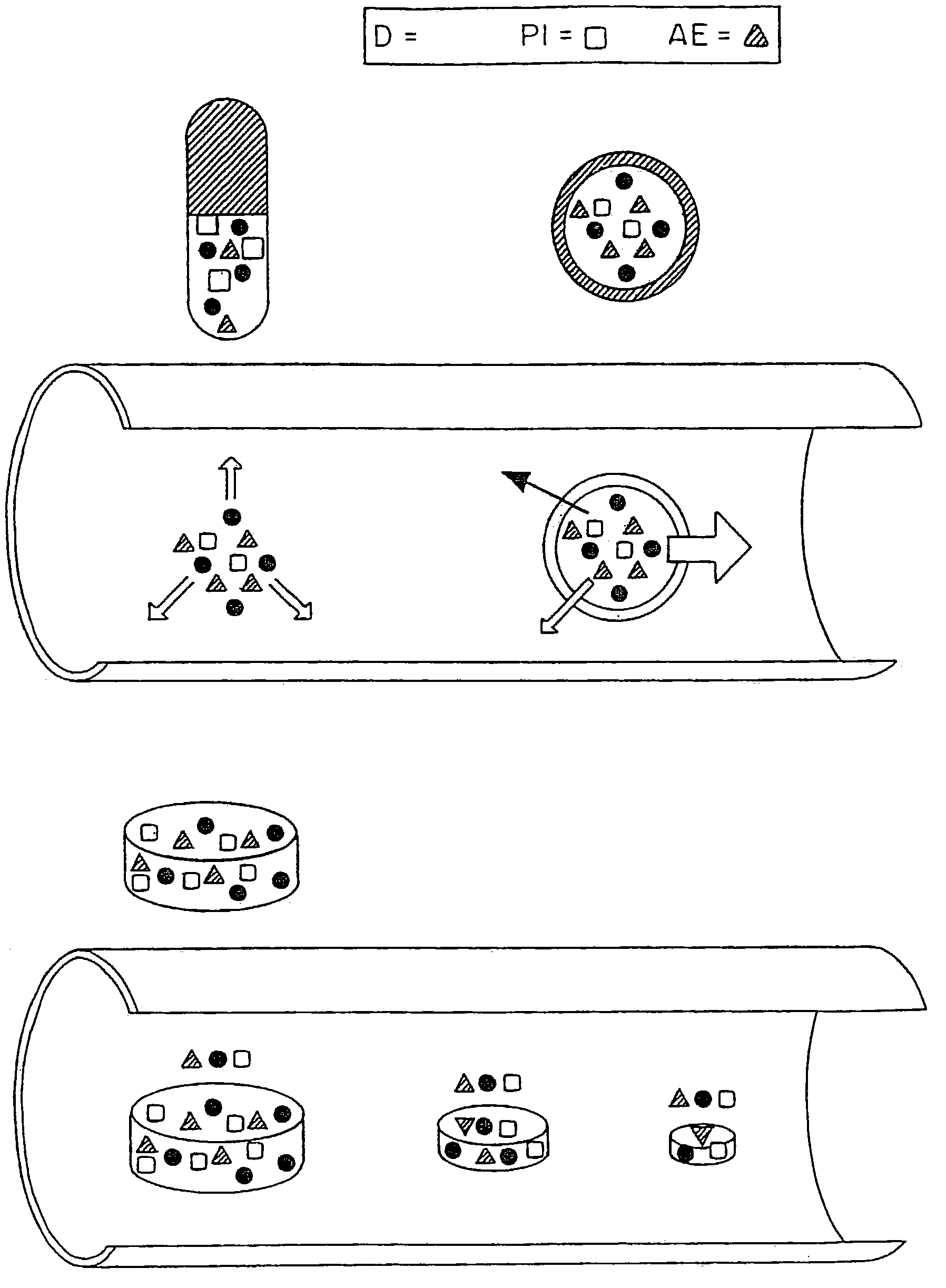
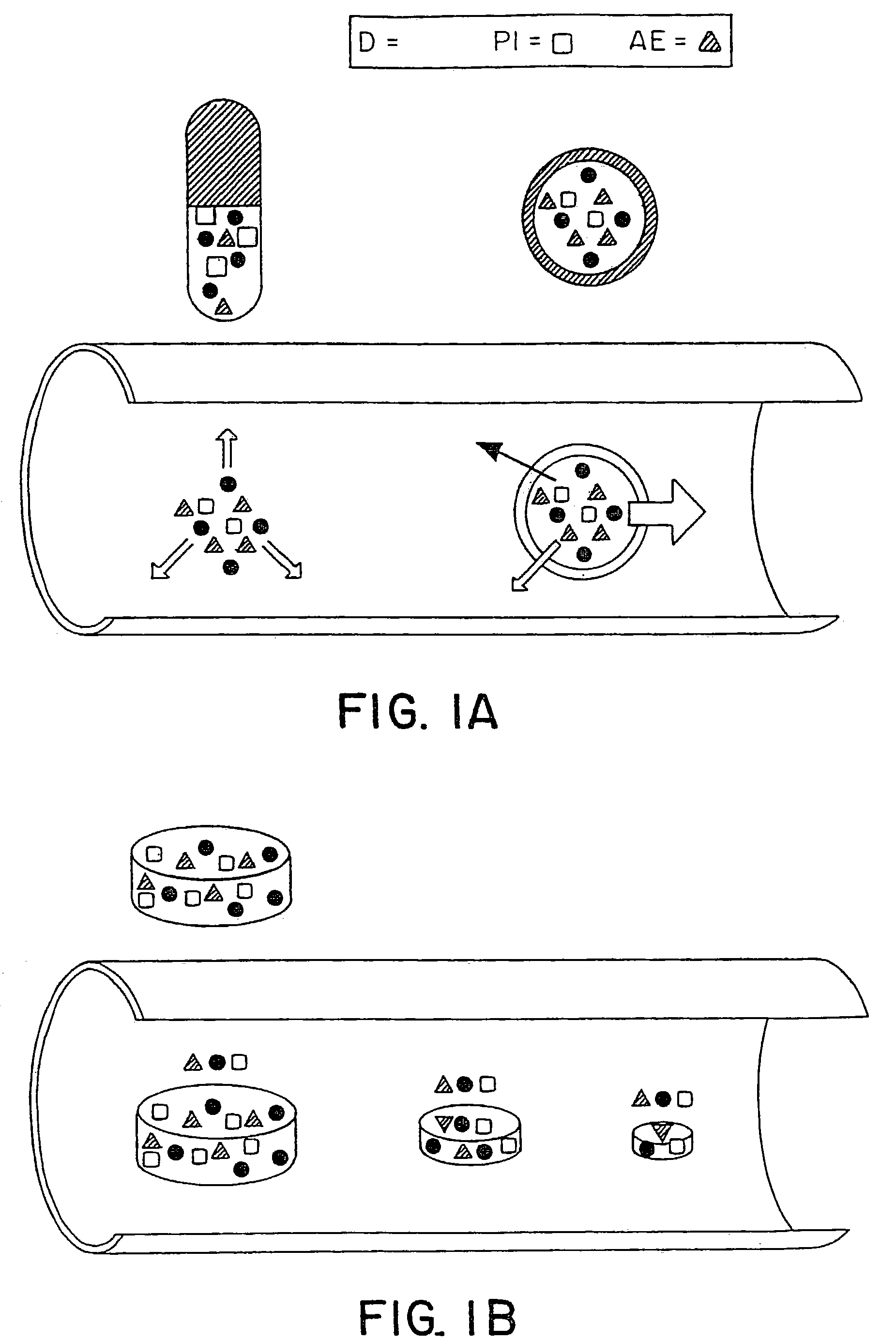
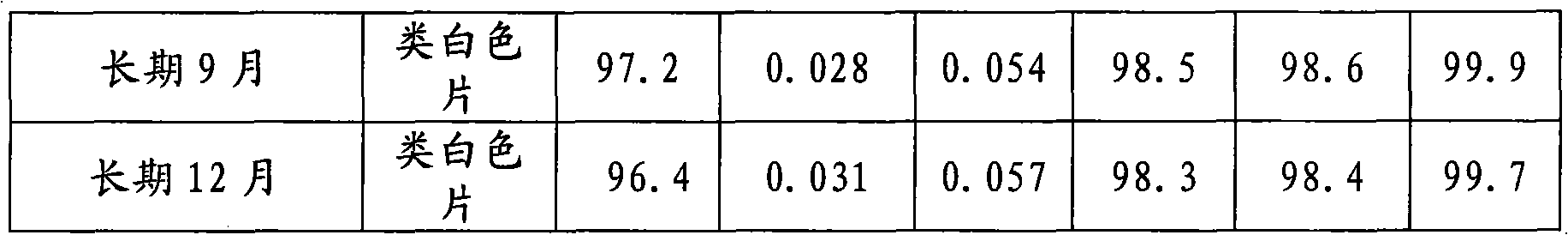
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
74 results about "Drug degradation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Forced degradation is a process that involves degradation of drug products and drug substances at conditions more severe than accelerated conditions and thus generates degradation products that can be studied to determine the stability of the molecule.
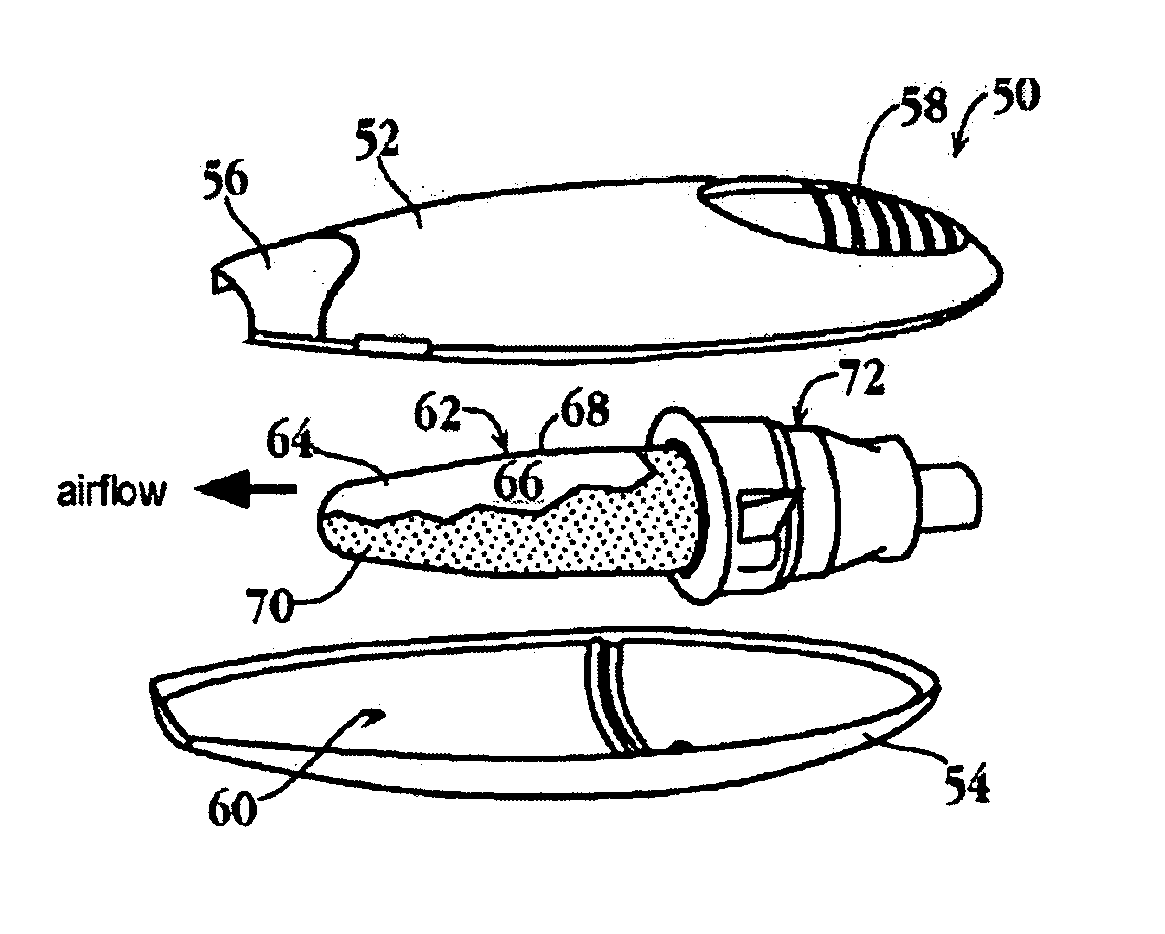
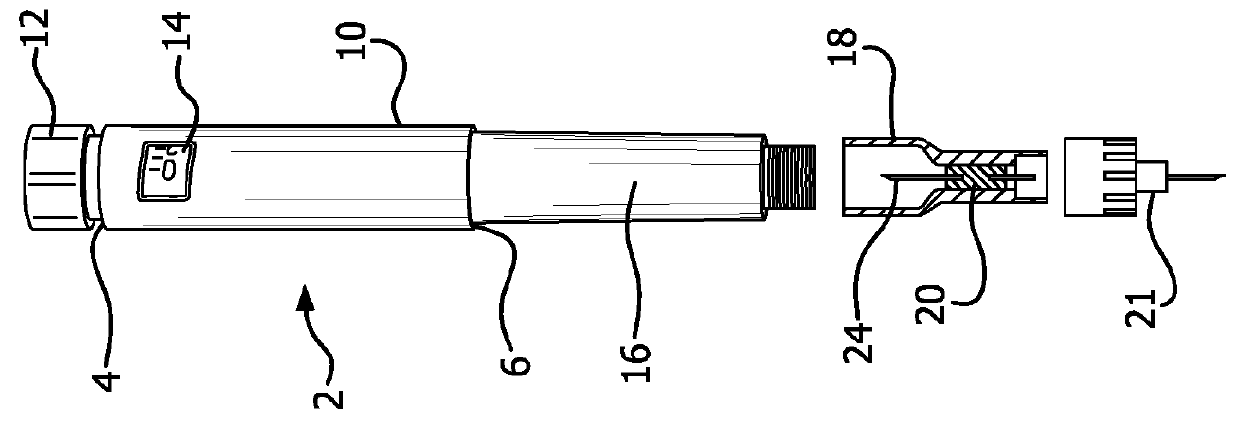
Substrates for drug delivery device and methods of preparing and use
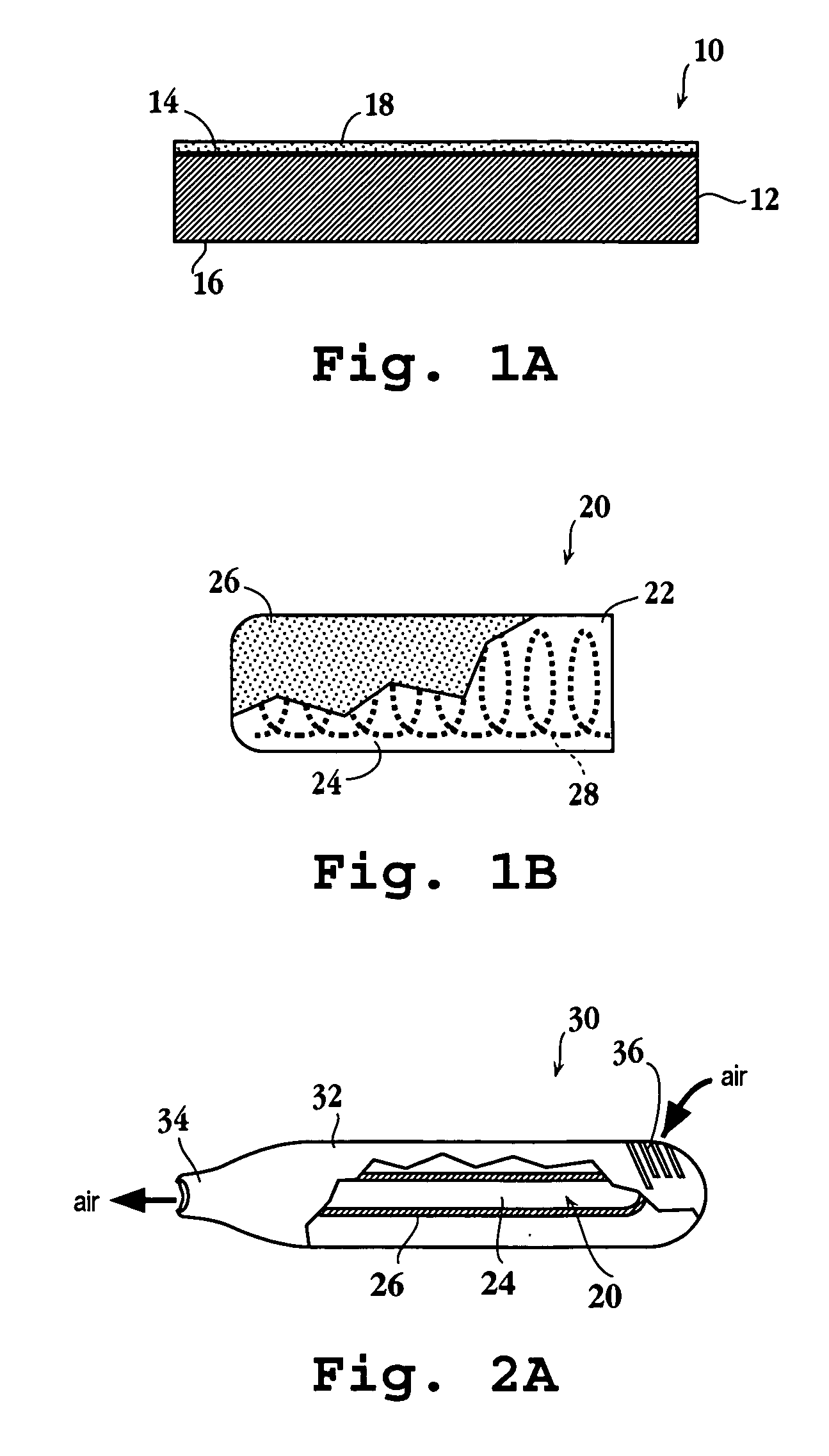
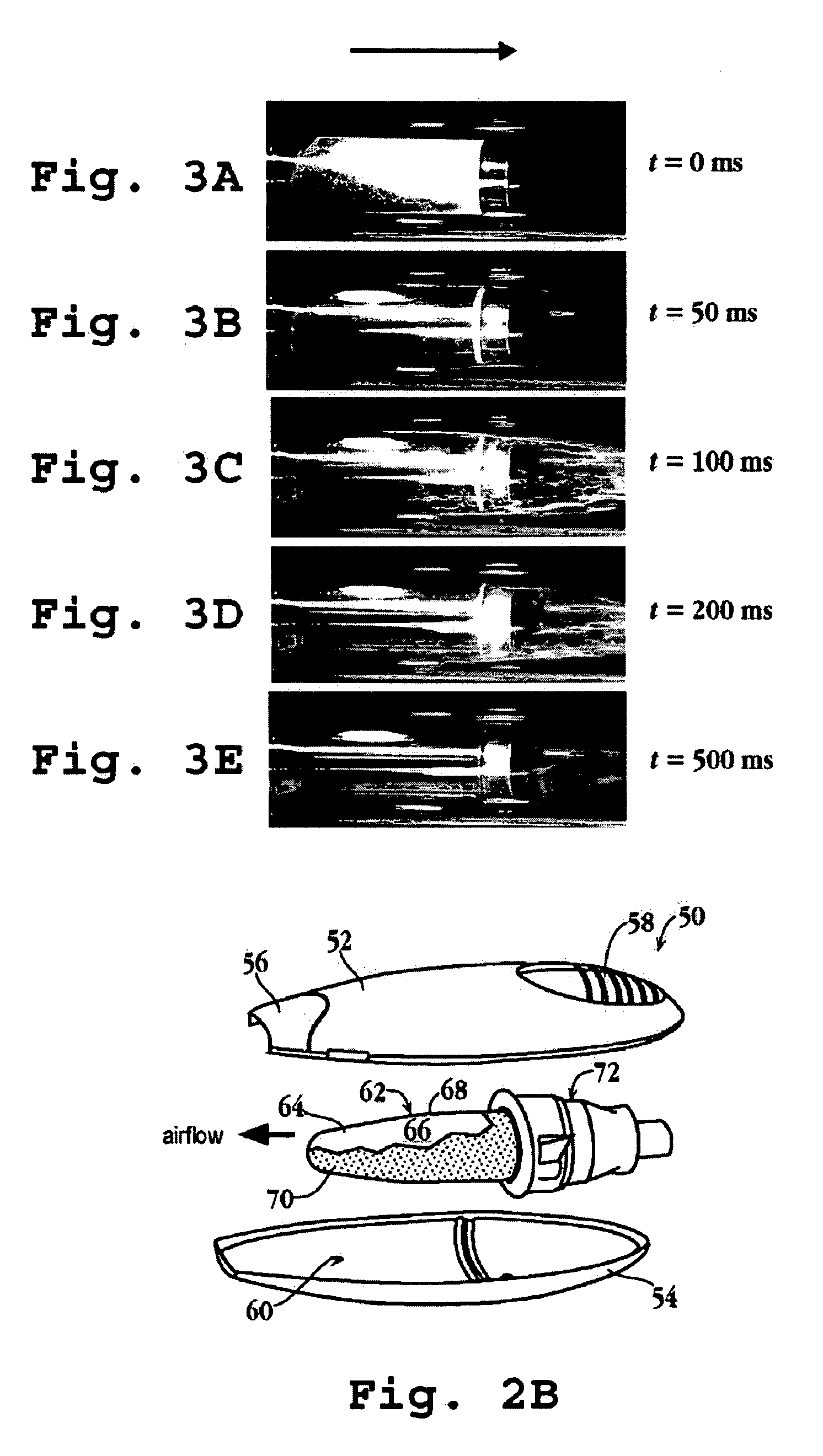
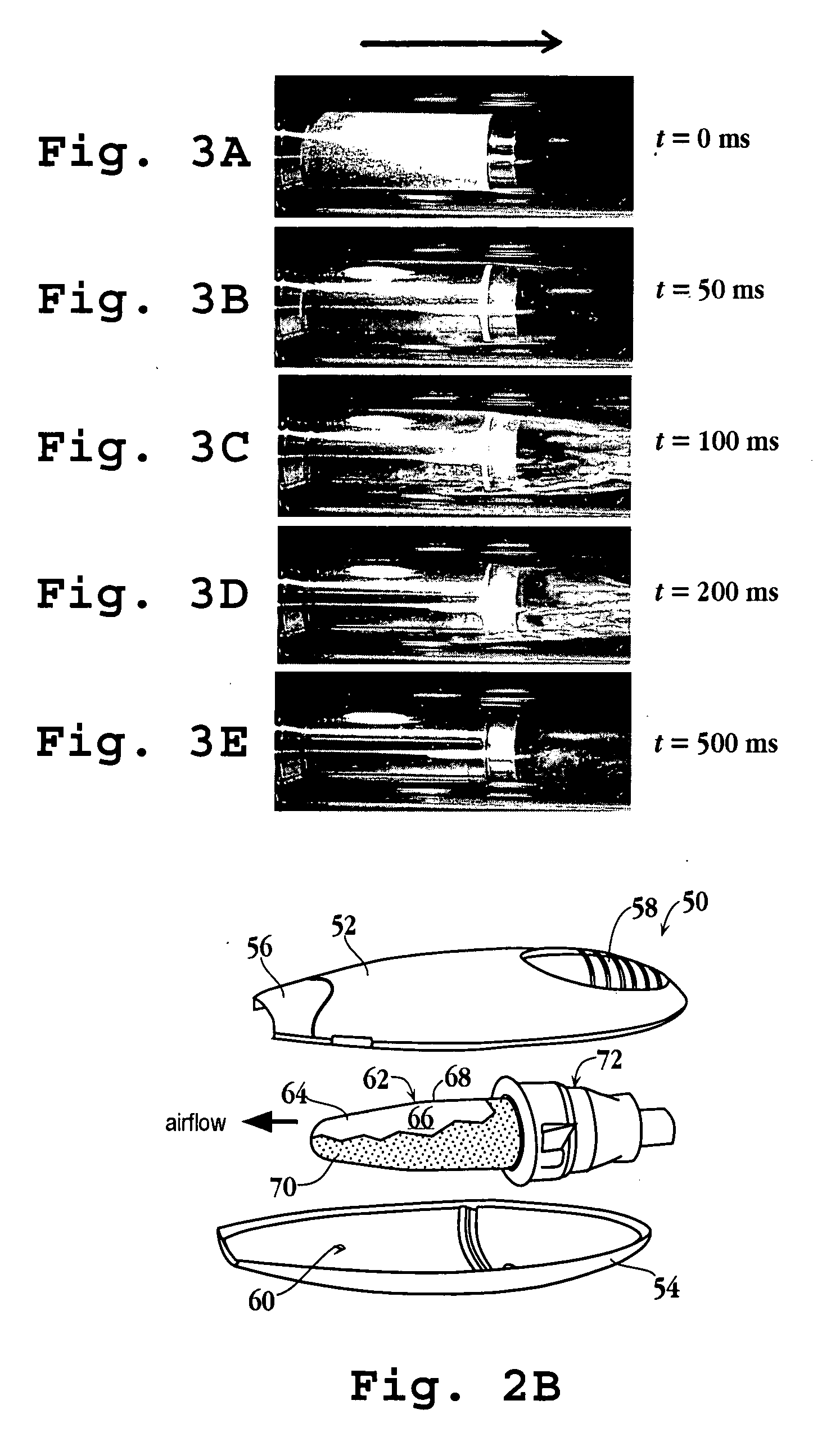
An assembly and method for producing a condensation aerosol are disclosed. The assembly includes a heat-conductive metal substrate with an oxidation resistant exterior surface and a drug composition film on the exterior surface and is for use in an aerosol device. The thickness of the film and the surface of the substrate is such that the aerosol formed by vaporizing and condensing the drug composition the aerosol contain 10% by weight or less drug-degradation products and at least 50% of the total amount of the drug composition in the film. The methods for treating the exterior surface include heat and chemical treatment and formation of a protective overcoat.
Owner:ALEXZA PHARMA INC
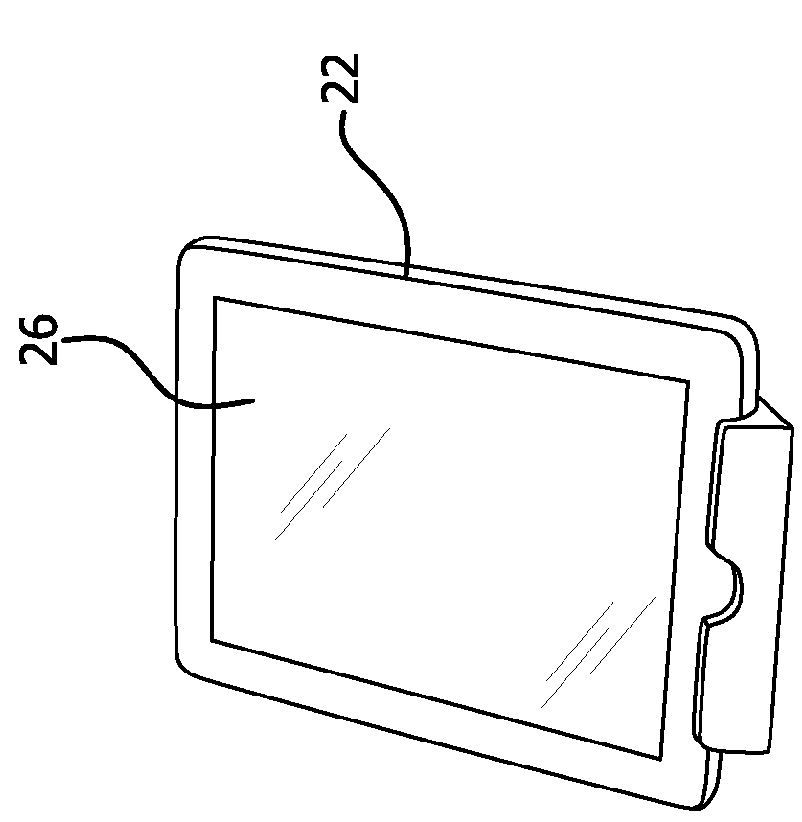
Smart adapter for infusion devices
ActiveUS20160030683A1Improve safety and efficacyInfusion syringesMicroneedlesPen InjectorDrug administration
Smart sensors are employed to determine one or more of drug identification, dose, flow rate, concentration, agglomeration, and degradation and / or other characteristics of drug administration that can be detected via sensing technology. A smart sensor(s) can be coupled to or retrofitted onto injection pen injectors and / or drug delivery cartridges and / or infusion sets or cannulae, enabling infusion sets, pen injector systems or drug delivery cartridges to improve tracking of drug self-administration and stop medication errors that occur primarily through self or automated injection (e.g., due to incorrect or incomplete dosing, excessive dose or rate, incorrect drug, or drug degradation).
Owner:BECTON DICKINSON & CO
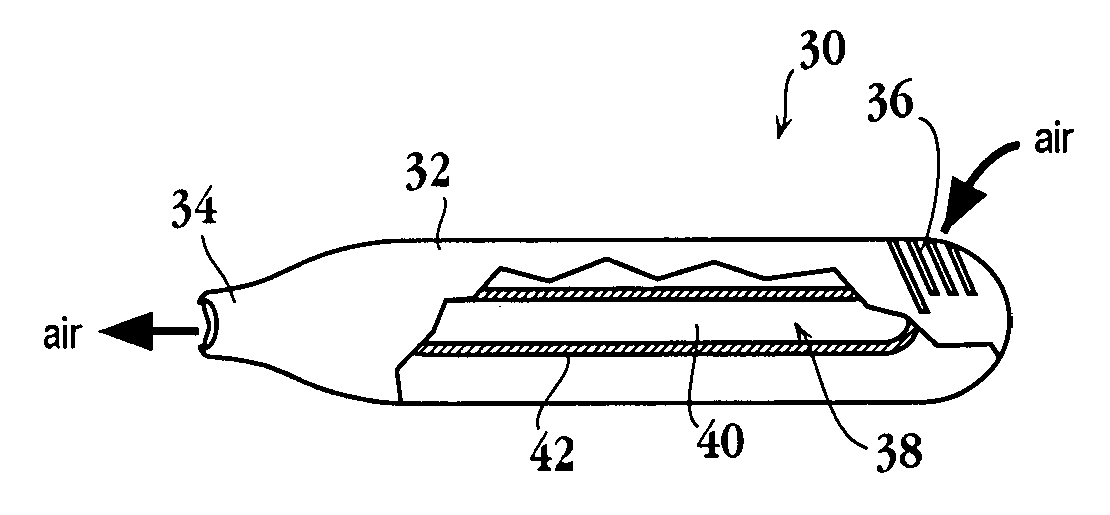
Thin-film drug delivery article and method of use
InactiveUS20070031340A1Reduce the amount requiredFast evaporationRespiratorsPowder deliveryDrug aerosolMedicine
An article for use in an aerosol device, for producing an aerosol of a drug composition is disclosed. The article includes a heat-conductive substrate having a surface with a selected surface area, and a drug composition film on the substrate surface having a selected film thickness of between 0.05 and 20 μm. The film thickness is such that an aerosol formed by vaporizing the drug composition by heating the substrate and condensing the vaporized compound contains 10% or less drug-degradation product and at least 50% of the total amount of drug composition contained in the film. The selected substrate surface area is such as to yield an effective human therapeutic dose of the drug aerosol. Also disclosed are methods of making and using the article.
Owner:ALEXZA PHARMA INC
Delivery of diazepam through an inhalation route
The present invention relates to the delivery of compounds for the treatment of anxiety disorders and symptoms of such disorders through an inhalation route. Specifically, it relates to aerosols containing that are used in inhalation therapy. In a method aspect of the present invention, diazepam is administered to a patient through an inhalation route. The method comprises: a) heating a composition, comprising diazepam to form a vapor; and, b) allowing the vapor to cool, thereby forming a condensation aerosol with less than 5% of the drug degradation products. In a kit aspect of the present invention, a kit for delivering diazepam through an inhalation route is provided which comprises: a) a thin coating of a diazepam composition; and, b) a device for dispending said thin coating as a condensation aerosol
Owner:ALEXZA PHARMA INC
Thin-film drug delivery article and method of use
InactiveUS7585493B2Improve the level ofReduce the amount requiredRespiratorsPowder deliveryDrug aerosolMedicine
An article for use in an aerosol device, for producing an aerosol of a drug composition is disclosed. The article includes a heat-conductive substrate having a surface with a selected surface area, and a drug composition film on the substrate surface having a selected film thickness of between 0.05 and 20 μm. The film thickness is such that an aerosol formed by vaporizing the drug composition by heating the substrate and condensing the vaporized compound contains 10% or less drug-degradation product and at least 50% of the total amount of drug composition contained in the film. The selected substrate surface area is such as to yield an effective human therapeutic dose of the drug aerosol. Also disclosed are methods of making and using the article.
Owner:ALEXZA PHARMA INC
Delivery of diazepam through an inhalation route
The present invention relates to the delivery of compounds for the treatment of anxiety disorders and symptoms of such disorders through an inhalation route. Specifically, it relates to aerosols containing diazepam that are used in inhalation therapy. In a method aspect of the present invention, diazepam is administered to a patient through an inhalation route. The method comprises: a) heating a thin layer of diazepam on a solid support to form a vapor; and, b) passing air through the heated vapor to produce aerosol particles having less than 5% drug degradation products. In a kit aspect of the present invention, a kit for delivering diazepam through an inhalation route is provided which comprises: a) a thin coating of a diazepam composition; and, b) a device for dispending said thin coating as a condensation aerosol.
Owner:ALEXZA PHARMA INC
Use of antioxidants to prevent oxidation and reduce drug degradation in drug eluting medical devices
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. The drugs, agents, and / or compounds may also be utilized to treat specific diseases, including vulnerable plaque. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices. In each of these instances, antioxidants are utilized to prolong product integrity.
Owner:WYETH LLC
Controlled release oral drug delivery system
The invention relates to synchronous drug delivery composition comprising a polymeric matrix which comprises hydrogel blended with a hydrophobic polymer, so as to form an erodible matrix, a drug, and, optionally, an agent which enhances intestinal drug absorption and / or an agent which inhibits intestinal drug degradation,wherein erosion of the erodible matrix, permits synchronous release of the drug, the hydrogel and the intestinal drug absorption agent and / or the agent which inhibits intestinal drug degradation.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
Delivery of diazepam through an inhalation route
The present invention relates to the delivery of compounds for the treatment of anxiety disorders and symptoms of such disorders through an inhalation route. Specifically, it relates to aerosols containing diazepam that are used in inhalation therapy. In a method aspect of the present invention, diazepam is administered to a patient through an inhalation route. The method comprises: a) heating a thin layer of diazepam on a solid support to form a vapor; and, b) passing air through the heated vapor to produce aerosol particles having less than 5% drug degradation products. In a kit aspect of the present invention, a kit for delivering diazepam through an inhalation route is provided which comprises: a) a thin coating of a diazepam composition; and, b) a device for dispending said thin coating as a condensation aerosol.
Owner:ALEXZA PHARMA INC
Alkylated cyclodextrin compositions and processes for preparing and using the same
The present invention related to low-chloride alkylated cyclodextrin compositions, along with processes for preparing and using the same. The processes of the present invention provide alkylated cyclodextrins with low levels of drug-degrading agents and chloride.
Owner:CYDEX PHARMACEUTICALS INC
Extrusion process for forming chemically stable drug multiparticulates
ActiveUS7625507B2Reducing drug degradation of drugImprove physical propertiesAntibacterial agentsPowder deliveryExtrusionDrug degradation
Owner:LONZA BEND INC
Extrusion process for forming chemically stable drug multiparticulates
ActiveUS20050181061A1Reducing drug degradation of drugImprove physical propertiesAntibacterial agentsPowder deliveryBiochemistryPharmaceutical Substances
Owner:LONZA BEND INC
Alkylated cyclodextrin compositions and processes for preparing and using the same
The present invention related to low-chloride alkylated cyclodextrin compositions, along with processes for preparing and using the same. The processes of the present invention provide alkylated cyclodextrins with low levels of drug-degrading agents and chloride.
Owner:CYDEX PHARMACEUTICALS INC
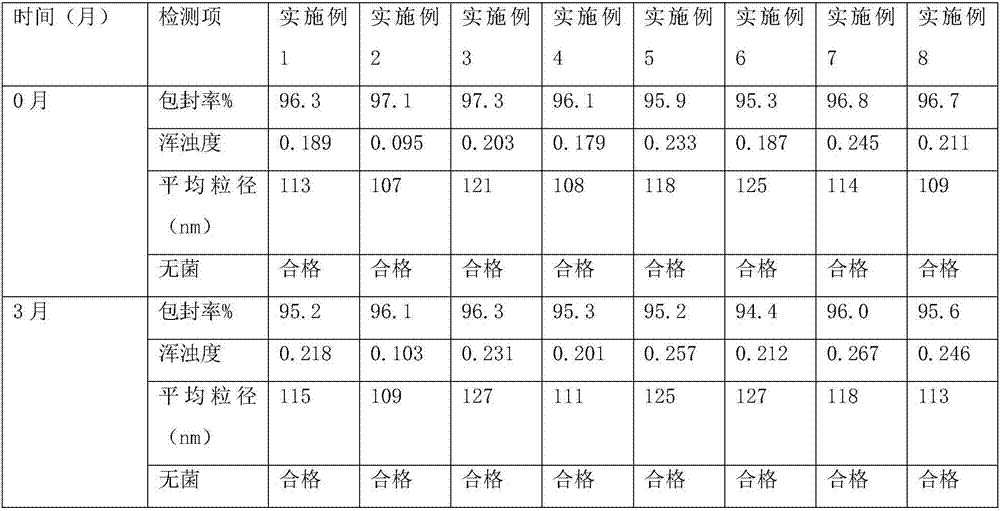
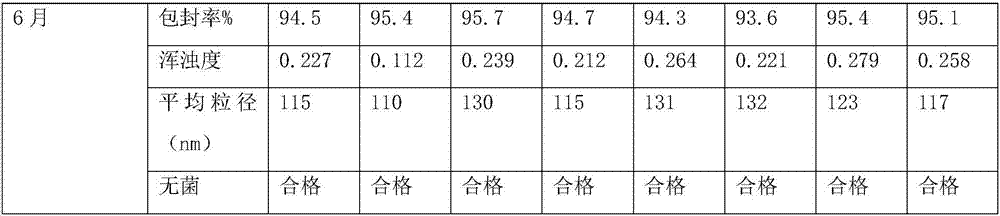
Nucleic acid lipid nano particle composition, pharmaceutical preparation containing same, and preparation method and application thereof
PendingCN112843019AImprove stabilityImprove bioavailabilityOrganic active ingredientsMacromolecular non-active ingredientsPharmaceutical formulationOrganic chemistry
The invention belongs to the field of biological medicine, and particularly relates to a nucleic acid lipid nano particle composition, a pharmaceutical preparation containing the same, and a preparation method and application thereof. The nucleic acid lipid nano particle composition comprises drug-loaded lipid nano particles and auxiliary materials, the mass ratio of the drug-loaded lipid nano particles to the auxiliary materials is (0.1-10): (90-99.9), the drug-loaded lipid nano particles comprise nucleic acid drugs and lipid carriers, the mass ratio of the nucleic acid drugs to the lipid carriers is 1: (2-30), the composition is good in stability, the nucleic acid drugs are not degraded due to too high temperature, the composition can be stored and transported at normal temperature, can be administered in various ways, and are quick in effect taking and high in bio-availability, large-scale production can be realized, the yield is high, and the repeatability is good.
Owner:SUZHOU CUREMED BIOMEDICAL TECH CO LTD
Topiramate sustained release preparation pharmaceutical composition
InactiveCN105326814AStable and effective plasma concentrationReduce incidenceOrganic active ingredientsNervous disorderAdhesiveTopiramate
The invention relates to a topiramate sustained release preparation pharmaceutical composition and a preparation method thereof. The topiramate sustained release preparation pharmaceutical composition is prepared from sustained release particles and preparation auxiliary materials; and the sustained release particles consist of 35%-45% of topiramate, 40%-50% of filler, 2.5%-6% of adhesives, 2%-10% of stabilizer, 5.0%-6.5% of sustained release coating materials, 2.0%-3.0% of porogen materials and 1%-2% of plasticizer. By the stabilizer, stability of the preparation can be improved obviously, and degradation of medicines is reduced. After a patient orally takes the topiramate sustained release preparation, stable and effective blood concentration can be maintained, occurrence rate of side reaction is reduced, and epilepsy is controlled well. In addition, the patient orally takes the preparation once every day, and compliance of the patient is greatly improved. Granulation is implemented at one step by a fluidized bed, special technologies are not required, and industrial production is facilitated.
Owner:BEIJING VENTUREPHARM BIOTECH
Solid preparation of clopidogrel and preparation method thereof
ActiveCN102106809AAvoid influenceImprove stabilityOrganic active ingredientsPharmaceutical delivery mechanismClopidogrelPharmaceutical formulation
The invention belongs to the field of medicinal preparations, and relates to a solid preparation and a preparation method thereof, in particular to a solid preparation of clopidogrel or pharmaceutically acceptable salt of the clopidogrel as well as a preparation method thereof. The solid preparation is mainly prepared by mixing the clopidogrel or the pharmaceutically acceptable salt thereof with dimeticone and then processing the obtained mixture according to a conventional solid preparation processing method. By adopting the dimeticone, the invention has the advantages of preventing a dextroisomer of the clopidogrel from converting into a levoisomer, preventing the clopidogrel from being degraded into clopidogrel acid, being applicable to the formula and process for large-scale production of the preparation, solving the problem of drug degradation, improving drug stability, solving sticking and picking phenomena during the tabletting process, and improving product quality.
Owner:QILU PHARMA
Smart adapter for infusion devices
ActiveUS10398852B2Improve safety and efficacyMicroneedlesMedical devicesPen InjectorDrug administration
Owner:BECTON DICKINSON & CO
Sewage mixing treatment device
InactiveCN110790429AStir wellWell mixedSpecific water treatment objectivesTreatment involving filtrationDrug additivePharmaceutical drug
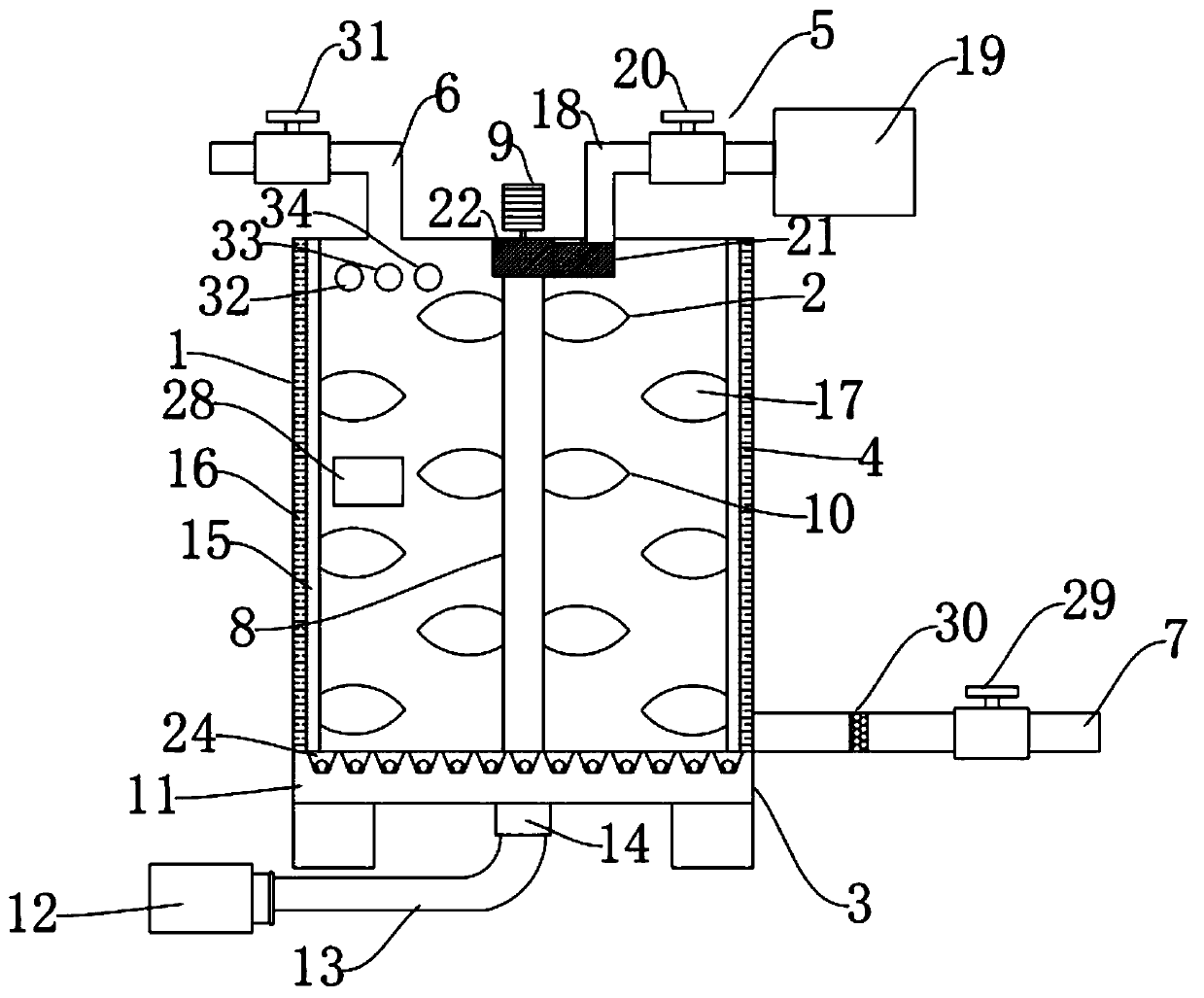
The invention discloses a sewage m .ing treatment device, and belongs to the technical filed of sewage treatment. The sewage mixing treatment device comprises a mixing box, a centrifugal stirring device, a pneumatic mixing device, an inner wall stirring device, a drug adding device, a sewage inlet pipe and a sewage outlet pipe; and the centrifugal stirring device is arranged in the mixing box, thepneumatic mixing device is arranged on the bottom wall of the mixing box, the inner wall stirring device is arranged on the inner wall of the mixing box, the drug adding device and the sewage inlet pipe are arranged at the top of the mixing box, and the sewage outlet pipe is arranged at the bottom of the side wall of the mixing box. According to the sewage mixing treatment device, sewage and a drug additive are stirred and mixed through centrifugal stirring, drug sedimentation and adhesion are avoided through the airflow impact and inner wall cleaning and brushing effects, by adding air and increasing the temperature, the efficiency of degrading impurities through the drug is improved, the sewage and the drug are fully mixed by adopting multiple procedures and devices for increasing the mixing degree of the sewage and the drug in the mode of drug administration at time intervals, and then the sewage purifying effect is improved.
Owner:广州诚毅科技咨询有限公司
Nanometer lipid emulsion
InactiveCN107441044AImprove stabilitySafe useEmulsion deliveryOil/fats/waxes non-active ingredientsSolubilitySide effect
The invention relates to a nanometer lipid emulsion which comprises a drug, oil, an emulgator and water, wherein the solubility of the drug is 0-0.0005g / ml and the drug is difficult to dissolve in water. The emulsion is clear and is capable of reducing the drug degradation and the emulsion droplet agglutination caused by ultrahigh pressure and high temperature. The drug is capable of being stably packed in the emulsion droplet, so that the toxic or side effect of the drug is reduced.
Owner:FUBICHENG SHANGHAI PHARMA TECH CO LTD
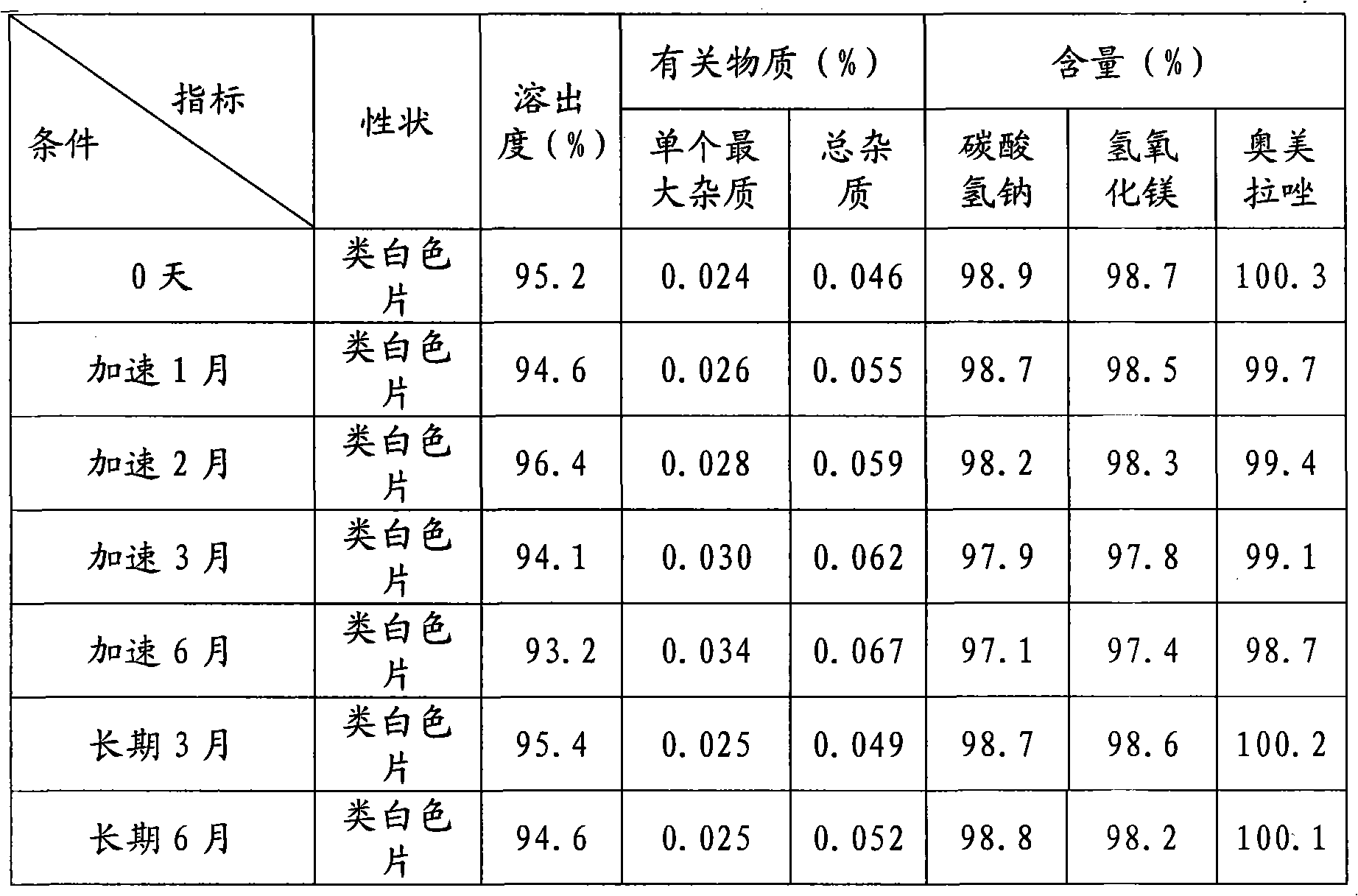
Compound Omeprazole tablets and preparation method thereof
InactiveCN102335148ASimple methodImprove stabilityOrganic active ingredientsDigestive systemSodium bicarbonateWater soluble
The invention discloses compound Omeprazole tablets, comprising the following ingredients: 20-40 weight portions of Omeprazole, 500-1000 weight portions of sodium bicarbonate, 200-652 weight portions of magnesium hydroxide, 10-30 weight portions of opacifier, 20-40 weight portions of water-soluble polymer material, 20-40 weight portions of disintegrating agent, and 5-10 weight portions of lubricant. The dissolution time of Omeprazole in the compound Omeprazole tablets is slightly lagged, so that antacid is dissolved in advance to neutralize the gastric acid, and the purposes of reducing drug degradation, gastricsolubility and quick release are achieved; and simultaneously, the light stability is increased, and the production operation and storage is convenient.
Owner:重庆健能医药开发有限公司
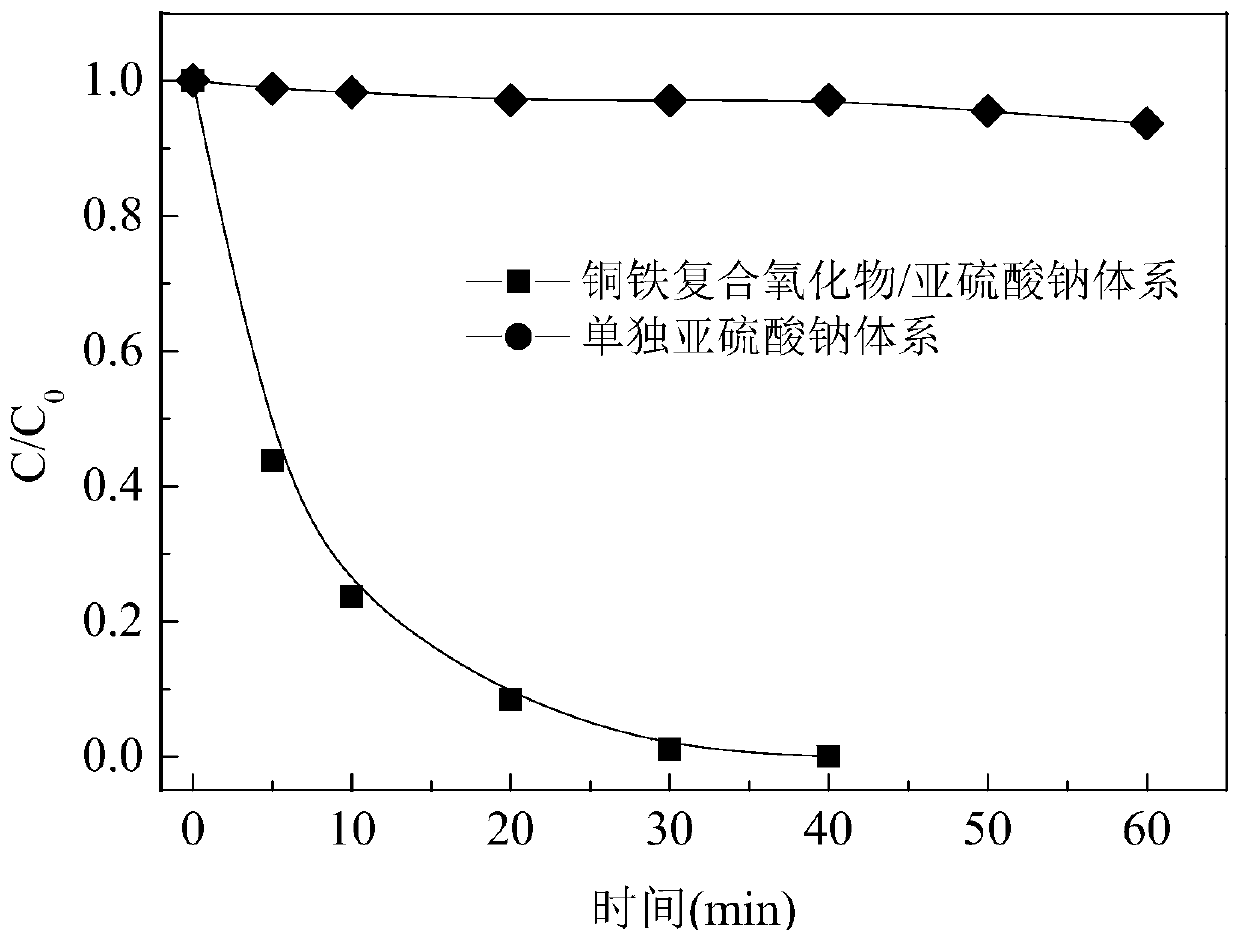
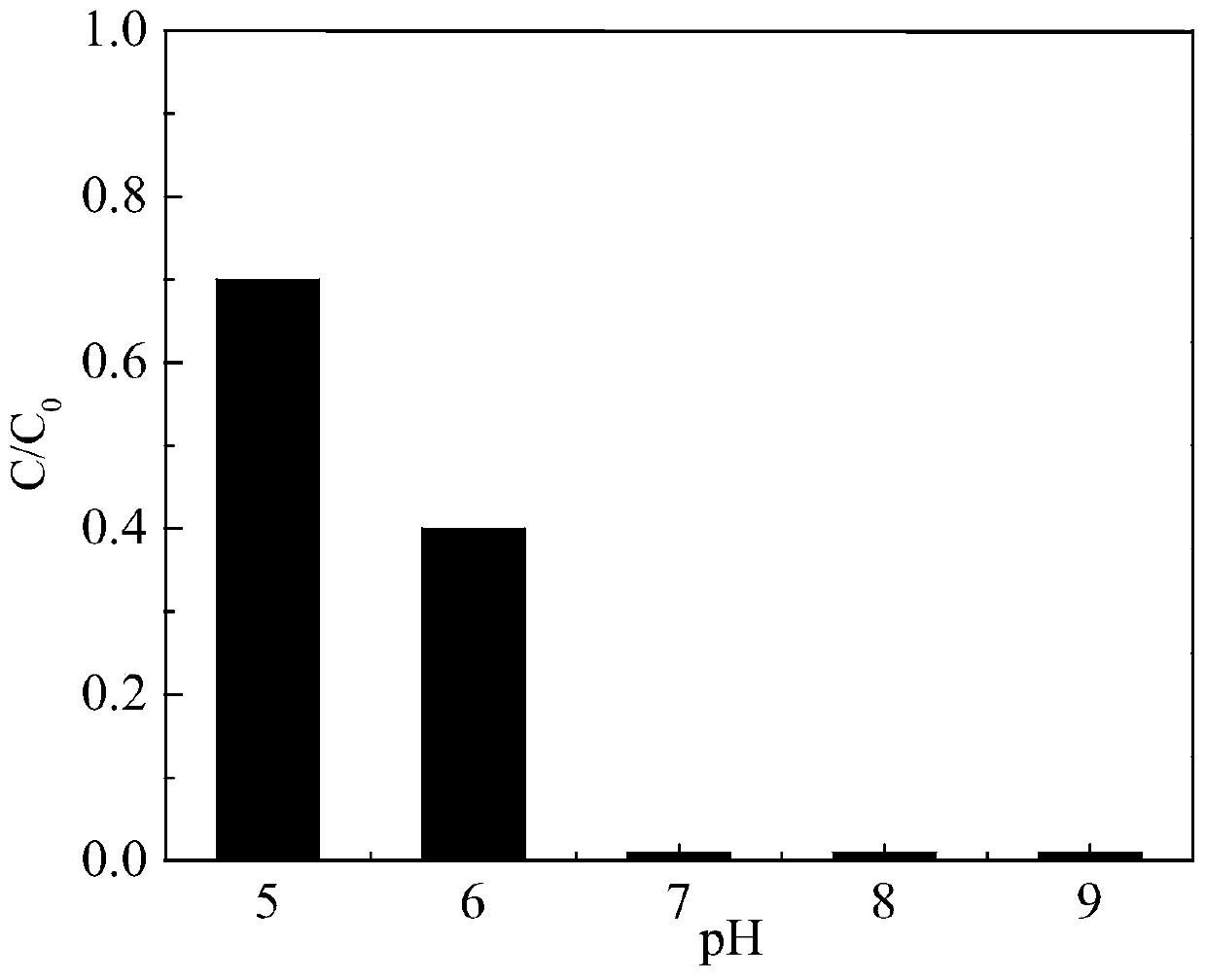
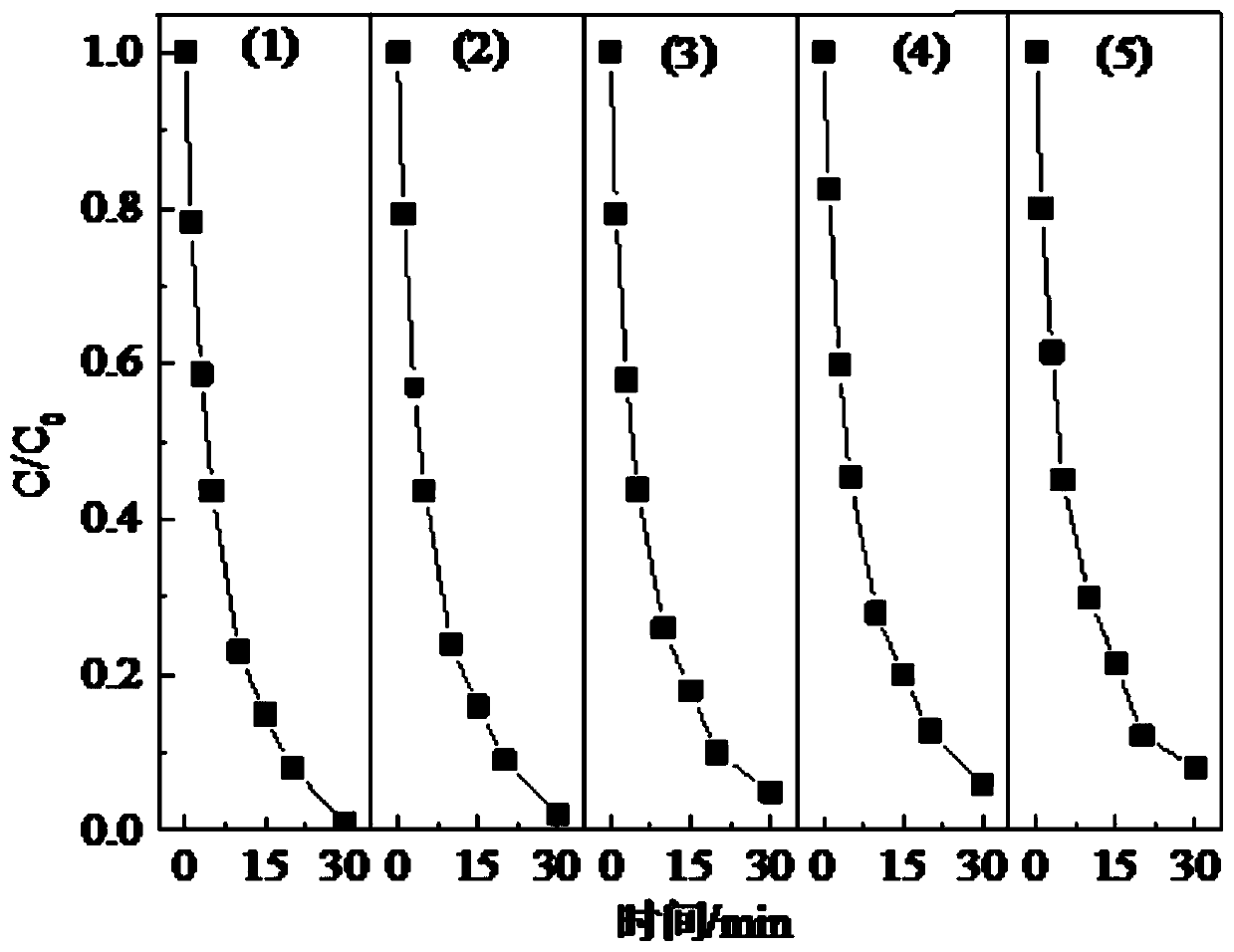
Iodine drug degradation method based on MOF template method
ActiveCN110272110AEasy to recycleAvoid secondary pollutionWater contaminantsIron compoundsSulfateSulfite
The invention discloses an iodine drug degradation method based on an MOF template method, and particularly relates to a method for preparing a copper-iron composite oxide to activate sulfite to generate sulfate free radicals to degrade iodine drugs in water on the basis of the MOF template method. According to the method, an MOF material can serve as a self-sacrificial template, the copper-iron composite oxide is prepared after calcination, the characteristics of high specific surface area and high catalytic activity of the copper-iron composite oxide are used for activating the sulfite to generate the sulfate free radicals to degrade the iodine drugs. The copper-iron composite oxide prepared on the basis of the MOF template method is easy to recycle, and still has a good activation effect after being recycled multiple times. The method is suitable for degradation of various iodine drugs, is high in activation efficiency and simple and feasible to operate, can efficiently activate the sulfite to degrade the iodine drugs under neutral conditions, and is relatively good in advantage.
Owner:HUAQIAO UNIVERSITY
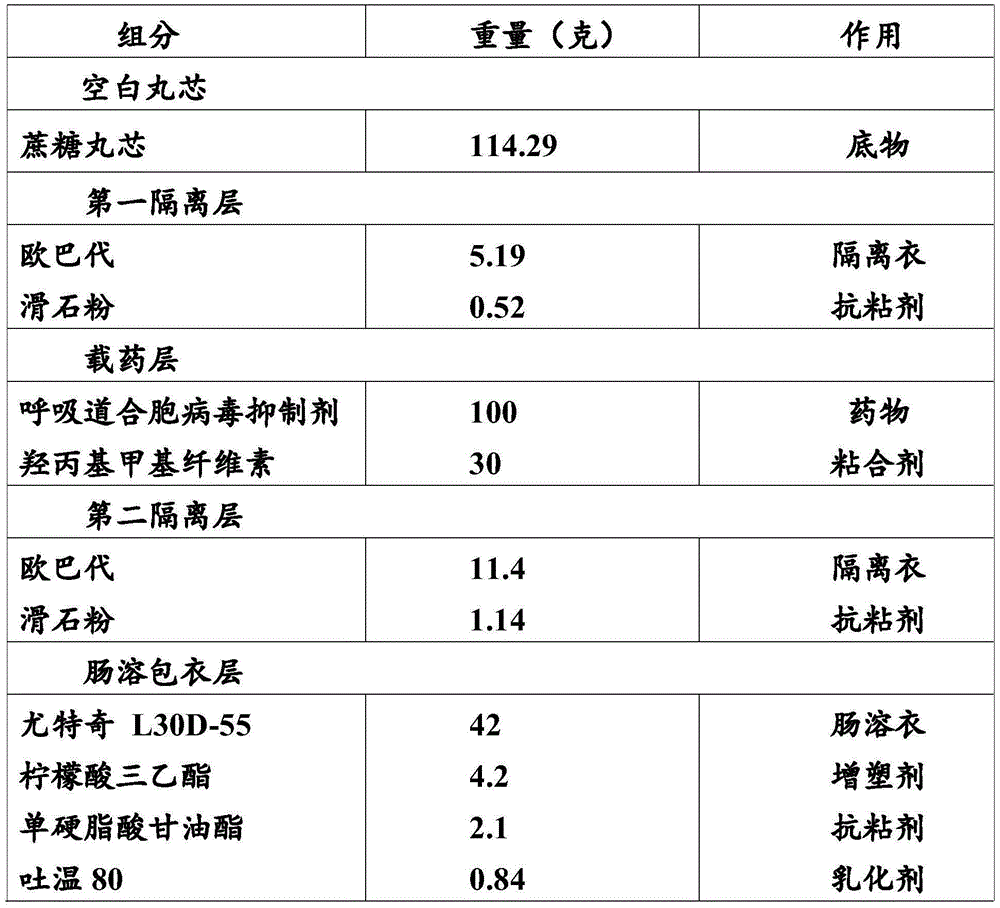
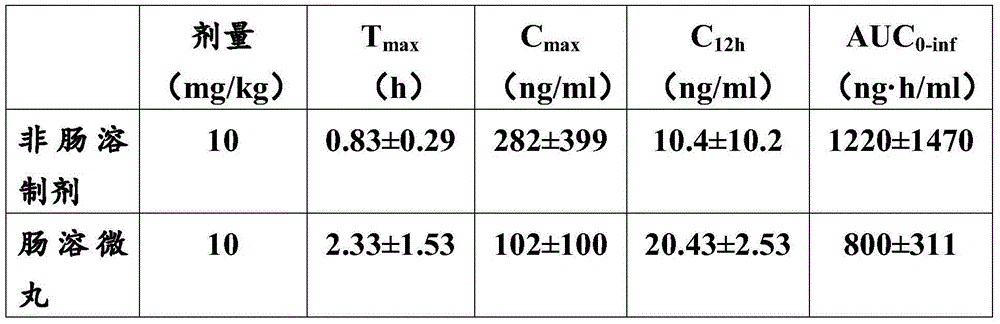
Enteric-coated pellet containing respiratory syncytial virus inhibitor and preparation method thereof
ActiveCN105726488AQuality improvementOptimizing Pharmacokinetic ParametersAntiviralsGranular deliveryIrritationMedicine
The invention relates to an enteric-coated pellet containing a respiratory syncytial virus inhibitor. The enteric-coated pellet comprises a blank pellet core, a first isolated layer, a drug carrying layer, a second isolated layer and an enteric coating layer from inside to outside. The weight gain of the first isolated layer is 2-10%, the weight gain of the drug carrying layer is 10-200%, the weight gain of the second isolated layer is 2-10% and the weight gain of the enteric coating layer is 5-50%. The first isolated layer and the second isolated layer comprise Opadry. The drug carrying layer comprises a respiratory syncytial virus inhibitor and a binder. The enteric coating layer comprises an enteric-soluble material, a plasticizer, an antiadherent and an emulsifier. The enteric-coated pellet prevents drug degradation in the gastric juice and irritation on the stomach, optimizes pharmacokinetic parameters, greatly improves a drug safety window and anti-RSV activity, can be prepared through simple processes and is suitable for large-scale production and application.
Owner:SHANGHAI ARK BIOSCI CO LTD
Mesoporous silicon nanoparticles capable of responding to X-rays to release drugs as well as preparation method and application of mesoporous silicon nanoparticles
InactiveCN112057434ADegradation rate regulationRegulated release rateMaterial nanotechnologyOrganic active ingredientsDrug carrierPharmaceutical drug
The invention discloses mesoporous silicon nanoparticles capable of responding to X-rays to release drugs as well as a preparation method and application of the mesoporous silicon nanoparticles, and belongs to the technical field of drug carriers. The preparation method of the mesoporous silicon nanoparticles capable of responding to X-rays to release drugs comprises the following steps: (1) preparing bis[3-(triethoxysilyl)propyl]diselenide; and (2) preparing double-selenium mesoporous silicon nanoparticles capable of responding to X-rays to release drugs. The prepared double-selenium mesoporous silicon is the first mesoporous silicon capable of responding to X-rays to generate skeleton degradation. Bis[3-(triethoxysilyl)propyl]diselenide and tetraethoxysilane are used as a mixed silicon source, the mesoporous silicon containing double-selenium bonds is synthesized by a copolycondensation method, and the prepared double-selenium mesoporous silicon can not only respond to X-rays to rapidly degradate and release drugs, but also respond to tumor redox microenvironments to slowly release drugs so as to regulate the drug degradation speed and the drug release speed.
Owner:SOUTH CHINA UNIV OF TECH
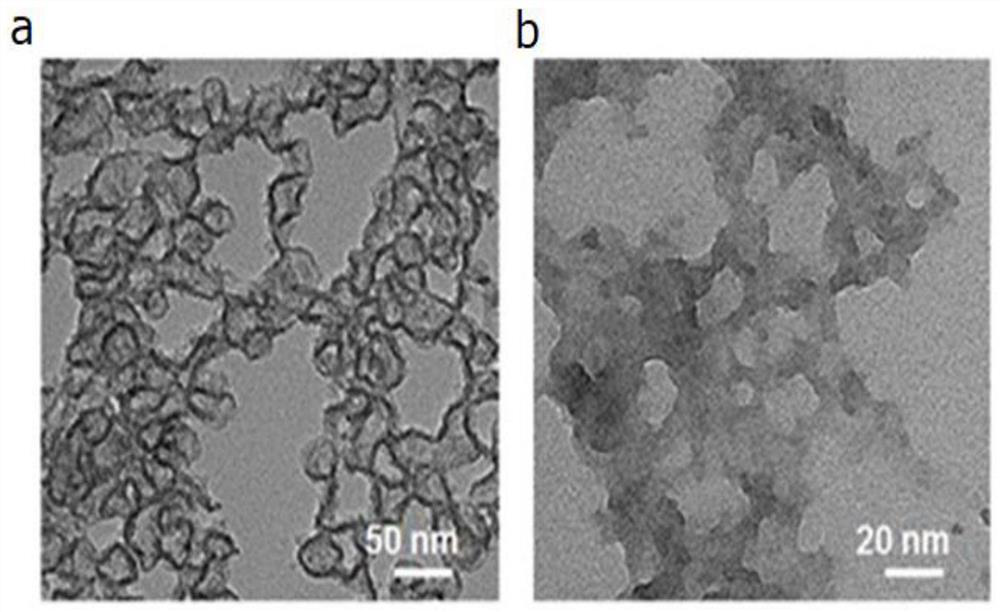
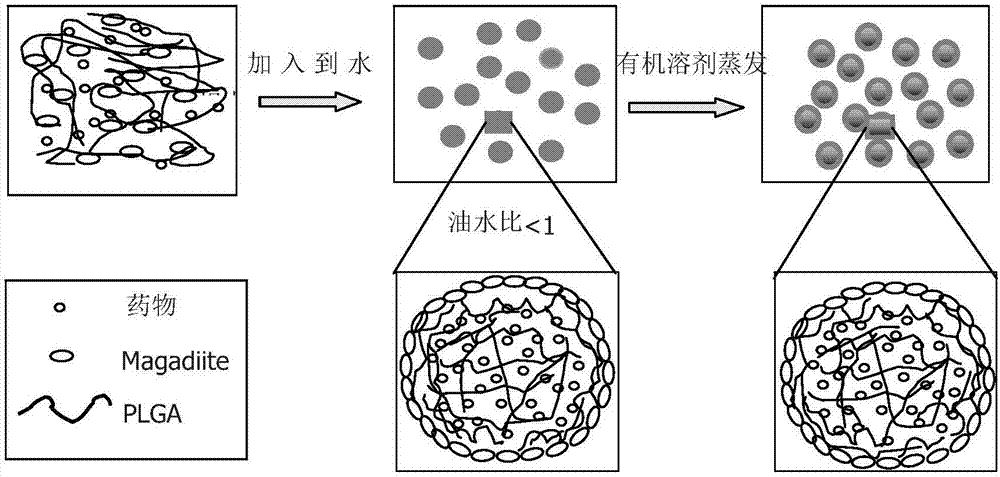
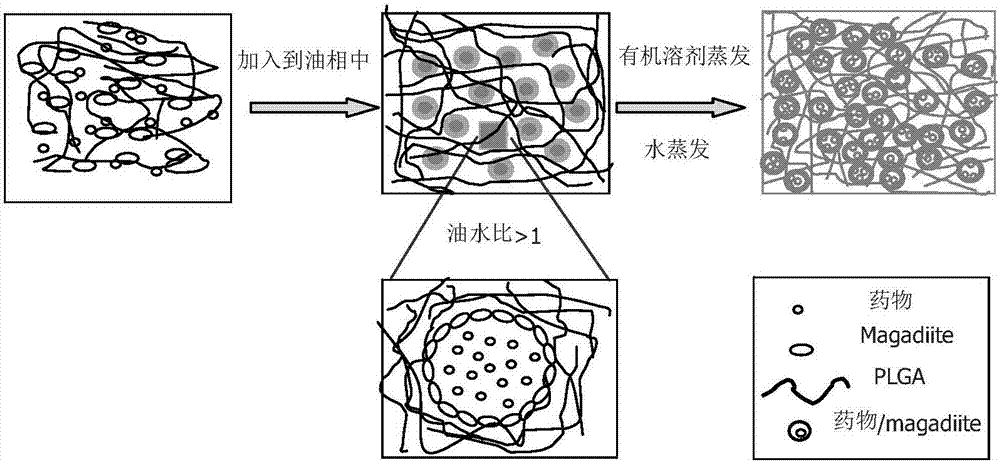
Nano-hybrid drug carrier prepared through Pickering emulsion template method with magadiite serving as emulsifier and preparation method of nano-hybrid drug carrier
InactiveCN107412193AMild preparation conditionsSimple and fast operationOrganic active ingredientsPharmaceutical non-active ingredientsNano hybridOil phase
Owner:SOUTH CHINA UNIV OF TECH
Use of antioxidants to prevent oxidation and reduce drug degradation in drug eluting medical devices
InactiveUS8007737B2Reduce degradationPrevent oxidationStentsAnalysis using chemical indicatorsBiological bodyAntioxidant
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. In each of these instances, antioxidants are utilized to prolong product integrity.
Owner:WYETH LLC
Drug carrier, drug preparation and preparation method
ActiveCN108187060AImprove stabilityUniform compositionPeptide/protein ingredientsMacromolecular non-active ingredientsSolubilityHalf-life
The invention relates to the field of medicinal accessories, and specifically provides a drug carrier, a drug preparation and a preparation method. The drug carrier provided by the invention is prepared from two polypeptides or one of the pharmaceutically acceptable derivatives of the two polypeptides or combination of at least two of the pharmaceutically acceptable derivatives. The drug carrier can from a stable drug complex with a polar drug. On the premise of not influencing the drug effect, the drug carrier greatly reduces the drug degradation speed, thus prolongs the in-vivo half-life period of the drug in a human body, and realizes a slow release function of the drug. Meanwhile, the drug carrier also can improve the drug solubility and is favorable for enhancing the absorption and effect of the drug, thereby lowering the medical cost. The drug preparation provided by the invention comprises the polar drug and the drug carrier, and has the advantages of high safety, long in-vivo half-life period and high solubility. The preparation method of the drug preparation provided by the invention is simple and easy to operate, imposes no special requirements on the equipment, and doesnot increase the production cost.
Owner:王琦
Composition for preventing scar adhesion, postoperative anti-adhesion material and application
PendingCN113577246AFast condensationLow swelling rateOrganic active ingredientsPeptide/protein ingredientsMitomycin CPolyethylene glycol
The invention relates to a composition for preventing scar adhesion, a postoperative anti-adhesion material and application. Epidural scar adhesion is an important cause of postoperative lumbar surgery failure syndrome, and the invention aims to provide a drug-loaded postoperative sealing material which can seal wounds and realize stable release of drugs at the same time. The invention firstly provides the mitomycin C-IFN gamma-sodium hyaluronate conjugate for preventing scar adhesion, the conjugate can effectively inhibit scar tissue formation and slow down the drug degradation speed, and when the conjugate is applied to a polyethylene glycol sealant, the sealing effects of low swelling and rapid condensation can be achieved. The product is especially suitable for spinal surgeries such as laminectomy and the like, and has good clinical application value.
Owner:SAIKE SAISI BIOTECH CO LTD
Stable mycophenolate mofetil for injection, and preparation method thereof
ActiveCN106943359AAvoid safety hazardsSimple processPowder deliveryOrganic active ingredientsFreeze-dryingTherapeutic effect
The present invention relates to a stable mycophenolate mofetil freeze-drying composition for injection, wherein the composition is prepared from mycophenolate mofetil, citric acid, polysorbate 80, disodium tetraborate, a pH value adjuster, and water for injection. The preparation method comprises: adding a prescription amount of mycophenolate mofetil, citric acid and polysorbate 80 to a proper amount of water for injection, uniformly stirring and mixing, adjusting the pH value of the solution to 2.6-3.4 with 1 M hydrochloric acid, adding a prescription amount of disodium tetraborate, carrying out volume metering to achieve the total amount, stirring the solution until achieving a colorless and clear liquid, filtering, filling, carrying out freeze drying, and carrying out visual inspection so as to obtain the finished product. According to the present invention, the product is stable, such that the safety risk caused by the treatment effect reducing and the impurity increasing due to the drug degradation can be avoided; and the method has characteristics of simple process and simple operation, and is suitable for industrial production.
Owner:SHANDONG NEWTIME PHARMA
Fentrazamide-containing weeding microemulsion
InactiveCN105052980AImprove elimination effectGood drug degradation effectBiocideAnimal repellantsBULK ACTIVE INGREDIENTSolvent
The invention discloses a fentrazamide-containing weeding microemulsion. The microemulsion comprises effective active ingredients, a surfactant, an emulsifier, namely, dioctylsulfosuccinate sodium salt, a cosolvent, namely, ethyl alcohol, water, an anti-freezing agent, namely, glycerin, and a synergist, namely, piperonyl butoxide, wherein the effective active ingredients comprise azimsulfuron, the fentrazamide and imazaquin; the surfactant is octaphenyl polyoxyethyiene sulfonate. The microemulsion has the very good eliminating effect on field weeds and is safe and efficient, the drug degradation effect is good, and few residues exist.
Owner:安徽新北卡化学有限公司
Salmon calcitonin-phospholipid complex and its lipid nanoparticle and preparation method
ActiveCN105919974AImprove long-term stabilityFor long-term storageNervous disorderPeptide/protein ingredientsRecombinant salmon calcitoninMembrane permeability
The invention belongs to the technical field of medicines and relates to a salmon calcitonin-phospholipid complex and its lipid nanoparticle and preparation method. The salmon calcitonin-phospholipid complex and its lipid nanoparticle can improve drug lipid solubility, have a certain intestinal adhesion, can be easily absorbed through intestinal mucosa and can prevent drug degradation caused by gastrointestinal tract protease. The lipid nanoparticle of the salmon calcitonin-phospholipid complex comprises the salmon calcitonin-phospholipid complex, a lipid material, a surfactant and water and is prepared through a film dispersion method. The water-soluble drug nanoparticle obtained through the preparation method has a high drug oil-liquid distribution coefficient and good membrane permeability and improves oral bioavailability. The preparation method has simple processes.
Owner:CHENGDU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com