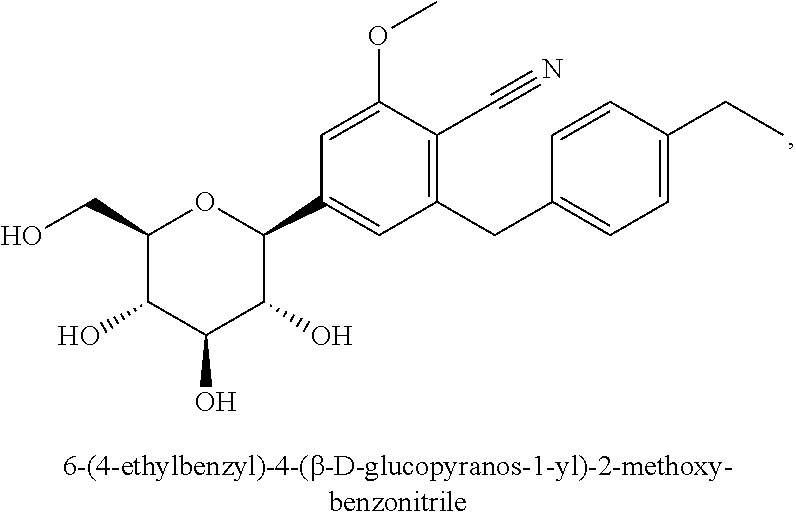
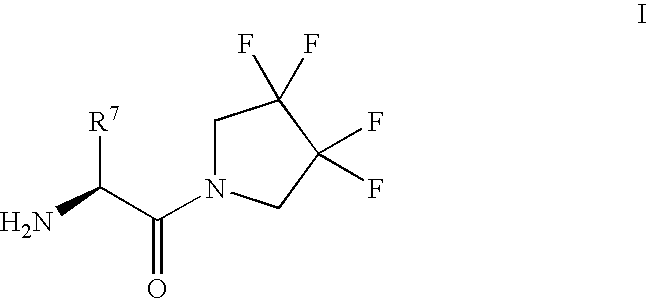
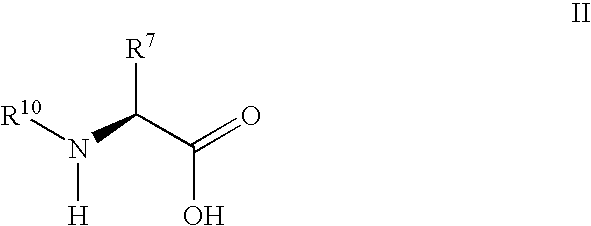
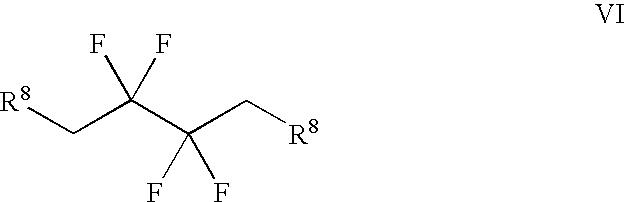
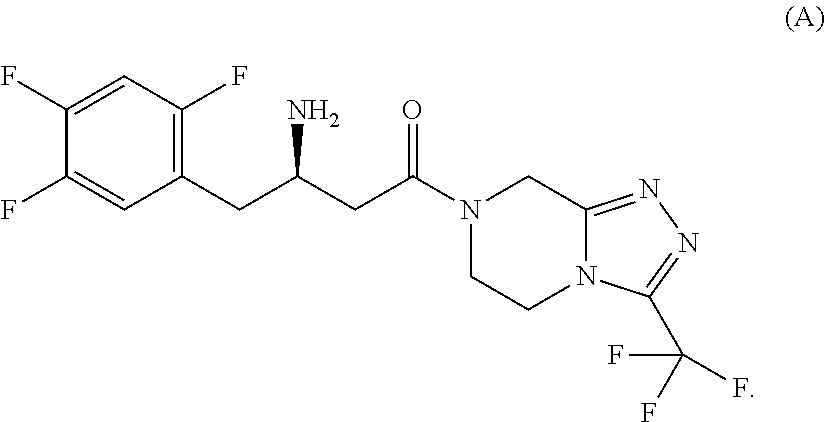
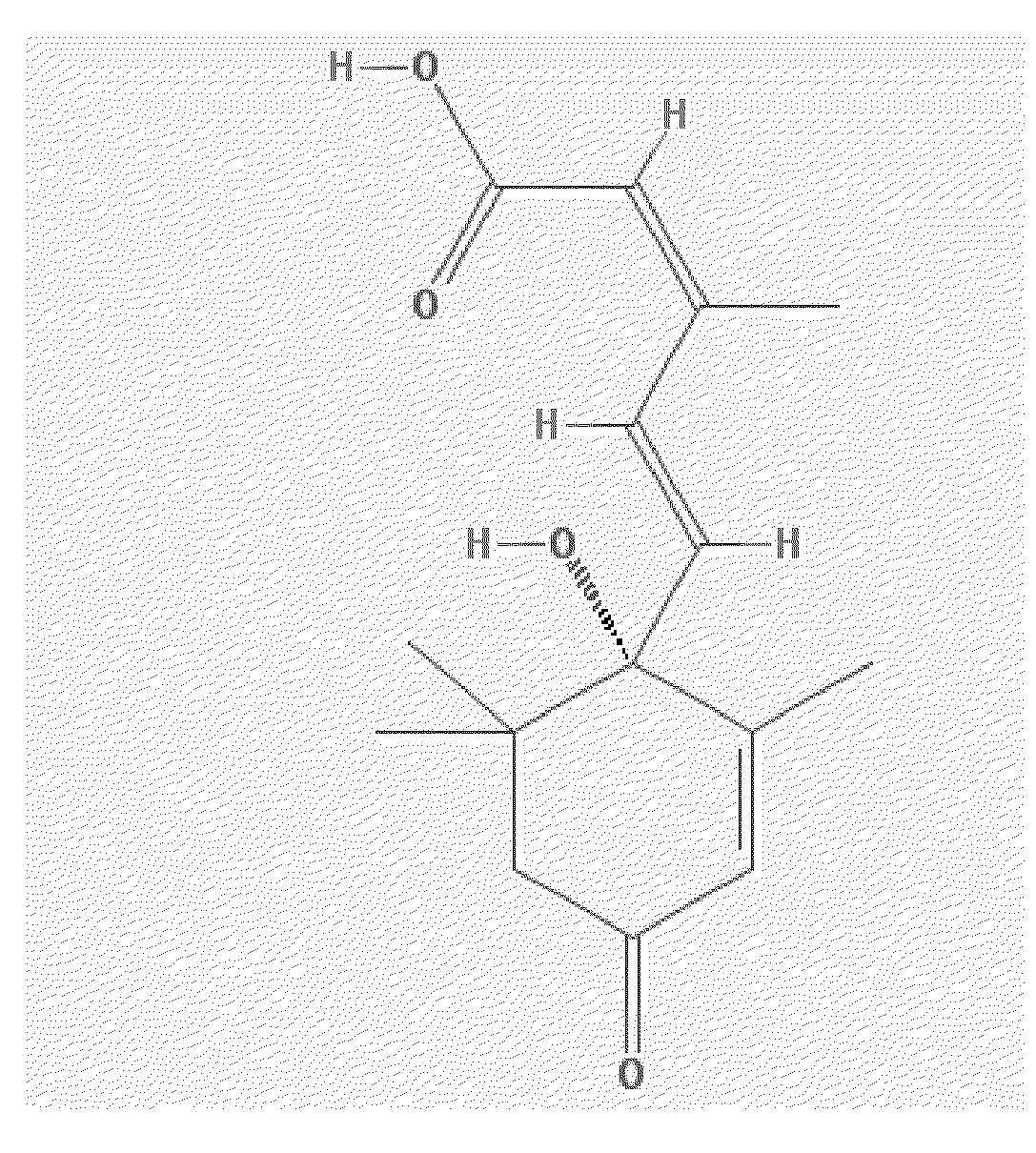
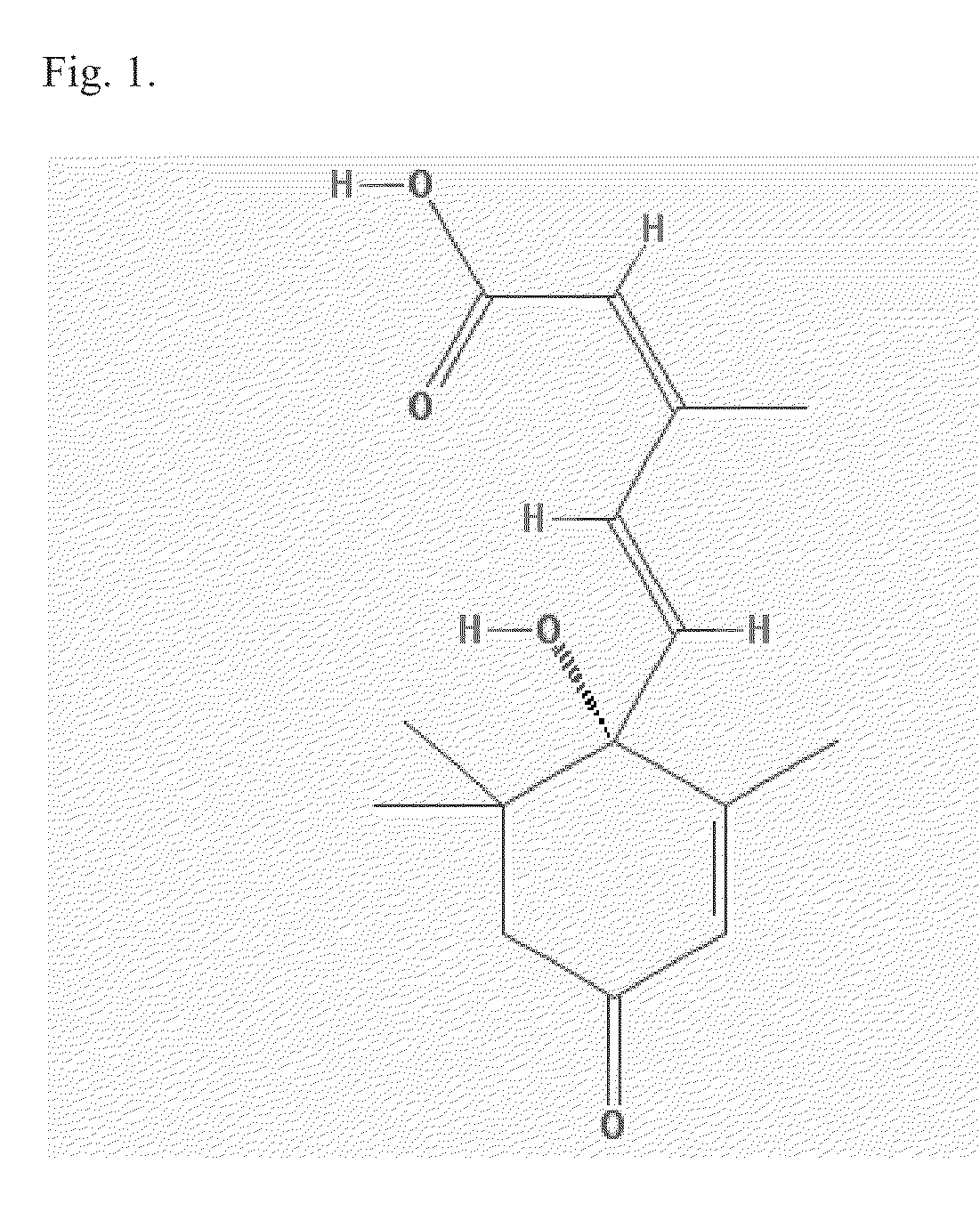
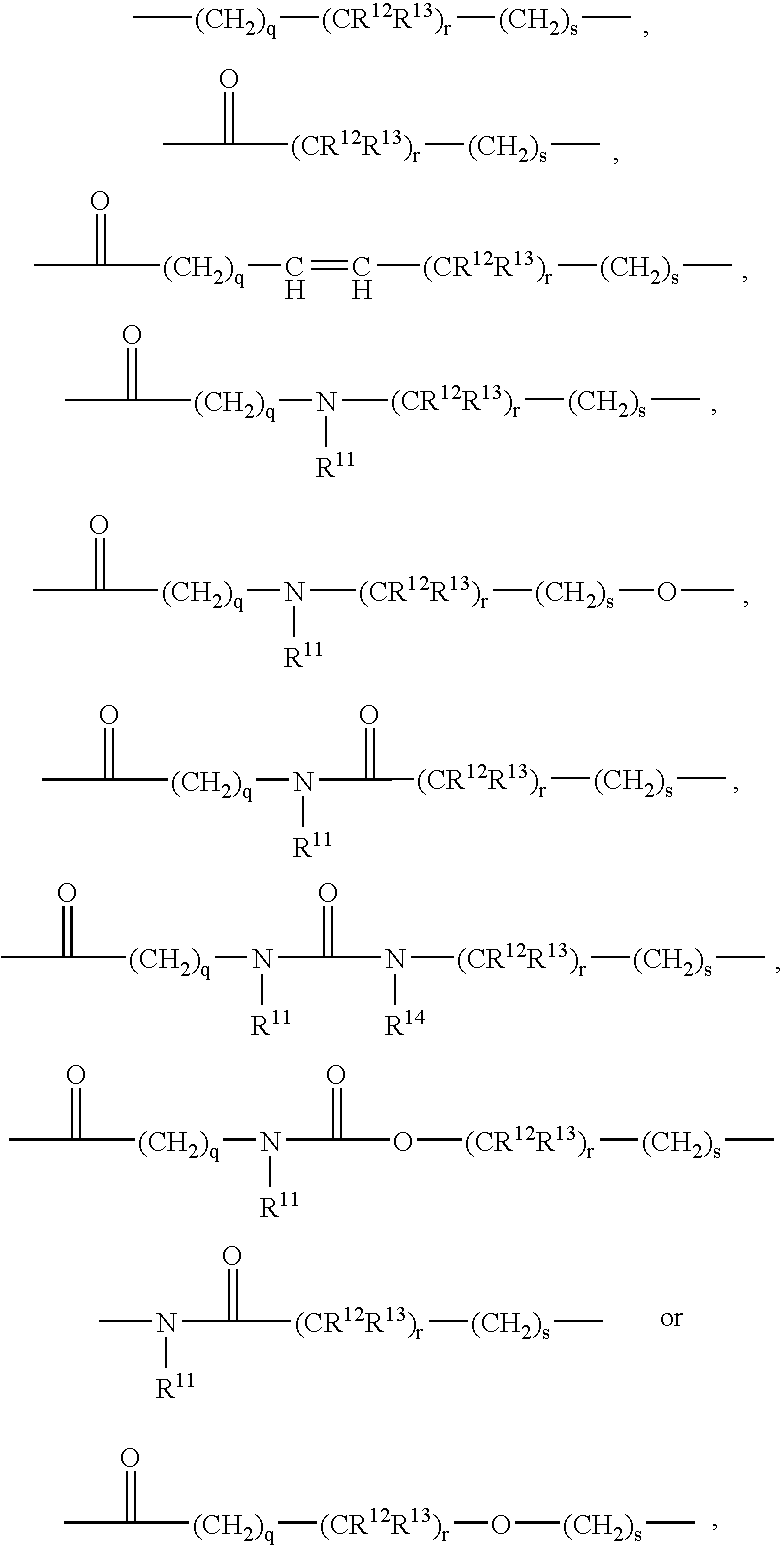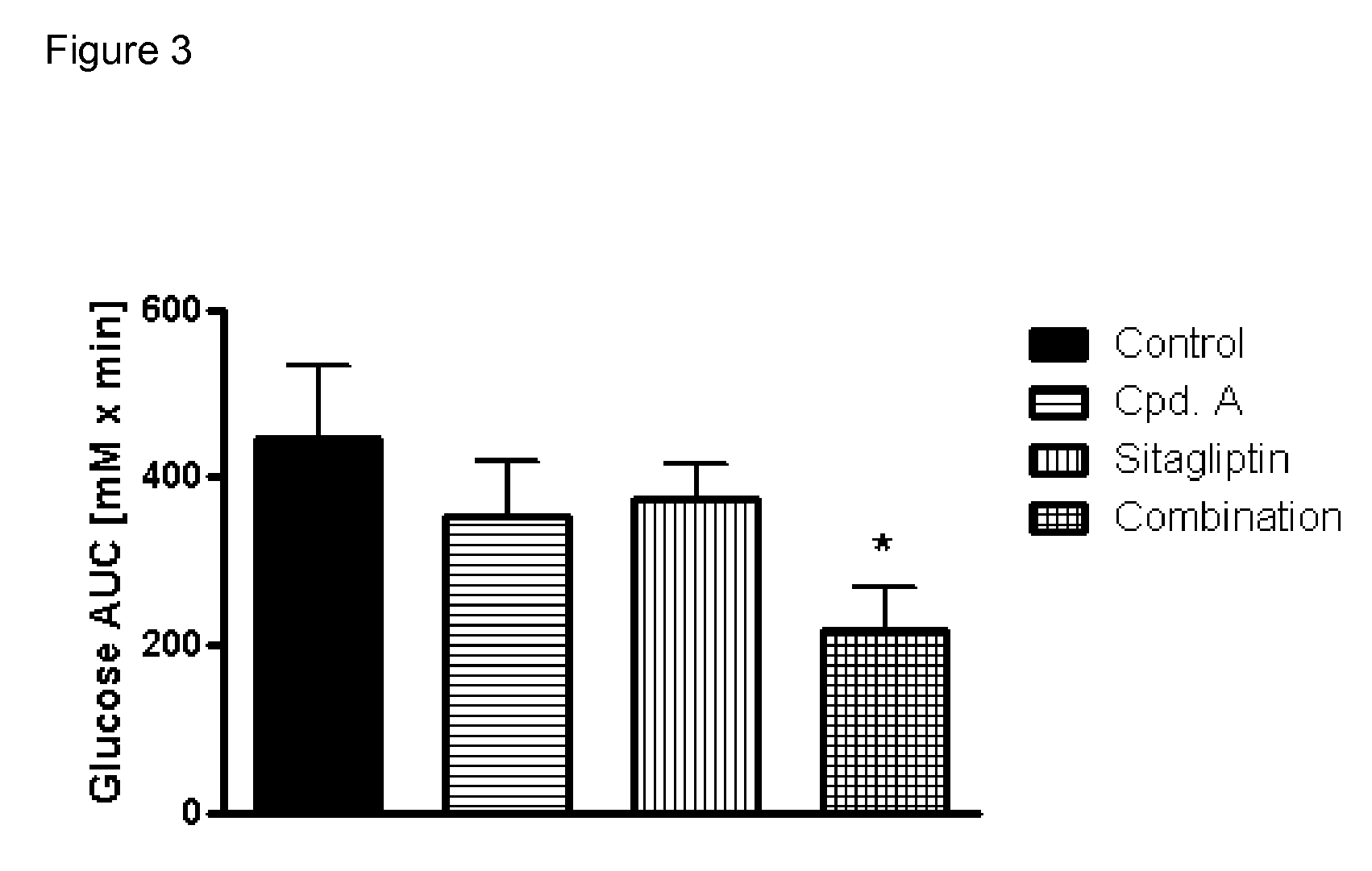Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
626 results about "Type 1 diabetes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A chronic condition where the pancreas produces little or no insulin.
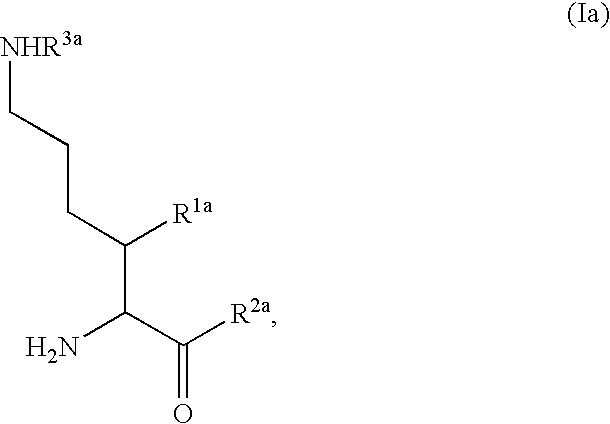
Fluorinated lysine derivatives as dipeptidyl peptidase IV inhibitors
InactiveUS20050043292A1Ease of preparation and detectabilityGood metabolic stabilityBiocideOrganic chemistryDiabetic retinopathyArthritis
The invention relates to new therapeutically active and selective inhibitors of the enzyme dipeptidyl peptidase-IV (“DPP-IV”), pharmaceutical compositions comprising the compounds and the use of such compounds for treating diseases that are associated with proteins that are subject to processing by DPP-IV, such as Type 2 diabetes, metabolic syndrome (syndrome X or insulin resistance syndrome), hyperglycemia, impaired glucose tolerance, glucosuria, metabolic acidosis, arthritis, cataracts, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, diabetic cardiomyopathy, Type 1 diabetes, obesity, conditions exacerbated by obesity, hypertension, hyperlipidemia, atherosclerosis, osteoporosis, osteopenia, frailty, bone loss, bone fracture, acute coronary syndrome, infertility due to polycystic ovary syndrome, short bowel syndrome, anxiety, depression, insomnia, chronic fatigue, epilepsy, eating disorders, chronic pain, alcohol addiction, diseases associated with intestinal motility, ulcers, irritable bowel syndrome, inflammatory bowel syndrome and to prevent disease progression in Type 2 diabetes. The invention also relates to a method of identifying an insulin secretagogue agent for diabetes.
Owner:PFIZER INC
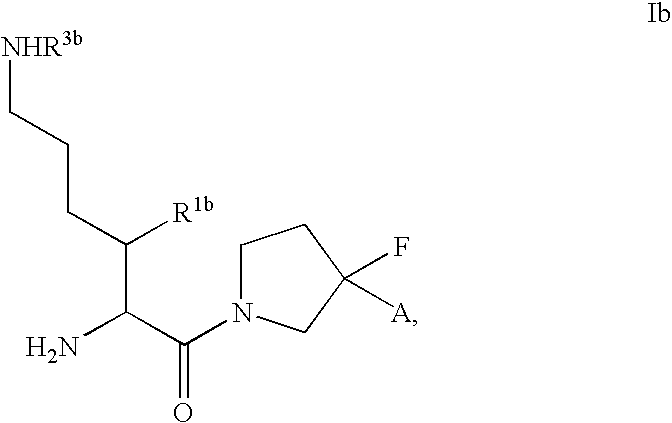
Fluorinated cyclic amides as dipeptidyl peptidase IV inhibitors
InactiveUS6710040B1Easy to prepareEase of detectabilityBiocideOrganic chemistryAcute coronary syndromeDisease progression
The invention relates to new therapeutically active and selective inhibitors of the enzyme dipeptidyl peptidase-IV, pharmaceutical compositions comprising the compounds and the use of such compounds for treating diseases that are associated with proteins that are subject to processing by DPP-IV, such as Type 2 diabetes mellitus, hyperglycemia, impaired glucose tolerance, metabolic syndrome (Syndrome X or insulin resistance syndrome), glucosuria, metabolic acidosis, cataracts, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, diabetic cardiomyopathy, Type 1 diabetes, obesity, conditions exacerbated by obesity, hypertension, hyperlipidemia, atherosclerosis, osteoporosis, osteopenia, frailty, bone loss, bone fracture, acute coronary syndrome, infertility due to polycystic ovary syndrome, short bowel syndrome, anxiety, depression, insomnia, chronic fatigue, epilepsy, eating disorders, chronic pain, alcohol addiction, diseases associated with intestinal motility, ulcers, irritable bowel syndrome, inflammatory bowel syndrome and to prevent disease progression in Type 2 diabetes. The invention also relates to a method of identifying an insulin secretagogue agent for diabetes.
Owner:PFIZER INC
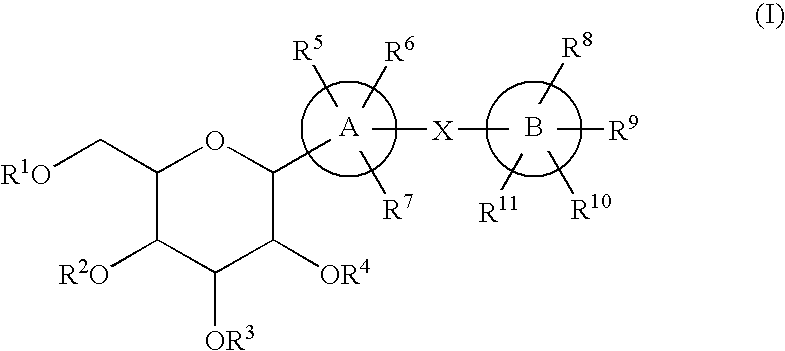
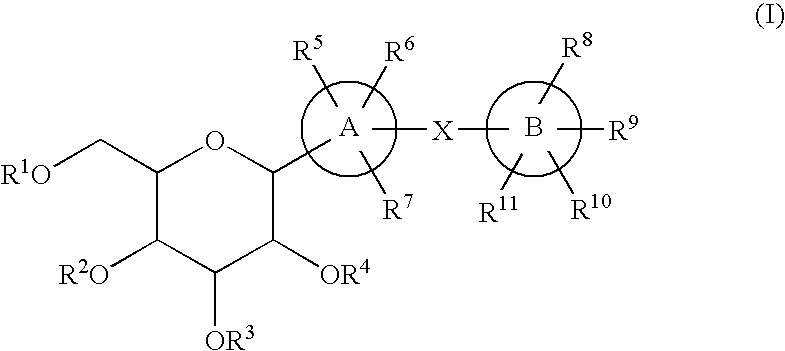
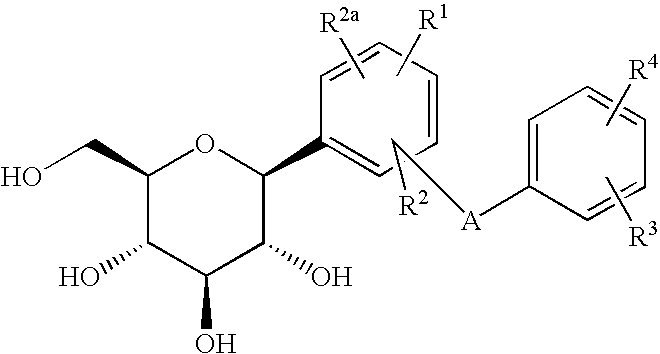
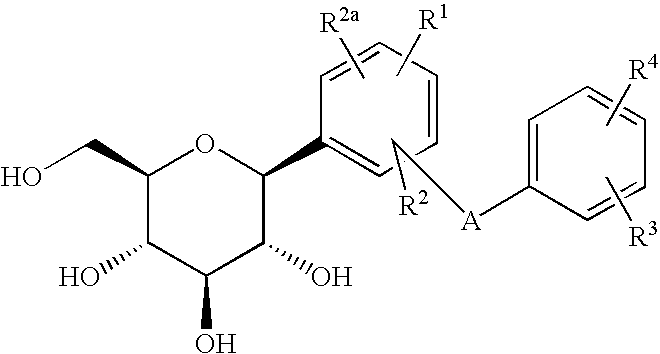
C-glycoside derivatives and salts thereof
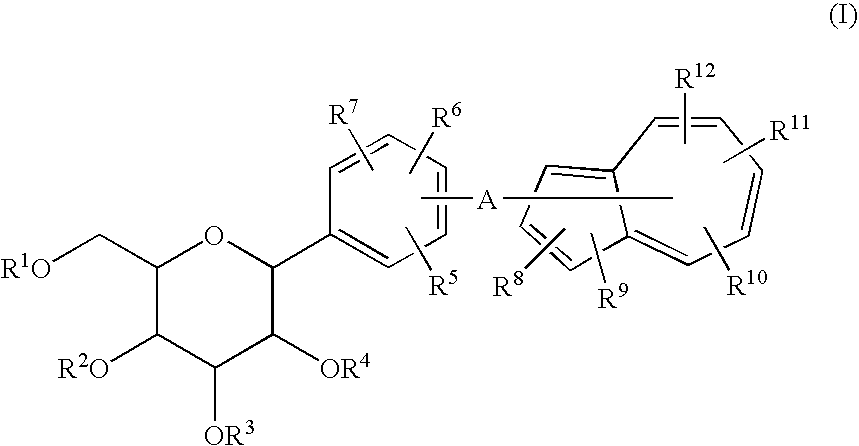
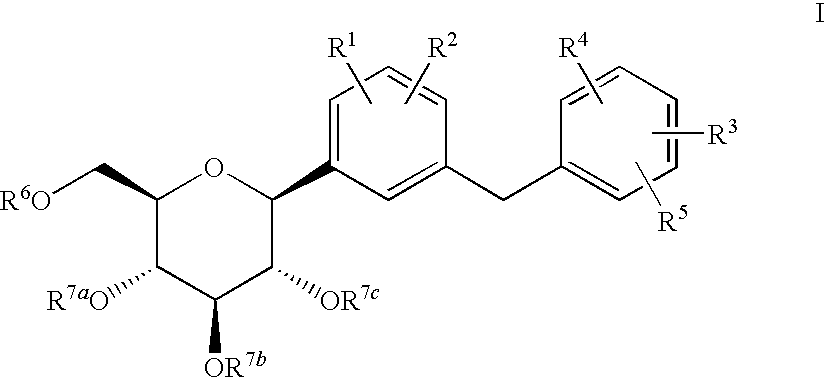
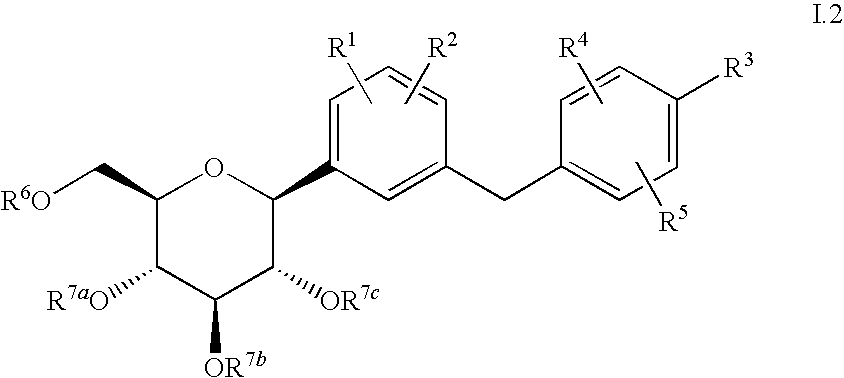
The present invention provides C-glycoside derivatives and salts thereof, wherein B ring is bonded to A ring via —X— and A ring is directly bonded to the glucose residue, and it is usable as a Na+-glucose cotransporter inhibitor, especially for a therapeutic and / or preventive agent for diabetes such as insulin-dependent diabetes (type 1 diabetes) and insulin-independent diabetes (type 2 diabetes), as well as diabetes related diseases such as an insulin-resistant diseases and obesity.
Owner:ASTELLAS PHARMA INC +1
C-glycoside derivatives and salts thereof
ActiveUS7202350B2Saccharide with heterocyclic radicalsSugar derivativesInsulin dependent diabetesDisease
The present invention provides C-glycoside derivatives and salts thereof, wherein B ring is bonded to A ring via —X— and A ring is directly bonded to the glucose residue, and it is usable as a Na+-glucose cotransporter inhibitor, especially for a therapeutic and / or preventive agent for diabetes such as insulin-dependent diabetes (type 1 diabetes) and insulin-independent diabetes (type 2 diabetes), as well as diabetes related diseases such as an insulin-resistant diseases and obesity.
Owner:ASTELLAS PHARMA INC +1
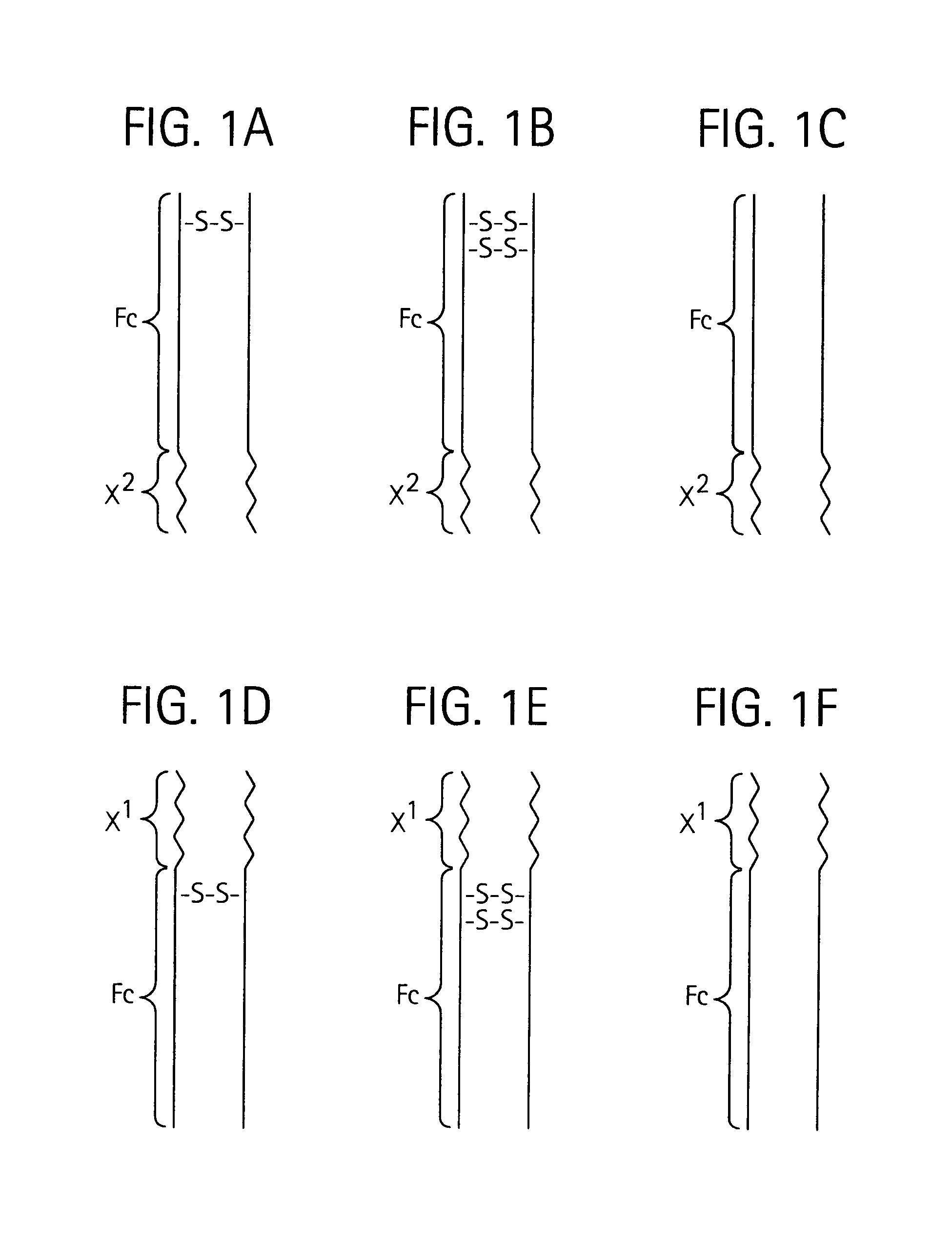
Toxin peptide therapeutic agents
ActiveUS20070071764A1Avoid it happening againRelieve symptomsNervous disorderAntipyreticHalf-lifeSjögren syndrome
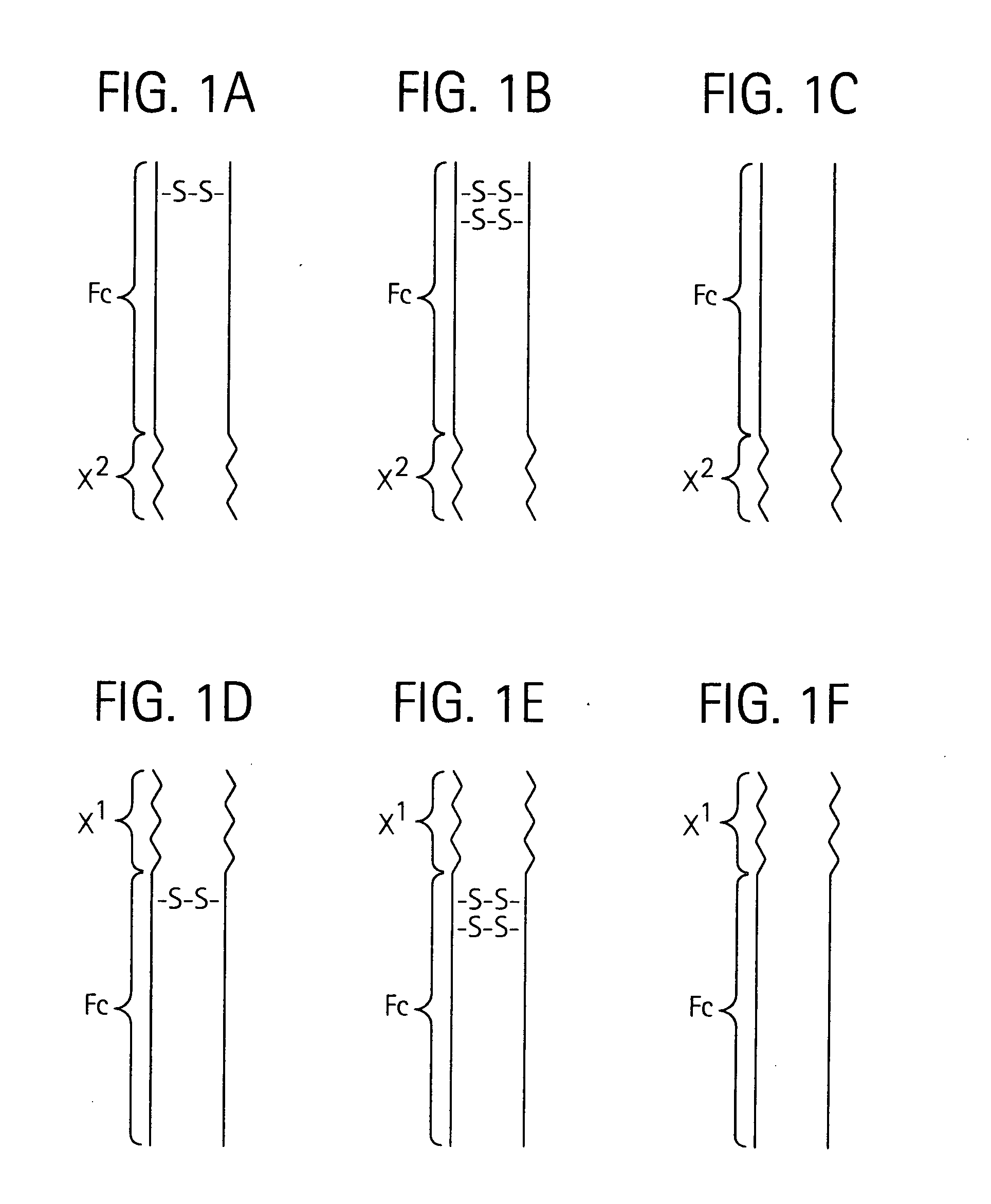
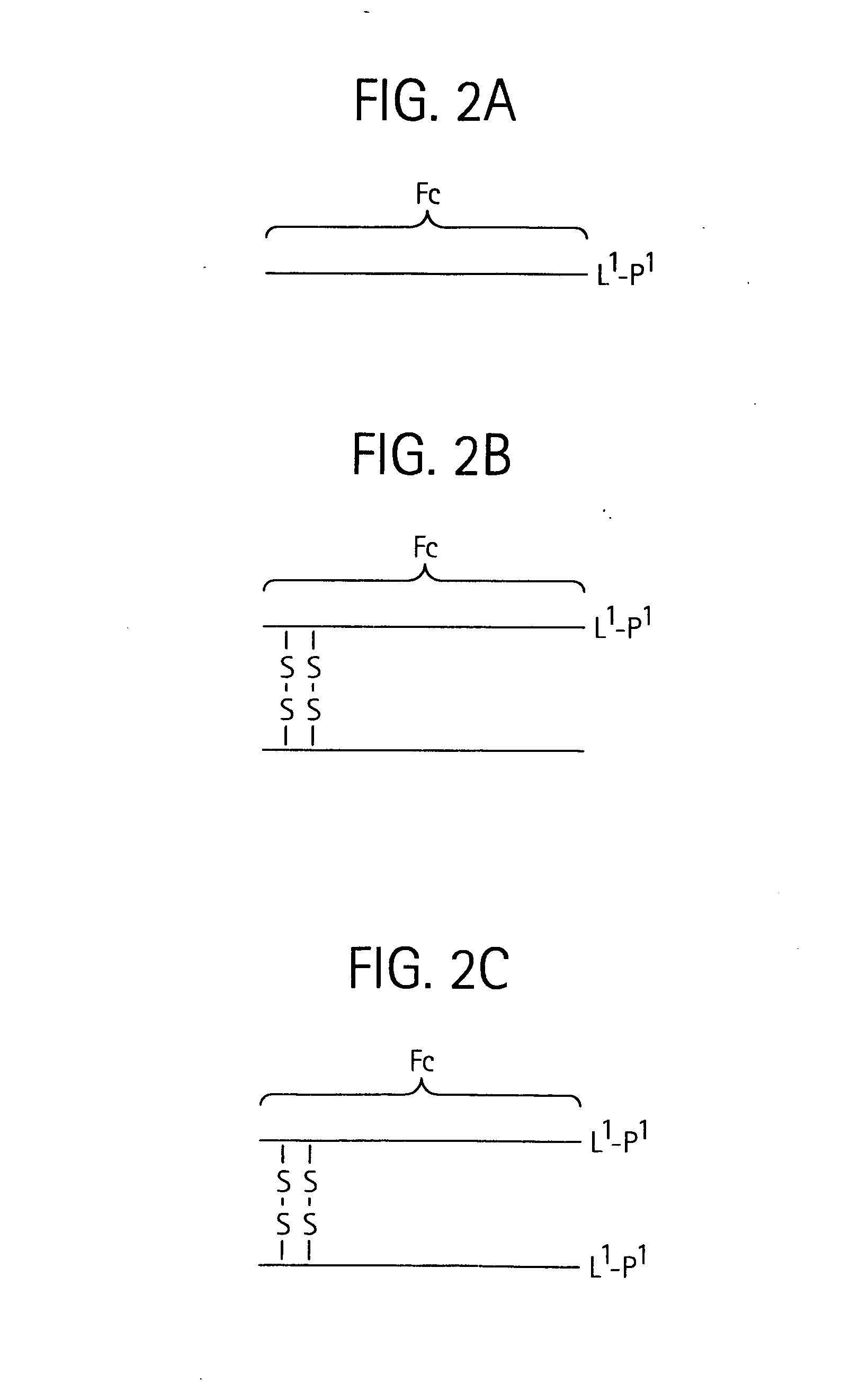
Disclosed is a composition of matter of the formula (X1)a—(F1)d—(X2)b—(F2)e—(X3)c (I) and multimers thereof, in which F1 and F2 are half-life extending moieties, and d and e are each independently 0 or 1, provided that at least one of d and e is 1; X1, X2, and X3 are each independently -(L)f-P-(L)g-, and f and g are each independently 0 or 1; P is a toxin peptide of no more than about 80 amino acid residues in length, comprising at least two intrapeptide disulfide bonds; L is an optional linker; and a, b, and c are each independently 0 or 1, provided that at least one of a, b and c is 1. Linkage to the half-life extending moiety or moieties increases the in vivo half-life of the toxin peptide, which otherwise would be quickly degraded. A pharmaceutical composition comprises the composition and a pharmaceutically acceptable carrier. Also disclosed are a DNA encoding the inventive composition of matter, an expression vector comprising the DNA, and a host cell comprising the expression vector. Methods of treating an autoimmune disorder, such as, but not limited to, multiple sclerosis, type 1 diabetes, psoriasis, inflammatory bowel disease, contact-mediated dermatitis, rheumatoid arthritis, psoriatic arthritis, asthma, allergy, restinosis, systemic sclerosis, fibrosis, scleroderma, glomerulonephritis, Sjogren syndrome, inflammatory bone resorption, transplant rejection, graft-versus-host disease, and lupus and of preventing or mitigating a relapse of a symptom of multiple sclerosis are also disclosed.
Owner:AMGEN INC
Method for diagnosing risk of type 1 diabetes and for preventing onset of type 1 diabetes
InactiveUS20130108598A1Early diagnosisAvoid seizuresBiocideMetabolism disorderIntestinal microorganismsType 1 diabetes
Owner:TEKNOLOGIAN TUTKIMUSKESKUS VTT
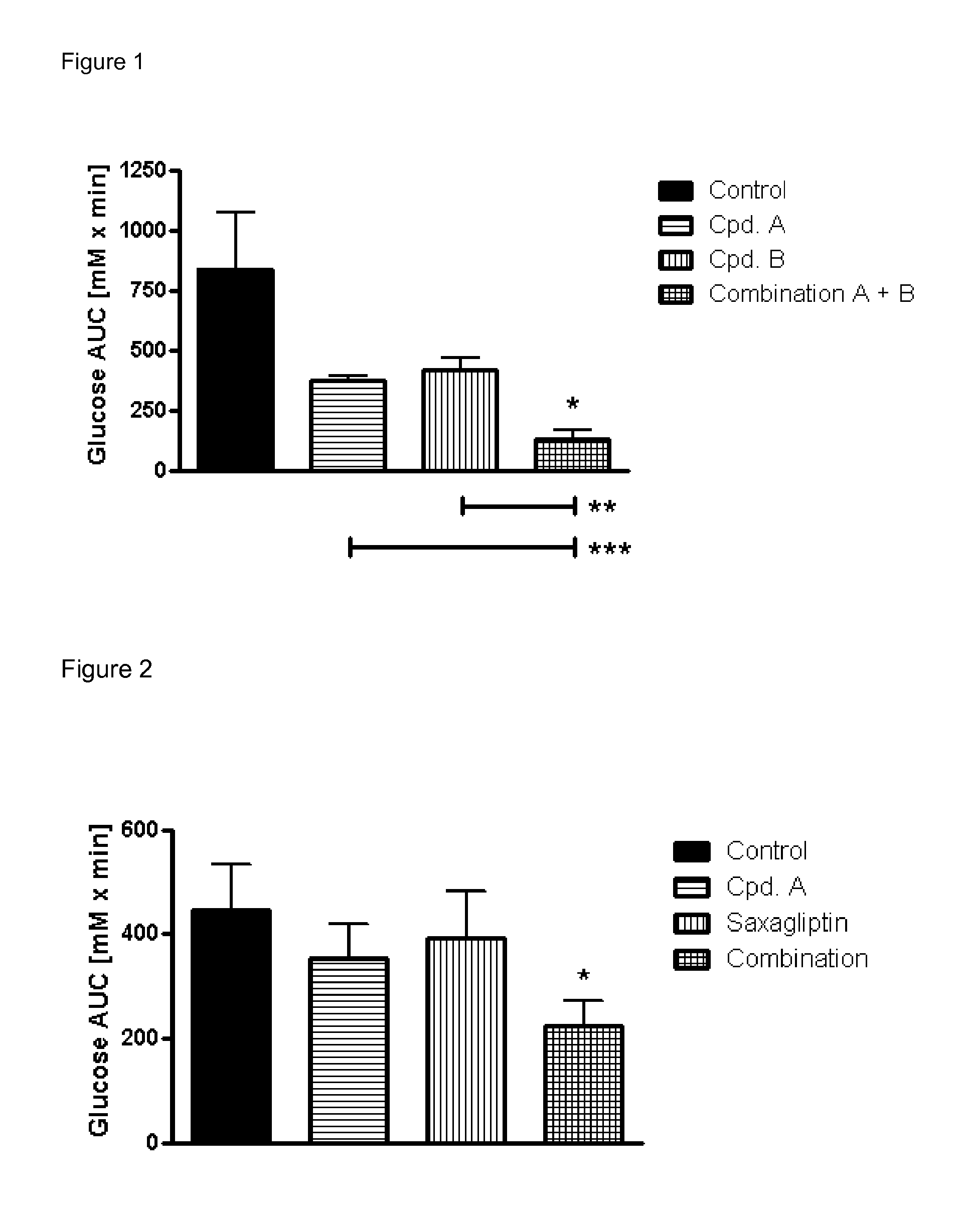
Pharmaceutical composition comprising a sglt2 inhibitor in combination with a dpp-iv inhibitor
InactiveUS20110098240A1Good effectBiocideSenses disorderIGT - Impaired glucose toleranceAcute hyperglycaemia
The invention relates to a pharmaceutical composition according to claim 1 comprising a SGLT2 inhibitor in combination with a DPP IV inhibitor which is suitable in the treatment or prevention of one or more conditions selected from type 1 diabetes mellitus, type 2 diabetes mellitus, impaired glucose tolerance and hyperglycemia. In addition the present invention relates to methods for preventing or treating of metabolic disorders and related conditions.
Owner:BOEHRINGER INGELHEIM INT GMBH
Pharmaceutical composition, methods for treating and uses thereof
InactiveUS20110046076A1Improve blood sugar controlPrevent and slow progressionBiocideMetabolism disorderAcute hyperglycaemiaIGT - Impaired glucose tolerance
The invention relates to a pharmaceutical composition according to the claim 1 comprising an SGLT2 inhibitor, a DPPIV inhibitor and a third antidiabetic agent which is suitable in the treatment or prevention of one or more conditions selected from type 1 diabetes mellitus, type 2 diabetes mellitus, impaired glucose tolerance and hyperglycemia. In addition the present invention relates to methods for preventing or treating of metabolic disorders and related conditions.
Owner:BOEHRINGER INGELHEIM INT GMBH
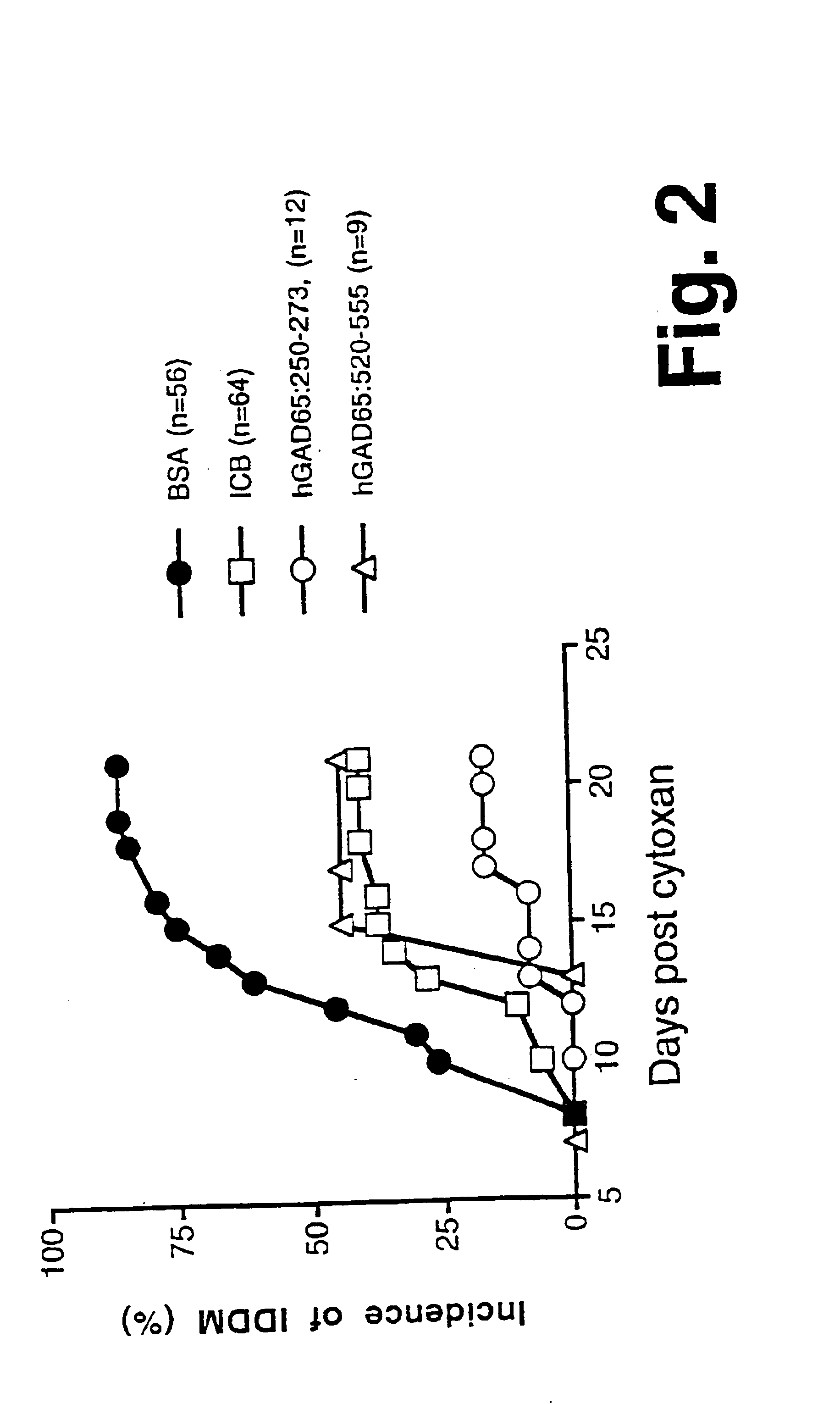
Chimeric proteins for diagnosis and treatment of diabetes
InactiveUS6982323B1Enhance the beneficial effectEasy diagnosisSugar derivativesPeptide/protein ingredientsImmunodominant EpitopesPancreas
Novel chimeric fusion proteins comprising immunodominant epitopes of GAD and insulin are provided. Also provided are immunomodulatory methods for the use of such proteins for both the prevention and treatment of Type 1 diabetes mellitus. The chimeric fusion proteins of the invention are useful in predicting risk of onset of Type 1 diabetes, determining prognosis of Type 1 diabetes patients early in disease progression, and in evaluating patients for suitability as recipients of transplants of pancreatic cells or tissues. The administration of the proteins of the invention in accordance with the immunomodulatory methods of the invention results in beneficial effects on disease development and severity in patients suffering from or predicted to be at risk of developing Type 1 diabetes, as well as on the outcome of transplants of pancreatic cells or tissues in Type 1 diabetes patients.
Owner:ALEXION PHARMA INC
Azulene derivatives and salts thereof
The present invention provides an azulene derivative and a salt thereof, wherein an azulene ring is bonded to a benzene ring directly or via a lower alkylene which may be substituted with a halogen atom and the benzene ring is directly bonded to the glucose residue, and it is usable as a Na+-glucose cotransporter inhibitor, especially for a therapeutic and / or preventive agent for diabetes such as insulin-dependent diabetes (type 1 diabetes) and insulin-independent diabetes (type 2 diabetes), as well as diabetes-related diseases such as insulin-resistant diseases and obesity.
Owner:ASTELLAS PHARMA INC +1
Azulene derivatives and salts thereof
The present invention provides an azulene derivative and a salt thereof, wherein an azulene ring is bonded to a benzene ring directly or via a lower alkylene which may be substituted with a halogen atom and the benzene ring is directly bonded to the glucose residue, and it is usable as a Na+-glucose cotransporter inhibitor, especially for a therapeutic and / or preventive agent for diabetes such as insulin-dependent diabetes (type 1 diabetes) and insulin-independent diabetes (type 2 diabetes), as well as diabetes-related diseases such as insulin-resistant diseases and obesity.
Owner:ASTELLAS PHARMA INC +1
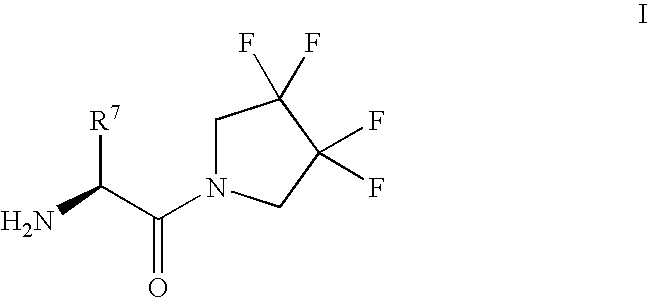
Synthesis of 3,3,4,4-tetrafluoropyrrolidine and novel dipeptidyl peptidase-IV inhibitor compounds
The present invention relates to a method of making novel dipeptidyl peptidase-IV ("DPP-IV') inhibitor compounds useful for treating, inter alia, diseases that are associated with proteins that are subject to processing by DPP-IV, such as Type 2 diabetes mellitus, metabolic syndrome (Syndrome X or insulin resistance syndrome), hyperglycemia, impaired glucose tolerance, glucosuria, metabolic acidosis, cataracts, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, diabetic cardiomyopathy, Type 1 diabetes, obesity, hypertension, hyperlipidemia, atherosclerosis, osteoporosis, osteopenia, frailty, bone loss, bone fracture, acute coronary syndrome, infertility due to polycystic ovary syndrome, short bowel syndrome and to prevent disease progression in Type 2 diabetes. The invention also relates to a method of making 3,3,4,4-tetrafluoropyrrolidine, a starting material utilized in the afore-mentioned method for preparing DPP-IV compounds.
Owner:PFIZER INC
Antidiabetic medications comprising a dpp-4 inhibitor (linagliptin) optionally in combination with other antidiabetics
InactiveUS20120094894A1Reduce weightAvoiding weight increaseAntibacterial agentsBiocideIGT - Impaired glucose toleranceAcute hyperglycaemia
The invention relates to antidiabetic medications which are suitable in the treatment or prevention of one or more conditions selected from type 1 diabetes mellitus, type 2 diabetes mellitus, impaired glucose tolerance and hyperglycemia, inter alia. In addition the present invention relates to methods for preventing or treating of metabolic disorders and related conditions. The medication is a mono treatment with a DPP-4 inhibitor <preferably linagliptin> or a combination treatment with a DPP-4 inhibitor and a second and / or third antidiabetic.
Owner:BOEHRINGER INGELHEIM INT GMBH
Synthesis of 3,3,4,4-tetrafluoropyrrolidine and novel dipeptidyl peptidase-IV inhibitor compounds
InactiveUS6812350B2Metabolism disorderPhosphorus organic compoundsDisease progressionDiabetic nephropathy
Owner:PFIZER INC
Glucagon antagonists/inverse agonists
A novel class of compounds, which act to antagonize the action of the glucagon hormone on the glucagon receptor. Owing to their antagonizing effect of the glucagon receptor the compounds may be suitable for the treatment and / or prevention of any glucagon-mediated conditions and diseases such as hyperglycemia, Type 1 diabetes, Type 2 diabetes and obesity.
Owner:PFIZER INC
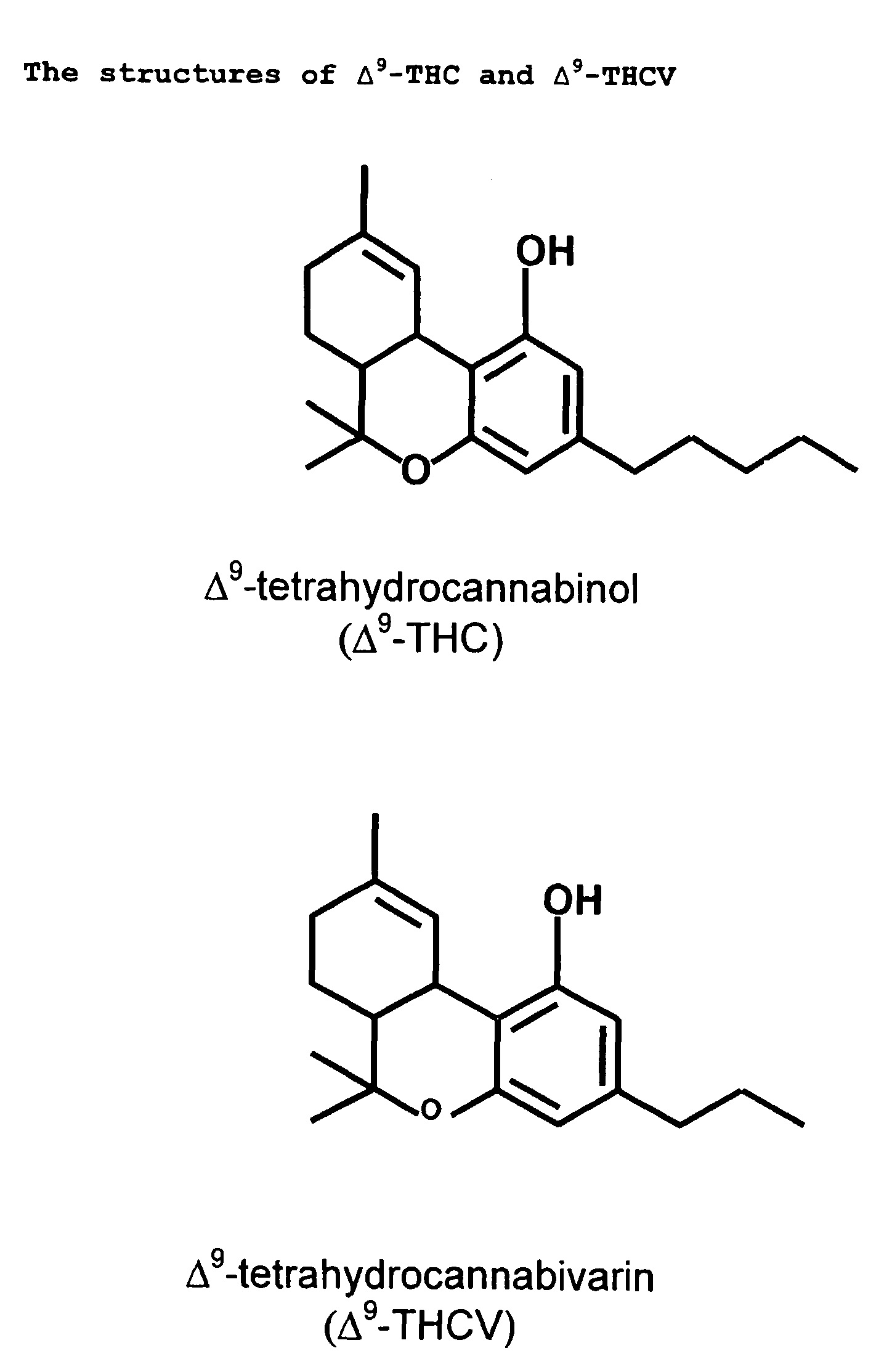
Use for cannabinoid
ActiveUS9168278B2High activityLess degree of activityBiocideNervous disorderDiseaseCannabinoid receptor
The invention relates to the use of one or more cannabinoids in the manufacture of medicaments for use in the treatment of diseases and conditions benefiting from neutral antagonism of the CB, cannabinoid receptor. Preferably the cannabinoid is tetrahydrocannabivarin (THCV). Preferably the diseases and conditions to be treated are taken from the group: obesity, schizophrenia, epilepsy, cognitive disorders such as Alzheimer's, bone disorders, bulimia, obesity associated with type II diabetes (non-insulin dependant diabetes) and in the treatment of drug, alcohol and nicotine abuse or dependency.
Owner:GW PHARMA LTD
Glucagon antagonists/inverse agonists
A novel class of compounds, which act to antagonize the action of the glucagon hormone on the glucagon receptor. Owing to their antagonizing effect of the glucagon receptor the compounds may be suitable for the treatment and / or prevention of any diseases and disorders, wherein a glucagon antagonistic action is beneficial, such as hyperglycemia, Type 1 diabetes, Type 2 diabetes, disorders of the lipid metabolism, such as dyslipidemia, and obesity.
Owner:PFIZER INC
Synthesis of 3,3,4,4-tetrafluoropyrrolidine and novel dipeptidyl peptidase-IV inhibitor compounds
InactiveUS20040002609A1Easy to cutMetabolism disorderPhosphorus organic compoundsDisease progressionDisease cause
The present invention relates to a method of making novel dipeptidyl peptidase-IV ("DPP-IV') inhibitor compounds useful for treating, inter alia, diseases that are associated with proteins that are subject to processing by DPP-IV, such as Type 2 diabetes mellitus, metabolic syndrome (Syndrome X or insulin resistance syndrome), hyperglycemia, impaired glucose tolerance, glucosuria, metabolic acidosis, cataracts, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, diabetic cardiomyopathy, Type 1 diabetes, obesity, hypertension, hyperlipidemia, atherosclerosis, osteoporosis, osteopenia, frailty, bone loss, bone fracture, acute coronary syndrome, infertility due to polycystic ovary syndrome, short bowel syndrome and to prevent disease progression in Type 2 diabetes. The invention also relates to a method of making 3,3,4,4-tetrafluoropyrrolidine, a starting material utilized in the afore-mentioned method for preparing DPP-IV compounds.
Owner:PFIZER INC
Implantable therapeutic device and methods of making
InactiveUS20130023823A1Peptide/protein ingredientsMetabolism disorderAcute hyperglycaemiaIGT - Impaired glucose tolerance
The subject invention pertains to an implantable therapeutic device for treating diabetes and methods of making. Upon implantation, the present device secretes insulin in response to blood glucose levels, exquisitely regulates blood glucose levels, reduces hyperglycemia, and includes β-cell regeneration in the host. It is useful for treating or ameliorating diabetes or diabetic conditions of a subject, including but not limited to, type 1 diabetes mellitus, hyperglycemia, impaired glucose tolerance, insulin deficiency, elevated glucose levels, and insulin resistance.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Chromium/biotin treatment of type II diabetes
Owner:NUTRITION 21 INC
Pharmaceutical composition, methods for treating and uses thereof
InactiveUS20110046087A1Prevent and slow progressionImprove blood sugar controlBiocideSenses disorderIGT - Impaired glucose toleranceAcute hyperglycaemia
The invention relates to the treatment or prevention of one or more conditions selected from type 1 diabetes mellitus, type 2 diabetes mellitus, impaired glucose tolerance and hyperglycemia using a SGLT-2 inhibitor. In addition the present invention relates to methods for preventing or treating of metabolic disorders and related conditions.
Owner:BOEHRINGER INGELHEIM INT GMBH
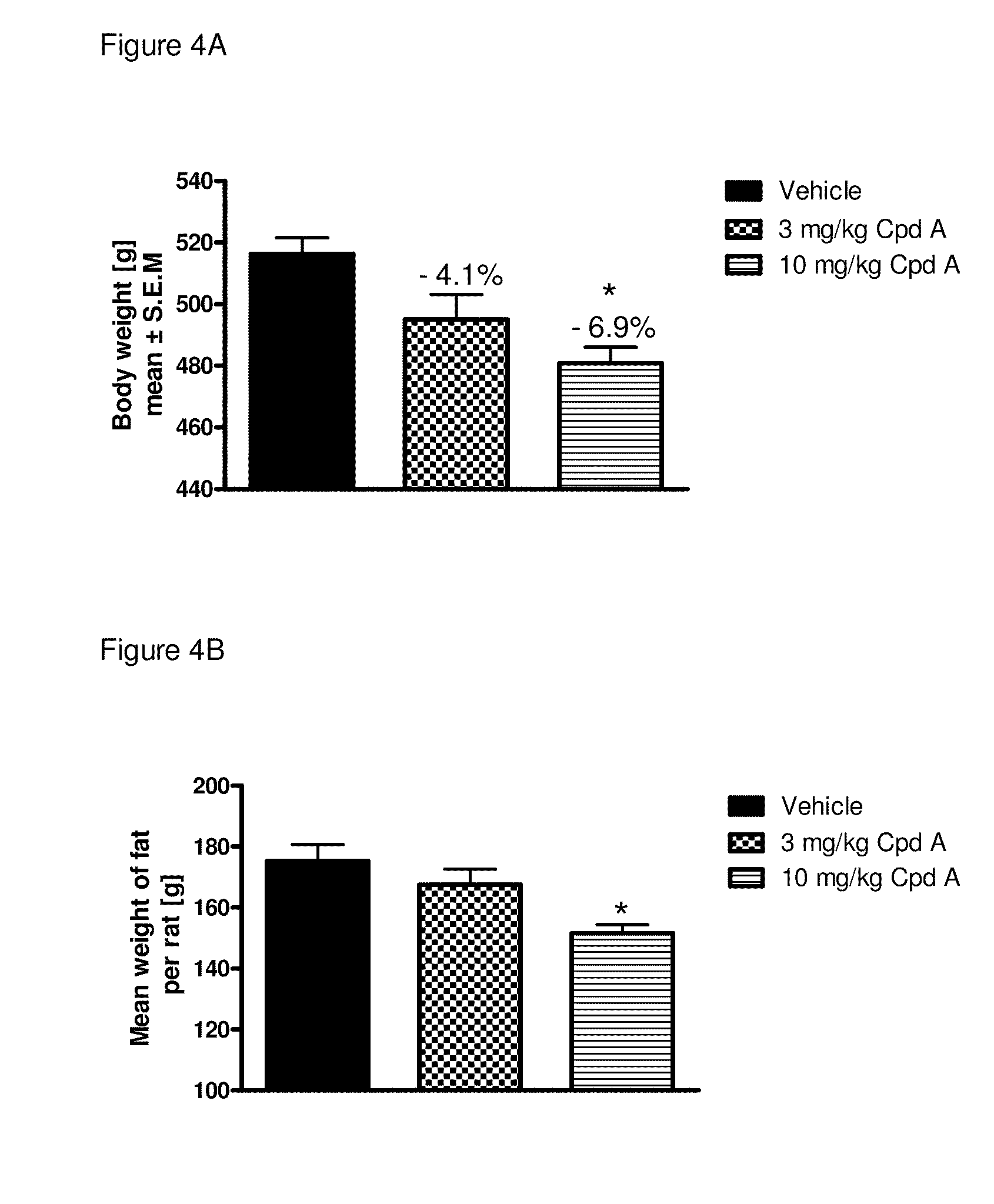
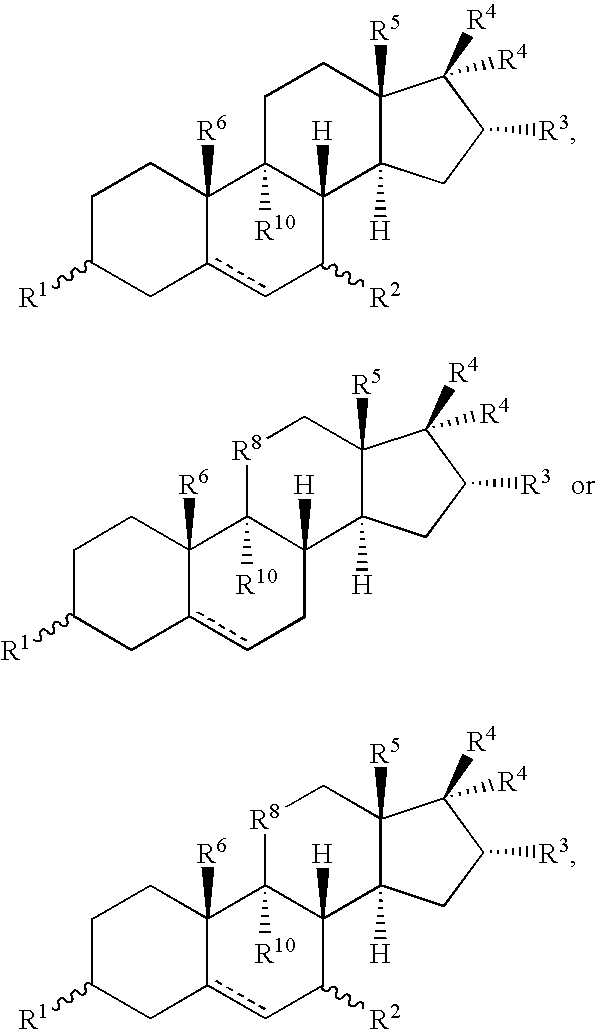
Metabolic Disease Treatments
The invention relates to the use of compounds to treat a number of conditions, such as a pre-diabetes condition, type 1 diabetes, type 2 diabetes, hyperglycemia, insulin resistance and glucose intolerance. Compounds that can be used in one or more of the treatment methods include 3β,7β,16α,17β-tetrahydroxyandrost-5-ene, 3α,7β,16α,17β-tetrahydroxyandrost-5-ene, 3β,7β,16α,17β-tetrahydroxyandrost-5-ene, 3β,16α,17β-trihydroxyandrost-5-ene-7-one, 3β,7β,17β-trihydroxy-17α-ethynylandrost-5-ene, 3β,17β-dihydroxy-17α-ethynylandrost-5-ene-7-one and 3β,7α,17β-trihydroxy-17α-ethynylandrost-5-ene.
Owner:HARBOR DIVERSIFIED +2
Combination therapy with sglt-2 inhibitors and their pharmaceutical compositions
InactiveUS20100298243A1Suitable for treatmentBiocidePeptide/protein ingredientsAcute hyperglycaemiaClass II obesity
The present invention is directed to a pharmaceutical composition comprised of one or more SGLT-2 inhibitor compound(s) in combination with one or more therapeutic agents which is suitable for the treatment of metabolic disorders including type 1 diabetes mellitus, type 2 diabetes mellitus, impaired glucose tolerance, hyperglycemia, postprandial hyperglycemia, overweight, obesity, including class I obesity, class II obesity, class III obesity, visceral obesity and abdominal obesity, and metabolic syndrome.
Owner:BOEHRINGER INGELHEIM INT GMBH
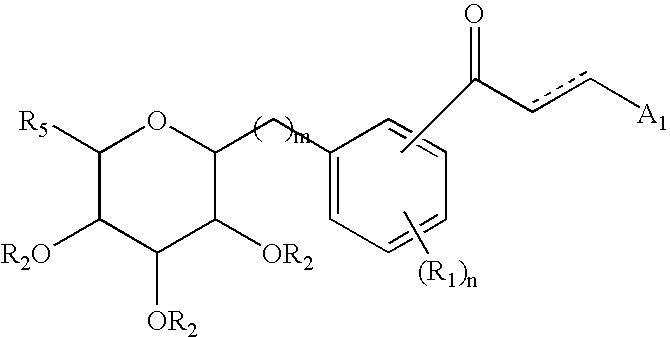
Pharmaceutical composition comprising a glucopyranosyl-substituted benzene derivate
ActiveUS8551957B2Good effectBiocidePowder deliveryIGT - Impaired glucose toleranceAcute hyperglycaemia
The invention relates to a pharmaceutical composition according to the claim 1 comprising a glucopyranosyl-substituted benzene derivative in combination with a DPP IV inhibitor which is suitable in the treatment or prevention of one or more conditions selected from type 1 diabetes mellitus, type 2 diabetes mellitus, impaired glucose tolerance and hyperglycemia. In addition the present invention relates to methods for preventing or treating of metabolic disorders and related conditions.
Owner:BOEHRINGER INGELHEIM INT GMBH
Lanthionine synthetase component c-like proteins as molecular targets for preventing and treating diseases and disorders
ActiveUS20110275558A1Organic active ingredientsPeptide/protein ingredientsThiazolidinedioneAutoimmune disease
The present invention relates to the field of medical treatments for diseases and disorders. More specifically, the present invention relates to the use of the lanthionine synthetase component C-like (LANCL) proteins as therapeutic targets for novel classes of anti-inflammatory, immune regulatory and antidiabetic drugs. This includes but it is not limited to abscisic acid (ABA), ABA analogs, benzimidazophenyls, repurposed drugs or drug combinations, including thiazolidinediones (TZDs); naturally occurring compounds such as conjugated diene fatty acids, conjugated triene fatty acids, isoprenoids, and natural and synthetic agonists of peroxisome proliferator-activated receptors that activate this receptor through an alternative mechanism of action involving LANCL2 or other membrane proteins to treat or prevent the common inflammatory pathogenesis underlying type 2 diabetes, atherosclerosis, cancer, some inflammatory infectious diseases such as influenza and autoimmune diseases including but not limited to inflammatory bowel disease (Crohn's disease and Ulcerative colitis), rheumatoid arthritis, multiple sclerosis and type 1 diabetes and other chronic inflammatory conditions.
Owner:VIRGINIA TECH INTPROP INC
Delivery scaffolds and related methods of use
InactiveUS20090238879A1Function increaseMaximizing graft functionBiocideOrganic active ingredientsDiseaseCell-Extracellular Matrix
The present invention relates to delivery systems. In particular, the present invention provides microporous scaffolds having thereon agents (e.g., extracellular matrix proteins, exendin-4) and biological material (e.g., pancreatic islet cells). In some embodiments, the scaffolds are used for transplanting biological material into a subject. In some embodiments, the scaffolds are used in the treatment of diseases (e.g., type 1 diabetes), and related methods (e.g., diagnostic methods, research methods, drug screening).
Owner:NORTHWESTERN UNIV
Delivery Scaffolds and Related Methods of Use
InactiveUS20140037749A1Increase capacityImprove blood sugar controlBiocideOrganic active ingredientsDiseaseCell-Extracellular Matrix
The present invention relates to delivery systems. In particular, the present invention provides microporous scaffolds having thereon agents (e.g., extracellular matrix proteins, exendin-4) and biological material (e.g., pancreatic islet cells). In some embodiments, the scaffolds are used for transplanting biological material into a subject. In some embodiments, the scaffolds are used in the treatment of diseases (e.g., type 1 diabetes), and related methods (e.g., diagnostic methods, research methods, drug screening).
Owner:NORTHWESTERN UNIV
Method of using abscisic acid to treat diseases and disorders
The present invention provides compositions and methods for treating and / or preventing diseases and disorders associated with expression of PPAR γ and / or infiltration of macrophages into skeletal muscle tissue and / or white adipose tissue. The method treats such diseases and disorders with abscisic acid (ABA). Exemplary diseases and disorders include diabetes, including type 2 diabetes, prediabetes, glucose intolerance insulin resistance, and diseases and disorders involving the immune system, such as inflammation, including obesity-related inflammation, inflammatory bowel disease, type 1 diabetes, multiple sclerosis, allergies, asthma, cardiovascular disease, and arthritis.
Owner:VIRGINIA TECH INTPROP INC
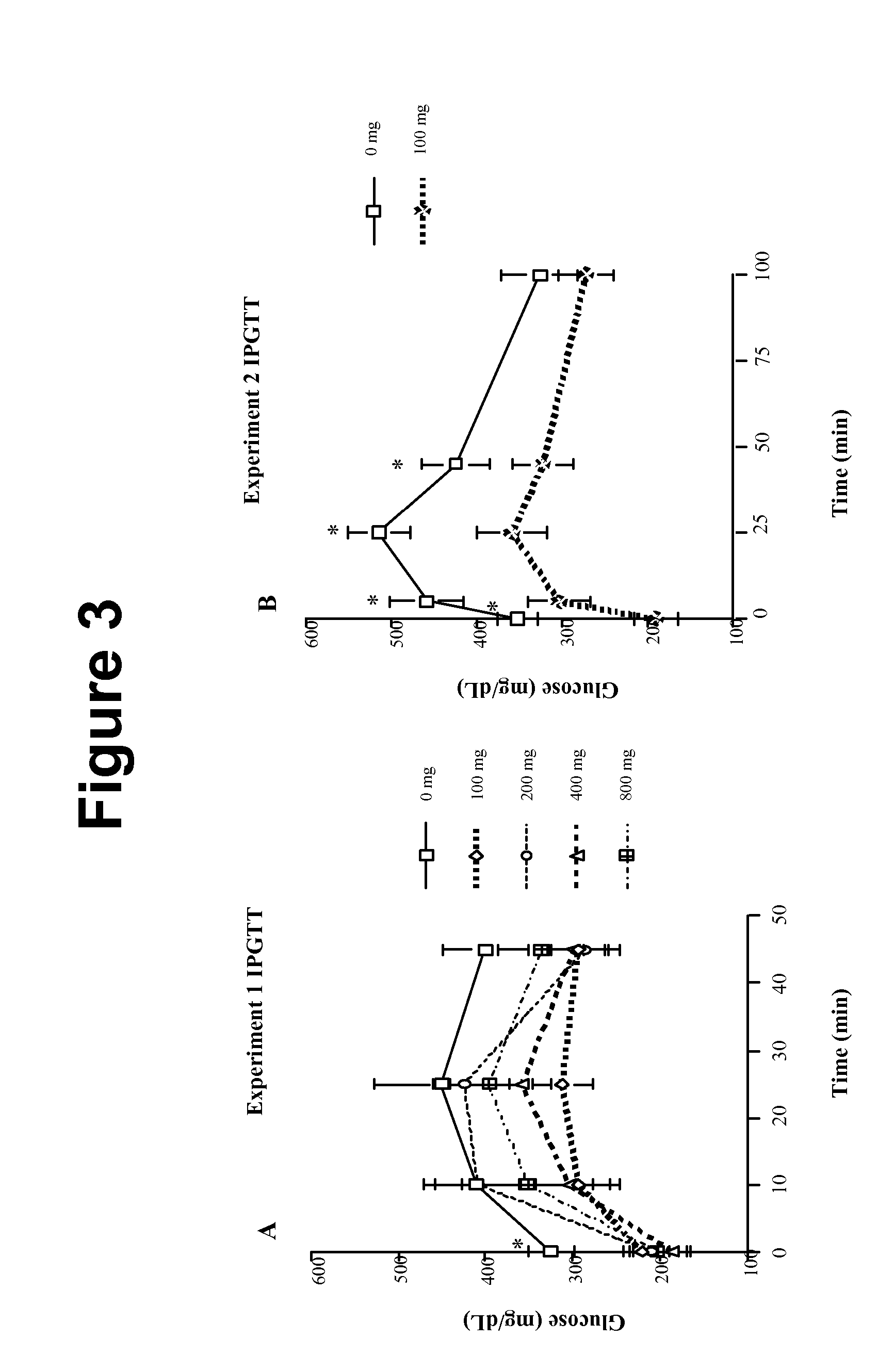
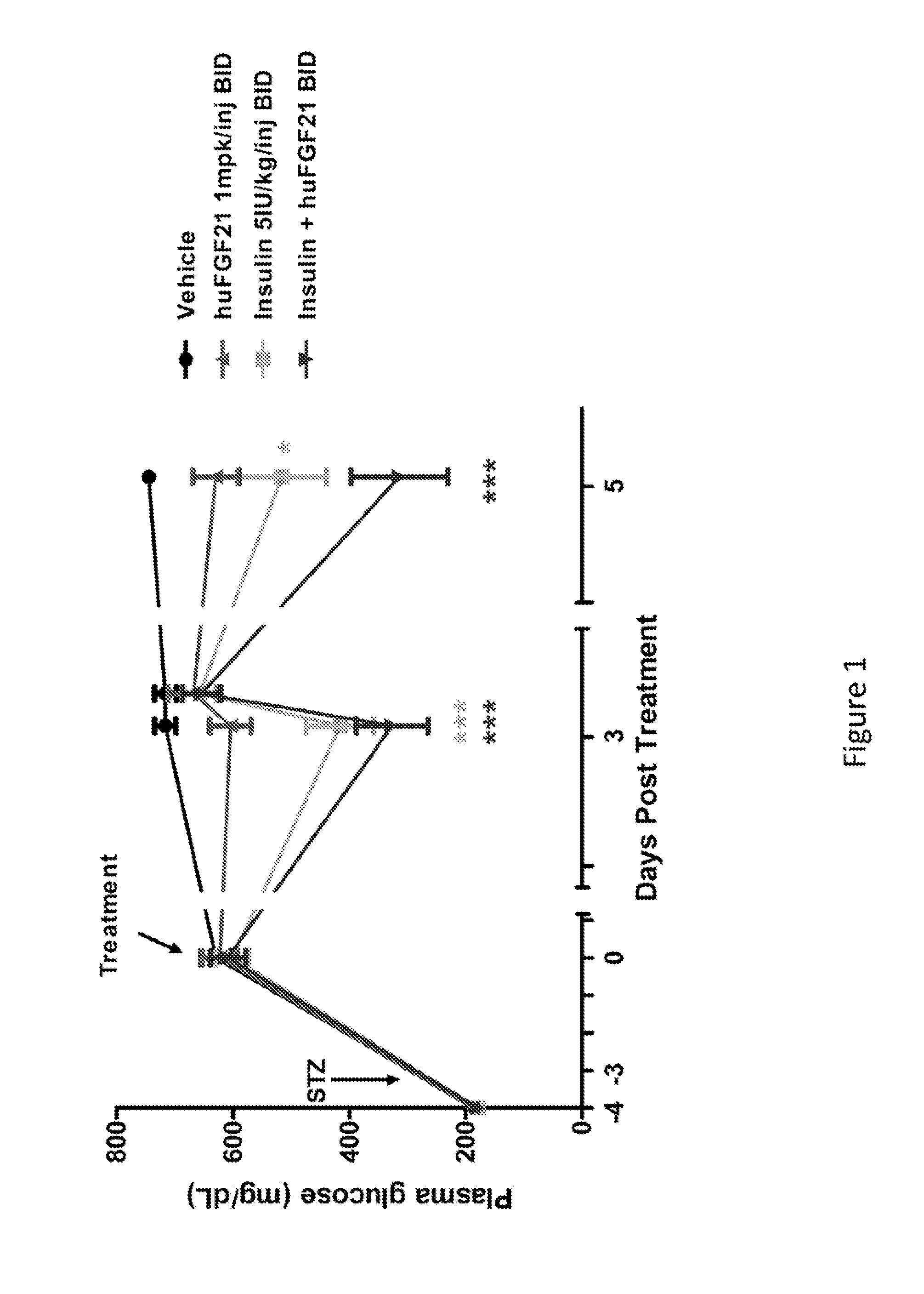
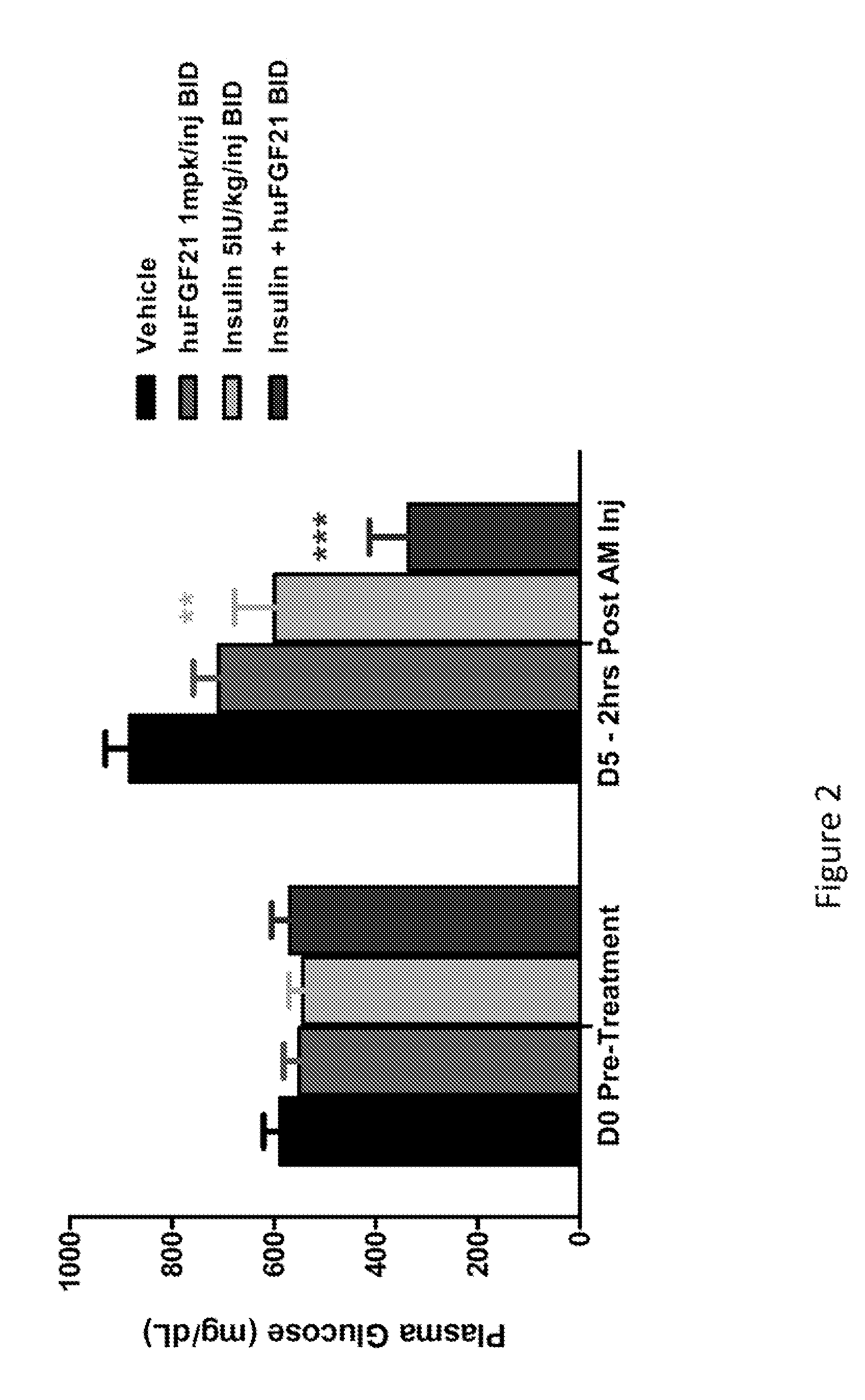
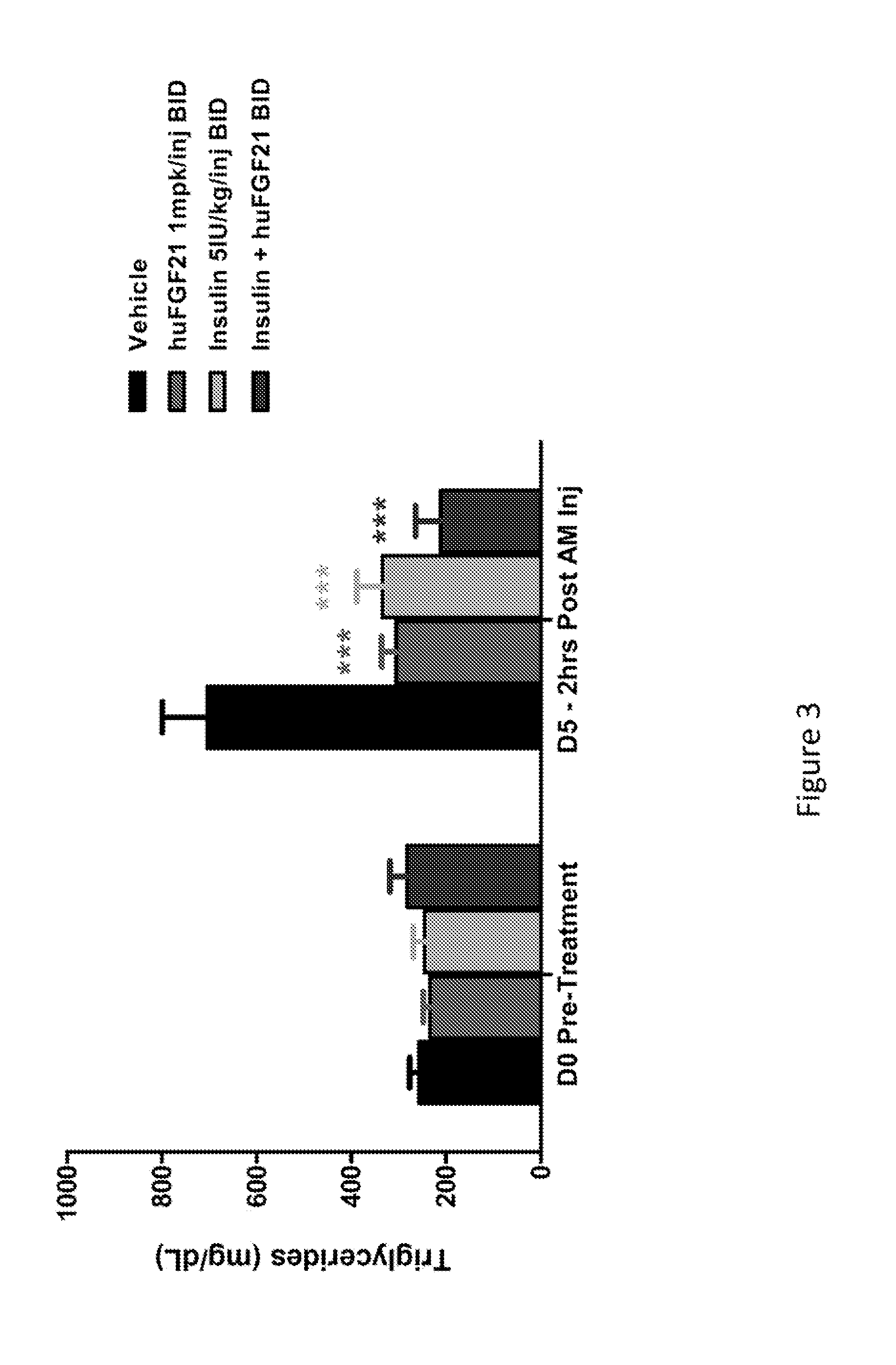
Method of Treating or Ameliorating Type 1 Diabetes Using FGF21
Methods of treating metabolic diseases and disorders using a FGF21 polypeptide are provided. In various embodiments the metabolic disease or disorder is type 1 diabetes, obesity, dyslipidemia, elevated glucose levels, elevated insulin levels, diabetic nephropathy, neuropathy, retinopathy, ischemic heart disease, peripheral vascular disease and cerebrovascular disease
Owner:AMGEN INC
Toxin peptide therapeutic agents
ActiveUS7833979B2Preventing and mitigating relapseAvoid it happening againNervous disorderAntipyreticHalf-lifeFibrosis
Disclosed is a composition of matter of the formula(X1)a—(F1)d—(X2)b—(F2)e—(X3)c (I)and multimers thereof, in which F1 and F2 are half-life extending moieties, and d and e are each independently 0 or 1, provided that at least one of d and e is 1; X1, X2, and X3 are each independently -(L)f-P-(L)g-, and f and g are each independently 0 or 1; P is a toxin peptide of no more than about 80 amino acid residues in length, comprising at least two intrapeptide disulfide bonds; L is an optional linker; and a, b, and c are each independently 0 or 1, provided that at least one of a, b and c is 1. Linkage to the half-life extending moiety or moieties increases the in vivo half-life of the toxin peptide, which otherwise would be quickly degraded. A pharmaceutical composition comprises the composition and a pharmaceutically acceptable carrier. Also disclosed are a DNA encoding the inventive composition of matter, an expression vector comprising the DNA, and a host cell comprising the expression vector. Methods of treating an autoimmune disorder, such as, but not limited to, multiple sclerosis, type 1 diabetes, psoriasis, inflammatory bowel disease, contact-mediated dermatitis, rheumatoid arthritis, psoriatic arthritis, asthma, allergy, restinosis, systemic sclerosis, fibrosis, scleroderma, glomerulonephritis, Sjogren syndrome, inflammatory bone resorption, transplant rejection, graft-versus-host disease, and lupus and of preventing or mitigating a relapse of a symptom of multiple sclerosis are also disclosed.
Owner:AMGEN INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com