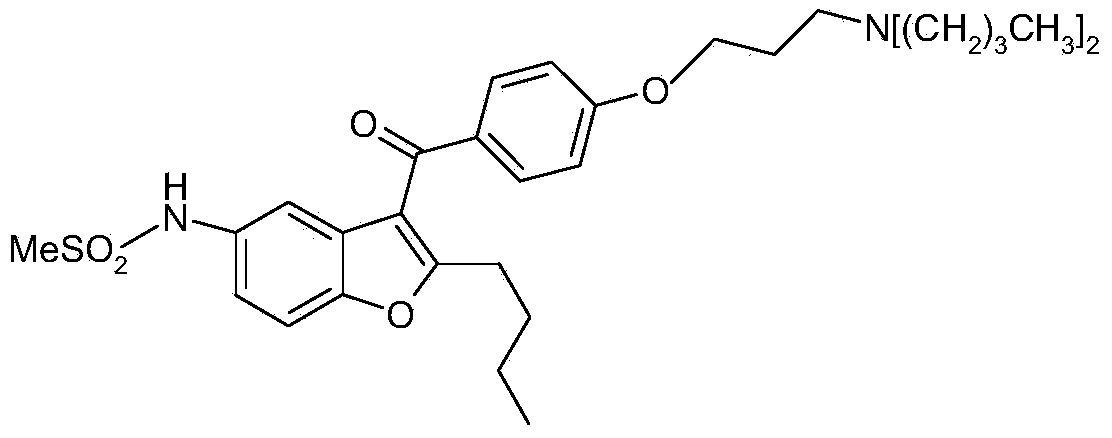
Pharmaceutical composition and solid galenic form having a high dronedarone content, and method for preparing same
A technology for dronedarone and composition, applied in the field of pharmaceutical compositions and solid galenic forms with high dronedarone content and their preparation, to achieve the effects of avoiding drying steps, saving time, and being easy to swallow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0107] The following examples describe the preparation of pharmaceutical compositions and galenic forms according to the invention. This example does not limit the invention and is only used to illustrate the invention.
[0108] Unless otherwise stated, the percentages of the compounds described below are expressed in weight percent relative to the total weight of the composition or galenic form in which the compound is located, as the case may be.
[0109] 1. Tablet composition of the present invention
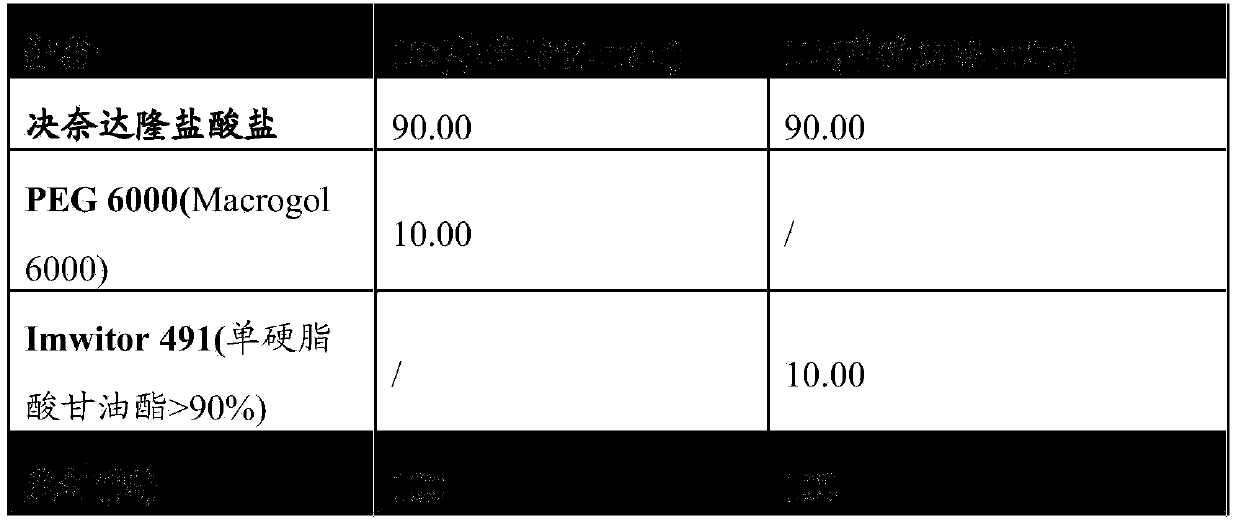
[0110] By way of example, pharmaceutical composition 1A and pharmaceutical composition 1B of the present invention comprising only dronedarone hydrochloride as active substance are illustratively shown in Table 1 below.
[0111] The properties of the components in these compositions are shown in the table, together with the concentrations of the components (expressed in % by weight of the components relative to the total amount of the composition).
[0112] Table 1
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting temperature | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com