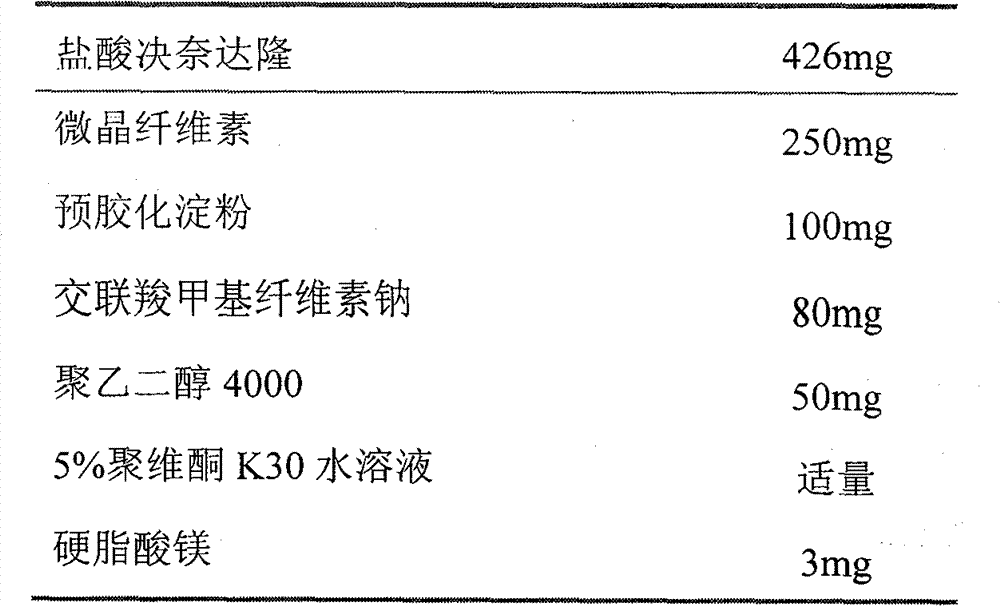
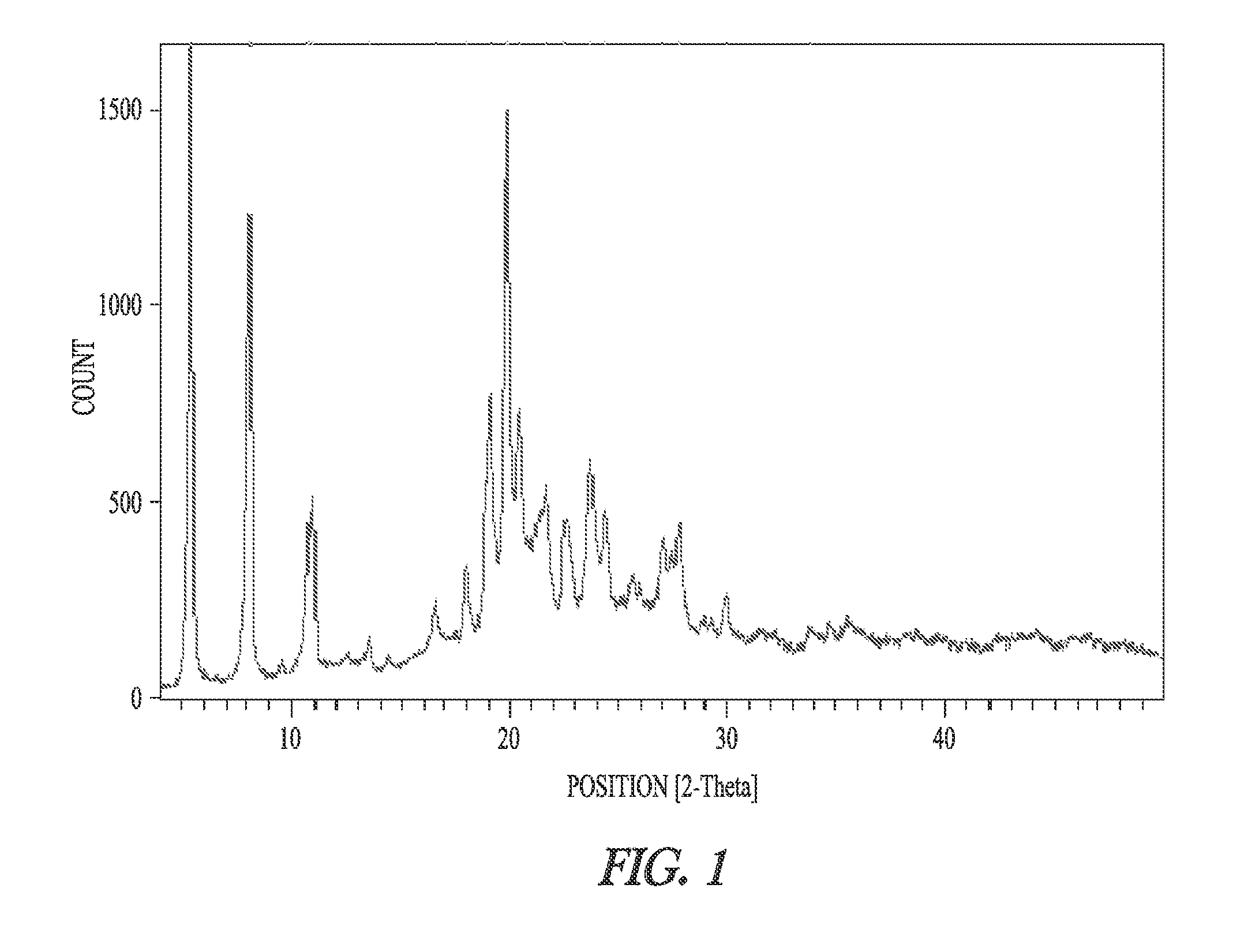
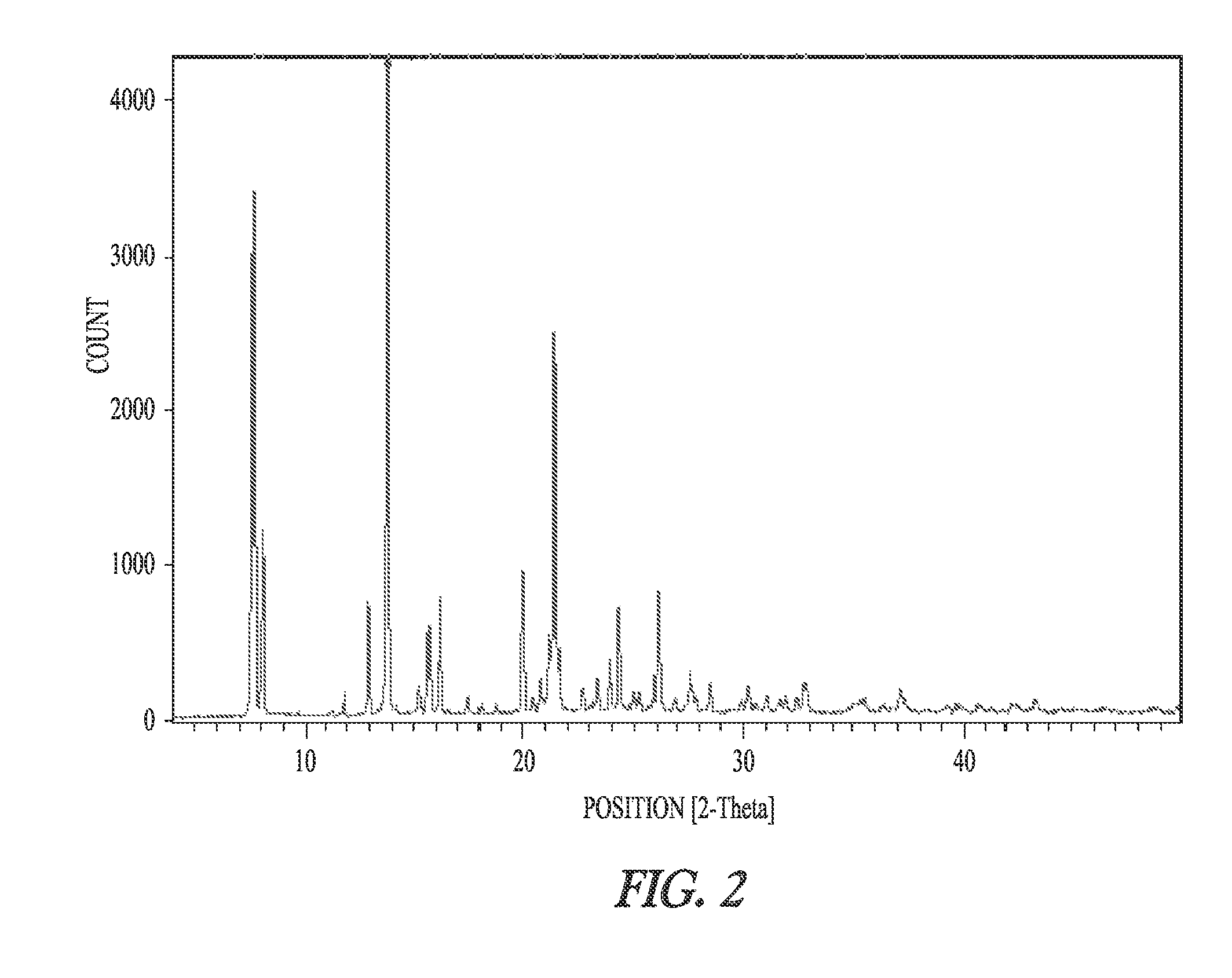
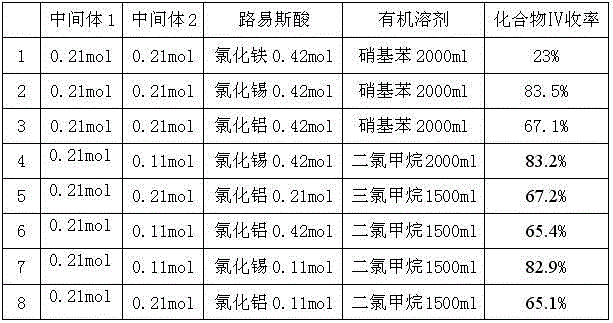
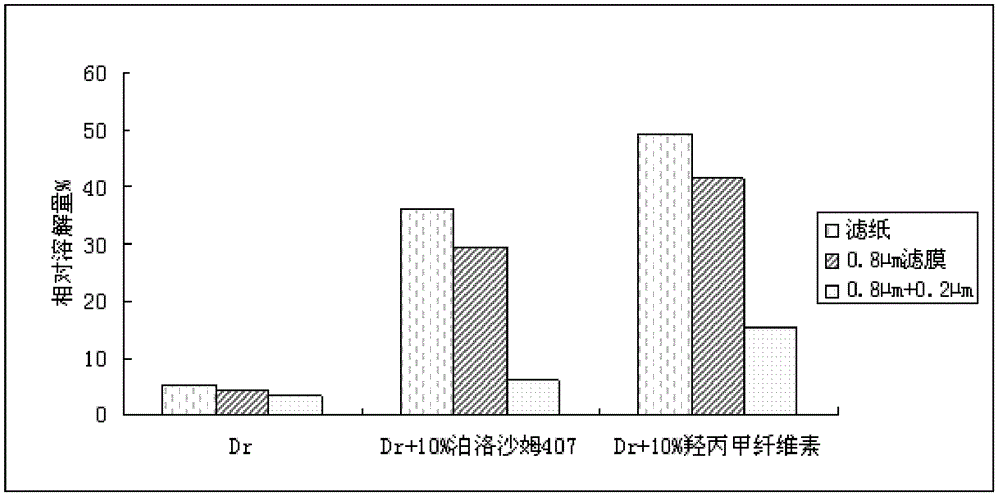
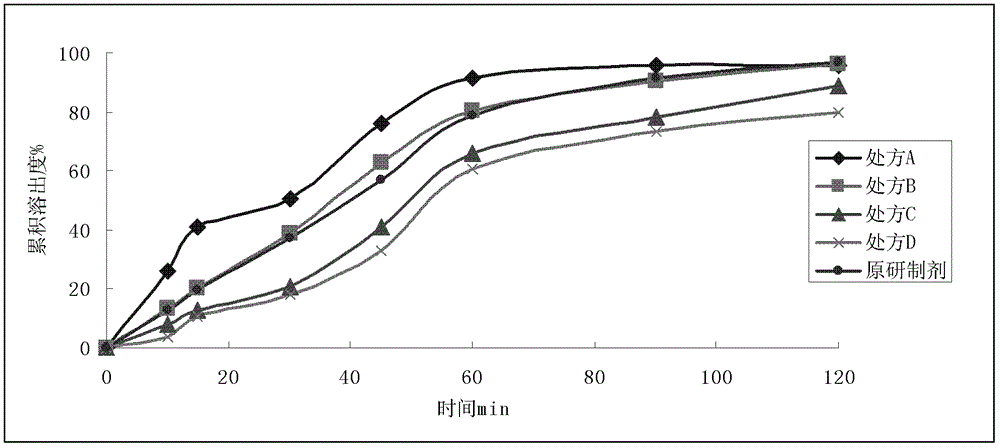
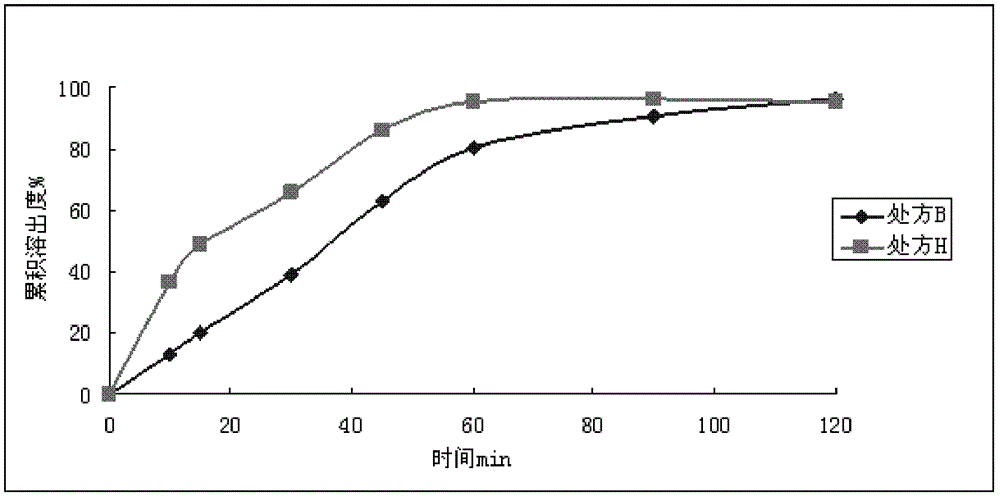
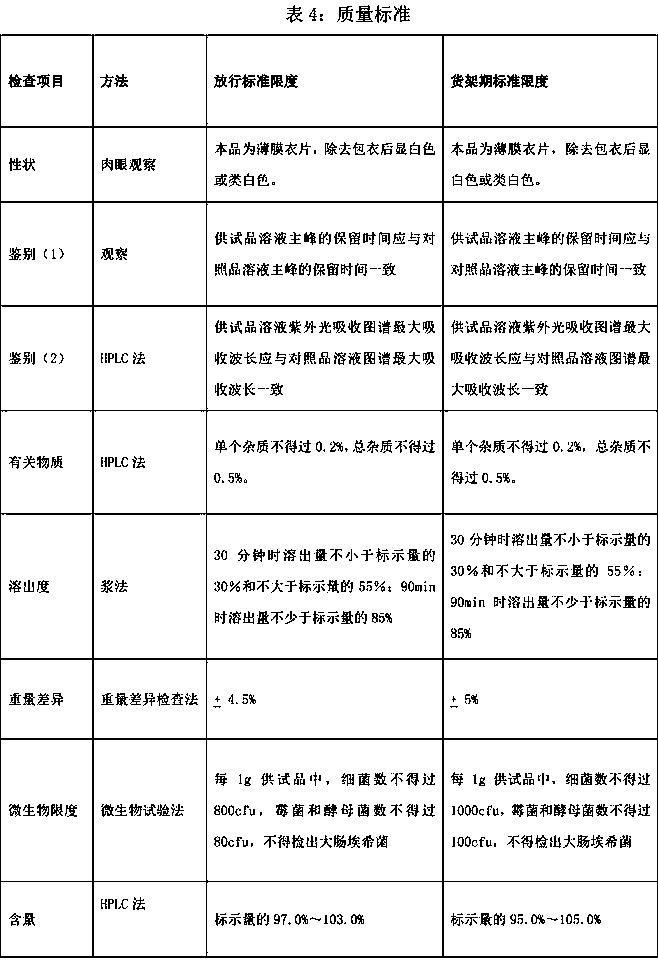
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
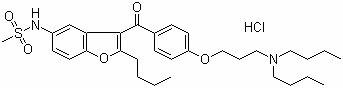
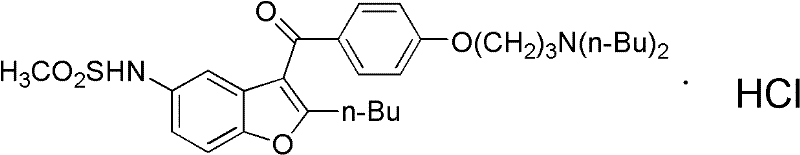
41 results about "Dronedarone hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
1. Sanofi-Aventis. Multaq (dronedarone hydrochloride) tablets prescribing information. Bridgewater, NJ; 2012 Jun. 2. Hohnloser SH, Crijns HJ, van Eickels M et al. Effect of dronedarone on cardiovascular events in atrial fibrillation.
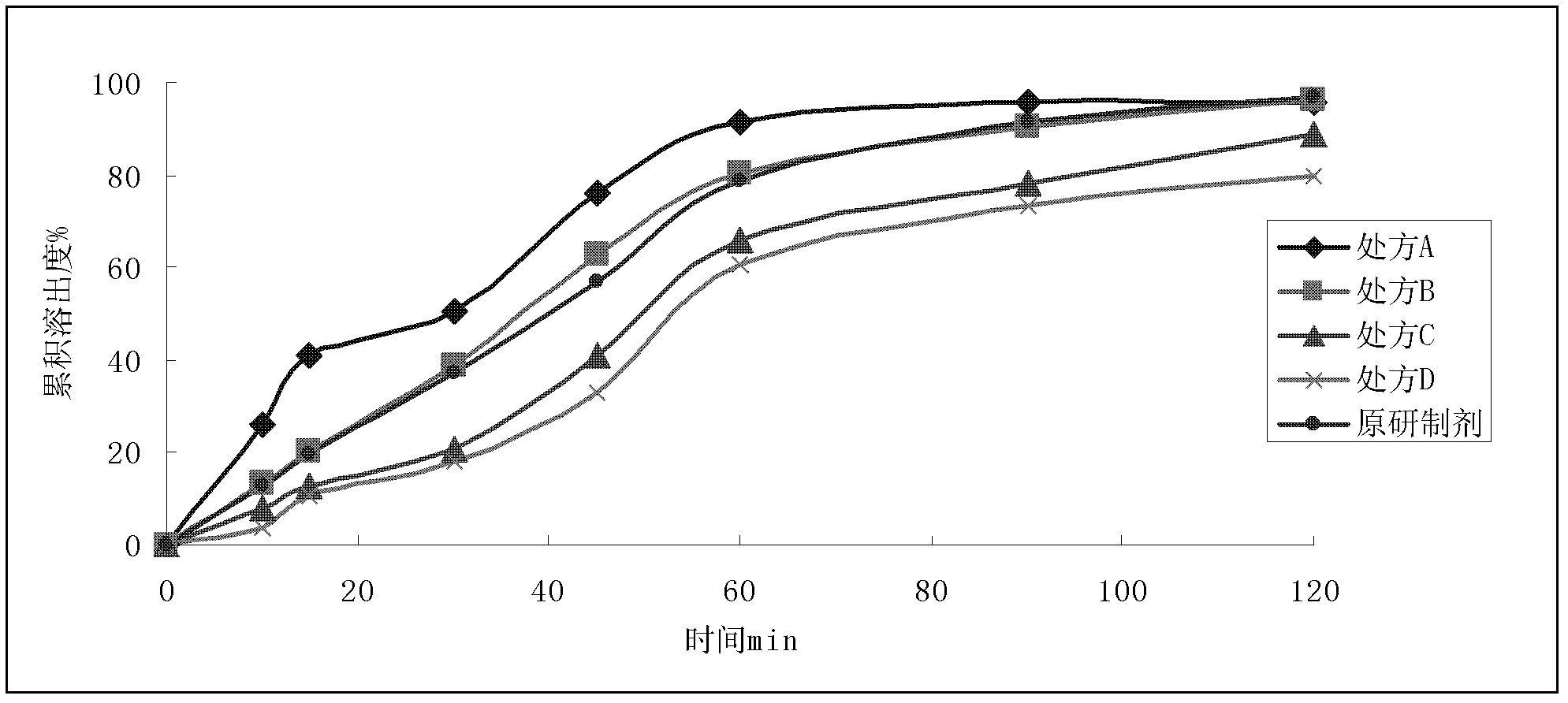
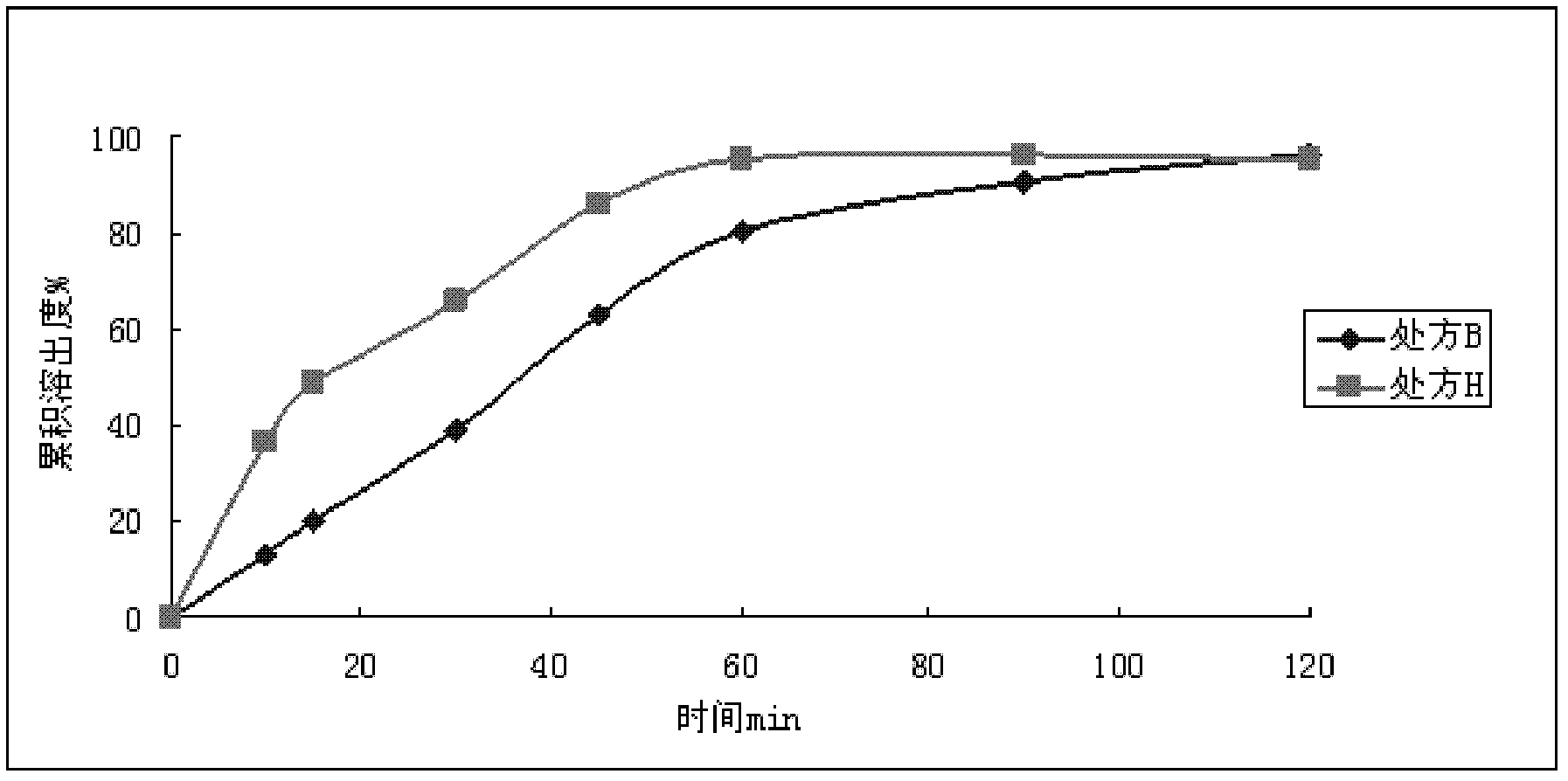
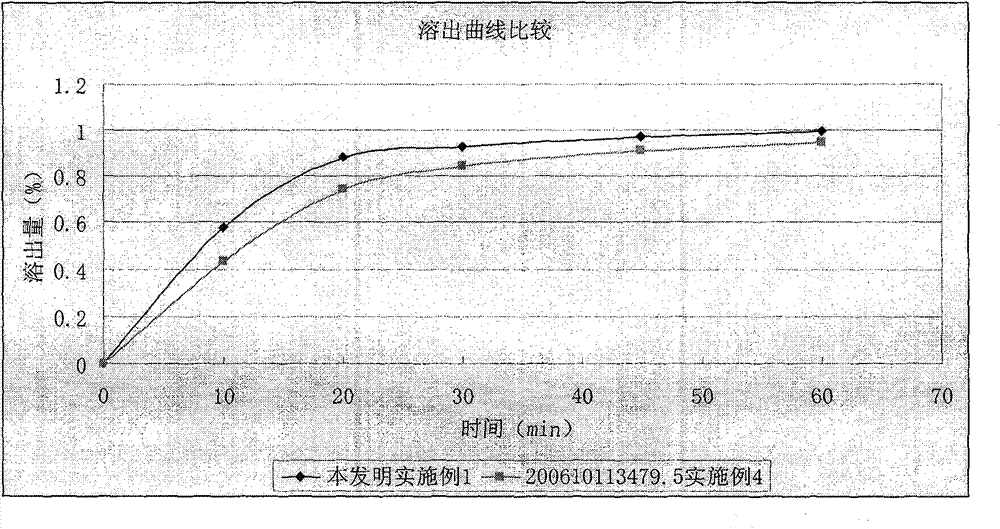
Dronedarone hydrochloride-containing oral solid medicinal composition and preparation method thereof
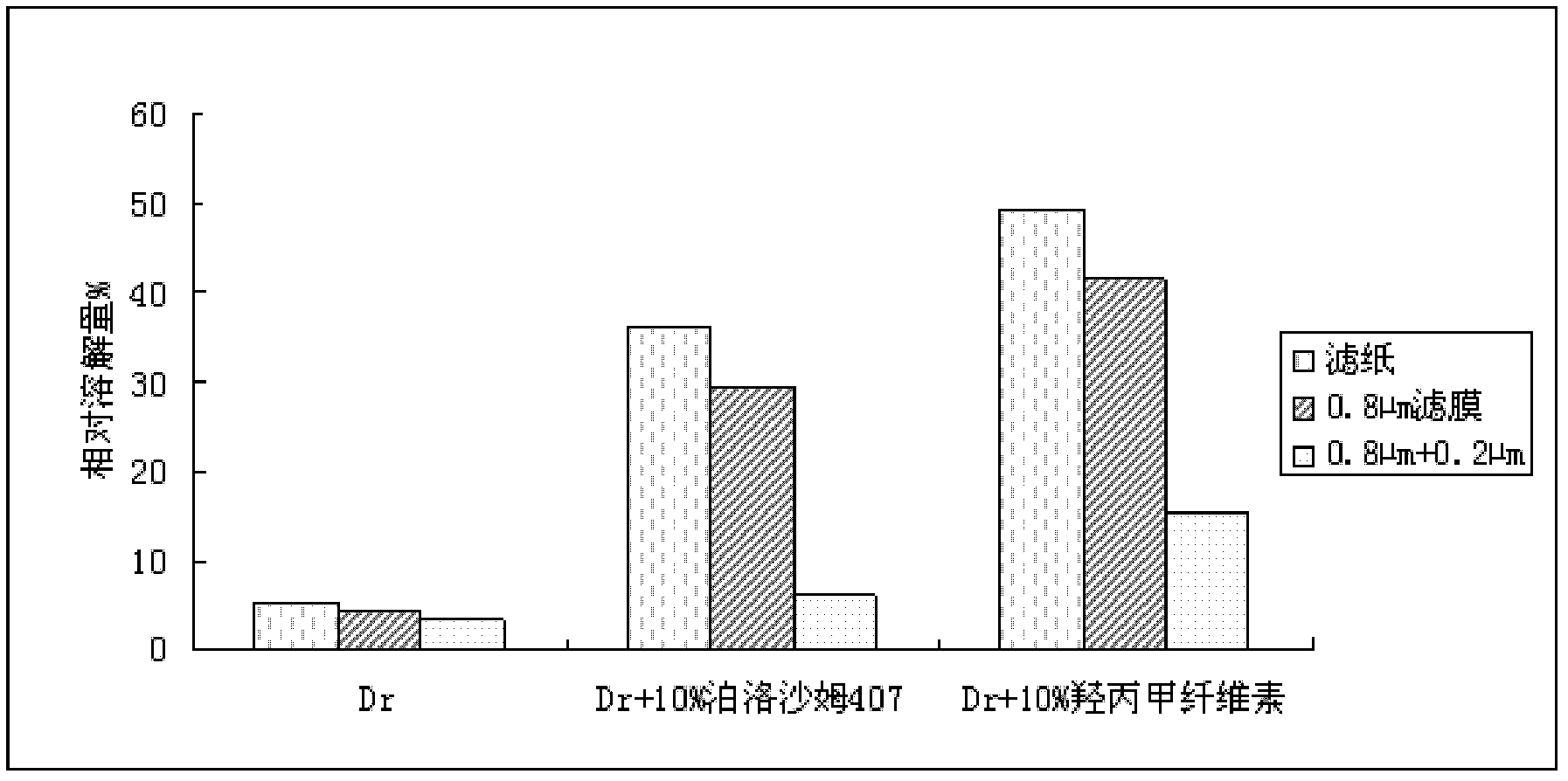
ActiveCN102908305AHigh dissolution rateReduce energy consumptionOrganic active ingredientsPharmaceutical delivery mechanismCelluloseMethyl cellulose
The invention relates to an oral solid medicinal composition, which is characterized by comprising pelletized dronedarone hydrochloride and hydrophilic gel matrix type slow release material hydroxypropyl methyl cellulose, wherein the pelletized dronedarone hydrochloride and hydrophilic gel matrix type slow release material hydroxypropyl methyl cellulose are selectively combined with one or more medicinal excipients. The oral solid medicinal composition is mainly used for treating arrhythmia.
Owner:PHARMA RES INST OF BENCAO TIANYUAN OF BEIJING
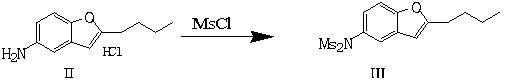
Preparation method of dronedarone hydrochloride and intermediate of dronedarone hydrochloride
InactiveCN102675267AInhibit side effectsImprove responseOrganic compound preparationAmino-hyroxy compound preparationPtru catalystAcyl group
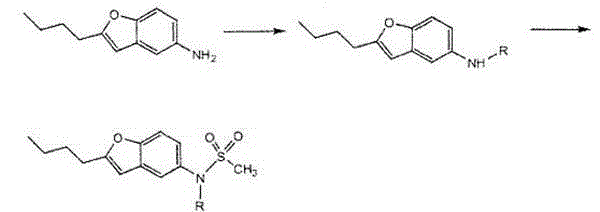
The invention discloses a preparation method of an intermediate of dronedarone hydrochloride, which comprises the step of reacting 2-butyl-5-amino benzofuran hydrochloride serving as a starting material with methylsulfonyl chloride in the presence of a basic catalyst and an organic solvent to produce the intermediate of dronedarone hydrochloride, wherein the intermediate is 2-butyl-5-((N, N-dimethyl sulfonyl) amido) benzofuran. The invention also discloses a method for preparing dronedarone hydrochloride by using the intermediate. The preparation of the intermediate is simple and easy to operate; amino is protected by methylsulfonyl, so that the side reaction possibly generated in the reaction for preparing the dronedarone hydrochloride at the later period can be avoided, and the difficulty in purification at the later period can be simplified; the dronedarone hydrochloride yield is high; the problems in the prior art that the preparation of dronedarone hydrochloride is complicated with high cost, the intermediate is difficult to purify and aftertreatment is complicated can be solved; and the preparation difficulty of dronedarone hydrochloride is greatly reduced.
Owner:山东富创医药科技有限公司
Method for preparing dronedarone hydrochloride
InactiveCN102382087ALess toxicLow process pollutionOrganic chemistrySulfonyl chlorideMethanesulfonyl chloride
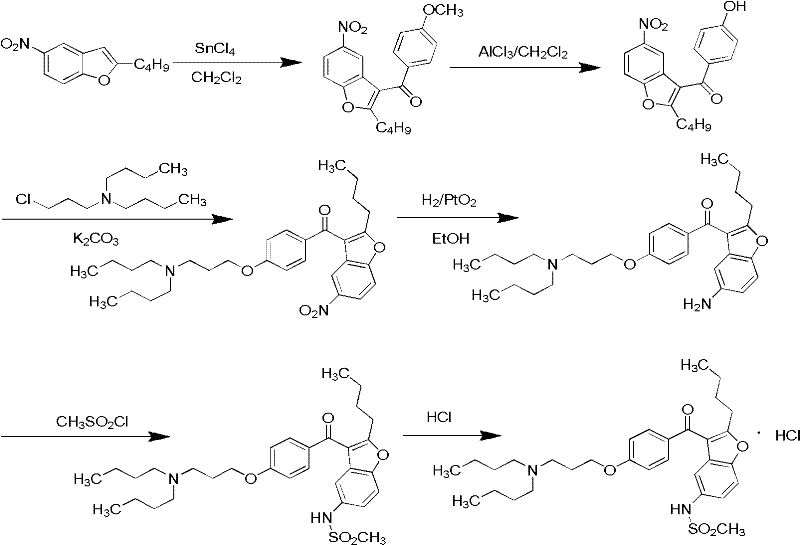
The invention relates to a method for preparing dronedarone hydrochloride, comprising the following concrete steps of: (1) carrying out etherification reaction on 2-butyl-3-(4-hydroxy benzoyl)-5-nitro benzofuran and 1-bromine-3-chloropropane in an organic solvent so as to obtain 2-n-butyl-3-[4-(3-chlorine propoxy) benzoyl]-5-nitro benzofuran; (2) reducing to 2-n-butyl-3-[4-(3-chlorine propoxy) benzoyl]-5-amido benzofuran by utilizing a reductant; (3) carrying out sulfamide reaction with methyl sulfonyl chloride in the organic solvent so as to obtain 2-n-butyl-3-[4-(3-chlorine propoxy) benzoyl]-5-methyl sulfonamide benzofuran; (4) carrying out N-hydrocarbon reaction with di-n-butylamine so as to obtain the dronedarone; and (5) salifying the dronedarone hydrochloride and obtaining the dronedarone hydrochloride. The method provided by the invention has the advantages that the reaction route is simple, the raw material is cheap and easy to obtain and the reaction condition is mild and easy to control and is suitable for industrialized production.
Owner:NANJING UNIV OF TECH
Dronedarone hydrochloride pharmaceutical composition and preparation method thereof
ActiveCN103054820ASmooth industrial productionPromote absorptionOrganic active ingredientsPill deliveryPatient complianceAdhesive
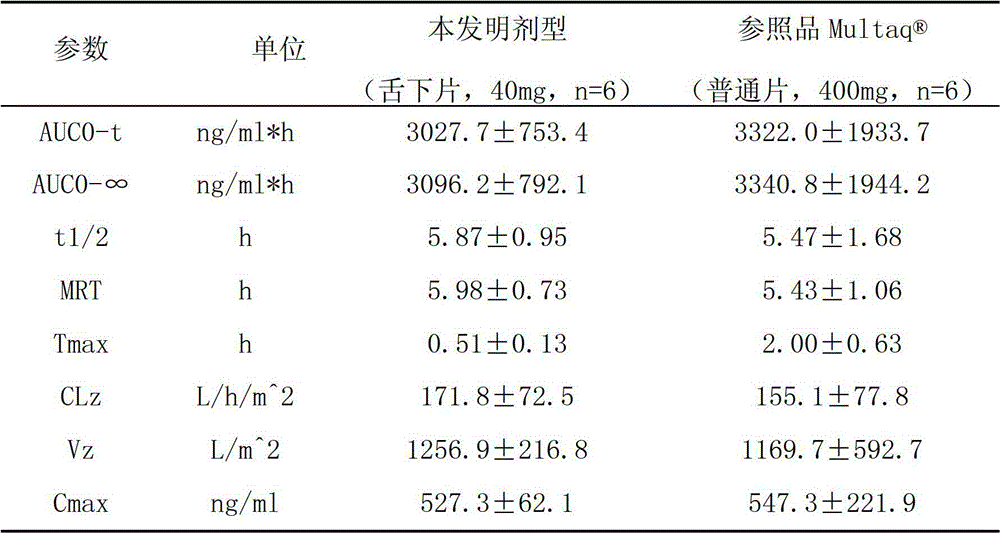
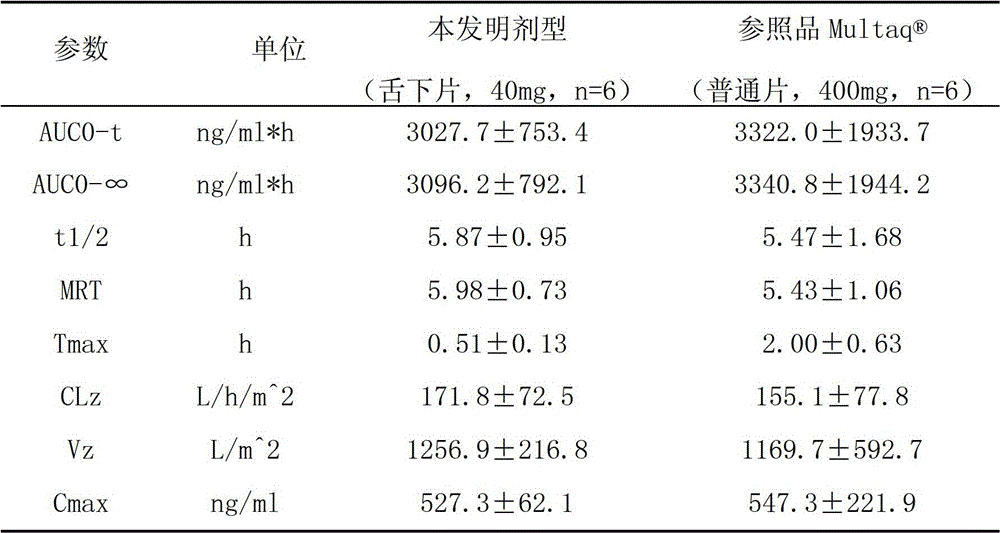
The invention provides a dronedarone hydrochloride pharmaceutical composition which is suitable for the sublingual administration, can quickly run, and can greatly improve the bioavailability and the curative effect. The pharmaceutical composition consists of 5-65 parts of dronedarone hydrochloride, 1-10 parts of a disintegrating agent, 10-80 parts of filler, 0.5-3 parts of antacid, 0-5 parts of corrigent, 1-10 parts of an adhesive and 0.3-2 parts of a lubricating agent. The invention further provides a preparation method suitable for the industrial production. The dronedarone hydrochloride pharmaceutical composition provided by the invention is directly absorbed by the sublingual mucosa, and the first-pass effect of oral medicaments can be avoided, and the gastrointestinal digestion, acidolysis and the like can be avoided, so that the bioavailability can be greatly improved. The epithelium of the sublingual mucosa is not cornified, so that the dronedarone hydrochloride pharmaceutical composition is large in superficial area, and high in infiltration capacity. The medicament can be quickly absorbed after being administrated, so that the dronedarone hydrochloride pharmaceutical composition is quick to run, and convenient to use; and compared with the other administrated preparations, the dronedarone hydrochloride pharmaceutical composition is convenient to administrate, and good in patient compliance.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
Dronedarone hydrochloride tablet and preparation method thereof
ActiveCN103565763AHigh dissolution rateHigh melting pointOrganic active ingredientsPharmaceutical non-active ingredientsMethyl celluloseSurfactant free
The invention belongs to the field of pharmaceutical preparations, and specifically relates to a surfactant-free dronedarone hydrochloride tablet. The dronedarone hydrochloride tablet comprises the following components in parts by weight: 400 parts of dronedarone hydrochloride, 100-1200 parts of hydroxypropyl methyl cellulose, 20-640 parts of disintegrating agent, 7-10 parts of lubricant, and zero surfactant. The technical scheme of the dronedarone hydrochloride tablet is characterized in that no surfactant is added, so that the safety is improved, and the problems of low melting point and easy sticking of the surfactant in the production process of the preparation are also avoided; and simultaneously, the dissolution rate of dronedarone hydrochloride is obviously improved. In the preparation process, the dronedarone hydrochloride and hydroxypropyl methyl cellulose are together ground until the particle size thereof is not greater than 1 micron, and then the obtained powder is formed into tablets with other suitable accessories; the process is quite simple, convenient to operate and suitable for industrial large-scale production.
Owner:SHANDONG NEWTIME PHARMA
Application of dronedarone hydrochloride in preparation of anti-digestive tract tumor drug
InactiveCN112137999AEnhanced inhibitory effectOrganic active ingredientsDigestive systemOncologyPharmacology
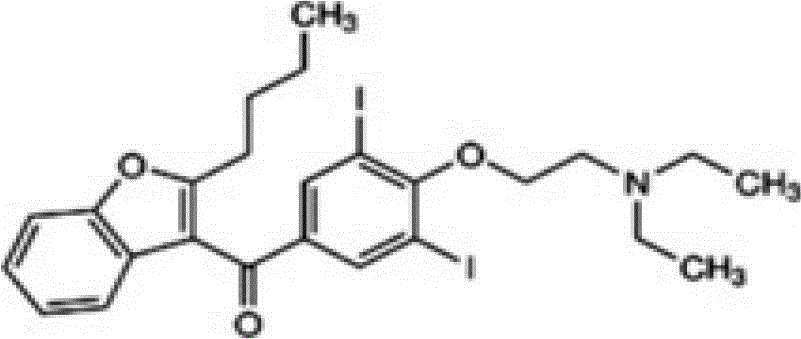
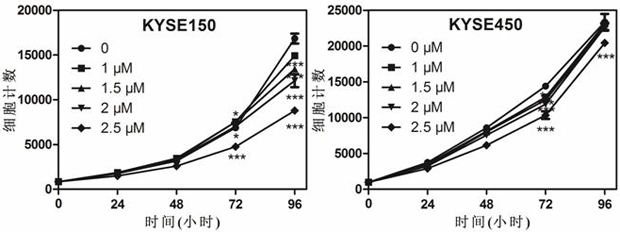
The invention discloses application of dronedarone hydrochloride in preparation of an anti-digestive tract tumor drug. The chemical name of dronedarone hydrochloride is N [2-butyl-3[4-[3- (dibutylamino) propoxy]phenyl]-5-benzofuran] methanesulfonamide. The application comprises the following steps: firstly, through the action of dronedarone hydrochloride on an esophageal cancer cell line, specifically evaluating the effect of dronedarone hydrochloride in an esophageal cancer in-vitro experiment. Secondly, through the action of dronedarone hydrochloride on the gastric cancer cell line, specifically evaluating the in-vitro inhibition effect of dronedarone hydrochloride on the gastric cancer cell line, and comprehensively evaluating the effect of dronedarone hydrochloride in digestive tract tumors. Results show that dronedarone hydrochloride with a proper concentration has a good inhibition effect in a digestive tract tumor cell line, and a new thought and a new basis are provided for treatment and prevention of tumors.
Owner:ZHENGZHOU UNIV
Sustained release preparation of dronedarone hydrochloride
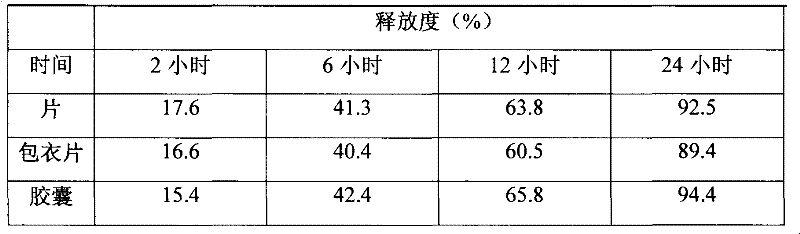
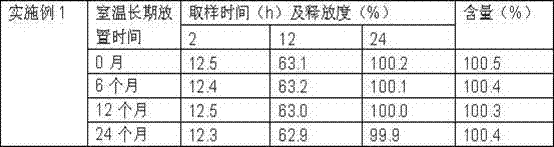
The invention discloses a sustained release preparation of dronedarone hydrochloride, aiming to maintain an optimal steady state plasma concentration better, improve a pharmaceutical effect and bring convenience for use and reduce hospitalization risk of patients. Active components are released slowly to obtain prospected drug release behavior in vivo and in vitro. Therefore, the sustained release preparation of dronedarone hydrochloride, disclosed by the invention, has the advantages of low drug use time, slow release of drug in vivo, steady plasma concentration, low fluctuation, high bioavailability and high safety.
Owner:CGENETECH (SUZHOU CHINA) CO LTD
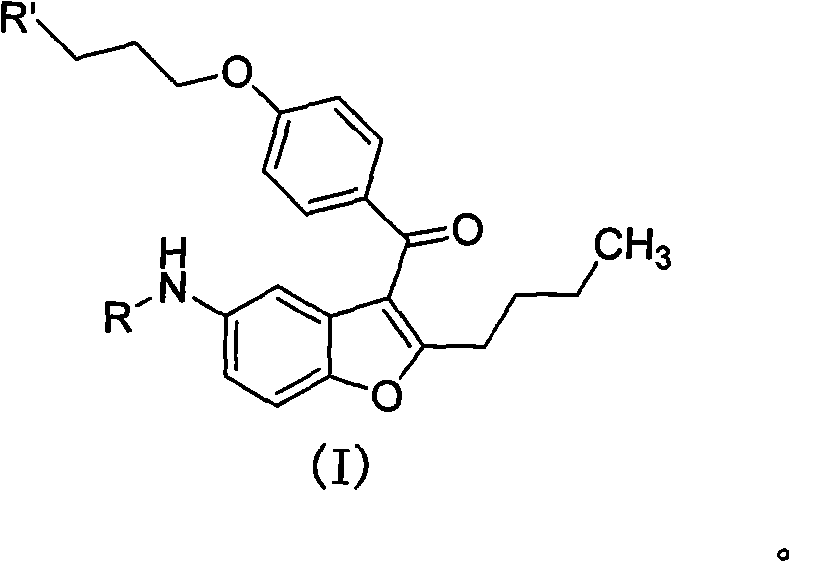
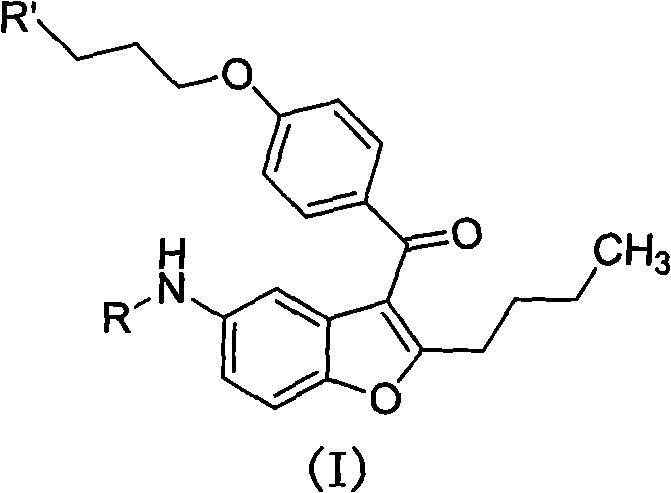
2-n-butyl-3-(4-subsitituted propylbenzoyl)-5-substituted amino benzofuran and application thereof
ActiveCN102070577AReduce manufacturing costAvoid using effectsOrganic chemistryPtru catalystPhenacyl
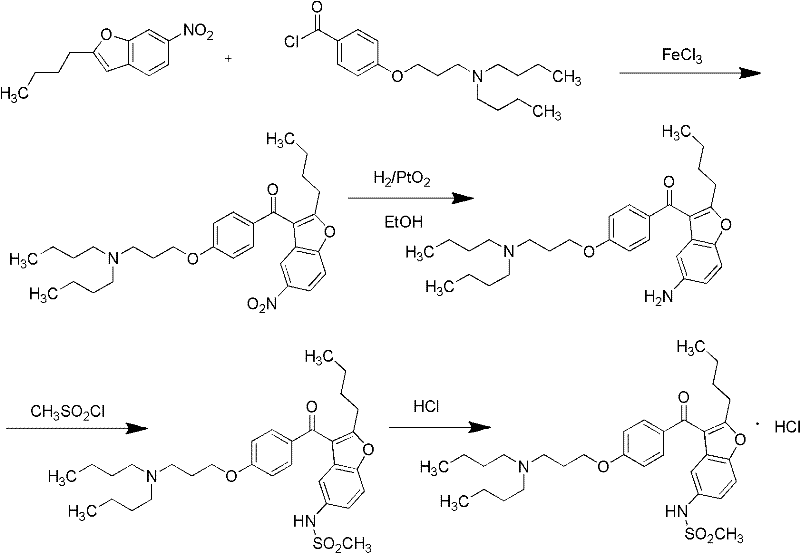
The invention discloses 2-n-butyl-3-(4-subsitituted propylbenzoyl)-5-substituted amino benzofuran and application thereof. The 2-n-butyl-3-(4-subsitituted propylbenzoyl)-5-substituted amino benzofuran can be used for preparing dronedarone and pharmaceutically acceptable salts of the dronedarone. The 2-n-butyl-3-(4-subsitituted propylbenzoyl)-5-substituted amino benzofuran has the characteristics that raw material is available, reaction conditions are mild, and operation is convenient, and the use of an expensive metal catalyst and a high-pressure hydrogenation operation are avoided; dronedarone hydrochloride is obtained from an intermediate (III) by the hydrolysis reaction, the salification, the purification, the sulfonylation, the salification and the purification. The purities of intermediates and target products in various steps are high, and the qualities are stable and controllable, and the preparation cost of the dronedarone is greatly reduced; and the 2-n-butyl-3-(4-subsitituted propylbenzoyl)-5-substituted amino benzofuran is suitable for a large amount of industrialization preparations. The general structure formula of the compound (I) is shown in the specification.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
Preparation method of dronedarone hydrochloride tablets
InactiveCN105412027ASolve the sticking problemPrevent precipitationOrganic active ingredientsPharmaceutical non-active ingredientsFiller ExcipientMagnesium stearate
The invention discloses a preparation method of dronedarone hydrochloride tablets. The preparation method includes the steps that firstly, poloxamer and Kollicoat IR are dissolved in ethanol, ethanol is removed through drying, screening is performed, and a poloxamer mixture (1) wrapped by Kollicoat IR is obtained; secondly, dronedarone hydrochloride, a disintegrating agent and a filling agent are evenly mixed, granulation is performed, drying is performed, and a mixture (2) is obtained; thirdly, the mixture (1) and the mixture (2) are evenly mixed, a proper amount of magnesium stearate is added, and the dronedarone hydrochloride tablets are obtained through mixing and tabletting.
Owner:QINGDAO HISER MEDICAL CENT
Medicine composition of dronedarone hydrochloride solid dispersion and preparation method thereof
ActiveCN102078307BReasonable prescriptionWorkmanship is feasibleOrganic active ingredientsPharmaceutical non-active ingredientsPharmaceutical drugTherapeutic effect
The invention discloses a medicine composition of dronedarone hydrochloride, which is a dispersible tablet. The medicine composition is a tablet which is prepared from dronedarone hydrochloride micronized by a solid dispersion technology and an auxiliary material. According to the medicine composition, the dissolubility of the primary medicine is improved, rapid disintegration of the medicine is facilitated, the absorbability of the medicine in the blood is increased, and the bioavailability of the primary medicine is improved. Moreover, the medicine composition has a favorable treatment effect on symptoms such as arrhythmia, atrial fibrillation and the like.
Owner:开封大宋制药有限公司
Synthesis of dronedarone and salts thereof
InactiveUS20120108828A1Simple and cost-effective and industrially viable processOrganic chemistryDronedarone hydrochlorideMedicinal chemistry
The present invention relates to a process for preparation of Dronedarone or pharmaceutically acceptable salts thereof. More particularly, the present invention provides a process for preparation of Dronedarone hydrochloride, without the isolation of Dronedarone base.
Owner:USV LTD
Content measuring method of dronedarone hydrochloride
InactiveCN107957451AAccurate and effective determinationReduce mistakesComponent separationPhosphoric acidDronedarone hydrochloride
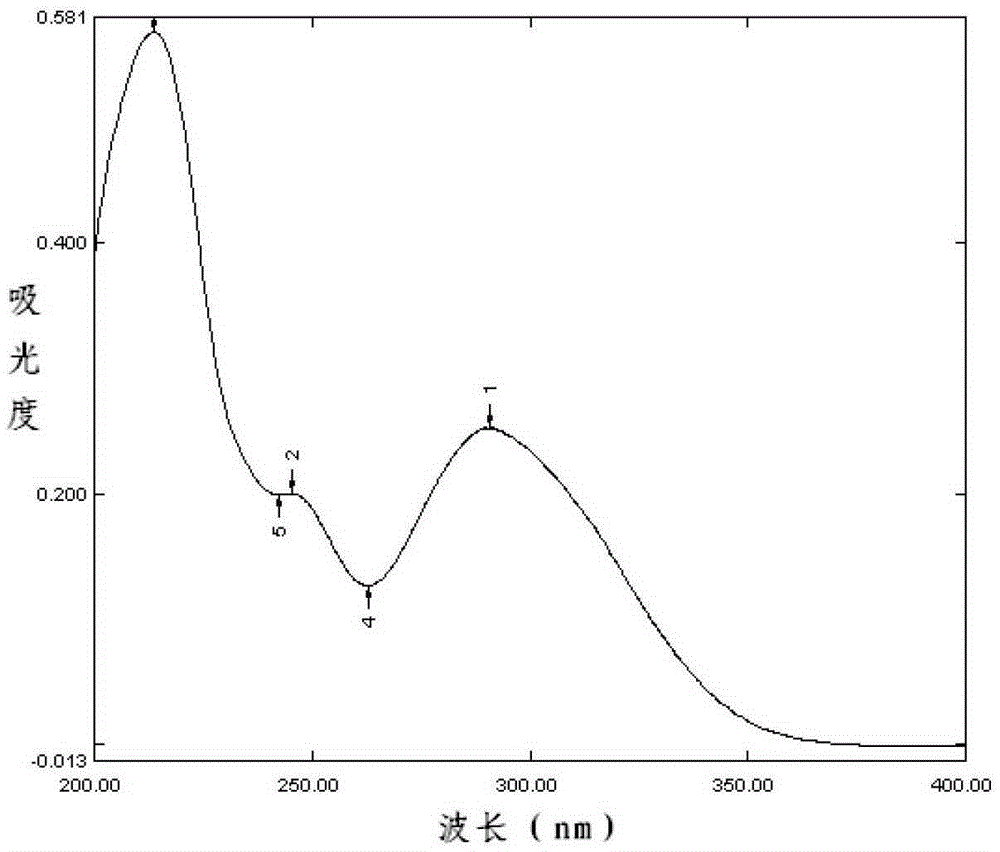
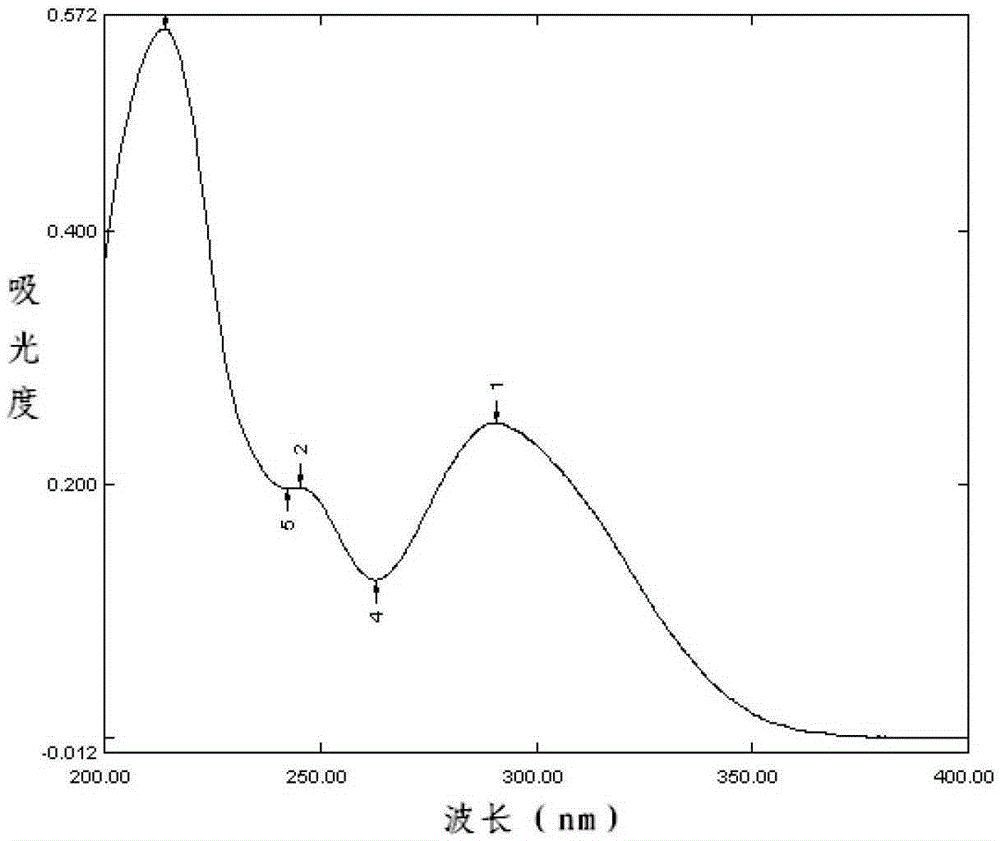
The invention relates to a content measuring method of dronedarone hydrochloride. Chromatographic conditions of the method include that a packed column is octadecyl silane bonded silica gel, a mobilephase is tetrahydrofuran-0,05% phosphoric acid solution, flow rate of the mobile phase is 1.0ml / min, column temperature is 35 DEG C, detection wavelength is 245nm, and sample size is 10ul. The methodis accurate and reliable in dronedarone hydrochloride content measuring result, simple, convenient and scientific, meets requirements of Chinese pharmacopoeia and is good in detection result.
Owner:朱隆娅
Preparation method of dronedarone hydrochloride and intermediate of dronedarone hydrochloride
InactiveCN102675267BInhibit side effectsImprove responseOrganic compound preparationAmino-hyroxy compound preparationPtru catalystAcyl group
The invention discloses a preparation method of an intermediate of dronedarone hydrochloride, which comprises the step of reacting 2-butyl-5-amino benzofuran hydrochloride serving as a starting material with methylsulfonyl chloride in the presence of a basic catalyst and an organic solvent to produce the intermediate of dronedarone hydrochloride, wherein the intermediate is 2-butyl-5-((N, N-dimethyl sulfonyl) amido) benzofuran. The invention also discloses a method for preparing dronedarone hydrochloride by using the intermediate. The preparation of the intermediate is simple and easy to operate; amino is protected by methylsulfonyl, so that the side reaction possibly generated in the reaction for preparing the dronedarone hydrochloride at the later period can be avoided, and the difficulty in purification at the later period can be simplified; the dronedarone hydrochloride yield is high; the problems in the prior art that the preparation of dronedarone hydrochloride is complicated with high cost, the intermediate is difficult to purify and aftertreatment is complicated can be solved; and the preparation difficulty of dronedarone hydrochloride is greatly reduced.
Owner:山东富创医药科技有限公司
Application of combination of dronedarone hydrochloride and 5-fluorouracil in preparation of antitumor drugs
PendingCN113925867AIncrease lethalityReduce financial burdenOrganic active ingredientsDigestive systemOncologyAnti neoplastic
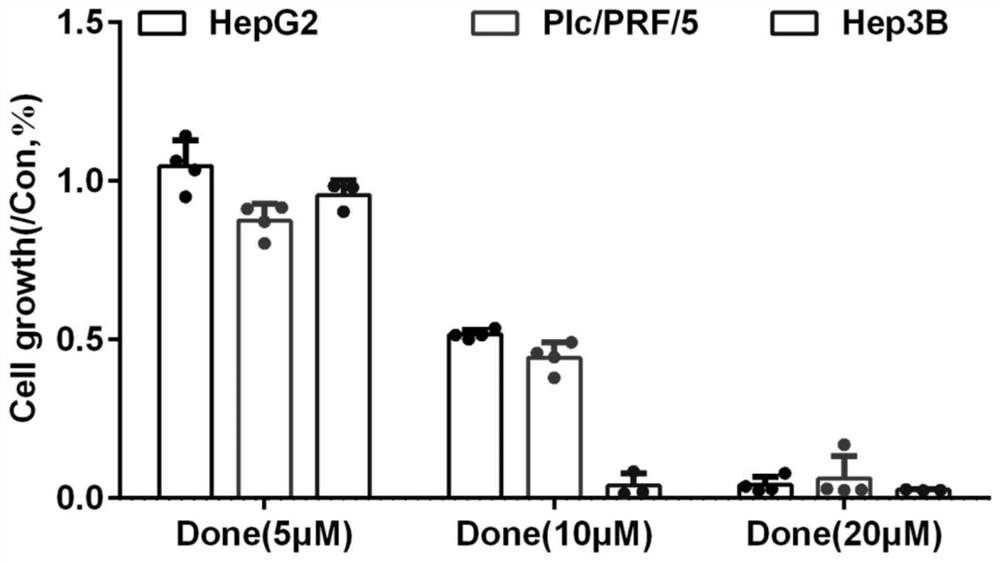
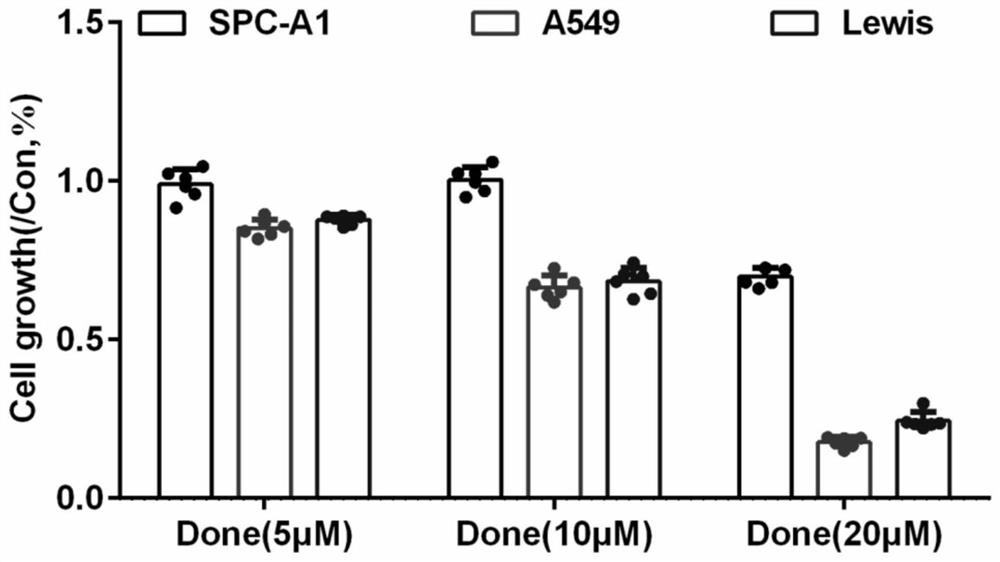
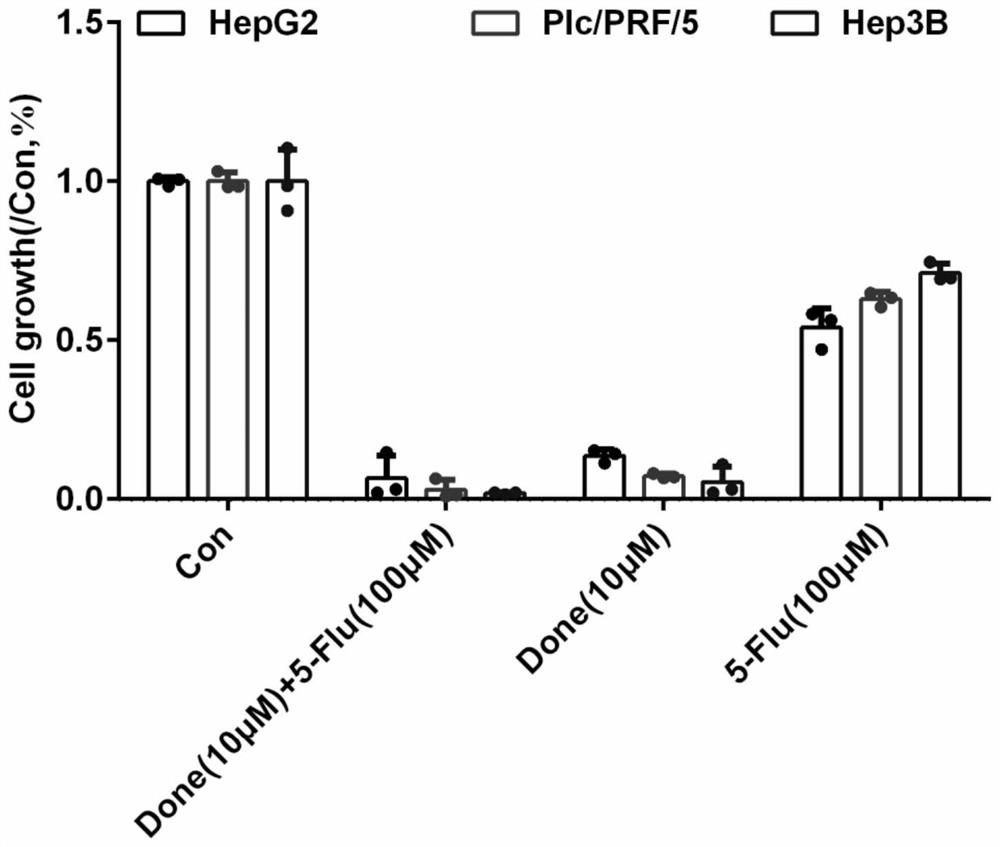
The invention discloses an application of combination of dronedarone hydrochloride and 5-fluorouracil in preparation of antitumor drugs. Pharmacodynamic experiments prove that the combination of dronedarone hydrochloride and 5-fluorouracil can effectively inhibit proliferation of human hepatoma cell lines HepG2, PLC / PRF / 5 and Hep3B. The single use of dronedarone hydrochloride can effectively inhibit the proliferation of human hepatoma cell lines HepG2, PLC / PRF / 5 and Hep3B, and the single use of dronedarone hydrochloride can effectively inhibit the proliferation of human lung cancer cell lines SPC-A1 and A549 and mouse lung cancer cell lines Lewis. In addition, dronedarone hydrochloride is combined with 5-fluorouracil, so that growth of tumors in a mouse liver cancer transplantation tumor model can be inhibited. The dronedarone hydrochloride combined antitumor drug has the characteristics of safety, effectiveness and the like when being used for treating liver cancer and lung cancer, can be used for preparing the drug for treating liver cancer and lung cancer, and provides a new treatment strategy for clinical treatment of liver cancer and lung cancer.
Owner:THE FIRST AFFILIATED HOSPITAL OF ARMY MEDICAL UNIV +1
Non-surfactant dronedarone hydrochloride tablet
InactiveCN108042501AHigh dissolution rateHigh simulationOrganic active ingredientsPharmaceutical non-active ingredientsLow-substituted hydroxypropylcelluloseDissolution
The invention provides a non-surfactant dronedarone hydrochloride tablet. The tablet is prepared from dronedarone hydrochloride, citric acid, magnesium stearate, hydroxypropylmethyl cellulose, low-substituted hydroxypropyl cellulose, croscarmellose sodium and sodium carboxymethyl starch. Compared with the prior art, the tablet has the following advantages that no surfactant is added, both the safety is improved and the problems of a low melting point and easy sticking of the surfactant during production of a preparation are avoided, the dissolution degree of dronedarone hydrochloride in tap water is significantly increased, and the in-vivo environment is better simulated.
Owner:GUANGDONG YIMING PHARMA
A kind of preparation method of dronedarone hydrochloride tablet
InactiveCN105412027BSolve the sticking problemPrevent precipitationOrganic active ingredientsPill deliveryFiller ExcipientMagnesium stearate
The invention discloses a preparation method of dronedarone hydrochloride tablets. The preparation method includes the steps that firstly, poloxamer and Kollicoat IR are dissolved in ethanol, ethanol is removed through drying, screening is performed, and a poloxamer mixture (1) wrapped by Kollicoat IR is obtained; secondly, dronedarone hydrochloride, a disintegrating agent and a filling agent are evenly mixed, granulation is performed, drying is performed, and a mixture (2) is obtained; thirdly, the mixture (1) and the mixture (2) are evenly mixed, a proper amount of magnesium stearate is added, and the dronedarone hydrochloride tablets are obtained through mixing and tabletting.
Owner:QINGDAO HISER MEDICAL CENT
Dronedarone hydrochloride self-microemulsion preparation and preparing method thereof
InactiveCN108042489AImprove solubilityIncrease dissolution rateOrganic active ingredientsPill deliveryOil phaseDronedarone hydrochloride
The invention discloses a dronedarone hydrochloride self-microemulsion preparation and a preparing method thereof. A liquid self-microemulsion preparation is prepared from dronedarone hydrochloride, an oil phase, an emulsifier and a co-emulsifier, or a solid self-microemulsion preparation is further prepared from the obtained liquid self-microemulsion preparation and an excipient.
Owner:佛山市弘泰药物研发有限公司
A dronedarone hydrochloride composition
ActiveCN105106152BMedium weightEasy to transportOrganic active ingredientsPharmaceutical product form changeBenzeneFuran
The invention relates to a solid preparation composition containing a benzofurane derivative and a preparation method thereof and belongs to the technical field of medicines. The technical scheme is that a dronedarone hydrochloride composition is characterized in that dronedarone hydrochloride and a surfactant undergo co-micronization to D90 5-10[mu]m. Through a co-micronization technology of dronedarone hydrochloride and the surfactant, a rational formula and a preparation method, a stable tablet is obtained. The tablet which is moderate in weight and convenient to transport and store is provided clinically.
Owner:DISHA PHARMA GRP
A dronedarone hydrochloride pharmaceutical composition and preparation method thereof
ActiveCN103054820BPromote absorptionIncrease surface areaOrganic active ingredientsPill deliveryEpitheliumPatient compliance
The invention provides a dronedarone hydrochloride pharmaceutical composition which is suitable for the sublingual administration, can quickly run, and can greatly improve the bioavailability and the curative effect. The pharmaceutical composition consists of 5-65 parts of dronedarone hydrochloride, 1-10 parts of a disintegrating agent, 10-80 parts of filler, 0.5-3 parts of antacid, 0-5 parts of corrigent, 1-10 parts of an adhesive and 0.3-2 parts of a lubricating agent. The invention further provides a preparation method suitable for the industrial production. The dronedarone hydrochloride pharmaceutical composition provided by the invention is directly absorbed by the sublingual mucosa, and the first-pass effect of oral medicaments can be avoided, and the gastrointestinal digestion, acidolysis and the like can be avoided, so that the bioavailability can be greatly improved. The epithelium of the sublingual mucosa is not cornified, so that the dronedarone hydrochloride pharmaceutical composition is large in superficial area, and high in infiltration capacity. The medicament can be quickly absorbed after being administrated, so that the dronedarone hydrochloride pharmaceutical composition is quick to run, and convenient to use; and compared with the other administrated preparations, the dronedarone hydrochloride pharmaceutical composition is convenient to administrate, and good in patient compliance.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
Composition for dronedarone hydrochloride tablet and preparation method thereof
InactiveCN104721178AFully absorbedImprove bioavailabilityOrganic active ingredientsPill deliveryActive componentPolyethylene glycol
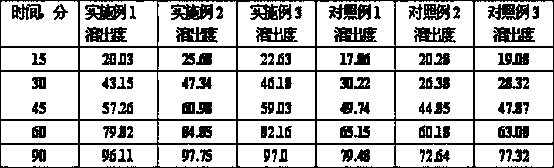
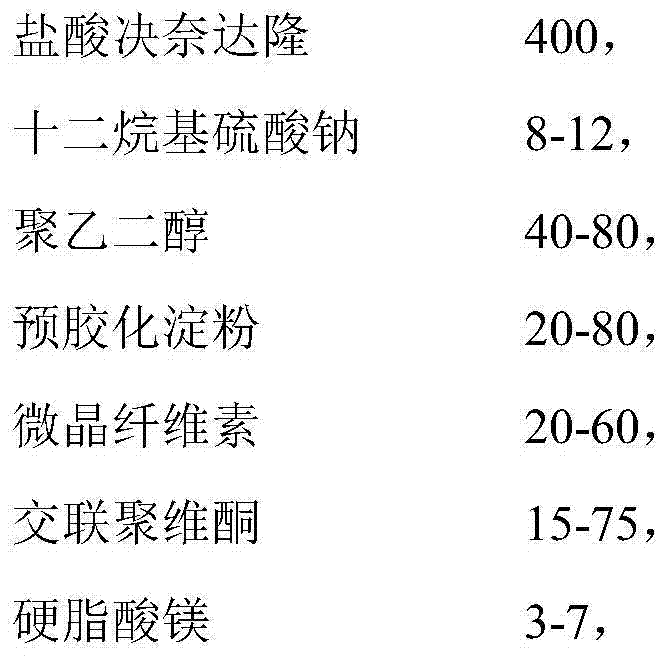
The invention provides a composition for a dronedarone hydrochloride tablet and a preparation method thereof. The dronedarone hydrochloride tablet is prepared from an active component, a surfactant, lauryl sodium sulfate and other pharmaceutically acceptable components, wherein the active component is dronedarone hydrochloride, and the surfactant is polyethylene glycol. The dronedarone hydrochloride tablet has high dissolution rate and is suitable for industrial production, and the preparation process of the dronedarone hydrochloride tablet is simple.
Owner:HEBEI RENHE YIKANG PHARMA
A method for detecting dronedarone hydrochloride
ActiveCN103901117BEasy to distinguishEasy to controlComponent separationColor/spectral properties measurementsDronedarone hydrochlorideImpurity
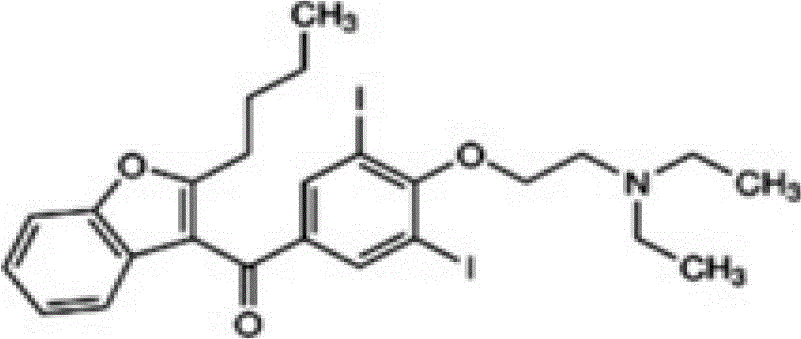
The invention provides a method for detecting N-(2-butyl-3-(4-(3-(dibutylamino)propoxy)benzoyl)benzofuran-5-yl)Methanesulfonamide hydrochloride (dronedarone hydrochloride). N-(2-Butyl-3-(4-(3-(dibutylaMino)propoxy)benzoyl)benzofuran-5-yl)MethanesulfonaMide hydrochloride is detected by employing high performance liquid chromatography. Under the high performance liquid chromatography conditions provided by the invention, N-(2-butyl-3-(4-(3-(dibutylamino)propoxy)benzoyl)benzofuran-5-yl)Methanesulfonamide hydrochloride can be relatively well isolated from concomitant impurities, and all impurities can be relatively well separated. The provided method has relatively high sensitivity, and therefore the provided method is capable of relatively accurately determining N-(2-butyl-3-(4-(3-(dibutylamino)propoxy)benzoyl)benzofuran-5-yl)Methanesulfonamide hydrochloride.
Owner:JIANGSU KANION PHARMA CO LTD
Novel method for refining dronedarone hydrochloride
The invention provides a novel method for refining dronedarone hydrochloride. According to the method, by virtue of a salt removing and forming manner, impurities can be effectively removed. Moreover,de-butyl degradation impurities are not produced, and the refined dronedarone hydrochloride with high purity is obtained.
Owner:BEIJING XINLINGXIAN MEDICAL TECH DEV CO LTD
Dronedarone hydrochloride osmotic pump type controlled release tablet
InactiveCN107281153AGood release performanceSlow and constant releaseOrganic active ingredientsPharmaceutical non-active ingredientsCellulose acetateSemipermeable membrane
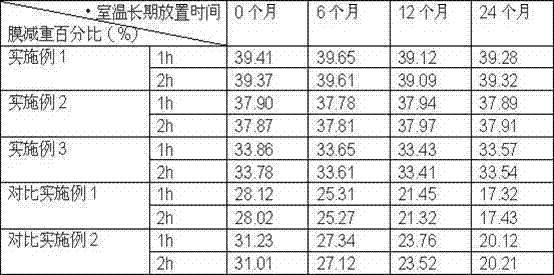
The invention provides a novel dronedarone hydrochloride osmotic pump-type controlled-release tablet, which adopts cellulose acetate and polycarbonate as semipermeable membrane film-forming materials, which can overcome the semipermeable membrane aging phenomenon, obtain a stable release rate, and Reduce drug residues. The dronedarone oral osmotic pump preparation provided by the present invention has excellent release performance and high stability, and there is no obvious aging phenomenon after being placed for a long time, which meets the market demand.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Dronedarone hydrochloride slow-release tablets and preparation method thereof
InactiveCN108014082ASimple processGood sustained release effectOrganic active ingredientsPill deliveryExtended release tabletsDronedarone hydrochloride
The invention belongs to the field of medicine preparations and discloses dronedarone hydrochloride slow-release tablets and a preparation method thereof. The slow-release tablets are prepared from, in percentage by mass, 10%-35% of dronedarone hydrochloride, 50%-90% of a hydrophobic framework material, 1%-5% of a binder and 0.5%-5% of a lubricant. The slow-release tables are prepared from the hydrophobic framework material and the like, medicine release can be well controlled, the process is simple, and expanded production is facilitated.
Owner:佛山市弘泰药物研发有限公司
A dronedarone hydrochloride tablet and preparation method thereof
ActiveCN103565763BHigh dissolution rateImprove securityOrganic active ingredientsPill deliveryMedicineMethyl cellulose
The invention belongs to the field of pharmaceutical preparations, in particular to a surfactant-free dronedarone hydrochloride tablet. Contains the following components by mass: 400 parts of dronedarone hydrochloride, 100-1200 parts of hydroxypropyl methylcellulose, 20-640 parts of disintegrant, 7-10 parts of lubricant, excluding Surfactant. The technical scheme of the present invention does not add any surfactant, which not only increases the safety, but also avoids the problem of low melting point of the surfactant and easy sticking during the production process of the preparation, and at the same time significantly improves the dissolution rate of dronedarone hydrochloride . During the preparation process, dronedarone hydrochloride and hydroxypropyl methylcellulose are pulverized together until the particle size is not greater than 1 μm, and then made into tablets with other suitable auxiliary materials. The process is relatively simple, the operation is convenient, and it is suitable for large-scale industrial production.
Owner:SHANDONG NEWTIME PHARMA
Slow-release dronedarone hydrochloride capsule and preparation method thereof
InactiveCN108066324AGood compatibilityPrevent infiltrationOrganic active ingredientsMicrocapsulesSide effectAdhesive
The invention discloses a slow-release dronedarone hydrochloride capsule and preparation method and application thereof. A slow-release dronedarone hydrochloride pellet comprises a medicated pellet core, an isolating coating layer, a slow-release coating layer and a protective coating layer from inside to outside, wherein the medicated pellet core comprises the following specific components in percent by weight: 10-25% of dronedarone hydrochloride, 10-30% of an adhesive and 10-60% of a sucrose blank pellet core. The slow-release preparation can prolong the duration of the treatment concentration of the dronedarone hydrochloride in a body to 12 hours, reduce side effects caused by excessive fluctuation in blood drug concentration, and reduce the medicine taking times.
Owner:佛山市弘泰药物研发有限公司
A dronedarone hydrochloride tablet and preparation method thereof
ActiveCN104771375BHigh dissolution rateHigh simulationOrganic active ingredientsPill deliveryIn vivoDronedarone hydrochloride
The invention discloses a dronedarone hydrochloride tablet and a preparation method thereof; the tablet contains citric acid and is prepared by the following method: mixing dronedarone hydrochloride, citric acid and pharmaceutically common auxiliary materials evenly, adding an aqueous solution, granulating, drying, adding a lubricant, mixing, and tabletting to obtain the product. Compared with the prior art, the dronedarone hydrochloride tablet has the dissolution rate of dronedarone hydrochloride in tap water significantly improved, and the in-vivo environment is better simulated; micronization, dispersion preparation and other complex operations are not needed, the preparation process is relatively simple, operations are convenient, addition of poloxamer and other surfactants are not needed, and the tablet is rapidly dissolved in a gastric juice.
Owner:SHANDONG NEWTIME PHARMA
A kind of preparation method of dronedarone hydrochloride
ActiveCN109384754BElimination reactionImprove responseOrganic chemistryMethanesulfonyl chlorideNucleophilic substitution
Owner:XINFA PHARMA
A kind of oral solid pharmaceutical composition containing dronedarone hydrochloride and preparation method thereof
ActiveCN102908305BUnexpected technical effectsLow costOrganic active ingredientsPharmaceutical delivery mechanismMethyl celluloseDronedarone hydrochloride
The invention relates to an oral solid medicinal composition, which is characterized by comprising pelletized dronedarone hydrochloride and hydrophilic gel matrix type slow release material hydroxypropyl methyl cellulose, wherein the pelletized dronedarone hydrochloride and hydrophilic gel matrix type slow release material hydroxypropyl methyl cellulose are selectively combined with one or more medicinal excipients. The oral solid medicinal composition is mainly used for treating arrhythmia.
Owner:PHARMA RES INST OF BENCAO TIANYUAN OF BEIJING
Method for preparing dronedarone hydrochloride tablets
PendingCN109908094AEfficient manufacturingKeep abreast of and controlOrganic active ingredientsPharmaceutical non-active ingredientsDronedarone hydrochlorideRaw material
The invention relates to the field of medicine preparation, in particular to a method for preparing dronedarone hydrochloride tablets. The method for preparing the dronedarone hydrochloride tablets comprises the following specific steps: S1, carrying out compatibility test on raw materials and auxiliary materials of dronedarone hydrochloride; S2, preliminarily selecting preparation auxiliary materials; S3, processing raw materials and auxiliary materials; S4, mixing raw materials and auxiliary materials; S5, pelletizing; S6, drying granules; S7, granulating; S8, total mixing; S9, tabletting; S10, coating; S11, internal coating; and S12, external coating. The feasibility and reproducibility of the production process determined in the invention can be reflected well; the tablets can be effectively prepared, intermediate products in different stages can be accurately understood and controlled in time in a processing and preparation process, the quality of the intermediate products in thewhole production process is guaranteed, and thus, it can be ensured that the final product meets the processing requirement.
Owner:山东希尔康泰药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com