Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
61results about How to "High enantiomeric purity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
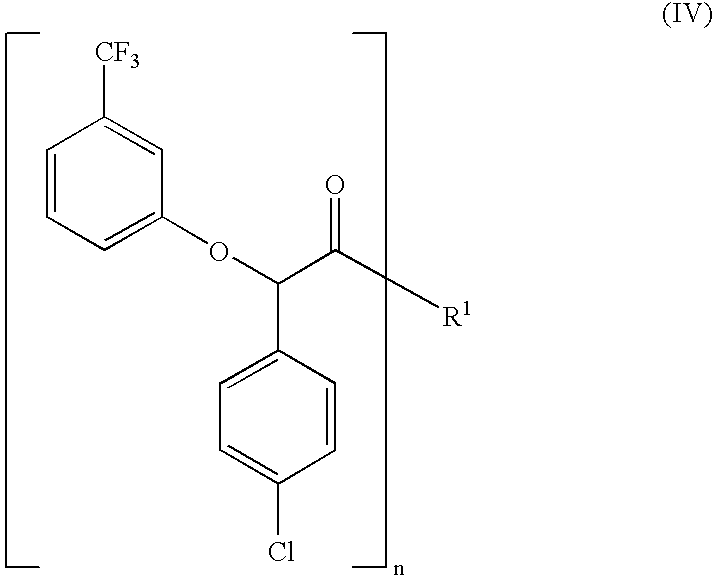
Process for the stereoselective preparation of (−)-halofenate and derivatives thereof
InactiveUS7714131B2High yieldHigh enantiomeric purityBiocideOrganic chemistryPhenylacetic acidHALOFENATE
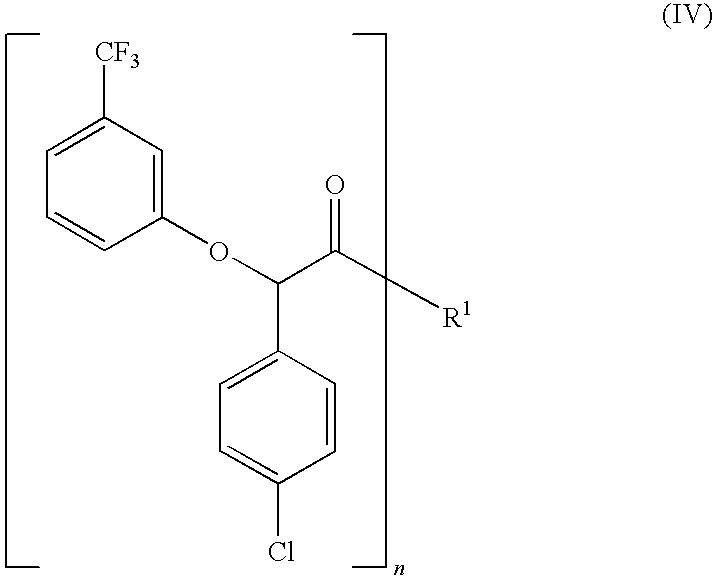
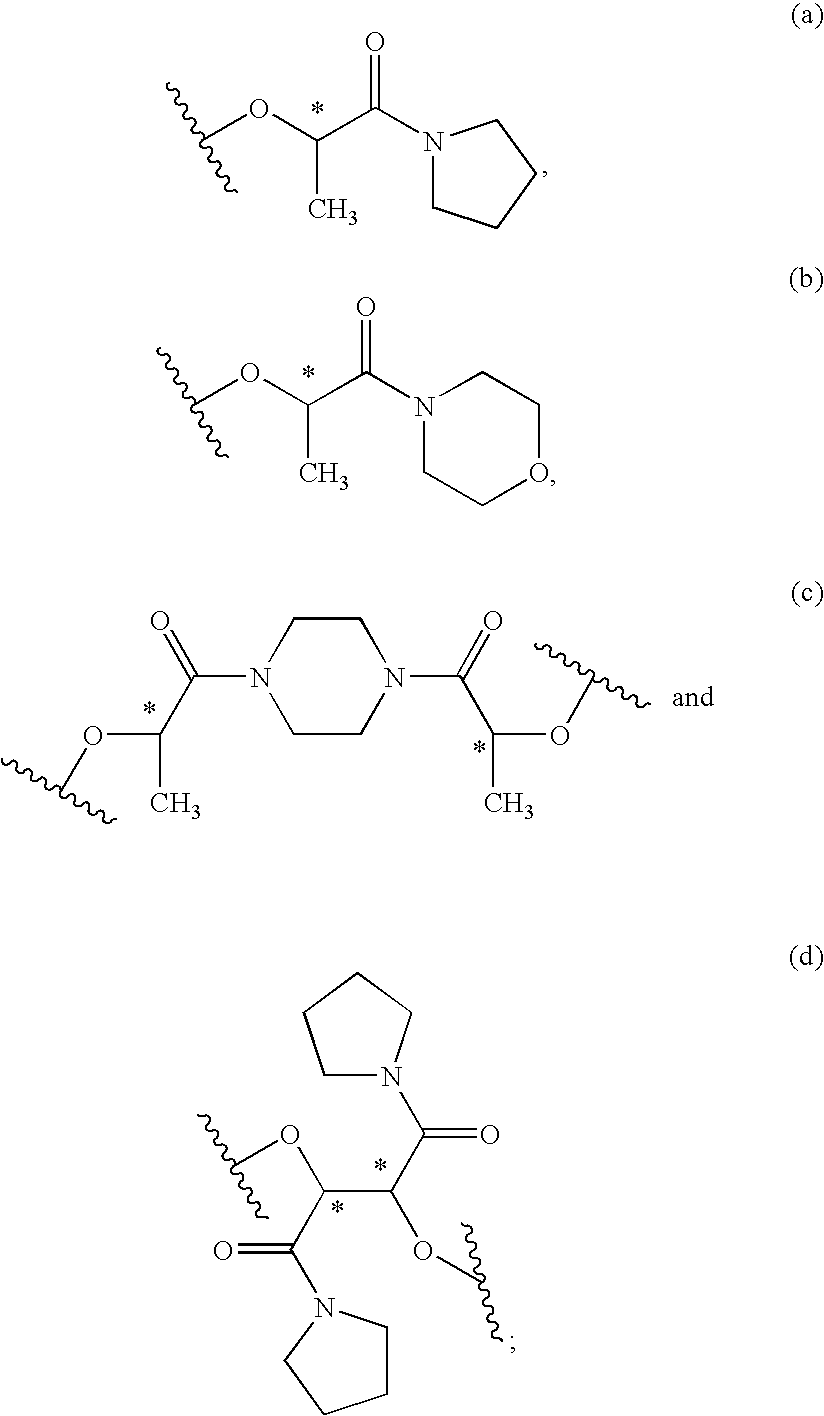
The present invention provides a compounds the formula (IV):and methods for producing an α-(phenoxy)phenylacetic acid compound of the formula:wherein R1 is a member selected from the group consisting of:each R2 is a member independently selected from the group consisting of (C1-C4)alkyl, halo, (C1-C4)haloalkyl, amino, (C1-C4)aminoalkyl, amido, (C1-C4)amidoalkyl, (C1-C4)sulfonylalkyl, (C1-C4)sulfamylalkyl, (C1-C4)alkoxy, (C1-C4)heteroalkyl, carboxy and nitro; the subscript n is 1 when R1 has the formula (a) or (b) and 2 when R1 has the formula (c) or (d); the subscript m is an integer of from 0 to 3; * indicates a carbon which is enriched in one stereoisomeric configuration; and the wavy line indicates the point of attachment of R1; and compounds.
Owner:DIATEX INC (US)
Process for the preparation of [S(-) amlodipine - L (+)- hemitartarate]
InactiveUS20030176706A1Good yieldHigh enantiomeric purityOrganic active ingredientsOrganic chemistryTartrateDimethyl sulfoxide
The present invention relates to a process for the preparation of [S(-)amlodipine-L(+)-hemi taratarte] from RS amlodipine base using L(+) tartaric acid in the presence of dimethyl sulfoxide.
Owner:COUNCIL OF SCI & IND RES
Method for synthesizing chiraltetrahydro naphthalenederivate through asymmetric hydrogenation on isoquinoline by means of iridium catalyst
The invention discloses a method for synthesizing a chiraltetrahydro naphthalenederivate through asymmetric hydrogenation on isoquinoline by means of an iridium catalyst. A catalysis system applied in the method is a chiral bi-phosphine complex of metal iridium. The reaction is carried out under the conditions that the temperature is 25-60 DEG C; the volume ratio of tetrahydrofuran to dichloromethane in a solvent, namely a mixed solvent of tetrahydrofuran and dichloromethane is 1:1; the pressure is 13-50 Mpa; the ratio of a substrate to a catalyst is 50:1; the catalyst is a coordination compound of a (1,5-cyclooctadiene) iridium chloridedipolymer and a chiral bi-phosphine ligand. The corresponding chiral 1-position or 3-position substituted tetrahydro naphthalenederivate through hydrogenation on isoquinoline, and the enantiomeric excess of the derivate can reach 96%. The method is simple and practical in operation, raw materials are easy to obtain, the enantioselectivity is high, the yield is high, the reaction has atom economy, and the environment is friendly.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
NOVEL CHIRAL N-ACYL-5,6,7(8-SUBSTITUTED)-TETRAHYDRO-[1,2,4]TRIAZOLO[4,3-a]PYRAZINES AS SELECTIVE NK-3 RECEPTOR ANTAGONISTS, PHARMACEUTICAL COMPOSITION, METHODS FOR USE IN NK-3 RECEPTOR MEDIATED DISORDERS AND CHIRAL SYNTHESIS THEREOF
ActiveUS20140275097A1Less electron-withdrawingLess labileBiocideNervous disorderDiseaseTherapeutic treatment
Owner:OGEDA SA
Synthetic method of bortezomib
The invention discloses a synthetic method of bortezomib, which comprises the following steps of: taking isovaleraldehyde as an initial raw material, taking (R)-methylpropane-2-sulfinamide as a chiral reagent, generating (R,E)-2-methyl-N-(3-methyl butylidene) propane-2-sulfinamide by a condensation and dehydration reaction, then carrying out a nucleophilic addition reaction with pinacol diboron so as to generate (R)-1-N-methylpropane sulfinyl-3-methyl butane-1-pinacol borate ester, afterwards hydrolyzing under an acidic condition so as to obtain pinacol-(R)-1-amino-3-methyl butane-1-borate ester hydrochloride, then reacting with (S)-3-phenyl-2-(pyrazine-2-formamido) propionic acid under the existence of a coupling agent and also hydrolyzing under the action of isobutyl borate so as to generate a final product of the bortezomib. According to the synthetic method of the bortezomib, the (R)-methylpropane-2-sulfinamide which is easy to obtain is used as the chiral induction reagent, so that an obtained intermediate enantiomorph has higher purity, and a bulk drug which is finally obtained has better quality.
Owner:HEFEI UNIV OF TECH
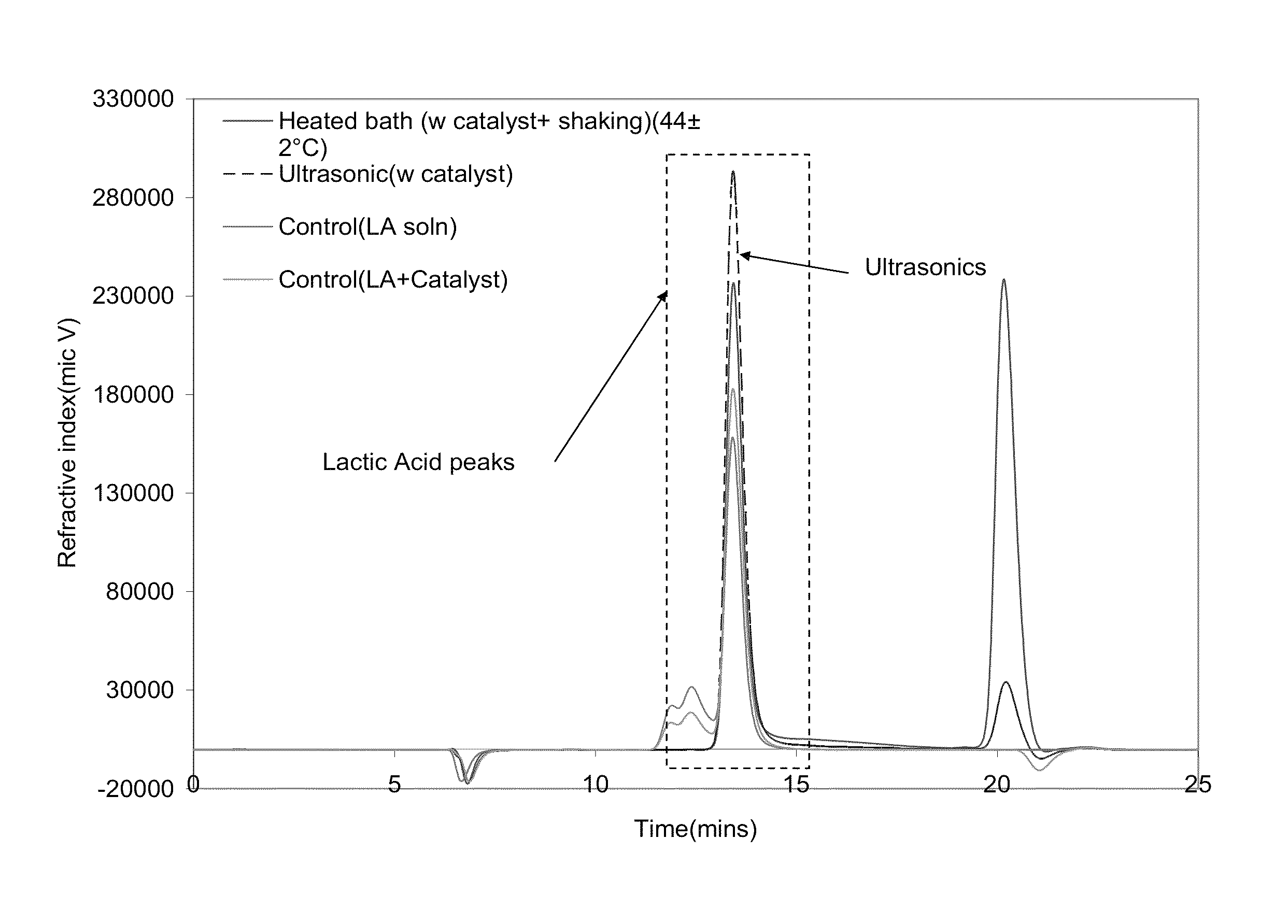
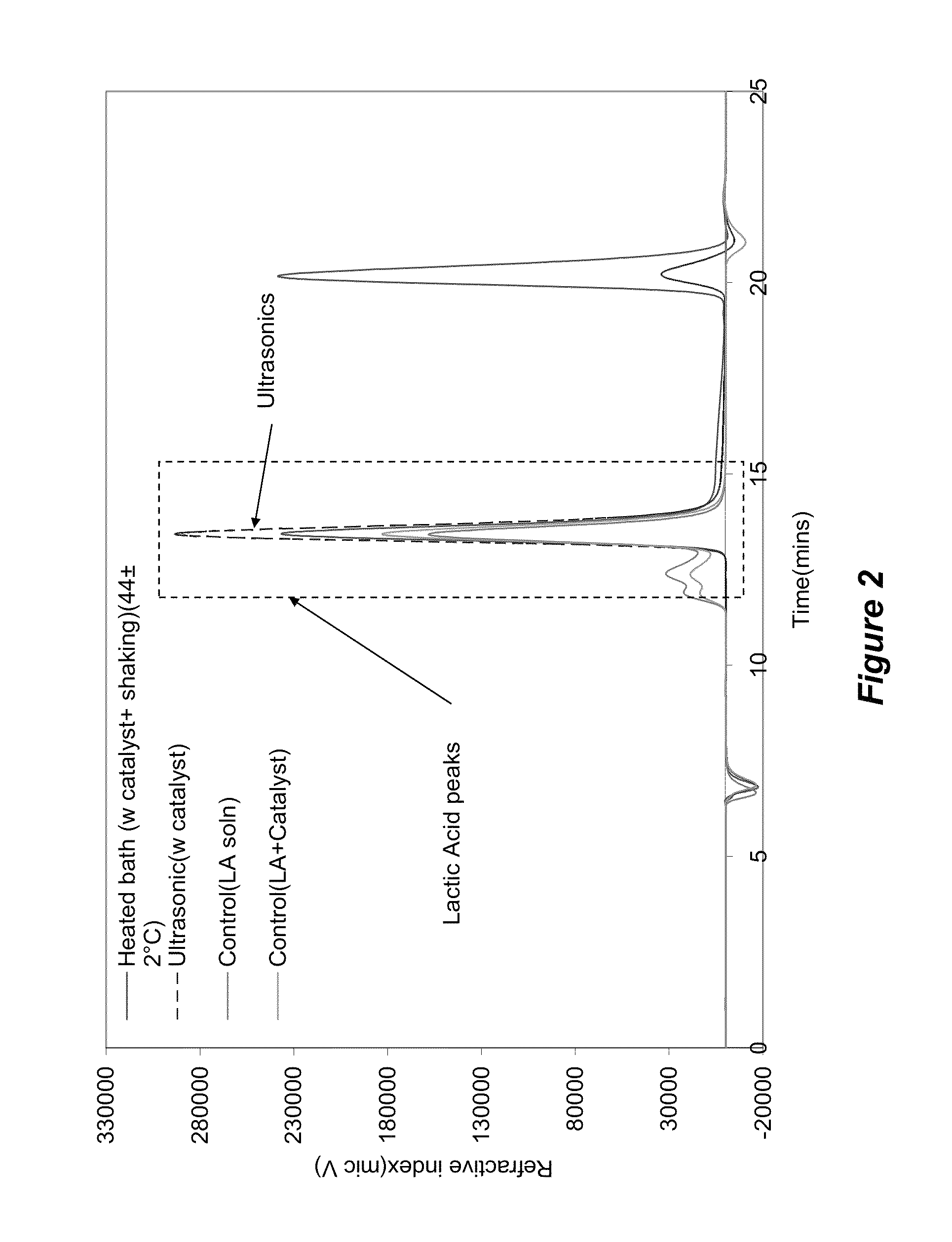
Depolymerization of polylactic acid
InactiveUS20130096342A1Lower energy requirementsReducing greenhouse gas emissionOrganic compound preparationPreparation from carboxylic acid esters/lactonesCyclic processPolyester
The invention provides energy efficient depolymerization of polyesters such as post-consumer polylactic acid. Ultrasonic induced implosions can be used to facilitate the depolymerization. The expanding market of polylactic acid-based plastic products, such as water bottles and packaging materials, has raised concerns of contaminating the recycling stream, which is largely filled with petroleum-based plastics. Thus the development of an energy efficient and economically viable PLA recycling process is urgently needed. Post consumer PLA was exposed to methanol as the suspension media in the presence of organic or ionic salts of alkali metals such a potassium carbonate and sodium hydroxide as depolymerization catalysts to provide high quality lactic acid monomers in high yield.
Owner:IOWA STATE UNIV RES FOUND
Process for the separation of racemic mixtures
InactiveUS6709597B1Increase load capacityHigh enantioselectivityIon-exchange process apparatusSilicaSilicon dioxideRacemic mixture
The present invention relates to a process for the separation of racemic mixtures comprising development of a denser molecular imprint on silica with a desired enantiomer by sol-gel protocol.
Owner:COUNCIL OF SCI & IND RES
Process For The Preparation Of 6,6-Dimethyl-3-Azabicyclo-[3.1.0]-Hexane Compounds ...
The present invention provides for a process for preparing racemic methyl 6,6-dimethyl-3-azabicyclo[3,1,0]hexane-2-carboxylate, its corresponding salt: (2S, 3R, 4S)-methyl 6,6-dimethyl-3-azabicyclo[3,1,0]hexane-2-carboxylate di-p-toluoyl-D-tartaric acid (“D-DTTA”) salt or a (2R, 3S, 4R)-methyl 6,6-dimethyl-3-azabicyclo[3,1,0]hexane-2-carboxylate di-p-toluoyl-L-tartaric acid salt (“L-DTTA”) in a high enantiomeric excess. This invention also provides for a process for preparing a (2S, 3R, 4S)-methyl 6,6-dimethyl-3-azabicyclo[3,1,0]hexane-2-carboxylate dibenzoyl-D-tartaric acid (“D-DBTA”) salt or a (2R, 3S, 4R)-methyl 6,6-dimethyl-3-azabicyclo[3,1,0]hexane-2-carboxylate L-tartaric acid (“L-DBTA”) salt in a high enantiomeric excess. Further, this invention provides a process for preparing intermediates II, IIB, III, IV, IV salt, V, VI, and VII.
Owner:MERCK SHARP & DOHME CORP
Resolution of racemates of methyl alpha-5-[4,5,6,7-tetrahydro[3,2-C]thienopyridyl]-(2-chlorophenyl) acetate
InactiveUS20050176960A1High enantiomeric purityExcellent characteristicsOrganic chemistryAntineoplastic agentsEnantiomerSolvent
A process for the resolution of each of the enantiomers of methyl-α-5-[4,5,6,7-tetrahydro[3,2-c]thienopyridyl]-(2-chlorophenyl)acetate and salts thereof by diastereomeric crystallization comprising the use of a single optically active resolving agent and at least one solvent.
Owner:APOTEX PHARMACHEN INC
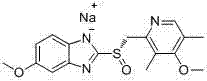
Method for purifying esomeprazole sodium
The invention relates to a method for purifying esomeprazole sodium, which comprises the following steps: after dissolving an esomeprazole sodium crude product in 0.5-10 times of an alcohol solvent, adding a poor solvent, filtering to obtain a solid, dissolving the solid in 0.5-10 times of acetone, crystallizing until the system becomes turbid, adding a poor solvent, filtering, and drying to finally obtain the sample. The method can remove abundant residual solvents which can not be easily removed in the prior art to obtain the high-purity low-solvent-residue high-yield product, and is suitable for industrial production.
Owner:四川尚锐生物医药有限公司
Methods and compositions for preparation of amphetamine conjugates and salts thereof
ActiveUS20120190880A1Reduce volatilityHigh enantiomeric purityCarbamic acid derivatives preparationOrganic compound preparationBenzphetamineMedicinal chemistry
The invention provides methods and compositions for preparing amphetamine conjugates, such as lisdexamfetamine, homoarginine-D-amphetamine, and salts thereof. In one embodiment, the invention provides methods of preparing an amphetamine conjugate from a chloramphetamine intermediate.
Owner:CAMBREX CHARLES CITY INC
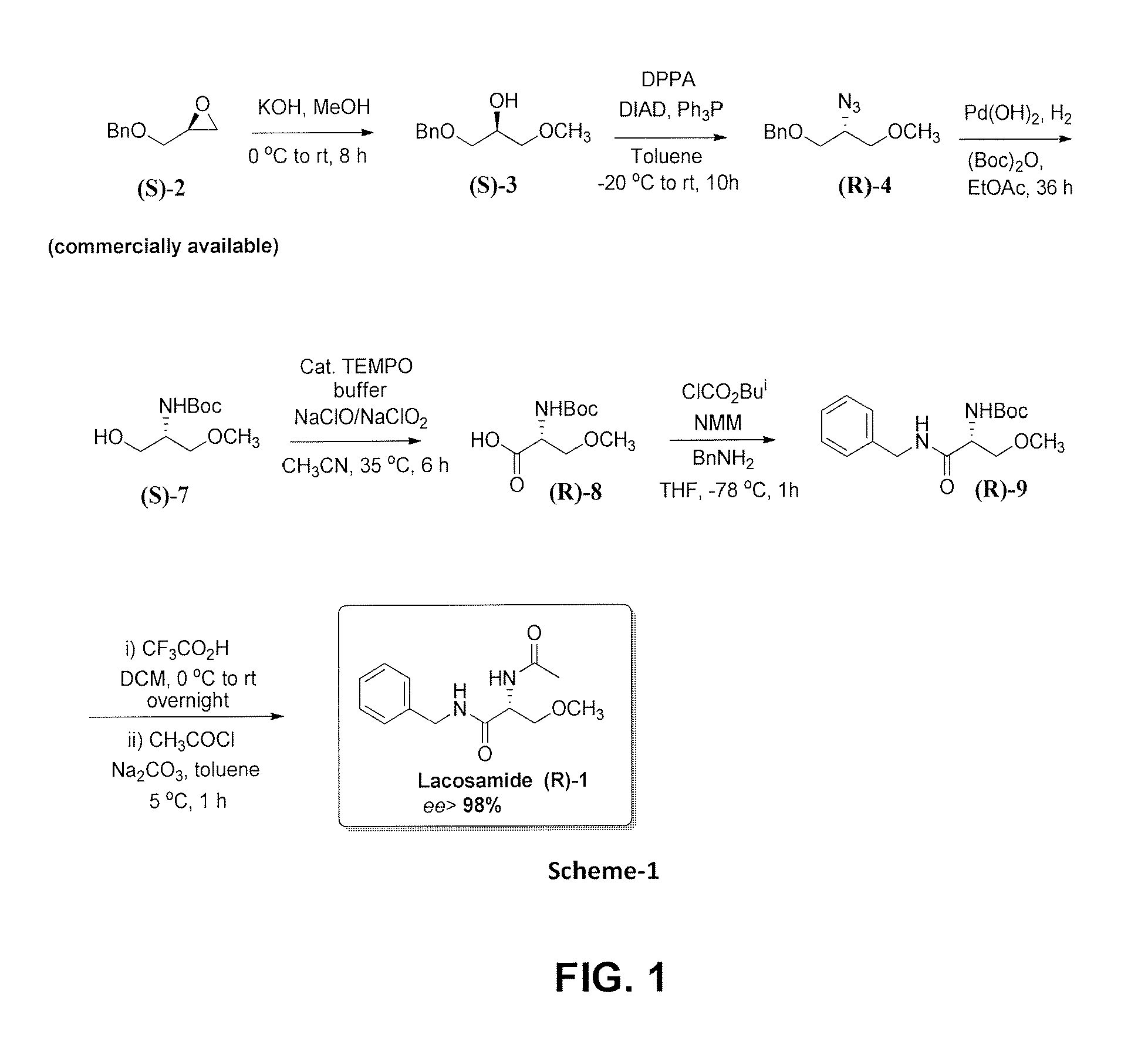
Process for the synthesis of antiepileptic drug lacosamide
ActiveUS20140012044A1Efficient and cost-effective and improvedHigh enantiopurityOrganic compound preparationCarboxylic acid amides optical isomer preparationAntiepileptic drugLacosamide
The present invention relates to the improved and efficient process for the synthesis of antiepileptic drug Lacosamide in high enantiopurity (>98% ee) and better yield. More particularly, the present invention relates to improved and efficient, cost effective process for synthesis of desired (R) isomer of Lacosamide starting from commercially available (S)-benzyl glycidyl ether.
Owner:COUNCIL OF SCI & IND SEARCH
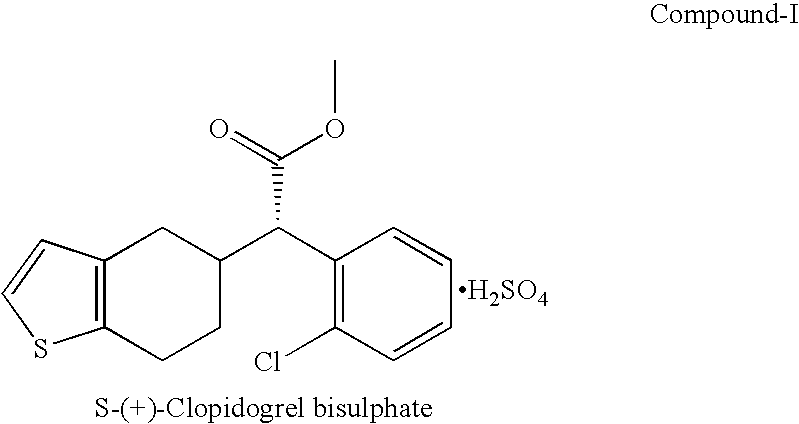
Process for preparation of clopidogrel bisulphate form-1
InactiveUS20070191609A1High isolation yieldHigh enantiomeric purityOrganic chemistryClopidogrel bisulphateSolvent
Disclosed herein is a cost effective and industrially feasible process for the preparation of (+) Clopidogrel bisulphate. The present invention further discloses a novel method of precipitation of (+) Clopidogrel bisulphate Form I directly from solvent mix of methanol and acetone in presence of sulfuric acid at a temperature of 25-40° C.
Owner:LEE PHARMA LTD
Chiral N-acyl-5,6,7(8-substituted)-tetrahydro-[1,2,4]triazolo[4,3-a]pyrazines as selective NK-3 receptor antagonists, pharmaceutical composition, methods for use in NK-3 receptor mediated disorders and chiral synthesis thereof
ActiveUS9475814B2Less electron-withdrawingLess labileNervous disorderOrganic chemistryDiseaseAcyl group
Owner:OGEDA SA
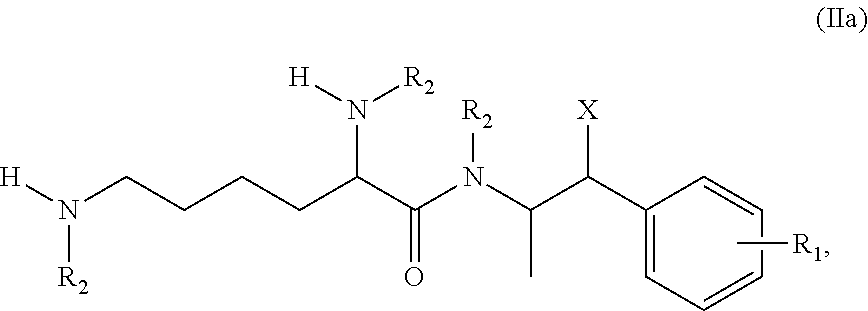
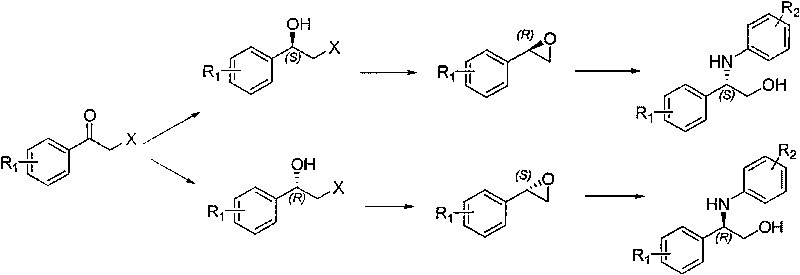
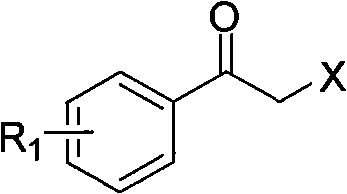
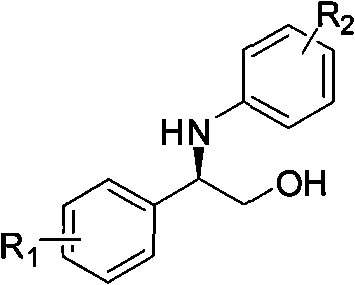
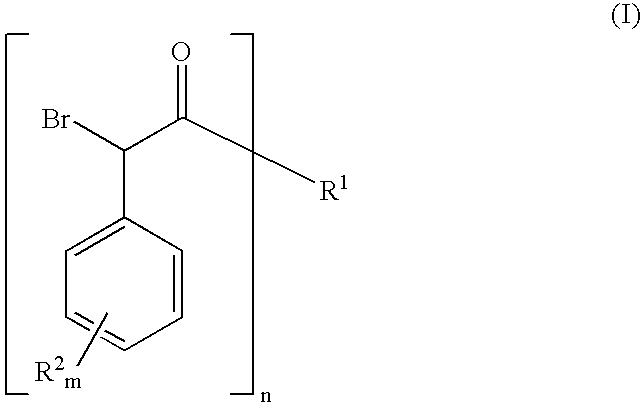
Synthesis method of derivative of chiral Beta-amino-alcohol and part of final products thereof
InactiveCN101747211AMeet the needs of large-scale productionNo racemization was foundPhysical/chemical process catalystsOrganic compound preparationAlcoholStereochemistry
The invention relates to a synthesis method of a derivative of chiral Beta-amino-alcohol and part of intermediate products and final products thereof. The method selects a raw material which is commercialized on a market or a raw material of Alpha-halogeno ketone with easy preparation as an initial raw material, wherein X is Br or Cl, the final product with the chemical formula as the accompanying drawing is obtained by reaction or wherein R1=H, 2-Cl, 3-Cl, 4-Cl, 2, 4-Cl, 4-Br, 4-F, 4-CF3 and 4-NO2; R2=H, 2-OMe, 3-OMe, 4-OMe, 4-F, 4-Cl, 4-Br, 3-Br, 3-F, 3-Cl, 2-Br, 2-Cl, 2F, 4-Me, 3-Me and 2-Me, and the chiral center is S or R; the intermediate product with the chemical formula is shown in the accompanying drawing and the chiral center is in S configuration or R configuration; and the final product with the chemical formula is shown in the accompanying drawing and the chiral center is in S configuration or R configuration.
Owner:ASYMCHEM LIFE SCI TIANJIN
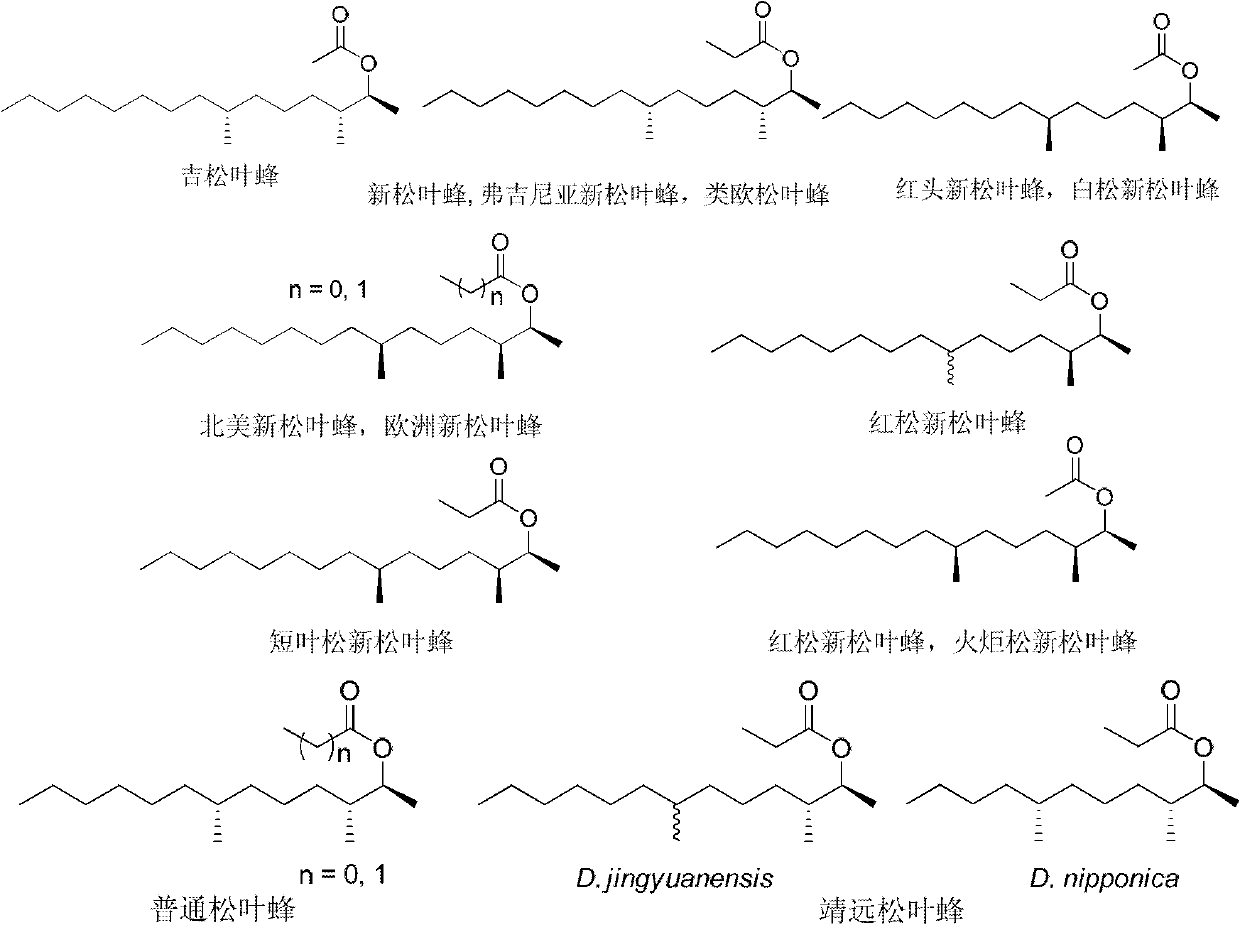
Method for synthesizing high antimer pure pine needle bee sex pharomone (2S, 3S, 7S)-3,7-dimethyl-2-pentadeca alcohol ester
InactiveCN1470490AHigh enantiomeric purityHigh step yieldOrganic compound preparationCarboxylic acid esters preparationAlcoholButanediol
The method for synthesizing high-antimer pure pine sawfly sex pheromone (2S, 3S, 7S)-3,7-dimethyl-2- pentadecyl alcohol ester is characterized by that it uses cheap easily-evailable natural (S)-malicacid with high-antimer purity as raw material, and can high-stereoselectively synthesize a series of important intermediate compounds of (S)-1,3- butanediol 4, (2S, 3S-2-methyl-1,3-butanediol 12, (S)-3-methyl-hendecyl alcohol 8 and (2S,3S,7S)-3,7- dimethyl-2-pentadecyl alcohol 15 of the pine sawfly sex pheromone, then makes the (2S,3S,7S)-3,7- dimethyl-2-pentadecyl alcohol 15 undergo the process of acrylation reaction to obtain the pine sawfly sex pheromone (2S,3S,7S)-3,7-dimethyl-2-pentadecyl alcohol carboxylic ester 16 and 17 with high antimer purity.
Owner:XIAMEN UNIV
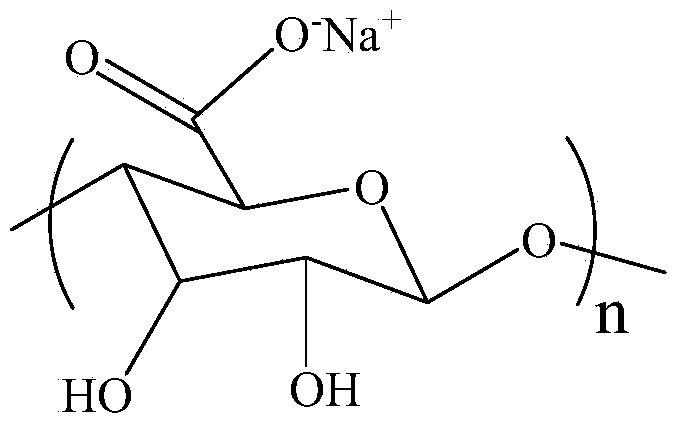
Sodium alginate chiral cross-linked membrane and applications thereof
InactiveCN103736402AHigh enantiomeric purityLow costDialysisCross linked membraneP-hydroxyphenylglycine
The invention discloses a sodium alginate chiral cross-linked membrane and applications thereof. The preparation method of the sodium alginate chiral cross-linked membrane comprises the following steps: weighing a certain amount of sodium alginate, dissolving the sodium alginate into a water solution of acetic acid, carrying out a supersonic treatment to remove the aggregated particle molecules, allowing the system to stand still so as to remove the bubbles; pouring the membrane-forming solution on a clean and smooth glass board at a room temperature, scrapping off the membrane from the glass board with a membrane-scrapping knife, drying for 4 days; putting the dry membrane into an acetone cross-linking agent containing glutaraldehyde and hydrochloric acid to carry out cross-linking reactions; and washing the cross-linked membrane with a large amount of acetone and ultrapure water until the membrane turns neutral so as to obtain the needed chiral cross-linked membrane. The cross-linked membrane is loaded into a normal dialysis device, and then separates D,L-p-hydroxyphenylglycine raceme aqueous solution under the driving of concentration difference, and the enantiomer purity of p-hydroxyphenylglycine in the permeate reaches 60% or more. The raw materials of the membrane are cheap, the purity of the obtained mono-enantiomer is high, and the preparation method of the membrane has the advantages of large throughput, environment-friendliness, low cost, and easiness in continuous operation and massive industrial production.
Owner:YUNNAN NORMAL UNIV
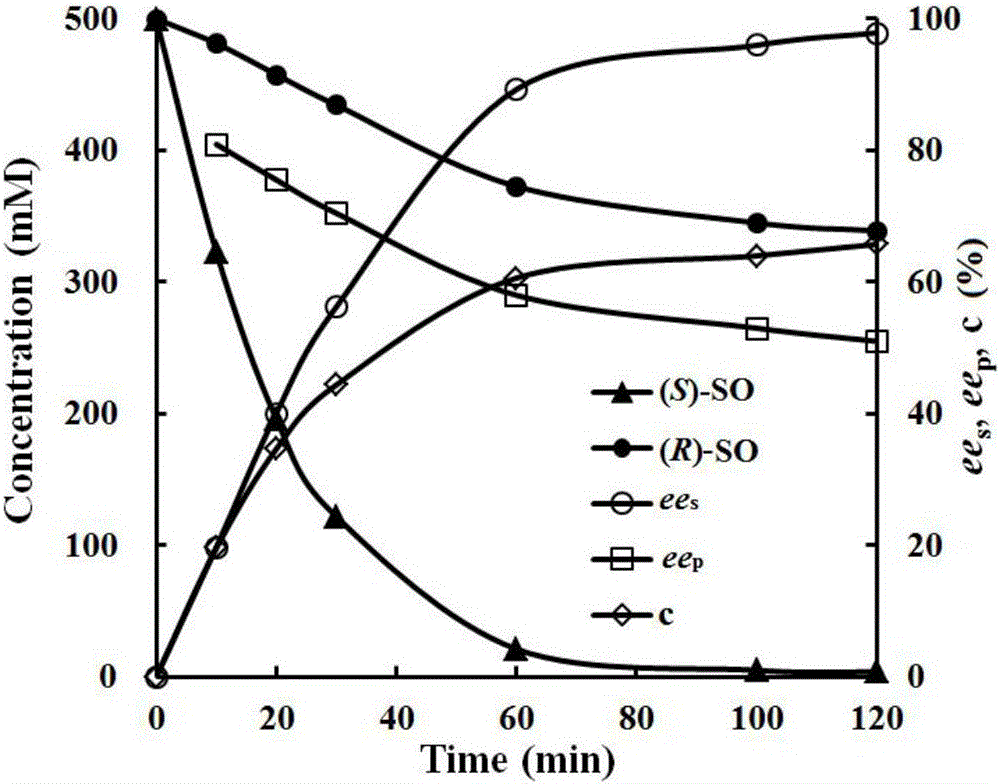
Method for preparing (S)-styrene oxide through enzyme method
ActiveCN105969837AIncrease concentrationHigh enantiomeric purityFermentationStyrene oxideEpoxide metabolism
The invention discloses a method for preparing (S)-styrene oxide through an enzyme method, which belongs to the technical field of biological catalysis. According to the method provided by the invention, in a two-phase system of hexyl alcohol / buffer solution, epoxide hydrolases from aspergillus usamii is utilized to catalyze hydrolytic kinetic resolution of racemization styrene oxide to prepare the (S)-styrene oxide. Compared with a single-phase reaction system, the concentration of the kinetic resolution rac-SO is improved to 120g / L from 24g / L; the space time yield is improved to 20.3g / L / h from 3.1g / L / h; an ee value for preparing the (S)-SO by kinetic resolution 120g / L rac-SO is improved to 98.3 percent from 36.8 percent, and the gram-scale preparation of the (S)-SO is realized. The method provided by the invention is simple in process, a product is high in enantiomer purity and yield, high in catalytic efficiency and environmental friendly, and the method has wider industrial application prospect.
Owner:南京科默生物医药有限公司
Depolymerization of polylactic acid
InactiveUS8895778B2Shorten the timeImprove economyOrganic compound preparationPreparation from carboxylic acid esters/lactonesCyclic processPolyester
The invention provides energy efficient depolymerization of polyesters such as post-consumer polylactic acid. Ultrasonic induced implosions can be used to facilitate the depolymerization. The expanding market of polylactic acid-based plastic products, such as water bottles and packaging materials, has raised concerns of contaminating the recycling stream, which is largely filled with petroleum-based plastics. Thus the development of an energy efficient and economically viable PLA recycling process is urgently needed. Post consumer PLA was exposed to methanol as the suspension media in the presence of organic or ionic salts of alkali metals such a potassium carbonate and sodium hydroxide as depolymerization catalysts to provide high quality lactic acid monomers in high yield.
Owner:IOWA STATE UNIV RES FOUND
Process for the synthesis of antiepileptic drug lacosamide
ActiveUS8748660B2Efficient and cost-effective and improvedHigh enantiomeric purityOrganic compound preparationCarboxylic acid amides optical isomer preparationAntiepileptic drugMedicinal chemistry
The present invention relates to the improved and efficient process for the synthesis of antiepileptic drug Lacosamide in high enantiopurity (>98% ee) and better yield. More particularly, the present invention relates to improved and efficient, cost effective process for synthesis of desired (R) isomer of Lacosamide starting from commercially available (S)-benzyl glycidyl ether.
Owner:COUNCIL OF SCI & IND SEARCH
Methods and compositions for preparation of amphetamine conjugates and salts thereof
ActiveUS8614346B2Reduce volatilityHigh enantiomeric purityCarbamic acid derivatives preparationOrganic compound preparationBenzphetamineAmphetamine use
The invention provides methods and compositions for preparing amphetamine conjugates, such as lisdexamfetamine, homoarginine-D-amphetamine, and salts thereof. In one embodiment, the invention provides methods of preparing an amphetamine conjugate from a chloramphetamine intermediate.
Owner:CAMBREX CHARLES CITY INC
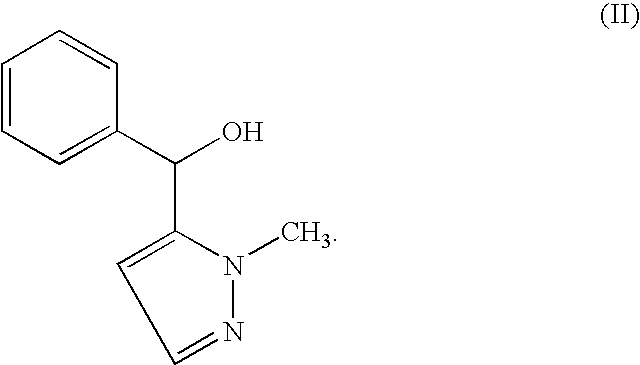
Process for obtaining enantiomers of thienylazolylalcoxyethanamines
InactiveUS7078531B2Speed up the conversion processAvoid problemsOrganic chemistryAlcoholEnantiomer
A process is described for the preparation of a precursor alcohol of (±)-2-[thienyl(1-methyl-1H-pyrazol-5-yl)methoxy]-N,N-dimethyletanamine and in general for thienylazolylalcoxyethanamines and their enantiomers. The process involves asymmetric addition of a metalated thienyl reagent to a pyrazolcarbaldehyde in the presence of a chiral ligand to yield chiral alcohols. The chiral alcohols are further O-alkylated to yield the corresponding pharmaceutically active thienylazolylalcoxyethanamines.
Owner:LAB DEL DR ESTEVE SA
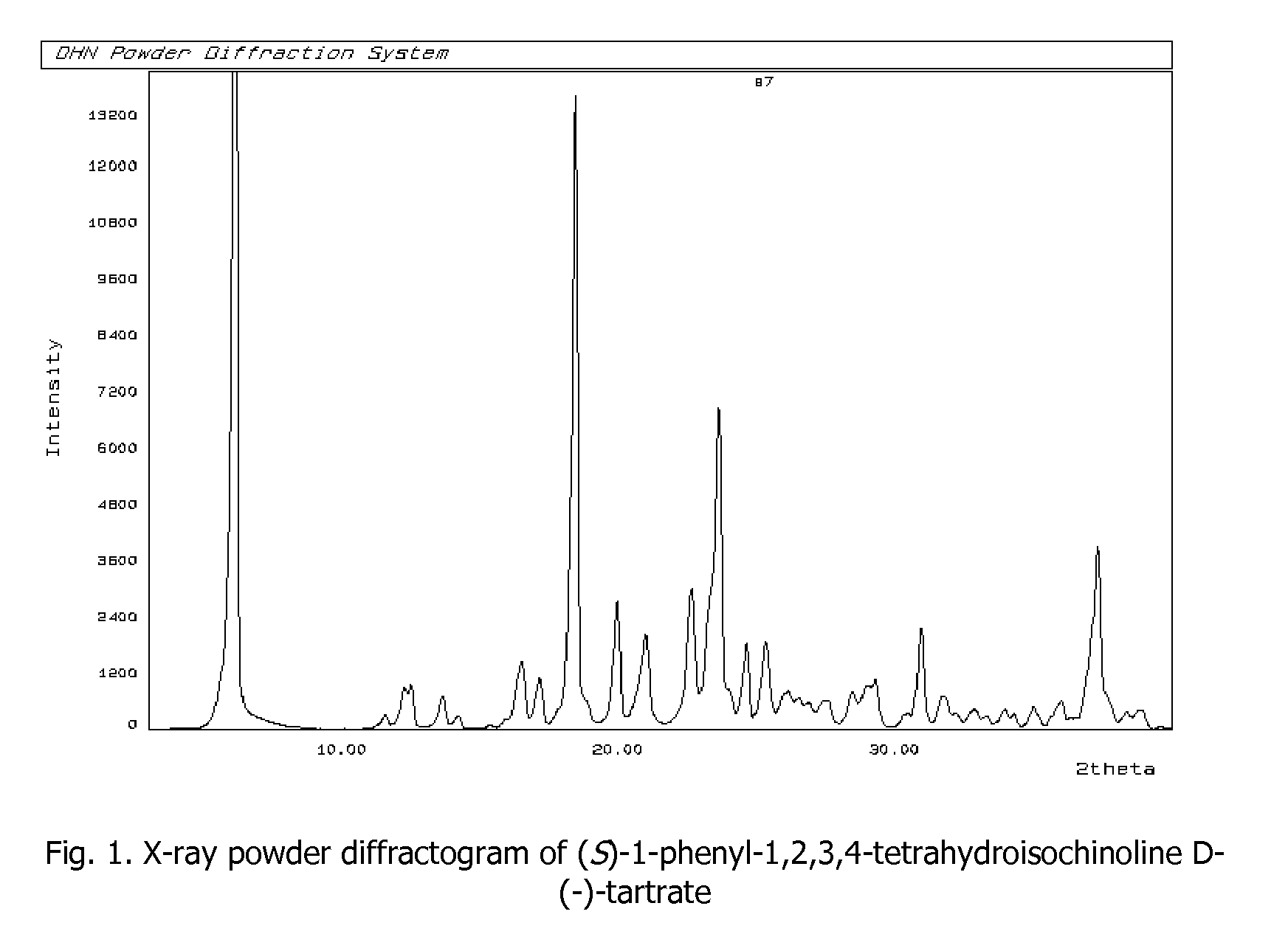
Process for preparation of enantiomerically pure (s)-1-phenyi-1,2,3,4- tetrahydroisoquinoline
InactiveUS20110077405A1High selectivity and yieldHigh selectivityOrganic chemistryPhenyl groupSolifenacin
Owner:ZAKLADY FARMACEUTYCZNE POLPHARMA SA
Process for obtaining Cizolirtine and its enantiomers
InactiveUS7109349B2Speed up the conversion processAvoid problemsOrganic chemistryAlcoholMethyl group
A process is described for the preparation of a precursor alcohol of Cizolirtine, (±)-2-[phenyl(1-methyl-1H-pyrazol-5-yl)methoxy]-N,N-dimethylethanamine and its enantiomers. The process involves the asymmetric addition of a metalated phenyl reagent to a pyrazolcarbaldehyde in the presence of a chiral ligand to yield chiral alcohols. The chiral alcohols are further O-alkylated to yield Cizolirtine or its enantiomers.
Owner:LAB DEL DR ESTEVE SA
Process
ActiveUS20200157589A1High chemical purityHigh enantiomeric purityOxidoreductasesFermentationCombinatorial chemistryPyrrolidine
Owner:ZANOPRIMA LIFESCI LTD
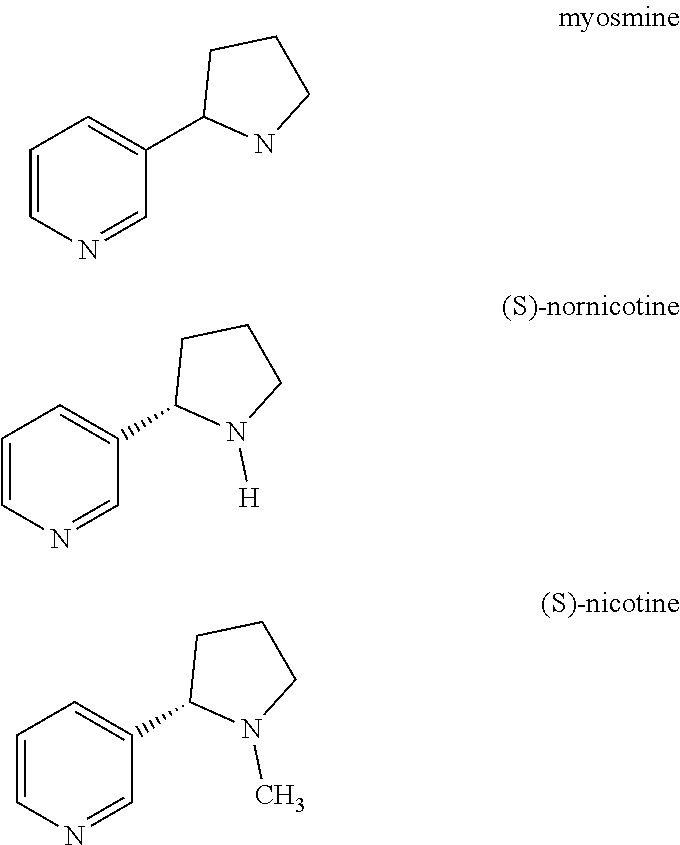
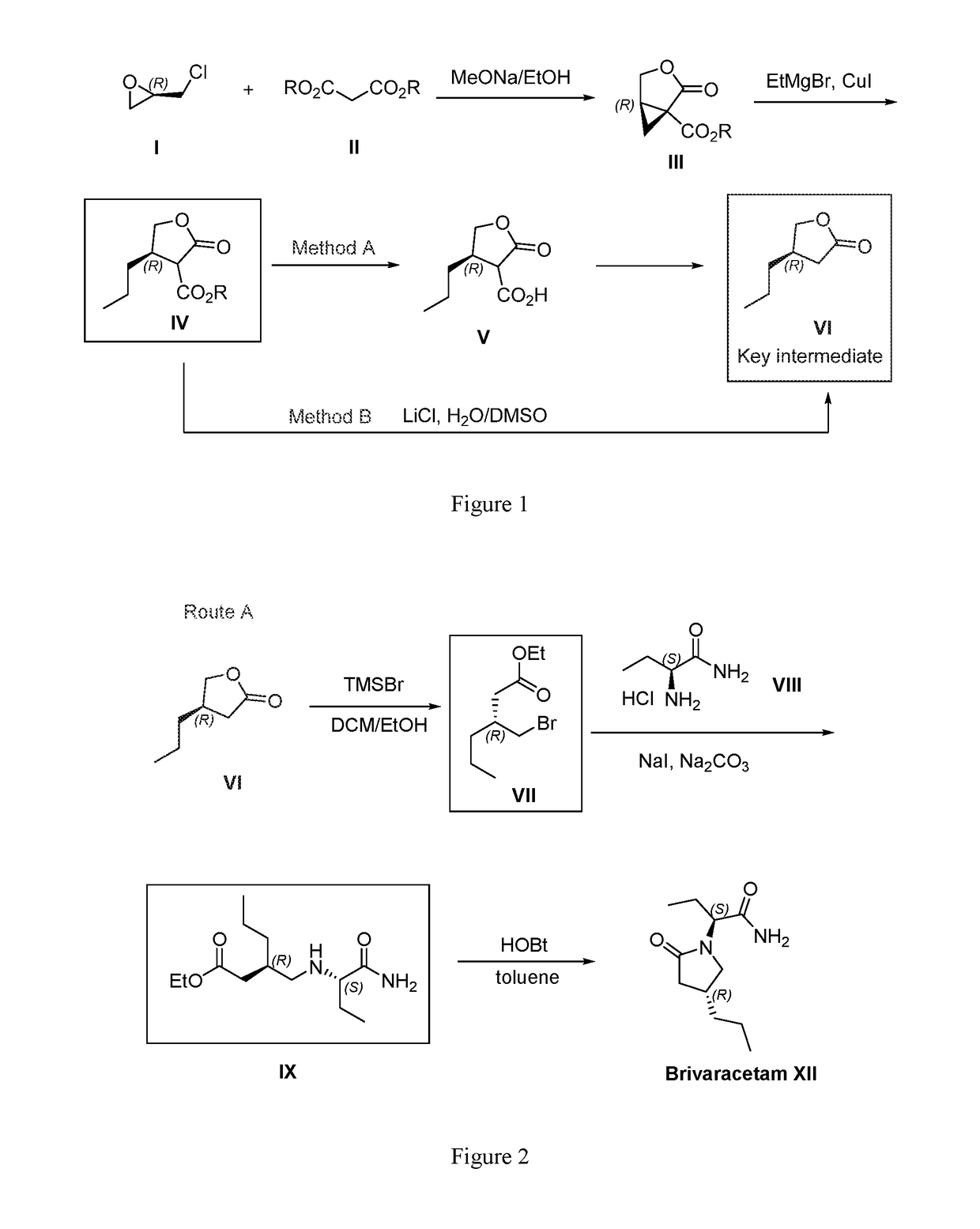
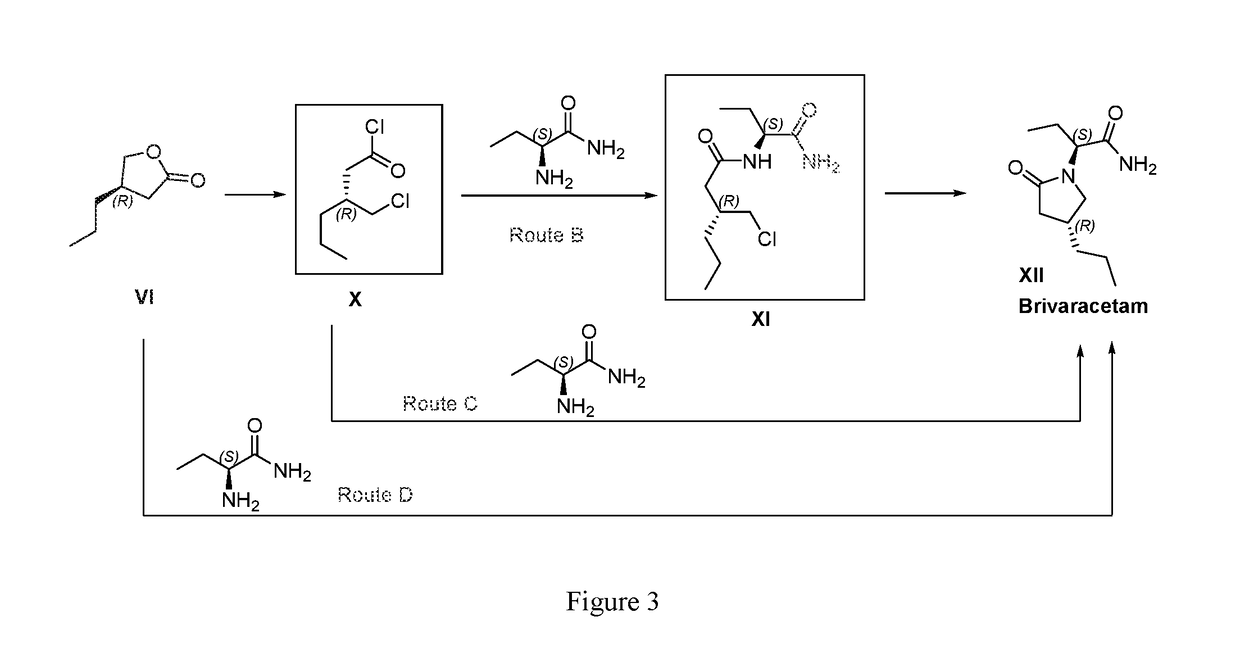
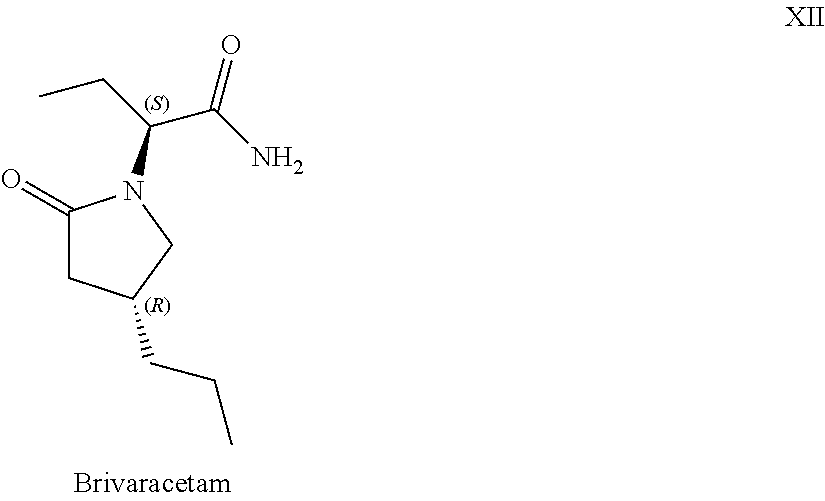
Processes to produce brivaracetam
ActiveUS10221134B2Cost effectiveHigh enantiomeric purityOrganic active ingredientsNervous disorderBrivaracetamRelated derivatives
The present invention provides a scalable synthesis of enantiomerically pure brivaracetam, and related derivatives.
Owner:SUZHOU PENGXU PHARM TECH CO LTD
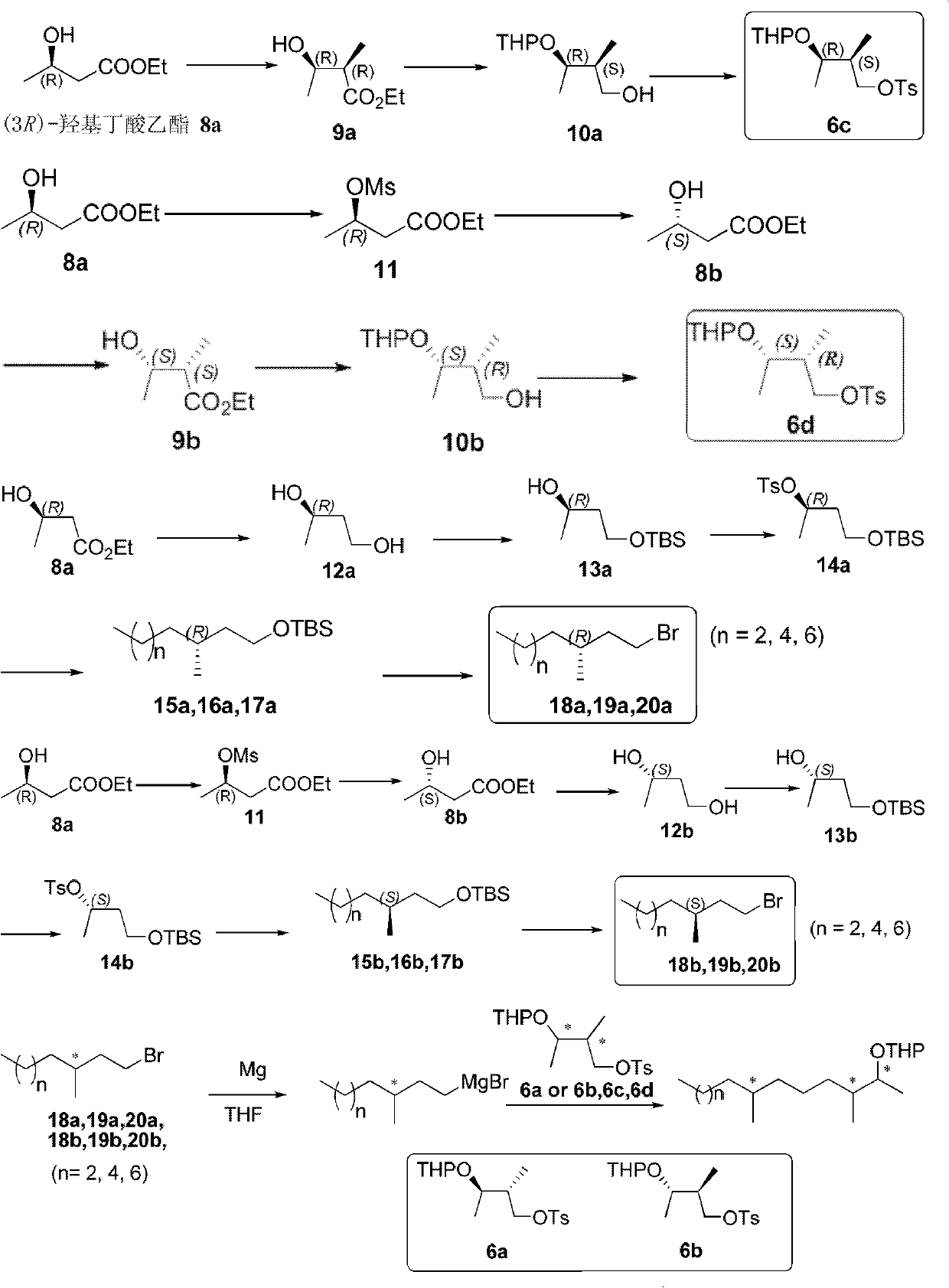
Method for synthesizing high-enantiomer-purity diprionidae pheromone and stereo isomer
InactiveCN102167666AHigh enantiomeric purityGood application prospectOrganic compound preparationCarboxylic acid esters preparationTridecyl alcoholOptically active
The invention discloses a method for synthesizing a high-enantiomer-purity diprionidae pheromone and a stereo isomer, relates to a diprionidae pheromone and provides a novel method for synthesizing an optically-active high-enantiomer-purity diprionidae pheromone and a stereo isomer. The method comprises the following steps of: selectively synthesizing the high-enantiomer-purity diprionidae pheromone, a homologous compound thereof and the stereo isomer by taking malic acid or (3R)-hydroxy ethyl butyrate as a raw material; and synthesizing a series of important intermediate compounds, including(R)-1,3-butanediol 12a, (S)-1,3-butanediol 12b, (R)-3-methyl-1-bromotridecane 20a (S)-3-methyl-1-bromotridecane 20b as well as chiral 3,7-dimethyl-2-pentadecyl alcohol 21-28, chiral 3,7-dimethyl-2-tridecyl alcohol 29-36 and chiral 3,7-dimethyl-2-undecyl alcohol 37-44. The diprionidae pheromone is obtained by acylating chiral alcohol 21-44.
Owner:XIAMEN UNIV
Process for the stereoselective preparation of (-)-halofenate and derivatives thereof
InactiveUS20070072858A1High yieldHigh enantiomeric purityBiocideOrganic chemistryCarboxyl radicalPhenylacetic acid
The present invention provides a compounds the formula (IV): and methods for producing an α-(phenoxy)phenylacetic acid compound of the formula: wherein R1 is a member selected from the group consisting of: each R2 is a member independently selected from the group consisting of (C1-C4)alkyl, halo, (C1-C4)haloalkyl, amino, (C1-C4)aminoalkyl, amido, (C1-C4)amidoalkyl, (C1-C4)sulfonylalkyl, (C1-C4)sulfamylalkyl, (C1-C4)alkoxy, (C1-C4)heteroalkyl, carboxy and nitro; the subscript n is 1 when R1 has the formula (a) or (b) and 2 when R1 has the formula (c) or (d); the subscript m is an integer of from 0 to 3; * indicates a carbon which is enriched in one stereoisomeric configuration; and the wavy line indicates the point of attachment of R1; and compounds
Owner:DIATEX INC (US)
Processes and Intermediates for Preparing a Macrocyclic Protease Inhibitor of HCV
InactiveUS20130005976A1High purityHigh enantiomeric purityPreparation from carboxylic acid saltsPreparation by ester-hydroxy reactionEphedrineOrganic chemistry
A process for preparing [(1R,2R)-4-oxo-1,2-cyclopentanedicarboxylic acid II, by the resolution of racemic 4-oxo-1,2-cyclopentanedicarboxylic acid (V), said process comprising:(a) reacting 4-oxo-1,2-cyclopentanedicarboxylic acid (V) with brucine or (1R,2S)-(−)-ephedrine, thus preparing the bis-brucine or bis-(1R,2S)-(−)-ephedrine salt of (V), and(b) precipitating selectively the bis-brucine or bis-(1R,2S)-(−)-ephedrine salt of (1R,2R)-4-oxo-1,2-cyclopentanedicarboxylic acid II, while the bis-brucine or bis-(1R,2S)-(−)-ephedrine salt of [(1S,2S)-4-oxo-1,2-cyclopentanedicarboxylic acid stays in solution;(c) liberating the acid II by removal of brucine or (1R,2S)-(−)-ephedrine from the precipitated salt obtained in step (b).
Owner:JANSSEN PHARMA INC
Process for preparing (3RS)-3-[(2SR)-(2-cyclopentyl-2-hydroxy-2-phenylacetyl)oxy]-1,1-dimethylp- yrrolidinium bromide
ActiveUS20170334848A1High yieldHigh purityOrganic chemistryRespiratory disorderIndustrial scaleBromide
The present invention relates to an efficient and environmentally friendly process for preparing (3RS)-3-[(2SR)-(2-cyclopentyl-2-hydroxy-2-phenylacetyl)oxy]-1,1-dimethylp- yrrolidinium bromide with high yield and purity suitable for industrial scale applications.
Owner:INKE SA (ES)
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

![Process for the preparation of [S(-) amlodipine - L (+)- hemitartarate] Process for the preparation of [S(-) amlodipine - L (+)- hemitartarate]](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/1c22eec7-1c05-499a-bb41-2f55da14435d/US20030176706A1-20030918-C00001.png)
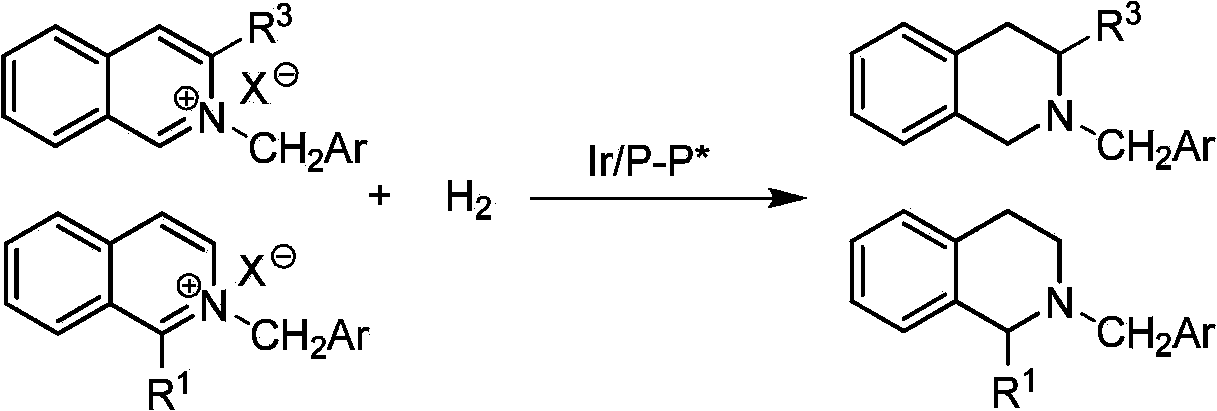
![NOVEL CHIRAL N-ACYL-5,6,7(8-SUBSTITUTED)-TETRAHYDRO-[1,2,4]TRIAZOLO[4,3-a]PYRAZINES AS SELECTIVE NK-3 RECEPTOR ANTAGONISTS, PHARMACEUTICAL COMPOSITION, METHODS FOR USE IN NK-3 RECEPTOR MEDIATED DISORDERS AND CHIRAL SYNTHESIS THEREOF NOVEL CHIRAL N-ACYL-5,6,7(8-SUBSTITUTED)-TETRAHYDRO-[1,2,4]TRIAZOLO[4,3-a]PYRAZINES AS SELECTIVE NK-3 RECEPTOR ANTAGONISTS, PHARMACEUTICAL COMPOSITION, METHODS FOR USE IN NK-3 RECEPTOR MEDIATED DISORDERS AND CHIRAL SYNTHESIS THEREOF](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/00896435-2a02-4e06-adde-bcc09c956cb6/US20140275097A1-20140918-C00001.png)
![NOVEL CHIRAL N-ACYL-5,6,7(8-SUBSTITUTED)-TETRAHYDRO-[1,2,4]TRIAZOLO[4,3-a]PYRAZINES AS SELECTIVE NK-3 RECEPTOR ANTAGONISTS, PHARMACEUTICAL COMPOSITION, METHODS FOR USE IN NK-3 RECEPTOR MEDIATED DISORDERS AND CHIRAL SYNTHESIS THEREOF NOVEL CHIRAL N-ACYL-5,6,7(8-SUBSTITUTED)-TETRAHYDRO-[1,2,4]TRIAZOLO[4,3-a]PYRAZINES AS SELECTIVE NK-3 RECEPTOR ANTAGONISTS, PHARMACEUTICAL COMPOSITION, METHODS FOR USE IN NK-3 RECEPTOR MEDIATED DISORDERS AND CHIRAL SYNTHESIS THEREOF](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/00896435-2a02-4e06-adde-bcc09c956cb6/US20140275097A1-20140918-C00002.png)
![NOVEL CHIRAL N-ACYL-5,6,7(8-SUBSTITUTED)-TETRAHYDRO-[1,2,4]TRIAZOLO[4,3-a]PYRAZINES AS SELECTIVE NK-3 RECEPTOR ANTAGONISTS, PHARMACEUTICAL COMPOSITION, METHODS FOR USE IN NK-3 RECEPTOR MEDIATED DISORDERS AND CHIRAL SYNTHESIS THEREOF NOVEL CHIRAL N-ACYL-5,6,7(8-SUBSTITUTED)-TETRAHYDRO-[1,2,4]TRIAZOLO[4,3-a]PYRAZINES AS SELECTIVE NK-3 RECEPTOR ANTAGONISTS, PHARMACEUTICAL COMPOSITION, METHODS FOR USE IN NK-3 RECEPTOR MEDIATED DISORDERS AND CHIRAL SYNTHESIS THEREOF](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/00896435-2a02-4e06-adde-bcc09c956cb6/US20140275097A1-20140918-C00003.png)




![Process For The Preparation Of 6,6-Dimethyl-3-Azabicyclo-[3.1.0]-Hexane Compounds ... Process For The Preparation Of 6,6-Dimethyl-3-Azabicyclo-[3.1.0]-Hexane Compounds ...](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/7e6ad494-c2a9-401b-b49b-4c10730f2e3f/US20090240063A1-20090924-C00001.png)
![Process For The Preparation Of 6,6-Dimethyl-3-Azabicyclo-[3.1.0]-Hexane Compounds ... Process For The Preparation Of 6,6-Dimethyl-3-Azabicyclo-[3.1.0]-Hexane Compounds ...](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/7e6ad494-c2a9-401b-b49b-4c10730f2e3f/US20090240063A1-20090924-C00002.png)
![Process For The Preparation Of 6,6-Dimethyl-3-Azabicyclo-[3.1.0]-Hexane Compounds ... Process For The Preparation Of 6,6-Dimethyl-3-Azabicyclo-[3.1.0]-Hexane Compounds ...](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/7e6ad494-c2a9-401b-b49b-4c10730f2e3f/US20090240063A1-20090924-C00003.png)







![Chiral <i>N</i>-acyl-5,6,7(8-substituted)-tetrahydro-[1,2,4]triazolo[4,3-a]pyrazines as selective NK-3 receptor antagonists, pharmaceutical composition, methods for use in NK-3 receptor mediated disorders and chiral synthesis thereof Chiral <i>N</i>-acyl-5,6,7(8-substituted)-tetrahydro-[1,2,4]triazolo[4,3-a]pyrazines as selective NK-3 receptor antagonists, pharmaceutical composition, methods for use in NK-3 receptor mediated disorders and chiral synthesis thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/35998279-441b-4541-8162-6ab3042b4ab0/US09475814-20161025-D00001.PNG)
![Chiral <i>N</i>-acyl-5,6,7(8-substituted)-tetrahydro-[1,2,4]triazolo[4,3-a]pyrazines as selective NK-3 receptor antagonists, pharmaceutical composition, methods for use in NK-3 receptor mediated disorders and chiral synthesis thereof Chiral <i>N</i>-acyl-5,6,7(8-substituted)-tetrahydro-[1,2,4]triazolo[4,3-a]pyrazines as selective NK-3 receptor antagonists, pharmaceutical composition, methods for use in NK-3 receptor mediated disorders and chiral synthesis thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/35998279-441b-4541-8162-6ab3042b4ab0/US09475814-20161025-D00002.PNG)
![Chiral <i>N</i>-acyl-5,6,7(8-substituted)-tetrahydro-[1,2,4]triazolo[4,3-a]pyrazines as selective NK-3 receptor antagonists, pharmaceutical composition, methods for use in NK-3 receptor mediated disorders and chiral synthesis thereof Chiral <i>N</i>-acyl-5,6,7(8-substituted)-tetrahydro-[1,2,4]triazolo[4,3-a]pyrazines as selective NK-3 receptor antagonists, pharmaceutical composition, methods for use in NK-3 receptor mediated disorders and chiral synthesis thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/35998279-441b-4541-8162-6ab3042b4ab0/US09475814-20161025-D00003.PNG)














![Process for preparing (3RS)-3-[(2SR)-(2-cyclopentyl-2-hydroxy-2-phenylacetyl)oxy]-1,1-dimethylp- yrrolidinium bromide Process for preparing (3RS)-3-[(2SR)-(2-cyclopentyl-2-hydroxy-2-phenylacetyl)oxy]-1,1-dimethylp- yrrolidinium bromide](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/68231bcf-534f-4f99-80d8-087c1c986fad/US20170334848A1-20171123-C00001.png)
![Process for preparing (3RS)-3-[(2SR)-(2-cyclopentyl-2-hydroxy-2-phenylacetyl)oxy]-1,1-dimethylp- yrrolidinium bromide Process for preparing (3RS)-3-[(2SR)-(2-cyclopentyl-2-hydroxy-2-phenylacetyl)oxy]-1,1-dimethylp- yrrolidinium bromide](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/68231bcf-534f-4f99-80d8-087c1c986fad/US20170334848A1-20171123-C00002.png)
![Process for preparing (3RS)-3-[(2SR)-(2-cyclopentyl-2-hydroxy-2-phenylacetyl)oxy]-1,1-dimethylp- yrrolidinium bromide Process for preparing (3RS)-3-[(2SR)-(2-cyclopentyl-2-hydroxy-2-phenylacetyl)oxy]-1,1-dimethylp- yrrolidinium bromide](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/68231bcf-534f-4f99-80d8-087c1c986fad/US20170334848A1-20171123-C00003.png)