Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
922 results about "Self-administration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Self-administration is, in its medical sense, the process of a subject administering a pharmacological substance to themself. A clinical example of this is the subcutaneous "self-injection" of insulin by a diabetic patient.
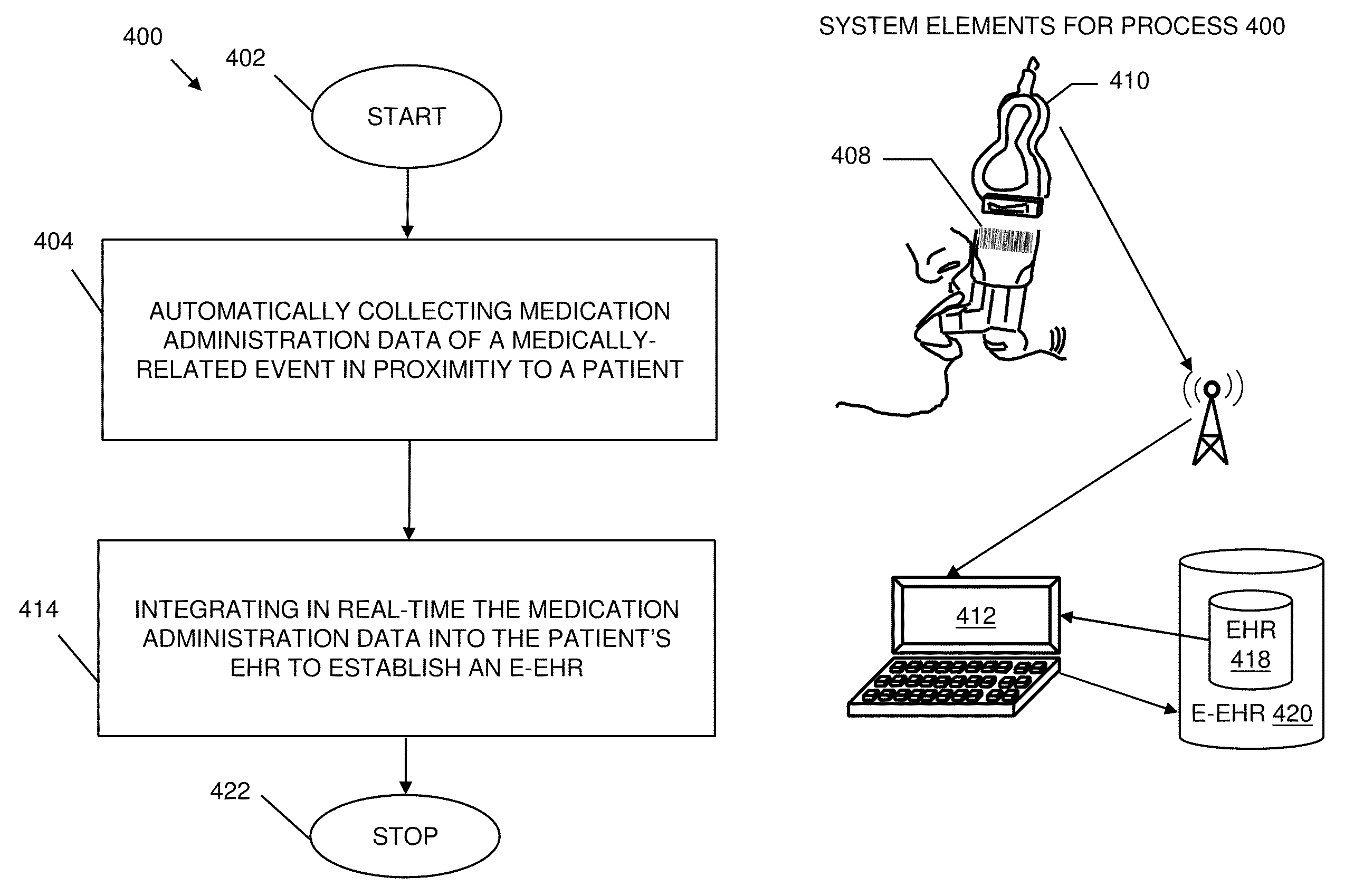
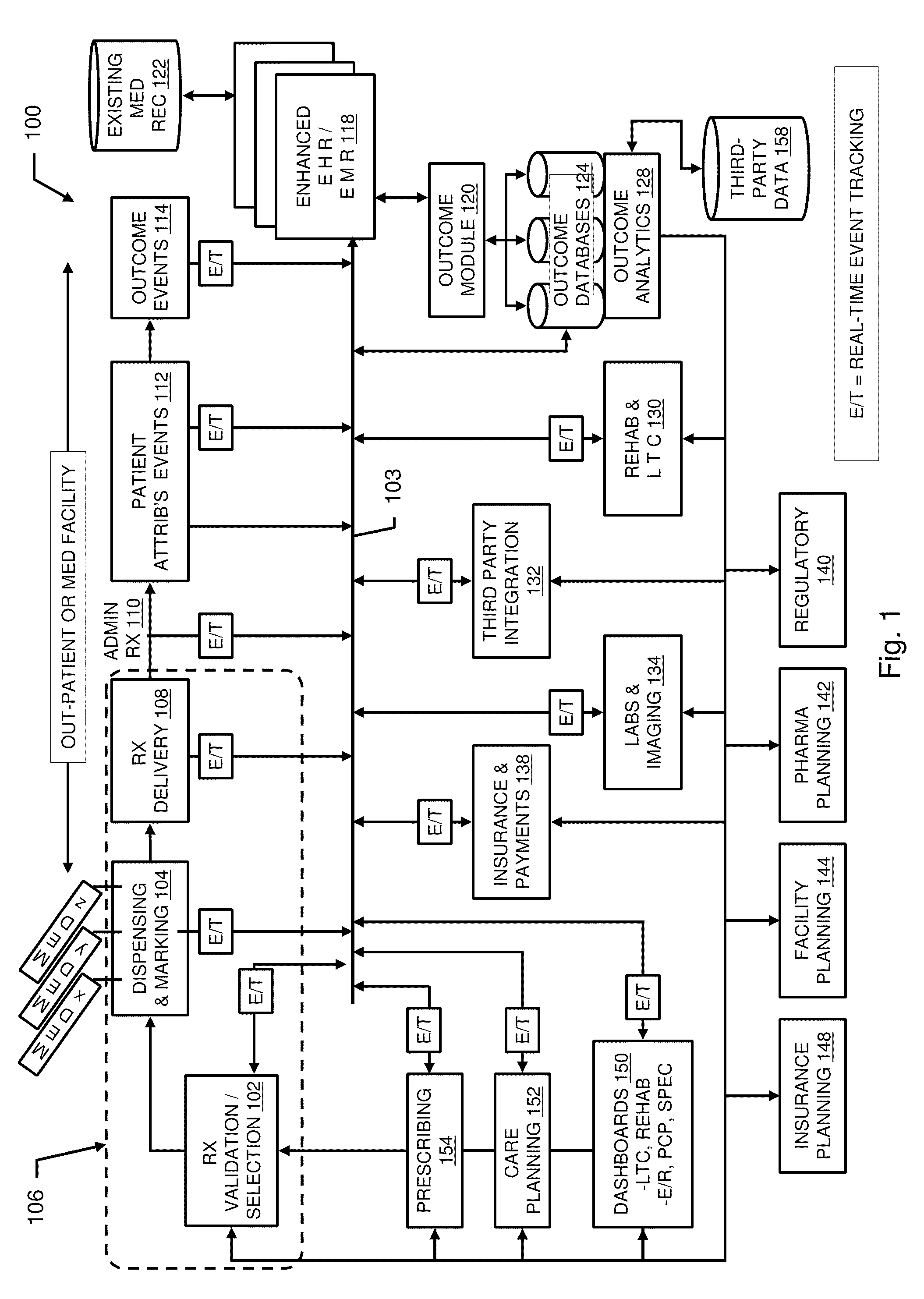
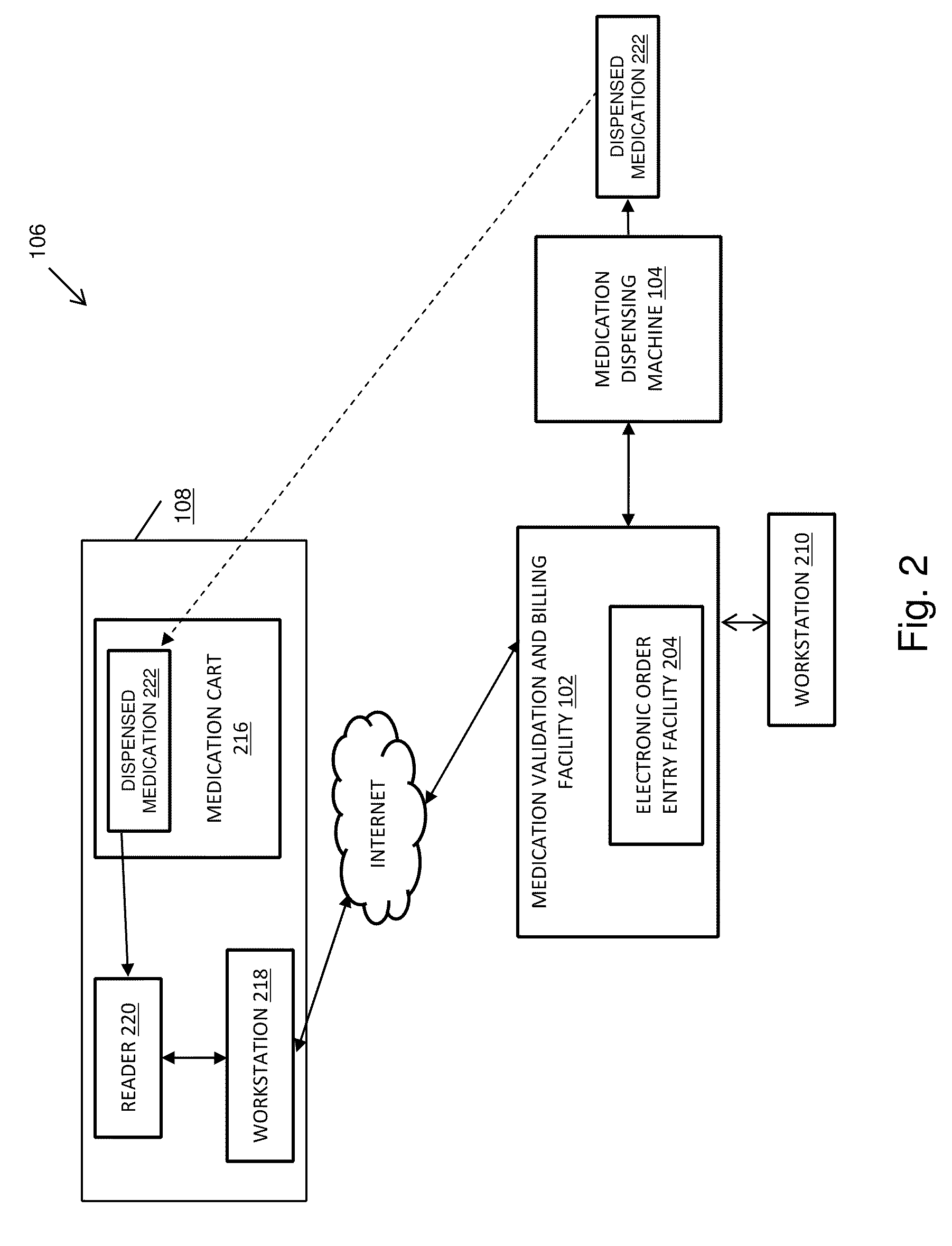
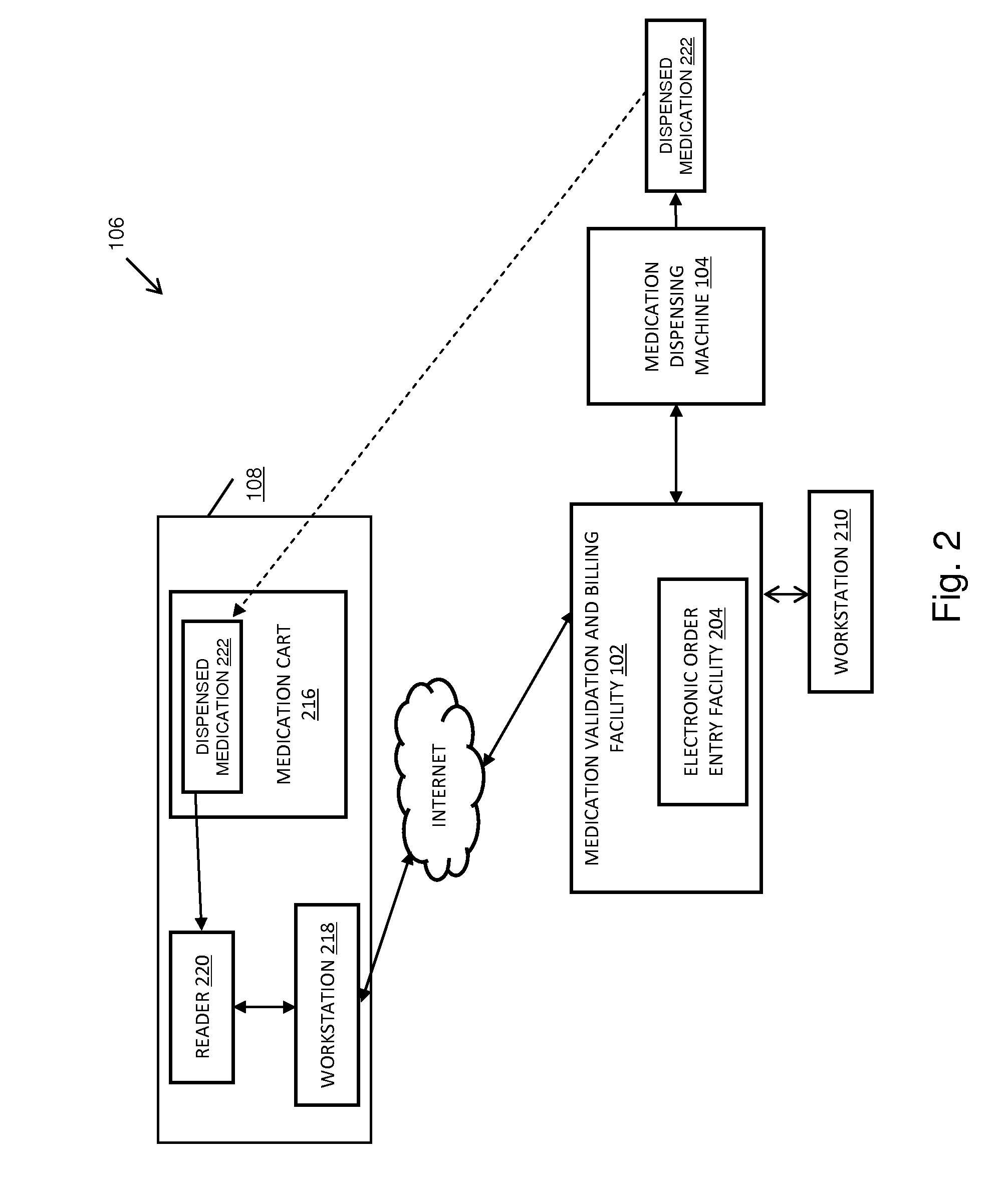
Pharmacy management and administration with bedside real-time medical event data collection
Methods and systems for automatically establishing an enhanced electronic health record (EHR) for a patient include an automatic data collection facility that collects data of a medically related event in proximity to a patient upon occurrence of the event. The collected data may include medication administration data such as medication, time of administration, administration of a dosage of medication, reaction data, and the like. The collected data is communicated to a real-time data integration facility that automatically integrates the data with a patient's electronic health record to establish an enhanced electronic health record.
Owner:MILLENNIUM PHARMACY SYST LLC
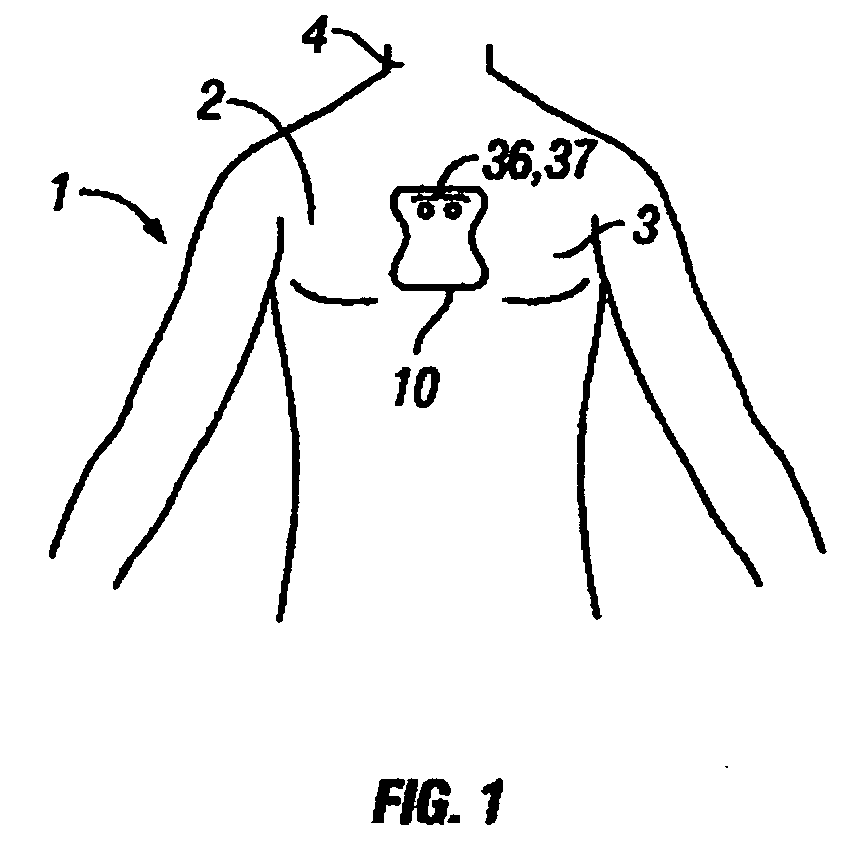
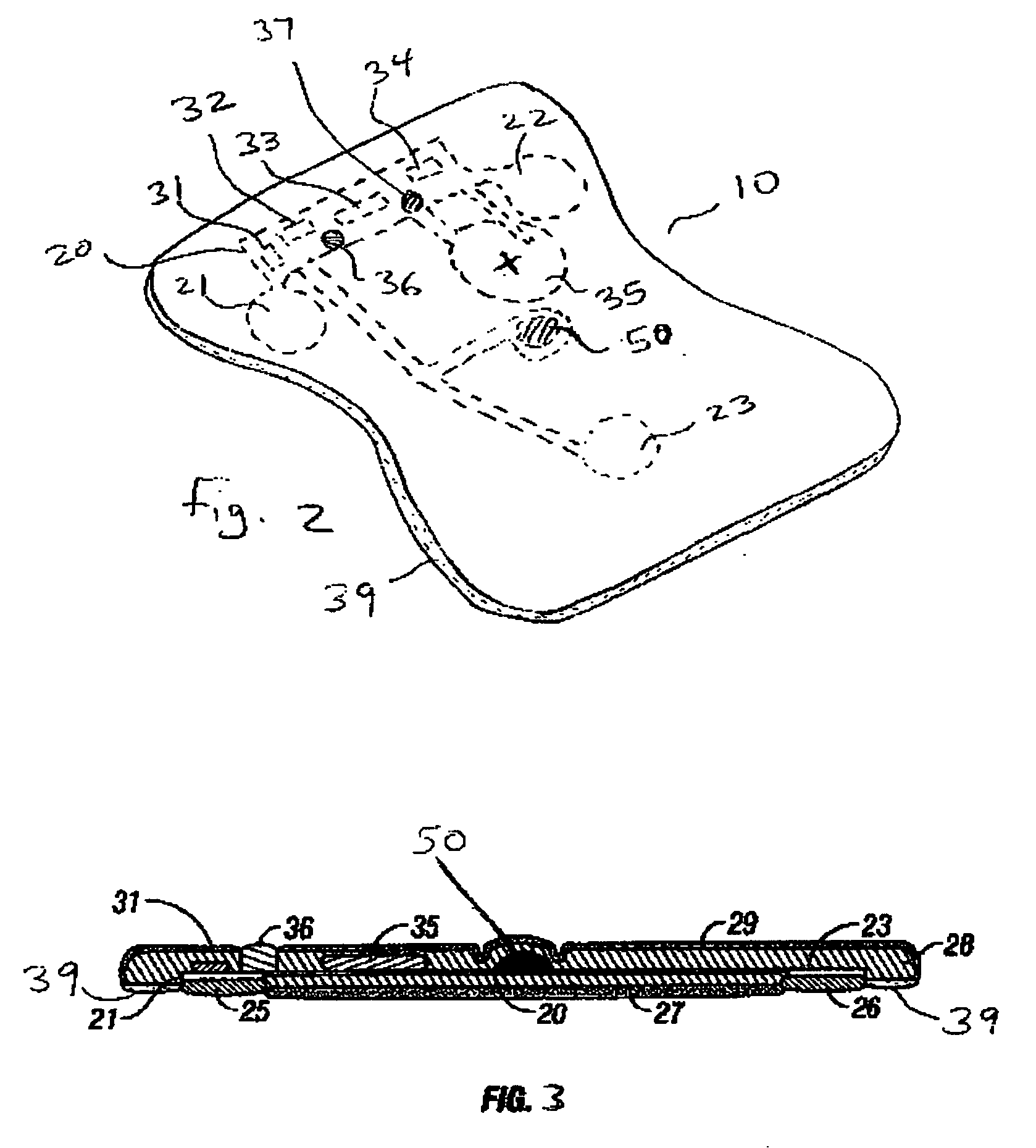
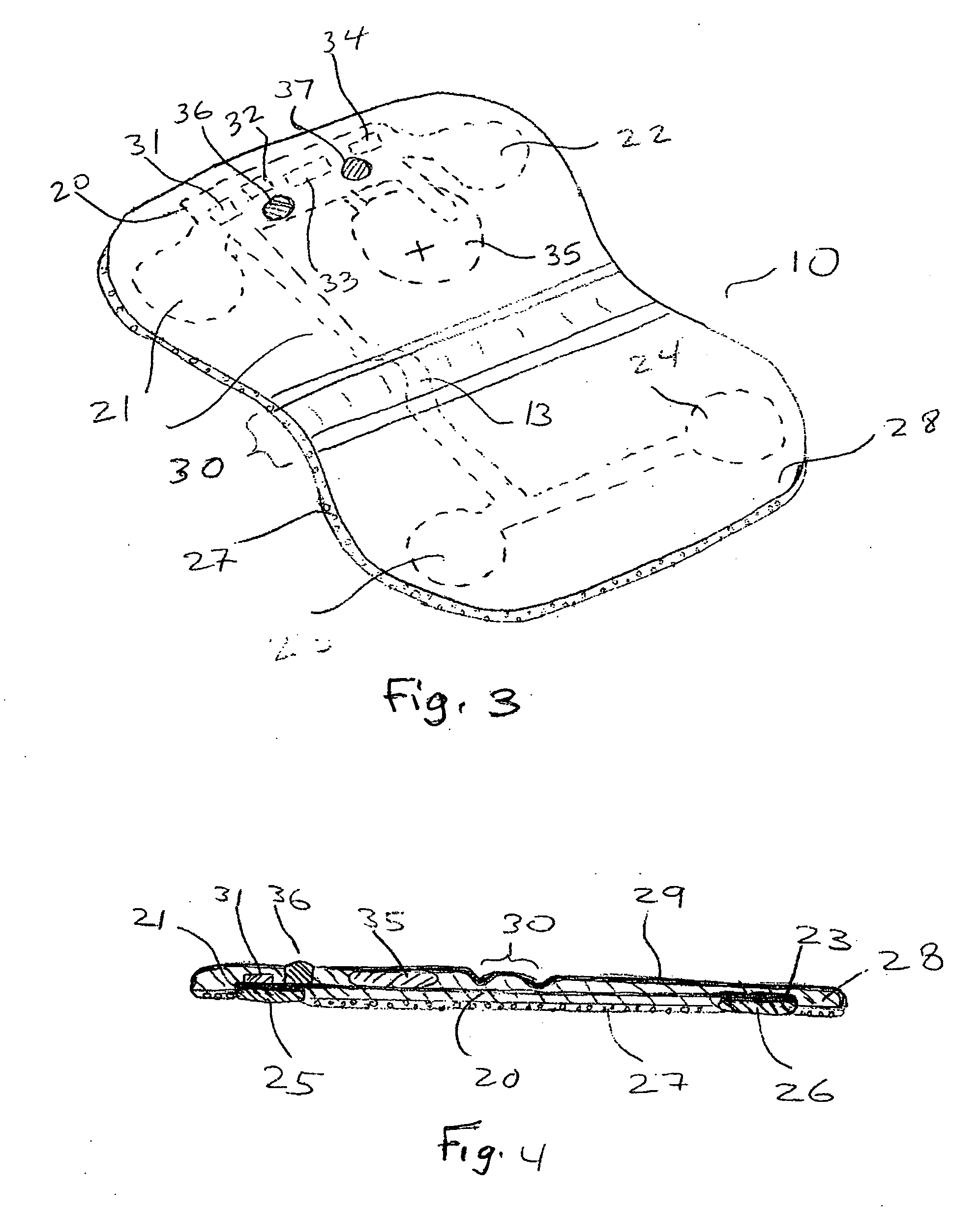
Disposable extended wear heart monitor patch
InactiveUS20060224072A1Easy diagnosisMinimizing contaminationElectrocardiographySensorsWireless transmissionNon invasive
The invention provides a disposable sensor patch for non-invasive monitoring and recording of infrequent cardiac events. The patch is thin and flexible for comfortable wear on the person's chest for automatic analysis and recording of ECG. The patch is inexpensive and simple for self-administration. The patch incorporates a battery, ECG amplifier, and a processor for analyzing ECG waveform and recording events. A software algorithm searches for a cardiac abnormality. The patch is designed for continuous long-term wear exceeding 3 days for diagnostic monitoring and exceeding 14 days for event detection. In one embodiment a preformatted report is automatically generated by the patch for wireless transmission to a reporting device such as a generic printer or a wireless network system. The patch may also incorporate a marker switch to correlate recorded ECG data with the patient's perception of a cardiac event.
Owner:CARDIOVU
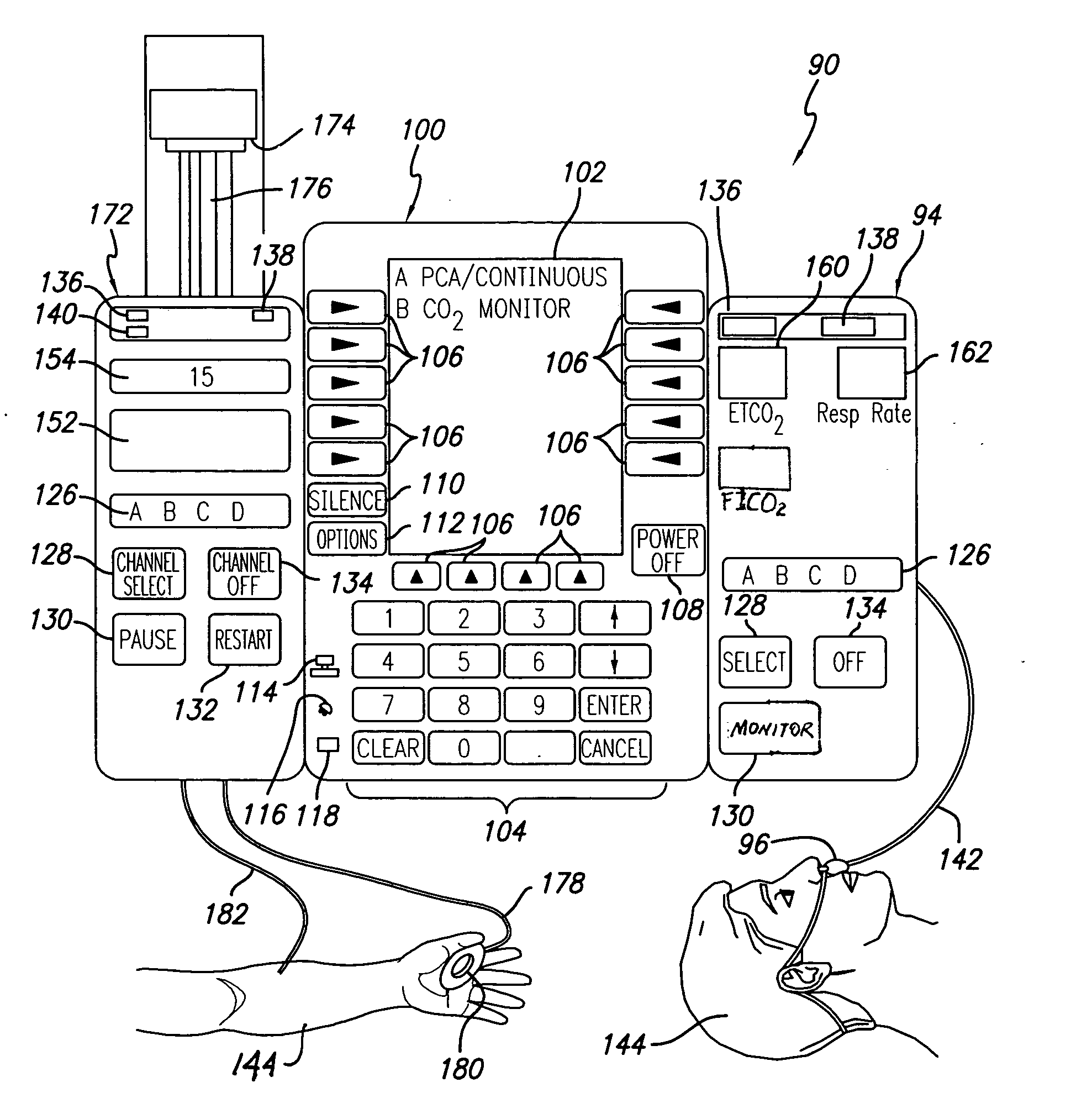
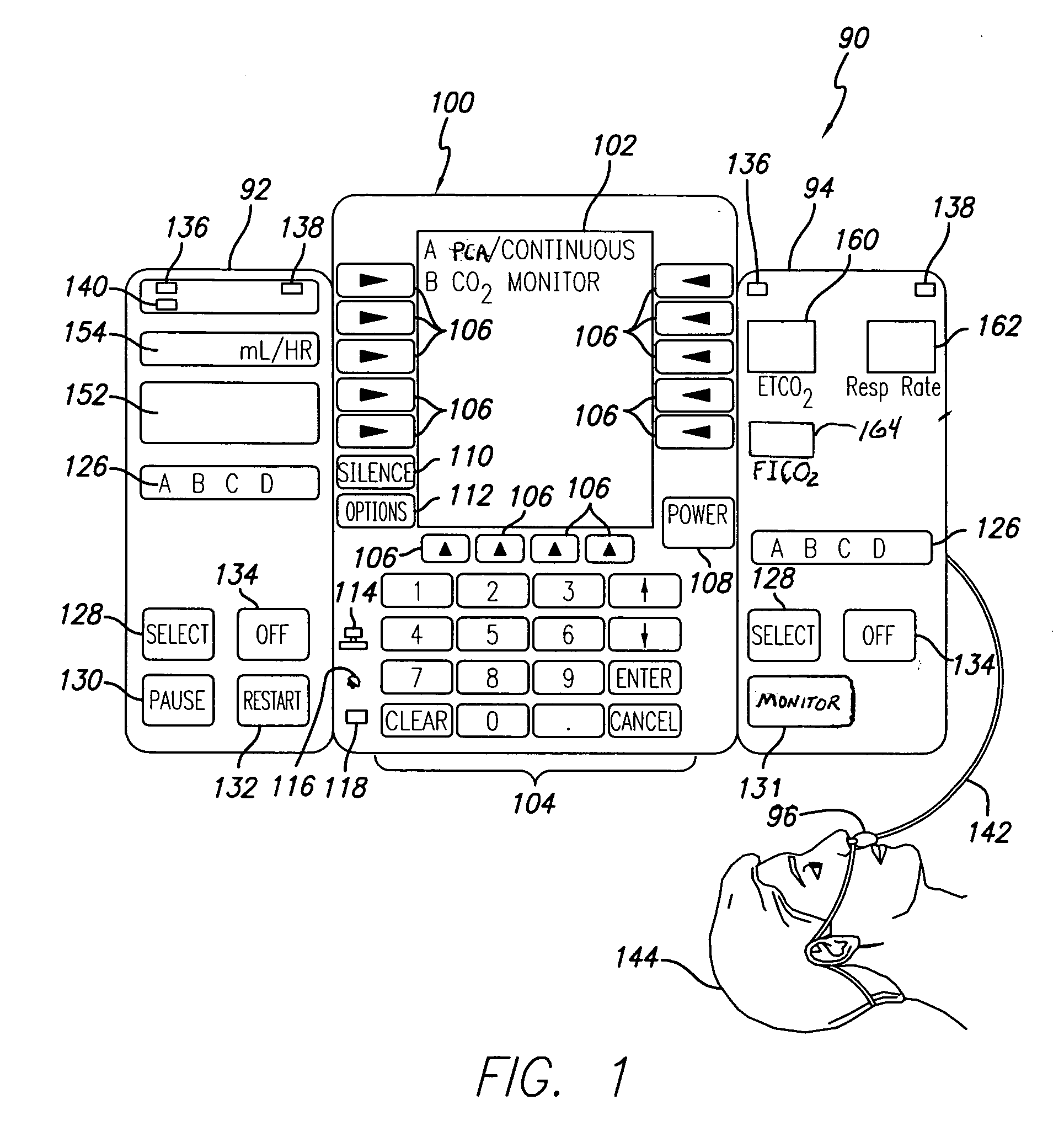
Patient-controlled analgesia with patient monitoring system and method
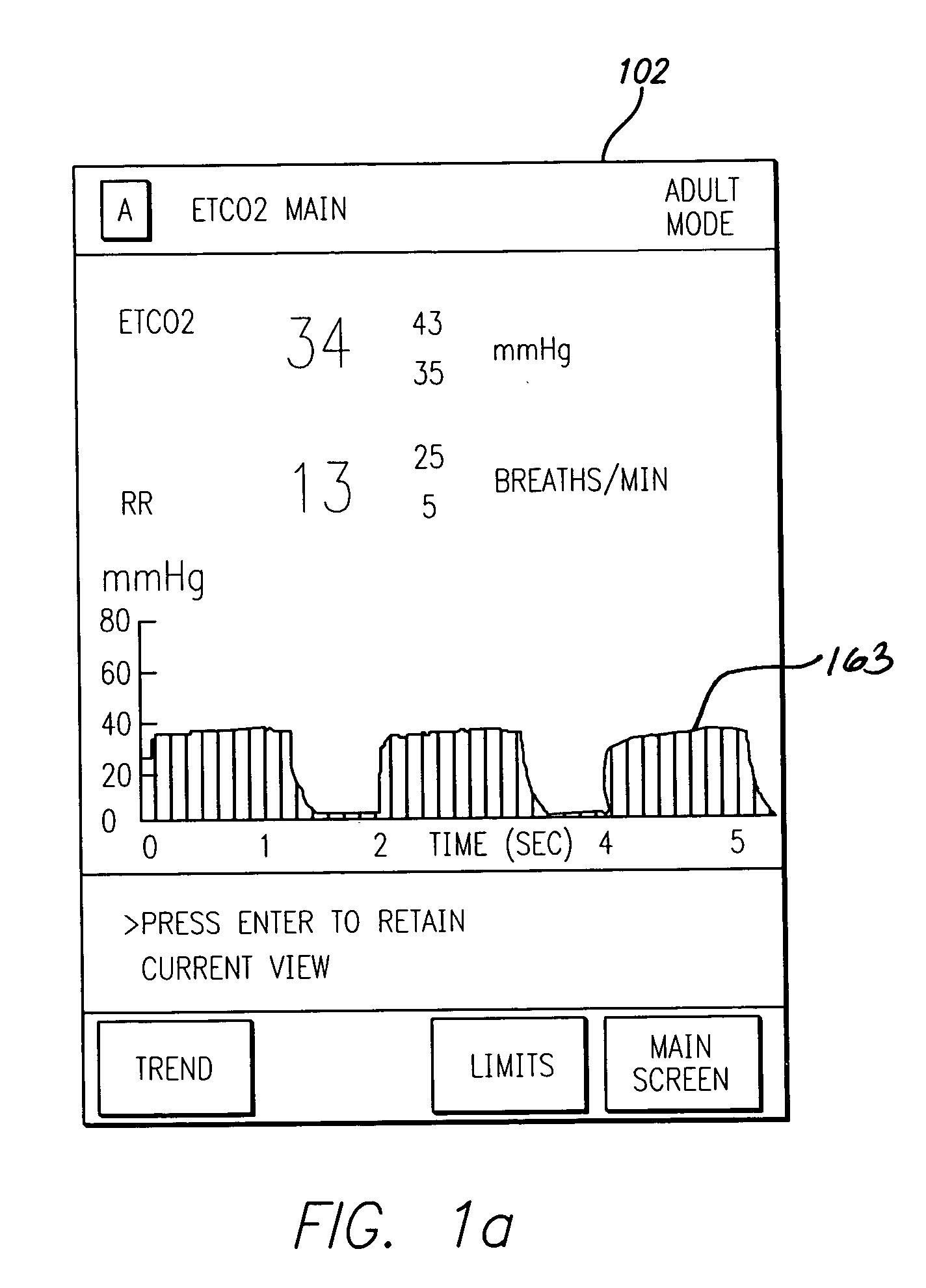
A patient care system in which a physiological parameter of a patient is monitored while the patient self-administers analgesic. A display presents a trend of the patient's physiological parameter along with the time of self-administration of the analgesic (“PCA”—patient controlled analgesic) such that the effect of the analgesic on the physiological parameter can be seen over selectable time periods. The physiological parameter may be ETCO2 or SpO2 or other. Also included is a drug library having acceptable pumping parameters as well as other PCA specific data. Should the operator program a pumping parameter that is outside an acceptable range, or should the patient attempt to self-administer more analgesic than the acceptable range permits, or should a patient's physiological parameter change during infusion such that a pumping parameter becomes outside an acceptable range, an indication of such will be given and action, such as stopping the pump, will be taken.
Owner:CAREFUSION 303 INC
Programmable multi-dose intranasal drug delivery device
InactiveUS6948492B2Avoid diversionAvoid abuseRespiratorsLiquid surface applicatorsNasal sprayBiological activation
An apparatus and method for the self-administration of a plurality of doses of an intranasal liquid pharmaceutical composition, including opioid analgesics, that includes a drug delivery device containing a plurality of sealed vials, each vial containing a predetermined volume of the pharmaceutical composition, a pump assembly for conveying the liquid pharmaceutical composition from the interior of the vial and discharging it as a nasal spray in response to manual activation by the patient, and programmable means for sequentially advancing a vial to the ready position after passage of a prescribed time interval following the last activation of the delivery device.
Owner:UNIV OF KENTUCKY RES FOUND
Fluid delivery device with collapsible needle cover
InactiveUS6126637AImprove reliabilityEasy to break awayPharmaceutical delivery mechanismMedical devicesStored energyEngineering
A fluid delivery device having a self-contained stored energy membrane for defining in conjunction with a base a fluid reservoir and for expelling fluids from the reservoir. The device includes novel reservoir filling syringe and filling adapter for conveniently filling the fluid reservoir of the device. Additionally, the device includes a unique crushable or collapsible needle cover which surrounds and protects the infusion cannula until time of use and then readily deforms as the device is connected to the patient so as to permit the needle to cleanly penetrate the patient's skin. The needle cover maintains the cannula in a substantially aseptic condition and enables self-administration by patients, such as young patients or needle adverse patients, which does not require their consciously inserting a needle into the skin.
Owner:PESCADERO BEACH HLDG
Omega-3 fatty acids and dyslipidemic agent for lipid therapy
InactiveUS20060211762A1Effective treatmentBiocideMetabolism disorderDyslipidemiaCoronary artery disease
A method and composition for blood lipid therapy by administering to the subject an effective amount of a dyslipidemic agent and omega-3 fatty acids. The method utilizes a single administration or a unit dosage of a combination of dyslipidemic agent and omega-3 fatty acids for the treatment of patients with hypertriglyceridemia, hypercholesterolemia, mixed dyslipidemia, coronary heart disease (CHD), vascular disease, artherosclerotic disease and related conditions, and the prevention or reduction of cardiovascular and vascular events.
Owner:RELIANT PHARMACEUTICALS INC +1
Laser probes for drug permeation
InactiveUS6389313B1Improve diffusivityImprove permeabilityElectrotherapyEar treatmentHigh concentrationLaser probe
Owner:SPECTRAL BIOSYST
Omega-3 Fatty Acids and Dyslipidemic Agent for Lipid Therapy
InactiveUS20090012167A1Effective treatmentBiocideAnimal repellantsDyslipidemiaCoronary artery disease
Owner:RELIANT PHARMACEUTICALS INC +1
Heart disease detection patch
InactiveUS20060030782A1Inexpensive and simple to useSuitable for self-administrationElectrocardiographySensorsFibrillationNon invasive
The invention provides a disposable sensor patch for the non-invasive detection of heart disease. The patch is placed on a person's chest area for automatic analysis of ECG. The heart condition is indicated via an indicator integrated within the patch. The patch is inexpensive and simple for self-administration. In one embodiment, the status of the heart is indicated via multiple LEDs. The detection and indication typically occurs, within 24 hours or sooner if a condition is readily identifiable. The patch is thin, flexible, and incorporates a battery, ECG amplifier, and a processor for analyzing ECG waveform and indicating the heart condition. A software algorithm searches for a cardiac abnormality such as arrhythmia, bradycardia, tachycardia, fibrillation, mycocardial infarction, ischemia, long-QT syndrome, blocks, late potentials, and premature contractions. In another embodiment, results and relevant ECG data are stored in memory for later retrieval.
Owner:CARDIOVU
Smart adapter for infusion devices
ActiveUS20160030683A1Improve safety and efficacyInfusion syringesMicroneedlesPen InjectorDrug administration
Smart sensors are employed to determine one or more of drug identification, dose, flow rate, concentration, agglomeration, and degradation and / or other characteristics of drug administration that can be detected via sensing technology. A smart sensor(s) can be coupled to or retrofitted onto injection pen injectors and / or drug delivery cartridges and / or infusion sets or cannulae, enabling infusion sets, pen injector systems or drug delivery cartridges to improve tracking of drug self-administration and stop medication errors that occur primarily through self or automated injection (e.g., due to incorrect or incomplete dosing, excessive dose or rate, incorrect drug, or drug degradation).
Owner:BECTON DICKINSON & CO
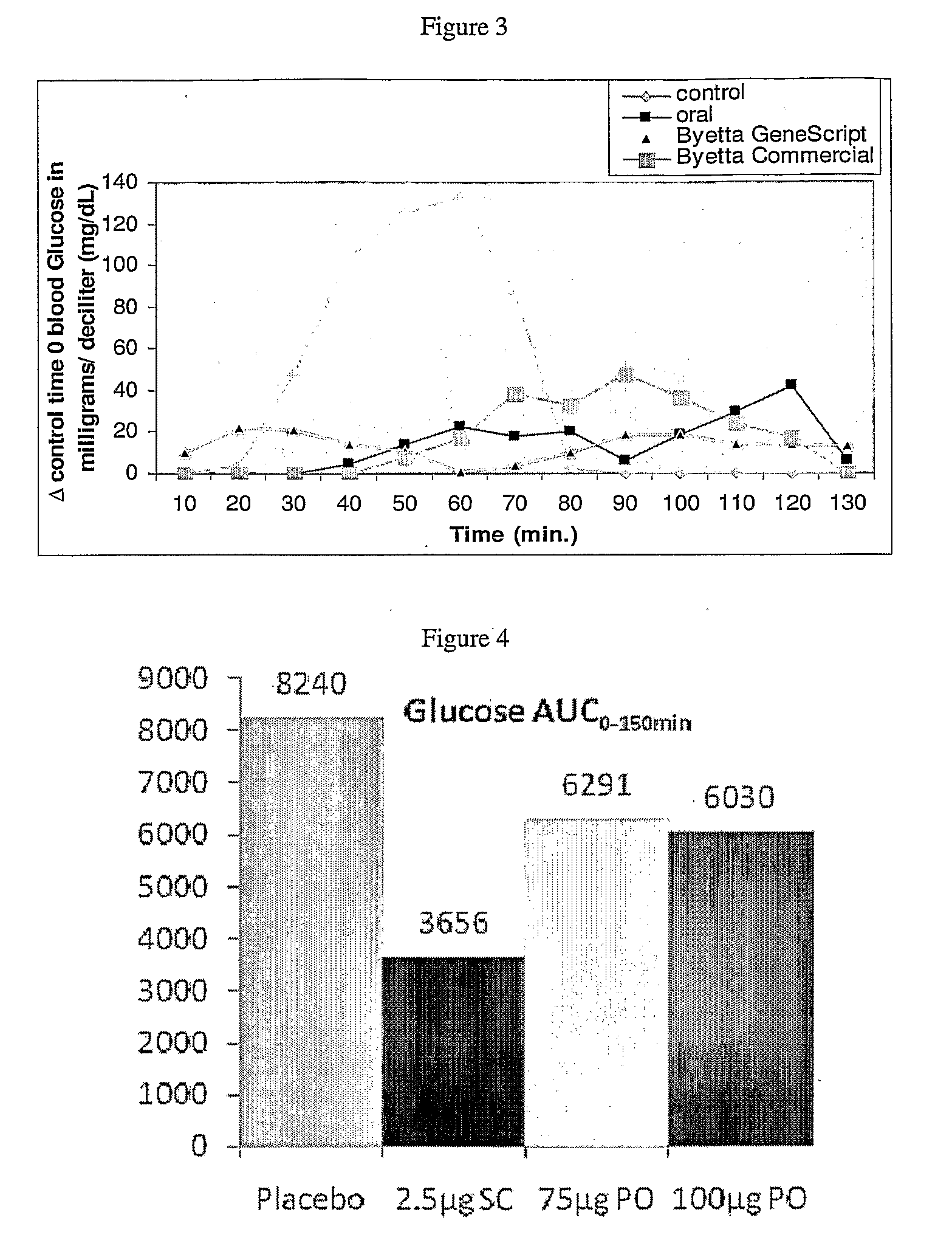
Methods and compositions for oral administration of exenatide
InactiveUS20110046053A1Reducing food intakeDecreased gastric motilityPeptide/protein ingredientsMetabolism disorderDiabetes mellitusOral medication
This invention provides compositions comprising a byetta, fish oil, and a protease inhibitor, method for treating diabetes mellitus, comprising administering same, and methods for oral or rectal administration of a byetta.
Owner:ORAMED
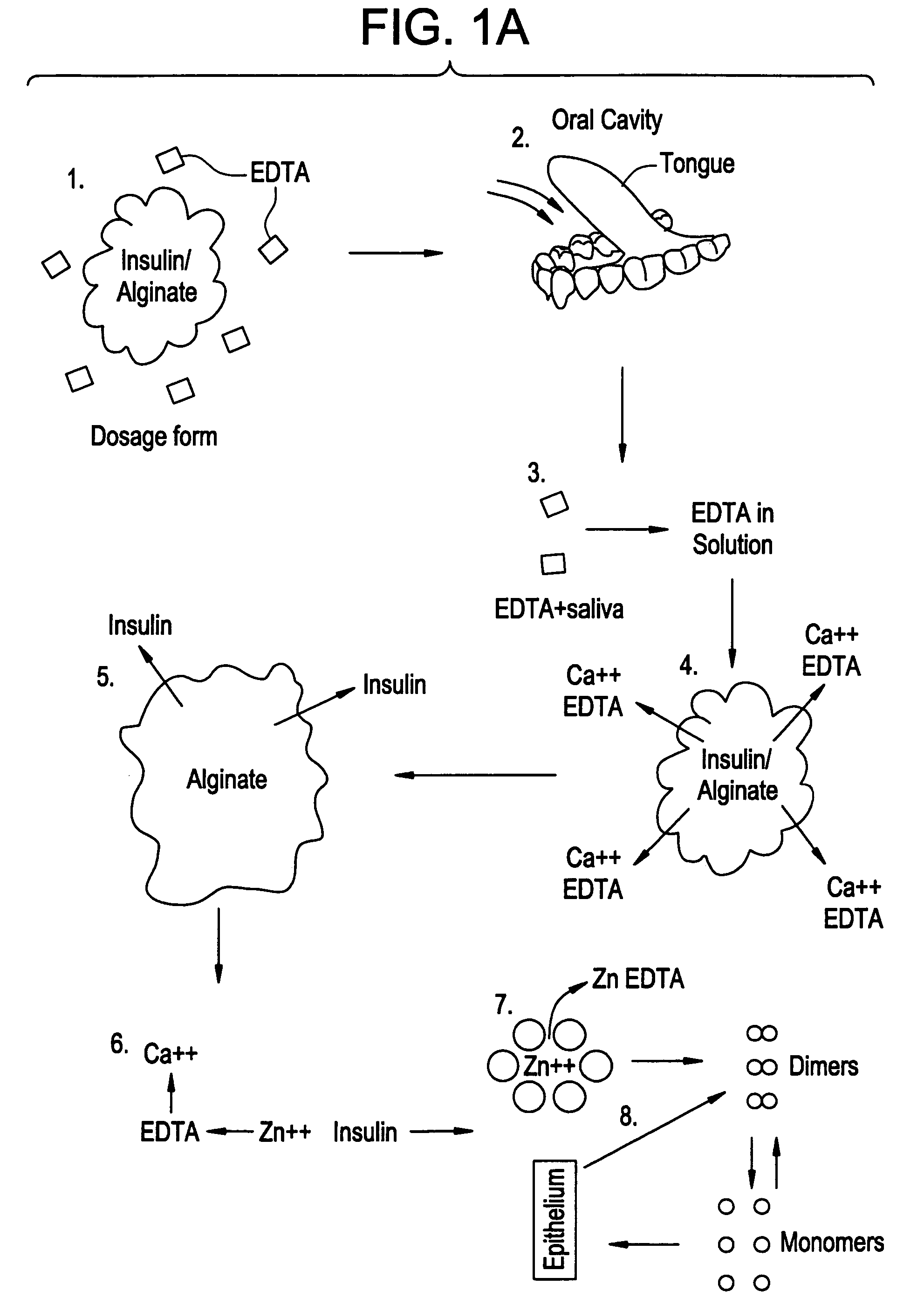
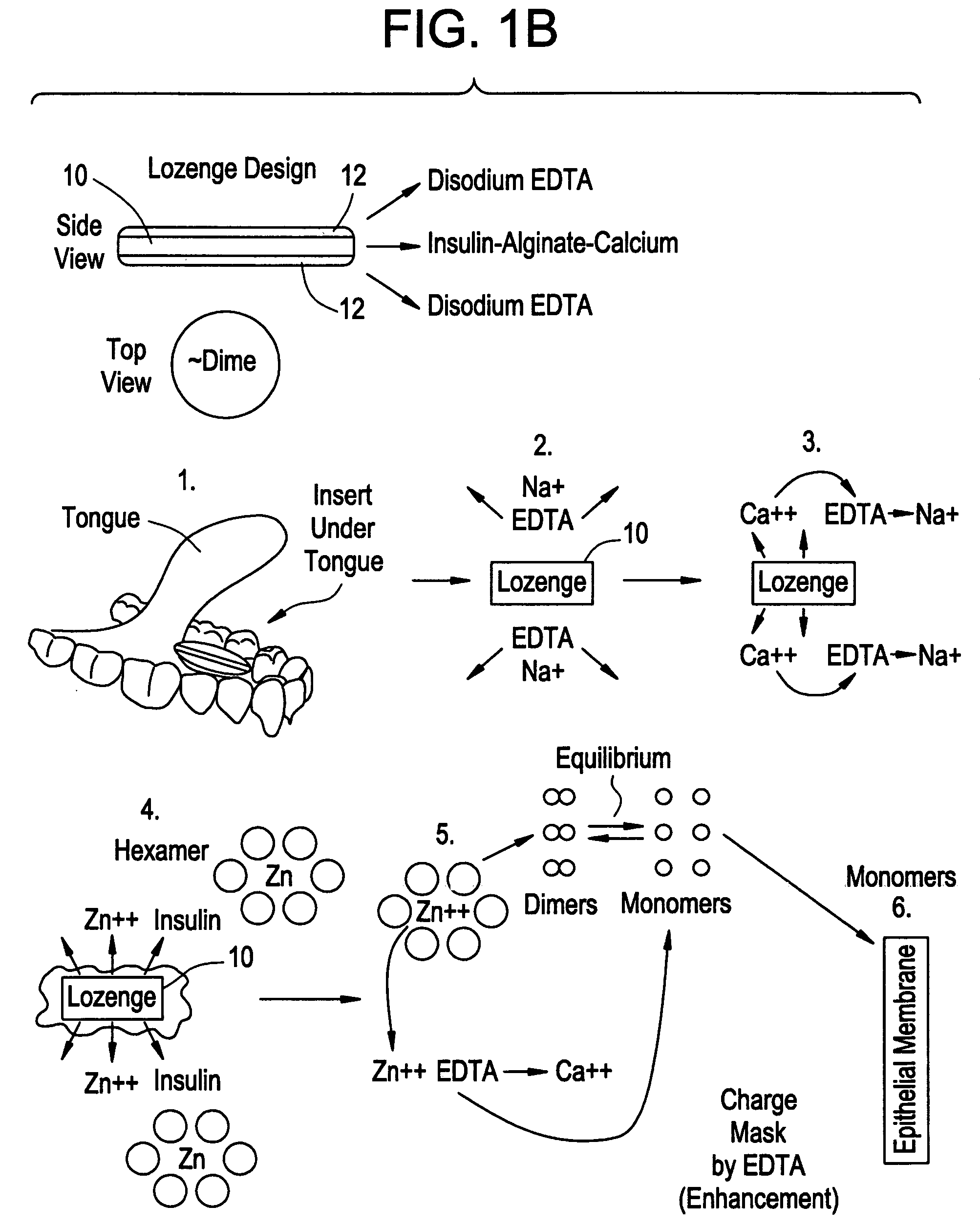
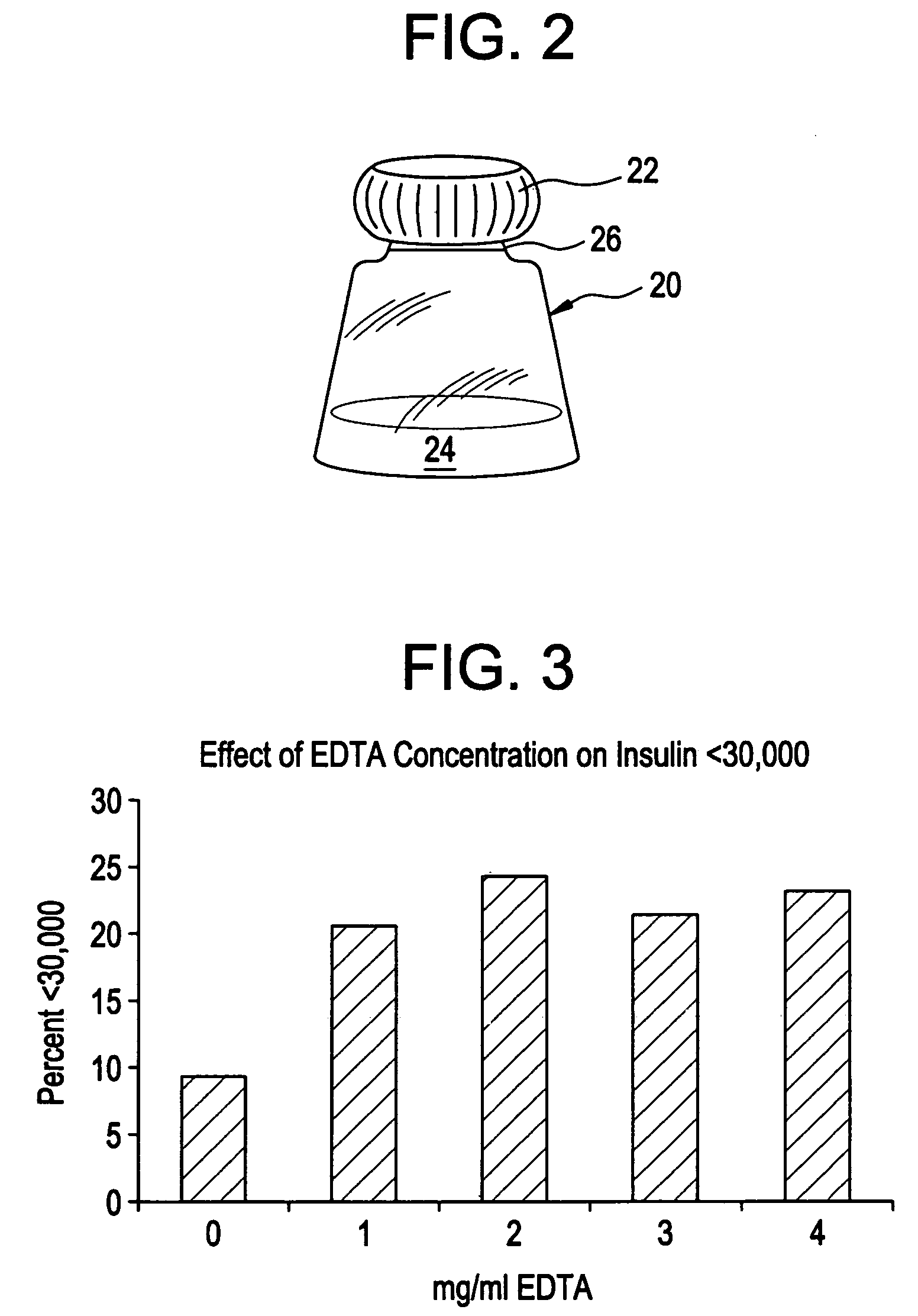
Rapid acting drug delivery compositions
Drug formulations for systemic drug delivery with improved stability and rapid onset of action are described herein. The formulations may be administered via buccal administration, sublingual administration, pulmonary delivery, nasal administration, subcutaneous administration, rectal administration, vaginal administration, or ocular administration. In the preferred embodiments, the formulations are administered sublingually or via subcutaneous injection. The formulations contain an active agent and one or more excipients, selected to increase the rate of dissolution. In the preferred embodiment, the drug is insulin, and the excipients include a metal chelator such as EDTA and an acid such as citric acid. Following administration, these formulations are rapidly absorbed by the oral mucosa when administered sublingually and are rapidly absorbed into the blood stream when administered by subcutaneous injection. In one embodiment, the composition is in the form of a dry powder. In another embodiment, the composition is in the form of a film, wafer, lozenge, capsule, or tablet. In a third embodiment, a dry powdered insulin is mixed with a diluent containing a pharmaceutically acceptable carrier, such as water or saline, a metal chelator such as EDTA and an acid such as citric acid. Devices for storing and mixing these formulations are also described.
Owner:ELI LILLY & CO
Delayed release formulations for oral administration of a polypeptide therapeutic agent and methods of using same
InactiveUS20040126358A1Increase ionic strengthReduced strengthAntipyreticAnalgesicsOral medicationWhite blood cell
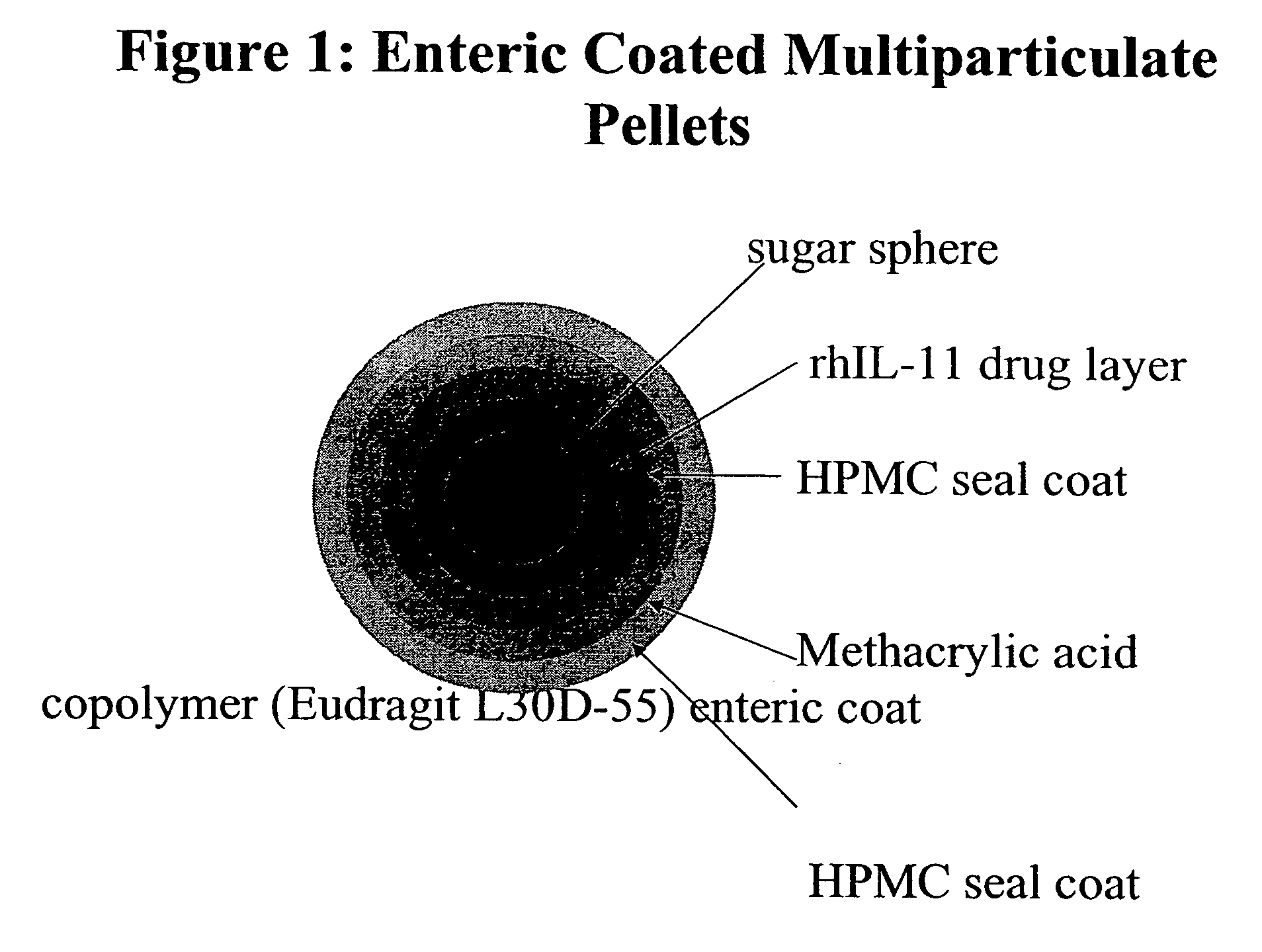
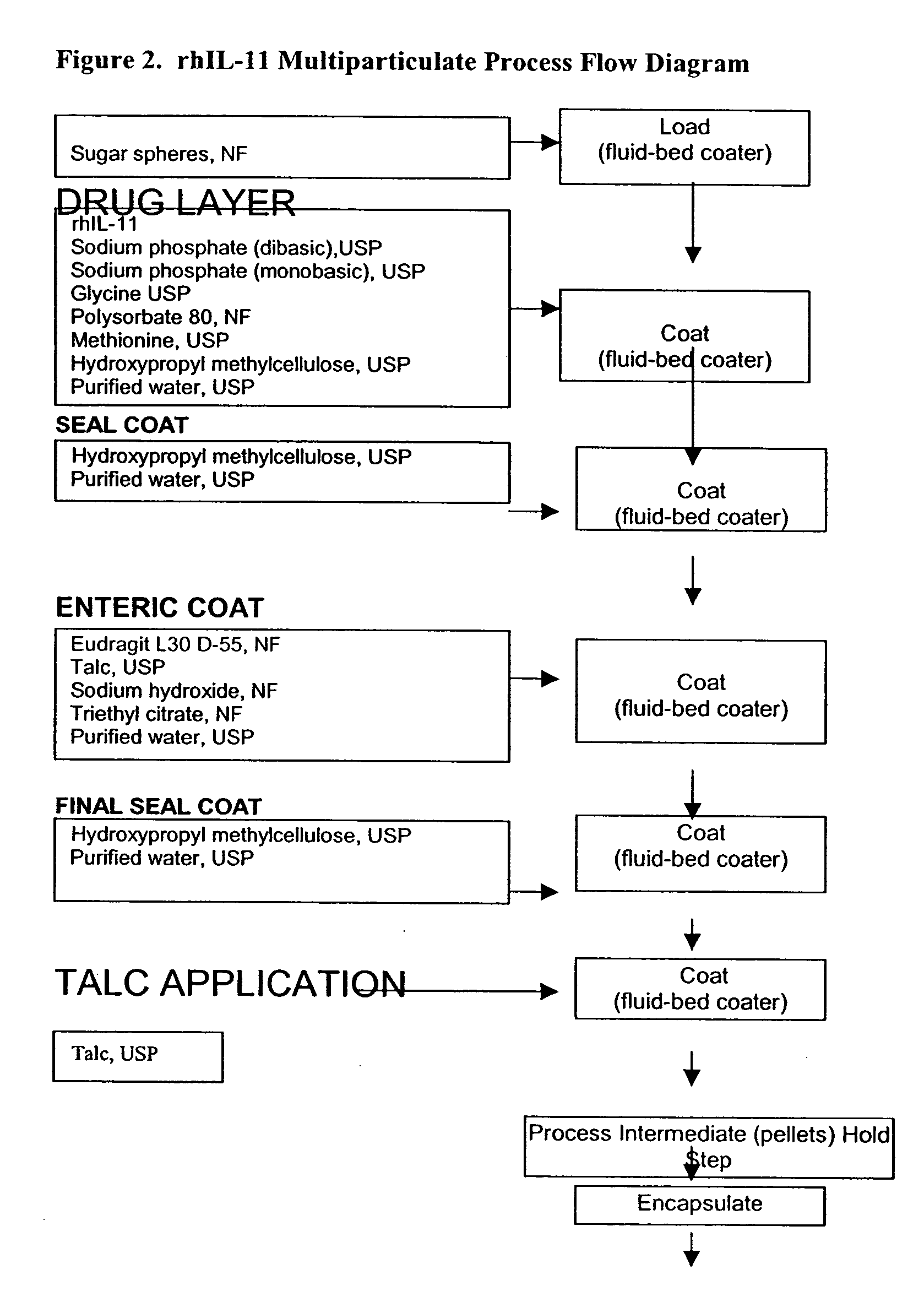
The invention provides compositions containing polypeptides, including therapeutic polypeptides such as interleukin-11, that are suitable for oral administration.
Owner:WYETH LLC
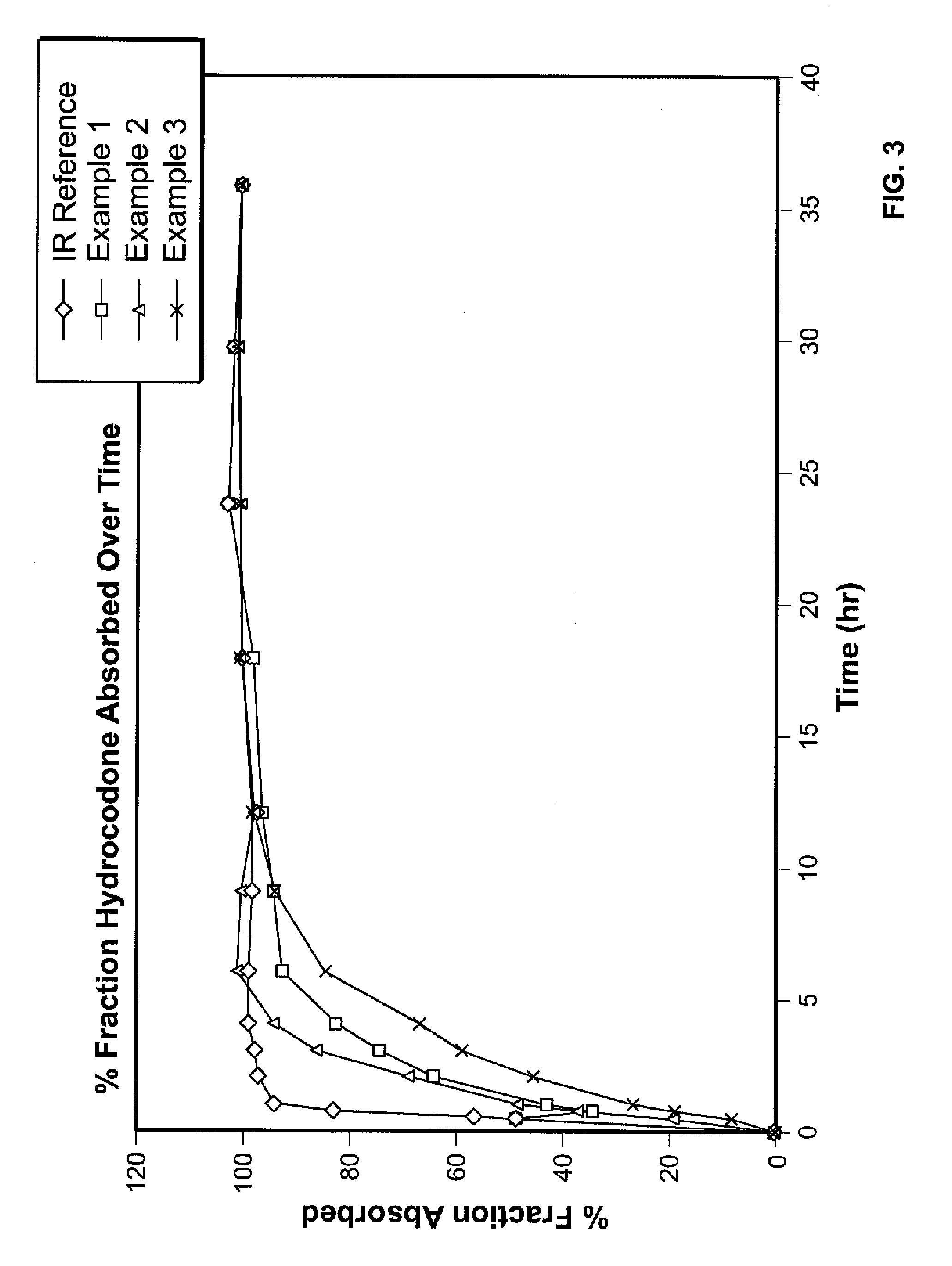
Controlled Release Hydrocodone Formulations
InactiveUS20110262532A1Improve efficiency and qualityGood effectPowder deliveryBiocideControlled releaseHuman patient
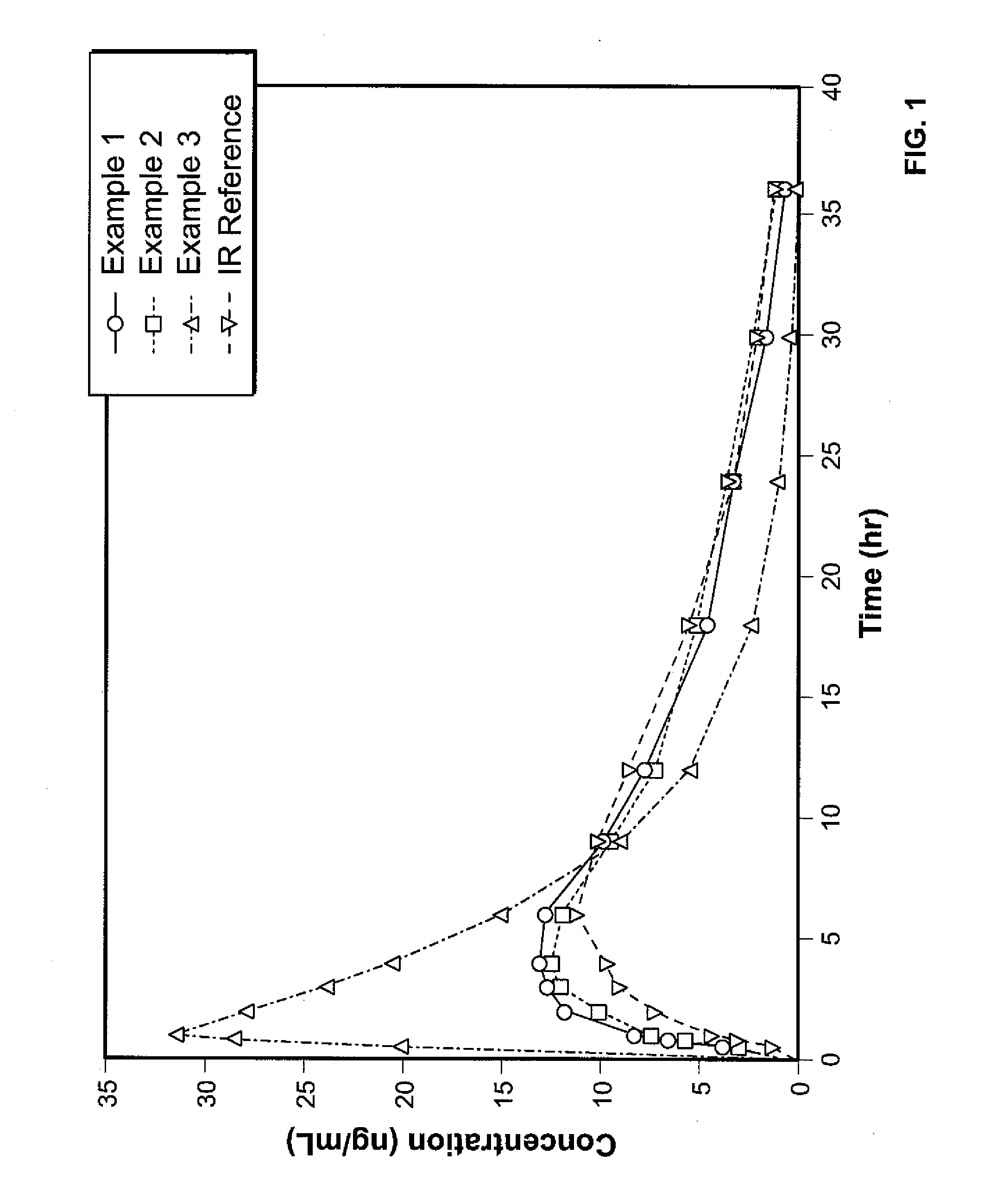
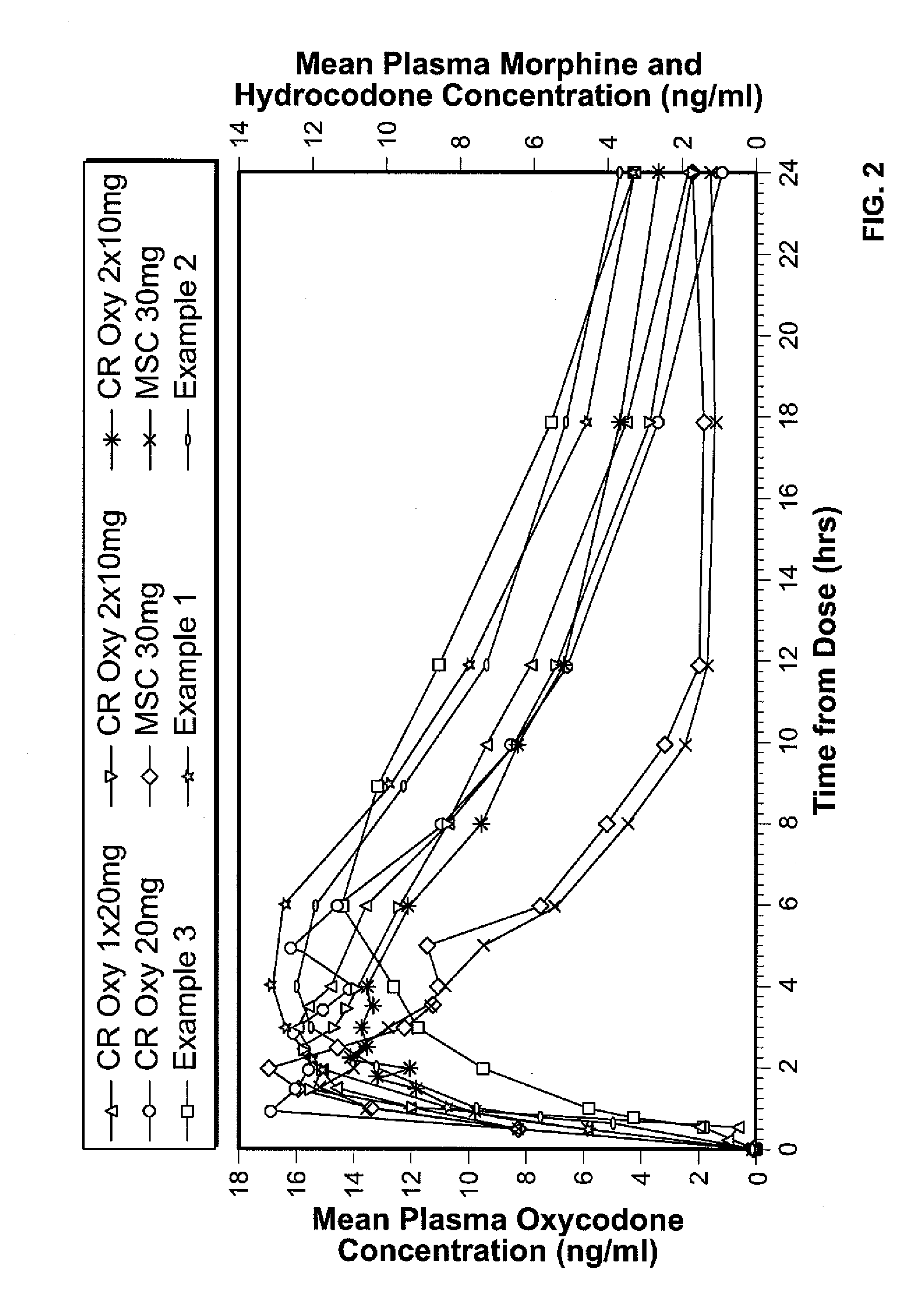
A solid oral controlled-release oral dosage form of hydrocodone is disclosed. The dosage form comprising an analgesically effective amount of hydrocodone or a pharmaceutically acceptable salt thereof, and a sufficient amount of a controlled release material to render the dosage form suitable for twice-a-day administration to a human patient, the dosage form providing a C12 / Cmax ratio of 0.55 to 0.85, said dosage form providing a therapeutic effect for at least about 12 hours.
Owner:PURDUE PHARMA LP
Split dose administration
The present disclosure provides, inter alia, formulation compositions comprising modified nucleic acid molecules which may encode a protein, a protein precursor, or a partially or fully processed form of the protein or a protein precursor. The formulation composition may further include a modified nucleic acid molecule and a delivery agent. The present invention further provides nucleic acids useful for encoding polypeptides capable of modulating a cell's function and / or activity.
Owner:MODERNATX INC
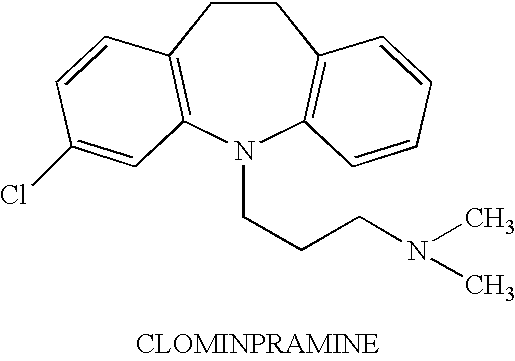
On demand administration of clomipramine and salts thereof to treat premature ejaculation
InactiveUS6495154B1Delaying the onset of ejaculationDelay the onset of ejaculationPowder deliveryAerosol deliveryActive agentWhole body
A method is provided for delaying the onset of ejaculation in an individual. The method involves systemic and on demand administration to an individual of a pharmaceutical formulation containing an amount of an active agent selected from the group consisting of clomipramine and pharmacologically acceptable acid addition salts thereof. Drug delivery may be accomplished via any route designed to provide systemic levels of the active agent effective to delay the onset of ejaculation. Pharmaceutical formulations and dosage forms are provided as well.
Owner:VIVUS
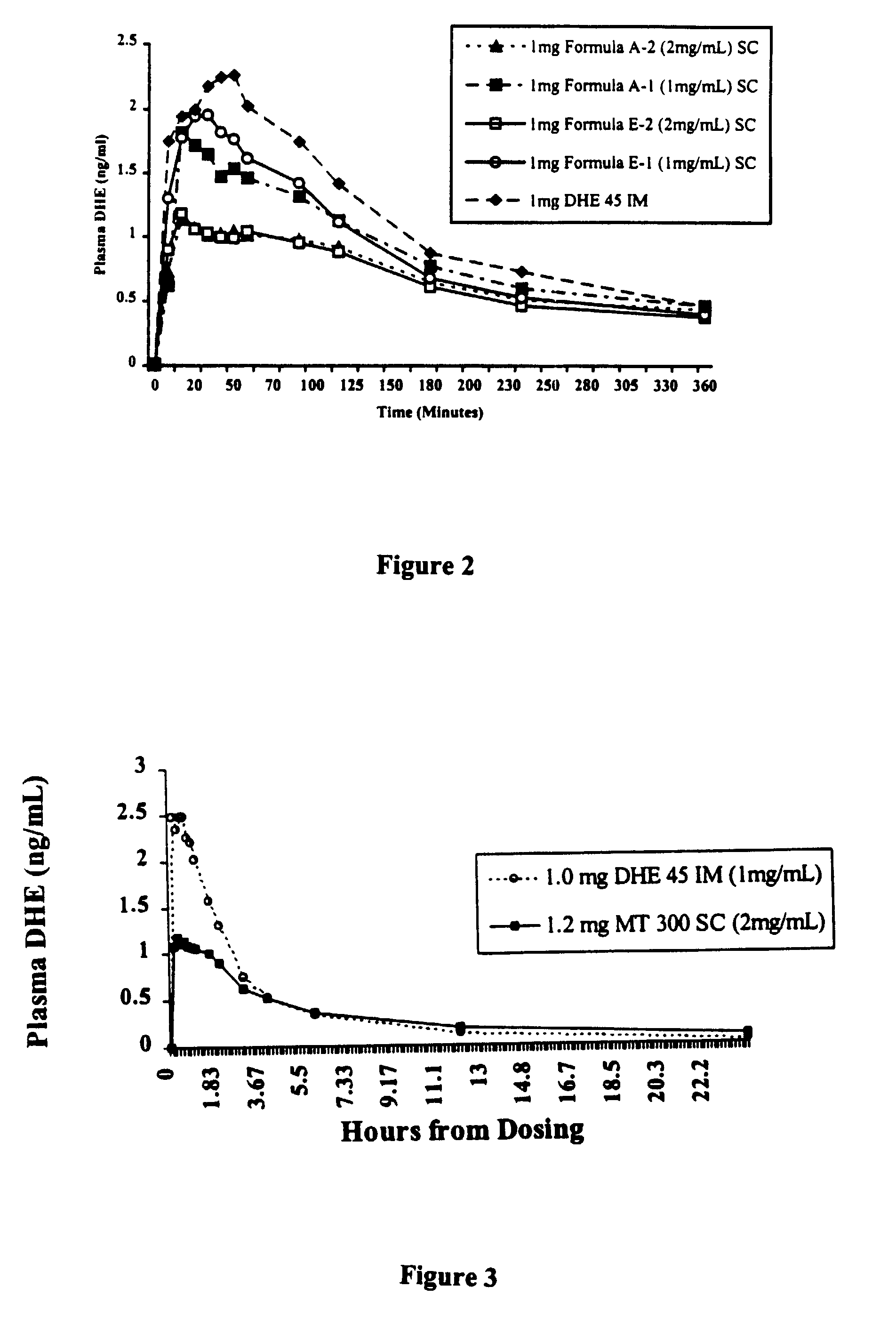
High potency dihydroergotamine compositions
InactiveUS7060694B2Improvement of side effectsEliminate side effectsBiocideNervous disorderDihydroergotamineHeadache severe
The present invention is directed to improved formulations for dihydroergotamine in which the drug is present at a concentration of at least 2.9 mM. The invention encompasses methods for using these formulations in treating patients for migraine headaches and the packaging of formulation into prefilled syringes for self-administration by patients.
Owner:POZEN INC
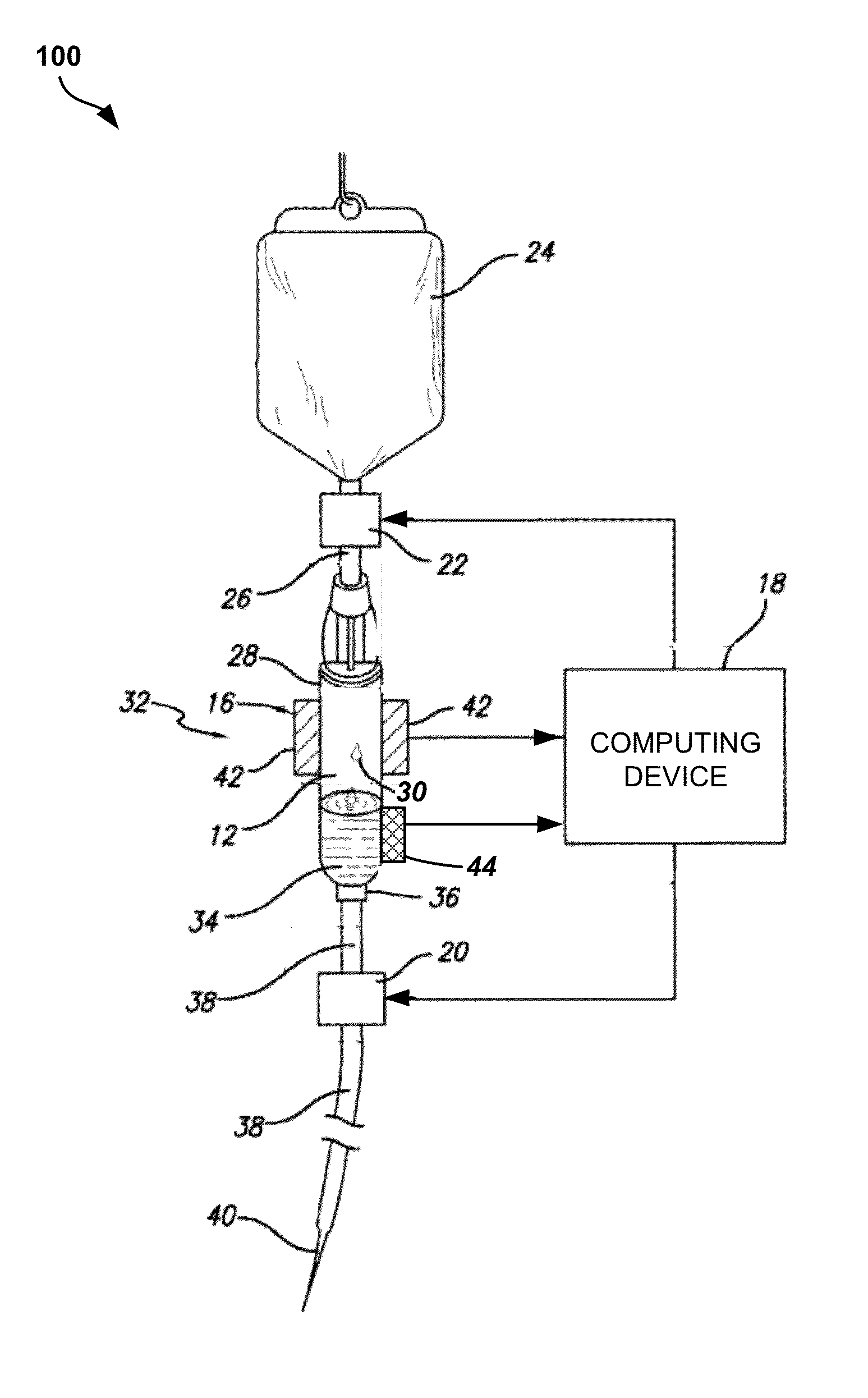
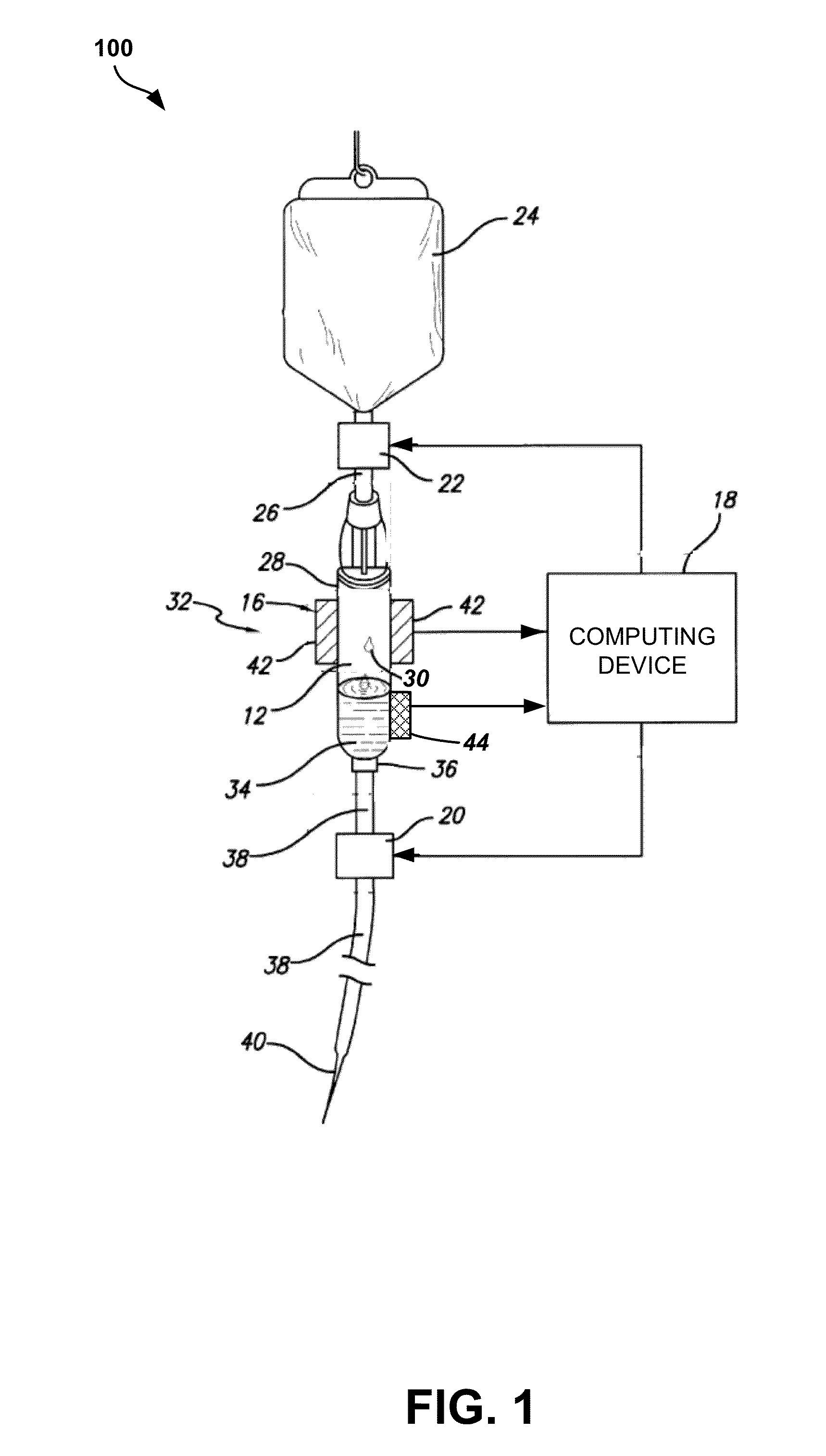
Apparatus and method for administration of IV liquid medication and IV flush solutions
InactiveUS6953450B2Reduce the number of stepsShorten the timeDiaphragm valvesYielding couplingInterconnectionBuccal administration
The present invention provides a medical liquid administration apparatus and administration method that are particularly apt for intravenous (IV) applications. More particularly, the administration apparatus and method may be employed in conjunction with the administration of liquid medication and one or more flush solutions from multi-dose sources, wherein fluid interconnections between at least one flush solution source and the administration apparatus may be established a single time at the outset of a given procedure. The administration apparatus may include a valve having a control member selectively positionable to provide any selected one of a plurality of closed flow paths through the valve, and a syringe interconnected to the control member for clockwise / counterclockwise co-rotation therewith (e.g. to establish the selected flow path).
Owner:BAXTER ENGLEWOOD
Controlled release pharmaceutical compositions comprising a fumaric acid ester
The present invention relates to controlled release pharmaceutical compositions comprising fumaric acid ester(s) as active substance(s). The compositions are suitable for use in the treatment of e.g. psoriasis or other hyperproliferative, inflammatory or autoimmune disorders and are designated to release the fumaric acid ester in a controlled manner so that local high concentrations of the active substance within the gastrointestinal tract upon oral administration can be avoided and, thereby, enabling a reduction in gastro-intestinal related side-effects.
Owner:BIOGEN SWISS MFG GMBH
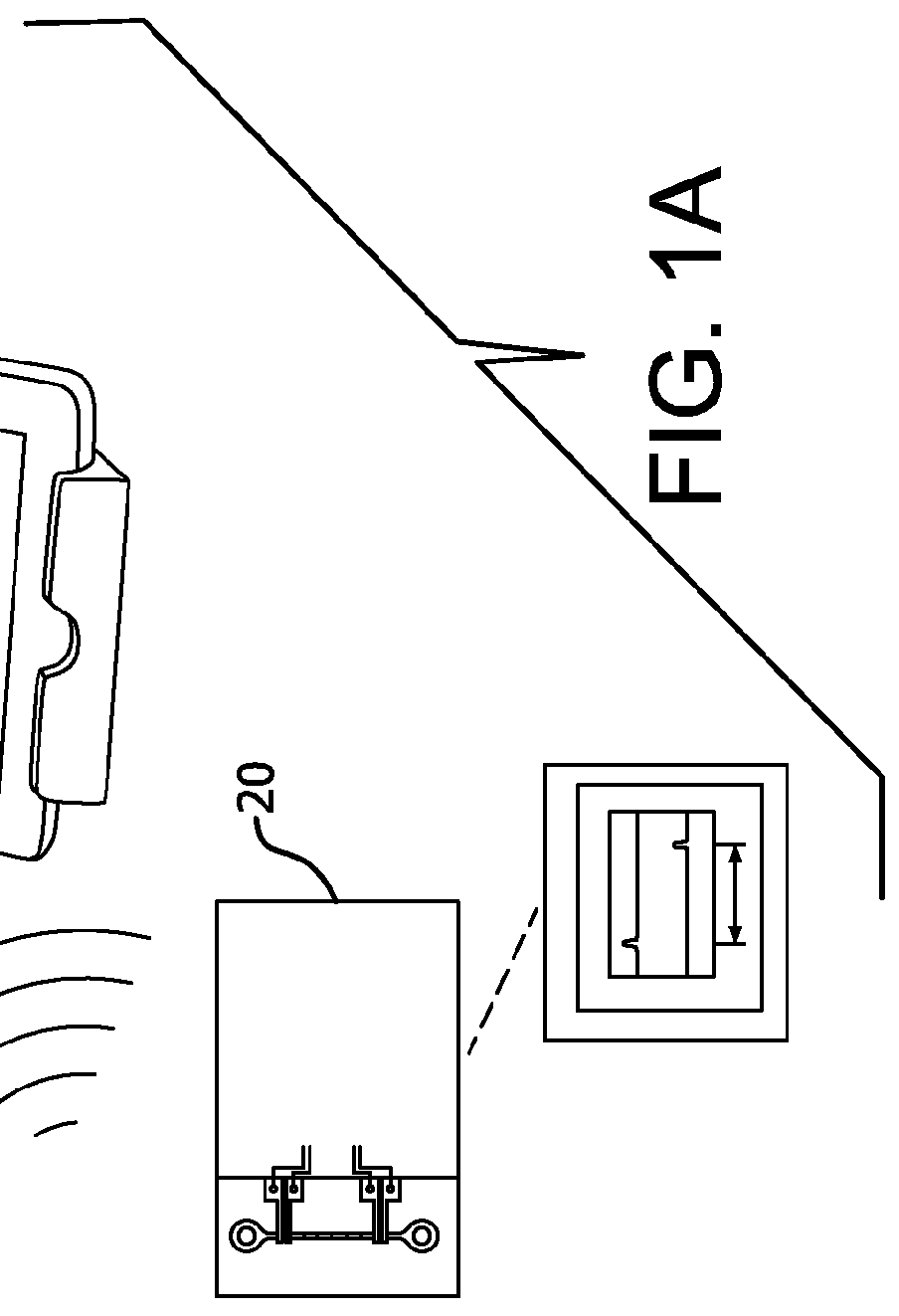
Self-administration injection system
Self-injection system allows a user to inject a drug from a cartridge carrying unique identification information, into any one of a plurality of injection sites. Tissue at each injection site is associated with at least one injection parameter, such as flow-rate, that is different for each site. A scanner reads the identification information of the cartridge and cooperates with a central processing unit to determine the validity of the drug in order to permit an injection procedure to commence. The central processing unit has a memory for storing the different injection parameters and controls a drive unit for driving fluid from the cartridge and through a needle into the selected tissue, at the injection parameter that is associated with the user selected tissue for the injection.
Owner:MILESTONE SCIENTIFIC INC
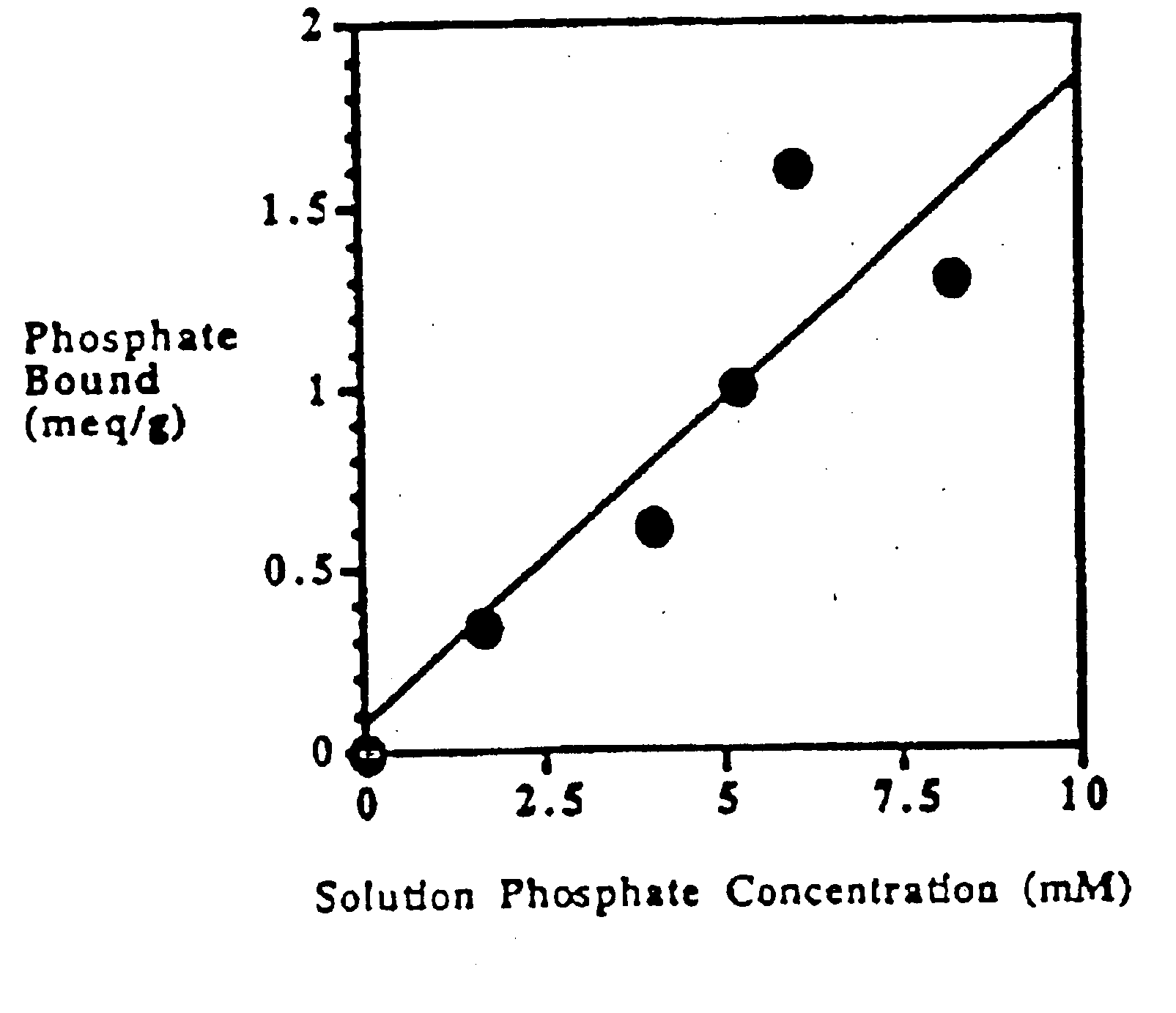
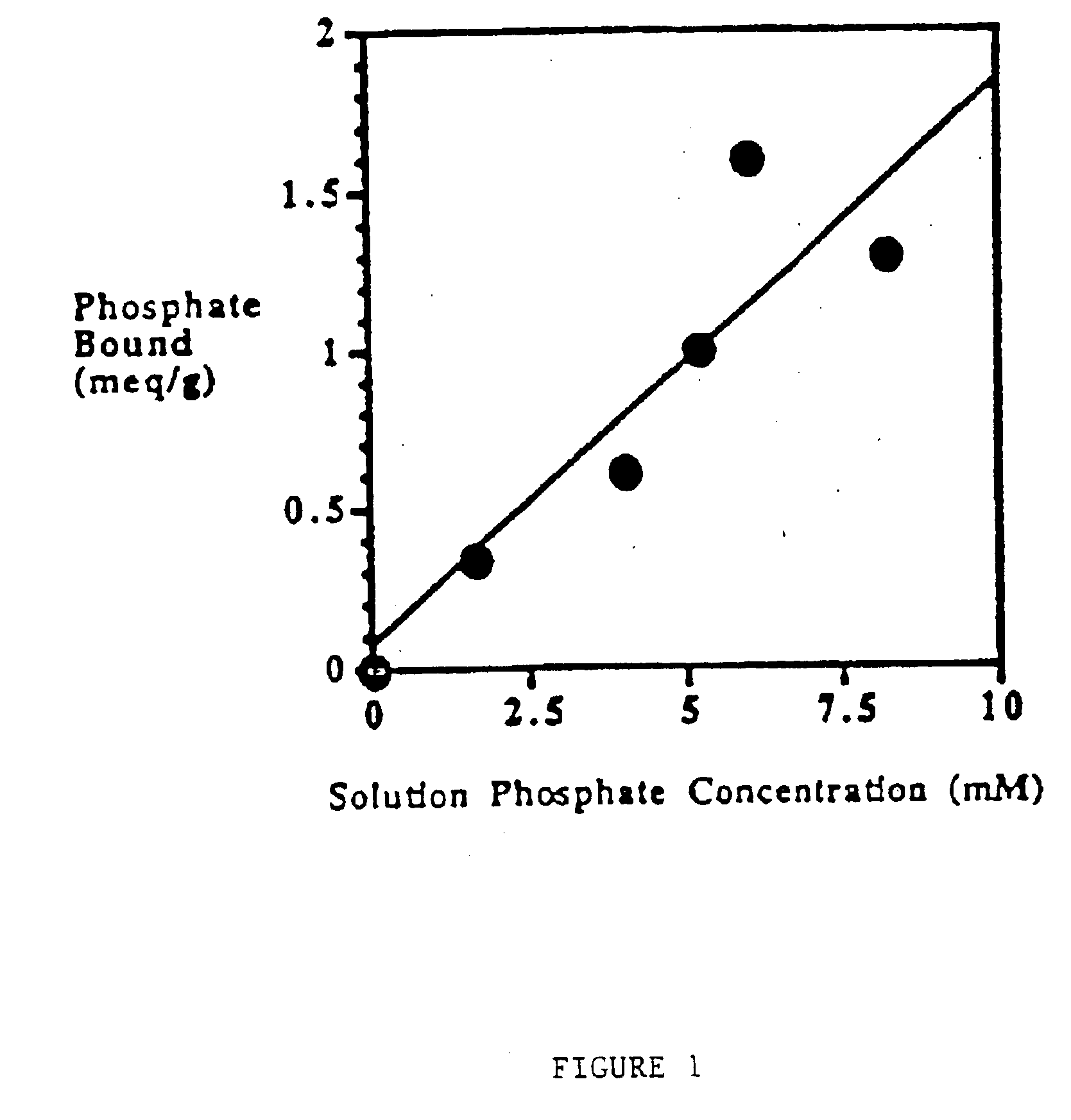
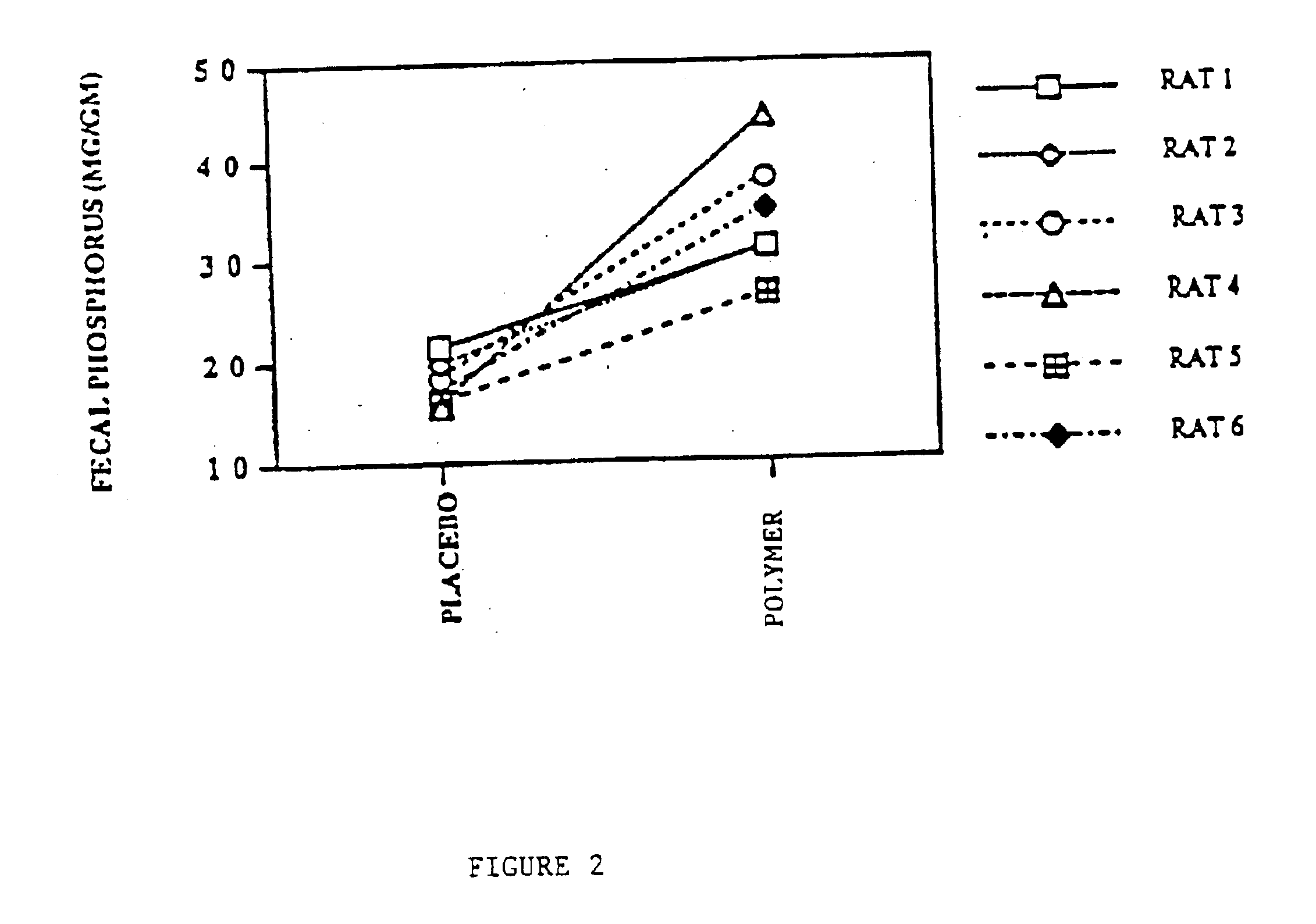
Method of making phosphate-binding polymers for oral administration
InactiveUS6858203B2Low serum levelsPromote absorptionPowder deliveryMetabolism disorderOral medicationBuccal administration
Phosphate-binding polymers are provided for removing phosphate from the gastrointestinal tract. The polymers are orally administered, and are useful for the treatment of hyperphosphatemia.
Owner:GENZYME CORP
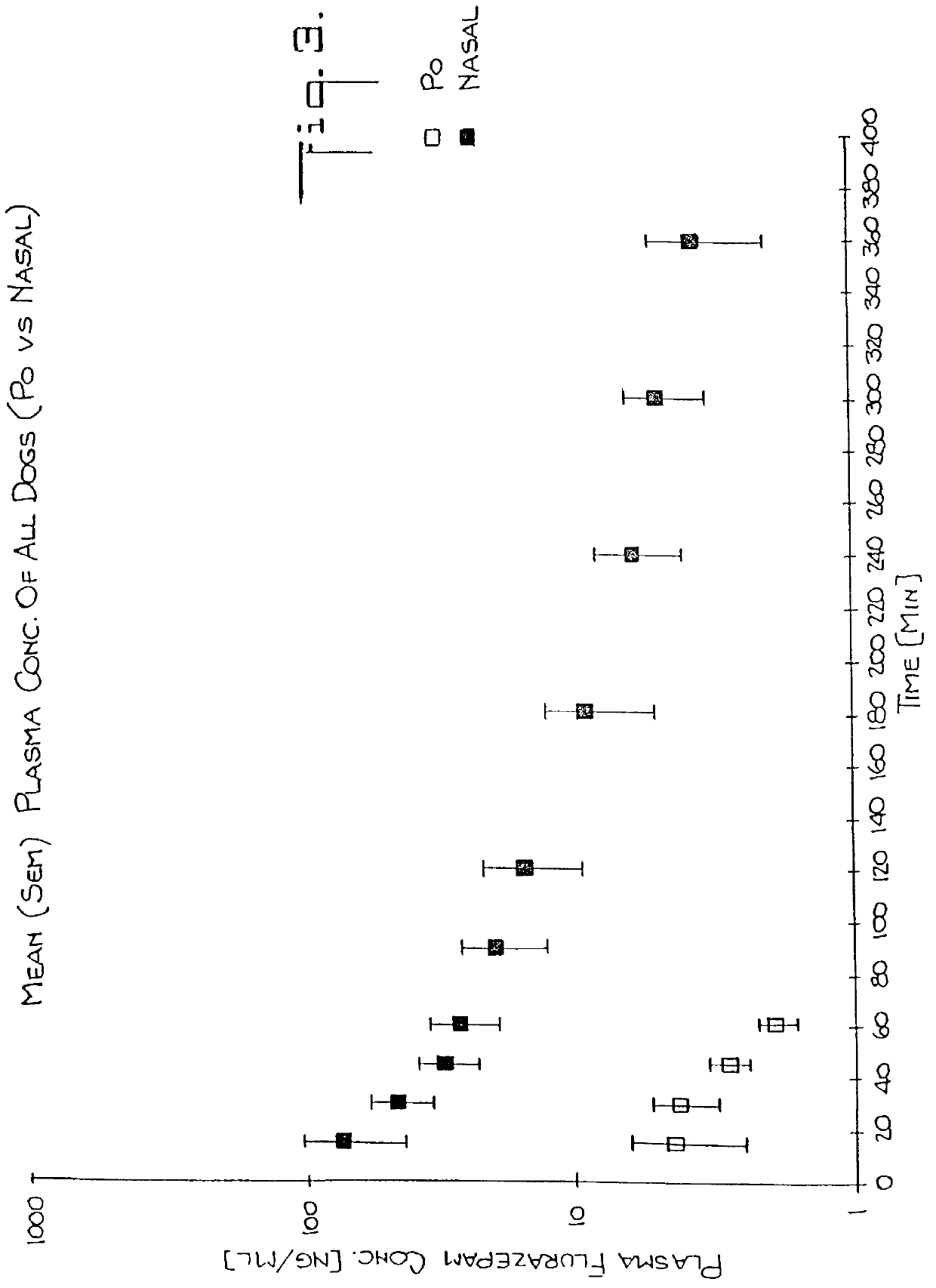
Nasal administration of benzodiazepine hypnotics
Nasal administration of benzodiazepines is described as providing improved therapeutic effects as compared to conventional delivery techniques. The compositions comprise a benzodiazepine hypnotic in a pharmaceutically acceptable nasal carrier.
Owner:QUESTCOR PHARMA
Pharmacy management and administration with bedside real-time medical event data collection
Owner:MILLENNIUM PHARMACY SYST LLC
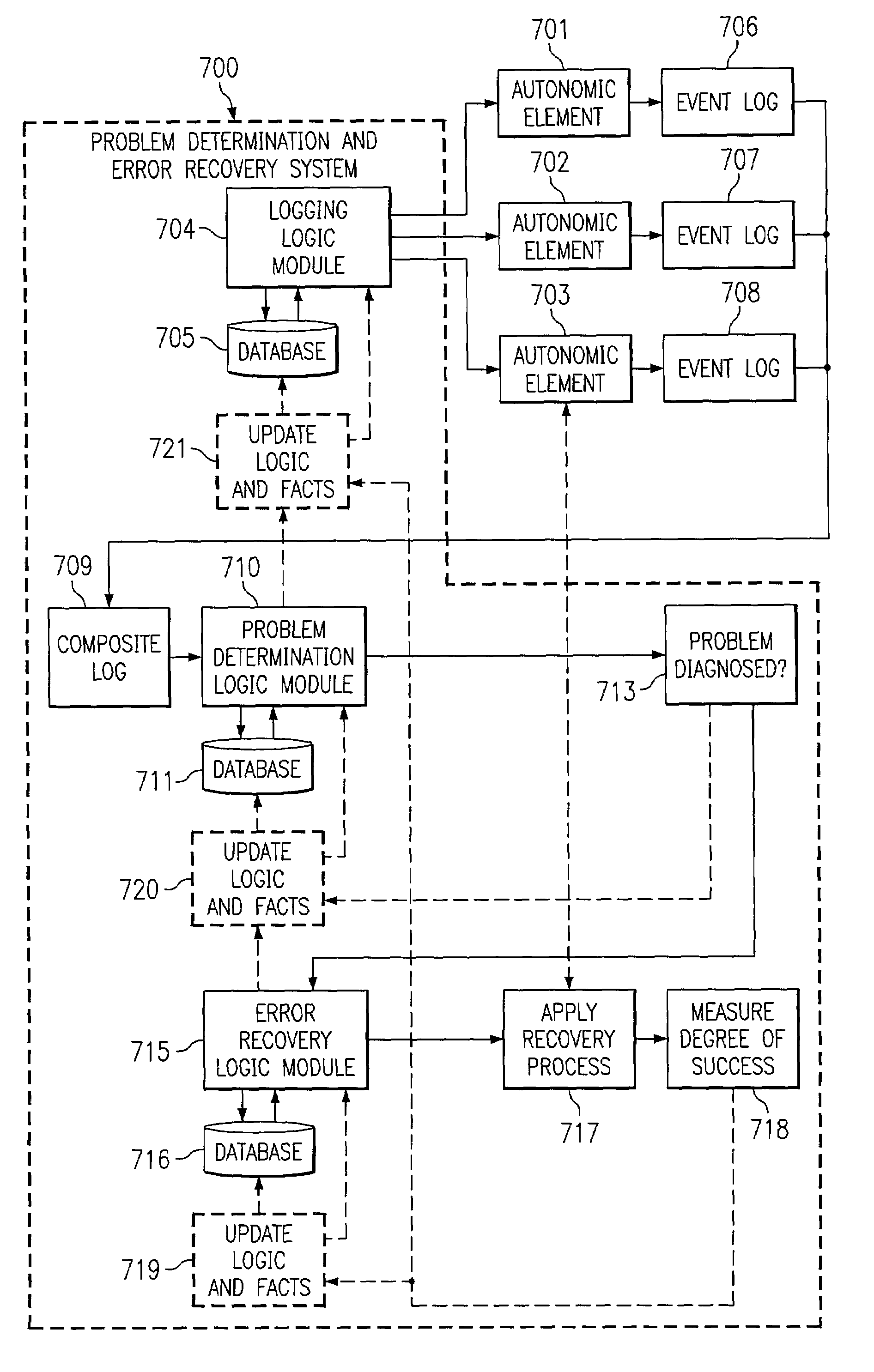
Adaptive problem determination and recovery in a computer system
InactiveUS7194445B2Reliability increasing modificationsError preventionSoftware engineeringSystem configuration
A method, computer program product, and data processing system for recognizing, tracing, diagnosing, and repairing problems in an autonomic computing system is disclosed. Rules and courses of actions to follow in logging data, in diagnosing faults (or threats of faults), and in treating faults (or threats of faults) are formulated using an adaptive inference and action system. The adaptive inference and action system includes techniques for conflict resolution that generate, prioritize, modify, and remove rules based on environment-specific information, accumulated time-sensitive data, actions taken, and the effectiveness of those actions. Thus, the present invention enables a dynamic, autonomic computing system to formulate its own strategy for self-administration, even in the face of changes in the configuration of the system.
Owner:LENOVO PC INT
Methods and apparatus for preparation and administration of training courses
ActiveUS6988239B2Electrical appliancesSpecial data processing applicationsComputer accessTraining course
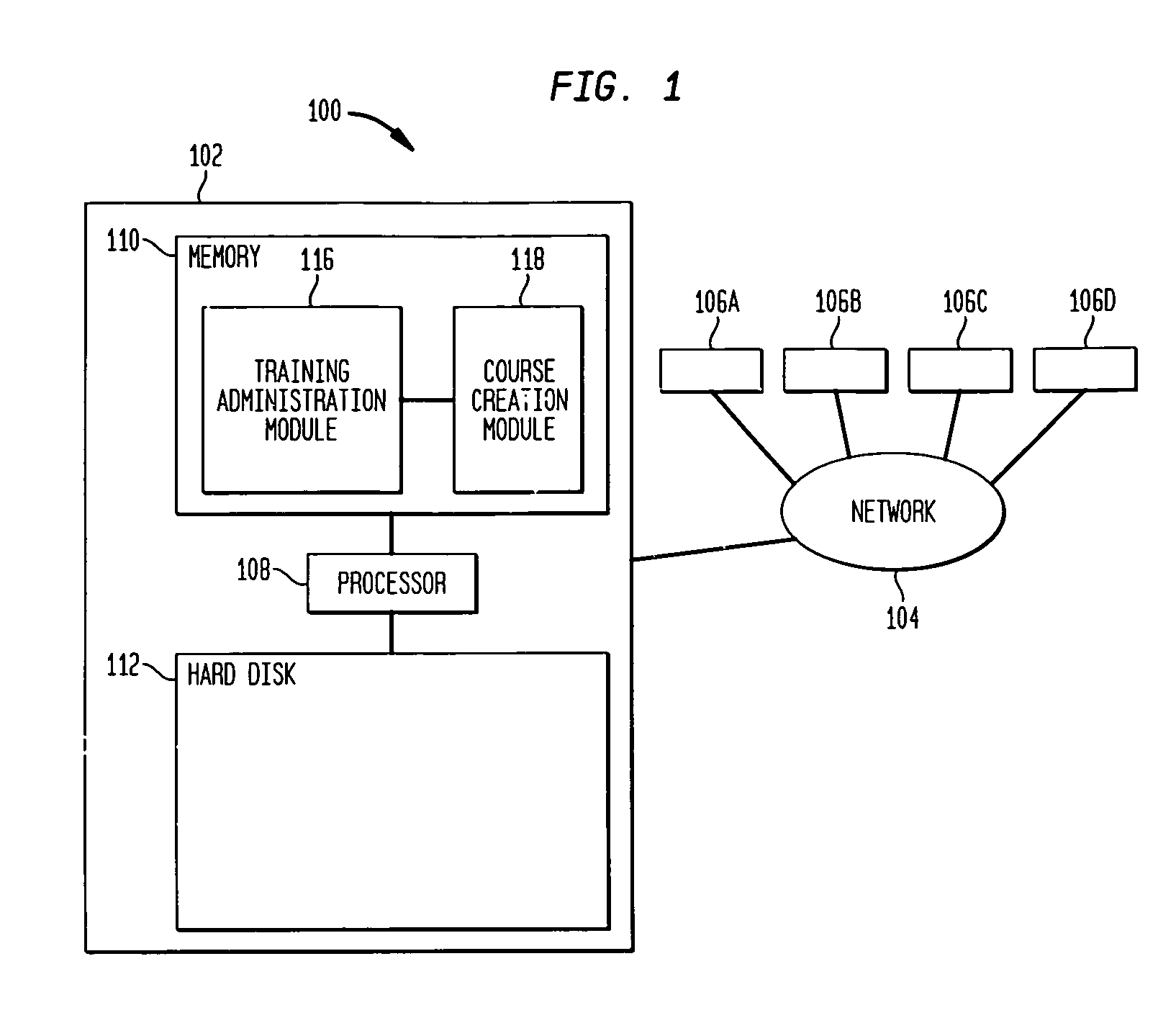
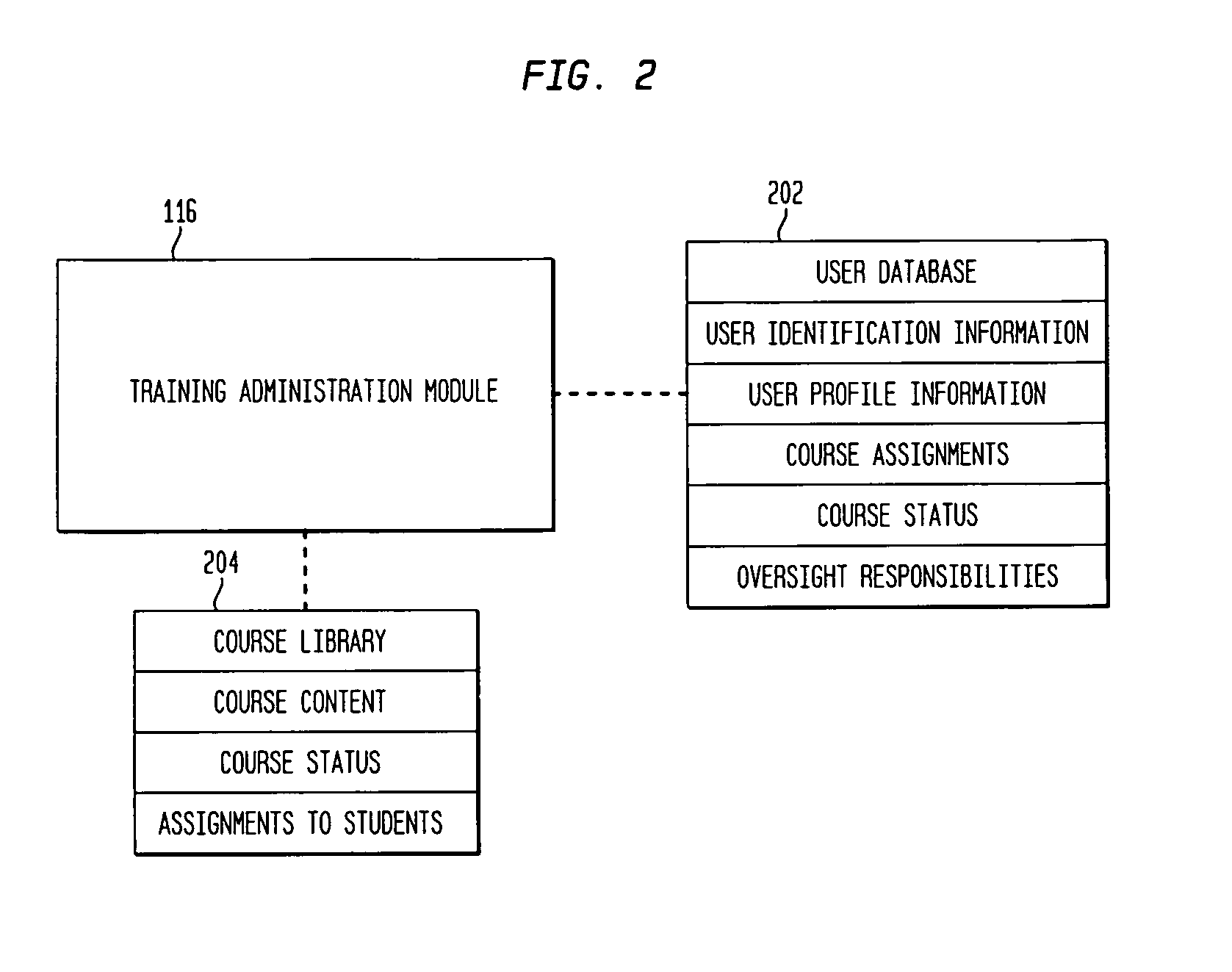
Techniques for creation and management of training courses are described. A system according to an aspect of the invention includes a central server accessible by one or more user computers. The central server hosts a training administration module which has access to a user database, a course library and a course creation module. The course creation module has access to a template database. The training administration module is able to retrieve information from the user database and the course library and to assign courses to students, to identify the status of students with respect to courses and of courses with respect to students, and to retrieve and display information relating to student progress in or completion of a course.
Owner:GE MORTGAGE HLDG
Controlled release metformin compositions
InactiveUS6866866B1Effective controlImprove bioavailabilityOrganic active ingredientsCoatingsCo administrationBlood plasma
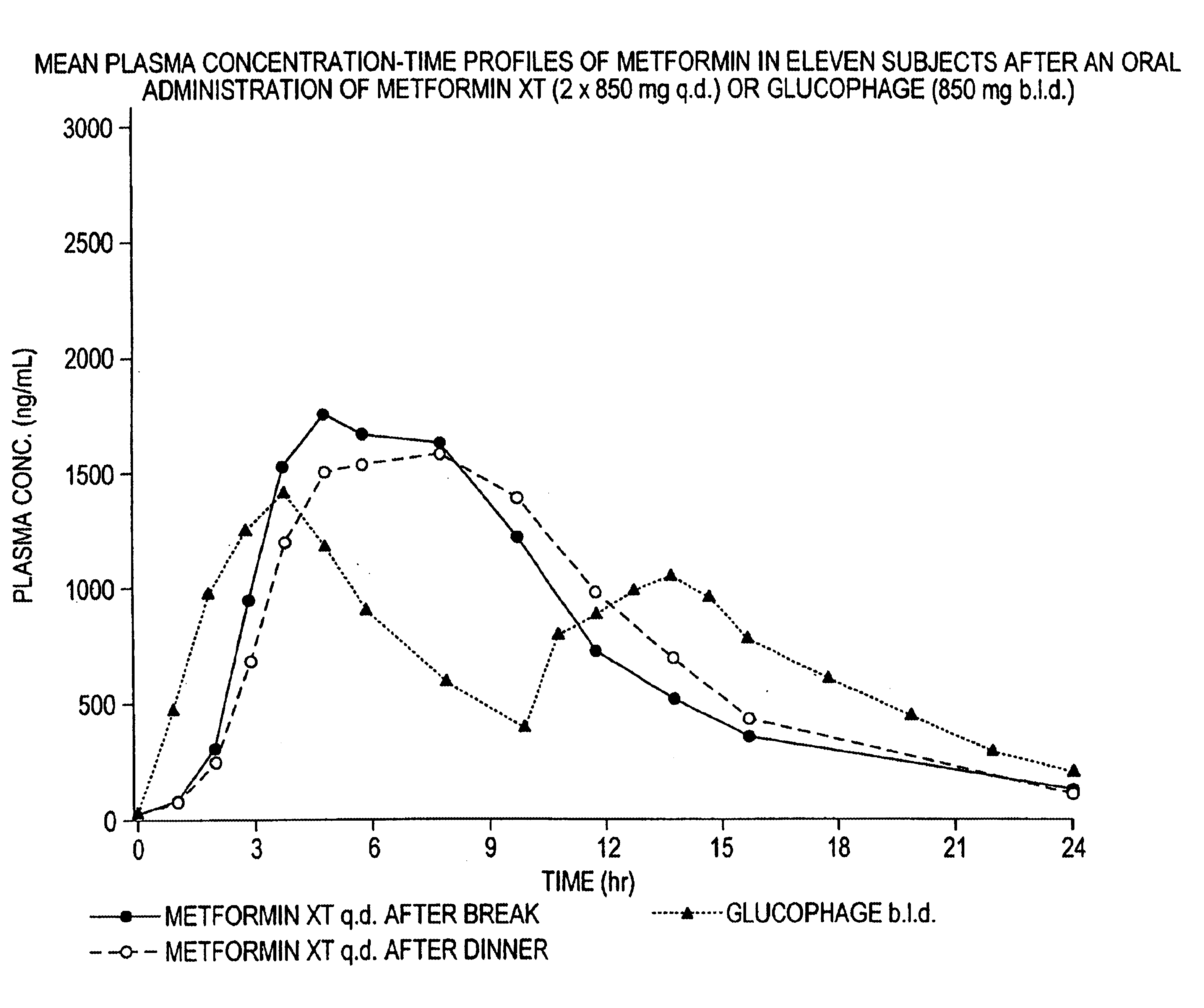
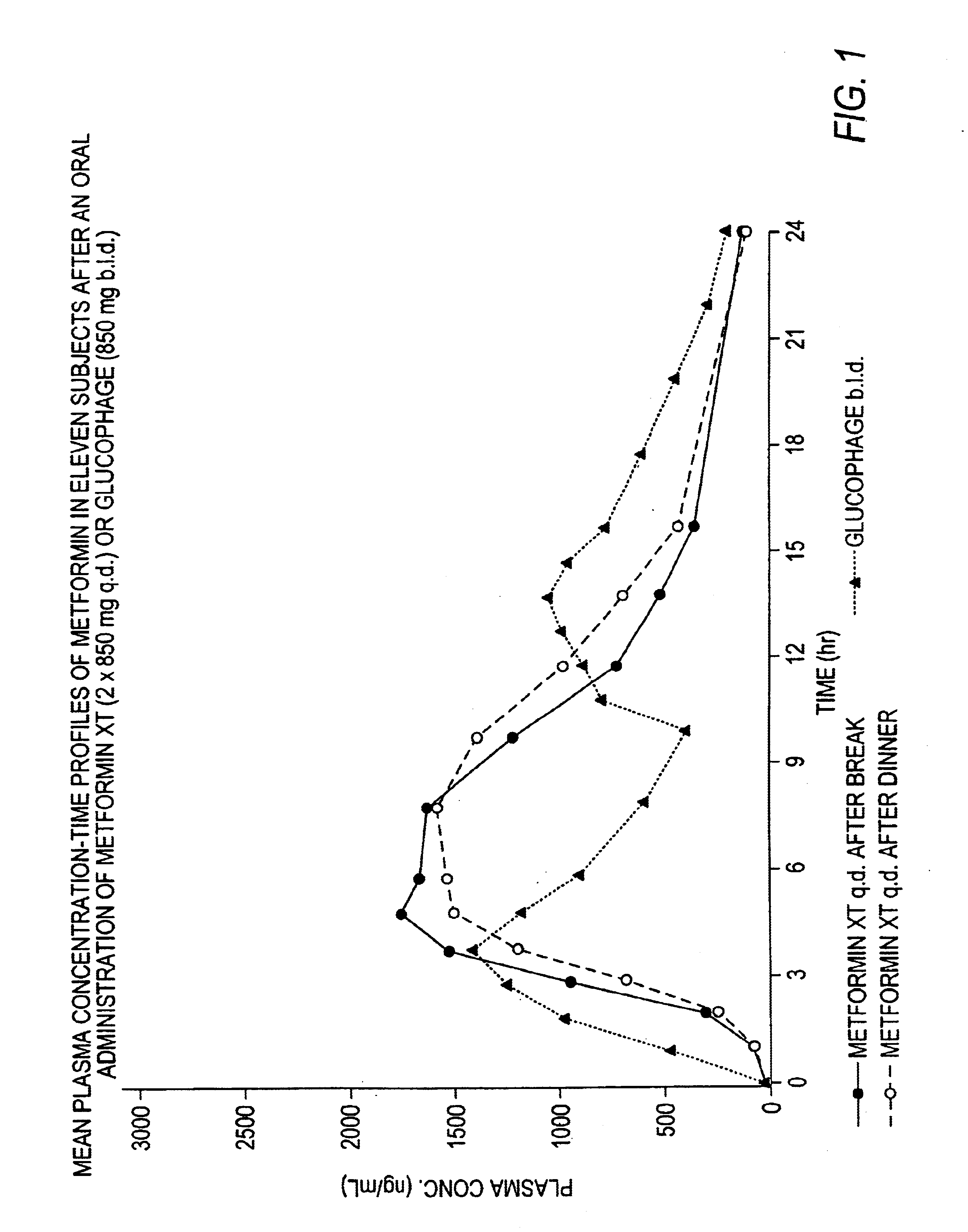
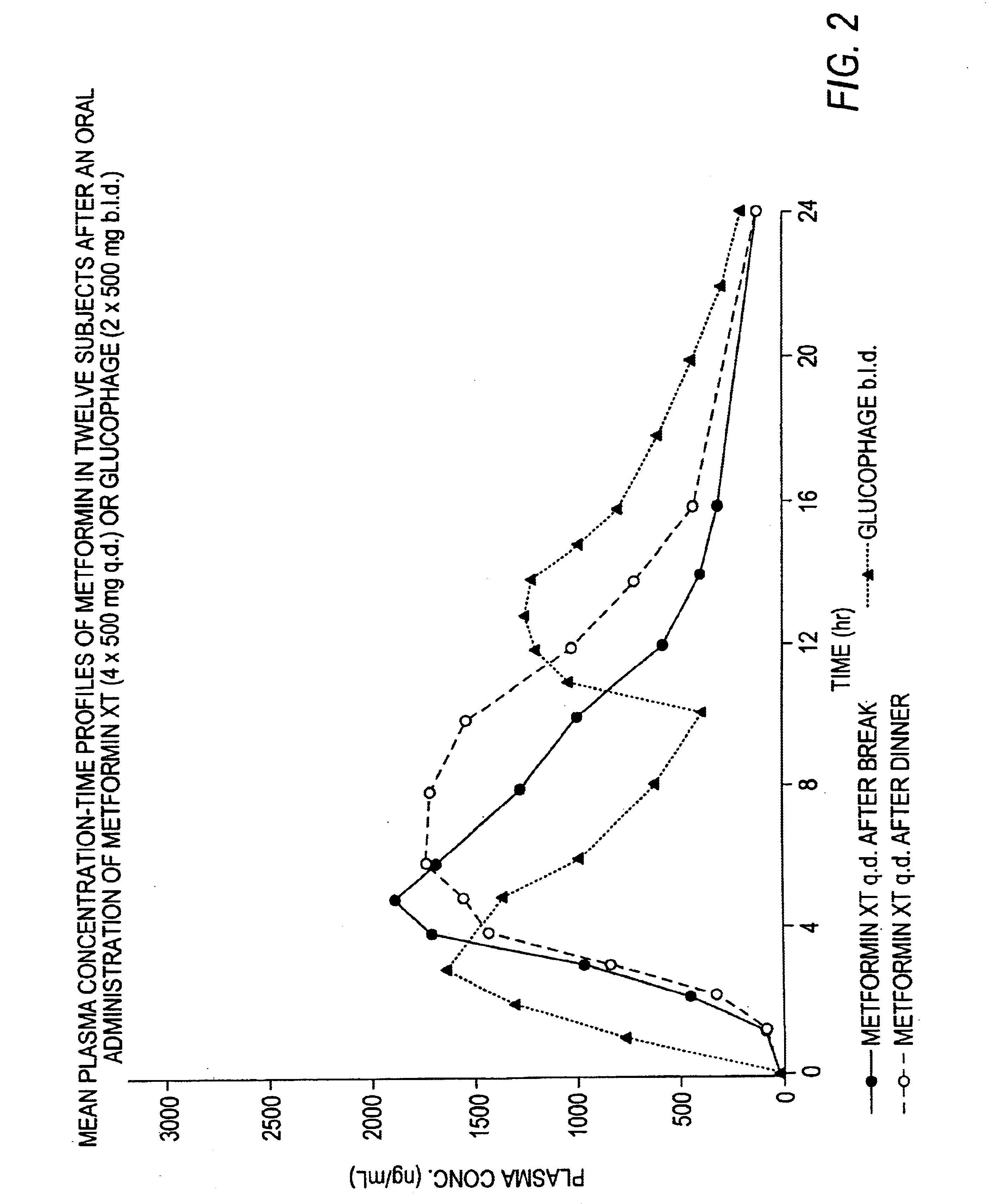
A composition for treating patients having non-insulin-dependent diabetes mellitus (NIDDM) by administering a controlled release oral solid dosage form containing preferably a biguanide drug such as metformin, on a once-a-day basis. The dosage form provides a mean time to maximum plasma concentration (Tmax) of the drug which occurs at 5.5 to 7.5 hours after oral administration on a once-a-day basis to human patients. Preferably, the dose of drug is administered at dinnertime to a patient in the fed state.
Owner:ANDRX LABS
Oral administration form for an acid liable active proton pump inhibitor
Novel administration form for acid-labile active compounds are described. The novel administration forms have no enteric layers and are suitable for oral administration.
Owner:TAKEDA GMBH
Intravenous Flow Rate Controller
Tilting of a drip chamber from its vertical axis during fluid administration can have negative effects upon the accuracy of systems configured for drop counting and / or for volumetric measurement of individual drops passing through the drip chamber. To address these negative effects, in accordance with one embodiment of the present disclosure, a fluid delivery system engages in a fluid control process that comprises determining an error parameter, based at least in part on a tilt signal, generating an error condition, and either holding the fluid flow at the present rate or stopping the flow.
Owner:ICU MEDICAL INC
Composition and method for inhibiting platelet aggregation
The present invention provides novel compounds of dinucleotide polyphosphates and the method of preventing or treating diseases or conditions associated with platelet aggregation. The method comprises administering systemically to a patient a pharmaceutical comprising a purinergic P2τ receptor antagonist, in an amount effective to elevate its extracellular concentration to bind to P2τ receptors and inhibit P2τ receptor-mediated platelet aggregation. Methods of systemic administration include injection by intravenous, intramuscular, intrasternal and intravitreal routes, infusion, transdermal administration, oral administration, rectal administration and intra-operative instillation.
Owner:INSPIRE PHARMA +1
Multimicroparticulate pharmaceutical forms for oral administration
InactiveUS20070264346A1Great therapeutic safetyGood effectOrganic active ingredientsPowder deliveryAlcohol freeMicroparticle
The object of the present invention is to minimize the risks of dose dumping associated with the concomitant consumption of alcohol and certain modified-release pharmaceutical or dietetic forms. The invention relates to an oral form comprising microparticles of the reservoir type for the modified release of at least one active principle (AP), characterized in that it is resistant to immediate dumping of the dose of AP in the presence of alcohol. In particular, the oral form according to the invention is characterized in that the time taken to release 50% of the AP in an alcoholic solution is not reduced more than 3-fold relative to the time taken to release 50% of the AP in an alcohol-free aqueous medium. The form comprises an agent D, which is a pharmaceutically acceptable compound whose hydration or solvation rate or capacity is greater in an alcohol-free aqueous medium than in alcoholic solution
Owner:FLAMEL IRELAND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com