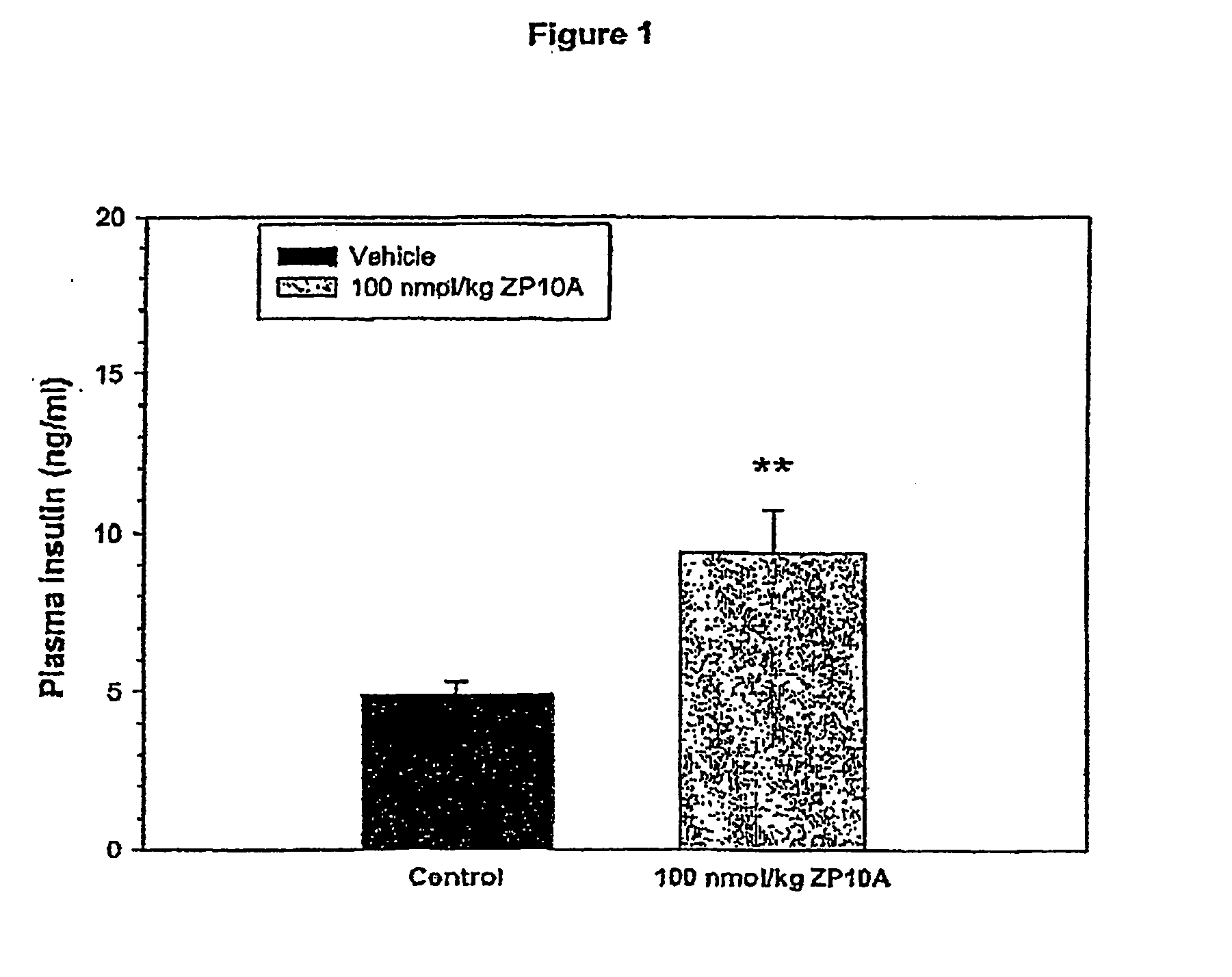
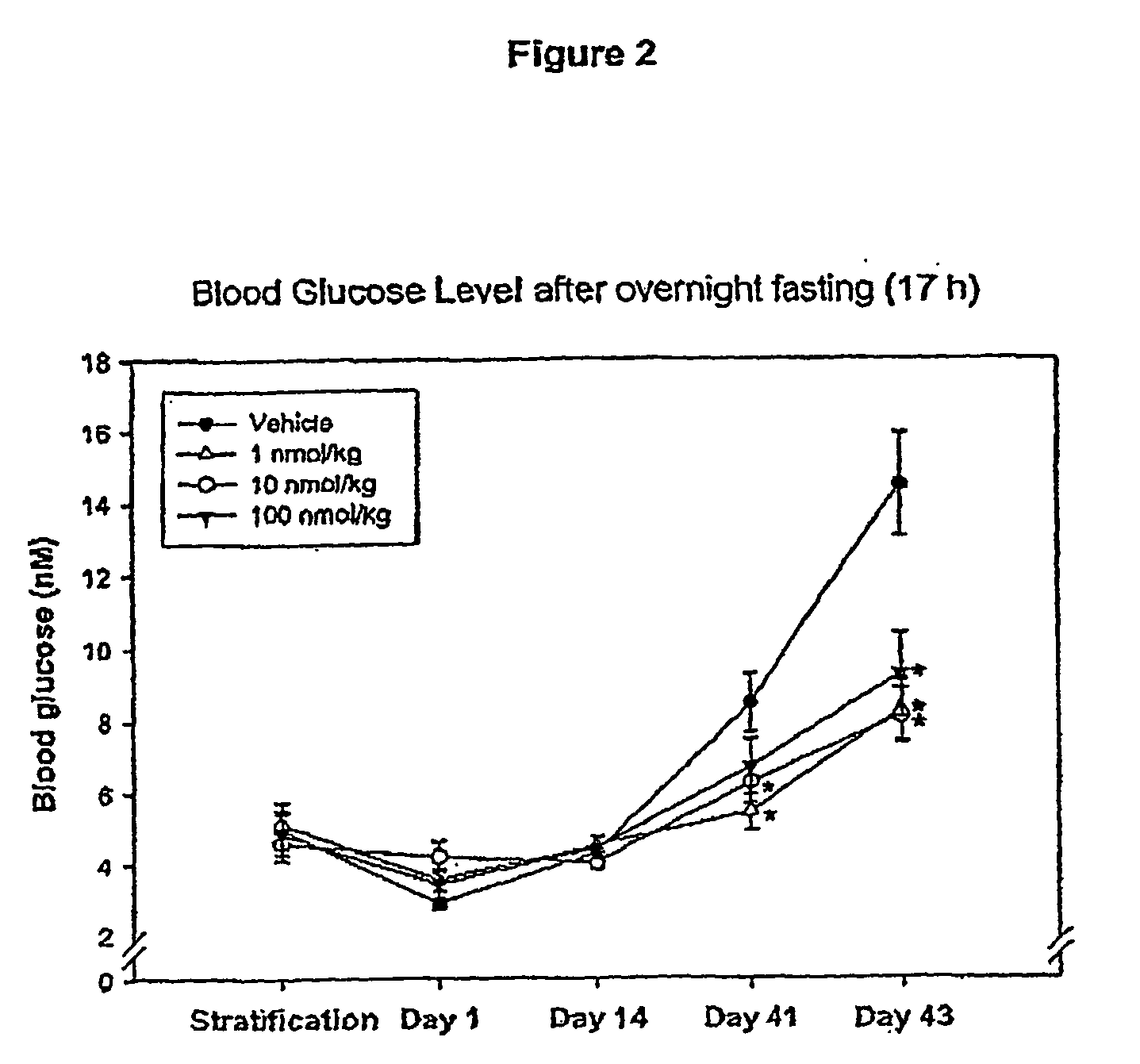
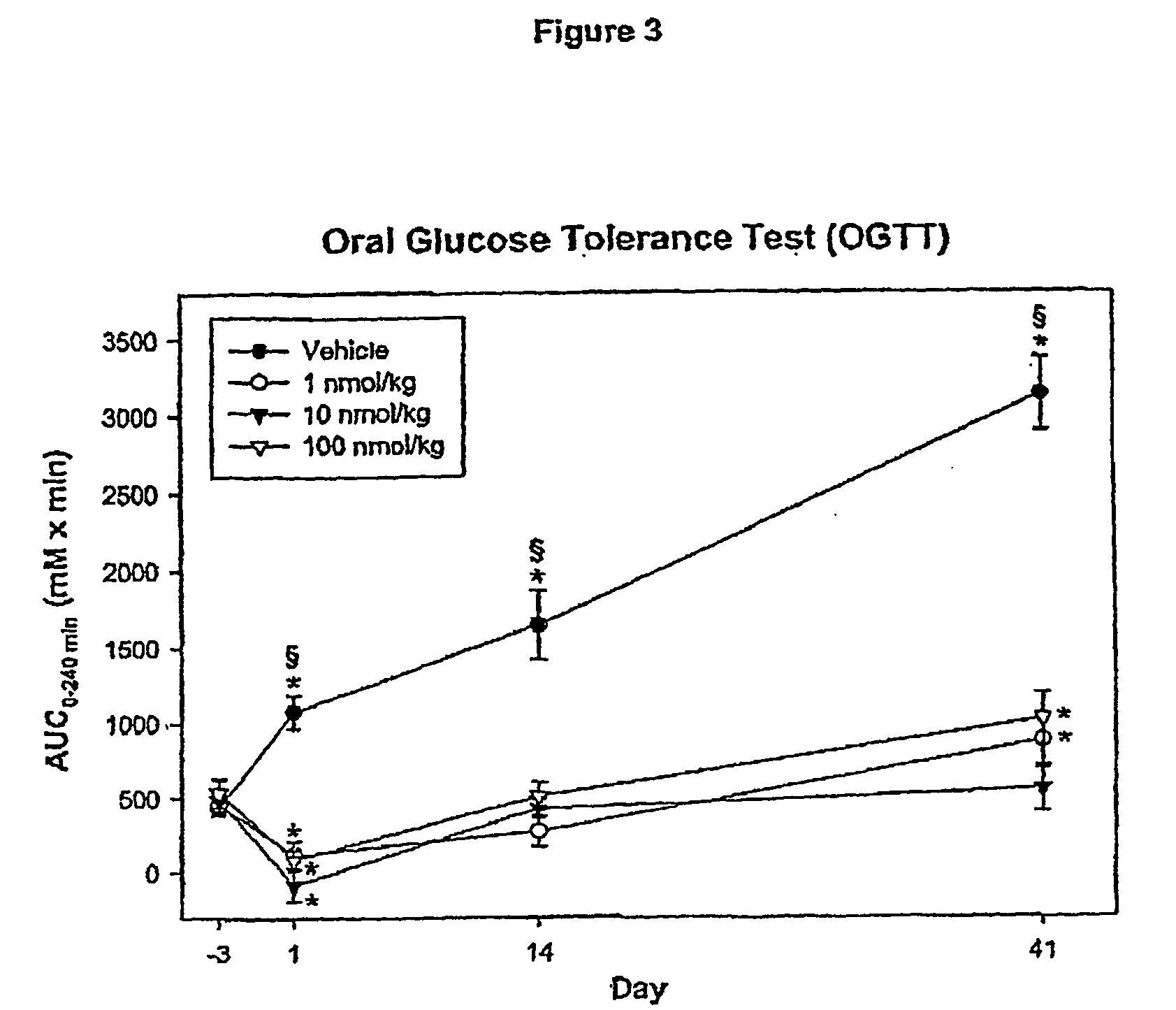
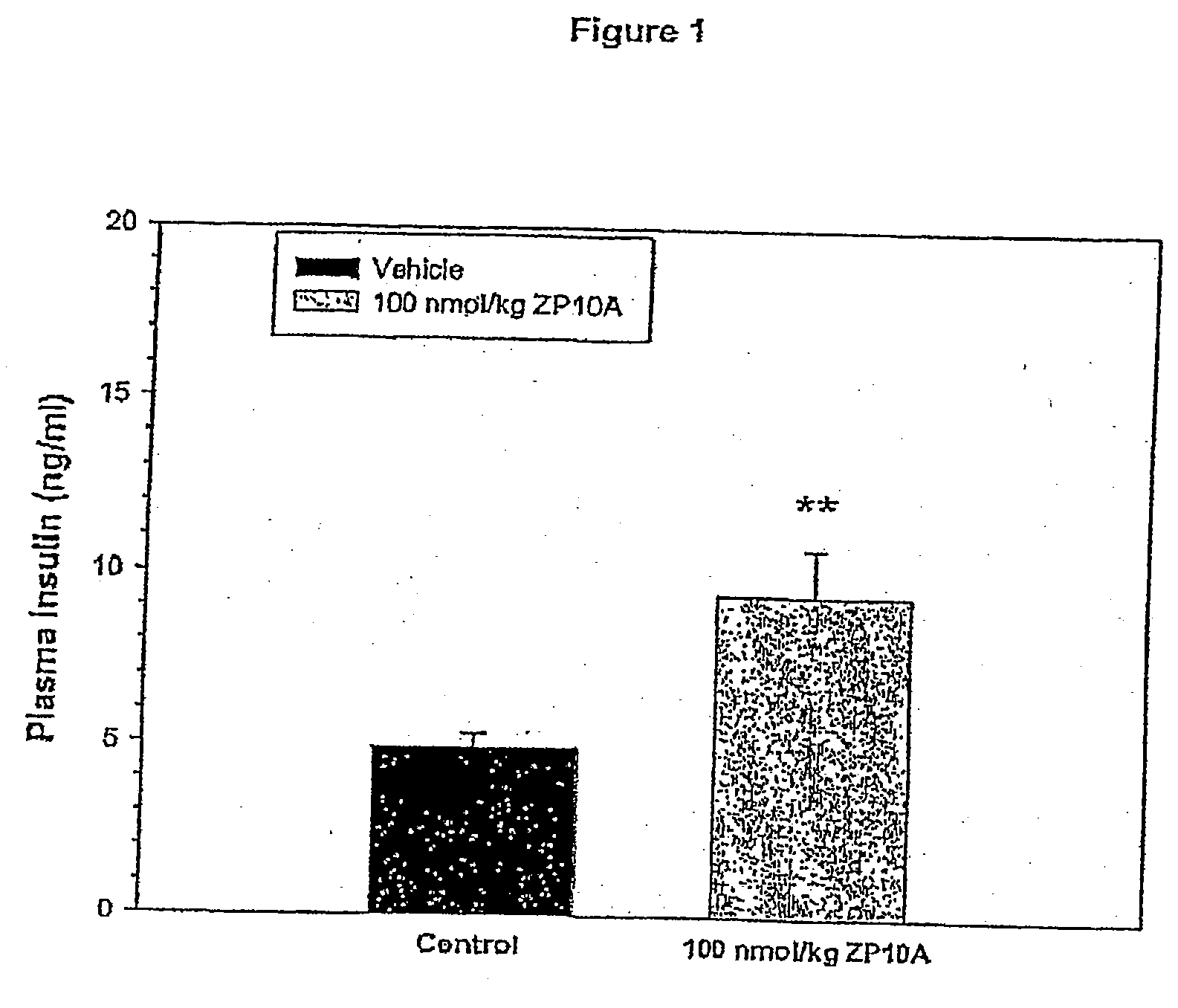
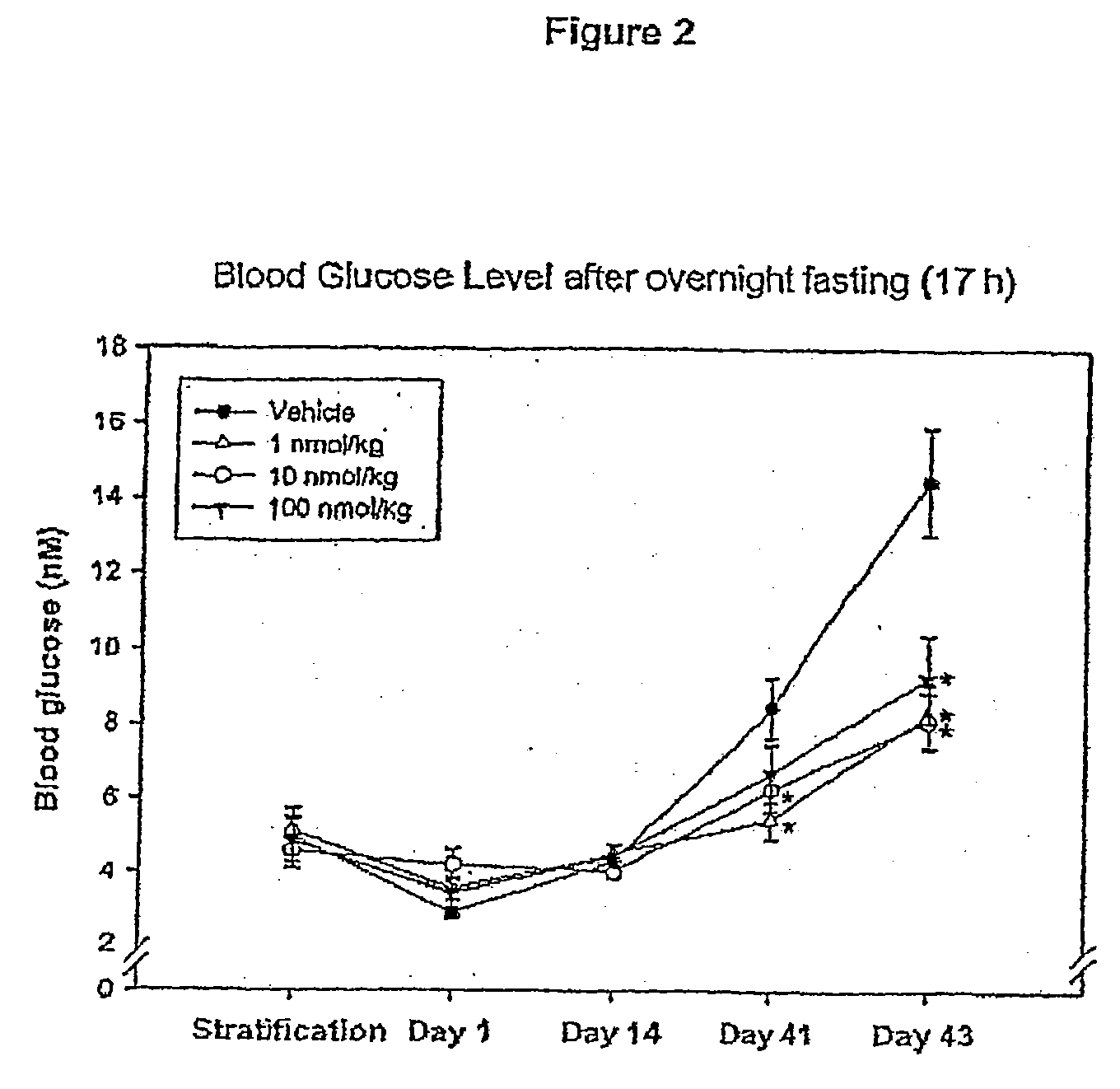
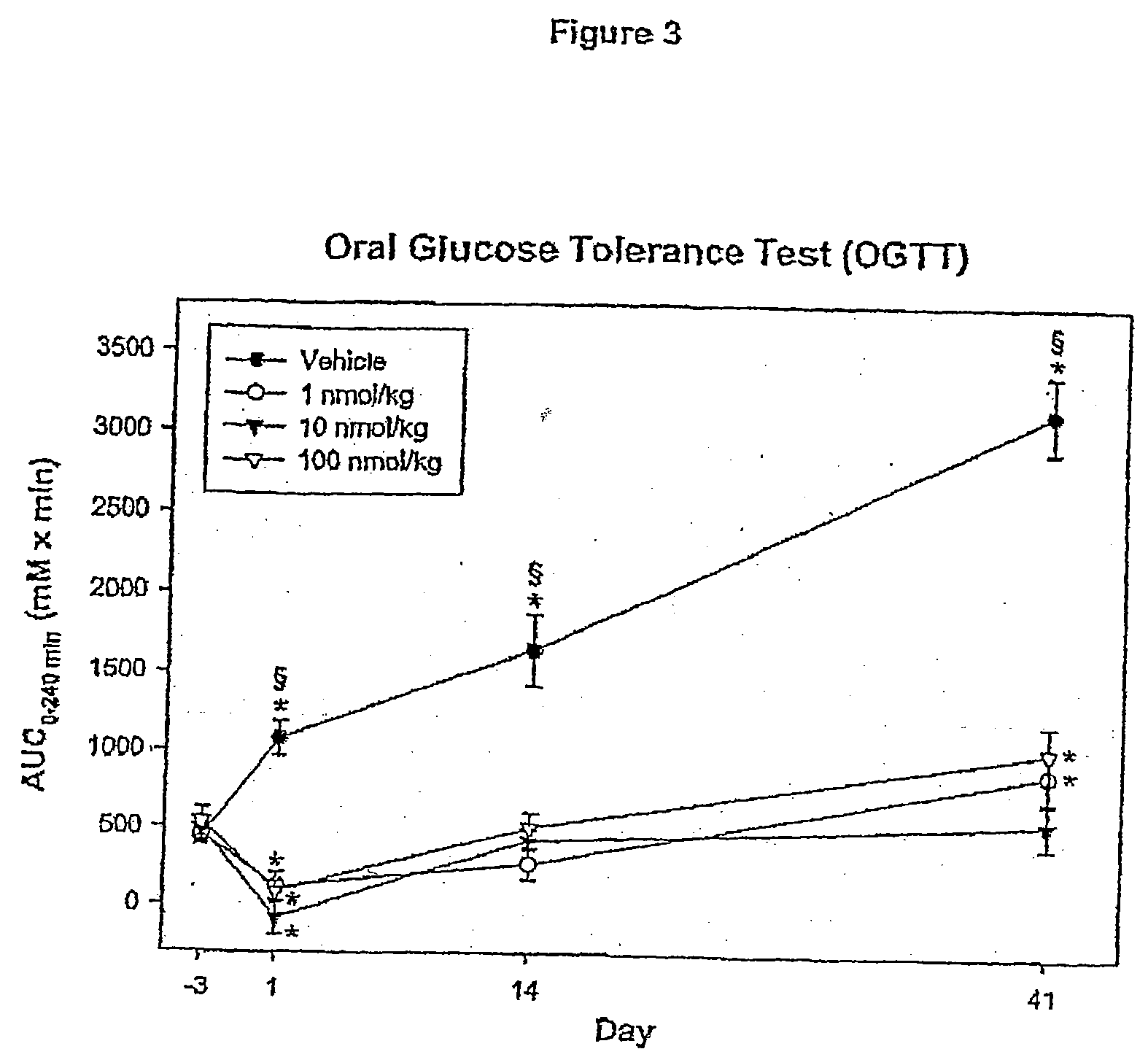
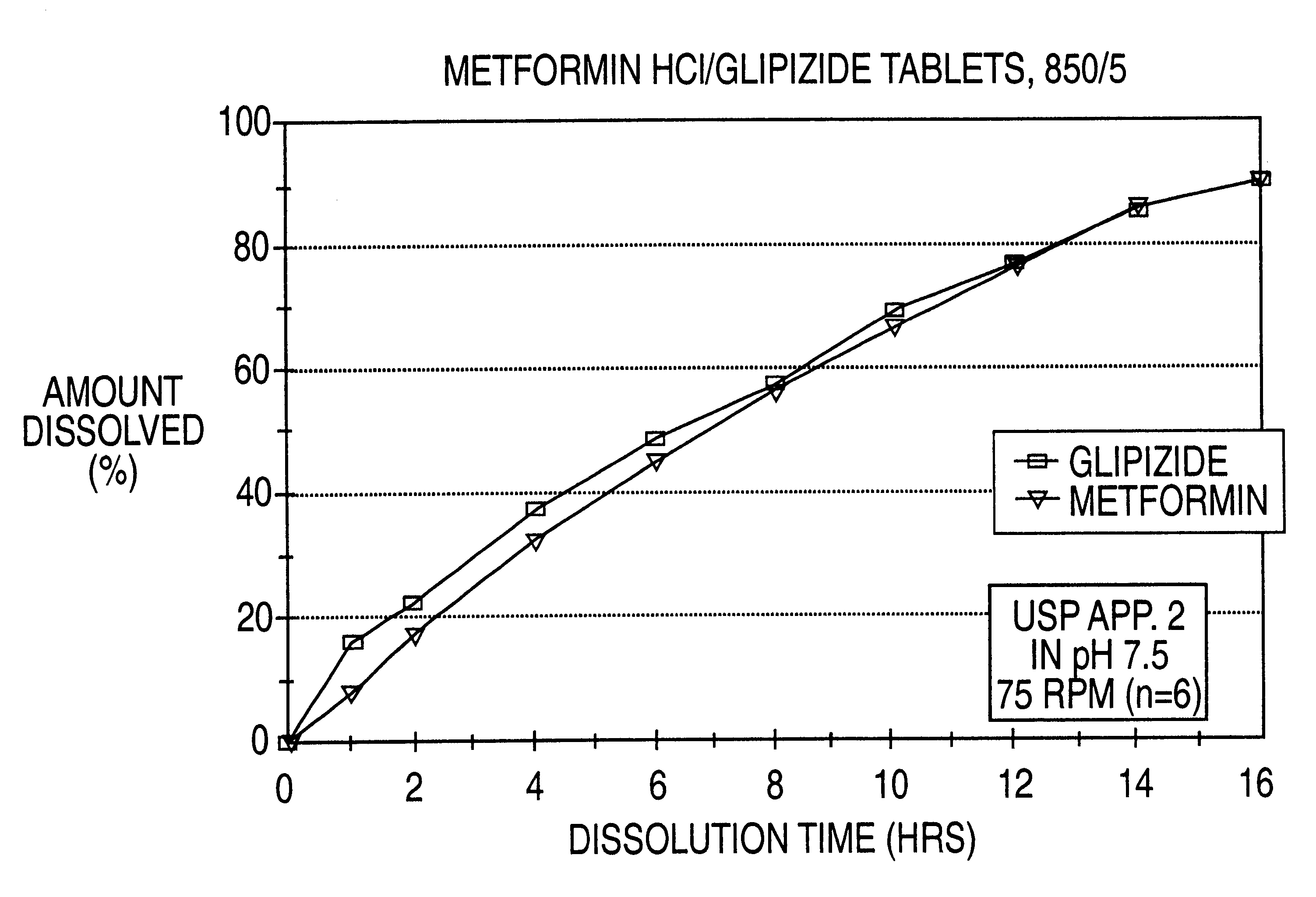
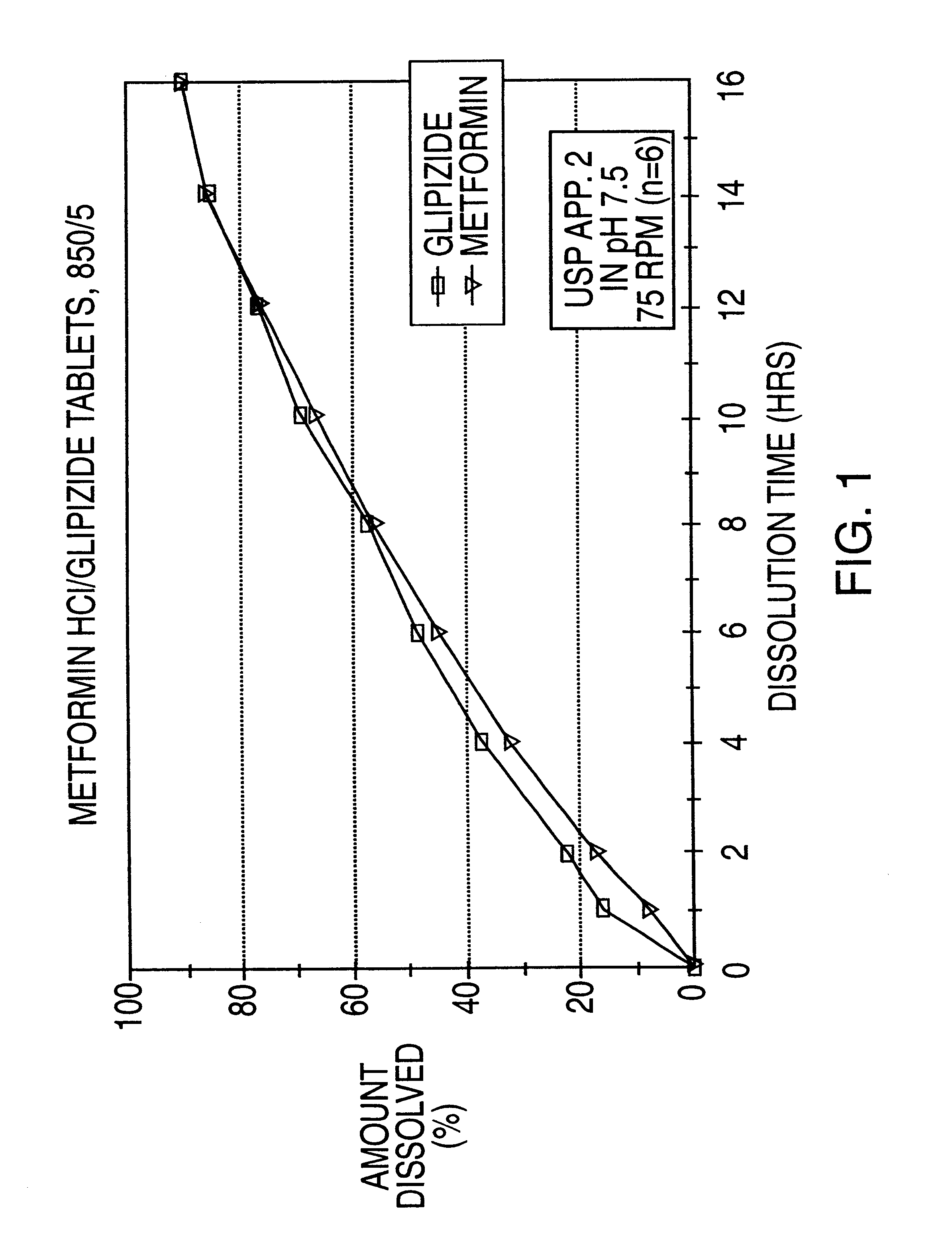
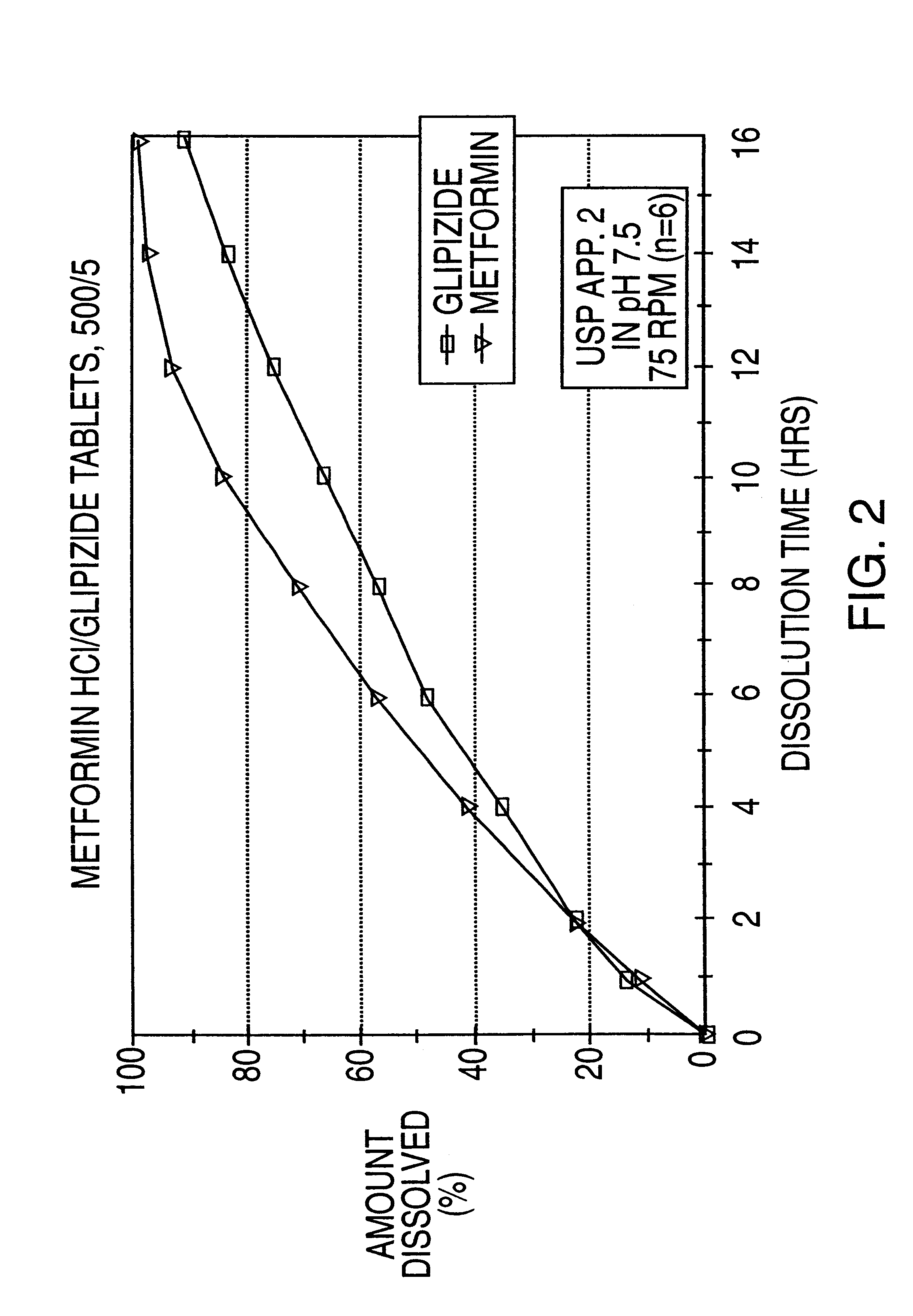
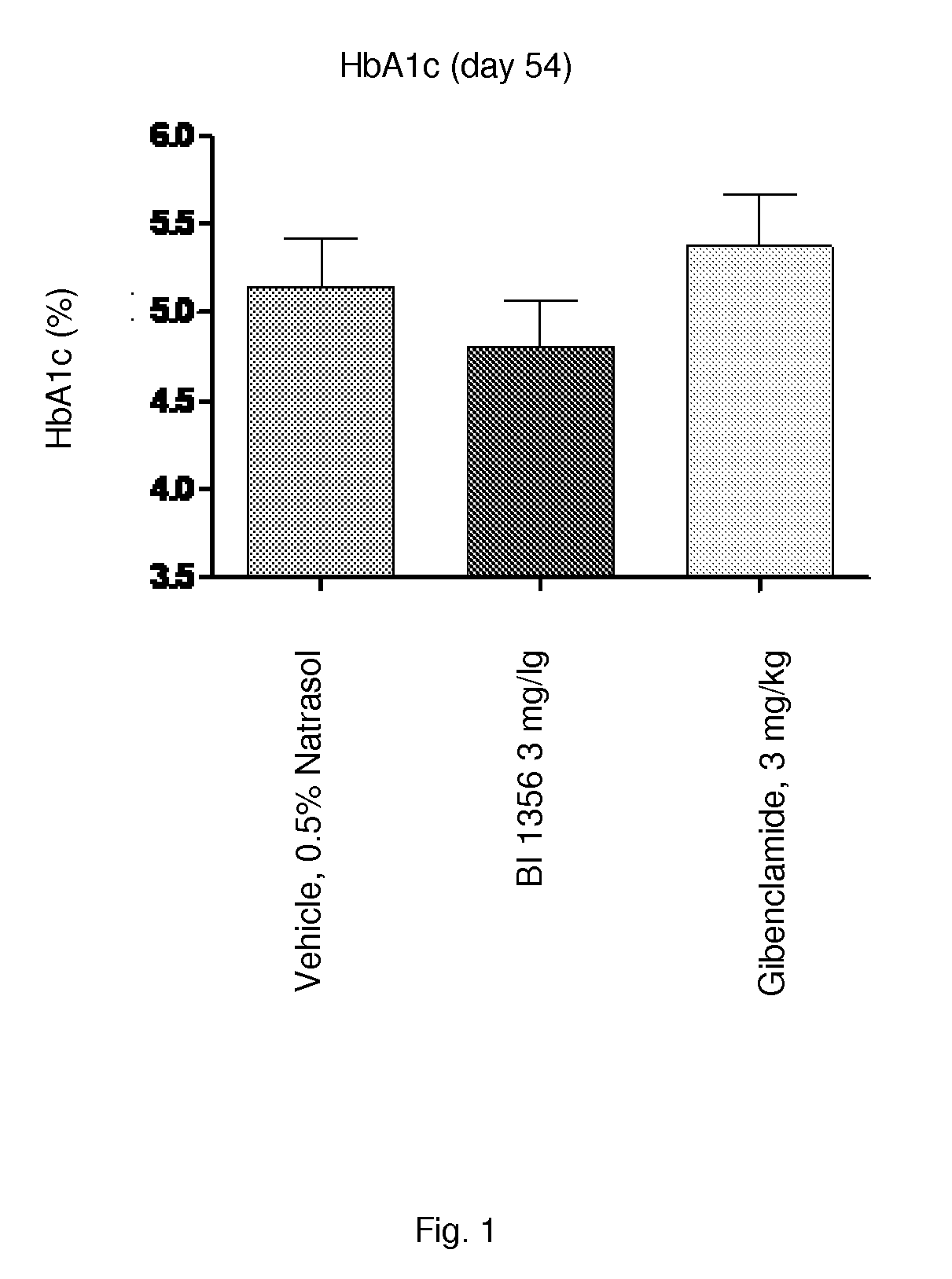
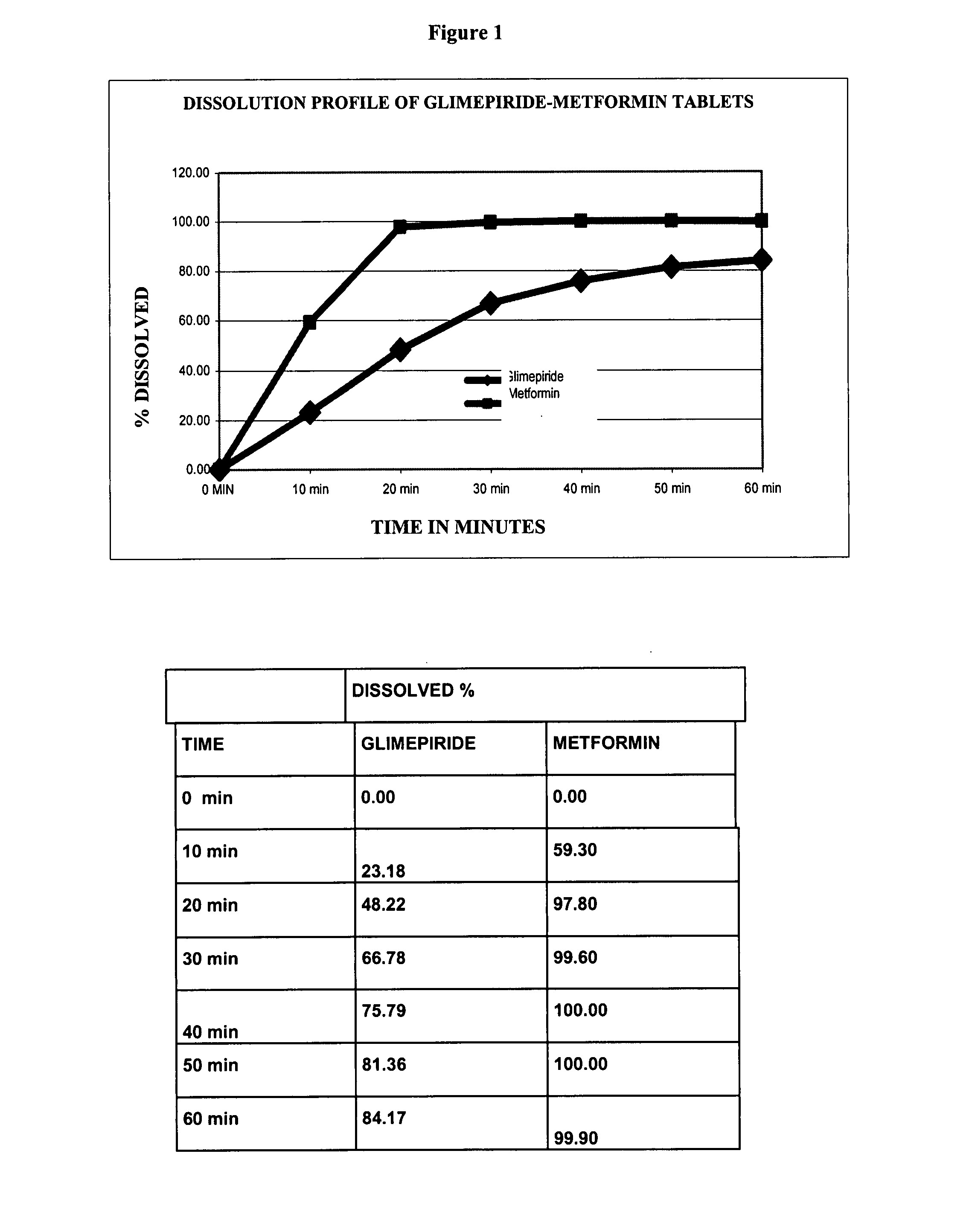
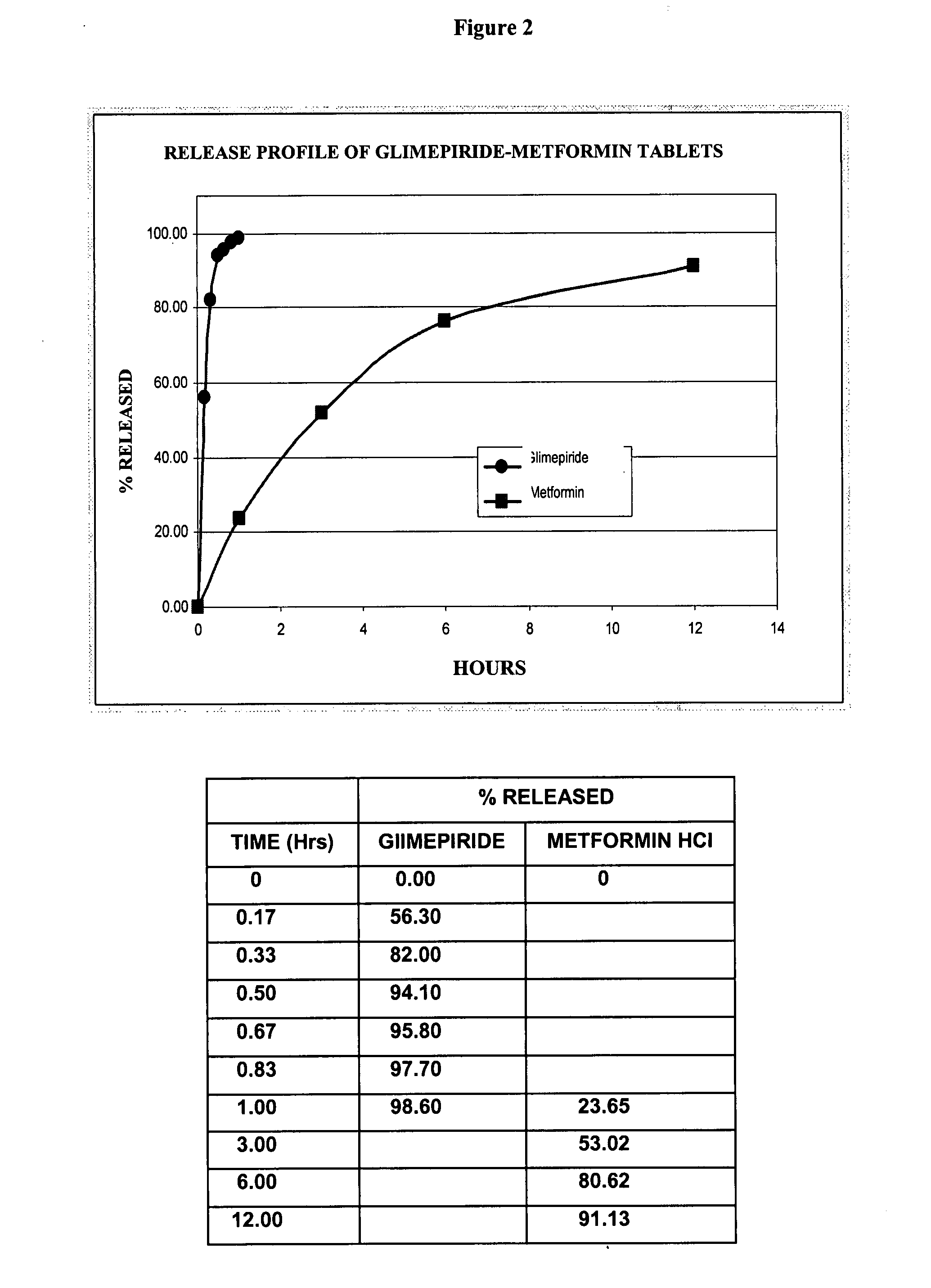
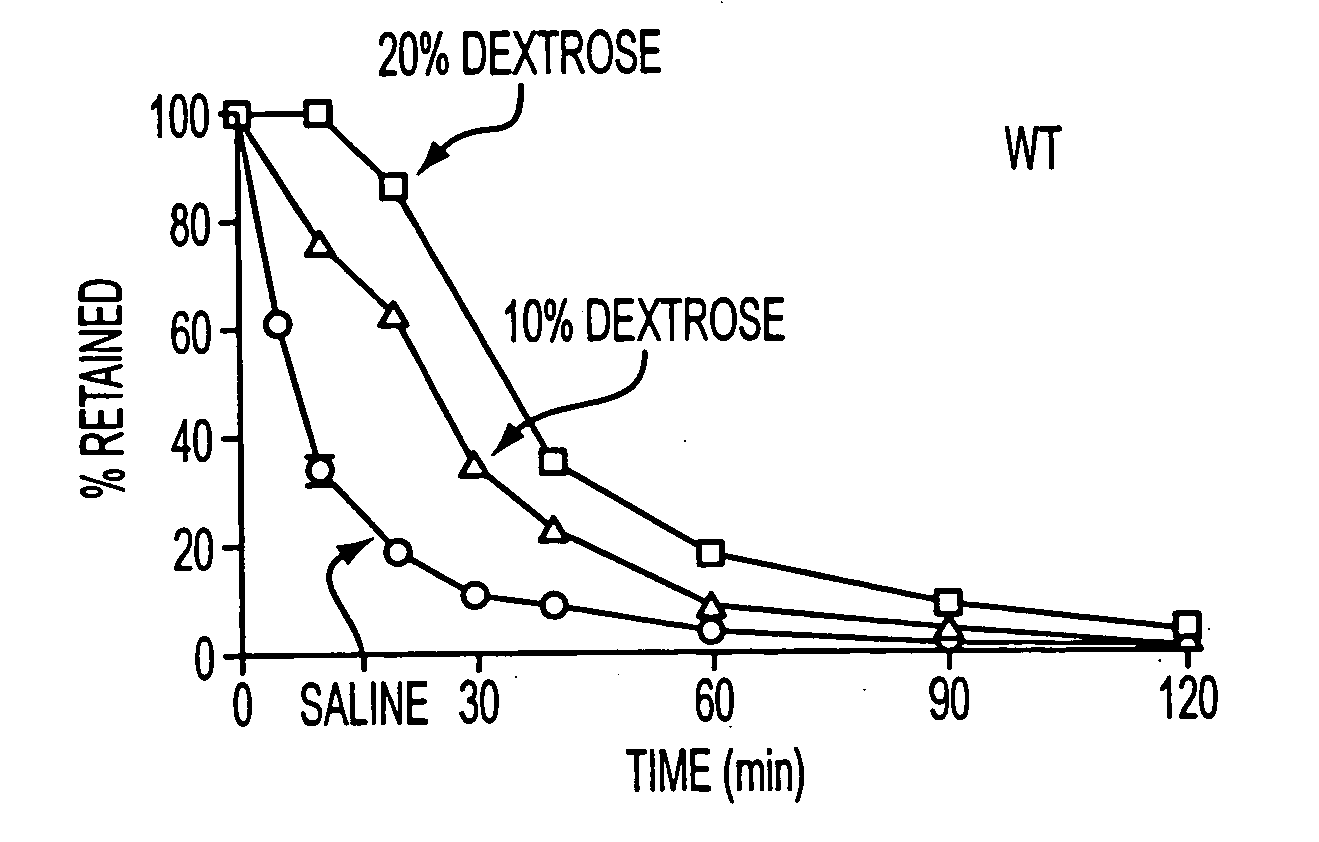
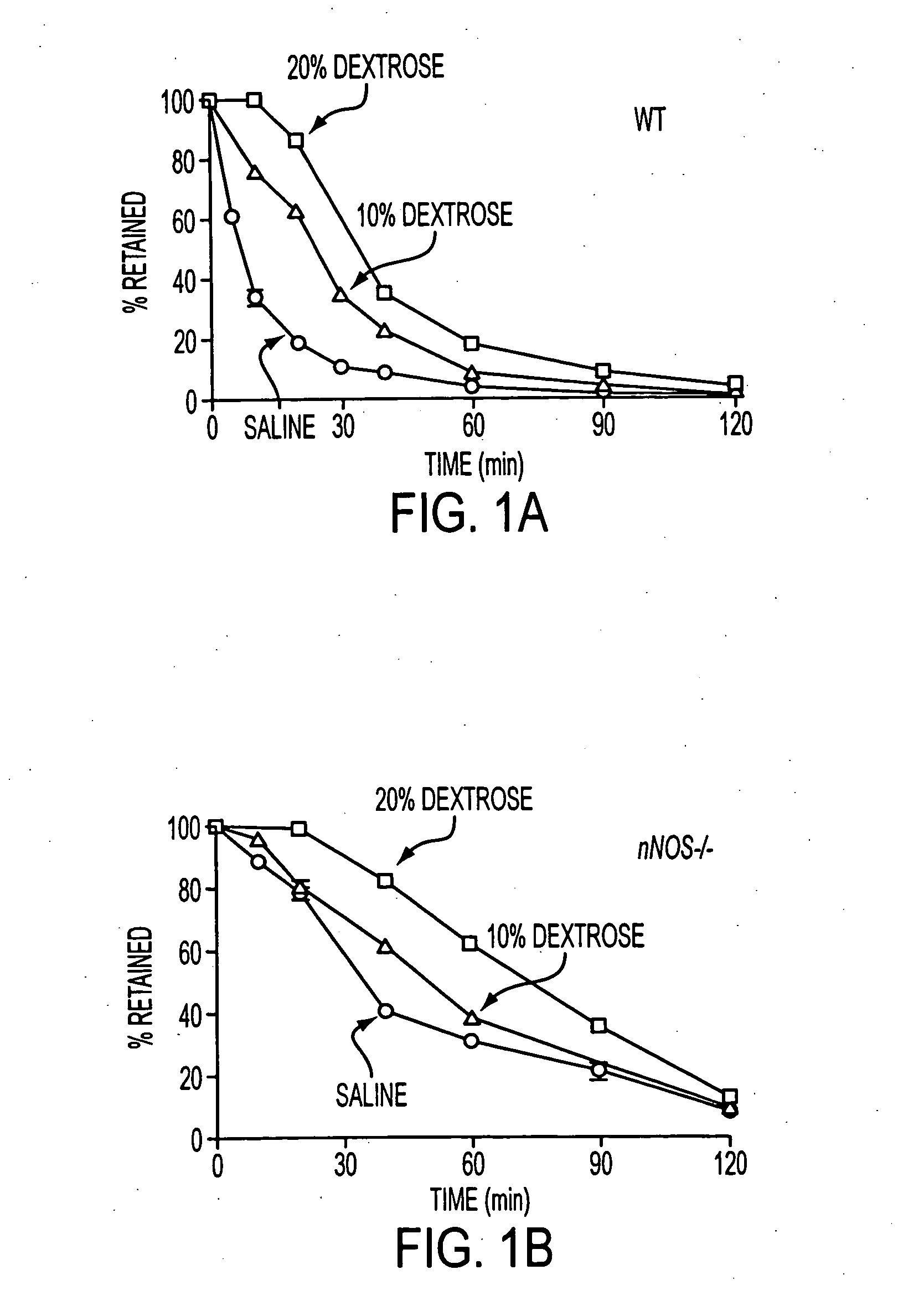
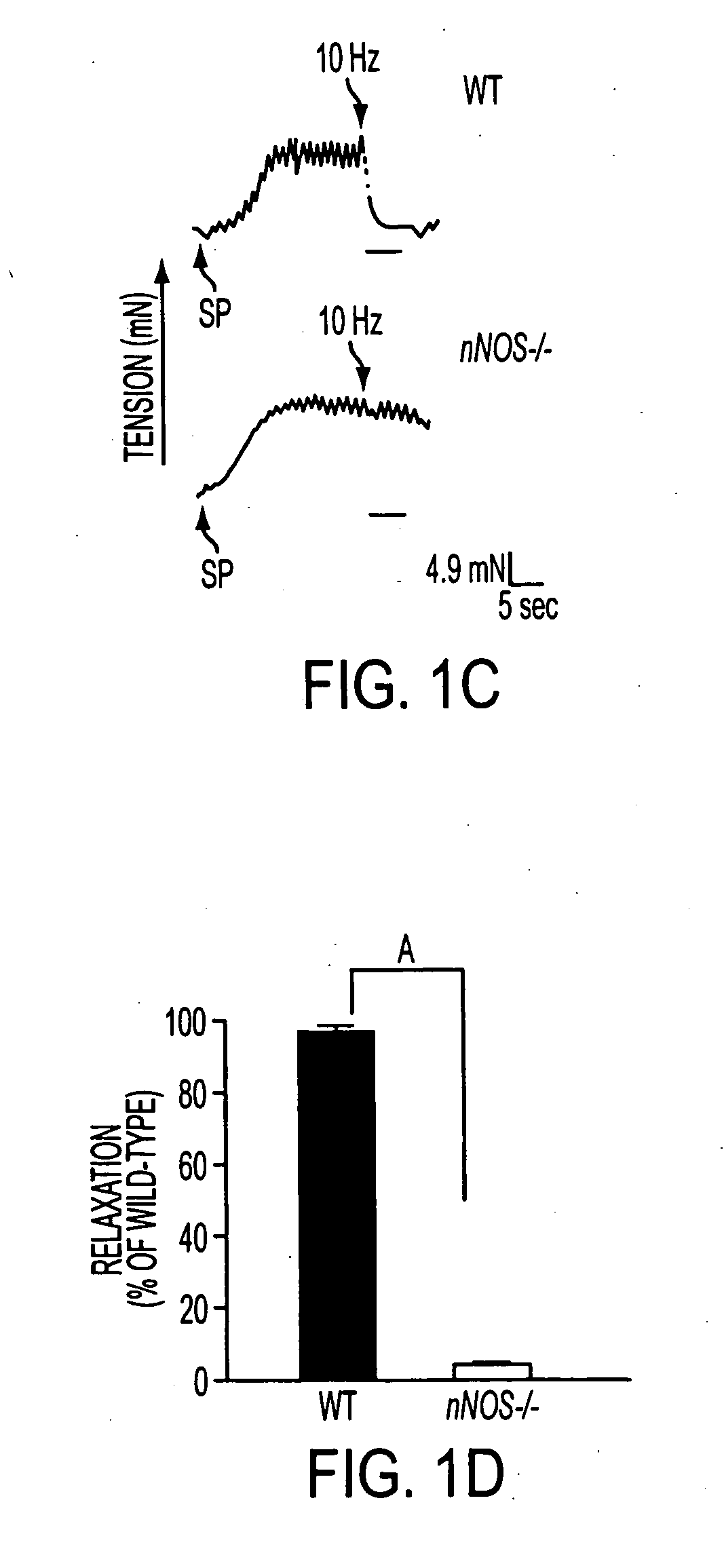
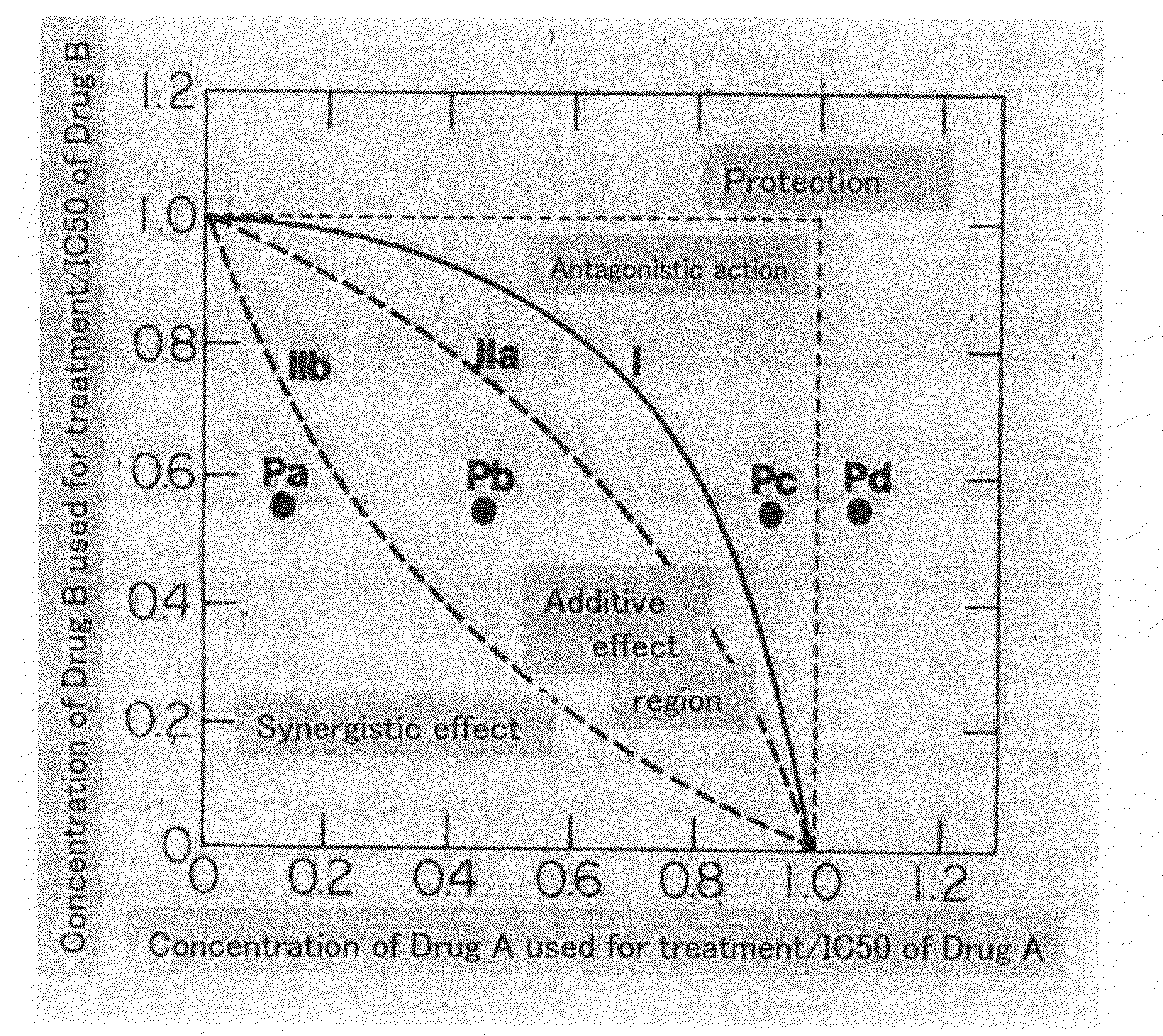
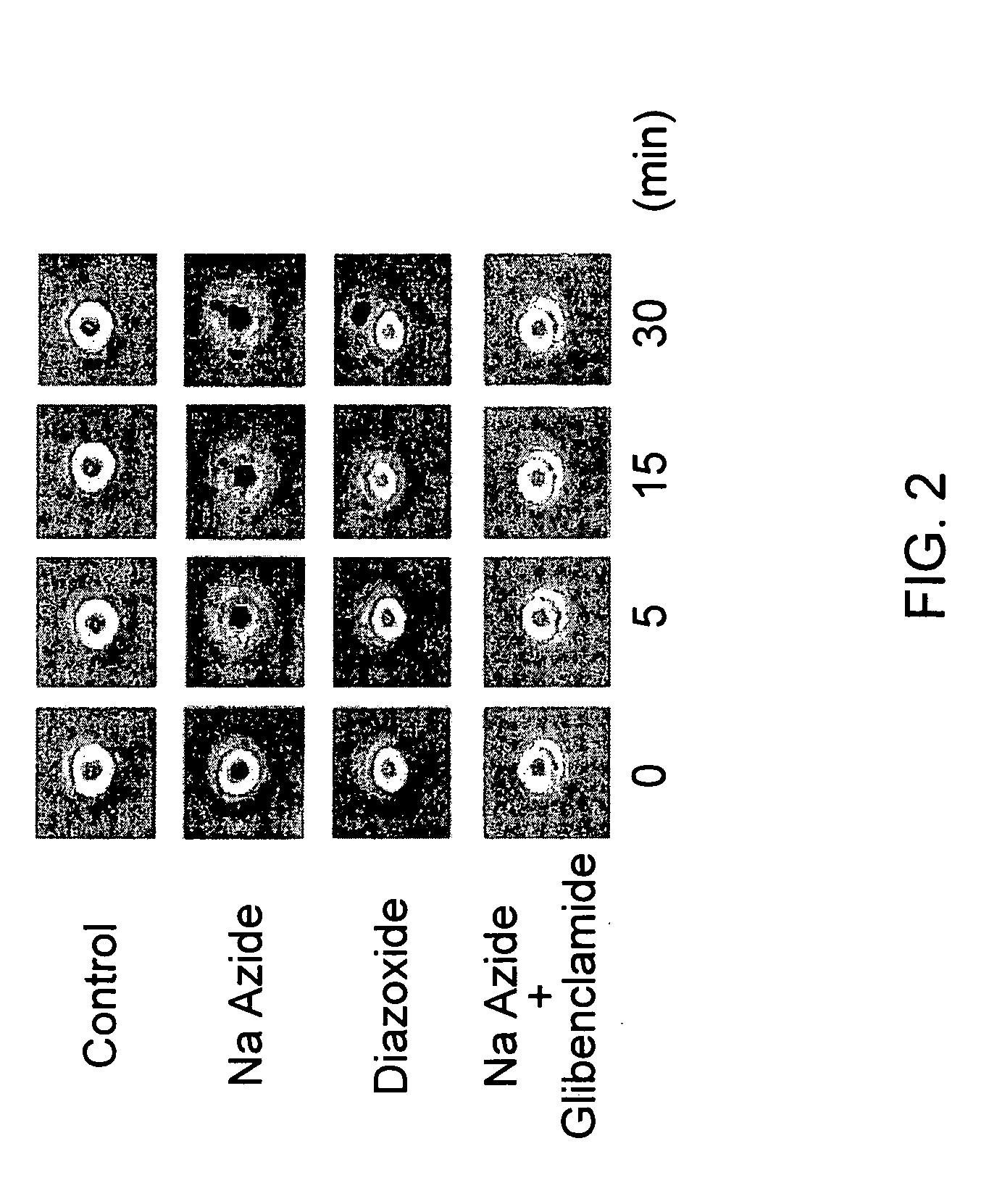
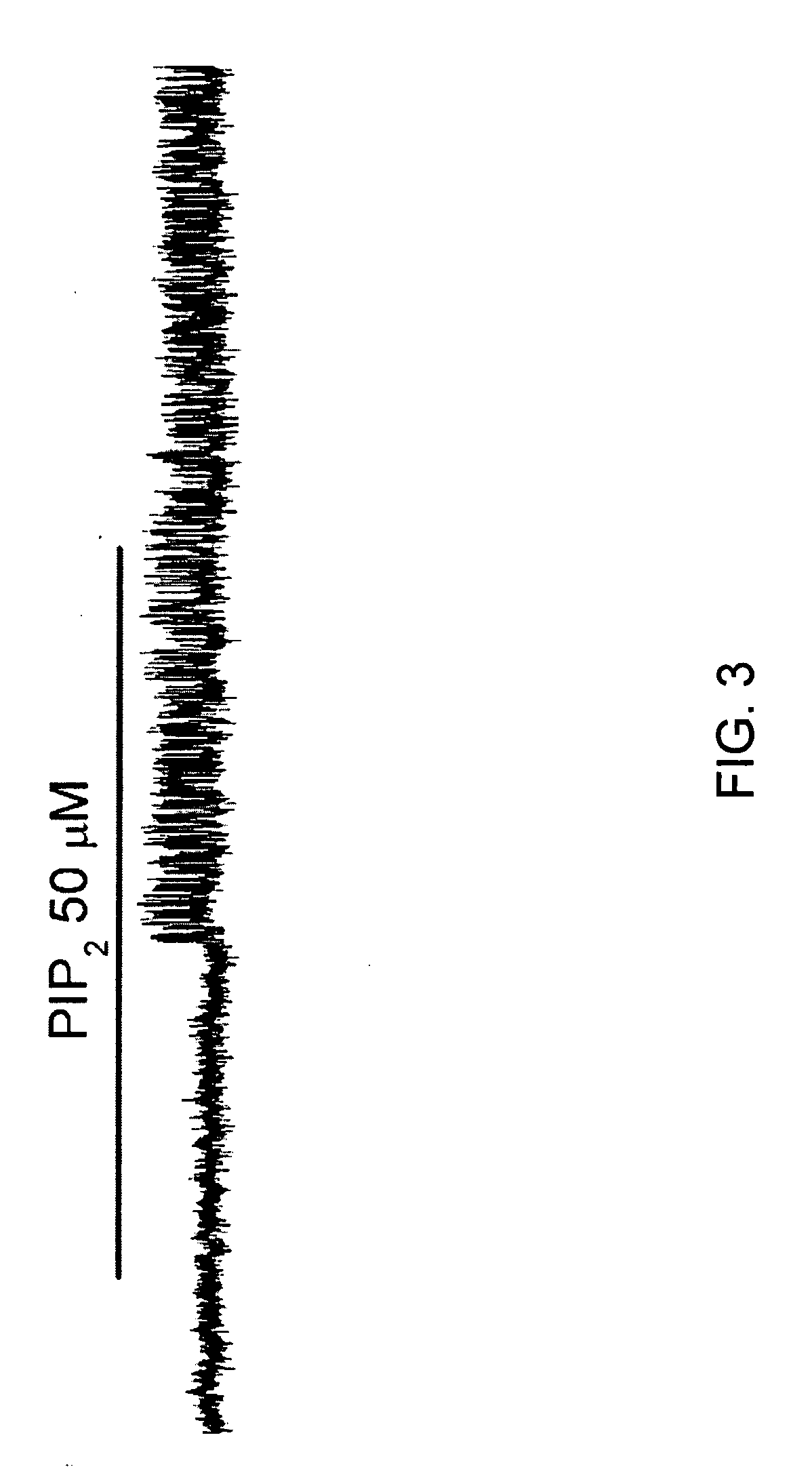
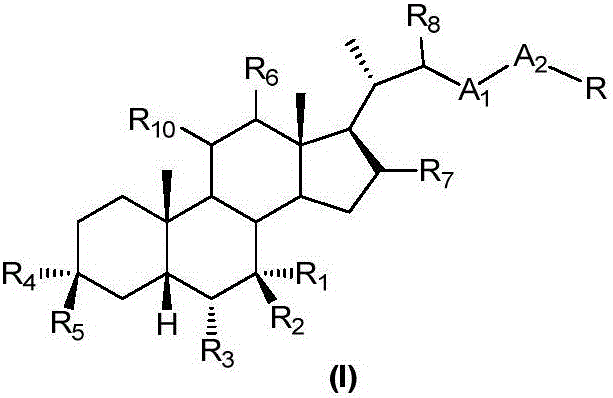
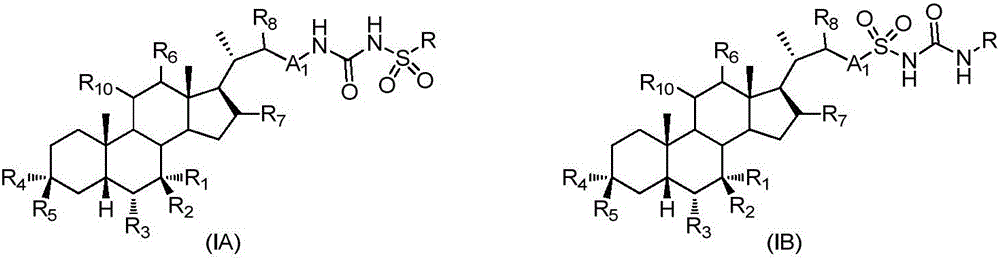
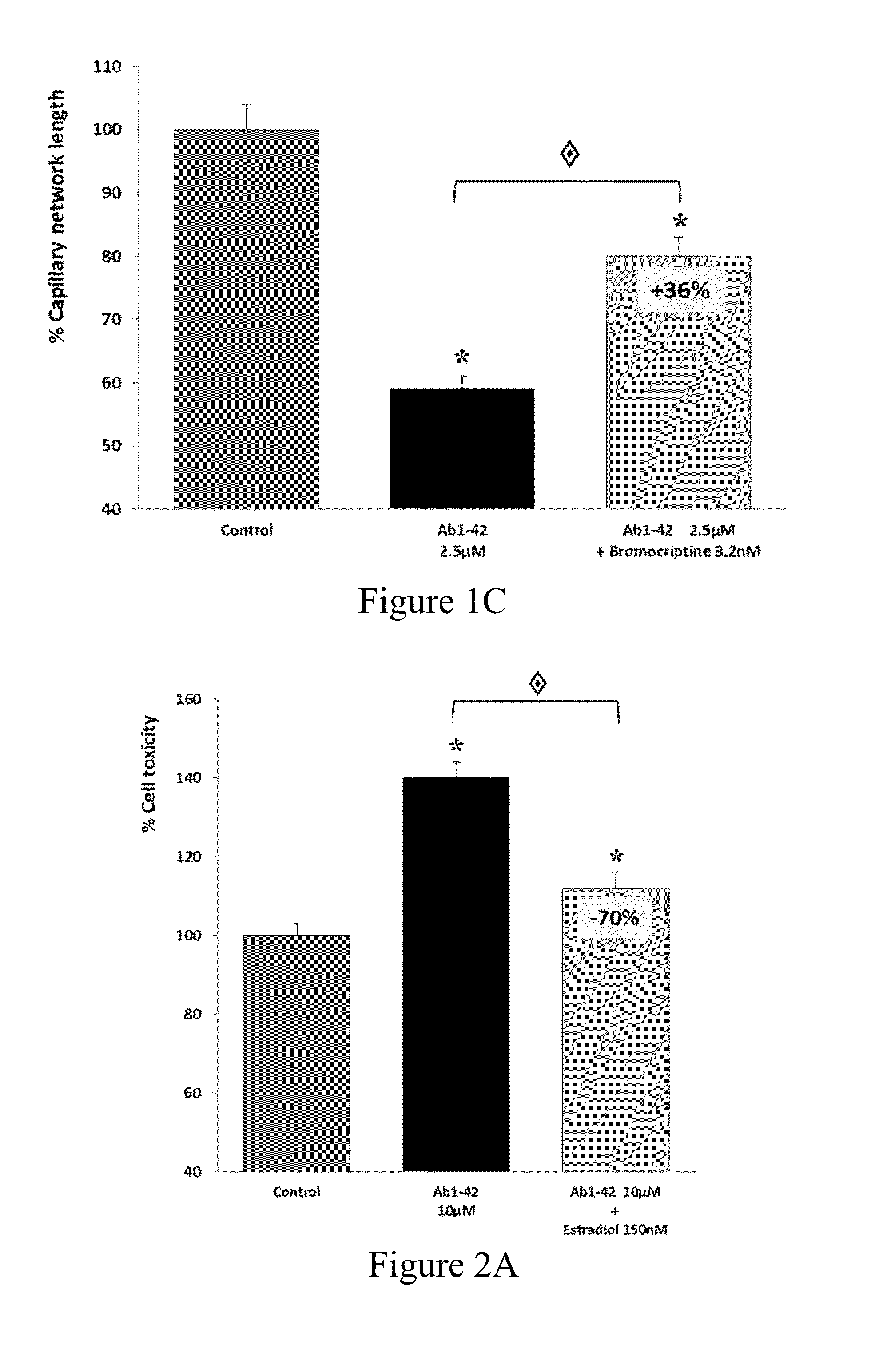
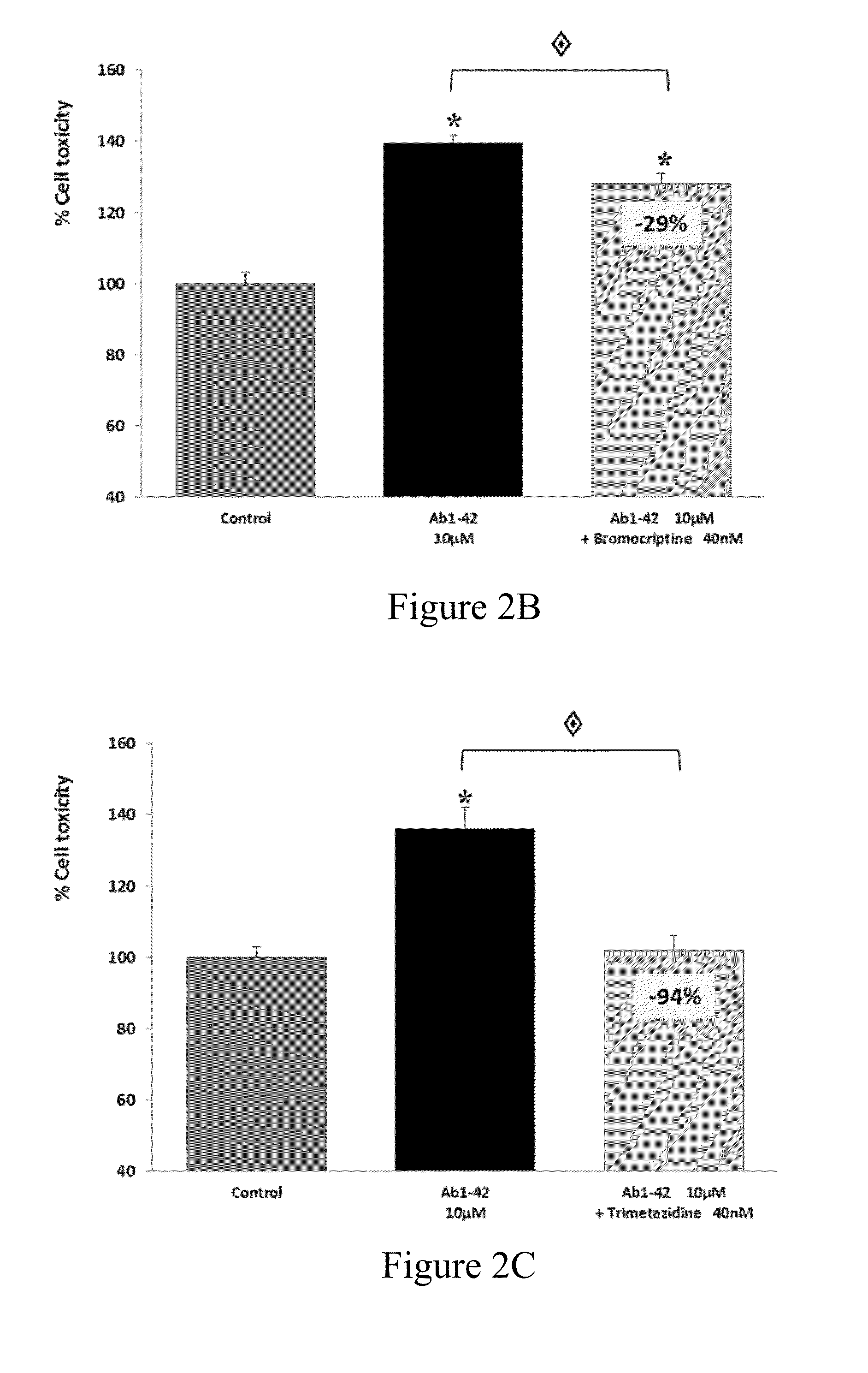
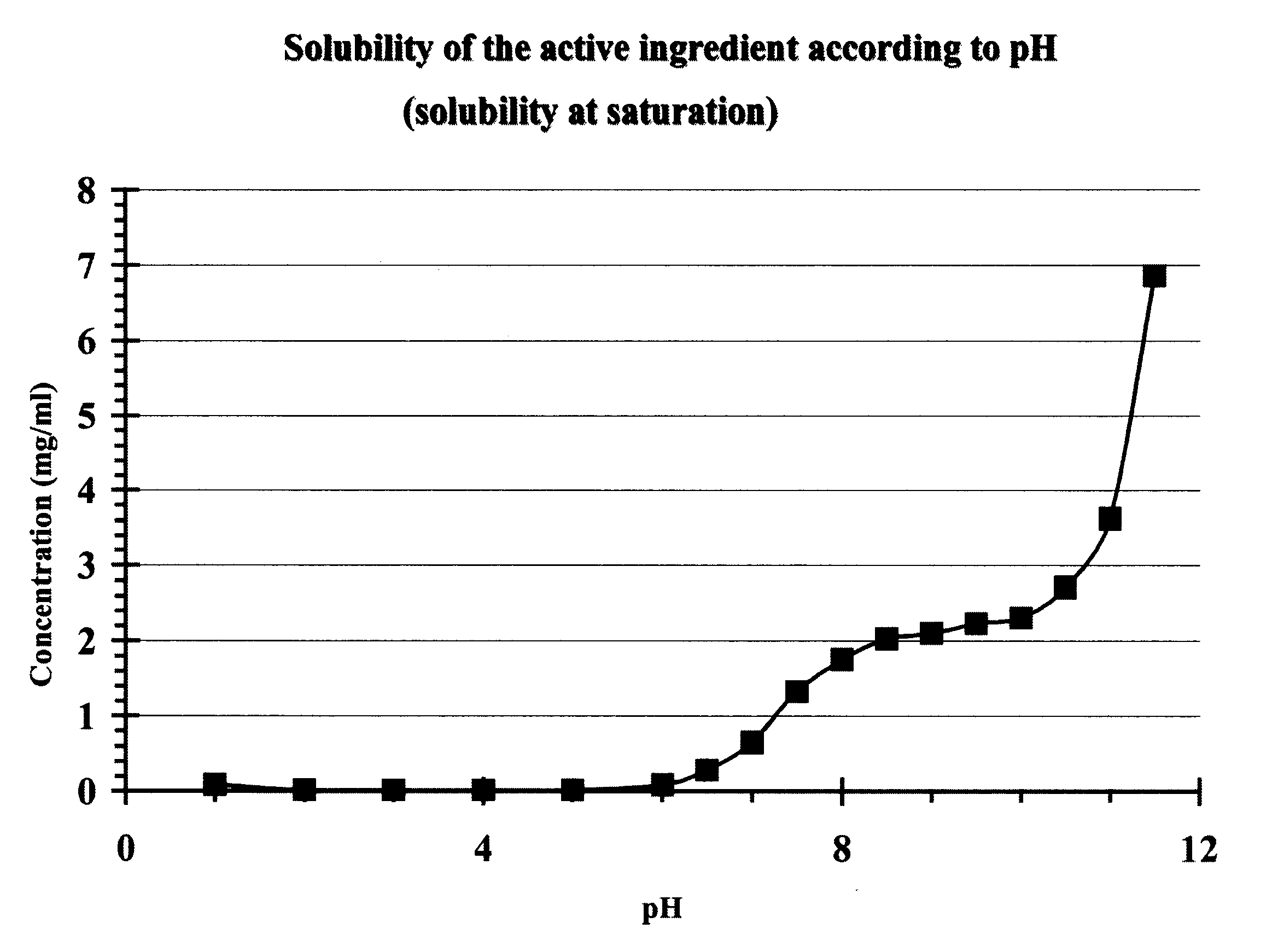
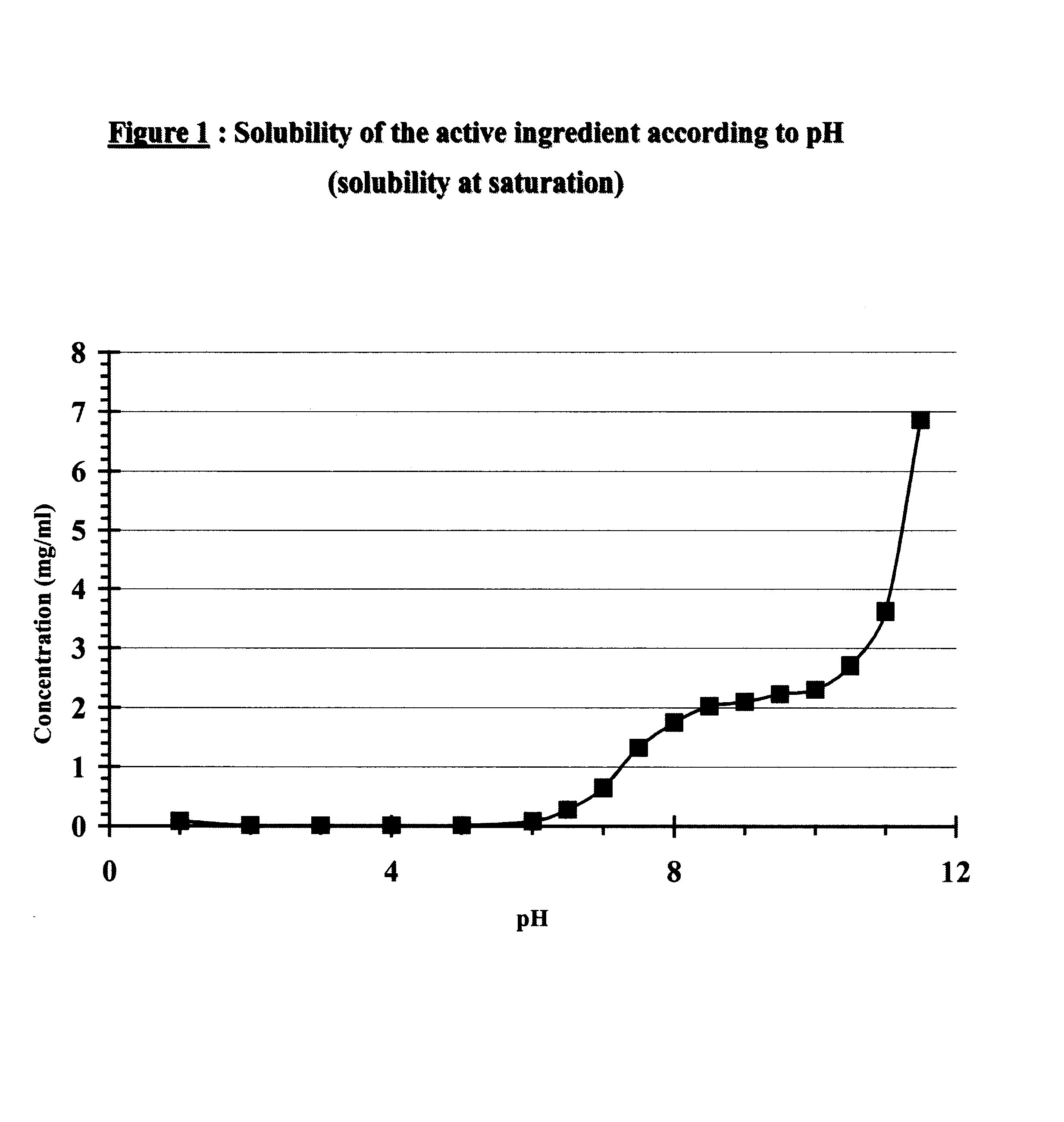
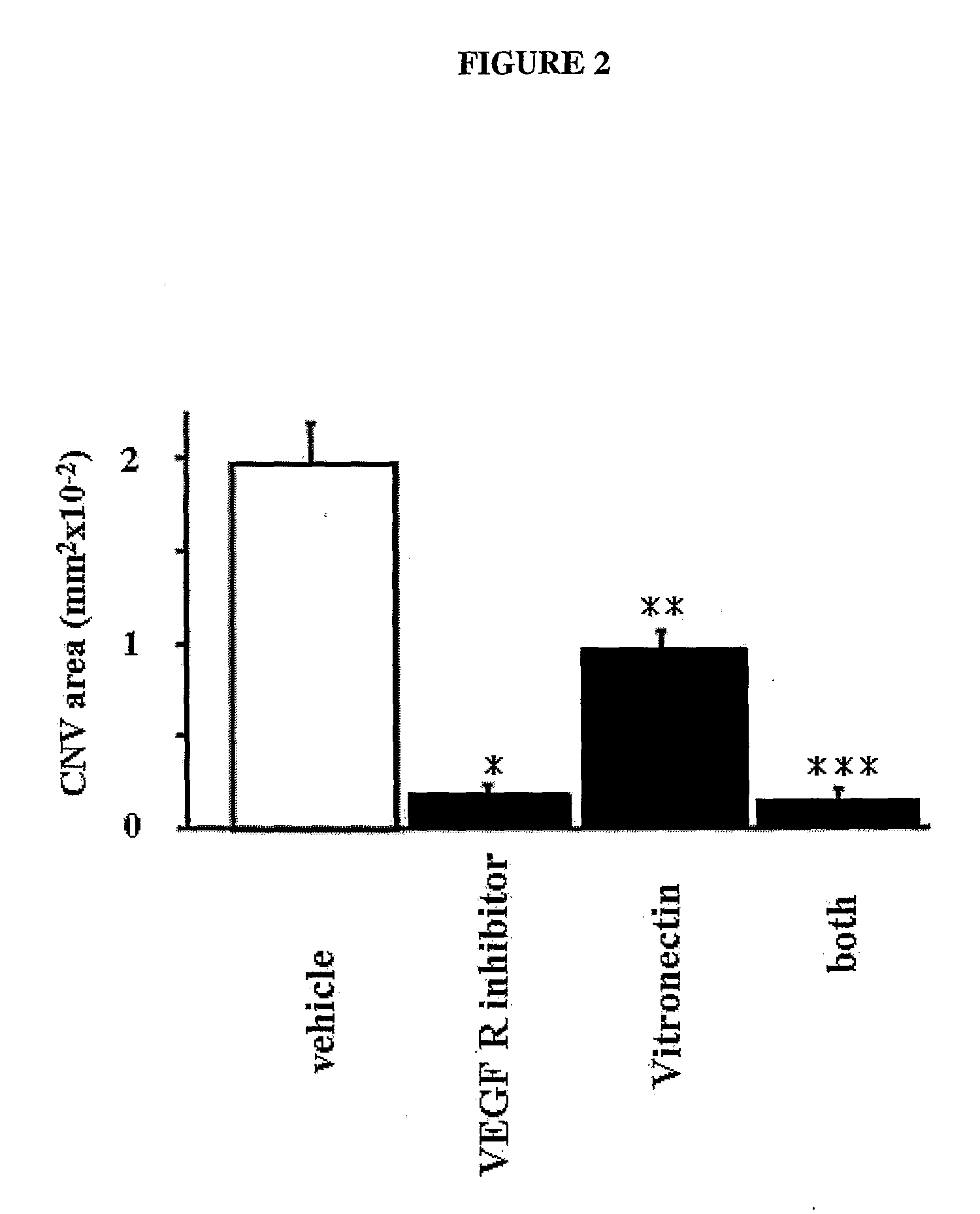
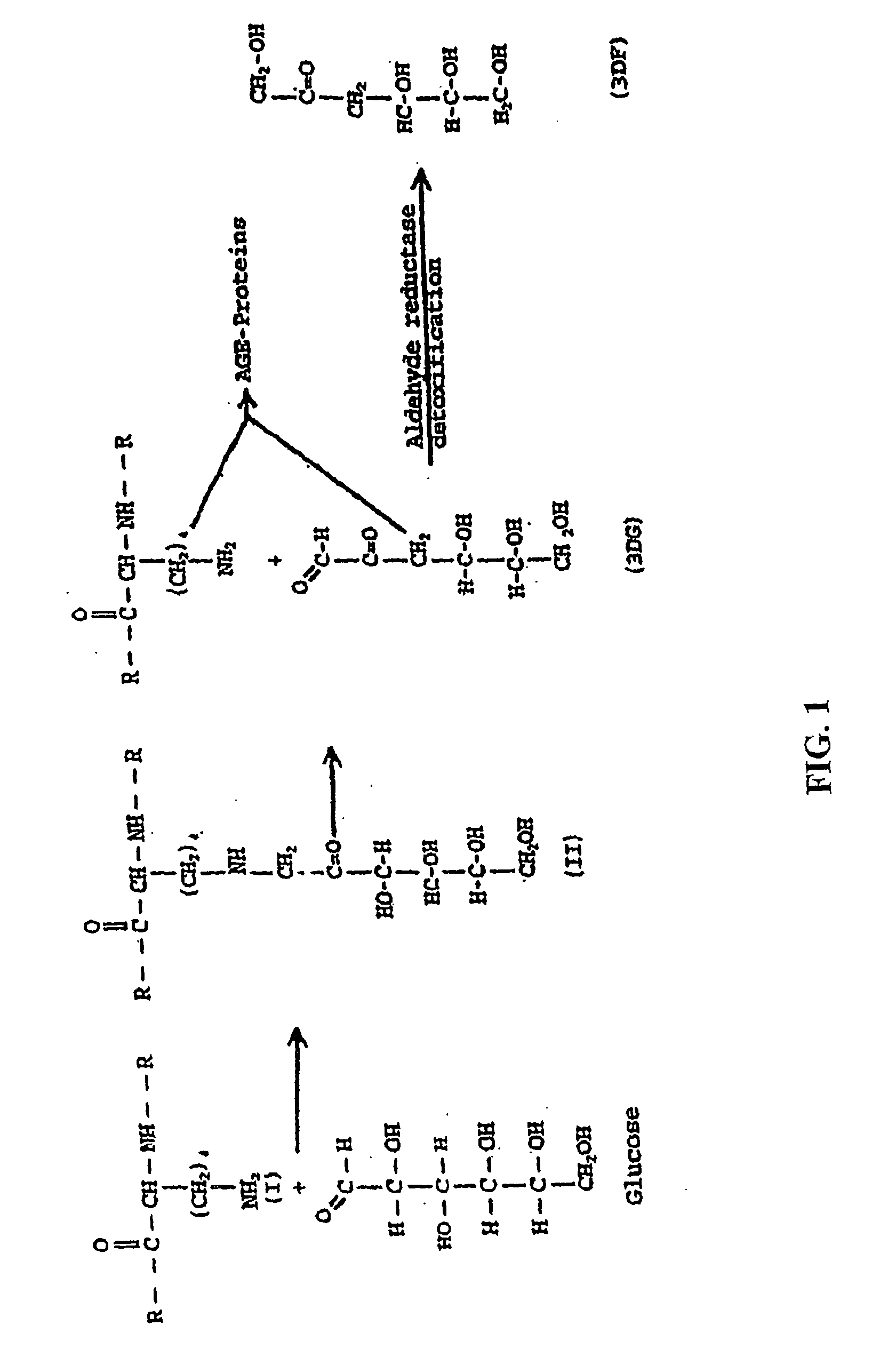
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
670results about "Sulfonylurea active ingredients" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Glp-1 and methods for treating diabetes
InactiveUS20060057137A1Prevent and treat diseasePrevent and treat and delay onset of and reduce symptomPeptide/protein ingredientsMetabolism disorderDiseaseDiabetes mellitus
The present invention relates to use of GLP-1 or a related molecule having GLP-effect for the manufacture of a medicament for preventing or treating diabetes in a mammal. The amount and timing of administration of said medicament are subsequently reduced to produce a “drug holiday”. Practice of the invention achieves effective therapy without continuous drug exposure and without continuous presence of therapeutic levels of the drug. The invention also discloses a method of treating diabetes and related disorders in a mammal by administering glucagons like peptide (GLP-1) or a related molecule having GLP-1 like effect and thereby providing a therapeutically effective amount of endogenous insulin.
Owner:ZEALAND PHARM AS
Glp-1 and methods for treating diabetes
InactiveUS20090088369A1Reduce the amount requiredPrevent and treat diseasePeptide/protein ingredientsMetabolism disorderDiabetes mellitusTreatment level
The present invention relates to use of GLP-1 or a related molecule having a GLP-effect for the manufacture of a medicament for preventing or treating diabetes in a mammal. The amount and timing of administration of said medicament are subsequently reduced to produce a “drug holiday.” Practice of the invention achieves effective therapy without continuous drug exposure and without continuous presence of therapeutic levels of the drug. The invention also discloses a method of treating diabetes and related disorders in a mammal by administering glucagon like peptide (GLP-1) or a related molecule having GLP-1 like effect and thereby providing a therapeutically effective amount of endogenous insulin.
Owner:ZEALAND PHARM AS
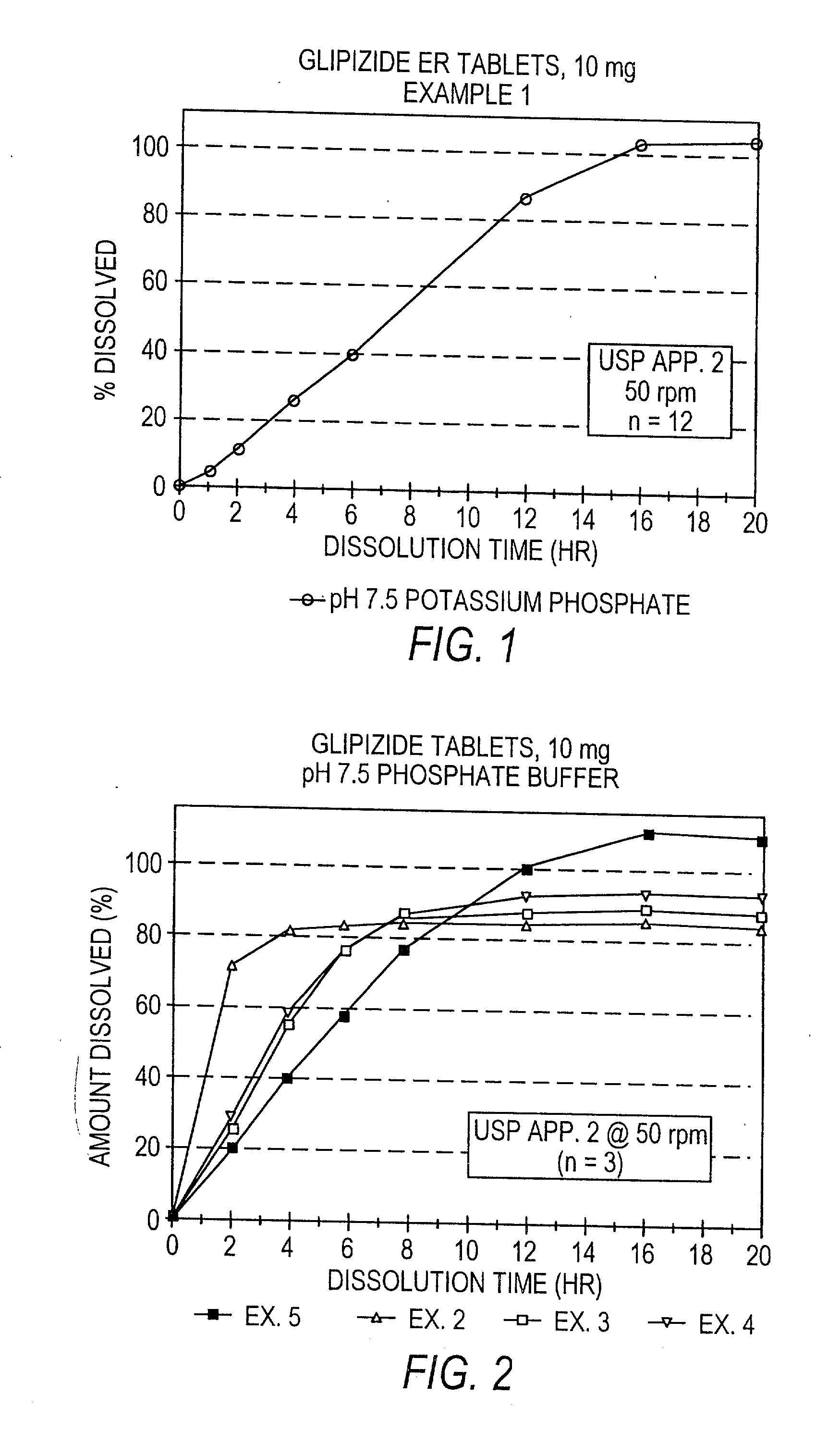
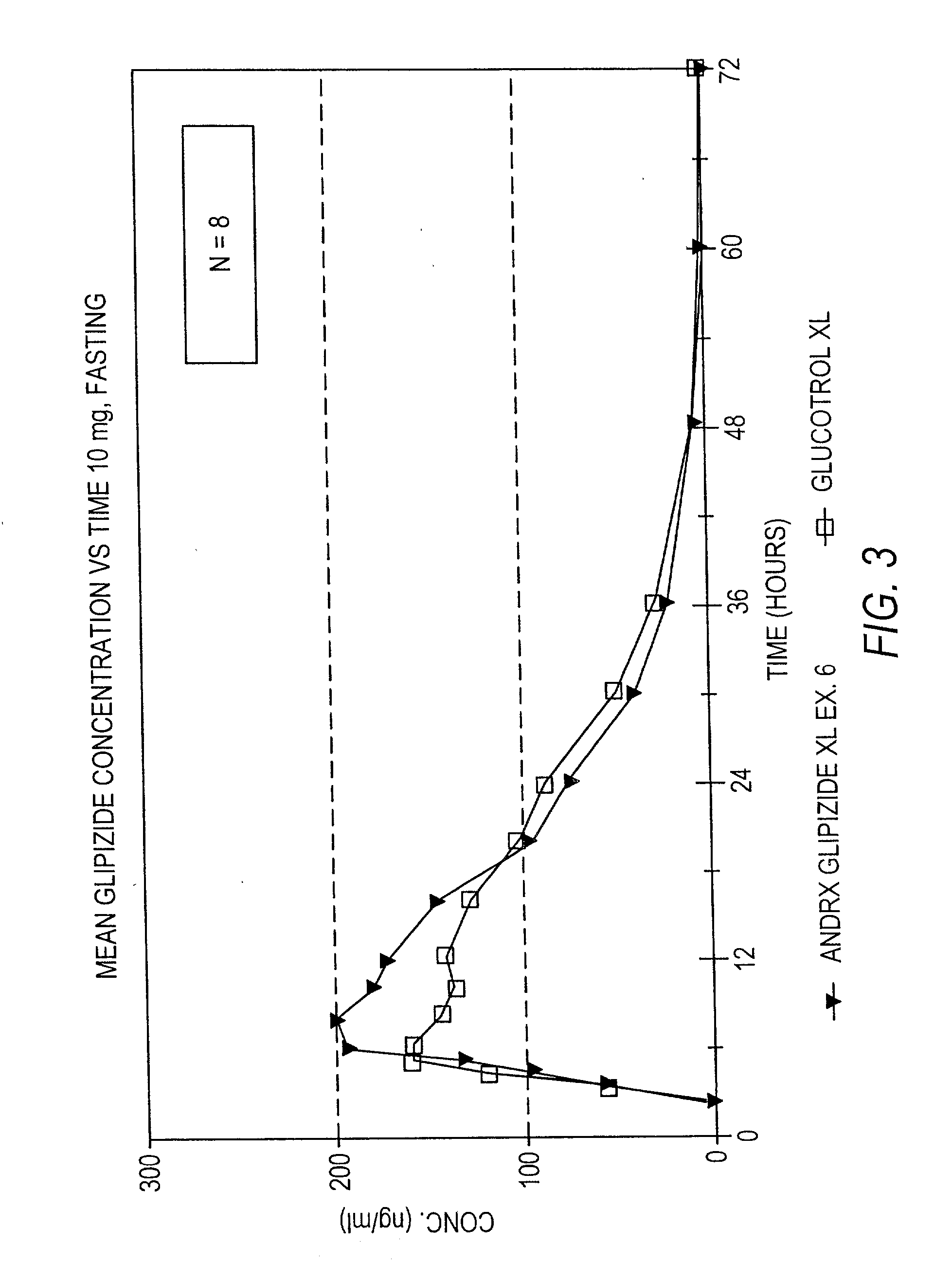
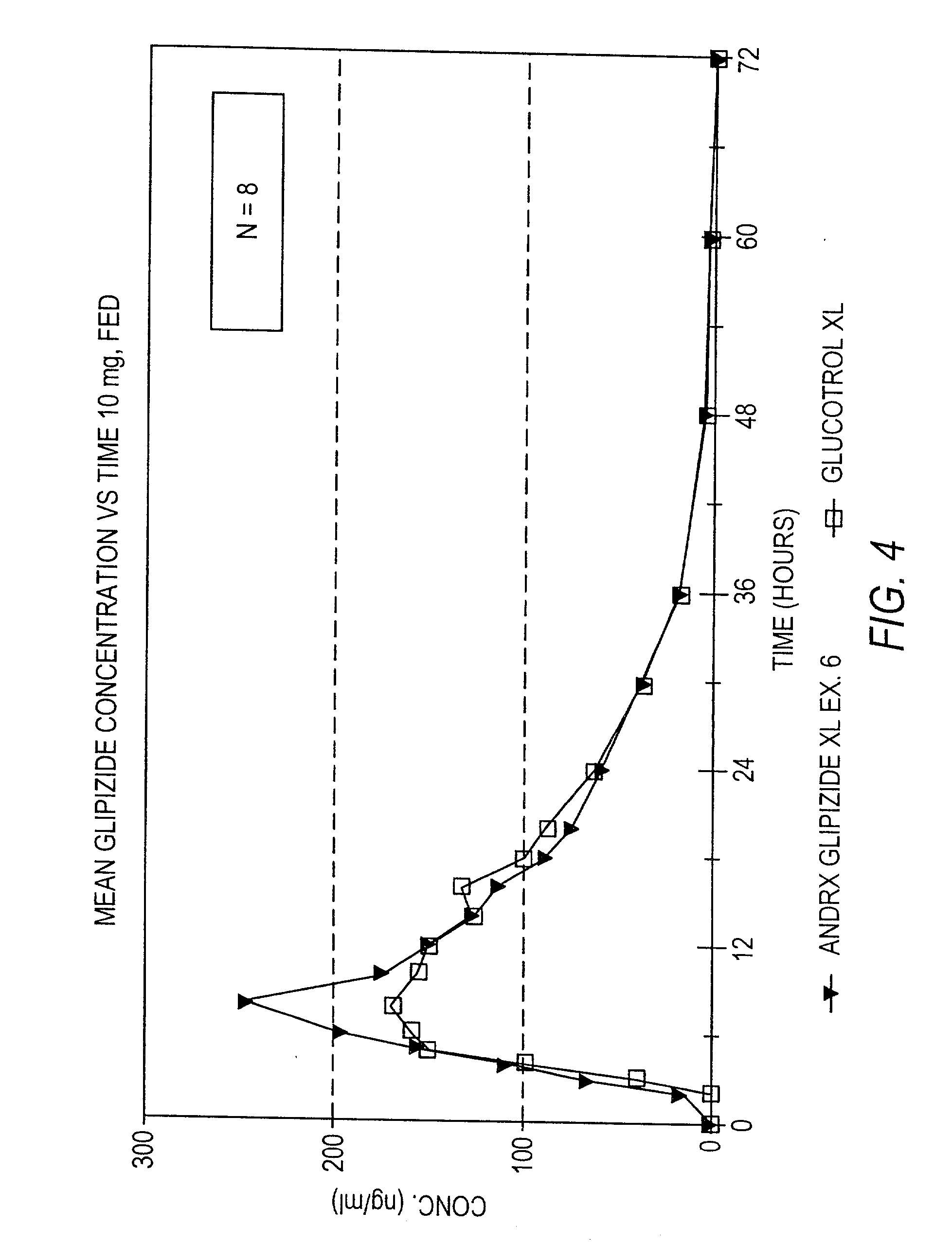
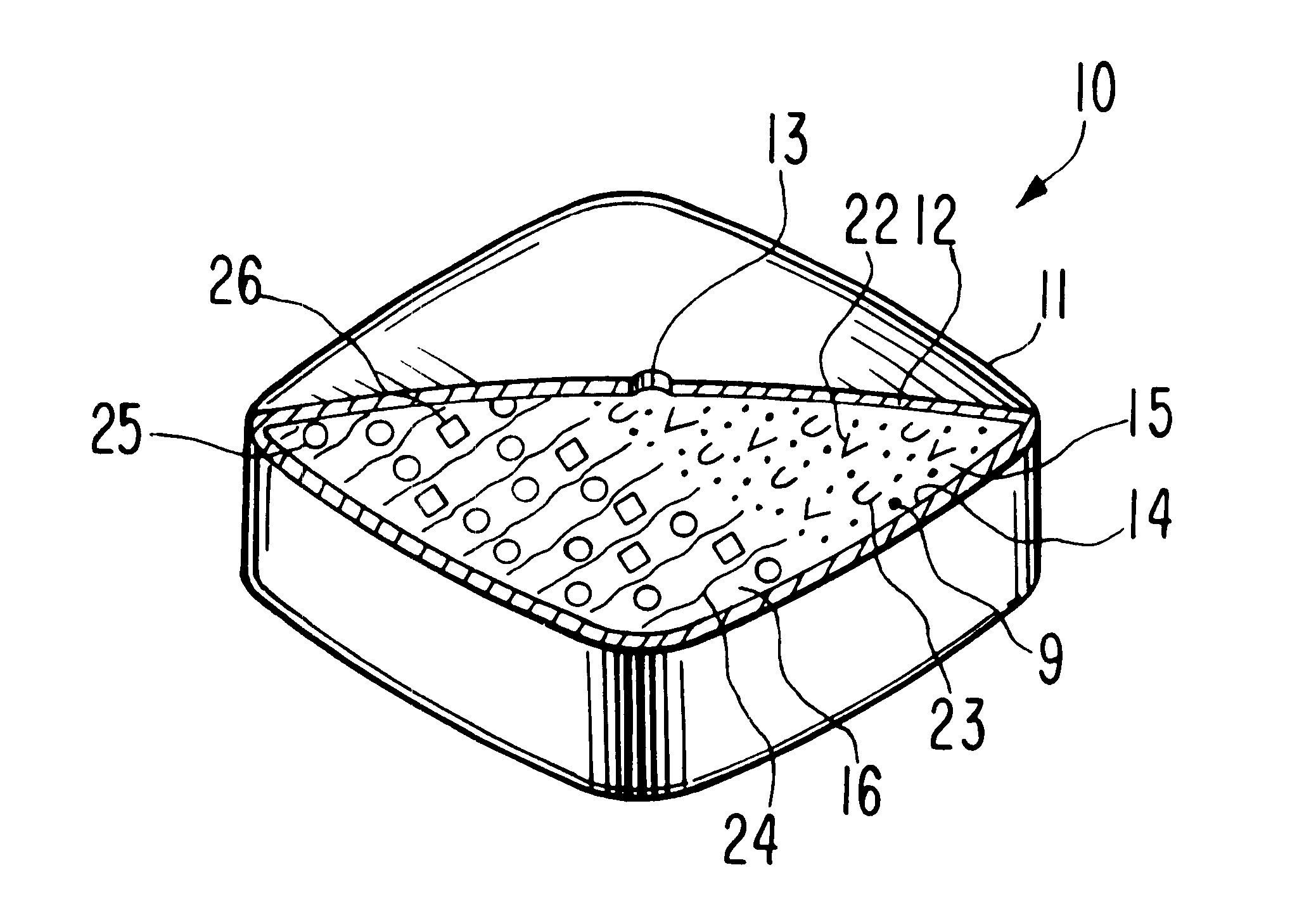
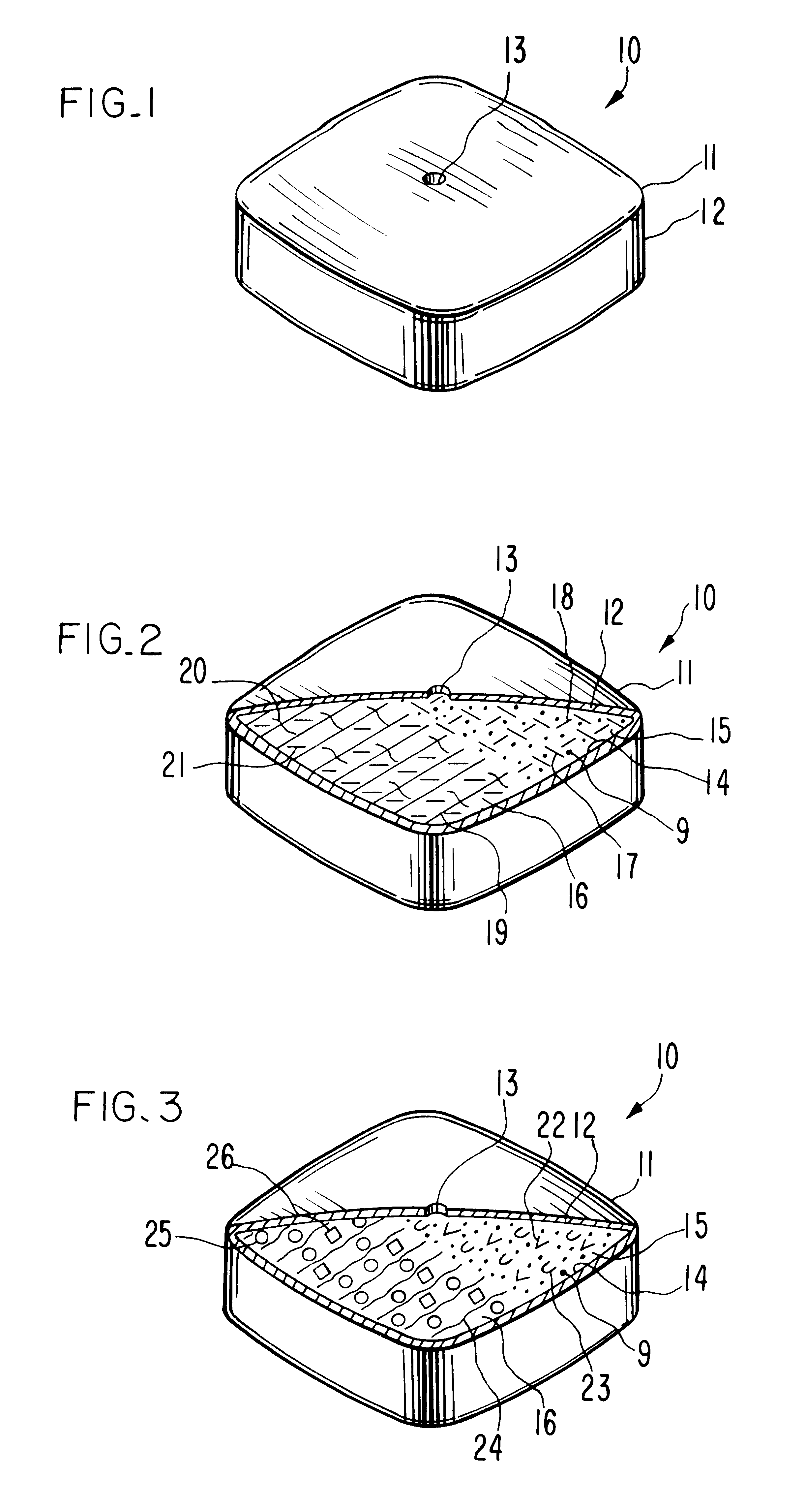
Controlled release tablet having a unitary core
InactiveUS6284275B1Metabolism disorderSulfonylurea active ingredientsControlled Release TabletControlled release drug
A controlled release pharmaceutical tablet containing antihyperglycemic drug and a hypoglycemic drug that does not contain an expanding or gelling polymer layer and comprising a core containing the antihyperglycemic drug and the hypoglycemic drug, a semipermeable coating membrane surrounding the core and at least one passageway in the membrane to allow the drugs to be released from the core.
Owner:ANDRX LABS
Treatment for diabetes in patients with insufficient glycemic control despite therapy with an oral or non-oral antidiabetic drug
Owner:BOEHRINGER INGELHEIM INT GMBH
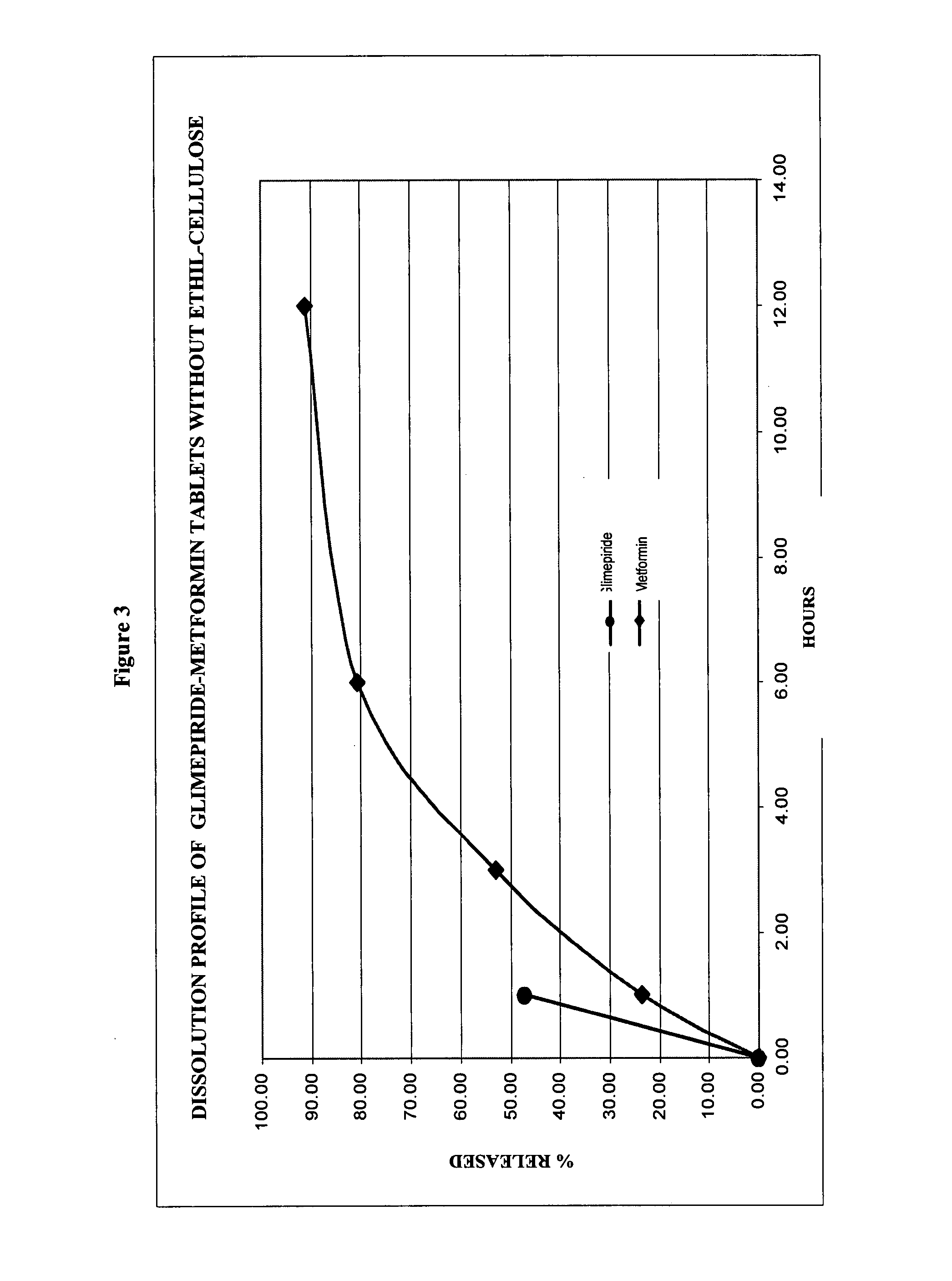
Stable pharmaceutical composition of immediate-release glimepiride and extended-release metformin
InactiveUS20070264331A1Avoid mixingBiocideMetabolism disorderImmediate releaseMetformin Hydrochloride
This invention is directed to a pharmaceutical composition in the form of a tablet with improved stability, as well as the process for obtaining said composition. The tablet of the present invention comprises two active ingredients comprising two oral hypoglycemic agents: one phase with a sulphonylurea, such as immediate release Glimepiride, and a second phase with a biguanide, such as extended-release Metformin hydrochloride (Metformin HCl). The biphasic tablet, which can include over 500 mg of Metformin HCl (i.e. up to 1,000 or 1,500 mg, depending on the daily requirements of each patient), is to be orally administered once or twice a day. The combination of these hypoglycemic agents has a synergic effect and therefore a greater effectiveness in controlling the blood glucose level in patients with diabetes mellitus, type 2.
Owner:LAB SILANES
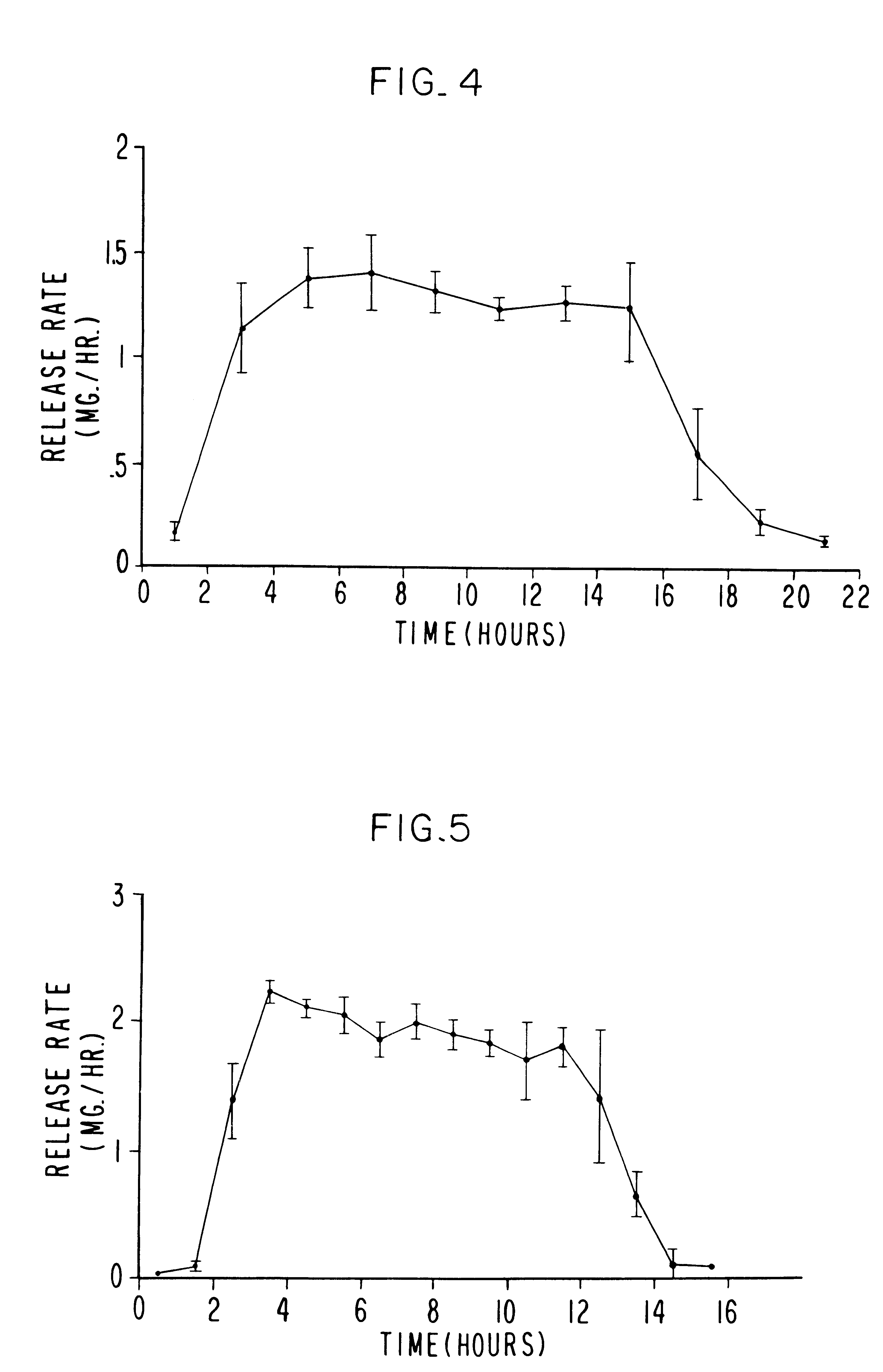
Controlled release sulfonylurea formulation
InactiveUS20030157166A1Effective controlBiocideSulfonylurea active ingredientsControl releaseSulfonylurea
Disclosed in a controlled release sulfonylurea formulation. In certain embodiments, the invention comprises: (a) a core comprising: (i) a sulfonylurea or a pharmaceutically acceptable salt thereof; (ii) a pharmaceutically acceptable polymer; (b) a membrane surrounding the core which is permeable to the sulfonylurea and gastrointestinal fluid, wherein said dosage form provides a mean time to maximum plasma concentration (Tmax) of said sulfonylurea.
Owner:ANDRX PHARMA INC
Methods for prevention and treatment of gastrointestinal disorders
InactiveUS20050004222A1High activityPrevent and reduce severity of and symptomBiocidePeptide/protein ingredientsGastrointestinal disorderHuman patient
Disclosed are methods for preventing or treating a gastrointestinal (GI) disorder in a mammal such as a human patient. In one embodiment, the methods include administering to the mammal a therapeutically effective amount of a compound that modulates a nitric oxide (NO) signaling pathway, particularly in GI neurons. Methods of the invention are particularly useful for the treatment (including prophylactic treatment) of diabetic gastropathies and other GI disorders.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Novel Combinational Use of Sulfonamide Compound
InactiveUS20090047278A1Remarkable anti-tumor effectStatistically significantBiocideSulfonylurea active ingredientsCancerDrug
The present invention relates to a pharmaceutical composition, a kit and a method for treating cancer, comprising a sulfonamide compound in combination with a substance having an EGF inhibitory activity.
Owner:EISIA R&D MANAGEMENT CO LTD
Multi-system therapy for diabetes, the metabolic syndrome and obesity
InactiveUS20050054731A1Symptoms improvedLower blood pressureBiocideDispersion deliveryLipid formationLipid lowering drug
A multi-system therapy which is adapted to treat diabetes, metabolic syndrome and obesity includes a hypoglycemic agent, a lipid lowering agent, a blood pressure lowering agent and, preferably, an anti-platelet agent. The composition can further include various vitamins and supplements such as vitamin B6, vitamin B12, arginin, a folate and other vitamins and minerals. Preferably, the hypoglycemic agent is a biguanide hypoglycemic agent without any additional hypoglycemic agent, making the composition suitable for treatment of individuals who are not hyperglycemic as well as those who are hyperglycemic.
Owner:FOLLI FRANCO +2
Treatment of genotyped diabetic patients with dpp-iv inhibitors such as linagliptin
ActiveUS20130196898A1Weight increaseLose weightBiocidePeptide/protein ingredientsPatient groupGenotype
Owner:BOEHRINGER INGELHEIM INT GMBH
Substituted quinazolin-4-ylamine analogues
Substituted quinazolin-4-ylamine analogues are provided. Such compounds are ligands that may be used to modulate specific receptor activity in vivo or in vitro, and are particularly useful in the treatment of conditions associated with pathological receptor activation in humans, domesticated companion animals and livestock animals. Pharmaceutical compositions and methods for using them to treat such disorders are provided, as are methods for using such ligands for receptor localization studies.
Owner:NEUROGEN
Method for lowering blood glucose
InactiveUS6361795B1Promote insulin secretionRaise insulin levelsMetabolism disorderSulfonylurea active ingredientsGlucose polymersD-Glucose
Owner:ALZA CORP
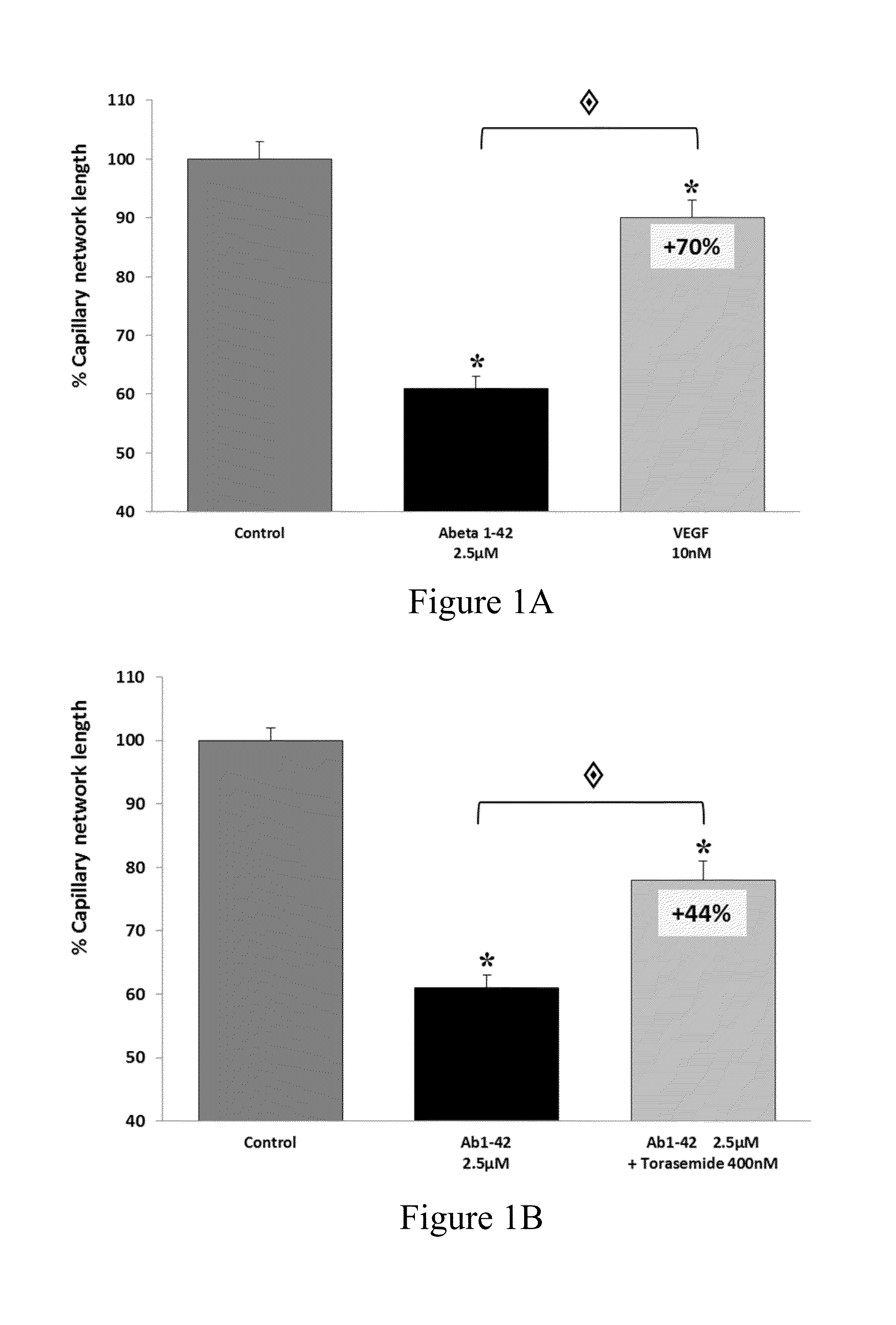
Therapeutic agents targeting the NCCa-ATP channel and methods of use thereof
ActiveUS20060100183A1Reduces and decrease and inhibits activationInhibition is effectiveBiocideNervous disorderMedicineNeuron
The present invention is directed to therapeutic compositions targeting the NCCa-ATP channel of an astrocyte, neuron or capillary endothelial cell and methods of using same. More specifically, antagonists of the NCCa-ATP channel are contemplated. The compositions are used to prevent cell death and to treat secondary damage associated with spinal cord injury.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
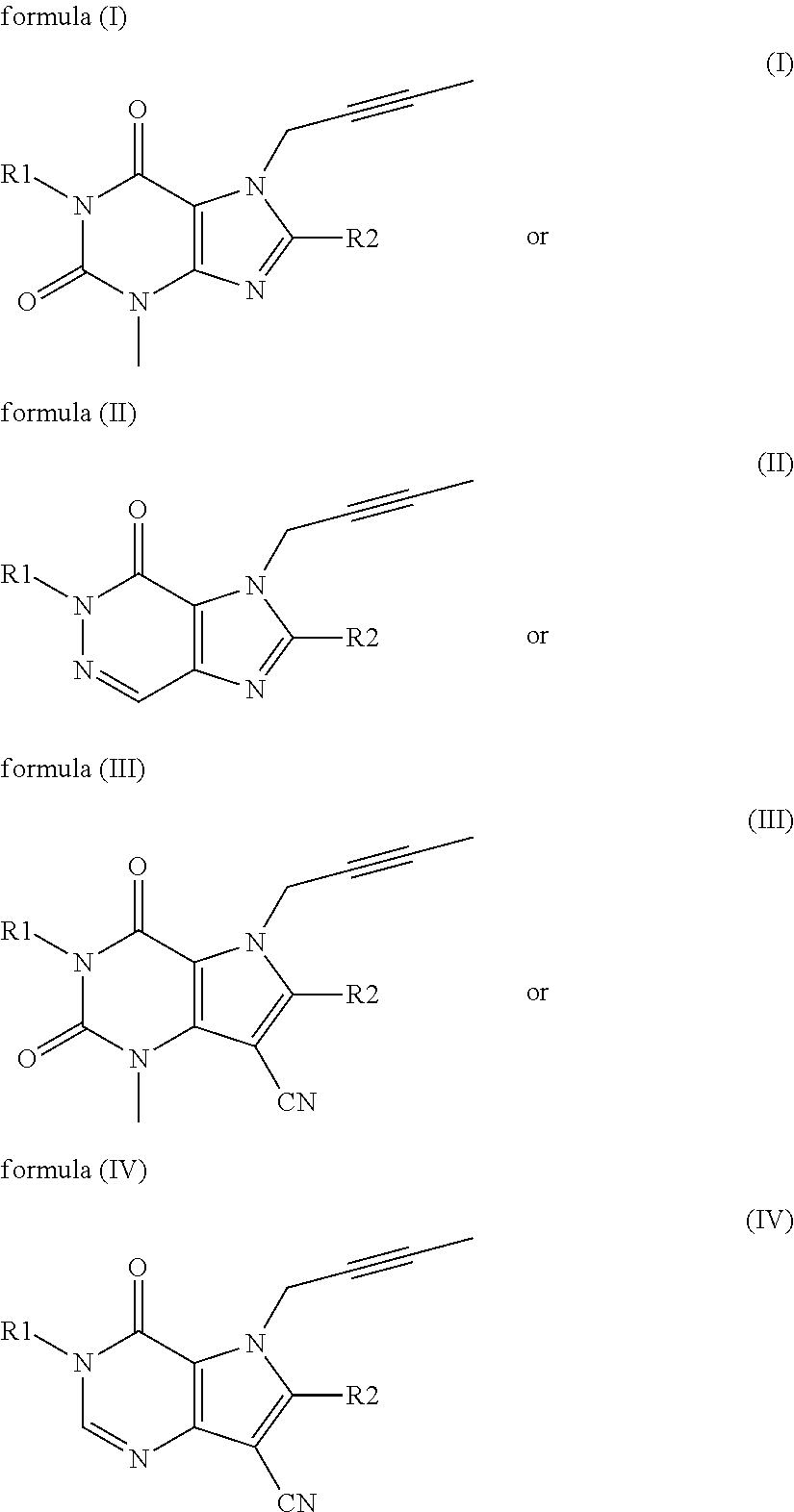
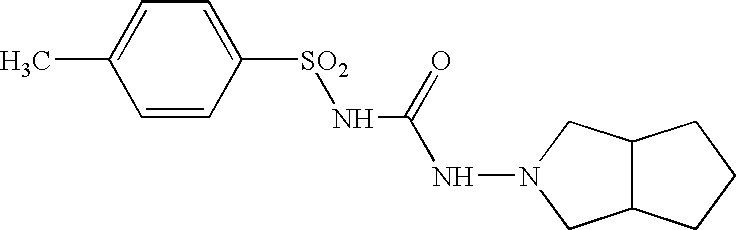
Sulfonylurea derivative and pharmaceutical composition and application thereof
The invention relates to a preparation method and application of a sulfonylurea compound and a composition containing the same component as FXR and / or TGR5 agonist, the FXR and / or TGR5 agonist is a compound shown as a formula (I), or a pharmaceutically acceptable salt, a solvate, a prodrug, an isomer and a stable isotope derivative thereof. The compounds can be used for treatment of FXR and / or TGR5 mediated diseases including primary biliary cirrhosis, nonalcoholic fatty liver, portal hypertension, bile acid diarrhea and cholestasis, type II diabetes and obesity and other field.
Owner:SHANGHAI DE NOVO PHARMA
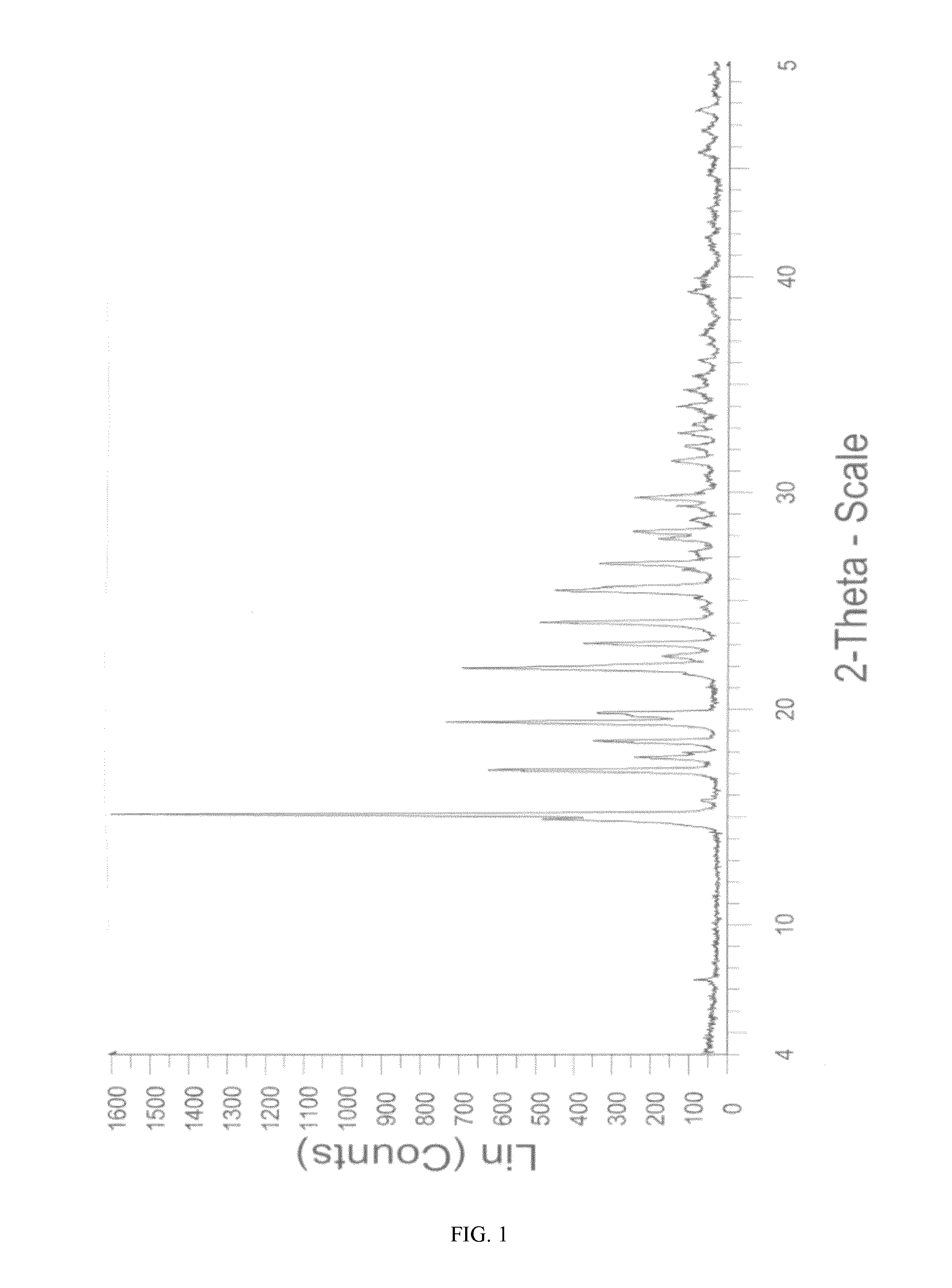
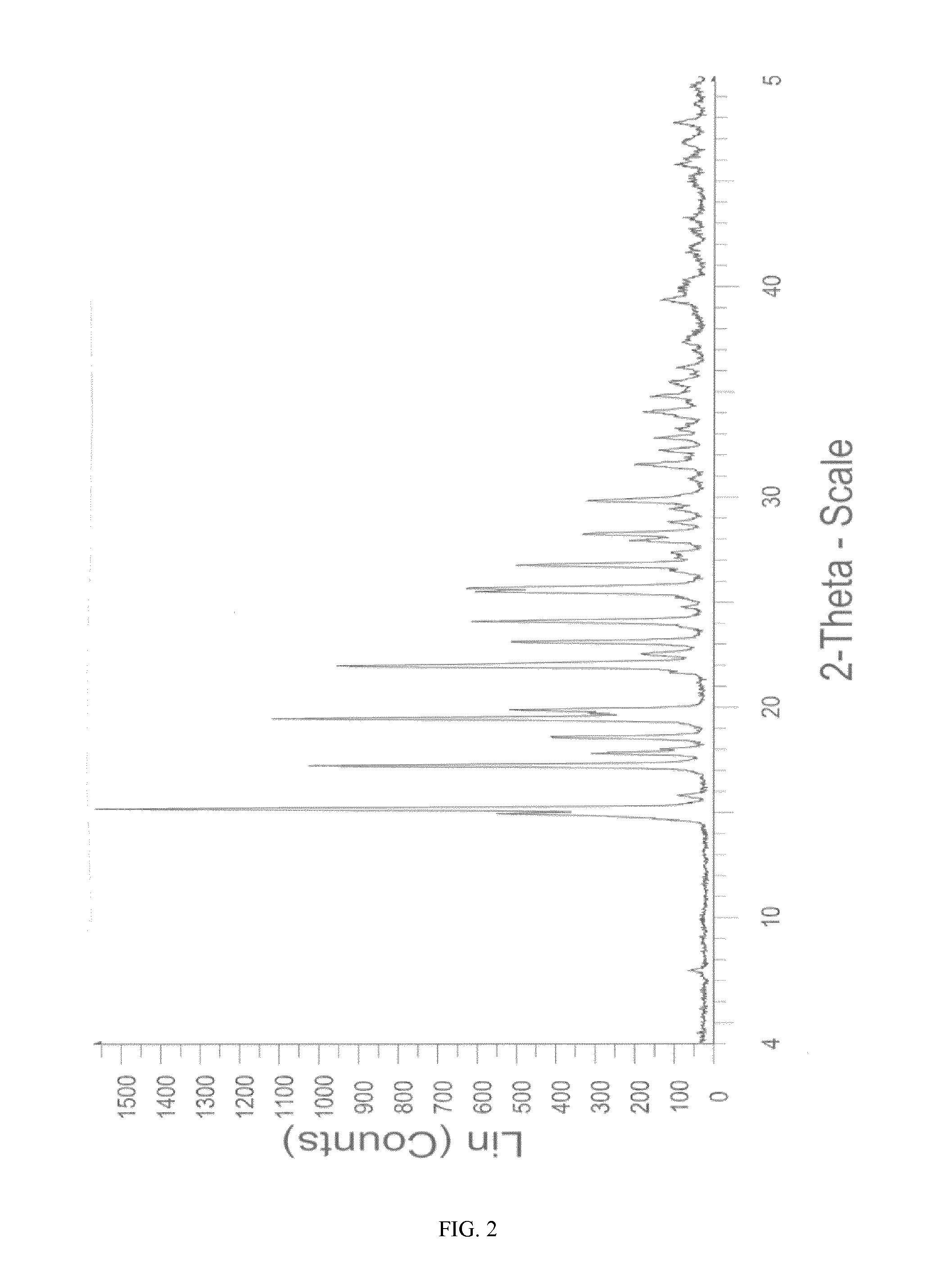
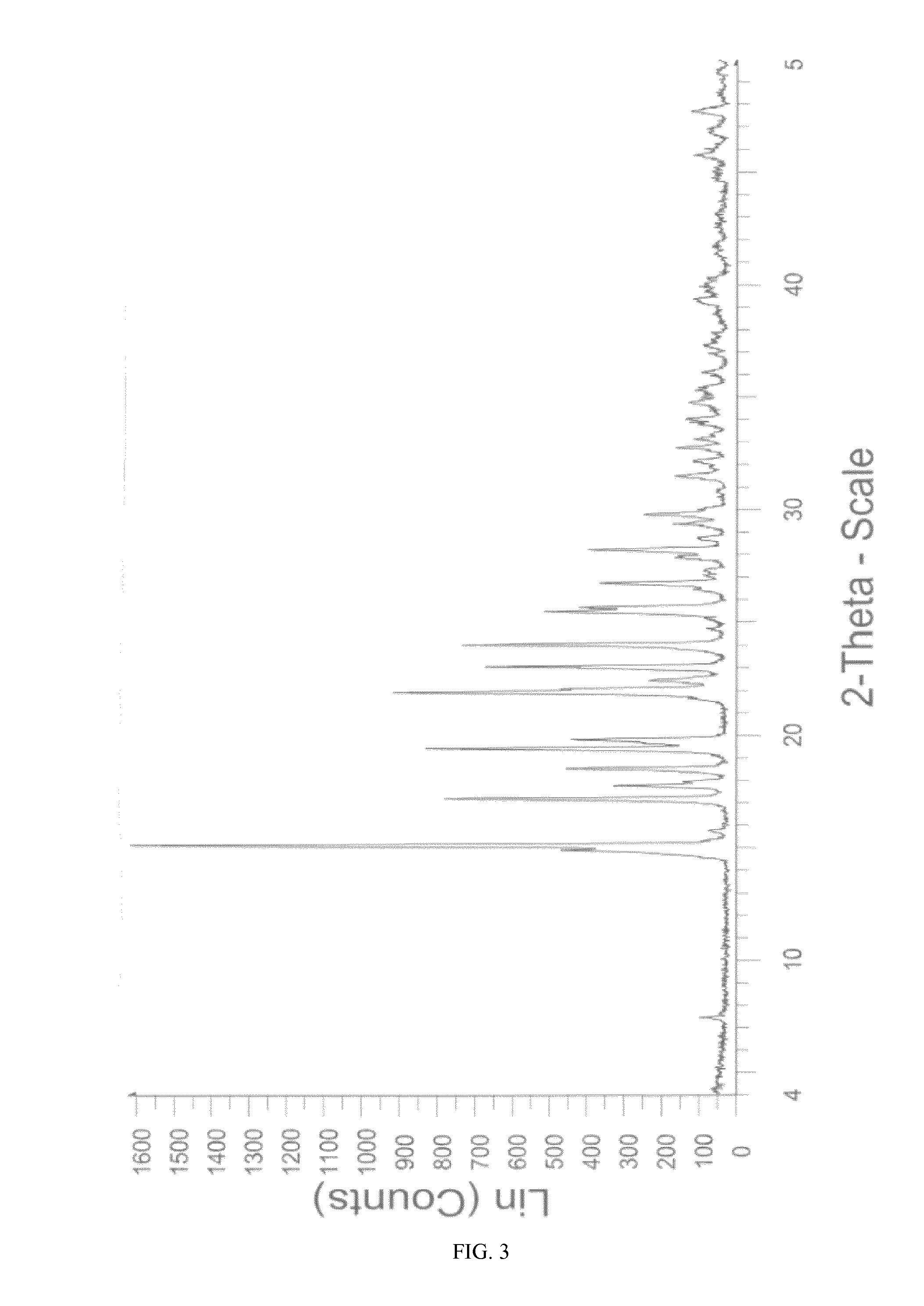
Novel crystalline form of sitagliptin sulfate
InactiveUS20150037406A1Physical improvementImprove pharmaceutical propertiesBiocideOrganic chemistrySitagliptinSulfate
A novel crystalline form of sitagliptin sulfate is provided. In addition, a method for obtaining the crystalline form, pharmaceutical compositions comprising the novel crystalline form and the crystalline form for use as a medicament are provided.
Owner:MOEHS IBERICA
Compositions for treating neurological disorders
InactiveUS20140038927A1Significant comprehensive benefitsLow dosBiocideNervous disorderHuntingtons choreaAlcoholisms
The present invention relates to compositions and methods for the treatment of neurological disorders related to glutamate excitotoxicity and Amyloid β toxicity. More specifically, the present invention relates to novel combinatorial therapies of Multiple Sclerosis, Alzheimer's disease, Alzheimer's disease related disorders, Amyotrophic Lateral Sclerosis, Parkinson's disease, Huntington's disease, neuropathic pain, alcoholic neuropathy, alcoholism or alcohol withdrawal, or spinal cord injury.
Owner:PHARNEXT
Platelet ADP receptor inhibitors
InactiveUS6906063B2Preventing and treating thrombosisBiocideOrganic chemistryGuanidine derivativesSulfonylurea
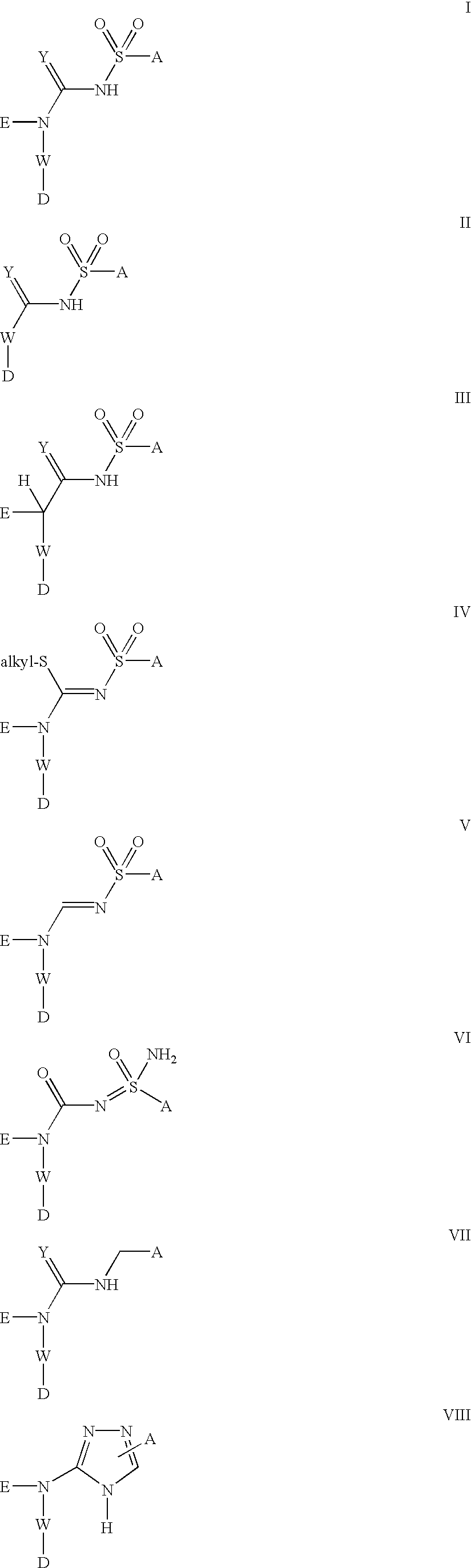
Novel compounds of formulae (I) to (VIII), which more particularly include sulfonylurea derivatives, sulfonylthiourea derivatives, sulfonylguanidine derivatives, sulfonylcyanoguanidine derivatives, thioacylsulfonamide derivatives, and acylsulfonamide derivatives which are effective platelet ADP receptor inhibitors. These derivatives may be used in various pharmaceutical compositions, and are particularly effective for the prevention and / or treatment of cardiovascular diseases, particularly those diseases related to thrombosis. The invention also relates to a method for preventing or treating thrombosis in a mammal comprising the step of administering a therapeutically effective amount of a compound of formulae (I) to (VIII), or a pharmaceutically acceptable salt thereof.
Owner:ALEXION PHARMA INC
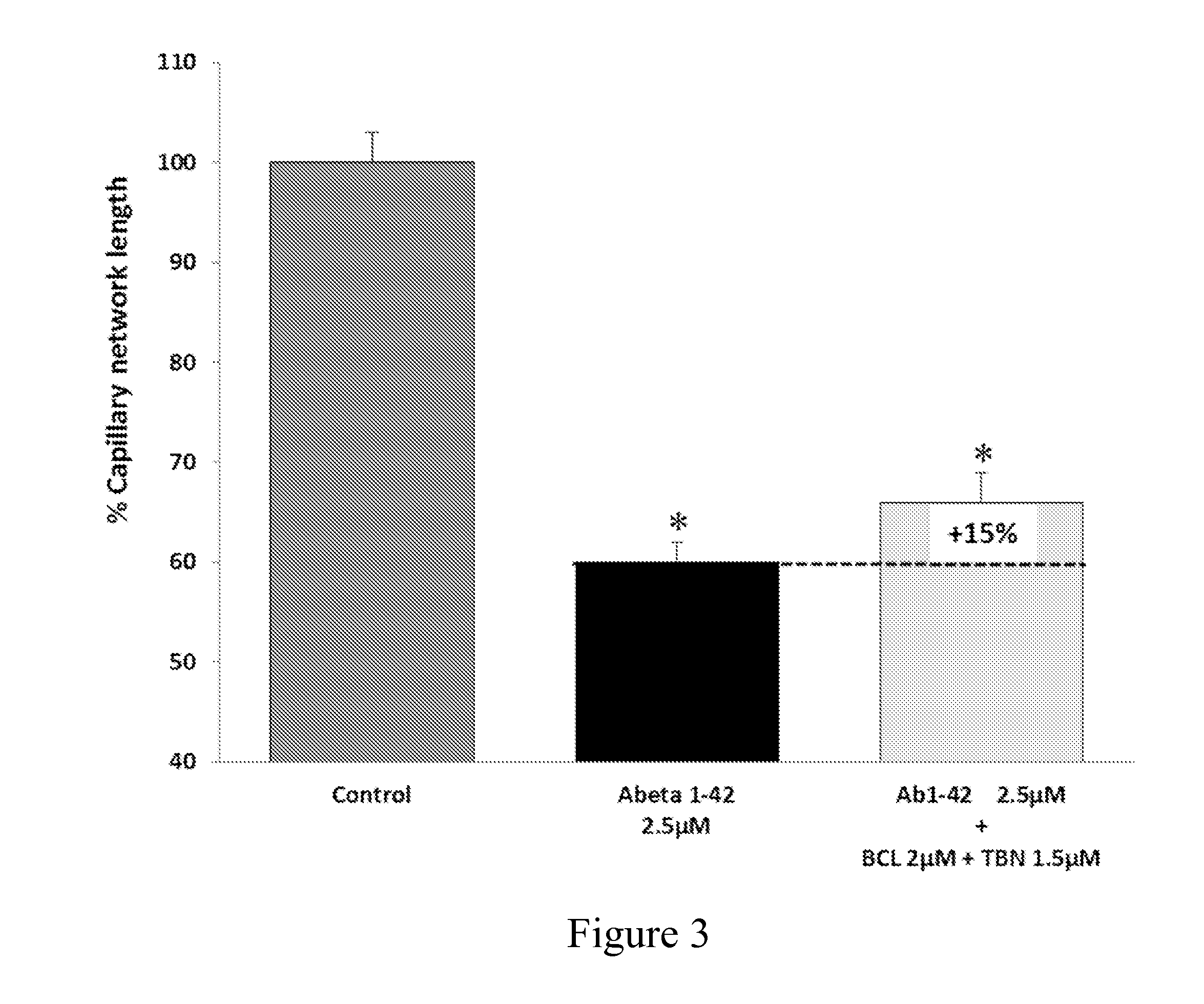
Baclofen and acamprosate based therapy of neurological disorders
The present invention relates to combinations and methods for the treatment of neurological disorders related to glutamate excitotoxicity and Amyloid β toxicity. More specifically, the present invention relates to novel combinatorial therapies of Multiple Sclerosis, Alzheimer's disease, Alzheimer's disease related disorder, Amyotrophic Lateral Sclerosis, Parkinson's disease, Huntington's disease, neuropathic pain, alcoholic neuropathy, alcoholism or alcohol withdrawal, or spinal cord injury, based on Baclofen and Acamprosate combination.
Owner:PHARNEXT
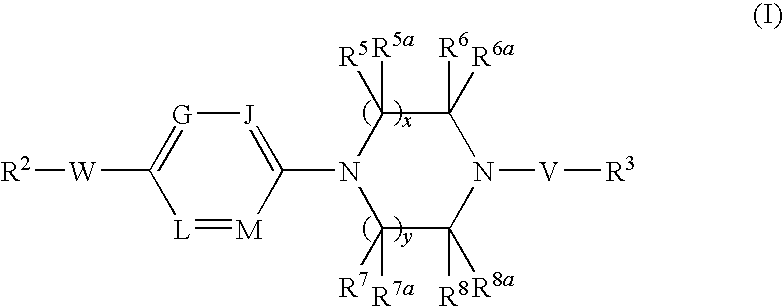
Combination therapy
InactiveUS20090131447A1Mitigating effect adverse weight gain sideAvoid weight gainBiocideNervous disorderPharmaceutical drugDepressant
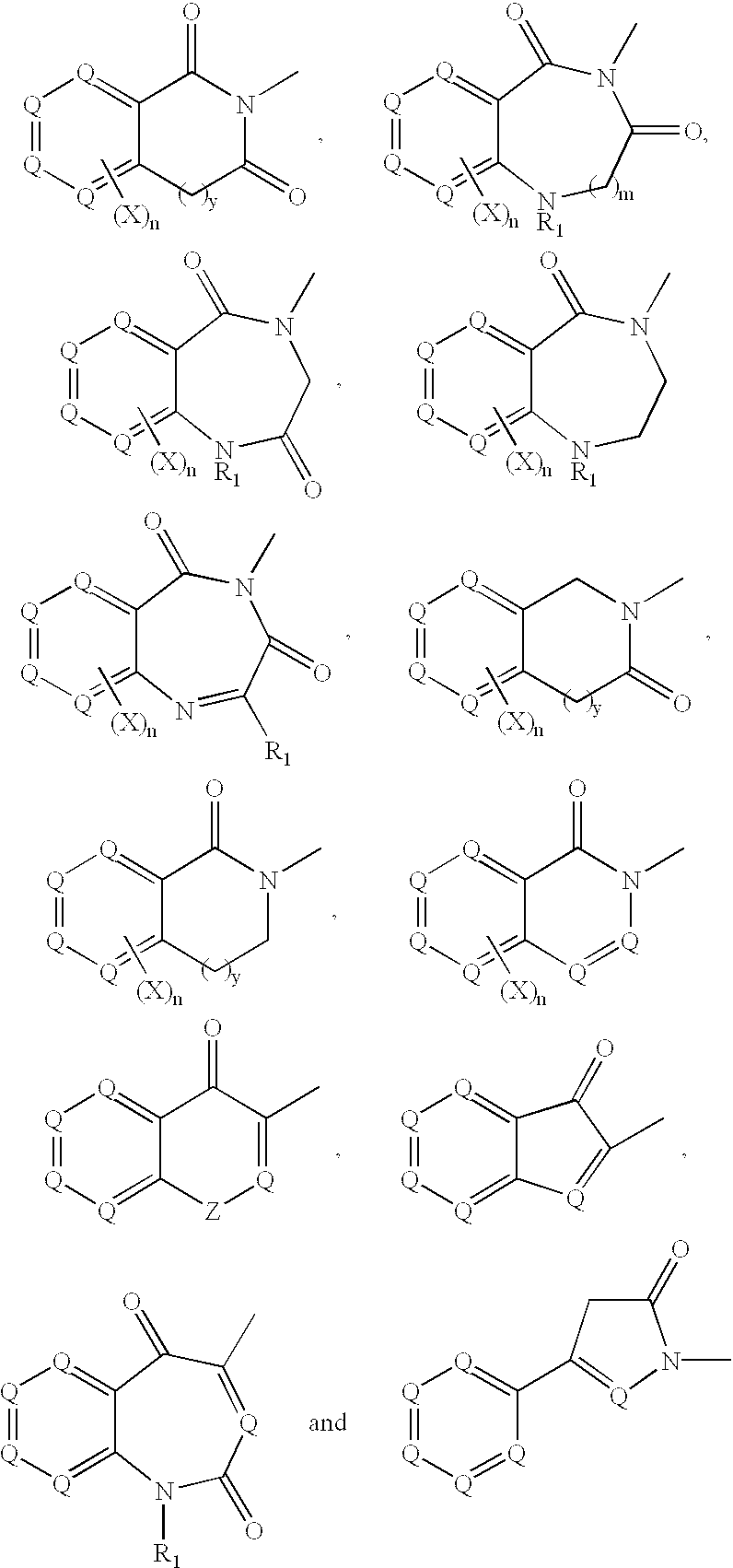
This invention is directed to the use of SCD-1 inhibitors of the formula (I): where x, y, V, W, G, J, L, M, R2, R3, R5, R5a, R6, R6a, R7, R7a, R8 and R8a are defined herein, in combination with other drug therapies to treat adverse weight gain.
Owner:XENON PHARMACEUTICALS INC
Core tablet for controlled release of gliclazide after oral administration
InactiveUS6733782B1Facilitated releaseIncrease concentrationPowder deliveryMetabolism disorderControlled releaseOral medication
The invention relates to a matrix tablet for the prolonged release of gliclazide which ensures continuous and consistent release of the active ingredient after administration by the oral route, the release being insensitive to variations in the pH of the dissolution medium.
Owner:LES LAB SERVIER
Methods and uses for modulating bile acid homeostasis and treatment of bile acid disorders and diseases
ActiveUS20170182123A1Improves bile acid homeostasisReduce doseAntibody mimetics/scaffoldsSulfonylurea active ingredientsDiseaseFGF19
Provided herein are methods of modulating bile acid homeostasis or treating a bile-acid related or associated disorder, comprising using variants and fusions of fibroblast growth factor 19 (FGF19), variants and fusions of fibroblast growth factor 21 (FGF21), fusions of FGF19 and / or FGF21, and variants or fusions of FGF19 and / or FGF21 proteins and peptide sequences (and peptidomimetics), in combination with agents effective in modulating bile acid homeostasis or treating a bile-acid related or associated disorder.
Owner:NGM BIOPHARMLS
Treatment Method
The present invention is directed to methods of treating an ocular neovascular disorder in a mammal by administration of pyrimidine derivatives, benzodiazepinyl derivatives and pharmaceutical compositions containing the same. The invention encompasses methods of treating an ocular neovascular disorder by administration of 5-[[4-[(2,3-Dimethyl-2H-indazol-6-yl)methylamino]-2-pyrimidinyl]amino]-2-methylbenzenesulfonamide, (S)-3-oxo-8-[3-(pyridin-2-ylamino)-1-propyloxy]-2-(2,2,2-trifluoroethyl)-2,3,4,5-tetrahydro-1H-2-benzazepine-4-acetic acid or salts or solvates thereof. Combination therapies for the treatment of ocular neovascular disorders are also encompassed.
Owner:SMITHKLINE BECKMAN CORP
Fructoseamine 3 kinase and the formation of collagen and elastin
InactiveUS20070065443A1Decrease in levelIncreases the flux through the Amadorase PathwayBiocideSenses disorderCompound (substance)Kinase
Owner:DYNAMIS THERAPEUTICS
Nanometer preparation and preparation method thereof
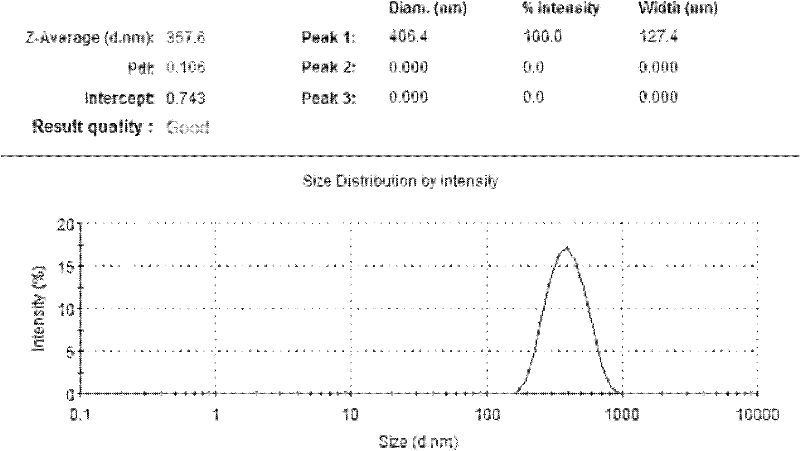
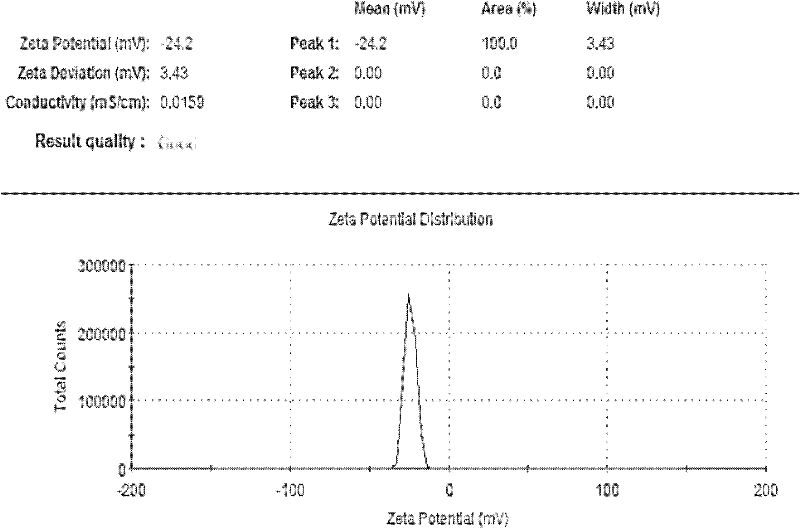
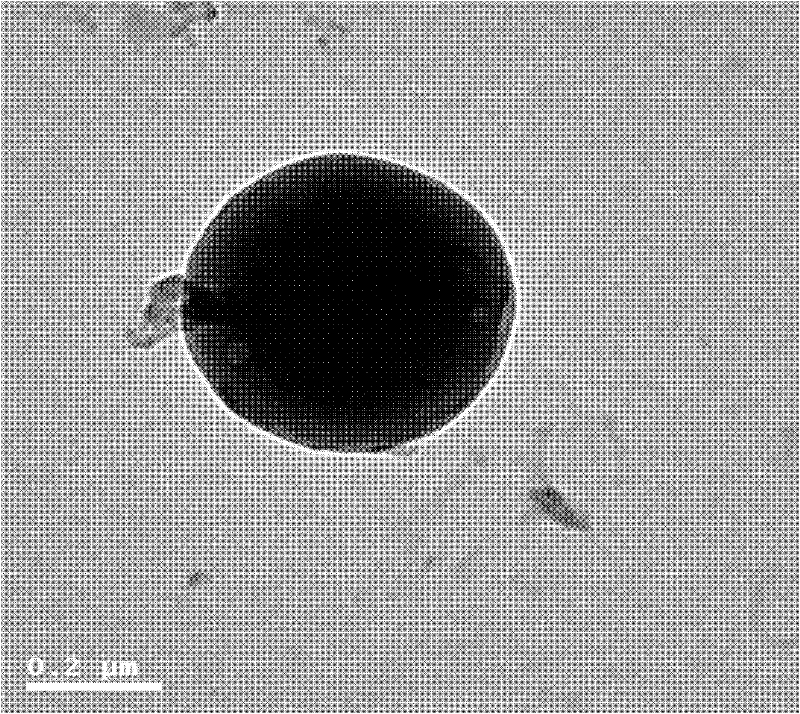
InactiveCN102232937ASulfonylurea active ingredientsSulfur/selenium/tellurium active ingredientsFluidized bedHigh pressure homogenization
The invention provides a nanometer suspension of a difficult-soluble drug and a granulated preparation thereof; the preparation is prepared by the following steps: preparing a nanometer suspension by a high-speed shearing and high-pressure homogenization method, and further preparing solidified granules by the nanometer suspension through a fluidized bed or a spray-drying method, wherein the granules can be further prepared into tablets or capsules. The invention also discloses a preparation method.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
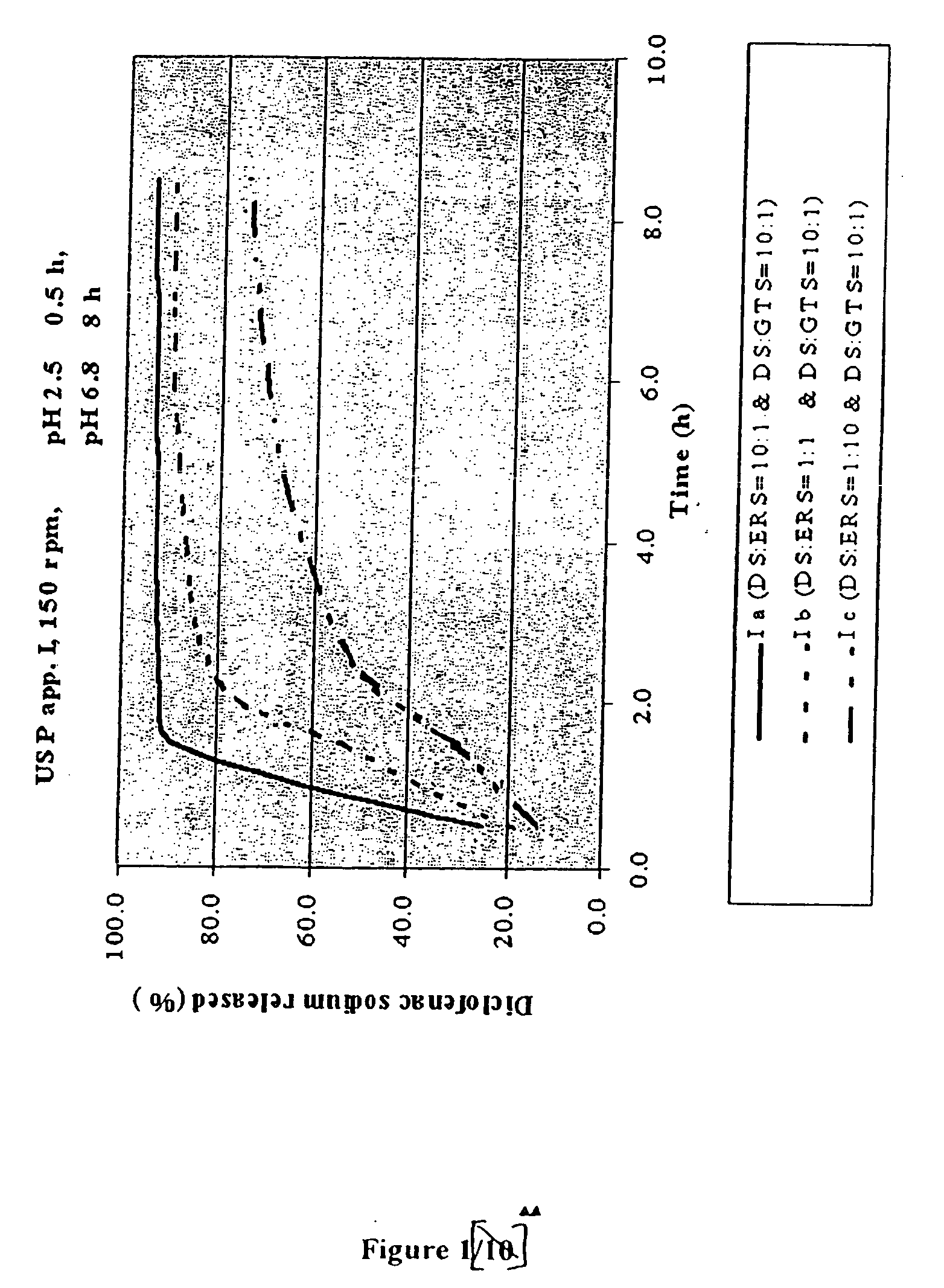
Sustained/controlled release solid formulation as a novel drug delivery system with reduced risk of dose dumping
A sustained / controlled release formulation with reduced risk of dose dumping and side effects combines two components: component (a) comprises a pharmaceutically active agent and a water-insoluble, but water-permeable polymer, whereas component (b) comprises a pharmaceutically active agent and a hydrophobic material. By changing the ratio of a pharmaceutically active agent and water-insoluble, but water-permeable polymer comprised in the component (a) and / or the ratio of the pharmaceutically active agent and hydrophobic material comprised in the component (b), and ideal release rate, with reduced risk of dose dumping and side effects, can easily be achieved.
Owner:PLIVA HRVATSKA D O O
Modified release, multiple unit drug delivery systems
The invention relates to novel modified release multiple unit systems, and methods of preparing these systems, which can be easily compressed into tablets or filled into capsules or sachets without affecting the desired release characteristics of the pharmaceutical active ingredients incorporated within the systems. The multiple unit tablet includes multiple units. Each unit includes at least one core having an outer surface, a first coating layer surrounding at least a portion of the outer surface of the core and having an outer surface, one or more rate controlling polymers, and one or more one active pharmaceutical ingredients. The coating layer includes one or both of the one or more active pharmaceutical ingredients and the one or more rate controlling polymers. The tablet may further include an outer layer on the outer surface of the unit which includes a material that is one or both of elastic and compressible. The material may be a wax materials, such as polyethylene glycol's (PEGS).
Owner:RANBAXY LAB LTD
Baclofen and acamprosate based therapy of neurological disorders
The present invention relates to combinations and methods for the treatment of neurological disorders related to glutamate excitotoxicity and Amyloid β toxicity. More specifically, the present invention relates to novel combinatorial therapies of Multiple Sclerosis, Alzheimer's disease, Alzheimer's disease related disorder, Amyotrophic Lateral Sclerosis, Parkinson's disease, Huntington's disease, neuropathic pain, alcoholic neuropathy, alcoholism or alcohol withdrawal, or spinal cord injury, based on Baclofen and Acamprosate combination.
Owner:PHARNEXT
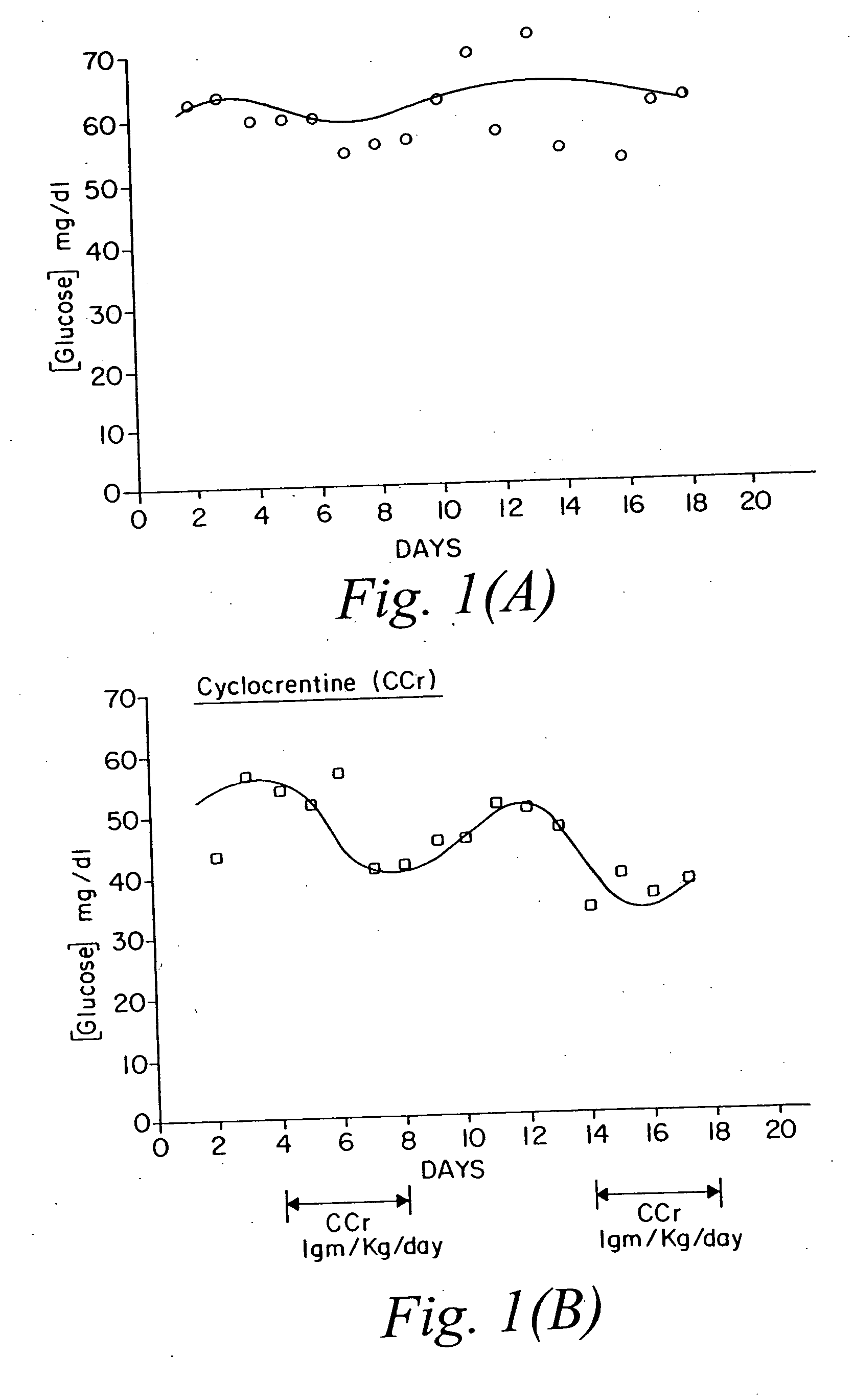
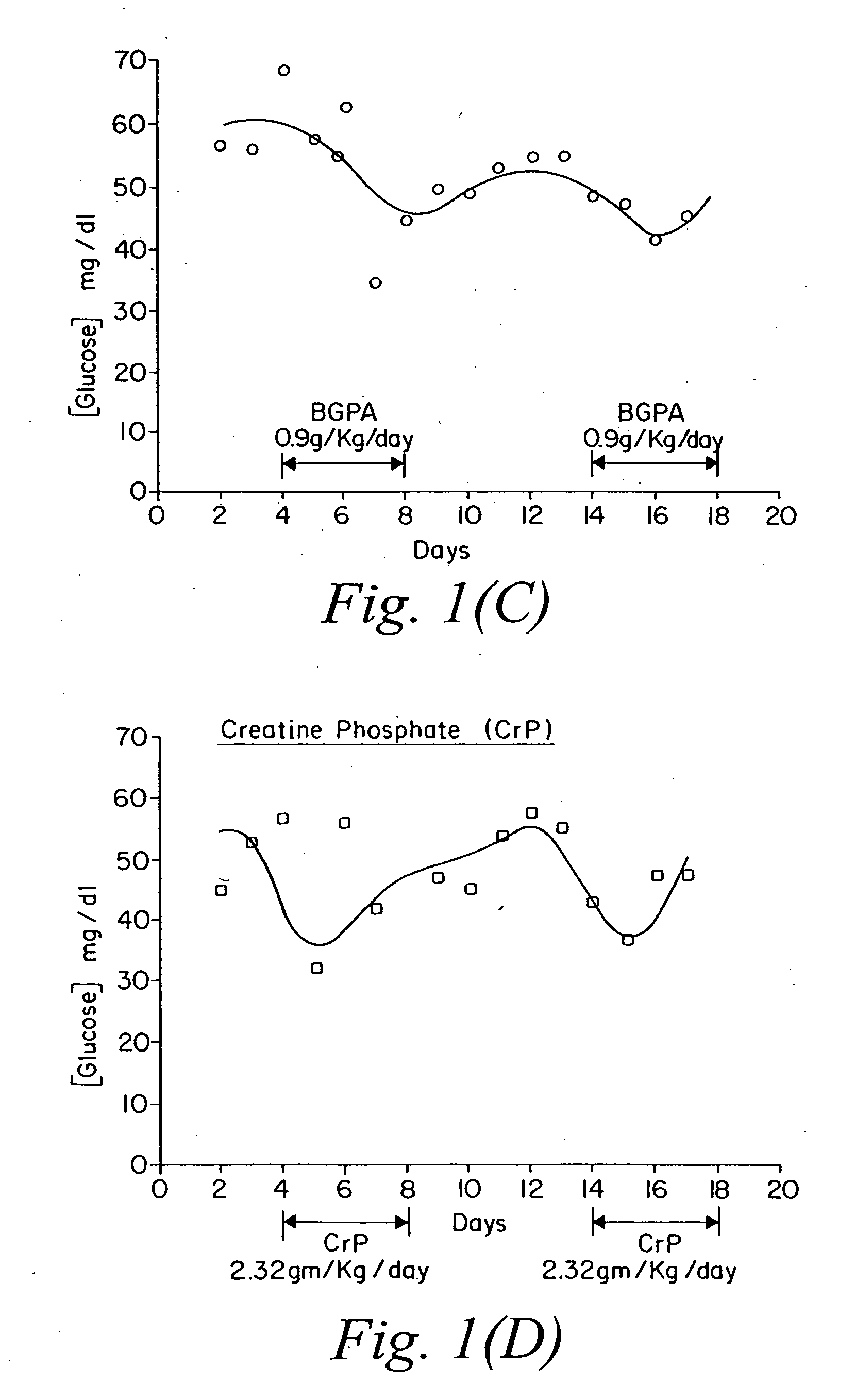
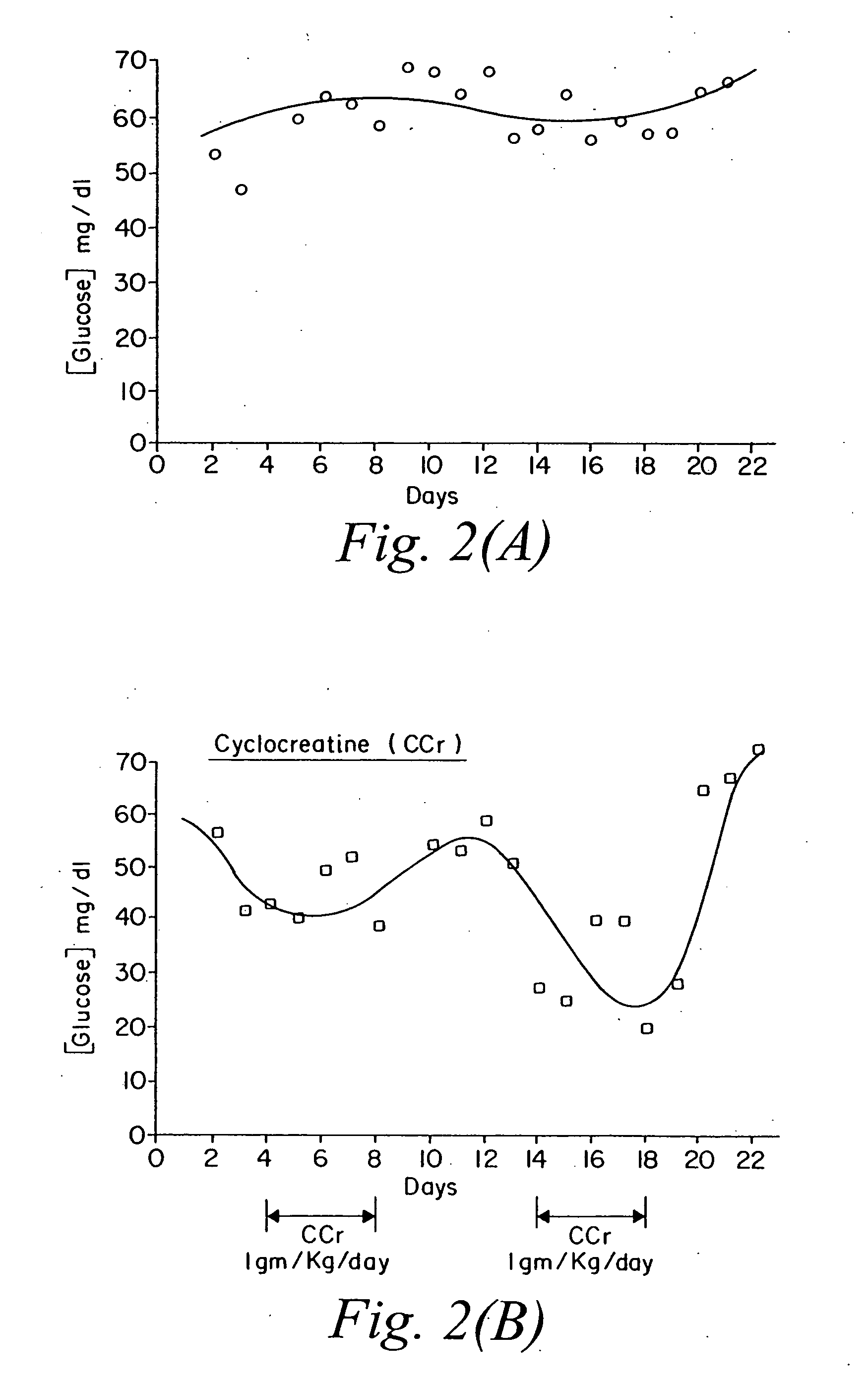
Use of creatine analogues and creatine kinase modulators for the prevention and treatment of glucose metabolic disorders
InactiveUS20050256134A1Affect glucose levelAlleviate and prevent symptomBiocidePeptide/protein ingredientsDiseaseAcute hyperglycaemia
The present invention relates to the use of creatine compounds including cyclocreatine and creatine phosphate for treating or preventing a metabolic disorder consisting of hyperglycemia, insulin dependent diabetes mellitus, impaired glucose tolerance, hyperinsulinemia, insulin insensitivity, diabetes related diseases in a patient experiencing said disorder. The creatine compounds which can be used in the present method include (1) analogues of creatine which can act as substrates or substrate analogues for the enzyme creatine kinase; (2) compounds which can act as activators or inhibitors of creatine kinase; (3) compounds which can modulate the creatine transporter (4) N-phosphocreatine analogues bearing transferable or non-transferable moieties which mimic the N-phosphoryl group. (5) compounds which modify the association of creatine kinase with other cellular components.
Owner:AVICENA GROUP +1
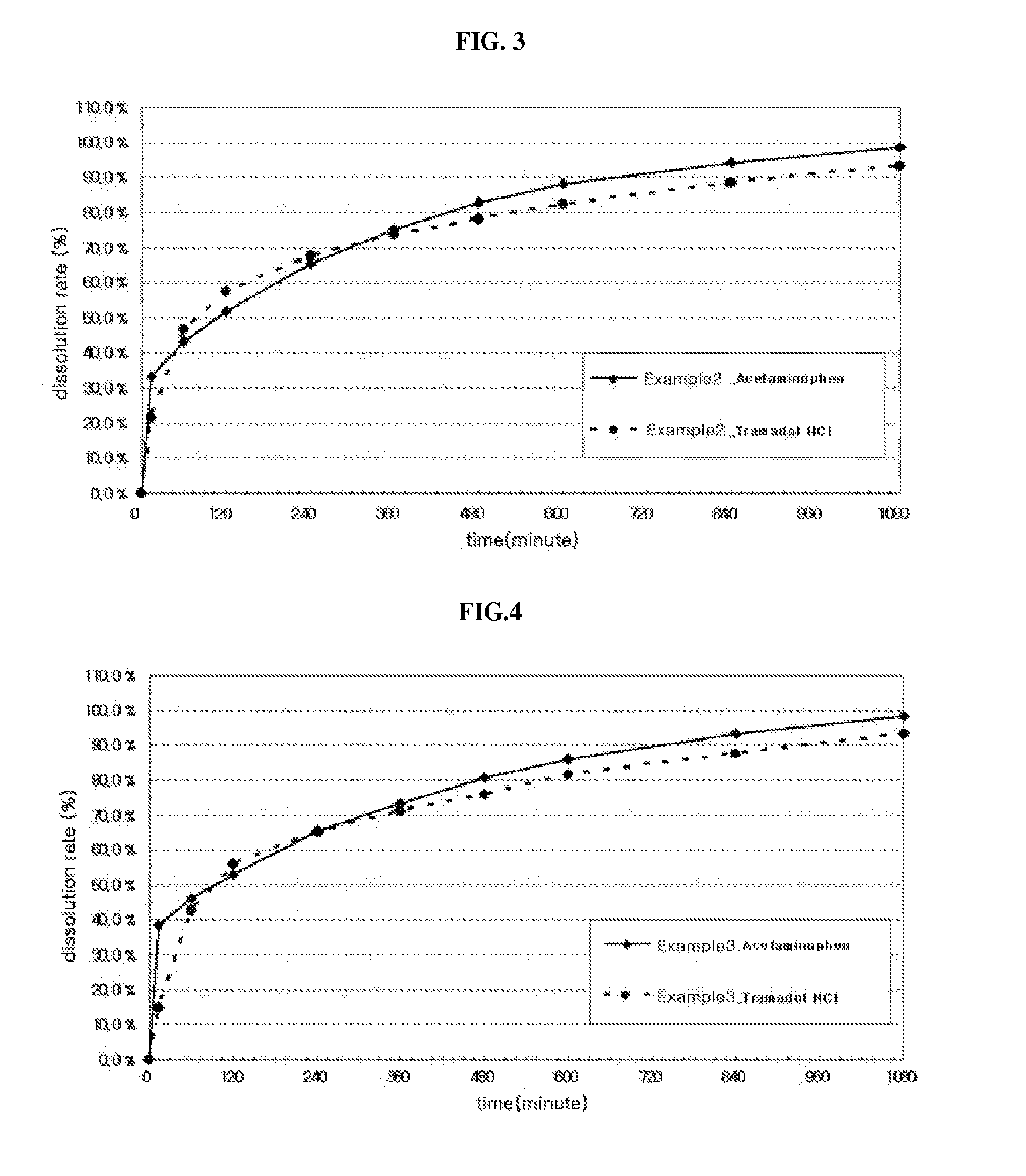
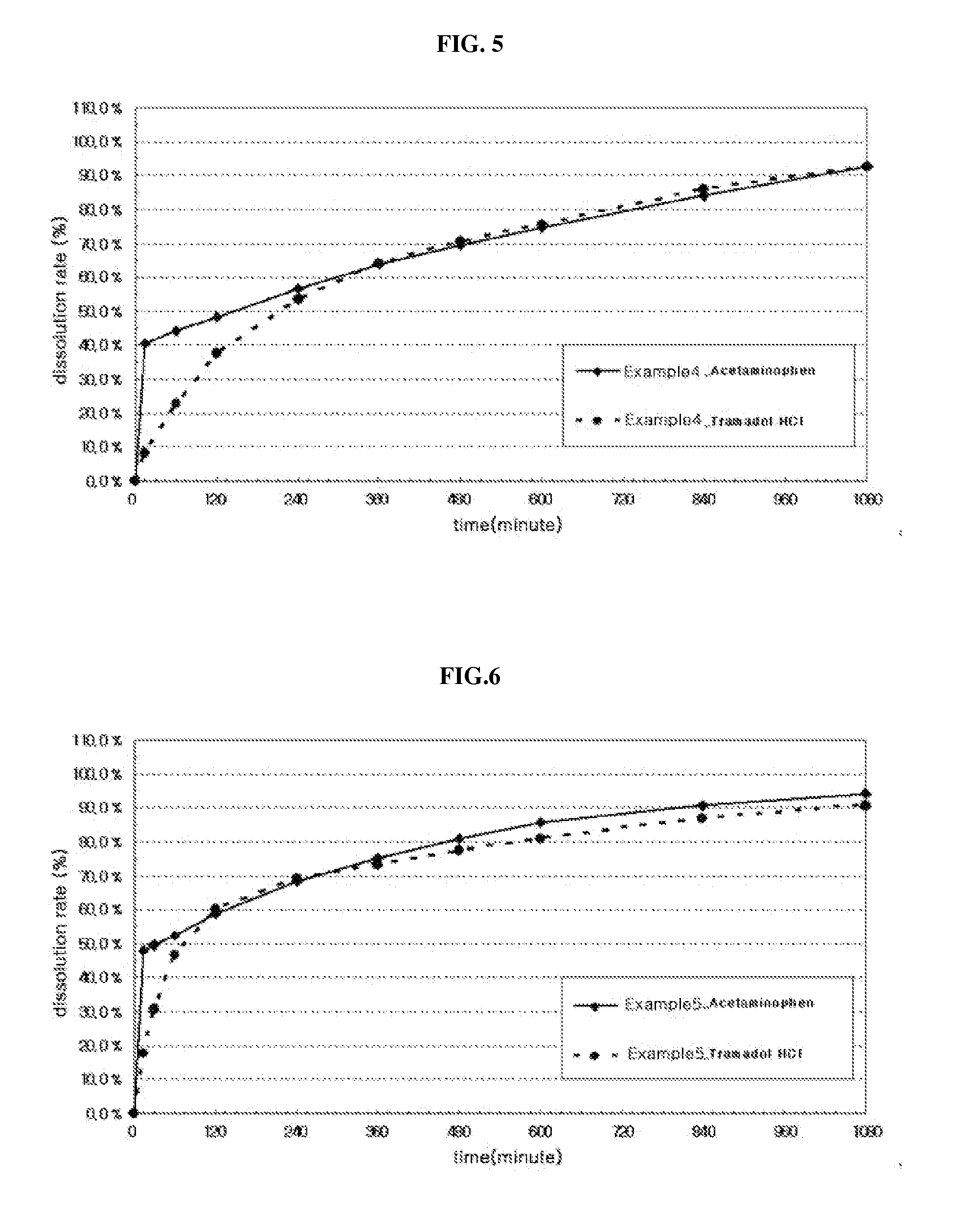
pharmaceutical composition simultaneously having rapid-acting property and long-acting property
Disclosed is a pharmaceutical composition simultaneously having a rapid acting property and a long-acting property, comprising a sustained-release part coated with a water-insoluble polymer on the surface, comprising a first active pharmaceutical ingredient, at least one release control base selected from the group consisting of water-insoluble polymer, and water-soluble viscous polymer, and a pharmaceutically acceptable carrier; and, an immediate release part comprising a second active pharmaceutical ingredient and a pharmaceutically acceptable carrier. The pharmaceutical composition exhibits independent release properties of the immediate release part and the sustained-release part by coating the surface of the sustained-release part comprising an active pharmaceutical ingredient, a release control base and a pharmaceutically acceptable carrier with a water-insoluble polymer to separate it from the immediate release part, and it may be prepared by a relatively simple process without specification limitation to the contents and the kinds of usable pharmaceutically active ingredients.
Owner:YUNGJIN PHARM CO LTD
Therapeutic Agents Targeting the NCCA-ATP Channel and Methods of Use Thereof
ActiveUS20090130083A1Expanding Therapeutic WindowAvoid depolarizationBiocideNervous disorderAbnormal tissue growthAntagonist
The present invention is directed to therapeutic compositions targeting the NCCa-ATP channel of an astrocyte, neuron or capillary endothelial cell and methods of using same. More specifically, agonists and antagonists of the NCCa-ATP channel are contemplated. The therapeutic compositions are used to treat cancer, more specifically, a metastatic brain tumor, wherein a tumor-brain barrier is present. Such treatments are contemplated in combination with conventional anti-cancer therapies. Alternatively, the compositions are used to prevent cell death and to treat cerebral edema that result from ischemia, due to interruption of blood flow, to tissue trauma or to increased tissue pressure.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com