Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
384results about How to "High chemoselectivity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
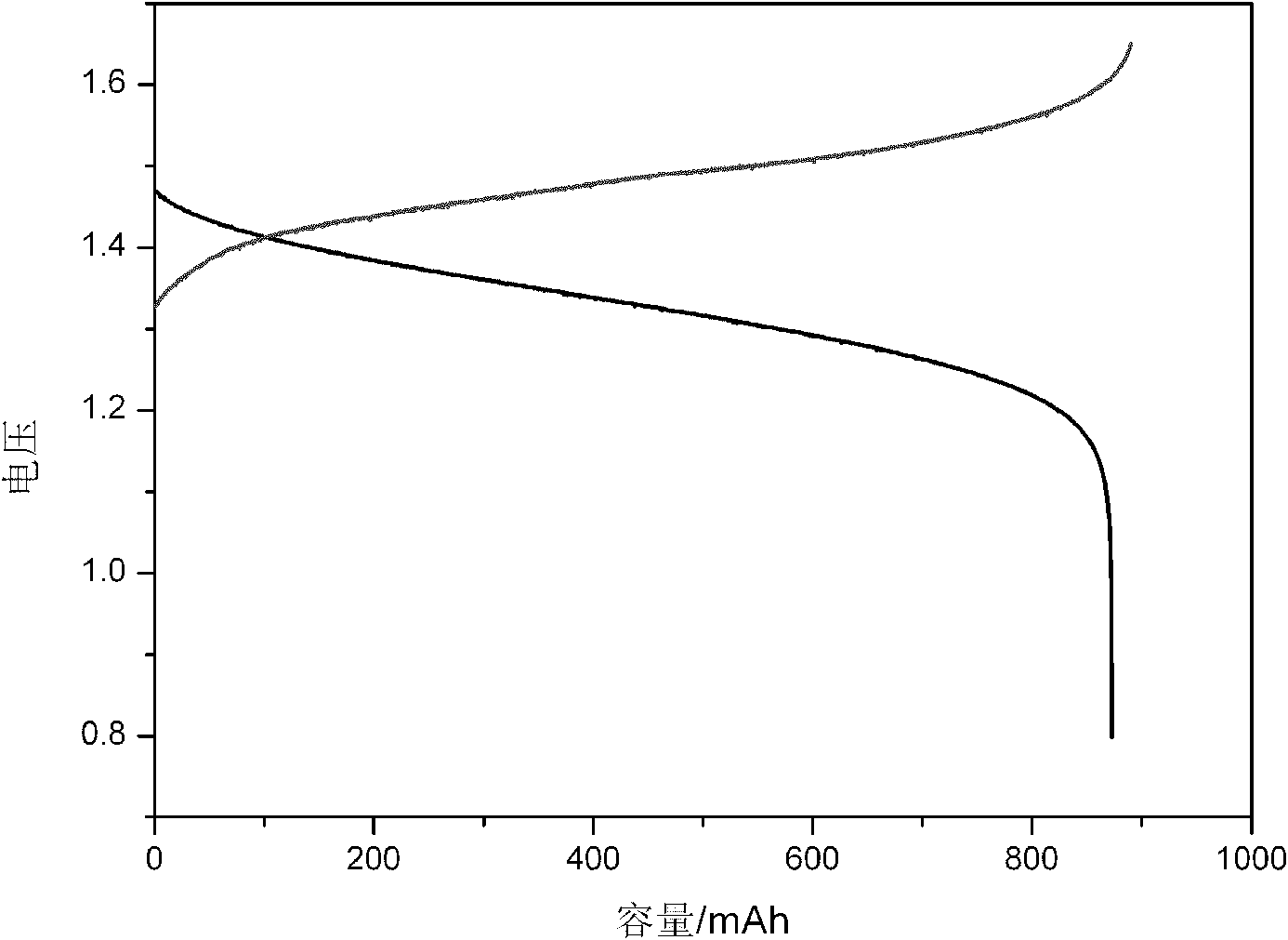
Microfabricated ion trap array
ActiveUS7154088B1High chemical selectivityResolution advantageStability-of-path spectrometersIsotope separationPhysicsIntegrated circuit
A microfabricated ion trap array, comprising a plurality of ion traps having an inner radius of order one micron, can be fabricated using surface micromachining techniques and materials known to the integrated circuits manufacturing and microelectromechanical systems industries. Micromachining methods enable batch fabrication, reduced manufacturing costs, dimensional and positional precision, and monolithic integration of massive arrays of ion traps with microscale ion generation and detection devices. Massive arraying enables the microscale ion traps to retain the resolution, sensitivity, and mass range advantages necessary for high chemical selectivity. The reduced electrode voltage enables integration of the microfabricated ion trap array with on-chip circuit-based rf operation and detection electronics (i.e., cell phone electronics). Therefore, the full performance advantages of the microfabricated ion trap array can be realized in truly field portable, handheld microanalysis systems.
Owner:NAT TECH & ENG SOLUTIONS OF SANDIA LLC
Preparation method for artificially synthesized transparent soil material
InactiveCN103969099AHigh purityWide range of gradingPreparing sample for investigationAlkaneVisual observation
The invention discloses a preparation method for an artificially synthesized transparent soil material. The transparent soil material comprises baked silica sand particles and colorless pore fluids which have the same refractive index, wherein the pore fluids are mixed fluids of 12, alkane and 15# white oil; during preparation, a glass bar is used to stir constantly so as to ensure intensive mixing; a vacuum chamber is adopted for vacuumizing so as to remove residual air in a mixture until the mixture is transparent; finally, a consolidometer is adopted for consolidation. According to the invention, the preparation method is simple and convenient to operate and easy to implement; compared with other preparation methods, the preparation method is wide in material sources, low in cost, pollution-free and non-hazardous and can achieve a better transparent effect; the prepared transparent soil can be effectively used for model tests, and metabolic un-embedding visual observation in soil bodies can be realized.
Owner:HOHAI UNIV
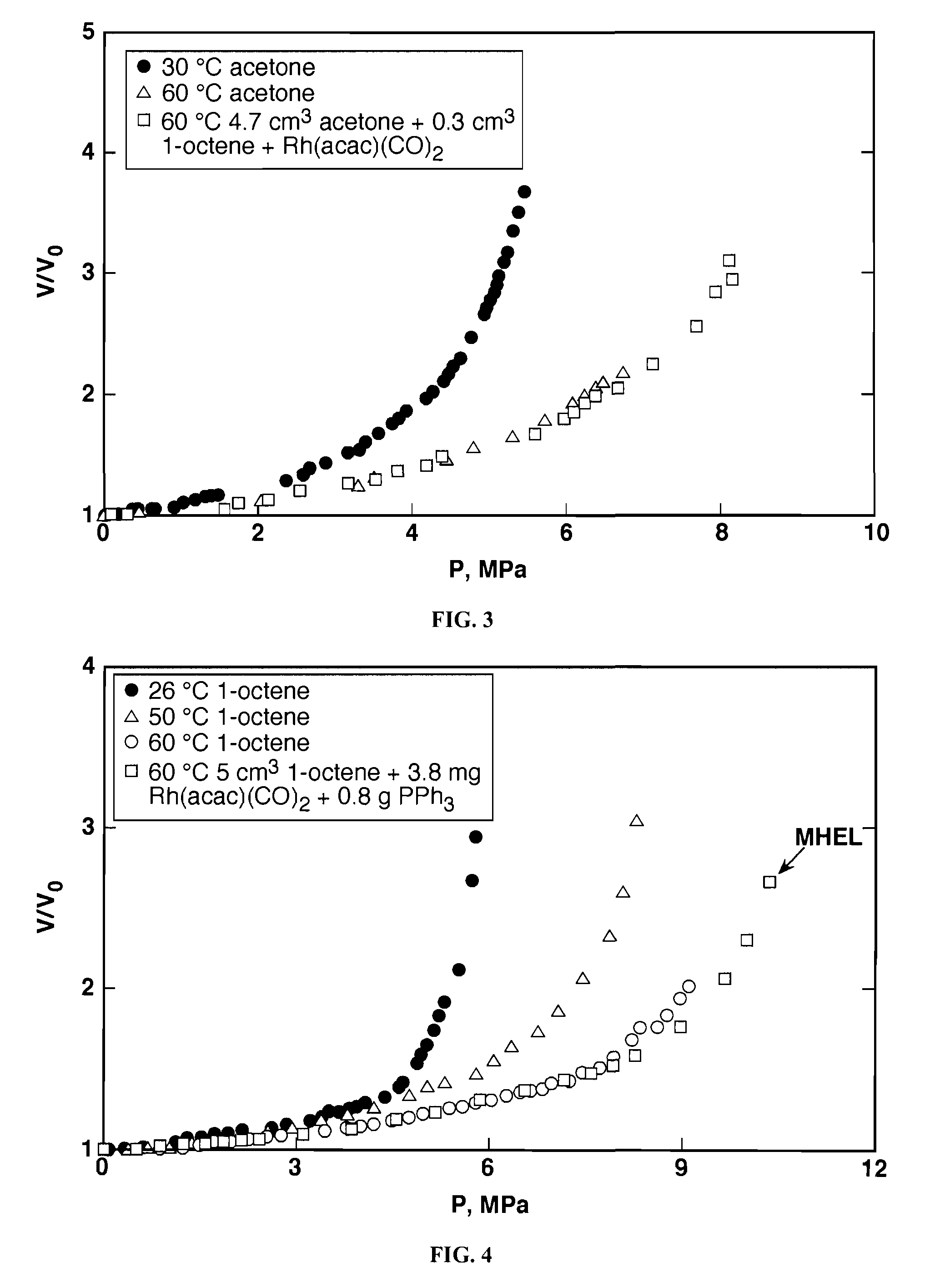
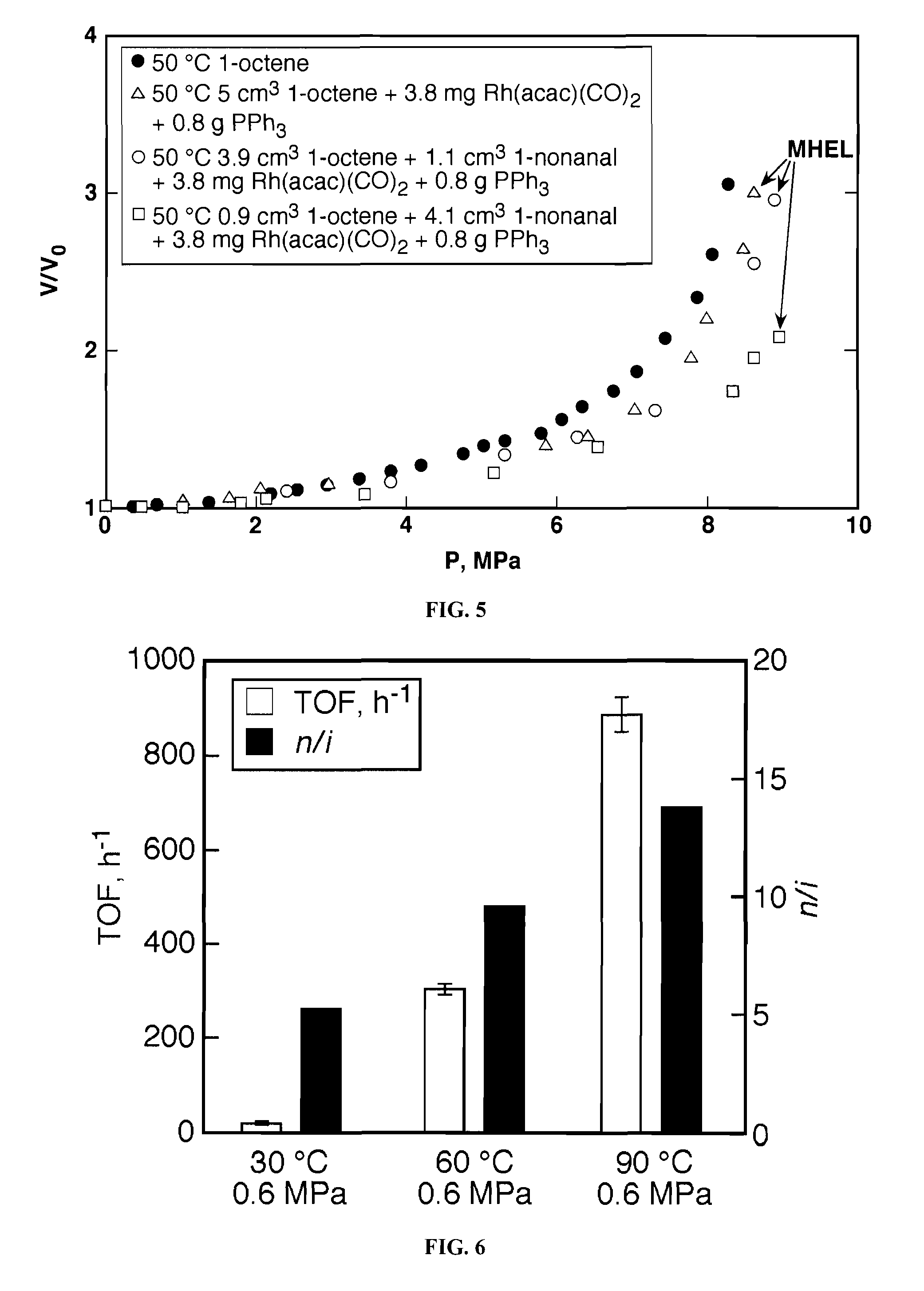
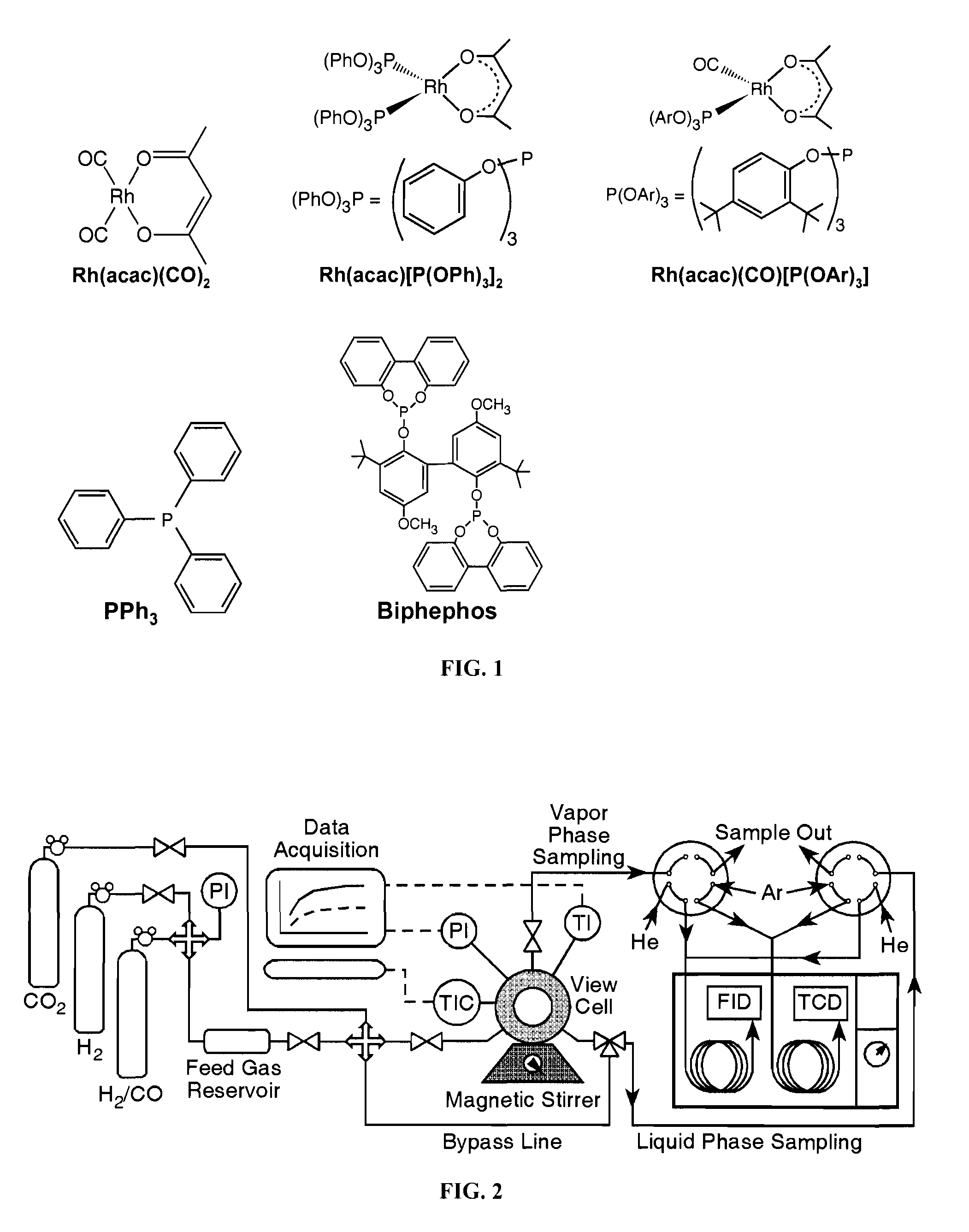
Tuning product selectivity in catalytic hydroformylation reactions with carbon dioxide expanded liquids
ActiveUS7365234B2Alters chemoselectivityAlters regioselectivityOrganic compound preparationPreparation by carbon monoxide reactionSupercritical carbon dioxideChemistry
An improved hydroformylation process is provided, which comprises reacting an olefin with CO and H2 in the presence of a hydroformylation catalyst in a liquid that has been volumetrically expanded with a compressed gas, such as supercritical carbon dioxide.
Owner:UNIVERSITY OF KANSAS
Process for the carbonylation of a conuugated diene
InactiveUS20060235241A1Improve rigidityPromote resultsOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsHydrogen atomDiphosphines
A process for the carbonylation of a conjugated diene, which involves reacting the conjugated diene with carbon monoxide and a co-reactant having a mobile hydrogen atom in the presence of a catalyst system including: (a) a source of palladium; and (b) a bidentate diphosphine ligand of formula II, R1R2>P1—R3m—R—R4n—P2<R5R6 (II) wherein P1 and P2 represent phosphorus atoms; R1, R2, R5 and R6 independently represent the same or different optionally substituted organic radical containing a tertiary carbon atom through which each radical is linked to the phosphorus atom; R3 and R4 independently represent the same or different optionally substituted methylene groups; R represents an organic group having the bivalent bridging group C1—C2 through which R is connected to R3 and R4; m and n independently represent a natural number in the range of from 0 to 4, wherein the rotation about the bond between the carbon atoms C1 and C2 of the bridging group is restricted a temperature in the range of from 0° C. to 250° C., and wherein the dihedral angle between the plane occupied by the three atom sequence composed of C1, C2 and the atom directly bonded to C1 in the direction of P1, and the plane occupied by the three atom sequence C1, C2 and the atom directly bonded to C2 in the direction of P2, is in the range of from 0 to 120°; and (c) a source of an anion.
Owner:SHELL OIL CO
Directed evolution of recombinant monooxygenase nucleic acids and related polypeptides and methods of use
InactiveUS20060051782A1Improve abilitiesIncrease rangeBacteriaLibrary screeningNitrobenzeneMonooxygenase
The present invention relates to novel monooxygenase nucleic acids and polypeptides created using mutagenesis, DNA shuffling, or both, in a single iteration or multiple iterations, and methods for their creation and use. The monooxygenase enzymes of the present disclosure have particular utility as biocatalysts in industrial chemical redox reactions, such as the oxidation of aromatic hydrocarbons, for example, toluene, benzene, or nitrobenzene, into industrially desirable products. The systems and processes of the present invention are especially useful for the coupled synthesis and recovery of catechols, methylcatechols, resorcinols, methylresorcinols, hydroquinones, methylhydroquinones, hydroxybenzenes, cresols, nitrobenzenes, and nitrohydroxyquinones.
Owner:UNIV OF CONNECTICUT
Process for preparing molybdenum nitride and use thereof as hydrogenation and desulfurizing catalyst
InactiveCN1401580AUniform crystal phaseHigh desulfurization activityMolybdenum halidesRefining to eliminate hetero atomsHydrodesulfurizationOxygen
A process for preparing molybdenum nitride from MoO3 includes multi-stage thermal reaction in the mixed atmosphere of N2 and H2, cooling in inert gas and purifying in oxygen. Said product can be used as hydrodesulfurizing catalyst for hdyrodesulfurizing at 250-450 deg.C and 0.2-20 MPa. Its advantages are simple process and low cost.
Owner:SHANXI INST OF COAL CHEM CHINESE ACAD OF SCI
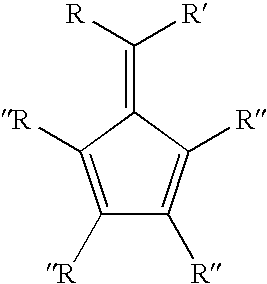
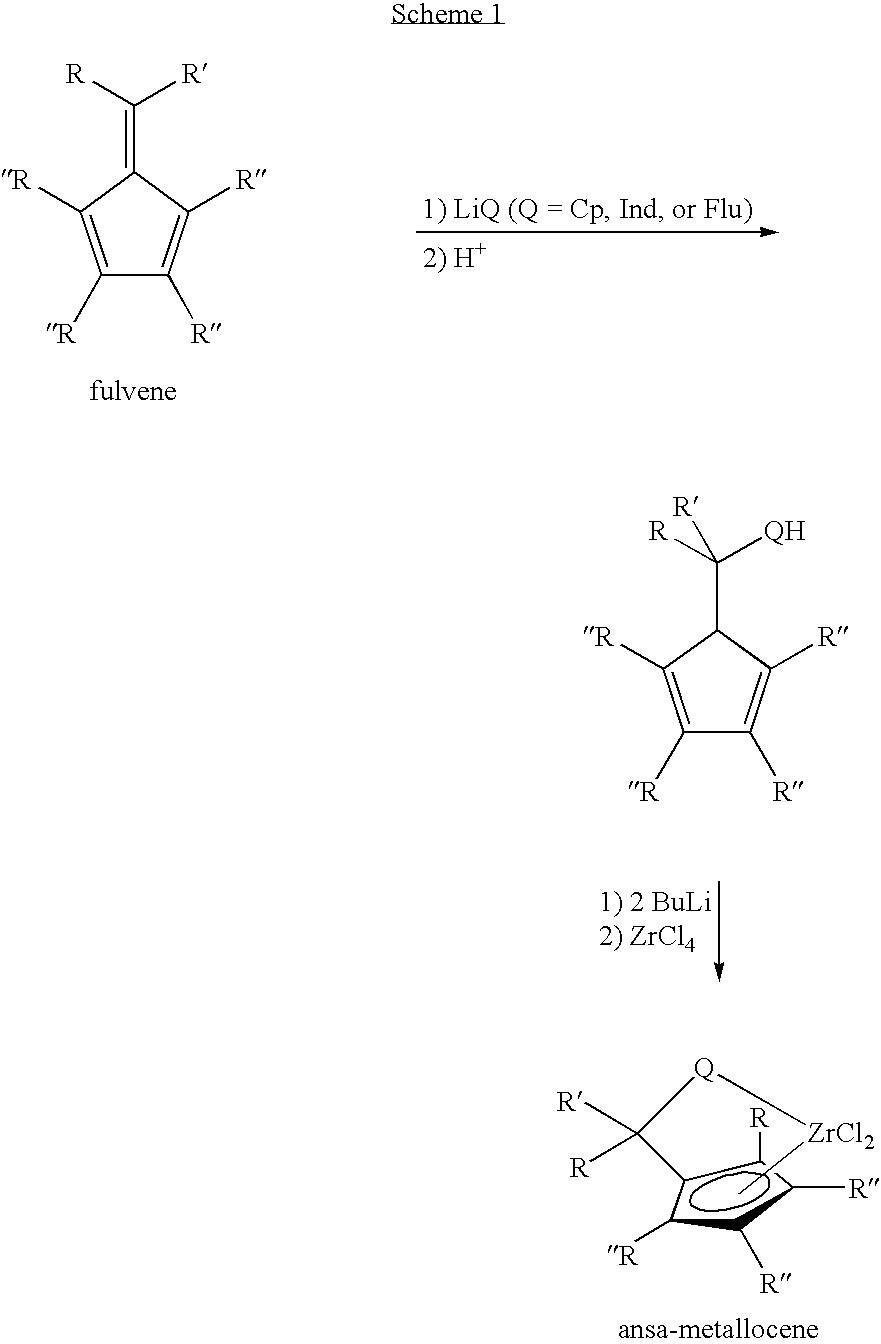
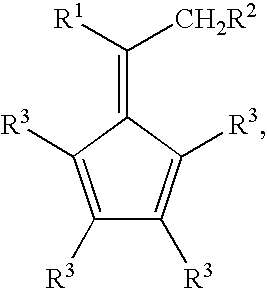
Synthesis of 6-aryl-6-alkyl fulvenes, 6-aryl-6-alkenyl fulvenes, and related compounds
ActiveUS20050288524A1High chemical selectivityHigh yieldSilicon organic compoundsHydrocarbon from oxygen organic compoundsSolventFulvenes
The present invention provides a method of making fulvenes, particularly 6-aryl-6-alkylfulvenes, 6-aryl-6-alkenylfulvenes, and related compounds, by combining alkyl- or alkenyl-arylketones with magnesium cyclopentadienyl reagents in nonprotic, including ethereal, solvents. The use of these compounds in preparing bis(cyclopentadienyl)methanes and related compounds, and ansa-metallocenes, is disclosed.
Owner:CHEVRON PHILLIPS CHEMICAL CO LP
Synthesis of 6-aryl-6-alkyl fulvenes, 6-aryl-6-alkenyl fulvenes, and related compounds
ActiveUS7420097B2High chemoselectivityHigh yieldHydrocarbon from oxygen organic compoundsHydrocarbon from halogen organic compoundsArylMetallole
The present invention provides a method of making fulvenes, particularly 6-aryl-6-alkylfulvenes, 6-aryl-6-alkenylfulvenes, and related compounds, by combining alkyl- or alkenyl-arylketones with magnesium cyclopentadienyl reagents in nonprotic, including ethereal, solvents. The use of these compounds in preparing bis(cyclopentadienyl)methanes and related compounds, and ansa-metallocenes, is disclosed.
Owner:CHEVRON PHILLIPS CHEMICAL CO LP
Catalysts for the synthesis of oxazolidinone compounds
InactiveUS20170088659A1Low color intensityHigh chemoselectivityOrganic chemistryOxazolidoneCombinatorial chemistry
The present invention relates to a method for the production of oxazolidinone compounds with low colour intensity, comprising the step of reacting an isocyanate compound with an epoxide compound in the presence of a catalyst which is free of halide anions. The invention further relates to a method for the production of oligooxazolidinone and / or polyoxazolidinone compounds, comprising the step of reacting a polyisocyanate compound with a polyepoxide compound in the presence of said catalyst. The invention further relates to oligooxazolidinone and / or polyoxazolidinone compounds with low colour intensity, obtainable by a method according to the invention.
Owner:COVESTRO DEUTSCHLAND AG
Iron-nitrogen co-doped mesoporous carbon and preparation method and application thereof
ActiveCN106914267AEasy to prepareImprove stabilityOrganic chemistryCatalyst activation/preparationIron saltsDissolution
The invention discloses iron-nitrogen co-doped mesoporous carbon and a preparation method and application thereof. The preparation method comprises taking citric acid monohydrate and magnesium nitrate hexahydrate, adding the citric acid monohydrate and magnesium nitrate hexahydrate into deionized water, stirring the solution for dissolution, then placing the solution in an oven, carrying out baking for a period of time to obtain foamy solids as precursors, mixing the precursors, a nitrogen source and an iron salt, firing the mixture in an atmosphere furnace in a nitrogen protective atmosphere, and cooling the product to the room temperature to obtain the iron-nitrogen co-doped mesoporous carbon NMC-Fe. The NMC-Fe has a flower-like structure, petals of the flower-like structure are multi-layer carbon sheets and the iron compound is distributed in the carbon sheets. The iron-nitrogen co-doped mesoporous carbon has high stability and can be used for preparation of aromatic azo compounds. The chemical selectivity of the catalytic reaction is very good so that the yield of the product azobenzene is 94%. Compared with the existing common preparation method, the preparation method provided by the invention has a higher yield.
Owner:永嘉跃龙密封件有限公司
Preparation method for optically-pure citronellol
ActiveCN105330515AHigh chemoselectivityHigh stereoselectivityOrganic compound preparationPreparation by hydrogenationAsymmetric hydrogenationCitronellol
The invention discloses a method for preparing optically-pure citronellol from neral or geranial. According to the method, an optically-pure transition metal catalyst is used, and neral or geranial is subjected to selective asymmetric hydrogenation for obtaining the optically-pure citronellol, the chemical selectivity reaches 98%-99.9%, and the stereoselectivity reaches 96%-99%, and the defects that raw materials are limited, the synthesis route is long, cost is high and the like in the process for synthesizing optically-pure citronellol in the prior art are solved.
Owner:WANHUA CHEM GRP CO LTD
Chiral pyrroline synthetic method by palladium-catalyzed asymmetric hydrogenation
InactiveCN102838522AAsymmetric hydrogenationHigh enantioselectivityOrganic chemistryHydrogen pressureEnantiomer
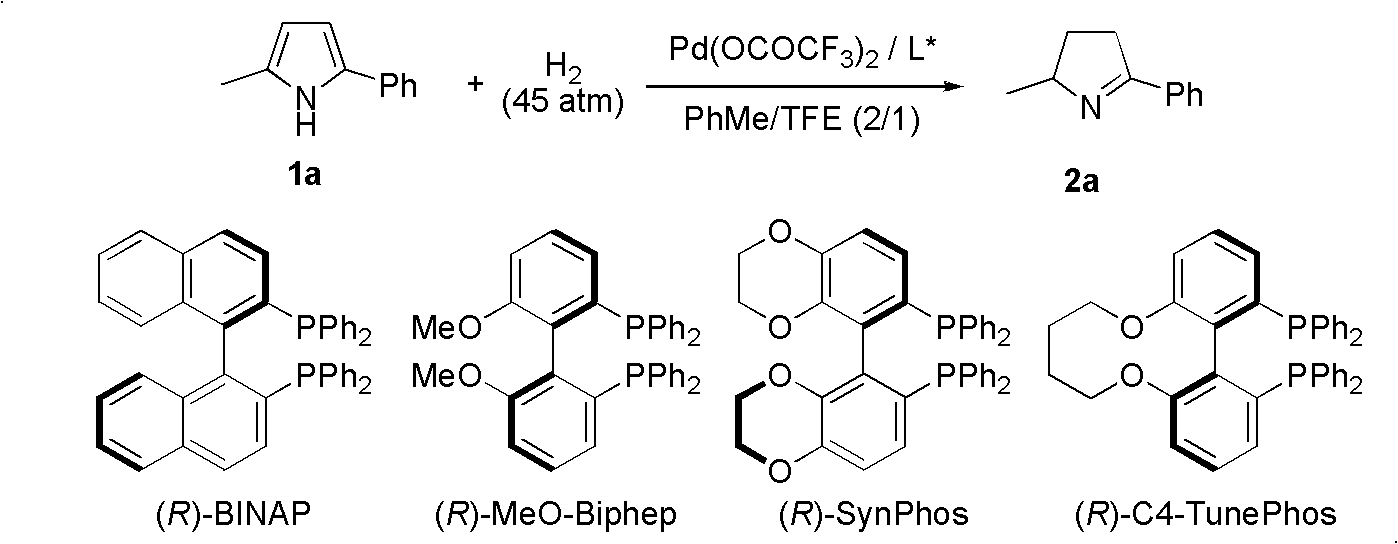
A chiral pyrroline synthetic method by palladium-catalyzed asymmetric hydrogenation is characterized by using a Bronsted acid as an activator, and a catalyst system of a chiral diphosphine complex of palladium. The reaction can be carried out under the following conditions: a reaction temperature at 20-70 DEG C; a reaction solvent of a mixed solvent containing toluene and 2, 2, 2-trifluoroethanol (a volume of PhMe to TFE is 2:1); hydrogen pressure at 13-45 atmospheric pressure; a ratio of a substrate to the catalyst being 50 / 1; a metal precursor of Pd (OCOCF3)2; a chiral ligand of a chiral diphosphine ligand; and the activator of ethyl sulfonic acid (EtSO3H). Unprotected simple 2, 5 bisubstituted pyrrole is subjected to hydrogenation to obtain a corresponding chiral pyrroline product (1-pyrroline), and the enantiomer of the chiral pyrroline product can reach an excessive amount of 92%. The invention has advantages of simple operation, practicality, easily available raw materials, good enantioselectivity and high yield; besides, the reaction product is convenient for subsequent conversion.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Preparation method of alpha-terpilenol
InactiveCN103044202AAvoid the reverse reactionHigh boiling pointOrganic compound preparationHydroxy compound preparationOrganic solventGas phase
The invention discloses a preparation method of alpha-terpilenol, which comprises the steps that firstly, alpha-pinene, water and an organic solvent are placed in a container, and subjected to heating reflux; evaporated vapor enters a rectifying tower; secondly, a gas-phase mixture from the top of the rectifying tower enters a condenser at the top end of the rectifying tower; a condensed liquid enters a packed tower, and hydrated by the catalysis of a solid acid catalyst to form alpha-terpilenol; and finally, a mixed liquid of the packed tower enters the container and is evaporated continuously; the evaporated vapor enters the rectifying tower and separated; alpha-terpilenol is left in the rectifying tower; and a gas phase mixture from the top end of the rectifying tower continuously enters the condenser. The preparation method adopts a rectification-coupling technology, and takes alpha-pinene as a raw material, performs alpha-pinene hydration directly, and produces alpha-terpilenol continuously, the yield of alpha-terpilenol is increased; the selectivity of alpha-terpilenol is improved; the production capacity is improved; the energy consumption and the production cost are lowered; and compared with the traditional technology, the automatization of a production process is easy to realize.
Owner:江苏宏邦化工科技有限公司
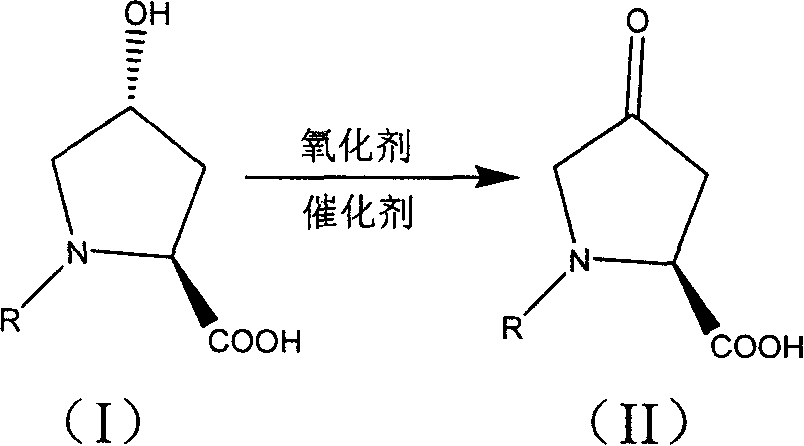
Production of 4-carbonyl-(S)-proline derivative
Synthesis of antihypertensive Lopli intermediate 4-carbonyl-(S)-proline derivative is carried out by taking 4-carbonyl-L-proline derivative as raw material, and reacting it with oxidant to obtain the final product under action of 2,2,6,6-tetramethyl nitro-oxide free radical piperidine compound. It's simple, has gentle reactive condition and high recovery rate, and can be used for industrial production.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
Synthesis method of (S)-nicotine
PendingCN113373188AEasy to operateHigh yieldOrganic chemistryOxidoreductasesPtru catalystCombinatorial chemistry
The invention relates to a synthesis method of (S)-nicotine, which comprises the following steps of: under the condition of a coenzyme circulation system, taking coenzyme as a hydrogen donor, taking imine reductase as a catalyst, carrying out catalytic reduction on myosmine to obtain (S)-nornicotine, and carrying out methylation reaction on the (S)-nornicotine to obtain the (S)-nicotine. The synthesis method of the (S)-nicotine disclosed by the invention is simple to operate, safe, reliable, high in yield and low in cost, and has excellent chemical selectivity; the purity of the product can reach 99.5% or above; the (S)-nicotine also has excellent enantioselectivity; and the optical purity can reach 99.5% or above.
Owner:PORTON FINE CHEM
Preparation of electrocatalysis electrode of Nano carbon fiber in use for organic electrochemical synthesis process
InactiveCN1958856AImprove current efficiencyHigh chemoselectivityElectrolytic organic productionElectrodesFiberCarbon fibers
This invention discloses a method for preparing carbon nanofiber electrode used in organic electrochemical synthesis. The method comprises: immersing carbon nanofibers in a solution containing metal precursor, and electrodepositing metal nanoparticles onto carbon nanofibers to obtain carbon nanofiber electrode. Compared with traditional high hydrogen-evolution overpotential metals such as Pb and Cd, the electrode has such advantages as high current efficiency, high chemical selectivity and low electrolytic tank voltage, and is environmentally friendly.
Owner:EAST CHINA UNIV OF SCI & TECH
Fluorine-containing heterocyclic compound and preparation method thereof
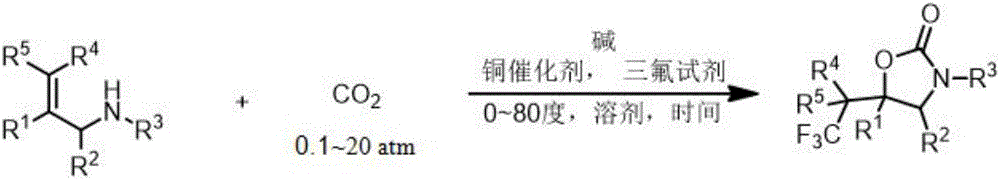
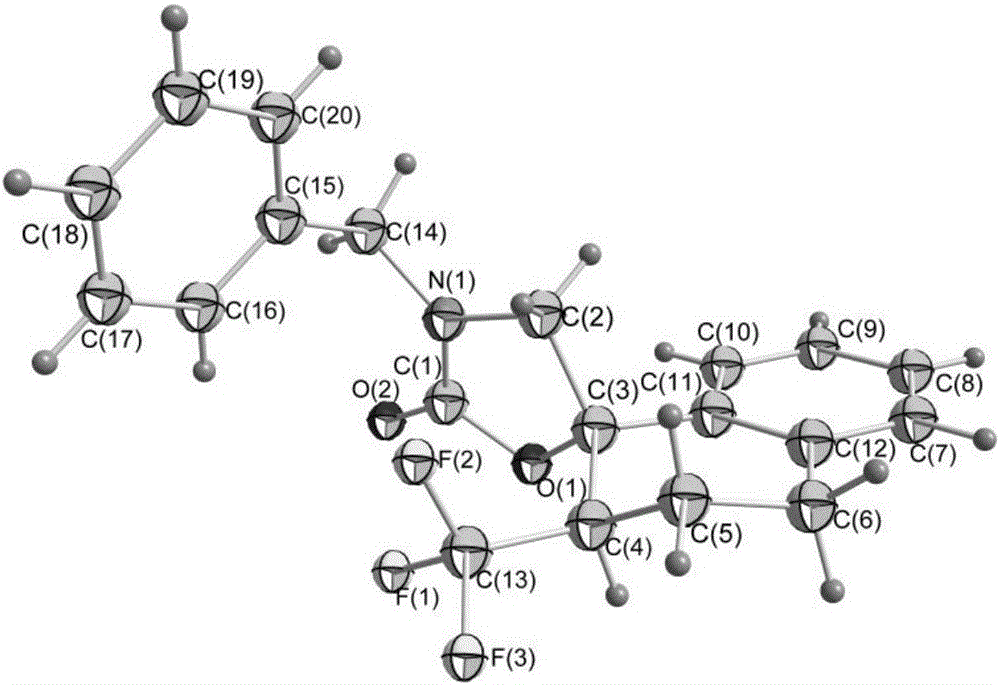
ActiveCN106220581AEasy to synthesizeReduce usageOrganic compound preparationCarboxylic acid amides preparationTrifluoromethylationAryl
The invention relates to a method for preparing a 2-oxazolinone compound containing a trifluoromethyl group from an allyl amine compound in virtue of carbon dioxide. Specifically, the allyl amine compound as shown in a formula 1 which is described in the specification reacts with CO2 in a solvent in the presence of a copper catalyst, alkali and a trifluoromethylation reagent so as to prepare the 2-oxazolinone compound as shown in a formula 2 which is described in the specification. In the formula 2, R<1>, R<2>, R<4> and R<5> are independently selected from a group consisting of H, halogen, a cyano group, an alkyl group, a carbonyl group, an ester group, an aryl group, a heteroaryl group and the like, R4 or R5 can optionally form a ring with R1, and the alkyl group, the carbonyl group, the ester group, the aryl group and the heteroaryl group can be optionally substituted by halogen, the alkyl group or the like; and R3 is H, halogen, an alkyl group, an aryl group, or the like.
Owner:SICHUAN UNIV
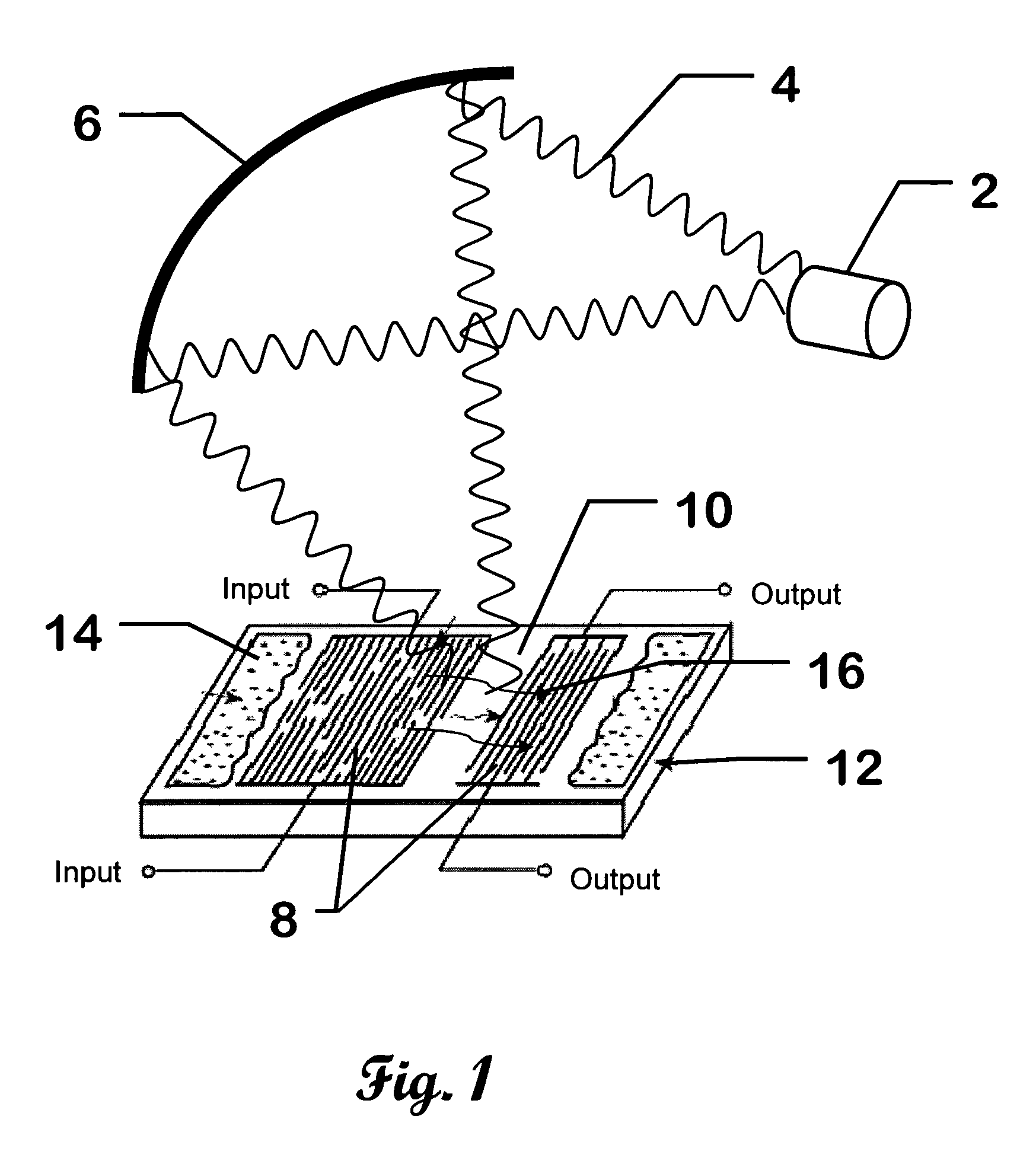
Surface wave chemical detector using optical radiation
InactiveUS7243548B2High sensitivityAvoid long termAnalysing fluids using sonic/ultrasonic/infrasonic wavesAnalysing solids using sonic/ultrasonic/infrasonic wavesOptical radiationLength wave
A surface wave chemical detector comprising at least one surface wave substrate, each of said substrates having a surface wave and at least one measurable surface wave parameter; means for exposing said surface wave substrate to an unknown sample of at least one chemical to be analyzed, said substrate adsorbing said at least one chemical to be sensed if present in said sample; a source of radiation for radiating said surface wave substrate with different wavelengths of said radiation, said surface wave parameter being changed by said adsorbing; and means for recording signals representative of said surface wave parameter of each of said surface wave substrates responsive to said radiation of said different wavelengths, measurable changes of said parameter due to adsorbing said chemical defining a unique signature of a detected chemical.
Owner:UT BATTELLE LLC
Green conjugated double bond reduction method
ActiveCN103467223AAchieve restorationReaction conditions greenOrganic reductionCarboxylic acid nitrile preparationDistillationOrganic layer
The invention relates to a green conjugated double bond reduction method capable of taking water as a solvent and dihydropyridine ester as a hydrogen source, and selectively reducing the conjugated double bond with strong electron-withdrawing groups. By adopting the method, conjugated carbon-carbon double bonds with strong electron-withdrawing groups are selectively reduced by taking water as the solvent and the dihydropyridine ester as the hydrogen source under the condition that no catalyst is needed. The method comprises the following steps: (1) adding a compound of conjugated carbon-carbon double bonds with strong electron-withdrawing groups and the dihydropyridine ester to water according to the molar ratio of (1:1) to (1:3), heating and warming to 60-100 DEG C; stirring and reacting for 5-24 hours at the temperature; (2) adding an organic solvent to the solution obtained in the step (1) to extract for at least three times according to the volume ratio of 2:5; (3) merging, drying and carrying out reduced pressure distillation on an organic layer of the product obtained in the step (2), and then carrying out column chromatography, so as to obtain the reduced product. The green conjugated double bond reduction method has the advantages of being non-toxic, mild in reaction condition, free of adding of a catalyst, high in chemical selectivity and the like.
Owner:无锡富泽药业有限公司
Tuning Product Selectivity in Catalytic Hydroformylation Reactions with Carbon Dioxide Expanded Liquids
ActiveUS20070219399A1Alters chemoselectivityAlters regioselectivityOrganic compound preparationPreparation by carbon monoxide reactionSupercritical carbon dioxideHydroformylation
An improved hydroformylation process is provided, which comprises reacting an olefin with CO and H2 in the presence of a hydroformylation catalyst in a liquid that has been volumetrically expanded with a compressed gas, such as supercritical carbon dioxide.
Owner:UNIVERSITY OF KANSAS
Method for preparing polyether based on three-component metal-free catalytic initiation system
The invention discloses a method for preparing polyether based on a three-component metal-free catalytic initiation system. The method comprises the steps of adding an epoxy monomer into a three-component metal-free catalytic initiation system containing hydroxy compounds, organic alkali and boron alkyl to react to obtain the polyether. The method is a currently known ethylene oxide room-temperature open-ring polymerization method with highest catalytic activity since the usage of organic alkali can be reduced to 40ppm and the conversion frequency can be 6000h / l. The polyether has the advantages of no metal residue and no cytotoxicity. The three-component catalytic initiation system adopts ethylene oxide open-ring polymerization, the chain transfer reaction toward the monomer and solvent can be completely avoided while the conversion frequency of 2720h / l can be obtained, and the polyether has the molecular weight of 0.1-400kg / mol, which can be accurately controlled. Furthermore, by utilizing the catalytic initiation system, the (poly) block polyether with controllable molecular weight, block sequence, block proportion and side base combination can be conveniently prepared by continuously adding materials.
Owner:SOUTH CHINA UNIV OF TECH
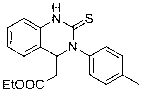
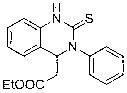
Method for preparing quinazoline-2-thioketone
InactiveCN103232400AHigh reactivityHigh yieldOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsChemical structureEthyl cinnamate
The invention discloses a method for preparing quinazoline-2-thioketone. The method comprises the steps of: under the catalysis of silicoamino rate-earth compound [(Me3Si)2N]3Ln(mu-Cl)Li(THF)3s erving as a catalyst; and preparing the quinazoline-2-thioketone by addition reaction of catalytic o-amino ethyl cinnamate and isothiocyanate, wherein Ln represents a triad rate-earth metal ion and is one of lanthanum, neodymium, samarium or ytterbium; (Me3Si)2N represents trimethyl silicoamino; mu- represents a bridged bond; THF represents tetrahydrofuran; the chemical structural formula of the o-amino ethyl cinnamate and isothiocyanate is shown in the specification; and the general formula of the chemical structure of the isothiocyanate is RNCS. In the method, the catalyst is good in chemical selectivity, high in reactivity, mild in reaction condition and in no need of a solvent, and the yield of a target product is high.
Owner:SUZHOU UNIV
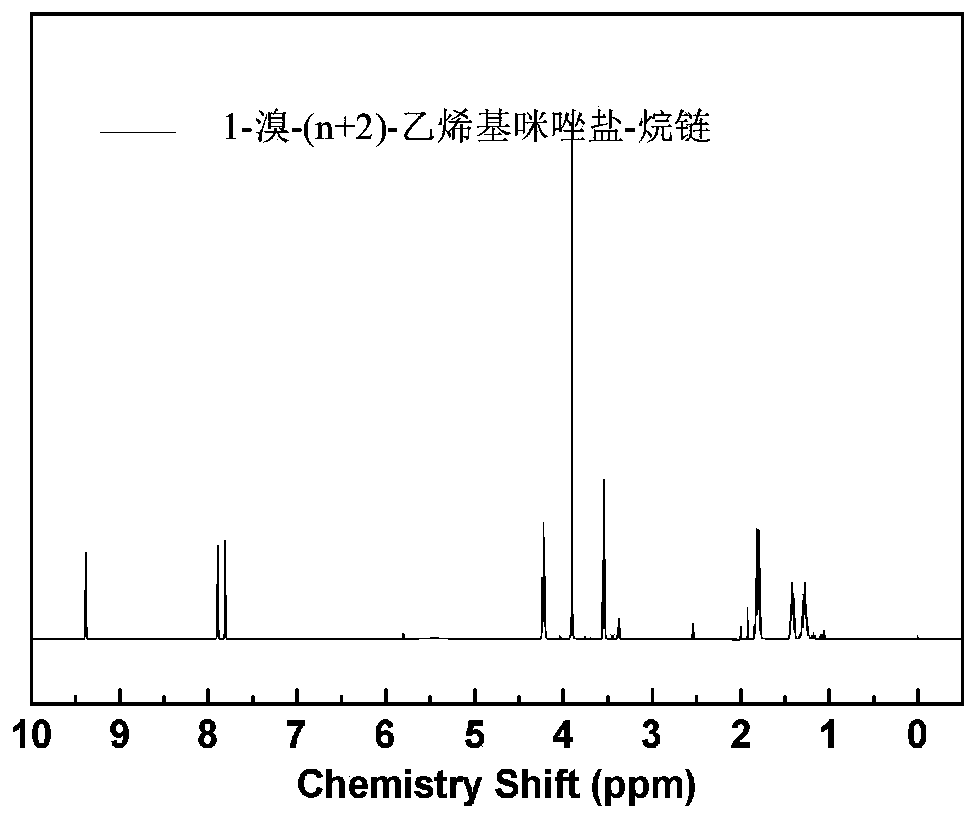
Ppreparation method for poly(aryl ether sulfone) anion exchange membrane with homogeneous cross-linked structure
The invention discloses a preparation method for a poly(aryl ether sulfone) anion exchange membrane with a homogeneous cross-linked structure. The method includes the following steps: step 1, performing a reaction on 1-vinylimidazole and dibromoalkane CH2Br(CH2)nCH2Br to obtain a 1-bromo-(n+2)-vinylimidazole salt-alkane chain represented by a formula (III) shown in the specification; step 2, dissolving amino-contained poly(aryl ether sulfone) represented by a formula (I) shown in the specification into a solvent, adding a 1-bromo-(n+2)-methylimidazole salt-alkane chain represented by a formula(II) shown in the specification, the 1-bromo-(n+2)-vinylimidazole salt-alkane chain represented by the formula (III) and a polymerization inhibitor, and performing a reaction at 20-90 DEG C for 8-24h to obtain imidazole-functionalized poly(aryl ether sulfone); and step 3, dissolving the imidazole-functionalized poly(aryl ether sulfone) into an organic solvent, adding azobisisobutyronitrile to obtain a casting solution, performing defoaming on the casting solution, performing drying at 40-150 DEG C to form the membrane, so as to obtain the poly(aryl ether sulfone) anion exchange membrane withthe homogeneous cross-linked structure. The anion exchange membrane prepared by the method has the characteristics of a low membrane resistance, good structural stability, and high monovalent anion permeability selectivity.
Owner:ZHEJIANG UNIV OF TECH +1
Sulfonated polyarylether polymer ion exchange membrane containing crosslinking groups and application thereof
InactiveCN102120874AHigh chemical stabilityImprove vanadium ion selectivityRegenerative fuel cellsCell component detailsIon-exchange membranesBatch production
The invention relates to a sulfonated polyarylether polymer ion exchange membrane containing crosslinking groups and application thereof to a liquid flow energy storage battery of acidic electrolyte. The ion exchange membrane provided by the invention is mild in preparation conditions and easy for batch production. The prepared ion exchange membrane has favorable mechanical performance and simultaneously has favorable proton conductivity and excellent performance of blocking vanadium ions from infiltrating in a full-vanadium liquid flow energy storage battery. In addition, the chemical stability and the vanadium blocking performance of the ion exchange membrane can be further improved through a crosslinking reaction.
Owner:DALIAN RONGKE POWER
Nucleophilic Acyl Substitutions of Acids or Esters Catalyzed by Oxometallic Complexes, and the Applications in Fabricating Biodiesel
InactiveUS20070017151A1Excellent chemical yieldProduction cost be reduceFatty oils/acids recovery from wasteFatty acid esterificationChemistryActinide
The present invention discloses a method of nucleophilic acyl substitution (NAS) of carboxylic acids or esters (hereinafter acids / esters) catalyzed by oxometallic complexes. According to the mentioned method, NAS reactions between acids / esters (R1COOH / R1—COO—R2) and protic nucleophile (R3-AH) can be catalyzed by oxxmetallic complexes, wherein A stands for O, S, or NH. The general formula of the mentioned oxometallic complexes is MOmL1yL2z, wherein M is selected from IVB, VB, VIB or actinide groups, m, y, z are integers, and m, y≧1, z≧0. A general catalytic equation is shown as follows:
Owner:NATIONAL TAIWAN NORMAL UNIVERSITY
Aminomethylated imidazo[1,2-a]pyridine compound and preparation method thereof
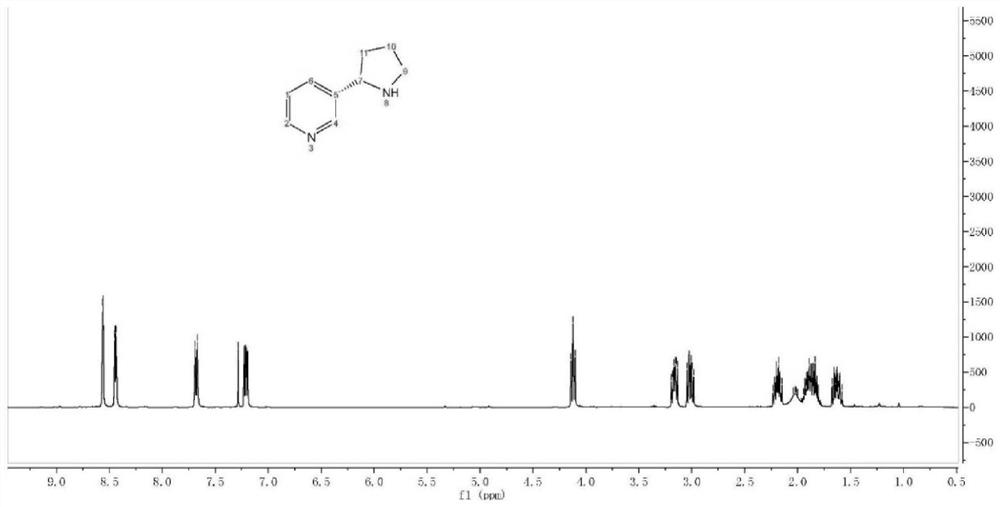
The invention discloses a synthesis method of an aminomethylated imidazo[1,2-a]pyridine compound; and according to the synthesis method, N-aryl glycine and imidazo[1,2-a]pyridine carry out decarboxylation coupling reactions in the presence of visible light. In an organic solvent, N-aryl glycine and imidazo[1,2-a]pyridine derivatives carry out reactions to prepare the aminomethylated imidazo[1,2-a]pyridine compound under the irradiation of visible light. The structure of the compound is tested and characterized by 1H NMR, 13C NMR, and HR-MS so as to determine the structure. The preparation method does not need any photo-sensitizer or additive, oxygen in the air is taken as the final oxidizing agent, and N-aryl glycine is induced by light to carry out decarboxylation, is oxidized, and reactswith imidazo[1,2-a]pyridine to prepare the aminomethylated imidazo[1,2-a]pyridine compound. The synthesis route is simple, convenient and efficient, the reaction conditions are middle, the operationis simple, and the synthesis method is environmentally friendly, can be applied to large scale production, and has a very good application prospect.
Owner:EAST CHINA UNIV OF TECH
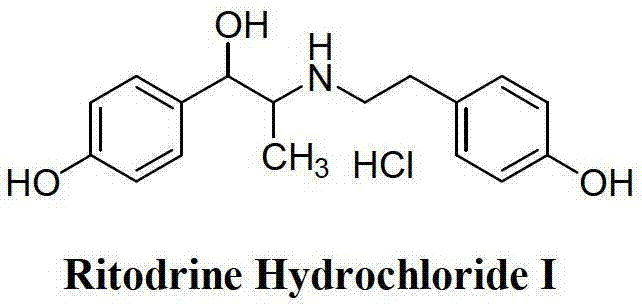
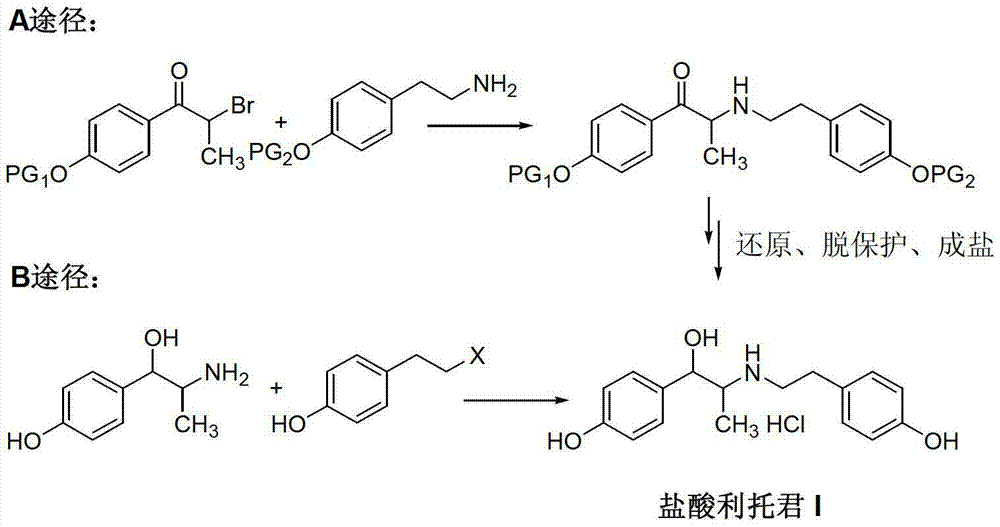
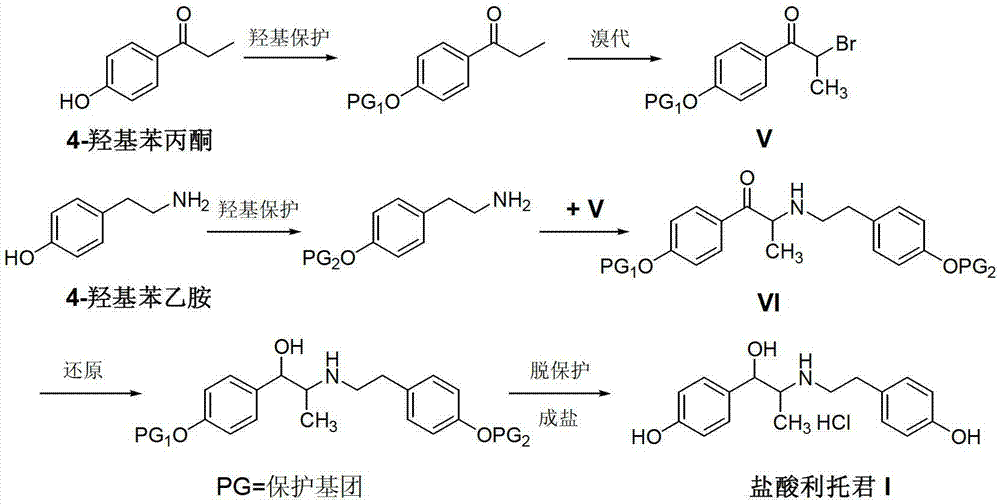
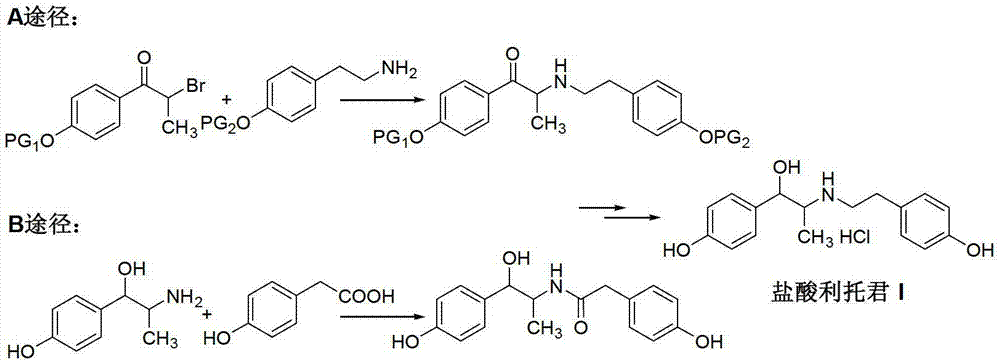
Preparation method of ritodrine hydrochloride
ActiveCN103113239AHigh chemoselectivityLow production costOrganic compound preparationAmino-hyroxy compound preparationPropanolHalogen
The invention discloses a preparation method of ritodrine hydrochloride (I). The preparation method comprises the following steps of: subjecting 2-amino-1-(4-hydroxyphenyl) propanol hydrochloride (II) and 4-(2-halogen ethanol) phenol (III) to condensation reaction in the presence of a catalyst to obtain ritodrine and then forming salt with ritodrine and hydrochloric acid to obtain ritodrine hydrochloride (I). The preparation method has the advantages that the production cost of ritodrine hydrochloride can be effectively controlled, the product quality is substantially improved and economic and technical development of the active pharmaceutical ingredient is promoted.
Owner:SUZHOU LIXIN PHARMA
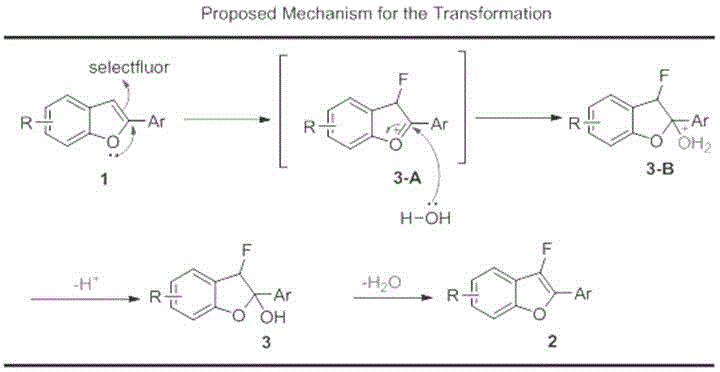
Synthesis method of 2-position aryl substituted benzofuran ring 3-position fluoro
InactiveCN105837539AEfficient conversionMild reaction conditionsOrganic chemistryTetrafluoroborateSynthesis methods
The invention relates to the technical field of fluorine organic compounds, in particular to a synthesis method of 2-position aryl substituted benzofuran ring 3-position fluoro. The method takes 1-chloromethyl-4-fluorodiazabicyclo[2, 2, 2]octane bis(tetrafluoroborate) (Selectfluor) as a fluorinating reagent, in a mixed solvent of acetonitrile and nucleophilic reagent water, a benzofuran compound is efficiently converted into corresponding 3-fluoro-2-ol-2, 3-dihydrobenzofuran, 3-fluoro-2-ol-2, 3-dihydrobenzofuran compound adopts pyridine and thionyl chloride as the dehydrating agent, and can be efficiently converted into a 3-position fluoro 2-position aryl substituted benzofuran compound. The method provided by the invention has the characteristics of mild reaction condition, easy operation, good chemical selectivity and functional group compatibility, and is an effective method for synthesis of 2-position aryl substituted benzofuran ring 3-position fluoro. (reaction formula).
Owner:FUDAN UNIV
Catalytic oxidation system and use thereof in tazobactam synthesis
ActiveCN101434609AIncrease profitMild reaction conditionsOrganic chemistryChemical recyclingHalogenRecyclable catalyst
The invention relates to a catalytic oxidation system and the application thereof in the synthesis of Tazobactam. The catalytic oxidation system formed a tungstate catalyst or a molybdate catalyst and a hydrogen peroxide oxidant is applied to the synthesis of Tazobactam to prepare a penicillanic acid sulfoxide intermediate I by catalytizing and oxidizing penicillanic acid. The reaction is characterized by high yield, good reaction selectivity, recyclable catalyst, and the like, required by green chemistry, wherein, R1 represents a carboxyl blocking group; R2 and R3 can be groups of the same pair or groups of different pair, such as H, halogen molecules, NH2, amide groups, and the like.
Owner:山东安信制药有限公司
Preparation method of ritodrine hydrochloride
ActiveCN103113237ALow production costPromote the development of economy and technologyOrganic compound preparationAmino-hyroxy compound preparationPropizochlorPtru catalyst
The invention discloses a preparation method of ritodrine hydrochloride (I). The preparation method comprises the following steps of: subjecting 2-amino-1-(4-hydroxyphenyl) propanol hydrochloride (II) and 4-hydroxyphenylacetic acid (III) to amidation and condensation reactions under the action of a catalyst to obtain an intermediate N-(2-(4-hydroxyphenyl)-2-hydroxy-1-methylethyl)-4-hydroxy phenylacetamide (IV) and obtaining ritodrine hydrochloride (I) through reduction reaction and salt forming reaction of the intermediate (IV). The preparation method has the advantages that the production cost of ritodrine can be effectively controlled, the product quality is substantially improved and economic and technical development of the active pharmaceutical ingredient is promoted.
Owner:SUZHOU LIXIN PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com














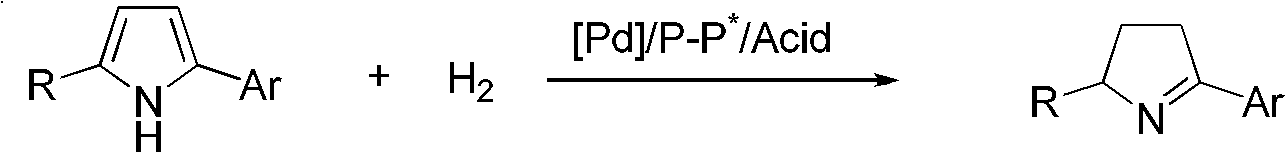














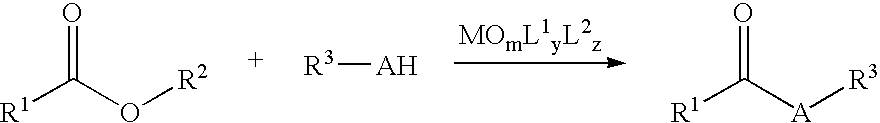


![Aminomethylated imidazo[1,2-a]pyridine compound and preparation method thereof Aminomethylated imidazo[1,2-a]pyridine compound and preparation method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/1ad7c474-0d15-4487-97c2-90197b3762a9/BDA0001934717740000021.png)
![Aminomethylated imidazo[1,2-a]pyridine compound and preparation method thereof Aminomethylated imidazo[1,2-a]pyridine compound and preparation method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/1ad7c474-0d15-4487-97c2-90197b3762a9/BDA0001934717740000022.png)
![Aminomethylated imidazo[1,2-a]pyridine compound and preparation method thereof Aminomethylated imidazo[1,2-a]pyridine compound and preparation method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/1ad7c474-0d15-4487-97c2-90197b3762a9/BDA0001934717740000031.png)