A method for detecting dronedarone hydrochloride
A hydrochloride and high-performance liquid chromatography technology, which is applied in the field of drug monitoring, can solve problems such as unfavorable production and treatment of diseases, low accuracy of results, and impact on the quality of dronedarone hydrochloride.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~4
[0165] According to the records of Appendix III of the Chinese Pharmacopoeia 2010 edition, the identification of obvious chlorides was carried out. Accurately weigh 10 mg of dronedarone hydrochloride samples purchased from Jiangsu Kangyuan Pharmaceutical Co., Ltd. and dronedarone hydrochloride tablets purchased from Sanofi-Aventis with batch numbers 110401, 110402 and 110403 , add 15 mL each of ethanol and water and 5 mL of ammonia test solution, shake, stand still, and filter, and operate the obtained filtrate according to the method in the Pharmacopoeia to identify the apparent chloride.
[0166] The results showed that the test results of the samples in Examples 1-4 were all positive, and negative without interference.
Embodiment 5~9
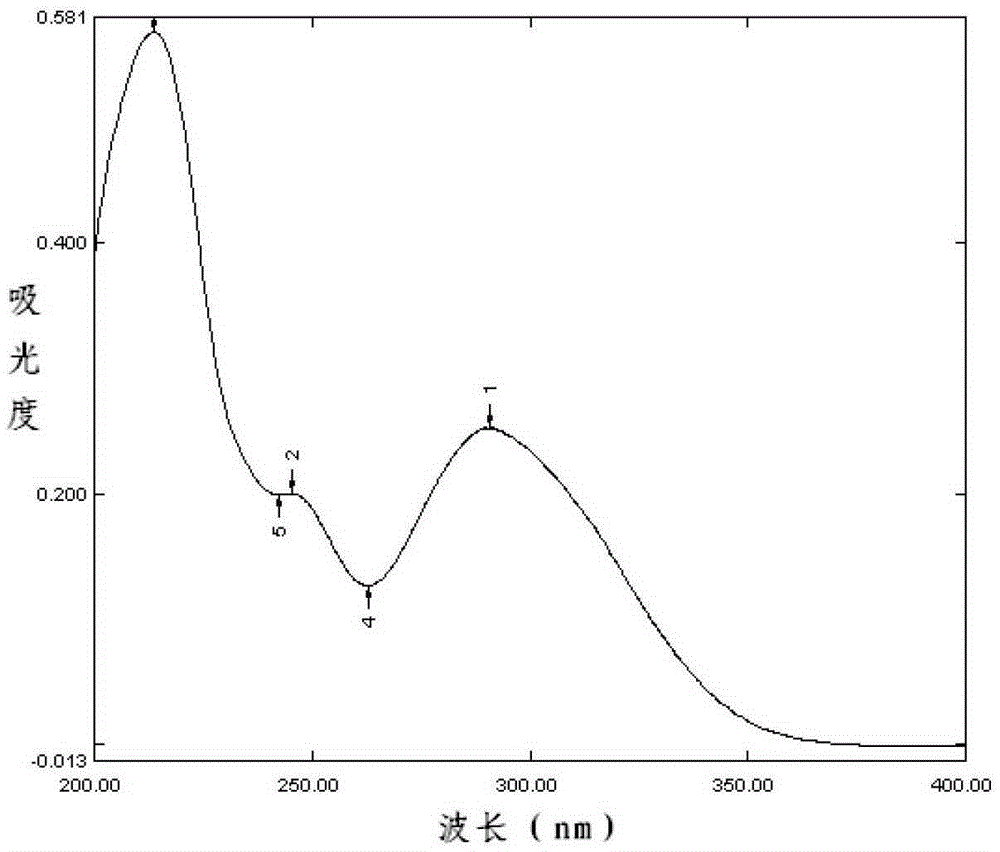
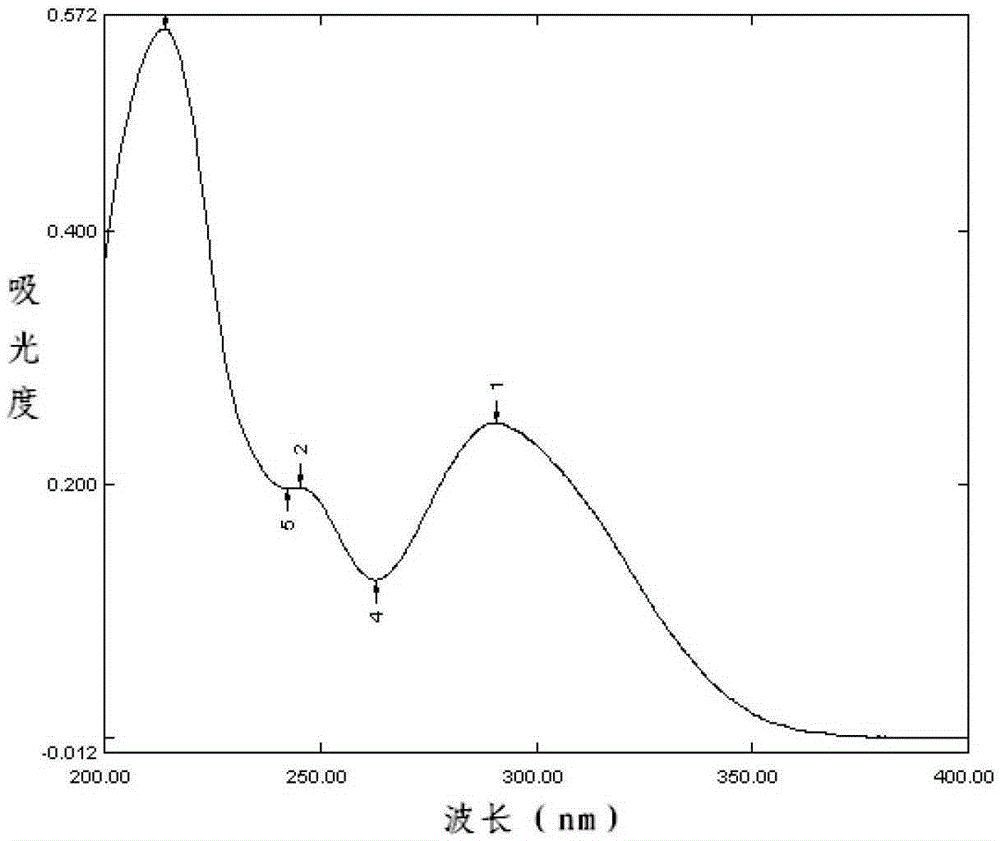
[0168] Accurately weighed 25mg samples of dronedarone hydrochloride purchased from Jiangsu Kangyuan Pharmaceutical Co., Ltd. and dronedarone hydrochloride purchased from Sanofi-Aventis (Sanofi-Aventis) with batch numbers 110401, 110402 and 110403 Tablets and blank excipients, put them in 50mL measuring bottles, add absolute ethanol to dissolve and dilute to the mark, shake well, let it stand, filter; accurately measure 1mL of the filtrate, put it in a 50mL volume In the bottle, add molar concentration to it and be 0.1mol / L hydrochloric acid solution to dissolve and dilute to every 1mL containing the solution of dronedarone hydrochloride 10 μ g, according to the ultraviolet-visible spectrophotometry method that Chinese Pharmacopoeia 2010 edition two appendix IVA record To measure.
[0169] The result is as Figure 1~5 as shown, Figure 1~4 Respectively, the ultraviolet spectrograms of the dronedarone hydrochloride tablets obtained in Examples 5 to 8 of the present invention, ...
Embodiment 10~12
[0174] Add the reference substance of dronedarone hydrochloride into the mobile phase, perform ultrasonic dissolution, and prepare a solution containing about 1 mg of dronedarone hydrochloride per 1 mL to obtain the solution to be tested. The mobile phase is a mixed solution of methanol-acetonitrile-phosphate buffer solution, wherein the phosphate buffer solution is prepared as follows: get 0.136g of potassium dihydrogen phosphate, add 200mL of water to dissolve, adjust the pH value with sodium hydroxide test solution To 7.5, add water to 1000mL, shake well, the volume ratio of methanol-acetonitrile-phosphate buffer solution in the mobile phase is 32:50:18.
[0175] Weigh about 30mg of the blank excipient, put it in a 50mL measuring bottle, add the above-mentioned mobile phase to dissolve and dilute to the mark, shake well to obtain the blank solution.
[0176] Precisely measure 10 μL each of the above mobile phase, blank solution and solution to be tested and inject it into t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com