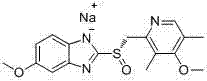
Method for purifying esomeprazole sodium
A technology of esomeprazole sodium and a purification method, which is applied in the field of purification of esomeprazole sodium, can solve the problems of not specifying too much the specific content of residual solvents, difficulty in removing residual solvents, and no mention of residual solvents, etc. Achieve the effect of low cost, high yield and high enantiomeric purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
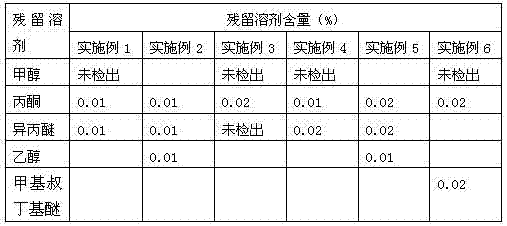
[0030] Weigh 3.0 kg of crude esomeprazole sodium, add 9.0 L of methanol, heat to dissolve at 65±5°C, add 0.1 kg of activated carbon for decolorization for 10 min, heat filter, add 0.03 kg of esomeprazole sodium seed crystals, wait After the system is turbid, add 9.0L of isopropyl ether, cool down to 0±5°C, continue to stir for 1h, filter, add 2.5L of acetone to the filter cake, stir and dissolve at room temperature, and when the system is cloudy, add 2.5L of isopropyl ether, filter, and filter the cake Vacuum-dried to obtain 2.5 kg of esomeprazole sodium refined product. After HPLC detection, the content is 99.5%, the largest single impurity is 0.03%, the total impurity is 0.06%, and the enantiomeric purity is 99.9%.
Embodiment 2
[0032] Weigh 3.0 kg of crude esomeprazole sodium, add 12.0 L of ethanol, heat to dissolve at 65±5°C, add 0.1 kg of activated carbon for decolorization for 10 min, heat filter, add 0.03 kg of esomeprazole sodium seed crystals, wait After the system is turbid, add 9.0L of isopropyl ether, cool down to 0±5°C, continue to stir for 1h, filter, add 2.5L of acetone to the filter cake, stir and dissolve at room temperature, and when the system is cloudy, add 2.5L of isopropyl ether, filter, and filter the cake Vacuum-dried to obtain 2.3 kg of esomeprazole sodium refined product. After HPLC detection, the content is 99.7%, the largest single impurity is 0.02%, the total impurity is 0.07%, and the enantiomeric purity is 99.8%.
Embodiment 3
[0034]Weigh 3.0 kg of crude esomeprazole sodium, add 9.0 L of methanol, heat to dissolve at 65±5°C, add 0.1 kg of activated carbon for decolorization for 10 min, heat filter, add 0.03 kg of esomeprazole sodium seed crystals, wait After the system is turbid, add 9.0L of isopropyl ether, cool down to 0±5°C, continue to stir for 1h, filter, add 1.5L of acetone to the filter cake, stir and dissolve at room temperature, and when the system is cloudy, add 3.0L of isopropyl ether, filter, and filter the cake Vacuum-dried to obtain 2.5 kg of esomeprazole sodium refined product. After HPLC detection, the content is 99.2%, the largest single impurity is 0.04%, the total impurity is 0.09%, and the enantiomeric purity is 99.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com