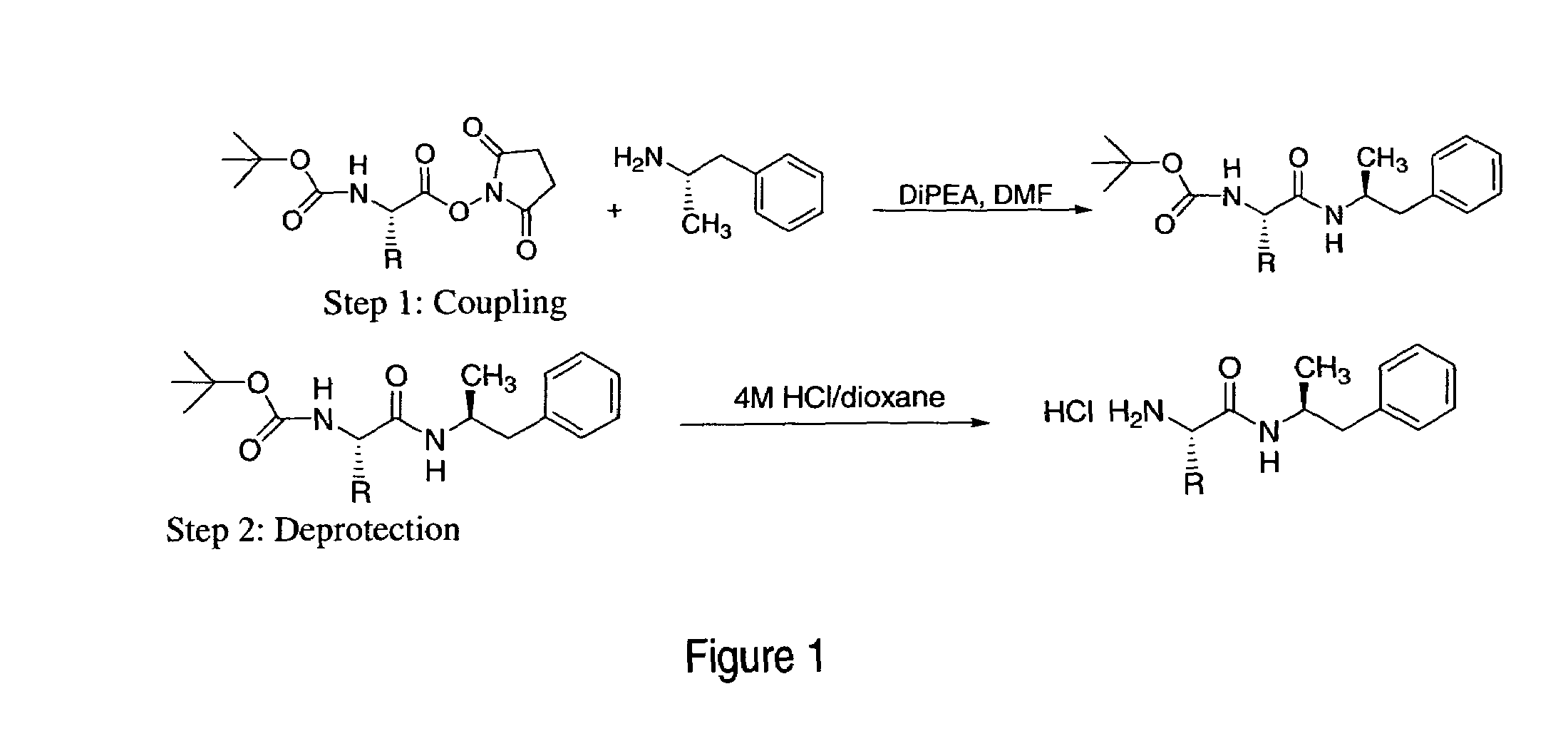
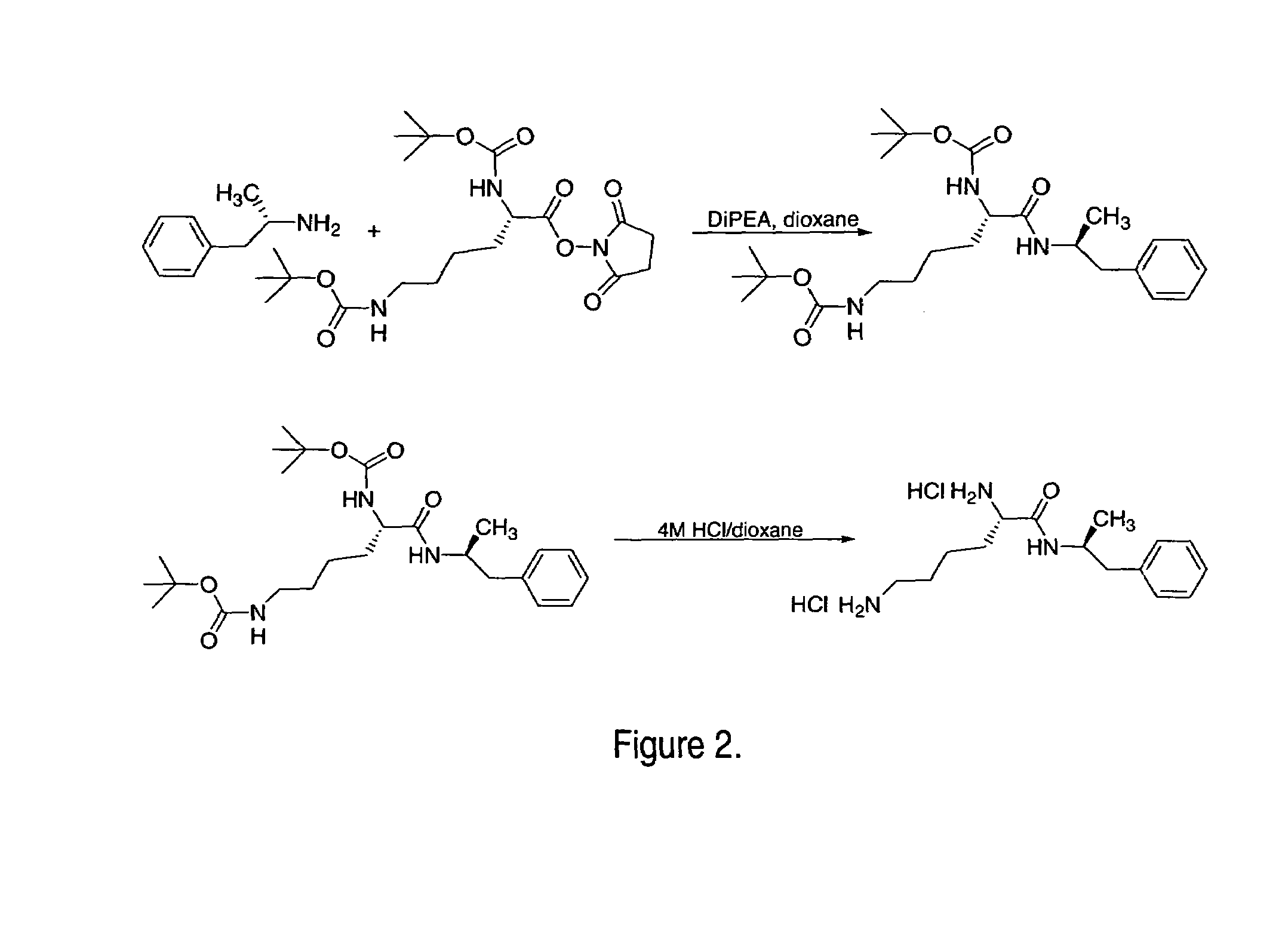
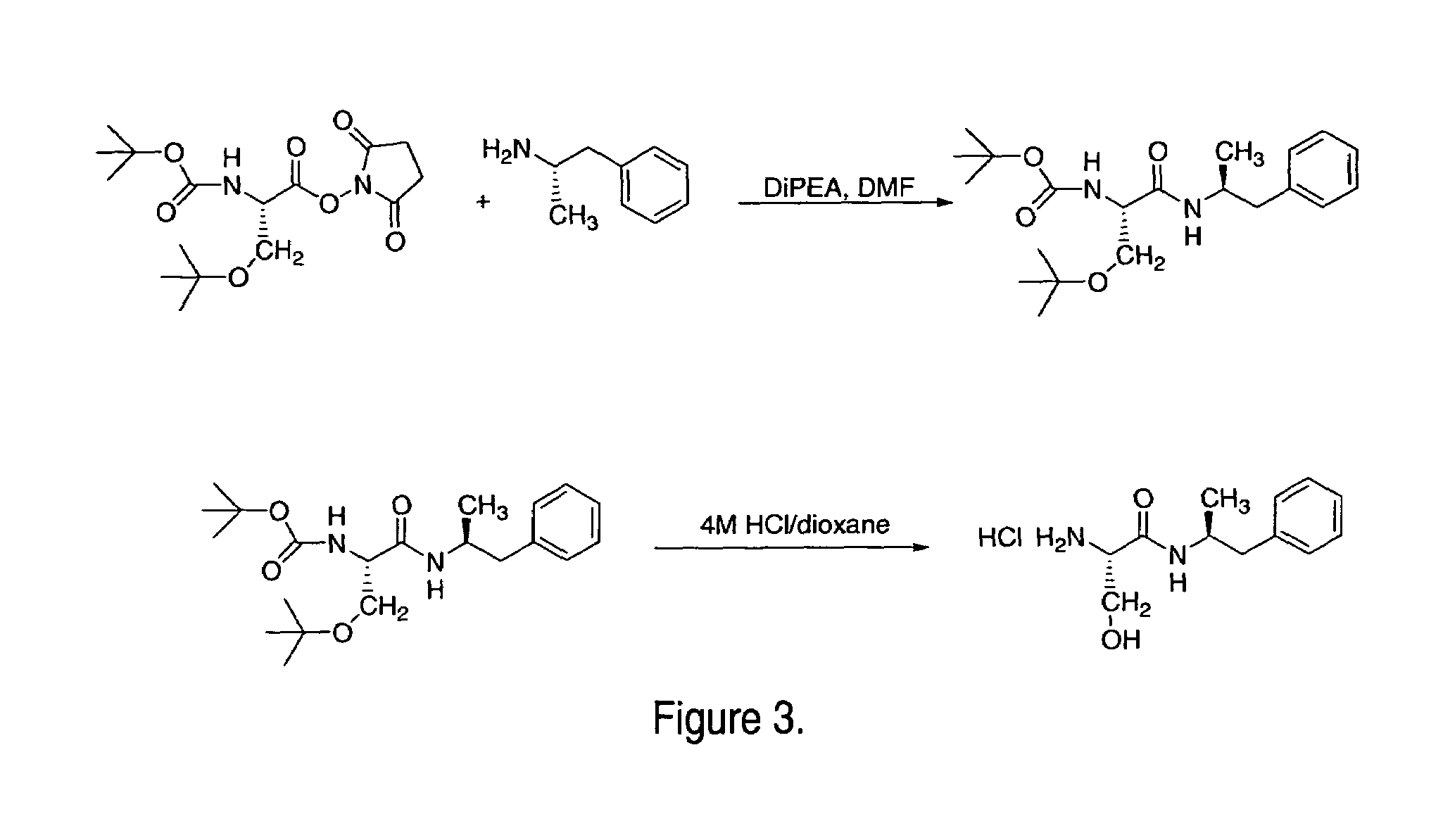
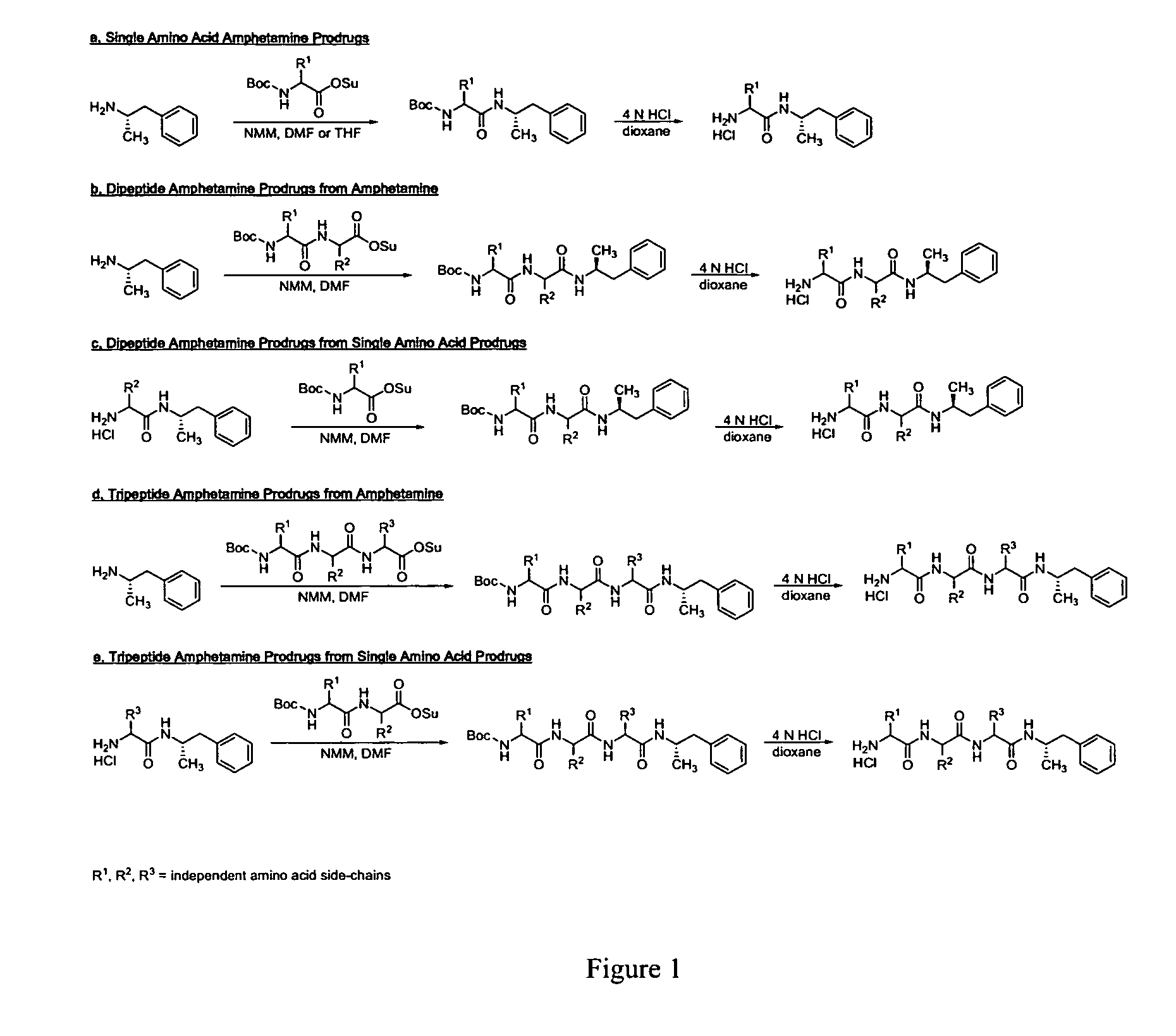
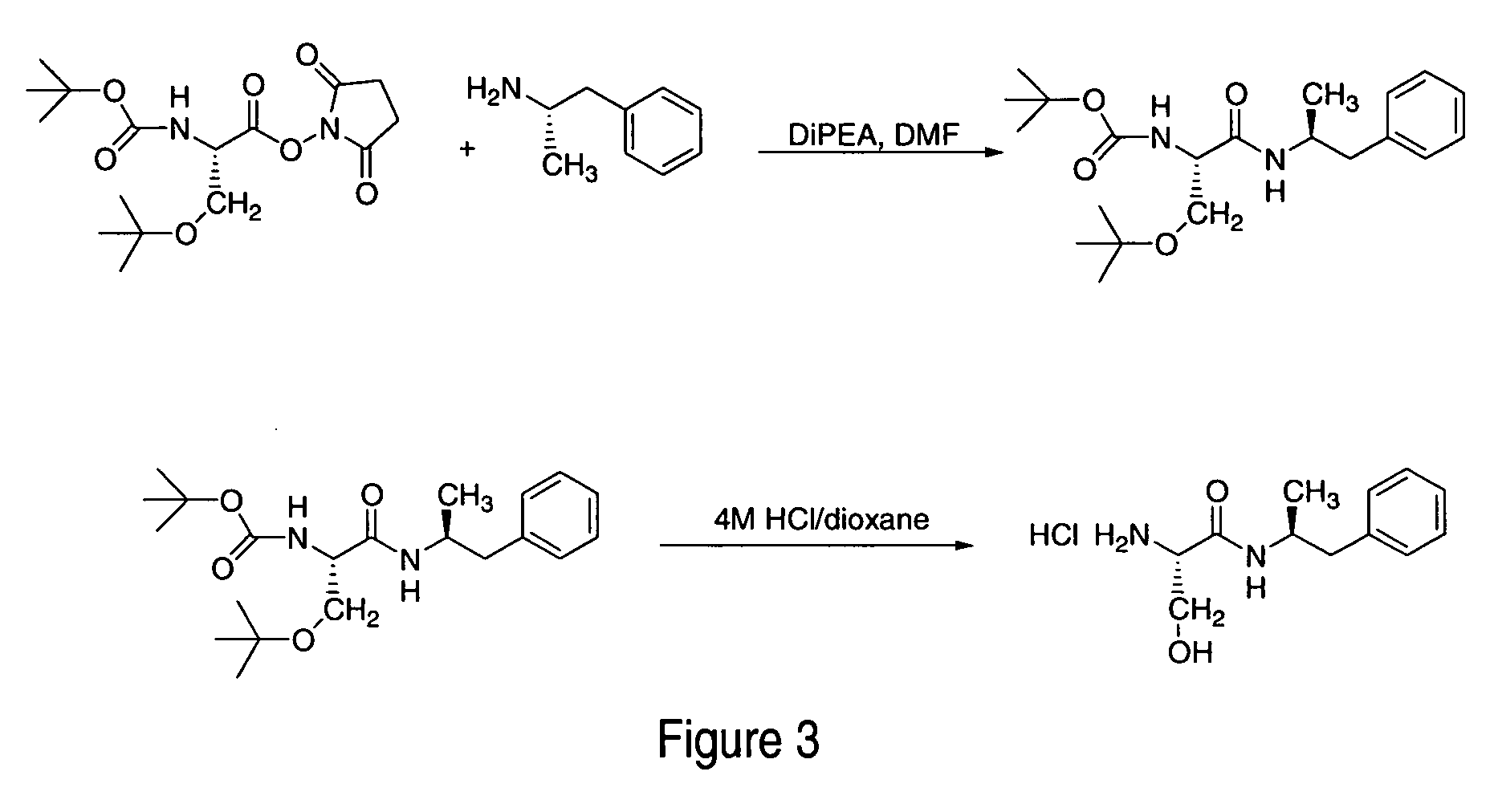
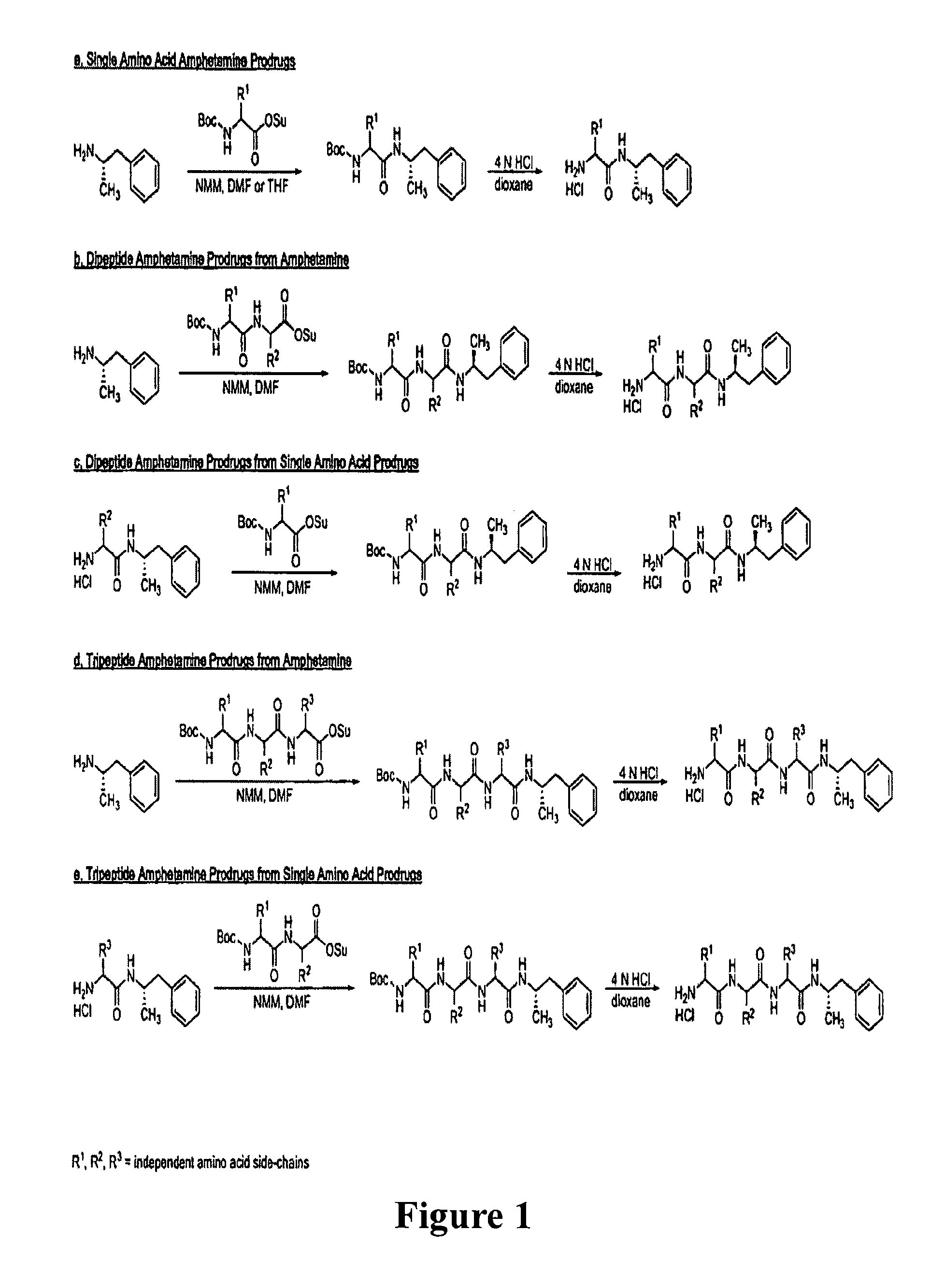
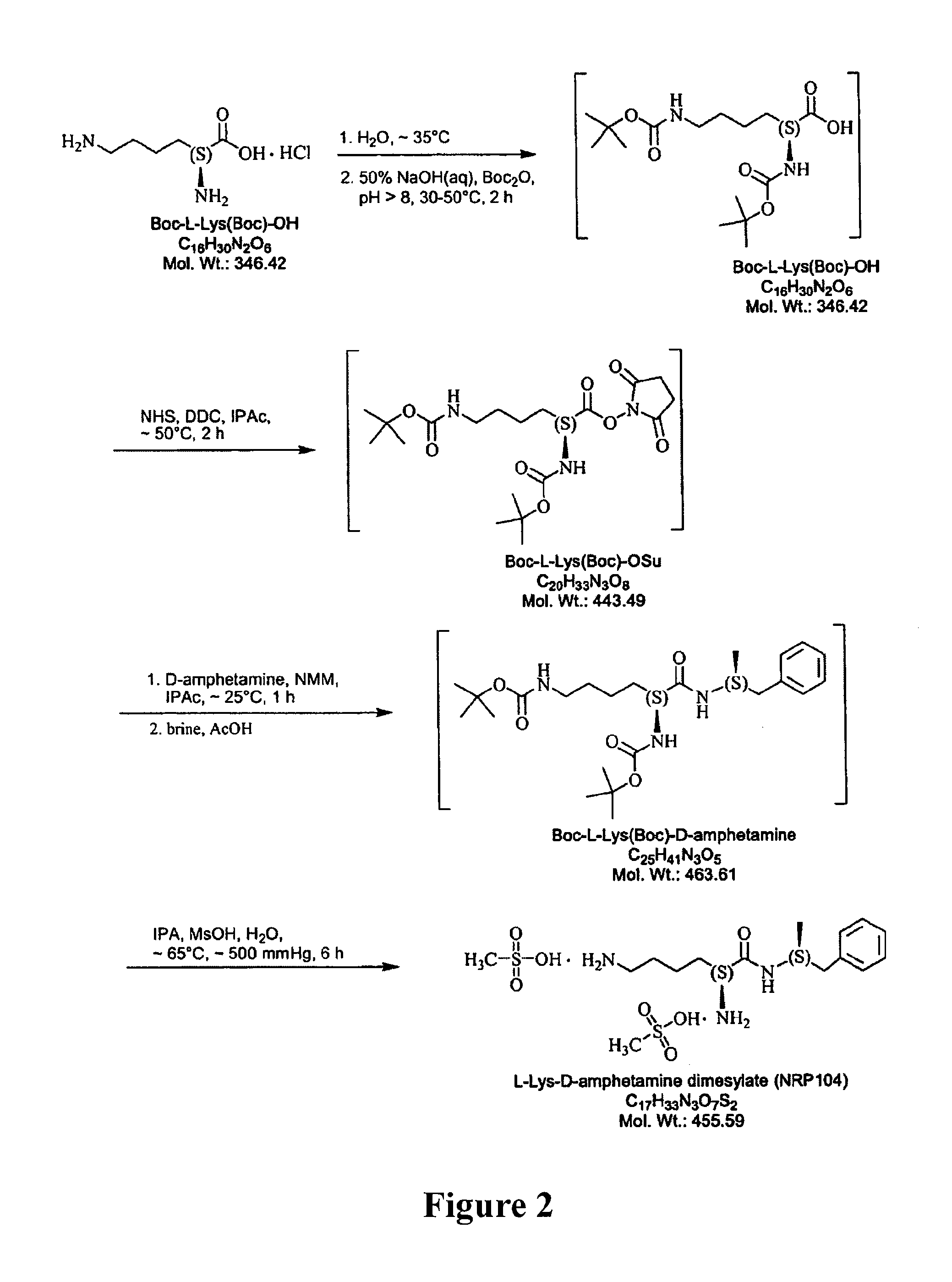
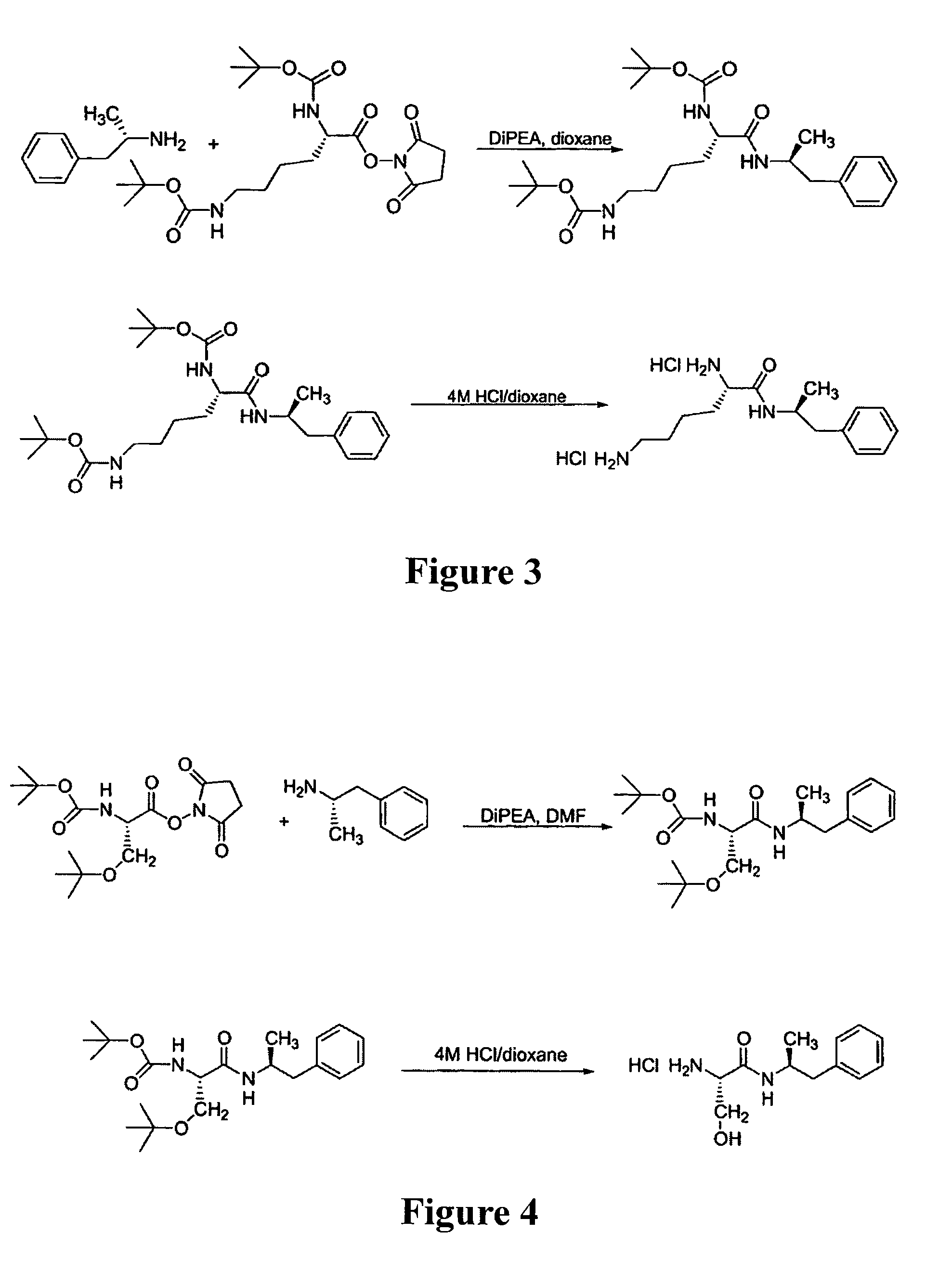
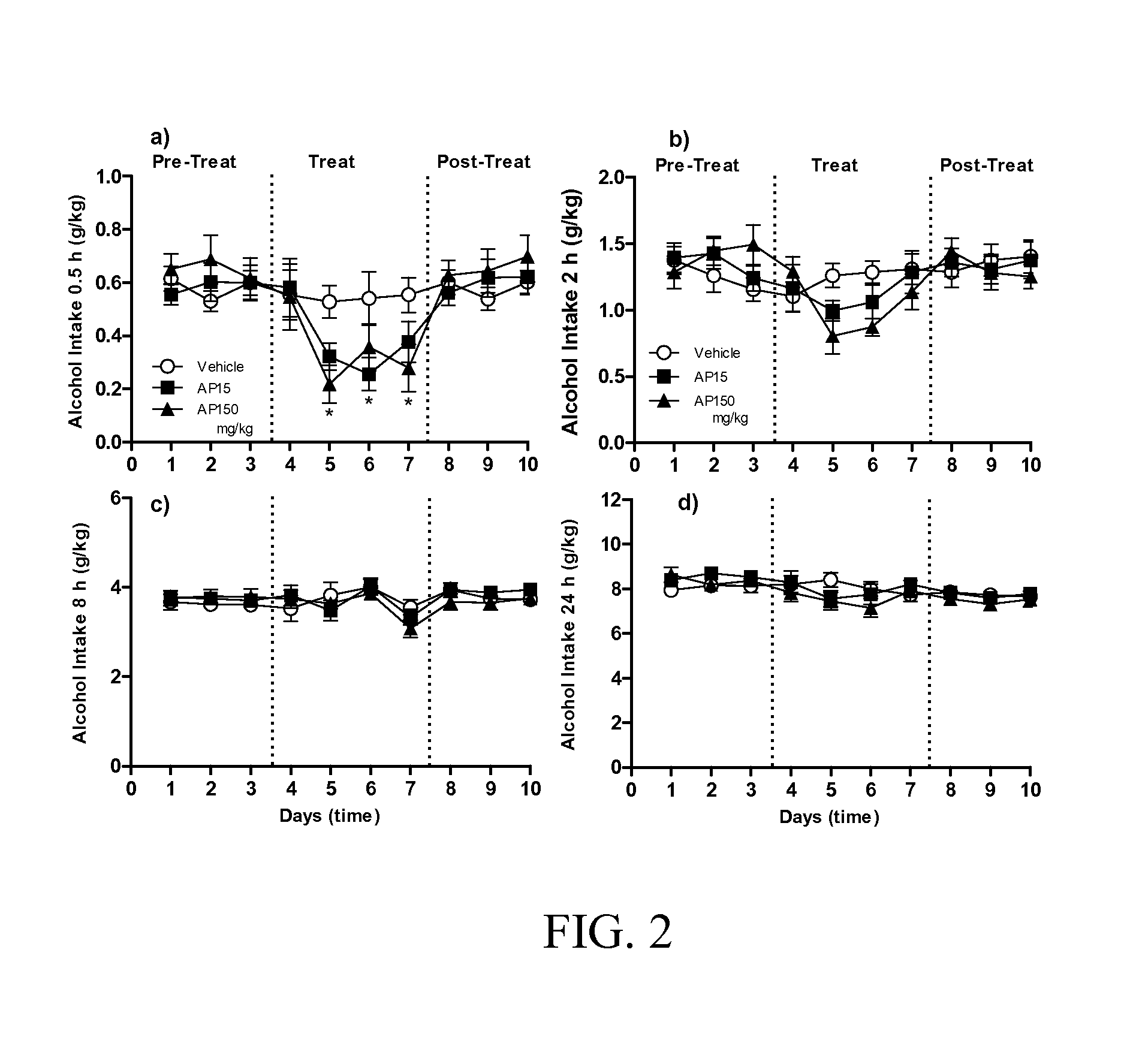
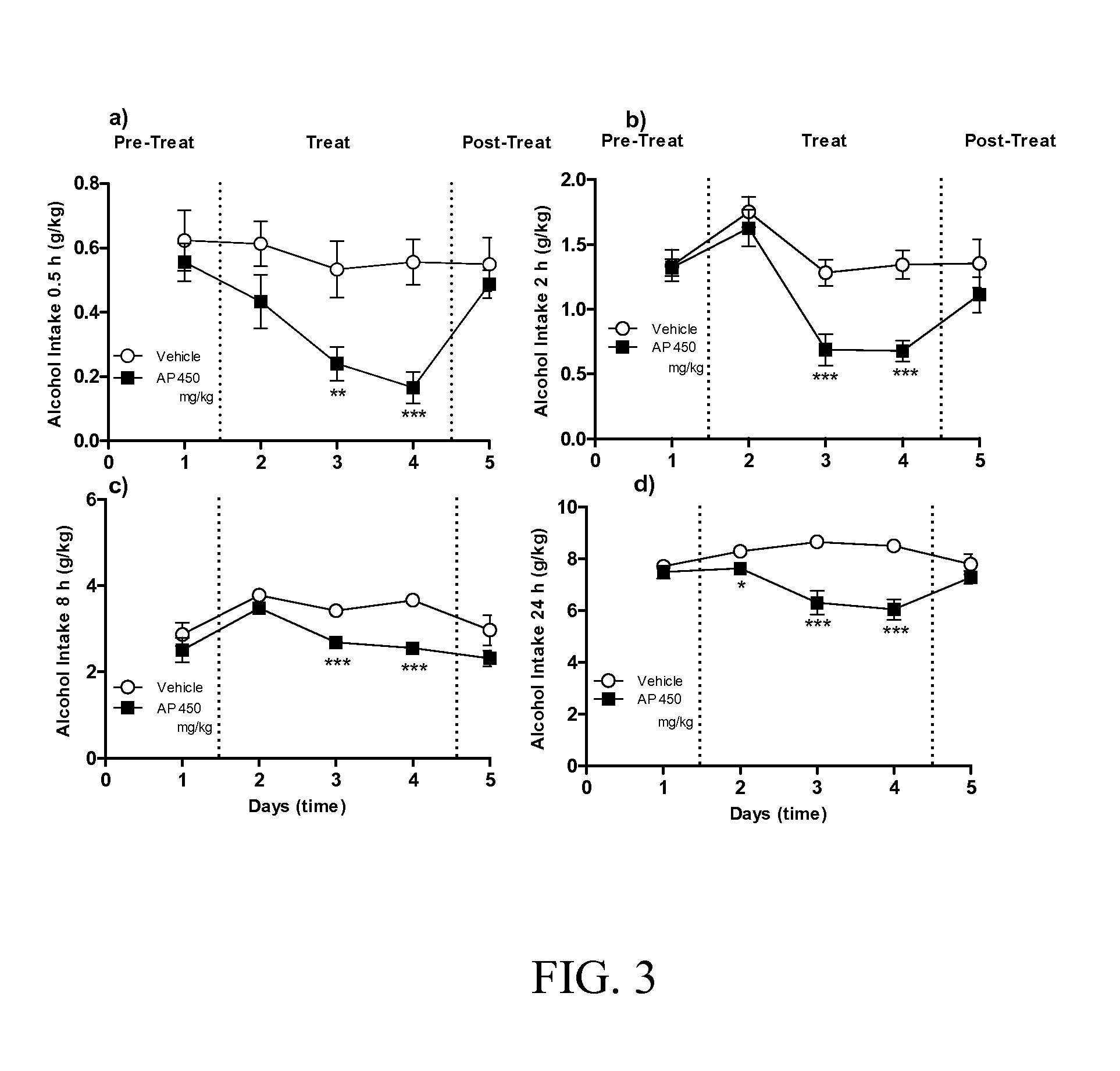
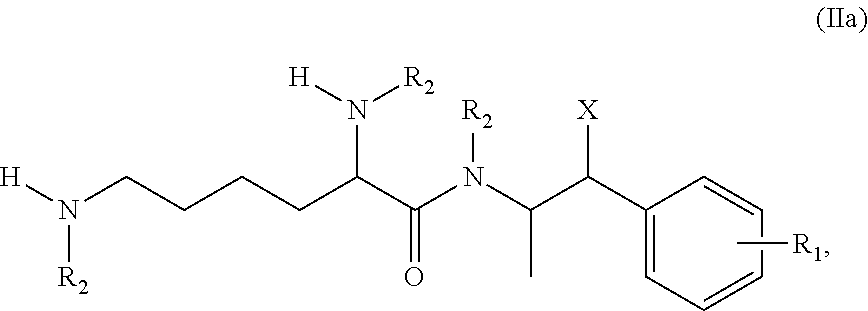
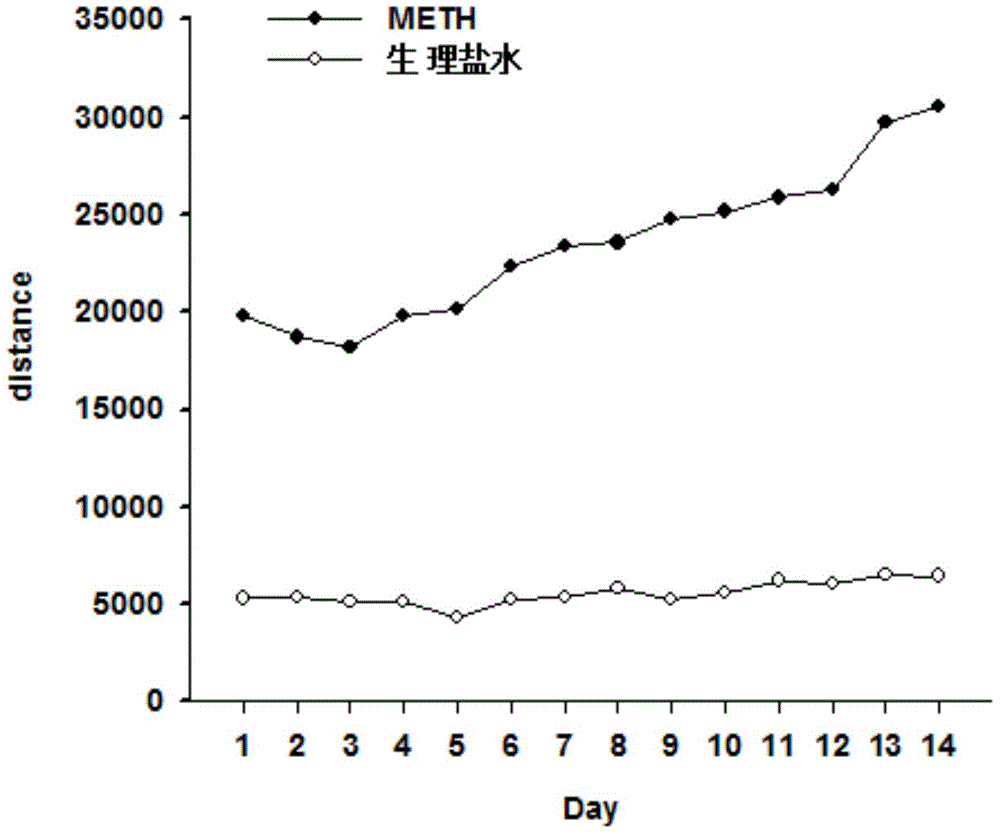
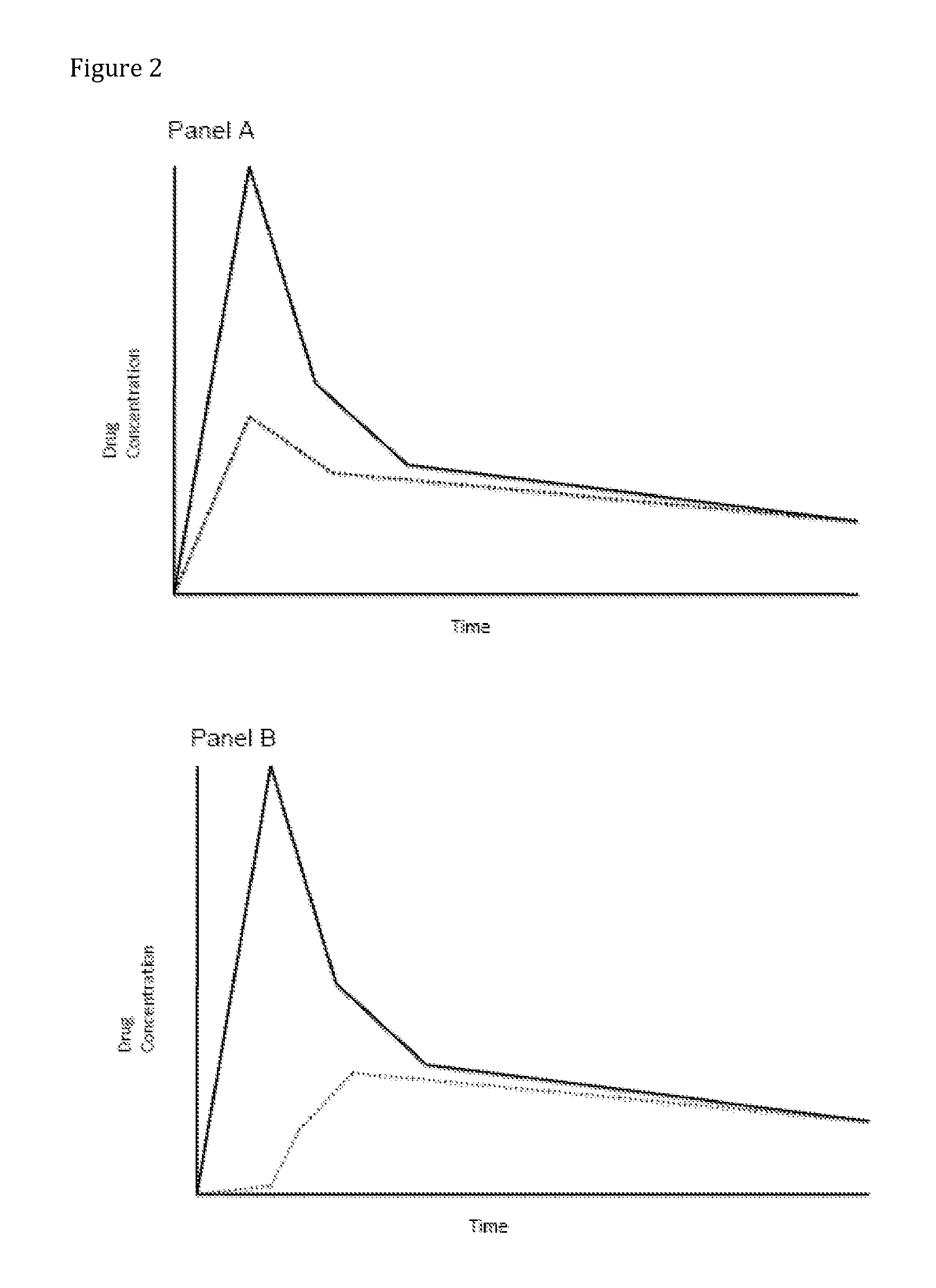
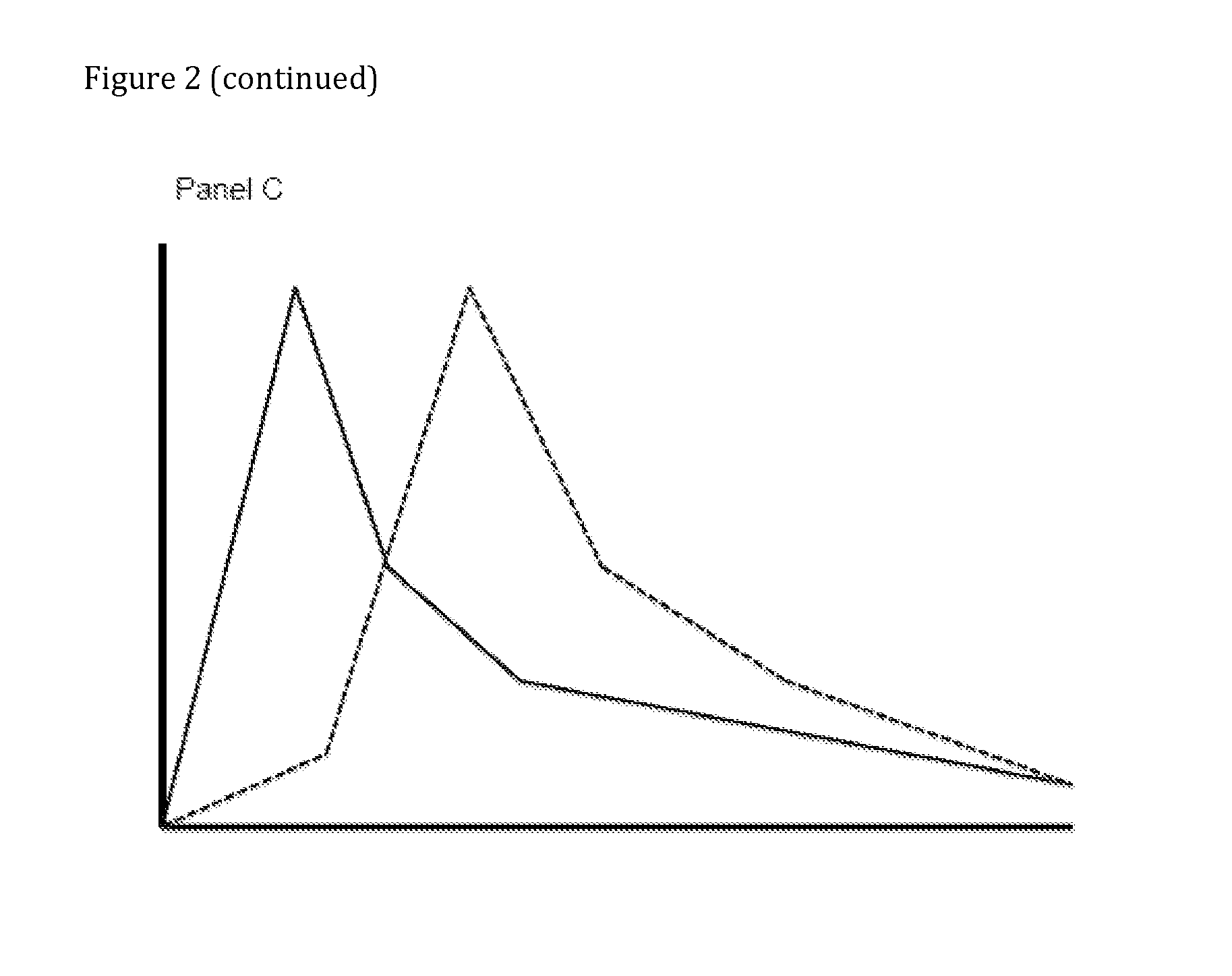Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36 results about "Benzphetamine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
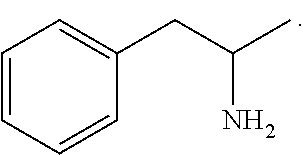
Benzphetamine is used short-term along with a doctor-approved, reduced-calorie diet, exercise, and behavior change program to help you lose weight. It is used in people who are significantly overweight (obese) and have not been able to lose enough weight with diet and exercise alone.
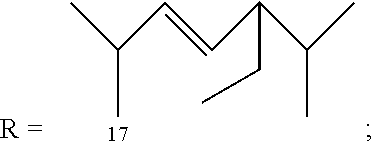
Abuse-resistant amphetamine compounds
InactiveUS7105486B2Reduced activityRelease is diminished and eliminatedOrganic active ingredientsPeptide/protein ingredientsChemical MoietyDisease
The invention describes compounds, compositions and methods of using the same comprising a chemical moiety covalently attached to amphetamine. These compounds and compositions are useful for reducing or preventing abuse and overdose of amphetamine. These compounds and compositions find particular use in providing an abuse-resistant alternative treatment for certain disorders, such as attention deficit hyperactivity disorder (ADHD), ADD, narcolepsy, and obesity. Oral bioavailability of amphetamine is maintained at therapeutically useful doses. At higher doses bioavailability is substantially reduced, thereby providing a method of reducing oral abuse liability. Further, compounds and compositions of the invention decrease the bioavailability of amphetamine by parenteral routes, such as intravenous or intranasal administration, further limiting their abuse liability.
Owner:TAKEDA PHARMA CO LTD
Abuse-resistant amphetamine prodrugs
The invention describes compounds, compositions, and methods of using the same comprising a chemical moiety covalently attached to amphetamine. These compounds and compositions are useful for reducing or preventing abuse and overdose of amphetamine. These compounds and compositions find particular use in providing an abuse-resistant alternative treatment for certain disorders, such as attention deficit hyperactivity disorder (ADHD), ADD, narcolepsy, and obesity. Oral bioavailability of amphetamine is maintained at therapeutically useful doses. At higher doses bioavailability is substantially reduced, thereby providing a method of reducing oral abuse liability. Further, compounds and compositions of the invention decrease the bioavailability of amphetamine by parenteral routes, such as intravenous or intranasal administration, further limiting their abuse liability.
Owner:TAKEDA PHARMA CO LTD
Abuse resistant lysine amphetamine compounds
InactiveUS7223735B2Reduced activityRelease is diminished and eliminatedOrganic active ingredientsNervous disorderDiseaseAlternative treatment
The present invention describes compounds, compositions and methods of using the same comprising lysine covalently attached to amphetamine. These compounds and compositions are useful for reducing or preventing abuse and overdose of amphetamine. These compounds and compositions find particular use in providing an abuse-resistant alternative treatment for certain disorders, such as attention deficit hyperactivity disorder (ADHD), ADD, narcolepsy, and obesity. Oral bioavailability of amphetamine is maintained at therapeutically useful doses. At higher doses bioavailability is substantially reduced, thereby providing a method of reducing oral abuse liability. Further, compounds and compositions of the invention decrease the bioavailability of amphetamine by parenteral routes, such as intravenous or intranasal administration, further limiting their abuse liability.
Owner:TAKEDA PHARMA CO LTD
Abuse-resistant amphetamine prodrugs
InactiveUS7659253B2Reducing patient to patient variabilityRisk minimizationBiocideOrganic chemistryDiseaseChemical Moiety
The invention describes compounds, compositions, and methods of using the same comprising a chemical moiety covalently attached to amphetamine. These compounds and compositions are useful for reducing or preventing abuse and overdose of amphetamine. These compounds and compositions find particular use in providing an abuse-resistant alternative treatment for certain disorders, such as attention deficit hyperactivity disorder (ADHD), ADD, narcolepsy, and obesity. Oral bioavailability of amphetamine is maintained at therapeutically useful doses. At higher doses bioavailability is substantially reduced, thereby providing a method of reducing oral abuse liability. Further, compounds and compositions of the invention decrease the bioavailability of amphetamine by parenteral routes, such as intravenous or intranasal administration, further limiting their abuse liability.
Owner:TAKEDA PHARMA CO LTD
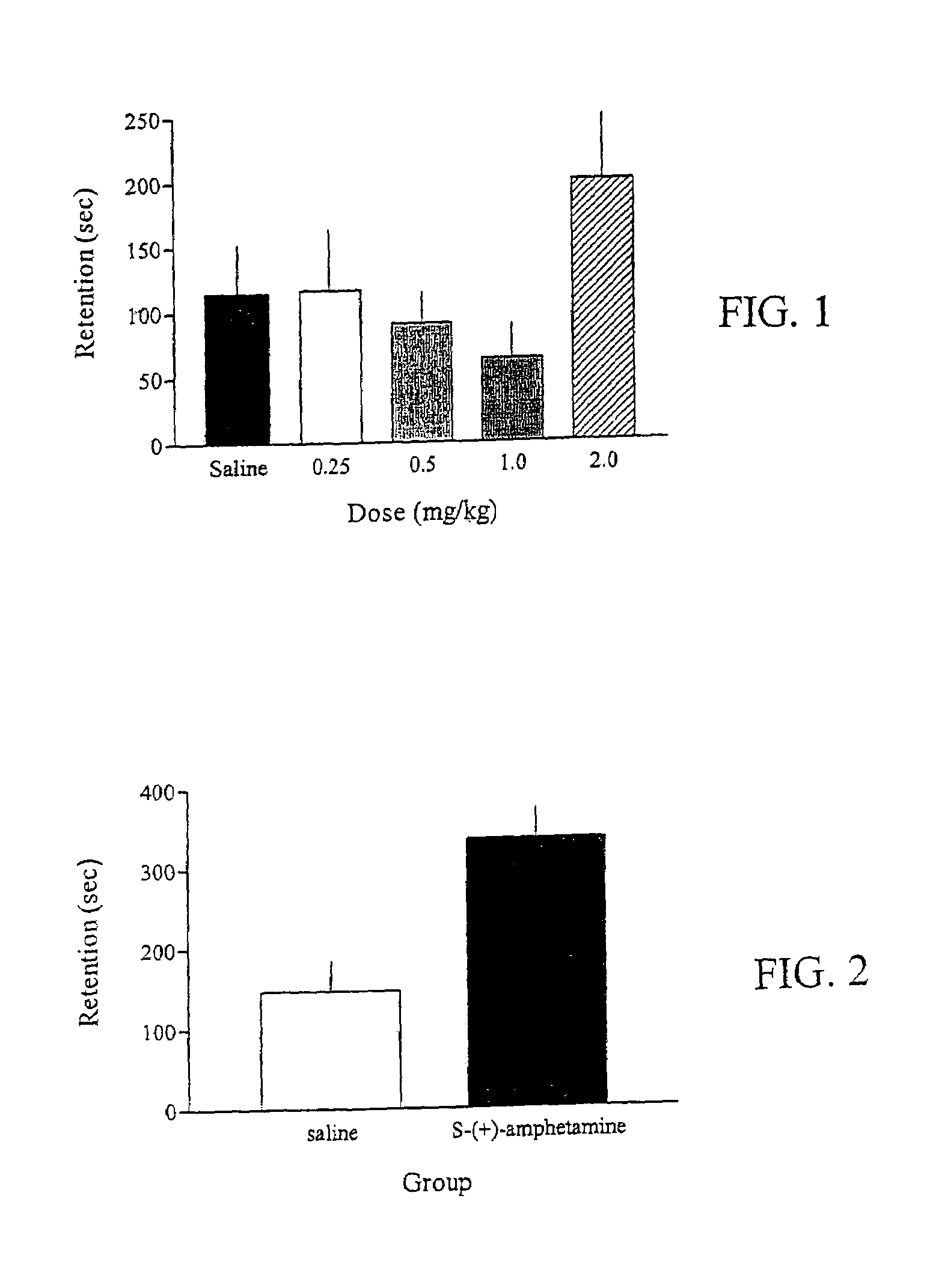
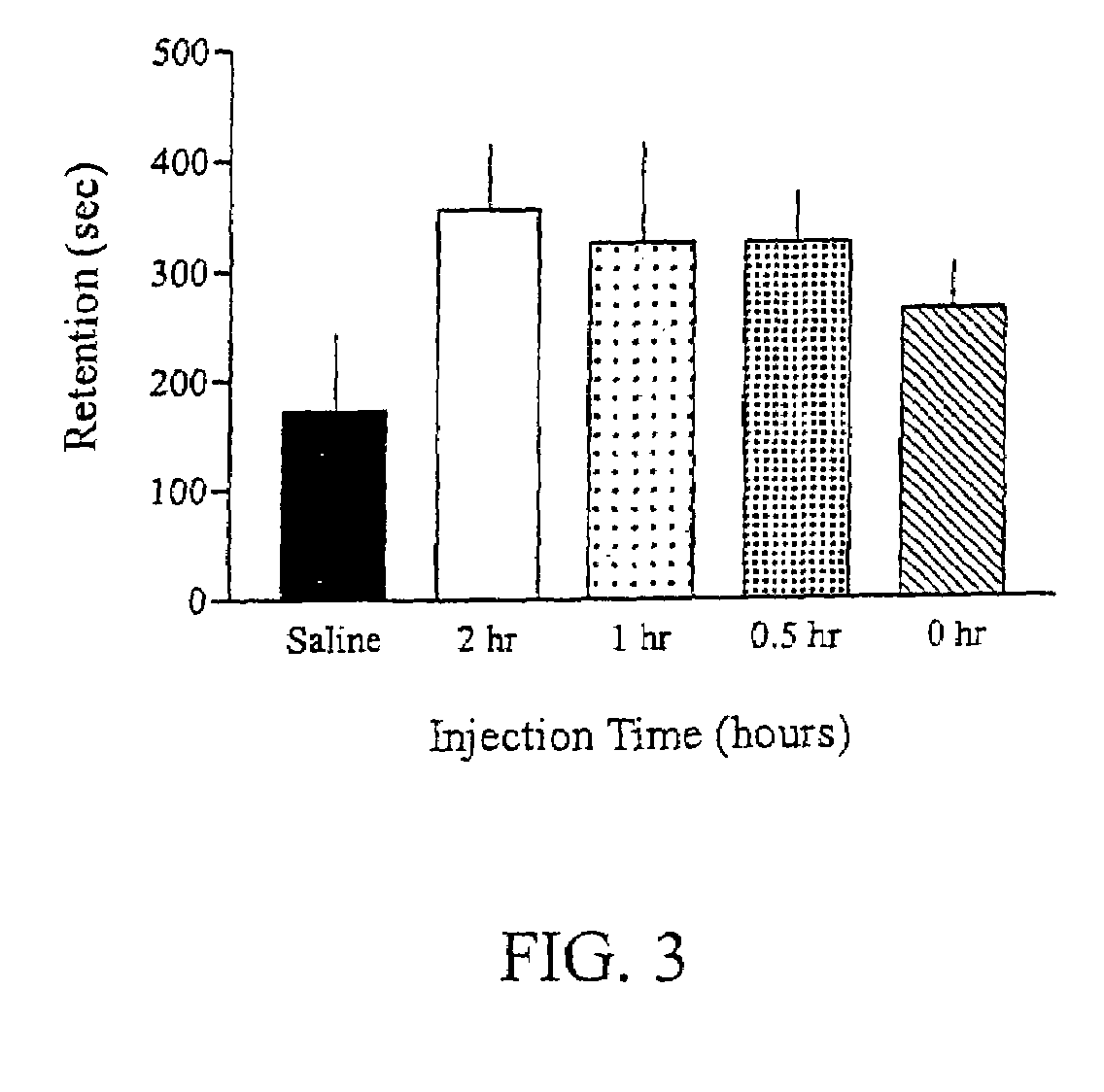
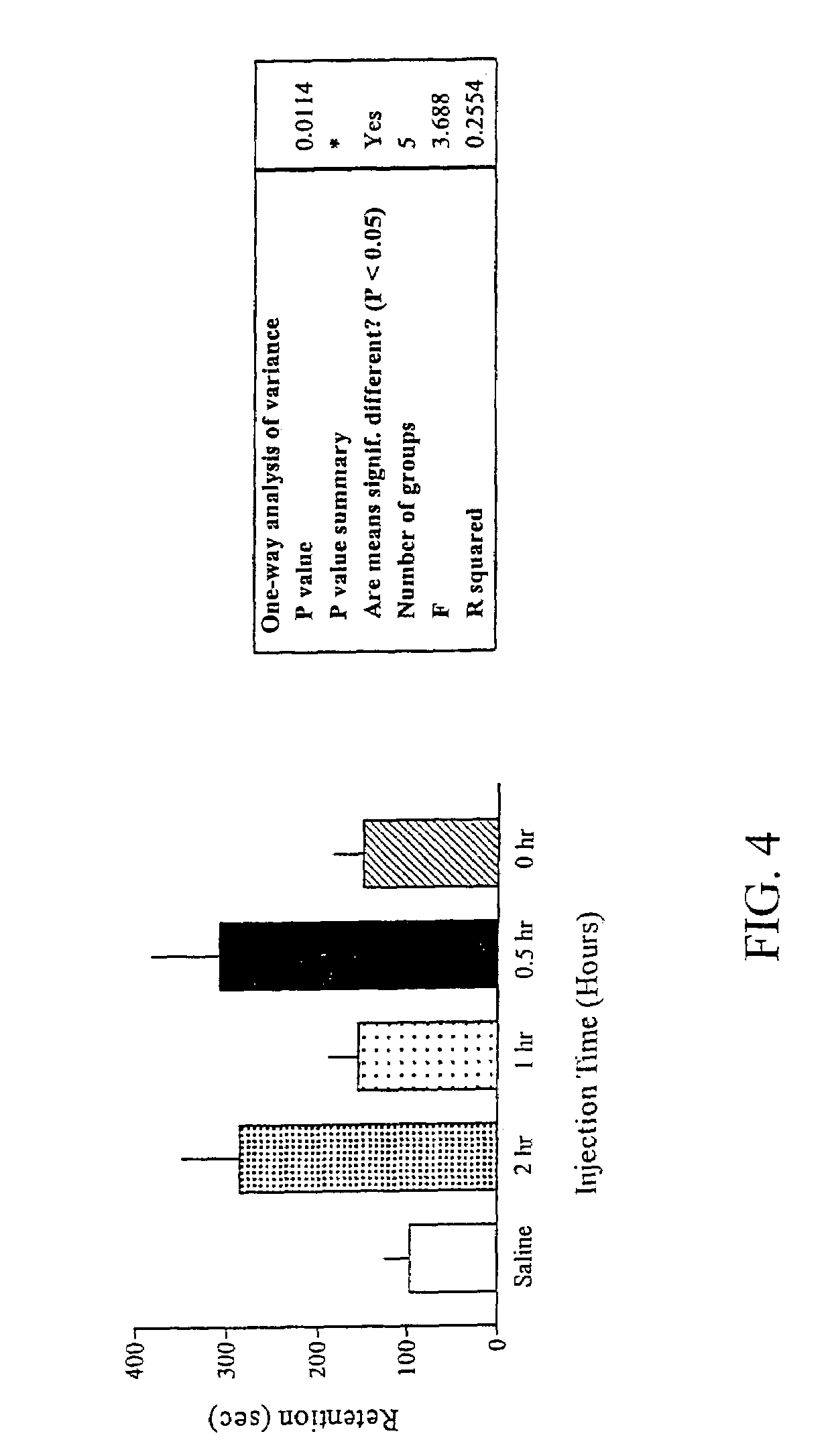
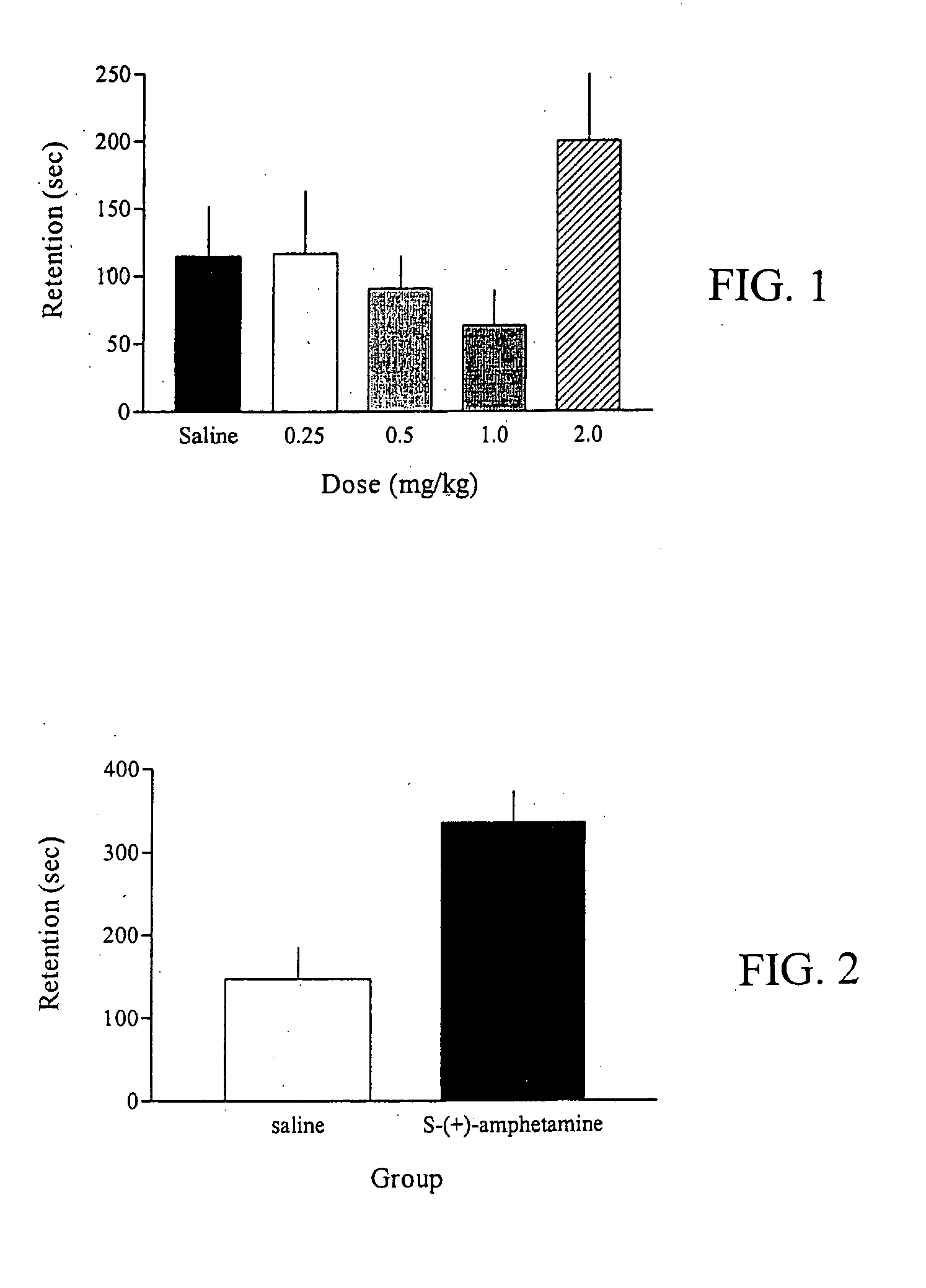
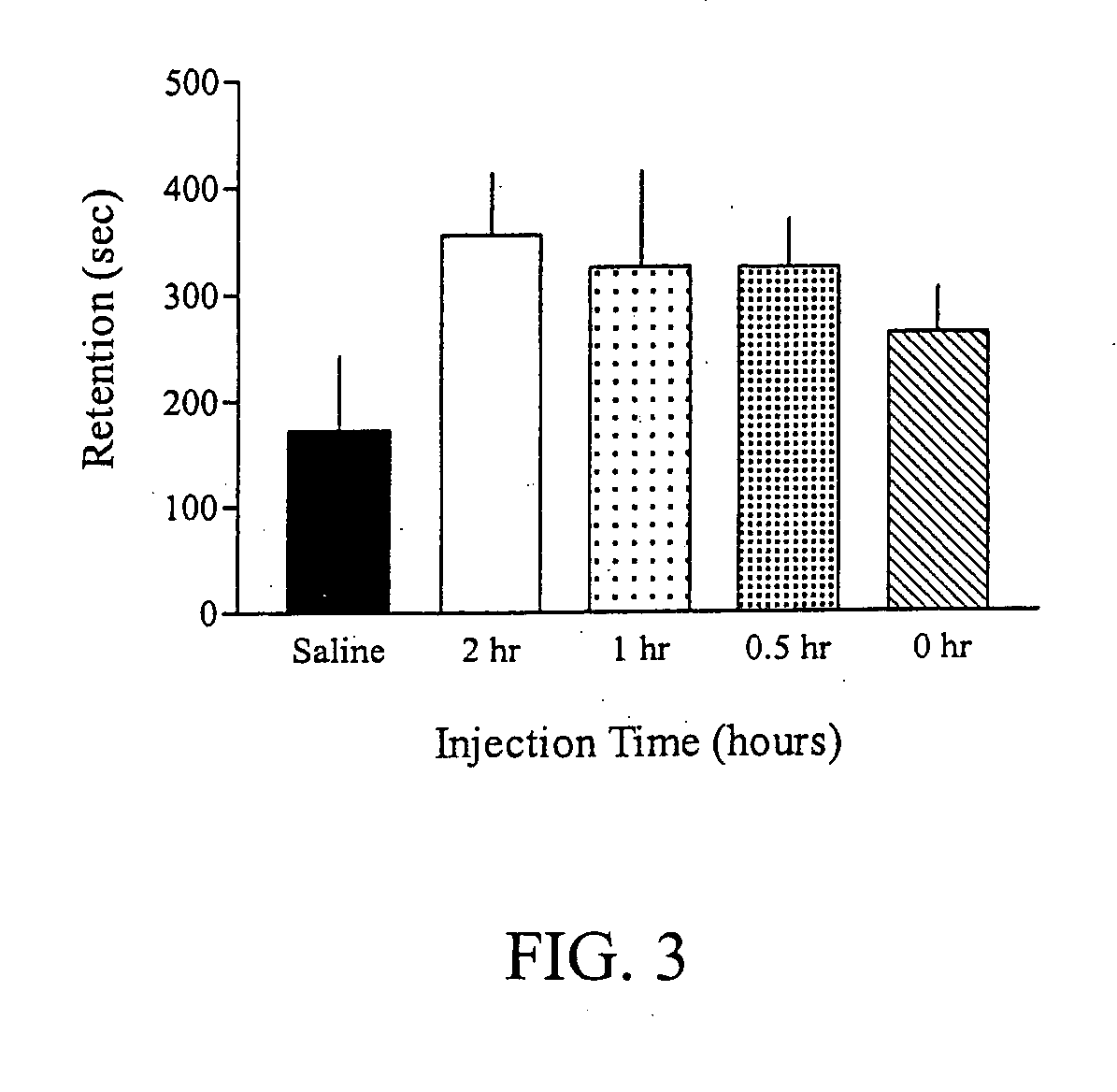
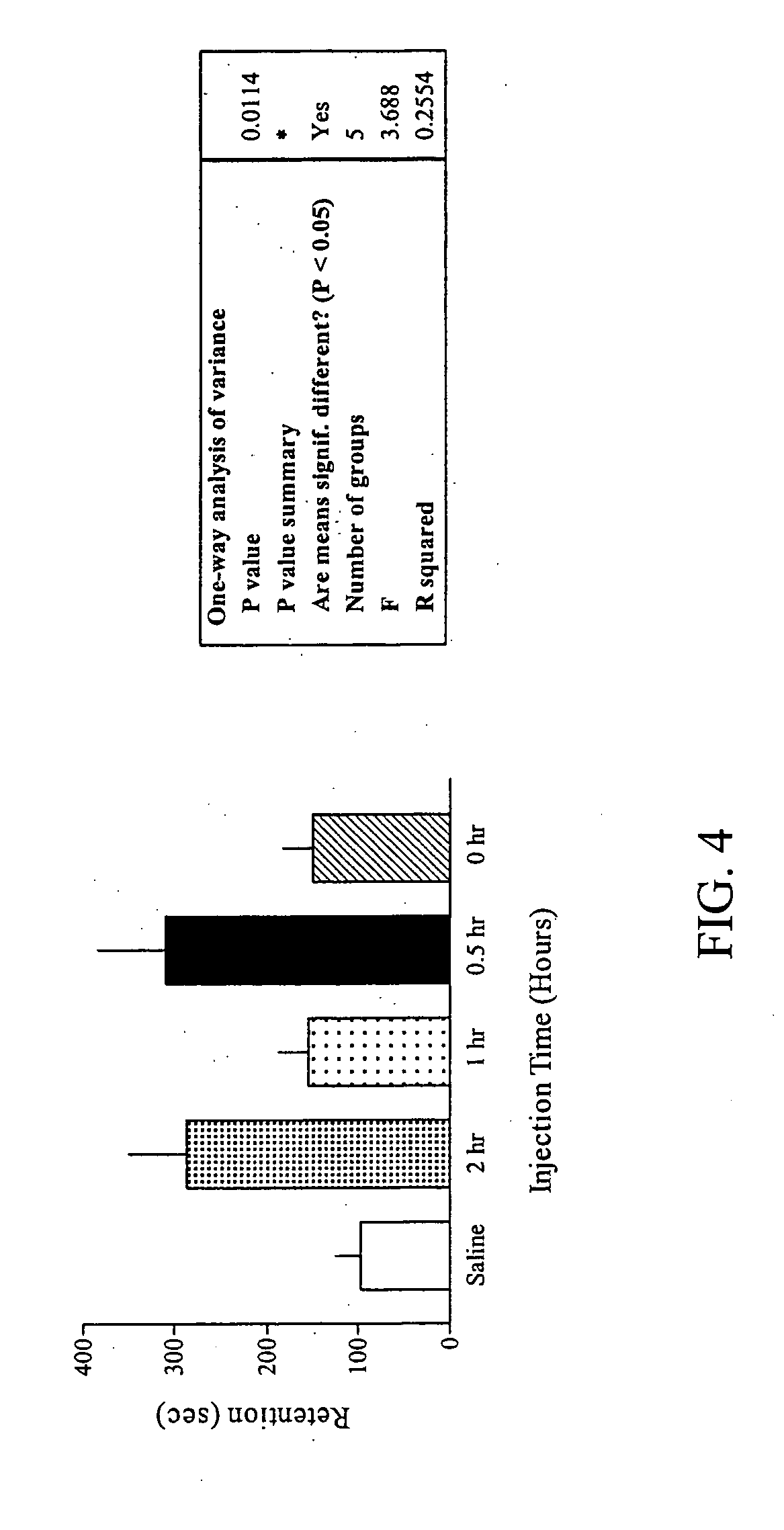
Methods for treating an impairment in memory consolidation
Impairments in memory consolidation are treated with an amphetamine compound. In one embodiment, the method includes administering an l-amphetamine compound. In another embodiment, the method includes administering an l-methamphetamine compound. The method can include determining memory consolidation before, during and / or after administering the amphetamine compound.
Owner:COGNITION PHARMA LLC +2
Method of treating binge eating disorder and obesity resulting from binge eating behavior
Owner:LUCERNE BIOSCI
Polar hydrophilic prodrugs of amphetamine and other stimulants and processes for making and using the same
ActiveUS7772222B2Eliminate the effects ofReduce stressBiocideNervous disorderChemical ligationStimulant
Owner:TAKEDA PHARMA CO LTD
Assays for amphetamine and methamphetamine using stereospecific reagents
Methods, compositions and kits are disclosed. Enzyme conjugates of Formula I may be employed in assays for the determination of an amphetamine and / or a methamphetamine. Immunogenic conjugates of Formula I may be employed to prepare antibodies for an amphetamine and / or for a methamphetamine for use in assays for the determination of an amphetamine and / or a methamphetamine. The enzyme conjugates may also be employed to screen antibodies for use in such methods.
Owner:SIEMENS HEALTHCARE DIAGNOSTICS INC
Methods of enhancing selective serotonin reuptake inhibitor effects in mammals
InactiveUS20100303903A1Convenient treatmentGood effectBiocideNervous disorderTreatment choicesEscitalopram
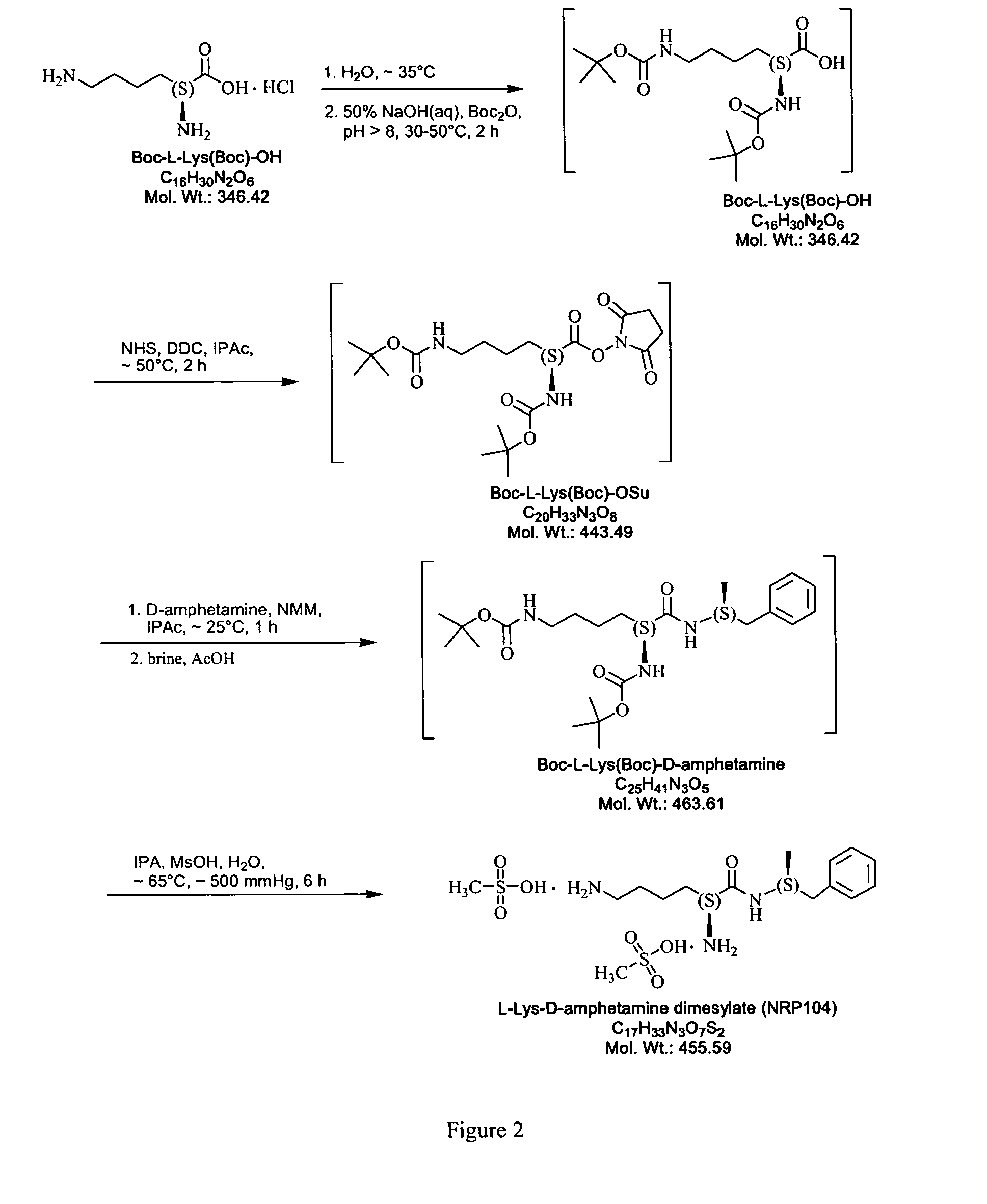
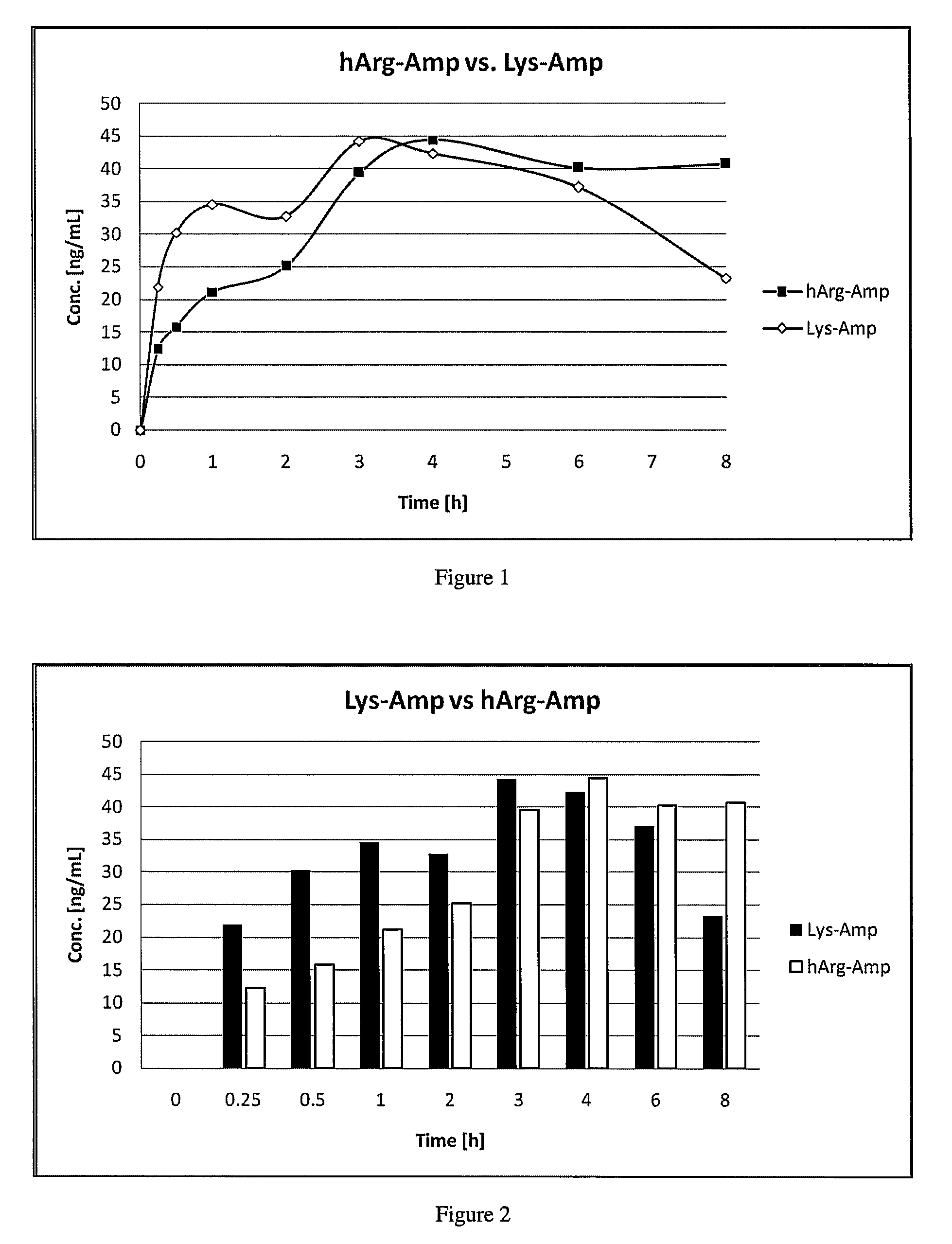
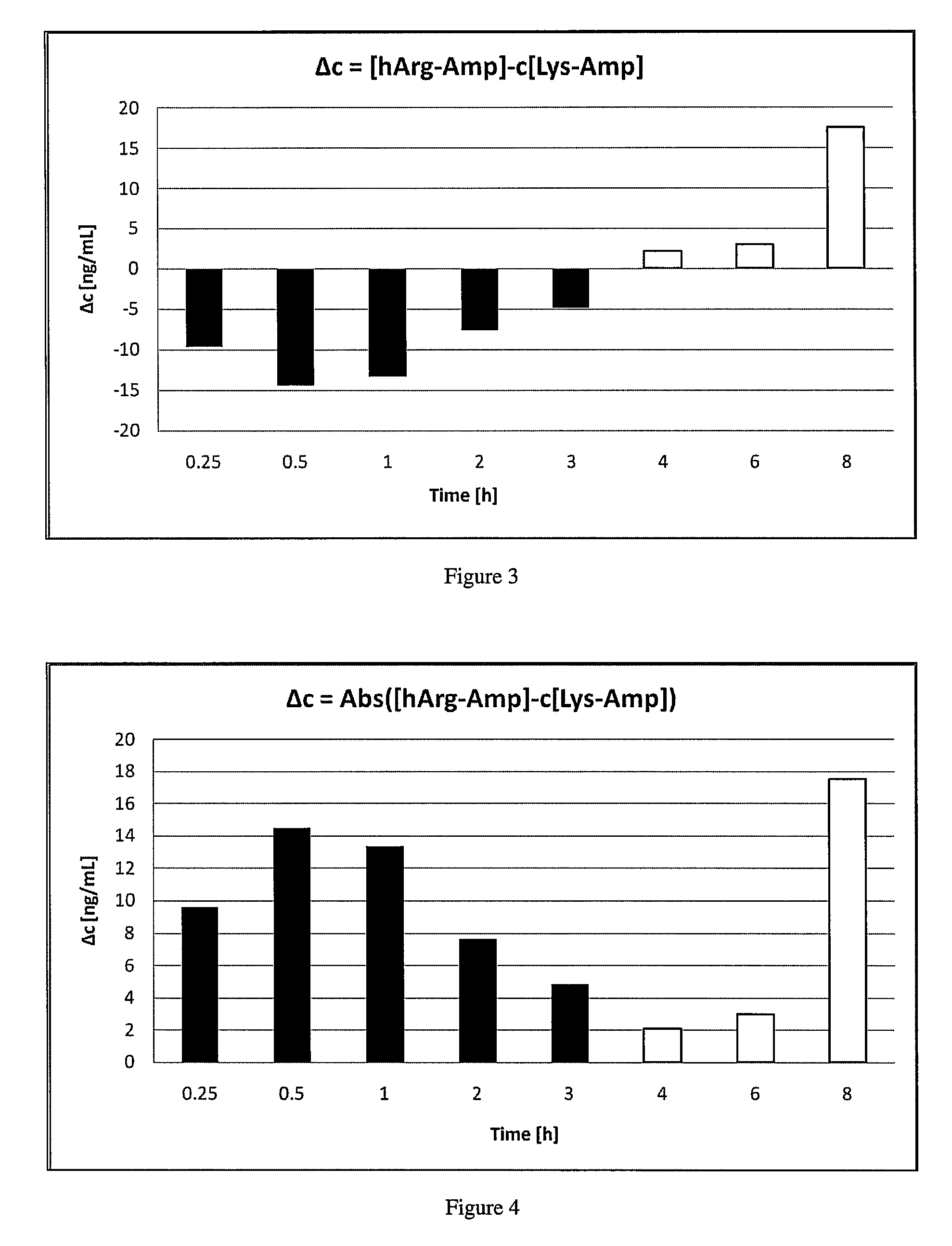
The invention relates to methods of enhancing selective serotonin reuptake inhibitor effects in mammals. In particular, the invention provides methods for treating selective serotonin reuptake inhibitor dependent conditions such as depression. More specifically, the present invention relates to a method of increasing the antidepressant activity of a selective serotonin reuptake inhibitor (“SSRI”) by administering L-lysine-d-amphetamine in combination with an SSRI and to formulates containing the same. In a preferred aspect, the combination is administered in connection with a method of treating depression. One preferred SSRI is escitalopram. The preferred amphetamine prodrug is L-lysine-d-amphetamine.
Owner:SHIRE PLC
Amphetamine addict craving evaluation method
The invention relates to an amphetamine addict craving evaluation method. Craving of an amphetamine addict for amphetamines is induced by virtual reality technology, and whether craving of the addict for the amphetamines is induced successfully or not is evaluated by calculating objective data of heart rate variability of the addict. By the aid of the theoretical framework of conditioned reflex, most significant end multimedia information input is added and physiological signals are acquired based on psychology, relevant managers can conveniently evaluate the addict at regular intervals, and the recurrence possibility of the addict is maximally reduced.
Owner:杭州赛翁思科技有限公司
Methods for treating mild cognitive impairment and alzheimer's disease
InactiveUS20050059743A1Avoid performanceImprove verbal memoryOrganic active ingredientsBiocideDiseaseCognitive diseases
Mild cognitive impairment and Alzheimer's disease are treated with an amphetamine compound. In one embodiment, the method includes administering an 1-amphetamine compound. In another embodiment, the method includes administering an 1-methamphetamine compound.
Owner:CNS ACQUISITIONS LLC
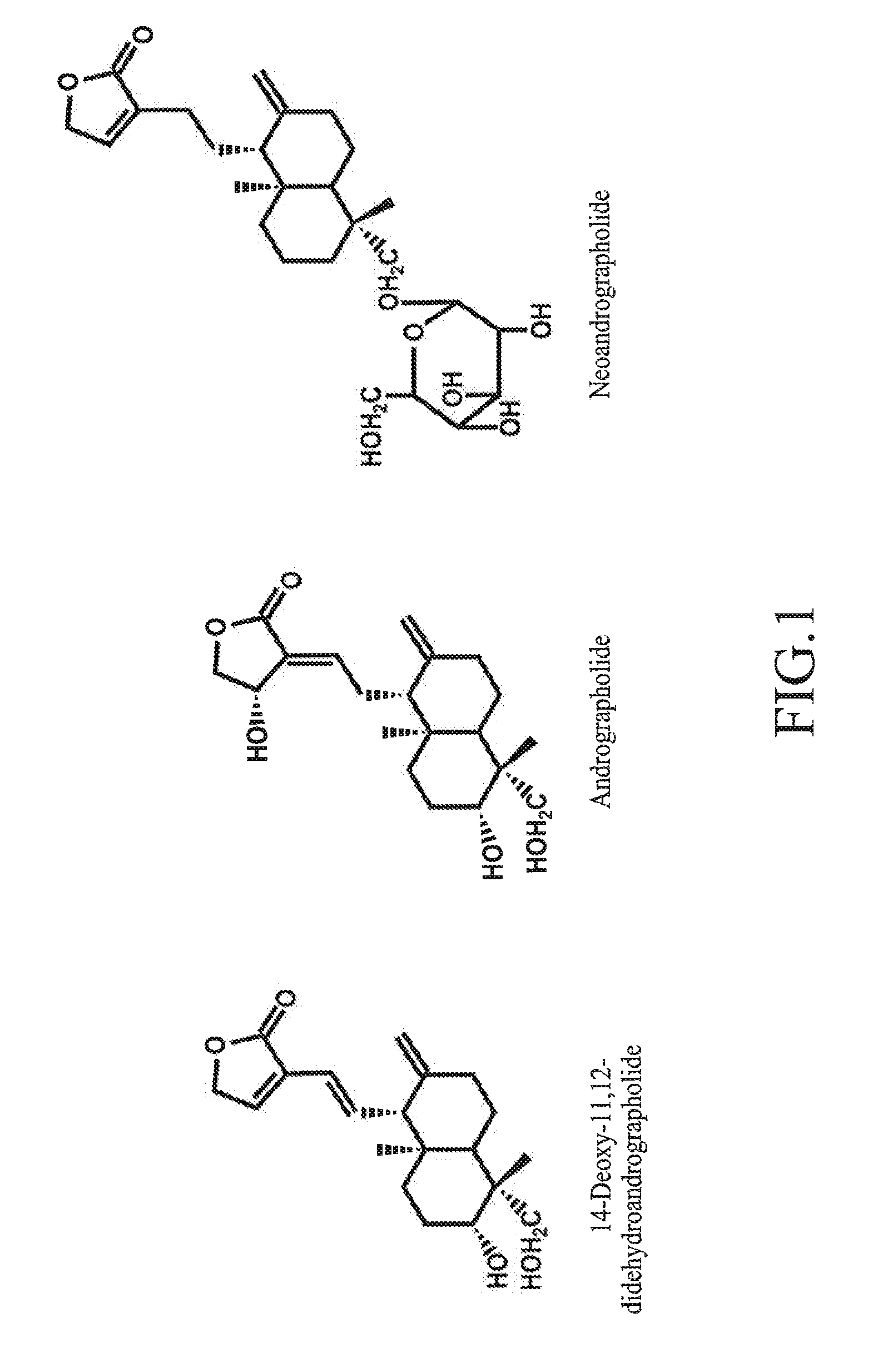
Andrographis Paniculata Compositions and Methods for Treatment of Addictions
InactiveUS20140072662A1Reduce the possibilityEffective treatmentBiocideNervous disorderCannabisOpioid Agonist
Methods and compositions for the treatment or prevention of addictions and impulse control disorders using Andrographis paniculata, active substances contained therein and extracts thereof, alone or in combination with other therapeutic agents. The methods and compositions of the invention are useful in the treatment or prevention of addiction to substances, including alcohol, nicotine, marijuana, opioid agonists cocaine, and amphetamines, as well as impulse control disorders, including pathological gambling and pathological overeating.
Owner:OMEROS CORP
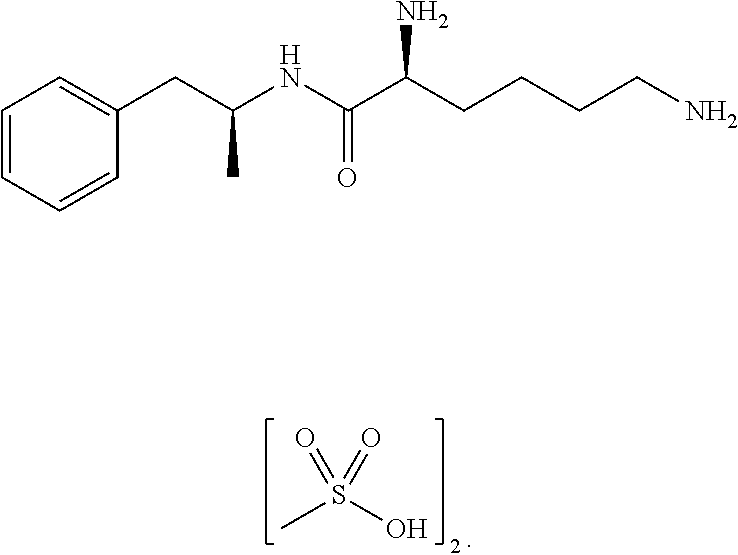
Methods and compositions for preparation of amphetamine conjugates and salts thereof
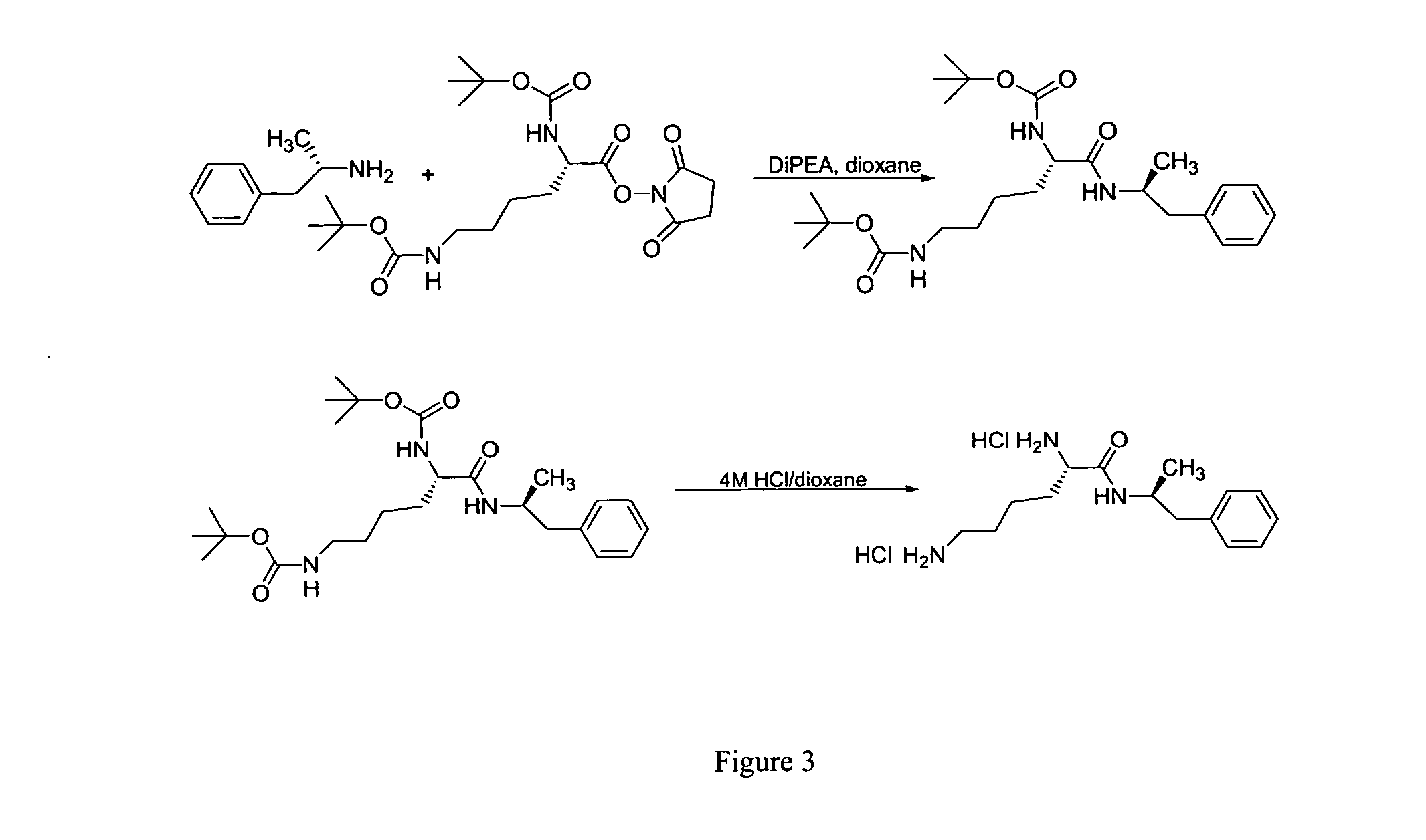
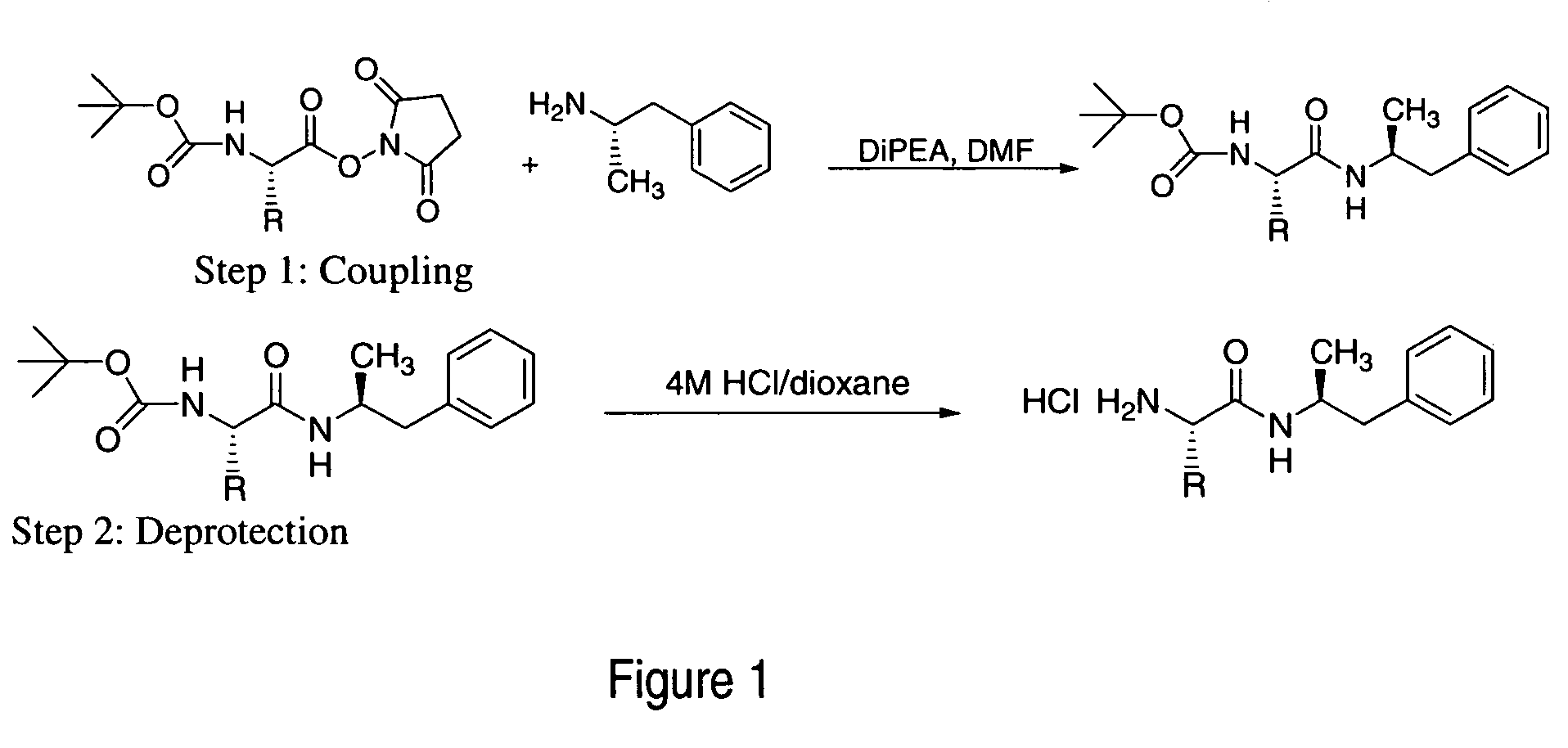
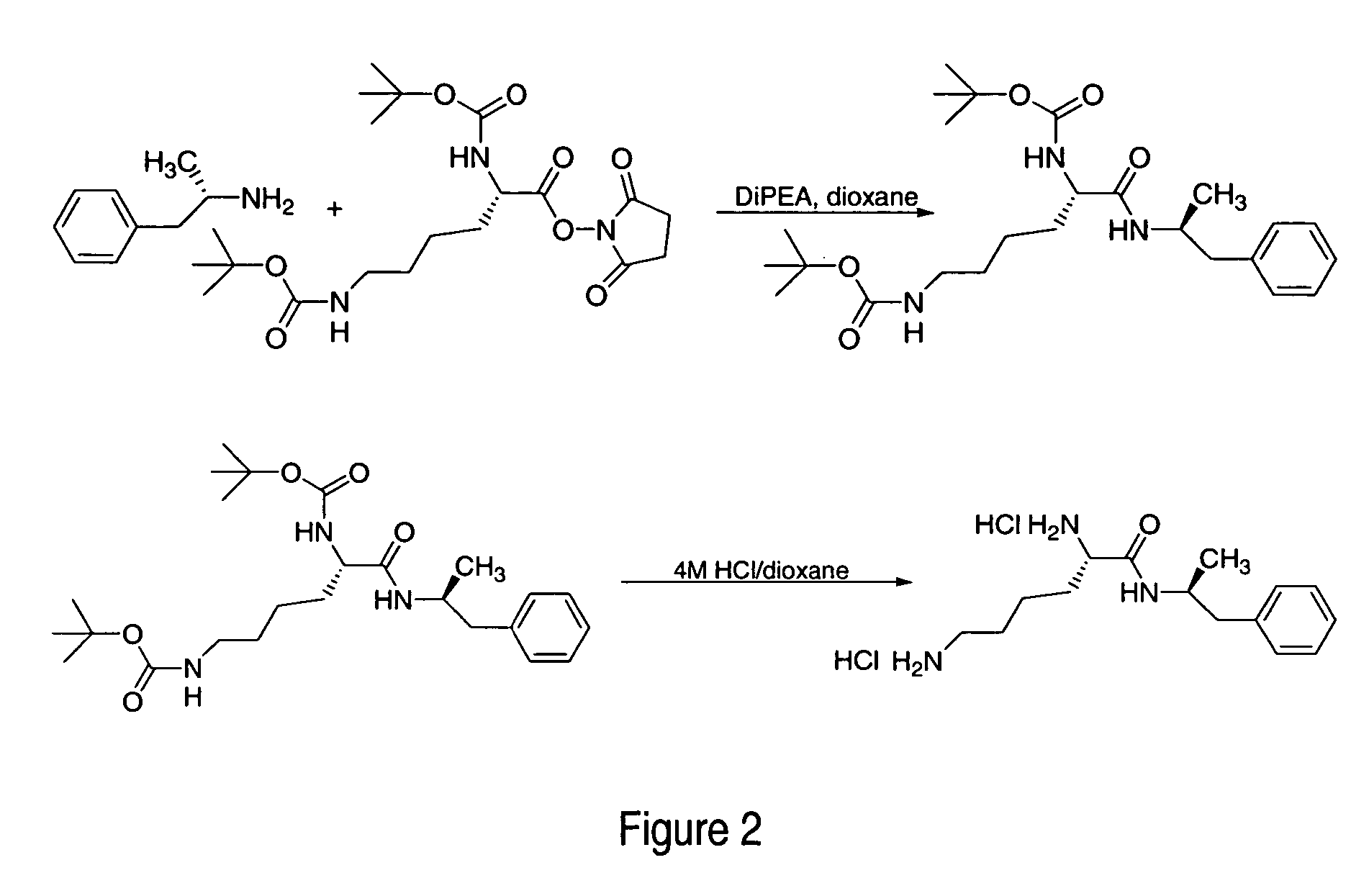
ActiveUS20120190880A1Reduce volatilityHigh enantiomeric purityCarbamic acid derivatives preparationOrganic compound preparationBenzphetamineMedicinal chemistry
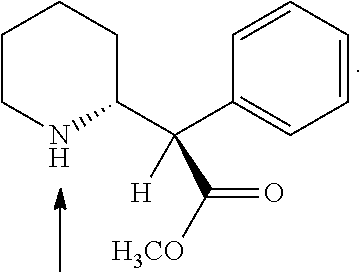
The invention provides methods and compositions for preparing amphetamine conjugates, such as lisdexamfetamine, homoarginine-D-amphetamine, and salts thereof. In one embodiment, the invention provides methods of preparing an amphetamine conjugate from a chloramphetamine intermediate.
Owner:CAMBREX CHARLES CITY INC
Medicament for treating amphetamine type stimulant dependency and mixed dependency of amphetamine type stimulants and opiates substances
InactiveCN103495172AGood effectControl withdrawal symptomsOrganic active ingredientsNervous disorderBenzodiazepineDrug withdrawal symptoms
The invention discloses a medicament for treating amphetamine type stimulant dependency and mixed dependency of amphetamine type stimulants and opiates substances. The medicament is prepared from the following components by weight percent: 2-95% of antipsychotics, 0.001-5% of alpha2 adrenergic agonists, 0-5% of anticholinergic agent, 0-80% of nonopioid analgesic, and 0-10% of benzodiazepine. The pharmaceutical composition disclosed by the invention achieves an ideal effect when the symptoms of a sufferer abusing stimulants such as benzedrine, or abusing the opiates substances in a merging manner are treated; the withdrawal symptom can be rapidly and obviously controlled; the detoxification recovery rate can be up to over 90%.
Owner:卢正堂 +1
Homoarginine prodrugs and/or conjugates of amphetamine and other stimulants and processes for making and using the same
Disclosed are homoarginine amphetamine prodrug and / or conjugate compositions, salts thereof, or a combination thereof that can reduce or prevent amphetamine side effects in a human subject, and methods to reduce or prevent amphetamine side effects in a human subject.
Owner:SHIRE PLC
Application of candesartan in preparing methamphetamine addiction drugs
InactiveCN104940196AEasy to learnEasy to rememberOrganic active ingredientsNervous disorderCandesartanInhibitory effect
The invention relates to the application of candesartan in preparing methamphetamine addiction drugs, wherein the role of candesartan in treating the rewarding effect of amphetamines addiction behavior incentives is studied. The result of the study shows that, candesartan facilitates the fading of the rewarding effect of the addiction behaviors of mice addicted to amphetamines, and also greatly improves the learning and remembering ability of mice addicted to amphetamines during the water maze training process. Meanwhile, candesartan is capable of obviously regulating the temperatures of mice after taking amphetamines, and inhibiting the methamphetamine rewarding incentives of mice. Moreover, candesartan obviously inhibits the methamphetamine-induced spontaneous activity behaviors of mice. That means, candesartan can be used for the withdrawal of methamphetamine addiction behaviors and the development of novel anti-toxicant drugs.
Owner:NINGBO UNIV
Polar Hydrophilic Prodrugs of Amphetamine and Other Stimulants and Processes for Making and Using the Same
InactiveUS20090239949A1Eliminate the effects ofReduce stressBiocideOrganic active ingredientsChemical ligationStimulant
Owner:SHIRE PLC
Amphetamine Prodrugs
InactiveUS20140073589A1Inhibition spikesIncrease plasma concentrationBiocideNervous disorderAmphetamine useBenzphetamine
Owner:SHIRE PLC
Method for treating aspects of alcoholism, addiction and psychosis
InactiveUS20050124963A1Reducing elevated amountHalogenated hydrocarbon active ingredientsInorganic active ingredientsDiseaseAlcoholisms
This application relates to a method for treating various aspects of alcoholism, including alcohol craving and relapse into alcoholism, by the administration of an effective amount of gaseous anaesthesia to a deep surgical anaesthesia. The method can be applied for the alleviation of addiction and craving associated with amphetamine use, and for the alleviation of psychosis in schizophrenia and the alleviation of psychosis in Alzheimer's disease. In addition, the state of addiction or the state of psychosis, or the state of alcohol withdrawal can be monitored in animal tissue in vitro or in humans in vivo by measuring the density of the high-affinity states of the dopamine D2 receptors in the striatum or other brains regions containing these receptors which are elevated in these conditions.
Owner:SEEMAN PHILIP
Drug for treating amphetamine-type exhilarant dependence syndrome and amphetamine-type exhilarant and opioid mixed dependence syndrome
ActiveCN101829328AGood effectControl withdrawal symptomsOrganic active ingredientsNervous disorderBenzodiazepineDrug withdrawal symptoms
The invention relates to a drug for treating amphetamine-type exhilarant dependence syndrome and amphetamine-type exhilarant and opioid mixed dependence syndrome, which comprises the following components in percent by weight: 2-95 percent of antipsychotics, 0.001-5 percent of a2 adrenergic receptor agonist, 0-5 percent of anticholinergic drug, 0-80 percent of non-opiates pain easing agent and 0-10 percent of benzodiazepines drugs. When used for treating symptoms occurring in patients abusing exhilarants of Benzedrine and the like or integrally abusing opioid, the drug achieves better effect, can rapidly and remarkably control abstinence syndrome, and has detoxification cure rate reaching over 90 percent.
Owner:卢正堂 +1
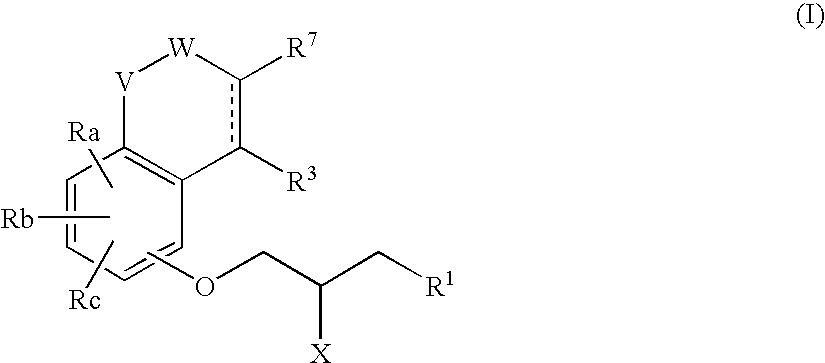
Phenoxypropylamine compounds
The present invention relates to a phenoxypropylamine compound of the formula (I)wherein each symbol is as defined in the specification, an optically active compound thereof or a pharmaceutically acceptable salt thereof and hydrates thereof, which simultaneously show selective affinity for and antagonistic activity against 5-HT1A receptor, as well as 5-HT reuptake inhibitory activity, and can be used as antidepressants quick in expressing an anti-depressive effect.
Owner:MITSUBISHI TANABE PHARMA CORP
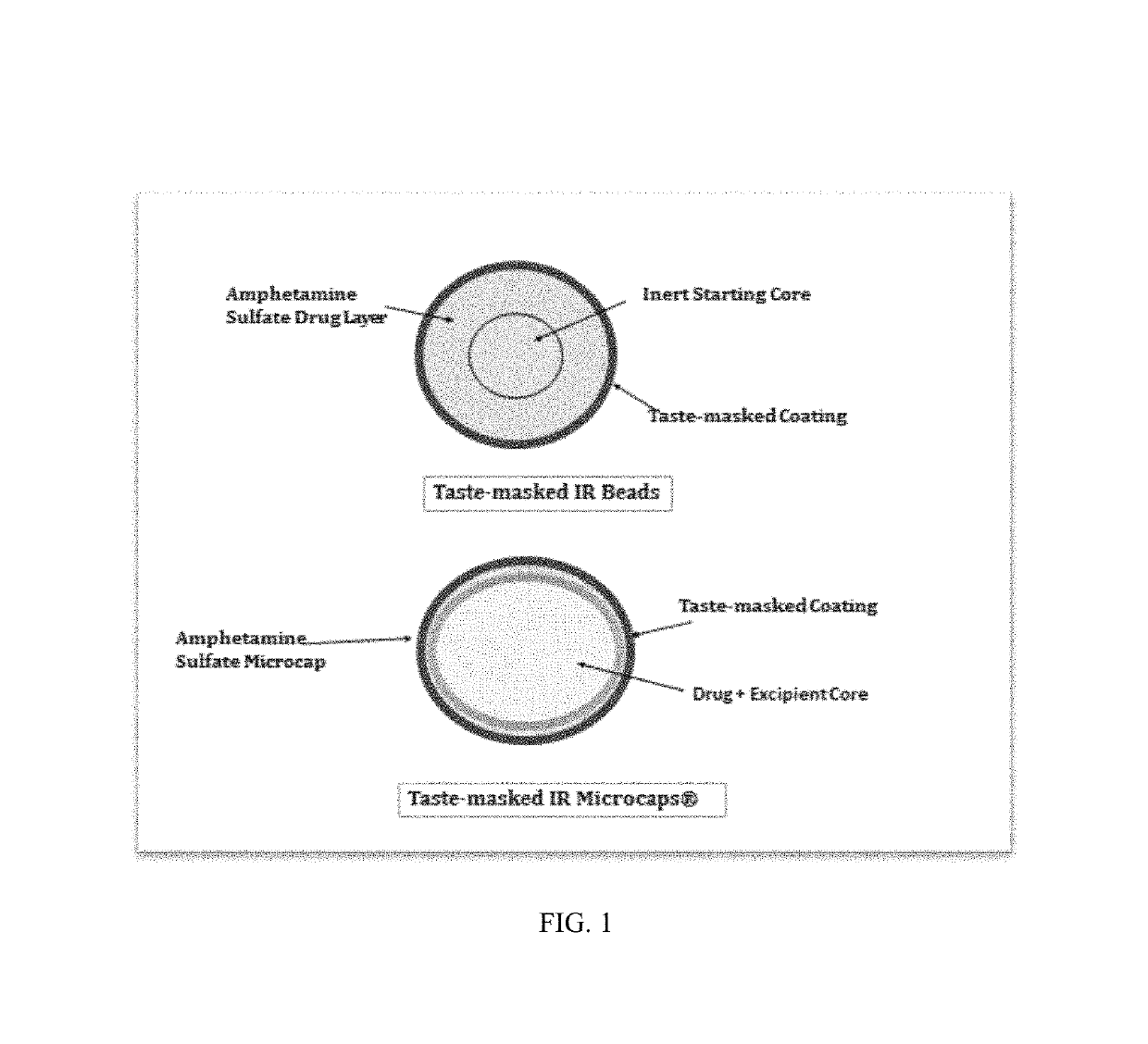
Oral amphetamine composition
ActiveUS10441554B2Oral administration is convenientRapid onsetOrganic active ingredientsPill deliveryAmphetamine SulfateWater soluble
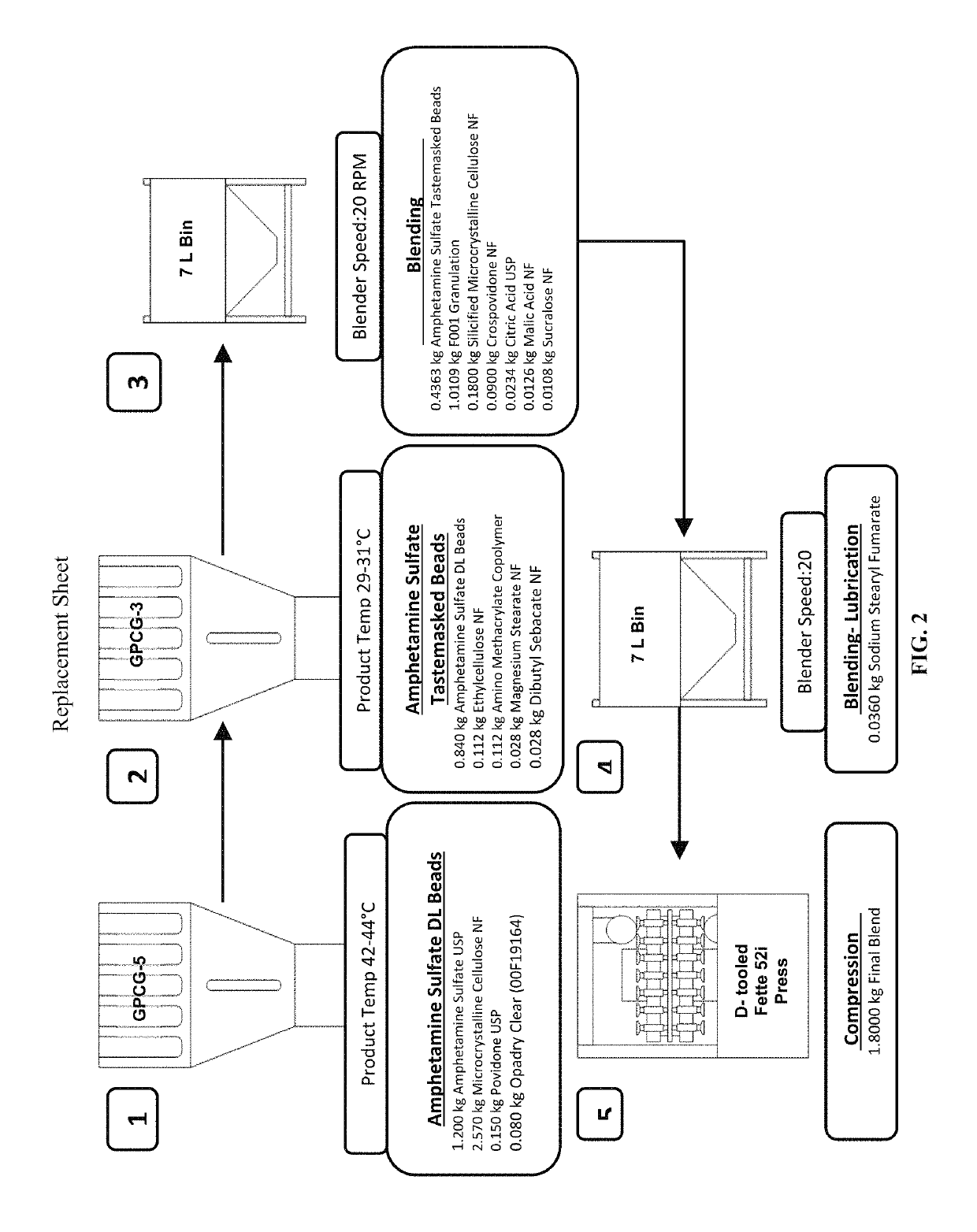
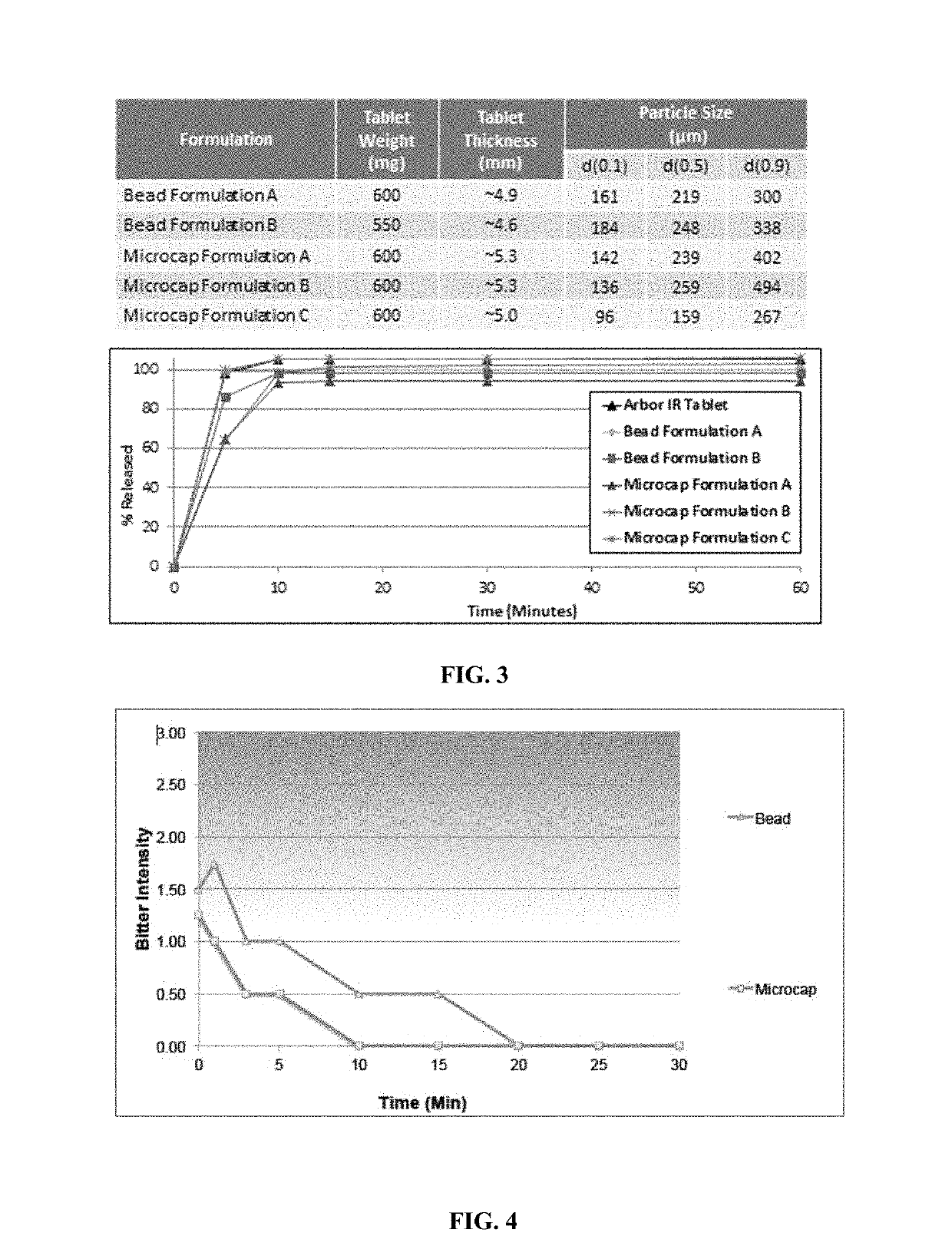
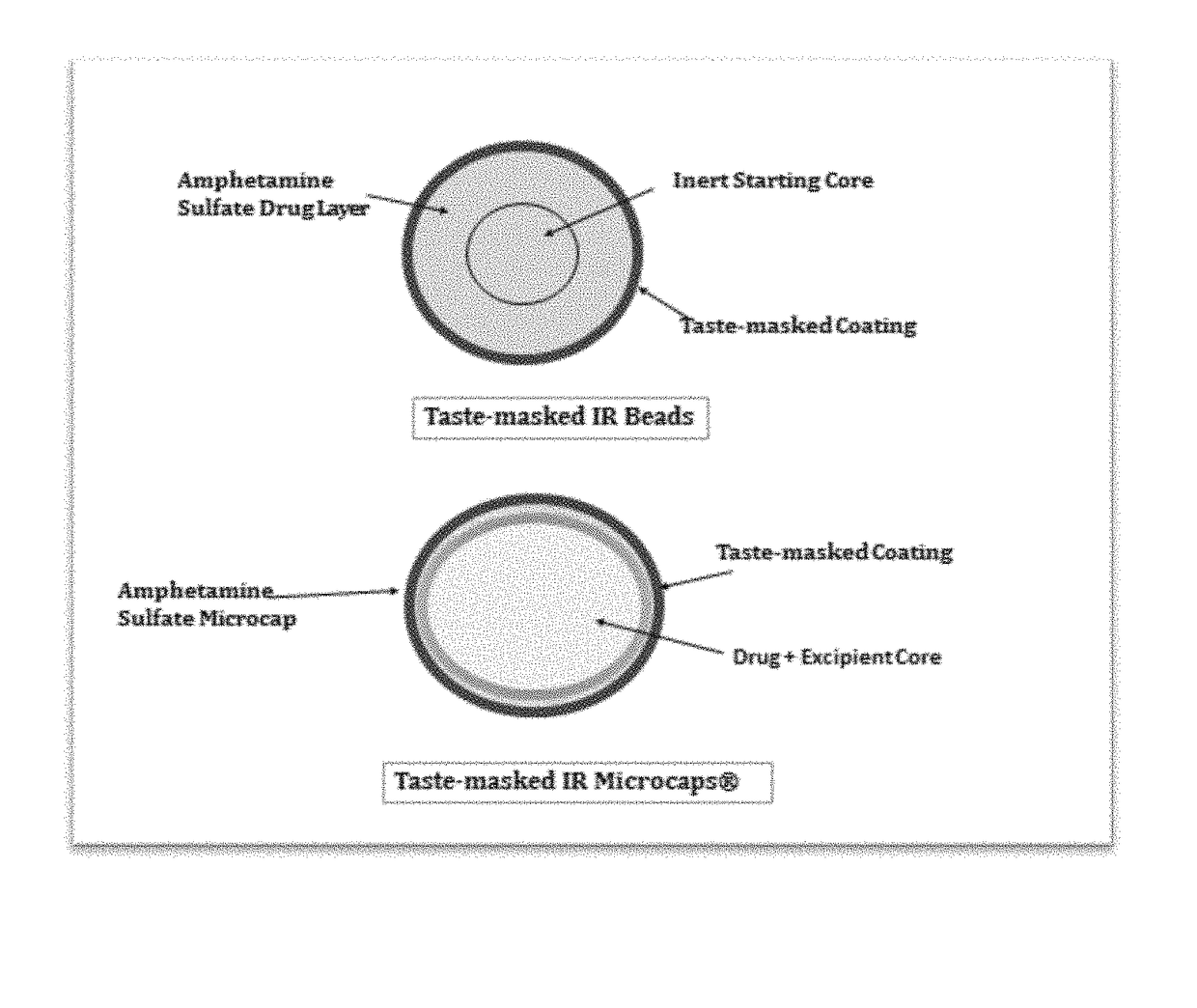
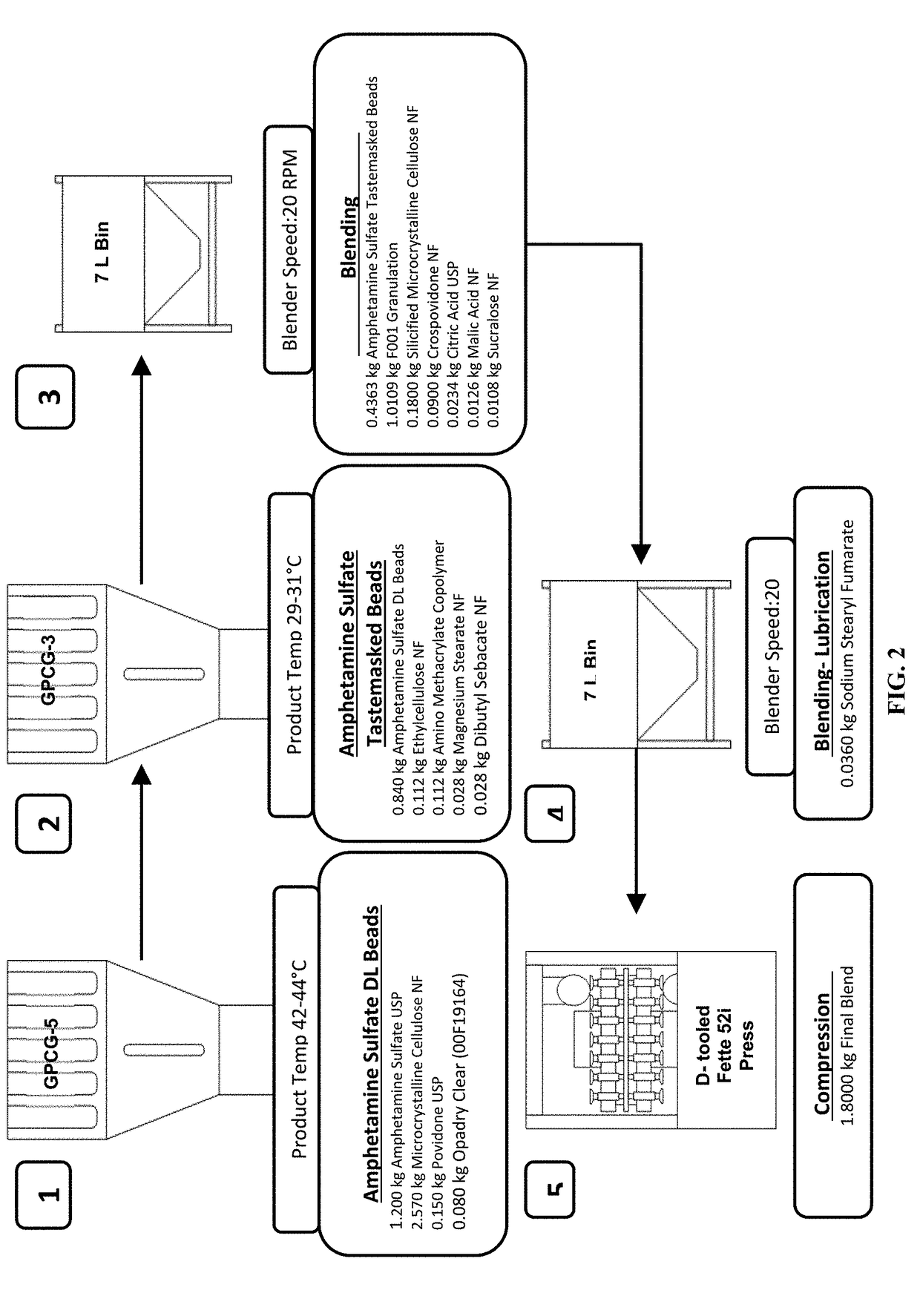
In various embodiments, the present invention is directed to oral pharmaceutical compositions. For example, in some embodiments, the present invention is directed to taste-masked compositions. In some embodiments, the taste masked compositions comprise a highly water soluble drug such as amphetamine, e.g., in the form of a salt such as amphetamine sulfate. In various embodiments, the present invention is directed to taste-masked, orally disintegrating compositions.
Owner:ADARE PHARM INC
Methods and compositions for preparation of amphetamine conjugates and salts thereof
ActiveUS8614346B2Reduce volatilityHigh enantiomeric purityCarbamic acid derivatives preparationOrganic compound preparationBenzphetamineAmphetamine use
The invention provides methods and compositions for preparing amphetamine conjugates, such as lisdexamfetamine, homoarginine-D-amphetamine, and salts thereof. In one embodiment, the invention provides methods of preparing an amphetamine conjugate from a chloramphetamine intermediate.
Owner:CAMBREX CHARLES CITY INC
Extract effective in treating drug addiction and preparation method therefor
ActiveUS20190151375A1Organic active ingredientsNervous disorderDrug withdrawal symptomsAcute toxicity testing
Disclosed are an extract that is effective in treating drug addiction and a preparation method therefor. An effective component of the extract has the following chemical structural characteristics: a cholestenol compound with hydroxyl (OH) at position 3 and a double bond between position 5 and position 6, or a cholestenol compound with hydroxyl (OH) at position 3, a double bond between position 5 and position 6 and a double bond between position 22 and position 23. The extract can be extracted from the traditional Chinese medicine Agriolima agrestis. The extract of the present invention is safe in acute toxicity, sedative and hypnotic without physical and psychic dependence, has an inhibitory effect on excitability in mouse caused by morphine and benzedrine and a detoxification treatment effect on the withdrawal symptoms in morphine-dependent rats, and is useful in the development of medications or food for treating drug addiction.
Owner:GUANGXI JIUFU BIOTECH CO LTD
Assays for amphetamine and methamphetamine using stereospecific reagents
Methods, compositions and kits are disclosed. Enzyme conjugates of Formula I may be employed in assays for the determination of an amphetamine and / or a methamphetamine. Immunogenic conjugates of Formula I may be employed to prepare antibodies for an amphetamine and / or for a methamphetamine for use in assays for the determination of an amphetamine and / or a methamphetamine. The enzyme conjugates may also be employed to screen antibodies for use in such methods.
Owner:SIEMENS HEALTHCARE DIAGNOSTICS INC
Oral amphetamine composition
ActiveUS20180311187A1Oral administration is convenientRapid onsetOrganic active ingredientsPharmaceutical non-active ingredientsAmphetamine SulfateMedicine
In various embodiments, the present invention is directed to oral pharmaceutical compositions. For example, in some embodiments, the present invention is directed to taste-masked compositions. In some embodiments, the taste masked compositions comprise a highly water soluble drug such as amphetamine, e.g., in the form of a salt such as amphetamine sulfate. In various embodiments, the present invention is directed to taste-masked, orally disintegrating compositions.
Owner:ADARE PHARM INC
Extended release suspensions of mixtures of dextro- and levo-amphetamines
InactiveUS20190183817A1Eliminating complex and lengthy coating processGood masking effectPowder deliveryOrganic active ingredientsIn vivoBlood plasma
The present invention relates to the pharmaceutical compositions of a mixture of dextro- and levo-amphetamines complexed with ion exchange resin and precursor resins. More particularly the invention relates to Attention Deficit Hyperactivity Disorder (ADHD) effective agent dosage forms that both facilitate oral ingestion and provide an effective treatment over a prolonged period oftime. In particular, the invention provides for pharmaceutical compositions having a first and second plurality of particles, where the first plurality of particles comprises drug-resin particles and the second plurality of particles comprises drug-precursor resin particles with or without any extended release coating, where the drug is an ADHD effective agent and the composition achieves an escalating in vivo plasma concentration of the ADHD effective agents selected from amphetamine and methylphenidate.
Owner:LUPIN INC
Abuse-resistant amphetamine prodrugs
InactiveUS20090131476A1Promote absorptionQuick eliminationBiocideNervous disorderDiseaseAlternative treatment
The invention describes compounds, compositions, and methods of using the same comprising a chemical moiety covalently attached to amphetamine. These compounds and compositions are useful for reducing or preventing abuse and overdose of amphetamine. These compounds and compositions find particular use in providing an abuse-resistant alternative treatment for certain disorders, such as attention deficit hyperactivity disorder (ADHD), ADD, narcolepsy, and obesity. Oral bioavailability of amphetamine is maintained at therapeutically useful doses. At higher doses bioavailability is substantially reduced, thereby providing a method of reducing oral abuse liability. Further, compounds and compositions of the invention decrease the bioavailability of amphetamine by parenteral routes, such as intravenous or intranasal administration, further limiting their abuse liability.
Owner:SHIRE PLC
Abuse-resistant amphetamine prodrugs
InactiveUS20090124831A1Promote absorptionQuick eliminationOrganic active ingredientsNervous disorderChemical MoietyAlternative treatment
The invention describes compounds, compositions, and methods of using the same comprising a chemical moiety covalently attached to amphetamine. These compounds and compositions are useful for reducing or preventing abuse and overdose of amphetamine. These compounds and compositions find particular use in providing an abuse-resistant alternative treatment for certain disorders, such as attention deficit hyperactivity disorder (ADHD), ADD, narcolepsy, and obesity. Oral bioavailability of amphetamine is maintained at therapeutically useful doses. At higher doses bioavailability is substantially reduced, thereby providing a method of reducing oral abuse liability. Further, compounds and compositions of the invention decrease the bioavailability of amphetamine by parenteral routes, such as intravenous or intranasal administration, further limiting their abuse liability.
Owner:TAKEDA PHARMA CO LTD
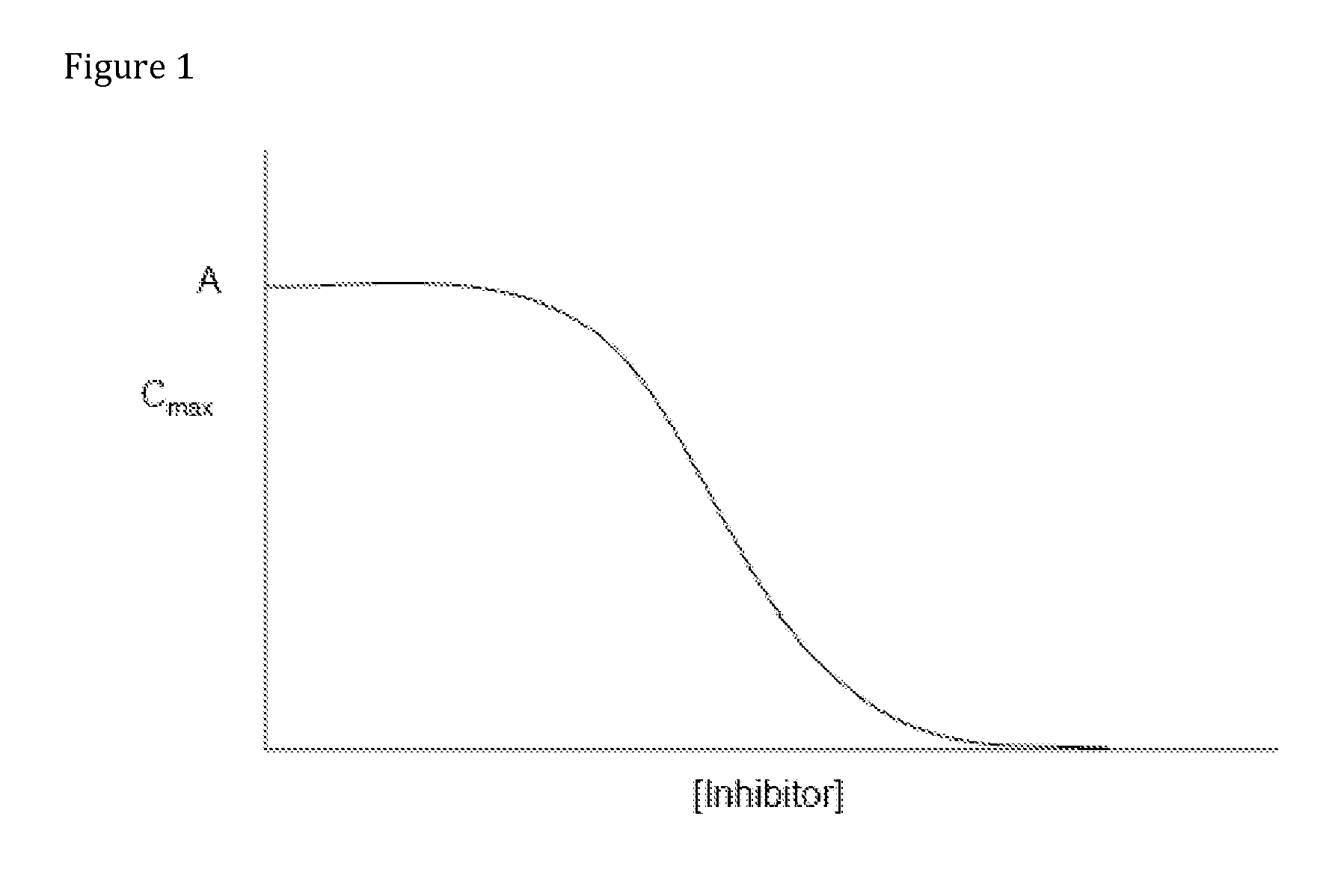
Compositions Comprising Enzyme-Cleavable Amphetamine Prodrugs and Inhibitors Thereof
ActiveUS20160235694A1Eliminate side effectsPatient compliance is goodOrganic chemistryPharmaceutical delivery mechanismControlled releaseAmphetamine
Pharmaceutical compositions and their methods of use are provided, where the pharmaceutical compositions comprise an amphetamine prodrug that provides enzymatically-controlled release of amphetamine or an amphetamine analog. The composition can further comprise an enzyme inhibitor that interacts with the enzyme(s) that mediates the enzymatically-controlled release of amphetamine or the amphetamine analog from the amphetamine prodrug so as to attenuate enzymatic cleavage of the amphetamine prodrug.
Owner:SIGNATURE THERAPEUTICS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com