Nanometer preparation and preparation method thereof
A technology of nanosuspension and solid preparation, which can be applied in the directions of pill delivery, pharmaceutical formulations, and medical preparations of inactive ingredients, etc., and can solve problems such as large individual differences.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
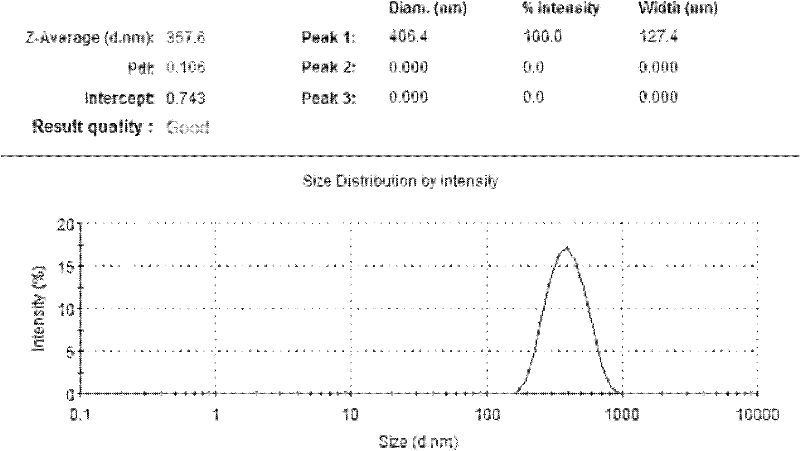
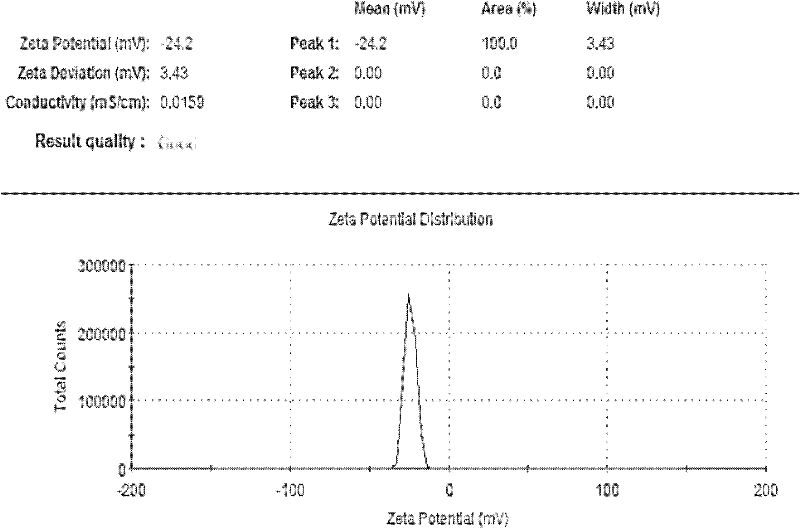
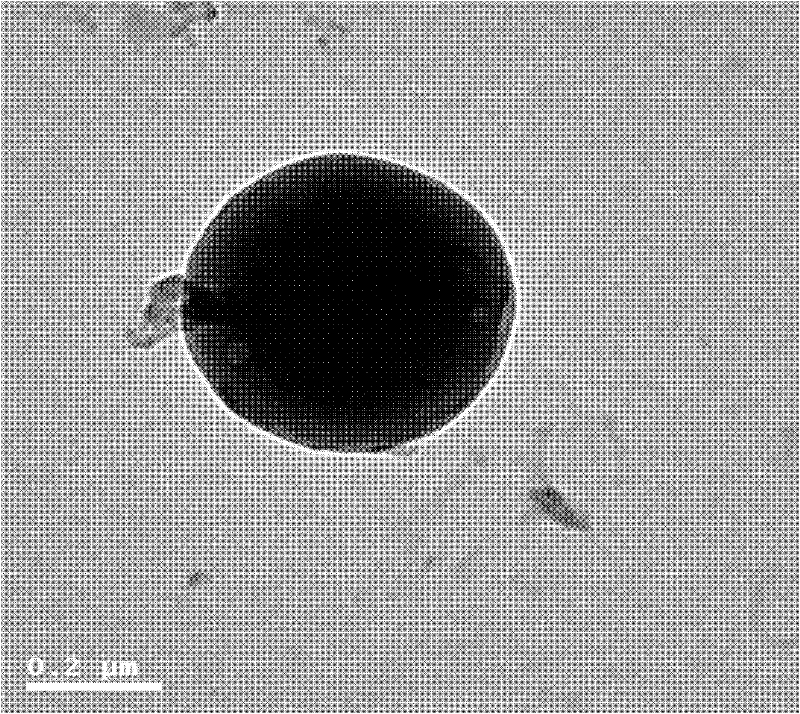
Image
Examples
Embodiment 1
[0072] The nanosuspension of present embodiment 1 contains following composition by weight percentage:
[0073] Probucol 9%
[0074] Glycerin 0.9%
[0075] Poloxamer 188 0.3%
[0076] pvp K30 0.9%
[0077] Water 88.9%
[0078] Preparation method: mix and grind probucol and glycerin; mix poloxamer 188, PVP K30 Dissolve in an appropriate amount of water, add to the above mixture, add the remaining amount of water, mix evenly, and use a high-speed dispersing homogenizer at 12000r.min -1 High-speed shear pre-emulsification for 5 minutes under high-speed conditions; then the resulting suspension was circulated with a high-pressure homogenizer at a pressure of 1500 bar for 5 cycles.
[0079] The nanosuspension solid particles of the present embodiment 1 contain the following components by weight percentage:
[0080] Probucol Nanosuspension 84%
[0081] Lactose 12%
[0082] 5% PVP by weight K30 Aqueous solution 4%
[0083] Preparation method: preheat the fl...
Embodiment 2
[0099] The nanosuspension of present embodiment 2 contains following composition by weight percentage:
[0100] Probucol 30%
[0101] Propylene Glycol 9%
[0102] Lecithin 5%
[0103] Glyceryl Behenate 5%
[0104] Water 51%
[0105] Preparation method: mix and grind probucol and propylene glycol; dissolve lecithin and glyceryl behenate in water, add to the above mixture, add the remaining amount of water, mix evenly, and use a high-speed dispersing homogenizer at 12000r. min -1 High-speed shear pre-emulsification for 5 minutes under high-speed conditions; then the resulting suspension was circulated with a high-pressure homogenizer at a pressure of 1000 bar for 6 cycles.
[0106] The nanosuspension solid particle of present embodiment 2 contains following composition by weight percentage:
[0107] Probucol Nanosuspension 50%
[0108] Glucose 21%
[0109] Sucrose 10%
[0110] Starch 10%
[0111] 3% PVP by weight K30 Aqueous solution 9%
[0112] Preparation method: p...
Embodiment 3
[0128] The nanosuspension of present embodiment 3 contains following composition by weight percentage:
[0129] Silybin 3%
[0130] Glycerin 0.1%
[0131] Poloxamer 188 0.1%
[0132] pvp K30 0.3%
[0133] Water 96.5%
[0134] Preparation method: mix and grind silybin and glycerin; mix poloxamer 188, PVP K30 Dissolve in water, add to the above mixture, add the remaining amount of water, mix evenly, use a high-speed dispersing homogenizer at 12000r.min -1 High-speed shear pre-emulsification for 5 minutes under high-speed conditions; then the resulting suspension was circulated with a high-pressure homogenizer at a pressure of 1500 bar for 5 cycles.
[0135] The nanosuspension solid particle of present embodiment 3 contains following composition by weight percentage:
[0136] Silibinin Nanosuspension 90%
[0137] Mannitol 5%
[0138] 3% PVP by weight K30 Aqueous solution 5%
[0139] Preparation method: preheat the fluidized bed at 40°C for half an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com