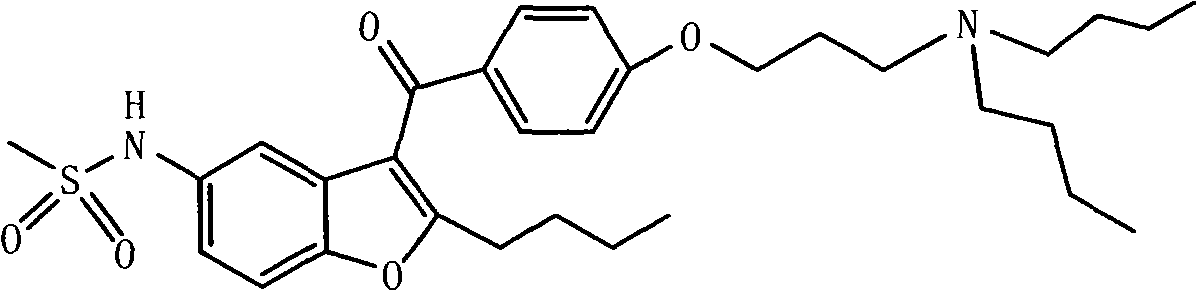
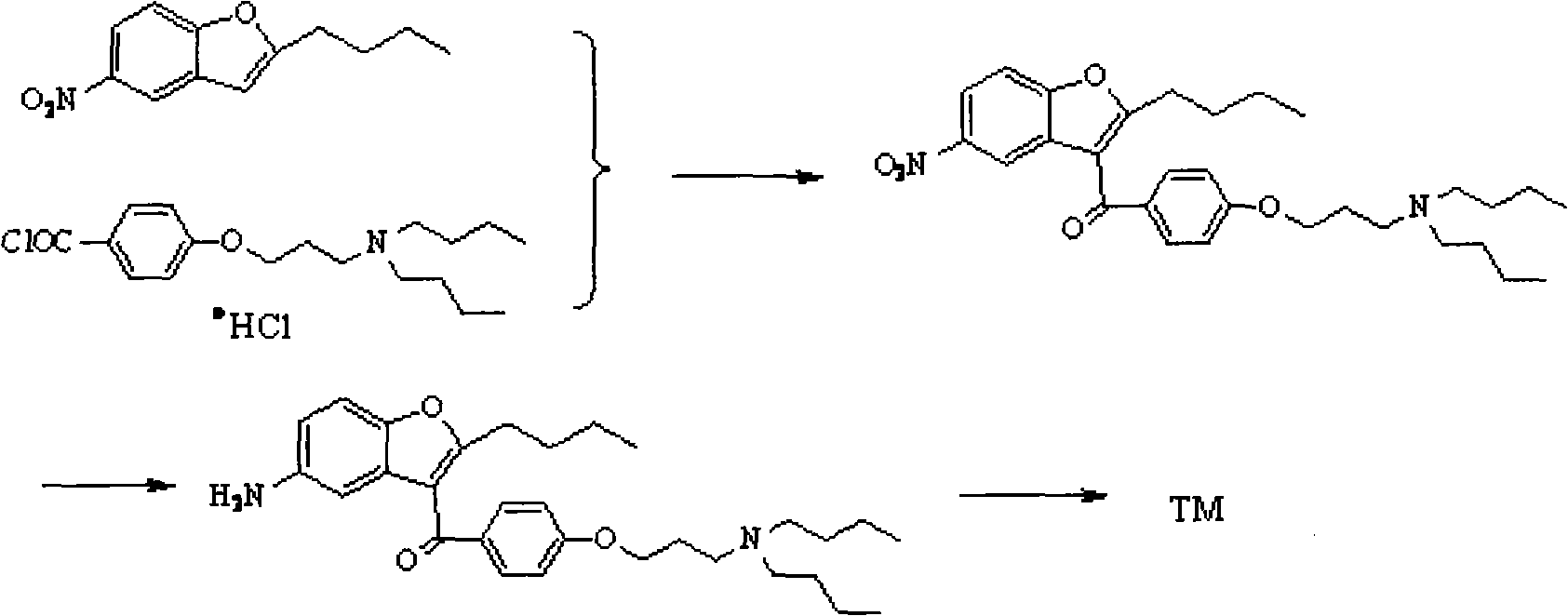
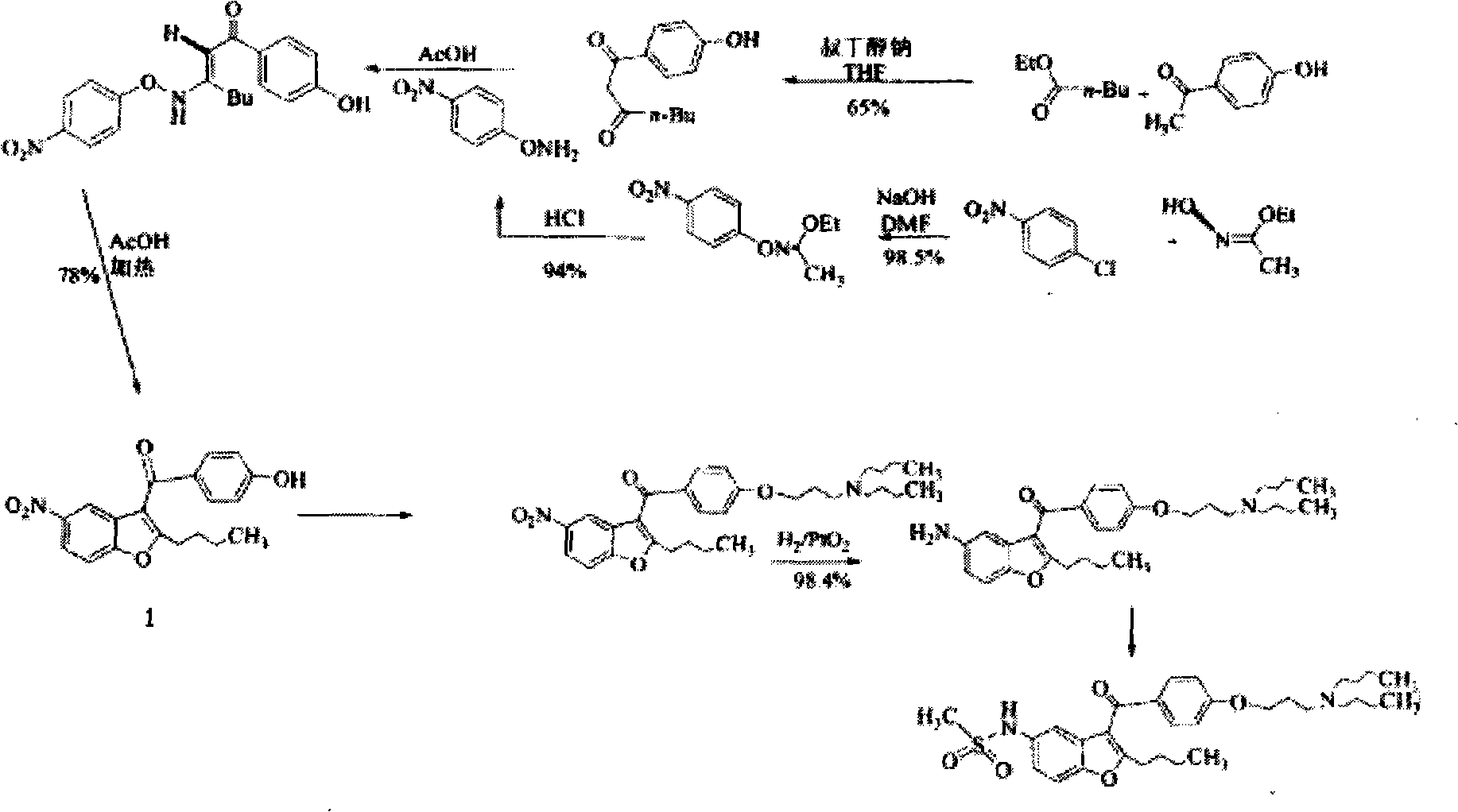
Novel method for synthesizing dronedarone key intermediate
A technology for dronedarone and intermediates, which is applied in the field of synthetic drug dronedarone, and can solve the problems of low purity and heavy process pollution, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: the synthesis of p-acetoxybenzoic acid A
[0028] Put 138g of p-hydroxybenzoic acid into a 2L three-necked flask, add 200ml of acetic anhydride dropwise and stir thoroughly, then slowly add 1ml of concentrated sulfuric acid, heat up vigorously, continue stirring for 10min, and the solid gradually dissolves. Then heat up to 85-90°C, react for 1h, TLC (chloroform:methanol 15:1) monitors the completion of the reaction of raw materials, remove the oil bath, cool and crystallize to 40-50°C, add 700ml of water, cool to room temperature, then ice-water bath (5-15°C) cool, stir for 1 hour, filter, and wash with water until neutral. Air-dried at 55°C for more than 6 hours to obtain 175 g of white solid with a yield of 97%. Melting point: 190~192℃
Embodiment 2
[0029] Embodiment 2: the synthesis of acetoxybenzoyl chloride B by oxalyl chloride method
[0030] At 10-20°C, suspend 90g of p-acetoxybenzoic acid (A) in 130ml of dichloromethane (insoluble state), then add dropwise a mixed solution of 50ml of oxalyl chloride and 20ml of dichloromethane (if bubbles are generated Slowly add 20 drops of DMF (1ml), the temperature is basically kept at 10-20°C, after the drop is completed, the temperature is raised to 25-30°C, and the reaction is until the solution becomes clear (note that adding CaCl 2 Drying tube, waterproof and let the CO, CO produced by the reaction 2 , HCl discharged in time) about 40 ~ 60min. The completion of the reaction was monitored by TLC, and the solvent was evaporated to dryness at 40° C. to obtain 98 g of light yellow oil, which was directly used in the next reaction.
Embodiment 3
[0031] Embodiment 3: Thionyl chloride method synthesizes p-acetoxybenzoyl chloride B
[0032] At 10-20°C, suspend 90g of p-acetoxybenzoic acid (A) in 130ml of dichloromethane (insoluble state), then dropwise add a mixed solution of 43ml of thionyl chloride and 20ml of dichloromethane (if any There are less air bubbles, you can speed up), slowly add 20 drops of DMF (1ml), the temperature is basically maintained at 10-20 ° C, after the drop, the temperature is raised to reflux reaction (note that CaCl is added to the reflux tube) 2 Drying tube, waterproof while letting the SO produced by the reaction 2 , HCl discharged in time), reacted for 3h until the solution became clear. The completion of the reaction was monitored by TLC, and the solvent was distilled off at 40° C. to obtain 99 g of light yellow oil, which was directly used in the next reaction.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com