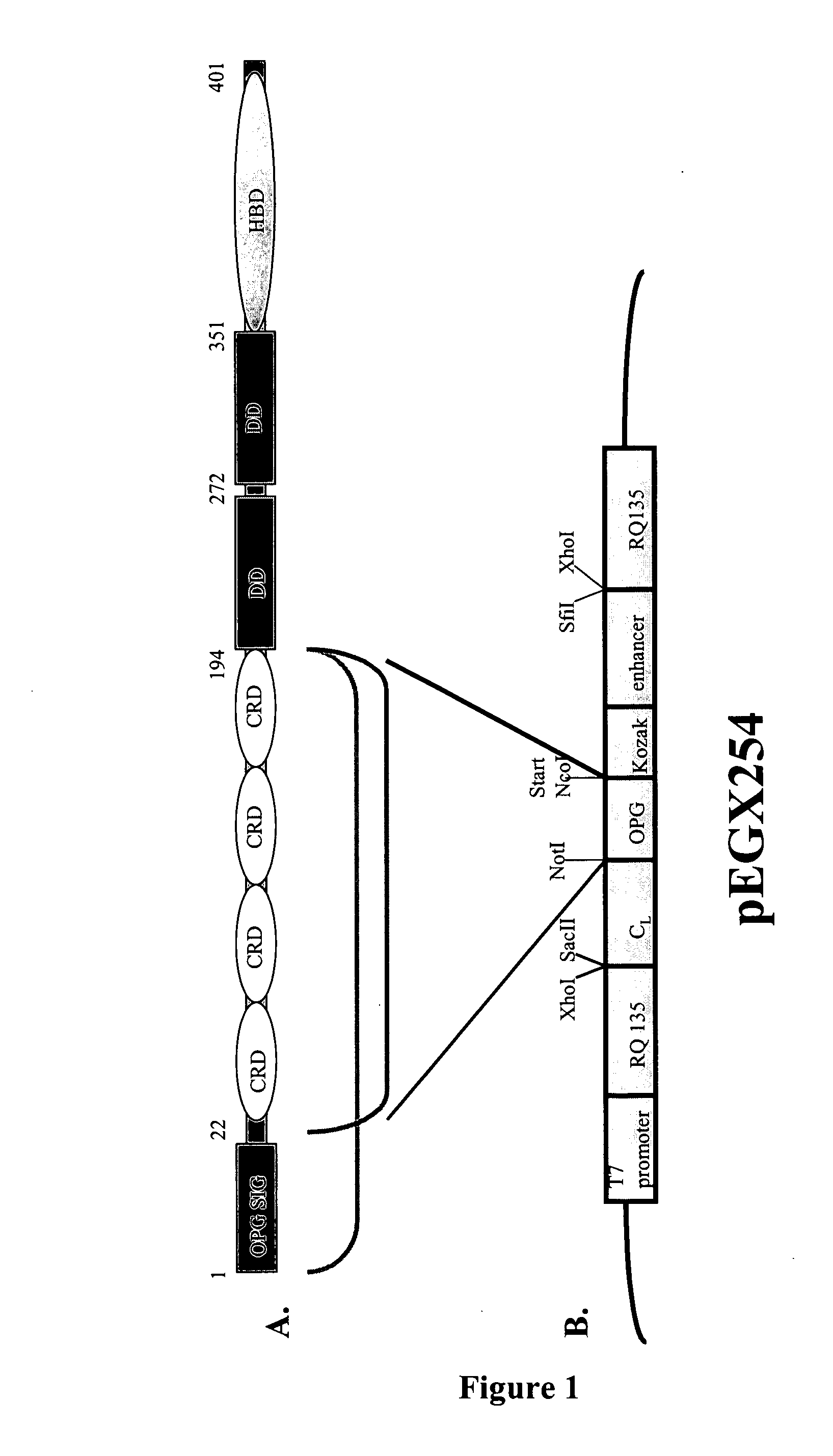
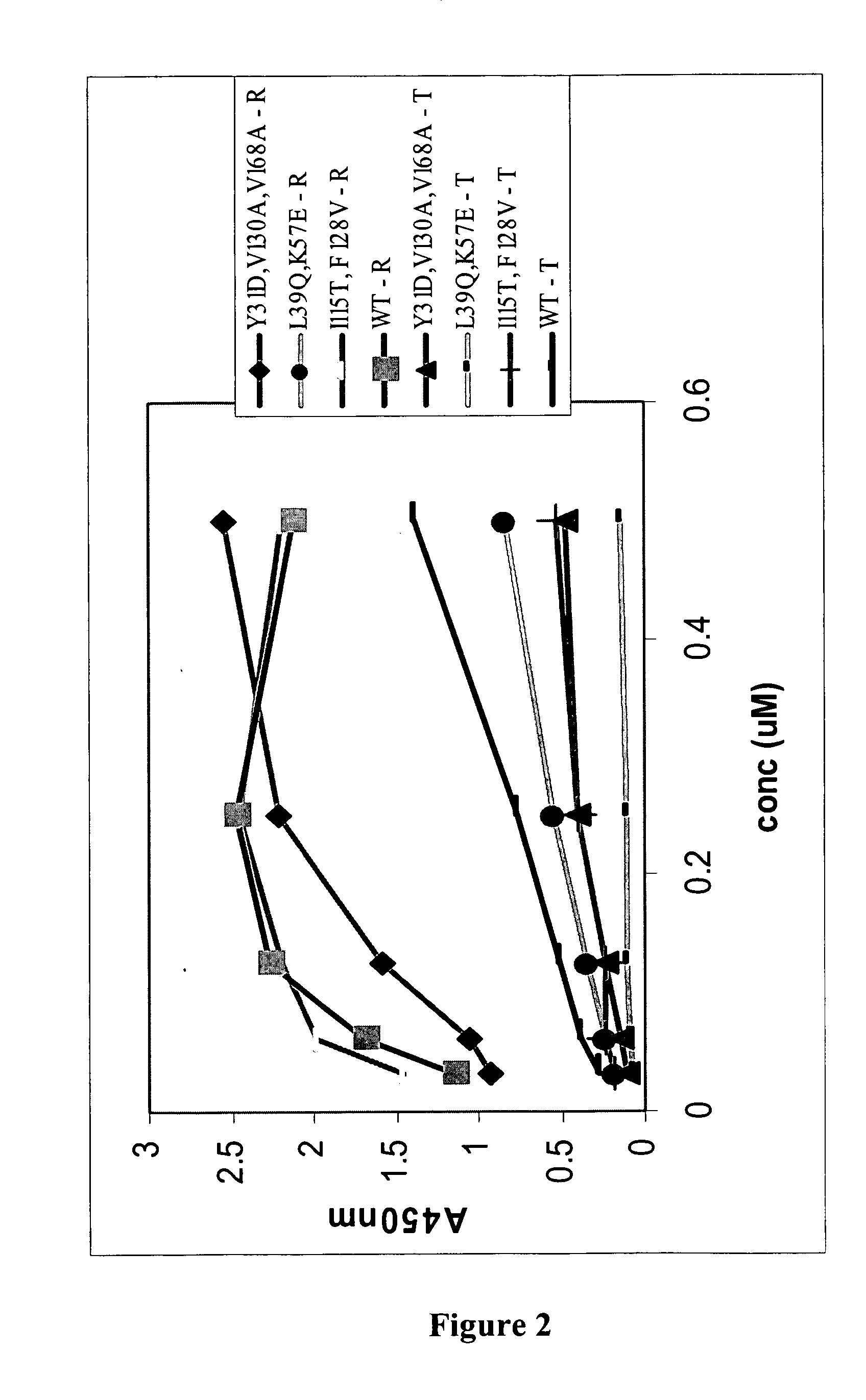
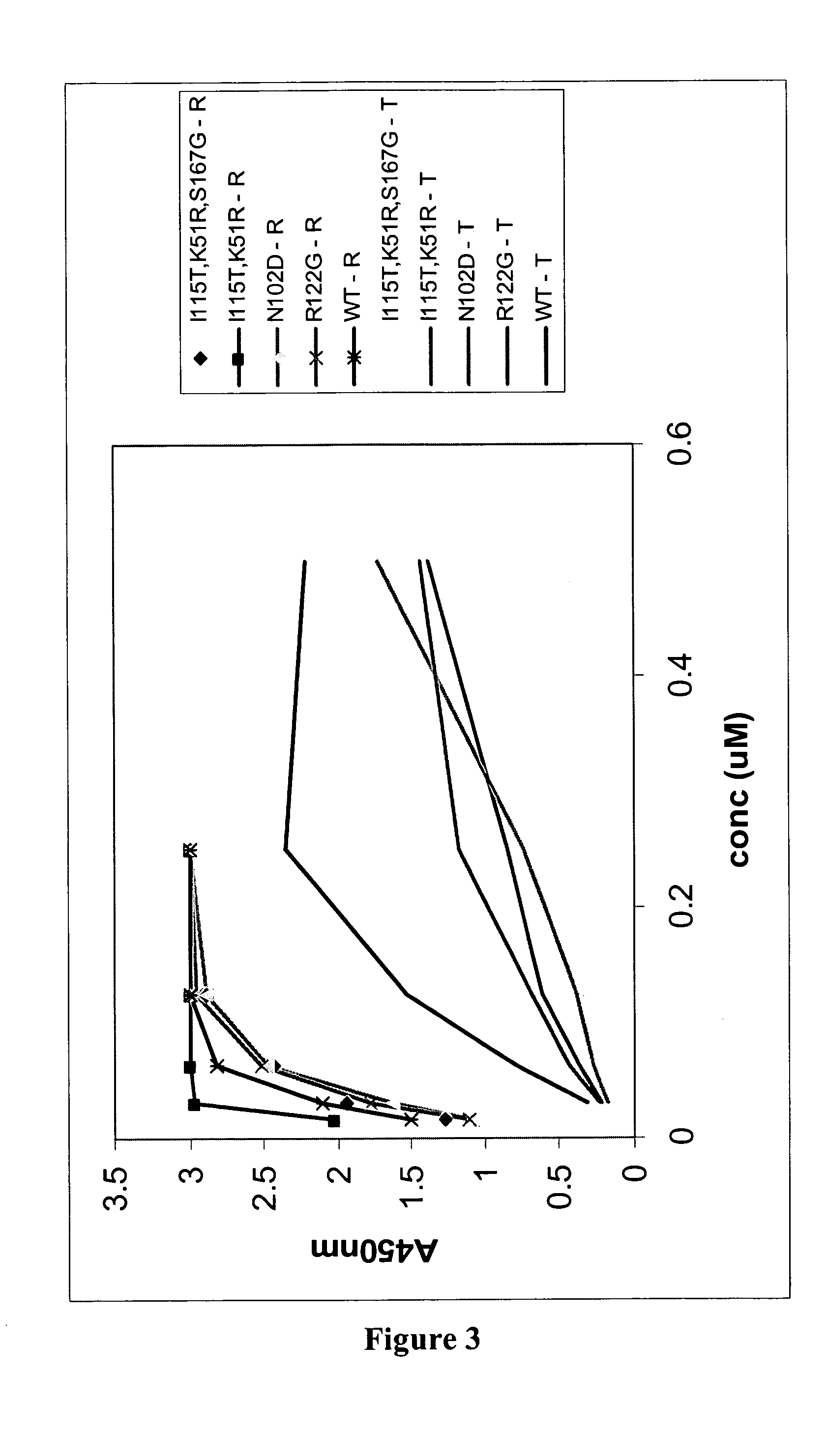
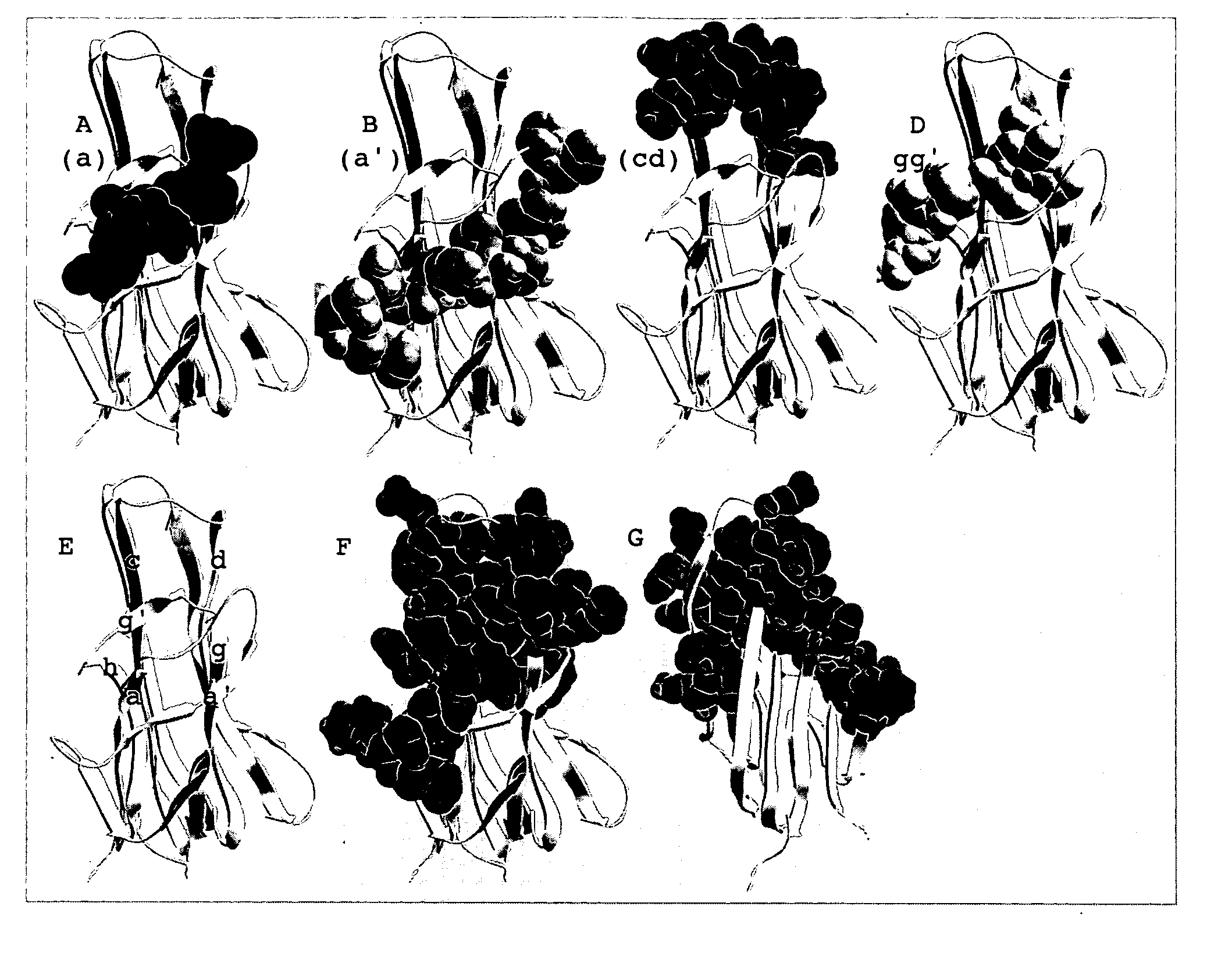
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
196 results about "Increased calcium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
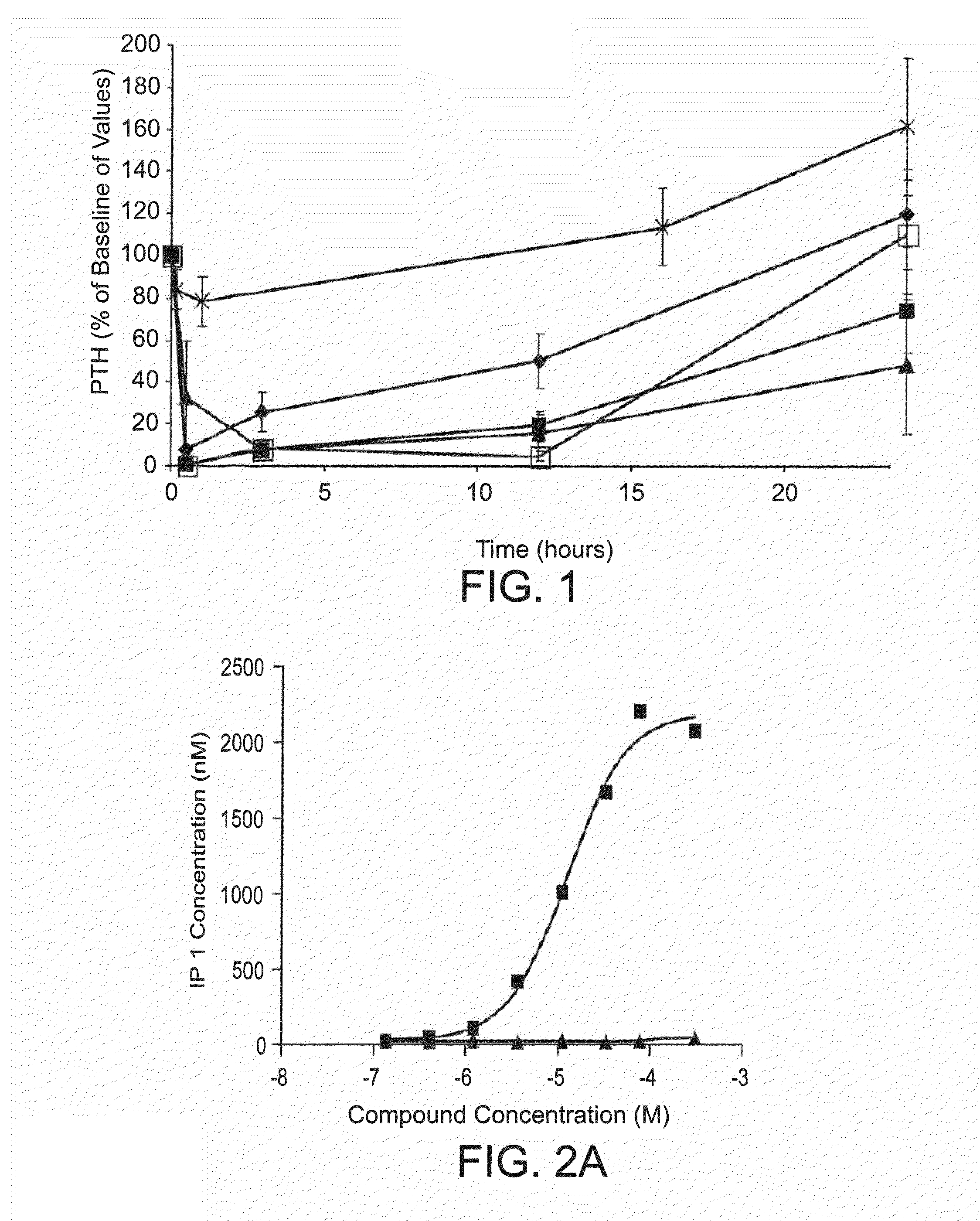
The neuromuscular symptoms of hypercalcemia are caused by a negative bathmotropic effect due to the increased interaction of calcium with sodium channels. Since calcium blocks sodium channels and inhibits depolarization of nerve and muscle fibers, increased calcium raises the threshold for depolarization.
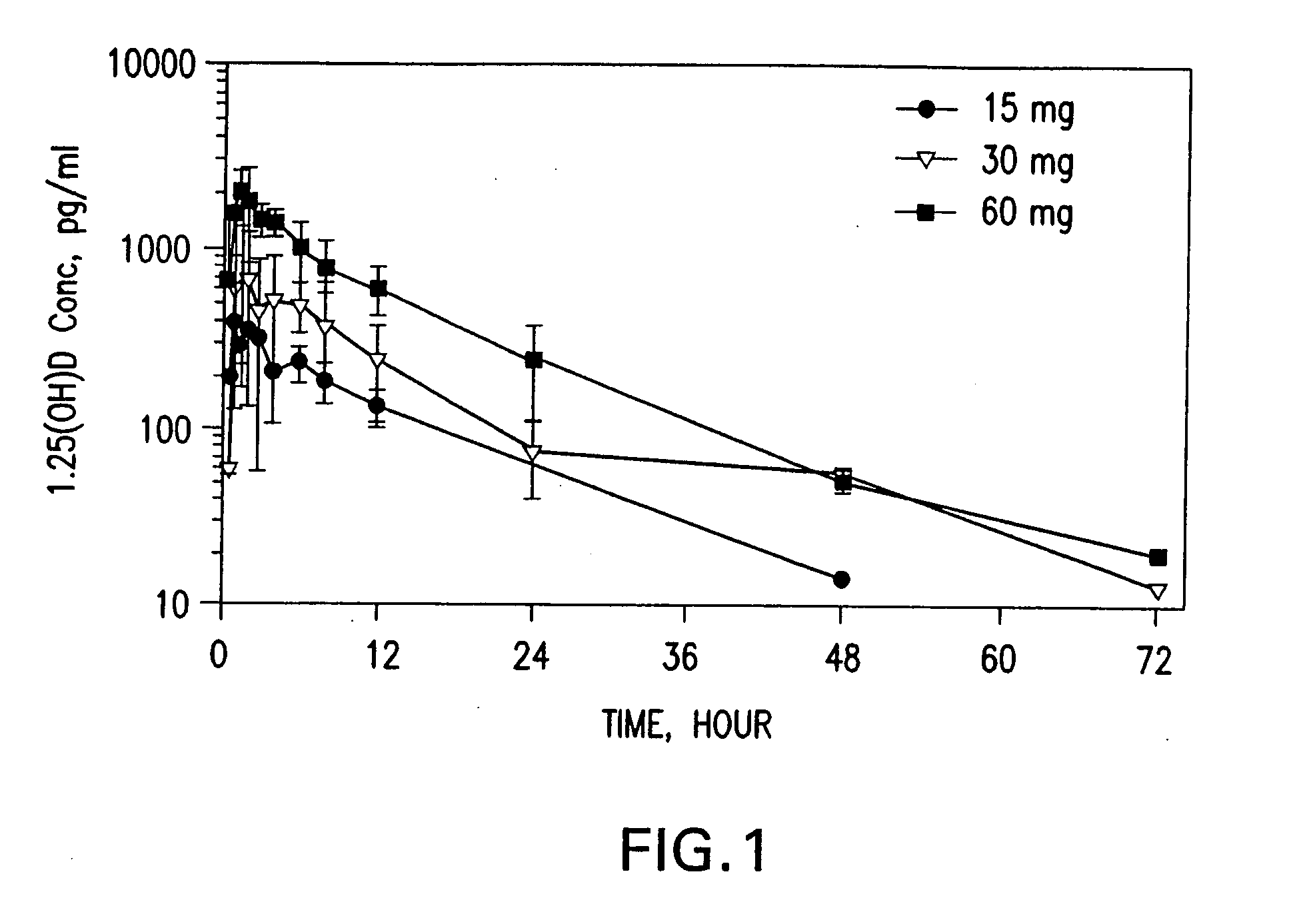
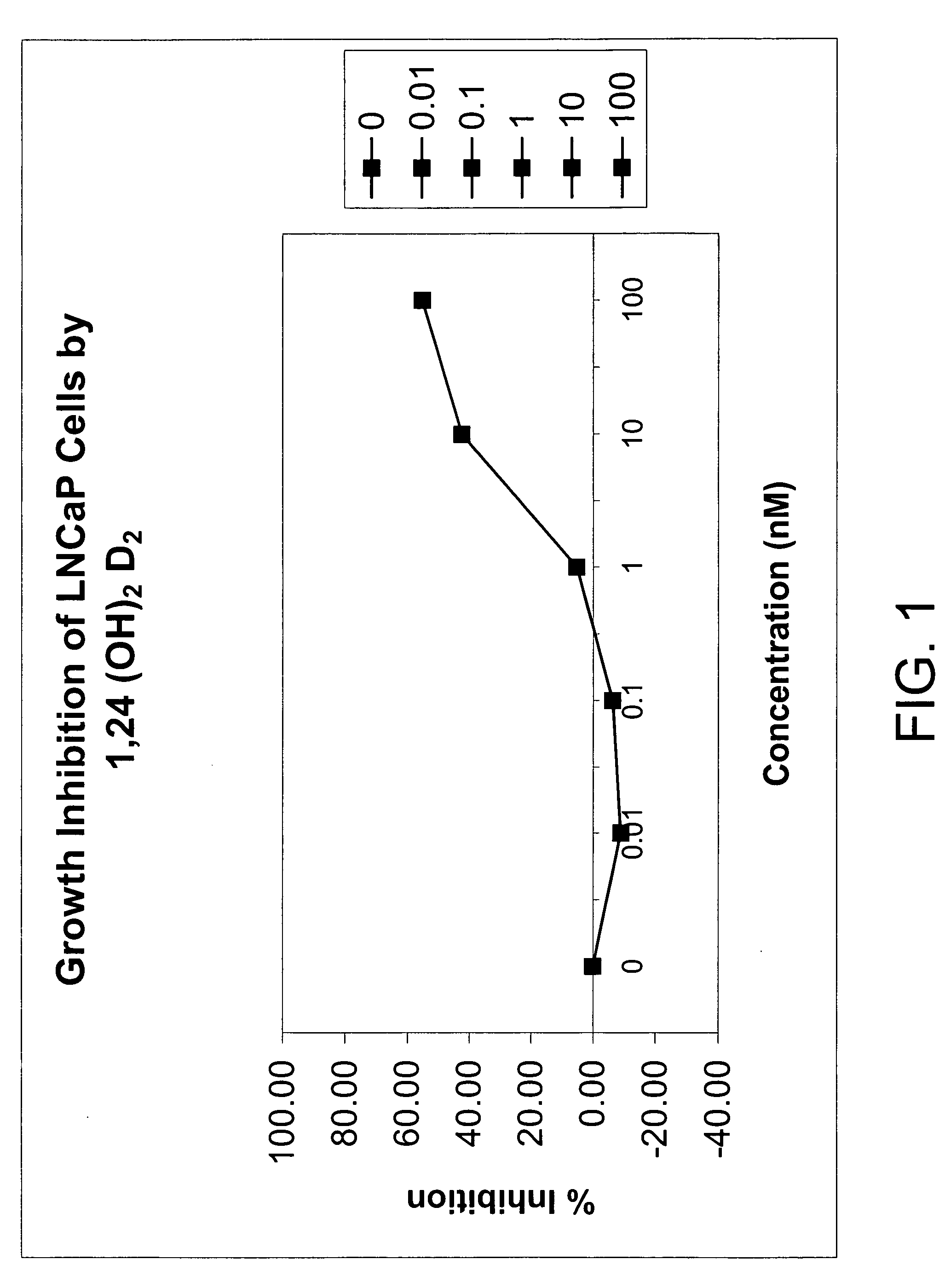
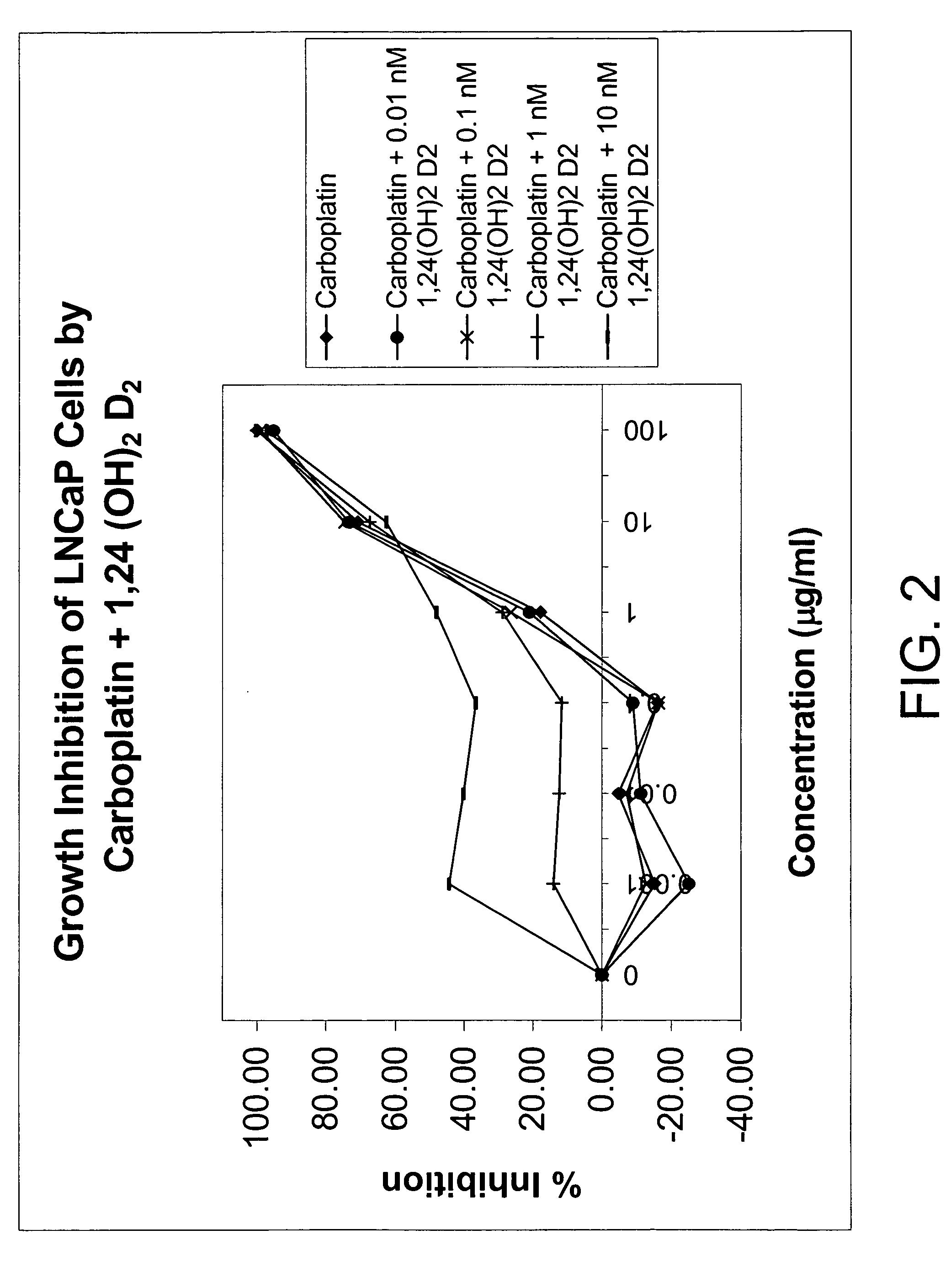
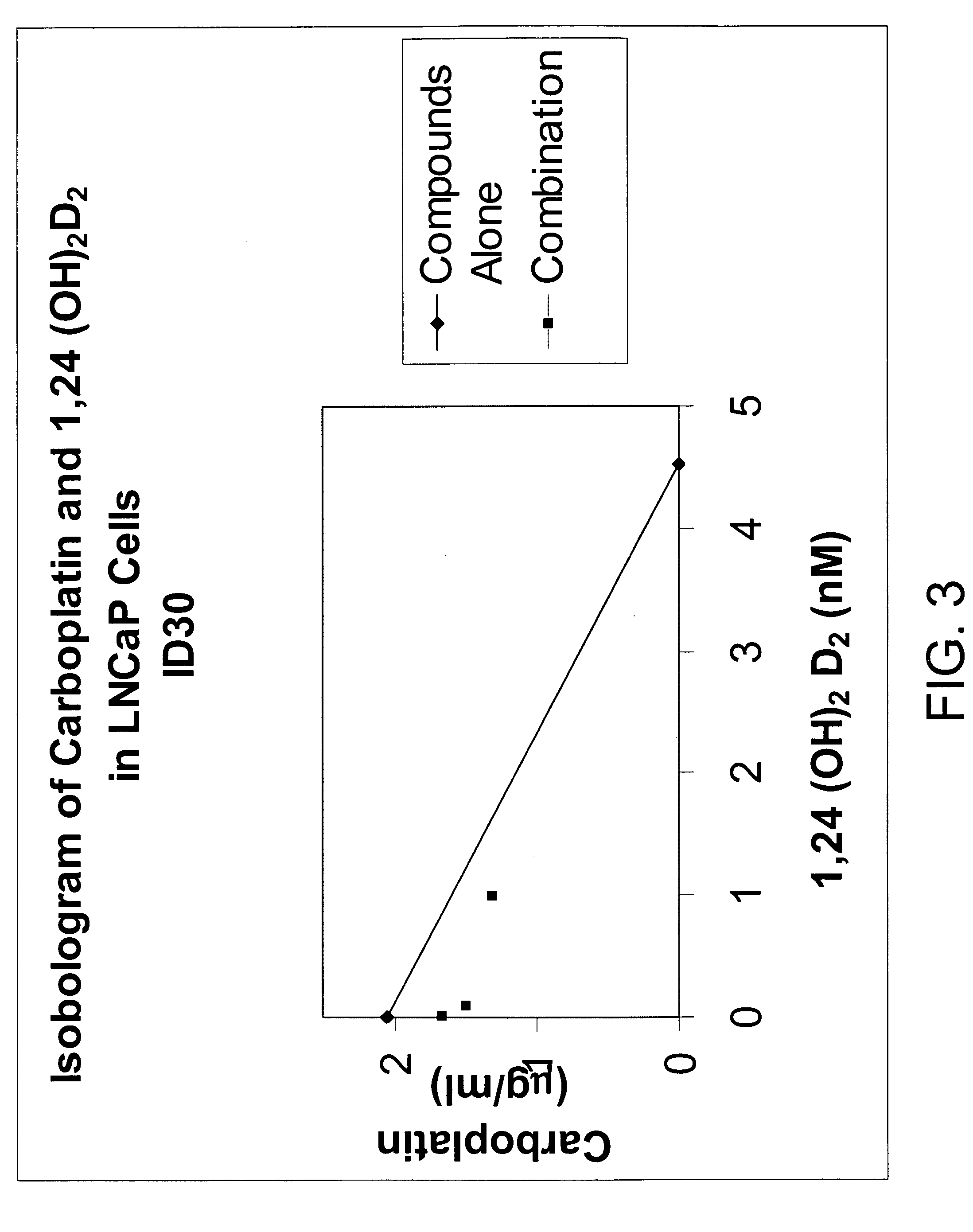
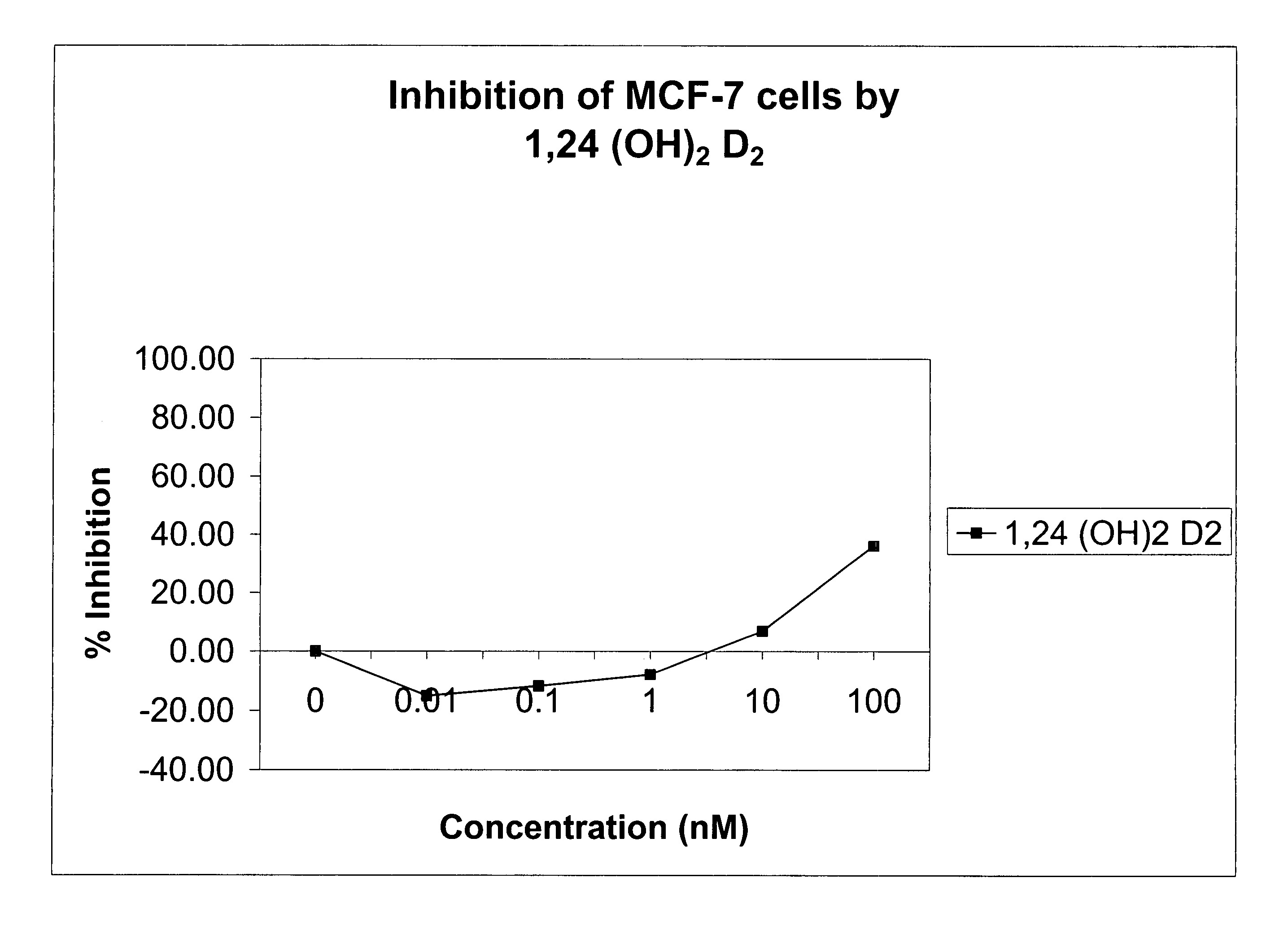
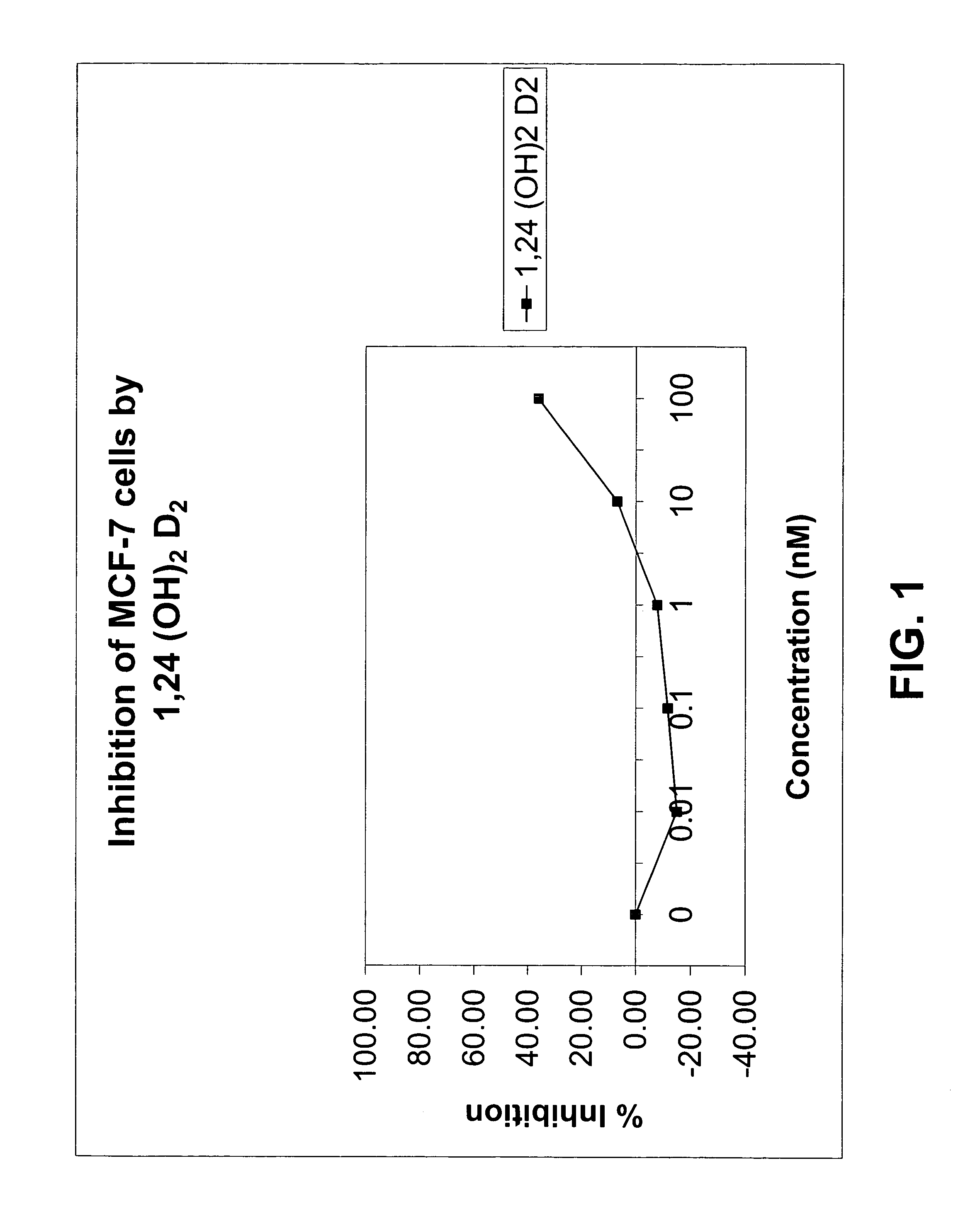
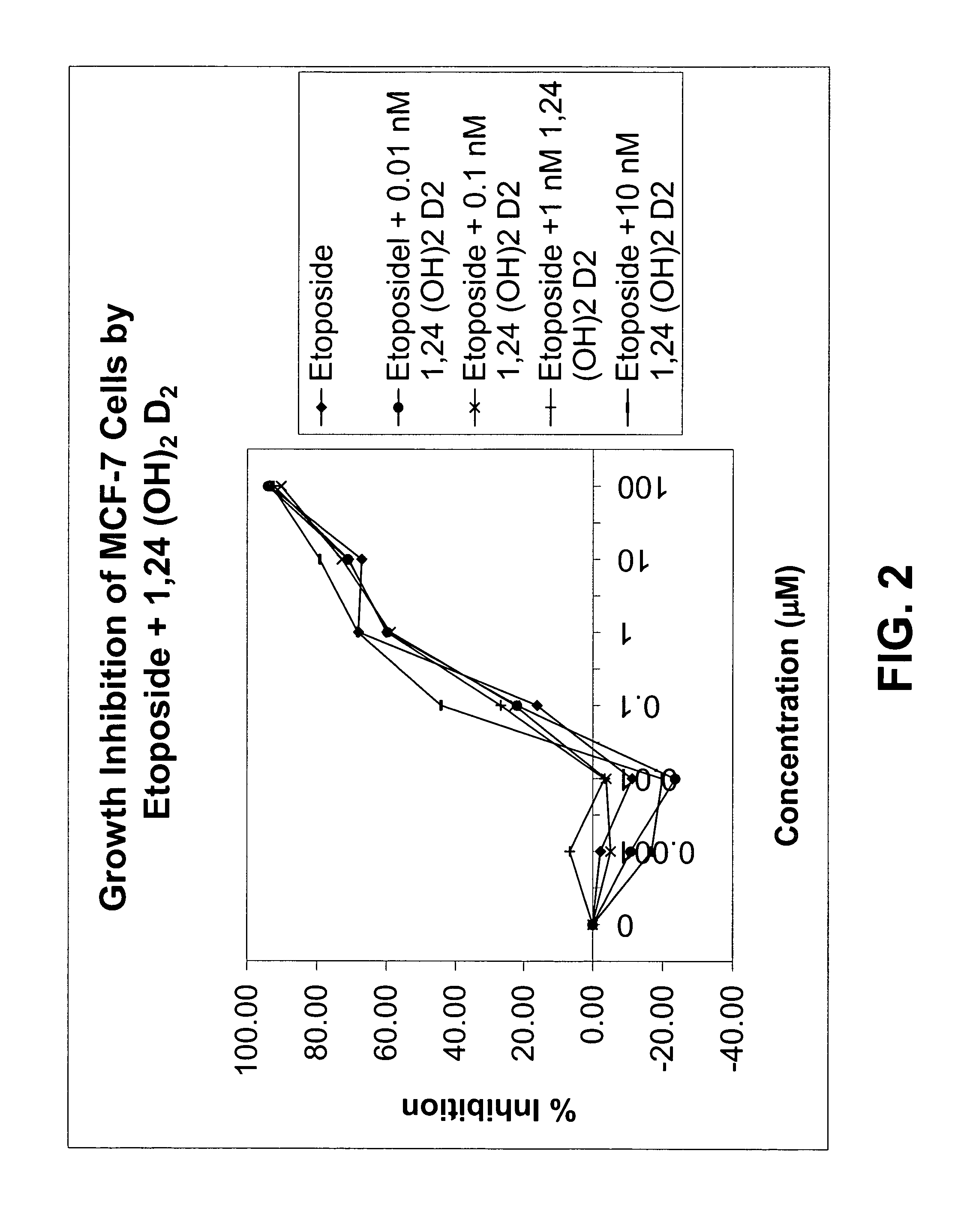
Methods of using vitamin D compounds in the treatment of myelodysplastic syndromes
InactiveUS20050101576A1Minimizing and avoiding effectUseful in treatmentOrganic active ingredientsBiocideActive agentHigh doses
Methods of treating MDS, or ameliorating a symptom thereof, are disclosed. Specific methods encompass the administration of one or more vitamin D compounds, or a pharmaceutically acceptable salt, solvate, hydrate, stereoisomer, clathrate, or prodrug thereof, alone or in combination with one or more additional active agents. Other methods include intermittent administration of a high dose of one or more vitamin D compounds, or a pharmaceutically acceptable salt, solvate, hydrate, stereoisomer, clathrate, or prodrug thereof, alone or in combination with one or more additional active agents. Such intermittent administration allows high doses of the vitamin D compounds to be administered while minimizing or eliminating hypercalcemia.
Owner:NOVACEA INC
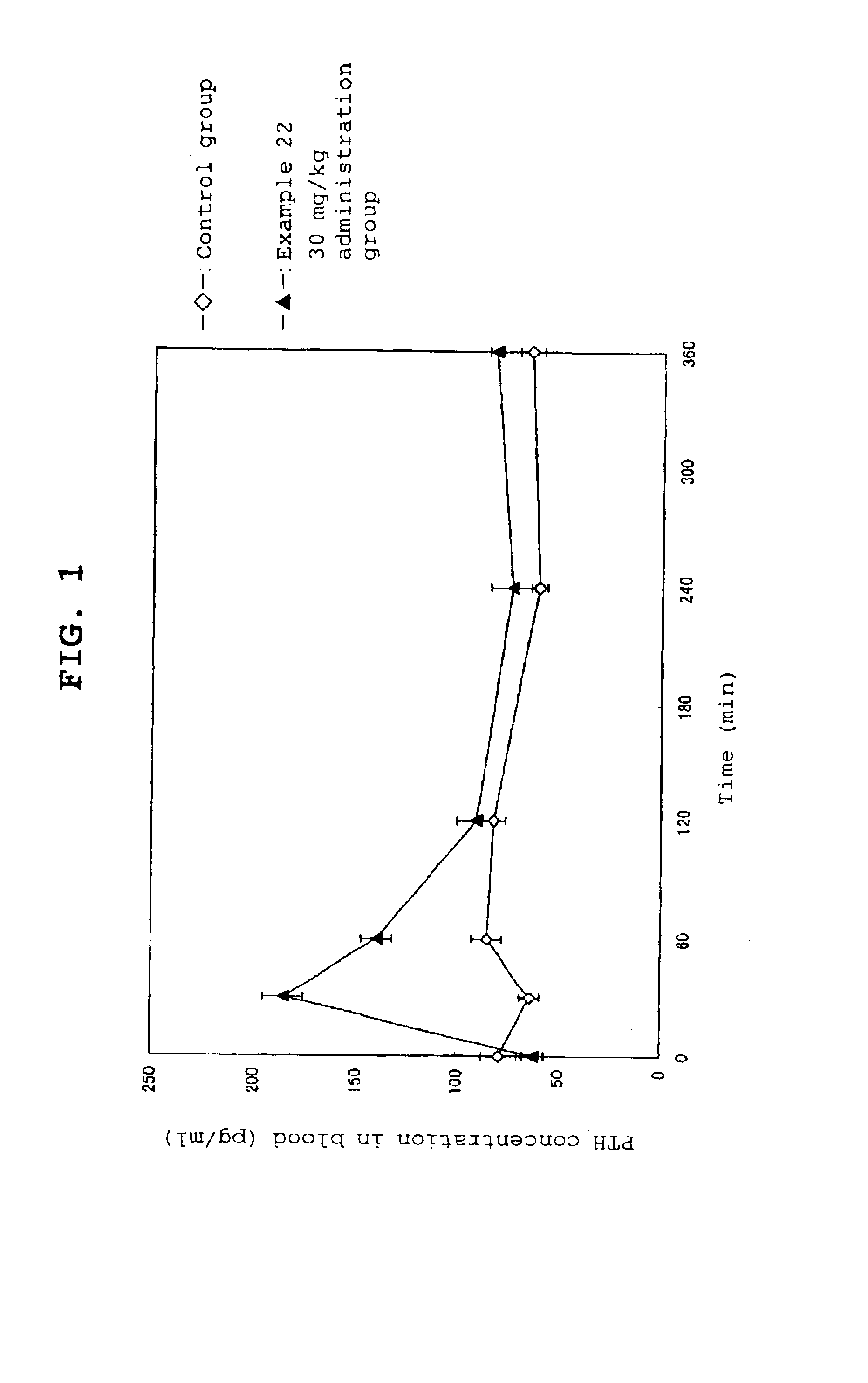
Method for inhibiting bone resorption
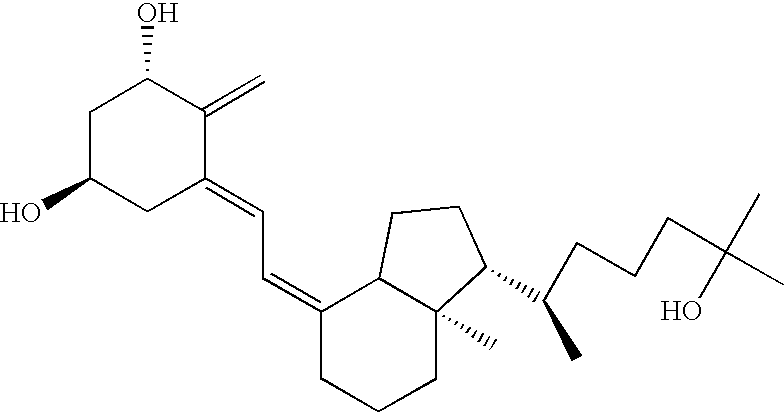
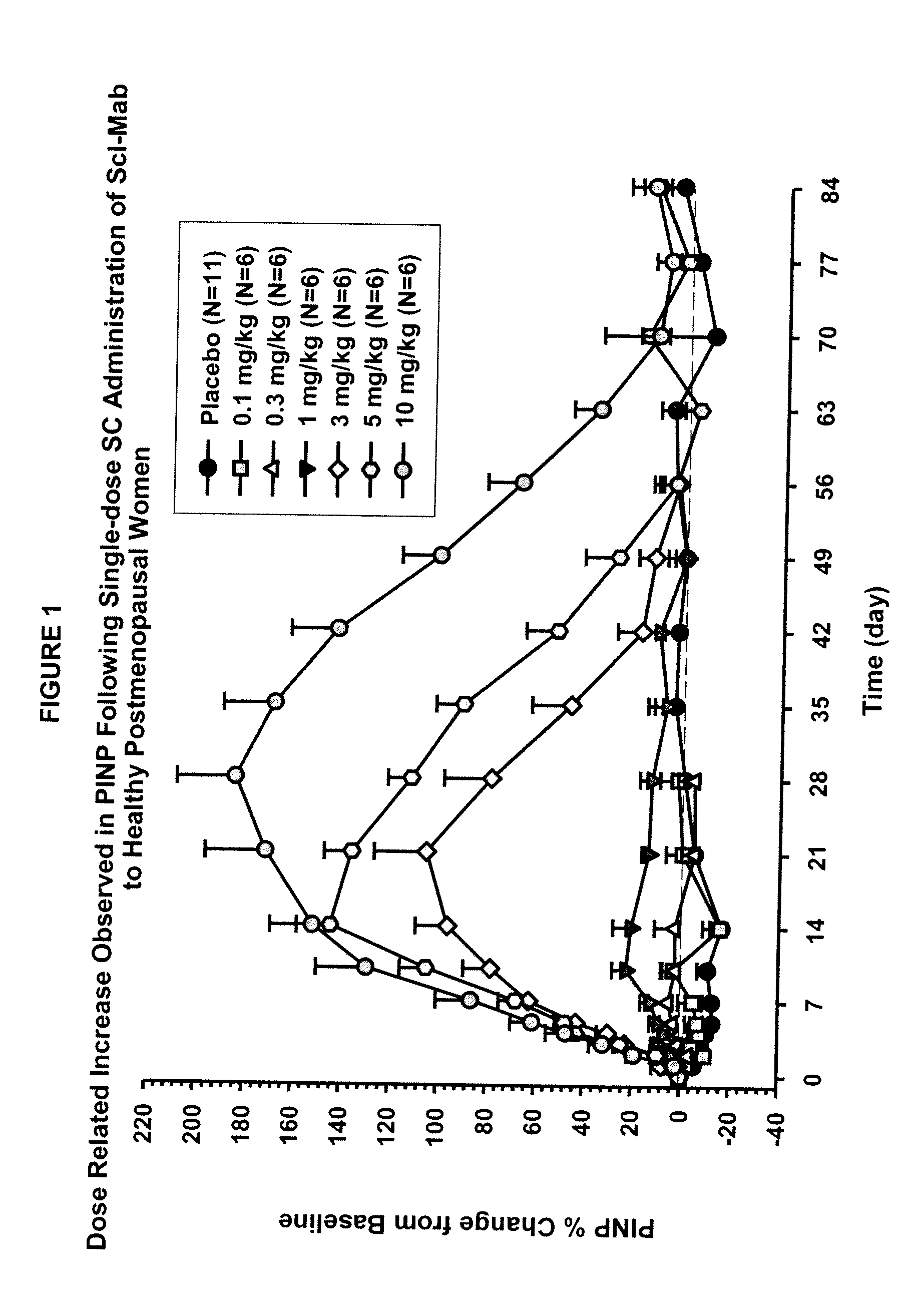
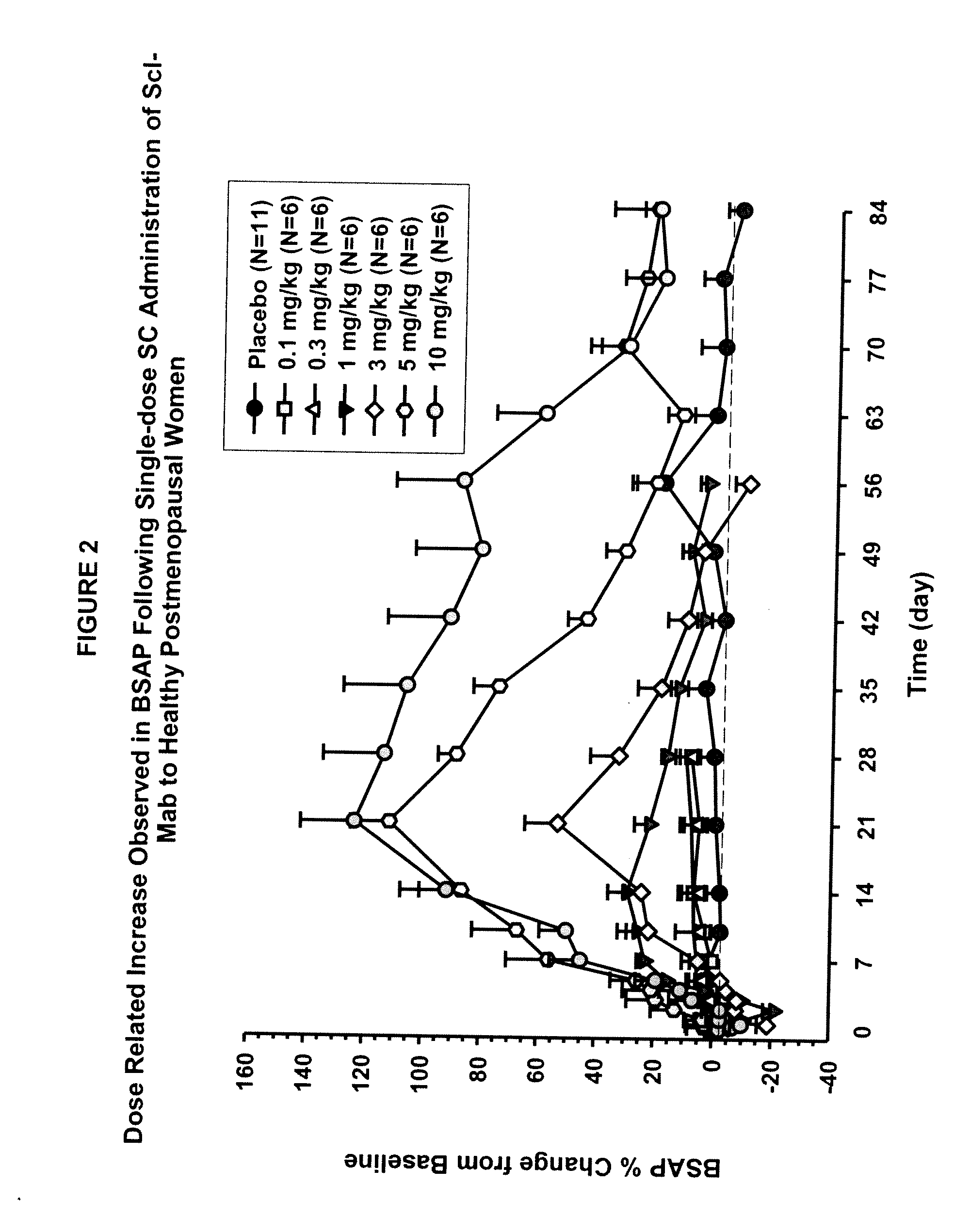
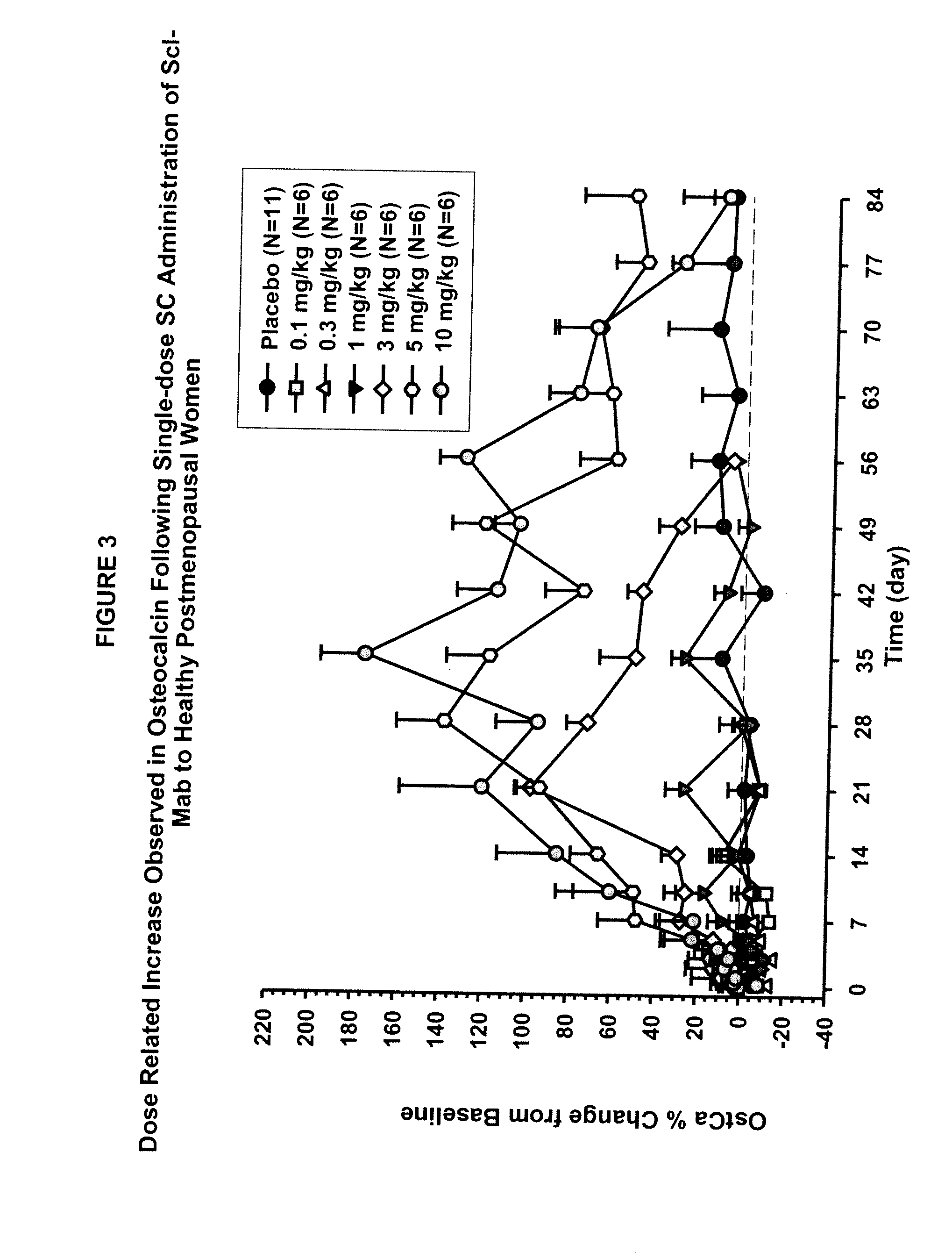
ActiveUS20090074763A1Inhibiting bone resorptionLower Level RequirementsAntibacterial agentsNervous disorderBone densityIncreased bone mineral density
The invention is directed to a method of inhibiting bone resorption. The method comprises administering to a human an amount of sclerostin inhibitor that reduces a bone resorption marker level for at least 2 weeks. The invention also provides a method of monitoring anti-sclerostin therapy comprising measuring one or more bone resorption marker levels, administering a sclerostin binding agent, then measuring the bone resorption marker levels. Also provided is a method of increasing bone mineral density; a method of ameliorating the effects of an osteoclast-related disorder; a method of treating a bone-related disorder by maintaining bone density; and a method of treating a bone-related disorder in a human suffering from or at risk of hypocalcemia or hypercalcemia, a human in which treatment with a parathyroid hormone or analog thereof is contraindicated, or a human in which treatment with a bisphosphonate is contraindicated.
Owner:AMGEN INC
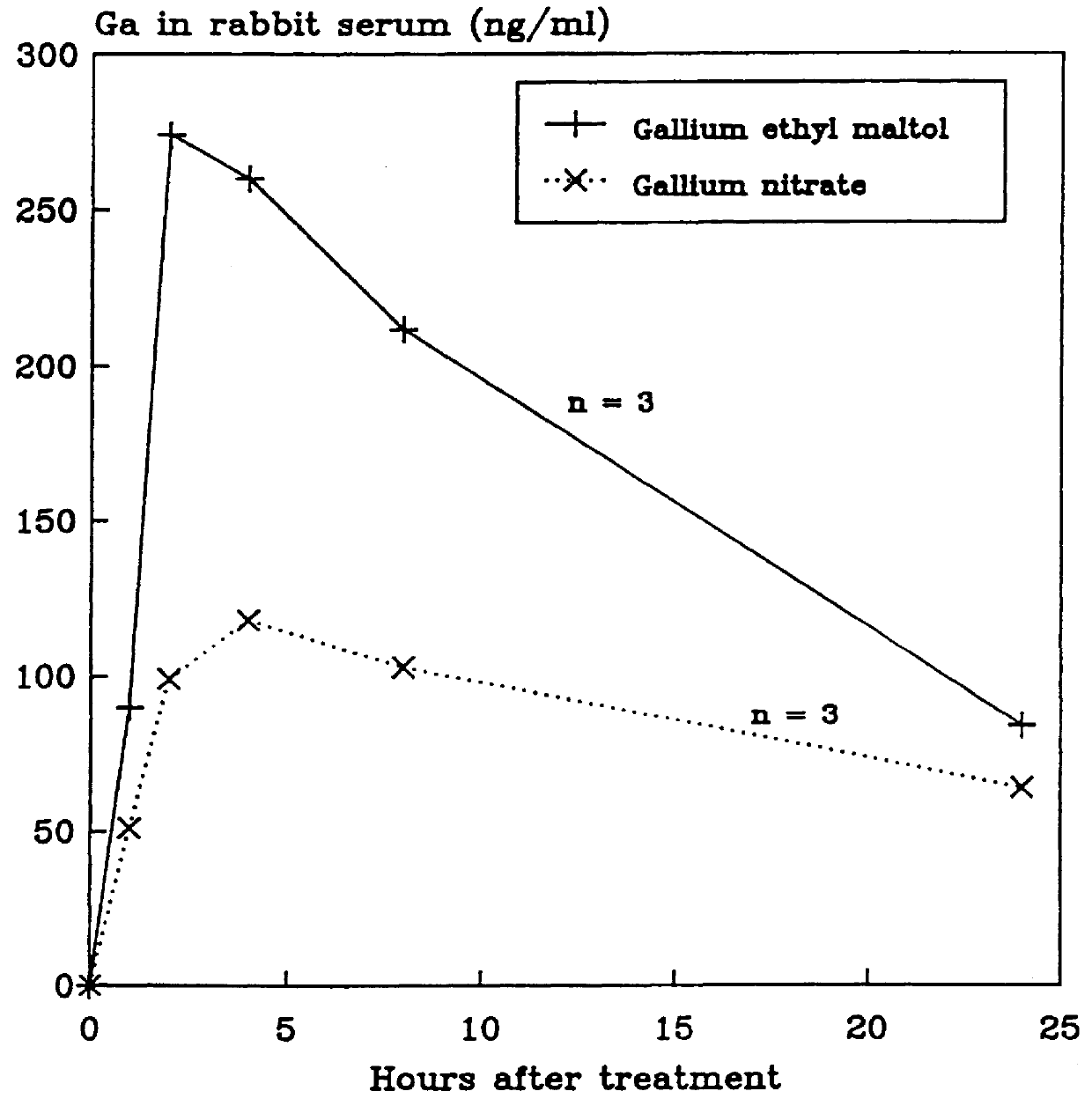
Gallium complexes of 3-hydroxy-4-pyrones to treat cancer
Disclosed are pharmaceutical compositions that comprise gallium complexes of 3-hydroxy-4-pyrones. These compositions provide enhanced gallium bioavailability particularly when orally administered as compared to the gallium bioavailability achieved by use of pharmaceutical compositions containing gallium salts. Compositions included in this invention are useful in providing gallium to humans and other mammals for a wide variety of medical and veterinary applications, including the treatment, prevention, or diagnosis of hypercalcemia, certain cancers, certain disorders of calcium homeostasis, and certain bone diseases including osteoporosis, osteopenia, and Paget's disease.
Owner:BERNSTEIN LAWRENCE RICHARD
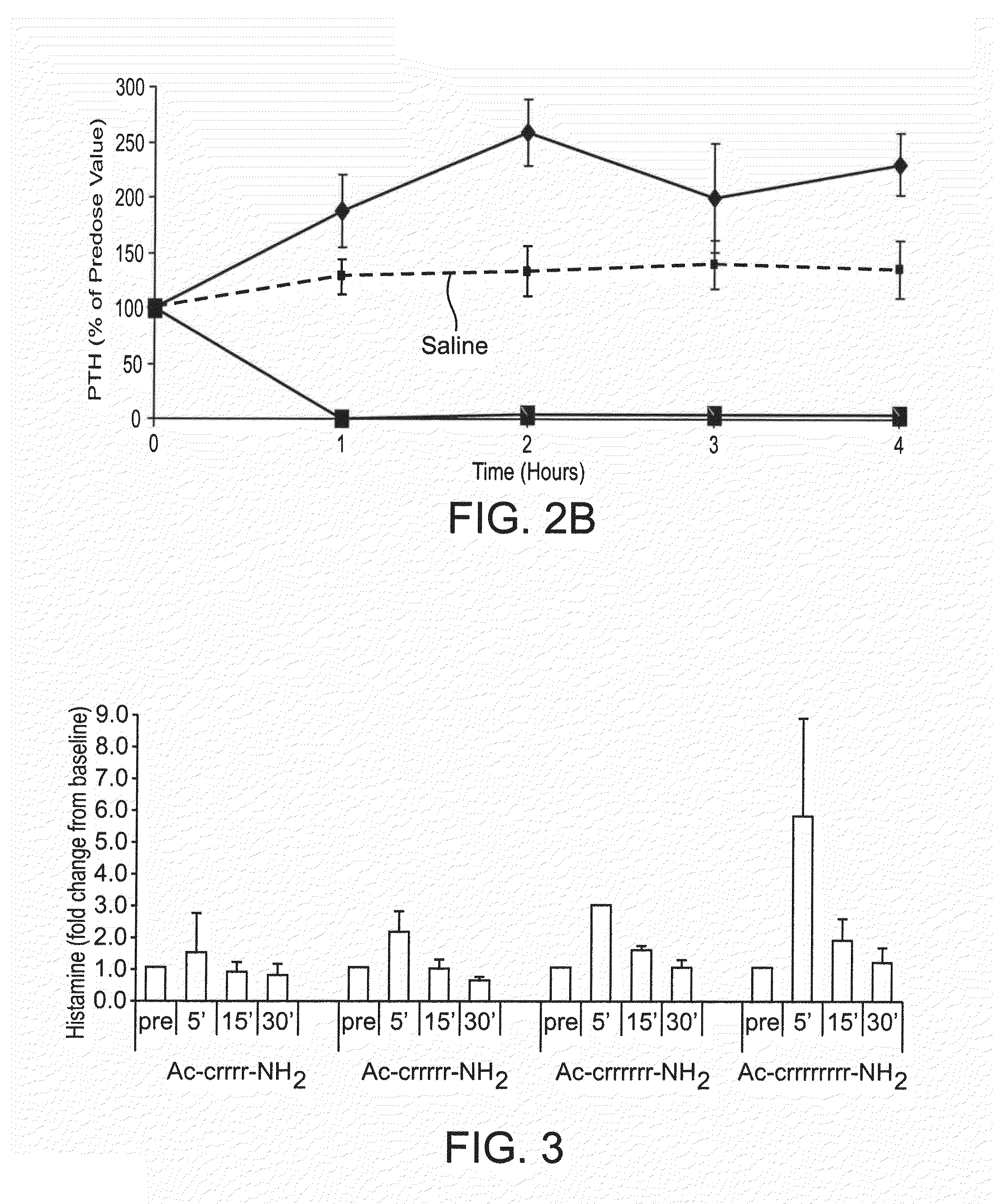
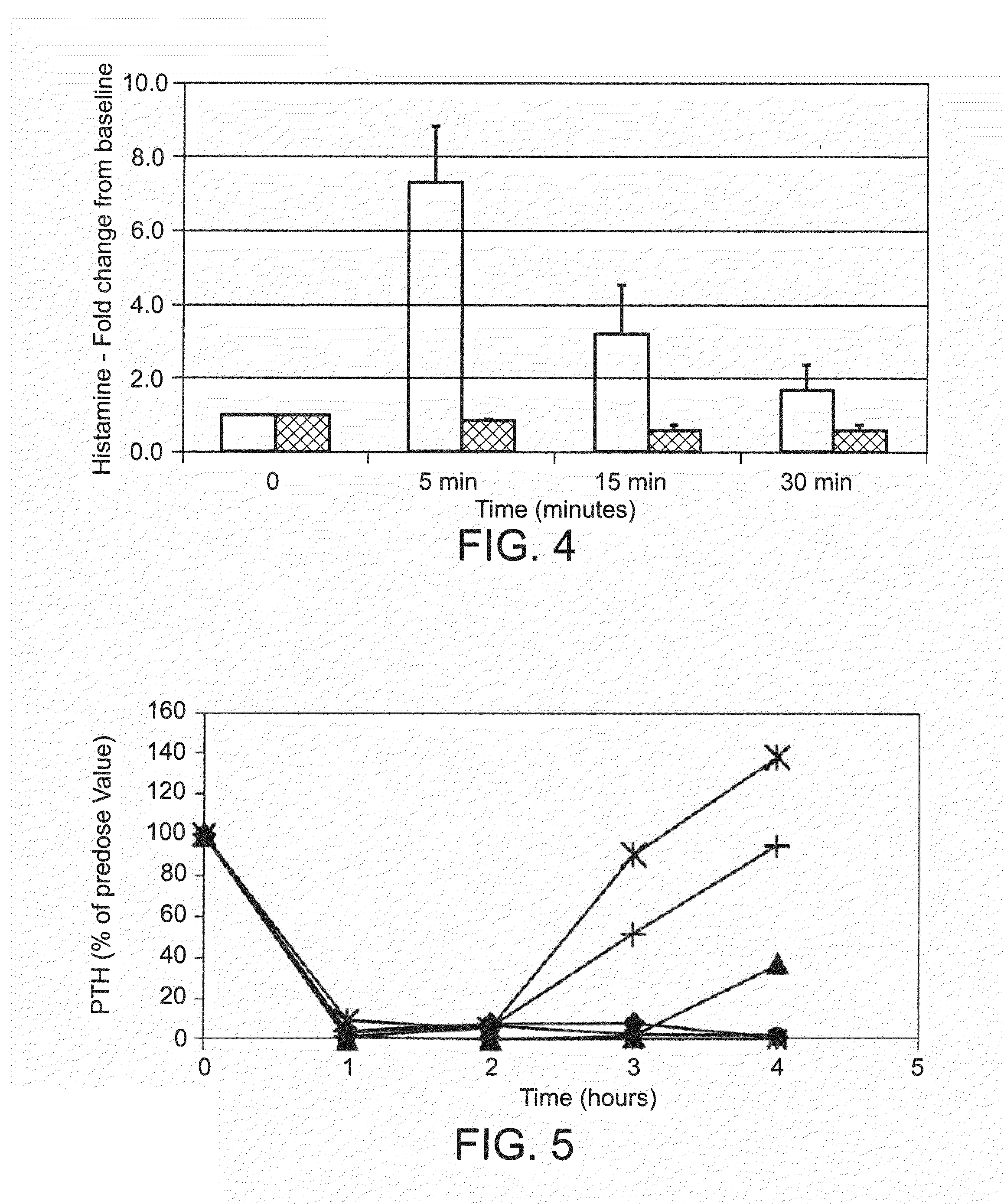
Polycationic calcium modulator peptides for the treatment of hyperparathyroidism and hypercalcemic disorders
ActiveUS20090023652A1Reduce serum PTHReduce serum calciumPeptide/protein ingredientsAntipyreticHypercalcemic disorderHyperparathyroidism
The present invention provides methods and kits for treating hyperparathyroidism, bone disease and / or hypercalcemic disorders. In particular, methods for lowering serum PTH and serum calcium using polycationic calcium modulator peptides are provided. The calcium modulator peptides can be used to treat subjects having, for example: primary, secondary or tertiary hyperparathyroidism; hypercalcemia of malignancy; metastatic bone disease; or osteoporosis.
Owner:KAI PHARMA
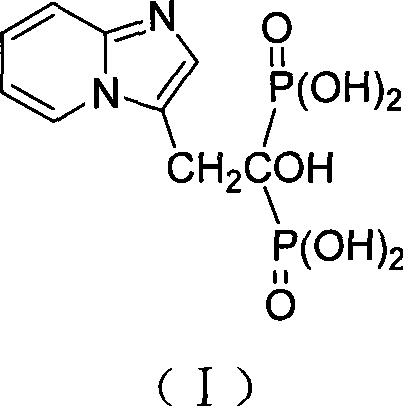
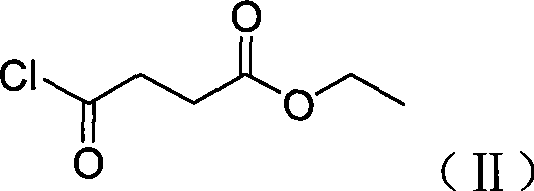
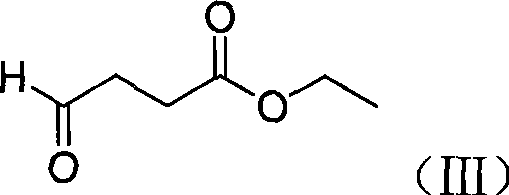
High-purity minodronic acid and preparation method thereof
ActiveCN101531681AFew reaction stepsReduce difficultyMetabolism disorderGroup 5/15 element organic compoundsPyridineIncreased calcium
The invention discloses a high-purity 1-hydroxyl-2-(imidazo(1,2-a) pyridine-3-group) ethylidene-1, 1-diphosphonic acid and a preparation method thereof. The target product of the invention can be applied to the treatment of osteoporosis and hypercalcemia caused by malignant tumor, with higher safety and effectiveness. The preparation method has mild reaction condition and simple post treatment, and can obtain high-purity final products and is easier to implement industrialized production.
Owner:AVENTIS PHARMA HAINAN
Therapeutic agents for reducing parathyroid hormone levels
ActiveUS20110028394A1Easy to transportReduce deliveryOrganic active ingredientsPeptide/protein ingredientsSerum igeThiol
Compounds having activity for lowering parathyroid hormone levels are described. In one embodiment, the compounds are comprised of a contiguous sequence of subunits, X1-X2-X3-X4-X5-X6-X7, wherein the X1 subunit comprises a thiol-containing moiety and the distribution of charge on the X2-X7 subunits provides the desired activity. Methods of using the compounds for treating hyperparathyroidism, bone disease and / or hypercalcemic disorders are also described, and in particular, methods for lowering plasma PTH and serum calcium are provided. The compounds can be used to treat subjects having, for example: primary, secondary or tertiary hyperparathyroidism; hypercalcemia of malignancy; metastatic bone disease; or osteoporosis.
Owner:KAI PHARMA
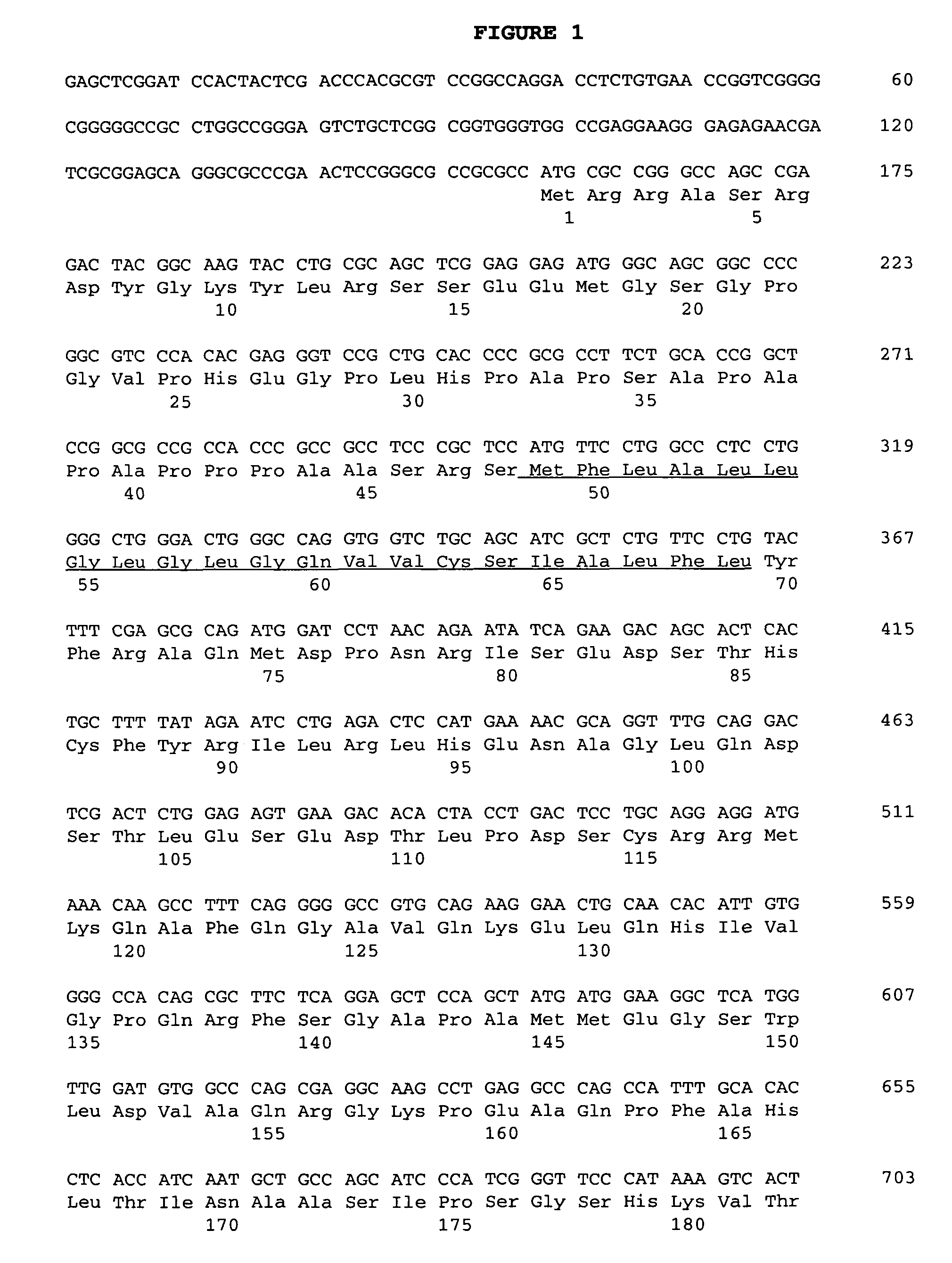
Nucleic acid molecules and polypeptides for a human cation channel polypeptide
InactiveUS20030096249A1Increase calciumAntibody mimetics/scaffoldsTissue cultureRisk strokeNeurological disorder
The present invention relates to novel human nucleic acid molecules encoding novel human cation channels, and proteins and polypeptides encoded by such nucleic acid molecules. More specifically, the nucleic acid molecules of the invention include the novel human gene designated HBMYCNG. The proteins and polypeptides of the invention represent a novel cation channel that may be therapeutically valuable targets for drug delivery in the treatment of human diseases which involve calcium, sodium, potassium or other ionic homeostatic dysfunction, such as central nervous system (CNS) disorders, e.g., stroke, anxiety and depression, or degenerative neurological disorders such as Alzheimer's disease or Parkinson's disease, or other disorders such as cardiac disorders, e.g., arrhythmia, diabetes, chronic pain, hypercalcemia, hypocalcemia, hypercalciuria, hypocalciuria, or ion disorders associated with immunological disorders, gasto-intestinal (GI) tract disorders, or renal or liver disease.
Owner:BRISTOL MYERS SQUIBB CO
Methods of using vitamin D compounds in the treatment of myelodysplastic syndromes
InactiveUS20070027120A1Fast concentrationQuick eliminationOrganic active ingredientsBiocideActive agentHigh doses
Owner:WHITEHOUSE MARTHA J +1
Acid stabilized calcium carbonate an method of making it
InactiveUS7033428B2Calcium/strontium/barium carbonatesPigmenting treatmentPrecipitated calcium carbonateWater soluble
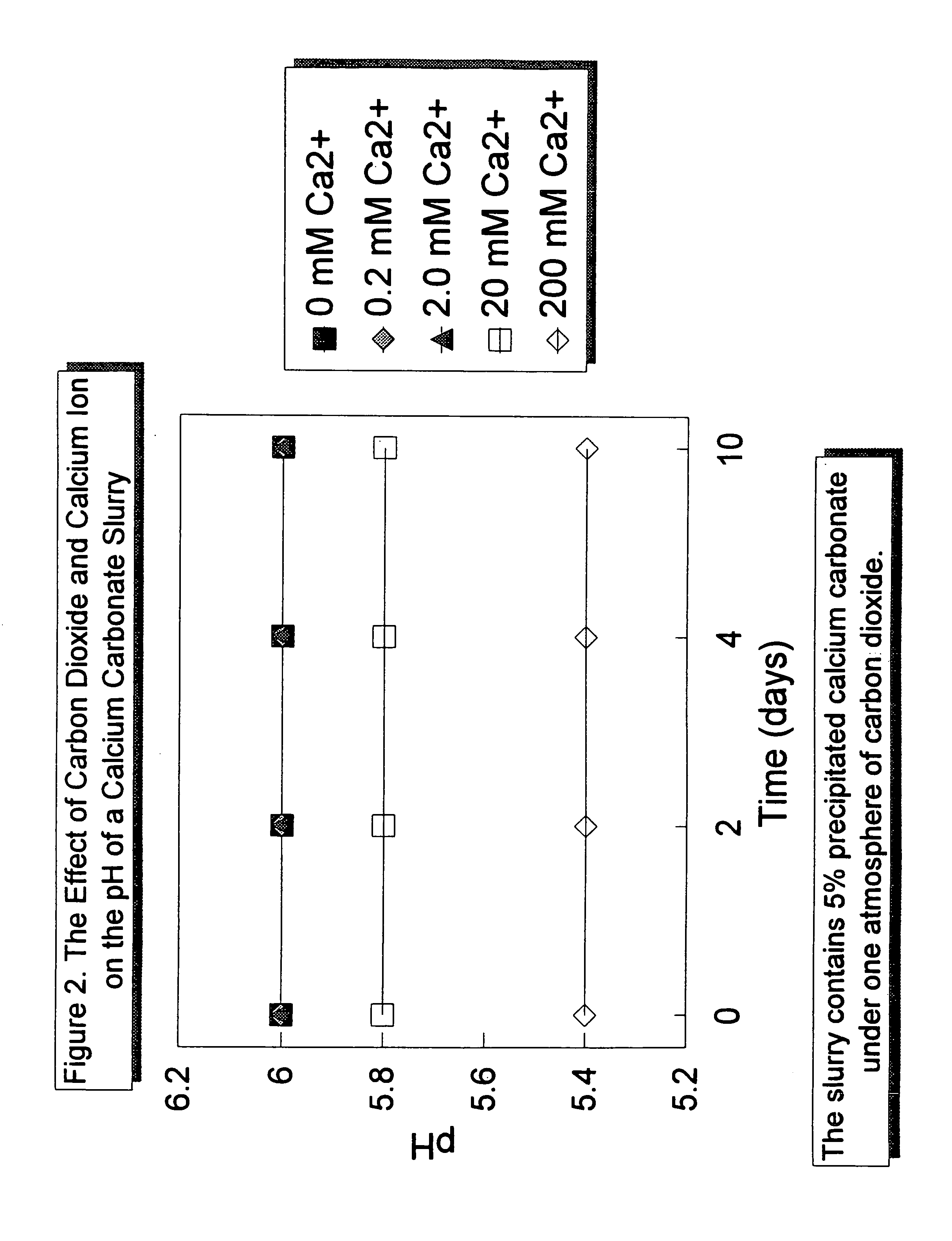
The present invention relates to an acid-stabilized calcium carbonate slurry having a pH of less than 7, preferably between about 6 and about 7, containing water, calcium carbonate, preferably precipitated calcium carbonate, and an acid-stabilizer of a water soluble calcium salt, a weak acid, a chelating agent, a weak acid capable of chelating calcium ion, or a mixture thereof. The acid-stabilizer is present in an amount sufficient to provide an aqueous calcium carbonate slurry having an increased calcium ion concentration and an acidic pH. In a typical acid-stabilized calcium carbonate slurry of the invention, the acid-stabilizer is present in an amount sufficient to provide a calcium ion concentration of about 1 millimolar to about 5 molar, preferably from about 1 to about 120 millimolar. The invention also relates to a method of making the acid-stabilized calcium carbonate slurry of the invention, to a method of forming a filled paper, that includes the step of adding the acid-stabilized calcium carbonate slurry of the invention to a papermaking pulp in a process for making acid paper, and to a filled paper produced according to the method of the invention.
Owner:MINERALS TECH
Heterocyclic Compounds For Preventing And Treating Disorders Associated With Excessive Bone Loss
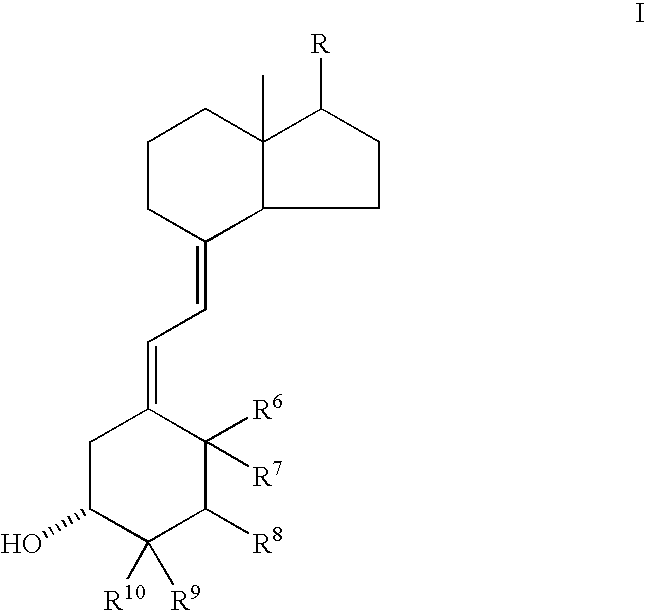
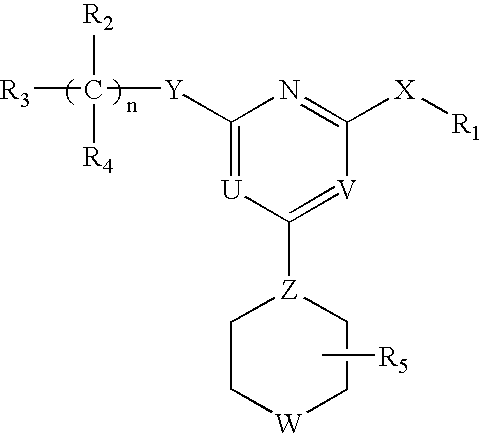
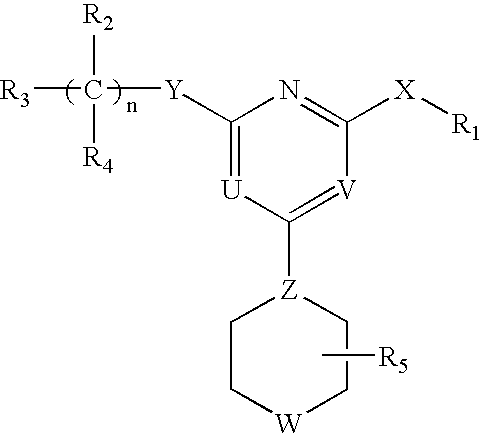
This invention relates to pyrimidine compounds of formula (I), formula (I′), and formula (I″):and pharmaceutically acceptable salts, solvates, clathrates, and prodrugs thereof, wherein R1, R2, R3, R4, R5, U, V, W, X, Y, Z, and n are defined herein. This invention also relates to compositions comprising these compounds and methods for using them. The compounds and compositions of this invention are useful to treat or prevent disorders associated with excessive bone loss, including, without limitation periodontal disease, non-malignant bone disorders (such as osteoporosis, Pagers-disease of bone, osteogenesis imperfecta, fibrous dysplasia, and primary hyperparathyroidism) estrogen deficiency, inflammatory bone loss, bone malignancy, arthritis, osteopetrosis, and certain cancer-related disorders (such as hypercalcemia of malignancy (HCM), osteolytic bone lesions of multiple myeloma and osteolytic bone metastases of breast cancer and other metastatic cancers).
Owner:SYNTA PHARMA CORP
Method of treating prostatic diseases using a combination of vitamin D analogues and other agents
InactiveUS20060003950A1Reduce incidenceReduce riskBiocideCarbohydrate active ingredientsAnticarcinogenActive Vitamin D
The invention provides therapeutic methods for inhibiting, ameliorating or alleviating the hyperproliferative cellular activity of diseases of the prostate, e.g., prostatic cancer and prostatic hyperplasia, which includes administering to a patient in need thereof an active vitamin D analogue and another anticancer agent. Cell differentiation is promoted, induced or enhanced without causing to the patient dose-limiting hypercalcemia and hypercalciuria.
Owner:BONE CARE INT
Methods for decreasing osteoclast formation or bone resorption using an antibody to osteoprotegerin binding protein
A novel polypeptide, osteoprotegerin binding protein, involved in osteolcast maturation has been identified based upon its affinity for osteoprotegerin. Nucleic acid sequences encoding the polypeptide, or a fragment, analog or derivative thereof, vectors and host cells for production, methods of preparing osteoprotegerin binding protein, and binding assays are also described. Compositions and methods for the treatment of bone diseases such as osteoporosis, bone loss due to arthritis or metastasis, hypercalcemia, and Paget's disease are also provided.
Owner:AMGEN INC
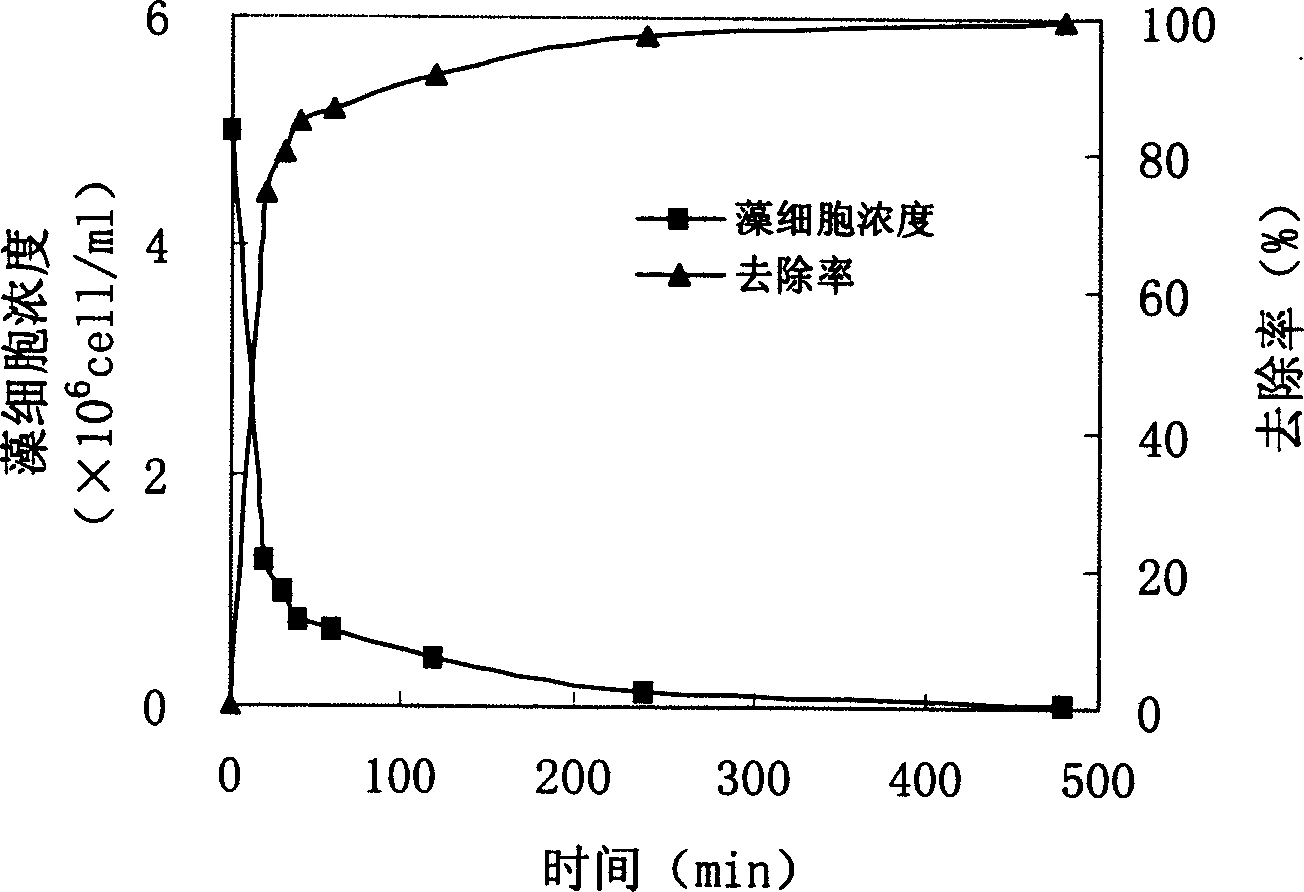
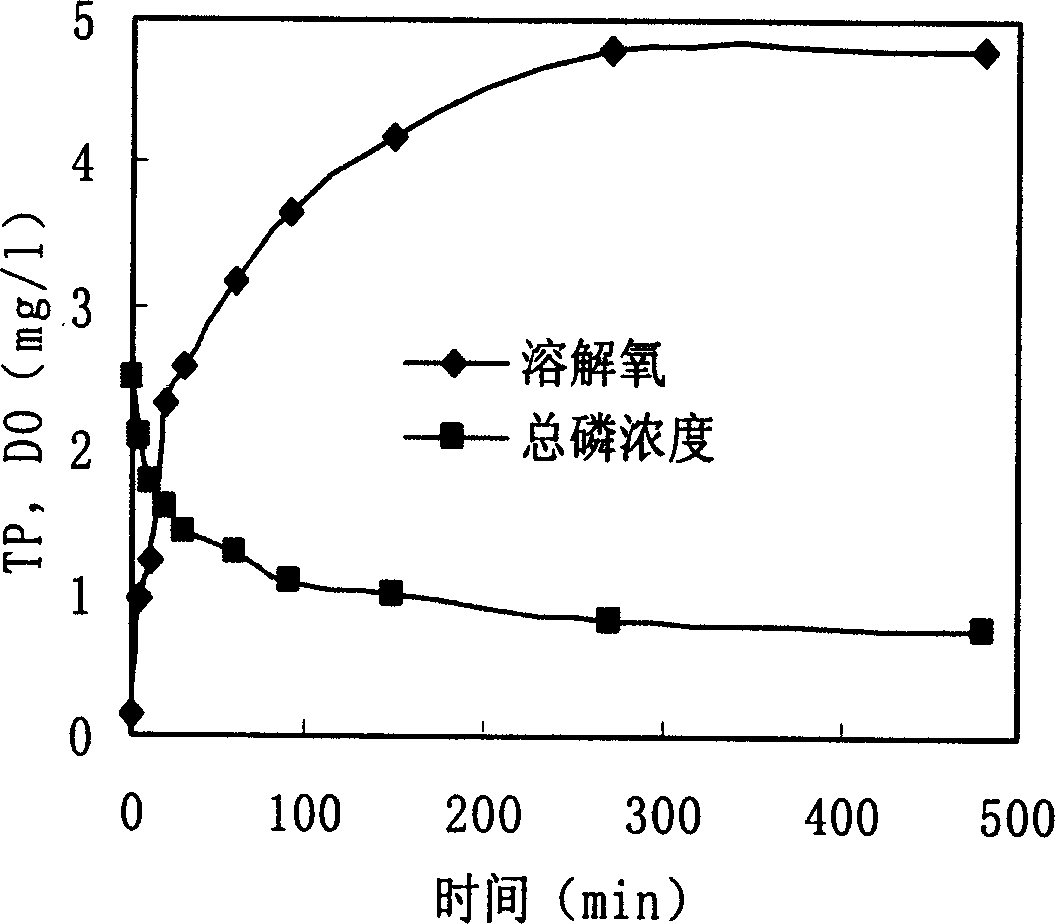
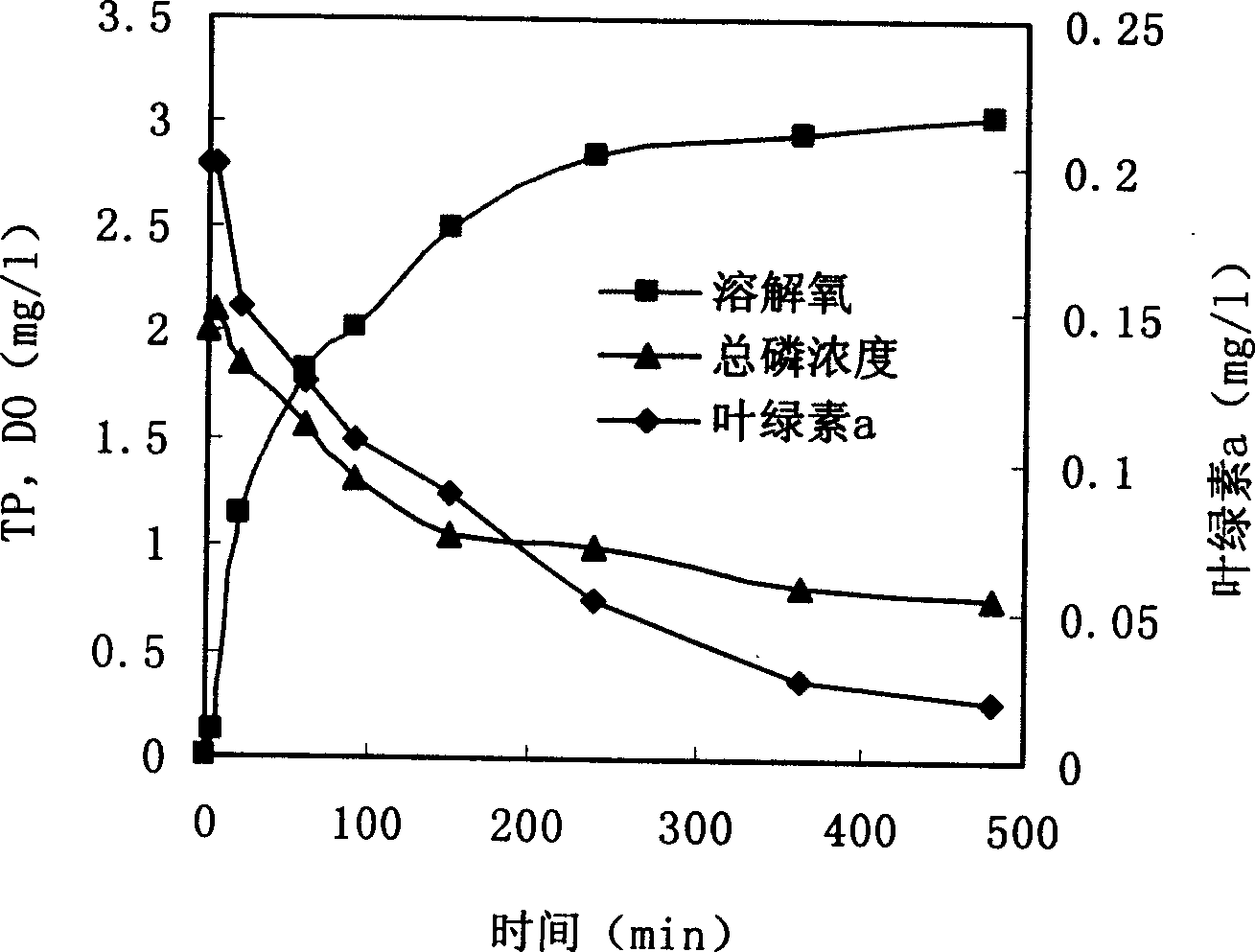
Technique for harnessing water bloom and bed mud secondary pollution using lake sediment
InactiveCN1541952AReduce concentrationIncrease oxygen concentrationWater/sewage treatment by flocculation/precipitationCalcium in biologyEutrophication
The present invention provides one comprehensive technology of utilizing eutrophic and algalbloom polluted precipitant in in-situ algae killing, ventilation and phosphate fixing. By means of the said technology, bed mud or bankside clay is used in treating algalbloom in low cost. The said technology can eliminate algae effectively, increase dissolved oxygen concentration in water, reduce H2S and NH3-N produced, improve water quality, speed the degradation of organic in water, increase calcium in water, make bottom matter loose and ventilating, minimize and fix water body, bed mud and deposited phosphor in algae cell for no further utilization by living things. The present invention may be used in comprehensive treatment of red tide in sea, water bloom in fresh water, etc.
Owner:RES CENT FOR ECO ENVIRONMENTAL SCI THE CHINESE ACAD OF SCI
Method of treating breast cancer using a combination of vitamin D analogues and other agents
ActiveUS7094775B2Good treatment effectReduce in quantityHeavy metal active ingredientsBiocideIncreased calciumActive Vitamin D
The invention provides therapeutic methods for inhibiting, ameliorating or alleviating the hyperproliferative cellular activity of diseases of the breast, e.g., breast cancer, which includes administering to a patient in need thereof an active vitamin D analogue and another anticancer agent. Cell differentiation is promoted, induced or enhanced without causing to the patient dose-limiting hypercalcemia and hypercalciuria.
Owner:BONE CARE INT
Co-administration of steroids and zoledronic acid to prevent and treat side effects from zoledronic acid infusion
ActiveUS20110263537A1Prevent and treat side effectBiocideOrganic active ingredientsPaget DiseaseSide effect
Zoledronic Acid is used for treatment of hypercalcemia of malignancy, for the treatment of bone metastasis associated with malignancies such as prostate and breast cancer, for the prevention of and treatment of osteoporosis and for the treatment of Paget's disease. Administration of Zoledronic Acid is complicated by what is described as “post-dosing syndrome” (PDS) and osteonecrosis of the jaw (ONJ). Inflammation may be the cause of these side effects, which could be decreased by the co-administration of steroids. This application is a method of use patent for the co-administration of steroids (oral, IV, IM, rectal, or by inhalation) with Zoledronic Acid and a composition of matter patent for mixing Methyl Prednisolone with Zoledronic Acid for infusion.
Owner:DESAI KETAN
Calcium receptor antagonist
InactiveUS6916956B2Superior calcium receptor antagonistic actionBiocideOrganic compound preparationNK1 receptor antagonistAbnormal calcium
A compound of the formula [I]wherein R1 is optionally substituted aryl group or optionally substituted heteroaryl group; R2 is optionally substituted C1-6 alkyl group, C3-7 cycloalkyl group and the like; R3 is hydrogen atom, C1-6 alkyl group, hydroxyl group and the like; R4 is hydrogen atom, C1-6 alkyl group and the like; R5 and R6 are each C1-6 alkyl group and the like; R7 is optionally substituted aryl group or optionally substituted heteroaryl group; X1, X2 and X3 are each C1-6 alkylene group and the like; and X4 and X5 are each a single bond, methylene group and the like, a salt thereof, a solvate thereof or a prodrug thereof, and a pharmaceutical composition containing the compound, particularly a calcium receptor antagonist and a therapeutic agent for osteoporosis, are provided. The compound of the present invention is useful as a therapeutic drug of diseases accompanied by abnormal calcium homeostasis, or osteoporosis, hypoparathyreosis, osteosarcoma, periodontal disease, bone fracture, steoarthrosis, chronic rheumatoid arthritis, Paget's disease, humoral hypercalcemia, autosomal dominant hypocalcemia and the like.In addition, an intermediate for the compound is provided.
Owner:JAPAN TOBACCO INC
Pyrrolidine derivative or salt thereof
InactiveUS20090062366A1Excellent CaSR agonistic activityHigh activityBiocideOrganic chemistryDiseaseRenal osteodystrophy
[Problem] To provide a compound which may be used in treating diseases in which a calcium sensing receptor (CaSR) is concerned, particularly hyperparathyroidism.[Means for Resolution] It was found that novel pyrrolidine derivatives which are characterized by the possession of aminomethyl group substituted with arylalkyl group or the like, or salts thereof, have excellent CaSR agonistic regulatory activity and also have excellent selectivity with CYP2D6 inhibitory activity having a possibility of causing drug interaction. Based on the above, these novel pyrrolidine derivatives are useful as therapeutic agents for treating diseases in which CaSR is concerned (hyperparathyroidism, renal osteodystrophy, hypercalcemia and the like).
Owner:ASTELLAS PHARMA INC
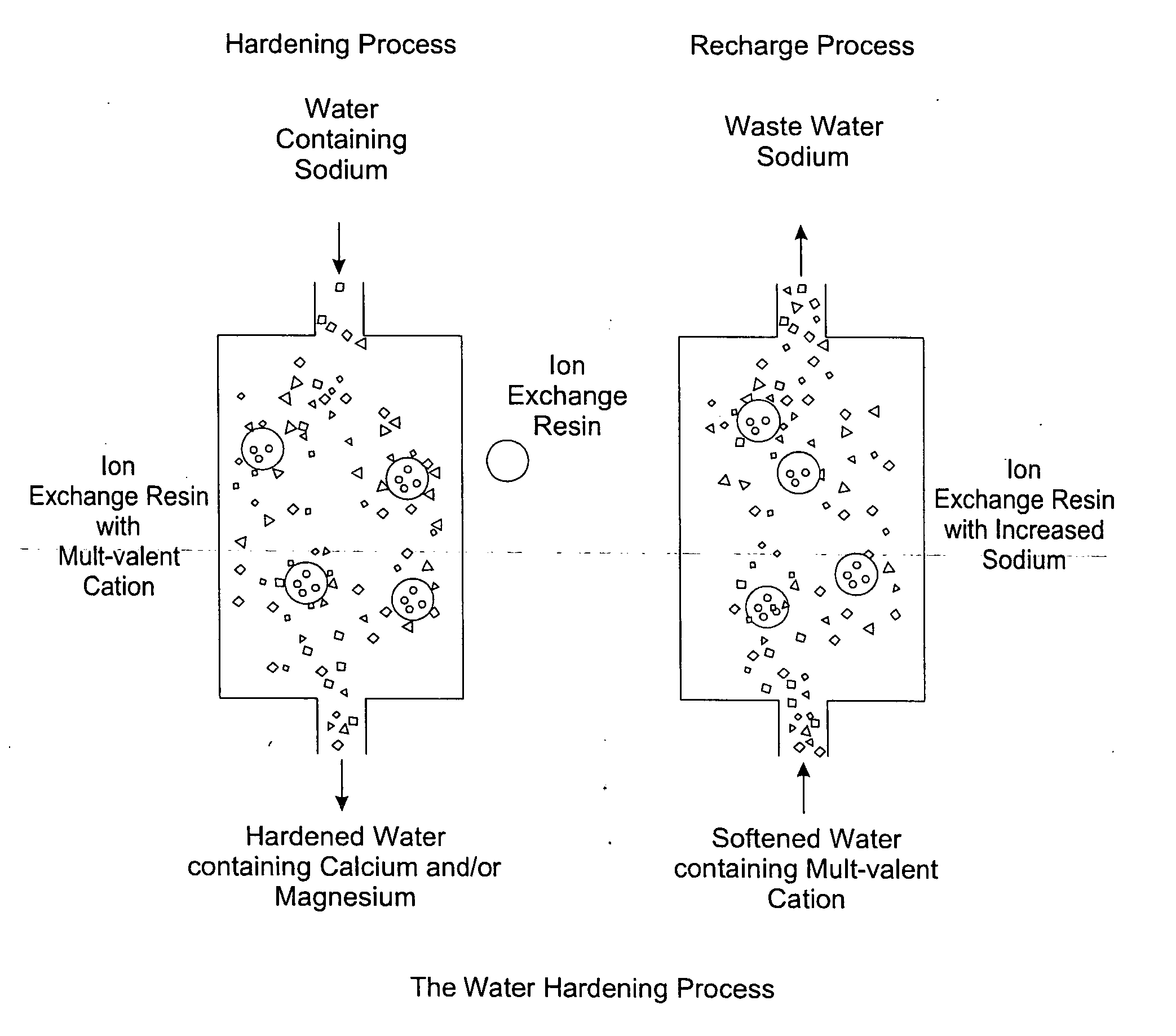
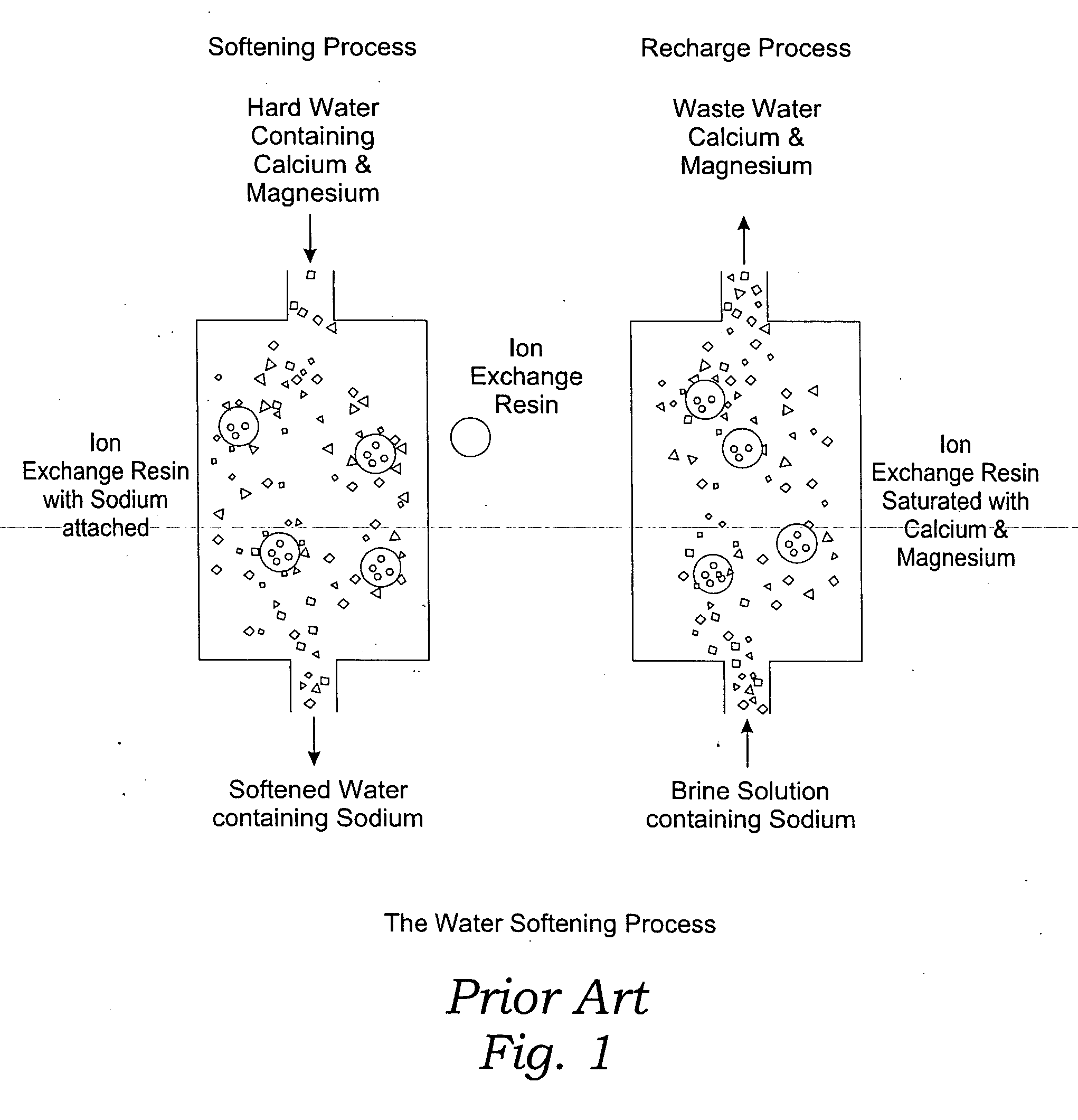
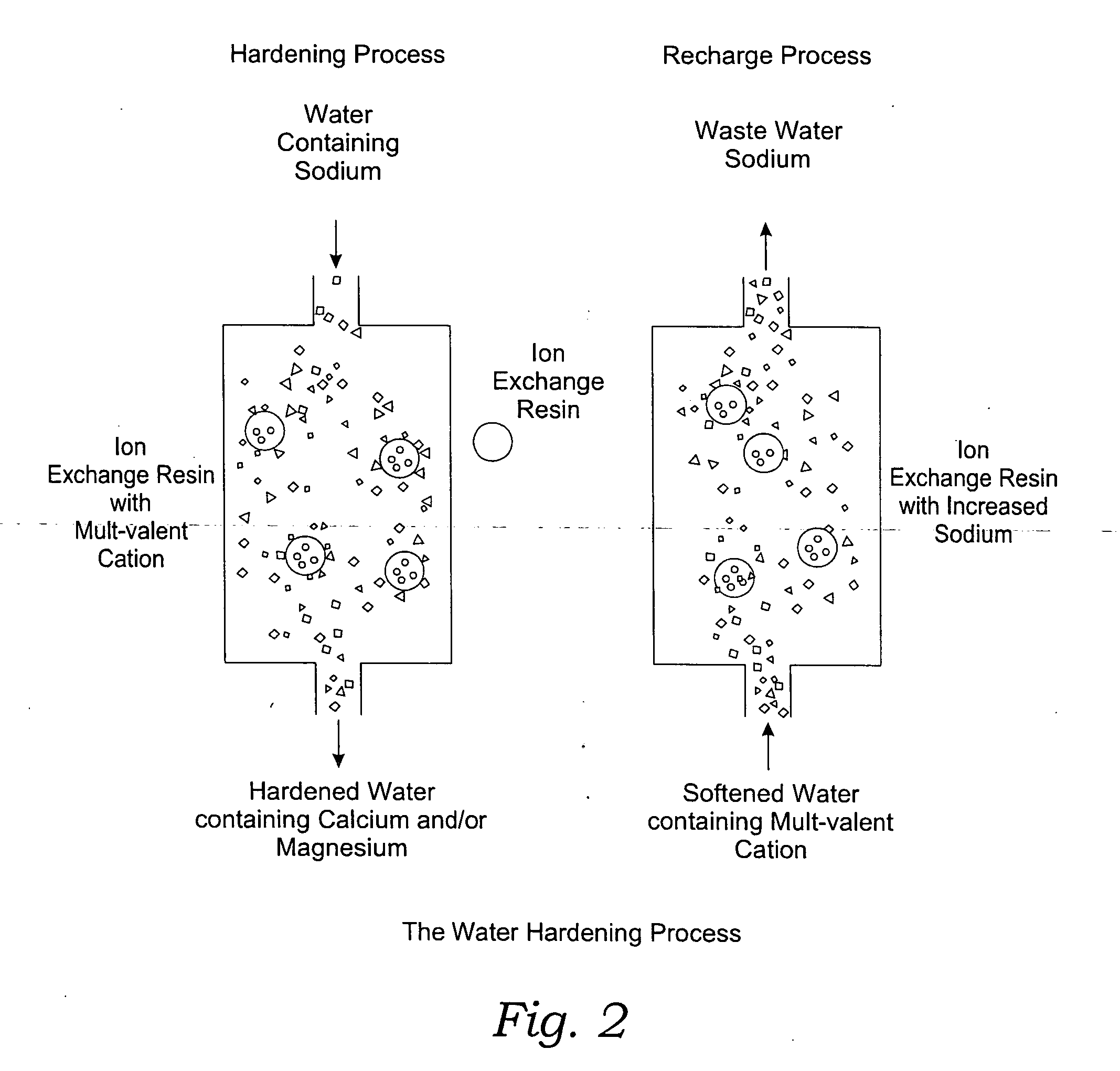
Methods of utilizing waste waters produced by water purification processing
InactiveUS20050210745A1Economically and efficiently processingLow sodium contentSpecific water treatment objectivesScale removal and water softeningDecreased sodiumHigh sodium
The invention relates to a process for treating unwanted moderately saline waters for producing waters acceptable for treating soil, such as for irrigation. The treated water is also suitable for human and livestock consumption. The process includes passing moderately saline waters having 0.05% or more by weight and less than 1.00% by weight of the salts of Na, K, Ca, Mg, Fe, Cl, SO4, or CO3 or combinations thereof through an ion exchange resin. The ion exchange resin is pre-treated to be saturated with multivalent cations. Preferred multivalent cations include calcium (Ca2+) or magnesium (Mg2+) ions, or combinations thereof. After passing through the ion exchange resin, the effluent has decreased sodium cations and increased calcium and / or magnesium cations compared to the pre-treated moderately saline water. As the moderately saline waters passes through the ion exchange resin, the sodium content of the resin rises as the multivalent cation content lowers until the resin is unacceptable for further water treatment in accordance with the present invention. To regenerate the ion exchange resin, the resin is flushed with a brine solution having more than 1.00% by weight of the salts of Na, K, Ca, Mg, Fe, Cl, SO4, or CO3. Preferably, the brine is particularly high in calcium and / or magnesium content and low in sodium. The brine solution is flushed through the ion exchange resin until the ion exchange resin is sufficiently saturated with multivalent cations to again process moderately saline water having high sodium content.
Owner:GROTT GERALD J
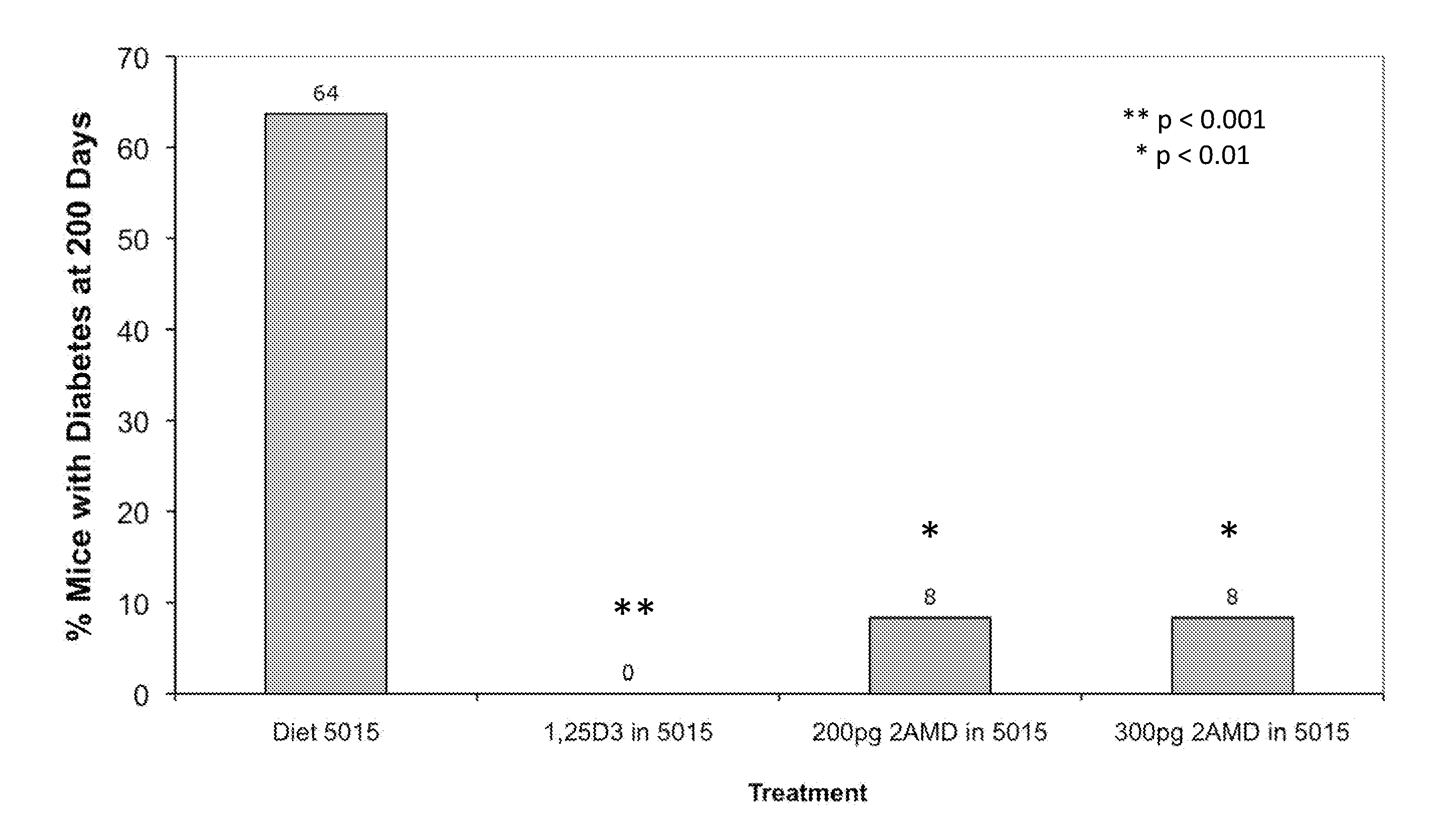
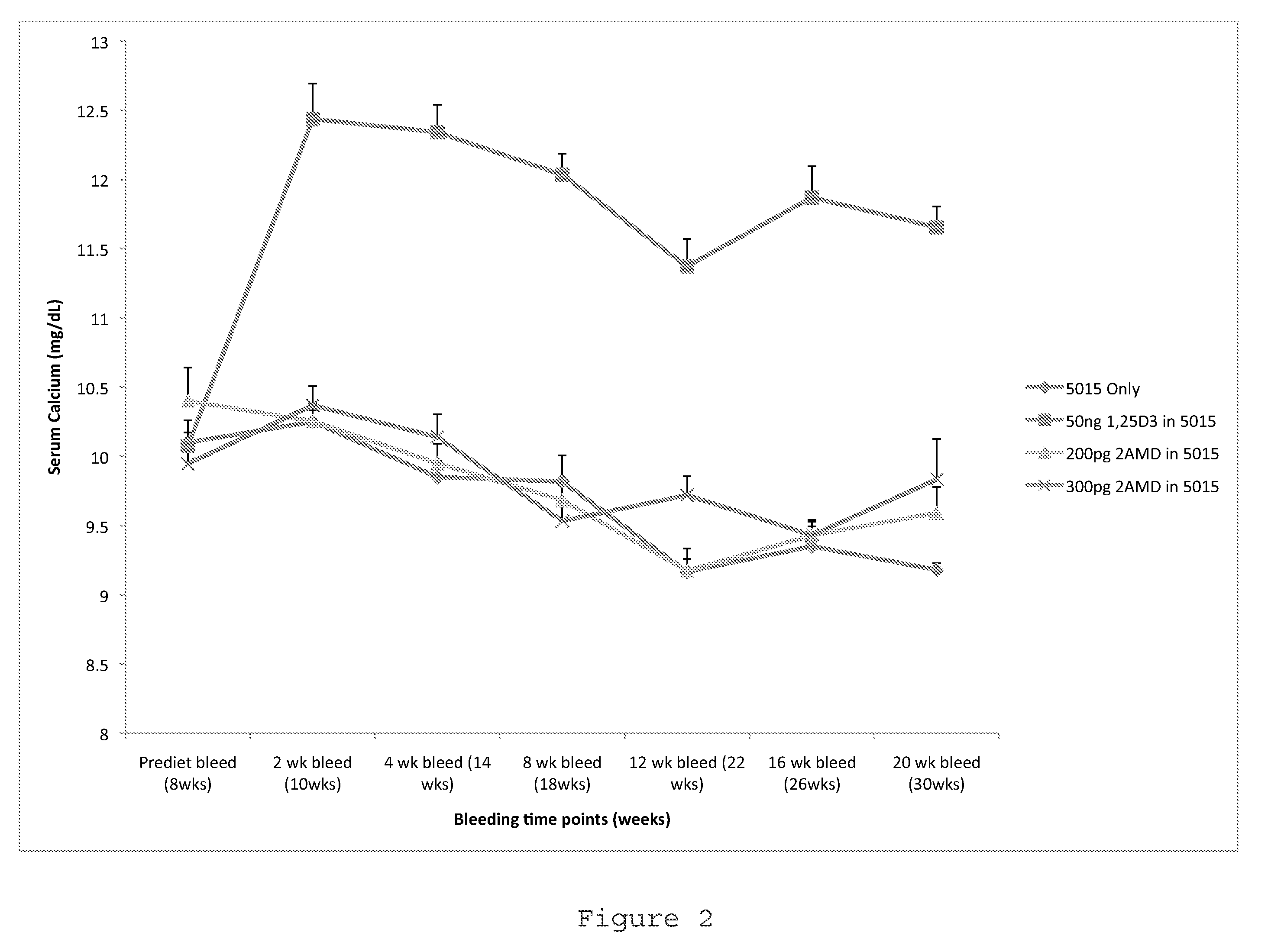
Method of Preventing Type I Diabetes
InactiveUS20110124609A1Avoid typingAvoid developmentOrganic active ingredientsBiocideType 1 diabetesDiabetic patient
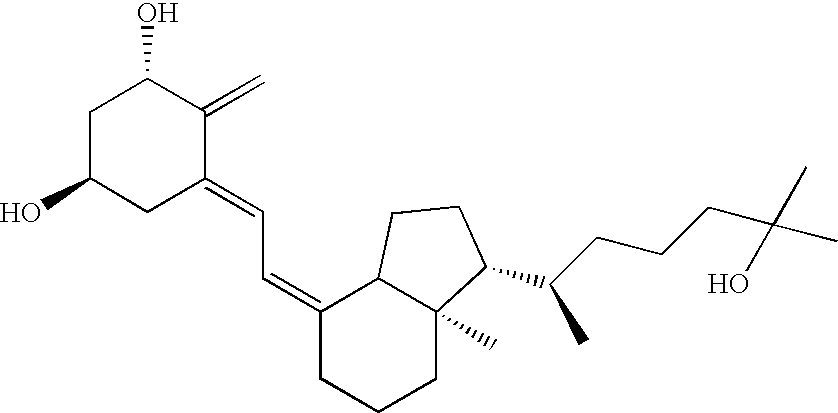
The invention relates to novel methods of using 2α-methyl-19-nor-20(S)-1α,25-dihydroxyvitamin D3 or 2-methylene-19-nor-20(S)-1,25-dihydroxyvitamin D3 to prevent Type 1 diabetes in a subject at risk of developing Type 1 diabetes without causing hypercalcemia in the subject.
Owner:WISCONSIN ALUMNI RES FOUND
Calcium phosphopeptide complexes
InactiveUS7312193B2Prevent demineralisationInhibition formationAntibacterial agentsBiocideDiseaseDietary supplement
Phosphopeptides containing the Ser(P) cluster sequence motif Ser(P)-Ser(P)-Ser(P)-Glu-Glu- can stabilize their own weight in amorphous calcium phosphate (ACP) [Ca3(PO4)1.87(HPO4)0.2xH2O] and amorphous calcium fluoride phosphate (ACFP) [Ca8(PO4)5F x H2O]. The amorphous phases stabilised by the phosphopeptides are an excellent delivery vehicle to co-localise Ca, F, and phosphate at the tooth surface in a slow-release amorphous form producing superior anticaries efficacy. These amorphous phases stabilised by the phosphopeptides also have utility as dietary supplements to increase calcium bioavailability and to help prevent diseases associated with calcium deficiencies.
Owner:UNIVERSITY OF MELBOURNE
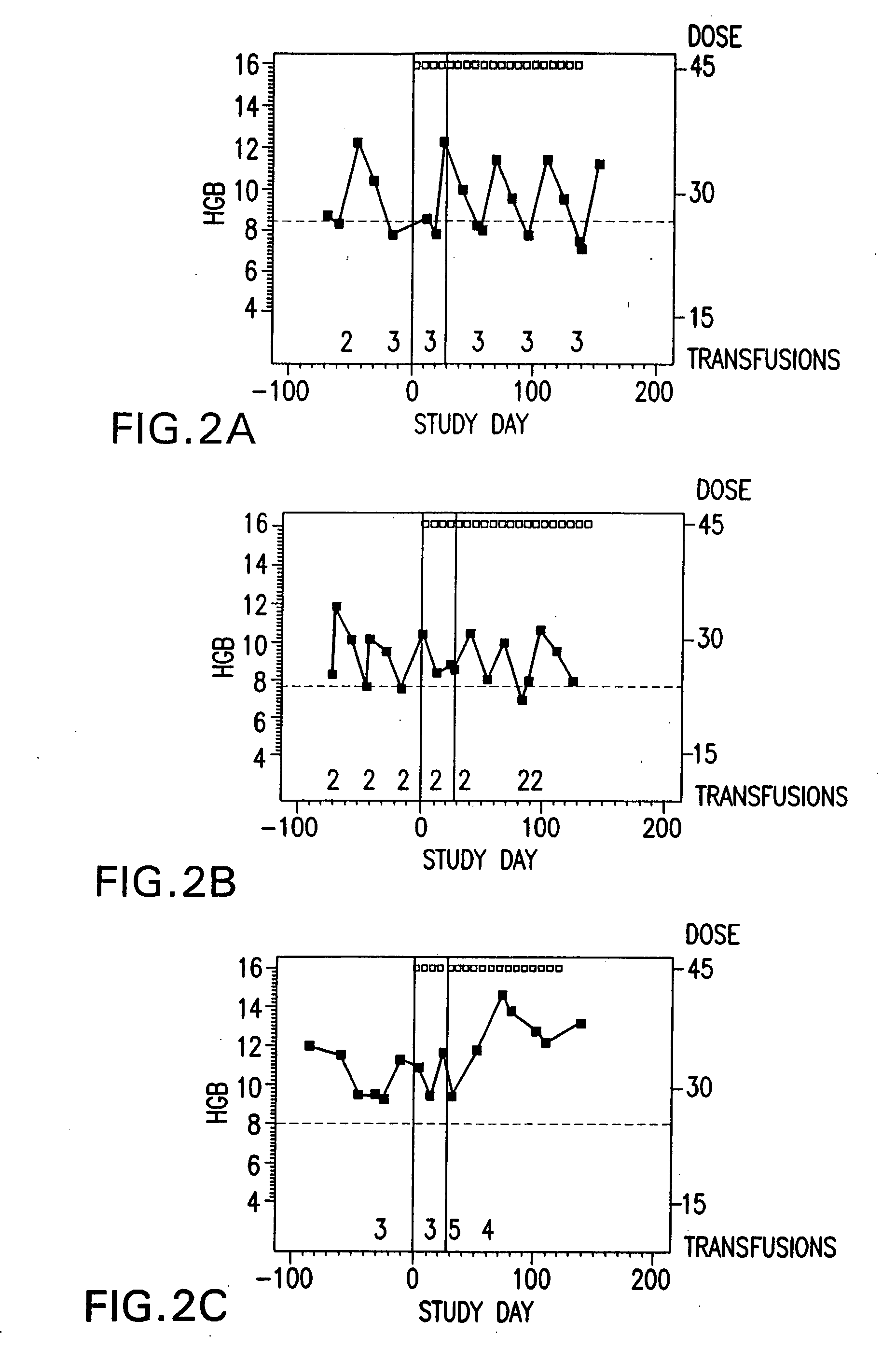
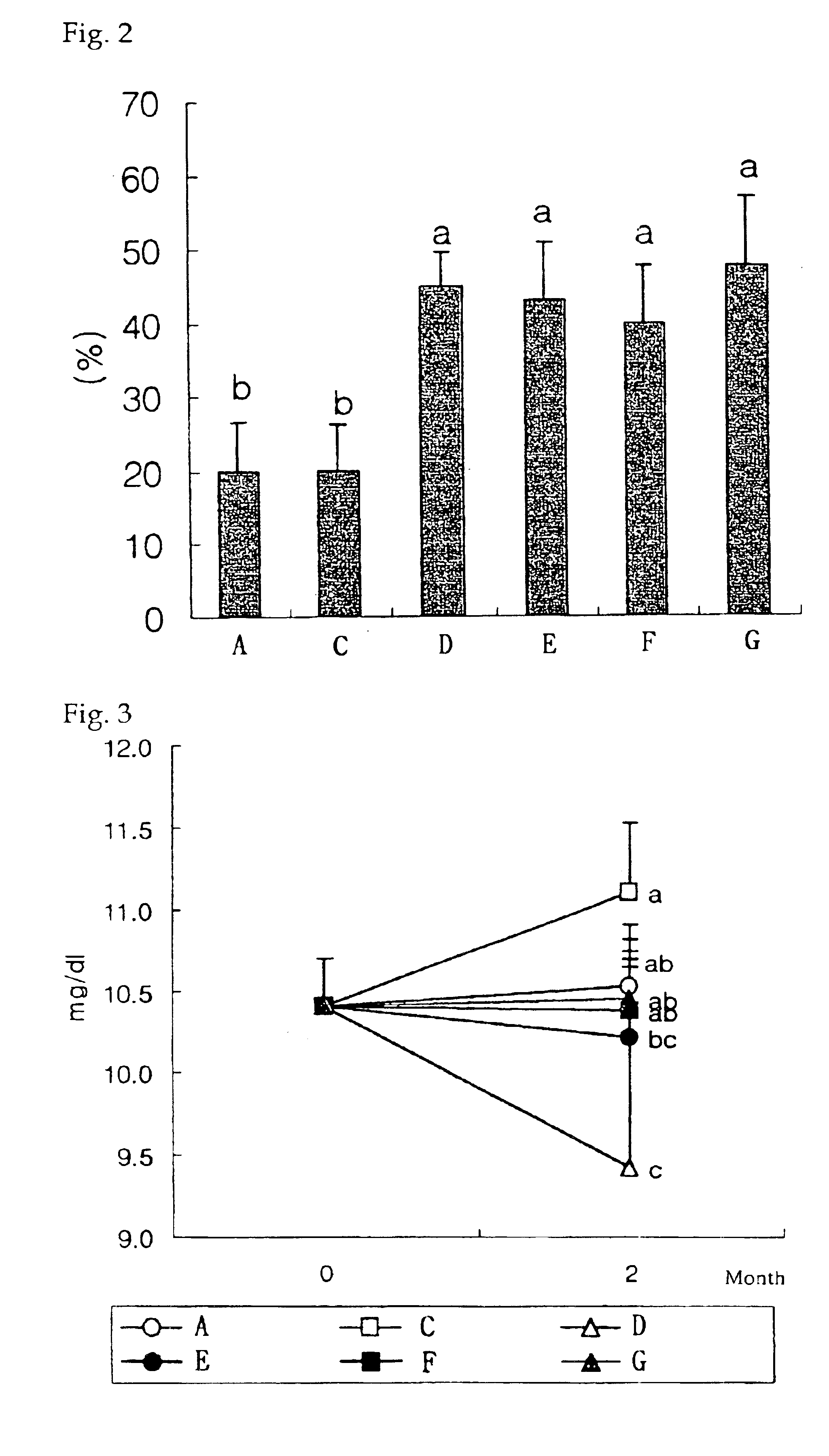
Pharmaceutical compositions and preparations for treatment of metabolic bone disease
The present invention relates to a pharmaceutical composition for the treatment of metabolic bone disease and the method of preparation thereof, and more particularly, to an improved pharmaceutical composition for the therapeutic treatment of metabolic disease and the method of preparation thereof, wherein said composition is prepared as a composite pharmaceutical agent which comprises calcitriol; which reduces the rate of spine fractures and increases bone density; alendronate, a bone resorption inhibitor, as two main active ingredients in an optimal mixing ratio to exert the greatest synergistic therapeutic effect; and adequate amount of other additives such as a resorption fortifier of alendronate. Thereof, the pharmaceutical composition according to the present invention can inhibit hypercalcemia caused when administered by calcitriol alone, compensate the inhibitory activity of bone remodeling caused by alendronate due to the presence of calcitriol, and improve drug compliance associated with the usual difficulty in administration as well as a side effect in esophagus, thus effectively preventing the occurence of osteoporosis.
Owner:YUYU IND
Hydrochloric acid process of producing feed level calcium diphosphate and gypsum coproduct
InactiveCN1772601AReduce the leaching of fluorineCalcium/strontium/barium sulfatesAccessory food factorsPhosphateIncreased calcium
The hydrochloric acid process of producing feed level calcium diphosphate and gypsum coproduct includes the steps of: acidolysis and depositing separation, filtering, the first section neutralization, the second section neutralization, preparing feed level calcium diphosphate, purification and concentrating calcium solution, synthesizing gypsum, recovering hydrochloric acid, and preparing gypsum product. The process adopts phosphate rock powder and sulfuric acid as main material, utilizes hydrochloric acid to acidolyze phosphate rock powder, recovers hydrochloric acid for reuse, controls the leaching out of fluorine and promotes it to precipitate for separating out, and has increased calcium solution purification step. The process has the features of high quality of feed level calcium diphosphate, P2O5 yield up to 90 %, production of gypsum with wide use, less environmental pollution and raised comprehensive utility.
Owner:自贡鸿鹤化工股份有限公司
Pyrrolidine derivative or salt thereof
[Problem] To provide a compound which may be used in treating diseases in which a calcium sensing receptor (CaSR) is concerned, particularly hyperparathyroidism. [Means for Resolution] It was found that novel pyrrolidine derivatives which are characterized by the possession of aminomethyl group substituted with arylalkyl group or the like, or salts thereof, have excellent CaSR agonistic regulatory activity and also have excellent selectivity with CYP2D6 inhibitory activity having a possibility of causing drug interaction. Based on the above, these novel pyrrolidine derivatives are useful as therapeutic agents for treating diseases in which CaSR is concerned (hyperparathyroidism, renal osteodystrophy, hypercalcemia and the like).
Owner:ASTELLAS PHARMA INC
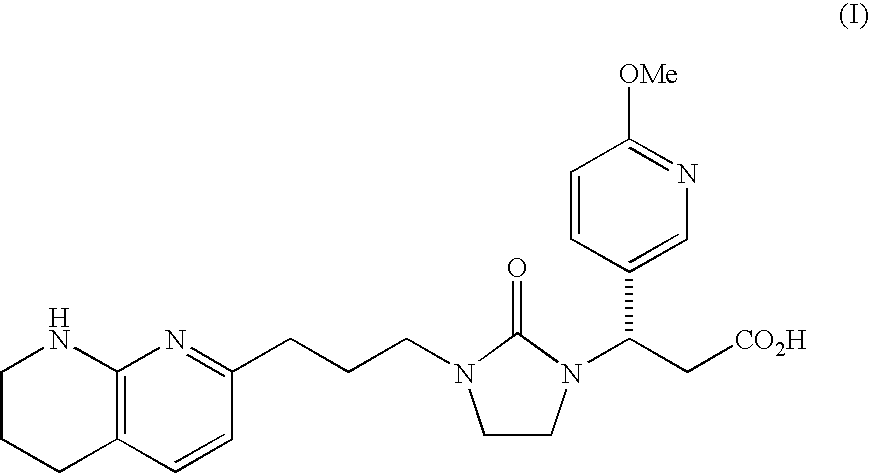
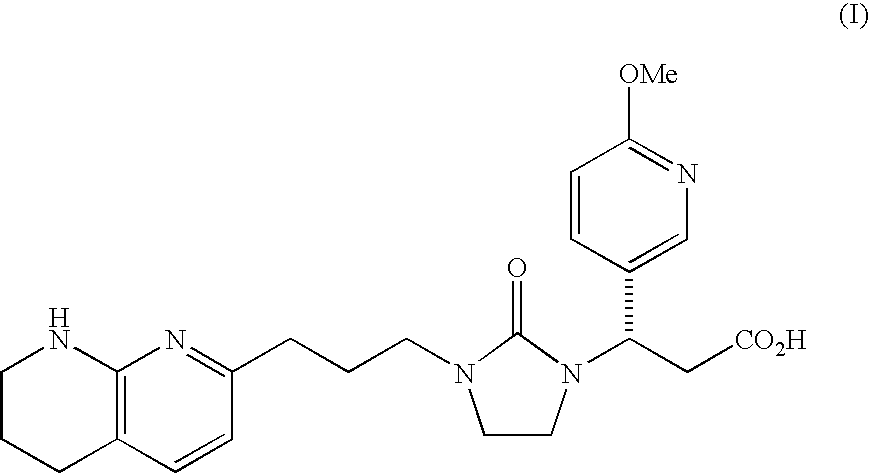
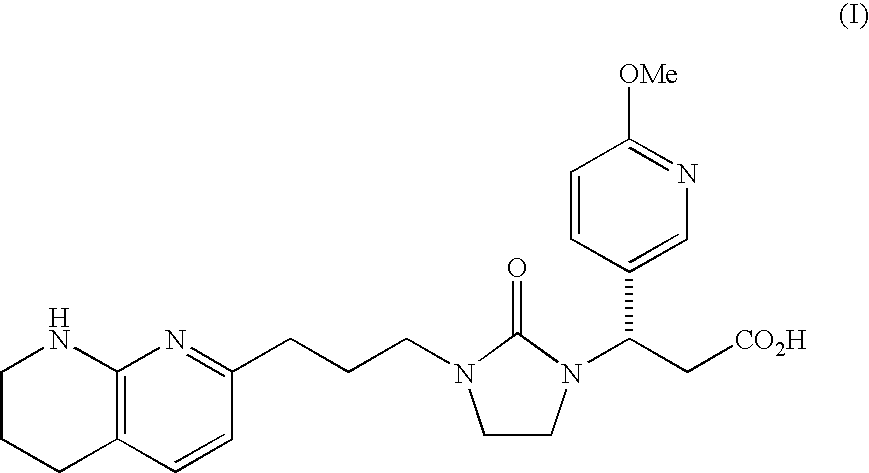
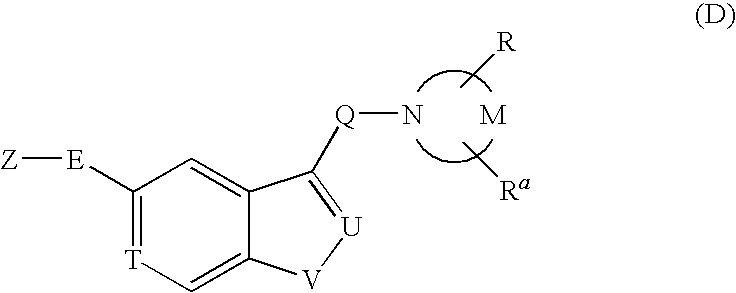
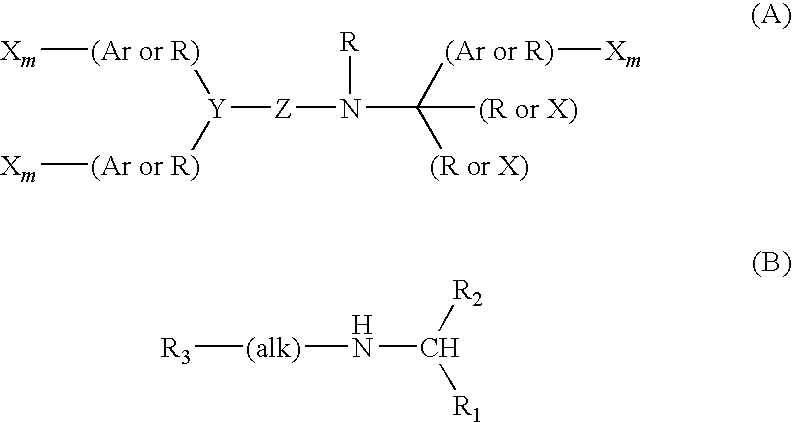
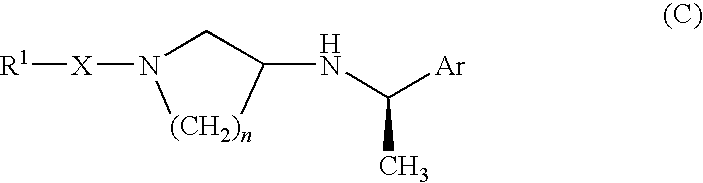
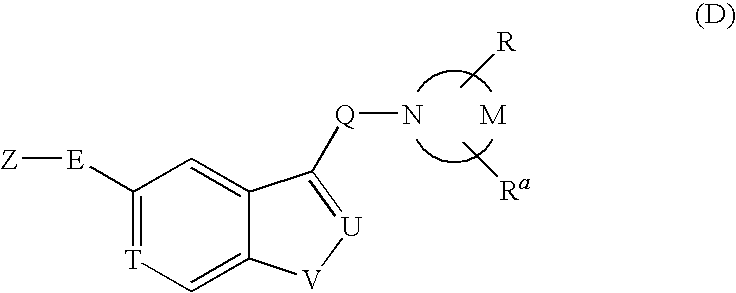
PYRIDO [4,3-d] PYRIMIDIN-4 (3H) -ONE DERIVATIVES AS CALCIUM RECEPTOR ANTAGONISTS
The present invention is directed to novel pyrido[4,3-d]pyrimidin-4(3H)-one derivatives and pharmaceutically acceptable salts thereof of structural formula I wherein the variables R1, R2, R3, R4 and R5 are as described herein. Also provided are pharmaceutical compositions comprising the compounds of formula I as well as methods of treatment employing compounds of formula I to treat a disease or disorder characterized by abnormal bone or mineral homeostasis such as hypoparathyroidism, osteoporosis, osteopenia, periodontal disease, Paget's disease, bone fracture, osteoarthritis, rheumatoid arthritis, and humoral hypercalcemia of malignancy.
Owner:PFIZER INC
Optoelectronic sensor
InactiveUS7090988B2Avoid pollutionBioreactor/fermenter combinationsBiological substance pretreatmentsAntigenCytosol
Owner:MASSACHUSETTS INST OF TECH
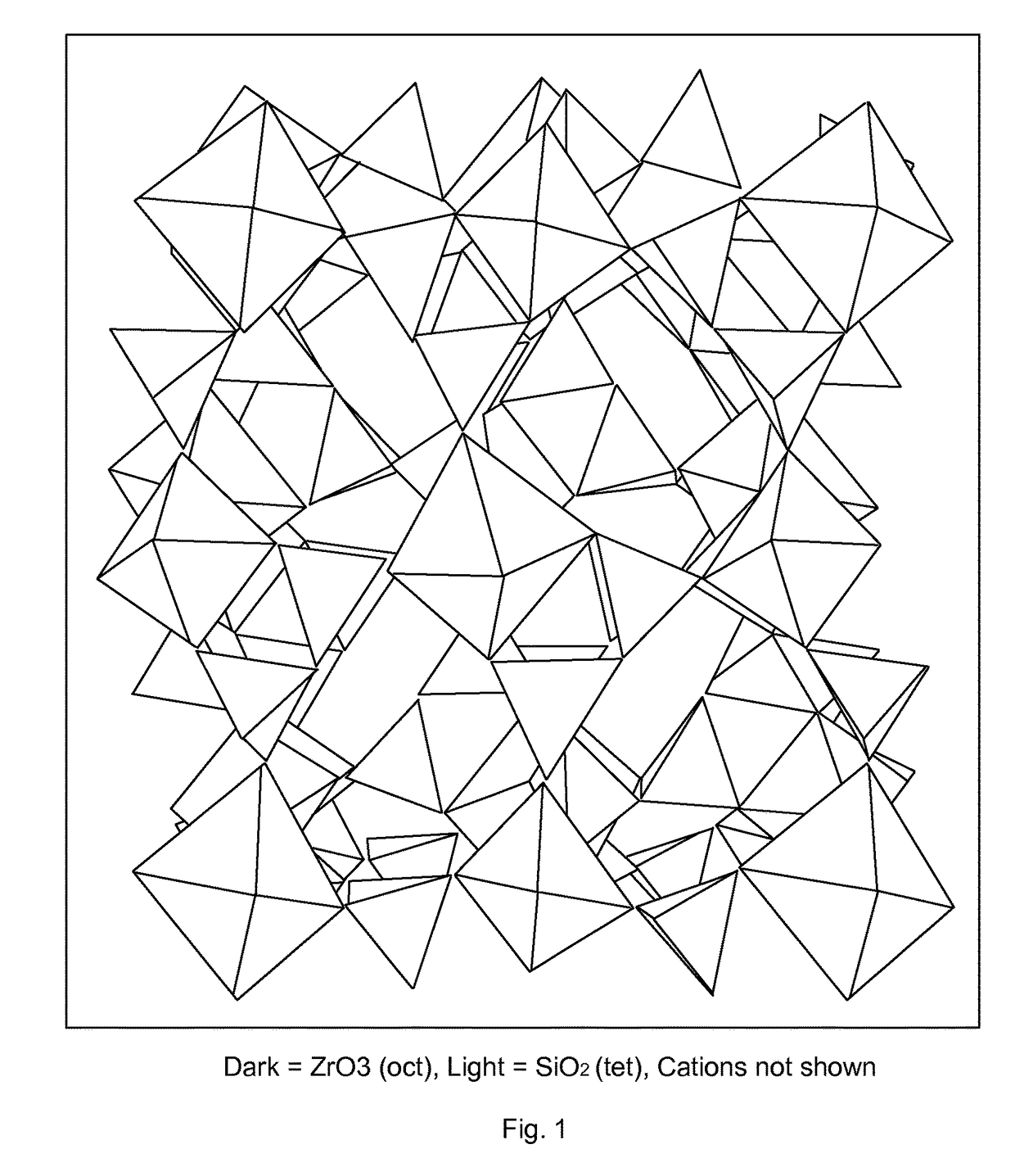
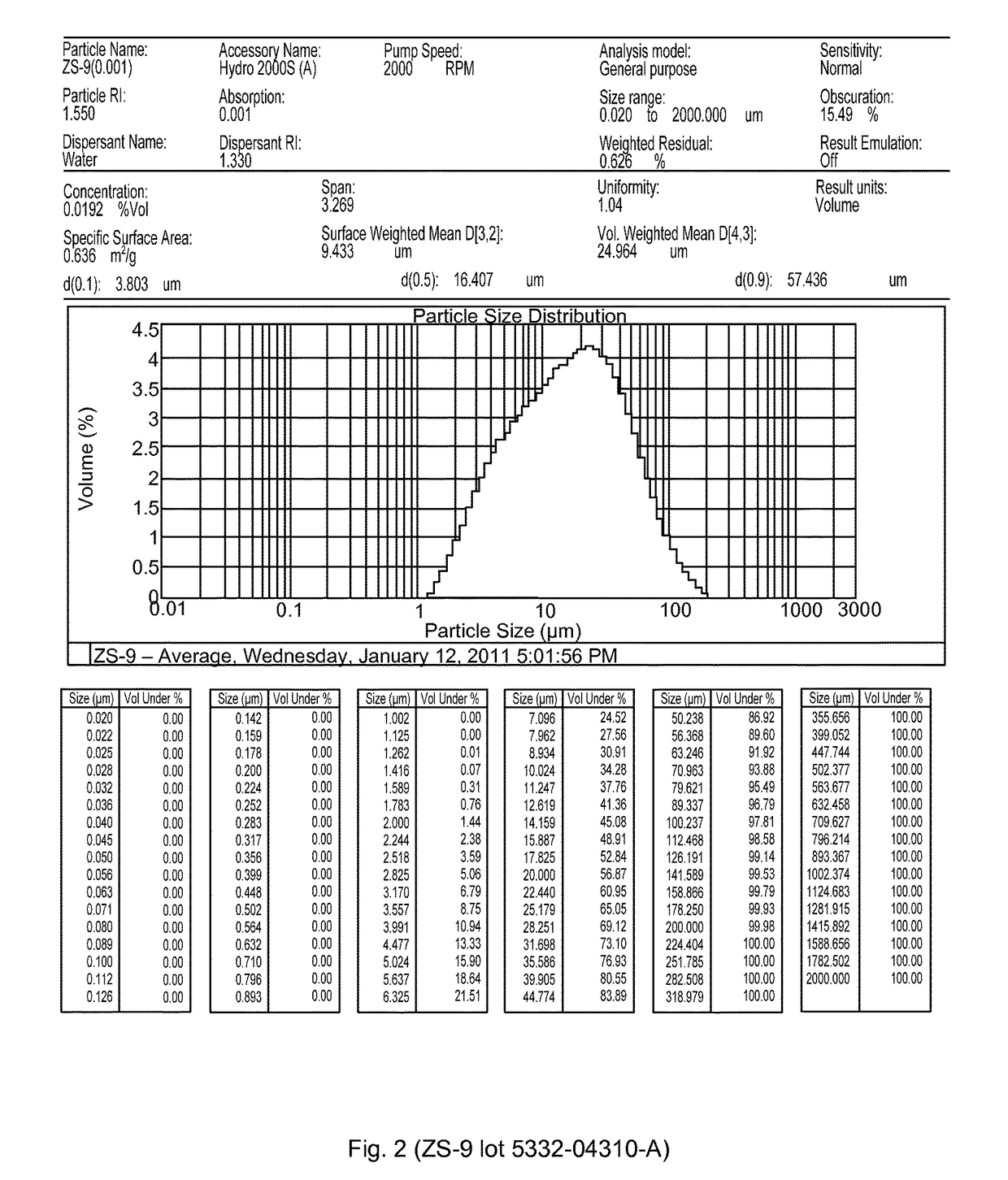
Microporous zirconium silicate for the treatment of hyperkalemia in hypercalcemic patients and improved calcium-containing compositions for the treatment of hyperkalemia
ActiveUS9707255B2High in calciumIncrease switching capacityHeavy metal active ingredientsSilicon organic compoundsPotassium ionsIncreased calcium
The present invention relates to novel calcium-containing microporous zirconium silicate compositions that are formulated to remove toxins, e.g. potassium ions, from the gastrointestinal tract at an elevated rate without removing calcium from the patient's body. Also disclosed are methods of using calcium-free or low calcium microporous zirconium silicate compositions for the treatment of hyperkalemia in patients also suffering from hypercalcemia.
Owner:ZS PHARMA
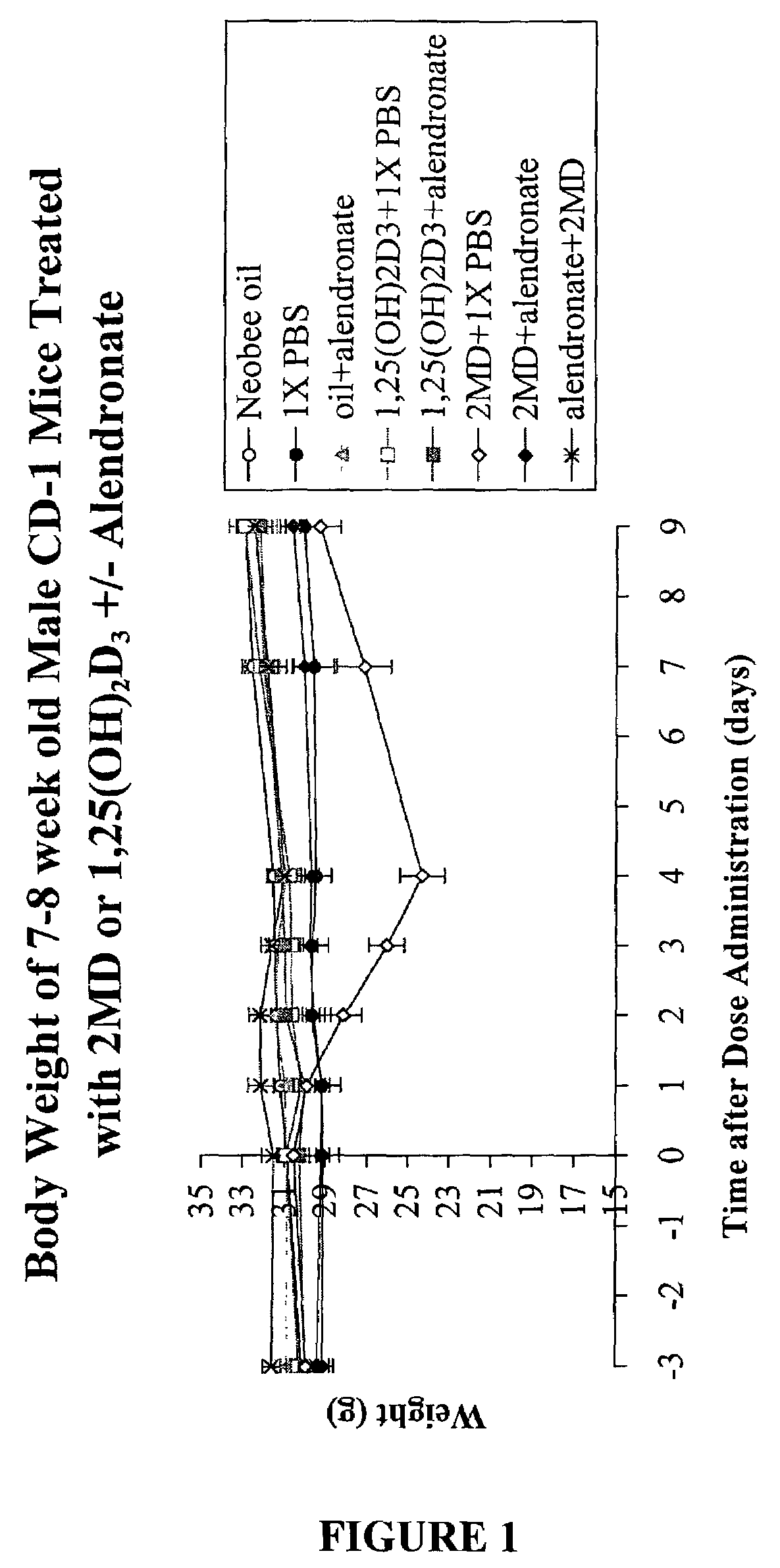
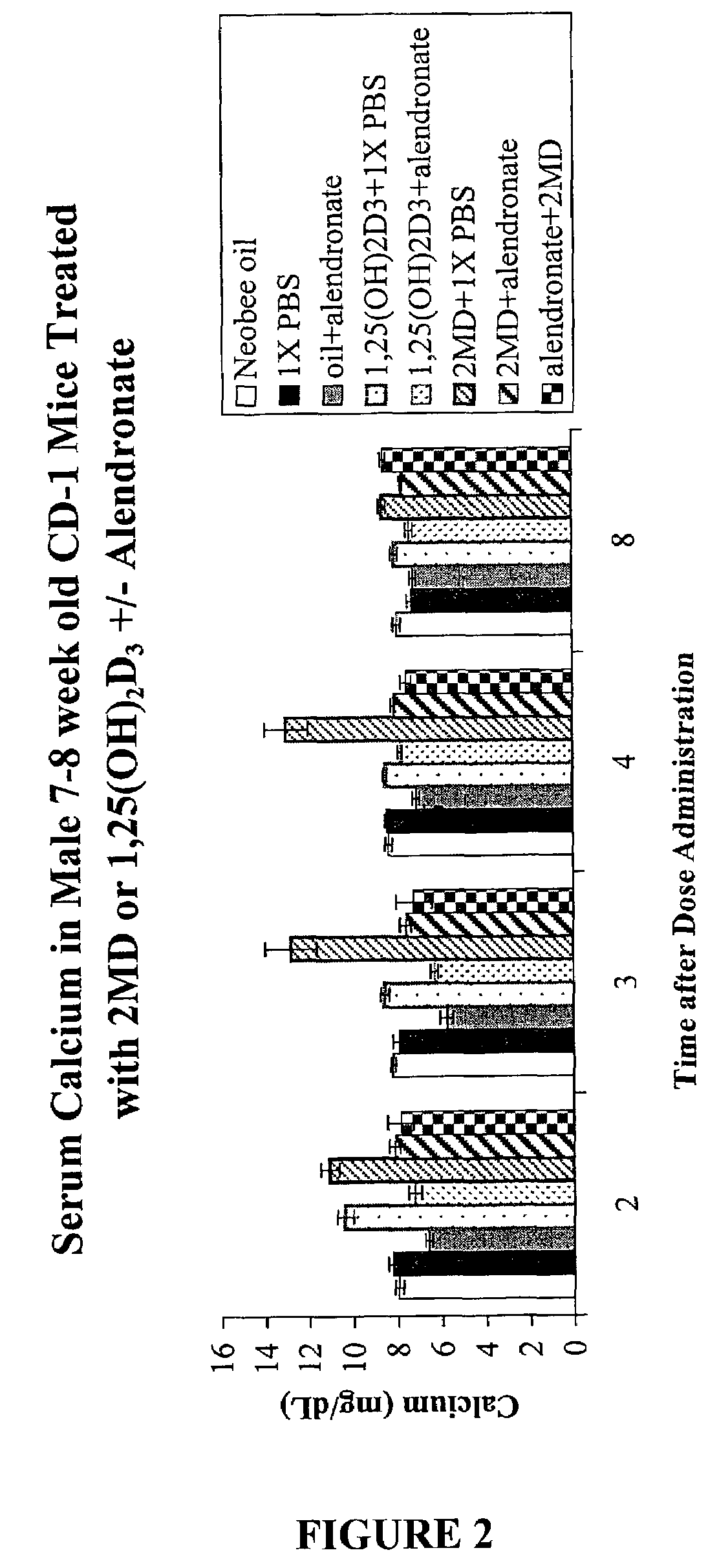
Method of extending the dose range of vitamin D compounds
InactiveUS7259143B2Inhibiting hypercalcemiaMinimal dangerBiocideOrganic active ingredientsDiseaseCalcification
Inhibitors of bone calcium resorption are administered to allow high doses of vitamin D compounds or mimetics to be given with the intent of treating non-calcium related diseases such as cancer, psoriasis, and autoimmune disease without the dangers of calcification of kidney, heart, and aorta. Inhibitors of bone calcium resorption include the bis-phosphonates, OPG (osteoprotegerin) or the soluble RANKL (receptor activator of NF-κB ligand) receptor known as sRANK (soluble RANK which is the protein expressed by the NF-κB gene), and function to block the availability of calcium from bone thereby preventing hypercalcemia and the resulting calcification of soft tissues. Thus, high doses of 1α,25-dihydroxyvitamin D3 (1,25-(OH)2D3), its analogs, prodrugs, or mimetics can be utilized with minimal risk to a patient. Specifically, alendronate is shown to block the bone calcium mobilization activity of both 1,25-(OH)2D3 and its very potent analog, 2-methylene -19-nor-(20S)-1α,25-dihydroxyvitamin D3.
Owner:WISCONSIN ALUMNI RES FOUND
Mannitol formulation for integrin receptor antagonist
Disclosed are pharmaceutical compositions of an integrin αvβ3 receptor antagonist containing mannitol as the binding agent. The compositions are prepared by wet granulation or direct compression tablet formulation. These pharmaceutical formulations are useful for inhibiting bone resorption associated with osteoporosis, metastatic bone disease, hypercalcemia of malignancy, and Paget's disease.
Owner:MERCK & CO INC
Osteoprotegerin variant proteins
InactiveUS20060189528A1Reduced binding affinityReduce capacityNervous disorderPeptide/protein ingredientsPAGET'S BONE DISEASEWild type
The present invention relates to novel osteoprotegerin variant proteins (OVPs) that demonstrate reduce binding affinity for their ligand TRAIL when compared to wild-type osteoprotegerin. Nucleic acids which encode these OVPs are also provided. Recombinant vectors and host cells expressing these OVPs are also encompassed as are methods of producing recombinant OVPs. The present invention also relates to compositions comprising these OVPs, and to methods of treating bone diseases characterised by increased bone turnover and / or loss. The OVPs of the invention are useful for preventing bone resorption and may be used to treat any condition resulting in abnormal bone turnover or bone loss such as osteoporosis, hypercalcemia, Paget's disease of bone, multiple myeloma, bone cancer and bone loss due to rheumatoid arthritis or osteomyelitis, and the like.
Owner:CEPHALON AUSTRALIA
Isolation of antibodies that cross-react and neutralize rankl originating from multiple species
InactiveUS20080107597A1Promote reproductionLittle or no activityIn-vivo radioactive preparationsPeptide/protein ingredientsAnticarcinogenGlucocorticoid
The invention provides specific binding members (e.g., antibodies or antigen-binding fragments thereof) which bind to RANKL originating from multiple species. An epitope recognized by the specific binding members can be selected from surface exposed loop domains that bind to and activate its cognate receptor, RANK (Receptor Activator of NFkB), on the surface of osteoclast precursors and other cell types. The invention provides peptides for generating such anti-RANKL antibodies, including murine sequences, other non-human sequences and cross-reactive peptides. The specific binding members are useful in the diagnosis and treatment of lytic bone diseases, including osteoporosis, rheumatoid arthritis, bone metastasis and hypercalcemia of malignancy, glucocorticoid-induced bone loss, a periodontal disease or condition, a cancer and Juvenile Paget's Disease. The binding members can also be used in therapy in combination with chemotherapeutics or anti-cancer agents and / or with other antibodies or antigen-binding fragments thereof.
Owner:ANAPTYSBIO INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com





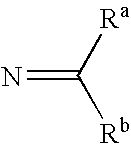




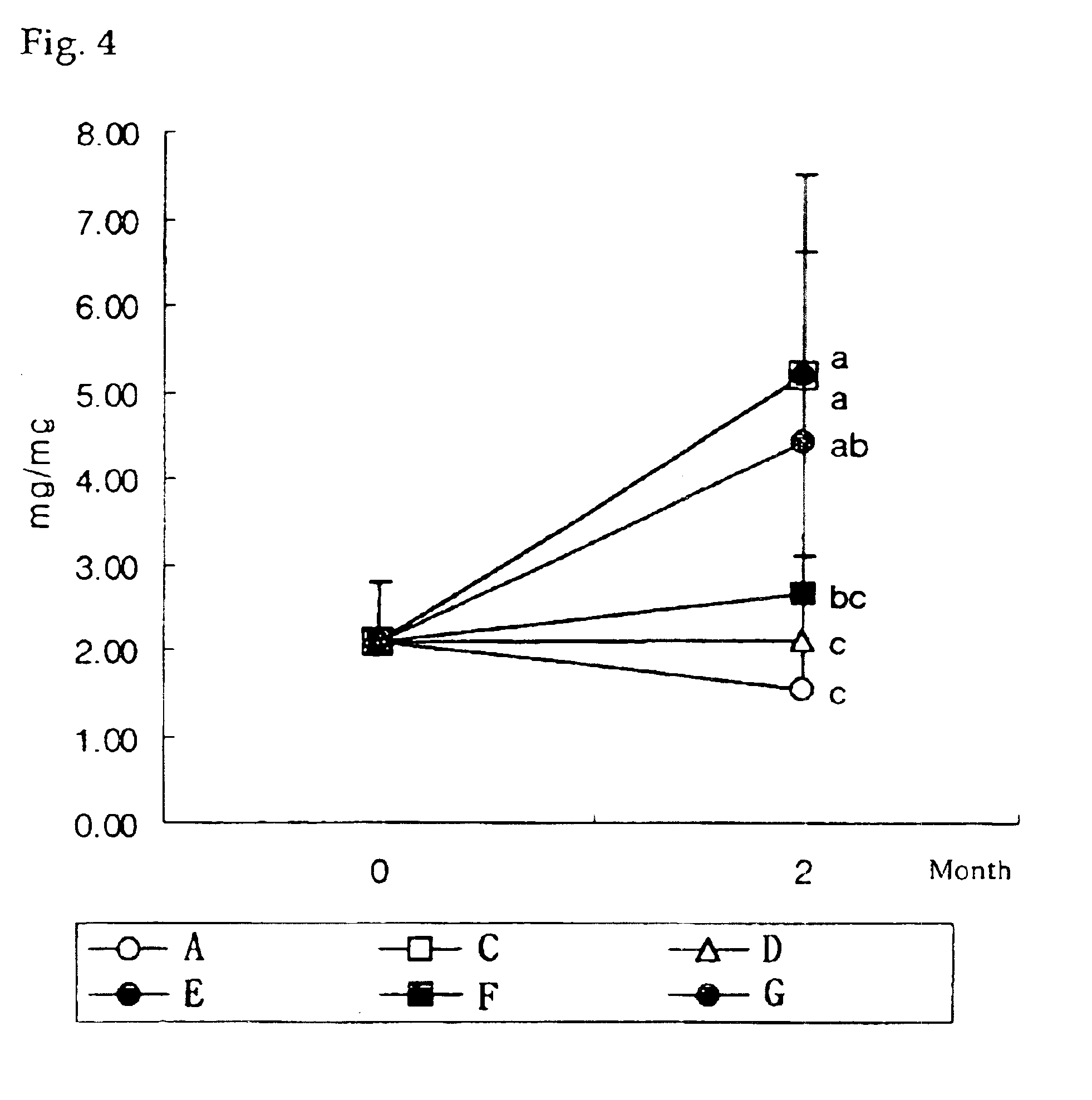
![PYRIDO [4,3-d] PYRIMIDIN-4 (3H) -ONE DERIVATIVES AS CALCIUM RECEPTOR ANTAGONISTS PYRIDO [4,3-d] PYRIMIDIN-4 (3H) -ONE DERIVATIVES AS CALCIUM RECEPTOR ANTAGONISTS](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/34f9b00a-7f53-4816-814c-6dbb659ca913/US20080085887A1-20080410-D00000.png)
![PYRIDO [4,3-d] PYRIMIDIN-4 (3H) -ONE DERIVATIVES AS CALCIUM RECEPTOR ANTAGONISTS PYRIDO [4,3-d] PYRIMIDIN-4 (3H) -ONE DERIVATIVES AS CALCIUM RECEPTOR ANTAGONISTS](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/34f9b00a-7f53-4816-814c-6dbb659ca913/US20080085887A1-20080410-D00001.png)
![PYRIDO [4,3-d] PYRIMIDIN-4 (3H) -ONE DERIVATIVES AS CALCIUM RECEPTOR ANTAGONISTS PYRIDO [4,3-d] PYRIMIDIN-4 (3H) -ONE DERIVATIVES AS CALCIUM RECEPTOR ANTAGONISTS](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/34f9b00a-7f53-4816-814c-6dbb659ca913/US20080085887A1-20080410-C00001.png)