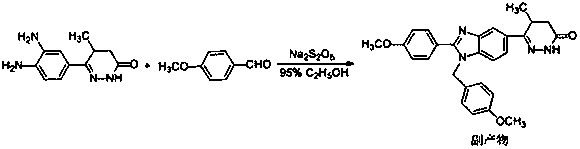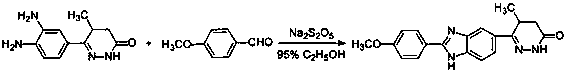Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31 results about "Pimobendan" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
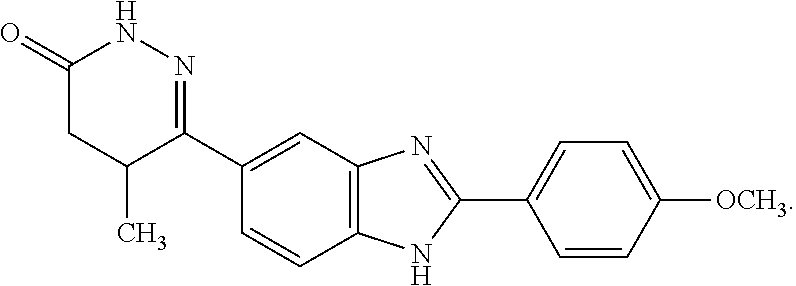
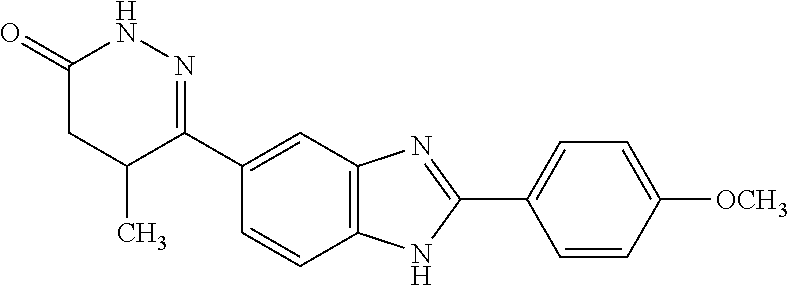
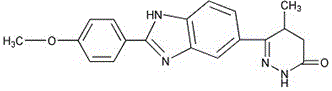
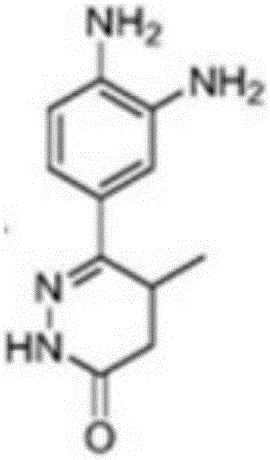
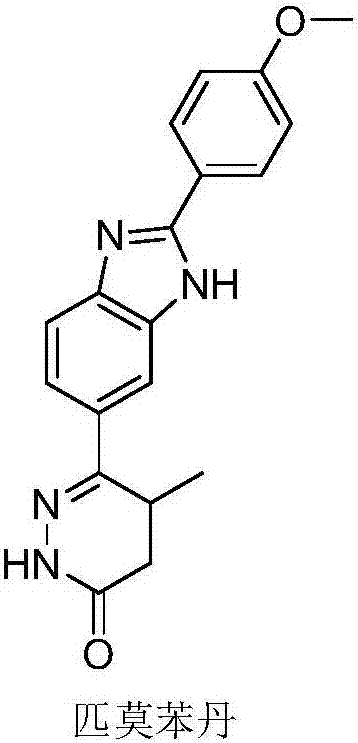
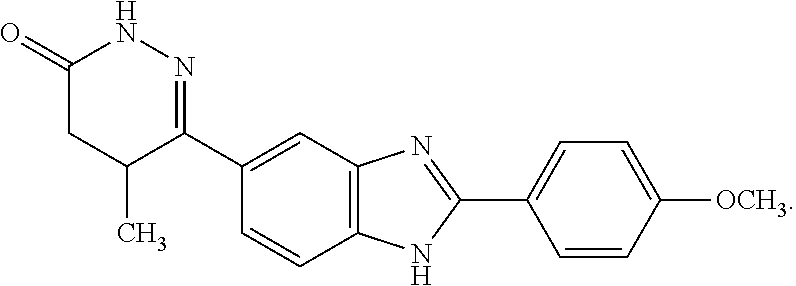
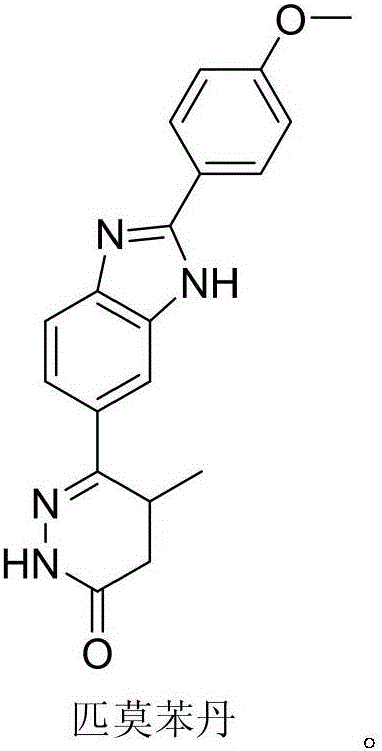
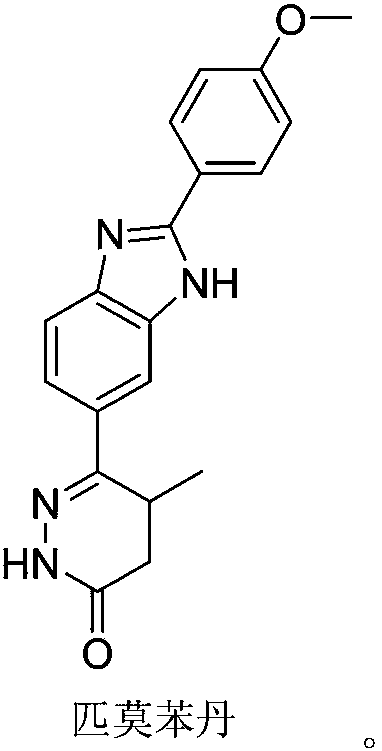
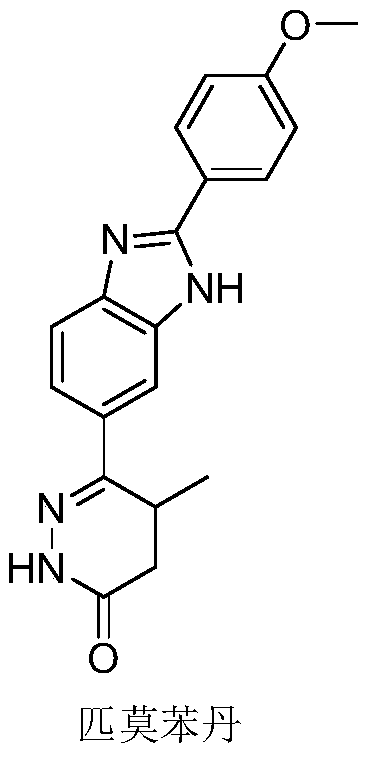
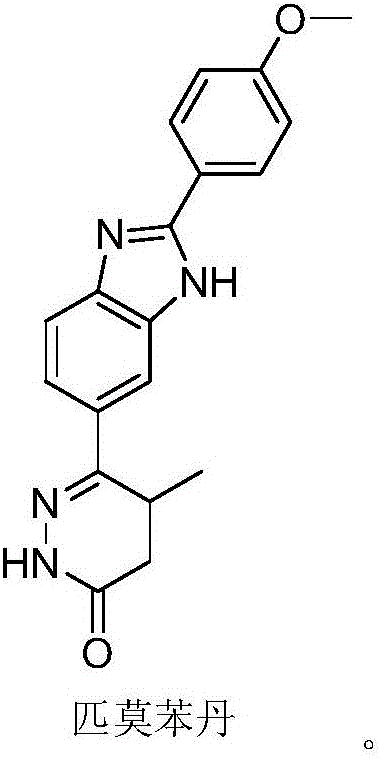
Pimobendan (INN, or pimobendane; tradenames Vetmedin, Acardi) is a veterinary medication. It is a calcium sensitizer and a selective inhibitor of phosphodiesterase 3 (PDE3) with positive inotropic and vasodilator effects.
Pharmaceutical composition comprising pimobendan
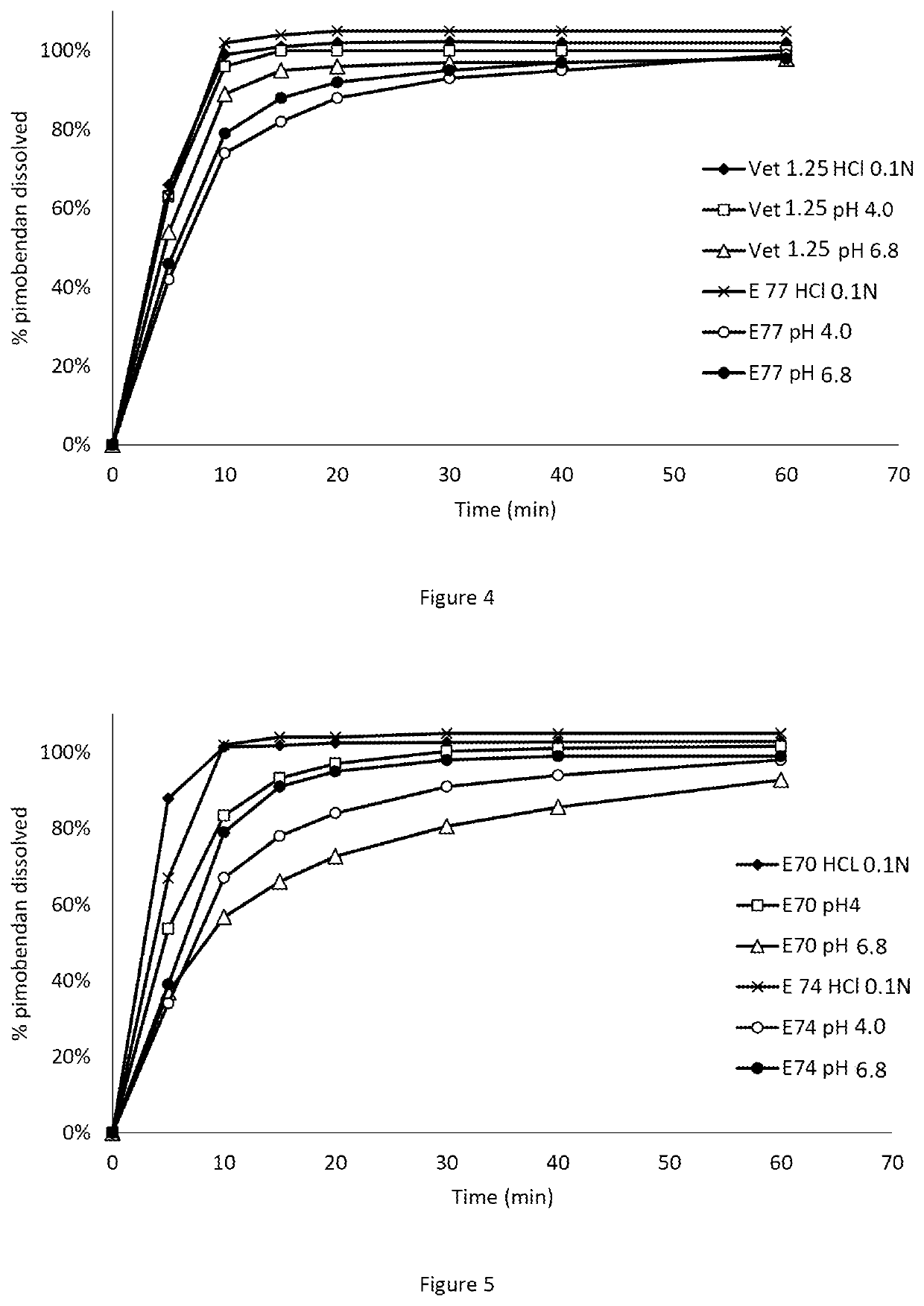
ActiveUS20050203097A1Decrease in dissolution characteristicReduce the amount requiredPowder deliveryPill deliveryPimobendanActive compound
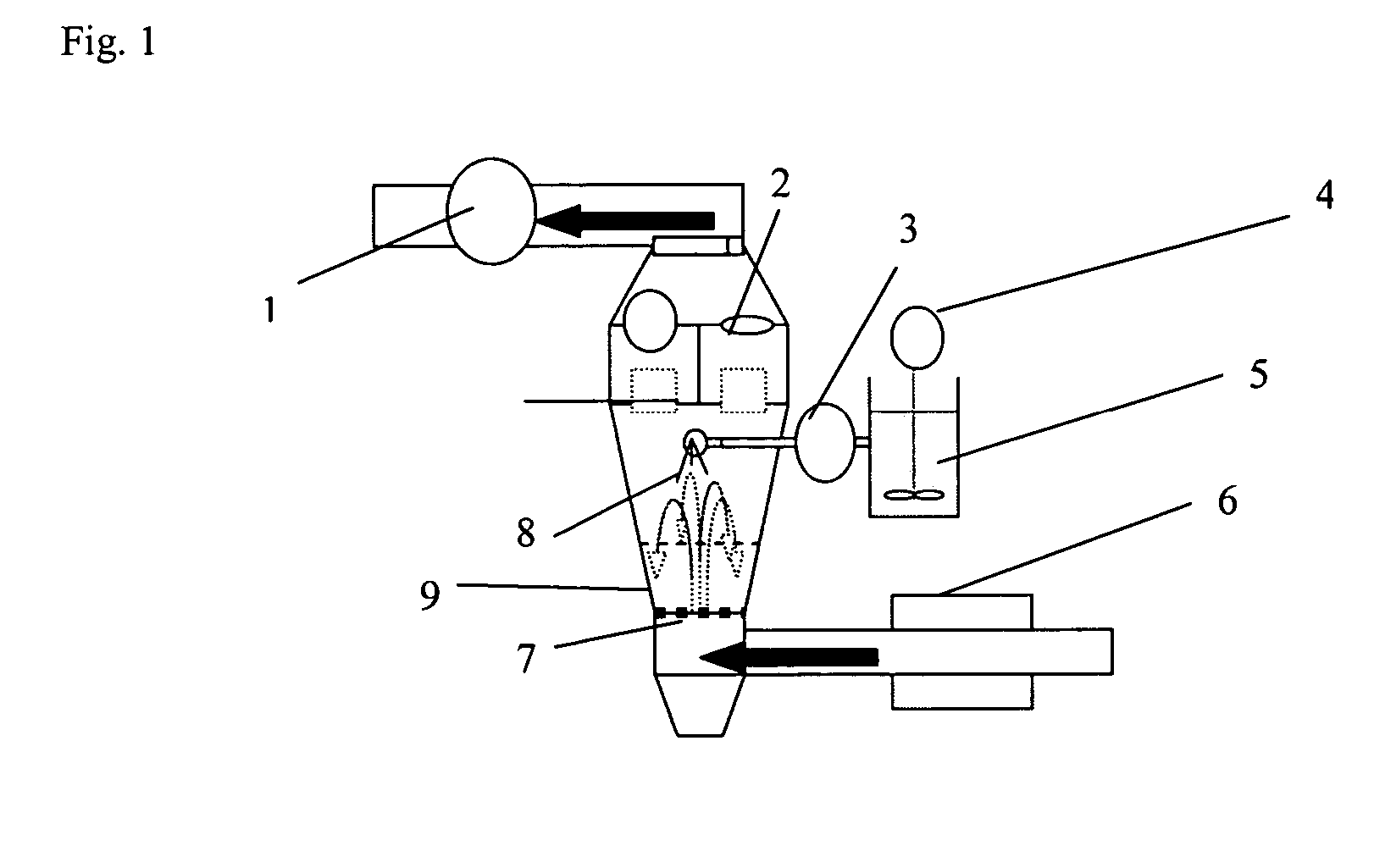
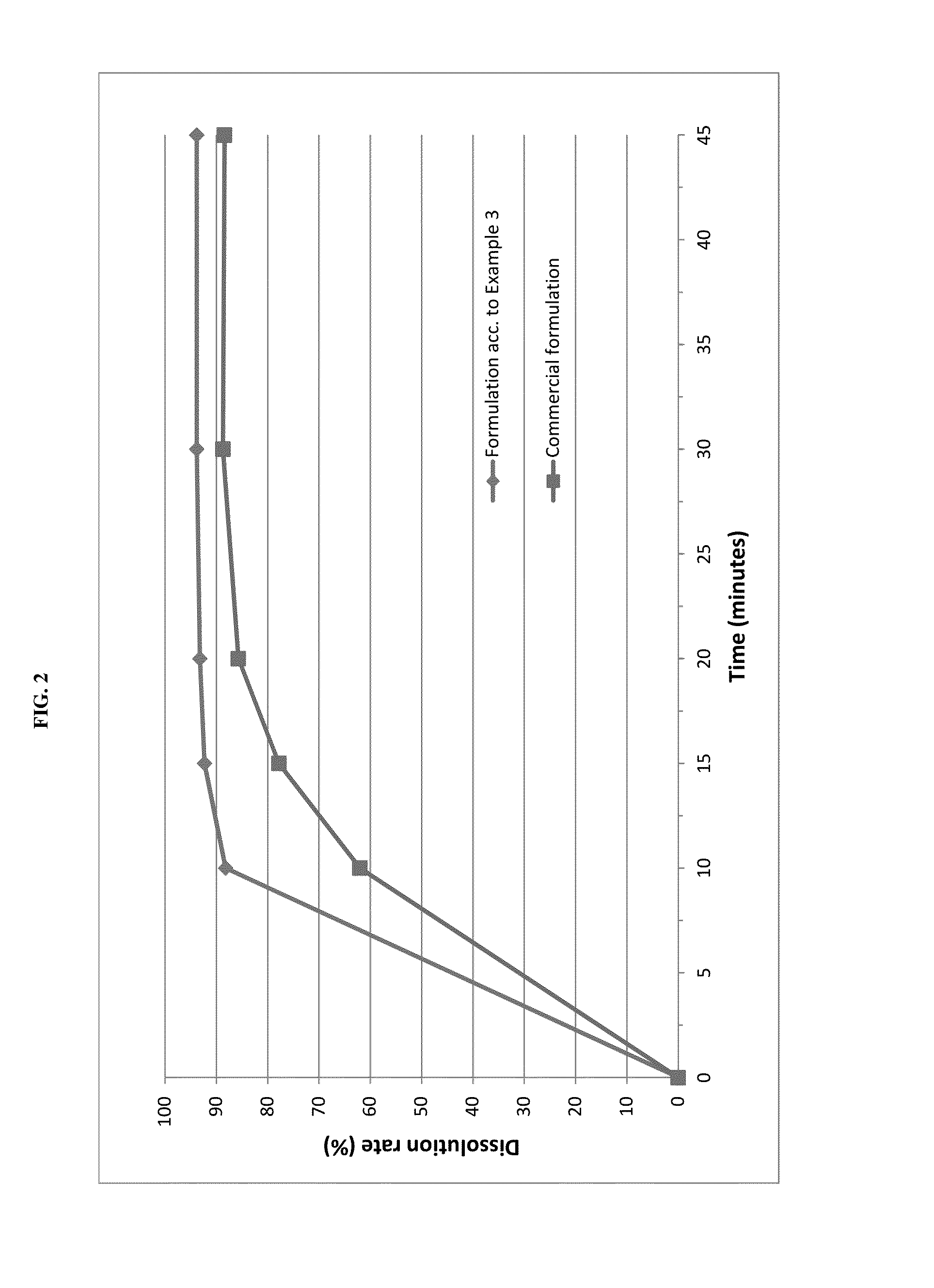
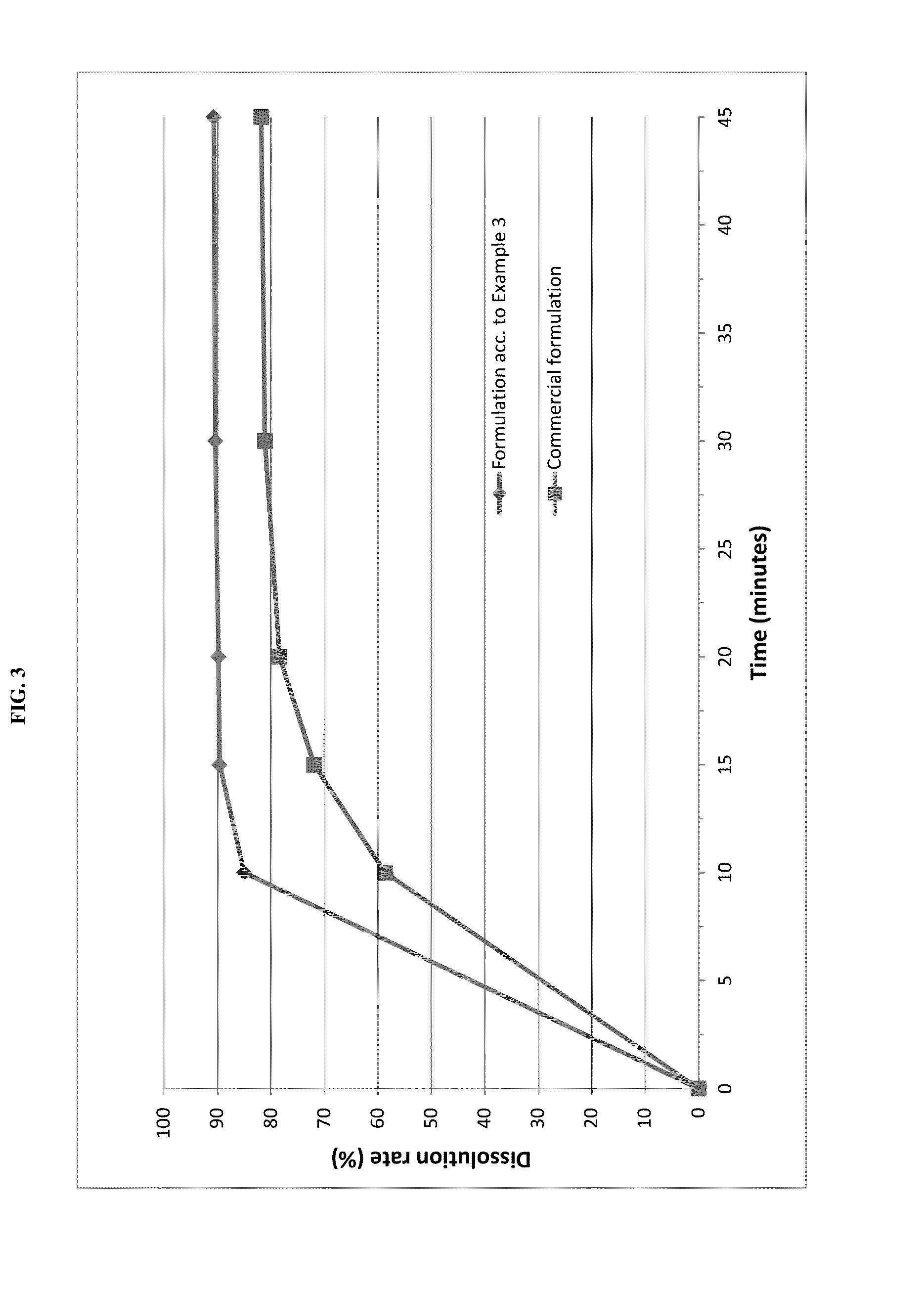
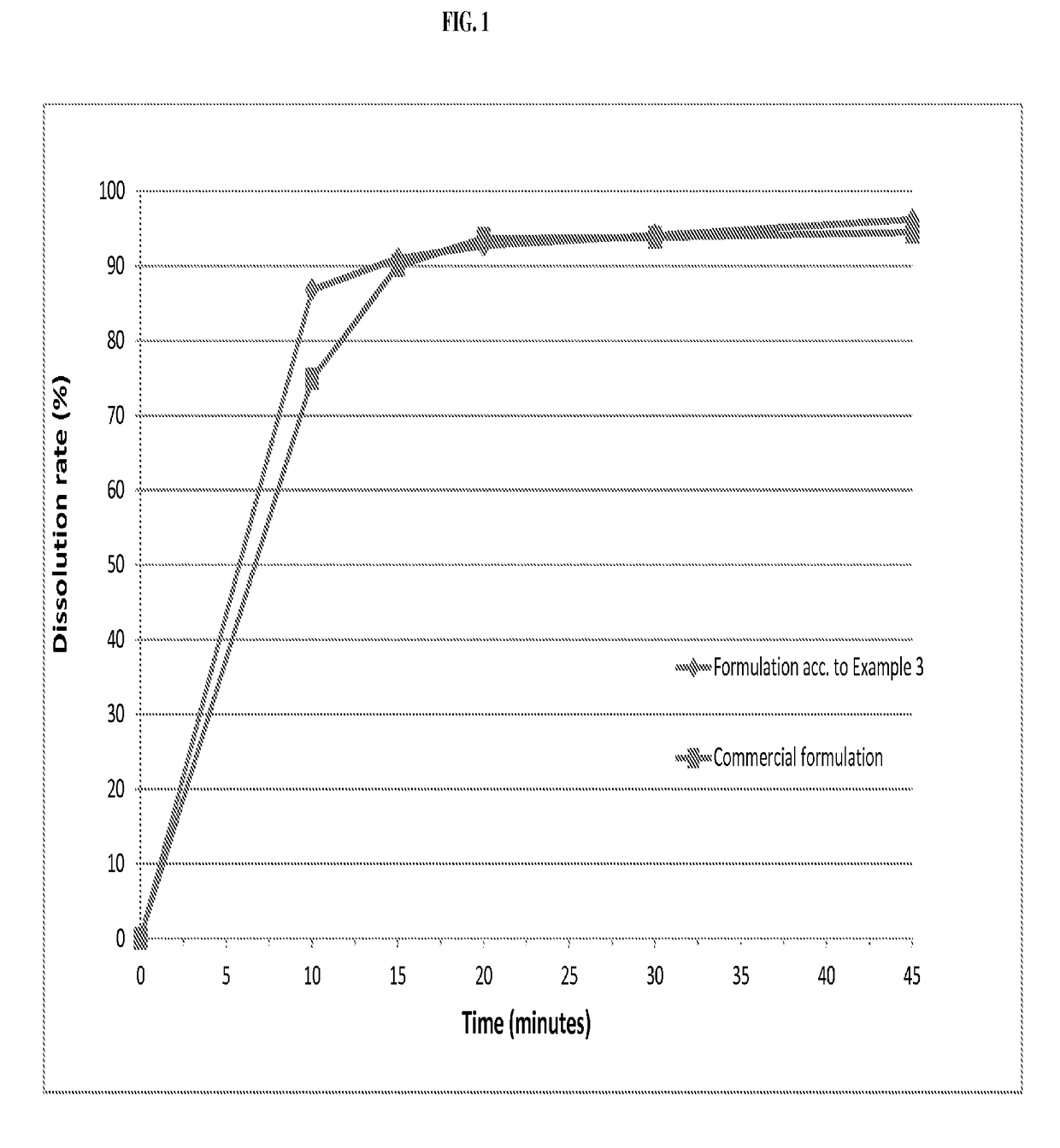
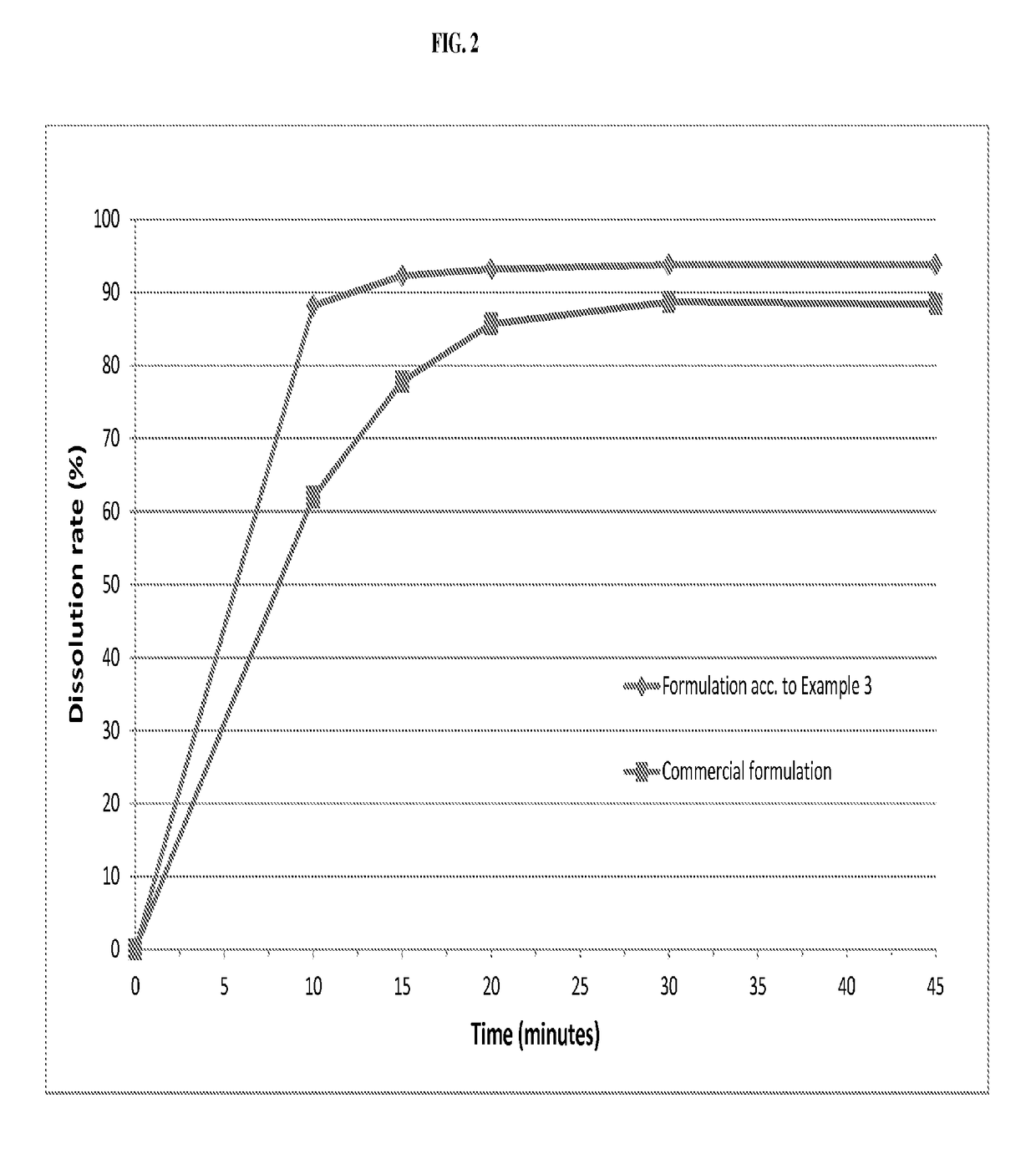
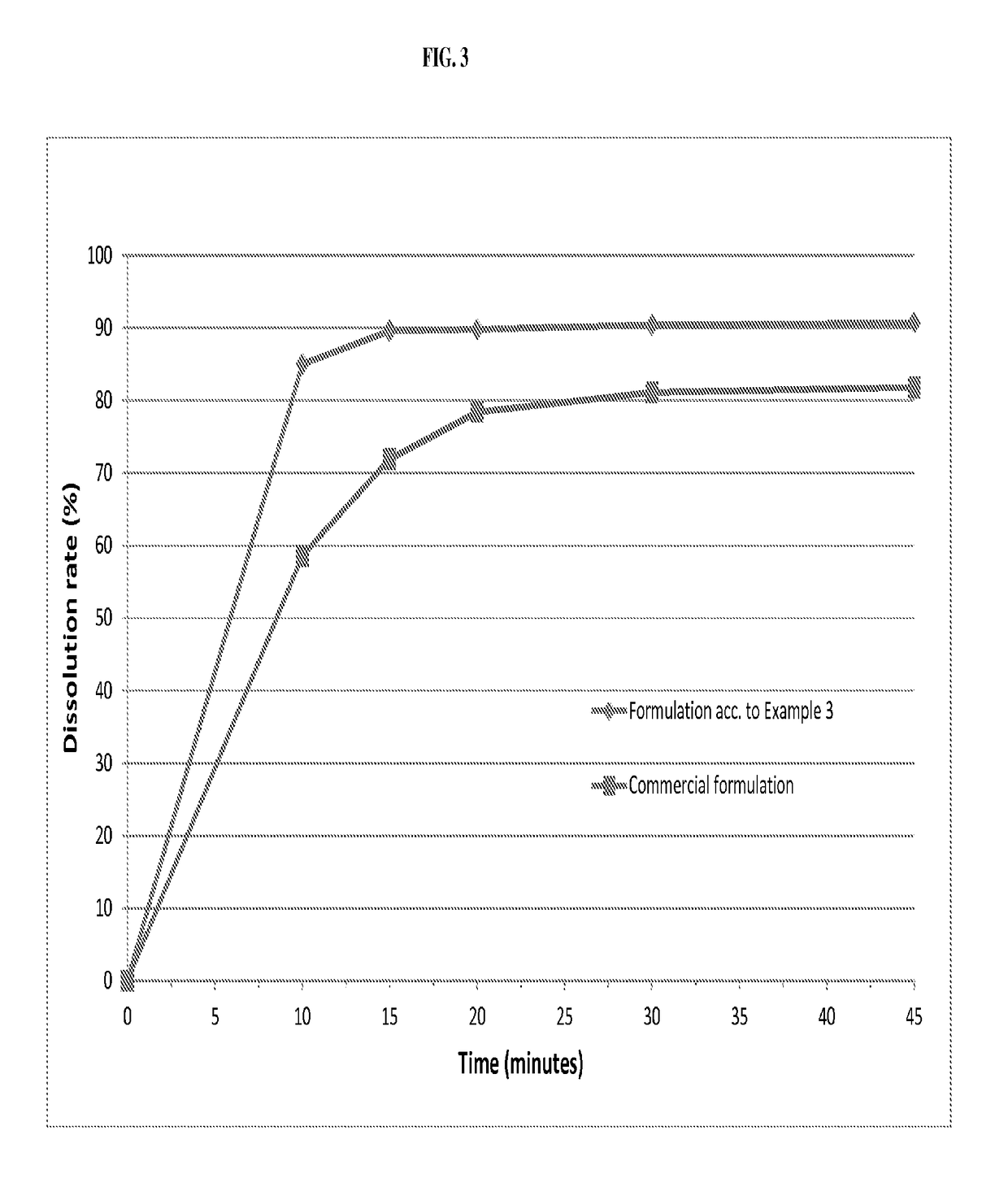
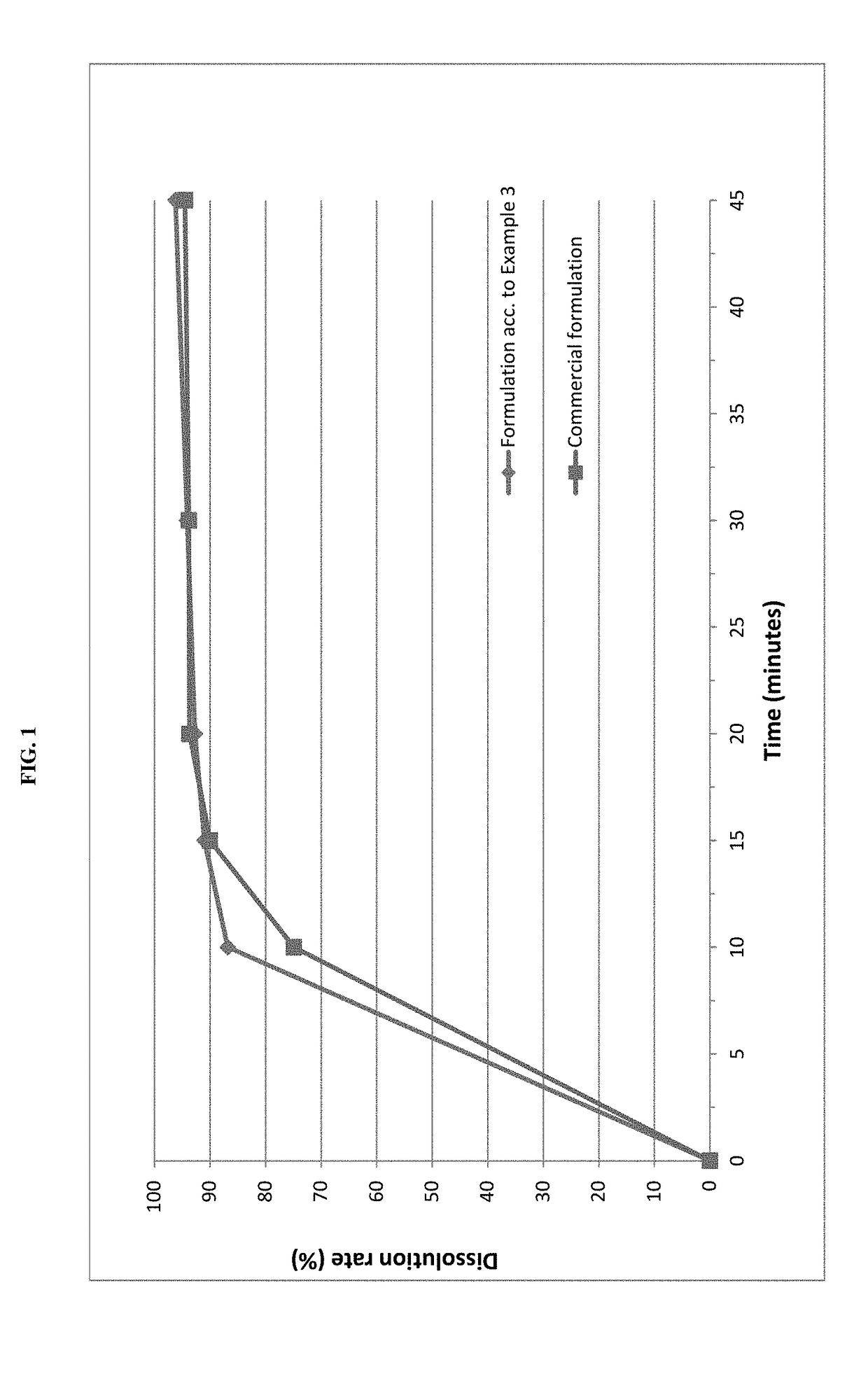
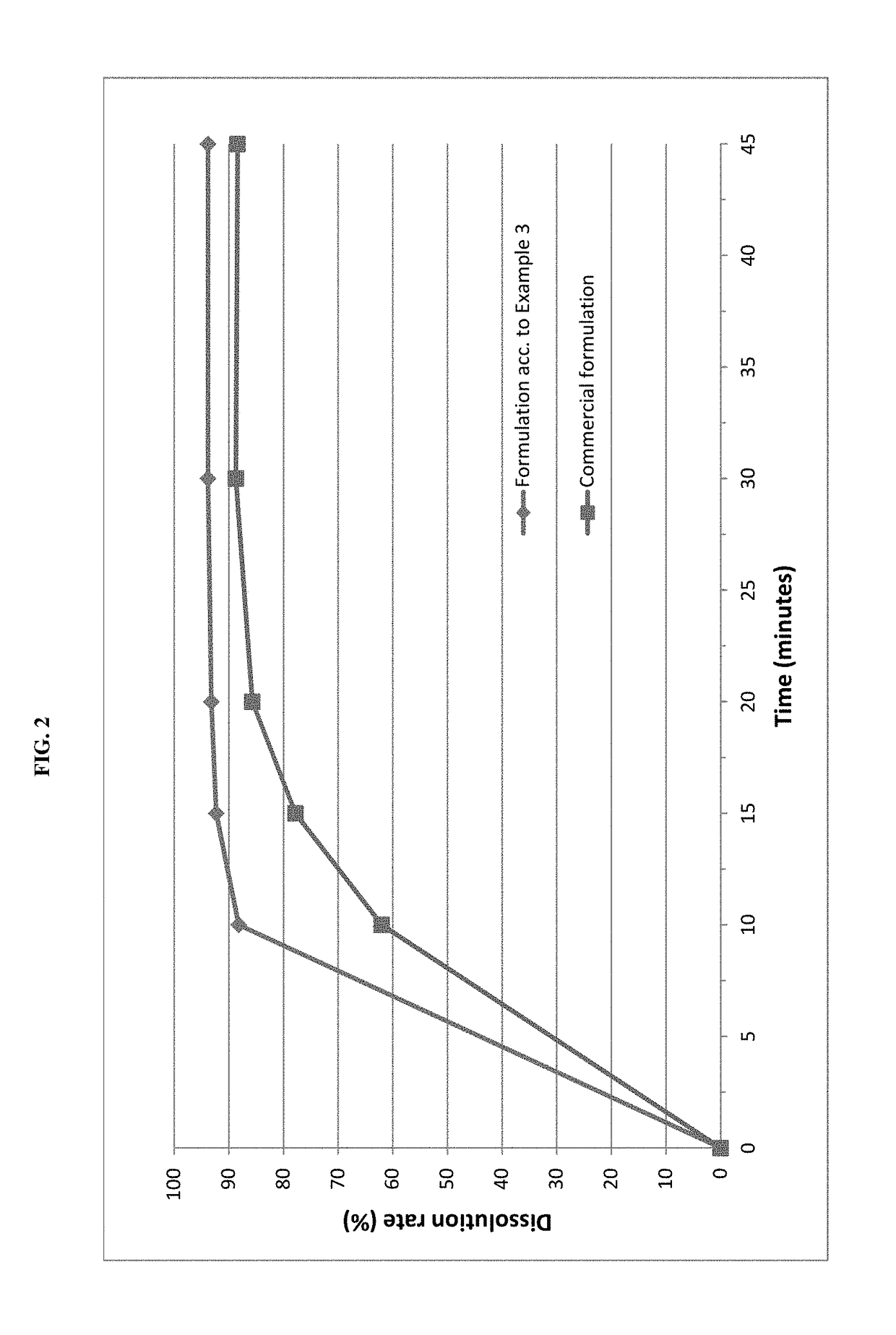
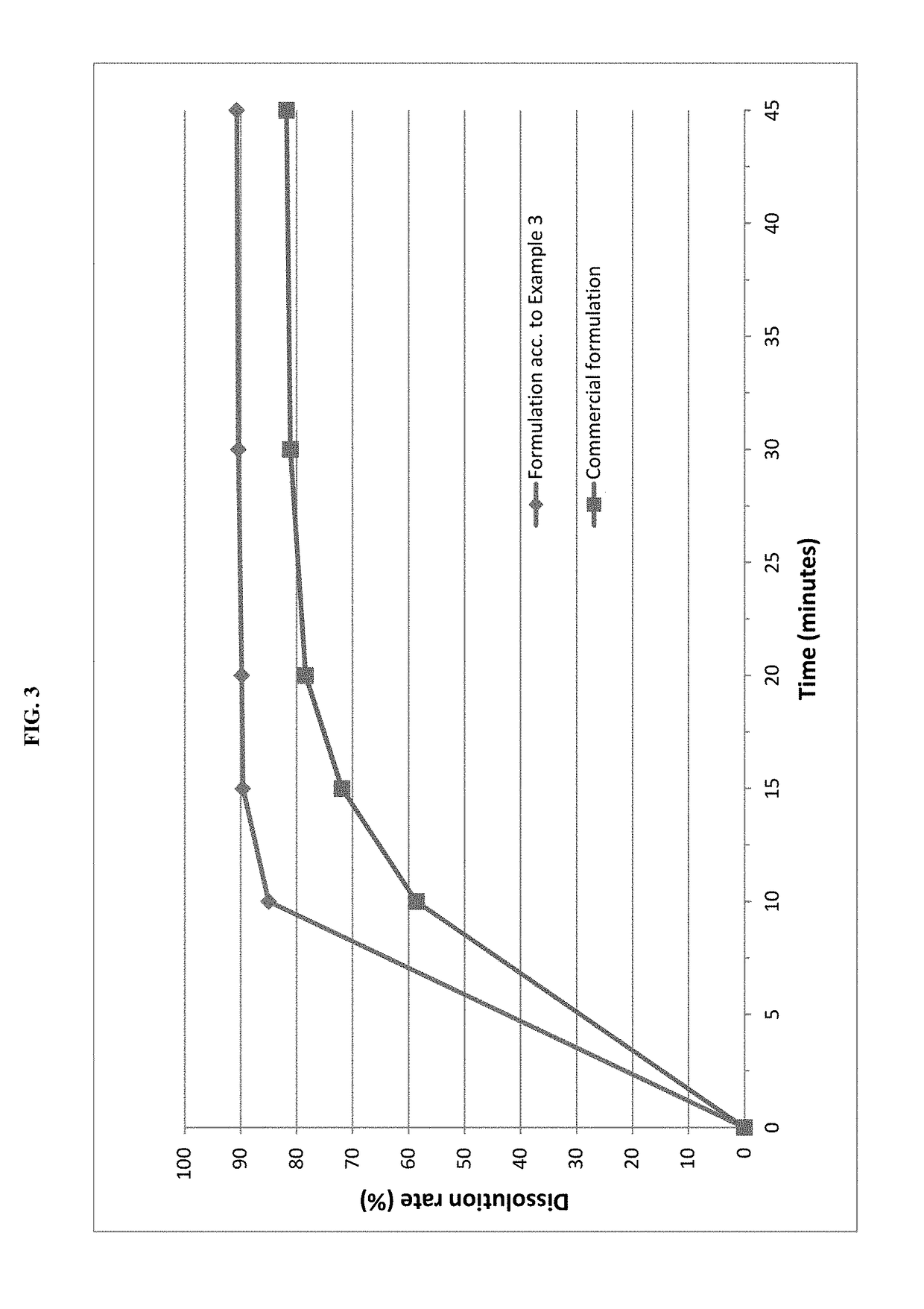
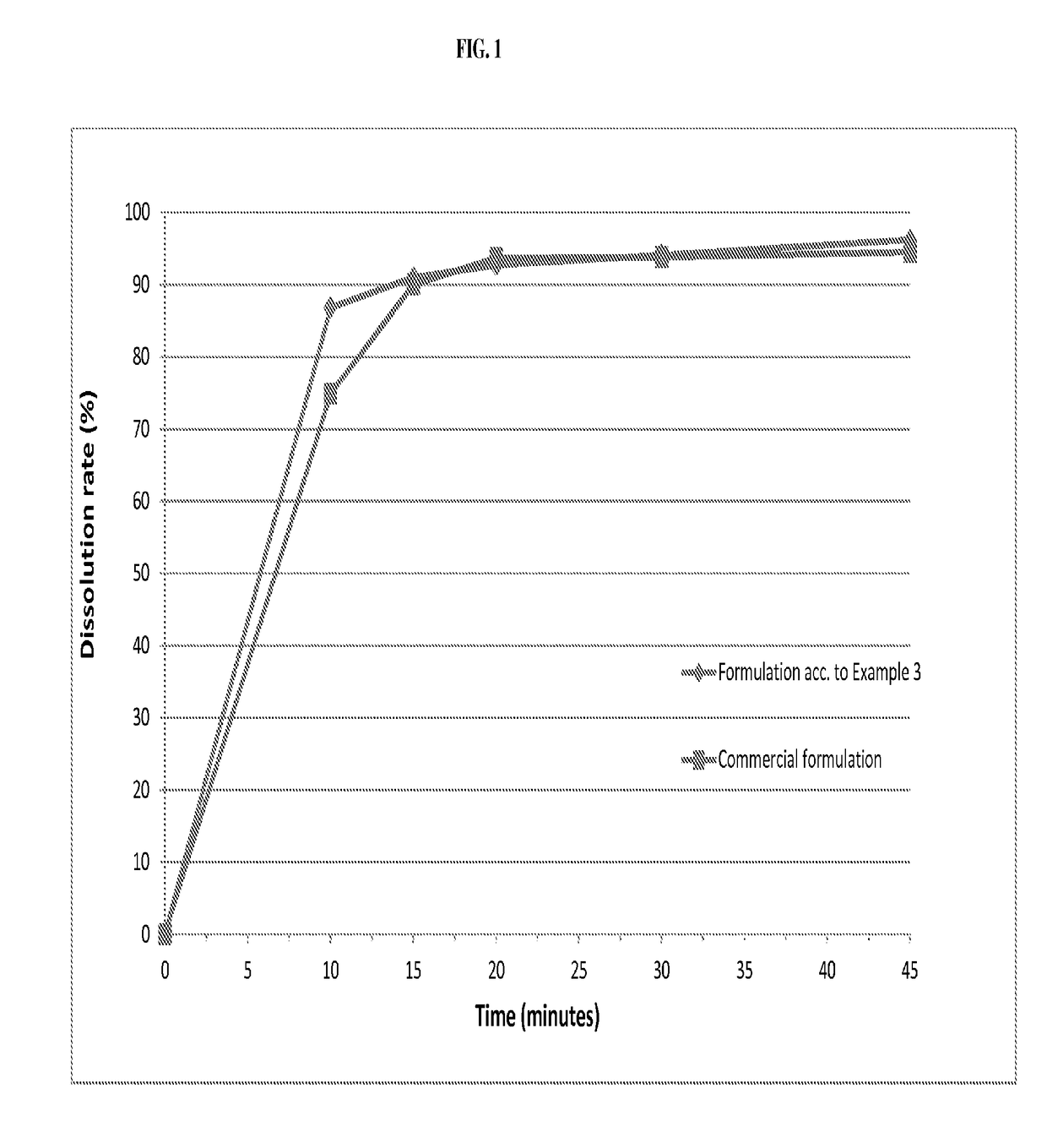
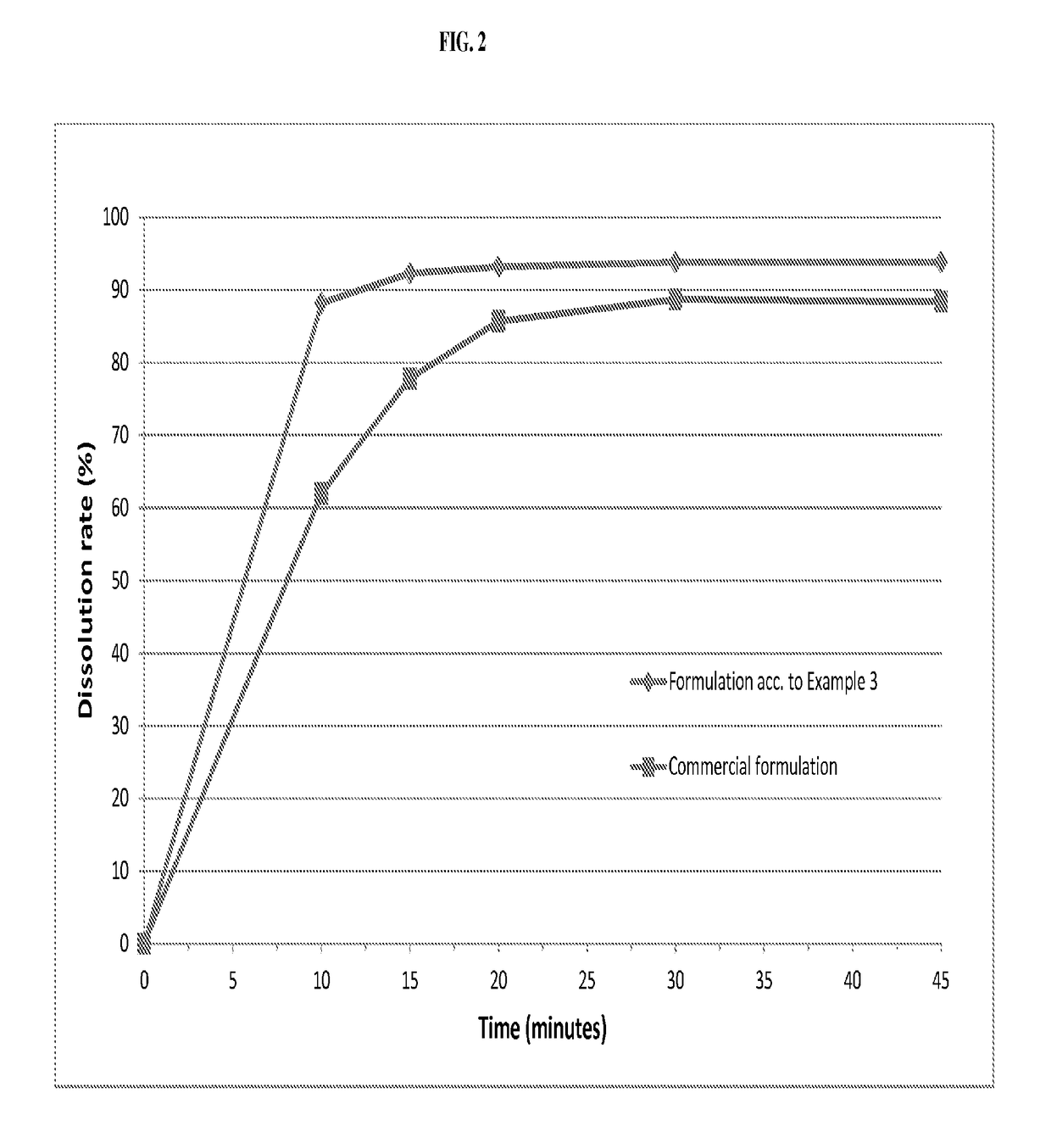
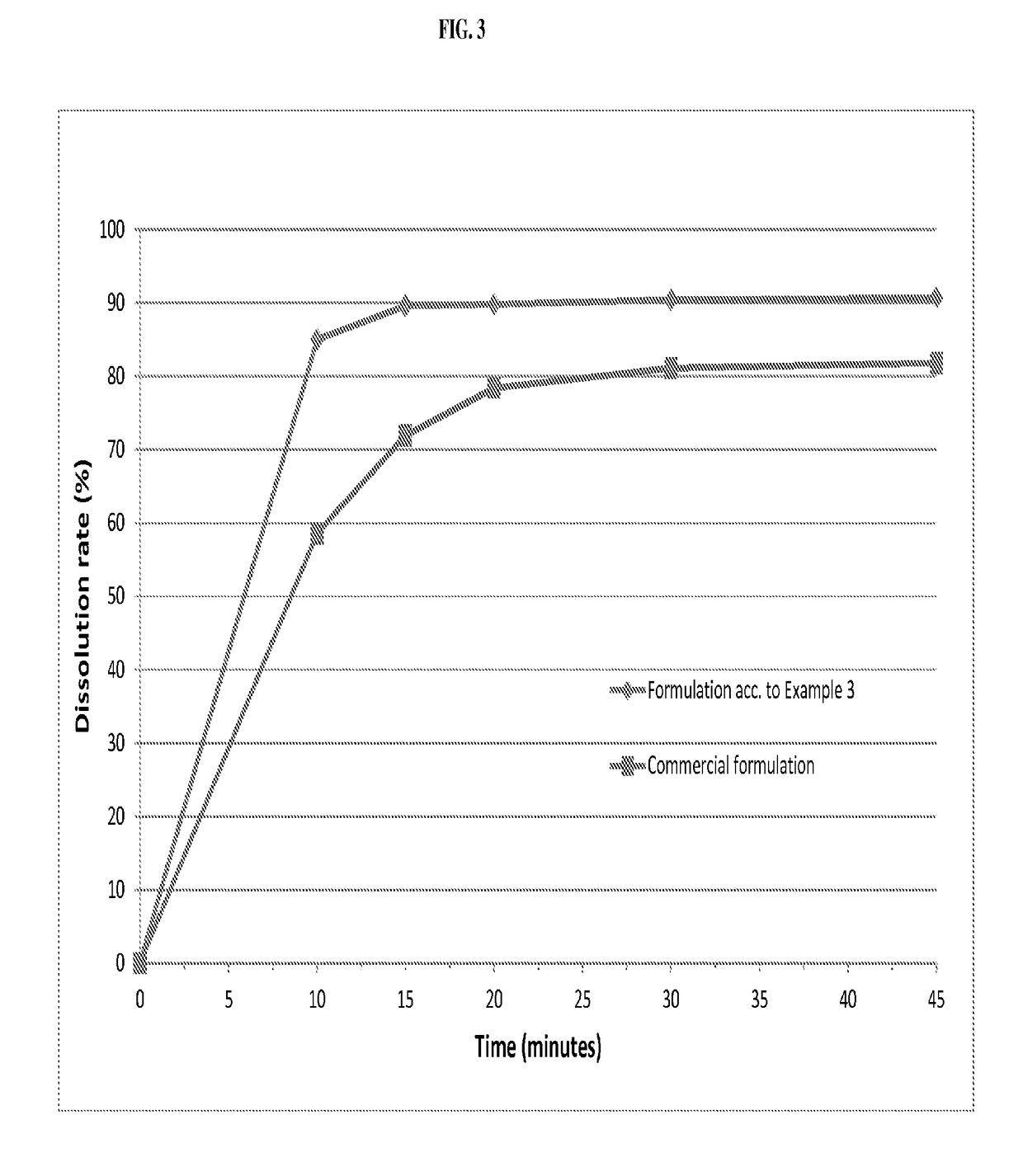
The invention relates to novel solid formulations comprising as pharmaceutically active compound pimobendan and to processes for producing such solid formulations. The invention furthermore relates to a method for manufacturing a medicament for the prevention and / or treatment of congestive heart failure, wherein the solid formulations according to the invention are used.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
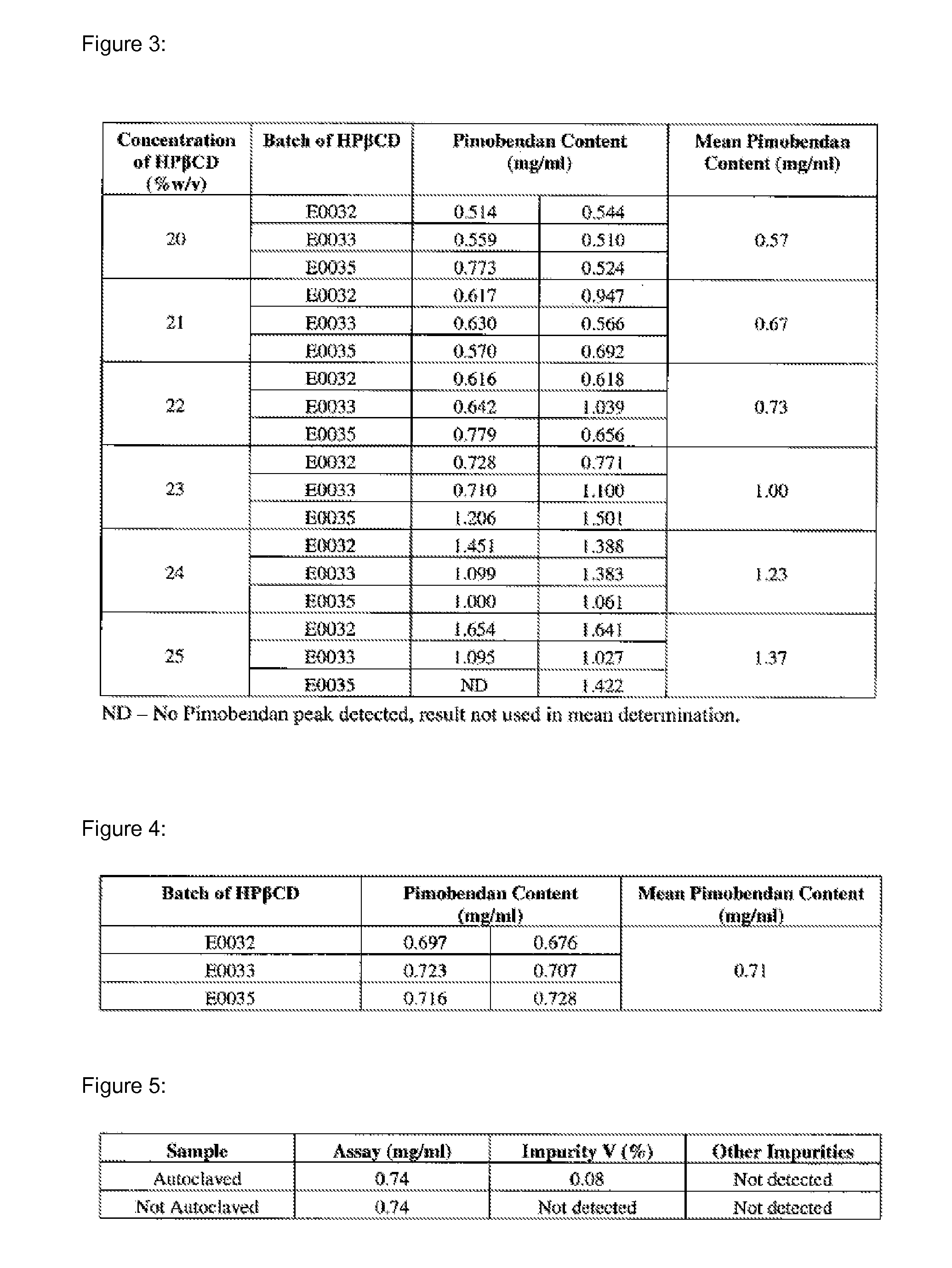
Pharmaceutical compositions of pimobendan
ActiveUS20150150820A1Fully absorbedReduce solubilityPowder deliveryBiocideDissolutionPharmaceutical formulation
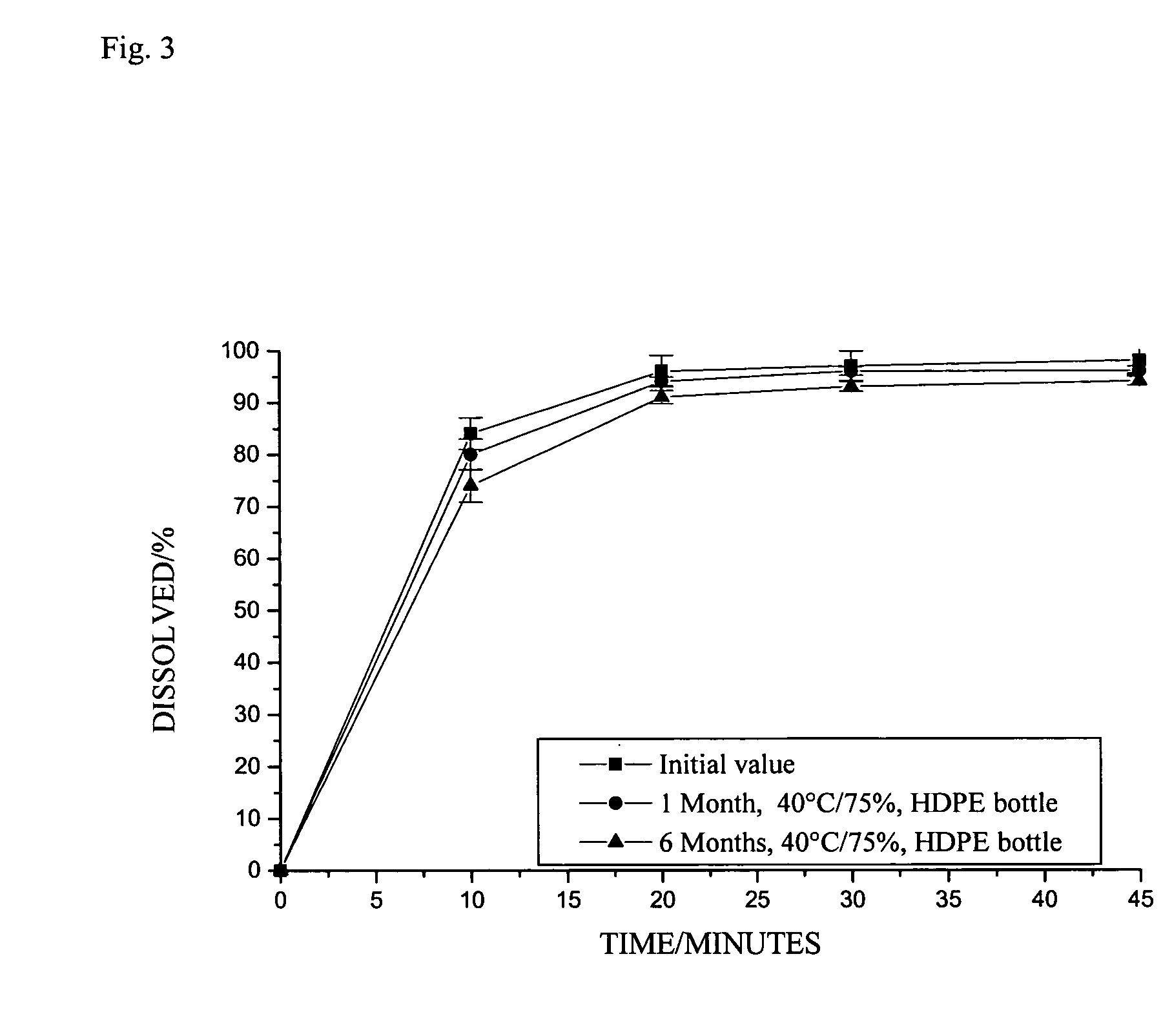
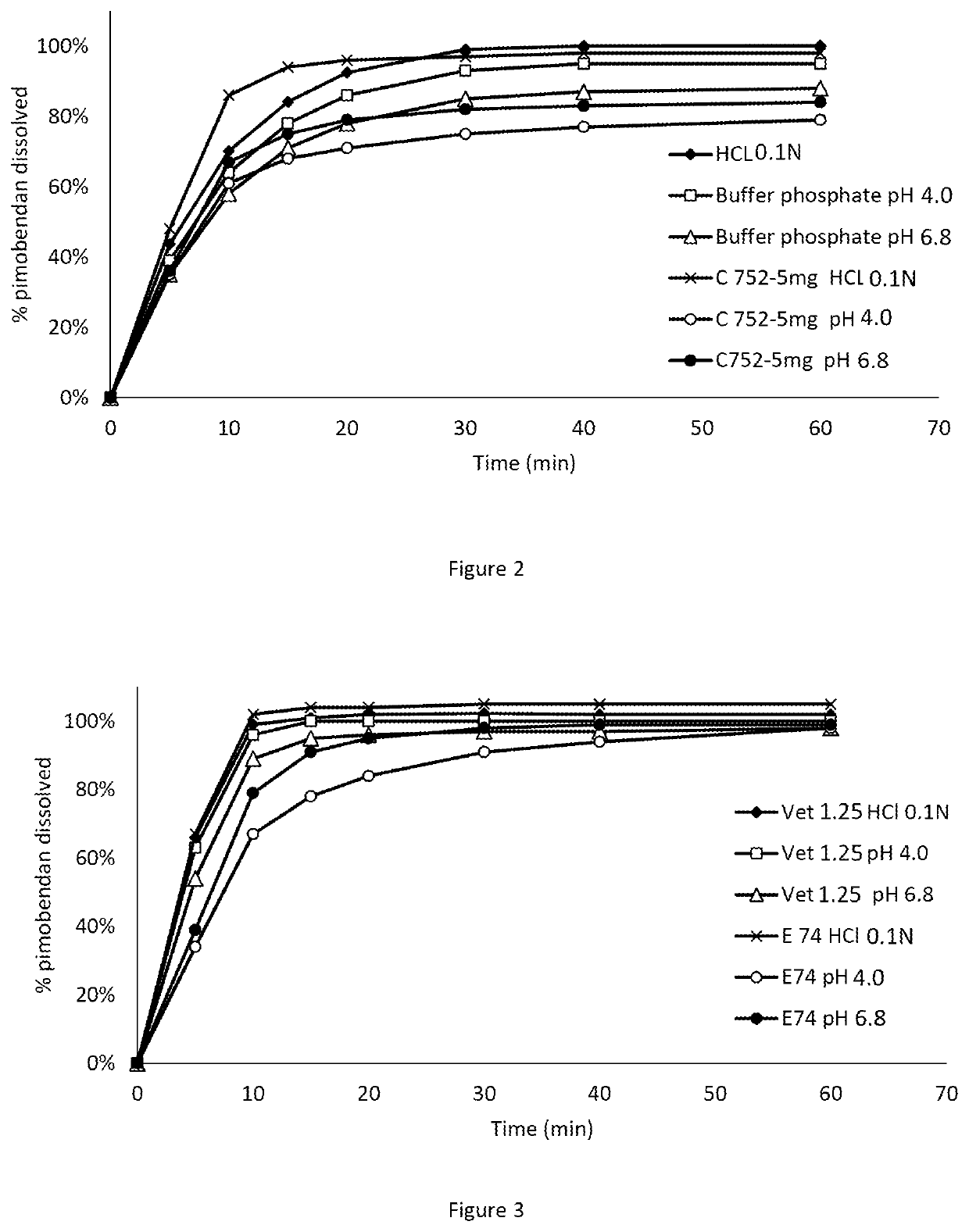
The present invention is directed to a composition comprising particles of pimobendan with an integral coating of a carrier matrix which serve to ensure a rapid dissolution of the active substance at each pH condition representing the gastrointestinal tract and therefore a reliable absorption, and a method of pimobendan microencapsulation using the spray congealing technology and incorporating the coated particles into oral formulations, for example into tablets.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
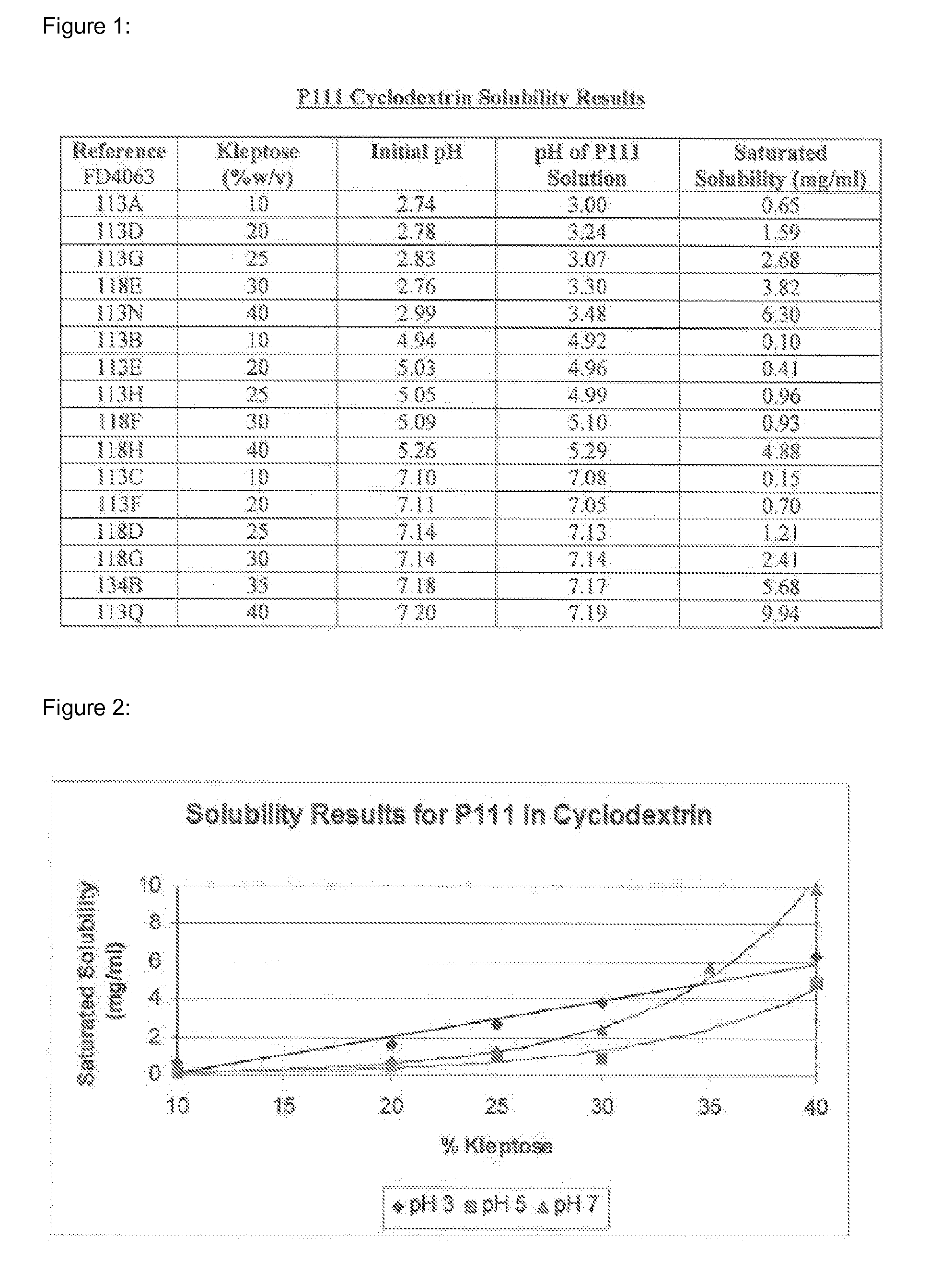
Preserved etherified cyclodextrin derivatives containing liquid aqueous pharmaceutical composition
ActiveUS20150025082A1Inhibit microbial growthRetaining its effectivenessAntibacterial agentsNervous disorderSodium acetrizoateSodium acetate
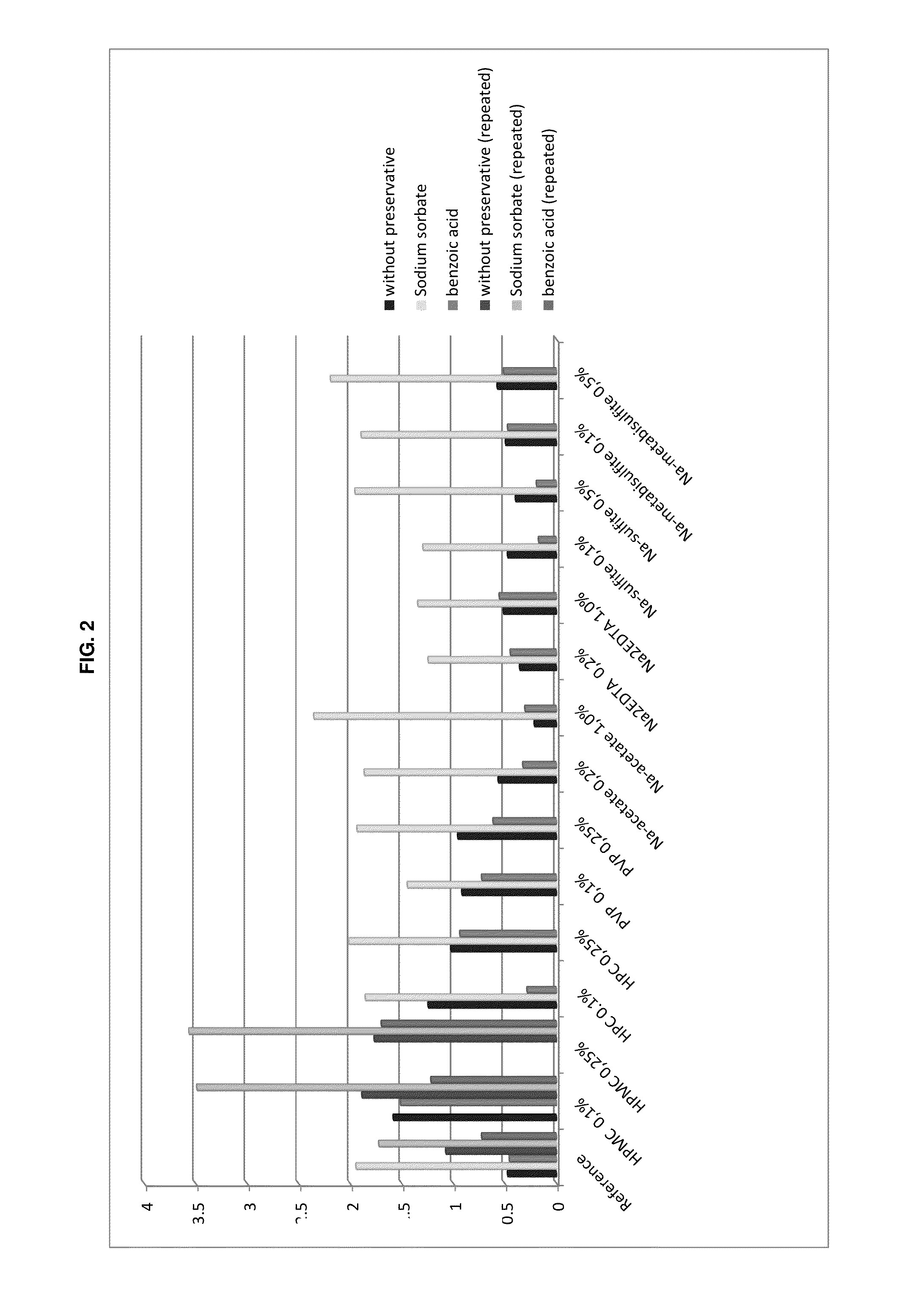
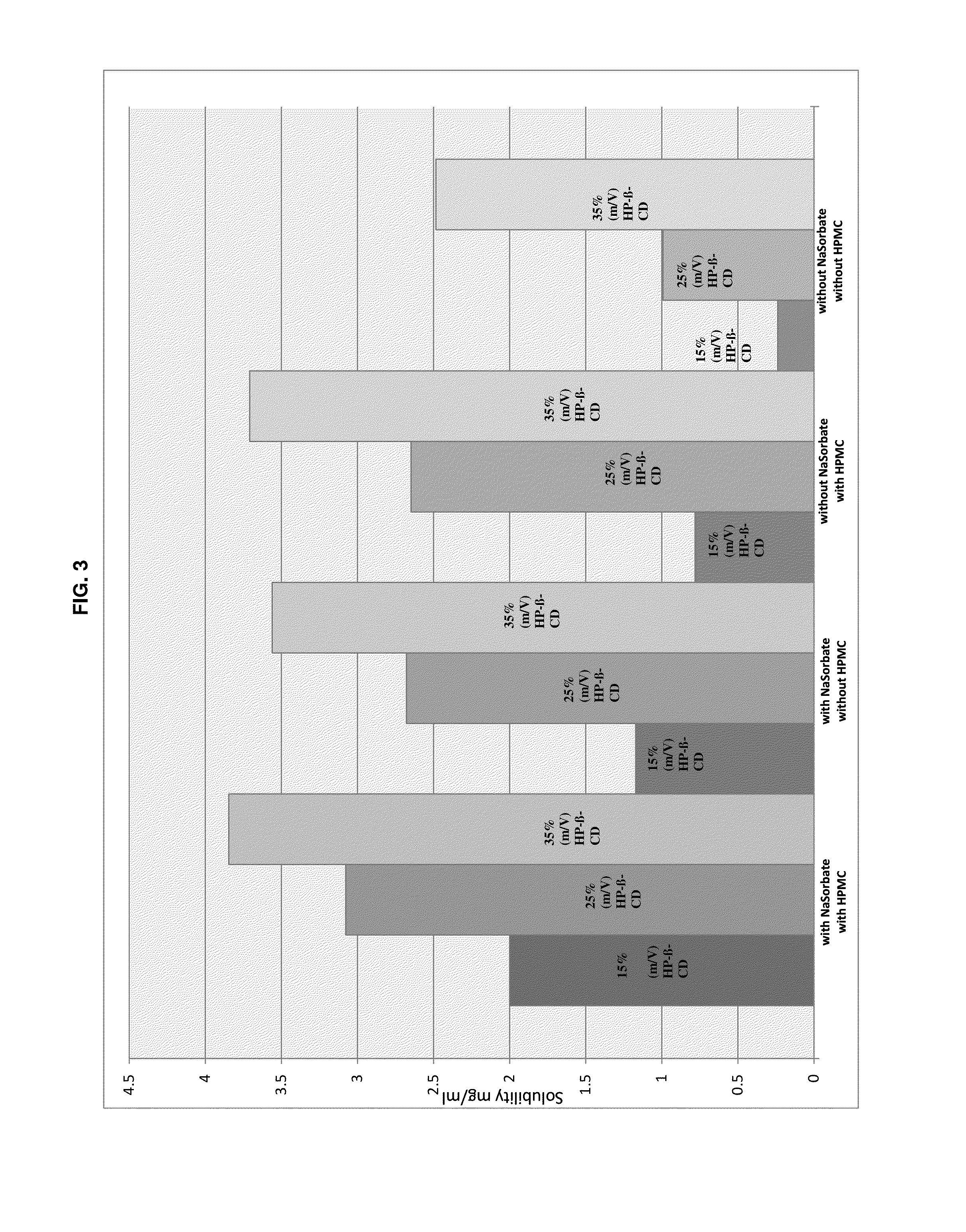
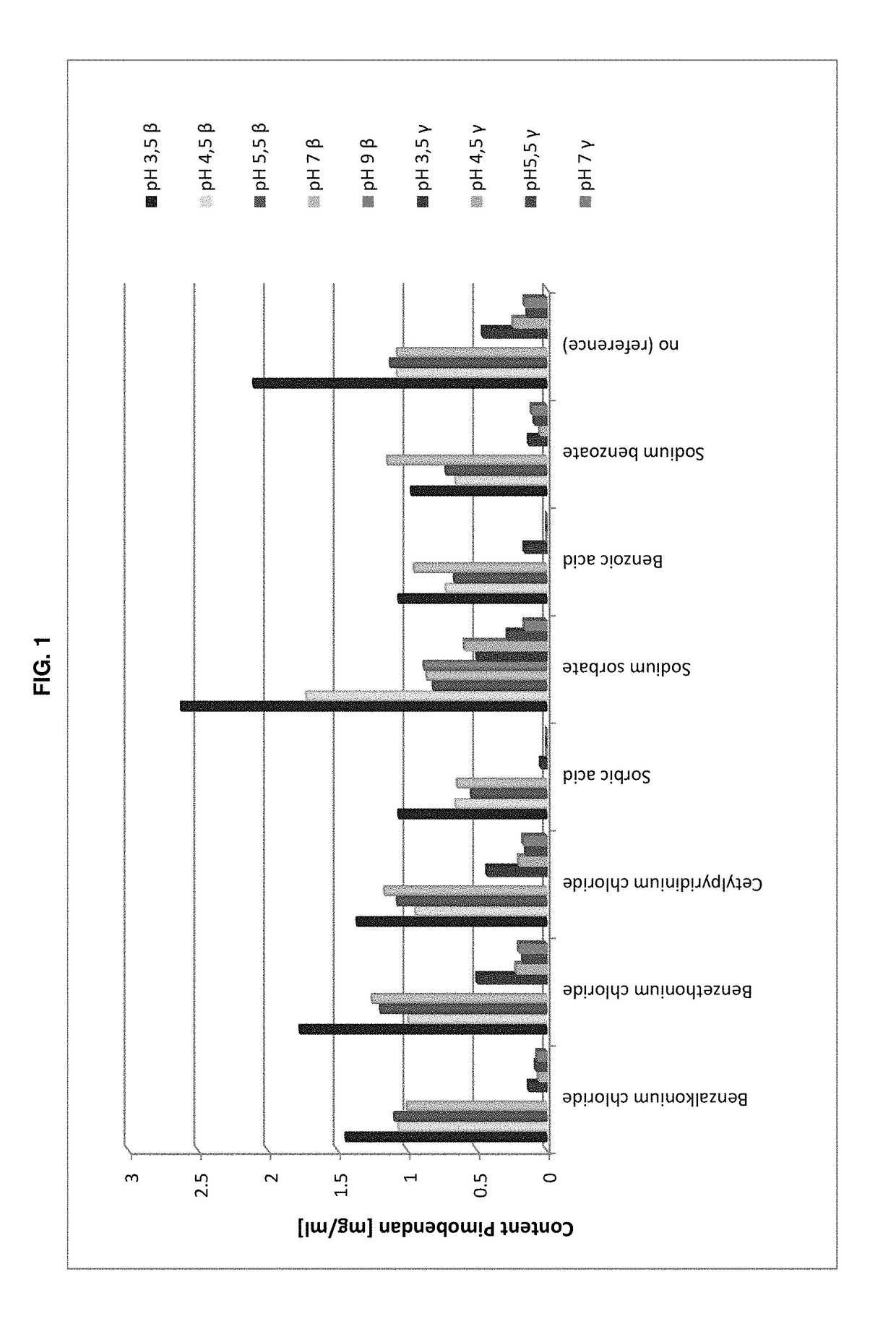
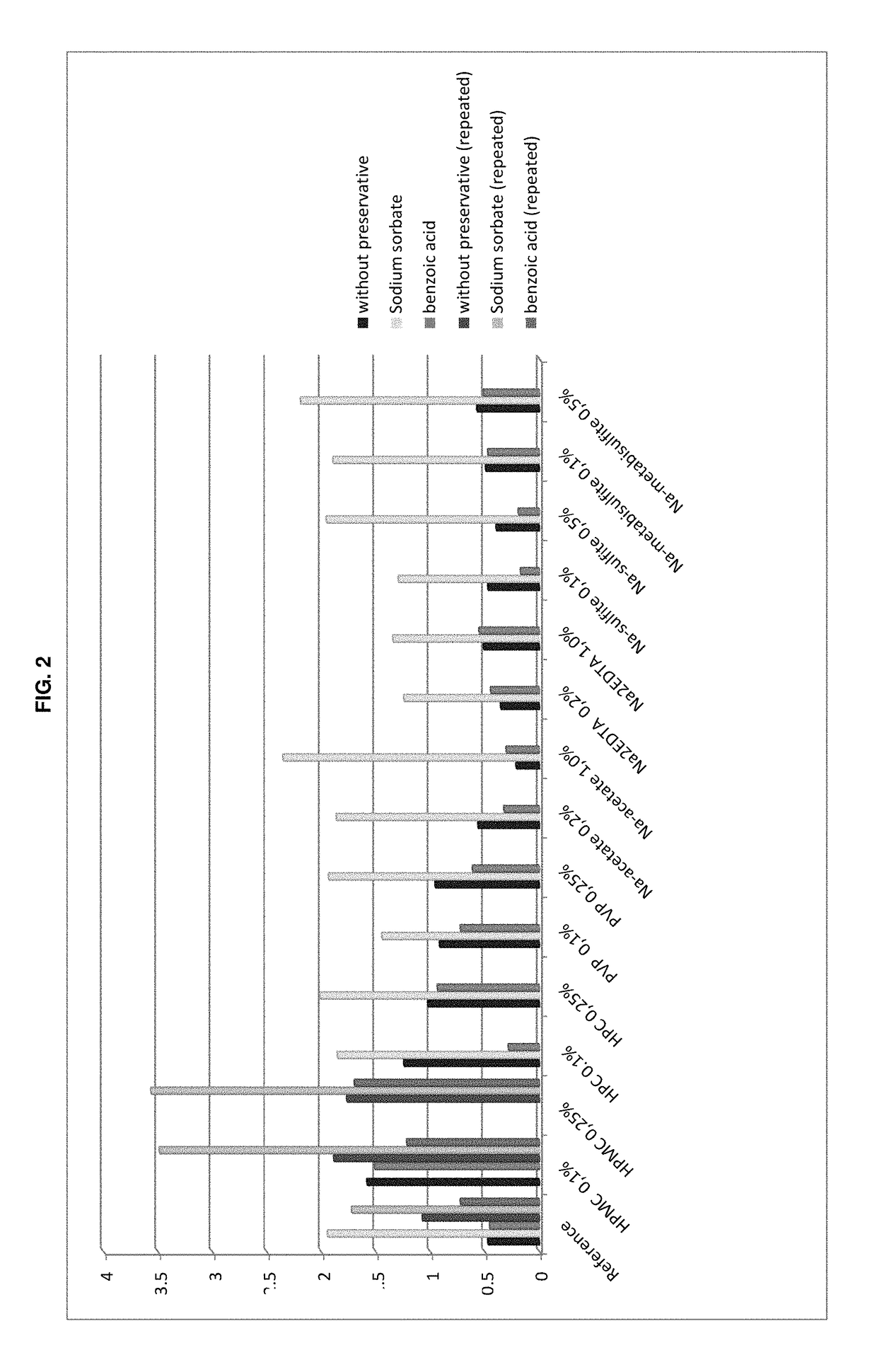
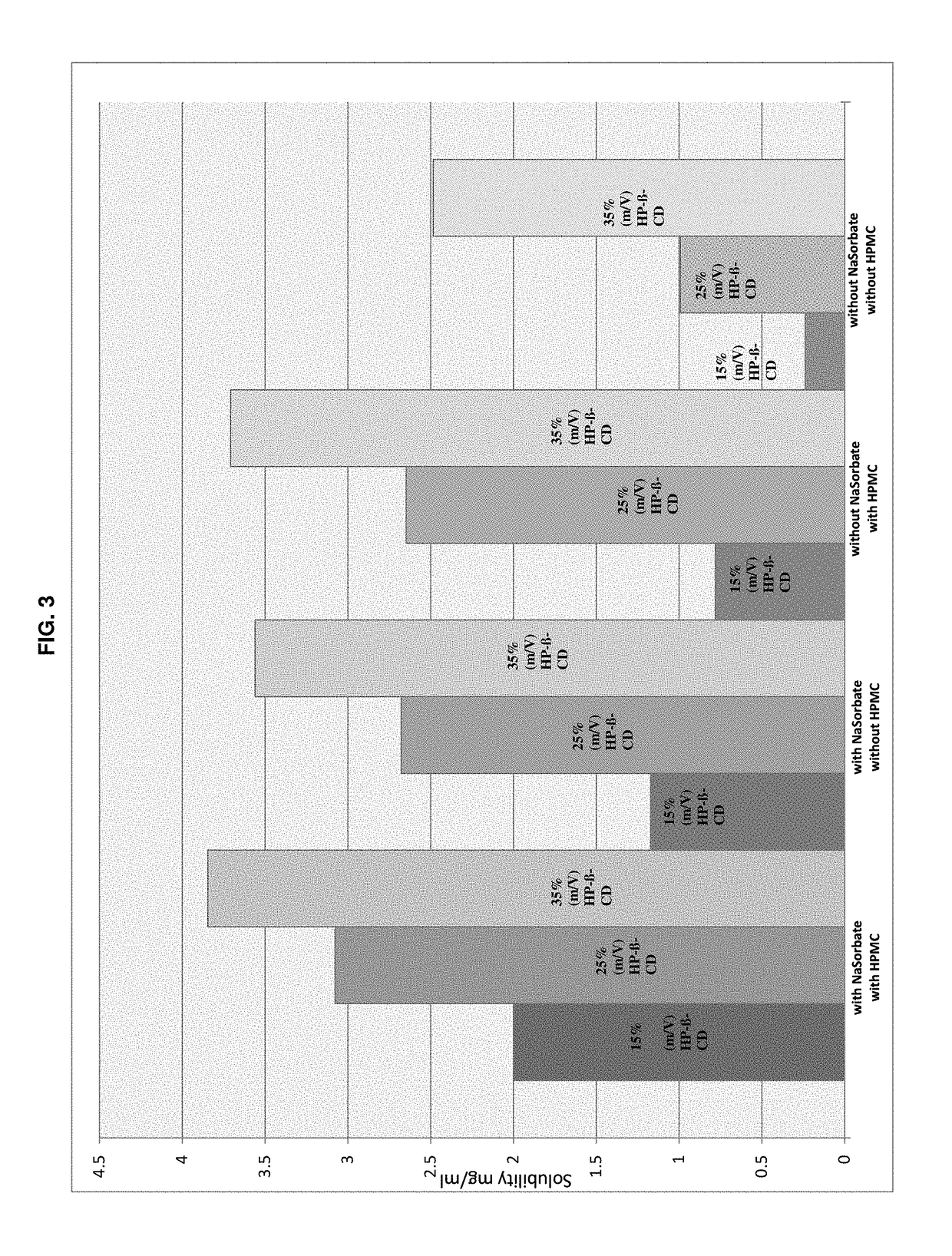
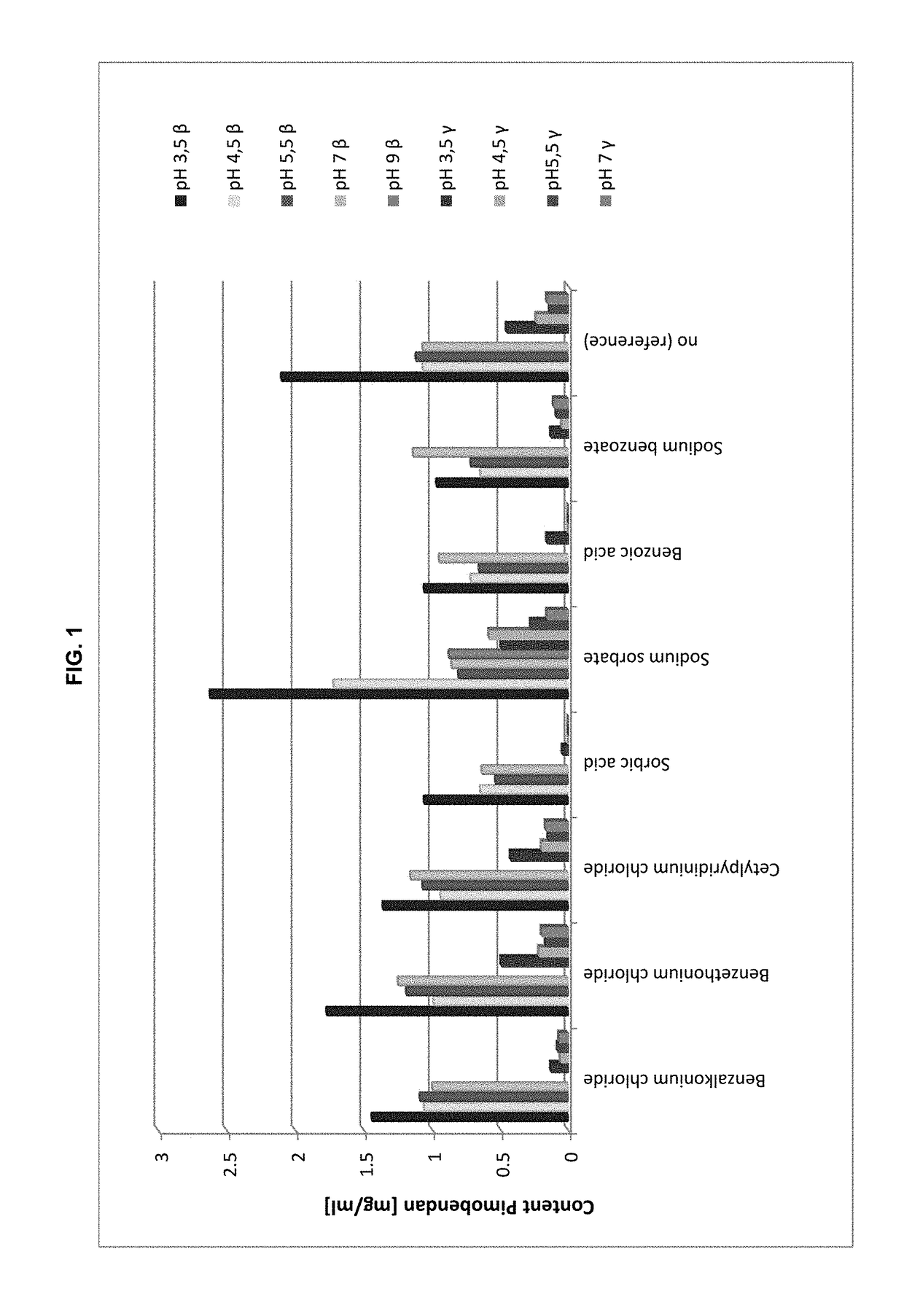
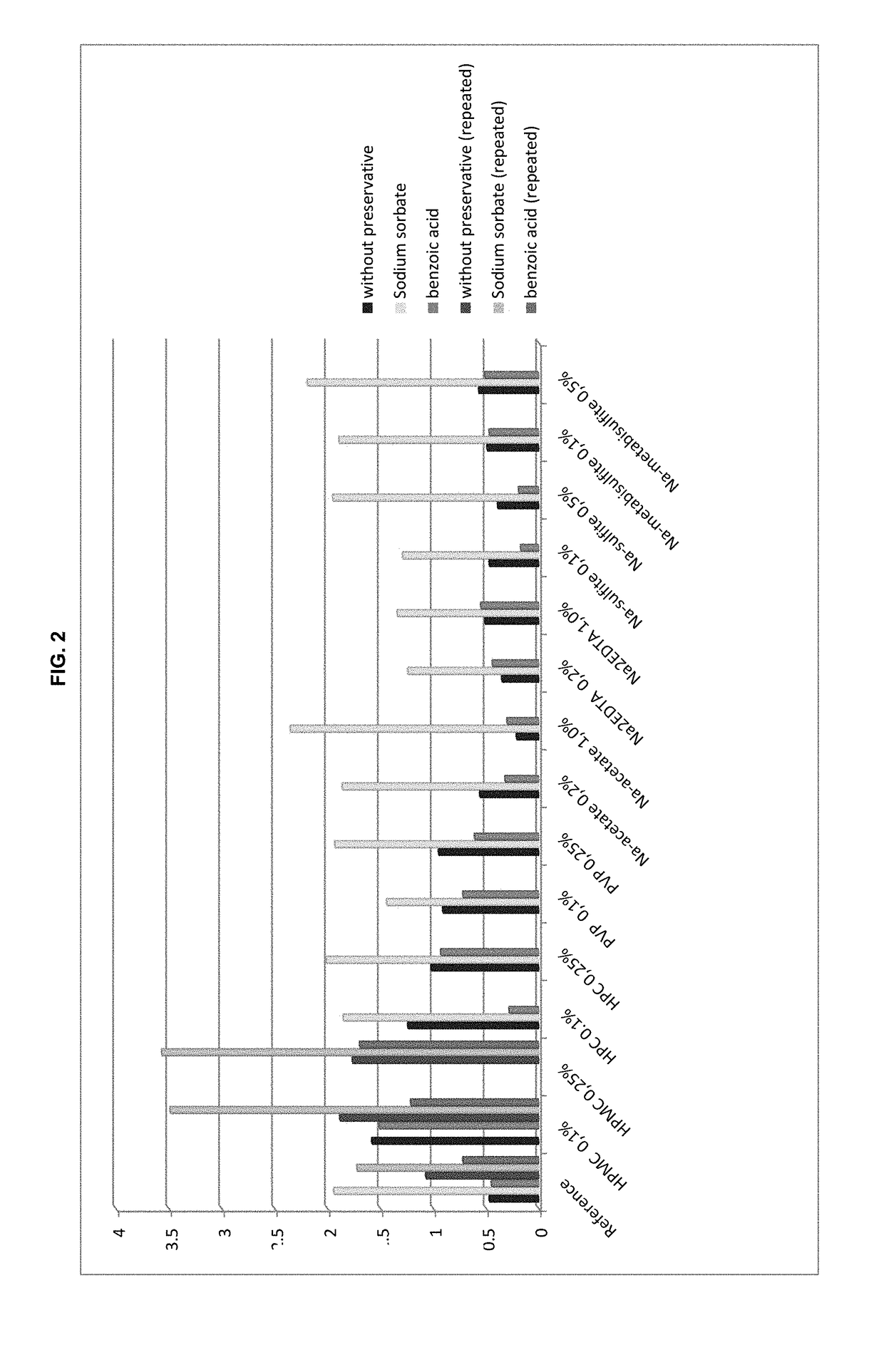
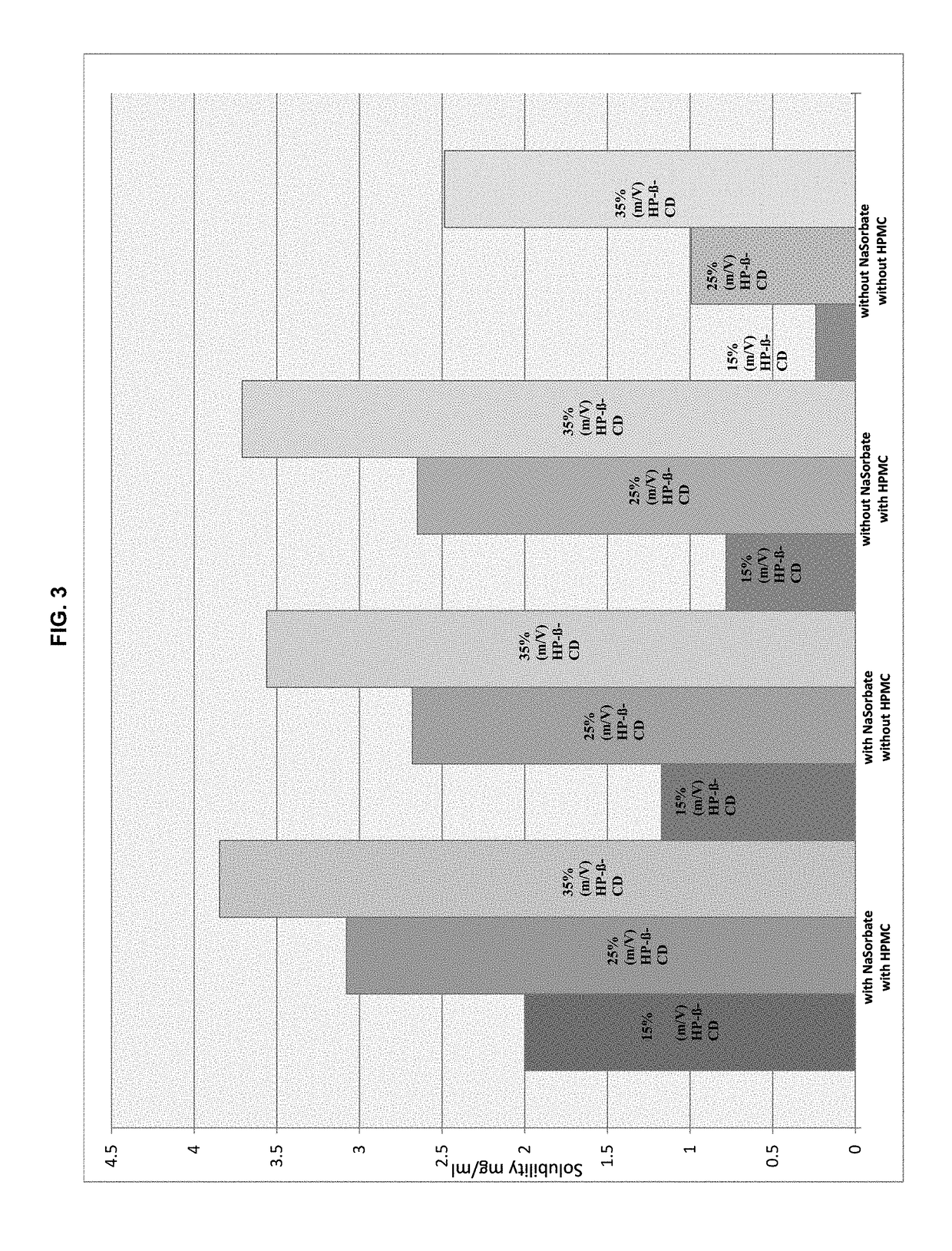
The present invention is directed to a preserved liquid aqueous pharmaceutical composition comprising one or more etherified cyclodextrin derivatives; one or more water-soluble preservatives; preferably selected from the group consisting of sorbic acid or salts thereof, preferably sodium sorbate, potassium sorbate, calcium sorbate; benzoic acid or salts thereof, preferably sodium benzoate; benzalkonium chloride; benzethonium chloride; cetylpyridinium chloride; sodium metabisulfite; sodium acetate; parabenes and salts thereof, preferably methylparabene, ethylparabene, propylparabene, butylparabene, butylparabene sodium; or combinations thereof; and at least one pharmaceutically active compound which is poorly water-soluble, very poorly water-soluble or water-insoluble. The liquid aqueous pharmaceutical composition provides an acceptable solubility of the pharmaceutically active compound, such as pimobendan, in aqueous solution whereby the water-soluble preservatives retain their effectiveness in the presence of the etherified cyclodextrin derivatives allowing the use in an oral administration form.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Use of pimobendan for the reduction of heart size and/or the delay of onset of clinical symptoms in patients with asymptomatic heart failure due to mitral valve disease
ActiveUS20170290829A1Reduction of heart sizeDelay is slowPill deliveryCardiovascular disorderCongestive heart failure chfCanis lupus familiaris
The invention relates to pimobendan for use in a method of reducing the heart size and / or delaying the onset of clinical symptoms in a patient suffering from asymptomatic (occult, preclinical) heart failure, preferably congestive heart failure, due to mitral valve disease (MVD), and / or delaying the onset of heart failure, preferably congestive heart failure, in a patient suffering from asymptomatic (occult, preclinical) heart failure, preferably congestive heart failure, due to mitral valve disease (MVD), wherein the patient is preferably a mammal, more preferably a human, a dog, a cat or a horse, and most preferably a dog.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Liquid preparation comprising pimobendan
A liquid preparation includes an etherified cyclodextrin derivative and a substituted benzimidazol, where the etherified cyclodextrin derivative is one of alpha-, beta, and gamma-cyclodextrin ether.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Chemical synthesis method of Pimobendan
InactiveCN106518850ALittle pollutionSuitable for industrial productionOrganic chemistryChemical synthesisHydrazine compound
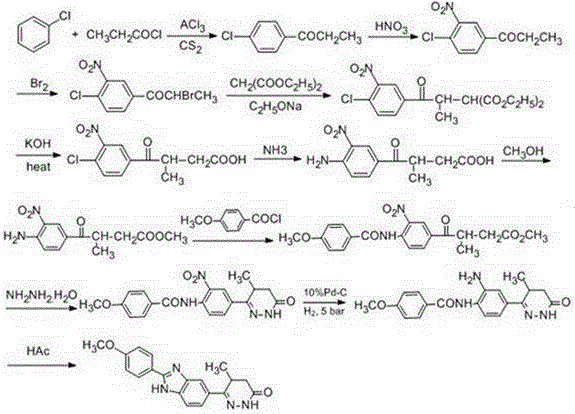
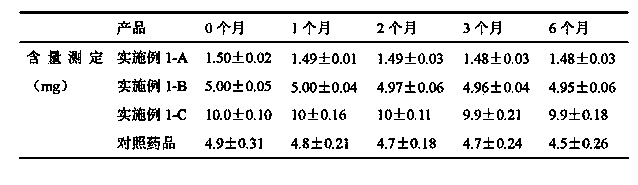
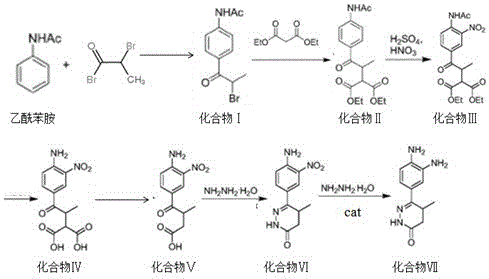
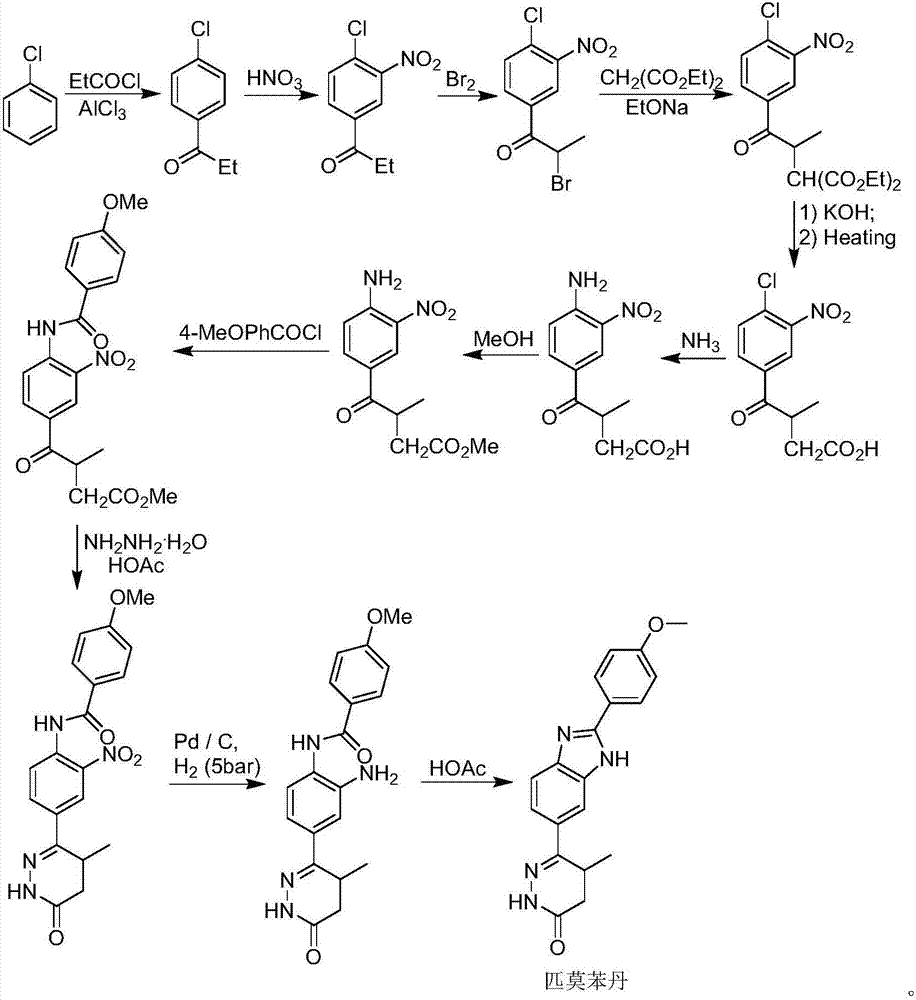
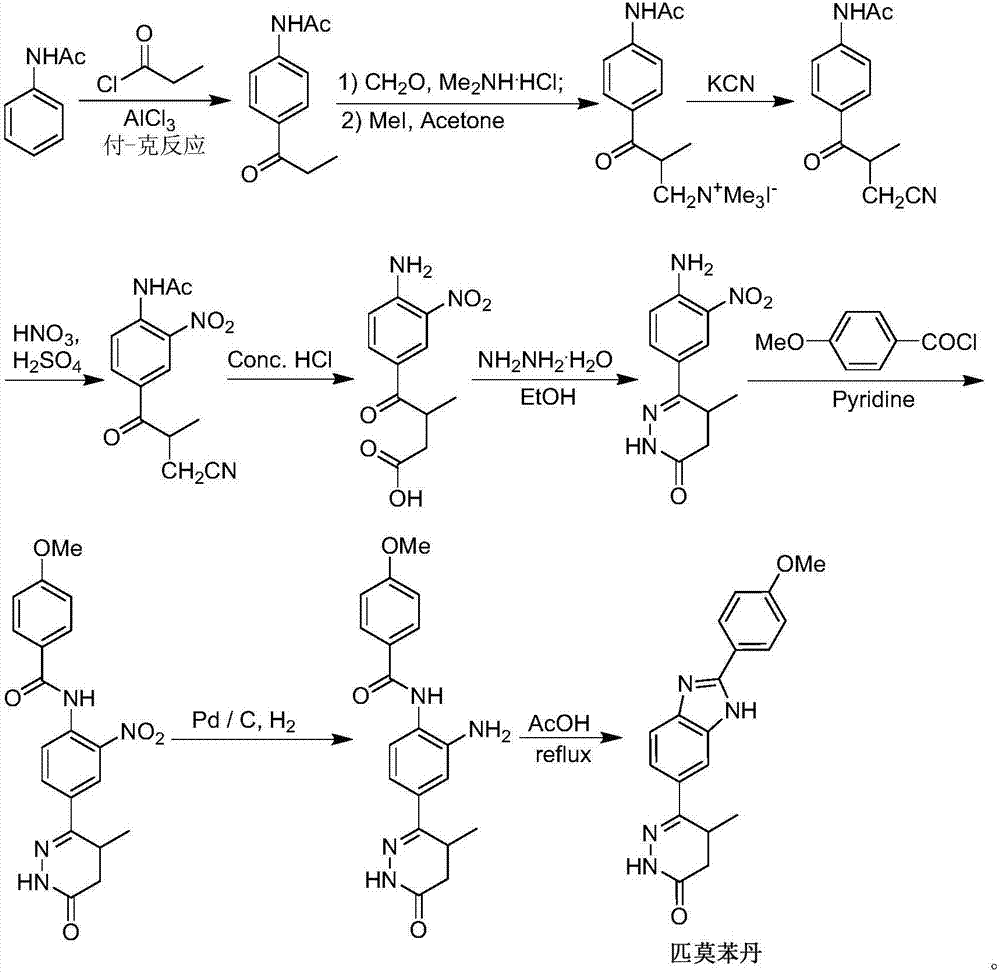
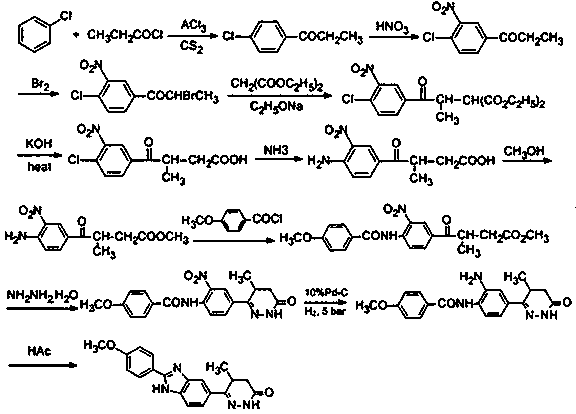
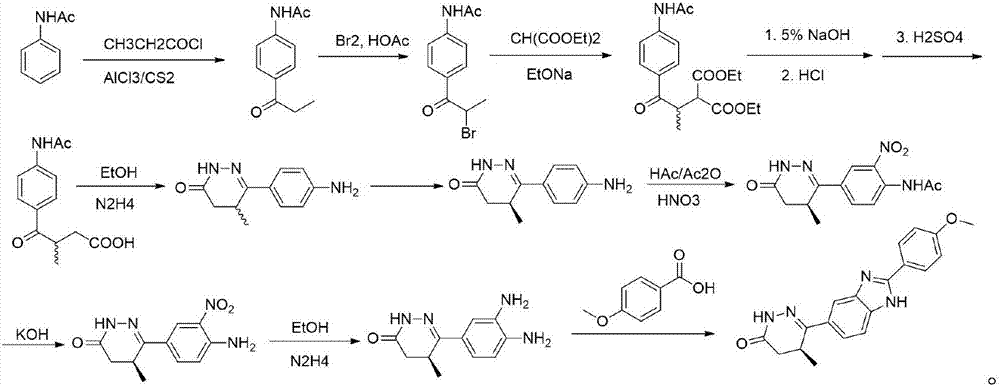
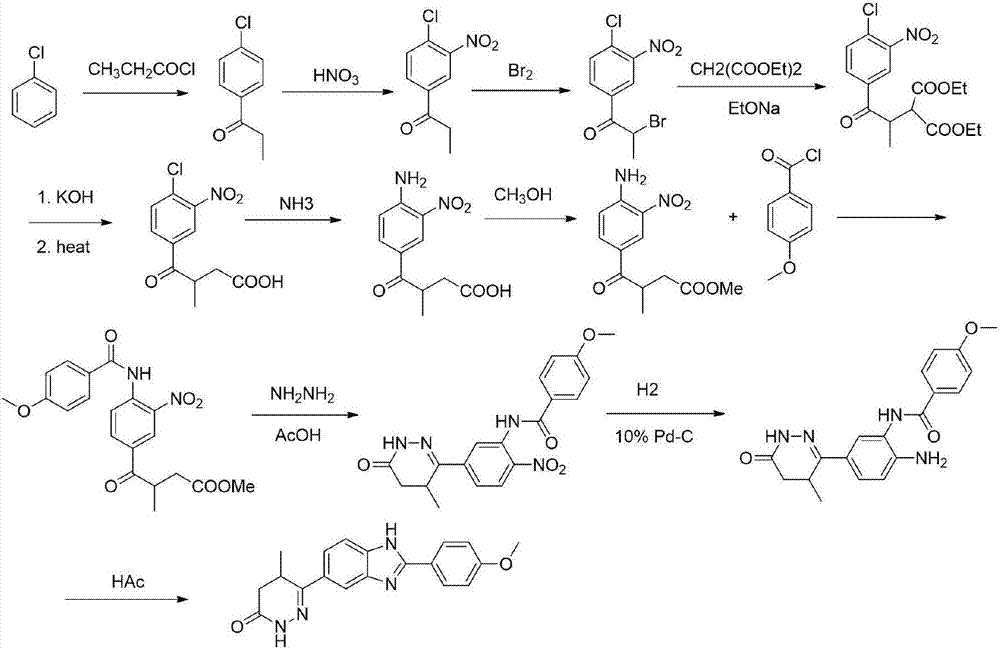
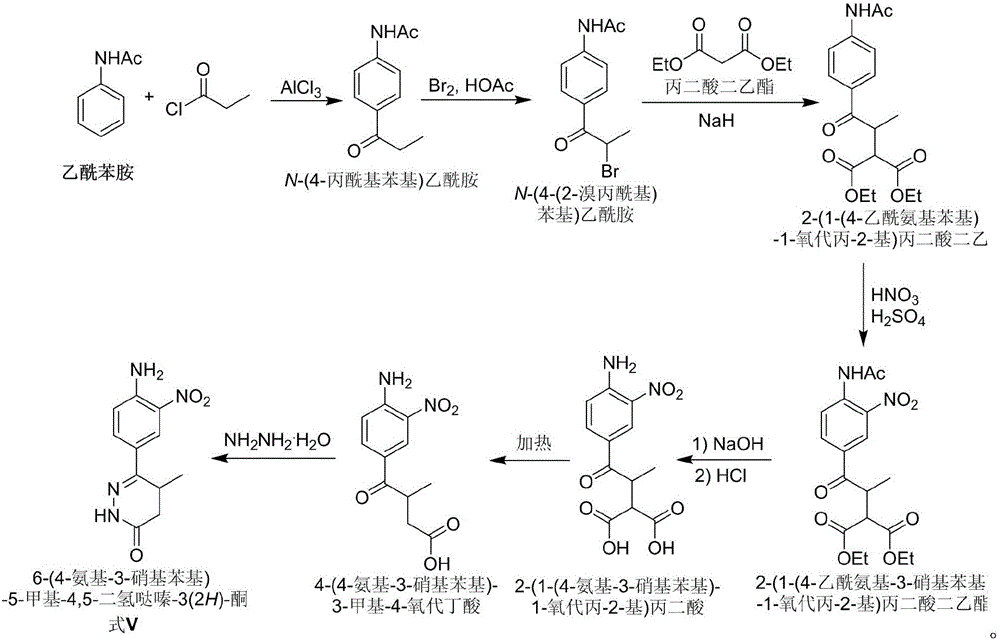
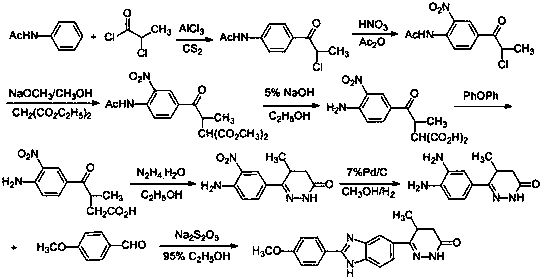
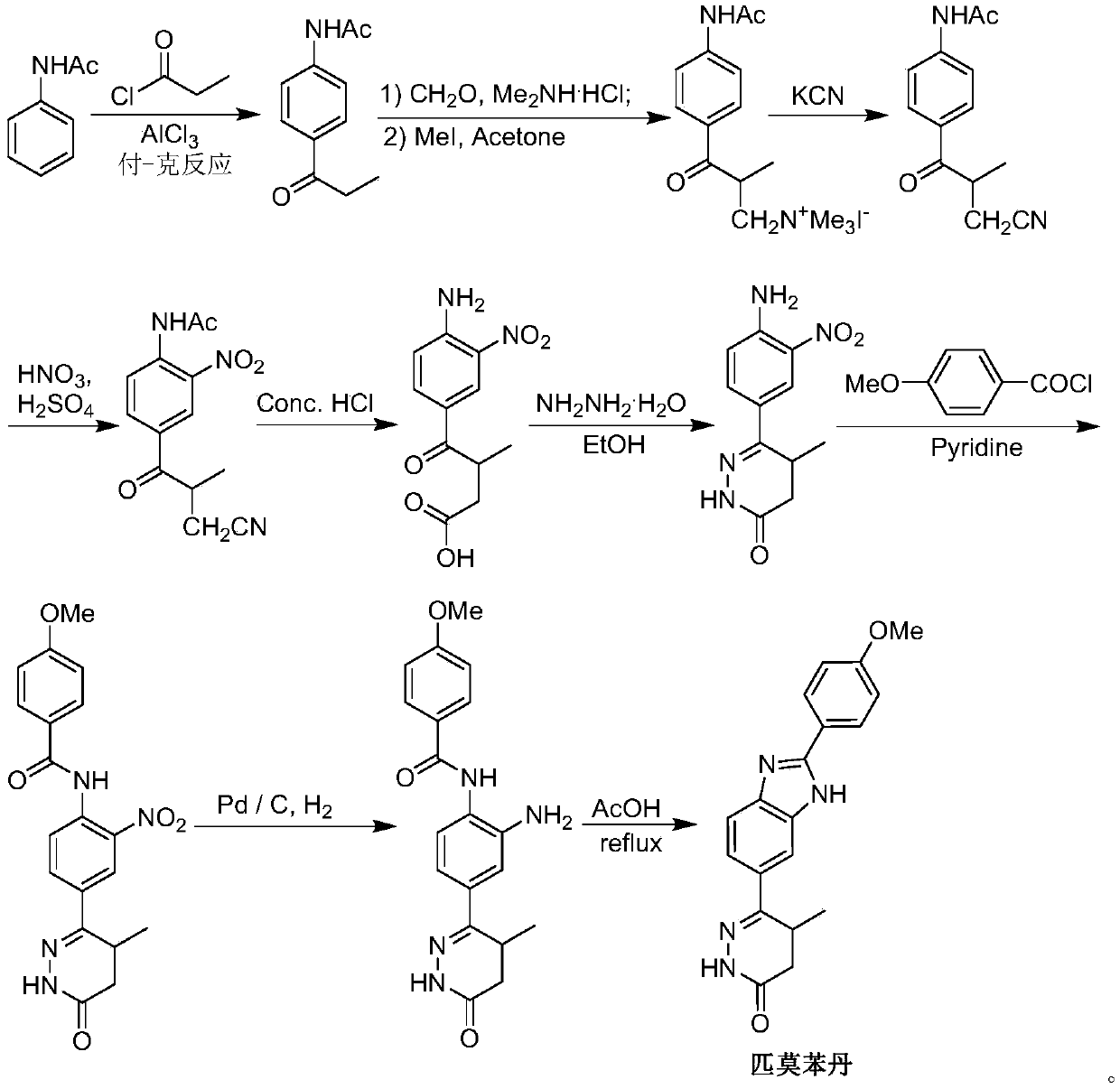
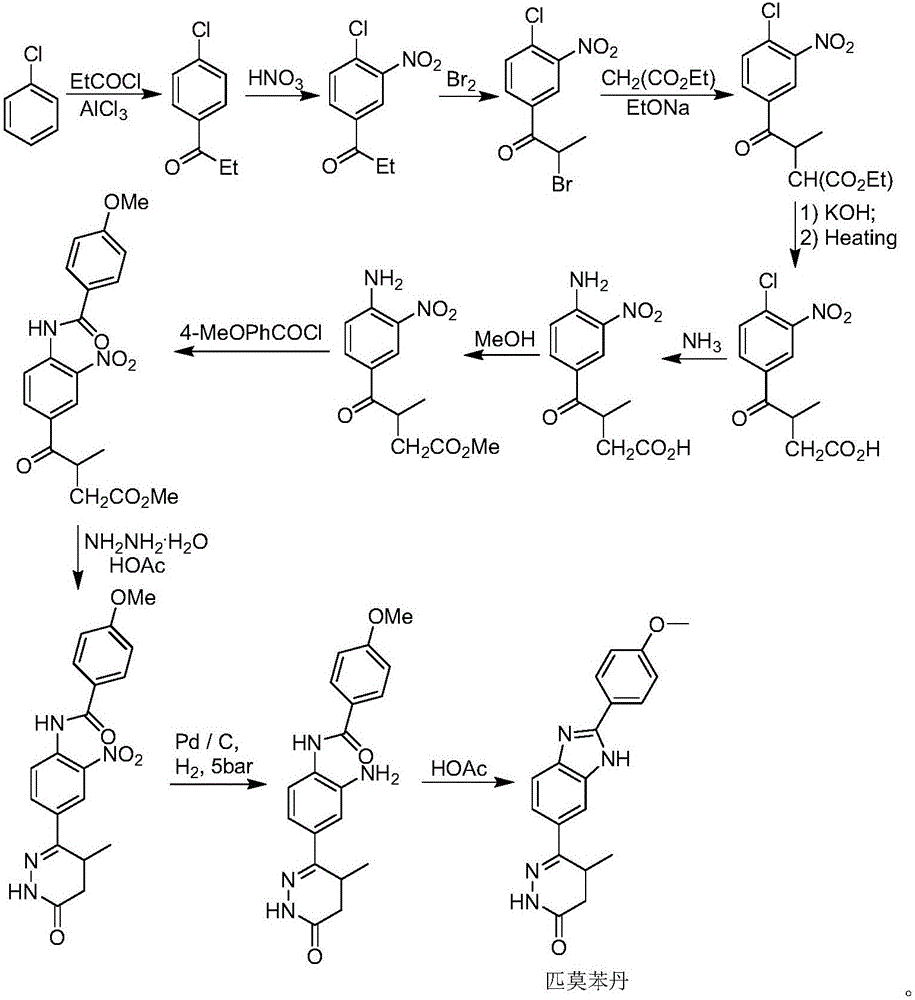
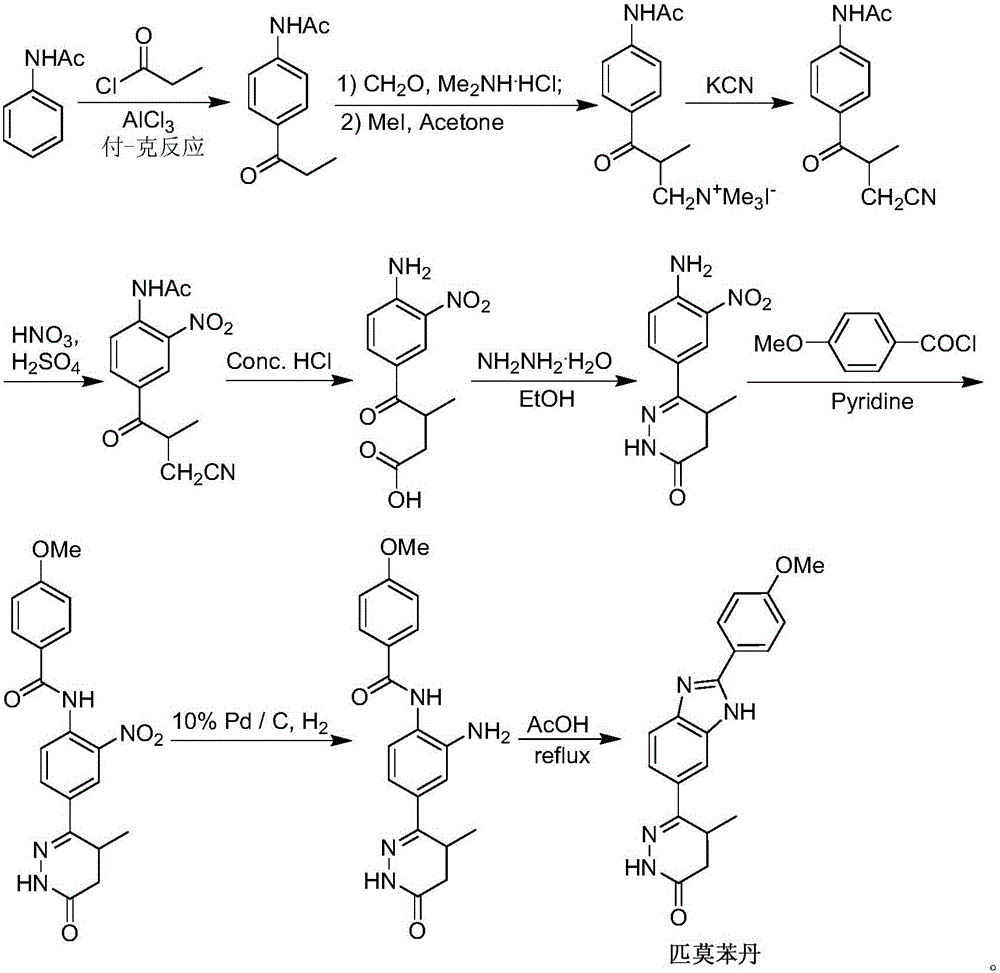
The invention discloses a chemical synthesis method of Pimobendan. The method comprises steps as follows: acetanilide and 2-methyl-3-(chloroformyl)propionate react to produce 3-p-acetylaminobenzoyl methyl butyrate in a mixed organic solvent under the action of a composite catalyst, 3-p-acetylaminobenzoyl methyl butyrate reacts with a nitration reagent, 3-(4-acetamido-3-nitrobenzoyl)-methyl butyrate is produced and subjected to a reflux reaction with alkali in an alcohol solvent to produce 3-(4-amino-3-nitrobenzoyl) butyrate, 3-(4-amino-3-nitrobenzoyl) butyrate and hydrazine hydrate are subjected to a reflux reaction, 4,5-dihydro-5-methyl-6-(4-amino-3-nitrophenyl)-3(2H)-pyridazinone is produced and reduced with zinc powder in absolute methanol, 4,5-dihydro-5-methyl-6-(3,4-diaminophenyl)-3(2H)-pyridazinone is produced and subjected to a reflux reaction with p-anisaldehyde, and Pimobendan is obtained. The method comprises a few reaction steps, operation is safe and convenient, and the cost is low.
Owner:QINGDAO AGRI UNIV
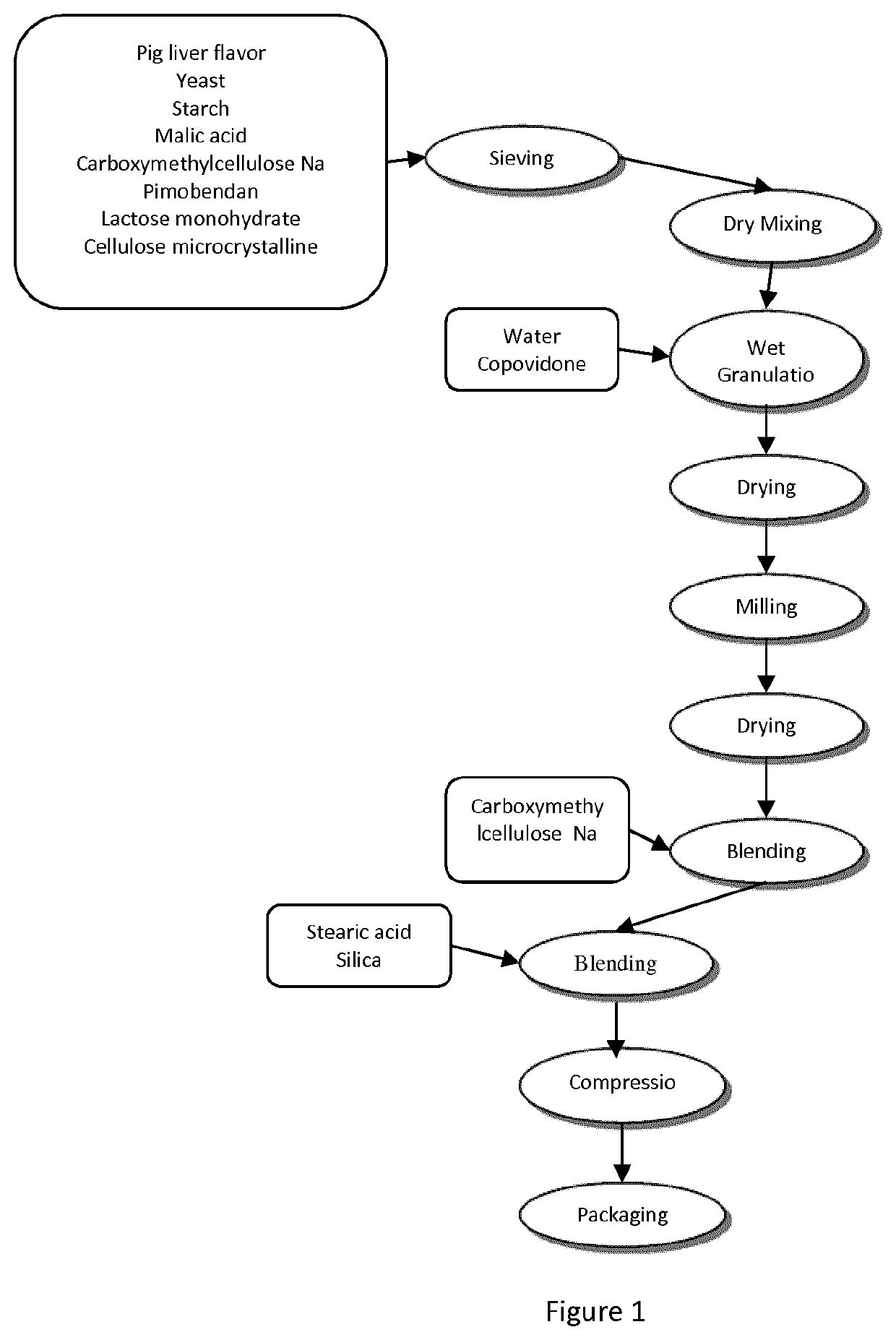
Pimobendan sustained-release tablets for pet and preparation method thereof
InactiveCN103655502AImprove palatabilityReduce excessive volatilityOrganic active ingredientsPharmaceutical delivery mechanismAdhesiveCurative effect
The invention discloses pimobendan sustained-release tablets for a pet and a preparation method thereof. The pimobendan sustained-release tablets disclosed by the invention are stable and safe to use and consist of a solid dispersion of pimobendan, a sustained-release material, a filling agent, an adhesive, a lubricant and a flavoring agent. The pimobendan sustained-release tablets disclosed by the invention have the characteristics of convenience in administration, lasting action and stable curative effect.
Owner:RINGPU TIANJIN BIOLOGICAL PHARMA
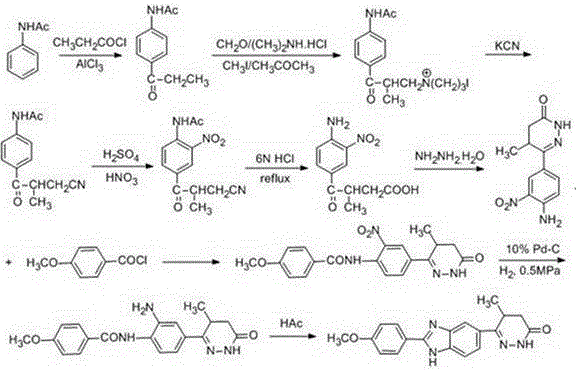
Preparation method of key intermediate of Pimobendan
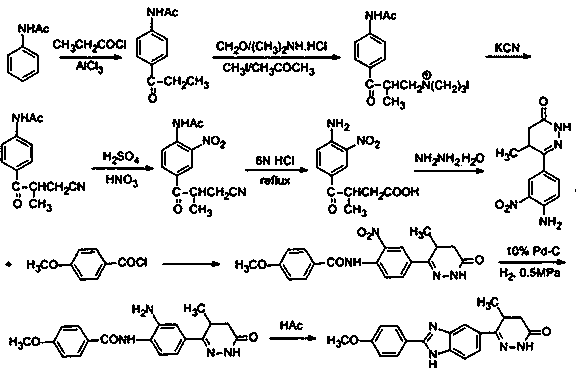
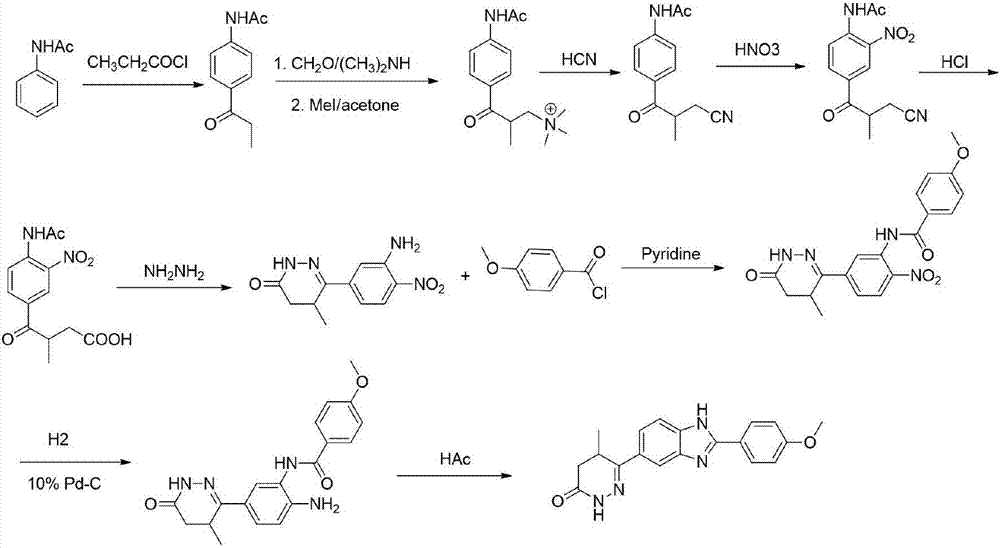
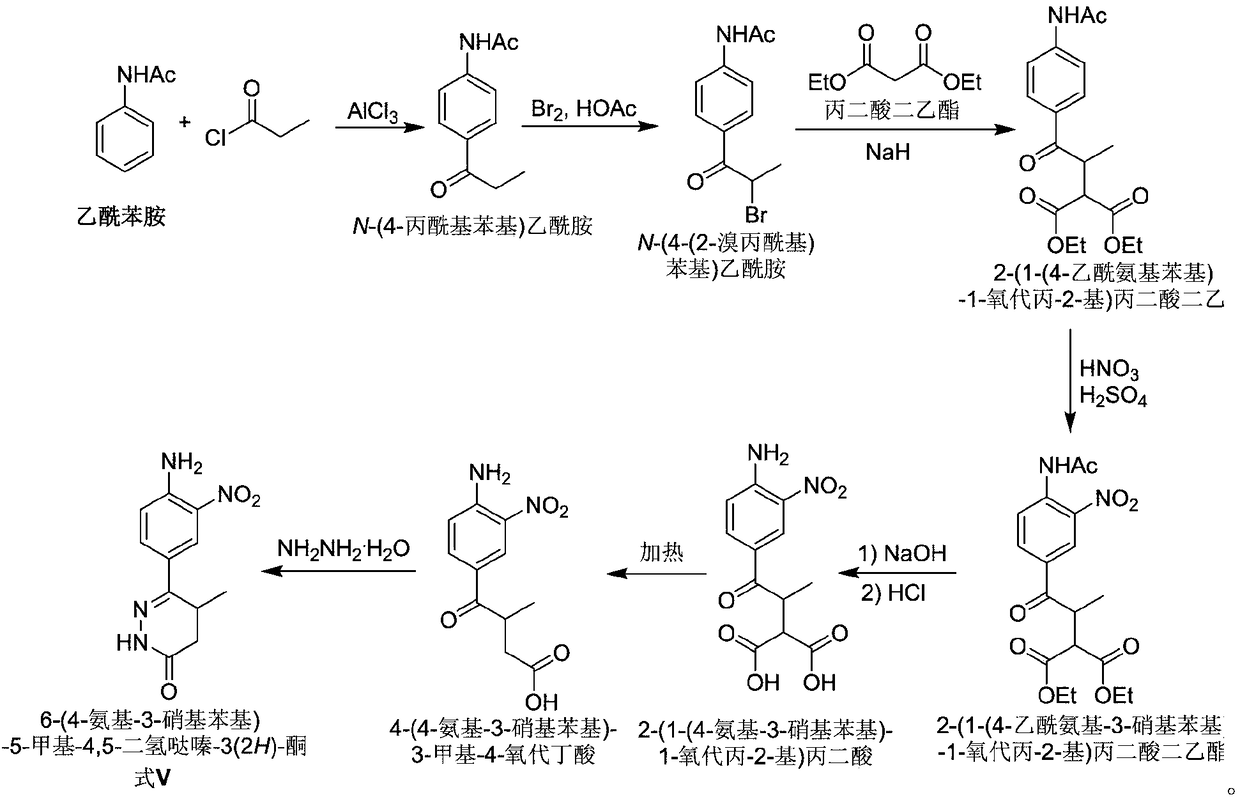
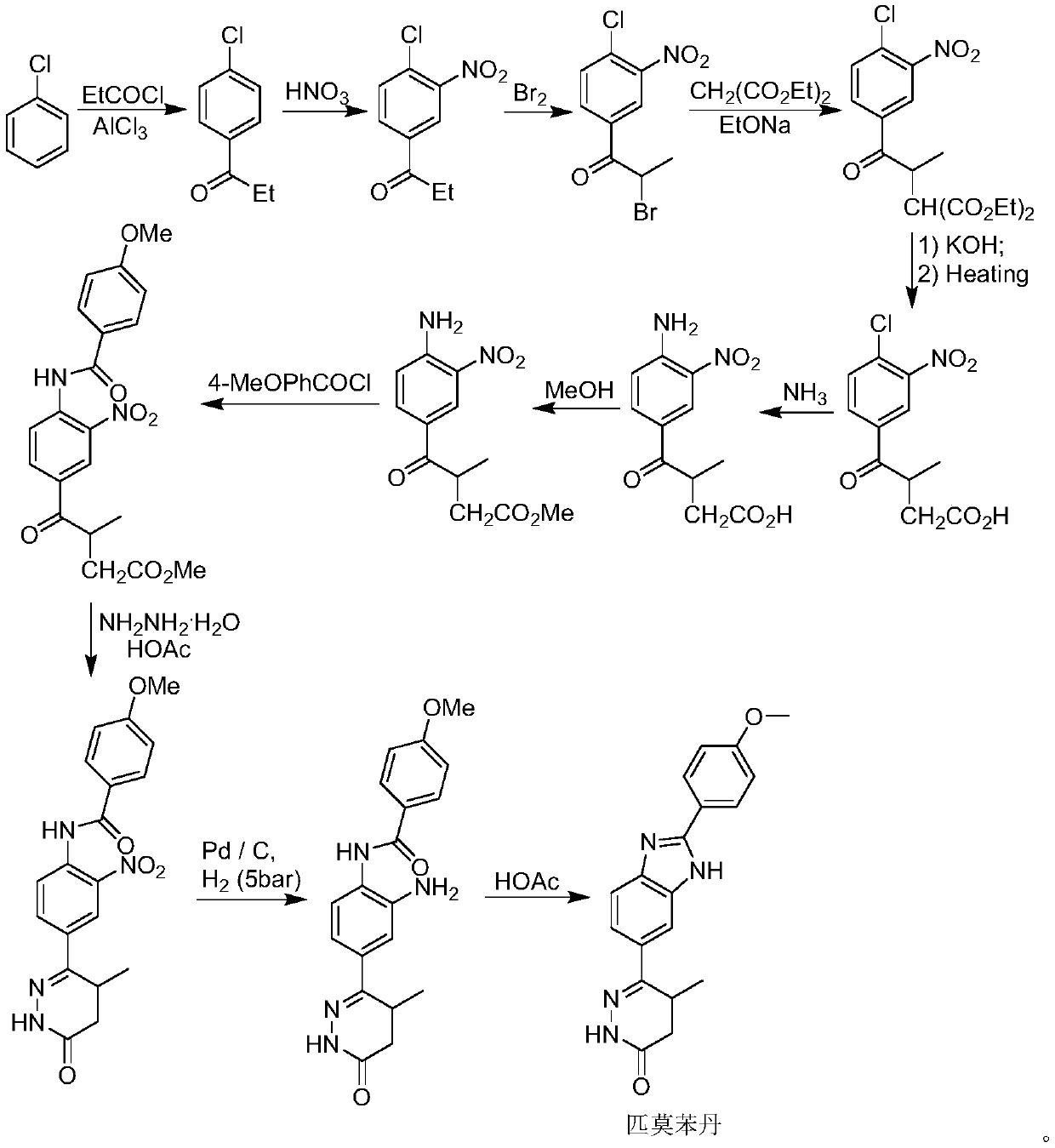
The invention discloses a preparation method of a key intermediate of Pimobendan. The method comprises steps as follows: (i) a compound I is synthesized; (ii) the compound I is subjected to nucleophilic substitution, and a compound II is produced; (iii) the compound II is subjected to a nitration reaction, and a compound III is produced; (iv) the compound III is subjected to a hydrolysis reaction, and a compound IV is produced; (v) the compound IV is subjected to a decarboxylation reaction, and a compound V is produced; (vi) the compound V and a hydrazine hydrate solution react, and a compound VI is produced; (vii) the compound VI and a hydrazine hydrate solution react, and a compound VII is produced. The invention provides the preparation method of the key intermediate of Pimobendan, namely, 4,5-dihydro-5-methyl-6-(3,4-diaminophenyl)-3(2H)pyridazinone. A few reaction steps are used, highly toxic and high-corrosivity raw materials such as liquid bromine, potassium cyanide and the like are not required to be used, and the method has low environmental pollution and facilitates industrial amplification.
Owner:天津长芦华信化工股份有限公司
Preparation of pimobendan
The invention relates to a novel preparation process of pimobendan. The process comprises short steps, does not refer to dangerous and highly toxic reagents such as Br2, KCN and NaH which have limitation in wide industrial use, is simple in operation and mild in reaction conditions and has obvious superiorities in industrial production.
Owner:WISDOM PHARM CO LTD +1
Pharmaceutical tablet formulation for the veterinary medical sector, method of production and use thereof
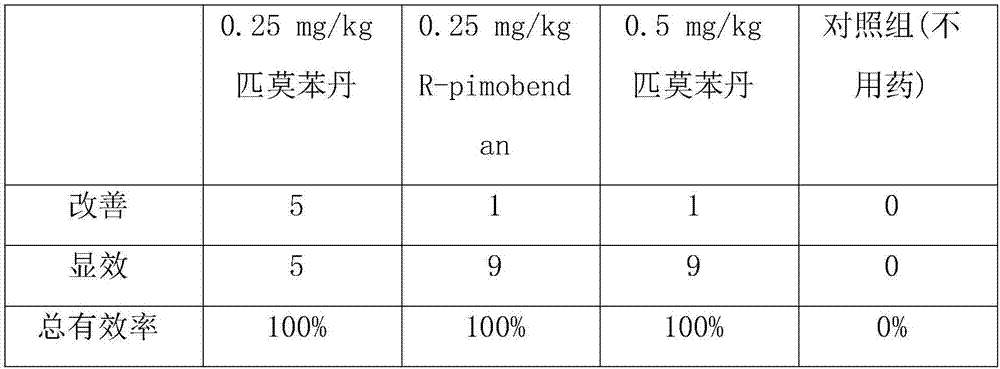
The invention is directed to a pharmaceutical tablet formulation for the veterinary medical sector containing an instable ACE inhibitor or a pharmaceutically acceptable salt thereof as a first pharmaceutically active substance, and pimobendan or a pharmaceutically acceptable salt thereof as a second pharmaceutically active substance, comprising granules which contain carrier core particles coated with at least one layer wherein the first pharmaceutically active substance is present, the granules being embedded in a tablet matrix wherein the second pharmaceutically active substance is present. It is provided a “fixed-dose-combination” which allows to ease the treatment and administration of the medication, improves the medication compliance by reducing the pill burden to the animal holder and enables the better observation of and adherence to the therapy by decreasing the number of tablets to be administered. The lower number of tablets leads to a lower treatment failure rate, minimizes dosage mistakes and avoids confusions by false dose intake and slower development of resistance.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
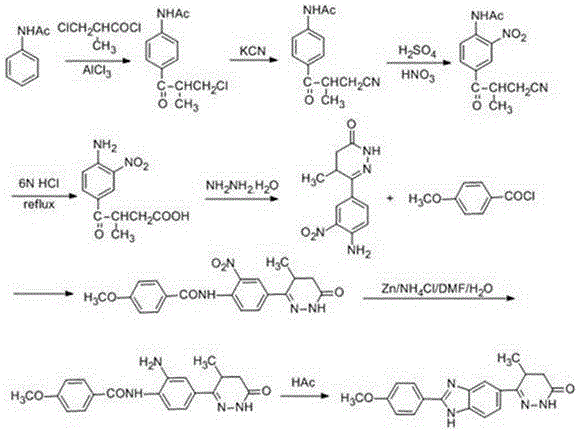
A kind of chemical synthesis method of pimobendan
InactiveCN106518850BLittle pollutionSuitable for industrial productionOrganic chemistryChemical synthesisNitration
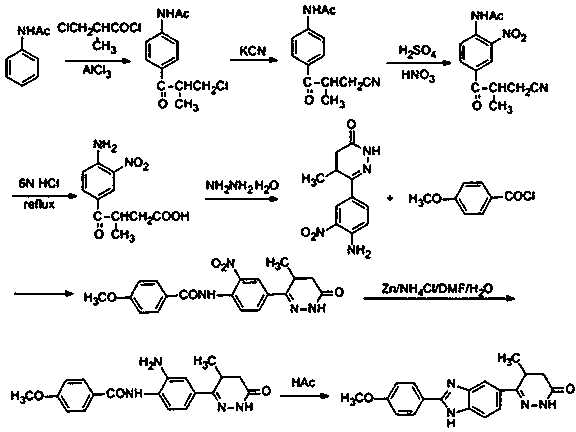
The invention discloses a chemical synthesis method of Pimobendan. The method comprises steps as follows: acetanilide and 2-methyl-3-(chloroformyl)propionate react to produce 3-p-acetylaminobenzoyl methyl butyrate in a mixed organic solvent under the action of a composite catalyst, 3-p-acetylaminobenzoyl methyl butyrate reacts with a nitration reagent, 3-(4-acetamido-3-nitrobenzoyl)-methyl butyrate is produced and subjected to a reflux reaction with alkali in an alcohol solvent to produce 3-(4-amino-3-nitrobenzoyl) butyrate, 3-(4-amino-3-nitrobenzoyl) butyrate and hydrazine hydrate are subjected to a reflux reaction, 4,5-dihydro-5-methyl-6-(4-amino-3-nitrophenyl)-3(2H)-pyridazinone is produced and reduced with zinc powder in absolute methanol, 4,5-dihydro-5-methyl-6-(3,4-diaminophenyl)-3(2H)-pyridazinone is produced and subjected to a reflux reaction with p-anisaldehyde, and Pimobendan is obtained. The method comprises a few reaction steps, operation is safe and convenient, and the cost is low.
Owner:QINGDAO AGRI UNIV
Medicinal composition containing pimobendan and preparation method of medicinal composition
InactiveCN106729723AImprove solubilityStrong positive muscle strengthOrganic active ingredientsPharmaceutical non-active ingredientsSolubilityAdhesive
The invention provides a medicinal composition containing pimobendan and a preparation method of the medicinal composition, relating to the field of veterinary drug preparations. The medicinal composition containing pimobendan is composed of pimobendan, a cosolvent, a filler, a disintegrating agent, a corrigent, an adhesive, a lubricant and the like, and the invention further provides the preparation method of the medicinal composition. The medicinal composition is cardiotonic drug, has the strong positive inotropic effect, strong vasorelaxation action and platelet aggregation effect, and is used for treating chronic and acute heart failure. A preparation process is simple, the cost is low, the stability is good, the solubility of pimobendan is increased through the micronization of pimobendan and the cosolvent, and thus the solubility is greatly improved.
Owner:QINGDAO AGRI UNIV
Preparation method and application of chiral pimobendan
InactiveCN107344933AImprove securityOrganic chemistry methodsCardiovascular disorderN dimethylformamideHypertrophic cardiomyopathy
The present invention relates to the preparation method and application of chiral pimobendan, comprising the following steps: preparing chiral intermediate 1 with chiral reagent, diammonium phthalate and aluminum trichloride; using chiral intermediate 1, Prepare intermediate 2 with base, N,N-dimethylformamide and malonic acid substituted ester; prepare intermediate 3 with intermediate 2 and a catalytic hydrogenation catalyst; prepare intermediate 4 with intermediate 3, alcohol and hydrazine hydrate; Intermediate 4, N,N-dimethylformamide and 4-methoxybenzaldehyde are prepared to obtain chiral pimobendan, and the method of the present invention only needs 5 steps to obtain chiral pimobendan, and the current The steps are shortened by more than half, and the starting materials are simple and easy to obtain; and the chiral pimobendan can be used to treat mild, moderate or severe congestive heart failure in dogs caused by atrioventricular valve insufficiency or myocardial hypertrophy , hypertrophic cardiomyopathy in pets, etc.
Owner:蒿莱医药技术(上海)有限公司
Pimobendan for the reduction of heart size and/or the delay of onset of clinical symptoms in patients with asymptomatic heart failure due to mitral valve disease
PendingUS20190008863A1Delay is slowSmall sizePill deliveryHeterocyclic compound active ingredientsDiseaseCongestive heart failure chf
Pimobendan is used for reducing the heart size and / or delaying the onset of clinical symptoms in a patient suffering from asymptomatic (occult, preclinical) heart failure, preferably congestive heart failure, due to mitral valve disease (MVD), and / or delaying the onset of heart failure, preferably congestive heart failure, in a patient suffering from asymptomatic (occult, preclinical) heart failure, preferably congestive heart failure, due to mitral valve disease (MVD), wherein the patient is preferably a mammal, more preferably a human, a dog, a cat or a horse, and most preferably a dog.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Preserved etherified cyclodextrin derivatives containing liquid aqueous pharmaceutical composition
ActiveUS10071162B2Improve solubilityInhibit microbial growthAntibacterial agentsNervous disorderSodium acetateBenzoic acid
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
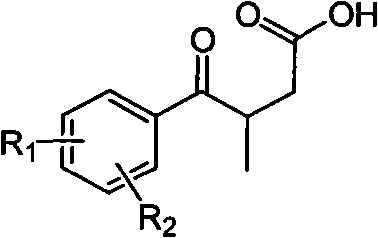
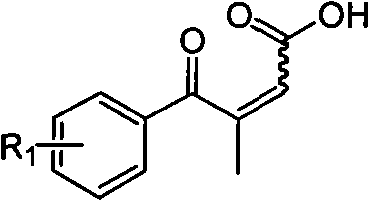
Novel process for preparing 3-(substituted benzoyl) butyric acid
InactiveCN101704740AOrganic compound preparationCarboxylic compound preparationAcetic acid2 Butenoic Acids
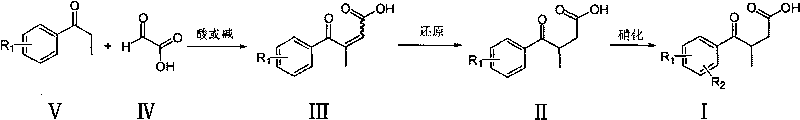
The invention provides a novel process, which takes substituted propiophenone and oxoacetic acid as starting raw materials, carries out condensation to obtain 3-(substituted benzoyl)-2-butenoic acid, and carries out reduction and nitrification to obtain a pimobendan intermediate 3-(substituted benzoyl) butyric acid. The method has the advantages of few steps, easy and simple operation, easy separation and purification of products, high yield and low cost, uses common reagents, and is the novel process suitable for industrialized production.
Owner:北京信益泰医药科技开发有限公司
Preparation method of pimobendan
The present invention relates to the preparation method of pimobendan, comprising the following steps: prepare the first intermediate with 2-chloropropionyl chloride, diammonium phthalate and aluminum trichloride; prepare the first intermediate with the first intermediate, alkali, N,N ‑Dimethylformamide and malonic acid substituted ester prepare the second intermediate; prepare the third intermediate with the second intermediate and a catalytic hydrogenation catalyst; prepare the fourth intermediate with the third intermediate, alcohol and hydrazine hydrate; The fourth intermediate, N,N-dimethylformamide and 4-methoxybenzaldehyde are prepared to obtain pimobendan, and the method of the present invention only needs 5 steps to obtain pimobendan, shortening the current steps more than half, and the starting materials are simple and easy to obtain.
Owner:蒿莱医药技术(上海)有限公司
Preparation of pimobendan key intermediate
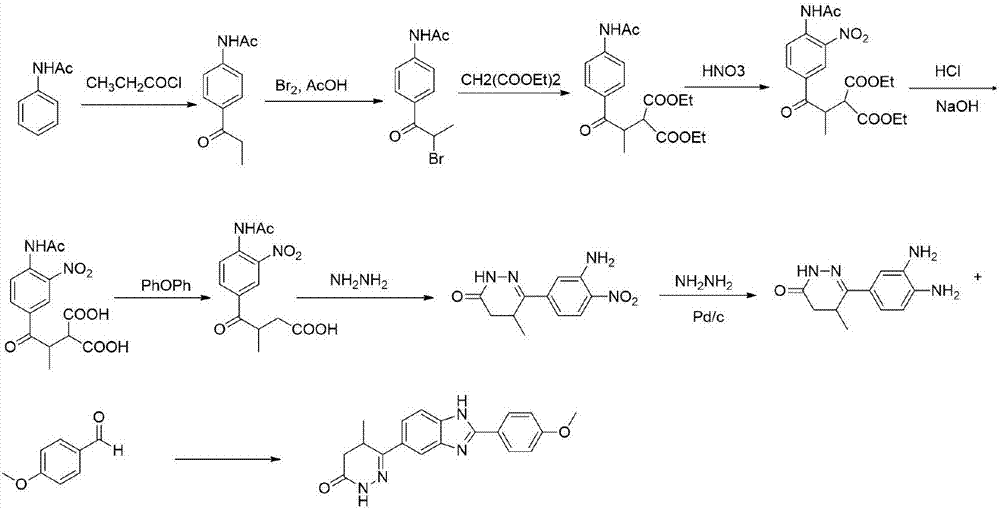
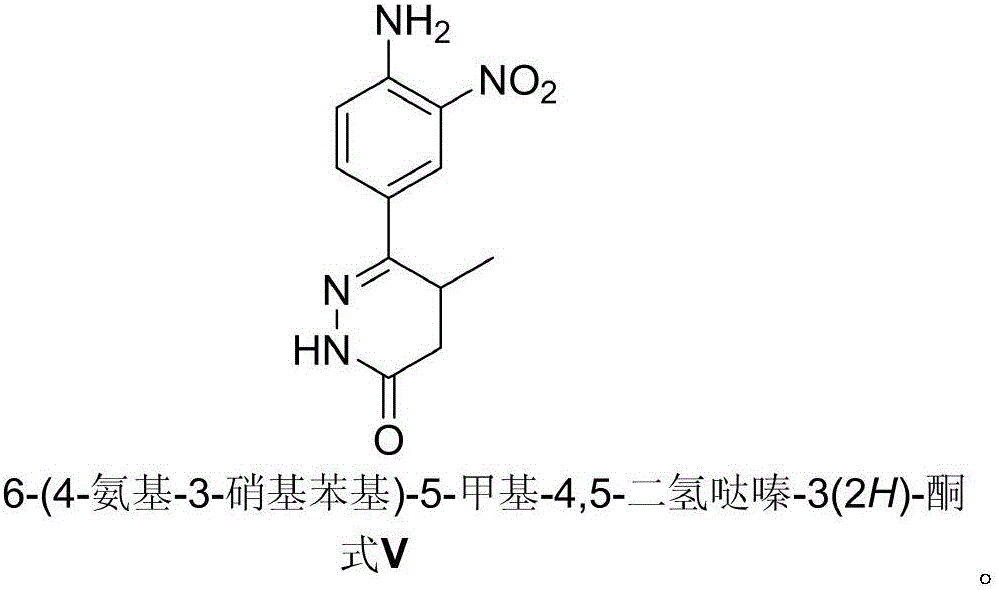
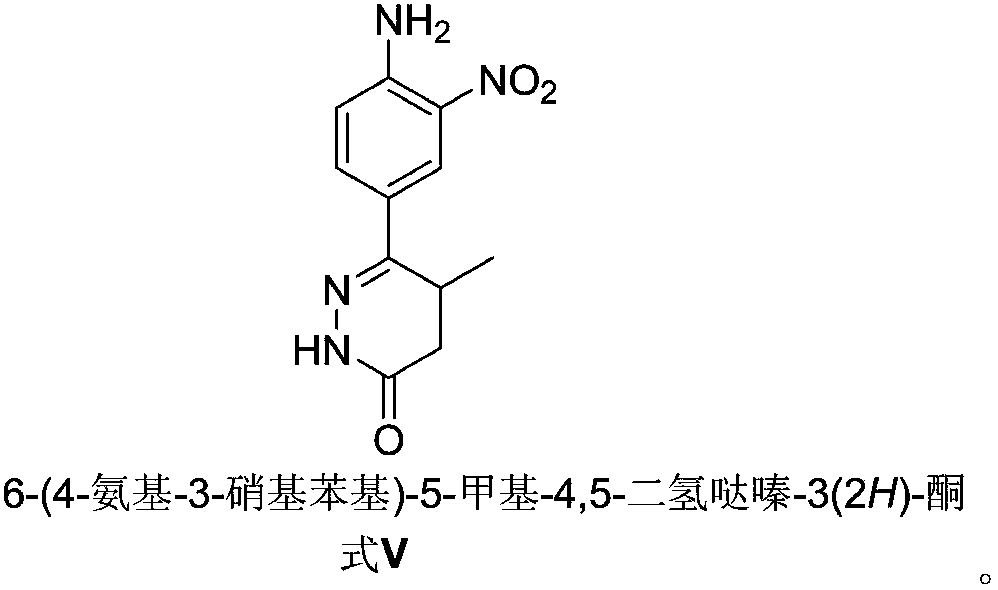
The invention relates to a preparation technology of a pimobendan key intermediate 6-(4-amino-3-nitro phenyl)-5-methyl-4, 5-dihydrogen pyridazine-3(2H)-ketone (formula V). The preparation technology is short in synthesis route and simple in operation, thus having large industrial production prospect. The chemical reaction formula is shown in the description.
Owner:WISDOM PHARM CO LTD
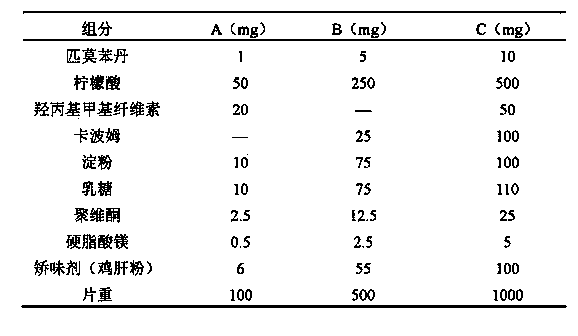
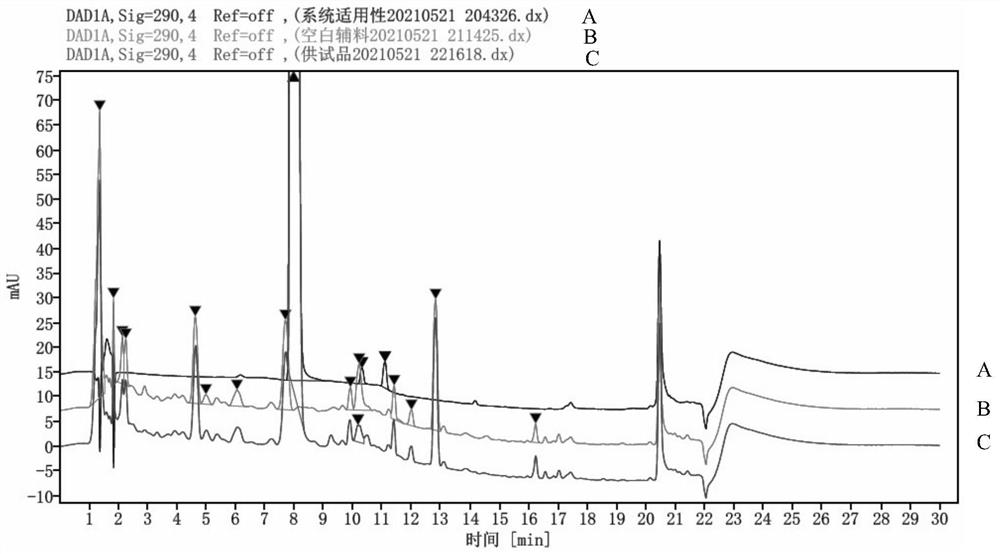
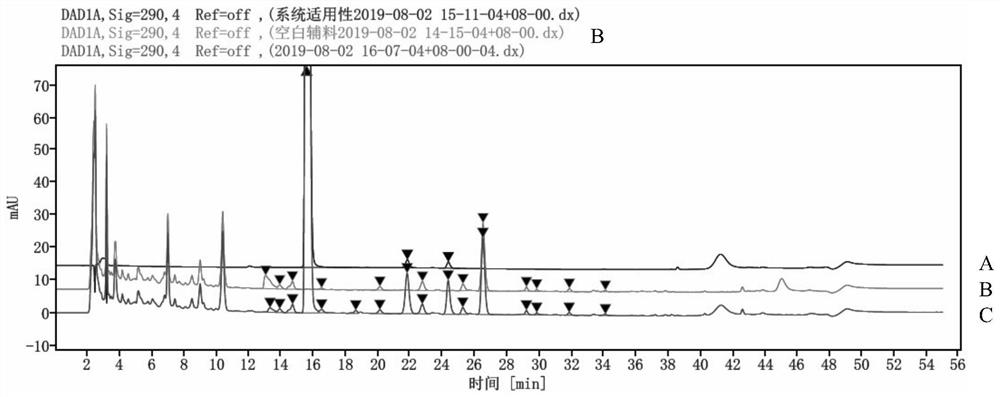
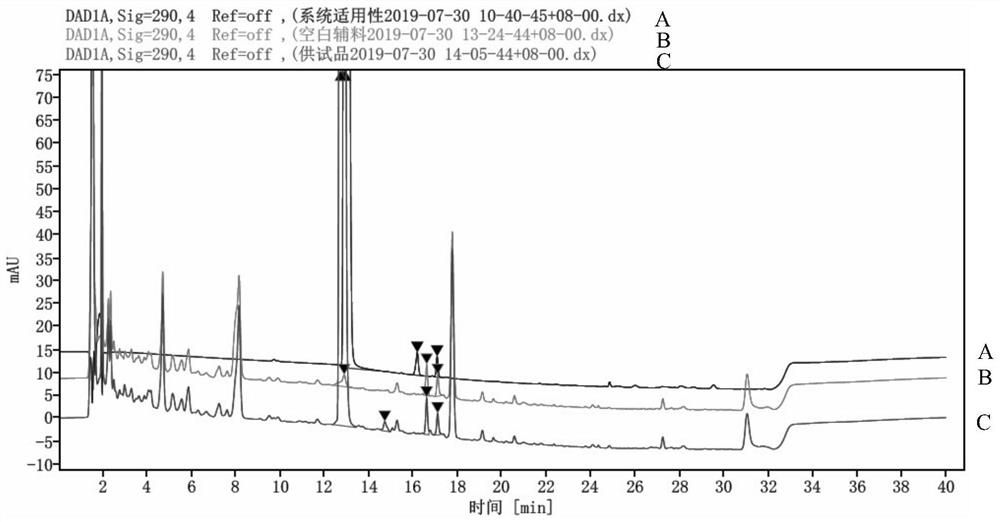
Impurity detection method for pimobendan soft chewing dosage form
PendingCN113804793AEasy to separateLong column lengthComponent separationPhosphoric acidHplc mass spectrometry
The invention discloses an impurity detection method for a pimobendan soft chewing dosage form, which is characterized in that three-phase high performance liquid chromatography is used for determination, and the chromatographic conditions comprise that a chromatographic column is C18, 4.6 mm * 250 mm, 4 [mu] m; a mobile phase A is a 2.6 g / L sodium dihydrogen phosphate solution, and the pH is adjusted to 3.0 by phosphoric acid; a mobile phase B is acetonitrile-methanol in a ratio of 80: 20; the column temperature is 45 DEG C; the detection wavelength is 290 nm; and the flow velocity is 1.0 ml / min. The pimobendan soft chewing dosage form solution is measured under the liquid chromatogram condition, and the impurity condition is judged according to the main peak and the impurity peak. In addition, under the method, a preservative in the pimobendan soft chewing dosage form can be detected. By adjusting the mobile phase system and optimizing the mobile phase gradient, the main peak of pimobendan appears at the gradient change mitigation position, the auxiliary material is completely separated from the main peak and the impurity peak, the auxiliary material does not interfere with impurity detection, the specificity is high, the sensitivity is high, and the impurity response is high.
Owner:BEIJING OUBOFANG MEDICAL TECH +1
Preserved etherified cyclodextrin derivatives containing liquid aqueous pharmaceutical composition
ActiveUS20180339054A1Improve solubilityInhibit microbial growthAntibacterial agentsOrganic active ingredientsSolubilityOral medication
A preserved liquid aqueous pharmaceutical composition includes one or more etherified cyclodextrin derivatives, at least one water-soluble preservative, and at least one pharmaceutically active compound which is poorly water-soluble, very poorly water-soluble or water-insoluble. The liquid aqueous pharmaceutical composition provides an acceptable solubility of the pharmaceutically active compound, such as pimobendan, in aqueous solution whereby the water-soluble preservatives retain their effectiveness in the presence of the etherified cyclodextrin derivatives allowing the use in an oral administration form.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Preparation of Pimobendan Key Intermediate
The invention relates to a preparation technology of a pimobendan key intermediate 6-(4-amino-3-nitro phenyl)-5-methyl-4, 5-dihydrogen pyridazine-3(2H)-ketone (formula V). The preparation technology is short in synthesis route and simple in operation, thus having large industrial production prospect. The chemical reaction formula is shown in the description.
Owner:WISDOM PHARM CO LTD
Novel method for preparing pimobendan from by-product for synthesizing pimobendan
ActiveCN108976200AReduce manufacturing costOrganic chemistryBulk chemical productionOrganic solventSolvent
The invention discloses a novel method for preparing pimobendan from a by-product for synthesizing the pimobendan. The method comprises the following steps that the by-product (the chemical name is (5RS)-6-[1-(4-methoxybenzyl)-2-(4-methoxyphenyl)-1H-benzimidazole-6-yl]-5-methyl-4,5-dihydro-3(2H)-pyridazinone) obtained in the pimobendan synthesizing process is dissolved into a mixed solvent and reacts with ceric ammonium nitrate (CAN) at 20-25 DEG C for 2-3 hours so as to obtain a pimobendan crude product; and the obtained pimobendan crude product is heated and refluxed in an organic solvent, and cooling, filtering and drying are carried out so as to obtain the pimobendan. According to the method, the unwanted by-product is converted into a required pimobendan product, waste is turned intowealth, and therefore the production cost is greatly lowered.
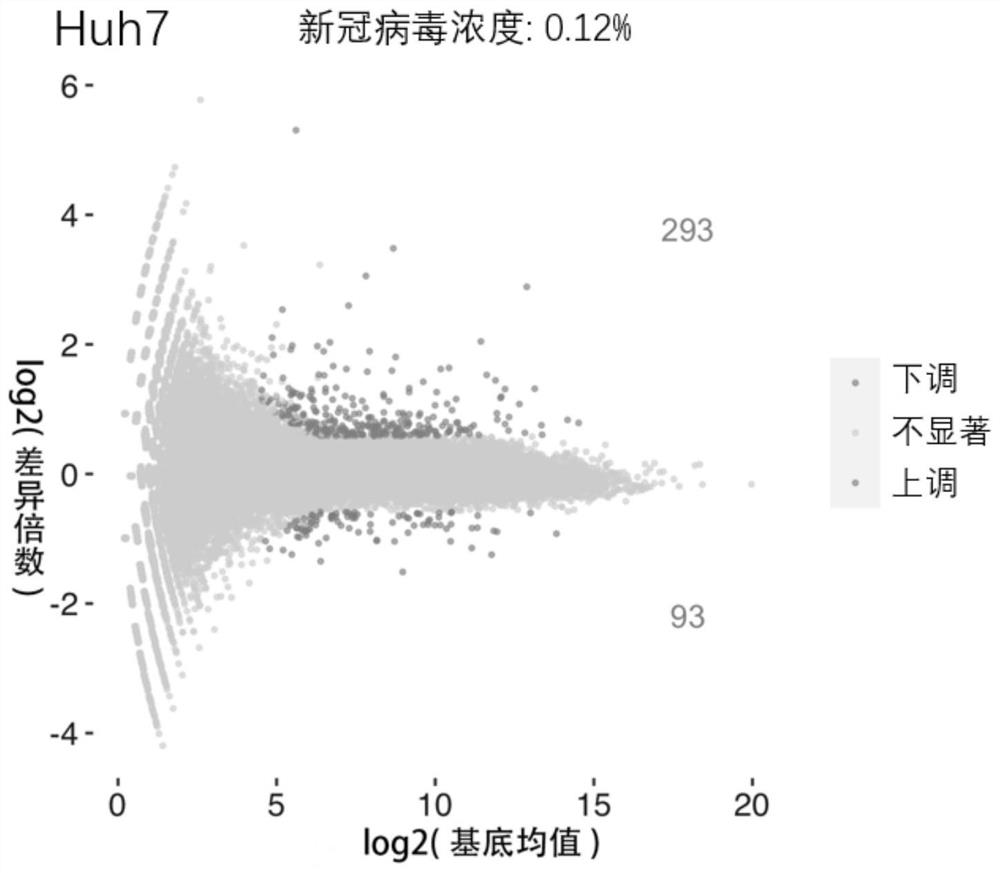
Application of phosphodiesterase inhibitor or pharmaceutical composition thereof in preparation of medicines for treating novel coronavirus pneumonia
PendingCN114053280APrevent proliferationStrong anti-coronavirus effectBoron compound active ingredientsAntiviralsZardaverinePhosphodiesterase inhibitor
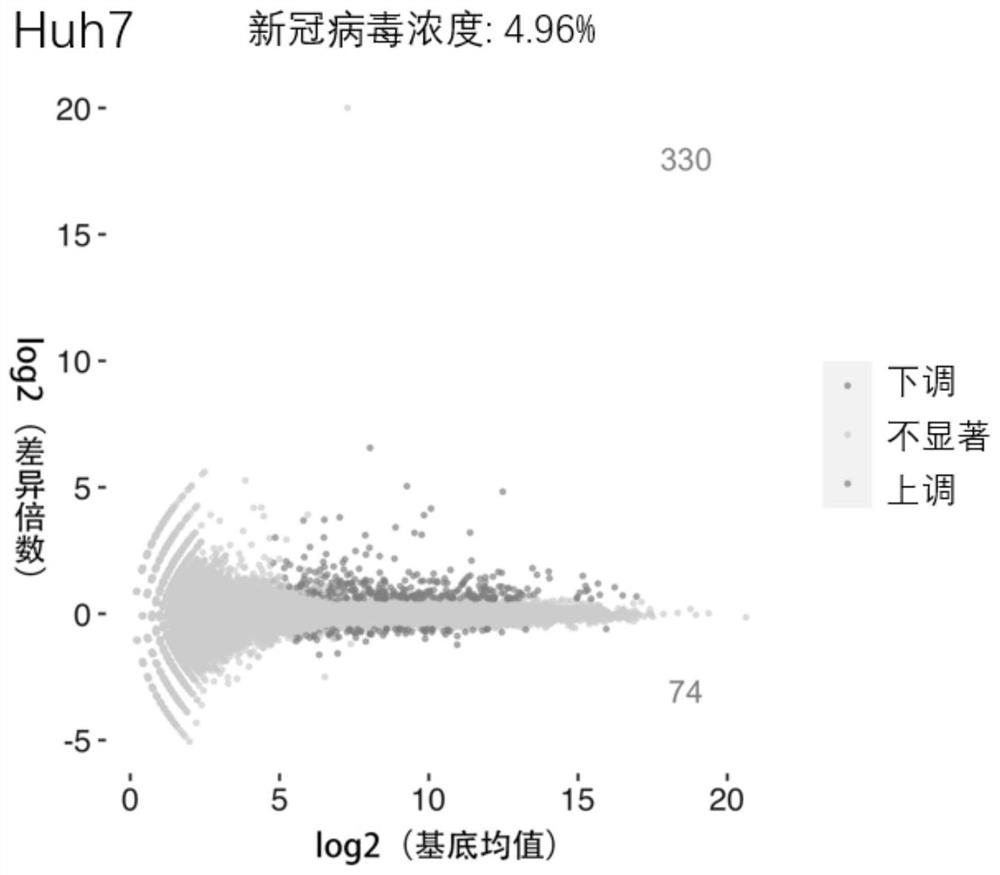
The invention provides the application of a phosphodiesterase inhibitor or a pharmaceutical composition of the phosphodiesterase inhibitor in preparation of medicines for treating novel coronavirus pneumonia, screening of systematic biology and cellular level experiments verify that zardaverine, Pimobendan and crisaborole can inhibit proliferation of novel coronavirus to different degrees on the cellular level; wherein zardaverine has the strongest anti-new coronavirus effect, Pimobendan has the second anti-new coronavirus effect, and zardaverine, Pimobendan and crisaborole can be used for preparing medicines for treating novel coronavirus pneumonia.
Owner:SOUTH UNIVERSITY OF SCIENCE AND TECHNOLOGY OF CHINA +1
Pharmaceutical composition comprising pimobendan
ActiveUS11246867B2Organic active ingredientsInorganic non-active ingredientsCombinatorial chemistryDrug activity
The invention relates to oral pharmaceutical compositions comprising pimobendan pharmaceutically active compound. It also relates to a method for preparing the same uses thereof.
Owner:CEVA SANTE ANIMALE
Preparation of pimobendan
Owner:WISDOM PHARM CO LTD +1
Pharmaceutical compositions of pimobendan
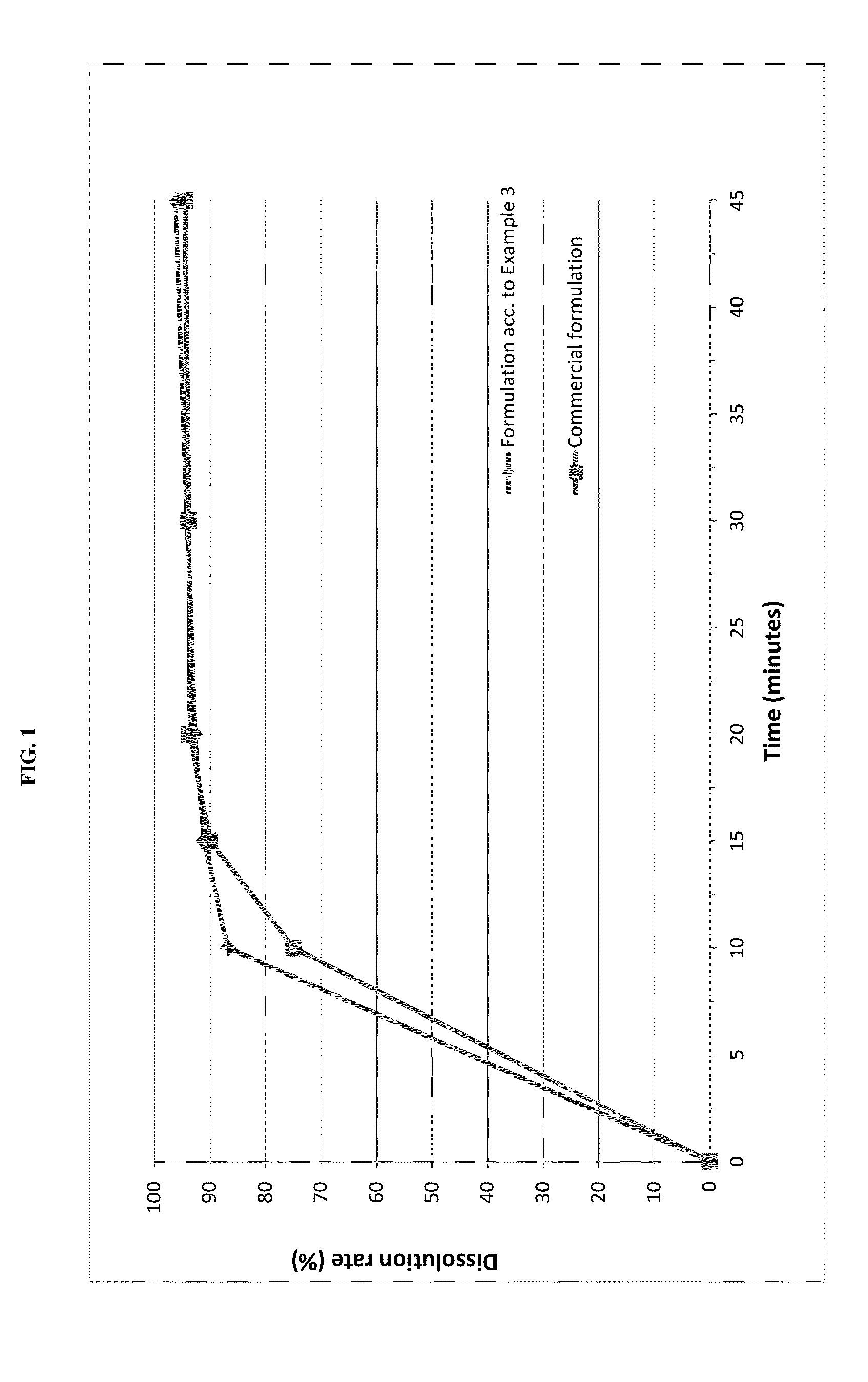
ActiveUS20190083411A1Rapid and substantial dissolutionEasily scaled-upPowder deliveryPill deliveryDissolutionPharmaceutical formulation
A composition includes particles of pimobendan with an integral coating of a carrier matrix which serve to ensure a rapid dissolution of the active substance at each pH condition representing the gastrointestinal tract and therefore a reliable absorption, and a method of pimobendan microencapsulation using the spray congealing technology and incorporating the coated particles into oral formulations, for example into tablets.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Pharmaceutical compositions of pimobendan
ActiveUS10172804B2Rapid and substantial dissolutionEasily scaled-upPowder deliveryPill deliveryDissolutionPimobendan
The present invention is directed to a composition comprising particles of pimobendan with an integral coating of a carrier matrix which serve to ensure a rapid dissolution of the active substance at each pH condition representing the gastrointestinal tract and therefore a reliable absorption, and a method of pimobendan microencapsulation using the spray congealing technology and incorporating the coated particles into oral formulations, for example into tablets.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Preparation of 6-(3,4-diamino phenyl)-5-methyl-4,5-dihydropyridazine-3(2H)-ketone key intermediate of 2-(4-methoxyphenyl)-5(6)-(5-methyl-3-oxo-4,5-dihydro-2H-6-pyridazinyl)benzimidazole
The invention relates to preparation of a 6-(3,4-diamino phenyl)-5-methyl-4,5-dihydropyridazine-3(2H)-ketone key intermediate for synthesizing 2-(4-methoxyphenyl)-5(6)-(5-methyl-3-oxo-4,5-dihydro-2H-6-pyridazinyl)benzimidazole. The technology is short in synthetic route and easy to operate and has an industrialized enlarged production prospect.
Owner:SOUTHEAST UNIV +1
Pharmaceutical compositions of pimobendan
ActiveUS20190083410A1Rapid and substantial dissolutionEasily scaled-upPowder deliveryPill deliveryMedicineDissolution
A composition includes particles of pimobendan with an integral coating of a carrier matrix which serve to ensure a rapid dissolution of the active substance at each pH condition representing the gastrointestinal tract and therefore a reliable absorption, and a method of pimobendan microencapsulation using the spray congealing technology and incorporating the coated particles into oral formulations, for example into tablets.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
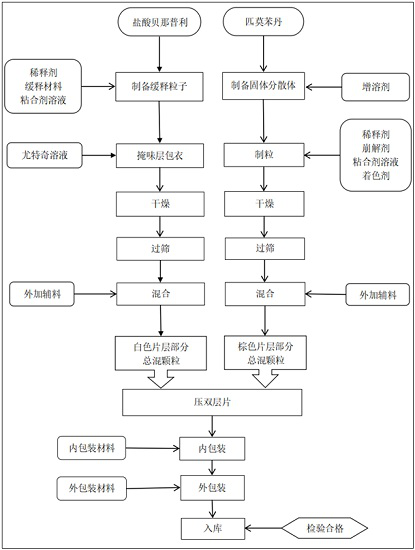
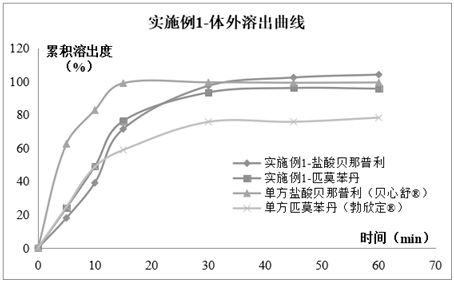
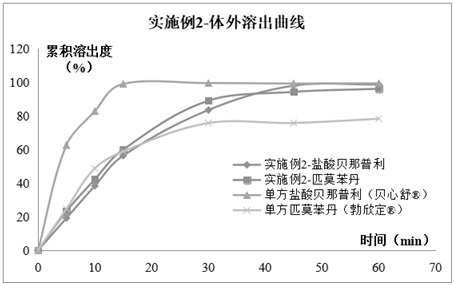
Compound pharmaceutical composition containing benazepril and pimobendan for pets and preparation method of compound pharmaceutical composition
ActiveCN112618505AEasy to administerLasting effectPharmaceutical non-active ingredientsPill deliverySolventCompanion animal
The invention relates to the technical field of pharmaceutical compositions, in particular to a compound pharmaceutical composition containing benazepril and pimobendan for pets and a preparation method of the compound pharmaceutical composition. The pharmaceutical composition comprises the following active pharmaceutical ingredients in percentage by weight: 1%-8.0% of benazepril hydrochloride, 0.5%-4.0% of pimobendan, 42%-71% of diluent, 1%-5% of sustained-release skeleton, 10%-20% of coating taste masking material, 1.5%-4% of disintegrating agent, 1%-5% of adhesive, 5%-15% of solubilizer, 1%-5% of flow aid, 1%-3% of lubricant , 0.5%-2% of corrigent and a small amount of pigment. The compound composition prepared by the invention has the characteristics of convenience in administration, lasting effect and stable curative effect, can relax blood vessels and reduce blood pressure so as to reduce heart burden, and also can improve myocardial contractility and improve the survival rate of sick dogs; the medicine is high in effect taking speed and high in bioavailability; moreover, a taste masking coating process and a proper amount of meat flavor flavoring agent are added, so that it has a pleasant taste for pets and the medicine taking compliance of animals is greatly improved.
Owner:南京朗博特动物药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com