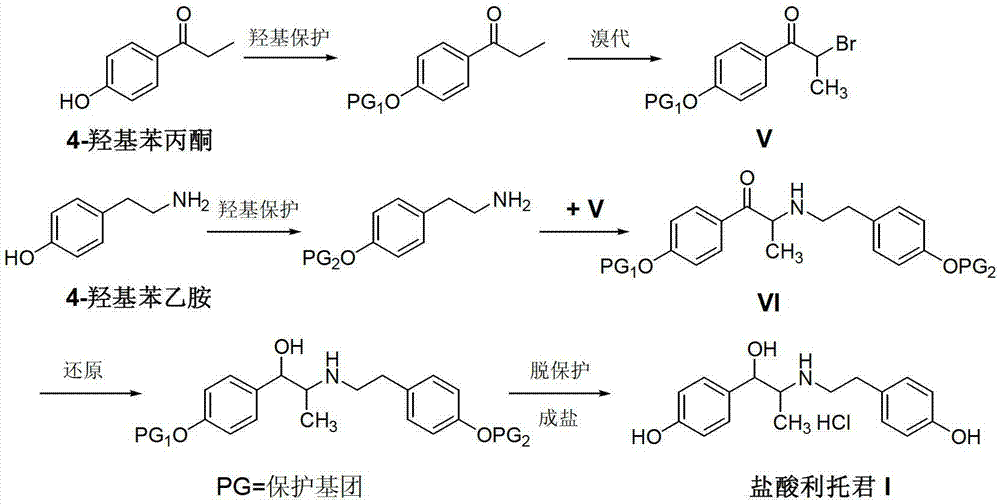
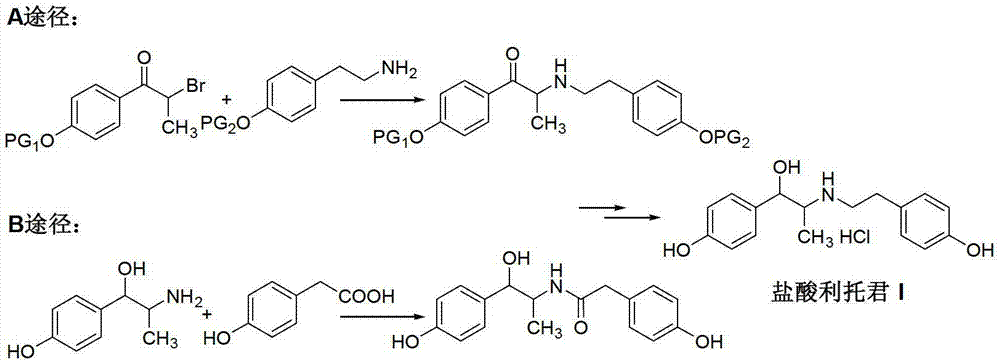
Preparation method of ritodrine hydrochloride
A technology for ritodrine hydrochloride and propanol hydrochloride, applied in the field of preparation of ritodrine hydrochloride, to achieve the effects of promoting economic and technological development, controlling production costs, and high chemical selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add 2-amino-1-(4-hydroxyphenyl)propanol hydrochloride (II) (50.2g, 0.25mol), triethylamine (25.0g, 0.25mol) and dichloromethane into a 500mL three-necked flask 250mL, stirred at room temperature until the system was dissolved uniformly. Add 4-hydroxyphenylacetic acid (III) (43.7g, 0.29mol) and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide (EDC) (39.2g, 0.25mol) to the system And N-hydroxybenzotriazole (HOBt) (3.37g, 0.1eq), be warming up to 35 ℃, react for 12 hours, TLC detects that the reaction ends. Cool down to room temperature, and remove insoluble matter by filtration. The filtrate was washed successively with saturated brine and water, dried and concentrated to recover the solvent to obtain 70.2 g of intermediate (IV) as an off-white solid with a yield of 93.3%.
Embodiment 2
[0030] Add 2-amino-1-(4-hydroxyphenyl)propanol hydrochloride (II) (50.2g, 0.25mol) and 250mL of N,N-dimethylformamide (DMF) into a 500mL three-necked flask, Stir at room temperature until the system dissolves uniformly. Add 4-hydroxyphenylacetic acid (III) (43.7g, 0.29mol), dicyclohexylcarbodiimide (DCC) (39.2g, 0.25mol) and 4-N,N-lutidine (DMAP) into the system (3.37g, 0.1eq), react at room temperature for 16 hours, and TLC detects that the reaction is complete. The reaction solution was poured into 500mL of dichloromethane, washed successively with 5% dilute hydrochloric acid, 10% sodium bicarbonate, saturated brine and water, dried and concentrated to recover the solvent to obtain 67.5 g of off-white solid as intermediate (IV), Yield 89.7%.
Embodiment 3
[0032] Add intermediate (IV) (60.2g, 0.2mol), 5% palladium carbon catalyst (6g, 10%w / w), 2mL of concentrated hydrochloric acid and 500mL of methanol into a 1L hydrogenation kettle, and keep the hydrogen pressure according to the hydrogenation reaction procedure 5KG and temperature 50 DEG C, to no longer absorbing hydrogen. The temperature was lowered, the catalyst was recovered by filtration, concentrated under reduced pressure, and the residue was recrystallized from a mixed solvent of n-hexane and ethyl acetate to obtain 52.5 g of ritodrine hydrochloride (I) as a white solid with a yield of 81.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com