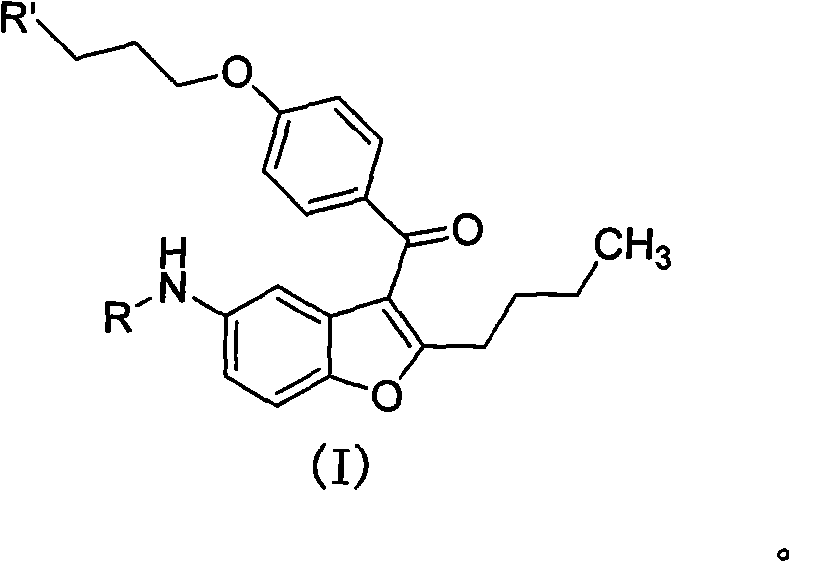
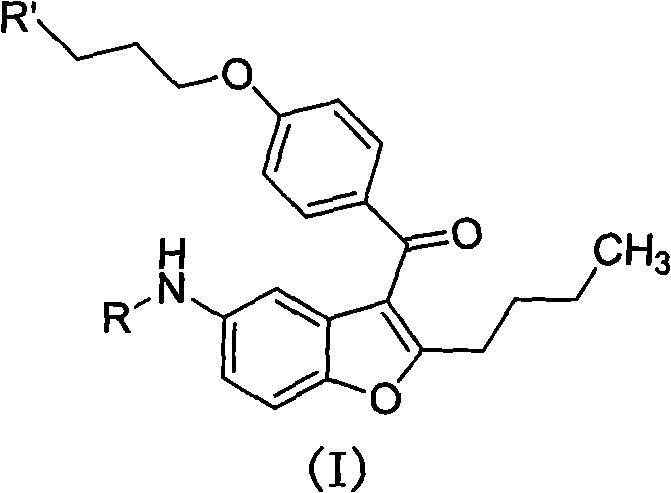
2-n-butyl-3-(4-subsitituted propylbenzoyl)-5-substituted amino benzofuran and application thereof
A technology of propoxybenzoyl group and benzoyl group is applied in the field of key intermediates for synthesizing dronedarone, which can solve the problems of being unsuitable for industrialized mass preparation and high preparation cost, avoiding the use of metal catalysts, reducing Preparation cost, avoid the effect of high pressure hydrogenation operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Preparation of 2-n-butyl-3-(4-(3-chloropropoxy)benzoyl)-5-acetamidobenzofuran (II-1)
[0071] Dissolve 2-n-butyl-5-acetylaminobenzofuran (0.02mol, 4.63g) in 40ml of dichloromethane, add 4-(3-chloropropoxy)-benzoyl chloride (0.02mol, 4.77g ), stirred for 10 minutes, cooled to 2±2°C in an ice-water bath, added anhydrous aluminum trichloride (0.044mol, 5.87g) in batches, and stirred at room temperature for 2 hours. According to the post-processing operation in General Method 1, the target compound II-1 was obtained, 6.31 g of white solid, and the yield was 73.7%.
[0072] ESI-MS[M+H] + :428.18
[0073] 1 H-NMR (CDCl 3 ): δ0.86(t, 3H, J=7.2Hz), 1.30-1.36(m, 2H), 1.68-1.76(m, 2H), 2.10(s, 3H), 2.25-2.30(m, 2H), 2.86(t, 2H, J=7.2Hz), 3.75(t, 2H, J=6.0Hz), 4.20(t, 2H, J=5.6Hz), 6.94(d, 2H, J=8.8Hz), 7.31( s, 1H), 7.33 (s, 1H, D 2 O exchange disappeared), 7.38 (d, 1H, J = 8.4Hz), 7.55 (d, 1H, J = 8.4Hz), 7.82 (d, 2H, J = 8.8Hz).
Embodiment 2
[0075] Preparation of 2-n-butyl-3-(4-(3-bromopropoxy)benzoyl)-5-acetamidobenzofuran (II-2)
[0076] Dissolve 2-n-butyl-5-acetylaminobenzofuran (0.02mol, 4.63g) in 40ml of dichloromethane, add 4-(3-bromopropoxy)-benzoyl chloride (0.02mol, 5.55g ), and stirred for 10 minutes. Cool down to 2±2°C in an ice-water bath, add anhydrous aluminum trichloride (0.044mol, 5.87g) in batches, control the temperature below 10°C and stir for reaction for 3 hours. According to the post-treatment operation in General Method 1, the target compound II-2 was obtained, 7.04 g of white solid, with a yield of 74.5%.
[0077] ESI-MS[M+H] + : 472.10
[0078] 1 H-NMR (CDCl 3 ): δ0.85(t, 3H, J=7.2Hz), 1.30-1.35(m, 2H), 1.65-1.76(m, 2H), 2.09(s, 3H), 2.14-2.25(m, 2H), 2.84(t, 2H, J=7.2Hz), 3.57(t, 2H, J=5.6Hz), 4.21(t, 2H, J=5.6Hz), 6.95(d, 2H, J=8.8Hz), 7.30( s, 1H), 7.35 (s, 1H, D 2 O exchange disappeared), 7.39 (d, 1H, J = 8.4Hz), 7.57 (d, 1H, J = 8.4Hz), 7.83 (d, 2H, J = 8.8Hz).
Embodiment 3
[0080] Preparation of 2-n-butyl-3-(4-(3-chloropropoxy)benzoyl)-5-propionylaminobenzofuran (II-3)
[0081]Dissolve 2-n-butyl-5-propionylaminobenzofuran (0.02mol, 4.91g) in 40ml of dichloromethane, add 4-(3-chloropropoxy)-benzoyl chloride (0.02mol, 4.77 g), stirring for 10 minutes, cooling down to 2±2°C in an ice-water bath, adding anhydrous aluminum trichloride (0.044mol, 5.87g) in batches, and stirring at room temperature for 2 hours. According to the post-processing operation in General Method 1, the target compound II-3 was obtained, 6.43 g of white solid, and the yield was 72.7%.
[0082] ESI-MS[M+H] + :442.17
[0083] 1 H-NMR (CDCl 3 ): δ0.82-0.90(t, 3H, J=7.2Hz), 1.05(t, 3H, J=7.6Hz), 1.31-1.36(m, 2H), 1.64-1.77(m, 2H), 2.12( q, 2H, J=7.6Hz), 2.15-2.27(m, 2H), 2.83(t, 2H, J=7.2Hz), 3.77(t, 2H, J=5.6Hz), 4.19(t, 2H, J =5.6Hz), 6.94(d, 2H, J=8.8Hz), 7.31(s, 1H), 7.38(d, 1H, J=8.4Hz), 7.49(s, 1H, D 2 O exchange disappeared), 7.57 (d, 1H, J = 8.4 Hz), 7.82 (d, 2H, J = ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com