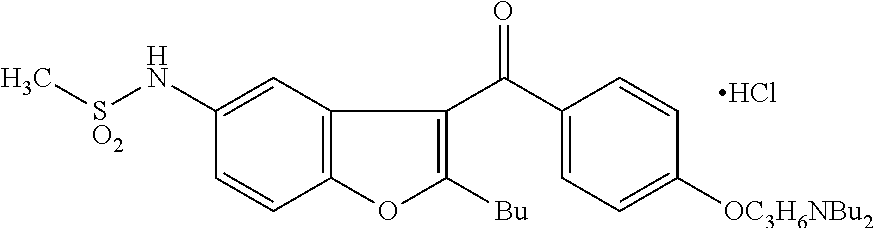
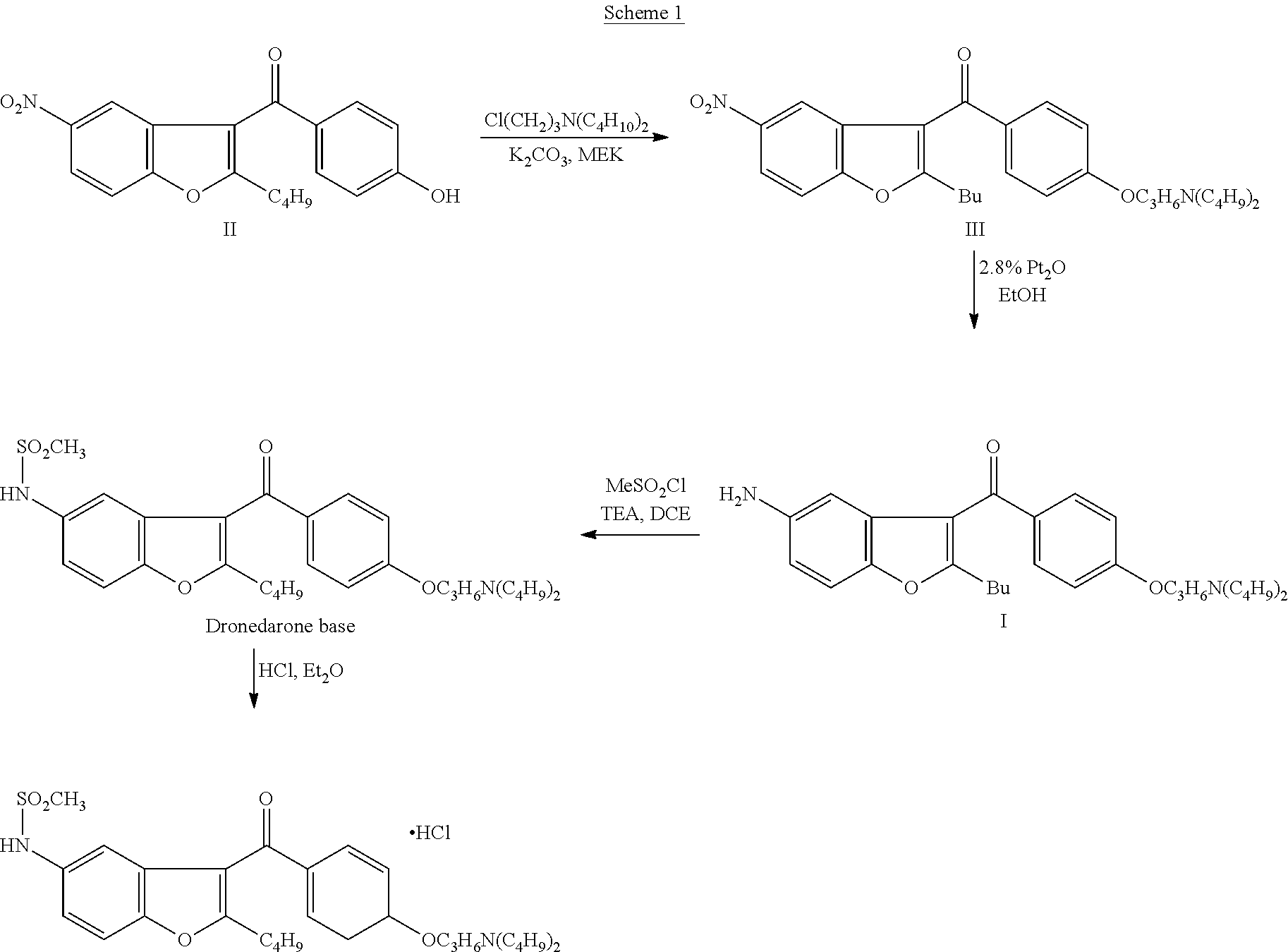
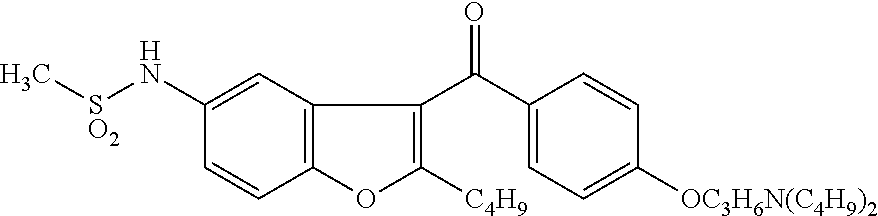
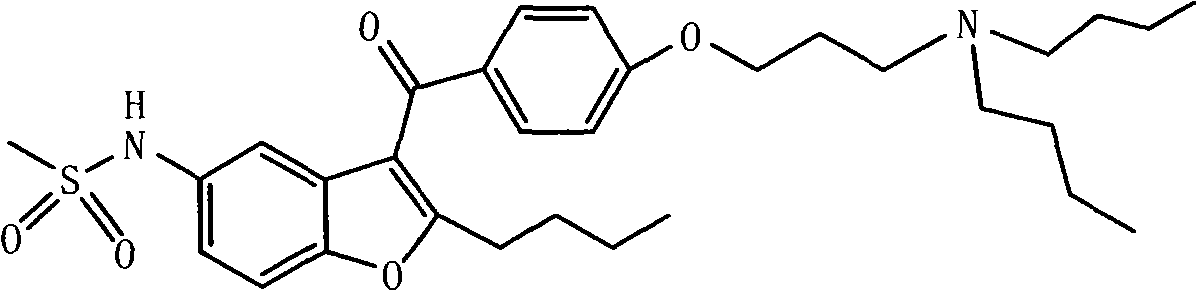
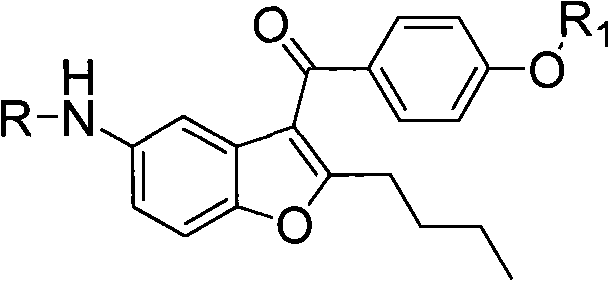
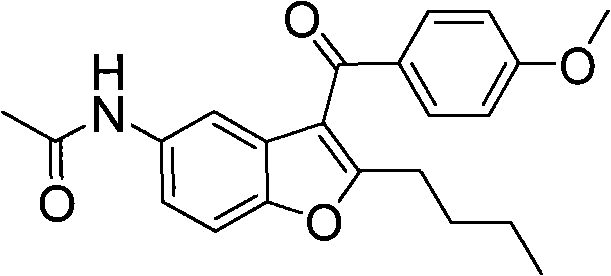
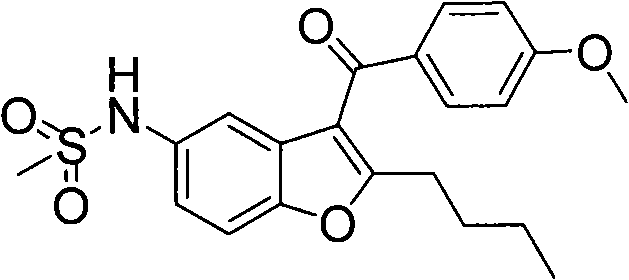
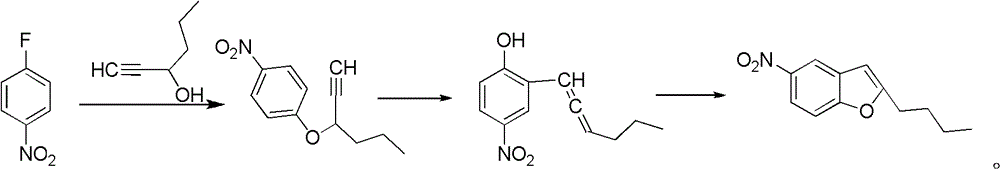
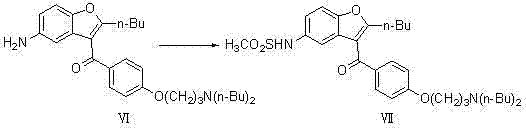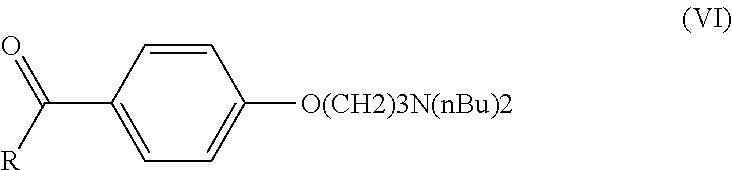Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
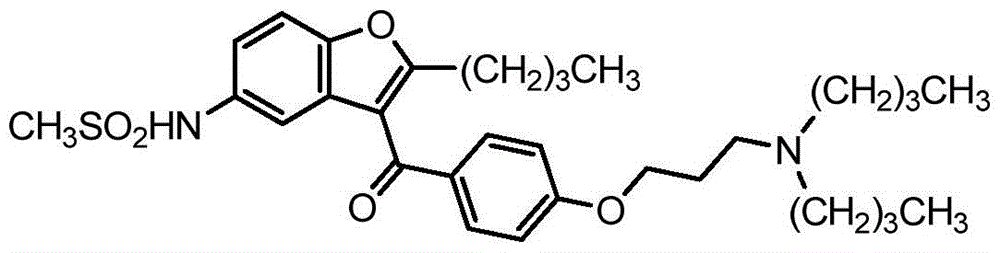
57 results about "Dronedarone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
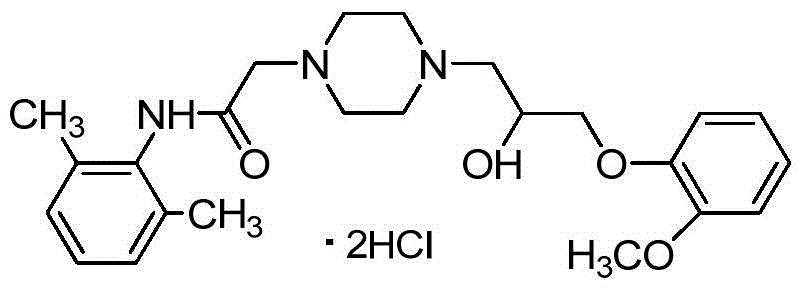
This medication is used if you have had certain types of irregular heartbeat (paroxysmal or persistent atrial fibrillation) in the past but now have a normal heart rhythm.
Method of treating atrial fibrillation
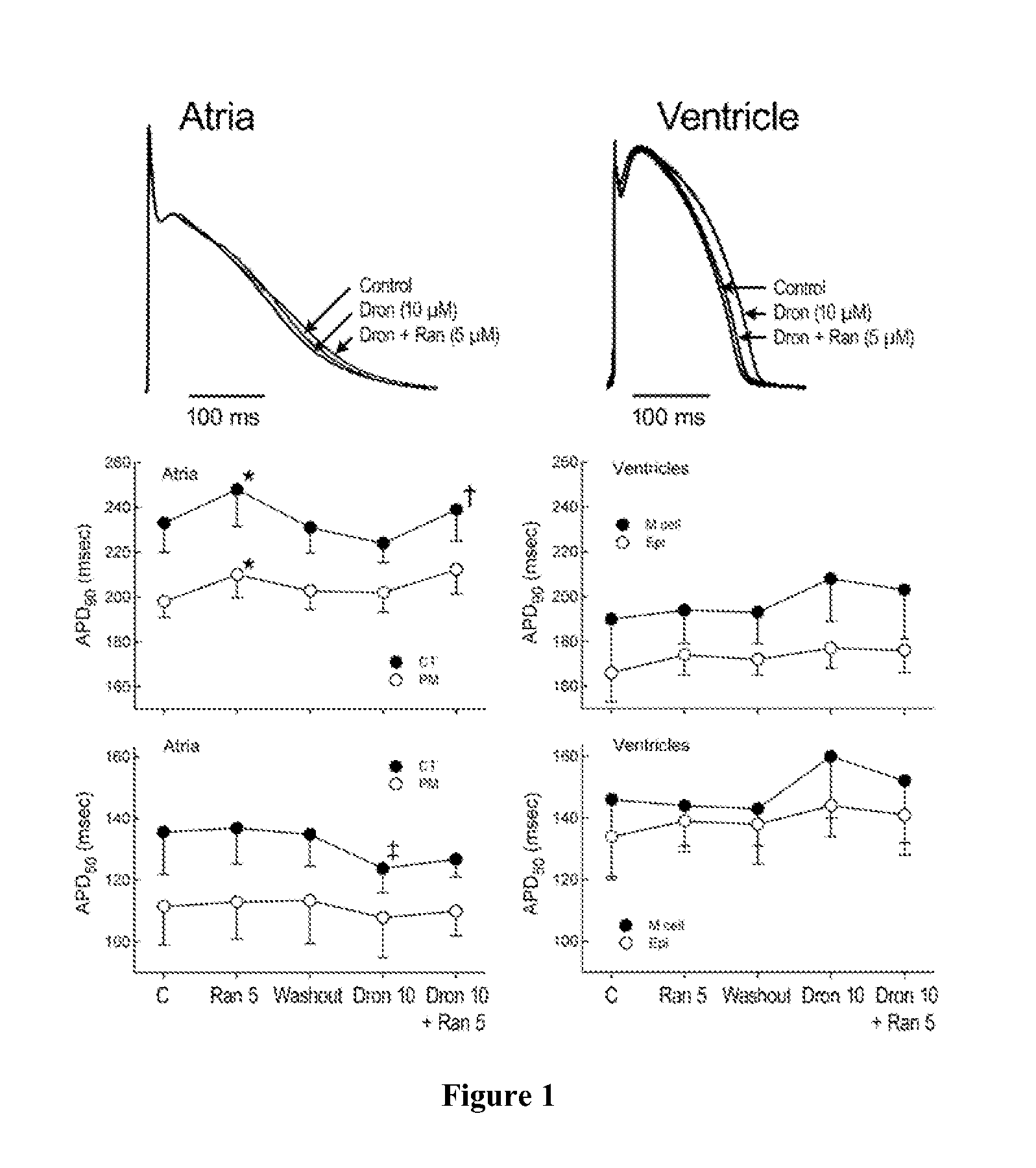
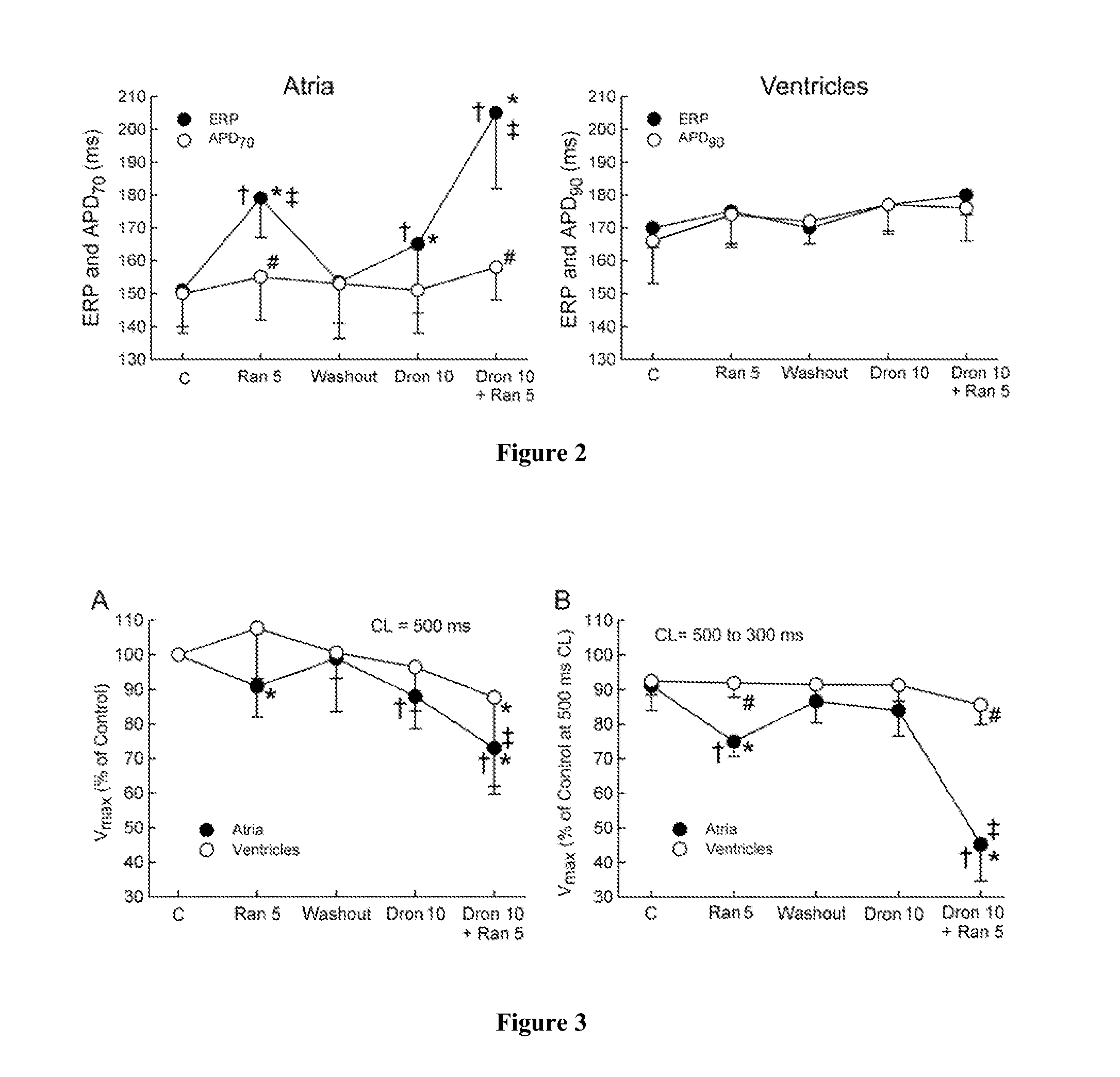
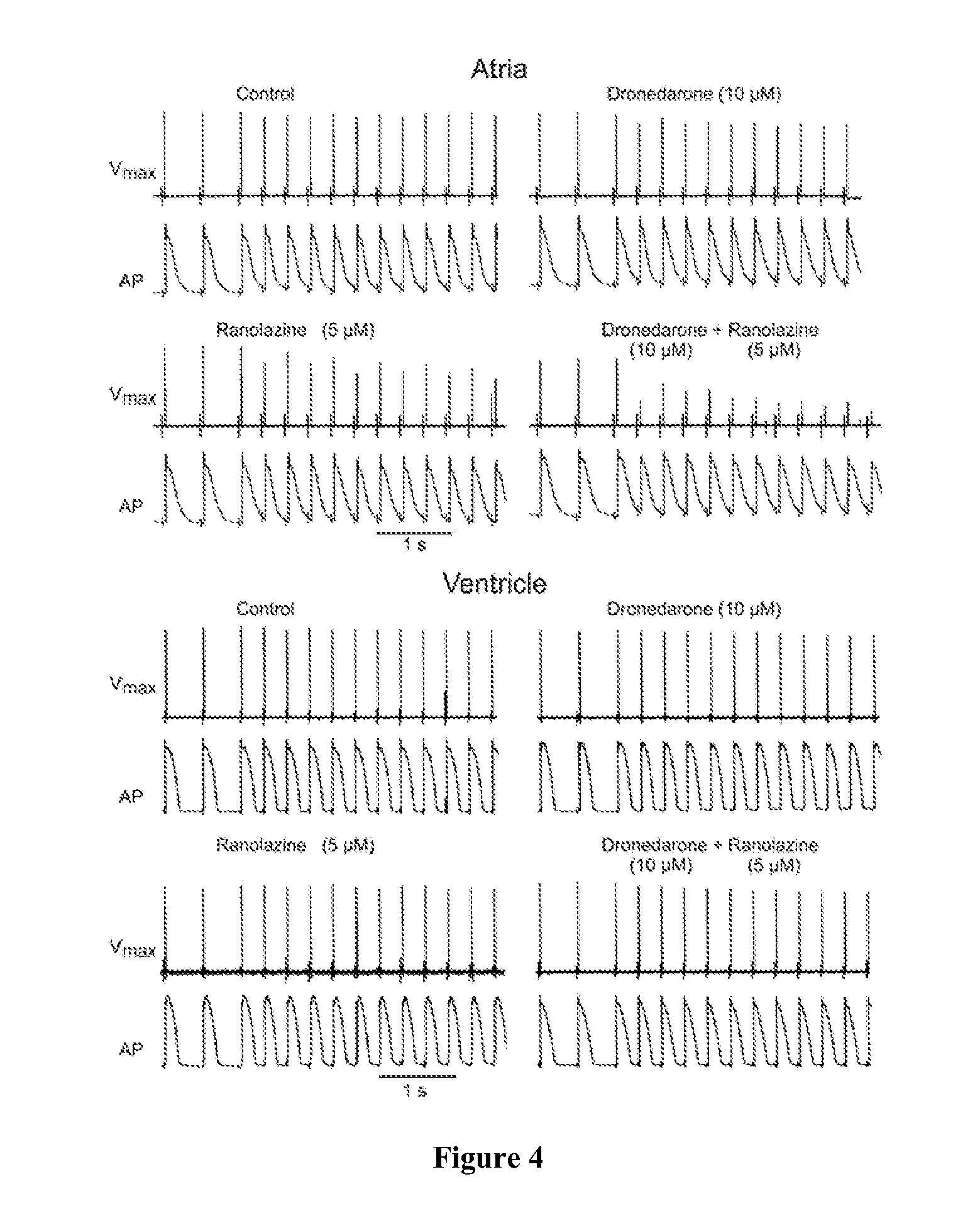
InactiveUS20110183990A1Reduce the amplitudeReduce adverse side effectsBiocideAnimal repellantsDronedaroneRanolazine
The present invention relates to a method for the treatment or prevention of atrial fibrillation and / or atrial flutter comprising coadministration of a synergistically therapeutic amount of dronedarone or a pharmaceutically acceptable salt or salts thereof and a synergistically therapeutic amount of ranolazine or a pharmaceutically acceptable salt or salts thereof. Also provided are methods for modulating ventricular and atrial rhythm and rate. This invention also relates to pharmaceutical formulations that are suitable for such combined administration.
Owner:GILEAD SCI INC
Hydrochloric acid dronedarone medicinal compositions for oral use and method for preparing the same
InactiveCN101152154ASolving Dissolution ProblemsOrganic active ingredientsPill deliveryDronedaroneSolvent
The invention provides a solid drug combination, consisting of micronized hydrochlorid Dronedarone, surfactant and hydrophilic polymer which is used as cosolvent. The invention is mainly used to cure arrhythmia.
Owner:BEIJING VENTUREPHARM BIOTECH
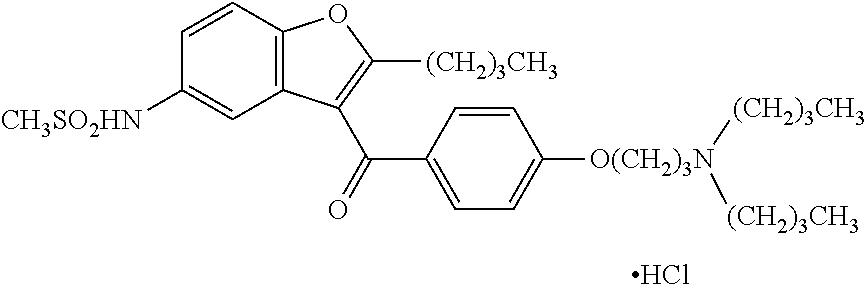
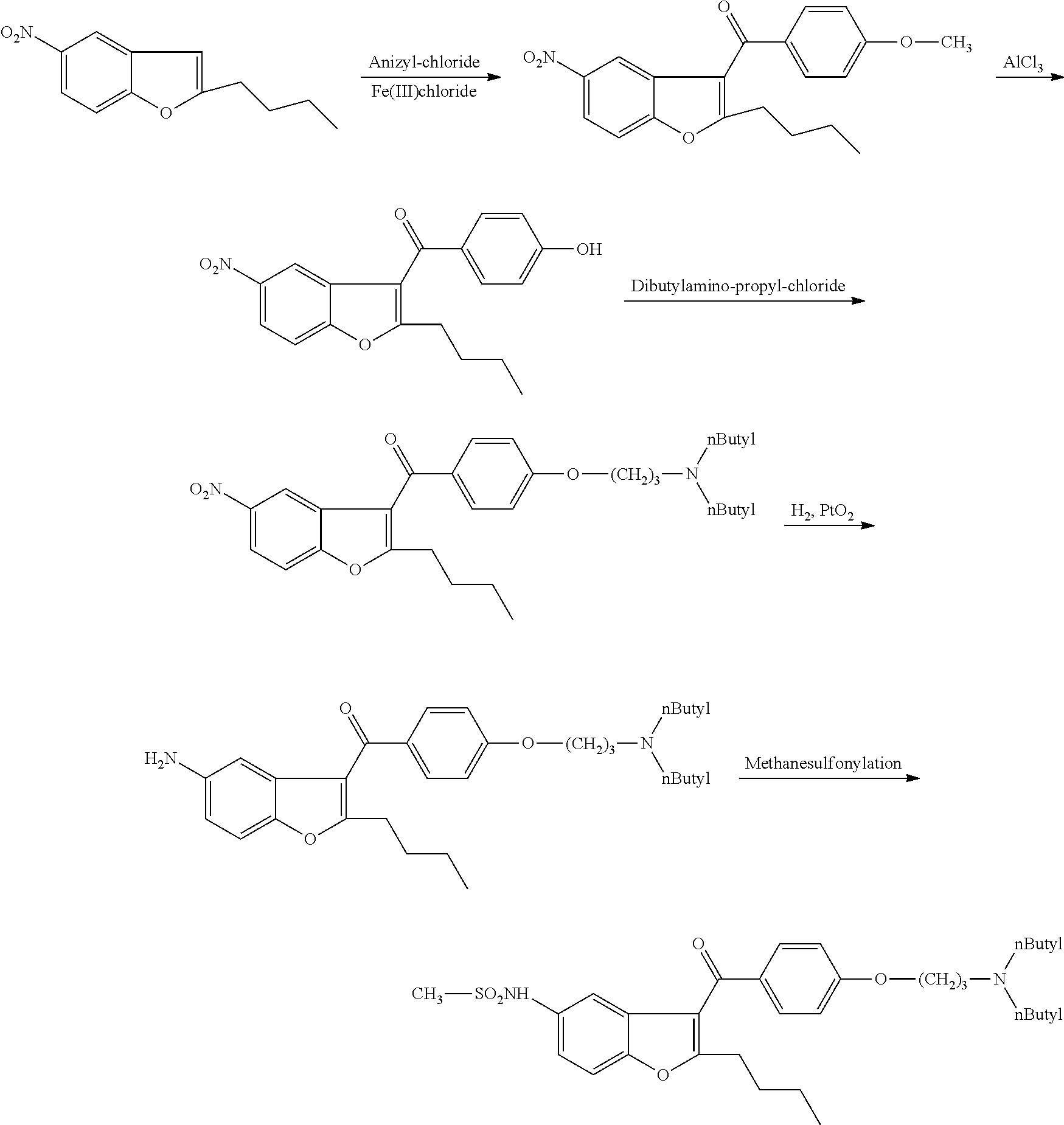
Process for the preparation of dronedarone
InactiveUS20050049302A1High yieldLow costBiocideCarbamic acid derivatives preparationAluminium chlorideDronedarone
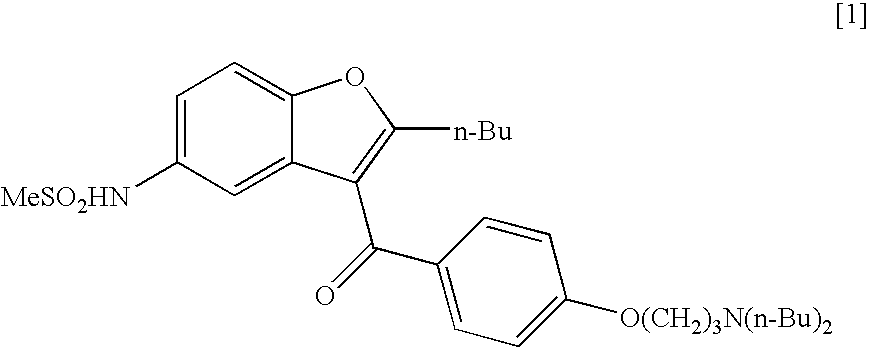
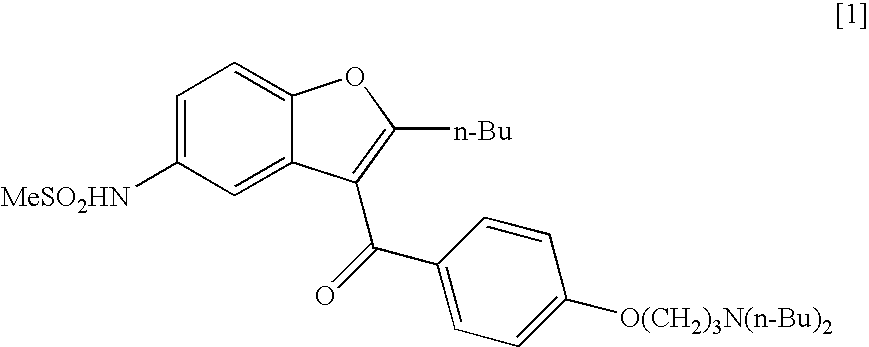
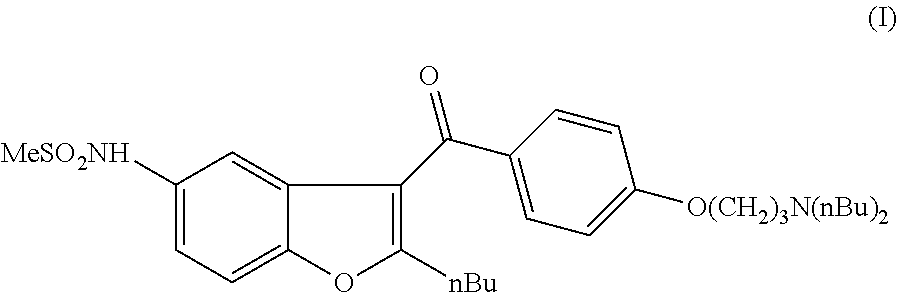
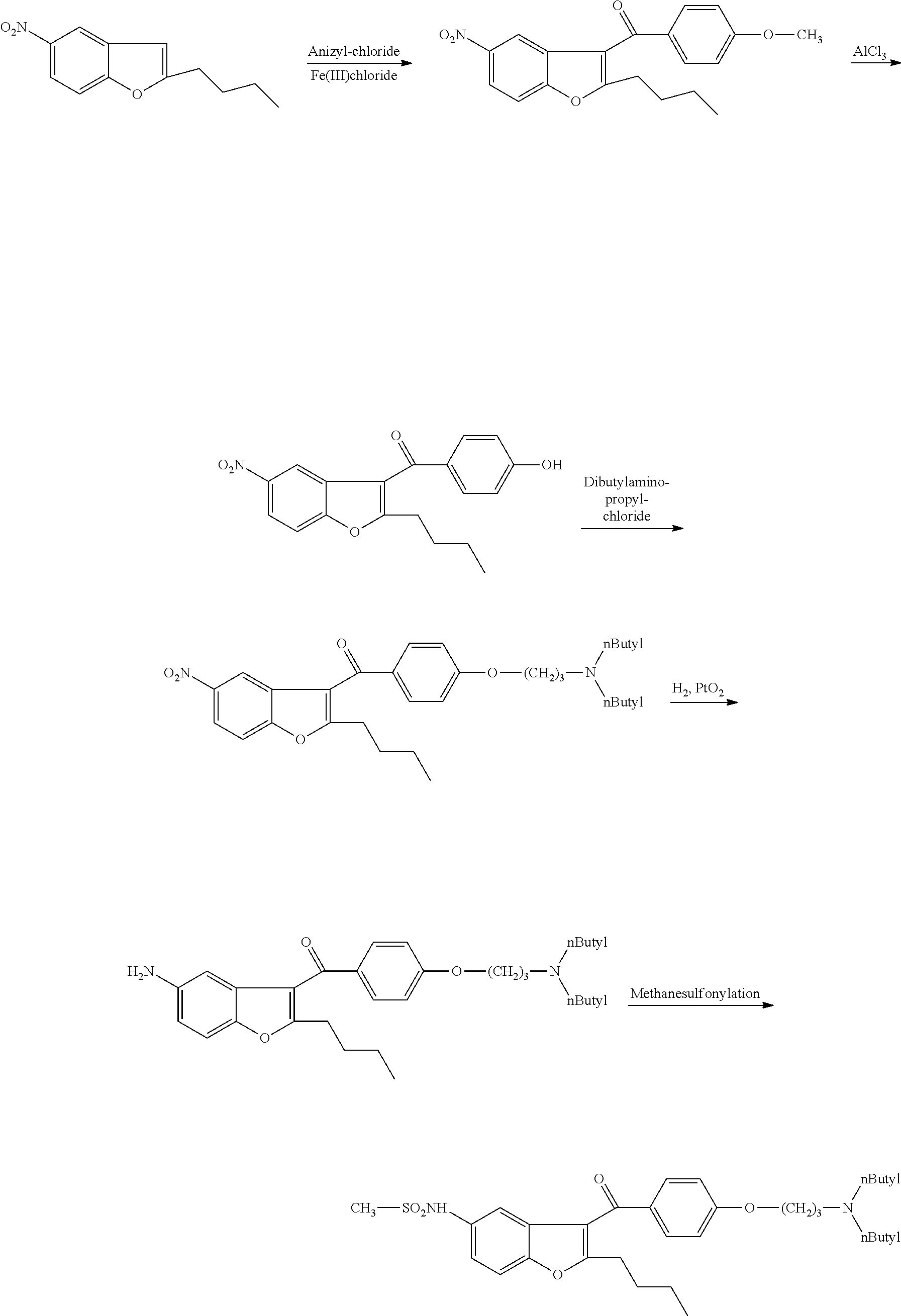
The present invention provides, according to an aspect thereof, a novel process for the preparation of dronedarone [1] and pharmaceutically acceptable salts thereof. According to a preferred embodiment, the process comprises N-acetylating of p-anisidine or p-phenetidine with acetic anhydride, reacting of the obtained N-(4-alkoxyphenyl)acetamide with 2-bromohexanoyl chloride or bromide in the presence of aluminum chloride or bromide to obtain N-[3-(2-bromohexanoyl)-4-hydroxyphenyl]acetamide [6a], converting the compound [6a] into 2-butyl-5-benzofuranamine hydrochloride [12a] and subsequently converting [12a] into [1] or pharmaceutically acceptable salts thereof. In accordance with another aspect of this invention, there are provided novel intermediates, inter alia the novel compounds [6a] and [12a]. The novel intermediates of the present invention are stable, solid compounds, obtainable in high yields, which can be easily purified by crystallization and stored for long periods of time.
Owner:ISP INVESTMENTS LLC
Process for the preparation of dronedarone
InactiveUS7312345B2High yieldLow costBiocideCarbamic acid derivatives preparationAluminium chlorideAcetic anhydride
The present invention provides, according to an aspect thereof, a novel process for the preparation of dronedarone [1] and pharmaceutically acceptable salts thereof. According to a preferred embodiment, the process comprises N-acetylating of p-anisidine or p-phenetidine with acetic anhydride, reacting of the obtained N-(4-alkoxyphenyl)acetamide with 2-bromohexanoyl chloride or bromide in the presence of aluminum chloride or bromide to obtain N-[3-(2-bromohexanoyl)-4-hydroxyphenyl]acetamide [6a], converting the compound [6a] into 2-butyl-5-benzofuranamine hydrochloride [12a] and subsequently converting [12a] into [1] or pharmaceutically acceptable salts thereof. In accordance with another aspect of this invention, there are provided novel intermediates, inter alia the novel compounds [6a] and [12a]. The novel intermediates of the present invention are stable, solid compounds, obtainable in high yields, which can be easily purified by crystallization and stored for long periods of time.
Owner:ISP INVESTMENTS LLC
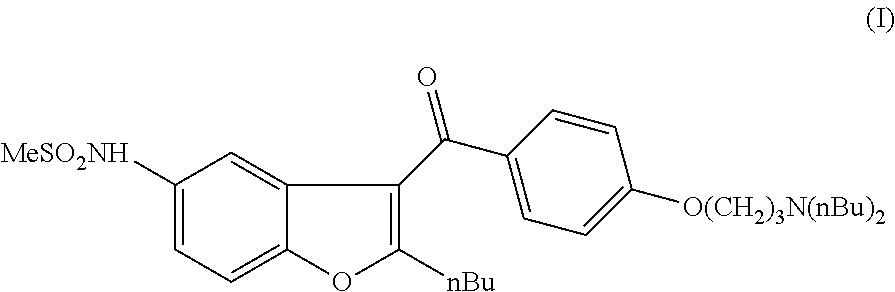
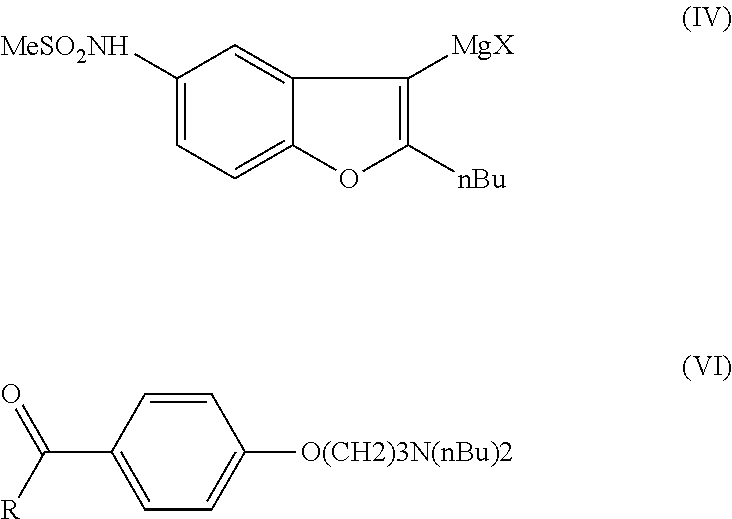
Process for the preparation of dronedarone by oxidation of a sulphenyl group
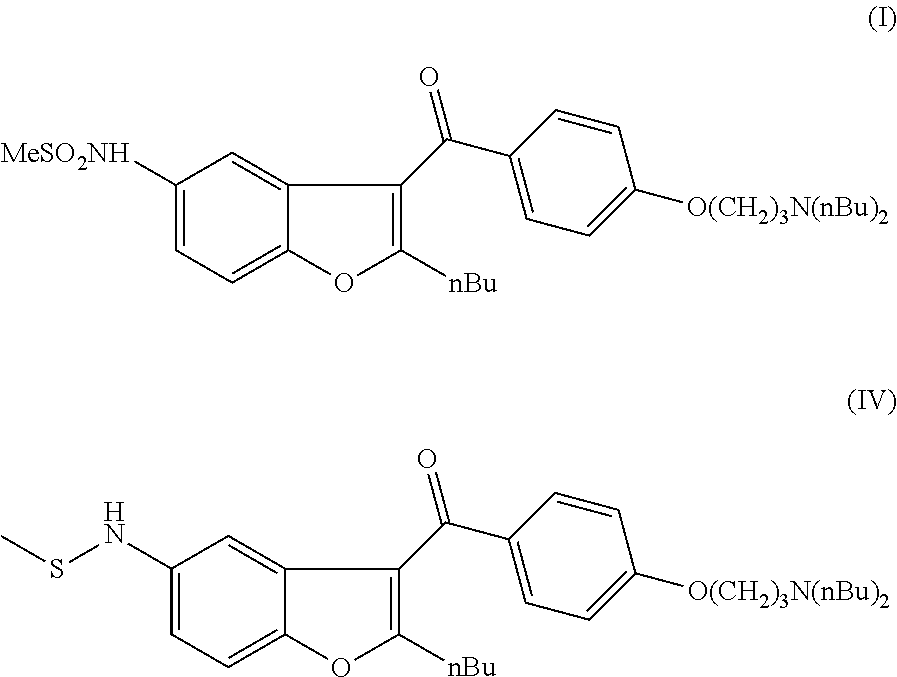
The invention relates to a novel process for the preparation of dronedarone (I) and pharmaceutically acceptable salts thereof which comprises oxidizing a compound of formula (IV) or a salt thereof with an oxidizing agent in an organic or inorganic solvent or solvent mixture, and isolating the obtained product and, if desired, converting it into a pharmaceutically acceptable salt thereof. Further aspects of the invention include the novel intermediary compound of formula (IV), and a process for the preparation thereof.
Owner:SANOFI SA
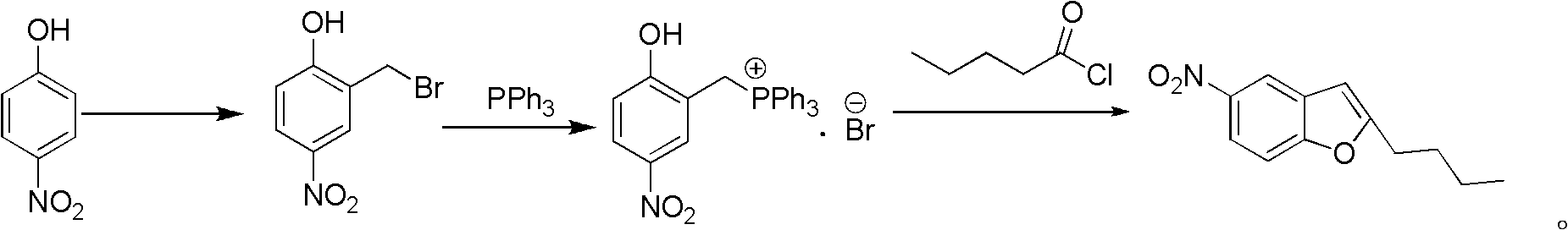
Method for synthesis of dronedarone
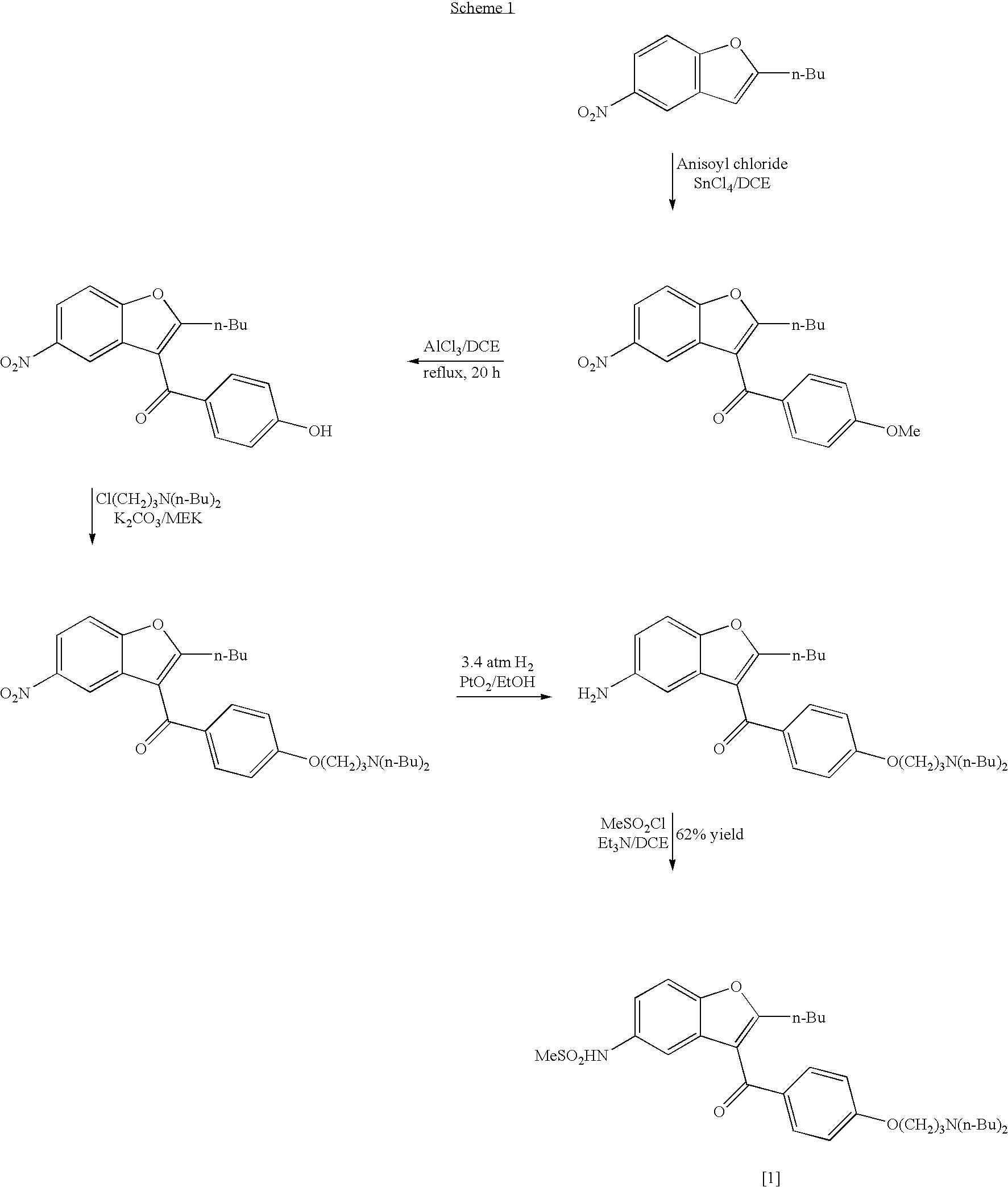
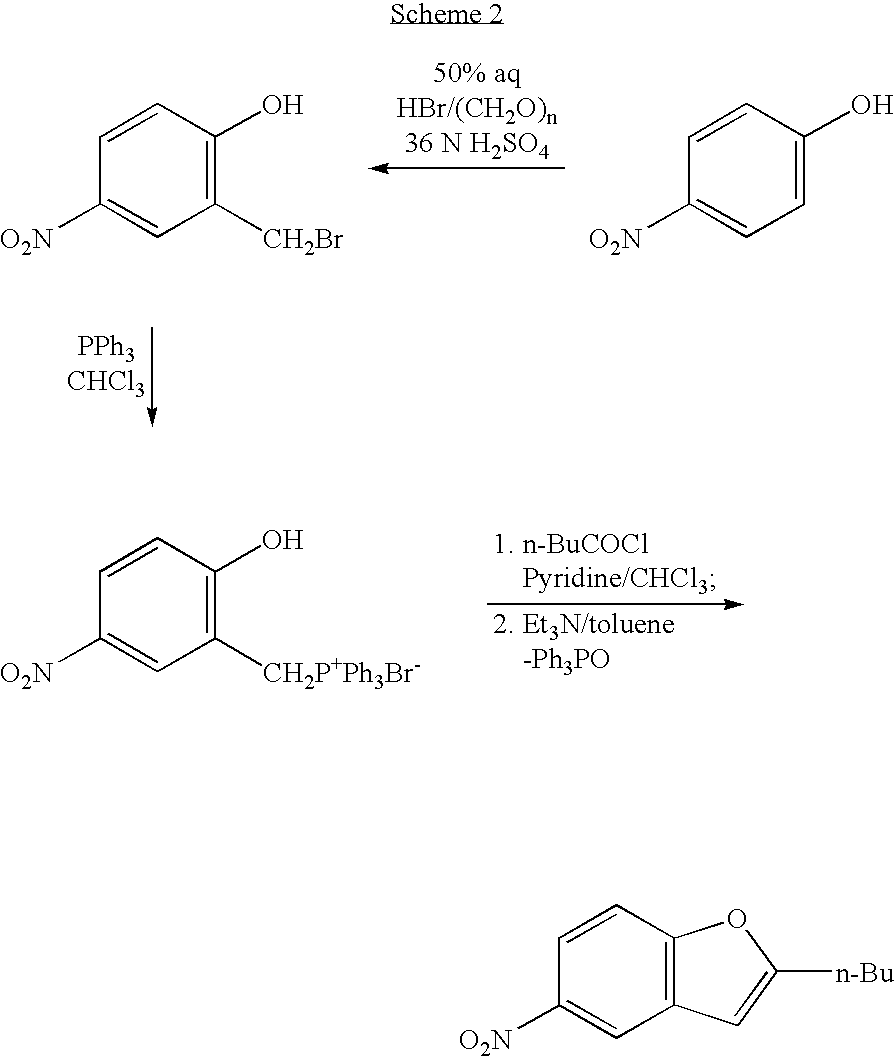
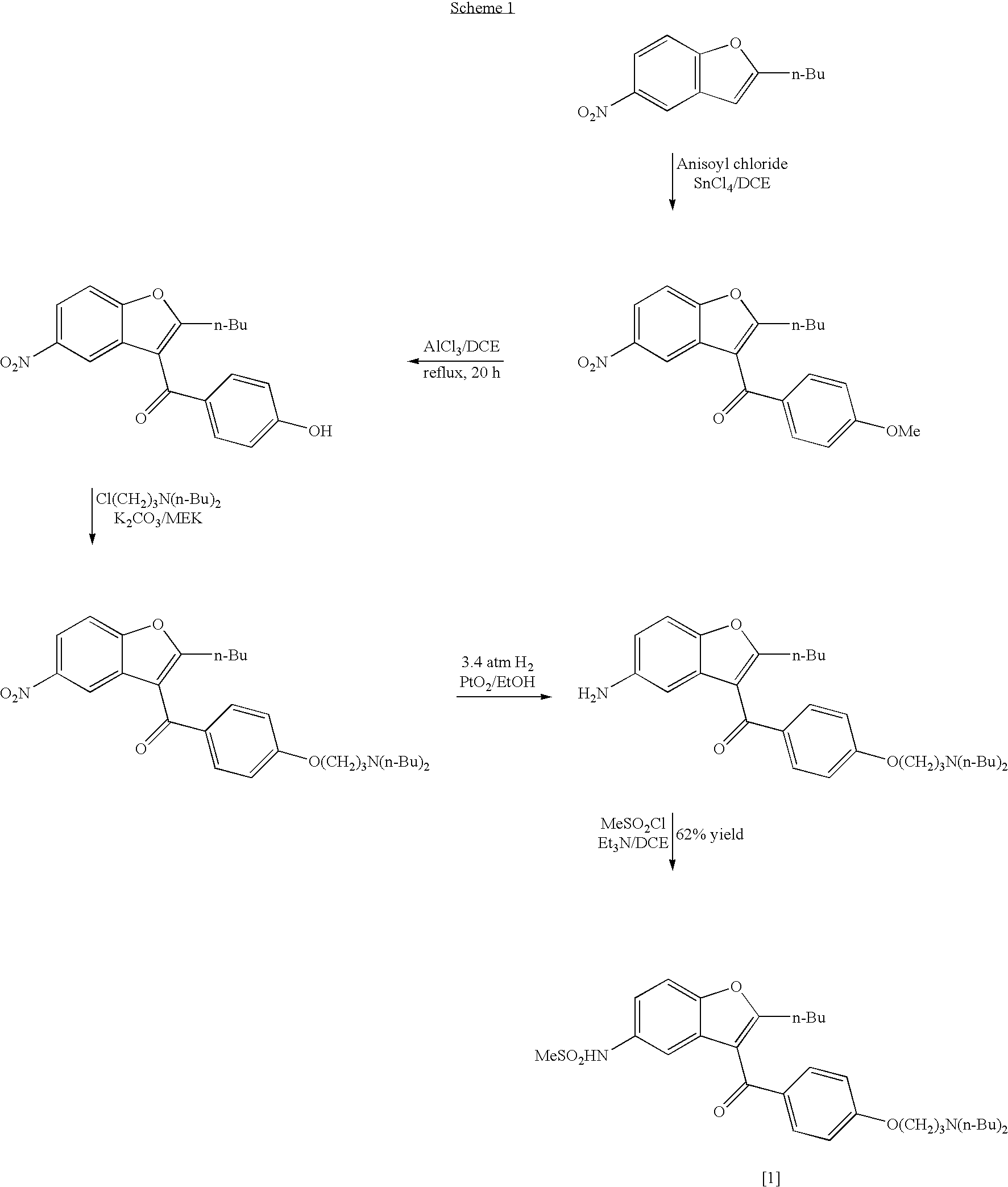
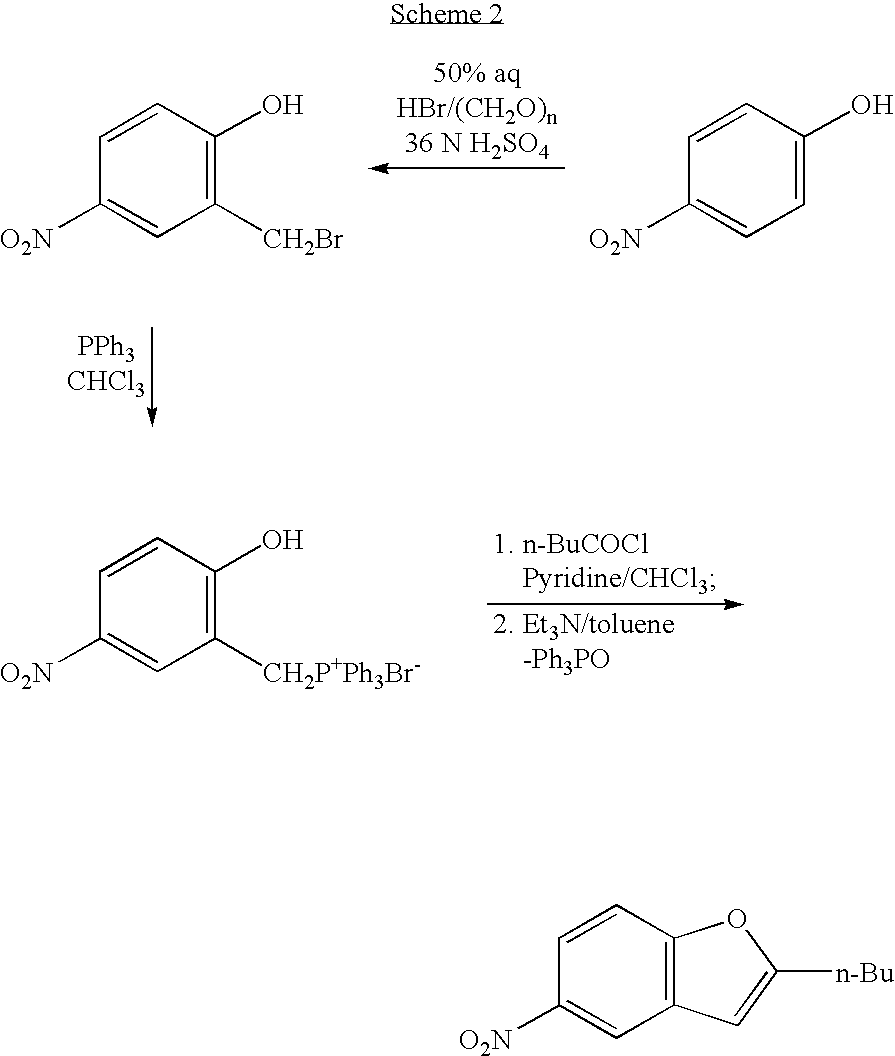
The invention relates to a method for synthesis of dronedarone. The method comprises the following steps that 1), p-nitrophenol, paraformaldehyde and concentrated hydrochloric acid as raw materials undergo a condensation reaction in the presence of concentrated hydrochloric acid or phosphoric acid as a catalyst to produce 2-chloromethyl-4-nitrophenol; 2), 2-chloromethyl-4-nitrophenol and triphenylphosphine undergo a reflux reaction in the presence of chloroform to produce 2-hydroxy-5-nitrobenzyl-triphenyl-phosphonium chloride, and 3), 2-hydroxy-5-nitrobenzyl-triphenyl-phosphonium chloride and n-valeryl chloride undergo a condensation reaction in a toluene solution in the presence of triethylamine and n-pentanoic acid as catalysts to produce 2-(n-butyl)-5-nitrobenzofuran.
Owner:FUJIAN COSUNTER PHARMA
Hydrochloric acid dronedarone medicinal compositions for oral use and method for preparing the same
InactiveCN100560067CSolving Dissolution ProblemsOrganic active ingredientsPill deliveryDronedaroneSolvent
The invention provides a solid drug combination, consisting of micronized hydrochlorid Dronedarone, surfactant and hydrophilic polymer which is used as cosolvent. The invention is mainly used to cure arrhythmia.
Owner:BEIJING VENTUREPHARM BIOTECH
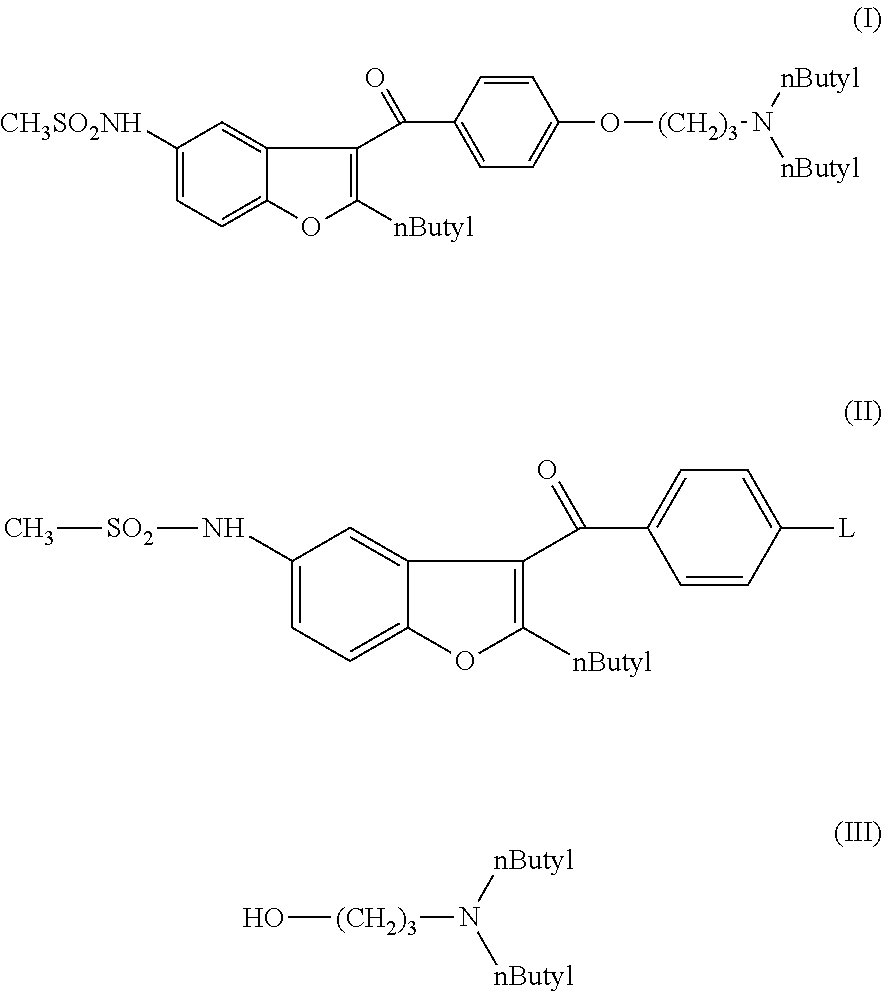
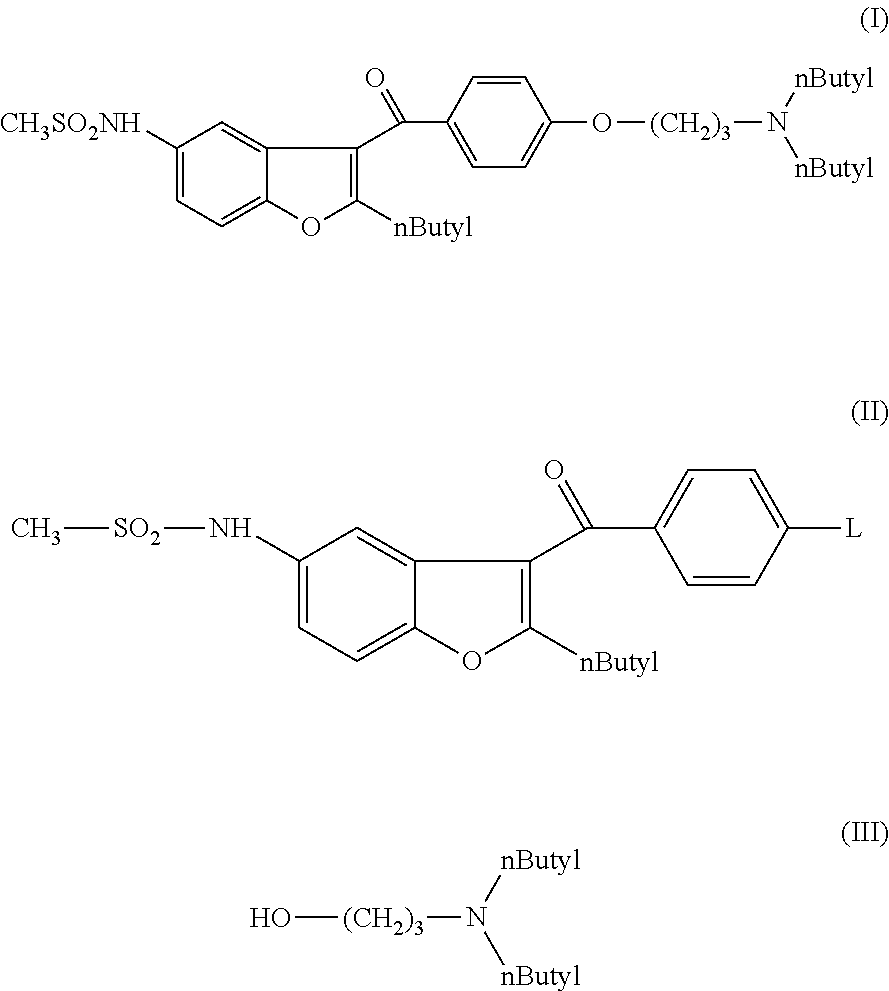
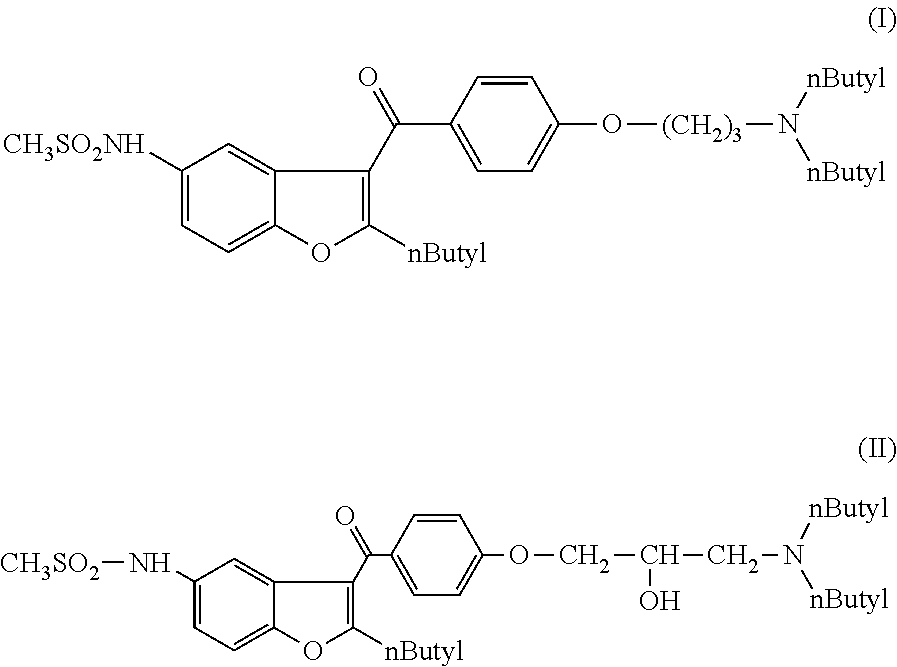
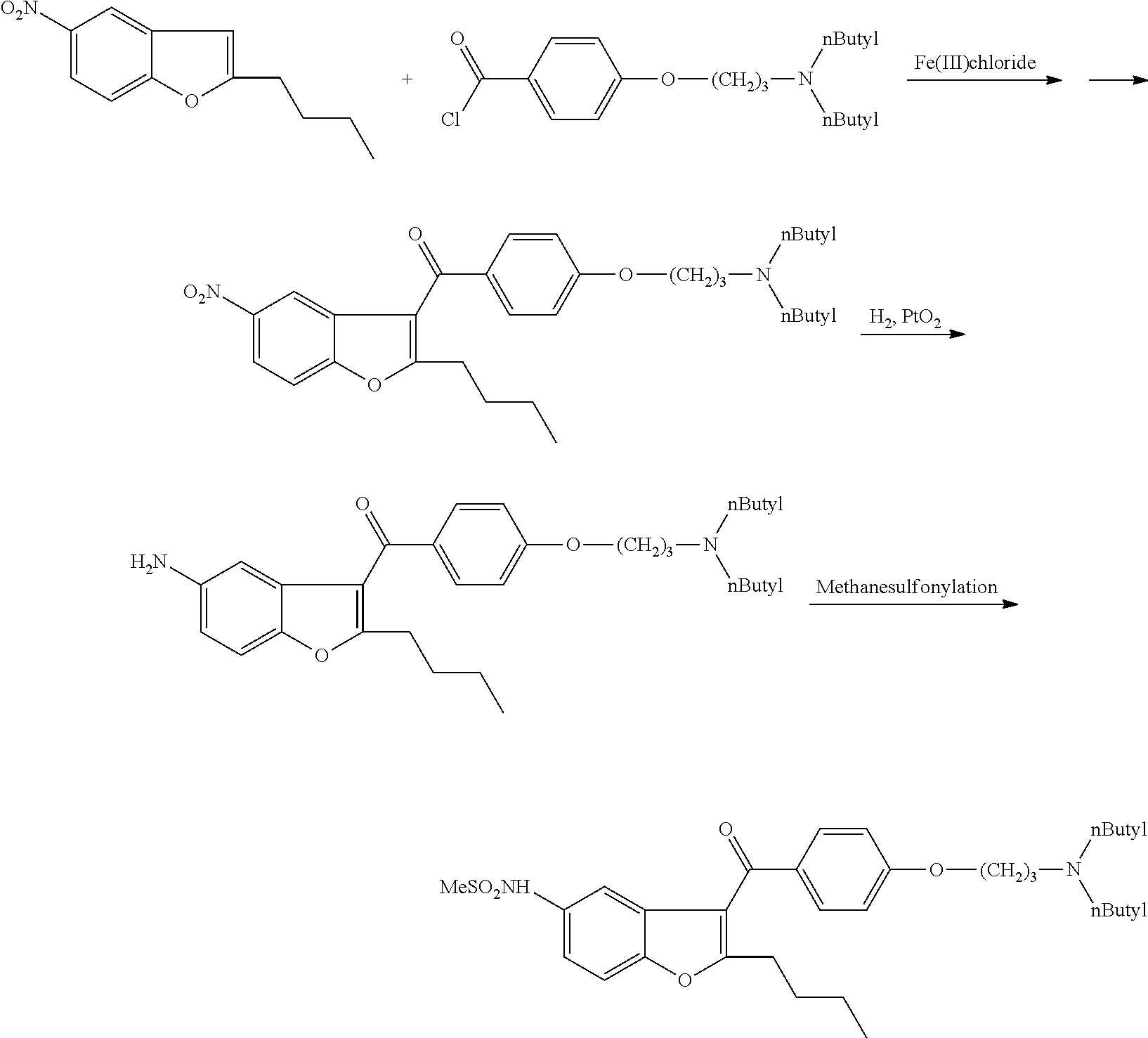
Process for preparation of dronedarone by the use of dibutylaminopropanol reagent
ActiveUS20150018568A1Easy to prepareInexpensive to purchaseOrganic chemistryDronedaroneLeaving group
The invention relates to a novel process for preparation of dronedarone of formula (I) and pharmaceutically acceptable salts thereof, characterized in that a compound of formula (II)—where L is leaving group—is reacted with compound of formula (III) and the obtained product is isolated and, if desired, converted into a pharmaceutically acceptable salt thereof.
Owner:SANOFI SA
Process for preparation of dronedarone by removal of hydroxyl group
The invention relates to a process for preparation of dronedarone of formula (I) and pharmaceutically acceptable salts thereof characterized in that from the compound of formula (II). the hydroxyl group is removed, and the obtained product is isolated and, if desired, converted into a pharmaceutically acceptable salt thereof.
Owner:SANOFI SA
Process for preparation of dronedarone by n-butylation
ActiveUS20140114081A1Avoid disadvantagesOrganic active ingredientsOrganic chemistryDronedaroneLeaving group
The invention relates to a novel process for preparation of dronedarone (I) and pharmaceutically acceptable salts thereof where the compound of formula (II) or salt thereof is reacted with a compound of formula L-(CH2)3—CH3 (III), where L is a leaving group, and isolating the obtained product and, if desired, converting it into a pharmaceutically acceptable salt thereof. The invention also relates to some novel intermediary compounds and the preparation thereof.
Owner:SANOFI SA
Composition containing dronedarone
ActiveCN102349889APromote dissolutionImprove absorption rateOrganic active ingredientsPharmaceutical non-active ingredientsDronedaroneBlood concentration
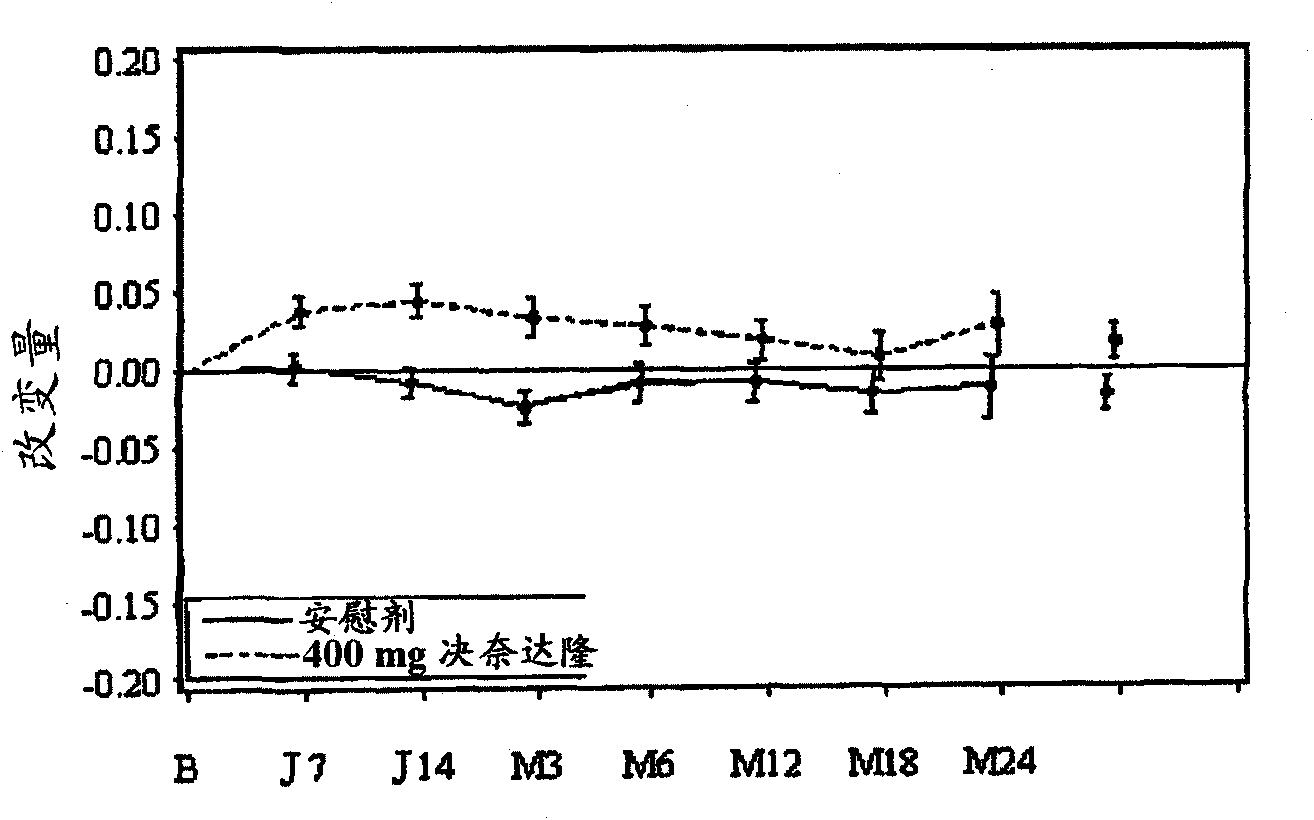
The invention relates to a pharmaceutical composition which comprises dronedarone or pharmaceutically acceptable salt thereof used as a preparation active ingredient, a phosphatide, an acidity regulator and one or more crystallization inhibitors. The composition is suitable for any oral drug forms, such as tablets, capsules or granules. The composition has the advantages that: the drug dissolution can be effectively increased, thereby increasing the in-vivo absorption rate and degree of the active ingredients; and more importantly, the areas below the in-vivo blood concentration-time curves of the Beagle dog under drug feeding / fasting conditions have no difference almost.
Owner:JILIN BODA PHARMA
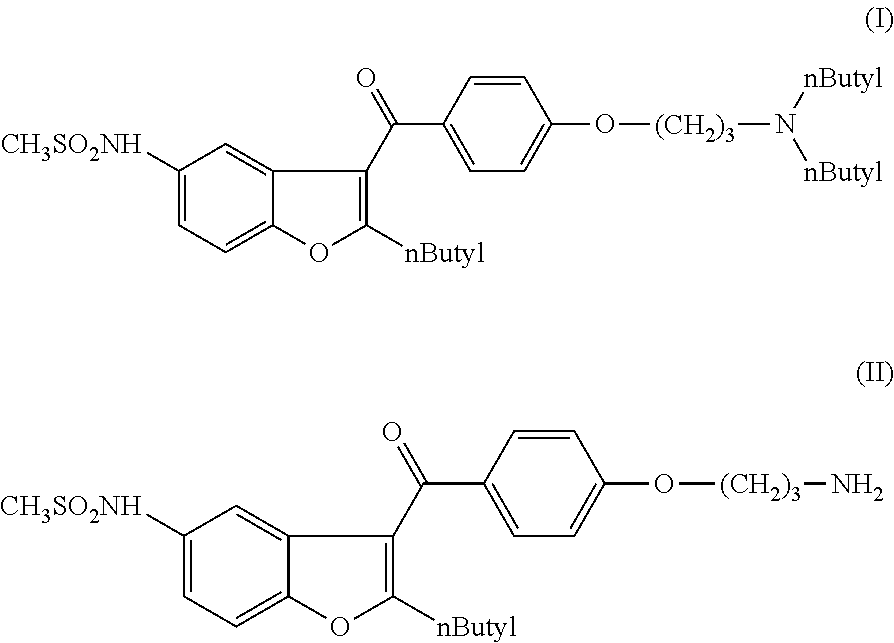
Process for obtaining dronedarone
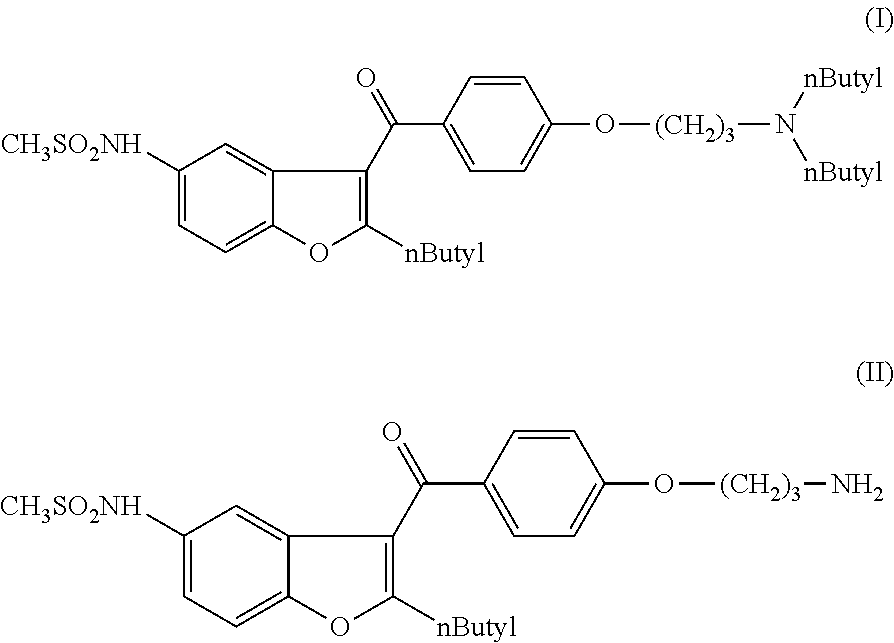
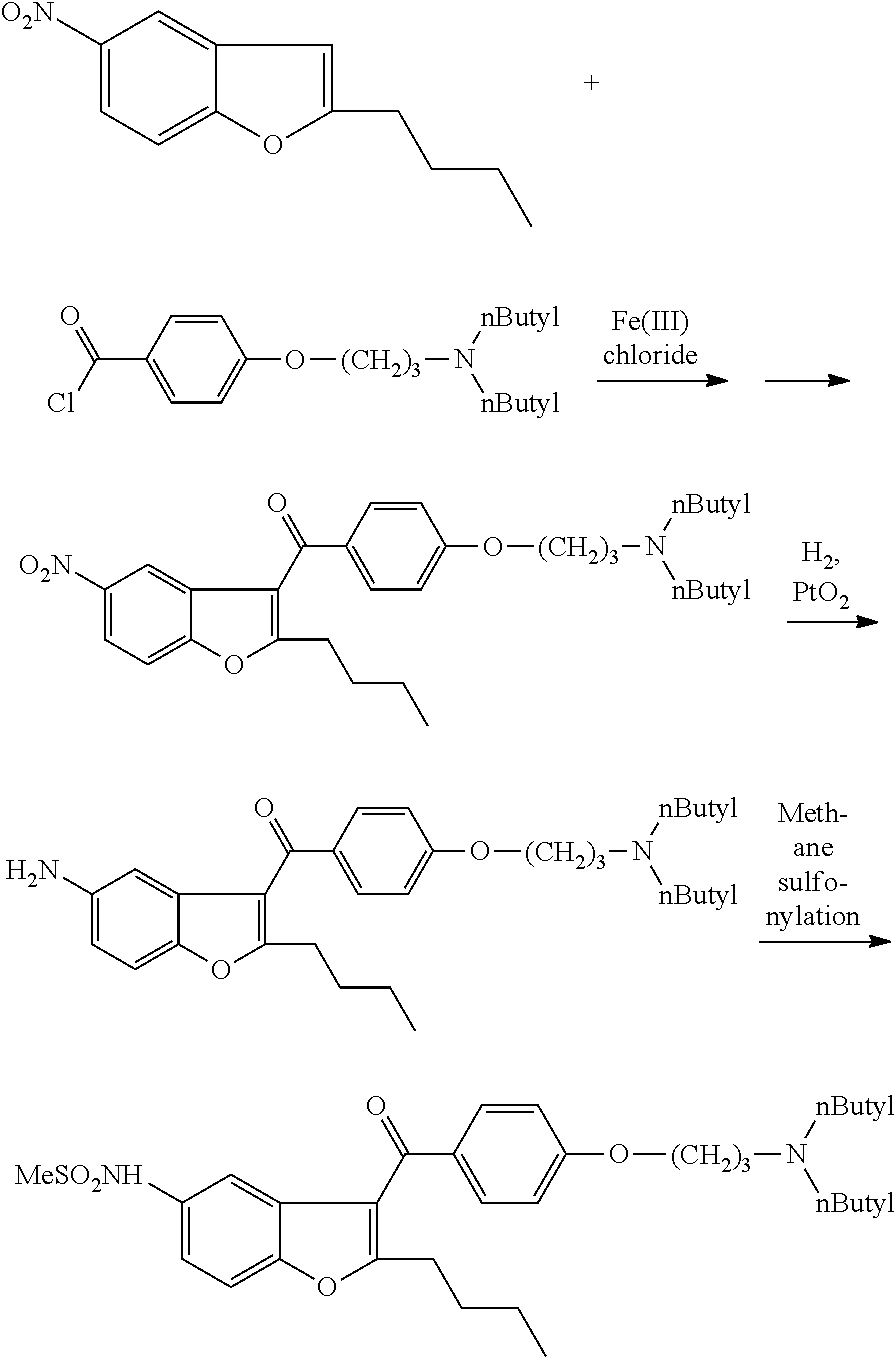
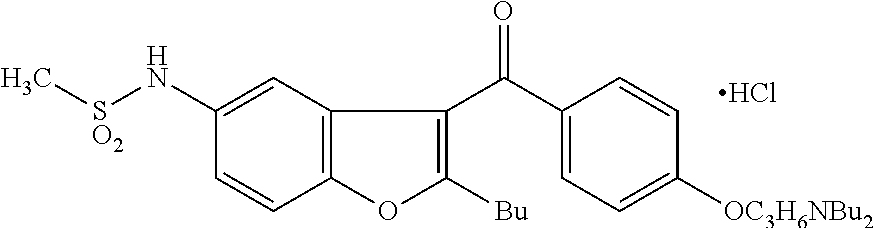
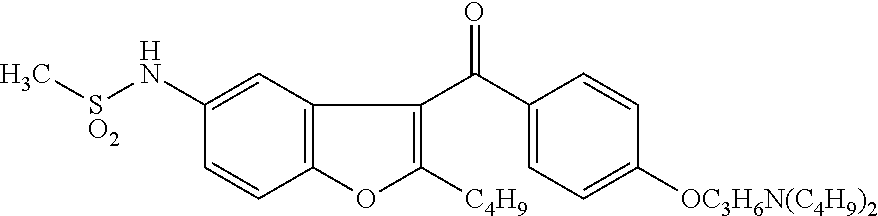
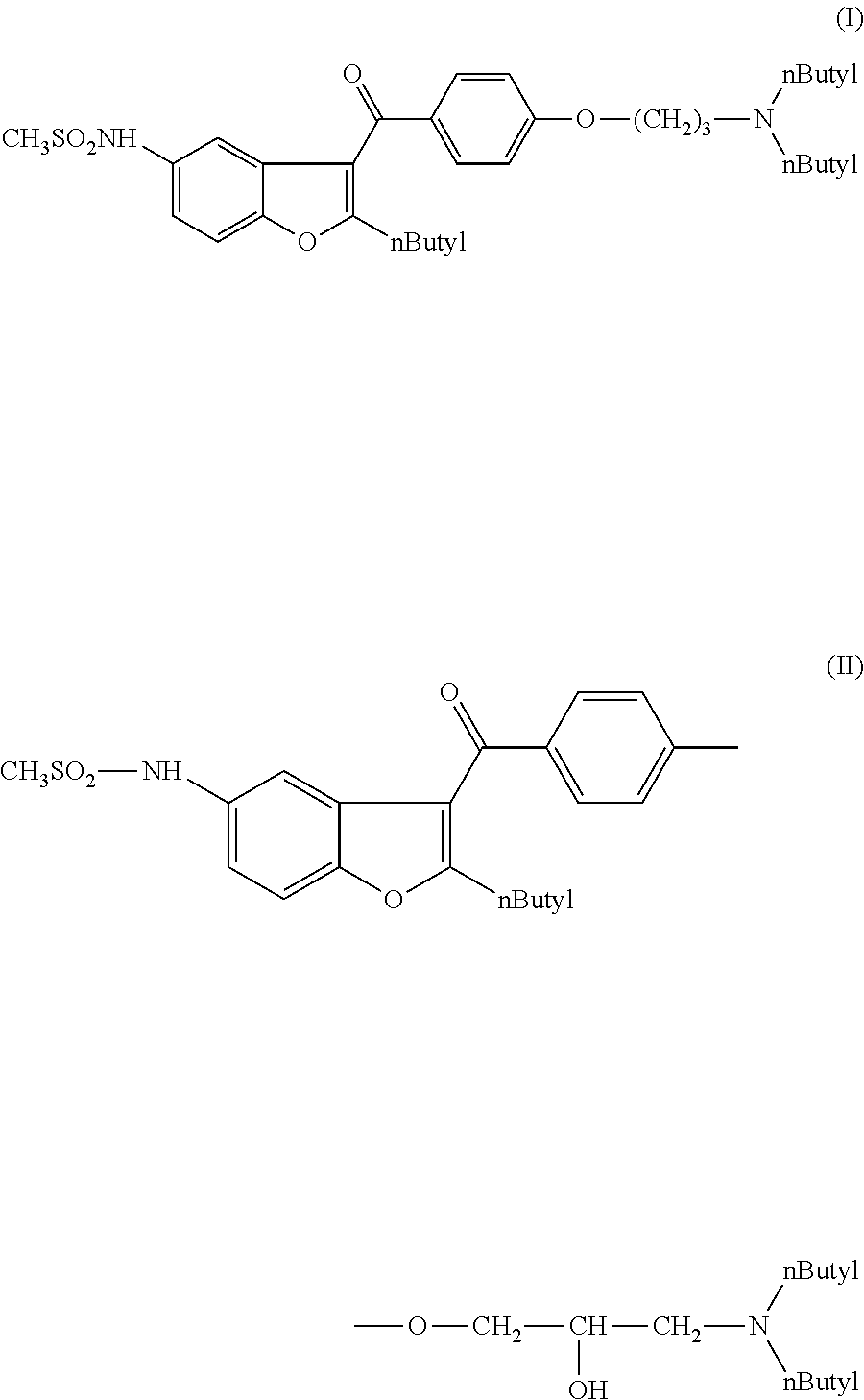
The present invention provides a process for obtaining dronedarone or salts thereof characterized in that in an organic phase comprising one or more non-polar solvents, 5-amino-3-[4-(3-di-n-butylaminopropoxy)benzoyl]-2-n-butyl-benzofuran is reacted with methane sulfonyl chloride without the addition of a base. The invention also provides a process for obtaining intermediates of dronedarone environmentally friendly and industrially viable.
Owner:INKE SA (ES)
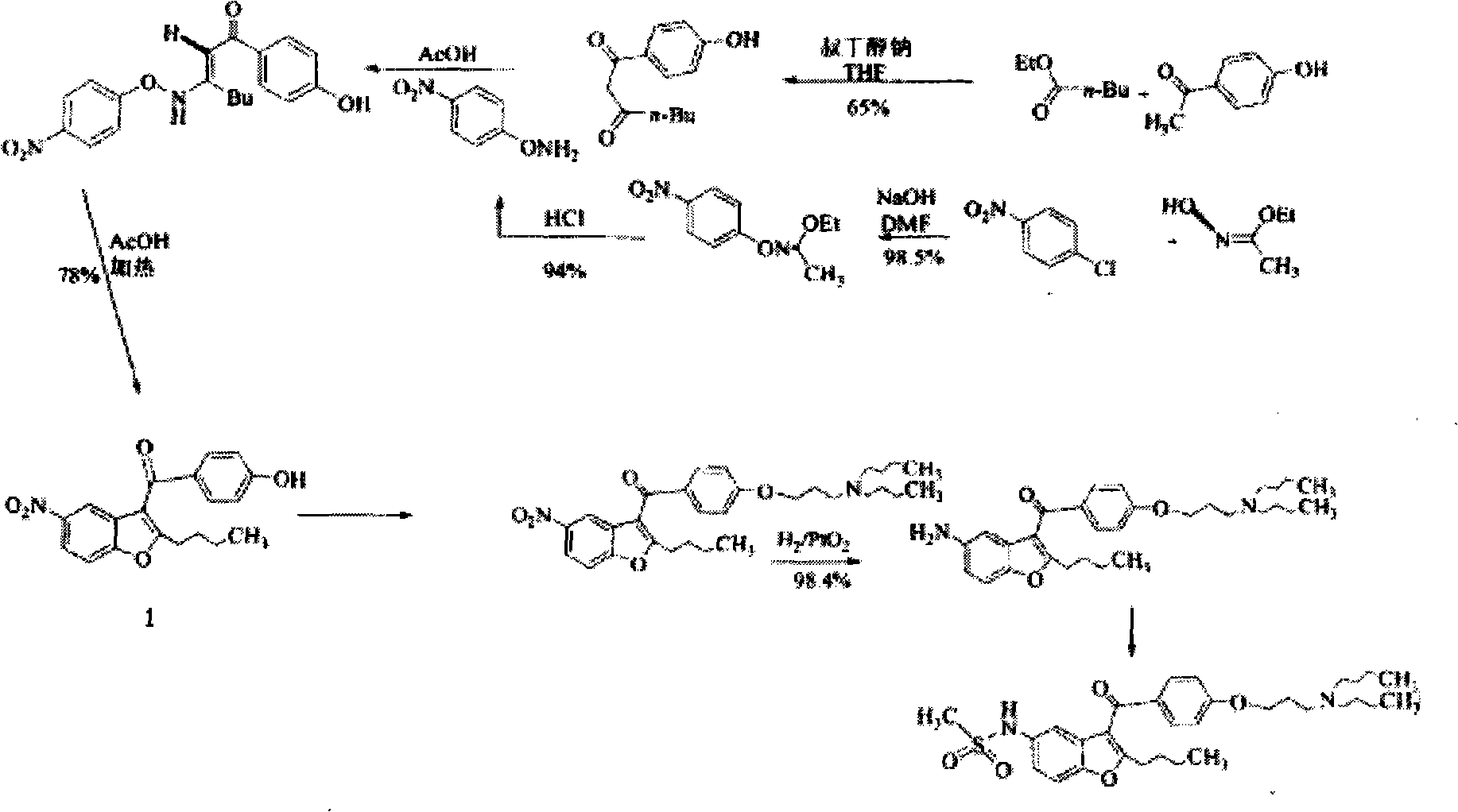
Novel method for synthesizing dronedarone key intermediate
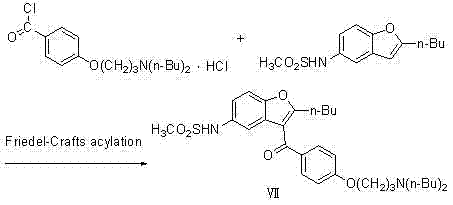
The invention relates to a novel method for synthesizing 2-butyl-3-(4-hydroxybenzene formoxyl)-5-nitryl benzfuran. An intermediate is a key intermediate of dronedarone serving as a novel medicament for treating atrial fibrillation. The method comprises the following steps of: undergoing a Fourier acylation reaction on 2-butyl-5-nitryl benzfuran serving as an initial raw material and p-acetoxyl benzoyl chloride to obtain 2-butyl-3-(4-hydroxybenzene formoxyl)-5-nitryl benzfuran; and hydrolyzing with sodium hydrate to obtain a target product.
Owner:北京欧格瑞化学科技有限公司
Process for preparation of dronedarone by N-butylation
ActiveUS9174959B2Avoid disadvantagesOrganic active ingredientsOrganic chemistryDronedaroneLeaving group
The invention relates to a novel process for preparation of dronedarone (I) and pharmaceutically acceptable salts thereof where the compound of formula (II) or salt thereof is reacted with a compound of formula L-(CH2)3—CH3 (III), where L is a leaving group, and isolating the obtained product and, if desired, converting it into a pharmaceutically acceptable salt thereof. The invention also relates to some novel intermediary compounds and the preparation thereof.
Owner:SANOFI SA
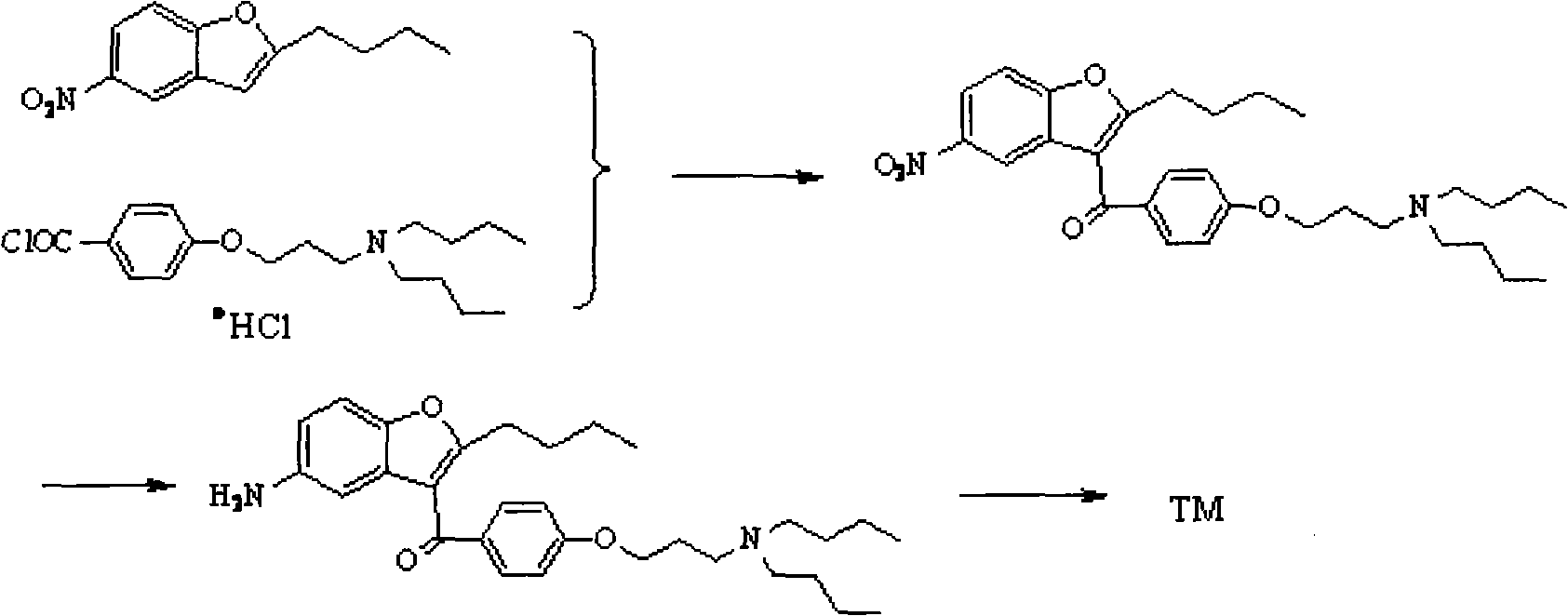
Preparation method for novel antiarrhythmic medicament of dronedarone
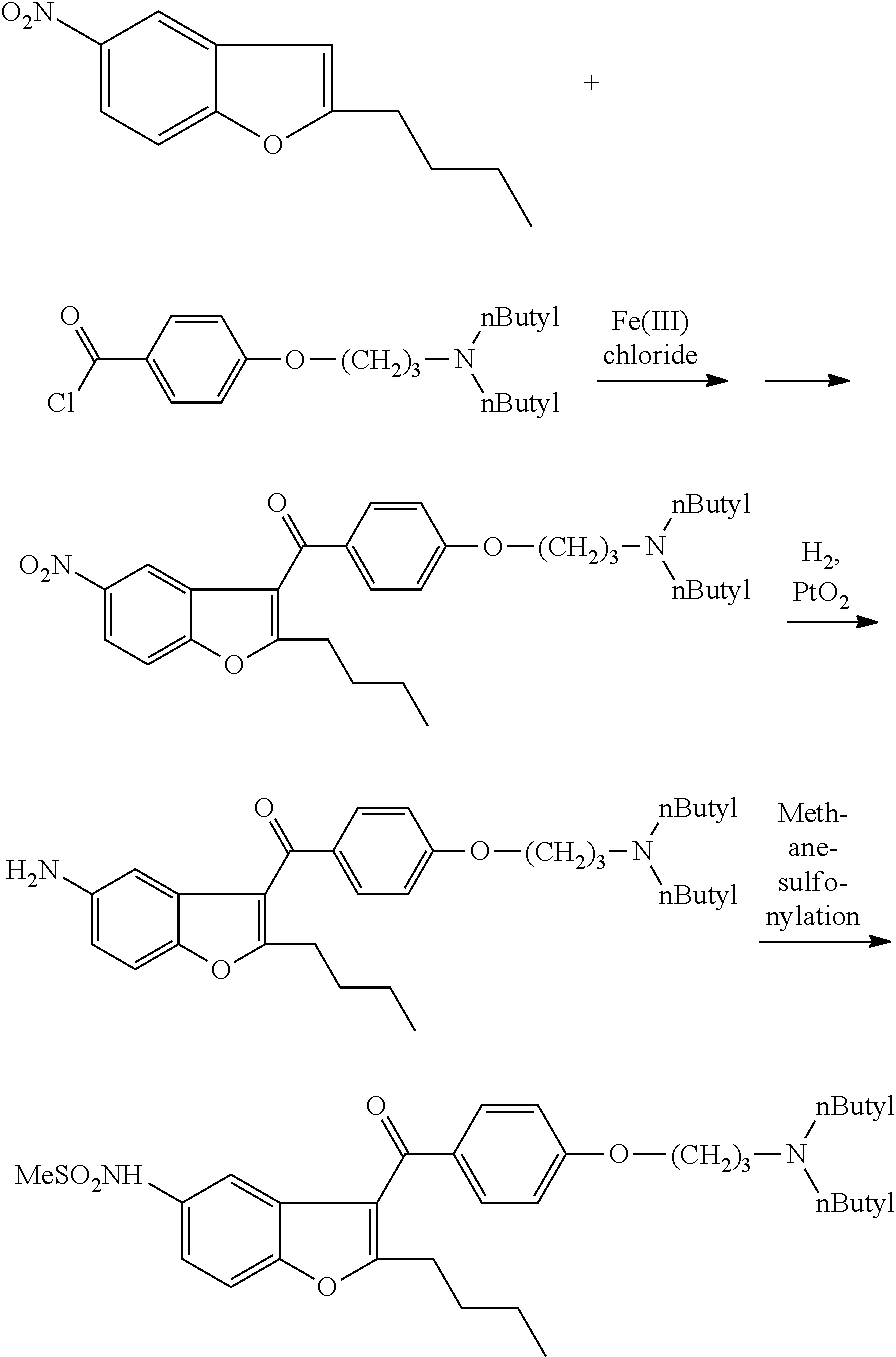
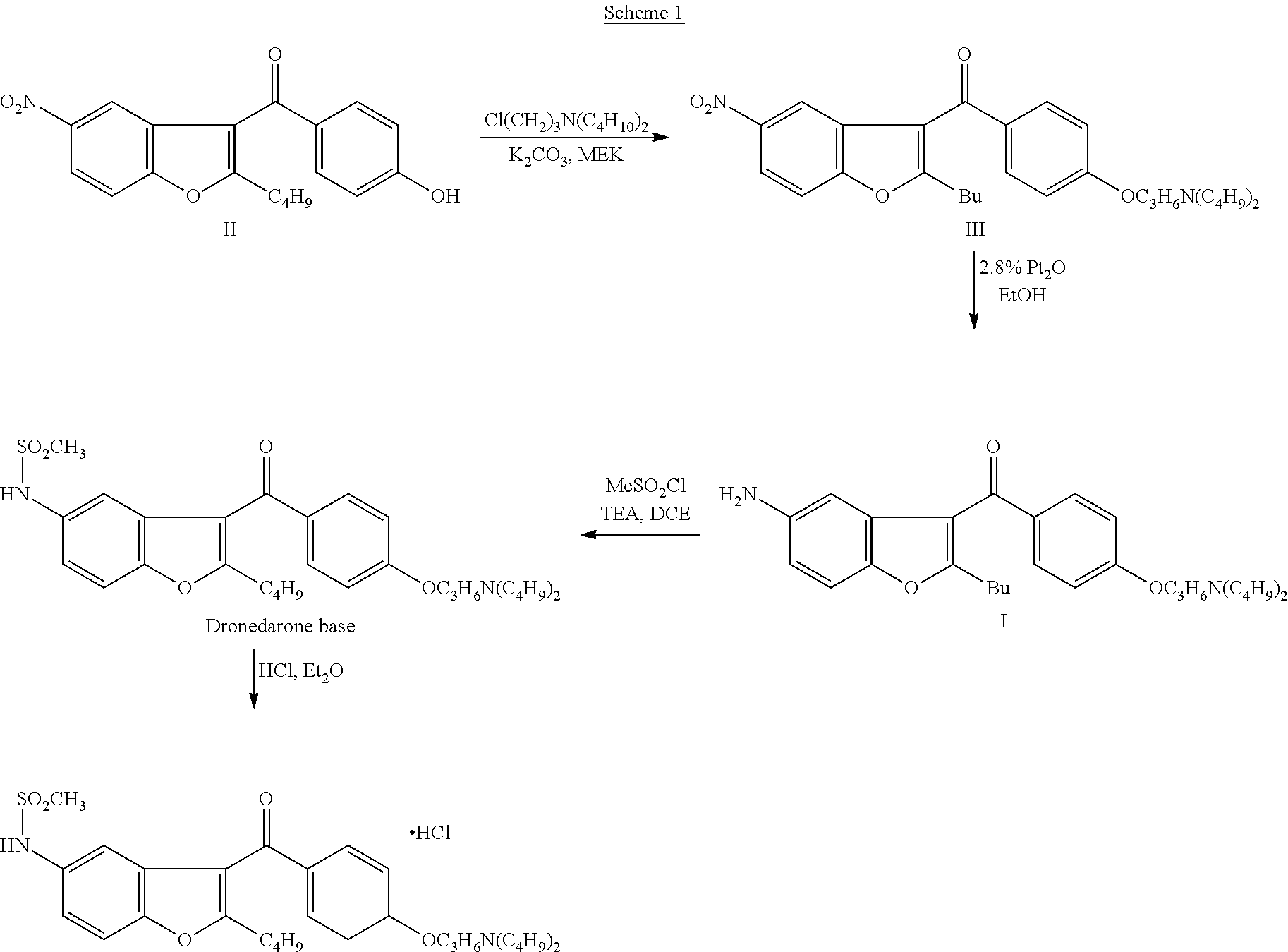
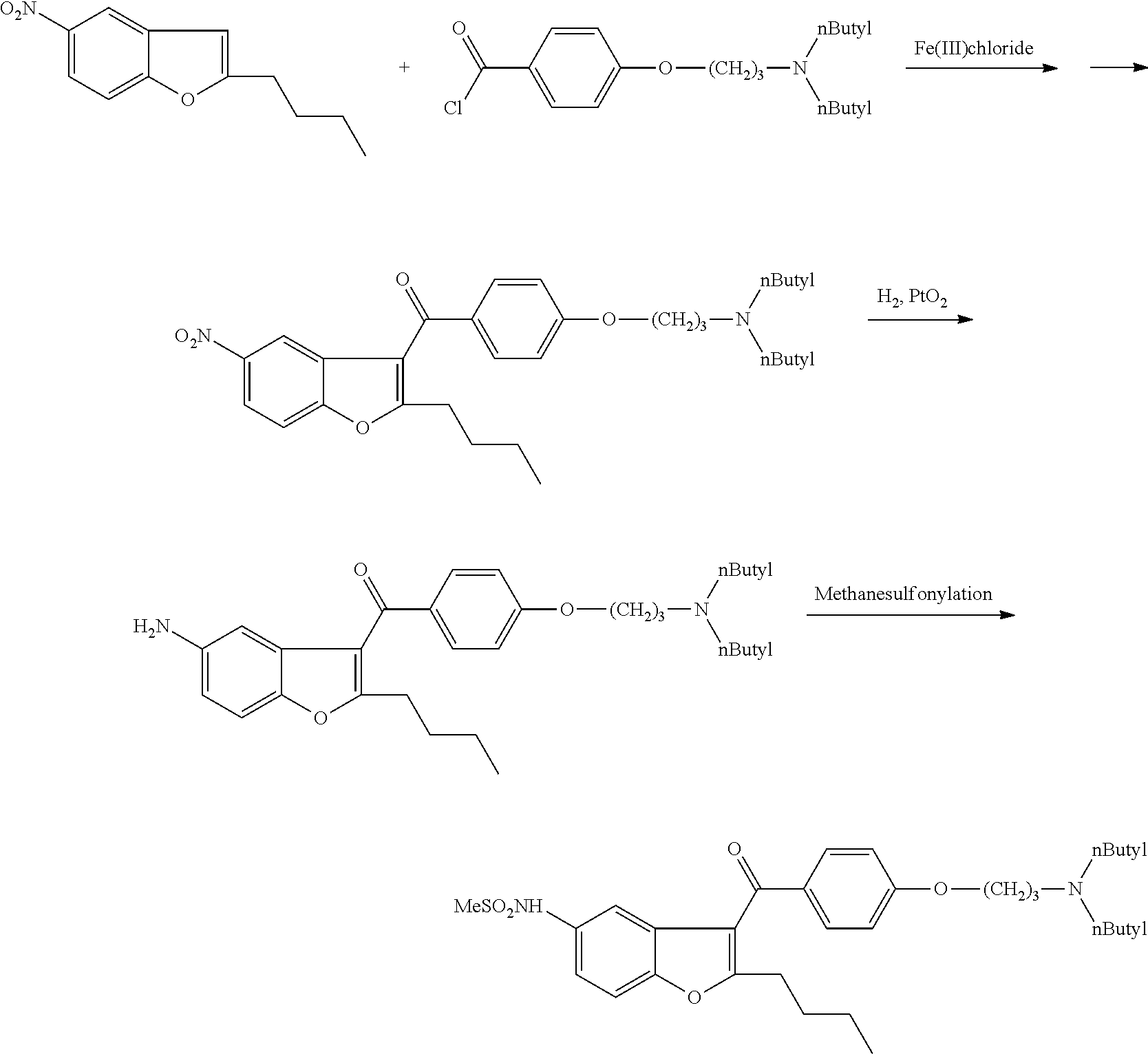
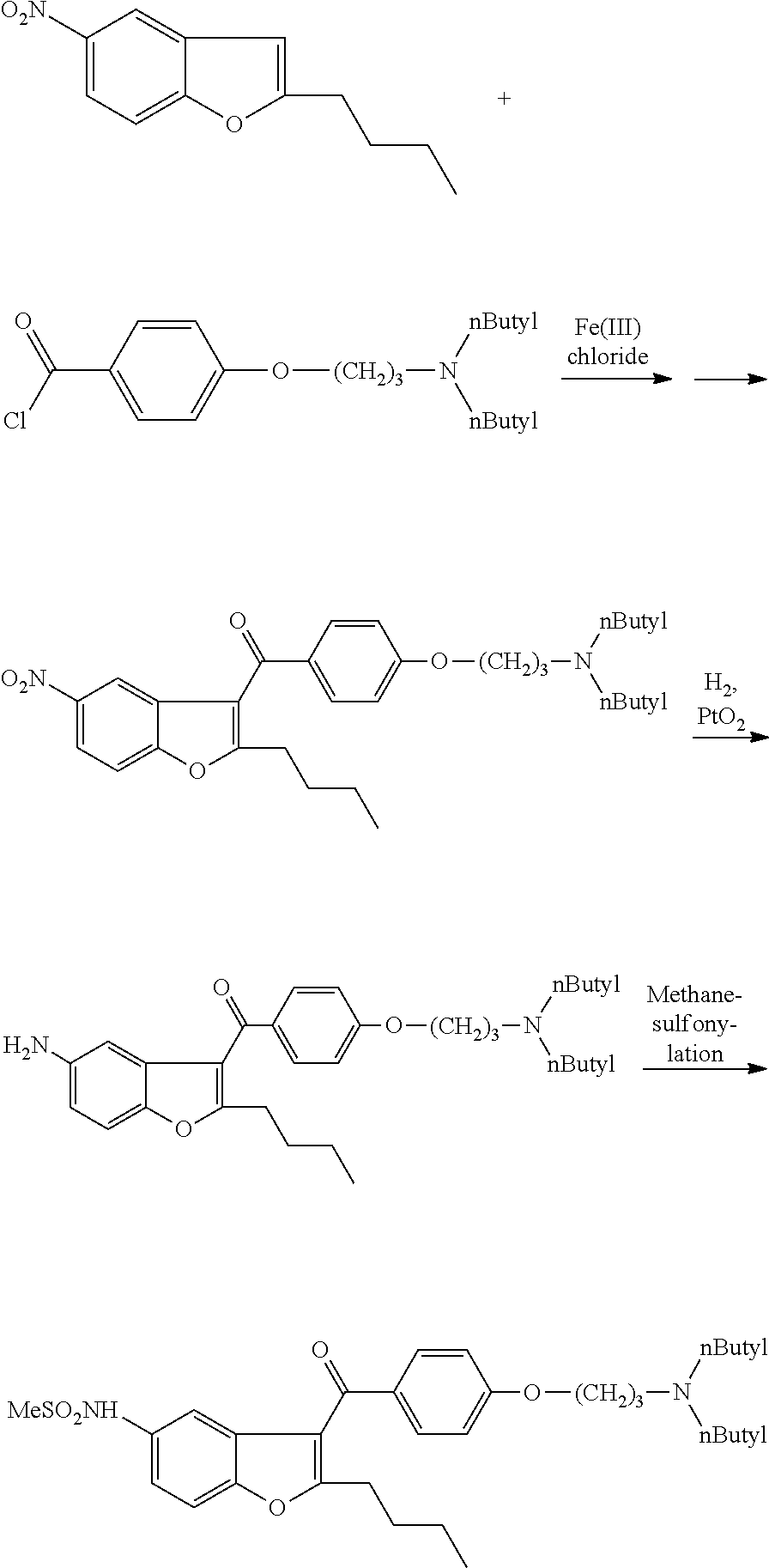
The invention discloses a preparation method for a novel antiarrhythmic medicament of dronedarone. The preparation method comprises the following steps of: (1) performing substitution reaction on a compound IV and dibutylamine in an organic solvent to obtain an intermediate compound V; (2) performing reduction reaction on the compound V in the organic solvent under the action of a catalyst to obtain an intermediate VI; and (3) performing sulfonylation reaction on the compound VI and methanesulfonyl chloride in the organic solvent to obtain the final product of dronedarone. The product prepared by the method has high purity, an operation method is simple, production cost is low, and the method has strong competitive power in markets, and is more suitable for industrialized production.
Owner:江苏万全特创医药生物技术有限公司
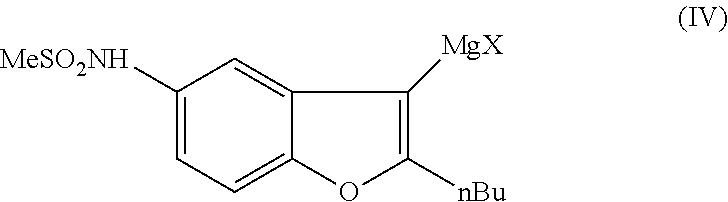
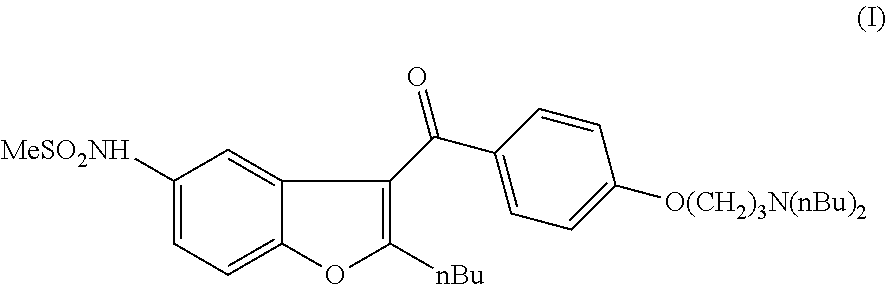
Process for preparation of dronedarone by Grignard reaction
ActiveUS9238636B2Organic compound preparationCarboxylic acid amides preparationDronedaroneGrignard reaction
The invention relates to a novel process for the preparation of dronedarone (I) and pharmaceutically acceptable salts thereof, which comprises reacting of compound of formula (IV) with compound of formula (VI) in a Grignard reaction, and the obtained product is isolated and, if desired, converted into a pharmaceutically acceptable salt thereof. The invention also relates to some novel intermediary compounds and processes for the preparation thereof.
Owner:SANOFI SA
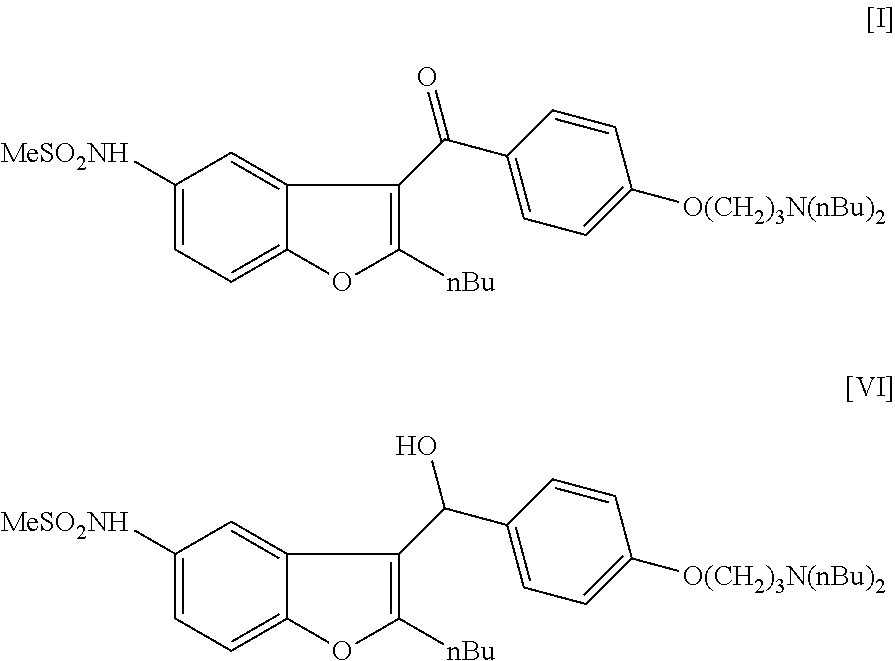
Process for preparation of dronedarone by oxidation of a hydroxyl group
The invention relates to a novel process for the preparation of dronedarone (I) and pharmaceutically acceptable salts thereof (formula I), which comprises oxidizing a compound of formula (VI), or a salt thereof and the obtained product is isolated and, if desired, converted into a pharmaceutically acceptable salt thereof. Further aspects of the invention include the novel intermediary compound of formula (VI) and process for the preparation thereof.
Owner:SANOFI SA
Process for obtaining dronedarone
The present invention provides a process for obtaining dronedarone or salts thereof characterized in that in an organic phase comprising one or more non-polar solvents, 5-amino-3-[4-(3-di-n-butylaminopropoxy)benzoyl]-2-n-butyl-benzofuran is reacted with methane sulfonyl chloride without the addition of a base. The invention also provides a process for obtaining intermediates of dronedarone environmentally friendly and industrially viable.
Owner:INKE SA (ES)
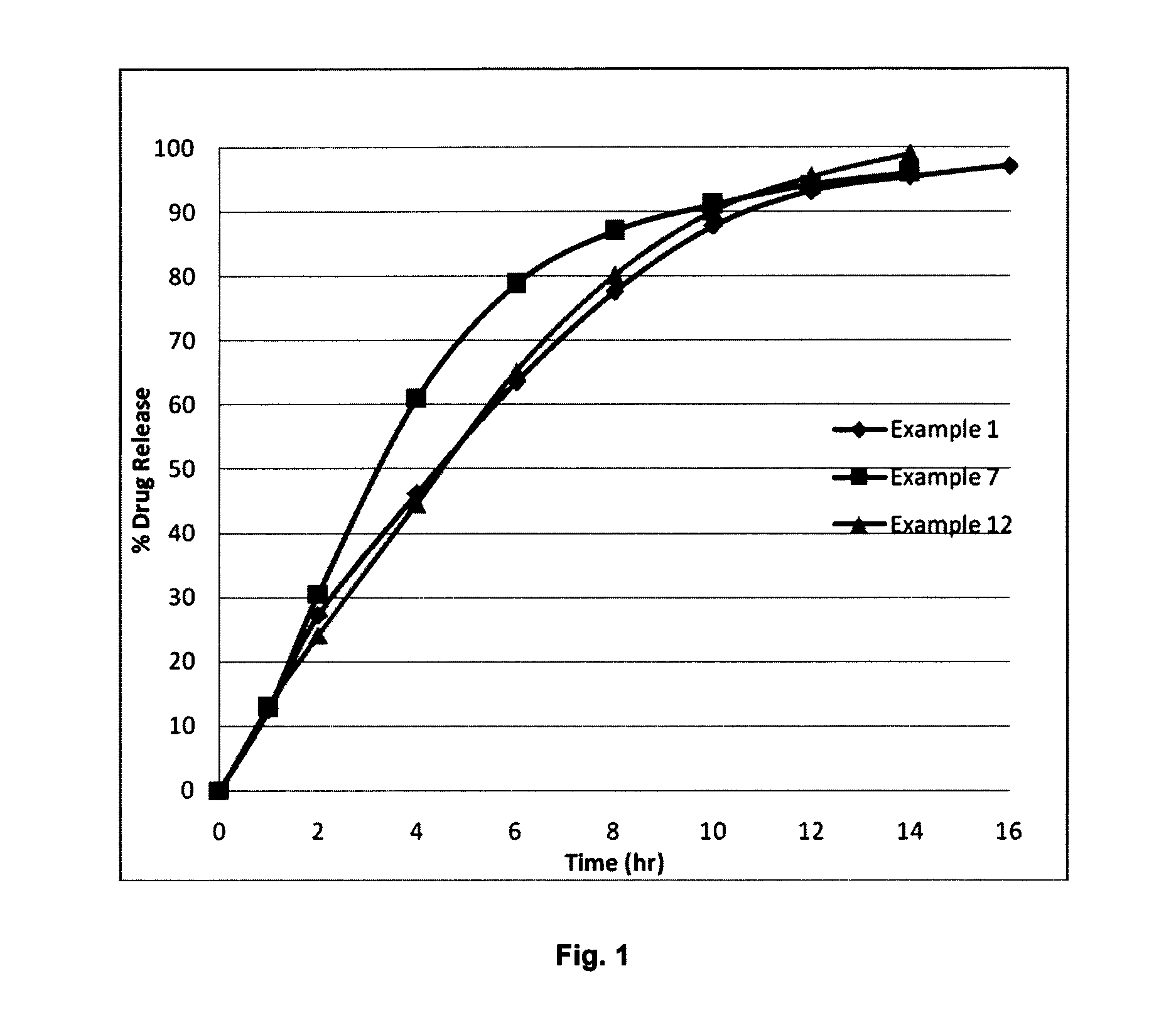
Controlled release formulations of dronedarone
The present invention relates to controlled release formulation of dronedarone or pharmaceutically acceptable salts, esters, metabolites, prodrugs or enantiomers thereof and controlled release polymers. The use of controlled release formulations of Dronedarone would improve the bioavailability and the patient compliance with reduction in number of dosages to be taken per day.
Owner:LUPIN LTD
Process for preparation of dronedarone by removal of hydroxyl group
The invention relates to a process for preparation of dronedarone of formula (I) and pharmaceutically acceptable salts thereof characterized in that from the compound of formula (II). The hydroxyl group is removed, and the obtained product is isolated and, if desired, converted into a pharmaceutically acceptable salt thereof.
Owner:SANOFI SA
Process for preparation of dronedarone by grignard reaction
ActiveUS20150274688A1Simple processOrganic compound preparationCarboxylic acid amides preparationDronedaroneGrignard reaction
The invention relates to a novel process for the preparation of dronedarone (I) and pharmaceutically acceptable salts thereof, which comprises reacting of compound of formula (IV) with compound of formula (VI) in a Grignard reaction, and the obtained product is isolated and, if desired, converted into a pharmaceutically acceptable salt thereof. The invention also relates to some novel intermediary compounds and processes for the preparation thereof.
Owner:SANOFI SA
Process for preparation of dronedarone by removal of hydroxyl group
The invention relates to a novel process for preparation of dronedarone of formula (I) and pharmaceutically acceptable salts thereof the hydroxyl group is removed, and the obtained product is isolated and, if desired, converted into a pharmaceutically acceptable salt thereof.
Owner:SANOFI SA
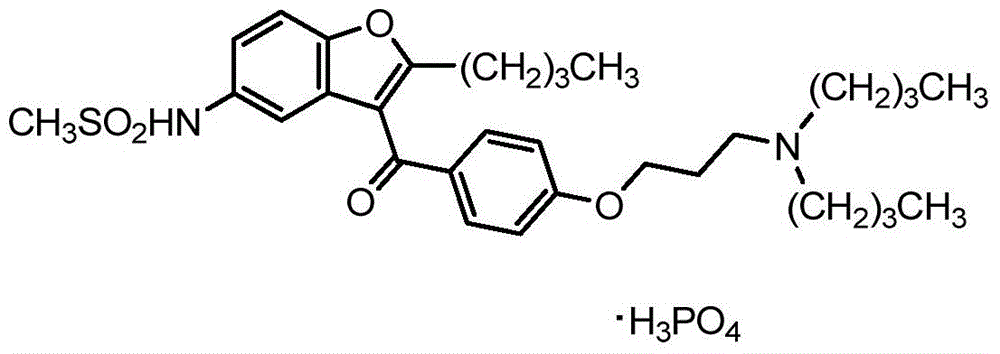
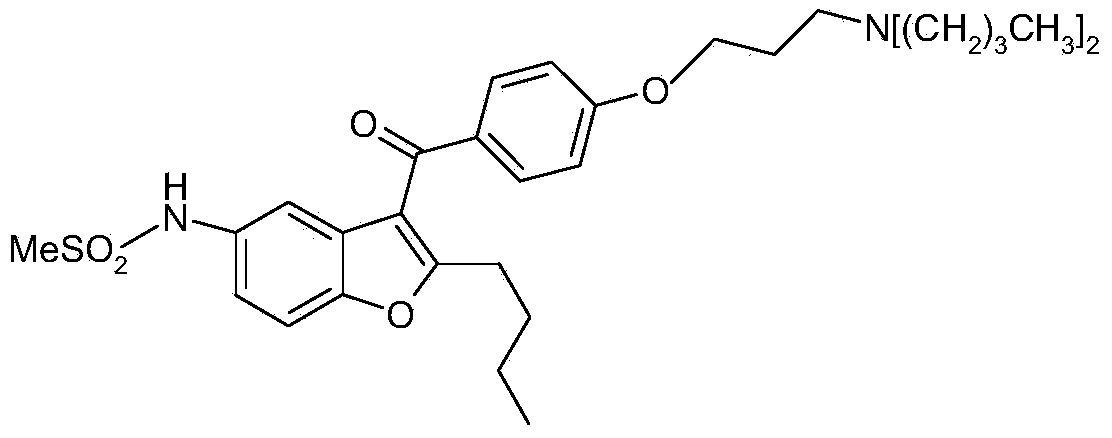
Pharmaceutical compositions of ranolazine and dronedarone
The present disclosure relates to a solid composition comprising ranolazine and a spray-dried phosphoric acid salt of dronedarone in a bilayer tablet.
Owner:GILEAD SCI INC
Use of dronedarone or a pharmaceutically acceptable salt thereof, for the preparation of a medicament for use in regulating the potassium level in the blood
Use of dronedarone or a pharmaceutically acceptable salt thereof, for the preparation of a medicament for use in regulating the potassium level in the blood.
Owner:SANOFI AVENTIS SA
Method of treating atrial fibrillation
The present invention relates to a method for the treatment or prevention of atrial fibrillation and / or atrial flutter comprising coadministration of a synergistically therapeutic amount of dronedarone or a pharmaceutically acceptable salt or salts thereof and a synergistically therapeutic amount of ranolazine or a pharmaceutically acceptable salt or salts thereof. Also provided are methods for modulating ventricular and atrial rhythm and rate. This invention also relates to pharmaceutical formulations that are suitable for such combined administration.
Owner:GILEAD SCI INC
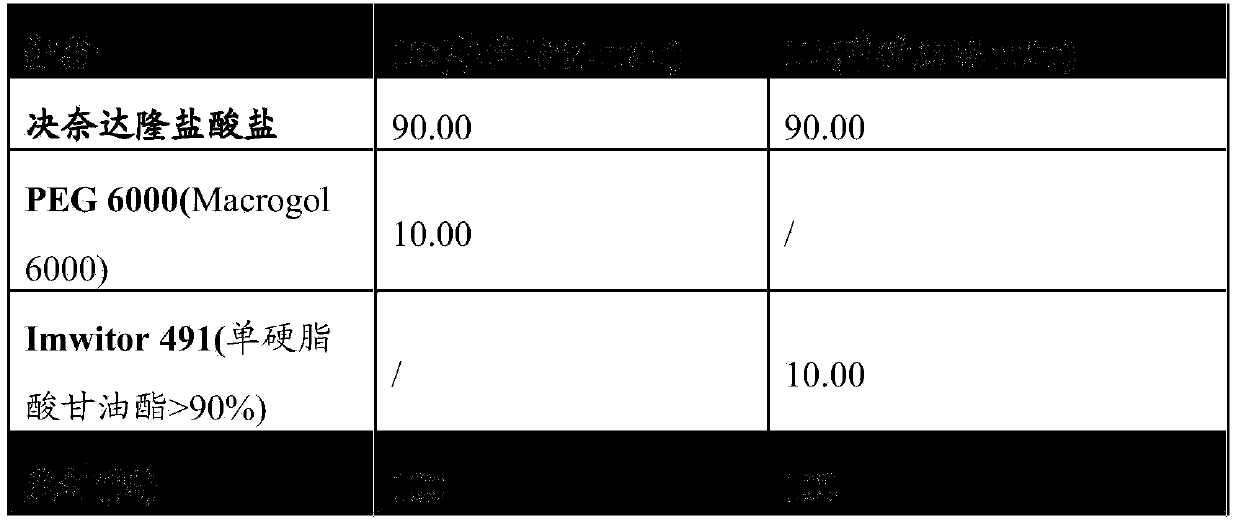
Pharmaceutical composition and solid galenic form having a high dronedarone content, and method for preparing same
InactiveCN103764126AReduce consumptionReduce water contentOrganic active ingredientsInorganic non-active ingredientsDronedaroneOral medication
The present invention relates to pharmaceutical compositions to be used, in a solid galenic form, for oral administration, and primarily including dronedarone and / or at least one of the derivatives thereof, as well as to solid galenic forms manufactured as such from said compositions, preferably in the form of tablets or capsules. The present invention also relates to a method for preparing such solid galenic forms using a hot-melt process.
Owner:SANOFI SA
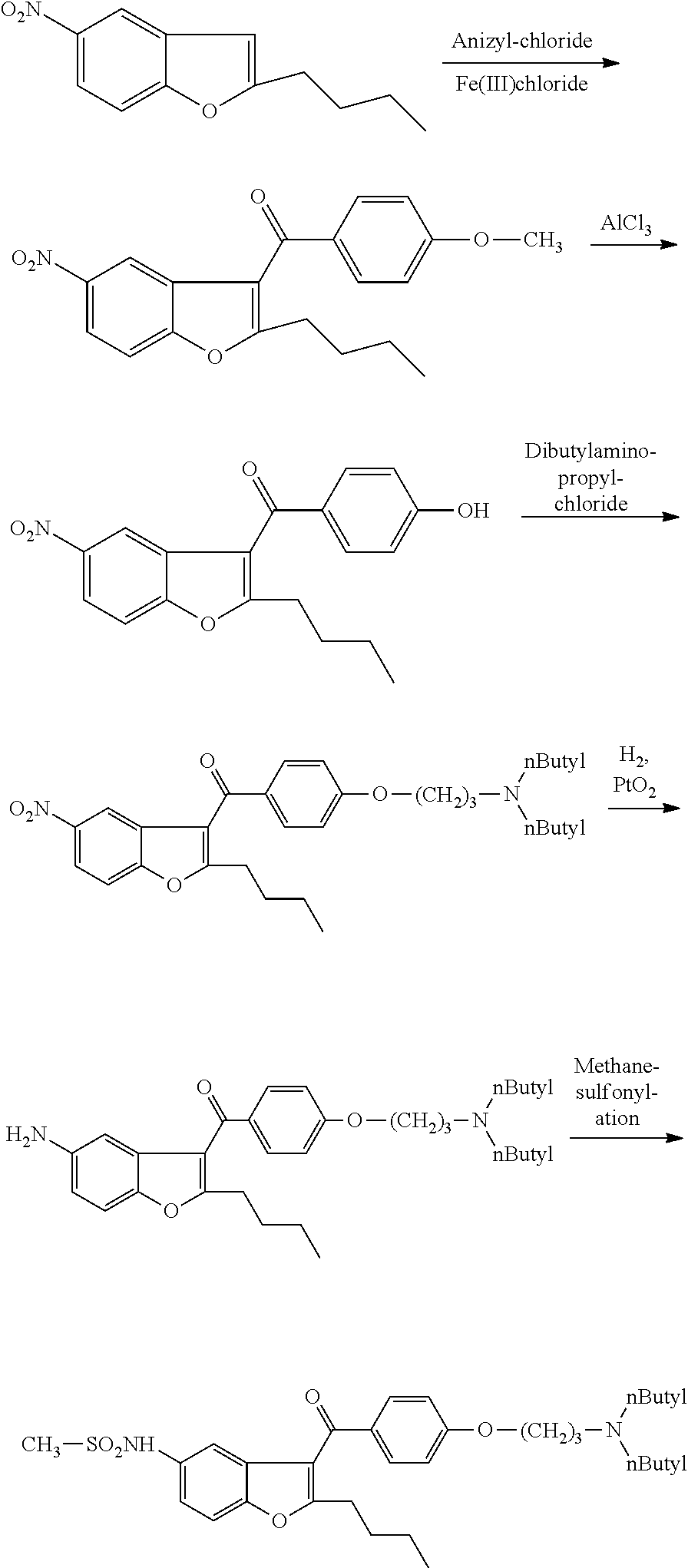
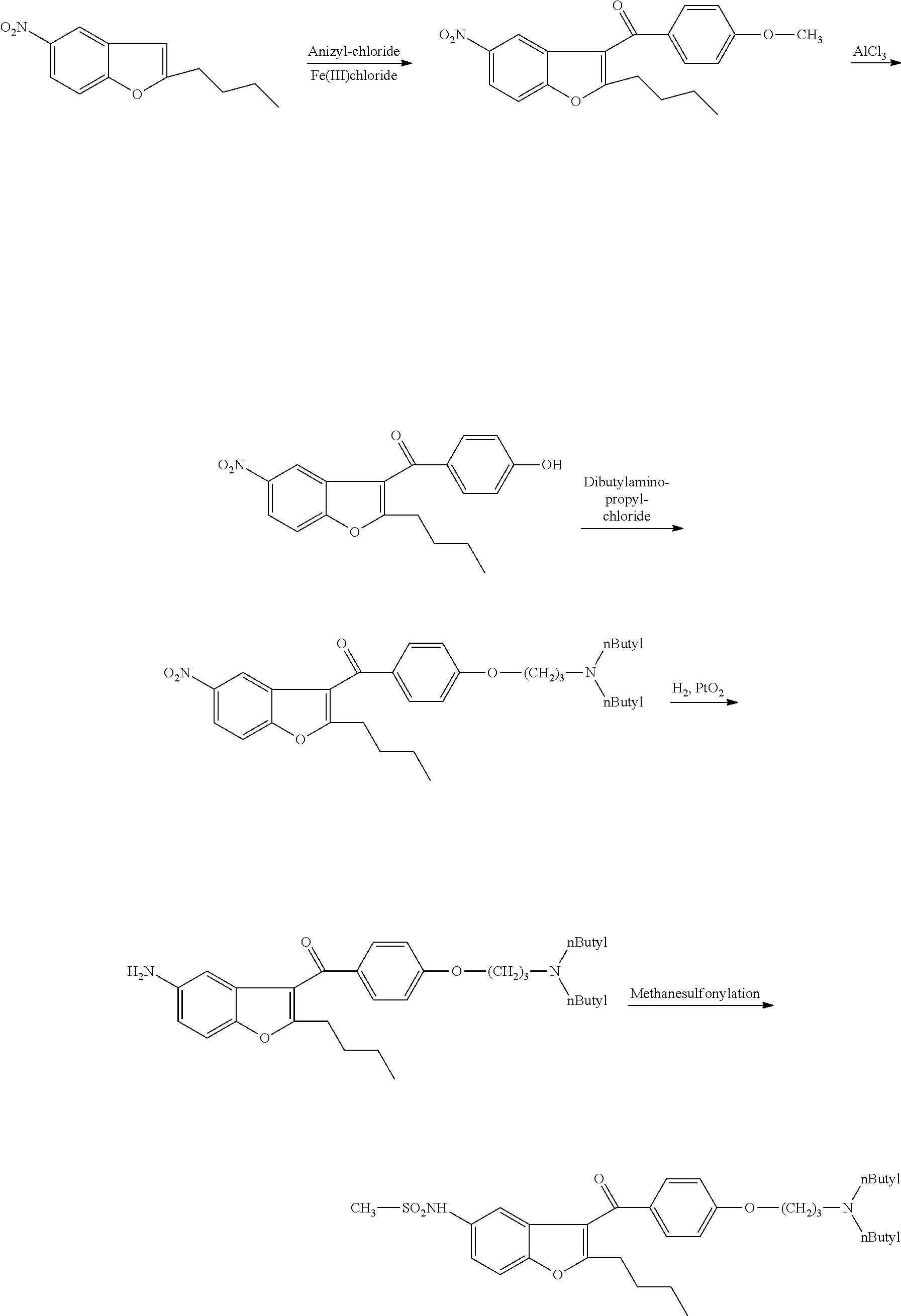
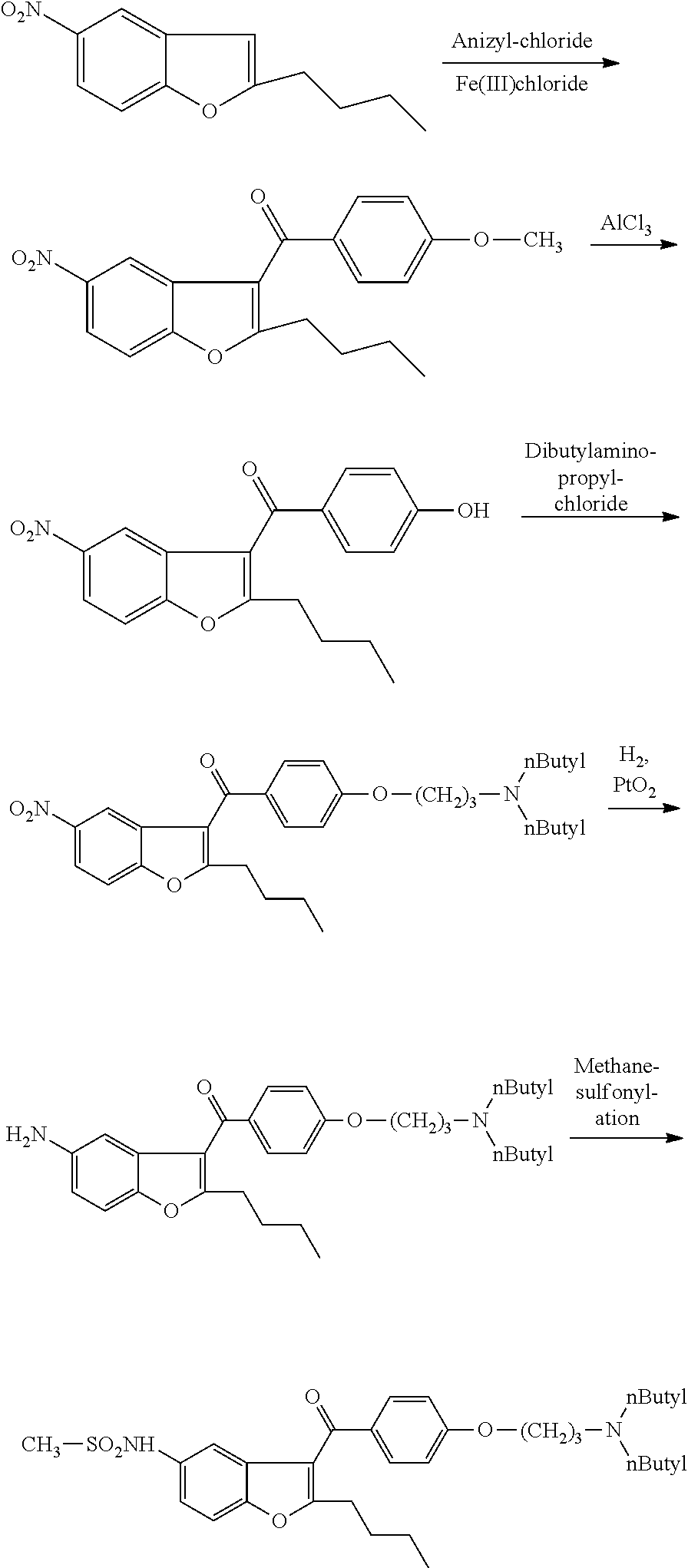
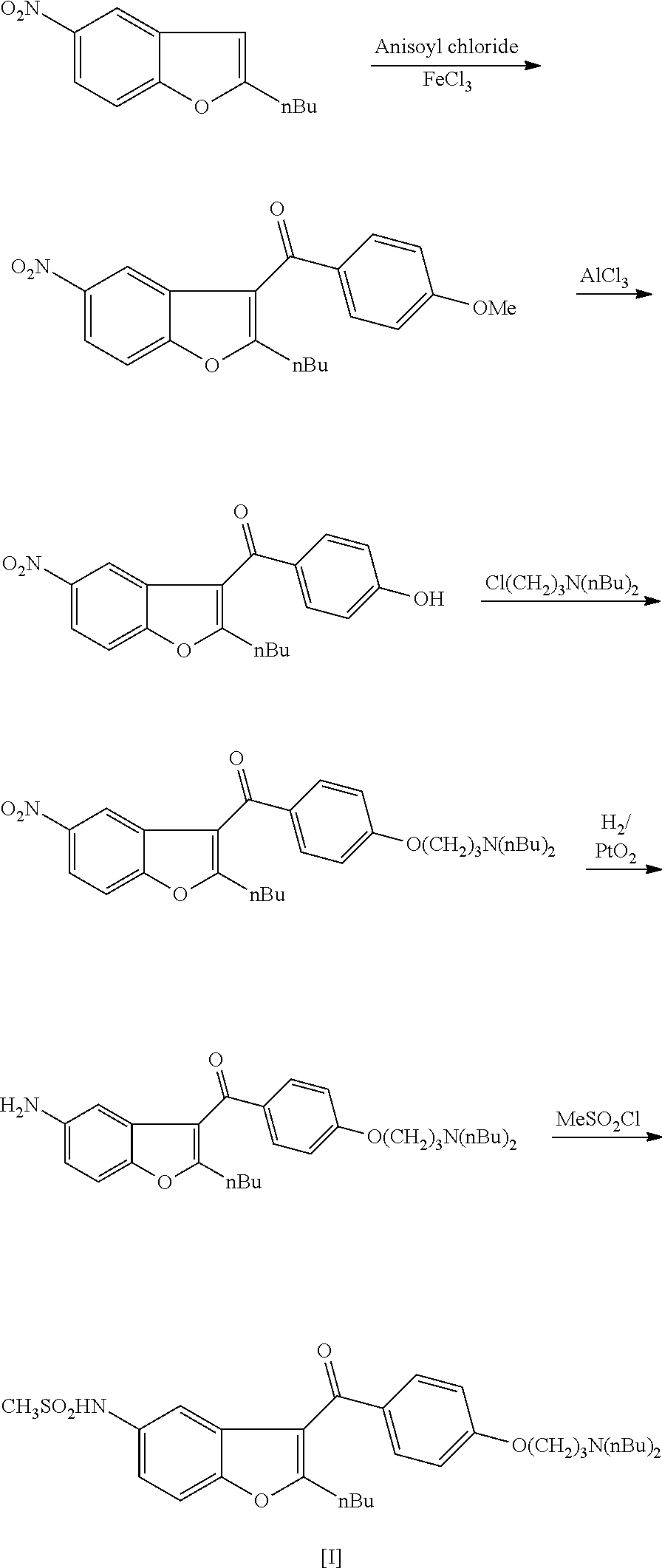
Intermediate and method used for preparing dronedarone
InactiveCN102532074AAvoid hydrogenation catalysisRaw materials are easy to getOrganic chemistryBulk chemical productionAnisoyl chlorideDronedarone
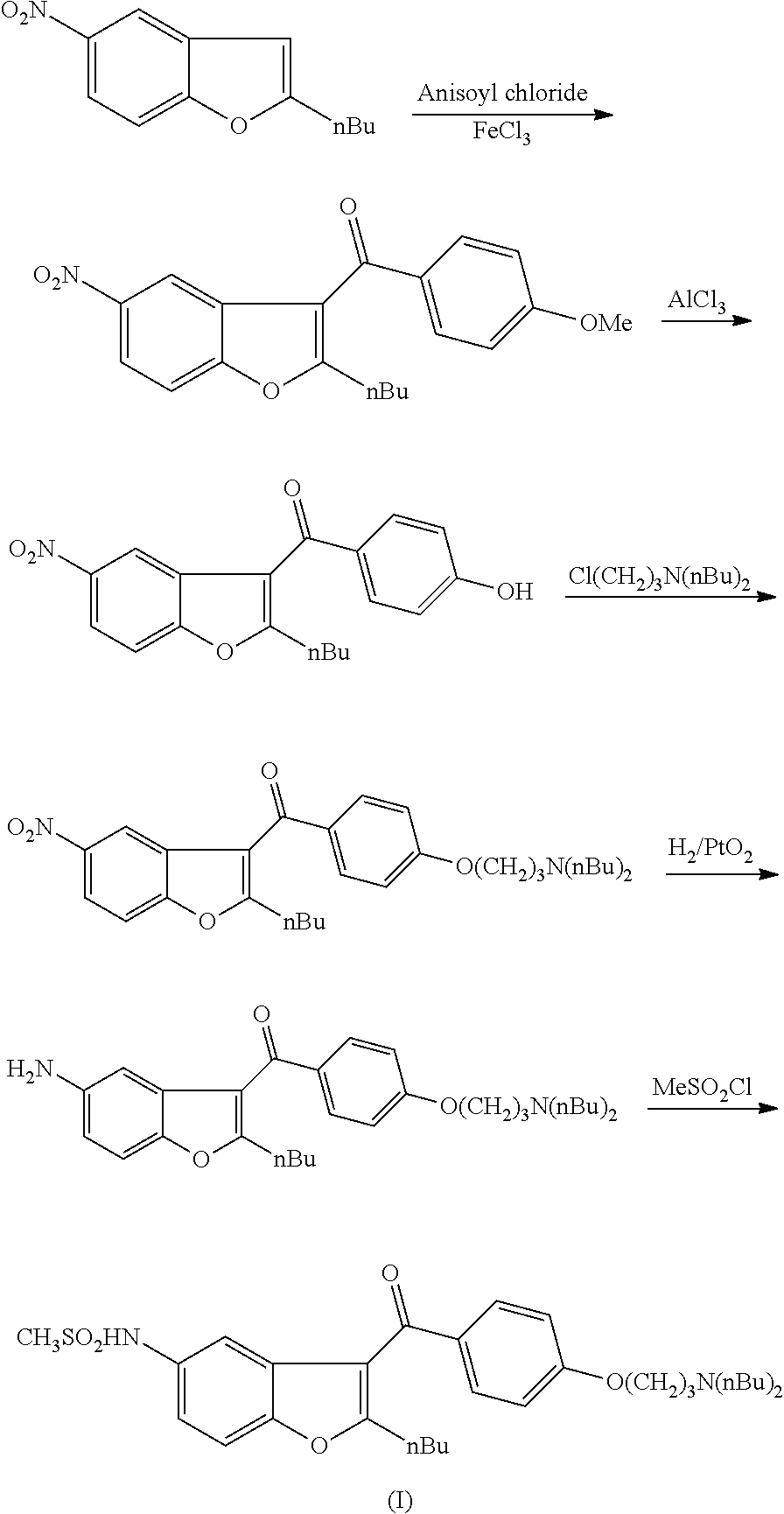
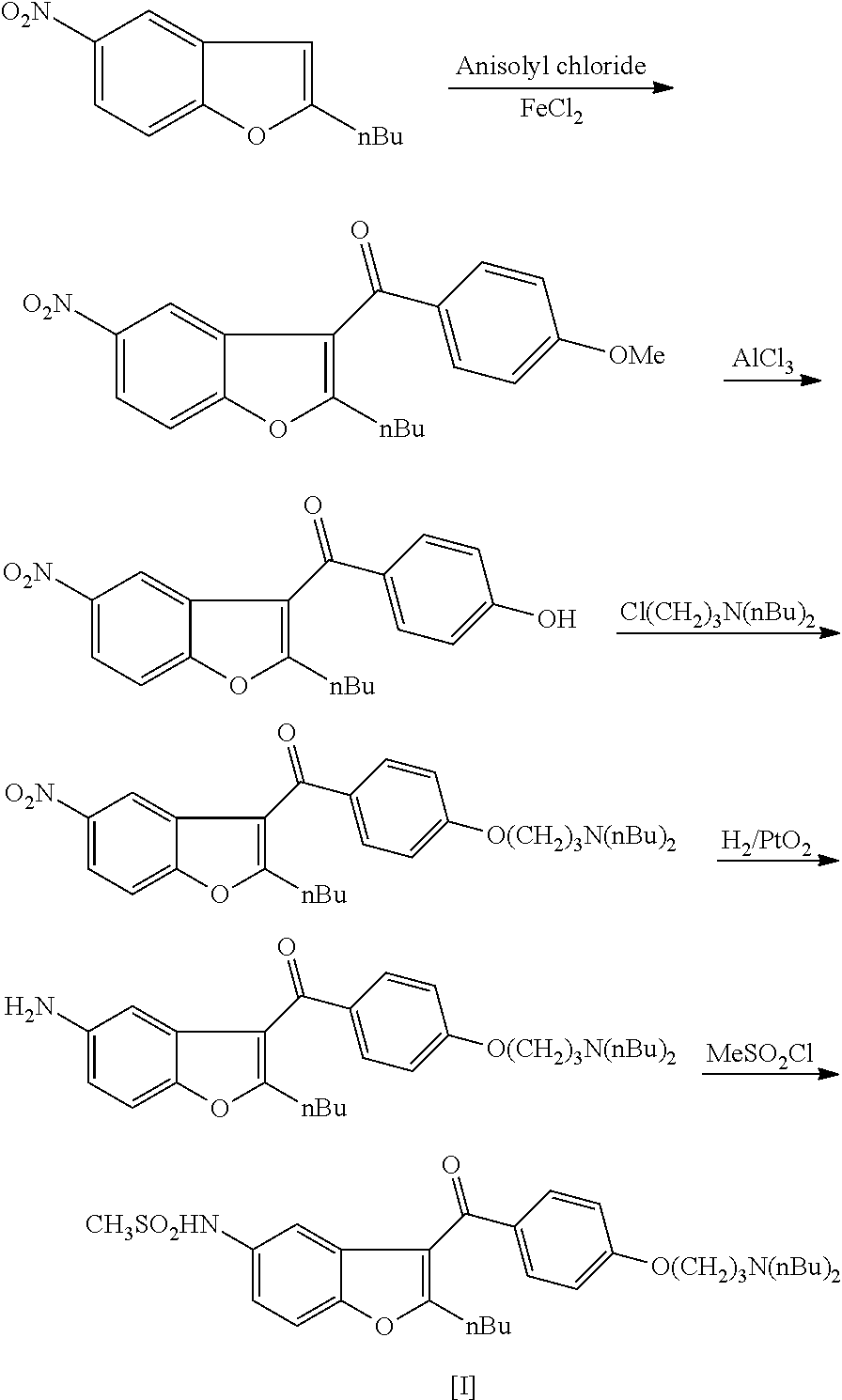
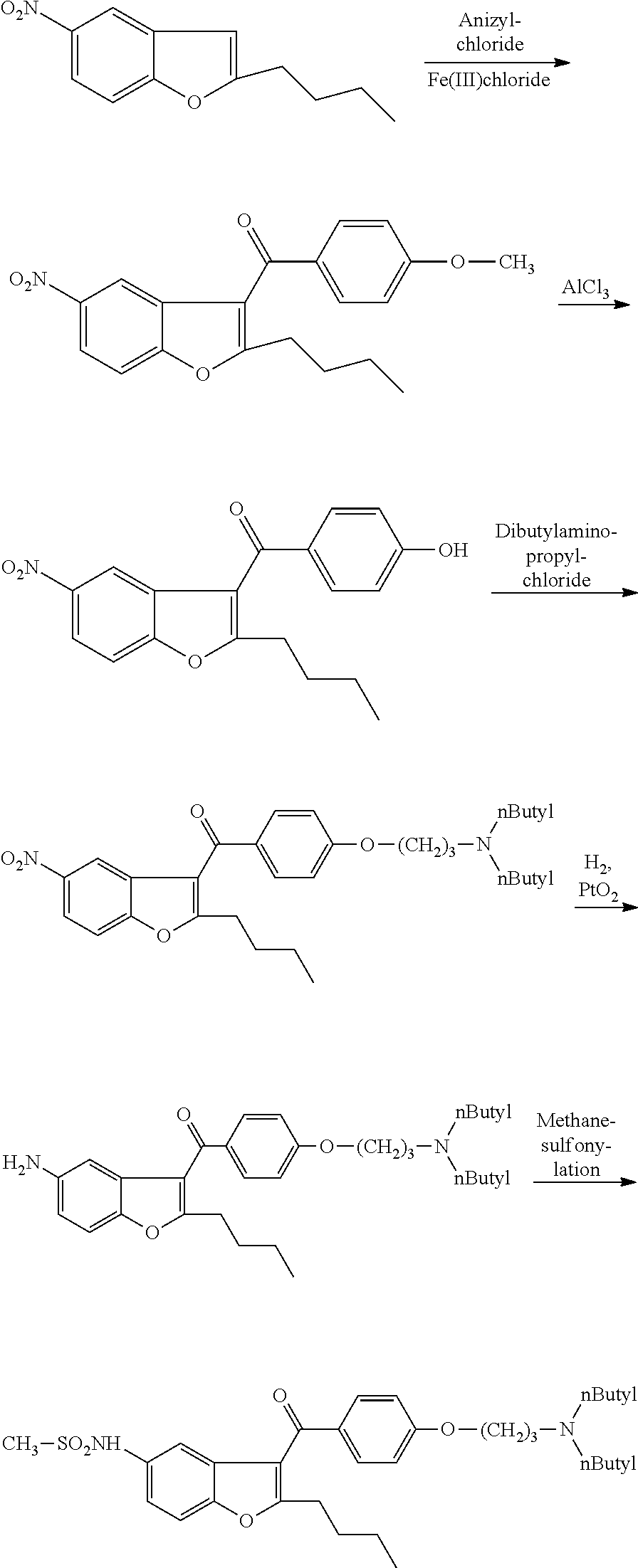
The invention relates to an intermediate and method used for preparing dronedarone and belongs to the technical field of cardiovascular drugs. The method comprises the following steps of: using 2-n-butyl-5-acetamidobenzobfuran to react with p-anisoyl chloride under the catalysis of Lewis acid and obtain 2-n-butyl-3-(4-methoxy-benzoyl)-5-acetamidobenzobfuran; acidizing to obtain 2-n-butyl-3-(4-methoxy-benzoyl)-5-benzofuranamin hydrochloride, reacting with methylsulfonyl chloride to obtain 2-n-butyl-3-(4-methoxy-benzoyl)-5-methylsulfonylamidobenzobfuran; obtaining 2-n-butyl-3-(4-hydroxyl-benzoyl)-5-methylsulfonylamidobenzobfuran under the action of the Lewis acid; and reacting with 1,3-dibromopropane to obtain 2-n-butyl-3-[4-(gamma-bromopropyl)hydroxyl-benzoyl]-5-methylsulfonylamidobenzobfuran, and then reacting with di-n-butylamine to obtain dronedarone. The method avoids the catalytic hydrogenation reaction and has the advantages of available raw materials, simple operation process, high yield, easiness in industrialization and the like.
Owner:TIANJIN KELIN CHEM
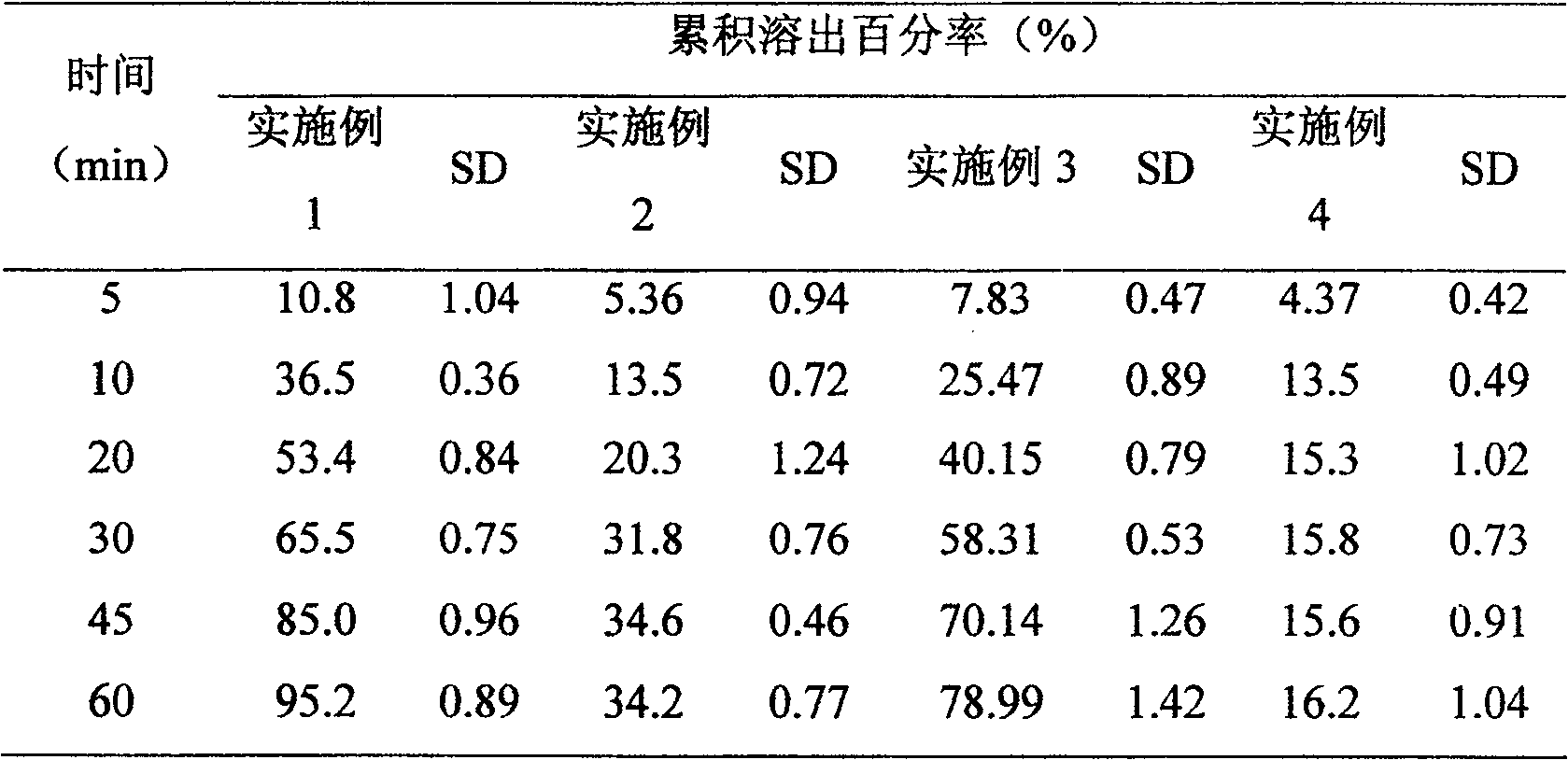
Antiarrhythmic medicinal composition and preparation method thereof
InactiveCN102579421AHigh dissolution rateImprove absorptionPowder deliveryOrganic active ingredientsDronedaroneMedicine
The invention relates to an antiarrhythmic medicinal composition and a preparation method thereof. According to the composition, active ingredients comprise dronedarone or pharmaceutically-acceptable salt thereof, and non-active ingredients comprise one or more of polymers and multiple common additives of preparations. The preparation process for the composition comprises the key step of preparing solid dispersoid by a hot-melting extrusion method, so that tablets, capsules and granules can be prepared. The invention has the advantages that: a preparation with a high dissolution rate can be prepared according to a proper formula by a simple and pollution-free process, and the preparation can be absorbed well in bodies; and samples are stable under the condition of accelerated tests.
Owner:JILIN BODA PHARMA +1
Method for synthesis of dronedarone
The invention relates to a method for synthesis of dronedarone. The method comprises the following steps that 1), p-nitrophenol, paraformaldehyde and concentrated hydrochloric acid as raw materials undergo a condensation reaction in the presence of concentrated hydrochloric acid or phosphoric acid as a catalyst to produce 2-chloromethyl-4-nitrophenol; 2), 2-chloromethyl-4-nitrophenol and triphenylphosphine undergo a reflux reaction in the presence of chloroform to produce 2-hydroxy-5-nitrobenzyl-triphenyl-phosphonium chloride, and 3), 2-hydroxy-5-nitrobenzyl-triphenyl-phosphonium chloride and n-valeryl chloride undergo a condensation reaction in a toluene solution in the presence of triethylamine and n-pentanoic acid as catalysts to produce 2-(n-butyl)-5-nitrobenzofuran.
Owner:FUJIAN COSUNTER PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com