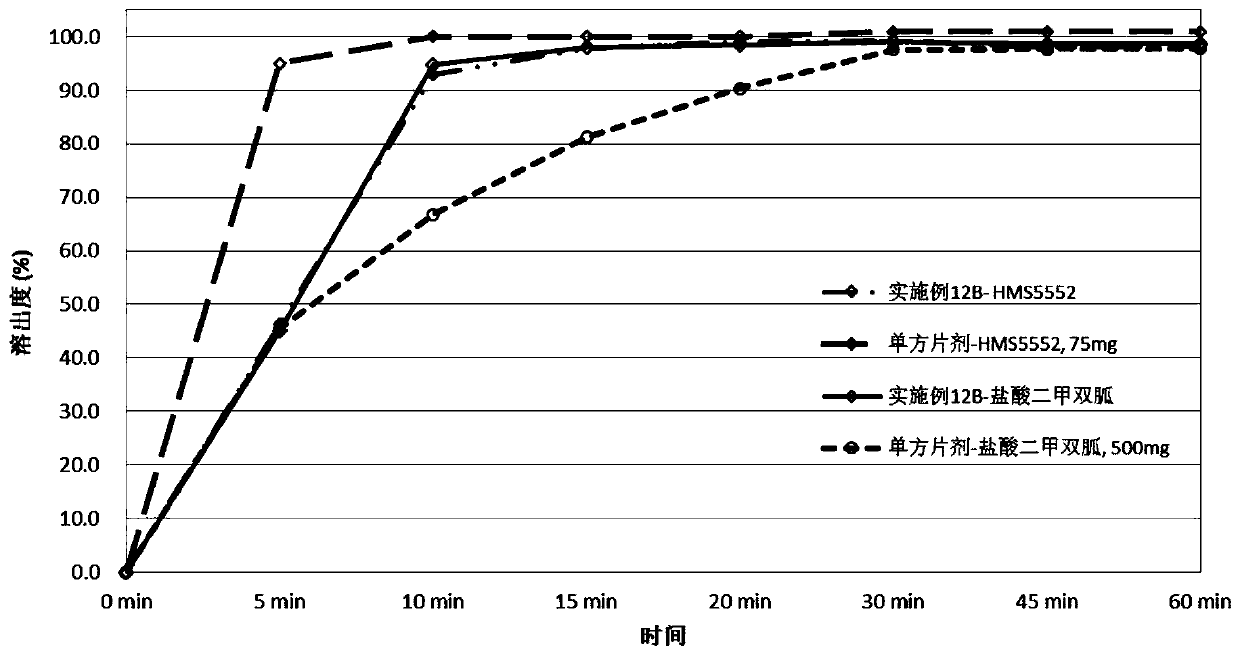
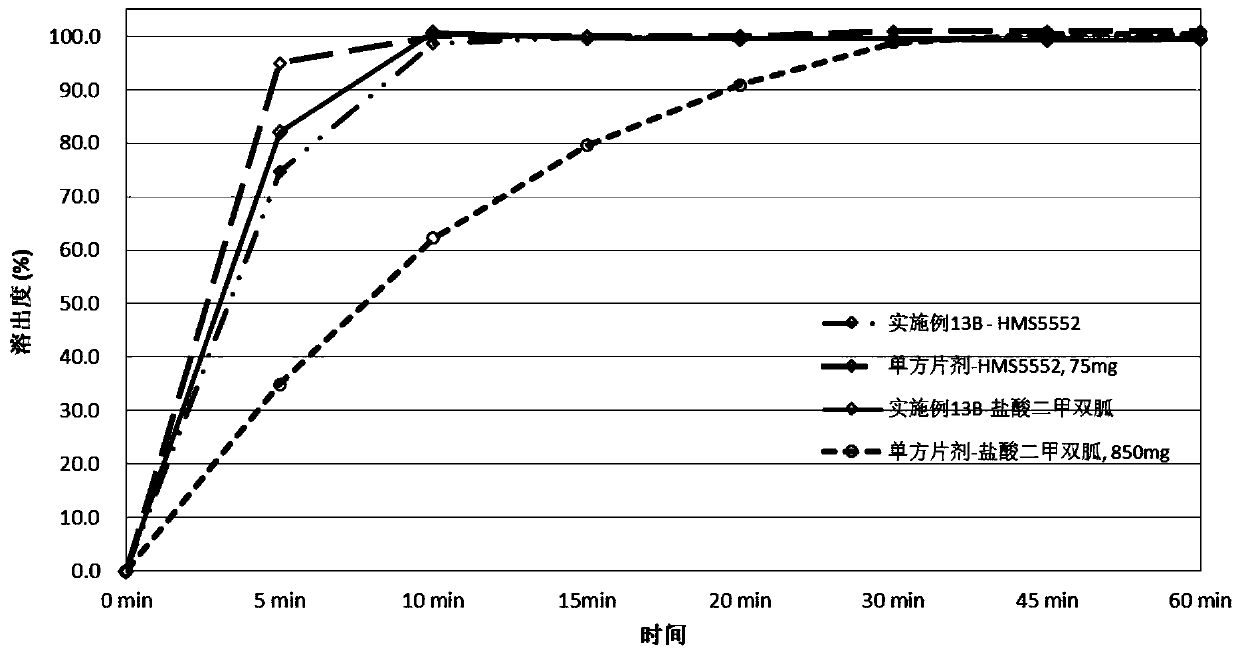
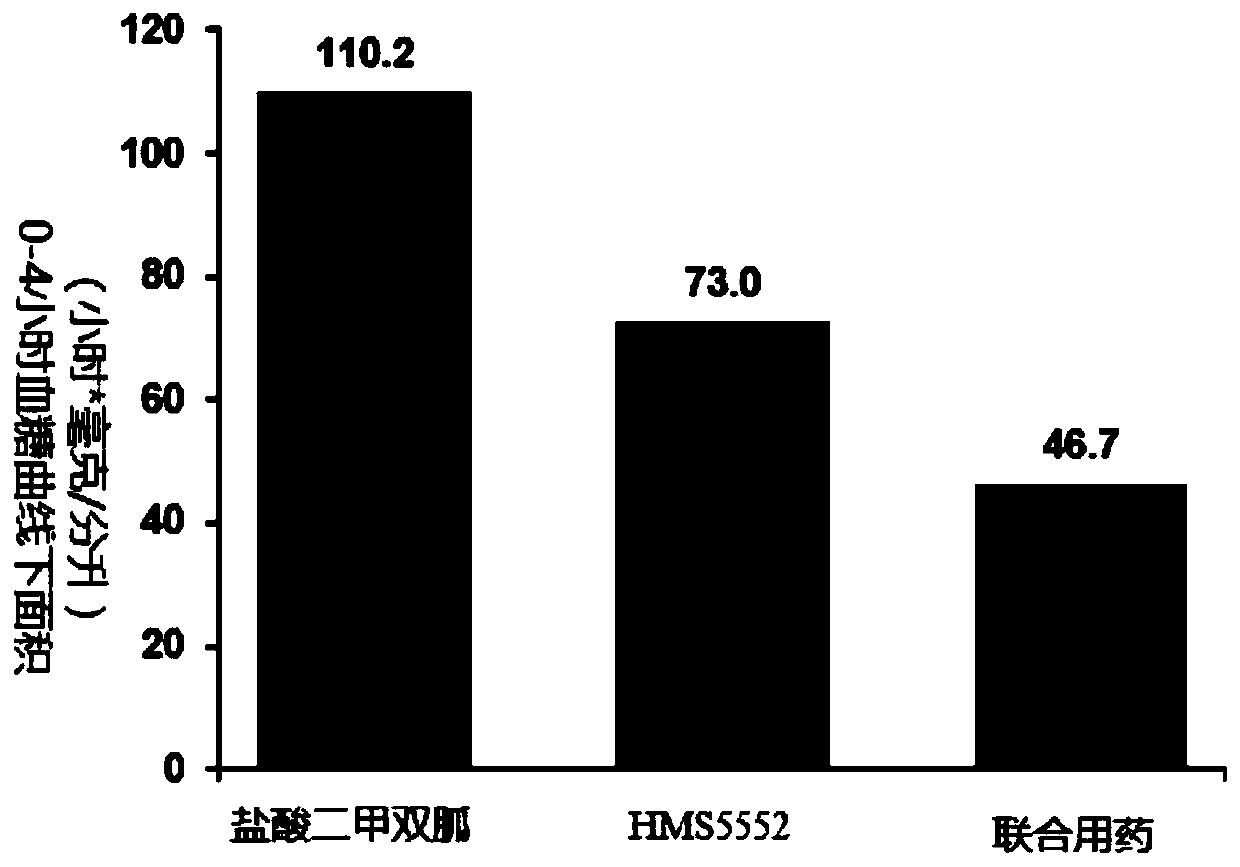
Drug combination containing glucokinase activator (GKA) and biguanide antidiabetic drug, composition and combination preparation and preparation methods and application thereof
A glucose-lowering technology of glucokinase and biguanides, which is applied in the field of oral solid preparations to treat and/or prevent one or more diseases and medical conditions, and can solve the problem of a single glucose-controlling organ, which cannot effectively treat the imbalance of blood glucose homeostasis balance system To achieve short disintegration time limit, treat diabetes and accompanying diseases and complications, and improve compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0352] Scheme 44. The method for preparing the pharmaceutical combination, pharmaceutical composition or fixed dose combination preparation according to scheme 43, wherein it is prepared by wet granulation (high shear and / or fluidized bed), or by dry processing (direct compression or dry process) pellets) preparation.
[0353] Item 45. The method for preparing a pharmaceutical combination, pharmaceutical composition or fixed dose combination according to any one of items 43-44, wherein the glucokinase activator is prepared in the form of a solid dispersion.
[0354] Scheme 46. The method for preparing a pharmaceutical combination, a pharmaceutical composition or a fixed-dose compound preparation according to any one of Scheme 43-45, wherein the glucokinase activator and the second or more active ingredients can also be prepared together into a compound solid Dispersion forms (ie solid dispersions containing 2 or more active ingredients).
[0355] Scheme 47. The pharmaceutical...
Embodiment
[0360] Preparation of compound tablet of glucokinase activator
[0361] The chemicals used in the present invention can be purchased from companies such as Shin-Etsu Japan, Evonik Germany, J.T. Baker US, SCR China, Ashland US, FMC US, JRS Germany, Colorcon US, Capsugel, BASF, revitalizing reagents, etc. Production equipment and analytical testing instruments etc. can be purchased from such companies as Sartorius, Nikon, Sympatec, Bruker, Gea Niro, Korsch, Erweka, Agilent, Quadro Engineering, Canada; Warters, US; TA, US; SOTAX, Switzerland; Mettler Toledo Instrument Newark, DE.
[0362] I. Preparation of Solid Dispersion of Glucokinase Activator
[0363] 1.1 Preparation of solid dispersion solution before spray drying
[0364] Example 1A (the weight ratio of active ingredient to polymer is 1:9)
[0365] Weigh 6.75 grams of Eudragit L100 (Evonik L100, Evonik Germany), add it to absolute ethanol (J.T.Baker) under stirring conditions, add 0.75 grams of compound HMS5552 after it...
Embodiment 2A
[0366] Example 2A (the weight ratio of active ingredient to polymer is 3:7)
[0367] Weigh 5.25 grams of Eudragit L100 (Evonik L100, Evonik Germany), add it into dehydrated ethanol (J.T.Baker) under stirring conditions, add 2.25 grams of compound HMS5552 after it is completely dissolved, add a sufficient amount of dehydrated ethanol and continue stirring to obtain 50ml solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com