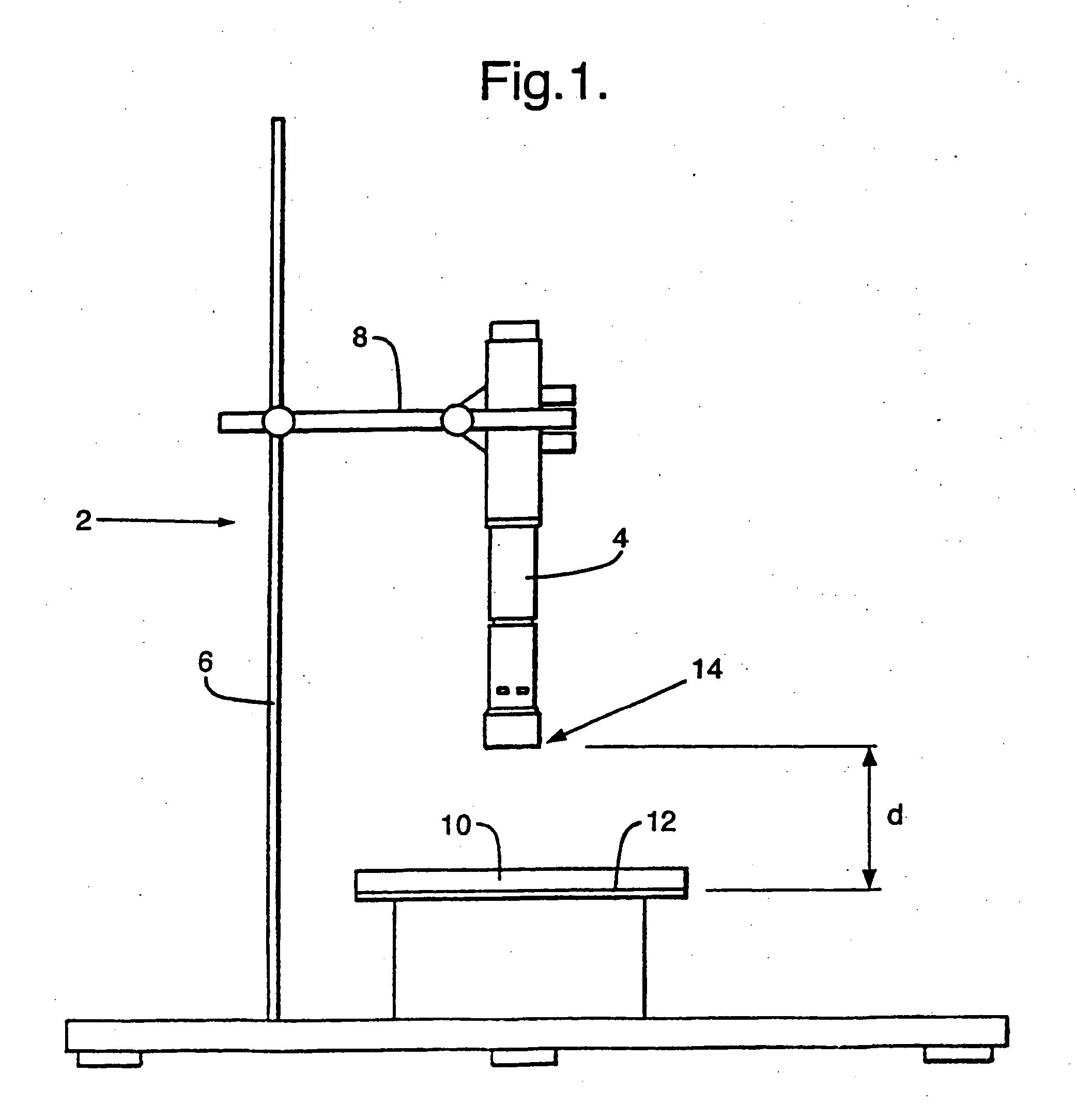
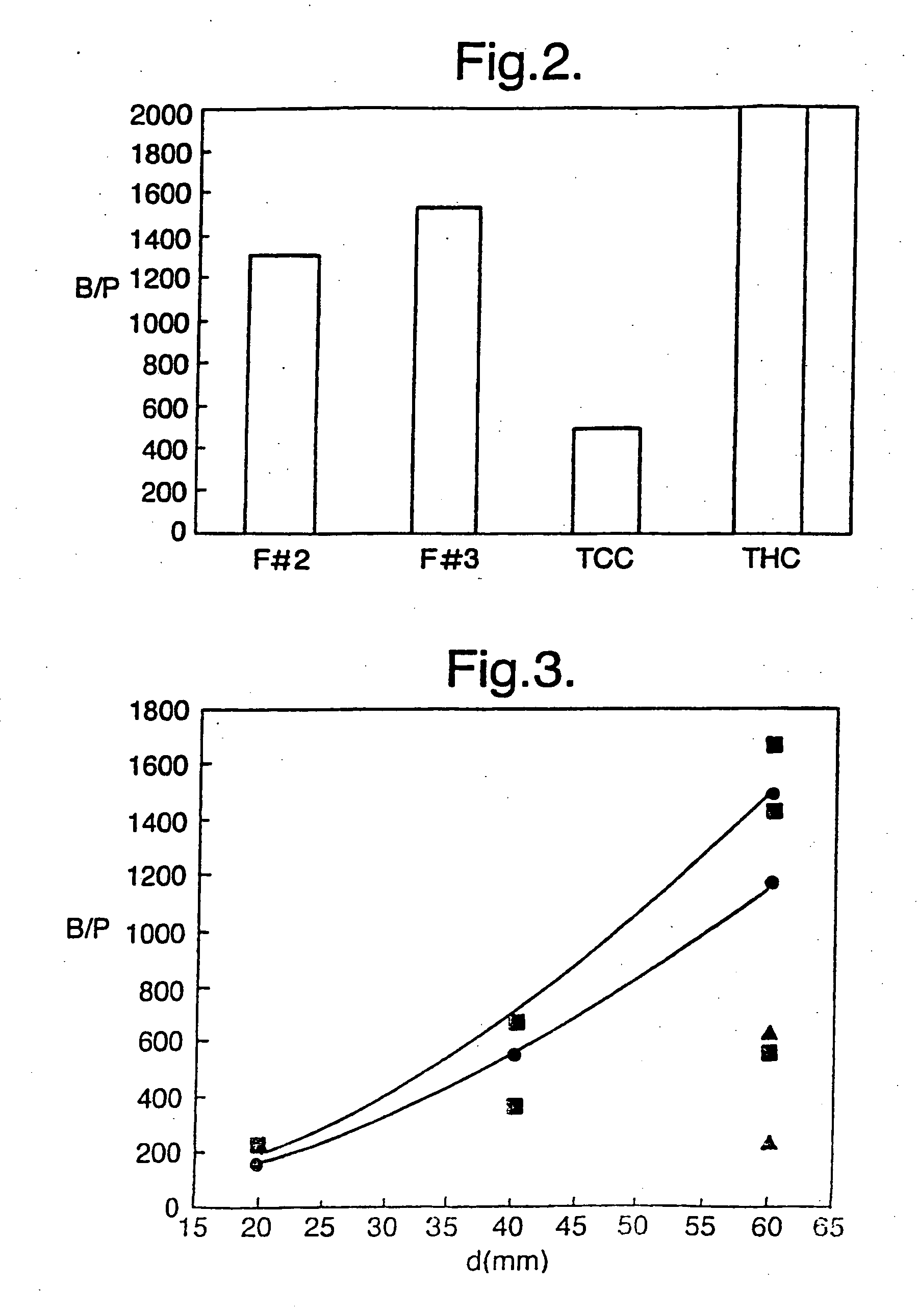
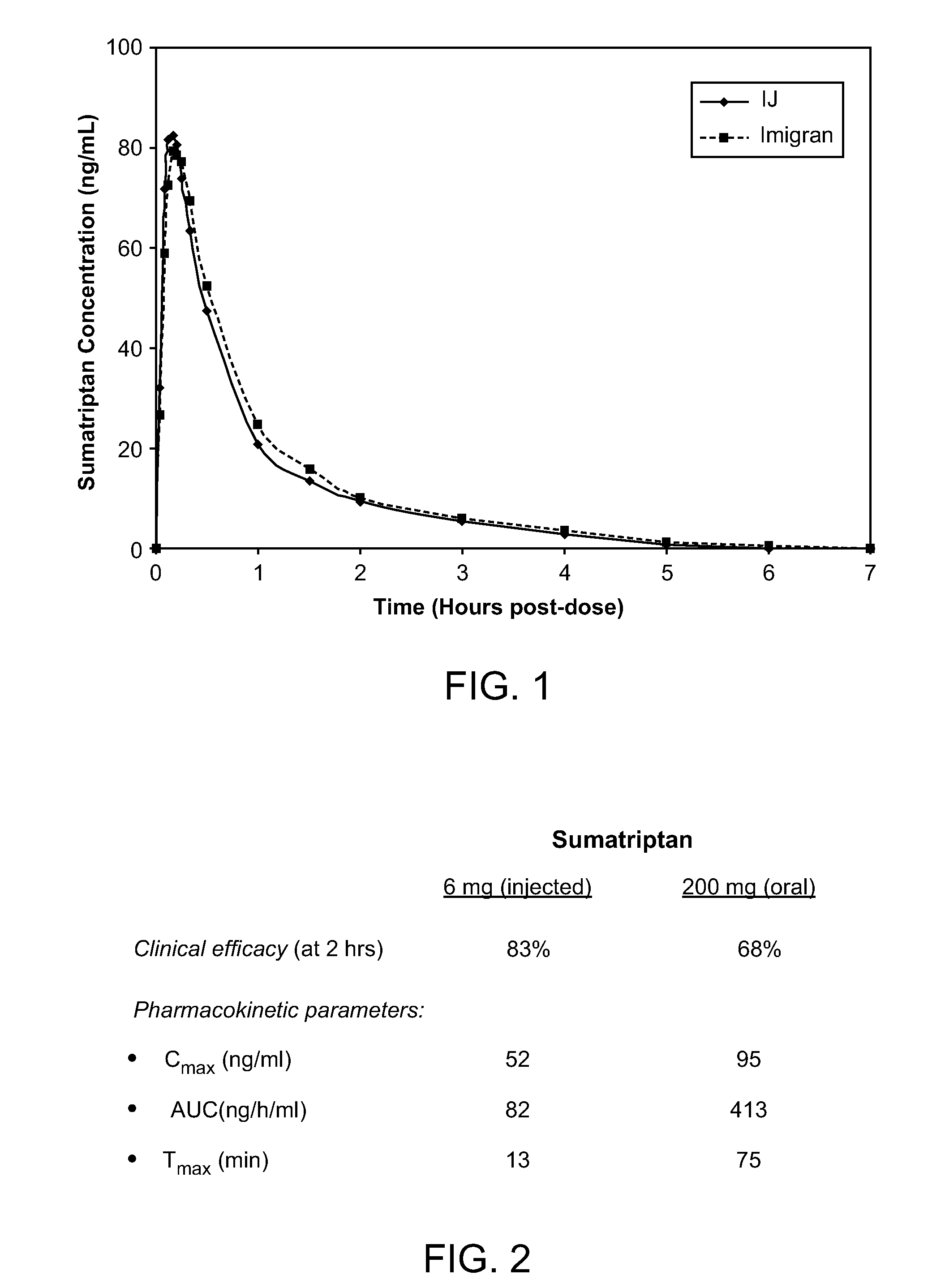
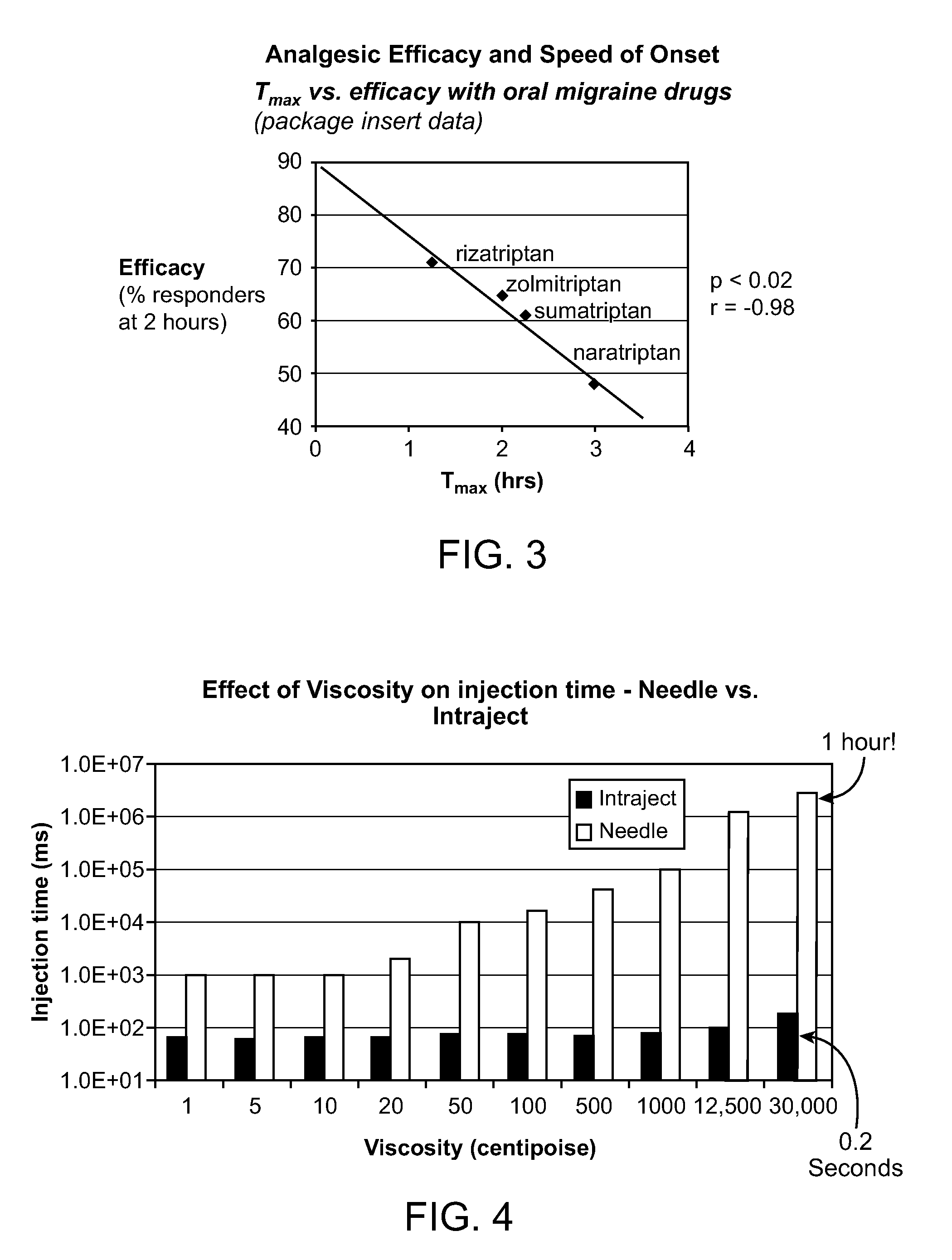
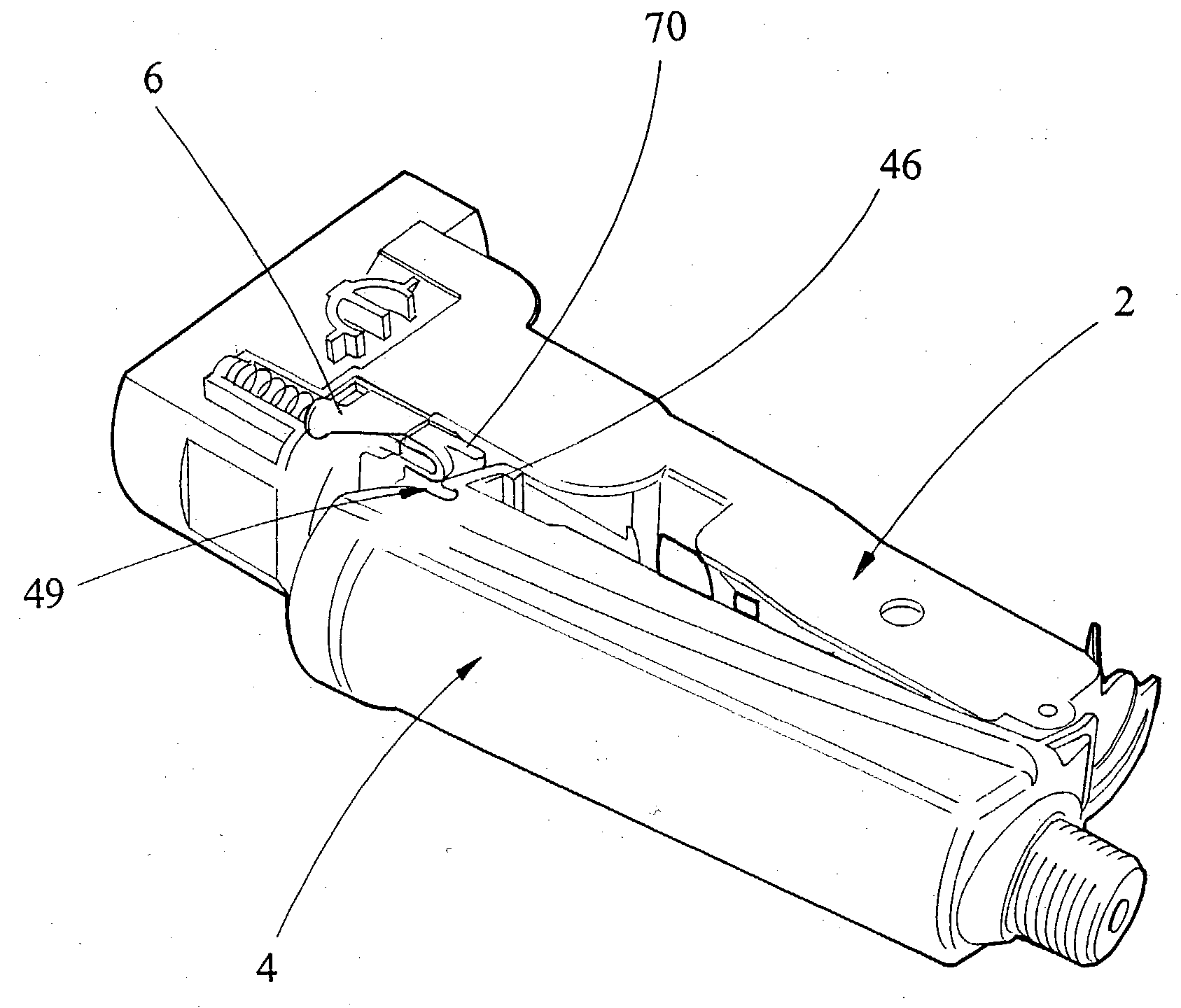
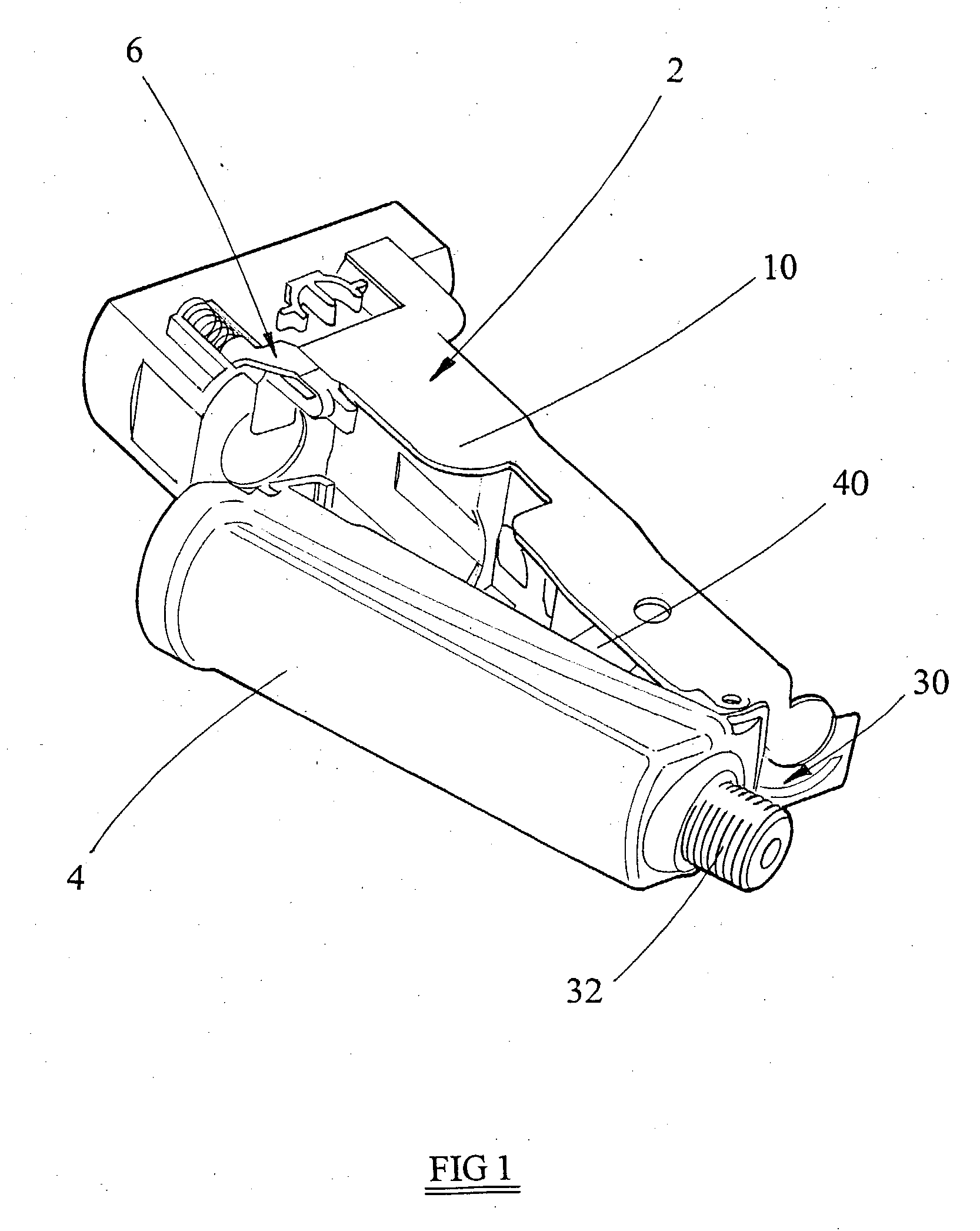
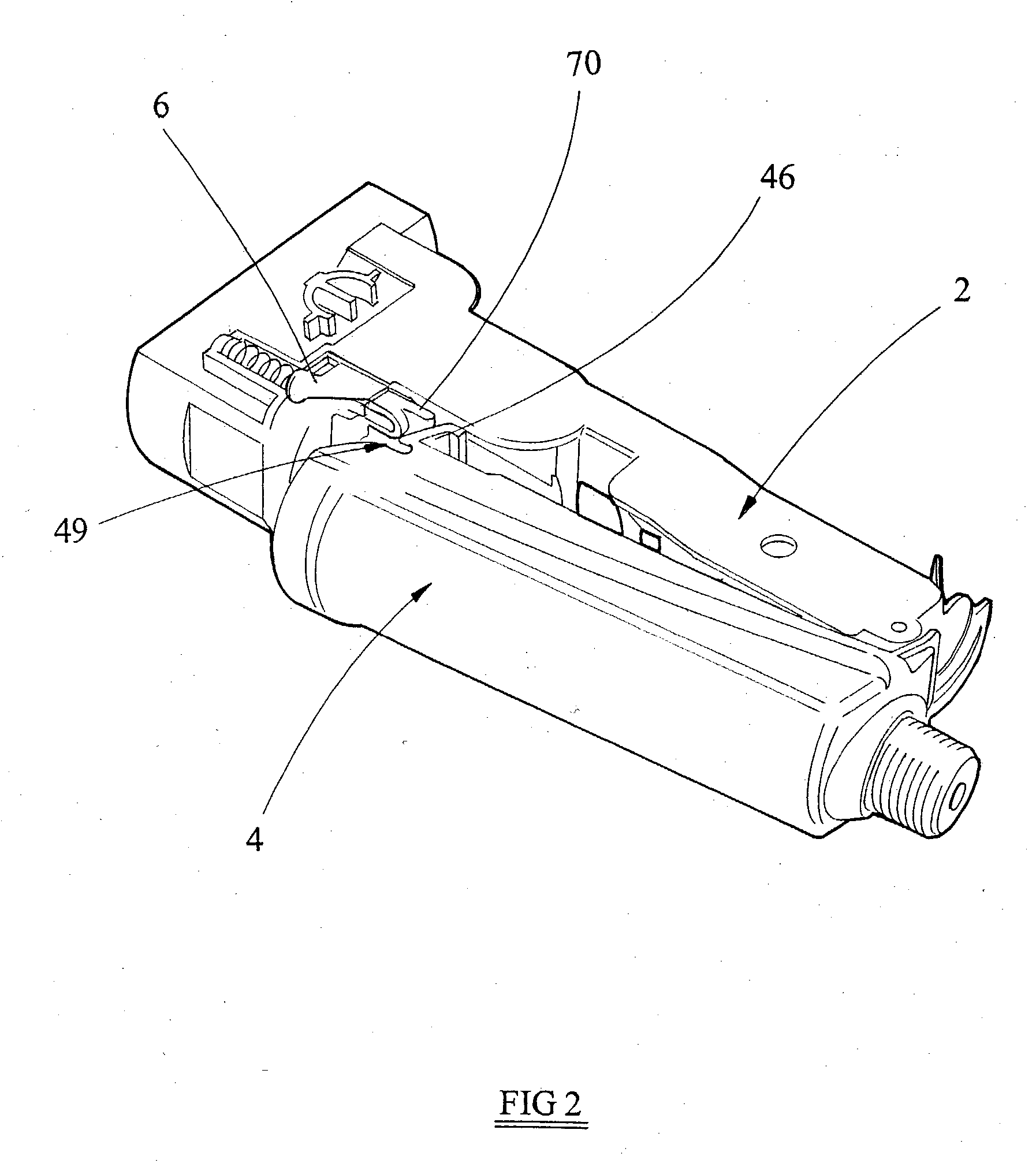
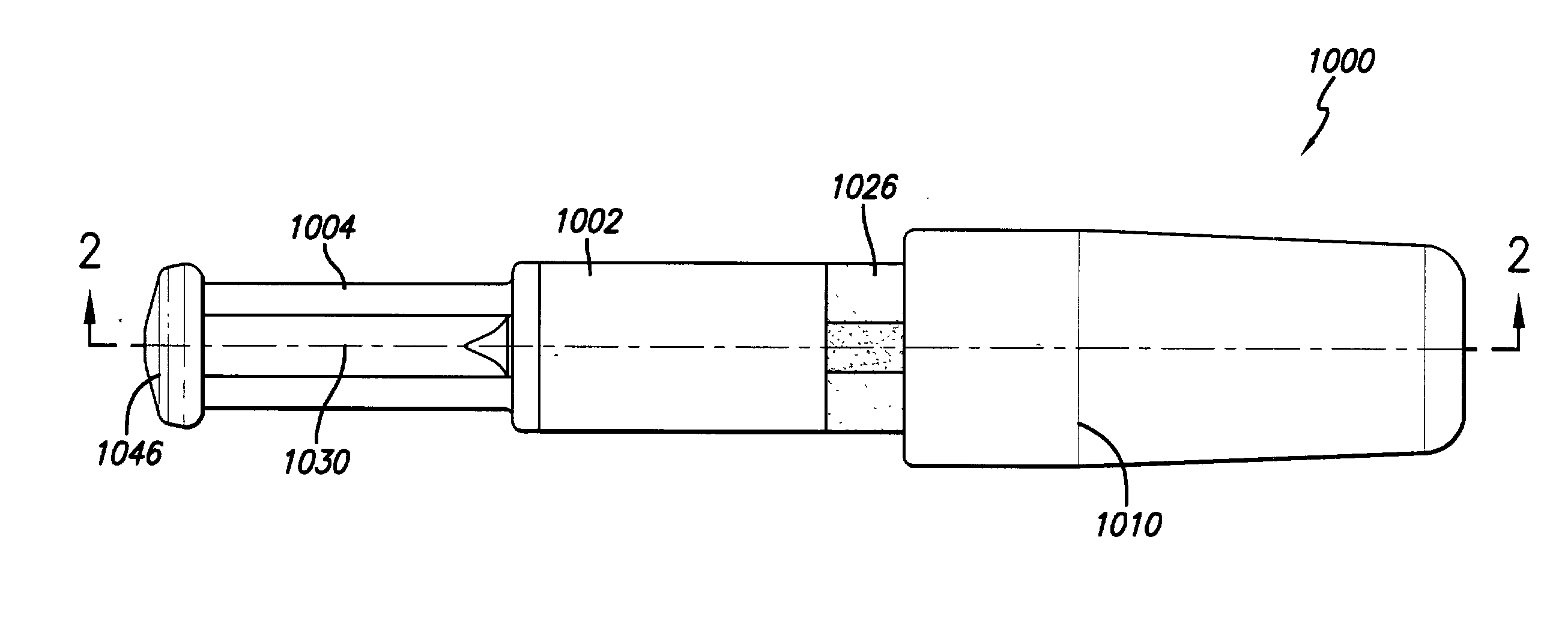
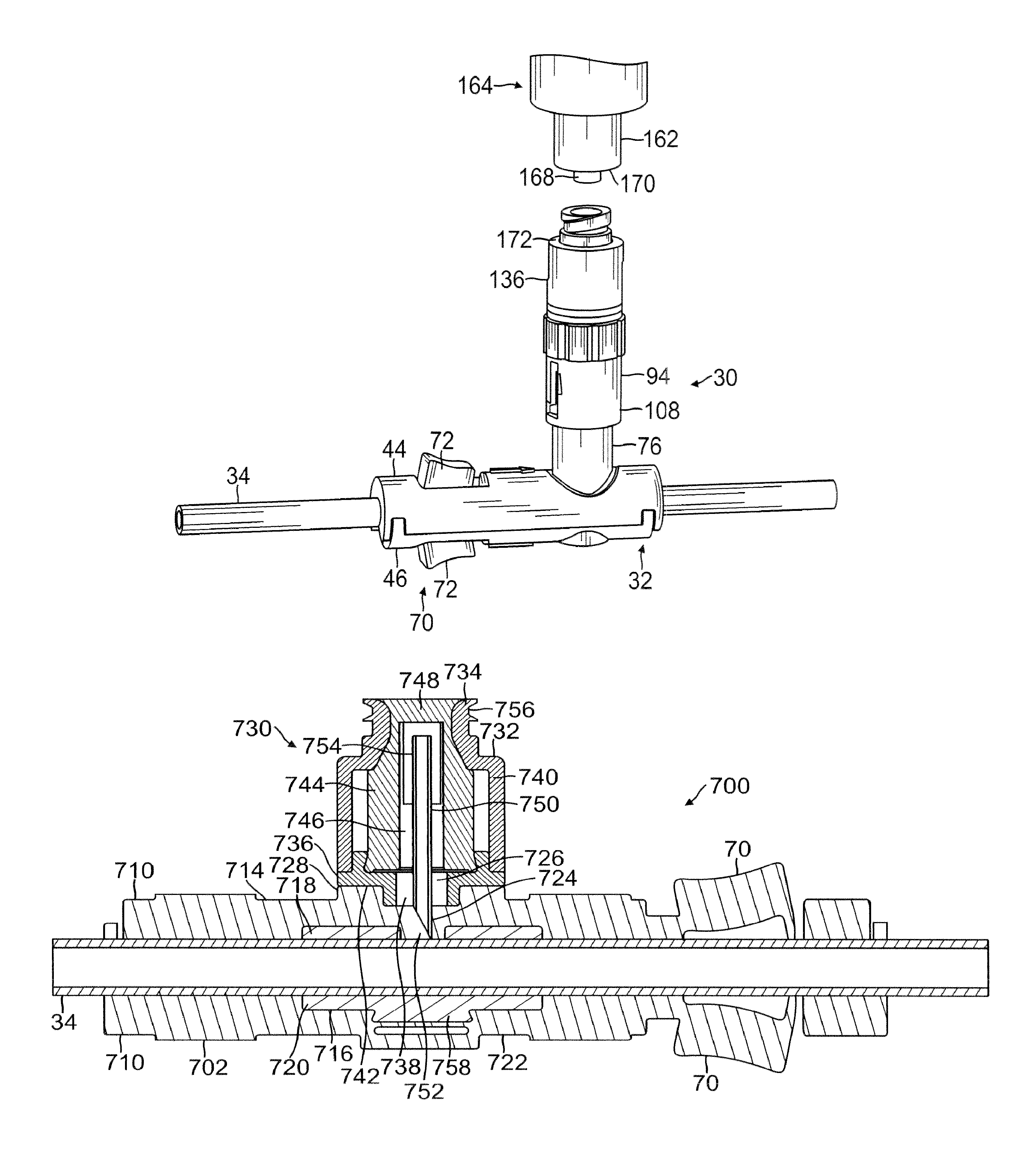
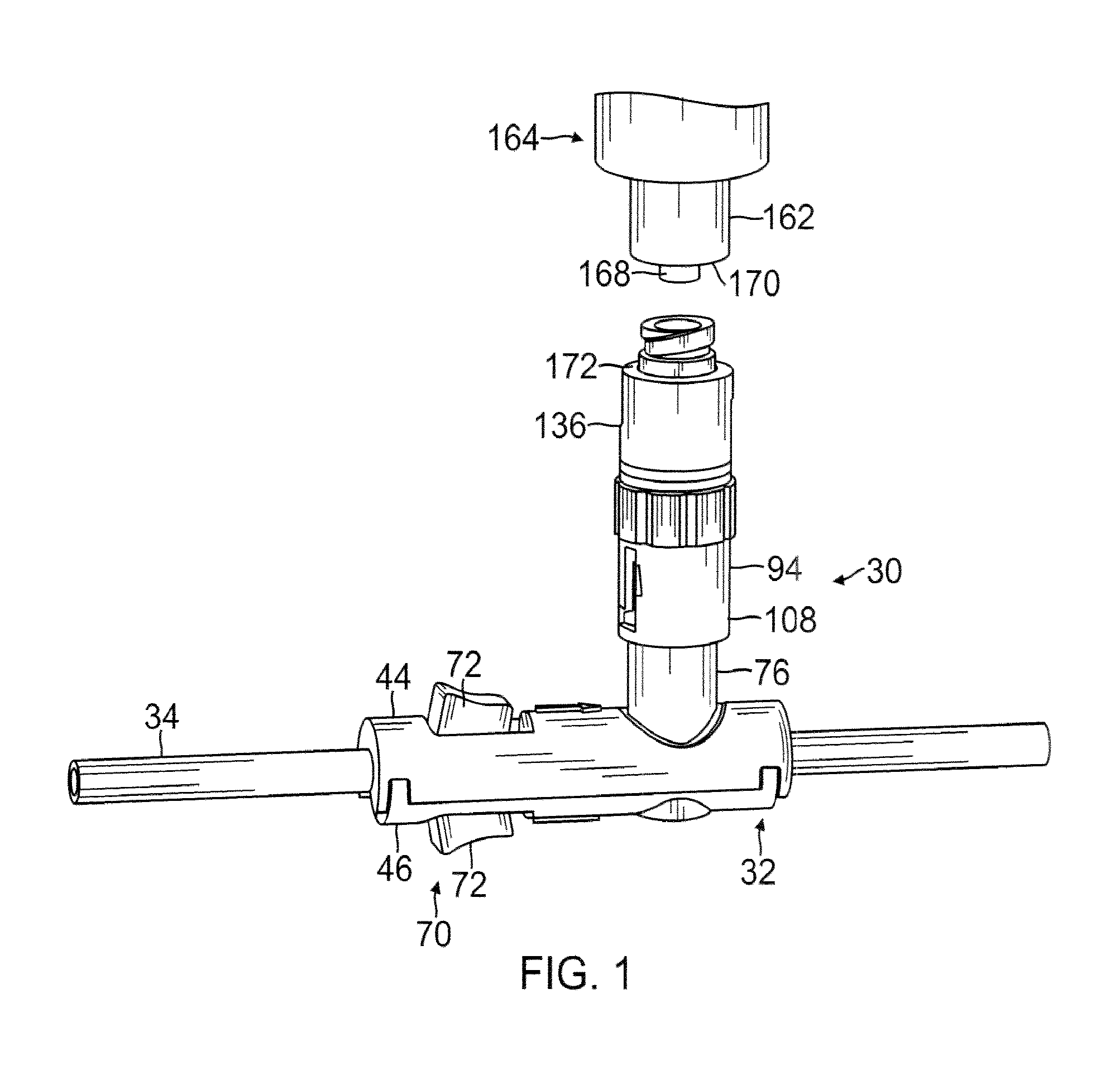
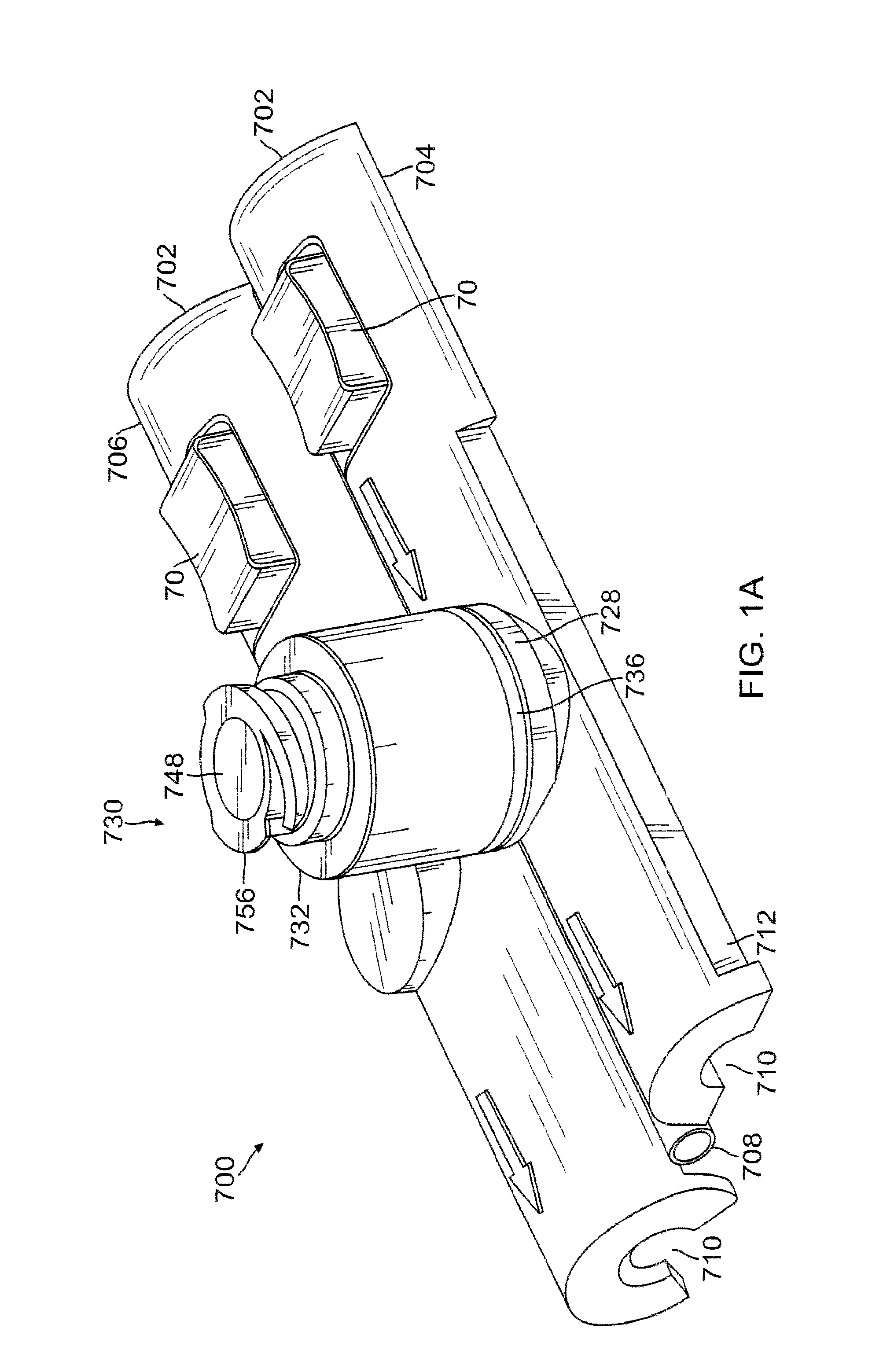
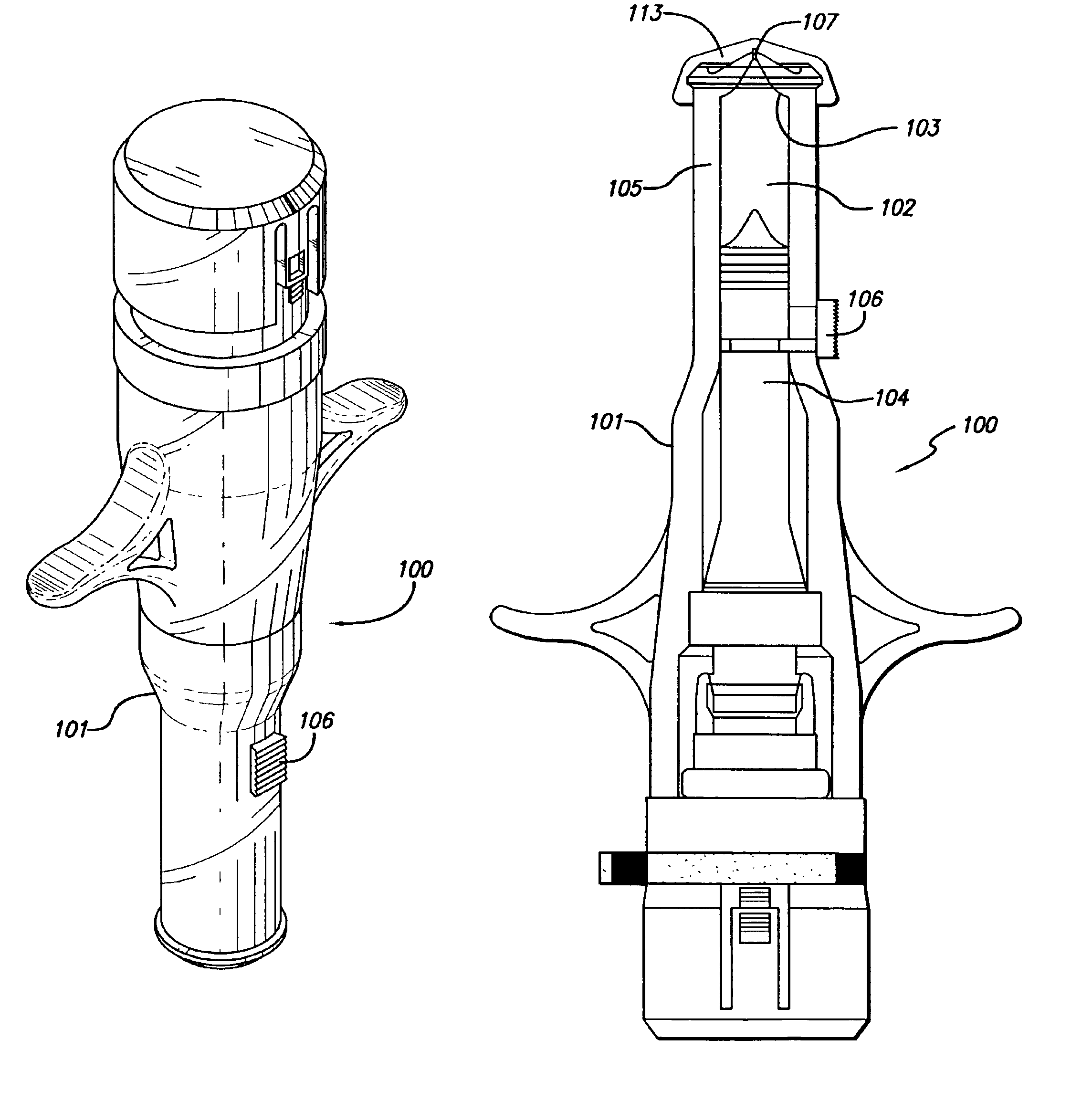
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
362 results about "Needle Free Injection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
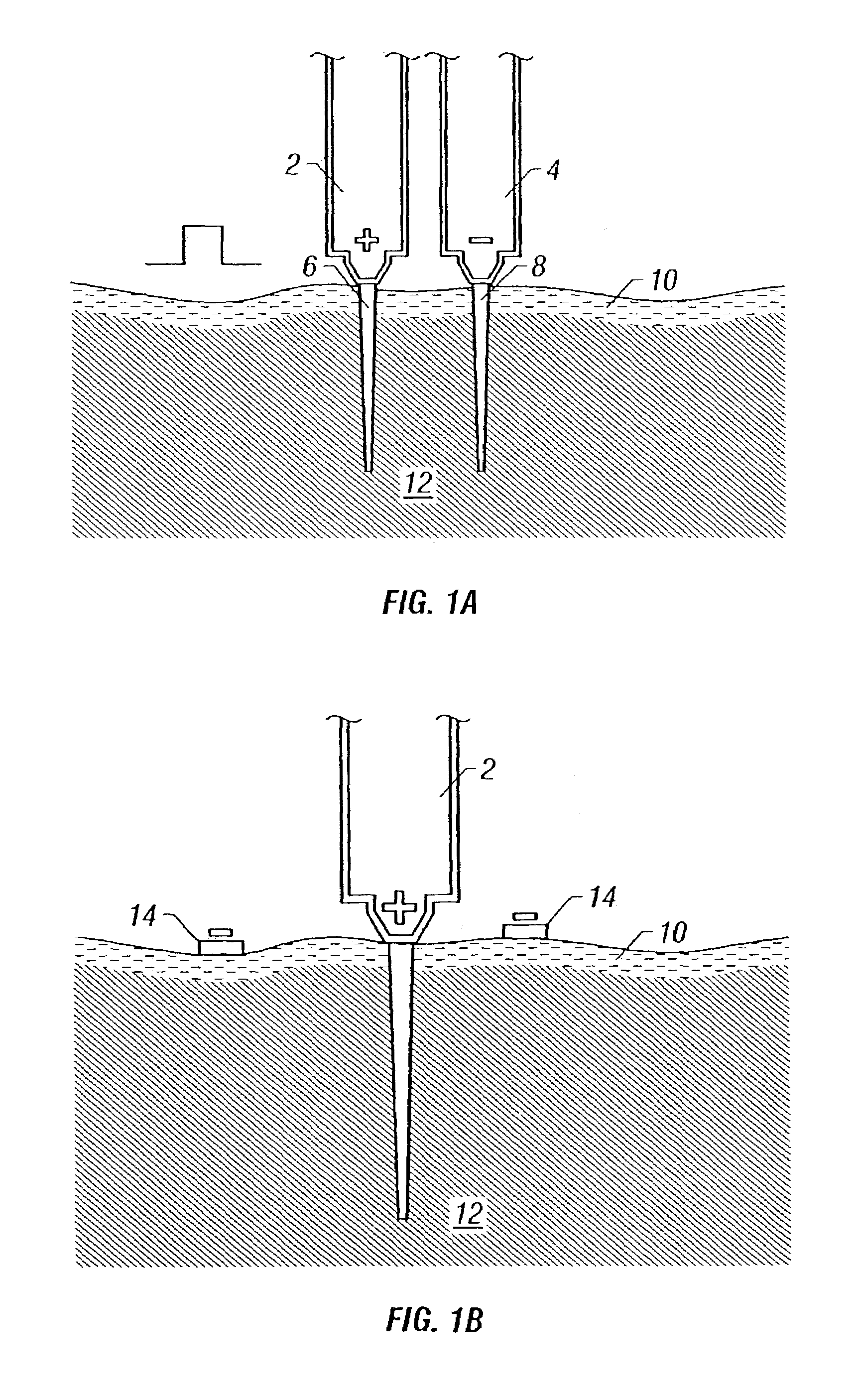
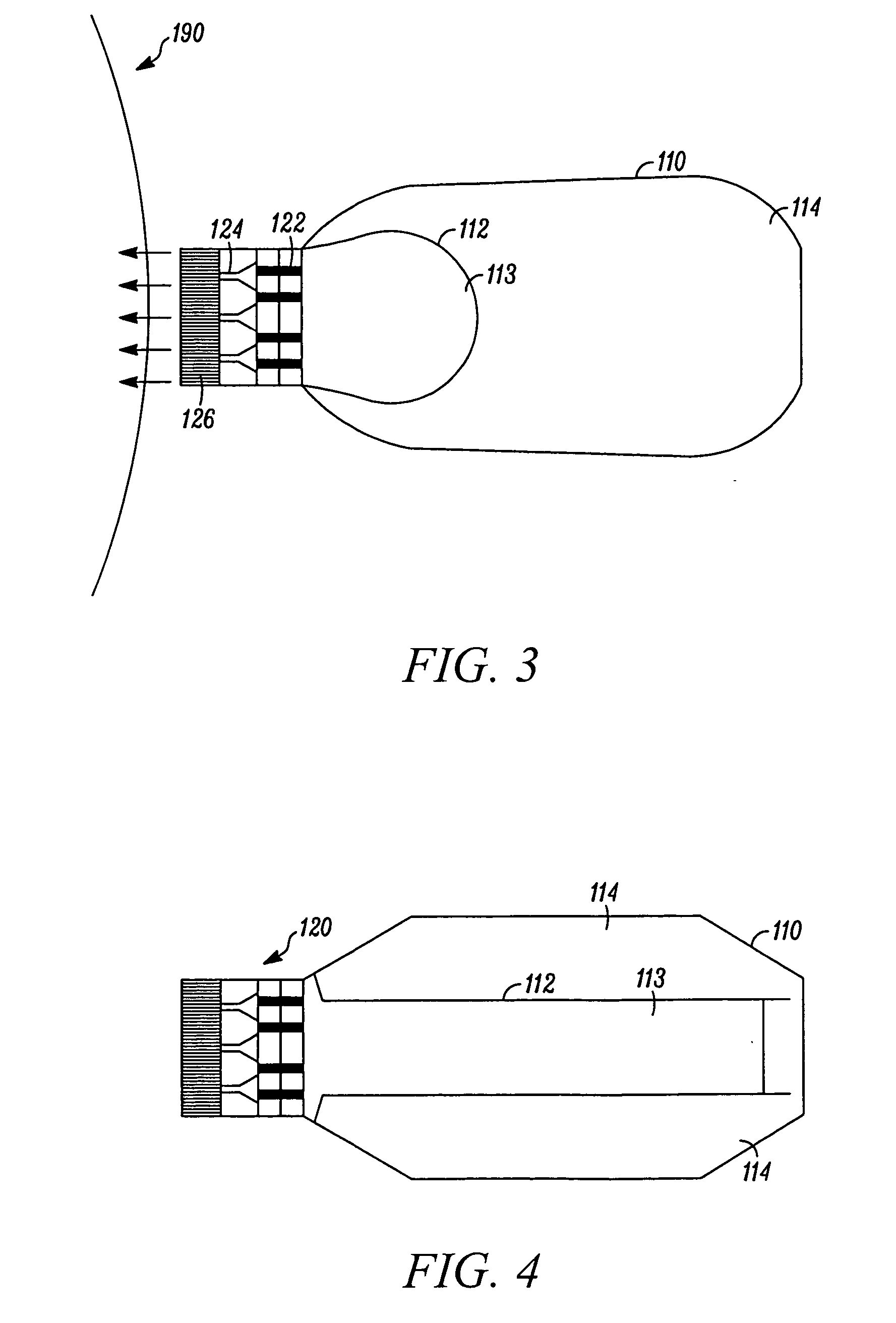
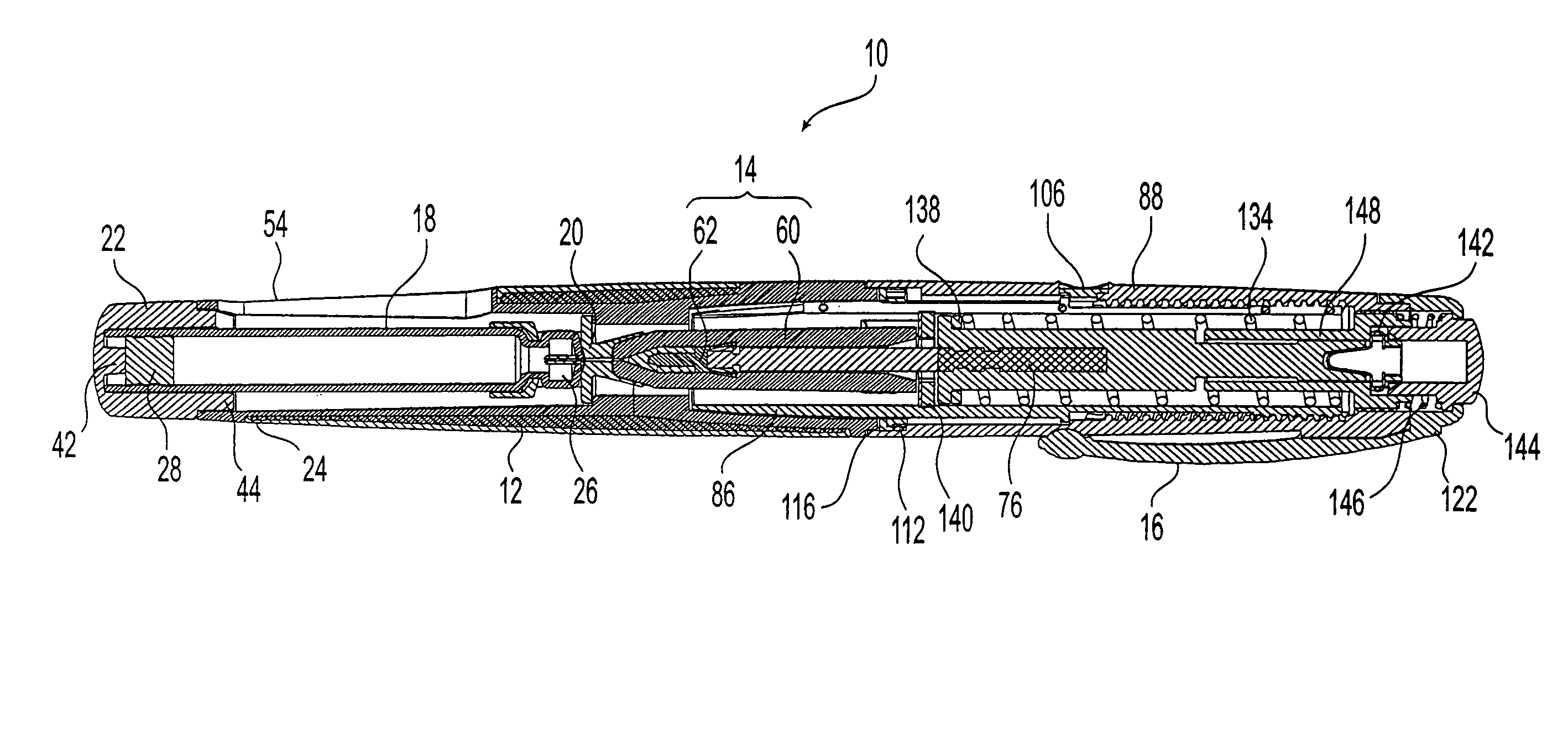
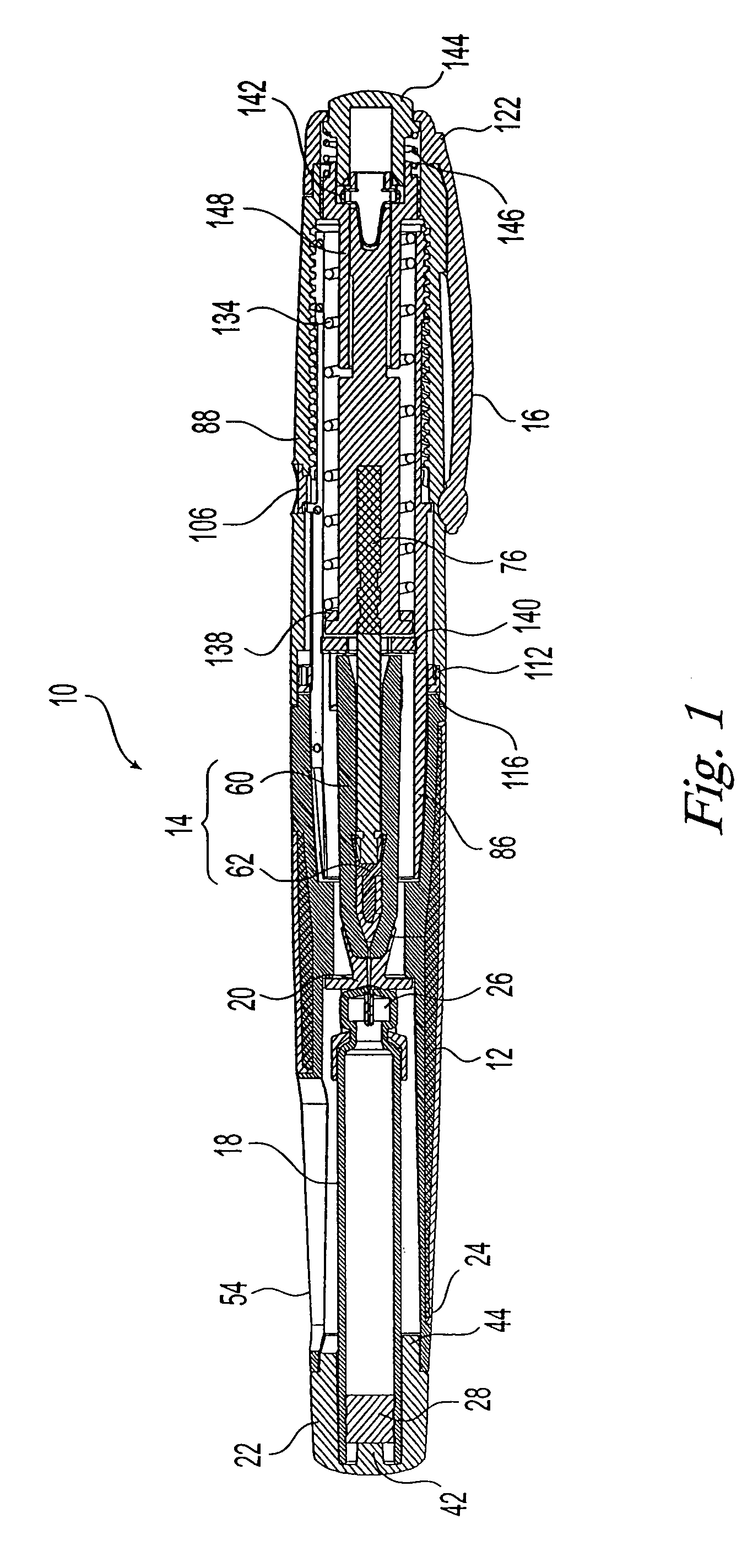
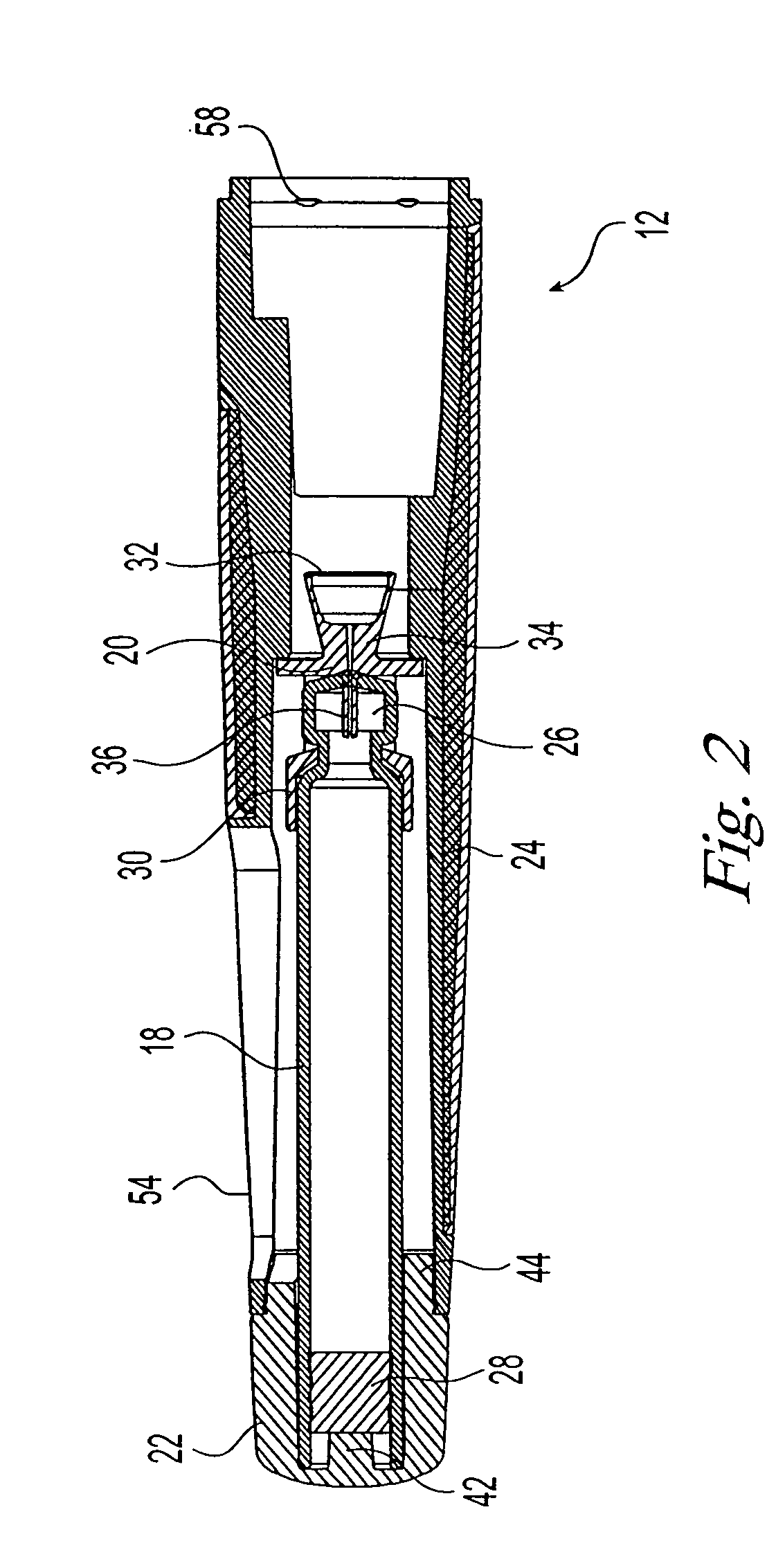
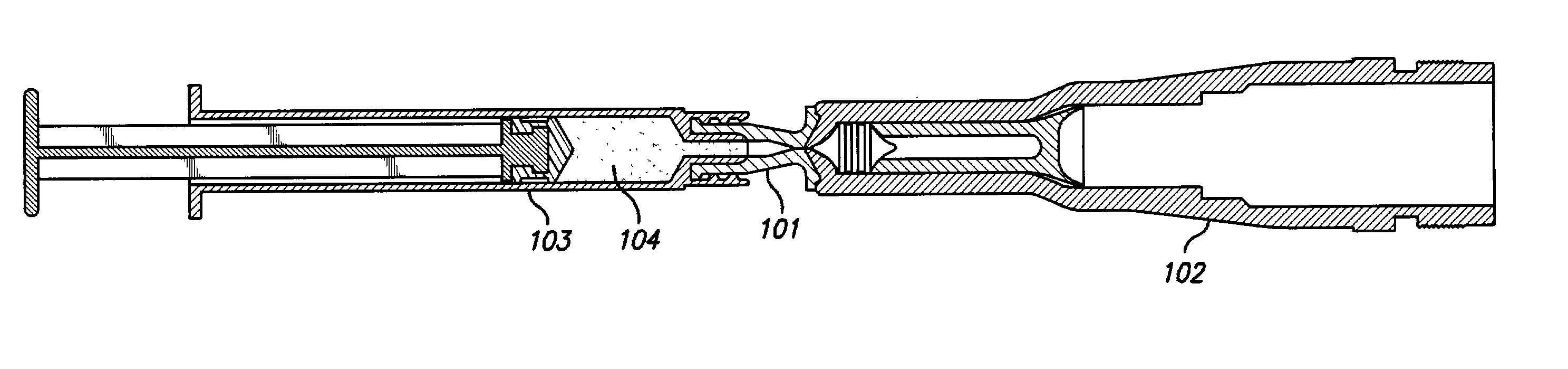
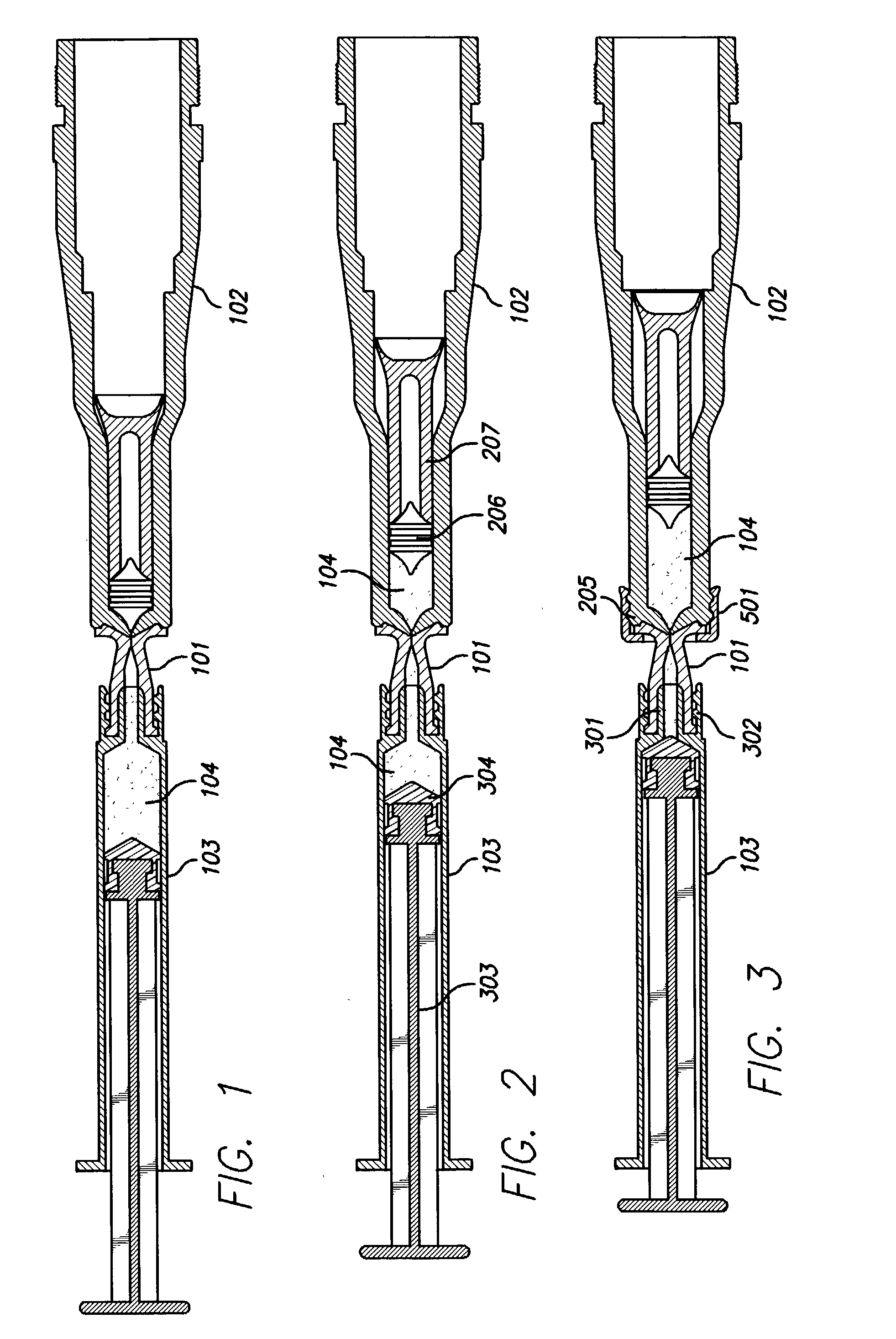
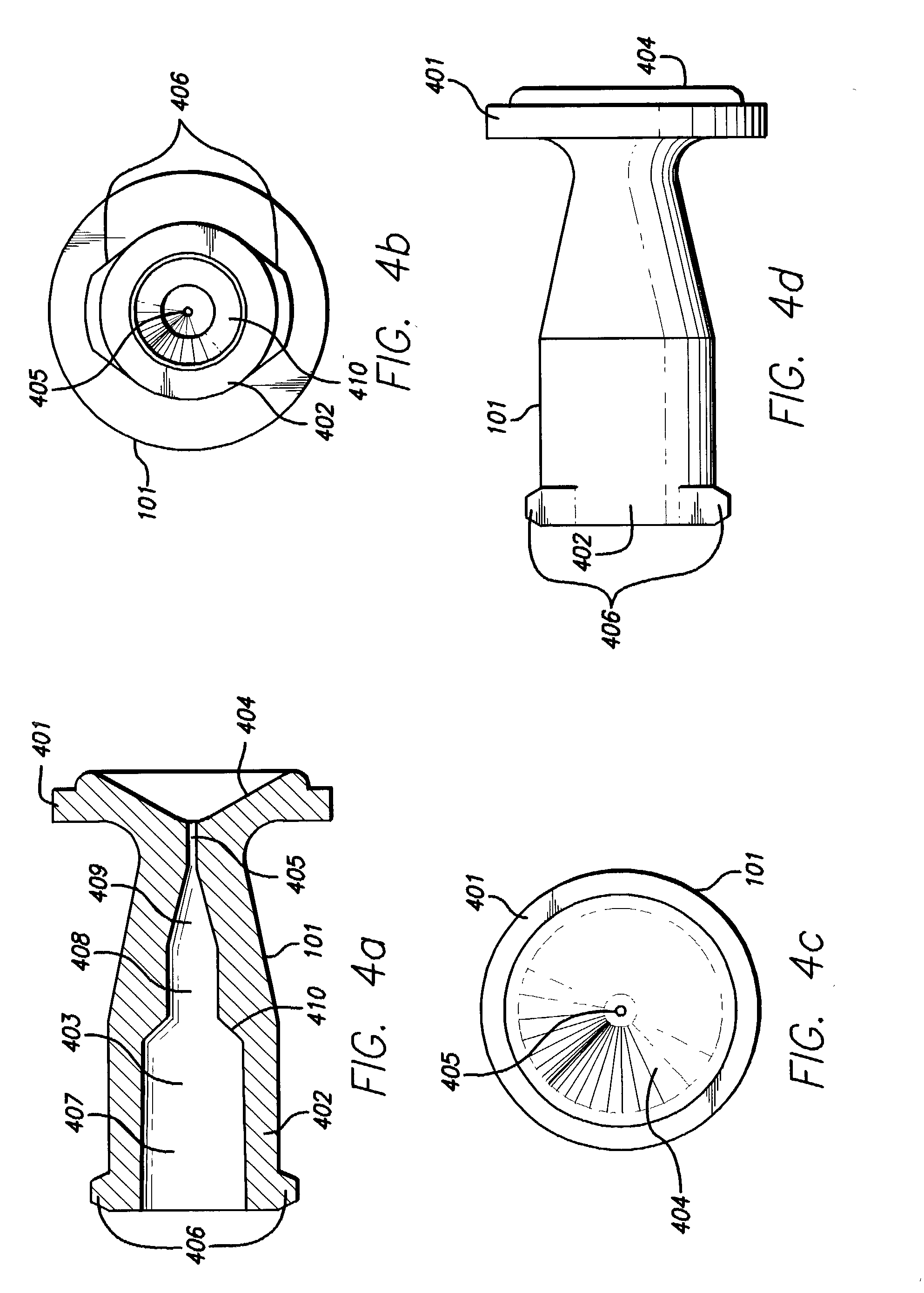
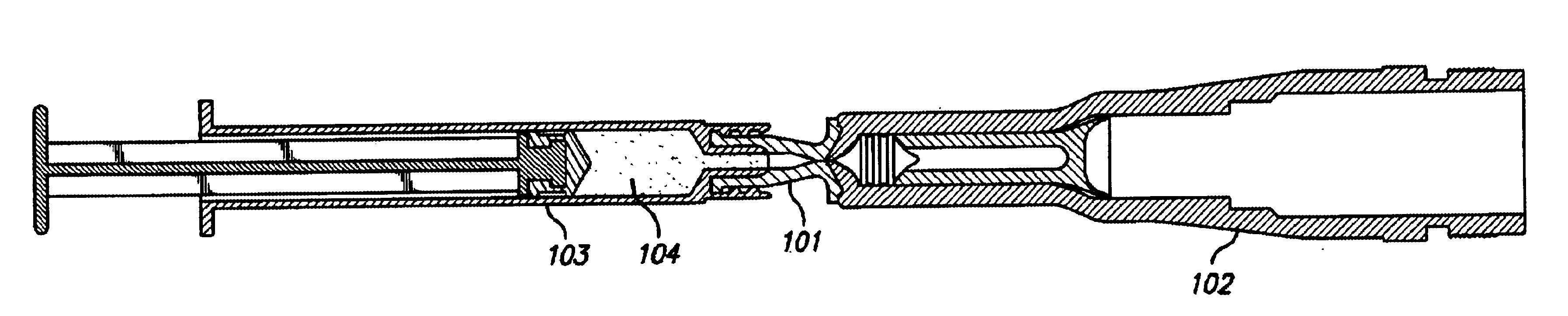
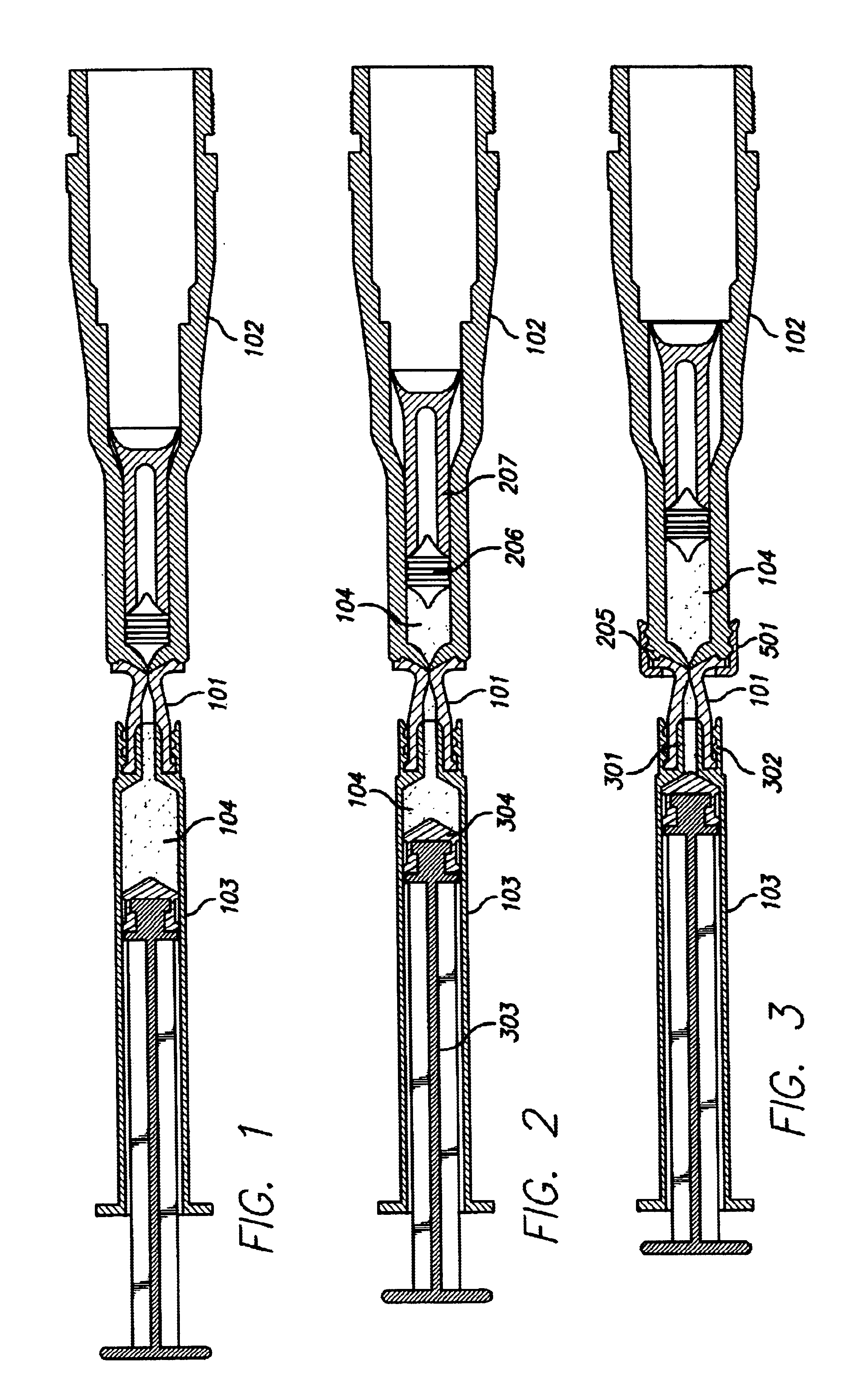
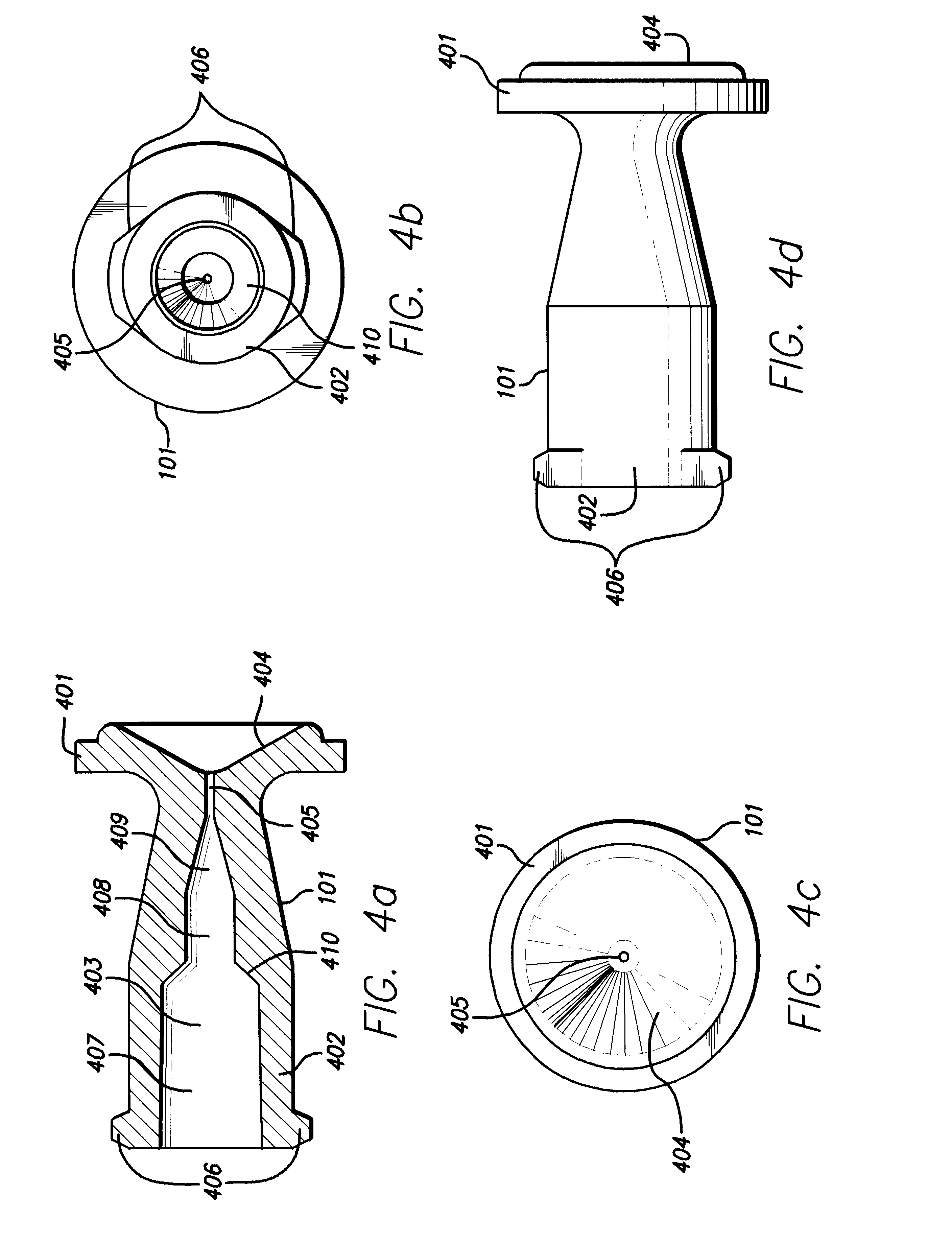
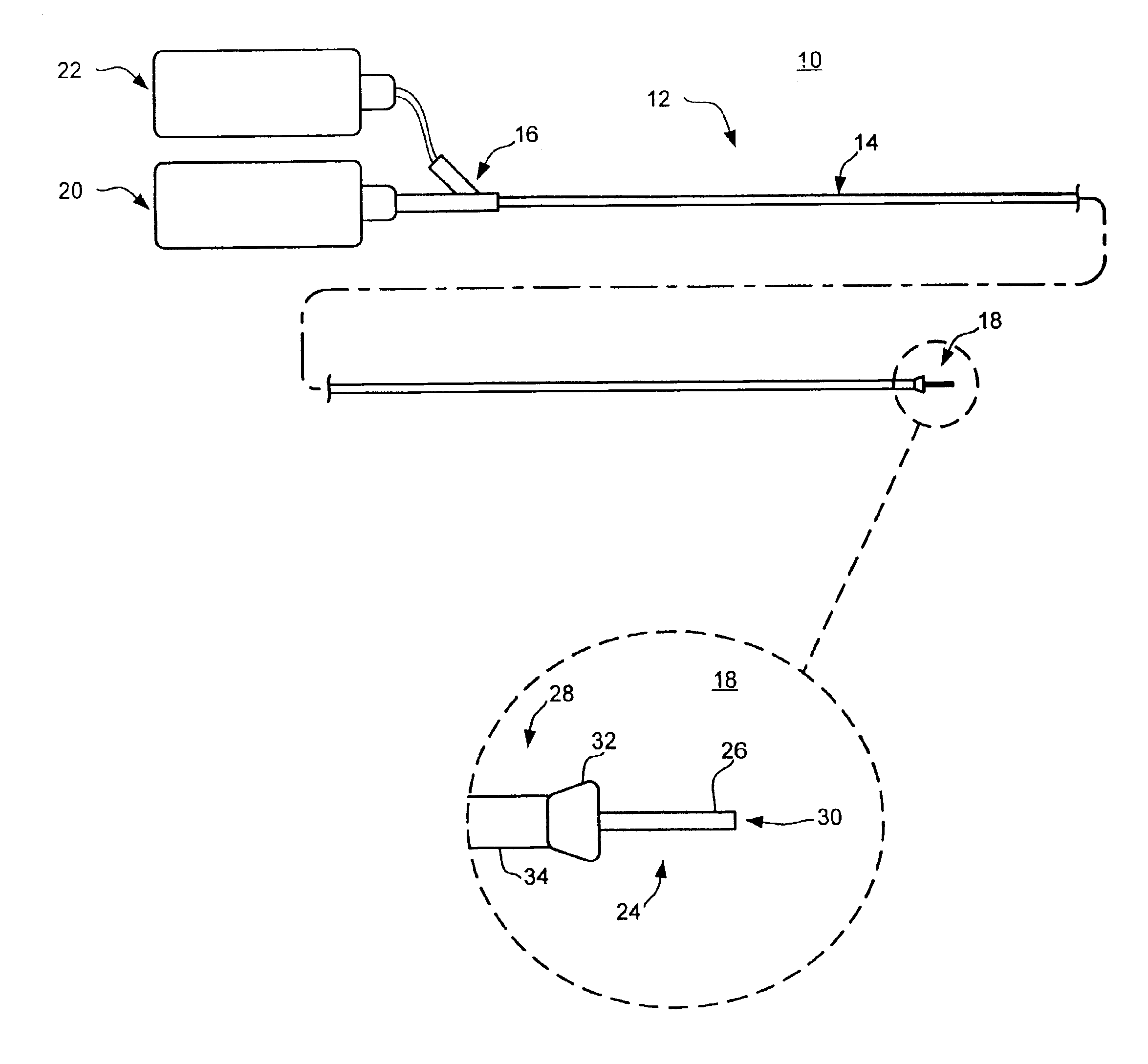
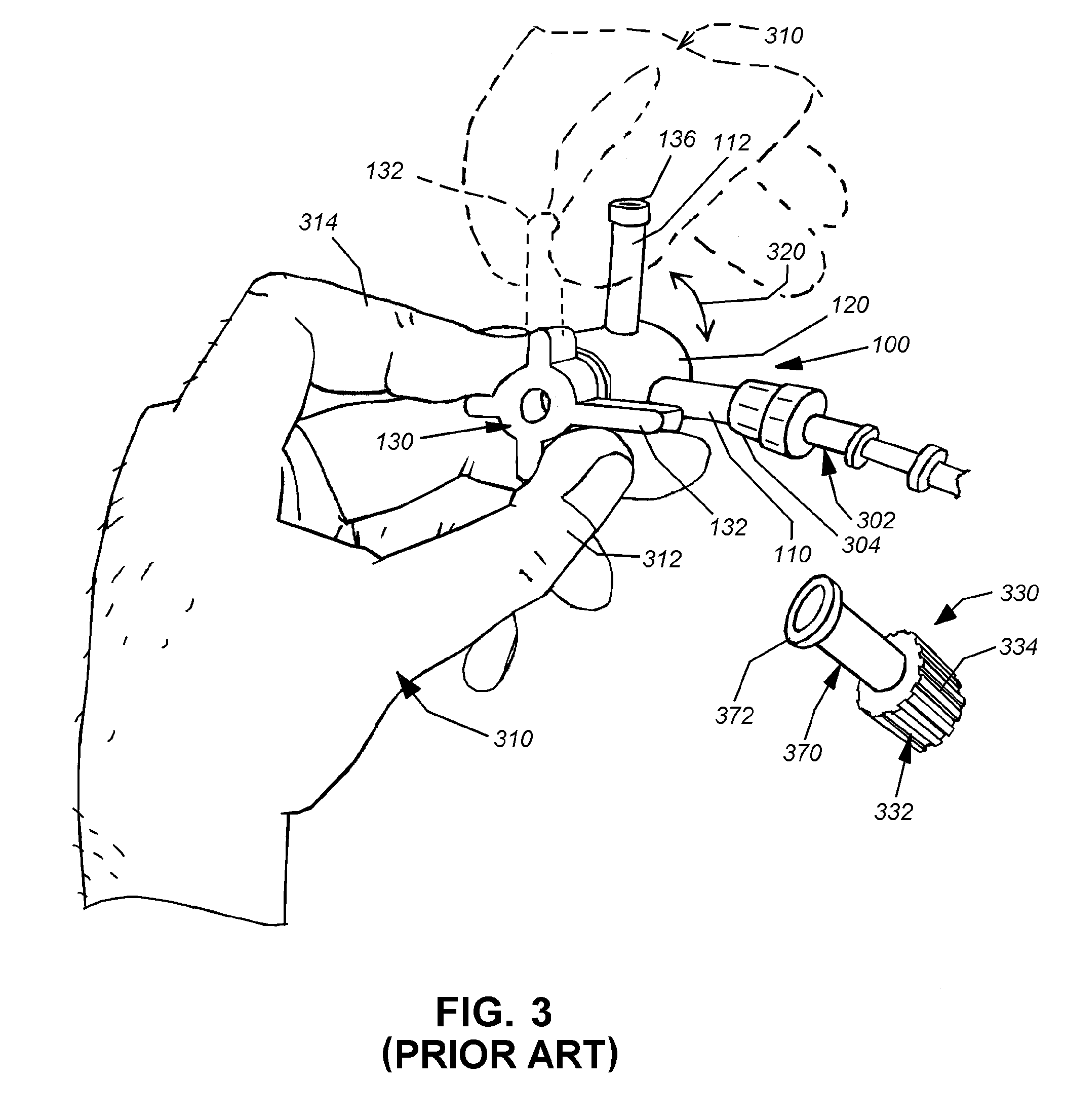
Needle-free injection is a method of injecting using a jet injector device like Comfort-in™, which injects liquid medicine, anaesthesia, vitamins, or other liquid injectables through the skin and into the subcutaneous tissue, via high pressure.
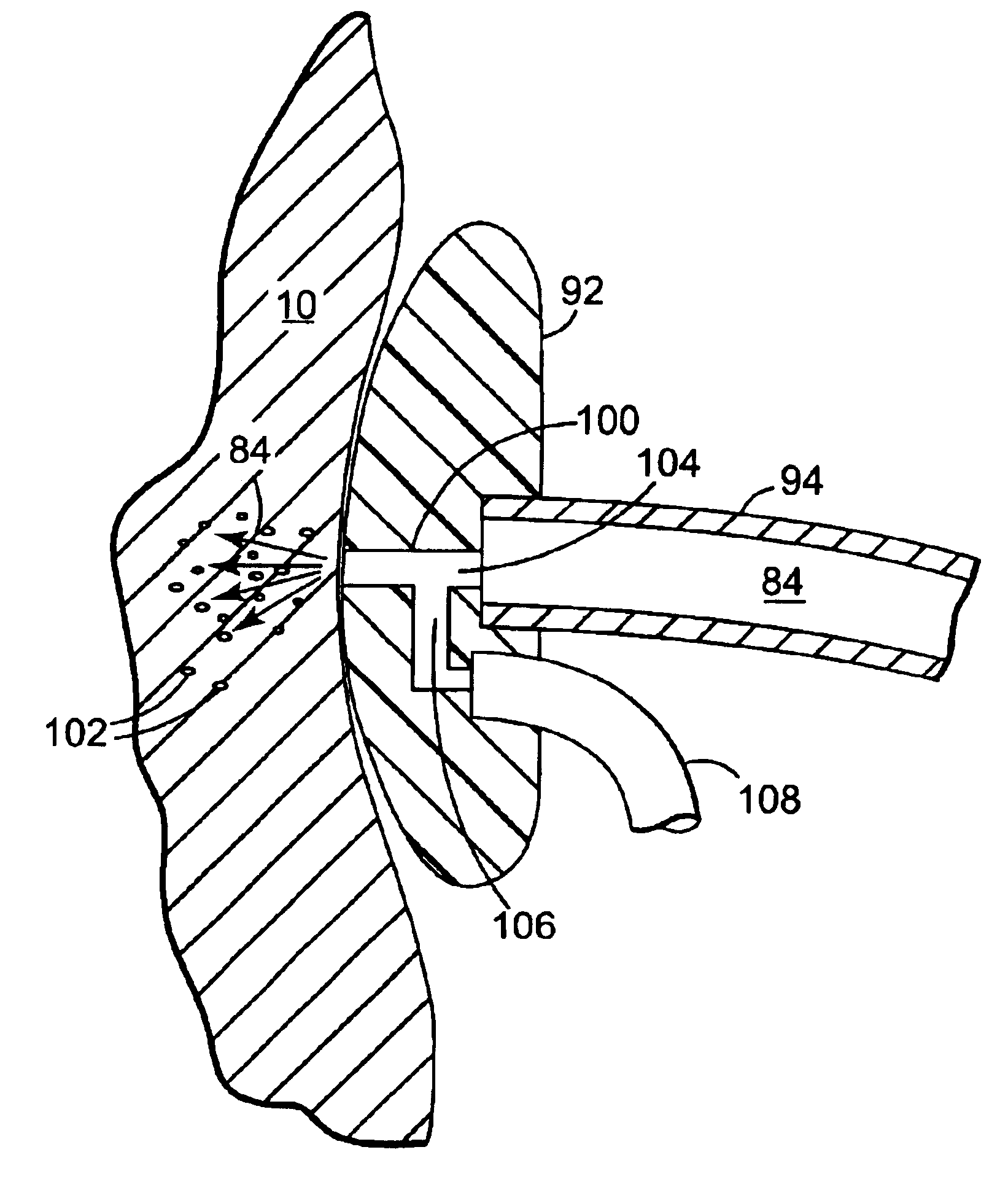
Method of injecting a drug and echogenic bubbles into prostate tissue
InactiveUS6905475B2Ultrasonic/sonic/infrasonic diagnosticsJet injection syringesNeedle Free InjectionEthanol Injection
Method and surgical instrument for treating prostate tissue including a surgical instrument having a main body, a needle deployment port, a needle, first and second handles and a lockout release mechanism to limit needle extension. Additionally, a kit includes the surgical instrument, together with a cystoscope, and optionally a syringe and reservoir of ethanol. The method includes needle-less injection and visualizing the ethanol injection by delivering both an echogenic agent and ethanol either by needle or needle-less injection or by providing an ultrasonically visible marker near the tip of the ethanol delivery cannula. The method also includes extending the needle transversely of the instrument housing using a link assembly.
Owner:BOSTON SCI SCIMED INC
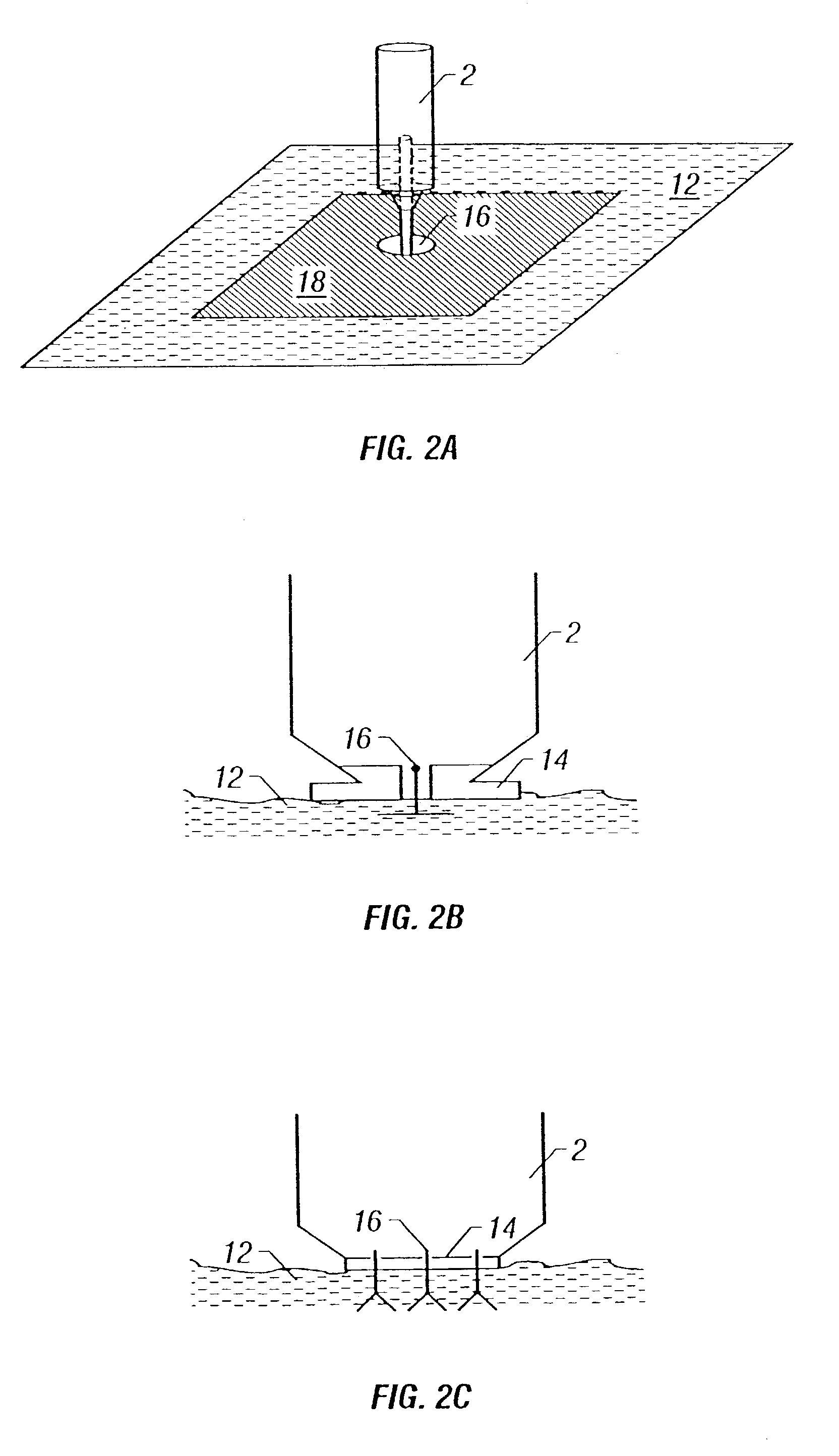
Intradermal delivery of active agents by needle-free injection and electroporation
Methods are proved for introducing a biologically active agent into cells of a subject by introducing the agent in a form suitable for electrotransport into a region of tissue of the subject using one or more needle-free injectors, and applying a pulsed electric field to the region of tissue, thereby causing electroporation of the region of tissue. The combination of needle-free injection and electroporation is sufficient to introduce the agent into cells in skin, muscle or mucosa. For example, the region of tissue can be contacted with two oppositely charged injectors, one acting as the donor electrode and one acting as the counter electrode, or a single injector and one or more electrodes can be used. In addition, needle-free injection may be used in combination with suitable non-invasive electrode configurations. The active agents delivered into cells using the invention method can be small molecules, polynucleotides, polypeptides, and the like.
Owner:ONCOSEC MEDICAL
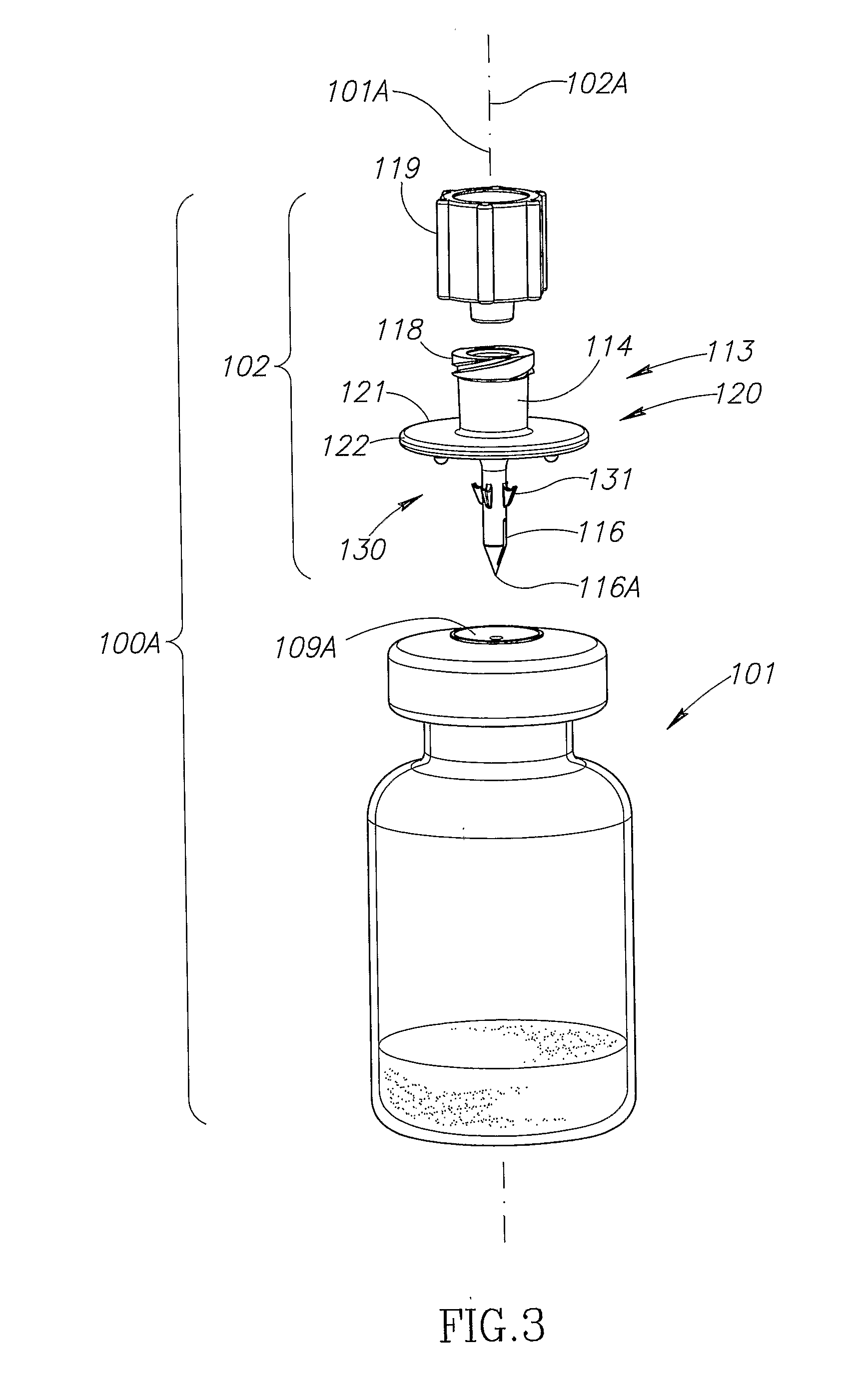
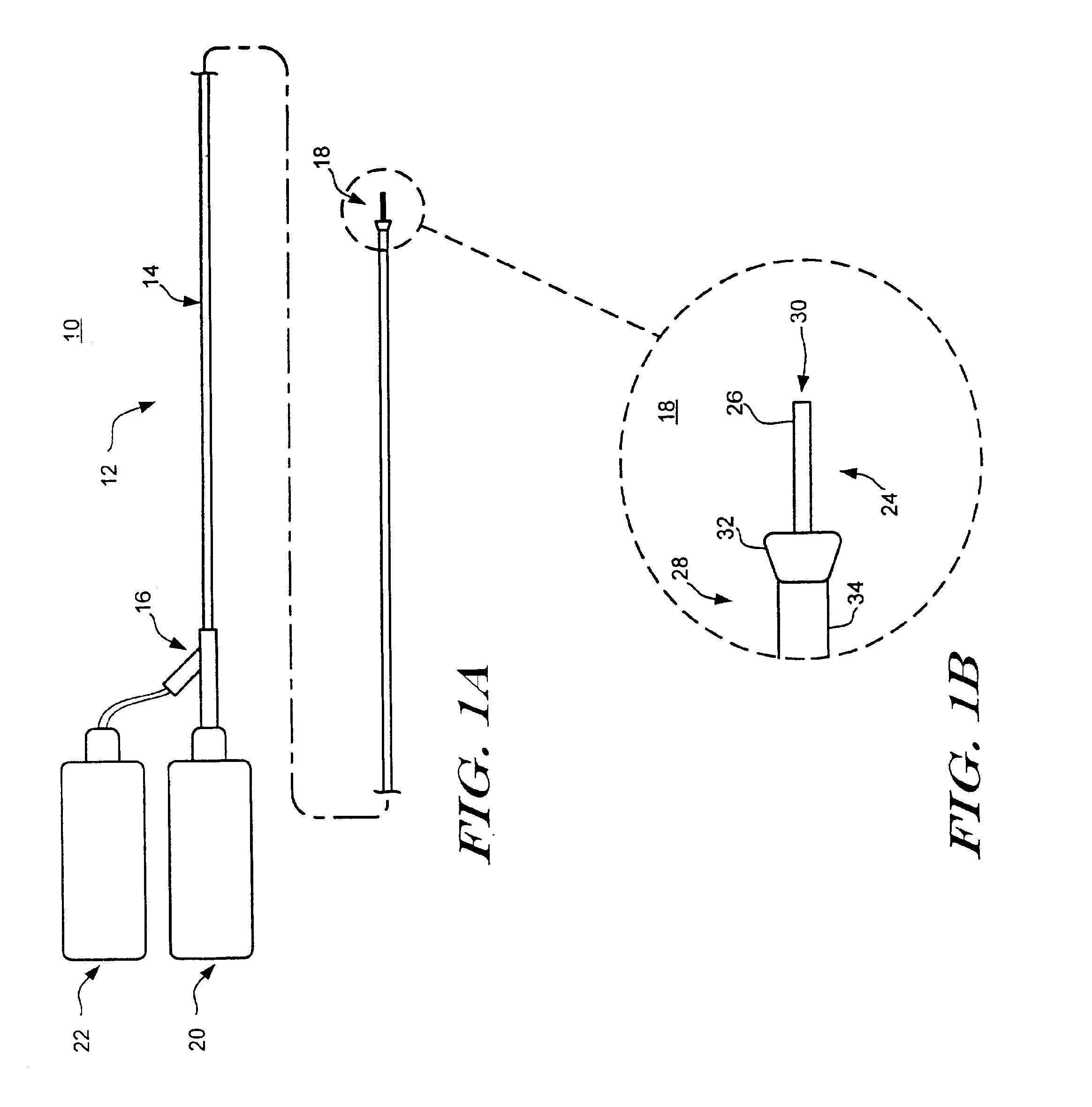
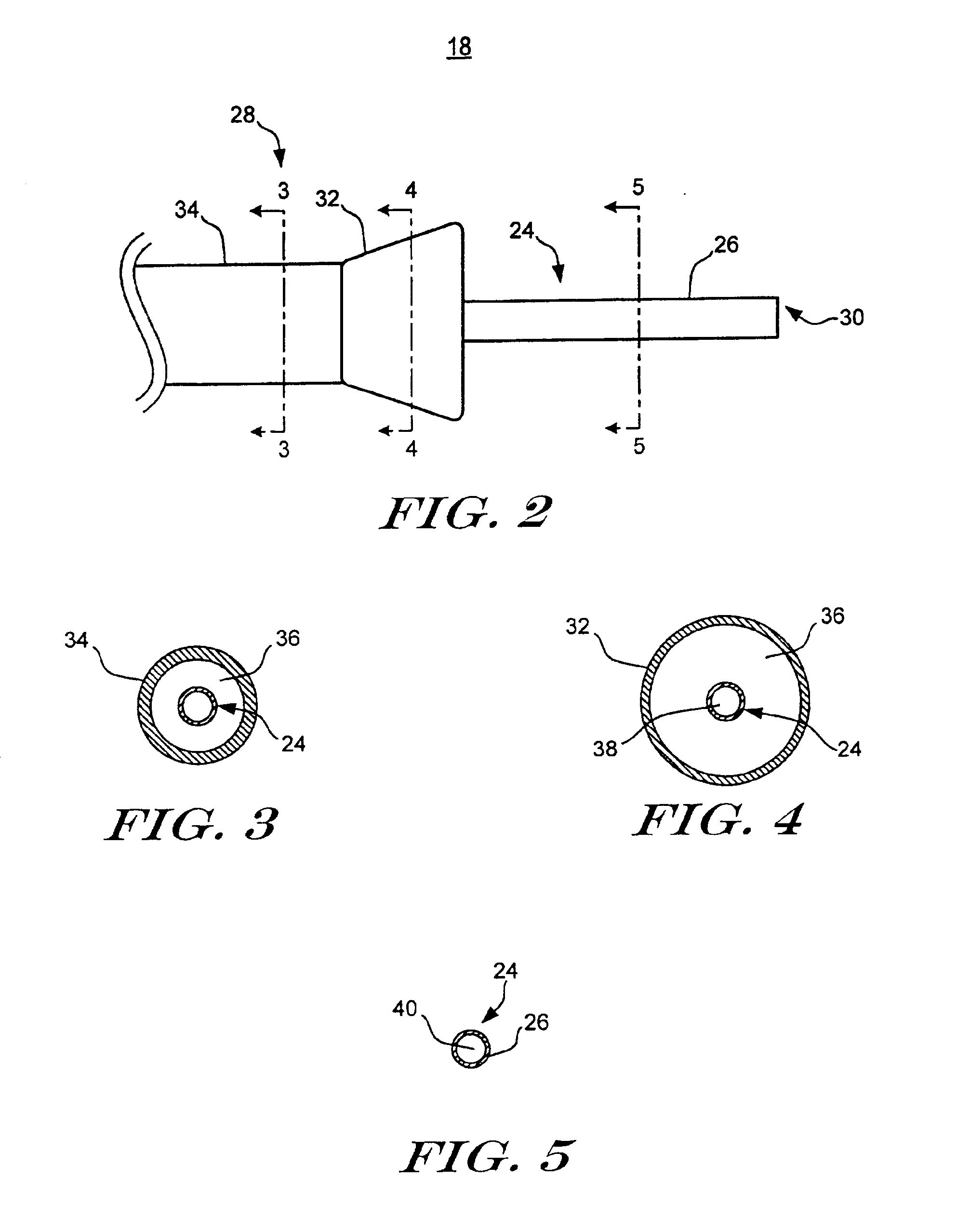
Vial adaptor
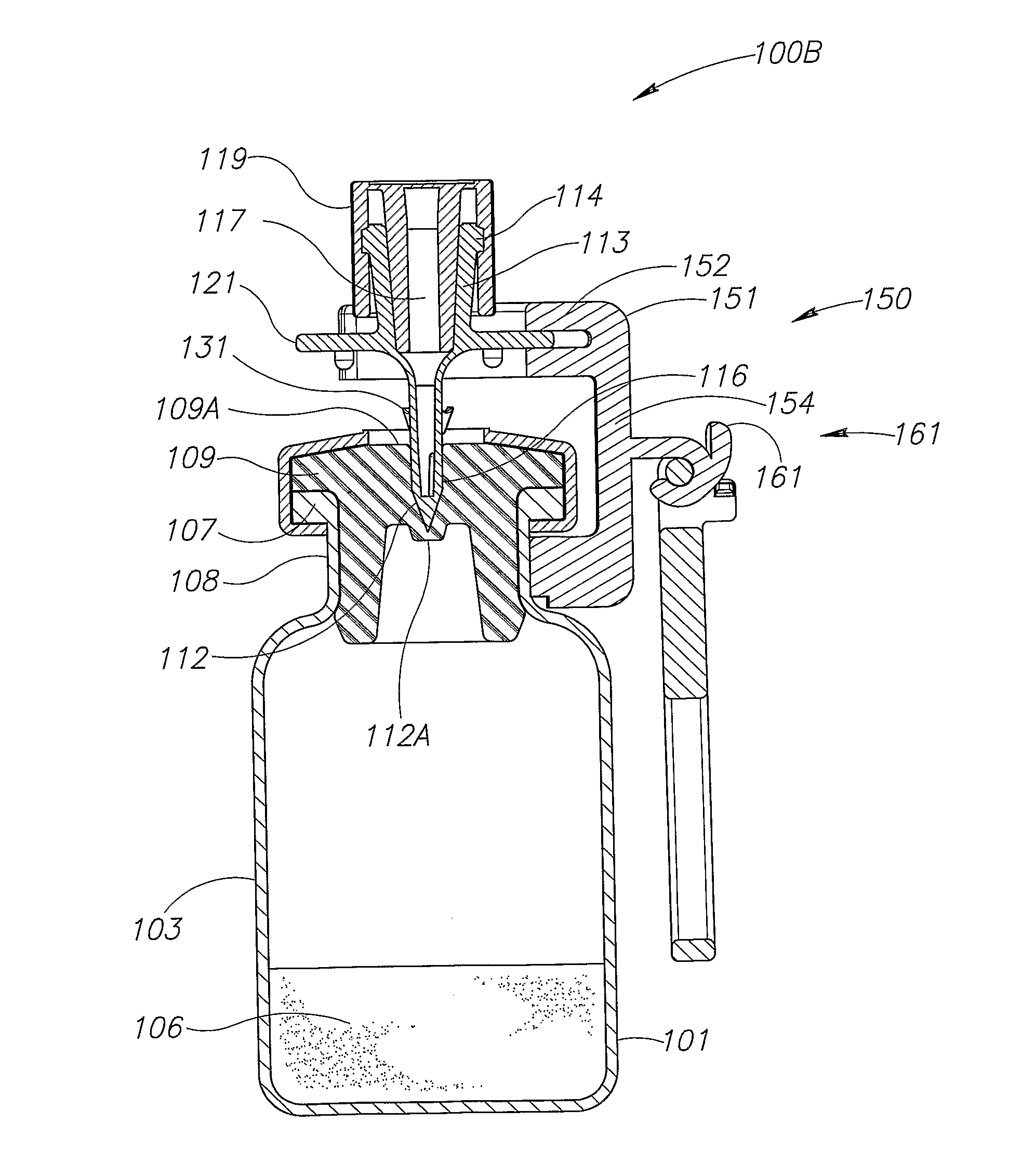
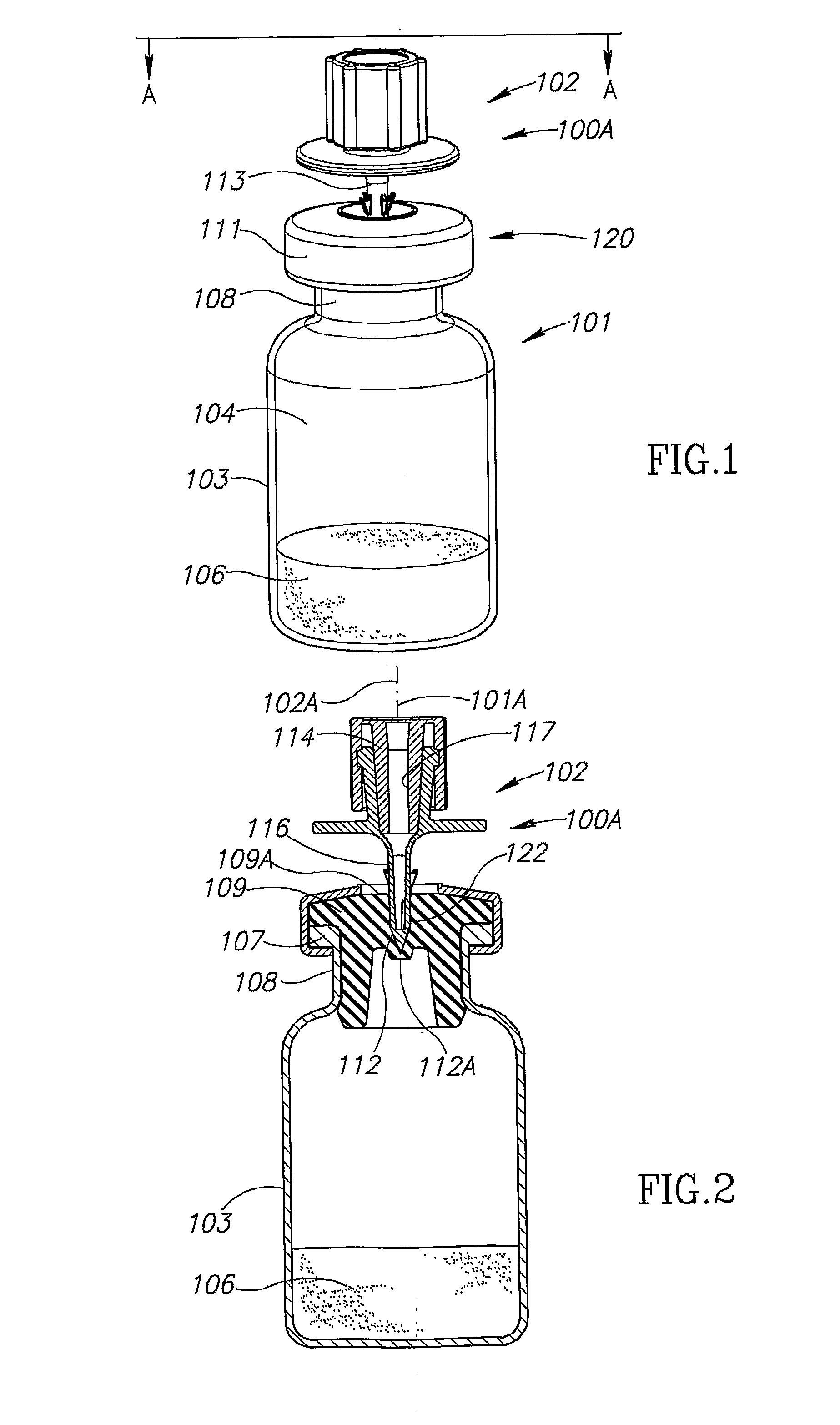
A vial adapter suitable for use in transferring fluid from a vial to a needleless syringe, the vial having a top end sealed with a septum. According to one embodiment, the vial adapter comprises (a) a body, the body having a top end, a bottom end and an inner cavity, the inner cavity being dimensioned to receive the vial, with the bottom end of the body extending below the bottom end of the vial; (b) a needle-bearing member mounted within the body, the needle-bearing member comprising a hollow needle extending downwardly into the inner cavity of the body for puncturing the septum of a vial disposed in the inner cavity; (c) a luer-lock-bearing member mounted on the top end of the body, the luer-lock-bearing member comprising a top portion and a bottom portion separated by a radial wall, the top portion being a female luer-lock, the bottom portion including a tubular structure in fluid communication with the hollow needle; and (d) a valve disposed within the luer-lock-bearing member for controlling fluid flow from the bottom portion to the top portion, the valve being opened by attachment of the needleless syringe to the vial adapter.
Owner:BOSTON SCI SCIMED INC
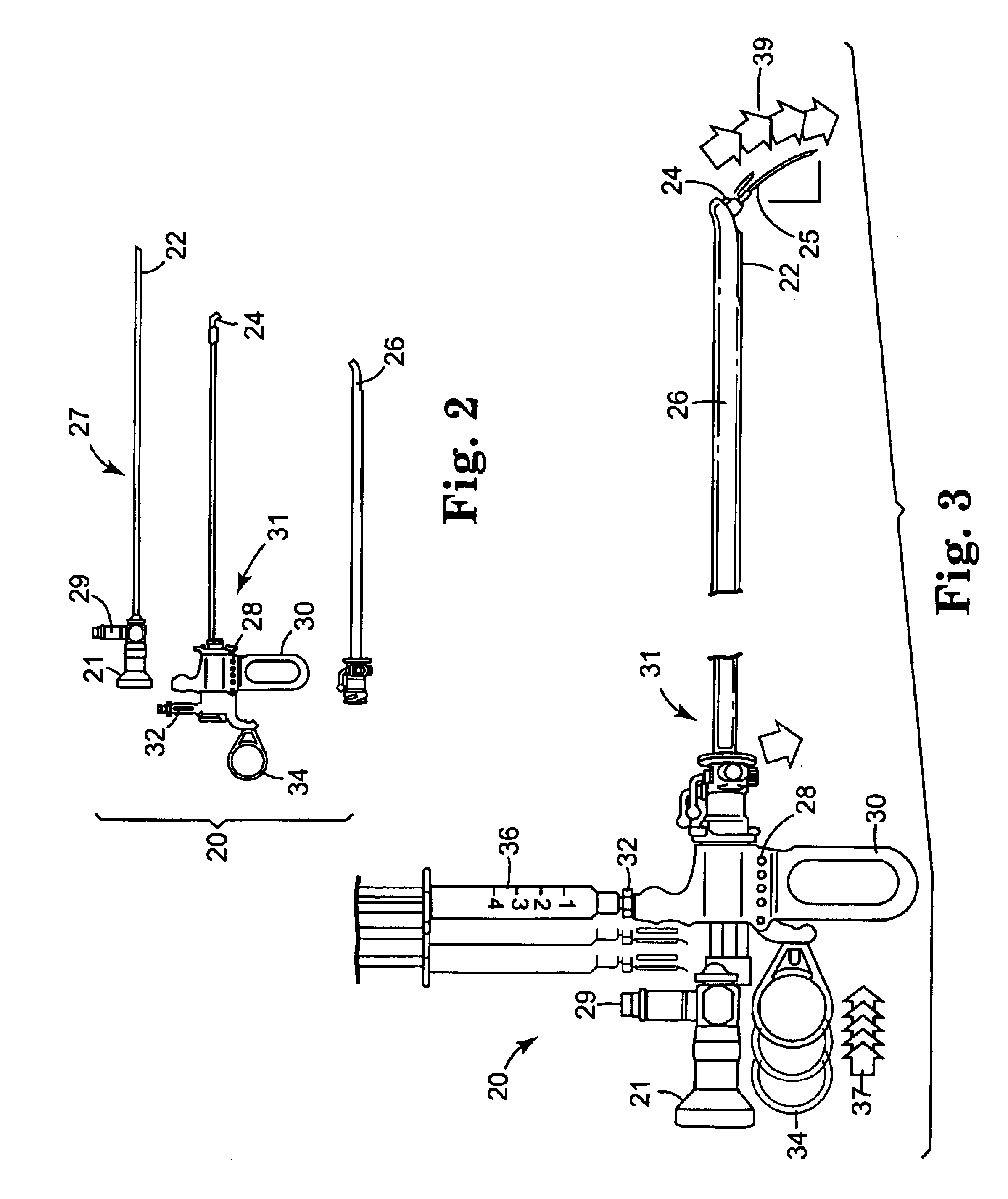
Method and apparatus for needle-less injection with a degassed fluid
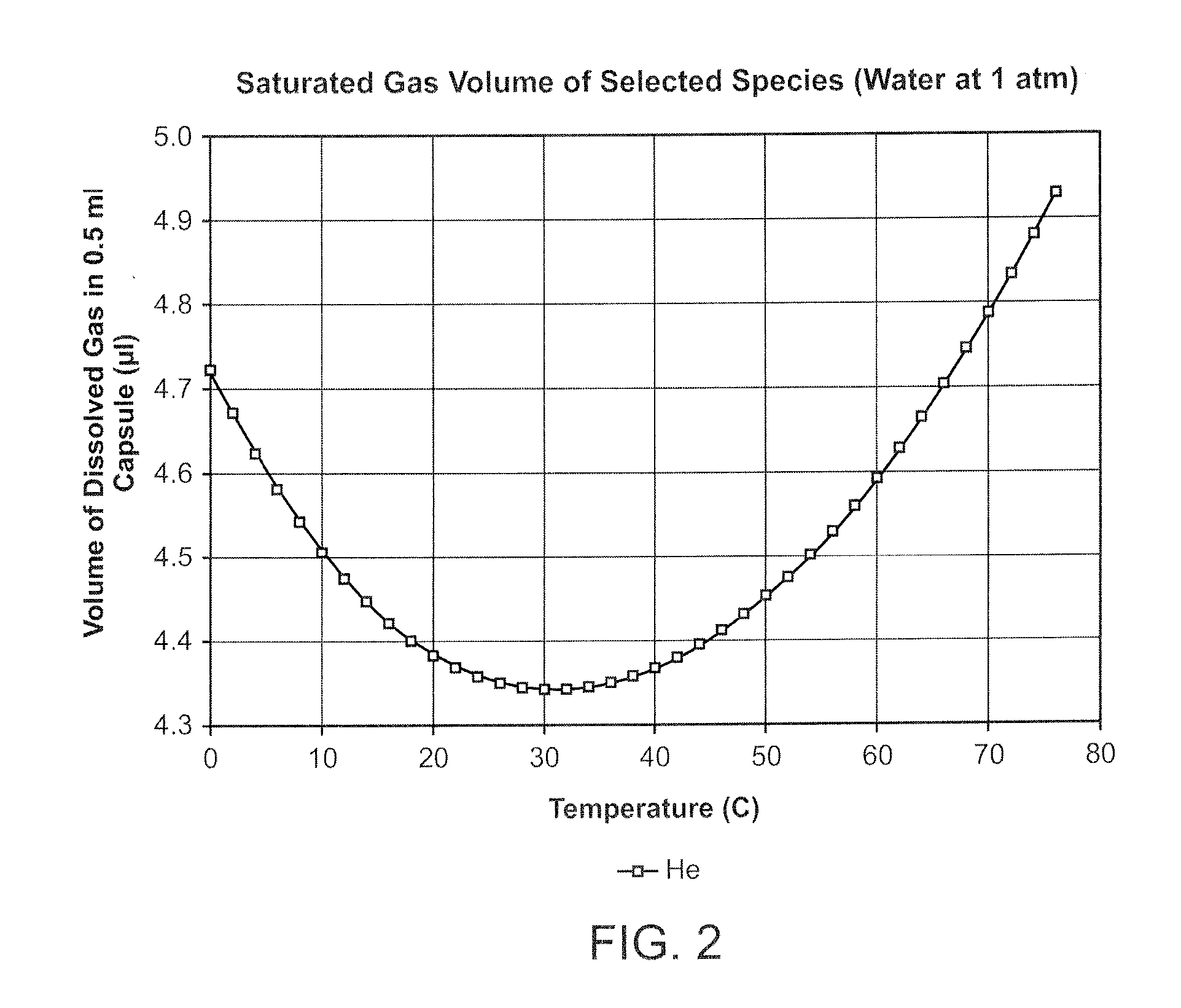
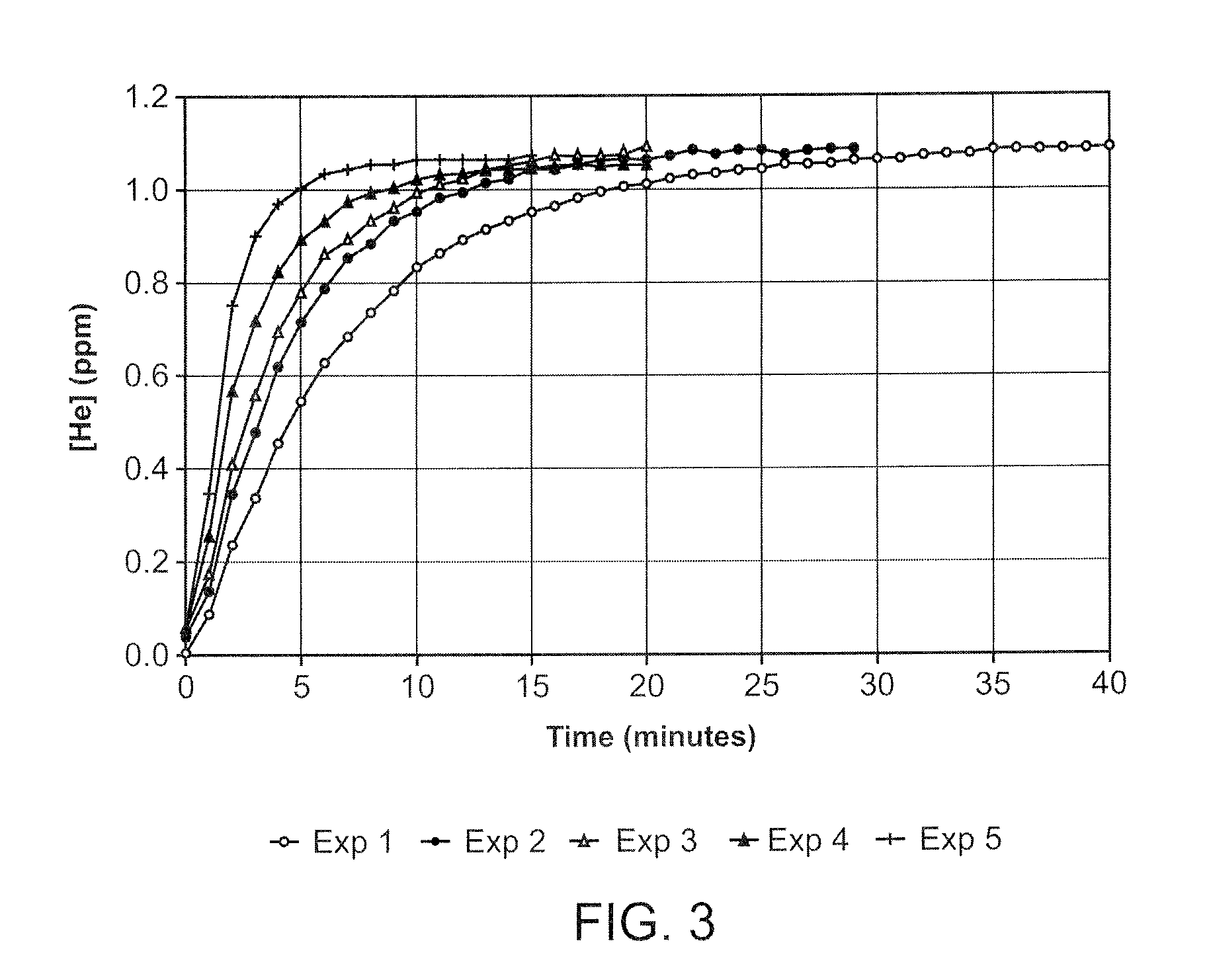
Apparatuses and methods are described for administering a needle-less injection of a degassed fluid. Prior to filling, or after filling but prior to administration of a needle-less injection, gas is removed from the fluid to create a degassed fluid. A needle-less injection may then be performed with a reduced risk of discomfort to the recipient of the injection and with lower potential for the creation of a subdermal hematoma as a result of the injection. A wide variety of needle-less injectors may be used in accordance with various embodiments of the present invention.
Owner:PENJET CORP
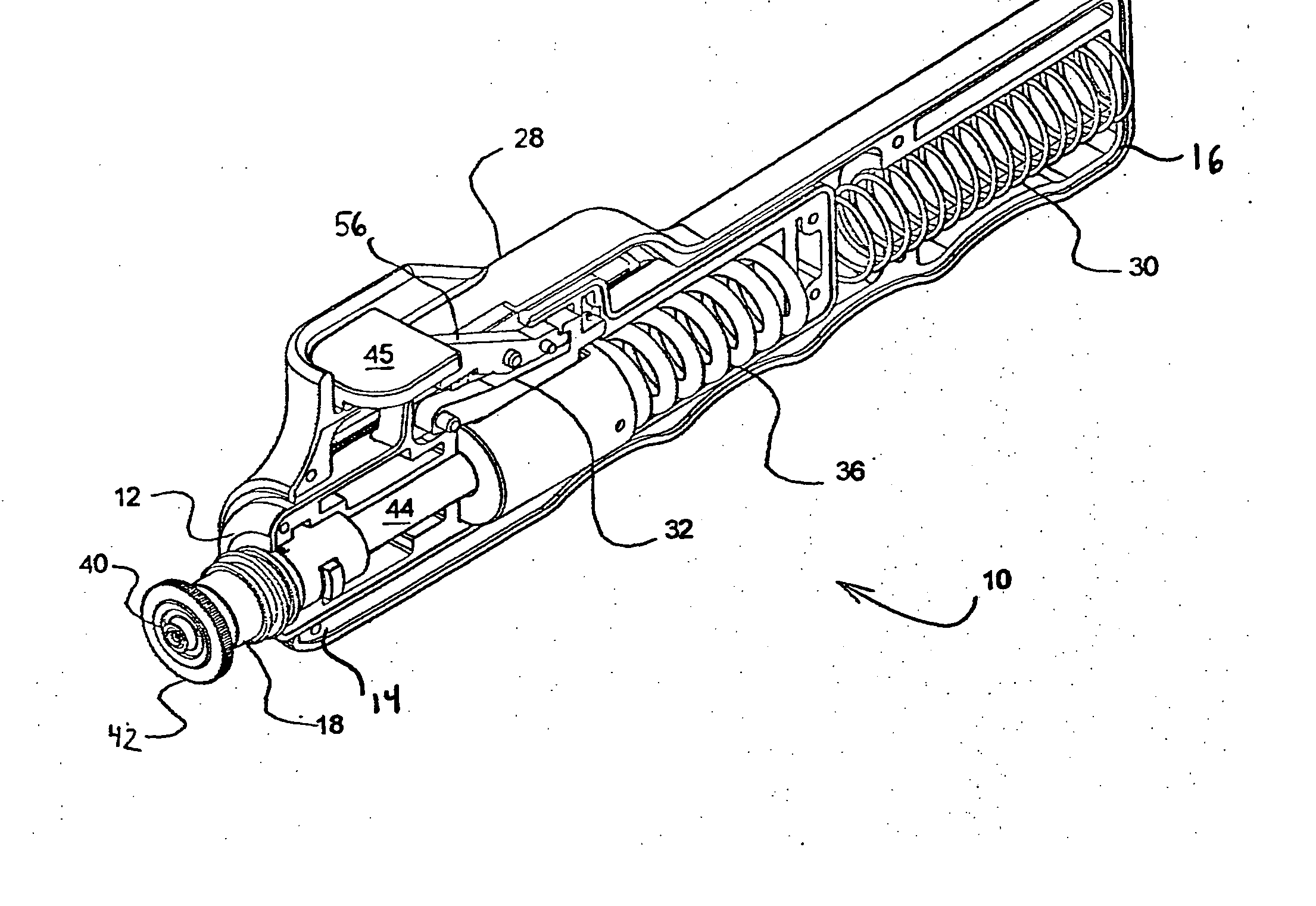
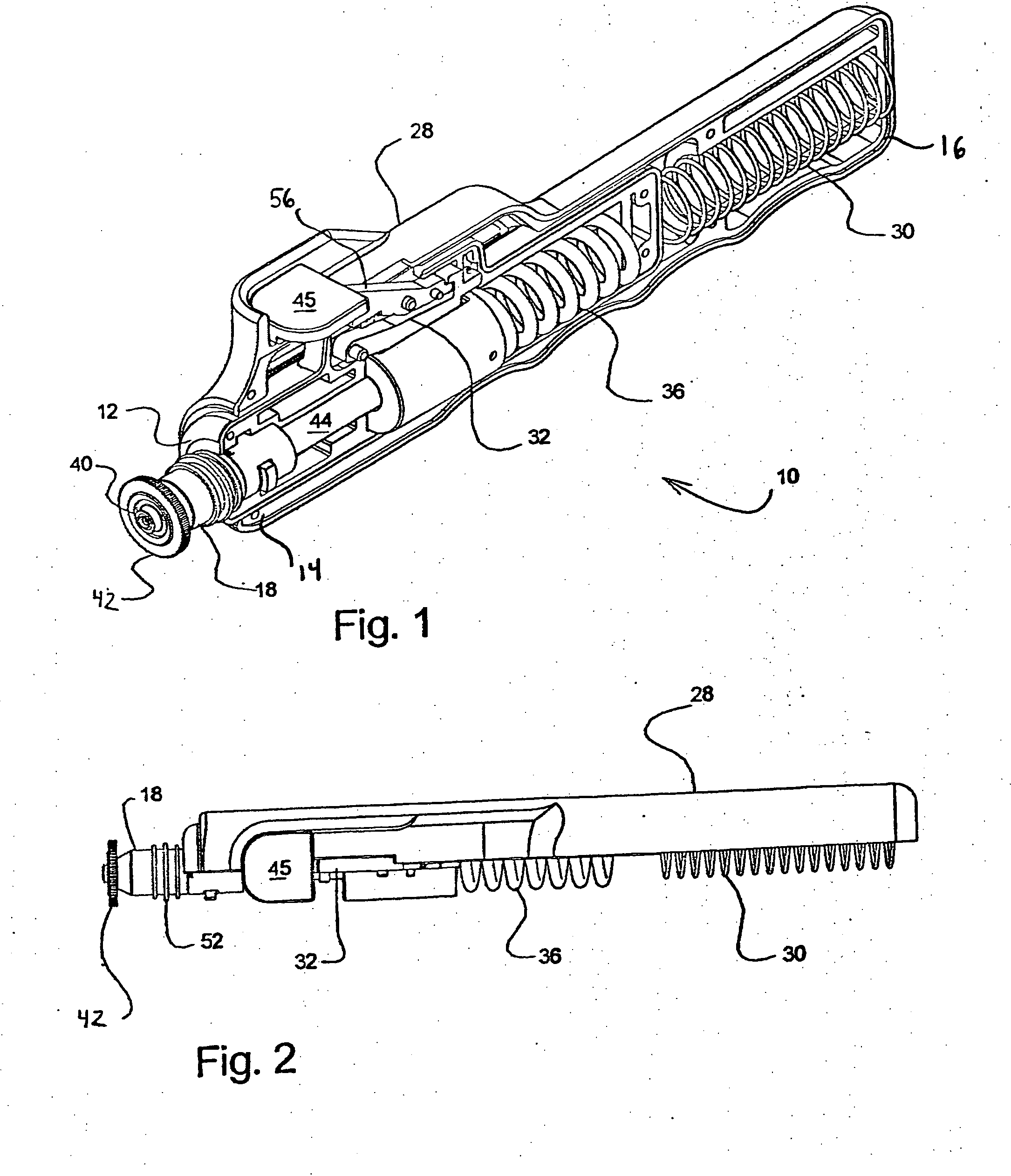
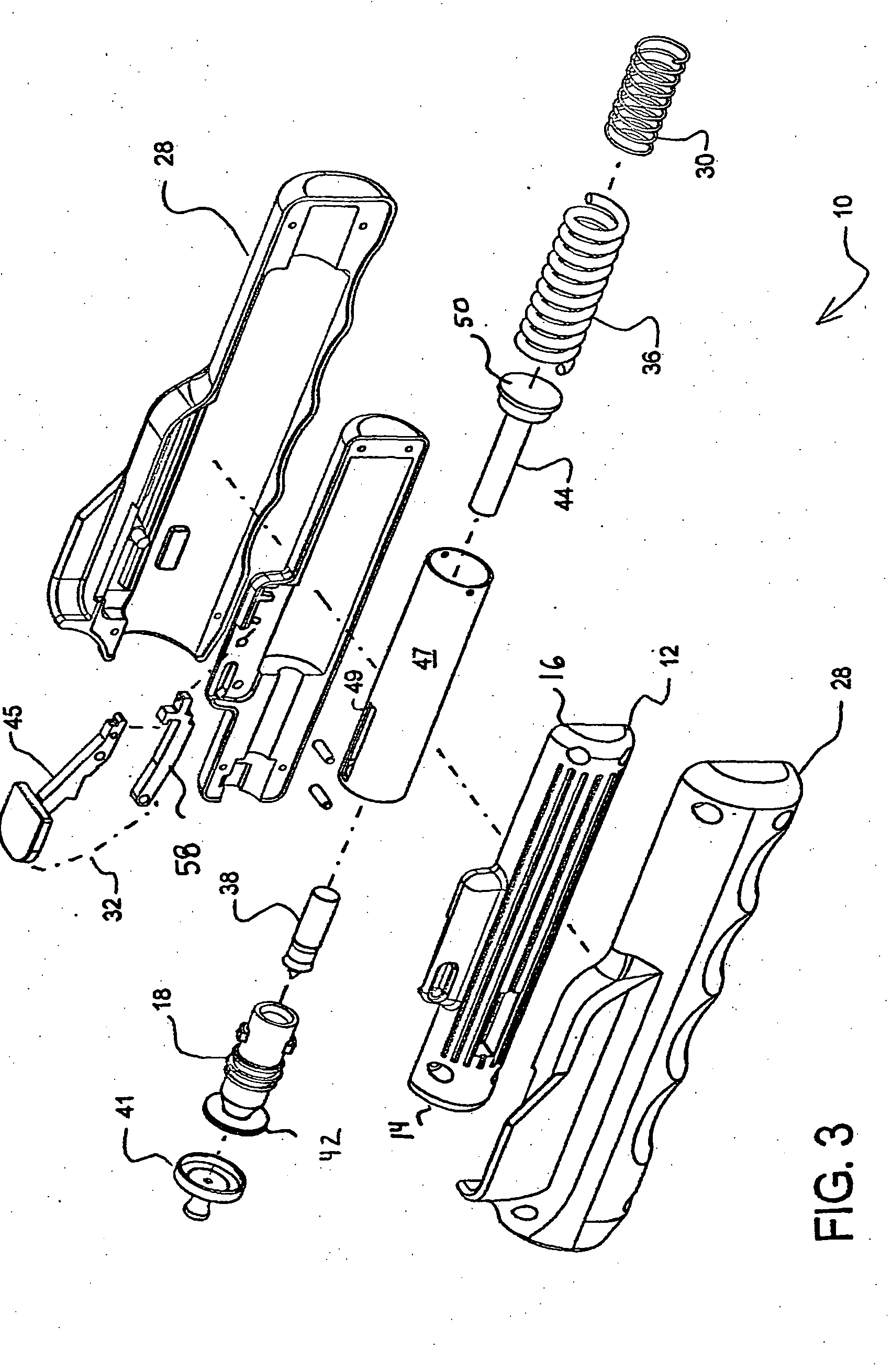
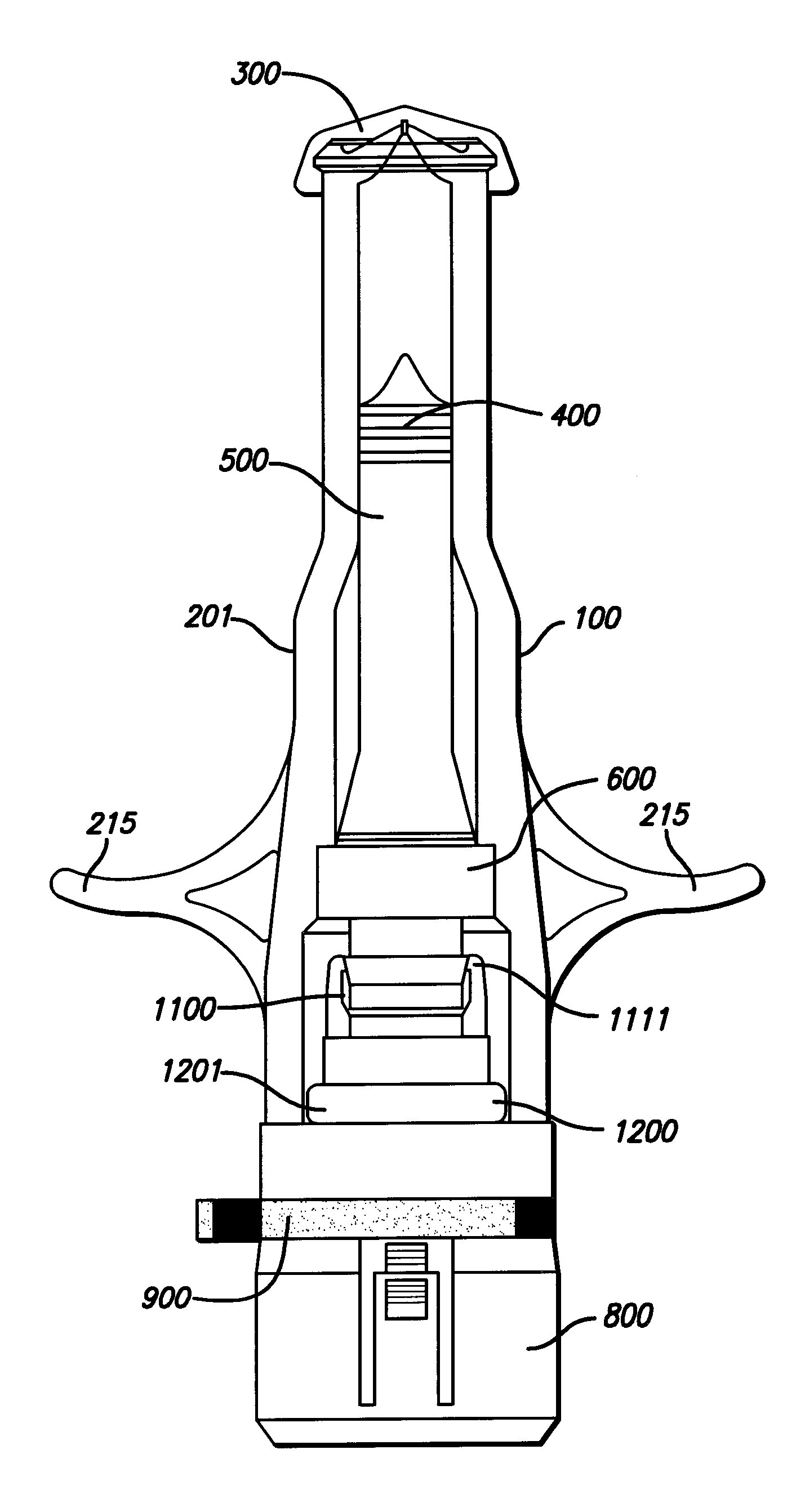
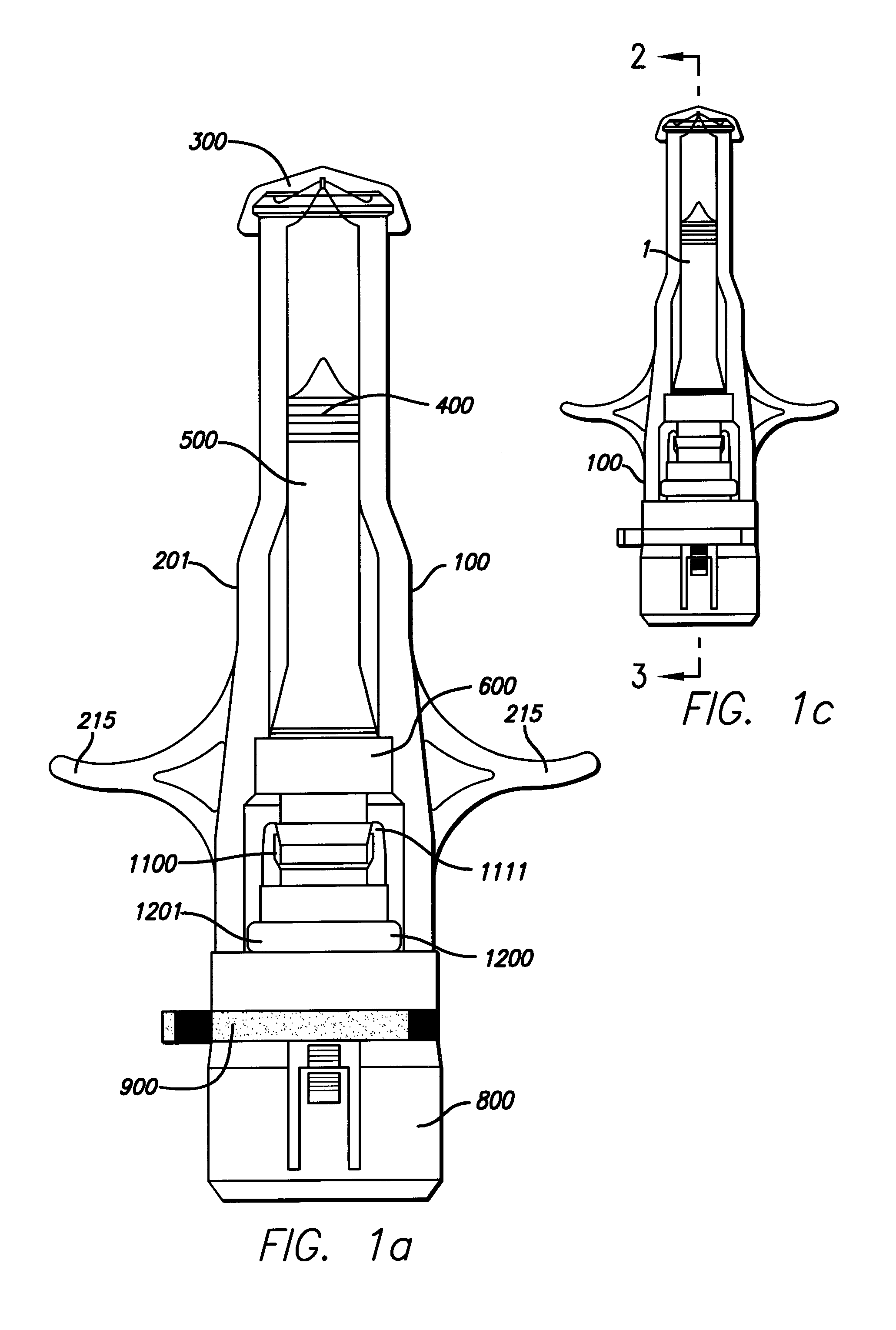
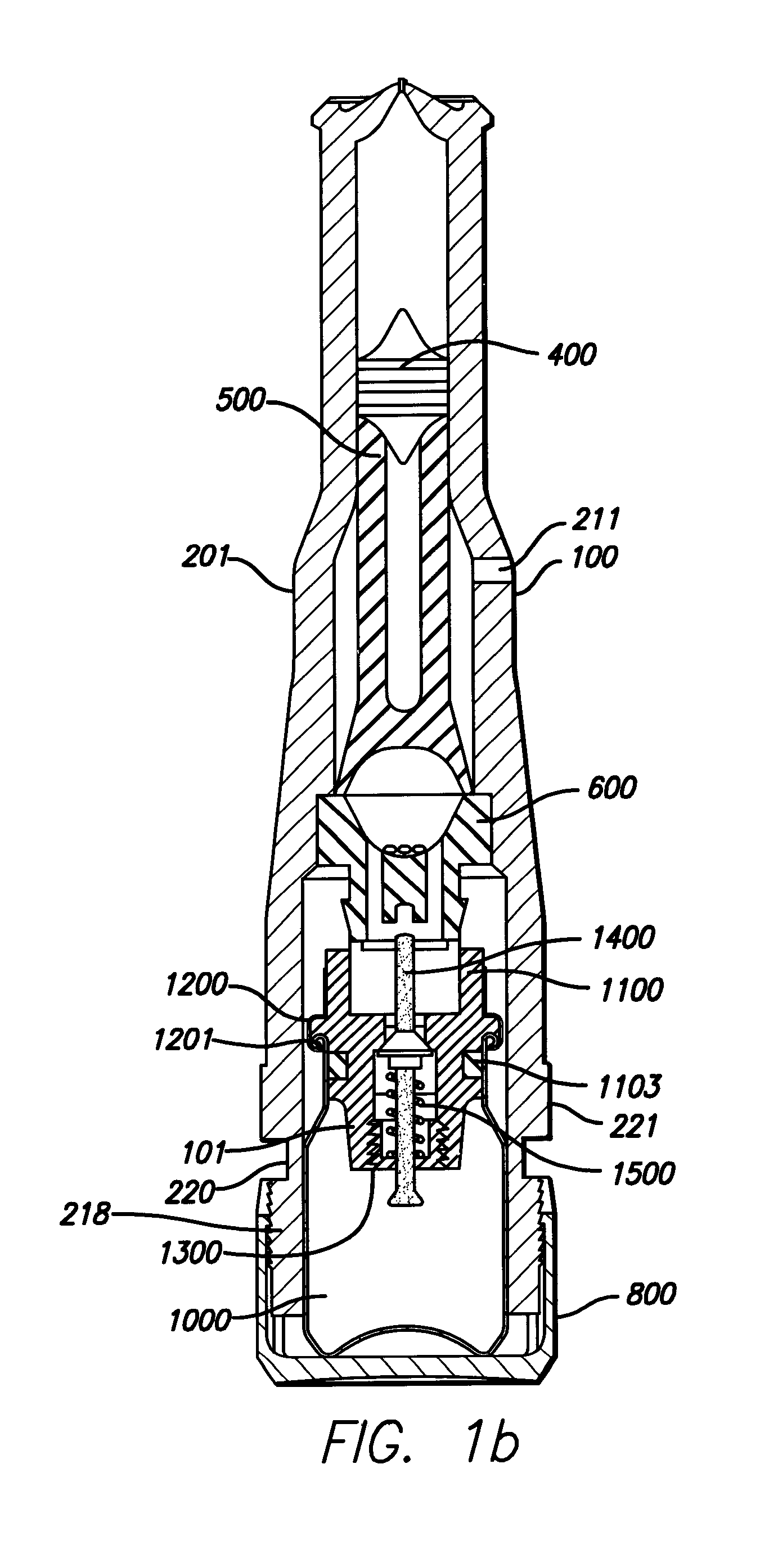
Needle-less injector and method of fluid delivery
ActiveUS20070118094A1Safe and reliableAvoid accidental dischargeJet injection syringesAutomatic syringesNeedle Free InjectionPlunger
A needle-less injector device for delivering a dose of fluid intradermally, subcutaneously or intramuscularly to an animal or human. The device includes an inner housing having opposed ends. A syringe is disposed in one end of the inner housing. The syringe includes a nozzle for delivering a dose of fluid held within the syringe. A plunger is movably disposed within the syringe. A spring powered hammer is movably disposed within the inner housing. The hammer cooperates with the plunger to drive the dose of medicament from the nozzle. An injection delivery spring for powering the hammer is positioned and compressed between the other end of the inner housing and the spring powered hammer. An outer housing slideably supports the inner housing. A skin tensioning spring is mounted between the inner housing and the outer housing, the skin tensioning spring biasing the nozzle of the syringe against the animal or human. A trigger mechanism is disposed in the outer housing, the trigger mechanism cooperating with the spring powered hammer to release the injection delivery spring, wherein the size of the injection delivery spring and the length of the hammer dictate the amount of dose delivered and whether the dose is delivered intradermally, subcutaneously or intramuscularly to an animal or human.
Owner:PHARMAJET INC
Microstream injector
InactiveUS20070043320A1Quick distinctionPrevent accidental contactJet injection syringesMicroneedlesNeedle Free InjectionInjection site
The present invention provides a micromachined or microsized component-based needleless injector for delivering a dose of a liquid formulation containing a biologically active agent to tissue by way of a high pressure liquid microstream that penetrates the skin and deposits the agent at an optimal depth in the tissue. The device is appropriate for subcutaneous, intramuscular, or mucosal injection sites, as well as intracellular injection. Embodiments include micromachined or microsized components such as valves, jets, and MEMS pumps. Some embodiments of the invention are modular, with interchangeable parts, other embodiments are integrated in a unitary design. Embodiments with a unitary design are typically single use, however modular features also create embodiments with components that provide multiple instances of use.
Owner:KENANY SAAD AL
Medical injector and medicament loading system for use therewith
InactiveUS7341575B2Eliminate adhesionImprove accuracyAmpoule syringesJet injection syringesNeedle Free InjectionNeedle free
The present invention discloses a medical injector and medicament loading system for use therewith. The medicament loading system includes cap for a medicament cartridge. The cap has a post for causing movement of the cartridge stopper toward the seal when the cap engages the medicament cartridge to thereby eliminate adhesion between the medicament chamber and the stopper. The medical injector according to the present invention includes the medicament loading system, i.e., a cartridge assembly, a needle free syringe assembly, and a power pack assembly.
Owner:FERRING INT CENT SA
Needleless syringe
ActiveUS7547292B2Reduce pressureWork moreJet injection syringesAutomatic syringesNeedle Free InjectionGas passing
A method of distributing particles in a flow of gas and a needleless syringe for use in the needleless injection of particles into the skin or mucosa of a vertebrate subject are disclosed. The syringe includes a convergence which reduces pressure of the gas flowing in the gas flow path due to the Venturi effect such that particles initially located outside of the gas flow path are drawn into the gas flow path under the action of the reduced pressure and become entrained in the gas. An exit nozzle accelerates the particles so entrained. In another aspect of the invention, there is provided a method of creating a gas flow in a needleless syringe which comprises flowing gas through a first convergence into a chamber to form a transsonic gas jet in the chamber and passing the gas jet from the chamber into a second convergence and along the nozzle.
Owner:POWDER PHARM INC
Needleless injector drug capsule and a method for filling thereof
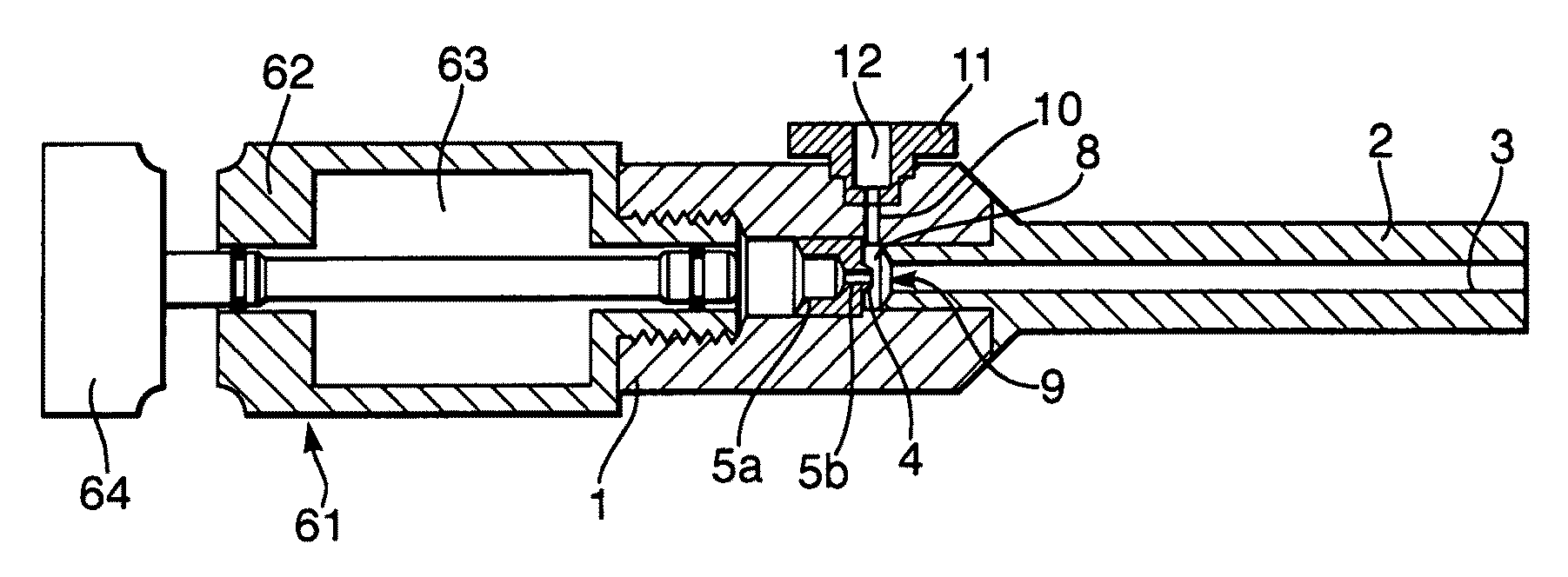
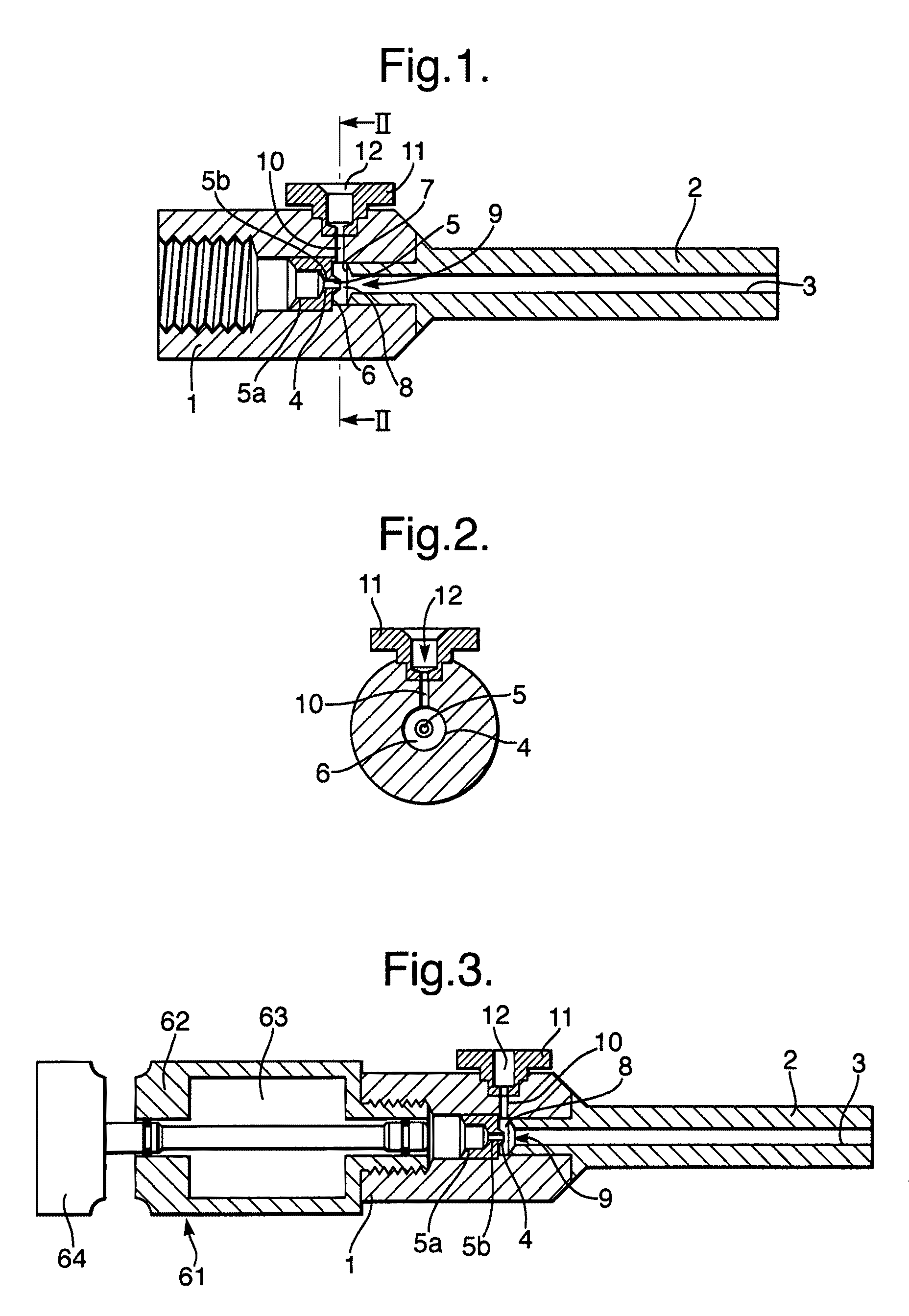
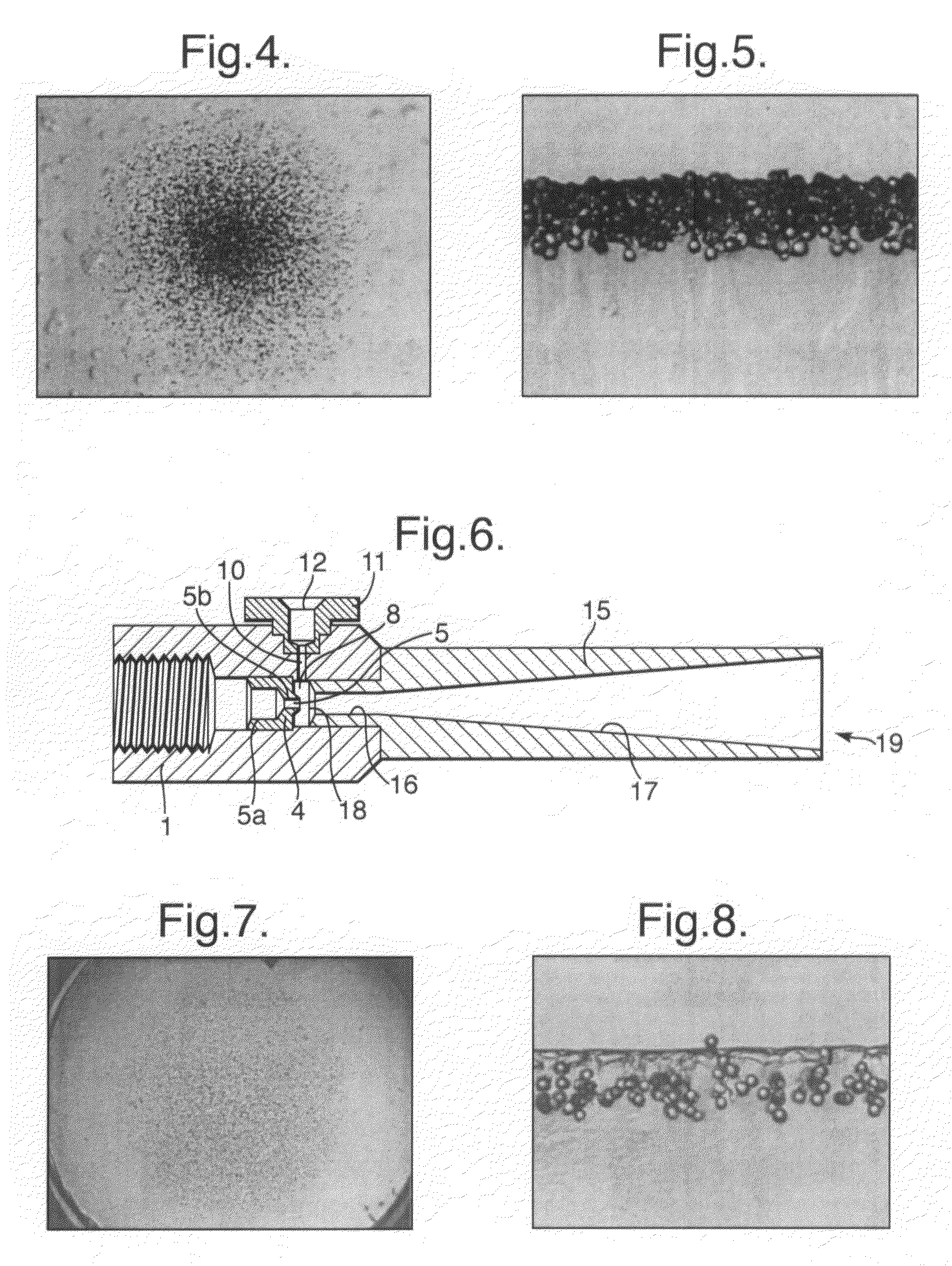
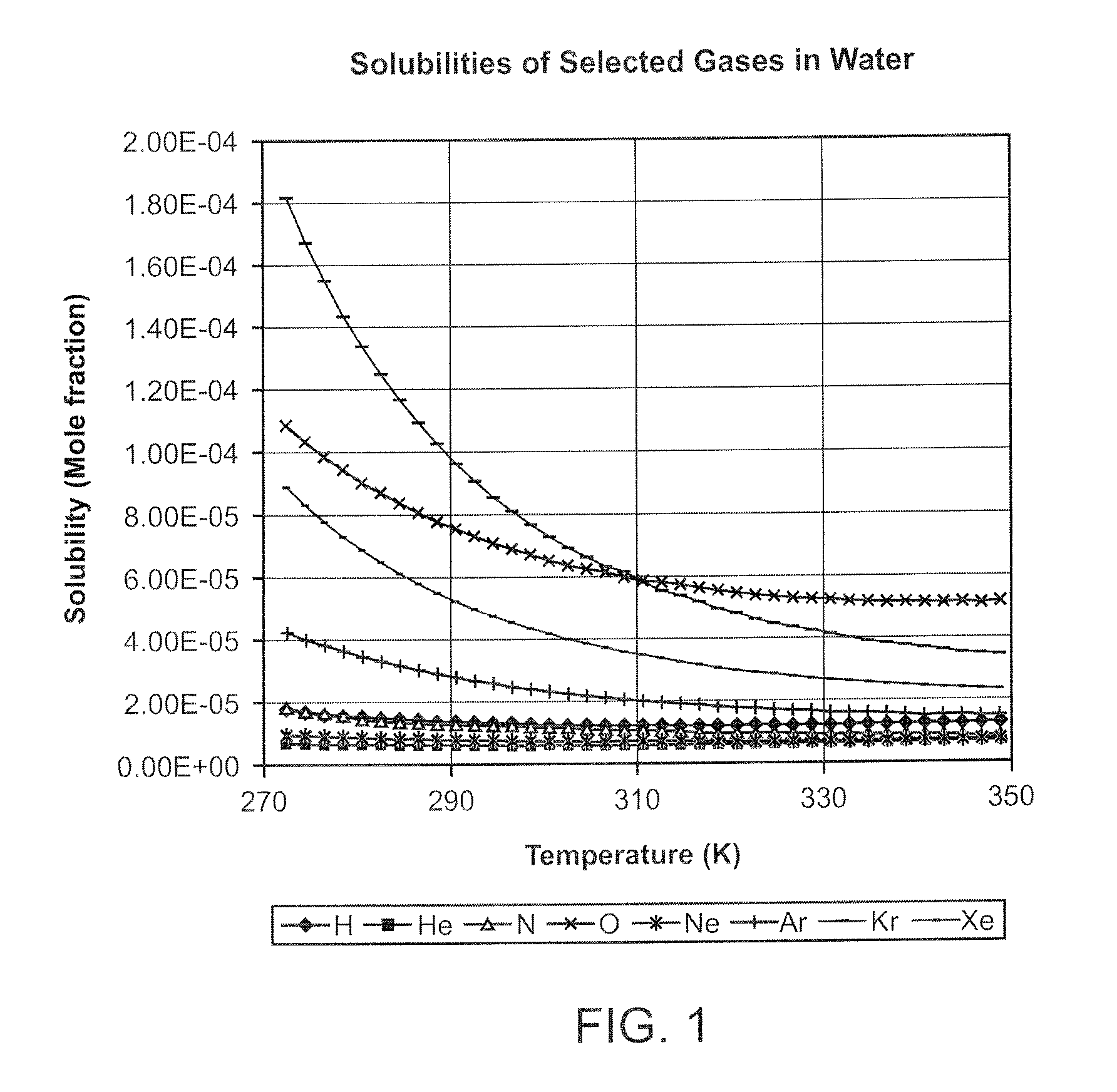
InactiveUS20080281260A1Reduce morbidityLiquid degasification with auxillary substancesJet injection syringesSolubilityDrug capsule
Owner:ZOGENIX INC
Vial assemblage with vial and pre-attached fluid transfer device
InactiveUS20120184938A1Avoid manual removalPrevent rotationClosuresDiagnosticsEngineeringBiomedical engineering
Vial assemblages having a vial and a pre-attached fluid transfer device for use with a needleless syringe for enabling flow communication between the syringe and the vial. The fluid transfer device includes an elongated tubular flow member having a connector for sealing flow communication with the needleless syringe and a spike having a spike end for puncturing the vial for enabling flow communication between the needleless syringe and vial interior. The spike end is in intimate sealing contact with the vial's stopper. The fluid transfer device also includes a manually removable closure for initially sealing the connector and, on removal, exposing the connector.
Owner:MEDIMOP MEDICAL PROJECTS
Modular gas-pressured needle-less injector
InactiveUS6613010B2Mitigate kickbackAvoid separationJet injection syringesAutomatic syringesNeedle Free InjectionEngineering
A needle-less injector suitable for injecting fluid through a surface includes a housing, a driver, an engine and a trigger. The housing contains a fluid and the engine contains a compressed gas. Upon application of sufficient force to the trigger, the compressed gas is released from the engine forcing the driver through the interior of the housing, expelling the liquid from the housing at a speed sufficient to pierce an injection surface. In one embodiment, the needle-less injector includes a mechanism for mitigating the kickback associated with releasing compressed gas from the engine. In another embodiment, the housing includes finger rests that provide stability and resistance to activate the device. In another embodiment, the engine is fitted with a reusable valve. In another embodiment, a safety clamp is included on the housing, preventing accidental activation of the device,
Owner:PENJET CORP
Method and apparatus for filling or refilling a needle-less injector
Devices and methods are described for filling or refilling a needle-less injector. A coupling device is attached to a vessel, and mated to the dispensing end of a needle-less injector. A volume of fluid may be transferred via the coupling device, from the vessel to the injector. Air may be removed from the injector prior to the injector, vessel, and coupling device being separated from one another. In alternate embodiments, the vessel may include filling equipment capable of filling or refilling multiple needle-less injectors simultaneously, in series, or both. In other embodiments, a coupling device may be used to withdraw a fluid from a storage vial into a vessel for filling a needle-less injector. A storage vial may alternately contain a product, and a fluid may be introduced therein via a coupling device to create a mixture, which may be subsequently withdrawn into a vessel for filling a needle-less injector.
Owner:PENJET CORP
Particle delivery techniques
InactiveUS20050271733A1Reduce deliveryPowder deliveryGenetic material ingredientsPresent methodBiology
A method is provided for in vivo or ex vivo delivery of a preparation of powdered nucleic acid molecules into vertebrate tissue for transformation of cells in the tissue using needleless injection techniques. The method can be used to deliver therapeutically relevant nucleotide sequences to cells in mammalian tissue to provide gene therapy, elicit immunity or to provide antisense or ribozyme functions. A method for providing densified processed pharmaceutical compositions is also described. The method is used to convert non-dense pharmaceutical powders or particulate formulations into densified particles optimally suited for transdermal delivery using a needleless syringe. The method is also used to optimize the density and particle size of powders and particulate formulations for subsequent transdermal delivery thereof. Densified pharmaceutical compositions formed by the present methods are also provided.
Owner:POWDERJECT RES LTD OXFORD (GB)
Novel formulations for treatment of migraine
InactiveUS20110118189A1Immediate pharmacological effectLonger effectBiocideSenses disorderNeedle freeHeadaches
Systems and methods are described for treating un-met medical needs in migraine and related conditions such as cluster headache. Included are treatments that are both rapid onset and long acting, which include sustained release formulations, and combination products. Also included are treatments for multiple symptoms of migraine, especially headache and nausea and vomiting. Systems that are self contained, portable, prefilled, and simple to self administer at the onset of a migraine attack are disclosed, and preferably include a needle-free injector and a high viscosity formulation, to eliminate such issues as fear of self administration with needles, and needle stick and cross contamination.
Owner:ZOGENIX INC
Medicament delivery device
An actuator is provided which is adapted, in conjunction with a cartridge, to form a needle-less injector, the cartridge being filled with a liquid to be injected in a subject, and having a liquid outlet and a free piston in contact with the liquid. The actuator comprises an impact member urged by a spring and temporarily restrained by a latch, the impact member being movable in a first direction under the force of the spring to first strike the free piston and then to continue to move the piston in the first direction to expel a dose of liquid through the liquid outlet, the spring providing a built-in energy store. A pressure pad surrounds the said liquid outlet and, in use, bears on the subject's skin. The actuator may be formed of two main housing components urged apart by an integral cantilever spring formed on one of them, a safety catch can be provided to prevent inadvertent actuation.
Owner:DCA DESIGN INTERNATIONAL LTD
Method and apparatus for needle-less injection with a degassed fluid
InactiveUS20040035491A1Minimize and avoid formationAmpoule syringesJet injection syringesNeedle Free InjectionProduct gas
Apparatuses and methods are described for administering a needle-less injection of a degassed fluid. Prior to filling, or after filling but prior to administration of a needle-less injection, gas is removed from the fluid. A needle-less injection may then be performed with a reduced risk of discomfort to the recipient of the injection and with lower potential for the creation of a subdermal hematoma as a result of the injection. A wide variety of needle-less injectors may be used in accordance with various embodiments of the present invention.
Owner:PENJET CORP
Refillable device with counting means
InactiveUS7080642B2Increase equipment costSimple mechanical structureRespiratorsMedical devicesNeedle Free InjectionMedical device
A refillable medical device comprising a base unit (4) adapted to be engaged with a refill unit (2), the device comprising means for counting the number of different refill units which are engaged with the base unit (4). The device may be in the form of a dry powder or pressurised aerosol inhaler, needleless injector, intravenous drip system etc. The device may comprise means to disable the device after a predetermined number of refill units have been used with the base.
Owner:3M INNOVATIVE PROPERTIES CO
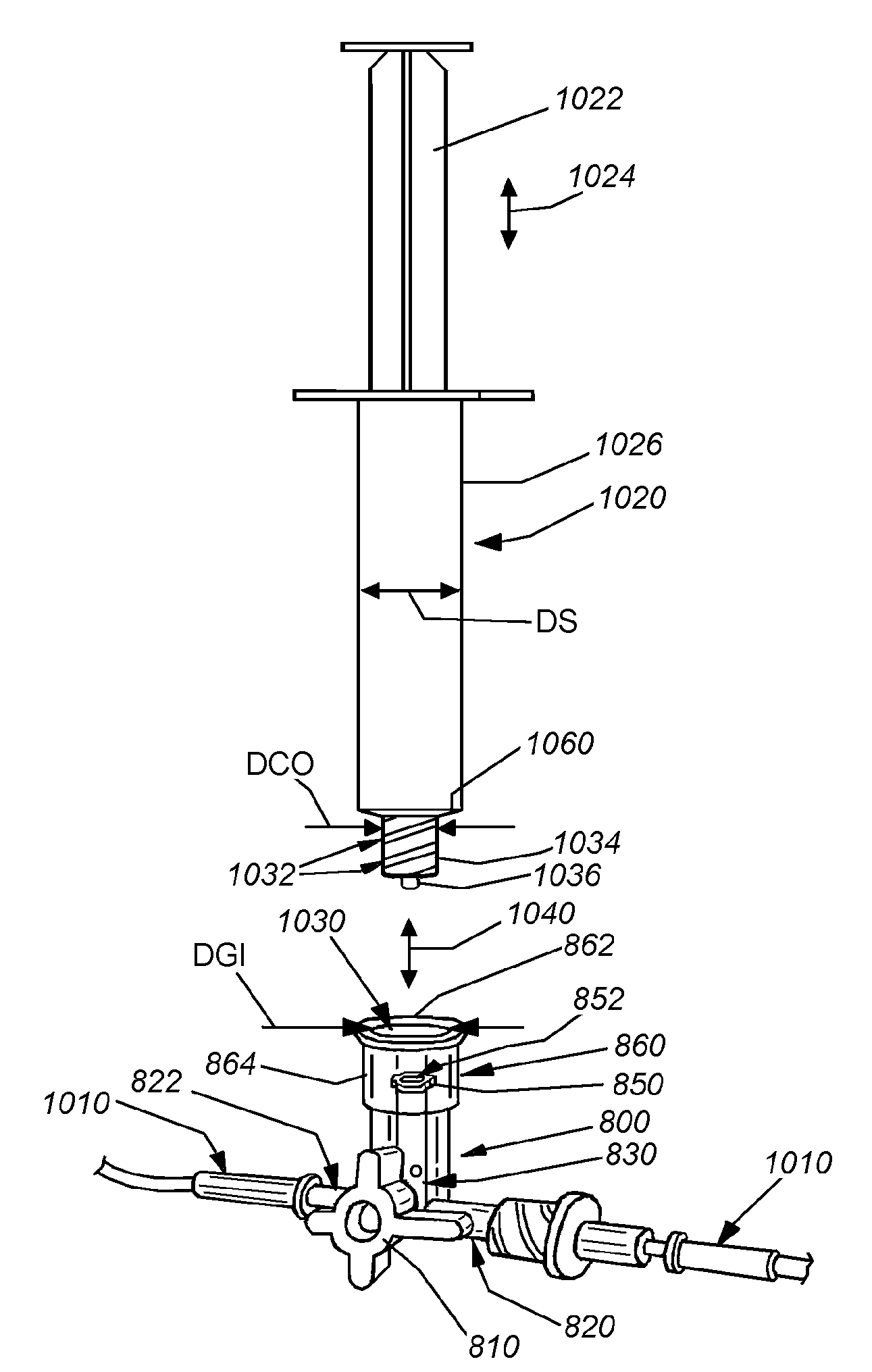
Methods and devices for delivering fluid to a reservoir of a fluid delivery device
Medical devices and methods for delivering therapeutic fluids transcutaneously to a body of a patient via a fluid delivery device and an adapter is provided. A Luer slip connector is provided at a first connecting end of the adapter. A therapeutic fluid container is connected with the second connecting end such that the container's neck is received in the second connecting end and a hollow penetrating member provided in the adapter punctures the septum of the container to admit the therapeutic fluid into a needle-less syringe from the container. After, fluid emerges from the tip of the penetrating member, the second connecting end of the adapter is attached to the fluid delivery device, thereby filling the reservoir with the fluid and pushing the syringe plunger forward to inject fluid into reservoir.
Owner:ROCHE DIABETES CARE INC
Method and apparatus for filling or refilling a needle-less injector
InactiveUS6755220B2Extended shelf lifeJet injection syringesPharmaceutical containersNeedle Free InjectionInjector
Devices and methods are described for filling or refilling a needle-less injector. A coupling device is attached to a vessel, and mated to the dispensing end of a needle-less injector. A volume of fluid may be transferred via the coupling device, from the vessel to the injector. Air may be removed from the injector prior to the injector, vessel, and coupling device being separated from one another. In alternate embodiments, the vessel may include filling equipment capable of filling or refilling multiple needle-less injectors simultaneously, in series, or both. In other embodiments, a coupling device may be used to withdraw a fluid from a storage vial into a vessel for filling a needle-less injector. A storage vial may alternately contain a product, and a fluid may be introduced therein via a coupling device to create a mixture, which may be subsequently withdrawn into a vessel for filling a needle-less injector.
Owner:PENJET CORP
Highly concentrated stable meloxicam solutions for needleless injection
Aqueous cyclodextrin-free solution of meloxicam suitable for administration by needleless injection, containing a pharmacologically acceptable meloxicam salt of an organic or inorganic base and one or more suitable excipients, the content of dissolved meloxicam salt being from 35 to 100 mg / ml. The formulation according to the invention has a shelf-life of up to 24 months or more.
Owner:FOLGER MARTIN ANDREAS +3
Needle-less injection apparatus and method
InactiveUS6964649B2Reduce traumaReduce liquid leakageJet injection syringesMedical devicesNeedle Free InjectionInjection port
A device and method for delivering and injecting fluid into heart tissue utilizing high pressure injection to increase injectate (fluid) retention in the heart tissue. The catheter includes a shaft having an infusion lumen extending therethrough, wherein the proximal end of the shaft connected to a pressurized fluid source capable of generating a transient pressure of more than 1000 psi. The distal end of the shaft includes a nozzle having an injection port in fluid communication with the infusion lumen such that fluid from the pressurized fluid source may be delivered to the heart tissue at a sufficiently high exit velocity to partially penetrate the heart tissue.
Owner:BOSTON SCI SCIMED INC
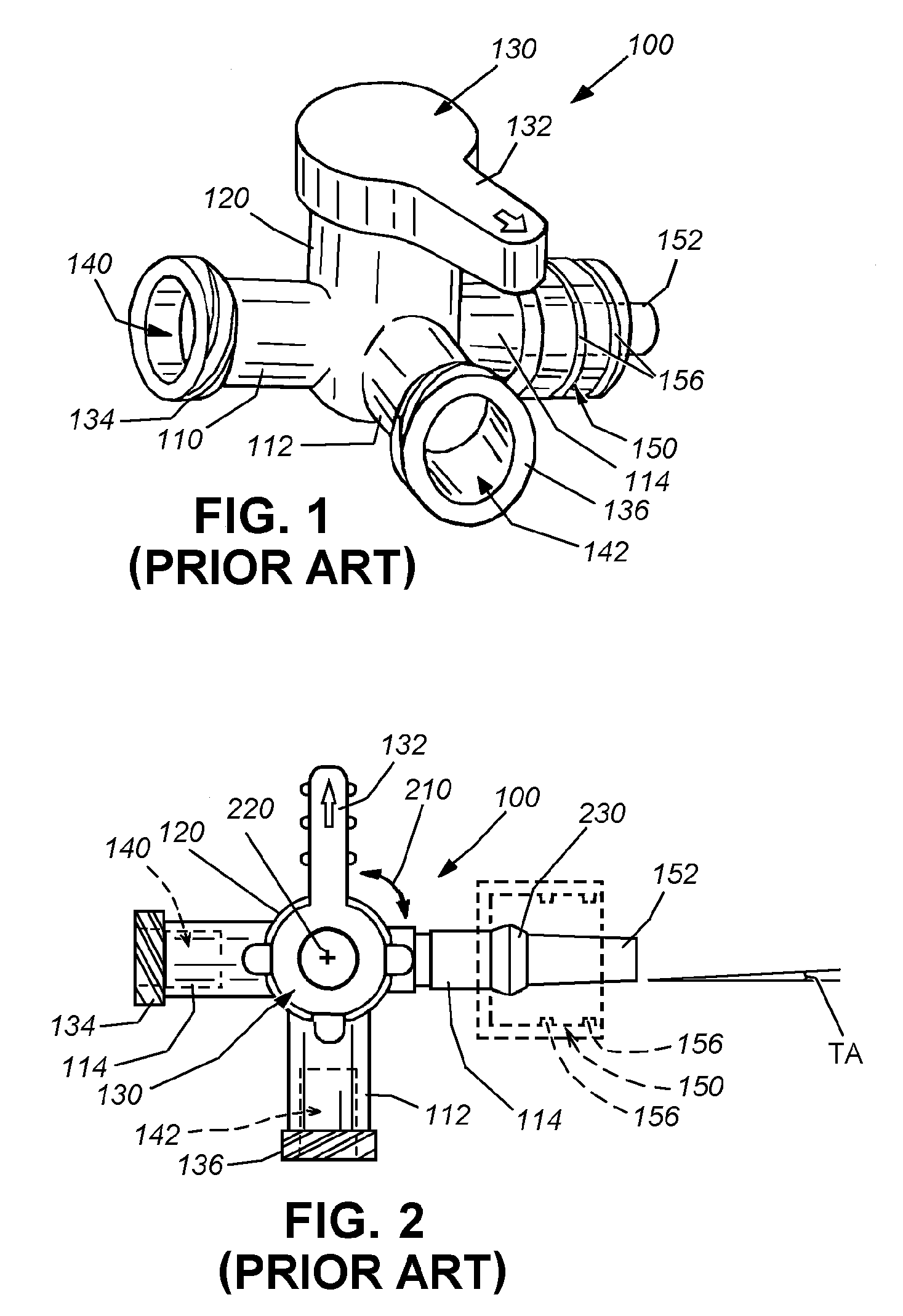
Medical fluid coupling port with guide for reduction of contamination
InactiveUS20090182309A1Prevent port exposureAvoid pollutionCatheterTube connectorsNeedle Free InjectionExtension set
This invention provides a female medical coupling port with an integrated port guide to enable more accurate and precise coupling of a male port coupling (such as the cannula of a syringe) and to prevent port exposure to non-sterile objects. The male and female ports can be arranged according to standard dimensions for male and female luer taper fittings recognized by ANSI and by ISO. This guide-shielded port is usable with the standard ANSI and ISO male cannula widely used in the medical field. In an embodiment the female port is used in medical fluid systems to receive a blunt male cannula, such as those found in the luer lock fitting of needle-less syringes and IV tubing systems to establish a mechanical coupling. Female ports allow coupling of devices (e.g. syringes and IV tubing) to a variety of medical applications including stopcocks, minimum fluid displacement medical couplings, female-to-female adapters, port dead-end caps, IV extension sets, pressure-monitoring devices, etc. The port guide can be constructed as a unitary part of the port, or can be a retrofittable structure that is either snapped into place on, for example, a female port stem, or slid onto a port, such as a minimum displacement fluid coupling. Appropriate drain ports can be provided in the port guide to prevent capture of excess fluid.
Owner:DARTMOUTH HITCHCOCK CLINIC
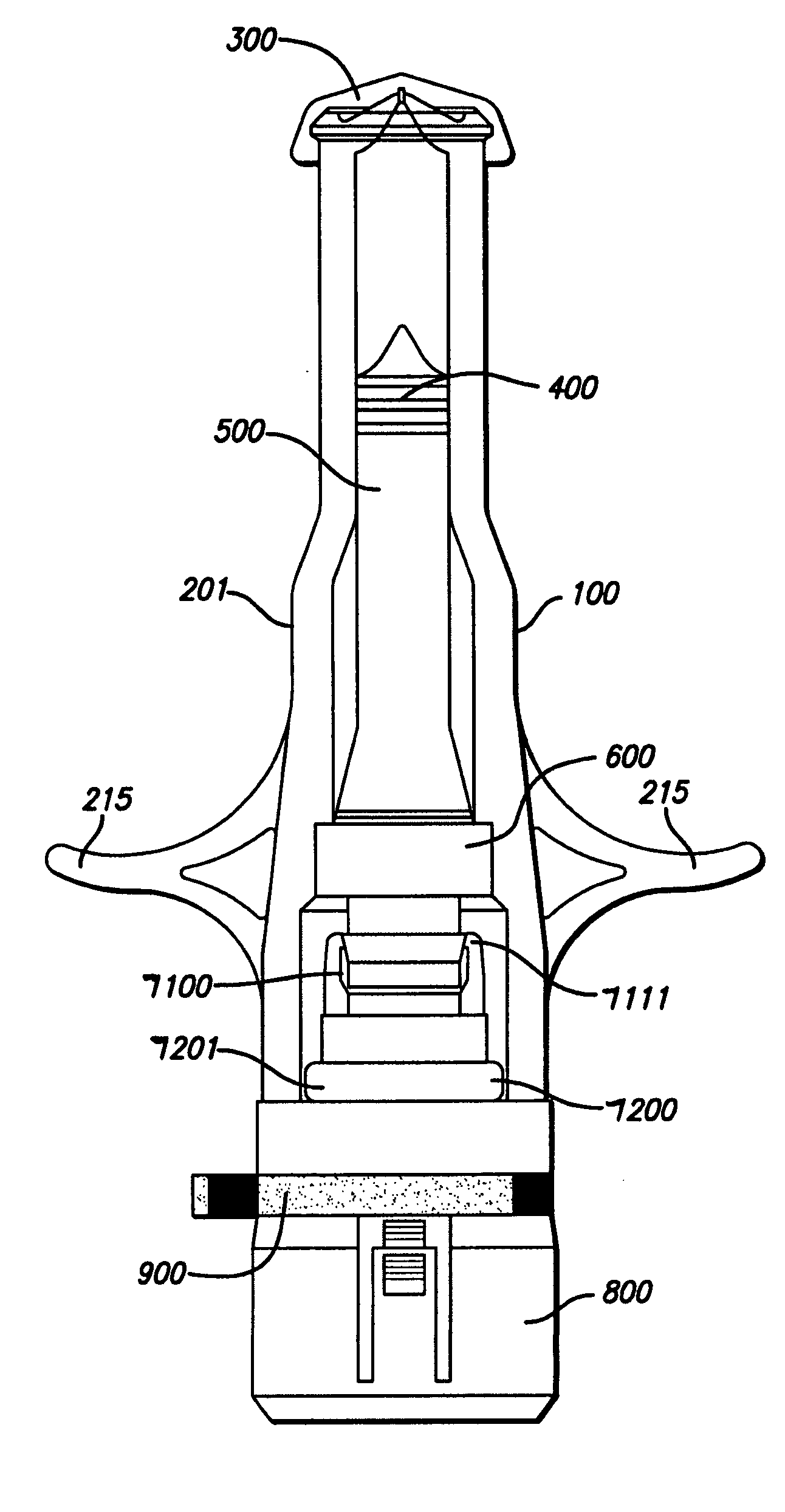
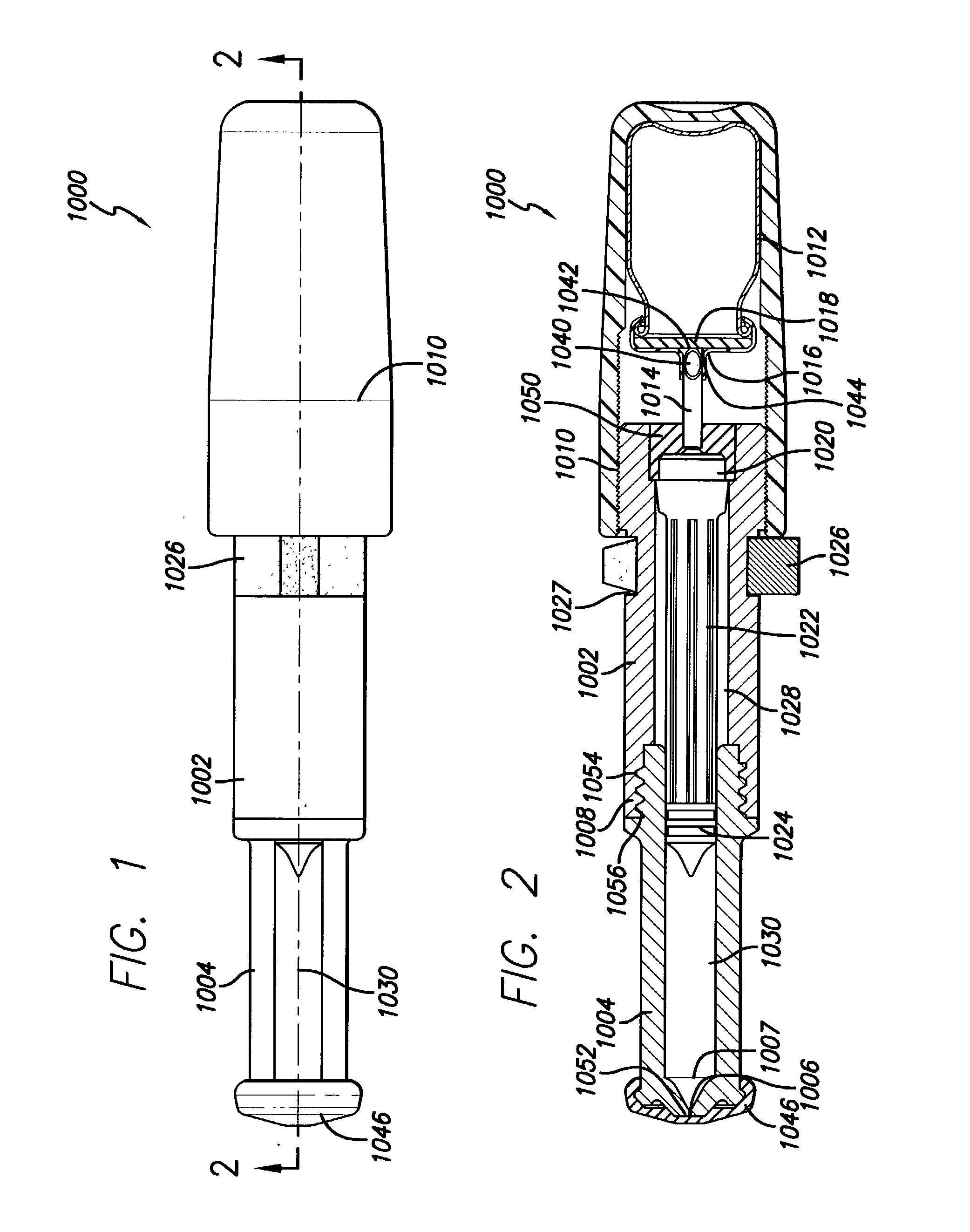
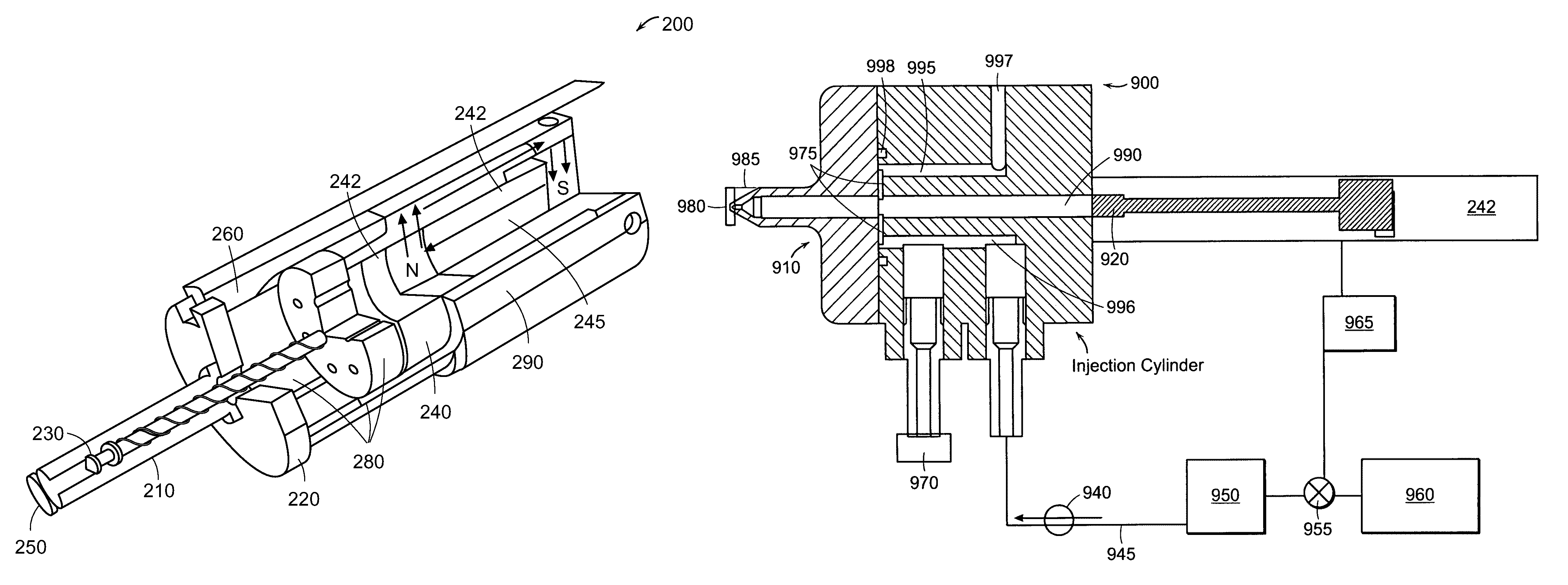
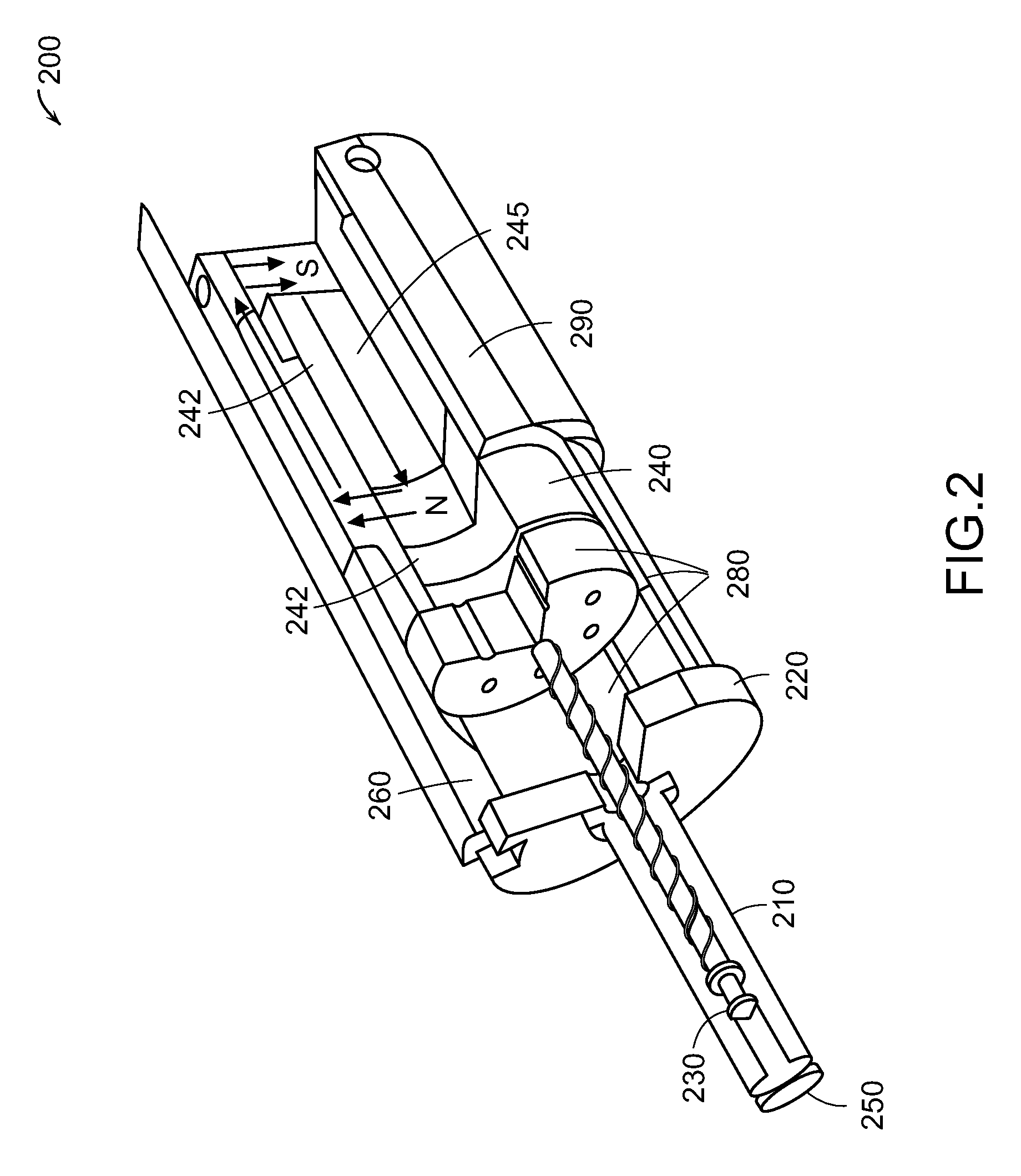
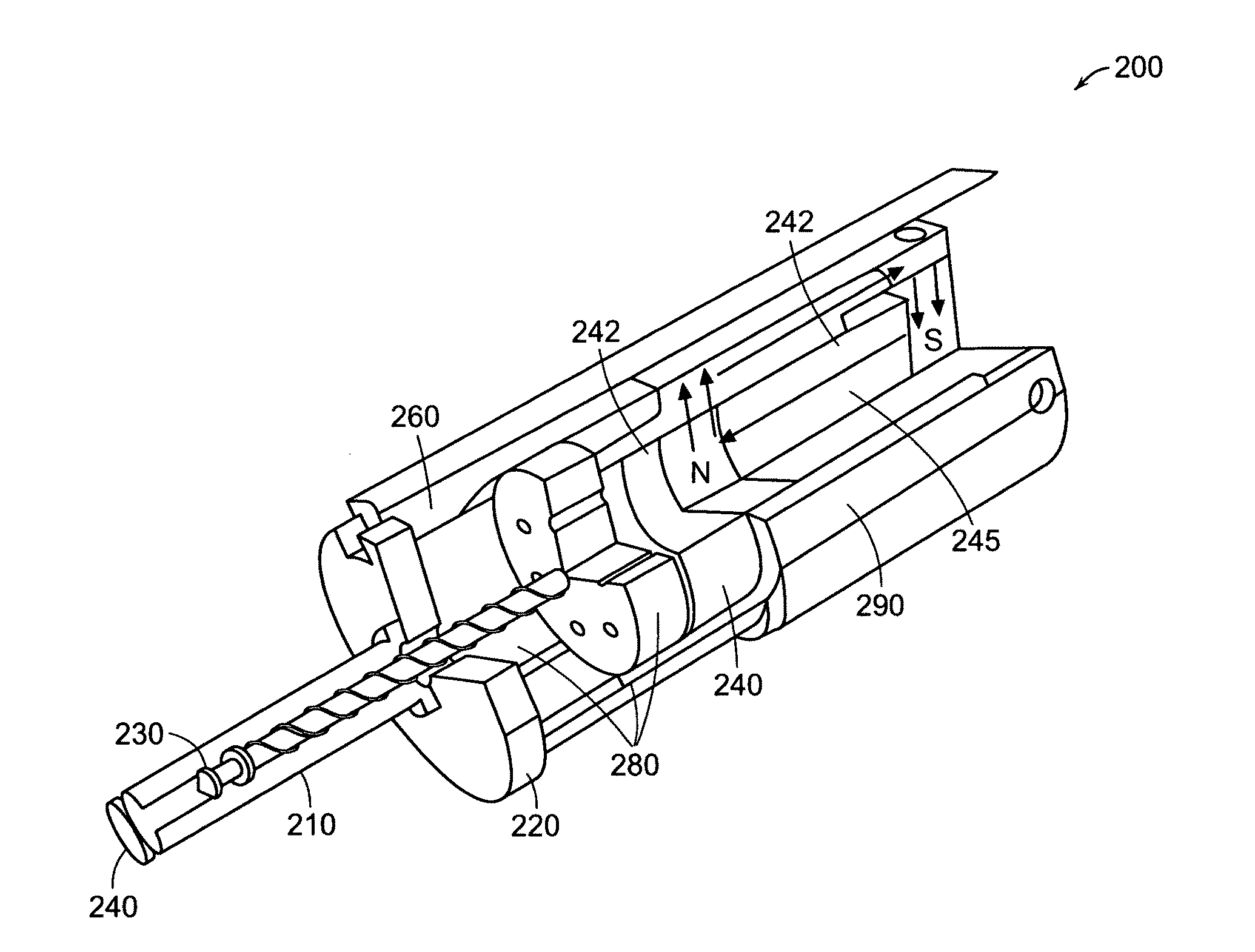
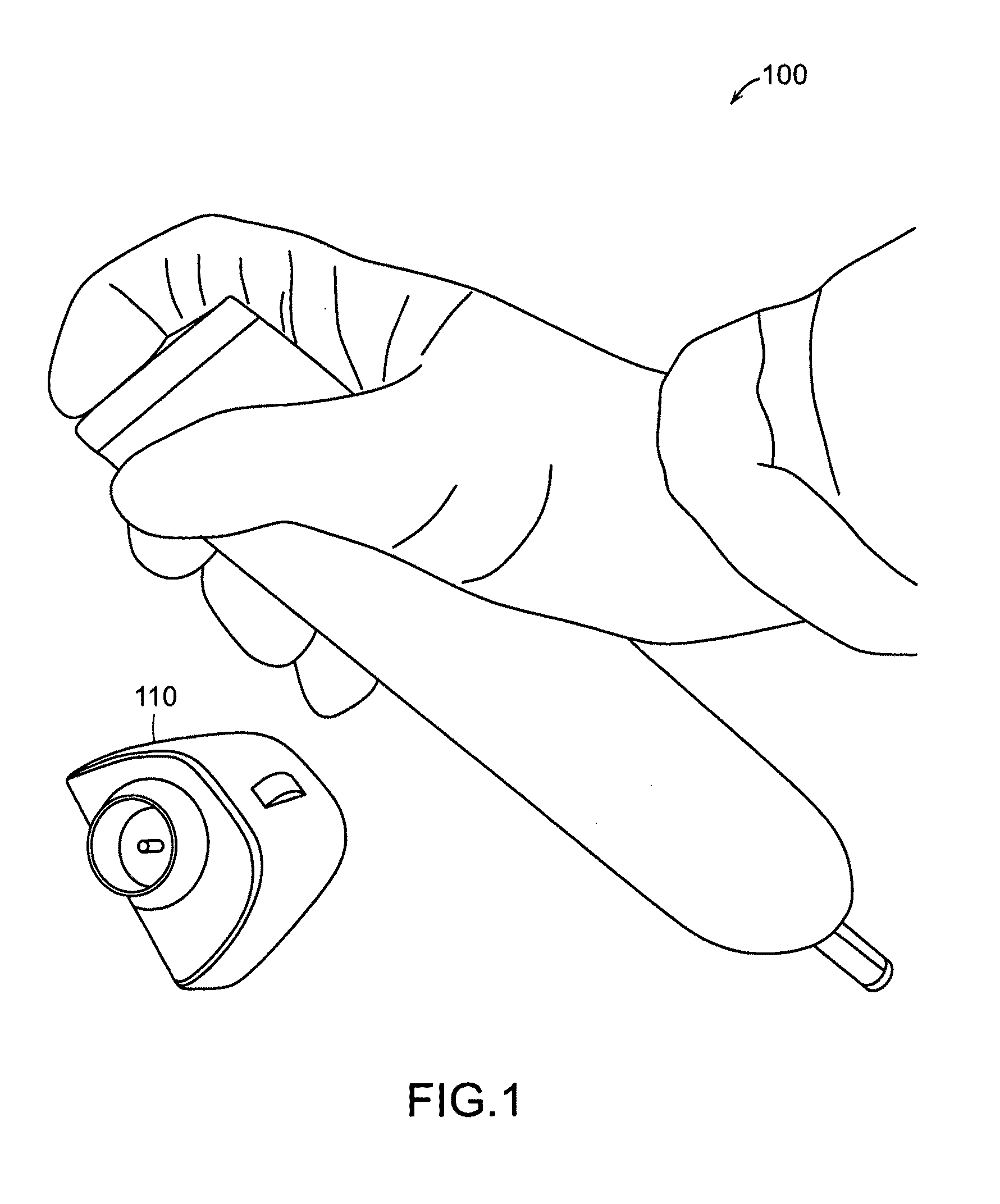
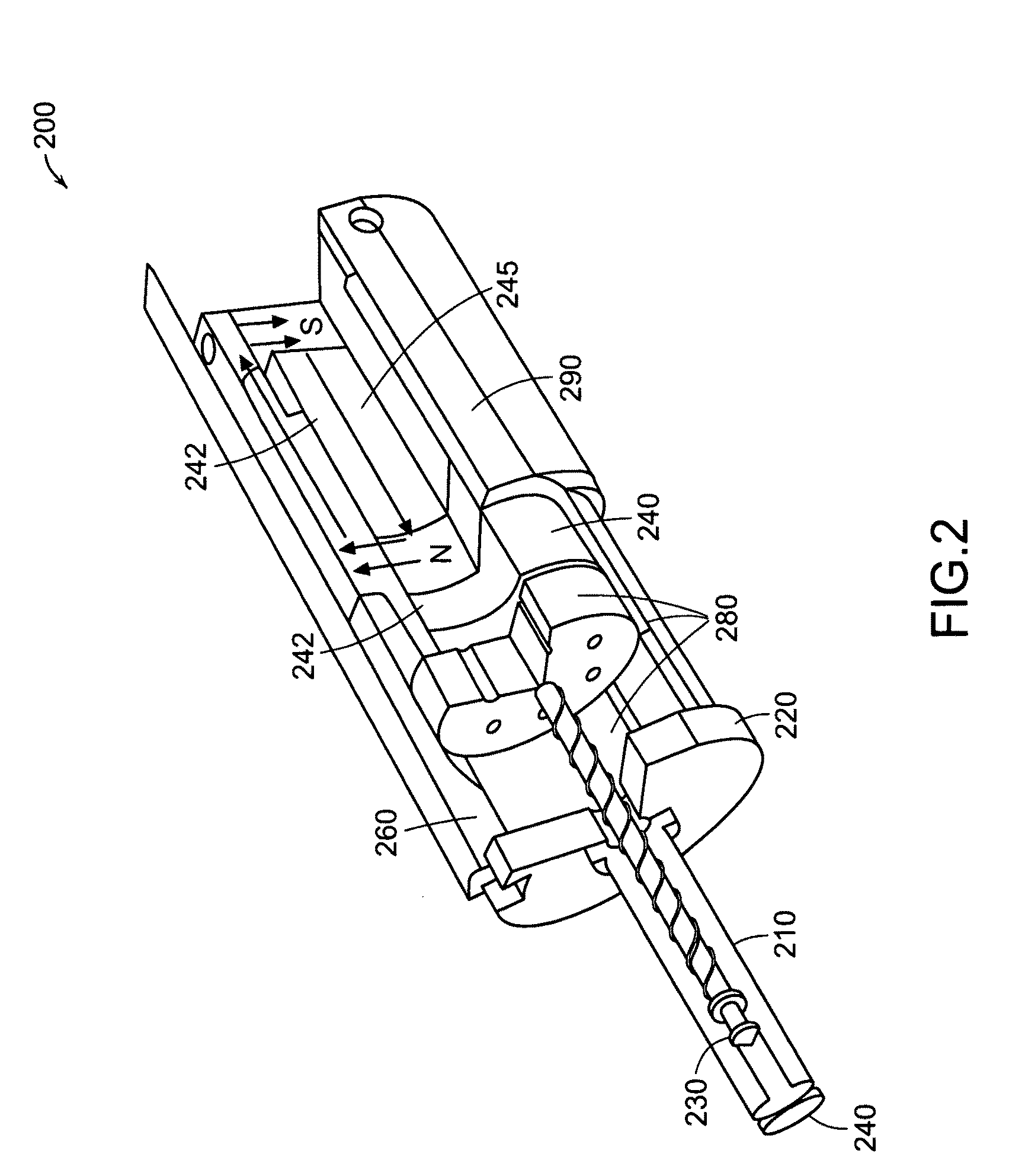
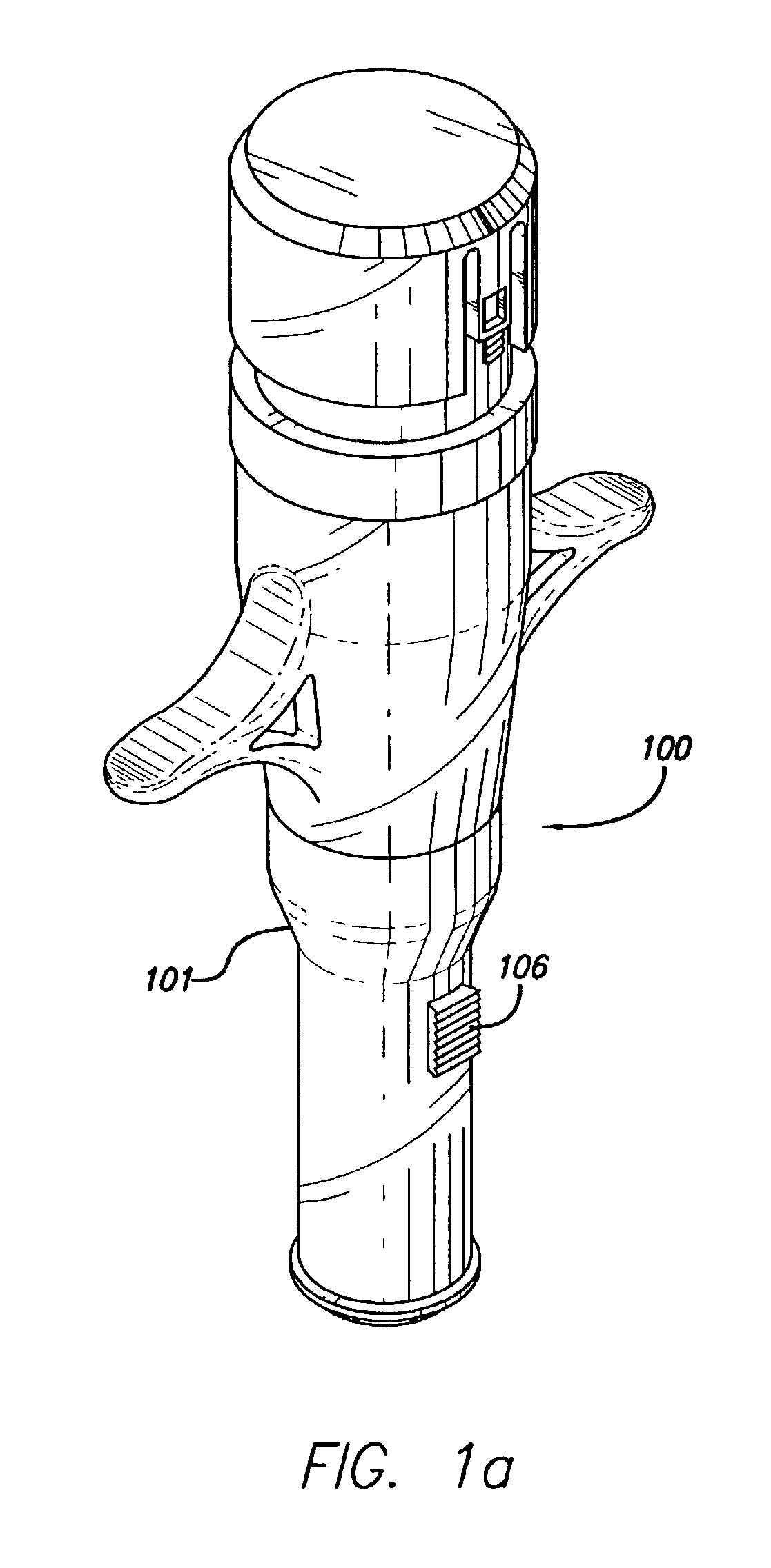
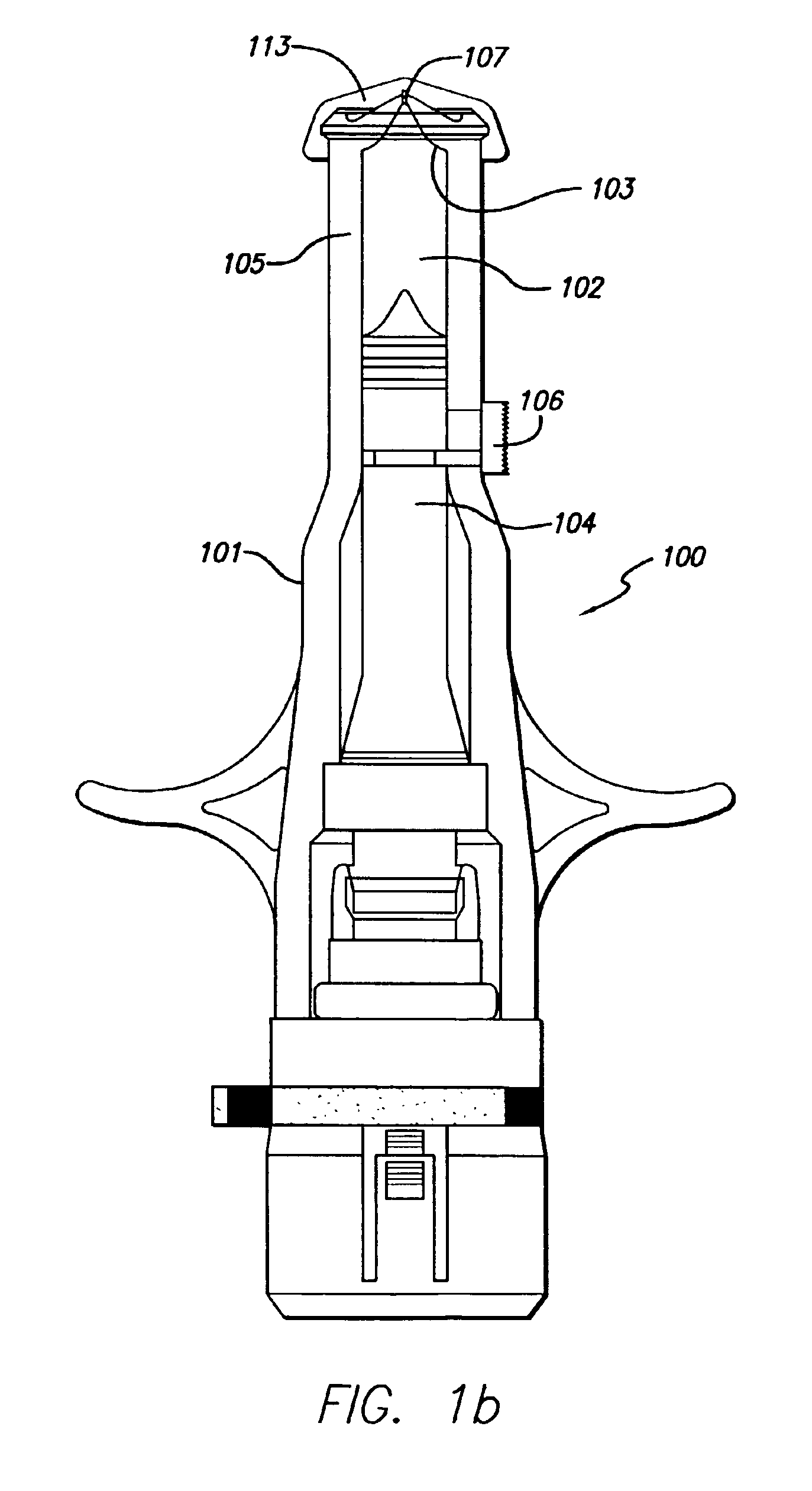
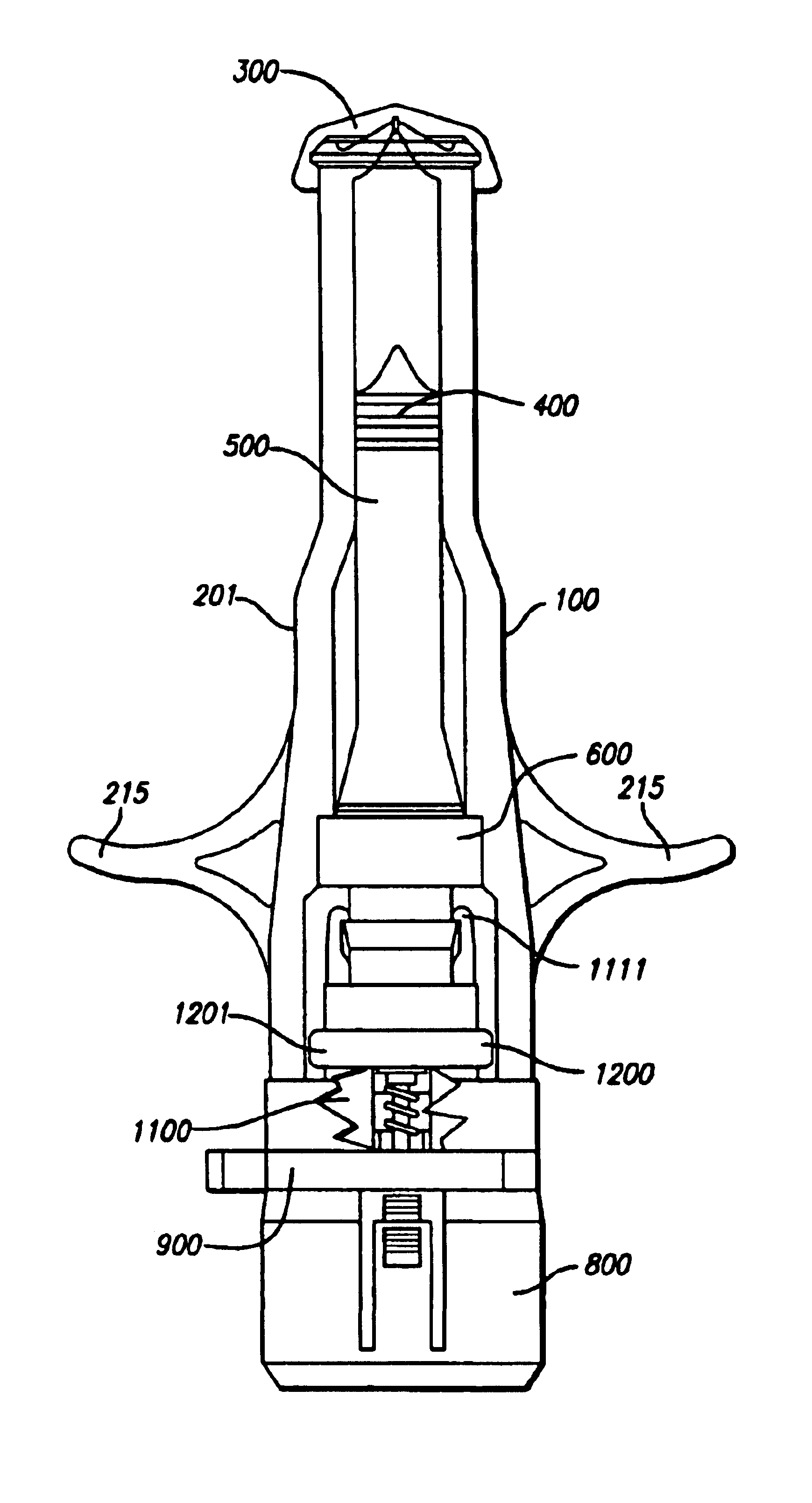
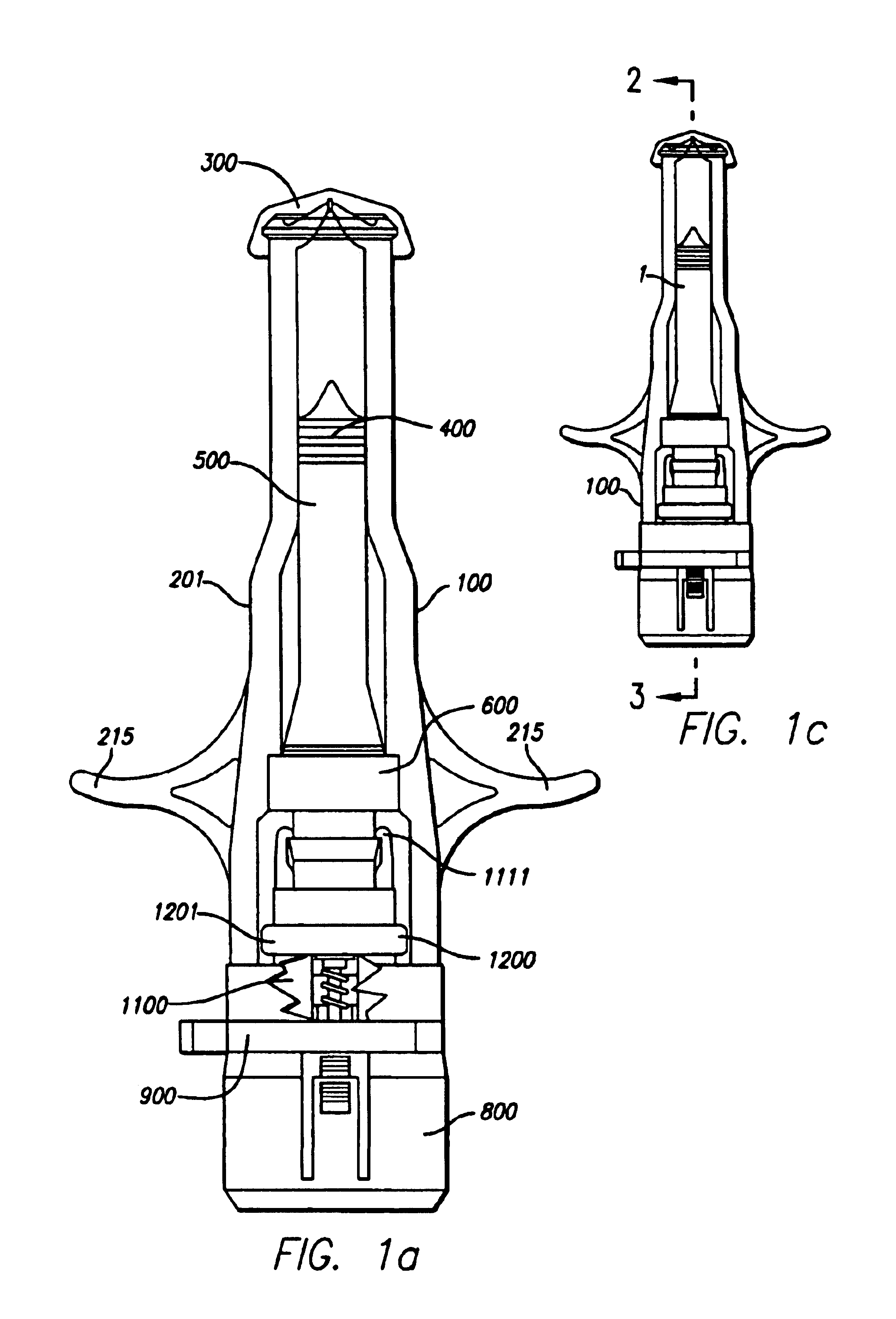
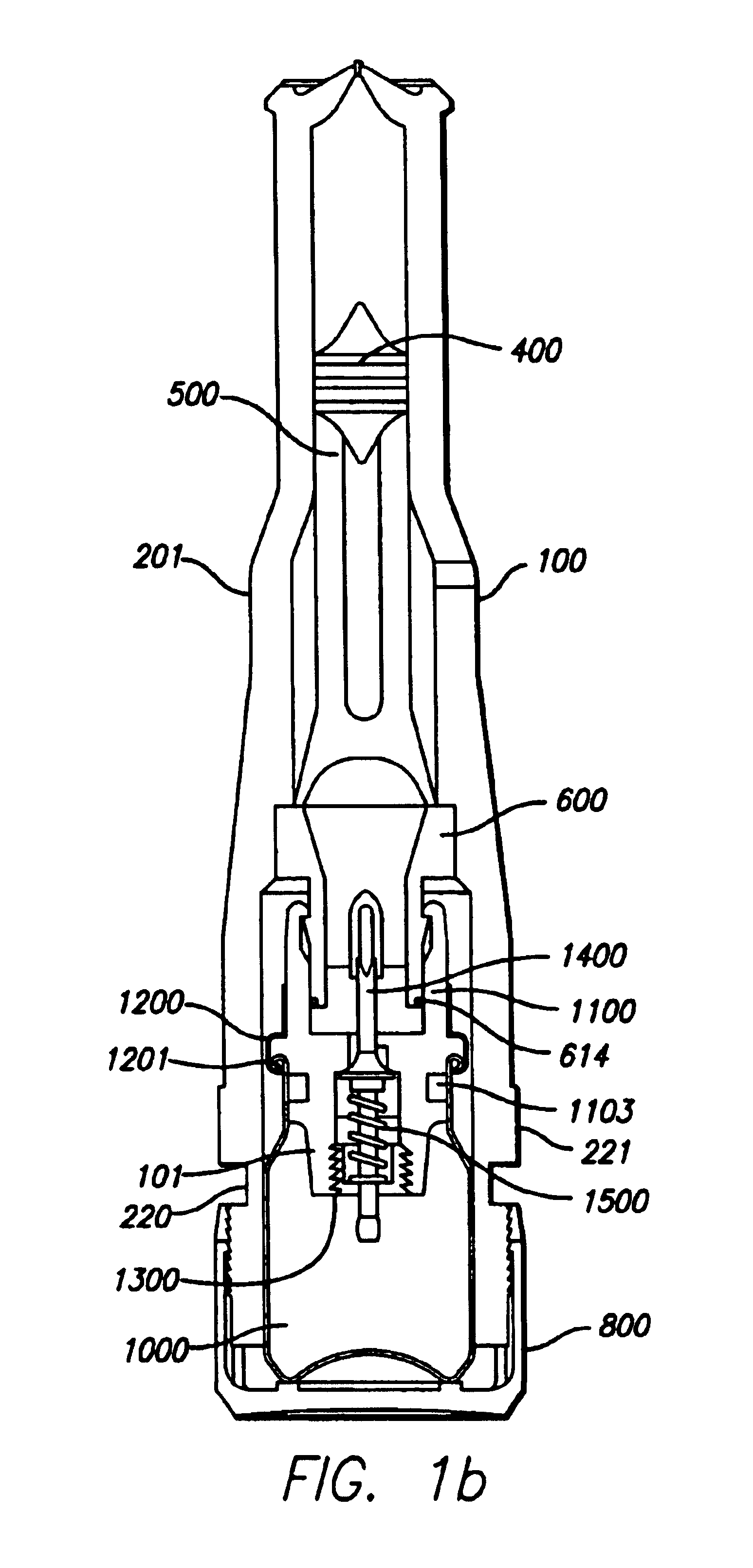
Needle-free injector device with autoloading capability
ActiveUS8172790B2Little controlProcess controlJet injection syringesMedical devicesNeedle freeNeedle Free Injection
A needle-free transdermal transport device includes a chamber (900) for holding the substance to be injected, a nozzle (910) in fluid communication with the chamber, and a drug reservoir (950) for storing the substance to be transferred to the chamber. The needle-free transdermal transport device also includes a controllable magnet and coil electromagnetic actuator (242) in communication with the chamber. The actuator receives an electrical input and generates in response a force. The force then causes a needle-free transfer of the substance from the chamber to the biological body. The force is variable responsive to variations in the received input during actuation. The actuator draws the substance from the drug reservoir or alternatively, the substance can be pressurized from the drug reservoir into the chamber by a pressure source.
Owner:MASSACHUSETTS INST OF TECH
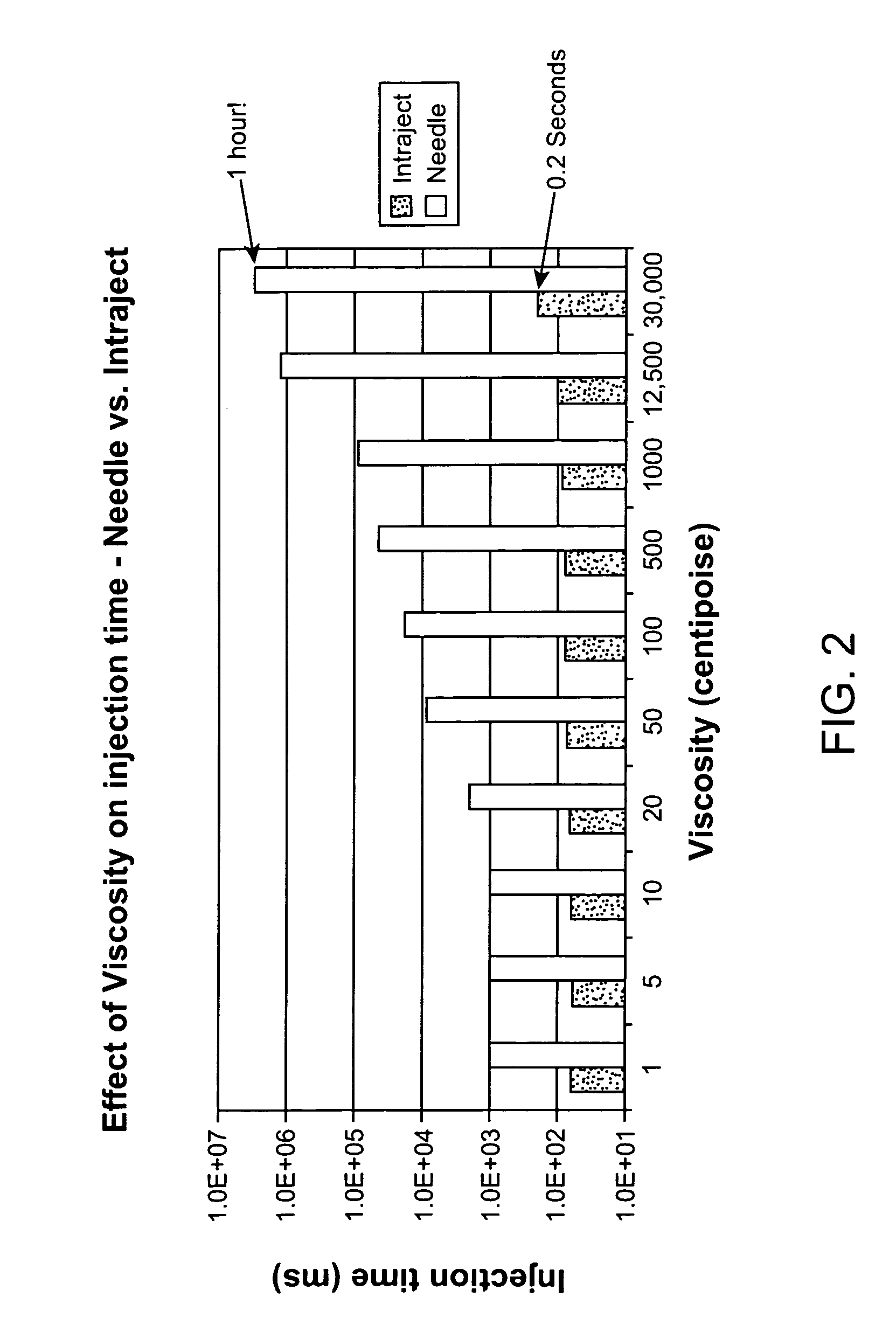
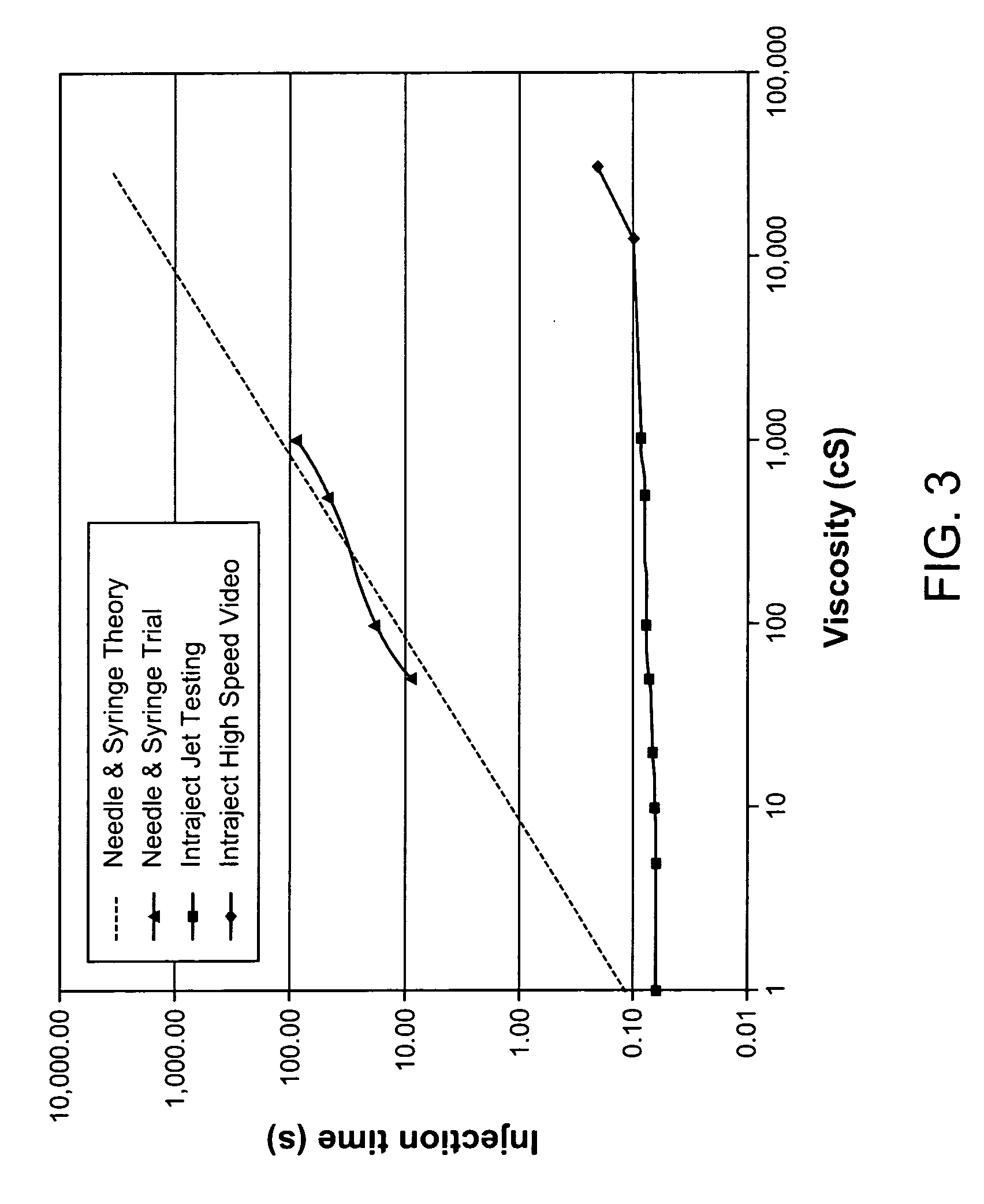
Viscous formulations and their use in needle-free injection
InactiveUS20080214995A1Improve stabilityInjection volume is smallNervous disorderJet injection syringesNeedle freeNeedle Free Injection
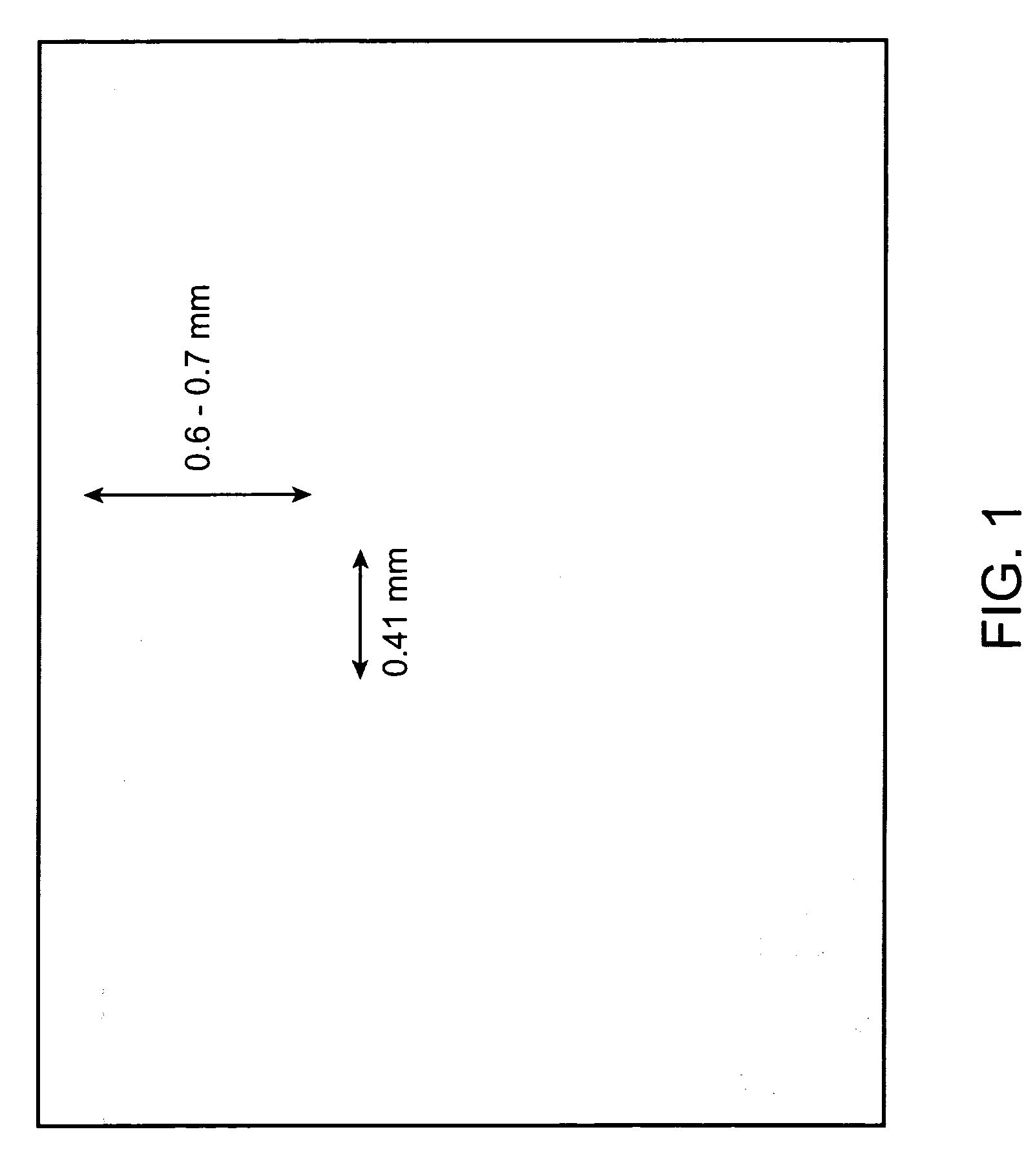
Formulations are described that are viscous and will benefit from needle-free delivery at high driving pressures. Conventional delivery of these viscous formulations by hypodermic syringes is inconvenient as well as painful. Formulations include those which have a viscosity of about 5 cS or more at about 20° C. and which can have 0.5 ml or more administered by a needle-free injector in about 0.1 second±0.02 seconds.
Owner:ZOGENIX INC
Needle-free injector device with autoloading capability
ActiveUS20100016827A1Process controlLittle control of pressureJet injection syringesMedical devicesNeedle freeBiological body
A needle-free transdermal transport device includes a chamber (900) for holding the substance to be injected, a nozzle (910) in fluid communication with the chamber, and a drug reservoir (950) for storing the substance to he transferred to the chamber. The needle-free transdermal transport device also includes a controllable magnet and coil electromagnetic actuator (242) in communication with the chamber. The actuator receives an electrical input and generates in response a force. The force then causes a needle-free transfer of the substance from the chamber to the biological body. The force is variable responsive to variations in the received input during actuation. The actuator draws the substance from the drug reservoir or alternatively, the substance can be pressurized from the drug reservoir into the chamber by a pressure source.
Owner:MASSACHUSETTS INST OF TECH
Apparatus for selectively establishing a needleless injection port on iv tubing, and associated methods
ActiveUS20120130305A1Avoid missing wasteMedical devicesCombustion enginesNeedle Free InjectionInjection port
Apparatus enables one or more needleless injection ports to be established on IV tubing as needed. An IV tubing-engaging portion secures the apparatus about the tubing. A puncturing member establishes fluid communication between the IV line and a sealing member on the apparatus. Connecting a syringe to the sealing member establishes fluid communication between the IV line and the syringe, enabling an injection to be made. When the syringe is withdrawn, the sealing member reseals to prevent fluid leakage from the IV line.
Owner:B BRAUN MELSUNGEN AG
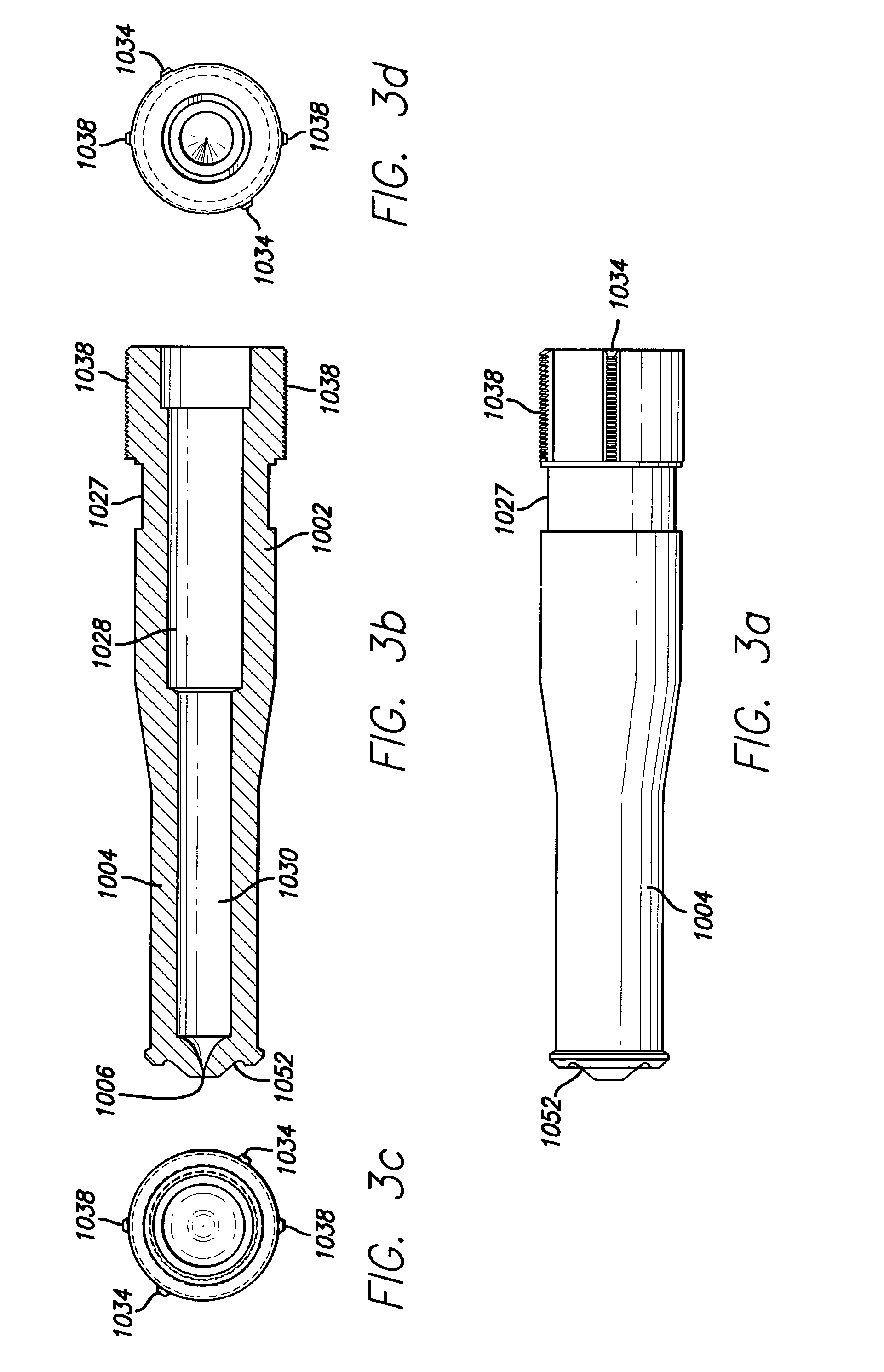
Needleless syringe for the delivery of therapeutic agents
A needleless syringe for subcutaneously delivering a therapeutic agent comprising a generally constant diameter elongate tubular nozzle and an inert gas reservoir thereto is described herein. The reservoir is advantageously mounted to the upstream end of the tubular nozzle through a contraction that is either gradual or sudden and wherein a membrane is positioned between the reservoir and the therapeutic agent.
Owner:SCOPRA SCI & GENIE SEC
Apparatus for selectively establishing a needleless injection port on IV tubing, and associated methods
ActiveUS9314604B2Avoid missing wasteMedical devicesCombustion enginesNeedle Free InjectionInjection port
Apparatus enables one or more needleless injection ports to be established on IV tubing as needed. An IV tubing-engaging portion secures the apparatus about the tubing. A puncturing member establishes fluid communication between the IV line and a sealing member on the apparatus. Connecting a syringe to the sealing member establishes fluid communication between the IV line and the syringe, enabling an injection to be made. When the syringe is withdrawn, the sealing member reseals to prevent fluid leakage from the IV line.
Owner:B BRAUN MELSUNGEN AG
Method and apparatus for adjusting the contents of a needle-less injector
InactiveUS7018356B2Lower the volumeJet injection syringesMedical devicesNeedle Free InjectionEngineering
A method and apparatus for adjusting the contents of a needle-less injector that contains an injectable product are described. A needle-less injector includes an adjustment switch in mechanical contact with the driver of a needle-less injector. A user may displace the adjustment switch to expel air or gas contained in the product section of the needle-less injector prior to administration of a needle-less injection with the same. The adjustment switch may alternatively or additionally be used to expel at least a portion of the injectable product contained in the needle-less injector to reduce the volume of the product to be injected with the needle-less injector.
Owner:WISE ROGER R +1
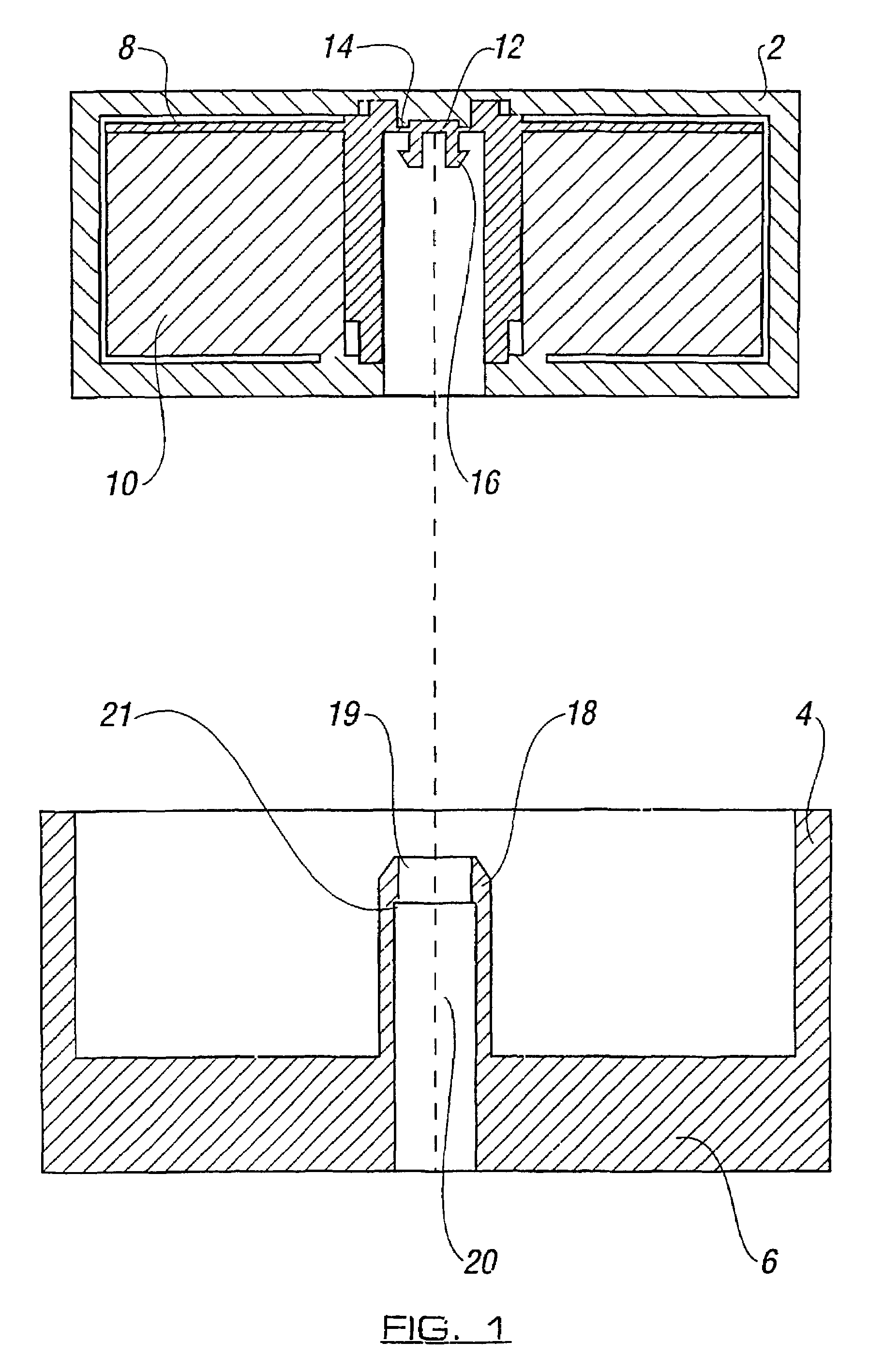
Engine and diffuser for use with a needle-less injector
InactiveUS6824526B2Facilitate in creation and maintenanceNegligible back pressureJet injection syringesAutomatic syringesNeedle Free InjectionDriver/operator
A needle-less injector suitable for injecting fluid through a surface includes a housing, a driver, an engine and a trigger. The housing contains a fluid and the engine contains a compressed gas. Upon application of sufficient force to the trigger, the compressed gas is released from the engine forcing the driver through the interior of the housing, expelling the fluid from the housing at a speed sufficient to pierce an injection surface. An aerodynamic diffuser maximizes air flow to the driver, allowing greater injection speed and mitigating pain associated with receiving an injection. Use of the injector is both silent and easy to activate, owing to an O-ring included about the circumference of the exterior of a diffuser operating within the engine. Further, the engine has safety features preventing a portion thereof from separating from the device under elevated temperatures and similar conditions. Engine leakage is obviated by the inclusion of a leakage ring therein.
Owner:PENJET CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com