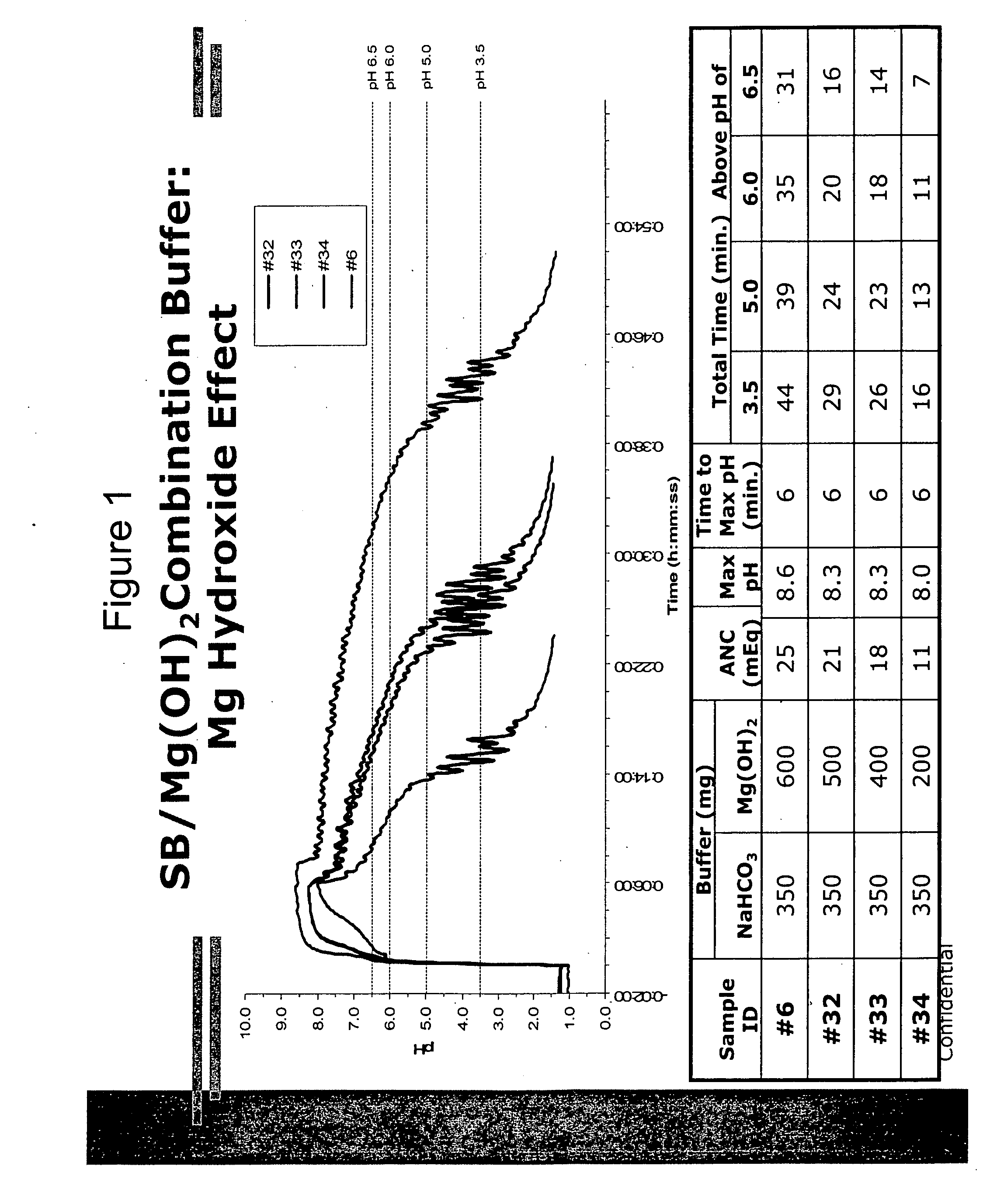
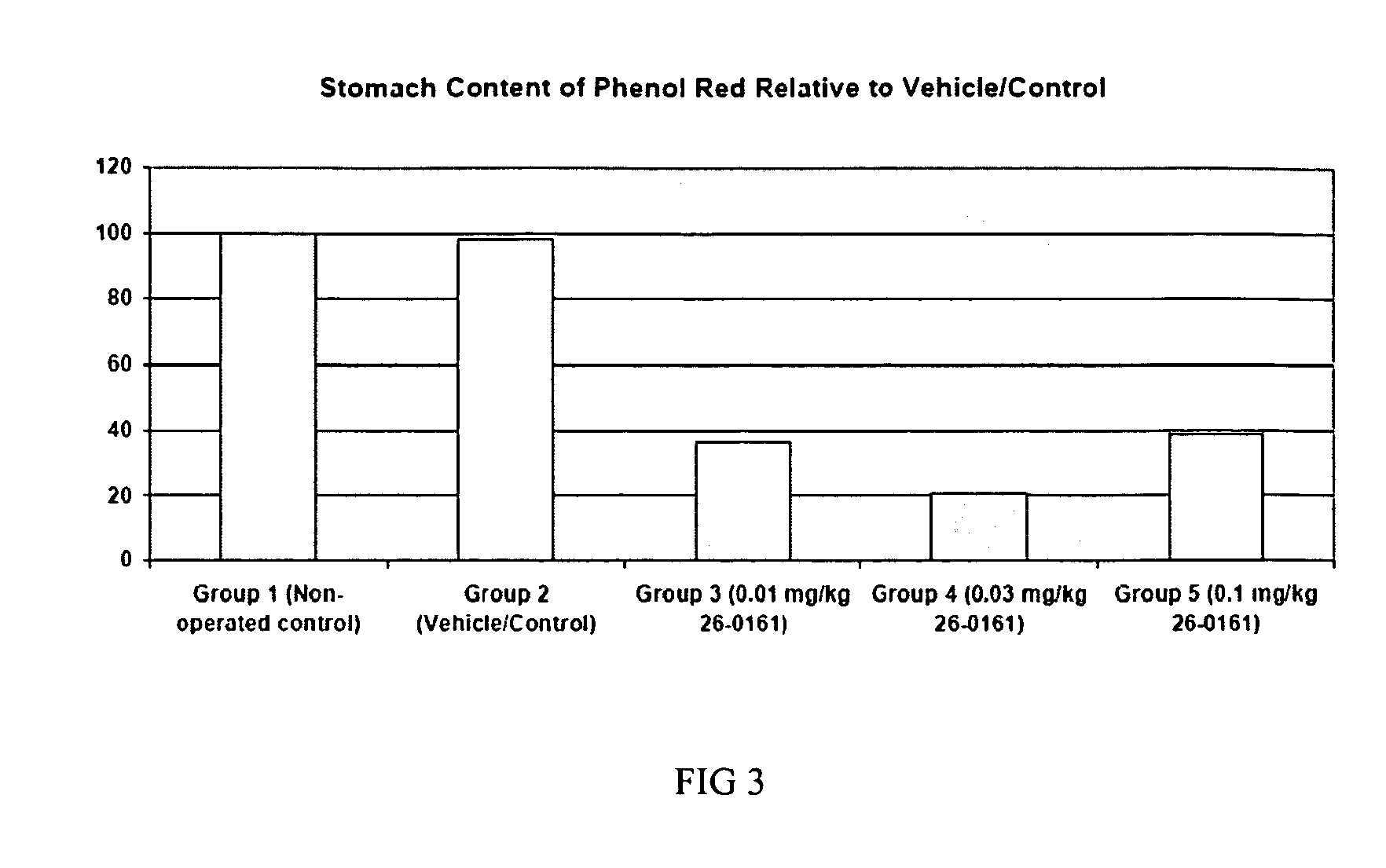
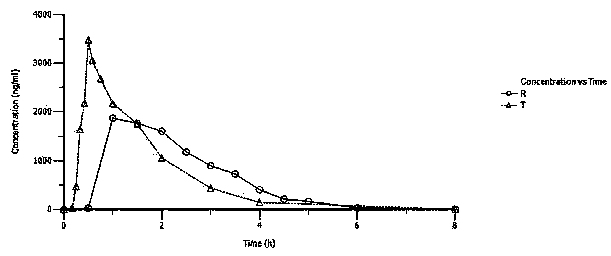
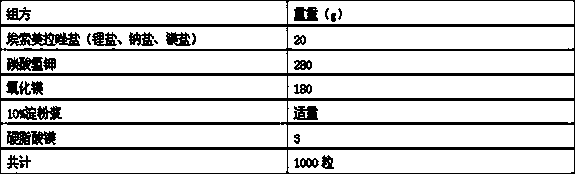
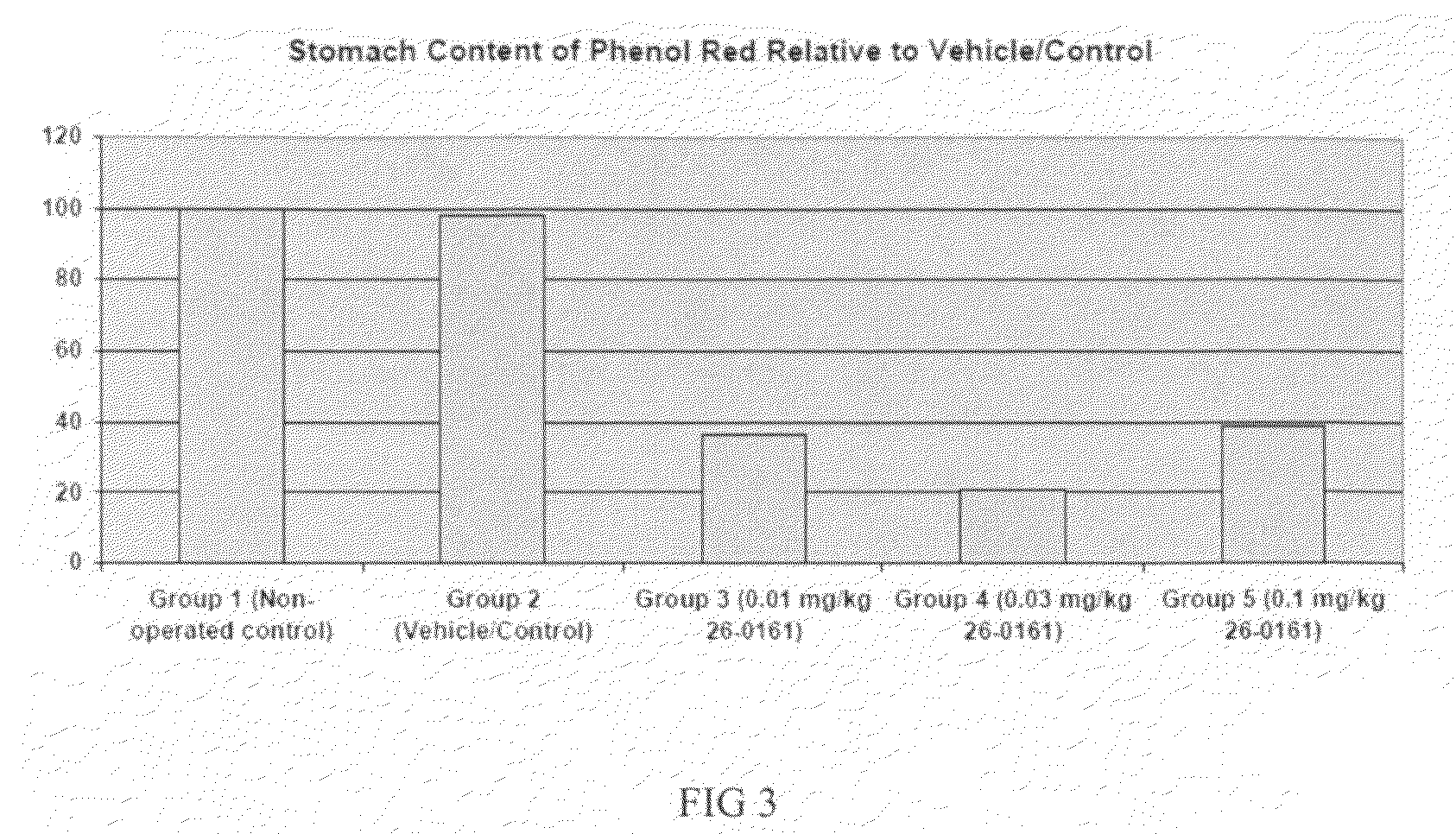
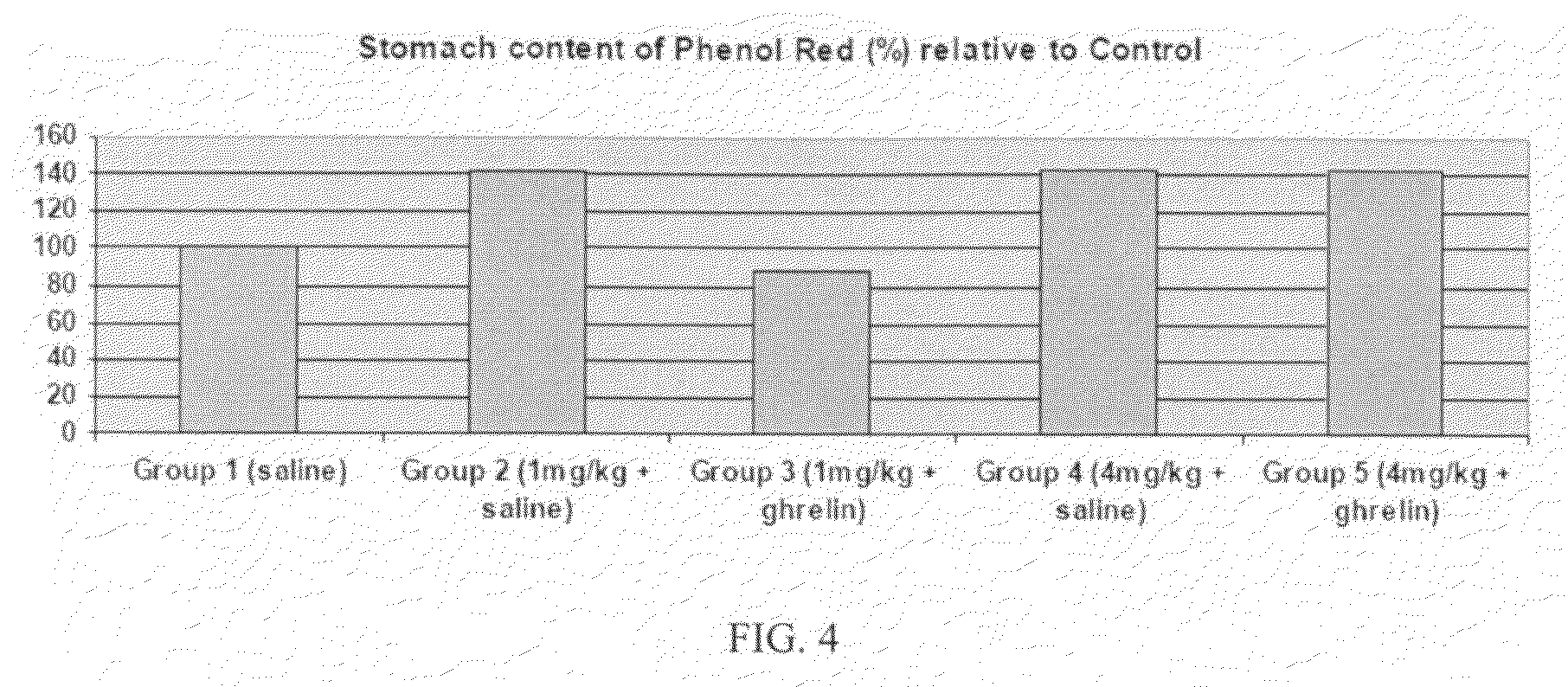
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
104 results about "Antacid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An antacid is a substance which neutralizes stomach acidity and is used to relieve heartburn, indigestion or an upset stomach.
Meloxicam compositions
InactiveUS20050038018A1Increase doseImprove efficacyCosmetic preparationsBiocideJoint arthralgiaStimulant
A pharmaceutical composition comprising: (a) meloxicam or a pharmaceutically acceptable salt thereof; and (b) one or more additional pharmaceutically active compounds selected from antacids, sedatives, and central nervous system stimulants, and the use of such composition of an inflammatory disease, symptoms of an inflammatory disease, including various symptoms thereof, and / or headache, toothache, ache after tooth extraction, sore throat, otalgia, arthralgia, neuralgia, lumbago, myalgia, muscle stiffness of shoulder, pain of contusion, pain of fracture, pain of sprain, menstrual pain, traumatic pain, chill, exothermic reaction and / or cold and various symptoms of cold such as sore throat, chill, pyrexia, headache, arthralgia and muscle pain.
Owner:BOEHRINGER INGELHEIM INT GMBH
Acid-free OCA and adhesive belt and fabrication method of acid-free OCA and adhesive belt
ActiveCN103031090AChange the phenomenon of whitishness and yellowingChange thicknessFilm/foil adhesivesEster polymer adhesivesAdhesive beltAntioxidant
The invention relates to an acid-free OCA (optical clear adhesive), an adhesive belt and a fabrication method of the acid-free OCA and the adhesive belt. The OCA comprises the following components in parts by weight: 100 parts of acrylate adhesive, 0.1-4.5 parts of soluble organometallic complex, 0.1-25.0 parts alkalescent antacid, 0.01-0.3 part of epoxy curing agent, and 0.01-0.5 part of antioxidant. Compared with the prior art and compared with the liquid OCA, the thickness of the prepared OCA is easy to control, the prepared OCA does not cause pollution easily and is convenient to operate when serving as a pressure sensitive adhesive; the adhesive belt has the advantages that the adhesive belt is excellent in adhesive following performance, and an edge does not raise easily. The adhesive belt is simple in production process, low in production cost and excellent in performance.
Owner:NINGBO SOKEN CHEM
Preparation method of blueberry wine
InactiveCN104232426AStable chemical propertiesShorten the brewing cycleAlcoholic beverage preparationMicroorganism based processesPectinaseFruit juice
The invention relates to a preparation method of a blueberry wine. The preparation method comprises the following steps of cleaning blueberry fruits, and then, smashing the blueberry fruits to form blueberry slurry; adding pectinase into the blueberry slurry to carry out enzymolysis, carrying out enzyme deactivation, cooling to the room temperature to obtain cooled blueberry slurry, adding alcohol and an active dry yeast for a wine to ferment, and when the sugar content is measured to be up to 1 BoX, ending the fermentation to obtain a raw blueberry wine; and adding an antacid into the raw blueberry wine, filtering, blending the raw wine by using the alcohol to ensure that the alcohol strength is up to 9-12 degrees, and then, filtering to obtain the blueberry wine. According to the method disclosed by the invention, insoluble substances such as pectin substances, cellulosics and polysaccharides in the raw wine are effectively dissolved by using a pectinase enzymolysis agent, so that fruit juice is clarified within short time; and the blueberry wine with stable chemical property is brewed by using processes such as fermenting by using an active dry yeast for a wine, ageing and filtering, and the brewing period is shortened from more than three years consumed by using the traditional method to three to five months, so that the working period is shortened, the utilization ratio of equipment is increased, and the labor cost is reduced.
Owner:天津绿丛洲农业发展有限公司
Pharmaceutical formulations useful for inhibiting acid secretion and methods for making and using them
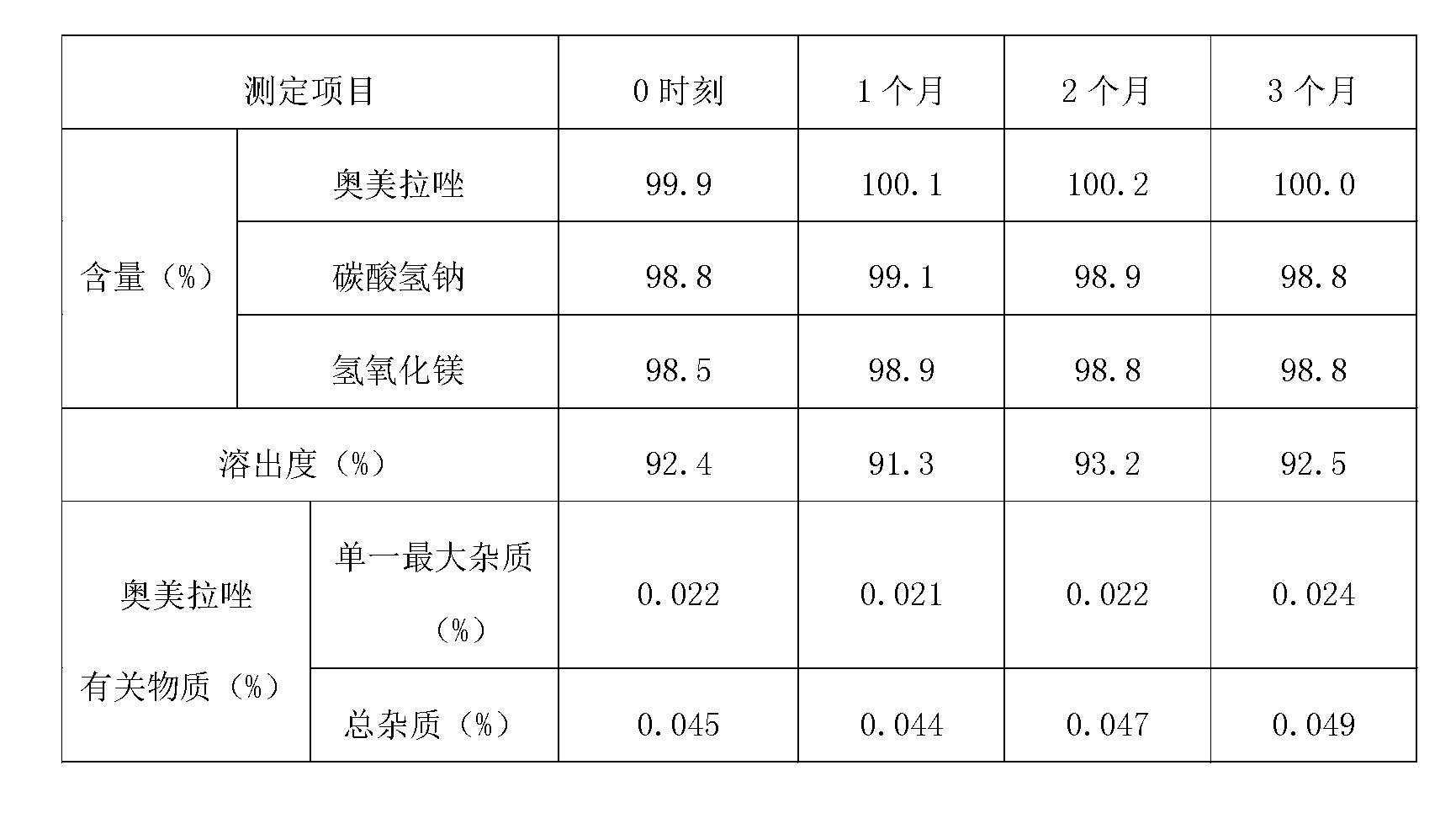
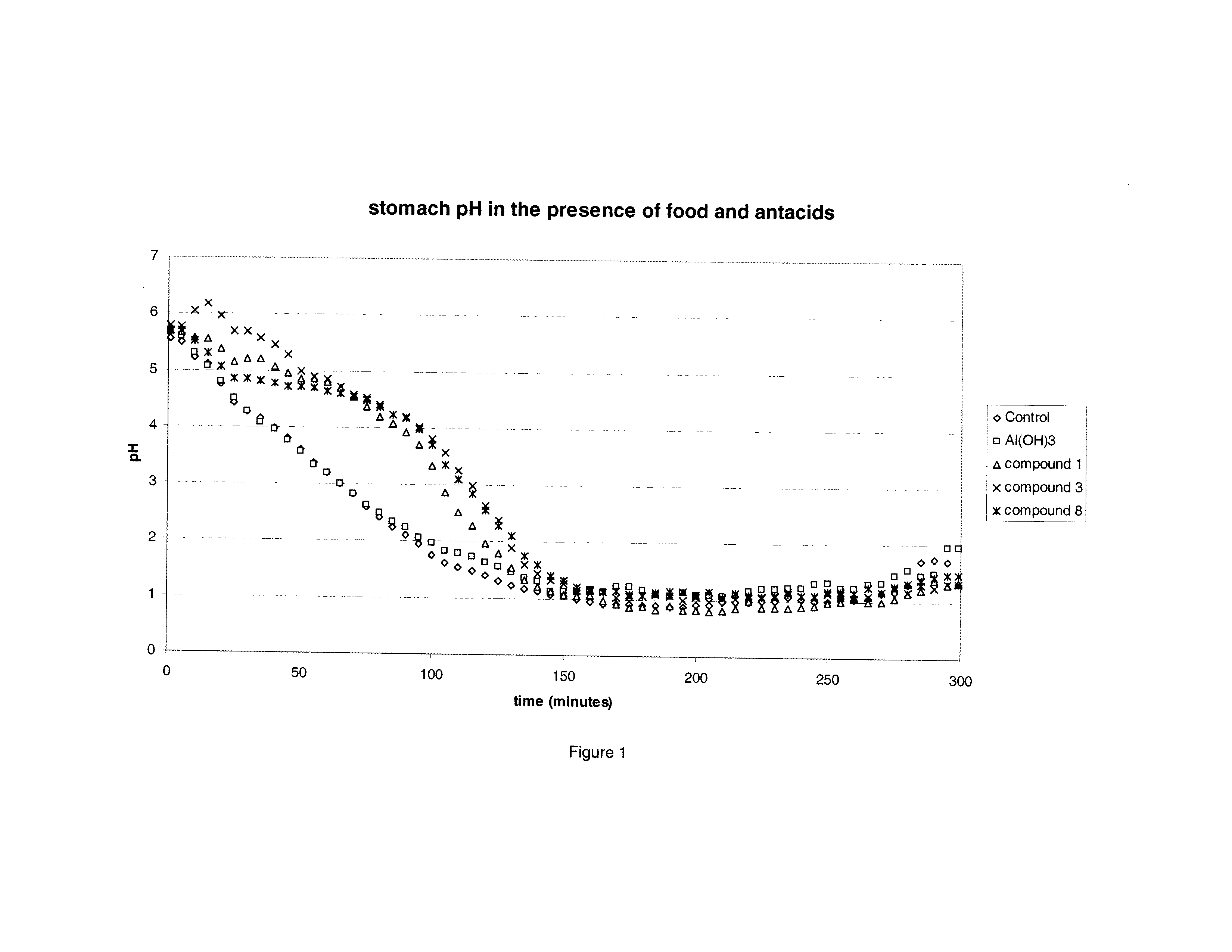
The present invention relates to pharmaceutical formulations comprising at least one acid-labile proton pump inhibiting agent and at least one antacid, which have improved bioavailability, chemical stability, physical stability, dissolution profiles, disintegration times, safety, as well as other improved pharmacokinetic, pharmacodynamic, chemical and / or physical properties. The present invention is directed to methods, kits, combinations, and compositions for treating, preventing or reducing the risk of developing a gastrointestinal disorder or disease, or the symptoms associated with, or related to, a gastrointestinal disorder or disease in a subject in need thereof.
Owner:SANTARUS
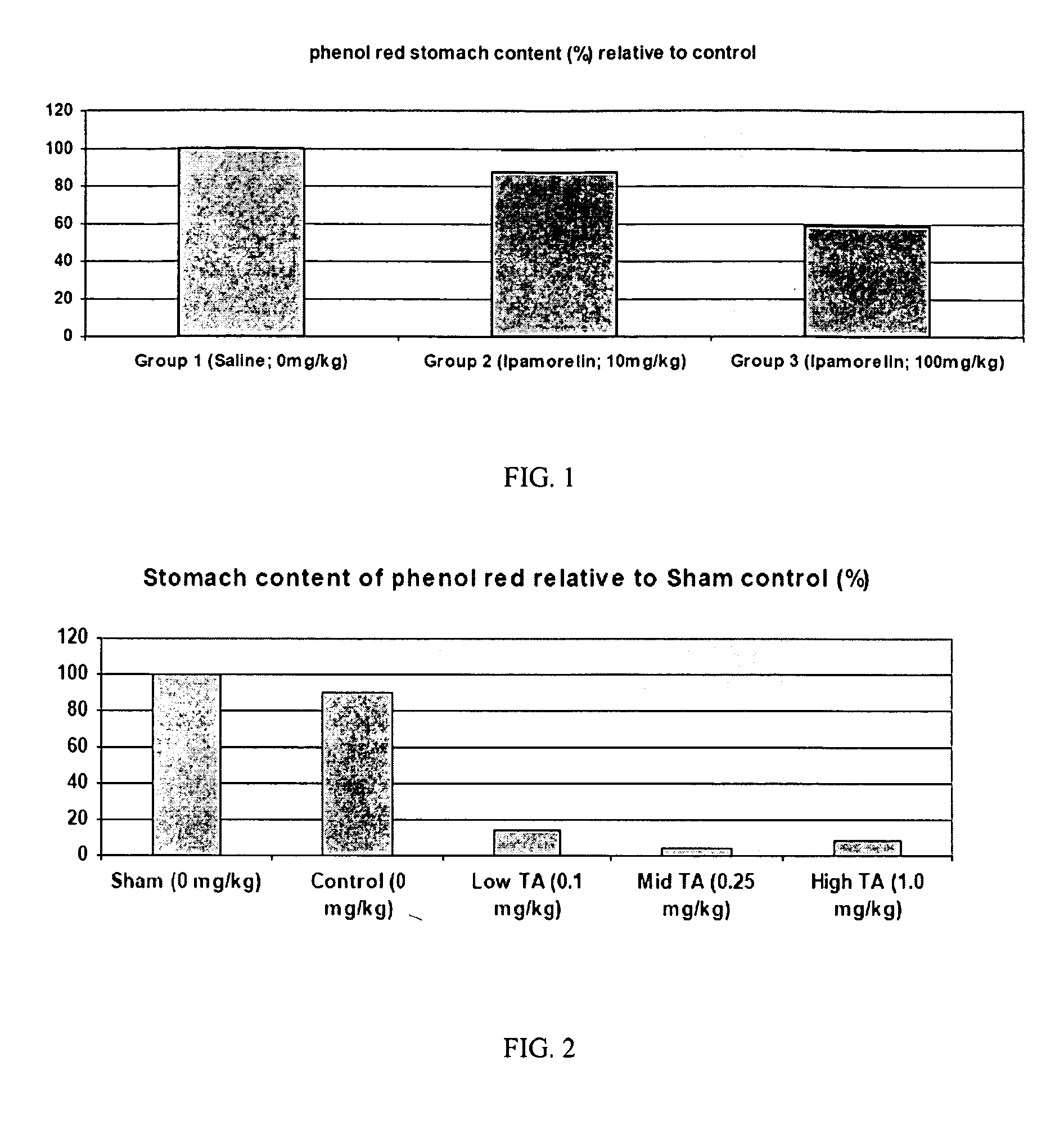
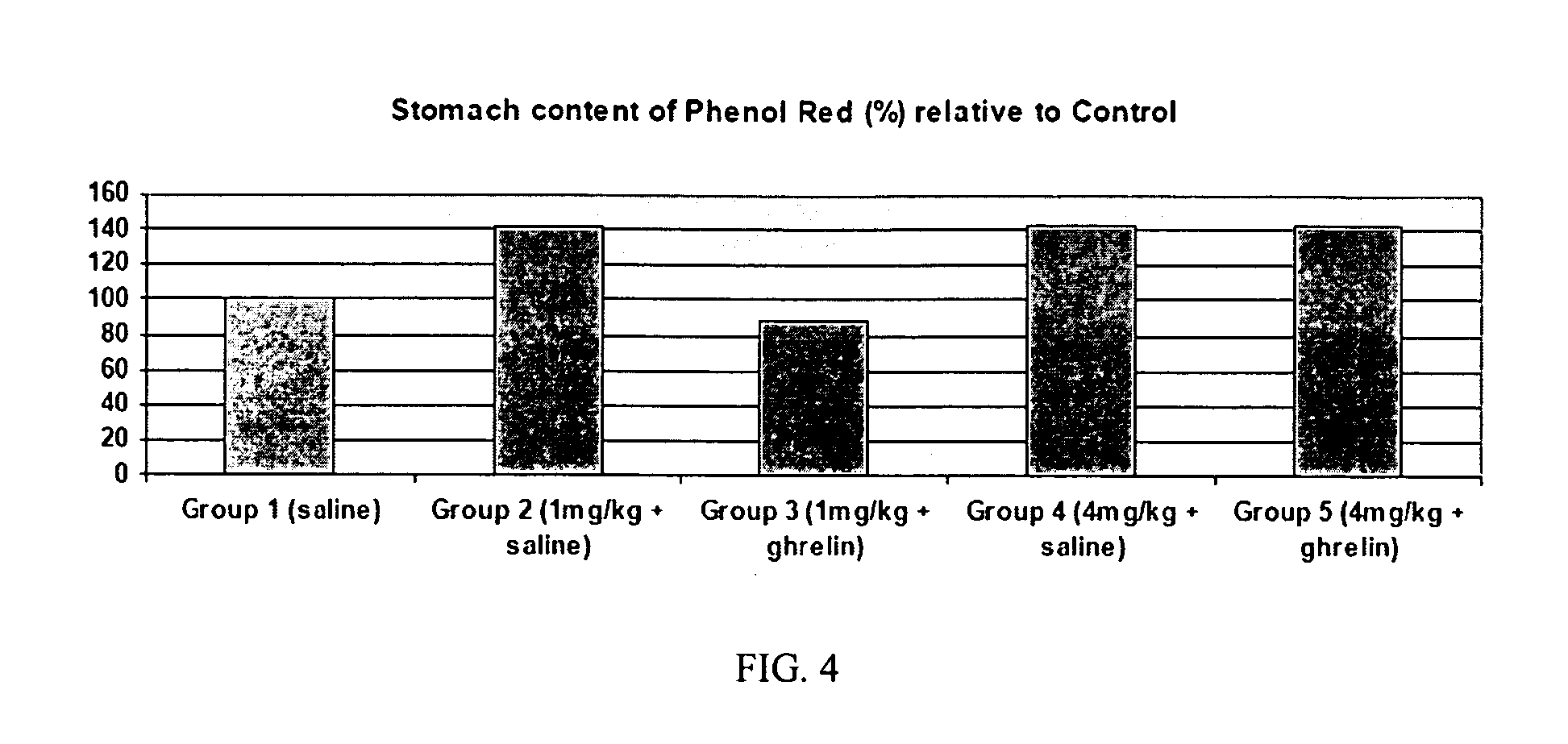
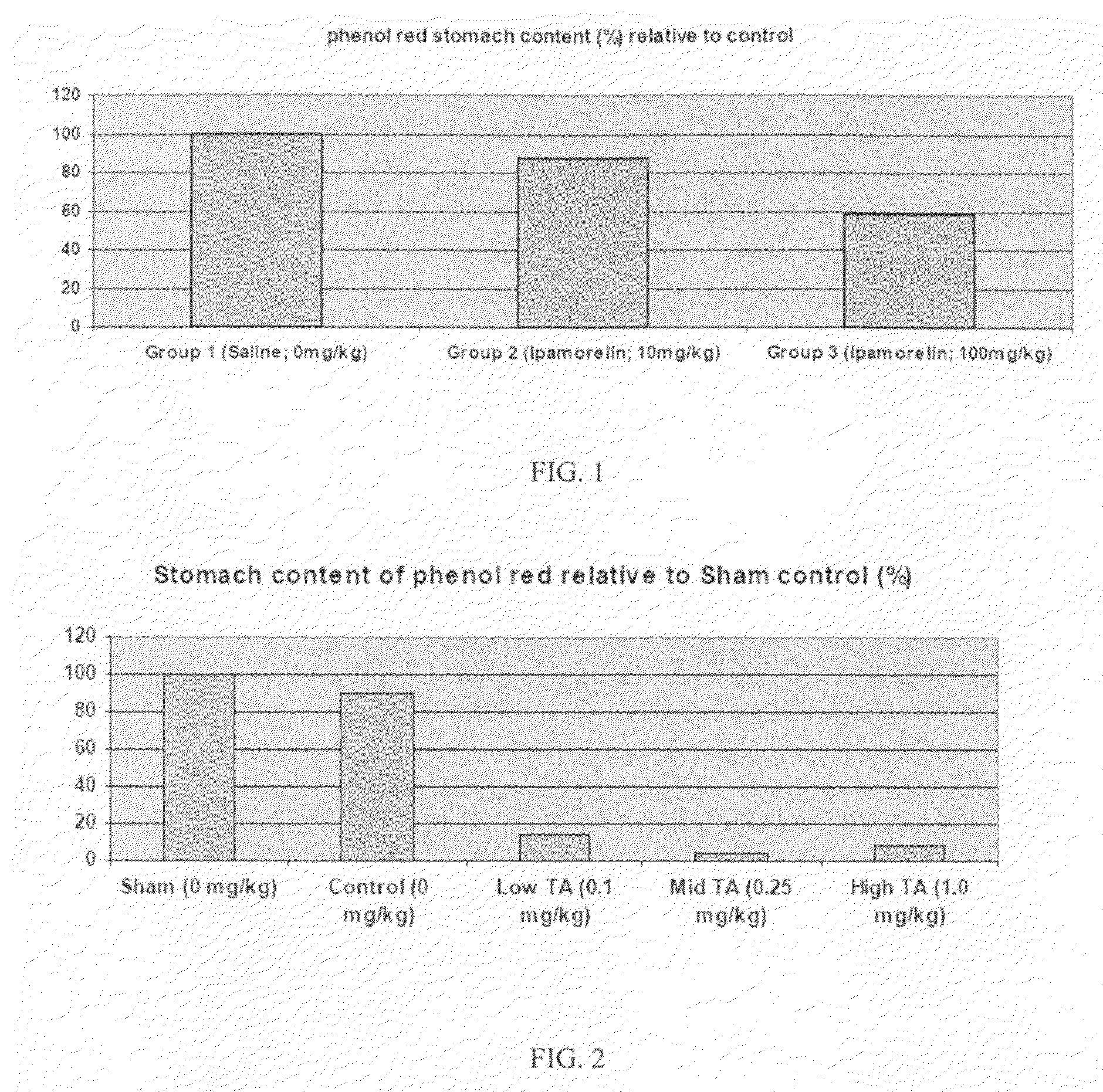
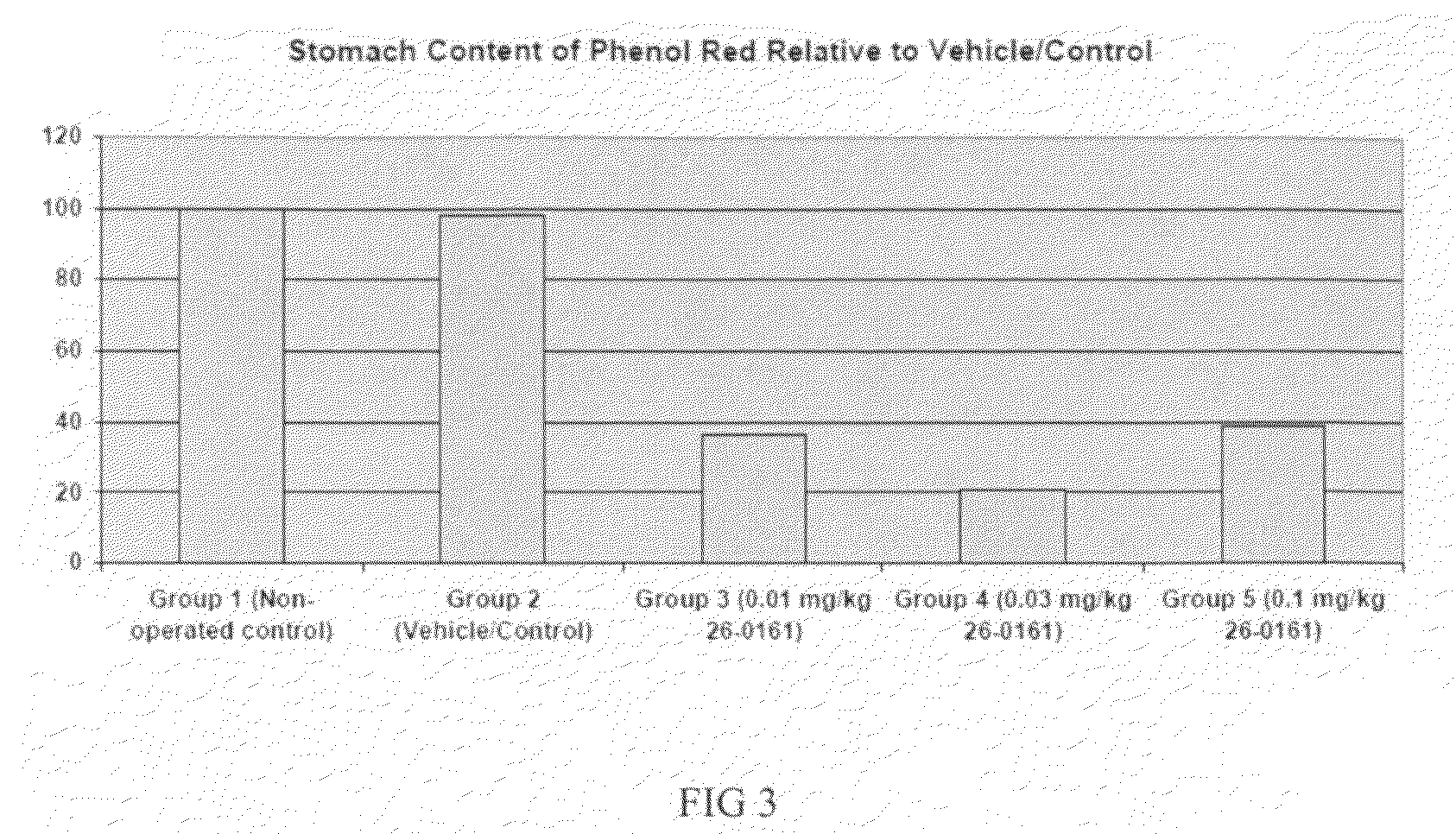
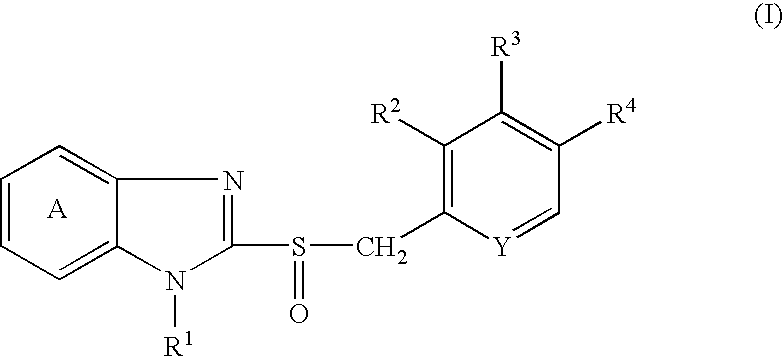
Method of stimulating the motility of the gastrointestinal system using ipamorelin
The present invention provides a method of stimulating the motility of the gastrointestinal system in a subject in need thereof, wherein the subject suffers from maladies (i.e., disorders, diseases, conditions, or drug- or surgery-induced dysfunction) of the gastrointestinal system, by administering to the subject a ghrelin mimetic, or pharmaceutically acceptable salt thereof. The invention also provides a method of treating a gastrointestinal malady by co-administering a ghrelin mimetic with a laxative, a H2 receptor antagonist, a serotonin receptor agonist, pure or mixed, an antacid, an opioid antagonist, a proton pump inhibitor, a motilin receptor agonist, dopamine antagonist, a cholinergic agonist, a cholinesterase inhibitor, somatostatin, octreotide, or any combination thereof.
Owner:HELSINN THERAPEUTICS (U S) INC
Chewable tablets containing mannitol and aspartame
InactiveUS6296868B1Increase surface areaLiquify rapidlyPowder deliveryConfectioneryAdditive ingredientDietary supplement
Chewable tablets and particulate food and pharmaceutical products are disclosed which are made from agglomerates comprising an alcohol sugar such as mannitol and a high intensity sweetener such as Aspartame from which agglomerate tablets may be directly compressed, and processes for making the agglomerates and tablets. The tablets or particulate product containing the agglomerate may contain active ingredients blended with the agglomerate or as part of the agglomerate structure. Tablets and particulate products according to the invention can contain active ingredients such as pharmaceuticals (e.g., antacids, analgesics, cough medicine, drugs, etc.) breath sweeteners, vitamins and dietary supplements, to name a few. The high intensity sweetener containing agglomerates can also be used to make solid food mix type products such as sugar free ice tea mixes.
Owner:ADVANCED TECH PHARMA CORP
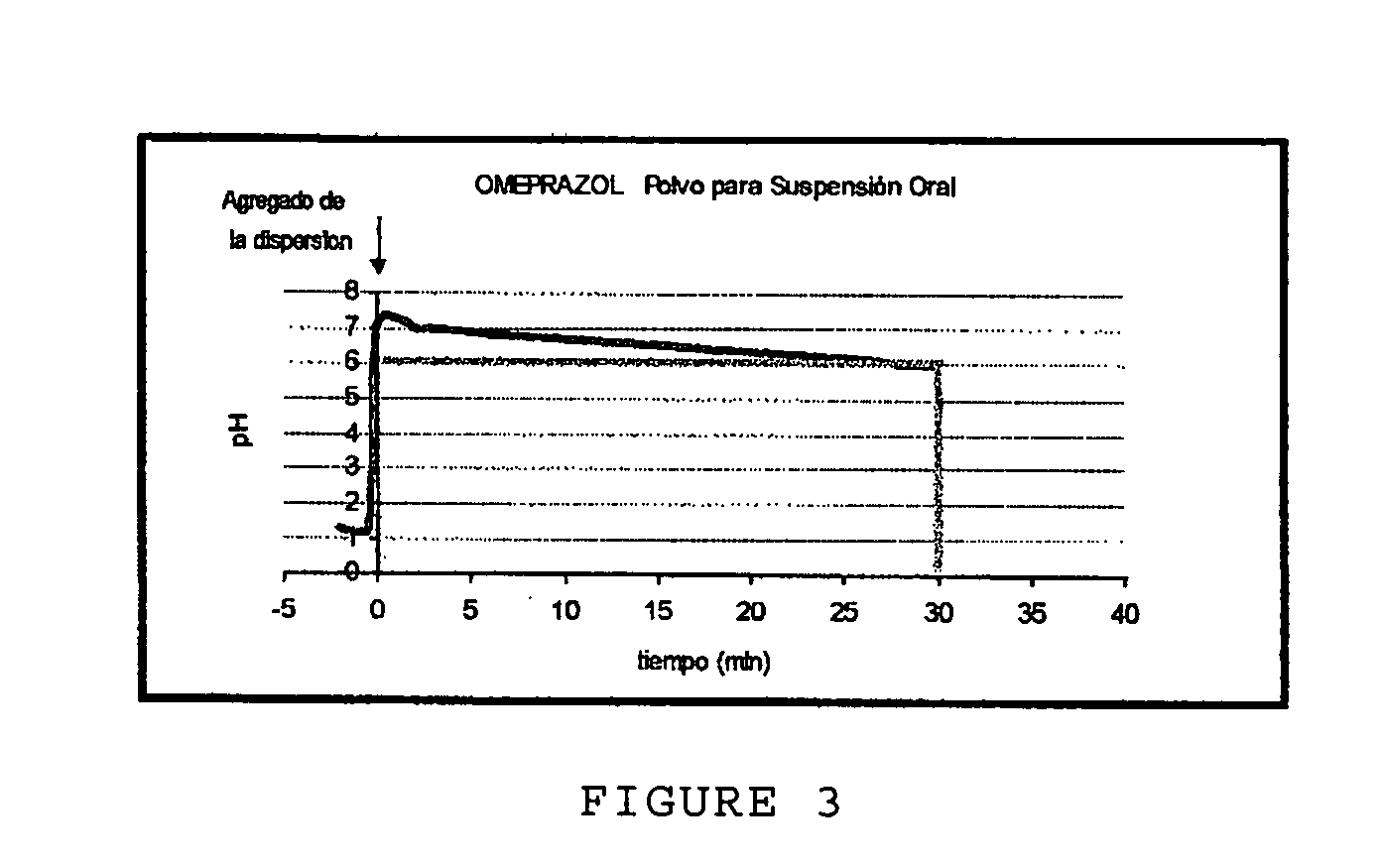
Anti-acid pharmaceutical composition in powder form and process for making it
ActiveUS20070281015A1Easy to controlImprove stabilityBiocideDispersion deliveryDrugs solutionDiluent
An anti-acid pharmaceutical composition for the rapid and prolonged neutralization of gastric acidity with mucosa-protecting activity in powder form to prepare, by dispersion in water, a pharmaceutical solution or suspension for oral use characterized in that the composition includes sodium alginate; an anti-acid soluble agent or a combination of anti-acids; an inhibitor of proton pump; diluent and sweetening agents, wherein a) at least 30% of sodium alginate present in the formulation along with the total of the inhibitor of proton pump are homogeneously distributed over the surface of the total soluble anti-acid agent or of the combination of anti-acids of the composition; and b) the rest, about 70%, of sodium alginate present in the formulation contains a percentage of humidity of less than 2%.
Owner:LAB BAGO
Controlled Release Solid Preparation
InactiveUS20090175959A1Improve stabilityRapidly pharmacological effectBiocideDigestive systemControlled releaseBULK ACTIVE INGREDIENT
The present invention provides a controlled release solid preparation superior in the stability of an active ingredient, which can exhibit pharmacological effects steadily and rapidly after administration, and shows a sustained pharmacological effect for a prolonged period of time: a controlled release solid preparation containing (1) an antacid, (2) an immediate-release part containing a compound unstable to acid and a basic substance, and (3) a sustained-release part containing a compound unstable to acid and a pH-independent material in combination.
Owner:TAKEDA PHARMA CO LTD
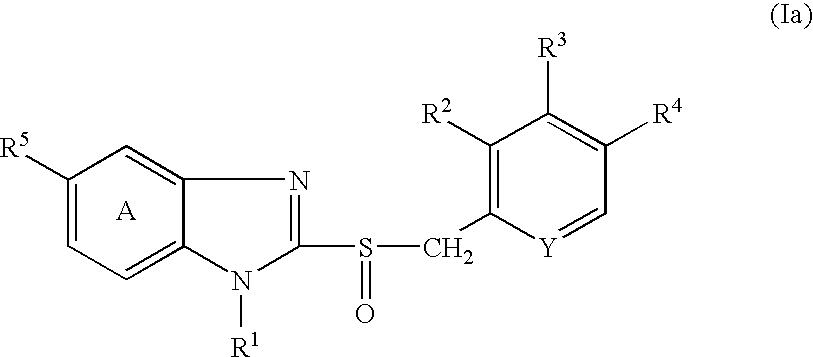
Pharmeceutical formulation comprising a proton pump inhibitor and antacids
InactiveUS20040219211A1Great tasteGuaranteed smooth progressBiocideDigestive systemEnteric-coated granulesPharmaceutical formulation
The present invention deals with a multiparticulate tablet, which disintegrates in the mouth containing: i) a proton pump inhibiting agent, in particular of the benzimidazole type, in the form of enteric coated microgranules, which enteric coated granules are overcoated with at least one barrier coating, such as for instance a methacrylic copolymer-based protective film; ii) at least one antacid in the form of granules, for instance based on CaCO3 and / or Mg(OH)2 and / or Al(OH)3; and, iii) a mixture of excipients comprising at least one disintegrating agent, one diluent agent, a lubricant, and optionally a swelling agent, a permeabilising agent, sweeteners, flavourings and colours. Furthermore, the present invention is directed to processes for the manufacture of the tablet and its use in the treatment of gastrointestinal disorders.
Owner:ASTRAZENECA AB
Montmorillonite loaded calcium hydroxide antacid
ActiveCN103961368AReduce solubilityWeaken Alkaline StrengthDigestive systemPharmaceutical non-active ingredientsGastric phBULK ACTIVE INGREDIENT
The invention aims to provide a montmorillonite loaded calcium hydroxide antacid which uses montmorillonite loaded calcium hydroxide as the antacid on the basis of acid activation of montmorillonite loaded calcium hydroxide, and solves the conventional problem caused by direct contact of active components of the antacid and the gastric mucosa, wherein the montmorillonite has a gastric mucosa protection function simultaneously. The montmorillonite loaded calcium hydroxide antacid is characterized in that the antacid takes medicinal montmorillonite as the raw material, the montmorillonite is taken as a carrier after acid activation, loaded calcium hydroxide serves as an active ingredient, fatty acid is adopted for surface modification, the carrier of the obtained antacid is stable and insoluble, calcium hydroxide serving as the active ingredient can be continuously and firmly loaded on the carrier, the alkaline intensity is weakened, excessive taking of the antacid cannot cause too high gastric pH, and further, the carrier improves the dispersibility of calcium hydroxide serving as the active ingredient and forms a protective barrier, so that mucosa stimulation is avoided.
Owner:GUANGXI UNIV
Method for making coated chewing gum products with a coating including an aldehyde flavor and a dipeptide sweetener
InactiveUS7115288B2Reduce debrisImproved pellet crunchContainers for annular articlesConfectioneryPolymer scienceDipeptide
A method of making coated chewing gum products comprises the steps of providing chewing gum cores; providing a first coating syrup comprising a bulk sweetener; providing an aldehyde flavor; providing a second coating syrup separate from the first coating syrup and comprising a dipeptide sweetener; and applying the first and second coating syrups to the cores and drying the syrups to produce a coating on the cores. Calcium carbonate or another antacid may be incorporated in the coating by being mixed into the first coating syrup or applied as a dry charge.
Owner:WM WRIGLEY JR CO
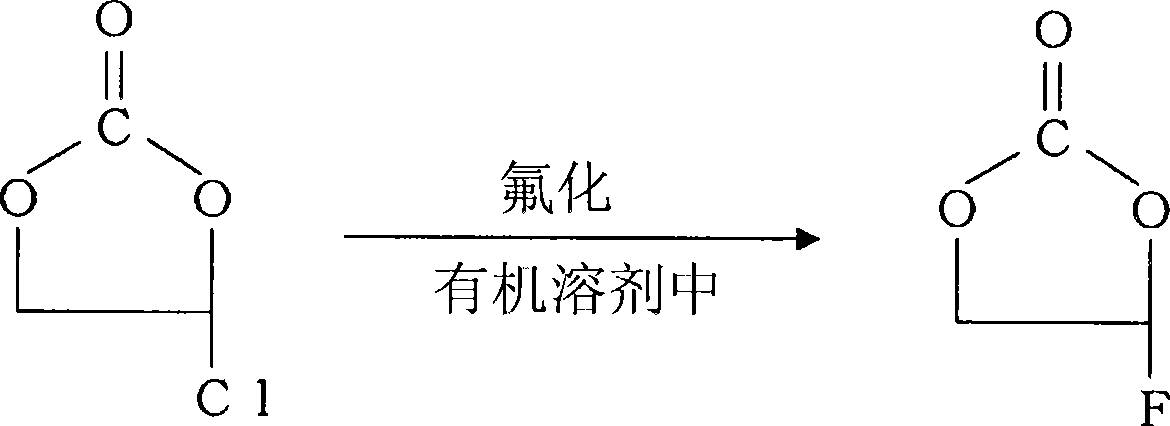
Preparation of 4-fluoro-1,3-dioxolane-2-one
The present invention provides a method for preparing 4-fluoro-1,3-dioxolane-2-one with reduced halide content at high yield. The preparation of 4-fluoro-1,3-dioxolane-2-one is characterized in that the preparation comprises: the process (A) of fluorizating 4-chloro-1,3-dioxolane-2-one by fluridizer in organic solvent; the process (B) of distilling reaction form solution 4-chloro-1,3-dioxolane-2-one; and the process (C) of treating by antacid.
Owner:DAIKIN IND LTD
Effervescence solid wine and preparation thereof
InactiveCN101298587AImprove solubilityEasy to storeWine preparationBeer brewingPreservative freePreservative
The invention discloses a solid wine, in particular to a solid effervescent wine, which is characterized in that: the solid wine adopts solid wine powder as raw material, and then auxiliary materials of antacids, alkaline agent, filler, adhesive, lubricant, sweetener, correction, toning agent, and stabilizer, etc. are added, to produce effervescent granules or effervescent tablets. When being put into the water, the solid effervescent wine disintegrates without any stirring, produces a great number of bubbles. Moving like the effervescent, the solid wine has the advantages of convenient storage, delivery and carrying, and no preservatives. The users can adjust the concentration of the wine in accordance with the needs. In addition, the solid wine is more suitable for travelling and for the elders and women who can not drink a lot.
Owner:张建武 +1
Pharmaceutical formulation and method for treating acid-caused gastrointestinal disorders
InactiveUS20050031700A1Good physical propertiesImprove bioavailabilityPowder deliveryBiocideGastrointestinal disorderSuspending Agents
Pharmaceutical formulations in the form of a powder for suspension comprising at least one proton pump inhibitor in micronized form; at least one antacid; and at lest one suspending agents are provided herein. Also provided herein are methods for making and using pharmaceutical formulations comprising at least one proton pump inhibitor and at least one antacid.
Owner:SANTARUS
Synergistic compositions and method for potentiating anti-oxidative activity
ActiveUS20070014876A1Protects against oxidative damageHigh activityPowder deliveryBiocidePharmacologyOxidative damage
The present invention relates to synergistic compositions for protection against oxidative damage, consisting essentially of antacids in conjunction with antioxidants. The invention further relates to method for preventing oxidative damage using the compositions of the invention.
Owner:THE STATE OF ISRAEL MINIST OF AGRI & RURAL DEV AGRI RES ORG ARO VOLCANI CENT
Method of stimulating the motility of the gastrointestinal system using ipamorelin
The present invention provides a method of stimulating the motility of the gastrointestinal system in a subject in need thereof, wherein the subject suffers from maladies (i.e., disorders, diseases, conditions, or drug- or surgery-induced dysfunction) of the gastrointestinal system, by administering to the subject a ghrelin mimetic, or pharmaceutically acceptable salt thereof. The invention also provides a method of treating a gastrointestinal malady by co-administering a ghrelin mimetic with a laxative, a H2 receptor antagonist, a serotonin receptor agonist, pure or mixed, an antacid, an opioid antagonist, a proton pump inhibitor, a motilin receptor agonist, dopamine antagonist, a cholinergic agonist, a cholinesterase inhibitor, somatostatin, octreotide, or any combination thereof.
Owner:HELSINN THERAPEUTICS (US) INC
Pharmaceutical formulation comprising a proton pump inhibitor and antacids
InactiveUS20110135722A1Guaranteed smooth progressSatisfactory enteric propertyBiocideHydroxy compound active ingredientsEnteric-coated granulesMicroparticle
Owner:CRIERE BRUNO +4
Antacid lozenge containing micronized particles
An organoleptically acceptable antacid lozenge including at least one antacid present in the form of micronized particles having a particle size of less than about 10 microns. The lozenge is a solid oral composition capable of dissolving slowly in the mouth for long lasting administration of an antacid at a sustained or slow release rate. The lozenge is non-gritty, smooth textured, and does not irritate the oral mucosa.
Owner:MCNEIL PPC INC
Controlled release solid preparation
InactiveUS20090104264A1Low toxicityImprove stabilityBiocideNervous disorderControl releaseChemical compound
The present invention provides a controlled release solid preparation superior in the stability of an active ingredient, which can exhibit pharmacological effects steadily and rapidly after administration, and shows a sustained pharmacological effect for a prolonged period of time: a controlled release solid preparation containing a combination of (1) an antacid, (2) an immediate-release part containing a compound unstable to acid and a basic substance, and (3) a sustained-release part containing a compound unstable to acid and a basic substance, and having a film that dissolves at pH 6.5 or above.
Owner:TAKEDA PHARMA CO LTD
Granular calcium carbonate for use as a dietary supplement and/or antacid
InactiveUS20020044974A1Improve featuresEasy to packBiocidePowder deliveryUpper gastrointestinalDietary supplement
A process for producing granular calcium carbonate for use as dietary supplements and / or antacids, which easily reconstitutes, upon mixing with a liquid or liquid-containing food prior to ingestion, into an ultra-fine calcium carbonate powder having improved non-gritty "mouthfeel". The process involves aggregating an ultra-fine calcium carbonate powder, having an average particle size of no more than 25 microns, into granular calcium carbonate particles. Methods for administering 100% of the adult RDA for calcium in a single dose, and for treating upper gastrointestinal tract disorders, using the granular calcium carbonate produced by the process of the present invention are also disclosed.
Owner:UNITED NUTRITION PROD
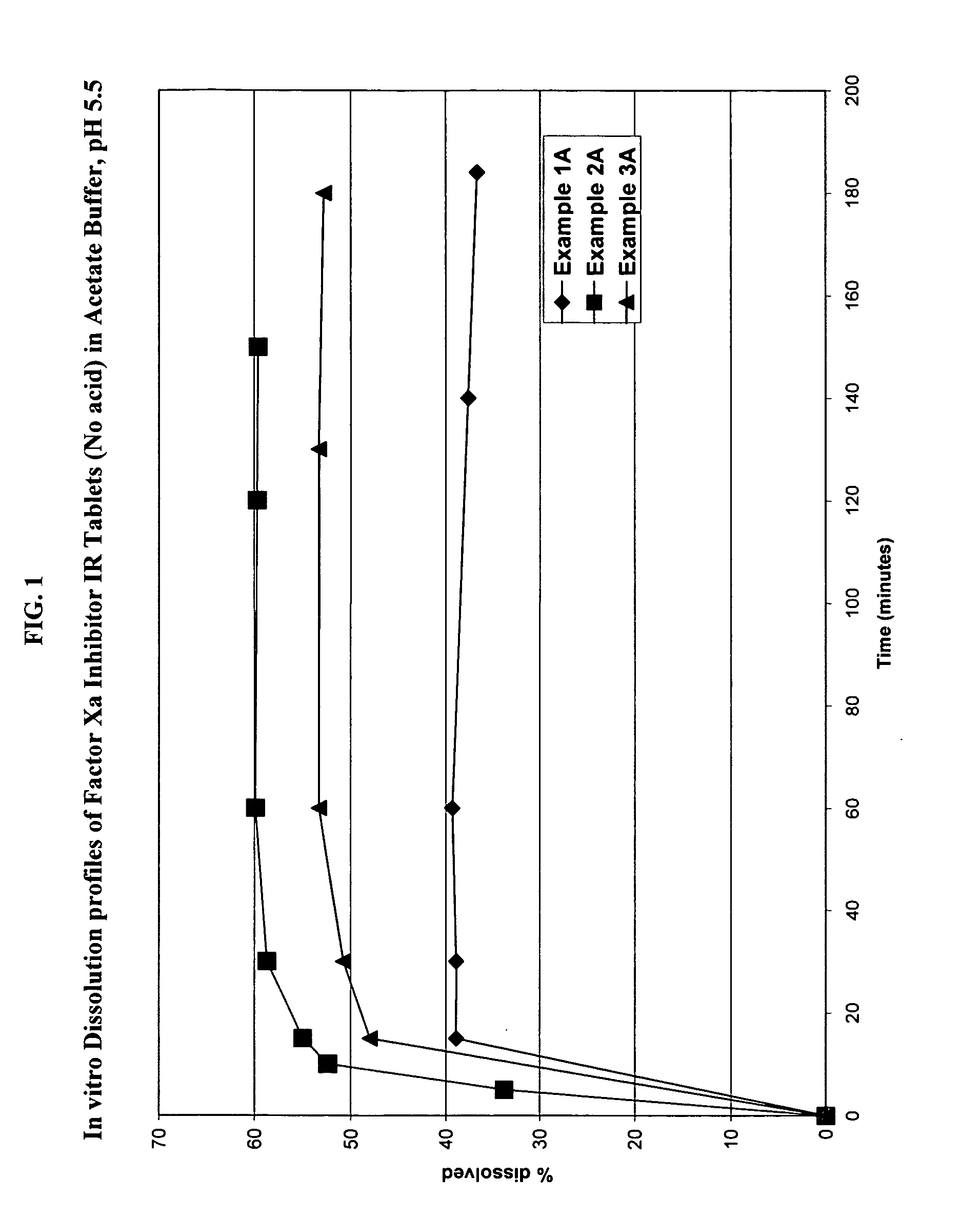
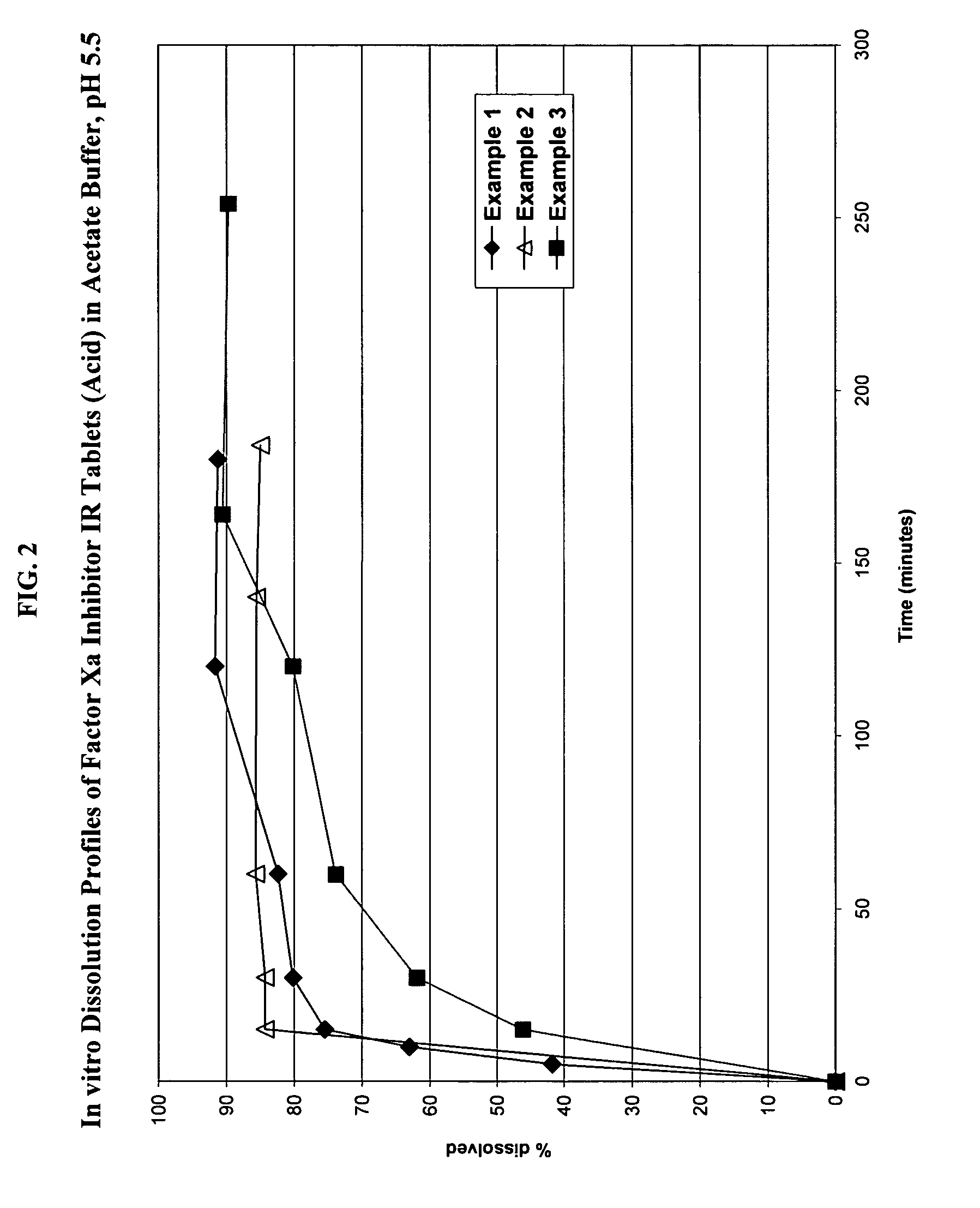
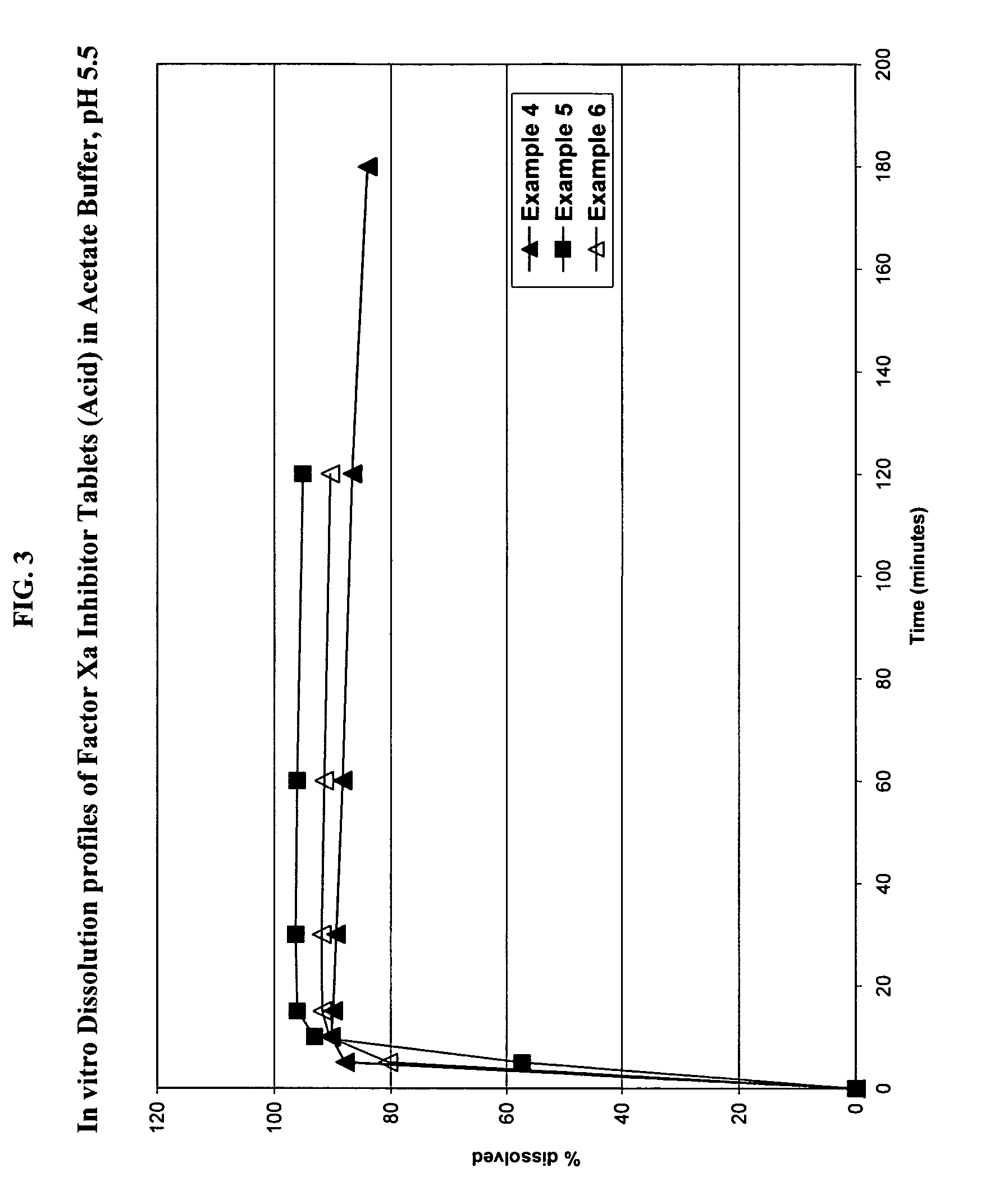
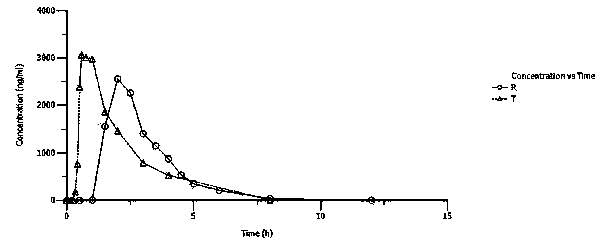
Solid dosage formulation containing a Factor Xa inhibitor and method
InactiveUS20050059719A1Improve oral bioavailabilityLow dissolutionBiocidePill deliverySolubilityCo administration
An oral solid dosage formulation is provided which contains a Factor Xa inhibitor for which oral bioavailability is not reduced by co-administration of antacids, H2 antagonists and proton pump inhibitors. Such solid dosage formulation includes the Factor Xa inhibitor of the structure, a pharmaceutically acceptable carrier, and an acid component, such as tartaric acid, whereby upon ingestion of the oral solid dosage formulation, the acid component increases solubility of the Factor Xa inhibitor in the local environment of the dissolving solid dosage formulation resulting in an otherwise lower degree of supersaturation of the Factor Xa inhibitor in such environment, than if the acid were not present. The result is that precipitation of the Factor Xa inhibitor in the form of its insoluble free base is minimized during dissolution of the Factor Xa inhibitor thereby increasing its oral bioavailability. A method for enhancing bioavailability of the Factor Xa inhibitor is also provided wherein an acid such as tartaric acid is incorporated with the solid dosage pharmaceutical carrier for the Factor Xa inhibitor.
Owner:BRISTOL MYERS SQUIBB CO
Antacid and laxative tablets
An antacid and laxative tablet comprising magnesium oxide particles as an effective component, wherein(i) the magnesium oxide particles contained in the tablet have an average secondary particle diameter measured by a laser diffraction scattering method of 0.5 to 10 μm,(ii) the content of magnesium oxide particles in the tablet is 88 wt % or more,(iii) the tablet does not become blackish and has substantially no tableting spot, and(iv) the disintegration time is 10 sec or less.The antacid and laxative tablet of the present invention has a high content of magnesium oxide particles, a short disintegration time, does not become blackish, has no tablet trouble and no tableting spot and is suitably administered orally.
Owner:KYOWA CHEM IND
Esomeprazole pharmaceutical composition and preparation thereof
InactiveCN103845734AAvoid degradation damageRaise the pHOrganic active ingredientsDigestive systemAluminium hydroxideStrong acids
The invention provides an esomeprazole pharmaceutical composition with stronger acid resistance and a preparation thereof. The esomeprazole pharmaceutical composition comprises esomeprazole, one or more antacids and common pharmaceutical adjuvants, wherein the esomeprazole comprises esomeprazole salt, particularly esomeprazole magnesium salt and esomeprazole lithium salt; and the antacids comprise sodium bicarbonate, sodium carbonate, potassium carbonate, potassium bicarbonate, calcium carbonate, aluminum carbonate, magnesium carbonate, magnesium hydroxide, magnesium oxide, aluminium hydroxide, magnesium aluminum carbonate and the like. The preparation of the esomeprazole pharmaceutical composition is prepared by adding the antacids via interior addition and exterior addition; and in an optimized preparation method, the weight ratio of the antacids in the interior addition and the exterior addition is 2: 3, so that the antacids play double efficacies, the dosage of acid neutralization agent is reduced and the acid resisting effect is better.
Owner:LIAONING YILING KECHUANG BIOLOGICAL MEDICAL TECH +1
Method of stimulating the motility of the gastrointestinal system using ipamorelin
InactiveUS20090143310A1Promote gastrointestinal motilityPeptide/protein ingredientsDigestive systemDiseaseOpioid antagonist
The present invention provides a method of stimulating the motility of the gastrointestinal system in a subject in need thereof, wherein the subject suffers from maladies (i.e., disorders, diseases, conditions, or drug- or surgery-induced dysfunction) of the gastrointestinal system, by administering to the subject a ghrelin mimetic, or pharmaceutically acceptable salt thereof. The invention also provides a method of treating a gastrointestinal malady by co-administering a ghrelin mimetic with a laxative, a H2 receptor antagonist, a serotonin receptor agonist, pure or mixed, an antacid, an opioid antagonist, a proton pump inhibitor, a motilin receptor agonist, dopamine antagonist, a cholinergic agonist, a cholinesterase inhibitor, somatostatin, octreotide, or any combination thereof.
Owner:HELSINN THERAPEUTICS (US) INC
New preparation method for aspirin-containing compound preparation
InactiveCN102198147AProne to decompositionGuaranteed disintegration release speedOrganic active ingredientsAntipyreticInsulation layerIrritation
The invention discloses a new preparation method for an aspirin-containing compound preparation, which is characterized in that: the aspirin-containing compound preparation is an internal and external multi-layer preparation consists of aspirin, and aluminum glycinate and heavy magnesium carbonate which are alkaline medicines; and an insulation layer is formed between the internal and externally-applied medicines to prevent the aspirin from contacting the alkaline medicines directly. The aluminum glycinate can neutralize gastric acid, relieve gastric acid increase, pain in upper abdomen and other clinic symptoms and has effects of astringing and stopping bleeding; and the heavy magnesium carbonate is an antiacid medicine and can reduce the adverse irritation of aspirin to the stomach, obviously reduce rate of gastric mucosal erosion and ulcer, thereby protecting the stomach.
Owner:JINAN RUIAN PHARMA DEV
Rapid-release compound omeprazole tablet and preparation method thereof
InactiveCN102793682AGuaranteed stabilityGuarantee function and effectOrganic active ingredientsDigestive systemCelluloseSodium bicarbonate
The invention relates to a rapid-release compound omeprazole tablet and a preparation method thereof; the tablet contains effective amounts of omeprazole, sodium bicarbonate and magnesium hydroxide, and selectively contains or does not contain one or more than one pharmaceutically accepted auxiliary material; the tablet is characterized in that the tablet contains a proton pump inhibitor of omeprazole, contains antacids of sodium bicarbonate and magnesium hydroxide which can neutralize gastric acid and change the pH environment in gastric juice, and also contains one or more than one of pharmaceutical auxiliary materials of hydroxy propyl cellulose, cross-linked sodium carboxymethyl cellulose, and sodium stearyl fumaryl. The tablet of the invention can effectively prevent degradation of omeprazole caused by acidic substances in gastric juice, and unexpectedly reach the purpose of rapid disintegration of the drug.
Owner:HUATUO MEDICINES CO LTD
Mixed metal compounds used as antacids
InactiveUS20100203152A1Reduce releaseImprove liquidityPowder deliveryHeavy metal active ingredientsBound waterCerium
There is provided use of a mixed metal compound in the manufacture of medicament for neutralising or buffering stomach acid, wherein the mixed metal compound contains at least one trivalent metal selected from iron (III) and aluminium and at least one divalent metal selected from of magnesium, iron, zinc, calcium, lanthanum and cerium, wherein (A) the mixed metal compound is of formula (I): MII1-aMIIIaObAn-c.zH2O (I) where MII is the at least one bivalent metal; MIII is the at least one trivalent metal; An- at least one n-valent anion; 2+a=2b+&Sgr;cn and &Sgr;cn<0.9a, and z is 2 or less, and / or (B) the mixed metal compound is provided in the form of a granular material comprising (i) at least 50% by weight, based on the weight of the granular material, of the mixed metal compound (ii) from 3 to 12% by weight, based on the weight of the granular material, of non-chemically bound water, and (iii) no greater than 47% by weight based on the weight of the granular material of excipient.
Owner:OPKO IRELAND GLOBAL HLDG LTD
Nitrosated and nitrosylated H2 receptor antagonist compounds, compositions and methods of use
InactiveUS6936627B2Increase ratingsLow recurrence rateAntibacterial agentsBiocideDiseaseGastrointestinal toxicity
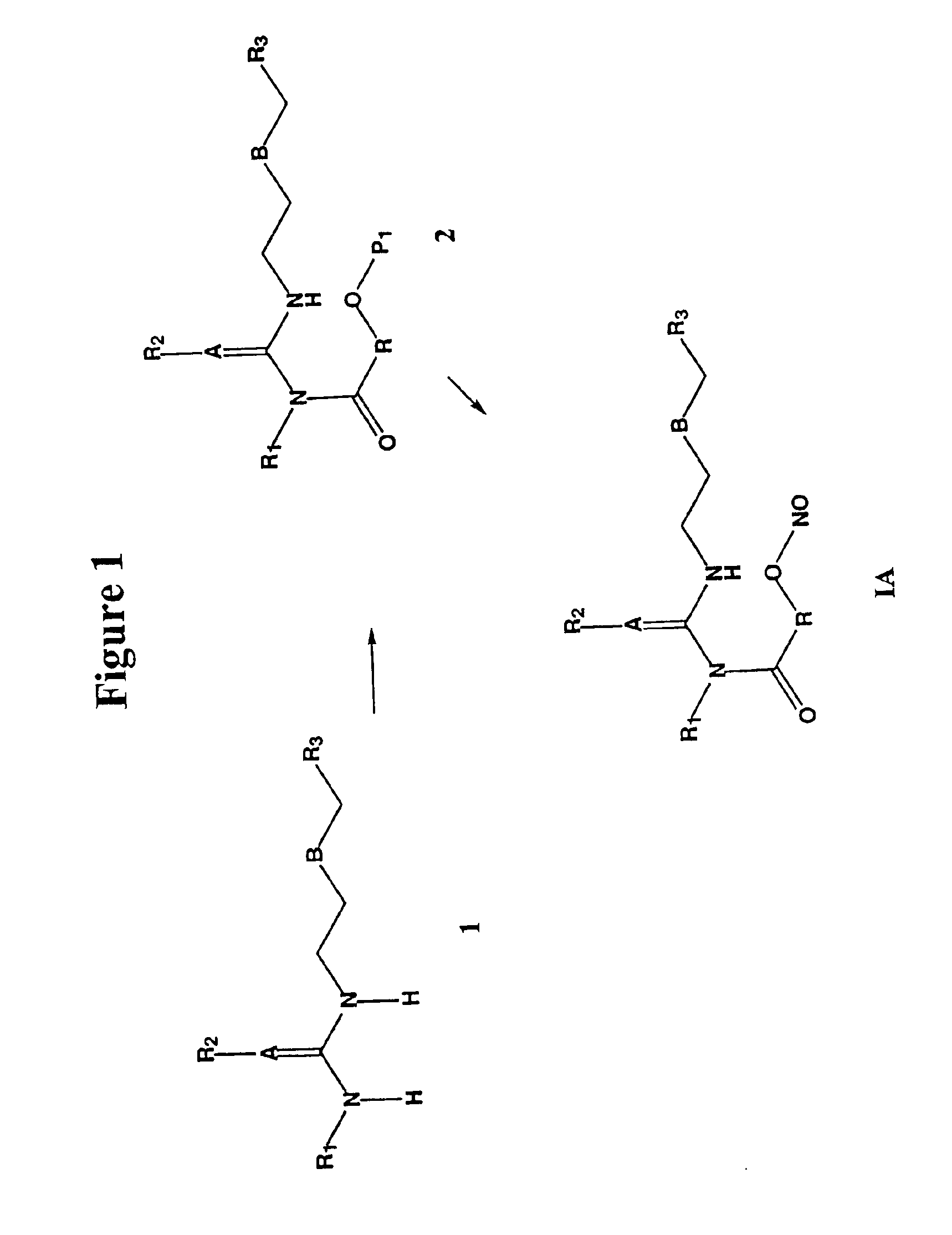
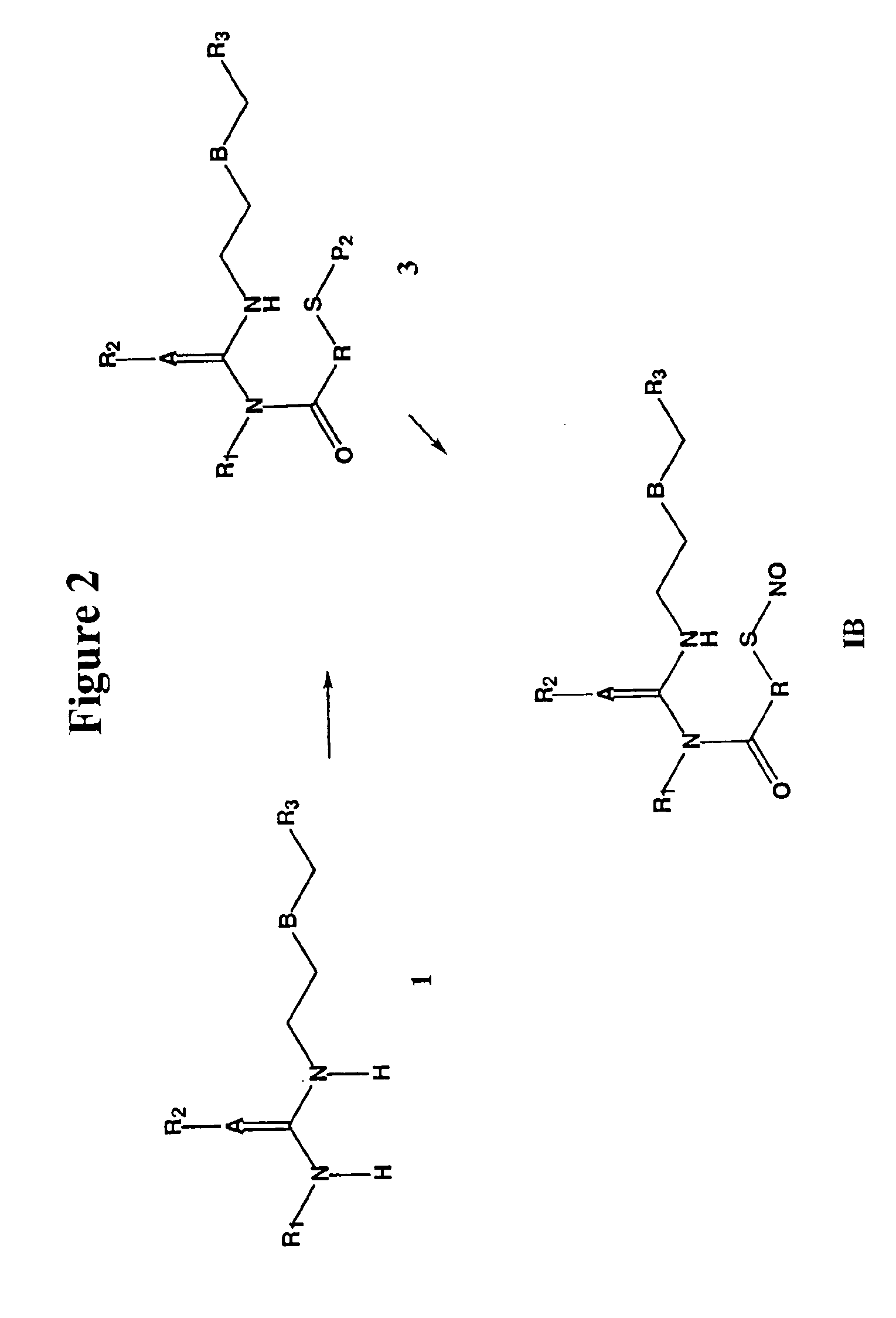
The invention describes novel nitrosated and / or nitrosylated H2 receptor antagonist compounds, and novel compositions comprising at least one H2 receptor antagonist compound that is optionally substituted with at least one NO and / or NO2 group, and, optionally, at least one compound that donates, transfers or releases nitric oxide, stimulates endogenous synthesis of nitric oxide, elevates endogenous levels of endothelium-derived relaxing factor or is a substrate for nitric oxide synthase and / or at least one nonsteroidal antiinflammatory drug, antacid, bismuth-containing reagent or anti-viral agent. The invention also describes methods for treating and / or preventing gastrointestinal disorders; improving gastroprotective properties of H2 receptor antagonists; decreasing the recurrence of ulcers; facilitating ulcer healing; preventing and / or treating inflammations and microbial infections, ophthalmic diseases and disorders, multiple sclerosis, and viral infections; and decreasing or reducing the gastrointestinal toxicity associated with the use of nonsteroidal antiinflammatory compounds.
Owner:NICOX SA
Compound Omeprazole tablets and preparation method thereof
InactiveCN102335148ASimple methodImprove stabilityOrganic active ingredientsDigestive systemSodium bicarbonateWater soluble
The invention discloses compound Omeprazole tablets, comprising the following ingredients: 20-40 weight portions of Omeprazole, 500-1000 weight portions of sodium bicarbonate, 200-652 weight portions of magnesium hydroxide, 10-30 weight portions of opacifier, 20-40 weight portions of water-soluble polymer material, 20-40 weight portions of disintegrating agent, and 5-10 weight portions of lubricant. The dissolution time of Omeprazole in the compound Omeprazole tablets is slightly lagged, so that antacid is dissolved in advance to neutralize the gastric acid, and the purposes of reducing drug degradation, gastricsolubility and quick release are achieved; and simultaneously, the light stability is increased, and the production operation and storage is convenient.
Owner:重庆健能医药开发有限公司
Dronedarone hydrochloride pharmaceutical composition and preparation method thereof
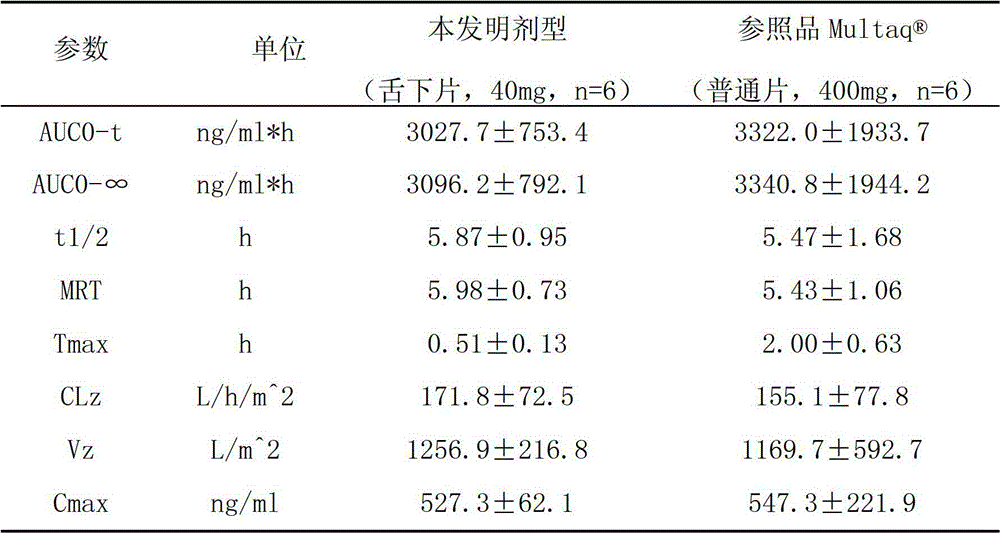
ActiveCN103054820ASmooth industrial productionPromote absorptionOrganic active ingredientsPill deliveryPatient complianceAdhesive
The invention provides a dronedarone hydrochloride pharmaceutical composition which is suitable for the sublingual administration, can quickly run, and can greatly improve the bioavailability and the curative effect. The pharmaceutical composition consists of 5-65 parts of dronedarone hydrochloride, 1-10 parts of a disintegrating agent, 10-80 parts of filler, 0.5-3 parts of antacid, 0-5 parts of corrigent, 1-10 parts of an adhesive and 0.3-2 parts of a lubricating agent. The invention further provides a preparation method suitable for the industrial production. The dronedarone hydrochloride pharmaceutical composition provided by the invention is directly absorbed by the sublingual mucosa, and the first-pass effect of oral medicaments can be avoided, and the gastrointestinal digestion, acidolysis and the like can be avoided, so that the bioavailability can be greatly improved. The epithelium of the sublingual mucosa is not cornified, so that the dronedarone hydrochloride pharmaceutical composition is large in superficial area, and high in infiltration capacity. The medicament can be quickly absorbed after being administrated, so that the dronedarone hydrochloride pharmaceutical composition is quick to run, and convenient to use; and compared with the other administrated preparations, the dronedarone hydrochloride pharmaceutical composition is convenient to administrate, and good in patient compliance.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com