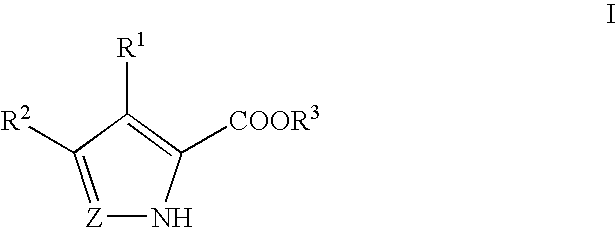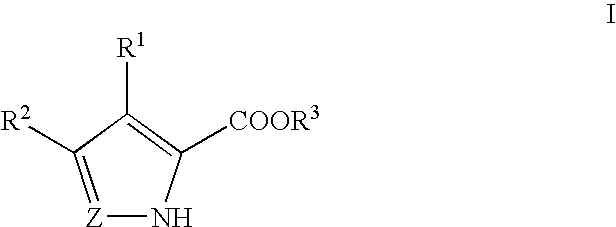Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
203results about How to "Reduce adverse side effects" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
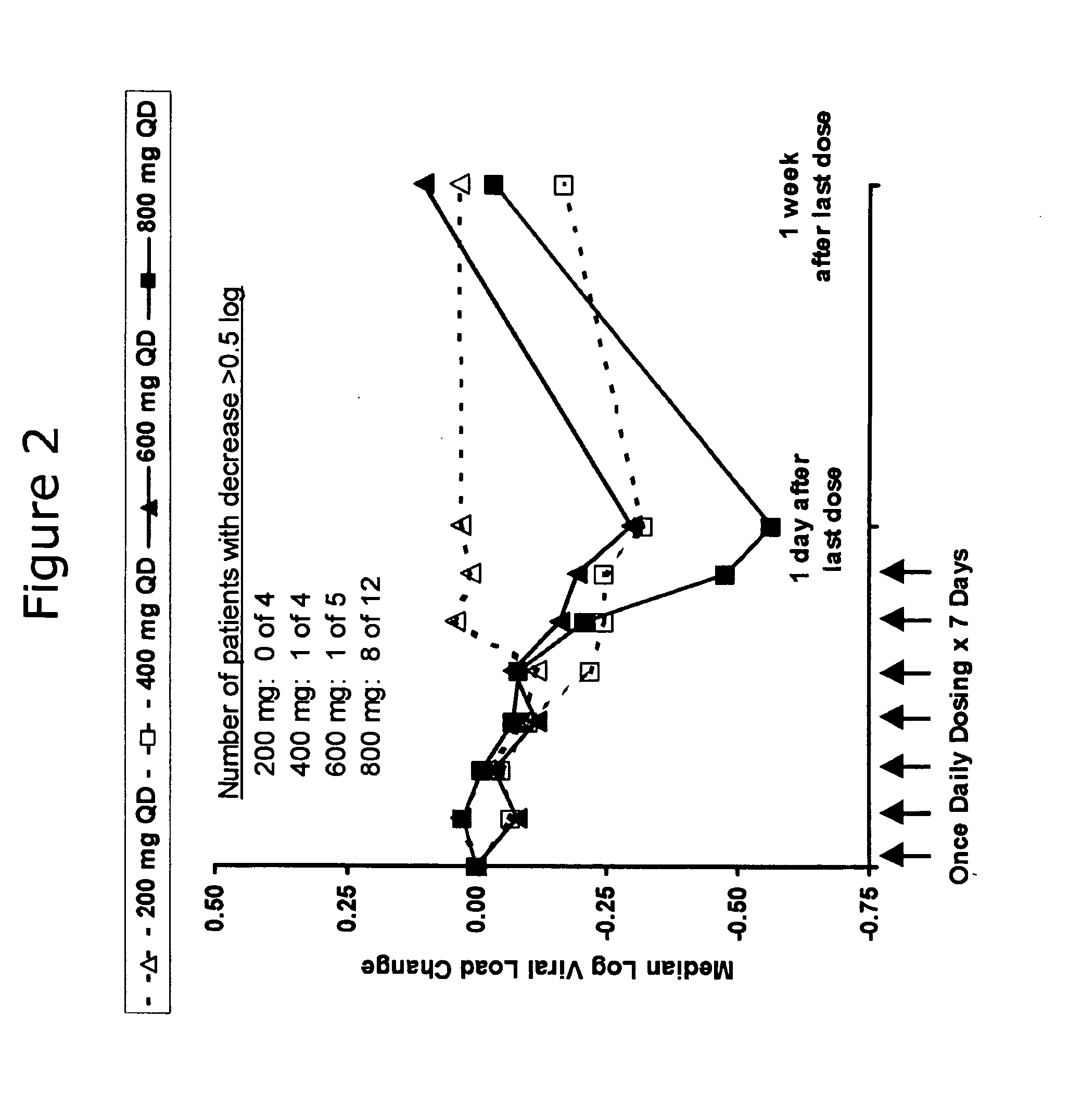
Administration of TLR7 ligands and prodrugs thereof for treatment of infection by hepatitis C virus
InactiveUS20050054590A1Reduce sensitivityAvoid spreadingBiocideDigestive systemHepatitis c viralSide effect
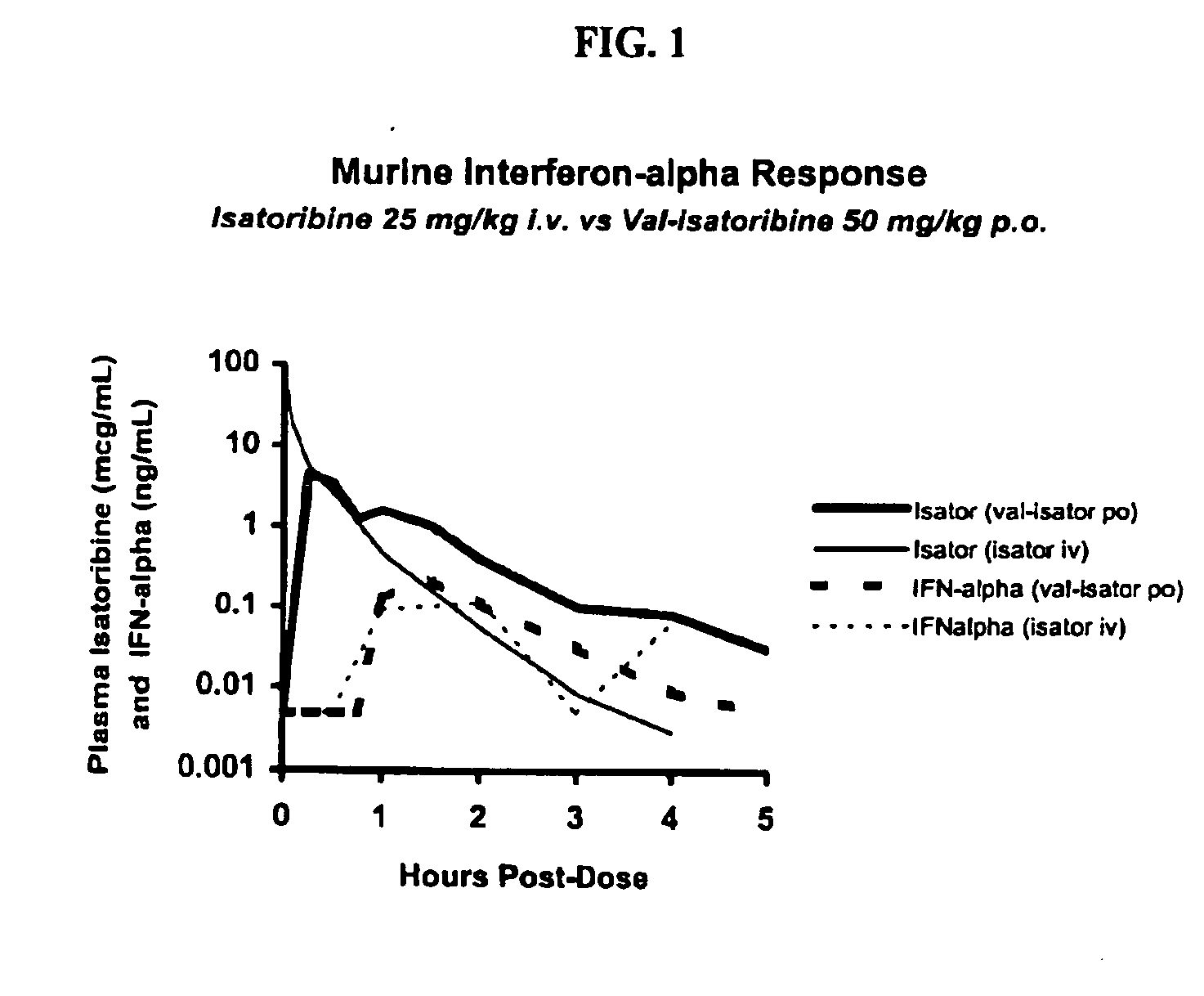
This invention relates to methods for treating or preventing hepatitis C virus infections in mammals using Toll-Like Receptor (TLR)7 ligands and prodrugs thereof. More particularly, this invention relates to methods of orally administering a therapeutically effective amount of one or more prodrugs of TLR7 ligands for the treatment or prevention of hepatitis C viral infection. Oral administration of these TLR7 immunomodulating ligands and prodrugs thereof to a mammal provides therapeutically effective amounts and reduced undesirable side effects.
Owner:ANDADYS PHARMA INC
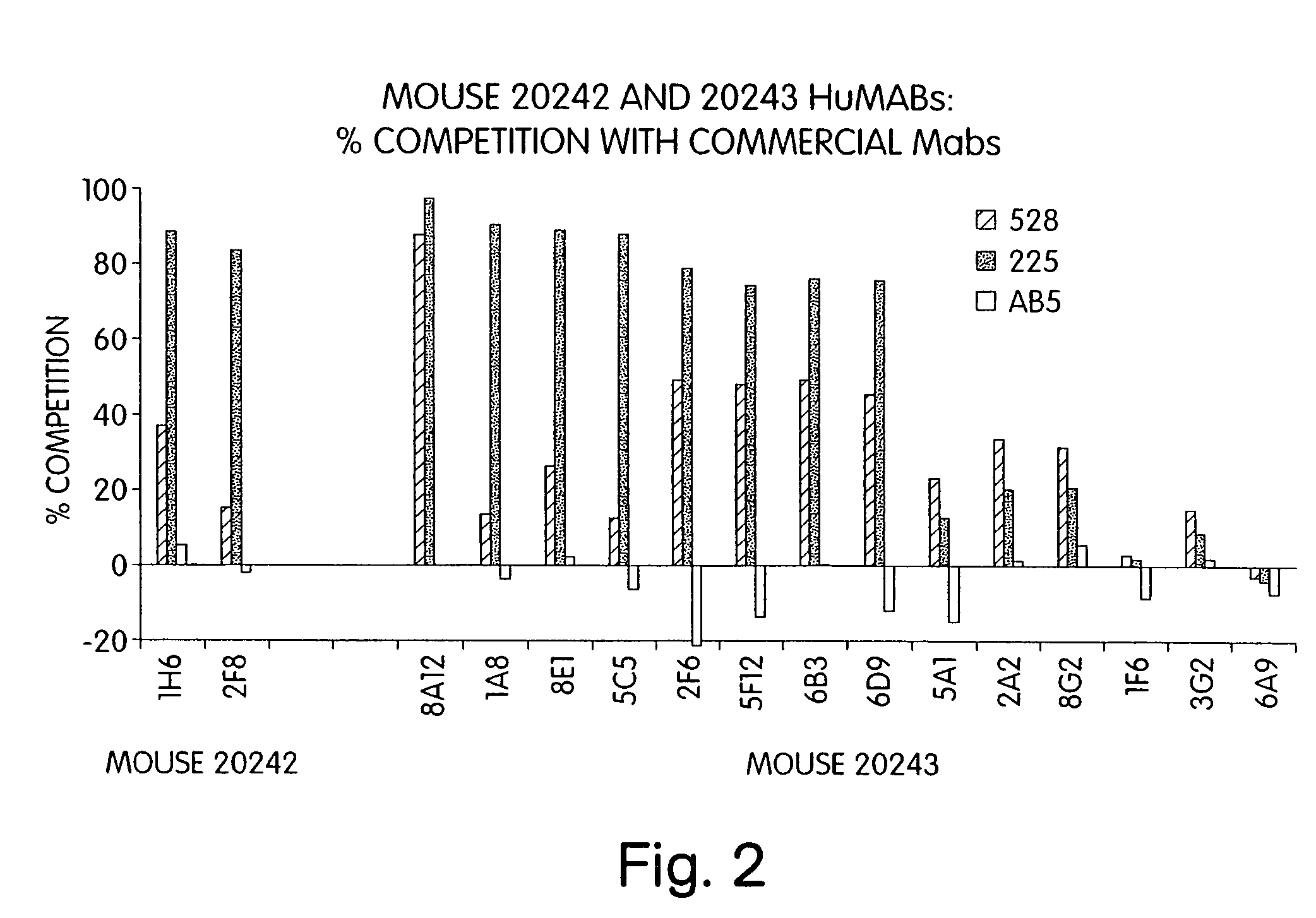
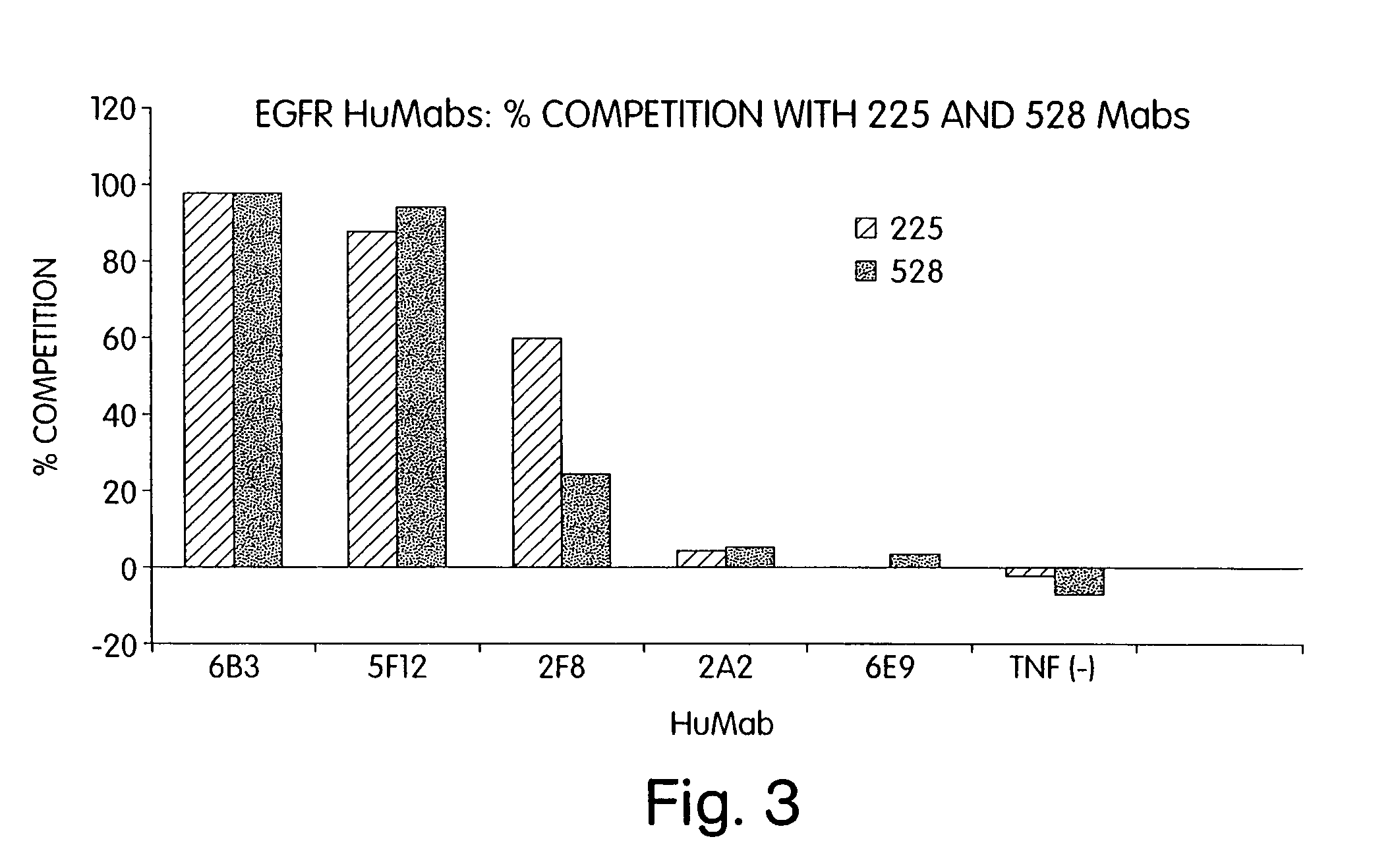
Human monoclonal antibodies to epidermal growth factor receptor (EGFR)
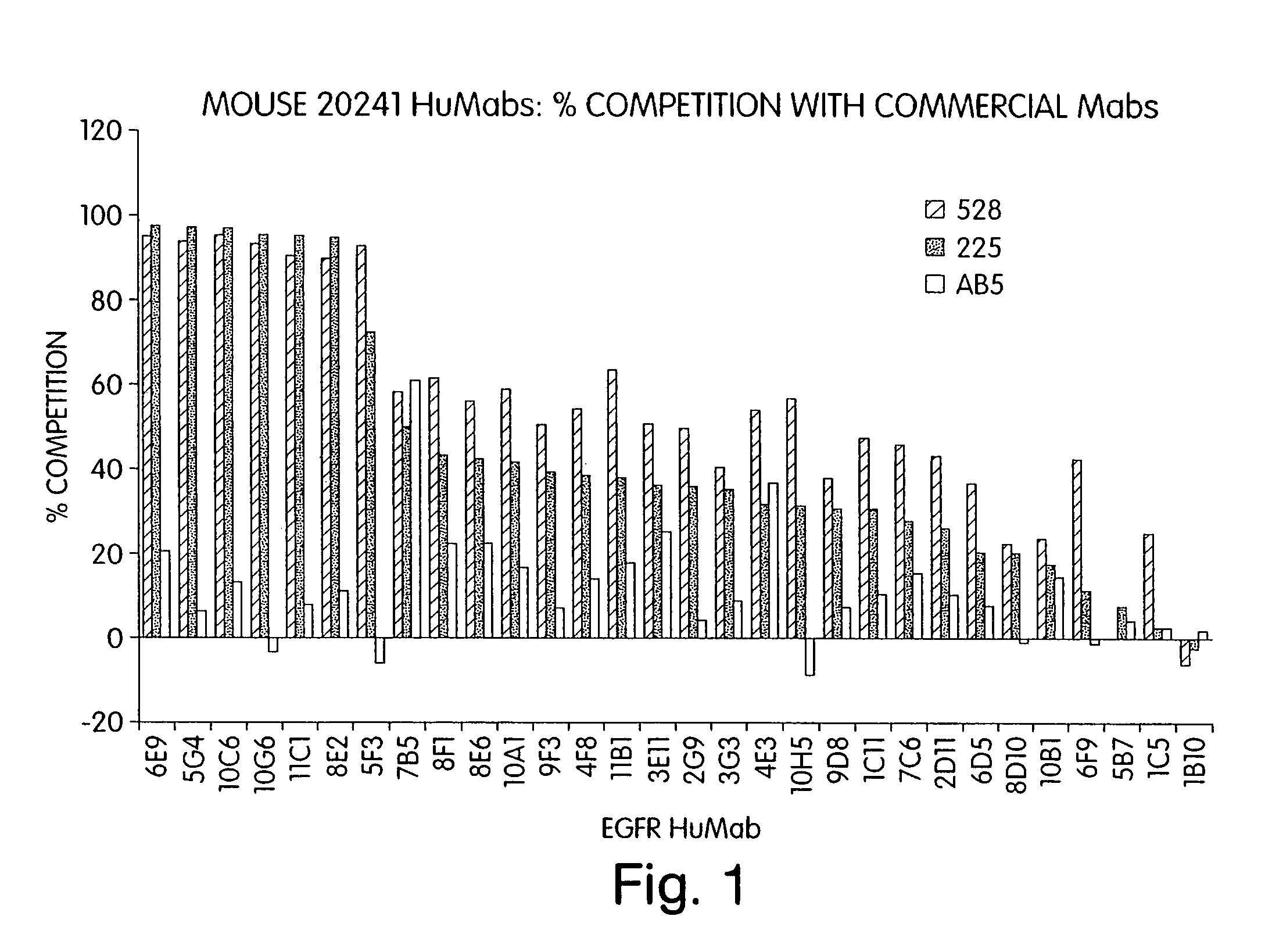
InactiveUS7247301B2Less immunogenicReduce adverse side effectsInorganic active ingredientsImmunoglobulins against cytokines/lymphokines/interferonsV(D)J recombinationHuman epidermal growth factor receptor
Isolated human monoclonal antibodies which specifically bind to human EGFR, and related antibody-based compositions and molecules, are disclosed. The human antibodies can be produced by a transfectoma or in a non-human transgenic animal, e.g., a transgenic mouse, capable of producing multiple isotypes of human monoclonal antibodies by undergoing V-D-J recombination and isotype switching. Also disclosed are pharmaceutical compositions comprising the human antibodies, non-human transgenic animals and hybridomas which produce the human antibodies, and therapeutic and diagnostic methods for using the human antibodies.
Owner:GENMAB INC
Compositions and methods for localized therapy of the eye
InactiveUS20050181017A1Reducing lens concentrationMitigate the cataractogenic potential of these drugsOrganic active ingredientsPowder deliveryTriamcinolone acetonidePolymer
Compositions, and methods of using such compositions, useful for injection into the posterior segments of human or animal eyes are provided. Such compositions include small particles of a poorly soluble therapeutic agent that facilitates formation of concentrated regions of the therapeutic agent in the retinal pigmented epithelium of an eye. The particles are formed by combining a therapeutic agent with an ophthalmically acceptable polymer component. The particles have sizes less than about 3000 nanometers, and in some cases, less than about 200 nanometers. One example of a composition includes particles of triamcinolone acetonide and hyaluronic acid have a size less than about 3000 nanometers.
Owner:ALLERGAN INC
Administration of TLR7 ligands and prodrugs thereof for treatment of infection by hepatitis C virus
InactiveUS7576068B2Reduce adverse side effectsReduce adverse effectsBiocideDigestive systemOral medicationSide effect
This invention relates to methods for treating or preventing hepatitis C virus infections in mammals using Toll-Like Receptor (TLR)7 ligands and prodrugs thereof. More particularly, this invention relates to methods of orally administering a therapeutically effective amount of one or more prodrugs of TLR7 ligands for the treatment or prevention of hepatitis C viral infection. Oral administration of these TLR7 immunomodulating ligands and prodrugs thereof to a mammal provides therapeutically effective amounts and reduced undesirable side effects.
Owner:ANDADYS PHARMA INC
Methods for administering weight loss medications
InactiveUS20080110792A1Good curative effectReduce adverse side effectsSmall article dispensingPill deliveryInternal medicineDrug
Methods and systems for administration of pharmaceuticals using a unit dosage package that includes a first unit dosage that has a first drug and a second drug, a second unit dosage that has the first drug and the second drug, where the second unit dosage includes a different amount of the second drug than the first unit dosage and a unit dosage package is configured to hold the first unit dosage and the second unit dosage. In preferred embodiments the methods and systems are used for administration of weight loss medications.
Owner:OREXIGEN THERAPEUTICS INC
Methods of administering/dosing CD2 antagonists for the prevention and treatment of autoimmune disorders or inflammatory disorders
InactiveUS20030068320A1Symptoms improvedGood curative effectAntipyreticAnalgesicsSide effectAutoimmune disease
The present invention provides compositions for the prevention or treatment of an autoimmune disorder or an inflammatory disorder in a subject comprising one or more CD2 antagonists. In particular, the invention provides methods for preventing or treating an autoimmune disorder or an inflammatory disorder in a subject comprising administering one or more CD2 binding molecules to said subject. The present invention provides doses of CD2 binding molecules and methods of administration that result in improved efficacy, while avoiding or reducing the adverse or unwanted side effects associated with the administration of an agent that induces the depletion of peripheral blood lymphocytes.
Owner:MEDIMMUNE LLC
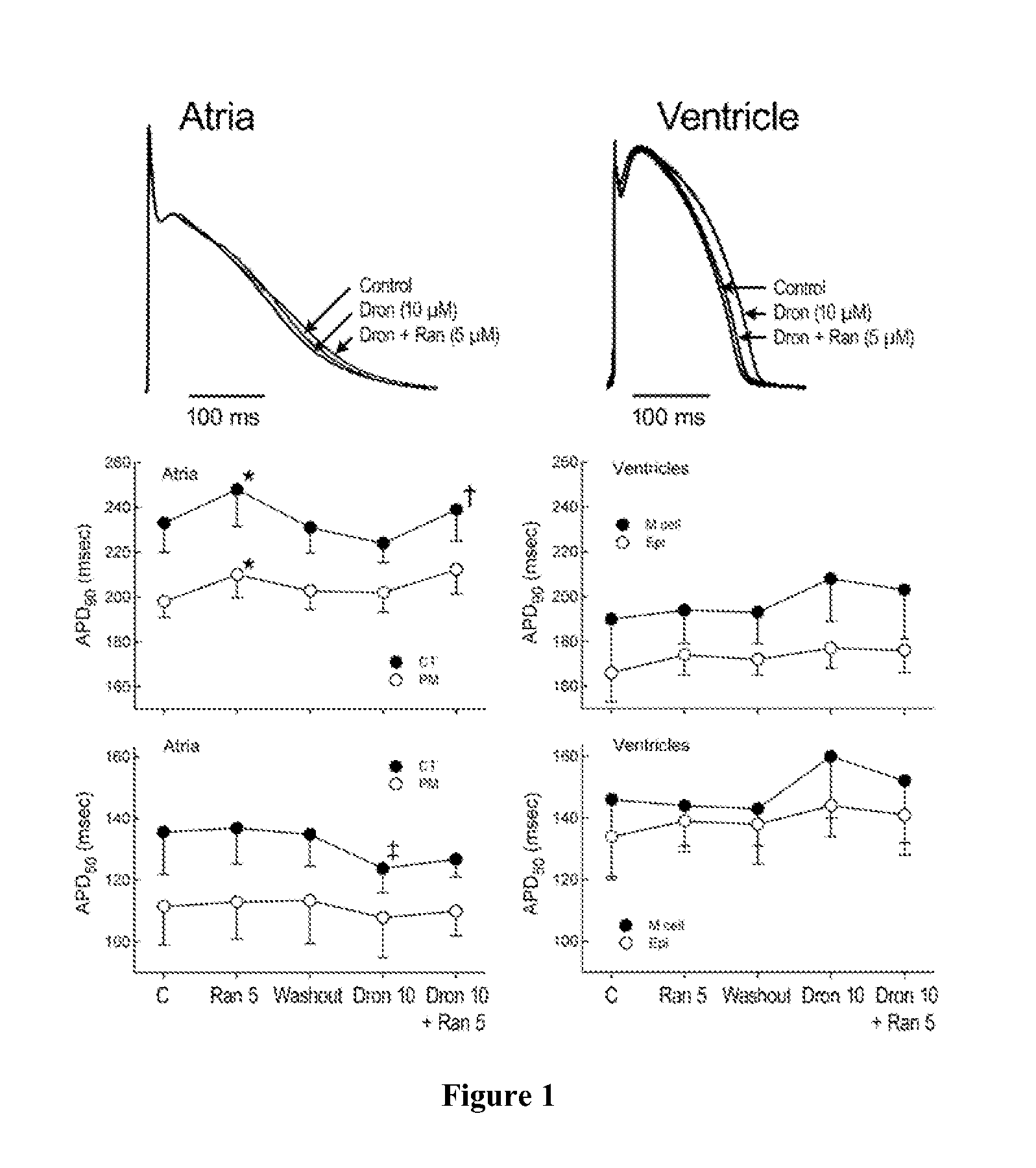
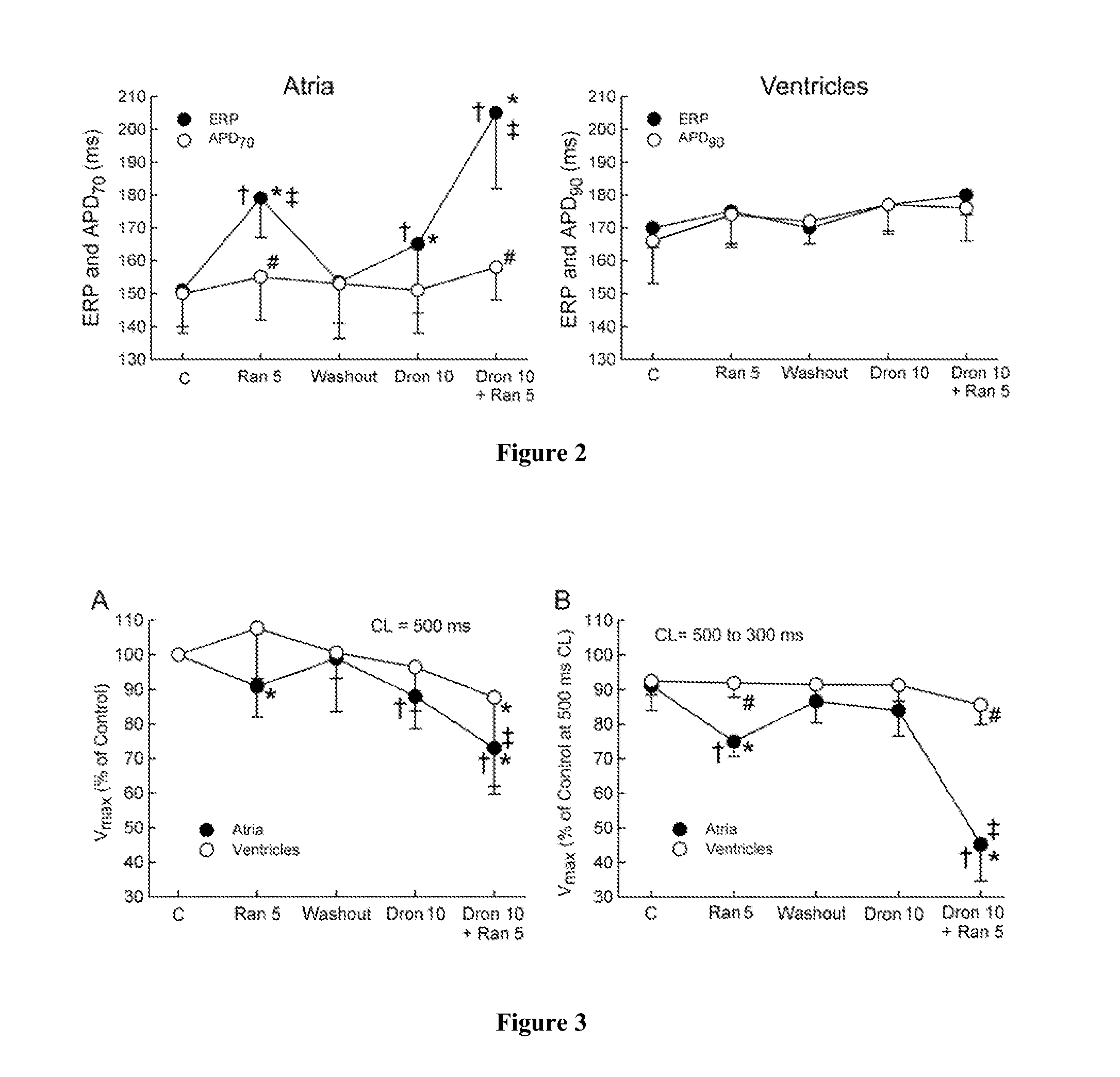
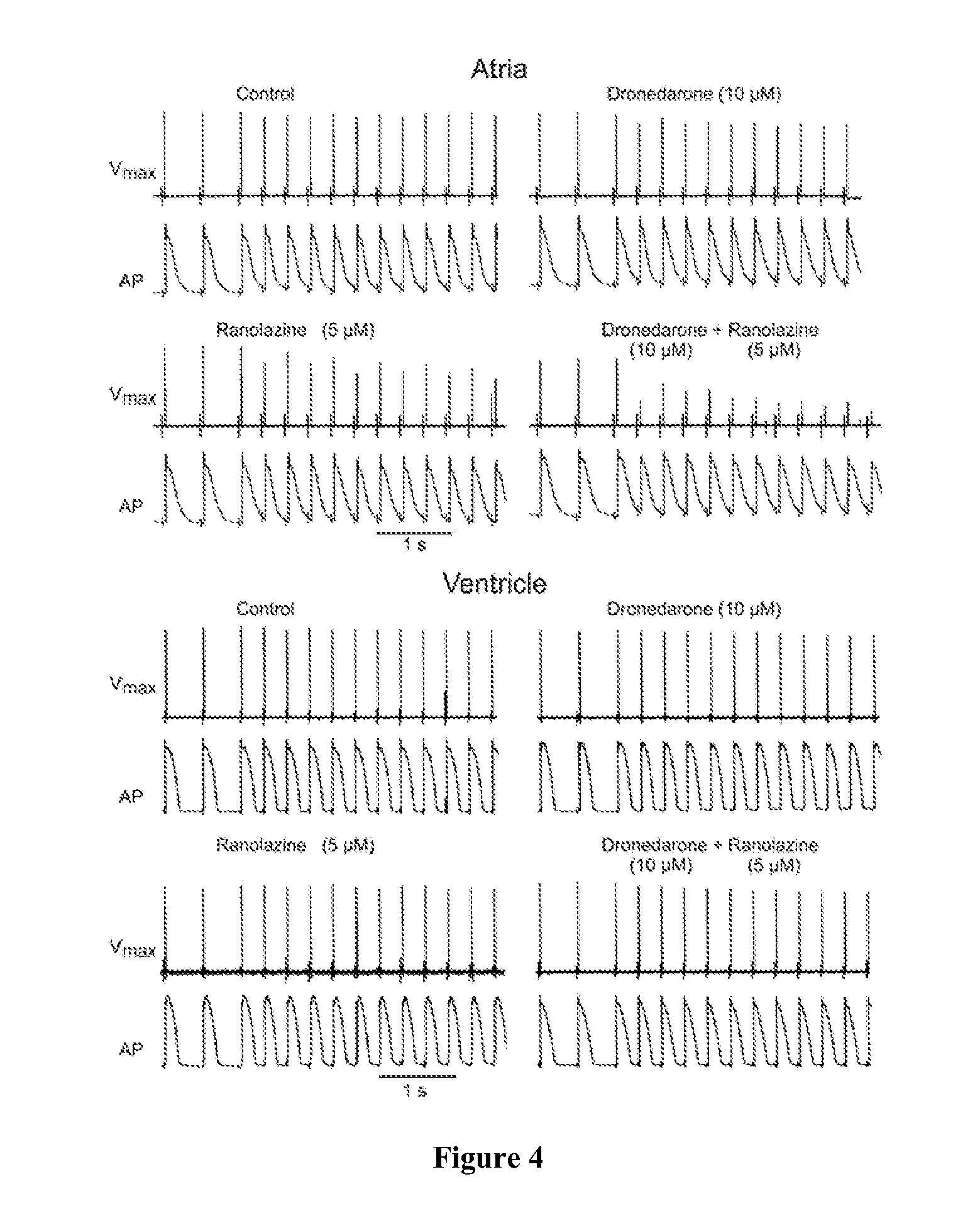
Method of treating atrial fibrillation
InactiveUS20110183990A1Reduce the amplitudeReduce adverse side effectsBiocideAnimal repellantsDronedaroneRanolazine
The present invention relates to a method for the treatment or prevention of atrial fibrillation and / or atrial flutter comprising coadministration of a synergistically therapeutic amount of dronedarone or a pharmaceutically acceptable salt or salts thereof and a synergistically therapeutic amount of ranolazine or a pharmaceutically acceptable salt or salts thereof. Also provided are methods for modulating ventricular and atrial rhythm and rate. This invention also relates to pharmaceutical formulations that are suitable for such combined administration.
Owner:GILEAD SCI INC
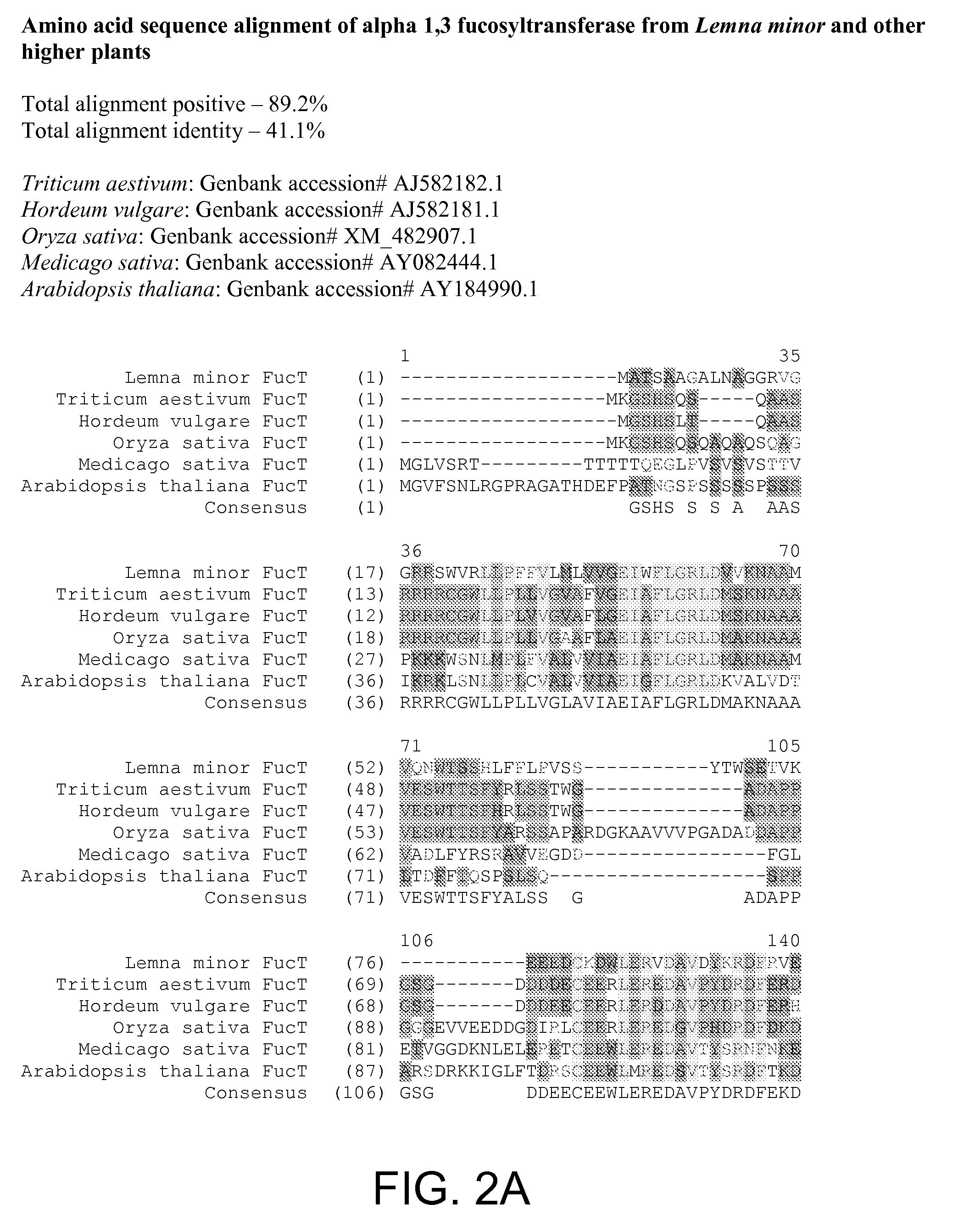
Compositions and methods for humanization and optimization of n-glycans in plants
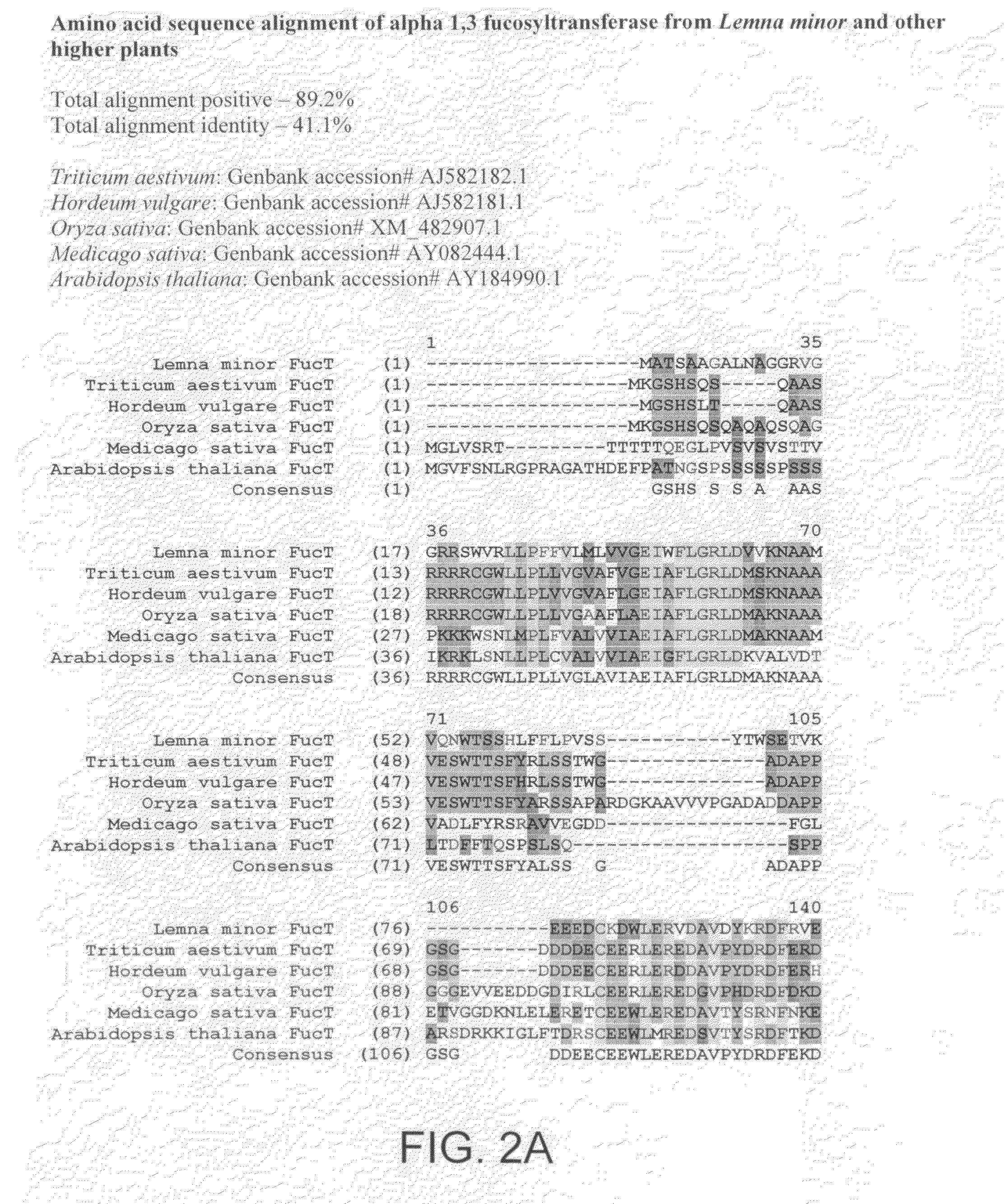
InactiveUS20080066200A1Function increaseHigh antibody activityAntipyreticAnalgesicsGrowth plantGlycan
Methods for altering the N-glycosylation pattern of proteins in higher plants are provided. The methods comprise introducing into the plant a recombinant construct that provides for the inhibition of expression of α1,3-fucosyltransferase (FucT) and β1,2-xylosyltransferase (XylT) in a plant. Use of these constructs to inhibit or suppress expression of both of these enzymes, and isoforms thereof, advantageously provides for the production of endogenous and heterologous proteins having a “humanized” N-glycosylation pattern without impacting plant growth and development. Stably transformed higher plants having this protein N-glycosylation pattern are provided. Glycoprotein compositions, including monoclonal antibody compositions, having substantially homogeneous glycosylation profiles, and which are substantially homogeneous for the G0 glycoform, are also provided.
Owner:SYNTHON BIOPHARMACEUTICALS BV +1
Unit Dose Drug Delivery Platform
ActiveUS20100331765A1Increase ease of administrationAdequate doseMedical devicesMedical applicatorsNoseDosing drugs
The delivery systems of the present disclosure are configurable to administer either single-dose or multiple-doses of one or more substances to a user, for example to the eye, nose, mouth, ear or rectum of the user. The precise and repeatable dosing features of the presently disclosed delivery systems overcome many of the disadvantages associated with known methods for dispensing substances to, for example, the eye of a user. The delivery systems administer precise doses of a substance to a precise location from unit dosage forms that may be single-dose or multiple-dose unit dosage forms, which may be externally or internally pierced.
Owner:MYSTIC PHARMA INC
Sustained release formulations
InactiveUS20060280789A1Reduce in quantityImprove complianceBiocidePill deliveryDonepezilCholinesterase
The invention provides sustained release formulations of basic drugs, stereoisomers of basic drugs, pharmaceutically acceptable salts of basic drugs, and pharmaceutically acceptable salts of stereoisomers of basic drugs. The basic drugs may be anti-dementia drugs, such as cholinesterase inhibitors or memantine. In one embodiment, the cholinesterase inhibitor is donepezil.
Owner:EISAI CO LTD
Glycan-optimized Anti-cd20 antibodies
InactiveUS20090060921A1High antibody activityEnhanced effector functionImmunoglobulins against animals/humansVaccinesAntigenFucosylation
Glycan-optimized monoclonal antibodies that specifically bind CD20 antigen and which have improved effector function are provided. The anti-CD20 antibodies of the invention have a glycosylation pattern that results in an antibody composition having predominately the G0 glycoform, and thus comprise N-glycans that lack fucose (i.e., afucosylated) and galactose residues attached thereto. In some embodiments, these anti-CD20 antibodies comprise the light chain and heavy chain sequences of the rituximab anti-CD20 antibody, and thus represent afucosylated rituximab. Methods for producing these glycan-optimized anti-CD20 antibodies are also provided.
Owner:SYNTHON BIOPHARMACEUTICALS BV
Plaster enclosing packaging bag
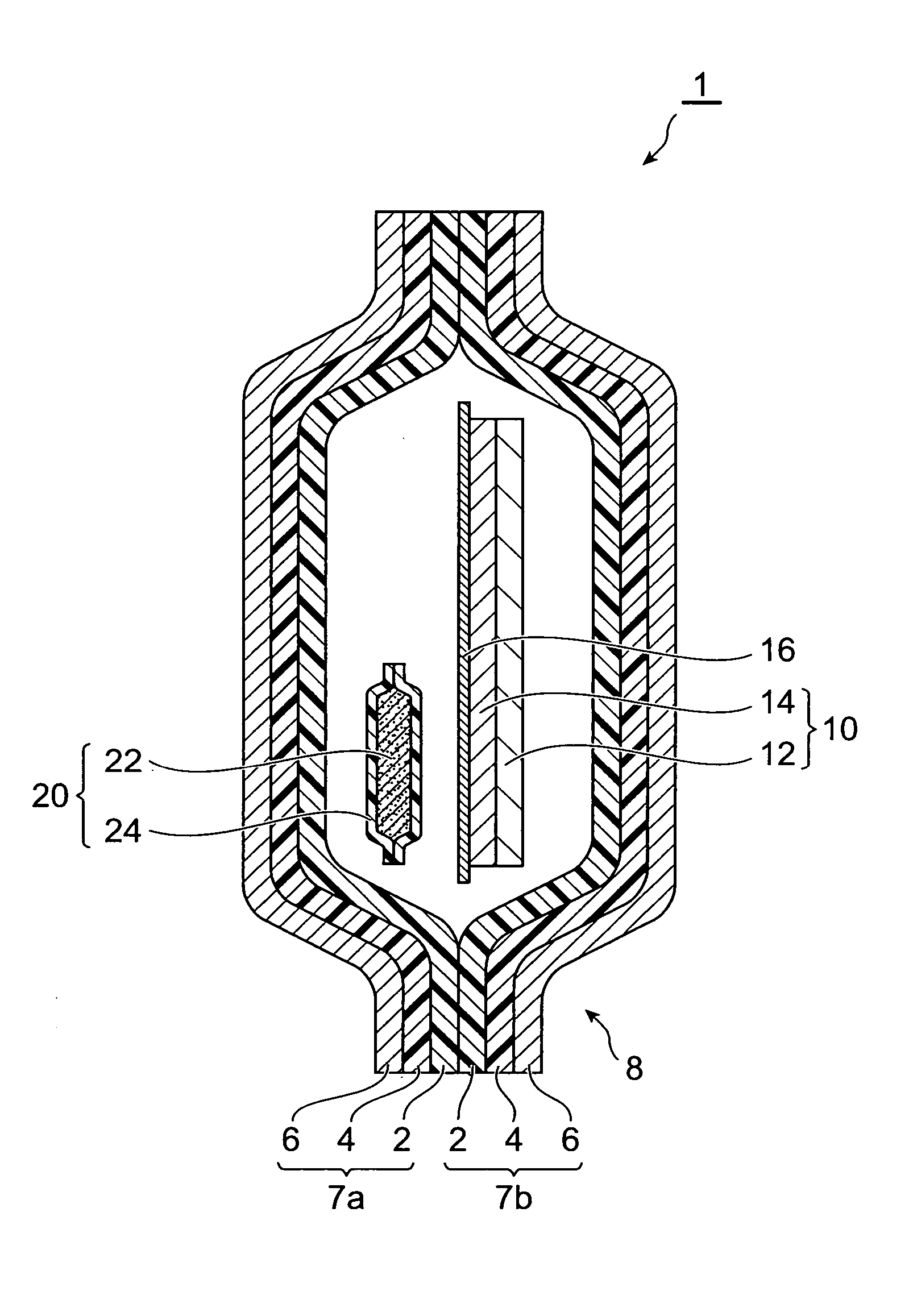
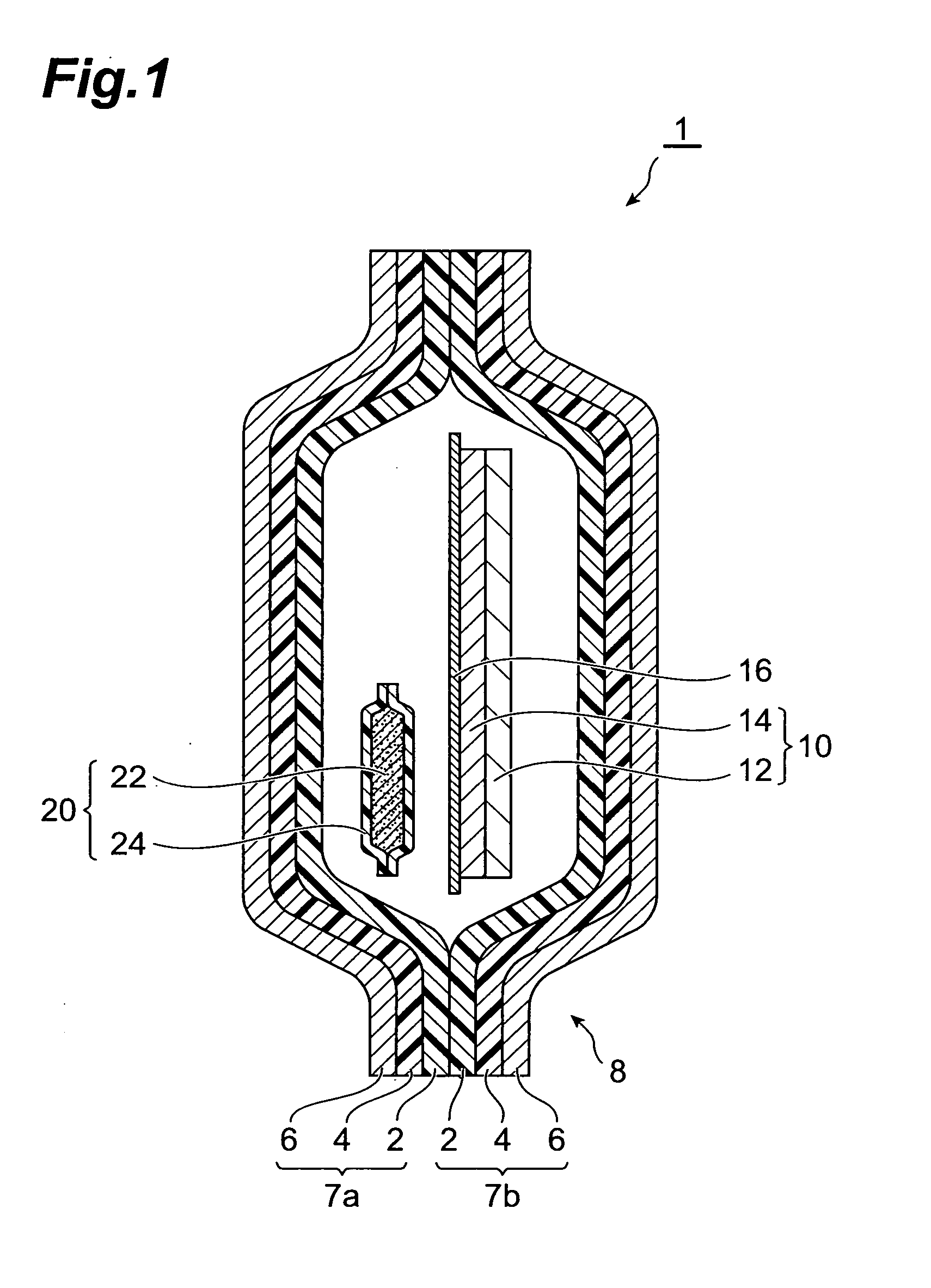
InactiveUS20070158227A1Satisfactory adhesionLittle skin irritationOrganic active ingredientsDiagnosticsDesiccantBiomedical engineering
An object of the present invention is to provide a patch-containing packaging pouch which enables stable storage of a patch containing bisoprolol in a pressure-sensitive adhesive layer. A packaged desiccant 1 of the present invention has a packaging pouch 8 composed of a pair of laminated package members 7a and 7b arranged in mutual opposition, and a patch 10 housed in the space within this packaging pouch 8. Patch 10 is provided with a support 12 and a pressure-sensitive adhesive layer 14 laminated substantially over the entire surface of one side of this support 12. In addition, the pressure-sensitive adhesive layer is composed of a pressure-sensitive adhesive composition containing a pressure-sensitive adhesive and bisoprolol or pharmaceutically acceptable salt thereof. Moreover, a packaged desiccant 20 is housed within packaging pouch 8. The relative humidity within packaging pouch 8 is maintained at 25% or less in a patch-containing packaging pouch 1 having this constitution.
Owner:HISAMITSU PHARM CO INC
Extended release of neuregulin for improved cardiac function
InactiveUS20070190127A1Good effectReduce adverse side effectsSenses disorderNervous disorderNeuregulinMedicine
The present invention provides extended release compositions comprising neuregulin for preventing, treating or delaying various diseases or disorders. The present invention also provides methods for preventing, treating or delaying various diseases or disorders by extended release of neuregulin.
Owner:ZENSUN (SHANGHAI) SCI & TECH CO LTD
Pharmaceutical composition for angiotensin II-mediated diseases
InactiveUS6228874B1Reduce adverse side effectsUndesirable side-effectBiocideAnimal repellantsPhenyl groupDisease
This invention relates to a pharmaceutical composition for angiotensin II-mediated diseases, which comprises a compound having angiotensin II antagonistic activity of the formulawherein R1 is H or an optionally substituted hydrocarbon residue; R2 is an optionally esterified carboxyl group; R3 is a group capable of forming an anion or a group convertible thereinto; X is a covalent bond between the 2 phenyl rings or a spacer having a chain length of 1 to 2 atoms as the linear moiety between the adjoining phenylene group and phenyl group; n is 1 or 2; the ring A is a benzene ring having 1 or 2 optional substituents in addition to R2; and Y is a bond, -O-, -S(O)m- (wherein m is 0, 1 or 2) or -N(R4)- (wherein R4 is H or an optionally substituted alkyl group), or a pharmaceutically acceptable salt thereof in combination with a compound having diuretic activity or a compound having calcium antagonistic activity.
Owner:TAKEDA PHARMACEUTICALS CO LTD
Titration dosing regimen for controlled release tramadol
ActiveUS7413749B2Reduce adverse side effectsImprove toleranceOrganic active ingredientsNervous disorderDosing regimenControlled release
A titration dosing regimen for the administration of controlled release tramadol analgesic to patients. The titration dosing regimen provides a significant reduction in the occurrence of adverse effects from the introduction of controlled released tramadol dosing, thus increasing patient compliance and medication tolerability.
Owner:PURDUE PHARMA LP
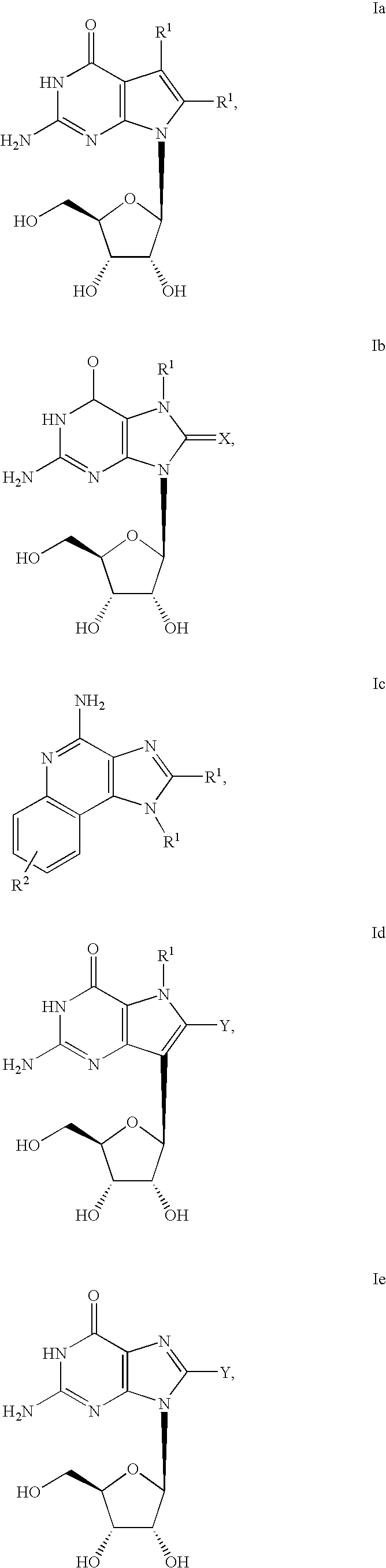
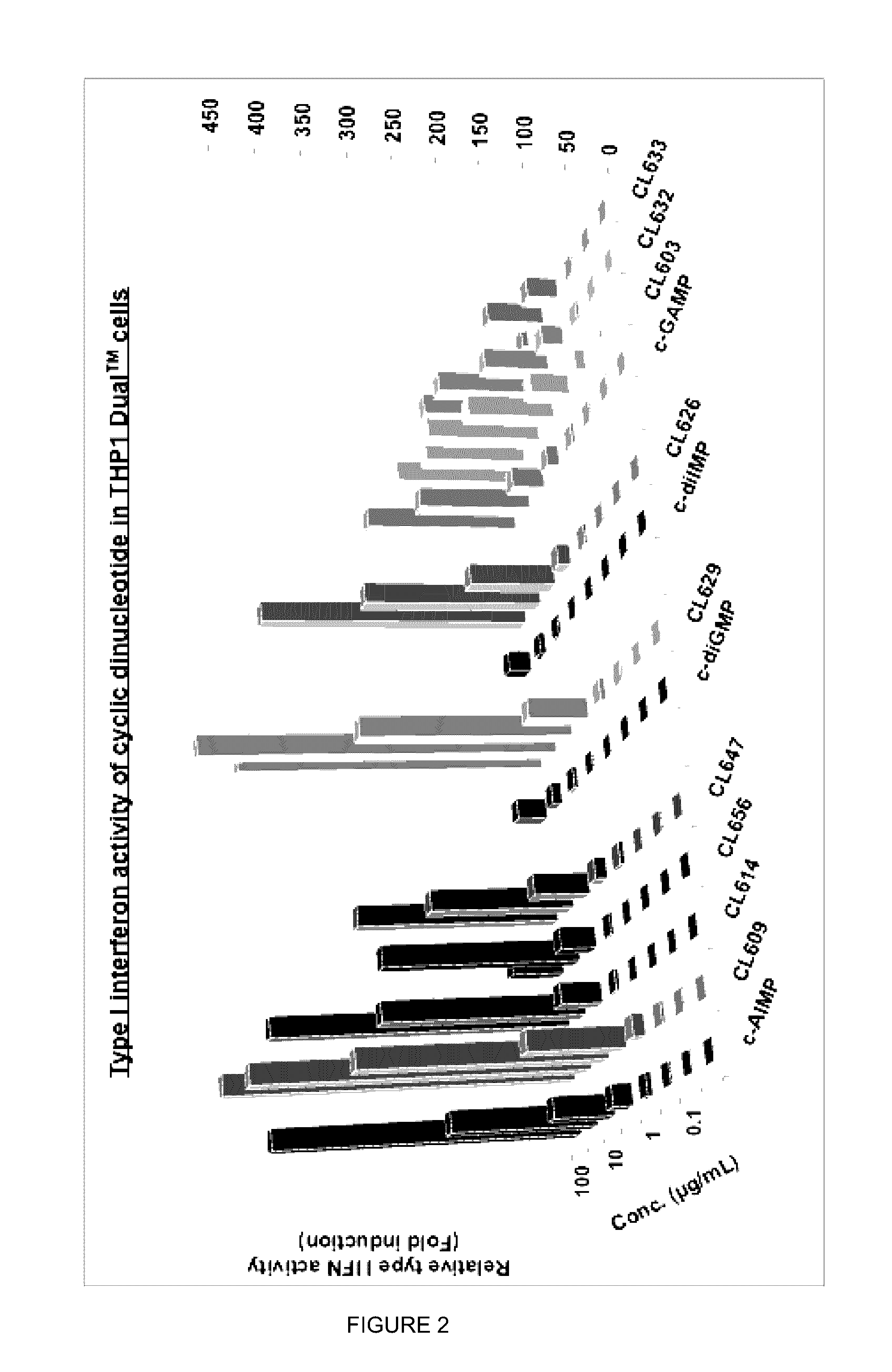
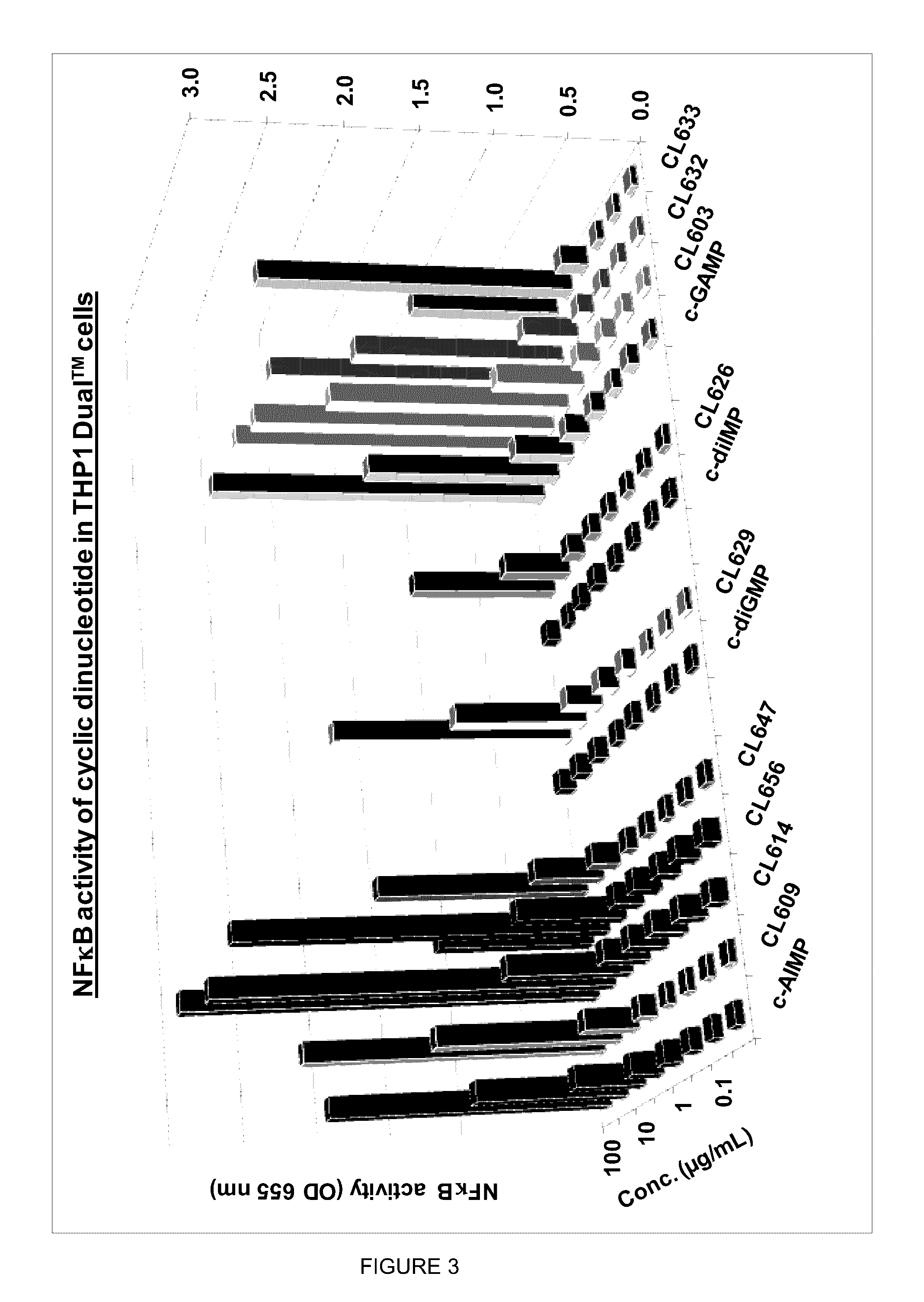
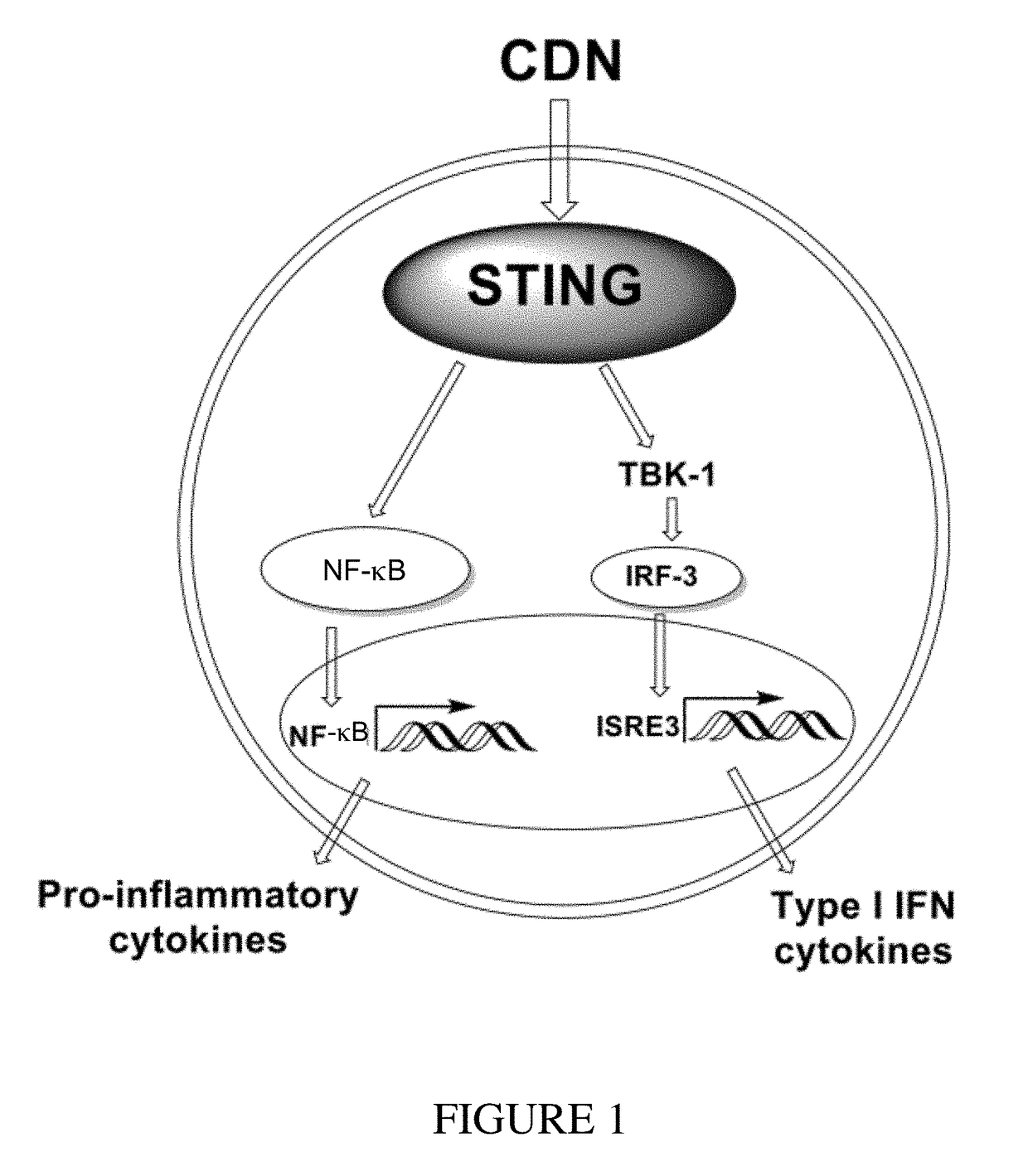
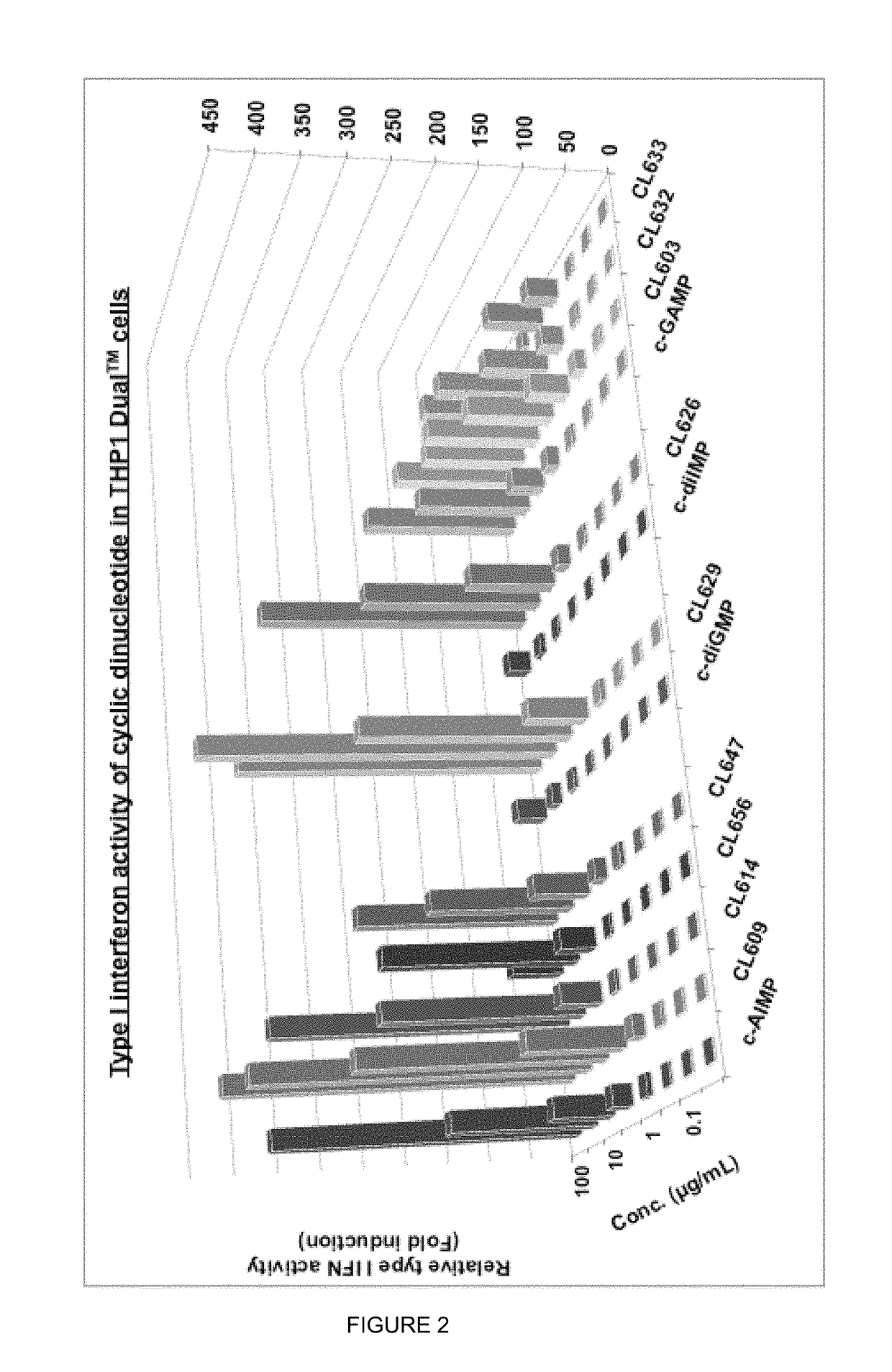
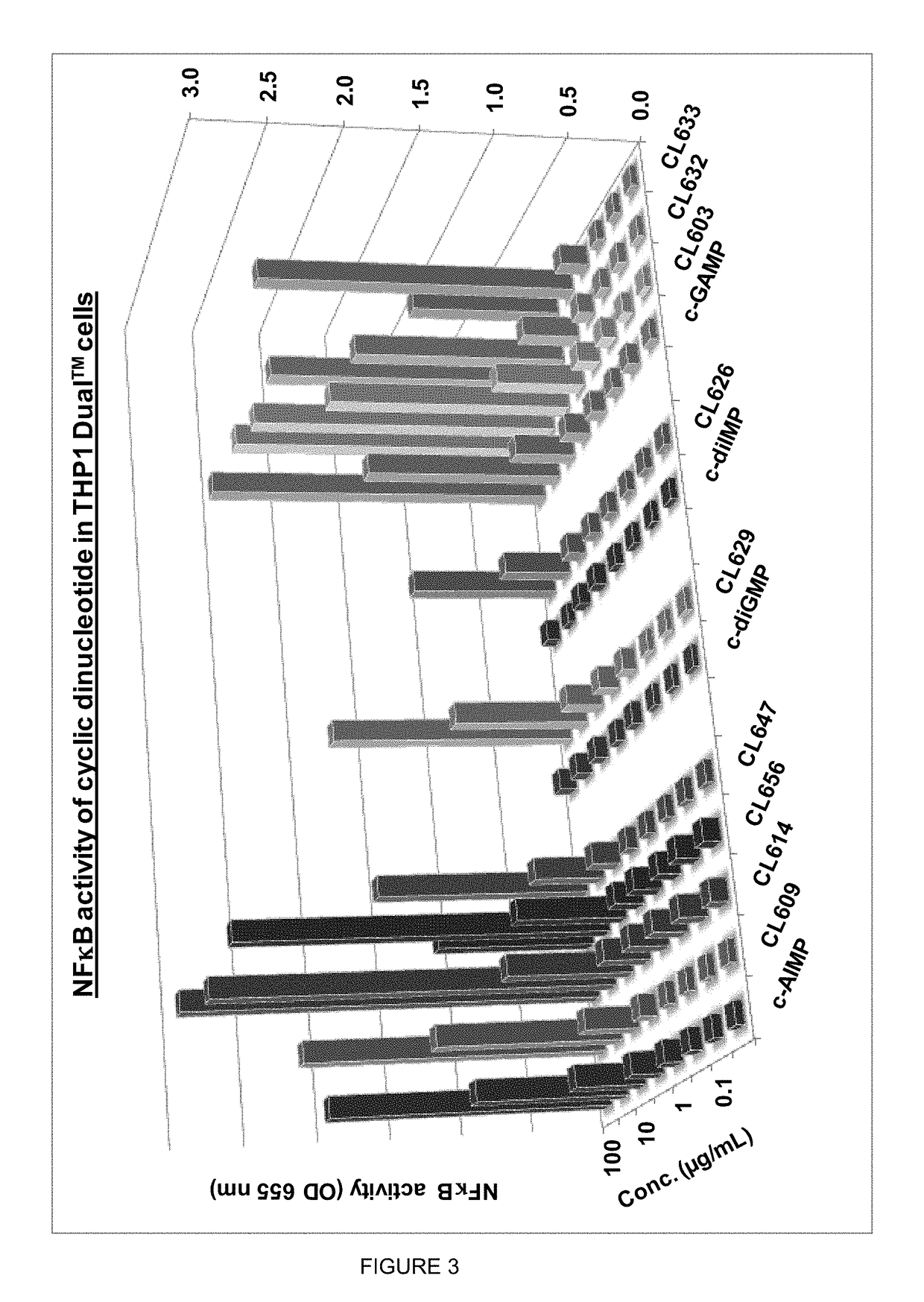
Cyclic dinucleotides for cytokine induction
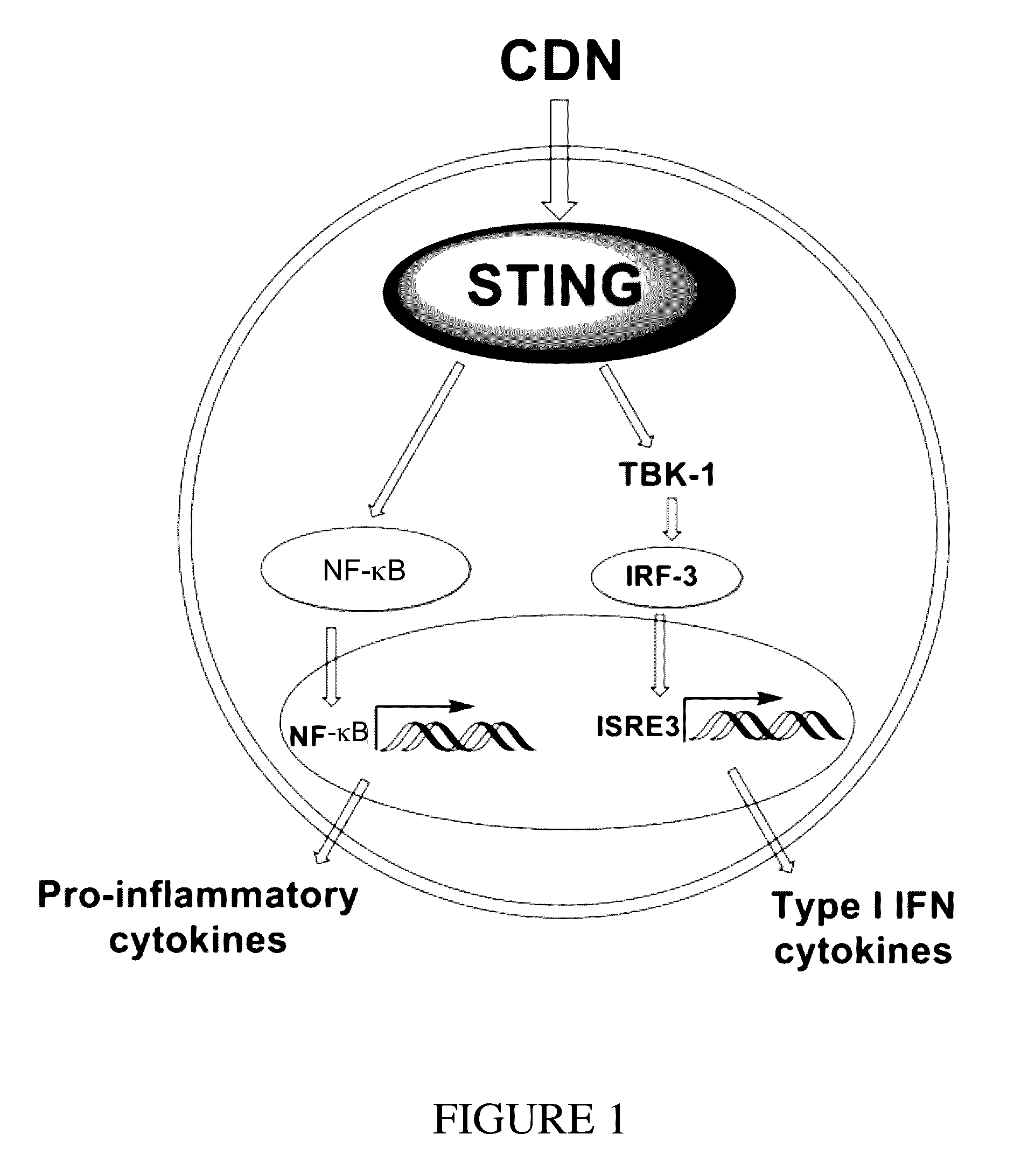
ActiveUS20160362441A1Improve the immunityDelay EliminationAntibacterial agentsOrganic active ingredientsPurineIn vivo
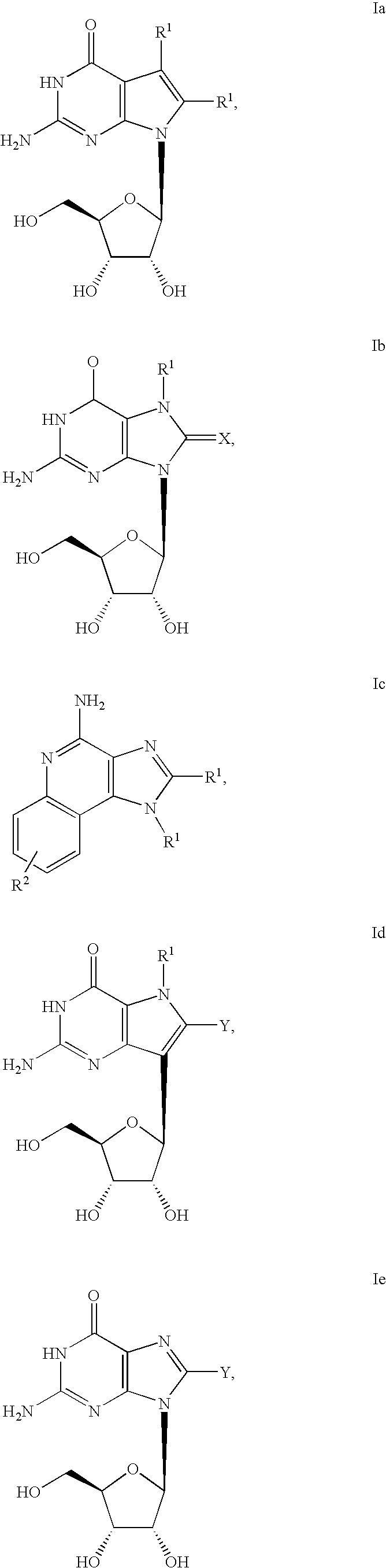
A cyclic dinucleotide compound of Formula (I):wherein X1 is H or F; X2 is H or F; at least one among X1 and X2 is a fluorine atom; Z is OH, OR1, SH or SR1, wherein: R1 is Na or NH4, or R1 is an enzyme-labile group which provides OH or SH in vivo such as pivaloyloxymethyl; B1 and B2 are bases chosen from Adenine, Hypoxanthine or Guanine, and B1 is a different base than B2 and a pharmaceutically acceptable salt thereof. Pharmaceutical compositions including the cyclic dinucleotide, as well as their use in the treatment of a bacterial infection, a viral infection or a cancer are also described.
Owner:KAYLA THERAPEUTICS
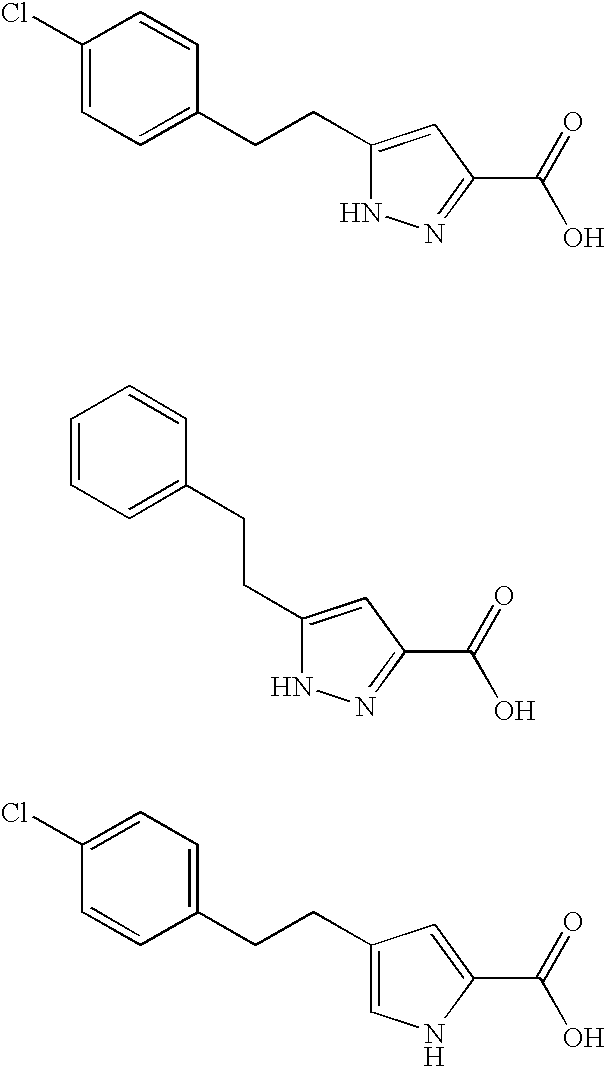
Pyrrole and pyrazole DAAO inhibitors
Methods for increasing D-Serine concentration and reducing concentration of the toxic products of D-Serine oxidation, for enhancing learning, memory and / or cognition, or for treating schizophrenia, Alzheimer's disease, ataxia or neuropathic pain, or preventing loss in neuronal function characteristic of neurodegenerative diseases involve administering to a subject in need of treatment a therapeutically effective amount of a compound of formula I, or a pharmaceutically acceptable salt or solvate thereof: wherein [0001]R1 and R2 are independently selected from hydrogen, halo, nitro, alkyl, acyl, alkylaryl, and XYR5; [0002]or R1 and R2, taken together, form a 5, 6, 7 or 8-membered substituted or unsubstituted carbocyclic or heterocyclic group; [0003]X and Y are independently selected from O, S, NH, and (CR6R7)n; [0004]R3 is hydrogen, alkyl or M+; M is aluminum, calcium, lithium, magnesium, potassium, sodium, zinc ion or a mixture thereof; [0005]Z is N or CR4; [0006]R4 is from selected from hydrogen, halo, nitro, alkyl, alkylaryl, and XYR5; [0007]R5 is selected from aryl, substituted aryl, heteroaryl and substituted heteroaryl; [0008]R6 and R7 are independently selected from hydrogen and alkyl; n is an integer from 1 to 6; [0009]at least one of R1, R2 and R4 is other than hydrogen; and [0010]at least one of X and Y is (CR6R7)n. D-serine or cycloserine may be coadministered along with the compound of formula I.
Owner:SEPACOR INC
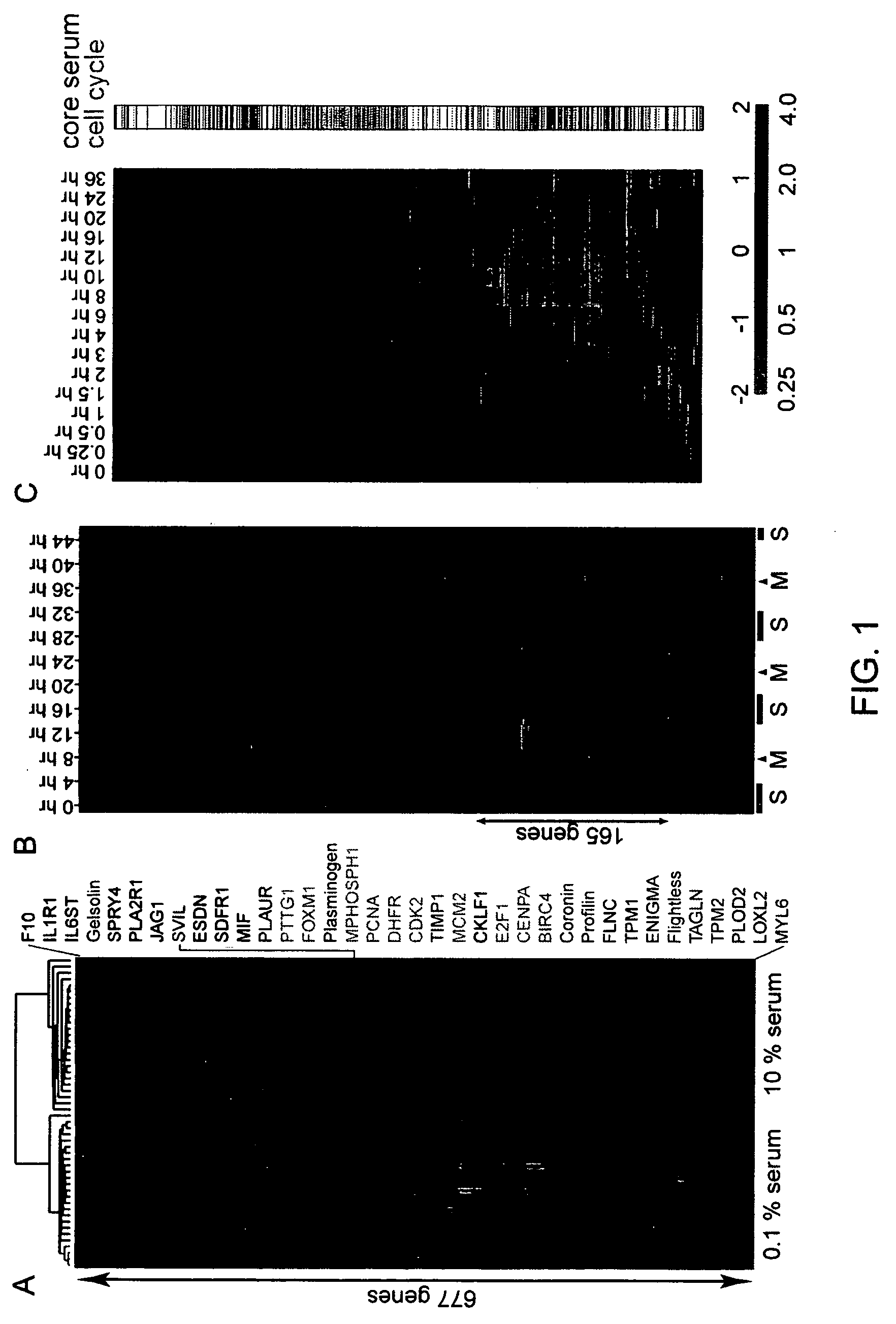
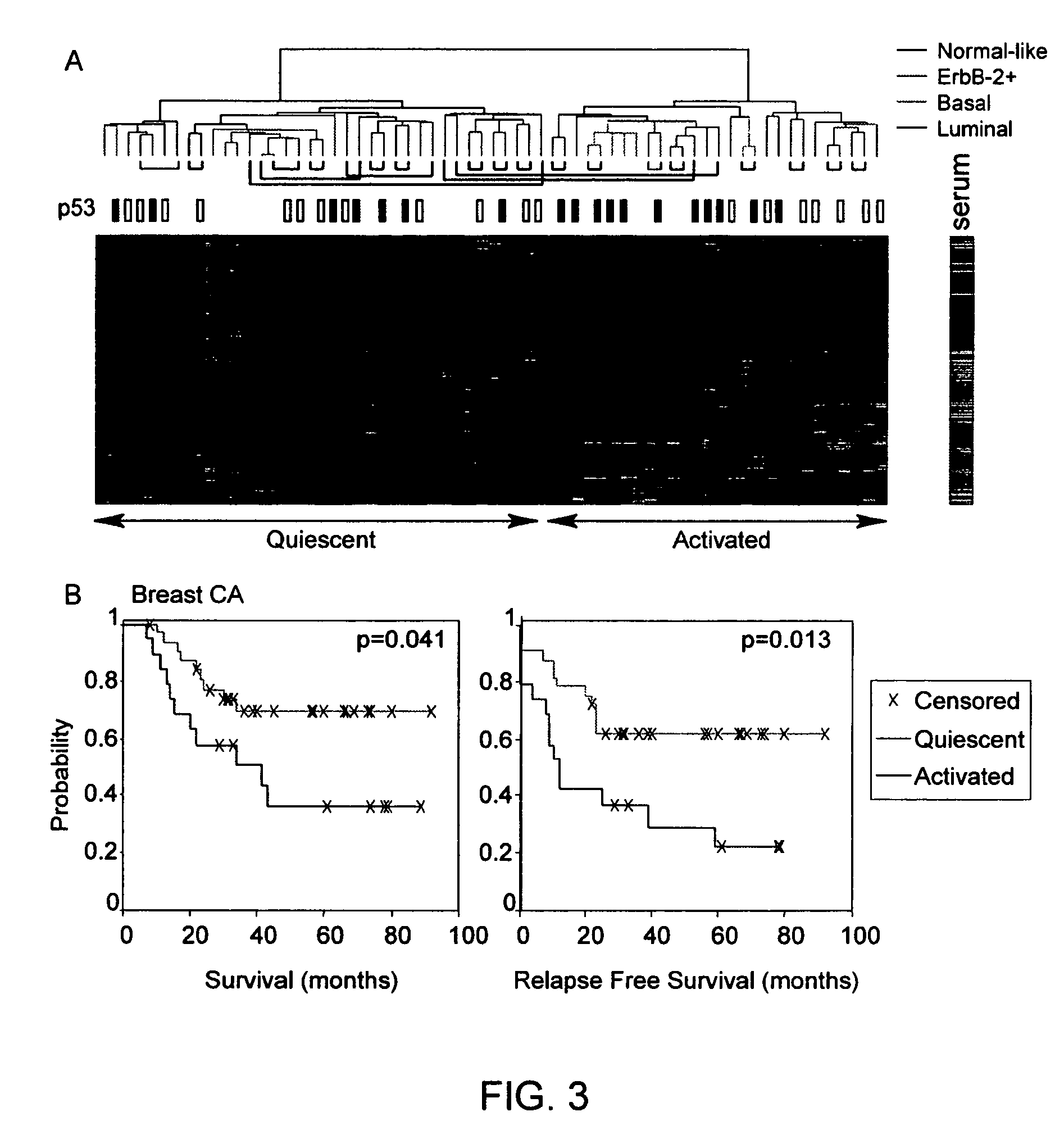
Gene expression signature for prediction of human cancer progression
InactiveUS20060183141A1Increase probabilityOptimize choiceSugar derivativesMicrobiological testing/measurementHuman cancerLymphatic Spread
Methods are provided for classification of cancers by the expression of a set of genes referred to as the core serum response (CSR), or a subset thereof. The expression pattern of the CSR in normal tissues correlates with that seen in quiescent fibroblasts cultured in the absence of serum, while cancer tissues can be classified as having a quiescent or induced CSR signature. Patients with the induced CSR signature have a higher probability of metastasis. Classification according to CSR signature allows optimization of treatment, and determination of whether on whether to proceed with a specific therapy, and how to optimize dose, choice of treatment, and the like.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
G protein coupled receptor agonists and antagonists and methods of activating and inhibiting G protein coupled receptors using the same
InactiveUS7696168B2Reduce accumulationReduce adverse side effectsOrganic active ingredientsNervous disorderG protein-coupled receptorAgonist
Owner:TUFTS MEDICAL CENTER INC
Methods of administering/dosing CD2 antagonists for the prevention and treatment of autoimmune disorders or inflammatory disorders
InactiveUS20070025990A1Symptoms improvedGood curative effectAntipyreticAnalgesicsSide effectAutoimmune disease
The present invention provides compositions for the prevention or treatment of an autoimmune disorder or an inflammatory disorder in a subject comprising one or more CD2 antagonists. In particular, the invention provides methods for preventing or treating an autoimmune disorder or an inflammatory disorder in a subject comprising administering one or more CD2 binding molecules to said subject. The present invention provides doses of CD2 binding molecules and methods of administration that result in improved efficacy, while avoiding or reducing the adverse or unwanted side effects associated with the administration of an agent that induces the depletion of peripheral blood lymphocytes.
Owner:MEDIMMUNE LLC
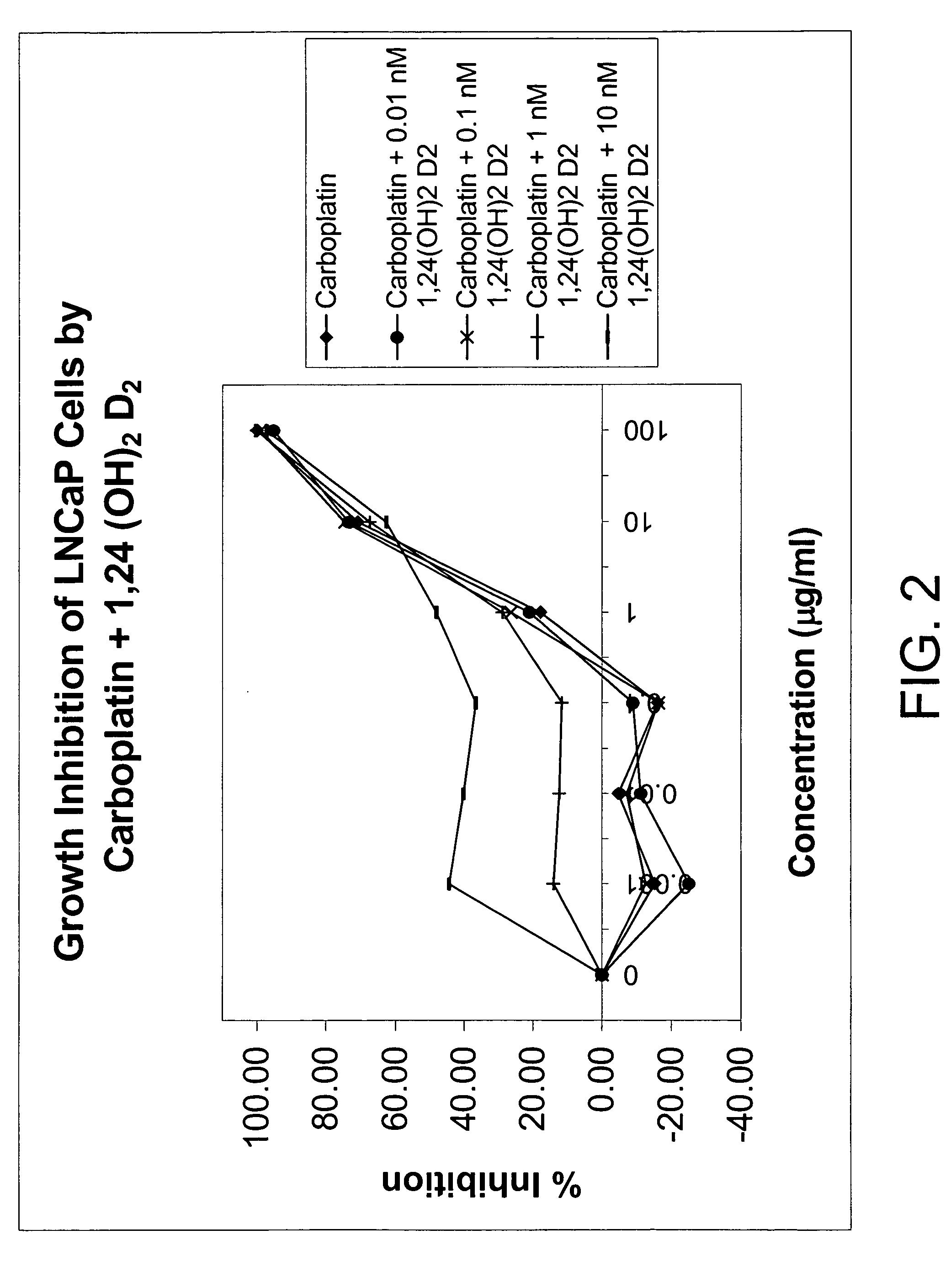
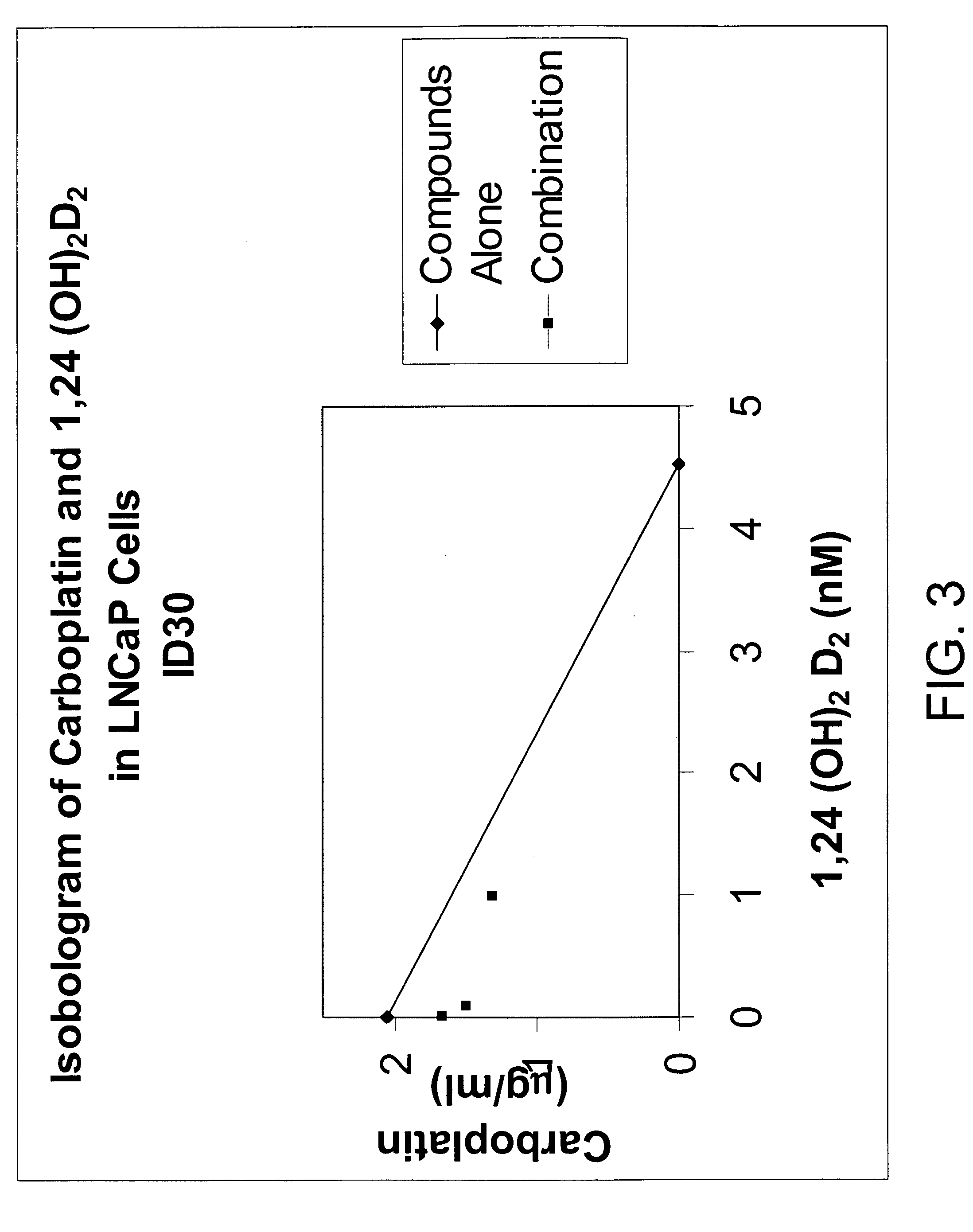
Method of treating prostatic diseases using a combination of vitamin D analogues and other agents
InactiveUS20060003950A1Reduce incidenceReduce riskBiocideCarbohydrate active ingredientsAnticarcinogenActive Vitamin D
The invention provides therapeutic methods for inhibiting, ameliorating or alleviating the hyperproliferative cellular activity of diseases of the prostate, e.g., prostatic cancer and prostatic hyperplasia, which includes administering to a patient in need thereof an active vitamin D analogue and another anticancer agent. Cell differentiation is promoted, induced or enhanced without causing to the patient dose-limiting hypercalcemia and hypercalciuria.
Owner:BONE CARE INT
Cyclic dinucleotides for cytokine induction
ActiveUS10011630B2High activityImprove bioavailabilityAntibacterial agentsOrganic active ingredientsPurineIn vivo
A cyclic dinucleotide compound of Formula (I):wherein X1 is H or F; X2 is H or F; at least one among X1 and X2 is a fluorine atom; Z is OH, OR1, SH or SR1, wherein: R1 is Na or NH4, or R1 is an enzyme-labile group which provides OH or SH in vivo such as pivaloyloxymethyl; B1 and B2 are bases chosen from Adenine, Hypoxanthine or Guanine, and B1 is a different base than B2 and a pharmaceutically acceptable salt thereof. Pharmaceutical compositions including the cyclic dinucleotide, as well as their use in the treatment of a bacterial infection, a viral infection or a cancer are also described.
Owner:KAYLA THERAPEUTICS
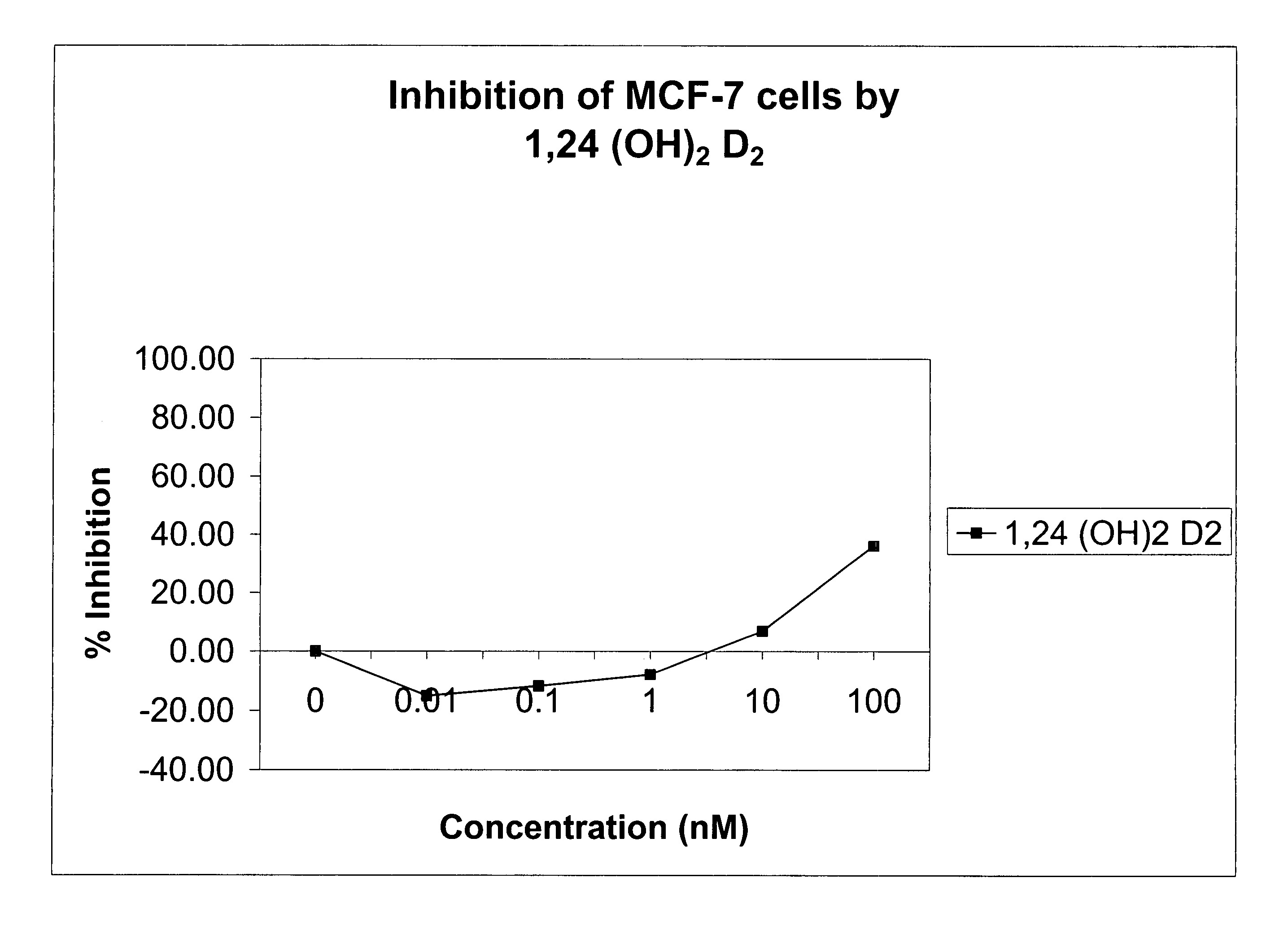
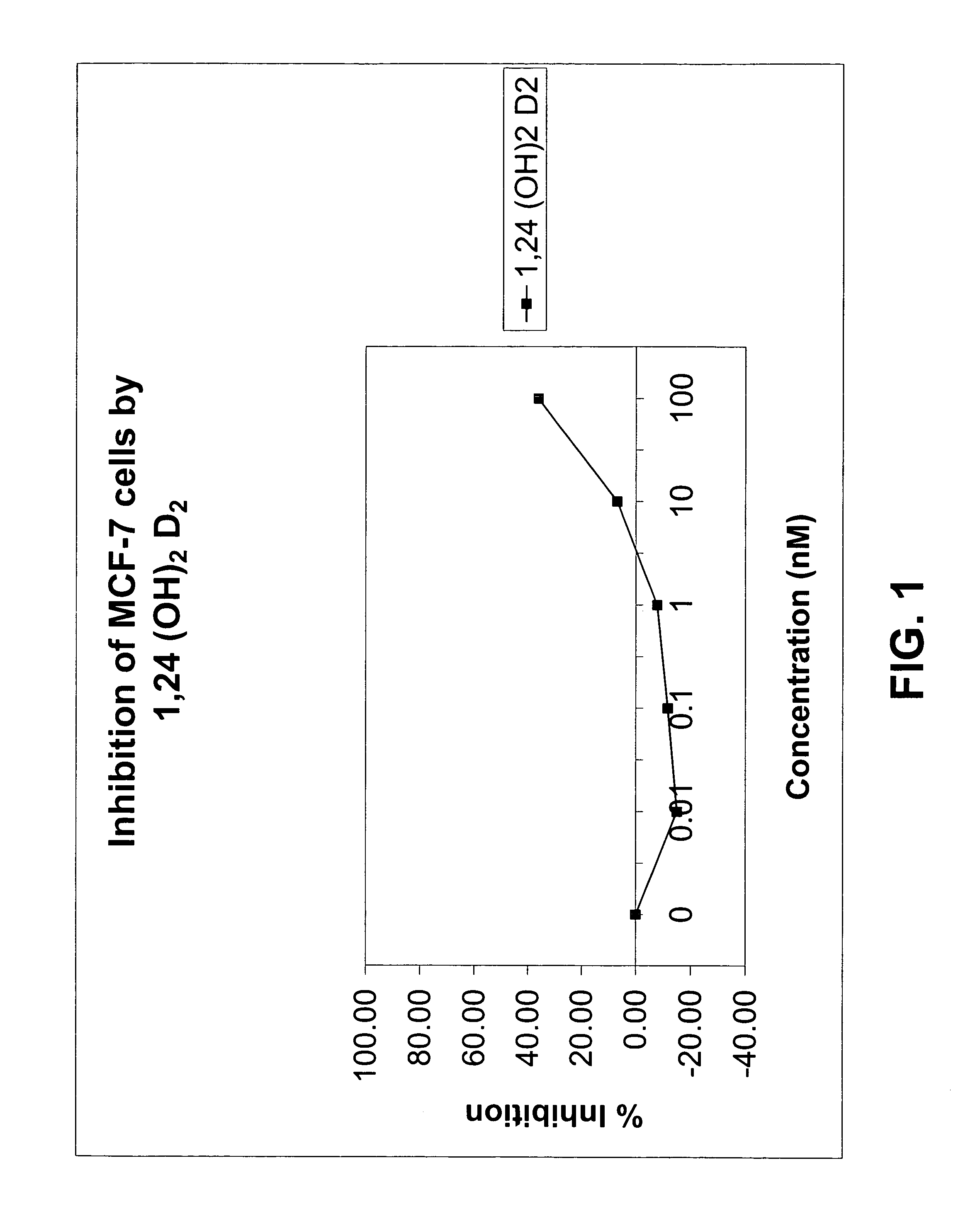
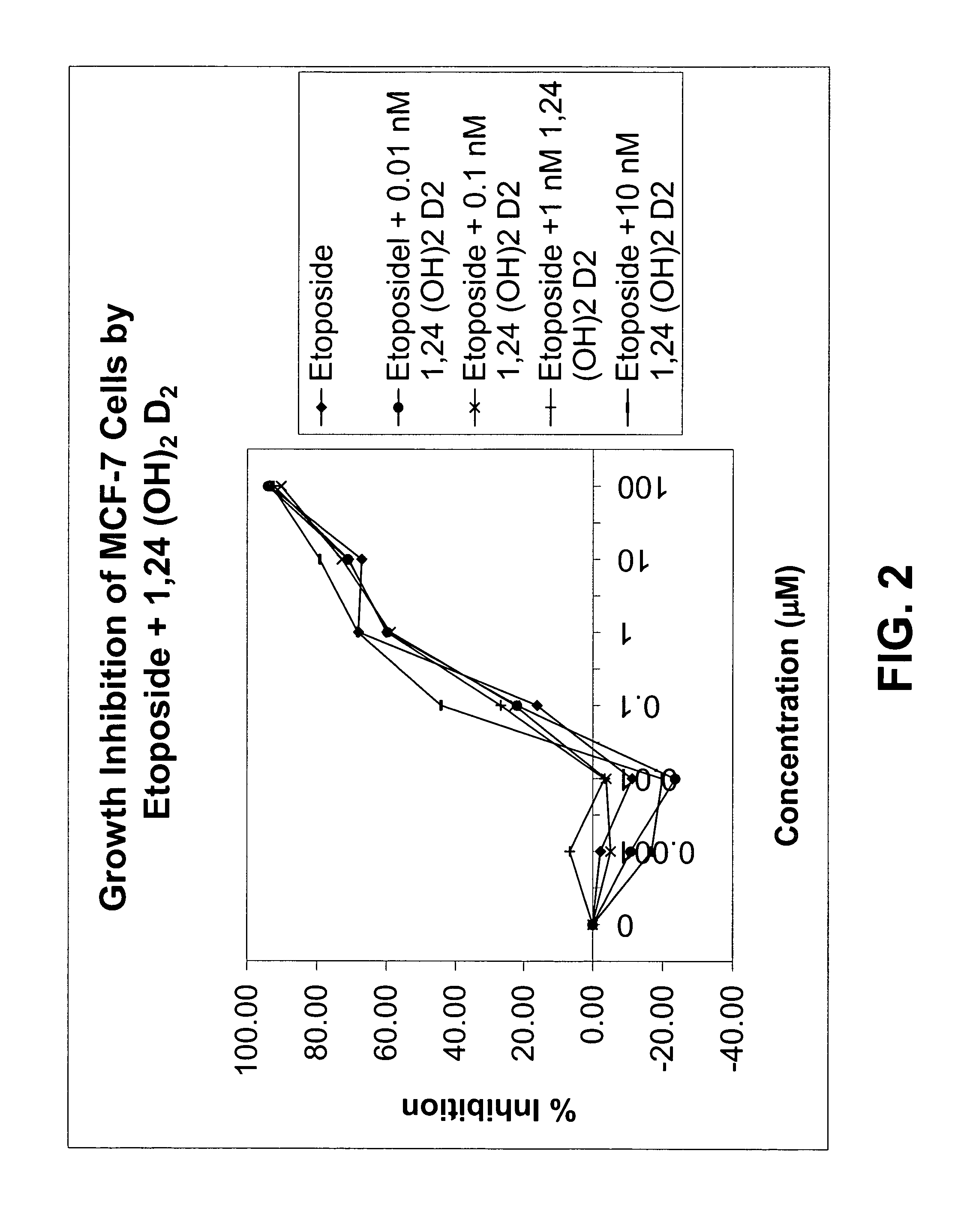
Method of treating breast cancer using a combination of vitamin D analogues and other agents
ActiveUS7094775B2Good treatment effectReduce in quantityHeavy metal active ingredientsBiocideIncreased calciumActive Vitamin D
The invention provides therapeutic methods for inhibiting, ameliorating or alleviating the hyperproliferative cellular activity of diseases of the breast, e.g., breast cancer, which includes administering to a patient in need thereof an active vitamin D analogue and another anticancer agent. Cell differentiation is promoted, induced or enhanced without causing to the patient dose-limiting hypercalcemia and hypercalciuria.
Owner:BONE CARE INT
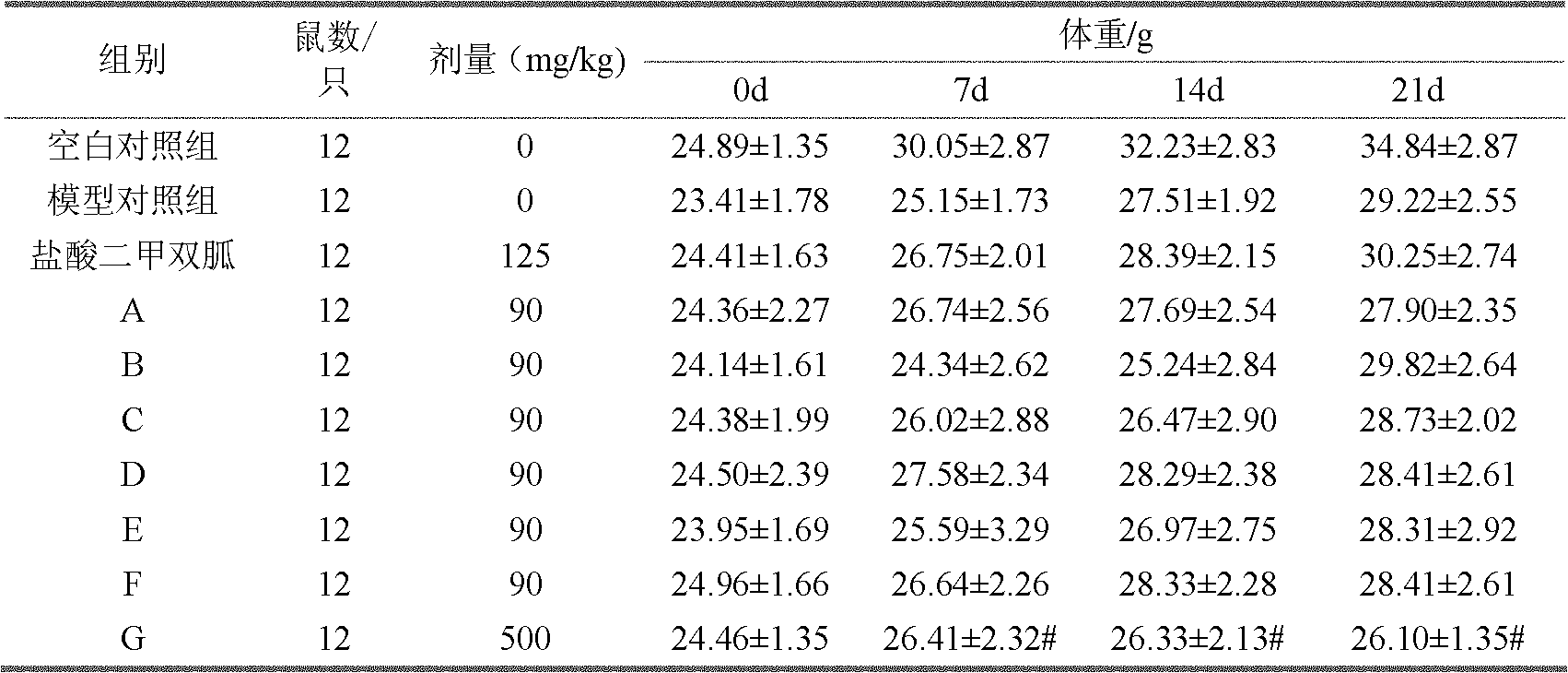
Fuscoporia obliqua active ingredients capable of lowering blood sugar and preparation method and application of fuscoporia obliqua active ingredients
InactiveCN102038720AReduce adverse side effectsStrong market competitive advantageMetabolism disorderFungi medical ingredientsCelluloseReduction Activity
The invention discloses fuscoporia obliqua active ingredients capable of lowering blood sugar and a preparation method and application of the fuscoporia obliqua active ingredients. The preparation method takes fuscoporia obliqua fruit body as raw material and comprises the following steps: respectively extracting, filtering and concentrating the fuscoporia obliqua fruit body with normal temperature water and high temperature water; adding alcohol into concentrate and depositing to obtain crude polysaccharide; respectively pouring the polysaccharide extracted with normal temperature water and the crude polysaccharide extracted with high temperature water to flow through a (diethylaminoethanol) DEAE-52 cellulose column; carrying out subsection elution by using distilled water and NaCl solutions with different concentrations; and collecting stepwise elution peak sugar solution. Internal blood sugar reduction activity experiment shows that 0.2mol / L NaCl-section eluted sugar of the crude polysaccharide extracted with normal temperature water and 0.2mol / L NaCl-section eluted sugar of the crude polysaccharide extracted with high temperature water both have obvious blood sugar reduction activity, same blood sugar reduction activity with the blood sugar reduction medicine of metformin hydrochloride, and no obvious toxic or side effect.
Owner:CHINA AGRI UNIV
Novel fused pyrimidine derivatives for inhibition of tyrosine kinase activity
ActiveUS20130116213A1Reduce adverse side effectsEffectively inhibits cancersBiocideNervous disorderImmunologic disordersKinase activity
The present invention relates to a novel fused pyrimidine derivative having an inhibitory activity for tyrosine kinases, and a pharmaceutical composition for preventing or treating cancers, tumors, inflammatory diseases, autoimmune diseases, or immunologically mediated diseases comprising same as an active ingredient.
Owner:HANMI SCI CO LTD
Method for detecting and/or removing protien comprising a cross-beta structure from a pharmaceutical composition
InactiveUS20070015206A1Induce side effectDecreasing and preventing side effectBiocidePeptide/protein ingredientsSide effectToxicity/Side Effects
The invention relates to the detection and / or removal of conformationally altered proteins and / or molecules comprising a cross-β structure from a pharmaceutical composition or any of its constituents comprising a protein. Disclosed iss that unwanted and / or toxic side effects of pharmaceuticals are caused by proteins present in the pharmaceutical and adopting a cross-β structure conformation. Further disclosed is a method for detecting a protein and / or peptide comprising a cross-β structure in a pharmaceutical composition or any of its constituents comprising a protein, the method comprising: contacting the pharmaceutical composition or any of its constituents comprising a protein with at least one cross-β structure-binding compound resulting in a bound protein and / or peptide comprising a cross-β structure and; detecting whether bound protein and / or peptide comprising a cross-β structure are present in the pharmaceutical composition or any of its constituents comprising a protein. Further described are methods for removing cross-β structures from a pharmaceutical composition and controlling the manufacture of a pharmaceutical composition.
Owner:CROSSBETA BIOSCIENCES BV
Method and composition for controlling oral pathogens
A composition and method of controlling oral and other human pathogens is disclosed. The composition and method utilize an antimicrobial or antibiotic and a berberine as active agents to treat mammals, including humans.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
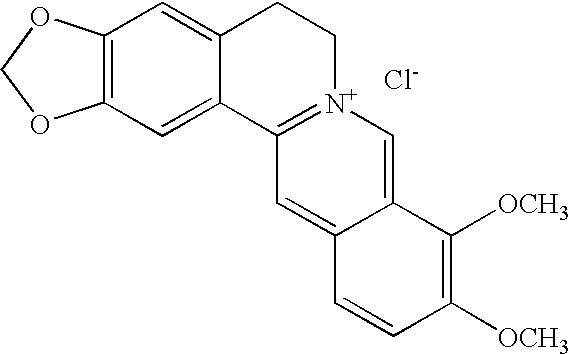
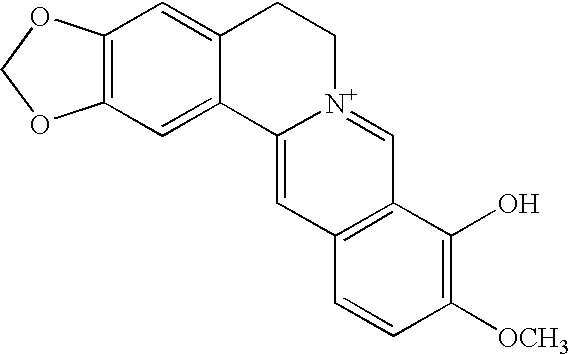
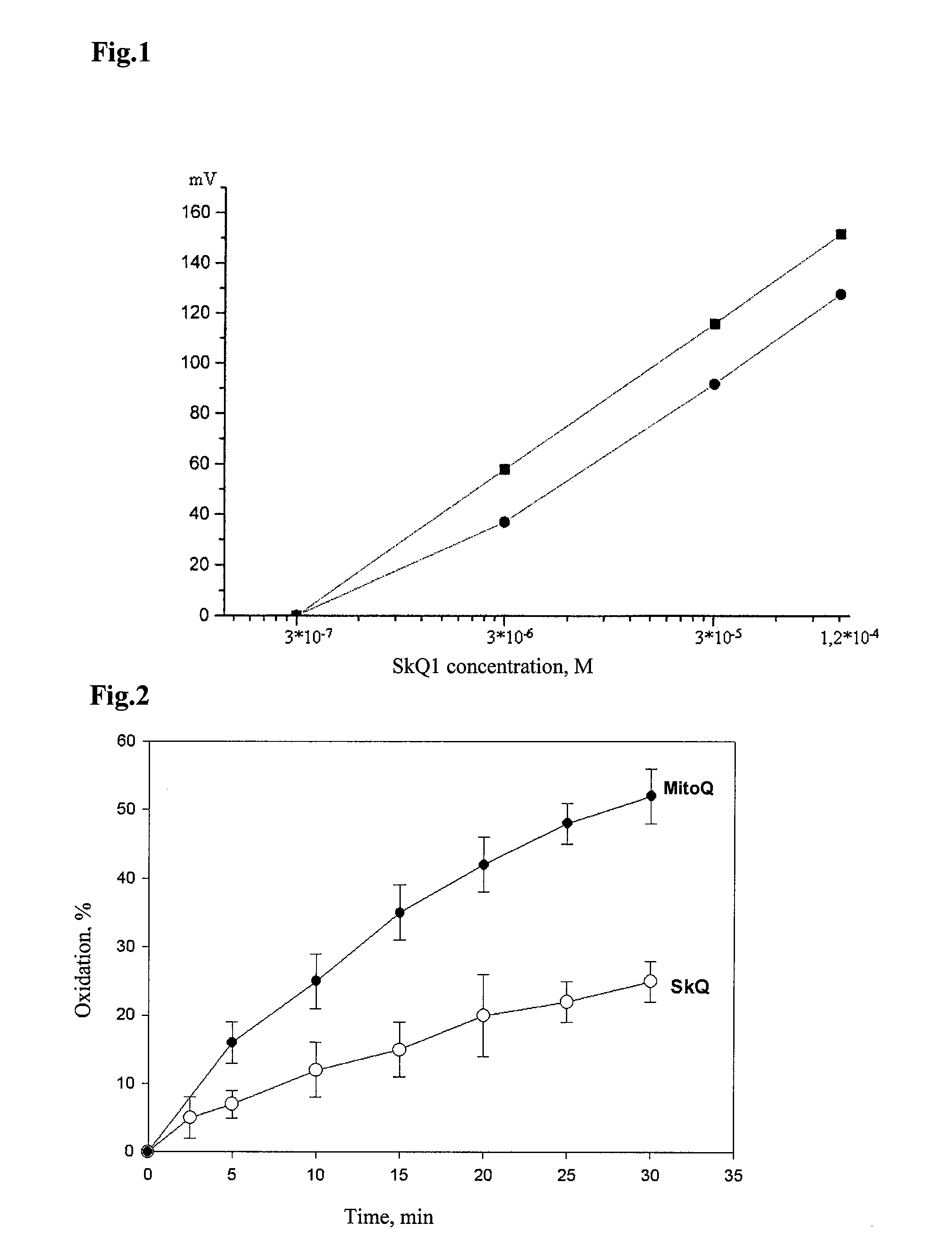
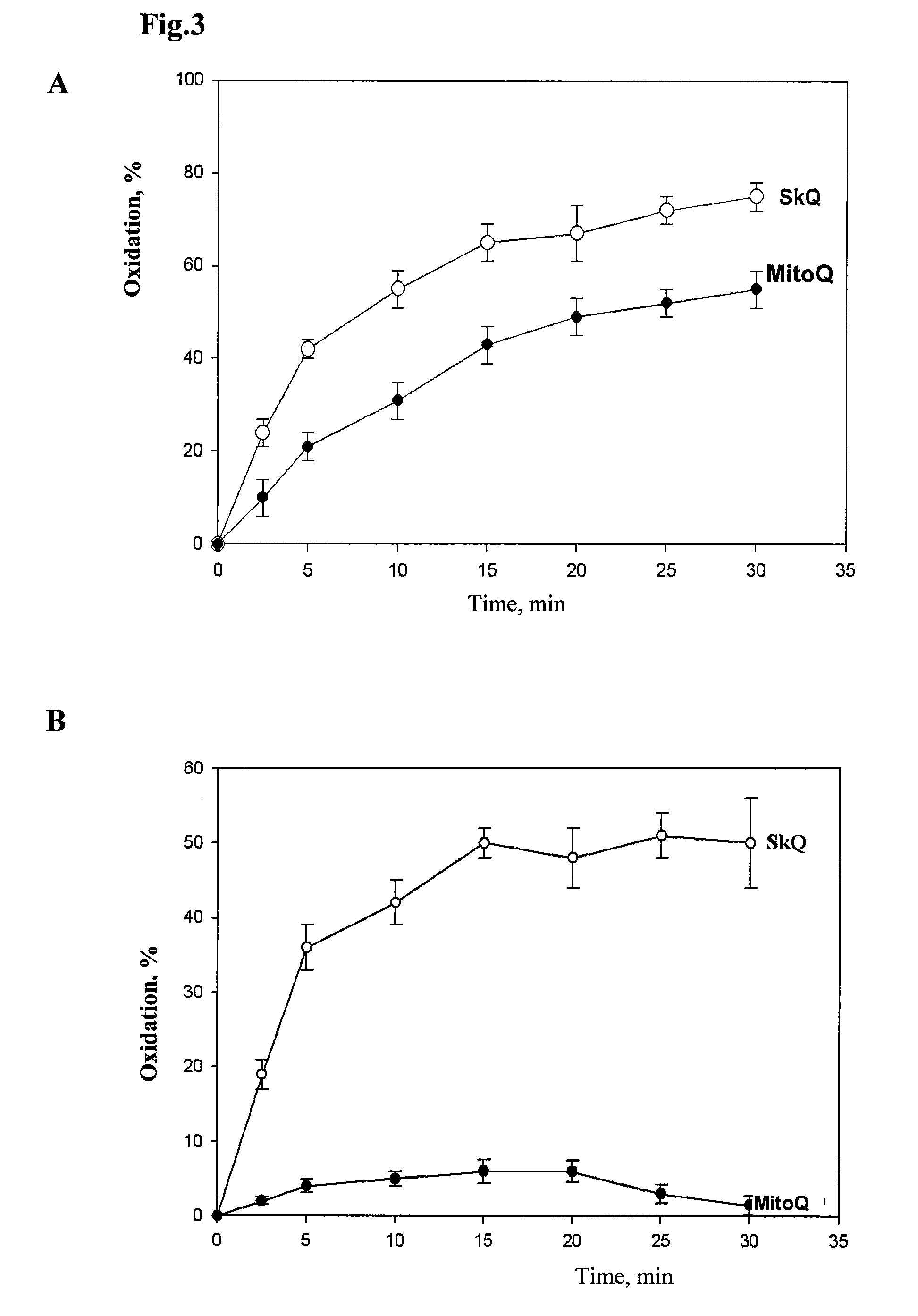
Method of treating organism by biologically active compounds specifically delivered into mitochondria, pharmaceutical composition required for the use of the method and a compound applicable for this purpose
ActiveUS20080176929A1High quantum yieldImprove processing efficiencyBiocidePeptide/protein ingredientsOxygenNormal functioning
This invention relates to biology and medicine and, in particular, can be used in medicine to make a pharmaceutical composition for targeted delivery of biologically active substances into mitochondria, driven by proton electro-chemical potential in the mitochondria. This invention also relates to the method to affect an organism by the targeted delivery of biologically active compounds to mitochondria. The invention can be useful in treatment of diseases or disorders associated with not normal functioning of mitochondria, in particular diseases associated with increased production of free radicals and reactive oxygen species.
Owner:MITOTECH SA
Compositions, Synthesis, and Methods of Using Quinolinone Based Atypical Antipsychotic Agents
ActiveUS20080293736A1Reduce adverse side effectsEffective treatmentOrganic active ingredientsNervous disorderBipolar mood disorderAtypical antipsychotic
The present invention provides novel quinolinone derivatives which can be advantageously used for treating schizophrenia and related psychoses such as acute manic, bipolar disorder, autistic disorder, and depression.
Owner:REVIVA PHARMA INC
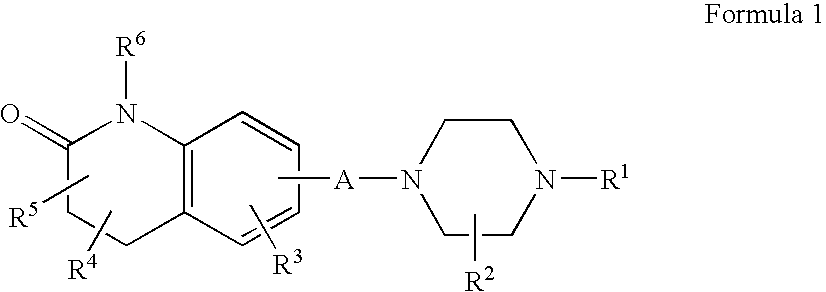
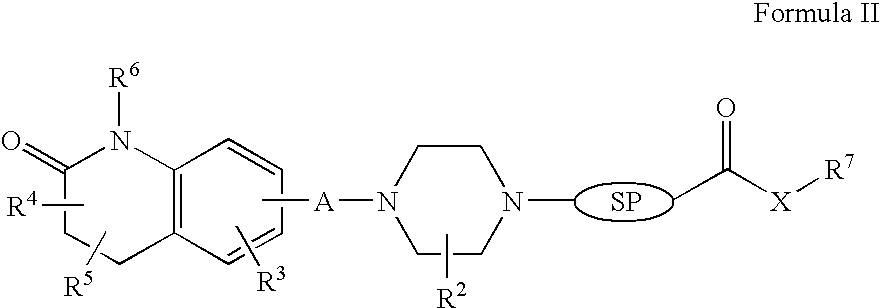
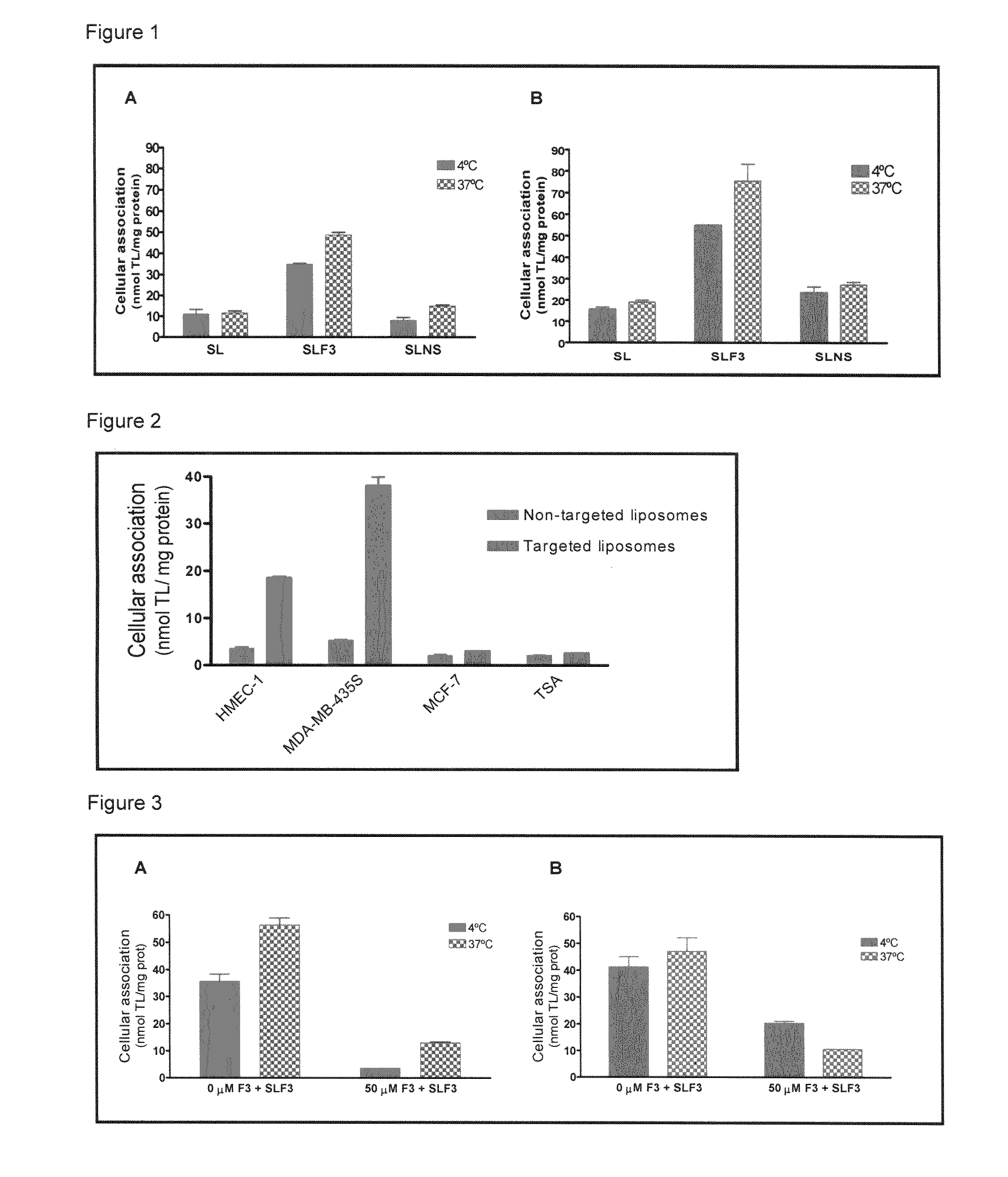
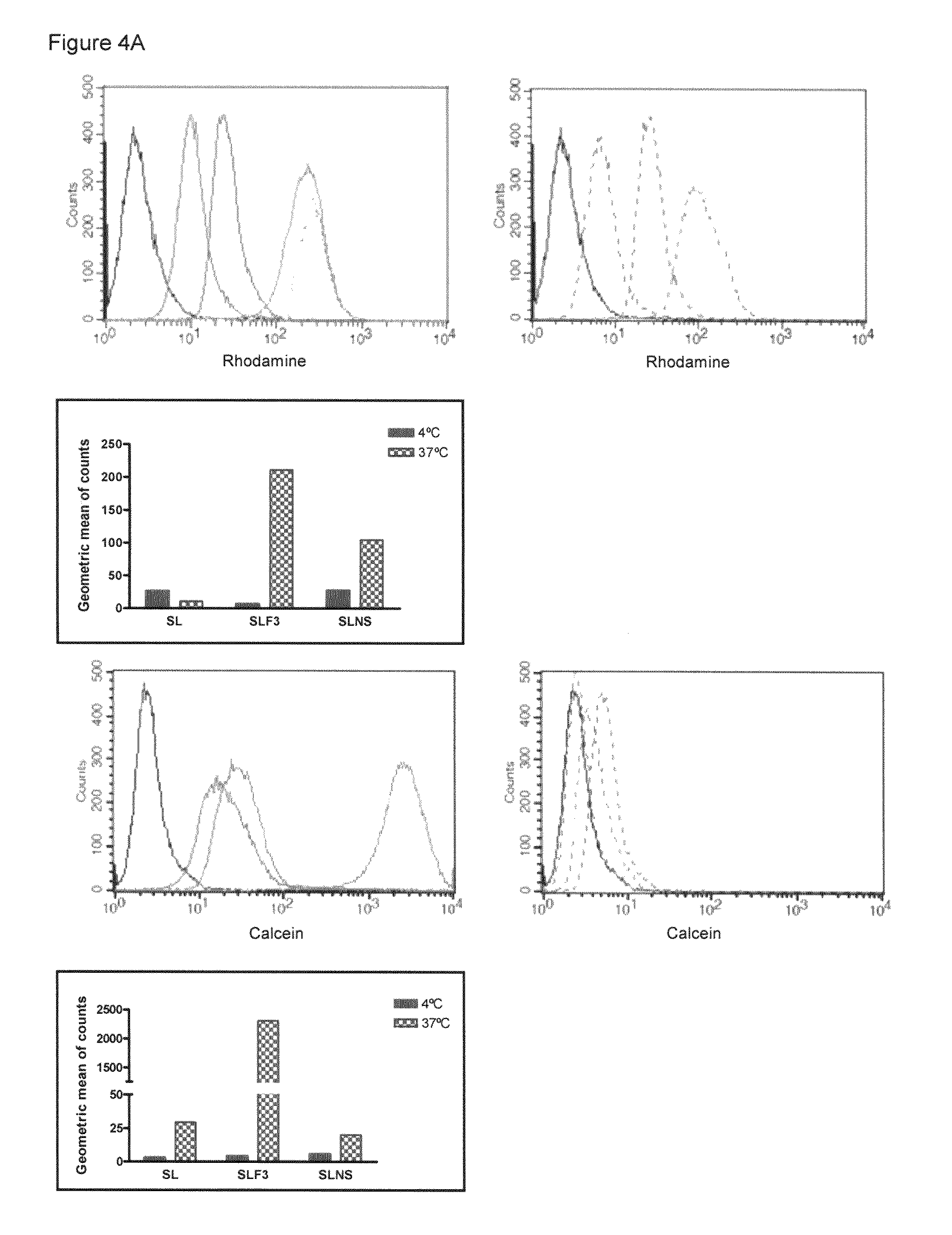
Targeted delivery to human diseases and disorders
ActiveUS20090291049A1Efficient intracellular releaseImprove concentrationBiocideHeavy metal active ingredientsAbnormal tissue growthHigh rate
The present invention provides a system presenting site-specific accumulation through a ligand that specifically targets a receptor overexpressed on the surface of specific cells within a target organ, like, for example, tumor cells and / or vascular cells of tumor blood vessels. Moreover, this invention provides a method where, upon internalization of the previous-mentioned system by the target cells, triggered release at a high rate of the associated agent takes place, permitting efficient intracellular delivery and, thus, increased concentration of the transported cargo at the target site. Overall, this invention provides a method for the diagnosis, prevention and treatment of human diseases and disorders.
Owner:UNIVE DE COIMBRA +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com