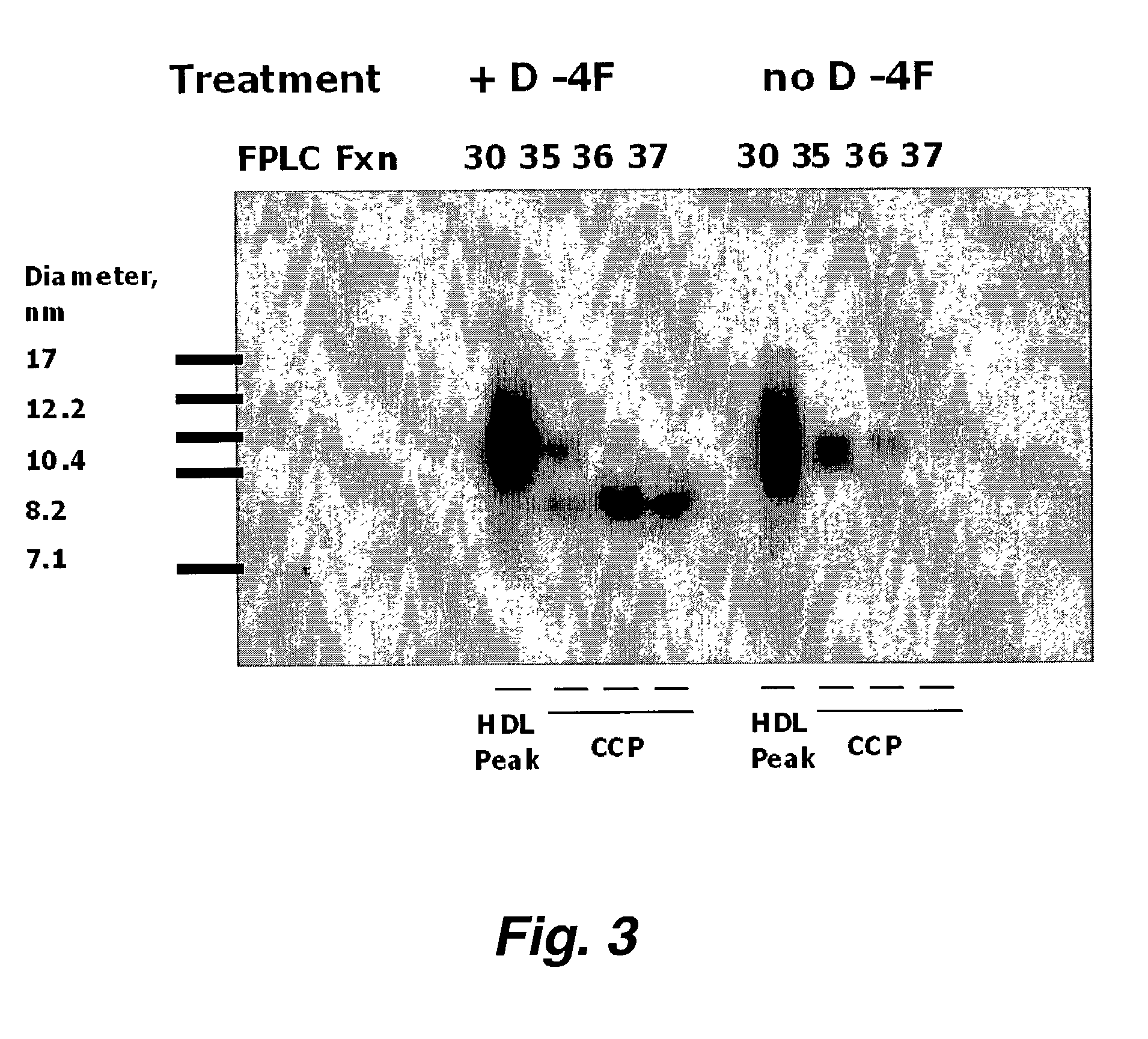
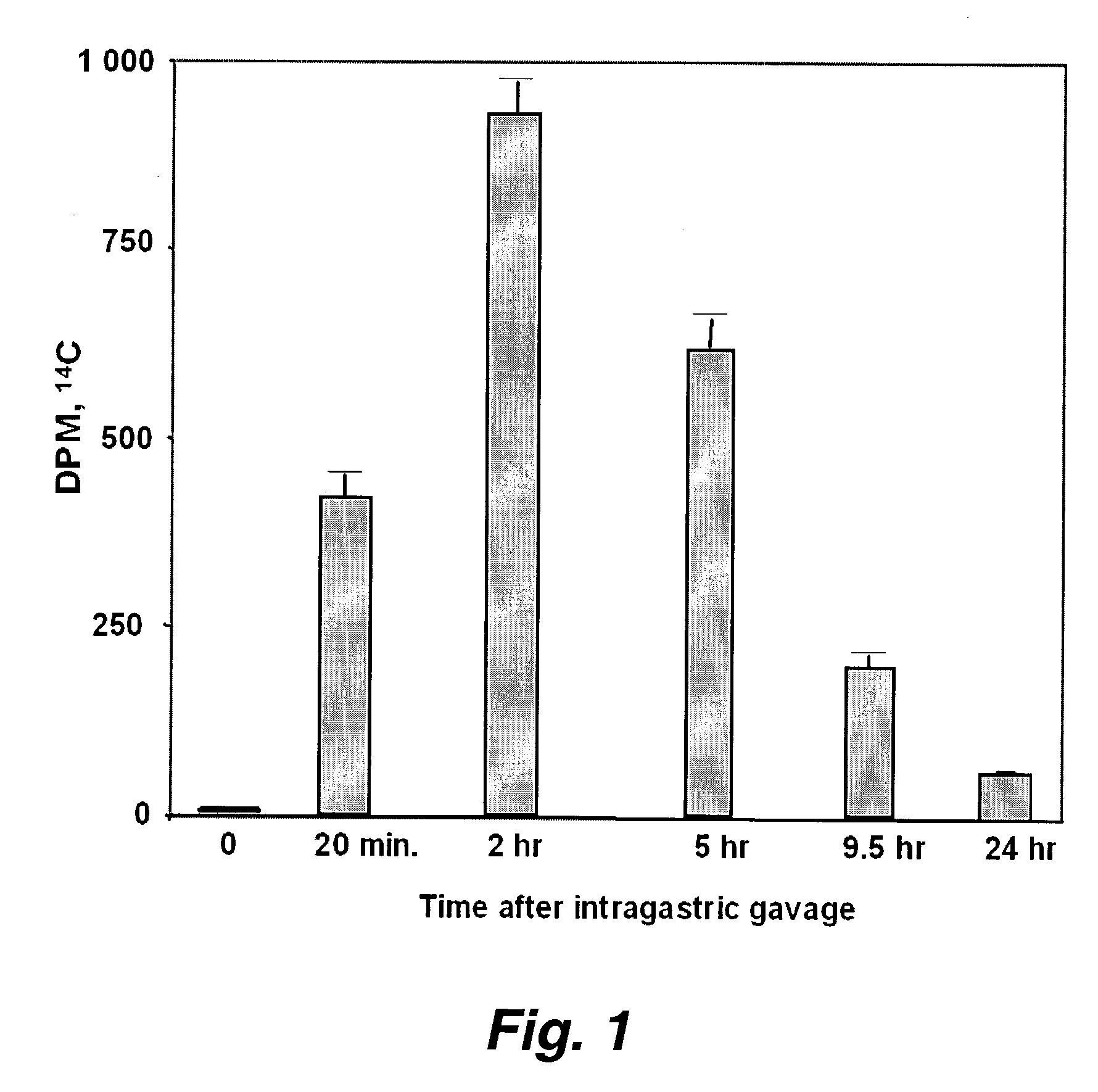
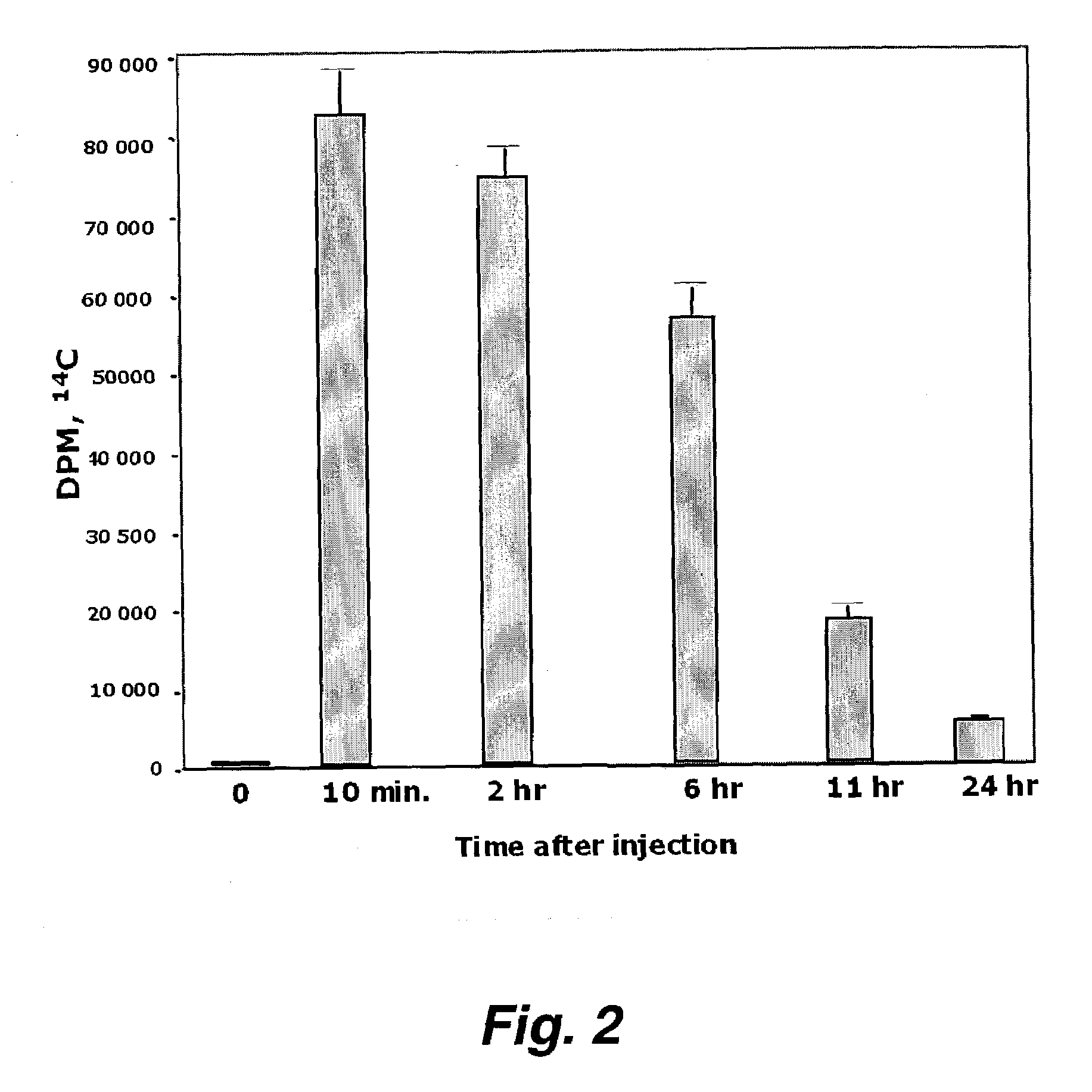
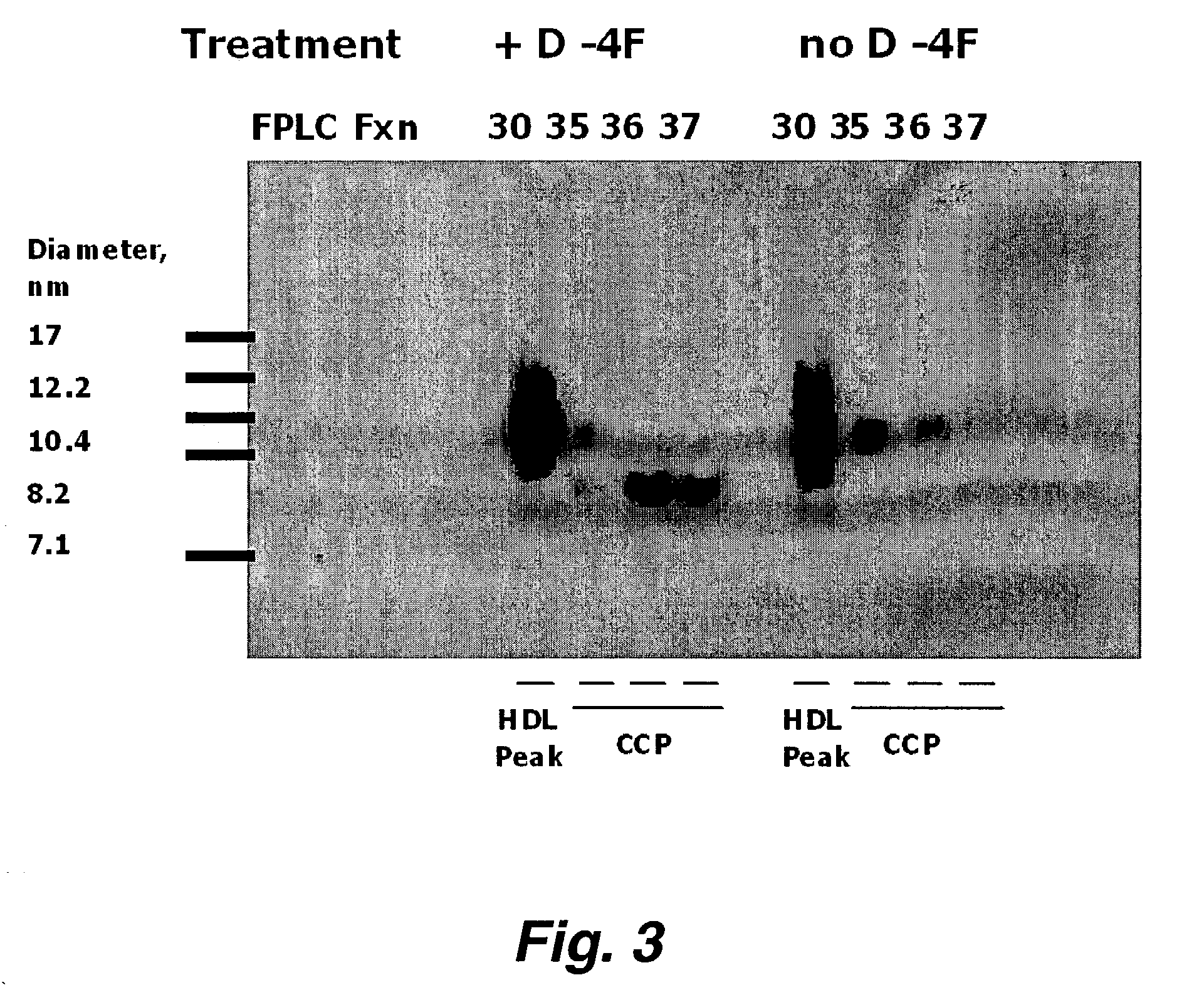
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
101 results about "Oral route" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The fecal–oral route (also called the oral–fecal route or orofecal route) describes a particular route of transmission of a disease wherein pathogens in fecal particles pass from one person to the mouth of another person.
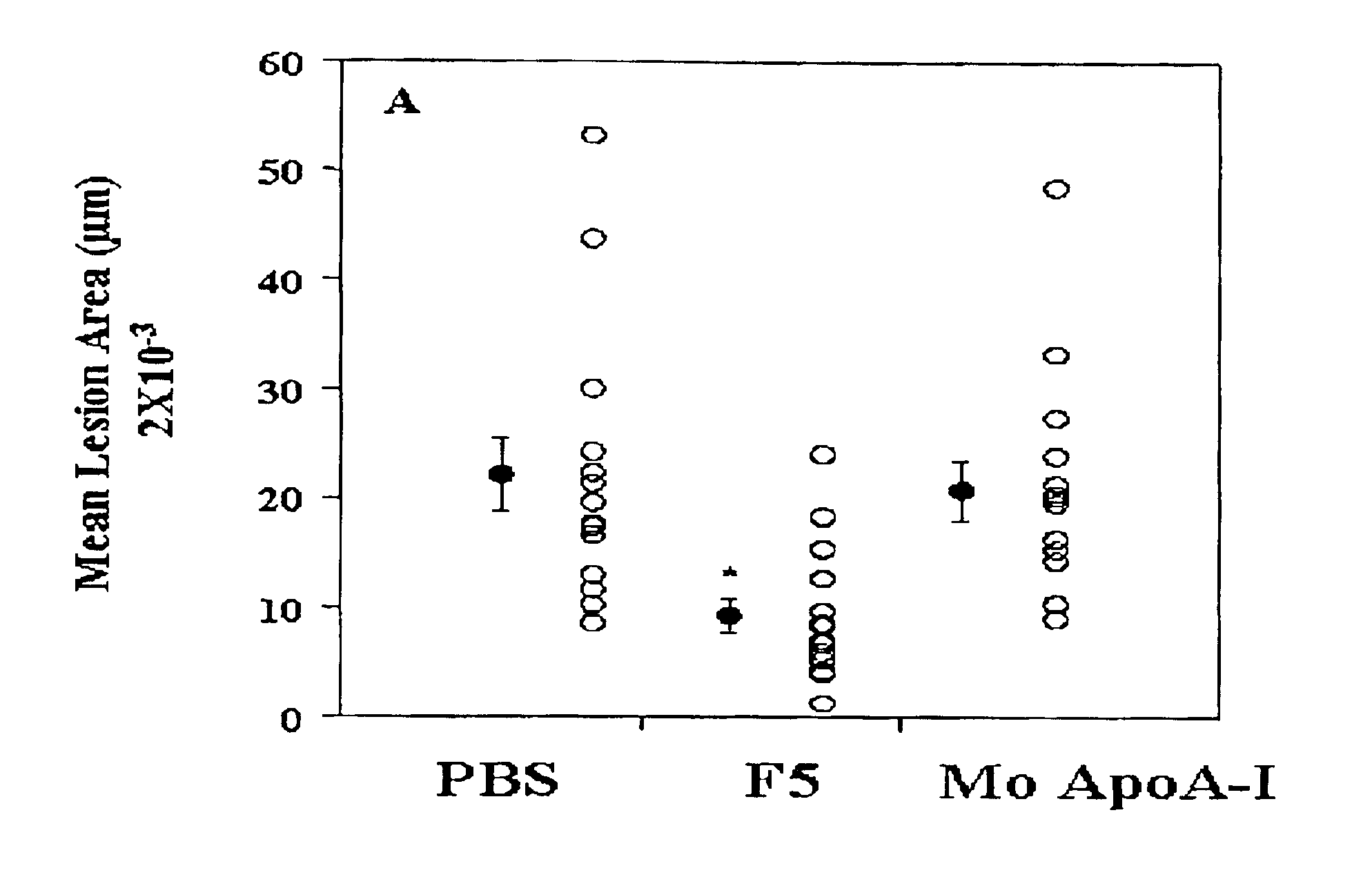
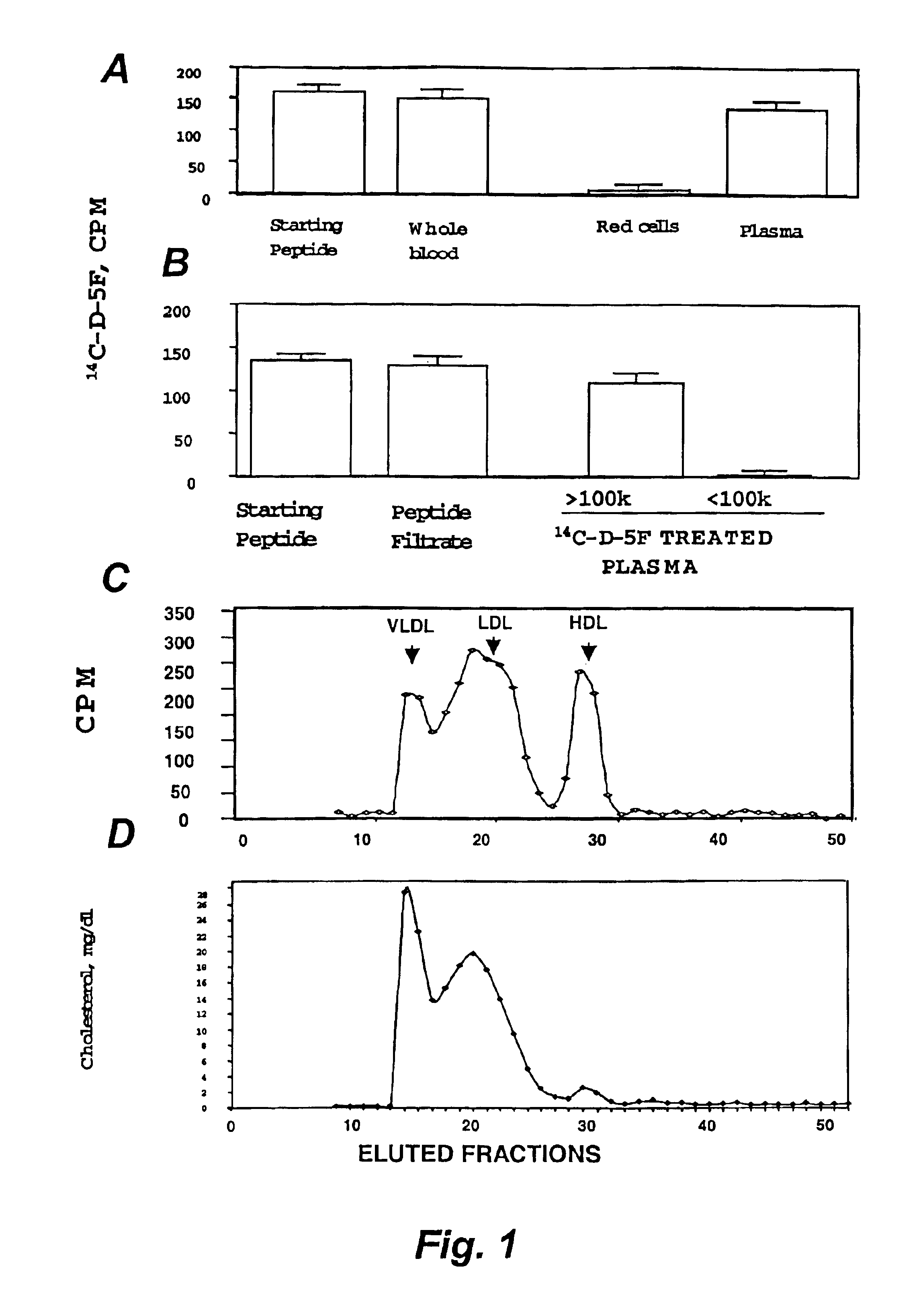
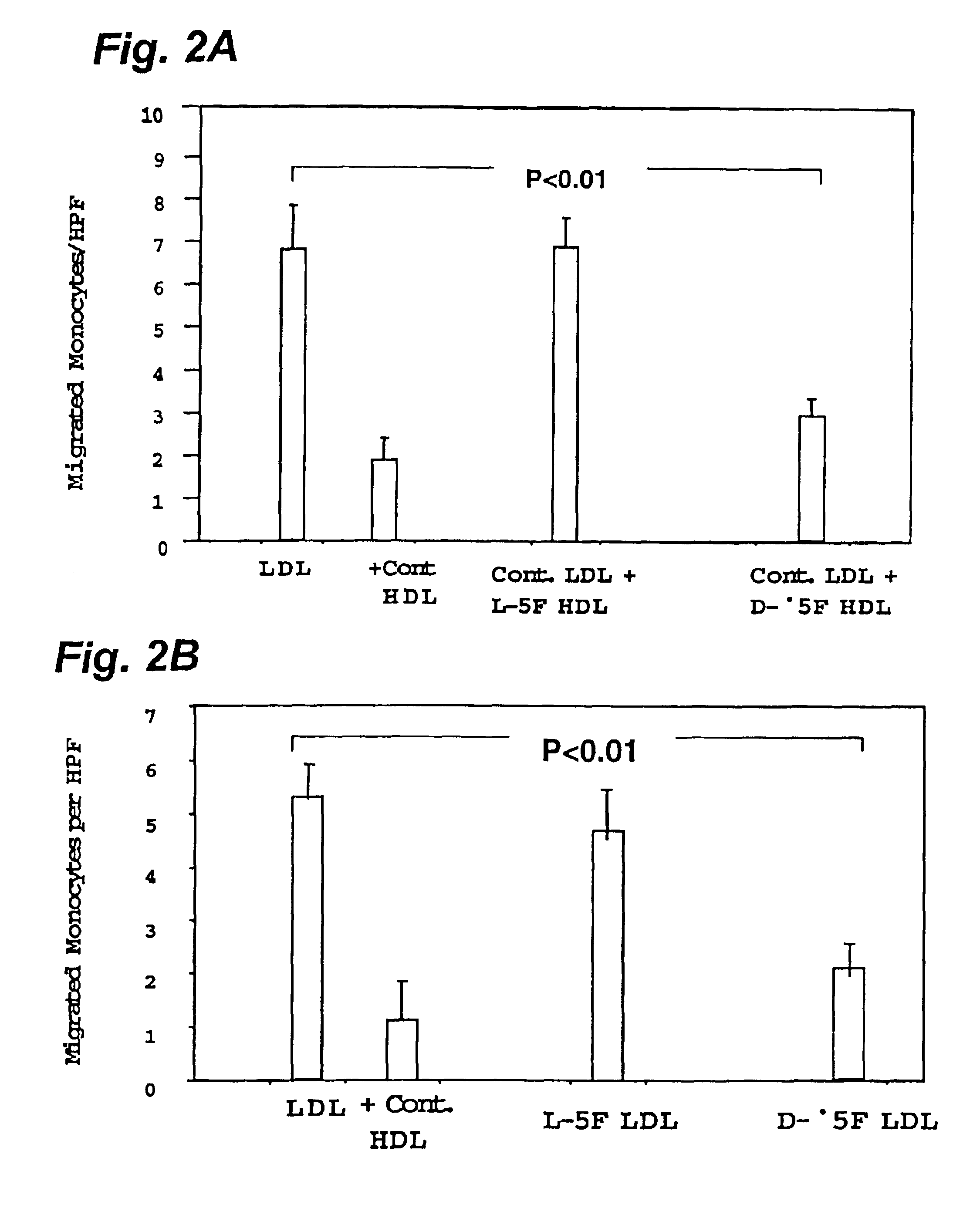
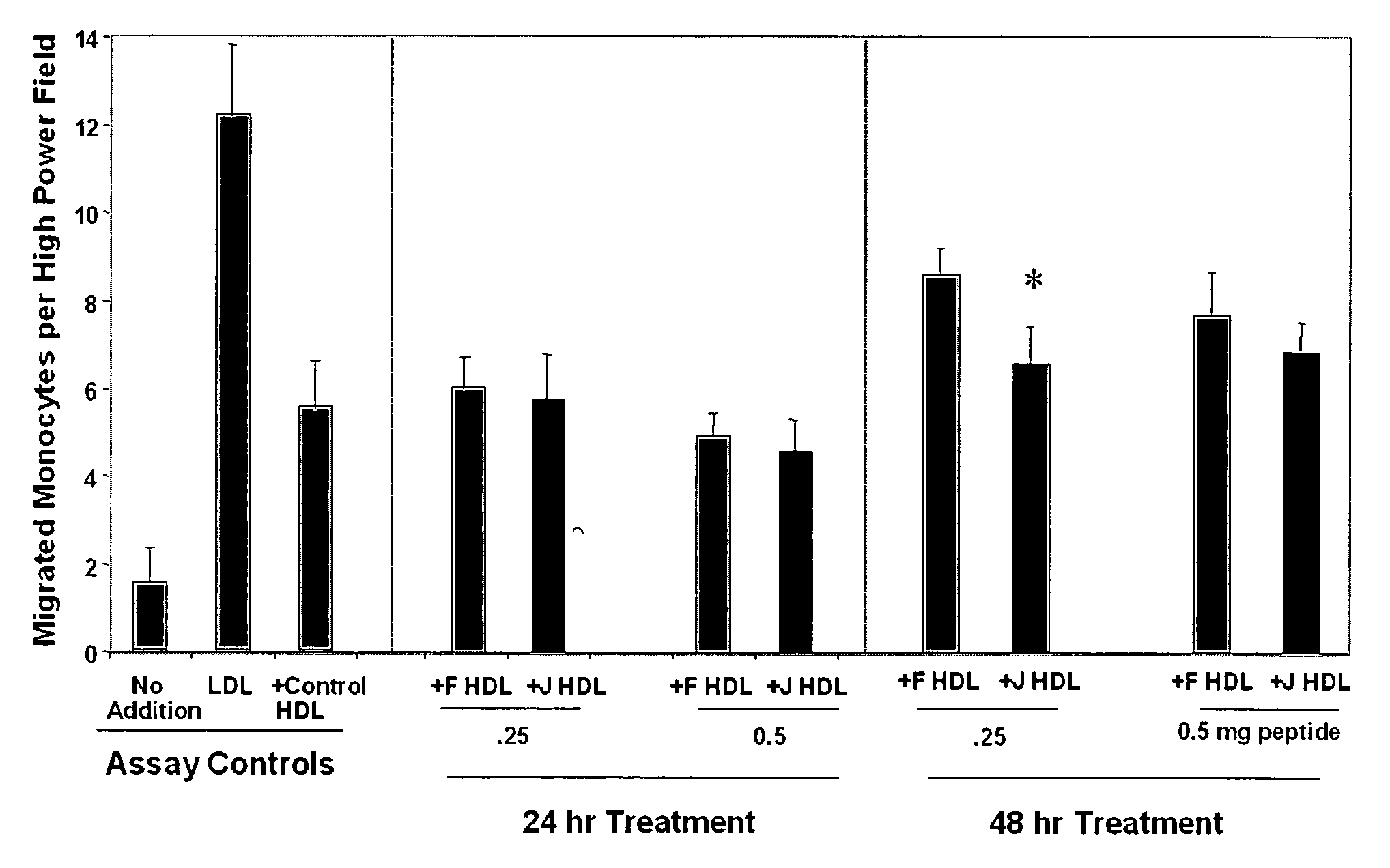
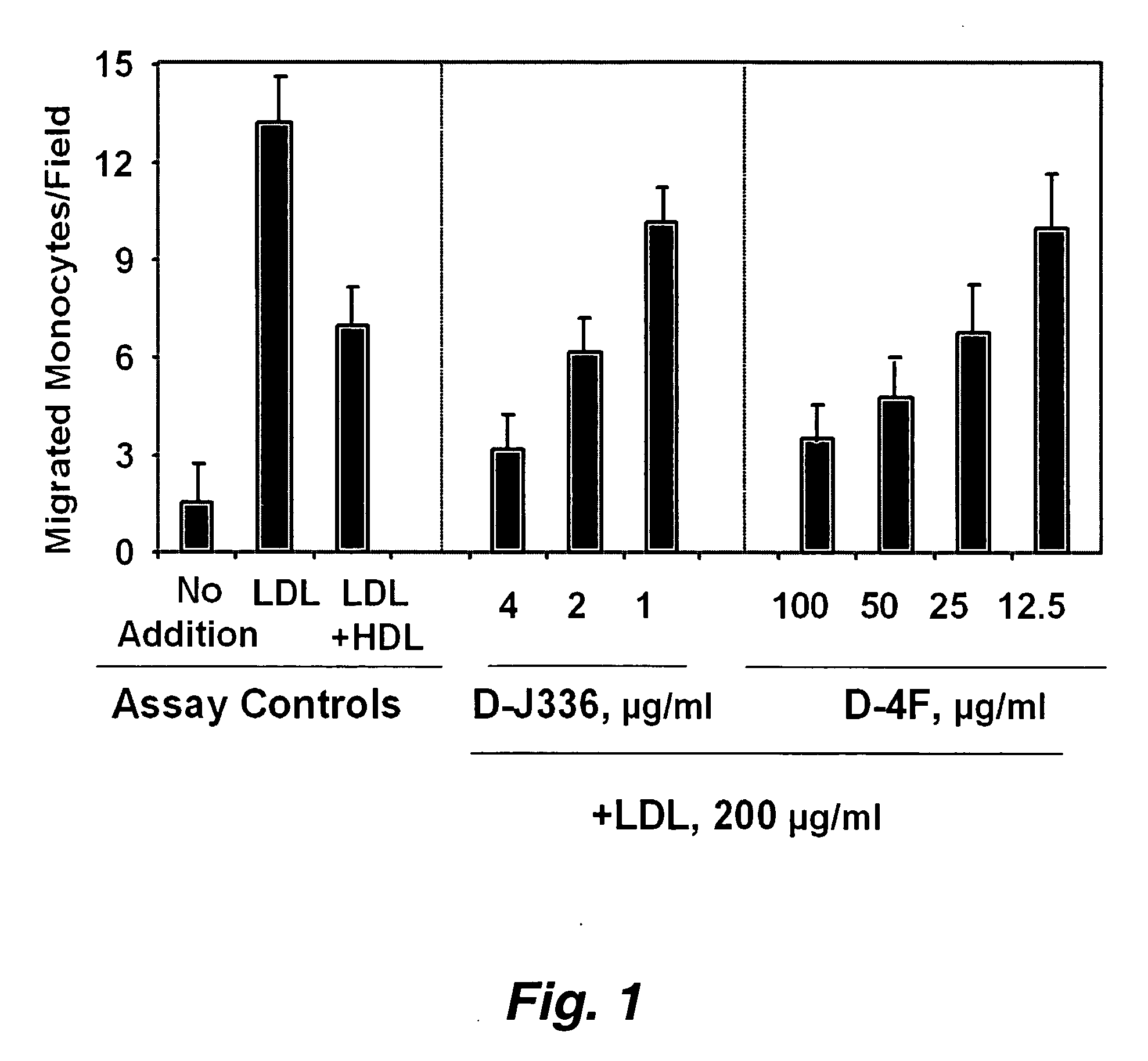
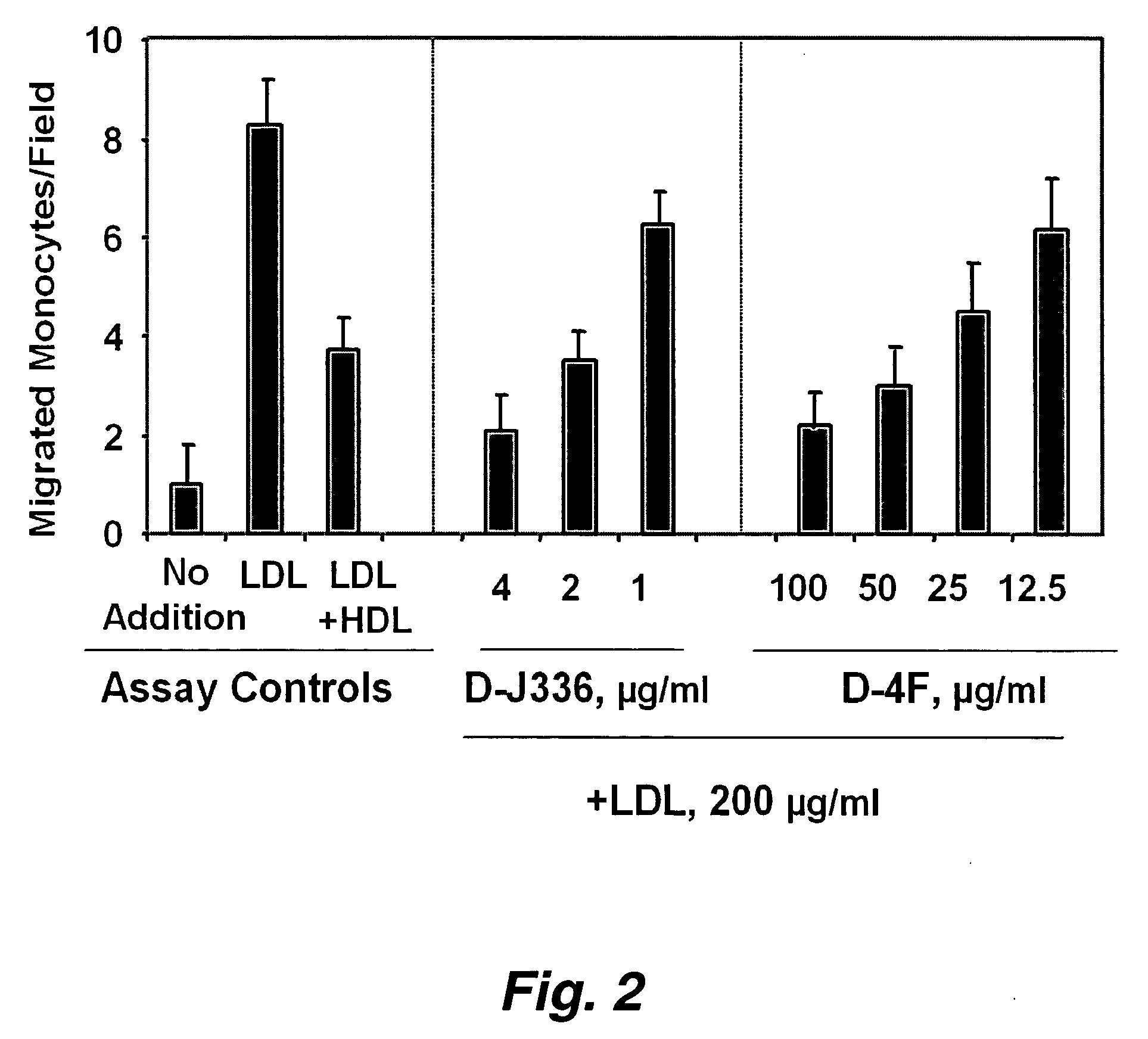
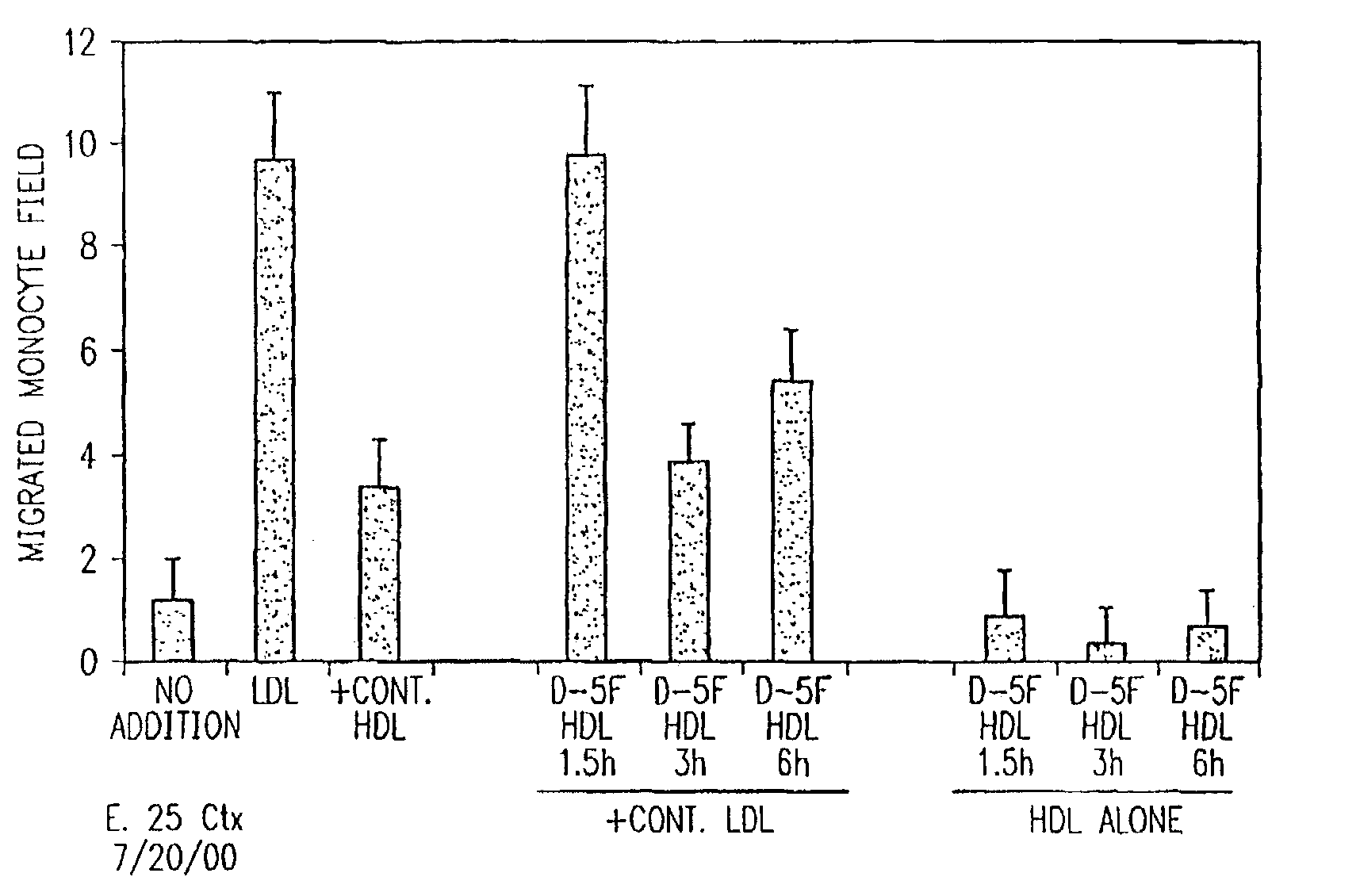
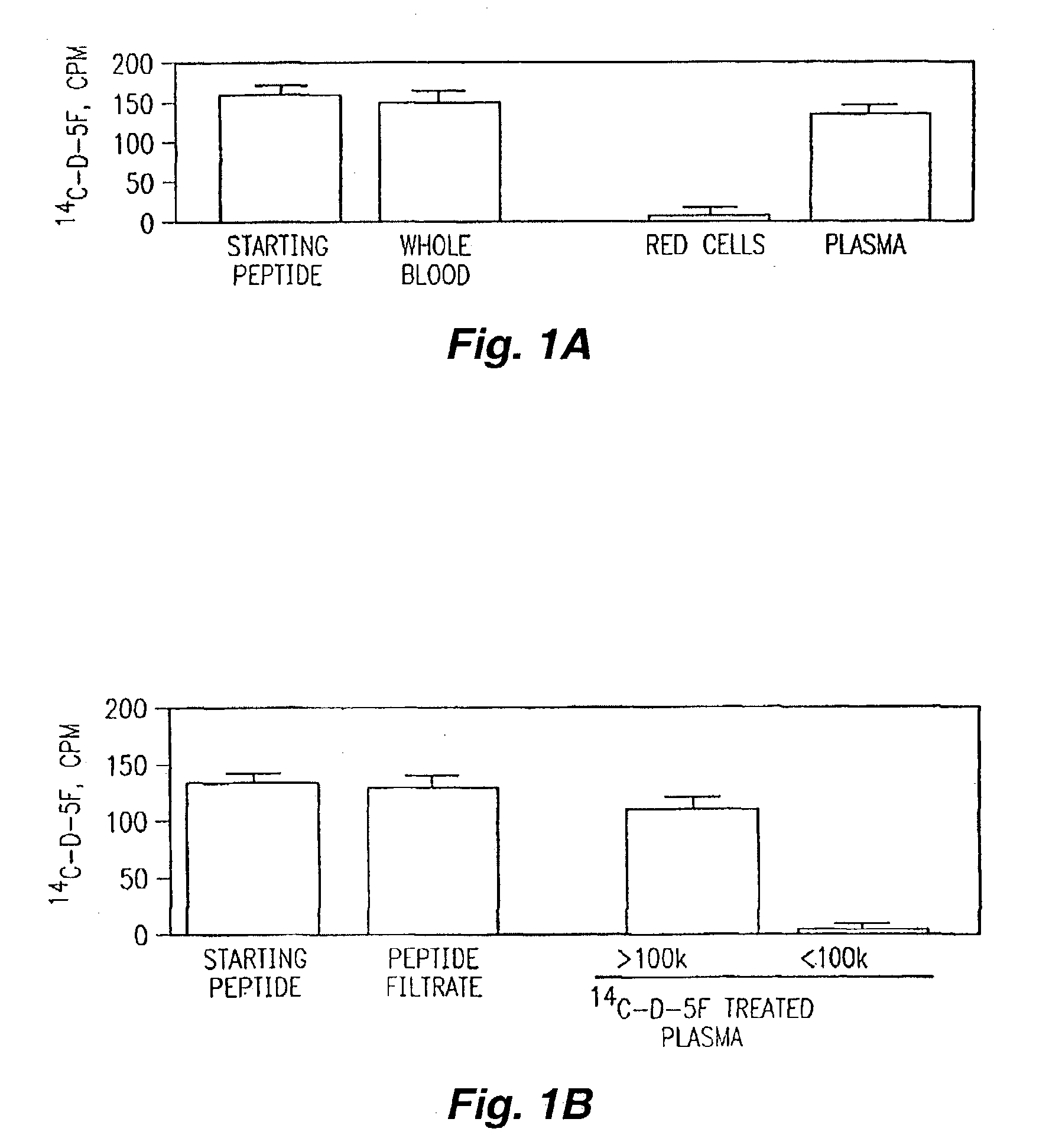
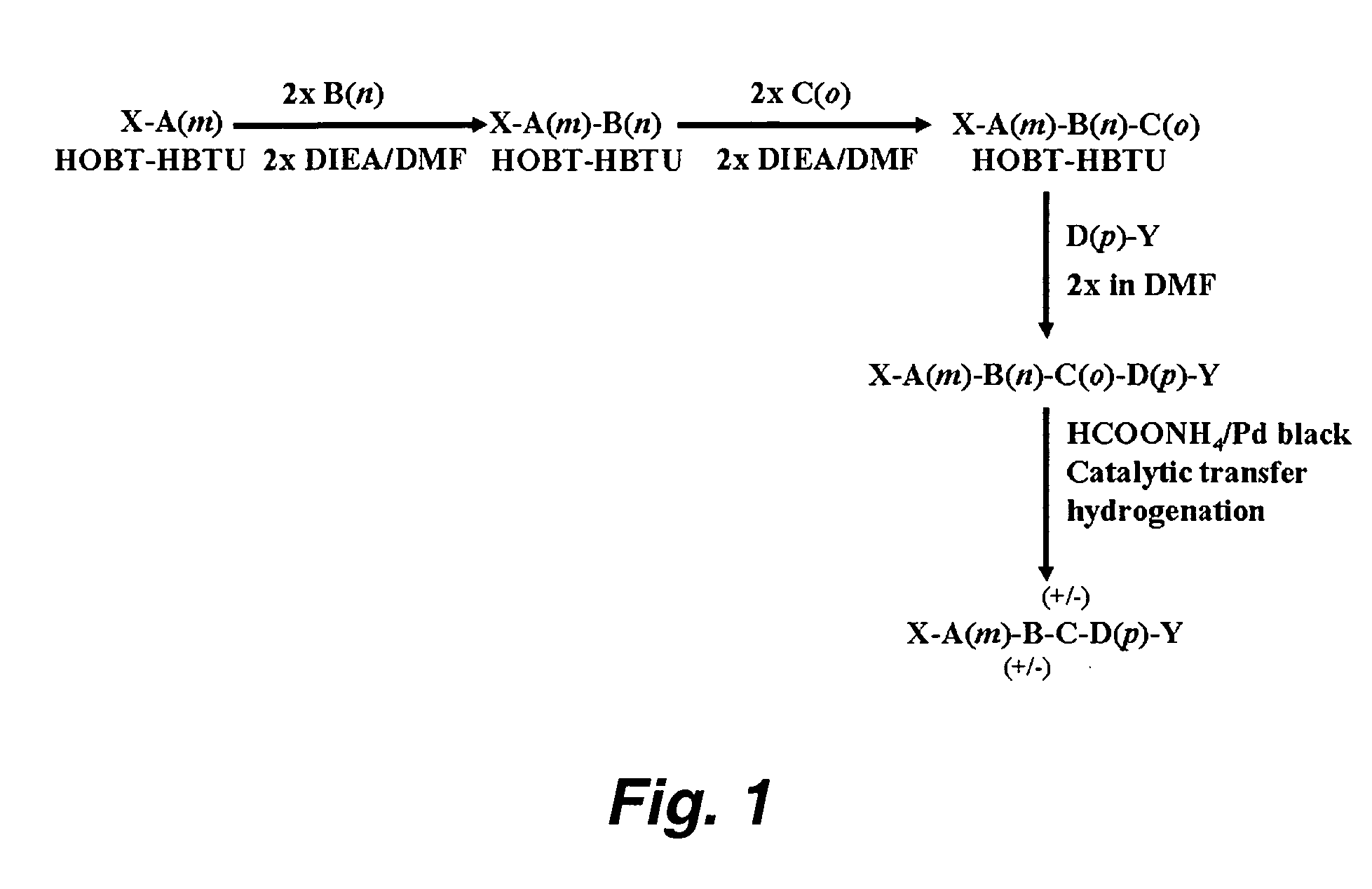
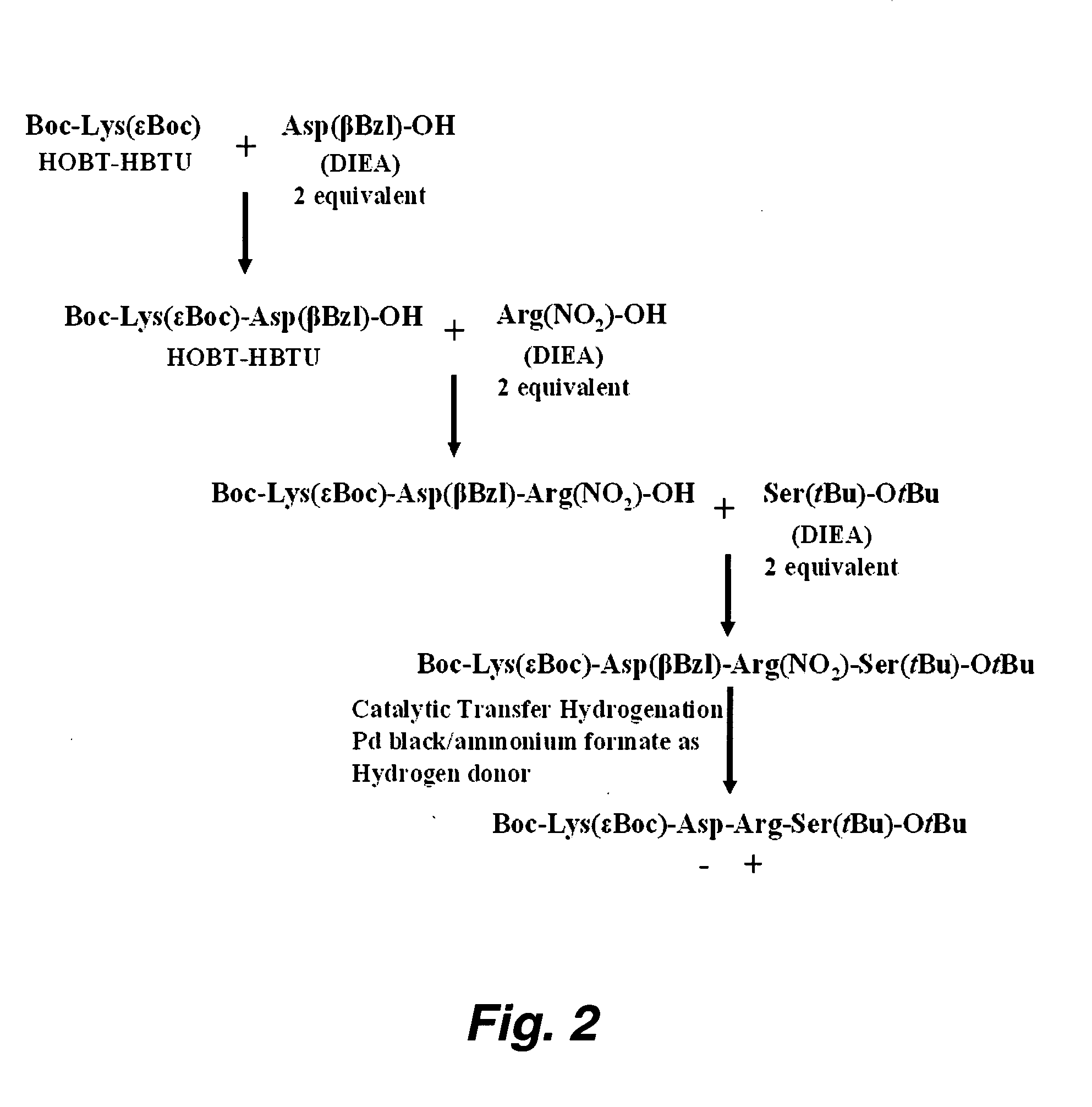
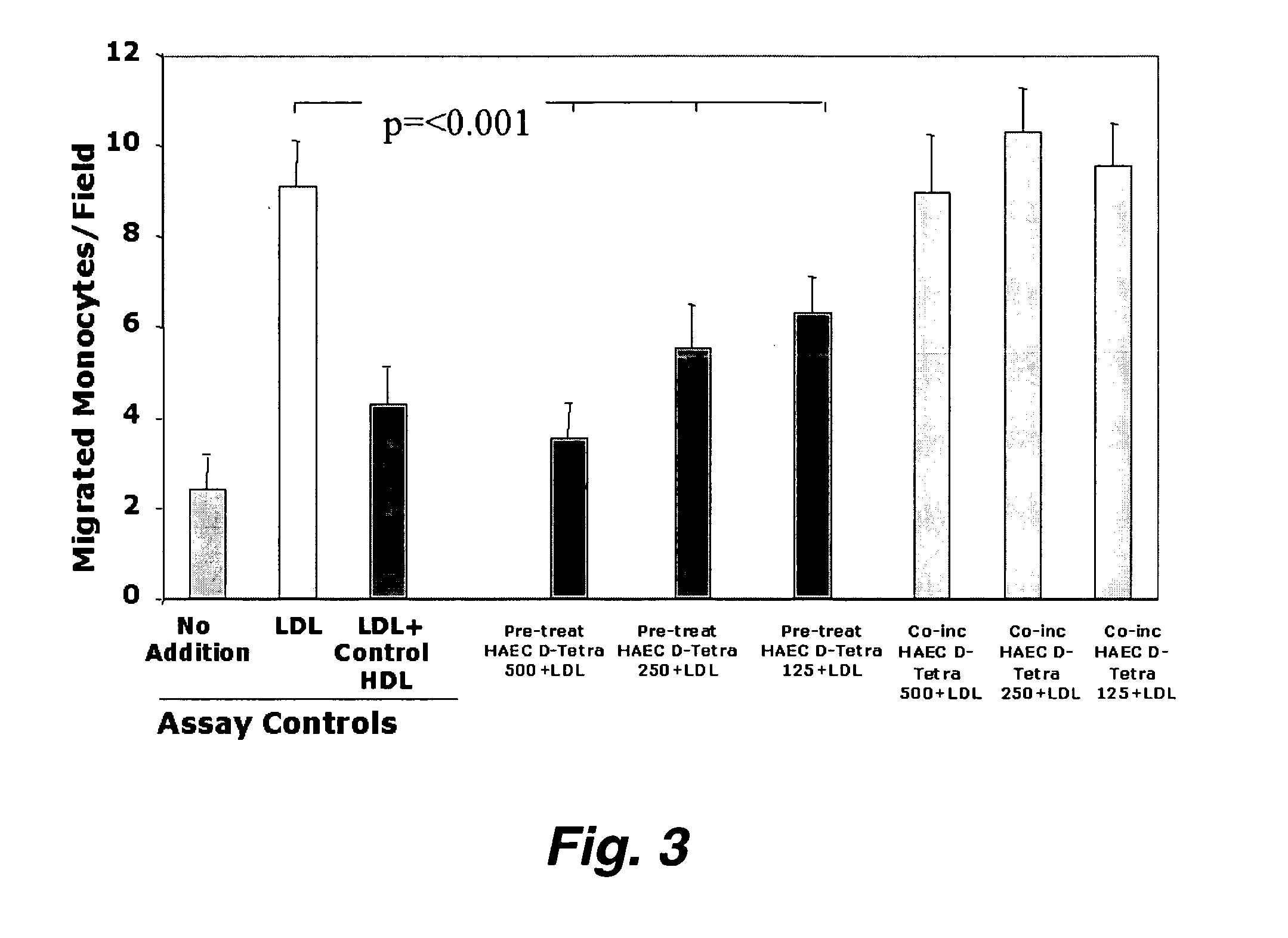
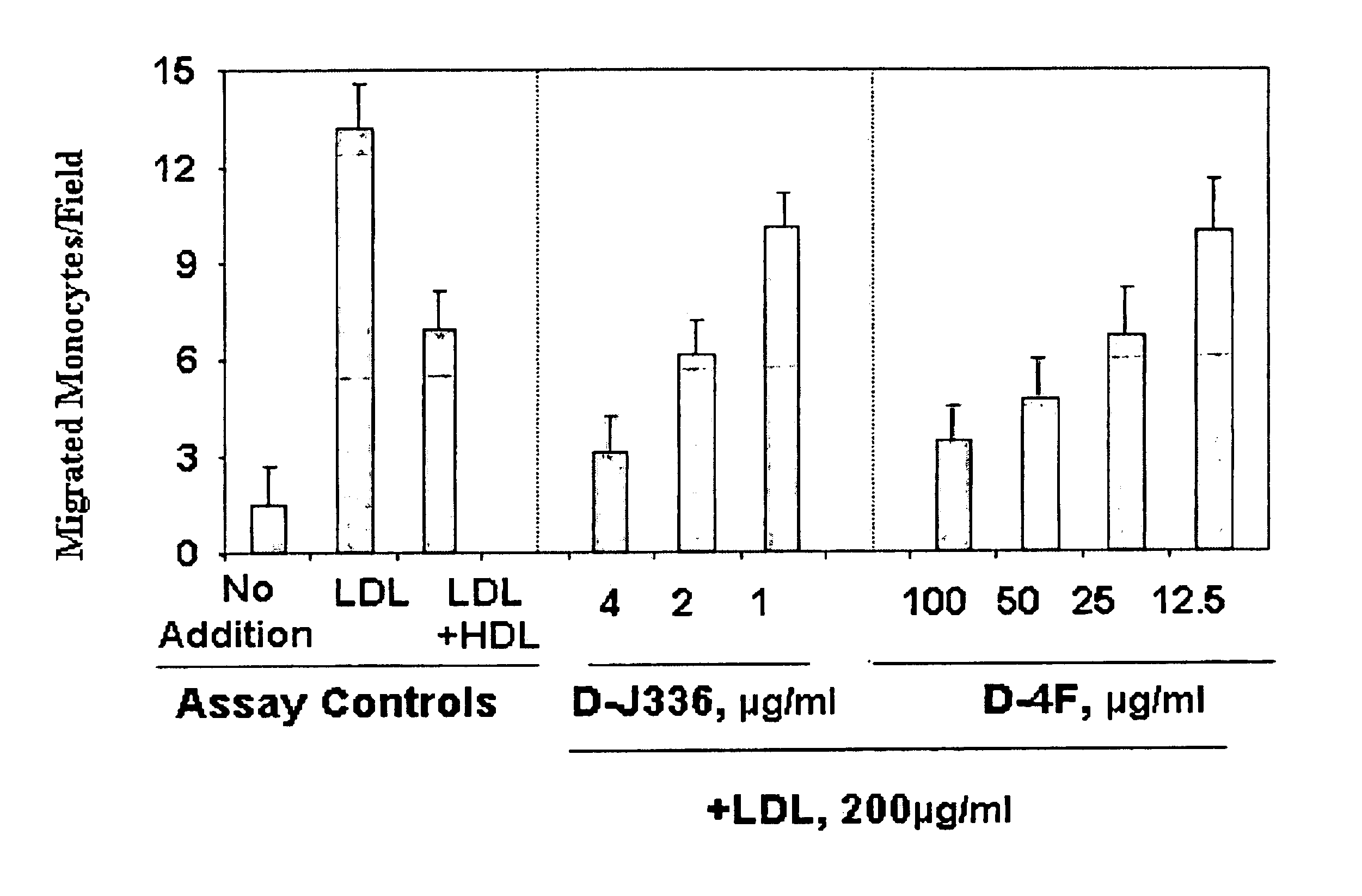
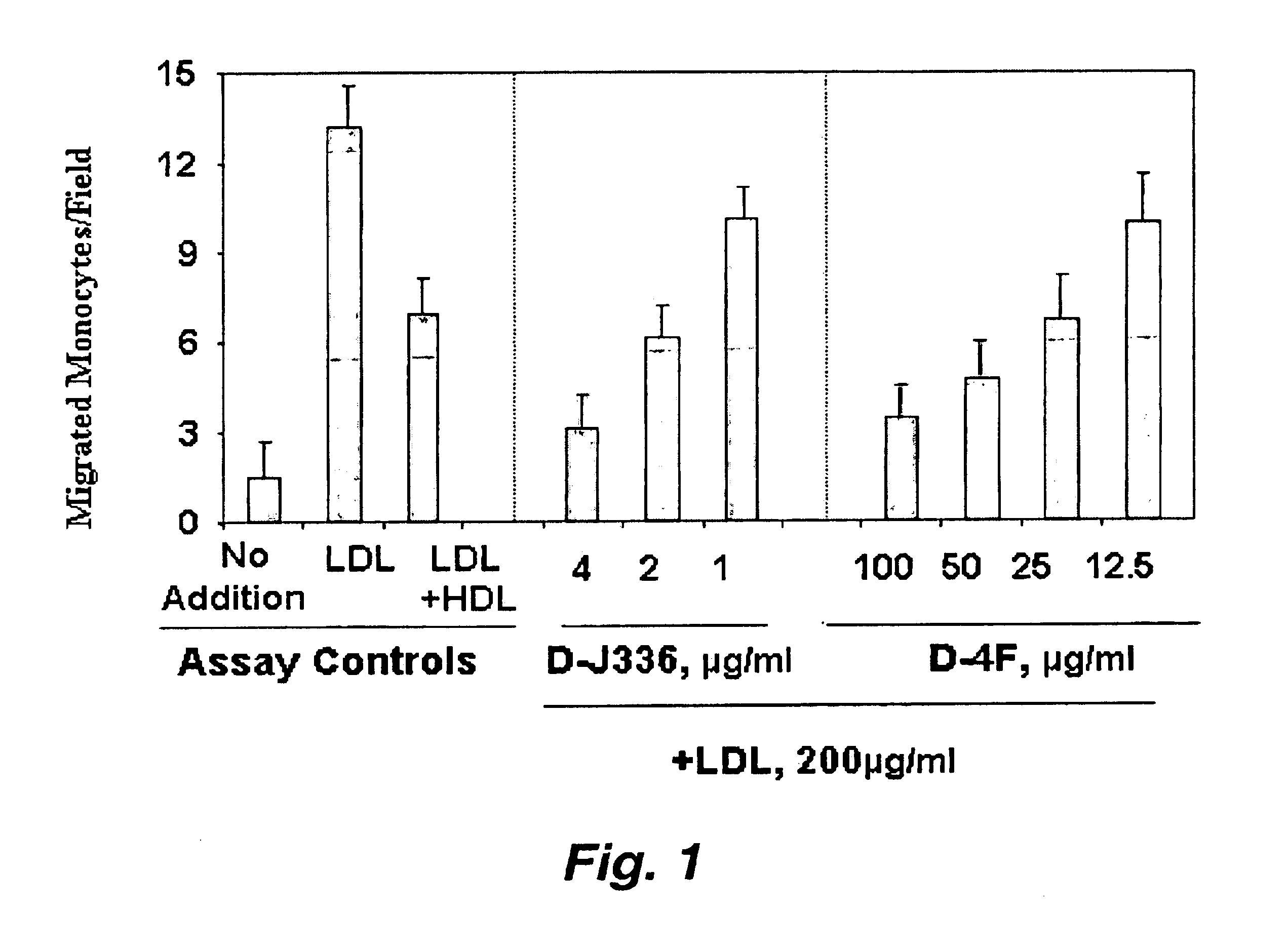
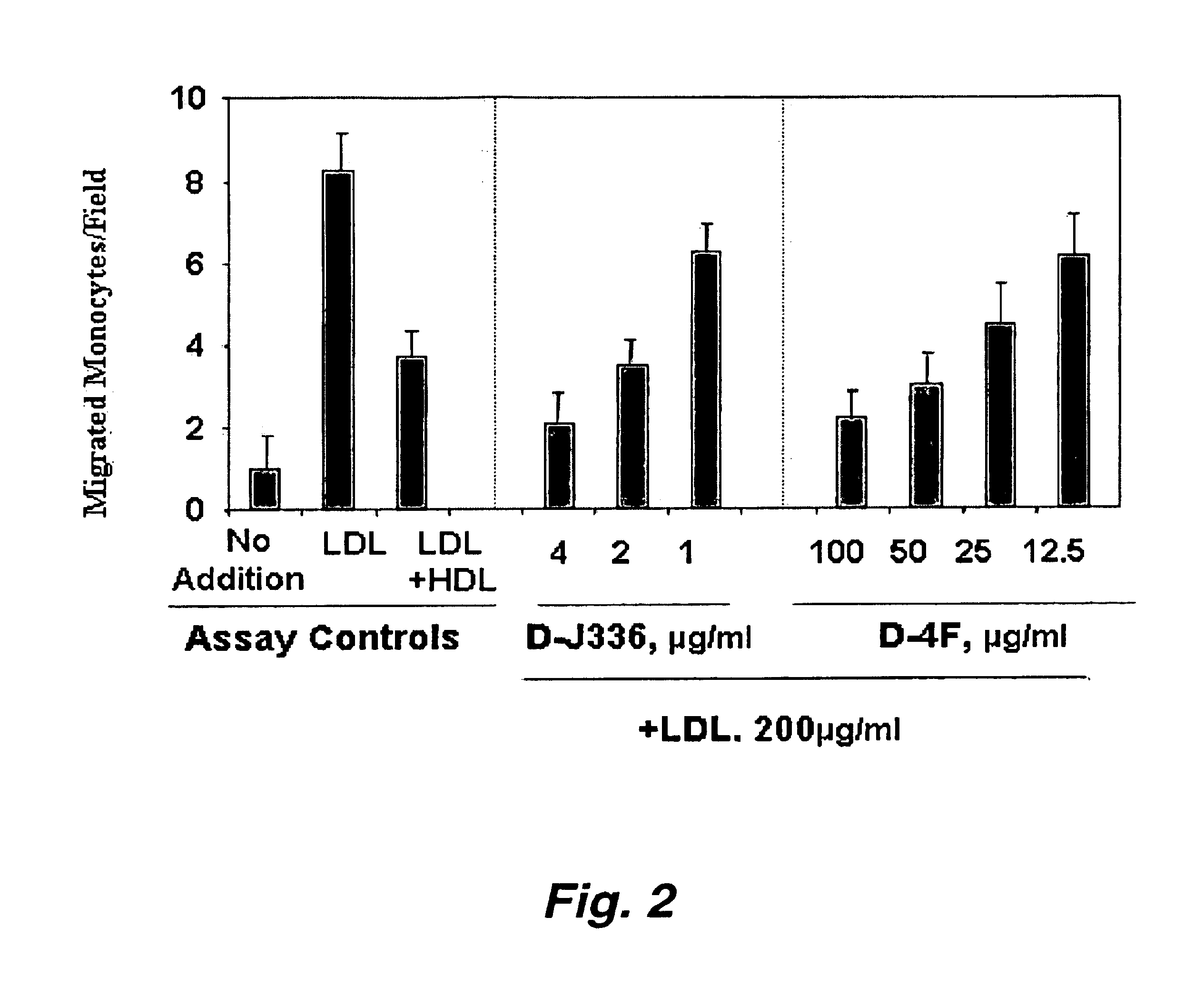
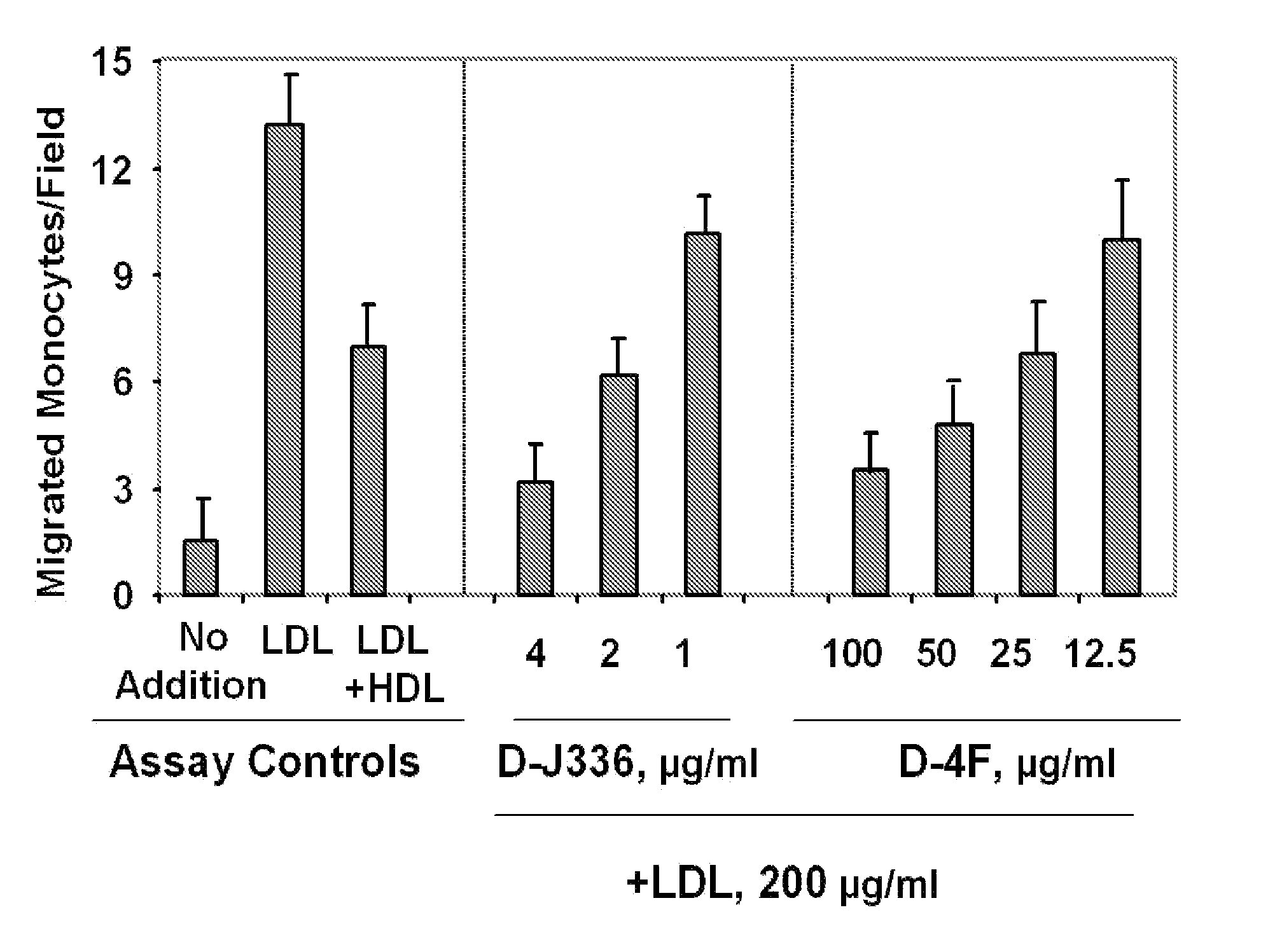
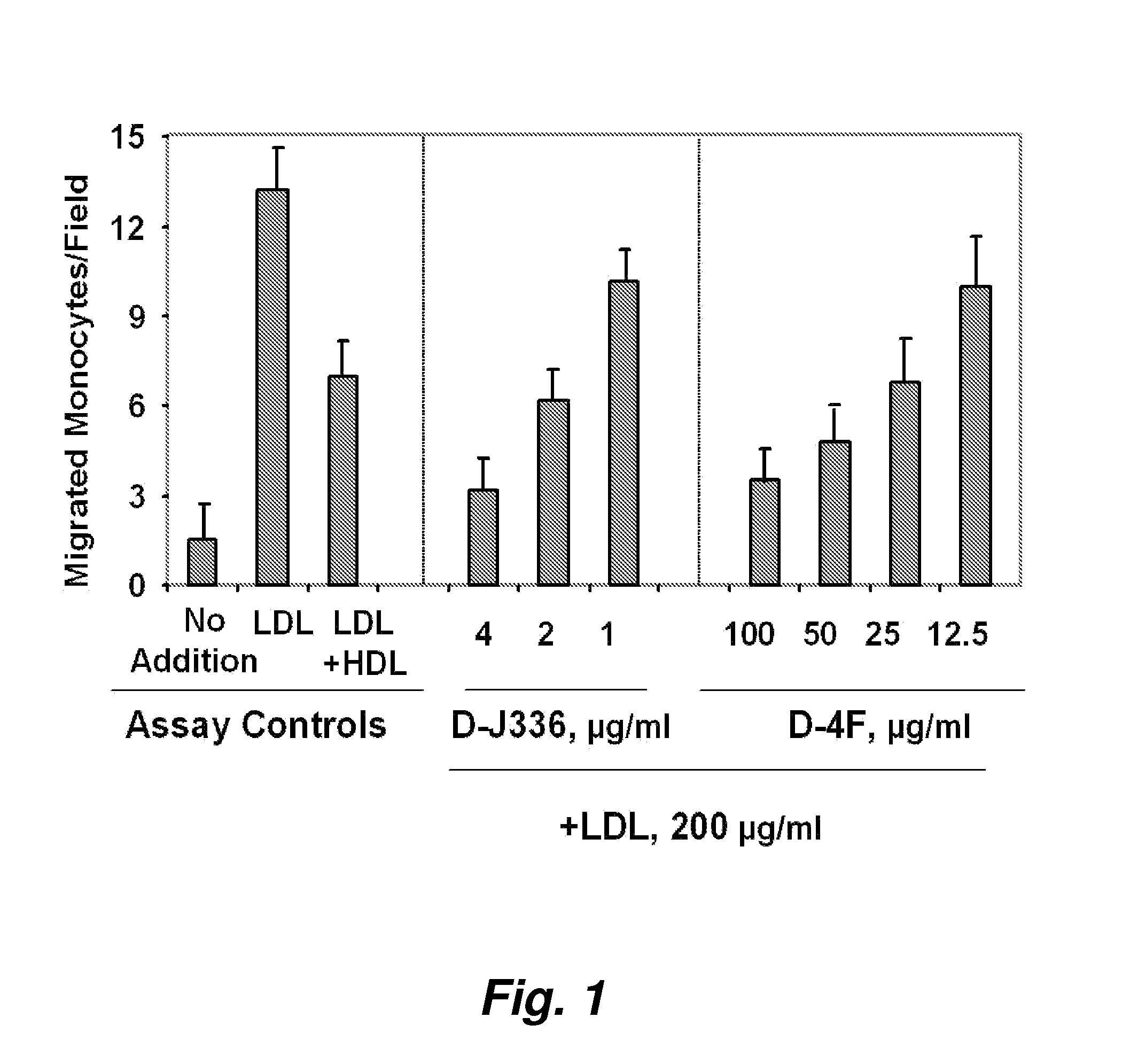
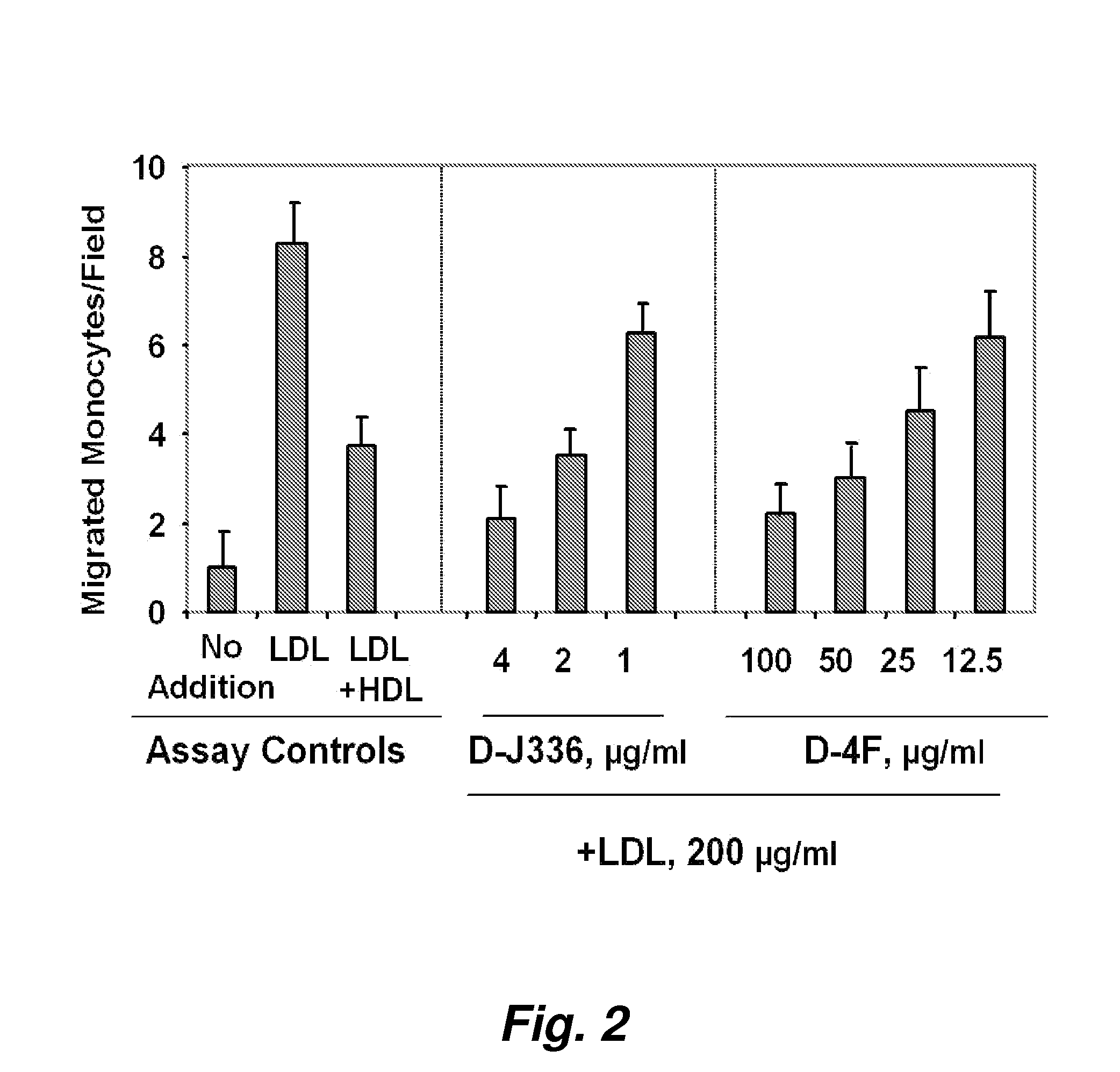
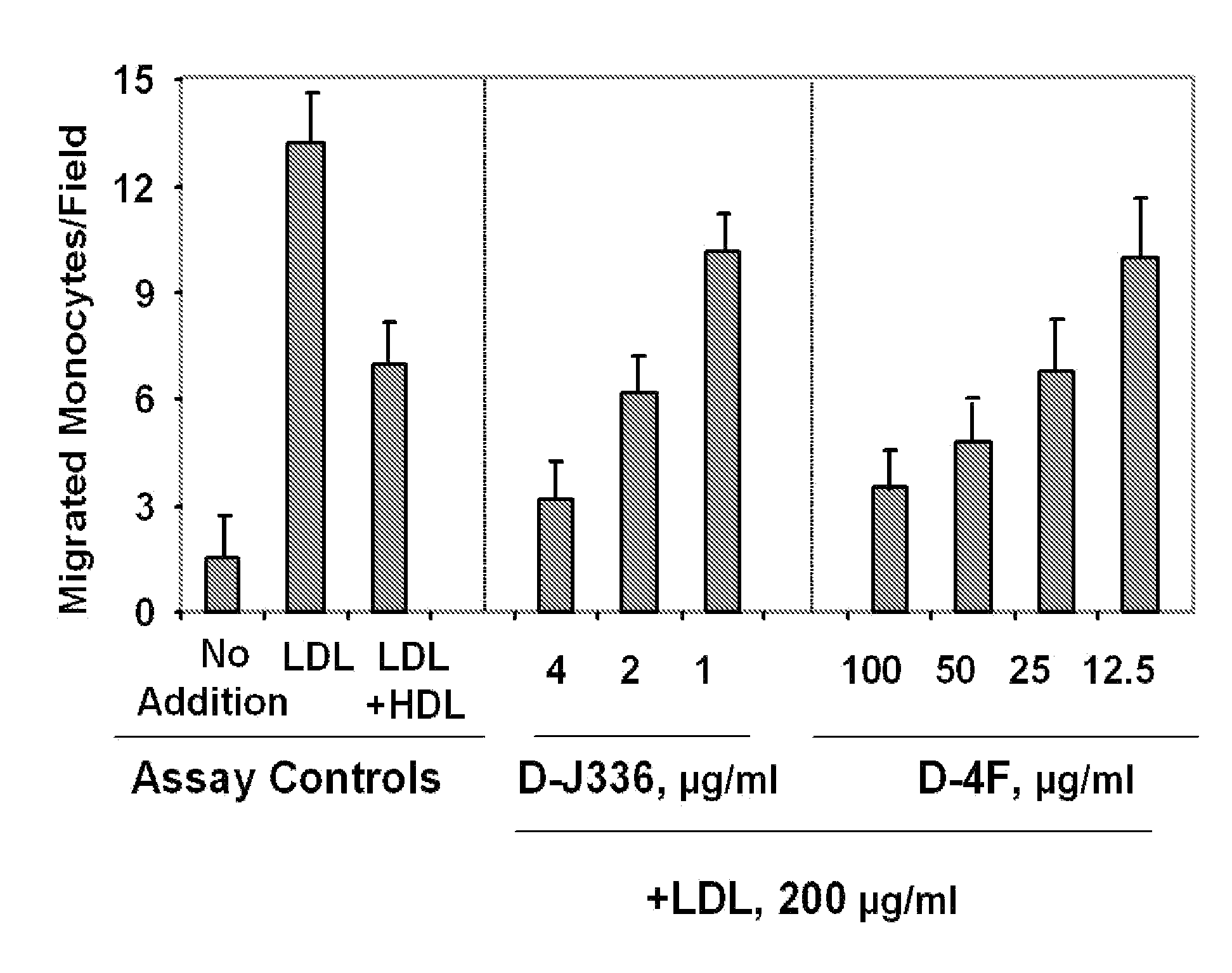
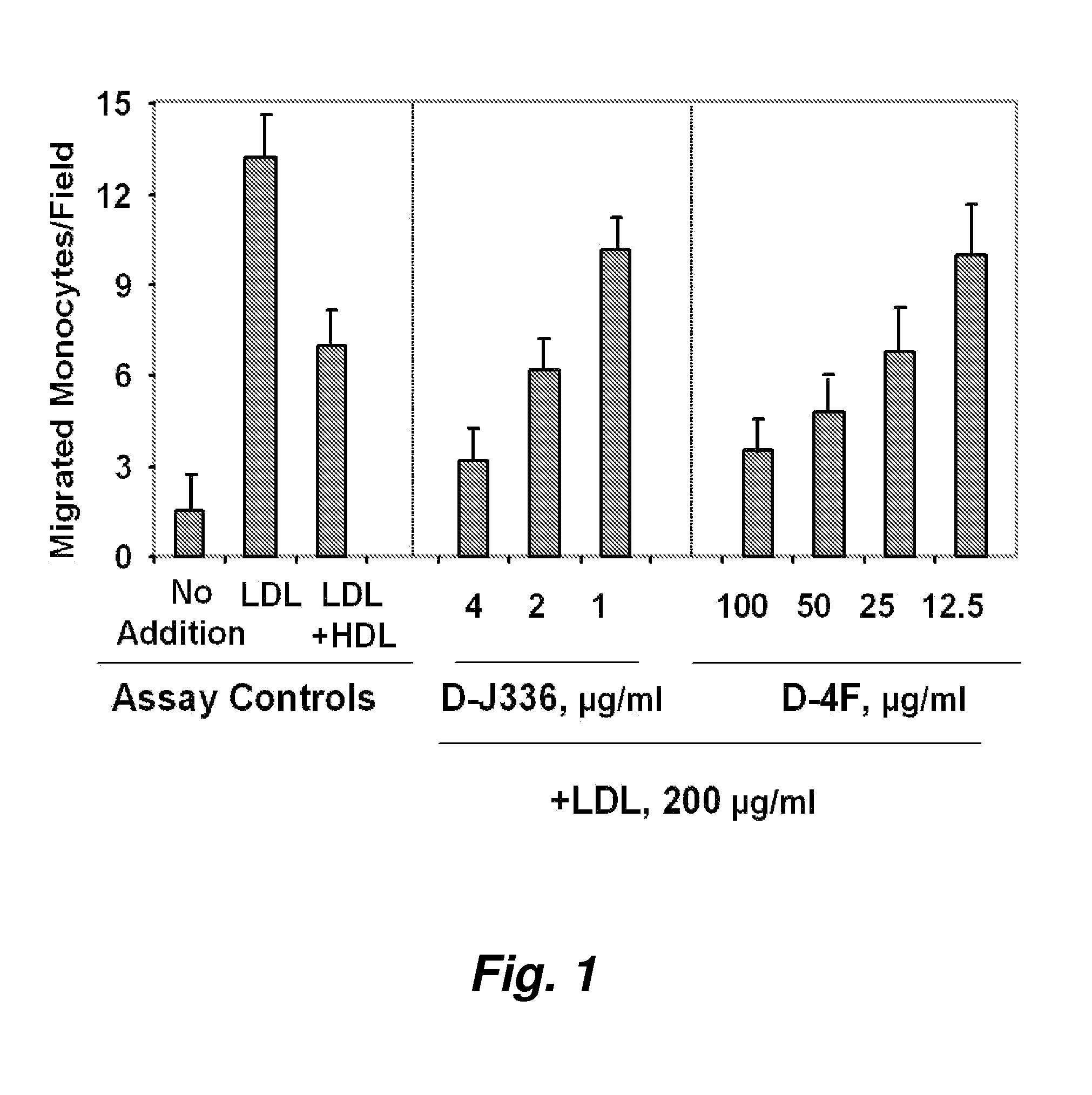
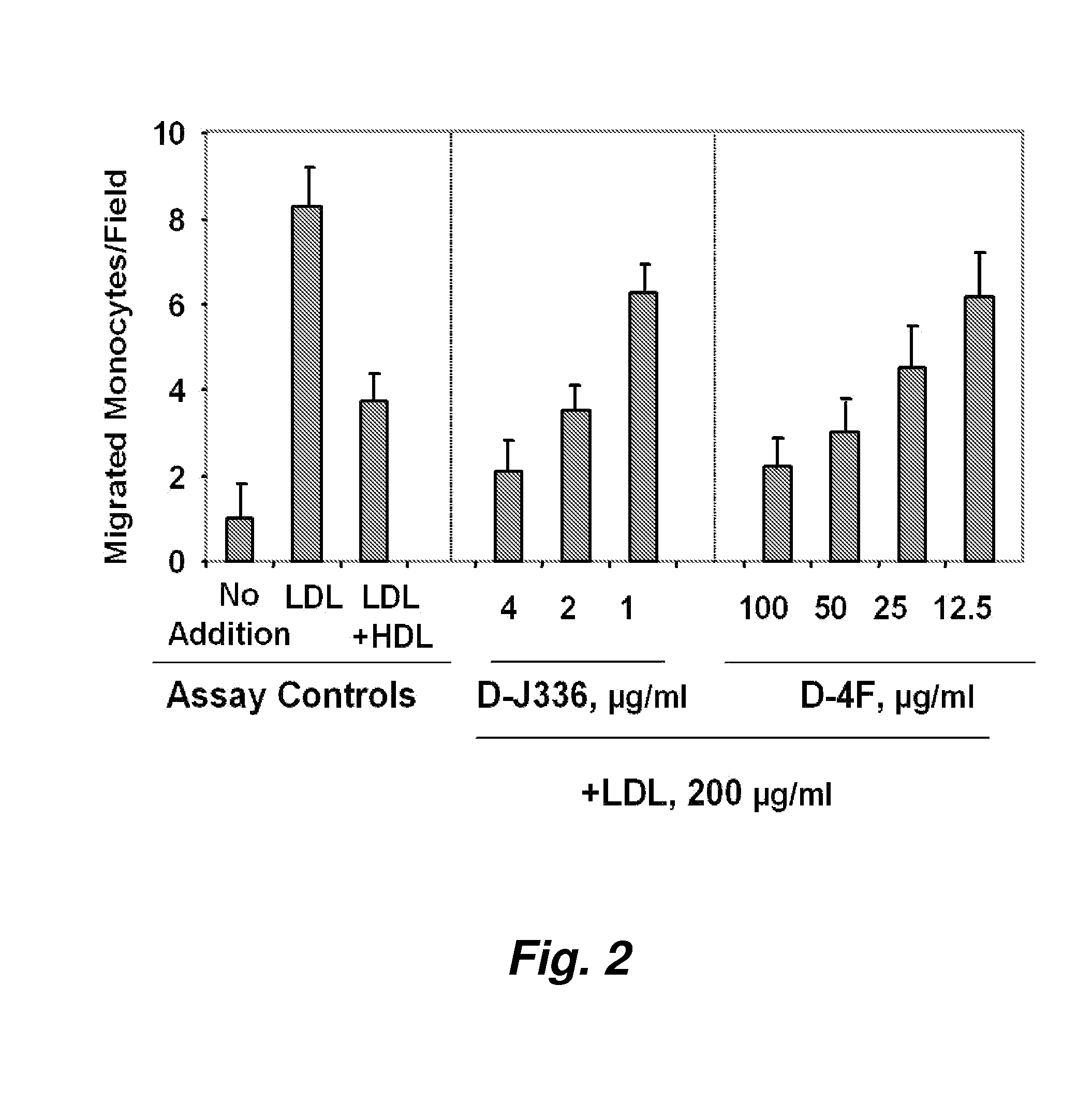
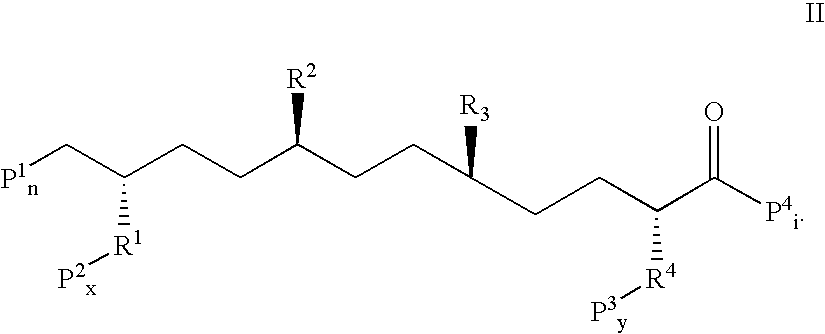
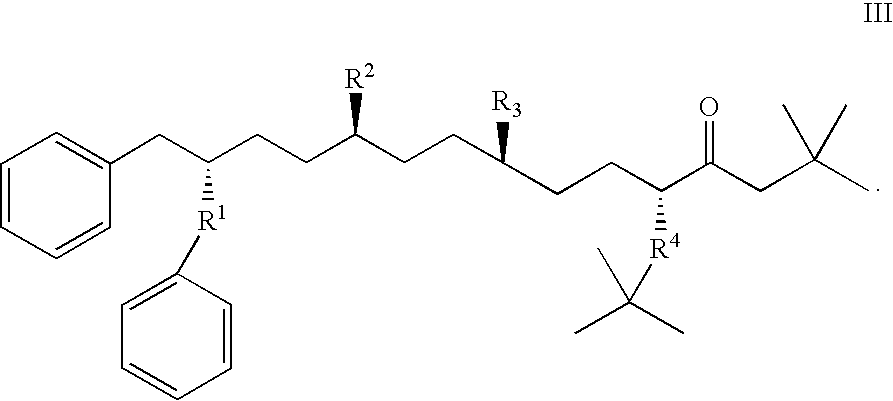
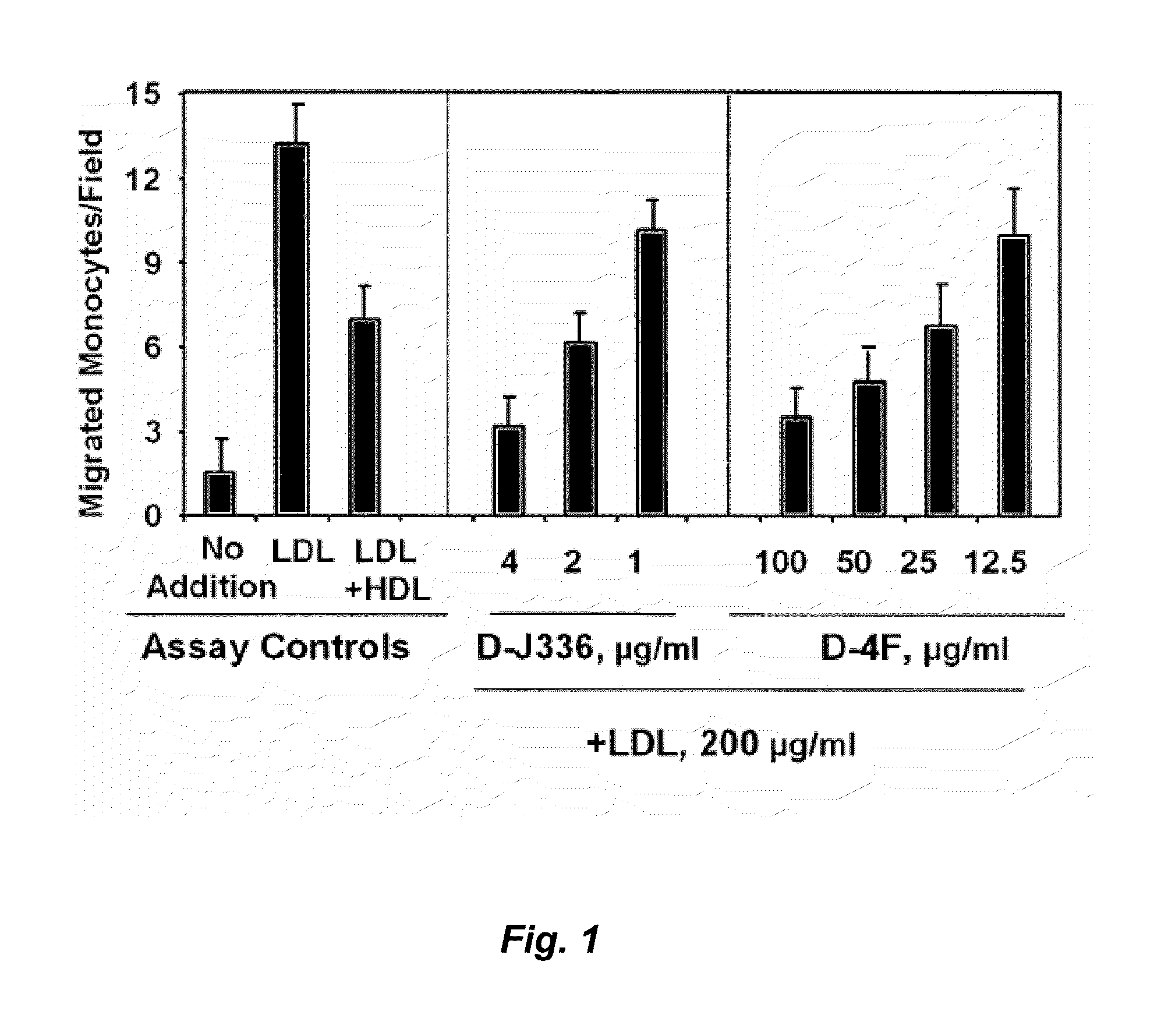
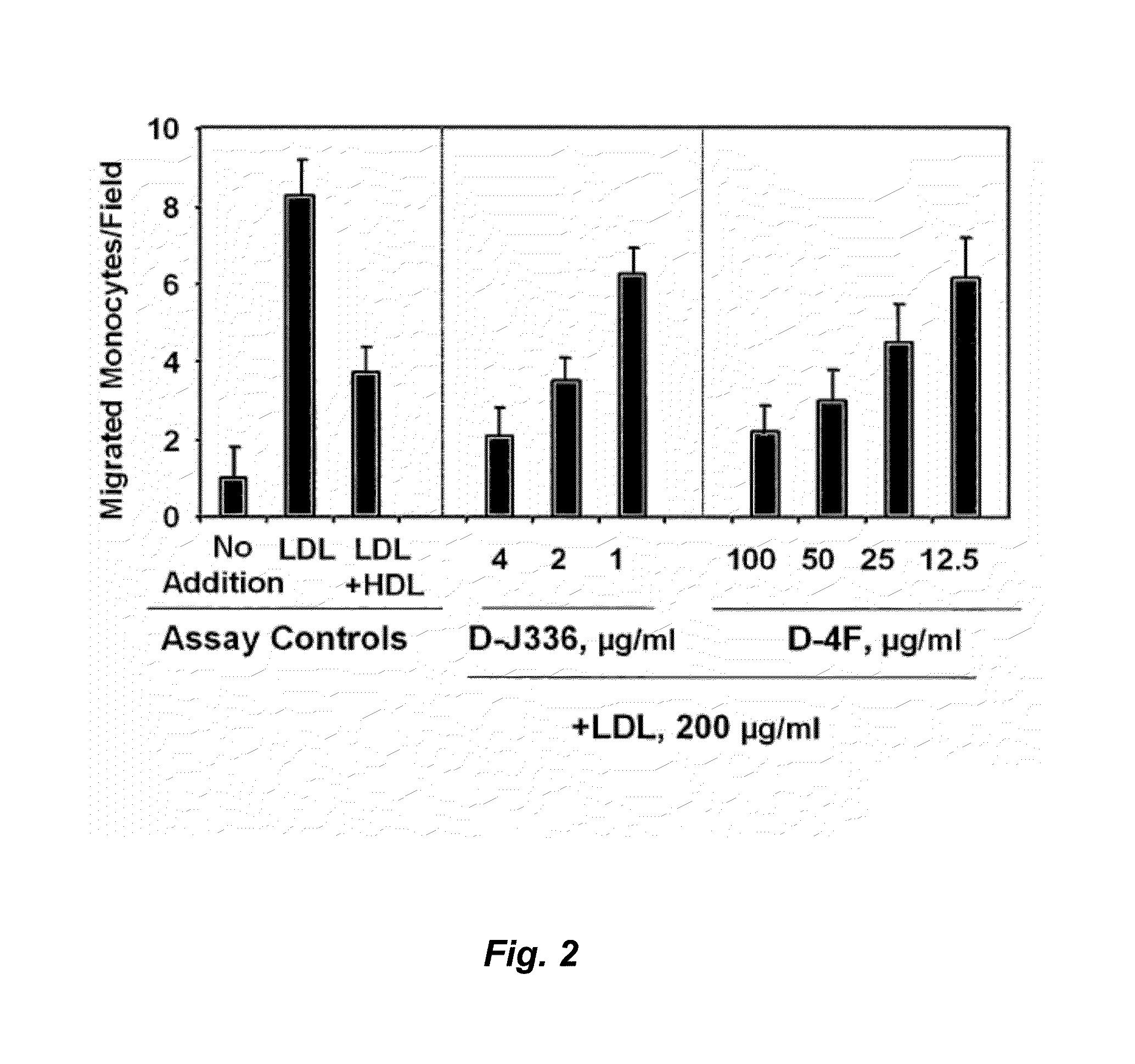
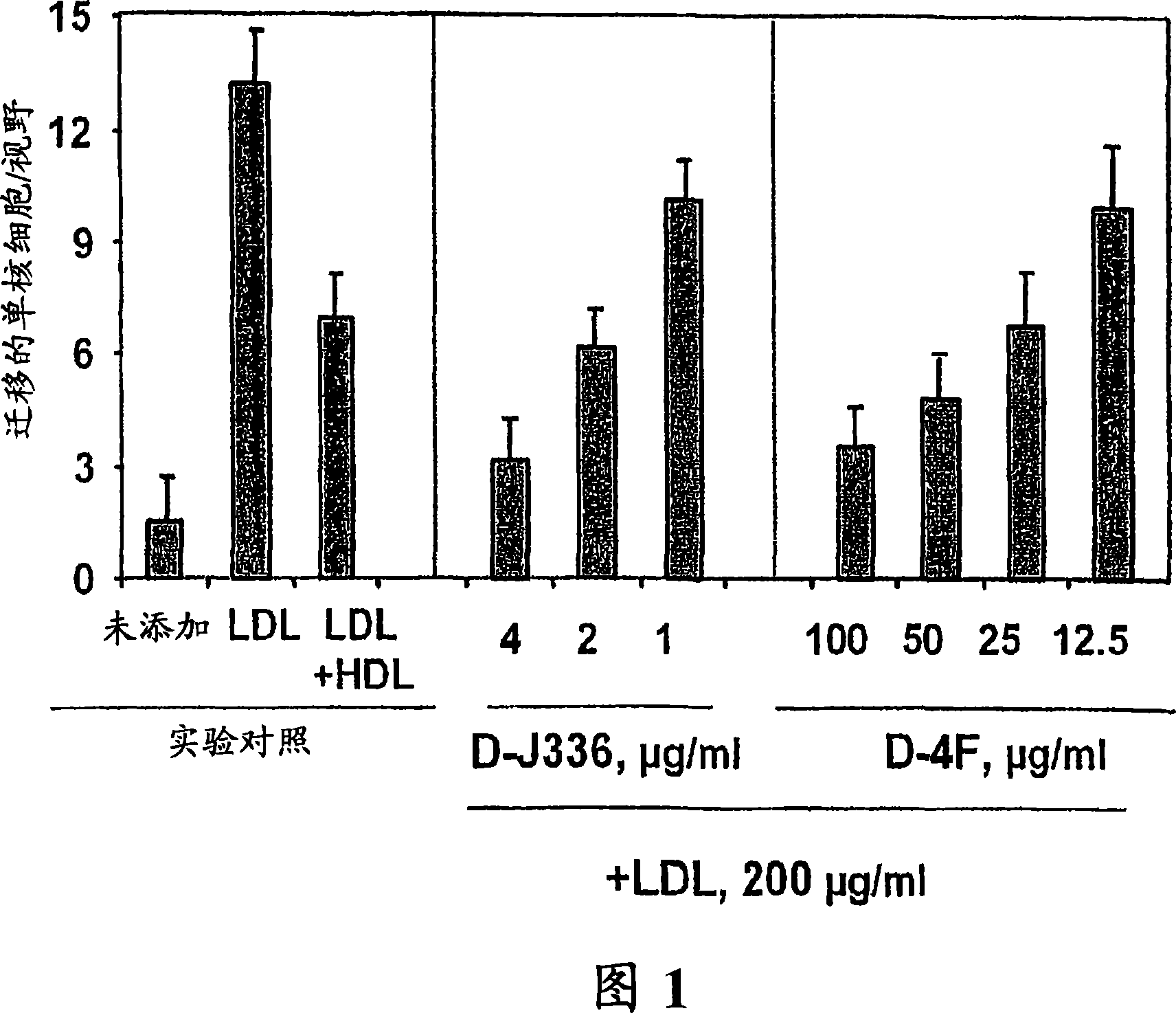
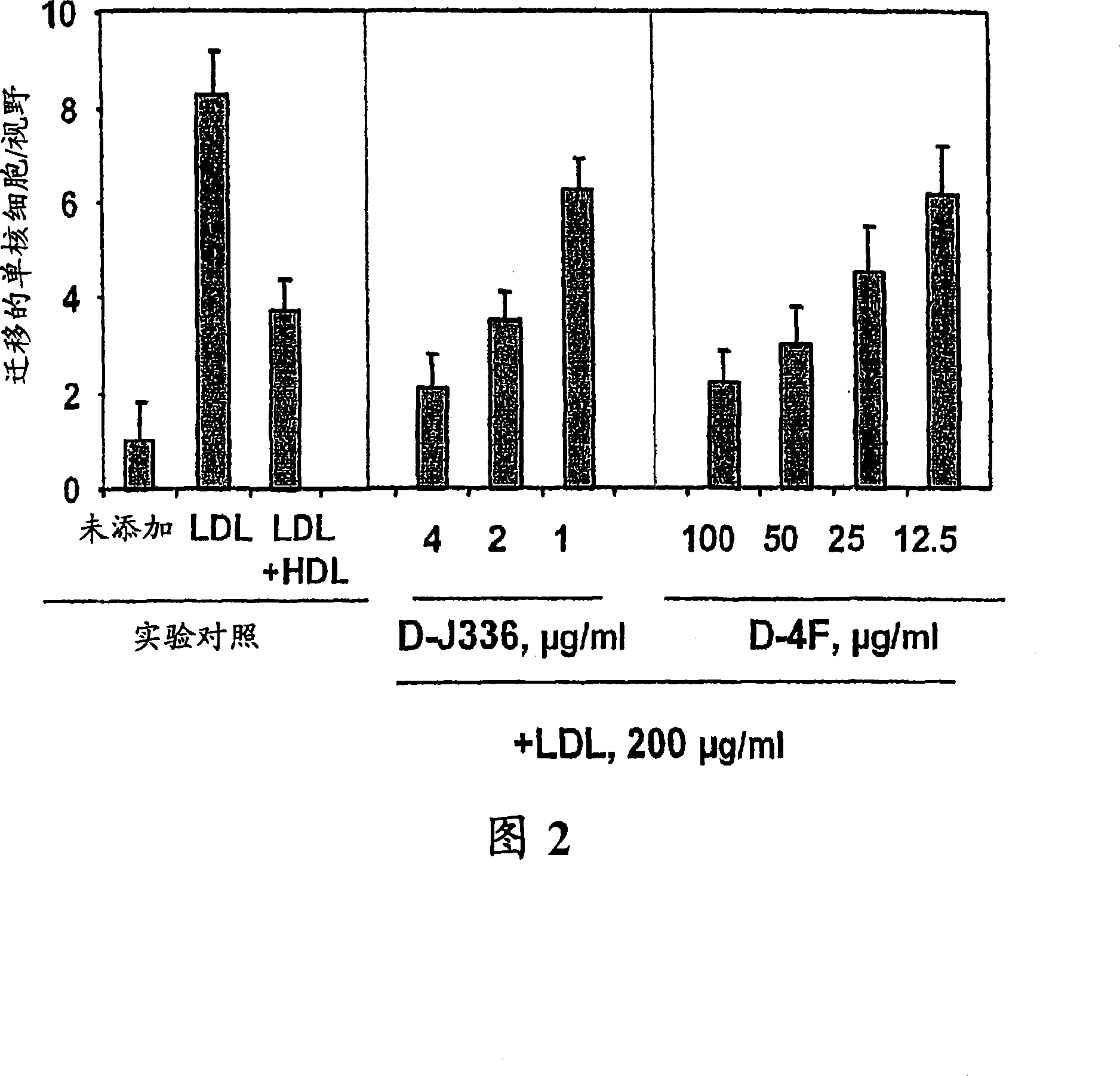
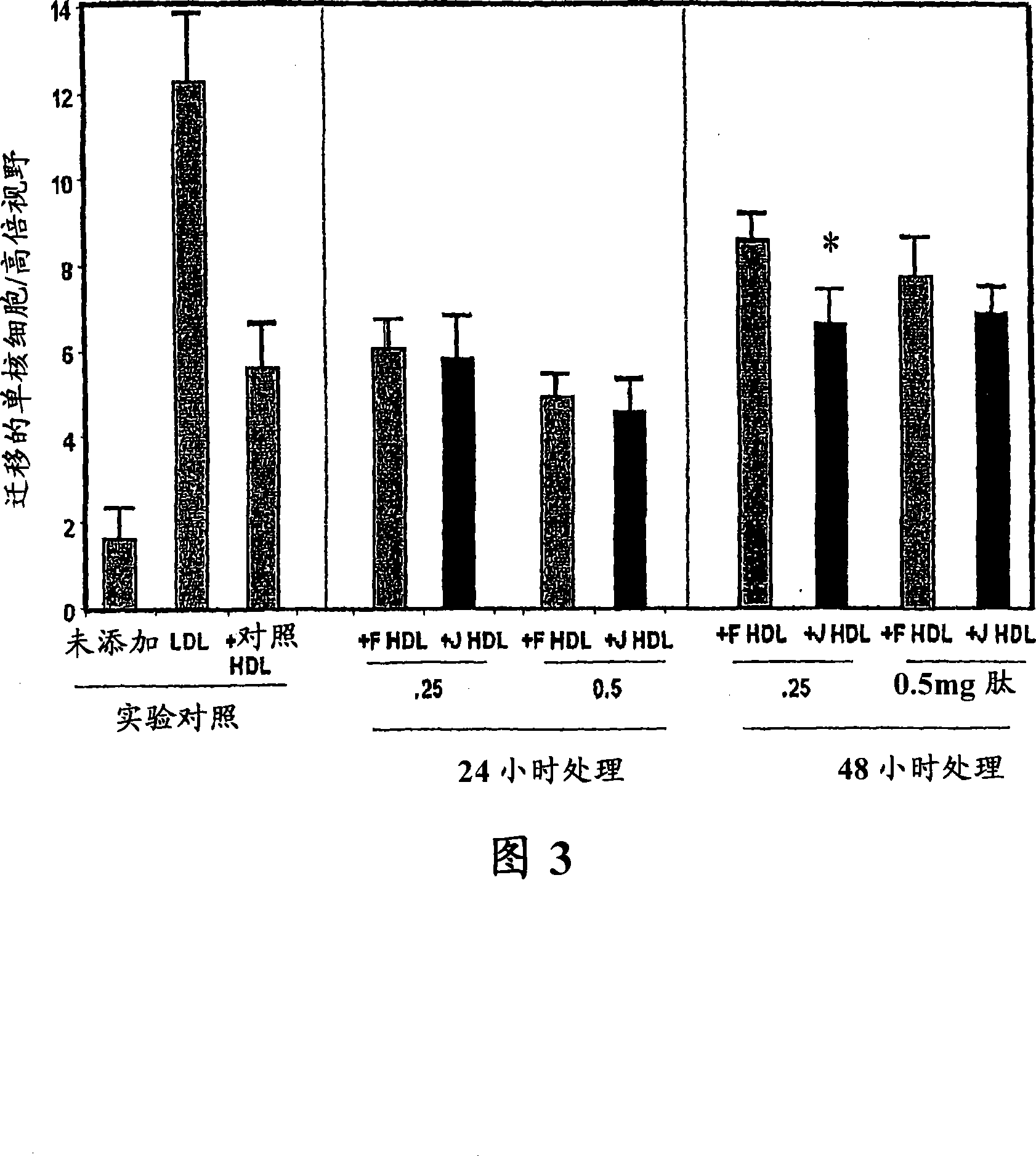
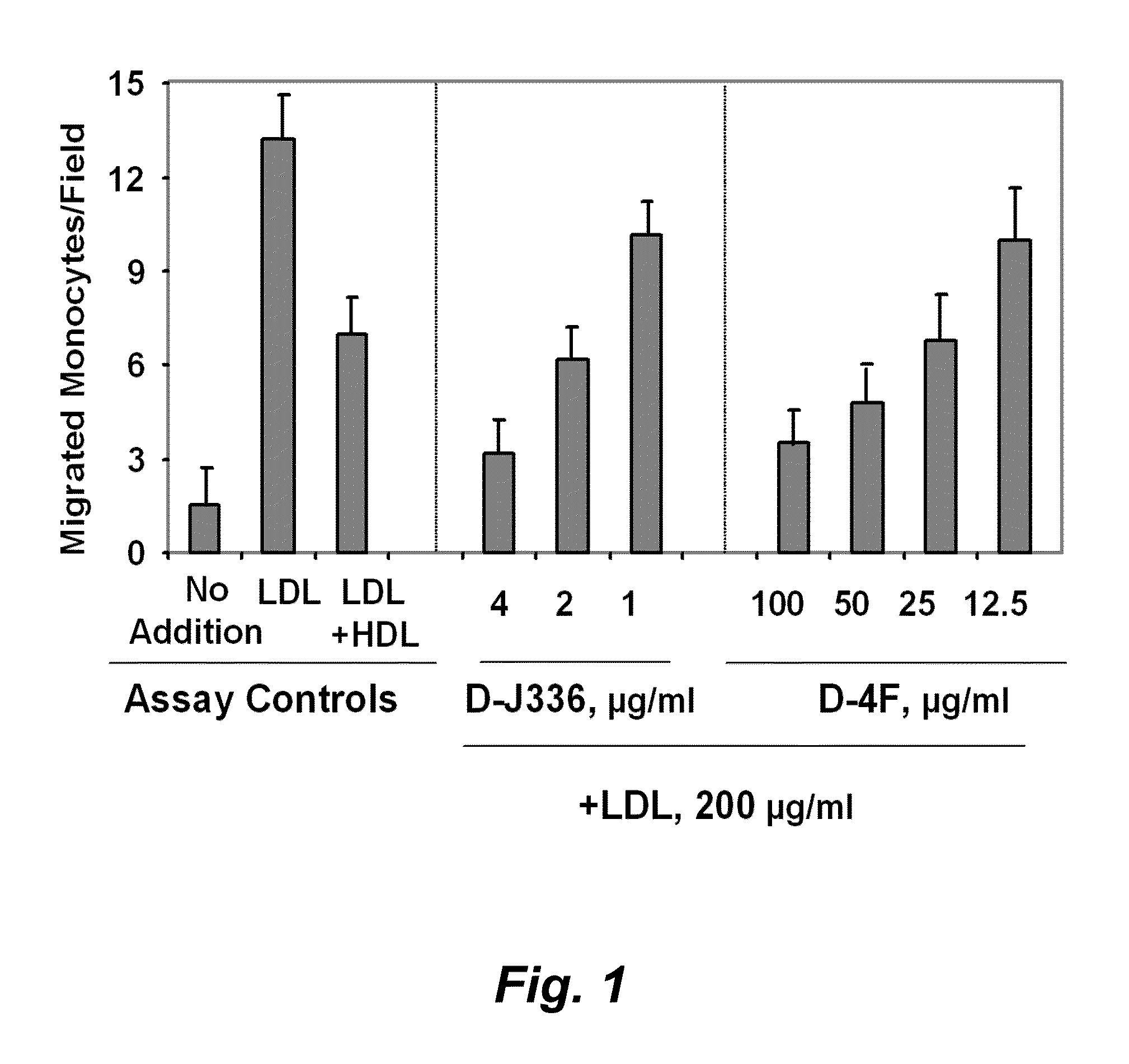
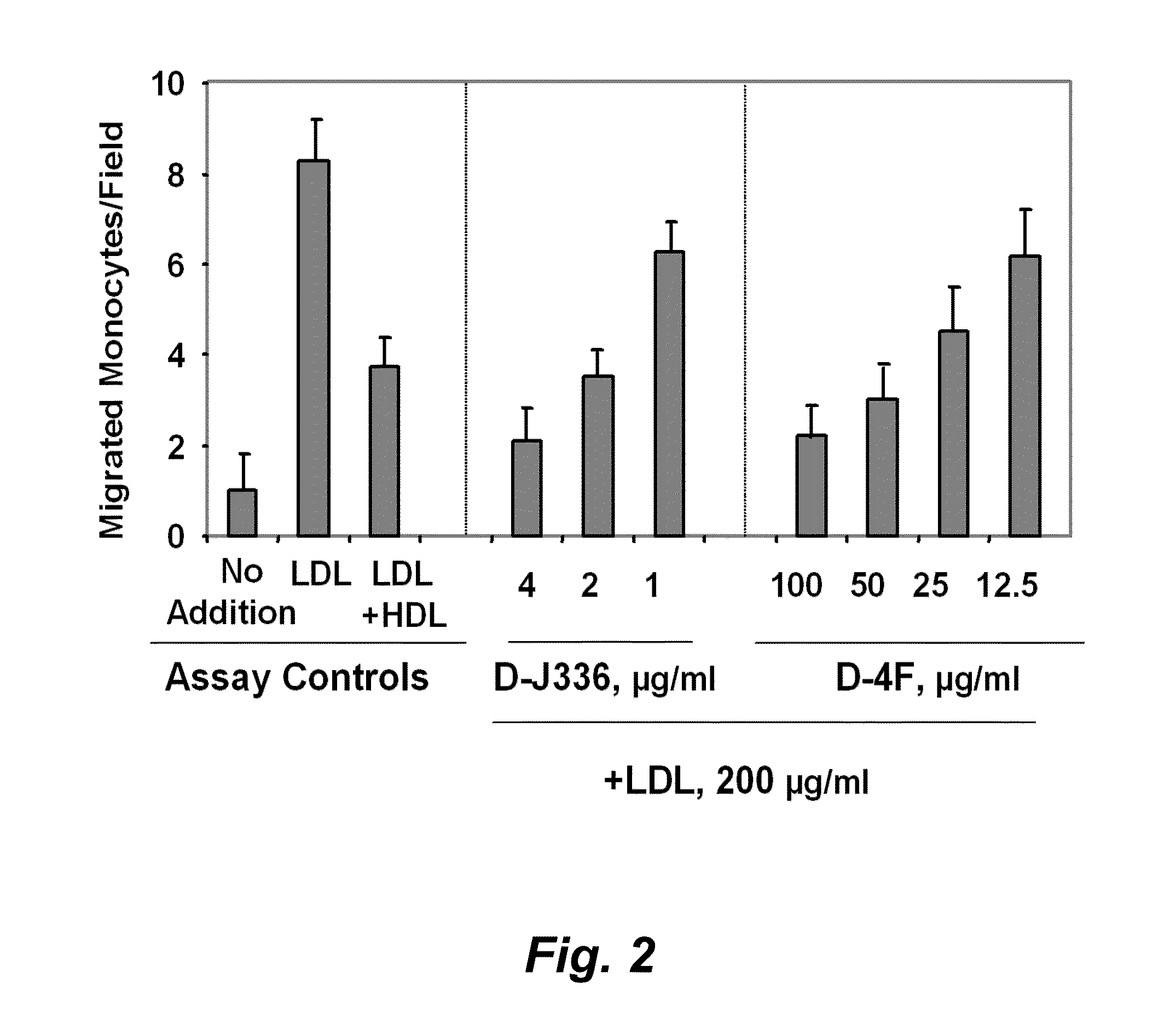
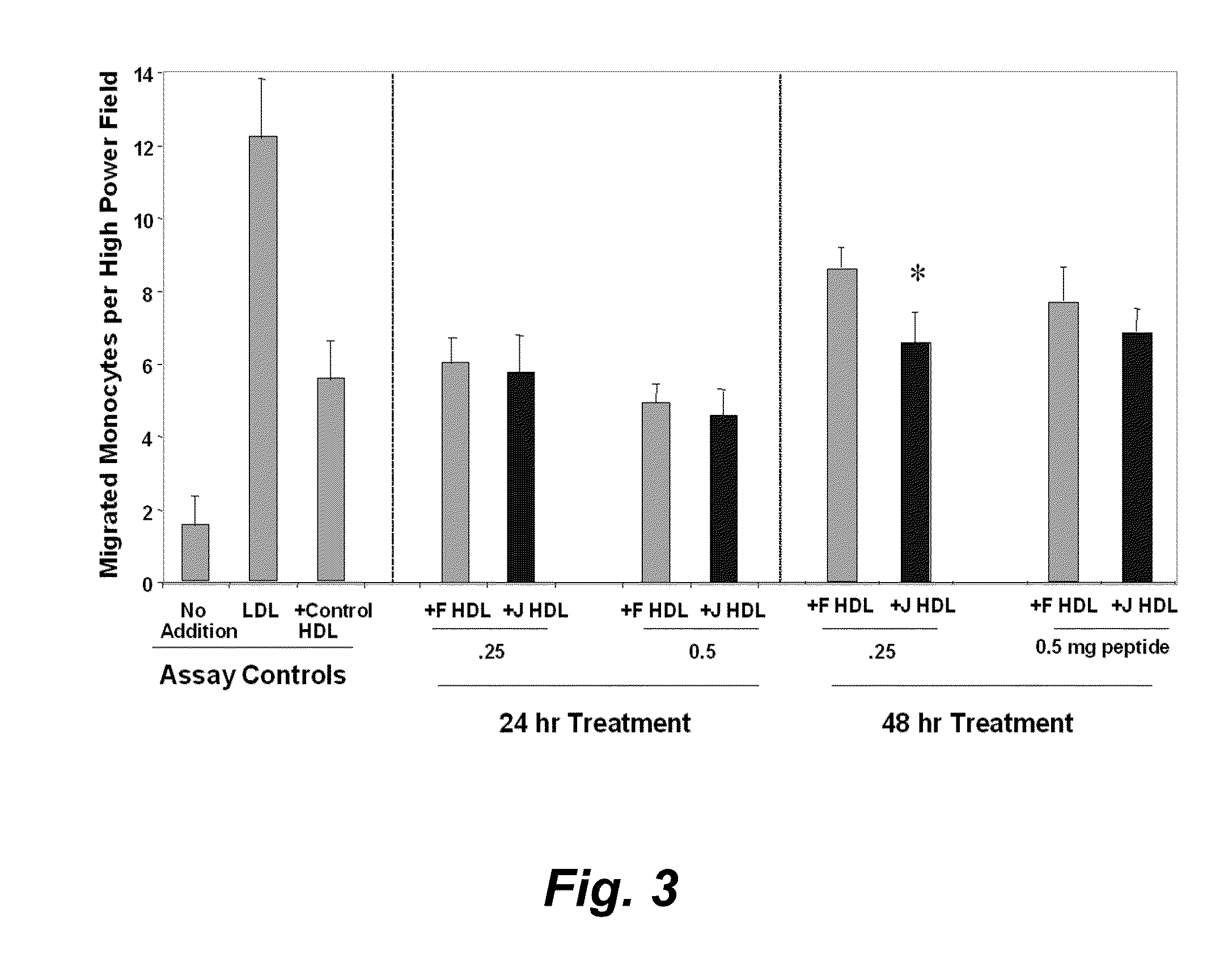
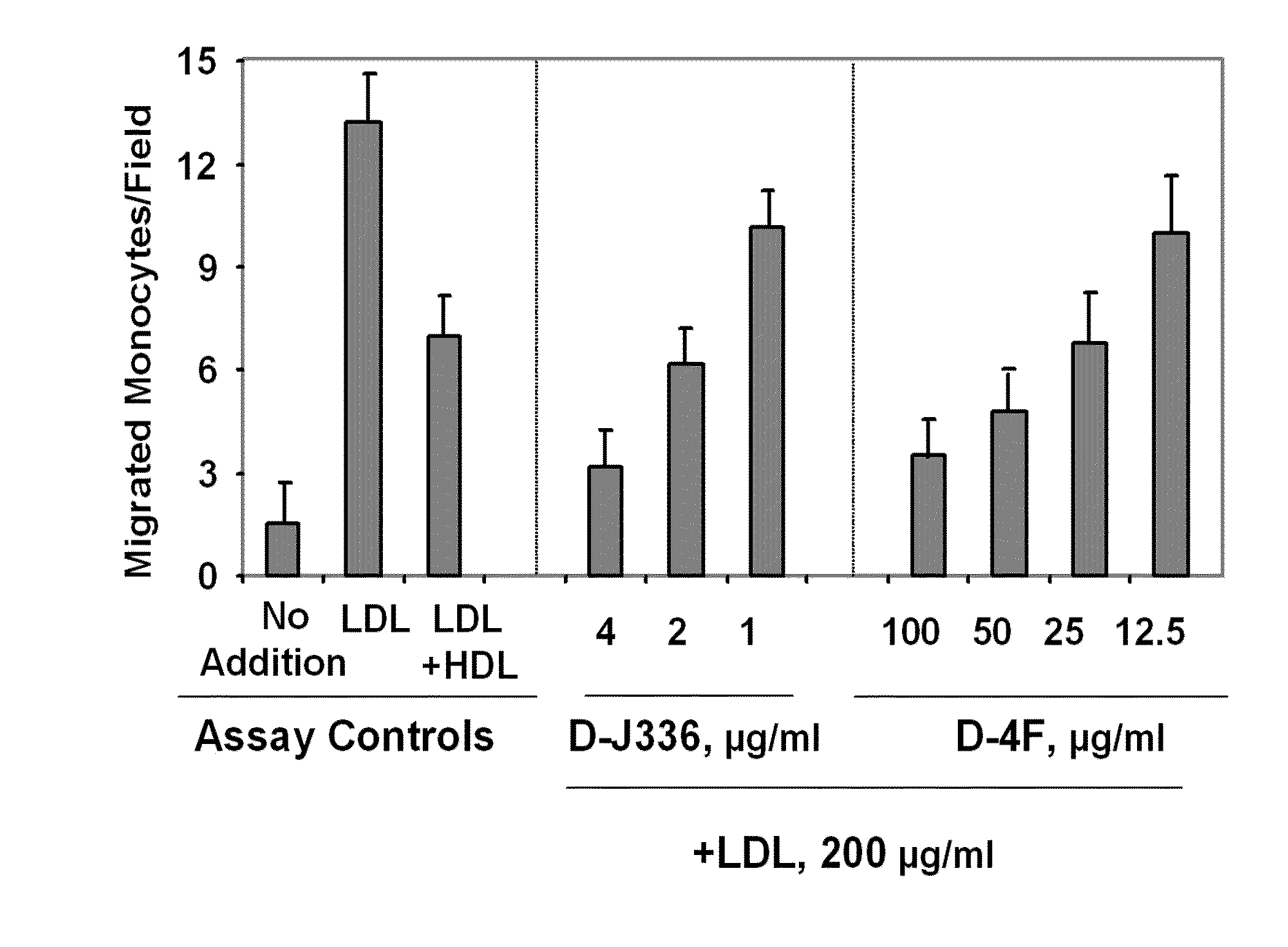
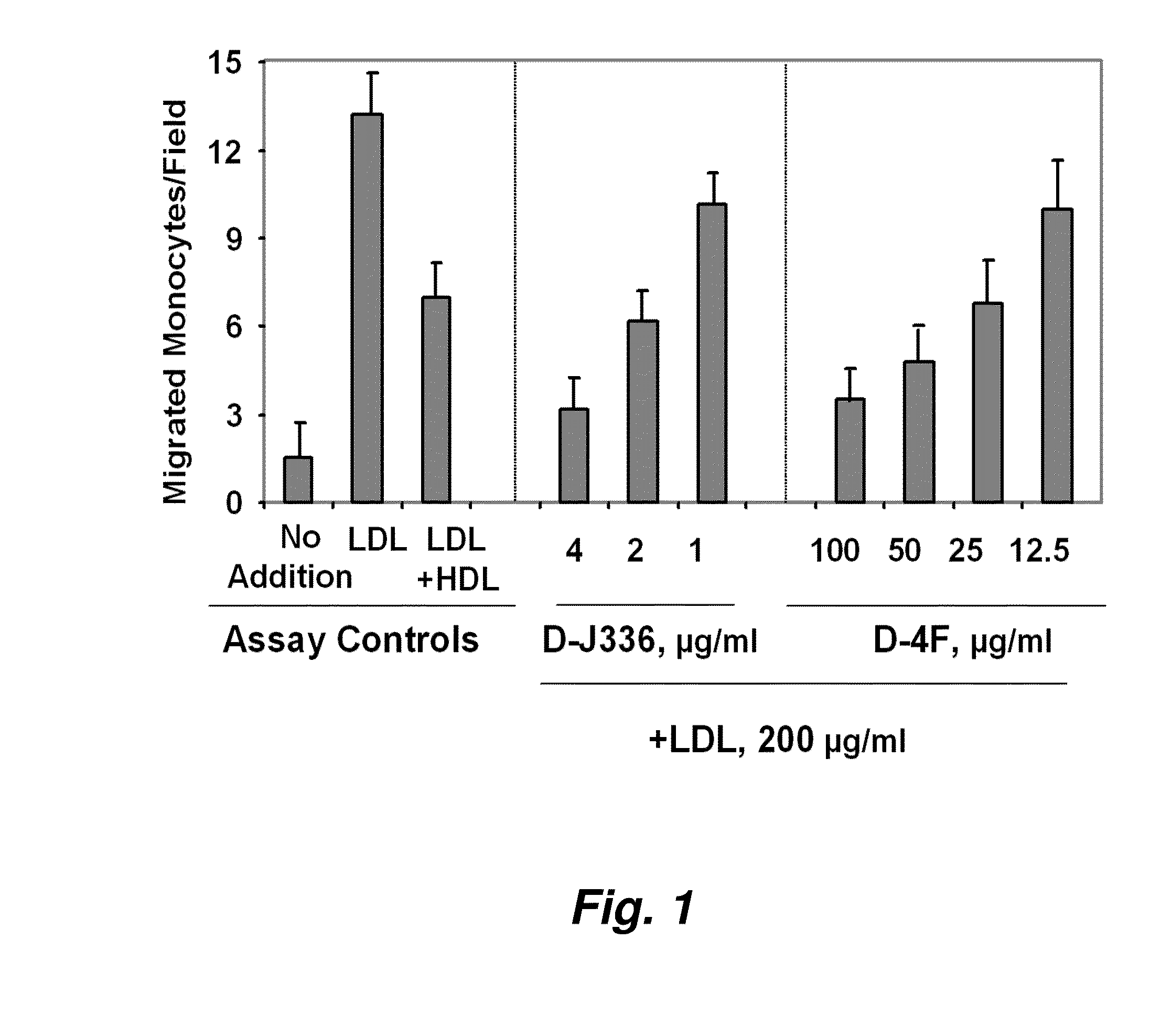
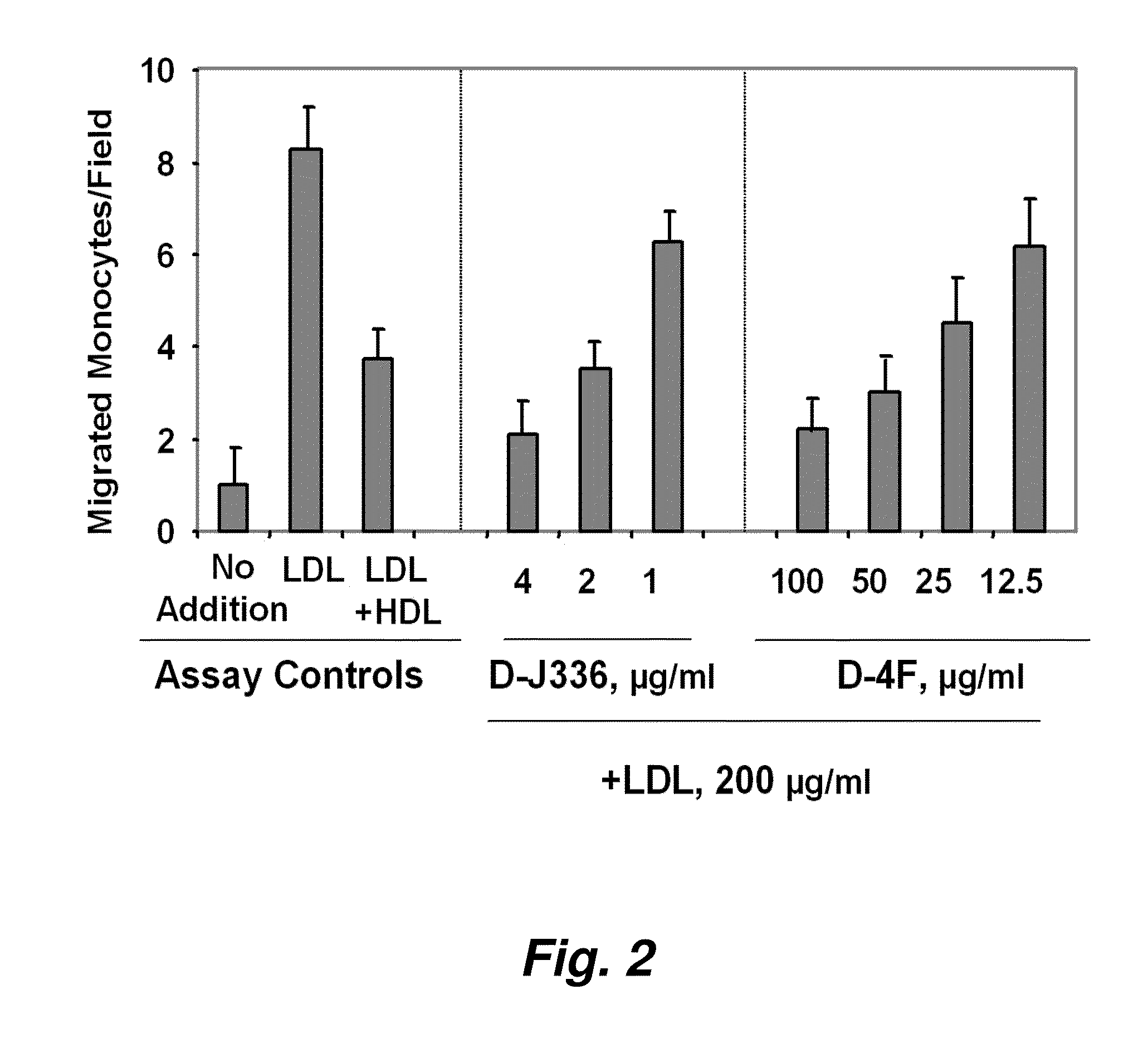
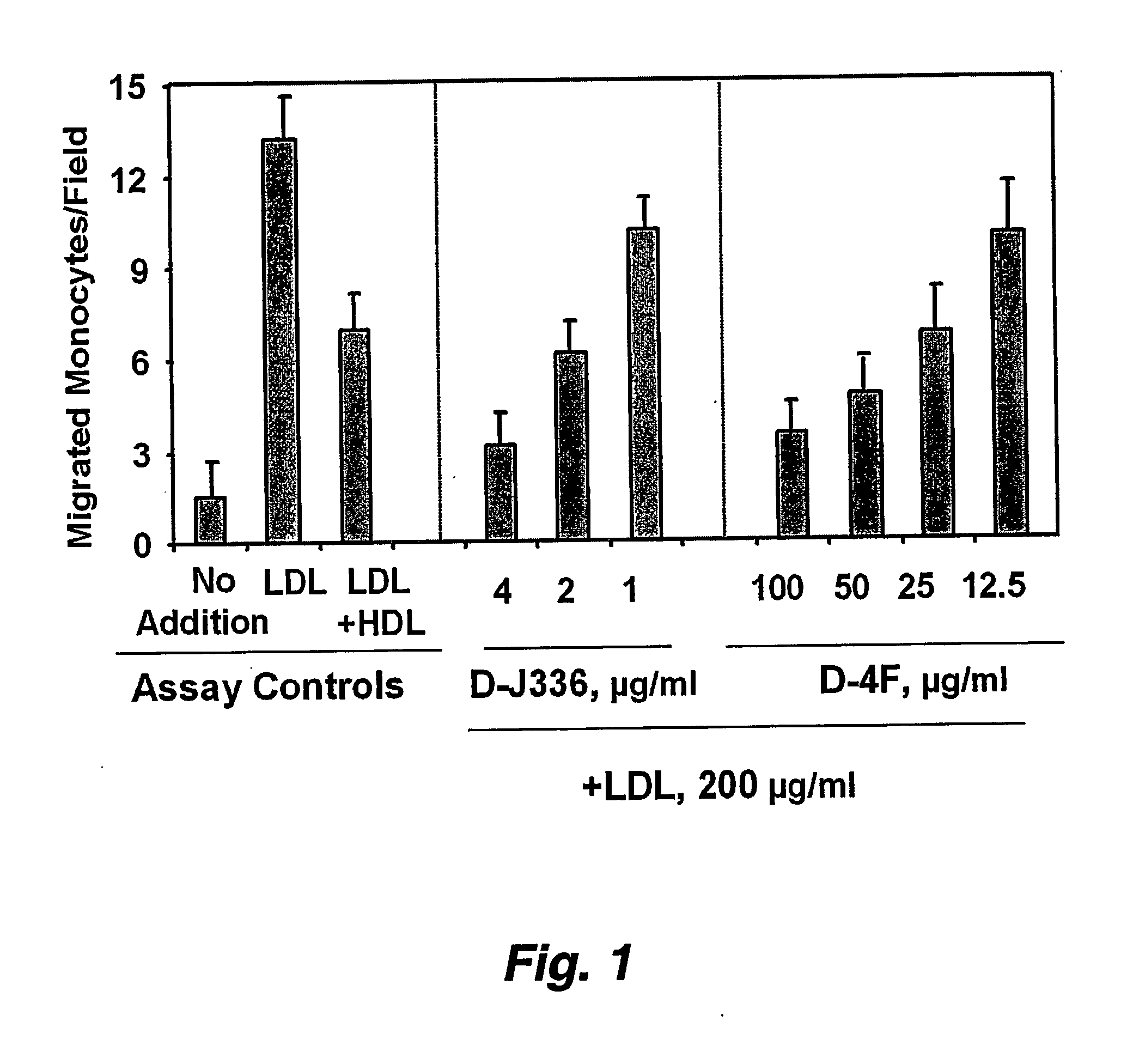
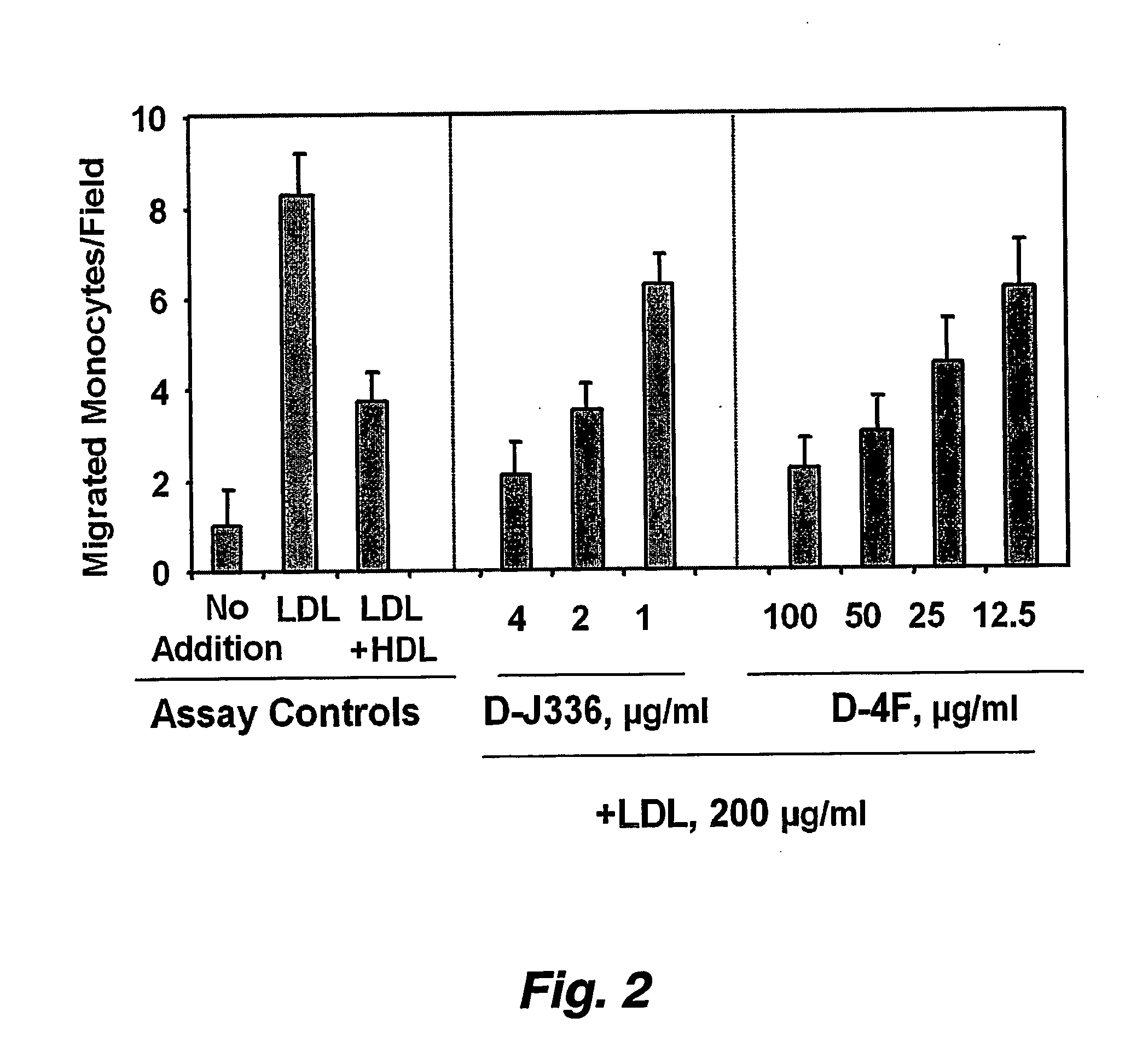
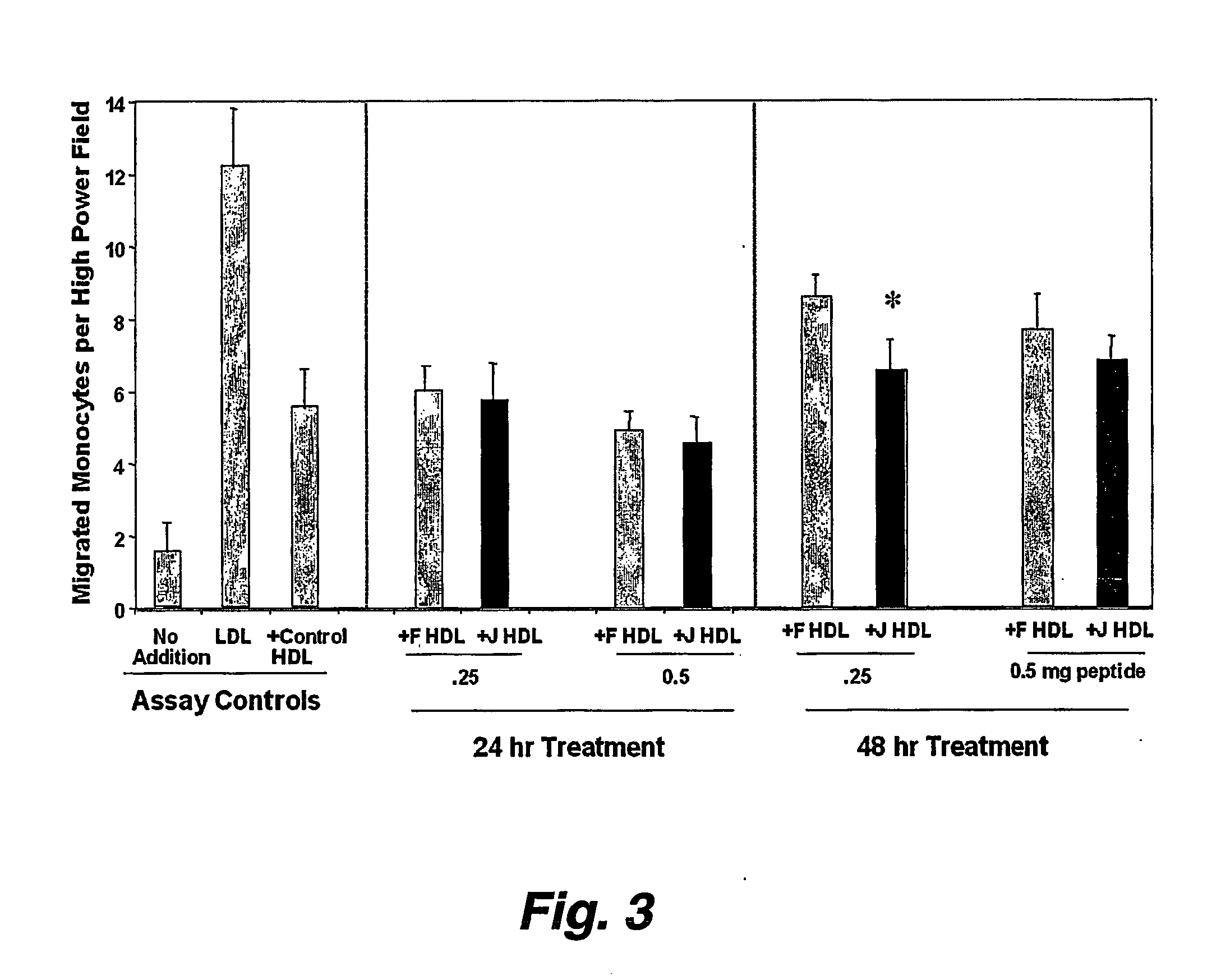
Orally administered peptides to ameliorate atherosclerosis
This invention provides novel peptides that ameliorate one or more symptoms of atherosclerosis. The peptides are highly stable and readily administered via an oral route.
Owner:UAB RES FOUND +1
Peptides and peptide mimetics to treat pathologies characterized by an inflammatory response
This invention provides novel active agents (e.g. peptides, small organic molecules, amino acid pairs, etc.) peptides that ameliorate one or more symptoms of atherosclerosis and / or other pathologies characterized by an inflammatory response. In certain embodiment, the peptides resemble a G* amphipathic helix of apolipoprotein J. The agents are highly stable and readily administered via an oral route.
Owner:RGT UNIV OF CALIFORNIA +1
Orally administered peptides to ameliorate atherosclerosis
InactiveUS7144862B2Readily taken up and deliveredMany symptomAntibacterial agentsBiocideAmphipathic helixMedicine
This invention provides novel peptides that ameliorate one or more symptoms of atherosclerosis. In certain embodiments, the peptide comprises an amino acid sequence that ranges in length from about 10 up to about 30 amino acids, that comprises at least one class A amphipathic helix, that bears at least one protecting group, that protects a phospholipid against oxidation by an oxidizing agent; and that is not the D-18A peptide. The peptides are highly stable and readily administered via an oral route.
Owner:UAB RES FOUND +1
pH-sensitive block copolymers for pharmaceutical compositions
The present invention relates to a pharmaceutical composition of a biologically active agent and a block copolymer composed of a poly(ethylene oxide) forming hydrophilic segment and a poly(butyl (alkyl)acrylate-co-(alkyl)acrylic acid) that is capable of forming supramolecular assemblies or micelles under favourable conditions. The supramolecular assemblies or micelles formed from said polymers associate or dissociate reversibly upon changes in the environmental pH. The pharmaceutical compositions of the present invention contain hydrophobic drugs, cations or polycationic compounds, which can be delivered to the body by oral route or other routes of administration.
Owner:PALADIN LABS INC
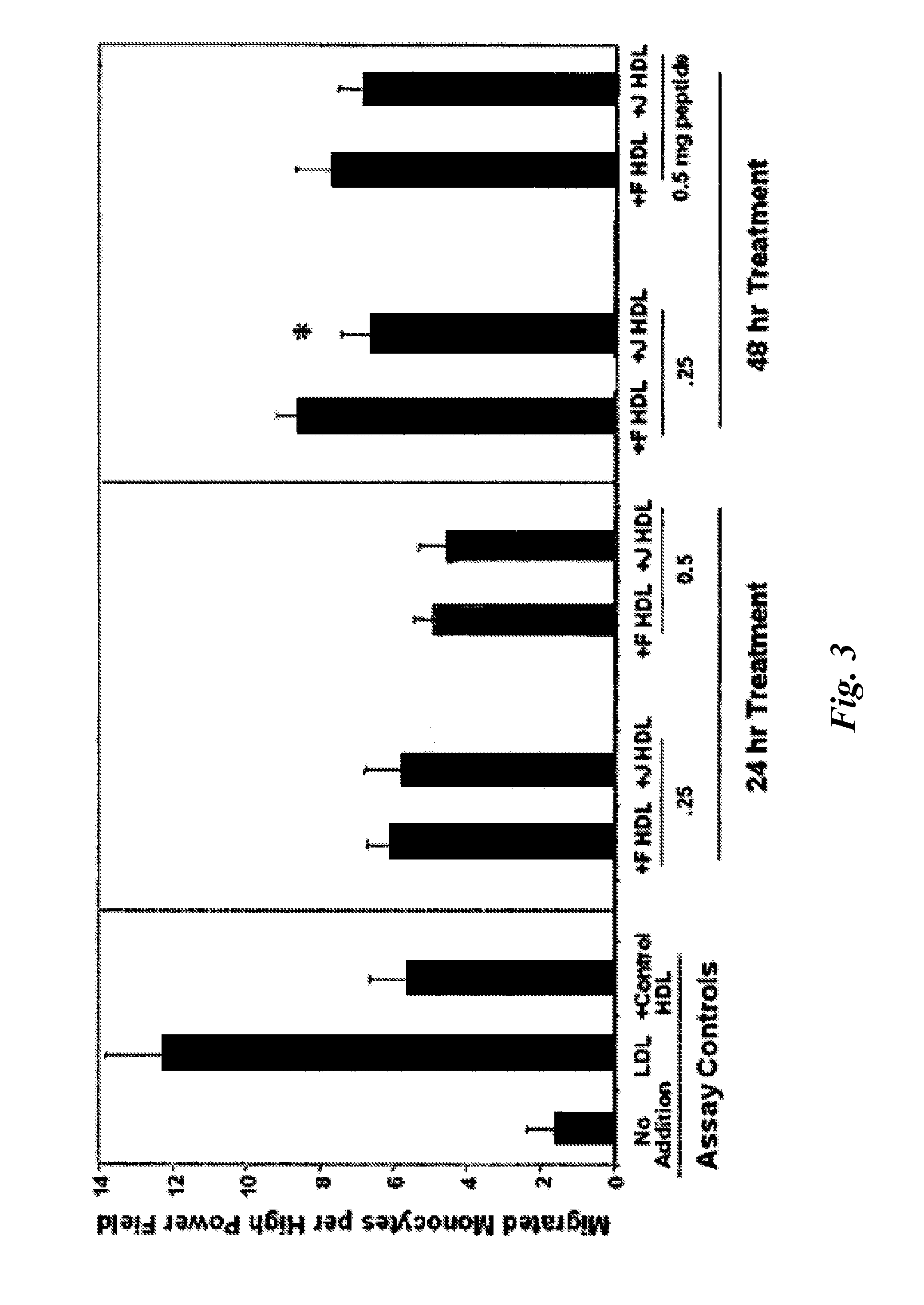
Orally administered peptides synergize statin activity
InactiveUS7166578B2Many symptomReadily takenNervous disorderApolipeptidesAmphipathic helixPhospholipid
This invention provides novel peptides that ameliorate one or more symptoms of atherosclerosis. The peptides are highly stable and readily administered via an oral route. In addition, the peptides inhibit osteoporosis. When administered with a statin, the peptides enhance the activity of the statin permitting the statin to be used at significantly lower dosages. In certain embodiments, the peptides range in length from about 10 up to about 30 amino acids, comprise at least one class A amphipathic helix, and protect a phospholipid against oxidation by an oxidizing agent.
Owner:ALABAMA RESEARCG FOUND UNIV OF THE +1
Orally administered peptides synergize statin activity
InactiveUS7199102B2Many symptomEasy to useNervous disorderPeptide/protein ingredientsAmphipathic helixLipid Transport
This invention provides novel peptides that ameliorate one or more symptoms of atherosclerosis. The peptides typically range in length up to about 30 amino acids, comprise at least one class A amphipathic helix, and protect a phospholipid against oxidation by an oxidizing agent. The peptides are highly stable and readily administered via an oral route. The peptides are effective to stimulate the formation and cycling of pre-beta high density lipoprotein-like particles and / or to promote lipid transport and detoxification. In addition, the peptides inhibit osteoporosis. When administered with a statin, the peptides enhance the activity of the statin permitting the statin to be used at significantly lower dosages and / or cause the statins to be significantly more anti-inflammatory at any given dose.
Owner:UAB RES FOUND +1
Orally administered small peptides synergize statin activity
InactiveUS20050164950A1Many symptomReadily takenNervous disorderDipeptide ingredientsLipid TransportSmall peptide
This invention provides novel peptides that ameliorate one or more symptoms of atherosclerosis. The peptides are highly stable and readily administered via an oral route. The peptides are effective to stimulate the formation and cycling of pre-beta high density lipoprotein-like particles and / or to promote lipid transport and detoxification. This invention also provides a method of tracking a peptide in a mammal. In addition, the peptides inhibit osteoporosis. When administered with a statin, the peptides enhance the activity of the statin permitting the statin to be used at significantly lower dosages and / or cause the statins to be significantly more anti-inflammatory at any given dose.
Owner:UNIV OF ALABAMA BIRMINGHAM RES FOUND +1
G-type peptides to ameliorate atherosclerosis
InactiveUS6930085B2Simplification of progressiveAlignment score can be increasedAntibacterial agentsAntimycoticsAmphipathic helixPeptide
This invention provides novel peptides that ameliorate one or more symptoms of atherosclerosis and / or other pathologies characterized by an inflammatory response. In certain embodiment, the peptides resemble a G* amphipathic helix of apolipoprotein J. The peptides are highly stable and readily administered via an oral route.
Owner:RGT UNIV OF CALIFORNIA
Peptides and peptide mimetics to treat pathologies characterized by an inflammatory response
Owner:UAB RES FOUND +1
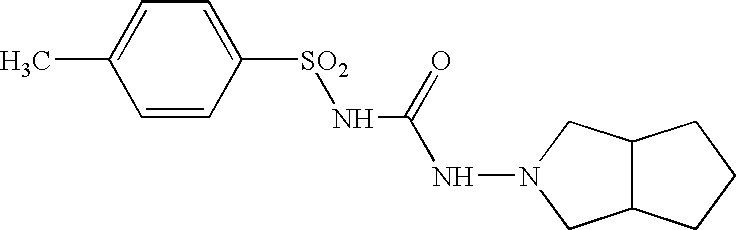
Core tablet for controlled release of gliclazide after oral administration
InactiveUS6733782B1Facilitated releaseIncrease concentrationPowder deliveryMetabolism disorderControlled releaseOral medication
The invention relates to a matrix tablet for the prolonged release of gliclazide which ensures continuous and consistent release of the active ingredient after administration by the oral route, the release being insensitive to variations in the pH of the dissolution medium.
Owner:LES LAB SERVIER
Use of methylnaltrexone and related compounds for treatment of gastrointestinal dysfunction induced by endogenous opioids
Owner:FOSS JOSEPH F +4
Peptides and peptide mimetics to treat pathologies characterized by an inflammatory response
InactiveUS20080293639A1Increase insulin sensitivityIncrease in adiponectinNervous disorderPeptide/protein ingredientsAmphipathic helixActive agent
This invention provides novel active agents (e.g. peptides, small organic molecules, amino acid pairs, etc.) peptides that ameliorate one or more symptoms of atherosclerosis and / or other pathologies characterized by an inflammatory response. In certain embodiment, the peptides resemble a G* amphipathic helix of apolipoprotein J. The agents are highly stable and readily administered via an oral route.
Owner:RGT UNIV OF CALIFORNIA
G-type peptides and other agents to ameliorate atherosclerosis and other pathologies
InactiveUS20060205669A1Simplification of progressiveAlignment score can be increasedPeptide/protein ingredientsAntipyreticAmphipathic helixIntensive care medicine
This invention provides novel peptides, and other agents, that ameliorate one or more symptoms of atherosclerosis and / or other pathologies characterized by an inflammatory response. In certain embodiment, the peptides resemble a G* amphipathic helix of apolipoprotein J. The peptides are highly stable and readily administered via an oral route.
Owner:ALABAMA RES FOUND UNIV OF THE +1
Orally administered peptides synergize statin activity
InactiveUS20070254839A1Readily taken up and deliveredMany symptomAntibacterial agentsNervous disorderLipid TransportMedicine
Owner:RGT UNIV OF CALIFORNIA +1
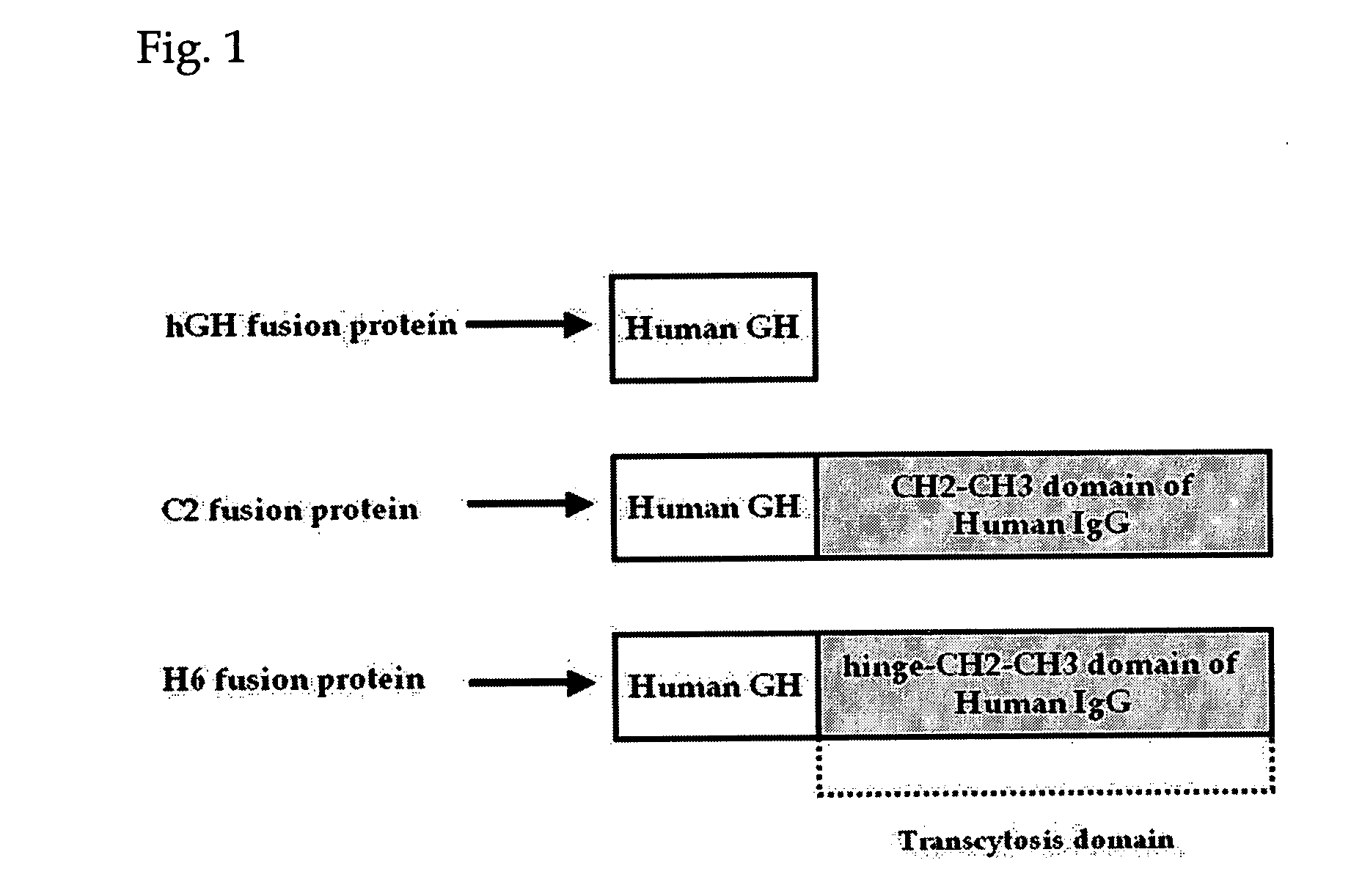
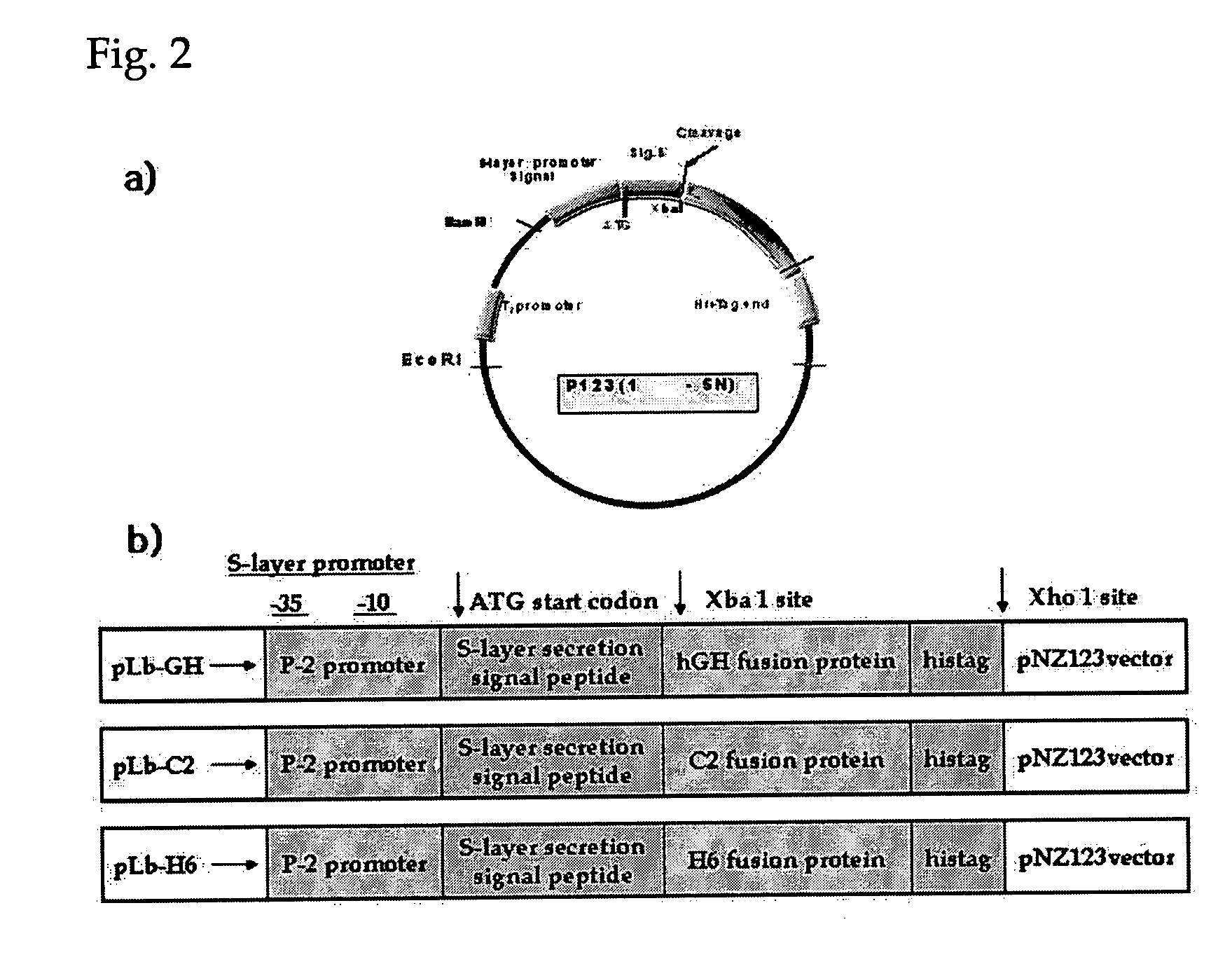
Probiotic microorganisms producing chimeric human growth hormone fused with Fc fragment of human IgG for oral delivery system and methods for producing them
The present invention relates to probiotic microorganisms producing chimeric human growth hormone for oral use and methods for preparing them. The invention provides probiotic Lactobacillus or yeast transformant expressing chimeric protein which is human growth hormone fused with Fc fragment of human IgG, in which the transformants are safely delivered into intestine though oral route. Also, the invention provides a chimeric protein-expressing vector which can induce transcytosis in intestine epithelial cells. Accordingly, the invention demonstrates that the chimeric protein for oral delivery system can be absorbed in intestine, and delivery of the chimeric protein by oral route using Lactobacillus has very excellent efficiency in vivo test in rats. Accordingly, the Lactobacillus of the present invention is an excellent deliverer of protein drugs.
Owner:INSILICO CO LTD
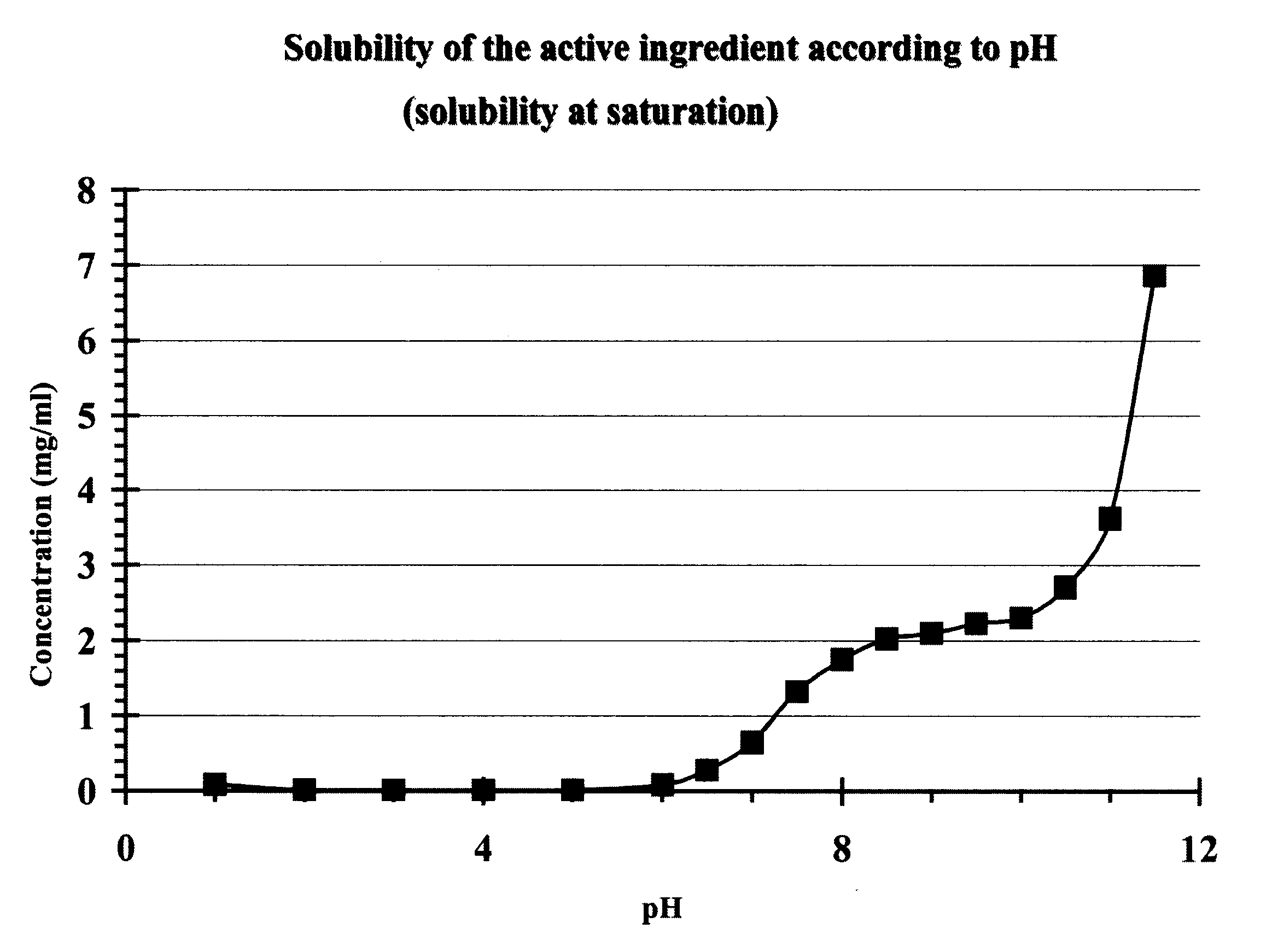
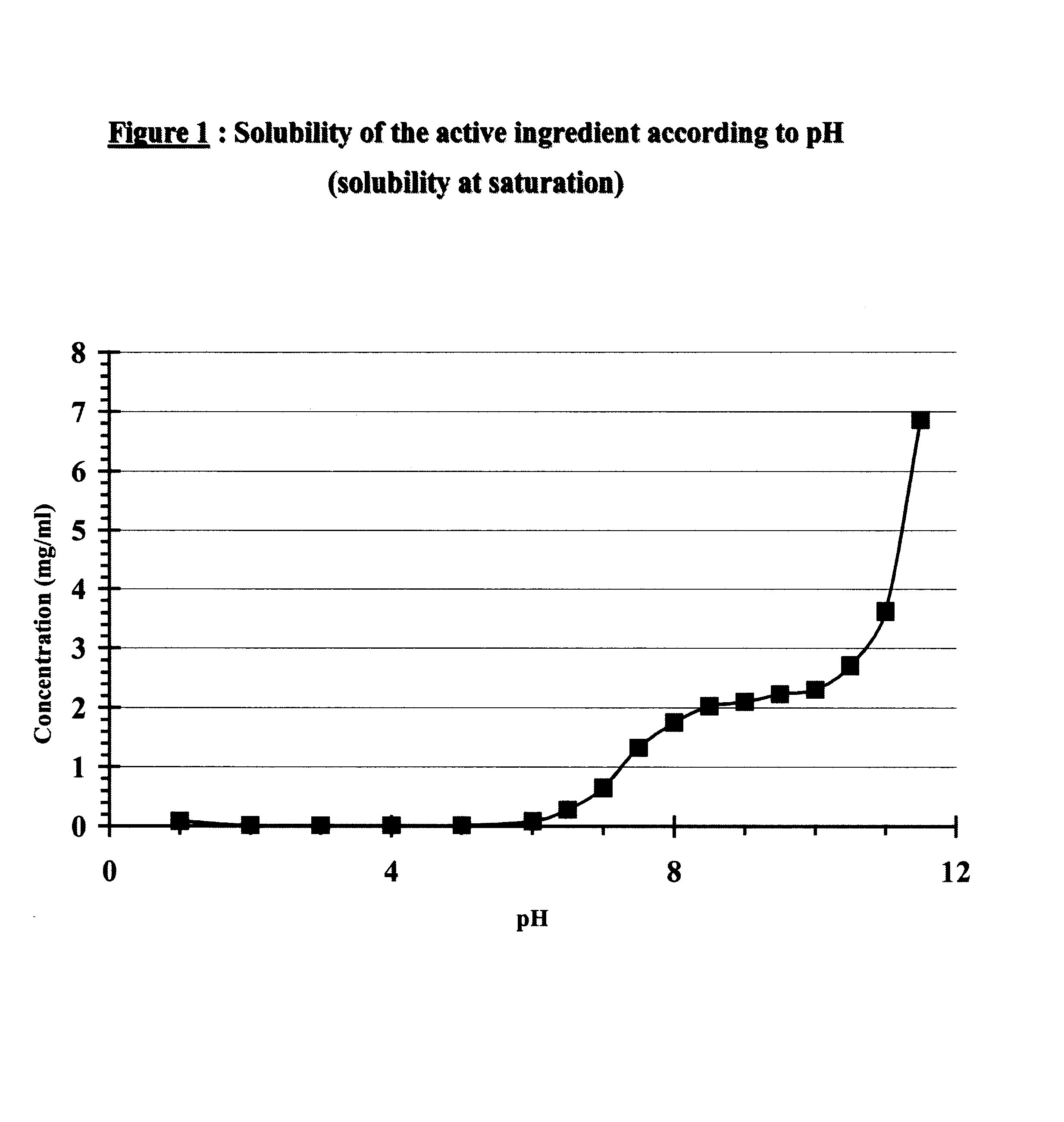
Pharmaceutical Compositions for Oral Administration in the Form of Stabilised Aqueous Suspensions
ActiveUS20080193548A1The process is convenient and fastEasy to manageDispersion deliverySolution deliveryOral medicationLiquid medium
The invention relates to a liquid pharmaceutical composition, in the form of a suspension of micronized powder of active substance in an acceptable physiological liquid medium, stabilised over time, for administration via the oral route.
Owner:CEVA SANTE ANIMALE
Ketamine or dextromethorphan formulations and methods of use
ActiveUS20150342947A1Act quicklyEliminating the digestive tractBiocideOrganic chemistryDiseaseRapid-acting antidepressant
The present invention provides a method of treating depression disease in a patient comprising administering to a mucosal membrane of a patient an effective amount of a pharmaceutically acceptable composition comprising an effective amount of ketamine or dextromethorphan, or both ketamine and dextromethorphan, wherein the mucosal administration of the ketamine or dextromethorphan containing composition allows for the mucosal absorption of the composition eliminating the digestive tract of the patient for effecting a rapid acting antidepressant treatment of the patient. Preferably, this method includes administering the composition to the oral cavity, and more preferably to the buccal cavity, of the patient. A pharmaceutically acceptable composition comprising ketamine or dextromethorphan and a vehicle is disclosed. A biomarker for identifying a depressive disease is set forth. A method of treating depressive illness in a patient using dextromethorphan via the oral route is provided.
Owner:WEST VIRGINIA UNIVERSITY
Compositions for the treatment of hemorrhoids and related diseases
InactiveUS20120183627A1Reduce inflammatory component characteristicEasy and quickening its relapseBiocideAntipyreticDiseaseNatural source
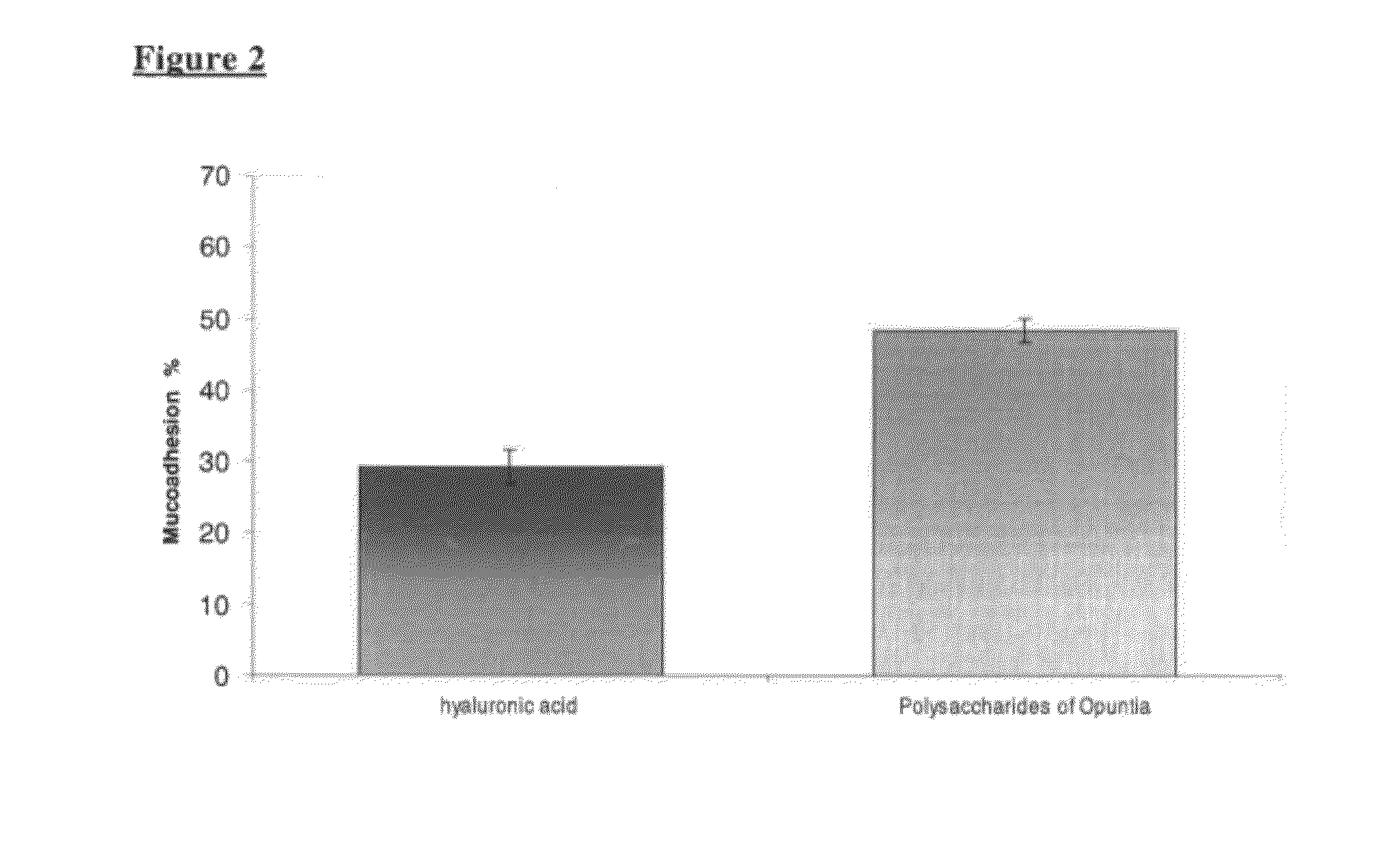
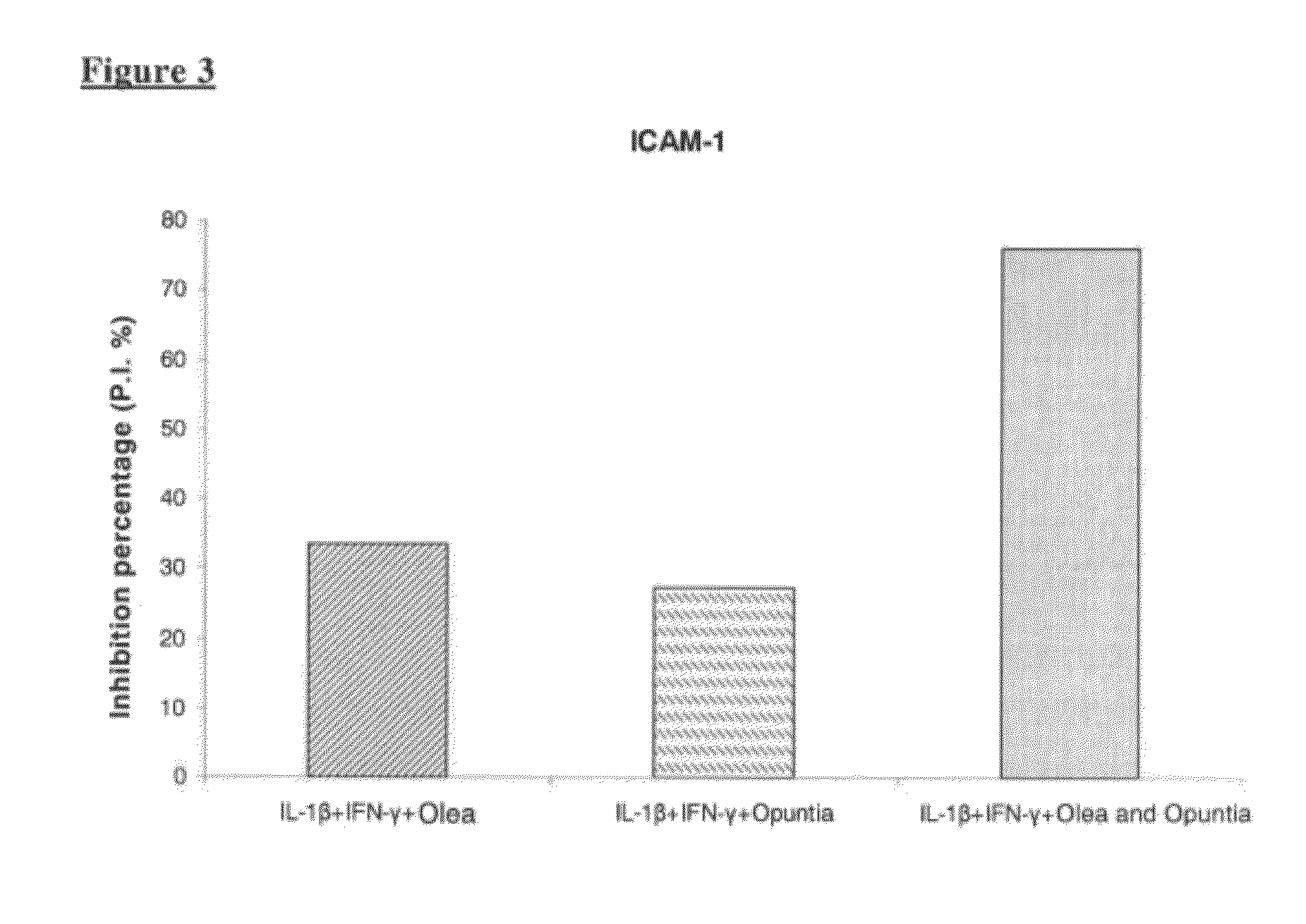
The present invention relates to pharmaceutical, nutraceutical, health foods and medical device compositions comprising polysaccharides from Opuntia Ficus Indica cladodes in combination with antioxidant and anti-inflammatory plant extracts as well as those from Olive leaf, Capparis Spinosa buttons, anthocianosides from red oranges, black rice or from other natural sources and their combinations, useful for prevention and therapy of hemorrhoidal disease and related diseases. The invention compositions may, be applied topically or administered by oral route.
Owner:BIONAP
Peptides and peptide mimetics to treat pathologies associated with eye disease
Owner:RGT UNIV OF CALIFORNIA
Peptides and peptide simulacrums to treat pathologies characterized by an inflammatory response
InactiveCN101227918APeptide/protein ingredientsAgainst vector-borne diseasesAmphipathic helixActive agent
The present invention provides novel active agent (eg, peptides, small organic molecules, amino acid pairs, etc.) peptides that ameliorate one or more symptoms of atherosclerosis and / or other pathologies characterized by an inflammatory response. In certain embodiments, the peptide is similar to the G of apolipoprotein J * Amphipathic helix. The active agent is very stable and easy to administer by oral route.
Owner:RGT UNIV OF CALIFORNIA
Oral pharmaceutical composition with improved bioavailability
InactiveUS7867517B2Improve bioavailabilityIncrease variabilityBiocidePowder deliveryIn vivoBioavailability
The present invention relates to prompt-release oral pharmaceutical compositions containing one or more active principles solubilised, suspended or embedded in a suitably formulated amphiphilic matrix for improving in vitro and in vivo bioavailability of medicaments sparingly absorbed through the oral route and / or with problems of high variability of absorption in the gastrointestinal tract.
Owner:FARMATRON
G-type peptides and other agents to ameliorate atherosclerosis and other pathologies
This invention provides novel peptides, and other agents, that ameliorate one or more symptoms of atherosclerosis and / or other pathologies characterized by an inflammatory response. In certain embodiment, the peptides resemble a G* amphipathic helix of apolipoprotein J. The peptides are highly stable and readily administered via an oral route.
Owner:RGT UNIV OF CALIFORNIA +1
Compositions of and method for preparing stable particles in a frozen aqueous matrix
InactiveUS7112340B2High viscositySlow down spontaneous degradationPowder deliveryCosmetic preparationsInhalationMedicine
The present invention discloses a composition of a stable suspension of a poorly water soluble pharmaceutical agent or cosmetic in the form of particles of the pharmaceutical agent or cosmetic suspended in a frozen aqueous matrix and method for its preparation. The composition is stable for a prolonged period of time, preferably six months or longer and is suitable for parenteral, oral, or non-oral routes such as pulmonary (inhalation), ophthalmic, or topical administration.
Owner:BAXTER INT INC
Peptides and peptide mimetics to treat cancer
InactiveUS20100227825A1Symptoms improvedAntibacterial agentsSenses disorderAmphipathic helixActive agent
This invention provides novel active agents (e.g. peptides, small organic molecules, amino acid pairs, etc.) peptides that ameliorate one or more symptoms of atherosclerosis and / or other pathologies characterized by an inflammatory response. In certain embodiment, the peptides resemble a G* amphipathic helix of apolipoprotein J. The agents are highly stable and readily administered via an oral route.
Owner:RGT UNIV OF CALIFORNIA
G-type peptides to ameliorate atherosclerosis
This invention provides novel peptides that ameliorate one or more symptoms of atherosclerosis and / or other pathologies characterized by an inflammatory response. In certain embodiment, the peptides resemble a G* amphipathic helix of apolipoprotein J. The peptides are highly stable and readily administered via an oral route.
Owner:RGT UNIV OF CALIFORNIA
Oral compositions for absorption of phosphorus compounds
InactiveUS7815898B2Convenient treatmentEasy to controlHeavy metal active ingredientsBiocidePhosphorus serum levelPhosphorus
Owner:CM&D PHARMA
Oral pharmaceutical composition with improved bioavailability
The present invention relates to prompt-release oral pharmaceutical compositions containing one or more active principles solubilised, suspended or embedded in a suitably formulated amphiphilic matrix for improving in vitro and in vivo bioavailability of medicaments sparingly absorbed through the oral route and / or with problems of high variability of absorption in the gastrointestinal tract.
Owner:FARMATRON
Sustained release pharmaceutical compounds to prevent abuse of controlled substances
The present invention provides methods for altering controlled substances in a manner that decreases their potential for abuse. The novel compounds may be combined in tablets with suitable excipients or formulated in solution for oral delivery. When delivered by the oral route the controlled substance is released in a time-dependent manner (sustained release) by acid hydrolysis and / or enzymatic cleavage. When administered by injection the controlled substance is released in a time-dependent manner (sustained release) by way of serum enzymes.
Owner:SHIRE PLC
Oral compositions and route of administration for the delivery of a thylakoid extract
InactiveUS20070036877A1Great tasteImprove scentBiocideAntipyreticChloroplast thylakoidsIngested food
The present invention provides a new use for a thylakoid extract, that is for oral route of administration, and a composition comprising the thylakoid extract in adjunction with an acceptable carrier for oral administration. Besides the pharmaceutical use, the thylakoid extract enters the composition of food or food supplements, for its inocuity and its capacity to provide a diet enriched in anti-oxidants and anti-inflammatory compounds. Therefore, in accordance with the present invention is provided the use of a thylakoid extract in the making of an oral composition for treating or preventing a disease or disorder involving the formation of reactive oxygen species or inflammation. Also is provided a method for treating or preventing a disease or disorder involving the formal of reactive oxygen species or inflammation in an individual, which comprises the step of orally administering an effective dose of a thylakoid extract. Further is provided an oral composition comprising a thylakoid extract and a vehicle for oral ingestion or oral administration. An oral composition comprising purified thylakoids and a carrier for oral ingestion or oral administration, with the proviso that the carrier does not essentially consists of water, physiological saline or propylene glycol is also provided as food or a food supplement, or as a pellet, or encapsulated granules or powder. The carrier may be present in an amount of 0.01% to 95% (w / w) of the total composition. The purified thylakoids are present in an amount which achieves a dosage of 0.1 to 10 mg per Kg of a subject's body weight.
Owner:PURECELL TECH
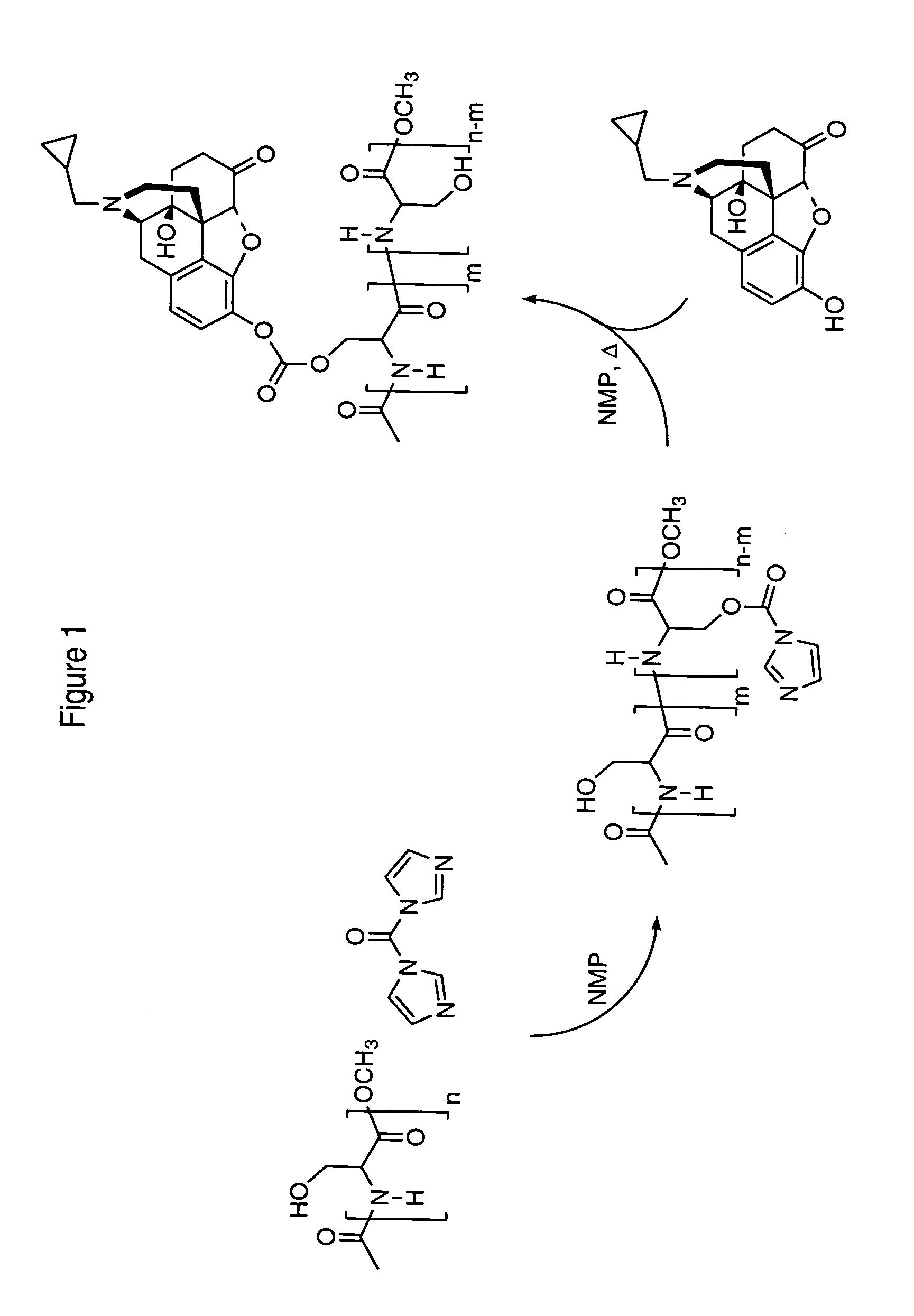
Polysubunit opioid prodrugs resistant to overdose and abuse
ActiveUS20170100390A1Reducing expected systemic exposureReduced systemic exposureOrganic active ingredientsNervous disorderPreventing painIn vivo
The invention provides compositions and methods for the treatment or prevention of pain. The invention provides constructs whereby hydrolysis of the construct by a specified gastrointestinal enzyme directly, or indirectly, releases an opioid when taken orally as prescribed. The gastrointestinal enzyme mediated release of opioid from constructs of the invention is designed to be attenuated in vivo via a saturation or inhibition mechanism when overdoses are ingested. The invention further provides constructs that are highly resistant to oral overdose, chemical tampering, and abuse via non-oral routes of administration.
Owner:ELYSIUM THERAPEUTICS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com