Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
4577 results about "Insomnia" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Trouble falling and/or staying asleep.
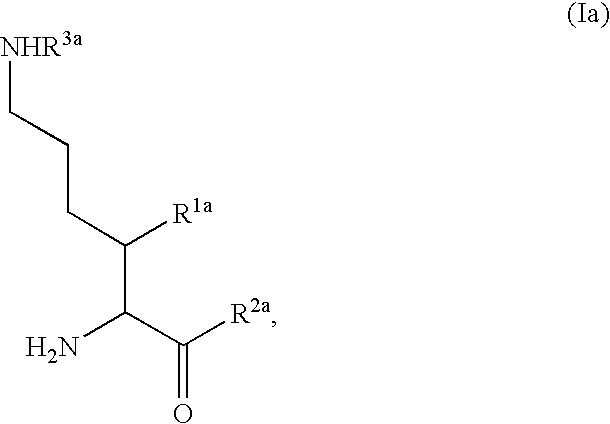
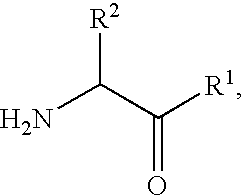
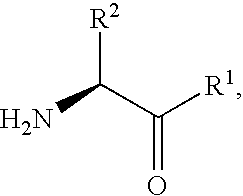
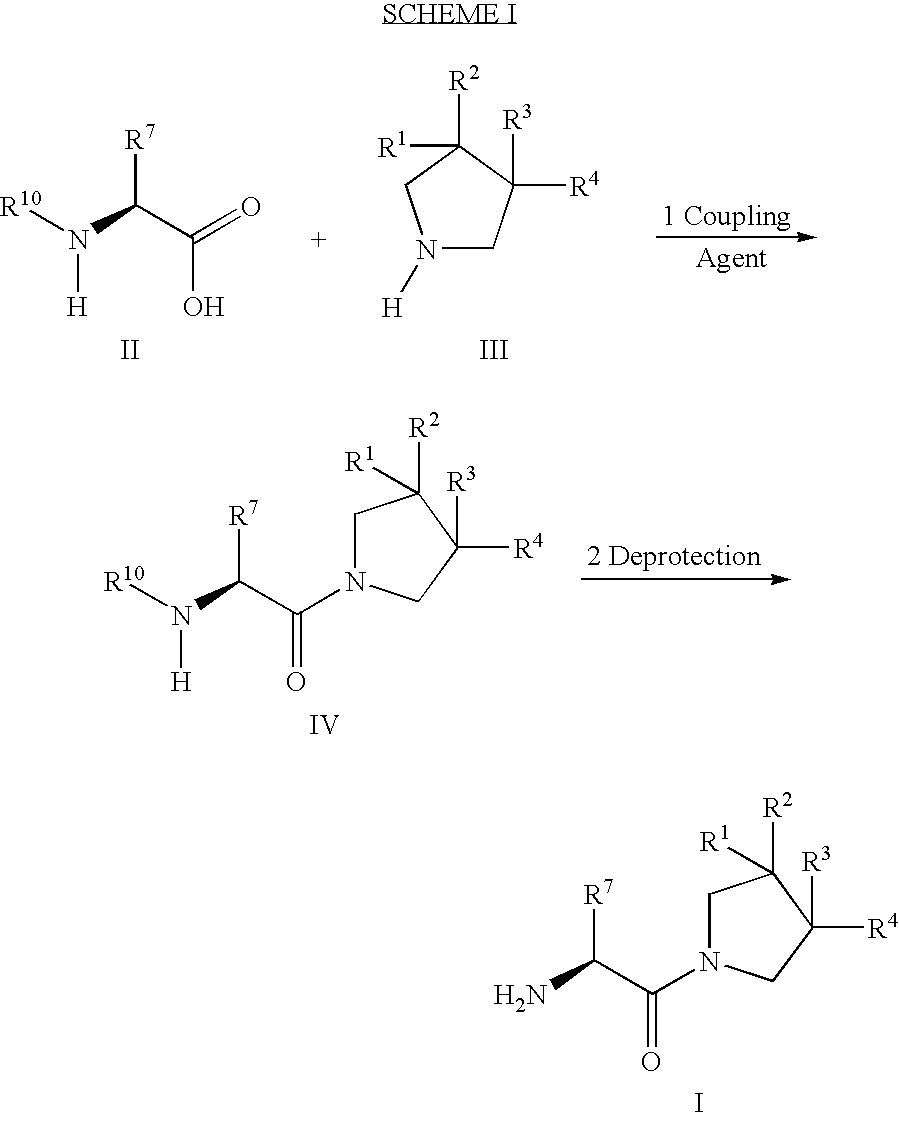
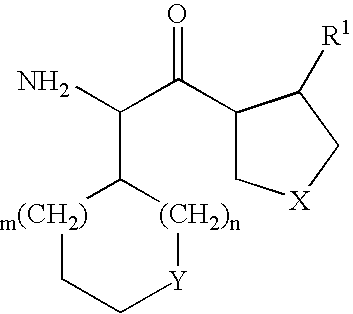
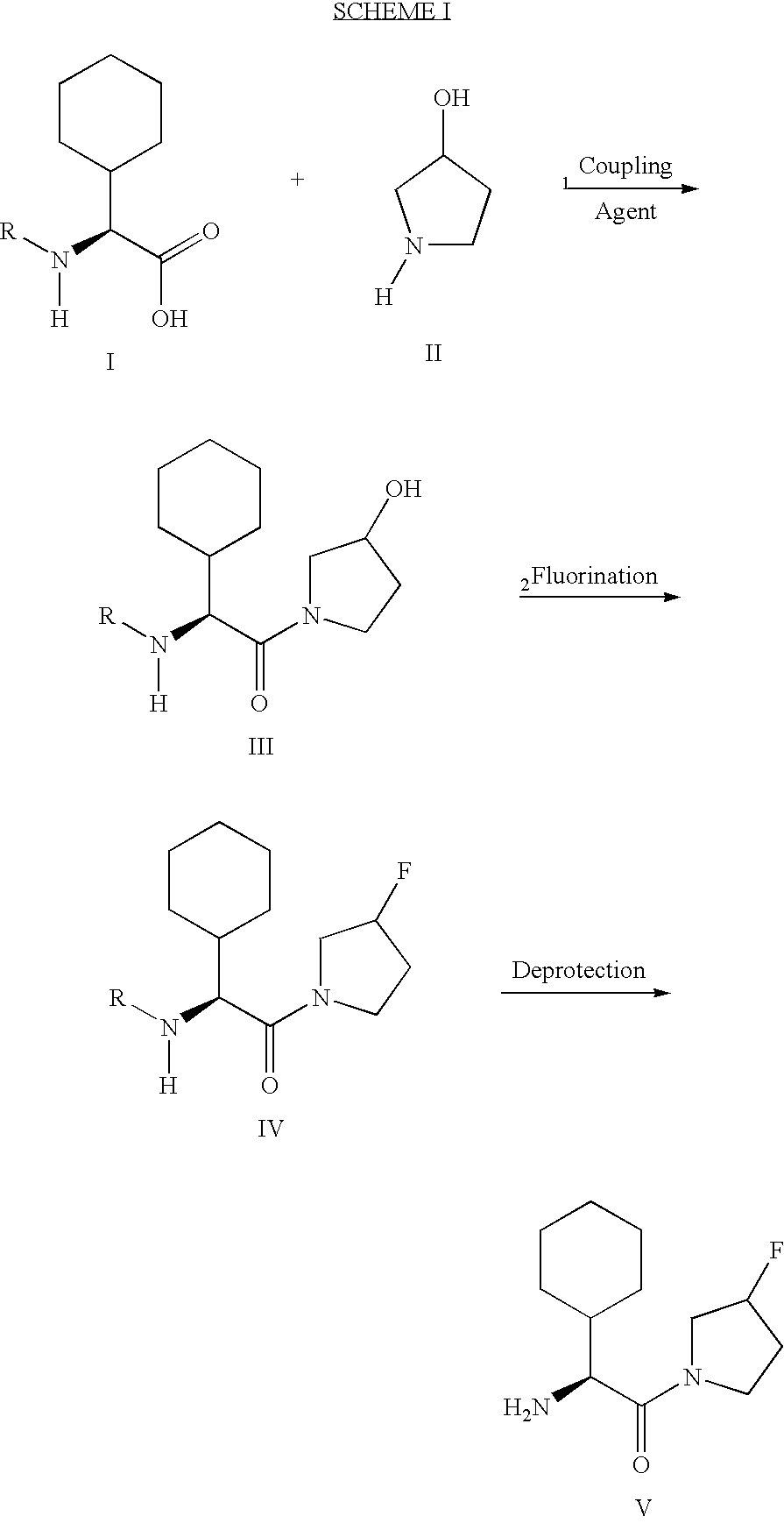
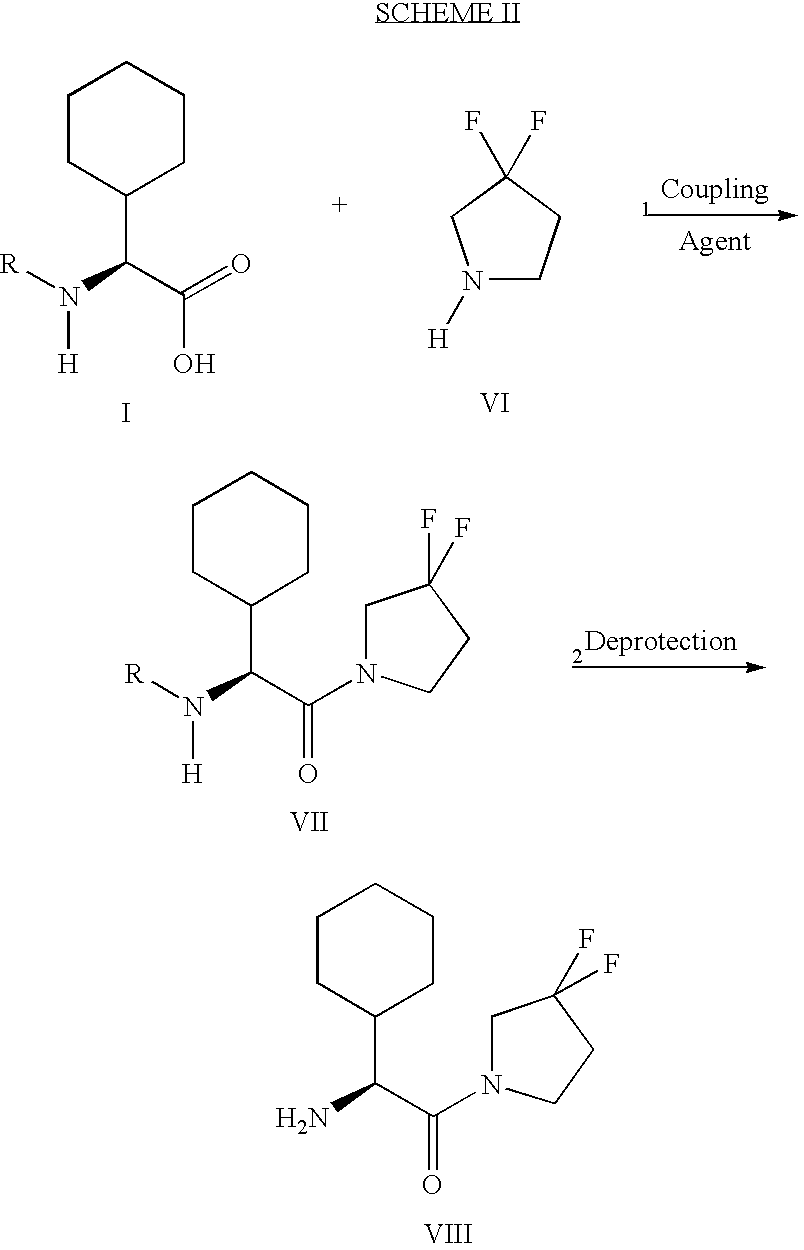
Fluorinated lysine derivatives as dipeptidyl peptidase IV inhibitors
InactiveUS20050043292A1Ease of preparation and detectabilityGood metabolic stabilityBiocideOrganic chemistryDiabetic retinopathyArthritis
The invention relates to new therapeutically active and selective inhibitors of the enzyme dipeptidyl peptidase-IV (“DPP-IV”), pharmaceutical compositions comprising the compounds and the use of such compounds for treating diseases that are associated with proteins that are subject to processing by DPP-IV, such as Type 2 diabetes, metabolic syndrome (syndrome X or insulin resistance syndrome), hyperglycemia, impaired glucose tolerance, glucosuria, metabolic acidosis, arthritis, cataracts, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, diabetic cardiomyopathy, Type 1 diabetes, obesity, conditions exacerbated by obesity, hypertension, hyperlipidemia, atherosclerosis, osteoporosis, osteopenia, frailty, bone loss, bone fracture, acute coronary syndrome, infertility due to polycystic ovary syndrome, short bowel syndrome, anxiety, depression, insomnia, chronic fatigue, epilepsy, eating disorders, chronic pain, alcohol addiction, diseases associated with intestinal motility, ulcers, irritable bowel syndrome, inflammatory bowel syndrome and to prevent disease progression in Type 2 diabetes. The invention also relates to a method of identifying an insulin secretagogue agent for diabetes.
Owner:PFIZER INC
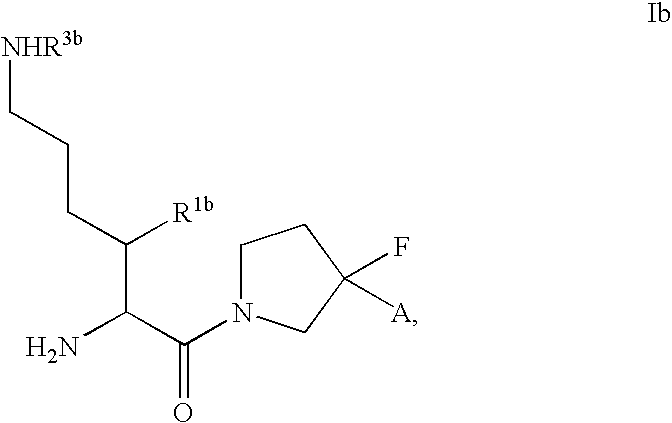
Fluorinated cyclic amides as dipeptidyl peptidase IV inhibitors
InactiveUS6710040B1Easy to prepareEase of detectabilityBiocideOrganic chemistryAcute coronary syndromeDisease progression
The invention relates to new therapeutically active and selective inhibitors of the enzyme dipeptidyl peptidase-IV, pharmaceutical compositions comprising the compounds and the use of such compounds for treating diseases that are associated with proteins that are subject to processing by DPP-IV, such as Type 2 diabetes mellitus, hyperglycemia, impaired glucose tolerance, metabolic syndrome (Syndrome X or insulin resistance syndrome), glucosuria, metabolic acidosis, cataracts, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, diabetic cardiomyopathy, Type 1 diabetes, obesity, conditions exacerbated by obesity, hypertension, hyperlipidemia, atherosclerosis, osteoporosis, osteopenia, frailty, bone loss, bone fracture, acute coronary syndrome, infertility due to polycystic ovary syndrome, short bowel syndrome, anxiety, depression, insomnia, chronic fatigue, epilepsy, eating disorders, chronic pain, alcohol addiction, diseases associated with intestinal motility, ulcers, irritable bowel syndrome, inflammatory bowel syndrome and to prevent disease progression in Type 2 diabetes. The invention also relates to a method of identifying an insulin secretagogue agent for diabetes.
Owner:PFIZER INC
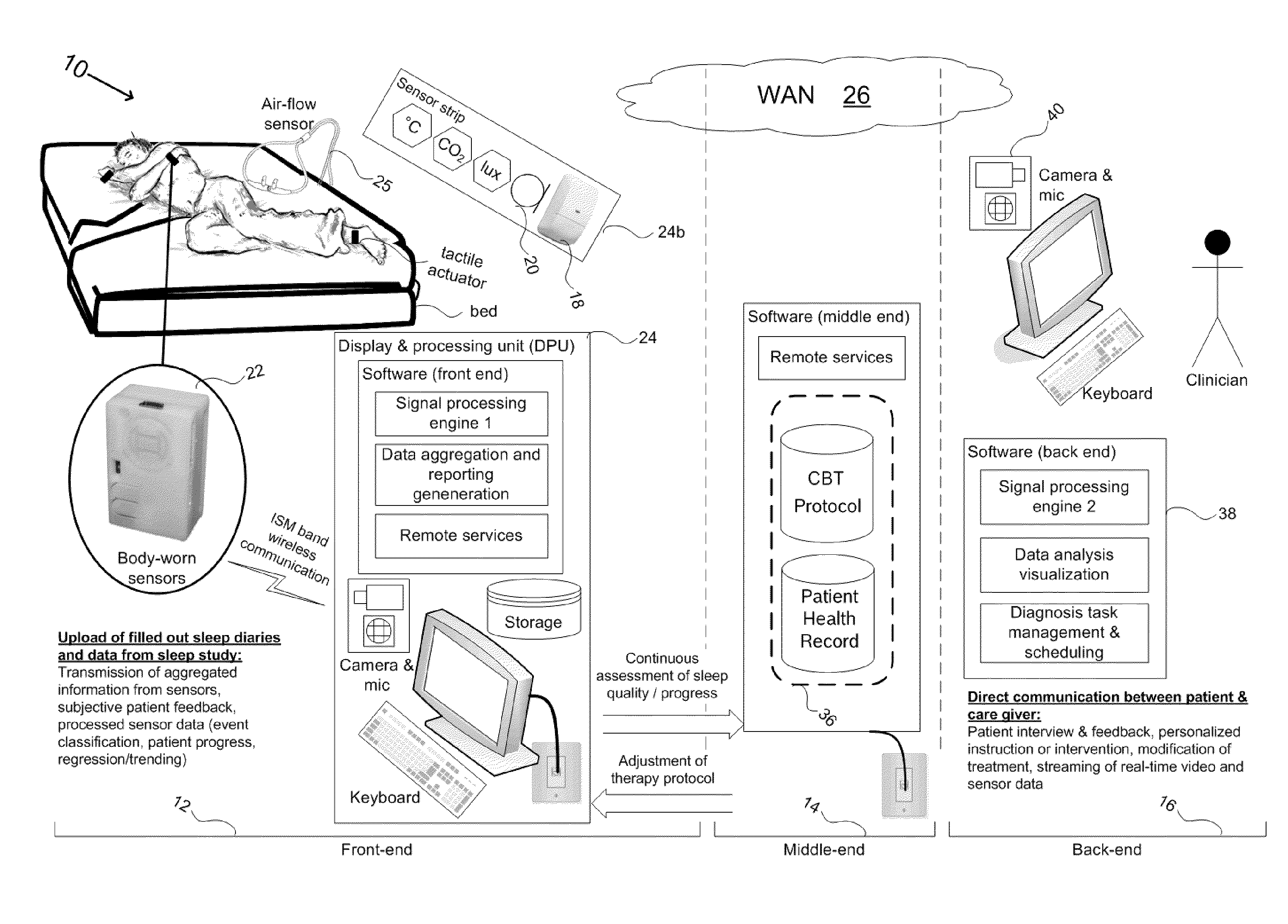
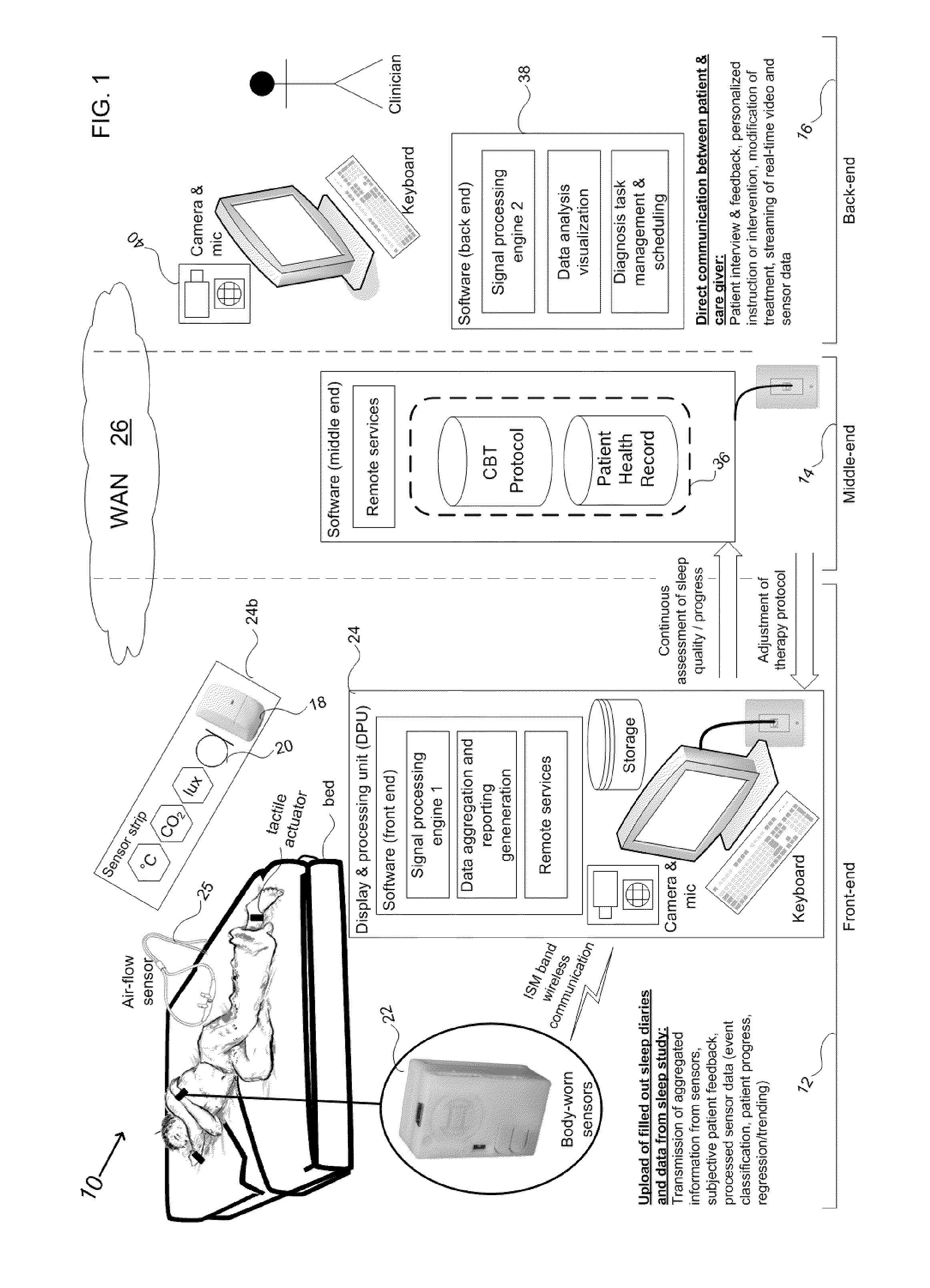
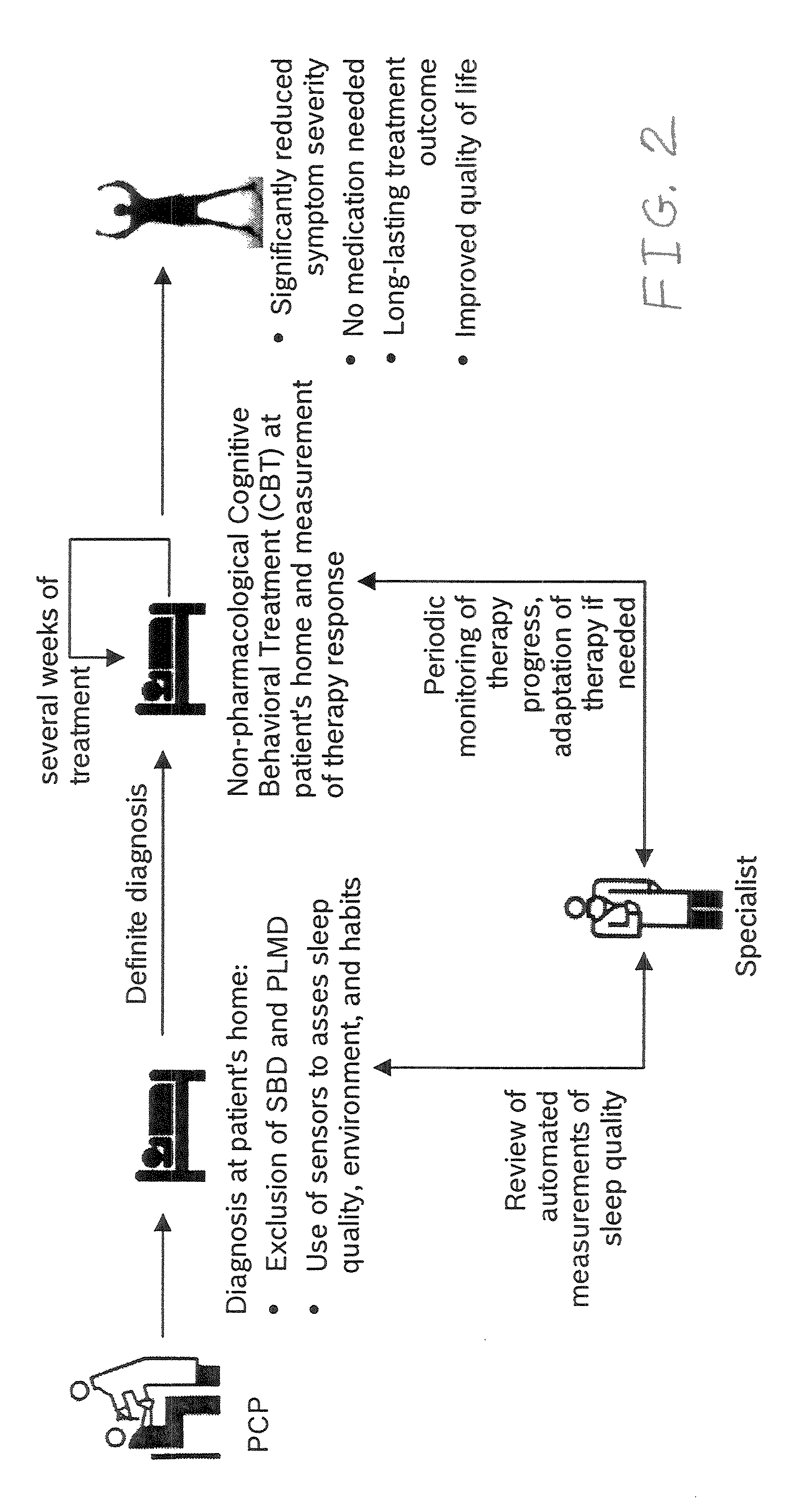
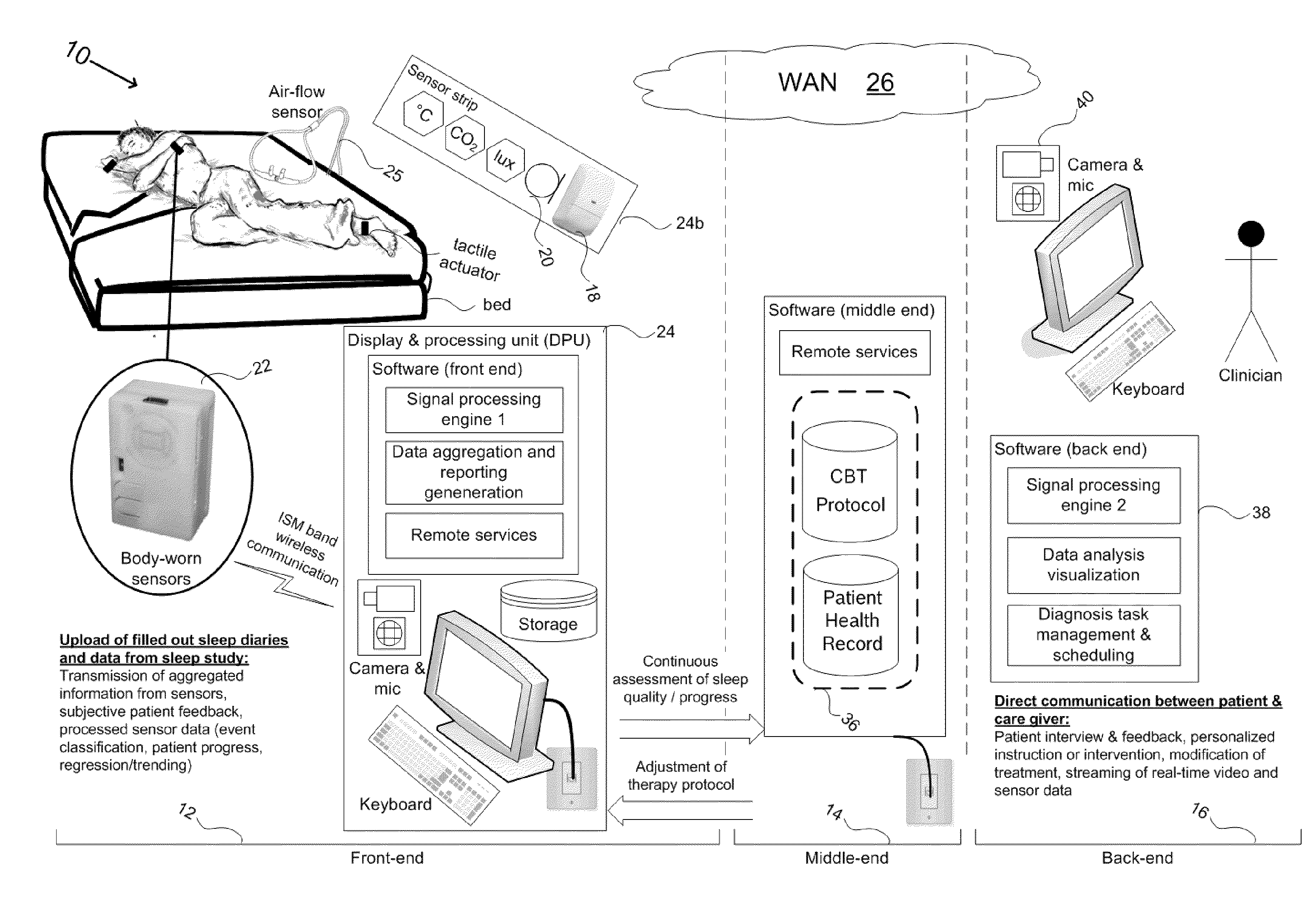
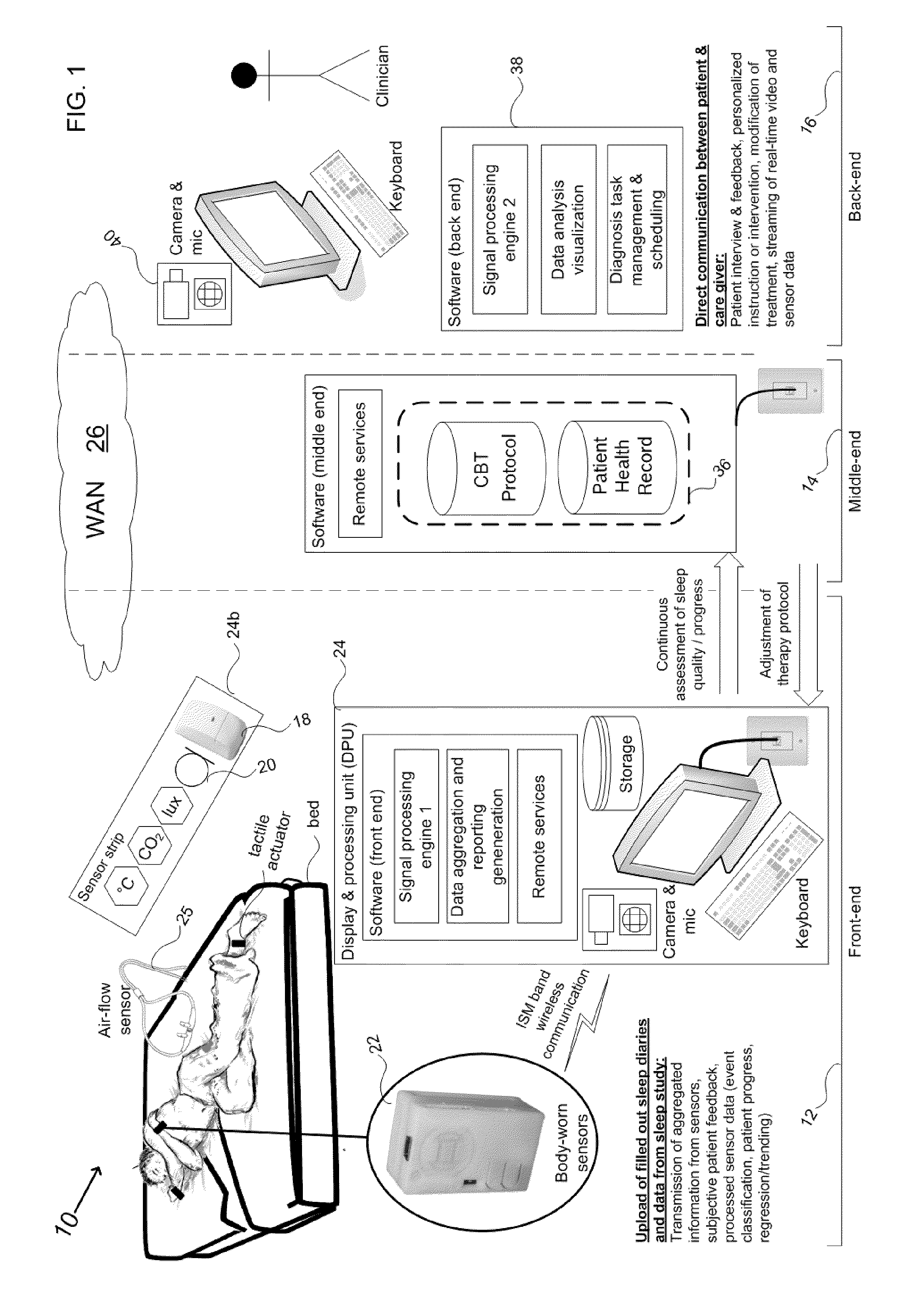
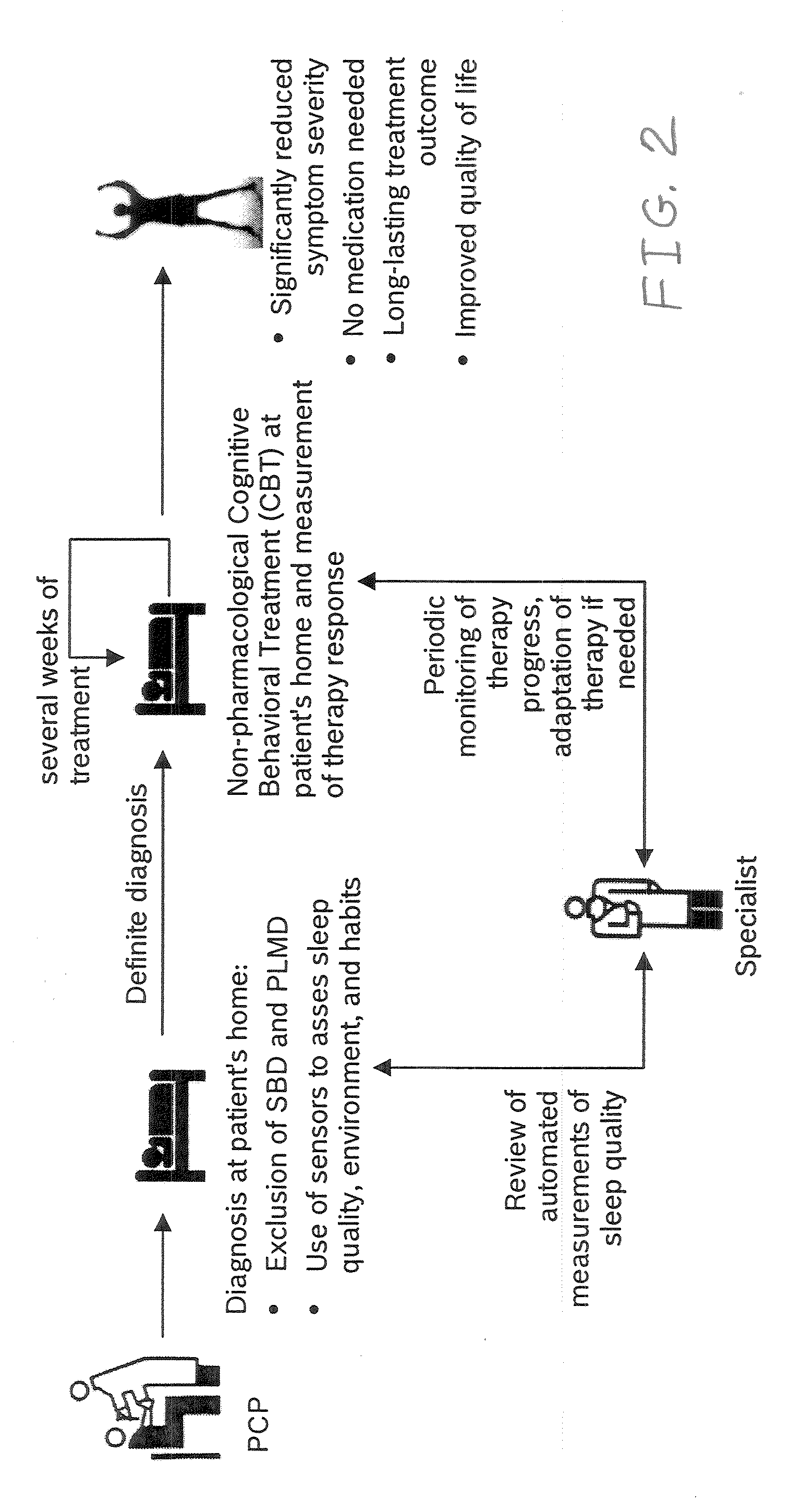
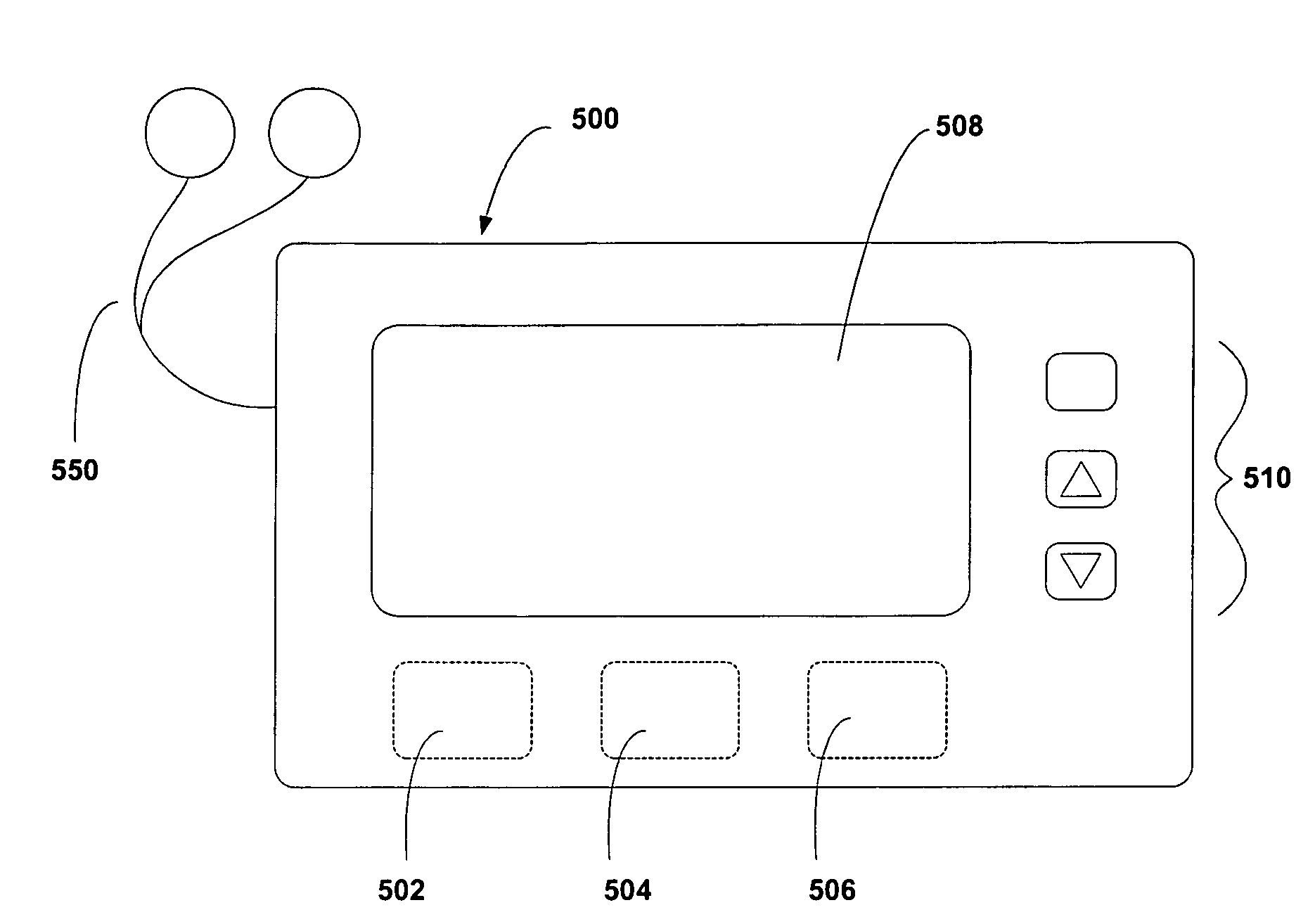
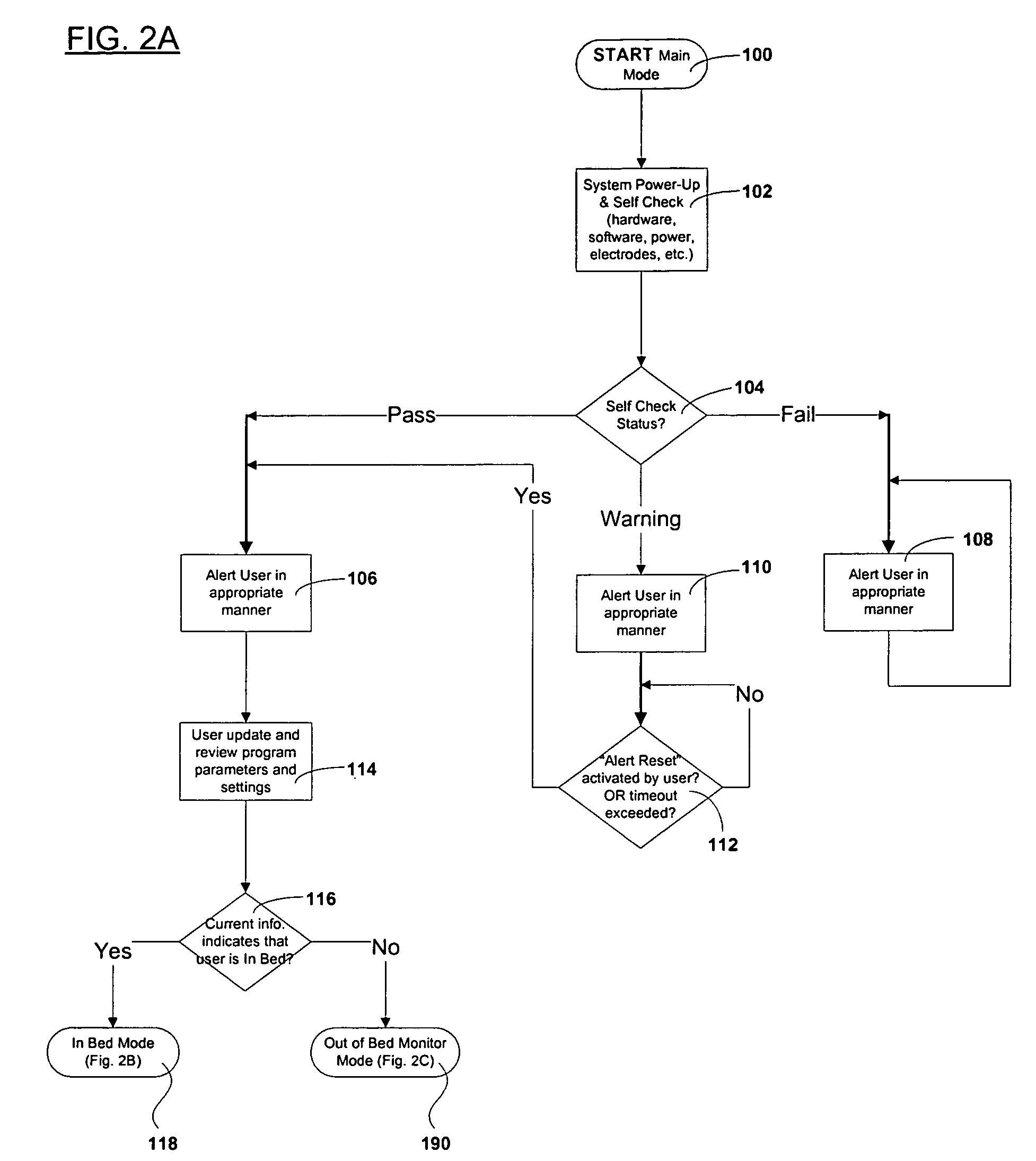
Device and method to monitor, assess and improve quality of sleep
A medical sleep disorder arrangement integrates into current diagnosis and treatment procedures to enable a health care professional to diagnose and treat a plurality of subjects suffering from insomnia. The arrangement may include both environmental sensors and body-worn sensors that measure the environmental conditions and the condition of the individual patient. The data may be collected and processed to measure clinically relevant attributes of sleep quality automatically. These automatically determined measures, along with the original sensor data, may be aggregated and shared remotely with the health care professional. A communication apparatus enables the healthcare professional to remotely communicate with and further assess the patient and subsequently administer the treatment. Thus, a more accurate diagnosis and more effective treatment is provided while reducing the required clinician time per patient for treatment delivery.
Owner:ROBERT BOSCH GMBH
Dipeptidyl peptidase IV inhibiting fluorinated cyclic amides
InactiveUS20040110817A1Ease of preparation and detectabilityGood metabolic stabilityBiocideSenses disorderDiabetic retinopathyDisease progression
The invention relates to new therapeutically active and selective inhibitors of the enzyme dipeptidyl peptidase-IV, pharmaceutical compositions comprising the compounds and the use of such compounds for treating diseases that are associated with proteins that are subject to processing by DPP-IV, such as Type 2 diabetes mellitus, hyperglycemia, impaired glucose tolerance, metabolic syndrome (Syndrome X or insulin resistance syndrome), glucosuria, metabolic acidosis, cataracts, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, diabetic cardiomyopathy, Type 1 diabetes, obesity, conditions exacerbated by obesity, hypertension, hyperlipidemia, atherosclerosis, osteoporosis, osteopenia, frailty, bone loss, bone fracture, acute coronary syndrome, infertility due to polycystic ovary syndrome, short bowel syndrome, anxiety, depression, insomnia, chronic fatigue, epilepsy, eating disorders, chronic pain, alcohol addiction, diseases associated with intestinal motility, ulcers, irritable bowel syndrome, inflammatory bowel syndrome and to prevent disease progression in Type 2 diabetes. The invention also relates to a method of identifying an insulin secretagogue agent for diabetes.
Owner:PFIZER INC
Device and method to monitor, assess and improve quality of sleep
ActiveUS20110190594A1Maximize individual convalescenceMaximize convalescenceRepeater circuitsMedical automated diagnosisTreatment deliveryMedicine
A medical sleep disorder arrangement integrates into current diagnosis and treatment procedures to enable a health care professional to diagnose and treat a plurality of subjects suffering from insomnia. The arrangement may include both environmental sensors and body-worn sensors that measure the environmental conditions and the condition of the individual patient. The data may be collected and processed to measure clinically relevant attributes of sleep quality automatically. These automatically determined measures, along with the original sensor data, may be aggregated and shared remotely with the health care professional. A communication apparatus enables the healthcare professional to remotely communicate with and further assess the patient and subsequently administer the treatment. Thus, a more accurate diagnosis and more effective treatment is provided while reducing the required clinician time per patient for treatment delivery.
Owner:ROBERT BOSCH GMBH
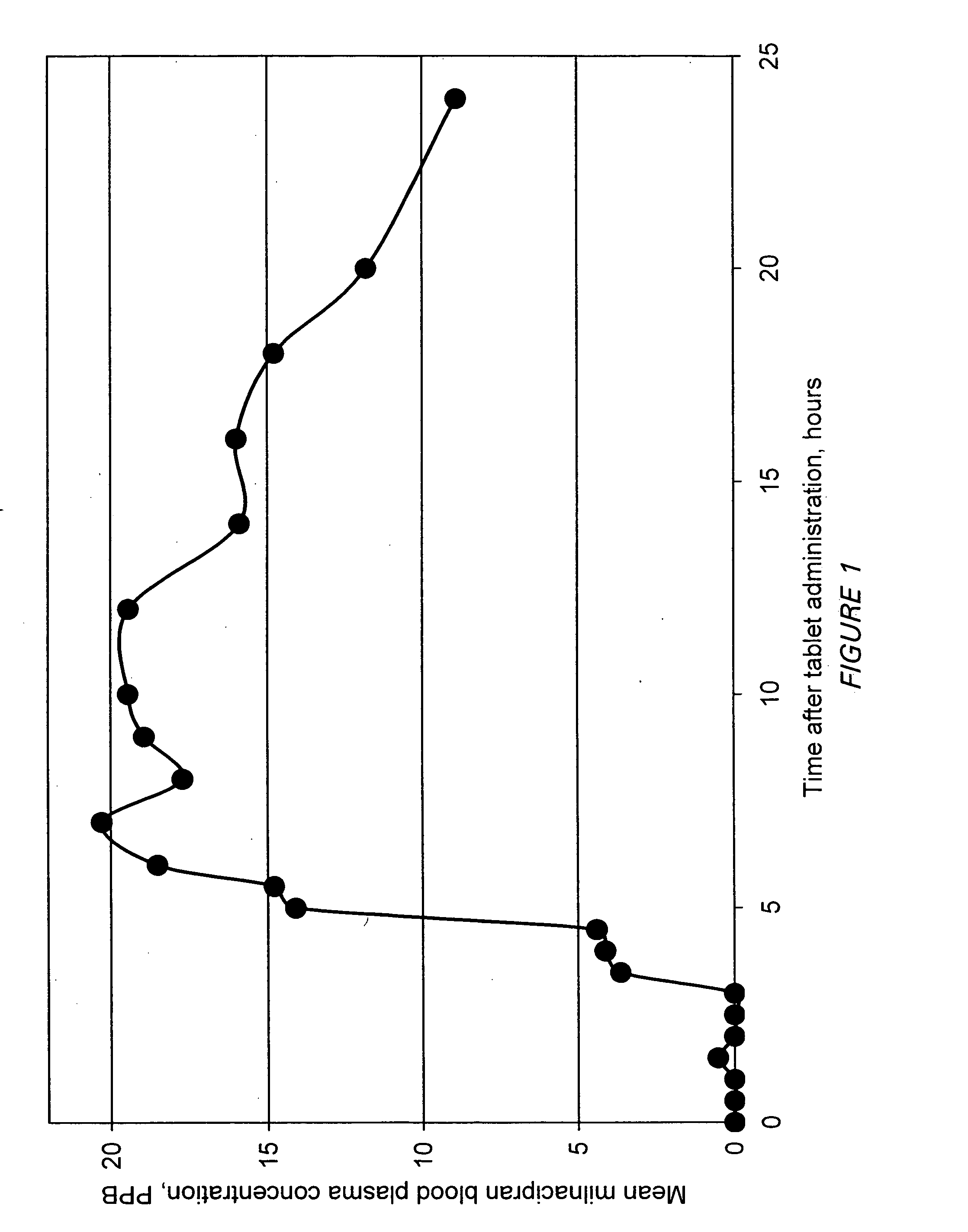
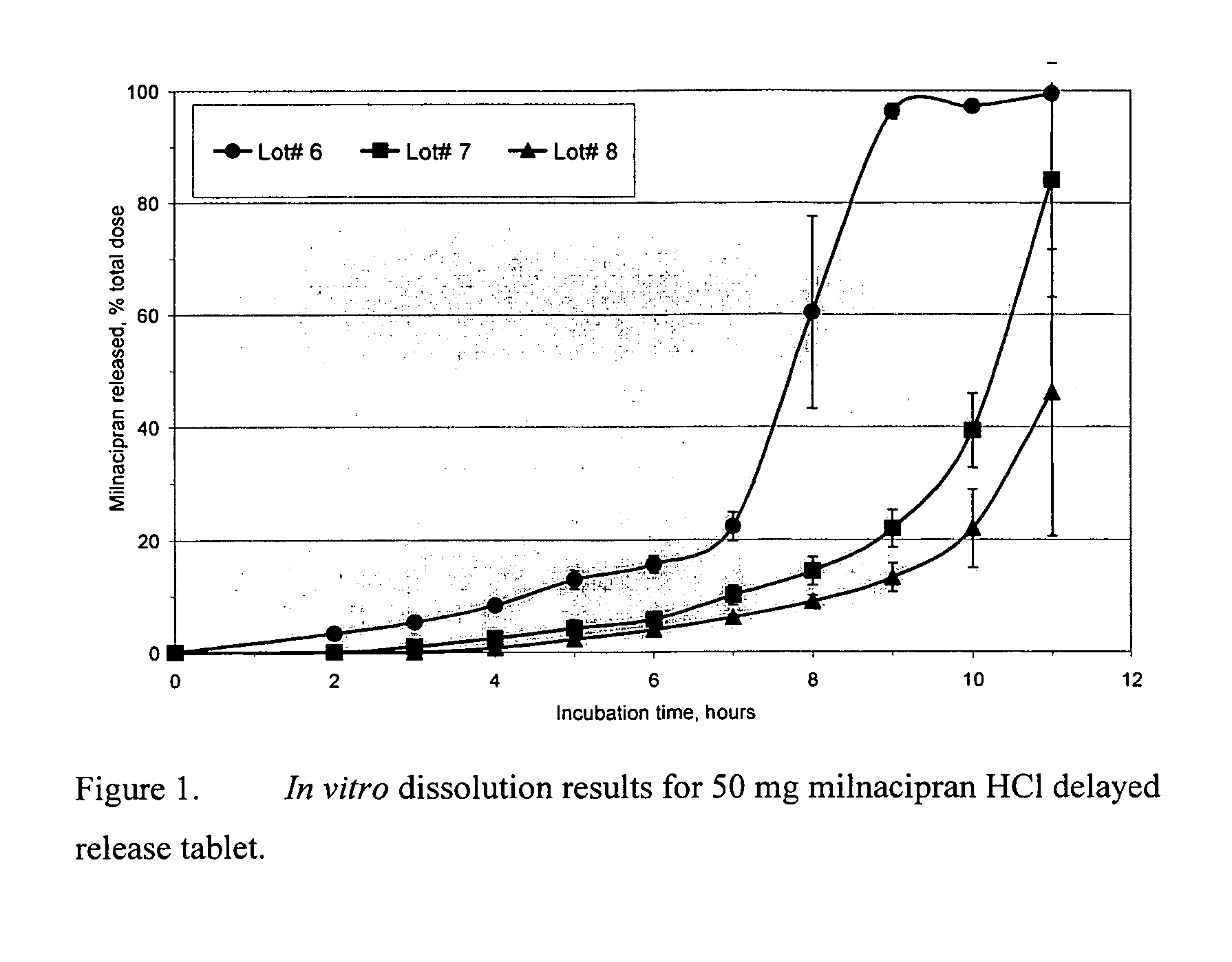
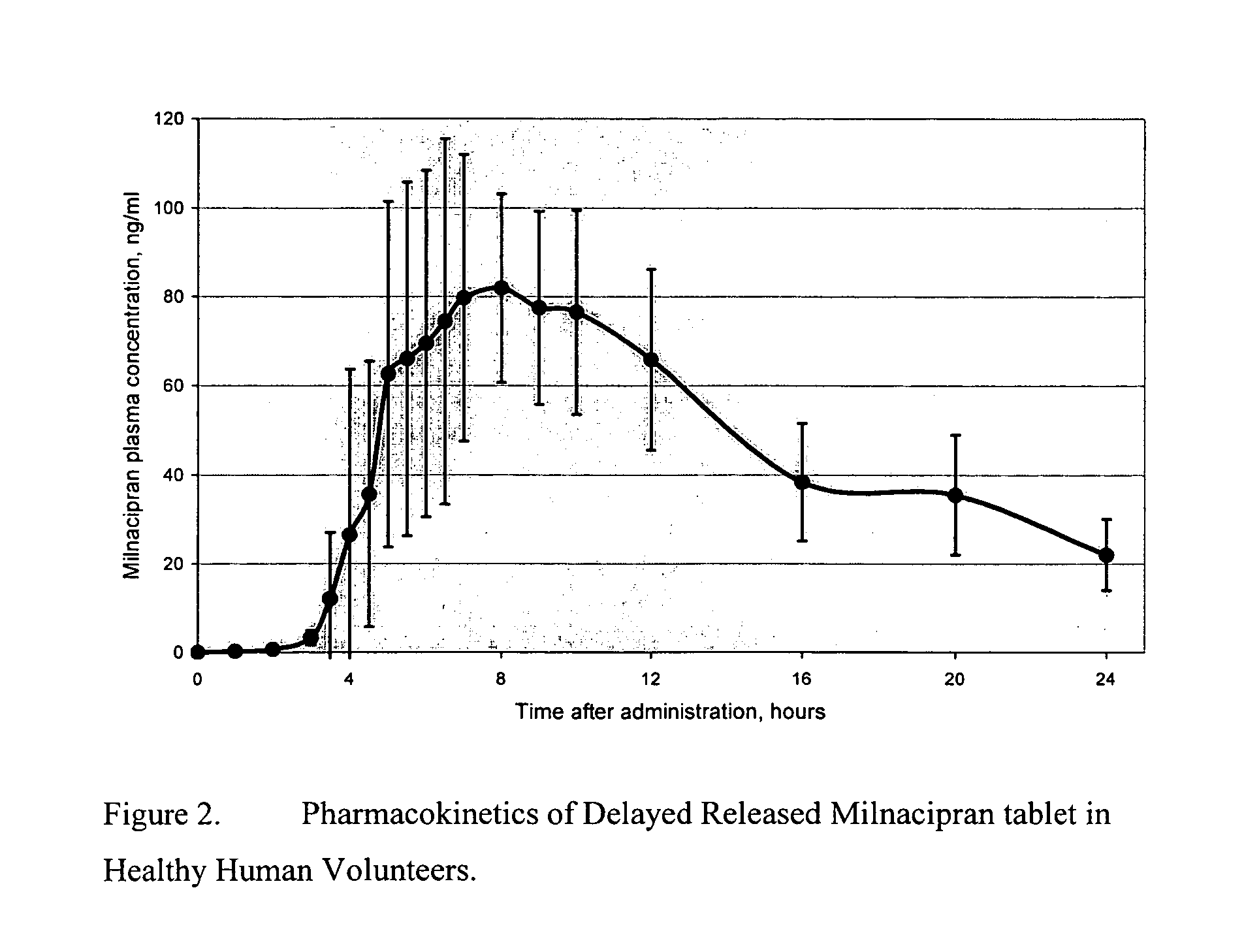
Modified release compositions of milnacipran
A once-a-day oral milnacipran modified release formulation has been developed. The formulation comprises an extended release dosage unit (optionally containing the immediate release portion) coated with delayed release coating. The milnacipran composition, when administered orally, first passes through the stomach releasing from zero to less than 10% of the total milnacipran dose and then enters the intestines where drug is released slowly over an extended period of time. The release profile is characterized by a 0.05-4 hours lag time period during which less than 10% of the total milnacipran dose is released followed by a slow or extended release of the remaining drug over a defined period of time. The composition provides in vivo drug plasma levels characterized by Tmax at 4-10 hours and an approximately linear drop-off thereafter and Cmax below 3000 ng / ml, preferably below 2000 ng / ml, and most preferably below 1000 ng / ml. The composition allows milnacipran to be delivered over approximately 24 hours, when administered to a patient in need, resulting in diminished incidence or decreased intensity of common milnacipran side effects such as sleep disturbance, nausea, vomiting, headache, tremulousness, anxiety, panic attacks, palpitations, urinary retention, orthostatic hypotension, diaphoresis, chest pain, rash, weight gain, back pain, constipation, vertigo, increased sweating, agitation, hot flushes, tremors, fatigue, somnolence, dyspepsia, dysoria, nervousness, dry mouth, abdominal pain, irritability, and insomnia.
Owner:COLLEGIUM PHARMA INC
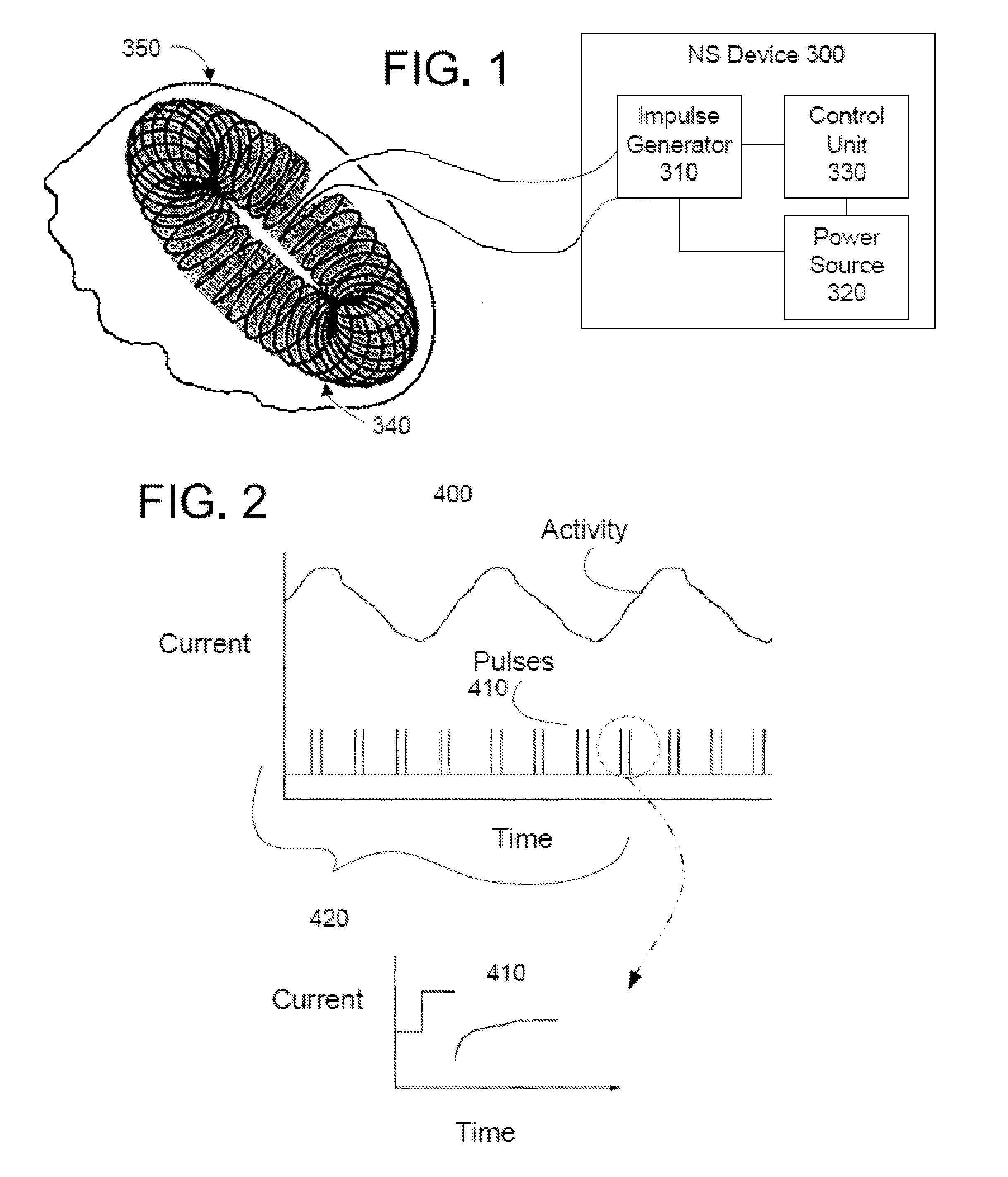
Non-invasive methods and devices for inducing euphoria in a patient and their therapeutic application
ActiveUS20110190569A1Reduced likelihoodReduce severityElectrotherapySleep inducing/ending devicesObsessive compulsiveAlcohol
A novel non-invasive magnetic stimulator is used to modulate electrical activity of a patient's vagus nerve. Parameters of the stimulation are selected in such a way as to induce a state of euphoria in the patient. The methods and devices may be used for anesthesia, or to treat insomnia, depression, or premenstrual syndromes. They may be used as substitution withdrawal tools for individuals who otherwise would depend on substances and behaviors to achieve a euphoric state of mind, particularly individuals who abusively consume drugs, alcohol or food, or who exhibit behavioral disorders such as compulsive gambling. The devices and methods may also be used to prevent, manage, or relieve stress.
Owner:ELECTROCORE
Treatment of transient and short term insomnia
The invention is directed to a method for the treatment of a patient suffering from transient or short term insomnia. The claimed method comprises the administration of a compound selected from the group consisting of the pharmaceutically acceptable forms of doxepin, amitriptyline, trimipramine, trazodone and mixtures thereof in dosages ranging from about 0.5 to about 20.0 milligrams.
Owner:PROCOM ONE
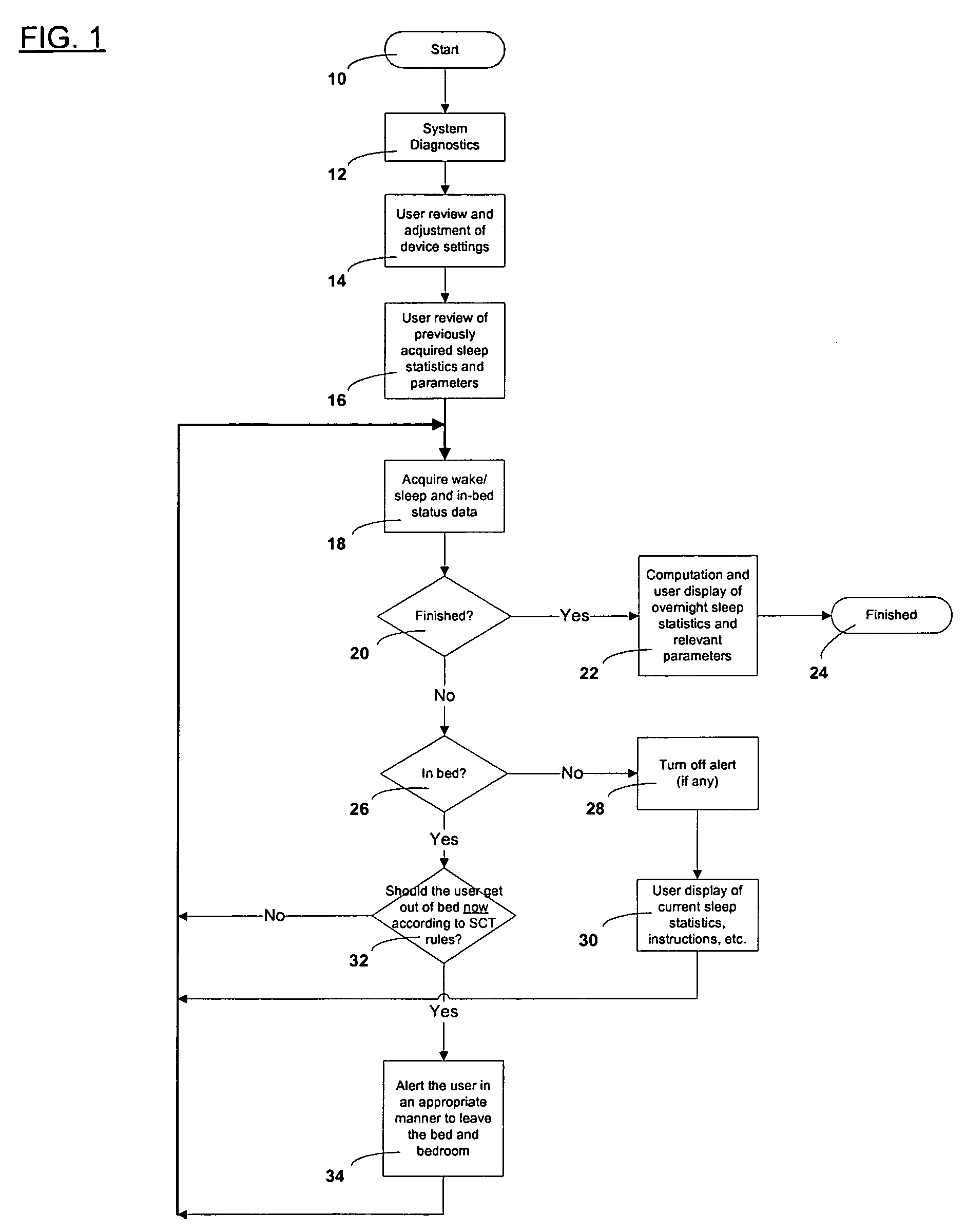
Automated treatment system for sleep
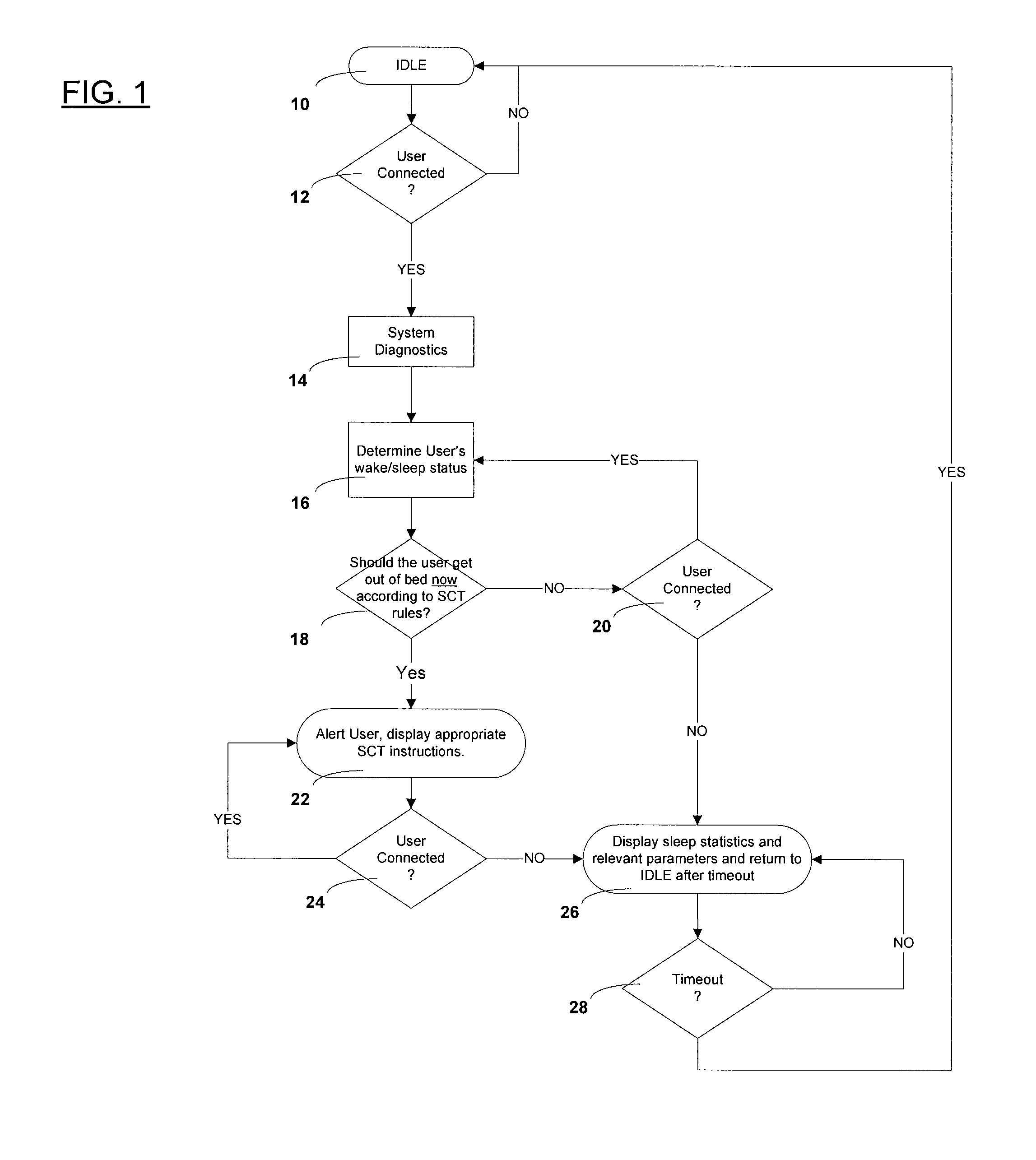
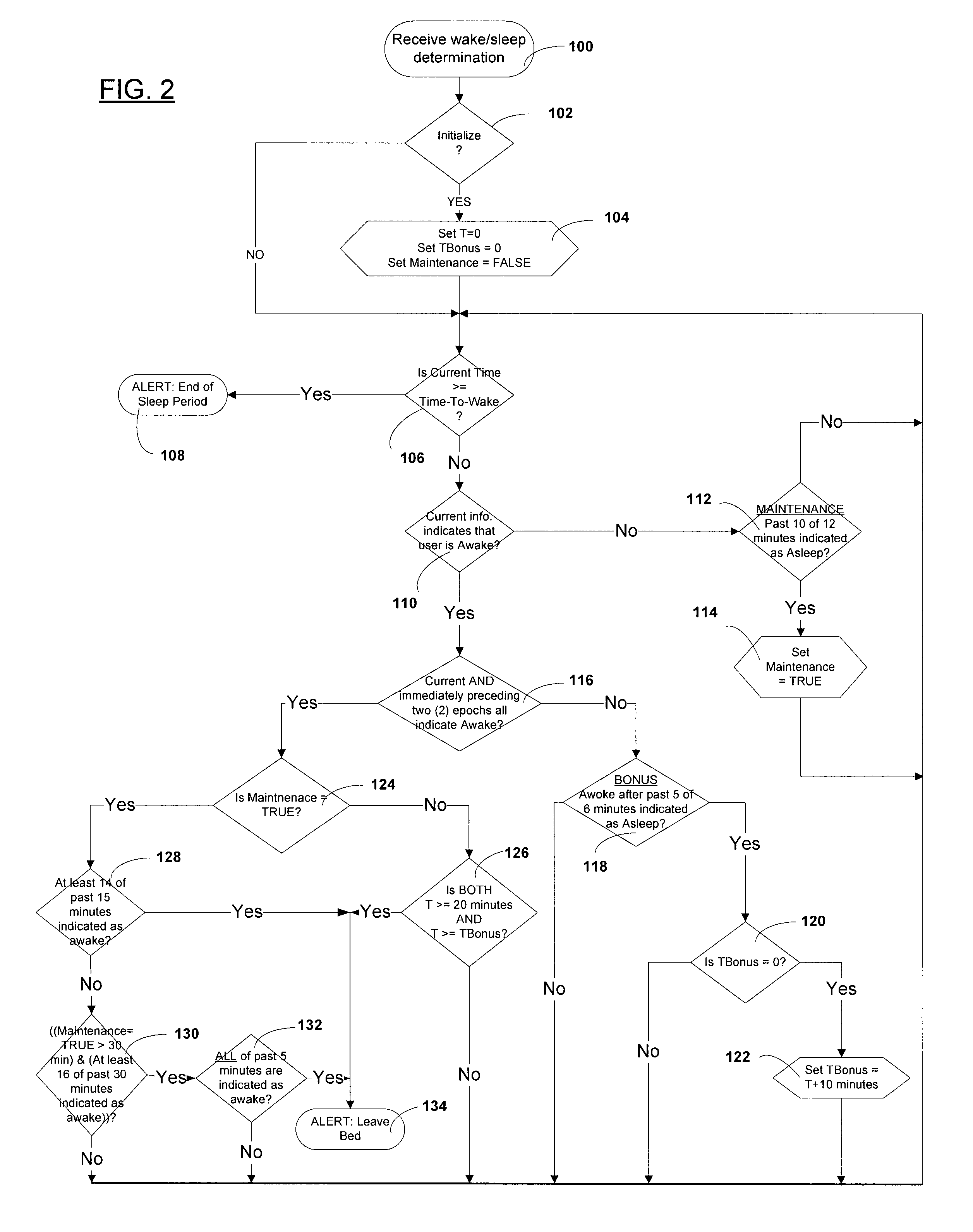
Automated behavioral methods and systems for treating Insomnia that use passive means for determining wake / sleep states.
Owner:CONSOL RES OF RICHMOND
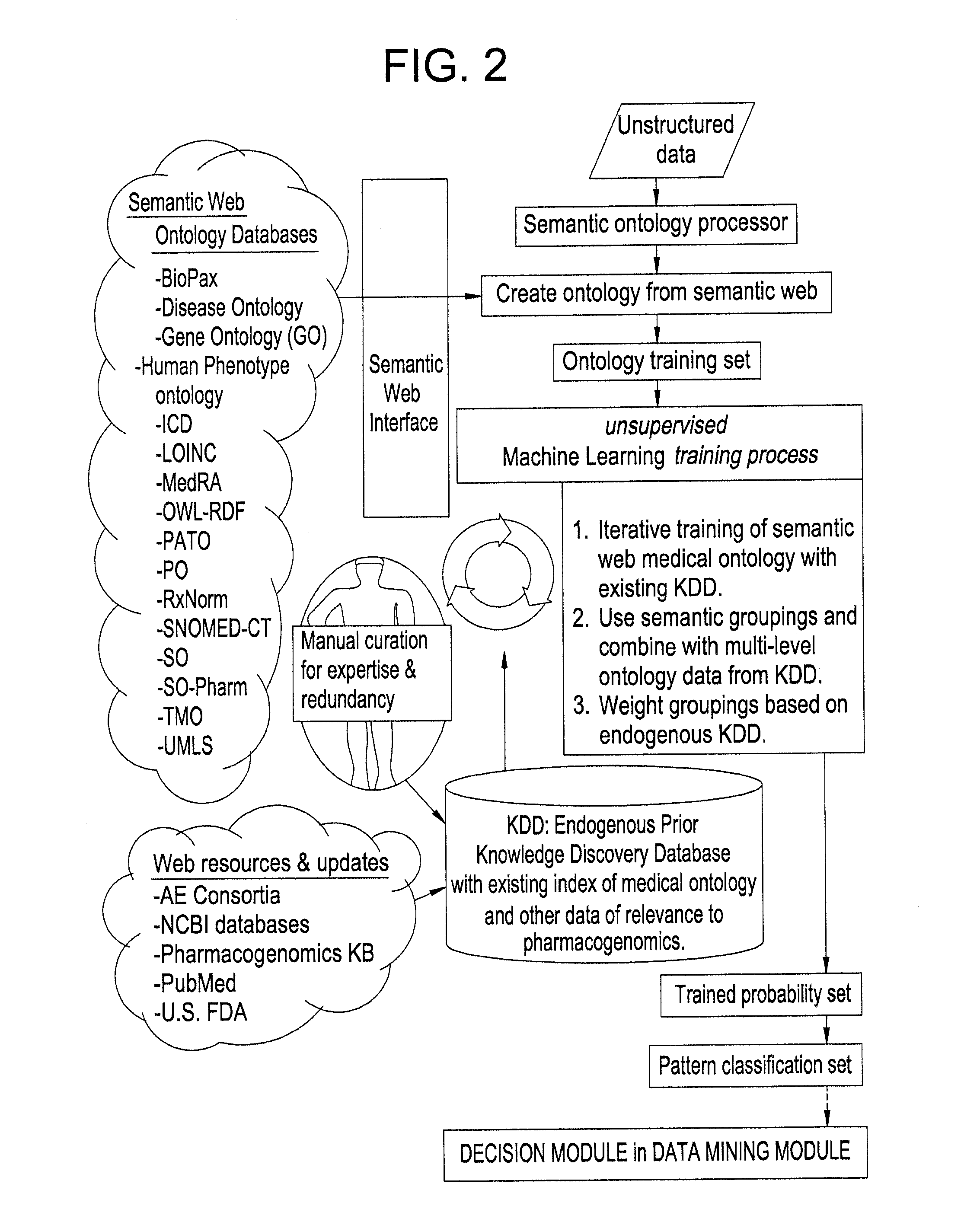
Systems and Methods for Pharmacogenomic Decision Support in Psychiatry
InactiveUS20140046696A1Medical simulationData processing applicationsClinical variablesDecision taking
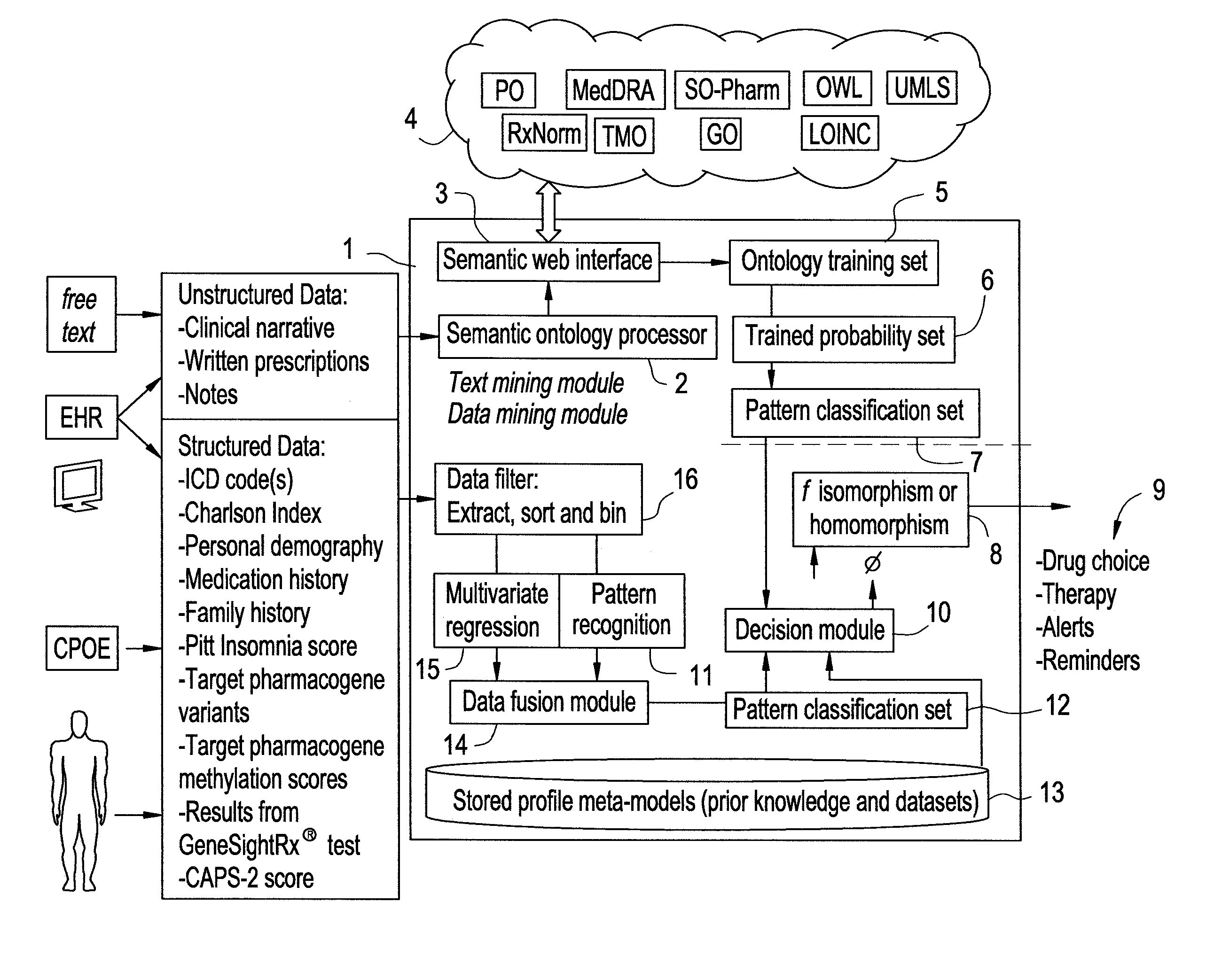
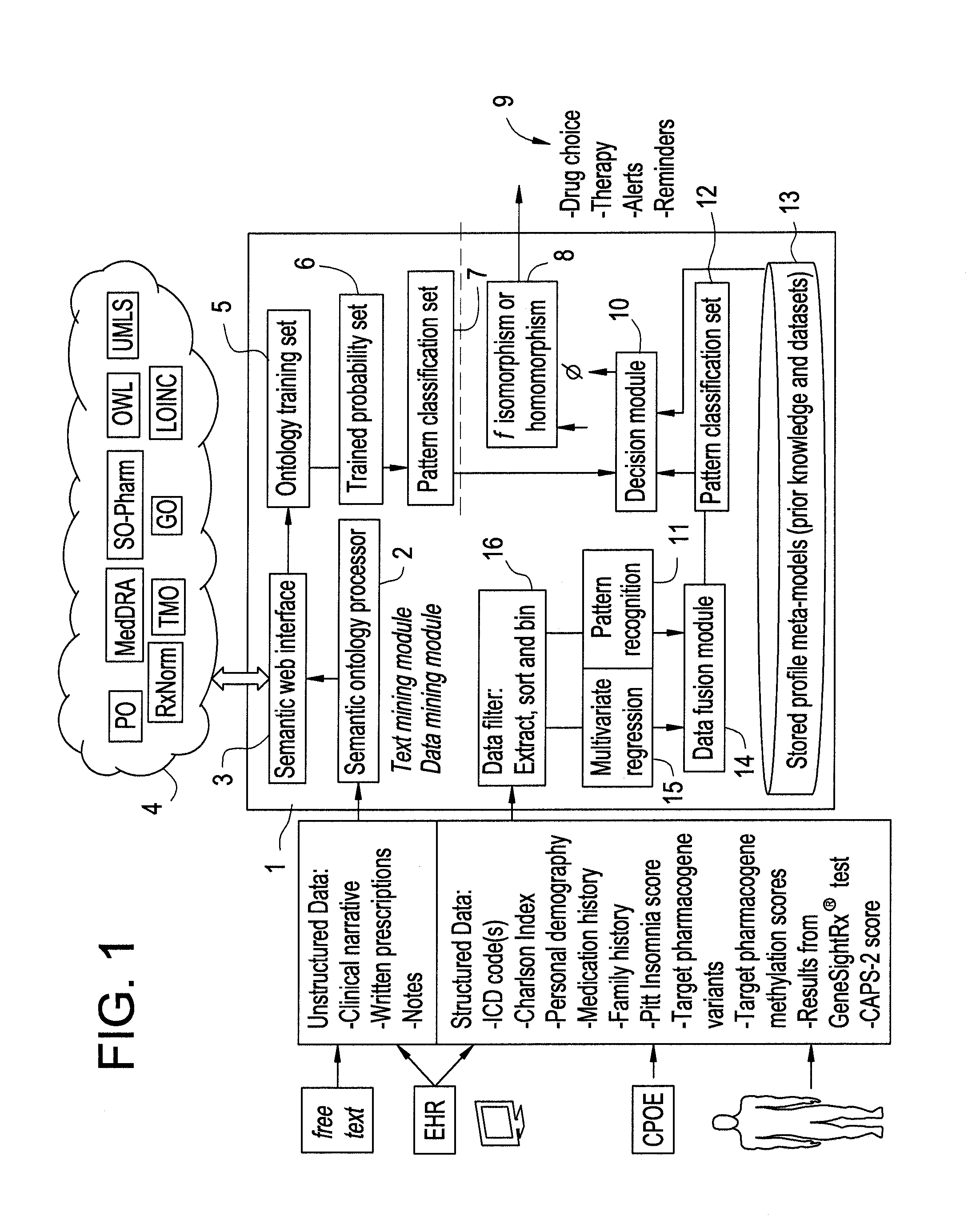
The present invention provides methods and systems or apparatuses, to analyze multiple molecular and clinical variables from an individual diagnosed with a psychiatric disorder, such as post-traumatic stress disorder (PTSD), in order to optimize medication selection for therapeutic response. Molecular co-variables include polymorphisms in genes including those involved in central control and mediation of the hypothalamic-pituitary axis (HPA) stress response, the density of methylation in regulatory regions of said polymorphic genes, polymorphisms in genes that encode cytochrome P450 enzymes responsible for drug metabolism, and drug-drug and drug-gene interactions. Clinical co-variables include but are not limited to the sex, age and ethnicity of that individual, medication history, family history, diagnostic codes, Pittsburgh insomnia rating score, and Charlson index score. The system makes a determination based on unstructured and structured data types derived from internal and external knowledge resources to determine psychotropic drug choice that best matches the molecular and clinical variation profile of an individual patient. The decision support system provides a therapeutic recommendation for a clinician based on the patient's variation profile.
Owner:ASSUREX HEALTH INC
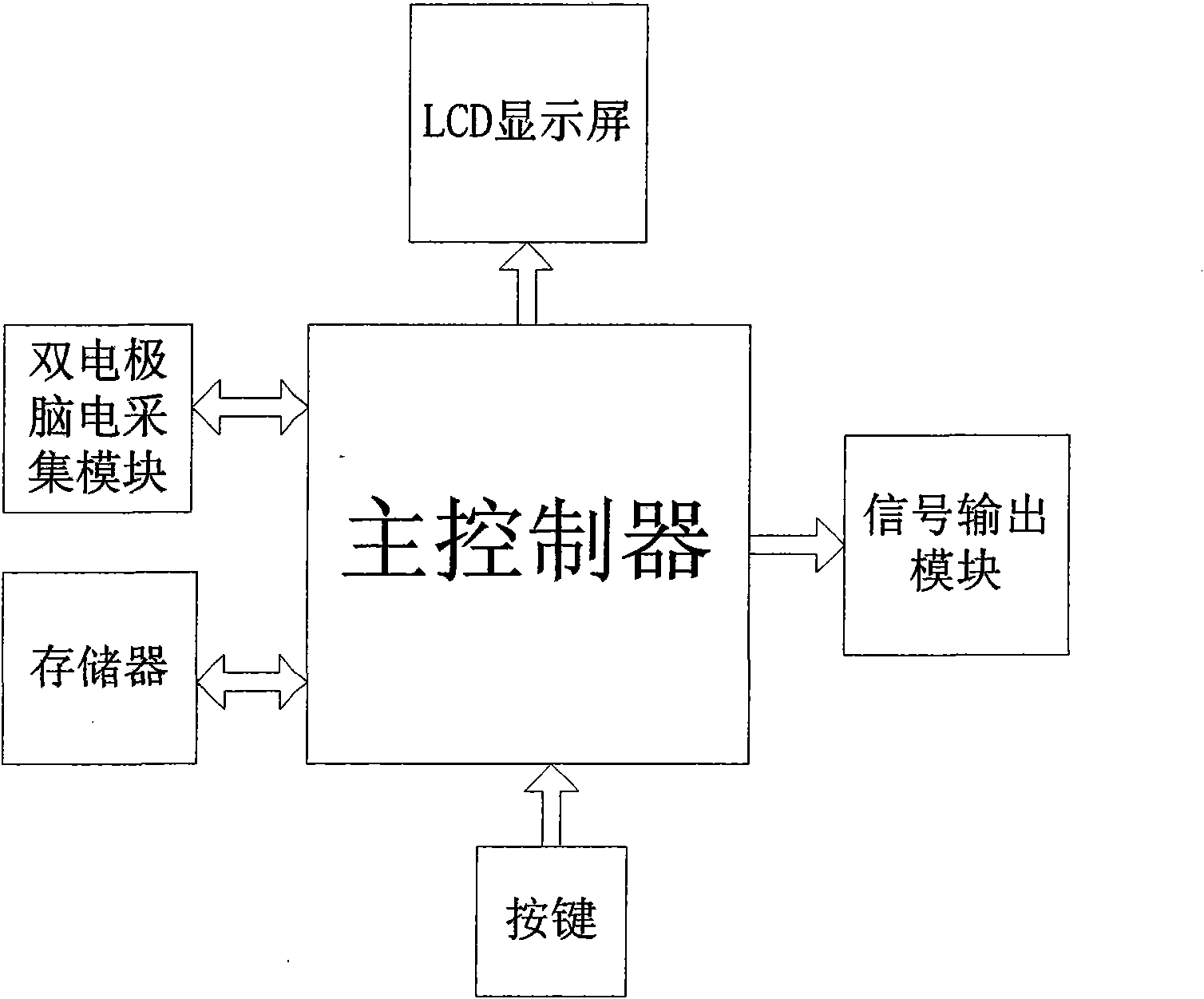
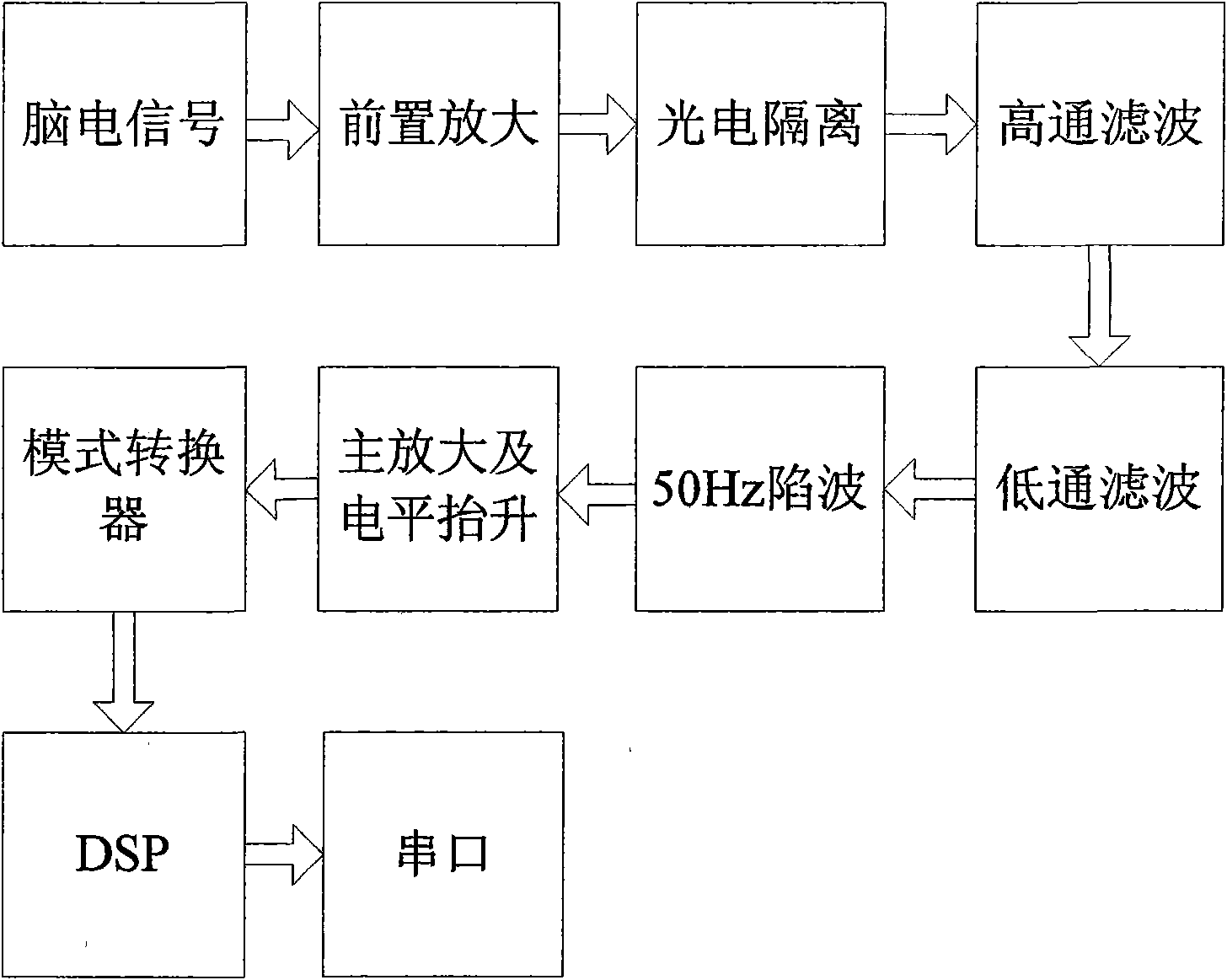
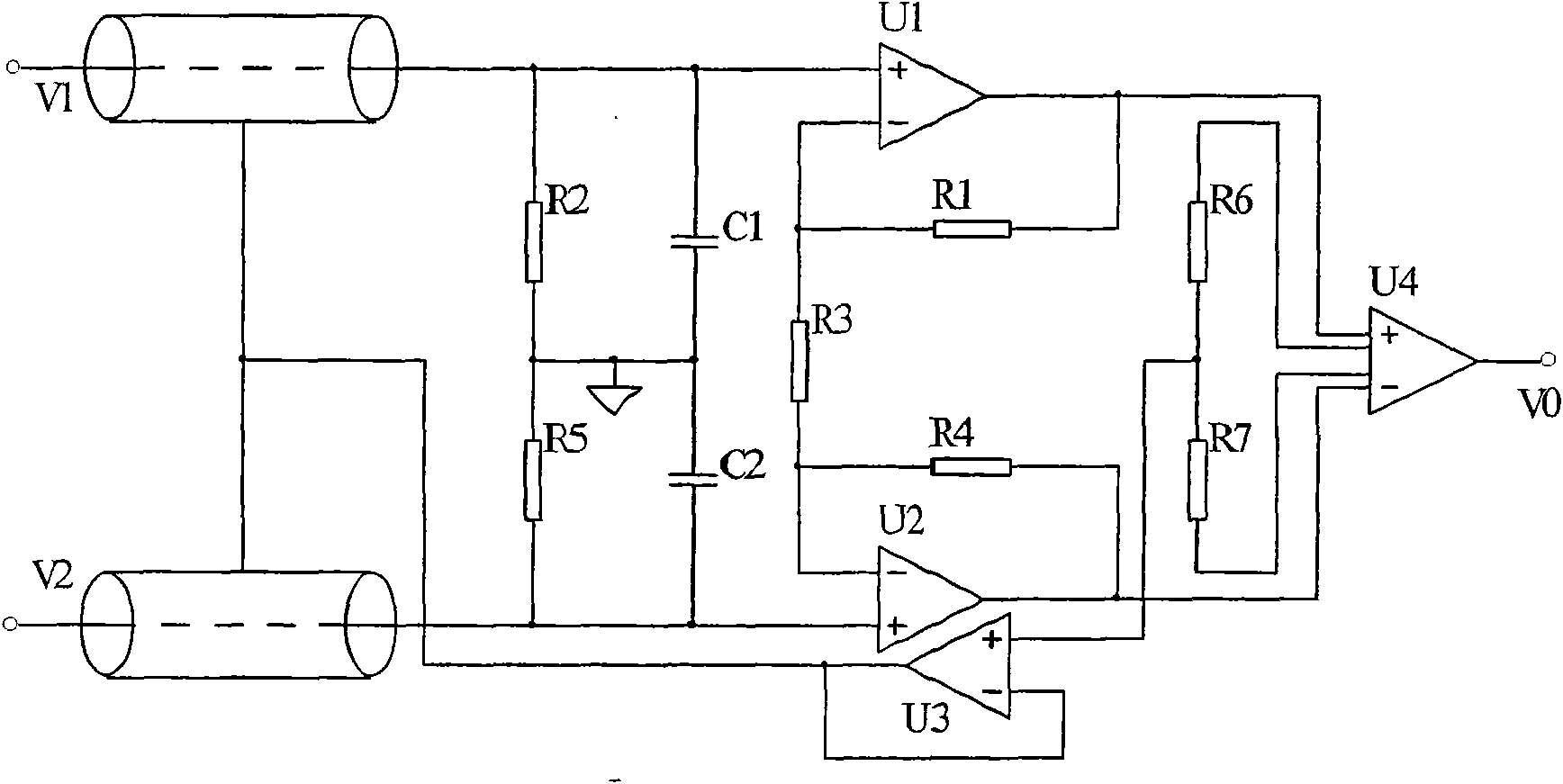
Intelligent insomnia therapeutic instrument
InactiveCN101559252AGood treatment effectElectrotherapyDiagnostic recording/measuringMedicineAnalog signal
The invention relates to an intelligent insomnia therapeutic instrument, aiming at providing a therapeutic instrument which can induce and regulate potential action of the sleep center of a patient so as to accelerate the falling asleep speed of the patient and improve the sleep quality or realize a natural timing awaking function. The technical proposal is as follows: the instrument comprises a signal output module generating time varying magnetic fields, a bipolar electrode brain wave acquiring module acquiring real-time brain waves of the patient and a main controller obtaining the brain waves and carrying out down-conversion or up-conversion, wherein the signal output module converts digital brain signals output by the main controller into analogue signals which are amplified so as to drive a magnetic field generator to generate time varying magnetic fields used for treating.
Owner:JIANGXI SHIMEILE BIOTECH DEV
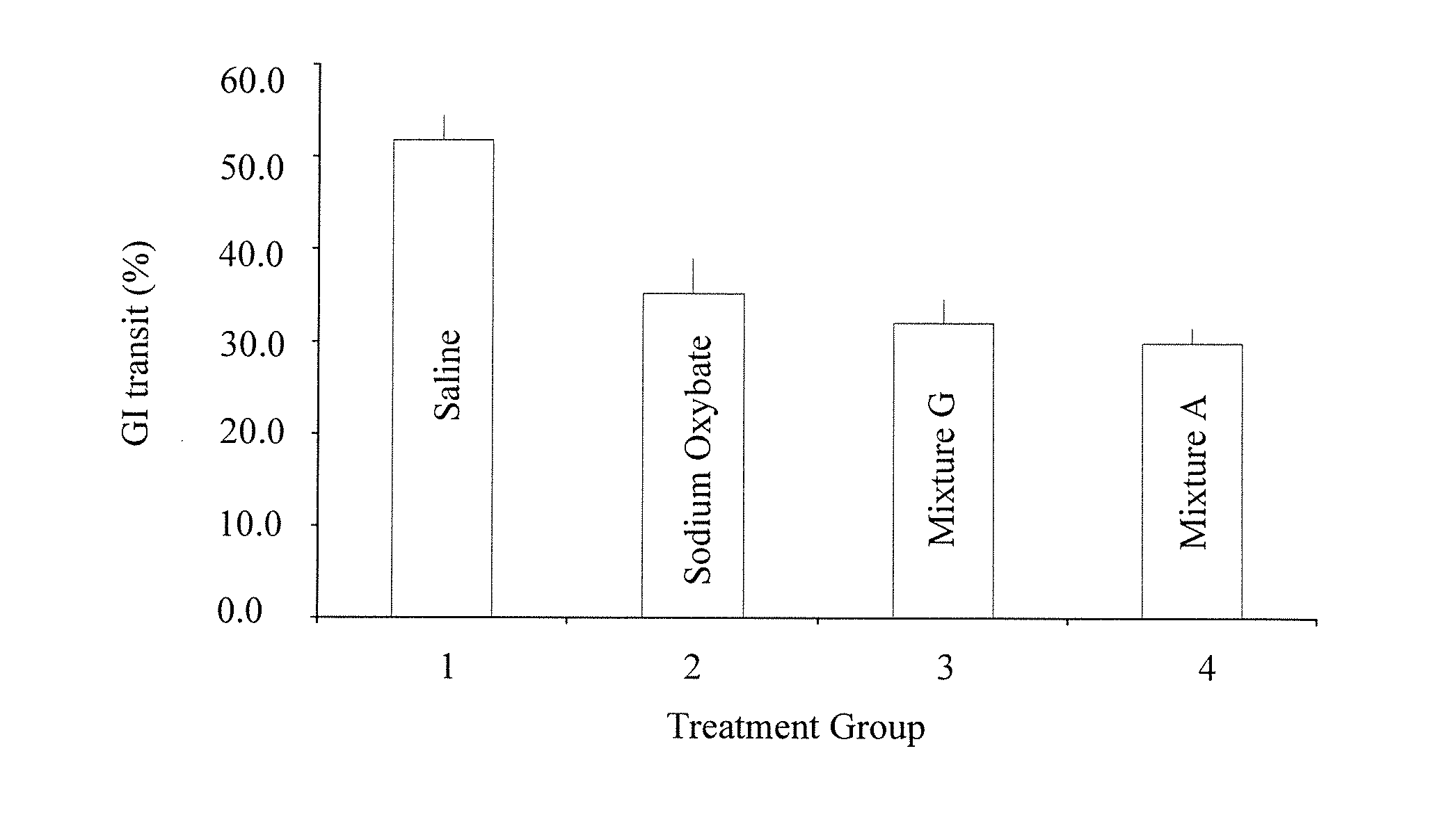
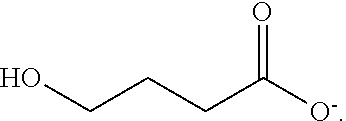
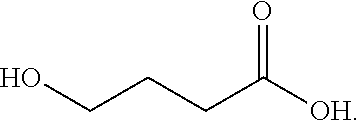
Gamma-hydroxybutyrate compositions and their use for the treatment of disorders
Provided herein are pharmaceutical compositions and formulations comprising mixed salts of gamma-hydroxybutyrate (GHB). Also provided herein are methods of making the pharmaceutical compositions and formulations, and methods of their use for the treatment of sleep disorders such as apnea, sleep time disturbances, narcolepsy, cataplexy, sleep paralysis, hypnagogic hallucination, sleep arousal, insomnia, and nocturnal myoclonus.
Owner:JAZZ PHARMA IRELAND LTD
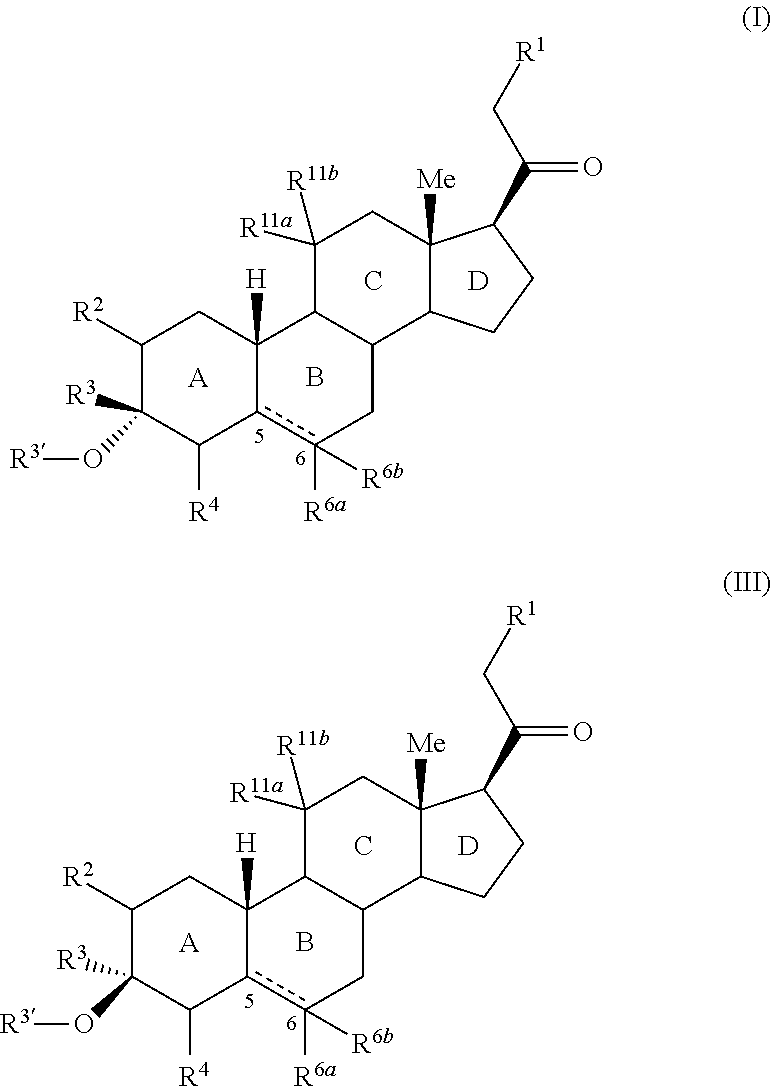
3,3 disubstituted 19-nor pregnane compounds, compositions, and uses thereof
InactiveUS20150291654A1Eliminate potentialInhibit metabolismSenses disorderNervous disorderInsomniaBrain traumas
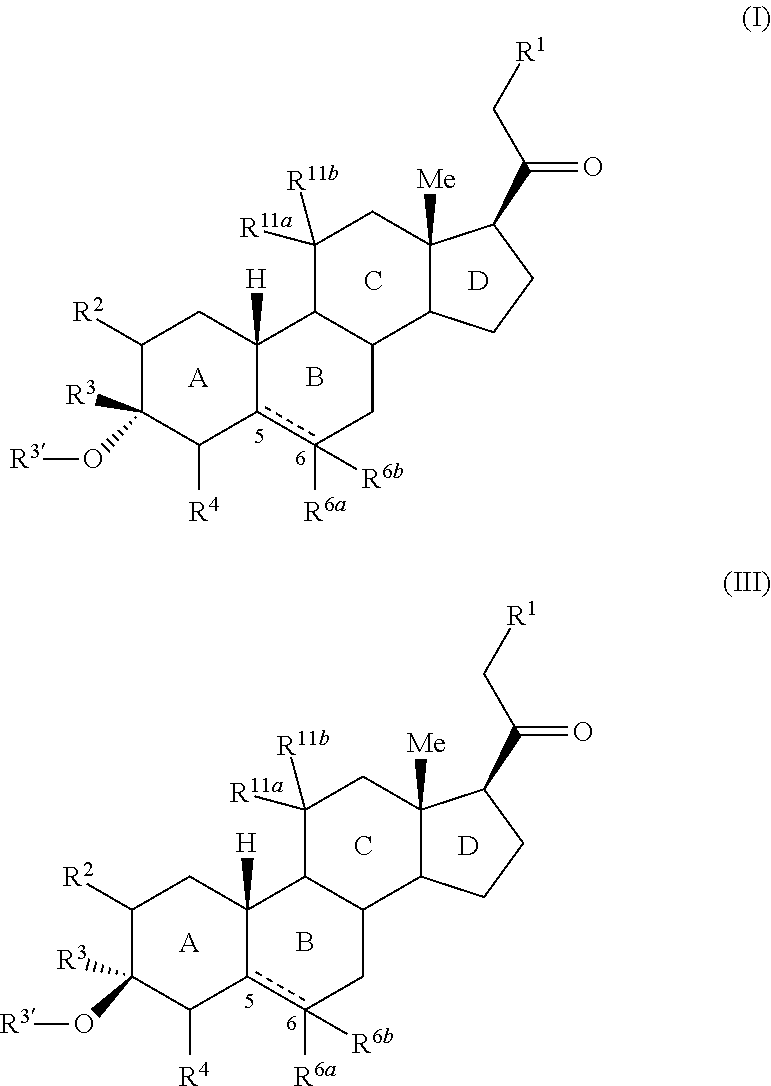
Provided herein are 3,3-disubstituted 19-nor-steroidal compounds according to Formula (I) and (III): where R1, R2, R3, R3′, R4, R6a, R6a, R11a, and R11b are as defined herein. Compounds of the present invention are contemplated useful for the prevention and treatment of a variety of CNS-related conditions, for example, treatment of sleep disorders, mood disorders, insomnia, anxiety, depression, traumatic brain injury (TBI), stress, and epilepsy.
Owner:SAGE THERAPEUTICS
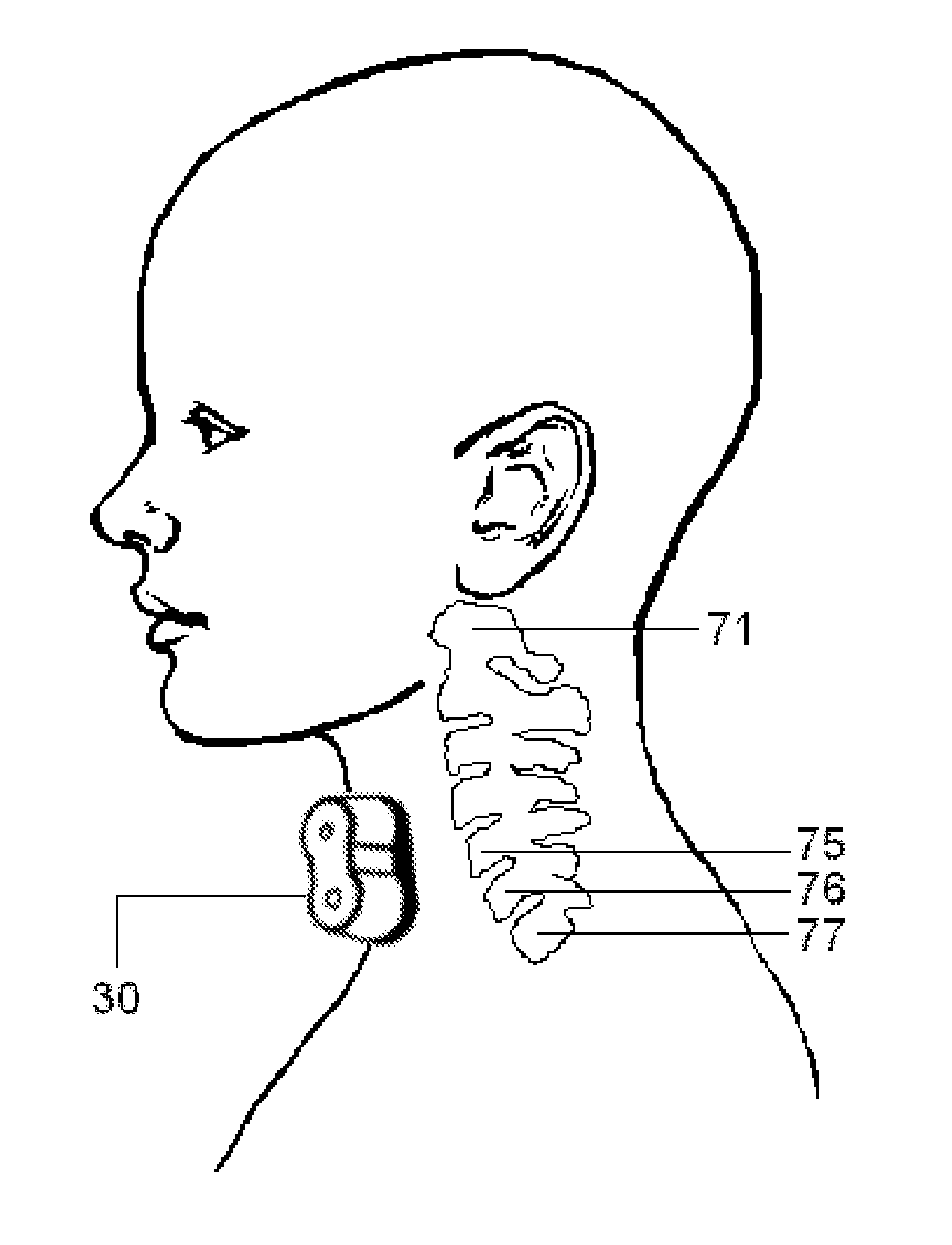
Methods, devices and systems for treating insomnia by inducing frontal cerebral hypothermia
ActiveUS20110125238A1Safe and comfortable to wearIncrease drowsinessAnaesthesiaSurgeryFrontal cortexNon invasive
Owner:UNIVERSITY OF PITTSBURGH
Methods and compositions for treating distress dysfunction and enhancing safety and efficacy of specific medications
InactiveUS20110159048A1Good treatment effectEliminate side effectsBiocideNervous disorderDiseaseNeurotransmitter systems
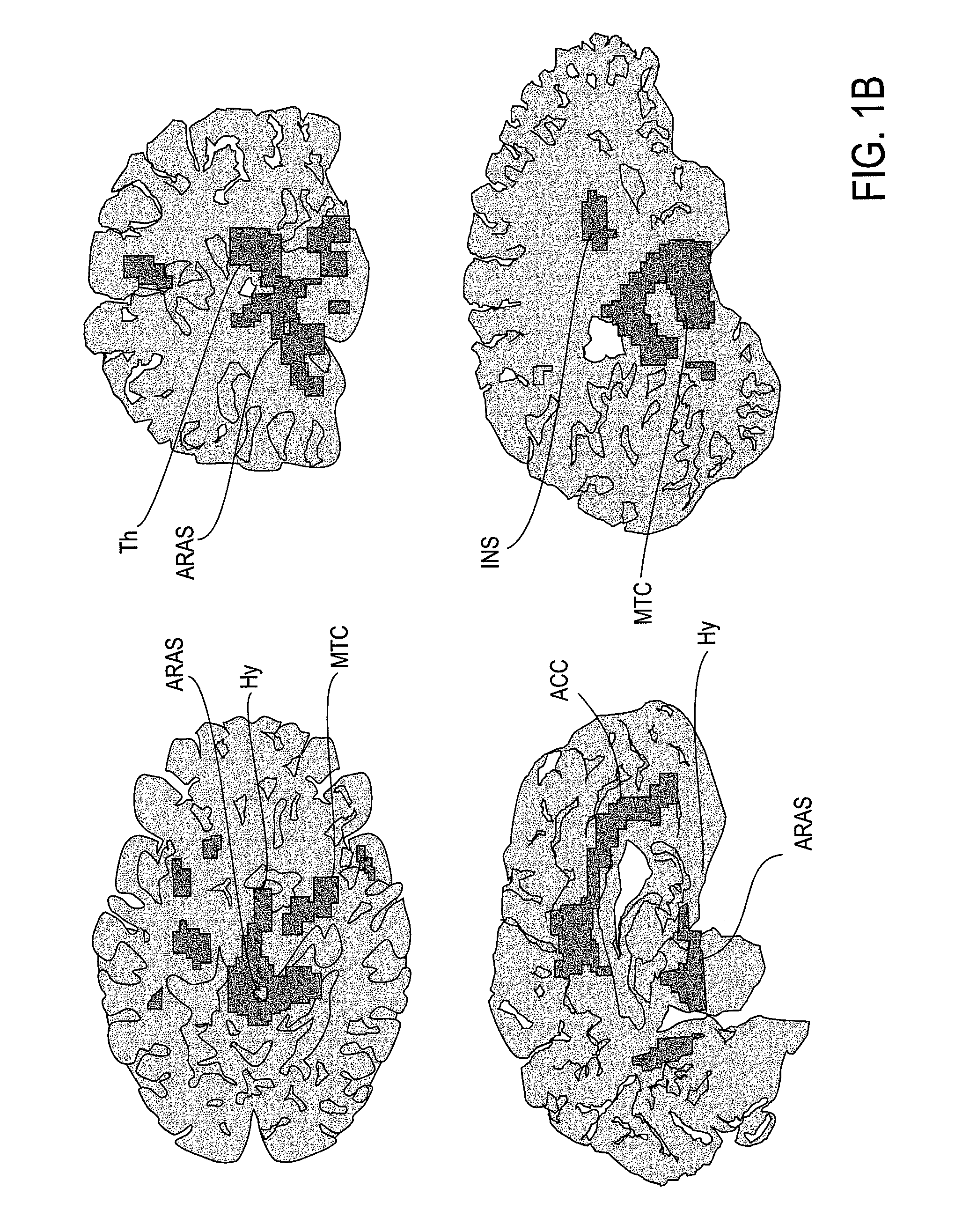
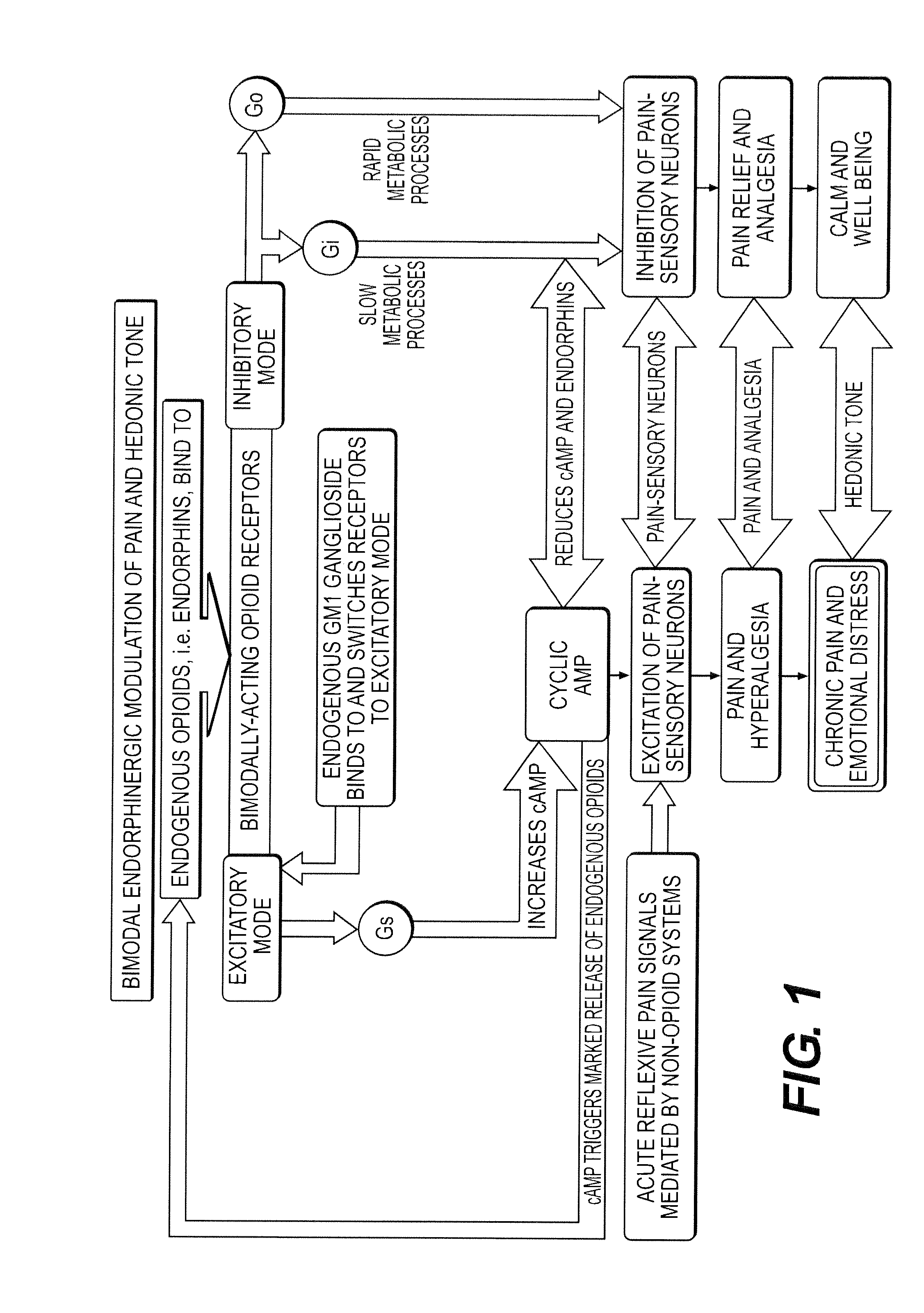
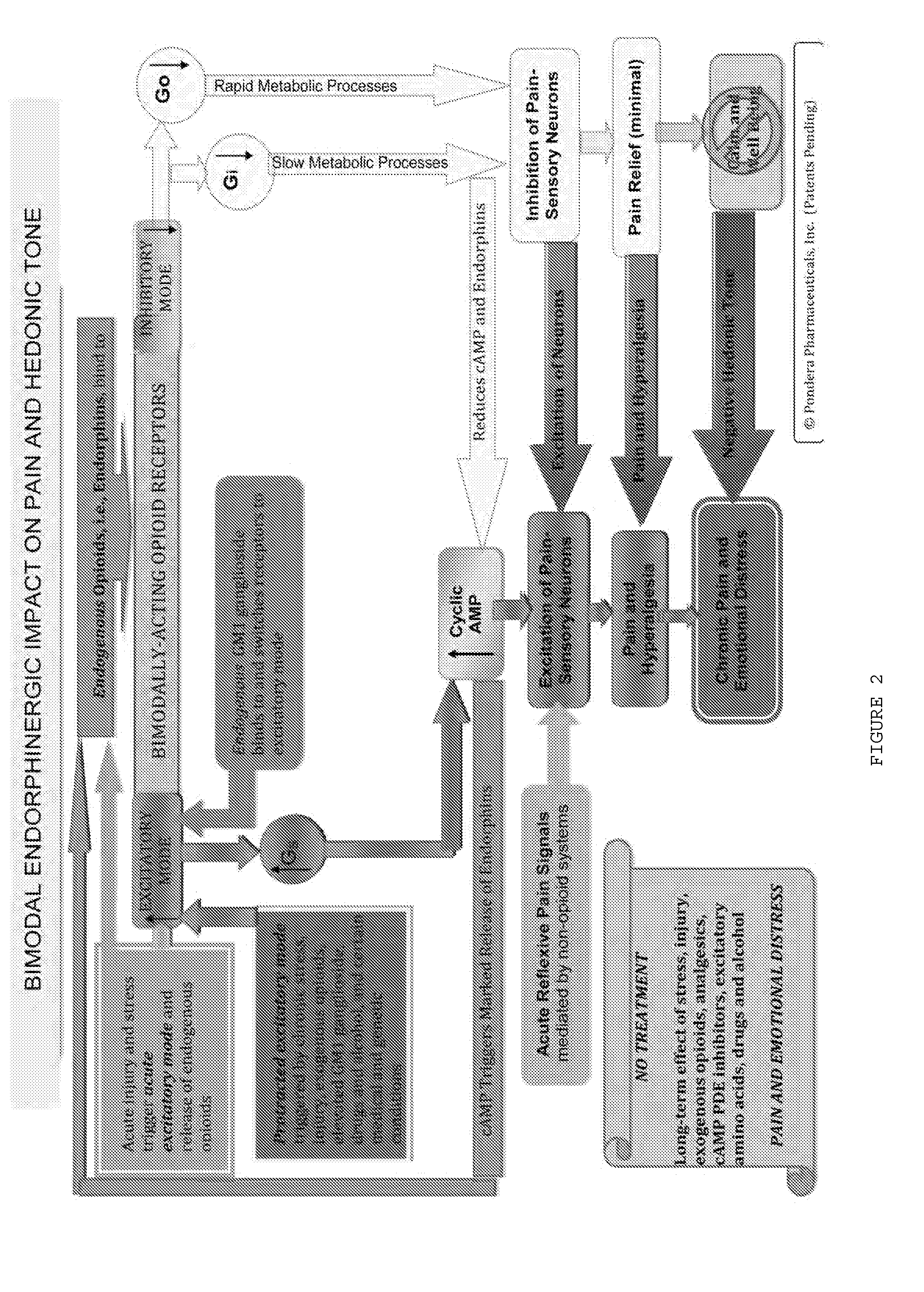
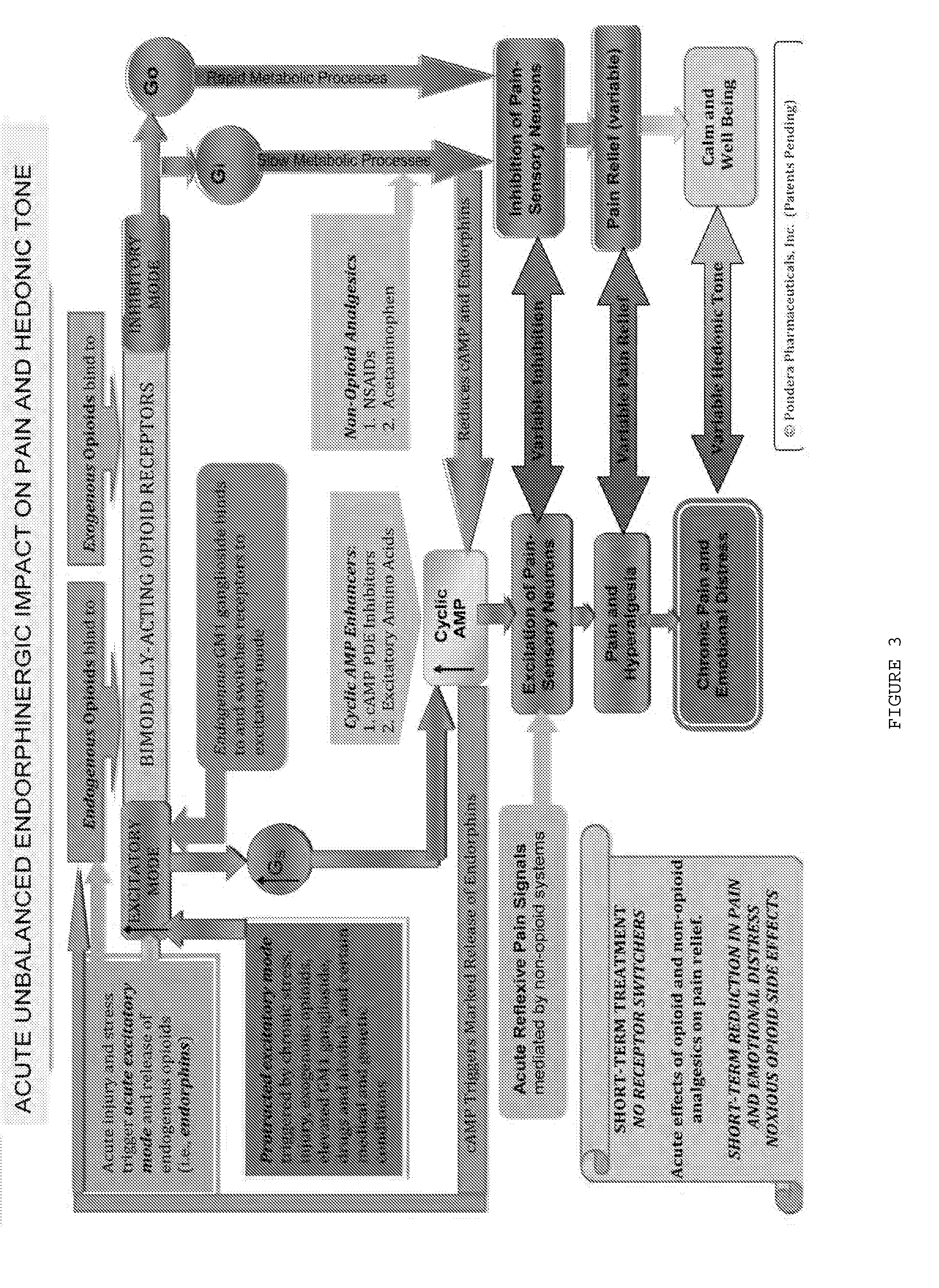
The present invention relates to methods and compositions for reducing Distress Dysfunction by restoring and maintaining homeostatic balance in the neurotransmitter systems underlying the Stress Response and the experience of distress and hedonic tone. Distress Dysfunction refers to the experience of dysfunctional emotional and physical distress that interferes with the individual's quality of life and functioning. A novel understanding of the bimodal opioid modulation of pain, and its impact, through serotonergic, dopaminergic, epinephrinergic, and norepinephrinergic processes, on hedonic tone, leads directly to new generation pharmaceutical formulations that are remarkably safe and effective for the treatment of a wide variety of Distress Dysfunctions, including anxiety, depression, anger, insomnia, mood disorders, eating disorders, sexual problems, pain, substance and behavioral addictions, gastrointestinal disorders, autistic spectrum disorders, attention-deficit and hyperactivity disorders, and other emotional and physical distress disorders. The foundation of this discovery is the power of Receptor Switchers, such as ultra-low-dose and very-low-dose opioid antagonists and GM1 ganglioside attenuators, in blocking acute and protracted excitatory opioid receptor signaling. Co-administration of Receptor Switchers with Endorphin Enhancers, such as specific cAMP PDE inhibitors and excitatory amino acids, is an excellent formulation for restoring healthy homeostatic balance to the endogenous opioid system, using the body's endorphins to reduce emotional and physical distress, and through synergistic and homeostatic processes, restoring positive hedonic tone. The addition of Synergistic Enhancers, such as amino acids, SSRI and SNRI agents, and non-opioid analgesics, as well as Exogenous Opioids, enhances and prolongs these therapeutic benefits. The novel principles discovered by this invention also teach a new generation of safe and effective formulations for the treatment of respiratory conditions, neuropathy, and nociceptive pain.
Owner:PONDERA BIOTECH
Scopolamine to Reduce or Eliminate Hot Flashes, Night Sweats, and Insomnia
InactiveUS20070010550A1Reduction in severity and frequencyAvoid problemsBiocideAnimal repellantsGynecologyHot flashes/flushes
Compositions and methods of treating or preventing hot flashes, night sweats, and / or insomnia in a subject that involve scopolamine are disclosed. For example, methods of treating hot flashes, night sweats, and / or insomnia in a subject that involve transdermal delivery of scopolamine are set forth herein.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
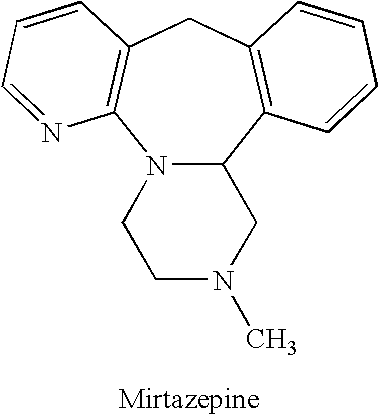
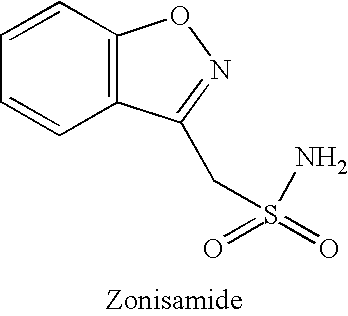
Methods for reducing the side effects associated with mirtzapine treatment
InactiveUS20060122127A1Eliminate side effectsBiocideCarbohydrate active ingredientsDiseaseNorepinephrine reuptake inhibitor
Compositions, and methods of use thereof, are provided for the prevention or treatment of side effects associated with the use of drugs that act as 5HT2 / 5HT3 serotonin receptor antagonists and alpha-2 adrenergic receptor antagonists (5HT2 / 5HT3 antagonist / alpha-2 antagonist). The method involves using dopamine-releasing compounds, such as amantadine, anticonvulsants, such as zonisamide, or dopamine / norepinephrine reuptake inhibitors, such as bupropion, in combination with 5HT2 / 5HT3 antagonist / alpha-2 antagonists, such as mirtazapine, to reduce the excessive daytime drowsiness and / or weight gain associated with 5HT2 / 5HT3 antagonist / alpha-2 antagonist use for the treatment of disorders, such as, depression, schizophrenia, anxiety disorders, sleep-related breathing disorders, insomnia, migraine headache, chronic tension-type headache, hot flashes, lower back pain, neuropathic pain and functional somatic syndromes. Formulations of dopamine-releasing compounds or anticonvulsants with 5HT2 / 5HT3 antagonist / alpha-2 antagonists are provided. In particular embodiments, combination therapy with mirtazapine and zonisamide provides relief from chronic low back pain, while reducing or avoiding side effects associated with monotherapy with mirtazapine or zonisamide.
Owner:CYPRESS BIOSCI
Automated insomnia treatment system
Automated behavioral methods and systems for treating insomnia that use passive means for determining wake / sleep states.
Owner:CONSOL RES OF RICHMOND
Traditional Chinese medicine composition for treating insomnia and preparation method thereof
InactiveCN101757568AImprove self-coordinationEffective treatmentHeavy metal active ingredientsNervous disorderAsparagus cochinchinensisClinical efficacy
The invention discloses a new traditional Chinese medicine composition for treating insomnia and a preparation method thereof. The traditional Chinese medicine composition mainly comprises the following raw Chinese medicinal herbal materials: cinnabar, nacre mother of pearl, cortex albiziae, semen boitae, tuber fleeceflower stem, polygala root, amber, fossil fragments, spina date seed, glossy ganoderma, shell of abalone, oyster, gastrodia elata, root of straight ladybell, ophiopogon japonicus, lucid asparagus, fruit of Chinese wolfberry, fushen, motherwort, Chinese angelica, radix paeoniae alba, cortex lycii radicis, agilawood, root of three-nerved spicebush, rhizoma cyperi and the like. The traditional Chinese medicine composition can be prepared into any one common oral preparation according to a conventional traditional Chinese medicine preparation method. The invention can remarkably improve symptoms of insomnia, difficulty falling asleep, easy awakening, difficulty falling asleep after awakening, alternation of falling asleep and awakening, difficulty falling asleep all through the night, doldrums, slow response, fatigue, even vexation and the like, and has the advantages of accurate clinical treatment effect, remarkable treatment effect, low cost, basically no toxic or side effects, and the like.
Herbal composition for treatment of insomnia and other related disorders and a method of preparing the same
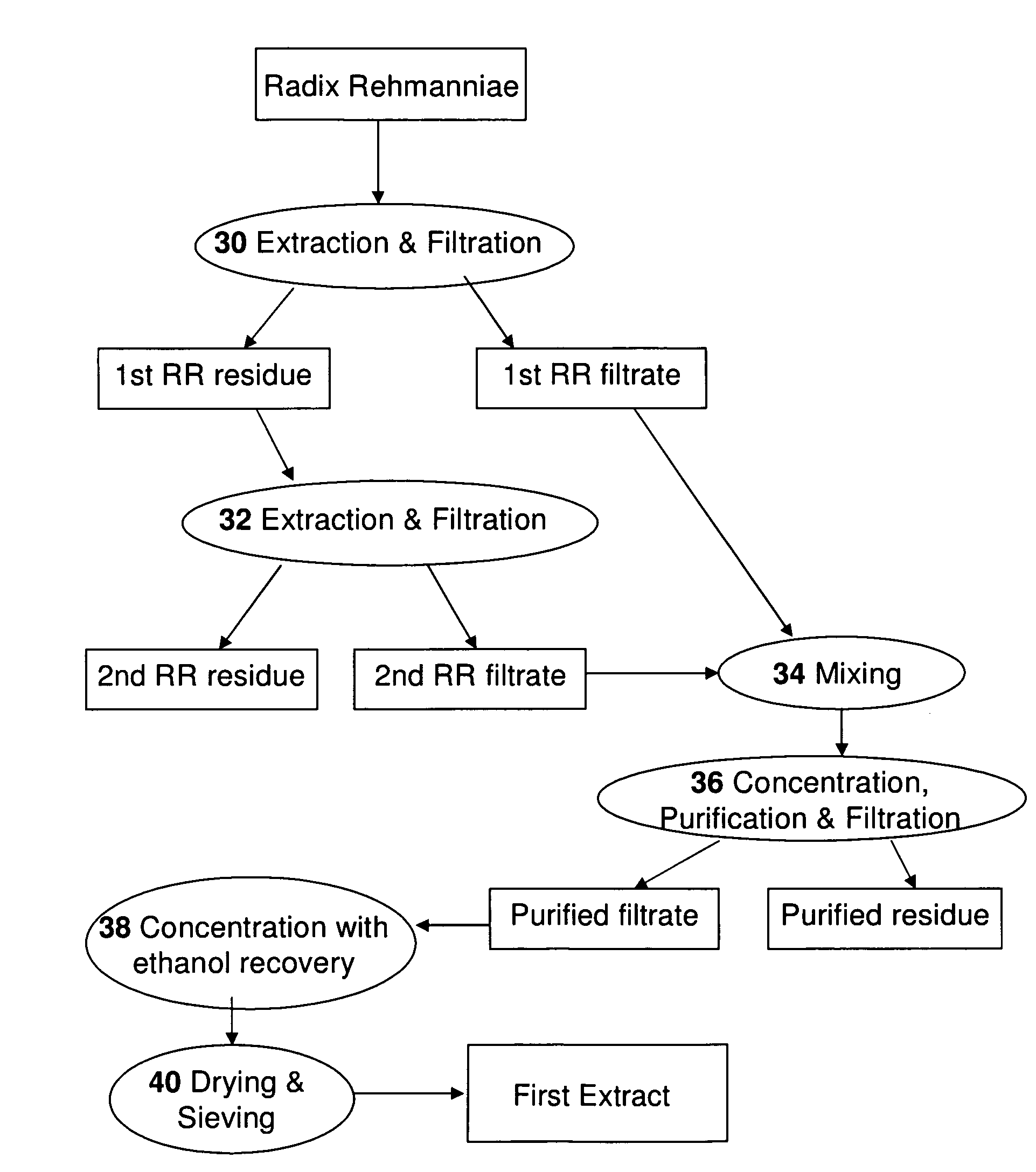
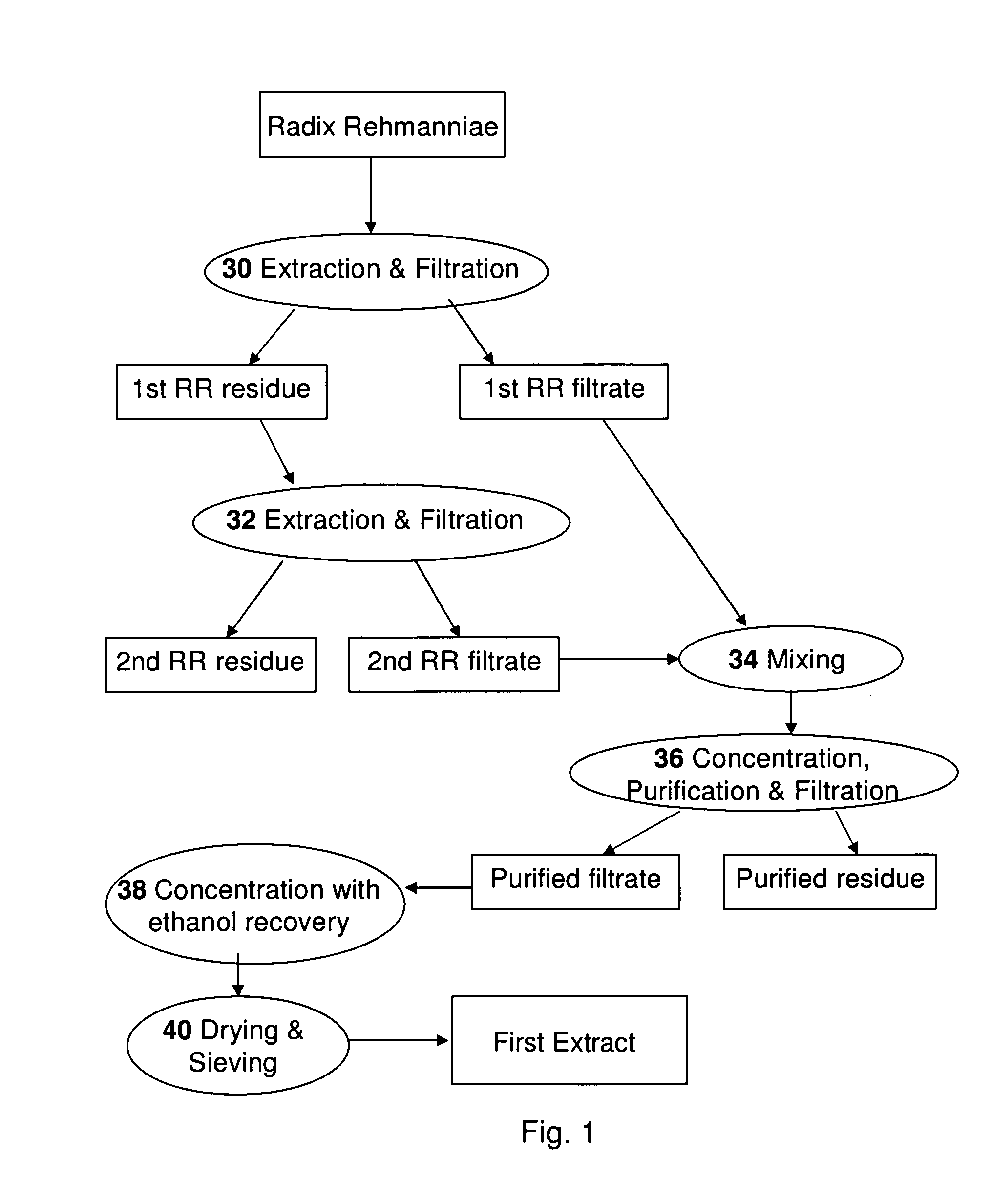
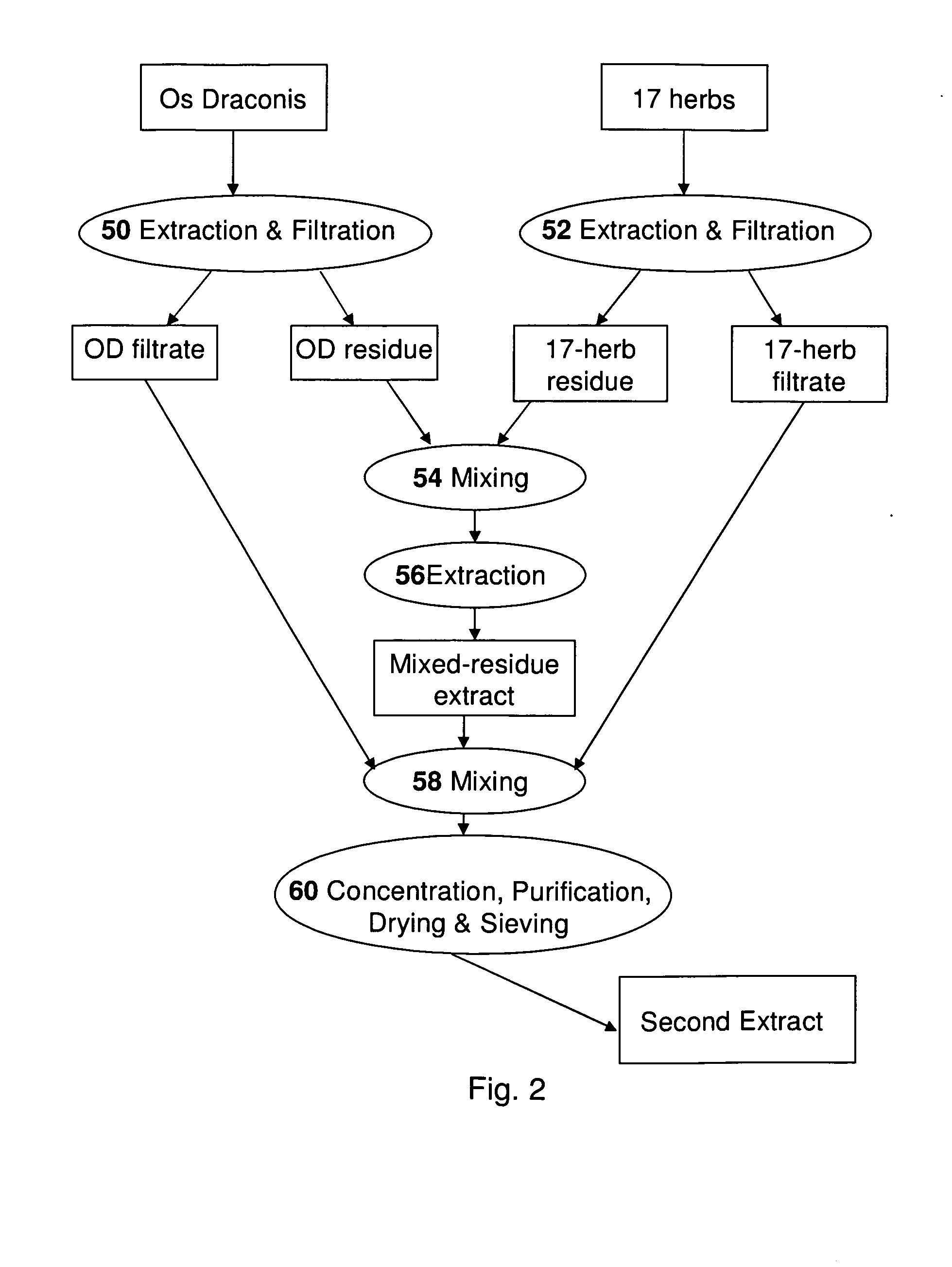
An herbal composition comprises an extract of Radix Rehmanniae, and an extract of Poria, Ganoderma Lucidum, Semen Ziziphi Spinosae, Semen Platycladi, Radix Rehmanniae Praeparata, Radix Angelicae Sinensis, Radix Et Rhizoma Salviae Miltiorrhizae, Rhizoma Chuanxiong, Rhizoma Atractylodis Macrocephalae, Fructus Gardeniae, Cortex Moutan, Radix Bupleuri, Radix Paeoniae Alba, Rhizoma Cyperi, Pericarpium Citri Reticulatae, Fructus Aurantii, Radix Glycyrrhizae, and Os Draconis. The herbal composition is prepared by separately extracting Radix Rehmanniae, and the remaining herbs. The composition is suitable for treatment of insomnia and other related disorders.
Owner:VITA GREEN HEALTH PRODS
Non-invasive methods and devices for inducing euphoria in a patient and their therapeutic application
ActiveUS9089719B2Reducing likelihood and severityGood conditionElectrotherapySleep inducing/ending devicesObsessive compulsiveAlcohol
Owner:ELECTROCORE
Pulsatile release compositions of milnacipran
InactiveUS20060003004A1Minimize exposureReduces milnacipran gastrointestinal side effectCapsule deliveryCoatingsPalpitationsPanic
A once-a-day oral milnacipran pulsatile release composition has been developed that releases the drug in spaced apart “pulses”. The dosage forms are comprised of first, second and optional third dosage units, with each dosage unit having a different drug release profile. This dosage form provides in vivo drug plasma levels characterized by Cmax below 3000 ng / ml, preferably below 2000 ng / ml, and most preferably below 1000 ng / ml. The composition provides pulsatile release of milnacipran to produce a therapeutic effect over approximately 24 hours, when administered to a patient in need, resulting in diminished incidence or decreased intensity of common milnacipran side effects such as sleep disturbance, nausea, vomiting, headache, tremulousness, anxiety, panic attacks, palpitations, urinary retention, orthostatic hypotension, diaphoresis, chest pain, rash, weight gain, back pain, constipation, vertigo, increased sweating, agitation, hot flushes, tremors, fatigue, somnolence, dyspepsia, dysoria, nervousness, dry mouth, abdominal pain, irritability, and insomnia.
Owner:COLLEGIUM PHARMA INC
Woman's shoe
A woman's shoe includes an insole surface layer, a sole under the insole surface layer and at least a thermoplastic rubber member. The sole is cut with at least a recess, for fitting the thermoplastic rubber member therein at a spot corresponding to a portion of a foot supporting a person's weight while walking or standing. Accordingly, the thermoplastic rubber member can absorb an impact force or a pressure as high as one third to two thirds of a body's weight while wearing such high-heel shoes. Moreover, it can soothe sourness of one's shoulders, soften the cervical vertebra and prevent insomnia, achieving a comfortable and healthy effect.
Owner:CHAN WEN CHIEH
Chinese medicament preparation for treating depression and anxiety as well as preparation method thereof
The invention discloses a traditional Chinese medicine preparation for treating depressive disorder and anxiety disorder and a preparation method thereof. The preparation comprises the following components in portion by weight: 10 to 30 portions of prepared rhizome of rehmannia, 10 to 30 portions of Flos Albiziae, 10 to 30 portions of shelled cedar seed, 10 to 30 portions of rhizoma cyperi, 10 to 30 portions of bupleurum, 10 to 30 portions of Chinese yam, 10 to 30 portions of prepared fleece-flower root, 10 to 30 portions of gynostemma pentaphylla, 10 to 30 portions of gardenia, 10 to 30 portions of radix codonopsitis, 10 to 30 portions of angelica, 10 to 30 portions of unprocessed oyster, 10 to 30 portions of hoantchy root, 10 to 30 portions of schisandra, 10 to 30 portions of epimedium, 10 to 30 portions of spina date seed, 10 to 30 portions of milkwort, 10 to 30 portions of phellodendron amurense, 10 to 30 portions of anoectochilus formosanus, and 10 to 30 portions of uncaria. The traditional Chinese medicine preparation utilizes the efficacy of each drug to generate synergic action, and has the efficacies of relieving restlessness and soothing the nerves, nourishing the mind and replenishing the deficiency, soothing the nerves and benefitting the intelligence, reducing phlegm and dredging acupuncture points, nourishing the mind and protecting the liver, promoting Qi circulation and removing obstruction in the collateral, and relieving melancholy, anxiety and insomnia so as to effectively treat the depressive disorder and the anxiety disorder.
Owner:杨颖
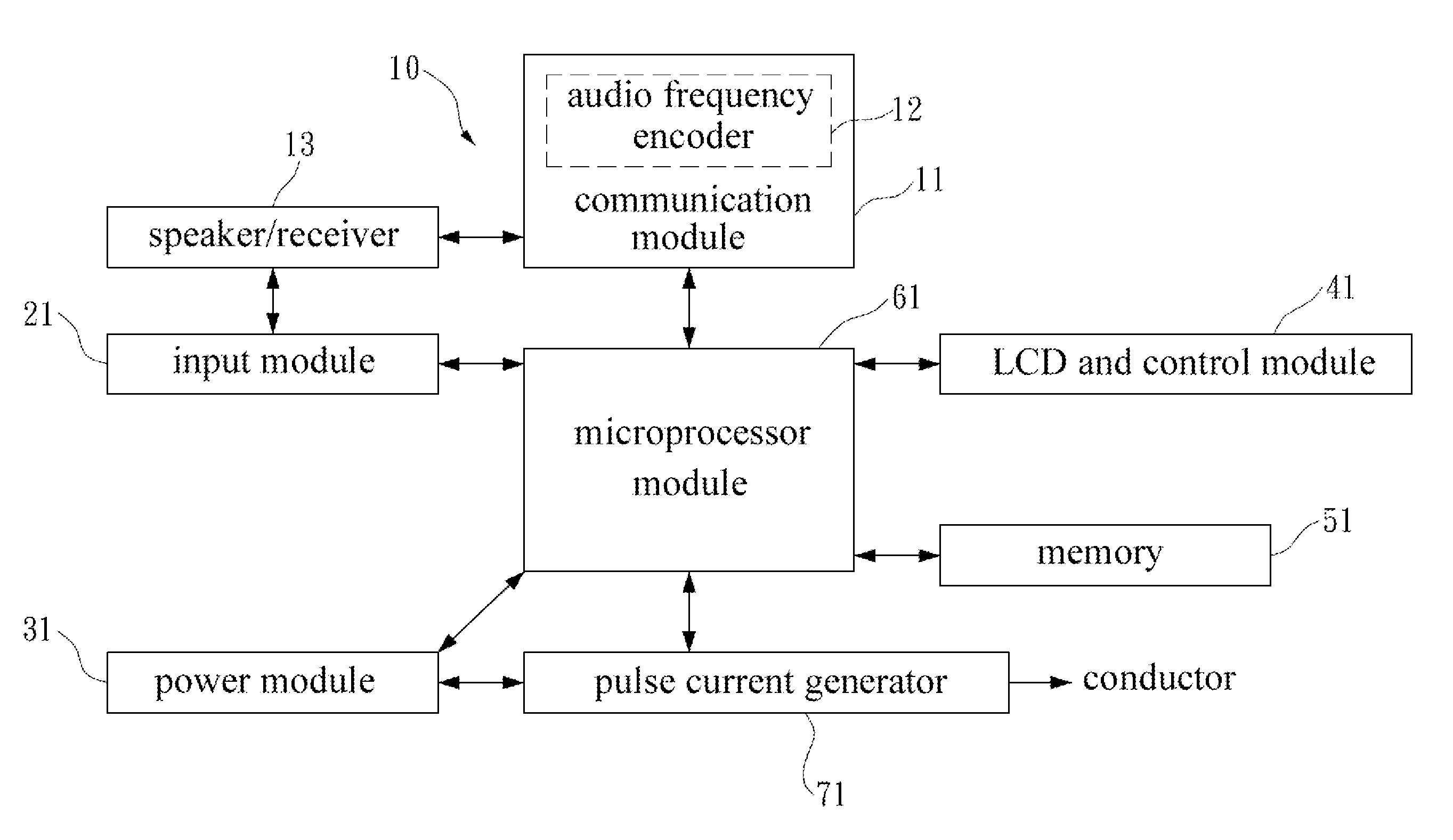
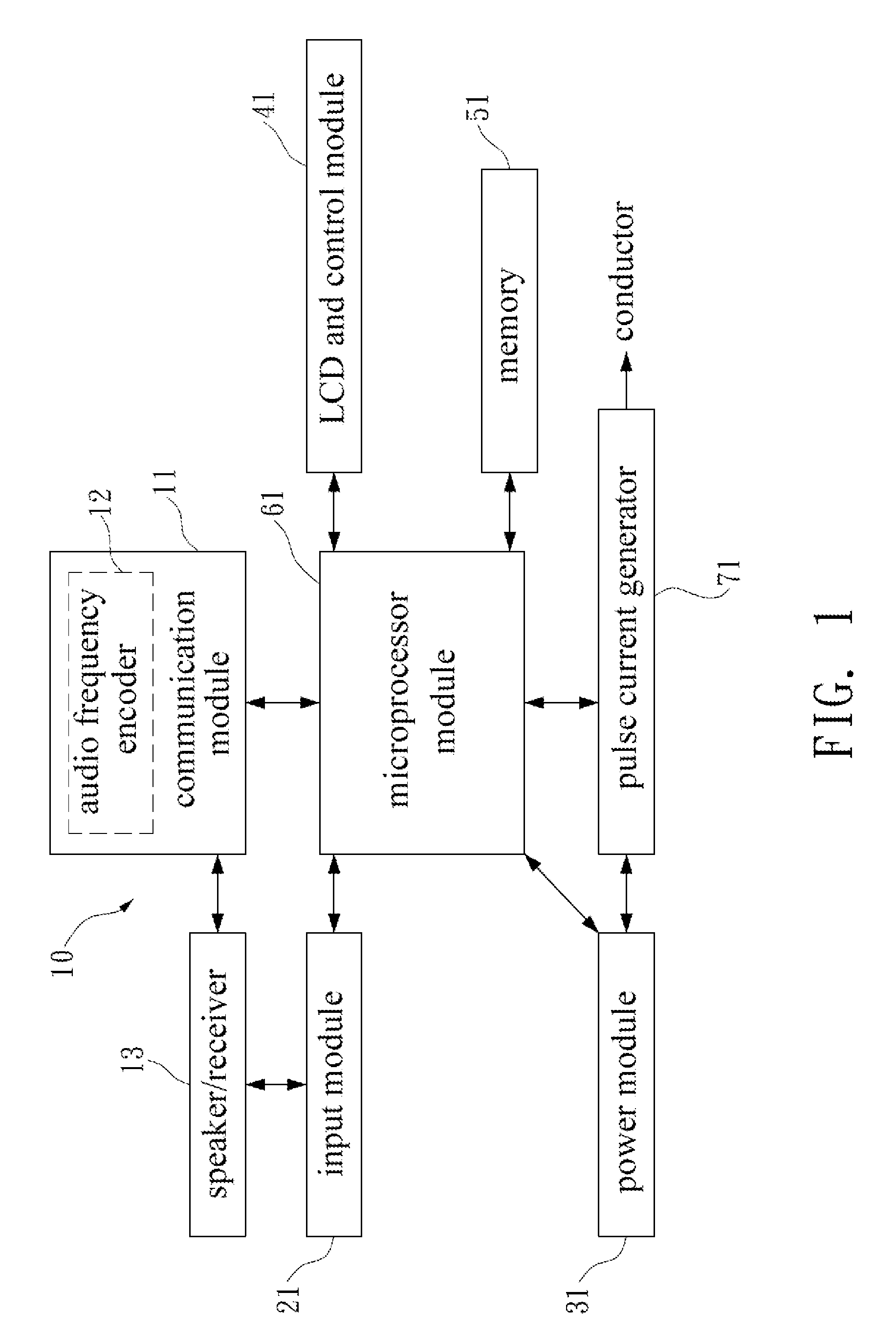
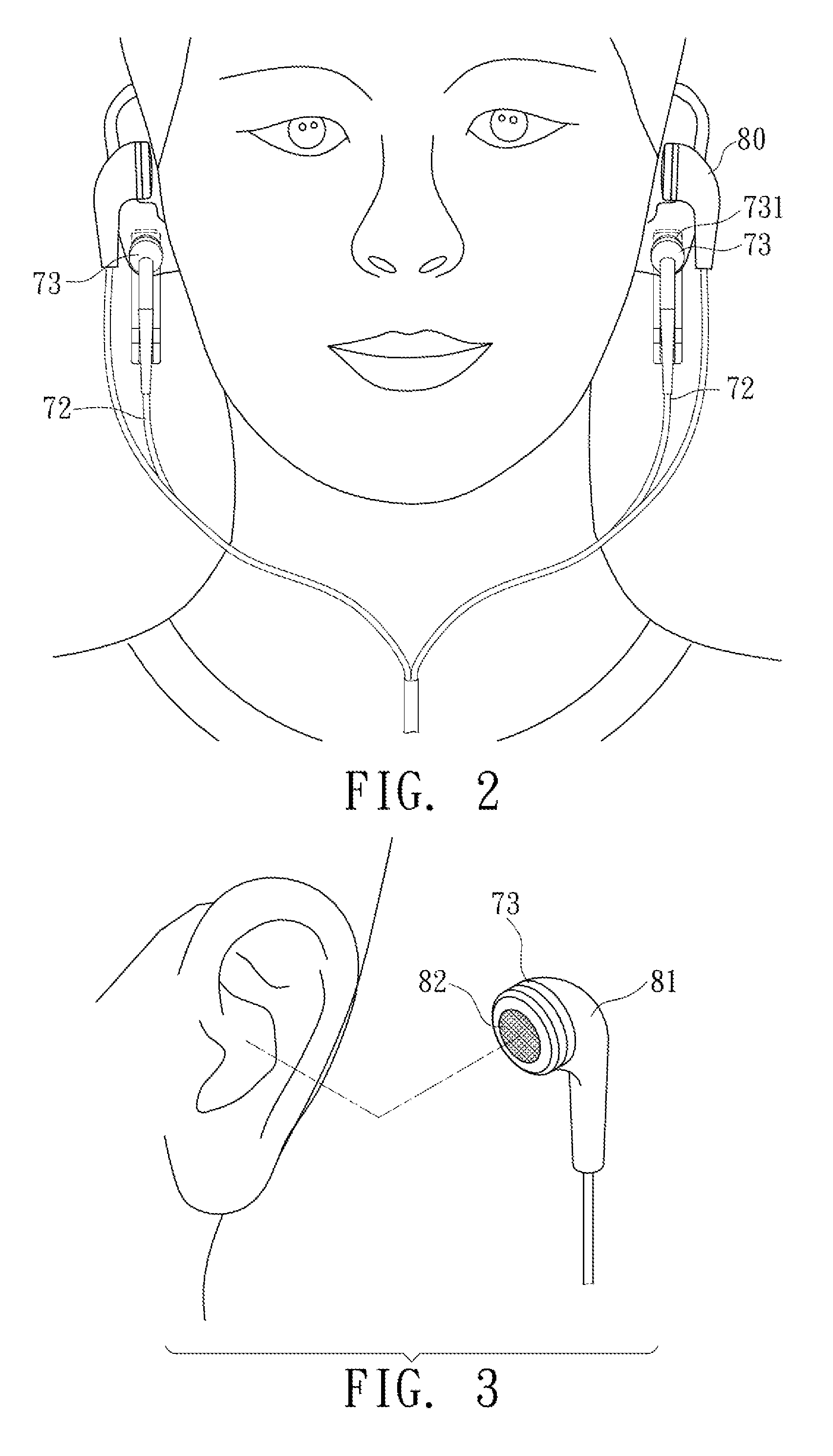
Av system with skin care and health care functions
InactiveUS20100324624A1Effect biological functionFacilitates synthesis of ATPElectrotherapyTransducer detailsElectrical conductorSide effect
This specification discloses an AV system with the skin care and health care functions. An AV system and a pulse current generator are integrated. A conductor transmits a pulse current output from the pulse current generator to a human body. The pulse current cures or alleviates the user in pain, melancholy, anxiety, and insomnia, accelerates the recovery of wound or bone, and reduces the side effects of radiotherapy and chemotherapy.
Owner:SYNHERB BIOTECH
Sleep-improving and health-care drug pillow
InactiveCN102058292ASimple preparation processLow costPillowsNervous disorderMedical productHeadaches
The invention provides a sleep-improving and health-care drug pillow, relating to a health-care medical product, in particular to making materials and process of the sleep-improving and health-care drug pillow. A drug core of the pillow mainly comprises the following raw materials in parts by weight: 1000 parts of cottonseeds, 500 parts of buckwheat shell, 20 parts of tea, 30 parts of spina date seed, 30 parts of schisandra, 20 parts of lily, 20 parts of ganoderma lucidum, 30 parts of akebia stem, 30 parts of tuber fleeceflower root, 10 parts of cordyceps, 10 parts of Chinese angelica, 30 parts of rehmannia root, 10 parts of safflower, 30 parts of red-rooted salvia root, 10 parts of longan, 30 parts of common clubmoss herb, 30 parts of garden balsam stem, 10 parts of Chinese atractylode rhizome, 10 parts of clematis root, 30 parts of pawpaw, 30 parts of divaricate saposhnikovia root, 30 parts of dried orange peel, 20 parts of costustoot, 20 parts of polygala root, 30 parts of Szechwan lovage rhizome, 10 parts of honeysuckle, 20 parts of glossy privet fruit, 10 parts of wild chrysanthemum, 10 parts of lonicera chrysantha flower, 10 parts of amomum shell, 20 parts of mint, 30 parts of galanga, 20 parts of bergamot, 20 parts of coptis, 20 parts of amur corktree bark, 10 parts of tuber fleeceflower stem, 10 parts of dwarf lilytruf tuber, 10 parts of dandelion, 30 parts of radix astragali, 30 parts of nutgrass galingale rhizome, 2000 parts of millet mature vinegar, 30 parts of honey and 30 parts of yellow wine and is refined by adopting the processes of soaking, roasting, brewing, mixing and the like. The sleeping-improving and health-care pillow has the advantages of simple making process and strong drug efficacy and has special curative effects on long-term insomnia, high blood pressure, headache, tinnitus and cervical pain.
Owner:栗全正 +1
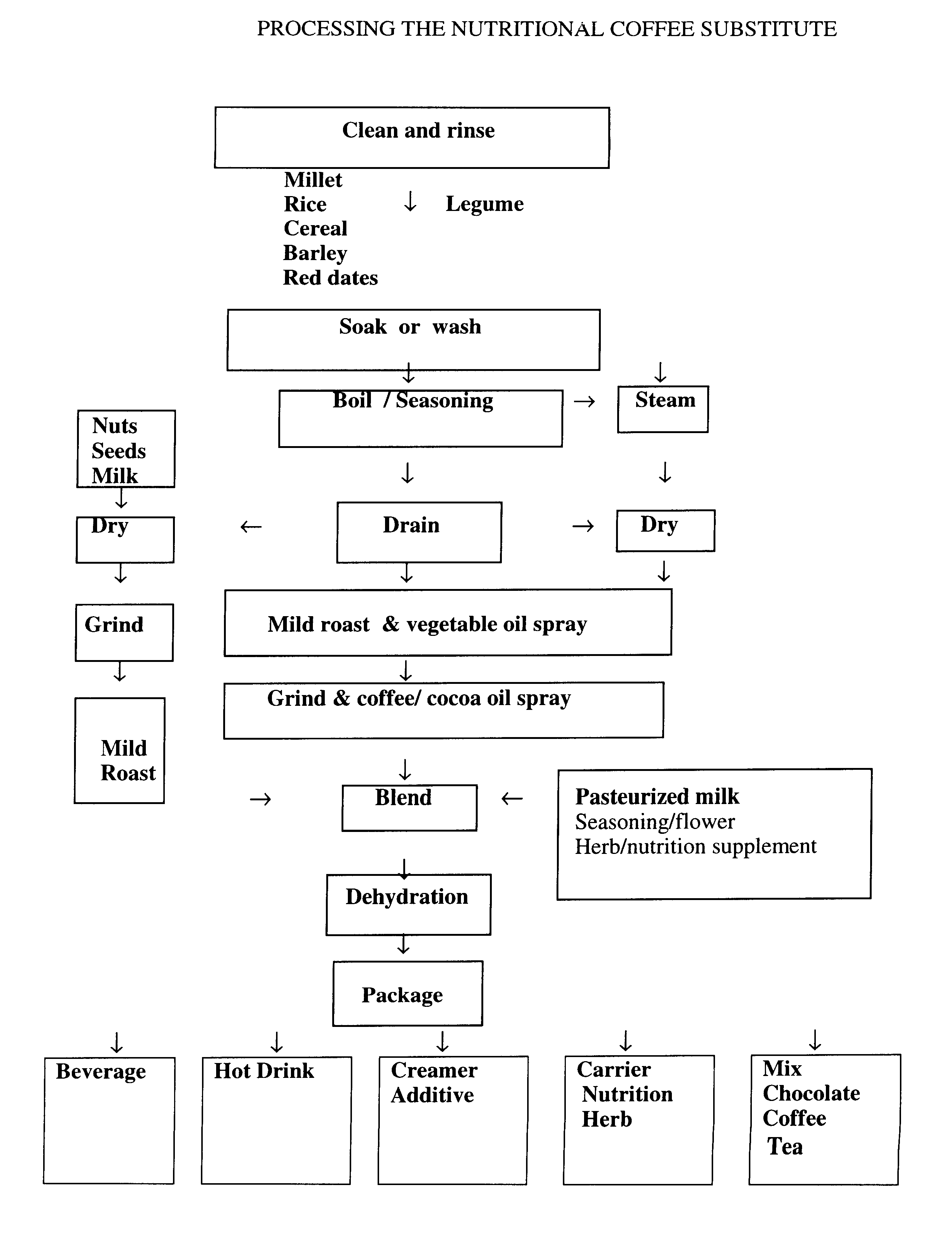
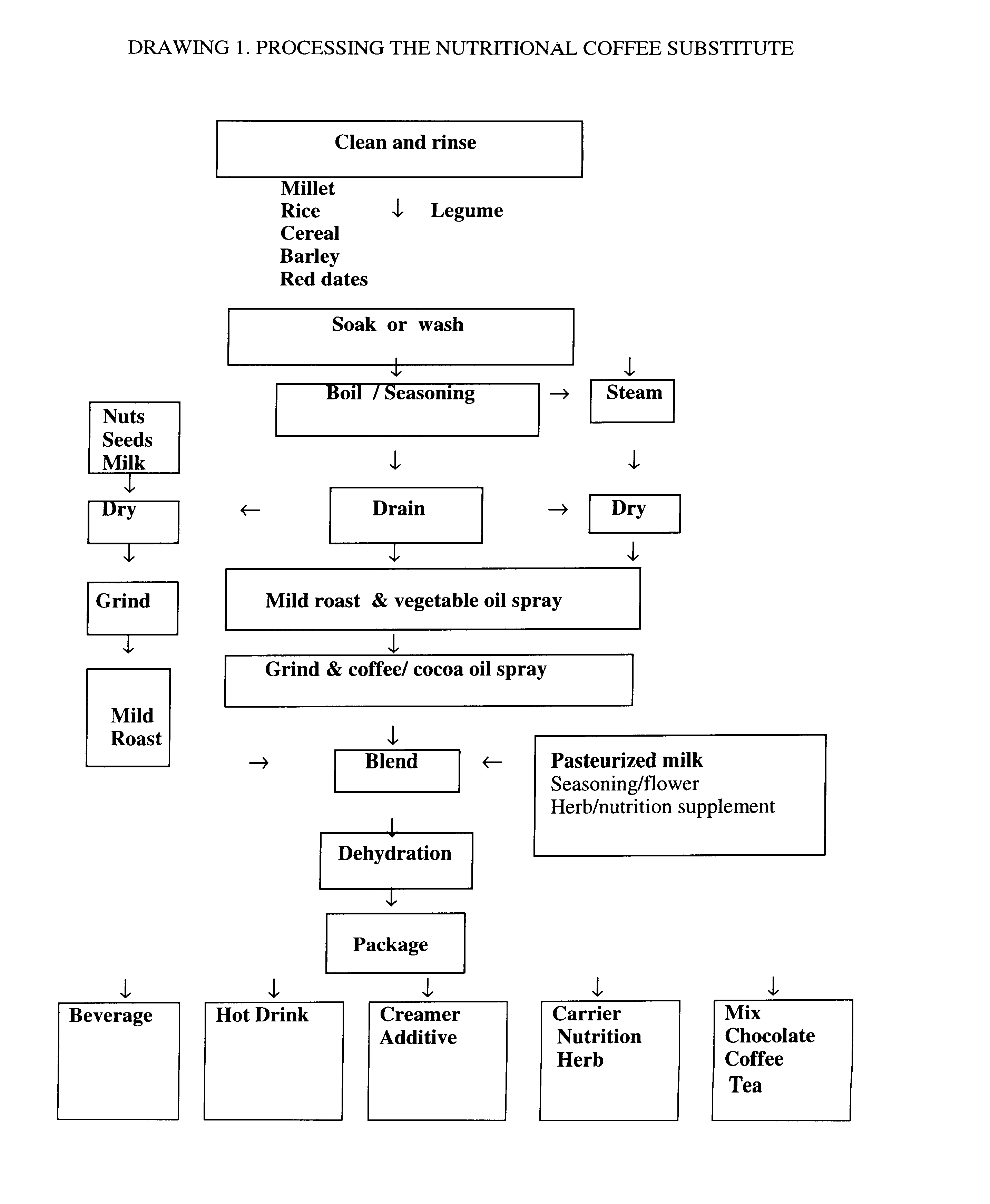
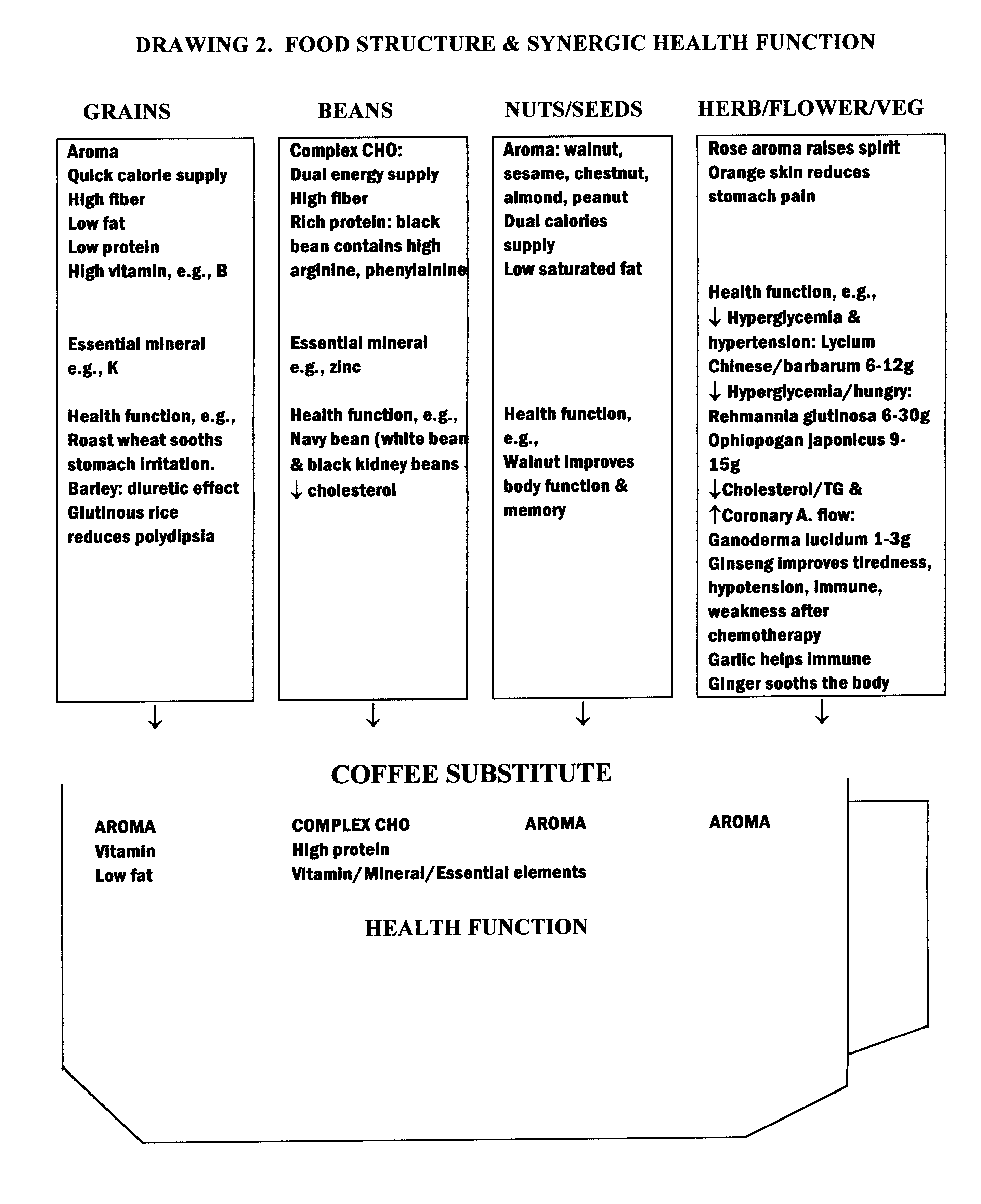
Coffee substitute
InactiveUS6171635B2Sufficient supplyEasy to acceptNatural extract food ingredientsFood preparationGastric irritationNutrition supplementation
A coffee-type beverage base is prepared by light roast method under 200° C. and originated from grain and legume. This coffee substitute has a pleasant aroma, and can be used as a carrier of nutritional supplement or herb therapy as well as an additive of coffee, tea, or chocolate. This novel drink is especially suitable for individuals who suffer from conditions making them coffee intolerant, e.g., pregnancy, or those who suffer form hypoglycemia, hypertension, arrhythmia, insomnia, or gastric irritation.
Owner:ZHAO IRIS G
Treatment of insomnia
The invention is directed to a method for the treatment of a patient suffering from insomnia. The claimed method comprises the administration of a compound selected from the group consisting of the pharmaceutically acceptable forms of dosage of mirtazapine, nortriptyline and mixtures thereof in dosages ranging from about 0.5 to about 10.0 milligrams.
Owner:PROMCOM ONE
Heterocyclic Compounds for the Treatment of Neurological and Psychological Disorders
ActiveUS20130096089A1Organic active ingredientsNervous disorderBipolar mood disorderRecurrent anxiety
Lactam compounds of Formula I and their use for the treatment of neurological and psychiatric disorders including schizophrenia, bipolar disorder, anxiety disorder and insomnia is disclosed.
Owner:ALKERMES PHARMA IRELAND LTD
Fluorinated cyclic amides as dipeptidyl peptidase IV inhibitors
InactiveUS20040132713A1Ease of preparation and detectabilityGood metabolic stabilityBiocideOrganic chemistryDisease progressionDiabetic nephropathy
The invention relates to new therapeutically active and selective inhibitors of the enzyme dipeptidyl peptidase-IV, pharmaceutical compositions comprising the compounds and the use of such compounds for treating diseases that are associated with proteins that are subject to processing by DPP-IV, such as Type 2 diabetes mellitus, hyperglycemia, impaired glucose tolerance, metabolic syndrome (Syndrome X or insulin resistance syndrome), glucosuria, metabolic acidosis, cataracts, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, diabetic cardiomyopathy, Type 1 diabetes, obesity, conditions exacerbated by obesity, hypertension, hyperlipidemia, atherosclerosis, osteoporosis, osteopenia, frailty, bone loss, bone fracture, acute coronary syndrome, infertility due to polycystic ovary syndrome, short bowel syndrome, anxiety, depression, insomnia, chronic fatigue, epilepsy, eating disorders, chronic pain, alcohol addiction, diseases associated with intestinal motility, ulcers, irritable bowel syndrome, inflammatory bowel syndrome and to prevent disease progression in Type 2 diabetes. The invention also relates to a method of identifying an insulin secretagogue agent for diabetes.
Owner:PFIZER INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com