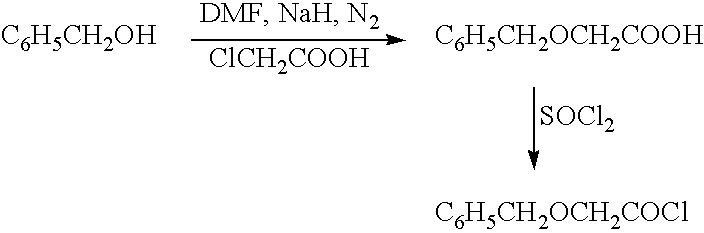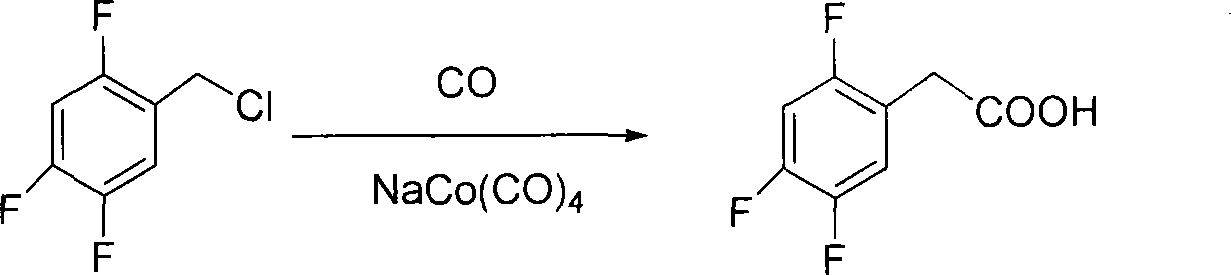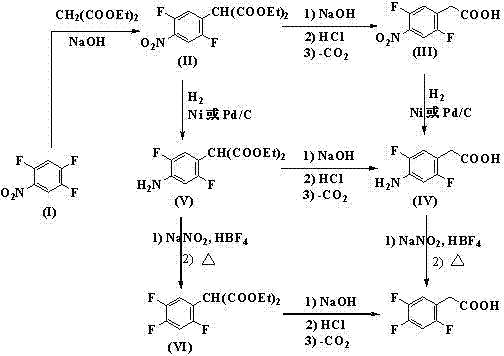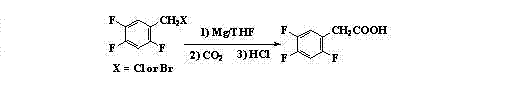Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
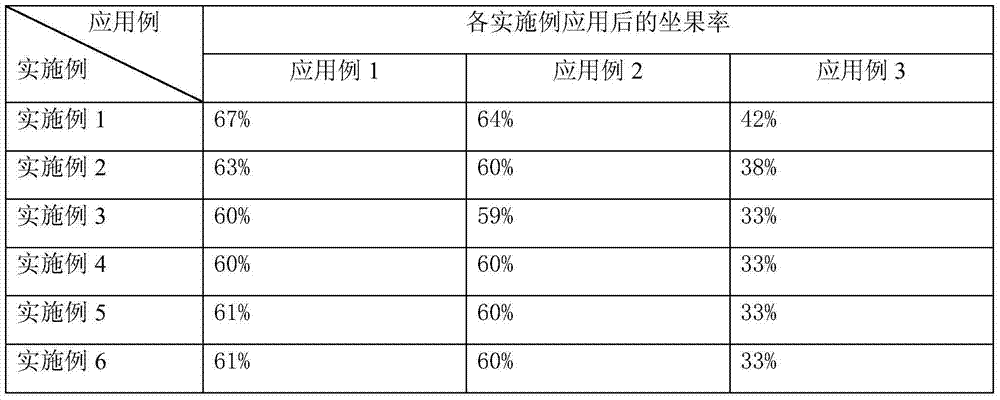
804 results about "Phenylacetic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
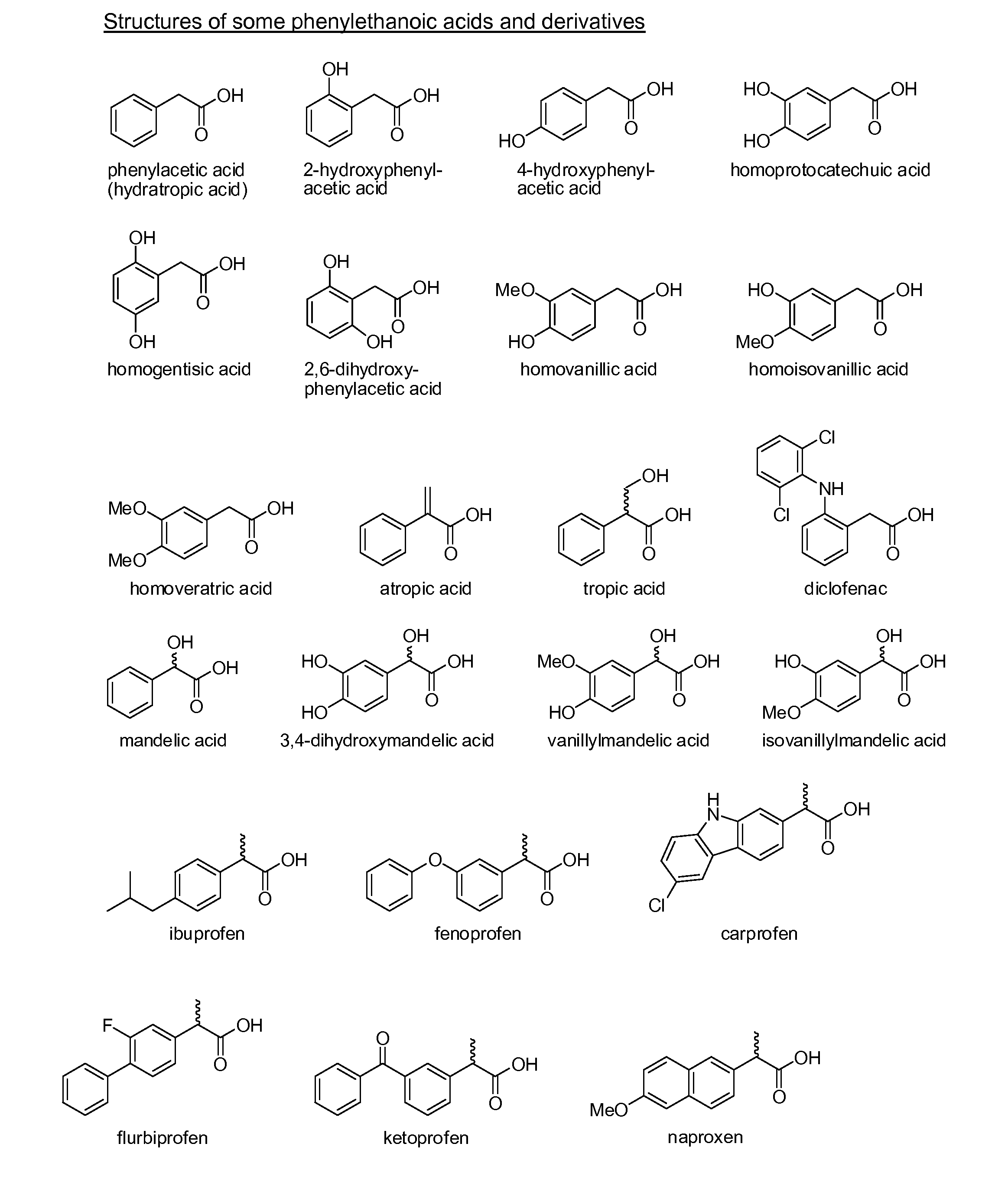
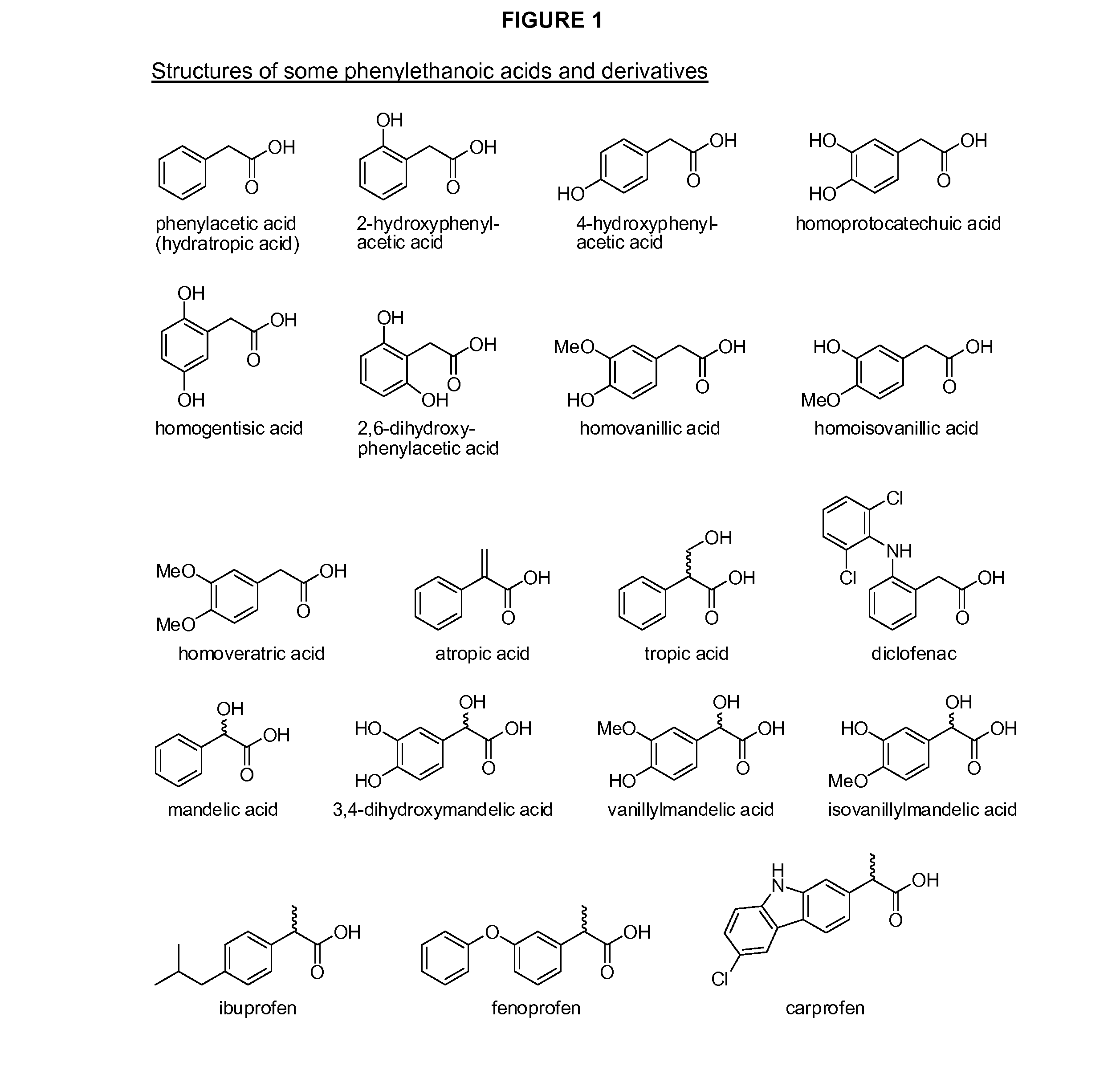
Phenylacetic acid (PAA) (conjugate base phenylacetate), also known by various synonyms, is an organic compound containing a phenyl functional group and a carboxylic acid functional group. It is a white solid with a strong honey-like odor. Endogeneously, it is a catabolite of phenylalanine. As a commercial chemical, because it can be used in the illicit production of phenylacetone (used in the manufacture of substituted amphetamines), it is subject to controls in countries including the United States and China.
Growth regulator for improving fruit setting rate of peaches and regulating method
ActiveCN103918650AReduce economic costsMake up for the lack of endogenous hormonesPlant growth regulatorsBiocideFruit setPeach orchard
The invention relates to the technical field of fruit growing and in particular relates to a growth regulator for improving the fruit setting rate of peaches and a regulating method. The growth regulator comprises the following components of gibberellin and auxin, wherein the auxin may be one of 1-Naphthalene acetic acid, sodium alpha-naphthyl acetate, indoleacetic acid, phenylacetic acid, indolebutyric acid, indoleacetic acid and 2,4-dichlorphenoxyacetic acid, and the growth regulator is sprayed after the peaches bloom. The growth regulator provided by the invention is easy to obtain and prepare and low in economic cost, and can effectively remedy deficiency of endogenous hormone caused by poor fertilization of peaches; the method for applying the growth regulator provided by the invention is simple and convenient and easy to operate and control, effectively solves the problem that in the growing of peaches, the fruit setting rate is influenced by abnormal weather in a flowering phase, shriveled flower buds, overly rapid warming and the like, improves the fruit setting rate by more than 34 times, and is obvious in effect and applicable to popularization and application in the production of peaches in common peach orchards, green houses and other facilities.
Owner:CHANGLI INST OF POMOLOGY HEBEI ACADEMY OF AGRI & FORESTRY SCI
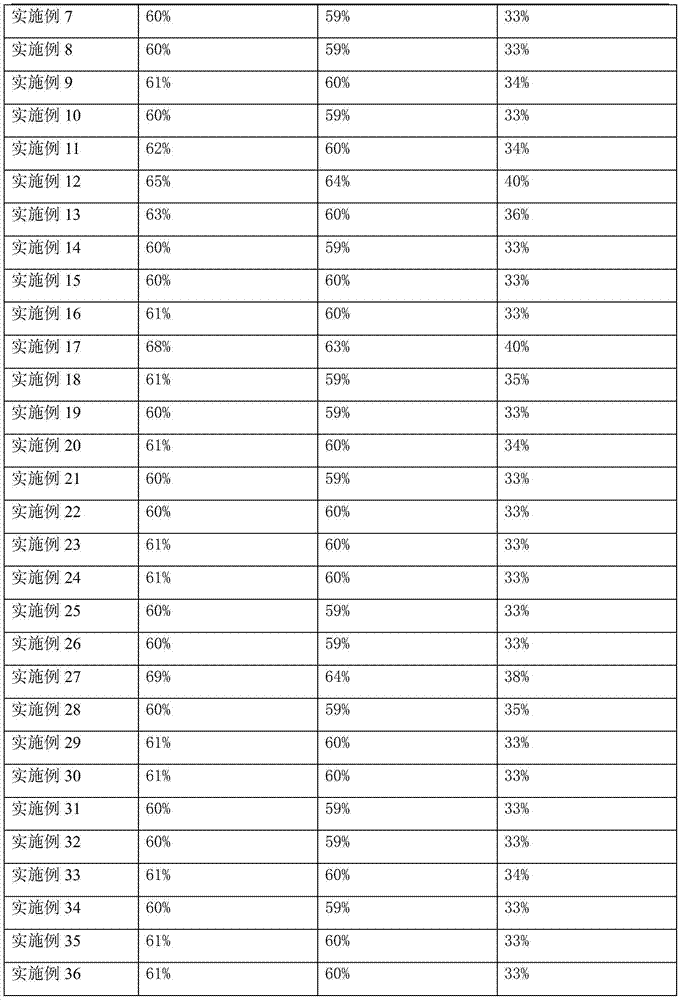
Phenylethanoic acid, phenylpropanoic acid and phenylpropenoic acid conjugates and prodrugs of hydrocodone, method of making and use thereof
ActiveUS20110002991A1Lower potentialReduce addictionBiocideNervous disorder3-phenylpropanoic acidEpoxy
The presently described technology provides phenylethanoic acid, phenylpropanoic acid, phenylpropenoic acid, a salt thereof, a derivative thereof or a combination thereof chemically conjugated to hydrocodone (morphinan-6-one, 4,5-alpha-epoxy-3-methoxy-17-methyl) to form novel prodrugs or compositions of hydrocodone which have a decreased potential for abuse of hydrocodone. The present technology also provides methods of treating patients, pharmaceutical kits and methods of synthesizing conjugates of the present technology.
Owner:KEMPHARM INC
Bromfenac ophthalmic formulations and methods of use
InactiveUS20070287749A1Minimal and no irritationBiocideOrganic active ingredientsPhenylacetic acidBromfenac
Owner:SENJU PHARMA CO LTD +1
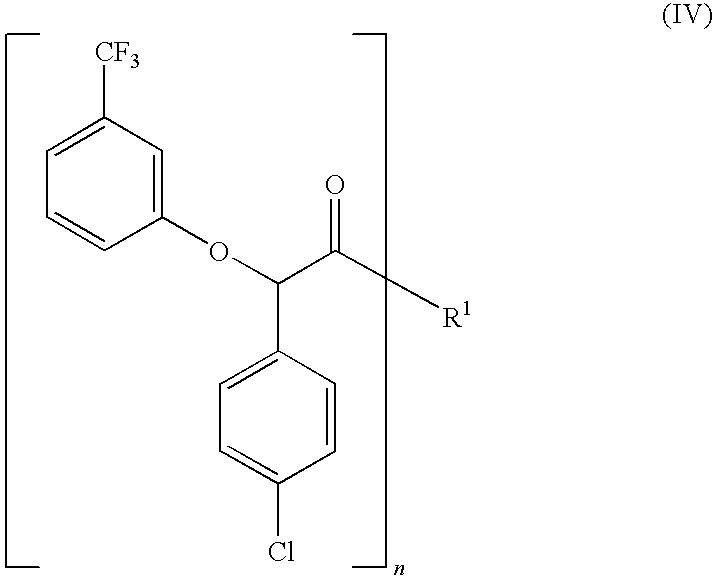
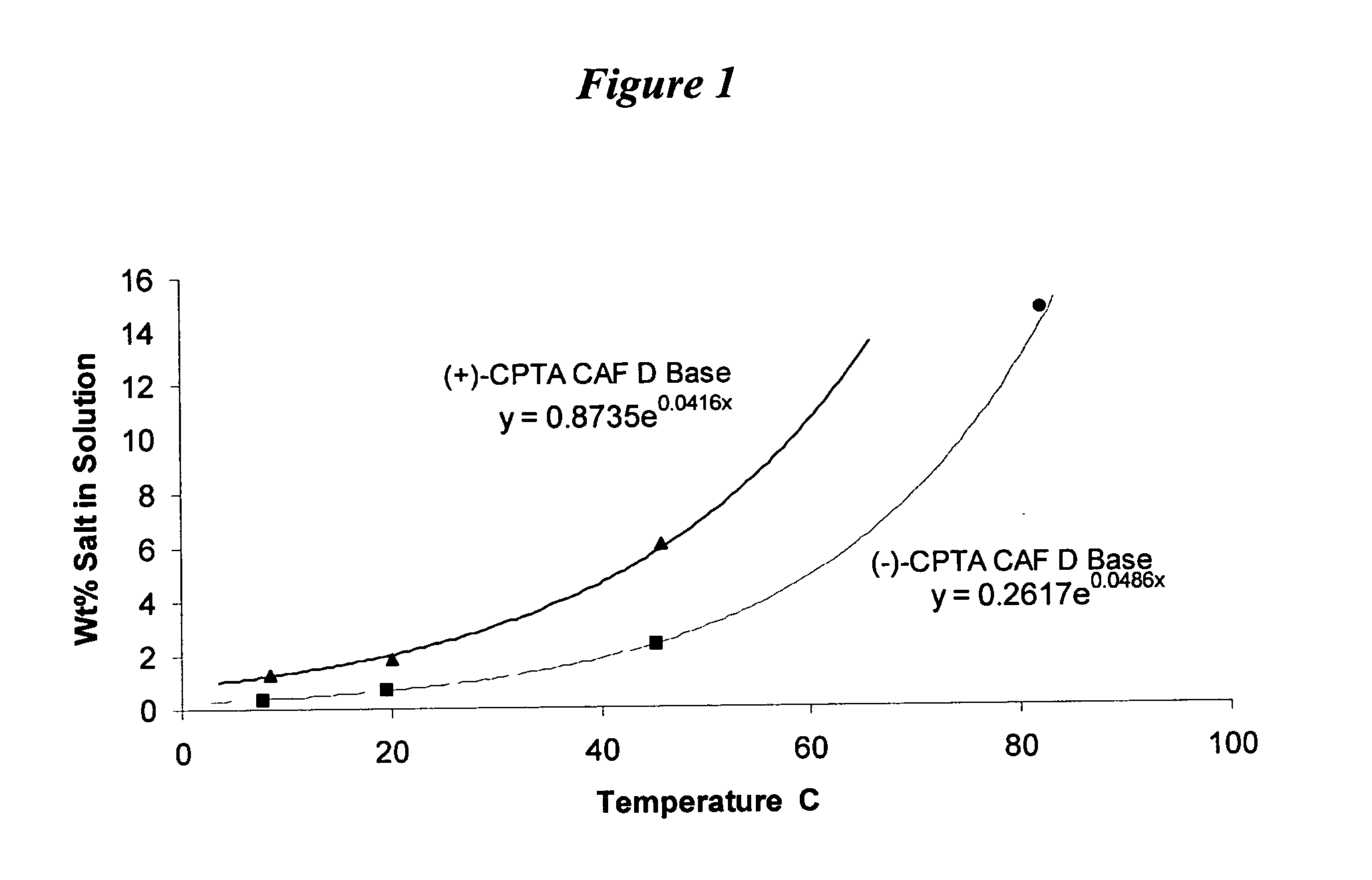
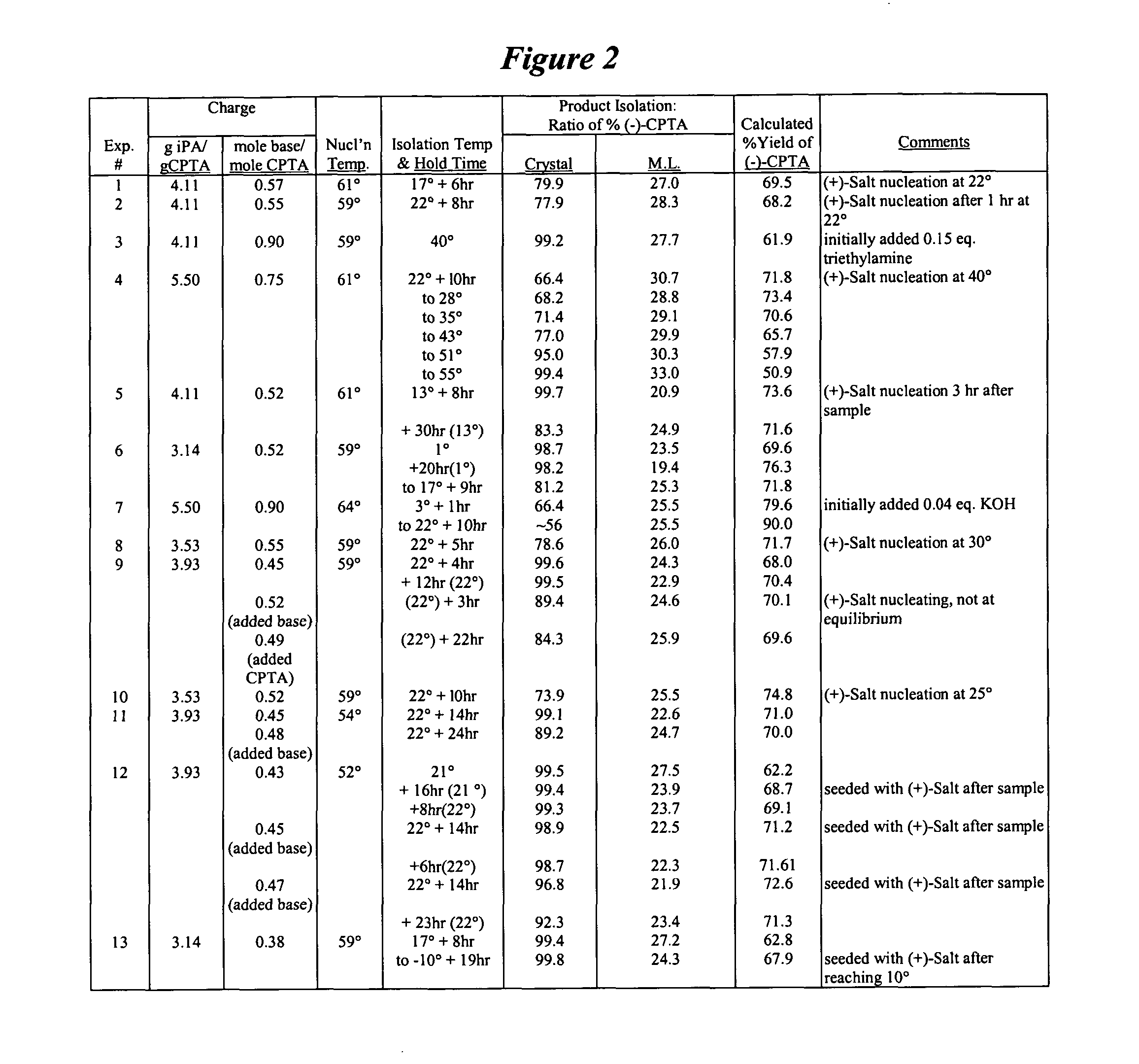
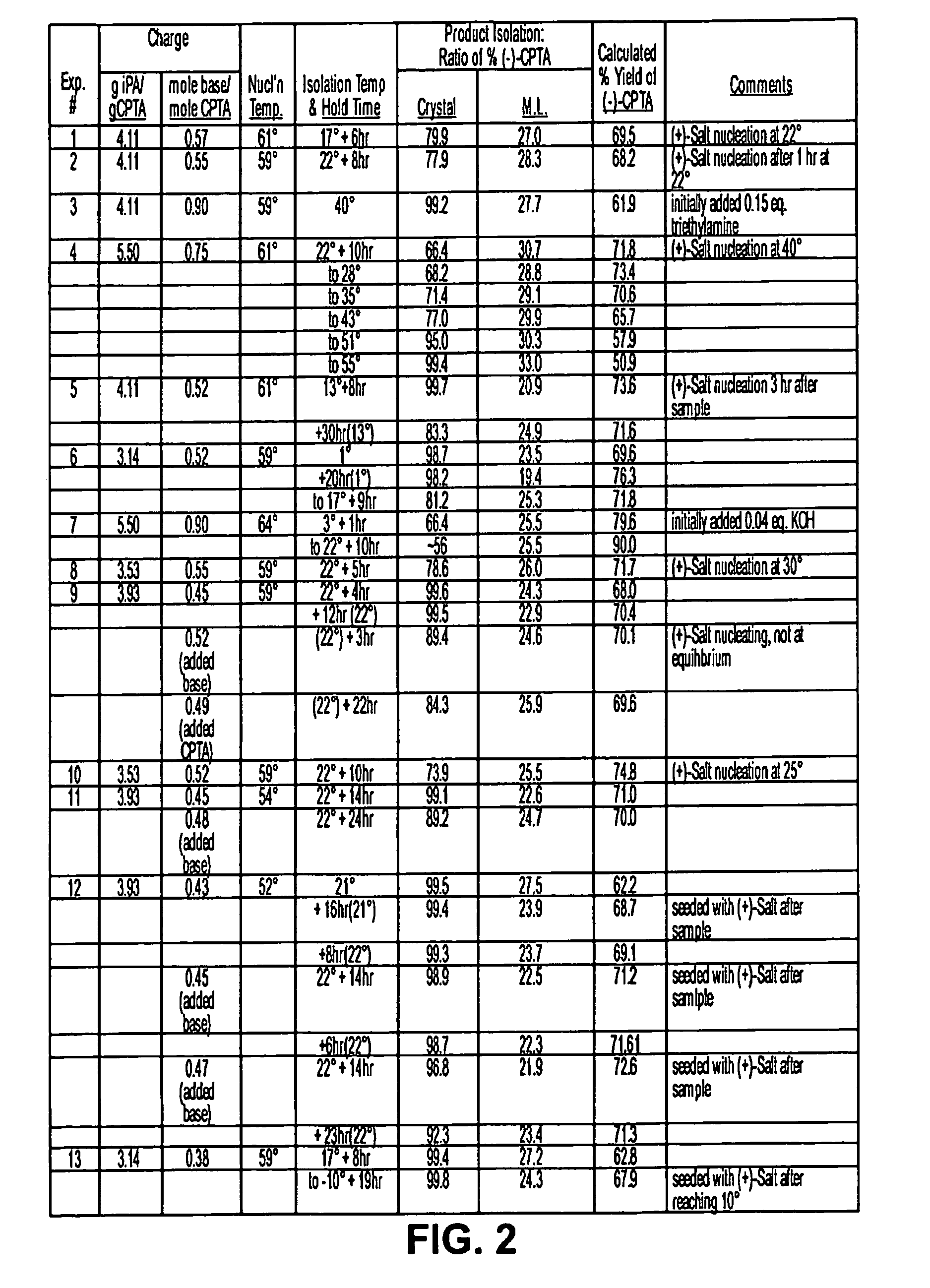
Process for the stereoselective preparation of (−)-halofenate and derivatives thereof
InactiveUS7714131B2High yieldHigh enantiomeric purityBiocideOrganic chemistryPhenylacetic acidHALOFENATE
The present invention provides a compounds the formula (IV):and methods for producing an α-(phenoxy)phenylacetic acid compound of the formula:wherein R1 is a member selected from the group consisting of:each R2 is a member independently selected from the group consisting of (C1-C4)alkyl, halo, (C1-C4)haloalkyl, amino, (C1-C4)aminoalkyl, amido, (C1-C4)amidoalkyl, (C1-C4)sulfonylalkyl, (C1-C4)sulfamylalkyl, (C1-C4)alkoxy, (C1-C4)heteroalkyl, carboxy and nitro; the subscript n is 1 when R1 has the formula (a) or (b) and 2 when R1 has the formula (c) or (d); the subscript m is an integer of from 0 to 3; * indicates a carbon which is enriched in one stereoisomeric configuration; and the wavy line indicates the point of attachment of R1; and compounds.
Owner:DIATEX INC (US)
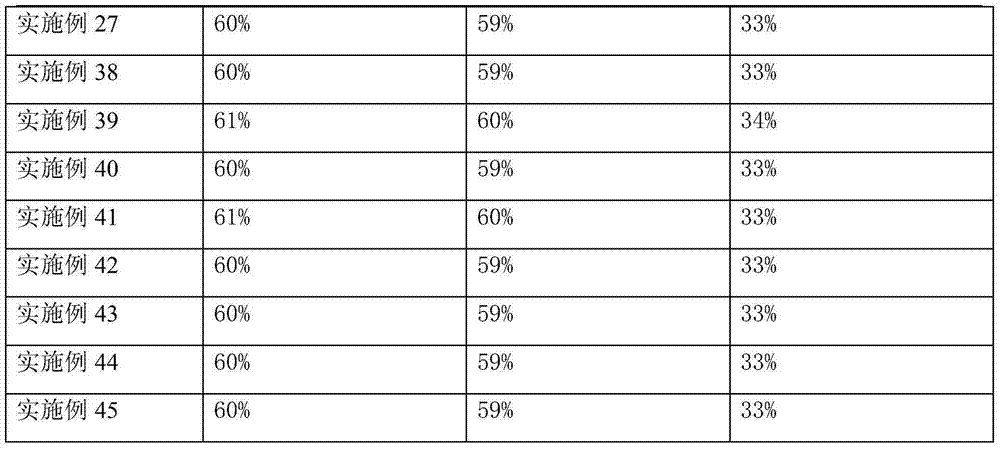
Functionalized phenolic esters and amides and polymers therefrom
InactiveUS20060173065A1Alter efficacyAlter valueBiocideOrganic chemistryBenzoic acidPhenylacetic acid
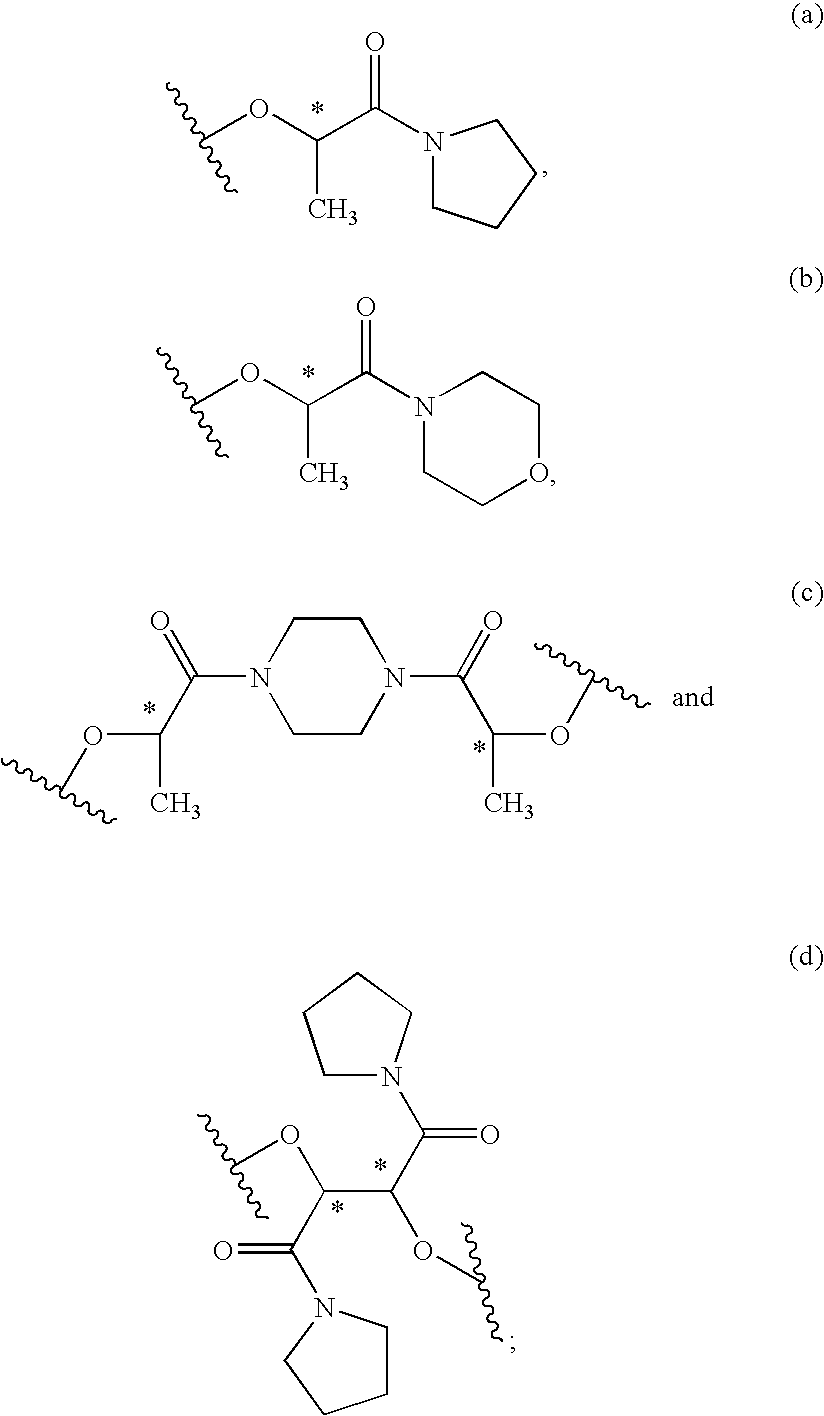
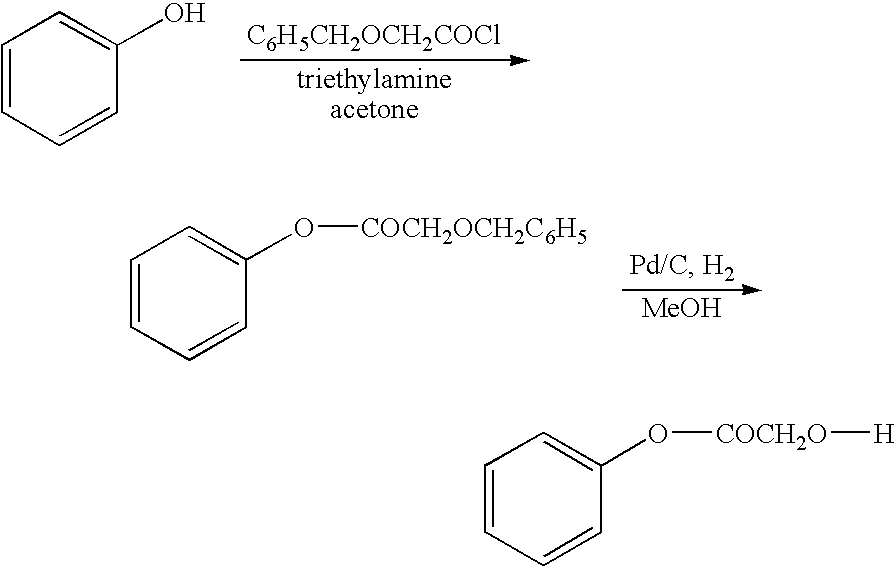
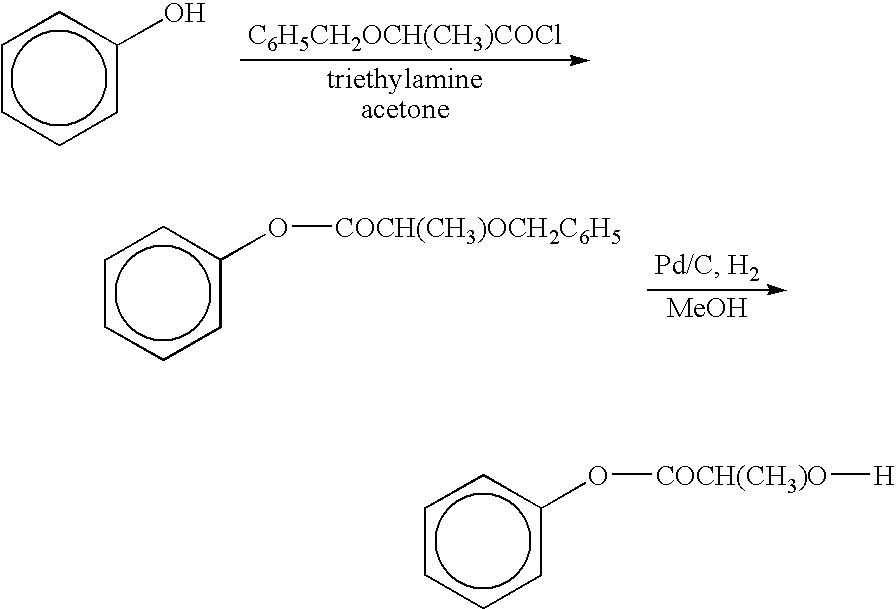
The present invention relates to a compound of the formula: R-AR—O—Y—R′Wherein R represents one or more members selected from H, alkoxy, benzyloxy, aldehyde, halogen, carboxylic acid, —NO2, —NH2, —NHCOCH3, and —NH—Y—R′, which is attached directly to AR or attached through an aliphatic chain. The carboxylic acid moiety in R includes but is not limited to the following carboxylic acids: benzoic acids, cinnamic acids, ferulic acid, caffeic acid, syringic acid, salicylic acid, vanillic acid, phenylacetic acids, phenylpropionic acids, and sinapinic acid. -AR—O— is a biologically active phenolic moiety comprising 1 to 6 substituted or unsubstituted aryl rings that are directly bonded to each other, fused together, or joined through a linking group. Y represents a member selected from: —COCH2O— (glycolic ester moiety) —COCH(CH3)O— (lactic ester moiety) —COCH2OCH2CH2O— (dioxanone ester moiety) —COCH2CH2CH2CH2CH2O— (caprolactone ester moiety) —CO(CH2)mO— where m is an integer between 2-4 and 6-24 inclusive —COCH2O(CH2CH2O)n— where n is an integer between 2 and 24, inclusive; and R′ is either hydrogen or a benzyl or an alkyl group, the alkyl group being either straight-chained or branched. The resultant functionalized phenolic compounds, used singly or in combinations, and their polymers have controllable degradation profiles, releasing the active component over a desired time range. The polymers are useful for biomaterials and biomedical devices, wherein said biologically active phenolic moiety is a residue of a phenolic compound.
Owner:BEZWADA BIOMEDICAL LLC
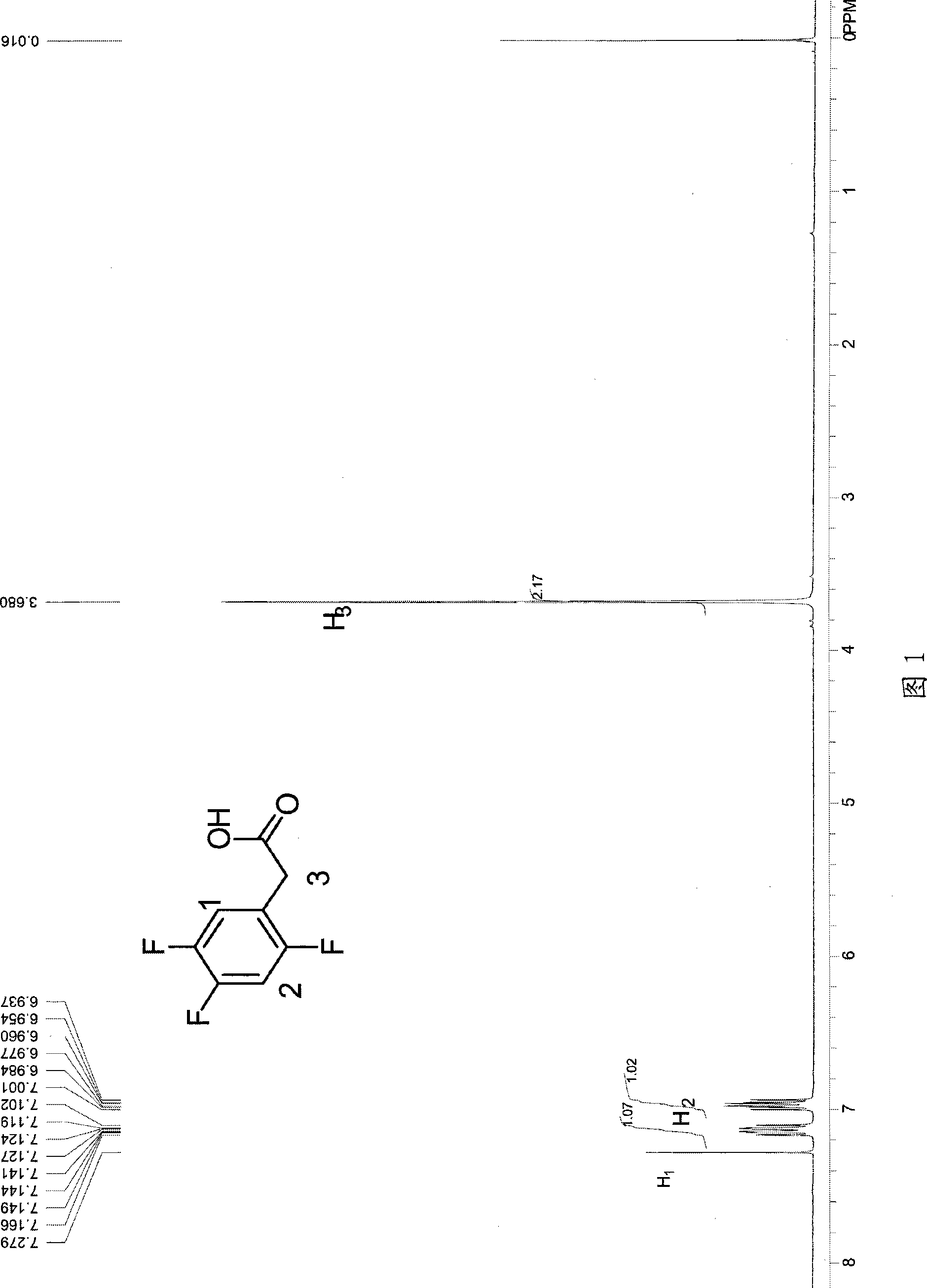
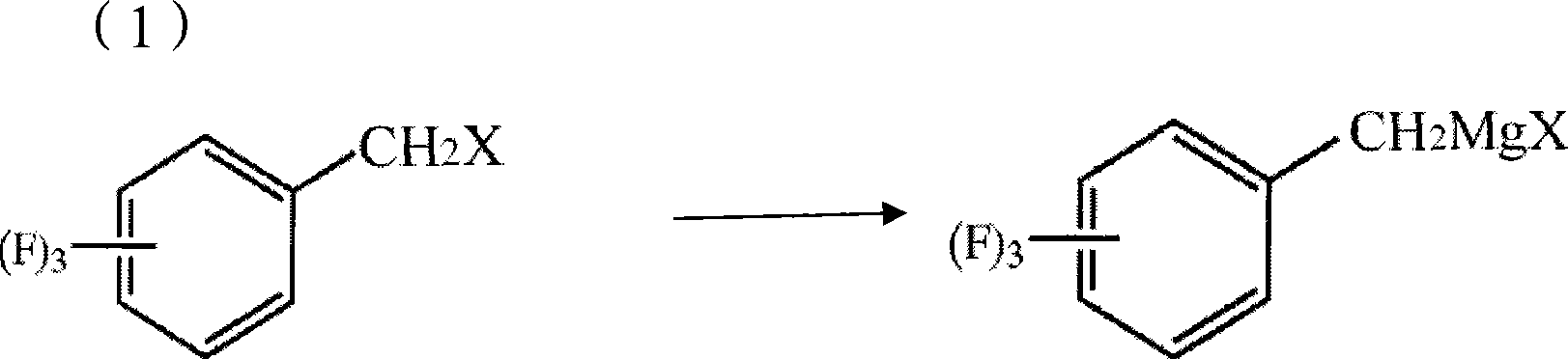
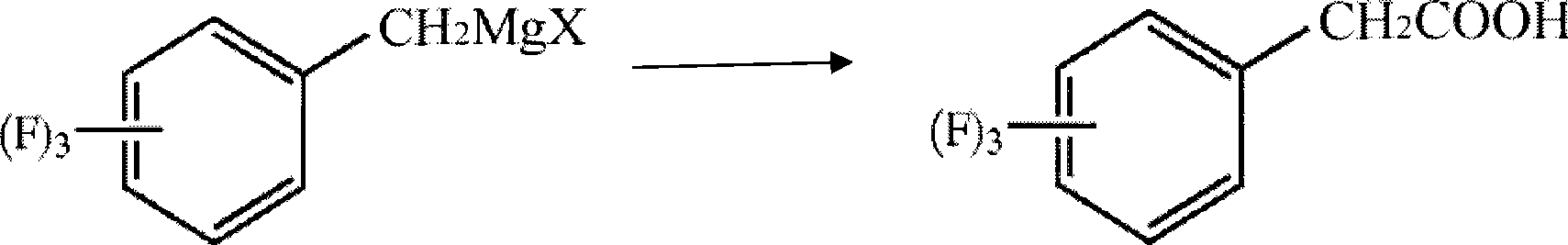
Process for producing trifluoro benzene acetic acid and sitagliptin
ActiveCN101429115AHigh process yieldHigh purityOrganic compound preparationCarboxylic compound preparationSitagliptinBenzene
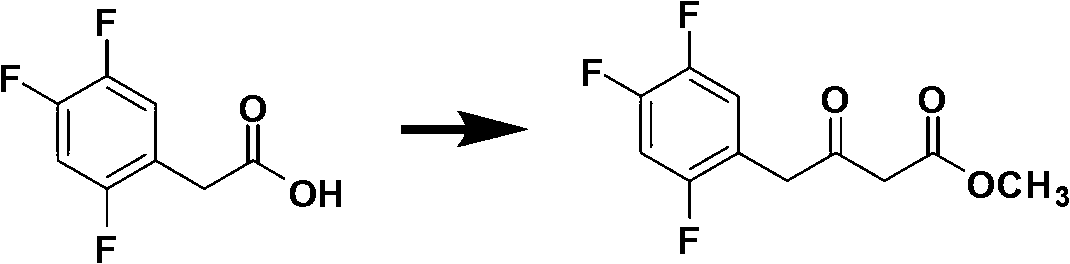
The invention discloses a method for preparing trifluoro-phenylacetic acid. The method comprises the following steps: (1) in the presence of an evocating agent, trifluoro-benzyl halides and magnesium in an organic solvent react to obtain a Grignard reagent; (2) carbon dioxide gas is introduced into the Grignard reagent for reaction; and (3) a product obtained in the step (2) is hydrolyzed to obtain the trifluoro-phenylacetic acid. The invention also discloses a method for preparing sitagliptin. The method has the characteristics of high yield, good purity, low cost, simple process, mild condition, few three wastes and good safety, and is suitable for industrialized production.
Owner:ZHEJIANG HISOAR PHARMA
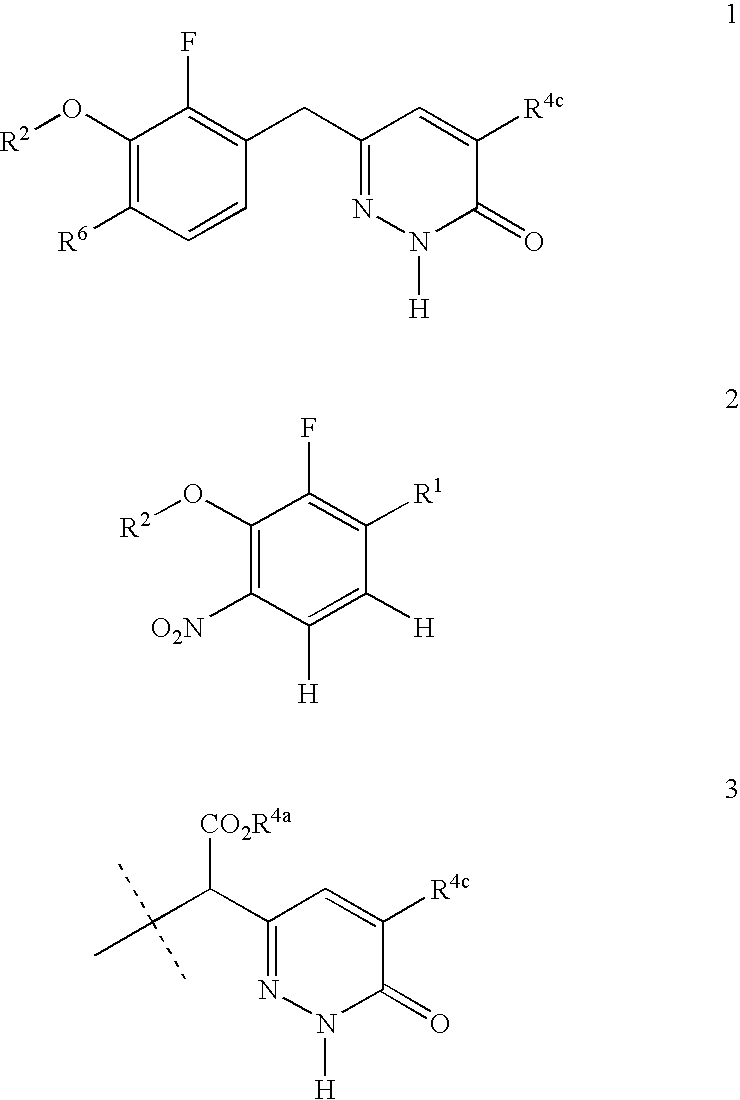
Process for preparing pyridazinone compounds
The present invention provides a process for the preparation 6-[3-(hetero)aryloxy-2-fluoro-benzyl]-2H-pyridazin-3-one compounds 1 where R2 is an optionally substituted aryl or an optionally substituted heteroaryl, R6 is NO2, NH2, alkyl, halogen, or a function group readily derived therefrom and R4c is hydrogen or alkyl. There also is provided a process for the preparation of phenylacetic acid compounds 2, wherein R2 and R6 are as defined previously and R5a is hydrogen or alkyl, which are useful for the preparation of pyridazinone compounds.
Owner:ROCHE PALO ALTO LLC
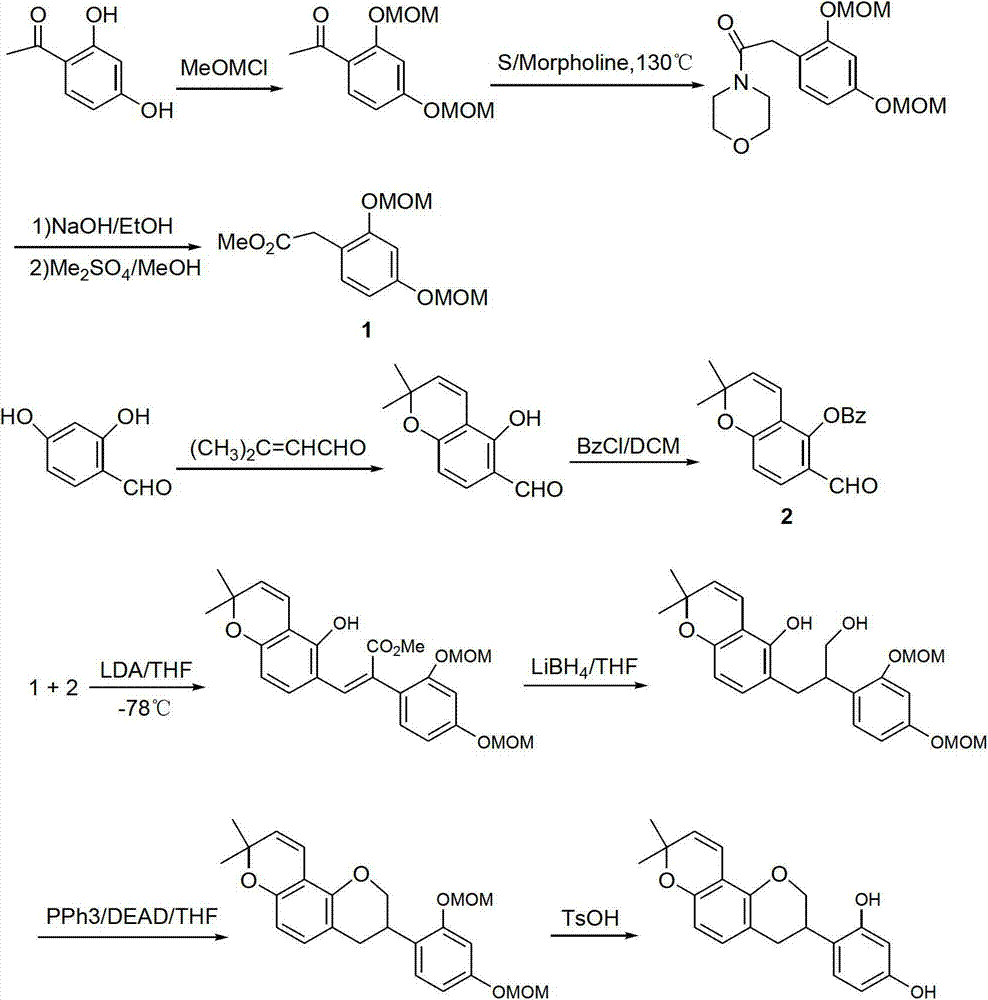
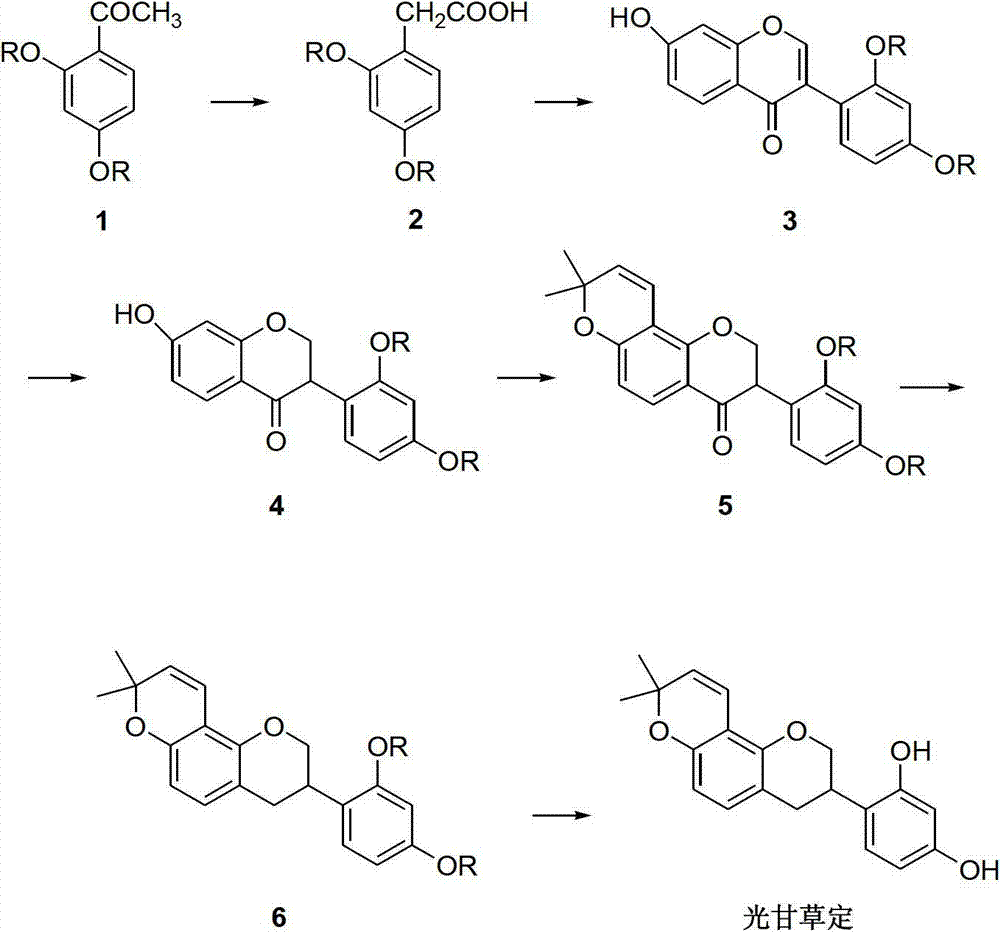
Method for synthesizing glabridin
The invention relates to a method for synthesizing glabridin. The method comprises the following steps: using acetophenone protected by phenol hydroxy as a raw material, carrying out a Willgerodt-Kindler reaction to obtain aryl phenylacetic acid, and carrying out a Friedel-Crafts reaction to obtain an isoflavones compound; causing the isoflavones compound to carry out Pd / C catalytic hydrogenation to obtain a isoflavanone compound; and causing the isoflavanone compound to carrying out a crclizationreaction, a conyl reduction reaction and a removing phenolic hydroxyl group protection group reaction to obtain the glabridin. The operation and the serparation of the steps of the method are simple, the yield is higher, the used reagents are common reagents, are cheap and are easily obtained, the path is shorter, and the tptal yield is not lower than 20 percent.
Owner:山东济清科技服务有限公司
Resolution of alpha-(phenoxy)phenylacetic acid derivatives
ActiveUS20050033084A1Increase productionOrganic compound preparationCarboxylic compound preparationPhenylacetic acidEnantiomer
The present invention provides a method for producing an enantiomerically enriched α-(phenoxy)phenylacetic acid compound of the formula: from its enantiomeric mixture, where R1 is alkyl or haloalkyl and X is halide.
Owner:DIATEX INC (US)
Synergistic flame retardant compositions and fiber blends including the same
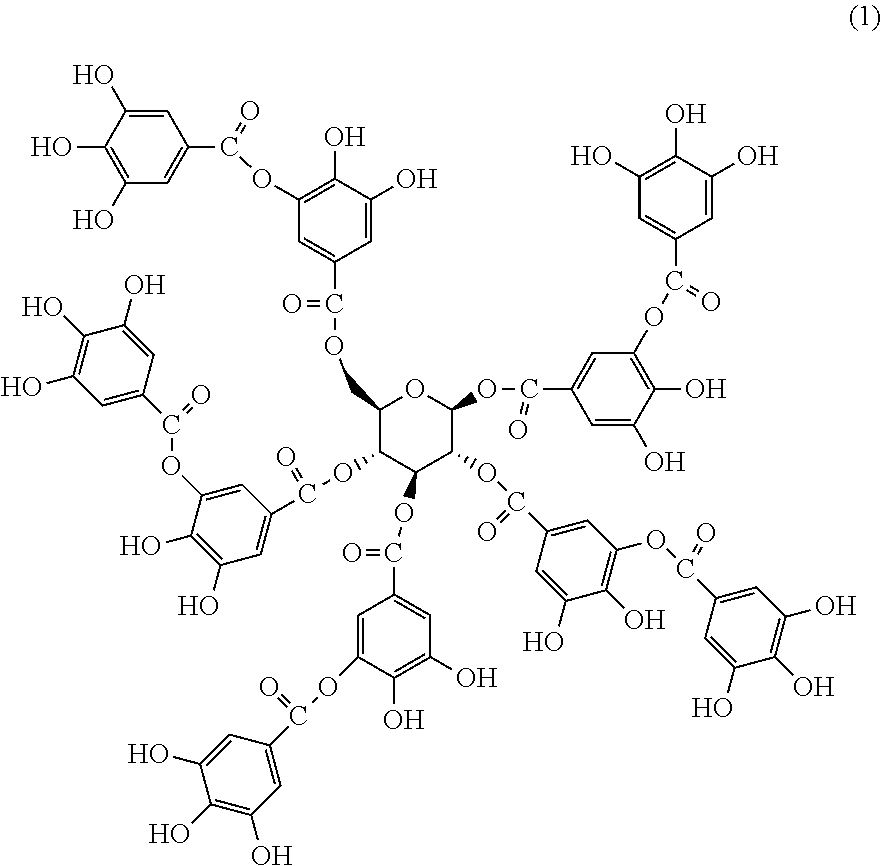
A synergistic flame retardant composition comprising a phenolic compound comprising condensed tannin, hydrolysable tannin, lignin, cardanol, quercetin, catechin, epicatechin, anthocyanidin, catechol, dopamine, hydroxytyrosol, adrenaline, 4-hydroxyphenylacetic acid, gallic acid, digallic acid, methyl gallate, ellagic acid, phloroglucinol, hexahydroxydiphenic acid, luteic acid, casuarictin, or a combination thereof; and a phosphorus-containing compound comprising a C5-7 carbocyclic polyol substituted with at least one phosphate group.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY +1
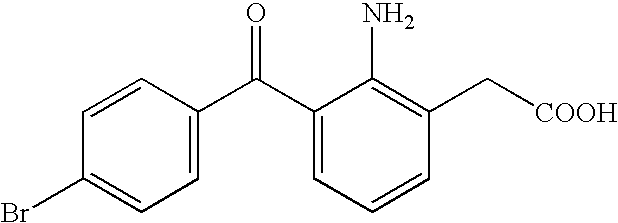
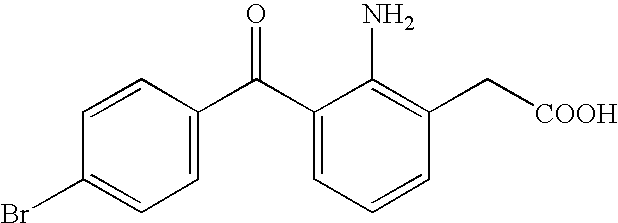
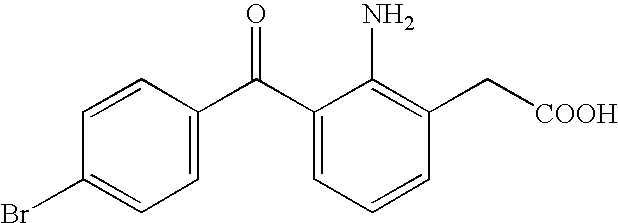
Aqueous liquid preparation containing 2-amino-3-(4-bromobenzoyl)phenylacetic acid
ActiveUS20050239895A1No irritationInhibit deteriorationBiocideOrganic active ingredientsScleritisPhenylacetic acid
An aqueous liquid preparation of the present invention containing 2-amino-3-(4-bromobenzoyl)phenylacetic acid or its pharmacologically acceptable salt or a hydrate thereof, an alkyl aryl polyether alcohol type polymer such as tyloxapol, or a polyethylene glycol fatty acid ester such as polyethylene glycol monostearate is stable. Since even in the case where a preservative is incorporated into said aqueous liquid preparation, the preservative exhibits a sufficient preservative effect for a long time, said aqueous liquid preparation in the form of an eye drop is useful for the treatment of blepharitis, conjunctivitis, scleritis, and postoperative inflammation. Also, the aqueous liquid preparation of the present invention in the form of a nasal drop is useful for the treatment of allergic rhinitis and inflammatory rhinitis (e.g. chronic rhinitis, hypertrophic rhinitis, nasal polyp, etc.).
Owner:SENJU PHARMA CO LTD
Aqueous liquid preparation containing 2-amino-3-(4-bromobenzoyl)phenylacetic acid
An aqueous liquid preparation of the present invention containing 2-amino-3-(4-bromobenzoyl)phenylacetic acid or its pharmacologically acceptable salt or a hydrate thereof, an alkyl aryl polyether alcohol type polymer such as tyloxapol, or a polyethylene glycol fatty acid ester such as polyethylene glycol monostearate is stable. Since even in the case where a preservative is incorporated into said aqueous liquid preparation, the preservative exhibits a sufficient preservative effect for a long time, said aqueous liquid preparation in the form of an eye drop is useful for the treatment of blepharitis, conjunctivitis, scleritis, and postoperative inflammation. Also, the aqueous liquid preparation of the present invention in the form of a nasal drop is useful for the treatment of allergic rhinitis and inflammatory rhinitis (e.g. chronic rhinitis, hypertrophic rhinitis, nasal polyp, etc.).
Owner:SENJU PHARMA CO LTD
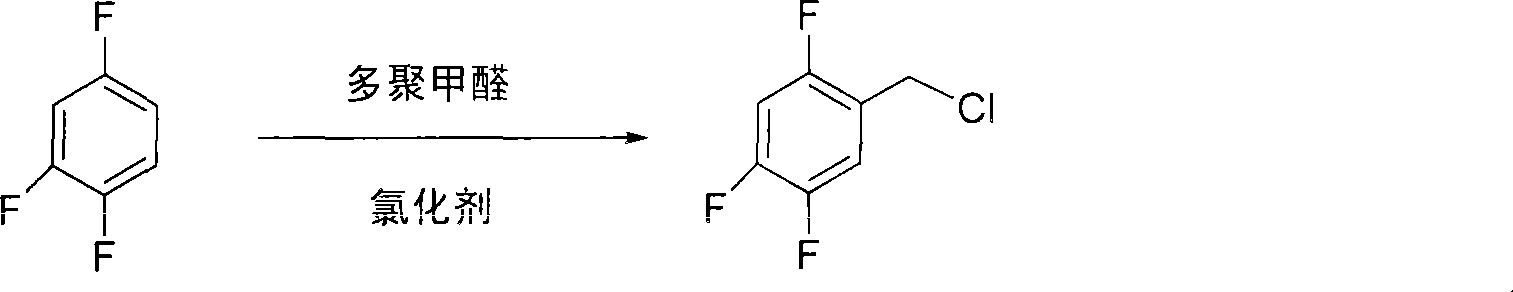
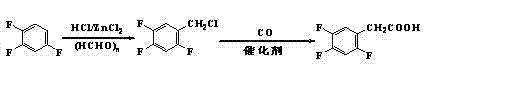
Method for preparing 2,4,5 trifluorobenzene acetic acid
ActiveCN101092345ALow priceFew reaction stepsCarboxylic preparation from carbon monoxide reactionAcetic acidPtru catalyst
This invention provides a method for preparing 2, 4, 5-trifluorophenylacetic acid. The method comprises: (1) reacting 1,2,4-trifluorobenzene and chlorinating agent in paraformaldehyde to obtain 2,4,5-trifluorobenzyl chloride; (2) performing carbonylation reaction with CO in the presence of catalyst to obtain 2,4,5-trifluorophenylacetic acid. The catalyst is alkali cobalt tetracarbonyl. The method has such advantages as few reaction procedures, and mild reaction conditions, and is suitable for industrial production of 2, 4, 5-trifluorophenylacetic acid.
Owner:ZHEJIANG YONGTAI TECH CO LTD
Synthetic method of hydroxytyrosol
InactiveCN103664536AMild reaction conditionsLower reaction costOrganic chemistryOrganic compound preparationChemical synthesisHydroxytyrosol
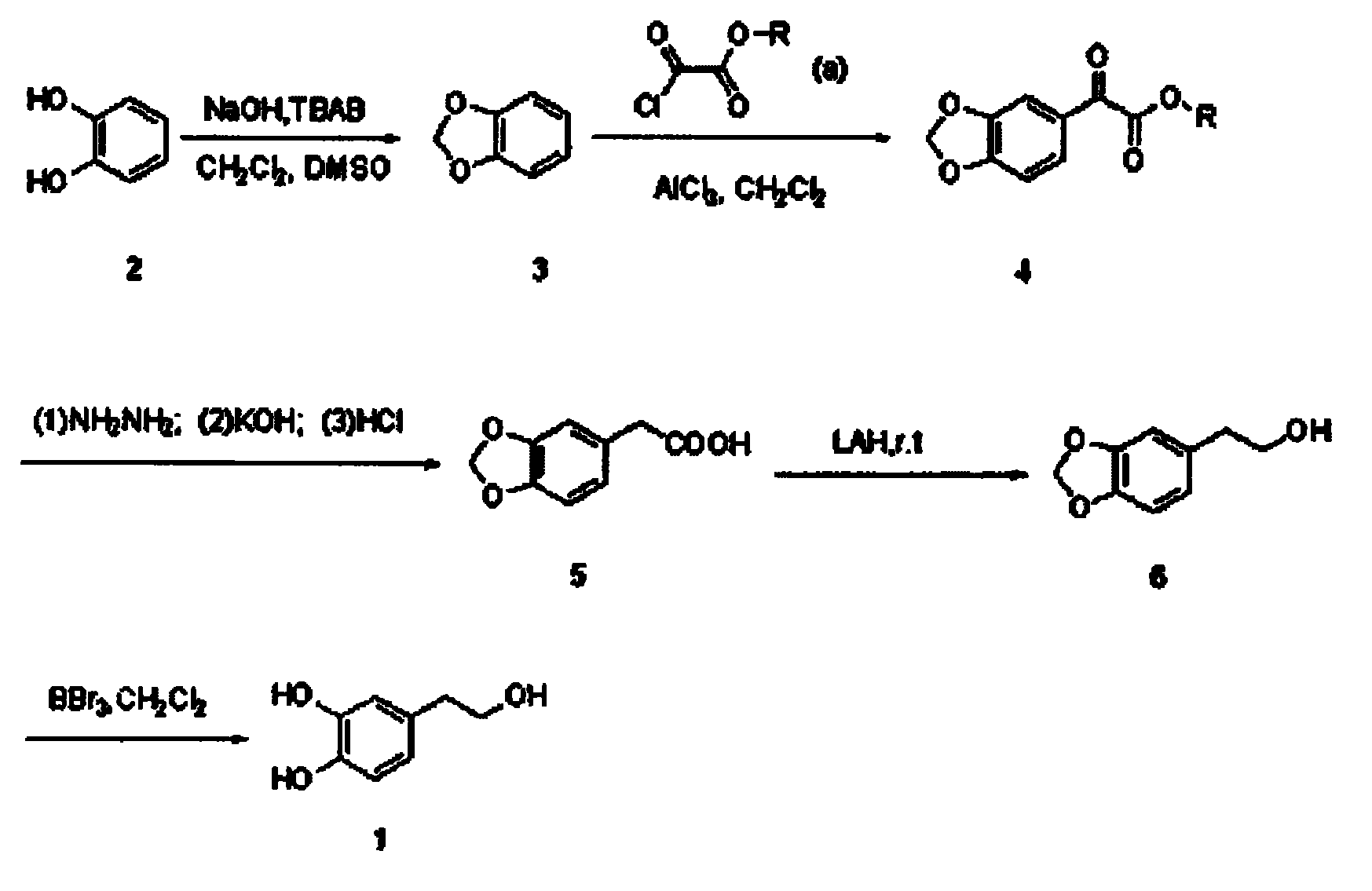
The invention belongs to the technical field of medicament synthesis, and in particular relates to a chemical synthetic method of hydroxytyrosol. The chemical synthetic method comprises the steps of (1) protecting two phenolic hydroxyl groups of catechol by using dichloromethane, and enabling catechol to react with dichloromethane to prepare 1,2-methylenedioxybenzene; (2) enabling 1,2-methylenedioxybenzene to react with various monoesters of oxalyl chloride to prepare 3,4-methylenedioxy phenylglyoxylic acid ester; (3) preparing 3,4-methylenedioxy phenylacetic acid by using 3,4-methylenedioxy phenylglyoxylic acid ester through a Wollff-kishner-Huang Minglong reduction reaction; and (4) reducing the 3,4-methylenedioxy phenylacetic acid by using lithium aluminum hydride, lithium borohydride or sodium borohydride to prepare 3,4-methylenedioxy phenethyl alcohol, and then removing methylene protection of the 3,4-methylenedioxy phenethyl alcohol by using boron tribromide or palladium on activated carbon to prepare hydroxytyrosol. A reactive reagent used in the synthetic method disclosed by the invention is easy to obtain and low in price, the reaction condition is mild, and the final yield of the whole reaction is 23%.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Preparation method of azoxystrobin intermediate
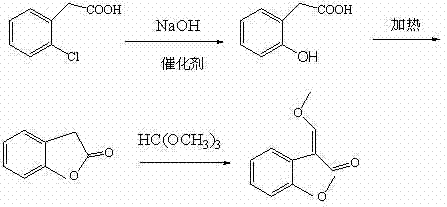
The invention relates to a preparation method of an azoxystrobin intermediate. The method comprises the following steps of: (1) synthesizing benzofuranone from o-hydroxyphenylacetic acid serving as a raw material; and (2) making benzofuranone react with trimethyl orthoformate to generate the azoxystrobin intermediate, wherein no solvent is used in the reaction in the step (1); and the reaction isperformed by the following steps of: putting o-hydroxyphenylacetic acid and 0-3 percent of catalyst into a reaction kettle; preserving heat in vacuum at the temperature 125-180 DEG C to react for 2-3hours; and evaporating water generated by the reaction in vacuum, wherein the residue in the reaction kettle is benzofuranone. The method has the advantages of simple process, high yield of the azoxystrobin intermediate and low production cost.
Owner:JIANGSU SEVENCONTINENT GREEN CHEM CO LTD
Formula of tobacco flavoring essence with sweet fragrance
ActiveCN102304428AGreat tasteAdd natural sweetnessTobacco preparationEssential-oils/perfumesBenzoic acidCyclopentene
The invention discloses a formula of tobacco flavoring essence with sweet essence. According to the formula, the tobacco flavoring essence consists of the following components in percentage by mass: 1.0 to 4.0 percent of ethyl maltol, 2.0 to 3.0 percent of plum extractum, 1.0 to 2.0 percent of phenethyl ethanol, 0.01 to 0.1 percent of damascenone, 0.1 to 0.5 percent of phenylacetic acid ethylester, 0.5 to 1.0 percent of methyl cyclopentenotone, 2.0 to 5.0 percent of red date extractum, 0.01 to 0.03 percent of linalool, 0.02 to 0.08 percent of 5-methylfurfural, 0.1 to 0.5 percent of benzoic acid, 0.05 to 0.15 percent of beta-irisone, 0.02 to 0.08 percent of 4-guaethol, 15 to 25 percent of glycerol, 45 to 55 percent of propylene glycol and 3 to 15 percent of purified water. By the formula, tobacco produces sweet fragrance, tobacco fragrance can be increased and taste of the tobacco can be adjusted, so that the taste of the tobacco is improved, the natural sweet fragrance of the tobacco is enhanced and dry, astringent and bitter mouthfeel of customers is improved and requirements of the customers are met.
Owner:GUANGZHOU AOJIAN PERFUME
Epoxy resin polymer mortar and production process thereof
InactiveCN104261765AImprove stress resistanceImprove impact resistancePolymer sciencePhenylacetic acid
The invention relates to epoxy resin polymer mortar and a production process thereof. The mortar is prepared from the following components in parts by weight: 15-20 parts of epoxy resin slurry, 2-4 parts of an active diluent, 6-8 parts of a curing mixture and 70-80 parts of a base material which are mixed, stirred and mixed, wherein the epoxy resin slurry is prepared from bisphenol A epoxy resin E51, modified epoxy resin, organic bentonite and dioctyl phthalate; the active diluent is prepared from epoxy chloropropane, fatty alcohol and trichloropropane; the curing mixture is prepared from 85 parts of modified phenolic aldehyde amine, hydroxy-terminated liquid nitrile rubber, 2-(3, 4-expoy cyclohexyl) ethyl triethoxyl silane, lauryl phenylacetate and resorcinol; the base material is prepared from ordinary portland cement, quartz sands, hydrophobic expanded perlite, refined graphite powder and sericite powder. The epoxy resin polymer mortar provided by the invention not only has excellent mechanical property and wear resistance, but also has a good adhesive performance, and can be integrally formed with substrate concrete.
Owner:XIAOLANGDI WATER CONSERVANCY & HYDROPOWER ENG CO LTD
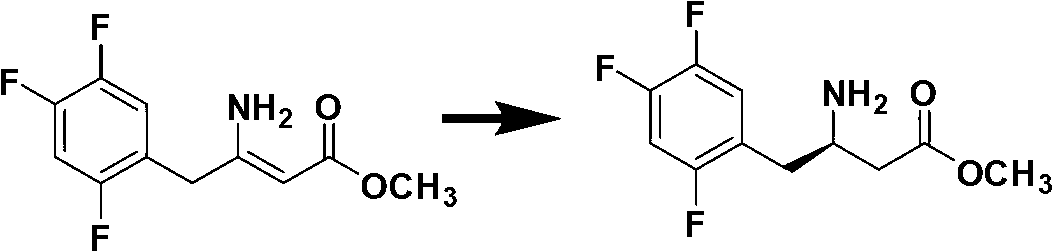
Preparation method of sitagliptin
The invention discloses a preparation method of sitagliptin, comprising the following steps: reacting 2, 4, 5-trifluoro-phenylacetic acid with malonic cyclo (sub) isopropyl ester; reacting 3-oxo-4-(2, 4, 5-trifluorophenyl) methyl butyrate with ammonium acetate; reacting 3-amino-4-(2, 4, 5-trifluorophenyl) methyl crotonate with hydrogen; performing hydrolysis reaction to (R)-3-amino-4-(2, 4, 5-trifluorophenyl) methyl butyrate; and reacting (R)-3-amino-4-(2, 4, 5-trifluorophenyl) butyric acid with 3-(trifluoromethyl)-5, 6, 7, 8-tetrahydro-[1, 2, 4] triazol [4, 3-a] pyrazine hydrochloride to obtain sitagliptin. According to the invention, EE value greater than 90% is obtained through high-efficiency catalytic hydrogenation; sitagliptin is prepared by only five steps; yield is higher; technical conditions are mild; operations are simple; cost is low; and yield and purity of products are high.
Owner:SUZHOU XINKAI BIOLOGICAL MEDICINE TECH
Resolution of alpha-(phenoxy)phenylacetic acid derivatives
ActiveUS7199259B2Increase productionOrganic compound preparationCarboxylic compound preparationPhenylacetic acidAlkyl
The present invention provides a method for producing an enantiomerically enriched α-(phenoxy)phenylacetic acid compound of the formula (I):from its enantiomeric mixture, where R1 is alkyl or haloalkyl R7 is heteroalkyl and X is halide.
Owner:DIATEX INC (US)
Rhodococcus ruber and application thereof in degradation of organic pollutants
The invention discloses rhodococcus ruber FQ-2 and an application thereof in degradation of acetone and other common industrial organic pollutants. The application method comprises the steps: an inorganic salt culture medium containing acetone and other common industrial pollutants is inoculated with the rhodococcus ruber FQ-2, a degradation reaction is carried out under the conditions of the temperature of 30 DEG C and the rotating speed of 160 r / min, and thus the organic pollutants are degraded; the common organic pollutants comprise acetone, n-hexane, carbon disulfide, chlorobenzene, butylacetate, ethyl acetate or alpha-pinene. The rhodococcus ruber FQ-2 is taken from activated sludge of an aeration tank of a pharmaceutical factory in Zhejiang province, has good degradation effect on VOCs organic pollutants, especially acetone, and can completely convert acetone into harmless substances such as CO2, H2O, cell biomass and the like; at the same time, the bacterial strain also can degrade common industrial pollutants such as carbon disulfide and chlorobenzene in different extent, so the bacterial strain has broad application prospects in biological purification of industrial wastegas and wastewater.
Owner:ZHEJIANG UNIV OF TECH
Fertilizer composition for improving stress resistance of land planted pear trees
ActiveCN103936500AStrong noveltyPracticalFertilizer mixturesArbuscular mycorrhizal fungiPhenylacetic acid
A fertilizer composition for improving stress resistance of land planted pear trees comprises arbuscular mycorrhizal fungi, betaine, salicylic acid, ethylenediamine-N,N'-bis(2-hydroxyphenylacetic acid) ferric-sodium complex (EDDHA-Fe), a chelate zinc fertilizer titanium zinc ethylenediaminetetraacetate, a promoter and fertilizers containing other elements. Contained arbuscular mycorrhizal fungi is capable of substantially improving stress resistance on drought, waterlogging disaster, salt and alkali, high temperature, heavy metal toxicity, toxic organics and the like of pear trees, and promoting absorption of soil mineral nutritional elements by soil. Betaine and salicylic acid are capable of inducing to improve crop resistance on adverse situations such as drought, freeze injury and the like.
Owner:SHANDONG INST OF POMOLOGY
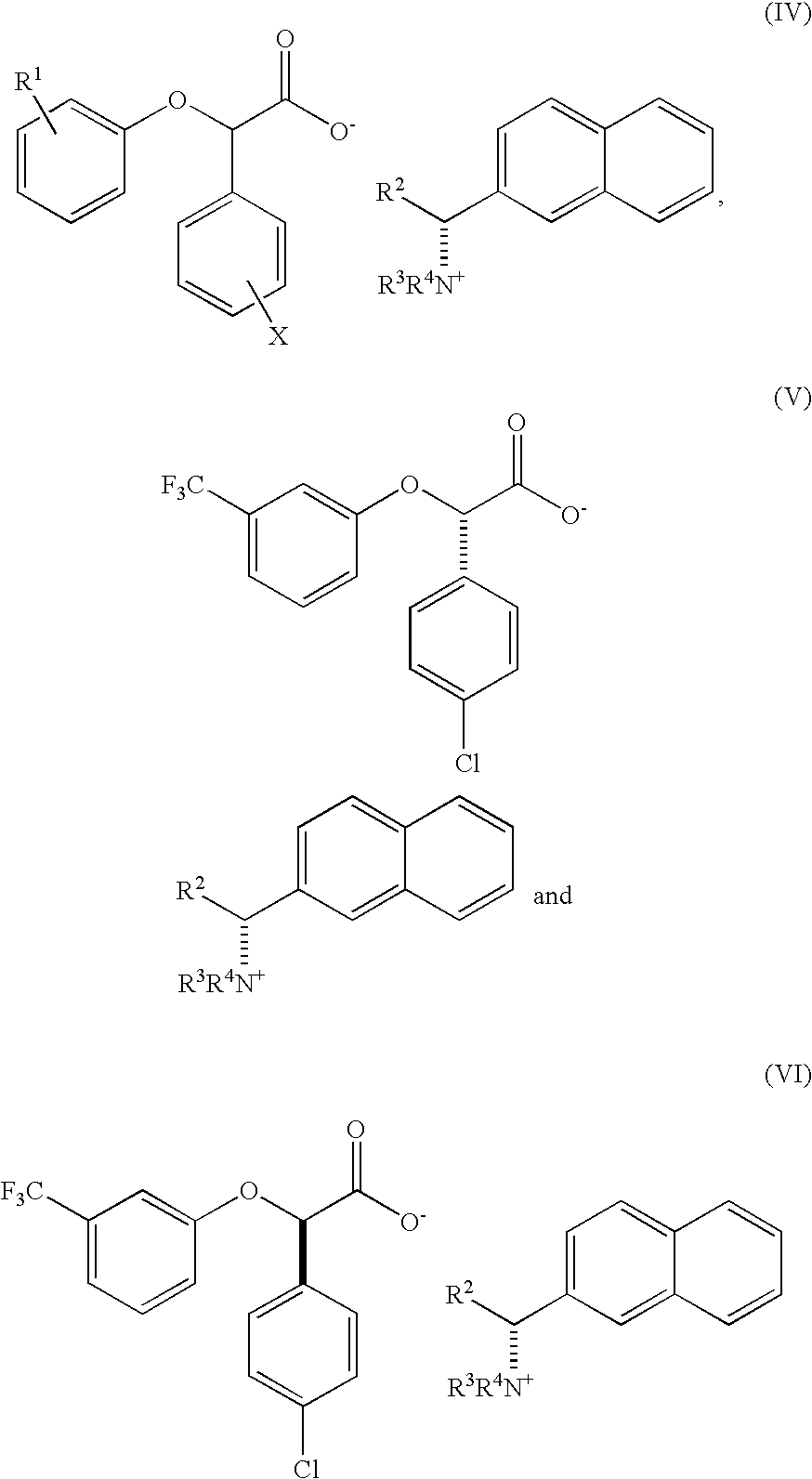
Resolution of alpha-(phenoxy) phenylacetic acid derivatives with naphthyl-alkylamines
InactiveUS7432394B2Reduce the ratioOrganic compound preparationCarboxylic acid amides preparationPhenylacetic acidEnantiomer
The present invention provides a methods and compounds for producing an enantiomerically enriched α-(phenoxy)phenylacetic acid compound of the formula:from a mixture of its enantiomers, where R1 is alkyl or haloalkyl and X is halide.
Owner:DIATEX INC (US)
Method for preparing 3-arylbenzofuran ketone compounds
ActiveCN101684111AOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsPolyolefinPhenylacetic acid
The invention relates to a method for preparing a 3-arylbenzofuran ketone compounds. The method comprises that: phenolic compounds and phenylacetic-acid structure compounds are subjected to alkylationin a mol ratio of 1:1-2 at a temperature between 60 and 270 DEG C; an alkylation catalyst is an acidized crosslinked montmorillonite catalyst prepared by acidizing, loading, crosslinking and activating montmorillonite; the weight ratio of phenolic compounds serving as reaction raw material to the montmorillonite alkylation catalyst is 1:0.01-0.2; an esterification catalyst is added; the mol ratioof the phenolic compounds serving as the reaction raw material to the esterification catalyst is 1:0.001-0.02; esterification is performed at a temperature between 60 and 170 DEG C; supported metal ions of the acidized crosslinked montmorillonite catalyst are 1 to 10 mmol / g of montmorillonite; and the mol ratio of silicon to aluminum is 2-10:1. The 3-arylbenzofuran ketone compound prepared is suitable for serving as organic polymer sensitive to oxidation and heat / light-induced degradation, particularly a polyolefin stabilizer.
Owner:PETROCHINA CO LTD
Process for producing 6-amino-penicillanic acid and phenylacetic acid
PCT No. PCT / ES97 / 00066 Sec. 371 Date Feb. 25, 1998 Sec. 102(e) Date Feb. 25, 1998 PCT Filed Mar. 14, 1997 PCT Pub. No. WO97 / 35029 PCT Pub. Date Sep. 25, 1997Alternative process for obtaining 6-aminopenicillanic acid. The process comprises replacing the stages of extraction with organic solvents and isolation and separation of the intermediate penicillin salt as a solid by a process of ultrafiltration of the culture broth in at least 2 successive stages. The first stage has a cut-off for molecular weights of 20,000 Dalton and the second, 2000 Dalton. Subsequent to the enzyme conversion stage the products from that stage are subjected to a series of anionic exchange chromatography steps.
Owner:ANTIBIOTICOS SA
Preparation method 2,4,5-trifluorophenylacetic acid
ActiveCN103012111AFew synthetic stepsMild reaction conditionsOrganic compound preparationCarboxylic preparation by ozone oxidationPhenylacetic acidNitrobenzene
The invention discloses a preparation method 2,4,5-trifluorophenylacetic acid. The method is characterized by consisting of four reaction steps of: A, reaction of 2,4,5-trifluoronitrobenzene (I) and diethyl malonate which are condensed to prepare 2,5-difloro-4-nitrobenzophenone diethyl malonate; B, hydrolysis, acidification and decarboxylic reaction of dibasic ester; C, reduction reaction of nitryl; and D, diazotization fluoridation of amino. The four reaction steps can be sequentially carried out according to A, B, C and D, or A, C, B and D, or A, C, D and B. According to the preparation method provided by the invention, condensation of 2,4,5-trifluoronitrobenzene (I) and diethyl malonate is easy to realize by means of high substituting activity of nitryl p-fluorine, and the raw material 2,4,5-trifluoronitrobenzene (I) is low in cost and easy to obtain and can be easily prepared by nitration and fluorination of 2,4-dichlor fluorbenzene. Compared with the prior art, the preparation method provided by the invention has the characteristics of low-cost and easily obtained raw materials, mild reaction condition, high total yield, low production cost and the like, and is comparatively suitable for industrialized production.
Owner:江苏中丽新材料有限公司
Method for preparing 4-hydroxyphenyl hydantoin
InactiveCN101973941AQuality improvementHigh synthetic yieldOrganic compound preparationAmino-carboxyl compound preparationPhenylacetic acidPhenol
The invention discloses a method for preparing 4-hydroxyphenyl hydantoin from a glyoxylic acid, phenol and urea by condensation under acid condition, which is characterized in that: the 4-hydroxyphenyl hydantoin is prepared in the presence of a sulfamic acid, the production of polymerization impurities is inhibited and simultaneously a phenylglycine byproduct is produced. The mol ratio of the reaction raw materials of the glyoxylic acid to the phenol to the urea to the sulfamic acid is 1:1.0-1.1:1-1.5:0.2-0.5. The method comprises the following steps of: adding dropwise glyoxylic acid solution at the relatively lower temperature of 50 to 60 DEG C to form a 2-ureidobenzeneacetic acid intermediate, preparing the 4-hydroxyphenyl hydantoin at the relatively higher temperature of 80 or 105 DEG C by ring formation and simultaneously producing the p-hydroxyphenylglycine byproduct, wherein reaction solution is subjected to post-treatment to obtain white 4-hydroxyphenyl hydantoin crystals with the purity of 99.6 percent and the yield of 62.7 percent; and mother solution is further treated to obtain white p-hydroxyphenylglycine crystals with the purity of 99.2 percent ad the yield of 10.1 percent. In the method, the main byproduct is p-hydroxyphenylglycine, the utilization rate of the synthesis raw materials is increased and the problems of unstable quality of product and high treatment cost of the mother solution in the prior art are solved.
Owner:TIANJIN VOCATIONAL INST
Method for preparing 2, 4, 5-trifluoro-phenylacetic-acid
ActiveCN101659611AEasy to separateMeet the requirementsOrganic-compounds/hydrides/coordination-complexes catalystsPreparation from nitrilesAcetic acidPhenylacetic acid
The invention provides a method for preparing 2, 4, 5-trifluoro-phenylacetic-acid. The method adopts 1, 2, 4-trifluoro-benzene to react with polyformaldehyde and chlorinating agent to obtain 2, 4, 5-trifluoro-benzyl chloride, and adopts the 2, 4, 5-trifluoro-benzyl chloride to react with cyaniding agent in ionic liquid to obtain 2, 4, 5-trifluoro-benzyl cyanide, and then the 2, 4, 5-trifluoro-benzyl cyanide is hydrolyzed in acid or alkalic conditions to obtain the 2, 4, 5-trifluoro-phenylacetic-acid. The preparation method for preparing 2, 4, 5-trifluoro-phenylacetic-acid requires cheap and available materials, and has moderate reaction conditions, less three-wastes and better safety; industrialized production can be easily realized, the product purity is high, and quality is stable, thuscompletely meeting the using requirement of 2, 4, 5-trifluoro-phenylacetic-acid as a pharmaceutical intermediate.
Owner:ZHEJIANG YONGTAI TECH CO LTD
Peach essence
ActiveCN104957584AReduce manufacturing costBroad sales marketFood ingredientsFood preparationAdditive ingredientPhenylacetic acid
The invention discloses a peach essence. The peach essence consists of the following ingredients: propanediol, hexanol, hexenyl phenylacetate, benzaldehyde, ethyl acetate, phenylacetic acid cis-3-ester hexane, methyl acetate, hexyl acetate, hexyl trans-2-ester hexane, ethyl maltol, leaf alcohol, hexanal, acetic acid, linalool, gamma-caprylolactone, theta-decalactone, gama-decalactone, gamma-undecalactone, 8-mercaptomenthone, 2-isopropyl-4-methylthiazole, beta- damascenone, ethyl caprate and ethyl benzoate. The peach essence is moderate in fragrance, saturated, thick, stable in quality, natural in flavor and approximat to the natural fragrance of peach.
Owner:ZHEJIANG GREEN CRYSTAL FLAVOR
Sulbenicillin sodium and sulbenicillin sodium used for injection
InactiveCN102161667AReduce contentReduce pollutionAntibacterial agentsPowder deliveryDichloromethanePhenylacetic acid
The invention provides a synthesis method of sulbenicillin sodium and sulbenicillin sodium used for injection, which comprises the concrete steps of: using 6-APA and BSA to synthesize an organic salt, and dissolving the organic salt into dichloromethane; using a sulphur phenylacetic acid triethylamine salt and pivaloyl chloride to make mixed anhydride; carrying out anhydrous condensation on the sulphur phenylacetic acid triethylamine salt and the pivaloyl chloride in the dichloromethane; and then forming a sodium salt, free-drying and obtaining a finished product. The new technical process is stable and feasible, better and stable in product quality and strong in operability of production, and reduces the environmental pollution.
Owner:辽宁科泰生物基因制药股份有限公司
Pharmaceutical composition
InactiveUS20070231382A1Organic active ingredientsPeptide/protein ingredientsPhenylacetic acidImmediate release
Disclosed herein are tablets and methods of treatment comprising the administration of such tablets. The tablets are immediate release tablets that comprise about 400 mg of 5-methyl-2-(2′-chloro-6′-fluoroanilino)phenylacetic acid or a pharmaceutically acceptable salt thereof, where the 5-methyl-2-(2′-chloro-6′-fluoroanilino)phenylacetic acid or a pharmaceutically acceptable salt thereof comprises between 60 and 70% by weight of the tablet. The methods involve the administration of the tablets of the invention to individuals in need of administration of such tablets.
Owner:KARNACHI ANEES ABDULQUADAR +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com