Peptides and peptide simulacrums to treat pathologies characterized by an inflammatory response
A disease and symptom technology, applied in the field of peptides and peptidomimetics for the treatment of pathologies characterized by inflammatory responses, to solve problems such as accelerated LDL oxidation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
Using ApoJ-related peptides to mediate atherosclerotic symptoms
Inhibit LDL-induced chemotactic activity of monocytes
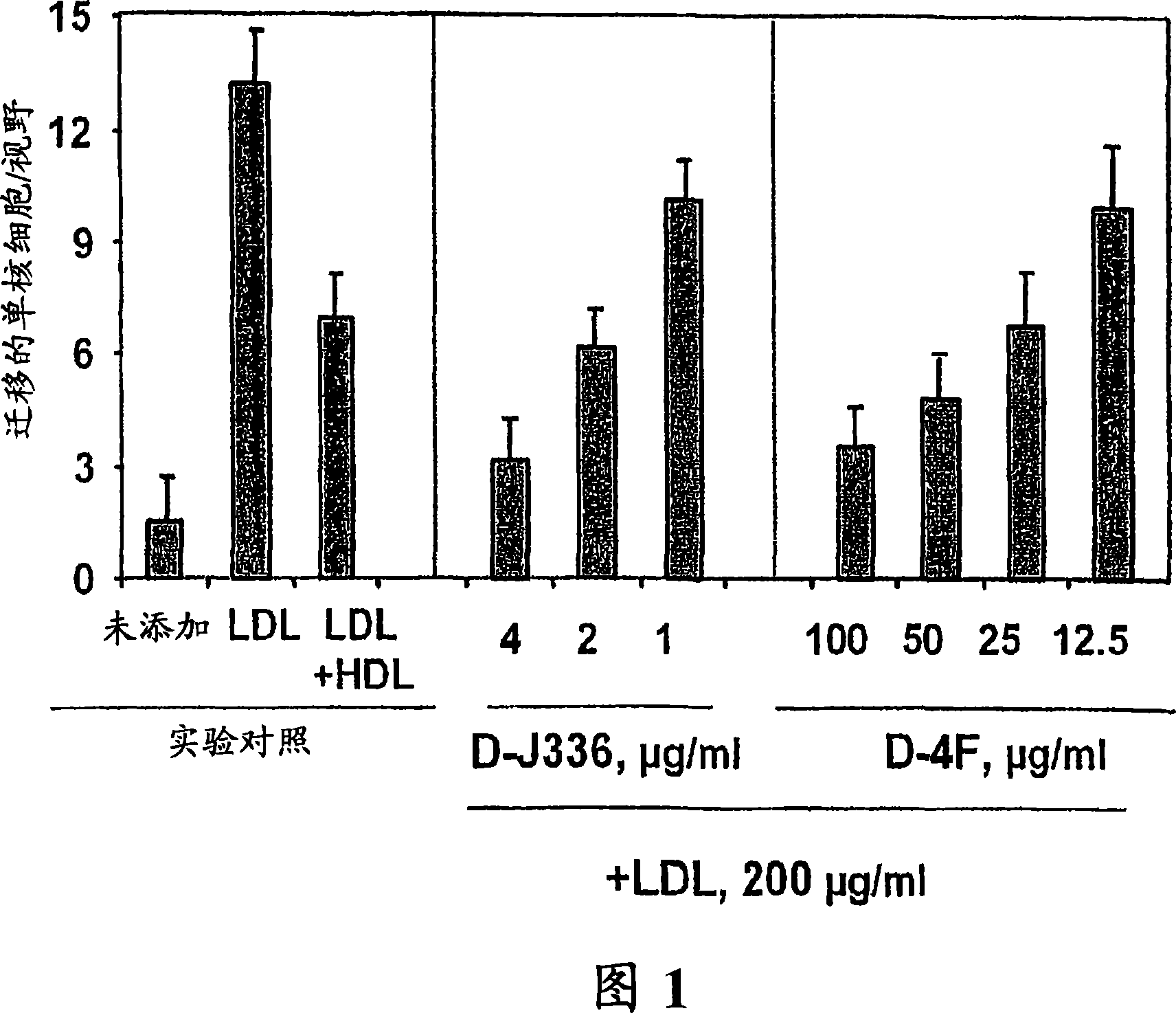
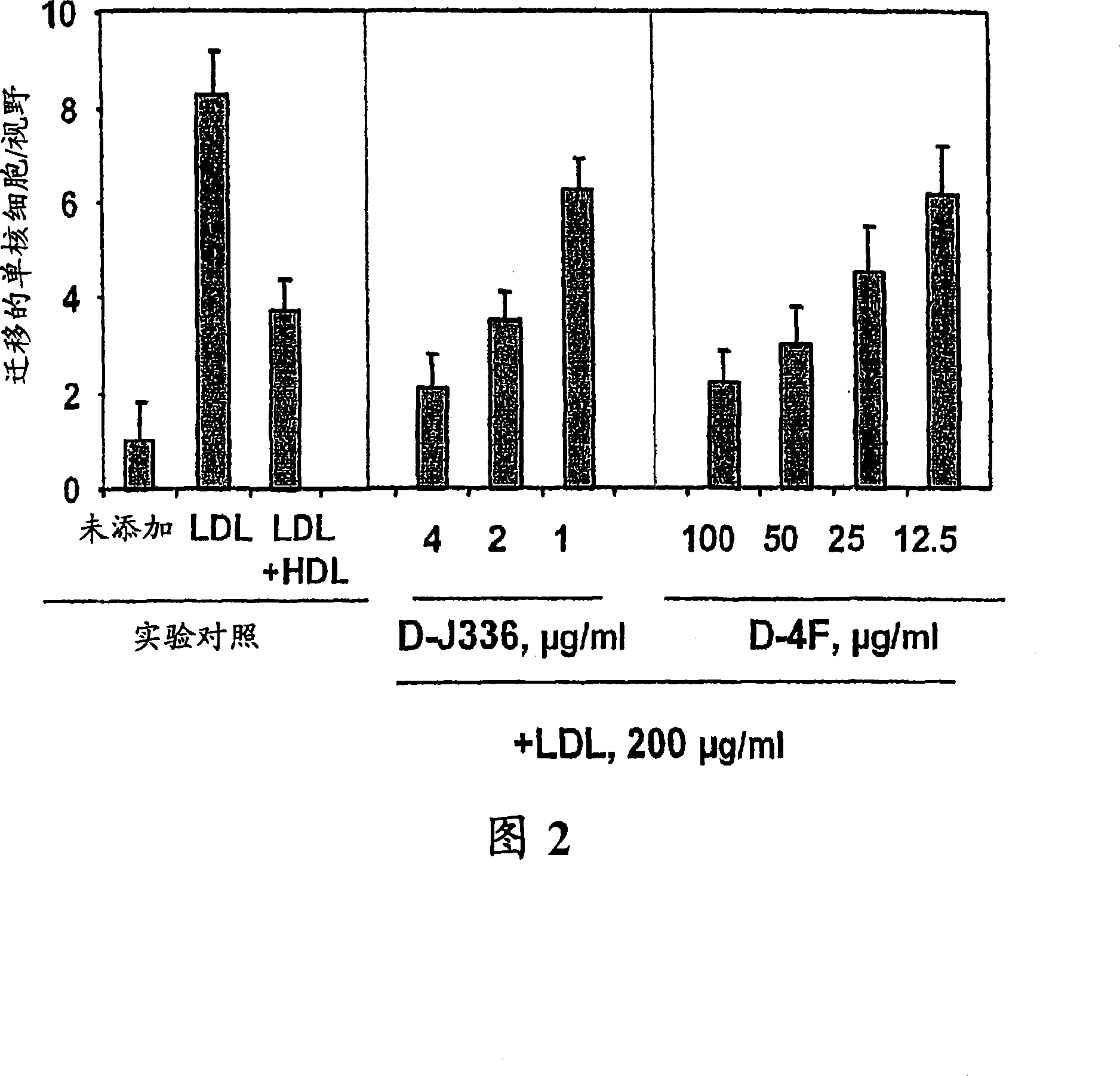
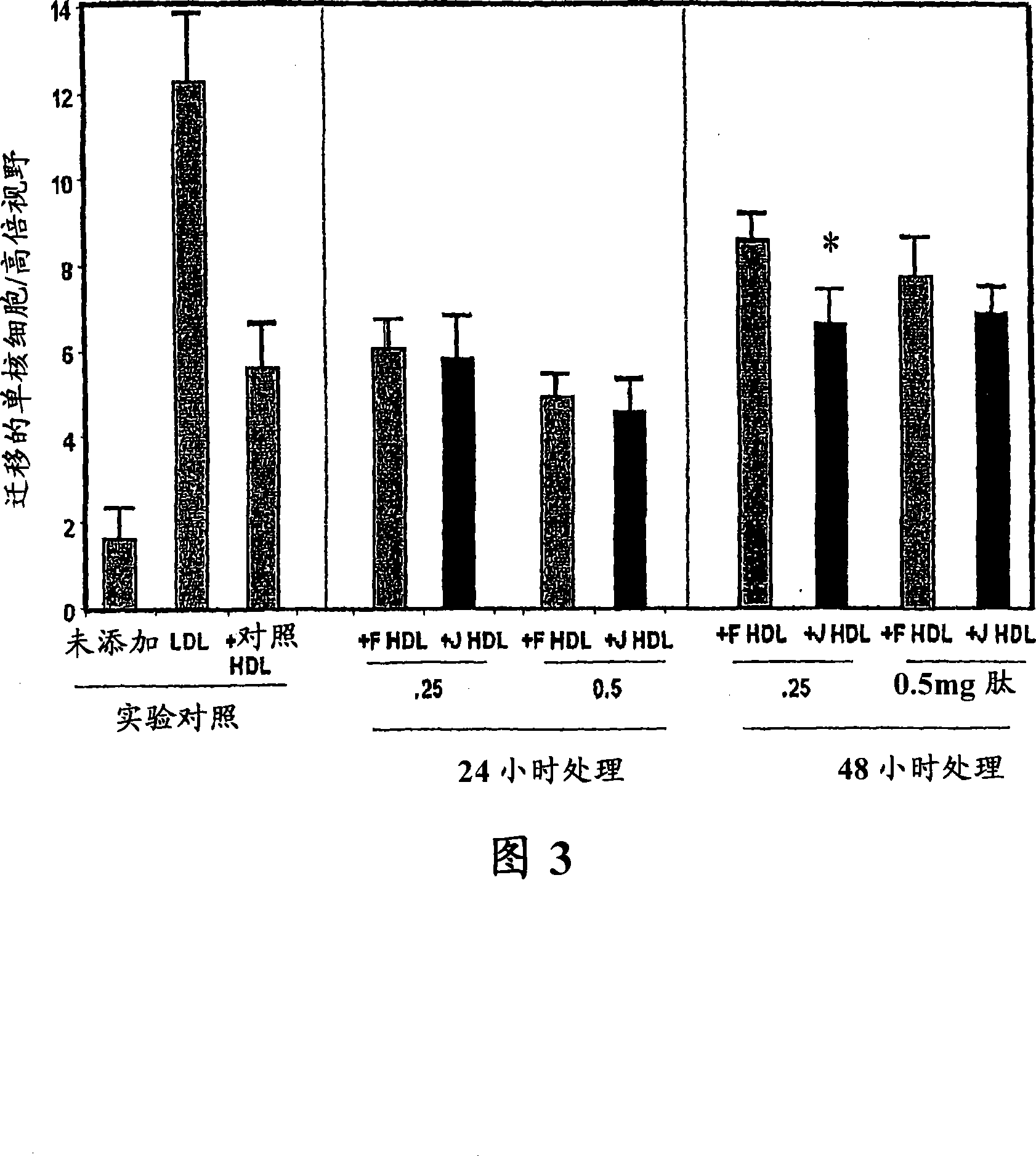
[0306] Figure 1 shows the effect of D-4F (Anantharamaiah et al. (2002) (2002) Circulation, 105: 290-292) on the inhibition of LDL-induced chemotaxis of monocytes during co-incubation and apoJ prepared with D amino acids Peptide (D-J336, Ac-LLEQLNEQFNWVSRLANLTQG-E-NH 2 , SEQ ID NO: 1177)) Comparison of effects. Human aortic endothelial cells were incubated with medium (not added) only, with control human LDL (200 μg protein / ml) or control human LDL + control human HDL (350 μg HDL protein / ml). Add D-J336 or D-4F in the specified concentration range plus control human LDL (200 μg protein / ml) to other wells. After overnight incubation, the monocyte chemotactic activity of the supernatant was determined. As shown in Figure 1, the in vitro concentration of the apoJ variant peptide that inhibits the chemotactic activity of monocytes induced by LDL induced by human art...
Embodiment 2
Apo E-deficient mice oral G * Peptides increase the protective ability of HDL
[0319] 4-month-old female apoE-deficient mice (n=4 per group) use G with the following amino acid sequence * Peptide treatment: peptide 113-122 = Ac-L V G R Q L E E F L-NH 2 (SEQ IDNO: 9), peptide 336-357=Ac-L L E Q L N E Q F N W V S R L A N L T Q GE-NH 2 (SEQ ID NO: 17) and peptide 377-390 = Ac-PS G V T E V V V K L F DS-NH 2 (SEQ ID NO: 19).
[0320] Each mouse was given 200 μg peptide by gastric tube. After 4 hours, blood was obtained, plasma was separated, lipoprotein was obtained, and the protective ability of HDL (25μg / ml) in human artery wall cell culture against LDL (100μg / ml) oxidation was determined. The data is shown in Figure 8. In mice, the peptide provides significant HDL protection to mice.
[0321] In another experiment, 4-month-old female apoE-deficient mice (n=4 per group) were treated with the 11 amino acid G* peptide 146-156 with the following sequence: Ac-Q Q T H ML D V M Q D-NH 2 (S...
Embodiment 3
Solution phase chemical synthesis of peptides
[0322] In certain embodiments, solution phase synthesis chemistry provides a more economical synthesis method for the peptides of the invention.
[0323] Before the synthesis method of the present invention, a total solid phase synthesis chemistry method is generally used for synthesis. The solid phase synthesis of peptides with less than 9 amino acids is more economical than the solid phase synthesis of peptides with more than 9 amino acids. Due to the separation of the extended amino acid chain on the resin, the synthesis of peptides larger than 9 amino acids resulted in severe material loss. Solid-phase synthesis of peptides containing less than 9 amino acids is more economical because the loss of extended chains on the resin is relatively small.
[0324] In some embodiments, the synthesis of 18 amino acid apoA-I mimic peptides, 4F (and other related peptides) is converted from full solid phase synthesis or conversion to full solu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| depth | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com