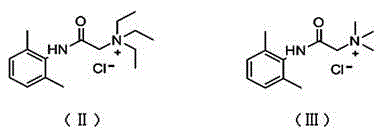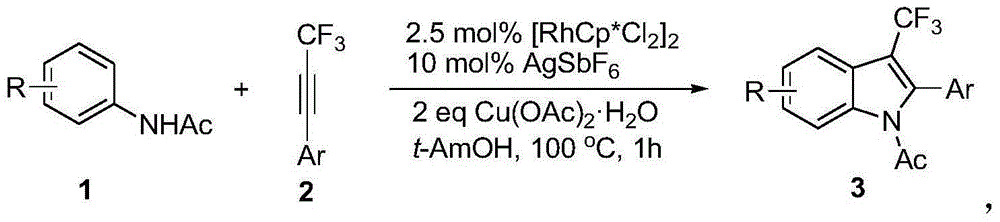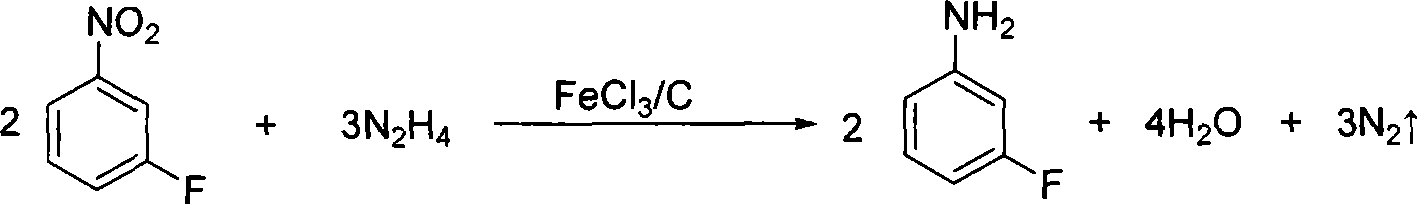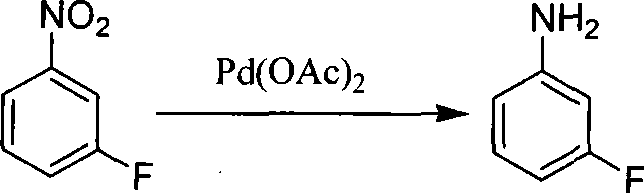Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
288 results about "Acetanilide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Acetanilide is an odourless solid chemical of leaf or flake-like appearance. It is also known as N-phenylacetamide, acetanil, or acetanilid, and was formerly known by the trade name Antifebrin.
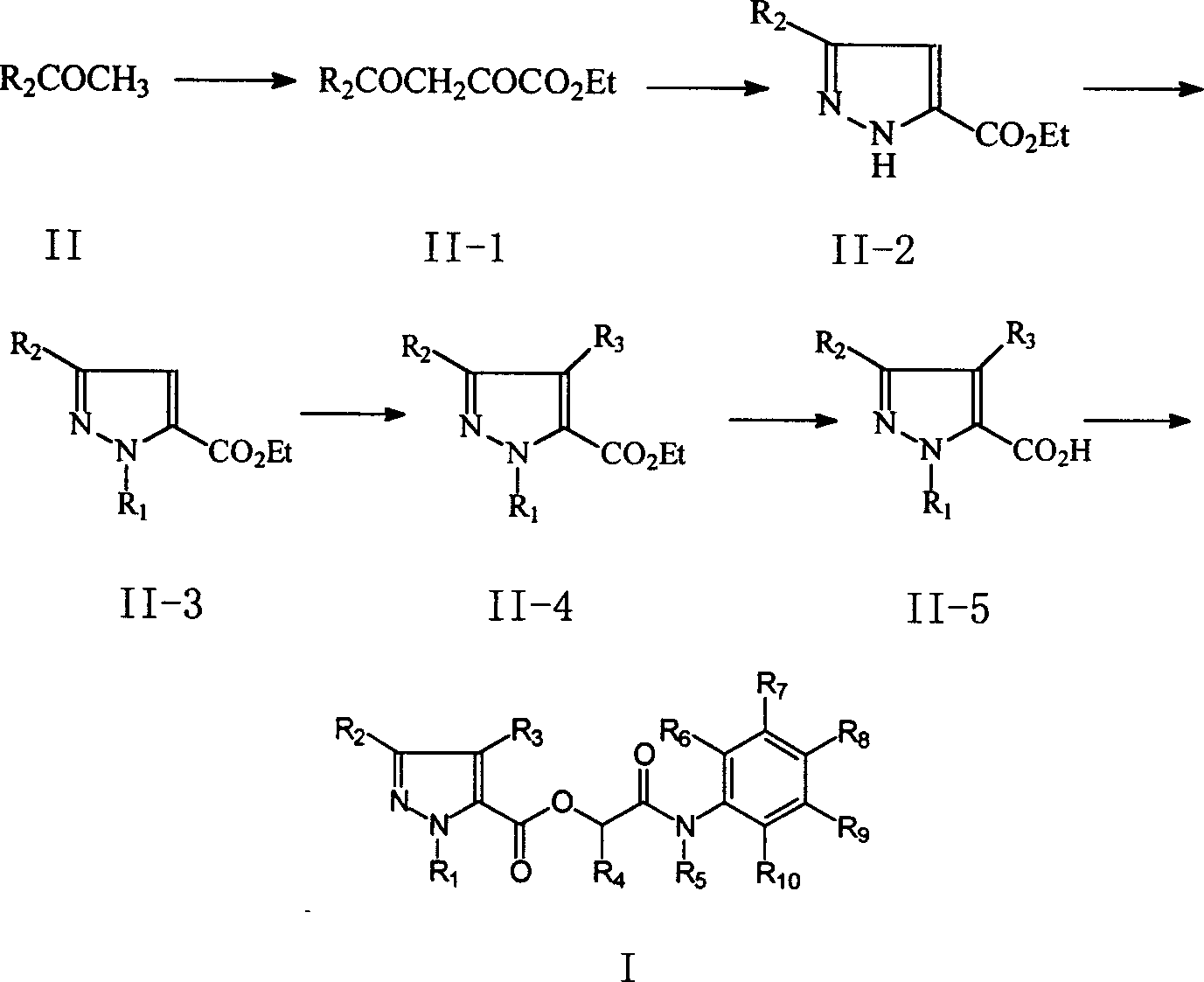
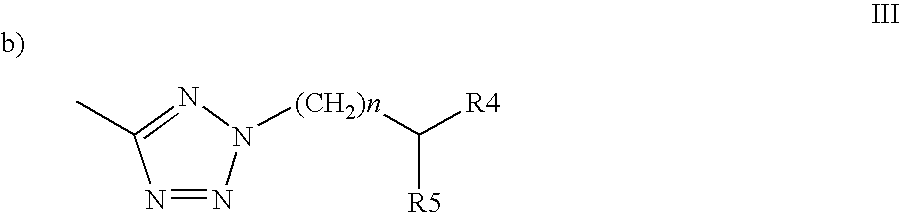
N[S(4-aryl-triazol-3-yl)α-mercaptoacetyl]-p-amino benozioc acids as HIV reverse transcriptase inhibitors
Owner:ARDEA BIOSCIENCES INC
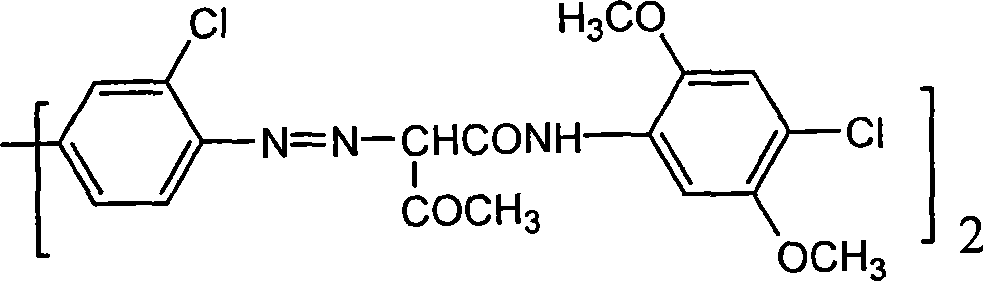
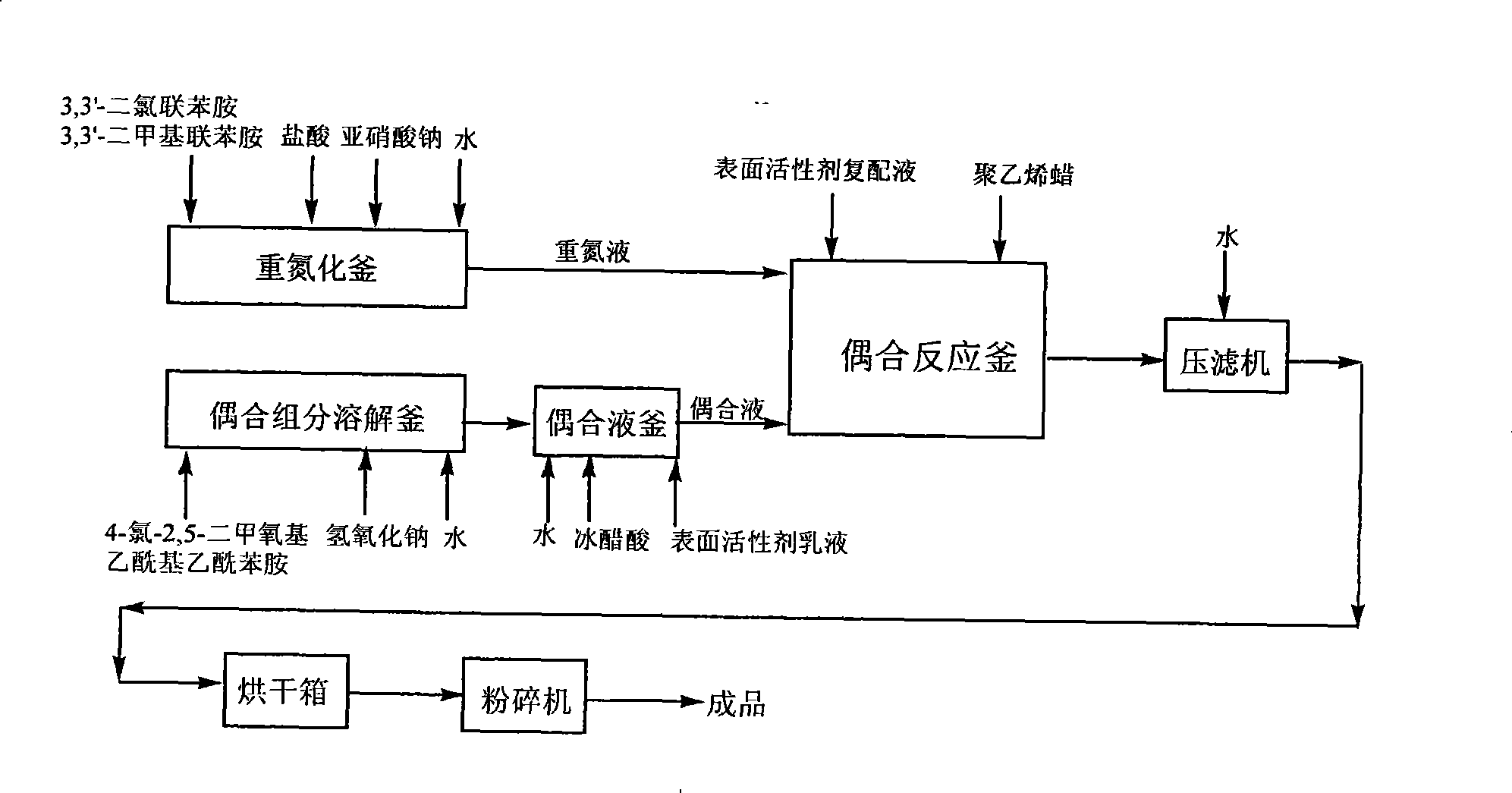
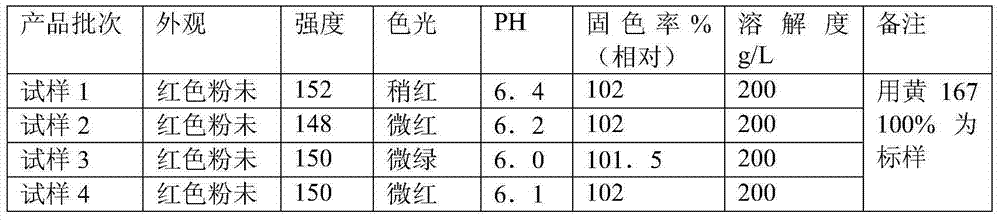
Method for preparing pigment yellow
The invention provides a method for preparing pigment yellow 83. The method comprises the following steps: 3, 3'-dichlorobenzidine, 3, 3'-dimethylbenzidine, hydrochloric acid and water are stirred, dissolved and added to sodium nitrite for diazotization, so as to obtain a diazonium solution; a sodium hydroxide solution containing 4-chlorine-2, 5-dimethoxy acetyl acetanilide is dripped into an acetic acid solution at the temperature between 20 and 40 DEG C, so as to prepare a coupling solution; the acetic acid solution contains an emulsion formed by well mixing anionic surfactant, nonionic surfactant, solvent and water; the diazonium solution is dripped into the coupling solution within 1 to 5 hours; the emulsion formed by compounding the anionic surfactant and non-polar hyper-dispersant is added to the coupling solution, reacts for 1 hour, and is heated to 90 to 100 DEG C; polyethylene wax is added in the mixture; and then the mixture, is insulated for 1 to 3 hours, pumped, filtered, washed, dried and ground, so as to obtain a finished product. The preparation method has the advantage of obtaining the pigment yellow 83 which is bright in color light, high in tinting strength, high in covering power and good in dispersibility.
Owner:宇虹颜料股份有限公司
Alpha-form or beta-form crystal of acetanilide derivative
ActiveUS7342117B2Surely and simply obtainedImprove solubilityOrganic active ingredientsBiocideAdditive ingredientDiabrezide
To provide novel crystals useful as an ingredient for the production of a diabetes remedy. The invention is concerned with α-form crystal and β-form crystal of (R)-2-(2-aminothiazol-4-yl)-4′-[2-[(2-hydroxy-2-phenyleth-yl)amino]ethyl]acetanilide. The α-form crystal does not exhibit hygroscopicity and has stability such that it can be used as a medicine, and is useful for mass synthesis in the industrial production. The β-form crystal does not relatively exhibit hygroscopicity and is also useful as a production intermediate of the α-form crystal.
Owner:ASTELLAS PHARMA INC
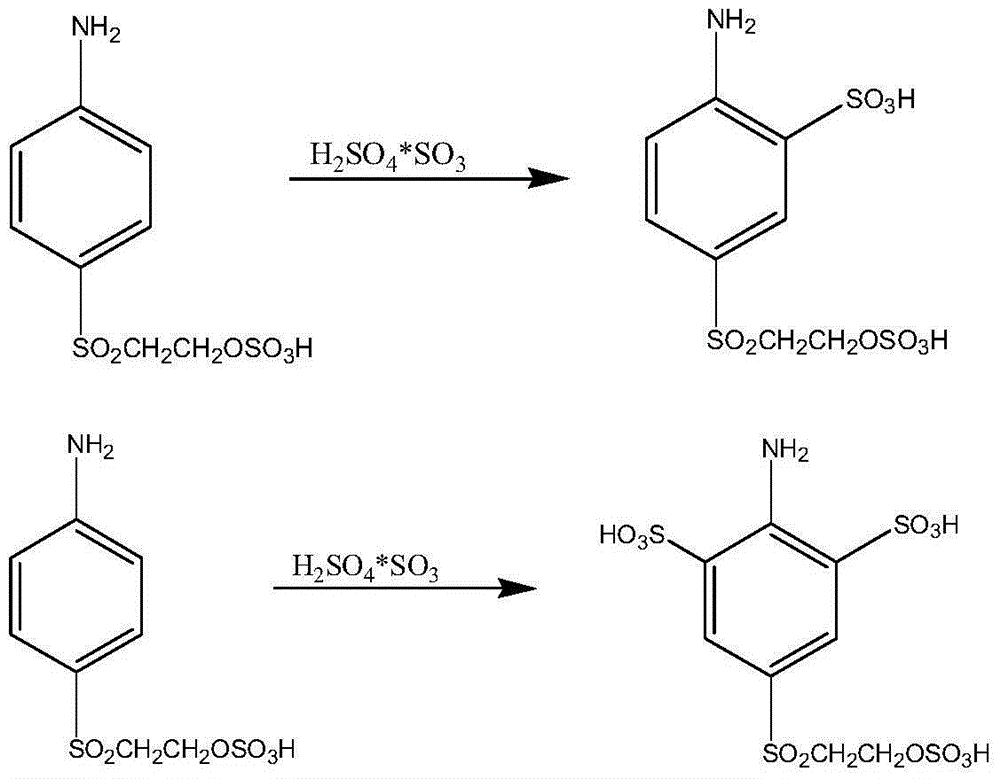
Active red dye preparation method
ActiveCN105524485AReduce salt contentPromote environmental protectionReactive dyesSalting outAcid value
The present invention discloses an active red dye preparation method comprising the following steps: (1) a sulfonated para-ester is obtained by successive sulfonation, hydrolysis, dilution and salting-out of p-hydroxyethyl sulfone acetanilide as a starting material; (2) the acid number of the sulfonated para-ester is detected, according to the detected acid number, hydrochloric acid is added, and after the reaction is completed, a sulfonated para-ester diazonium salt is obtained; (3) a first-coupling fluid is obtained by first coupling of the sulfonated para-ester diazonium salt obtained by the step (2) and a J acid; and (4) an active red dye is obtained by secondary coupling of the first-coupling fluid obtained by the step (3) and a cresidine para-ester diazonium salt and processing. According to the method, on the basis of the acid number of the prepared sulfonated para-ester diazonium salt, the addition amount of the hydrochloric acid is determined, and the obtained active red dye has better strength.
Owner:ZHEJIANG JINGGUANG IND
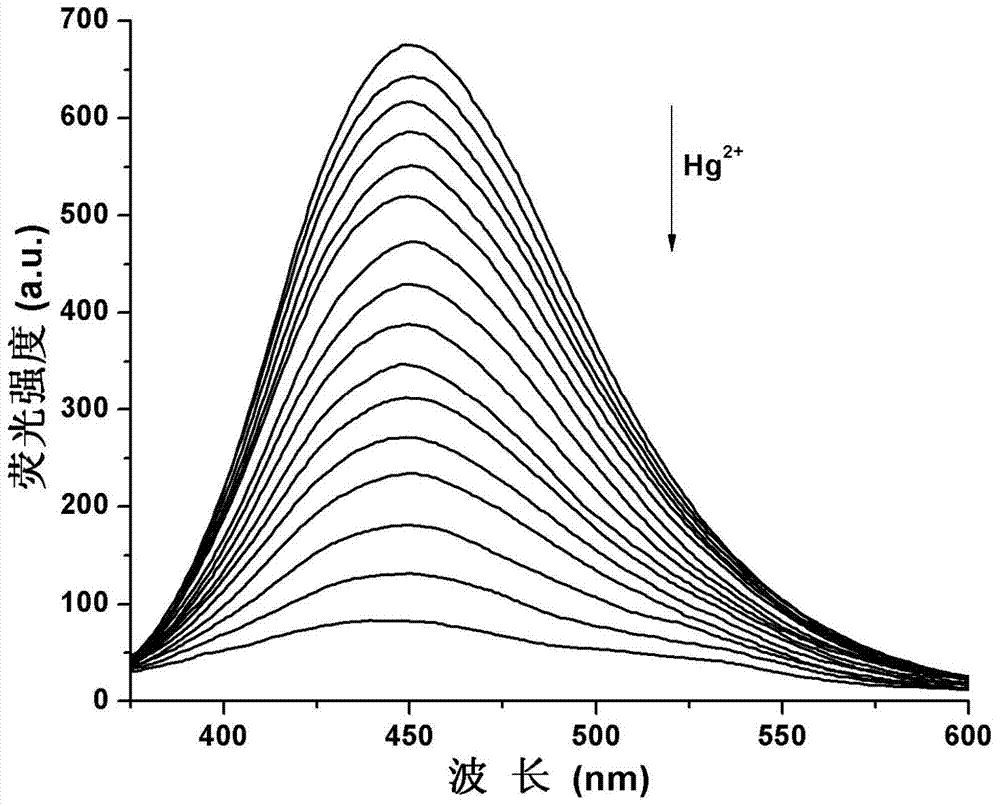
2-quinolinone compound and preparation method and application thereof
InactiveCN103570678ALow costRaw materials are easy to getOrganic chemistryFluorescence/phosphorescenceFluorescenceQuinoline
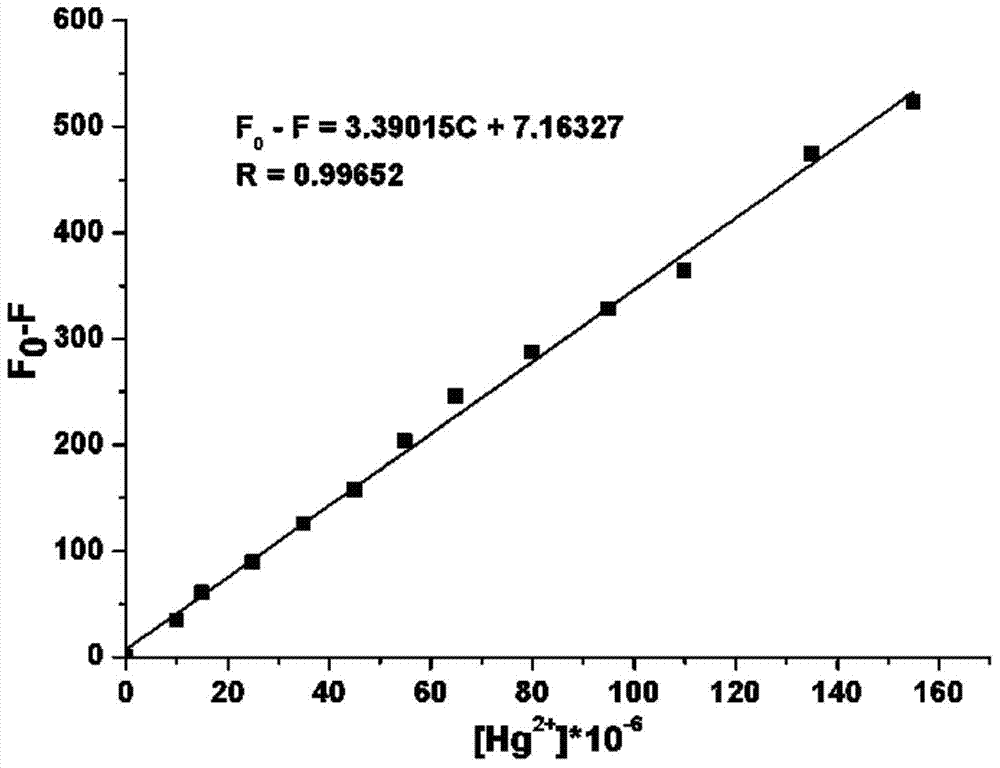
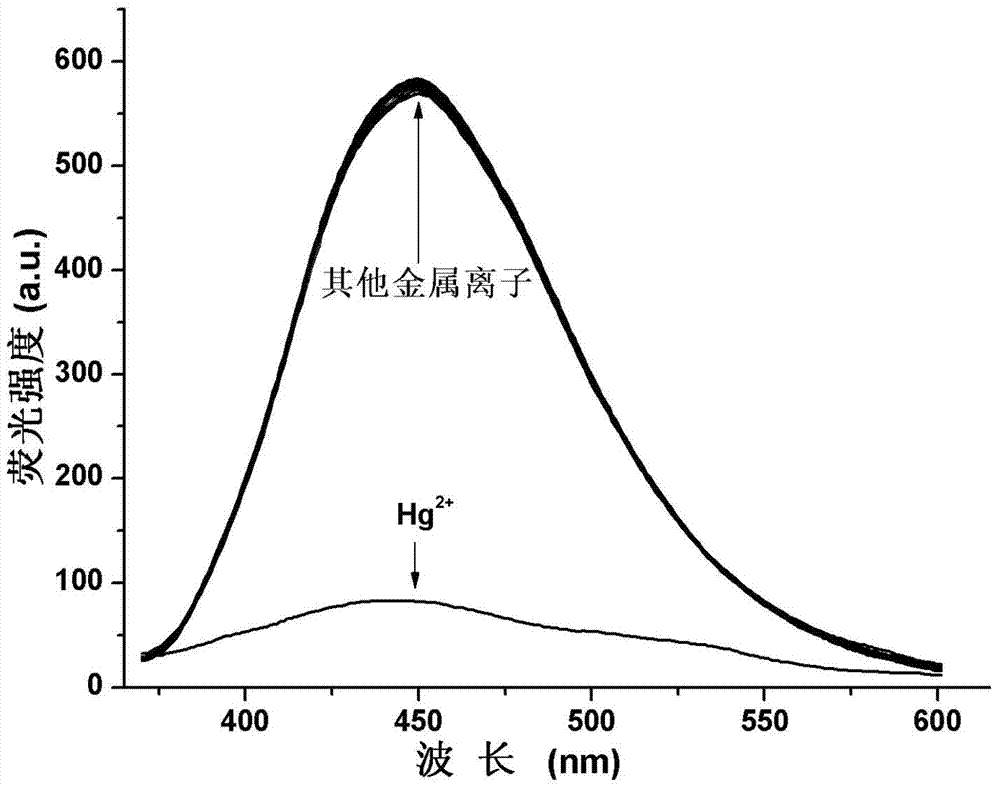
The invention provides a 2-quinolinone compound and a preparation method and application thereof. The 2-quinolinone compound is 3-(2-benzimidazolyl)-6, 7-difluoro-2-quinolinone. The preparation method comprises the following steps: taking 3, 4-difluoro acetanilide as an initial raw material, reacting with POC13 (phosphorus oxychloride) and DMF (dimethyl formamide) to obtain 2-chlorine-6, 7-difluoro quinoline-3-formaldehyde, then hydrolyzing to generate 3-aldehyde group-6, 7-difluoro-2-quinolinone, then reacting with o-phenylenediamine to obtain the 2- quinolinone compound. The 2-quinolinone compound can be used as a fluorescent probe for quantitative detection of the content of Hg<2+>, and shows high sensitivity and selectivity, the detection process is simple and rapid, and the detection result is accurate.
Owner:SHANXI UNIV
Synthetic method of m-amino acetanilide
InactiveCN101328133ASimple processShorten the production cycleOrganic compound preparationCarboxylic acid amides preparationHydrogen chlorideEnergy consumption
The invention provides a method for synthesizing m-Aminoacetanilide. Compared with the prior method for synthesizing m-Aminoacetanilide, the method is characterized in that chlorine hydride gas replaces hydrochloric acid with a content of 31 percent, the step of concentrating a mother liquid in the subsequent steps is avoided, a large number of production resources and the cost are saved, the final reaction liquid is pumped and filtered to remove the product, the filter liquor can be reused for the next reaction immediately, and the step of concentrating the mother liquid is not needed; and the process is simple, the production cycle is short and the energy consumption is low.
Owner:浙江龙盛染料化工有限公司 +2
Method for synthesizing p-acetamido benzene sulfonyl chloride by phosphorus pentachloride
InactiveCN101613308AHigh yieldReduce dosageSulfonic acid preparationSulfonyl chlorideChlorosulfuric acid
The invention relates to a method for synthesizing p-acetamido benzene sulfonyl chloride by phosphorus pentachloride, relating to the preparation method for sterilization and mould inhibition midbody of sulfonamides. The invention takes acetanilide and chlorosulfonic acid as raw materials, uses phosphorus pentachloride as chlorinating agent; under the action of organic dissolvent, the raw materials are sulfonated, chloridized, separated, and washed to obtain the product. The invention has the effects of little chlorosulfonic acid usage, high product yield, few generated waste acid,, completely cycling dissolvent, recycled by-products, low manufacturing cost, being convenient to popularize and apply, and the like. The products prepared by the method can be widely applied to the preparation of the sterilization and mould inhibition of sulfonamides and industries such as coating, plastics, pesticides, etc.
Owner:CHONGQING UNIV
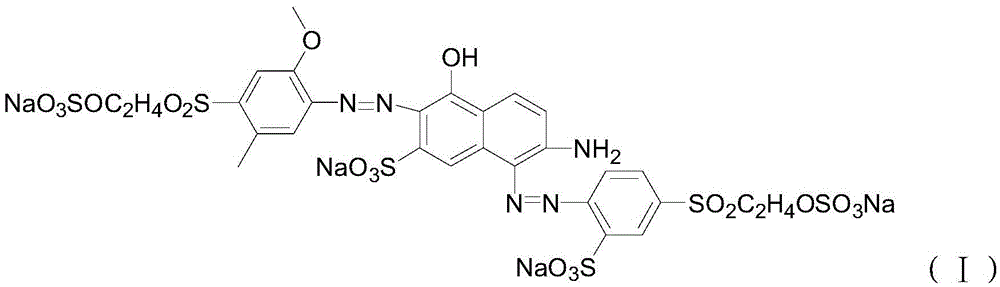
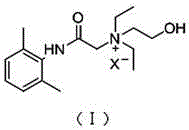
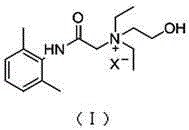
Application of N-acetanilide cationic compound in preparation of local nerve blocking drug
The invention discloses an application of an N-acetanilide cationic compound in preparation of a local nerve blocking drug. The cationic compound is a quaternary ammonium salt compound and has a structure as shown in a formula (I), wherein X is a halogen atom. When used alone, the compound has the characteristics of good safety performance, strong nerve blocking effect and the like, can take a reversible and durable local anaesthesia effect in vivo and can be used as a local anaesthesia drug or an analgesic drug which is long-acting and / or capable of realizing selective blocking. Particularly, a composition composed of the compound and other local anaesthesia drugs has the remarkable characteristics of high effect taking speed, strong effect, long acting time, little nerve injury and the like when used for nerve blocking.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV +1
Continuous sulfonating process for synthesizing p-aminophenyl-beta-hydroxyethyl sulfone sulphate
ActiveCN104496866AImprove conversion rateThe reaction temperature is easy to controlSulfonic acid preparationMolten stateChlorosulfuric acid
The invention discloses a continuous sulfonating process for synthesizing p-aminophenyl-beta-hydroxyethyl sulfone sulphate. The process comprises the steps: performing reaction on chlorosulfonic acid and acetanilide which serve as raw materials; respectively atomizing chlorosulfonic acid and molten-state acetanilide and contacting with each other during feeding to perform reaction. According to the continuous sulfonating process for synthesizing p-aminophenyl-beta-hydroxyethyl sulfone sulphate, the molten-state acetanilide is used as a raw material, and the reaction on the acetanilide in an atomizing state and chlorosulfonic acid which is also in an atomizing state are carried out and the reaction temperature is easily controlled, so that the production of byproducts such as parachloroaniline is avoided, and the conversion rate of acetanilide serving as a raw material is high. The process is convenient to operate, short in production period, controllable in reaction process, less in labor demands, low in cost and easy for industrial production.
Owner:ZHEJIANG QICAI ECO TECH CO LTD
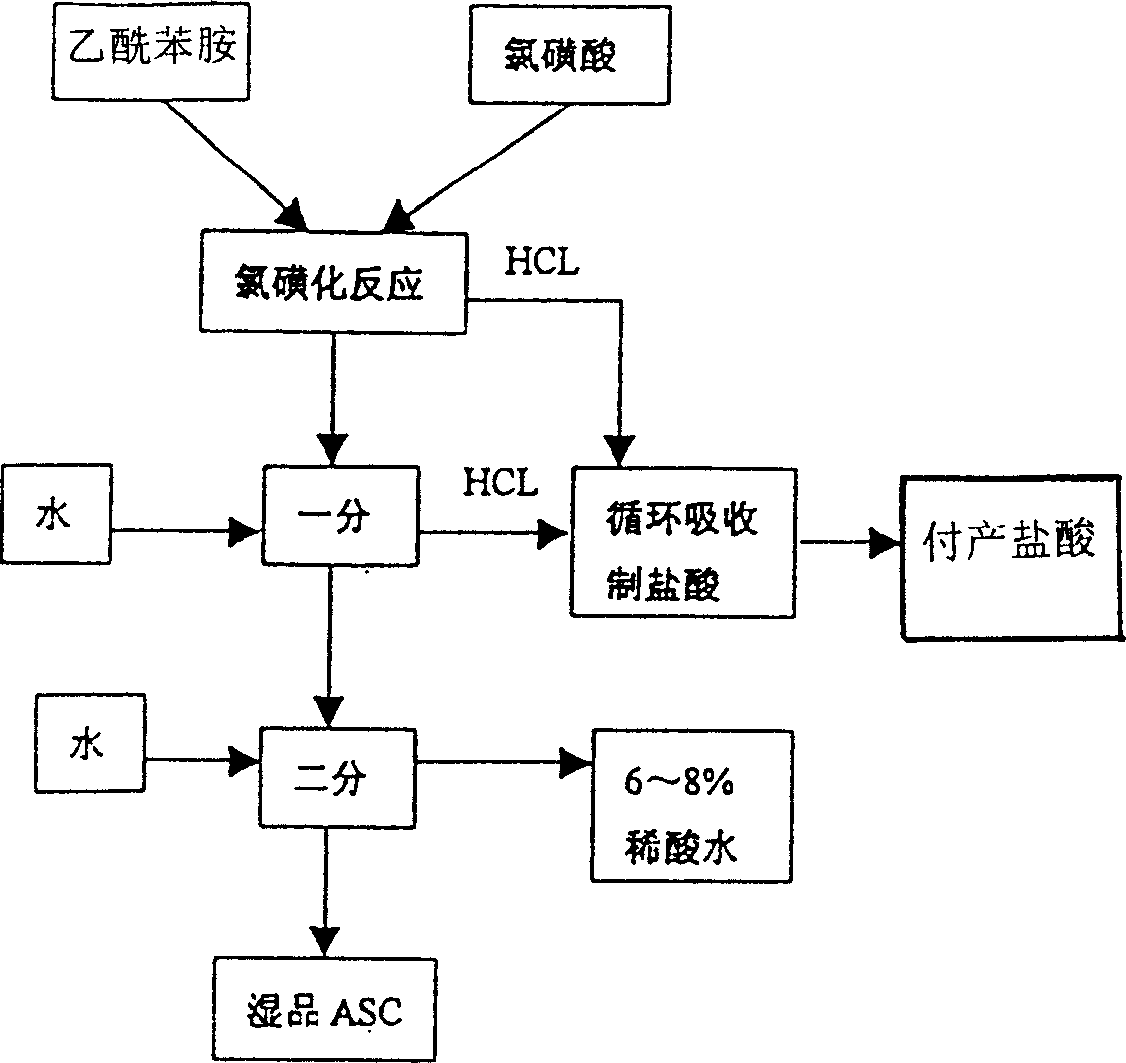
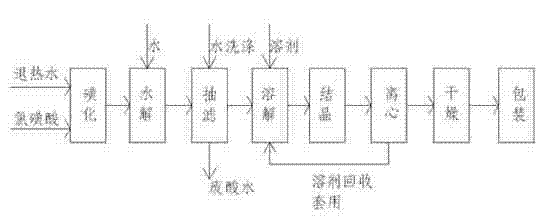
Process for producing sulfanilic amide medicine mother substance p-acetamido benzene sulfonyl chloride
InactiveCN1683331ATo achieve the goal of zero emissionsConserve waterSulfonic acid preparationSulfonyl chlorideChlorosulfuric acid
The production process of p-acetamido benzene sulfonyl chloride as intermediate for sulfanilamide medicine includes the following steps: chlorosulfonating acetylaminobenzene as main material with chlorosulfonic acid to produce sulfonated oil and absorbing hydrogen chloride gas to prepare hydrochloric acid; separating sulfonated oil, adding water and decomposing chlorosulfonic acid to obtain separated oil while absorbing produced hydrogen chloride gas; separating the separated oil and adding water to deposit out p-acetamido benzene sulfonyl chloride; further separating, eliminating p-acetamido benzene sulfonyl chloride mother liquid, water washing the crystal while side producing sulfuric acid; using the side produced sulfuric acid in recovering p-amido benzene sulfonic acid; and reusing crystal eliminating water in the separation. The present invention realizes the comprehensive utilization, protects environment and lowers the cost.
Owner:黄升
Preparation method for N-acetyl acetanilide
ActiveCN103224455APrevent mutual condensationReduce the amount of waterOrganic compound preparationCarboxylic acid amide separation/purificationBiotechnologyEthenone
The invention relates to a preparation method for N-acetyl acetanilide. The method comprises the following steps of adding N-acetyl acetanilide crystal seeds and an emulsifying agent to deionized water at a temperature of 0-10 DEG C to carry out a reaction; then dropwise adding diketene and aniline simultaneously under the control of a certain temperature; keeping the reaction at the temperature; cooling to a temperature of 0 DEG C; filtering and drying to obtain the N-acetyl acetanilide. The method has the advantages of effectively preventing product caking, improving product appearance and performance, increasing product yield and greatly reducing wastewater quantity.
Owner:NANTONG ACETIC ACID CHEM
Compound in alpha (pyrazole formyloxy) acetanilide class of possessing fungicidal property
An alpha-(pyrazole-formyl) acetylphenylamine compound with high bactericiding activity and its formula are disclosed.
Owner:中国中化股份有限公司 +1
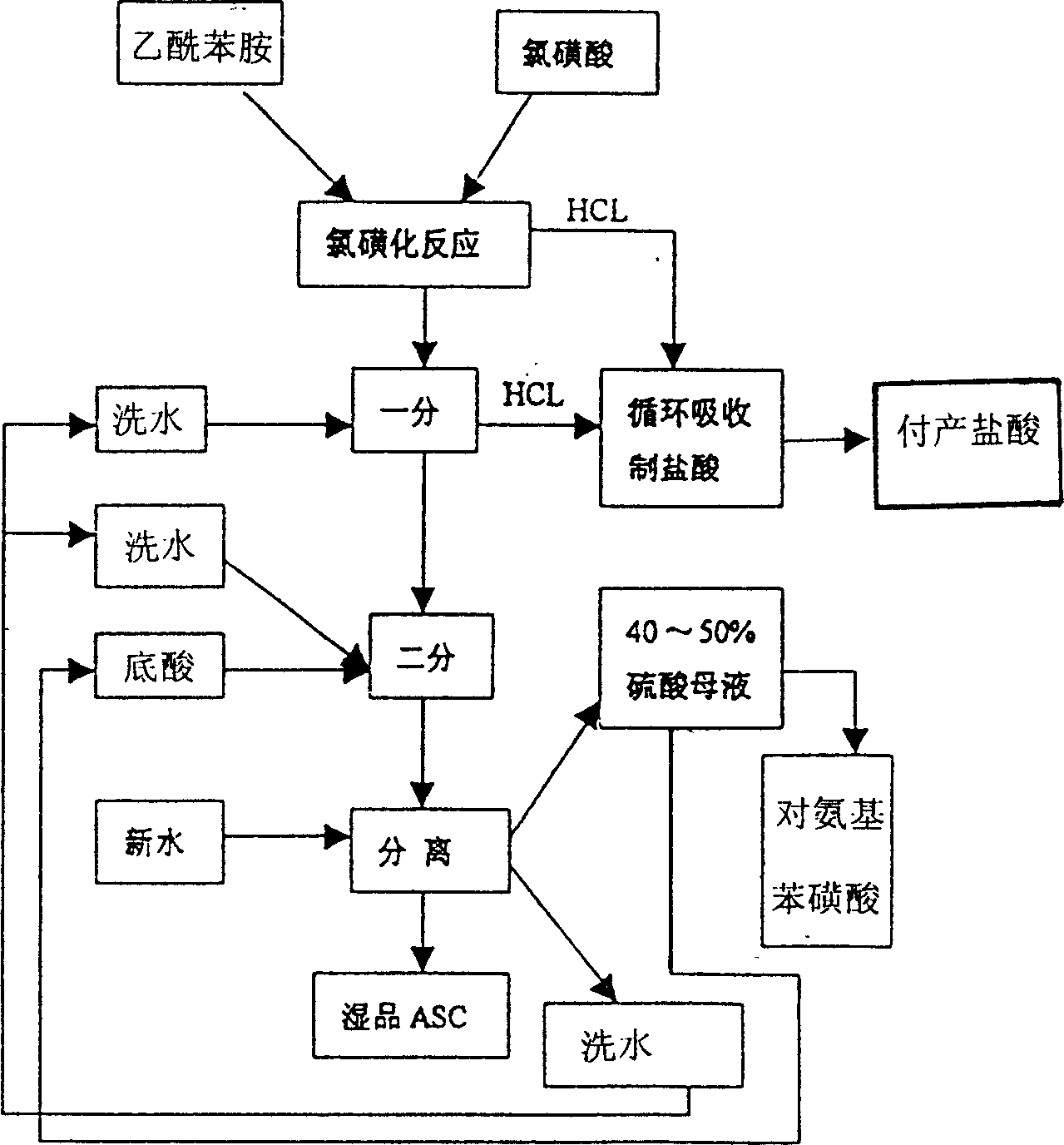
Para-acetylsulfanilamide synthesis method combined with waste water treatment
InactiveCN102320997ASolving Governance ChallengesAchieve recyclingOrganic compound preparationSulfonic acid amide preparationHigh concentrationSynthesis methods
The invention relates to a para-acetylsulfanilamide synthesis method combined with waste water treatment. The method comprises the following steps of: hydrolyzing reaction liquid (commonly called sulfonated oil) generated by chlorosulfonation of acetanilide and chlorosulfonic acid at low temperature; directly adding the reaction liquid hydrolyzed at low temperature into ammonia water to perform amination; and neutralizing and filtering to obtain solid para-acetylsulfanilamide (p-ASN), wherein the filtrate contains p-aminobenzene sulfonic acid and ammonium sulfate; the p-aminobenzene sulfonic acid in the filtrate is recovered by an adsorption method; and the ammonium sulfate is obtained by concentrating, crystallizing, filtering and drying the residual adsorption solution. The method has the advantages that: all the sulfuric acid generated after the sulfonated oil is hydrolyzed is converted into the ammonium sulfate, so the treatment problem of a large amount of high-concentration acidic waste water is solved from the source; the quantity of the liquid to be treated in the new process is 25 percent of that of the liquid to be treated in the conventional process, so a great amount of water resource and capital are saved; the byproducts such as the ammonium sulfate and the p-aminobenzene sulfonic acid can be recovered, so resources can be utilized circularly and comprehensively; and the steamed water in the concentration process of the ammonium sulfate and the p-aminobenzene sulfonic acid is neutral and can serve as industrial water to be recycled.
Owner:丁同富
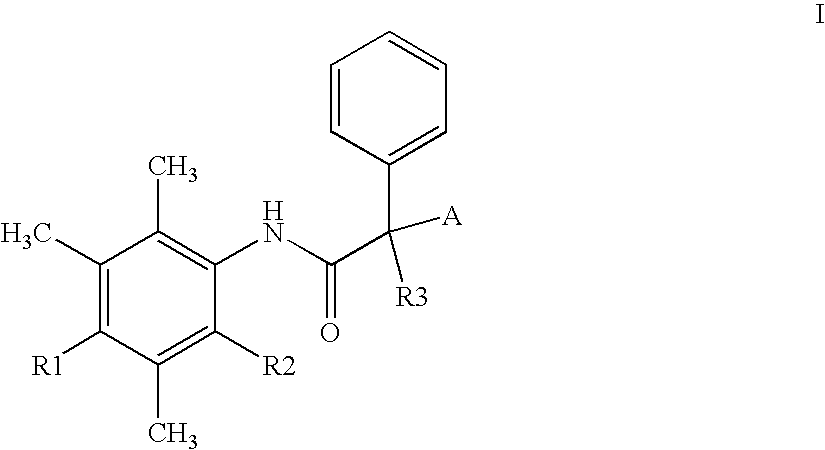
Alpha-phenyl acetanilide derivatives having an acat inhibiting activity and the therapeutic application thereof
InactiveUS20060135785A1Improve bioavailabilityImprove stabilityOrganic chemistryMetabolism disorderCholesterol bloodEnantiomer
Owner:PIERRE FABRE MEDICAMENT SAS
Production process of azoic disperse dye
InactiveCN1754920AIncrease concentrationReduce the amount requiredAzo dye preparationCoupling reaction in azo dyesDisperse dyeAniline
The invention discloses a preparation technique for azo disperse dyes, which comprises preparing esterification solution and coupling reacting. Wherein, the esterification solution comprises mainly one of: 1) 3-N, N-acetoxylated amido-4-methoxyl-1-acetanilide; 2) N- cyanoethyl-N-acetoxylated amido aniline; 3) 3-N, N-acetoxylated amido-1-acetanilide. This invention increases concentration of esterification solution from 65% to about 90% to decrease COD in waste water more than 60%, and has high efficiency.
Owner:ZHEJIANG LONGSHENG GROUP
Synthetic method of p-aminobenzenesulfonamide
InactiveCN105237446APrecisely control the amount addedReduce dosageSulfonic acid amide preparationCentrifugationP-Aminobenzenesulfonamide
Owner:SUZHOU WUGAN PHARMA
Process for producing p-acetamidobenzene sulfonyl chloride
InactiveCN102304070AGuaranteed stabilityFill the gap in the marketSulfonic acid preparationSulfonyl chlorideAcetamido-CNU
The invention relates to a process for producing p-acetamidobenzene sulfonyl chloride. The process comprises: adding chlorosulfonic acid and antifebrine into acetanilide serving as a raw material to perform chlorosulfonation to obtain sulfonated oil; adding water into the obtained sulfonated oil to perform a hydrolysis reaction; subjecting the product of the hydrolysis of sulfonated oil to vacuum filtration; circularly absorbing one hydrogen chloride gas product to prepare diluted hydrochloric acid; and dissolving the other p-acetamidobenzene sulfonyl chloride product in a dissolving kettle, crystallizing, centrifuging and drying to obtain finished p-acetamidobenzene sulfonyl chloride. The process is characterized in that: dichloroethane is added in the dissolution process as a solvent, the finished product obtained after centrifugation is dried by air flow and then packed under vacuum, and thus, the finished product is obtained. In the production process disclosed by the invention, the mass ratio of the added dichloroethane solvent and the acetanilide raw material is (4-8):1, air flow is used for drying, the quality guarantee period of the prepared finished product is as long as 180 days, and the finished product is more stable.
Owner:SUZHOU WUGAN PHARMA
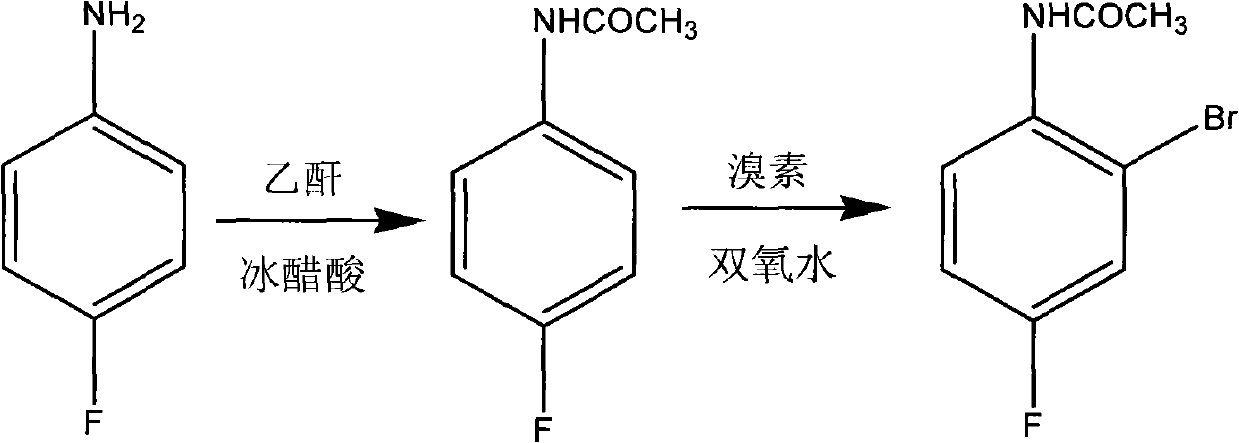
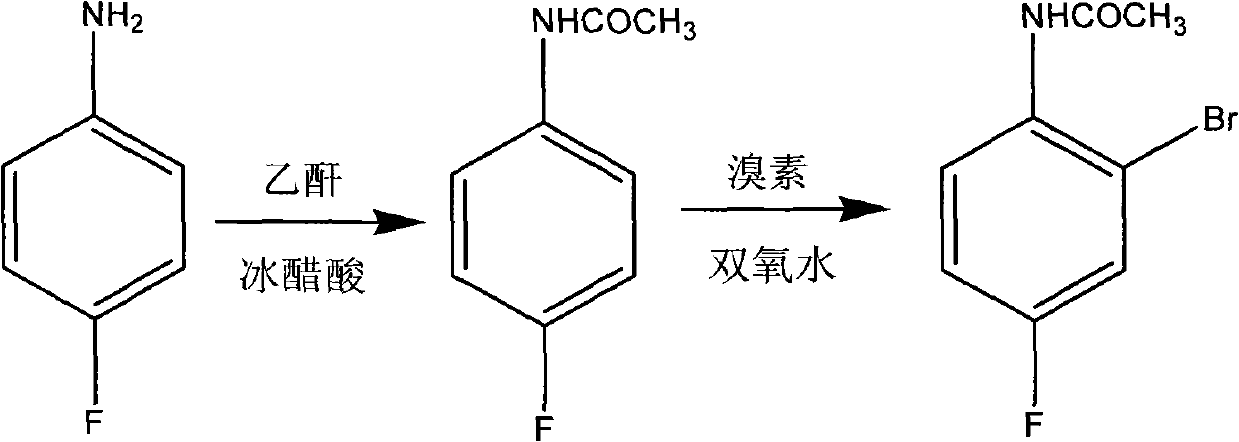
Preparation method of 2-br-4-fluoacetanilide
InactiveCN102120723AHigh reaction conversion rateHigh purityOrganic compound preparationCarboxylic acid amides preparationChemical reactionAcetic anhydride
The invention belongs to preparation of compound products of fine chemical engineering and particularly relates to a preparation method of 2-br-4-fluoacetanilide, which comprise the steps of: acetylation: adding para-fluoro aniline and glacial acetic acid into a reaction flask, heating to 50 DEG V, dropping acetic anhydride, carrying out reaction for 1-3h under the condition of 55-100 DEG C to prepare an intermediate para-fluoro acetanilide; and (2) bromination: dropping bromine at 45-55 DEG C, carrying out reaction for 1-3h under the condition of 50-60 DEG C, then dropping hydrogen peroxide at 40-55 DEG C, carrying out reaction for 1-3h under the condition of 50-60 DEG C, decolorizing and crystallizing with sodium hydrogensulfite, recrystallizing with an ethanol water solution to obtain a finished product of the 2-br-4-fluoacetanilide. The 2-br-4-fluoacetanilide has a chemical reaction formula shown in the specification, the whole process is shorter in reaction time and simple in operation, the reaction conversion rate for the acetylation and the bromination is up to above 90%, a finally obtained product has high purity, and the preparation method is suitable for enlarged industrial production; and thus, the production cost is reduced.
Owner:KINGCHEM LIAONING CHEMICAL CO LTD
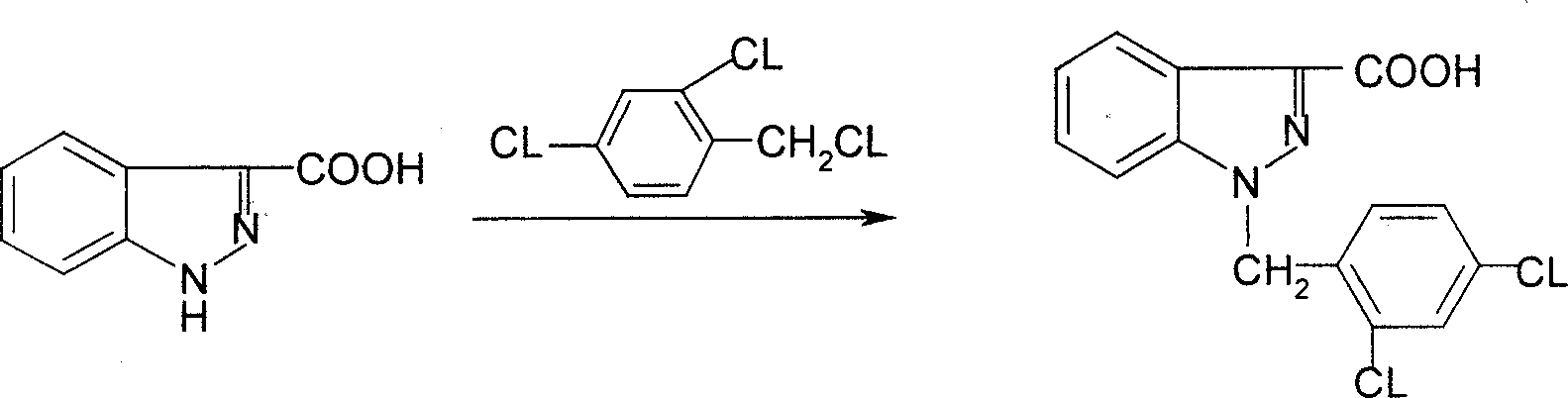
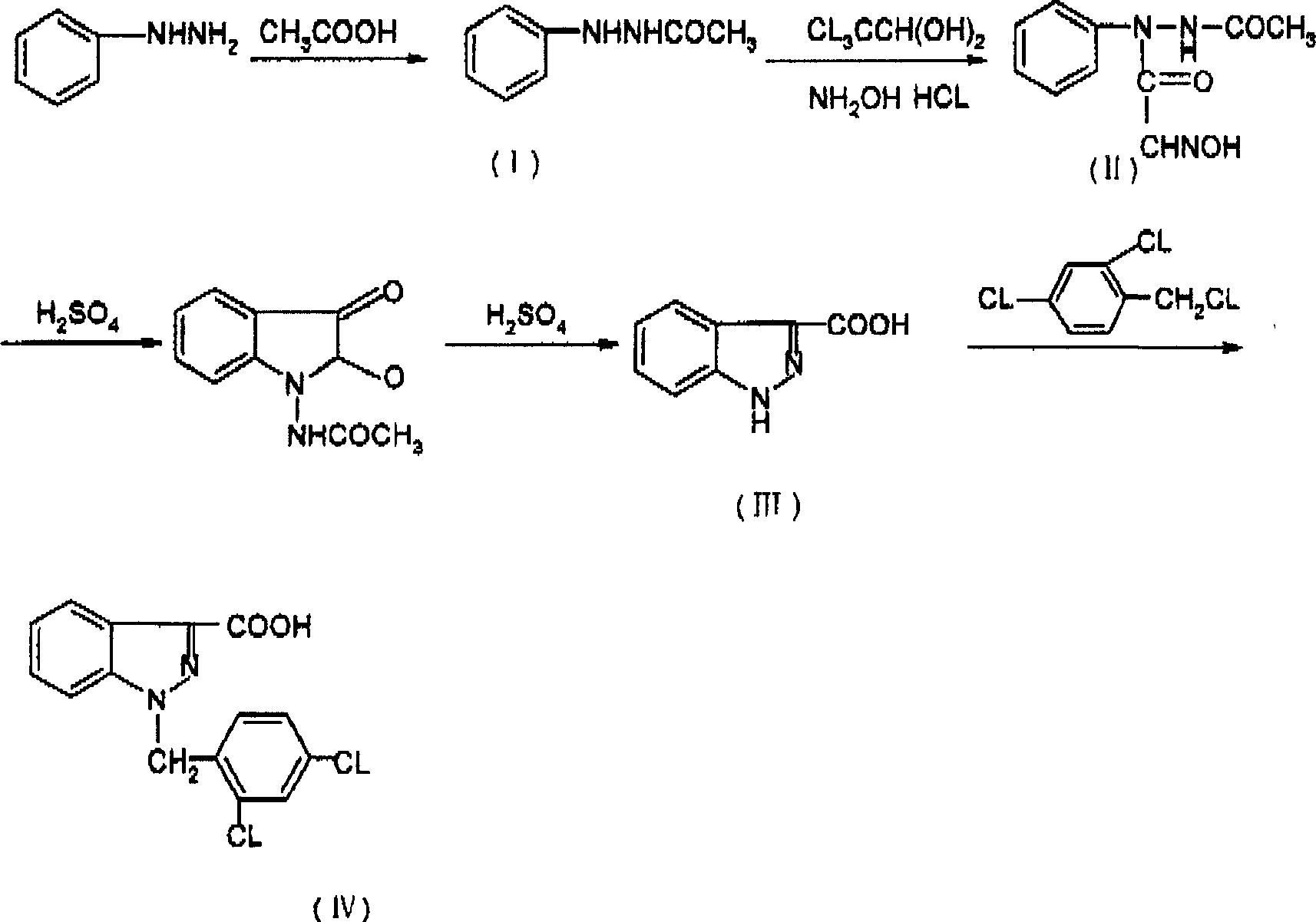
Process for synthesis of lonidamine
ActiveCN1594297AReduce pollutionLow costOrganic chemistryAntineoplastic agentsSynthesis methodsCarboxylic acid
The invention relates to a process for synthesizing 1-[(2,4-dichlorobenzene) methyl]-1H-indazole-3-carboxyl acid which consists of, using phenylhydrazine as starting raw material, producing beta-acetylphenylhydrazine through reaction with glacial acetic acid, reacting with hydrated chloral and hydroxylamine hydrochloride, obtaining N-acetamido-isonitro-acetanilide, preparing 1H-indazole-3-carboxylic acid under the condition of concentrated sulfuric acid, finally subjecting 1H-indazole-3-carboxylic acid with 2,4-dichlorin benzyl chloride.
Owner:SHANGHAI ZHAOHUI PHARMA +1
Para-ester synthesis method
ActiveCN107056663AReduce dosageReduce generationOrganic compound preparationSulfonic acid preparationP-chloroacetanilideChlorosulfuric acid
The invention relates to the technical field of dye intermediates, in particular to a para-ester synthesis method. According to the synthesis method, two reactions of a single sulfonation reaction and chlorination reaction are adopted to replace a chlorylation reaction with chlorosulfonic acid directly used in the traditional reaction; on one hand, the dosage of the chlorosulfonic acid can be reduced, and the production of waste acid is reduced; on the other hand, due to the presence of fuming sulfuric acid, the reactivity of the reacting chlorosulfonic acid and acetanilide can be changed, and the formation of chloroacetanilide by-products can be reduced. In addition, the para-ester synthesis method has the advantages that reaction raw materials are easy to obtain; the reaction condition is mild; the product quality is stable; the method is suitable for large-scale industrial production.
Owner:新乡市锦源化工有限公司
Synthesis method of environmental-friendly p-(beta-sulfatoethylsulfonyl) aniline
ActiveCN104193657AAltered sulfonation activityReduce production processOrganic compound preparationSulfonic acid preparationP-chloroacetanilideSynthesis methods
The invention discloses a synthesis method of environmental-friendly p-(beta-sulfatoethylsulfonyl) aniline. The synthesis method comprises the following steps: (1) in the presence of a chlorination inhibitor, carrying out sulfonation reaction on acetanilide and chlorosulfonic acid, and then carrying out chlorination reaction on the obtained product and thionyl chloride, so that a chlorosulfonation substance is obtained; and (2) sequentially carrying out condensation reaction, sulfonating reaction and hydrolysis reaction on the chlorosulfonation substance obtained in the step (1), so that environmental-friendly p-(beta-sulfatoethylsulfonyl) aniline is obtained. According to the preparation method, by changing a production formula, introducing the chlorination inhibitor, and changing the reaction reactivity of reaction raw materials, p-chloroacetanilide byproducts are greatly reduced, so that an effect that a para-ester product parachloroaniline meets the standard is finally achieved.
Owner:ZHEJIANG JINGGUANG IND
Treatment method for p-beta hydroxyethyl sulfone acetanilide mother liquor waste water
ActiveCN103756359AImprove dyeing effectPromote emission standardsReactive dyesWater/sewage treatmentHigh concentrationAfter treatment
The invention discloses a treatment method for p-beta hydroxyethyl sulfone acetanilide mother liquor waste water. The treatment method comprises the following steps of performing pretreatment on p-beta hydroxyethyl sulfone acetanilide mother liquor water to recover p-beta hydroxyethyl sulfone acetanilide in mother liquor waste water, and then sequentially performing diazo reaction, coupling, primary condensation and secondary condensation to prepare a yellow reactive dye. According to the treatment method disclosed by the invention, p-beta hydroxyethyl sulfone acetanilide in the p-beta hydroxyethyl sulfone acetanilide mother liquor waste water produced in the production process of para-ester is reasonably utilized, and p-beta hydroxyethyl sulfone acetanilide in the waste water is used for synthesizing an intermediate of the reactive dye after hydrolysis, so that the waste is turned into treasure, the high-concentration waste water produced in the production process of the para-ester can achieve up-to-standard emission after treatment at the relatively low cost, and the para-ester and the related industry of the para-ester can realize sustained development.
Owner:ZHEJIANG JINGGUANG IND
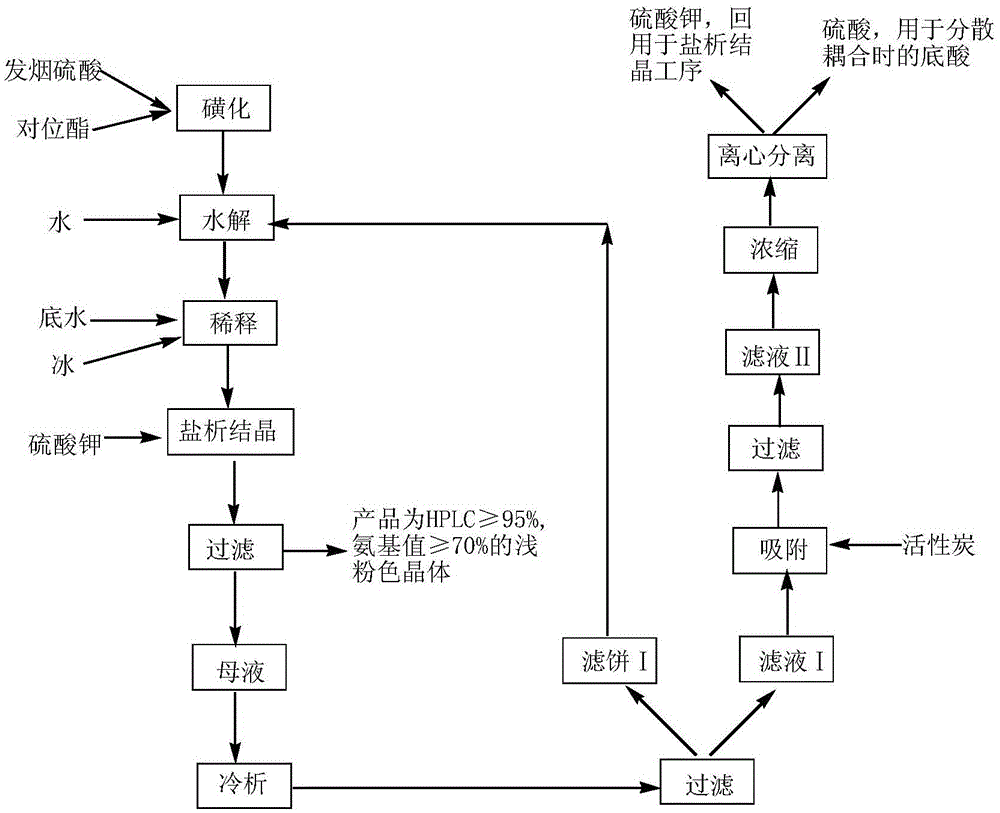
Clean production process for dye intermediate sulfonated para-ester
ActiveCN105130858AReduce wasteSimple processOrganic chemistryOrganic compound preparationHydrolysateSorbent
The invention discloses a clean production process for a dye intermediate sulfonated para-ester. The process comprises the following steps: (1) sulfonation: carrying out a reaction on p-beta-ethoxyl sulfonyl acetanilide and sulfuric acid to obtain a sulfonated body; (2) hydrolyzation: carrying out hydrolysis reaction on the sulfonated body and water to obtain a hydrolysate; (3) dilution: adding water into the hydrolysate to dilute the hydrolysate to obtain a diluted liquid; (4) salting-out crystallization: adding potassium sulfate into the diluted liquid for salting-out crystallization and filtering the liquid to obtain sulfonated para-ester and mother liquor wastewater; and (5) resource recycling of the mother liquor wastewater: cooling and cold-separating the mother liquor wastewater and filtering the mother liquor wastewater to obtain a filtrate I and a filter cake I, adding an adsorbent into the filtrate I for adsorption, filtering the filtrate to obtain a filtrate II, and then concentrating the filtrate II, carrying out cooling crystallization and separation on the concentrated filtrate II to obtain diluted sulfuric acid and solid potassium sulfate. According to the clean production process, useful intermediate product, potassium sulfate and sulfuric acid in the wastewater are effectively recovered by adding the potassium sulfate for salting-out crystallization, and meanwhile, COD of the wastewater is reduced.
Owner:JIANGSU YUANZHENG CHEM
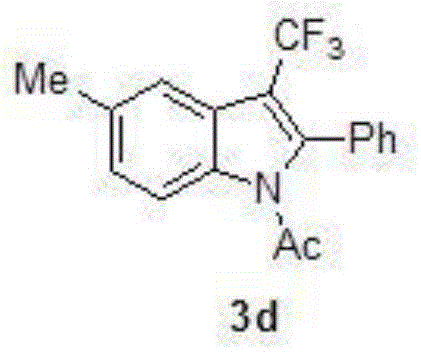
Preparation method for 3-substituted trifluoromethyl indole
InactiveCN105130872AHigh chemoselectivityHigh regional selectivityOrganic chemistryRegioselectivityAniline
The invention discloses a preparation method for 3-substituted trifluoromethyl indole. Various substituted acetanilides are taken as a reaction substrate with a medium to excellent reaction yield, excellent reaction chemical selectivity, and high area selectivity; another isomer (2-substituted trifluoromethyl indole) is monitored to be not detected in detection; the conditions are gentle; the substrate is wide in scope of application (wherein R is H or various electron-donating groups such as CH3, OCH3, SCH3 and the like, as well as various electron withdrawing groups such as NO2, Cl and the like, and Ar is various substituted benzene rings); the preparation method is simple and convenient in operation, relatively low in cost, less in side reaction, high in product purity, convenient in separation and purification, and suitable for large-scale preparation; and a product prepared by the preparation method has a very good application prospect in the biomedicine field.
Owner:JIANGXI NORMAL UNIV
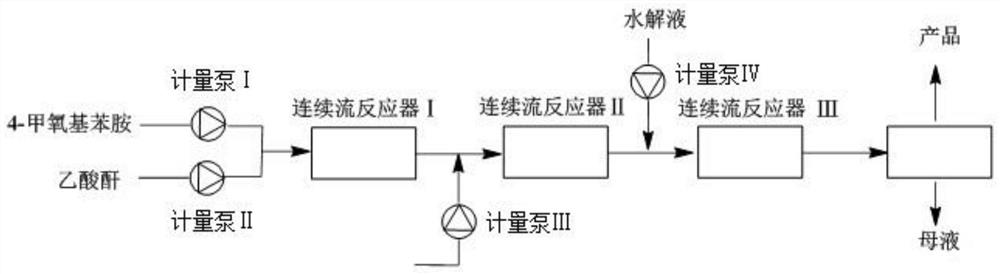
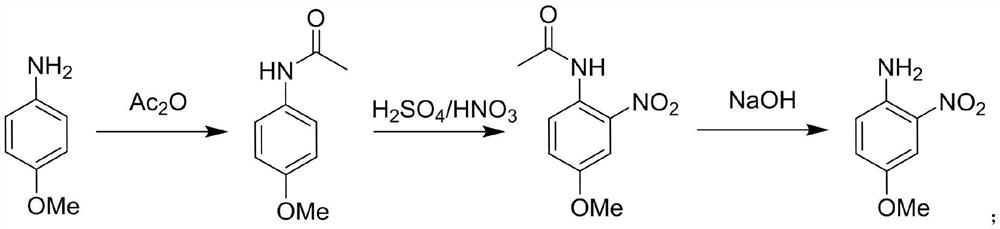
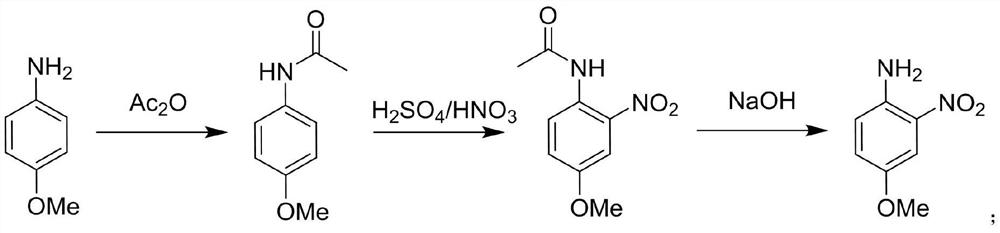
Method for synthesizing 4-methoxy-2-nitroaniline by adopting continuous flow reactor
PendingCN111704555ANo purification requiredThere will be no quizzesOrganic compound preparationCarboxylic acid amides preparationNitroacetanilideAcetic anhydride
The invention relates to the technical field of organic synthesis, and discloses a method for synthesizing 4-methoxy-2-nitroaniline by using a continuous flow reactor. The method comprises the following steps: S1, respectively adding a 4-methoxyaniline solution and acetic anhydride into a continuous flow reactor I, and carrying out an acetylation reaction to obtain a reaction solution I containing4-methoxyacetanilide; S2, respectively adding a nitration reagent and the reaction solution I into a continuous flow reactor II, and carrying out a nitration reaction to obtain a reaction solution IIcontaining 4-methoxy-2-nitroacetanilide; S3, respectively adding hydrolysate and the reaction solution II into a continuous flow reactor III, and carrying out a hydrolysis reaction to obtain a reaction solution III containing 4-methoxy-2-nitroaniline; and S4, carrying out post-treatment on the reaction solution III to obtain 4-methoxy-2-nitroaniline. The method is high in reaction speed, small inamount of byproduct 4-methoxy-3-nitroaniline, high in heat and mass transfer efficiency, high in reaction safety, high in selectivity, high in yield and purity and convenient in after-treatment.
Owner:ASTATECH CHENGDU BIOPHARM CORP
Preparation method of light-resistant and waterproof paint
InactiveCN103937364AImprove the defect of "hot sticky and cold brittle"Improve waterproof performanceCoatingsPolymer scienceEmulsion
The invention relates to a preparation method of light-resistant and waterproof paint, which comprises the following steps: adding water, emulsifier and acrylate, heating to 45 DEG C, and stirring for 45 minutes; adding a monomer A, emulsifying for 40-60 minutes, and heating to 75 DEG C; introducing return water, heating to 80 DEG C, keeping the temperature, dropwisely adding initiator, and reacting for 2 hours to obtain a core layer emulsion; dropwisely adding a monomer B and the initiator into the obtained core layer emulsion simultaneously for 1-3 hours, then keeping the temperature at 75-85 DEG C, and reacting for 2 hours; adding 10-20 parts by weight of light-resistant material, keeping the temperature at 75-85 DEG C, and reacting at 1-3 hours; adding 0.5-1.0 part by weight of metal salt, regulating the pH value to 4.0, keeping the temperature at 75-85 DEG C, and reacting for 1-2 hours; cooling to 50 DEG C, adding 0.2 part by weight of crosslinking agent, and reacting for 30 minutes; adding 0.3 part by weight of organic silicon and 0.1 part by weight of acetanilide, and reacting for 60 minutes; and adding ammonia water to regulate the pH value to 5, thus obtaining the light-resistant and waterproof paint. The obtained acrylate resin has favorable light resistance and water resistance, and the production process is environment-friendly.
Owner:FOSHAN SHUNDE HESHENG CHEMICAL INDUSTRIAL CO LTD
Alpha-form or beta-form crystal of acetanilide derivative
ActiveUS20050004190A1Good hygroscopicityImprove stabilityOrganic active ingredientsBiocideAdditive ingredientDiabrezide
To provide novel crystals useful as an ingredient for the production of a diabetes remedy. The invention is concerned with α-form crystal and β-form crystal of (R)-2-(2-aminothiazol-4-yl)-4′-[2-[(2-hydroxy-2-phenyleth-yl)amino]ethyl]acetanilide. The α-form crystal does not exhibit hygroscopicity and has stability such that it can be used as a medicine, and is useful for mass synthesis in the industrial production. The β-form crystal does not relatively exhibit hygroscopicity and is also useful as a production intermediate of the α-form crystal.
Owner:ASTELLAS PHARMA INC
Preparation method of m-fluoroaniline
InactiveCN101367738ASimple processLow costOrganic compound preparationAmino compound preparationDecompositionSteam distillation
The present invention relates to a preparation method of m-fluoroaniline. Water and m-amino acetanilide hydrochloride are mixed, cooled to be at the temperature of minus 5 DEG C and 0 DEG C, and added into NaOH aqueous solution to be neutralized; borofluoric acid aqueous solution is added; and the temperature of the mixed solution is maintained between minus 5 DEG C and 0 DEG C. Then NaNO2 aqueous solution is dropped for diazo reaction, and the borofluoric diazonium salt is prepared. The diazonium salt is added into a reaction bottle to be heated and decomposed until nearly no gas is emitted; and the decomposition and fluorination are stopped. NaOH aqueous solution are added into the reaction bottle, acetyl is eliminated through mixing and hydrolysis, steam distillation and static demixing are completed, and the product m-fluoroaniline is in the oil layer. The main raw materials for synthesis, m-amino acetanilide hydrochloride, NaNO2, borofluoric acid and NaOH are all cheaper and easily accessible, the technologies of diazo reaction, decomposition and fluorination are simpler, the cost is low, and the present invention is easy for industrialization.
Owner:TIANJIN UNIV
Method for synthesizing n-acetylsulfanilyl chloride by using sulfur trioxide as sulfonating agent
ActiveCN106866466AGuaranteed qualityAvoid it happening againSulfonic acid preparationAcetic acidWastewater
The invention relates to a method for synthesizing n-acetylsulfanilyl chloride by using sulfur trioxide as a sulfonating agent. The method comprises the following steps: (a) sequentially adding acetanilide and glacial acetic acid into a reaction kettle, stirring and warming to 40 to 70 DEG C; (b) inflating sulfur trioxide gas into the reaction kettle, reacting for 3 to 15 hours at the temperature of 40 to 70 DEG C to enable acetanilide to be completely converted and stopping inflating the sulfur trioxide gas; (c) warming temperature in the reaction kettle to 65 to 80 DEG C and decompressing to recycle the glacial acetic acid; (d) controlling temperature in the reaction kettle as 60 to 90 DEG C, dropwise adding thionyl chloride to react for 2 to 4 hours, decompressing to recycle unreacted thionyl chloride and cooling. Therefore, quality of n-acetylsulfanilyl chloride is guaranteed, waste water and waste acid are prevented from being generated, processing cost is saved, and environmental friendliness is facilitated.
Owner:吴赣药业(苏州)有限公司
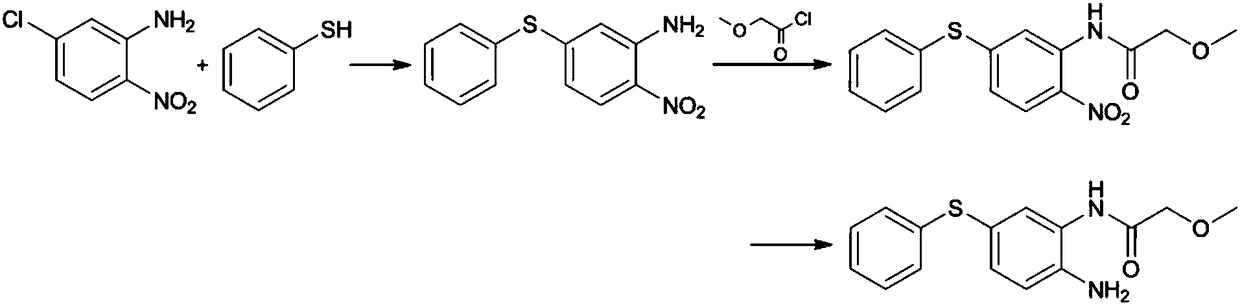
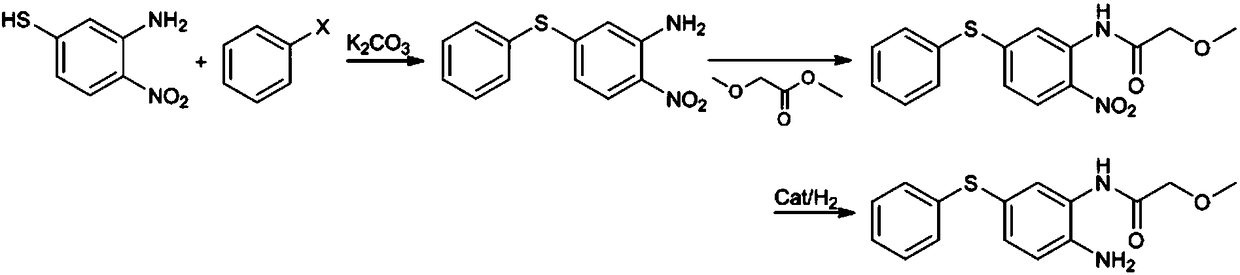
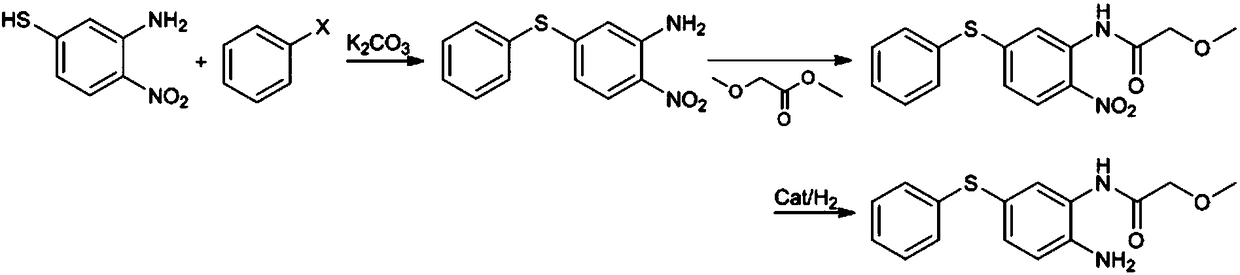
Preparation method of 2-amino-5-phenylthio-(2-methoxy)acetanilide
ActiveCN108299259AGood reaction selectivityReduce usageSulfide preparationMethyl methoxyacetateOrganic synthesis
The invention belongs to the technical field of organic synthesis and particularly relates to a preparation method of 2-amino-5-phenylthio-(2-methoxy)acetanilide. According to the method, 2-nitro-5-(phenylthio)aniline is prepared from 2-nitro-5-mercaptoaniline and benzene halide and then subjected to a reaction with methyl methoxyacetate, 2-nitro-5-phenylthio-(2-methoxy)acetanilide is generated and subjected to catalytic hydrogenation reduction finally, and the target product is obtained. The preparation method has good reaction selectivity, and the obtained target product is high in purity and yield; use of high-toxicity materials is avoided, requirements for equipment and operation conditions are reduced, and safety and stability of the preparation method are improved; doses of the reaction materials are optimized, and excess benzene halide and methyl methoxyacetate can be recycled after being recovered simply; little solid waste is produced, waste treatment cost is reduced greatly,and the requirement for environmental protection is met.
Owner:珠海优润医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![N[S(4-aryl-triazol-3-yl)α-mercaptoacetyl]-<i>p</i>-amino benozioc acids as HIV reverse transcriptase inhibitors N[S(4-aryl-triazol-3-yl)α-mercaptoacetyl]-<i>p</i>-amino benozioc acids as HIV reverse transcriptase inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0efc470b-2453-496c-a38c-02b8a1dc5ec7/US07435752-20081014-C00001.png)
![N[S(4-aryl-triazol-3-yl)α-mercaptoacetyl]-<i>p</i>-amino benozioc acids as HIV reverse transcriptase inhibitors N[S(4-aryl-triazol-3-yl)α-mercaptoacetyl]-<i>p</i>-amino benozioc acids as HIV reverse transcriptase inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0efc470b-2453-496c-a38c-02b8a1dc5ec7/US07435752-20081014-C00002.png)
![N[S(4-aryl-triazol-3-yl)α-mercaptoacetyl]-<i>p</i>-amino benozioc acids as HIV reverse transcriptase inhibitors N[S(4-aryl-triazol-3-yl)α-mercaptoacetyl]-<i>p</i>-amino benozioc acids as HIV reverse transcriptase inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0efc470b-2453-496c-a38c-02b8a1dc5ec7/US07435752-20081014-C00003.png)