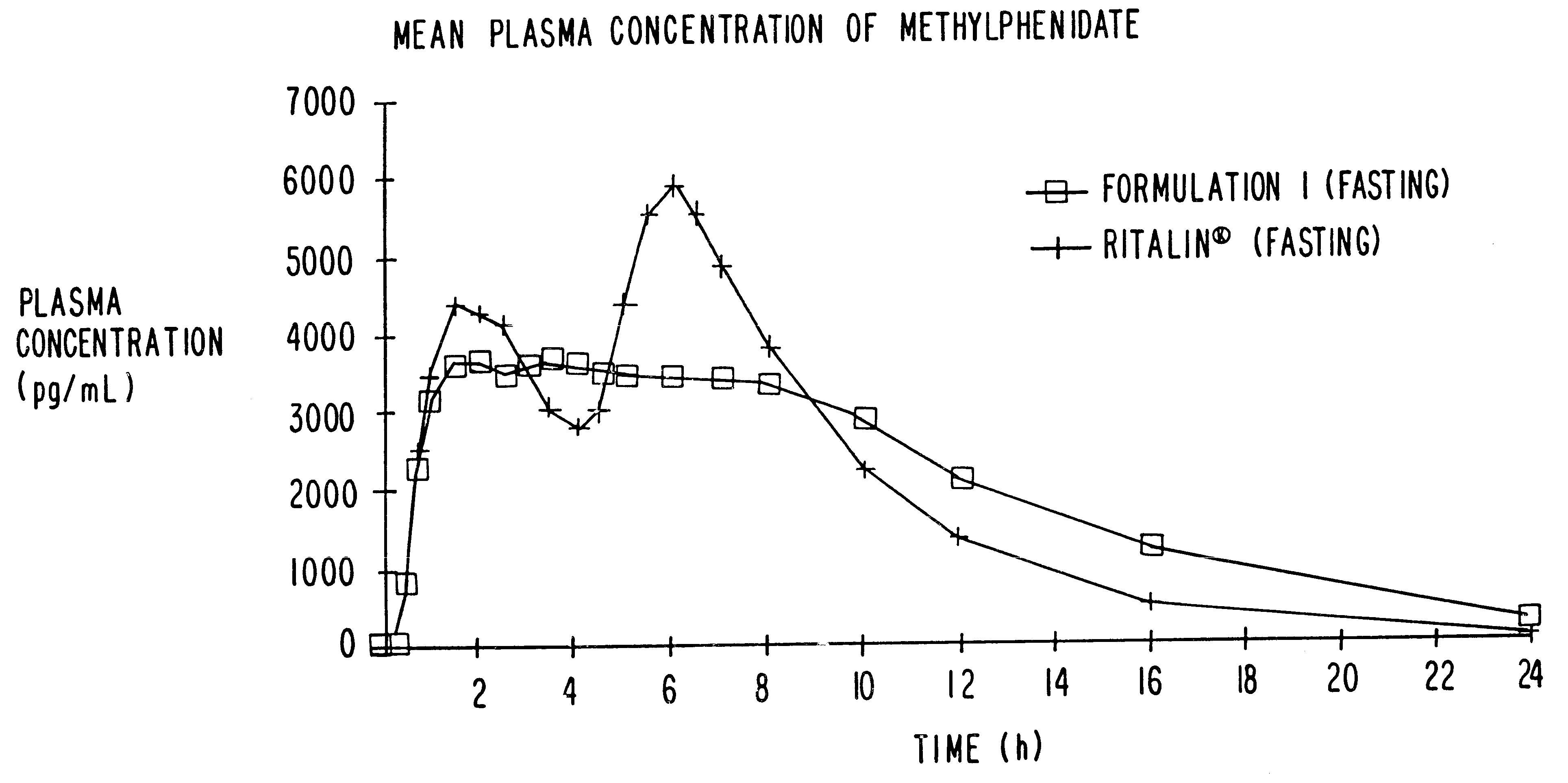
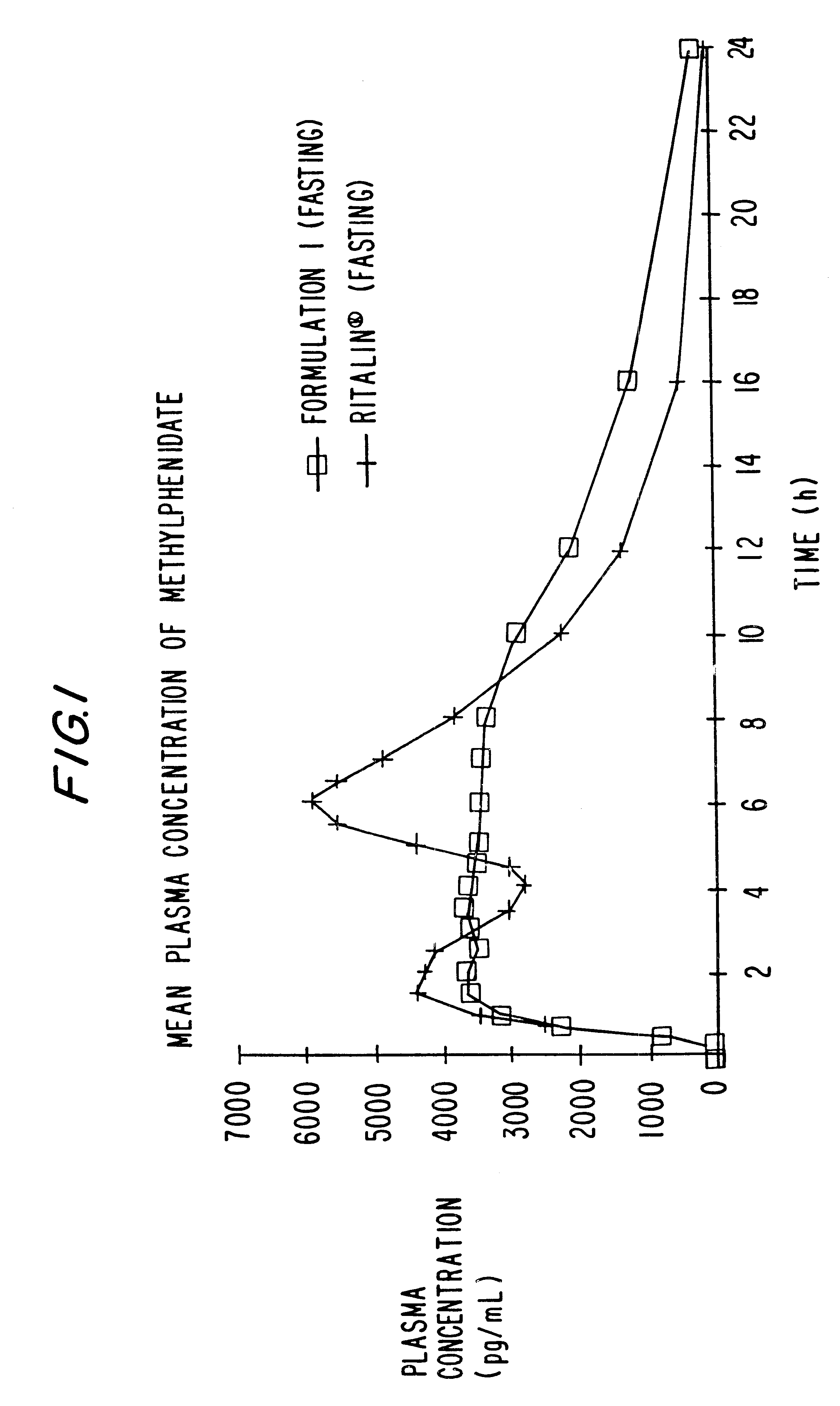
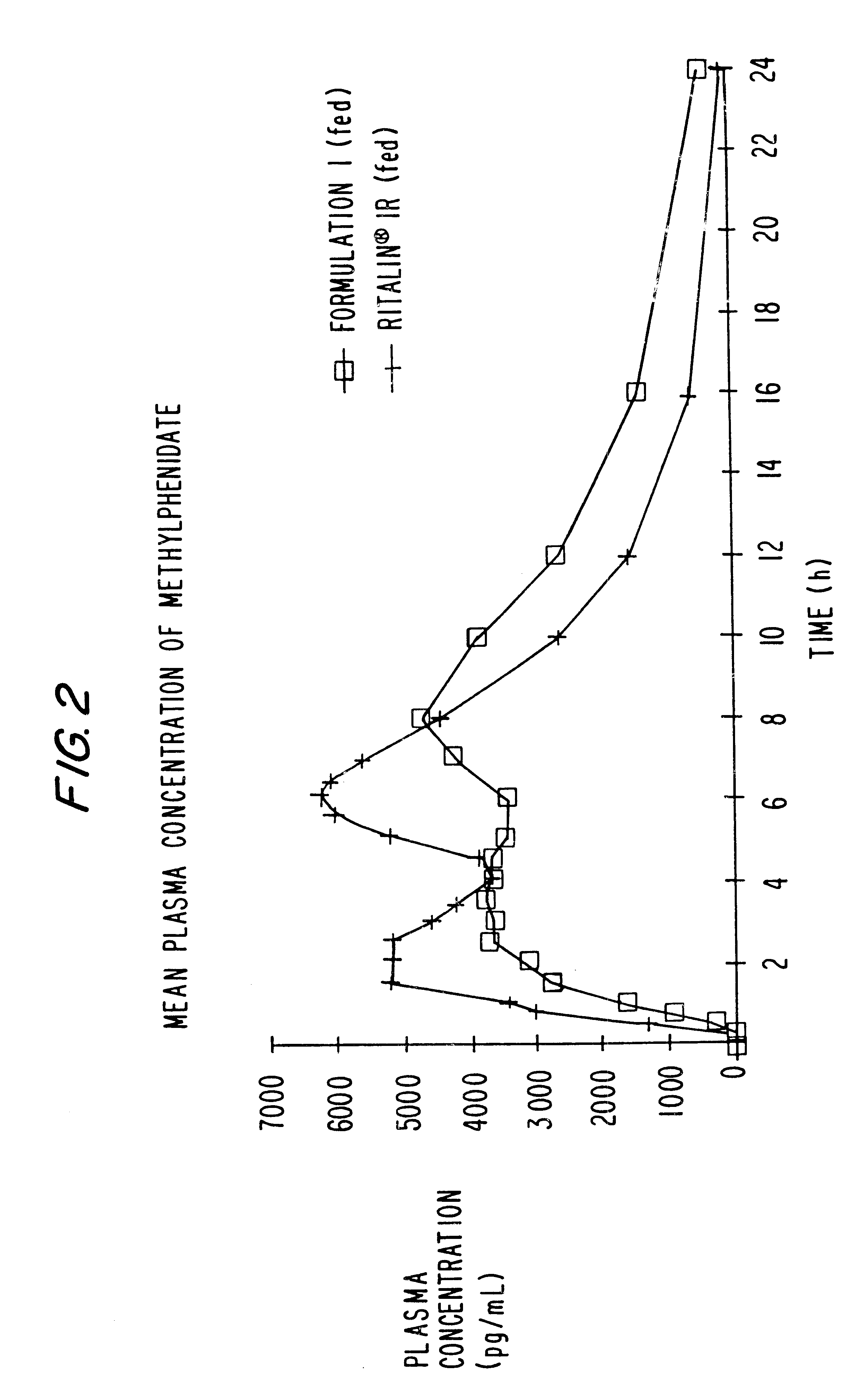
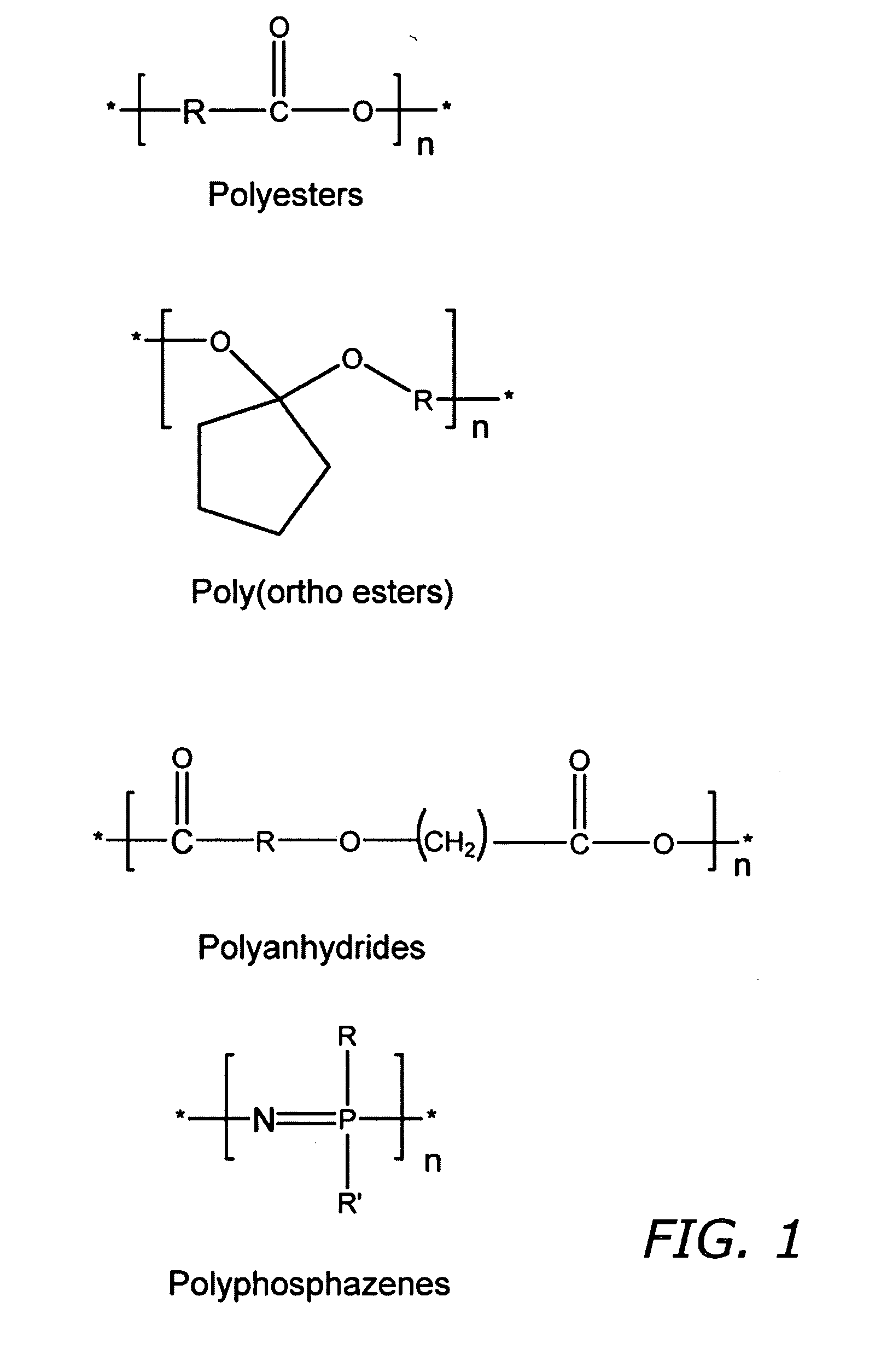
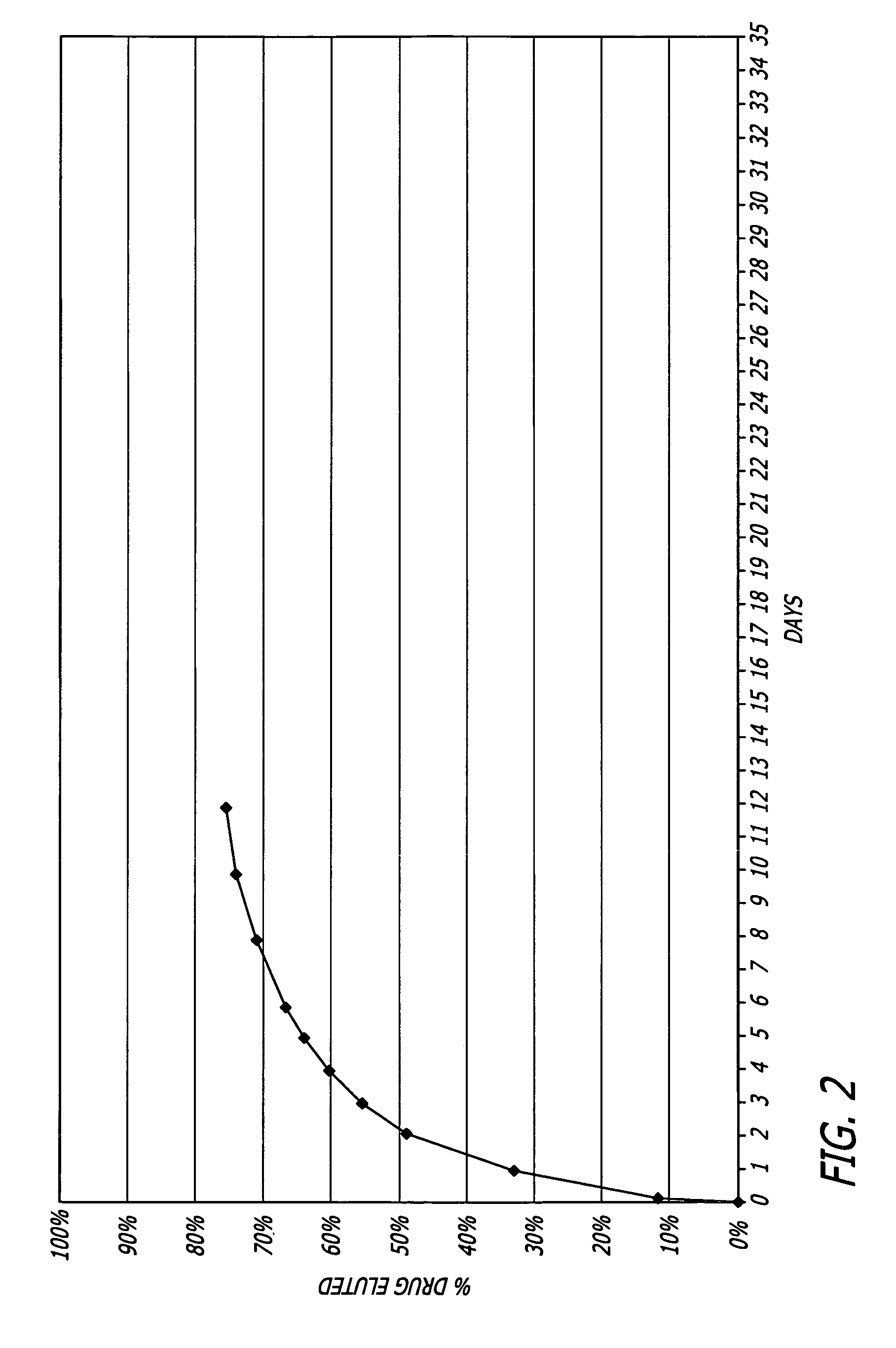
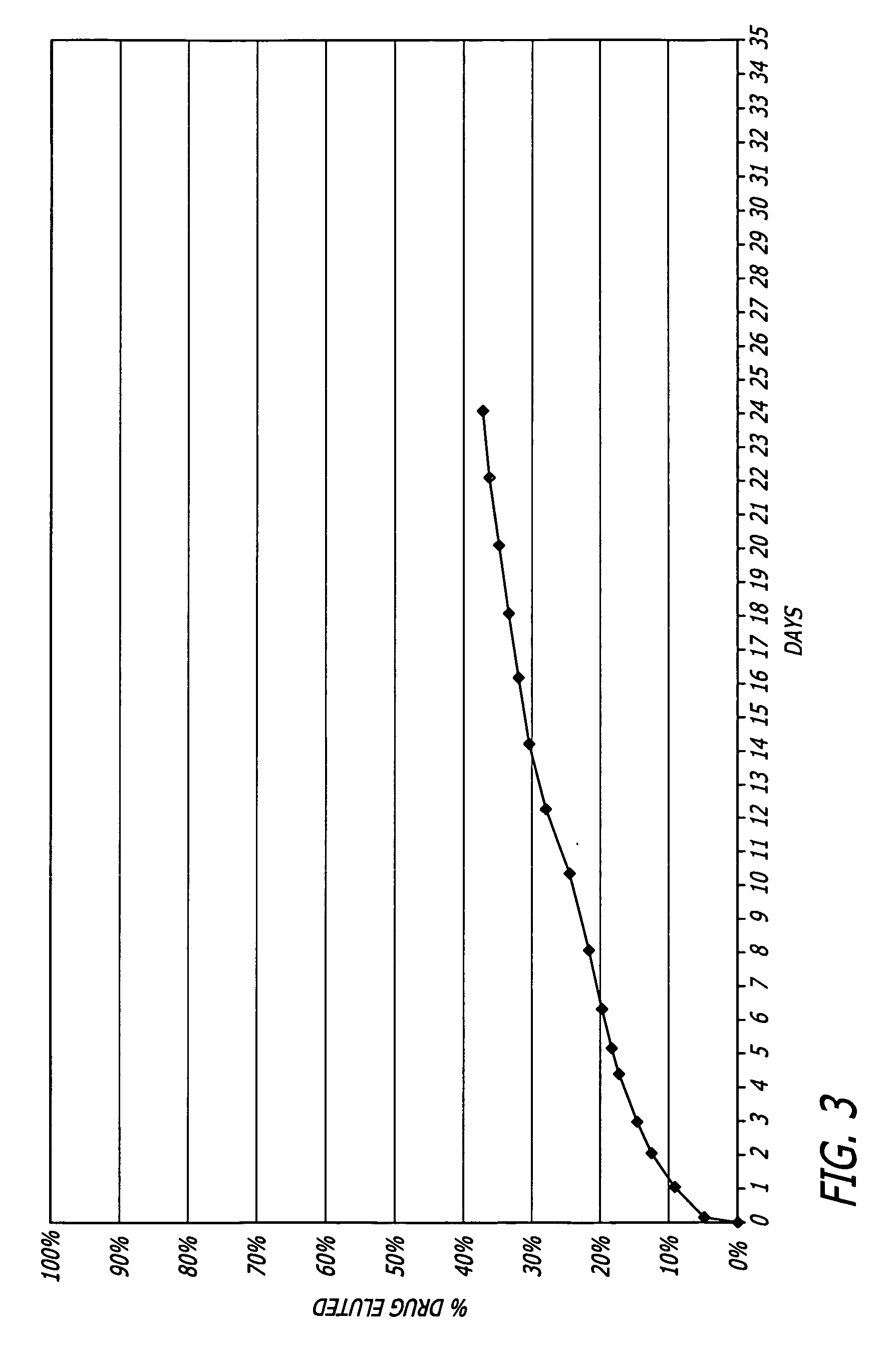
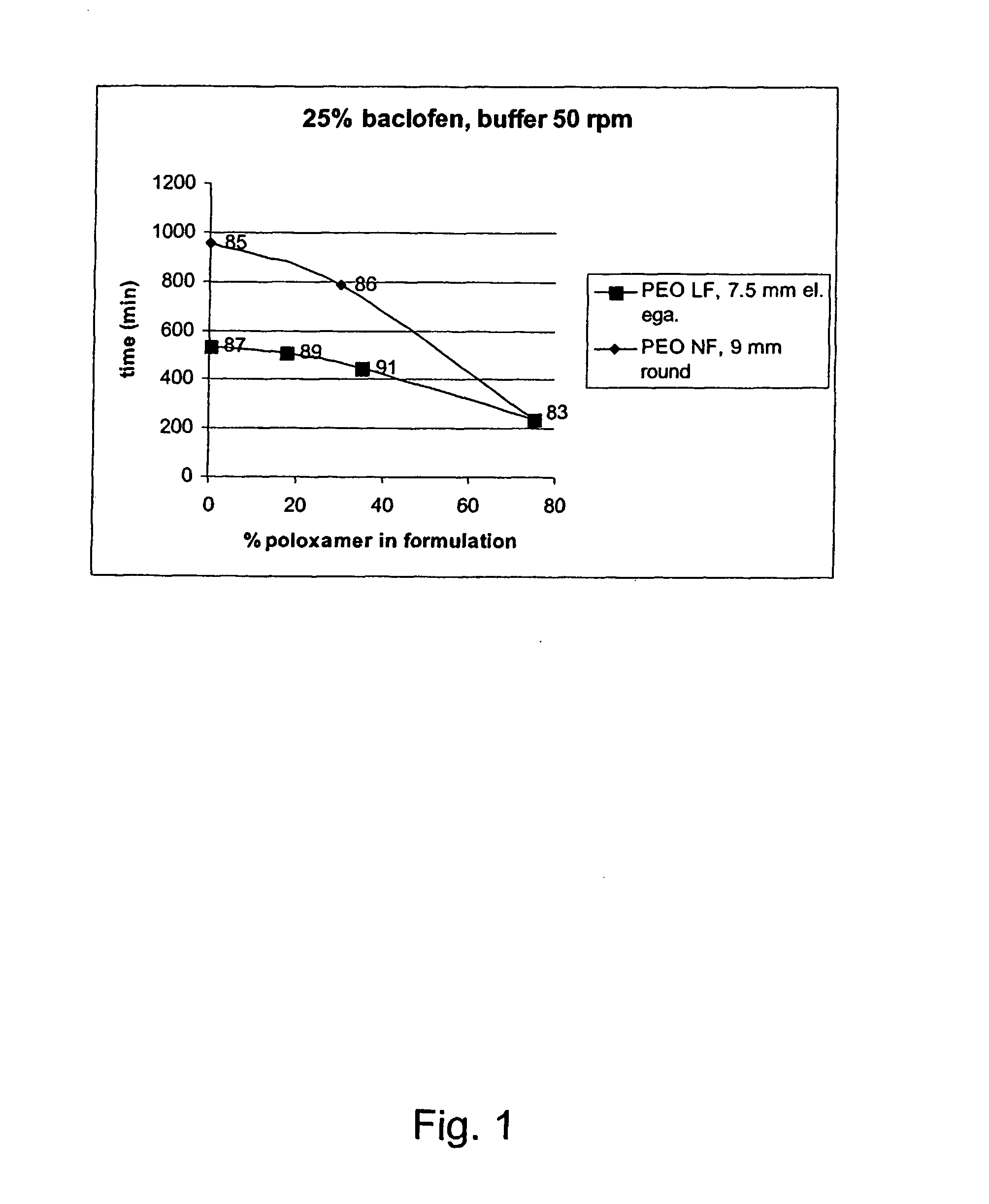
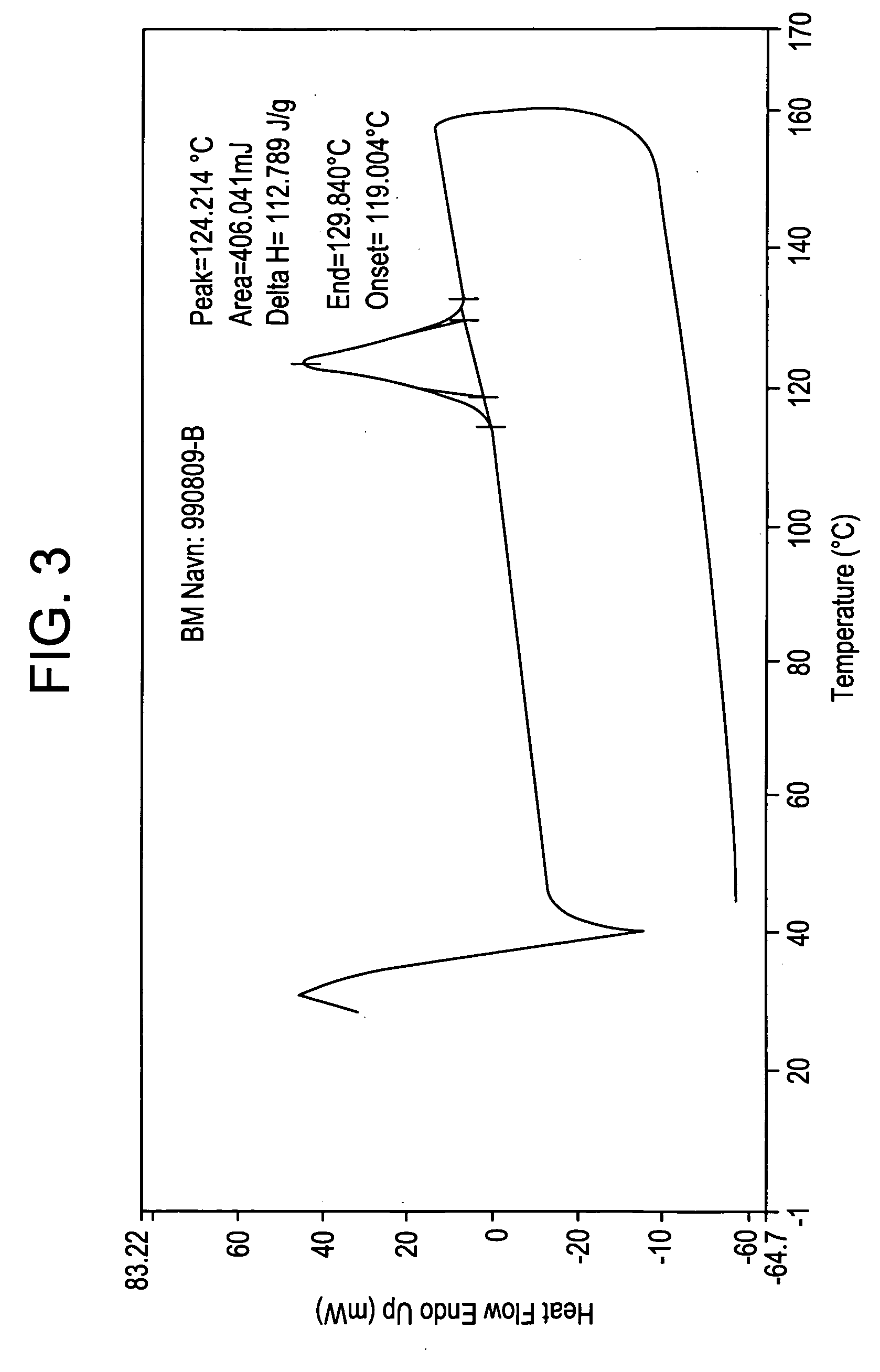
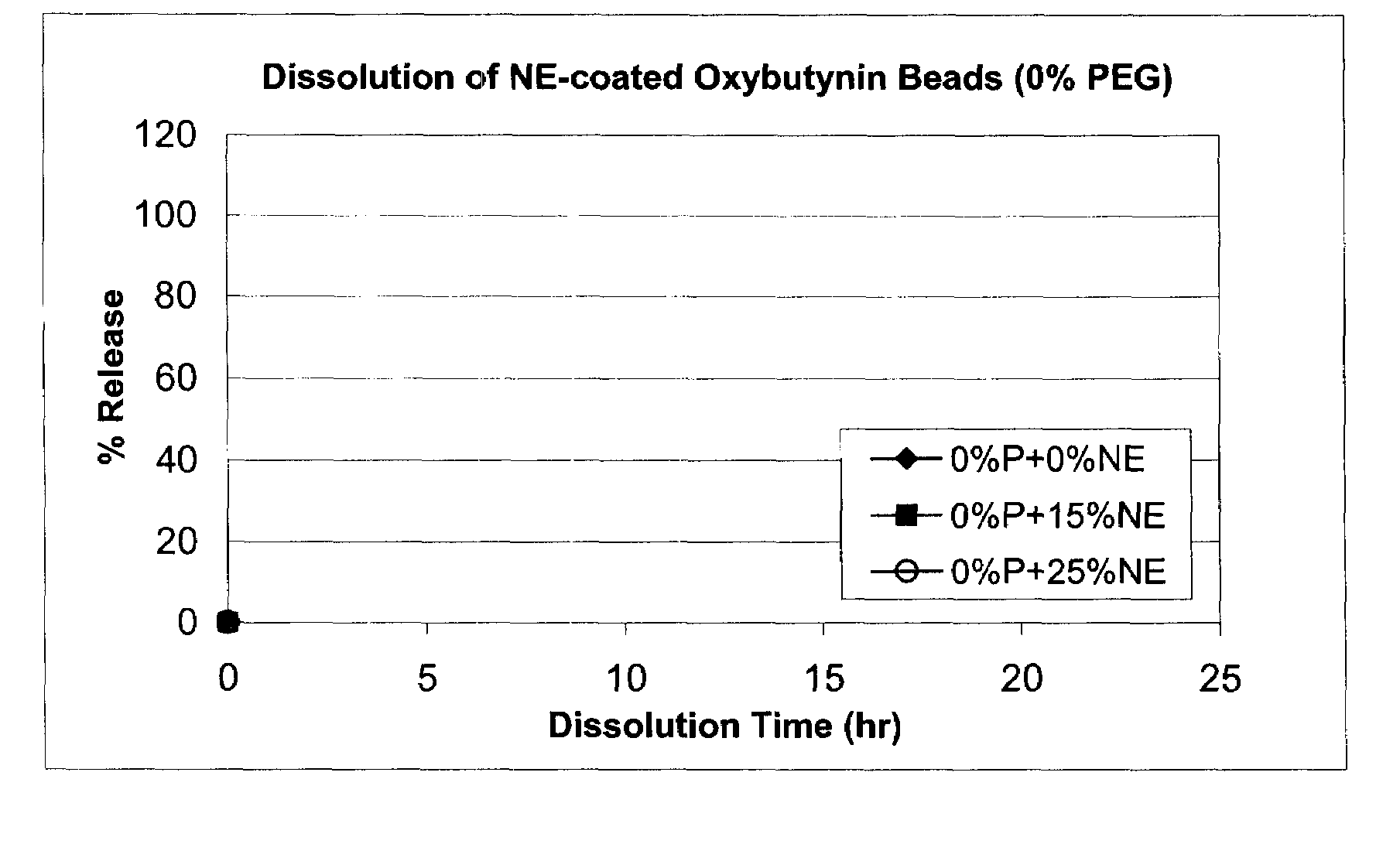
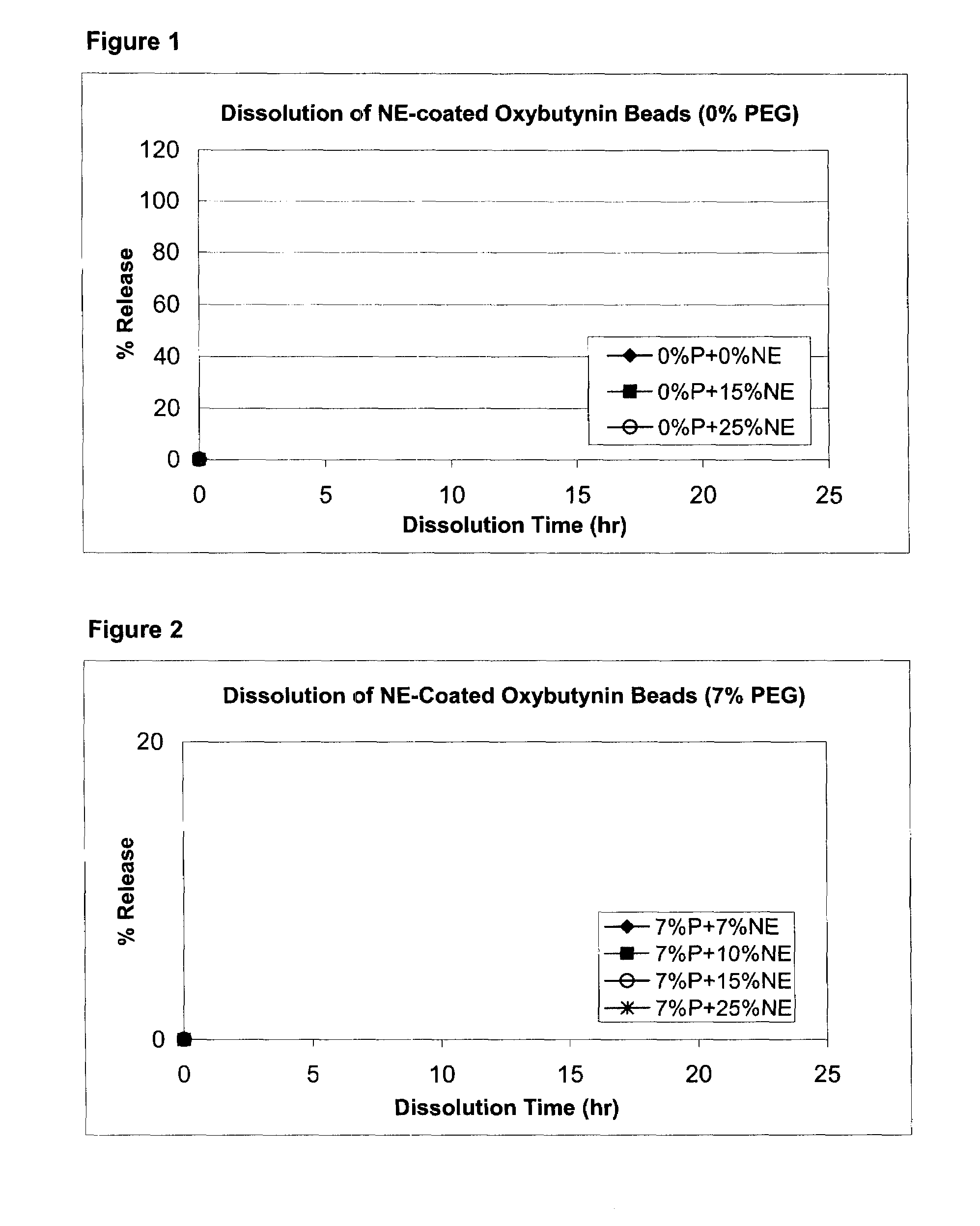
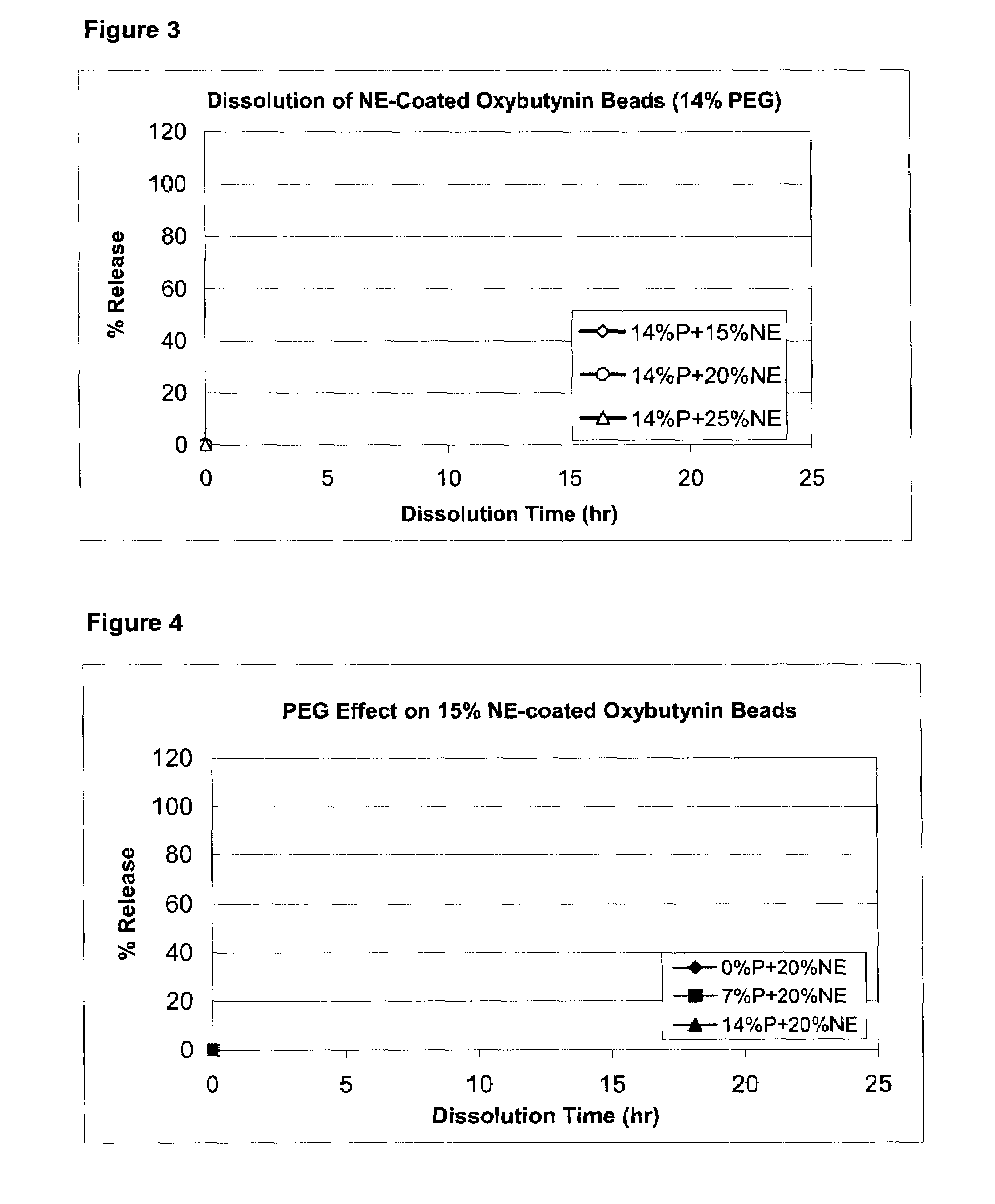
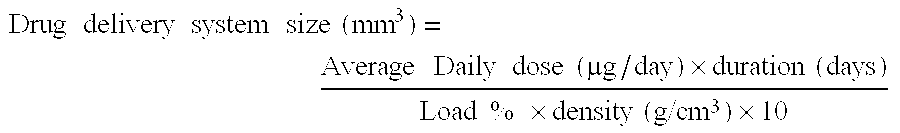Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
319 results about "Controlled release drug" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
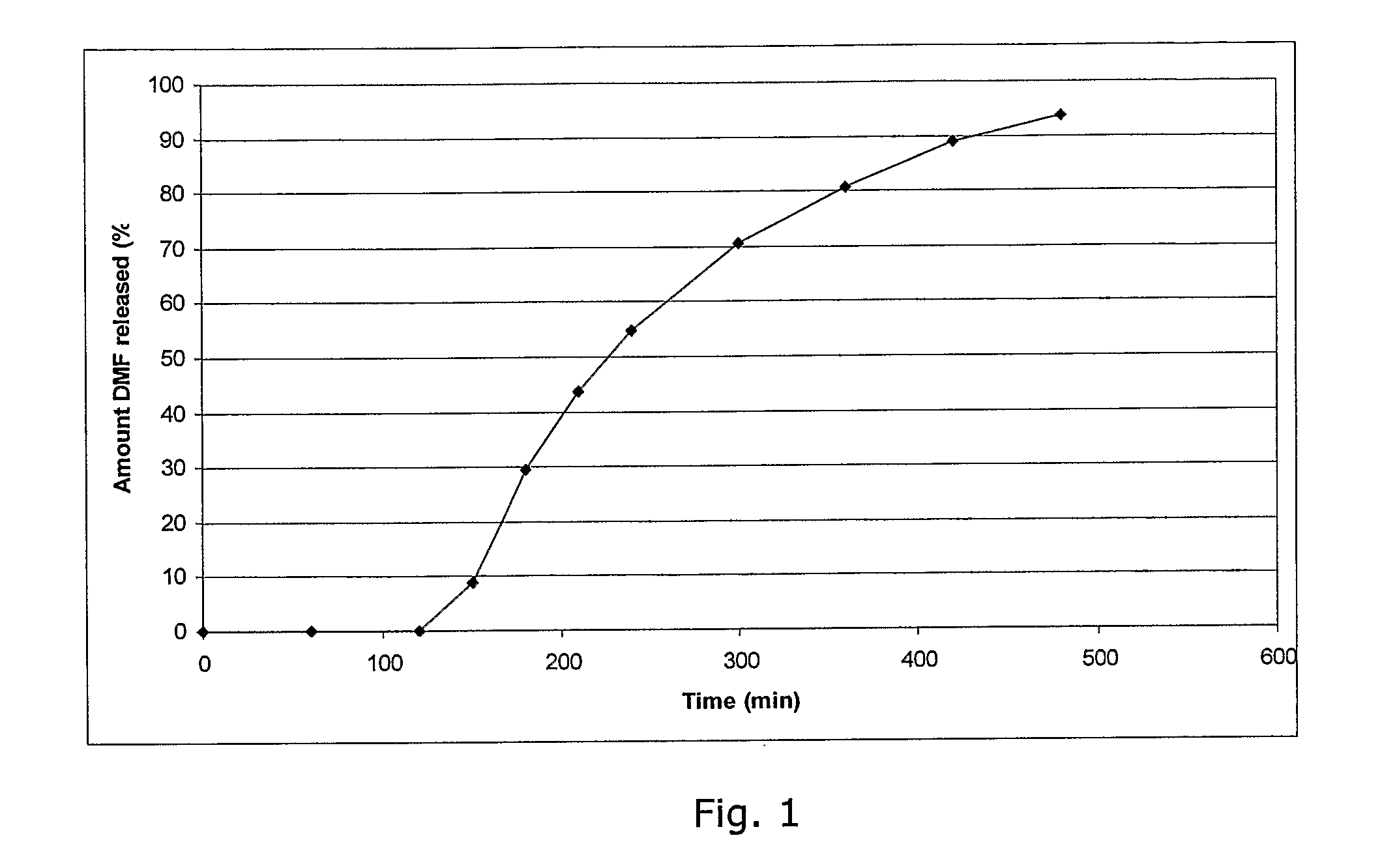
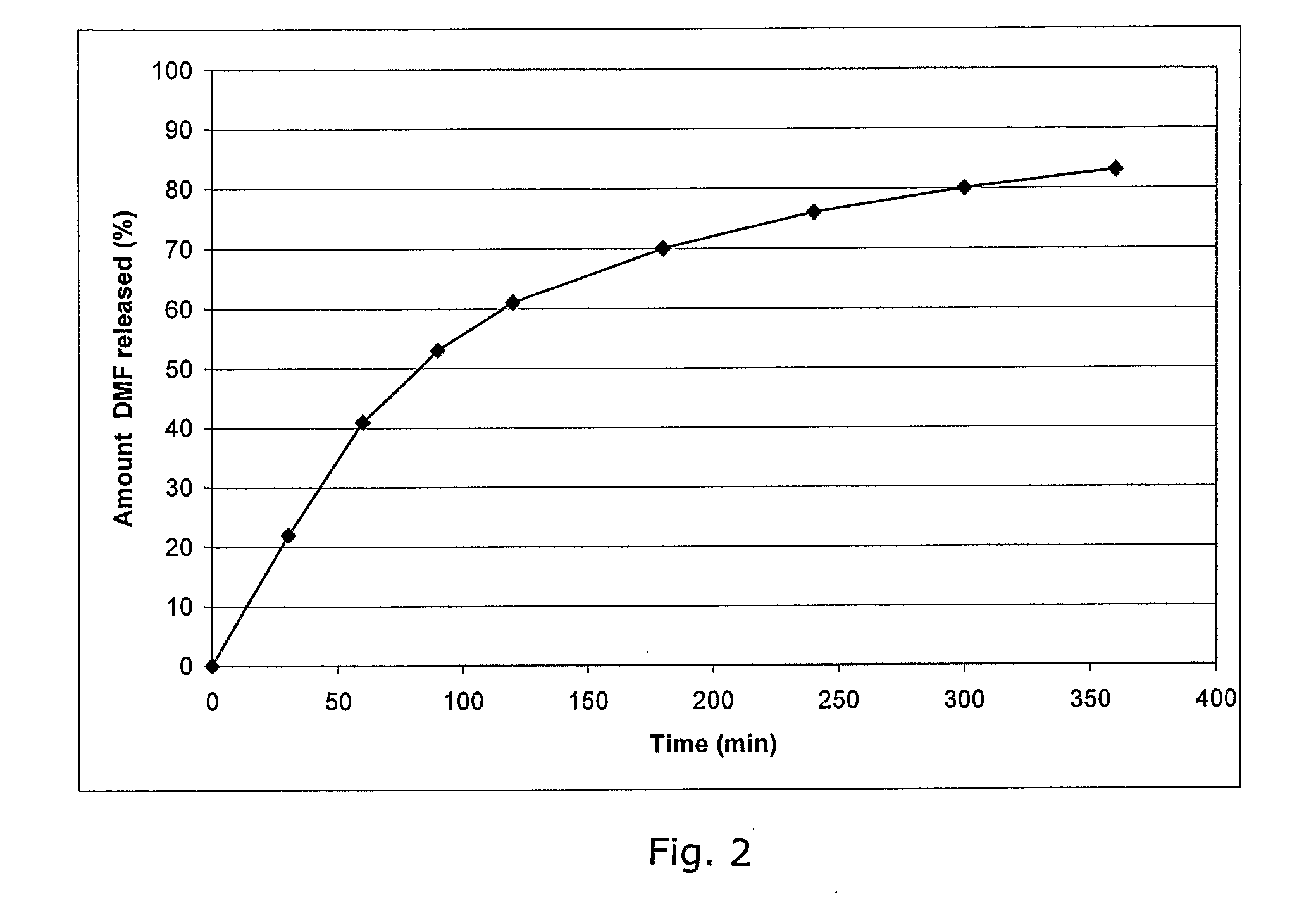
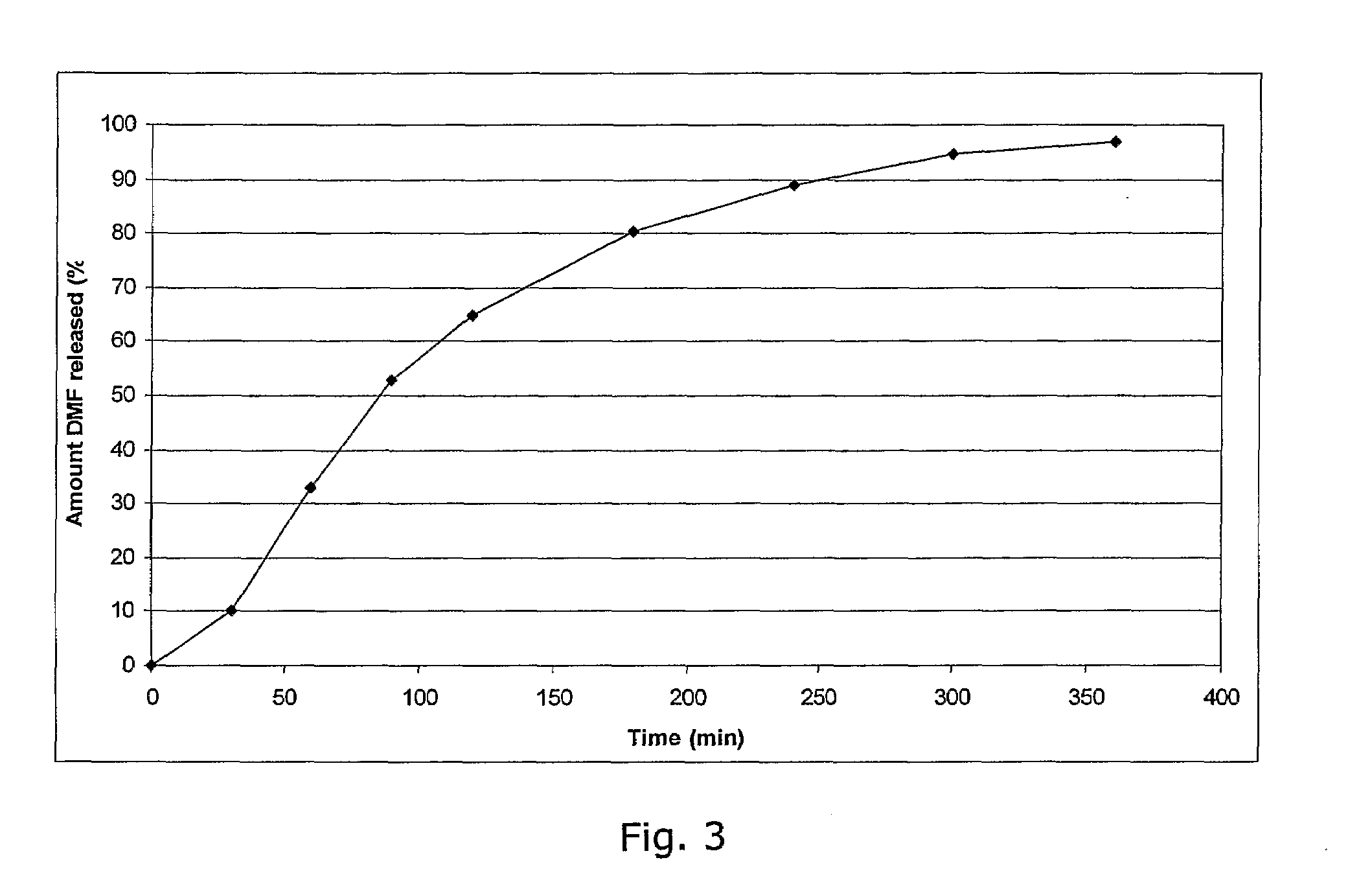
A controlled release drug, which is also called a time release drug, is any type of pill that has been engineered to release the medication inside slowly rather than all at once.
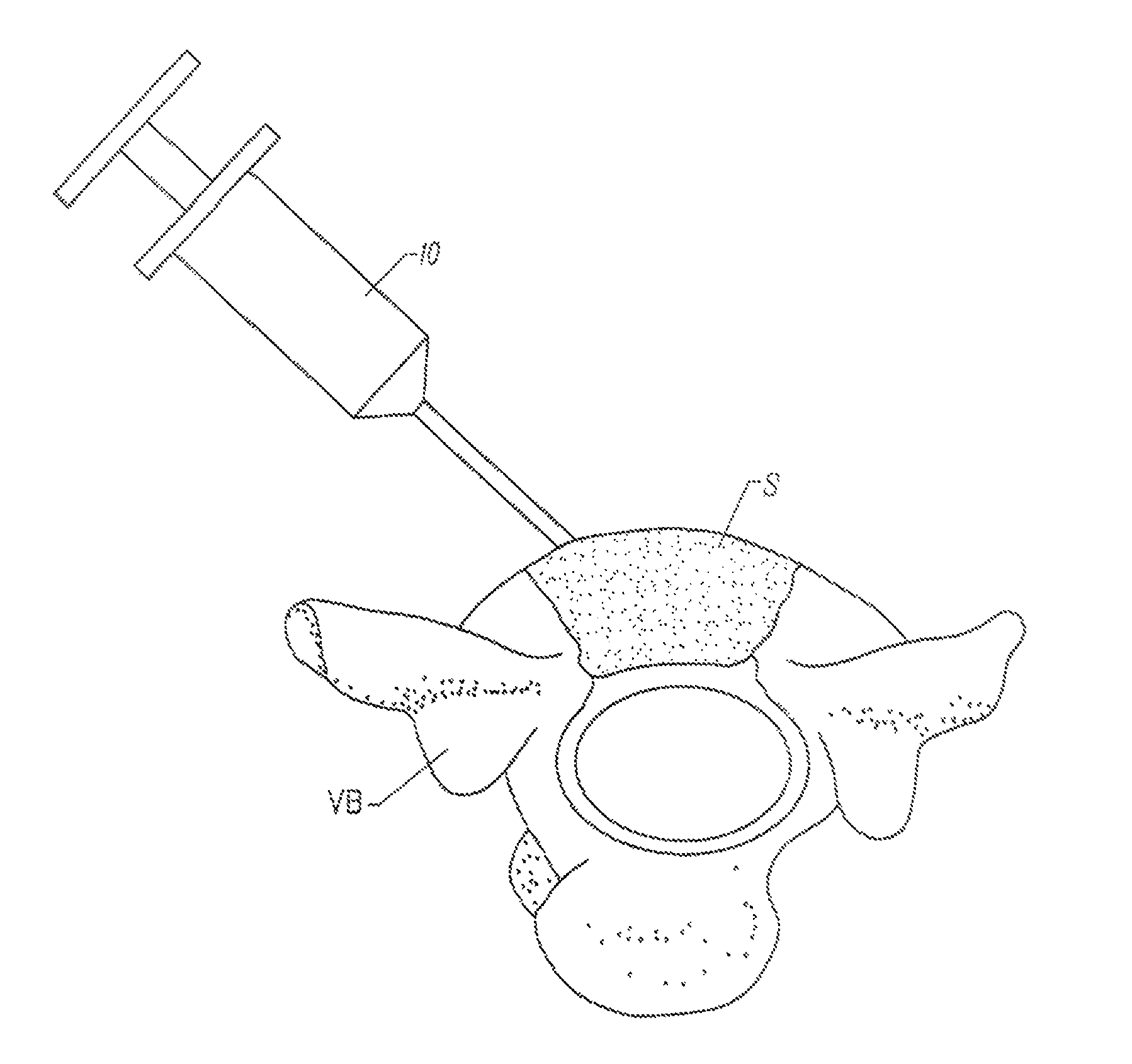
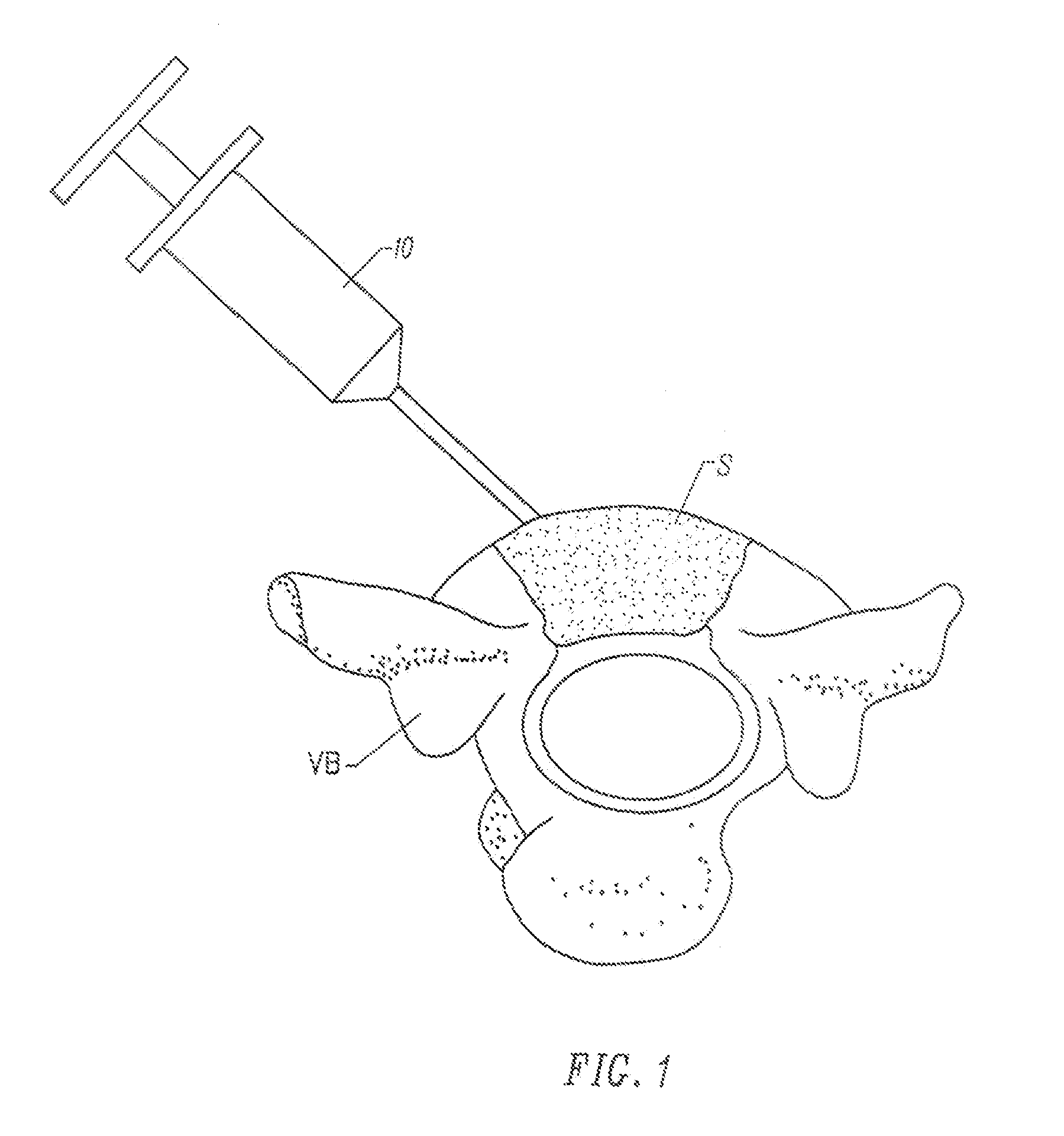
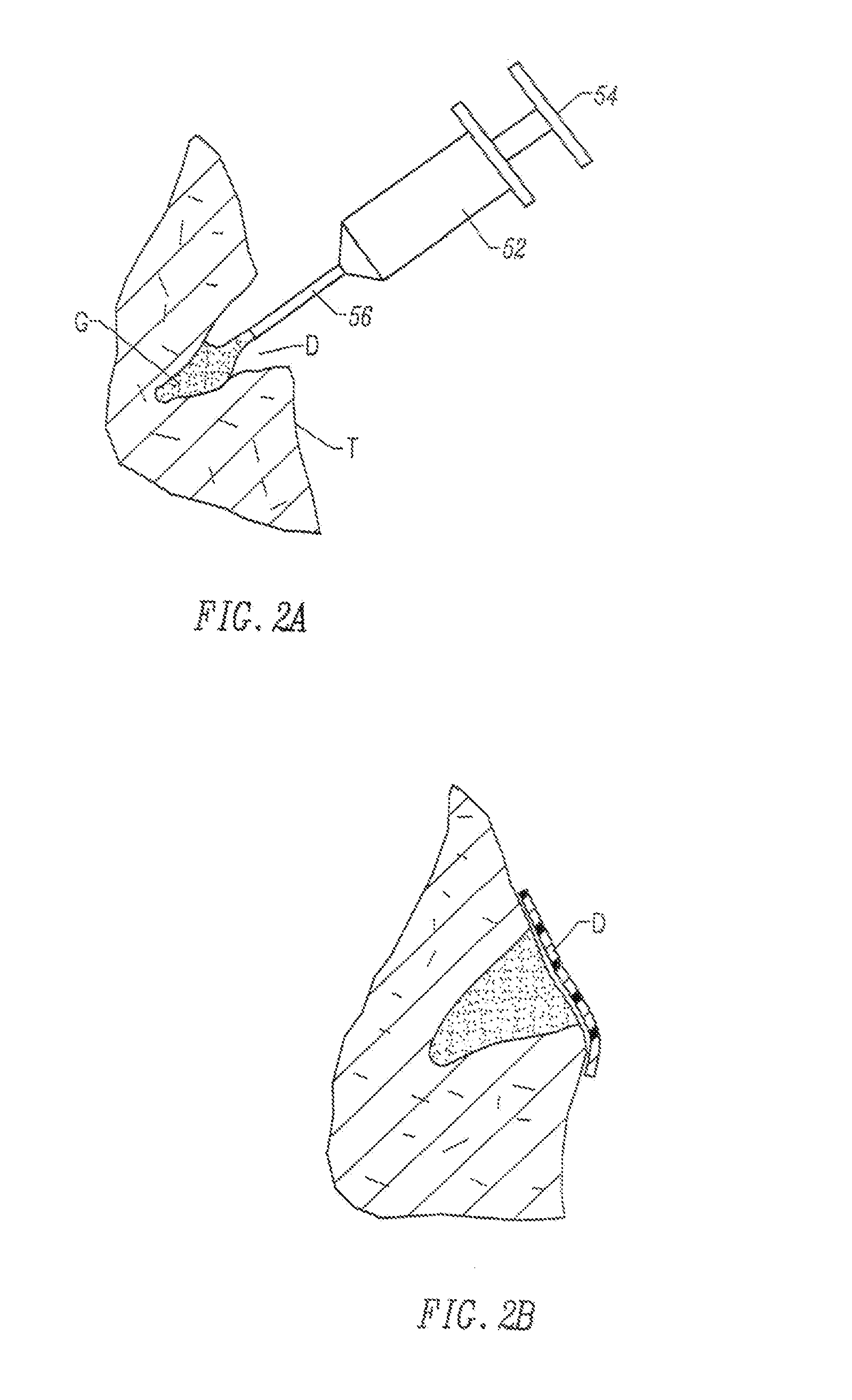
Methods and drug delivery systems for the treatment of orofacial diseases
This invention relates to methods of treating various orofacial diseases involving inflammation, infection and / or pain, using intratissue controlled release drug delivery systems. More particularly, the invention relates to methods for localized or targeted administration of a sustained release formulation of an agent such as an anti-inflammatory agent to a specified tissue location within the orofacial environment.
Owner:HALLUX
Controlled release formulations having rapid onset and rapid decline of effective plasma drug concentrations
InactiveUS6419960B1Patient compliance is goodGood retarding effectPowder deliveryOrganic active ingredientsImmediate releasePlasma drug concentration
The invention is directed to oral modified / controlled release drug formulations which provide a rapid initial onset of effect and a prolonged duration of effect. Preferably, the peak concentration is lower than that provided by the reference standard for immediate release formulations of the drug, and the duration of effect falls rapidly at the end of the dosing interval.
Owner:RHODES PHARMA LP
Controlled release pharmaceutical compositions for prolonged effect
InactiveUS20100239667A1Simple wayFew stepsOrganic active ingredientsAntipyreticEfferalganControl release
Layered pharmaceutical composition suitable for oral use in the treatment of diseases where absorption takes place over a large part of the gastrointestinal tract. The composition comprising A) a solid inner layer comprising i) an active substance, and ii) one or more disintegrants / exploding agents, one of more effervescent agents or a mixture thereof. the solid inner layer being sandwiched between two outer layers B1) and B2), each outer layer comprising iii) a substantially water soluble and / or crystalline polymer or a mixture of substantially water soluble and / or crystalline polymers, the polymer being a polyglycol in the form of one of a) a homopolymer having a MW of at least about 100,000 daltons, and b) a copolymer having a MW of at least about 2,000 daltons, or a mixture thereof, and iv) an active substance, which is the same as in said solid inner layer A), and layer A being different from layer B, the layered composition being coated with a coating C) that has at least one opening exposing at least one surface of said outer layer, the coating being substantially insoluble in and impermeable to fluids and comprising a polymer, and the composition having a cylindrical form optionally with one or more tapered ends, wherein the ratio between the surface area of one end surface of the cylinder and the length of the cylinder is in a range of from 0.02 to 45 mm.
Owner:EGALET LTD
Plasticized bioerodible controlled delivery system
InactiveUS6372245B1Easy to useExtend posting timeSolution deliveryDrug compositionsControlled releaseSufficient time
A controlled release medicament delivery system comprises a plasticized bioerodible polymer, such as a polyorthoester. Medicament desirably is entrapped in the plasticized polymer. The resulting delivery system is able to release the medicament in a controlled and sustained manner. The formulation is particularly advantageous for use as a once-a-day eyedrop. During preparation, the polymer may be heated to an elevated temperature for a sufficient time to substantially reduce its molecular weight.
Owner:INSITE VISION
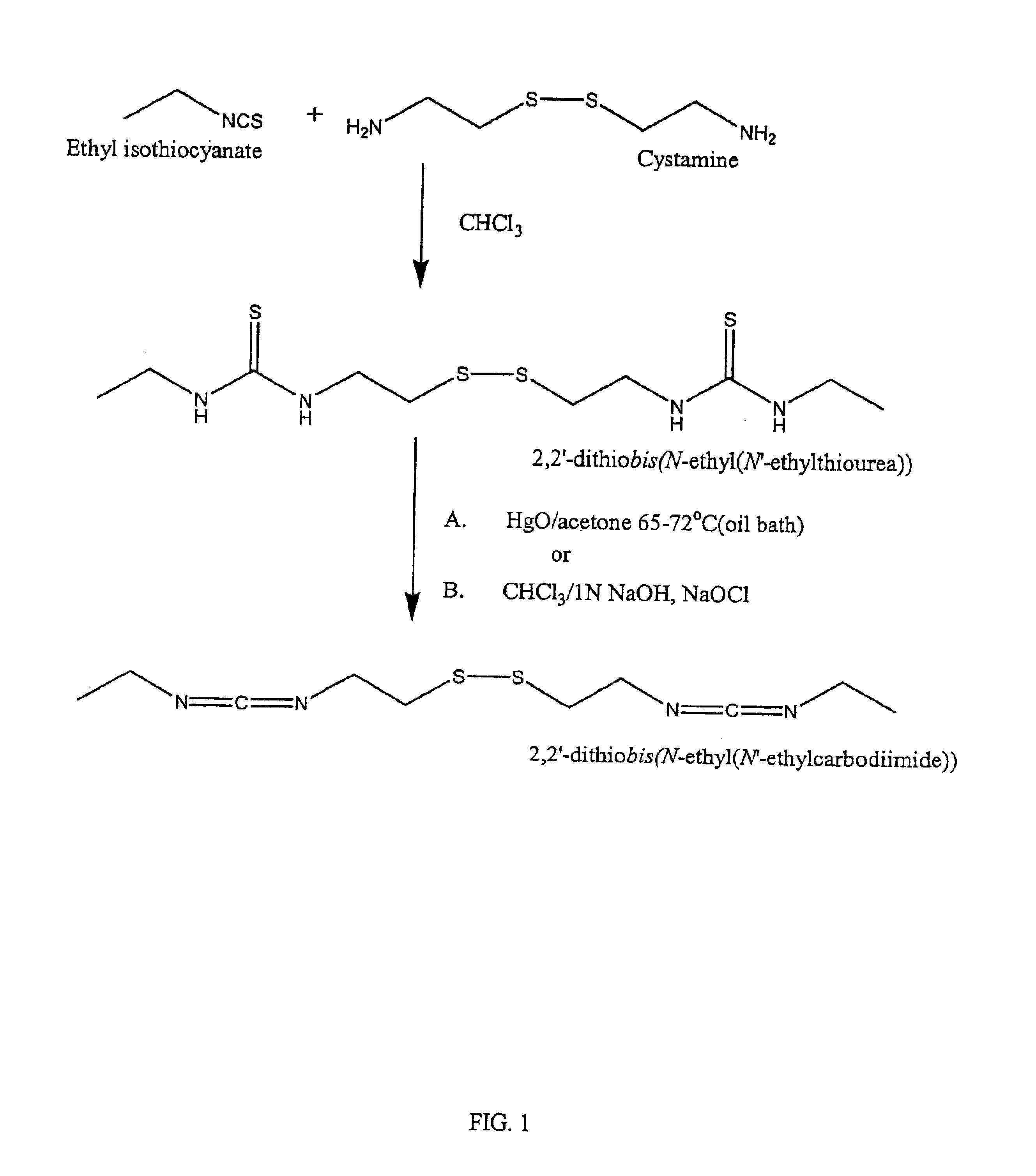
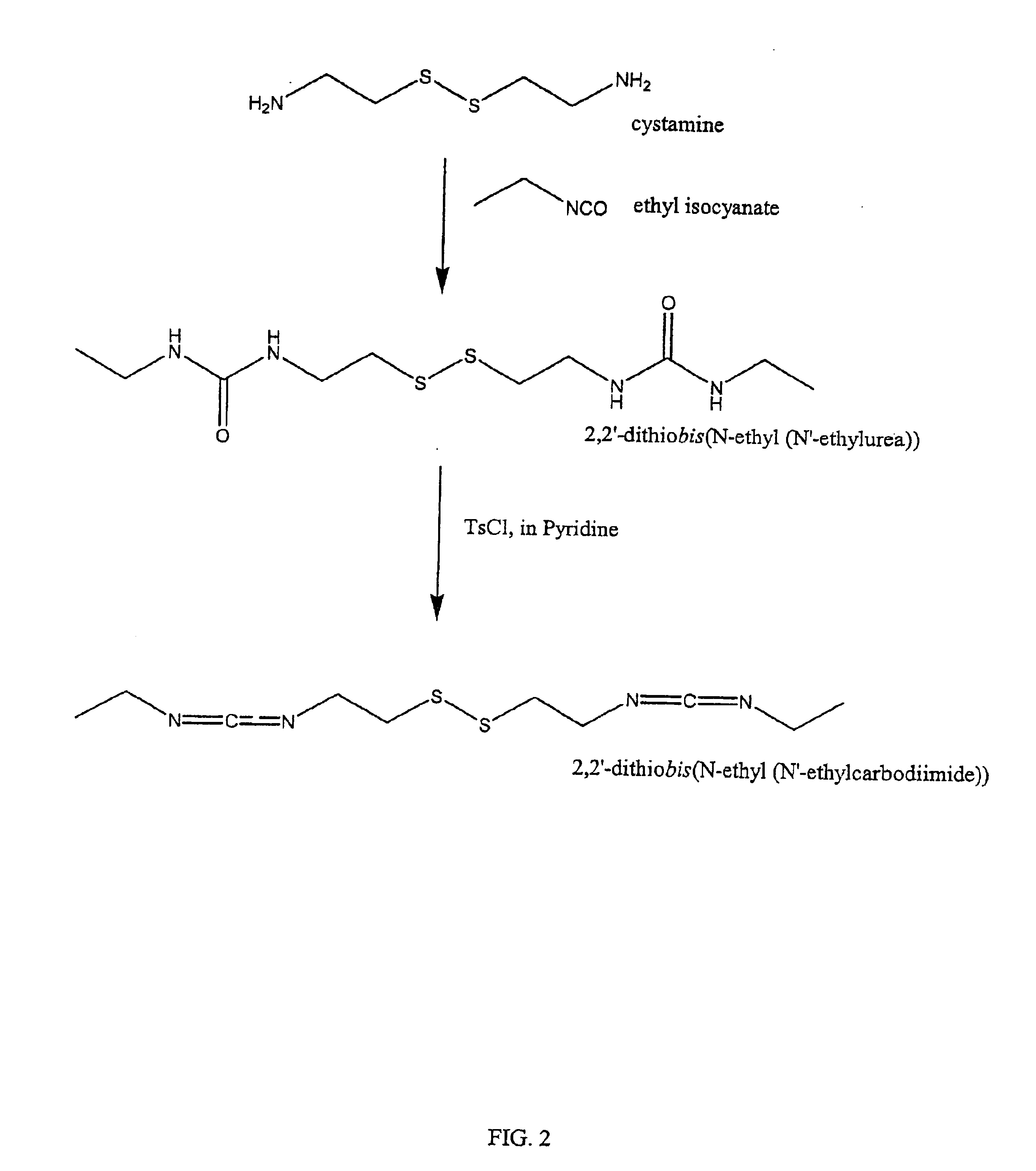
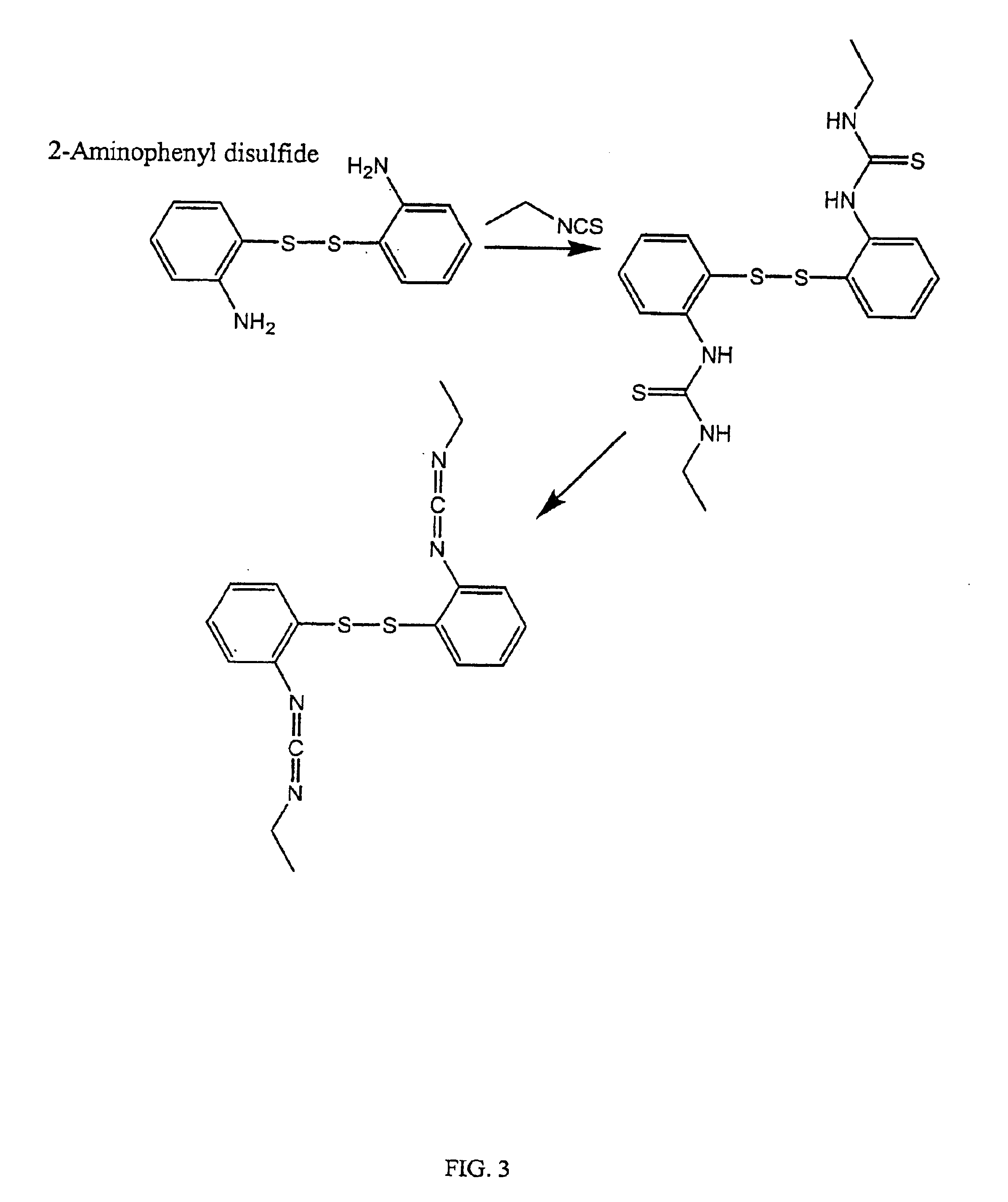
Thiol-modified hyaluronan
InactiveUS6884788B2High activityControl moreBiocideOrganic active ingredientsUrea derivativesCross-link
The present invention relates to biscarbodiimides, thiourea derivatives, urea derivatives, and cross-linked hyaluronan derivatives having at least one intramolecular disulfide bond, and methods of preparation thereof. The invention also includes thiolated hyaluronan derivatives and salts thereof having at least one pendant thiol group or a modified pendant thiol group, and methods of preparation thereof. An example of a modified pendant thiol group is a sulfhydryl group linked to a small molecule such as a bioactive agent, for example a drug or pharmaceutically active moiety. A hyaluronan derivative having a sulfhydryl group linked to a pharmaceutically active moiety is useful as a sustained or controlled release drug delivery vehicle. Compositions containing the hyaluronan derivatives of the invention can reversibly viscosify in vivo or in vitro, in response to mild changes in condition, and are thus useful in ophthalmic surgery and in tissue engineering.
Owner:ANIKA THERAPEUTICS INC
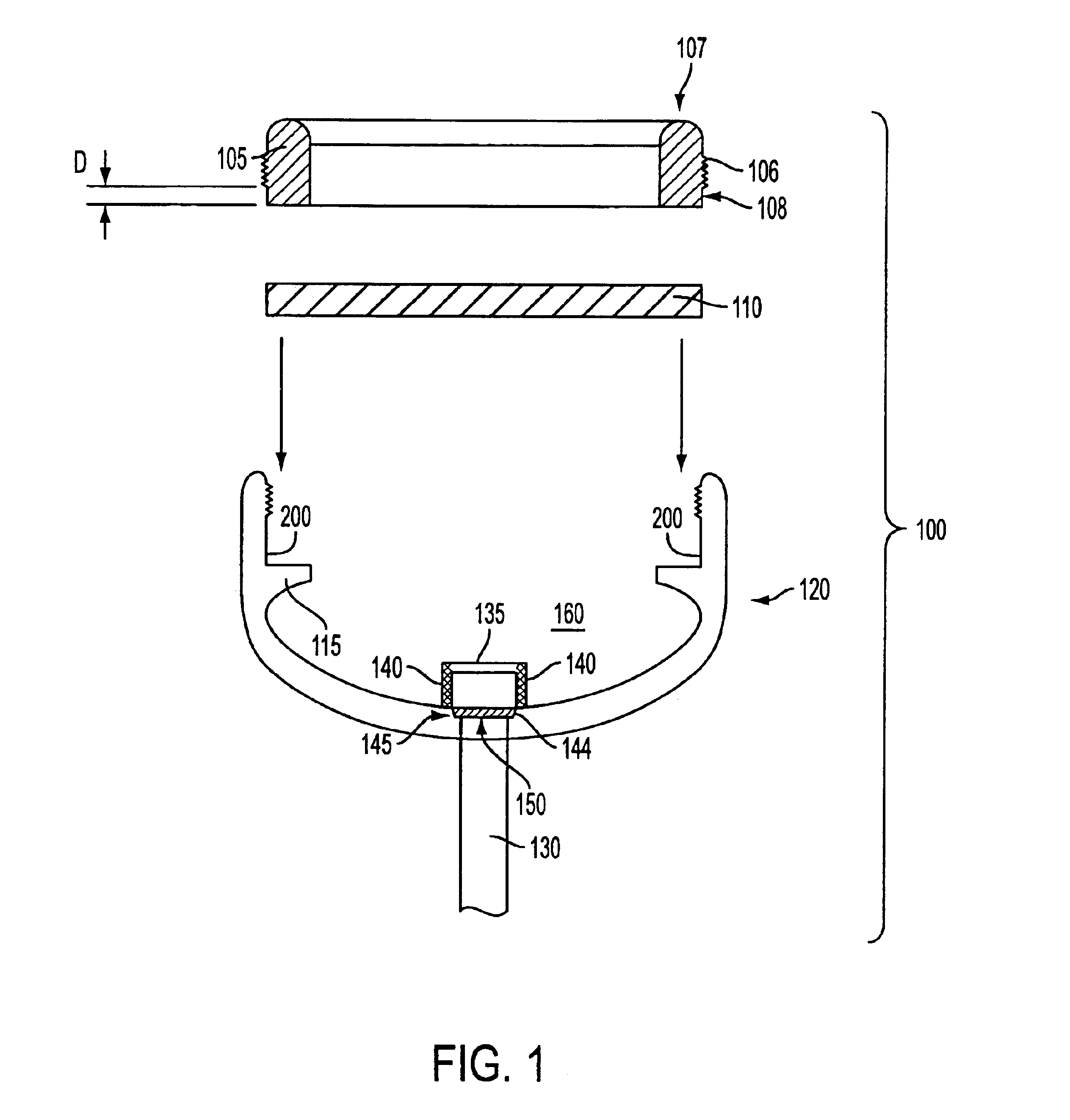
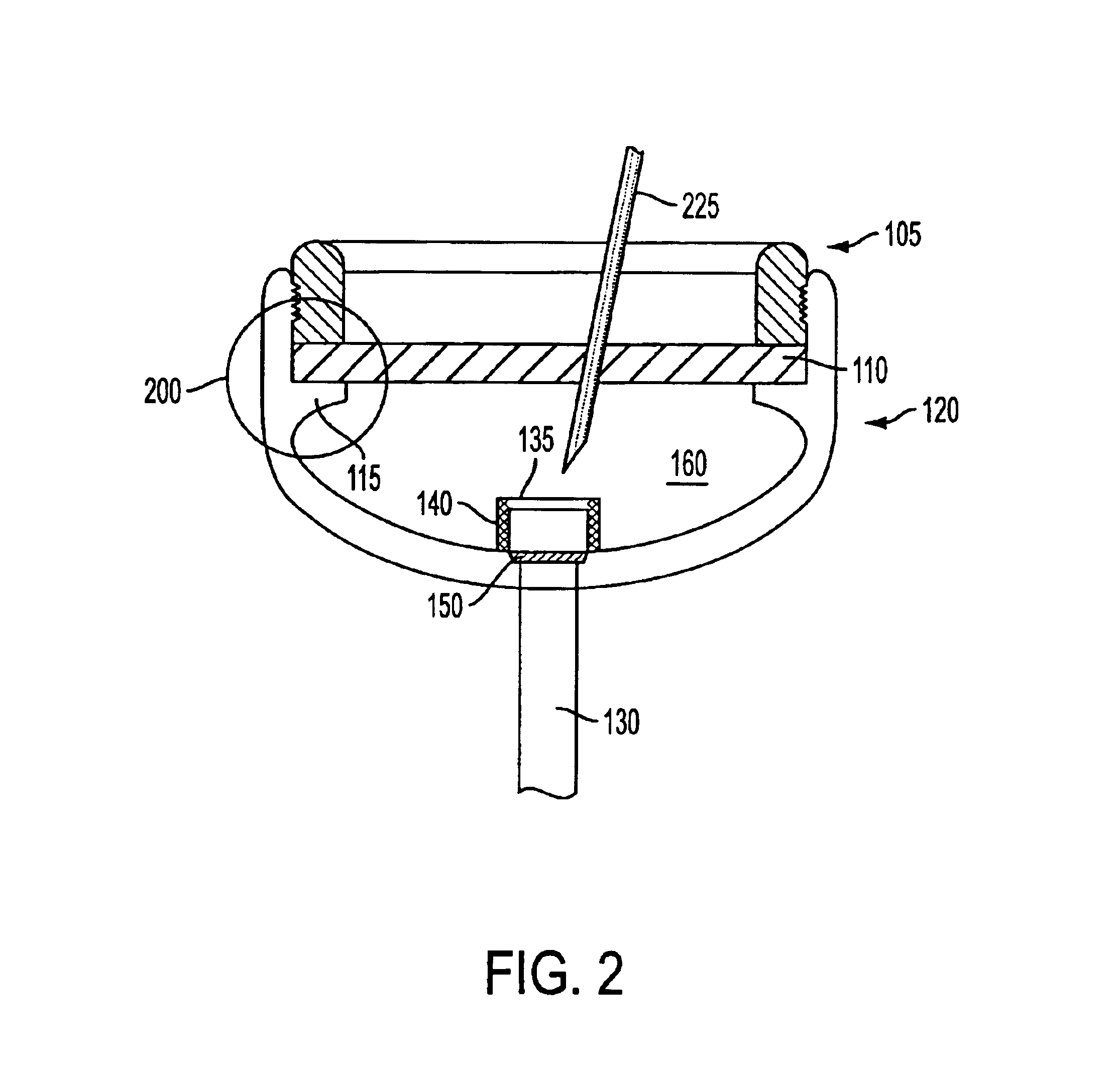
Implantable refillable and ported controlled release drug delivery device
InactiveUS6852106B2Prevent leakageEnhanced advantageSurgeryMedical devicesRate limitingDifferential pressure
An implantable, refillable, rate controlled drug delivery device is disclosed that includes a base structure having at least a first opening and a second opening, the base structure defining a chamber, a septum covering the first opening and configured to substantially prevent leakage from the first opening to an exterior of the device, a drug delivery tube comprising a first and second distal end, wherein the first distal end of the tube communicates with the chamber through the second opening, and at least one rate-limiting permeable membrane disposed across a passage between the base structure and the second distal end of the drug delivery tube, which membrane passively regulates drug delivery. The drug delivery device is used to provide controlled drug delivery to an internal portion of the body and is advantageously leak-proof and does not rely on a pressure differential to drive the drug from the device.
Owner:CONTROL DELIVERY SYST
Metering and packaging of controlled release medication
InactiveUS7404968B2Powder deliveryPeptide/protein ingredientsControlled releaseBiomedical engineering
Controlled quantities of powdered medication are formed in controlled release packages using electrostating metering. Also provided are combination medication therapy delivery packages comprising two or more active pharmaceuticals segregated from one another in a single delivery package.
Owner:MICRODOSE THERAPEUTX INC
Controlled release pharmaceutical compositions comprising a fumaric acid ester
The present invention relates to controlled release pharmaceutical compositions comprising fumaric acid ester(s) as active substance(s). The compositions are suitable for use in the treatment of e.g. psoriasis or other hyperproliferative, inflammatory or autoimmune disorders and are designated to release the fumaric acid ester in a controlled manner so that local high concentrations of the active substance within the gastrointestinal tract upon oral administration can be avoided and, thereby, enabling a reduction in gastro-intestinal related side-effects.
Owner:BIOGEN SWISS MFG GMBH
Nitric oxide-releasing polymers derived from modified polymers
InactiveUS20070053952A1Treat and inhibit restenosisSurgerySynthetic polymeric active ingredientsPolymer modifiedVulnerable plaque
Modified polyimines and derivatives thereof suitable as implantable medical devices and coatings therefore are provided. Specifically, implantable medical devices and / or coatings comprise amphiphilic polymers derived from modified polyimines. The medical devices and coatings of the present invention can also be used for in situ nitric oxide release / controlled release drug delivery and are useful for treating or preventing medical conditions such as restenosis, aneurysms and vulnerable plaque.
Owner:MEDTRONIC VASCULAR INC
Nanoporous Drug Delivery System
Disclosed herein are controlled release drug delivery systems. The systems comprise a medical device at least one nonoporous surface, at least one bioactive agent and optionally a biodegradable polymer. The nanoporous surfaces of the medical devices contain nanopores capable of acting as reservoirs for drugs that are controllably released.
Owner:MEDTRONIC VASCULAR INC
Matrix compositions for controlled delivery of drug substances
InactiveUS20070042044A1Improve solubilityImprove oral bioavailabilityBiocidePowder deliveryPolyethylene oxidePEG-PLGA-PEG
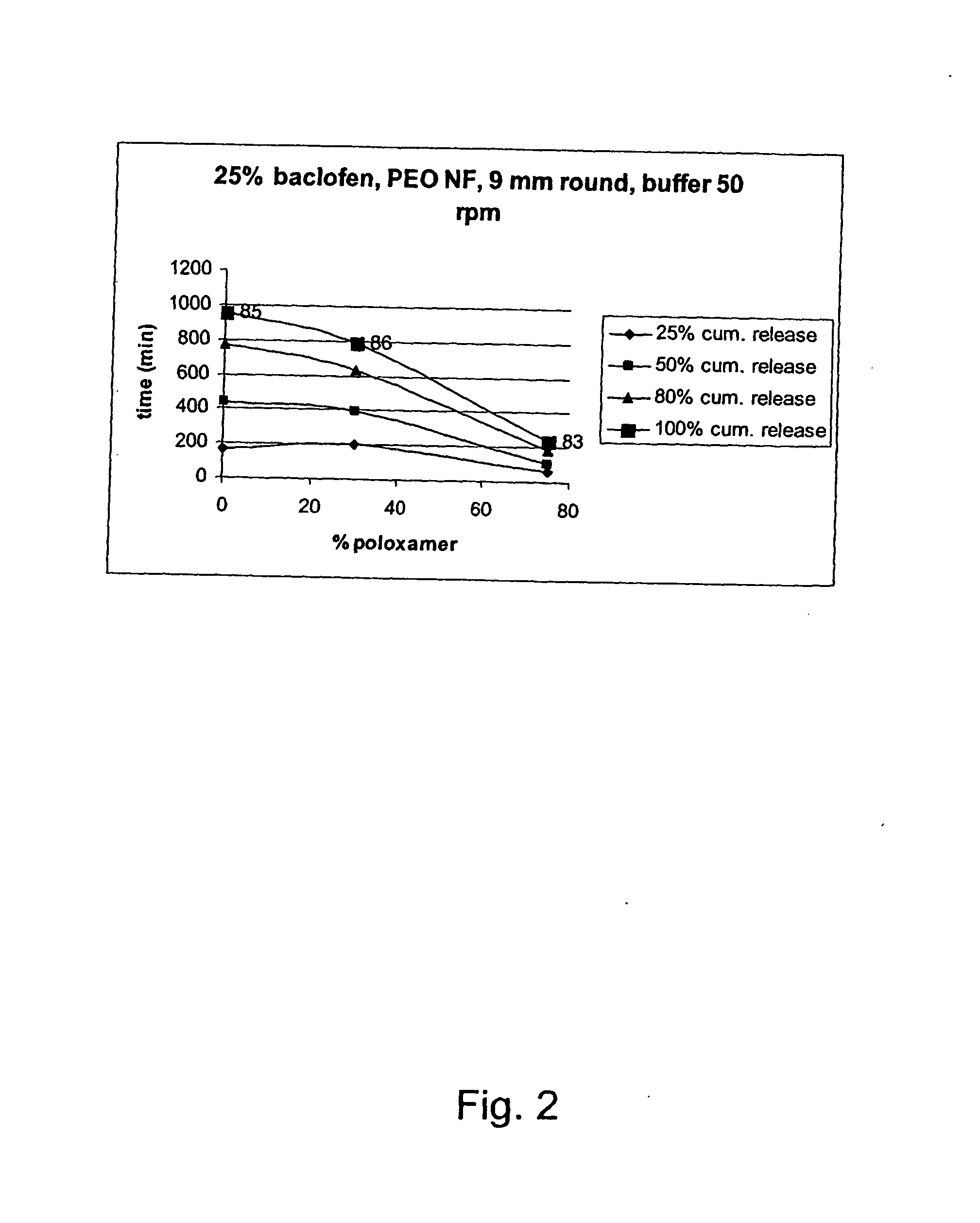
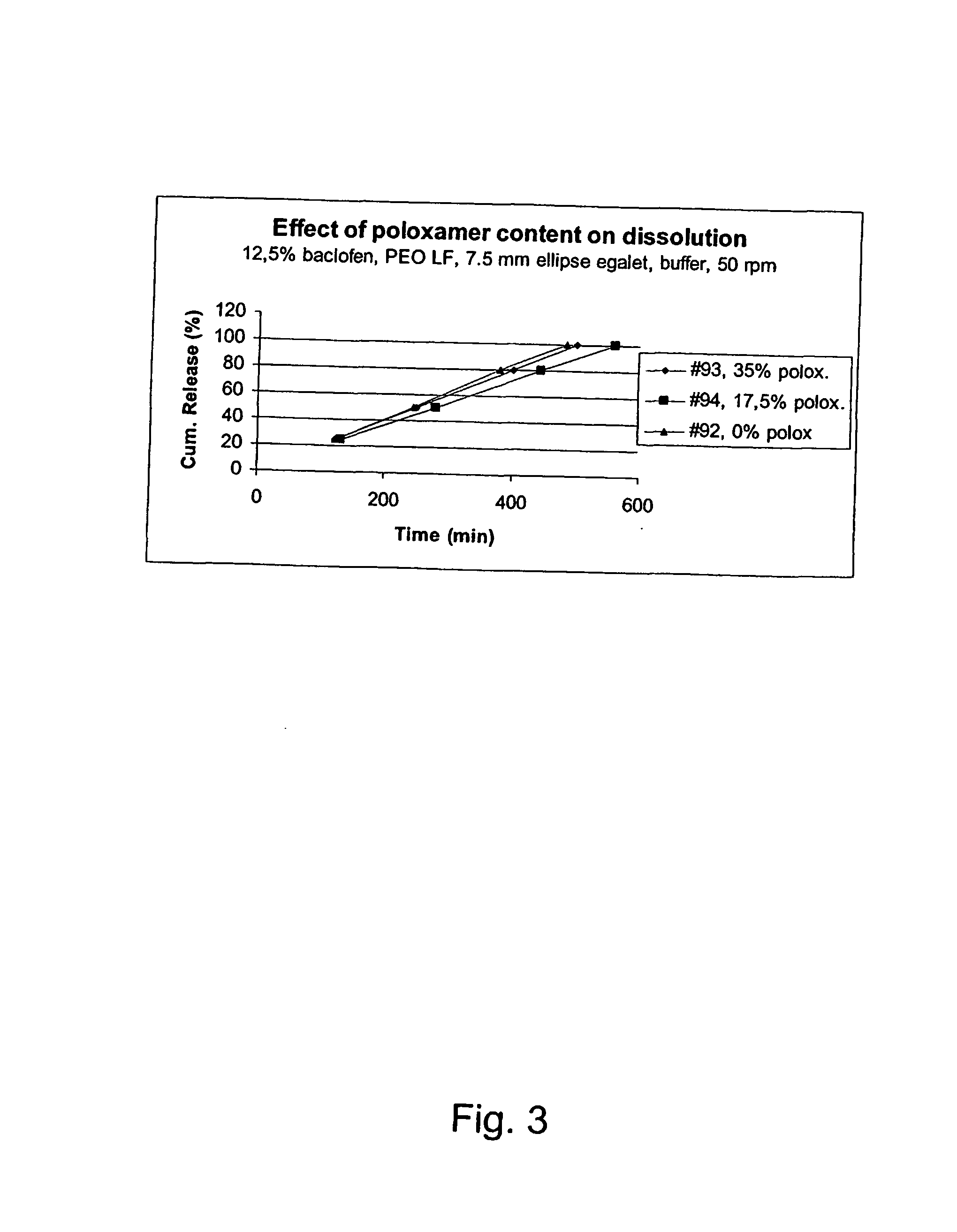
A novel matrix composition for pharmaceutical use. The matrix composition has been designed so that it is especially suitable in those situation where an improved bioavailability is desired and / or in those situation where a slightly or insoluble active substance is employed. Accordingly, a controlled release pharmaceutical composition for oral use is provided in the form of a coated matrix composition, the matrix composition comprising i) a mixture of a first and a second polymer that have plasticizing properties and which have melting points or melting intervals of a temperature of at the most 200° C., the first polymer being selected from the group consisting of polyethylene glycols and polyethylene oxides, and the second polymer being selected form block copolymer of ethylene oxide and propylene oxide including poly(ethylene-glycol-b-(DL-lactic acid-co-glycolic acid)-b-ethylene glycol (PEG-PLGA PEG), poly((DL-lactic acid-co-glycolic acid)-g-ethylene glycol) (PLGA-g-PEG), poloxamers and polyethylene oxide-polypropylene oxide (PEO-PPO), ii) a therapeutically, prophylactically and / or diagnostically active substance, the matrix composition being provided with a coating having at least one opening exposing at one surface of said matrix, wherein the active substance is released with a substantially zero order release.
Owner:EGALET LTD
Controlled release solid dispersions
InactiveUS20080234352A1Different equipmentInhibition strengthBiocideAnimal repellantsSolubilityPolyethylene oxide
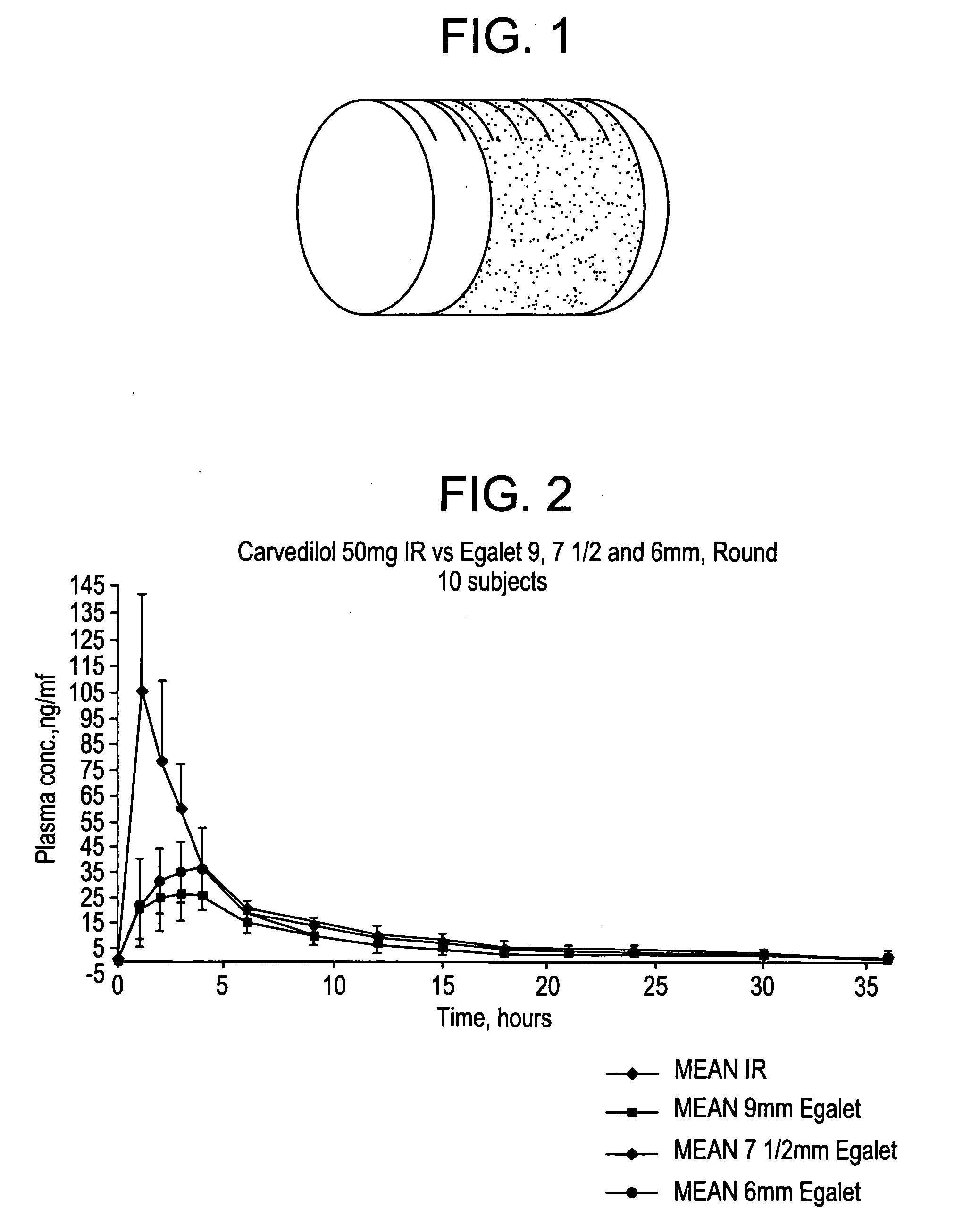
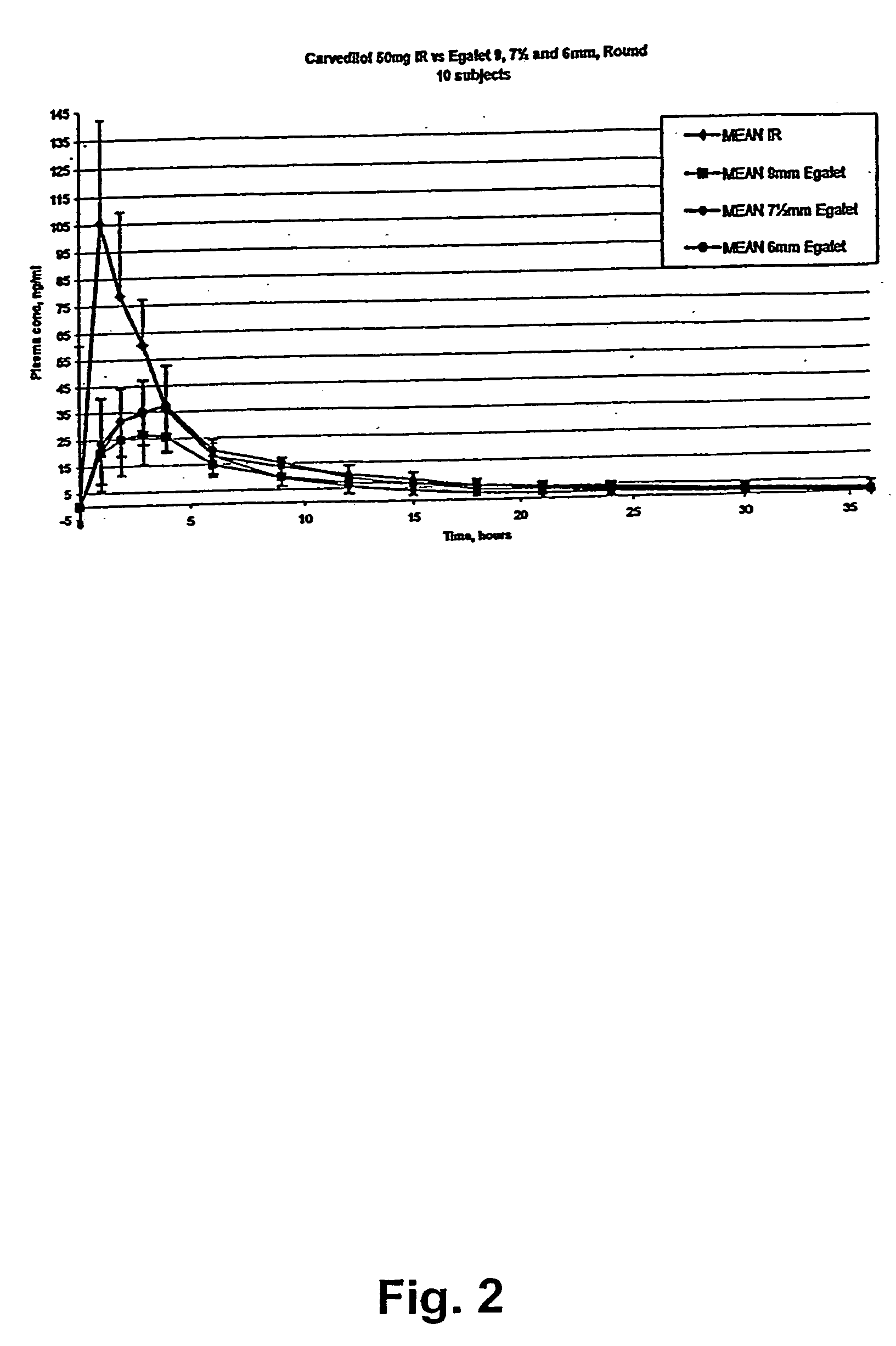
A controlled release pharmaceutical composition for oral use comprising a solid dispersion of i) at least one therapeutically, prophylactically and / or diagnostically active substance, which at least partially is in an amorphous form, ii) a pharmaceutically acceptable polymer that has plasticizing properties, and iii) optionally a stabilizing agent, the at least one active substance having a limited water solubility, and the composition being designed to release the active substance with a substantially zero order release. The polymer is typically a poly ethylene glycol and / or polyethylene oxide having a molecular weight of at least about 20,000 in crystalline and / or amorphous form or a mixture of such polymers, and the active substance is typically carvedilol. The composition may comprise a coated matrix, the coating comprising a first cellulose derivative which is substantially insoluble in the aqueous medium, and at least one of a) a second cellulose derivative which is soluble or dispersible in water, b) a plasticizer, and c) a filler.
Owner:EGALET LTD
Polymer coated drug-ion exchange resins and methods
Included are compositions, and methods of making, coated controlled-release drug and ion exchange resin form complexes. Methacrylate coatings, which can be free of plasticizers particularly with Eudragit® NE type polymer, are preferred to enhance the control of drug release from the drug-resin complexes. Liquid formulations including the coated resin forms and a chelating agent to inhibit degradation are also included.
Owner:WOCKHARDT EU OPERATIONS SWISS
Topiramate pharmaceutical composition
InactiveUS20060121112A1Improve bioavailabilityDifferential bioavailabilityBiocideCarbohydrate active ingredientsDiseaseGeneralized seizure
A once daily controlled-release pharmaceutical formulation which contains therapeutic amounts of topiramate and which is capable of being administered to specific regions along the gastrointestinal tract used to treat various types of conditions, for example, partial seizures with or without secondarily generalized seizures, primary generalized tonic-clonic seizures, seizures associated with Lennox Gastaut Syndrome, migraines, and obesity.
Owner:ALKERMES PHARMA IRELAND LTD
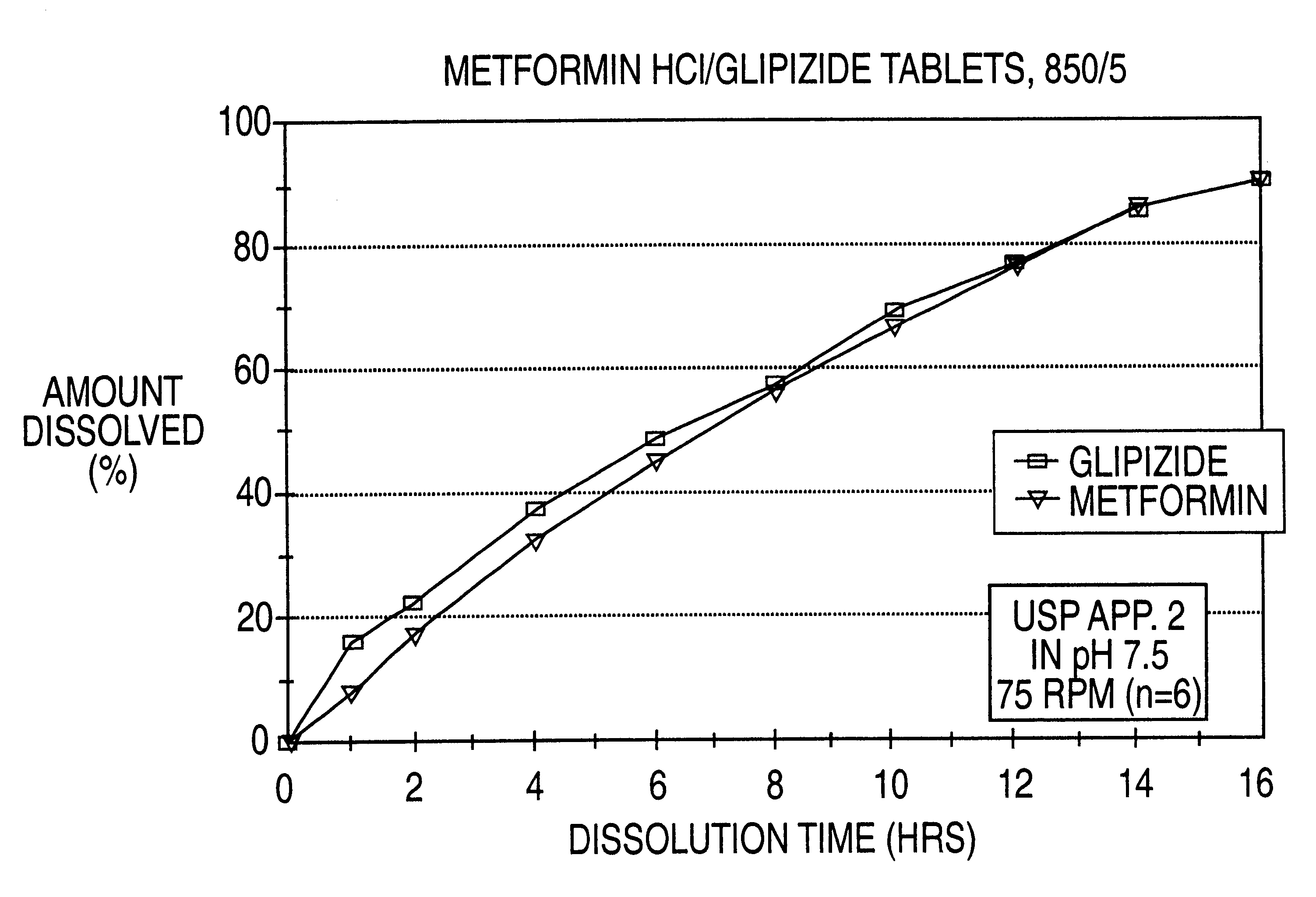
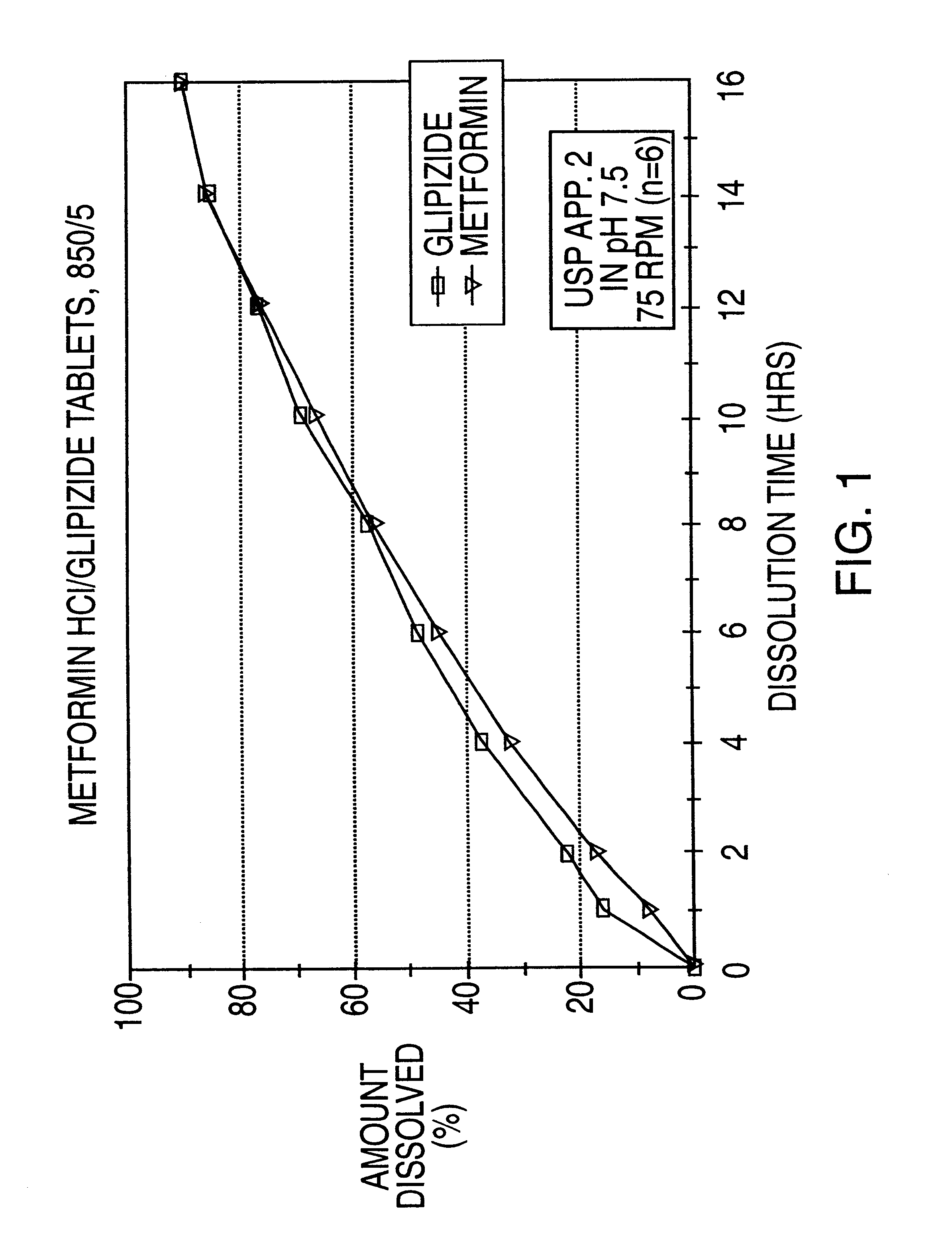
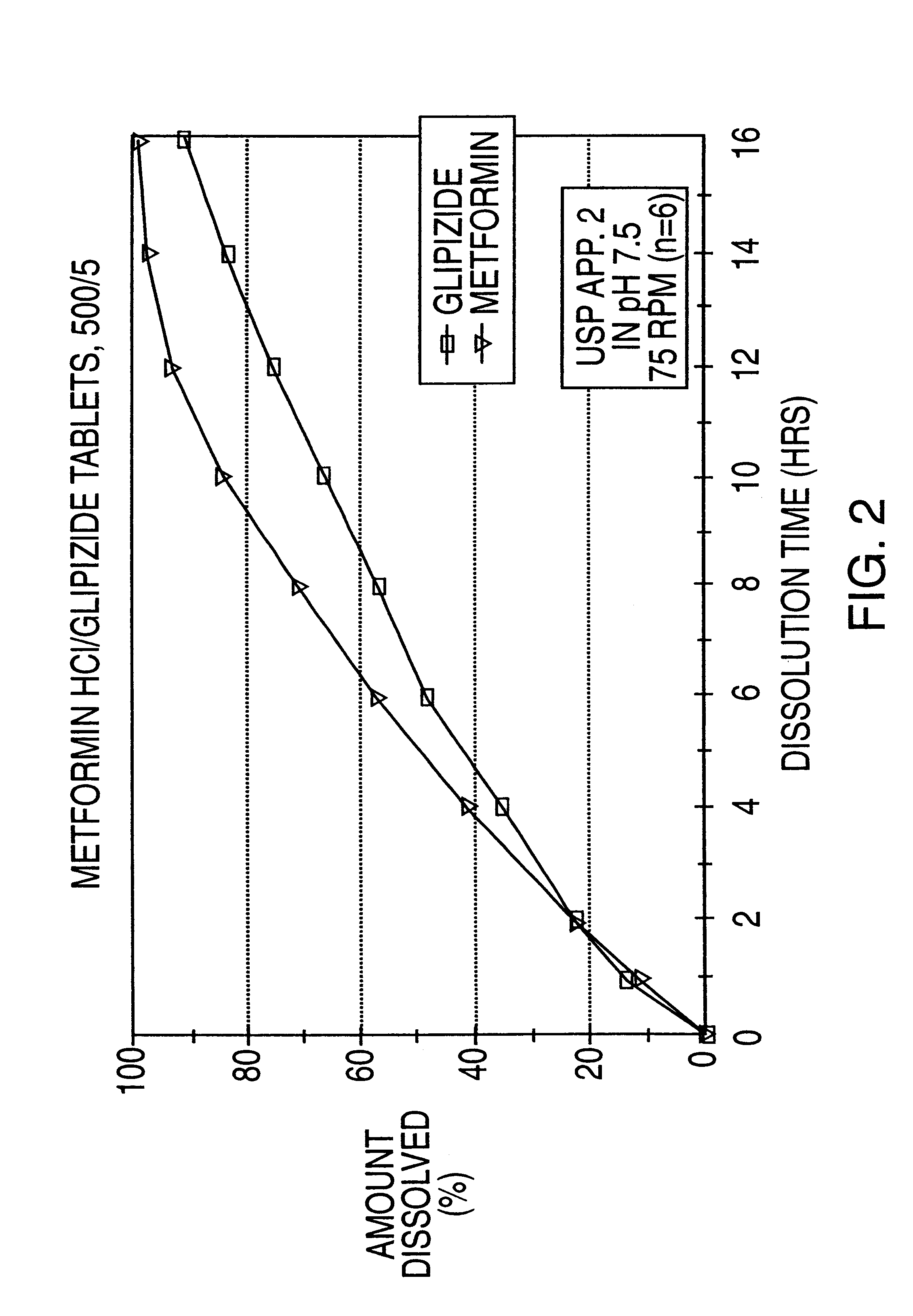
Controlled release tablet having a unitary core
InactiveUS6284275B1Metabolism disorderSulfonylurea active ingredientsControlled Release TabletControlled release drug
A controlled release pharmaceutical tablet containing antihyperglycemic drug and a hypoglycemic drug that does not contain an expanding or gelling polymer layer and comprising a core containing the antihyperglycemic drug and the hypoglycemic drug, a semipermeable coating membrane surrounding the core and at least one passageway in the membrane to allow the drugs to be released from the core.
Owner:ANDRX LABS
Linker-Based Lecithin Microemulsion Delivery Vehicles
ActiveUS20080139392A1Improve solubilityPromote absorptionBiocideTransportation and packagingHigh concentrationSide effect
The present invention relates to biocompatible microemulsion systems designed for controlled release drug delivery applications formulated with phospholipids such as lecithin (surfactant), a lipophilic additive (linker) containing 9 or more carbons in their alkyl group and hydrophilic-lipophilic balance (HLB) of 5 or less, and a surfactant-like hydrophilic additive (linker) containing between 6 to 9 carbon atoms in their alkyl tail. The combination of linkers and phospholipids produce formulations capable of delivering high concentrations of poorly soluble drugs into epithelial tissue using low surfactant concentrations, with minimum cytotoxic side effects.
Owner:ACOSTA ZARA EDGAR JOEL +1
Targeted transscleral controlled release drug delivery to the retina and choroid
InactiveUS20050208103A1Good curative effectReduce impactSenses disorderPeptide/protein ingredientsDiagnostic agentMedicine
The invention provides methods for delivering a therapeutic or diagnostic agent to the eye of a mammal. The method involves contacting sclera with a therapeutic or diagnostic agent so as to permit its passage through the sclera into the choroidal and retinal tissues. The sclera may be contacted with a therapeutic or diagnostic agent together with a device for enhancing transport of the agent through the sclera.
Owner:ADAMIS ANTHONY P +2
Amniotic membrane covering for a tissue surface and devices facilitating fastening of membranes
ActiveUS7494802B2Reduce inflammationPromote wound healingBioreactor/fermenter combinationsBiological substance pretreatmentsBandage contact lensDelivery vehicle
The present invention relates to a biopolymer covering for a tissue surface including, for example, a dressing, a bandage, a drape such as a bandage contact lens, a composition or covering to protect tissue, a covering to prevent adhesions, to exclude bacteria, to inhibit bacterial activity, or to promote healing or growth of tissue. An example of such a composition is an amniotic membrane covering for an ocular surface. Use of a covering for a tissue surface according to the invention eliminates the need for suturing. The invention also includes devices facilitating the fastening of a membrane to a support, culture inserts, compositions, methods, and kits for making and using coverings for a tissue surface and culture inserts. Compositions according to the invention may include cells grown on a membrane or attached to a membrane, and such compositions may be used as scaffolds for tissue engineering or tissue grafts. A method of preparing and using an amniotic membrane covering for a tissue surface as a controlled release drug delivery vehicle is also disclosed.
Owner:TISSUETECH INC
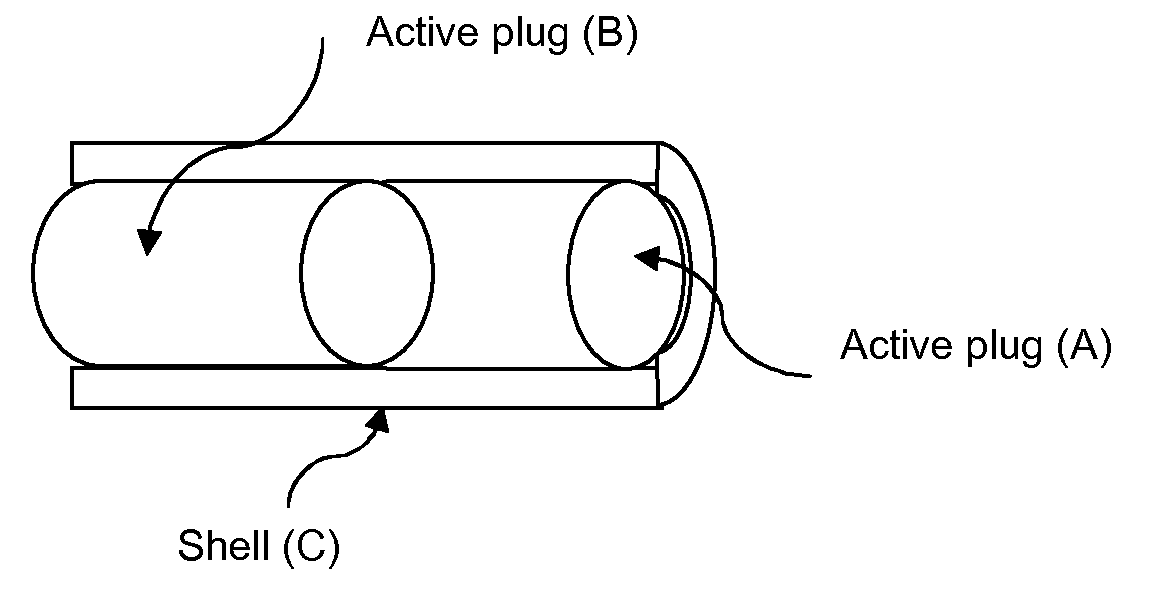
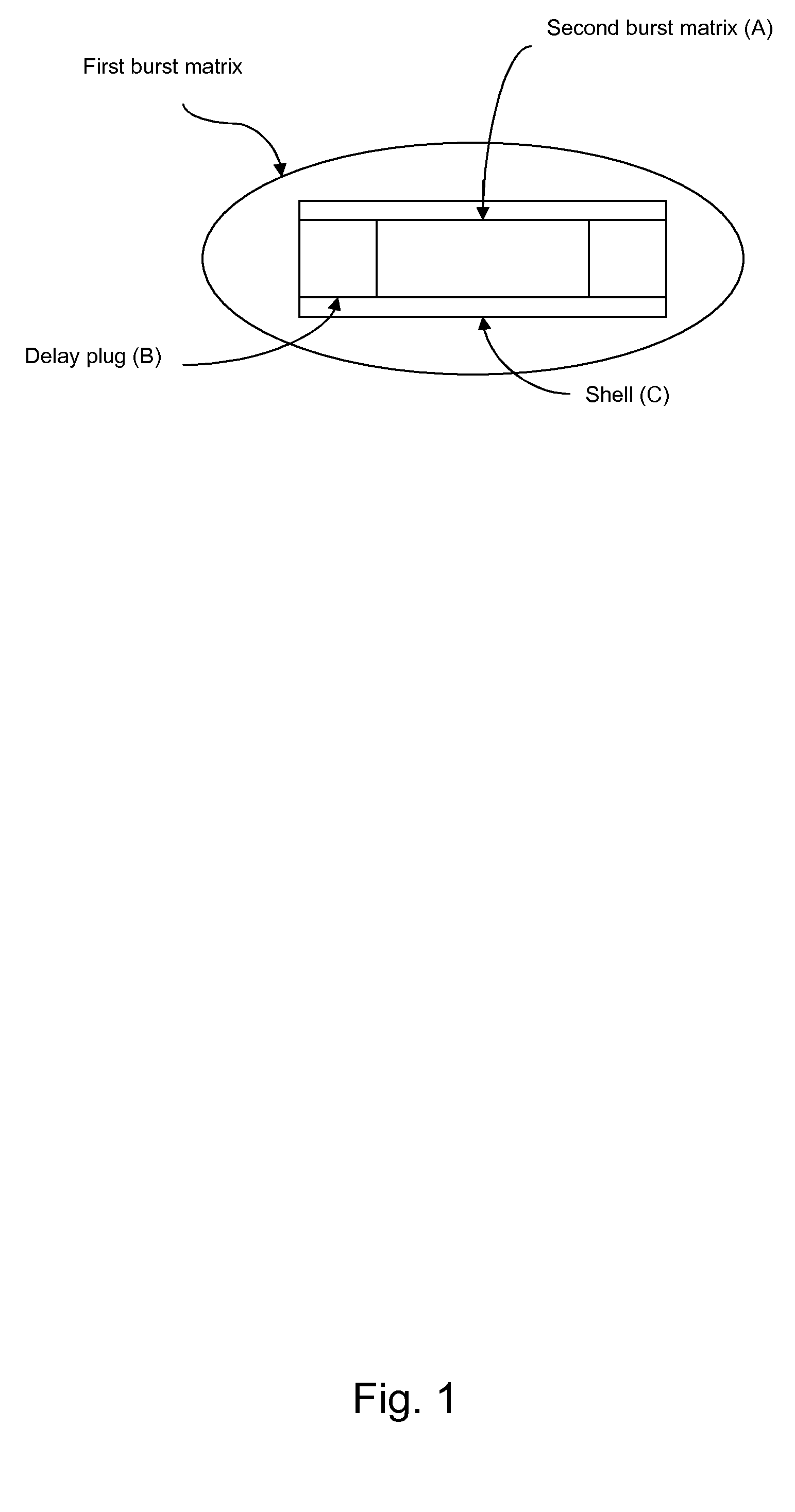
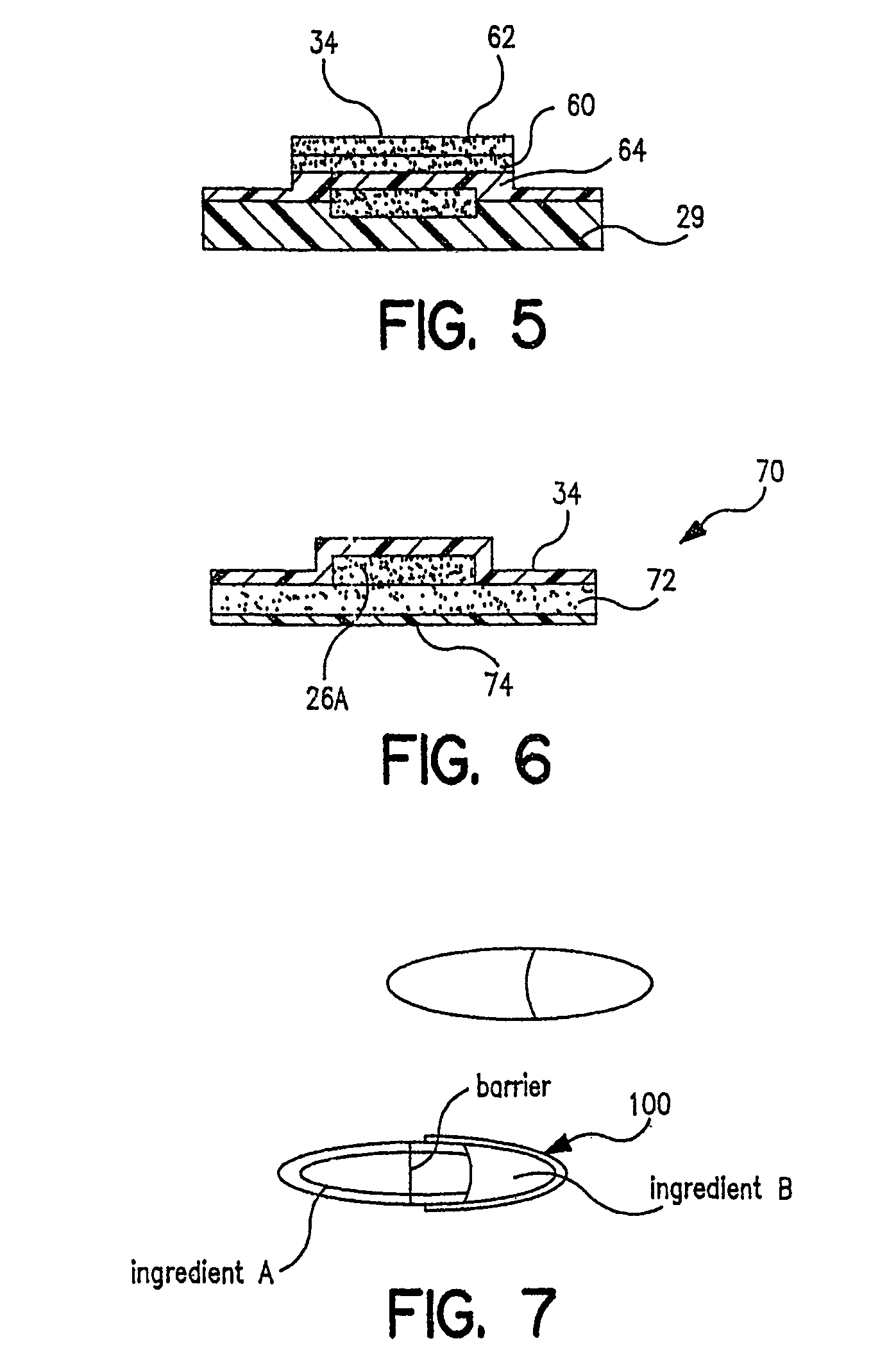
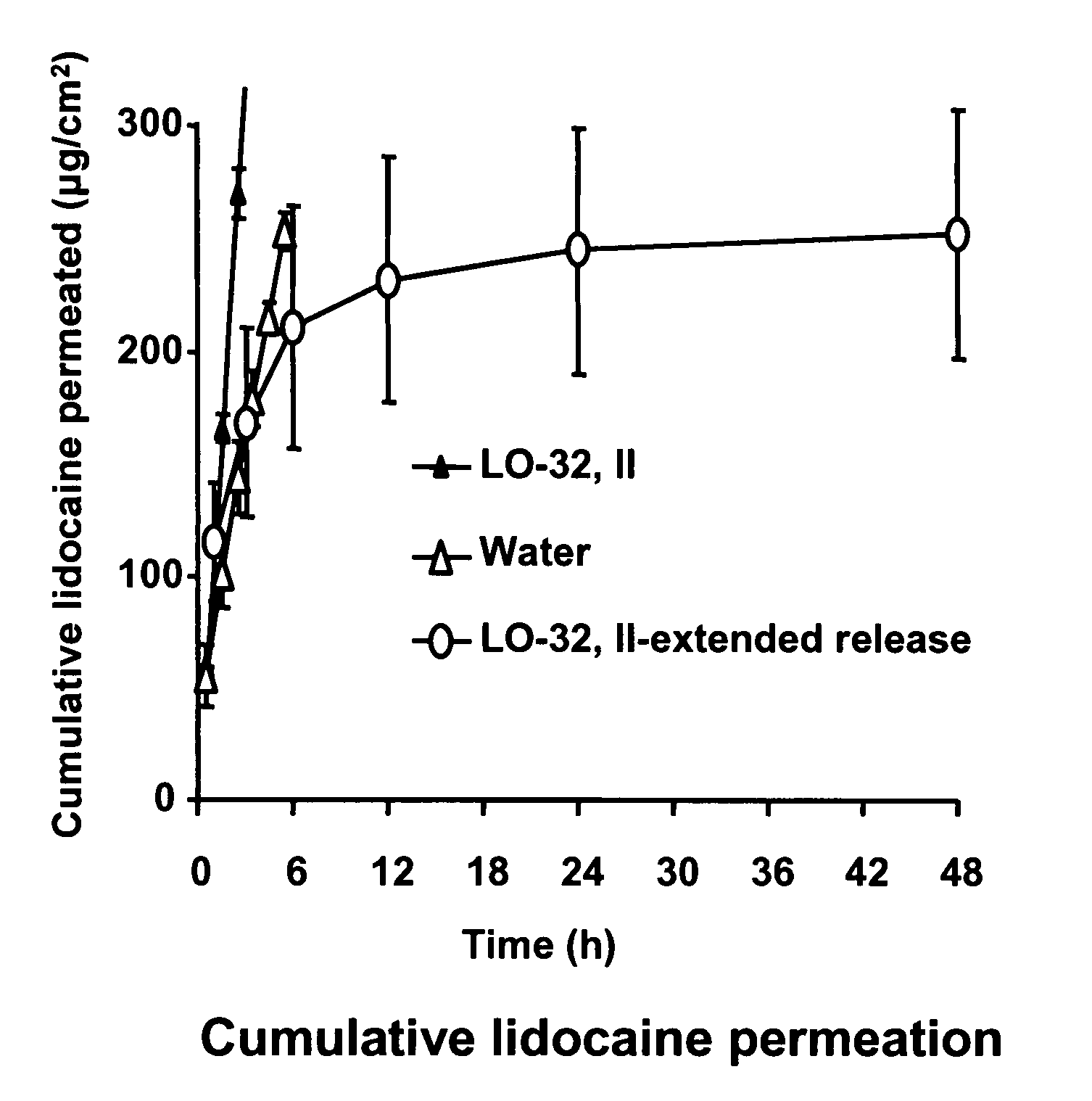
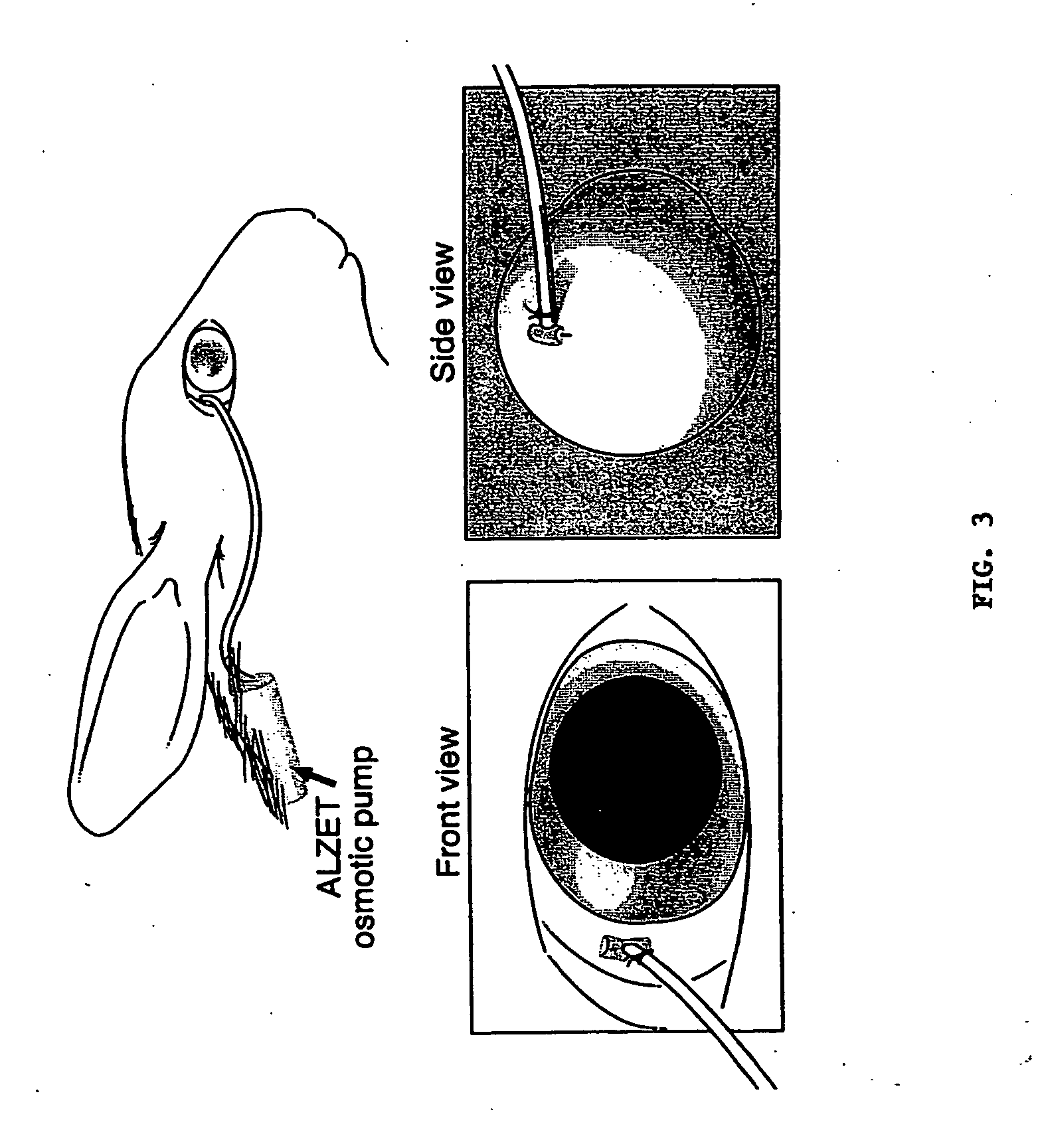
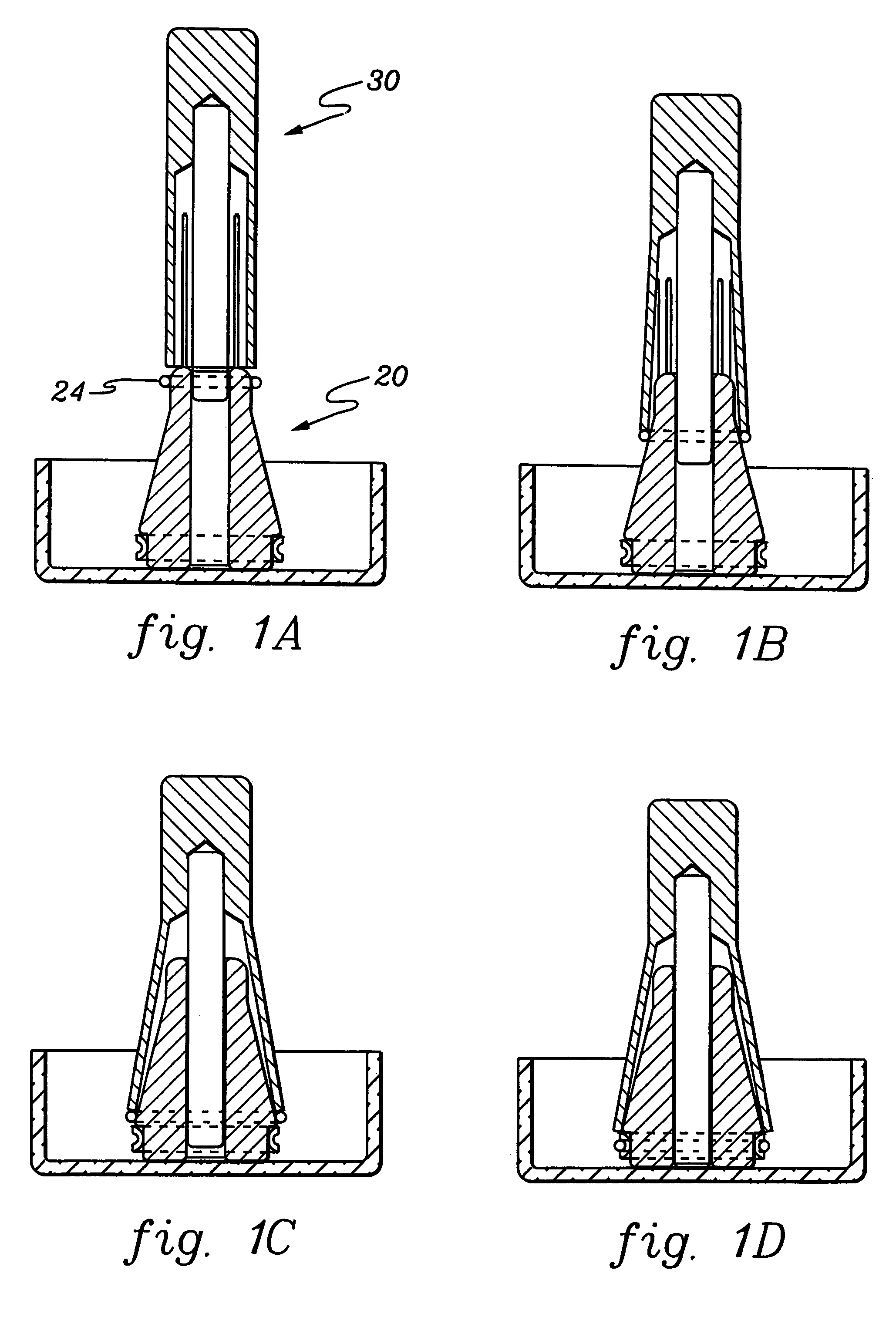
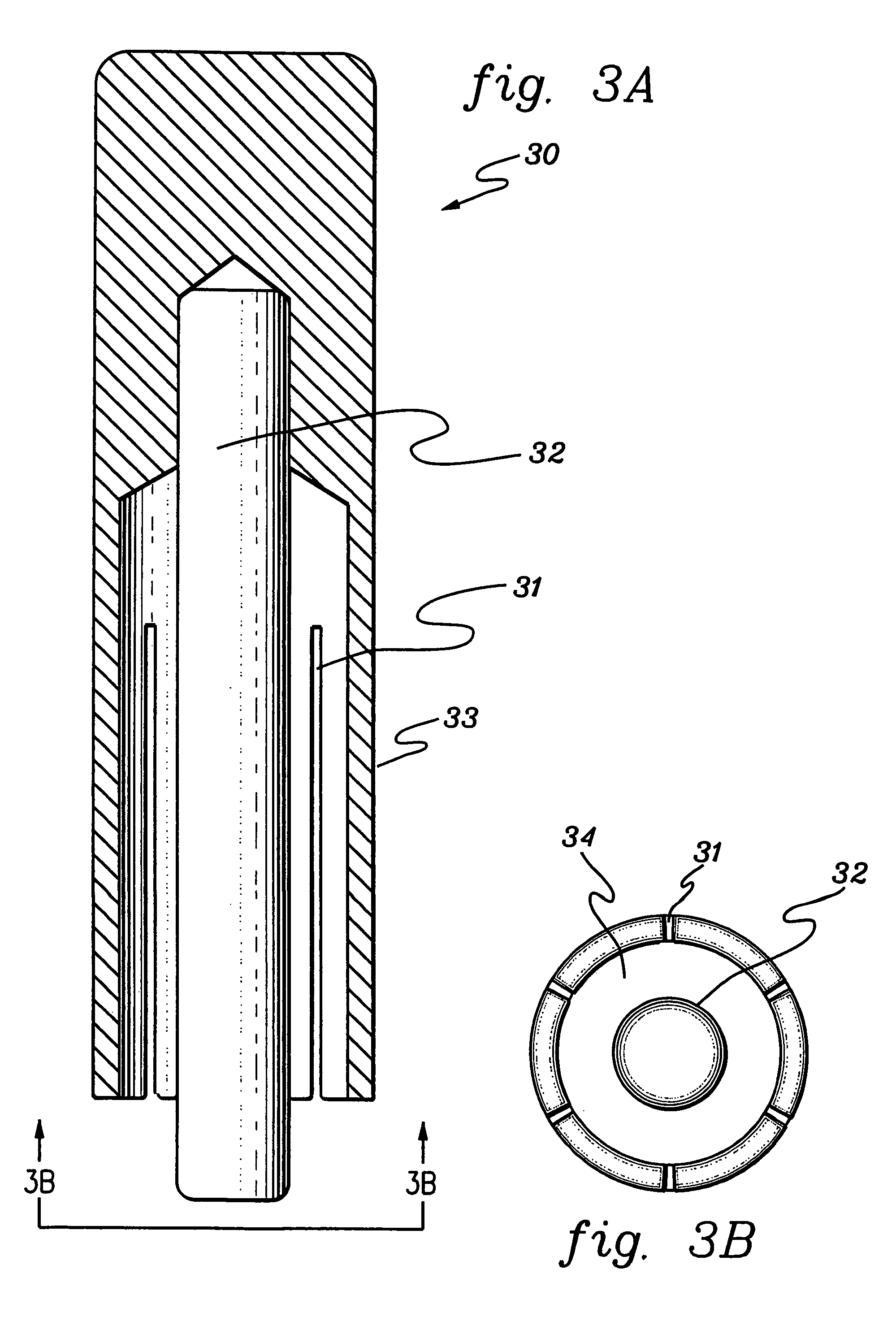
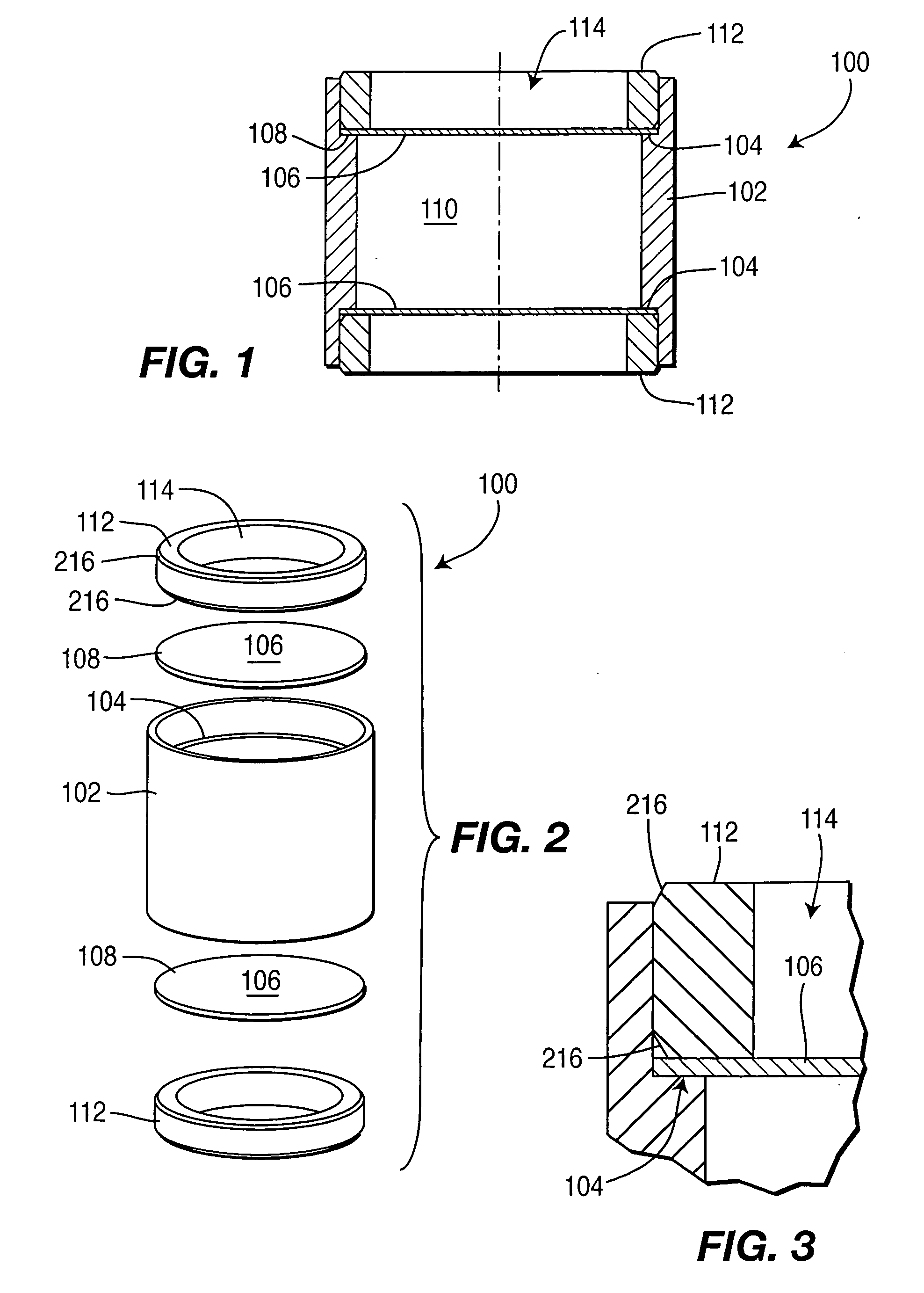
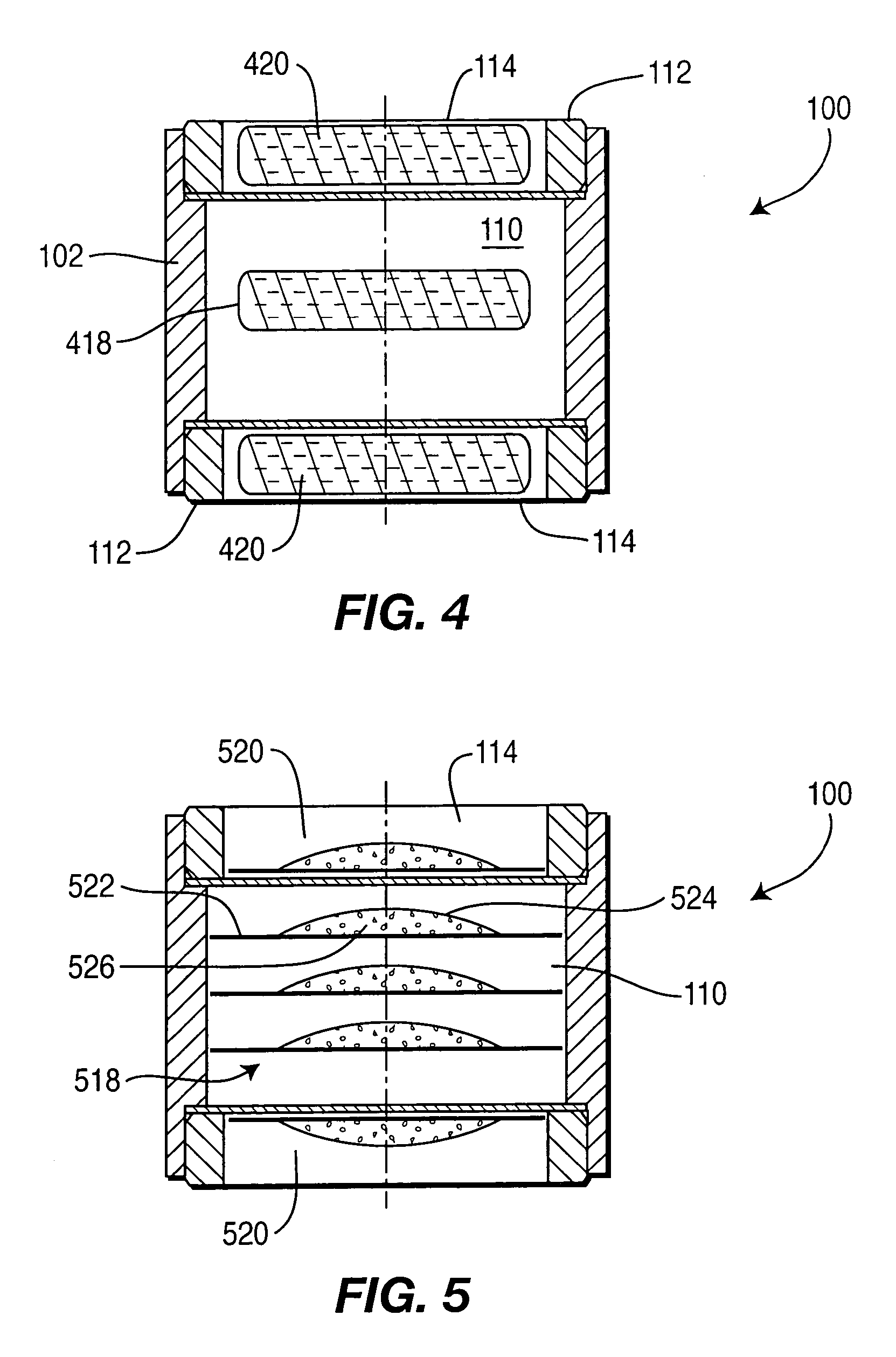
Controlled-release drug delivery system
A controlled-release drug delivery system advantageously includes an open-ended, inflexible sleeve, at least two controlled-release layers and two open-center caps. Each controlled-release layer abuts a sealing surface that is located within and near each end of the sleeve. The caps seal each controlled-release layer against the abutting sealing surface. One or more dose units of drug are disposed in a region that is formed between the controlled-release layers. The controlled-release layers dissolve, at a predetermined rate, by the action of body fluids that are in contact with those layers through the center of the caps. Release of drug is delayed at least until the controlled-release layers dissolve. The dose unit itself, which is advantageously a core, can be tailored to provide an extended period of drug release. One or more dose units that provide an immediate release component can also be disposed near each end of the sleeve.
Owner:SARNOFF CORP
In situ controlled release drug delivery system
ActiveUS20060188583A1Less frequent administrationAvoidance of surgical interventionNervous disorderAntipyreticAbnormal tissue growthMicrosphere
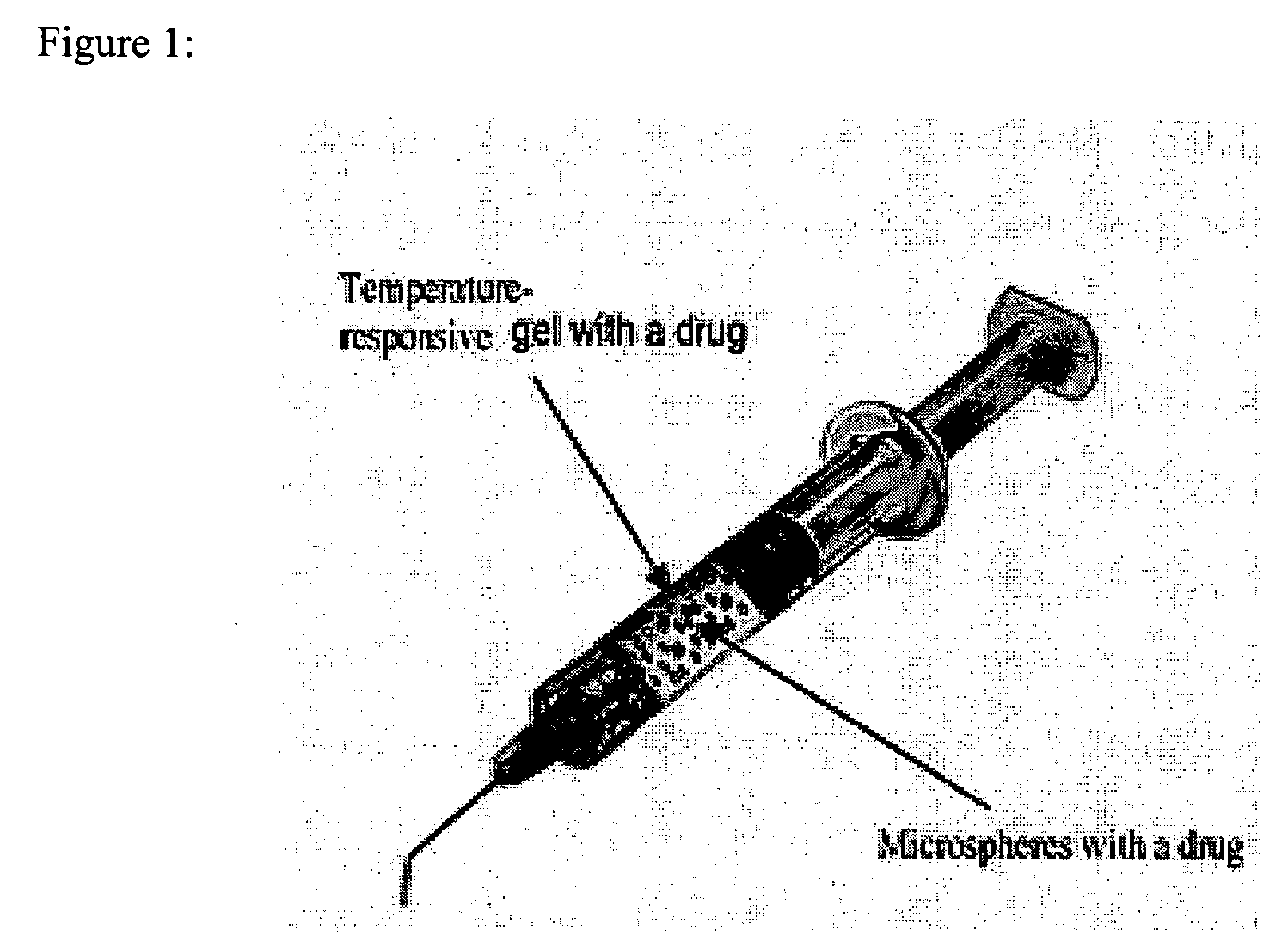
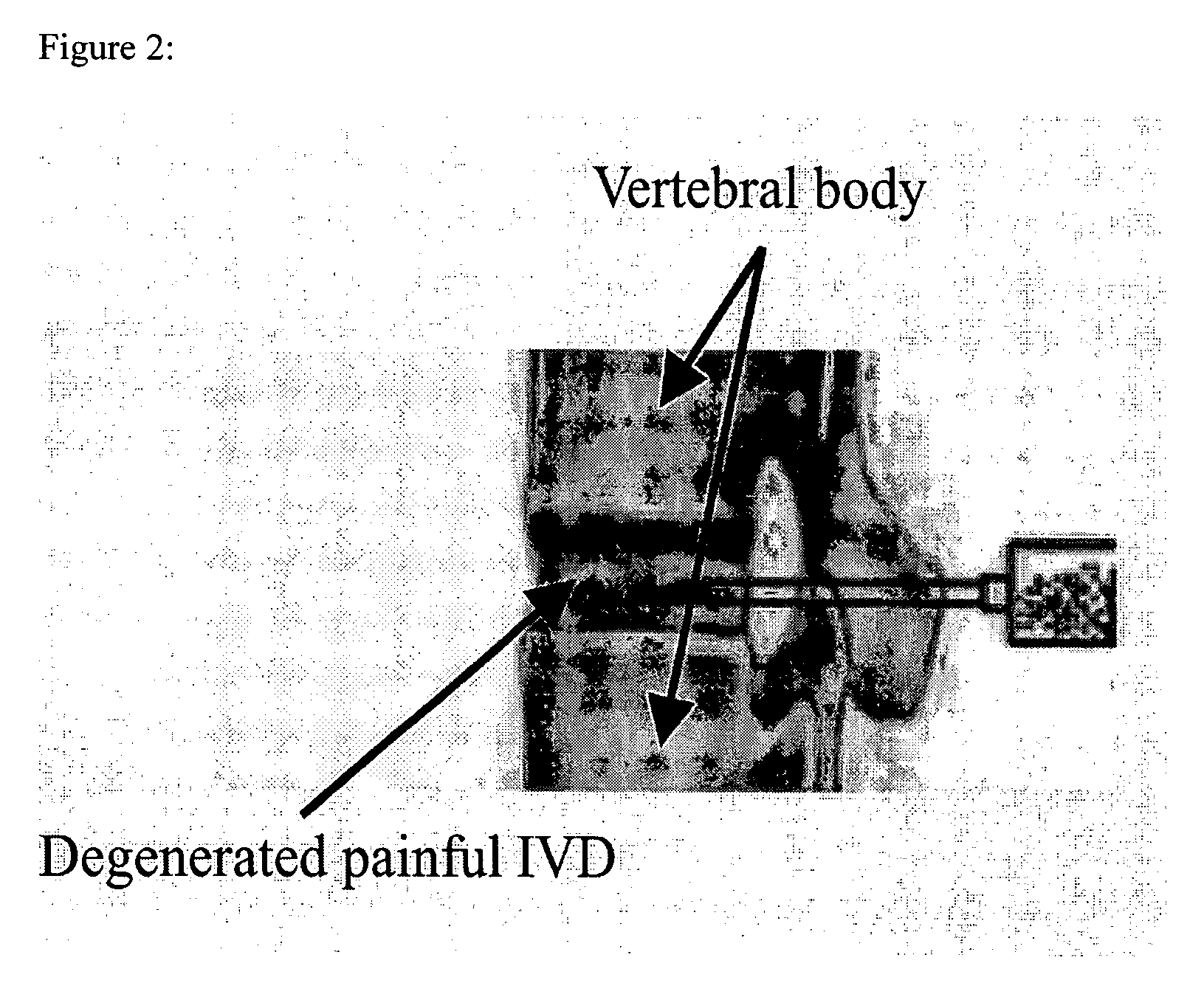
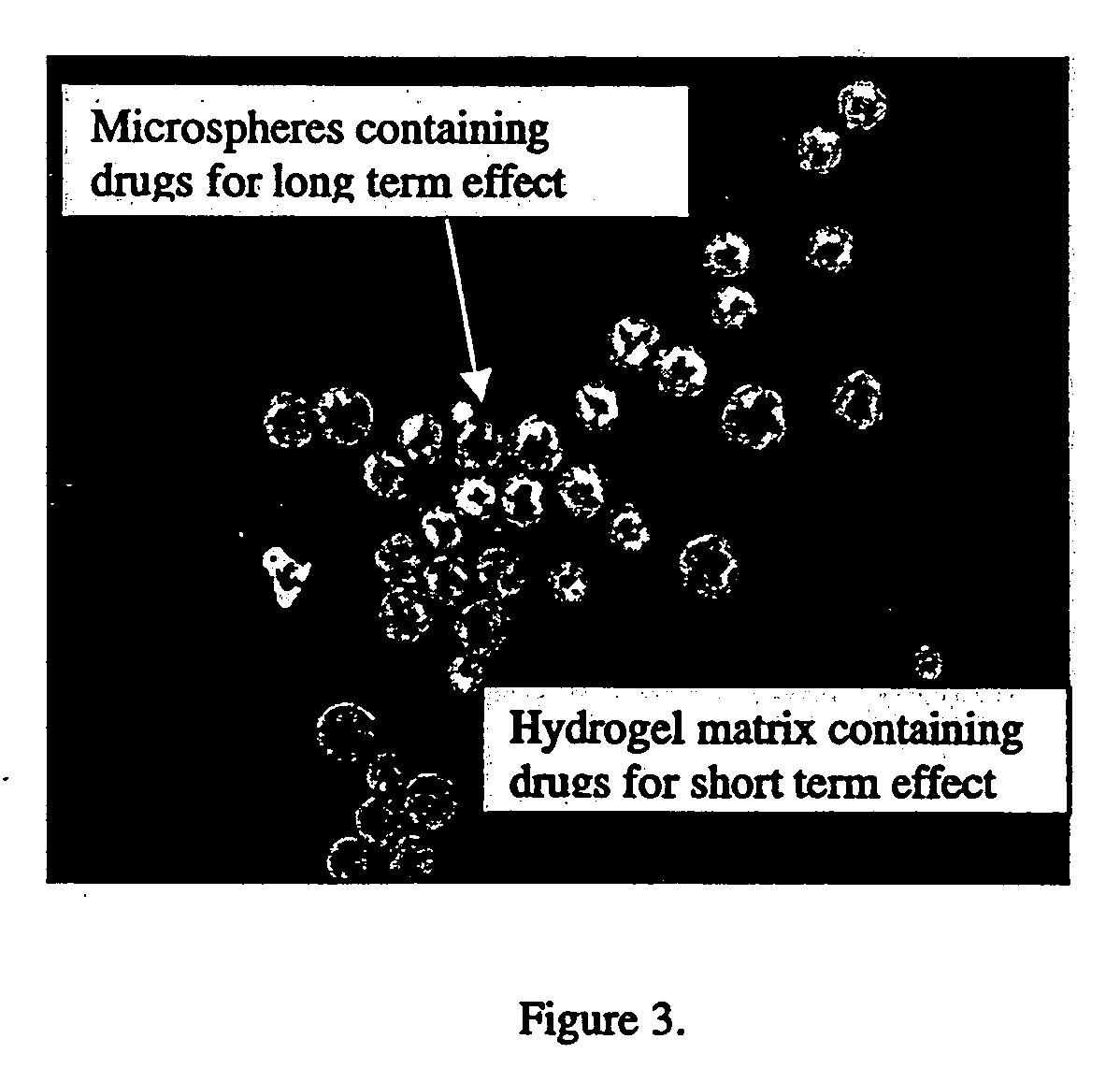
A system is described for long-term controlled release delivery of a drug or a therapeutic agent. According to the invention, one or more drugs or therapeutic agents contained in microspheres are mixed with a temperature sensitive hydrogel which is then introduced directly to the desired situs of the drug or therapeutic agent. The temperature sensitive hydrogel may also contain a drug or a therapeutic agent, for example, a pain relieving drug, for a short-term controlled release. The temperature sensitive hydrogel is in liquid state at room temperature, but upon injection, shortly becomes gelatinous. This system is particularly suitable for treatment of diseases, disorders, or conditions, for example, tumors, discogenic back pain, or arthritis, warranting localized administration of a drug or a therapeutic agent. In addition, the specification provides a method for production of a drug—or therapeutic agent-containing microspheres.
Owner:UNIV OF IOWA RES FOUND
Aqueous sustained-release drug delivery system for highly water-soluble electrolytic drugs
InactiveUS20050013792A1Change in permeabilityPowder deliveryNervous disorderPolyelectrolyteSustained release drug
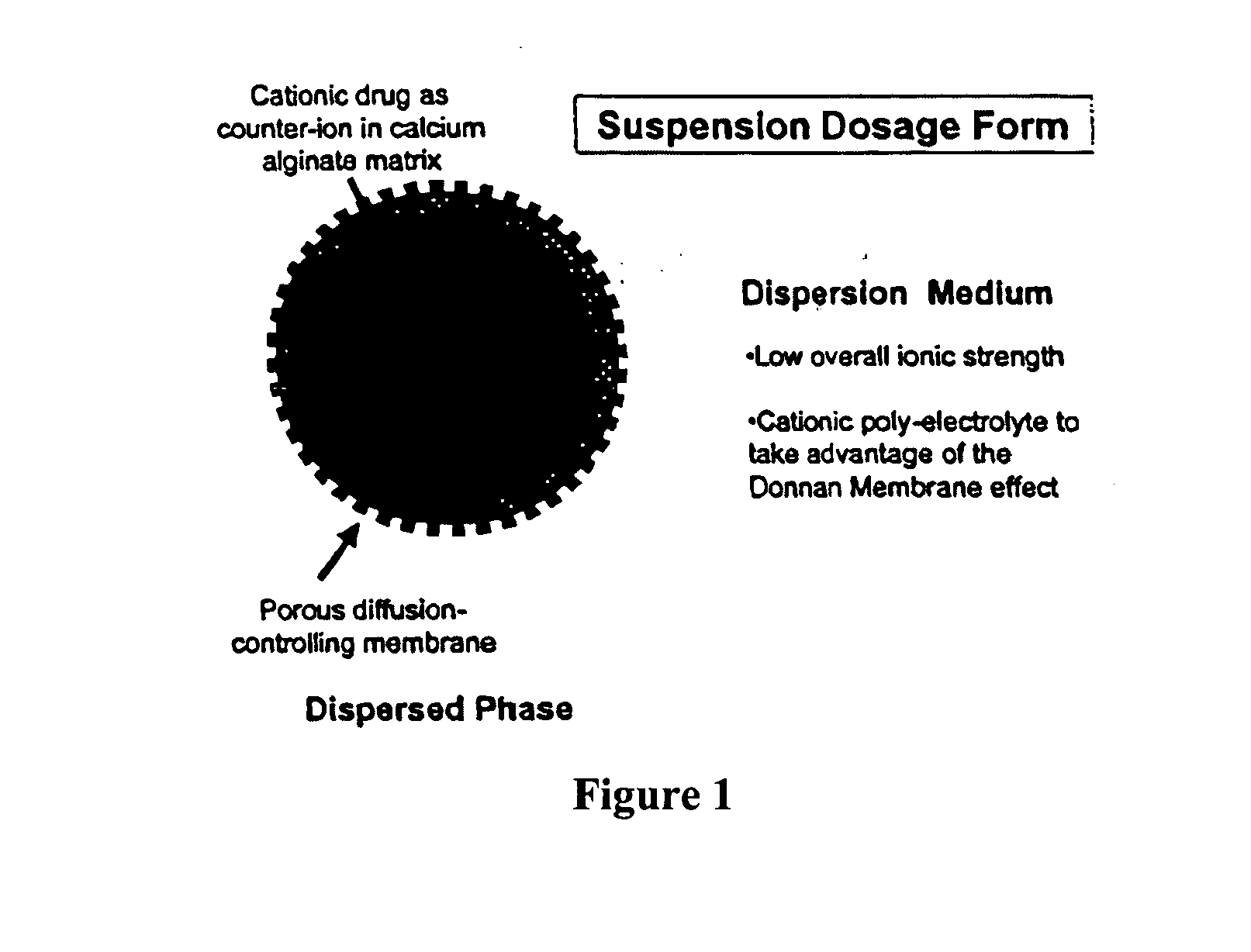
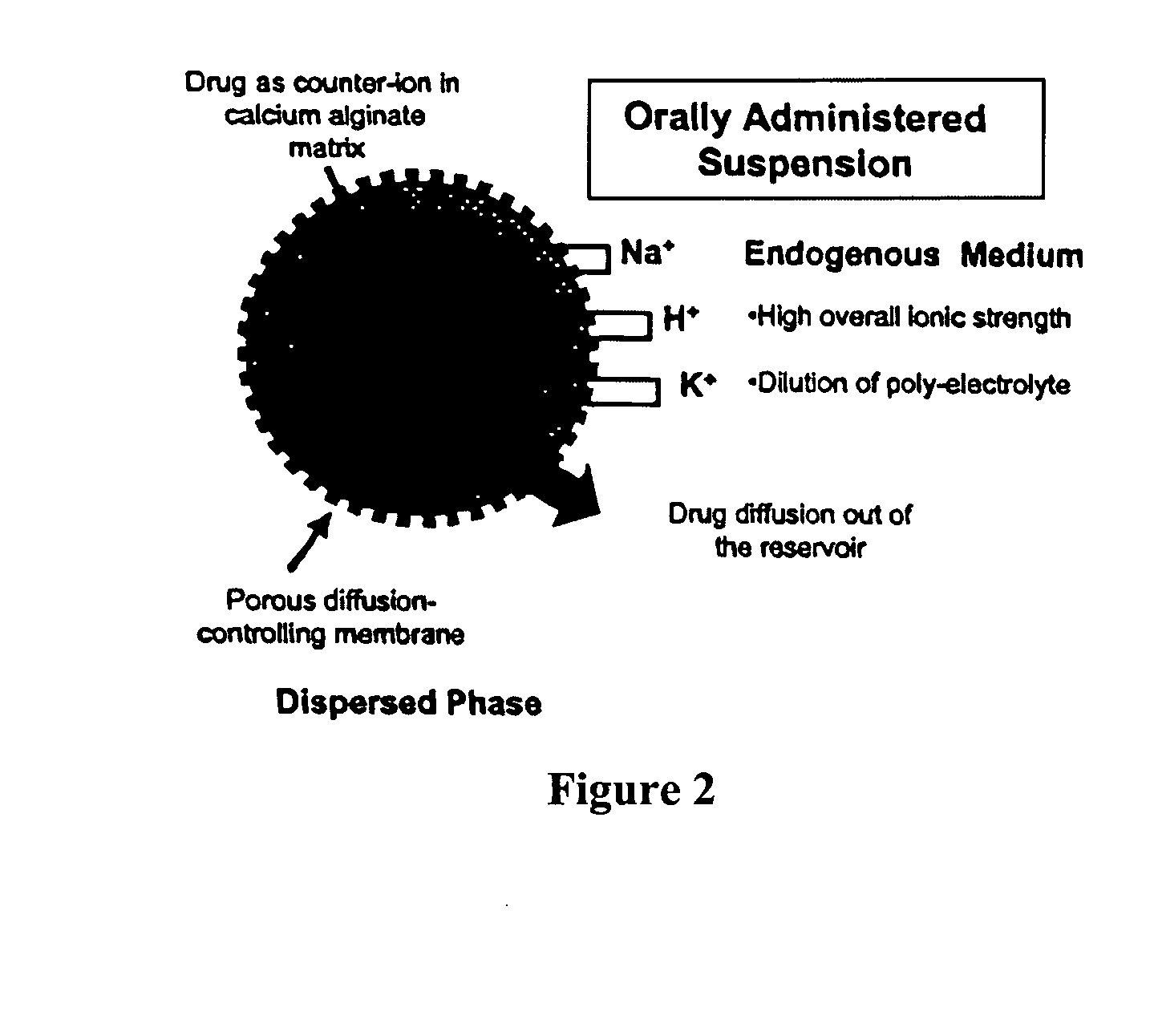
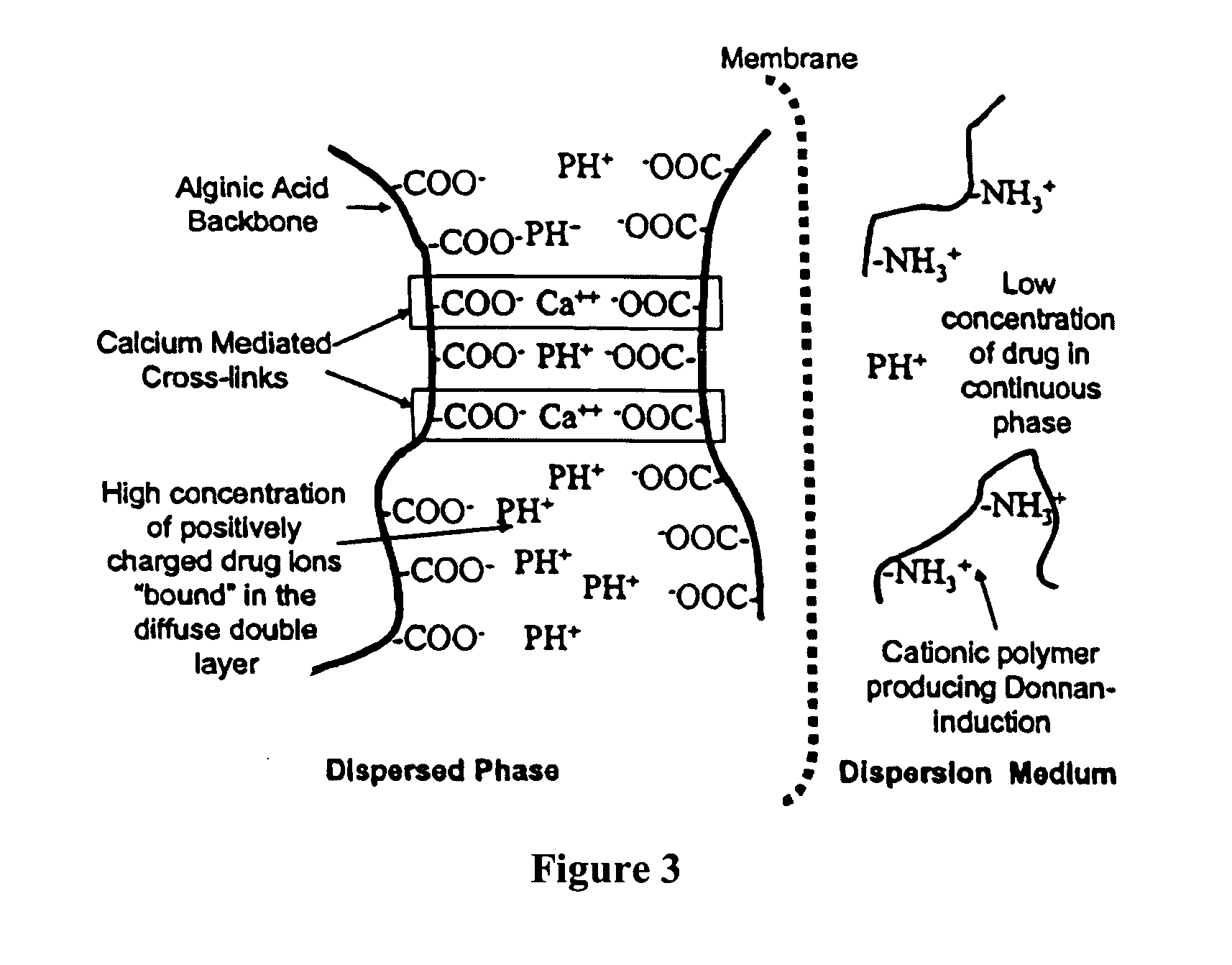
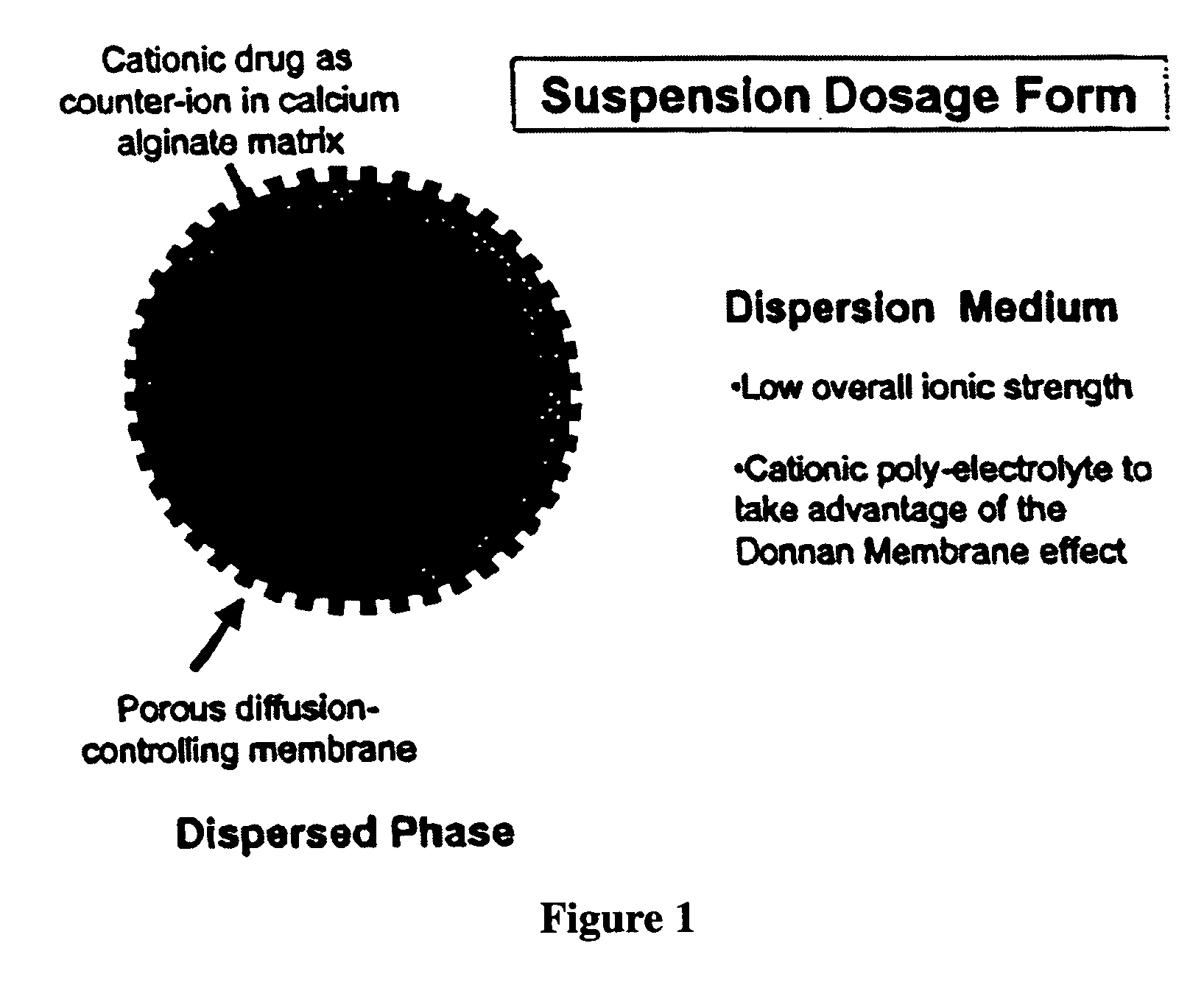
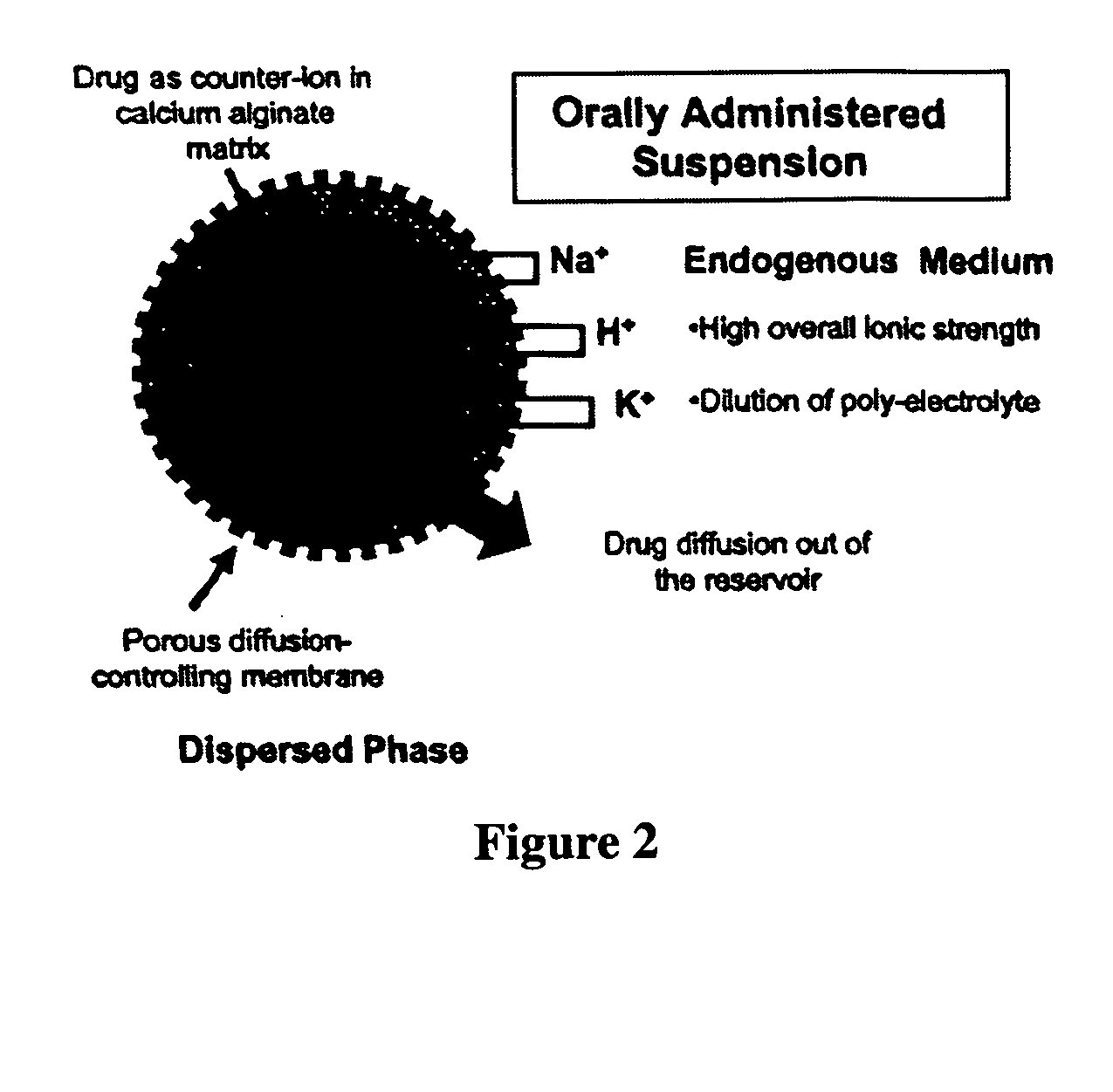
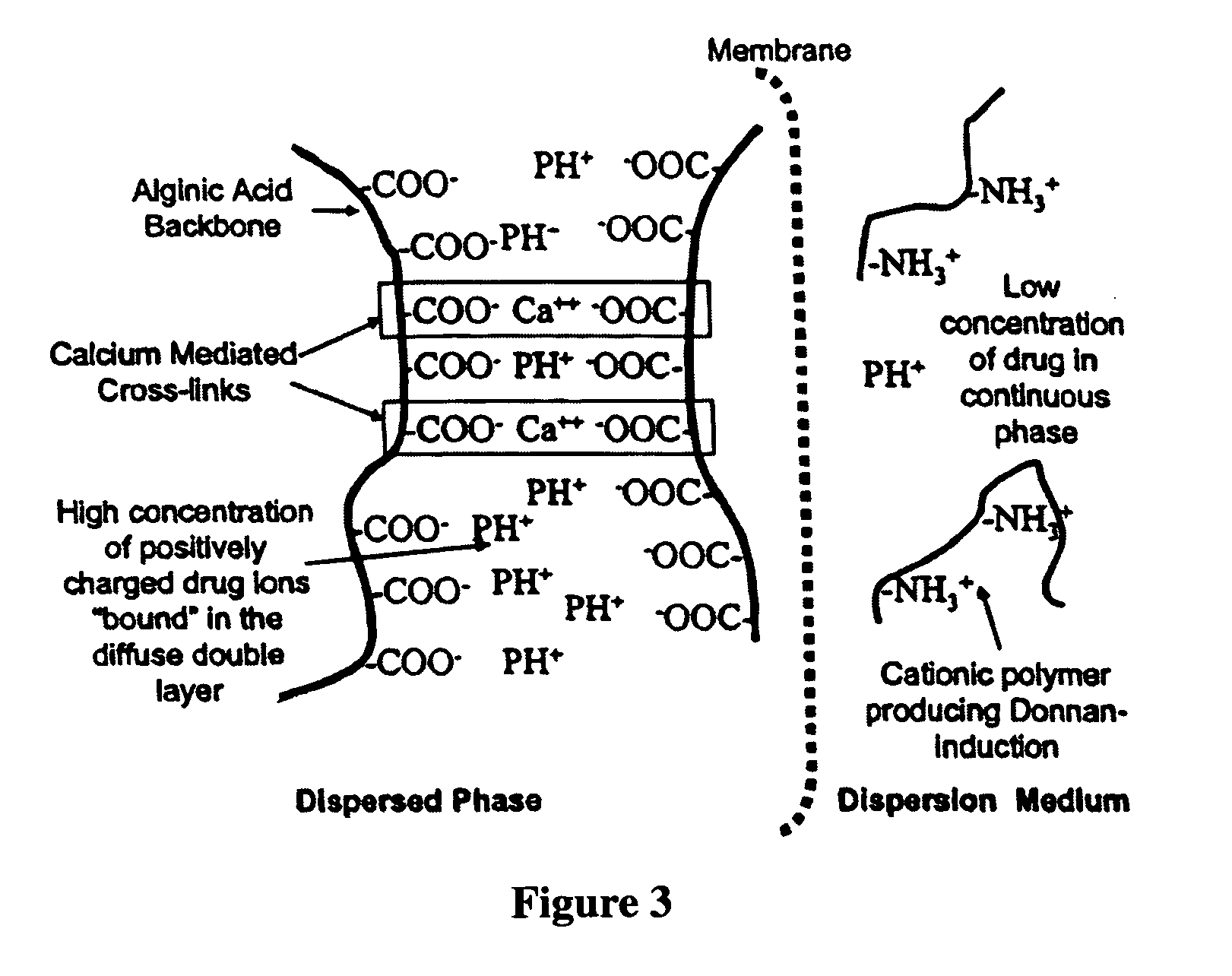
The present invention relates to liquid sustained release suspension dosage forms comprising ionized forms of water-soluble drugs. In particular, the invention encompasses a liquid form controlled release drug composition comprising a dispersed phase comprising an ion-exchange matrix drug complex comprising a pharmaceutically acceptable ion-exchange matrix and a water-soluble electrolytic drug associated with the ion-exchange matrix, wherein the surface charge of the ion-exchange matrix is opposite that of the electrolytic drug and a dispersion medium substantially free of diffusible counterions, further comprising a polyelectrolyte having the same charge as the electrolytic drug. The invention also provides methods for preparing such compositions and methods of treatment.
Owner:MARYLAND UNIV OF BALTIMORE +1
Aqueous sustained-release drug delivery system for highly water-soluble electrolytic drugs
InactiveUS20060134148A1Reduce molecular weightQuick releasePowder deliveryPharmaceutical non-active ingredientsElectrolysisIon exchange
Owner:HOLLENBECK R GARY
Controlled release solid dispersions
InactiveUS20050019399A1Suitable shelf-lifeImprove solubilityBiocideAnimal repellantsSolubilityPolyethylene oxide
A controlled release pharmaceutical composition for oral use comprising a solid dispersion of: i) at least one therapeutically, prophylactically and / or diagnostically active substance, which at least partially is in an amorphous form, ii) a pharmaceutically acceptable polymer that has plasticizing properties, and iii) optionally, a stabilizing agent, the at least one active substance having a limited water solubility, and the composition being designed to release the active substance with a substantially zero order release. The polymer is typically a polyethylene glycol and / or polyethylene oxide having a molecular weight of at least about 20,000 in crystalline and / or amorphous form or a mixture of such polymers, and the active substance is typically carvedilol. The composition may comprise a coated matrix, the coating comprising a first cellulose derivative which is substantially insoluble in the aqueous medium, and at least one of a) a second cellulose derivative which is soluble or dispersible in water, b) a plasticizer, and c) a filler.
Owner:EGALET LTD
In situ gelling drug delivery system
The invention provides liquid controlled-release drug delivery compositions which gel upon injection into the body to form, in situ, controlled-release drug implants. The compositions of the invention feature a gel-forming polymer that is insoluble in water, a polyethylene glycol solvent in which the polymer is dissolved, and the drug substance to be delivered.
Owner:PSIVIDA INC
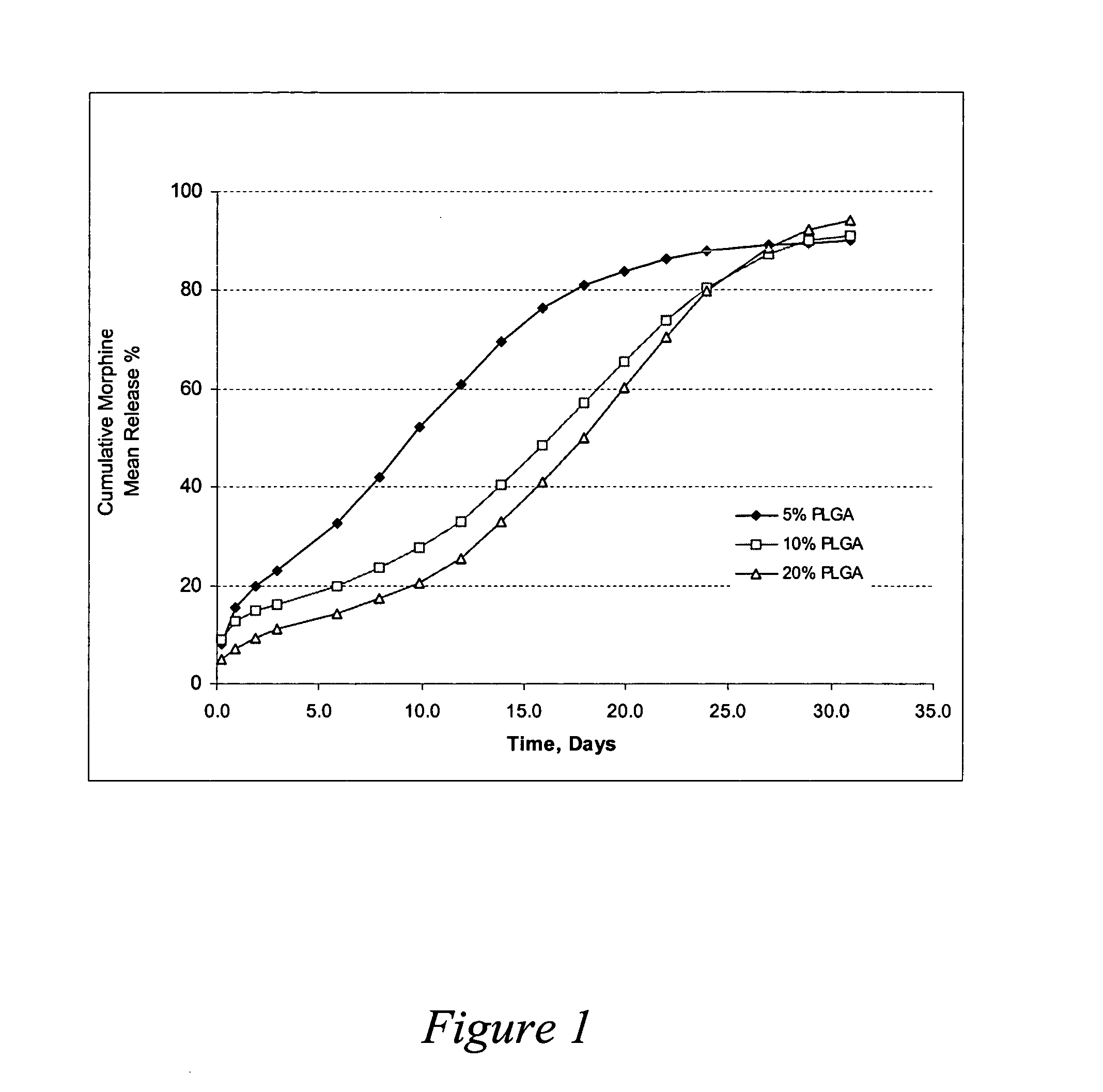
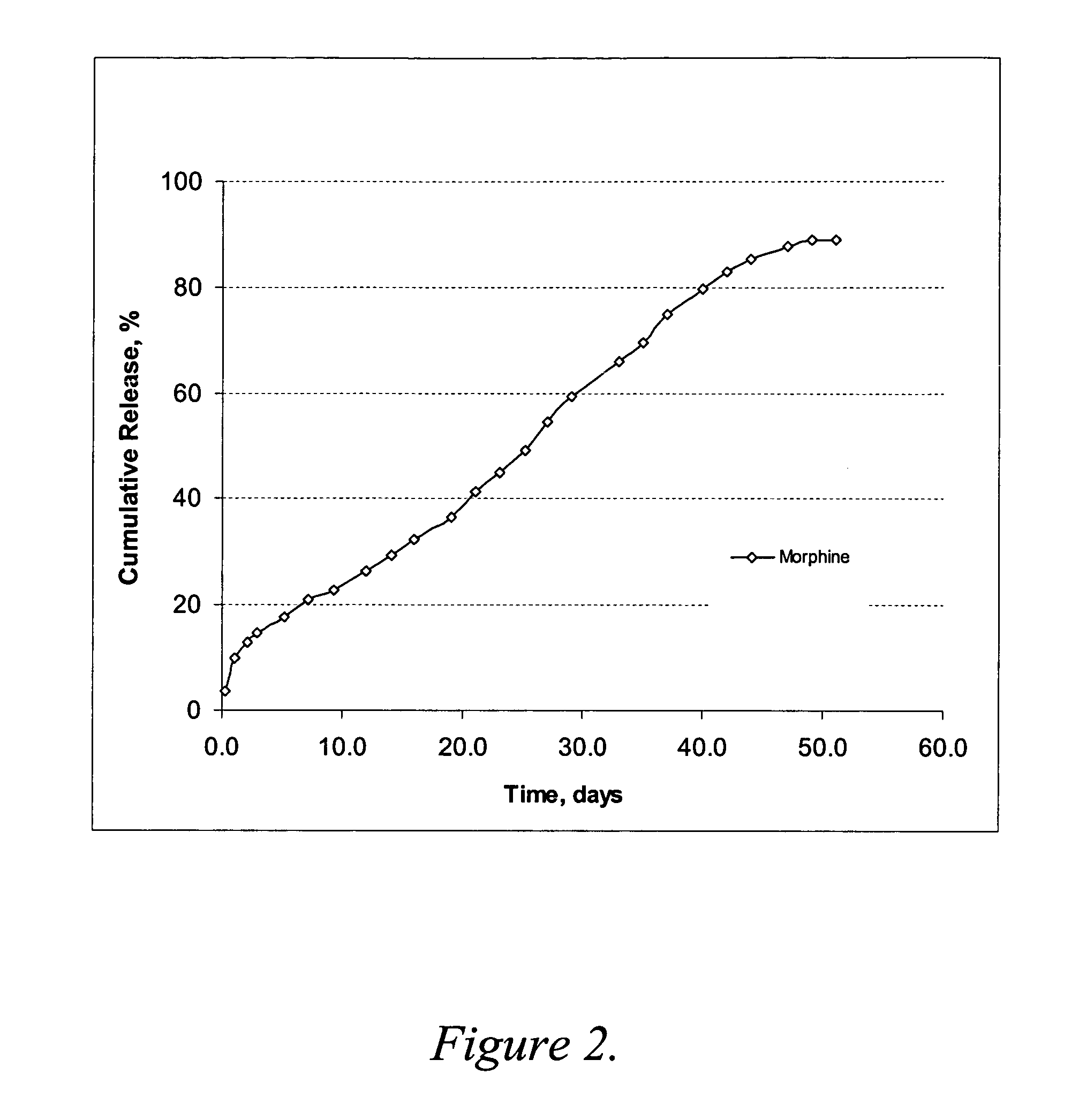
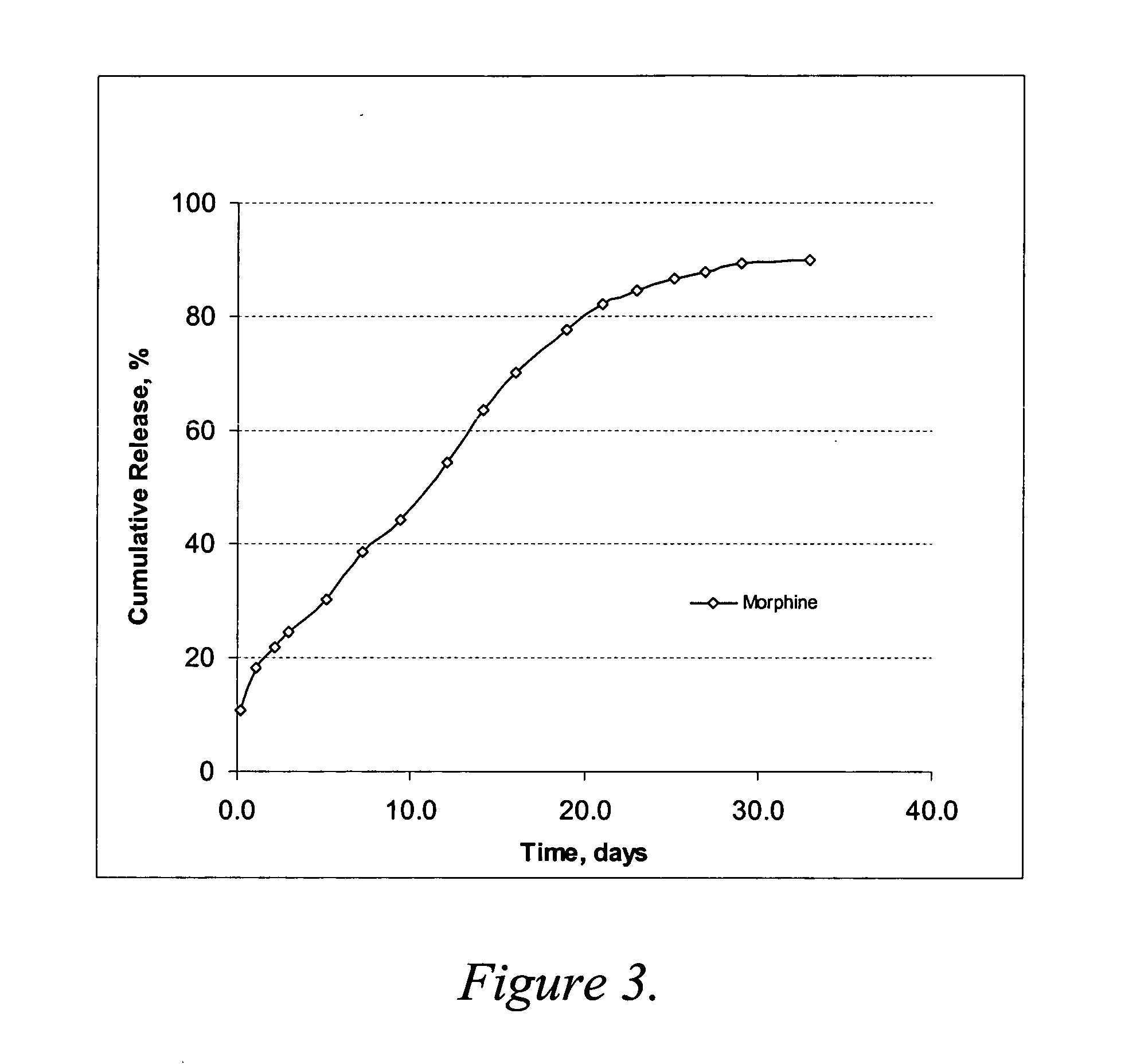
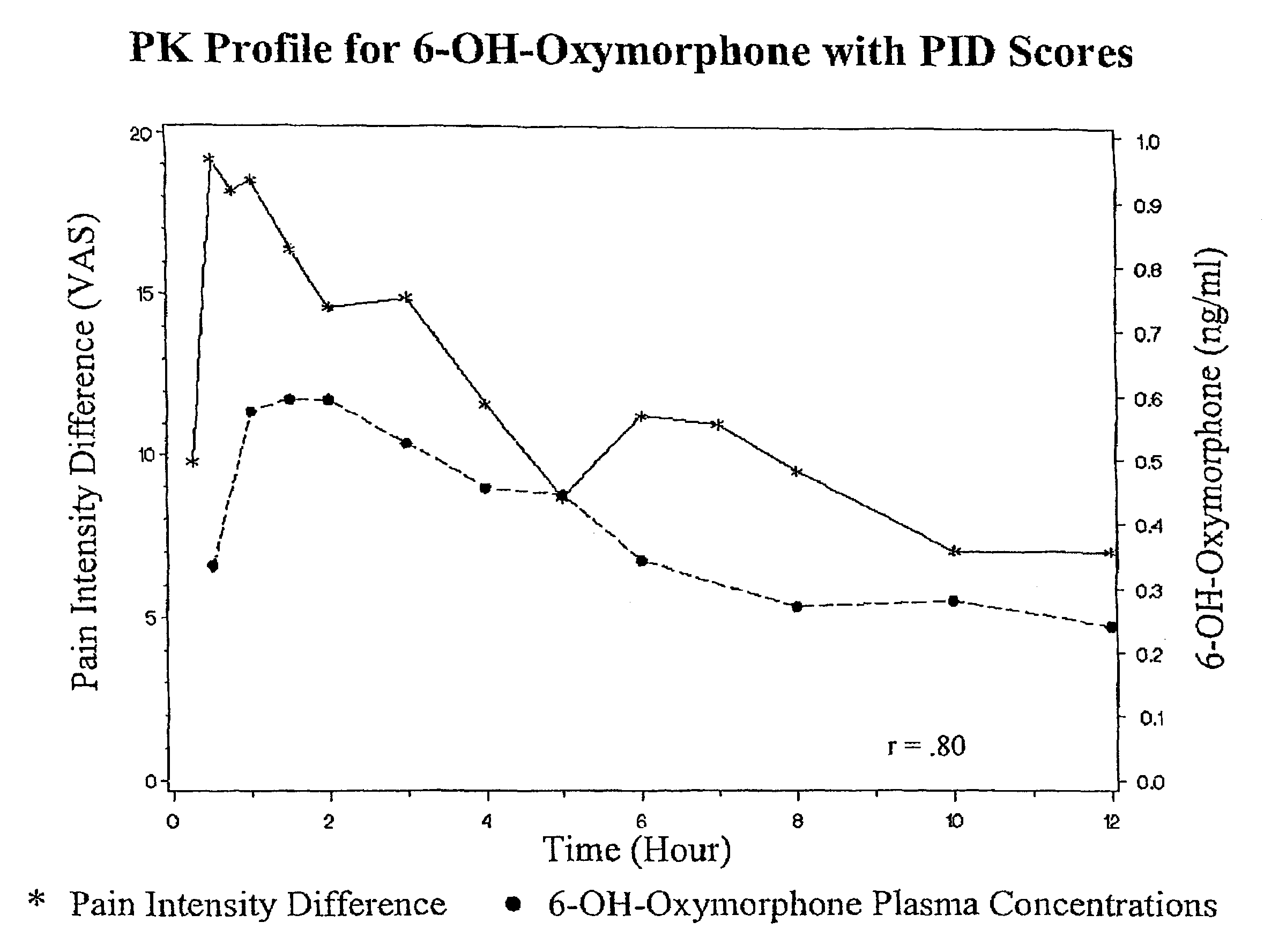
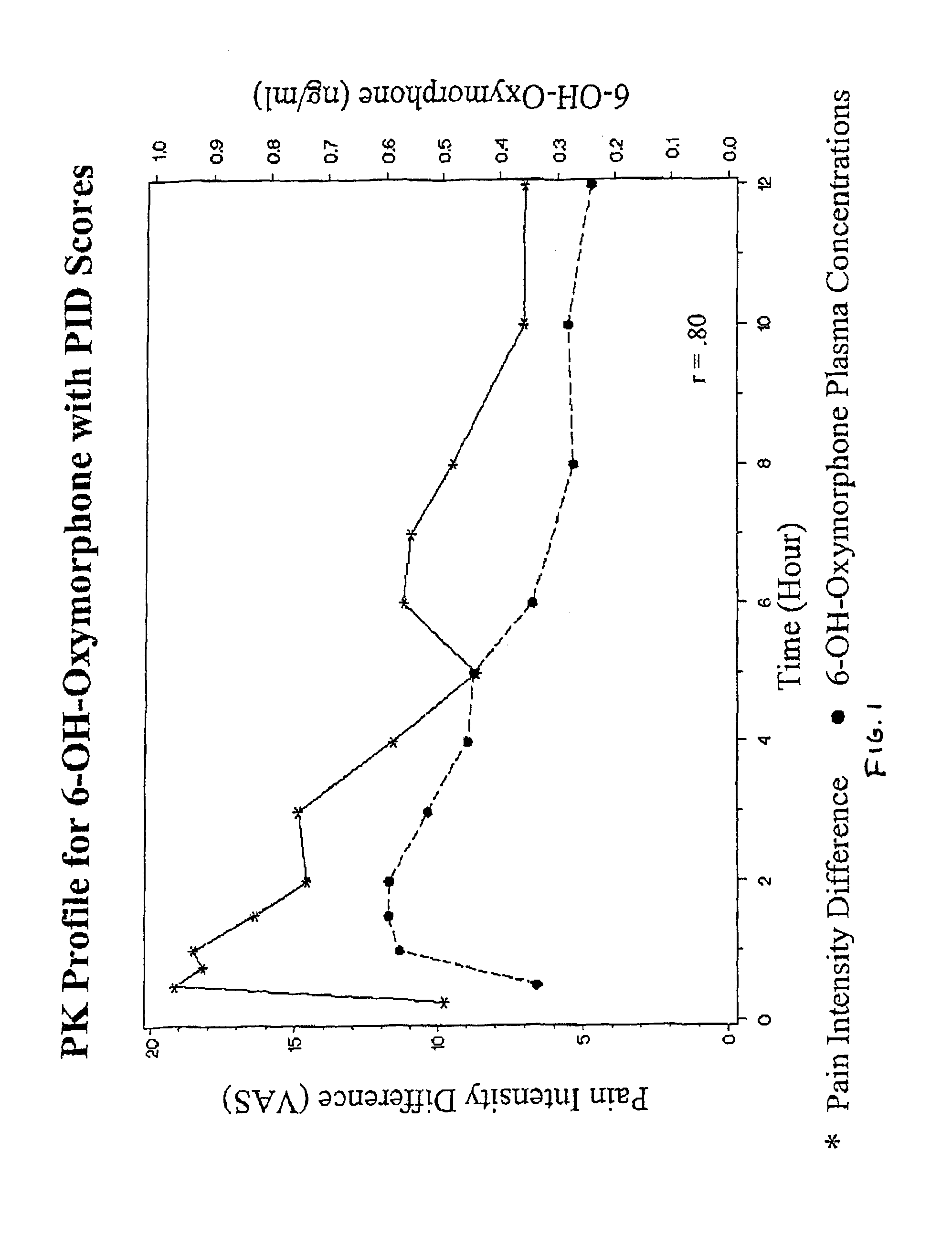
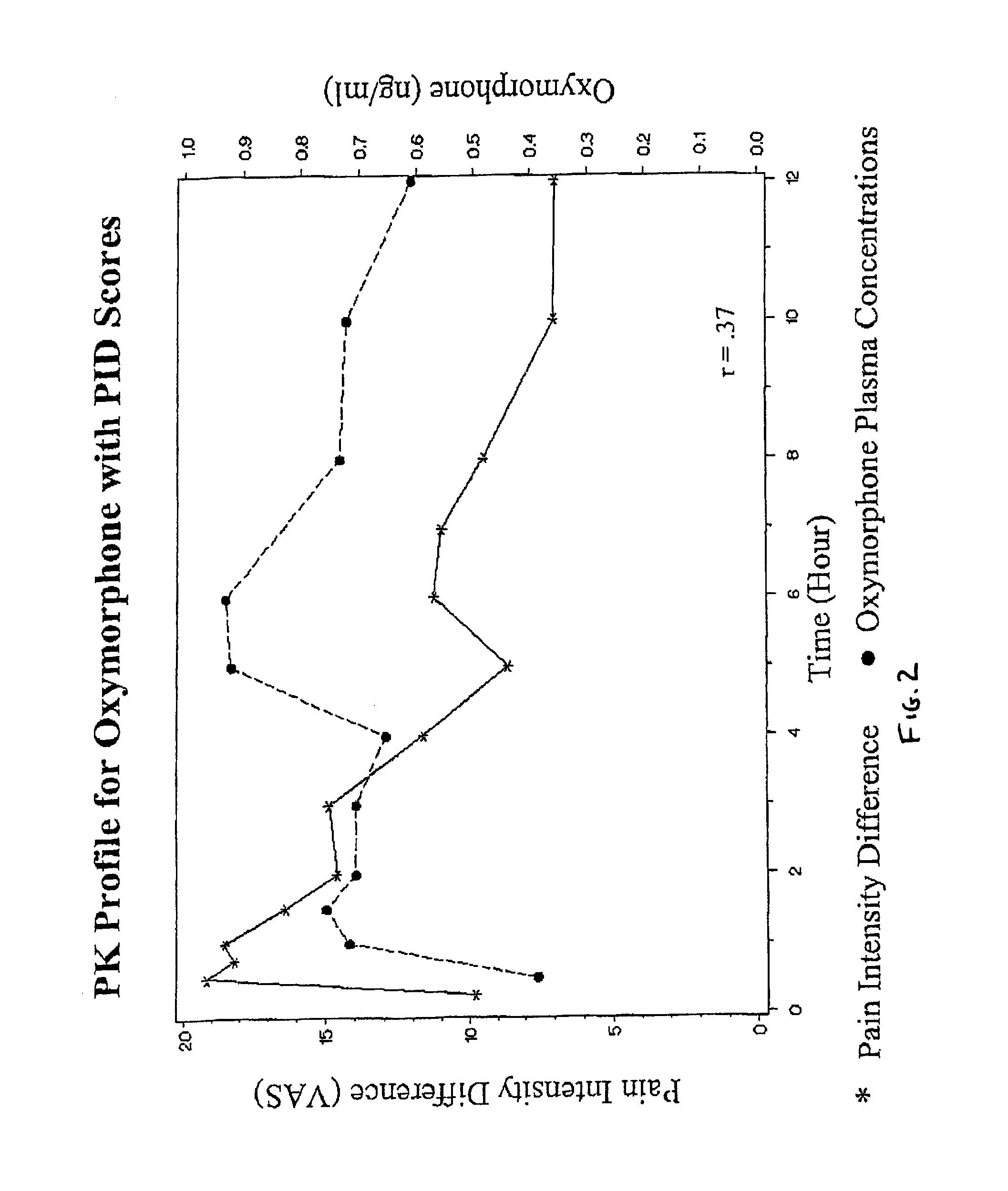
Oxymorphone controlled release formulations
The invention pertains to a method of relieving pain by administering a controlled release pharmaceutical tablet containing oxymorphone which produces a mean minimum blood plasma level 12 to 24 hours after dosing, as well as the tablet producing the sustained pain relief.
Owner:ENDO PHARMA INC
Fragmented polymeric compositions and methods for their use
InactiveUS8357378B2Improve liquidityHydration can be adjusted very simplyPowder deliverySurgical adhesivesBiopolymerIn vivo
Owner:BAXTER INT INC +1
Stable pharmaceutical compositions without a stabilizer
InactiveUS6893660B2Need can be overcomeAvoid rapid degradationOrganic active ingredientsBiocideControlled releaseBULK ACTIVE INGREDIENT
Stabilized controlled release pharmaceutical preparations are disclosed in which active ingredient degradation is prevented without the use of a stabilizer. The active ingredient is sealed away from excipients that can adversely affect stability by sealing the excipients rather than the active ingredient. The preparations are substantially unaffected by exposure to storage conditions of elevated temperature and / or elevated relative humidity.
Owner:ANDRX PHARMA INC
Matrix compositions for controlled delivery of drug substances
InactiveUS20100166866A1Improve solubilityImprove oral bioavailabilityPowder deliveryBiocidePolyethylene oxidePEG-PLGA-PEG
Owner:EGALET LTD
Fragmented polymeric compositions and methods for their use
InactiveUS8303981B2Improve liquiditySimple and convenient fashionPowder deliverySurgical adhesivesBreast implantBiopolymer
Cross-linked hydrogels comprise a variety of biologic and non-biologic polymers, such as proteins, polysaccharides, and synthetic polymers. Such hydrogels preferably have no free aqueous phase and may be applied to target sites in a patient's body by extruding the hydrogel through an orifice at the target site. Alternatively, the hydrogels may be mechanically disrupted and used in implantable articles, such as breast implants. When used in vivo, the compositions are useful for controlled release drug delivery, for inhibiting post-surgical spinal and other tissue adhesions, for filling tissue divots, tissue tracts, body cavities, surgical defects, and the like.
Owner:BAXTER INT INC +1
Controlled release drug delivery system of pravastatin
InactiveUS20050089572A1Minimizes instabilityGood formulation stabilityBiocideAnimal repellantsControlled releaseDelivery system
The present invention relates to an oral drug delivery system comprising pravastatin or its pharmaceutically acceptable salts such that the system provides enhanced stability in the acidic environment of the stomach and exhibits controlled release of the drug.
Owner:RANBAXY LAB LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com