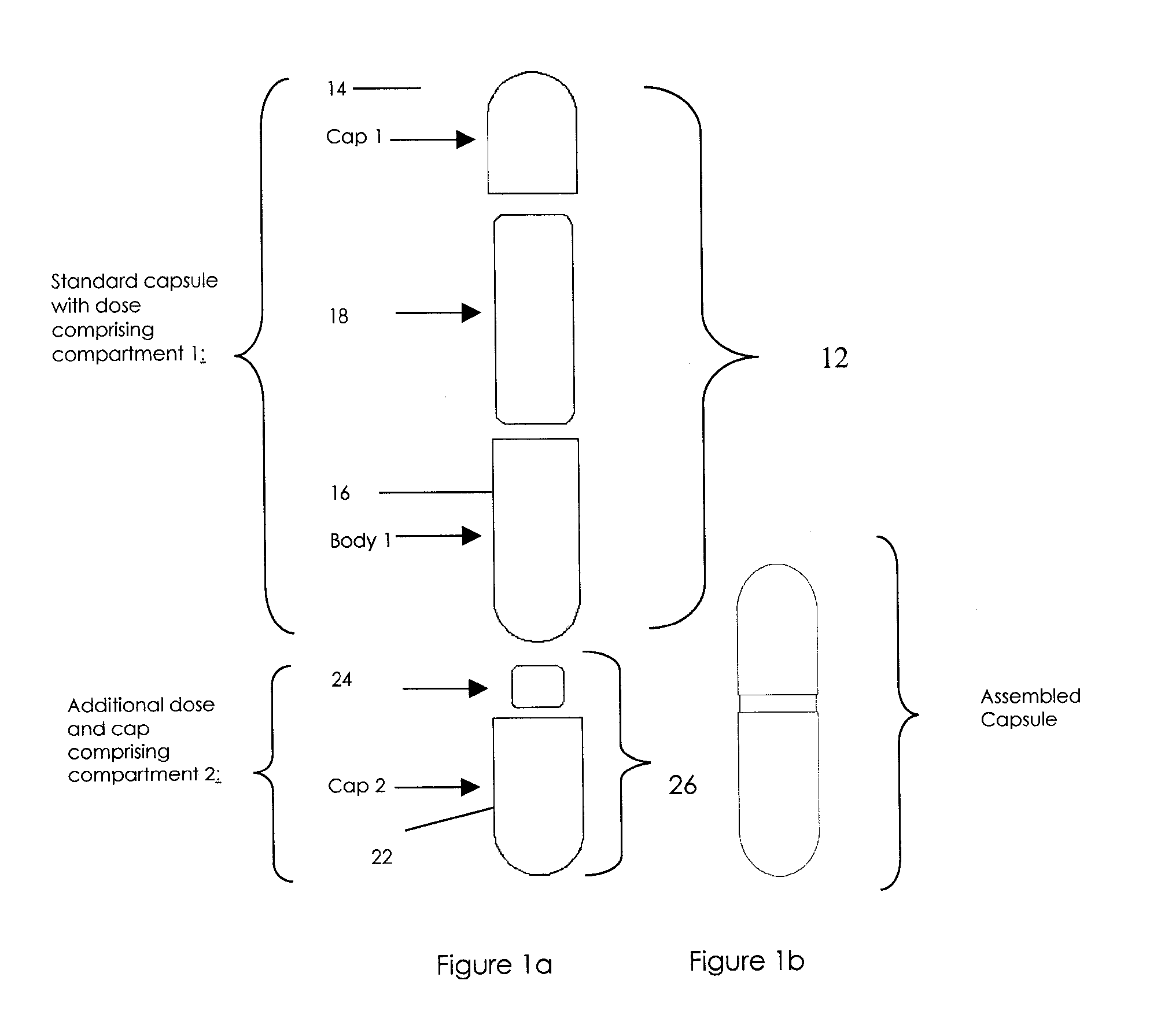
Pharmaceutical fixed dose combination products are formed by merging a fixed dose of a first pharmaceutical formulation from primary module, with a fixed dose of a second pharmaceutical formulation from a secondary module. In a preferred embodiment the first and second pharmaceutical formulations are separated from one another in a three piece capsule, a capsule-in-a-capsule or a tablet-in-a-capsule, and the primary and secondary modules are interchangeable.
Owner:MICRODOSE THERAPEUTX INC
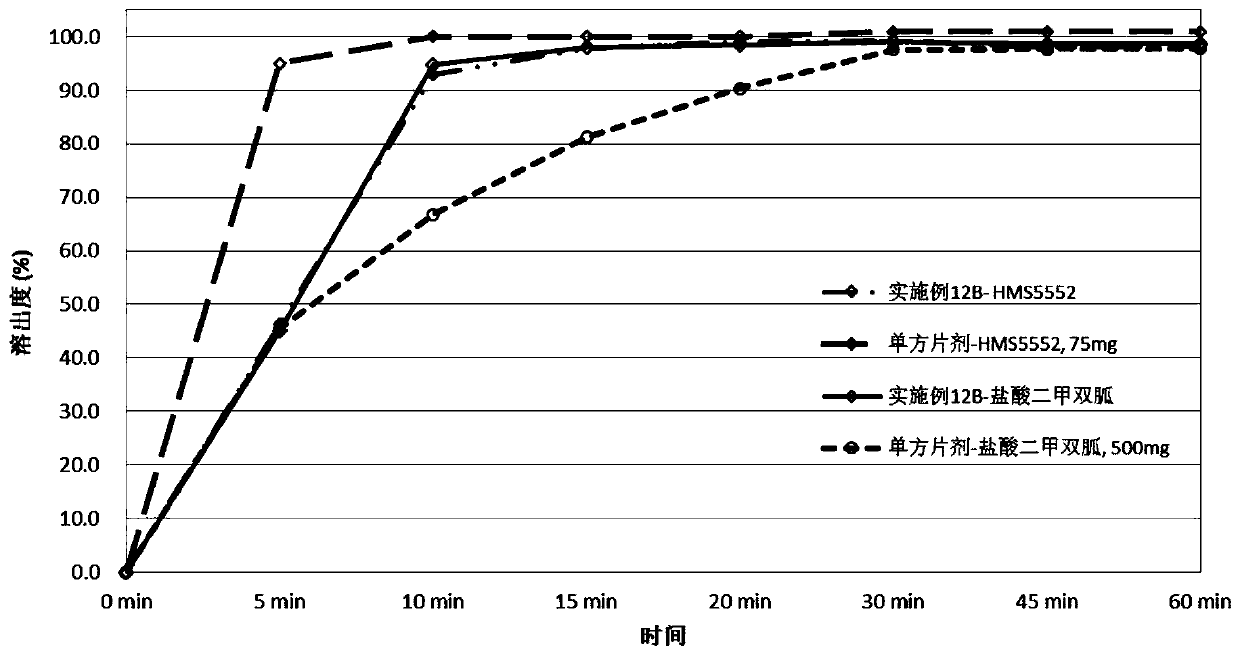
Pharmaceutical compositions of a combination of metformin and a dipeptidyl peptidase-iv inhibitor
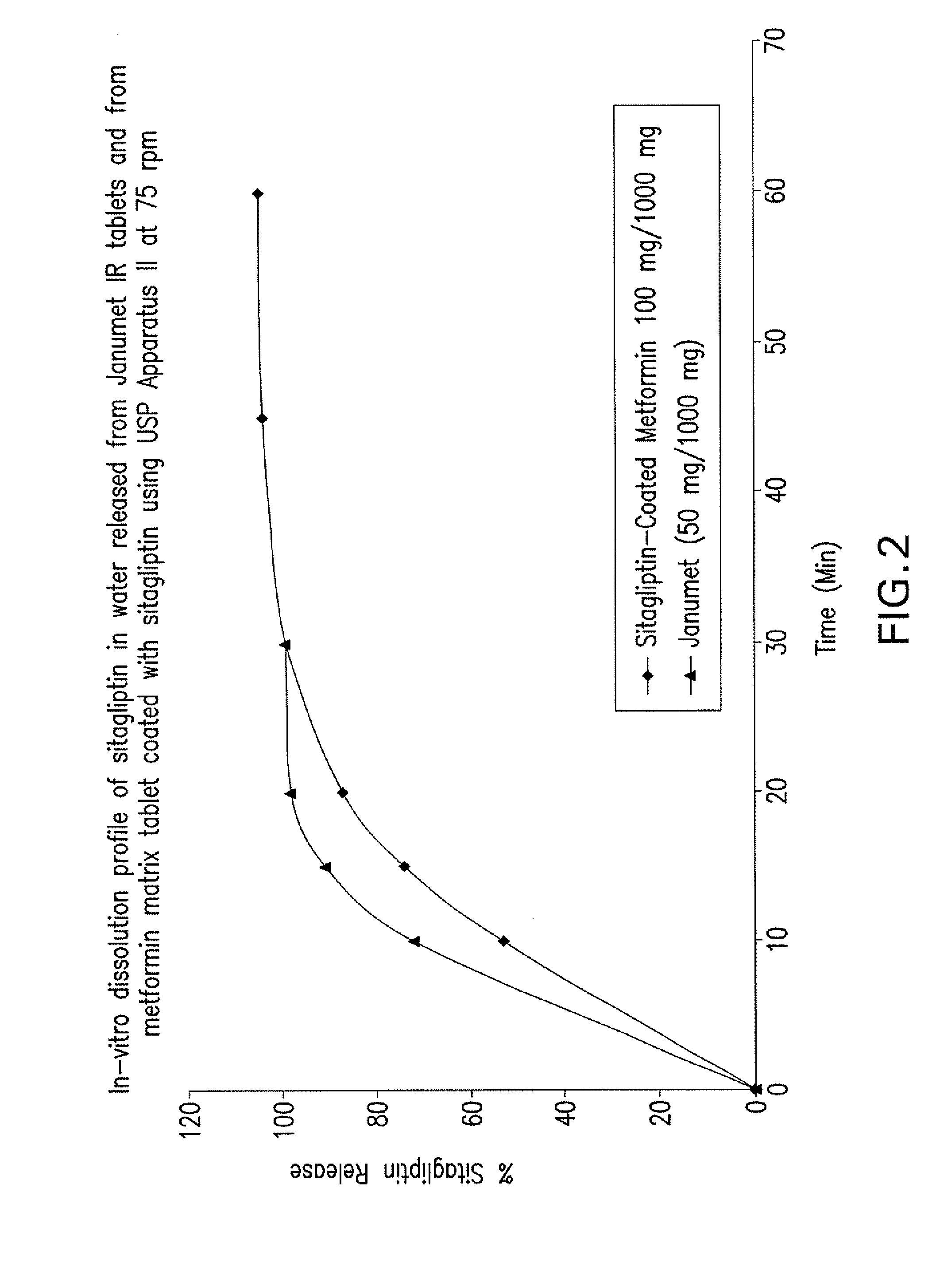
Disclosed are pharmaceutical compositions comprising fixed-dose combinations of an extended-release form of metformin, or a pharmaceutically acceptable salt thereof, coated with an immediate-release form of the DPP-4 inhibitor sitagliptin, or a pharmaceutically acceptable salt thereof.
Owner:MERCK SHARP & DOHME CORP
Stable Pharmaceutical Composition Comprising a Fixed Dose Combination of Fenofibrate and an Hmg-Coa Reductase Inhibitor
InactiveUS20080131503A1Avoid interactionImprove stabilityBiocideDrug compositionsHMG-CoA reductaseAdditive ingredient
Owner:VELOXIS PHARMA
Oral dosage combination pharmaceutical packaging
InactiveUS20090232886A1Increased riskDevelopment costAntibacterial agentsBiocidePharmaceutical packagingPharmaceutical formulation
Pharmaceutical fixed dose combination products are formed by merging a fixed dose of a first pharmaceutical formulation from primary module, with a fixed dose of a second pharmaceutical formulation from a secondary module In a preferred embodiment the first and second pharmaceutical formulations are separated from one another in a three piece capsule, a capsule-in-a-capsule or a tablet-in-a-capsule, and the primary and secondary modules are interchangeable.
Owner:SISON RAYMUNDO A
Pharmaceutical compositions of a combination of metformin and a dipeptidyl peptidase-iv inhibitor
Disclosed are pharmaceutical compositions comprising fixed-dose combinations of an extended-release form of metformin, or a pharmaceutically acceptable salt thereof, coated with an immediate-release form of the DPP-4 inhibitor sitagliptin, or a pharmaceutically acceptable salt thereof.
Owner:MERCK SHARP & DOHME CORP
Pharmaceutical Compositions of Combinations of Dipeptidyl Peptidase-4 Inhibitors With Metformin
Disclosed are pharmaceutical compositions comprising fixed-dose combinations of a dipeptidyl peptidase-4 inhibitor and metformin, methods of preparing such pharmaceutical compositions, and methods of treating Type 2 diabetes with such pharmaceutical compositions.
Owner:MERCK SHARP & DOHME LLC
Pharmaceutical compositions of combinations of dipeptidyl peptidase-4 inhibitors with metformin
ActiveCN101365432APeptide/protein ingredientsMetabolism disorderDipeptidyl peptidaseDipeptidyl peptidase-4 inhibitor
Disclosed are pharmaceutical compositions comprising fixed-dose combinations of a dipeptidyl peptidase-4 inhibitor and metformin, methods of preparing such pharmaceutical compositions, and methods of treating Type 2 diabetes with such pharmaceutical compositions.
Owner:MERCK SHARP & DOHME BV
Blister package for patient compliance
InactiveUS20140183095A1Patient compliance is goodEasy to useSmall article dispensingOrganic active ingredientsPatient complianceBULK ACTIVE INGREDIENT
This invention relates to a compliance blister package comprising a matrix of blisters arranged in a plurality of columns and plurality of rows which comprises a combination of one of the fixed dose combination dosage form comprising two or more therapeutically active ingredients loaded into blisters of one of the columns and the other dosage form which comprises one or more of therapeutically active ingredient form loaded into the successive column blisters for once a day administration or concurrent administration.
Owner:WOCKHARDT LTD
Compositions Comprising Statins, Biguanides and Further Agents for Reducing Cardiometabolic Risk
ActiveUS20150196509A1Reduced gastrointestinal complicationReduce riskBiocideOrganic chemistryVascular diseaseActive agent
Compositions and methods comprising at least one biguanide compound and at least one statin combined with at least one additional active agent in fixed dose combinations are provided for reducing cardiometabolic risk, and for the treatment of cardiovascular disease, wherein the biguanide compound is formulated for delayed release.
Owner:ANJI PHARMA INC
Blister package for patient compliance
InactiveUS9408777B2Patient compliance is goodEasy to useOrganic active ingredientsPharmaceutical containersPatient complianceMedicine
Owner:WOCKHARDT LTD
Methods for treating cardiovascular disorders
There is provided a once-a-day therapeutically synergistic pharmaceutical dosage form for treatment of cardiovascular disorders, wherein the dosage form comprises a fixed dose combination of metoprolol in extended release form and one or more calcium channel blocker, angiotensin II receptor blocker or angiotensin converting enzyme inhibitor along with one or more rate controlling excipient.
Owner:WOCKHARDT LTD
Pharmaceutical tablet formulation for the veterinary medical sector, method of production and use thereof
ActiveUS20150064249A1Reduce adhesionSmall surface areaBiocidePill deliveryDrugFixed-dose combination
The invention is directed to a pharmaceutical tablet formulation for the veterinary medical sector containing an instable ACE inhibitor or a pharmaceutically acceptable salt thereof as a first pharmaceutically active substance, and pimobendan or a pharmaceutically acceptable salt thereof as a second pharmaceutically active substance, comprising granules which contain carrier core particles coated with at least one layer wherein the first pharmaceutically active substance is present, the granules being embedded in a tablet matrix wherein the second pharmaceutically active substance is present. It is provided a “fixed-dose-combination” which allows to ease the treatment and administration of the medication, improves the medication compliance by reducing the pill burden to the animal holder and enables the better observation of and adherence to the therapy by decreasing the number of tablets to be administered. The lower number of tablets leads to a lower treatment failure rate, minimizes dosage mistakes and avoids confusions by false dose intake and slower development of resistance.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
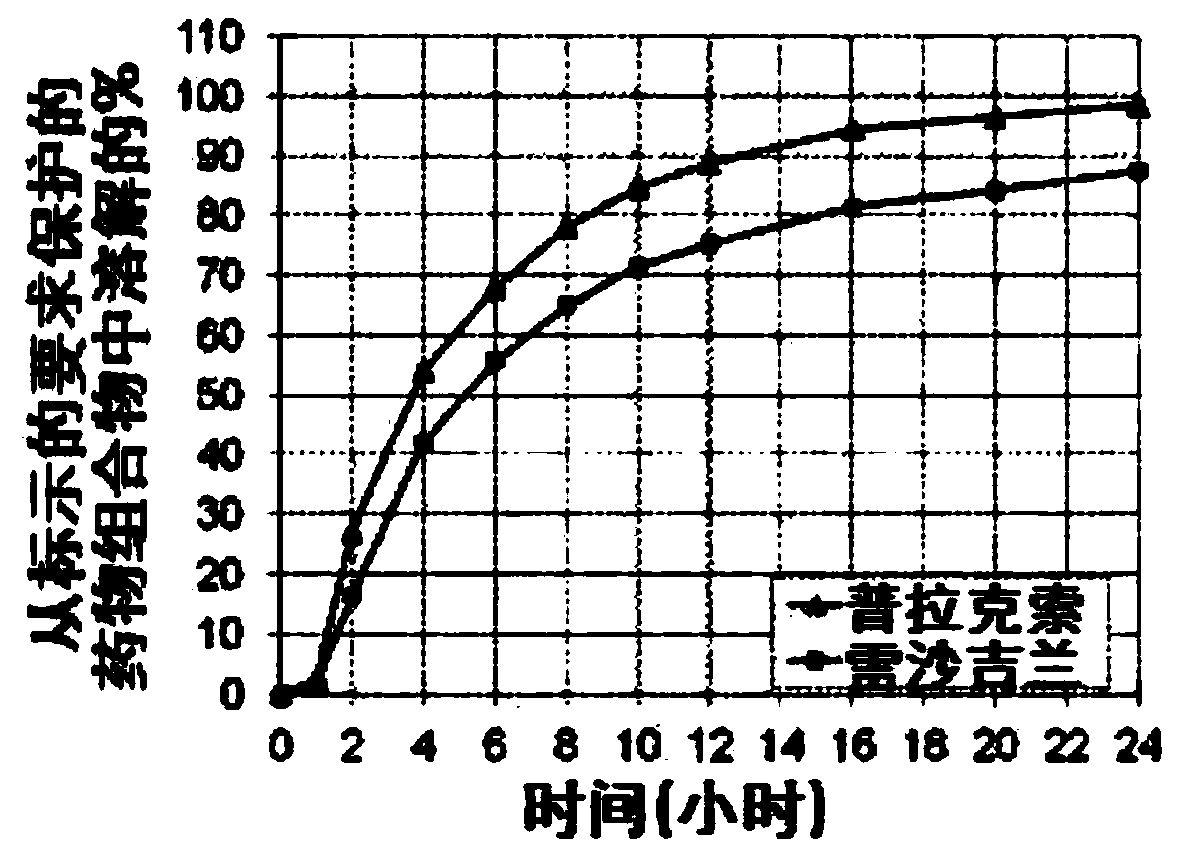
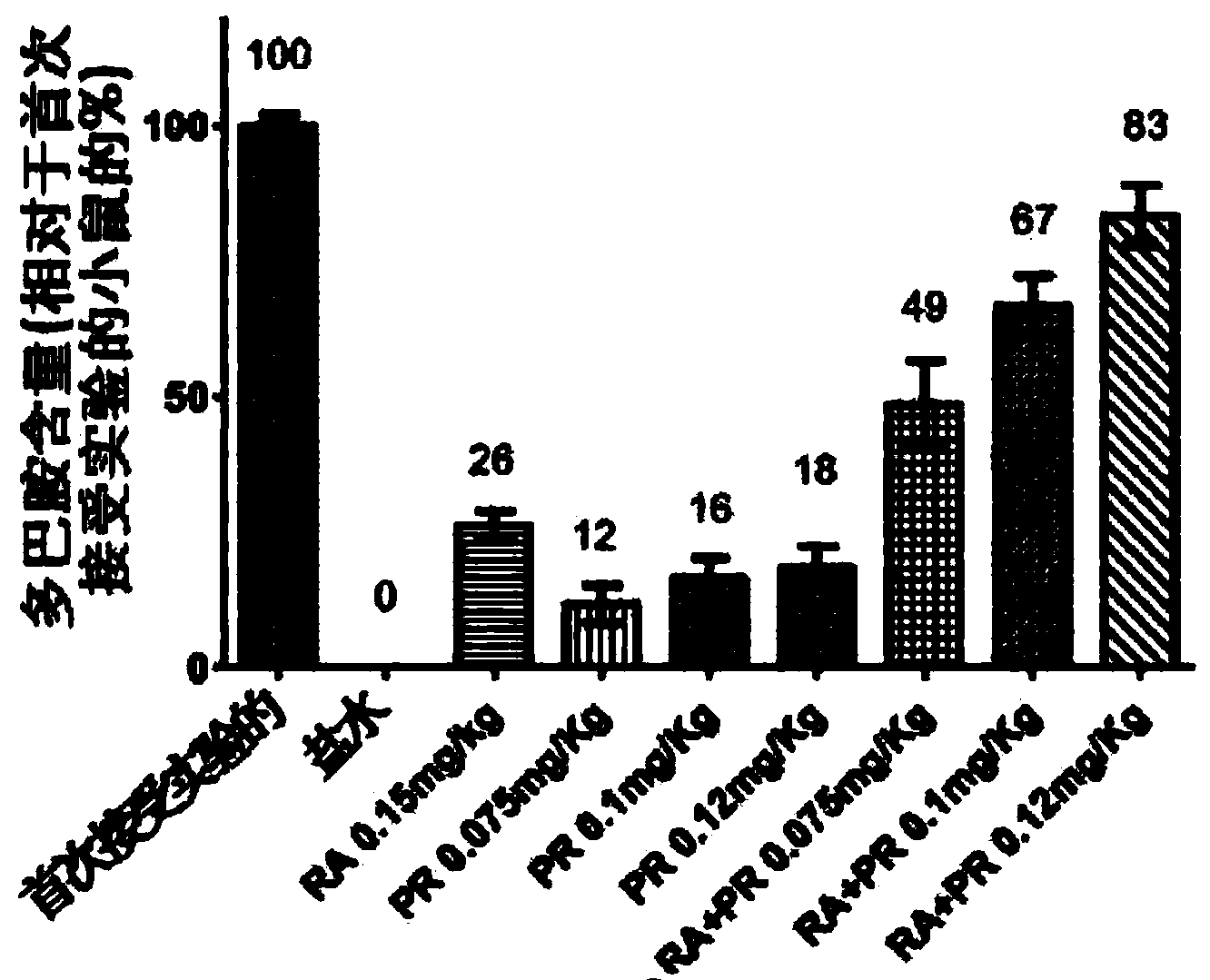
Pharmaceutical compositions for treatment of parkinsons disease
Pharmaceutical compositions are provided for treatment of Parkinson's disease comprising a pharmaceutically acceptable carrier and a fixed dose combination of two active agents selected from compounds having either neuroprotective or symptomatic effects, or both, in Parkinson's disease patients, wherein the molar ratio of the two compounds is in the range of 1:1 to 1:100. The compositions are formulated for immediate release, controlled release, or both immediate and controlled release
Owner:PHARMA TWO B
Pharmaceutical compositions of combinations of dipeptidyl peptidase-4 inhibitors with simvastatin
InactiveUS20140093564A1BiocideMetabolism disorderDipeptidyl peptidaseDipeptidyl peptidase-4 inhibitor
The present invention is directed to novel pharmaceutical compositions comprising fixed dose combinations of a dipeptidyl peptidase-4 inhibitor (DPP-4 inhibitor), or a pharmaceutically acceptable salt thereof, and simvastatin, or pharmaceutically acceptable salt thereof, methods of preparing such pharmaceutical compositions, and methods of treating Type 2 diabetes and hypercholesterolemia with such pharmaceutical compositions. In particular, the invention is directed to pharmaceutical compositions comprising fixed-dose combinations of sitagliptin phosphate and simvastatin.
Owner:MERCK SHARP & DOHME LTD
A stable pharmaceutical composition comprising a fixed dose combination of fenofibrate and an HMG-CoA reductase inhibitor
A pharmaceutical composition for oral administration comprising a fixed dose combination of a first solid pharmaceutical composition containing fenofibrate as the active substance and second solid pharmaceutical composition containing an HMG-CoA reductase inhibitor such as a statin as the active substance, wherein the first and the second pharmaceutical compositions are present in separate entities in a single solid dosage form. For example a multilayer tablet, a two-layer tablet, or capsules or sachets containing the active ingredients in separate granulates or beads, either granulate or bead optionally being coated with a protective coating or an entero-coating.
Owner:LIFECYCLE PHARMA AS
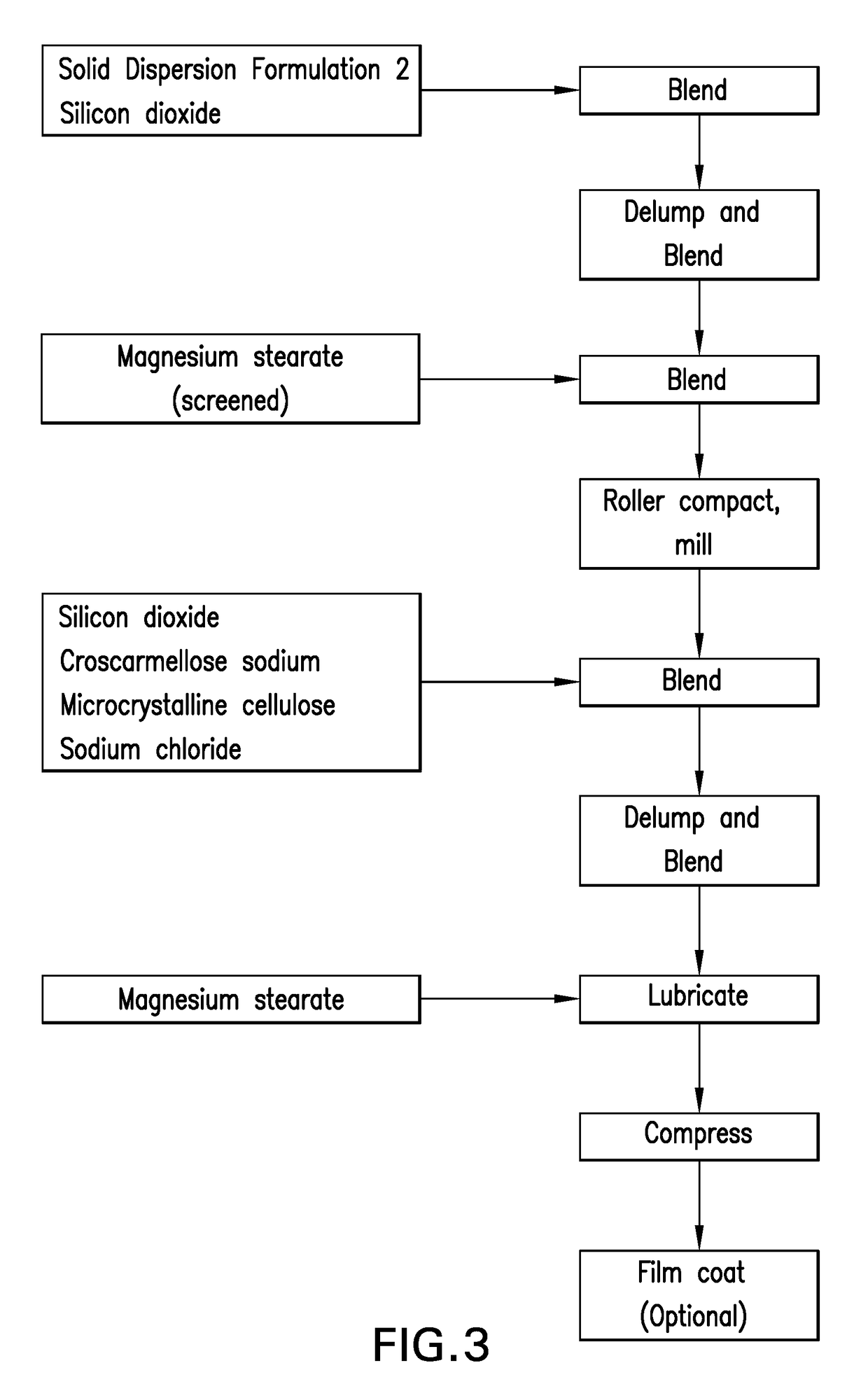
Fixed-dose combinations of antiviral compounds
InactiveUS20180228826A1Improve bioavailabilityIncrease insensitivityOrganic active ingredientsPowder deliveryDepressantExcipient
The present disclosure is directed to compositions comprising blended materials comprising a substantially crystalline HCV nucleotide polymerase inhibitor; a first solid dispersion formulation, which comprises an HCV NS5a inhibitor or a pharmaceutically acceptable salt thereof, one or more pharmaceutically acceptable polymers or a mixture thereof; and optionally one or more pharmaceutically acceptable surfactants or a mixture thereof; and optionally one or more excipients; and a second solid dispersion formulation, which comprises an HCV NS3 inhibitor or a pharmaceutically acceptable salt thereof, one or more pharmaceutically acceptable polymers or a mixture thereof; and optionally one or more pharmaceutically acceptable surfactants or a mixture thereof; and optionally one or more excipients. The present disclosure is also directed to oral dosage forms, such as tablets or capsules comprising the disclosed blended compositions comprising the disclosed solid dispersion formulations, and the methods for making these solid dispersion formulations and pharmaceutical compositions.
Owner:MERCK SHARP & DOHME CORP
Fixed dose combination of bimatoprost and brimonidine
InactiveUS20130023536A1Improve their wellbeingRelieve symptomsBiocideSenses disorderBrimonidineTreatment glaucoma
The present invention is directed to compositions comprising combinations of brimonidine and bimatoprost useful for lowering intraocular pressure in a patient and for the treatment of glaucoma
Owner:ALLERGAN INC
Pharmaceutical compositions of a combination of metformin and a dipeptidyl peptidase-IV inhibitor
Disclosed are pharmaceutical compositions comprising fixed-dose combinations of an extended-release form of metformin, or a pharmaceutically acceptable salt thereof, coated with an immediate-release form of the DPP-4 inhibitor sitagliptin, or a pharmaceutically acceptable salt thereof.
Owner:MERCK & CO INC
Methods for treating cardiovascular disorders
There is provided a once-a-day therapeutically synergistic pharmaceutical dosage form for treatment of cardiovascular disorders, wherein the dosage form comprises a fixed dose combination of metoprolol in extended release form and one or more calcium channel blocker, angiotensin II receptor blocker or angiotensin converting enzyme inhibitor along with one or more rate controlling excipient.
Owner:WOCKHARDT LTD
Pharmaceutical composition, pharmaceutical dosage form, process for their preparation, methods for treating and uses thereof
ActiveUS10610489B2Good effectFew complianceOrganic active ingredientsBiocideDiseasePharmaceutical drug
The present invention relates to pharmaceutical compositions comprising fixed dose combinations of a SGLT-2 inhibitor drug and a partner drug, processes for the preparation thereof, and their use to treat certain diseases.
Owner:BOEHRINGER INGELHEIM INT GMBH
Compositions comprising statins, biguanides and further agents for reducing cardiometabolic risk
Compositions and methods comprising at least one biguanide compound and at least one statin combined with at least one additional active agent in fixed dose combinations are provided for reducing cardiometabolic risk, and for the treatment of cardiovascular disease, wherein the biguanide compound is formulated for delayed release.
Owner:ANJI PHARMA INC
Fixed dose combination for pain relief without edema
InactiveUS20160120885A1Without increase risk of edemaConvenient treatmentBiocideOrganic active ingredientsArthritic painsCOX-2 inhibitor
Owner:AUTOTELIC
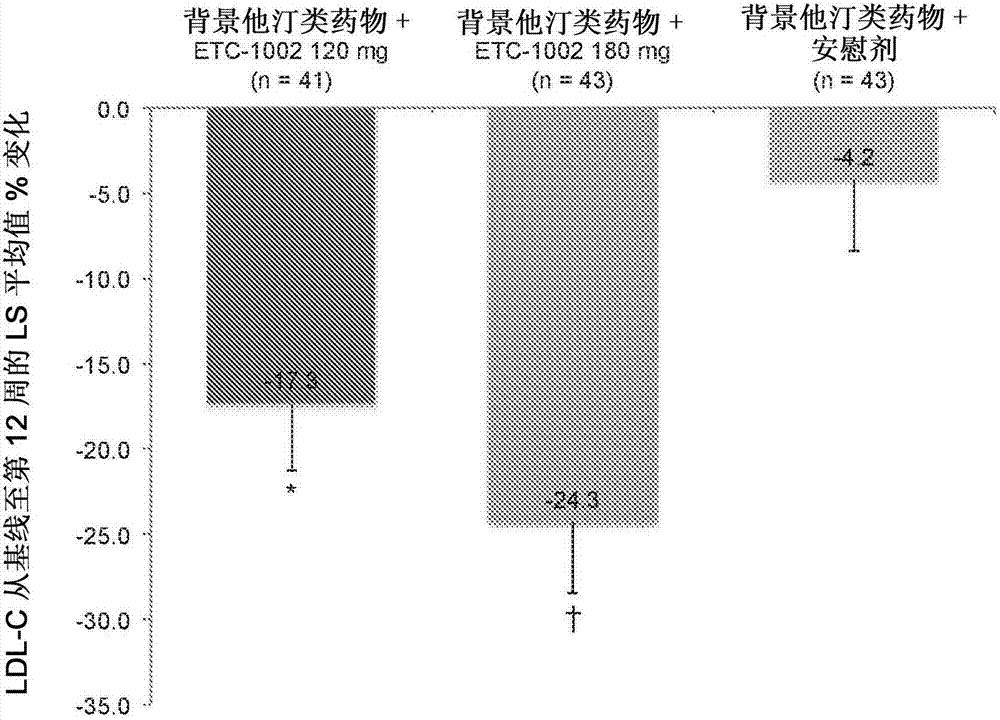
Fixed dose combinations comprising ETC1002 and one or more statins for treating or reducing cardiovascular risk
PendingCN107530308AReduce cholesterolMetabolism disorderEster active ingredientsVascular diseaseStatine
Disclosed herein are compositions comprising fixed doses of ETC-1002 and one or more statins. Also disclosed herein are methods for using fixed doses of ETC-1002 and one or more statins. Uses includemethods of treating cardiovascular disease or reducing the risk of cardiovascular disease in a subject. Uses also include methods of treating hypercholesterolemia in a subject.
Owner:ЕСПЕРІОН ТЕРАПЕУТІКС ІНК
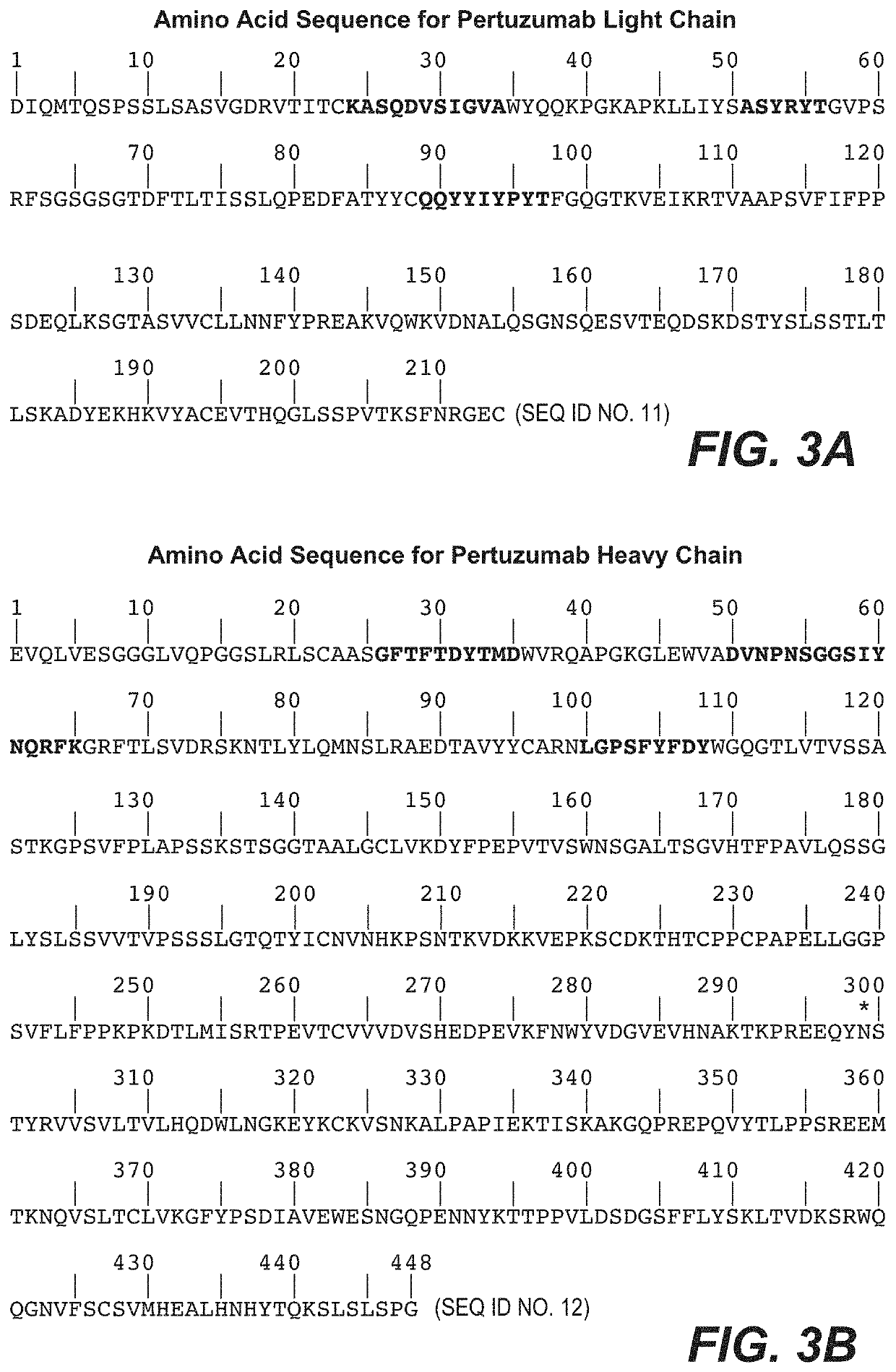
Pertuzumab plus trastuzumab fixed dose combination
PendingUS20210403599A1Easy to manageOvercome inconveniencePeptide/protein ingredientsAntibody ingredientsTrial drugEfficacy
The invention disclosed concerns a fixed dose combination (FDC) of pertuzumab, trastuzumab, and, optionally, recombinant human hyaluronidase (rHuPH20), which is administered subcutaneously to patients. The final efficacy and safety data for the FeDeriCa clinical trial, United States Prescribing Information (USPI) (including home-use) methods, and primary analysis of the PHranceSCa clinical trial are disclosed and claimed.
Owner:GENENTECH INC +1
Formulations and methods for treatment of metabolic syndrome
ActiveUS20160213694A1Reduced pill burdenImprove efficacyPeptide/protein ingredientsHeterocyclic compound active ingredientsHypertension medicationsDisease
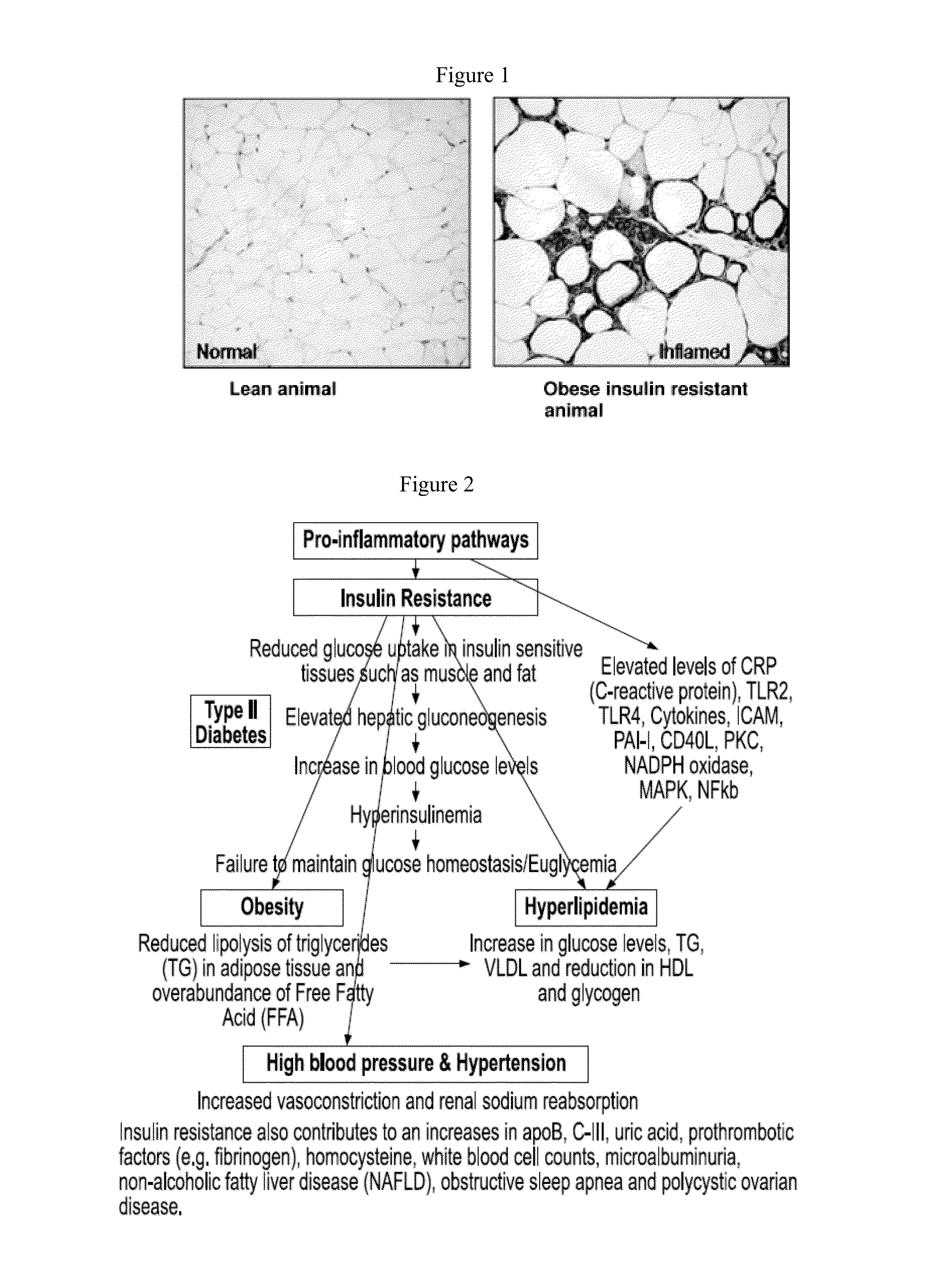
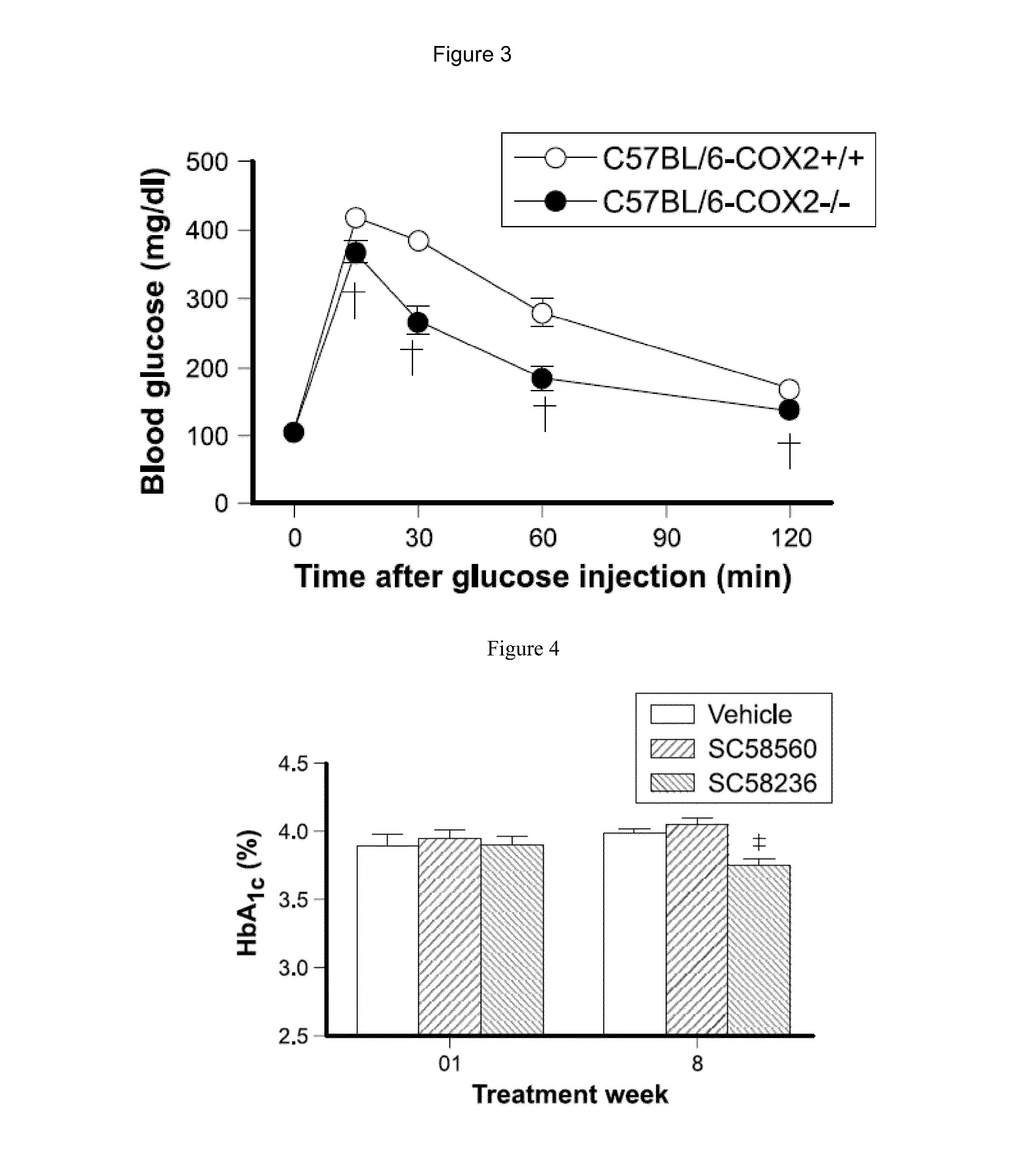
Formulations and methods of providing an orally-active anti-metabolic disease Fixed Dose Combinations (FDC) for use as personalized medicine to treat different components of the Metabolic Syndrome or Insulin resistance syndrome such as Type II diabetes, Hypertension, Hyperlipidemia and Obesity are disclosed. Pharmaceutical compositions of anti-inflammatory and pancreatic beta-cell centric drug formulations and methods comprising of NSAIDS in general and selective Cox-2 inhibitors in particular and one or more anti-T2DM or anti-hypertensive or anti-hyperlipidemic or anti-obesity drugs formulated to exhibit pre-determined modified release kinetics to achieve therapeutic as well as kinetic synergies are disclosed.
Owner:ARKAY THERAPEUTICS
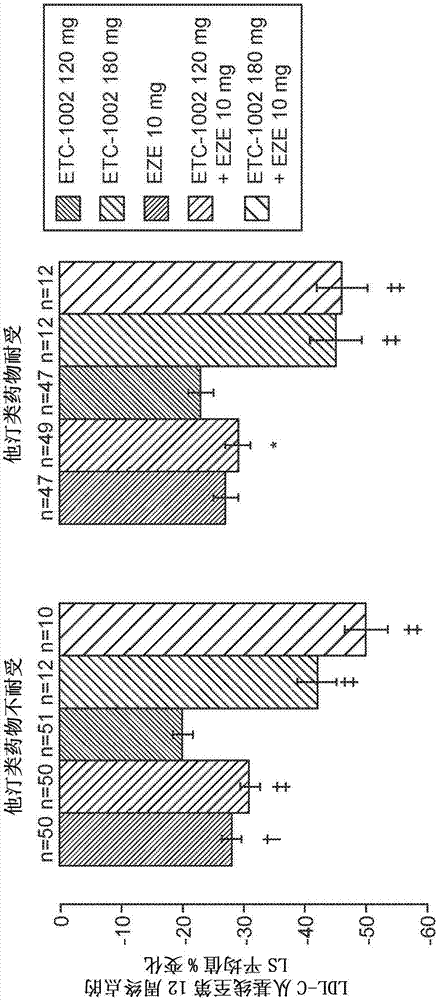
Fixed dose combinations and formulations comprising etc1002 and ezetimibe and methods of treating or reducing the risk of cardiovascular disease
PendingCN107530307AReduce cholesterolReduce riskMetabolism disorderAnhydride/acid/halide active ingredientsVascular diseaseEzetimibe
Disclosed herein are compositions comprising fixed doses of ETC-1002 and Ezetimibe. Also disclosed herein are methods for using fixed doses of ETC-1002 and Ezetimibe. Uses include methods of treatingcardiovascular disease or reducing the risk of cardiovascular disease in a subject. Uses also include methods of treating hypercholesterolemia in a subject.
Owner:ESPERION THERAPEUTICS INC
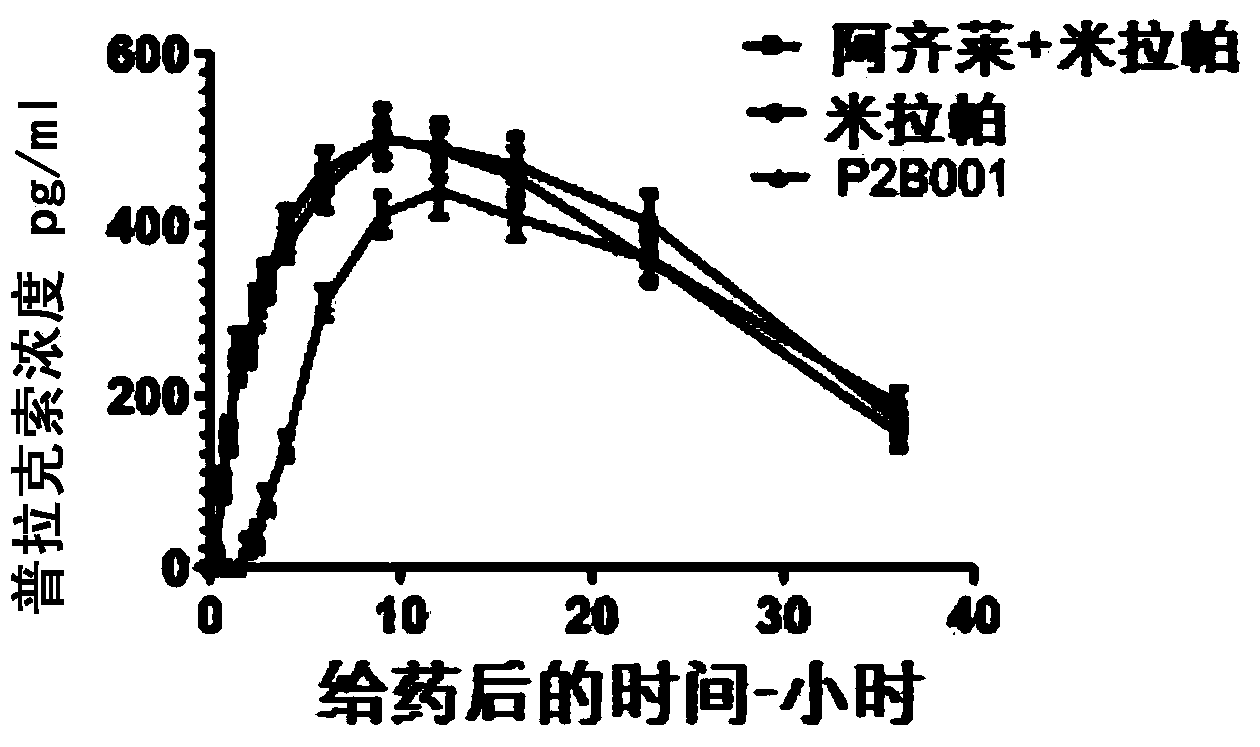
Fixed dose combination therapy of parkinson's disease
ActiveCN104168896AExtended release formulationOrganic active ingredientsCosmetic preparationsPramipexoleCombination therapy
A pharmaceutical composition for use in treatment of Parkinson's disease is provided comprising a pharmaceutically acceptable carrier and a fixed dose combination of pramipexole and rasagiline, wherein the fixed dose combination contains a subtherapeutic dose of pramipexole and a subtherapeutic dose of rasagiline, and the dose of pramipexole is lower than or equal to the dose of rasagiline.
Owner:图必制药公司
Drug combination containing glucokinase activator (GKA) and biguanide antidiabetic drug, composition and combination preparation and preparation methods and application thereof
ActiveCN110548146AReduce doseImprove multiple organ functionPowder deliveryNervous disorderEnantiomerIsotope
The invention relates to a drug combination. The drug combination comprises a glucokinase activator (GKA), a pharmaceutical salt, an isotope marker and a crystalline form of the GKA, a hydrate, a solvate, a diastereoisomer or an enantiomer form and a biguanide antidiabetic drug. The invention further relates to a drug composition, a fixed dose combination (FDC) preparation and a preparation methods and application of the drug combination and the FDC preparation.
Owner:HUA MEDICINE (SHANGHAI) LIMITED
Maintenance therapy regime/regimen for the treatment of acne
ActiveUS20110144003A1Avoiding potential bacterial resistanceAvoid developmentBiocideHydroxy compound active ingredientsDiseaseMaintenance therapy
A novel maintenance therapy regime / regimen for the treatment of acne related diseases includes administering an oral antibiotic with a topical fixed-dose combination of a retinoid, such as adapalene, and an anti-bacterial agent, such as benzoyl peroxide.
Owner:GALDERMA RES & DEV SNC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com