Solid preparation of clopidogrel and preparation method thereof
A technology of pidogrel solid and clopidogrel, which is applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, which can solve problems such as stickiness and impact on product quality, and avoid Effect of sticking phenomenon, increasing fluidity of raw materials, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
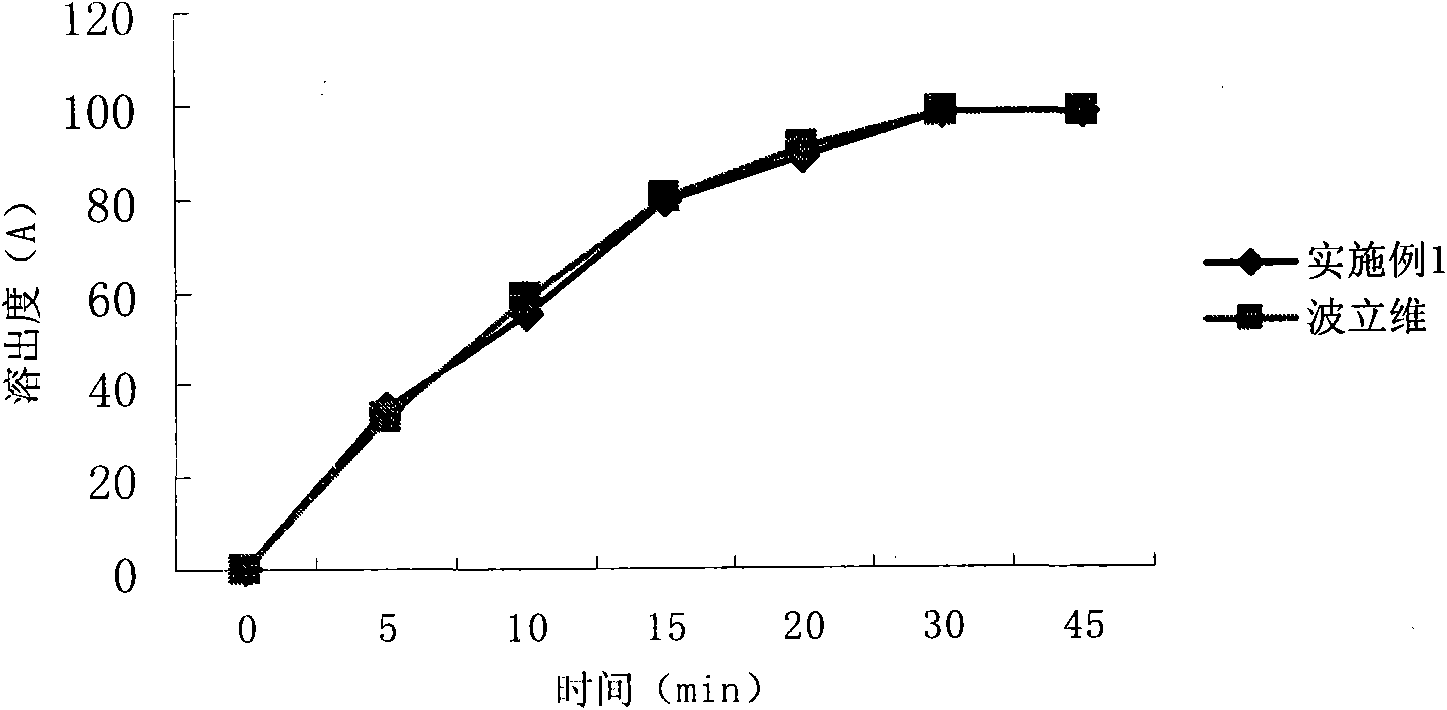
Embodiment 1
[0048] Name of raw material Amount / g
[0049] Clopidogrel Sulfate 75
[0050] Simethicone 5
[0051] Mannitol 115
[0052] Microcrystalline Cellulose 24
[0053] Hypromellose 14
[0054] Stearic acid 1.5
[0055] Glyceryl behenate 3
[0056] 1. Tablet or capsule after dry granulation:
[0057] A, the clopidogrel sulfate is pulverized through an 80 mesh sieve and mixed evenly with simethicone;
[0058] B. Add mannitol, microcrystalline cellulose and hydroxypropyl cellulose to the mixed powder obtained in step A, mix evenly, dry press into a tablet, grind the obtained tablet and granulate with a 20-mesh sieve;
[0059] C, add stearic acid, glyceryl behenate, mix well;
[0060] D. Tablet pressing, control hardness ≥ 5.0kg / mm 2 , tableting speed 8-10 tablets / hour;
[0061] E. Coat the plain tablet obtained in step D with Opadry 85G68918 coating powder, control the coating temperature at 40°C-50°C, and spray the coating solution according to the weight gain of 3%.
[006...
Embodiment 2
[0071] Name of raw material Amount / g
[0072] Clopidogrel Sulfate 75
[0073] Lactose 80
[0074] Microcrystalline Cellulose 64
[0075] Hypromellose 14
[0076] Stearic acid 1.5
[0077] Hydrogenated castor oil 3
[0078] 1. Tablet or capsule after dry granulation:
[0079] A. Mix clopidogrel sulfate, lactose, microcrystalline cellulose, and hydroxypropyl cellulose uniformly, dry press into tablets, and granulate with a 20-mesh sieve after the resulting tablets are pulverized;
[0080] B, add stearic acid, hydrogenated castor oil, mix well;
[0081] C. Tablet pressing, control hardness ≥ 5.0kg / mm 2 , the tableting speed is 80,000-100,000 tablets / hour;
[0082] D. Coat the plain tablet obtained in step C with Opadry 85G68918 coating powder, control the coating temperature at 40°C-50°C, and spray the coating solution according to the weight gain of 3%.
[0083] If preparing capsules, the granules obtained in step B can be directly poured into capsules.
[0084] 2. Aft...
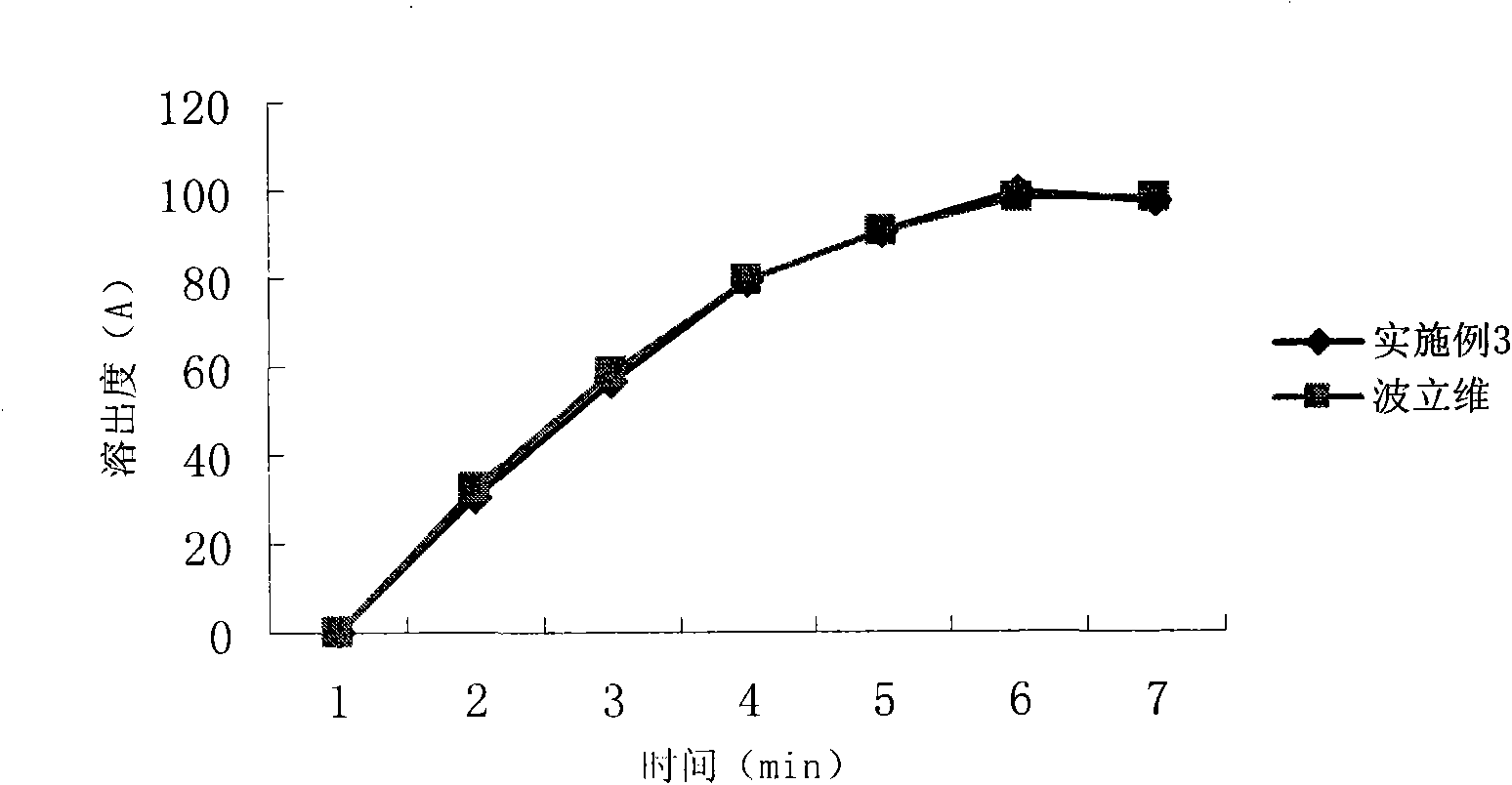
Embodiment 3
[0091] Name of raw material Amount / g
[0092] Clopidogrel Sulfate 75
[0093] Simethicone 4
[0094] Lactose 70
[0095] Pregelatinized starch 26
[0096] Microcrystalline Cellulose 38
[0097] Crospovidone 20
[0098] Stearic acid 1.5
[0099] Hydrogenated castor oil 3
[0100] 1. Tablet or capsule after dry granulation:
[0101] A. After crushing clopidogrel sulfate through an 80-mesh sieve and mixing it evenly with simethicone, first mix it with 50% pregelatinized starch;
[0102] B. Add lactose, the remaining 50% pregelatinized starch, microcrystalline cellulose and crospovidone to the mixed powder obtained in step A, mix evenly, dry press into tablets, grind the obtained tablets and granulate with a 20-mesh sieve;
[0103] C, add stearic acid, hydrogenated castor oil, mix well;
[0104] D. Tablet pressing, control hardness ≥ 5.0kg / mm 2 , the tableting speed is 80,000-100,000 tablets / hour;
[0105] E. Coat the plain tablet obtained in step D with Opadry 85G68918...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com