Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
110results about How to "Elimination reaction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
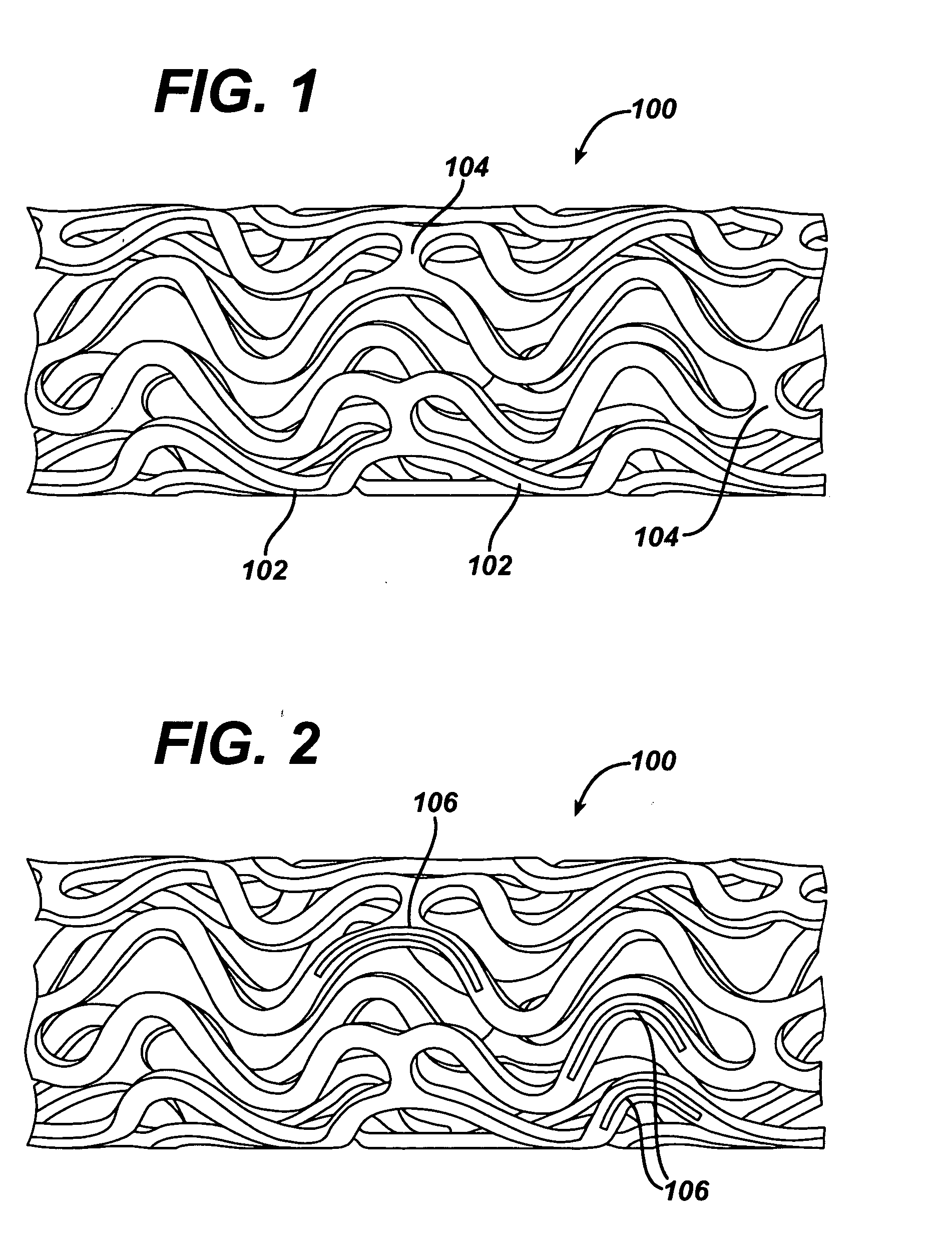
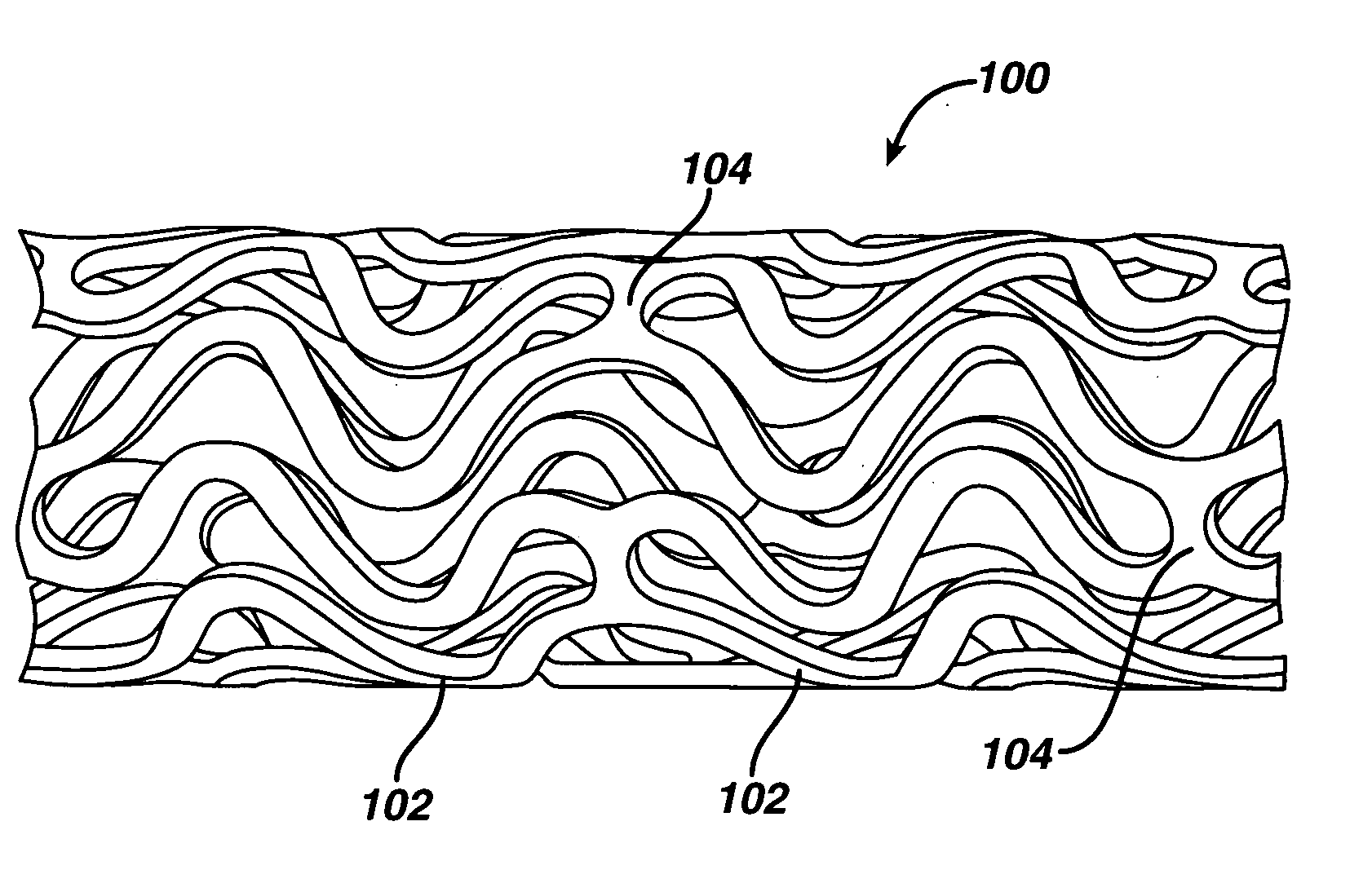
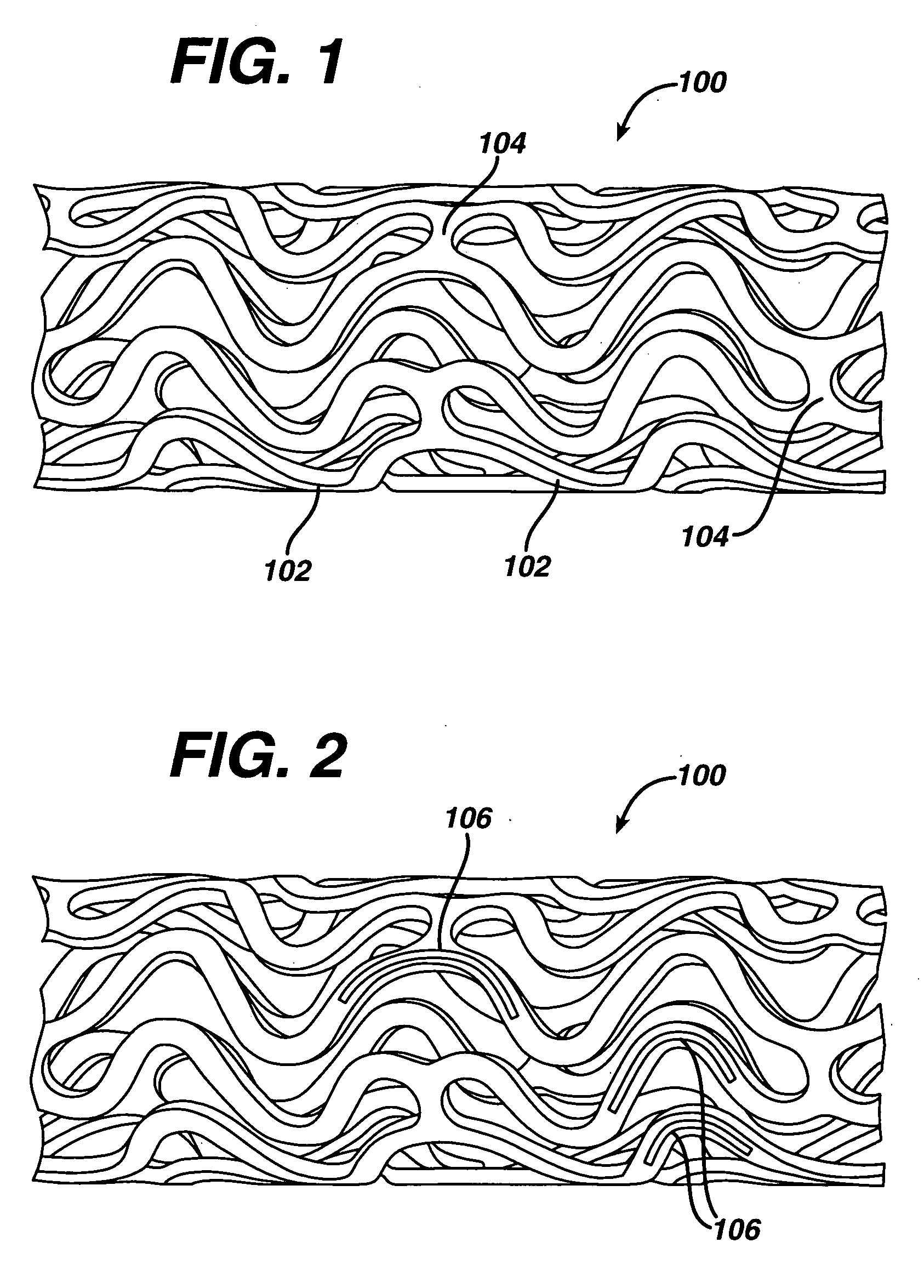
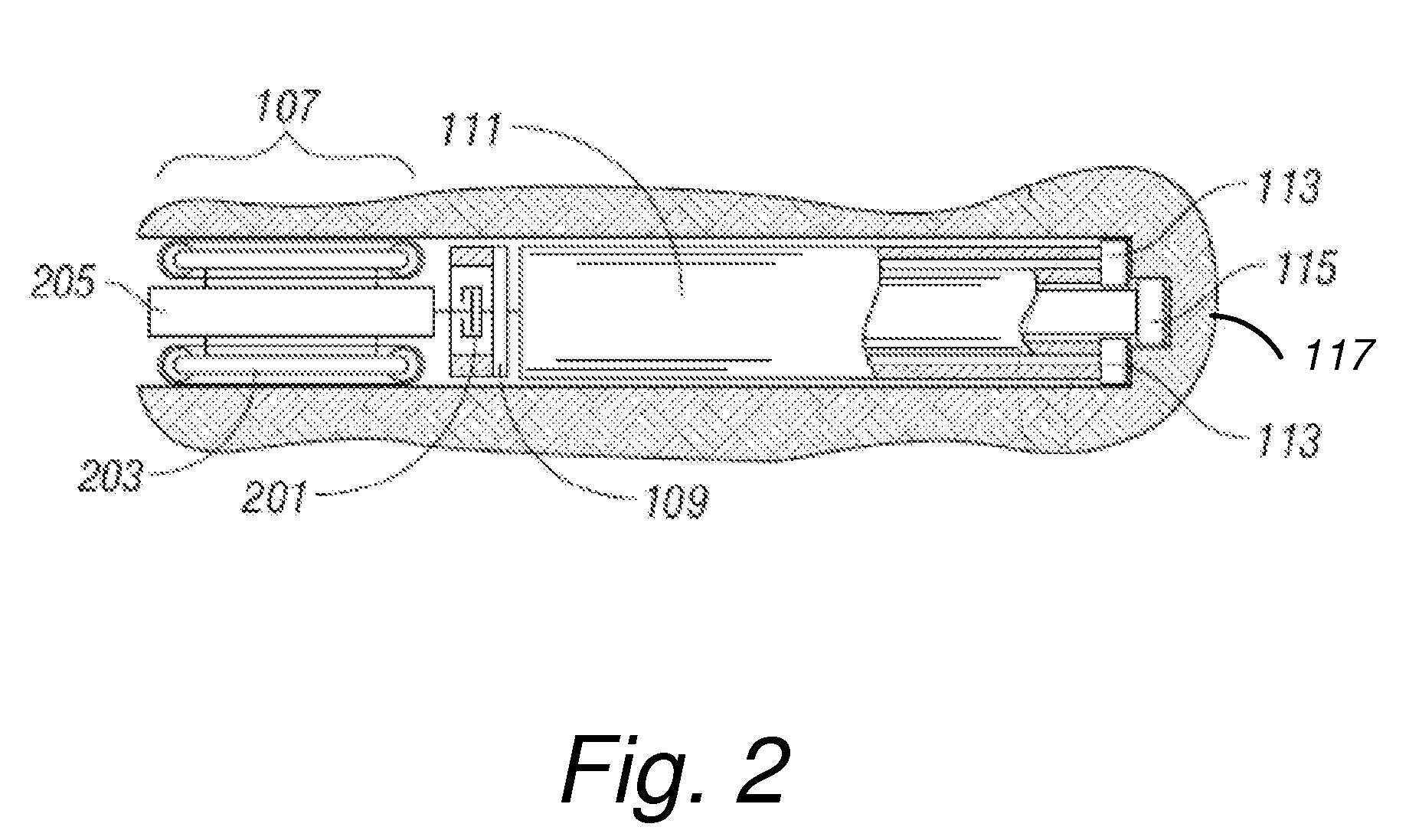
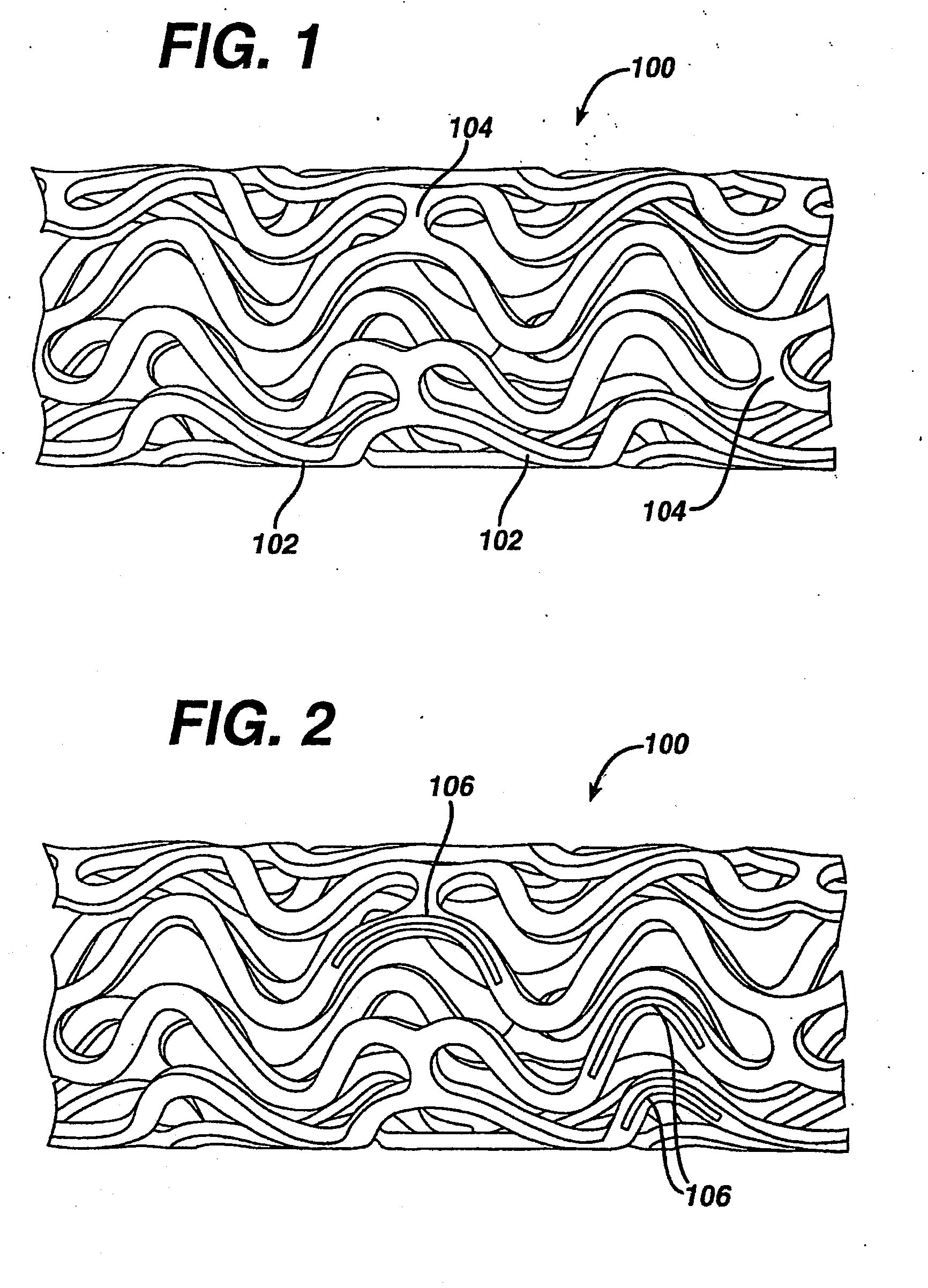
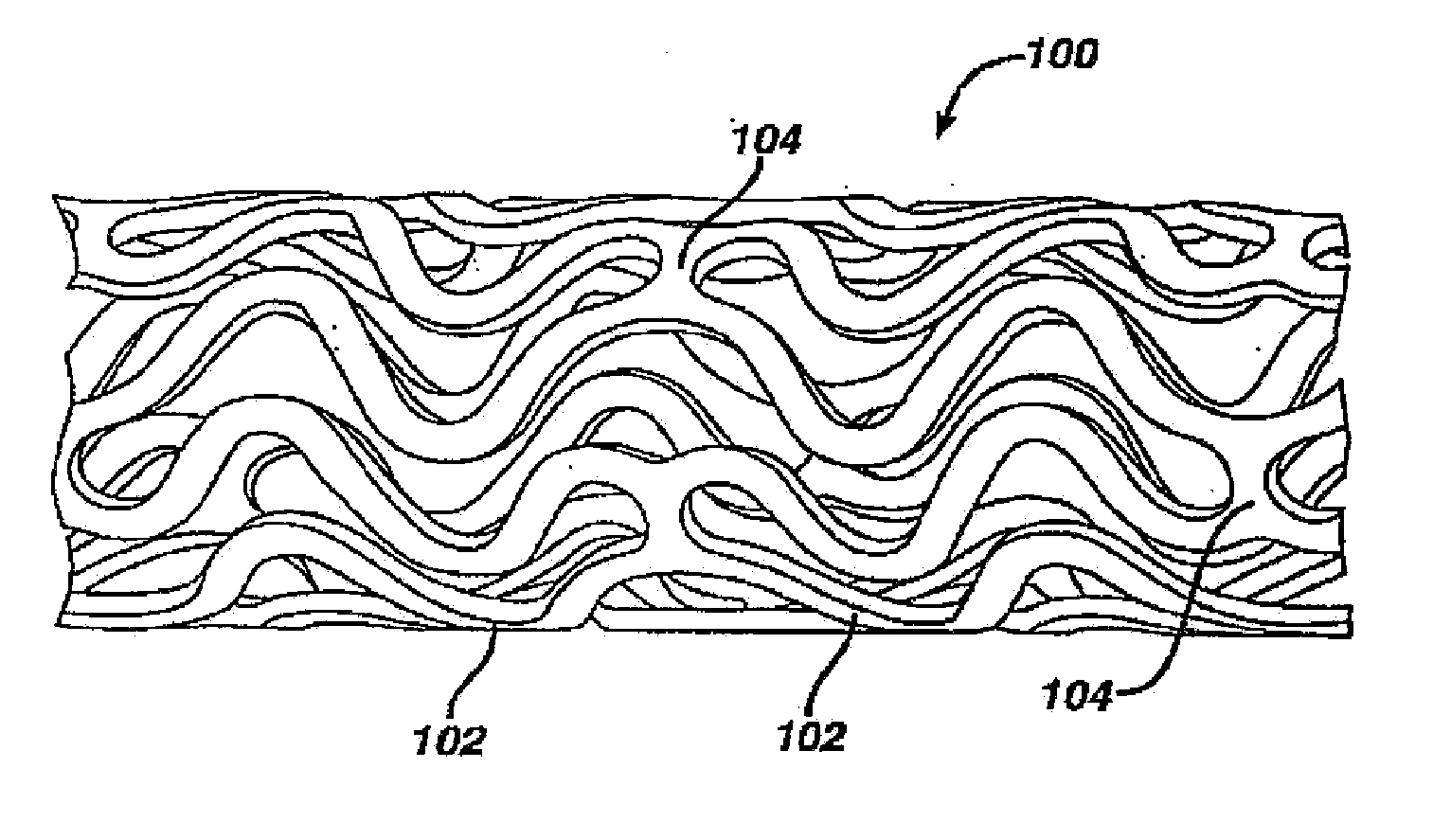
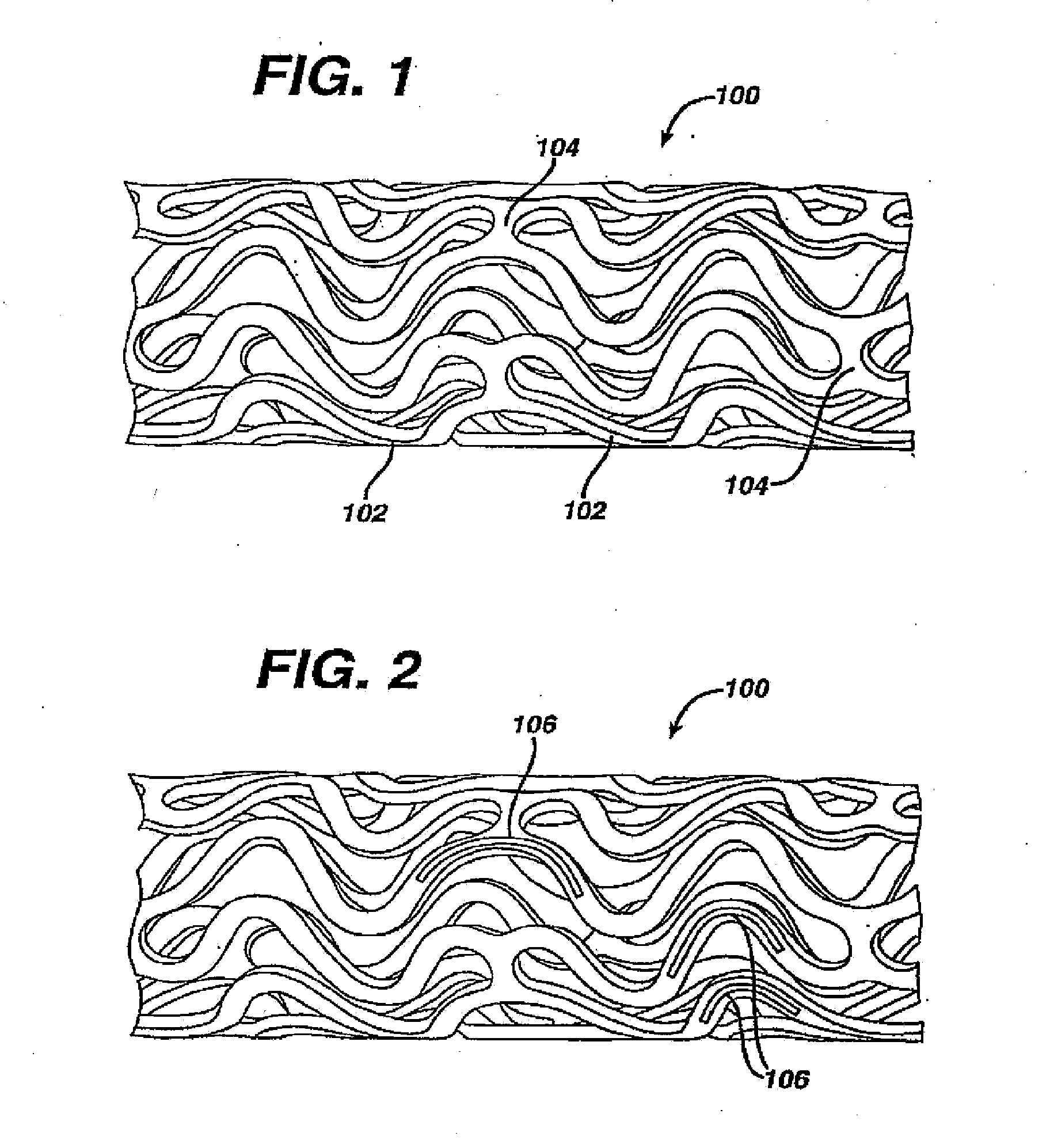
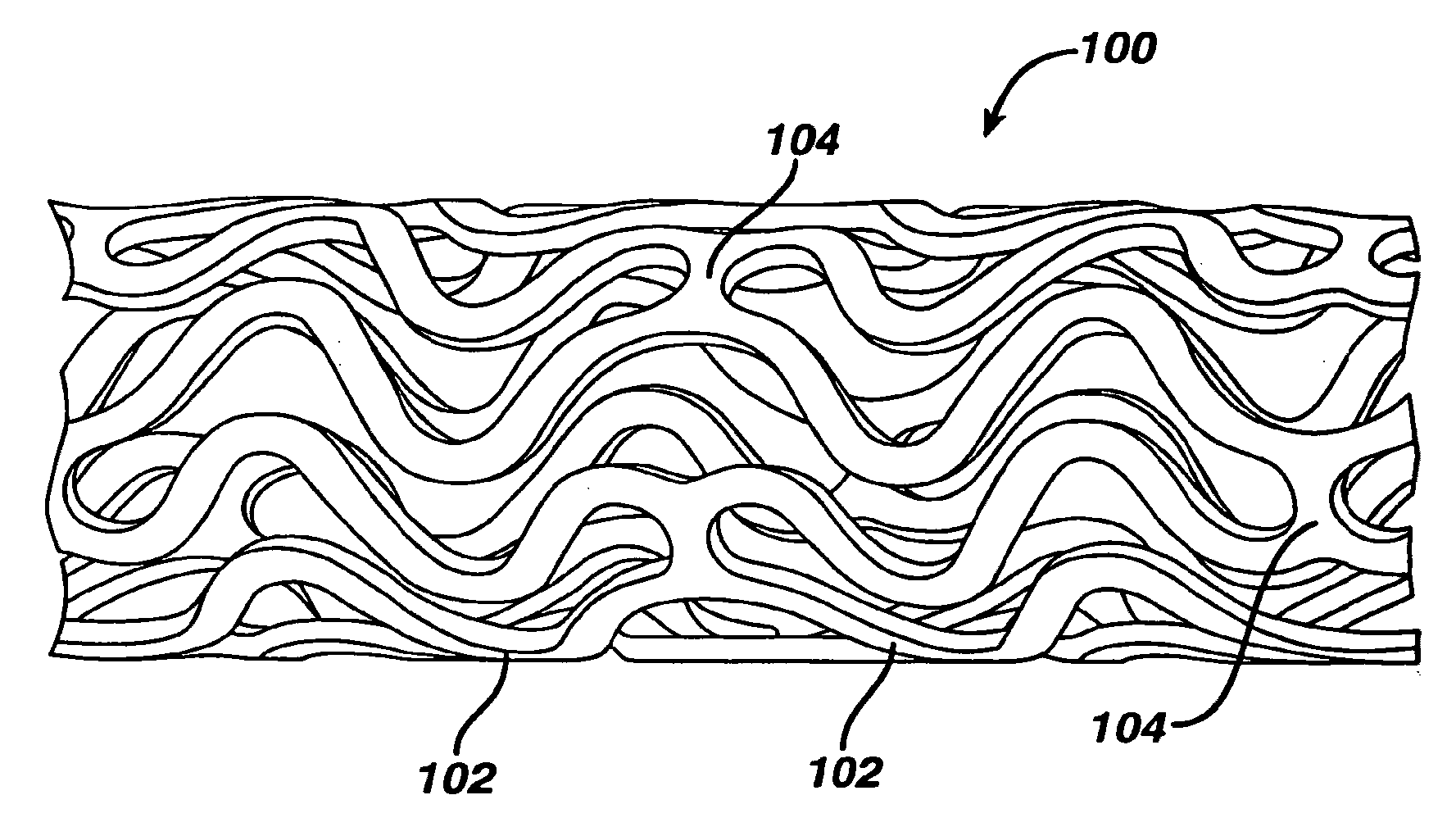
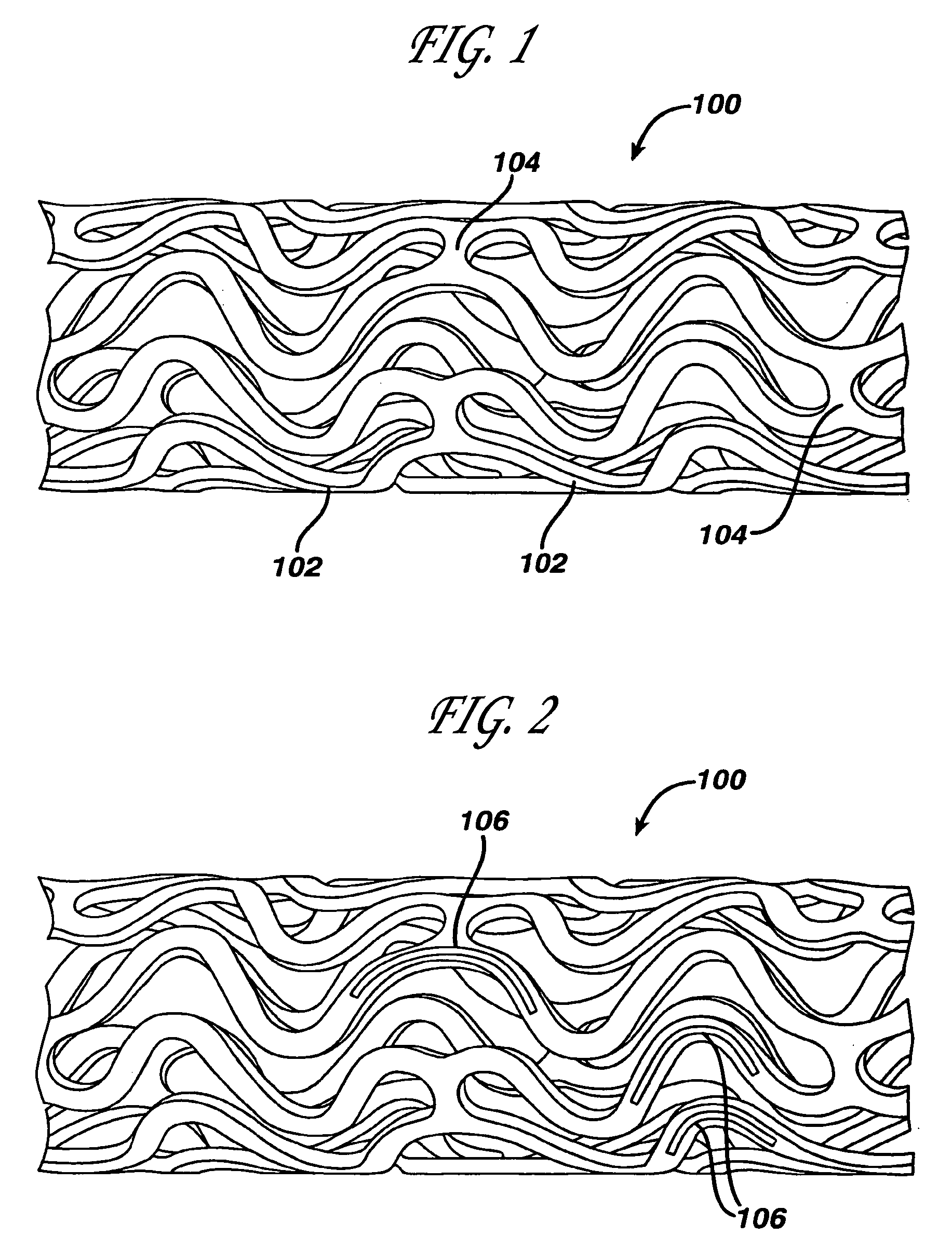
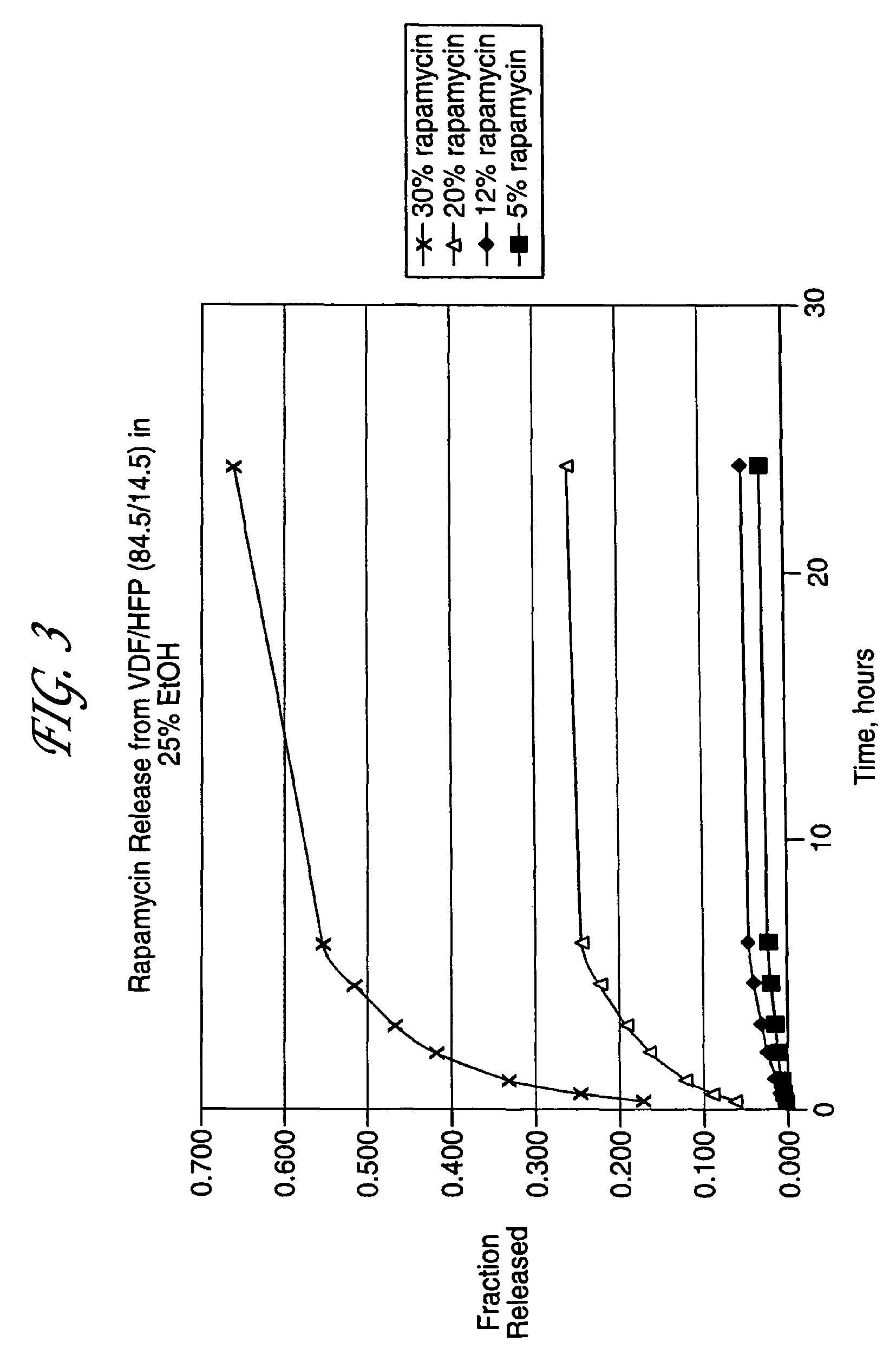
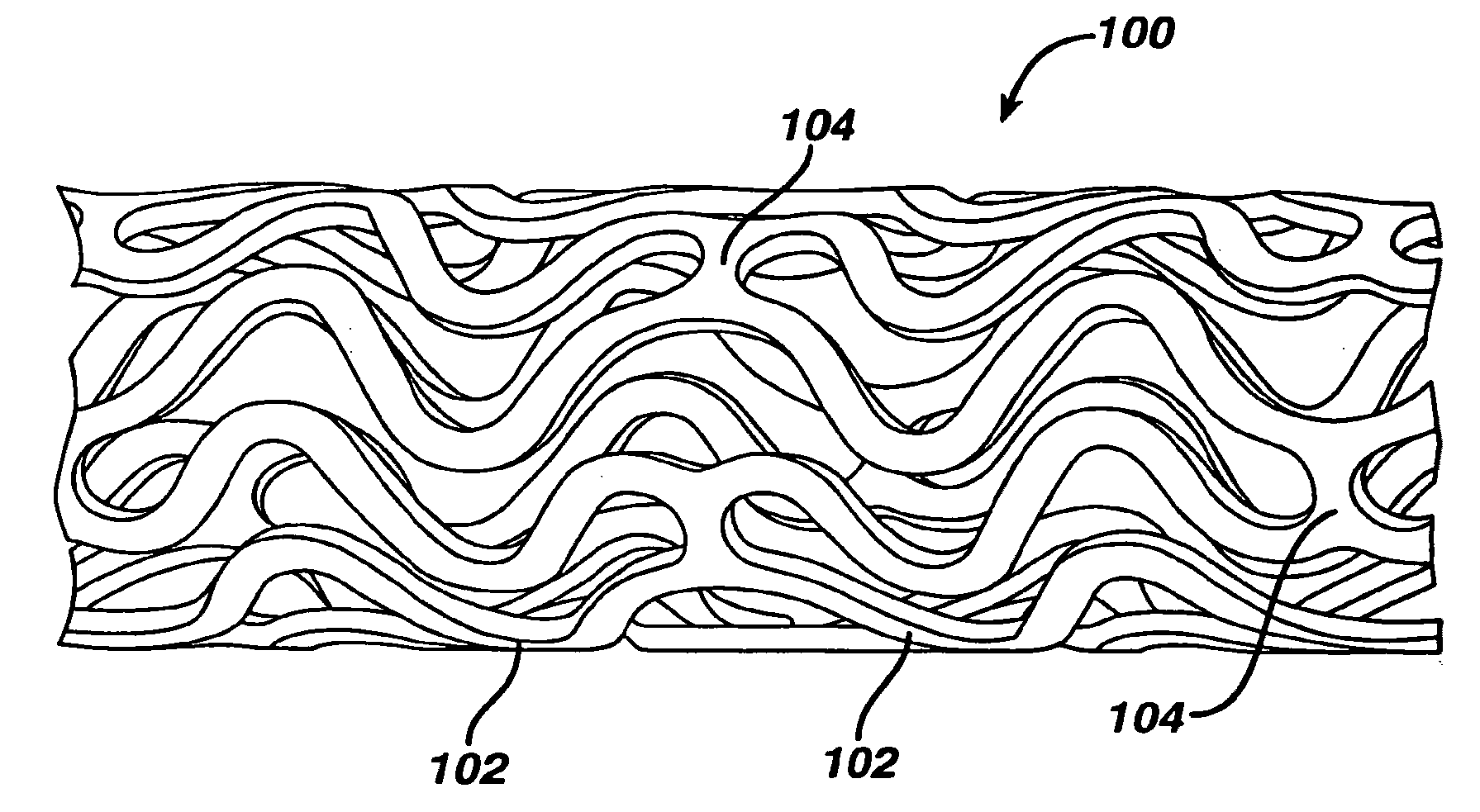
Coated endovascular AAA device
InactiveUS6852122B2Reduce drug toxicityGood curative effectSuture equipmentsStentsBiological bodyReady to use
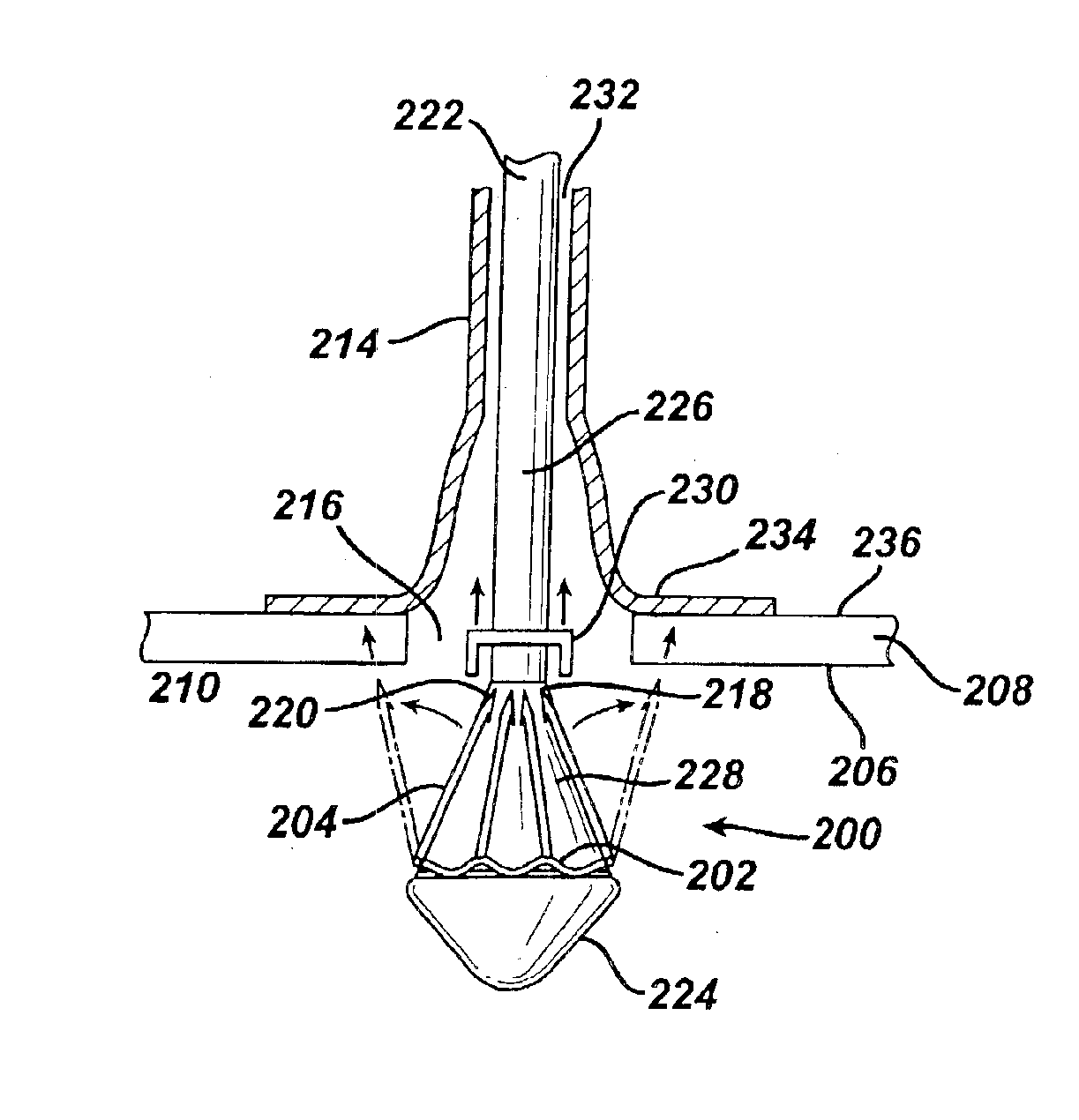
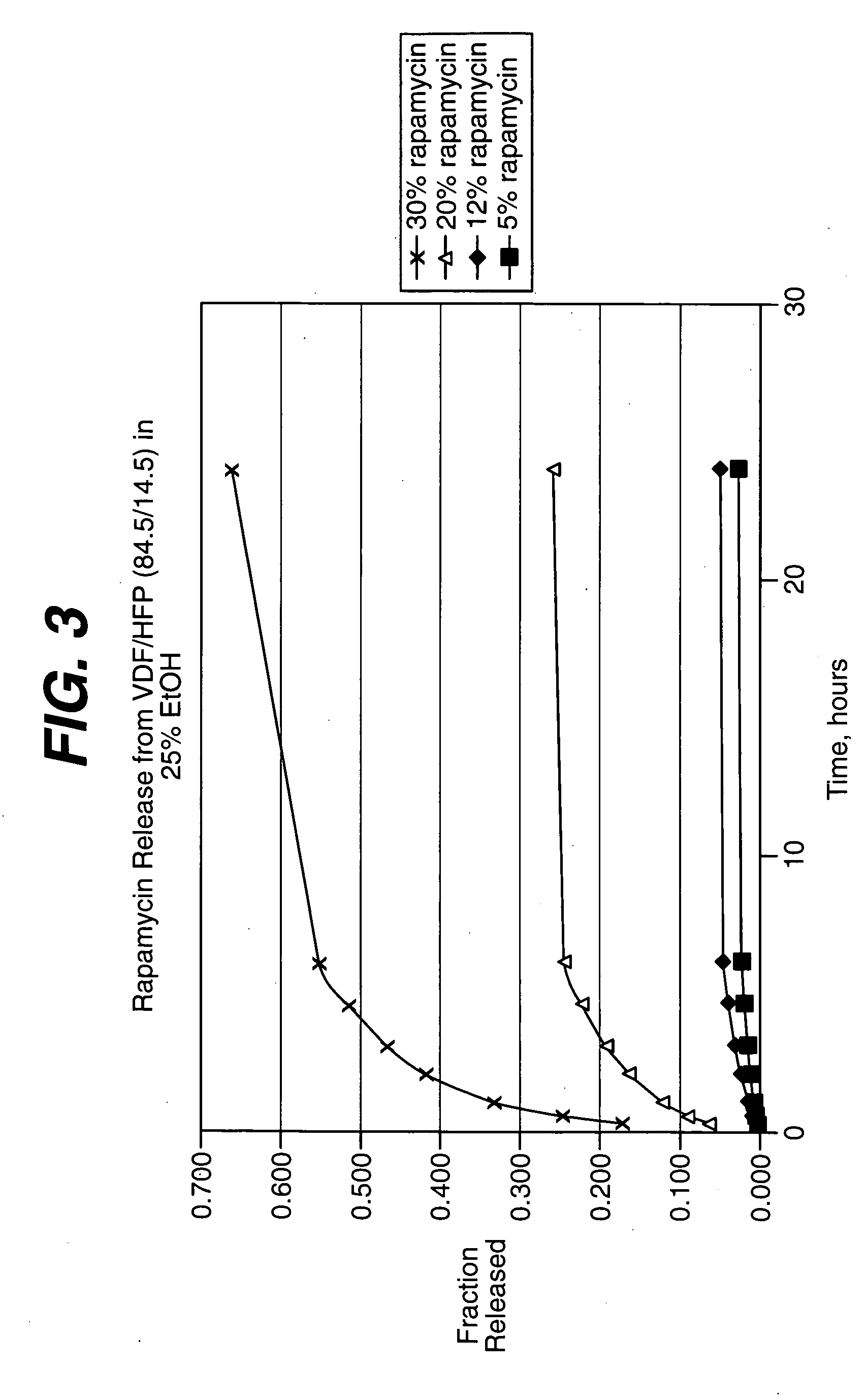
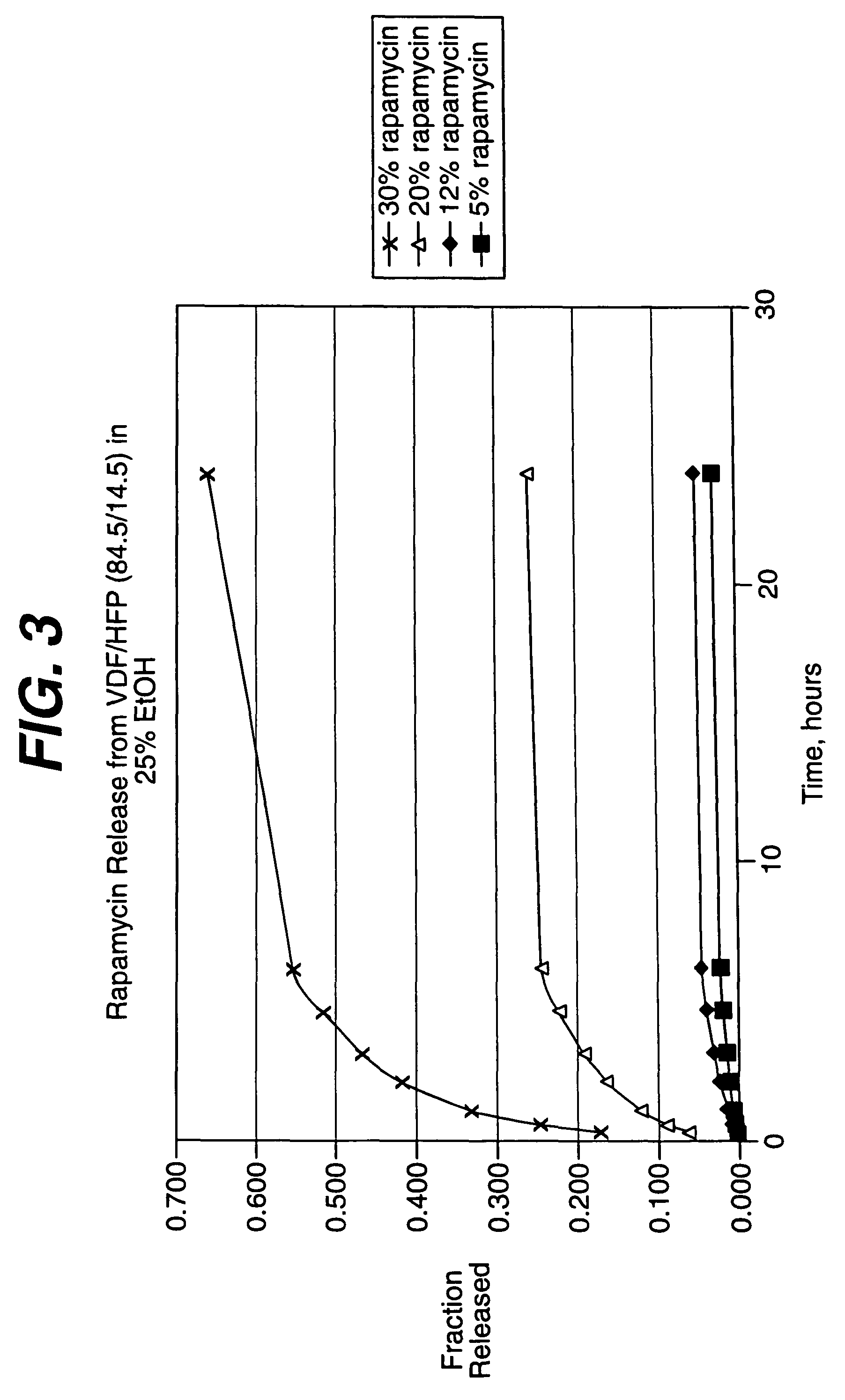
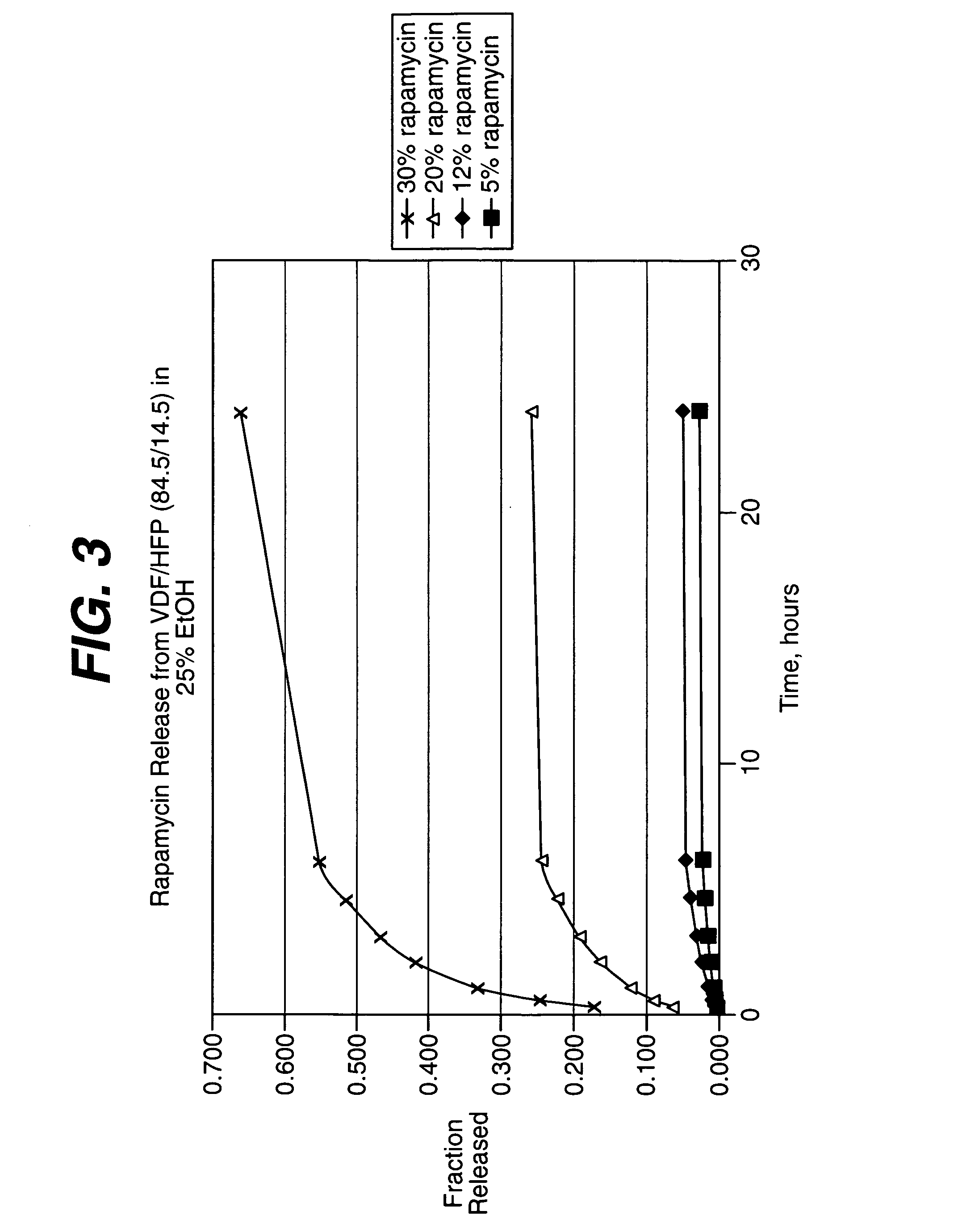
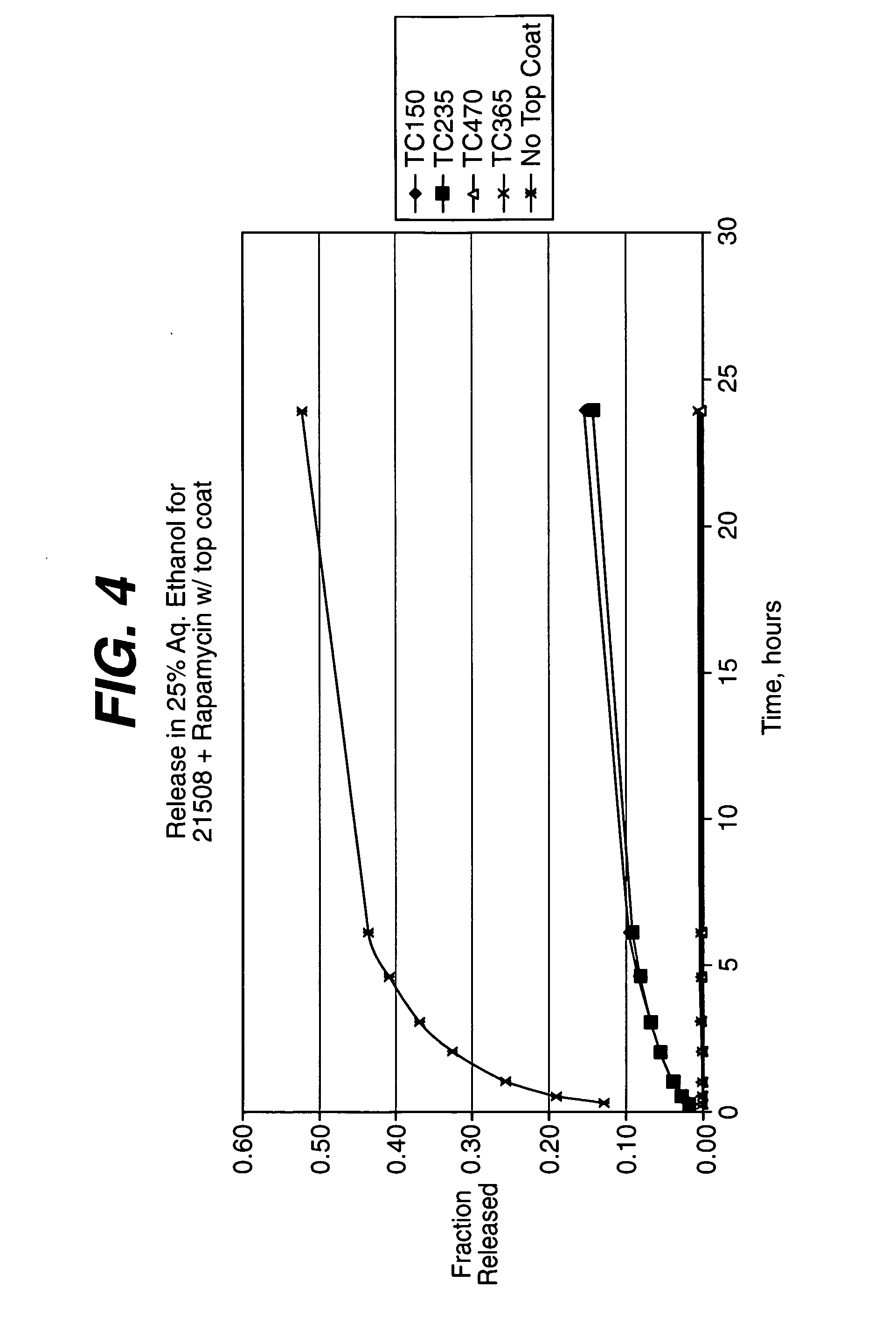
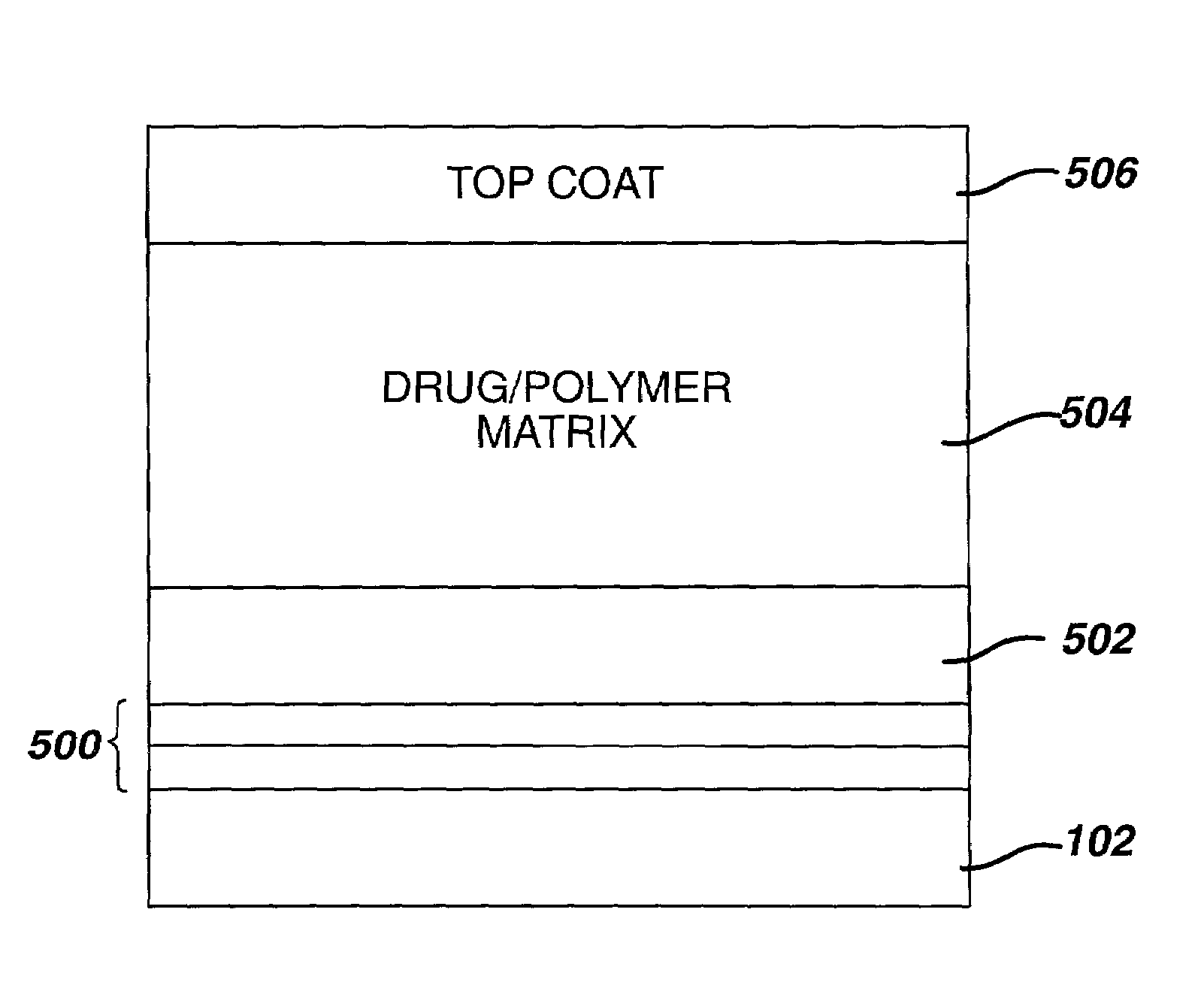
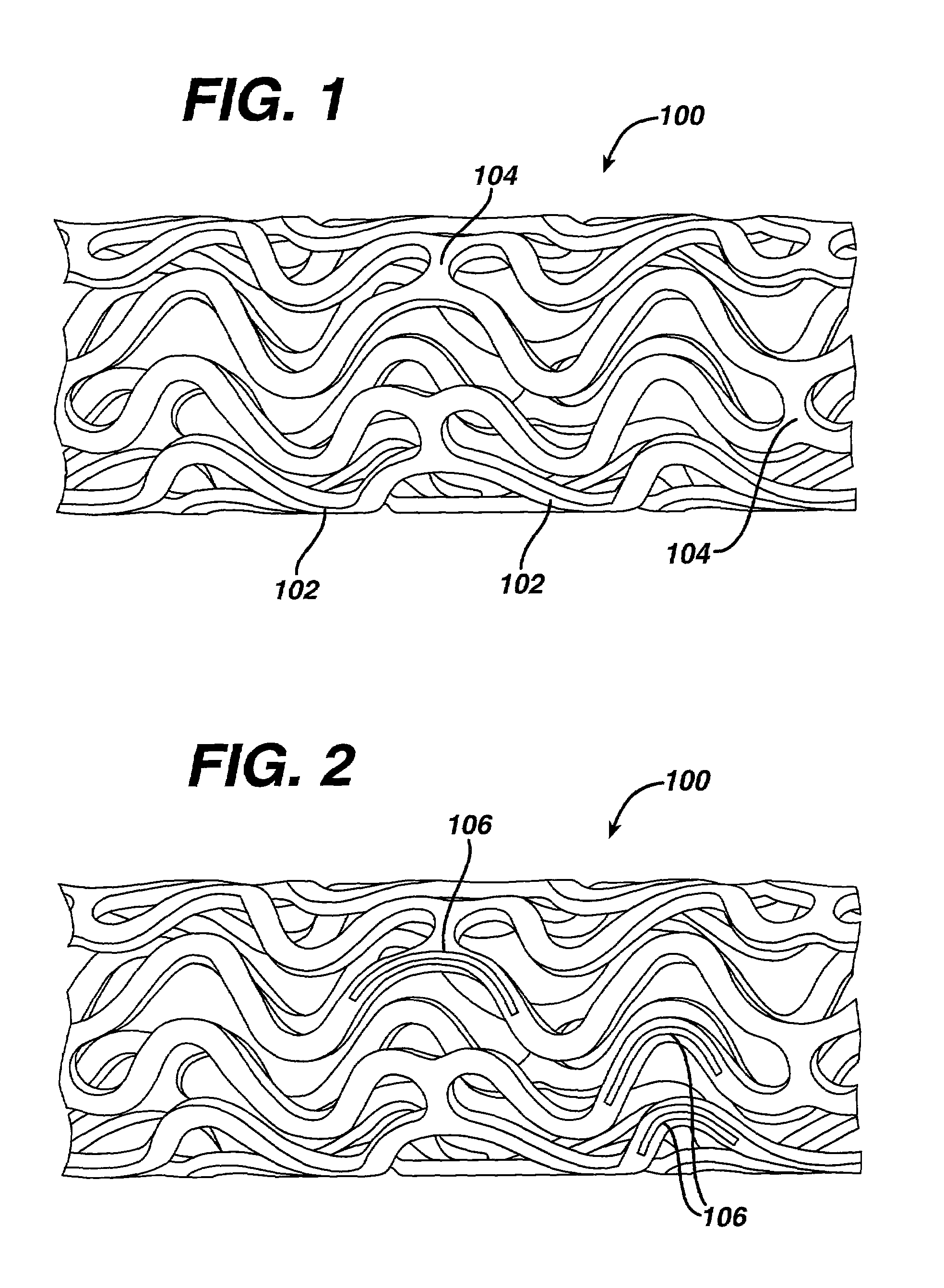
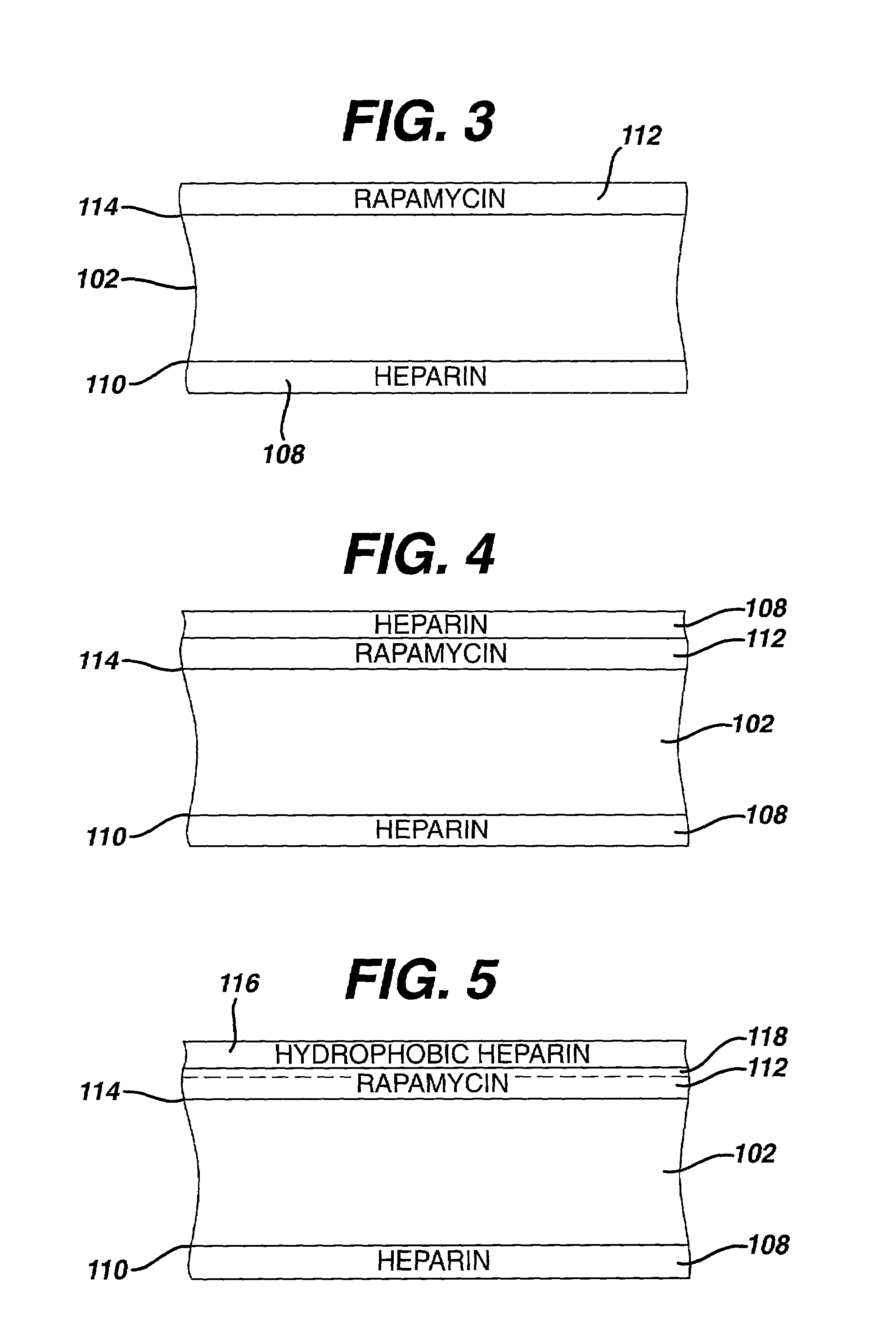
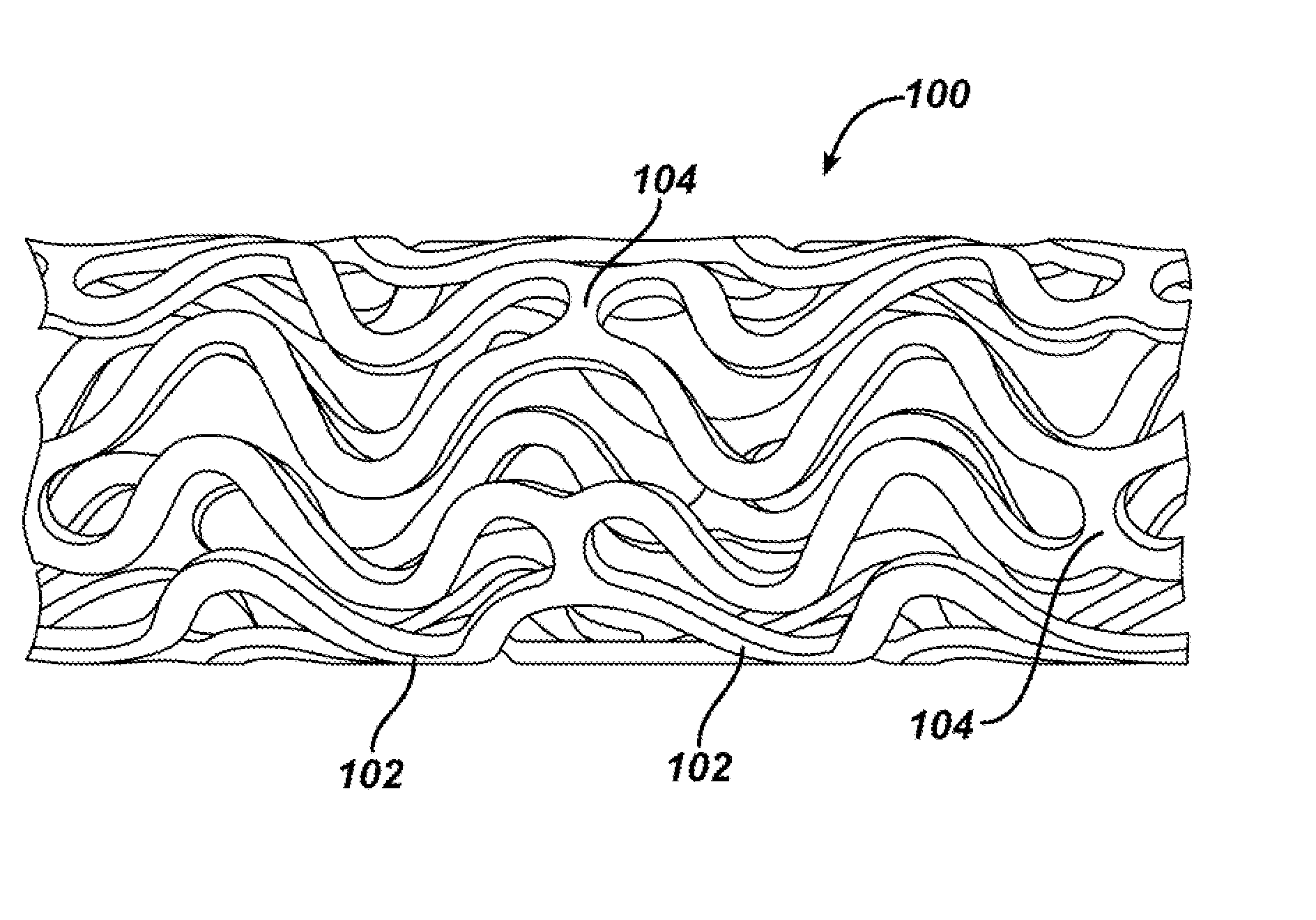
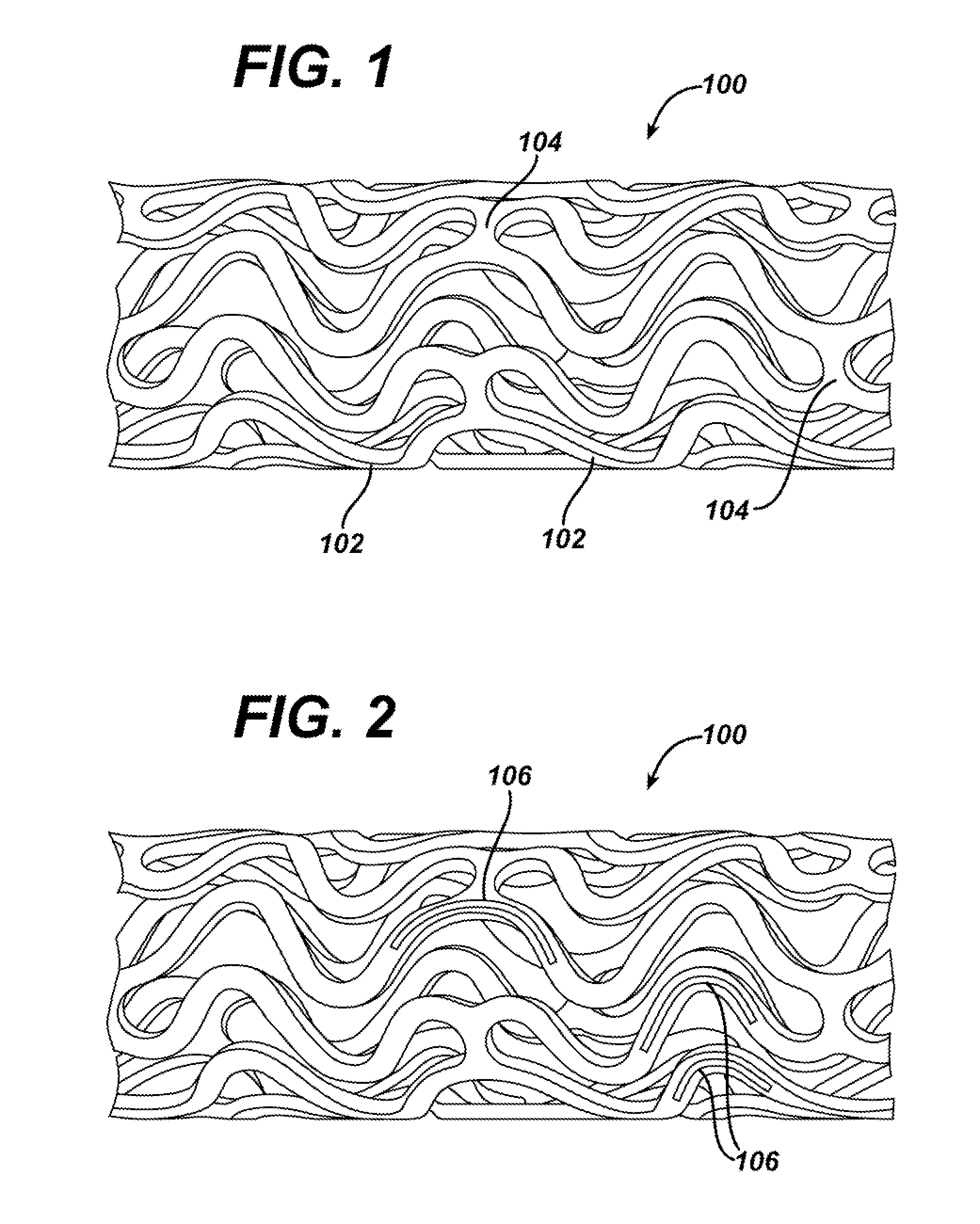
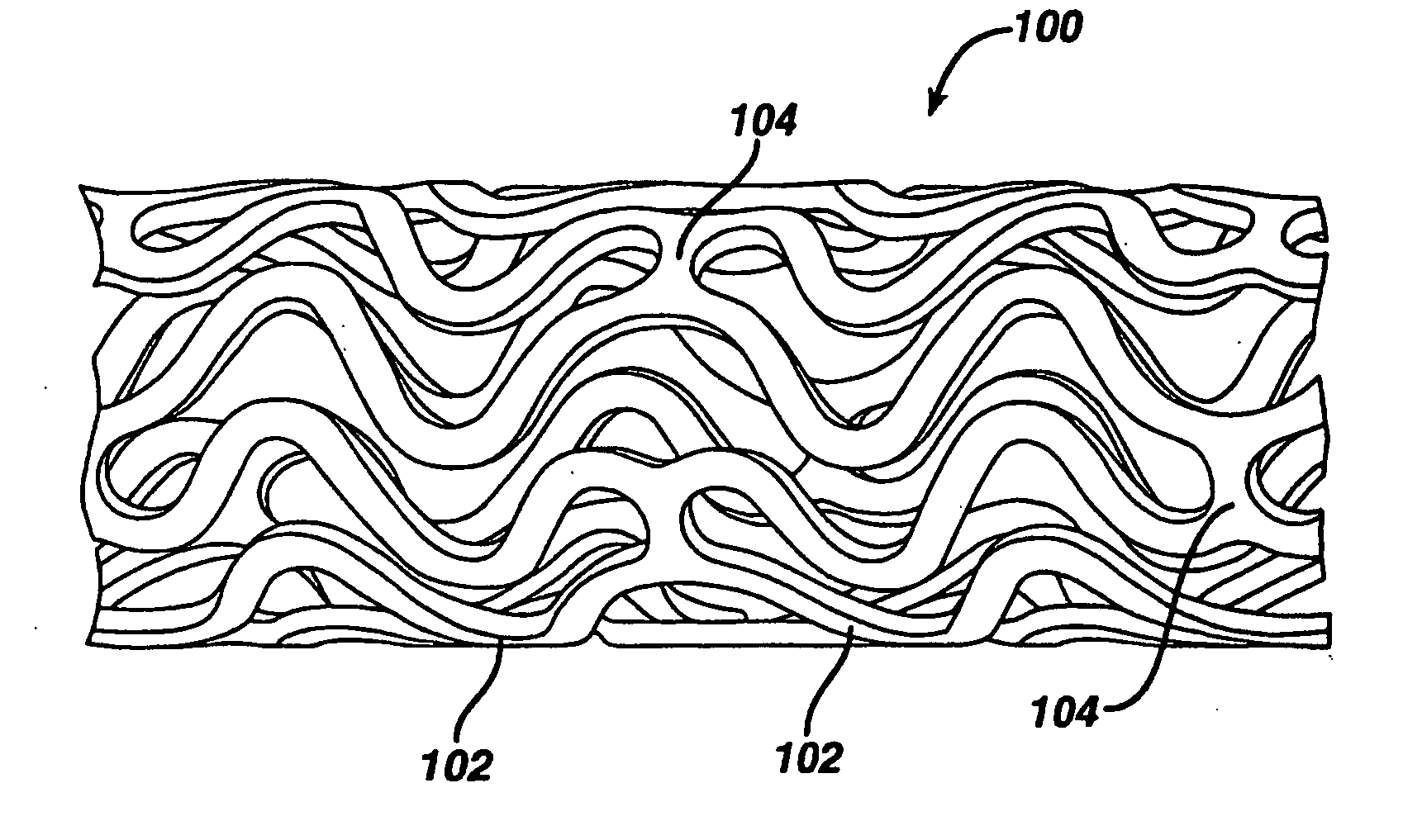
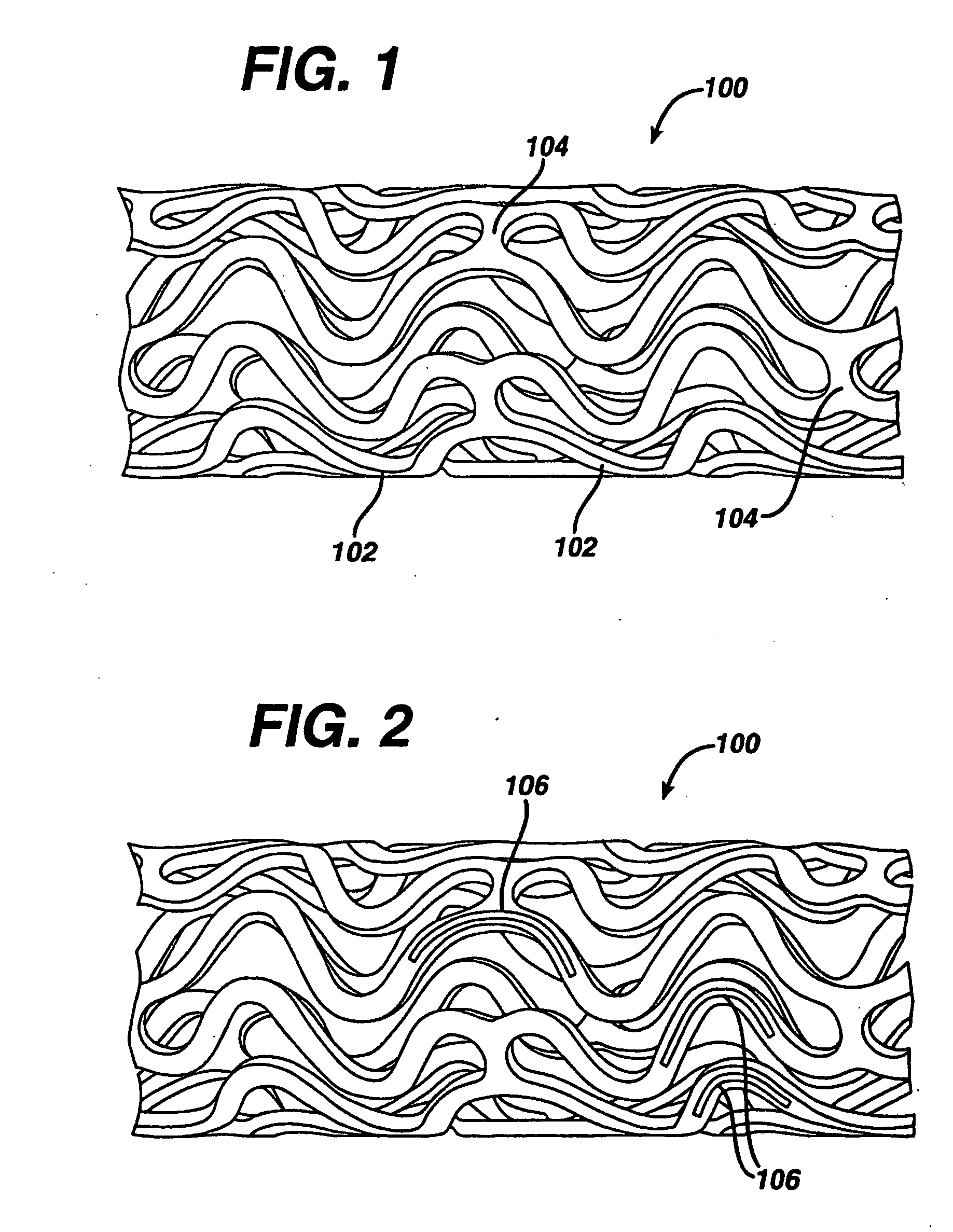
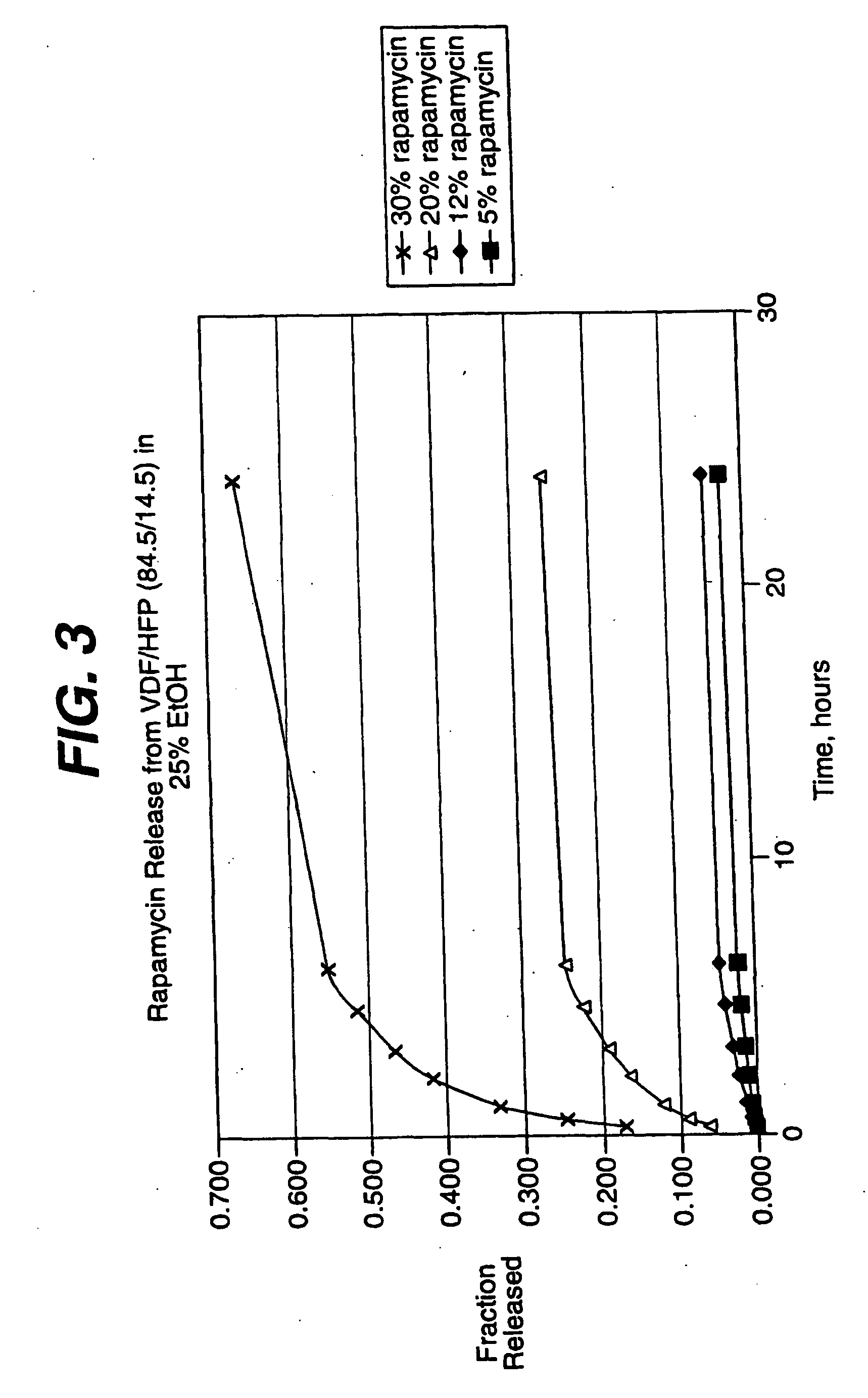
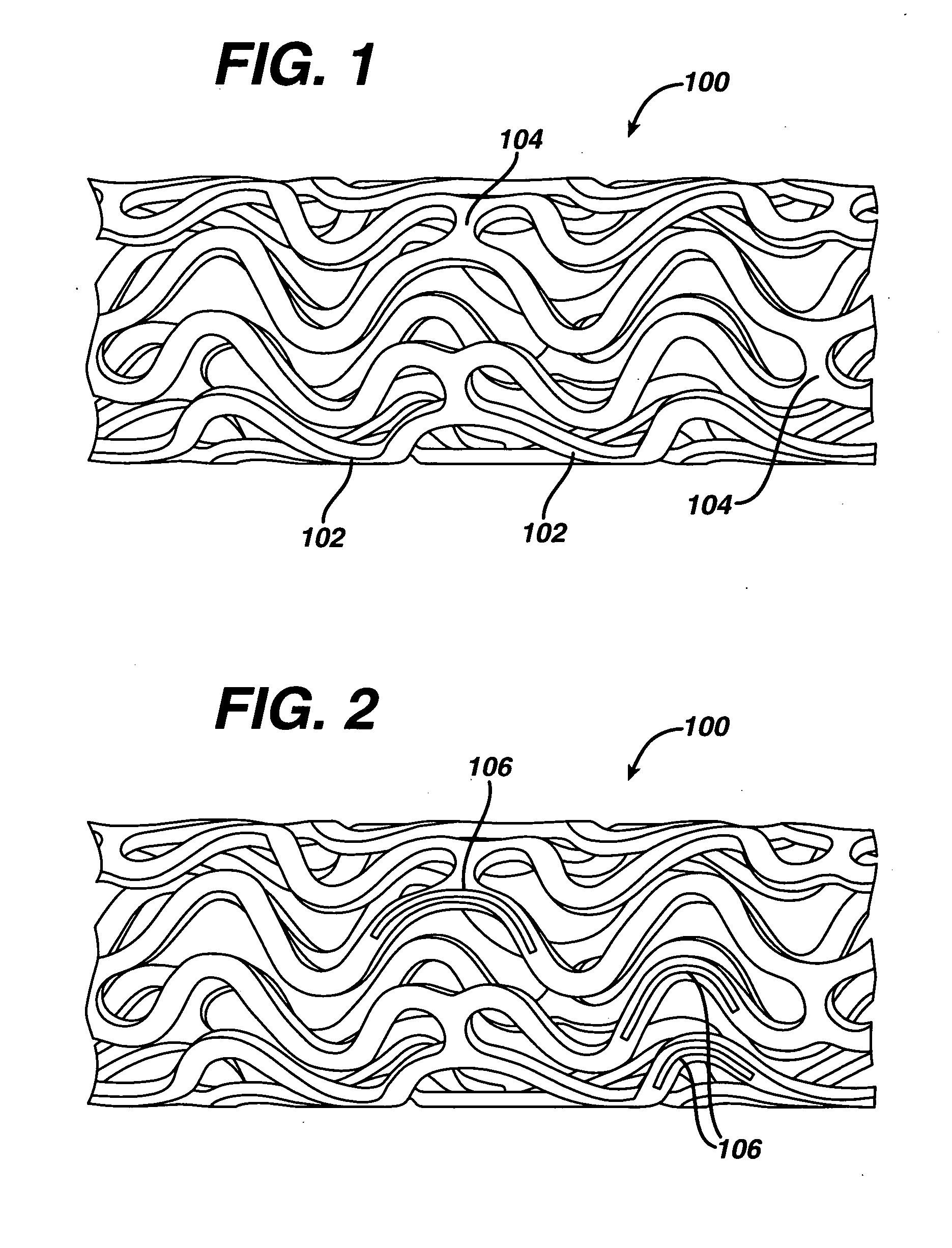
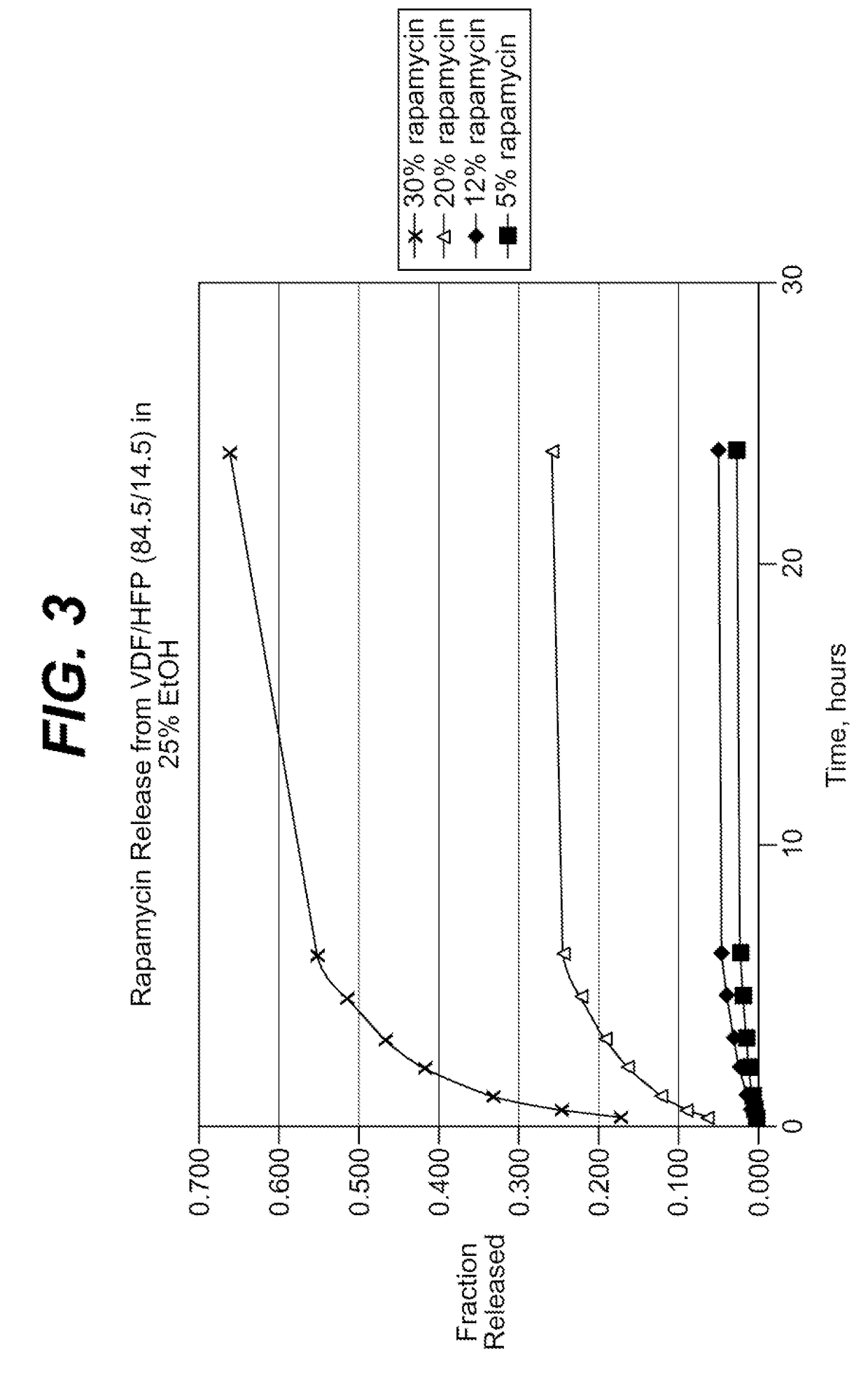
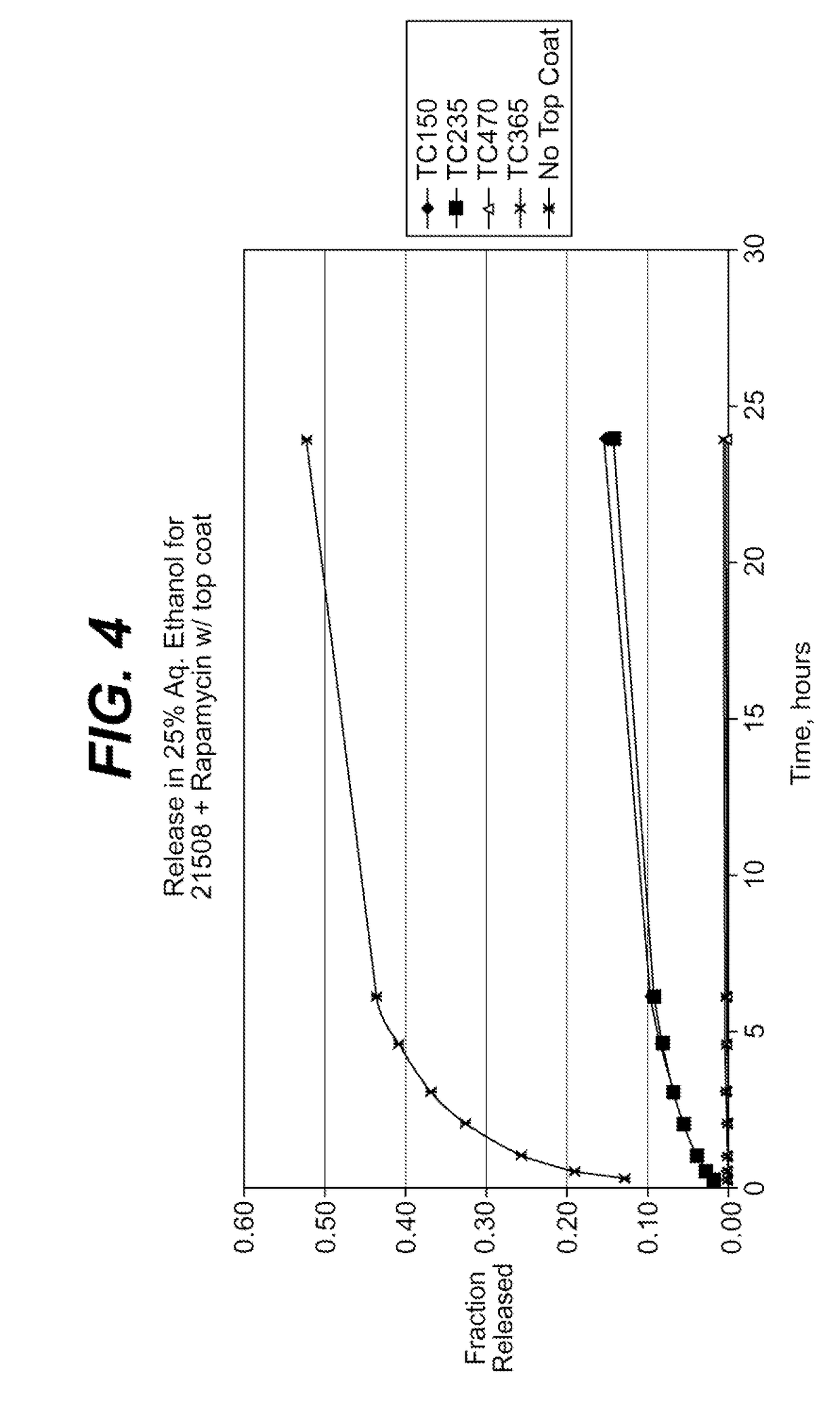
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Endovascular graft with differentiable porosity along its length
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device such as a stent-graft. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. A stent-graft fabricated from a thin-walled, high strength material provides for a more durable and lower profile endoprosthesis. The stent-graft comprises one or more stent segments covered with a fabric formed by the weaving, knitting or braiding of a biocompatible, high tensile strength, abrasion resistant, highly durable yarn such as ultra high molecular weight polyethylene. The one or more stent segments may be balloon expandable or self-expanding. The fabric may be attached to the stent segments utilizing any number of known materials and techniques. In addition, the pore size of the graft material may be varied.
Owner:CORDIS CORP
Intraluminal device and therapeutic agent combination for treating aneurysmal disease
InactiveUS20070083258A1Avoid dilationReduce drug toxicityStentsSurgeryCompound (substance)Biocompatible material
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. The drugs, agents, and / or compounds may also be utilized to treat specific diseases, including vulnerable plaque. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:CORDIS CORP
Medical devices, drug coatings and methods for maintaining the drug coatings thereon
InactiveUS7056550B2Reduce drug toxicityGood curative effectSuture equipmentsOrganic active ingredientsBiological bodyMedical device
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:WYETH LLC +1
Coated aneurysmal repair device
InactiveUS20050249776A1Reduce drug toxicityGood curative effectBiocideStentsBlood vesselDisease cause
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. The drugs, agents, and / or compounds may also be utilized to treat specific diseases, including vulnerable plaque. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:WYETH LLC
Local drug delivery devices and methods for maintaining the drug coatings thereon
InactiveUS7261735B2Reduce drug toxicityGood curative effectSuture equipmentsOrganic active ingredientsMedical deviceDrug coating
Local drug delivery medical devices are utilized to deliver therapeutic dosages of drugs, agents or compounds directly to the site where needed. The local drug delivery medical devices utilize various materials and coating methodologies to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:WYETH LLC
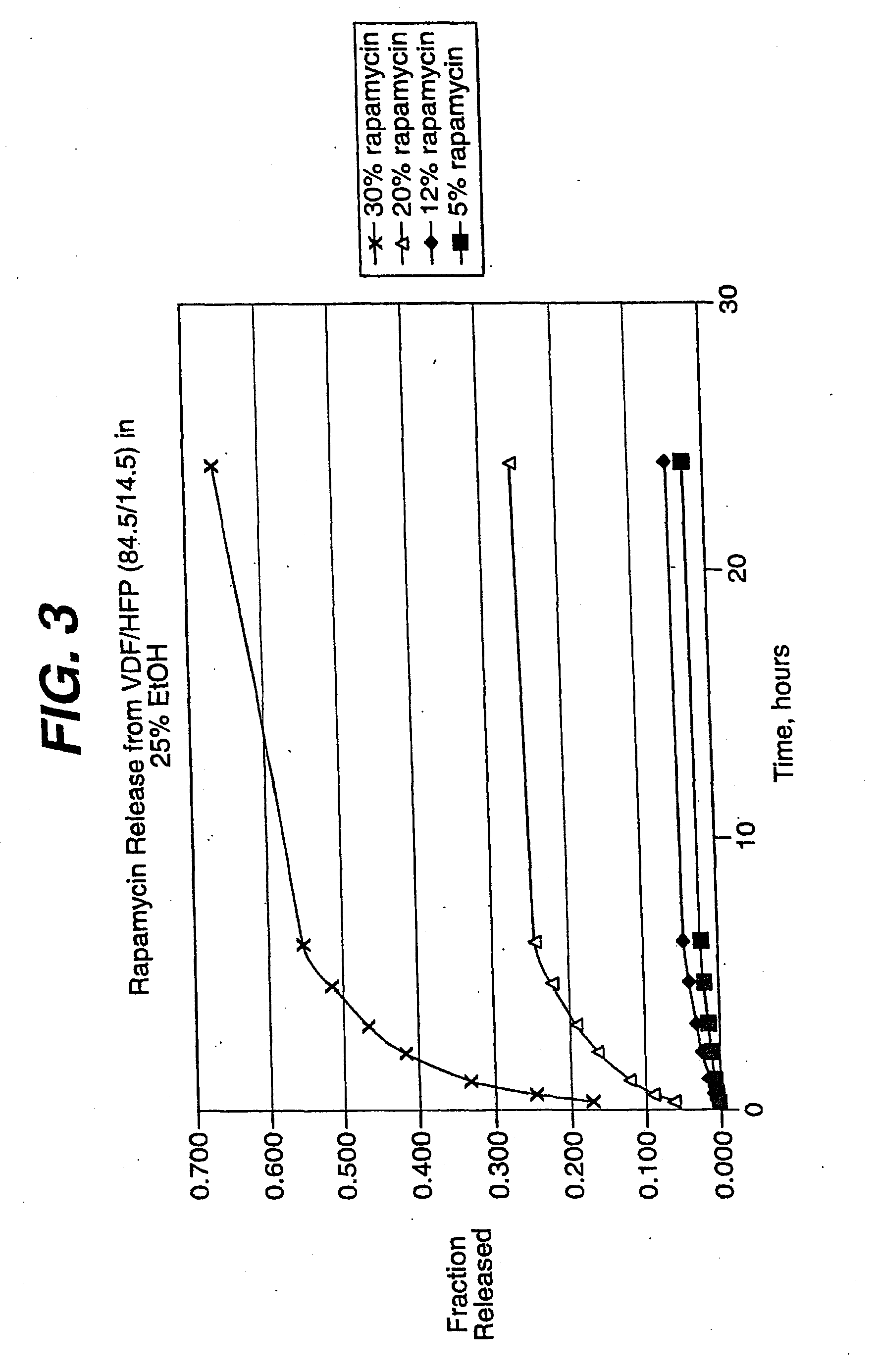
Device for local and/or regional delivery employing liquid formulations of therapeutic agents
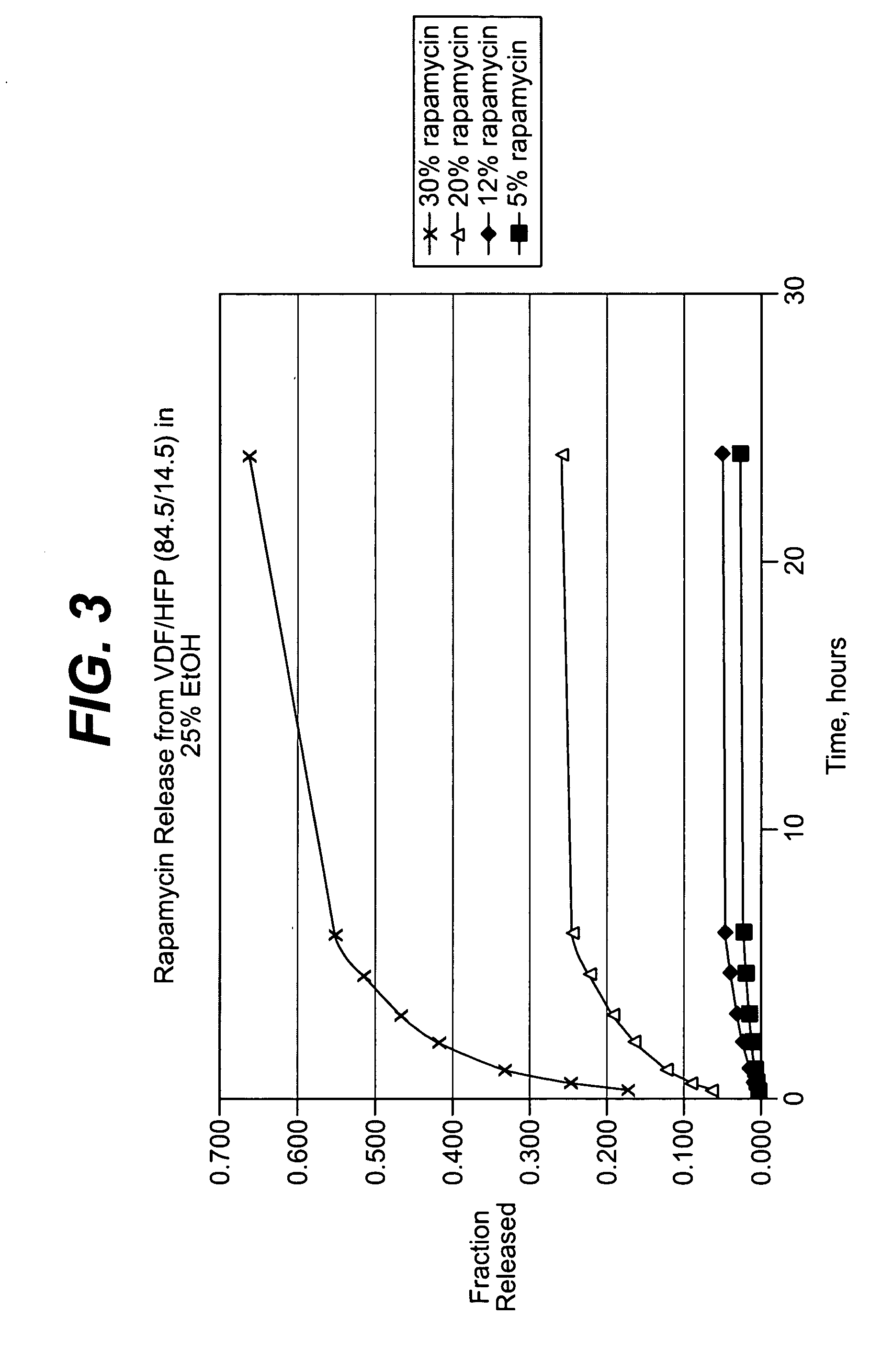
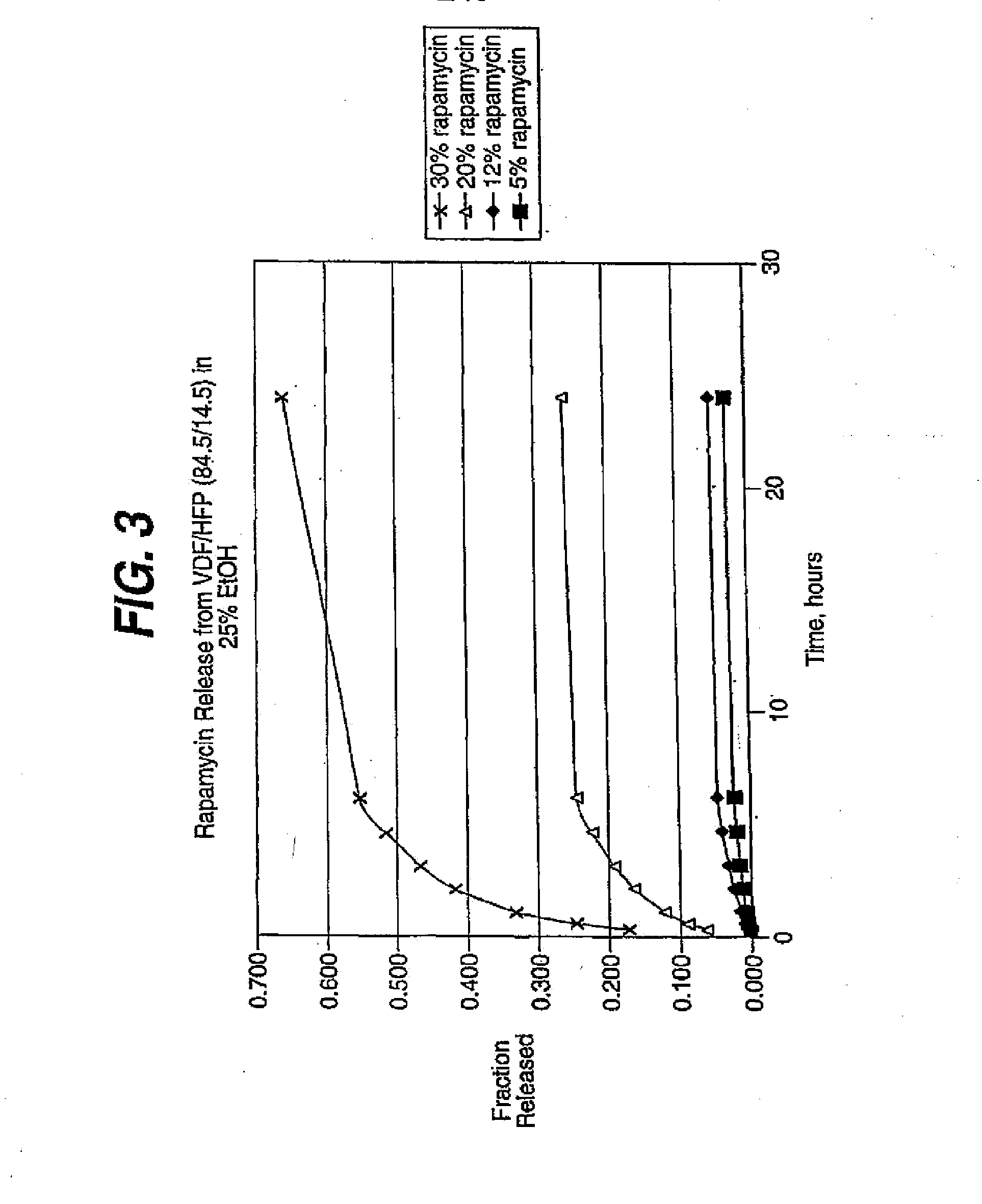
InactiveUS20080181927A1Elimination reactionReduce riskBiocideSurgeryDiseasePercent Diameter Stenosis
Medical devices may be utilized for local and regional therapeutic agent delivery. These therapeutic agents or compounds may reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the prevention of thrombosis. The drugs, agents, and / or compounds may also be utilized to treat specific disorders, including restenosis, vulnerable plaque, and atherosclerosis in type 2 diabetic patients. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Various materials and coating methodologies may be utilized to maintain the agents or compounds on the medical device until delivered and positioned.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
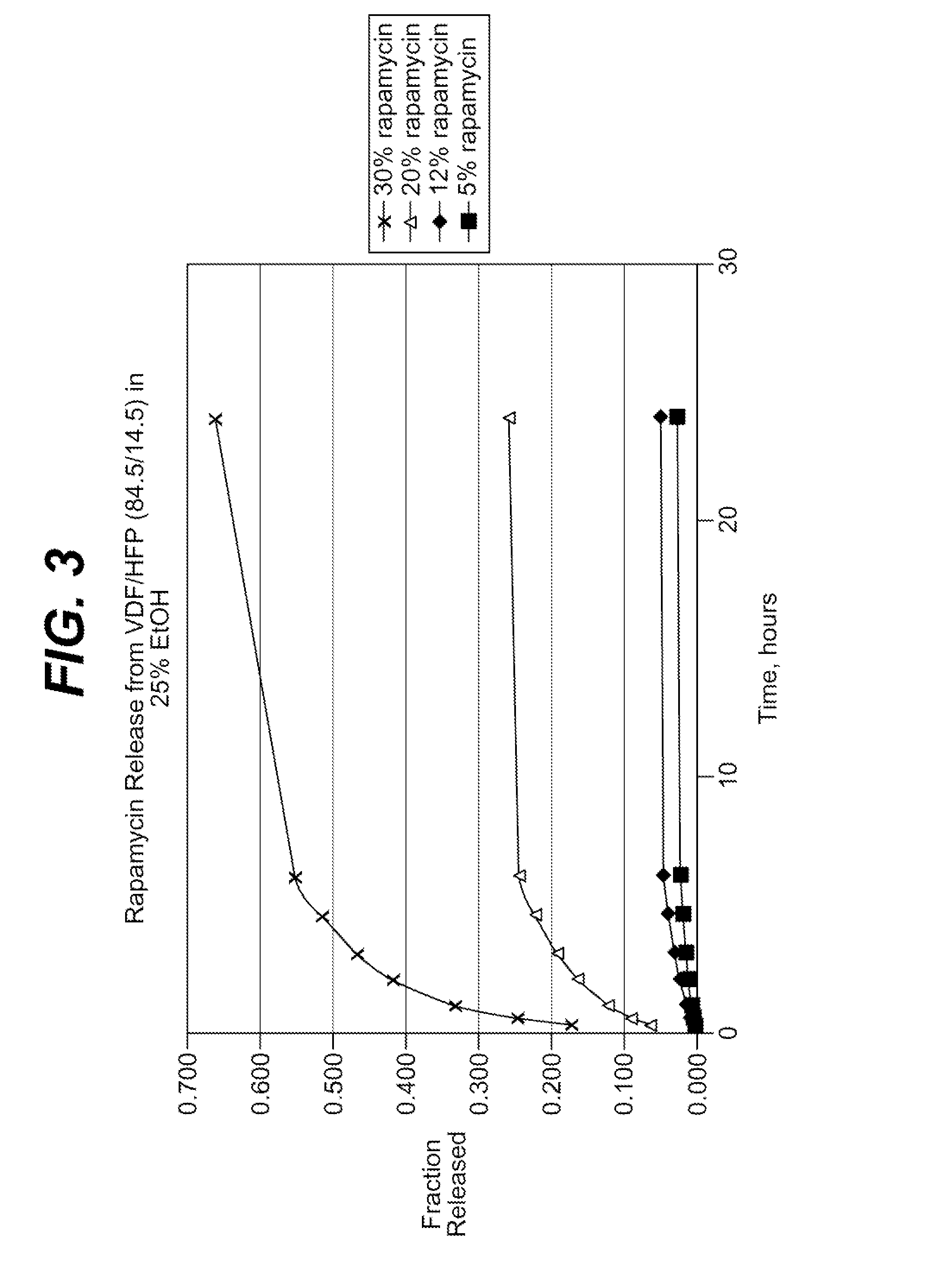
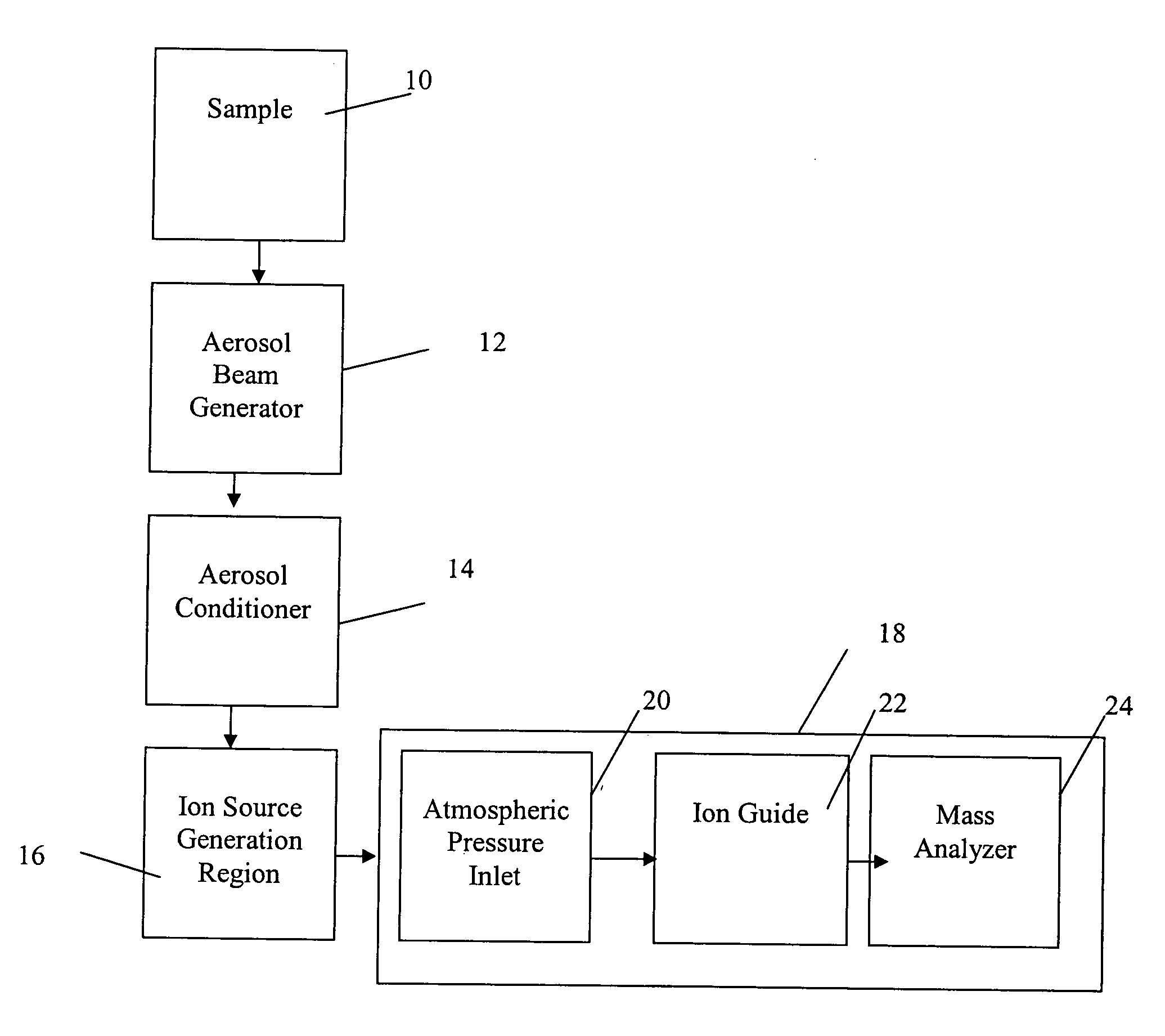
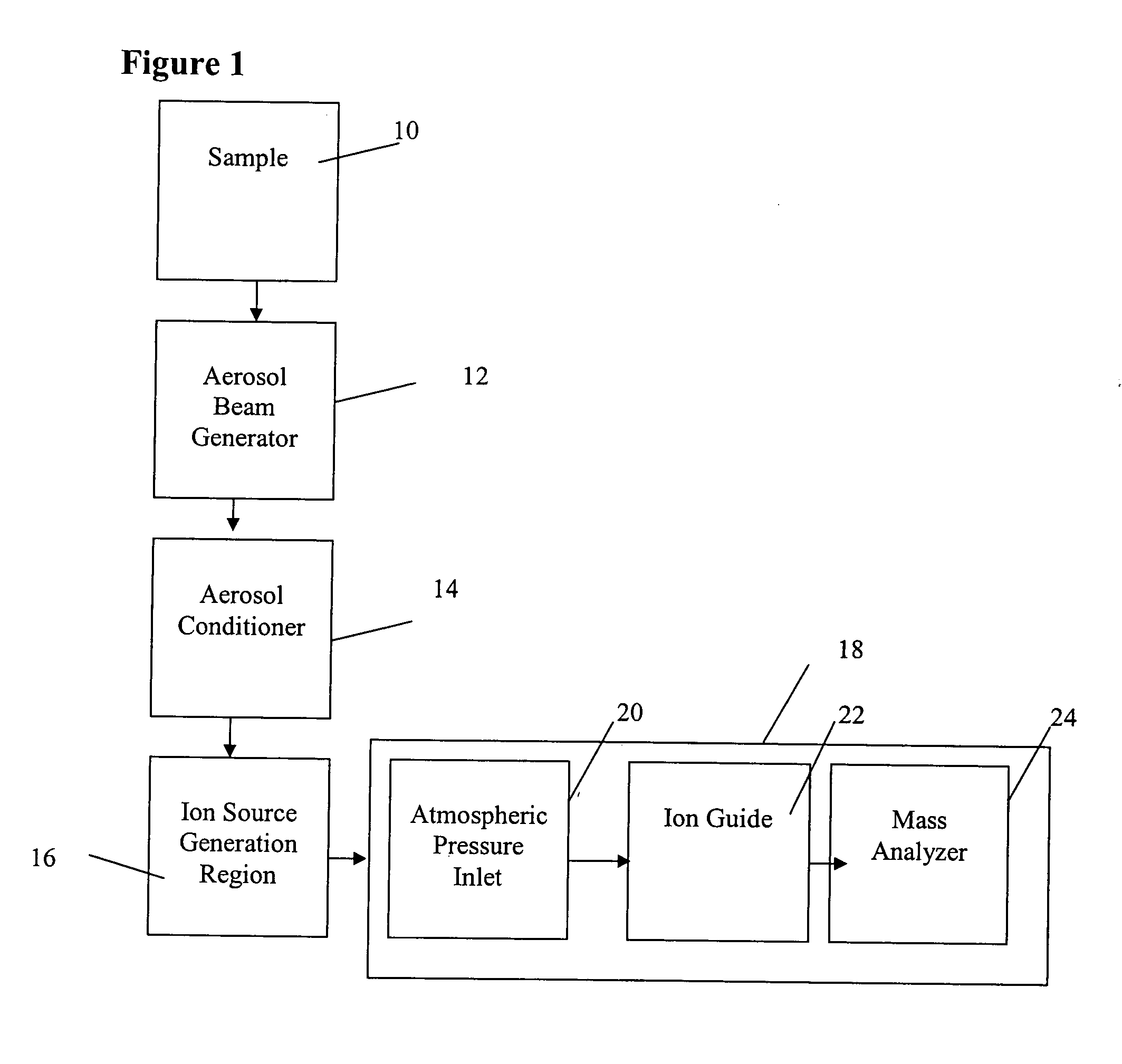
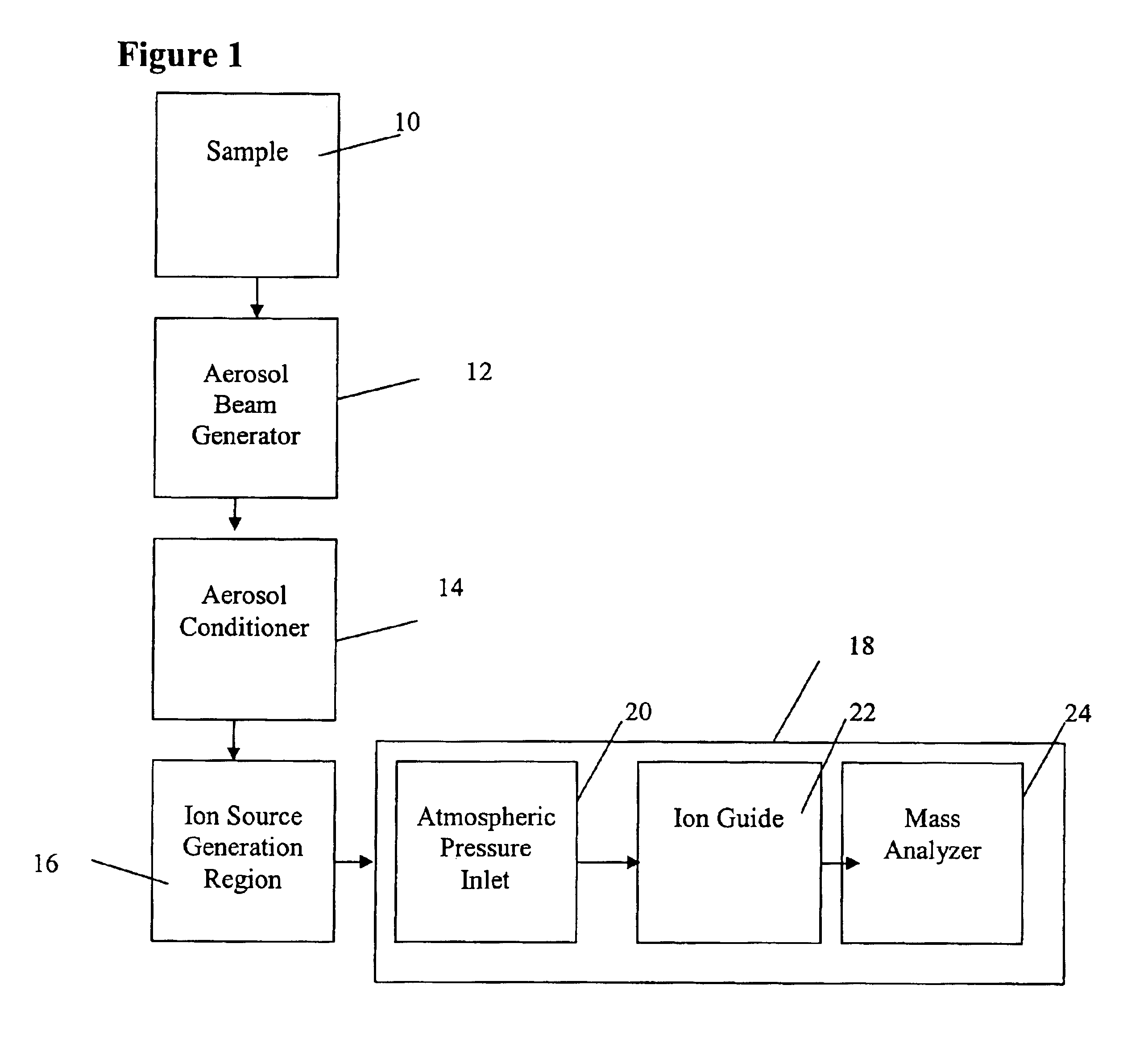
Method and apparatus for mass spectrometry analysis of aerosol particles at atmospheric pressure
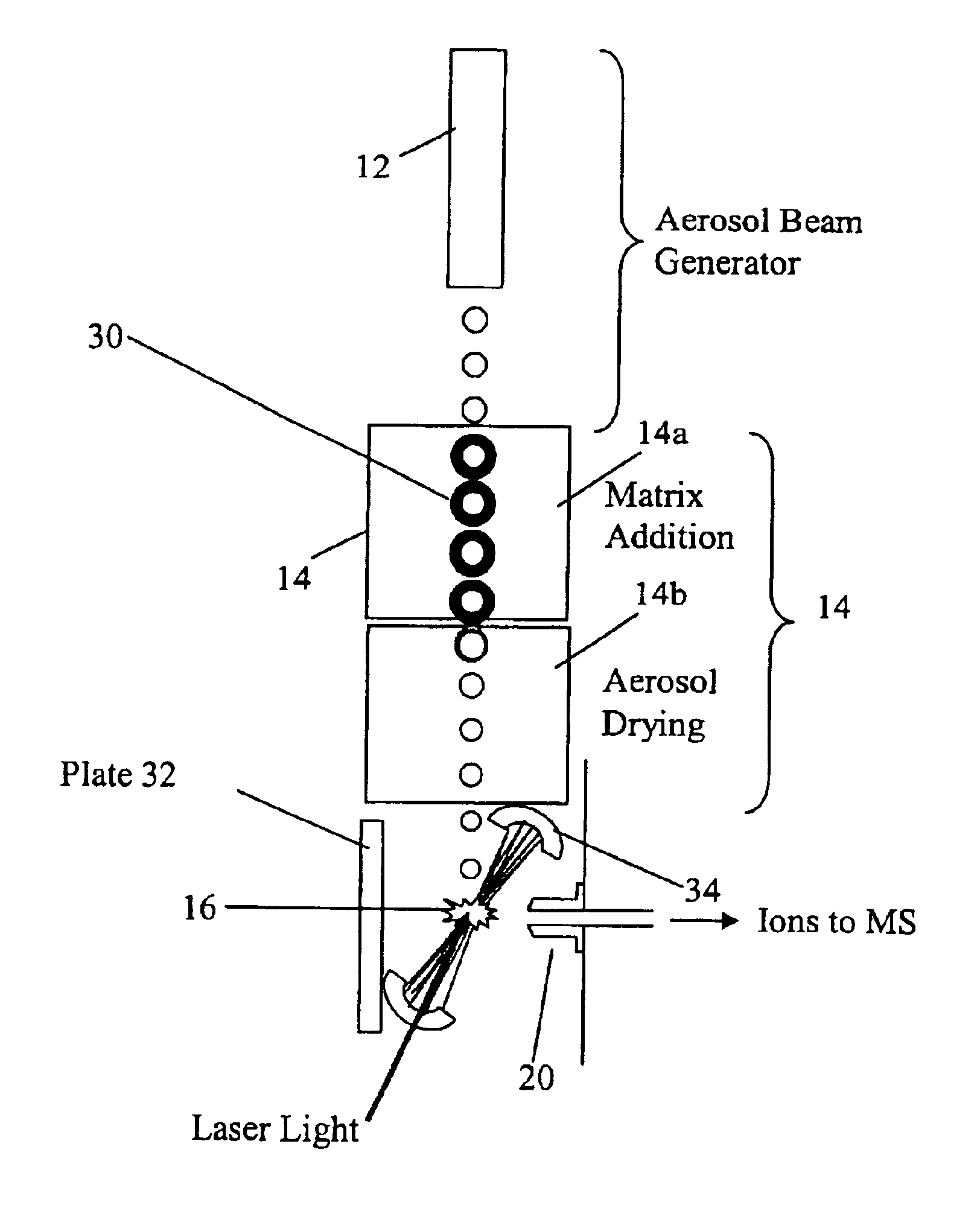
InactiveUS20050035285A1Maximize efficiencyMinimizes sample lossRadiation pyrometryTime-of-flight spectrometersPhysicsAerosol beam
An apparatus and method for generating ions from an aerosol and transferring the ions into a mass analyzer. In the apparatus and method, an aerosol beam is generated, the aerosol beam is directed to a spatial volume outside the mass analyzer, particles in the aerosol beam are ionized to produce the ions, and the ions are collected into the mass analyzer. As such the apparatus includes respectively an aerosol beam generator, an ion source generator, and an ion collector.
Owner:SCI & ENG SERVICES
Medical devices, drug coatings and methods of maintaining the drug coatings thereon
InactiveUS20060222756A1Reduce drug toxicityGood curative effectSuture equipmentsOrganic active ingredientsBiological bodyReady to use
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:WYETH LLC
System for treating aneurysmal disease
InactiveUS20070026042A1Reduce drug toxicityGood curative effectSuture equipmentsBiocideCompound (substance)Biocompatible material
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. The drugs, agents, and / or compounds may also be utilized to treat specific diseases, including vulnerable plaque. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:WYETH
Apparatus for eliiminating net drill bit torque and controlling drill bit walk
InactiveUS20080135292A1Prevent rotationElimination reactionDrilling rodsConstructionsDrill bitPetroleum engineering
A drilling apparatus controls or eliminates reaction torque from drill bits thereby preventing loss of penetration due to undesired rotation of the drilling apparatus or controls drilling direction by intentionally manipulating reaction torque thereby inducing desired drill bit walk. The drilling apparatus has a concentrically divided drill bit in which an inner drill bit rotates simultaneously in the opposite direction from an outer drill bit. The inner drill bit can be moved axially forward from or back toward the outer drill bit. Forces produced by the inner and outer drill bits are controlled to eliminate or adjust reaction torque.
Owner:SCHLUMBERGER TECH CORP
Medical Devices, Drug Coatings and Methods for Maintaining the Drug Coatings Thereon
InactiveUS20080051885A1Reduce drug toxicityGood curative effectStentsSurgeryBiological bodyCompound (substance)
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:CORDIS CORP
Lithium Ion Rocking Chair Rechargeable Battery
InactiveUS20060234125A1Improve cycle lifeLower resistanceFinal product manufactureSecondary cellsLithiumElectrolysis
An electrochemical cell for a lithium ion rechargeable battery. The electrochemical cell comprises an anode including anode active material having a reduction potential of at least about 1.0 volt, a cathode including cathode active material having an oxidation potential of no more than about 3.7 volts, and an electrolyte separator separating the anode and the cathode.
Owner:BATHIUM CANADA
Apparatus for eliminating net drill bit torque and controlling drill bit walk
InactiveUS7610970B2Prevent rotationElimination reactionDrilling rodsConstructionsDrill bitPetroleum engineering
A drilling apparatus controls or eliminates reaction torque from drill bits thereby preventing loss of penetration due to undesired rotation of the drilling apparatus or controls drilling direction by intentionally manipulating reaction torque thereby inducing desired drill bit walk. The drilling apparatus has a concentrically divided drill bit in which an inner drill bit rotates simultaneously in the opposite direction from an outer drill bit. The inner drill bit can be moved axially forward from or back toward the outer drill bit. Forces produced by the inner and outer drill bits are controlled to eliminate or adjust reaction torque.
Owner:SCHLUMBERGER TECH CORP
Plating media for the identification of Salmonella
InactiveUS7150977B2Reduce and eliminate responseElimination reactionMicrobiological testing/measurementAdditive ingredientBeta-Galactosidases
An isolation plating medium for the identification of Salmonella bacteria in a sample containing a plurality of different bacteria comprising a mixture of a carbohydrate capable of being a metabolic source for Salmonella bacteria and supporting colonies of Salmonella bacteria, a pH indicator dye that changes the color of the plating medium to a first color different from the color of the medium responsive to a change in the pH of the medium, a first substrate that does not react with Salmonella bacteria and injects color into the medium of a second color responsive to the presence of beta-galactosidase, the second color contrasting with the first color and the color of the medium, a second substrate that does not react with Salmonella bacteria and injects color into the medium of substantially the same color as the second color responsive to the presence of beta=galactosidase, and an ingredient for thickening the mixture in sufficient quantity to solidify the mixture.
Owner:R&F PROD
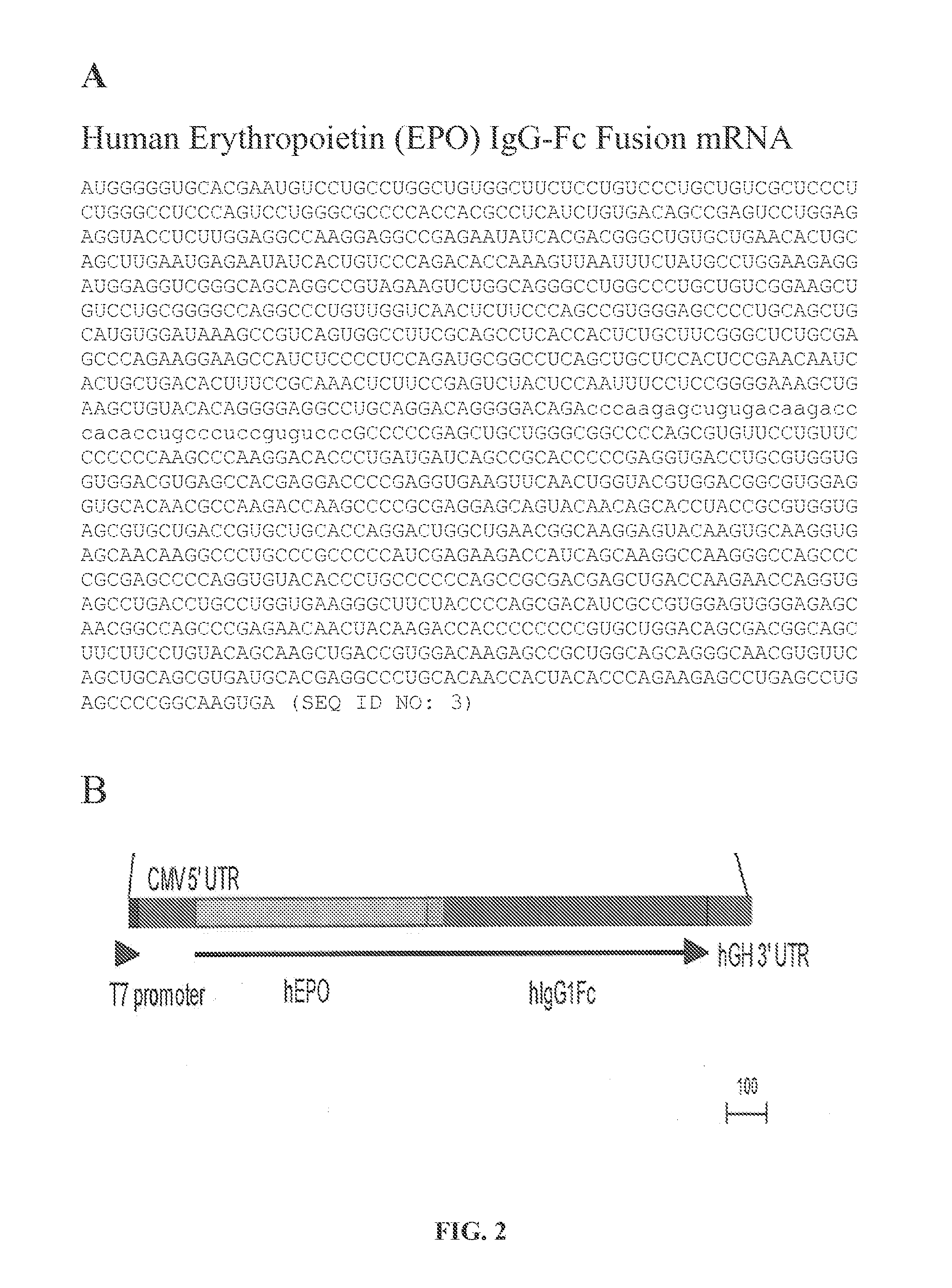
mRNA therapeutic compositions and use to treat diseases and disorders
InactiveUS20160184458A1Reduce immunogenicitySimplification and improvement of processAntibody mimetics/scaffoldsMicroencapsulation basedTherapeutic proteinDisease
Disclosed are compositions and methods for producing therapeutic fusion proteins in vivo. The compositions and methods disclosed herein are capable of ameliorating diseases by providing therapeutic protein delivery.
Owner:TRANSLATE BIO INC
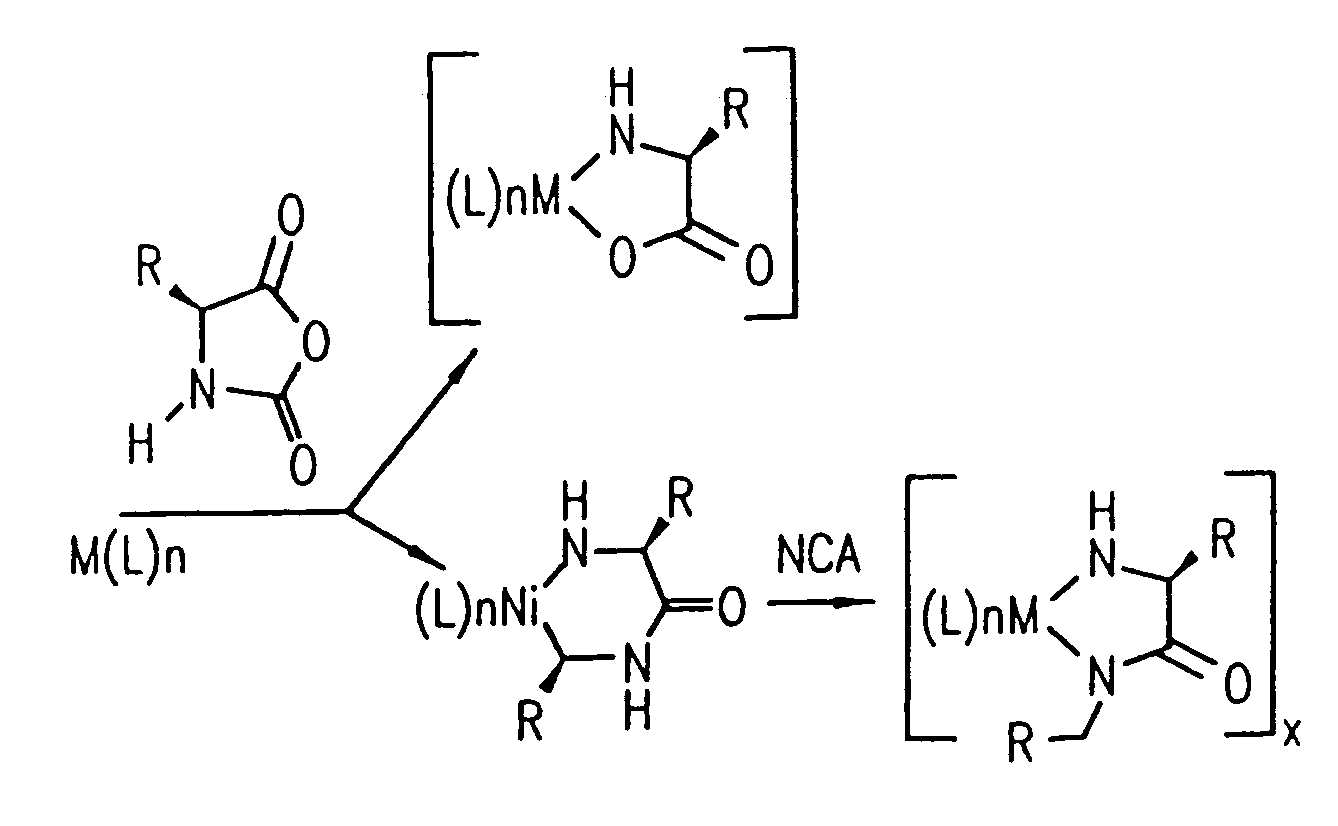
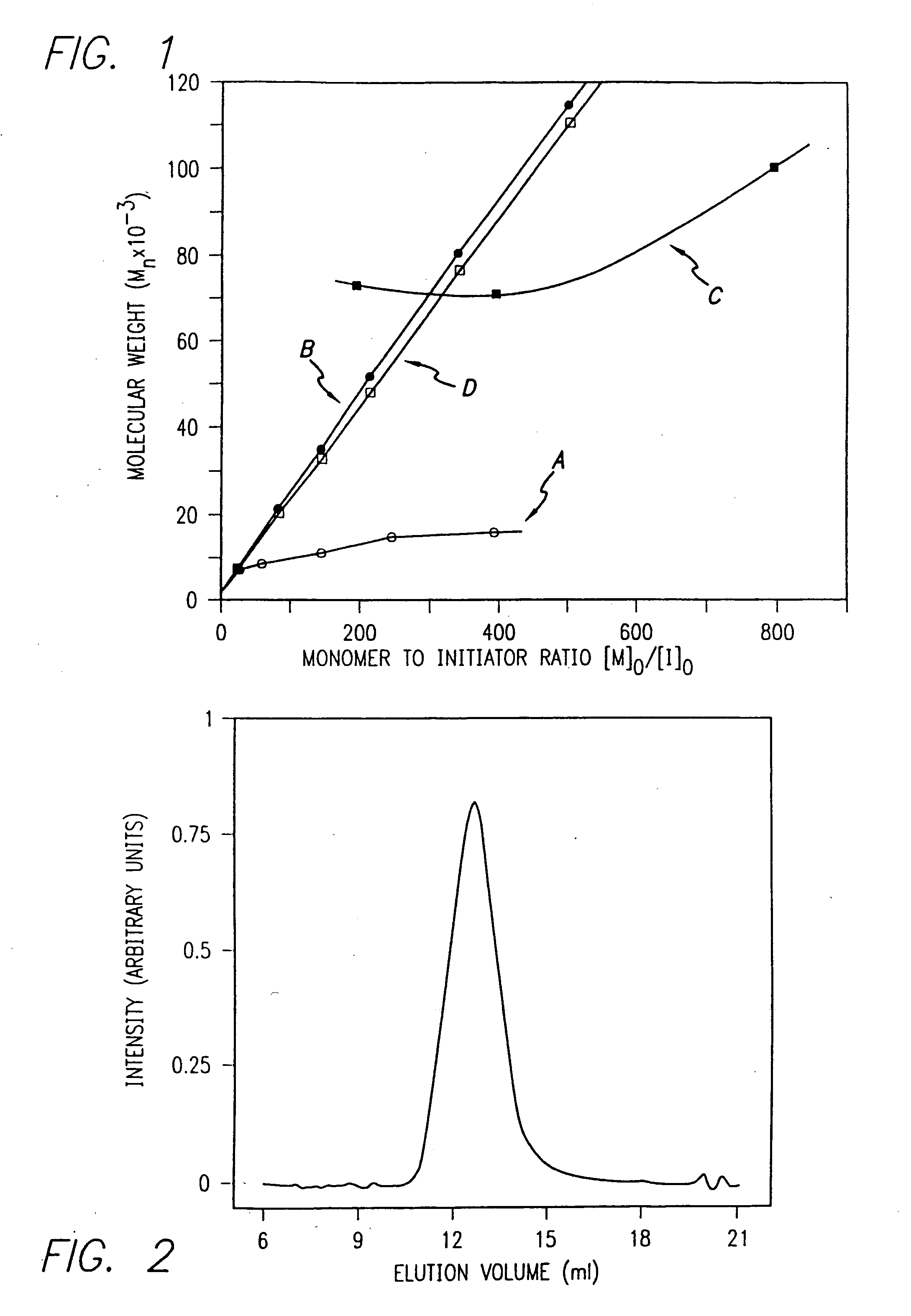
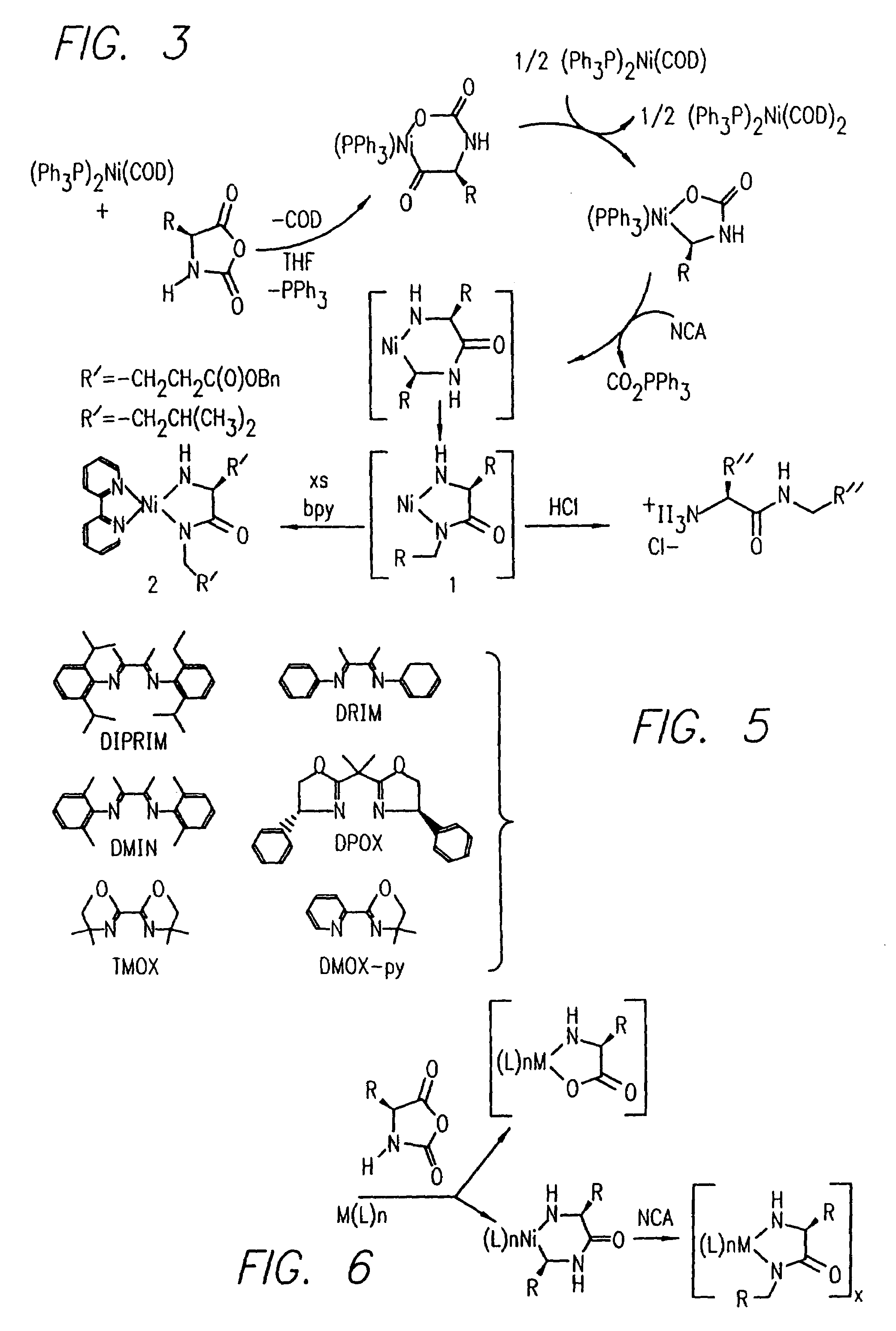
Methods and compositions for controlled polypeptide synthesis
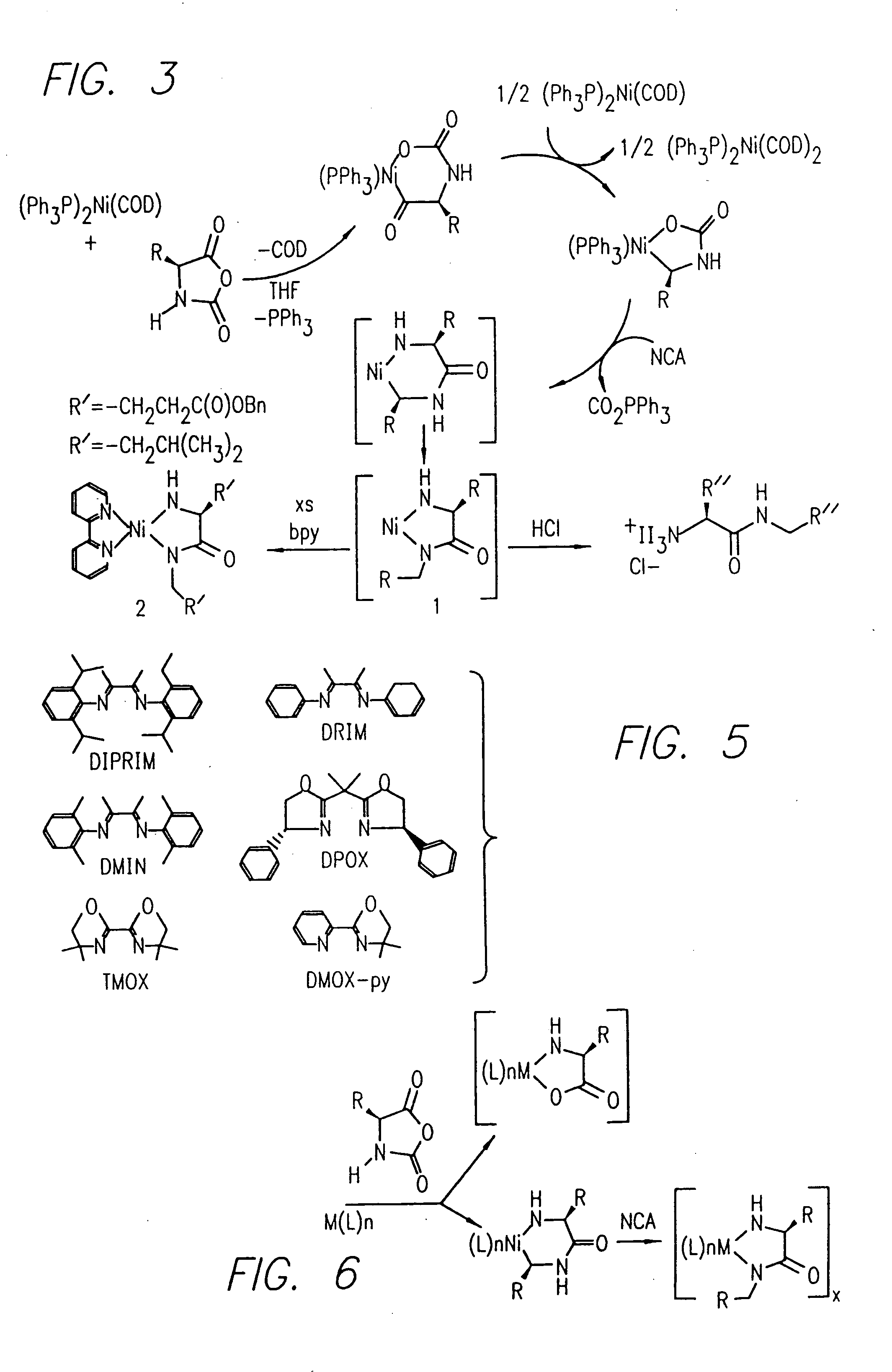
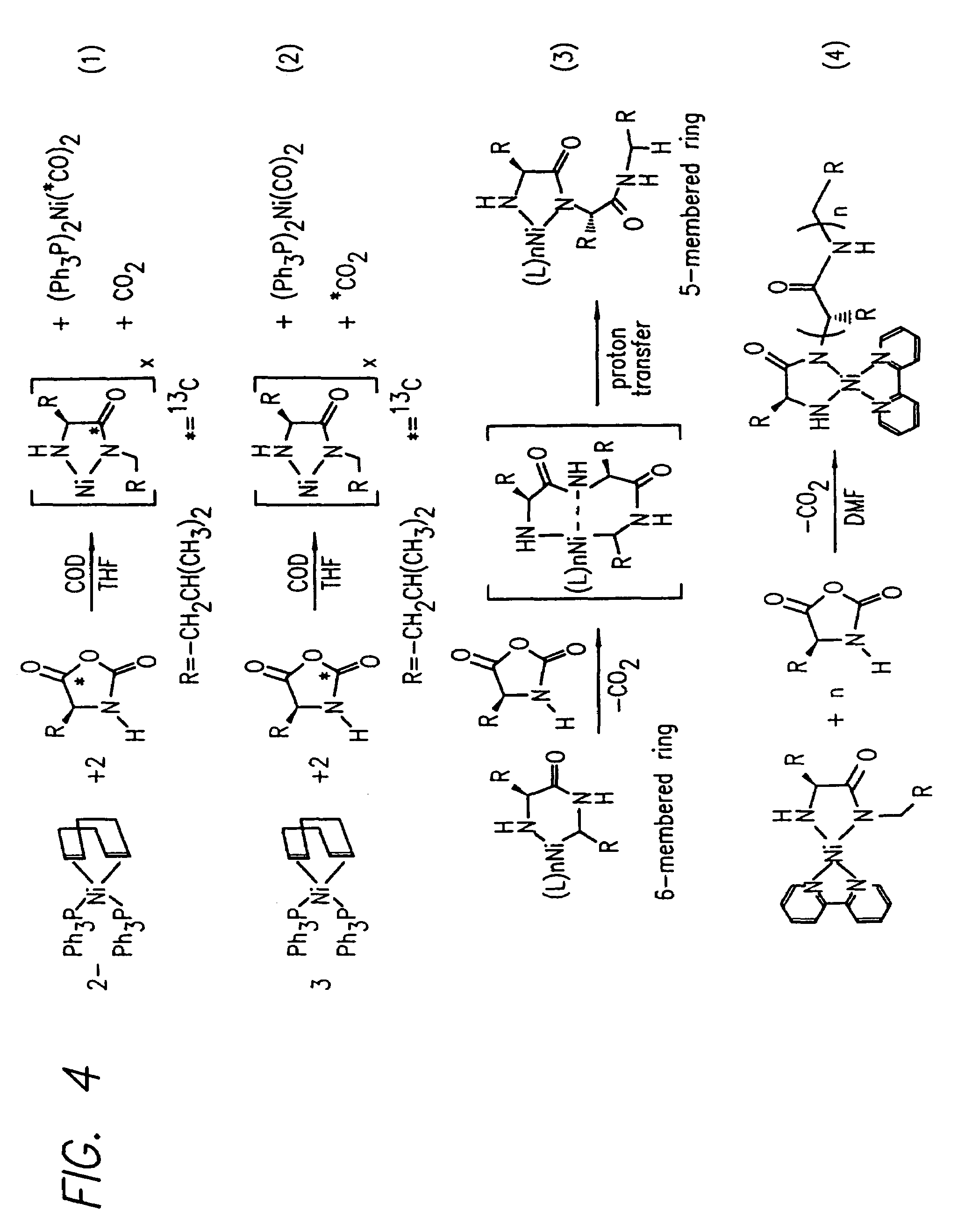
InactiveUS20080125581A1Elimination reactionEasy to controlPeptide/protein ingredientsPeptide preparation methodsSynthesis methodsEnd-group
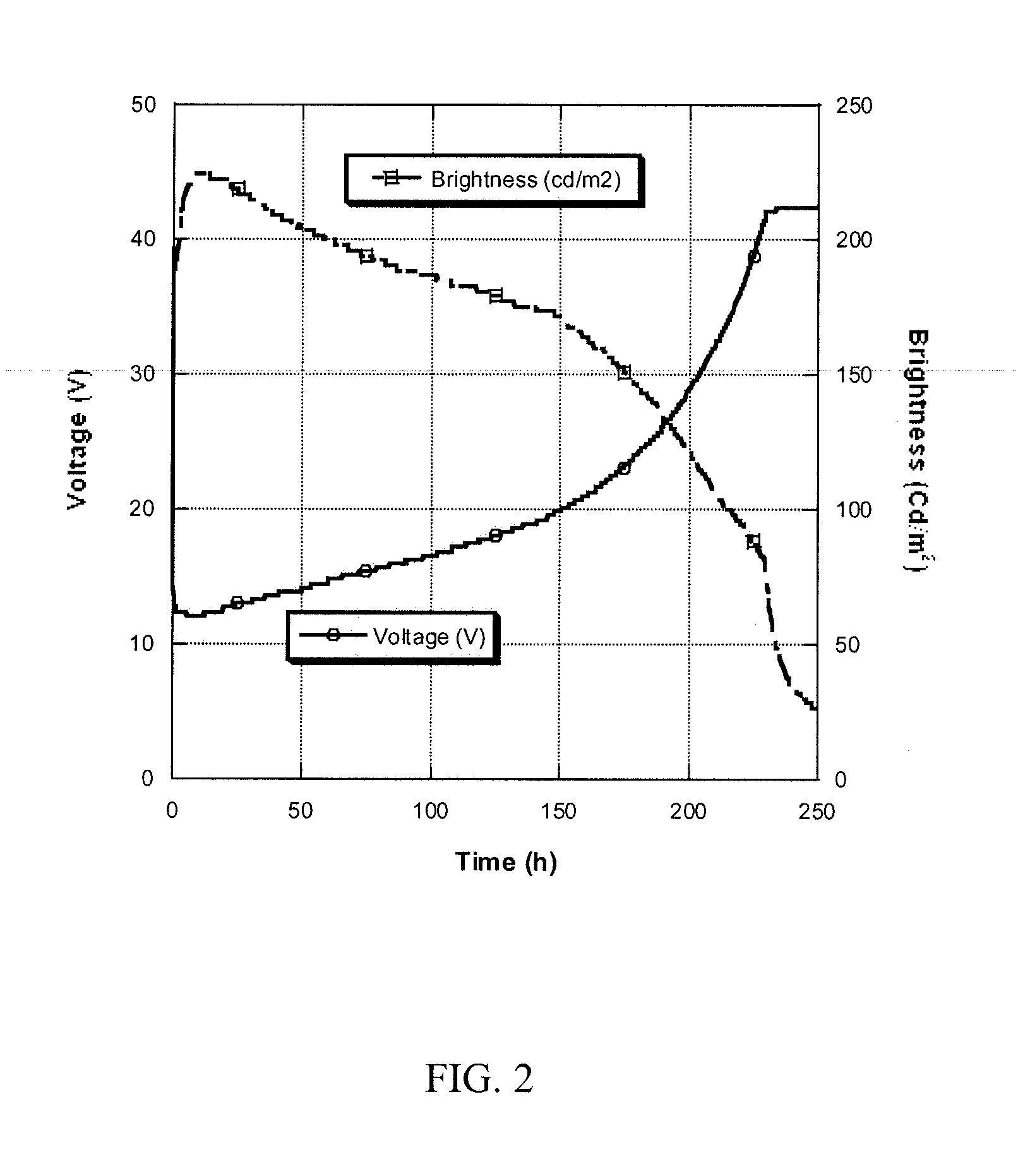
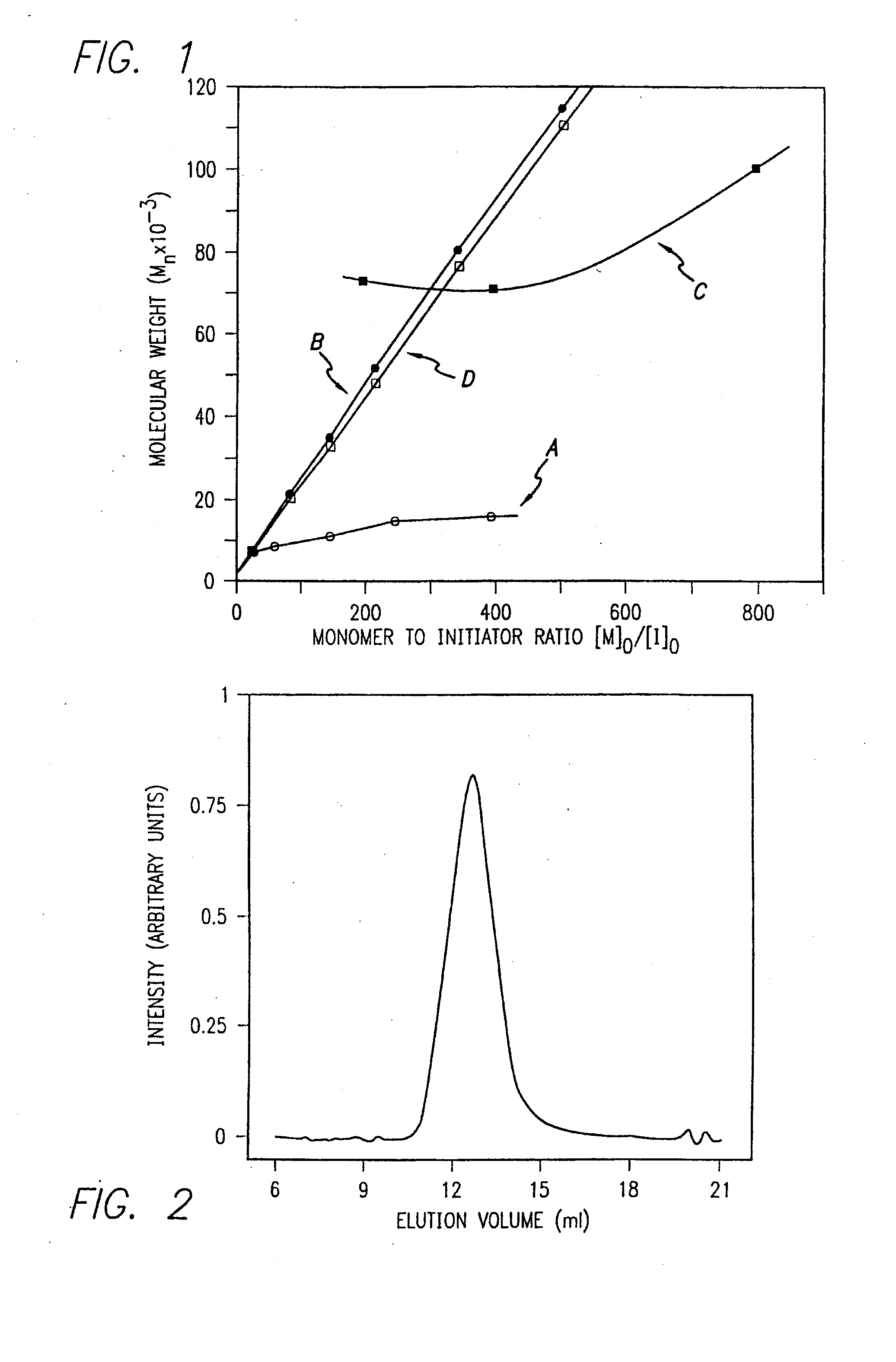
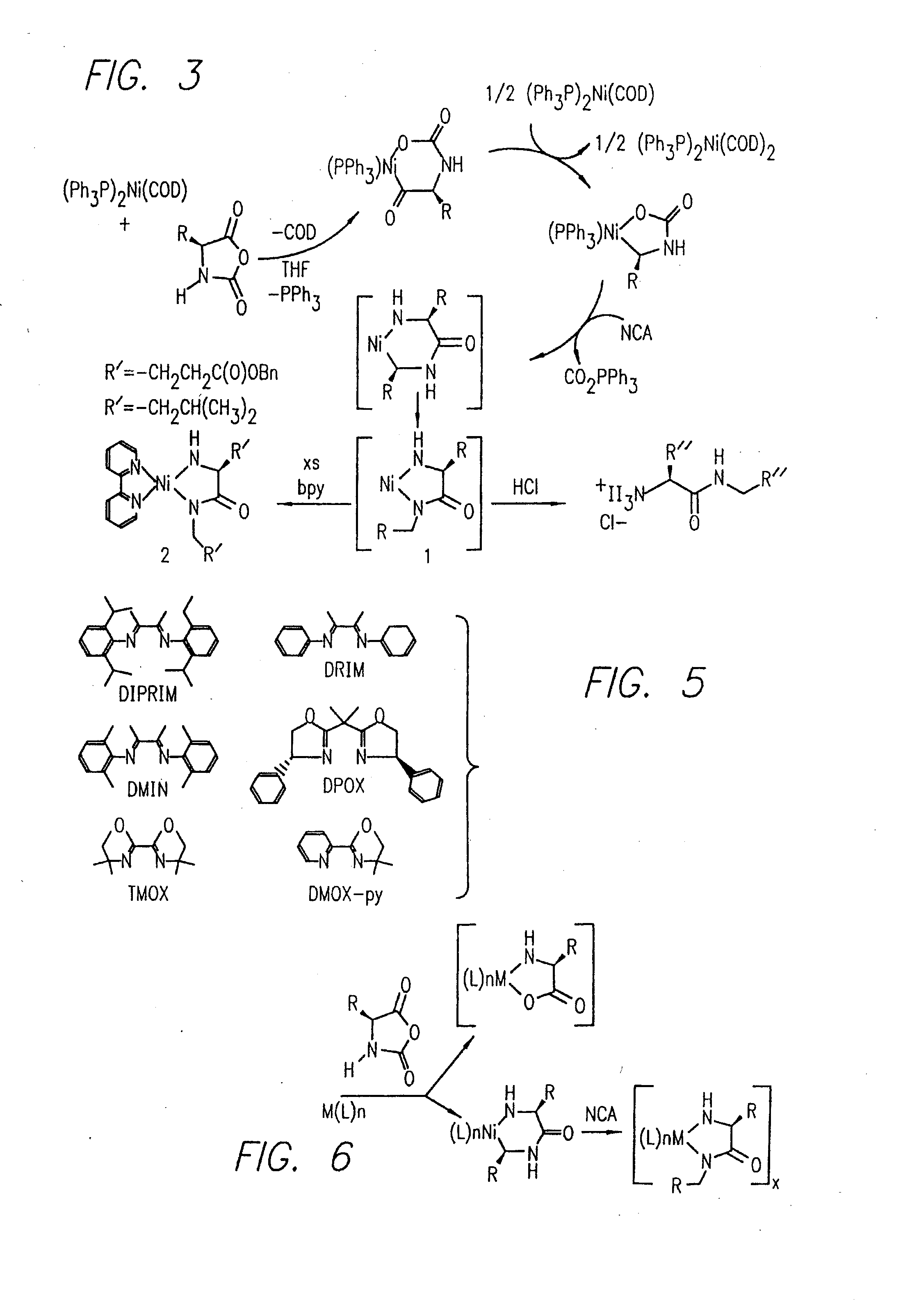
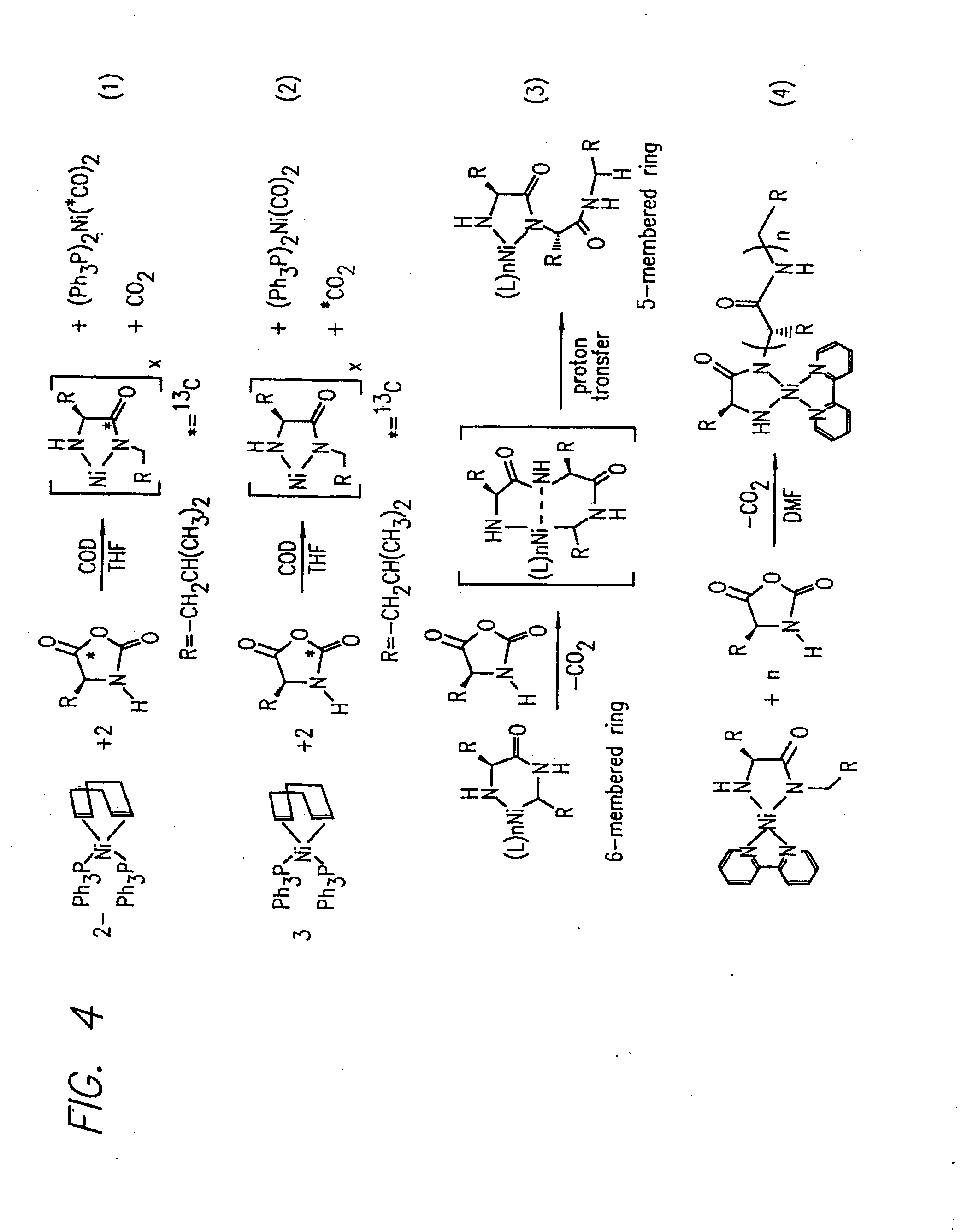
Methods and compositions for the generation of polypeptides having varied material properties are disclosed herein. Methods include means for initiating the polymerization of aminoacid-N-carboxyanhydride (NCA) monomer by combining the monomer with an amido-containing metallacycle, for making self assembling amphiphilic block copolypeptides and related protocols for adding oligo(ethyleneglycol) functionalized aminoacid-N-carboxyanhydrides (NCAs) to polyaminoacid chains. Additional methods include means of adding an end group to the carboxy terminus of a polyaminoacid chain by reacting an alloc-protected amino acid amide with a transition metal-donor ligand complex to forming an amido-amidate metallacycle for use in further polymerization reactions. Novel compositions for use in peptide synthesis and design including five and six membered amido-containing metallacycles and block copolypeptides are also disclosed.
Owner:RGT UNIV OF CALIFORNIA
Method and apparatus for mass spectrometry analysis of aerosol particles at atmospheric pressure
InactiveUS6943346B2Minimizes sample lossHigh sensitivityRadiation pyrometryTime-of-flight spectrometersMass Spectrometry-Mass SpectrometryIon transfer
An apparatus and method for generating ions from an aerosol and transferring the ions into a mass analyzer. In the apparatus and method, an aerosol beam is generated, the aerosol beam is directed to a spatial volume outside the mass analyzer, particles in the aerosol beam are ionized to produce the ions, and the ions are collected into the mass analyzer. As such the apparatus includes respectively an aerosol beam generator, an ion source generator, and an ion collector.
Owner:SCI & ENG SERVICES
Layer-by-layer stereocomplexed polymers as drug depot carriers or coatings in medical devices
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the prevention of thrombosis. The drugs, agents, and / or compounds may also be utilized to treat specific disorders, including vulnerable plaque, and atherosclerosis in type 2 diabetic patients. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Various materials and coating methodologies may be utilized to maintain the agents or compounds on the medical device until delivered and positioned.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Medical Devices, Drug Coatings and Methods for Maintaining the Drug Coatings Thereon
InactiveUS20070179594A1Reduce drug toxicityGood curative effectSuture equipmentsOrganic active ingredientsBiological bodyReady to use
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:ETHICON INC
Medical Devices, Drug Coatings and Methods for Maintaining the Drug Coatings Thereon
InactiveUS20070276473A1Reduce drug toxicityGood curative effectStentsSurgeryBiological bodyCompound (substance)
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:CORDIS CORP
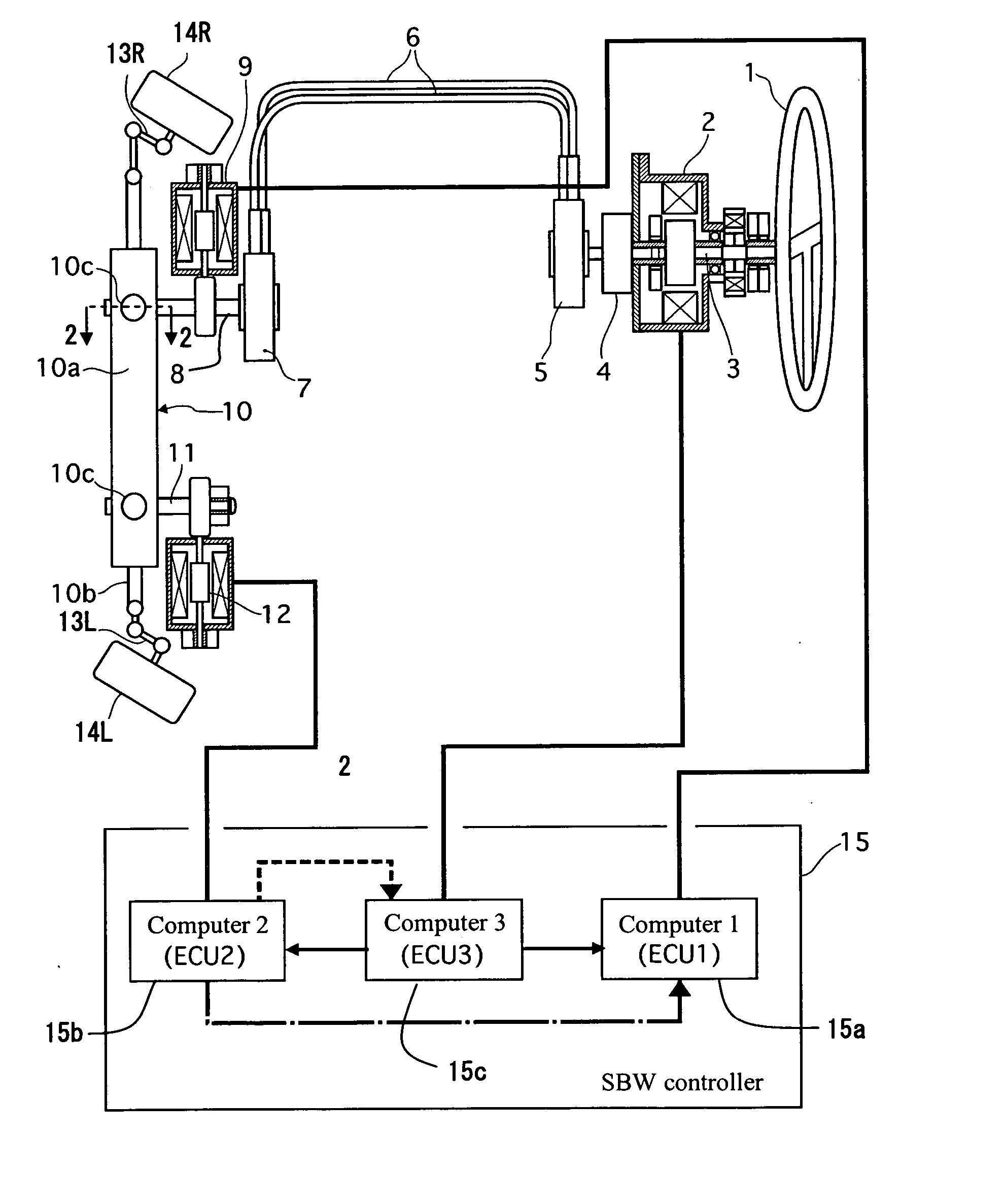
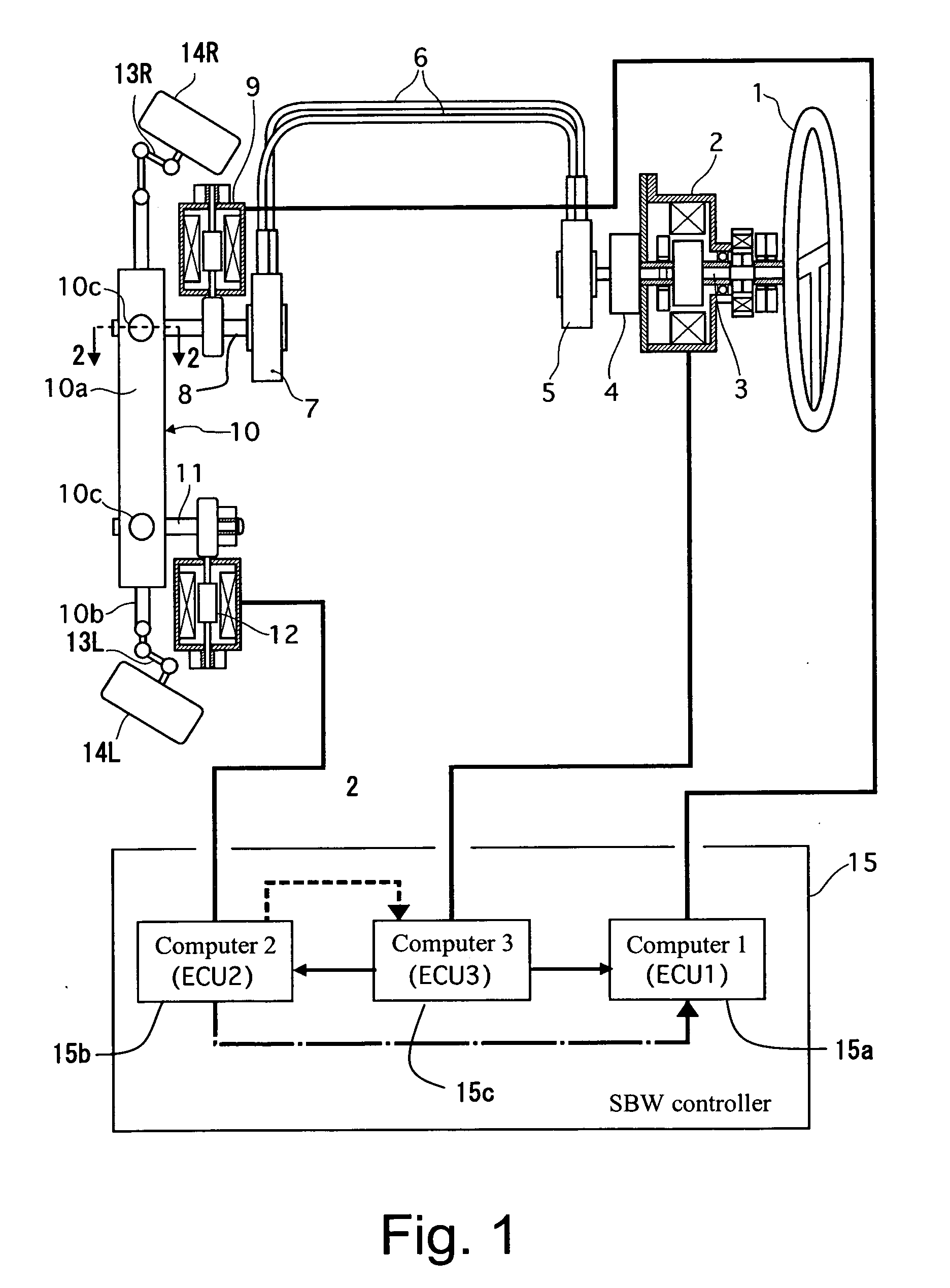
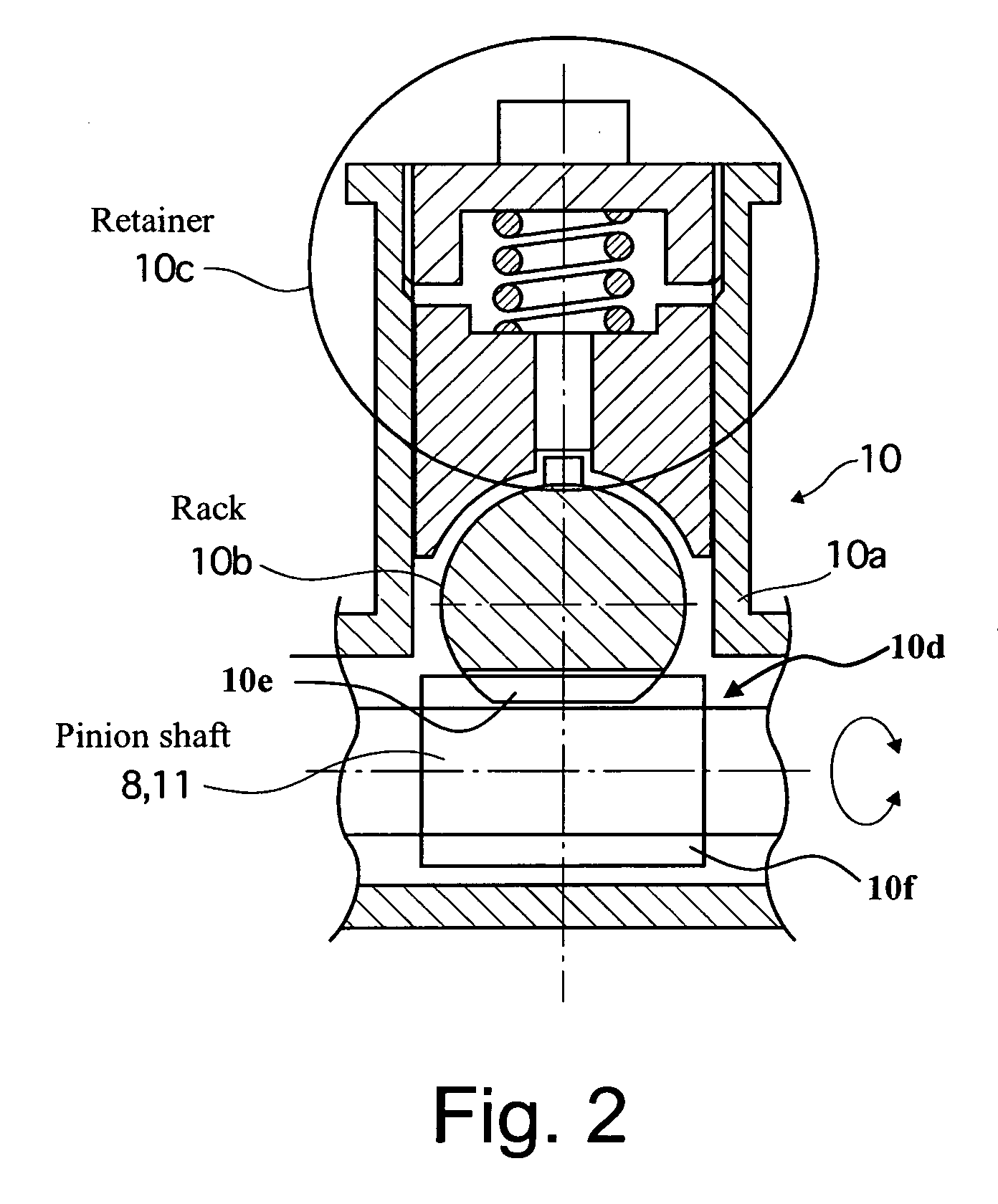
Vehicle steering control
ActiveUS20070131476A1Reducing unpleasant sensationAdvanced technologyMechanical steeringFluid steeringDriver/operatorSteering control
A steer-by-wire system is provided with a driver operating unit, a turning unit, a backup mechanism, a steering reaction actuator, a wheel turning actuator and a controller. The backup mechanism mechanically connects the driver operating unit to the turning unit via a first shaft to transmit an input torque from the driver operating unit to the turning unit when the driver operating unit and the turning unit are mechanically connected by the backup mechanism. The steering reaction actuator applies a steering reaction torque to the driver operating unit. The wheel turning actuator applies a wheel turning torque to the turning unit via a second shaft. The controller controls the wheel turning actuator and the steering reaction actuator. The controller is configured to control the steering reaction actuator based on an operating parameter of the wheel turning actuator.
Owner:NISSAN MOTOR CO LTD
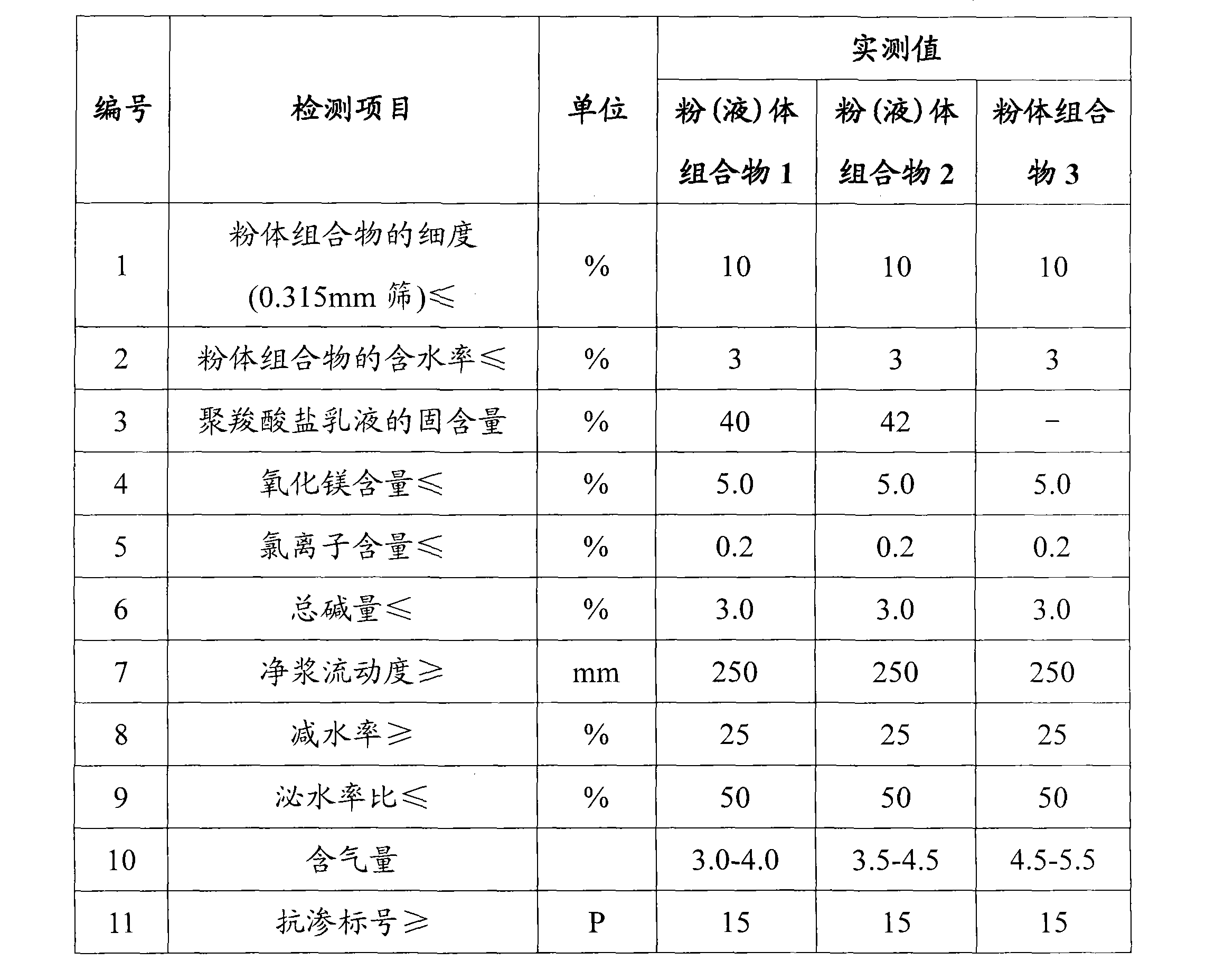
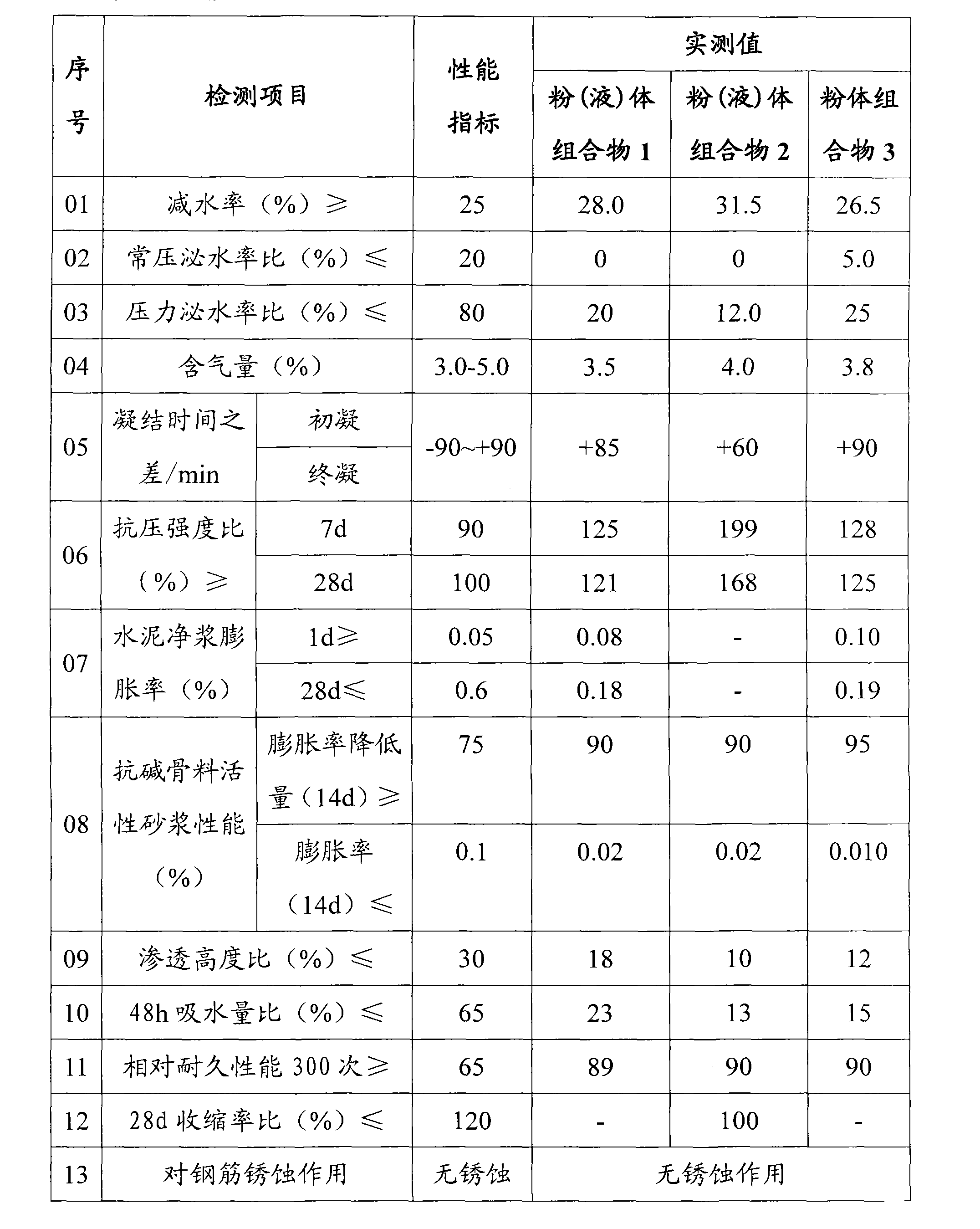
Composition for inhibiting concrete alkali aggregate reaction and preparation thereof
The invention belongs to the technical field of concrete engineering, and in particular relates to a composition for inhibiting concrete alkali-aggregate reaction and a preparation method thereof. The composition for inhibiting the concrete alkali-aggregate reaction comprises the following compositions: 10 to 50 percent of plasticizing agent, 10 to 70 percent of aluminate and 10 to 30 percent of alkali metal salt or alkaline-earth metal salt, wherein the alkali metal salt or the alkaline-earth metal salt is selected from at least one group of lithium compounds, barium compounds or calcium-magnesium compounds, and the total content of various compositions is 100 percent. The composition can cover or coat active elements of aggregate, prevent or isolate contact reaction between alkali metal ions such as K<+> and Na<+> and the active elements of the aggregate, prevent generation of soluble swelled gel and generation of insoluble compounds, and further eliminate alkali-aggregate reaction and improve the durability of concrete.
Owner:苏笮斌
Diluent for norovirus or sapovirus specimen and method for detecting virus
ActiveUS20060216695A1High detection sensitivityEliminating non-specific factorsMicrobiological testing/measurementImmunoglobulinsFecesDiluent
The present invention relates to a diluent for a Norovirus or Sapovirus specimen, the diluent containing an alkaline buffer of pH 9.0 to 10.0, and to a method for detecting a Norovirus or a Sapovirus through use of the diluent. According to the present invention, a Norovirus or a Sapovirus can be detected in a Norovirus- or Sapovirus-containing specimen such as stool, vomit, body fluid, blood, body tissue, or food, in a convenient manner without use of a special machine such as a centrifuge, with an improved detection sensitivity, and with complete elimination of non-specific factors.
Owner:DENKA CO LTD
Methods and compositions for controlled polypeptide synthesis
InactiveUS7329727B2Elimination reactionEasy to controlPeptide/protein ingredientsGroup 8/9/10/18 element organic compoundsEnd-groupSynthesis methods
Methods and compositions for the generation of polypeptides having varied material properties are disclosed herein. Methods include means for initiating the polymerization of aminoacid-N-carboxyanhydride (NCA) monomer by combining the monomer with an amido-containing metallacycle, for making self assembling amphiphilic block copolypeptides and related protocols for adding oligo(ethyleneglycol) functionalized aminoacid-N-carboxyanhydrides (NCAs) to polyaminoacid chains. Additional methods include means of adding an end group to the carboxy terminus of a polyaminoacid chain by reacting an alloc-protected amino acid amide with a transition metal-donor ligand complex to forming an amido-amidate metallacycle for use in further polymerization reactions. Novel compositions for use in peptide synthesis and design including five and six membered amido-containing metallacycles and block copolypeptides are also disclosed.
Owner:RGT UNIV OF CALIFORNIA
Flow system method for preparing substantially pure nanoparticles, nanoparticles obtained by this method and use thereof
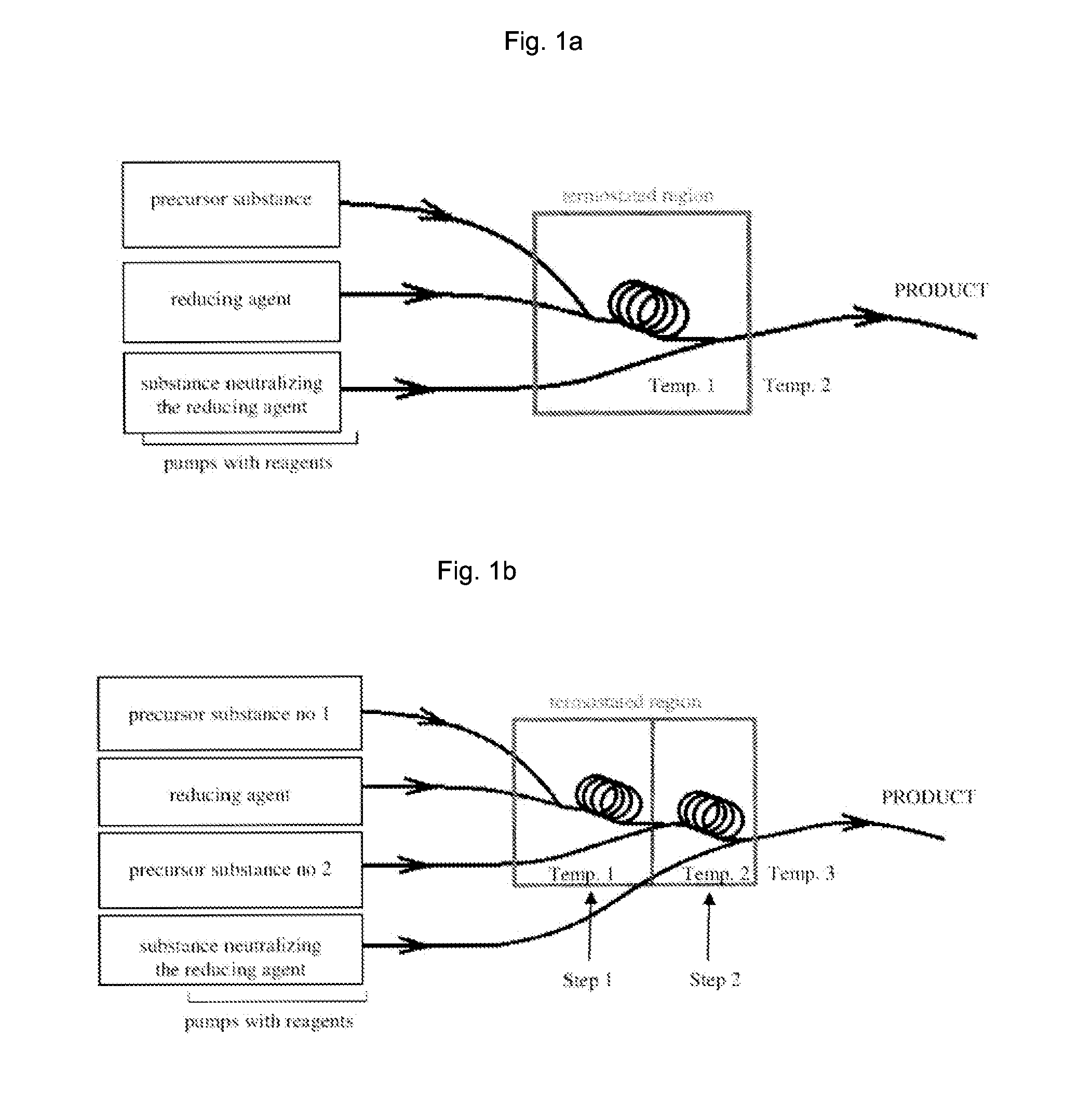
ActiveUS20150011655A1Improve stabilityEliminate needPowder deliveryOrganic active ingredientsNanoparticleContinuous flow
The invention relates to a method of synthesis of substantially pure nanoparticles in a continuous-flow system, in which a precursor substance solution undergoes reduction reaction using a reducing agent solution and nanoparticles are produced, wherein the reduction reaction is terminated by adding an agent neutralizing the reducing agent and a stable nanoparticle colloid is produced. In the method of the invention a need for using surfactants or other organic molecules for nanoparticle stabilization has been eliminated.
Owner:UNIWERSYTET WARSZAWSKI
Medical Devices, Drug Coatings and Methods for Maintaining the Drug Coatings Thereon
InactiveUS20080051884A1Reduce drug toxicityGood curative effectStentsSurgeryBiological bodyCompound (substance)
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:CORDIS CORP
Medical Devices, Drug Coatings and Methods for Maintaining the Drug Coatings Thereon
InactiveUS20080051883A1Reduce drug toxicityGood curative effectStentsSurgeryBiological bodyCompound (substance)
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:CORDIS CORP
Organic additives for improved lifetimes in organic and solution processible electronic devices
InactiveUS20120049120A1Improvement of lifetime characteristicEfficiently reactElectrical apparatusElectroluminescent light sourcesScavengerAntioxidant
Organic additives are used to improve the lifetimes of organic electronic devices, such as electroluminescent devices fabricated from polymer luminescent ink. These additives include moisture getters, thermally-activated organic / inorganic hybrids, radical scavengers, antioxidants, UV stabilizers, and photoretarders. For water and oxygen scavengers, activation at elevated temperatures or through another activation method is preferred. This allows for the handling of the device materials containing the scavenger under a lower temperature condition in air where higher levels of ambiently-supplied water or oxygen may also be present. The invention also improves operational lifetimes as getters, scavengers and similar acting additives serve to reduce detrimental reactive species that transport into the device, are generated during operation, or become reactive during operation due to the presence of excited states or external stimulation by electrical, optical or other means.
Owner:SUMITOMO CHEM CO LTD
Methods and compositions for controlled polypeptide synthesis
InactiveUS20120088848A1Eliminate chain-breaking side reactionEasy to controlSugar derivativesIron group organic compounds without C-metal linkagesEnd-groupSynthesis methods
Methods and compositions for the generation of polypeptides having varied material properties are disclosed herein. Methods include means for initiating the polymerization of aminoacid-N-carboxyanhydride (NCA) monomer by combining the monomer with an amido-containing metallacycle, for making self assembling amphiphilic block copolypeptides and related protocols for adding oligo(ethyleneglycol) functionalized aminoacid-N-carboxyanhydrides (NCAs) to polyaminoacid chains. Additional methods include means of adding an end group to the carboxy terminus of a polyaminoacid chain by reacting an alloc-protected amino acid amide with a transition metal-donor ligand complex to forming an amido-amidate metallacycle for use in further polymerization reactions. Novel compositions for use in peptide synthesis and design including five and six membered amido-containing metallacycles and block copolypeptides are also disclosed.
Owner:RGT UNIV OF CALIFORNIA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com