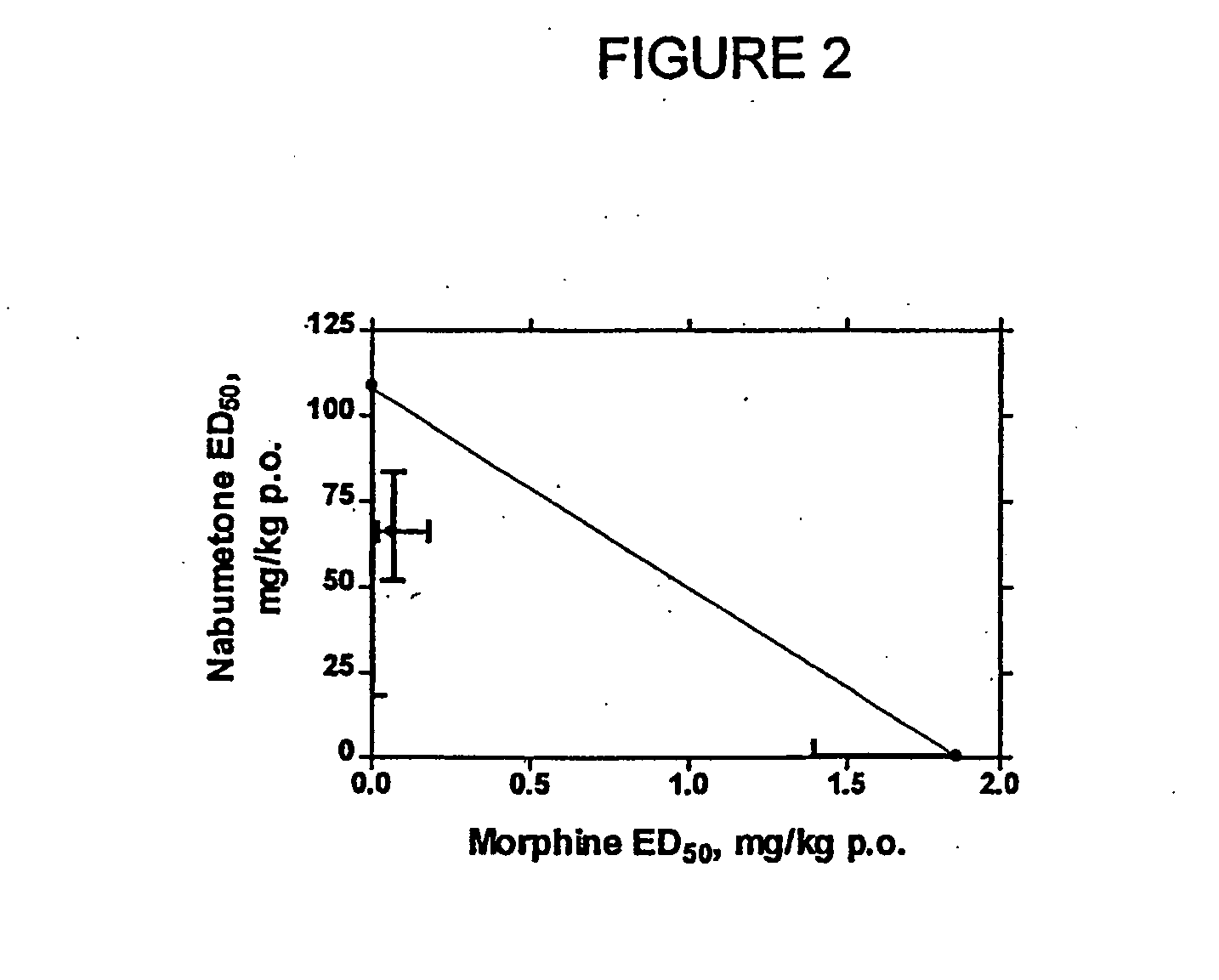
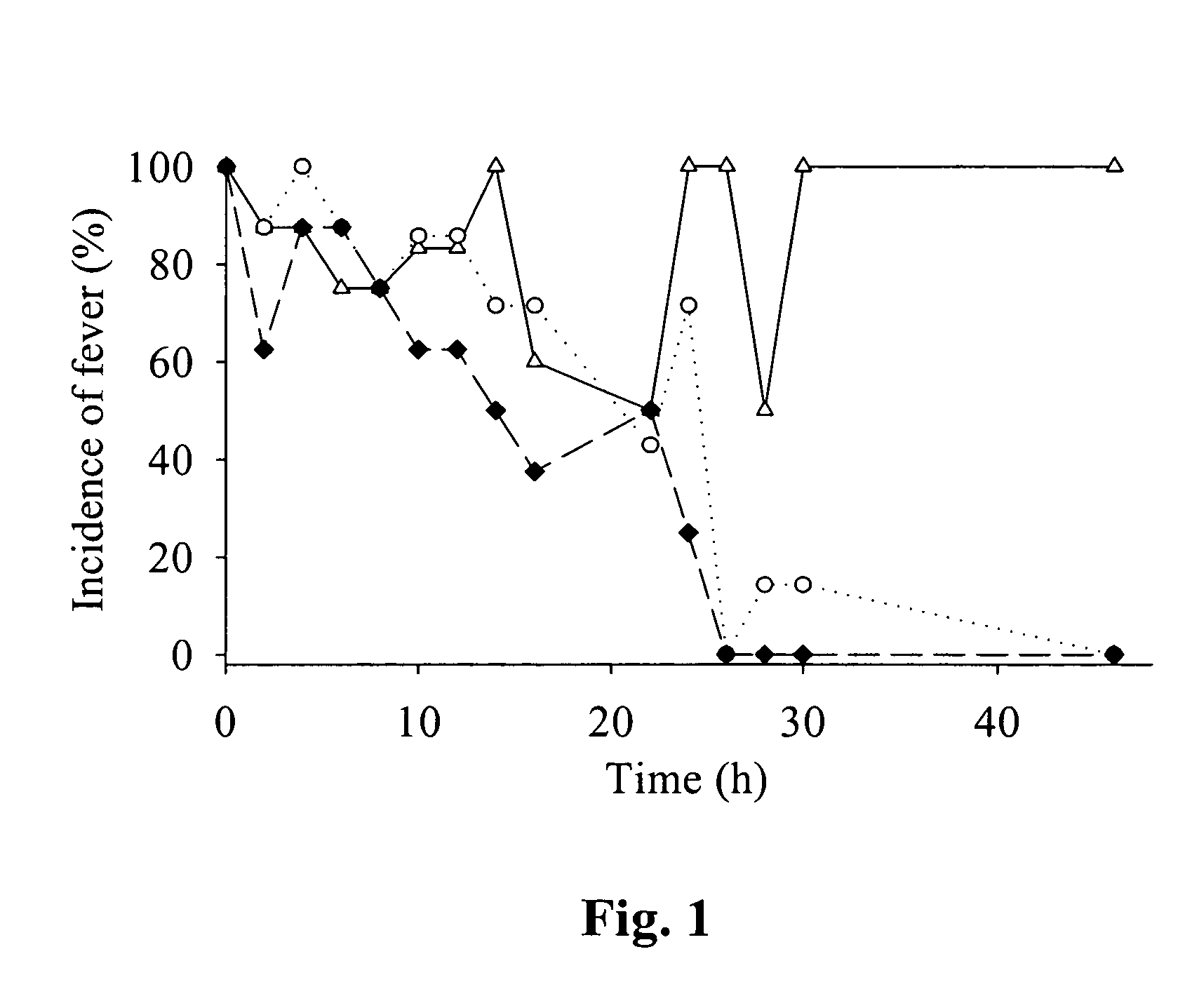
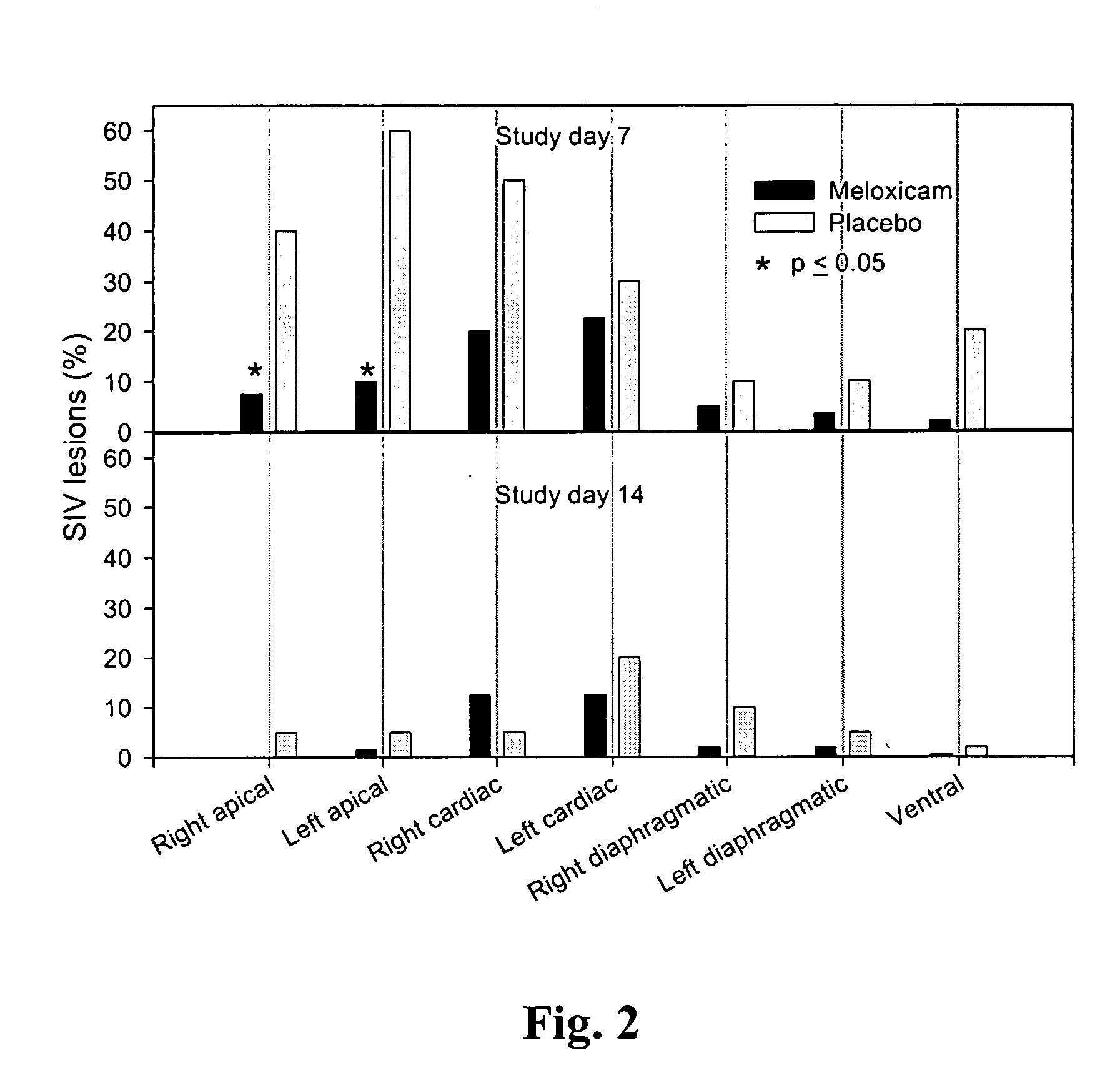
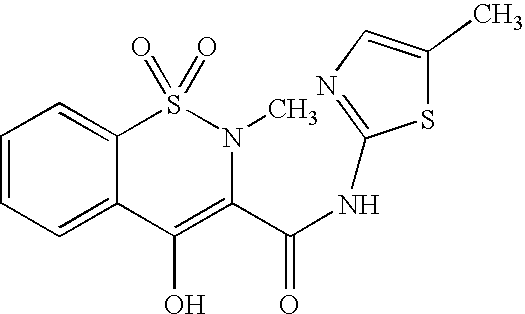
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
175 results about "Meloxicam" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
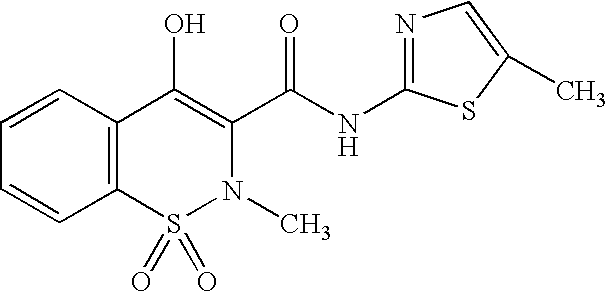
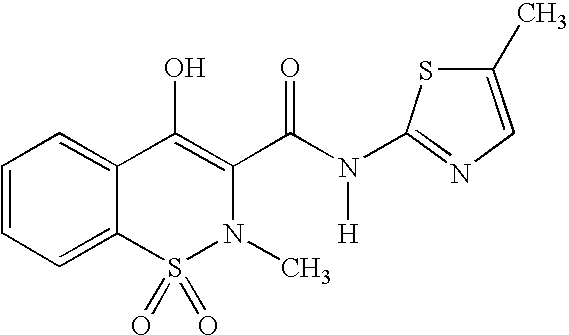
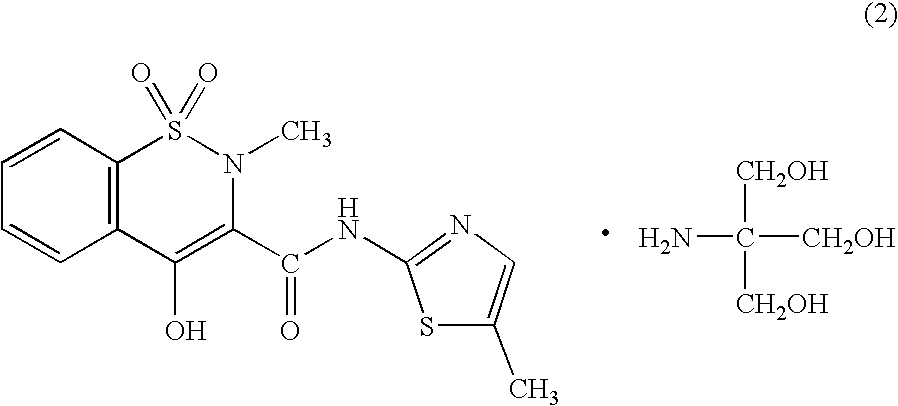
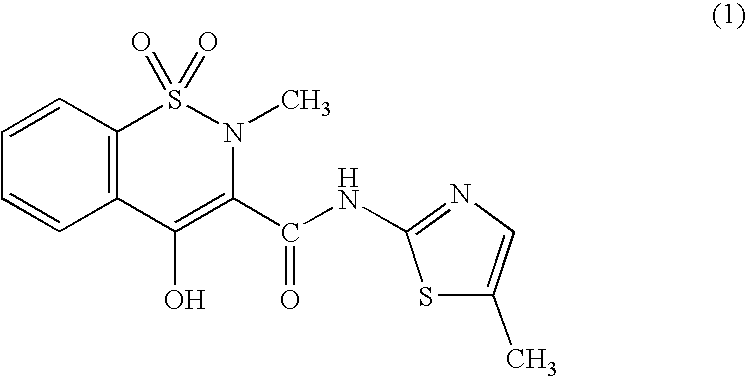
Meloxicam is used to treat arthritis.
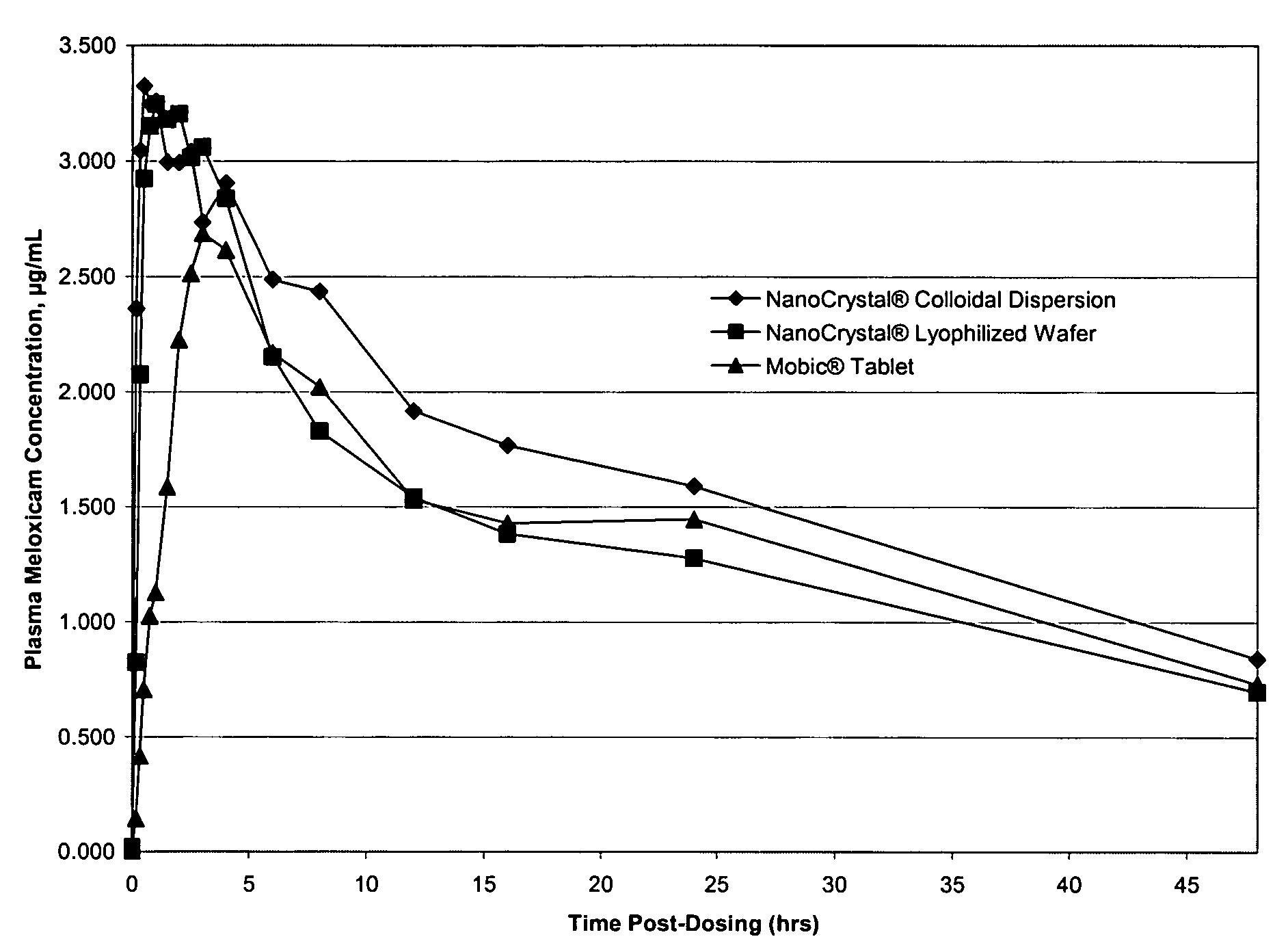
Nanoparticulate meloxicam formulations
InactiveUS8512727B2Improve bioavailabilitySuperior Cmax profileSenses disorderNervous disorderMeloxicamNanoparticle
Owner:ALKERMES PHARMA IRELAND LTD +1
Anti-inflammatory and analgesic compositions and related methods
Methods and compositions for delivering a meloxicam compound are disclosed and described. In one aspect, a method may include perorally administering to a subject a therapeutically effective amount of a meloxicam compound that provides a meloxicam plasma concentration within 1 hour which is at least about 40% of the maximum plasma concentration attained by the formulation. In another aspect, a composition may include a therapeutically effective amount of a meloxicam compound in a pharmaceutically acceptable carrier including at least one of an alkalizer or a solubilizer, with the meloxicam compound having a solubility in the carrier that is greater than about 1.0 mg / gm.
Owner:LIPOCINE
In vivo studies of crystalline forms of meloxicam
ActiveUS20090203680A1Improve bioavailabilityHigh dissolution rateOrganic chemistryAntipyreticMeloxicamDissolution
The invention is directed to novel crystalline forms of meloxicam. These novel crystalline forms of meloxicam have improved bioavailability, an enhanced rate of dissolution and shorter time to Cmax in blood, as compared to pure meloxicam.
Owner:THAR PHARMA
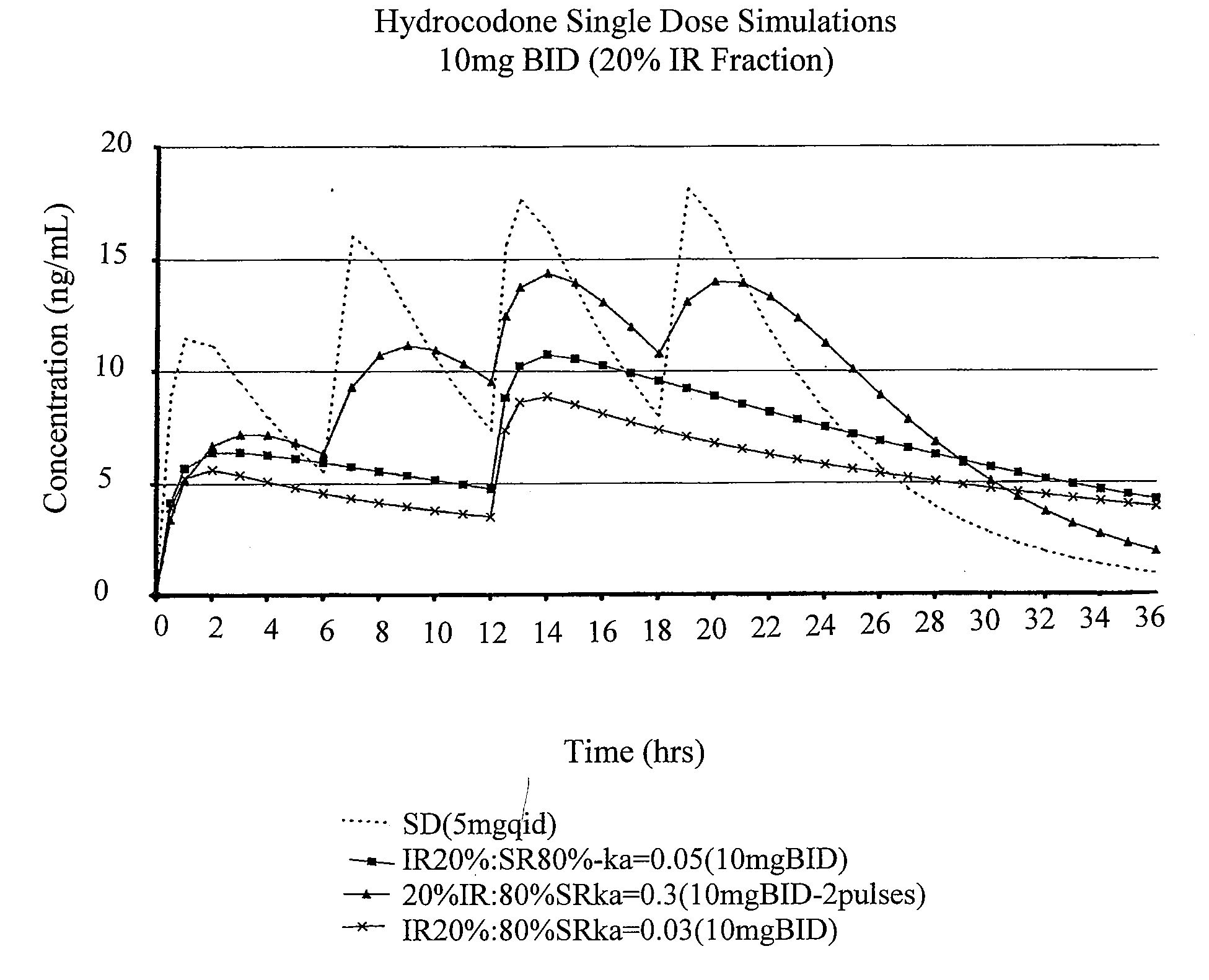
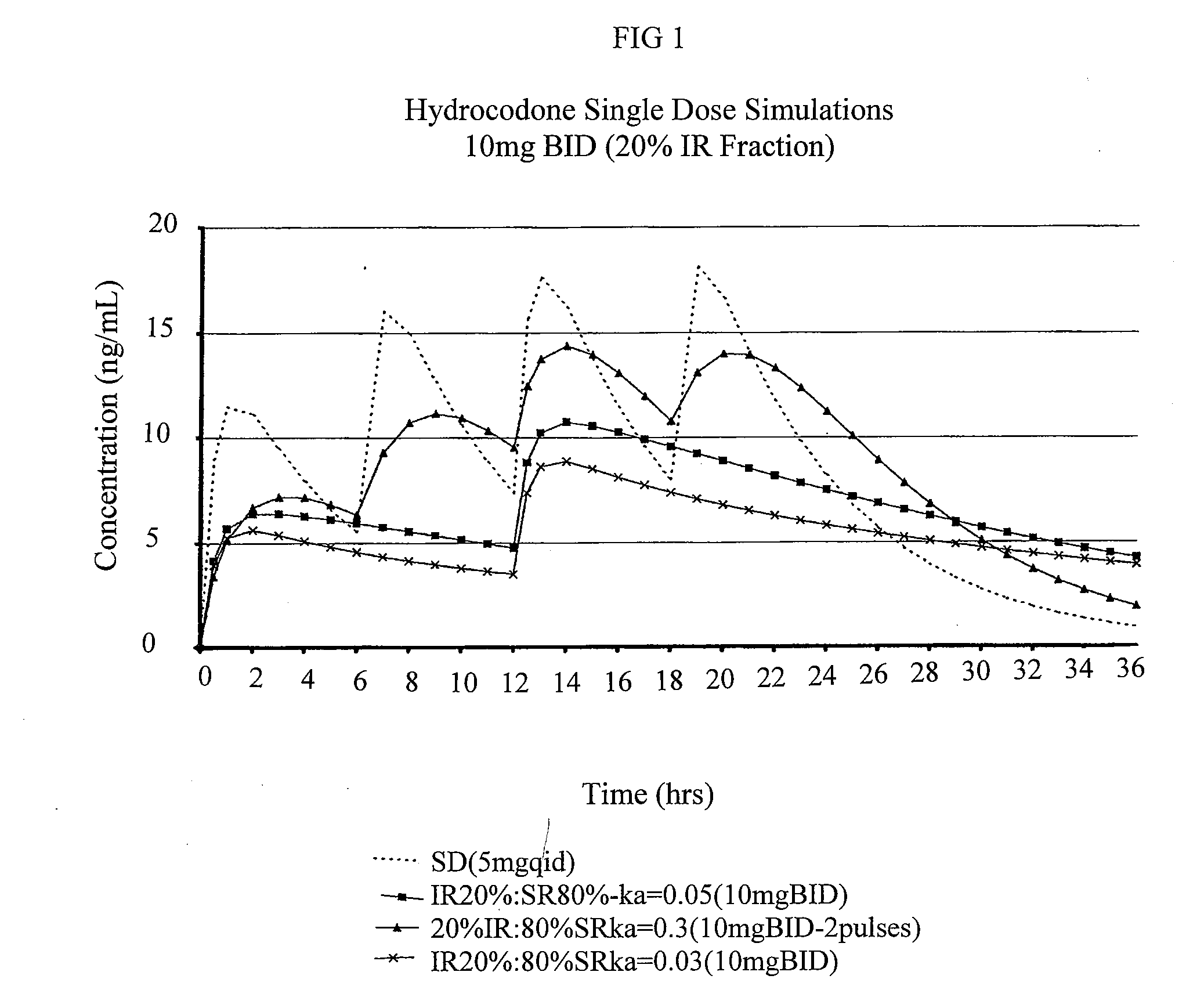
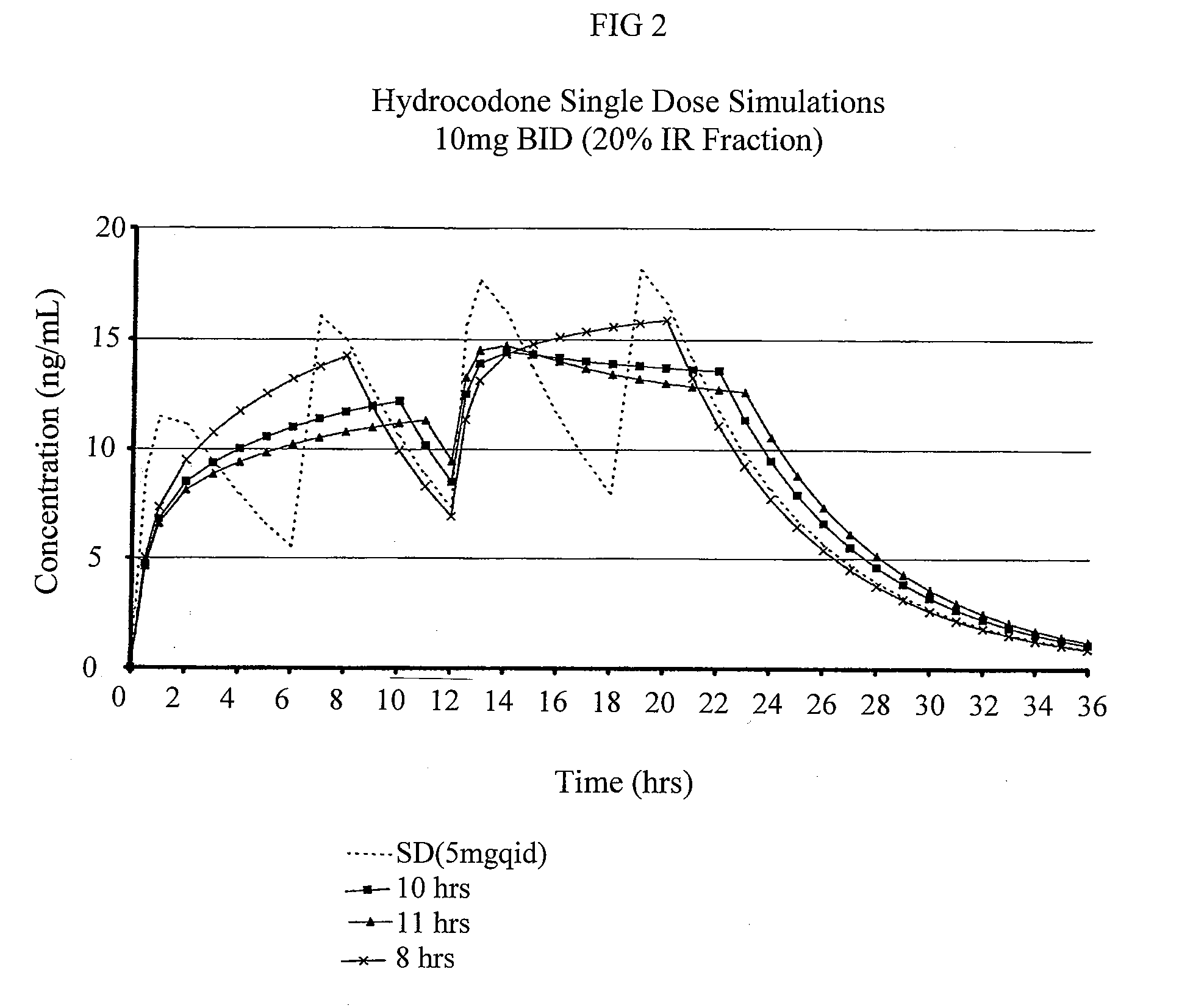
Compositions comprising nanoparticulate meloxicam and controlled release hydrocodone
InactiveUS20080102121A1Increasing patient convenienceImprove complianceBiocidePowder deliveryMeloxicamControl release
The invention relates to a compositions comprising a nanoparticulate meloxicam composition in combination with a multiparticulate modified release hydrocodone composition that, upon administration to a patient, delivers a hydrocodone in a bimodal or multimodal manner. The multiparticulate modified release composition comprises a first component and at least one subsequent component; the first component comprising a first population of hydrocodone-comprising particles and the at least one subsequent component comprising a second population of hydrocodone-comprising particles, wherein the combination of the components exhibit a bimodal or multimodal release profile. The invention also relates to a solid oral dosage form comprising such a combination composition.
Owner:ELAN PHRMA INT LTD
Oral suspension of pharmaceutical substance
InactiveUS6184220B1Low viscosityHigh viscosityDispersion deliverySolution deliveryOral suspensionsMeloxicam
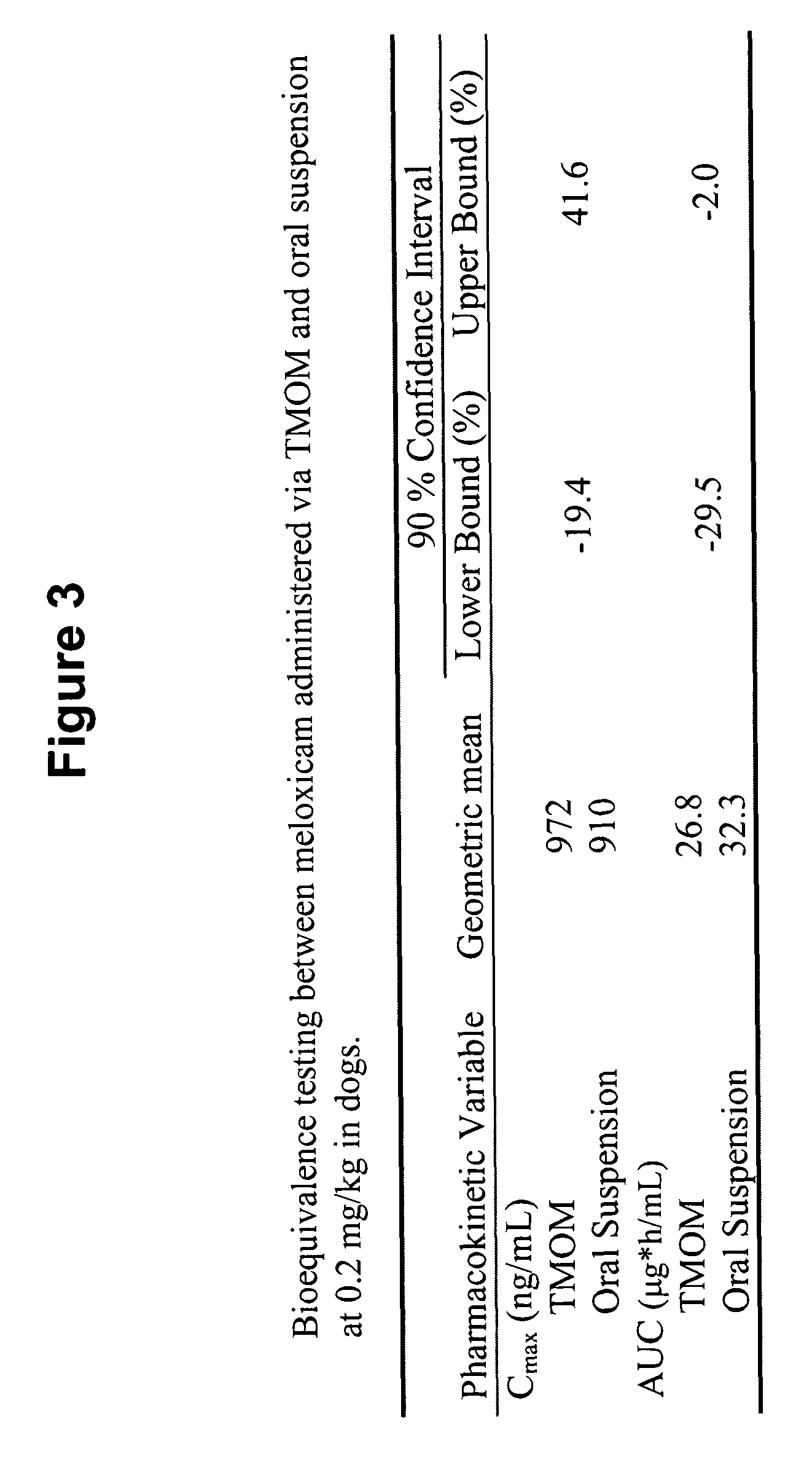
The present invention relates to orally administered suspensions of pharmaceutical active substances of the NSAID type, particularly the antirheumatic agent Meloxicam, which are stabilized by the addition of small amounts of highly dispersed silicon dioxide using high shear forces and adding small amounts of hydrophilic polymers to form a three-dimensional siloid structure, and a process for the preparation thereof.
Owner:BOEHRINGER INGELHEIM PHARM KG
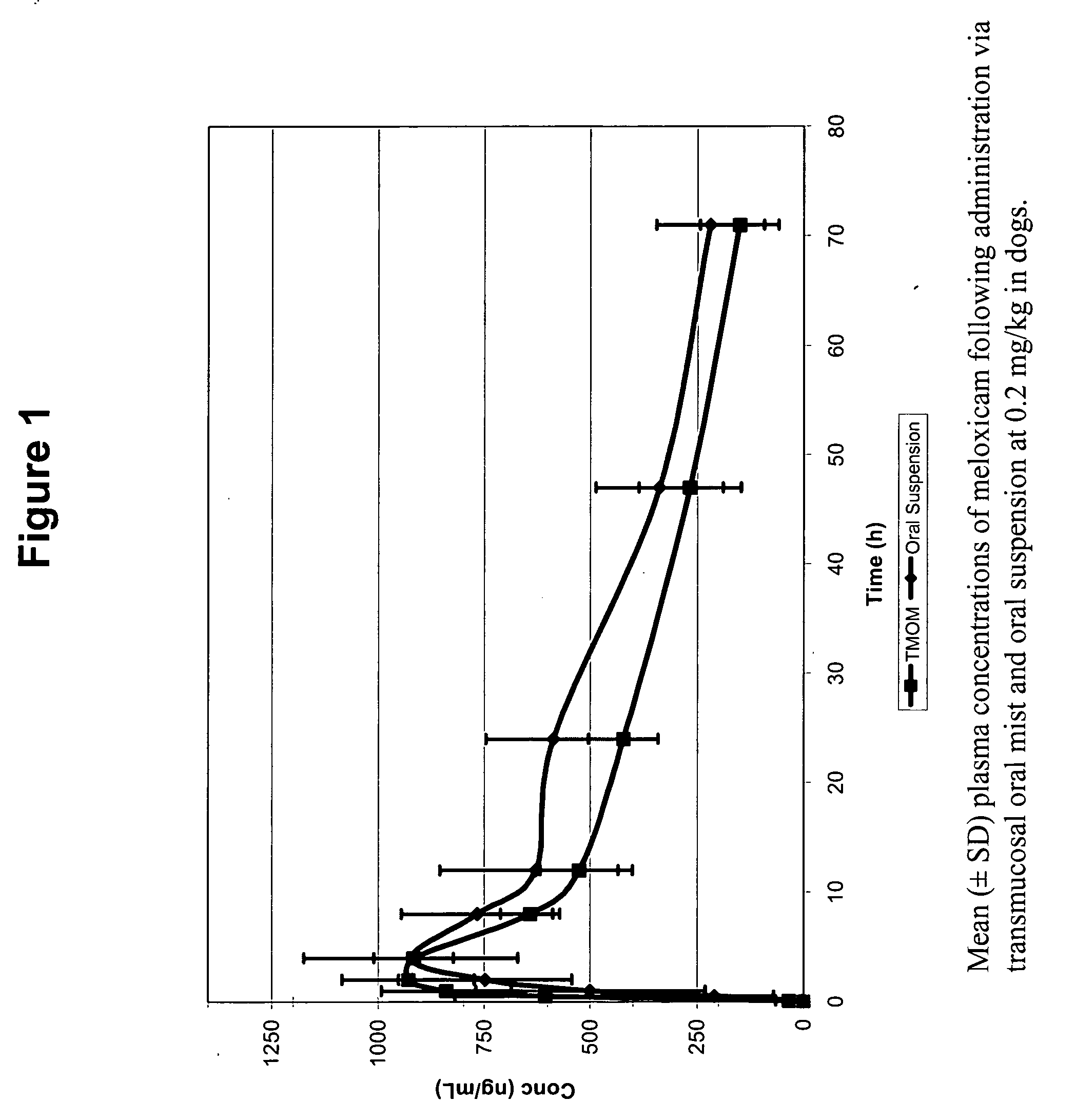
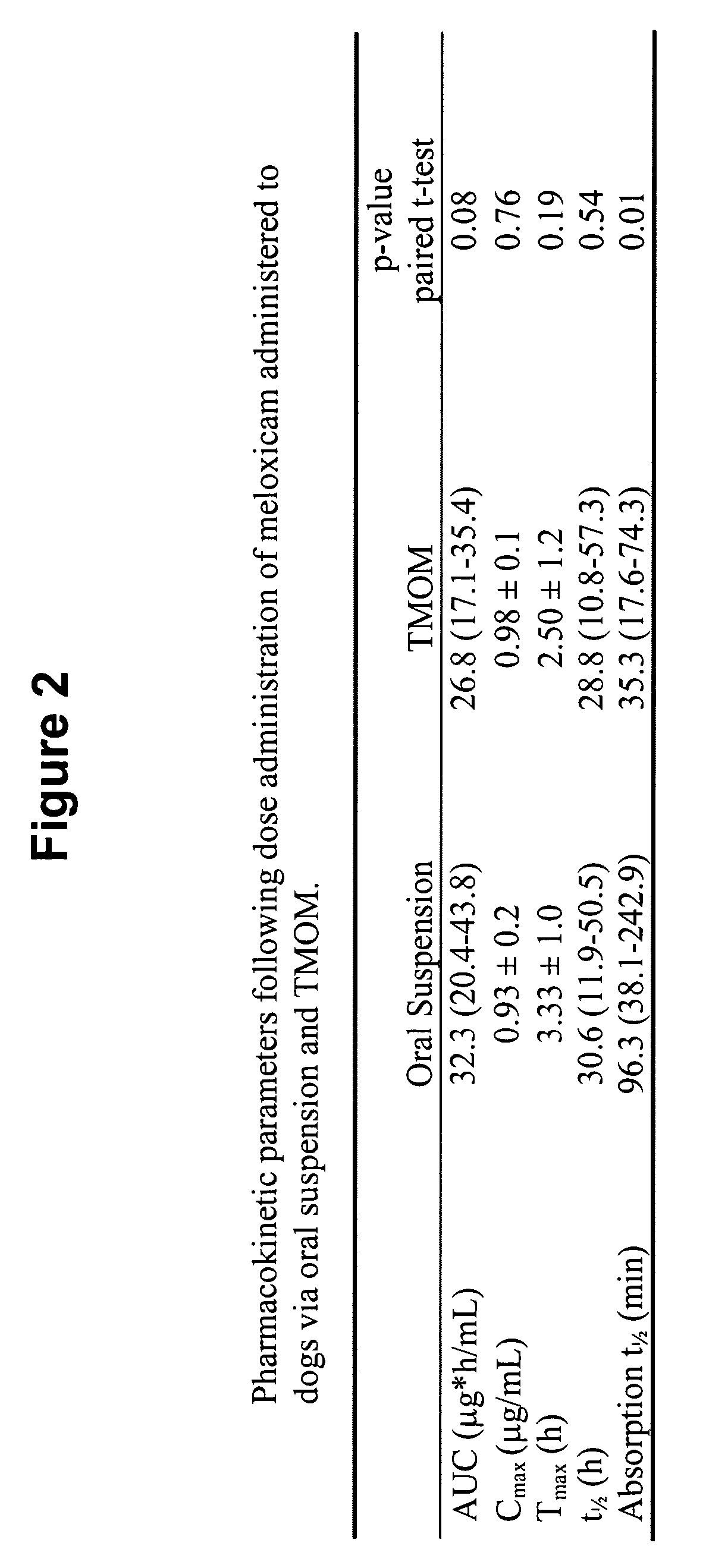
Transmucosal administration of drug compositions for treating and preventing disorders in animals
The invention includes compositions for transmucosal administration to an animal comprising at least one active agent and a pharmaceutically acceptable carrier. A preferred active agent is selected from the group consisting of meloxicam, carprofen, enrofloxacin, clemastine, diphenhydramine, digoxin, levothyroxine, cyclosporine, ondansetron, lysine, zolpidem, propofol, nitenpyram, ivermectin, milbemycin, and pharmaceutically acceptable salts, solvates and esters thereof. In another embodiment, the invention includes methods of treating or preventing a condition in an animal comprising transmucosally administering a composition comprising a therapeutically or prophylactically effective amount of an active agent and a pharmaceutically acceptable carrier.
Owner:ZOTTIS BELGIUM
Analgesic combination of tramadol and meloxicam
InactiveUS20080050427A1Reduce concentrationEfficient managementBiocidePowder deliveryMeloxicamPharmaceutical drug
Disclosed is a pharmaceutical composition, comprising a combination of a dose of meloxicam or a pharmaceutically acceptable salt thereof and a dose of oxycodone or a pharmaceutically acceptable salt thereof, said combination in an amount sufficient to provide an analgesic effect in a human patient. Also disclosed is a method of effectively treating pain in humans or other mammals, comprising administering to the patient a combination of a dose of meloxicam or a pharmaceutically acceptable salt thereof and a dose of oxycodone or a pharmaceutically acceptable salt thereof such that the dosing interval of the meloxicam overlaps with the dosing interval of the oxycodone, said combination in an amount sufficient to provide an analgesic effect in a human patient.
Owner:PURDUE PHARMA LP
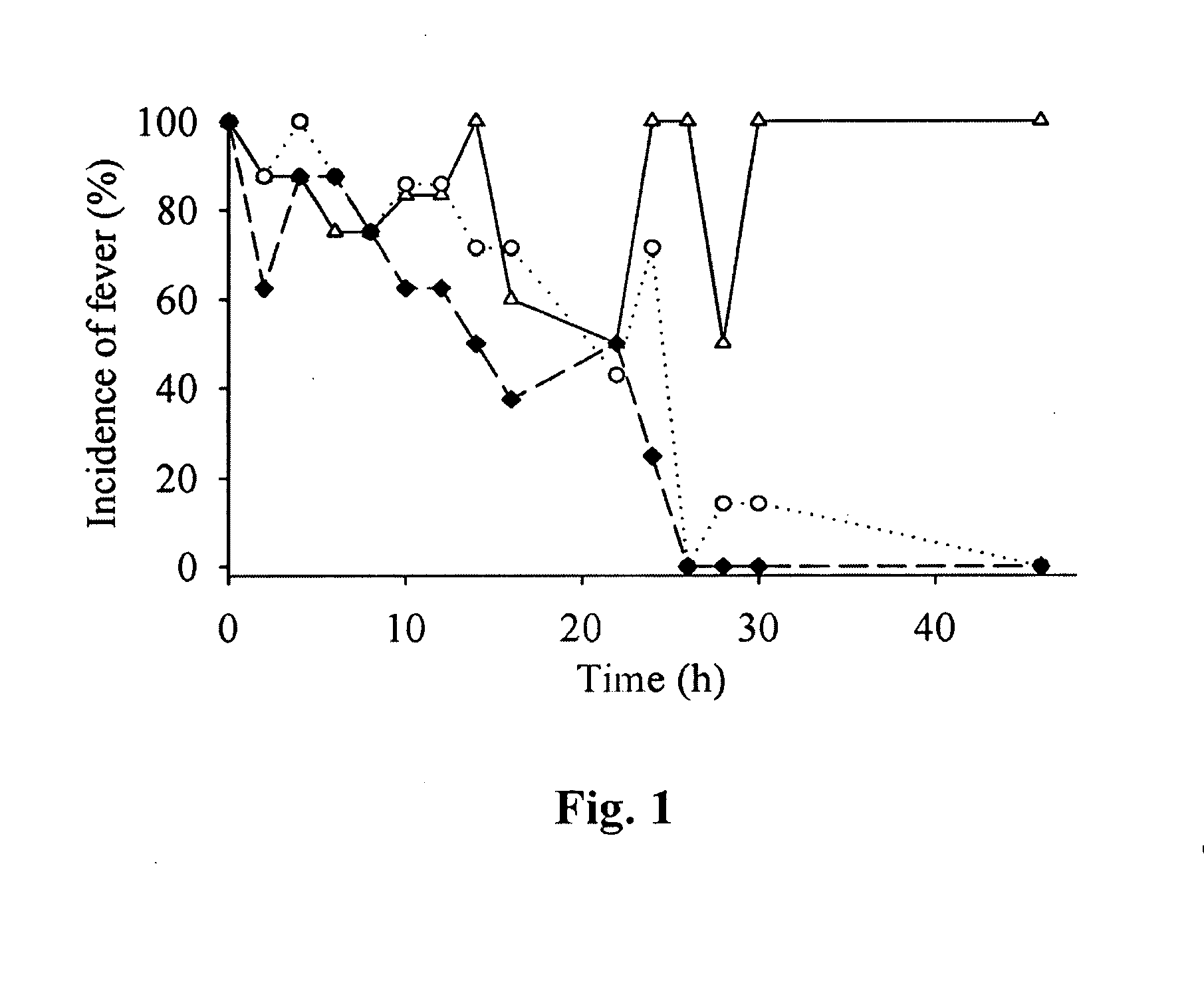
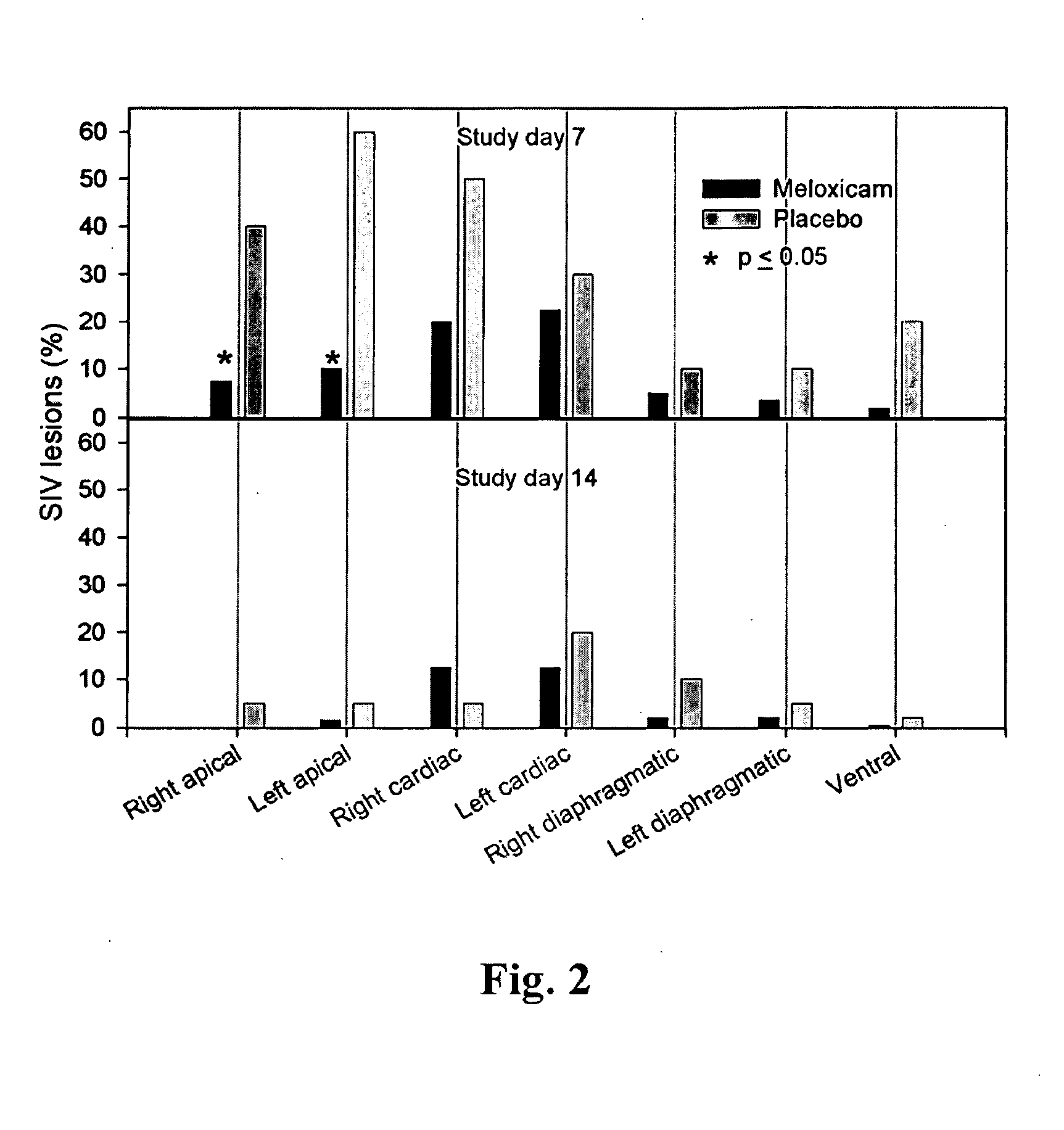
Meloxicam for the treatment of respiratory diseases in pigs
A method of treating or preventing a respiratory disease in a pig, the method comprising administering to the pig in need thereof an effective amount of meloxicam or a pharmaceutically acceptable salt thereof.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Highly concentrated stable meloxicam solutions for needleless injection
Aqueous cyclodextrin-free solution of meloxicam suitable for administration by needleless injection, containing a pharmacologically acceptable meloxicam salt of an organic or inorganic base and one or more suitable excipients, the content of dissolved meloxicam salt being from 35 to 100 mg / ml. The formulation according to the invention has a shelf-life of up to 24 months or more.
Owner:FOLGER MARTIN ANDREAS +3
Composition and method for compounded therapy
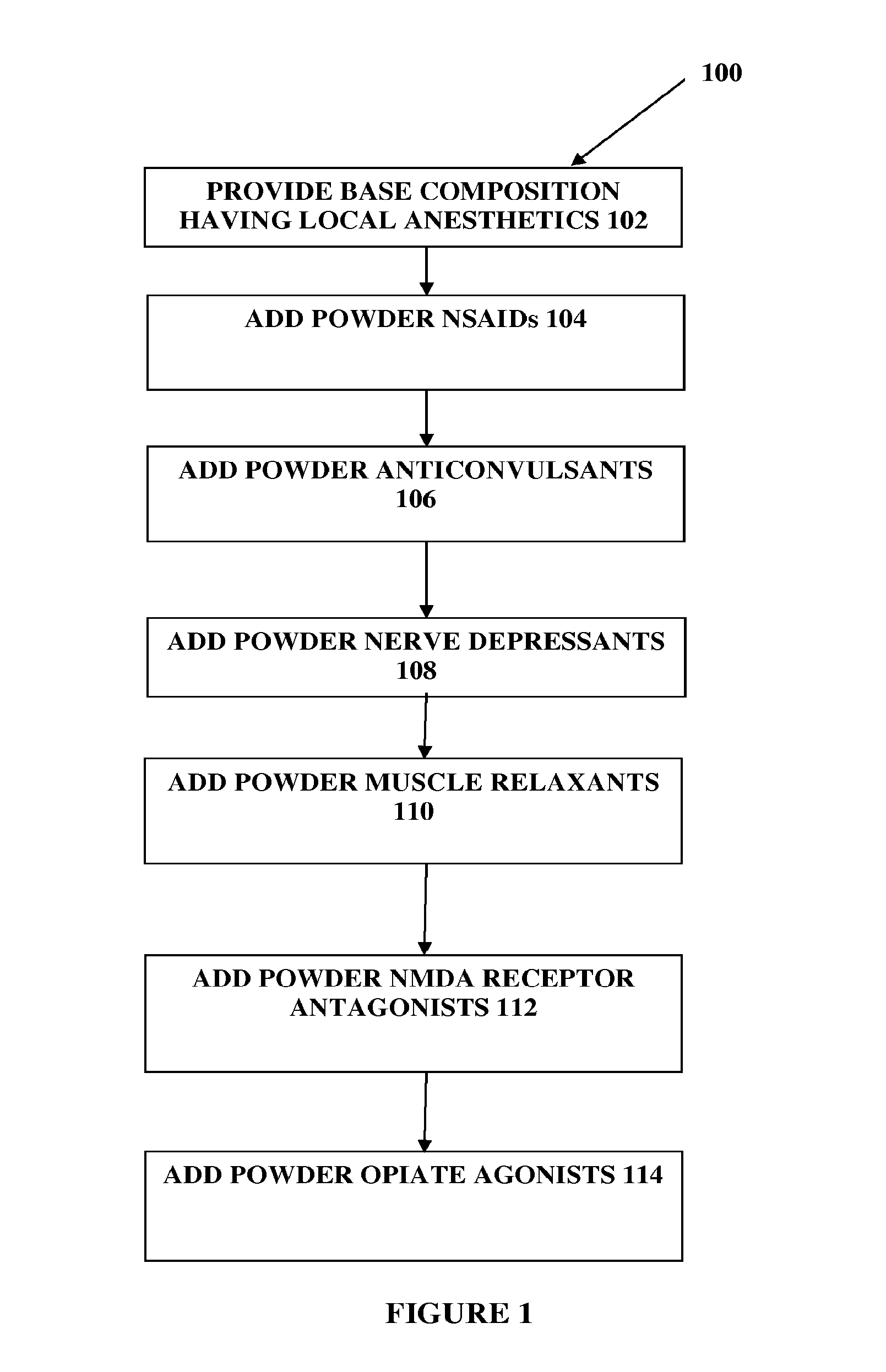
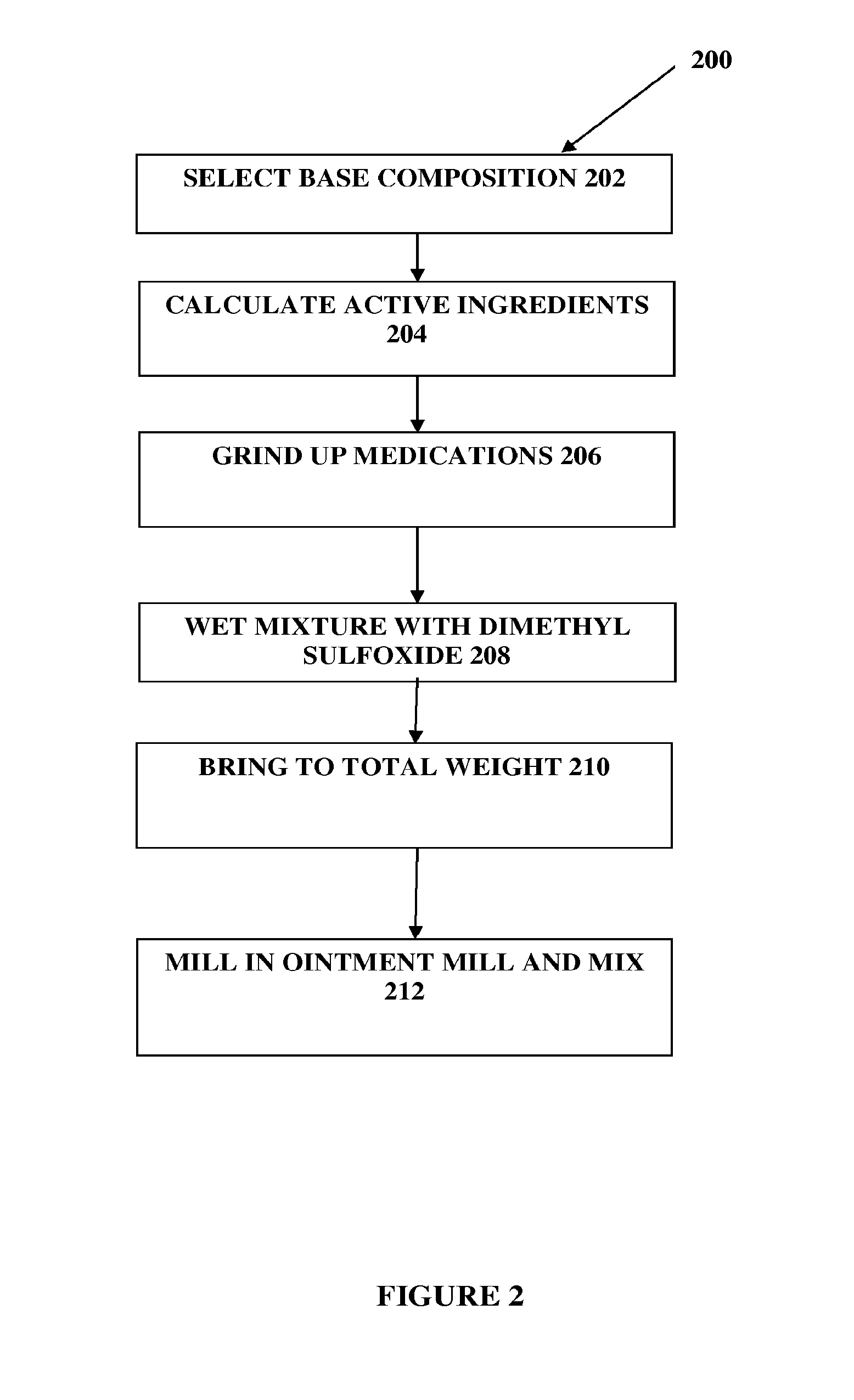
The present embodiments relate to topically delivered compounded medications. A transdermal cream may provide the effective topical administration of multiple medications simultaneously; may include low concentrations of local anesthetics, a NSAID, an anticonvulsant, and / or other active ingredients; and may include lidocaine, prilocaine, meloxicam, and lamotrigine and / or topiramate. Alternatively, the transdermal cream may include a lidocaine / prilocaine base cream to which is added a fine powder of one or more ground up medications to form a compounded medication. The compounded medication in powder form may be generated from grinding up tablets of NSAIDs, anticonvulsants, nerve depressants, antidepressants, muscle relaxants, NMDA receptor antagonists, opiate or opioid agonists, and / or other agents. The compounded medication in powder form may include meloxicam, lamotrigine, topiramate, other active ingredients, and DMSO or Sterile Water for Irrigation. In another aspect, the present embodiments relate to methods of compounding medications and transdermal creams or gels.
Owner:CMPD LICENSING
Highly concentrated stable meloxicam solutions
Aqueous cyclodextrin-free solution of meloxicam for administration by oral or parenteral route, containing a pharmacologically acceptable meloxicam salt of an organic or inorganic base and one or more suitable excipients, the content of dissolved meloxicam salt being more than 10 mg / mL. The formulation according to the invention has a shelf-life of up to 24 months or more.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Ophthalmic aqueous pharmaceutical preparation
InactiveUS7105512B2Good anti-inflammatory effectLess stimulationBiocideSenses disorderMeloxicamDrugs preparations
The present invention provides an ophthalmic aqueous pharmaceutical preparation, which is excellent in anti-inflammatory effect, which is less stimulative and which has high safety and excellent storage stability. The ophthalmic aqueous pharmaceutical preparation comprises meloxicam and trometamol.
Owner:WAKAMOTO PHARMA
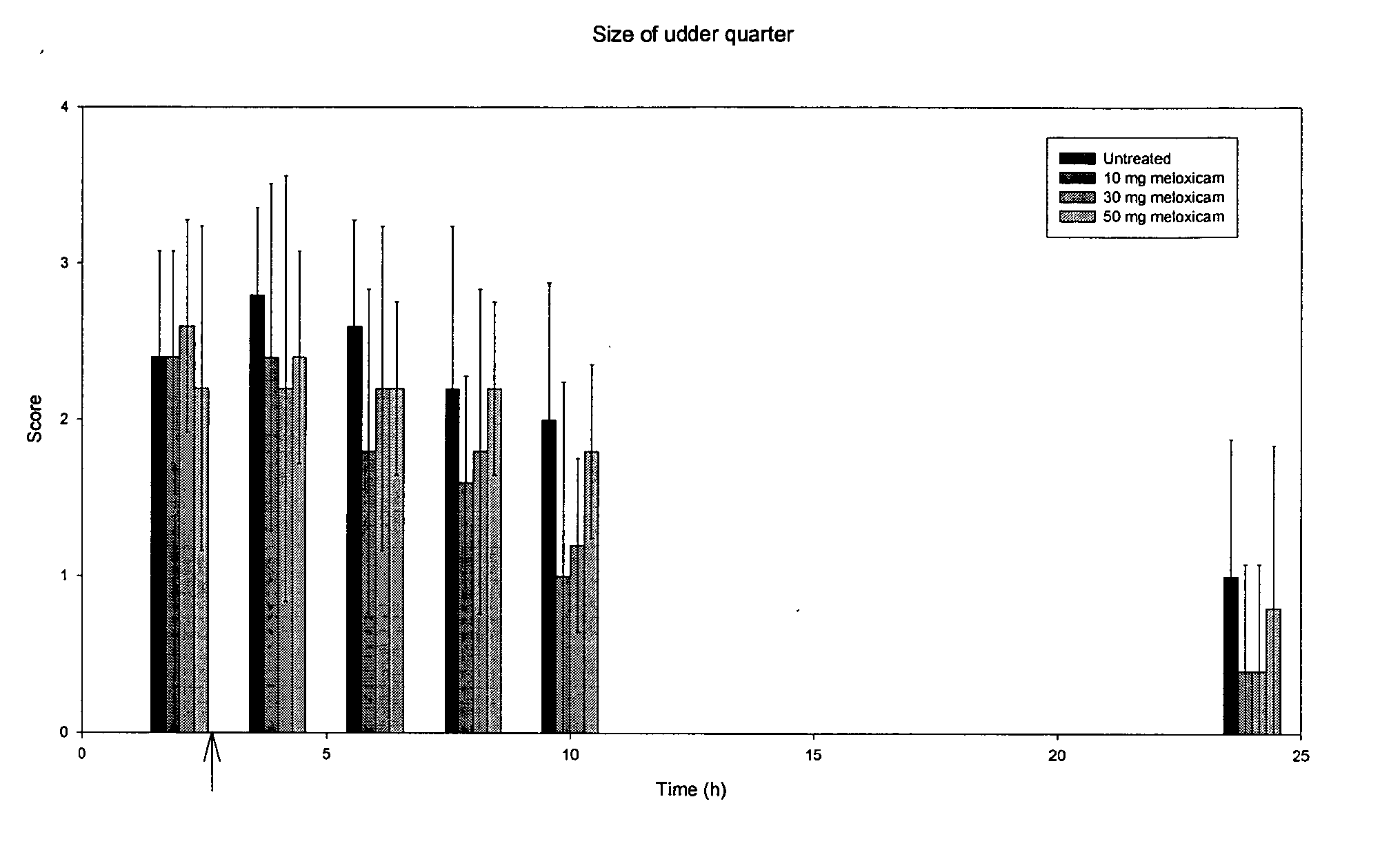
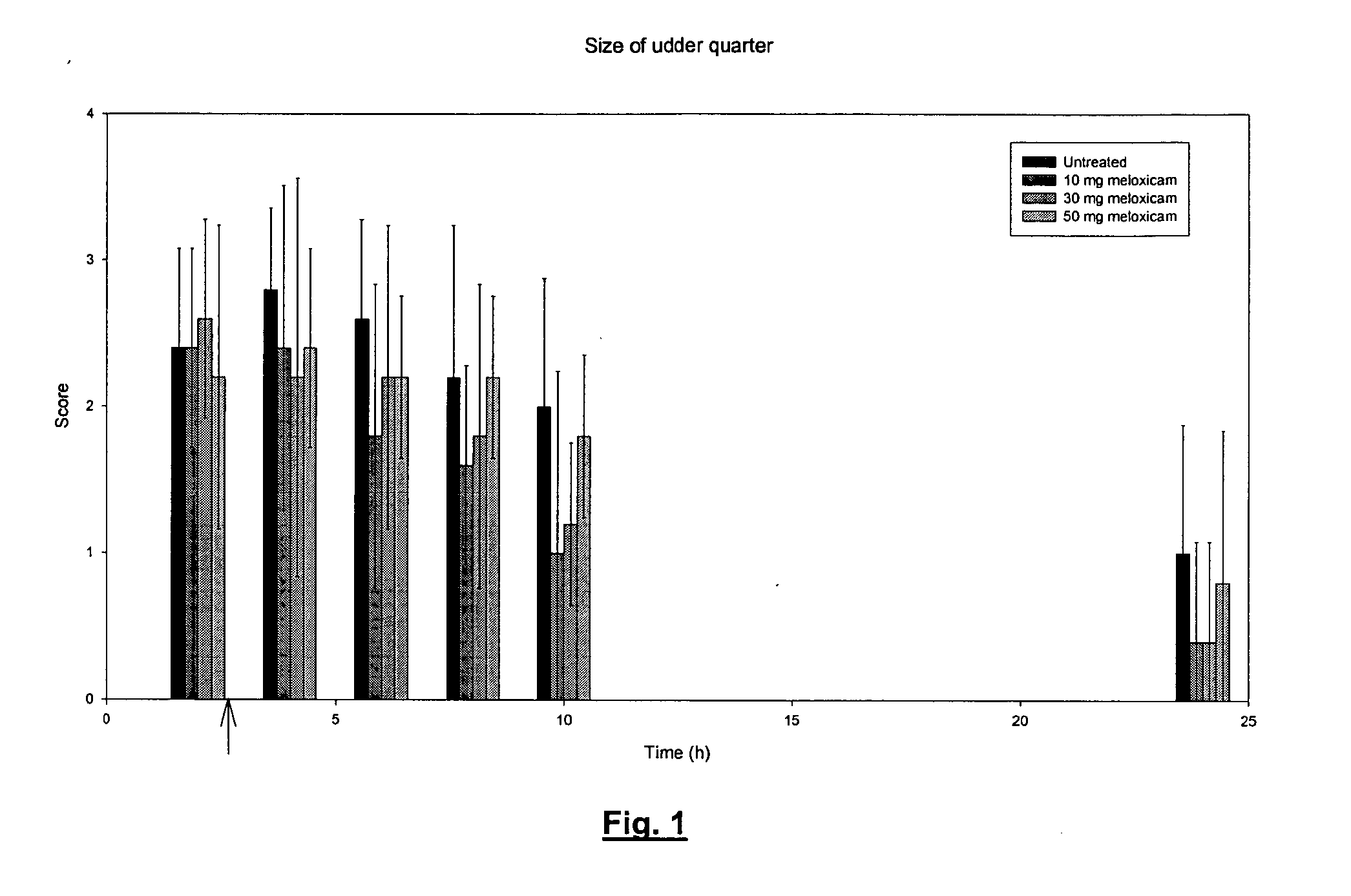
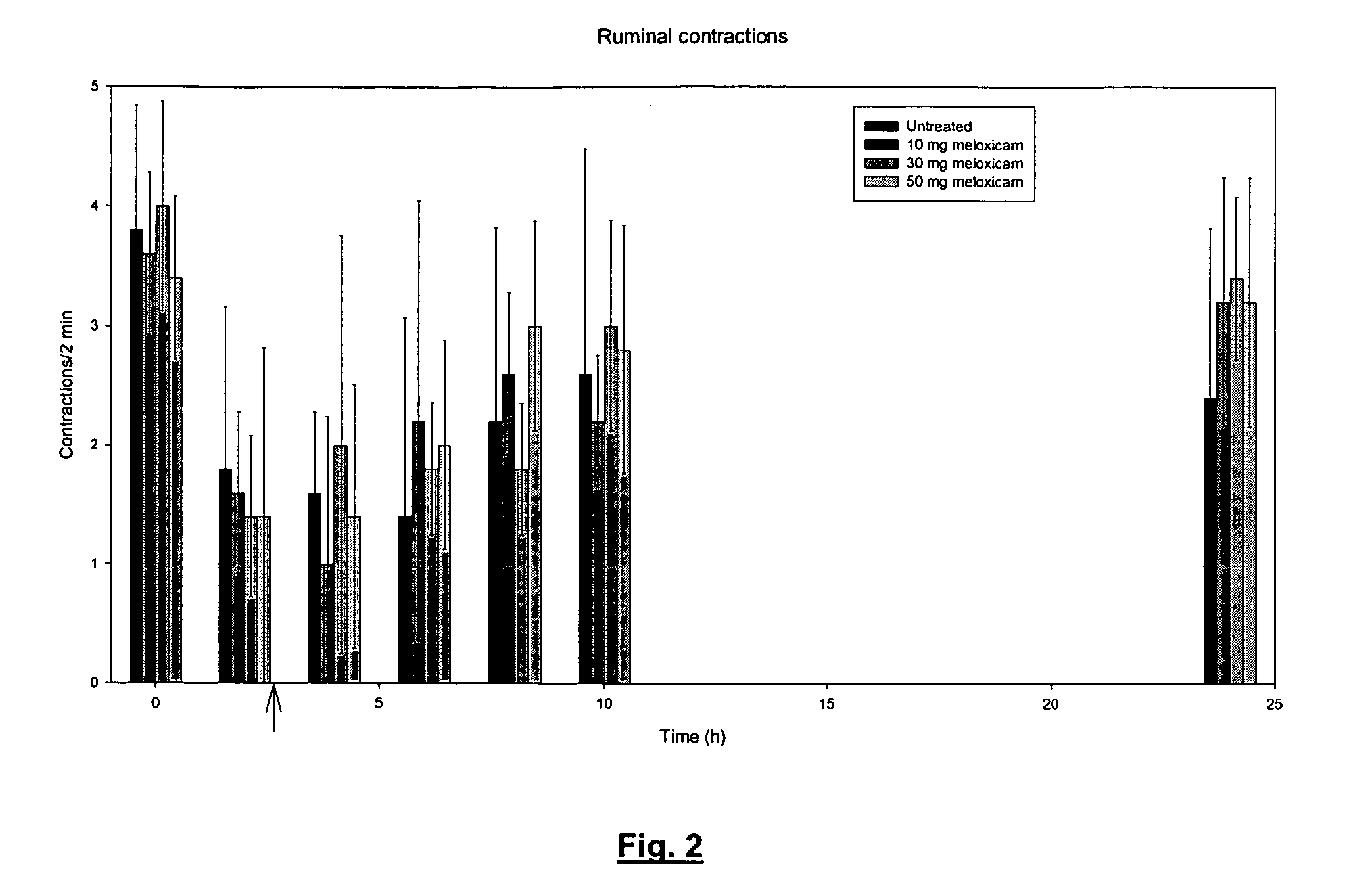
Meloxicam in veterinary medicine
A veterinary pharmaceutical formulation containing meloxicam or a pharmacologically acceptable meloxicam salt of an organic or inorganic base and one or more vehicles having analgesic efficacy for the treatment of inflammatory painful diseases, particularly for the treatment of mild or moderate mastitis cases. The treatment leads to an effective long lasting reduction of a hypersensitive state associated with inflammatory pain in mild or moderate mastitis cases, particularly chronic states thereof.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Pharmaceutical formulations of meloxicam
This invention is a novel pharmaceutical formulation of aqueous EDTA (Ethylene diamine tetraacetic acid) free solution of meloxicam in combination with meglumin for administration by oral or parenteral route, comprising one or more pharmaceutically acceptable excipients which is comprising N,N dimethylacetamide and propylene glycol for treating mammals, preferably animals.
Owner:SANOVEL ILAC SANAYI & TICARET ANONIM SIRKETI +1
Nanoparticulate meloxicam formulations
The present invention is directed to nanoparticulate compositions comprising meloxicam particles having an effective average particle size of less than about 2000 nm.
Owner:ALKERMES PHARMA IRELAND LTD
Meloxicam compositions
InactiveUS20050038018A1Increase doseImprove efficacyCosmetic preparationsBiocideJoint arthralgiaStimulant
A pharmaceutical composition comprising: (a) meloxicam or a pharmaceutically acceptable salt thereof; and (b) one or more additional pharmaceutically active compounds selected from antacids, sedatives, and central nervous system stimulants, and the use of such composition of an inflammatory disease, symptoms of an inflammatory disease, including various symptoms thereof, and / or headache, toothache, ache after tooth extraction, sore throat, otalgia, arthralgia, neuralgia, lumbago, myalgia, muscle stiffness of shoulder, pain of contusion, pain of fracture, pain of sprain, menstrual pain, traumatic pain, chill, exothermic reaction and / or cold and various symptoms of cold such as sore throat, chill, pyrexia, headache, arthralgia and muscle pain.
Owner:BOEHRINGER INGELHEIM INT GMBH
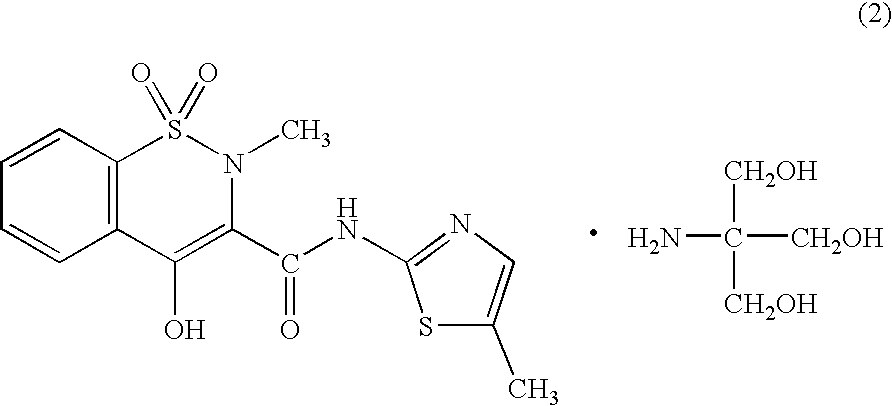
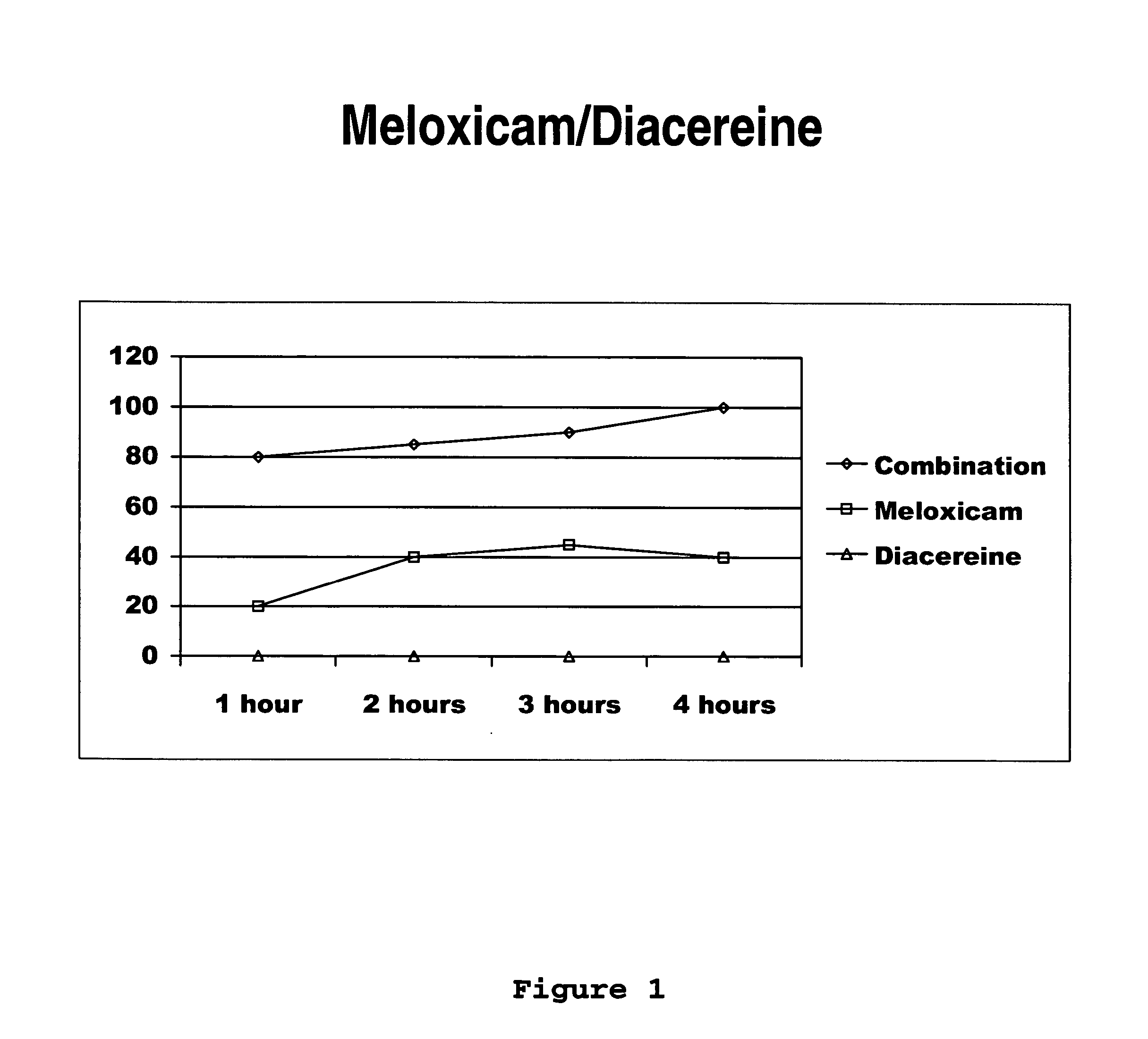
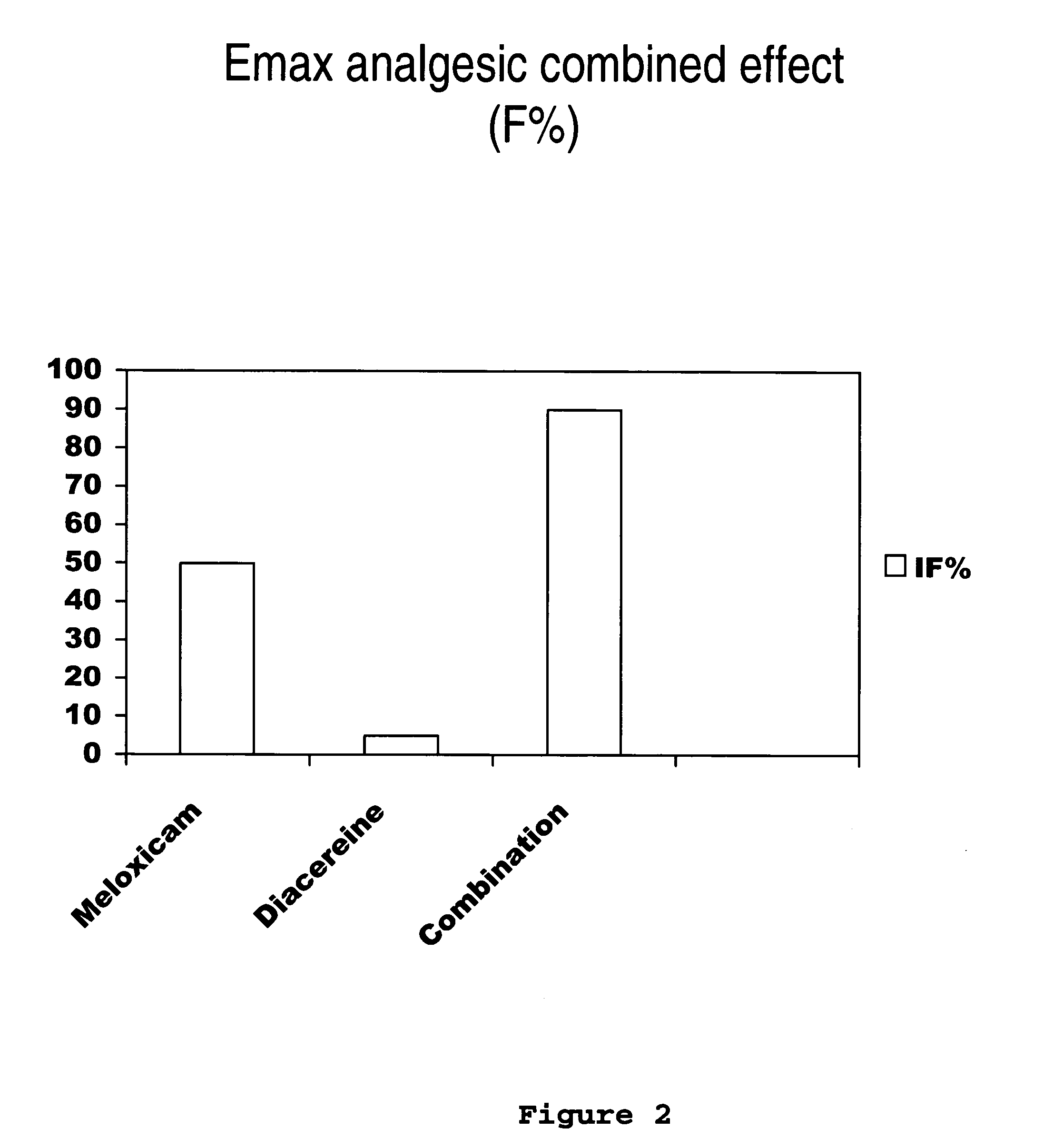
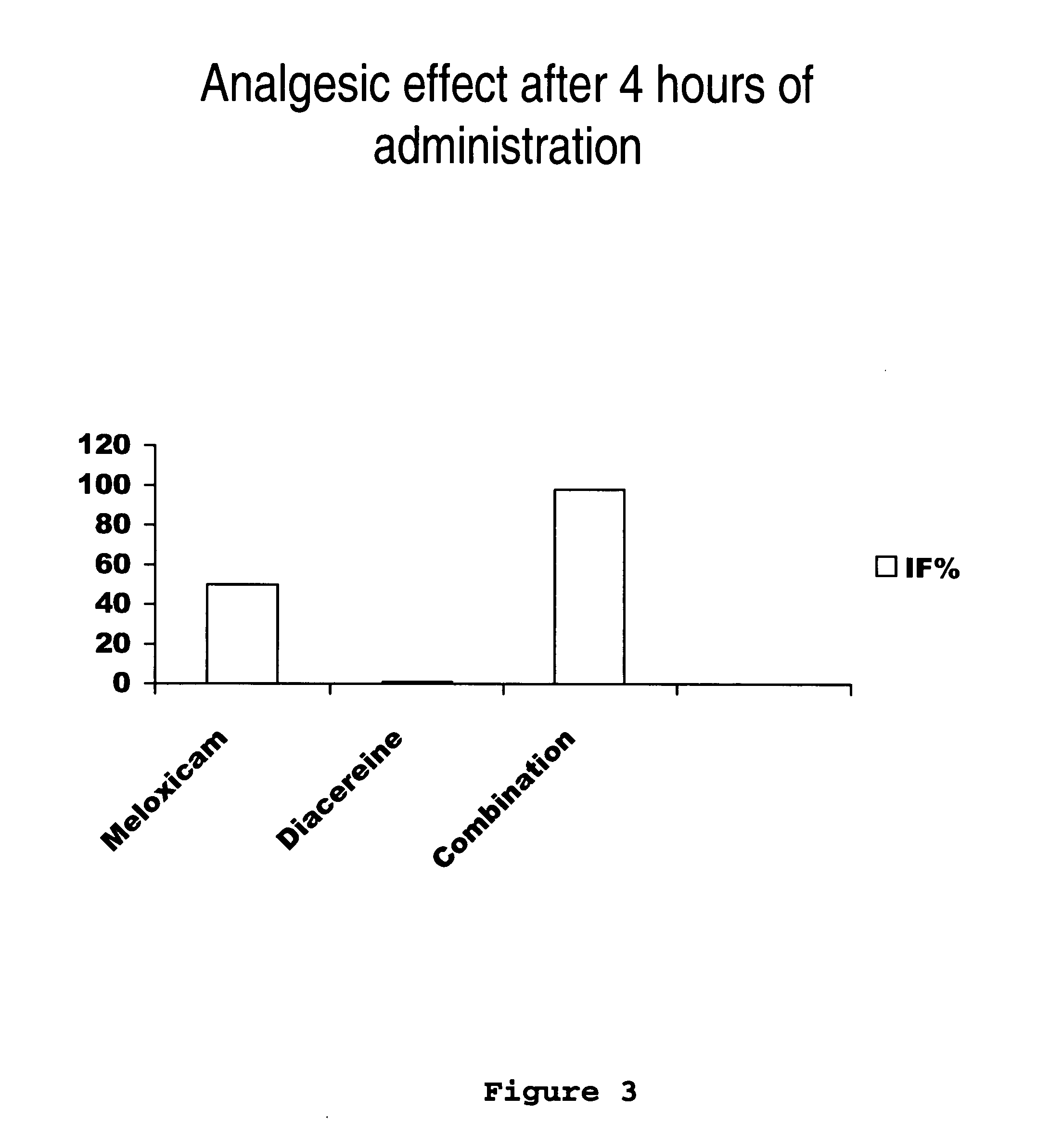
Solid pharmaceutical formulations comprising Diacereine and Meloxicam
InactiveUS20060074079A1Relieve painModifier of cartilage structureBiocideAntipyreticEtiologyGout arthritis
This invention relates to formulations in solid pharmaceutical forms containing diacereine and meloxicam. The present invention provides novel formulations comprising: (a) Diacereine, (b) Meloxicam, (c) one or more anti-adherent agents, (d) one or more disintegrating agents, (e) one or more binder agents, (f) one or more lubricants, (g) one or more diluents, (h) one or more solvents, and (i) any other additive which assists in formulation. The present invention also provides a method for treatment of osteoarthritis, rheumatoid arthritis, gout arthritis, multiple sclerosis, amyotrophic lateral sclerosis and related diseases, in addition of inflammatory processes originated from various etiologies, by administering suitable doses.
Owner:ESPINOSA ABDALA LEOPOLDO
Use of meloxicam formulations in veterinary medicine
The invention is directed to the use of a formulation containing meloxicam or a pharmacologically acceptable meloxicam salt of an organic or inorganic base, one or more vehicles and one or more suitable additives for preparing a veterinary medical composition for intramammary treatment of inflammatory diseases in mammals, particularly mastitis. The intramammary administration leads to an effective concentration in the target tissue, which is achieved very quickly.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
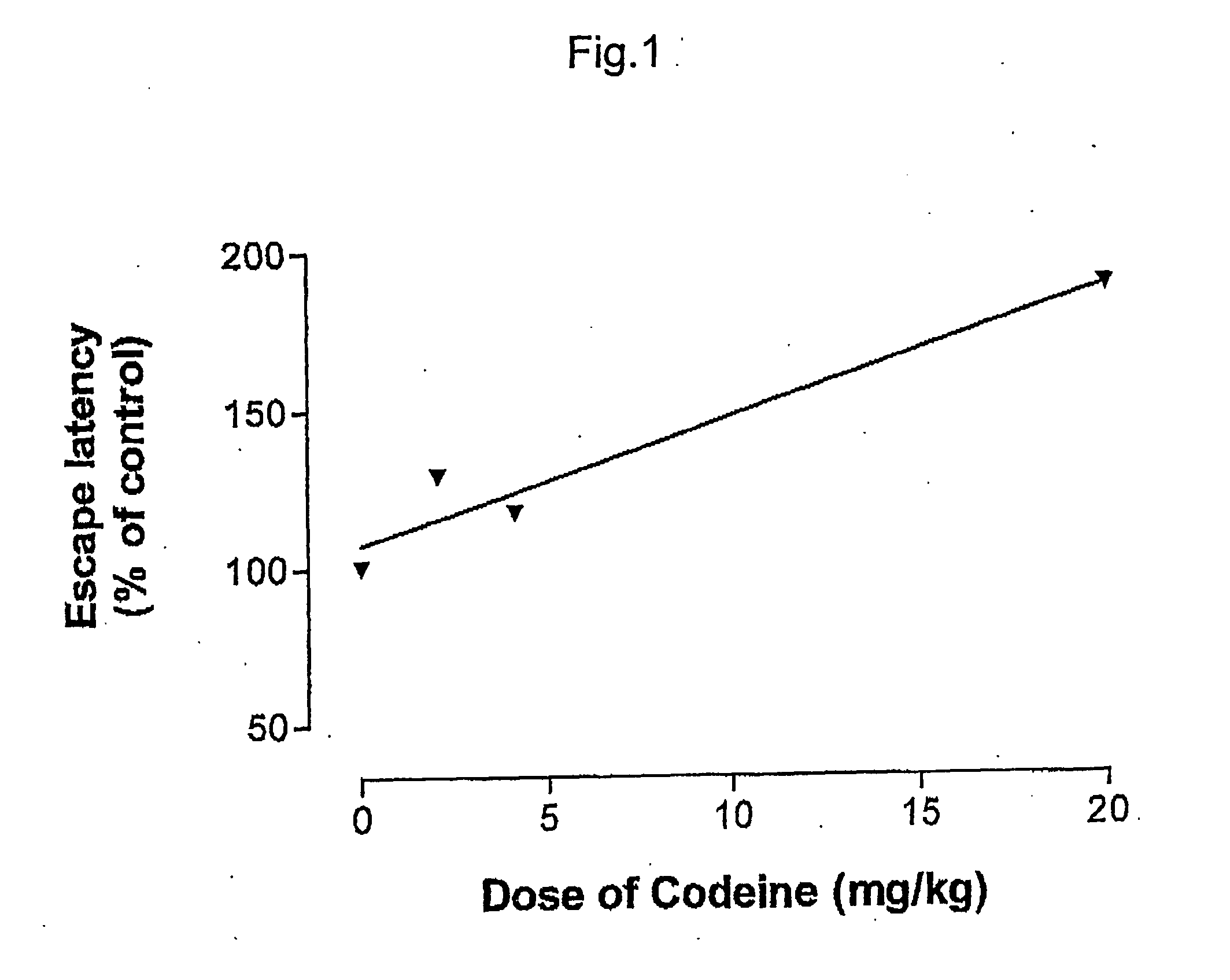
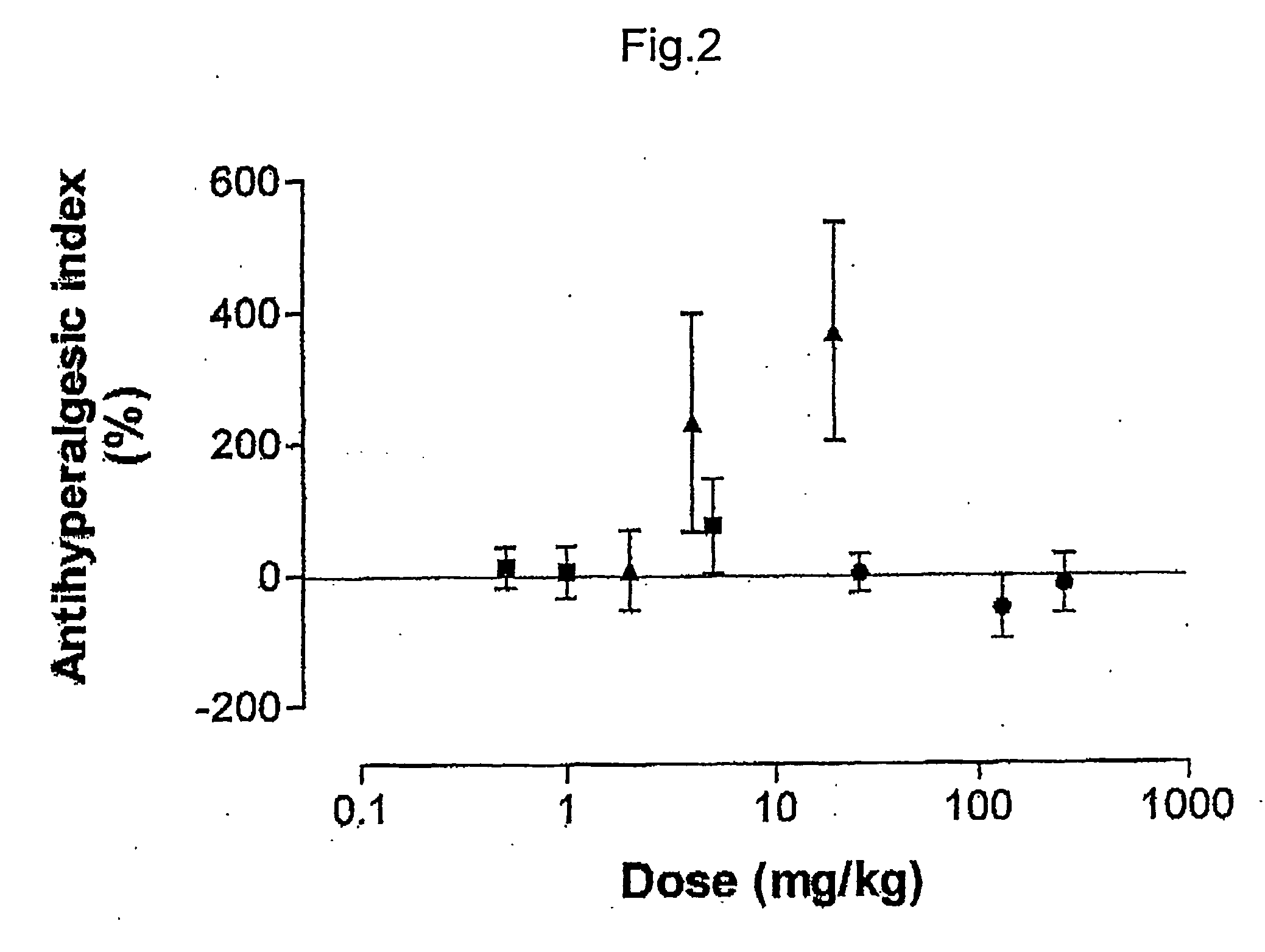
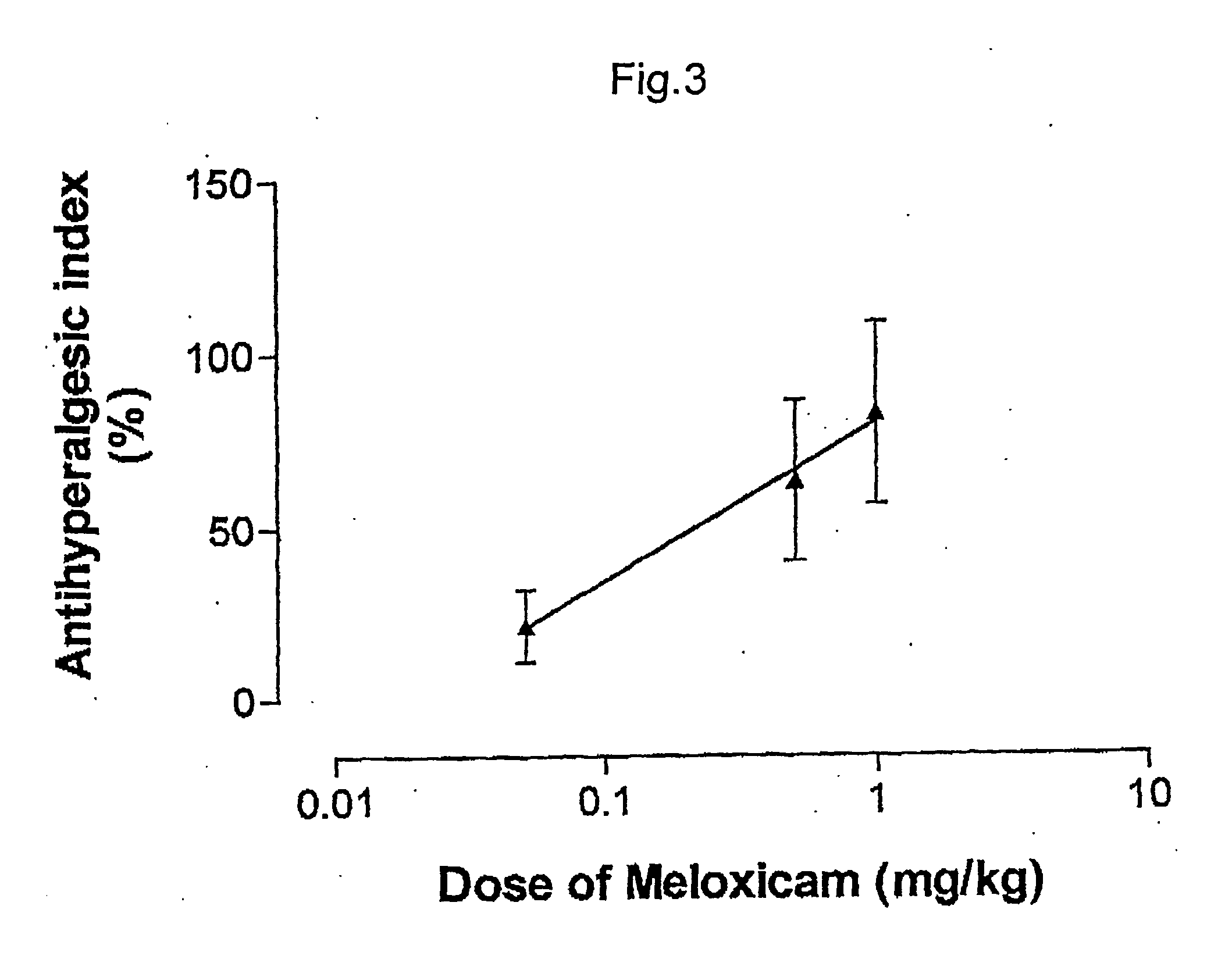
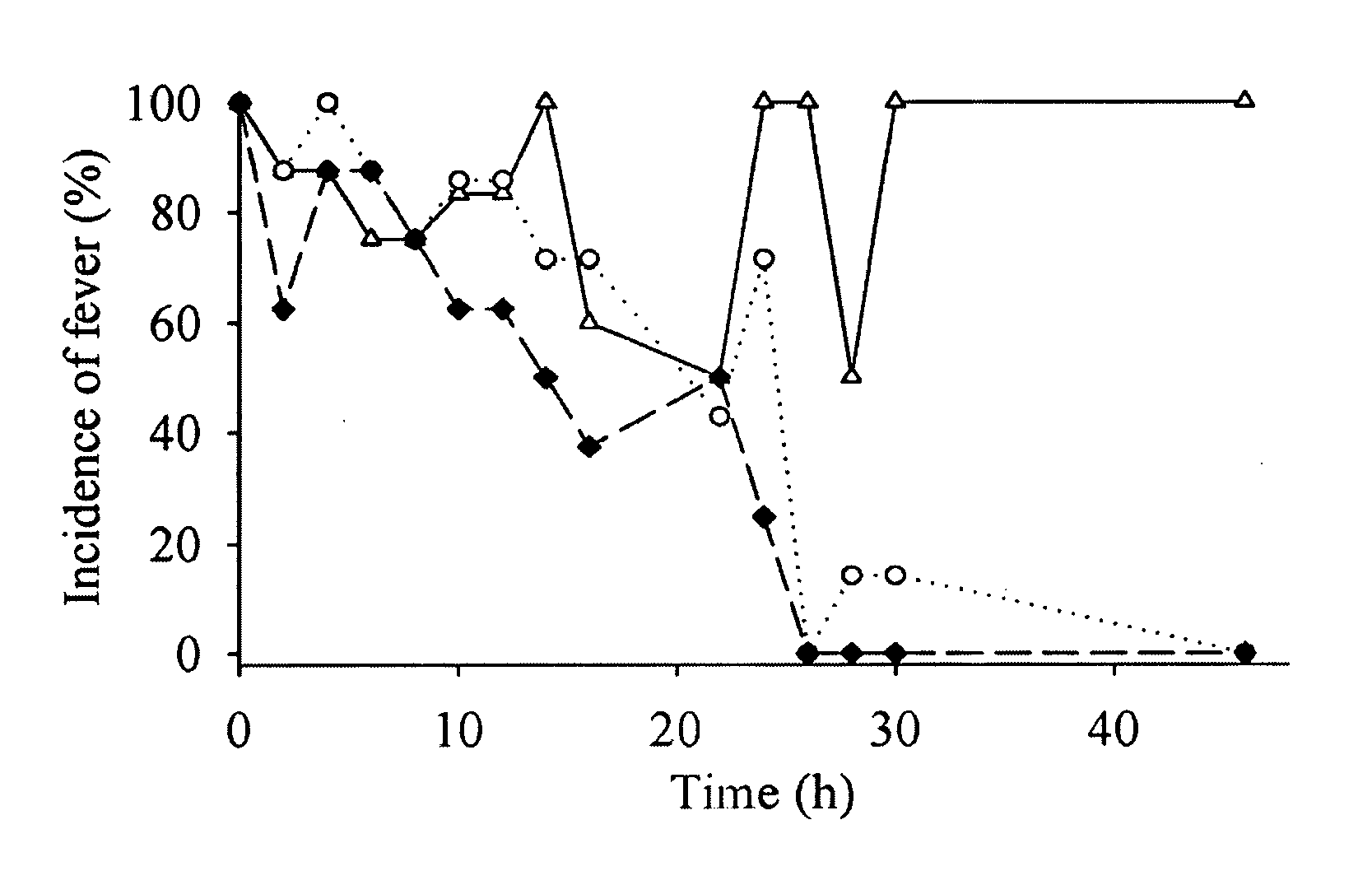
Pharmaceutical combinations of cox-2 inhibitors and opiates
ABSTRACTA pharmaceutical composition comprises a combination of a selective or specific COX 2 inhibitor or a pharmaceutically acceptable salt or derivative thereof and an opiate or a pharmaceutically acceptable salt or derivative thereof, for example a combination of meloxicam and codeine, as active ingredients, and a pharmaceutically acceptable carrier. It may include a centrally-acting cyclo-oxygenase inhibitor such as paracetamol or its pharmaceutically acceptable salts or derivatives. The pharmaceutical compositions are used in methods of providing symptomatic relief or treatment of pain, in an algesic and / or hyperalgesic state, with or without fever, in particular that associated with inflammation such as that associated with trauma, osteoarthritis, rheumatoid arthritis, non-inflammatory myalgia or dysmenorrhoea
Owner:ADCOCK INGRAM LTD
Use of meloxicam in combination with an antiplatelet agent for treatment of acute coronary syndrome and related conditions
InactiveUS20050197332A1Reducing risk of cardiovascular eventEffective amountSalicyclic acid active ingredientsHeterocyclic compound active ingredientsMeloxicamAnti platelet
The invention relates to a method of treatment or prevention of acute coronary syndrome or related conditions or reducing the risk of cardiovascular events comprising the administration of a therapeutically or prophylactically effective amount of meloxicam in combination with a therapeutically or prophylactically effective amount of an antiplatelet agent to a patient in need of such treatment. The invention also provides pharmaceutical compositions comprising meloxicam and an antiplatelet agent as a combined preparation suitable for use in these indications. Furthermore, the invention provides the use of meloxicam for manufacture of a pharmaceutical composition for treatment or prevention of acute coronary syndrome and related conditions when used in combination with an antiplatelet agent.
Owner:BOEHRINGER INGELHEIM INT GMBH
Use of meloxicam in combination with an antiplatelet agent for treatment of acute coronary syndrome and related conditions
InactiveUS20060160793A1Reducing risk of cardiovascular eventIncrease blockingSalicyclic acid active ingredientsHeterocyclic compound active ingredientsMeloxicamAnti platelet
The invention relates to a method of treatment or prevention of acute coronary syndrome or related conditions or reducing the risk of cardiovascular events comprising the administration of a therapeutically or prophylactically effective amount of meloxicam in combination with a therapeutically or prophylactically effective amount of an antiplatelet agent to a patient in need of such treatment. The invention also provides pharmaceutical compositions comprising meloxicam and an antiplatelet agent as a combined preparation suitable for use in these indications. Furthermore, the invention provides the use of meloxicam for manufacture of a pharmaceutical composition for treatment or prevention of acute coronary syndrome and related conditions when used in combination with an antiplatelet agent.
Owner:BOEHRINGER INGELHEIM INT GMBH
Meloxicam for the treatment of respiratory diseases in pigs
A method of treating or preventing a respiratory disease in a pig, the method comprising administering to the pig in need thereof an effective amount of meloxicam or a pharmaceutically acceptable salt thereof.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Use of meloxicam in combination with an antiplatelet agent for treatment of acute coronary syndrome and related conditions
InactiveUS20070099907A1Reducing risk of cardiovascular eventIncrease blockingSalicyclic acid active ingredientsHeterocyclic compound active ingredientsMeloxicamAnti platelet
The invention relates to a method of treatment or prevention of acute coronary syndrome or related conditions or reducing the risk of cardiovascular events comprising the administration of a therapeutically or prophylactically effective amount of meloxicam in combination with a therapeutically or prophylactically effective amount of an antiplatelet agent to a patient in need of such treatment. The invention also provides pharmaceutical compositions comprising meloxicam and an antiplatelet agent as a combined preparation suitable for use in these indications. Furthermore, the invention provides the use of meloxicam for manufacture of a pharmaceutical composition for treatment or prevention of acute coronary syndrome and related conditions when used in combination with an antiplatelet agent.
Owner:ALTMAN RAUL DR
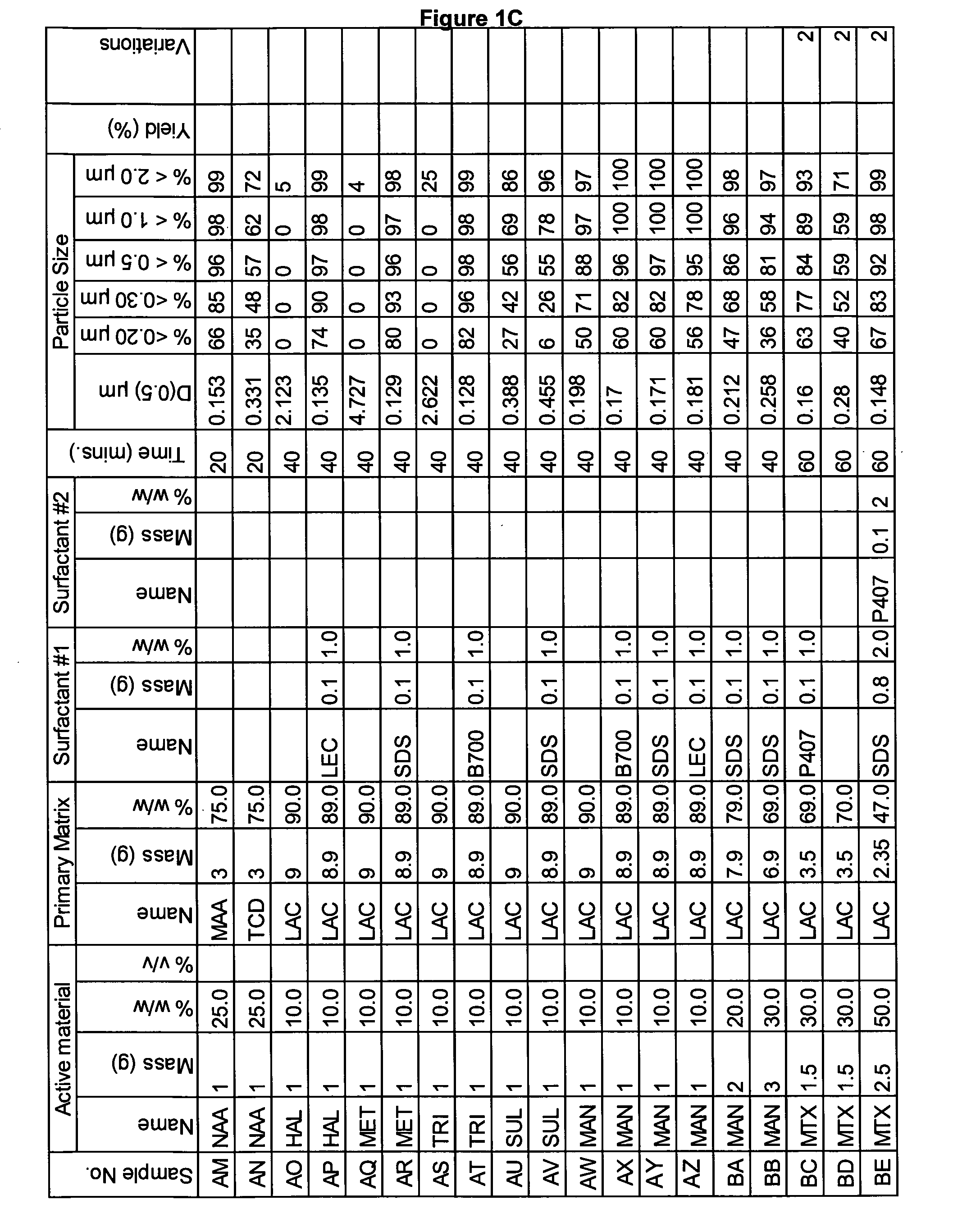
Novel formulation of meloxicam
InactiveUS20120141548A1Appropriate useEasy to separatePowder deliveryNervous disorderParticulatesMeloxicam
The present invention relates to methods for producing particles of meloxicam using dry milling processes as well as compositions comprising meloxicam, medicaments produced using meloxicam in particulate form and / or compositions, and to methods of treatment of an animal, including man, using a therapeutically effective amount of meloxicam administered by way of said medicaments.
Owner:ICEUTICA PTY LTD
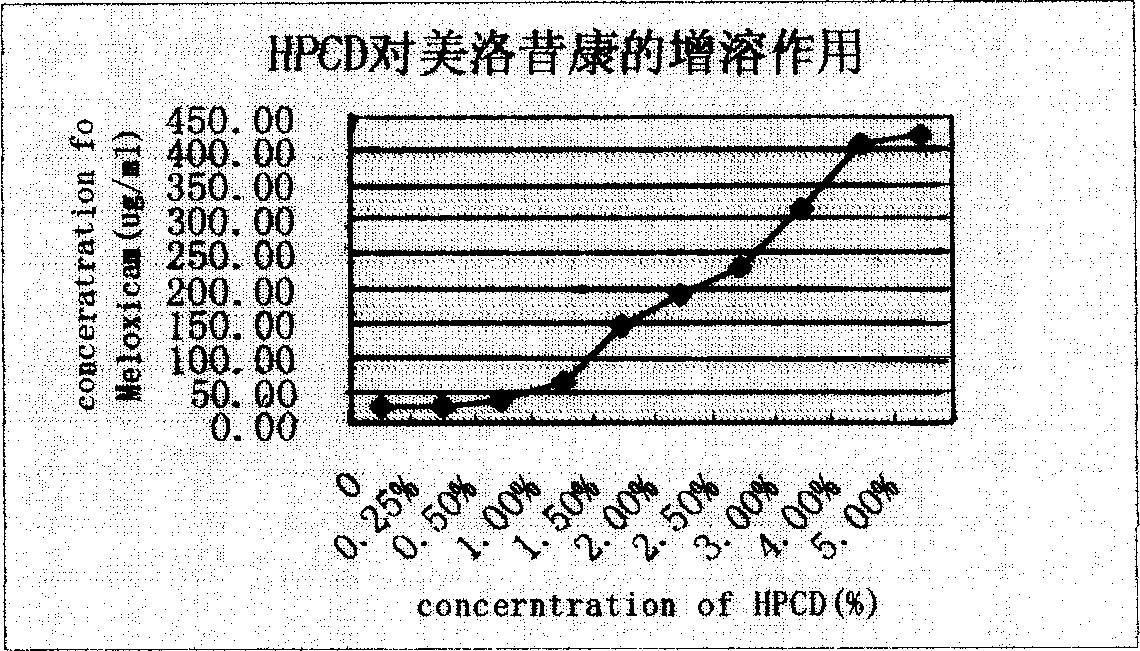
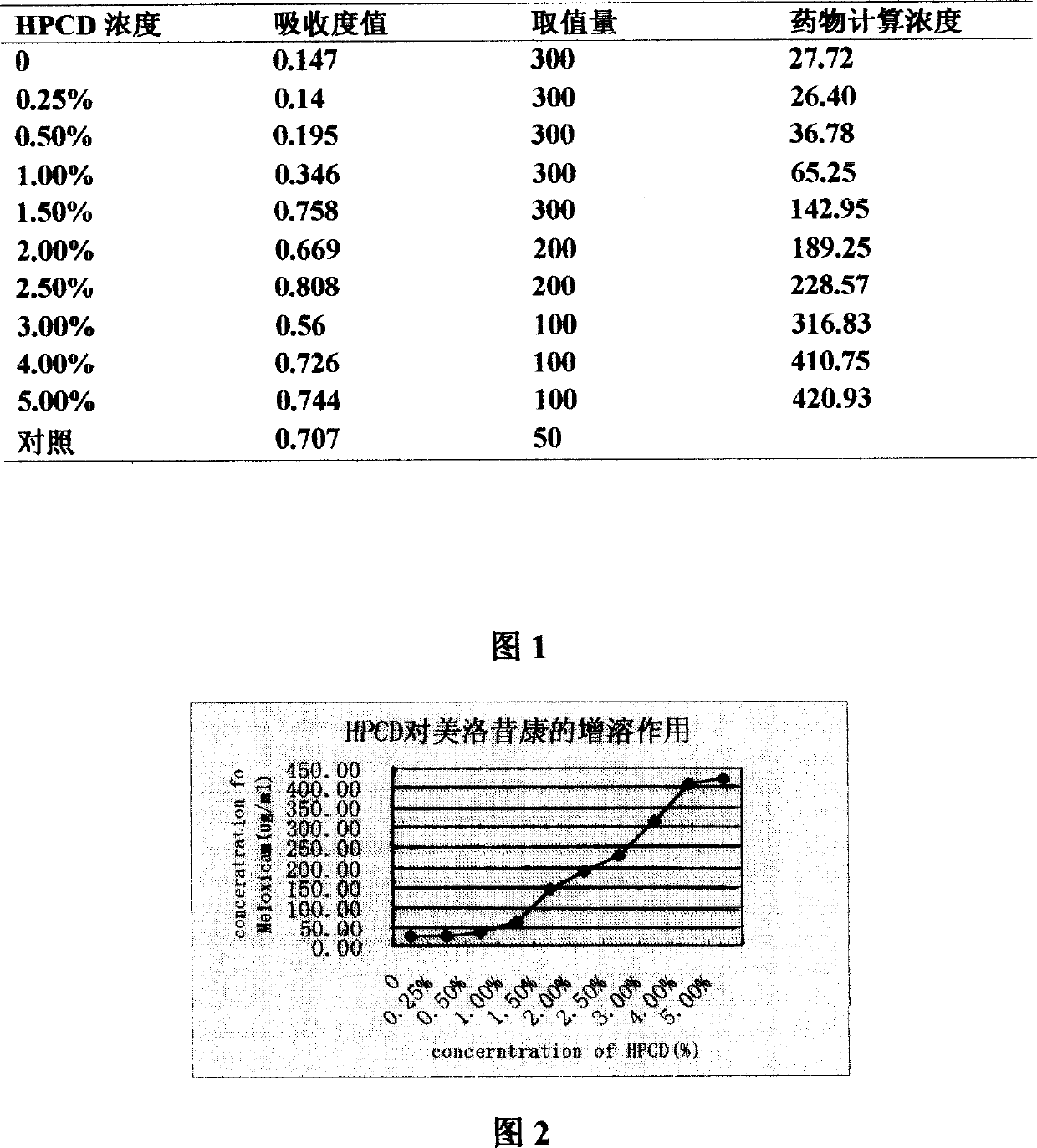
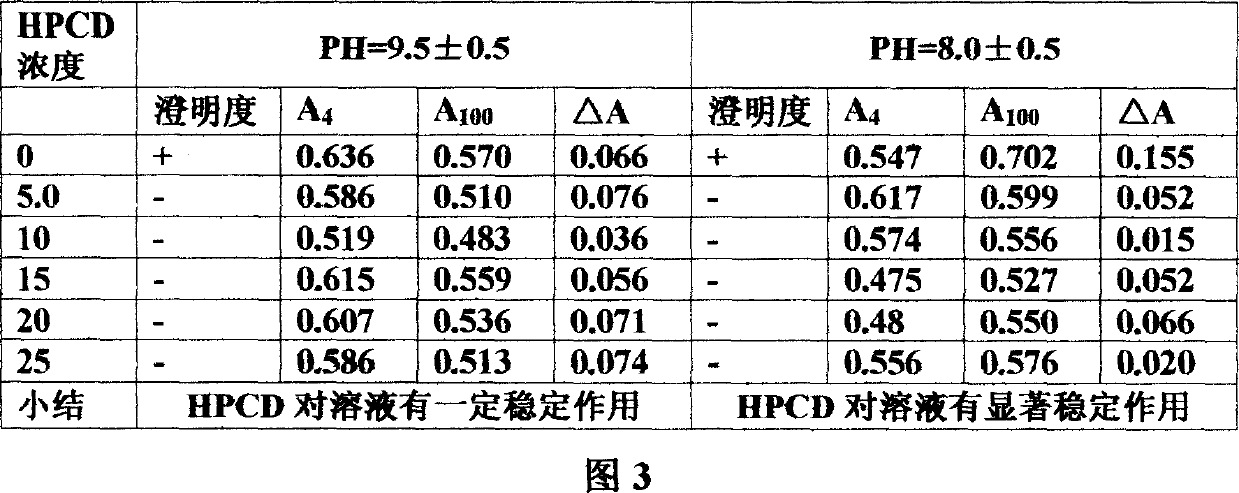
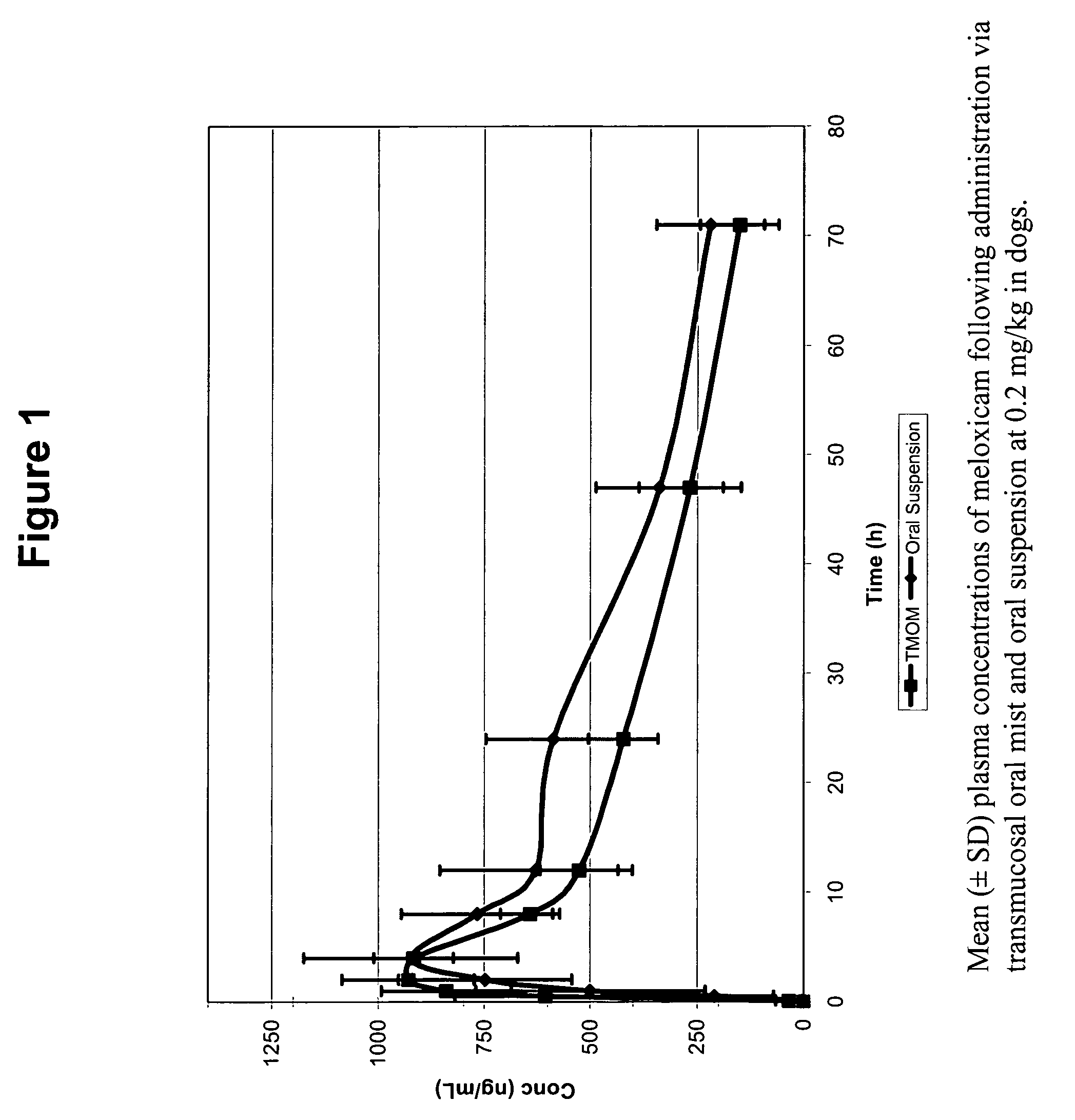
Mezloxicon liquid preparation using HPCD as solubilizing and stabilizing agent and its preparation method
InactiveCN1493292ASmall individual differencesAvoid gastrointestinal side effectsOrganic active ingredientsAntipyreticMeloxicamAdditive ingredient
A liquid preparation of meloxican is prepared from the meloxicam or its medical salt and water-soluble cyclodextrin derivative HP-beta-CD used as solubilizer and stabilizer, proportionally mixing, adding cosolvent, pH regulator and water for injection, heating and membrane filtering. It has long storage time at low temp.
Owner:SHENYANG PHARMA UNIVERSITY
Highly Concentrated Stable Meloxicam Solutions for Needleless Injection
A method for treating pain, inflammation, fever and respiratory complaints in mammals comprising administering by needleless injection to a mammal in need of such treatment an aqueous cyclodextrin-free solution of meloxicam containing a pharmacologically acceptable meloxicam salt of an organic or inorganic base.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Highly concentrated stable meloxicam solutions
Aqueous cyclodextrin-free solution of meloxicam for administration by oral or parenteral route, containing a pharmacologically acceptable meloxicam salt of an organic or inorganic base and one or more suitable excipients, the content of dissolved meloxicam salt being more than 10 mg / mL. The formulation according to the invention has a shelf-life of up to 24 months or more.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Transmucosal administration of meloxicam compositions for treating and preventing disorders in non-human domesticated animals
The invention includes compositions for transmucosal administration to an animal comprising at least one active agent and a pharmaceutically acceptable carrier. A preferred active agent is selected from the group consisting of meloxicam, carprofen, enrofloxacin, clemastine, diphenhydramine, digoxin, levothyroxine, cyclosporine, ondansetron, lysine, zolpidem, propofol, nitenpyram, ivermectin, milbemycin, and pharmaceutically acceptable salts, solvates and esters thereof. In another embodiment, the invention includes methods of treating or preventing a condition in an animal comprising transmucosally administering a composition comprising a therapeutically or prophylactically effective amount of an active agent and a pharmaceutically acceptable carrier.
Owner:ZOTTIS BELGIUM
Meloxicam tablet, production technology and purposes thereof
ActiveCN101618026AGood anti-inflammatory and analgesic effectReduce the number of dosesOrganic active ingredientsAntipyreticDuration periodSolubility
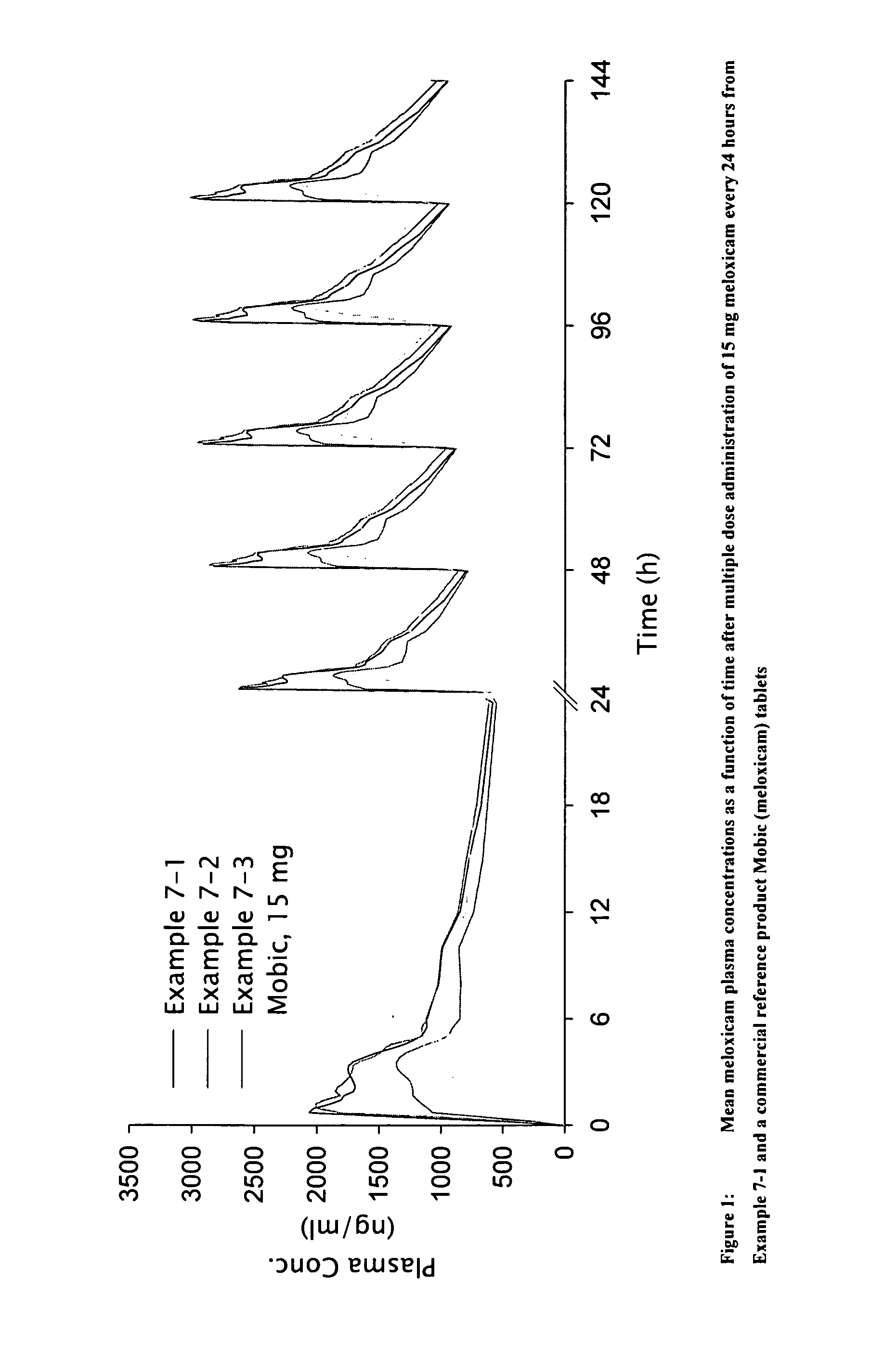
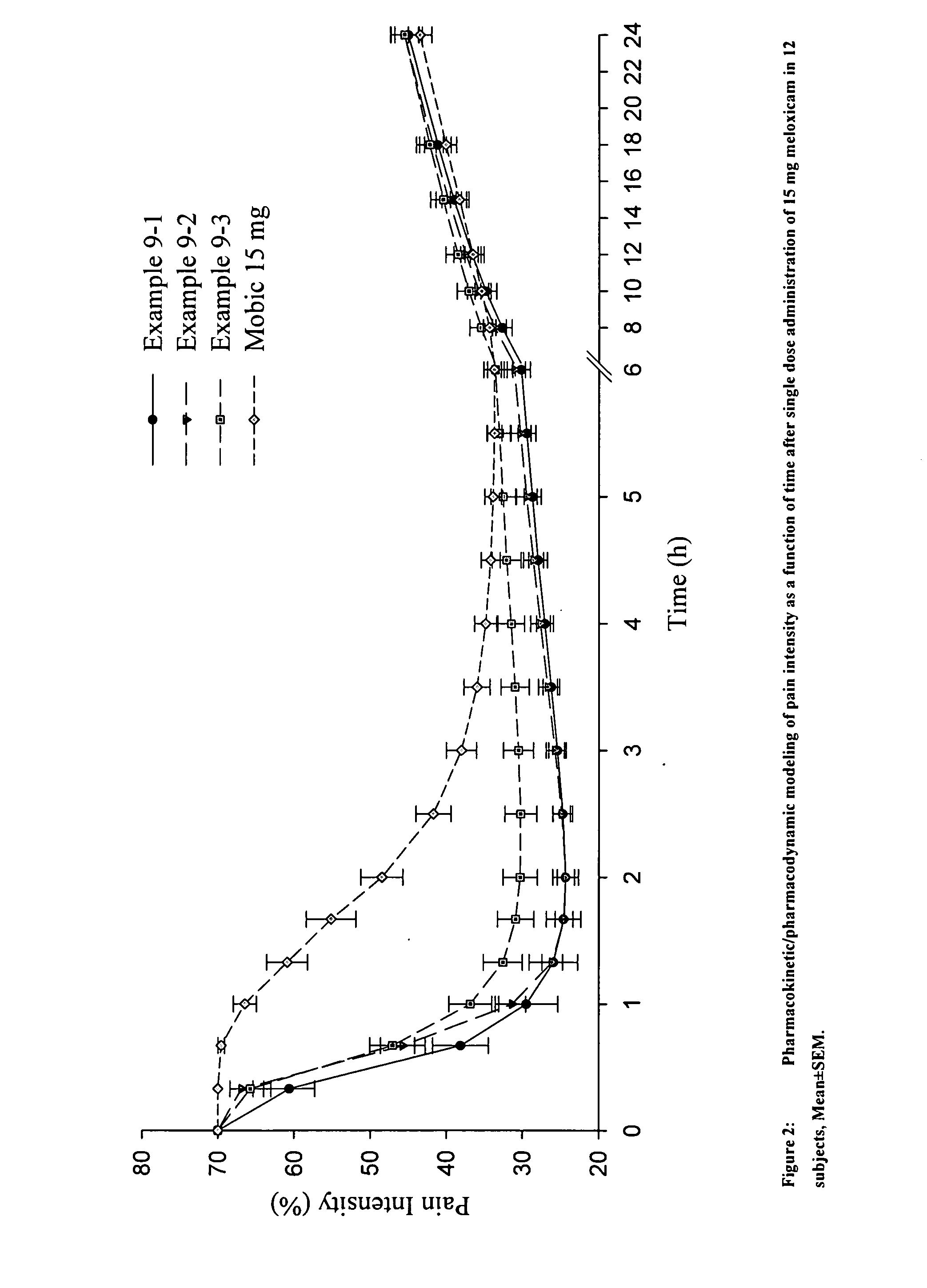
The invention discloses a Meloxicam tablet, a production technology and purposes thereof. The Meloxicam tablet consists of Meloxicam, lactose, pregelatinized starch, low-substitute hyprolose, polysorbate 80, 12% of pregelatinized starch for flushing syrup, superfine silica gel powder, magnesium stearate, alcohol and gastric-solubility film coating premixer. The production method of the Meloxicam tablet comprises the following steps: manufacturing tablet cores and coatings, and the like. The Meloxicam tablet treats 125 cases of rheumatoid arthritis, and the anti-inflammatory and pain-relieving curative effects are good, while the curative effect on blood sedimentation is little, and the total effective rate achieves 84%. The invention has the characteristics of few medical taking times, stable blood and medicine concentration, long duration period, and the like.
Owner:FEIMA PHARMA NANTONG CITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com