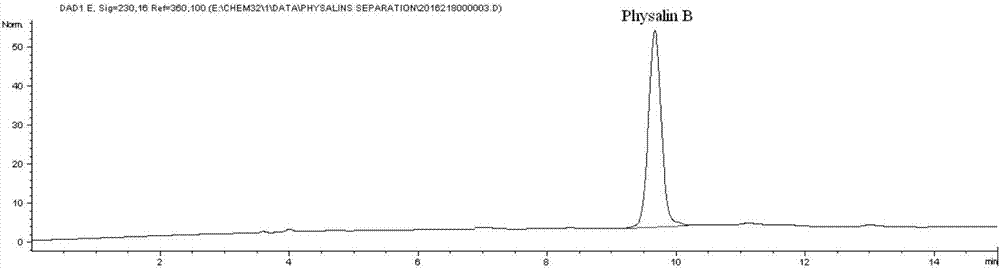
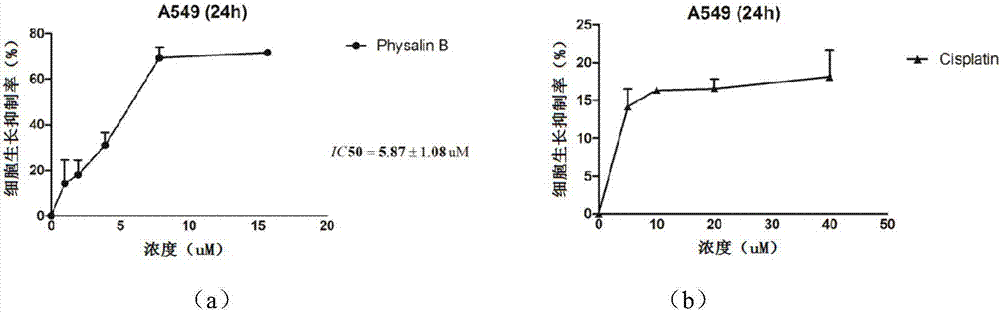
Preparation method for physalin B and application thereof in anti-lung tumor drug
A technology of Physalis bitterin and tumor drugs, which is applied in the field of traditional Chinese medicine, can solve the problems of lack of drugs and difficulty in large-scale production, and achieve the effects of simplified preparation process, simple operation and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The preparation of embodiment 1 Physalin B:
[0021] (1) Extraction:
[0022] Take 5 kg of dried calyx of Physalis alkekengi, add it to 15 L of 60vol% ethanol and carry out hot reflux extraction twice, each time for 2 hours, combine the two extracts, then concentrate under reduced pressure to no alcohol, add 1 times the volume of ethyl acetate was extracted twice, and then the two extracts were combined, and the ethyl acetate layer was evaporated to dryness under reduced pressure to obtain 560 g of extract.
[0023] (2) Purification:
[0024] The medicinal extract that step (1) is obtained is dissolved completely with ethyl acetate, after D101 macroporous adsorption resin adsorption, successively with the water of 10 times of column volumes, 20vol% ethanol, 40vol% ethanol, 50vol% ethanol, 60vol% ethanol and 70vol% ethanol was eluted, and the 70vol% ethanol eluted fraction was collected, concentrated under reduced pressure, and evaporated to dryness to obtain 375kg of ...
Embodiment 2
[0025] The preparation of embodiment 2 Physalin B:
[0026] (1) Extraction:
[0027] Take 5kg of dried calyx of Physalis alkekengi, add it to 25L of 60vol% ethanol and carry out hot reflux extraction twice, each time reflux for 2 hours, combine the two extracts, then concentrate under reduced pressure to no alcohol, add Three times the volume of ethyl acetate was extracted twice, and then the two extracts were combined, and the ethyl acetate layer was evaporated to dryness under reduced pressure to obtain 582 g of extract.
[0028] (2) Purification:
[0029] The extract was dissolved in ethyl acetate, adsorbed with D101 macroporous adsorption resin, and then eluted with 20 times column volume of water, 20vol% ethanol, 40vol% ethanol, 50vol% ethanol, 60vol% ethanol and 70vol% ethanol, and collected 70vol% The fraction eluted with % ethanol was concentrated under reduced pressure and evaporated to dryness to obtain 381 kg of powder. Load the sample at a ratio of 3:1 (weight o...
Embodiment 3
[0030] Embodiment 3 structure identification:
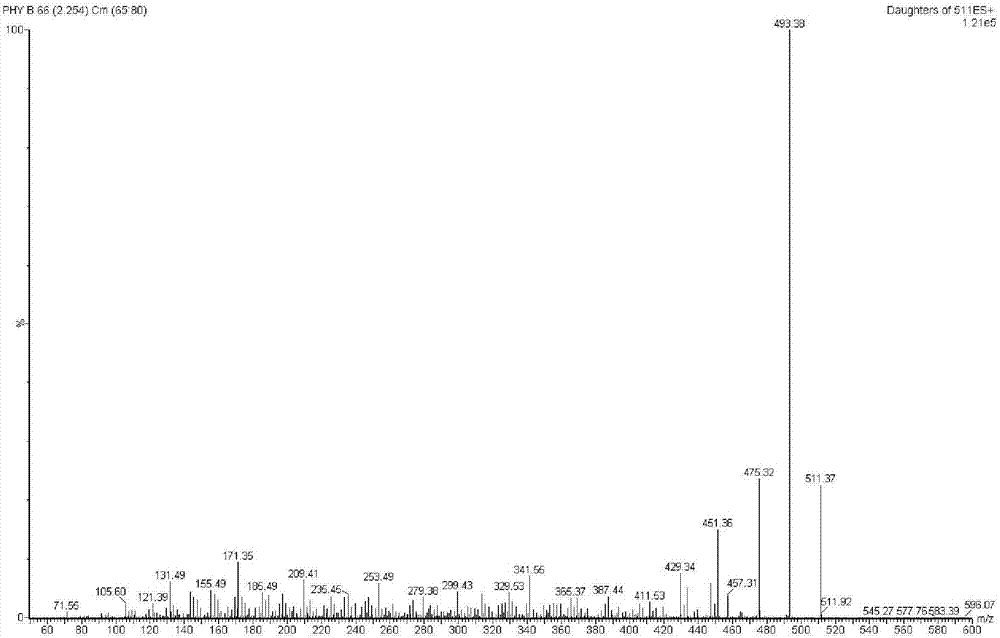
[0031] The molecular weight of the compound was analyzed by ESI-TOFMS as m / z: 510.1897, and the corresponding molecular formula was C 28 h 30 o 9 , and its MS / MS spectrum shows ( figure 2 Shown) the main product ions are 493, 475, 451 and 171 etc. 13 C-NMR spectrum (100MHz, DMSO-d 6 ) gives 28 carbon signals, δ CThere are 4 carbon signals above 160, suggesting that there may be 4 carbonyl groups: δ C 202.4 is the carbonyl carbon signal (C-1) of α and β unsaturated ketones on the six-membered ring, δ C 171.8, 167.3 are ester carbonyl carbon signals (C-18, C-26), δ C 209.4 is the carbon signal of ketone. δ C 106.3 is the ketal carbon, and this signal is a characteristic signal in the carbon spectrum of physalis compounds, which is preliminarily considered to be consistent with the spectral characteristics of physalis compounds. δ C 126.7, 146.1 are △ conjugated with carbonyl 2,3 ;δ C 135.5, 123.4 is △ 5,6 ; 1 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com