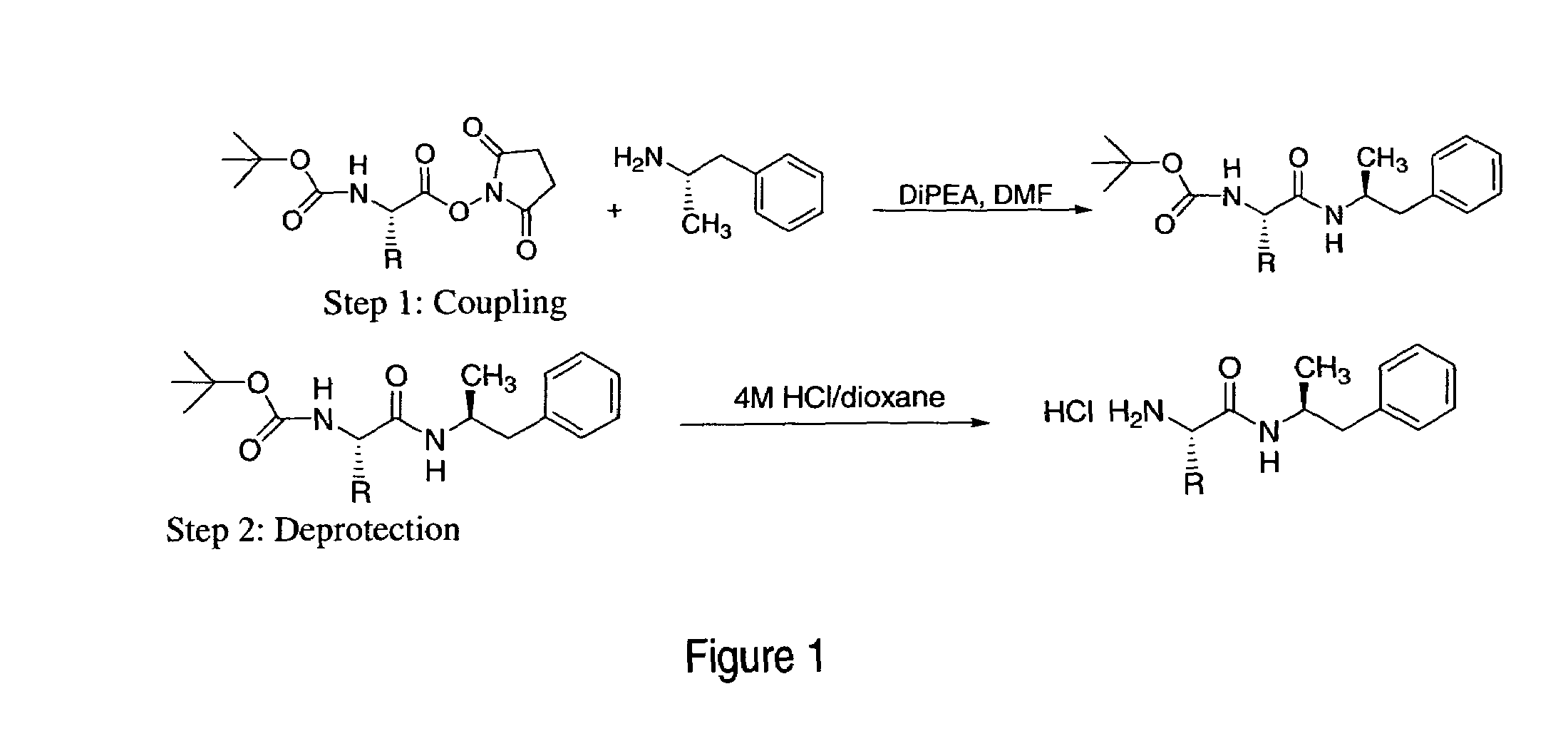
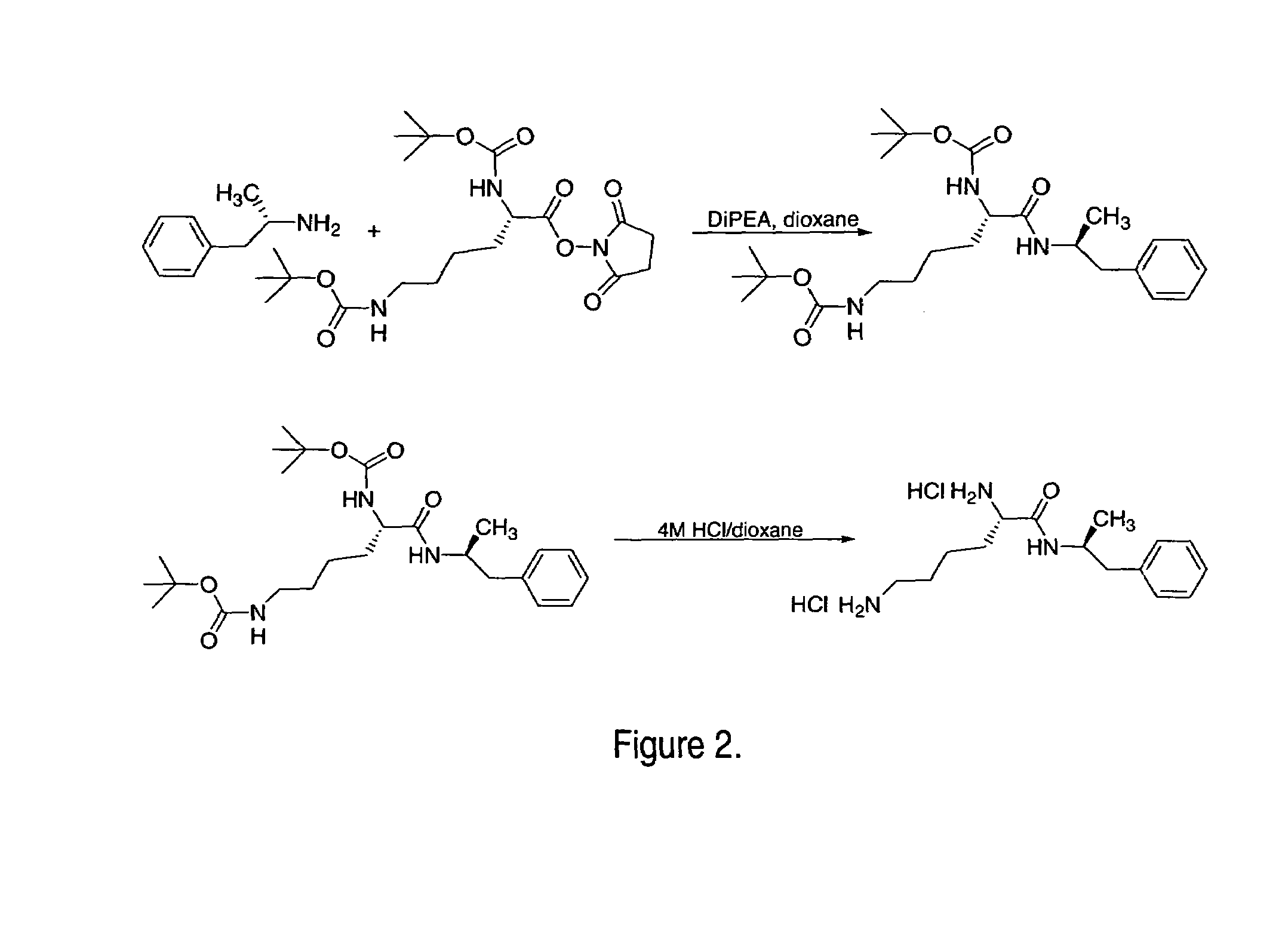
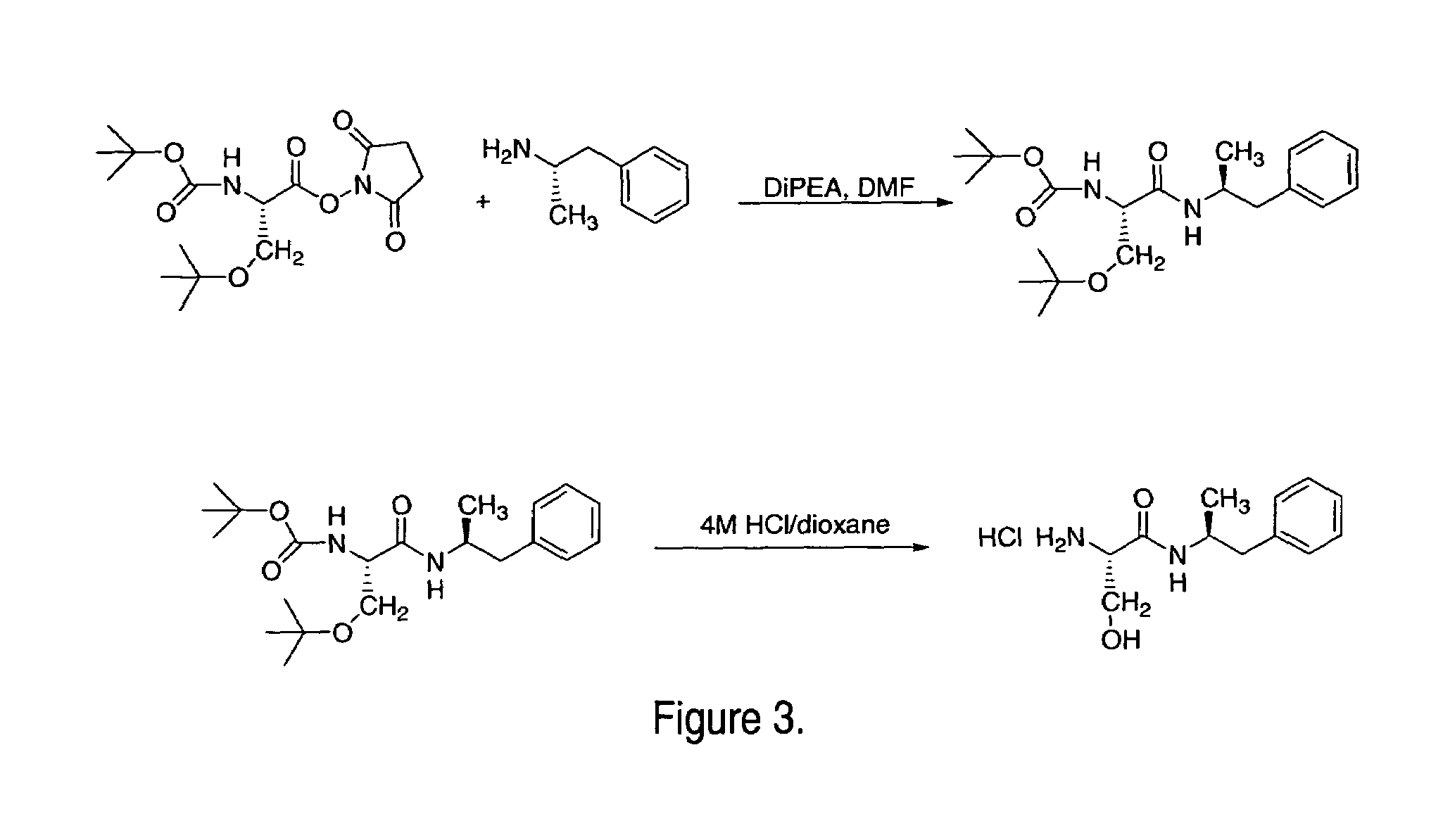
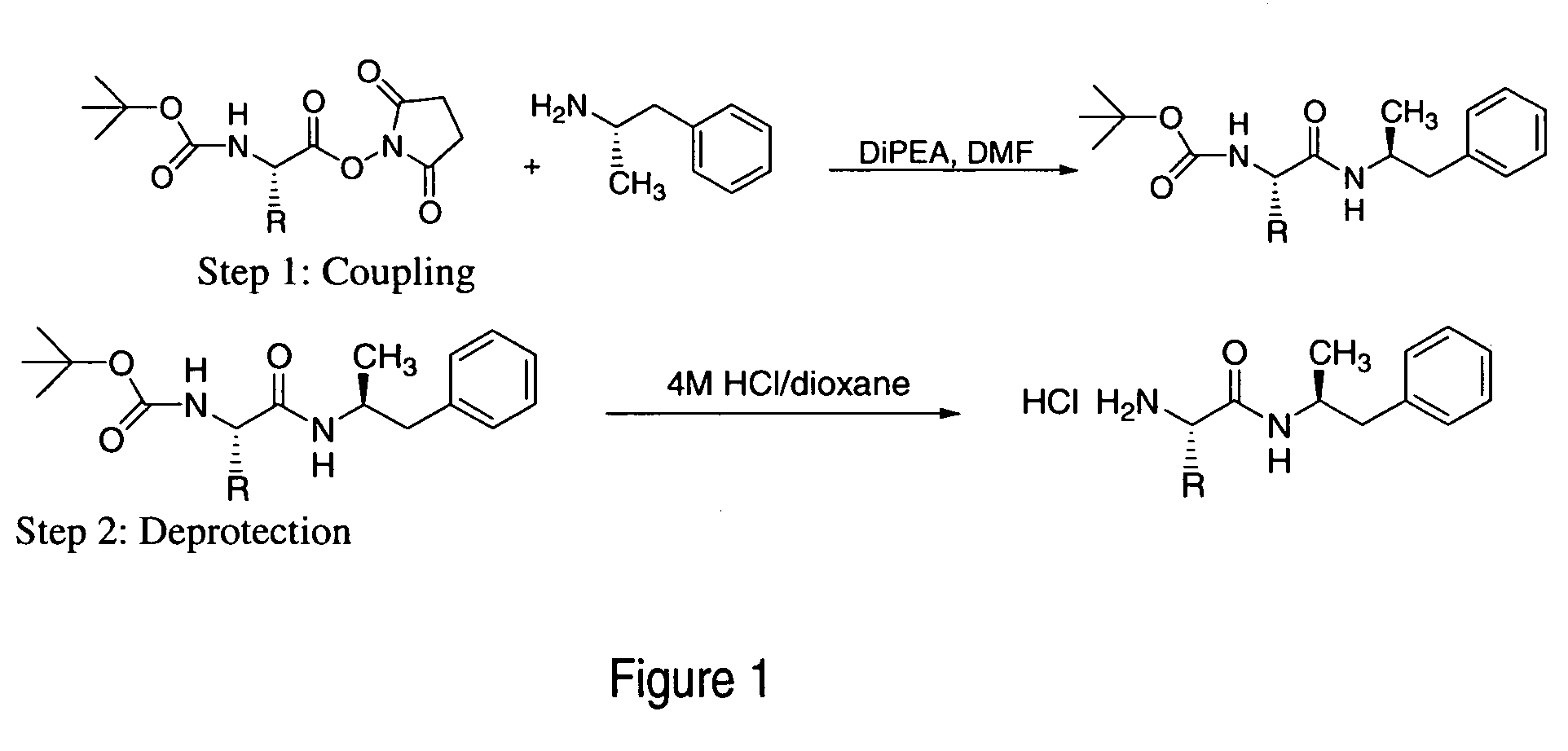
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
49 results about "Parenteral route" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
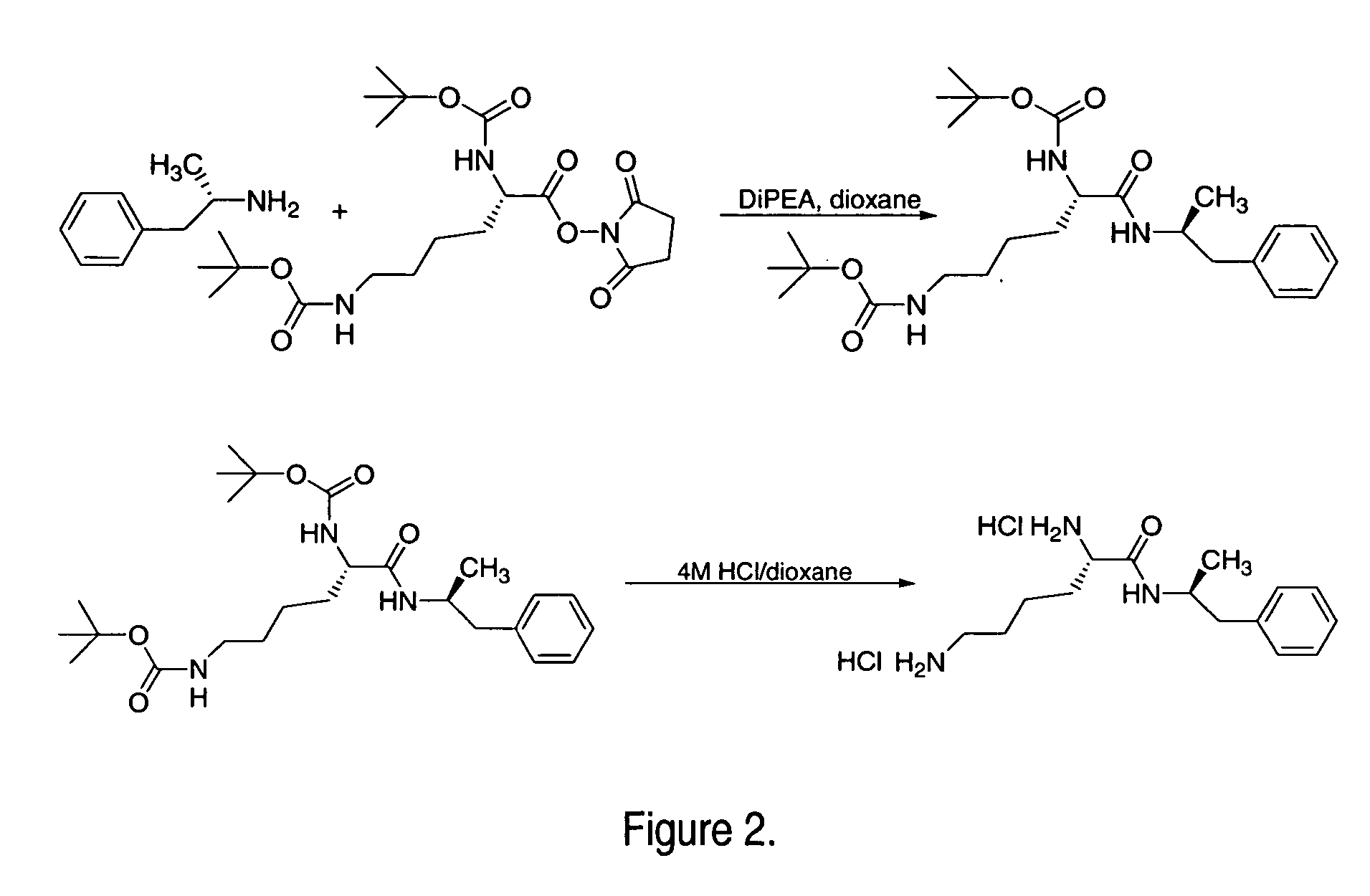
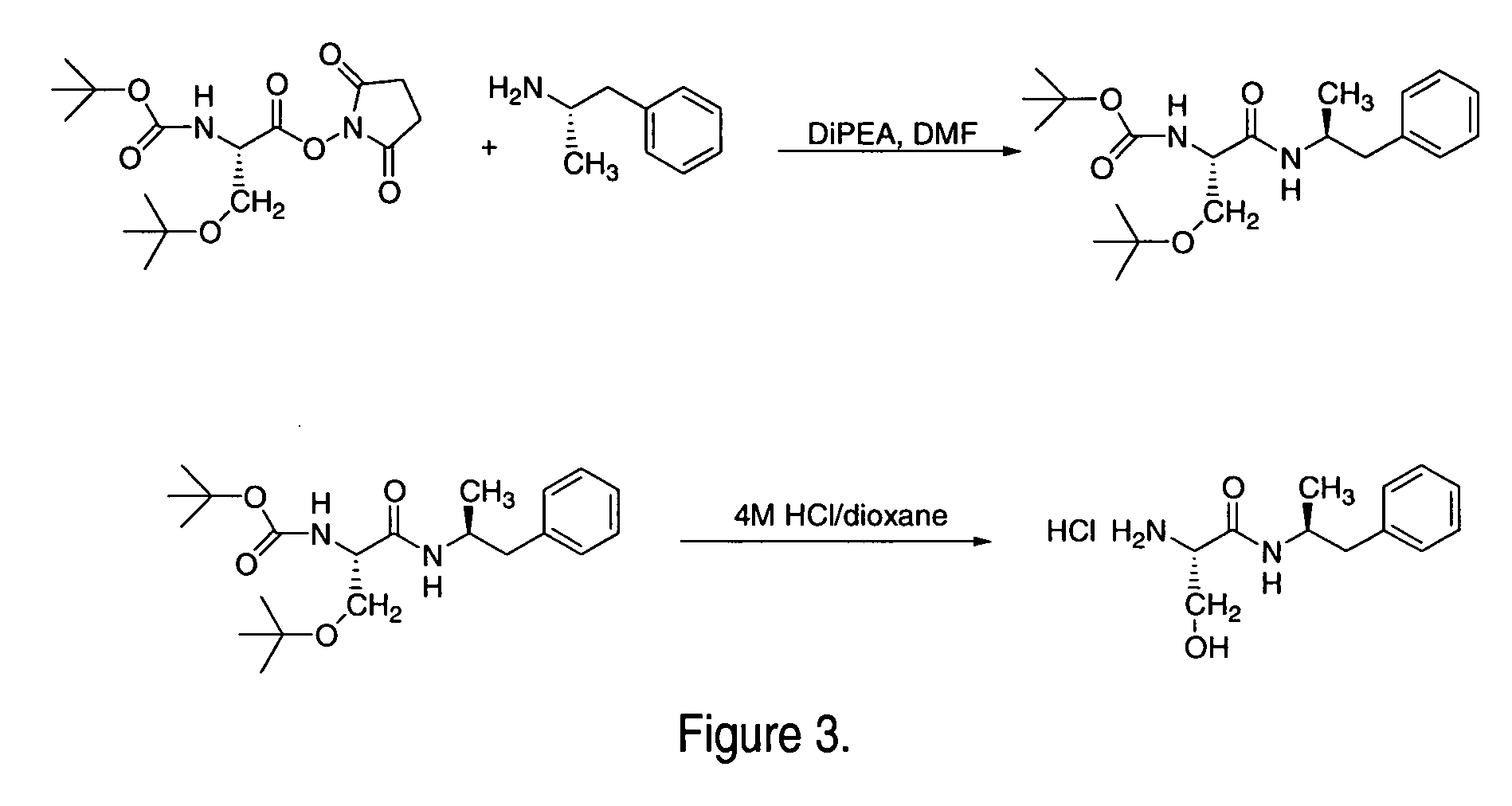
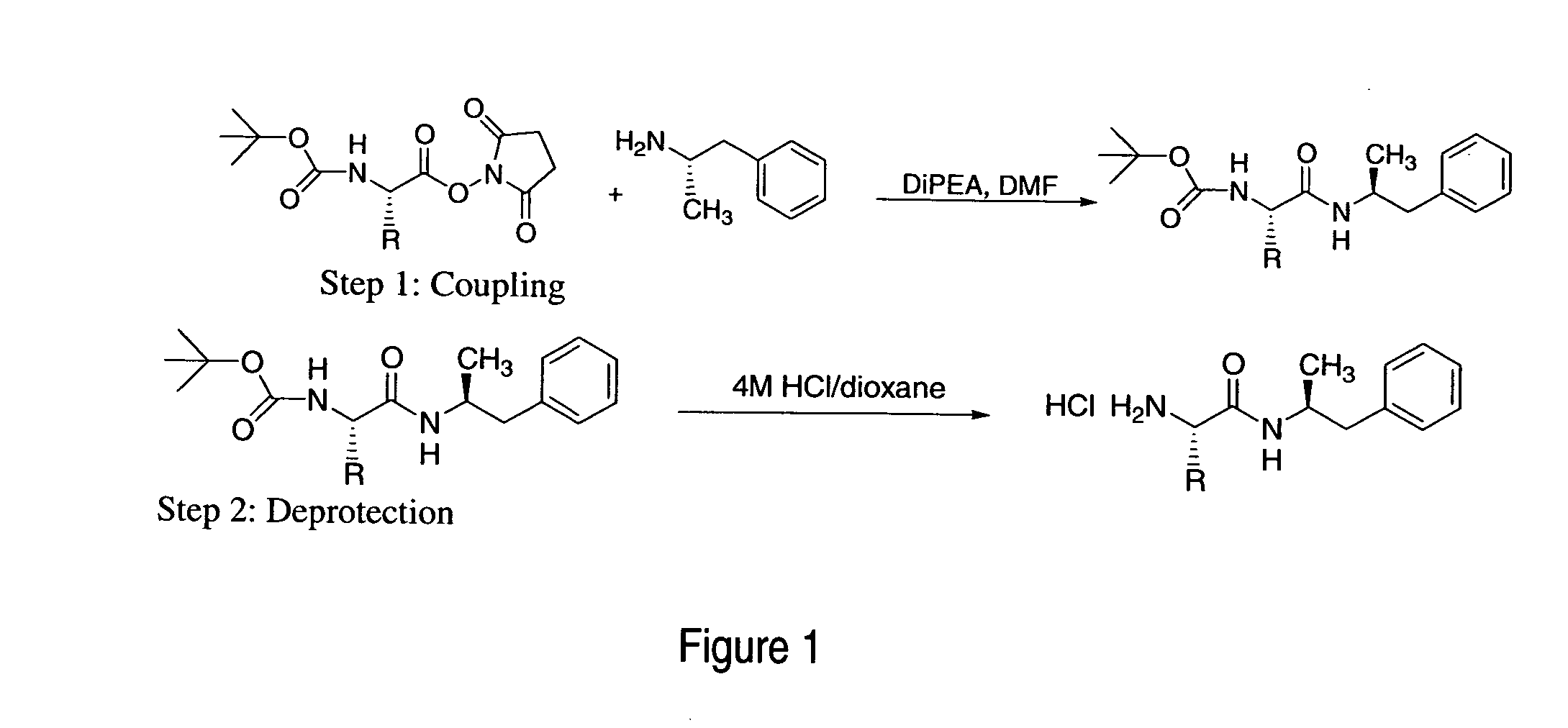
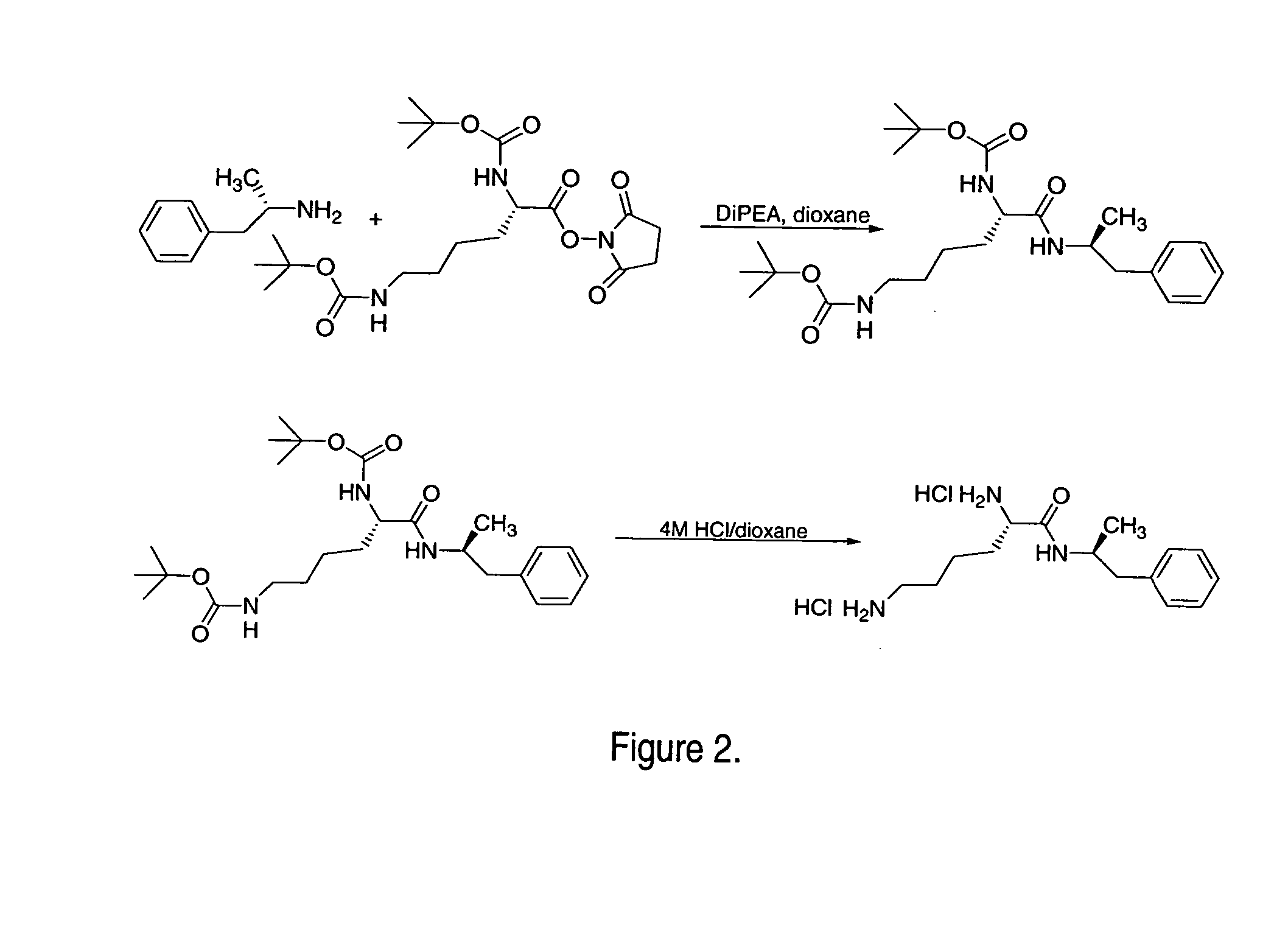
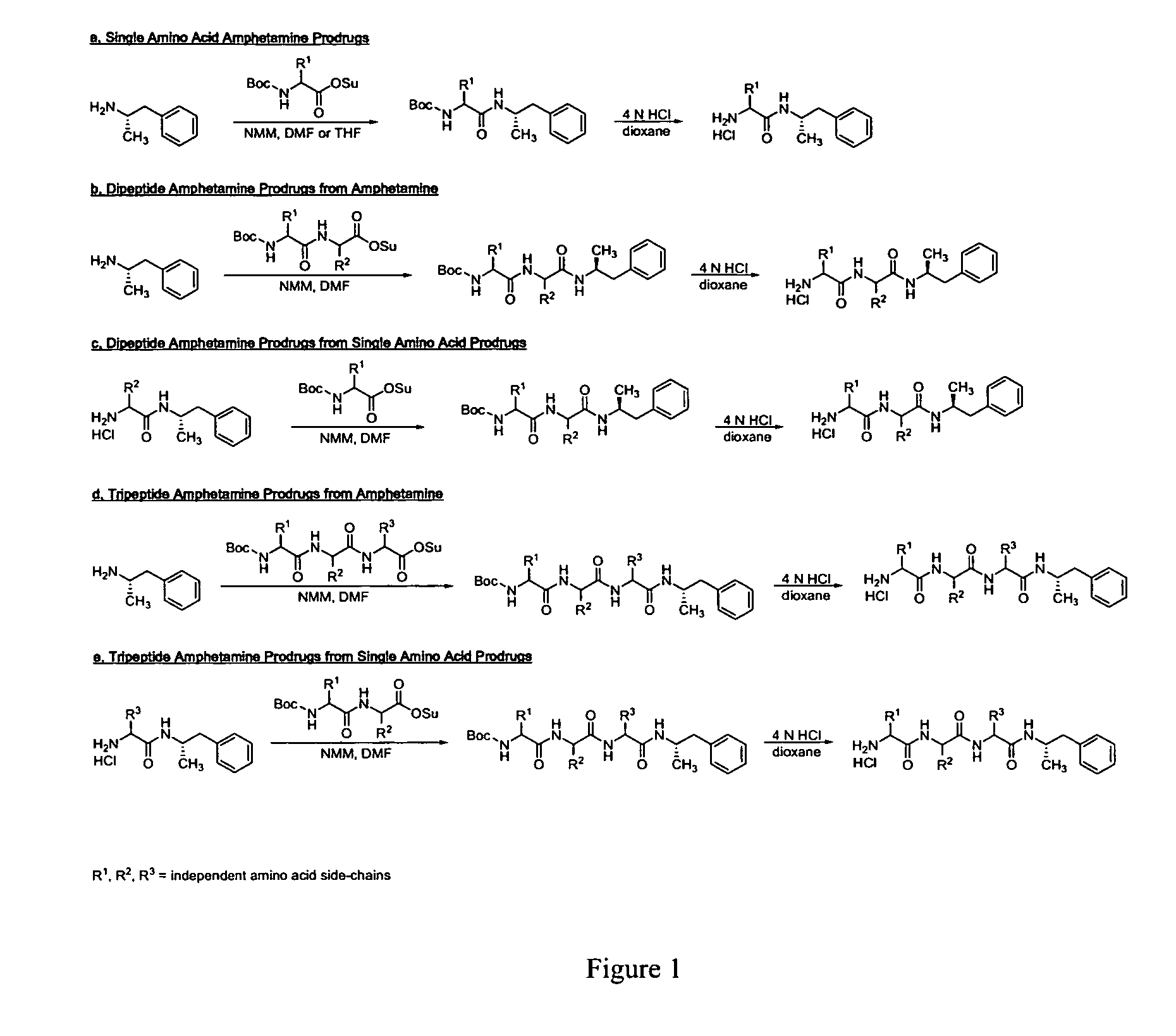
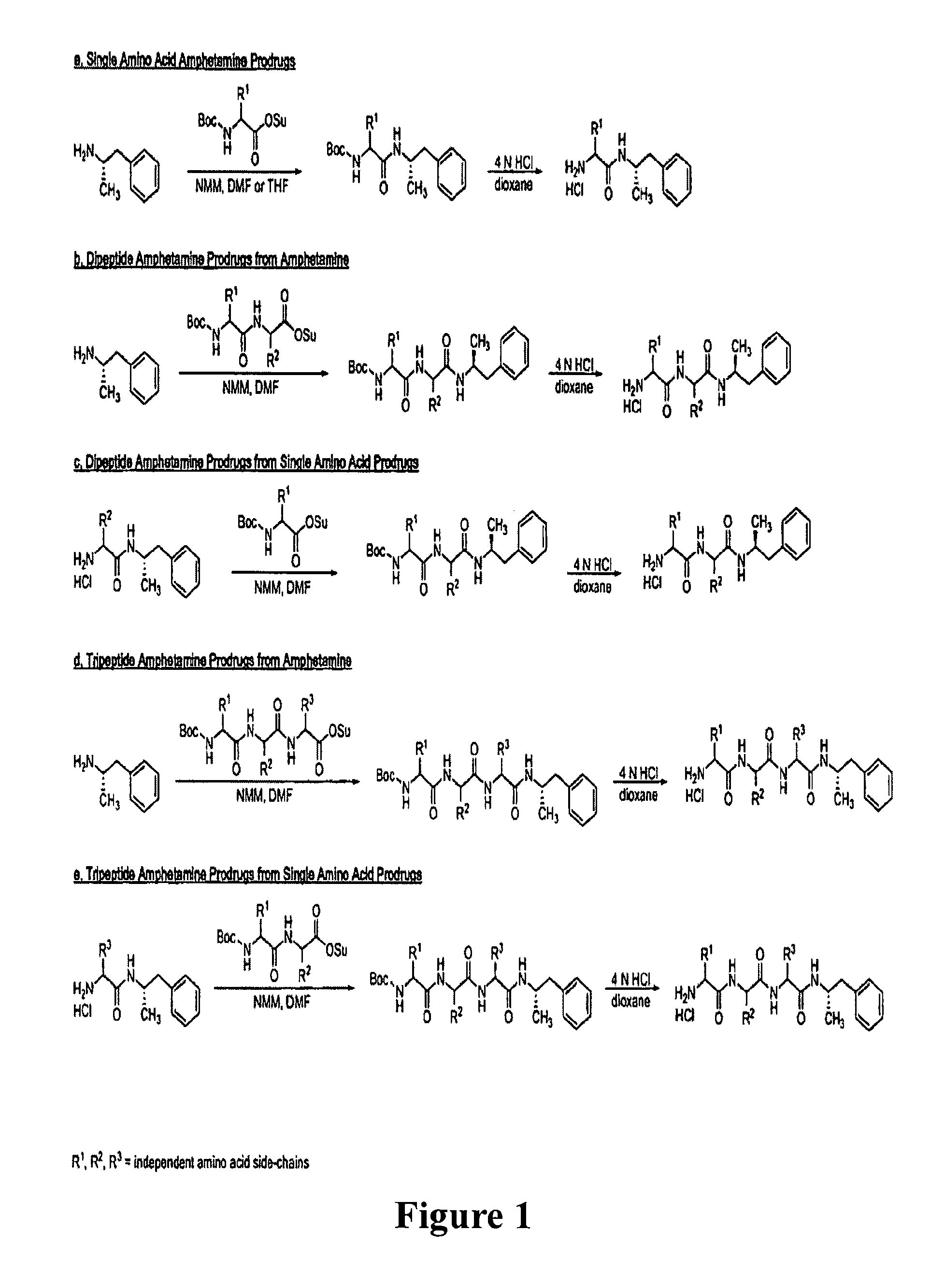
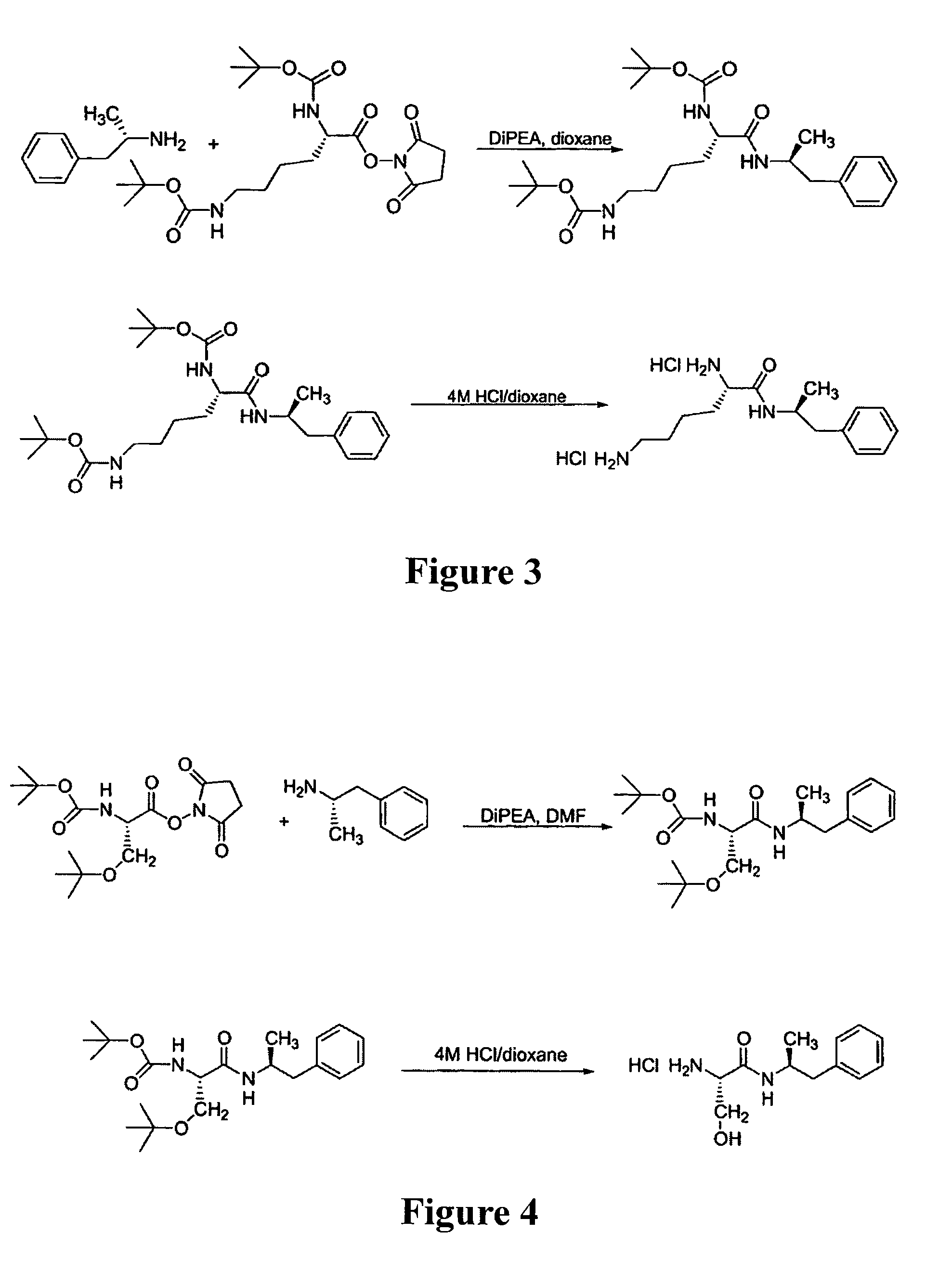
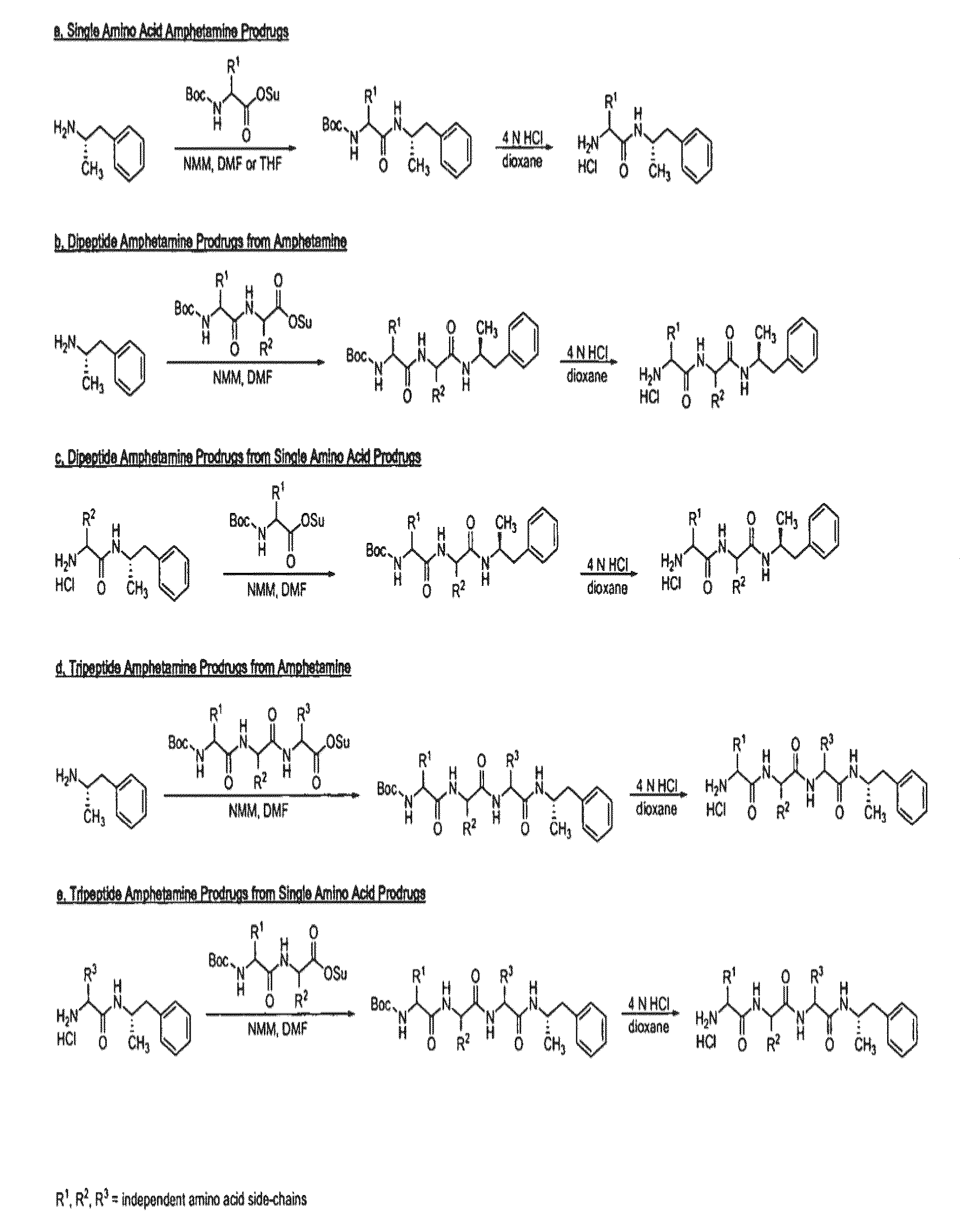
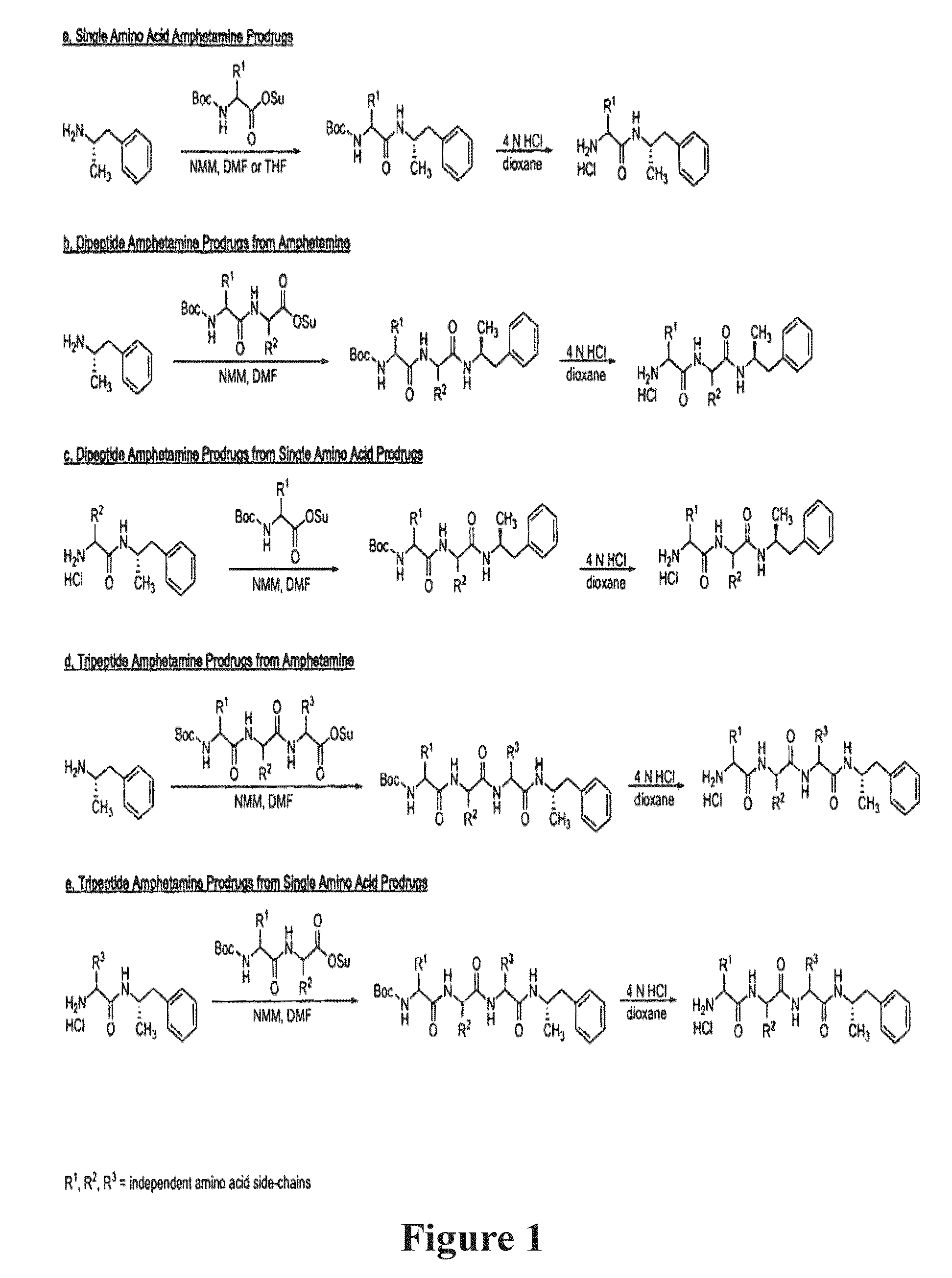
Abuse-resistant amphetamine compounds
InactiveUS7105486B2Reduced activityRelease is diminished and eliminatedOrganic active ingredientsPeptide/protein ingredientsChemical MoietyDisease
The invention describes compounds, compositions and methods of using the same comprising a chemical moiety covalently attached to amphetamine. These compounds and compositions are useful for reducing or preventing abuse and overdose of amphetamine. These compounds and compositions find particular use in providing an abuse-resistant alternative treatment for certain disorders, such as attention deficit hyperactivity disorder (ADHD), ADD, narcolepsy, and obesity. Oral bioavailability of amphetamine is maintained at therapeutically useful doses. At higher doses bioavailability is substantially reduced, thereby providing a method of reducing oral abuse liability. Further, compounds and compositions of the invention decrease the bioavailability of amphetamine by parenteral routes, such as intravenous or intranasal administration, further limiting their abuse liability.
Owner:TAKEDA PHARMA CO LTD
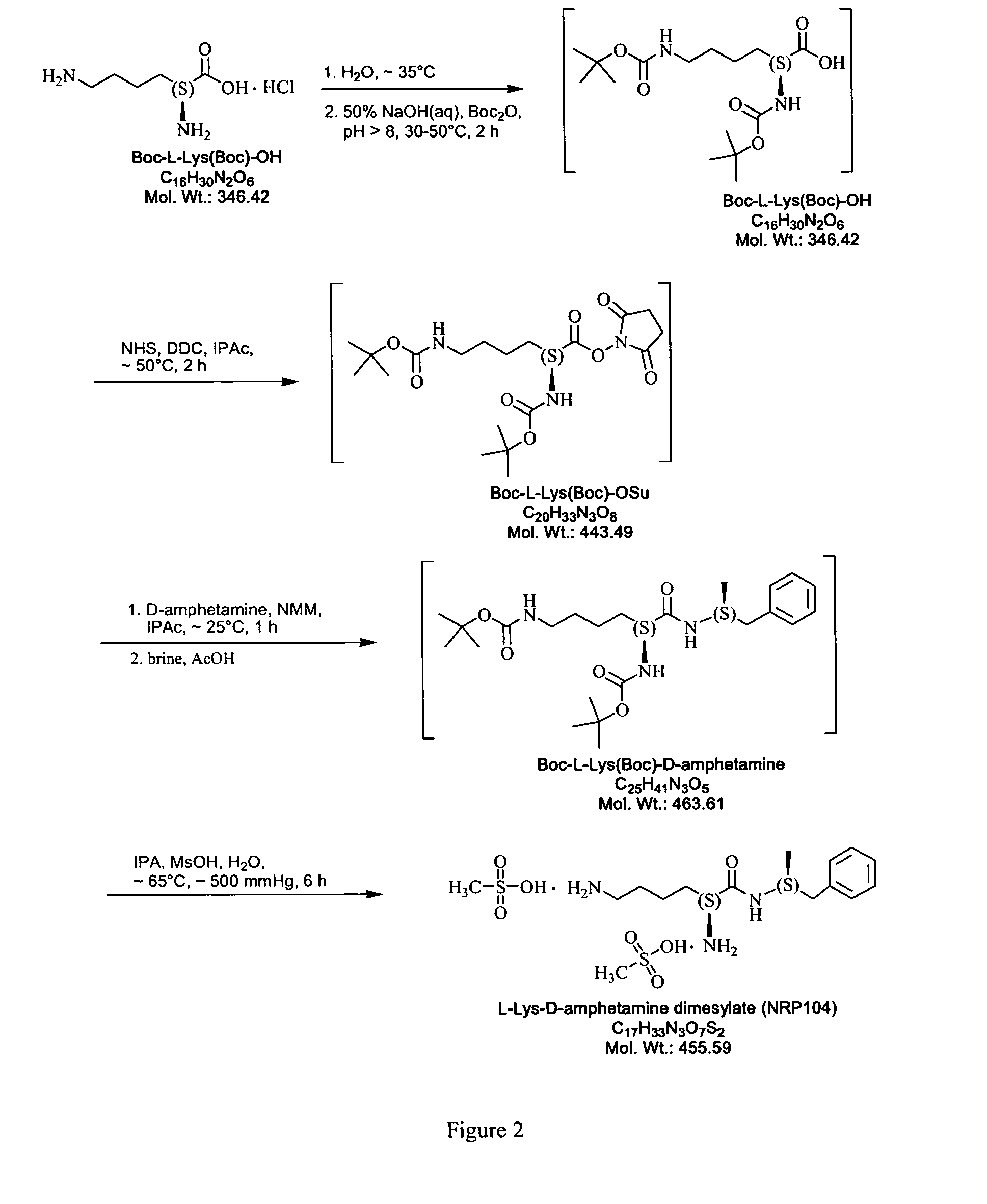
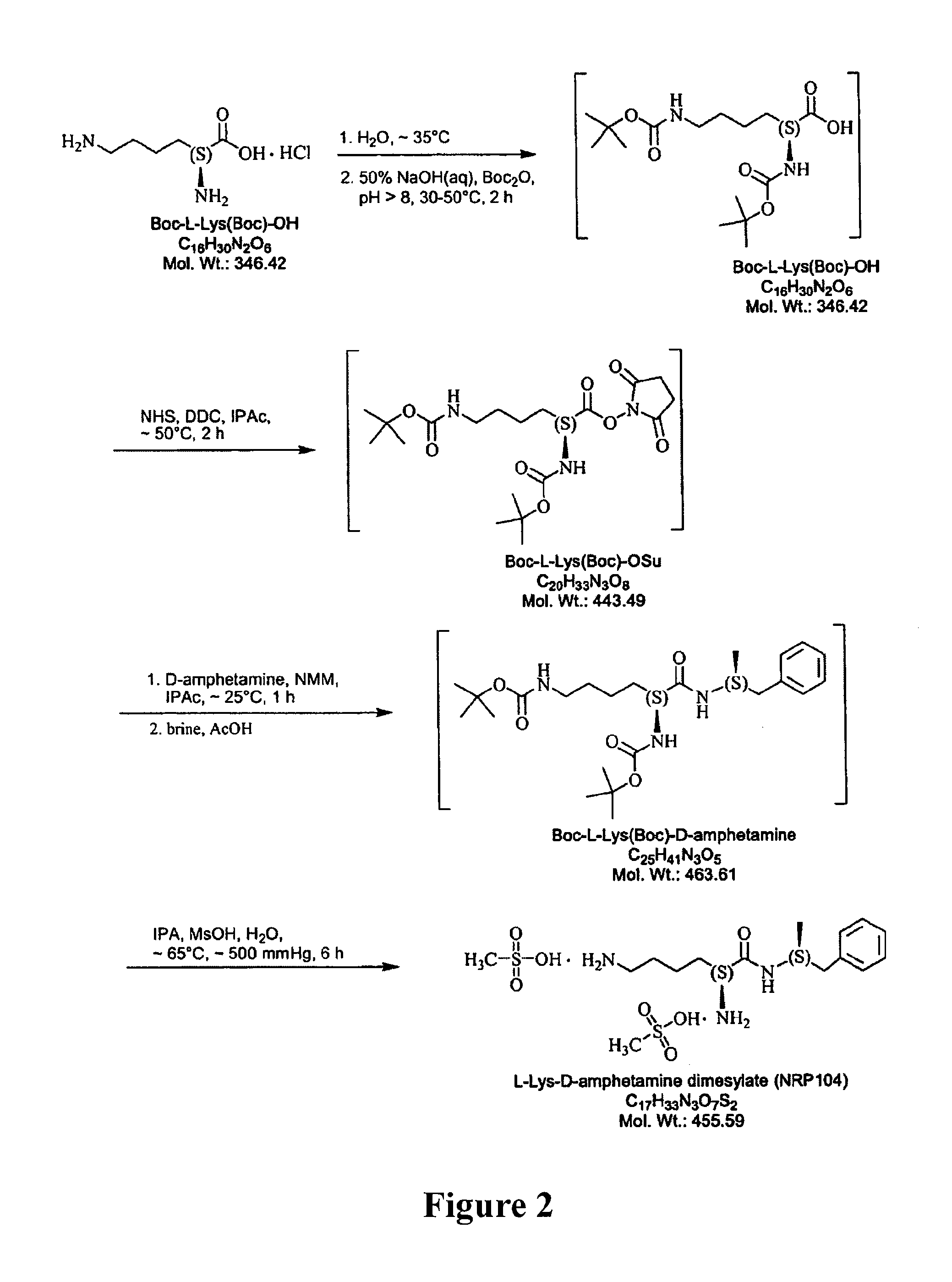
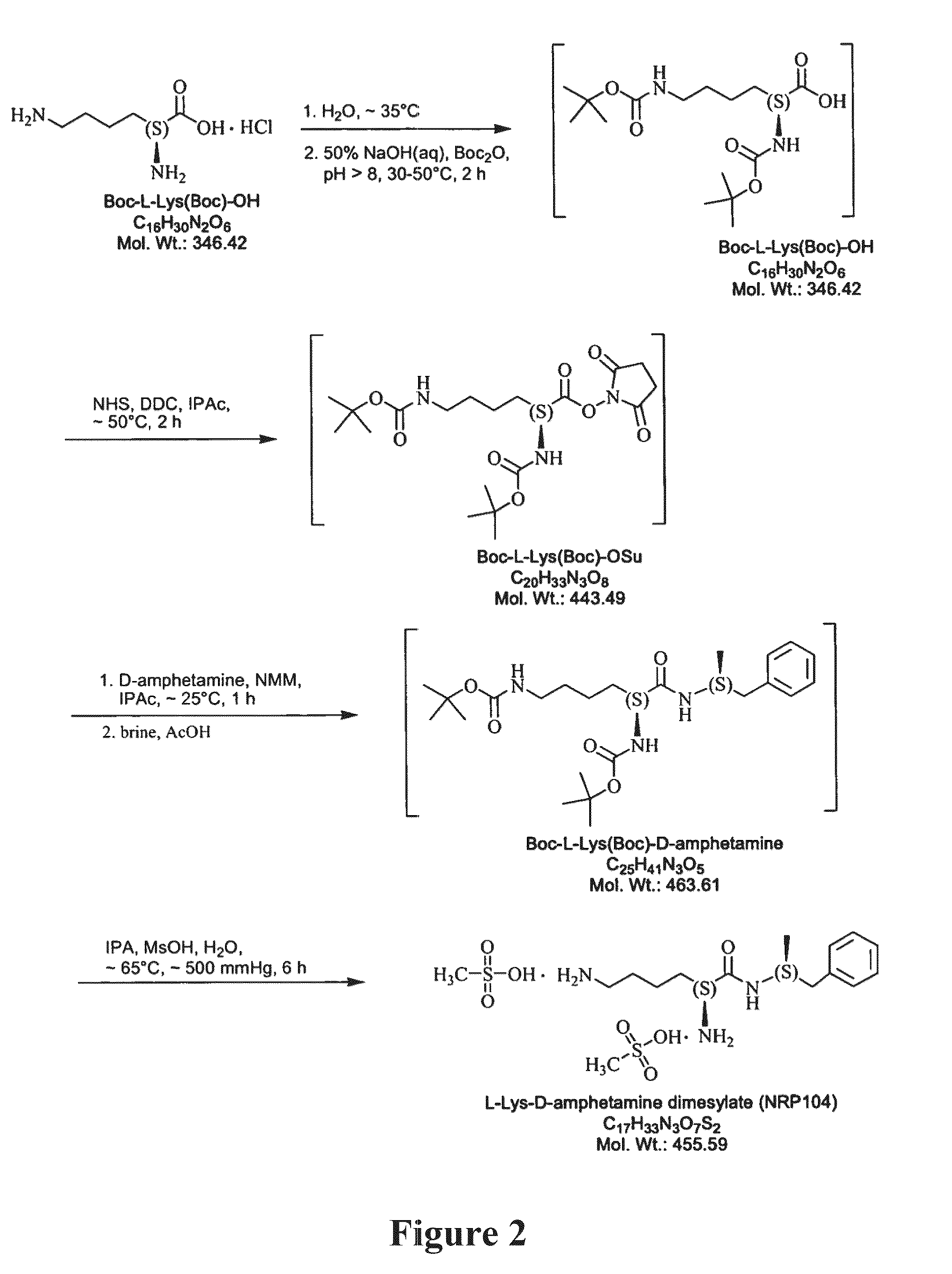
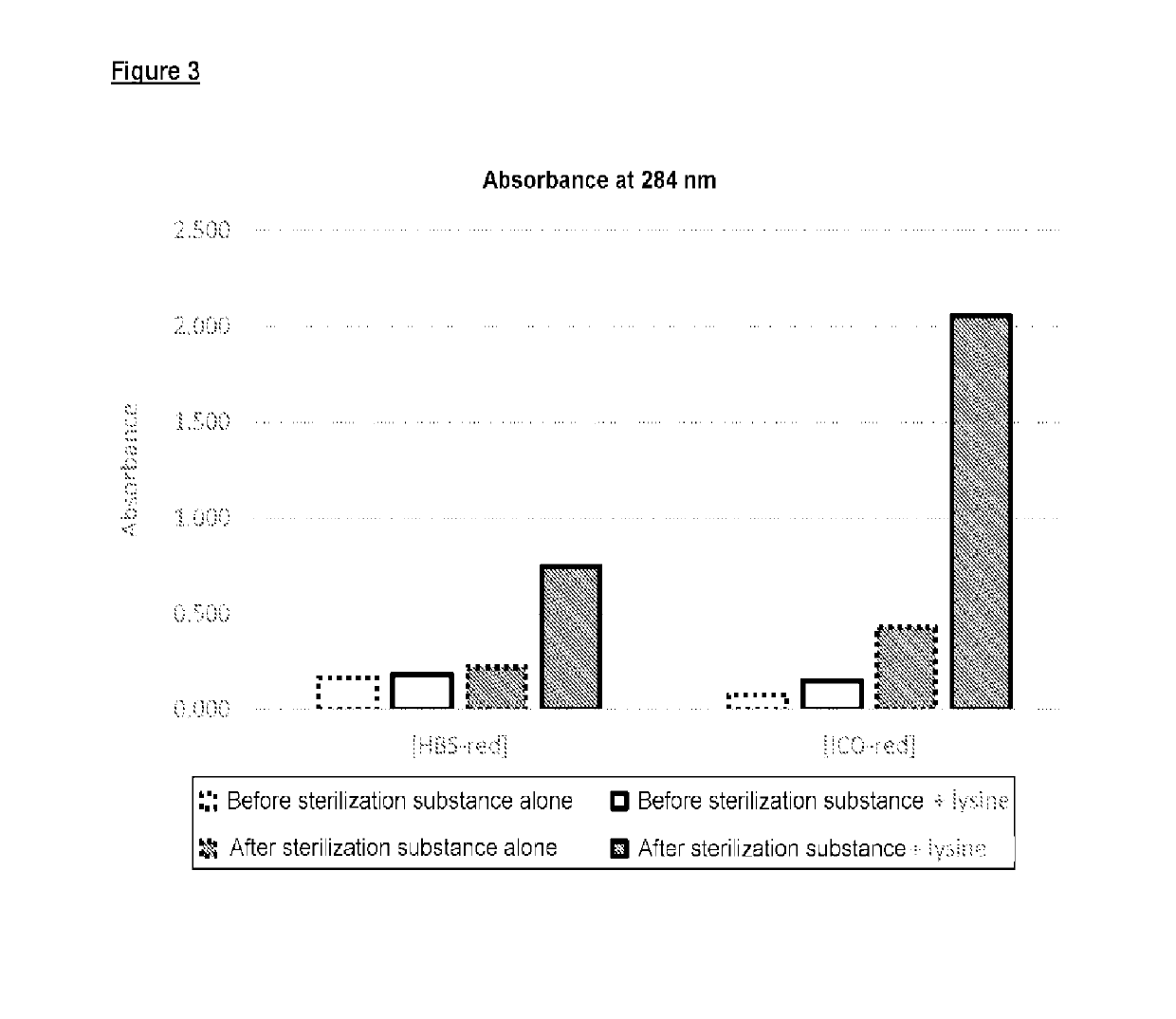
Abuse resistant lysine amphetamine compounds
ActiveUS20050038121A1Prevents euphoriaLower potentialOrganic active ingredientsBiocideDiseaseAlternative treatment
The present invention describes compounds, compositions and methods of using the same comprising lysine covalently attached to amphetamine. These compounds and compositions are useful for reducing or preventing abuse and overdose of amphetamine. These compounds and compositions find particular use in providing an abuse-resistant alternative treatment for certain disorders, such as attention deficit hyperactivity disorder (ADHD), ADD, narcolepsy, and obesity. Oral bioavailability of amphetamine is maintained at therapeutically useful doses. At higher doses bioavailability is substantially reduced, thereby providing a method of reducing oral abuse liability. Further, compounds and compositions of the invention decrease the bioavailability of amphetamine by parenteral routes, such as intravenous or intranasal administration, further limiting their abuse liability.
Owner:TAKEDA PHARMA CO LTD
Abuse-resistant amphetamine compounds
InactiveUS20050054561A1Prevents euphoriaLower potentialBiocidePeptide/protein ingredientsChemical MoietyDisease
The invention describes compounds, compositions and methods of using the same comprising a chemical moiety covalently attached to amphetamine. These compounds and compositions are useful for reducing or preventing abuse and overdose of amphetamine. These compounds and compositions find particular use in providing an abuse-resistant alternative treatment for certain disorders, such as attention deficit hyperactivity disorder (ADHD), ADD, narcolepsy, and obesity. Oral bioavailability of amphetamine is maintained at therapeutically useful doses. At higher doses bioavailability is substantially reduced, thereby providing a method of reducing oral abuse liability. Further, compounds and compositions of the invention decrease the bioavailability of amphetamine by parenteral routes, such as intravenous or intranasal administration, further limiting their abuse liability.
Owner:TAKEDA PHARMA CO LTD
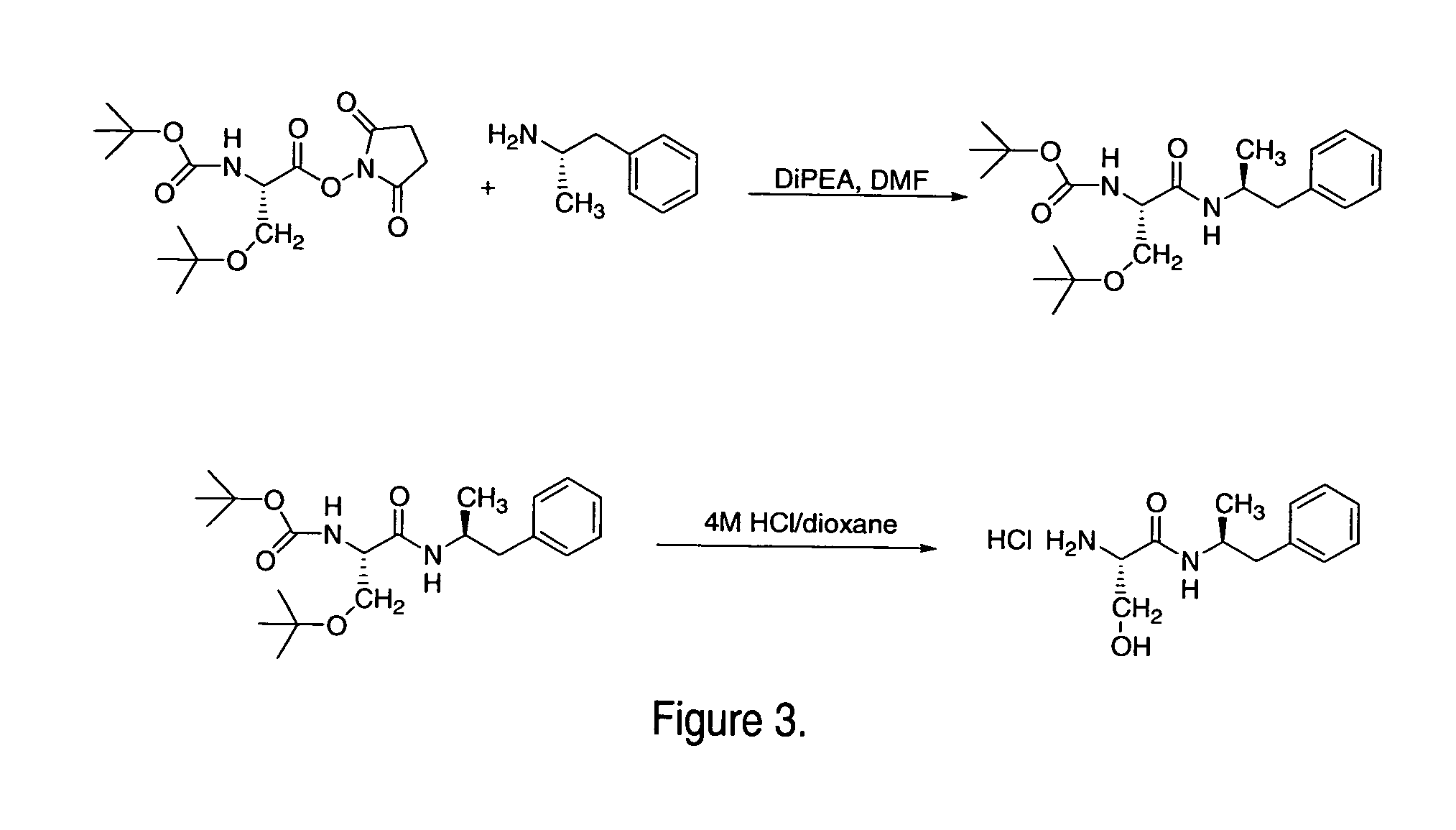
Abuse-resistant amphetamine prodrugs
The invention describes compounds, compositions, and methods of using the same comprising a chemical moiety covalently attached to amphetamine. These compounds and compositions are useful for reducing or preventing abuse and overdose of amphetamine. These compounds and compositions find particular use in providing an abuse-resistant alternative treatment for certain disorders, such as attention deficit hyperactivity disorder (ADHD), ADD, narcolepsy, and obesity. Oral bioavailability of amphetamine is maintained at therapeutically useful doses. At higher doses bioavailability is substantially reduced, thereby providing a method of reducing oral abuse liability. Further, compounds and compositions of the invention decrease the bioavailability of amphetamine by parenteral routes, such as intravenous or intranasal administration, further limiting their abuse liability.
Owner:TAKEDA PHARMA CO LTD
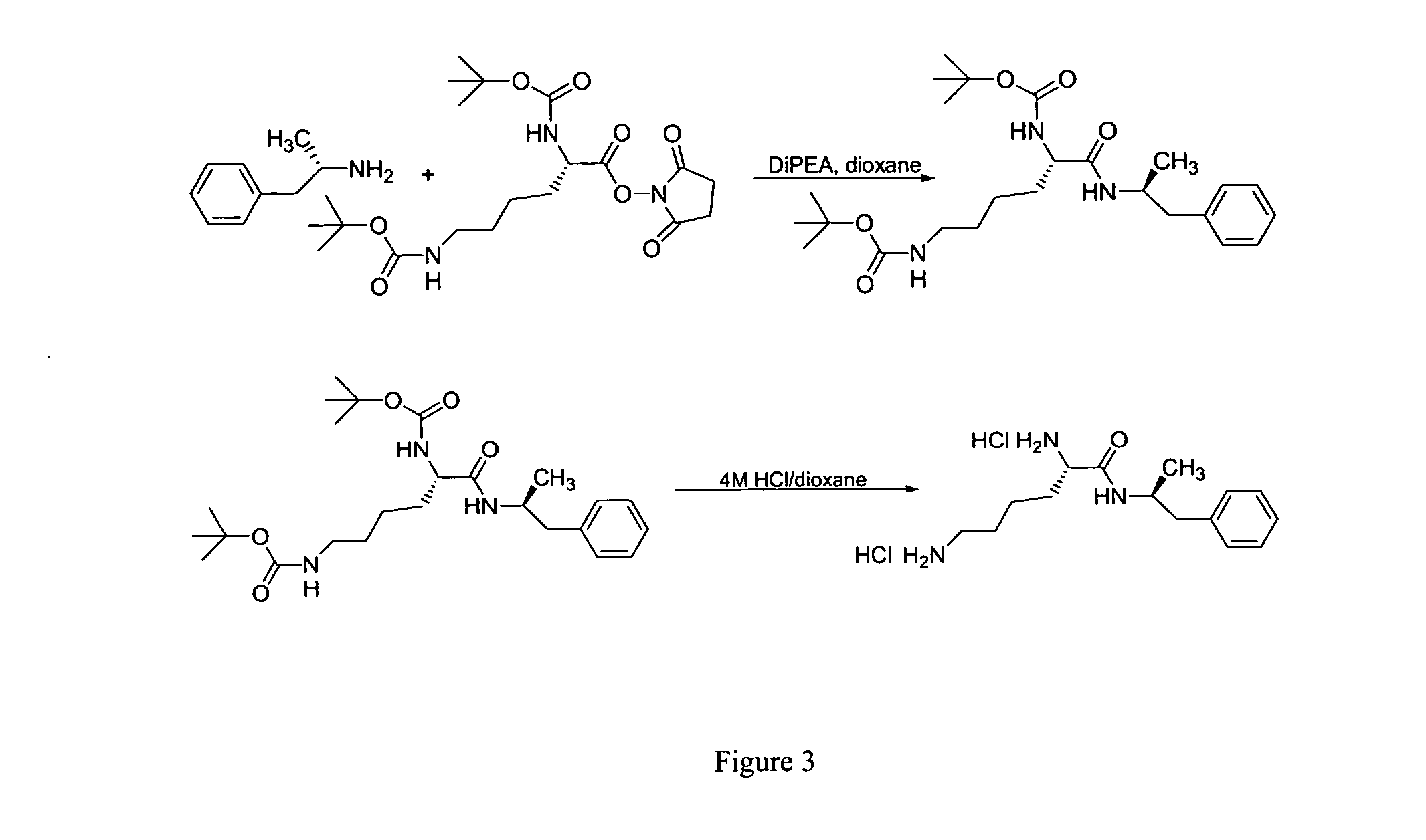
Abuse resistant lysine amphetamine compounds
InactiveUS7223735B2Reduced activityRelease is diminished and eliminatedOrganic active ingredientsNervous disorderDiseaseAlternative treatment
The present invention describes compounds, compositions and methods of using the same comprising lysine covalently attached to amphetamine. These compounds and compositions are useful for reducing or preventing abuse and overdose of amphetamine. These compounds and compositions find particular use in providing an abuse-resistant alternative treatment for certain disorders, such as attention deficit hyperactivity disorder (ADHD), ADD, narcolepsy, and obesity. Oral bioavailability of amphetamine is maintained at therapeutically useful doses. At higher doses bioavailability is substantially reduced, thereby providing a method of reducing oral abuse liability. Further, compounds and compositions of the invention decrease the bioavailability of amphetamine by parenteral routes, such as intravenous or intranasal administration, further limiting their abuse liability.
Owner:TAKEDA PHARMA CO LTD
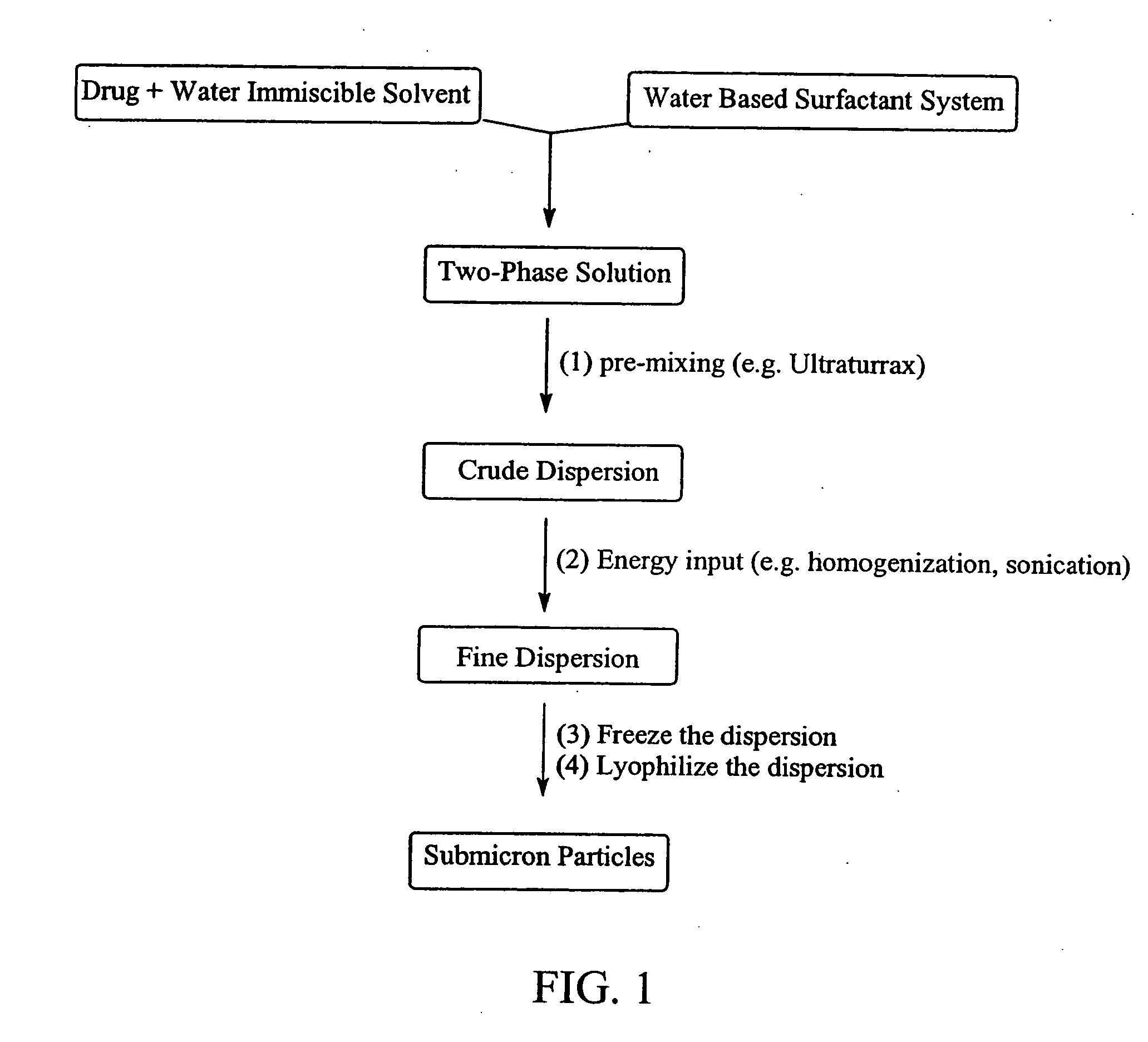
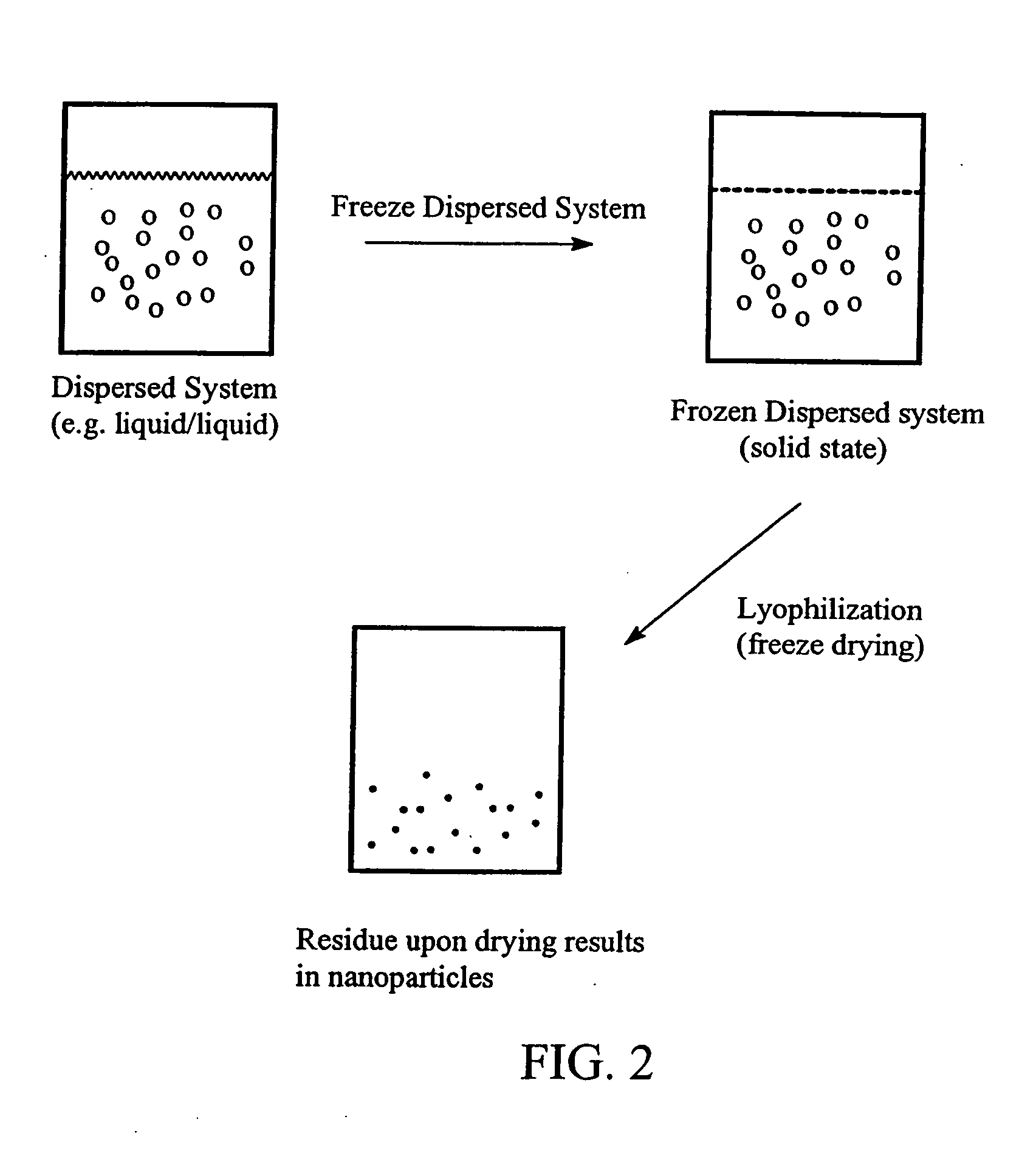
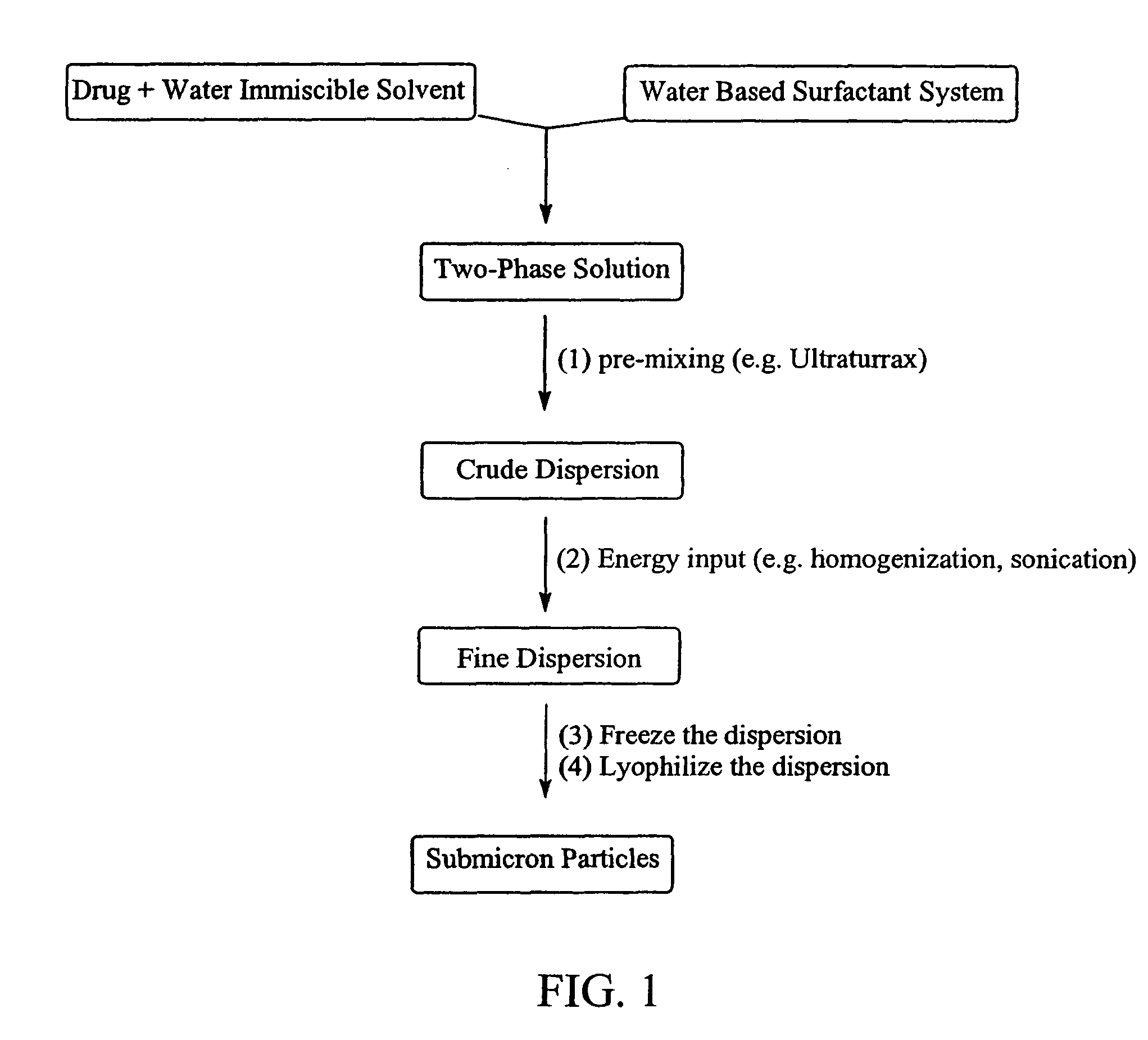
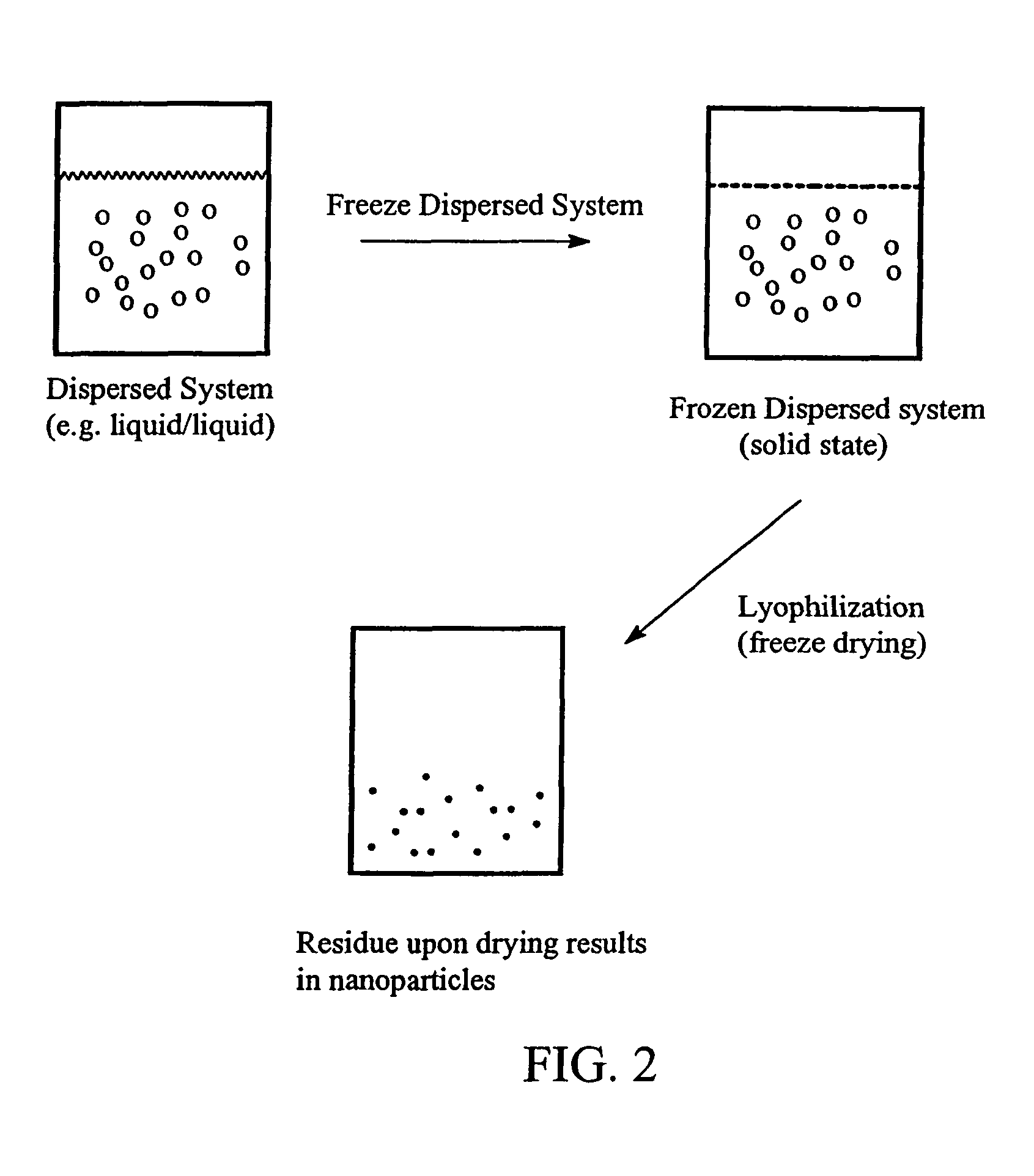
Preparation of submicron sized nanoparticles via dispersion lyophilization
InactiveUS20050013868A1Powder deliveryOrganic active ingredientsSimple Organic CompoundsNanoparticle
The present invention relates to a process for preparing submicron sized nanoparticles of a poorly water soluble compound by lyophilizing a dispersion or microdispersion of a multiphase system having an organic phase and an aqueous phase, the organic phase having the poorly water soluble organic compound therein. The method is preferably used to prepare nanoparticles of a poorly water soluble, pharmaceutically active compound suitable for in vivo delivery, particularly by parenteral routes.
Owner:BAXTER INT INC
Abuse-resistant amphetamine prodrugs
InactiveUS7659253B2Reducing patient to patient variabilityRisk minimizationBiocideOrganic chemistryDiseaseChemical Moiety
The invention describes compounds, compositions, and methods of using the same comprising a chemical moiety covalently attached to amphetamine. These compounds and compositions are useful for reducing or preventing abuse and overdose of amphetamine. These compounds and compositions find particular use in providing an abuse-resistant alternative treatment for certain disorders, such as attention deficit hyperactivity disorder (ADHD), ADD, narcolepsy, and obesity. Oral bioavailability of amphetamine is maintained at therapeutically useful doses. At higher doses bioavailability is substantially reduced, thereby providing a method of reducing oral abuse liability. Further, compounds and compositions of the invention decrease the bioavailability of amphetamine by parenteral routes, such as intravenous or intranasal administration, further limiting their abuse liability.
Owner:TAKEDA PHARMA CO LTD
Highly concentrated stable meloxicam solutions
Aqueous cyclodextrin-free solution of meloxicam for administration by oral or parenteral route, containing a pharmacologically acceptable meloxicam salt of an organic or inorganic base and one or more suitable excipients, the content of dissolved meloxicam salt being more than 10 mg / mL. The formulation according to the invention has a shelf-life of up to 24 months or more.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Formulations for amylin agonist peptides
InactiveUS7312196B2Loss of biological activityLong-term stabilityPowder deliveryPeptide/protein ingredientsPrefilled SyringePharmaceutical formulation
The present invention is concerned with a pharmaceutical formulation comprising an amylin agonist and optionally a buffer, a tonicifier or stabilizer, and a preservative in a container, for example, a vial, prefilled cartridge, prefilled syringe or disposable pen. This formulation may be in liquid, gel, solid or powdered form for delivery, for example, via nasal, pulmonary, oral, sublingual, buccal, transdermal, or parenteral routes. Formulation with biocompatible polymers and release modifiers, such as sugars, can facilitate controlled release after injection, minimizing the number of administrations to a patient. These formulations maintain stability upon storage under refrigerated or room temperature conditions. Such formulations can be further combined with insulin for administration to a patient.
Owner:ASTRAZENECA PHARMA LP
Therapy for Treating Resistant Bacterial Infections
The invention relates to an improved therapy for treating resistant bacterial infections caused by extended-spectrum β-lactamase (ESBLs)-producing strains in a warm-blooded animal, adjuvant step down therapy, and pharmaceutical compositions for such therapies. The invention also relates to a method for inhibiting bacterial resistance in ESBLs-producing strains so as to have better control over the therapy; achieve reduced hospital stay and adjuvant step down therapy so as to avoid recrudescence. In particular, the therapy includes antibacterial combination of cefepime with sulbactam via parenteral route, followed by oral third generation cephalosporin with a suitable β lactamase inhibitor.
Owner:PATEL MAHESH VITHALBHAI +5
Pharmaceutical formulations of meloxicam
This invention is a novel pharmaceutical formulation of aqueous EDTA (Ethylene diamine tetraacetic acid) free solution of meloxicam in combination with meglumin for administration by oral or parenteral route, comprising one or more pharmaceutically acceptable excipients which is comprising N,N dimethylacetamide and propylene glycol for treating mammals, preferably animals.
Owner:SANOVEL ILAC SANAYI & TICARET ANONIM SIRKETI +1
Formulations for amylin agonist peptides
InactiveUS20090018053A1Retain stabilityShort term mixing compatibilityPowder deliveryPeptide/protein ingredientsPrefilled SyringePharmaceutical formulation
The present invention is concerned with a pharmaceutical formulation comprising an amylin agonist and optionally a buffer, a tonicifier or stabilizer, and a preservative in a container, for example, a vial, prefilled cartridge, prefilled syringe or disposable pen. This formulation may be in liquid, gel, solid or powdered form for delivery, for example, via nasal, pulmonary, oral, sublingual, buccal, transdermal, or parenteral routes. Formulation with biocompatible polymers and release modifiers, such as sugars, can facilitate controlled release after injection, minimizing the number of administrations to a patient. These formulations maintain stability upon storage under refrigerated or room temperature conditions. Such formulations can be further combined with insulin for administration to a patient.
Owner:AMYLIN PHARMA INC
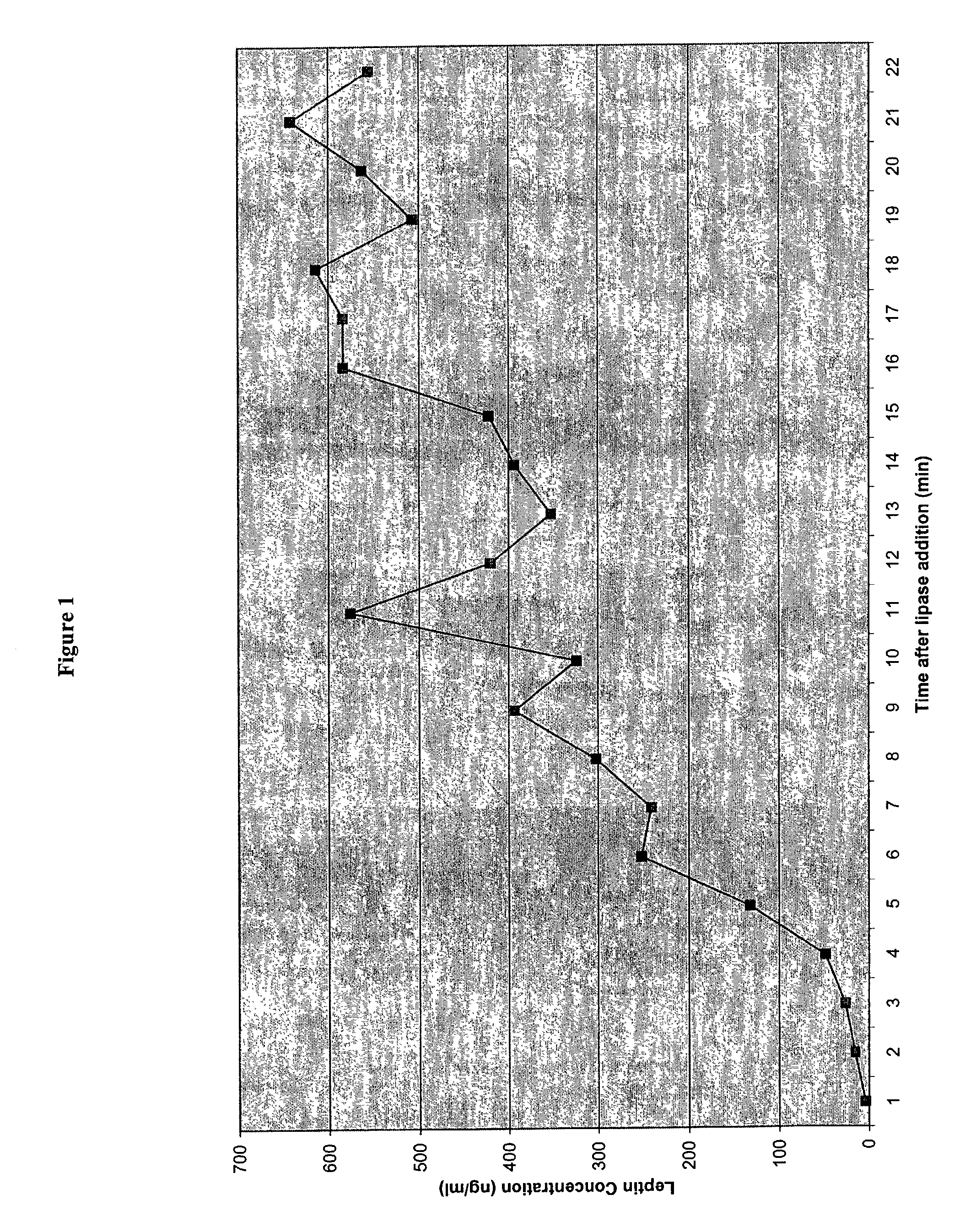
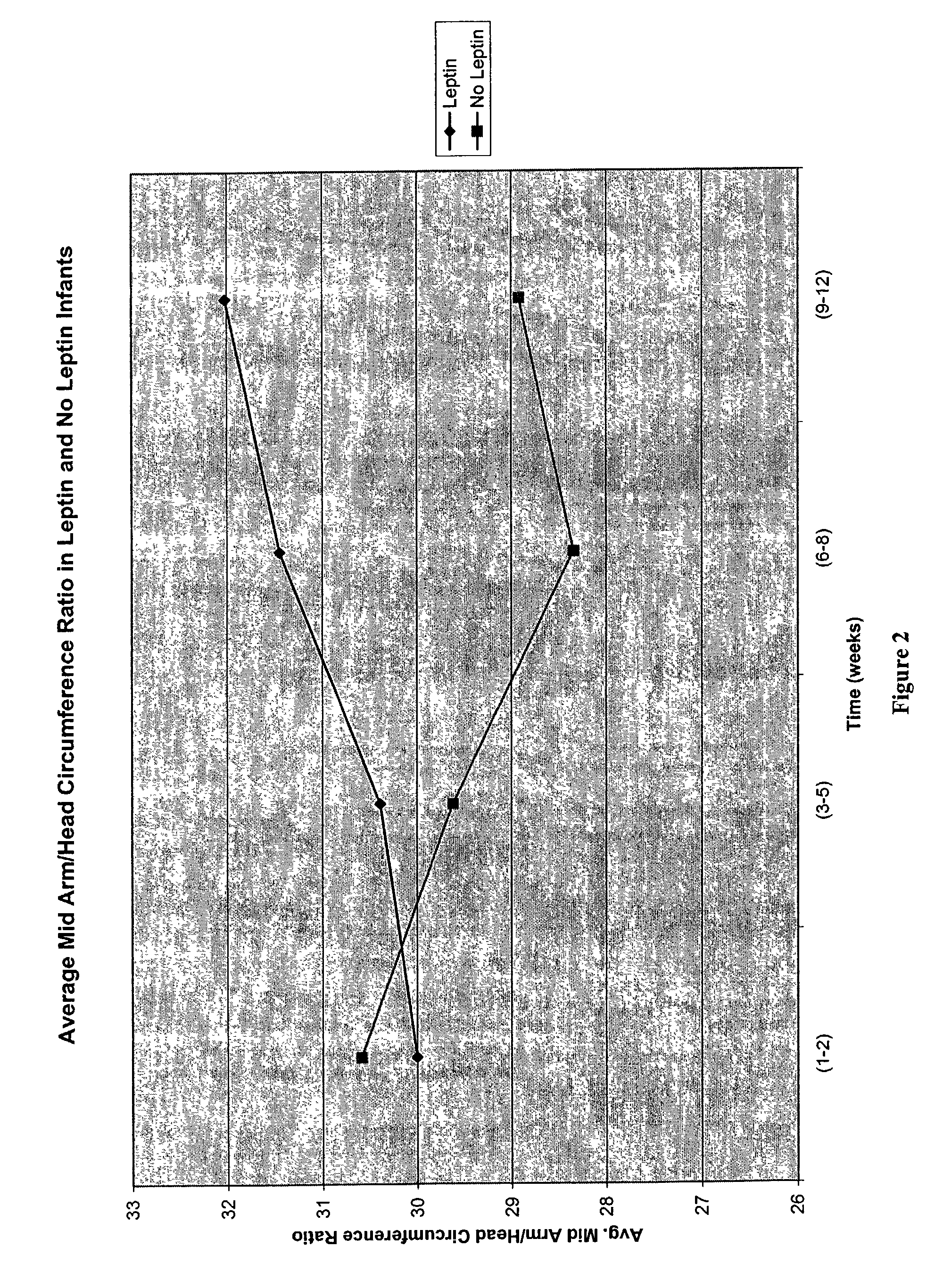
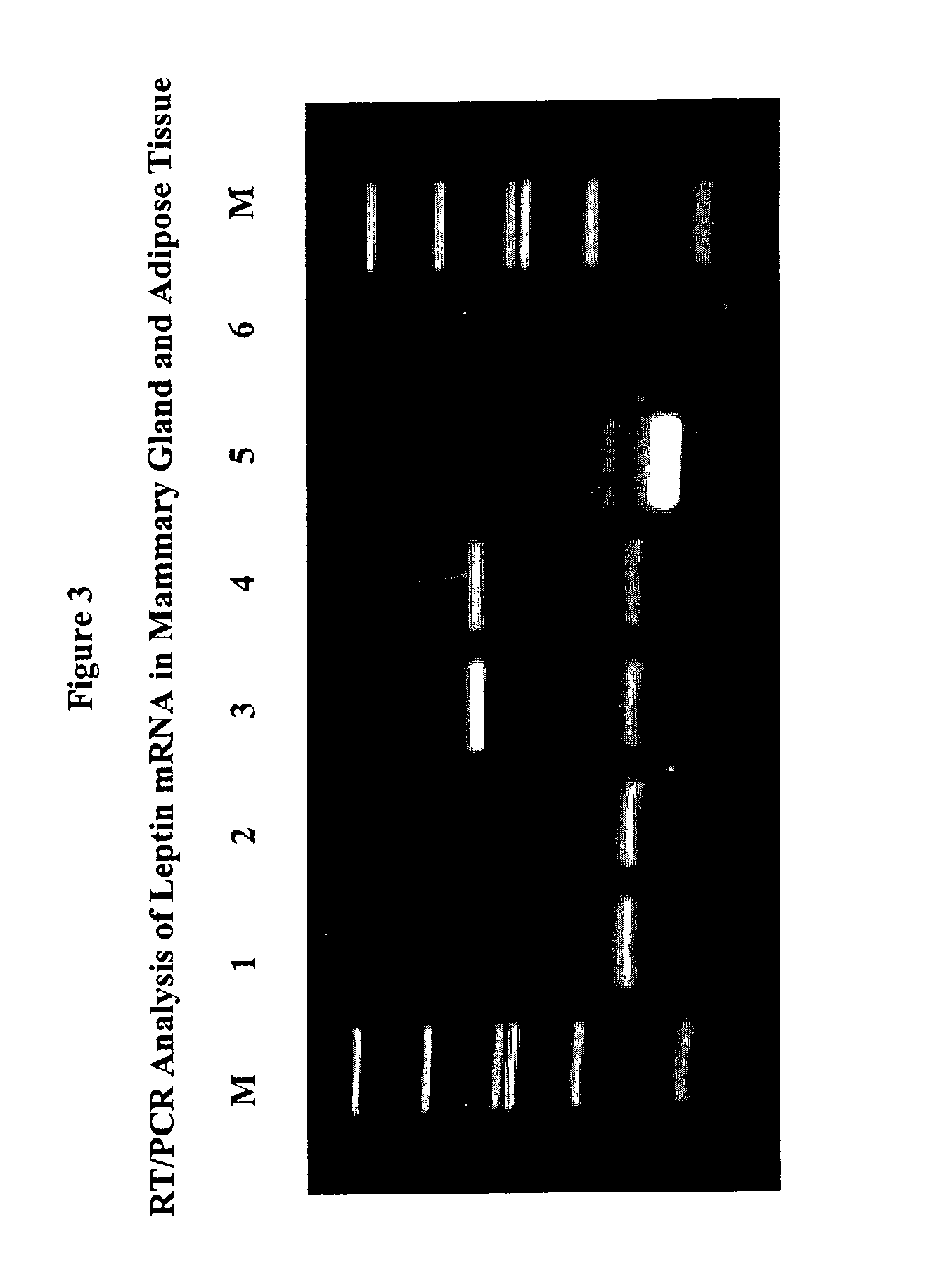
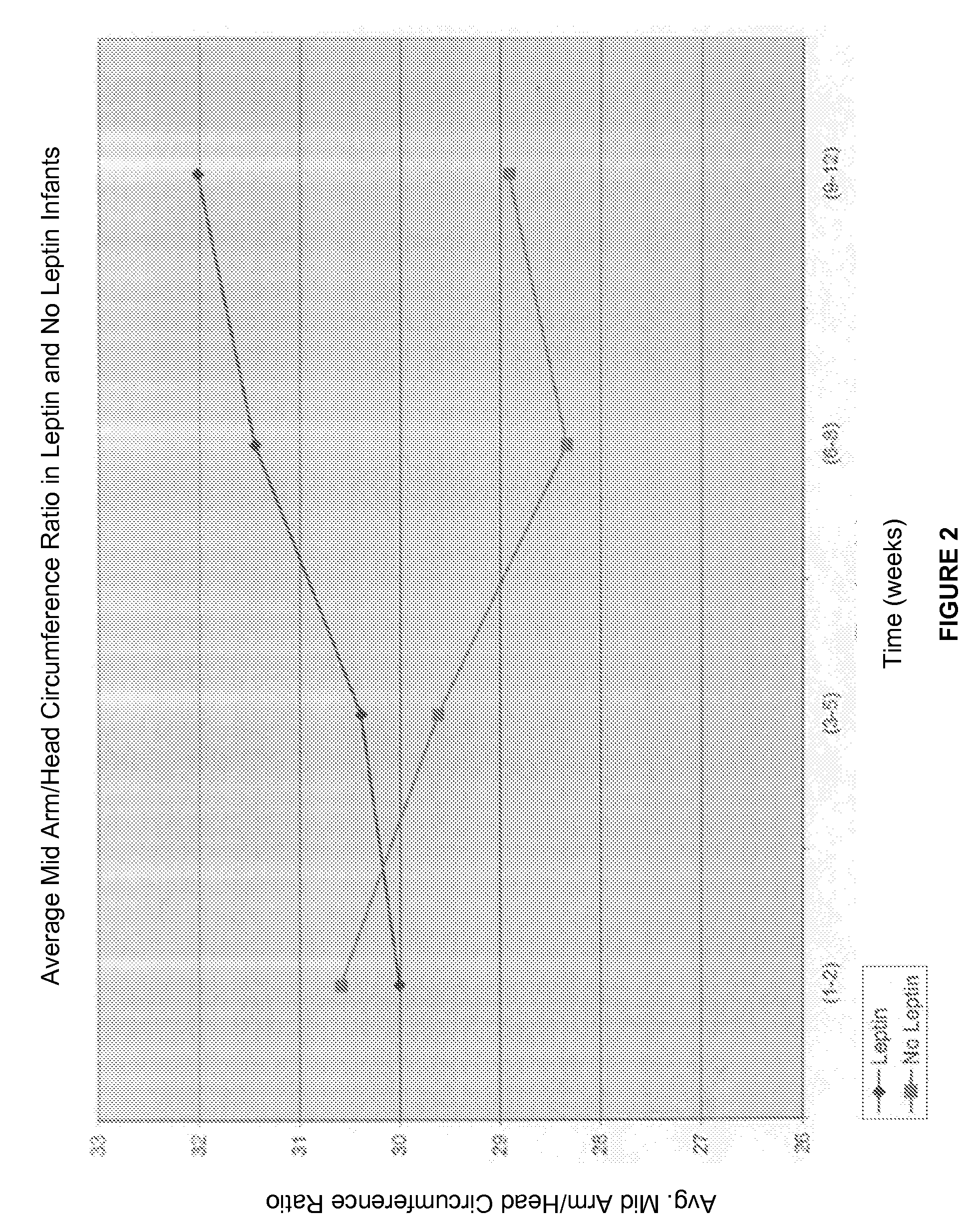
Administration of leptin
A method and composition for administering leptin to a subject. The invention includes suspending isolated native leptin-containing milk fat globules in a suitable medium for administering to a subject. The suspended milk fat globules may be administered orally as well as by intravenous, intramuscular, intraperitoneal, other enteral routes of administration, and other parenteral routes of administration. The invention includes a method for treating growth or maturational-related disorders in newborns as well as subjects having conditions that can be treated by the administration of leptin.
Owner:THE NEMOURS FOUND
Highly concentrated stable meloxicam solutions
Aqueous cyclodextrin-free solution of meloxicam for administration by oral or parenteral route, containing a pharmacologically acceptable meloxicam salt of an organic or inorganic base and one or more suitable excipients, the content of dissolved meloxicam salt being more than 10 mg / mL. The formulation according to the invention has a shelf-life of up to 24 months or more.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
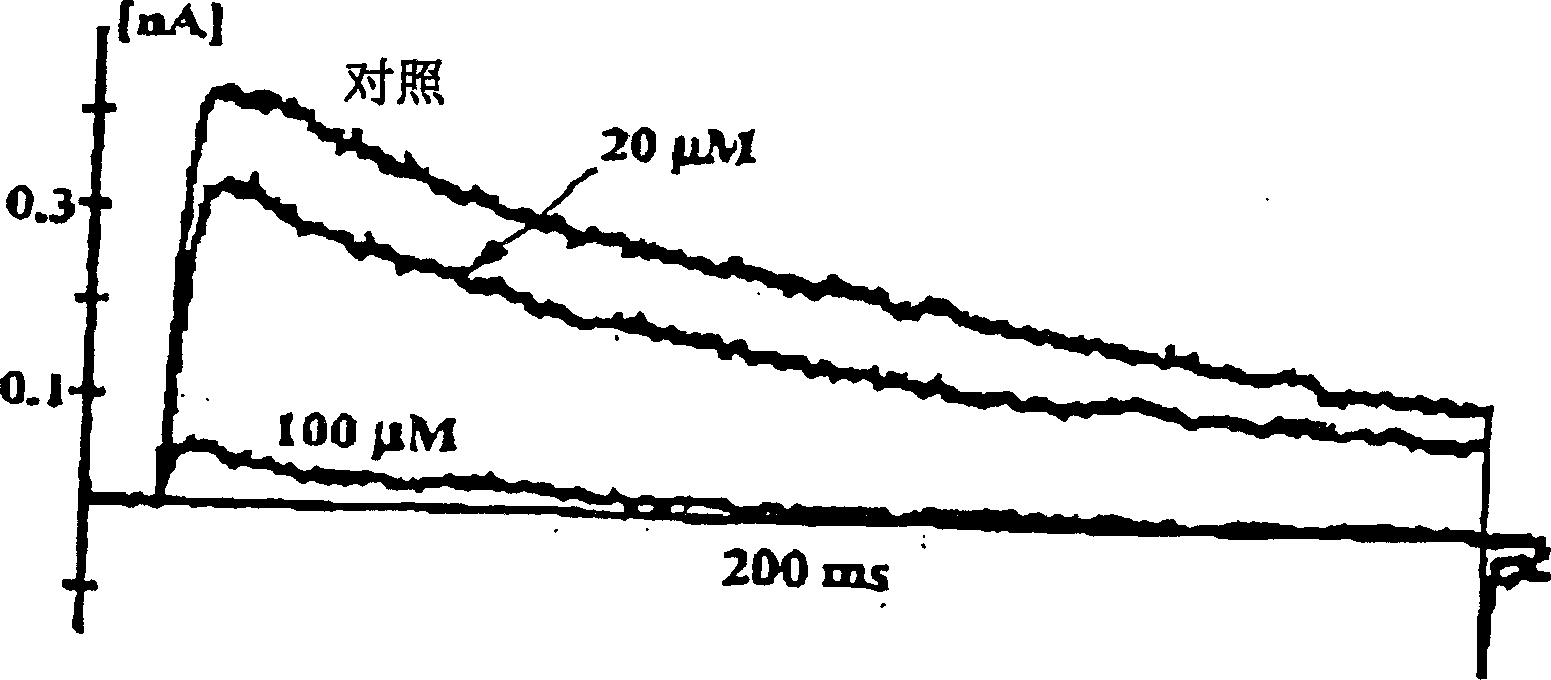
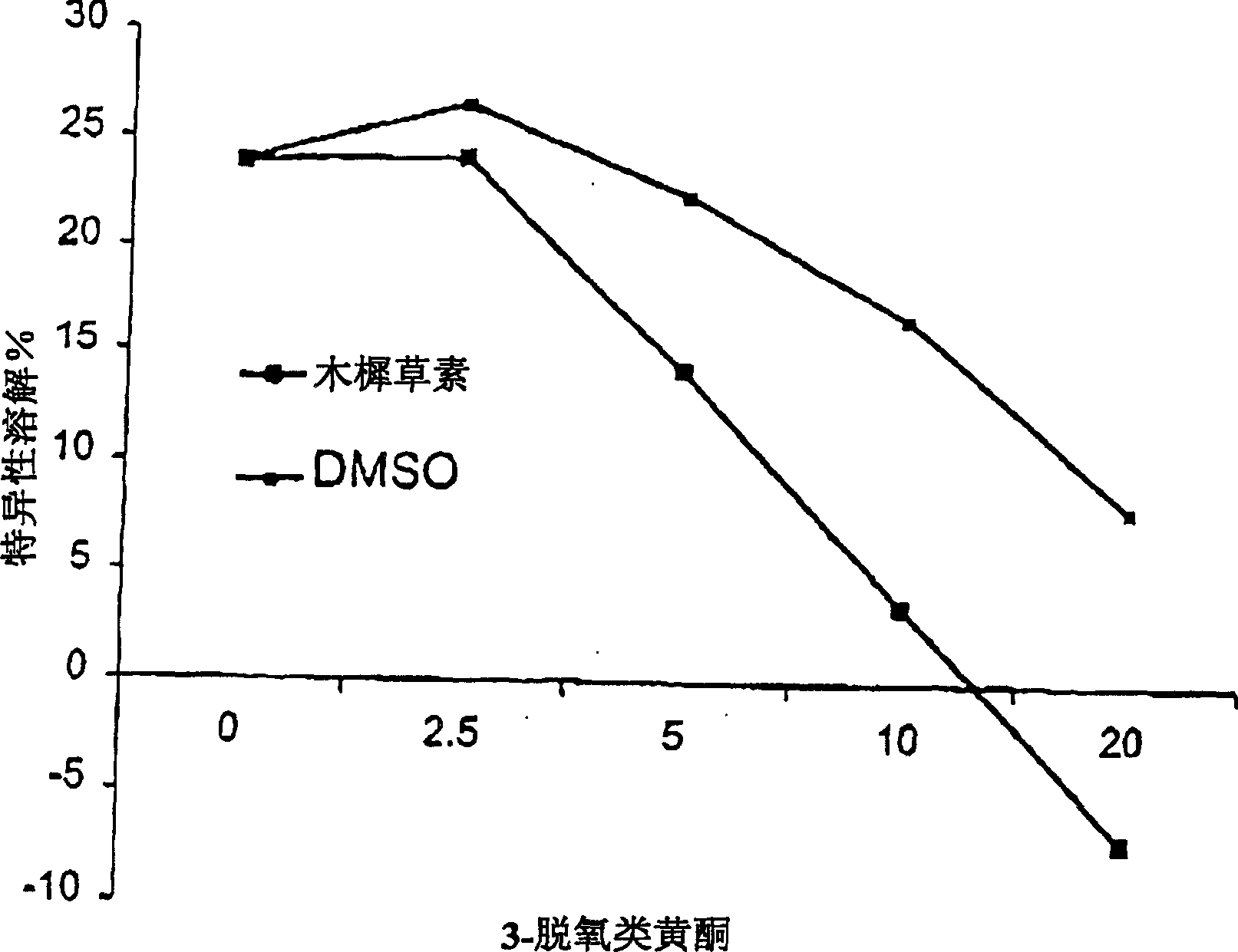
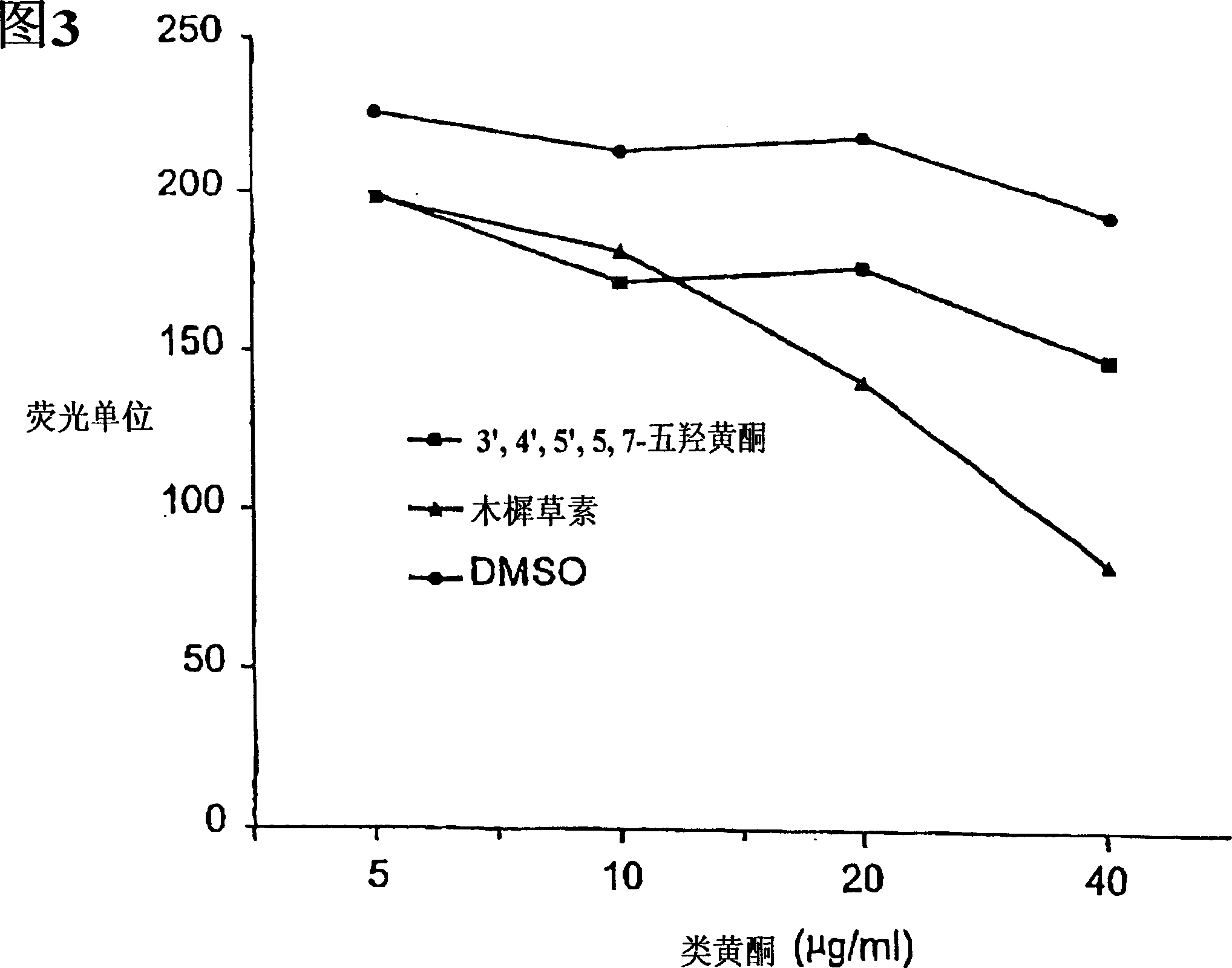
Inhibition by 3-deoxyflavonoids of t-lymphocyte activation and therapies related thereto
InactiveCN1668287AHalogenated hydrocarbon active ingredientsMetabolism disorderAutoimmune diseaseRutin
3-Deoxyflavonoid compounds and methods for inhibiting T-cell activity and treating diseases and disorders (e.g., autoimmune disorders, inflammatory disorders, diabetes, ALS, MS, rheumatoid arthritis, etc.). In some cases the efficacy and / or duration of action of luteolin and / or other 3-dioxyflavinoid compounds may be increased by administering such compounds along with Rutin, a Rutin congener and / or a Rutin derivative. Also, in some cases, first pass metabolism of luteolin or other 3-deoxyflavinoids may be avoided by administering such compounds by parenteral routes (e.g., sublingual, buccal, intranasal, injection, etc.).
Owner:SYNORX
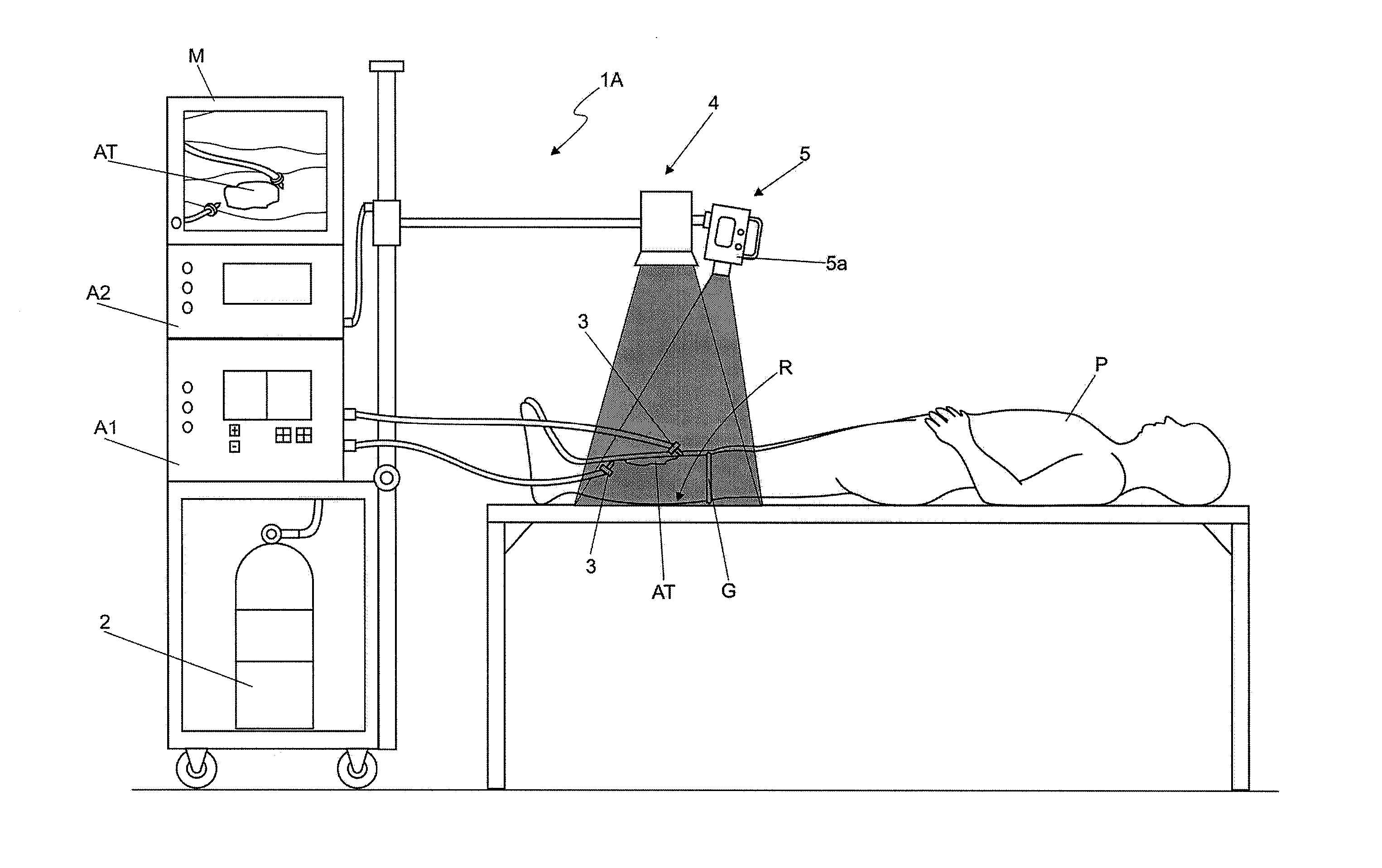
Method and apparatus that associate the parenteral injection of medical grade carbon dioxide (CO2) concomitantly with the application of infrared radiation from thermal and/or light sources using control by means of cutaneous and/or body thermometry
InactiveUS20080058709A1Maintain densityExpected effectMedical devicesPharmaceutical delivery mechanismBody temperature measurementMedicine
A method and apparatus that associate a new parenteral injection of medical grade carbon dioxide concomitantly with the application of infrared radiation from thermal and / or light sources using control by means of cutaneous and / or body thermometry, associating the concomitant application of infrared radiation from different light or thermal sources, to the direct injections of medical grade carbon dioxide CO2 into parenteral routes via carbon dioxide infusion regulating apparatuses and infrared radiation emitting apparatuses controlled by cutaneous and / or body thermometry, either direct or indirect.
Owner:DA SILVA FREITAS MARIO AUGUSTO
Cationic lipid-mediated enhancement of nucleic acid immunization of cats
InactiveUS7314627B2Enhance immune responseSsRNA viruses negative-senseOrganic active ingredientsLipid formationParenteral route
The present invention relates to a method to introduce a nucleic acid molecule into a felid by administration of a nucleic acid-cationic lipid complex composition. The method includes the step of administering to the felid, by a parenteral route, a nucleic acid-cationic lipid complex to elicit and / or enhance an immune response. In one embodiment, this method enhances the immune response in a felid compared to a method in which a naked DNA vaccine is administered to a felid. Also provided is a method to deliver a nucleic acid to a felid. This method comprises parenterally administering to the felid a composition that includes a nucleic acid molecule complexed with a cationic lipid.
Owner:HESKA
Biodegradable targetable microparticle delivery system
InactiveUS20050163745A1Easy to packSuitable propertyOrganic active ingredientsDrug compositionsAntigenPolyester
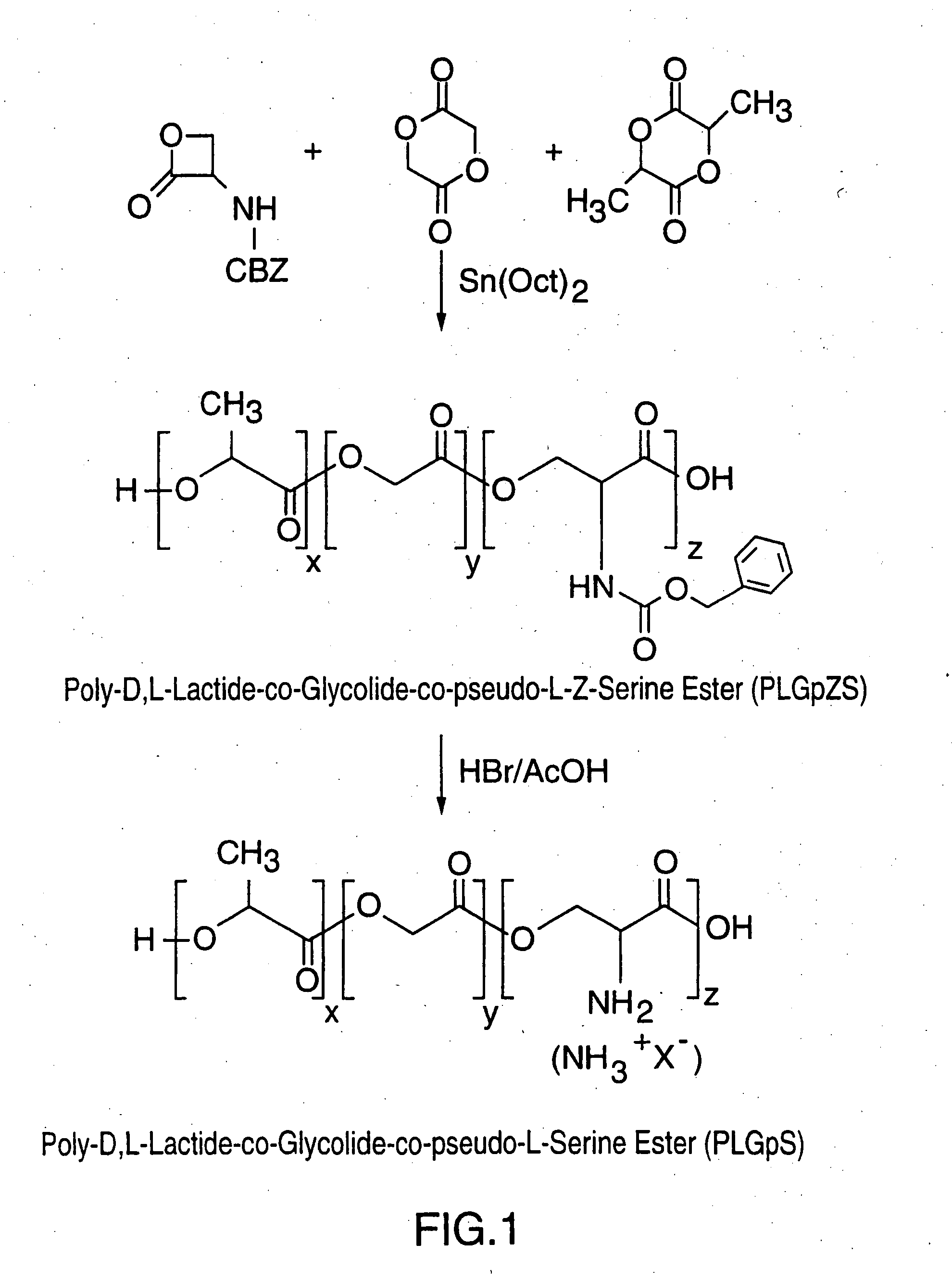
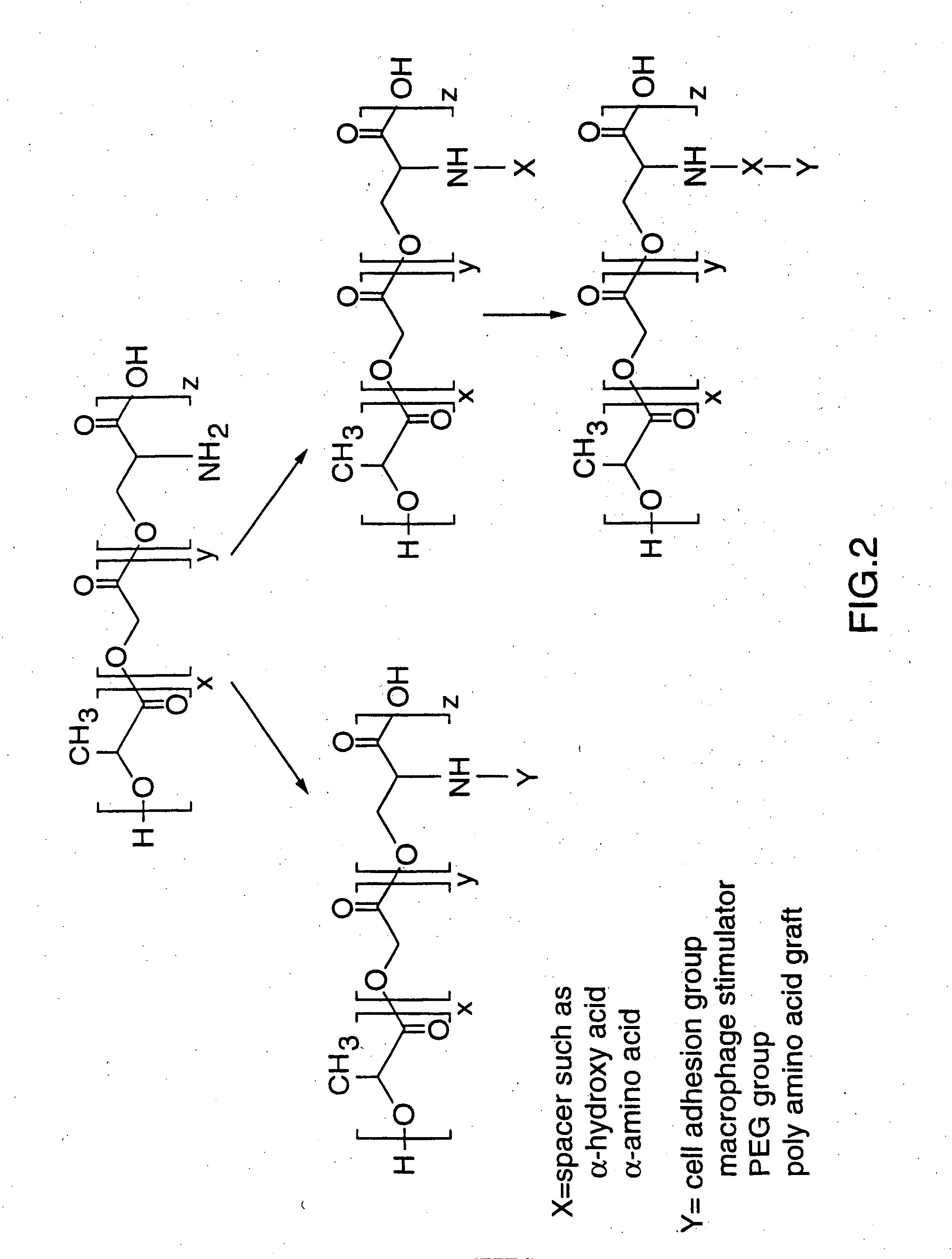
Copolymers designed for use as particulate carriers containing functionalizable amino acid subunits for coupling with targeting ligands are described. The copolymers are polyesters composed of α-hydroxy acid subunits such as D,L-lactide and pseudo-α-amino acid subunits which may be derived from serine or terpolymers of D,L-lactide and glycolide and pseudo-α-amino acid subunits which may be derived from serine. Stable vaccine preparations useful as delayed release formulations containing antigen or antigens and adjuvants encapsulated within or physically mixed with polymeric microparticles are described. The particulate carriers are useful for delivering agents to the immune system of a subject by mucosal or parenteral routes to produce immune responses, including antibody and protective responses.
Owner:SOKOLL KENNETH K +2
Administration of leptin
A method and composition for administering leptin to a subject. The invention includes suspending isolated native leptin-containing milk fat globules in a suitable medium for administering to a subject. The suspended milk fat globules may be administered orally as well as by intravenous, intramuscular, intraperitone41, other enteral routes of administration, and other parenteral routes of administration. The invention includes a method for treating growth or maturational-related disorders in newborns as, well as subjects having conditions that can be treated by the administration of leptin.
Owner:THE NEMOURS FOUND
Protectant for UV-induced skin damage
The present invention provides a method for protecting against UV radiation-induced skin damage. Specifically, compositions including dapsone are administered to provide UV protection. The dapsone compositions may be administered orally, or by other parenteral routes, such as topically, transdermally, by inhalation, and the like.
Owner:ALLERGAN INC
Abuse and alcohol resistant drug composition
ActiveUS20140010860A1Reduced drug abuse potentialNo abuse potentialPowder deliveryOrganic active ingredientsSodium BentoniteNasal route
A liquid or semi-solid matrix or magma or paste which is non-newtonian, thixotropic and pseudoplastic and composed of one or more controlled release agent, and / or one or more clays such as bentonite and / or one or more fillers in a non aqueous vehicle, and optionally materials selected from disintegrants, humectants, surfactants and stabilizers. The composition and physicochemical properties makes it harder or prevents dose dumping of narcotic analgesics in the presence of alcohol and harder to abuse opiod agonists or narcotic analgesics and discourages drug abuse via crushing, milling or grinding the dosage form to powder or heating the dosage form to vapour and snorting or inhalation by the nasal route or dissolving to abuse via the parenteral route.
Owner:INTELLIPHARMACEUTICS
Formulations comprising a nucleic acid in a high concentration
PendingCN111132663AImprove toleranceOrganic active ingredientsCosmetic preparationsHypoviscosityAcute myocardial ischaemia
Low-viscosity, high concentration nucleic acid compositions that can be administered by multiple parenteral routes may allow for less frequent dosing than nucleic acid products currently on the market. In particular, low-viscosity defibrotide formulations for subcutaneous, intramuscular, and intraperitoneal administration are more convenient to the patient and / or are administered outside of the hospital setting. Formulations of the invention may be used for the treatment of numerous conditions including for example, treatment of peripheral arteriopathies, treatment of acute renal insufficiency, treatment of acute myocardial ischemia, and treatment and prevention of sinusoidal obstruction syndrome or VOD.
Owner:JAZZ PHARMA IRELAND LTD
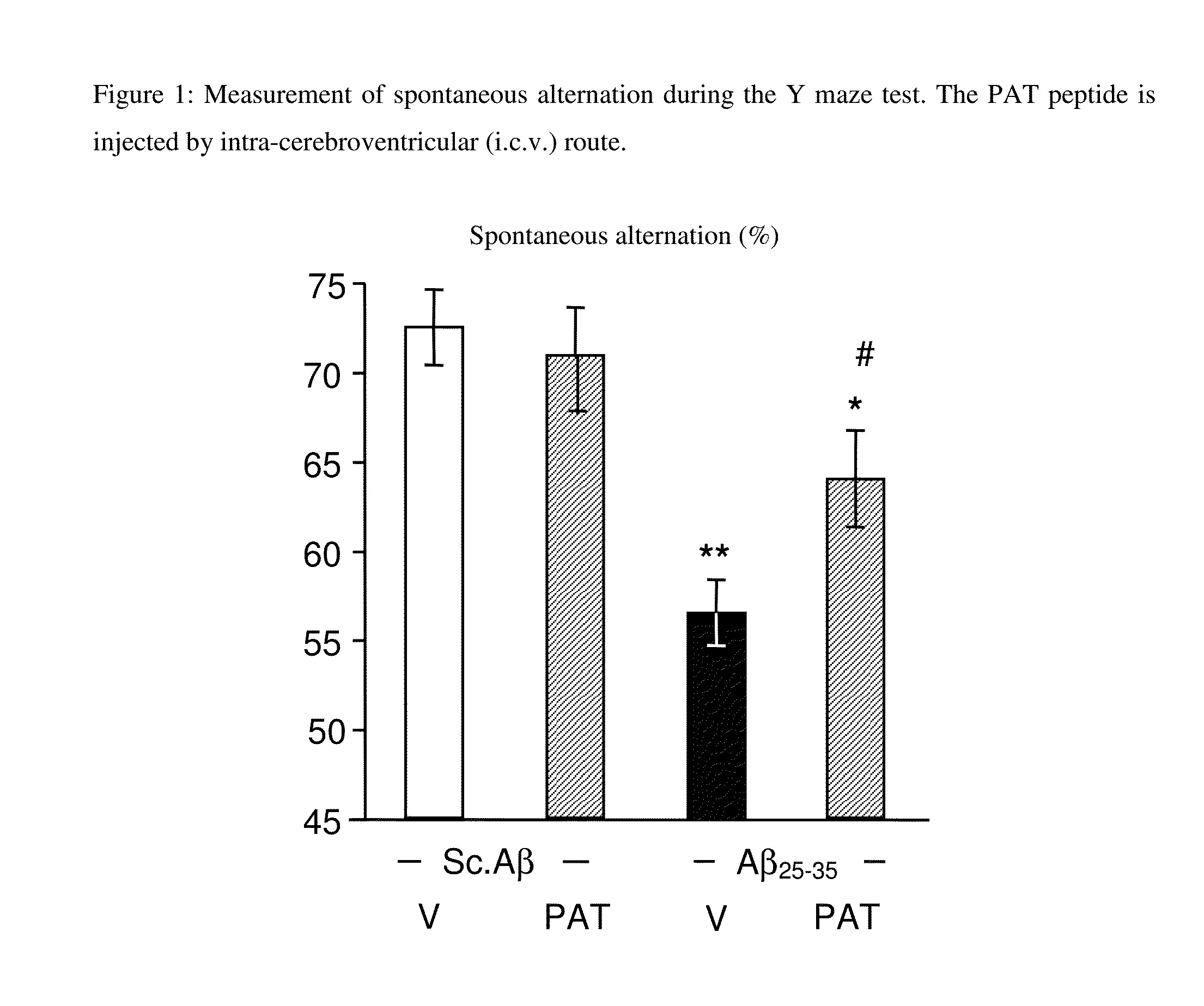
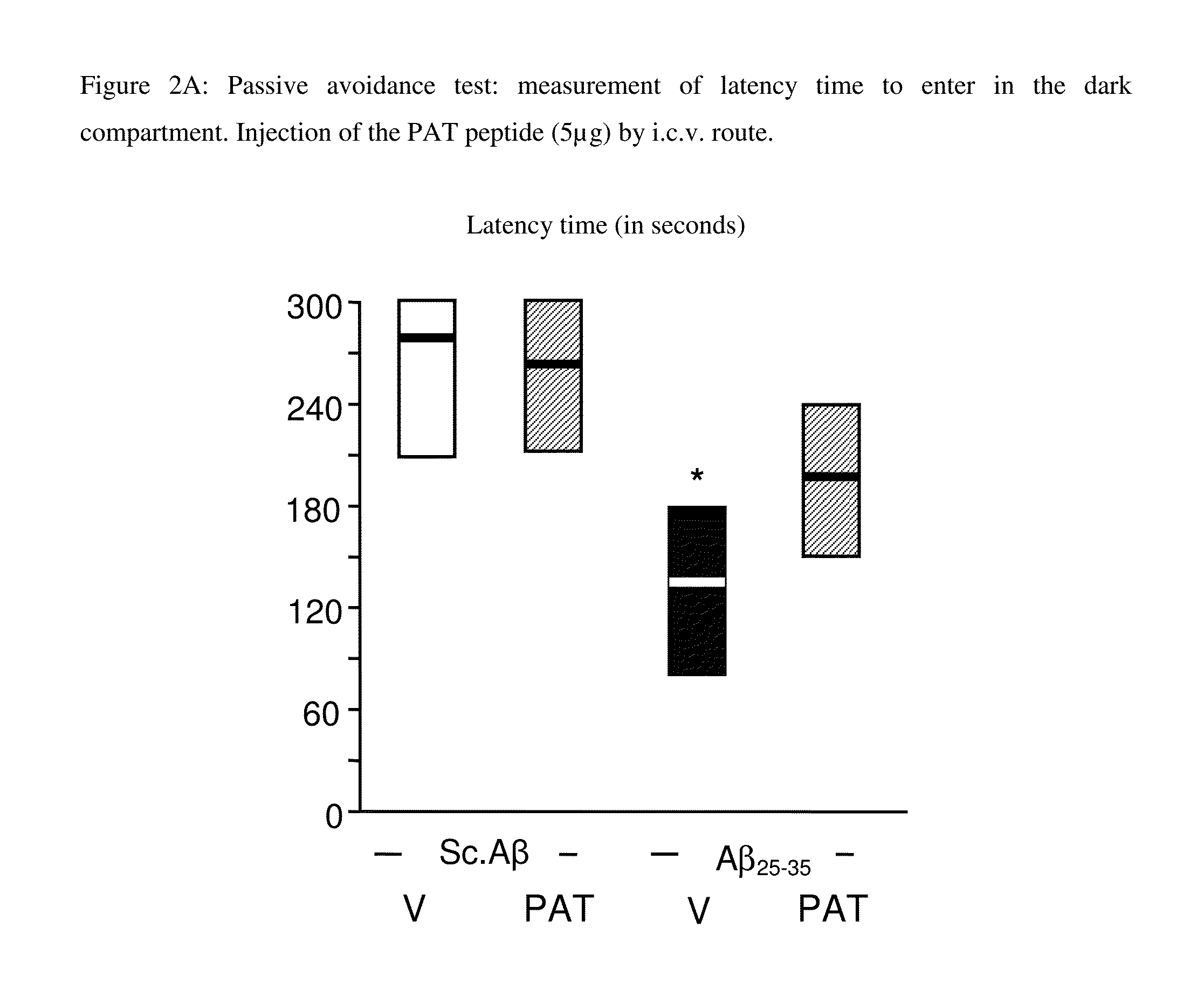
Use of the pat nonapeptide in the treatment and prevention of neurodegenerative diseases
InactiveUS20160074463A1Diffusion slowNervous disorderPeptide/protein ingredientsHuntingtons choreaAmyotrophic lateral sclerosis
A PAT nonapeptide of formula EAKSQGGSD can be used to treat or prevent neurodegenerative diseases such as Alzheimer's disease, Parkinson's disease, Huntington's disease and amyotrophic lateral sclerosis. Pharmaceutical compositions containing the PAT nonapeptide can be formulated for administration by parenteral route, including the subcutaneous, intraperitoneal, intravenous or intranasal routes.
Owner:ORPHIT
Preparation of submicron sized nanoparticles via dispersion lyophilization
The present invention relates to a process for preparing submicron sized nanoparticles of a poorly water soluble compound by lyophilizing a dispersion or microdispersion of a multiphase system having an organic phase and an aqueous phase, the organic phase having the poorly water soluble organic compound therein. The method is preferably used to prepare nanoparticles of a poorly water soluble, pharmaceutically active compound suitable for in vivo delivery, particularly by parenteral routes.
Owner:BAXTER INT INC
Abuse-resistant amphetamine prodrugs
InactiveUS20090131516A1Promote absorptionQuick eliminationBiocideNervous disorderChemical MoietyAlternative treatment
The invention describes compounds, compositions, and methods of using the same comprising a chemical moiety covalently attached to amphetamine. These compounds and compositions are useful for reducing or preventing abuse and overdose of amphetamine. These compounds and compositions find particular use in providing an abuse-resistant alternative treatment for certain disorders, such as attention deficit hyperactivity disorder (ADHD), ADD, narcolepsy, and obesity. Oral bioavailability of amphetamine is maintained at therapeutically useful doses. At higher doses bioavailability is substantially reduced, thereby providing a method of reducing oral abuse liability. Further, compounds and compositions of the invention decrease the bioavailability of amphetamine by parenteral routes, such as intravenous or intranasal administration, further limiting their abuse liability.
Owner:TAKEDA PHARMA CO LTD
Pharmaceutical composition of microspheres for preventing diabetic foot amputation
InactiveCN103933552AHeal fastGood treatment effectPeptide/protein ingredientsMetabolism disorderDiabetic footPharmaceutical Substances
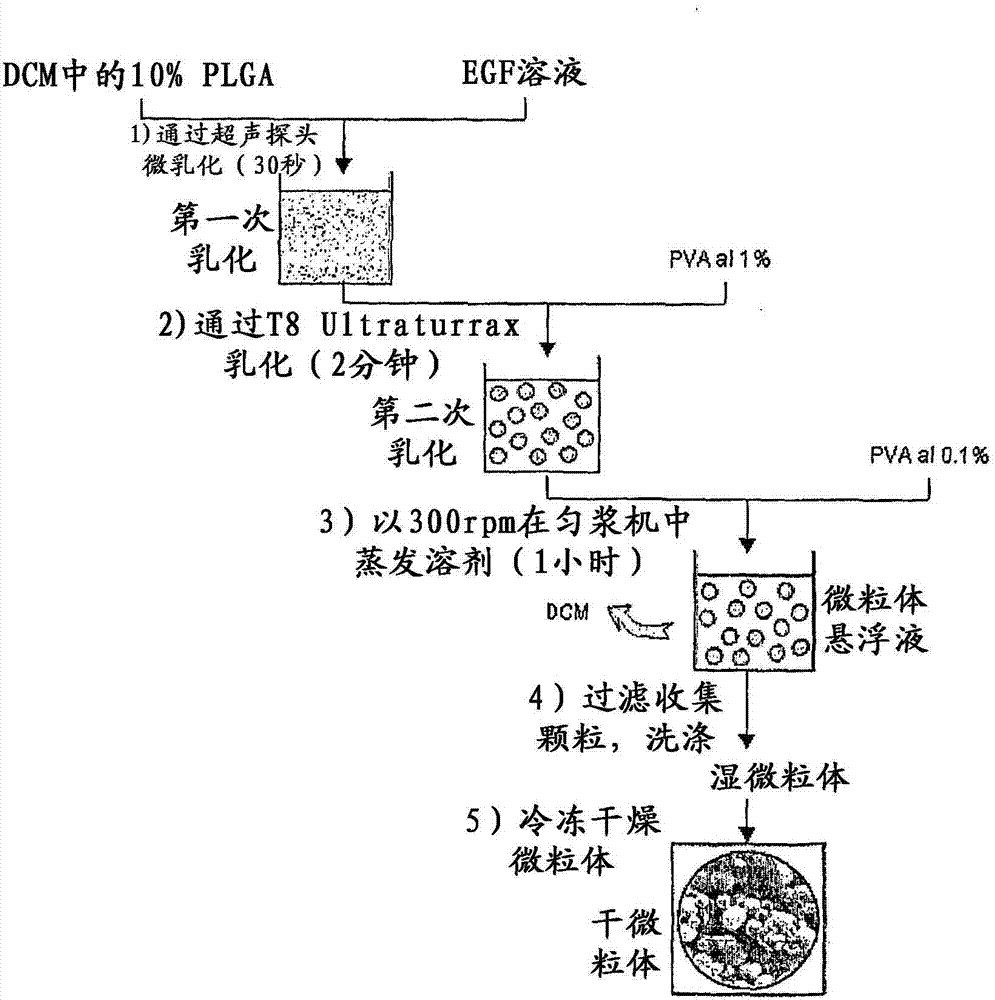
The present invention relates to a pharmaceutical composition that comprises polymeric microspheres containing epidermal growth factor (EGF) for the application, by the parenteral route, into the lower limbs of diabetic patients with cutaneous chronic ischemic ulcerative wounds. The pharmaceutical composition described herein, in contrast with the state of the art, is useful because reduce the administration frequency during the treatment and allows for the healing of the ulcerative wounds in a shorter time interval with respect to the injection of equivalent quantities of non-encapsulated EGF.
Owner:CENT DE ING GENETICA & BIOTECNOLOGIA
Particles for Treatment of Pulmonary Infection
InactiveUS20080213373A1Maintain good propertiesImprove stabilityAntibacterial agentsPowder deliveryPulmonary infectionMeningococcal meningitis
Formulations have been developed to treat or reduce the spread of respiratory infections, especially chronic or drug resistant infections, particularly tuberculosis (TB), severe acute respiratory syndrome (SARS), meningococcal meningitis, Respiratory syncytial virus (RSV), influenza, and small pox. Formulations include a drug or vaccine in the form of a microparticle, nanoparticle, or aggregate of nanoparticles, and, optionally, a carrier, which can be delivered by inhalation. Giving the drugs via an inhaler sidesteps the problems associated with oral or injectable drugs by bypassing the stomach and liver, and delivering the medication directly into the lungs. In one embodiment, the particle containing the agent is a large porous aerosol particle (LPPs). In another embodiment, the particles are nanoparticles, which can be administered as porous nanoparticle aggregates with micron diameters that disperse into nanoparticles following administration. Optionally, the nanoparticles are coated, such as with a surfactant or protein coating. The formulation may be administered as a powder or administered as a solution or via an enteral or non-pulmonary parenteral route of administration. The formulation is preferably administered as a pulmonary formulation. In the preferred embodiment for treatment of TB, the vaccine is a BCG vaccine that is stable at room temperature, or is an antibiotic effective against TB, such as capreomycin or PA-824, loaded at a very high percentage into the microparticles or nanoparticles. In one embodiment, a patient is treated with formulations delivering both antibiotic and vaccine.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Novel glucose polymers for peritoneal dialysis
ActiveUS20190300558A1Without impairing hyperbranched structureSugar derivativesMetabolism disorderPeritoneal dialysisMedicine
The invention relates to a novel glucose polymer which is particularly useful for administration by the parenteral route, and to the method for the production thereof. The invention also relates to compositions comprising such a glucose polymer, and to the methods for the production thereof. The invention further relates to the use thereof as a medicament, for example as an osmotic agent for peritoneal dialysis.
Owner:ROQUETTE FRERES SA
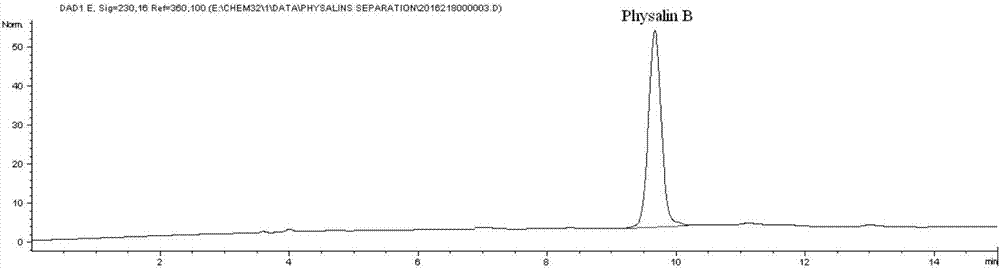
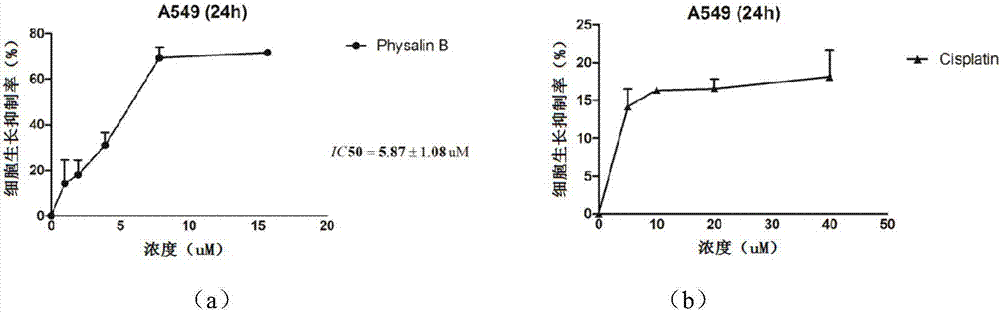
Preparation method for physalin B and application thereof in anti-lung tumor drug
InactiveCN107011357AHigh anti-lung tumor activitySimple preparation processOrganic active ingredientsOrganic chemistryDiseaseTreatment of lung cancer
The invention discloses a preparation method of physalicin B and its application in anti-lung tumor pharmacy, belonging to the technical field of medicine. After process optimization, the extraction and purification yield of Physalisin B is about 0.07%, and the purity of the obtained monomer is above 97%. Physalisin B can be well enriched in the lungs after intravenous administration, and it has a significant inhibitory effect on lung cancer cells, which is significantly stronger than cisplatin (positive drug), and it is concentration-dependent; The physin B cannot be effectively absorbed into the body by the body, so an effective amount of physin B and pharmaceutically acceptable adjuvant components can be prepared into a solid or liquid preparation for parenteral administration Drug pathway for the treatment of lung tumor diseases.
Owner:ZHEJIANG UNIV
Protectant for UV-induced skin damage
The present invention provides a method for protecting against UV radiation-induced skin damage. Specifically, compositions including dapsone are administered to provide UV protection. The dapsone compositions may be administered orally, or by other parenteral routes, such as topically, transdermally, by inhalation, and the like.
Owner:ALLERGAN INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com