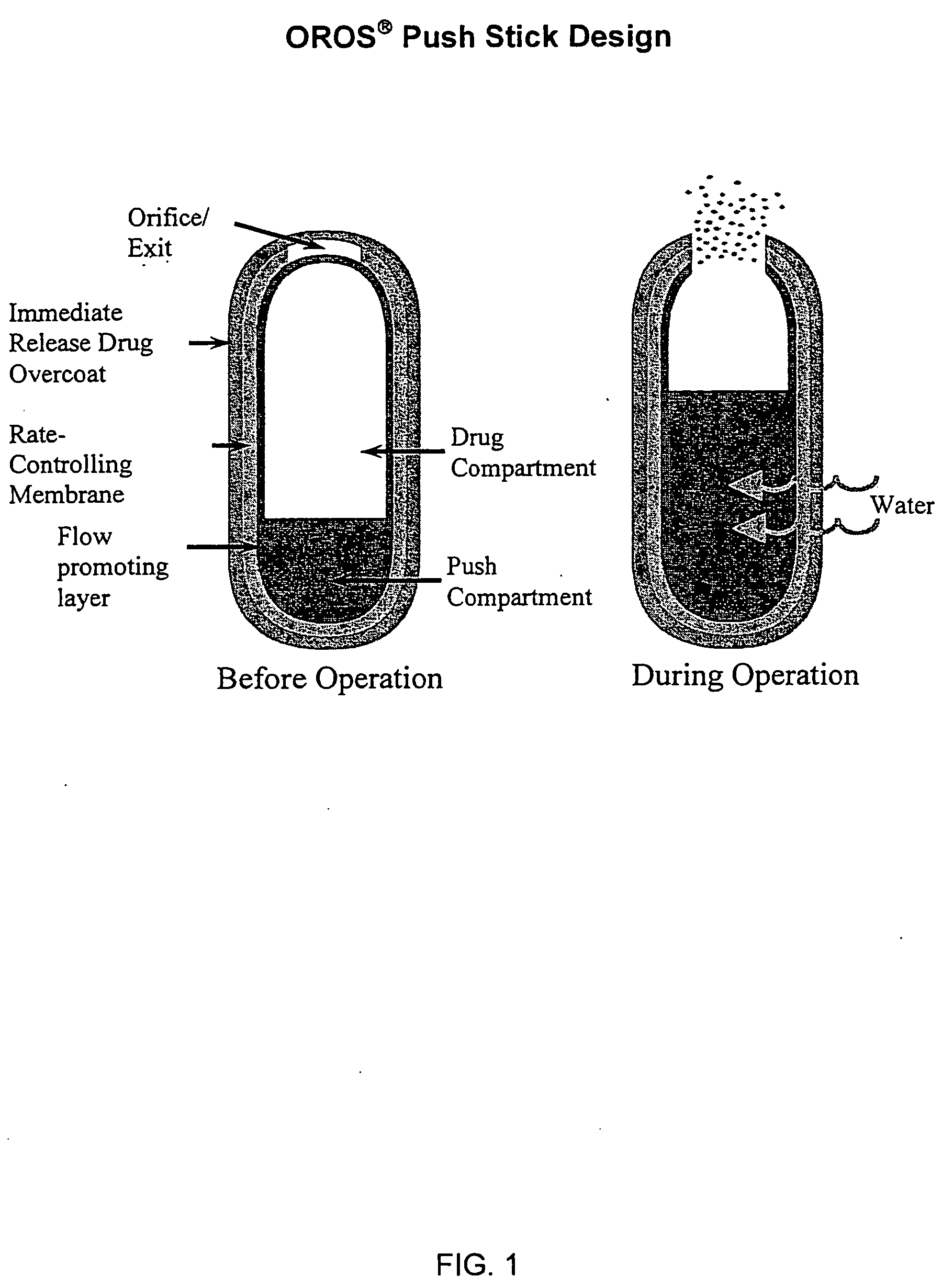
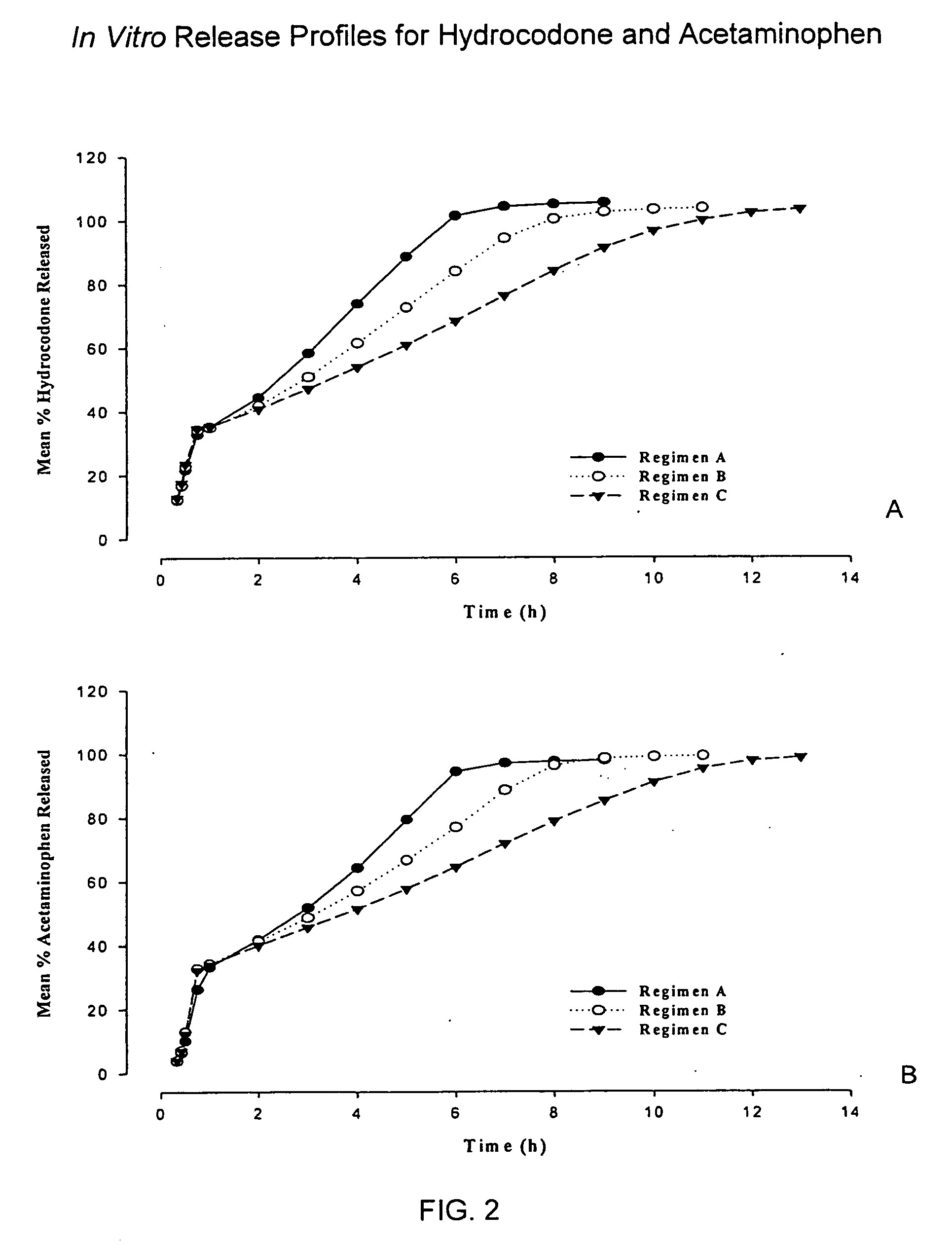
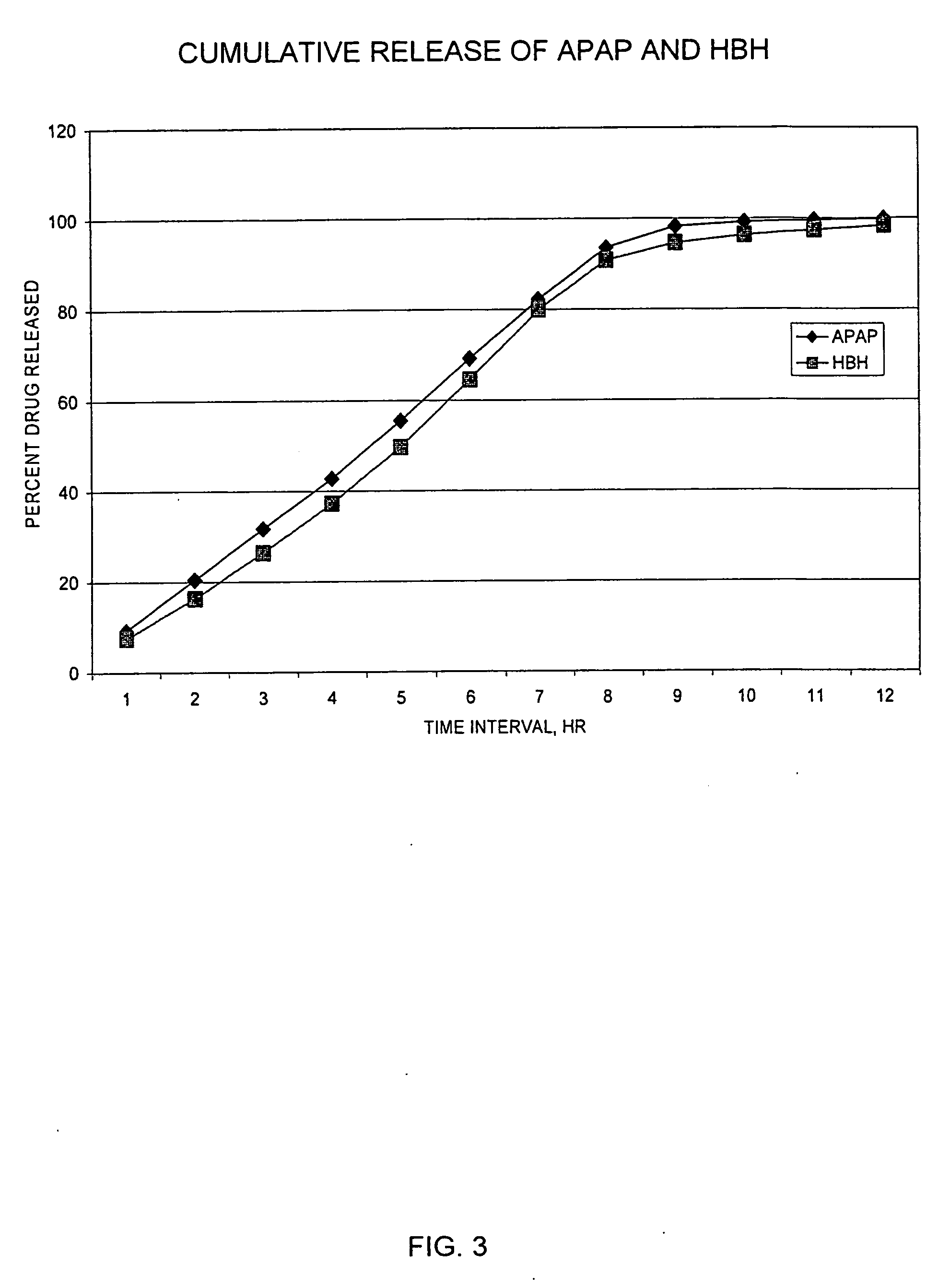
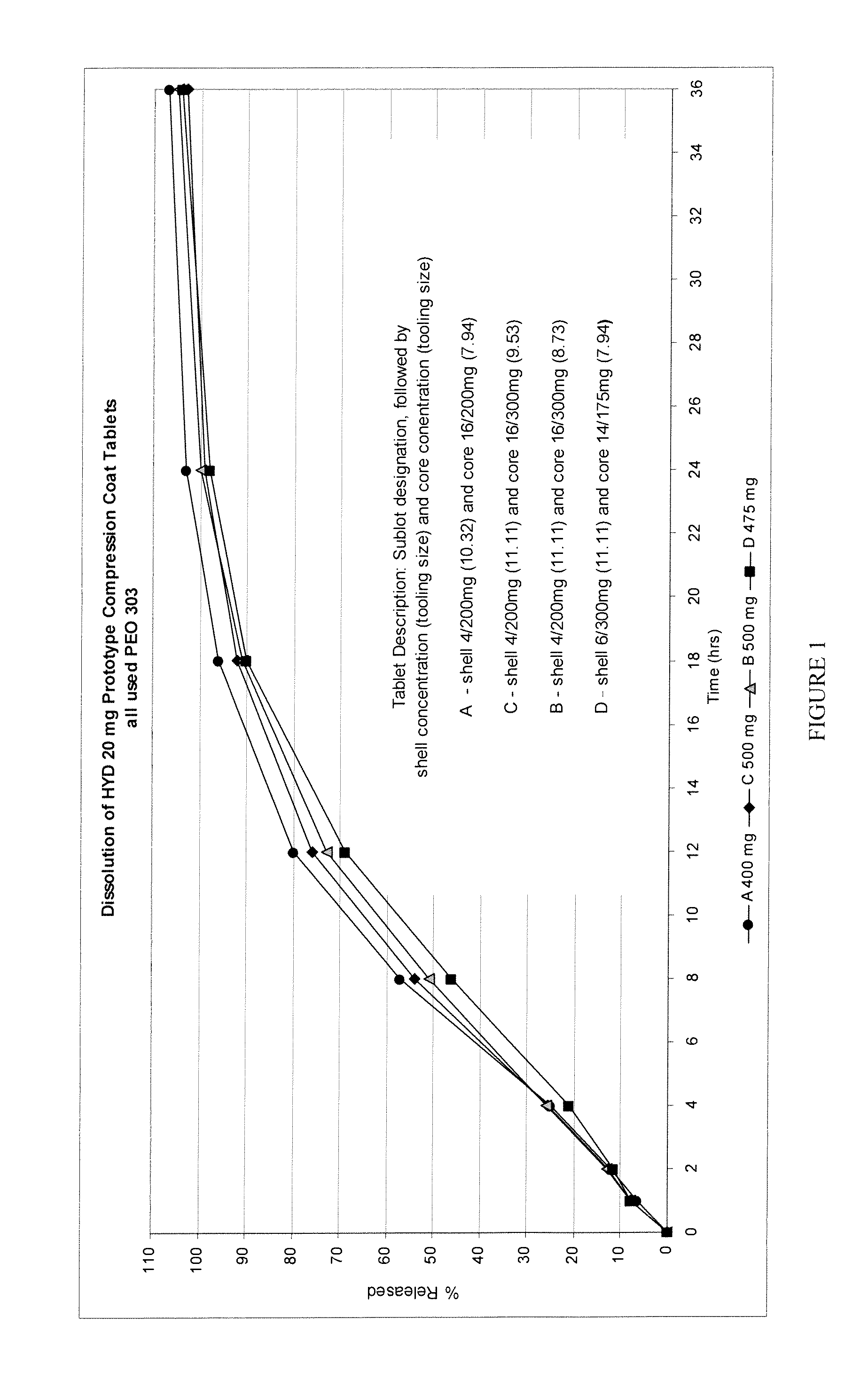
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
134 results about "Opioid analgesics" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method of preventing abuse of opioid dosage forms
InactiveUS6228863B1Reducing parenteral abuse potential of dosage formBiocideNervous disorderOpioid antagonistOpioid Agonist
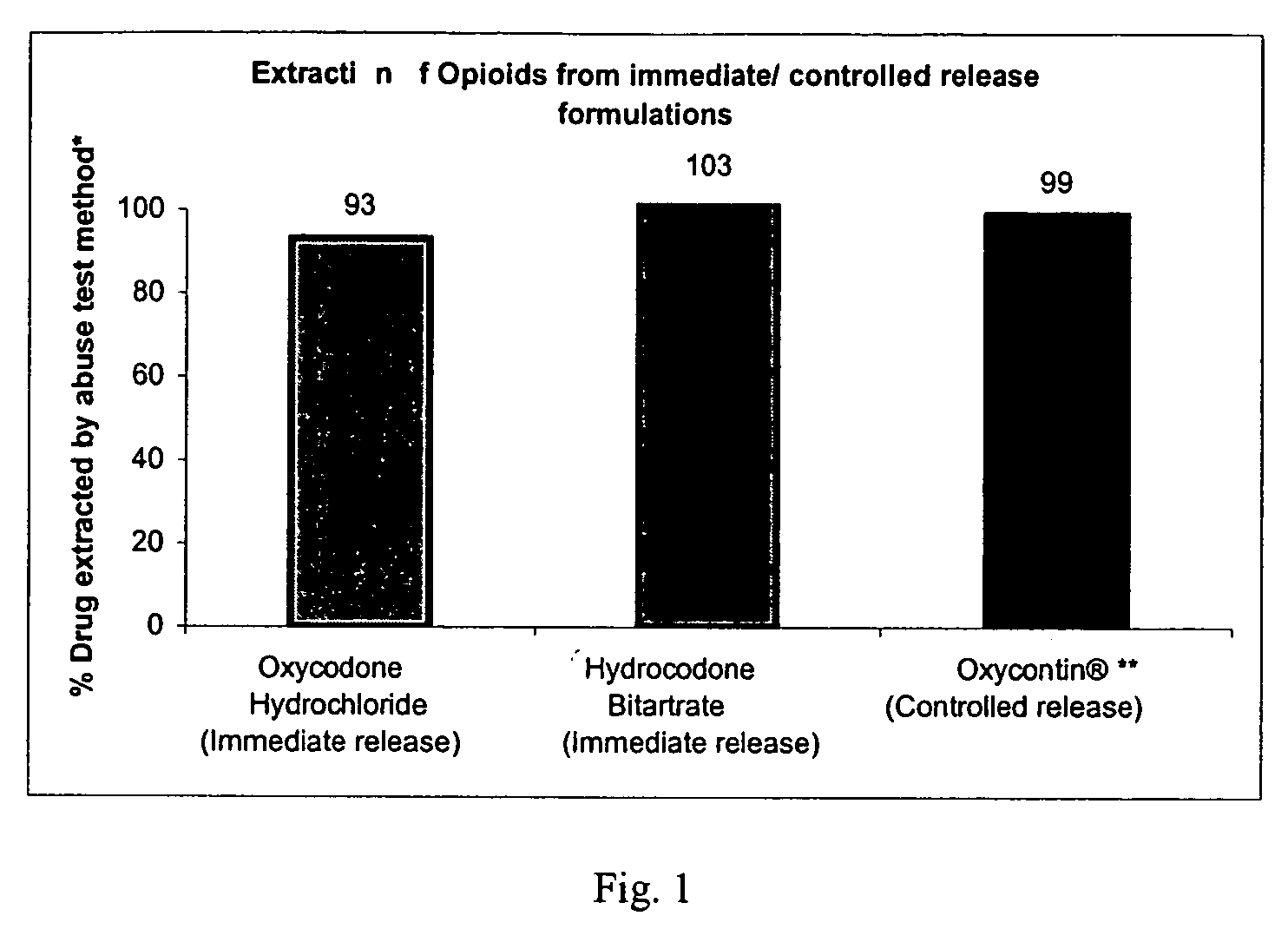
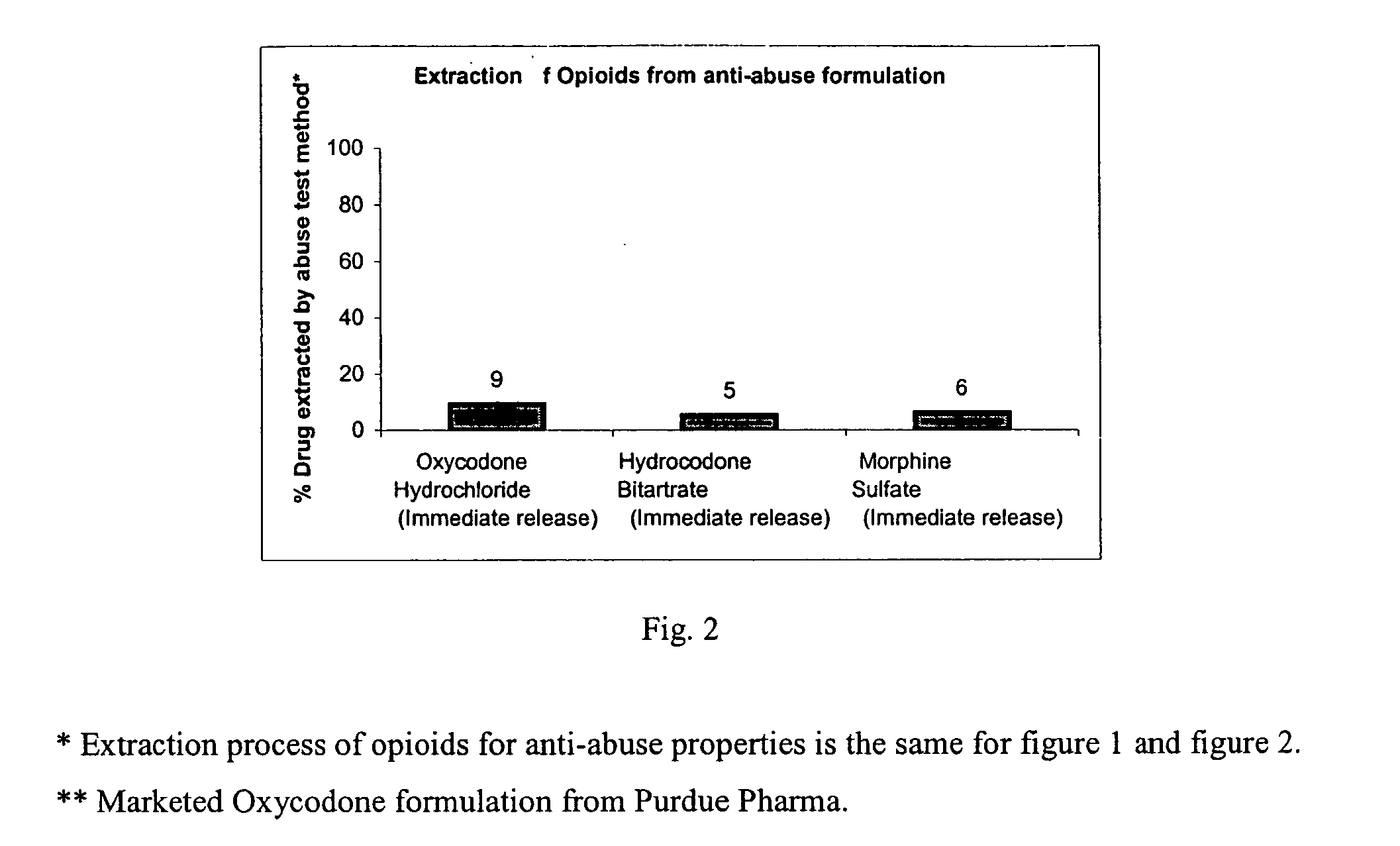
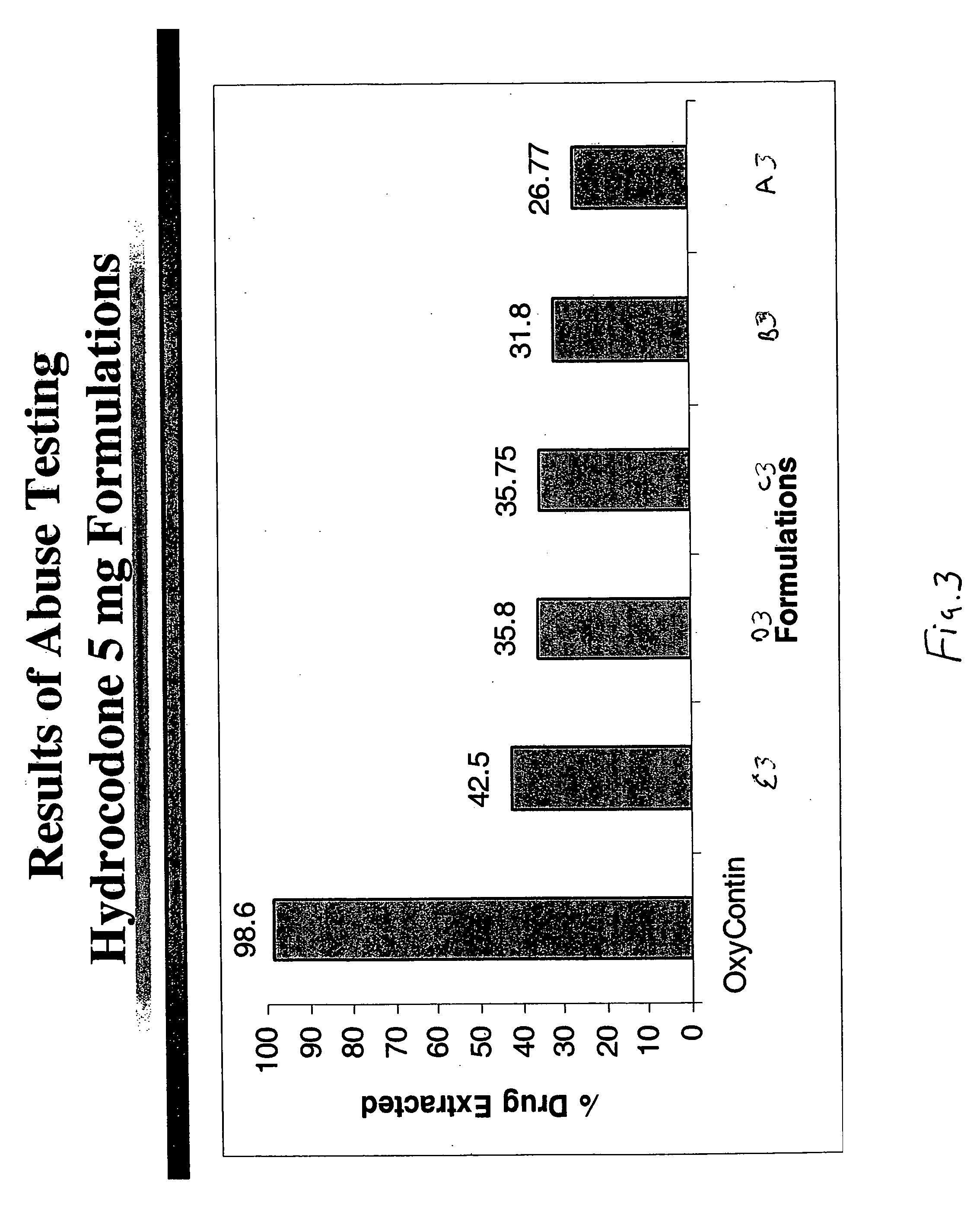
The invention relates in part to a method of reducing the abuse potential of an oral dosage form of an opioid analgesic, wherein an analgesically effective amount of an orally active opioid agonist is combined with an opioid antagonist into an oral dosage form which would require at least a two-step extraction process to be separated from the opioid agonist, the amount of opioid antagonist including being sufficient to counteract opioid effects if extracted together with the opioid agonist and administered parenterally.
Owner:PURDUE PHARMA LP
Pharmaceutical formulation containing opioid agonist, opioid antagonist and irritant
ActiveUS20030068392A1Reduce and eliminate effectInhibition effectBiocideNervous disorderOpioid AgonistOpioid antagonist
Disclosed in certain embodiments is an oral dosage form comprising: a therapeutically effective amount of an opioid analgesic; an opioid antagonist; and an irritant in an effective amount to impart an irritating sensation to an abuser upon administration of the dosage form after tampering.
Owner:PURDUE PHARMA LP
Pharmaceutical formulation containing opioid agonist,opioid antagonist and gelling agent
InactiveUS20030068371A1Reduce and eliminate effectInhibition effectBiocideNervous disorderOpioid antagonistOpioid Agonist
Disclosed in certain embodiments is an oral dosage form comprising a therapeutically effective amount of an opioid analgesic, an opioid antagonist and one or more pharmaceutically acceptable excipients; the dosage form further including a gelling agent in an effective amount to impart a viscosity unsuitable for administration selected from the group consisting of parenteral and nasal administration to a solubilized mixture formed when the dosage form is crushed and mixed with from about 0.5 to about 10 ml of an aqueous liquid.
Owner:PURDUE PHARMA LP
Combination sustained release-immediate release oral dosage forms with an opioid analgesic and a non-opioid analgesic
InactiveUS20030092724A1Long durationConstant plasma levels of opioid and non-opioid analgesicsBiocidePill deliveryImmediate releaseTherapeutic effect
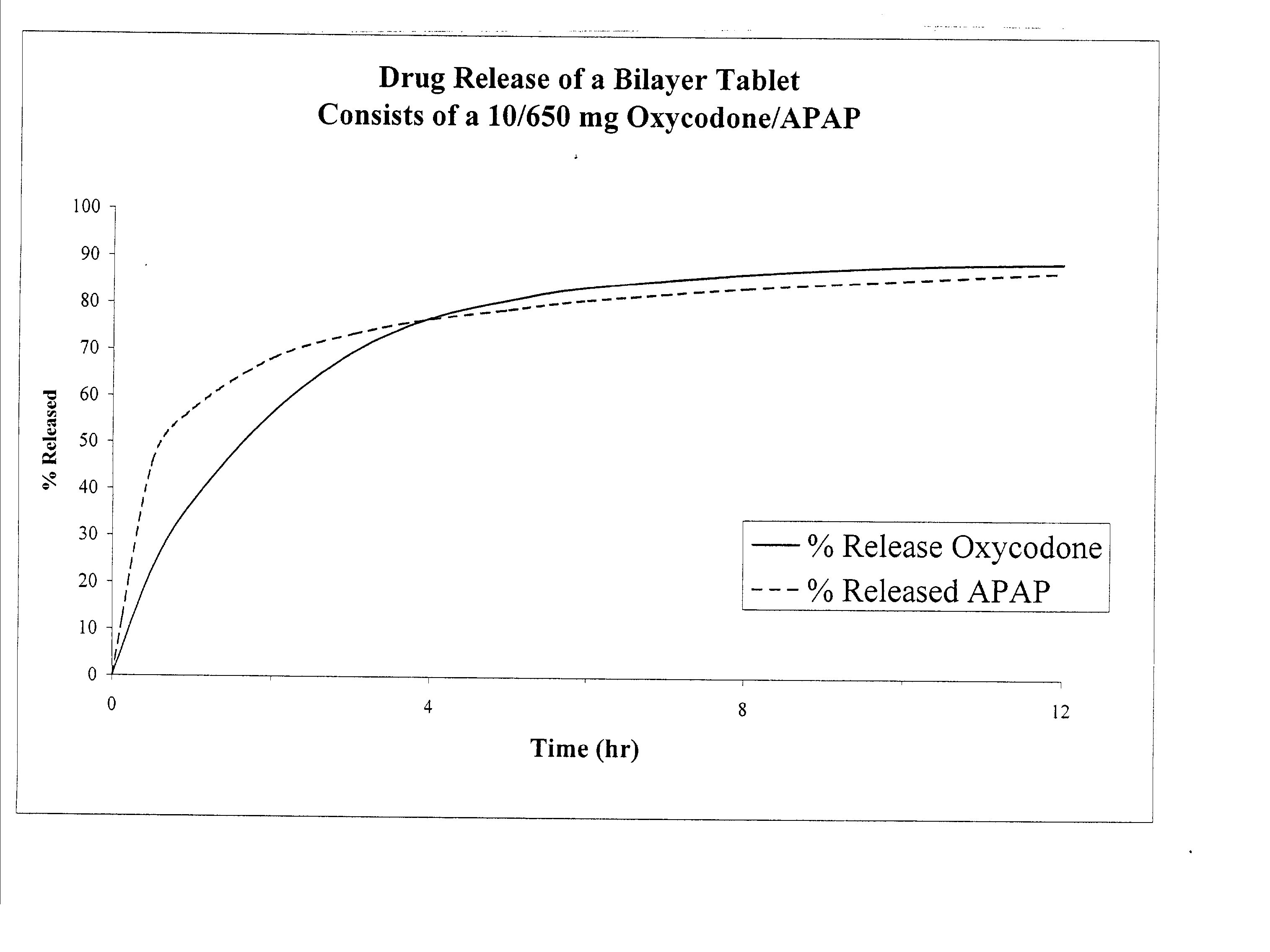
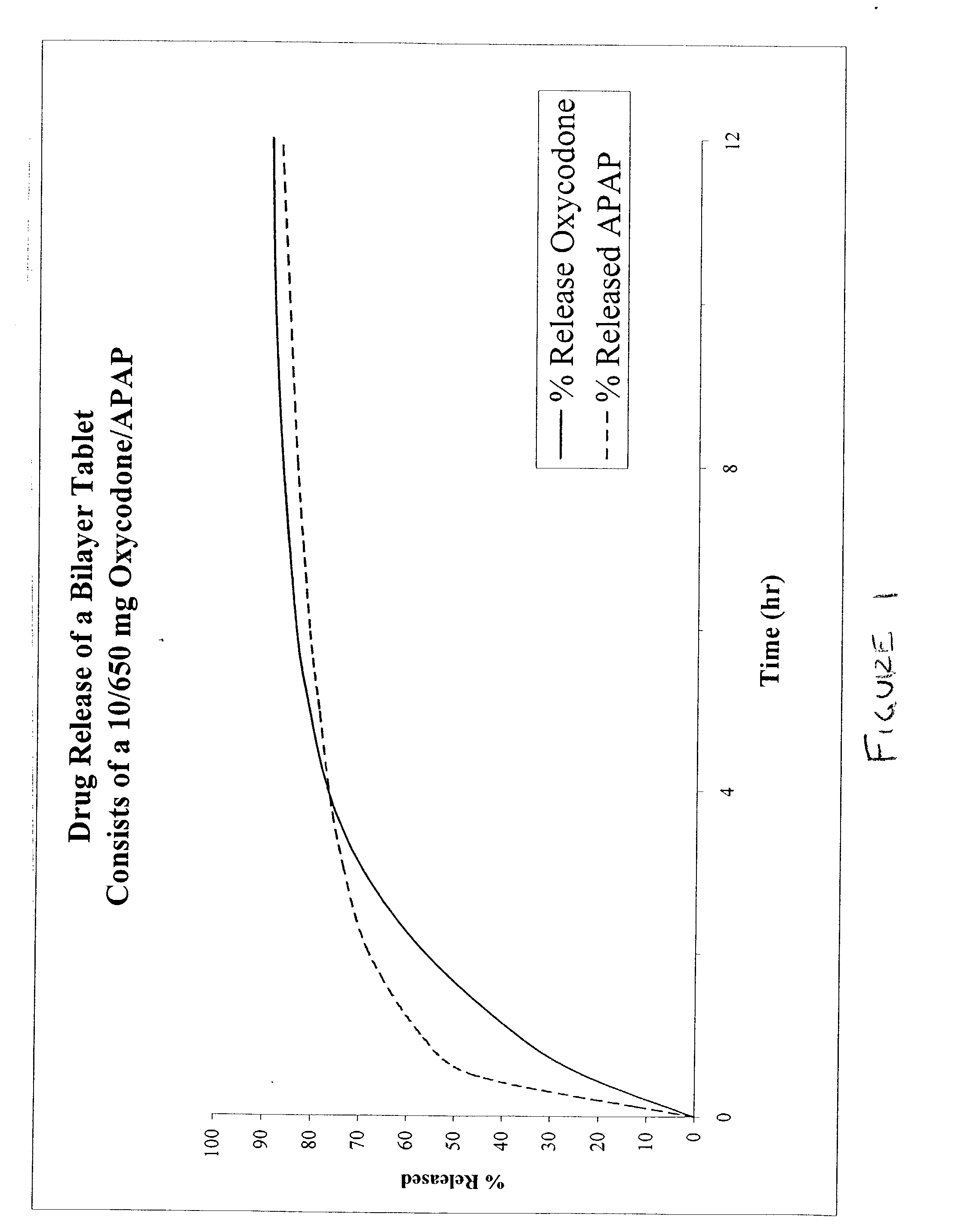
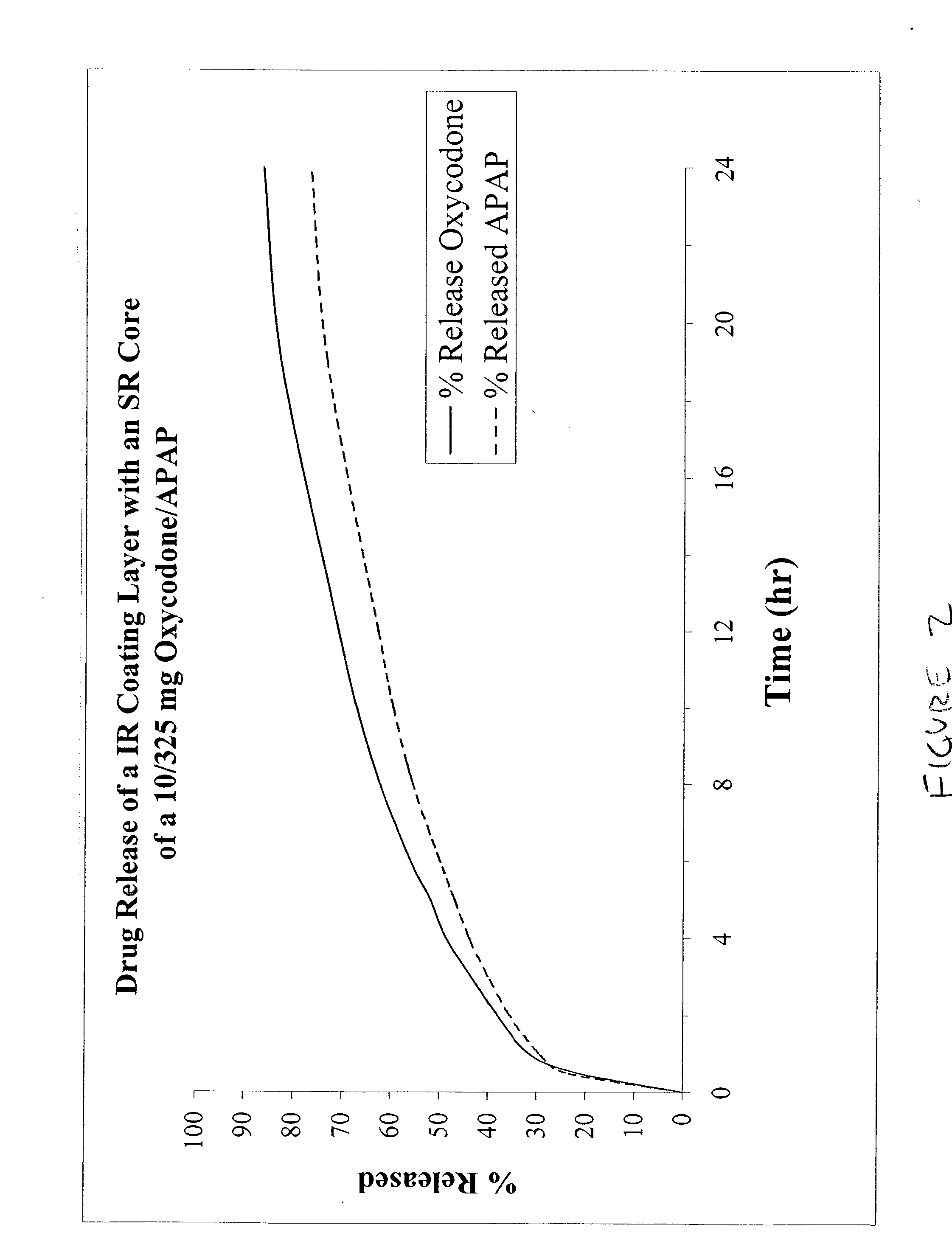
The present invention relates to new and useful oral tablet compositions which include an immediate release portion having an opioid analgesic and a non-opioid analgesic, providing for a rapid onset of therapeutic effect, and a sustained release portion of an opioid analgesic and a non-opioid analgesic, providing for a relatively longer duration of therapeutic effect. A multilayer oral dosage form containing a sustained release layer, which includes oxycodone and APAP, hydrocodone and APAP, or oxymorphone and APAP, and an immediate release layer containing the same active ingredients as the sustained release layer, is also disclosed. Also disclosed are oral tablet compositions, containing a sustained release core, which includes oxycodone and APAP, hydrocodone and APAP, or oxymorphone and APAP, and an immediate release coating containing the same active ingredients as the sustained release core, are also disclosed. In addition, methods of making and using such oral tablet compositions are disclosed.
Owner:ENDO PHARMA INC
Orally administrable opioid formulations having extended duration of effect
InactiveUS6294195B1Effective steady-state blood levelPowder deliveryBiocideBlood levelOral medication
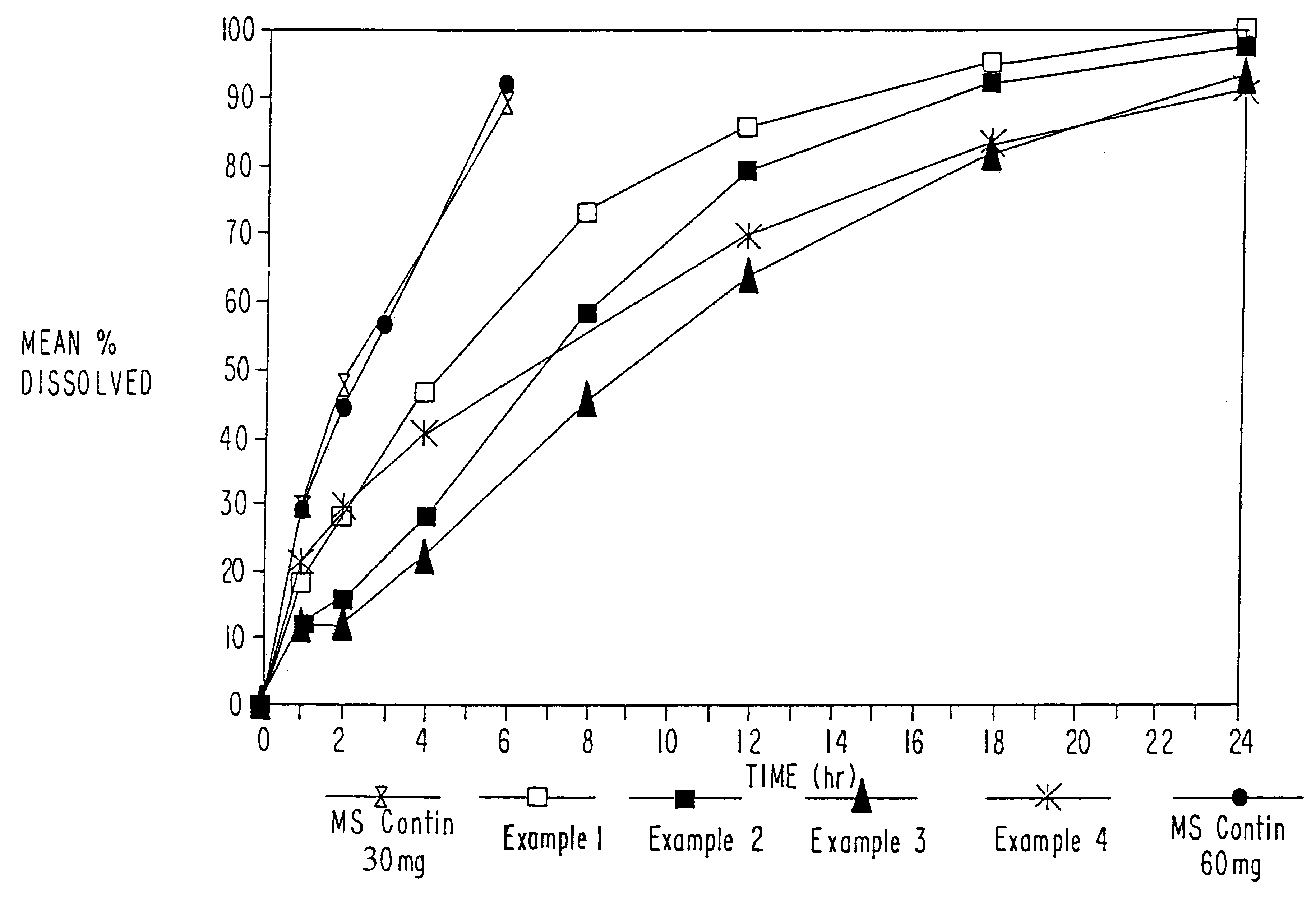
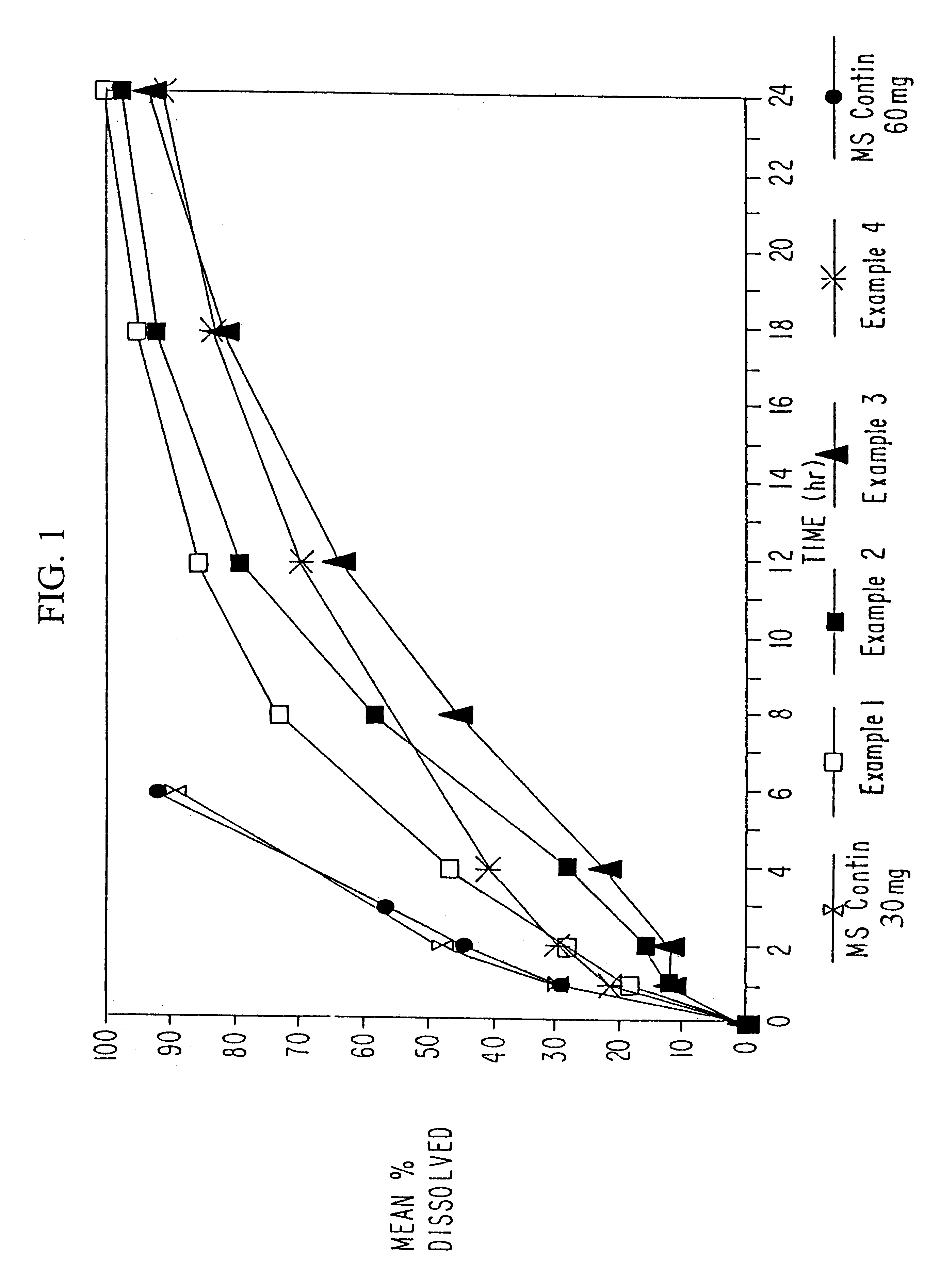
Sustained release oral solid dosage forms of opioid analgesics are provided as multiparticulate systems which are bioavailable and which provide effective blood levels of the opioid analgesic for at least about 24 hours. A unit dose of the opioid analgesic contains a plurality of substrates including the opioid analgesic in sustained release form. The substrates have a diameter from about 0.1 mm to about 3 mm.
Owner:PURDUE PHARMA LP
Methods and compositions for deterring abuse of opioid containing dosage forms
This invention relates to an abuse deterrent dosage form of opioid analgesics, wherein an analgesically effective amount of opioid analgesic is combined with a polymer to form a matrix.
Owner:HALSEY DRUG
Tamper resistant dosage forms
ActiveUS20090081290A1Reduces and prevents stickingBiocidePowder deliveryOpioid analgesicsDosage form
The present invention relates to pharmaceutical dosage forms, for example to a tamper resistant dosage form including an opioid analgesic, and processes of manufacture, uses, and methods of treatment thereof.
Owner:PURDUE PHARMA LP
Controlled release formulations of opioid and nonopioid analgesics
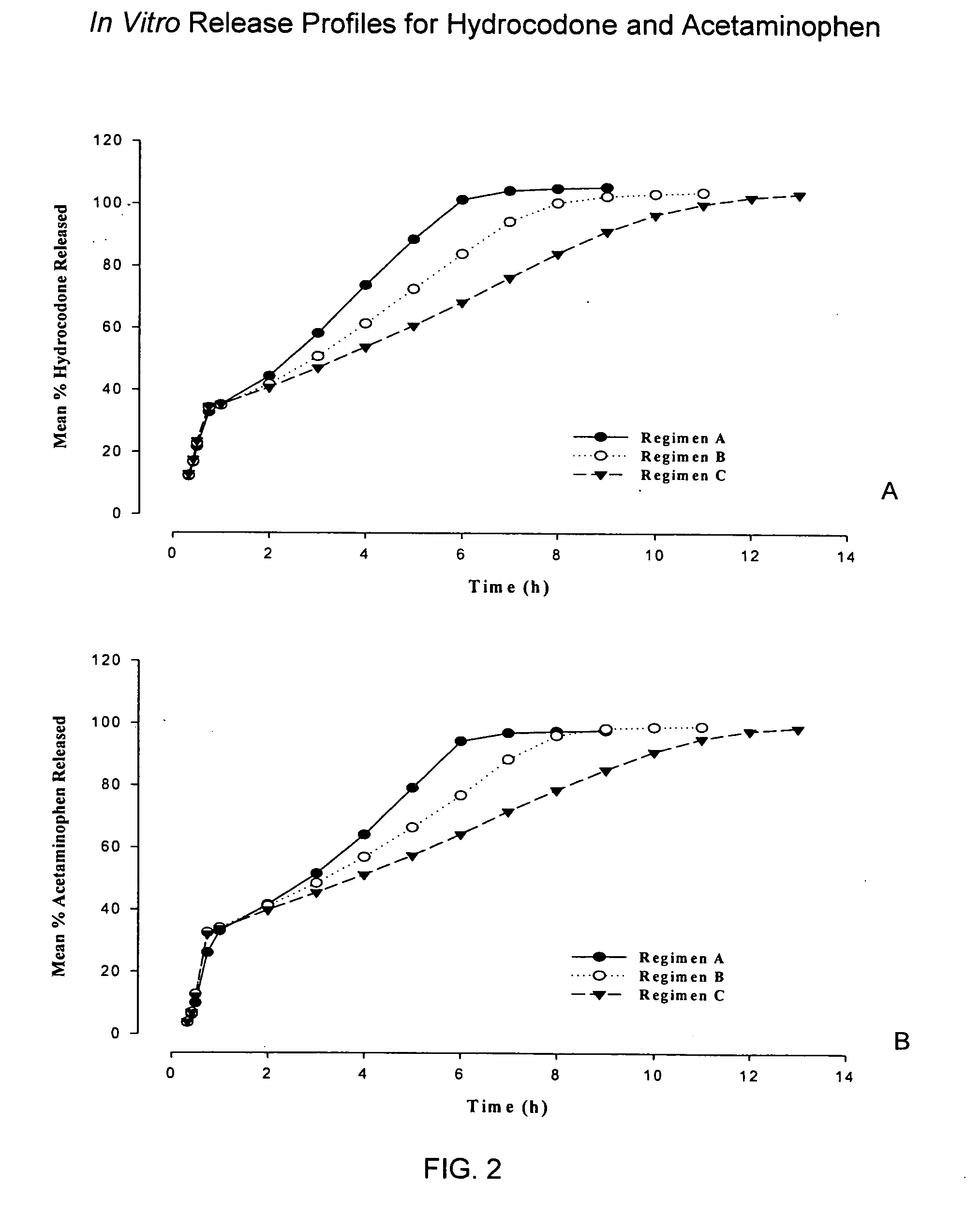
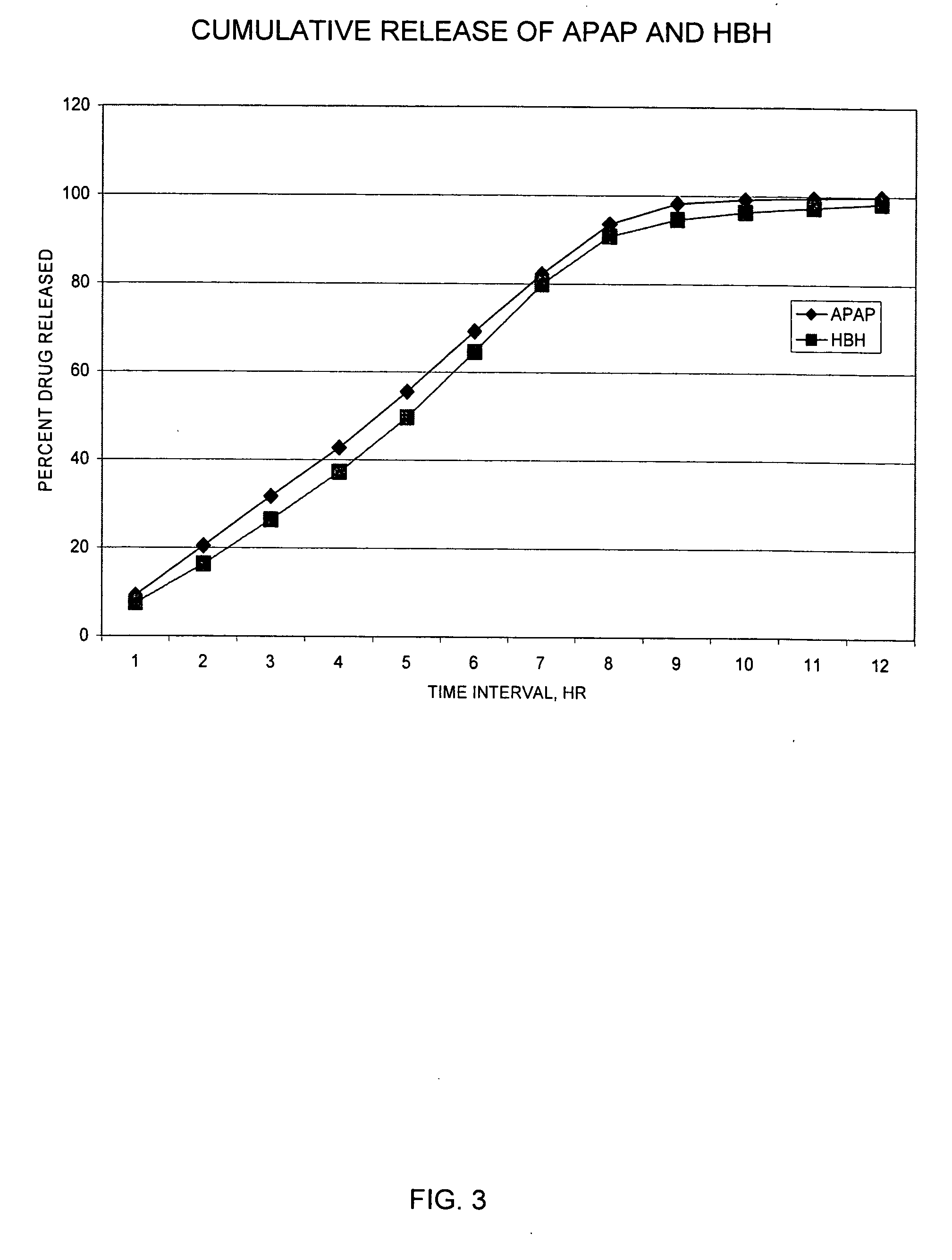
InactiveUS20050158382A1Reduce the maximumRapid rise in plasma concentrationBiocideNervous disorderImmediate releaseAnalgesic agents
Sustained release dosage forms for twice daily oral dosing to a human patient for providing relief from pain are provided. The sustained release dosage form comprises an immediate release component and a sustained release component, wherein the immediate release component and the sustained release component collectively contain a therapeutically effective amount of an opioid analgesic and a therapeutically effective amount of nonopioid analgesic. In a preferred embodiment, the nonopioid analgesic is acetaminophen and the opioid analgesic is hydrocodone and pharmaceutically acceptable salts thereof, and in preferred embodiments, the pharmaceutically acceptable salt is bitartrate. The dosage forms produce plasma profiles in a patient characterized by a Cmax for hydrocodone of between about 0.6 ng / mL / mg to about 1.4 ng / mL / mg and an AUC for hydrocodone of between about 9.1 ng*hr / mL / mg to about 19.9 ng*hr / mL / mg (per mg hydrocodone bitartrate administered) and a Cmax for acetaminophen of between about 2.8 ng / mL / mg and 7.9 ng / mL / mg and an AUC for acetaminophen of between about 28.6 ng*hr / mL / mg and about 59.1 ng*hr / mL / mg (per mg acetaminophen administered) after a single dose.
Owner:ALZA CORP
Pharmaceutical formulation containing opioid agonist, opioid antagonist and gelling agent
InactiveUS7842307B2Reducing abuse potential of dosage formLower potentialBiocideNervous disorderOpioid antagonistOpioid Agonist
Disclosed in certain embodiments is an oral dosage form comprising a therapeutically effective amount of an opioid analgesic, an opioid antagonist and one or more pharmaceutically acceptable excipients; the dosage form further including a gelling agent in an effective amount to impart a viscosity unsuitable for administration selected from the group consisting of parenteral and nasal administration to a solubilized mixture formed when the dosage form is crushed and mixed with from about 0.5 to about 10 ml of an aqueous liquid.
Owner:PURDUE PHARMA LP
Programmable multi-dose intranasal drug delivery device
InactiveUS6948492B2Avoid diversionAvoid abuseRespiratorsLiquid surface applicatorsNasal sprayBiological activation
An apparatus and method for the self-administration of a plurality of doses of an intranasal liquid pharmaceutical composition, including opioid analgesics, that includes a drug delivery device containing a plurality of sealed vials, each vial containing a predetermined volume of the pharmaceutical composition, a pump assembly for conveying the liquid pharmaceutical composition from the interior of the vial and discharging it as a nasal spray in response to manual activation by the patient, and programmable means for sequentially advancing a vial to the ready position after passage of a prescribed time interval following the last activation of the delivery device.
Owner:UNIV OF KENTUCKY RES FOUND
Pharmaceutical formulation containing opioid agonist, opioid antagonist and bittering agent
InactiveUS7144587B2Reducing abuse potential of dosage formLower potentialPowder deliveryPill deliveryOpioid antagonistOpioid Agonist
Disclosed in certain embodiments is an oral dosage form comprising a therapeutically effective amount of an opioid analgesic; an opioid antagonist; and a bittering agent in an effective amount to impart a bitter taste to an abuser upon administration of the dosage form after tampering.
Owner:PURDUE PHARMA LP
Formulations of nonopioid and confined opioid analgesics
The preferred exemplary embodiments in the present application provide formulations and methods for the delivery of drugs, particularly drugs of abuse, having an abuse-relevant drug substantially confined in the core and a non-abuse relevant drug in a non-core region. These formulations have reduced potential for abuse. In the formulation, preferably the abuse relevant drug is an opioid and the non-abuse relevant drug is acetaminophen or ibuprofen. More preferably, the opioid is hydrocodone, and the non-abuse relevant analgesic is acetaminophen. In certain preferred embodiments, the dosage forms are characterized by resistance to solvent extraction; tampering, crushing or grinding. Certain embodiments of the inventions provide dosage forms that provide an initial burst of release of drug followed by a prolonged period of controllable drug release.
Owner:ABBVIE DEUTSHLAND GMBH & CO KG
Controlled release formulations of opioid and nonopioid analgesics
InactiveUS20060251721A1Improved ability to treat painLess attentionBiocideNervous disorderImmediate releasePharmaceutical medicine
Sustained release dosage forms for twice daily oral dosing to a human patient for providing relief from pain are provided. The sustained release dosage form comprises an immediate release component and a sustained release component, wherein the immediate release component and the sustained release component collectively contain a therapeutically effective amount of an opioid analgesic and a therapeutically effective amount of nonopioid analgesic. In a preferred embodiment, the nonopioid analgesic is acetaminophen and the opioid analgesic is hydrocodone and pharmaceutically acceptable salts thereof, and in preferred embodiments, the pharmaceutically acceptable salt is bitartrate. The dosage forms produce plasma profiles in a patient characterized by a Cmax for hydrocodone of between about 0.6 ng / mL / mg to about 1.4 ng / mL / mg and an AUC for hydrocodone of between about 9.1 ng*hr / mL / mg to about 19.9 ng*hr / mL / mg (per mg hydrocodone bitartrate administered) and a Cmax for acetaminophen of between about 2.8 ng / mL / mg and 7.9 ng / mL / mg and an AUC for acetaminophen of between about 28.6 ng*hr / mL / mg and about 59.1 ng*hr / mL / mg (per mg acetaminophen administered) after a single dose.
Owner:ALZA CORP
Encased Tamper Resistant Controlled Release Dosage Forms
ActiveUS20120164220A1Reducing abuse potential of dosage formBiocideNervous disorderGastric fluidEnzyme
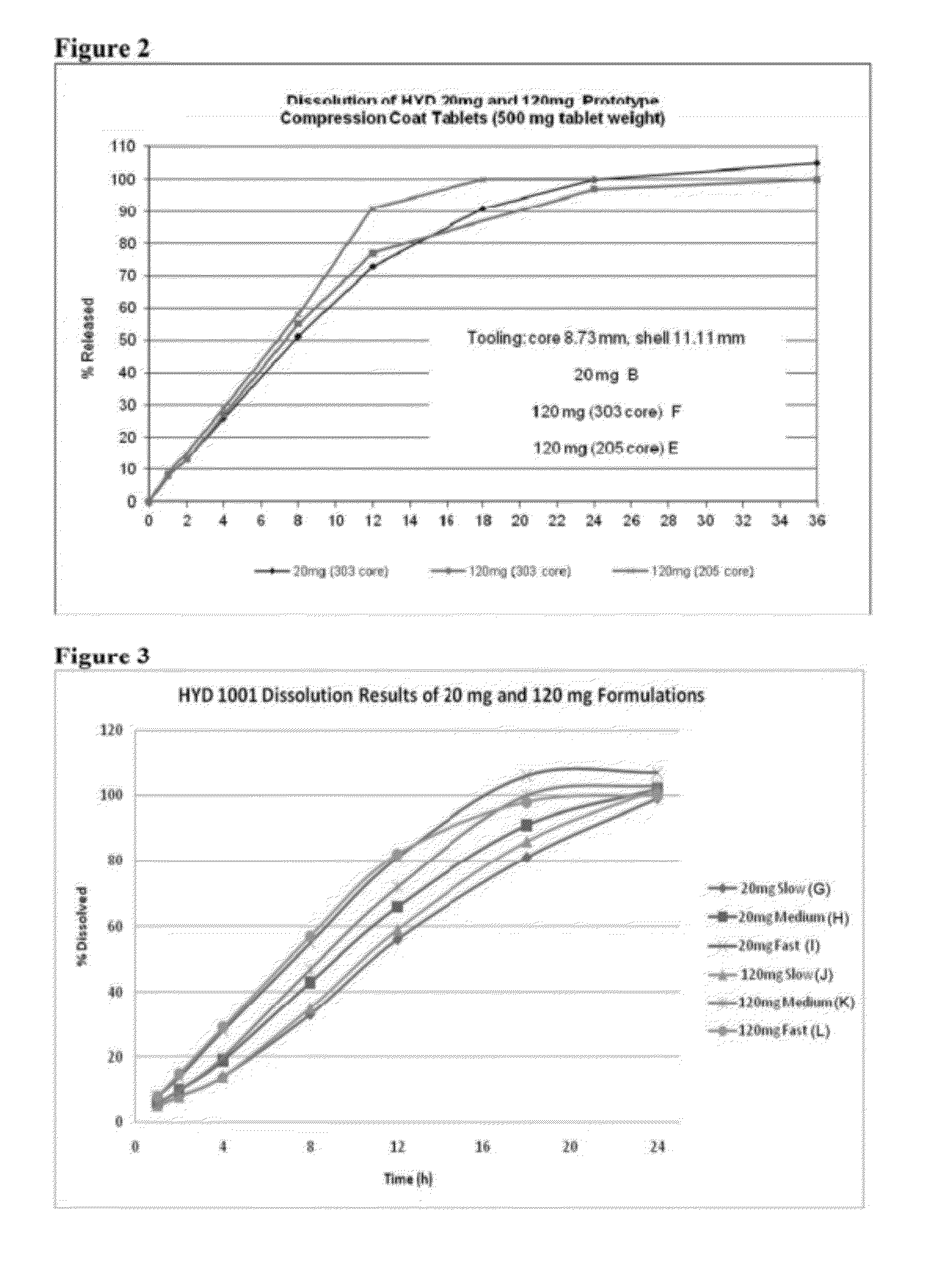
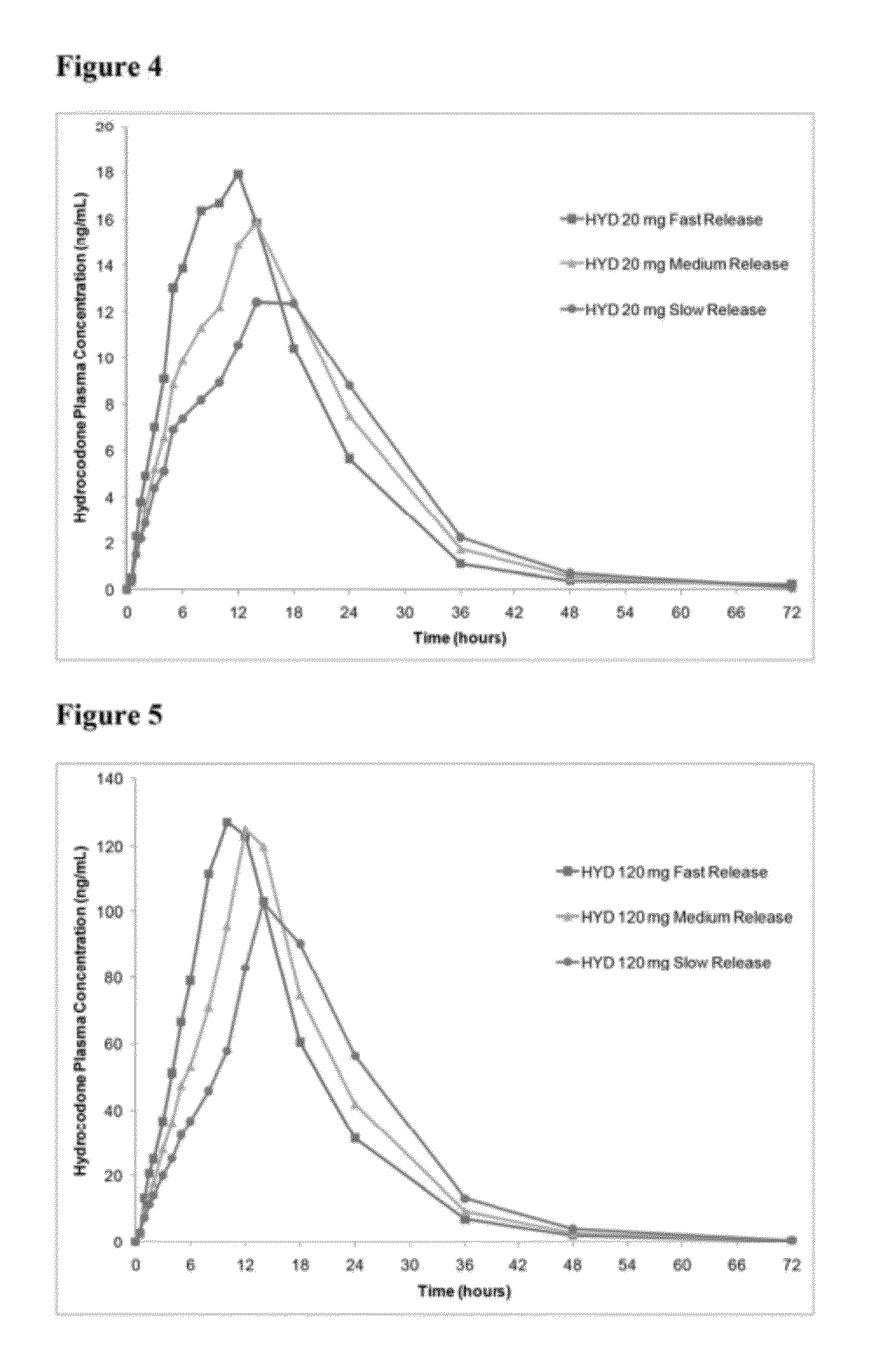
In certain embodiments, the present invention is directed to a solid controlled release dosage form comprising: a core comprising a first portion of an opioid analgesic dispersed in a first matrix material; and a shell encasing the core and comprising a second portion of the opioid analgesic dispersed in a second matrix material; wherein the amount of opioid analgesic released from the dosage form is proportional within 20% to elapsed time from 8 to 24 hours, as measured by an in-vitro dissolution in a USP Apparatus 1 (basket) at 100 rpm in 900 ml simulated gastric fluid without enzymes (SGF) at 37 C.
Owner:PURDUE PHARMA LP
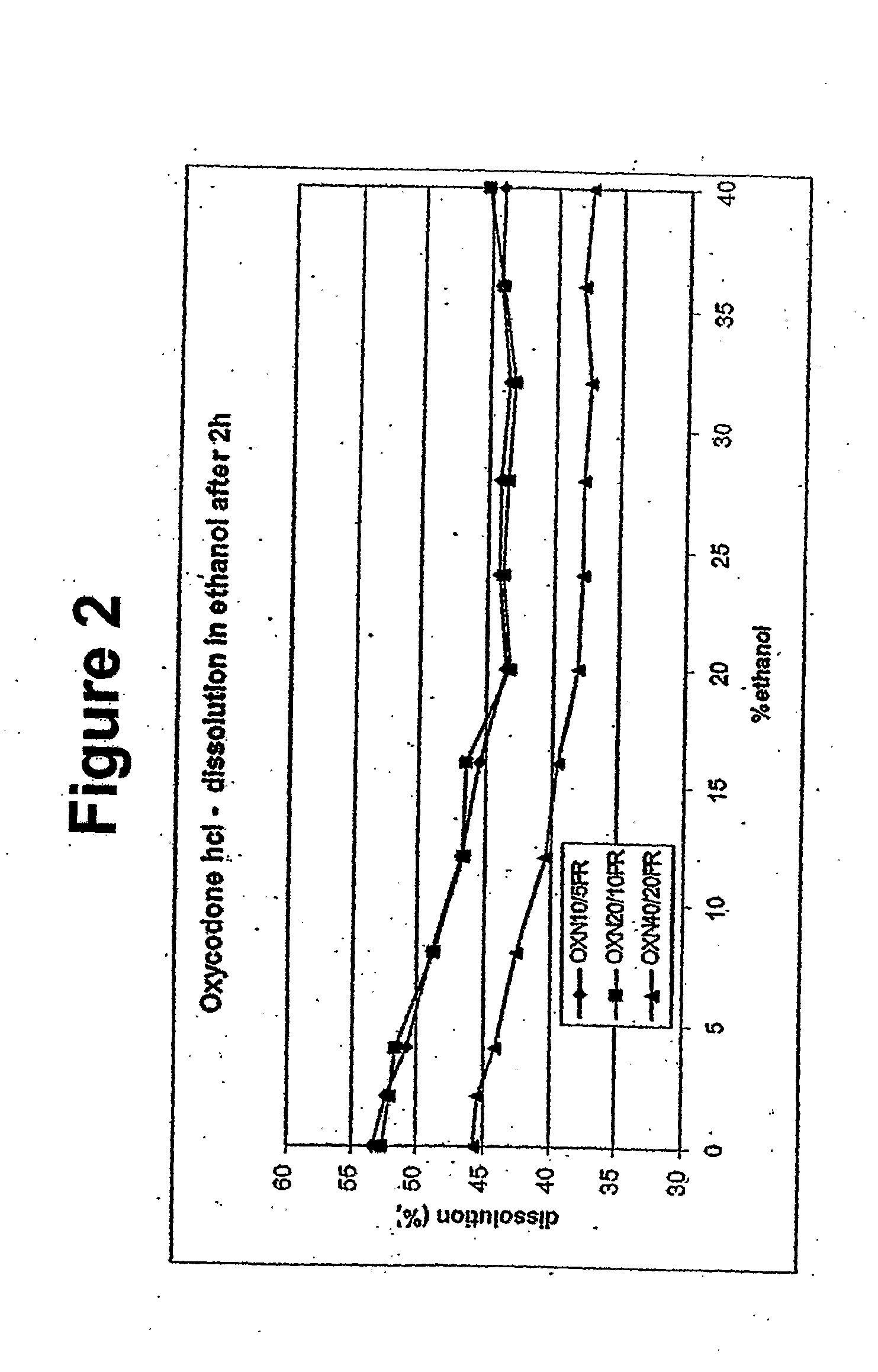
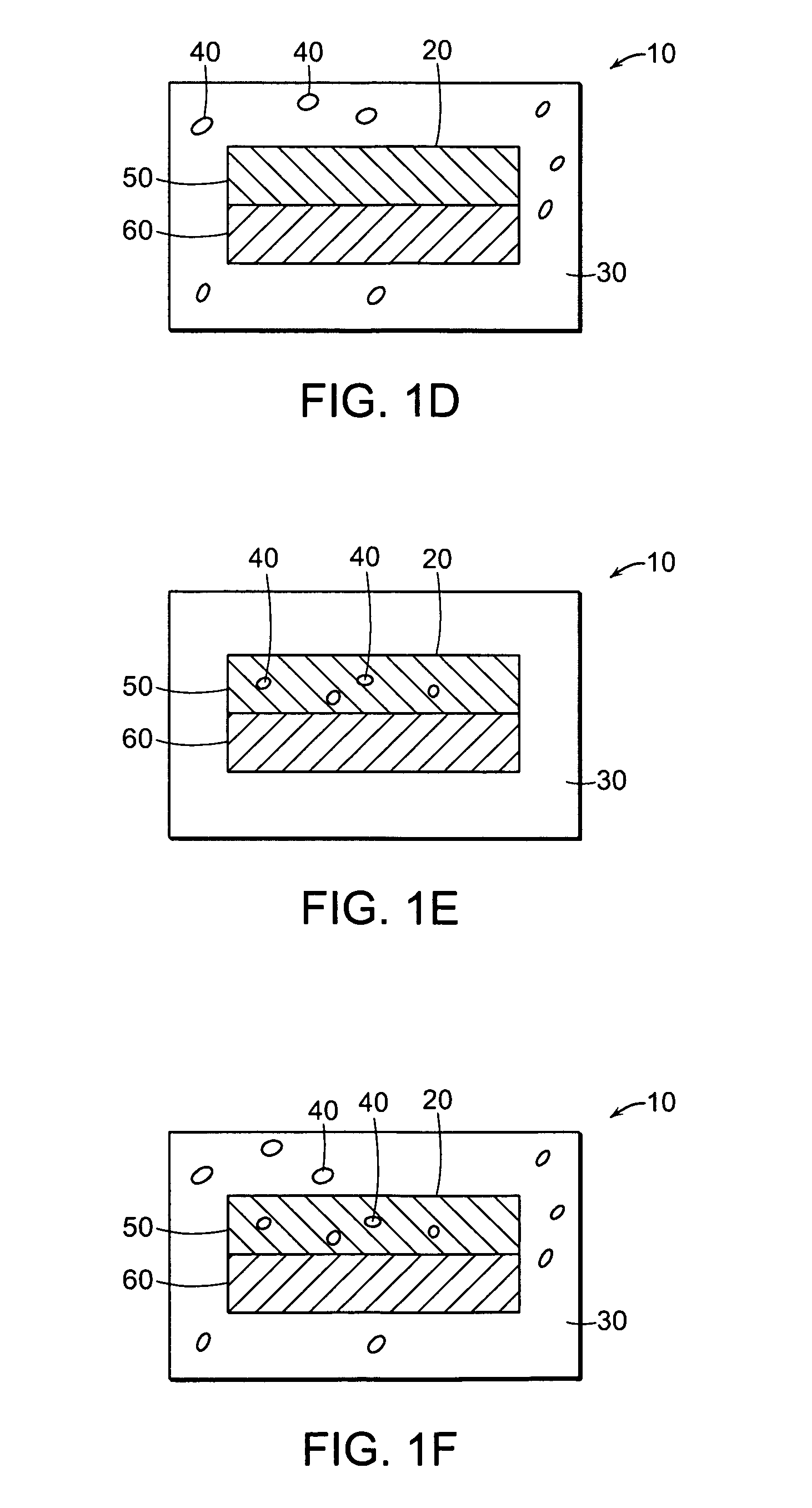
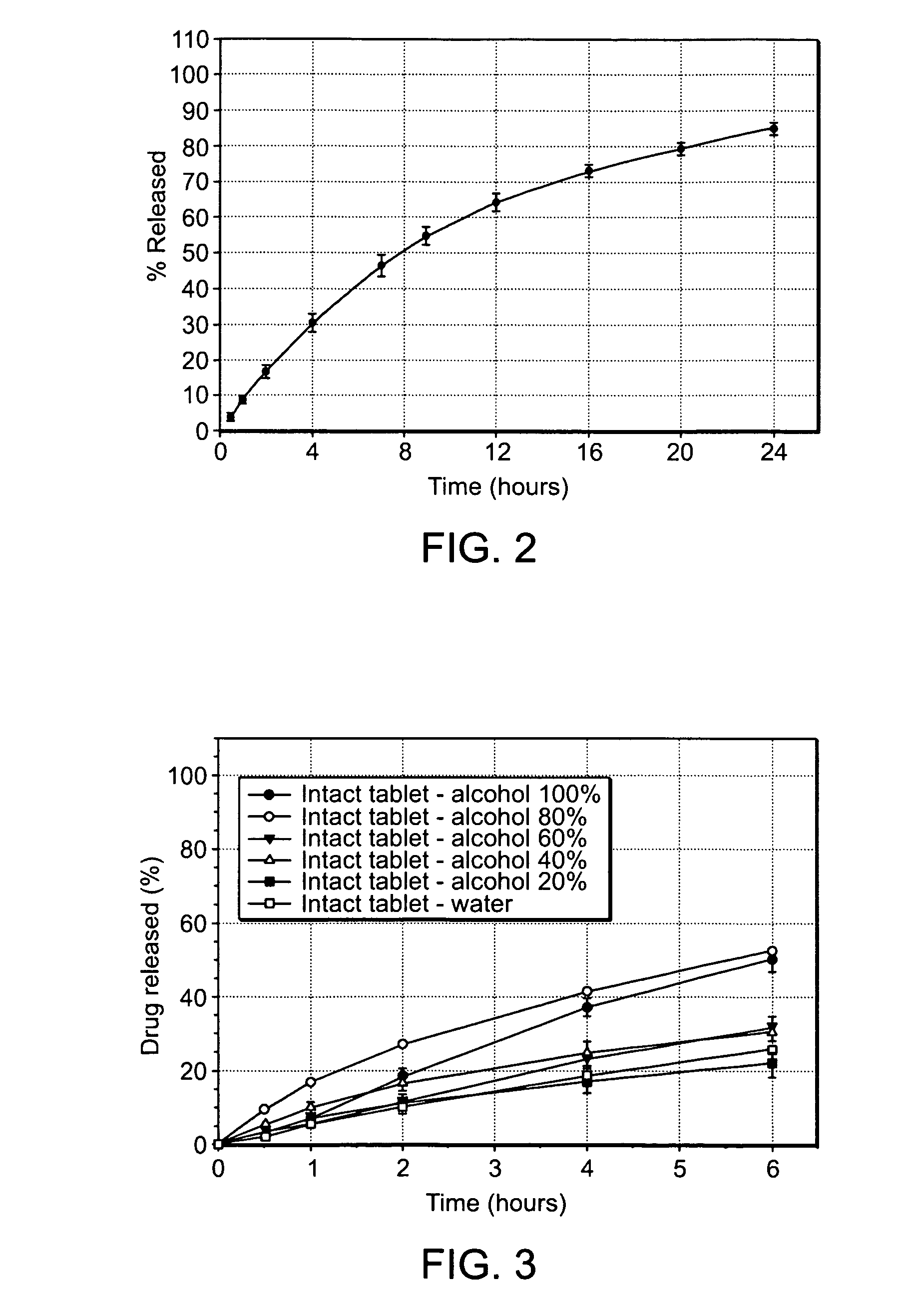
Alcohol Resistant Dosage Forms
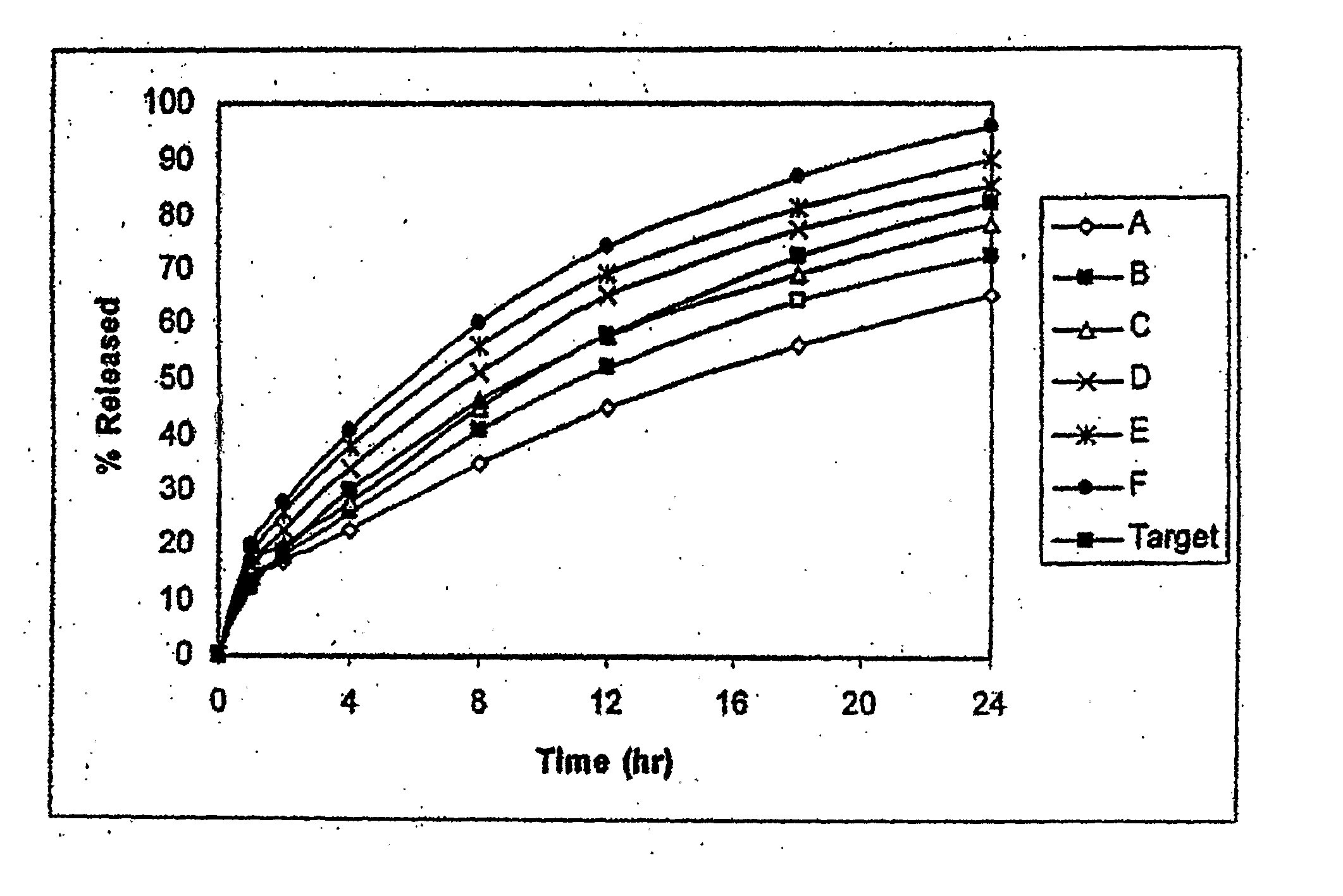
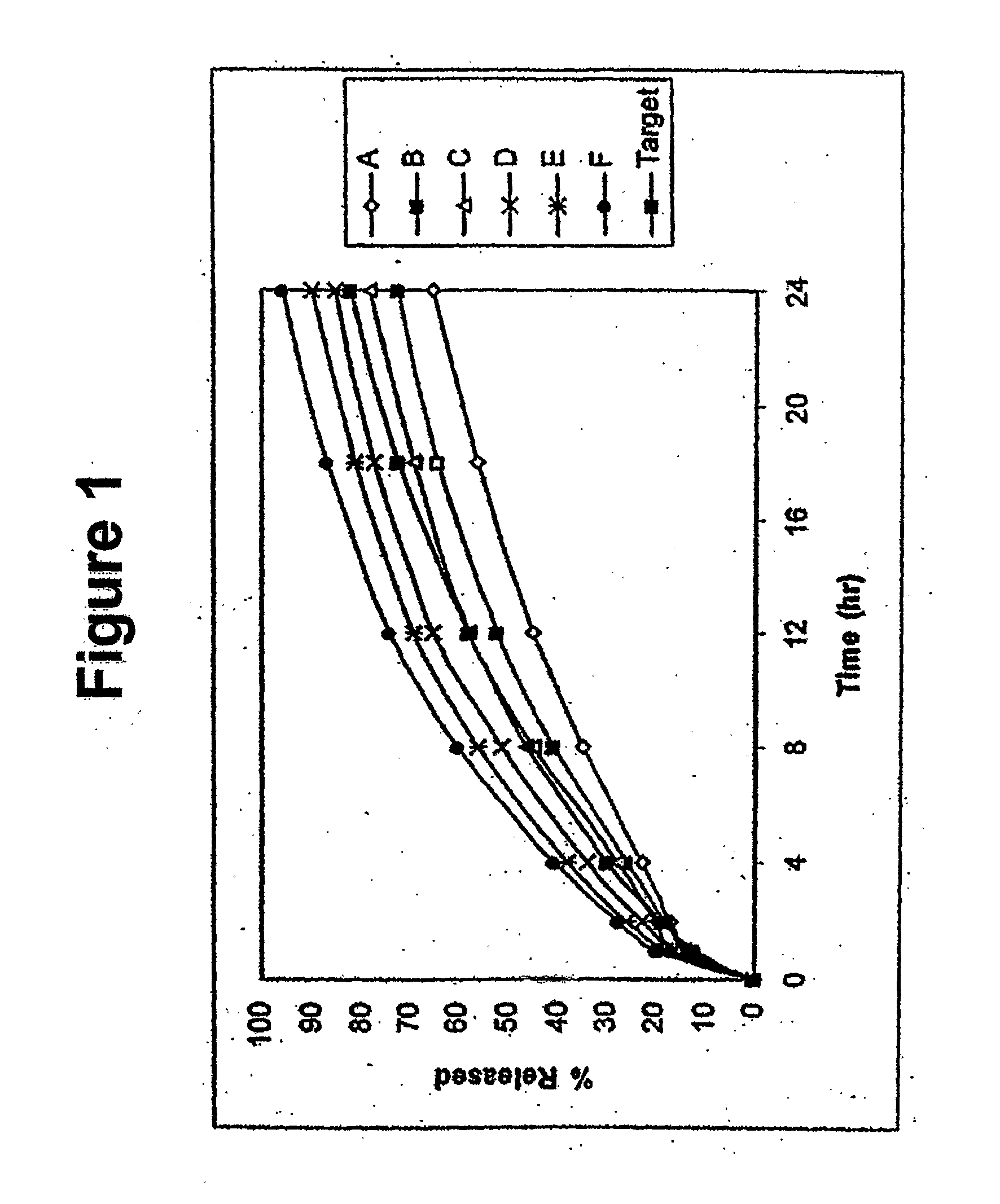
Disclosed in certain embodiments is a controlled release dosage form comprising a matrix comprising a pharmaceutically acceptable salt of an opioid analgesic in a controlled release material; wherein less than 25% of the opioid salt is released after 1 hour of in-vitro dissolution of the dosage form in 900 ml of Simulated Gastric Fluid with 20% ethanol using a USP Apparatus I (basket) apparatus at 100 rpm at 37 degrees C.°.
Owner:PURDUE PHARMA LP
Dosage forms for administering combinations of drugs
The present invention is directed to dosage forms that can be used in therapeutic methods involving the oral co-administration of a combination of at least two drugs, one of which impairs gastrointestinal absorption and one of which does not. The dosage forms are designed so that the drug impairing absorption is not released into the gastrointestinal tract of a patient until after the drugs that do not impair absorption have been released and substantially absorbed. The invention may be used in treatment of migraine using a combination of triptans and NSAIDs or in the treatment of pain using a combination of NSAIDs and opioid analgesics.
Owner:POZEN INC
Misuse Preventative, Controlled Release Formulation
ActiveUS20090175937A1Control releasePowder deliveryOrganic active ingredientsActive agentMicroparticle
Disclosed is a misuse preventative, controlled release formulation comprising a core comprising a superabsorbent material (for example, polycarbophil), a controlled release coat surrounding the core, and a plurality of controlled release microparticles having a pharmaceutically active agent (for example, an opioid analgesic) disposed within the core, the coat, or both the core and the coat. When crushed, either intentionally or accidentally, and exposed to an aqueous medium, the superabsorbent material present in the core swells to encapsulate the microparticles, which remain substantially intact thereby retarding the release of the pharmaceutically active agent from the formulation. Also disclosed is a method of using the misuse preventative, controlled release formulation to deliver a pharmaceutically active agent to a mammal, for example, a human, in need thereof.
Owner:LABOPHARM BARBADOS LTD 36646
Dosage forms for administering combinations of drugs
InactiveUS20070207200A1Efficient and rapid deliveryImpairs absorptionOrganic active ingredientsNervous disorderCo administrationGastrointestinal absorption
The present invention is directed to dosage forms that can be used in therapeutic methods involving the oral co-administration of a combination of at least two drugs, one of which impairs gastrointestinal absorption and one of which does not. The dosage forms are designed so that the drug impairing absorption is not released into the gastrointestinal tract of a patient until after the drugs that do not impair absorption have been released and substantially absorbed. The invention may be used in treatment of migraine using a combination of triptans and NSAIDs or in the treatment of pain using a combination of NSAIDs and opioid analgesics.
Owner:POZEN INC
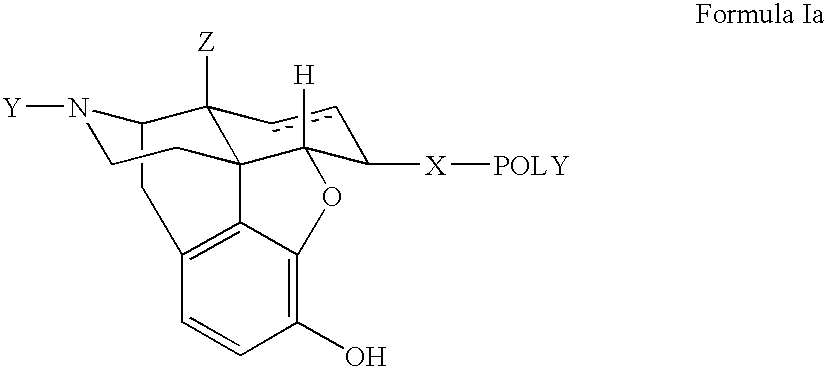
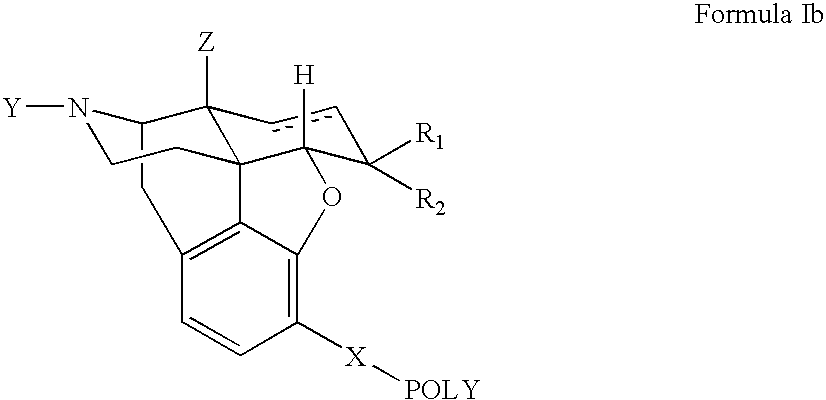
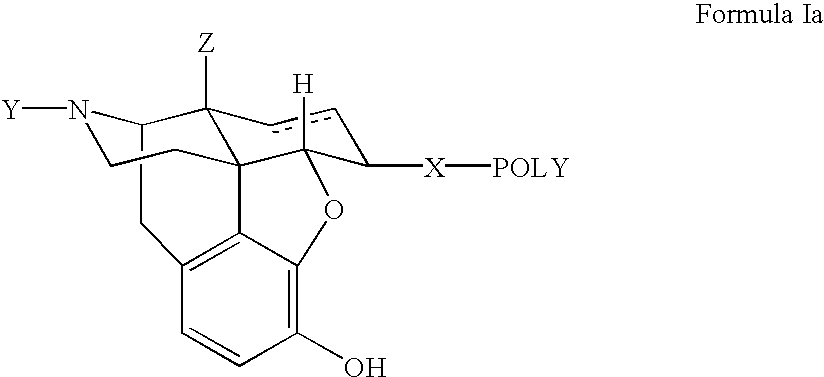
Polymer conjugates of opioid antagonists
The invention provides polymer conjugates of opioid antagonists comprising a polymer, such as poly(ethylene glycol), covalently attached to an opioid antagonist. The linkage between the polymer and the opioid antagonist is preferably hydrolytically stable. The invention also includes a method of treating one or more side effects associated with the use of opioid analgesics, such as constipation, nausea, or pruritus, by administering a polymer conjugate of the invention.
Owner:NEKTAR THERAPEUTICS INC
Programmable multi-dose intranasal drug delivery service
InactiveUS20060021614A1InterestingEasily interfaceRespiratorsLiquid surface applicatorsMedicineNasal spray
An apparatus and method for the self-administration of a plurality of doses of an intranasal liquid pharmaceutical composition, including opioid analgesics, that includes a drug delivery device containing a plurality of sealed vials, each vial containing a predetermined volume of the pharmaceutical composition, a pump assembly for conveying the liquid pharmaceutical composition from the interior of the vial and discharging it as a nasal spray in response to manual activation by the patient, and programmable means for sequentially advancing a vial to the ready position after passage of a prescribed time interval following the last activation of the delivery device.
Owner:UNIV OF KENTUCKY RES FOUND
Orally administrable opioid formulations having extended duration of effect
InactiveUS20020081333A1Effective steady-state blood levelPowder deliveryBiocideBlood levelOral medication
Owner:PURDUE PHARMA LP
Methods and compositions for treating distress dysfunction and enhancing safety and efficacy of specific medications
InactiveUS20110159048A1Good treatment effectEliminate side effectsBiocideNervous disorderDiseaseNeurotransmitter systems
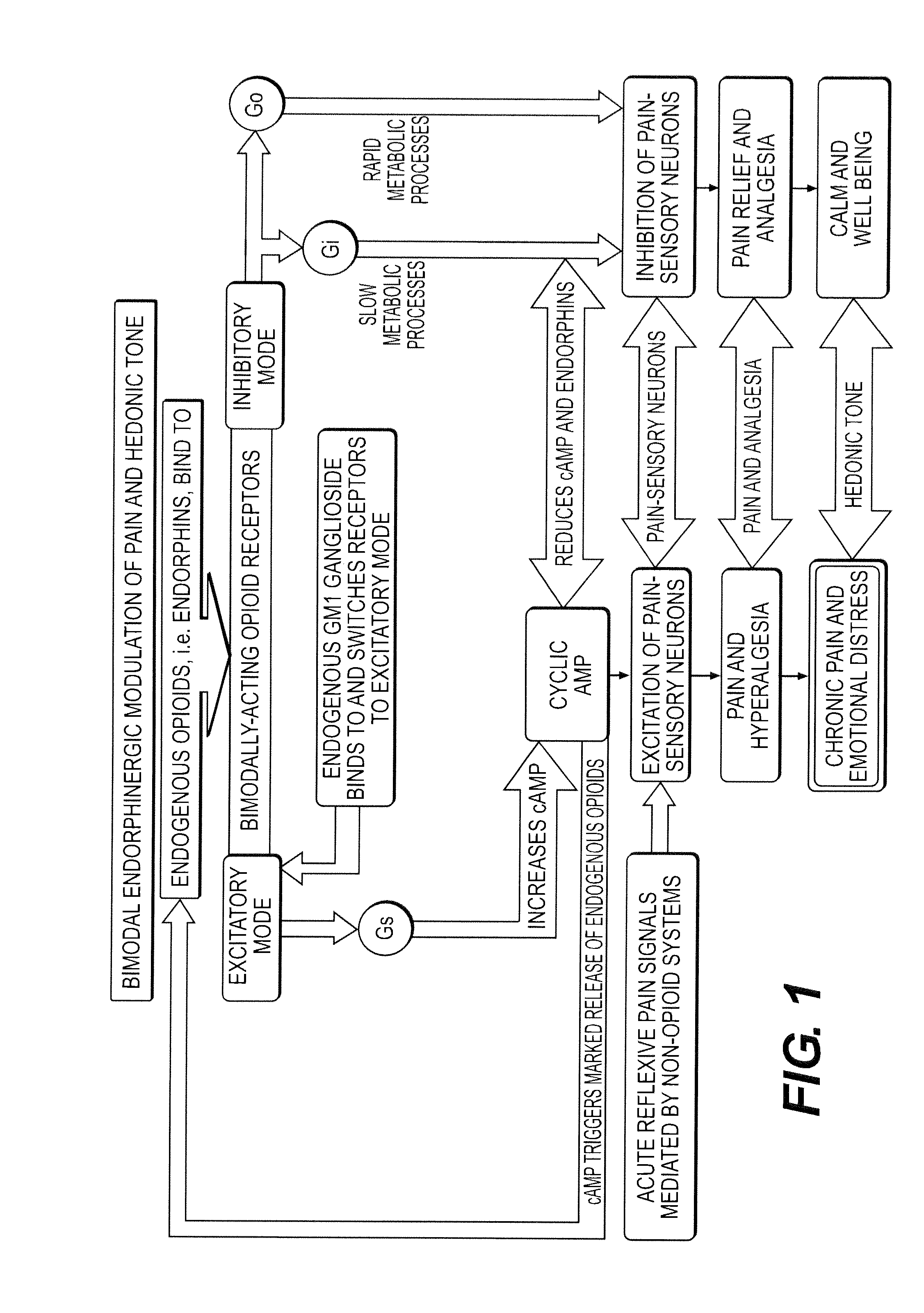
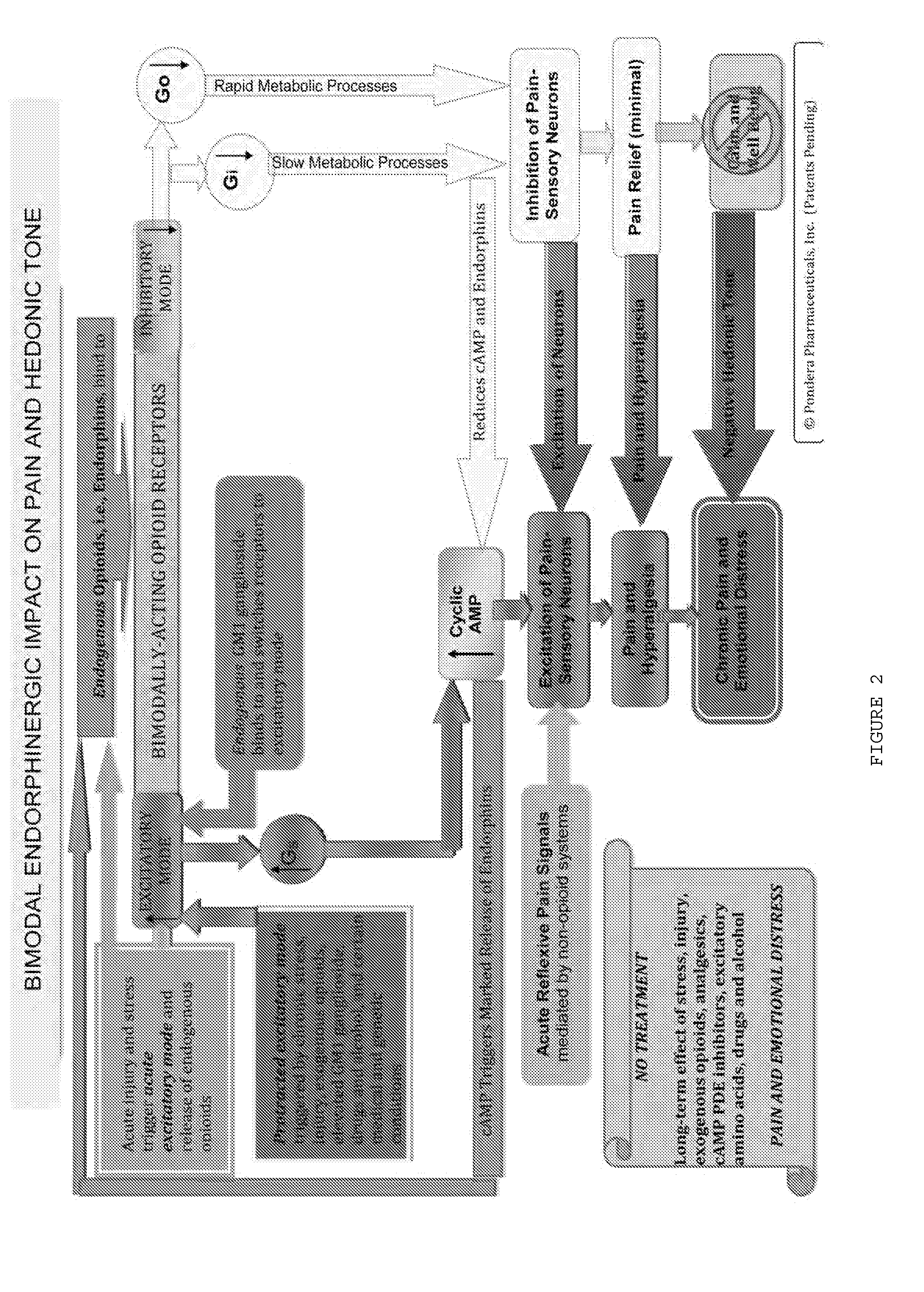
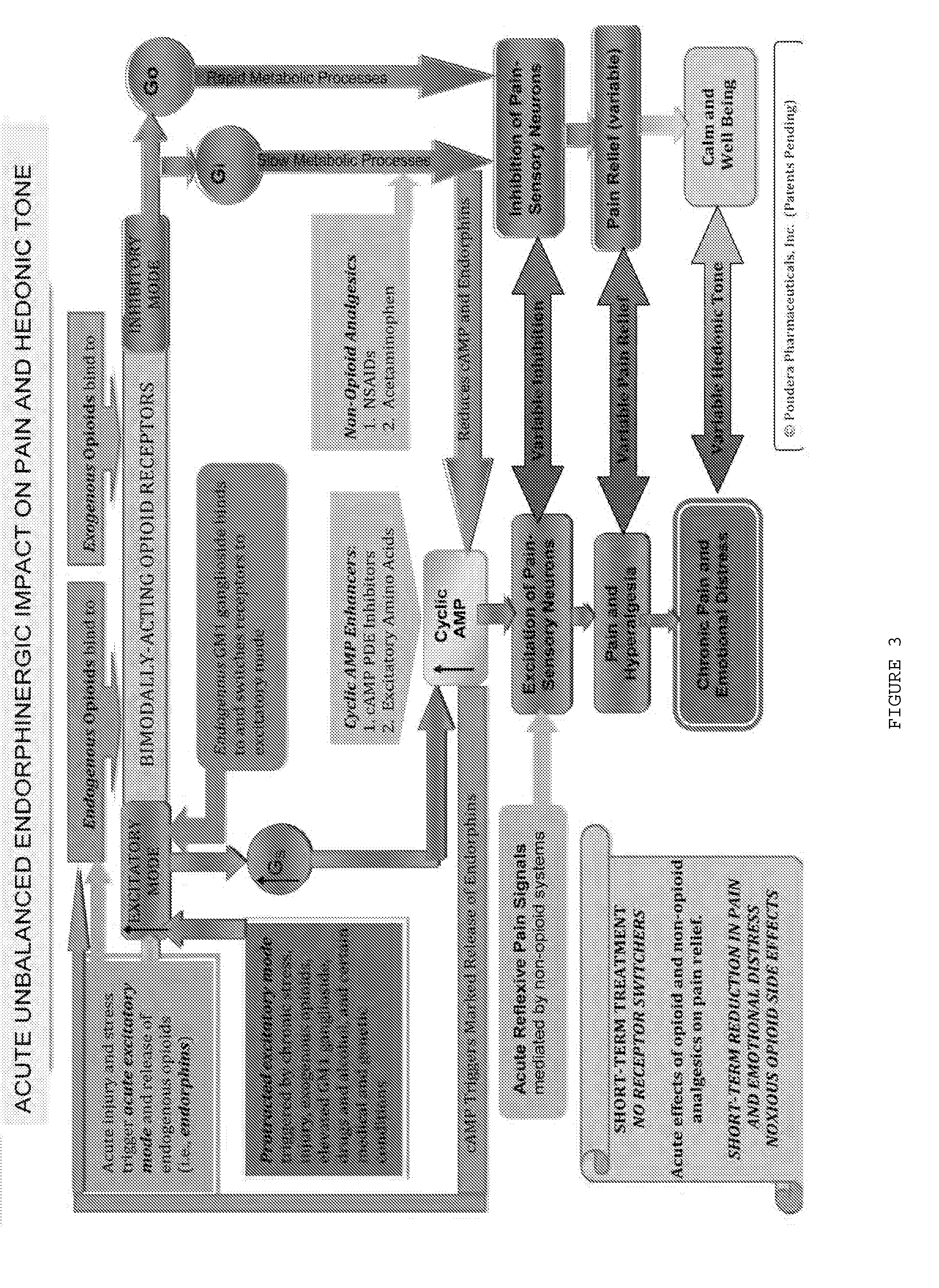
The present invention relates to methods and compositions for reducing Distress Dysfunction by restoring and maintaining homeostatic balance in the neurotransmitter systems underlying the Stress Response and the experience of distress and hedonic tone. Distress Dysfunction refers to the experience of dysfunctional emotional and physical distress that interferes with the individual's quality of life and functioning. A novel understanding of the bimodal opioid modulation of pain, and its impact, through serotonergic, dopaminergic, epinephrinergic, and norepinephrinergic processes, on hedonic tone, leads directly to new generation pharmaceutical formulations that are remarkably safe and effective for the treatment of a wide variety of Distress Dysfunctions, including anxiety, depression, anger, insomnia, mood disorders, eating disorders, sexual problems, pain, substance and behavioral addictions, gastrointestinal disorders, autistic spectrum disorders, attention-deficit and hyperactivity disorders, and other emotional and physical distress disorders. The foundation of this discovery is the power of Receptor Switchers, such as ultra-low-dose and very-low-dose opioid antagonists and GM1 ganglioside attenuators, in blocking acute and protracted excitatory opioid receptor signaling. Co-administration of Receptor Switchers with Endorphin Enhancers, such as specific cAMP PDE inhibitors and excitatory amino acids, is an excellent formulation for restoring healthy homeostatic balance to the endogenous opioid system, using the body's endorphins to reduce emotional and physical distress, and through synergistic and homeostatic processes, restoring positive hedonic tone. The addition of Synergistic Enhancers, such as amino acids, SSRI and SNRI agents, and non-opioid analgesics, as well as Exogenous Opioids, enhances and prolongs these therapeutic benefits. The novel principles discovered by this invention also teach a new generation of safe and effective formulations for the treatment of respiratory conditions, neuropathy, and nociceptive pain.
Owner:PONDERA BIOTECH
Polymer conjugates of opioid antagonists
The invention provides polymer conjugates of opioid antagonists comprising a polymer, such as poly(ethylene glycol), covalently attached to an opioid antagonist. The linkage between the polymer and the opioid antagonist is preferably hydrolytically stable. The invention also includes a method of treating one or more side effects associated with the use of opioid analgesics, such as constipation, nausea, or pruritus, by administering a polymer conjugate of the invention.
Owner:NEKTAR THERAPEUTICS INC
Abuse-resistant opioid solid dosage form
InactiveUS20060073102A1Prevent and discourage abuseGood curative effectOrganic active ingredientsPill deliveryN-methyl-D-aspartate Receptor AntagonistsOpioid abuse
The present invention pertains to a solid dosage form comprising an analgesically effective amount of opioid analgesic and an opioid abuse-deterring amount of a nontoxic N-methyl-D-aspartate receptor antagonist contained in a carrier which isolates, or separates, the antagonist from the opioid analgesic. The nontoxic N-methyl-D-aspartate receptor antagonist is released and made available only when the dosage form is misused, as would be the case when the dosage form is crushed or dissolved and thereafter administered in a manner other than that indicated, e.g., by injection or intranasally.
Owner:ENDO PHARMA INC
Pharmaceutical Formulation Containing Opioid Agonist, Opioid Antagonist and Gelling Agent
InactiveUS20150031718A1Avoid abuseReduce releaseBiocidePharmaceutical non-active ingredientsOpioid antagonistPharmaceutical formulation
Disclosed in certain embodiments is an oral dosage form comprising a therapeutically effective amount of an opioid analgesic, an opioid antagonist and one or more pharmaceutically acceptable excipients; the dosage form further including a gelling agent in an effective amount to impart a viscosity unsuitable for administration selected from the group consisting of parenteral and nasal administration to a solubilized mixture formed when the dosage form is crushed and mixed with from about 0.5 to about 10 ml of an aqueous liquid.
Owner:PURDUE PHARMA LP
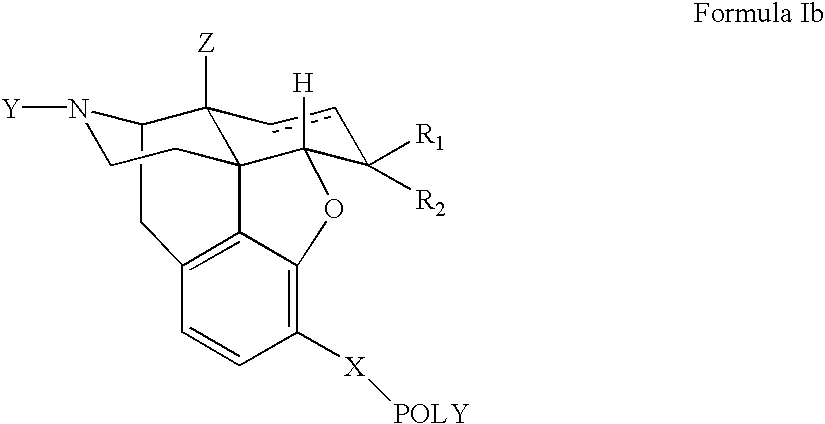
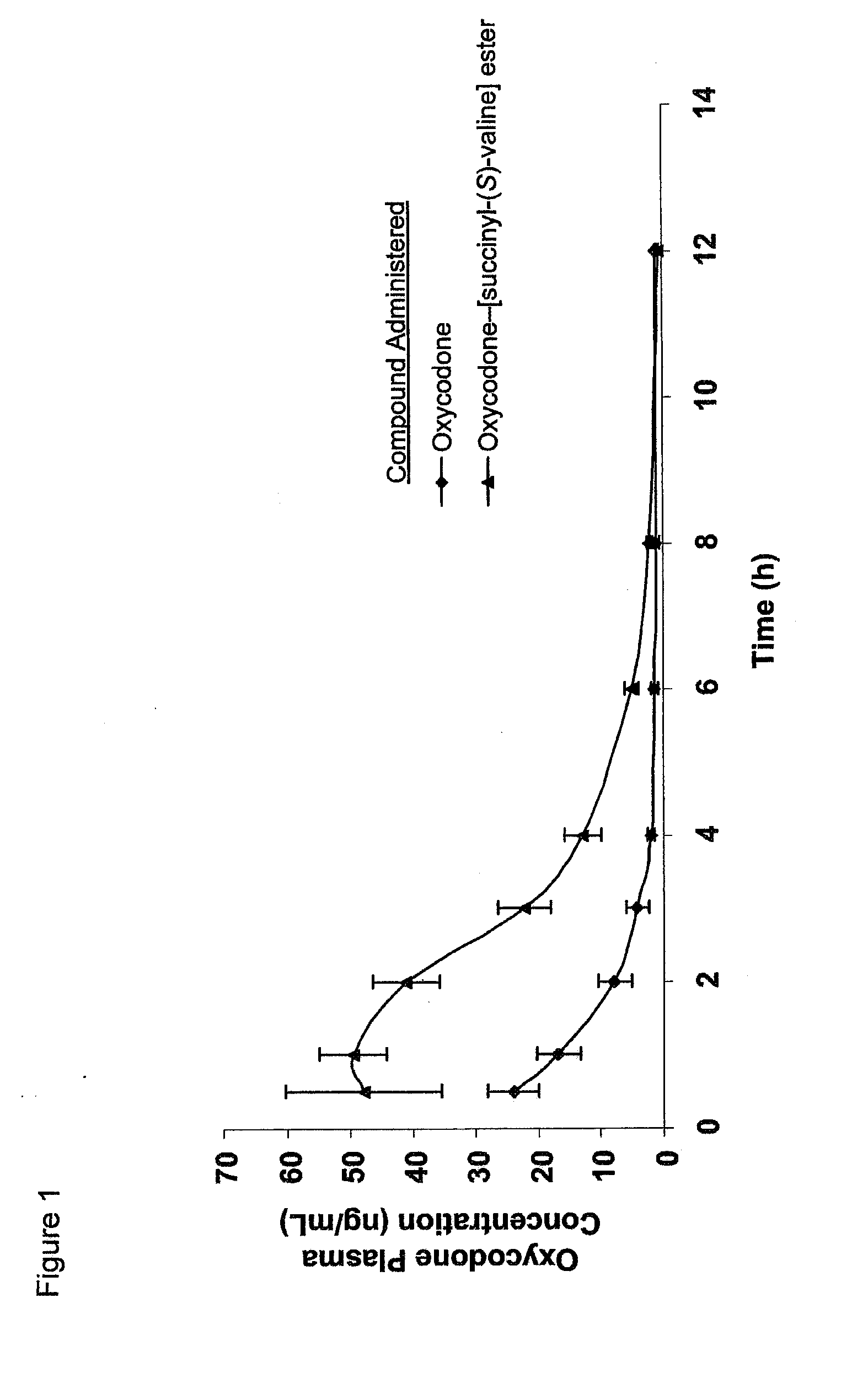
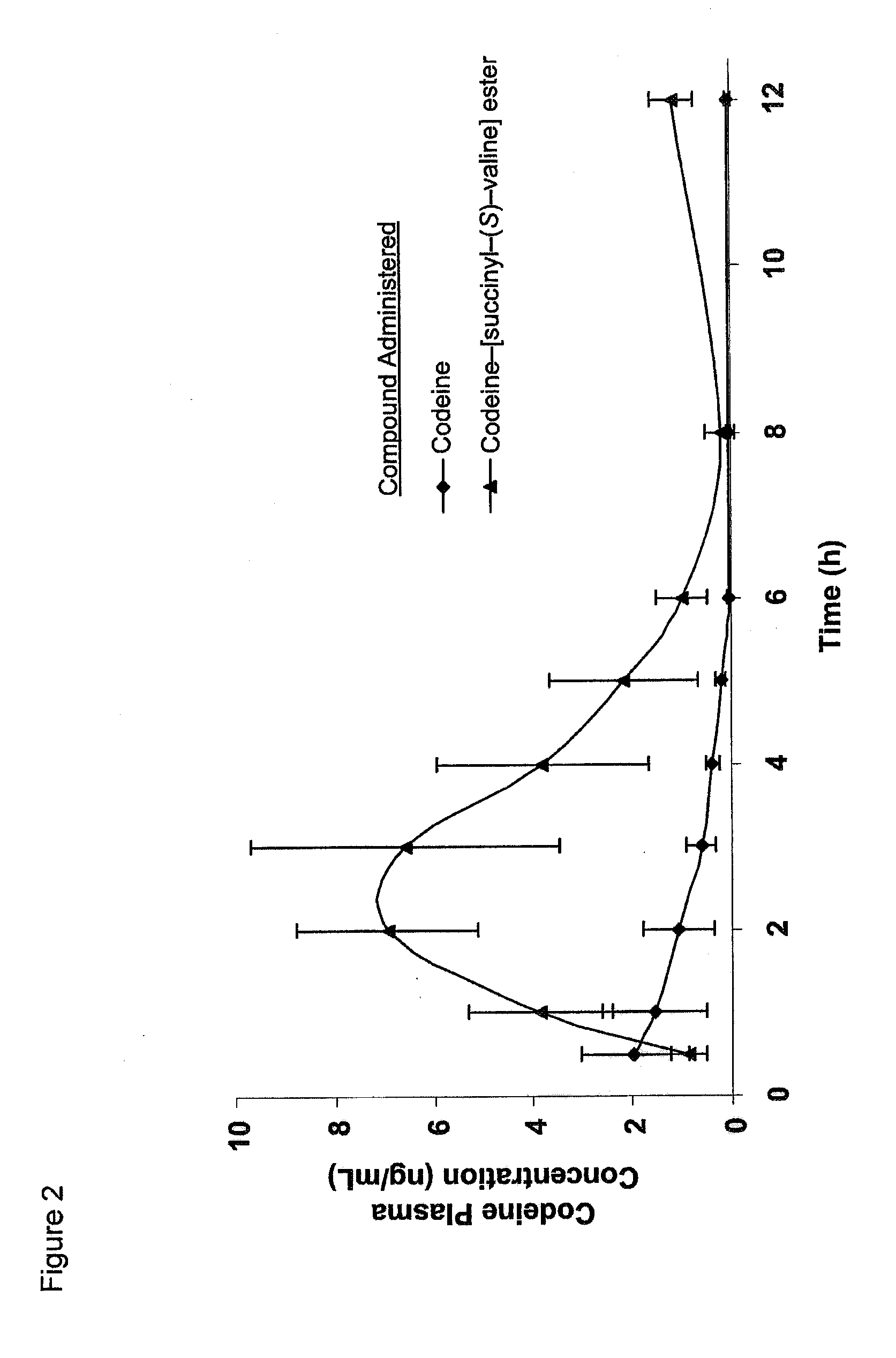
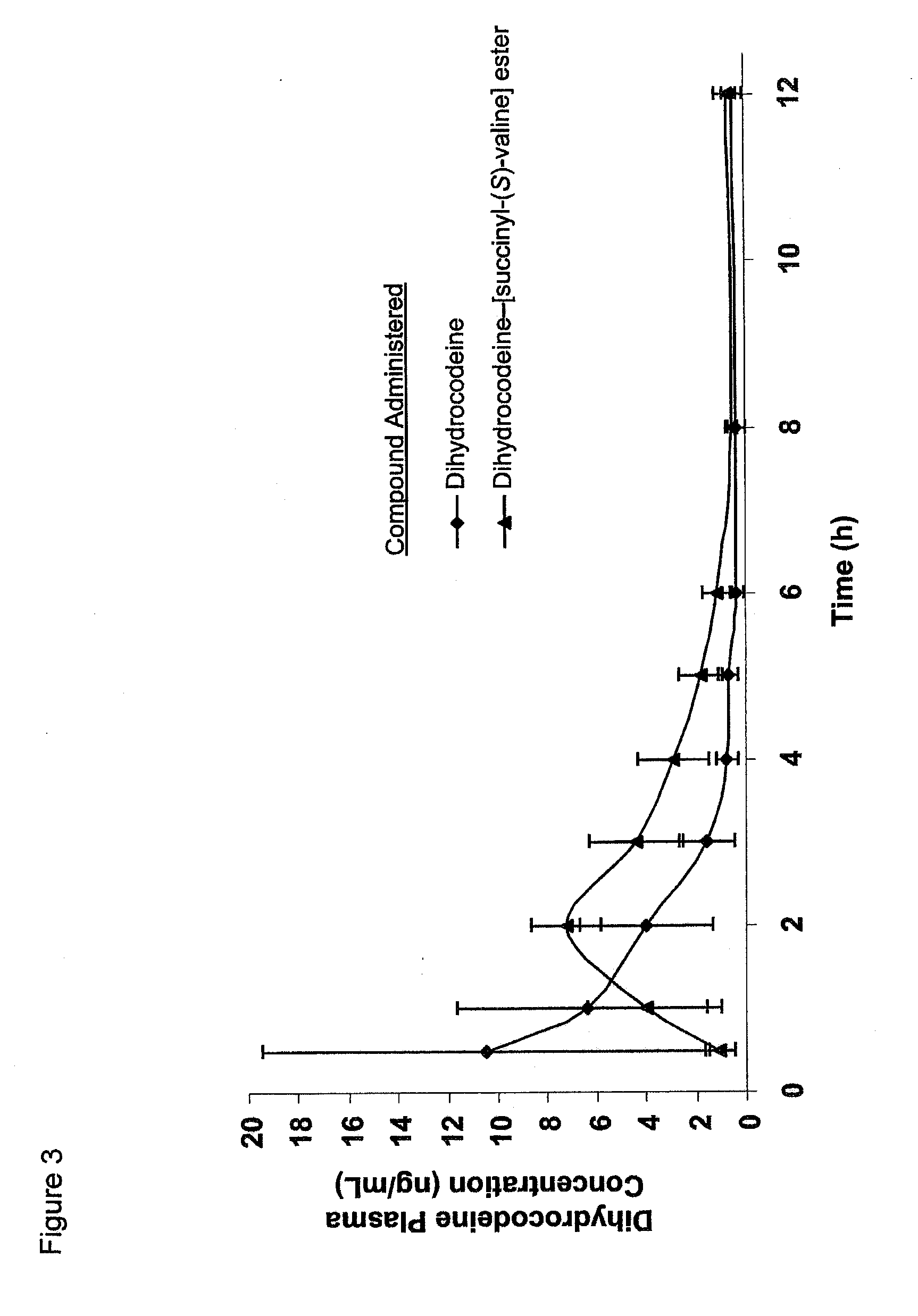
Novel dicarboxylic acid linked amino acid and peptide prodrugs of opioids and uses thereof
InactiveUS20100286186A1Low variabilityReduction and elimination of painBiocideNervous disorderSide effectAmino acid side chain
The present invention concerns dicarboxylic acid linked amino acid and peptide prodrugs of opioid analgesics and pharmaceutical compositions containing such prodrugs. Methods for providing pain relief, decreasing the adverse GI side effects of the opioid analgesic and increasing the bioavailability of the opioid analgesic with the aforementioned prodrugs are also provided. In one embodiment, prodrugs having the amino acid side chains of valine, leucine, isoleucine and glycine; and mono-, di- and tripeptides thereof are provided.
Owner:SHIRE PLC
Tamper resistant dosage forms
InactiveUS20130251796A1Reduces and prevents stickingPowder deliveryBiocideOpioid analgesicsDosage form
The present invention relates to pharmaceutical dosage forms, for example to a tamper resistant dosage form including an opioid analgesic, and processes of manufacture, uses, and methods of treatment thereof.
Owner:PURDUE PHARMA LP
Misuse preventative, controlled release formulation
ActiveUS8486448B2Control releasePowder deliveryOrganic active ingredientsControl releaseActive agent
Disclosed is a misuse preventative, controlled release formulation comprising a core comprising a superabsorbent material (for example, polycarbophil), a controlled release coat surrounding the core, and a plurality of controlled release microparticles having a pharmaceutically active agent (for example, an opioid analgesic) disposed within the core, the coat, or both the core and the coat. When crushed, either intentionally or accidentally, and exposed to an aqueous medium, the superabsorbent material present in the core swells to encapsulate the microparticles, which remain substantially intact thereby retarding the release of the pharmaceutically active agent from the formulation. Also disclosed is a method of using the misuse preventative, controlled release formulation to deliver a pharmaceutically active agent to a mammal, for example, a human, in need thereof.
Owner:LABOPHARM BARBADOS LTD 36646
Tamper resistant dosage forms
InactiveUS20130251801A1Reduces and prevents stickingBiocideNervous disorderOpioid analgesicsDosage form
The present invention relates to pharmaceutical dosage forms, for example to a tamper resistant dosage form including an opioid analgesic, and processes of manufacture, uses, and methods of treatment thereof.
Owner:PURDUE PHARMA LP
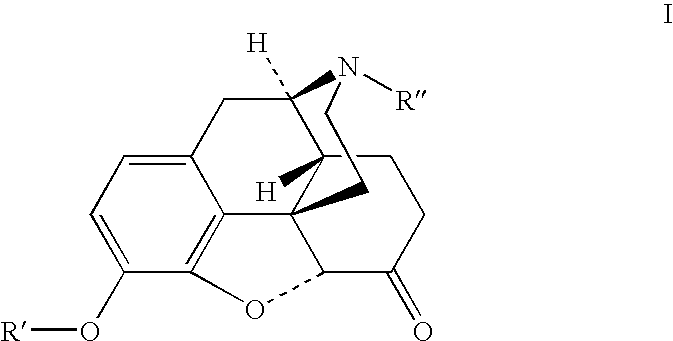
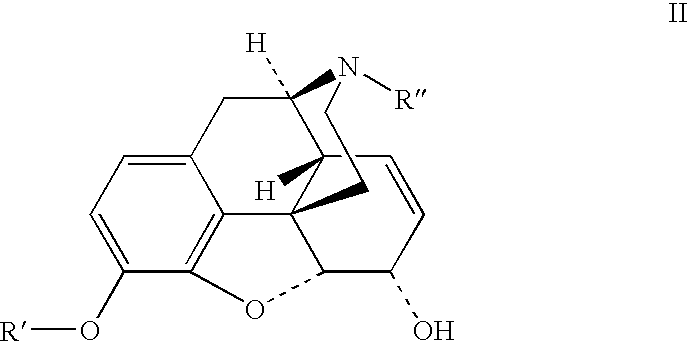
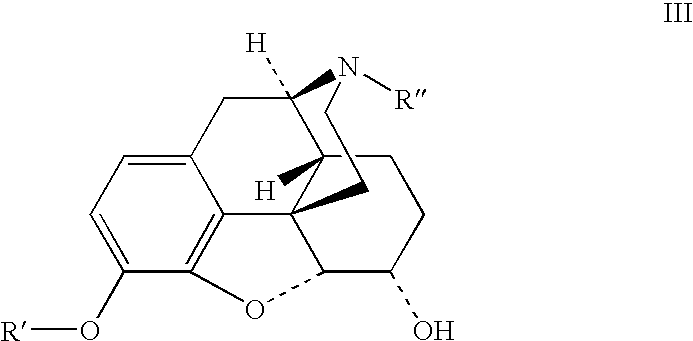
Preparation of opioid analgesics by a one-pot process
A one-pot process for preparing opioid analgesics such as hydrocodone, hydromorphone, and analogues thereof by reacting codeine, morphine, and analogues thereof with hydrogen in a solvent system of benzophenone and neutral solvent in the presence of a metal catalyst followed by oxidation in the presence of potassium tert-alkylate.
Owner:ACURA PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com