Nucleic acid lipid nano particle composition, pharmaceutical preparation containing same, and preparation method and application thereof
A technology of lipid nanoparticles and pharmaceutical preparations, applied in the field of biomedicine, can solve problems such as single large-scale clinical application of nucleic acid drug administration routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0080] The present invention also provides a preparation method of the pharmaceutical preparation described in the second aspect, comprising the following steps:
[0081] The nucleic acid drug is mixed with the lipid carrier to prepare the drug-loaded lipid nanoparticle, and then an auxiliary material is added to form a pharmaceutical preparation.
[0082] In some specific embodiments of the present invention, for the preparation of dry powder, the present invention provides the following steps:
[0083] The nucleic acid drug and the lipid carrier are mixed to prepare a drug-loaded lipid nanoparticle solution, and then the aqueous solution of auxiliary materials is added, and the nucleic acid lipid nanoparticle dry powder is formed by spray drying.
[0084] In some specific embodiments of the present invention, when the nucleic acid drug is mixed with the lipid carrier, the mixing method is selected from microfluidic method, mechanical stirring method, high-pressure emulsion h...
Embodiment 1
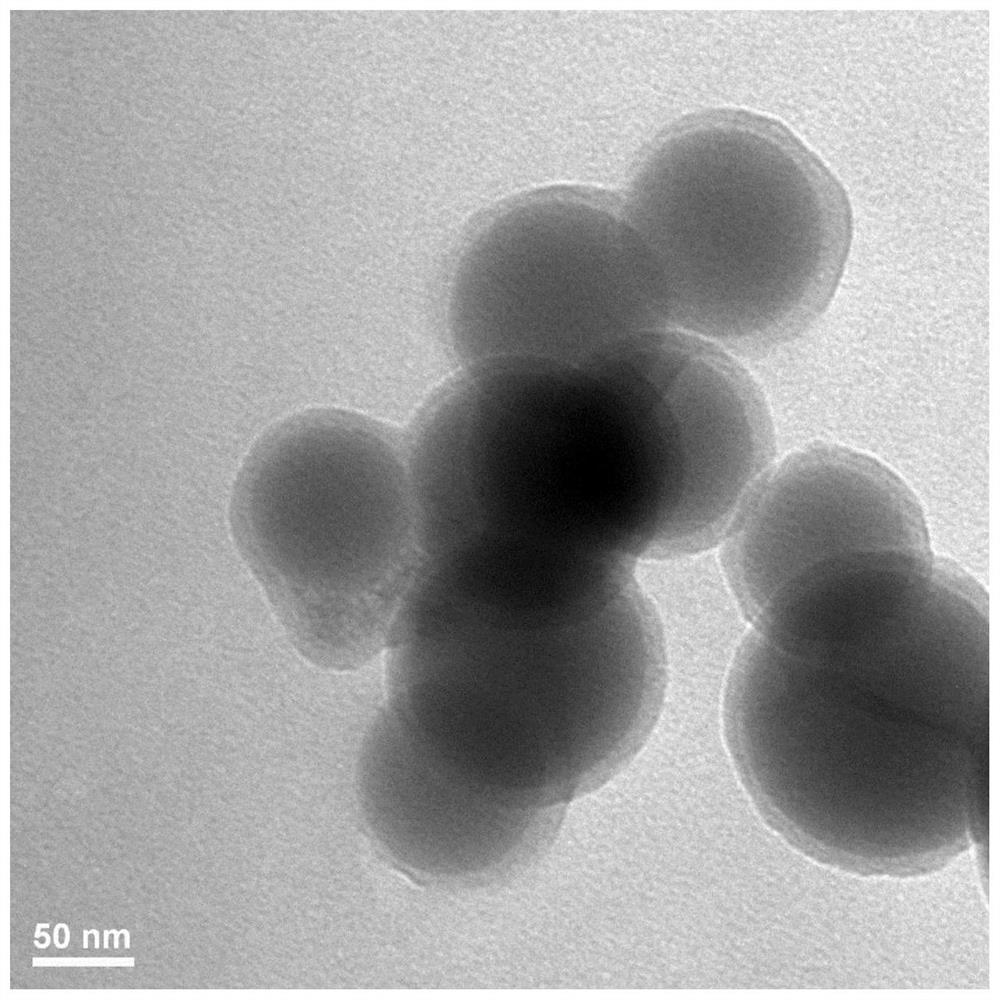
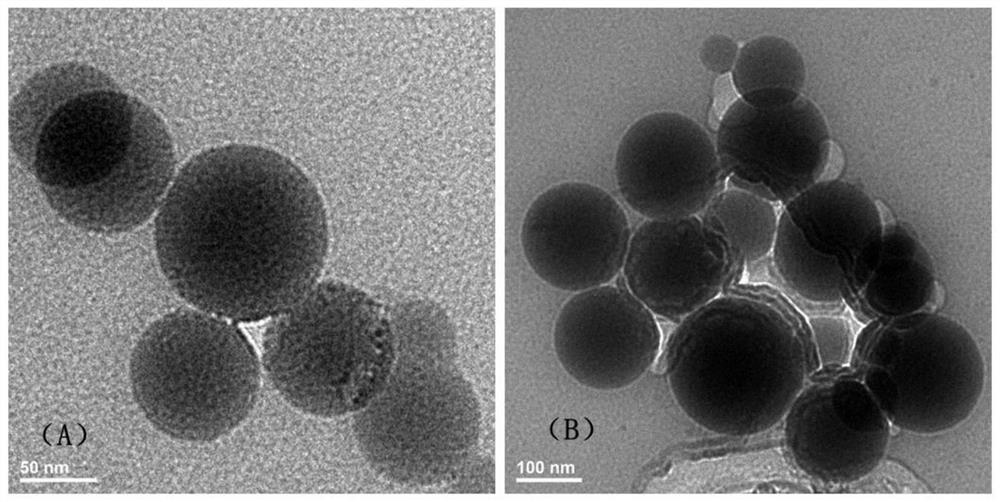
[0094] DOTAP, DSPC, cholesterol and PEG-DMG with a total mass of 24.4 mg were dissolved in 1 mL of ethanol in a molar ratio of 50:38.5:10:1.5; 1 mg of mRNA was dissolved in 3 mL of citrate buffer (50 mM, pH=4.0 ), rapidly mixed with the above-mentioned ethanol solution by microfluidic technology, and then dialyzed to remove ethanol to obtain an LNP solution loaded with mRNA. Dissolve 1 g of mannitol in 46 mL of water to obtain an aqueous solution of excipients. After fully dissolving with the LNP solution, it is sprayed out of a spray drying tower at normal temperature, and a two-fluid atomization device is used to form a dry powder of nucleic acid lipid nanoparticles. Among them, the inlet air temperature is 45°C, the liquid flow rate is 0.3L / h, and the compressed air pressure is 0.2Mpa. The D50 of the dry powder particle size of this group is 5 μm.
Embodiment 2
[0096] DOTAP, DSPC, cholesterol and PEG-DMG with a total mass of 24.4 mg were dissolved in 1 mL of ethanol in a molar ratio of 50:38.5:10:1.5; 1 mg of mRNA was dissolved in 3 mL of citrate buffer (50 mM, pH=4.0 ), rapidly mixed with the above-mentioned ethanol solution by microfluidic technology, and then dialyzed to remove ethanol to obtain an LNP solution loaded with mRNA. Dissolve 1g of mannitol and 1g of lecithin in 46mL of water to obtain an aqueous solution of excipients. After fully dissolving with the LNP solution, spray it out through a freezing spray tower, use a two-fluid atomization device, and freeze-dry to form nucleic acid lipid nanoparticle dry powder. Among them, the temperature in the tower is -40°C, the liquid flow rate is 1L / h, and the compressed air pressure is 1Mpa.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| hydrodynamic diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com