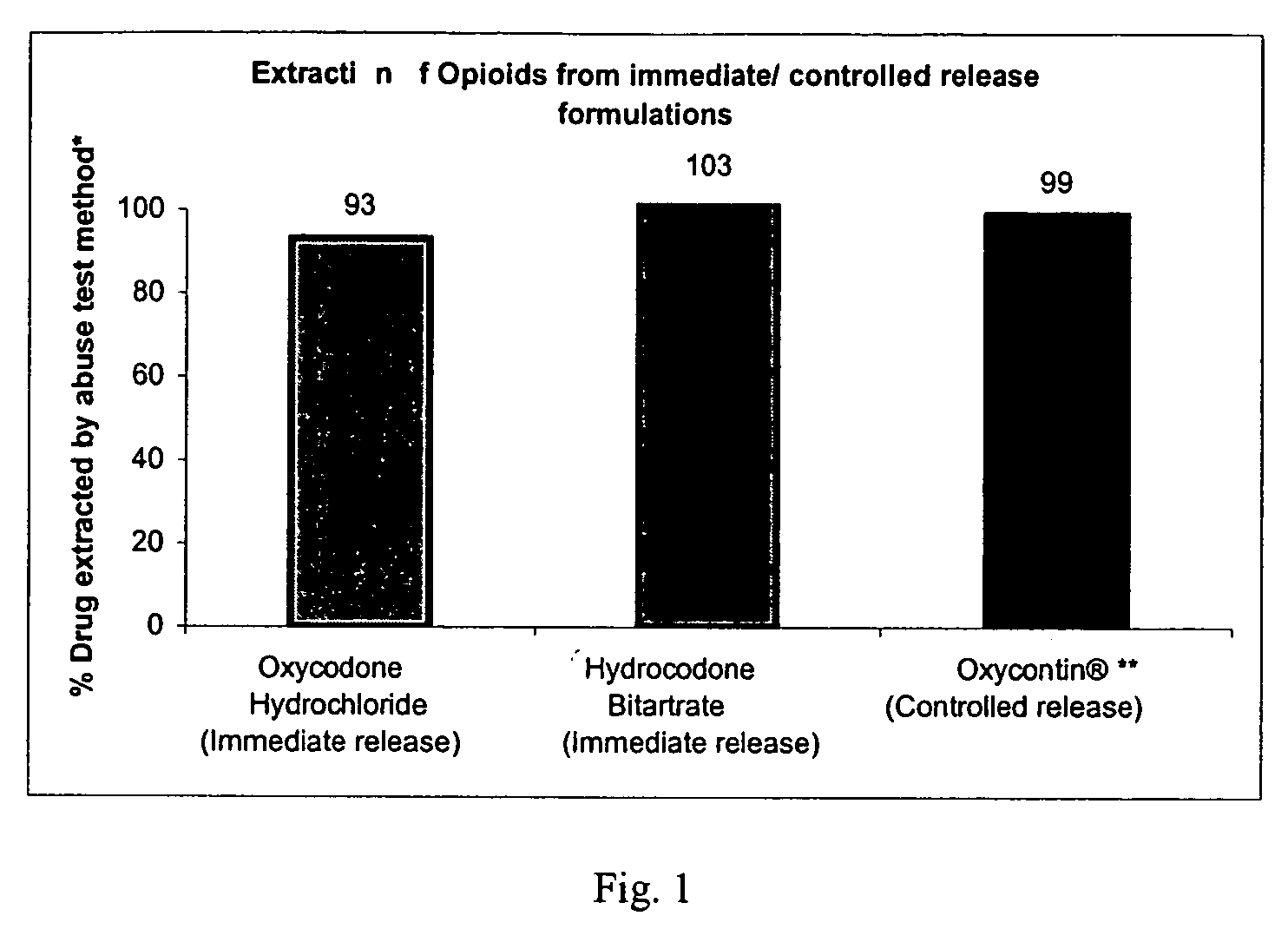
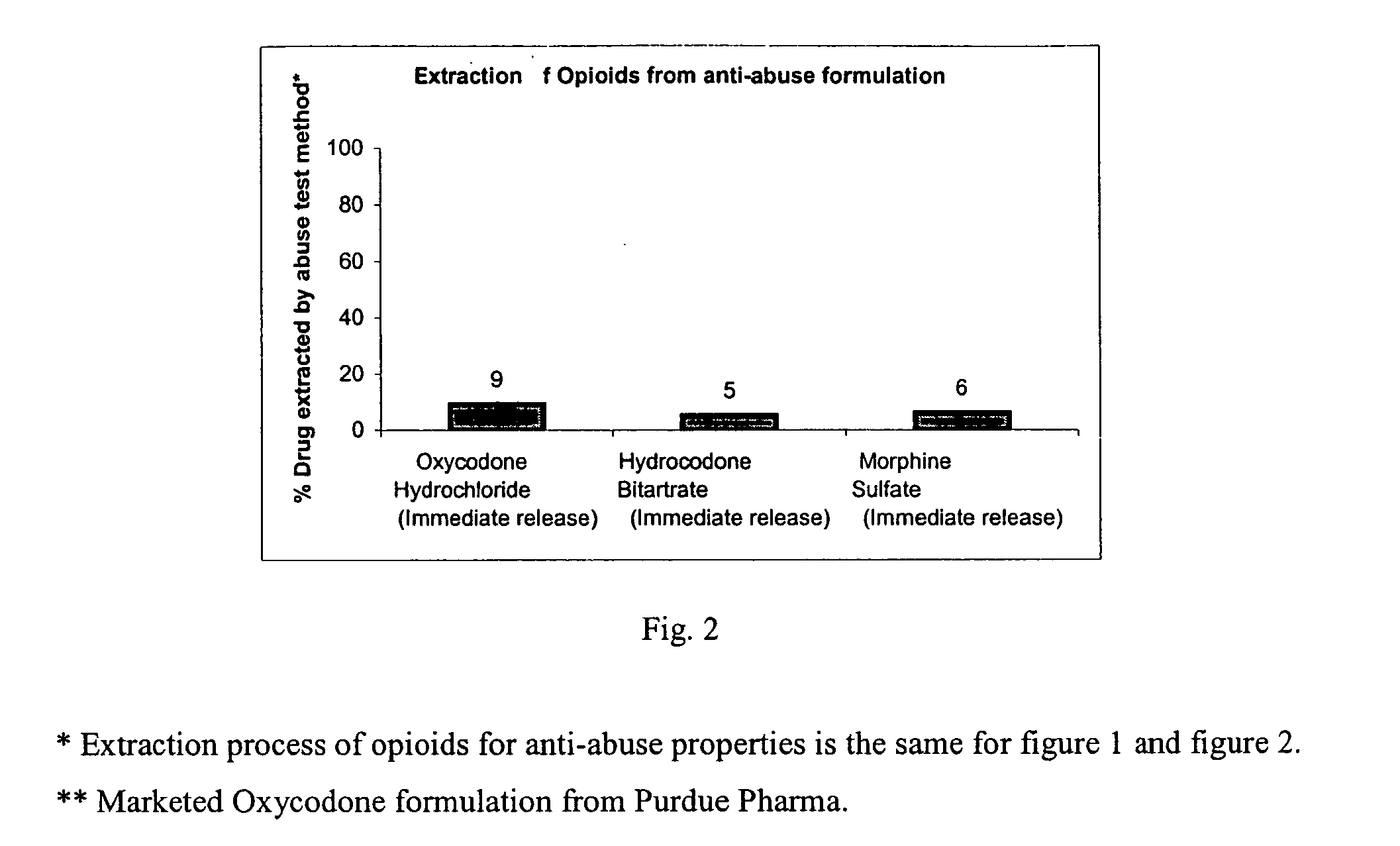
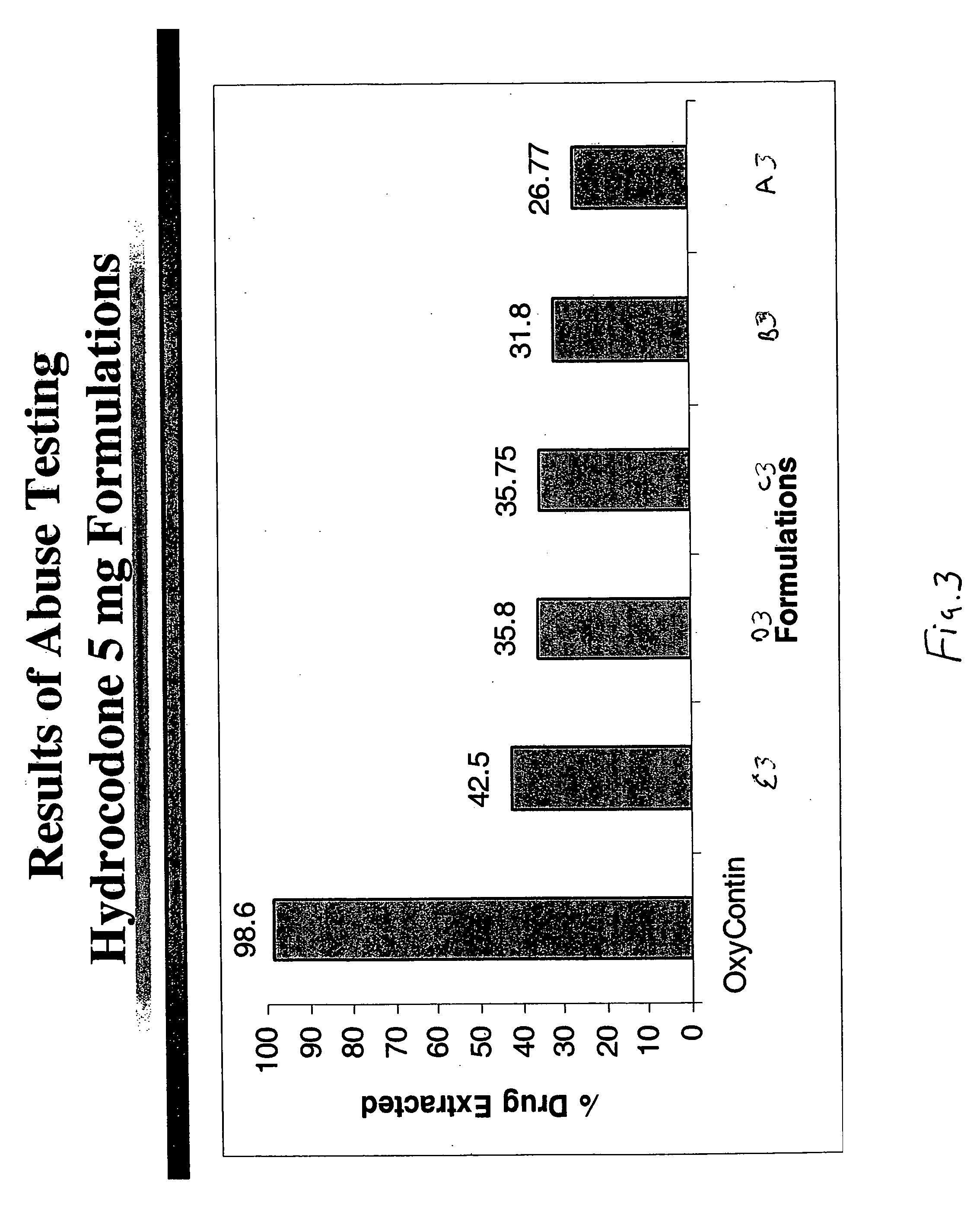
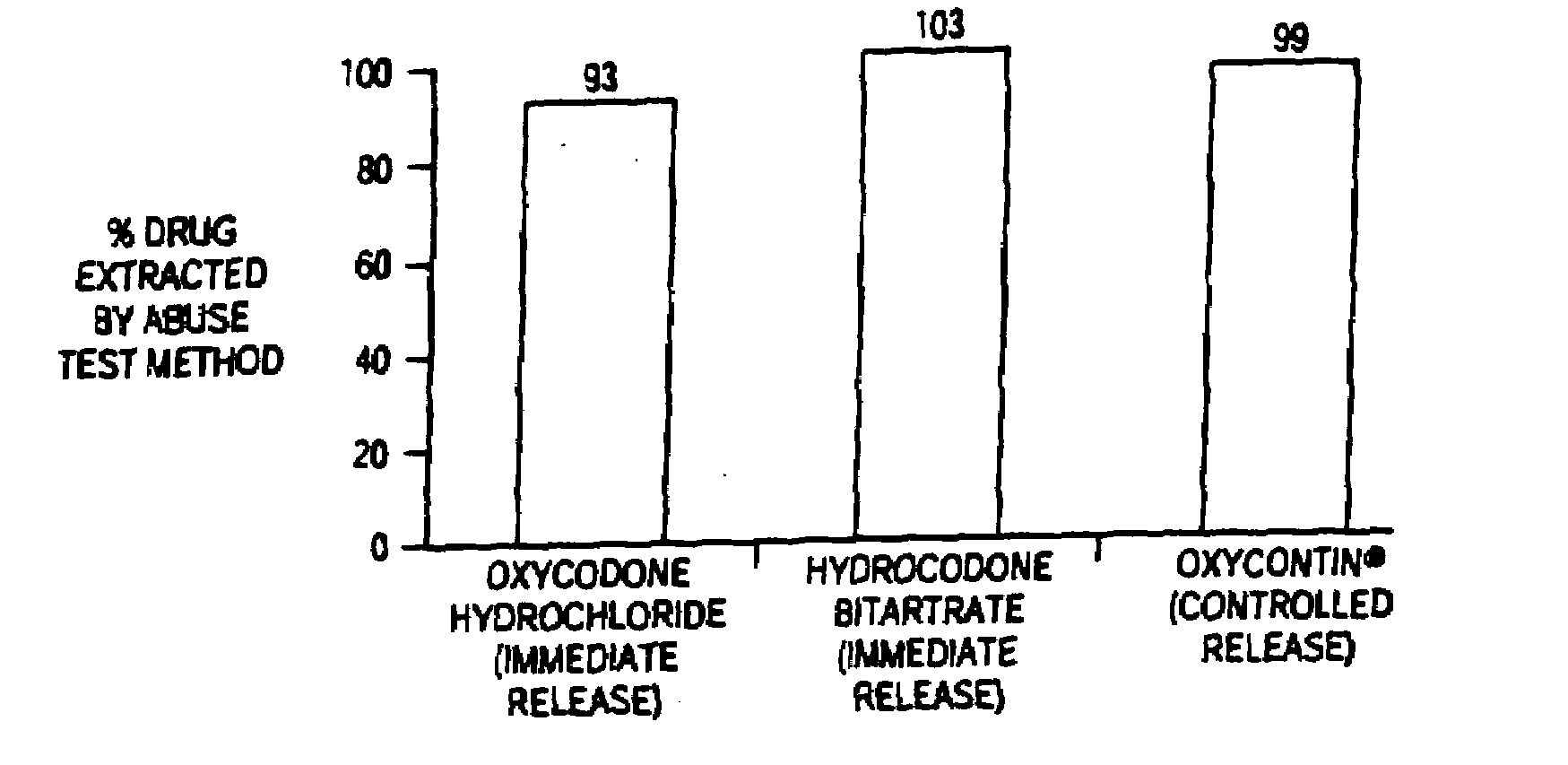
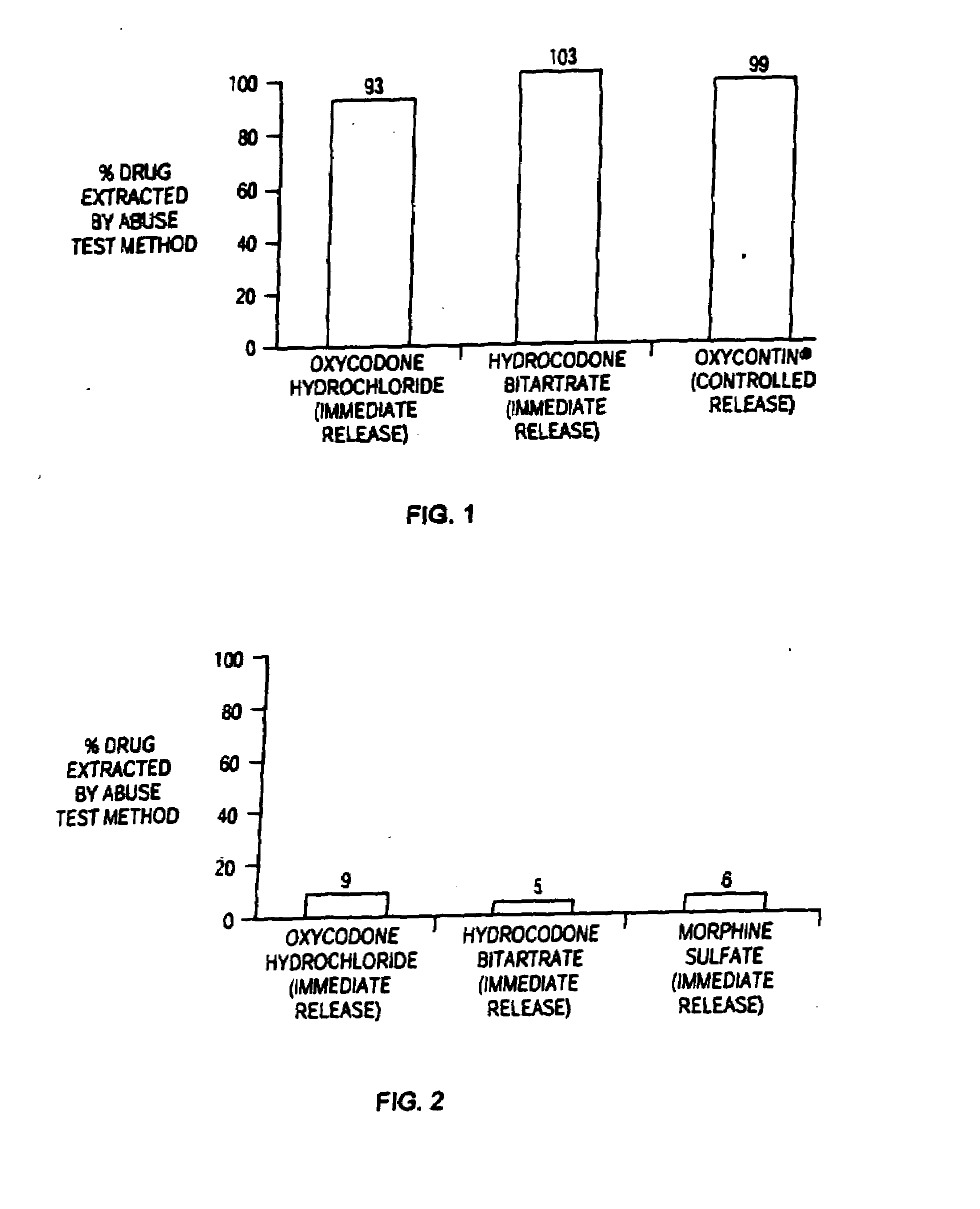
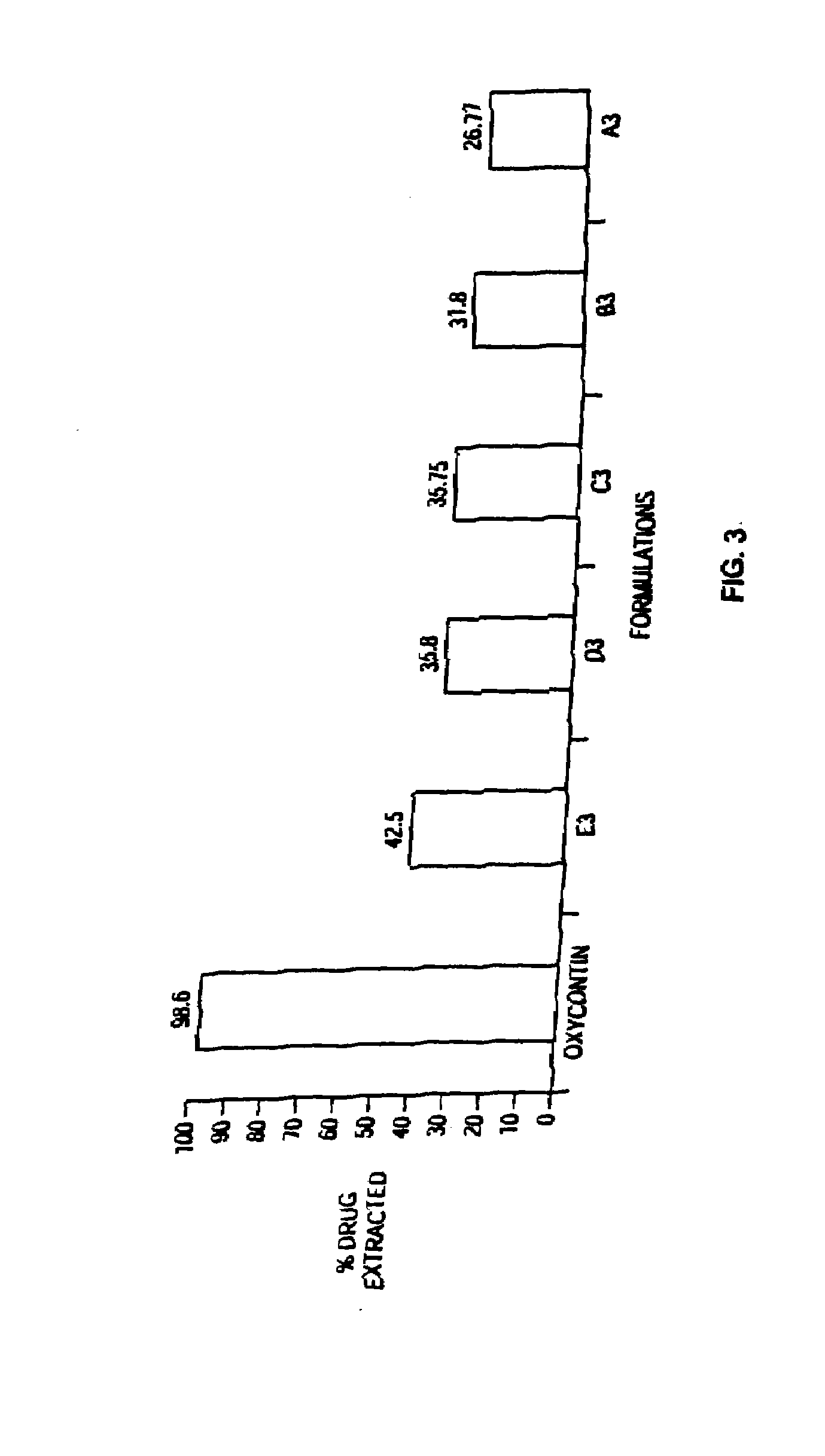
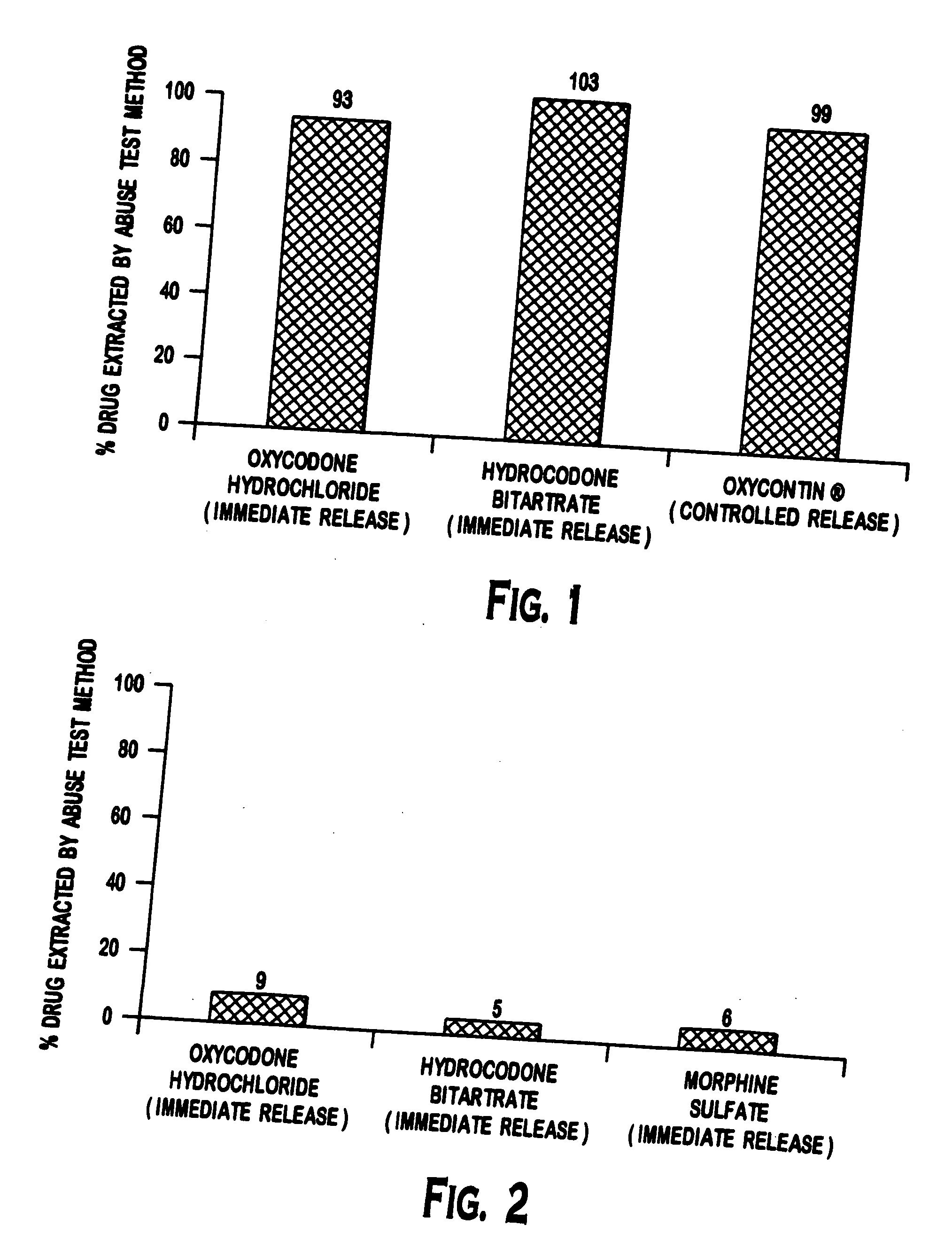
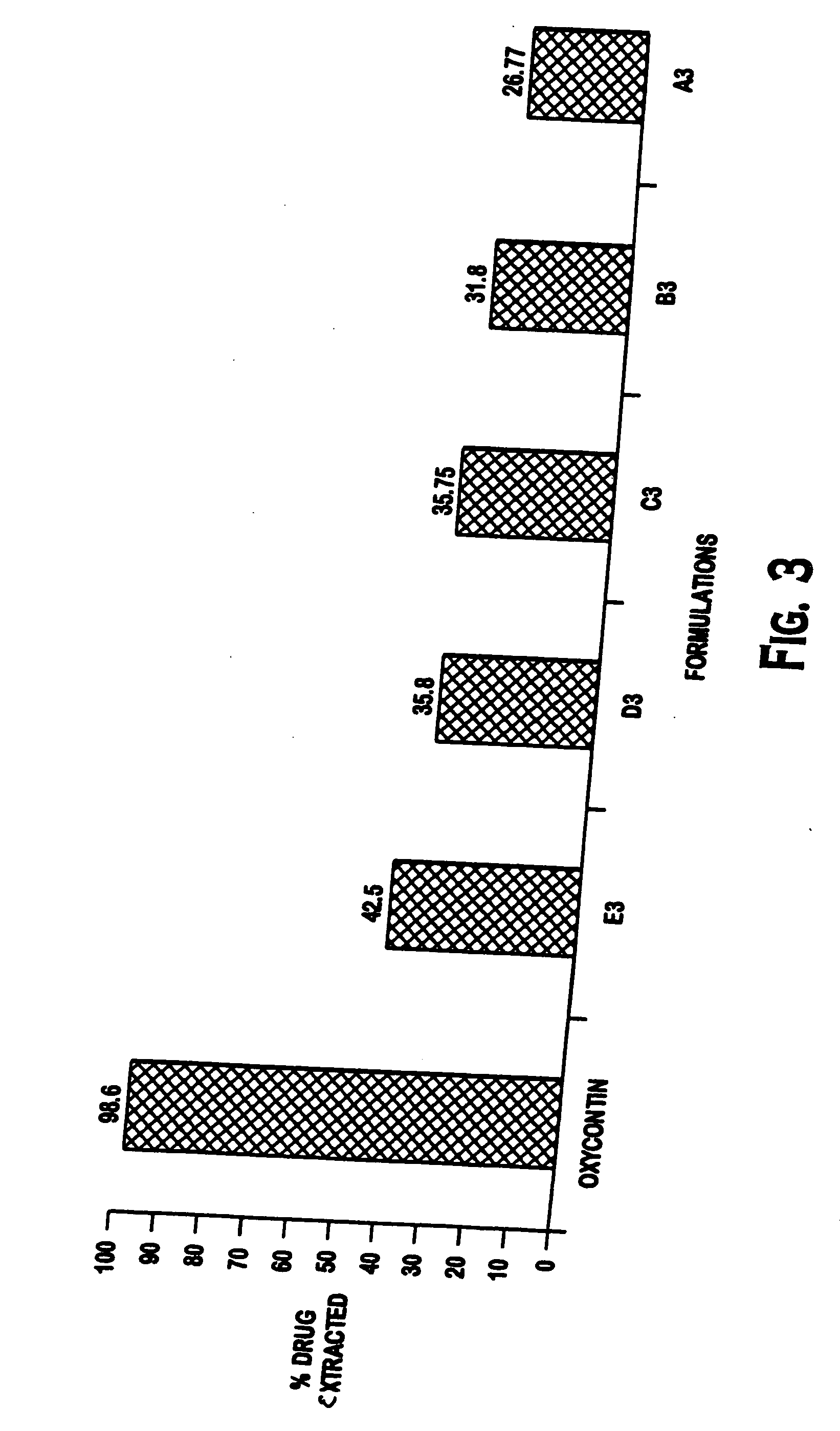
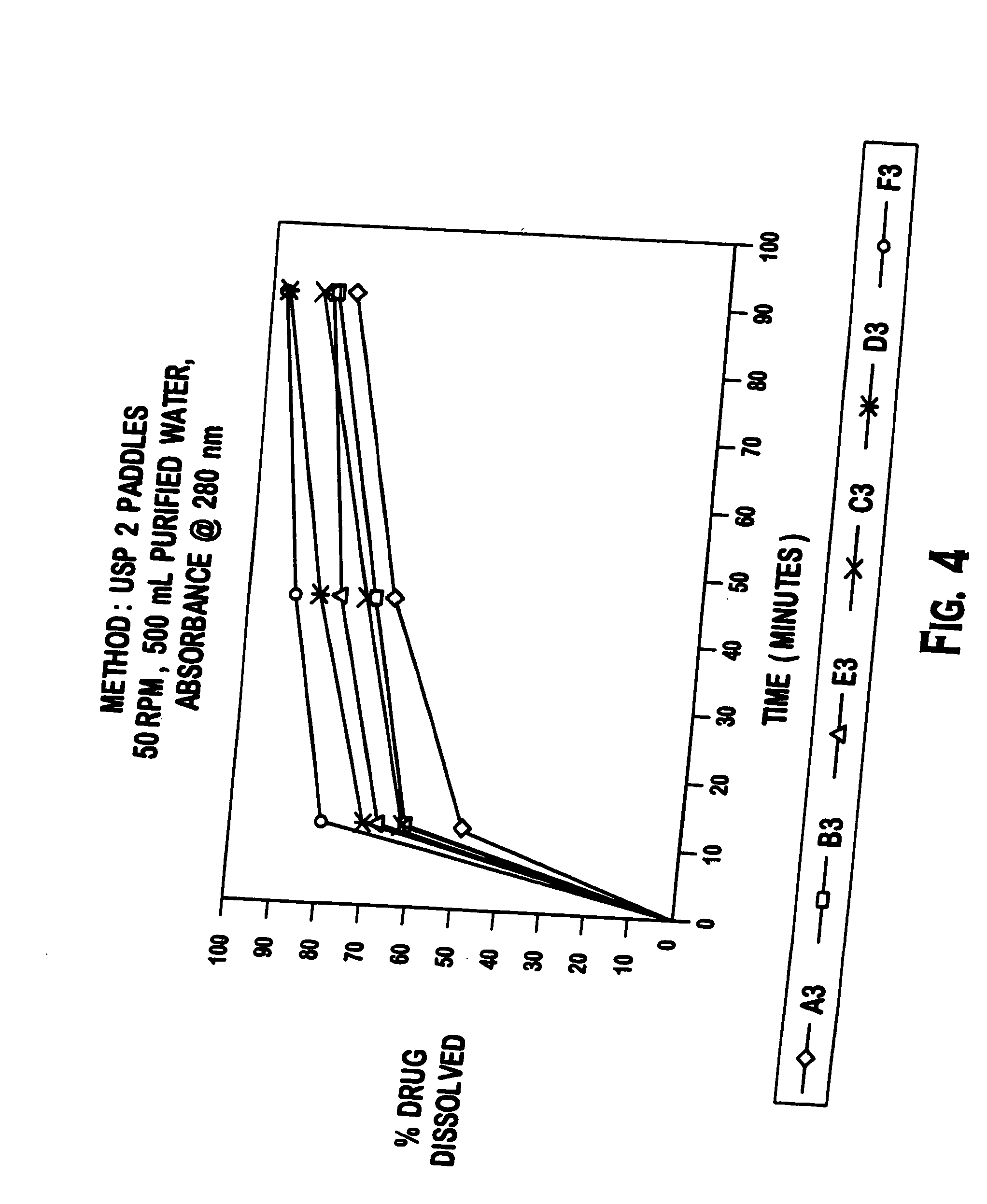
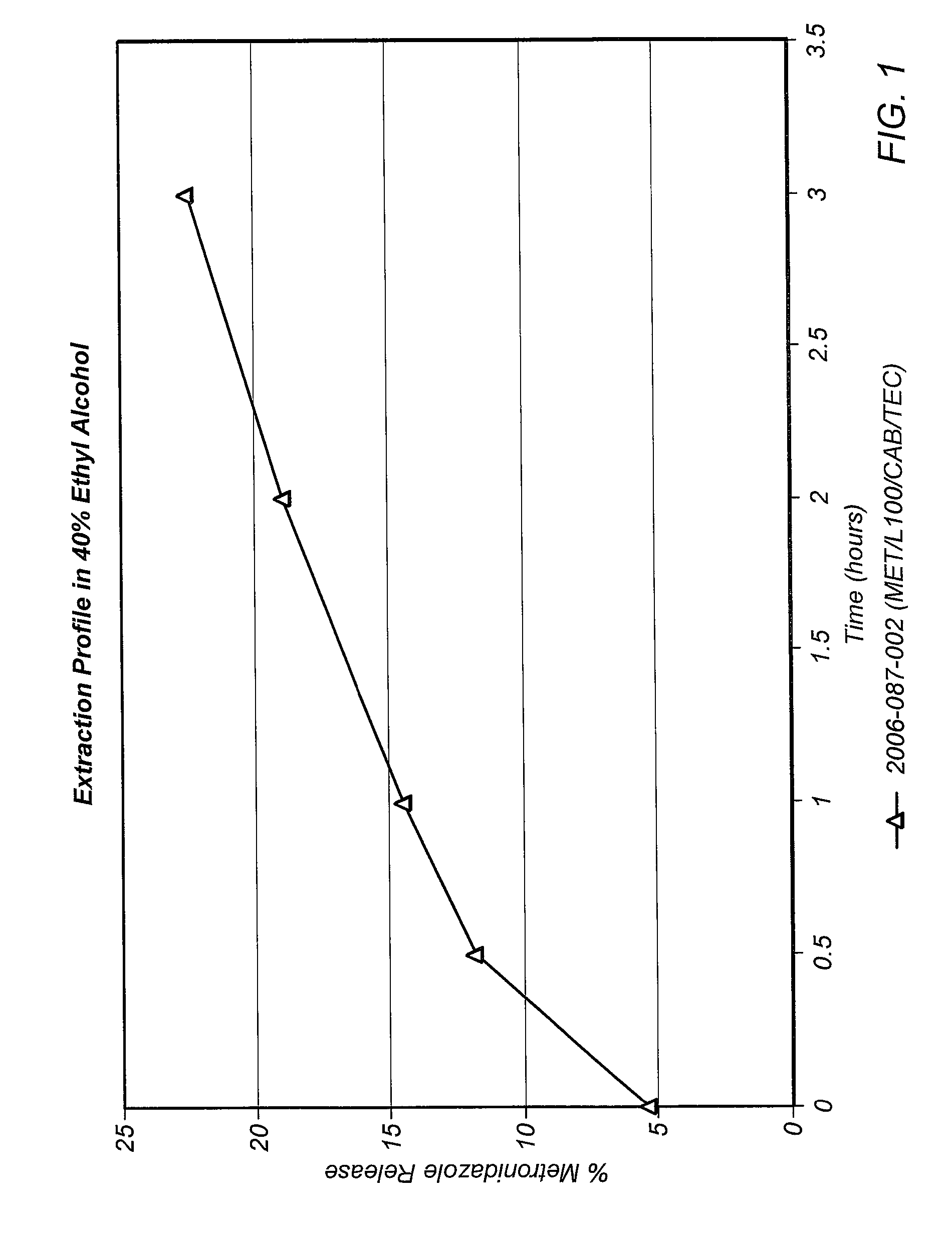
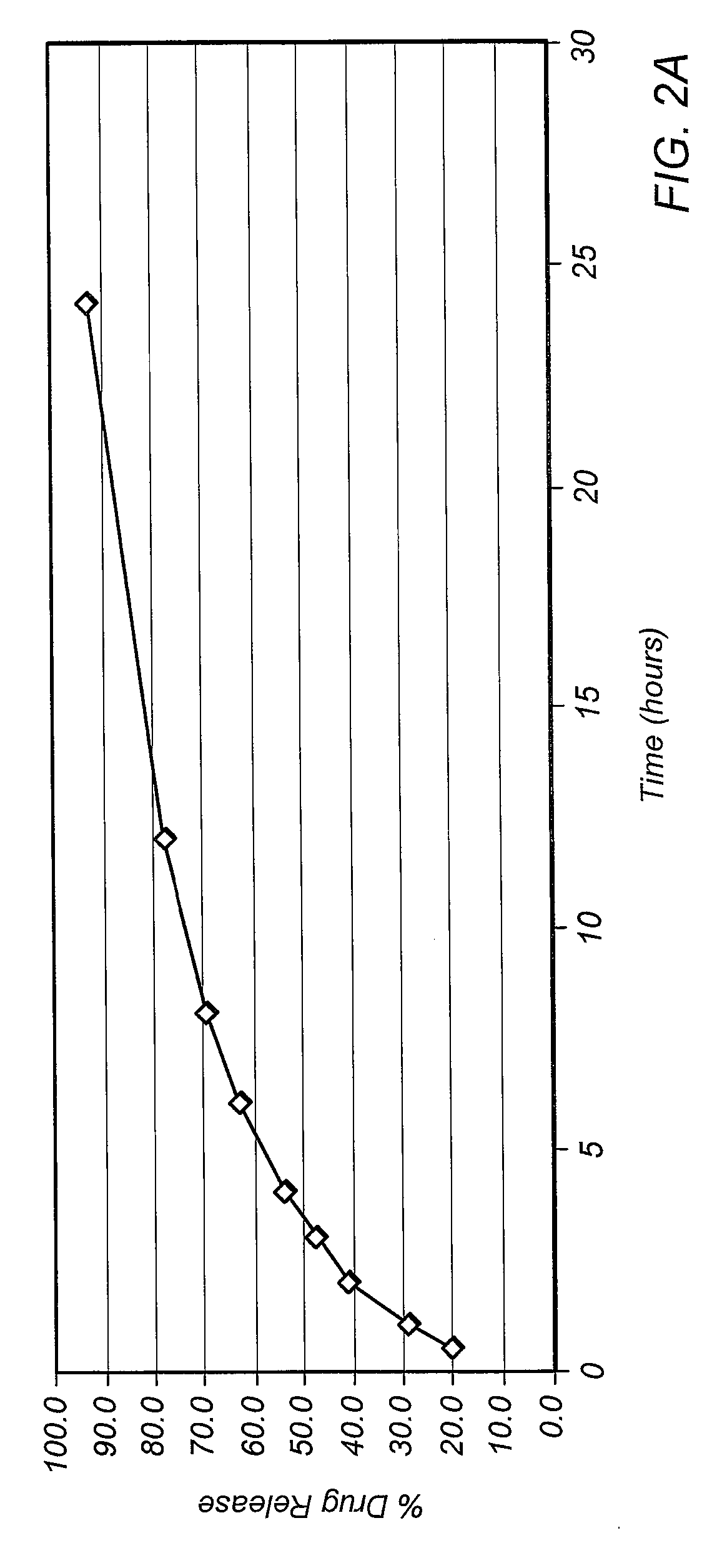
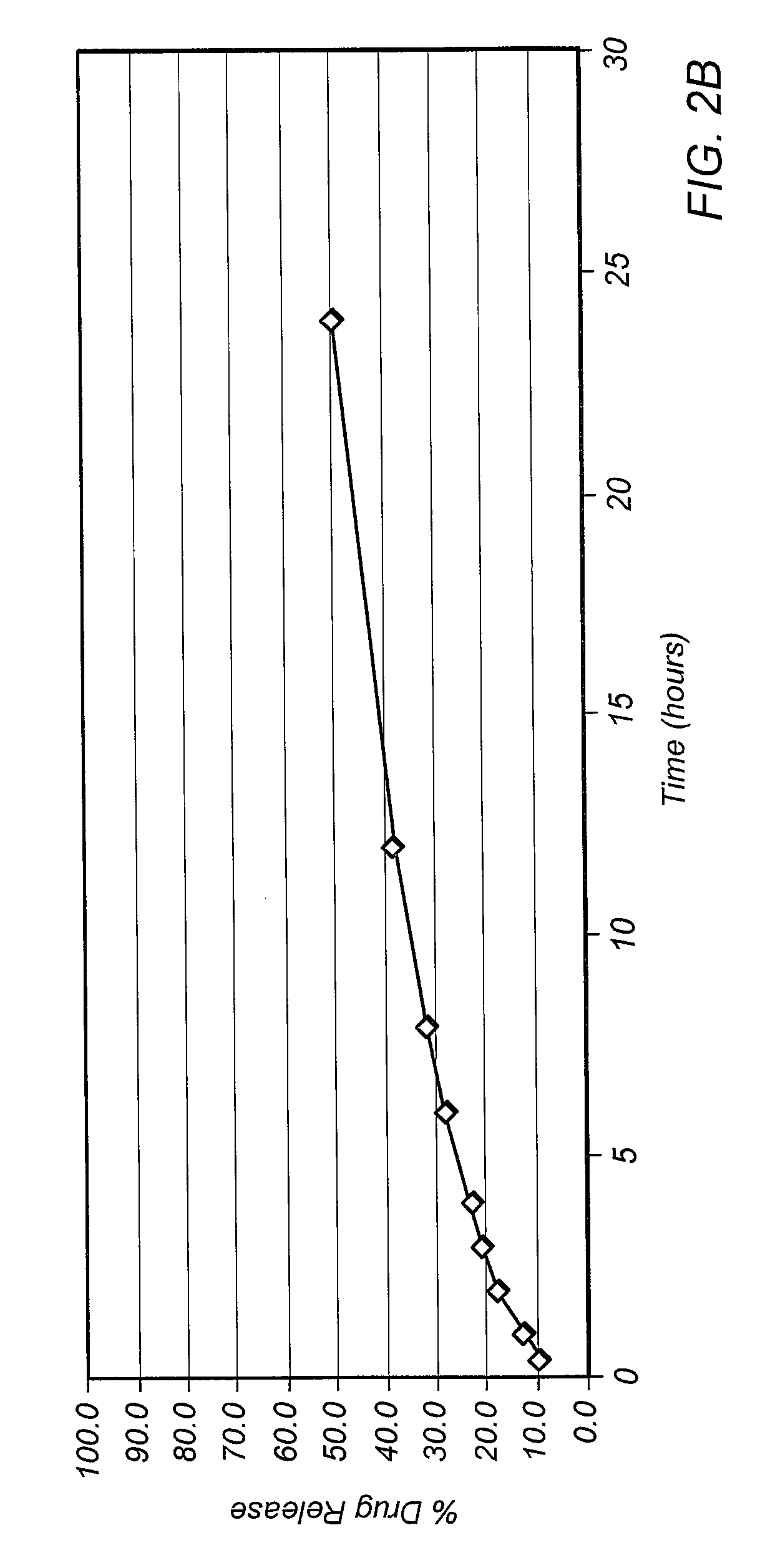
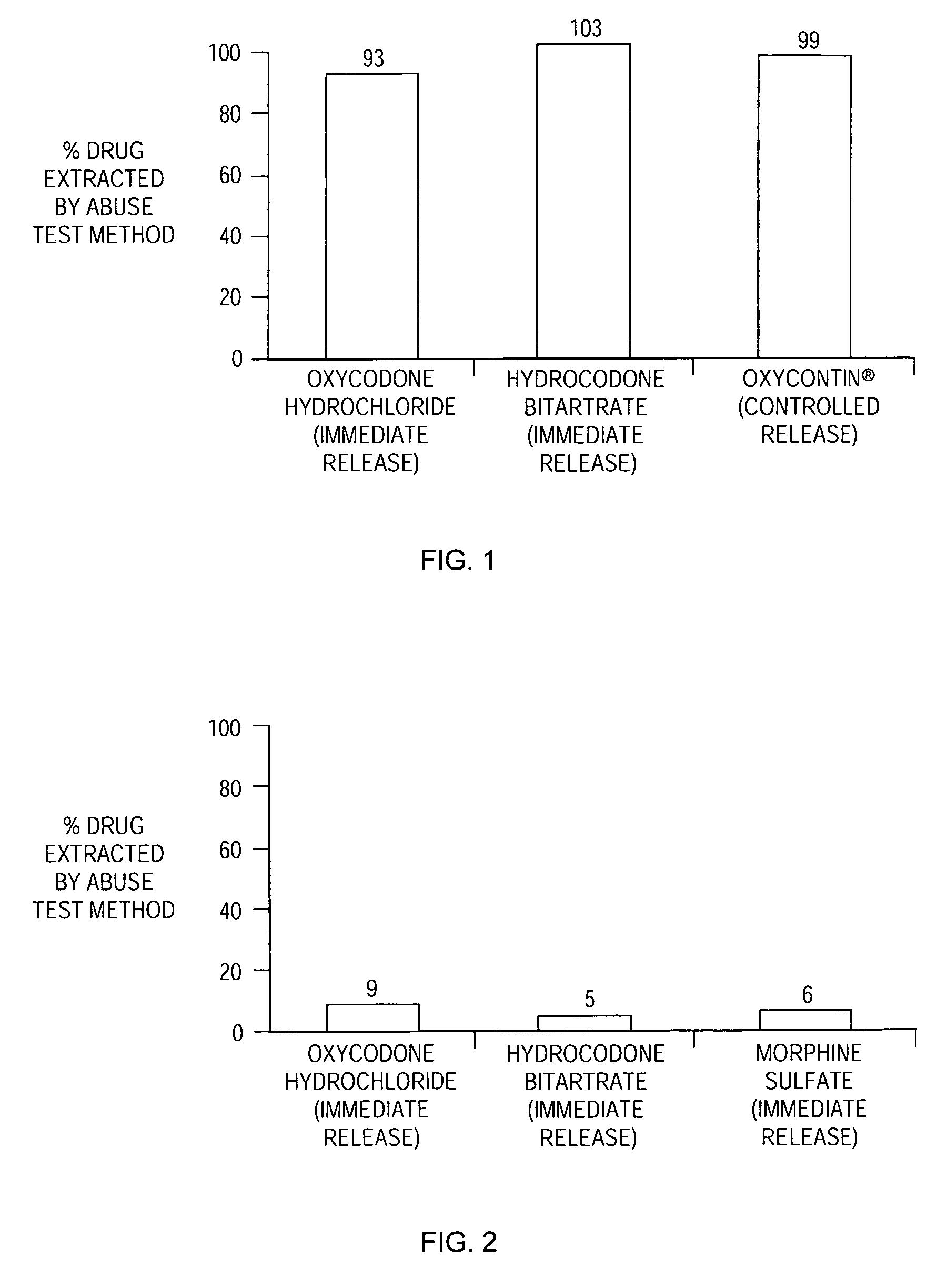
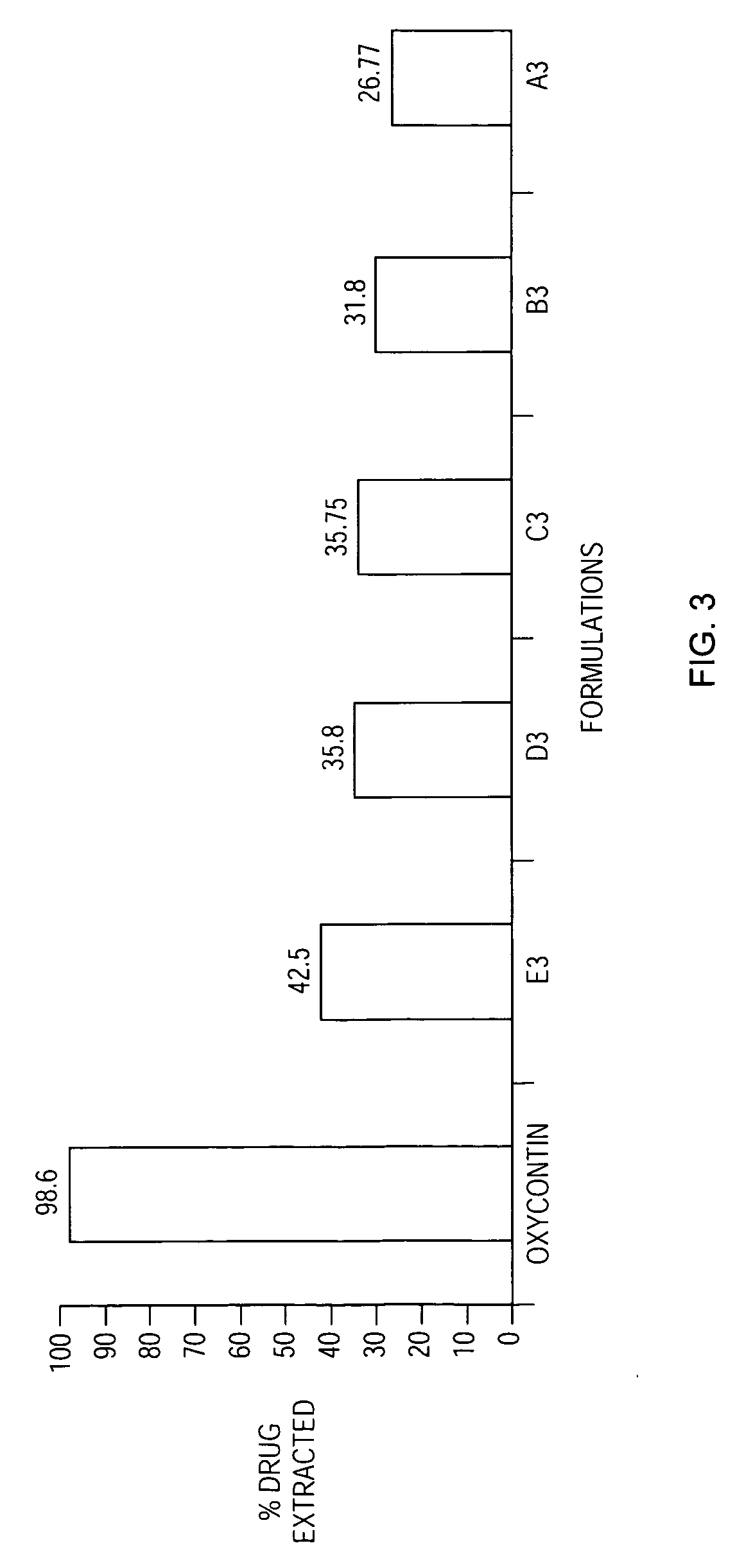
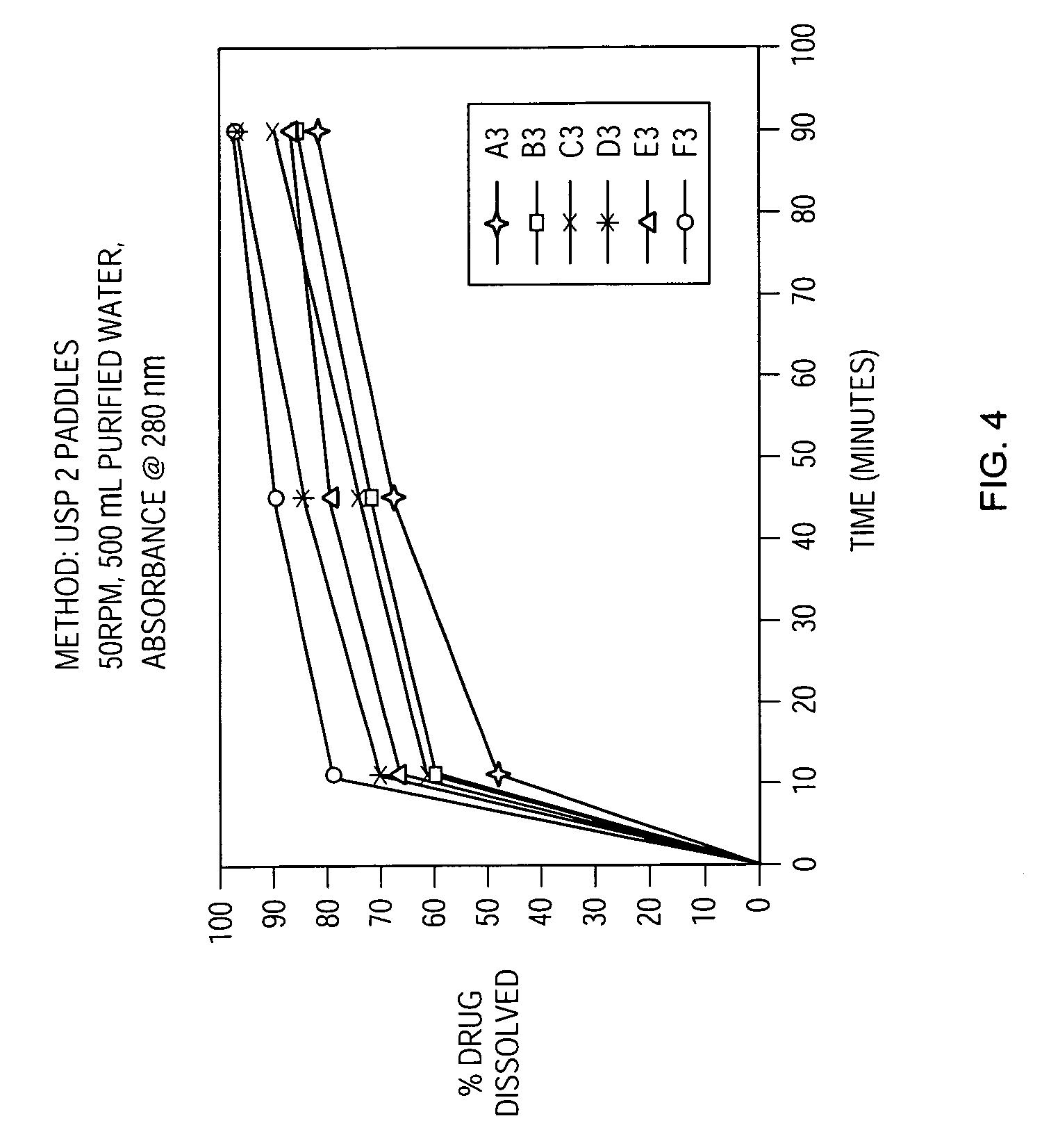
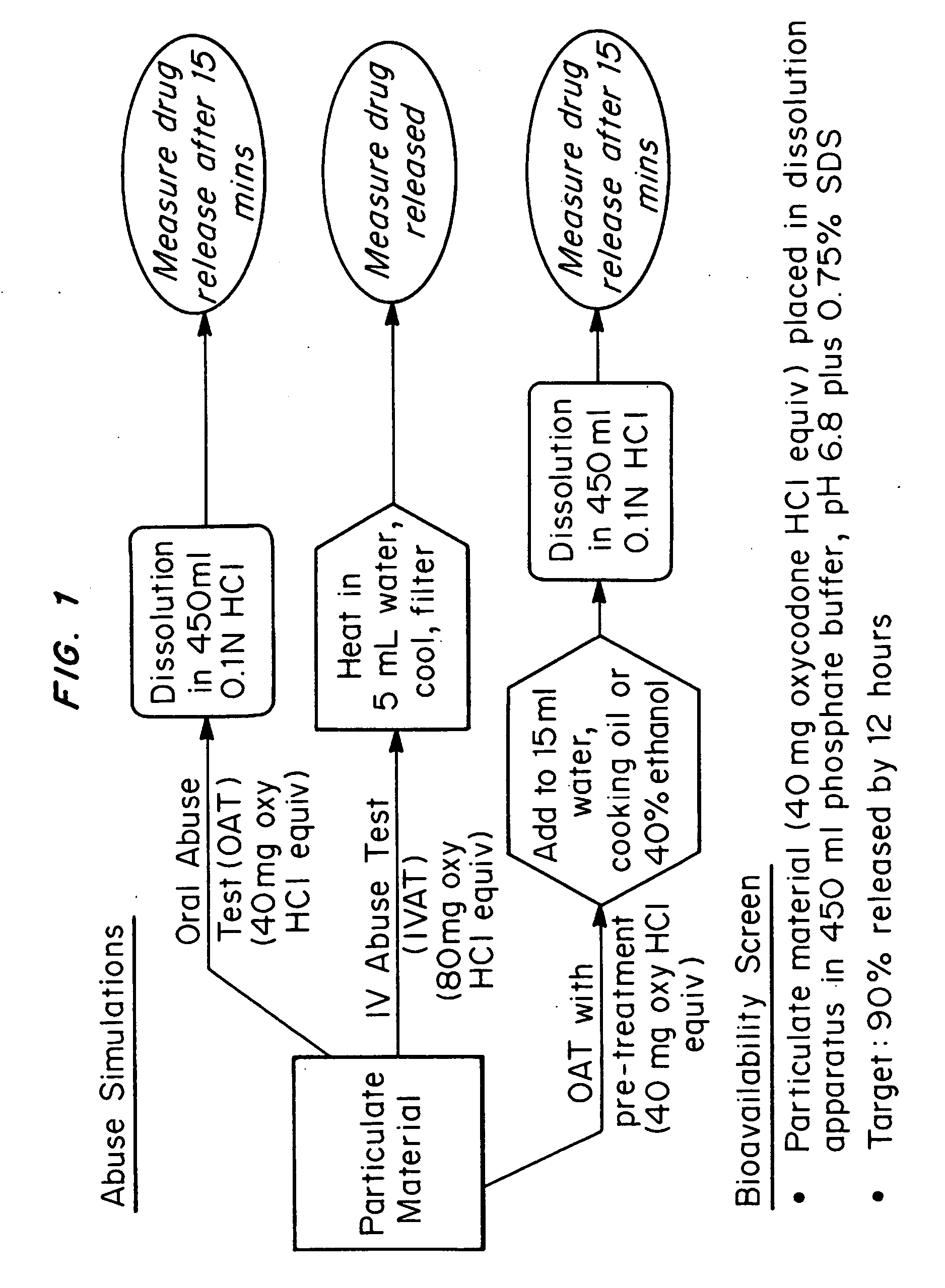
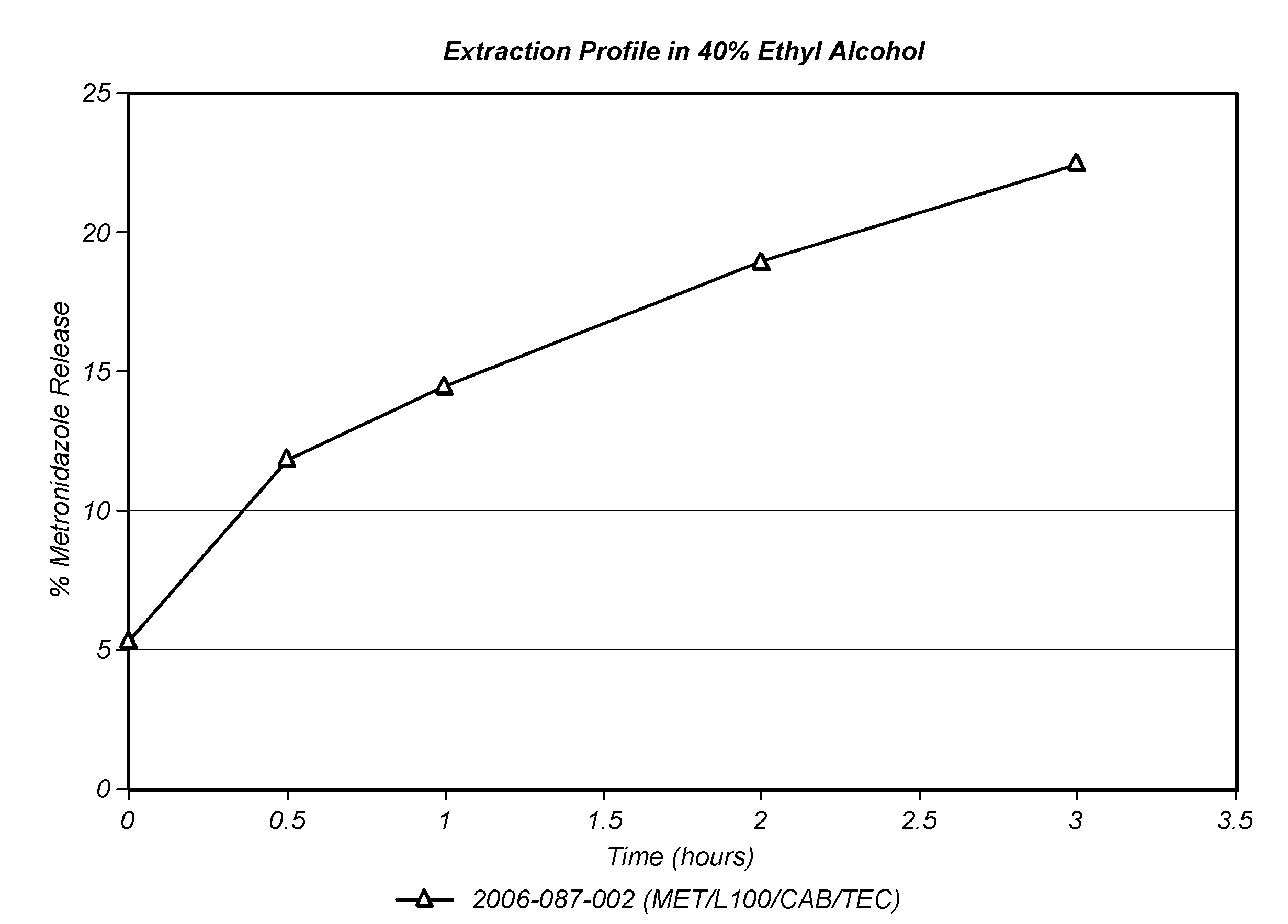
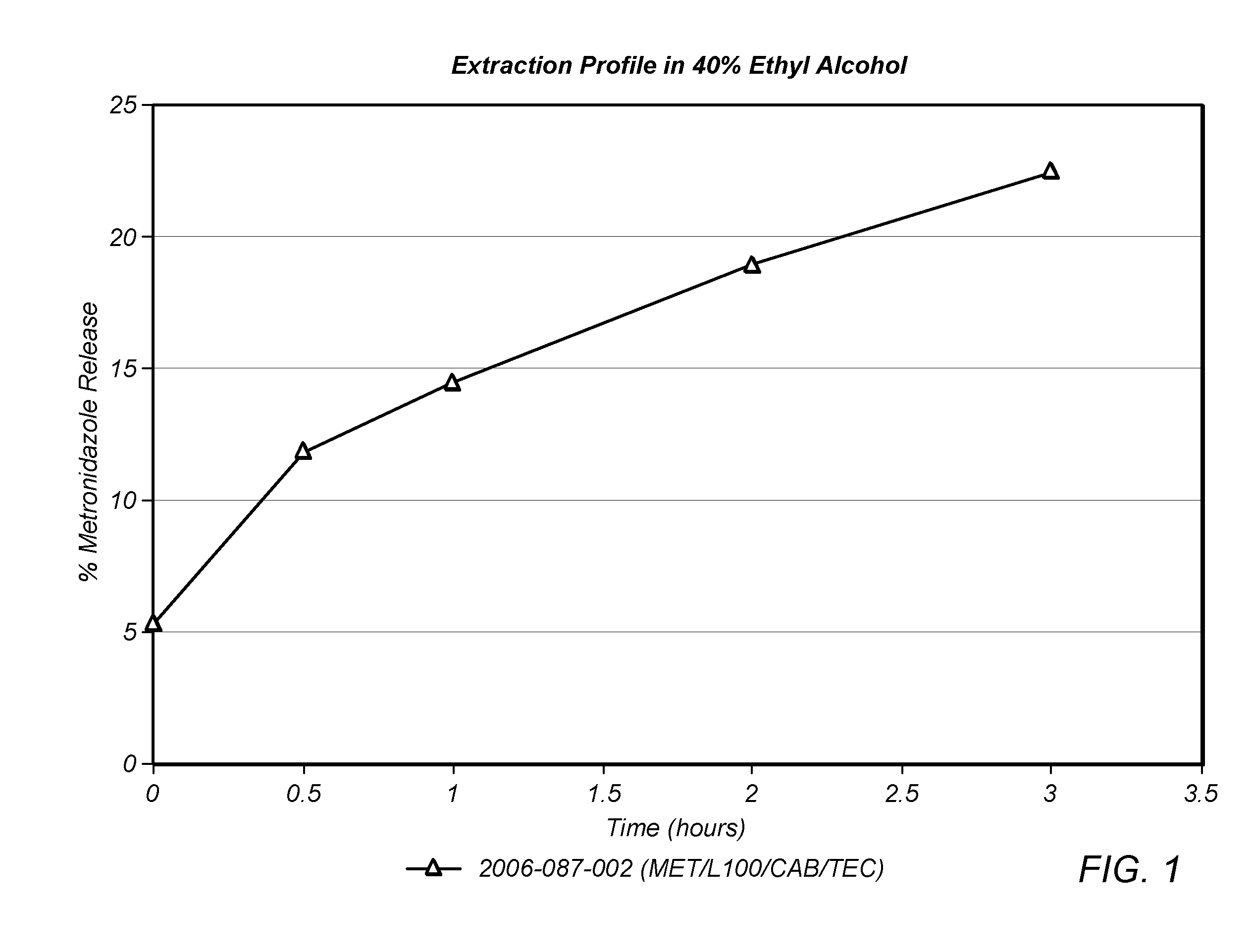
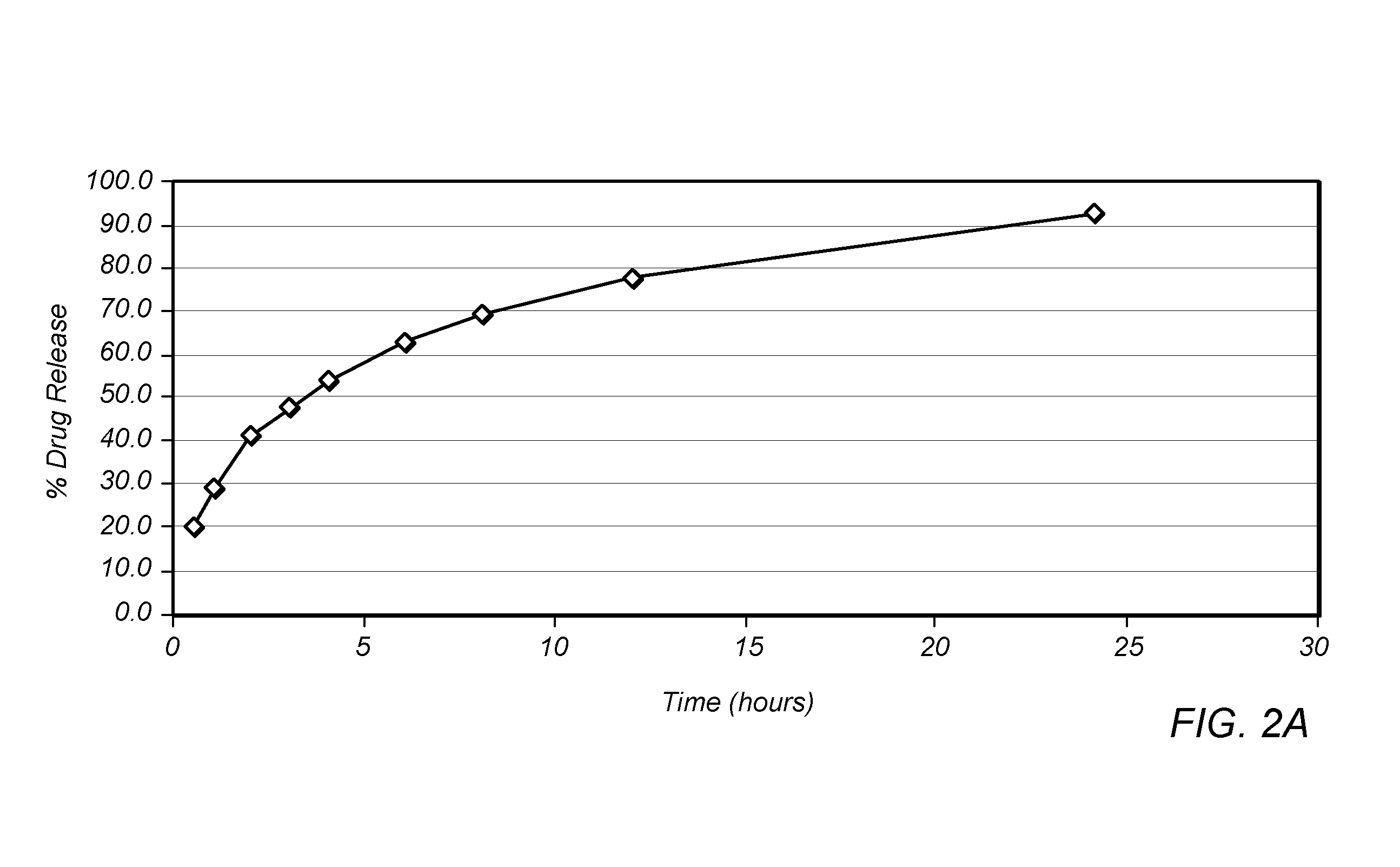
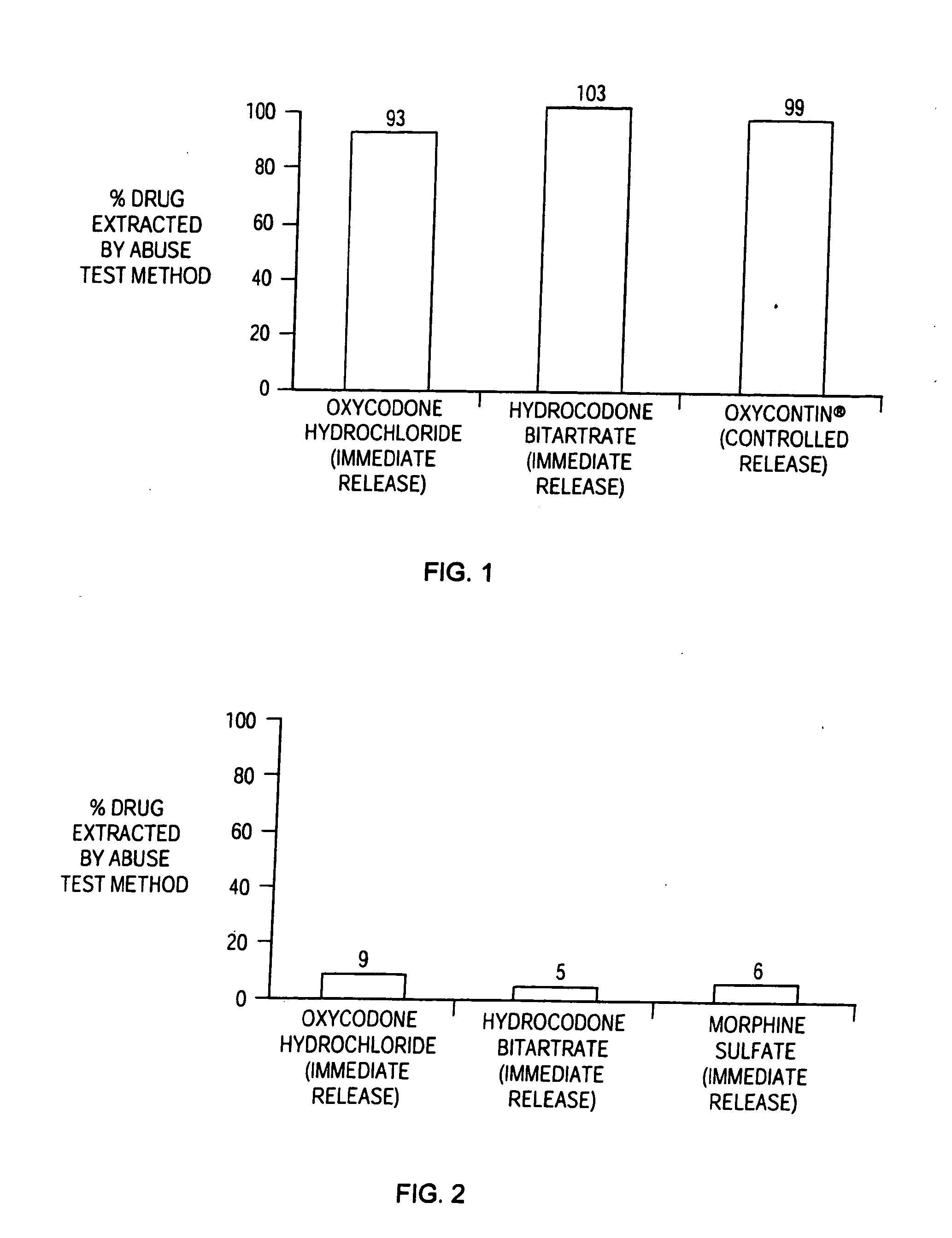
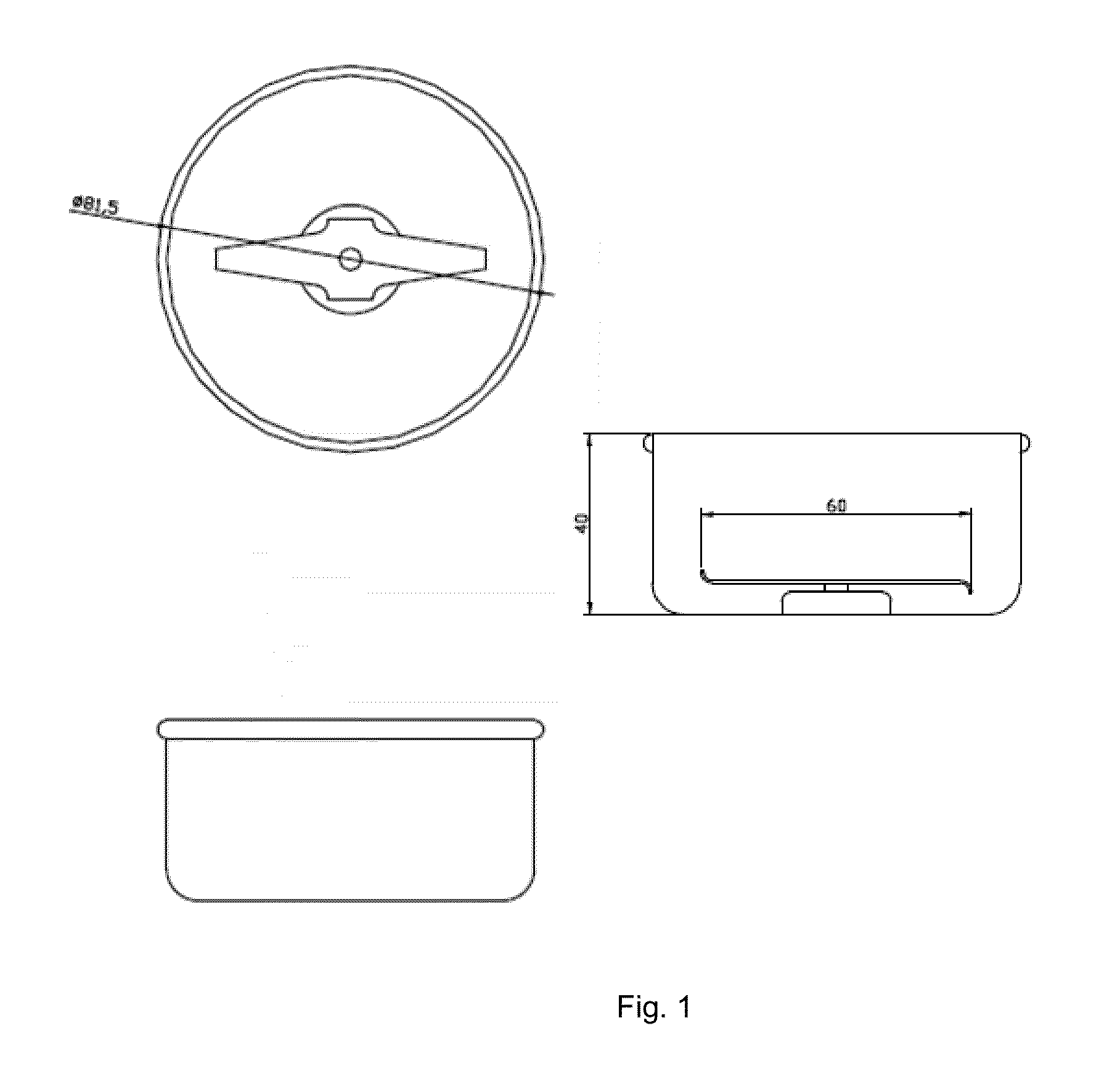
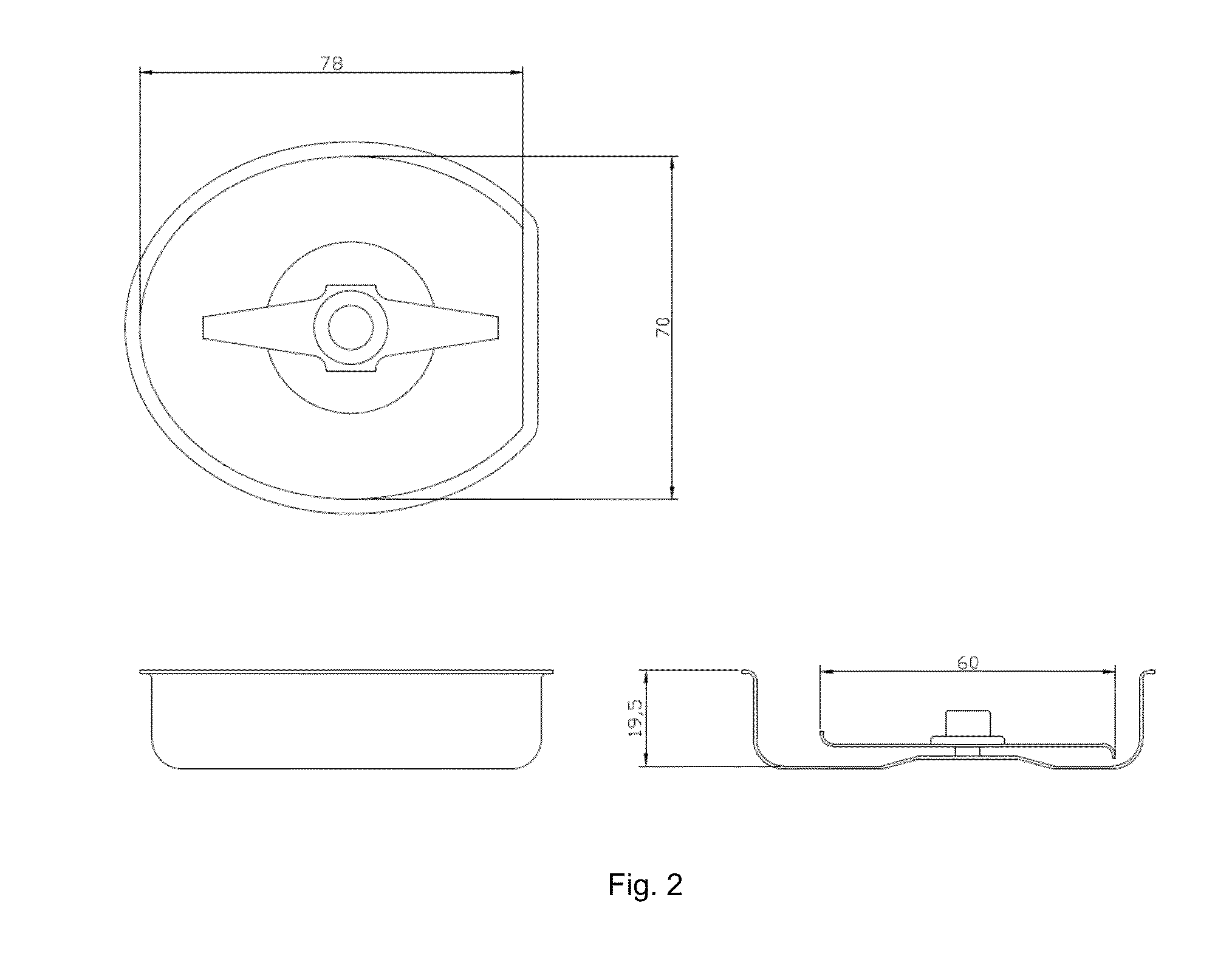
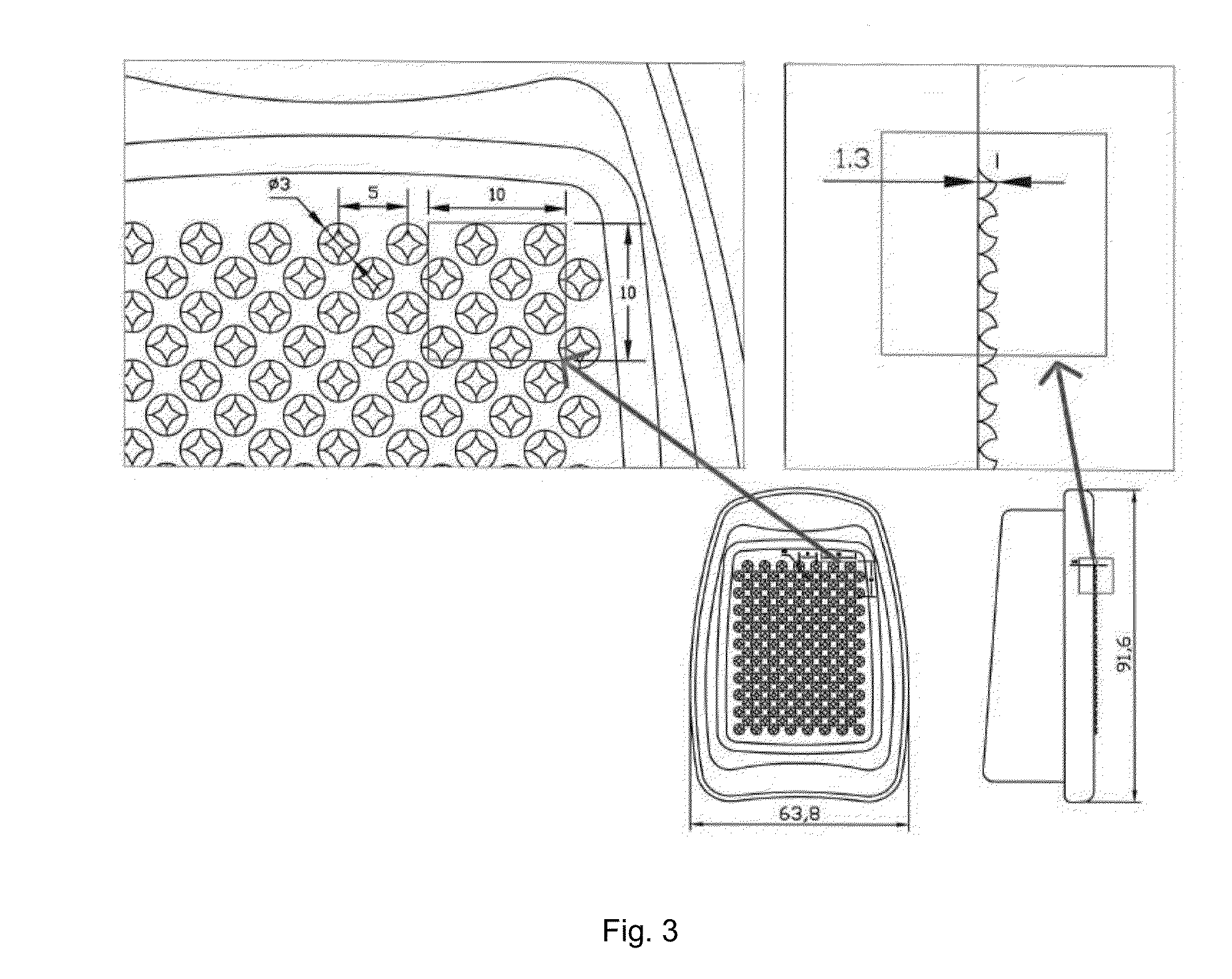
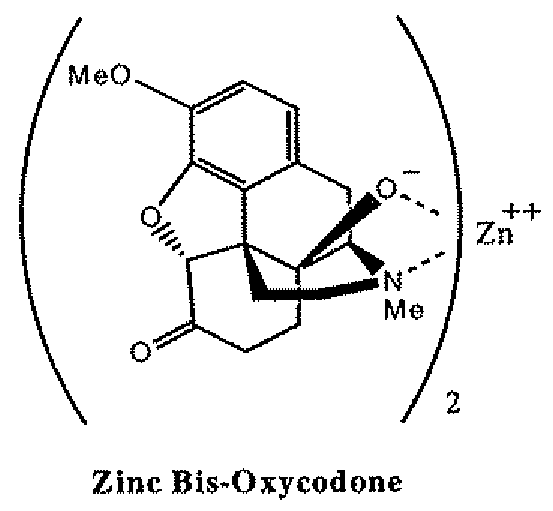
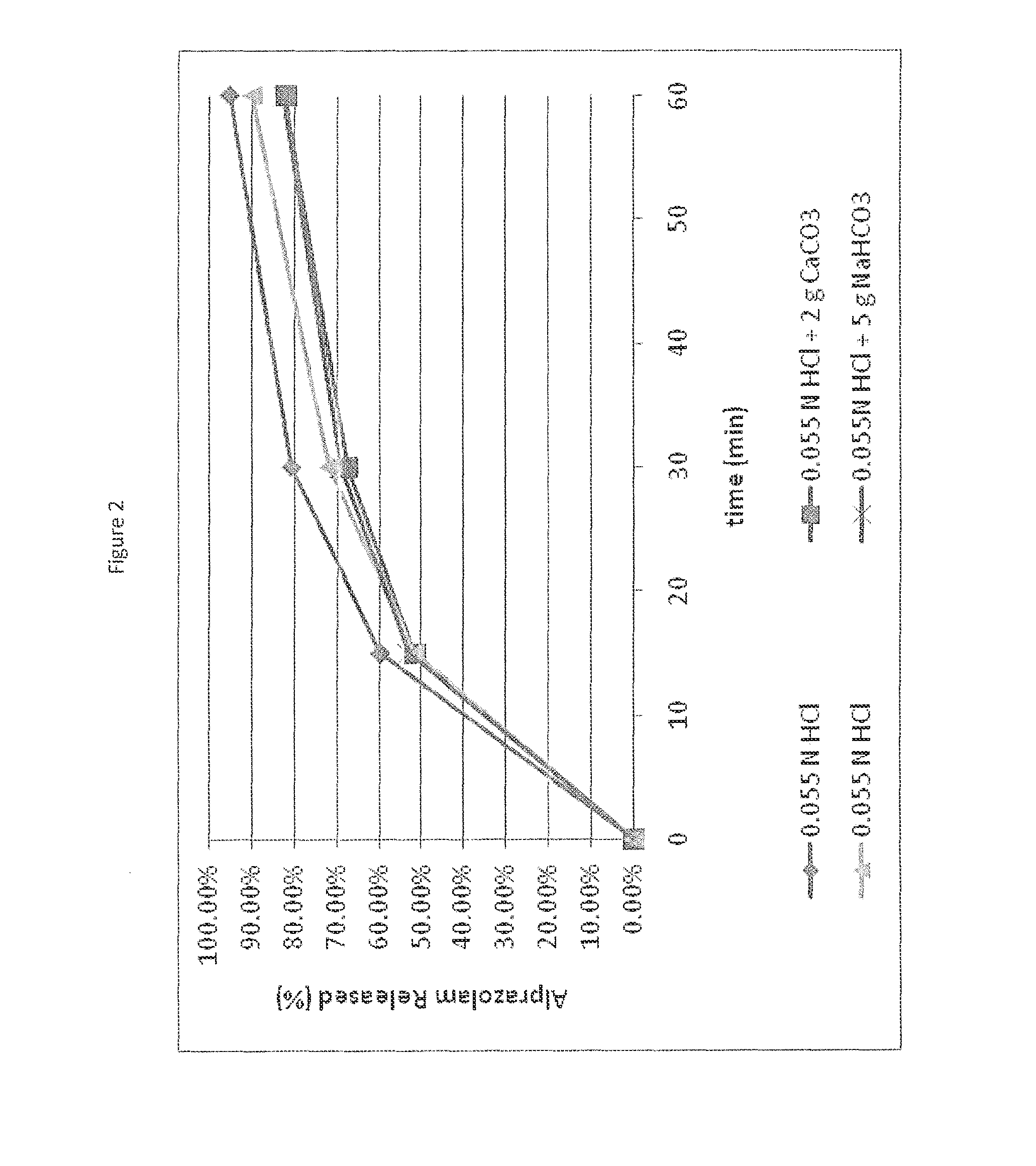
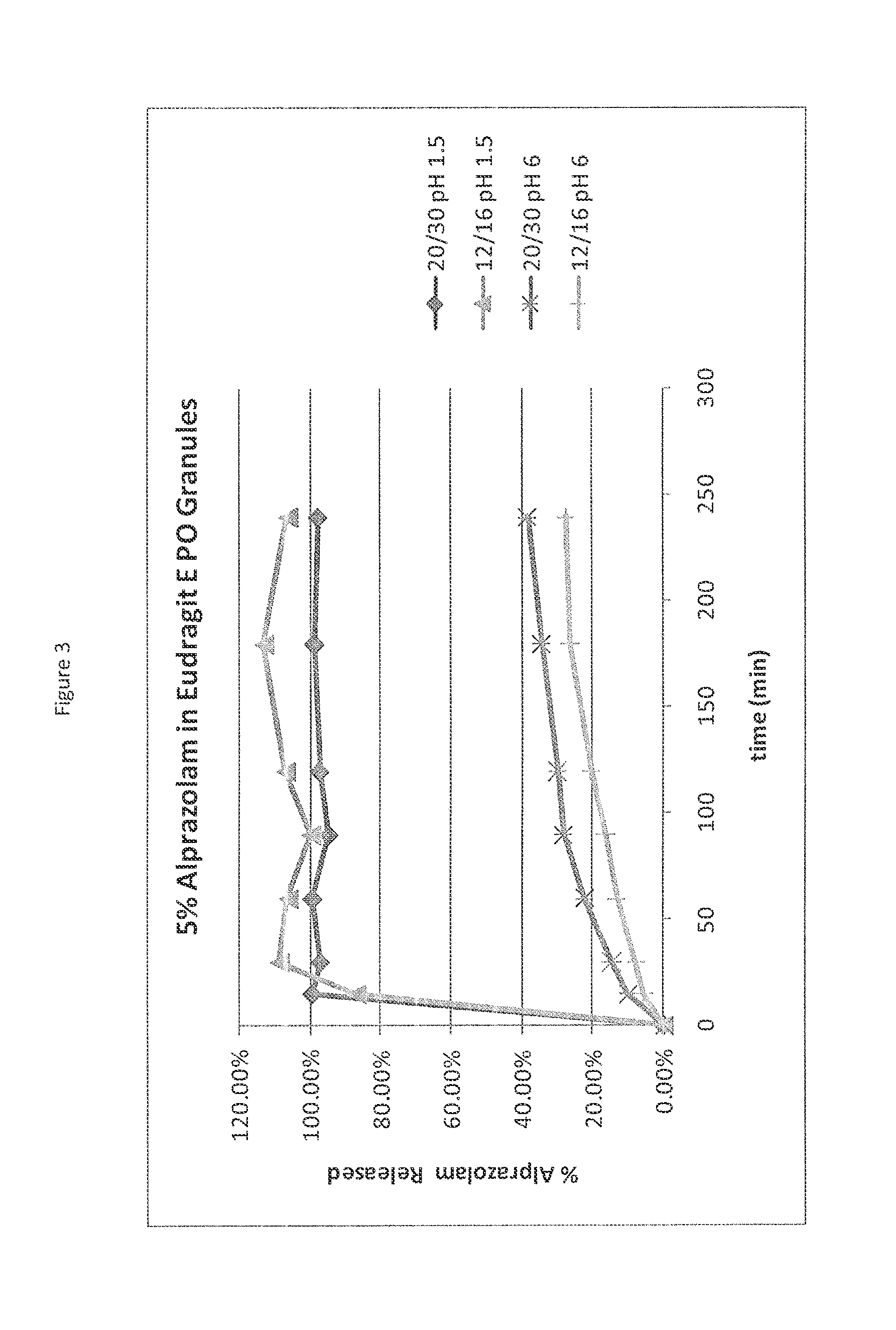
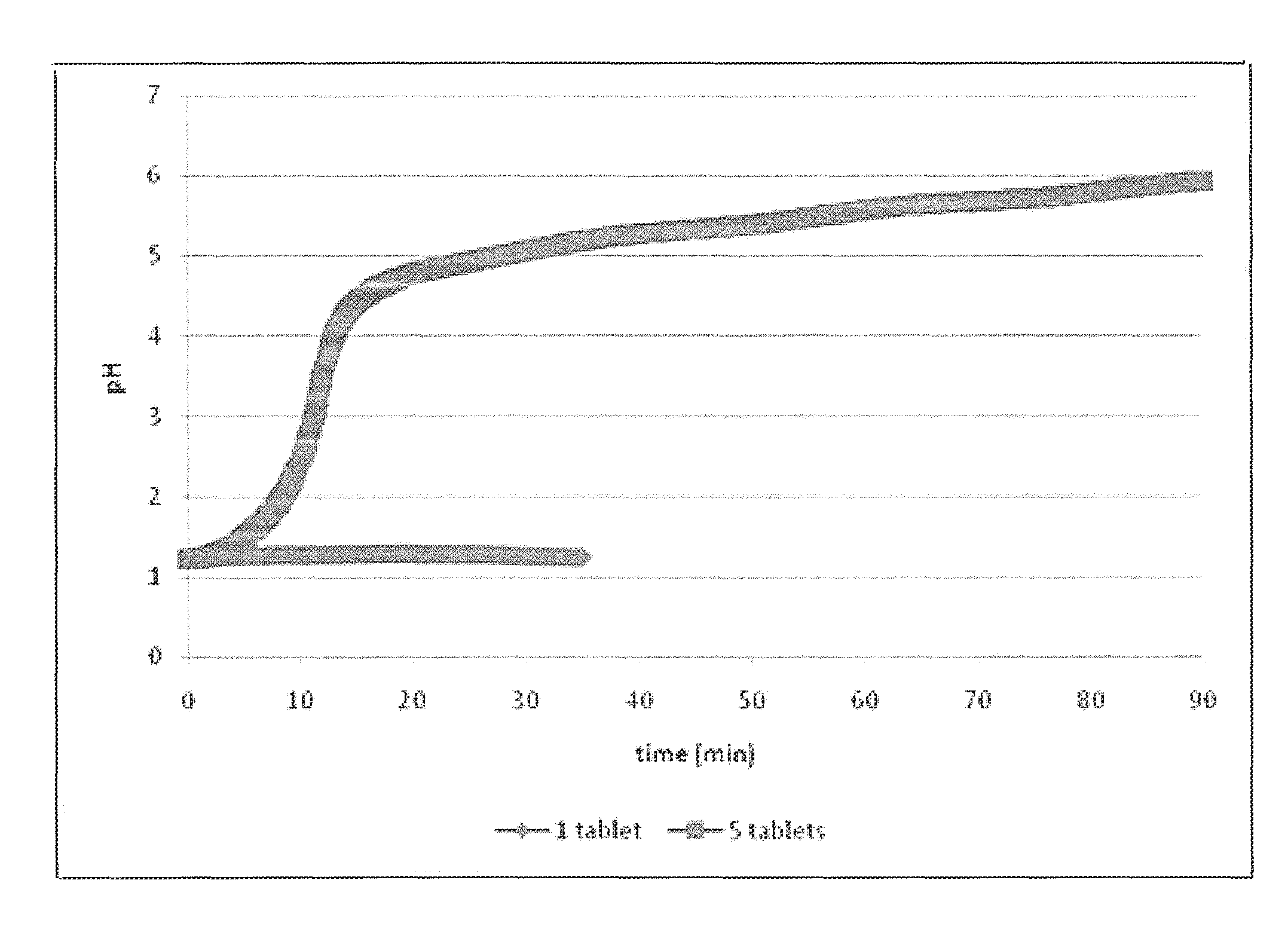
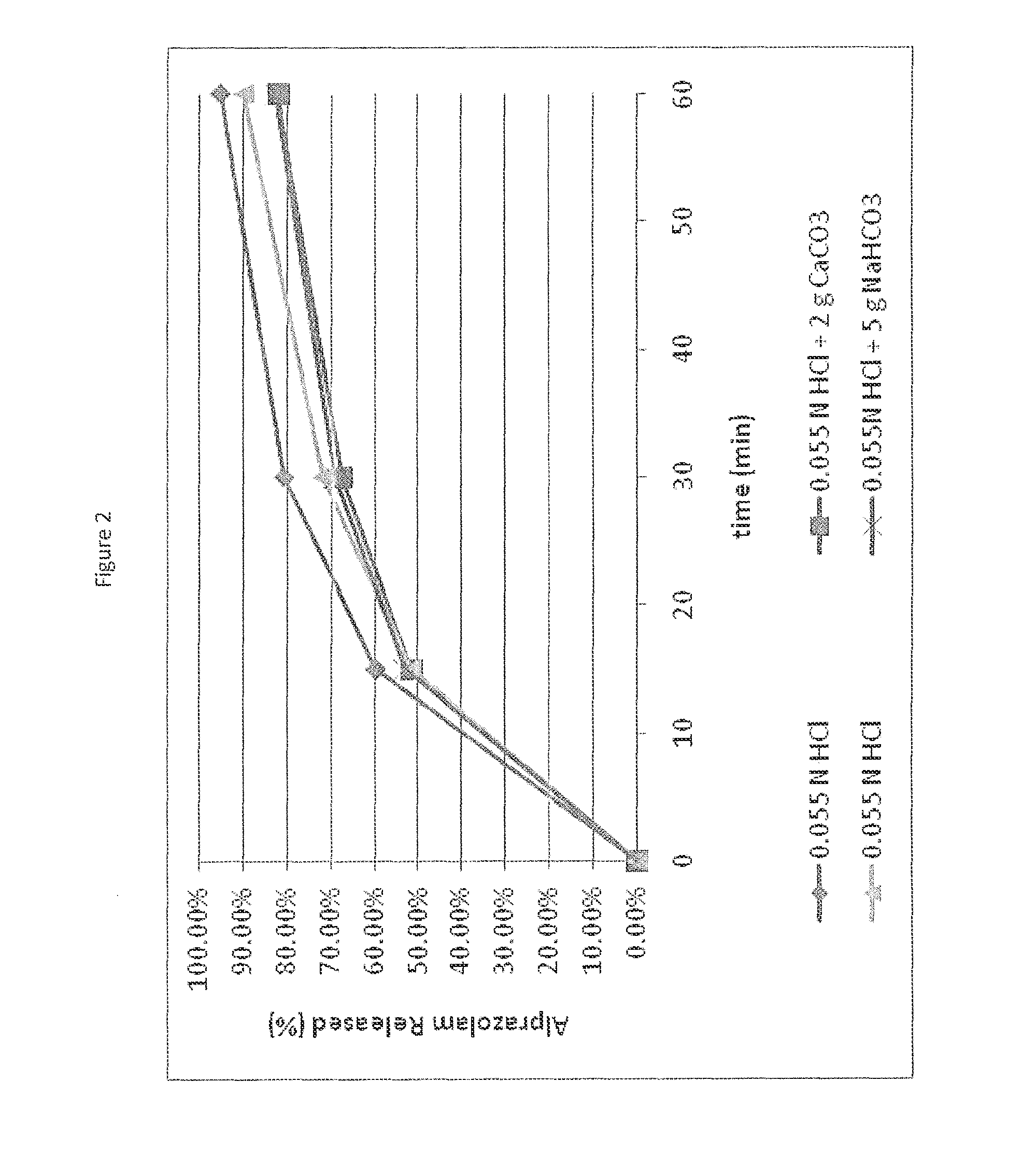
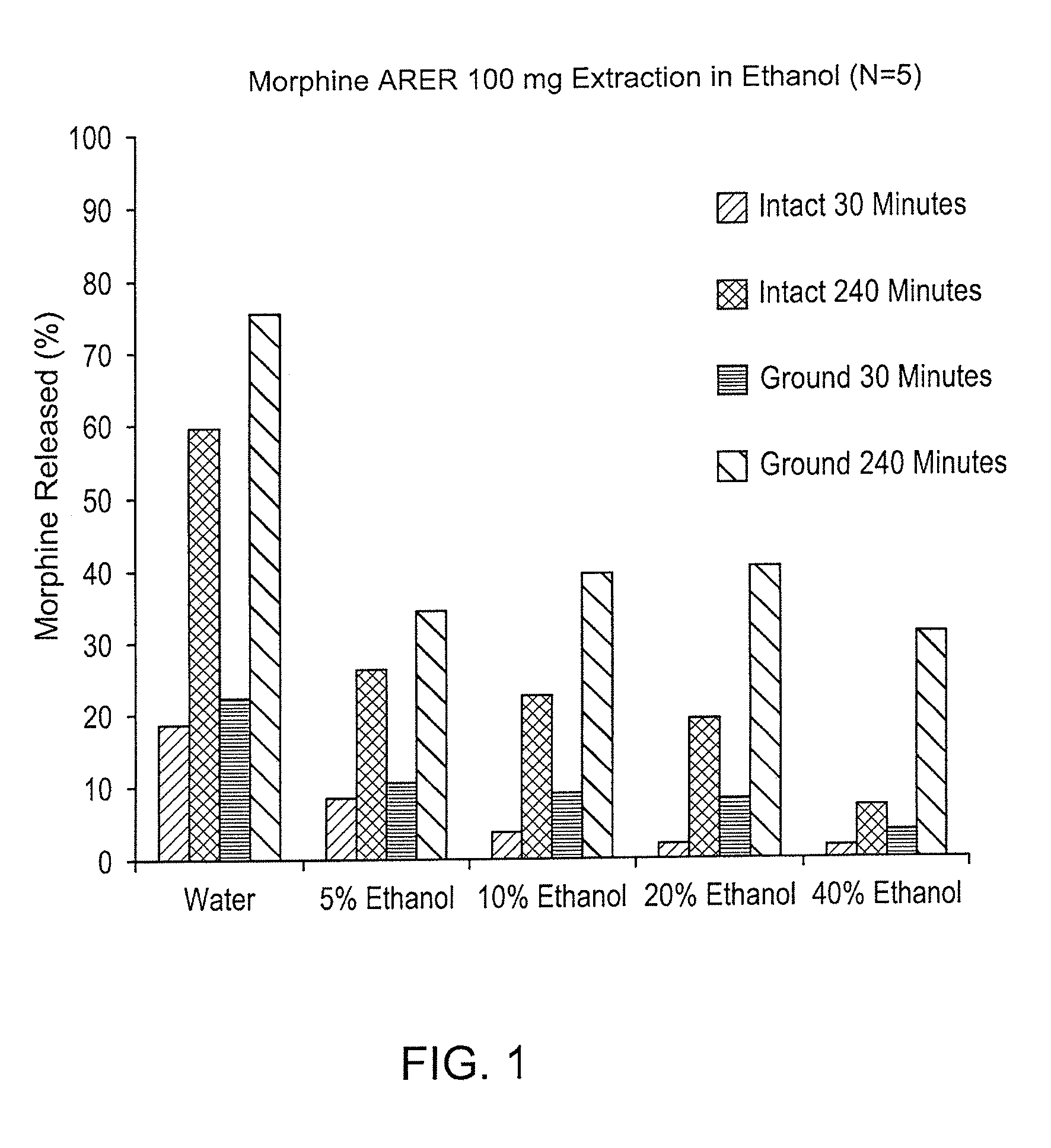
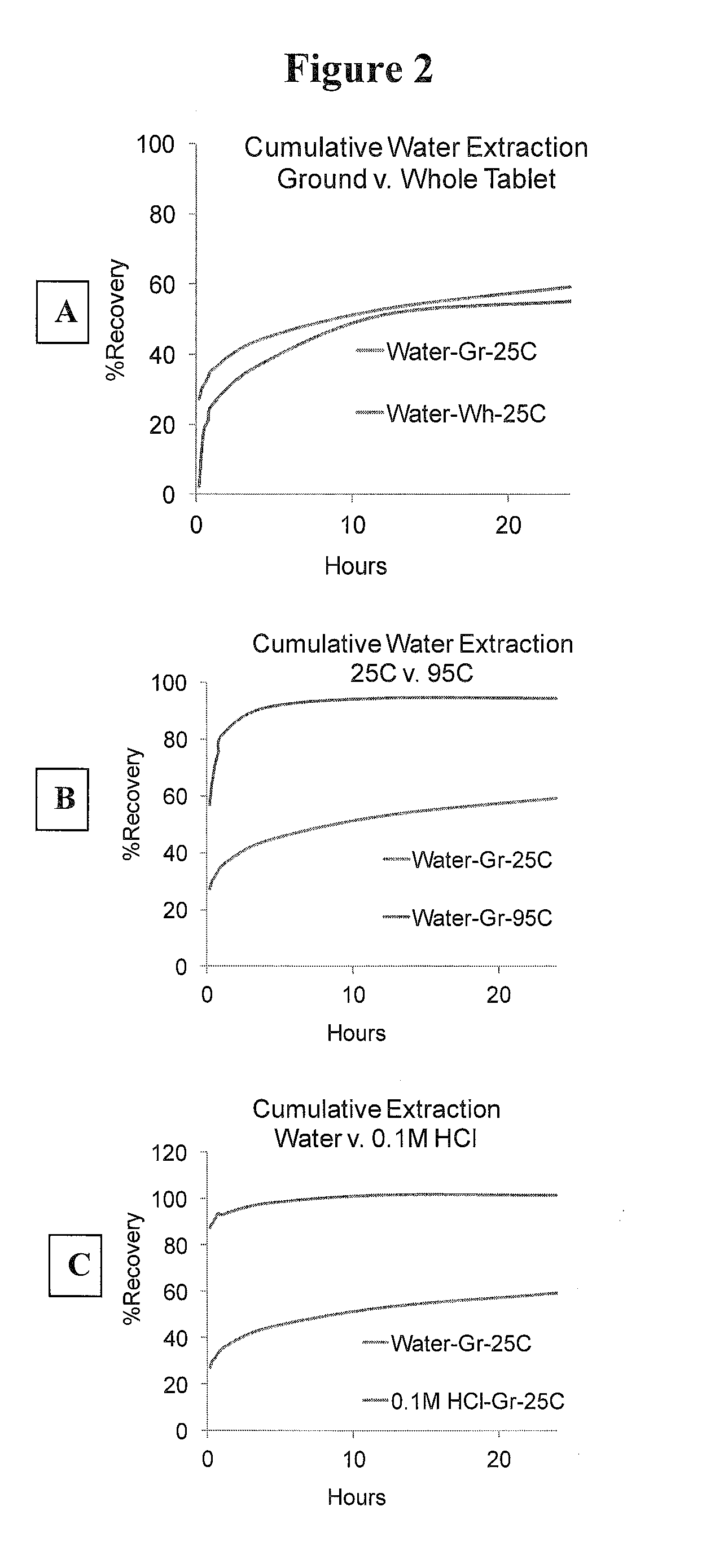
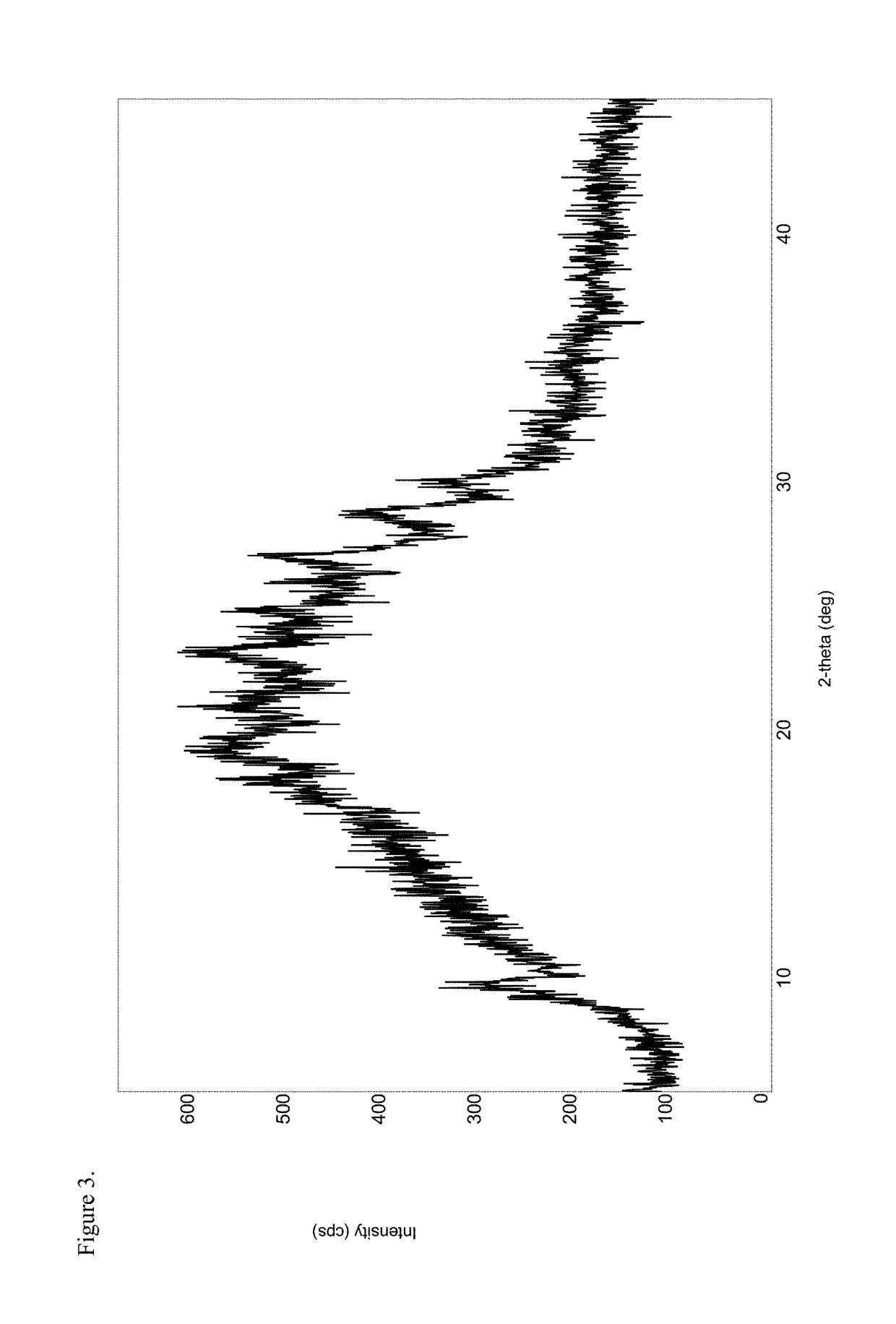
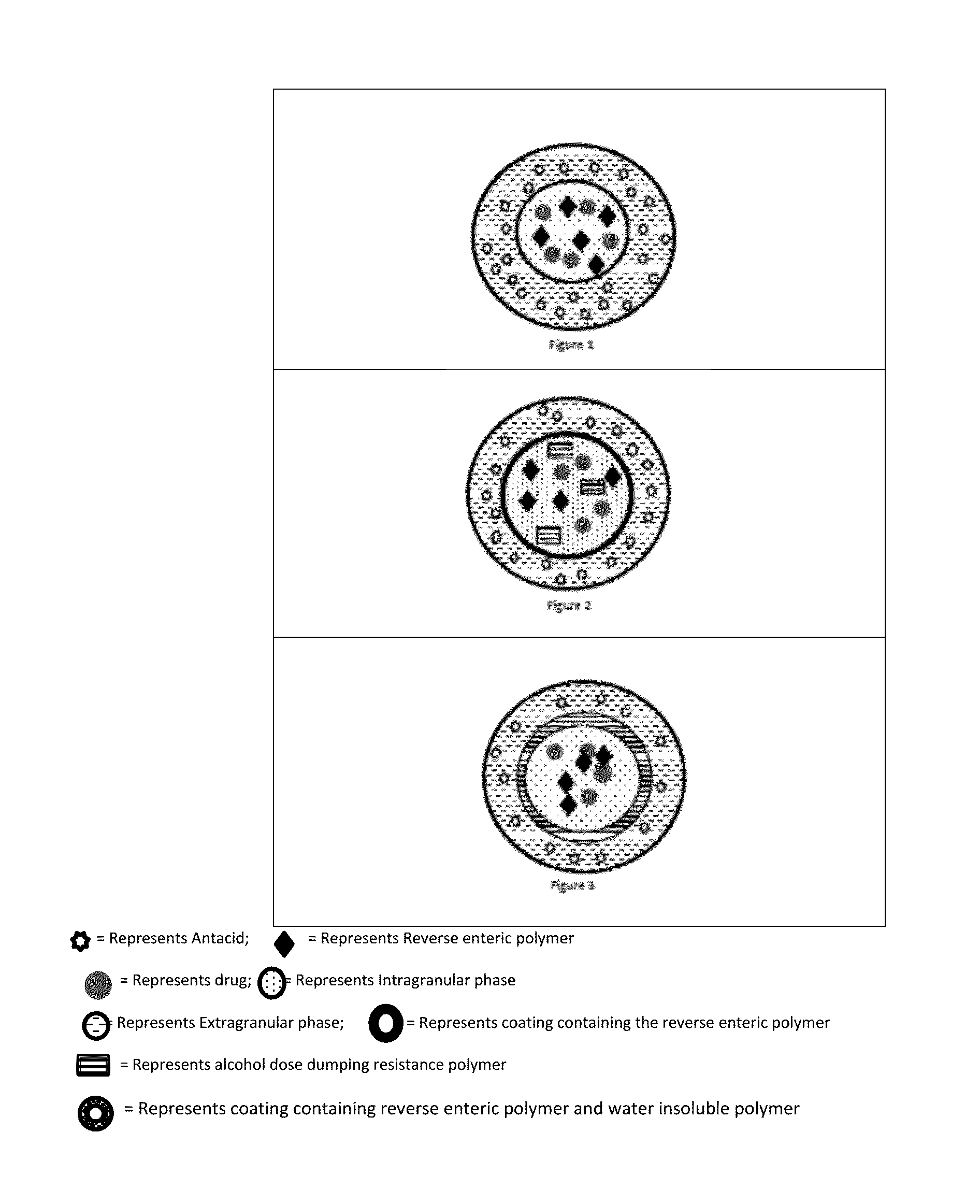
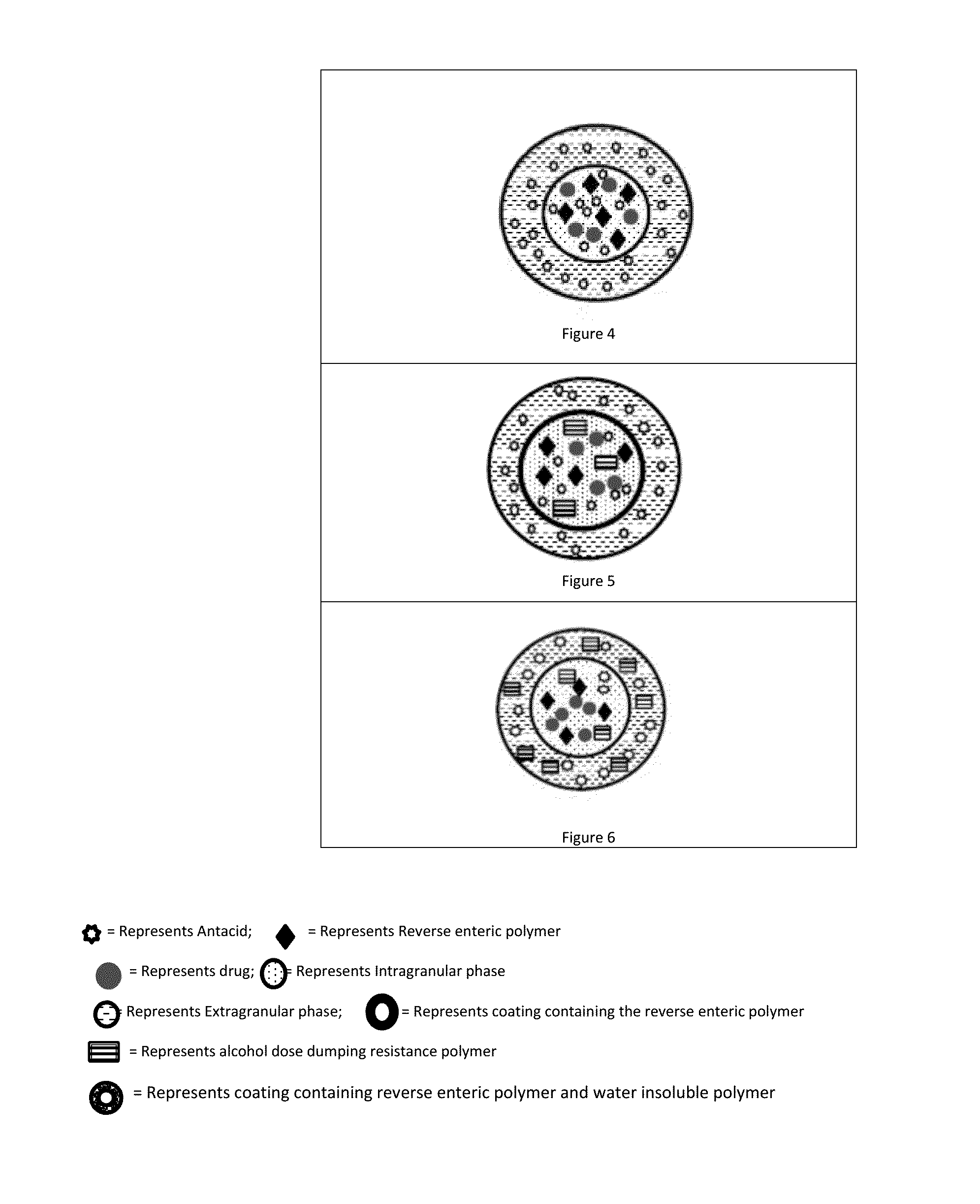
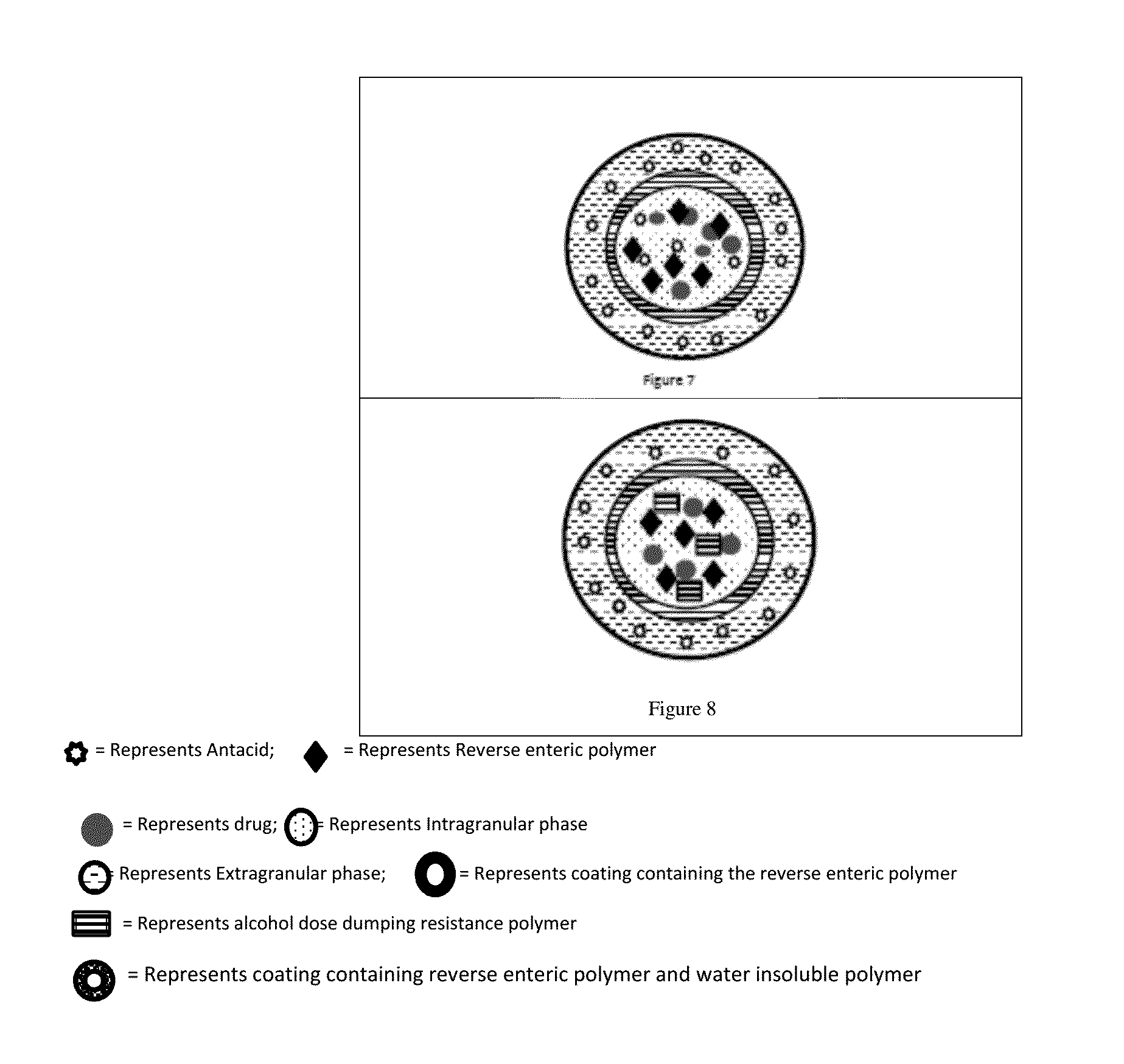
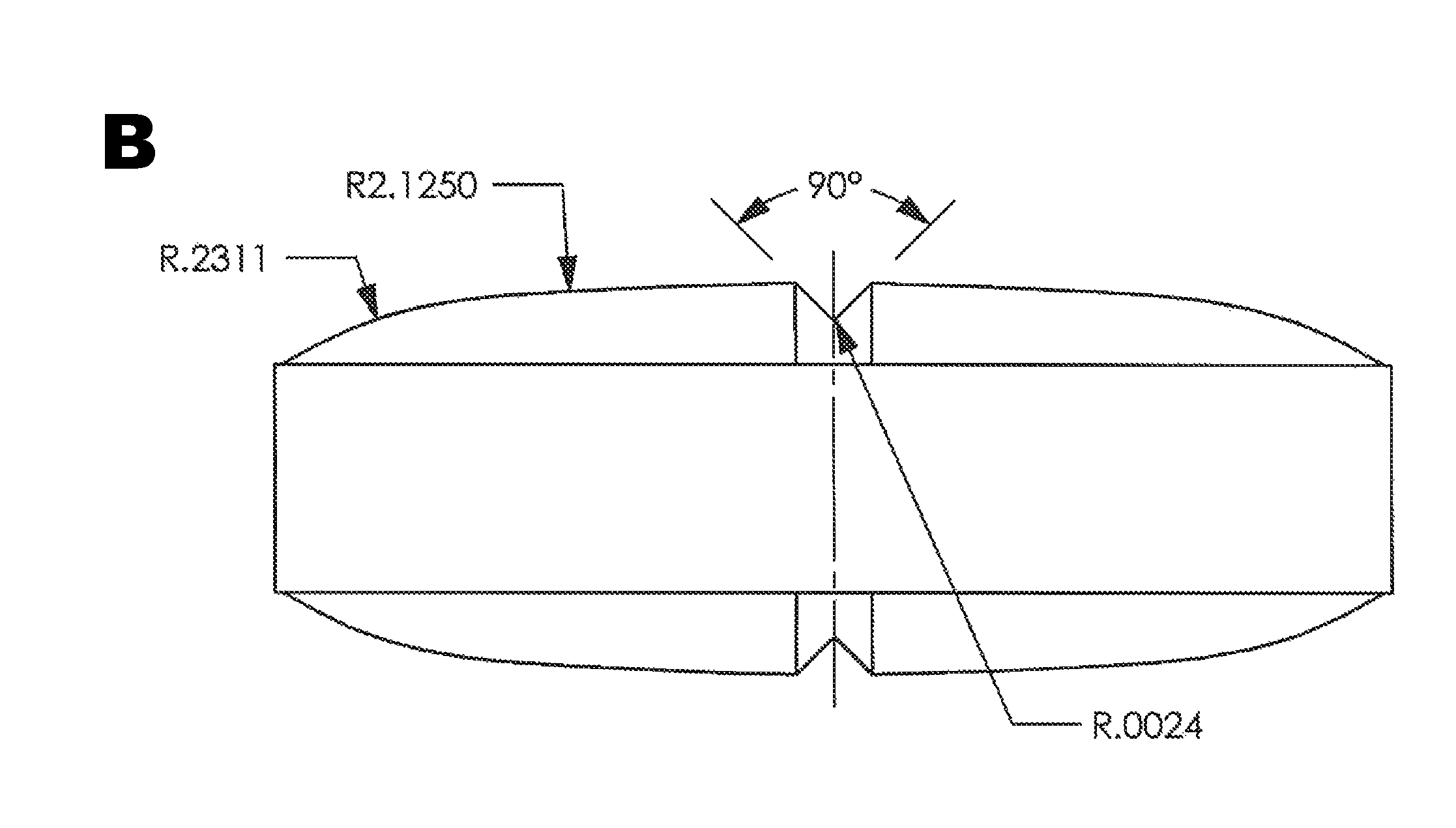
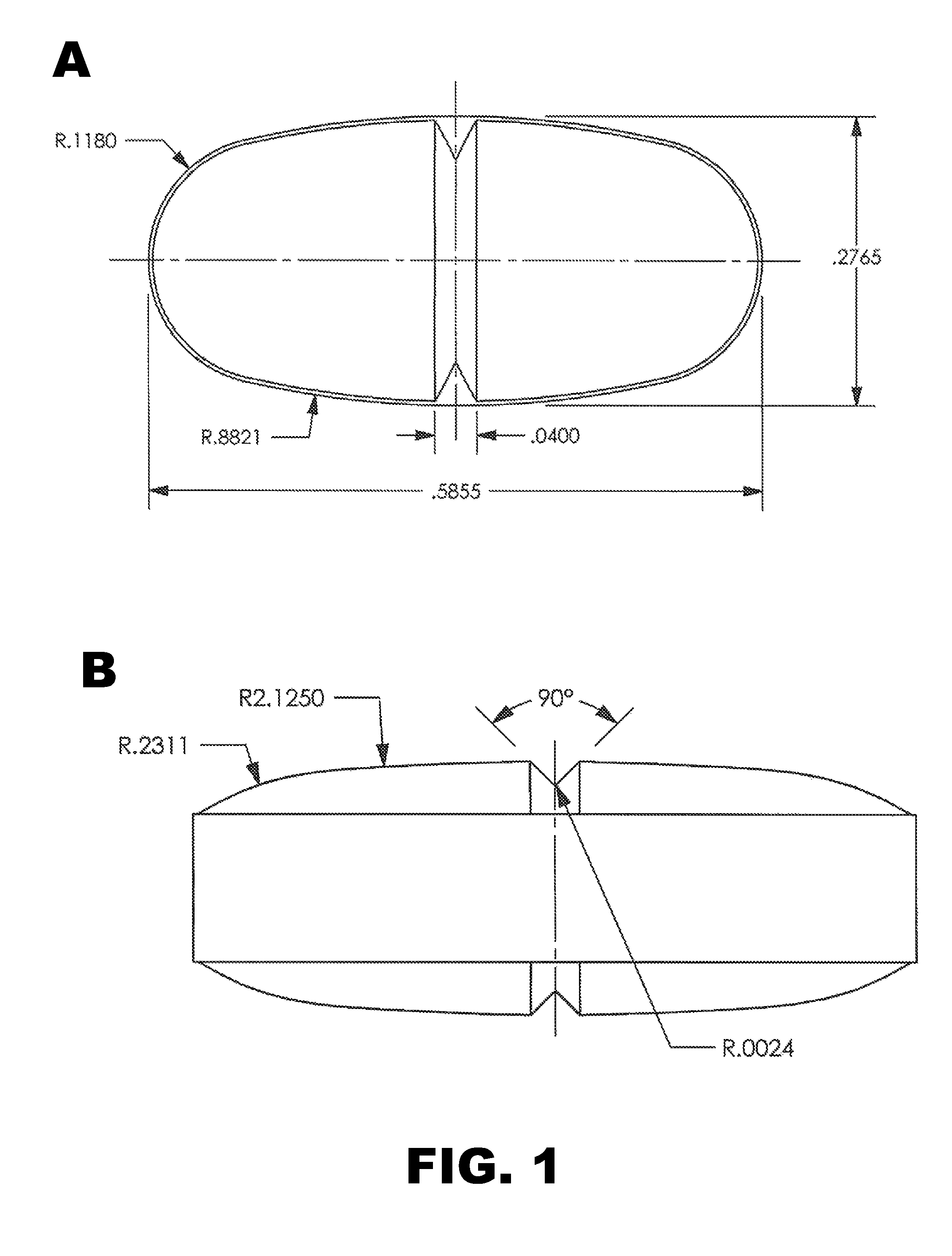
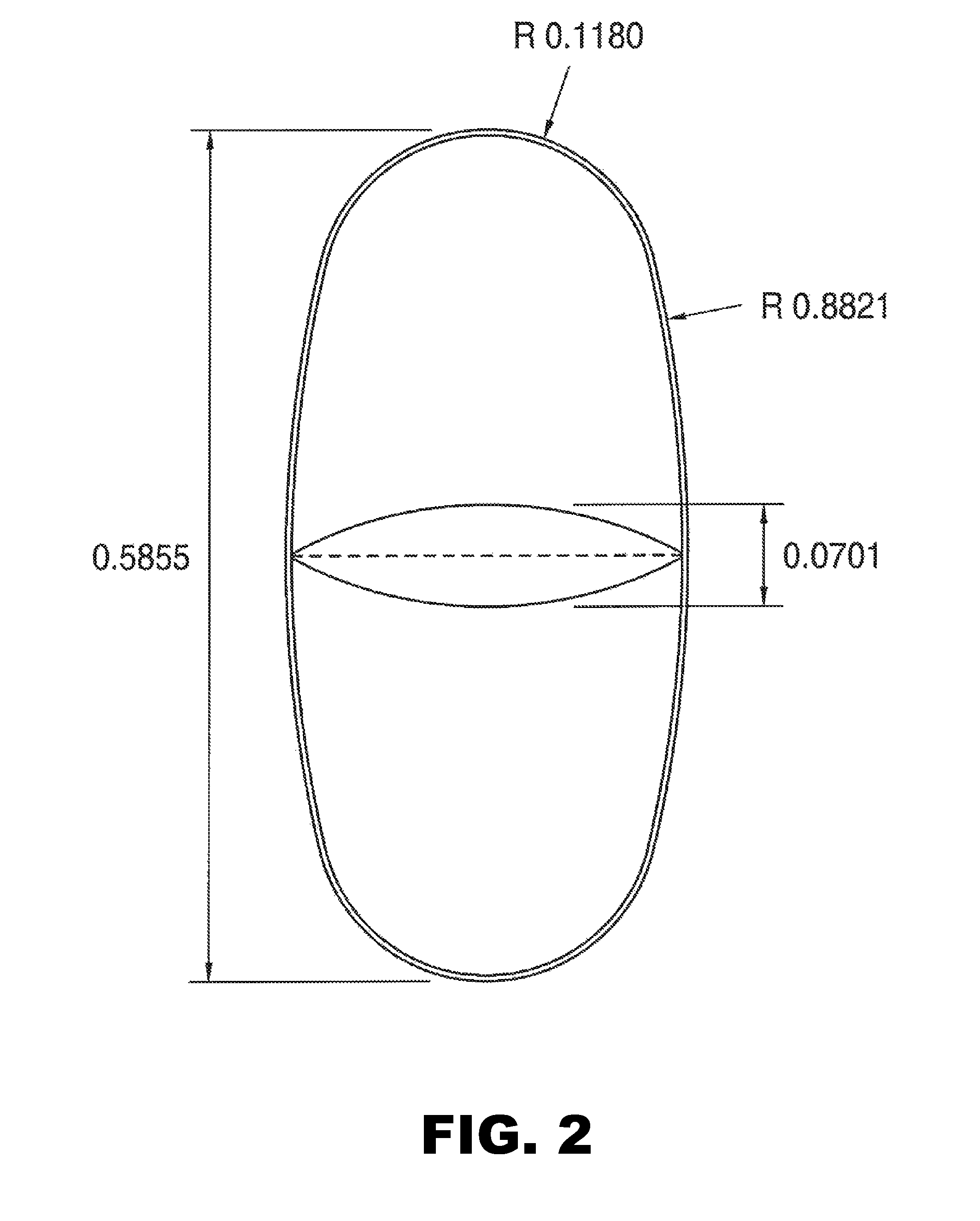
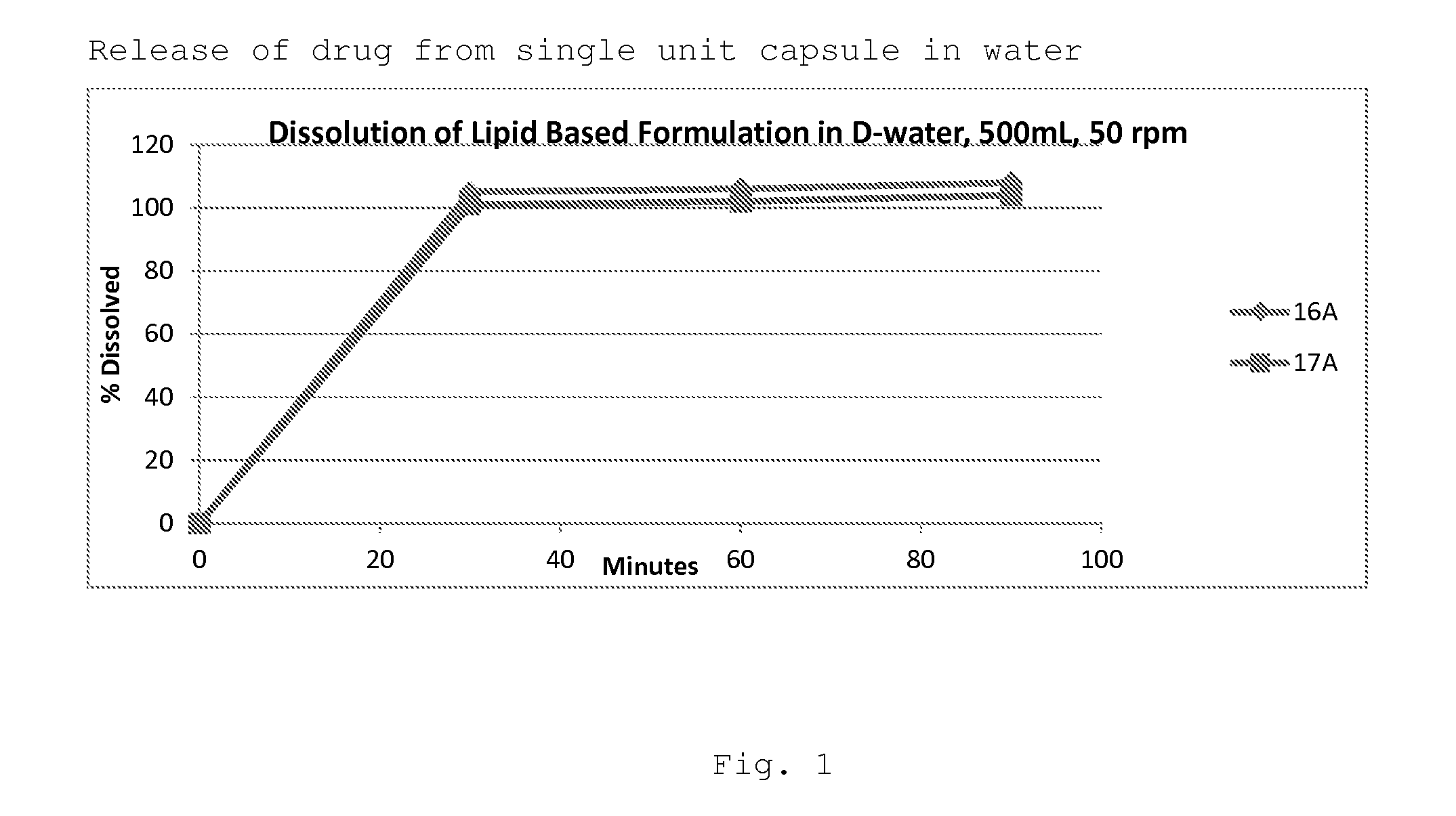
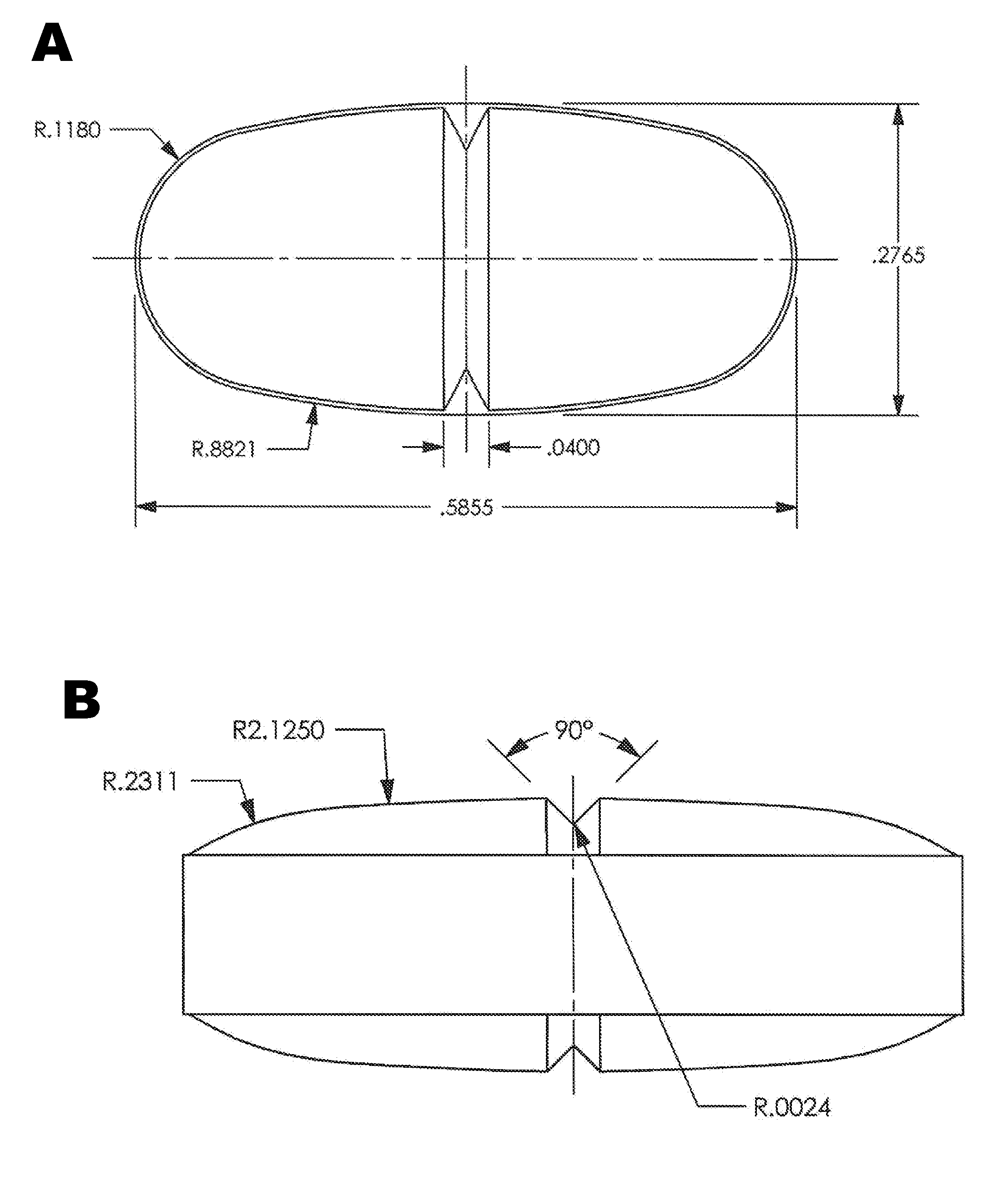
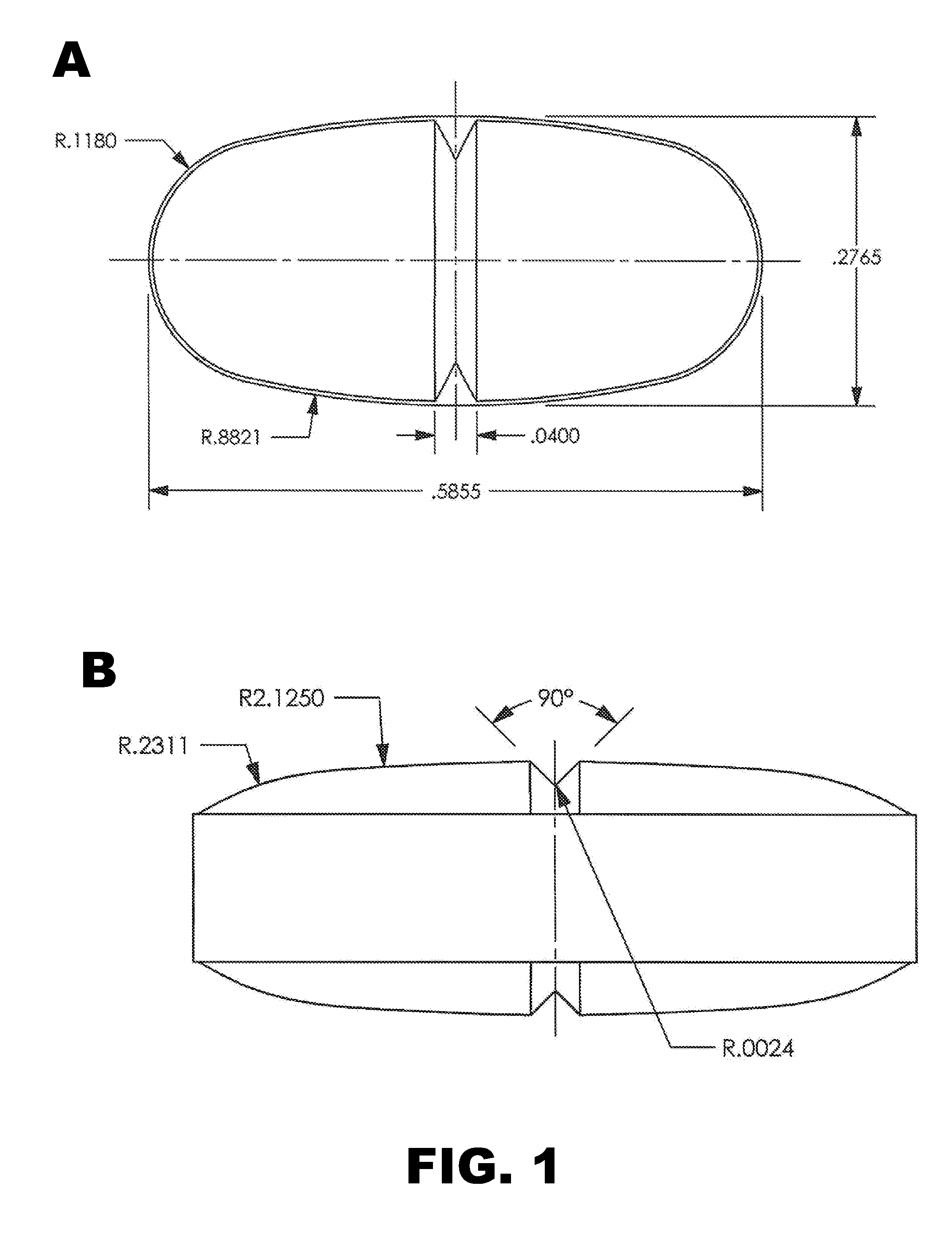
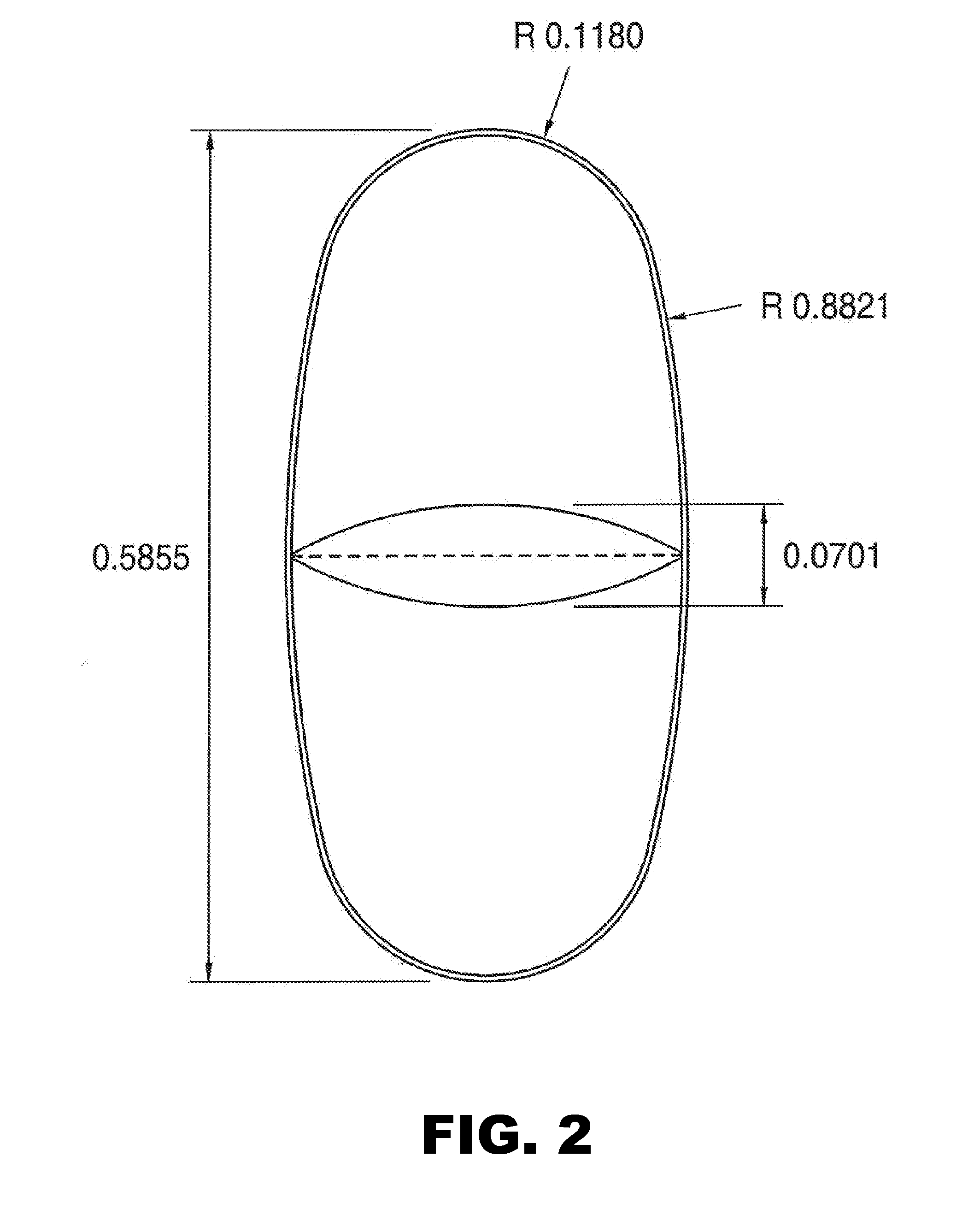
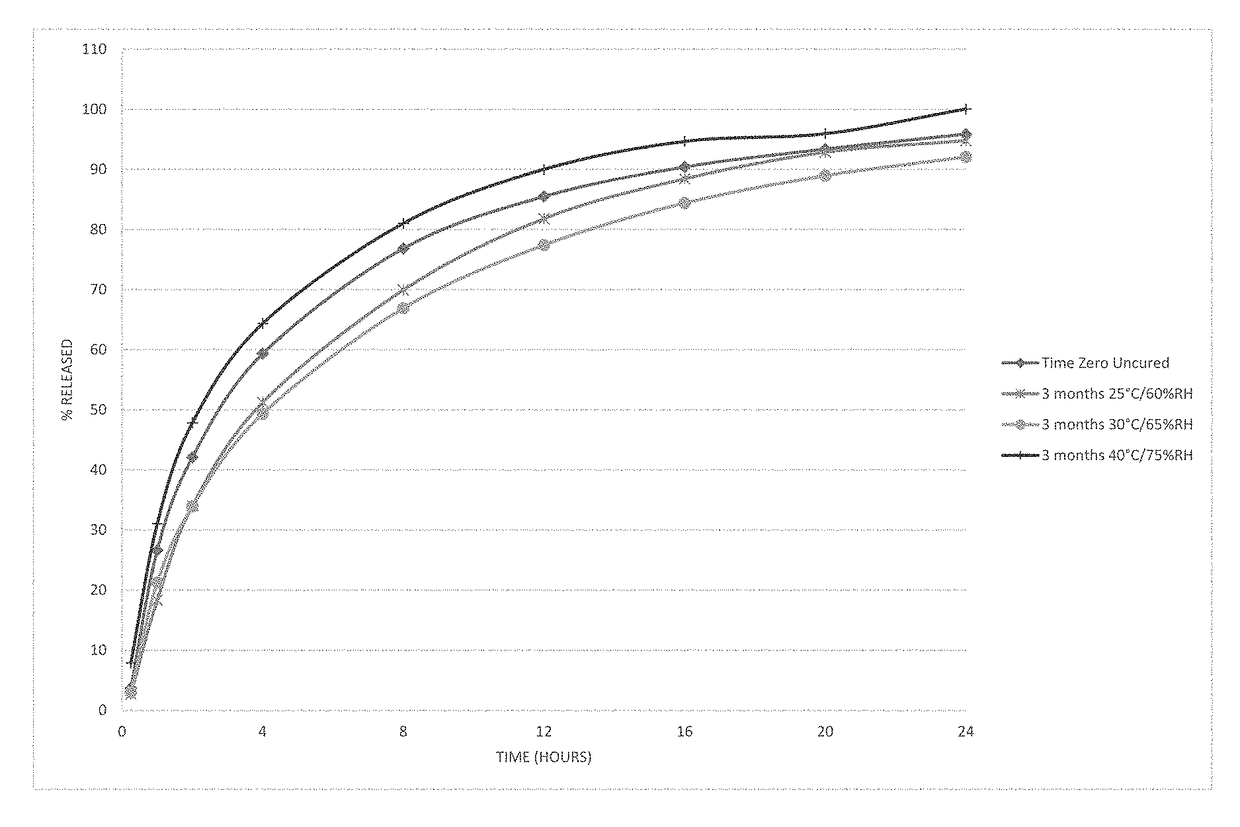
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
96 results about "Abuse deterrent" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Abuse-deterrent opioid formulations (ADFs) are among the various strategies aimed at reducing opioid misuse and abuse. Although ADFs were introduced to the US market in 2010, they are not yet widely used. In a study published in Expert Opinion on Drug Delivery, investigators explored potential barriers to the widespread use of ADFs. 1.
Methods and compositions for deterring abuse of opioid containing dosage forms
This invention relates to an abuse deterrent dosage form of opioid analgesics, wherein an analgesically effective amount of opioid analgesic is combined with a polymer to form a matrix.
Owner:HALSEY DRUG
Abuse-deterrent pharmaceutical compositions of opioids and other drugs
ActiveUS7399488B2Good treatment effectSmall dosePowder deliveryNervous disorderAdditive ingredientWater insoluble
An abuse-deterrent pharmaceutical composition has been developed to reduce the likelihood of improper administration of drugs, especially drugs such as opiods. In the preferred embodiment, a drug is modified to increase its lipophilicity. In preferred embodiments the modified drug is homogeneously dispersed within microparticles composed of a material that is either slowly soluble or not soluble in water. In some embodiments the drug containing microparticles or drug particles are coated with one or more coating layers, where at least one coating is water insoluble and preferably organic solvent insoluble, but enzymatically degradable by enzymes present in the human gastrointestinal tract. The abuse-deterrent composition retards the release of drug, even if the physical integrity of the formulation is compromised (for example, by chopping with a blade or crushing) and the resulting material is placed in water, snorted, or swallowed. However, when administered as directed, the drug is slowly released from the composition as the composition is broken down or dissolved gradually within the GI tract by a combination of enzymatic degradation, surfactant action of bile acids, and mechanical erosion.
Owner:COLLEGIUM PHARMA INC
Methods and compositions for deterring abuse of orally administered pharmaceutical products
This invention relates to an abuse deterrent formulation of an oral dosage form of a therapeutically effective amount of any active drug substance that can be subject to abuse combined with a gel forming polymer, a nasal mucosal irritating surfactant and a flushing agent. Such a dosage form is intended to deter abuse of the active drug substance via injection, nasal inhalation or consumption of quantities of the dosage unit exceeding the usual therapeutically effective dose.
Owner:ACURA PHARMA
Methods and compositions for deterring abuse of orally administered pharmaceutical products
This invention relates to an abuse deterrent formulation of an oral dosage form of a therapeutically effective amount of any active drug substance that can be subject to abuse combined with a gel forming polymer, a nasal mucosal irritating surfactant and a flushing agent. Such a dosage form is intended to deter abuse of the active drug substance via injection, nasal inhalation or consumption of quantities of the dosage unit exceeding the usual therapeutically effective dose.
Owner:ACURA PHARMA
Hydrophobic abuse deterrent delivery system
Disclosed herein are oral dosage forms of therapeutic agents that are resistant to abuse and methods of their formulation. In particular, oral dosage forms that are resistant to dissolution in aqueous solutions of ethanol are described.
Owner:LAB INT
Methods and compositions for deterring abuse of orally administered pharmaceutical products
This invention relates to an abuse deterrent formulation of an oral dosage form of a therapeutically effective amount of any active drug substance that can be subject to abuse combined with a gel forming polymer, a nasal mucosal irritating surfactant and a flushing agent. Such a dosage form is intended to deter abuse of the active drug substance via injection, nasal inhalation or consumption of quantities of the dosage unit exceeding the usual therapeutically effective dose.
Owner:ACURA PHARMA
Abuse-deterrent drug formulations
ActiveUS20050281748A1Reduce the possibilityImprove lipophilicityTelevision system detailsPowder deliveryImmediate releaseActive agent
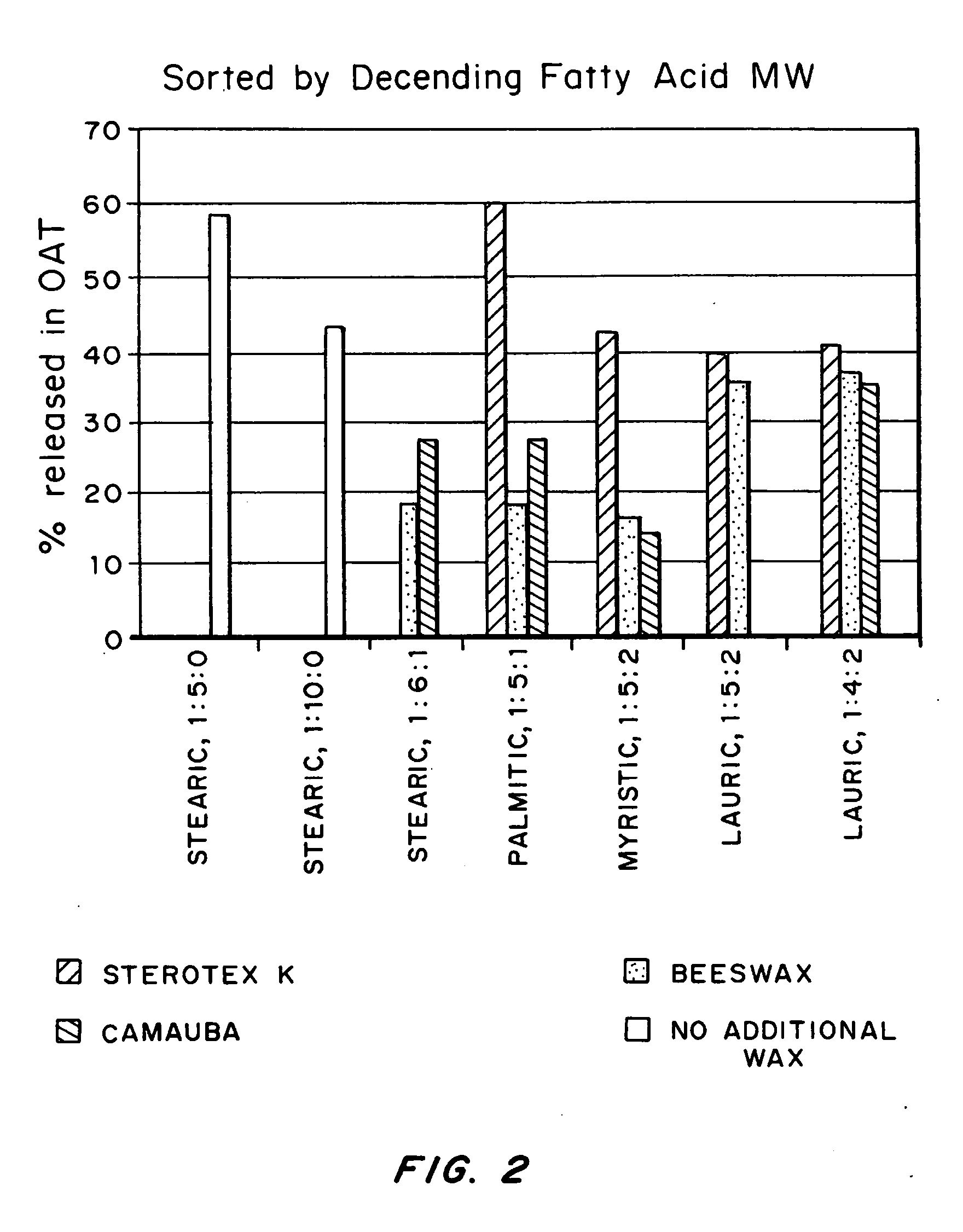
An abuse-deterrent pharmaceutical composition has been developed to reduce the likelihood of improper administration of drugs, especially drugs such as opiods. In the preferred embodiment, the drug is modified to increase its lipophilicity by forming a salt between the drug and one or more fatty acids wherein the concentration of the one or more fatty acids is one to 15 times the molar amount of the active agent, preferably two to ten times the molar amount of the active agent. In one embodiment the modified drug is homogeneously dispersed within microparticles composed of a material that is either slowly soluble or not soluble in water. In some embodiments the drug containing microparticles or drug particles are coated with one or more coating layers, where at least one coating is water insoluble and preferably organic solvent insoluble. The abuse-deterrent composition prevents the immediate release of a substantial portion of drug, even if the physical integrity of the formulation is compromised (for example, by chopping with a blade or crushing) and the resulting material is placed in water, snorted, or swallowed. However, when administered as directed, the drug is slowly released from the composition as the composition is broken down or dissolved gradually within the GI tract by a combination of enzymatic degradation, surfactant action of bile acids, and mechanical erosion.
Owner:COLLEGIUM PHARMA INC
Abuse-deterrent pharmaceutical compositions of opioids and other drugs
ActiveUS20090297617A1Reduce the possibilityImprove lipophilicityBiocidePowder deliveryAs DirectedOrganic solvent
An abuse-deterrent pharmaceutical composition has been developed to reduce the likelihood of improper administration of drugs, especially drugs such as opioids. In a preferred embodiment, a drug is modified to increase its lipophilicity. In some embodiments the modified drug is homogeneously dispersed within spherical microparticles composed of a material that is either slowly soluble or not soluble in water. In some embodiments the drug containing microparticles or drug particles are coated with one or more coating layers, where at least one coating is water insoluble and / or organic solvent insoluble. The abuse-deterrent composition retards the release of drug, even if the physical integrity of the formulation is compromised (for example, by chopping with a blade or crushing) and the resulting material is placed in water, snorted, or swallowed. However, when administered as directed, the drug is slowly released from the composition as the composition is passes through the GI tract.
Owner:COLLEGIUM PHARMA INC
Drug formulations
InactiveUS20120202838A1Improve fracture toughnessImprove fracture resistancePowder deliveryBiocideHigh fractureMedicine
In one embodiment, the present invention relates to abuse-deterrent drug formulations comprise a plurality of discrete domains uniformly dispersed in a pharmaceutically acceptable matrix, wherein said domains have high fracture toughness and comprise at least one polymer and at least one abuse-relevant drug. In another embodiment, the present invention relates to a formulation comprising a plurality of discrete mechanically reinforcing particles uniformly dispersed in a pharmaceutically acceptable matrix, wherein said matrix has high fracture toughness and comprises at least one polymer and at least one active agent, at least one abuse-relevant drug or a combination of at least one active agent and at least one abuse-relevant drug.
Owner:ABBVIE INC
Technology for preventing abuse of solid dosage forms
InactiveUS20120321716A1Excessive amountReduce probabilityBiocidePowder deliveryAbuse deterrentPharmaceutical formulation
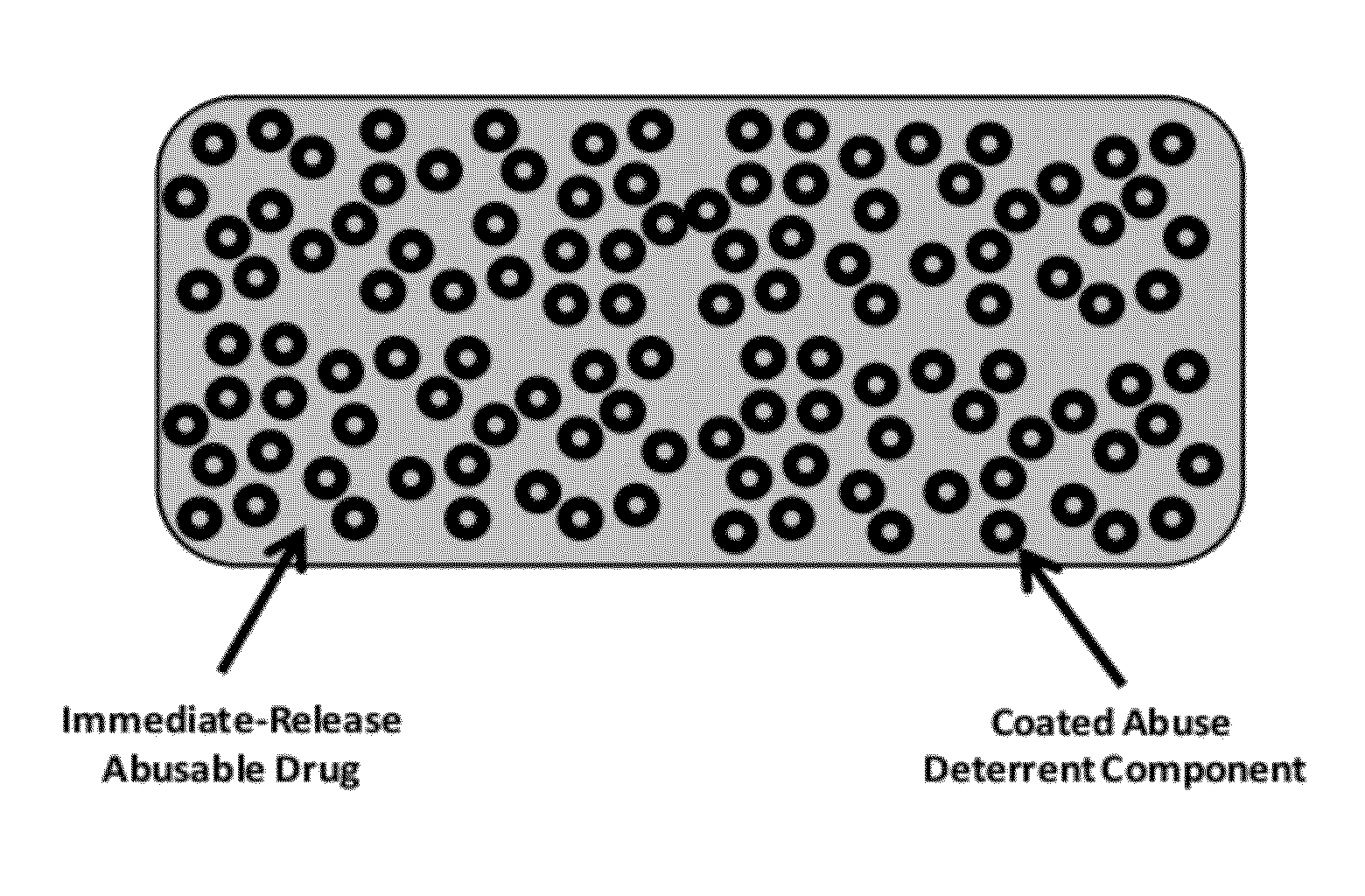
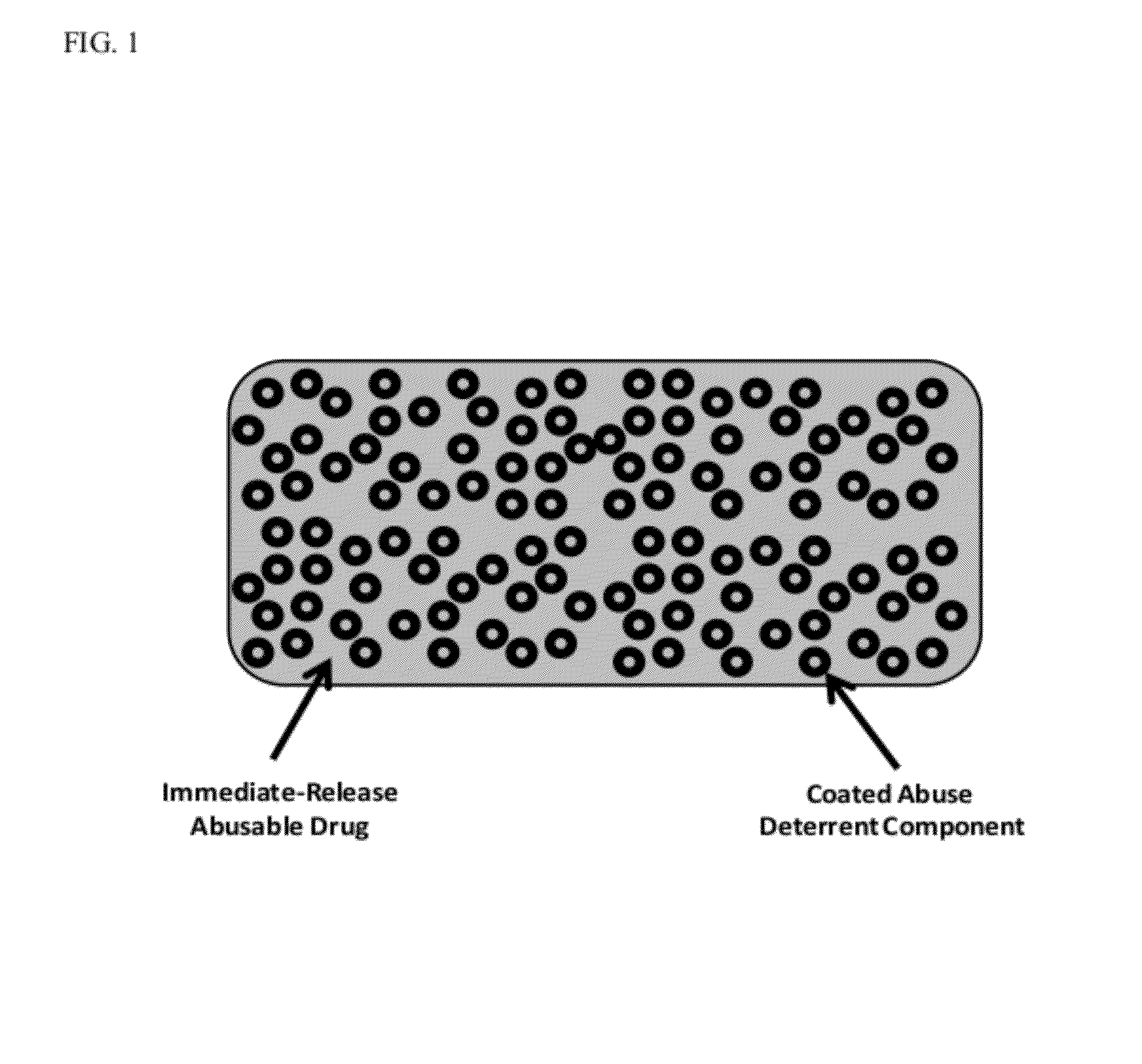
Abuse resistant pharmaceutical formulations are provided that contain one or more abusable drugs and one or more abuse deterrent components. The abuse deterrent component(s) prevent the abusable drug(s) from being removed / extracted to an appreciable extent and / or rate. The abuse deterrent component(s) may be in the form of pellets, beads, beadlets, granules, powders, or the like, and may comprise a core that contains a material that is both hydrophilic and hydrophobic, and optionally a pH-dependent coating.
Owner:VACHON MICHAEL +1
Hydrophobic abuse deterrent delivery system for hydromorphone
Disclosed herein are oral dosage forms of hydromorphone that are resistant to abuse and methods of their formulation. In particular, oral dosage forms that are resistant to dissolution in aqueous solutions of ethanol are described.
Owner:LAB INT
Methods and compositions for deterring abuse of orally administered pharmaceutical products
This invention relates to an abuse deterrent formulation of an oral dosage form of a therapeutically effective amount of any active drug substance that can be subject to abuse combined with a gel forming polymer, a nasal mucosal irritating surfactant and a flushing agent. Such a dosage form is intended to deter abuse of the active drug substance via injection, nasal inhalation or consumption of quantities of the dosage unit exceeding the usual therapeutically effective dose.
Owner:ACURA PHARMA
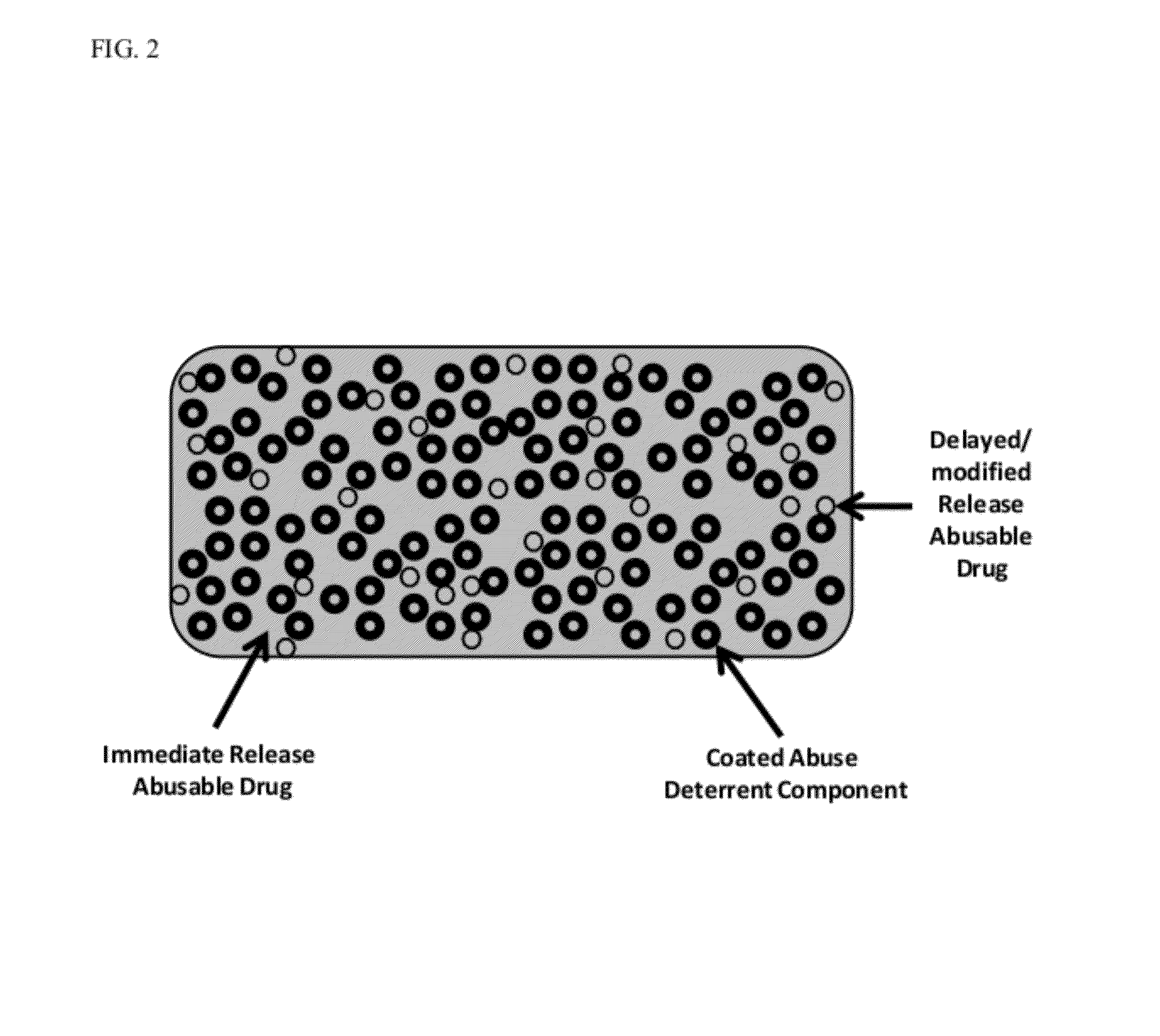
Immediate Release, Abuse Deterrent Pharmaceutical Compositions
The present disclosure provides pharmaceutical compositions and processes for making solid dosage form pharmaceutical compositions that provide immediate release of active ingredients and have abuse deterrent properties. The pharmaceutical compositions provided herein comprise at least one pharmaceutically active ingredient, at least one low molecular weight hydrophilic polymer, at least one high molecular weight hydrophilic polymer, and an effervescent system.
Owner:SPECGX LLC
Abuse deterrent pharmaceutical compositions for controlled release
The present disclosure relates to pharmaceutical compositions that are abuse resistant and may also provide controlled release. The present disclosure also relates to the use of pharmaceutical compositions in the treatment of pain.
Owner:EGALET LTD
Abuse-deterrent pharmaceutical compositions of opioids and other drugs
InactiveUS20080199530A1Reduce the possibilityImprove lipophilicityPowder deliveryNervous disorderAdditive ingredientWater insoluble
Owner:COLLEGIUM PHARMA INC
Extended Release, Abuse Deterrent Pharmaceutical Compositions
The present disclosure provides pharmaceutical compositions comprising at least one active pharmaceutical ingredient or a pharmaceutically acceptable salt thereof, at least one hydrophilic plastomer, optionally at least one hydrophilic elastomer, and at least one deliquescent plasticizer, wherein the pharmaceutical compositions provide extended release of the API and have abuse deterrent properties. Also provided are methods for preparing the pharmaceutical compositions in which the components of the composition are humidified such that the deliquescent plasticizer deliquesces, thereby plasticizing the hydrophilic polymers.
Owner:SPECGX LLC
Abuse-deterrent pharmaceutical compositions of opioids and other drugs
ActiveUS8557291B2Reduce the possibilityImprove lipophilicityPowder deliveryBiocideOrganic solventAs Directed
An abuse-deterrent pharmaceutical composition has been developed to reduce the likelihood of improper administration of drugs, especially drugs such as opioids. In a preferred embodiment, a drug is modified to increase its lipophilicity. In some embodiments the modified drug is homogeneously dispersed within spherical microparticles composed of a material that is either slowly soluble or not soluble in water. In some embodiments the drug containing microparticles or drug particles are coated with one or more coating layers, where at least one coating is water insoluble and / or organic solvent insoluble. The abuse-deterrent composition retards the release of drug, even if the physical integrity of the formulation is compromised (for example, by chopping with a blade or crushing) and the resulting material is placed in water, snorted, or swallowed. However, when administered as directed, the drug is slowly released from the composition as the composition is passes through the GI tract.
Owner:COLLEGIUM PHARMA INC
Safety and abuse deterrent improved device
InactiveUS20090211576A1Inhibition of activationAvoid accessRespiratorsMedical devicesMedicineBiological activation
Modifications of an inhaler and method of use thereof are described. In some embodiments, an inhaler may include one or more systems and / or methods that function to inhibit a child from accessing medicant from an inhaler. A system may function to inhibit a child from accessing an opening of an inhaler from which medicants are dispensed. In some embodiments, a system may inhibit a child from removing a cap for an inhaler. A system may function to inhibit a child from actuating an activation mechanism for an inhaler. A system may function to inhibit a user and / or a child from accidentally actuating an activation mechanism for an inhaler multiple times possibly resulting in an overdose. A system may function to inhibit an activation mechanism for an inhaler from actuating after the activation mechanism has been actuated a predetermined number of times. An inhaler may include a security fitting which functions to permanently plug an inhaler once a prescribed user is finished with the inhaler.
Owner:LEHTONEN TIMO +4
Methods and compositions for self-regulated release of active pharmaceutical ingredient
An abuse deterrent pharmaceutical composition including a pharmaceutically active ingredient; an acid soluble ingredient; and a buffering ingredient; wherein the acid soluble ingredient and the buffering ingredient retard release of the active pharmaceutical ingredient when the composition is ingested in excess of an intended dosage.
Owner:ACURA PHARMA
Methods and compositions for self-regulated release of active pharmaceutical ingredient
ActiveUS9101636B2Reduce releaseOrganic active ingredientsNervous disorderDrug activityBULK ACTIVE INGREDIENT
Owner:ACURA PHARMA
Abuse-deterrent dosage forms
ActiveUS20140271848A1Abuse potentialLow dosage formBiocideAnimal repellantsOpioid AgonistBenzodiazepine
Abuse-deterrent dosage forms (e.g., orally administered dosage forms, transdermal dosage forms, suppositories, etc.), processes for formulating pharmaceutical dosage forms comprising an active agent susceptible to abuse (e.g., an opioid agonist, a benzodiazepine, a stimulant) such that the resulting dosage forms are abuse-deterrent are described. Also described are methods of deterring abuse of pharmaceutical dosage forms.
Owner:PURDUE PHARMA LP
Abuse deterrent compositions and methods of use
InactiveUS20150005334A1Reduce intensityReduce frequencyBiocideAnimal repellantsOral medicationMedicine
Orally administrable pharmaceutical compositions, methods of administration, and methods of making the same are provided. The pharmaceutical compositions provide abuse deterrent properties.
Owner:OHEMO LIFE SCI INC
Opioid receptor modulator dosage formulations
ActiveUS20140271854A1Extended shelf lifeImprove stabilityBiocideOrganic active ingredientsBenzoic acidMixed Opioid Agonist/Antagonist
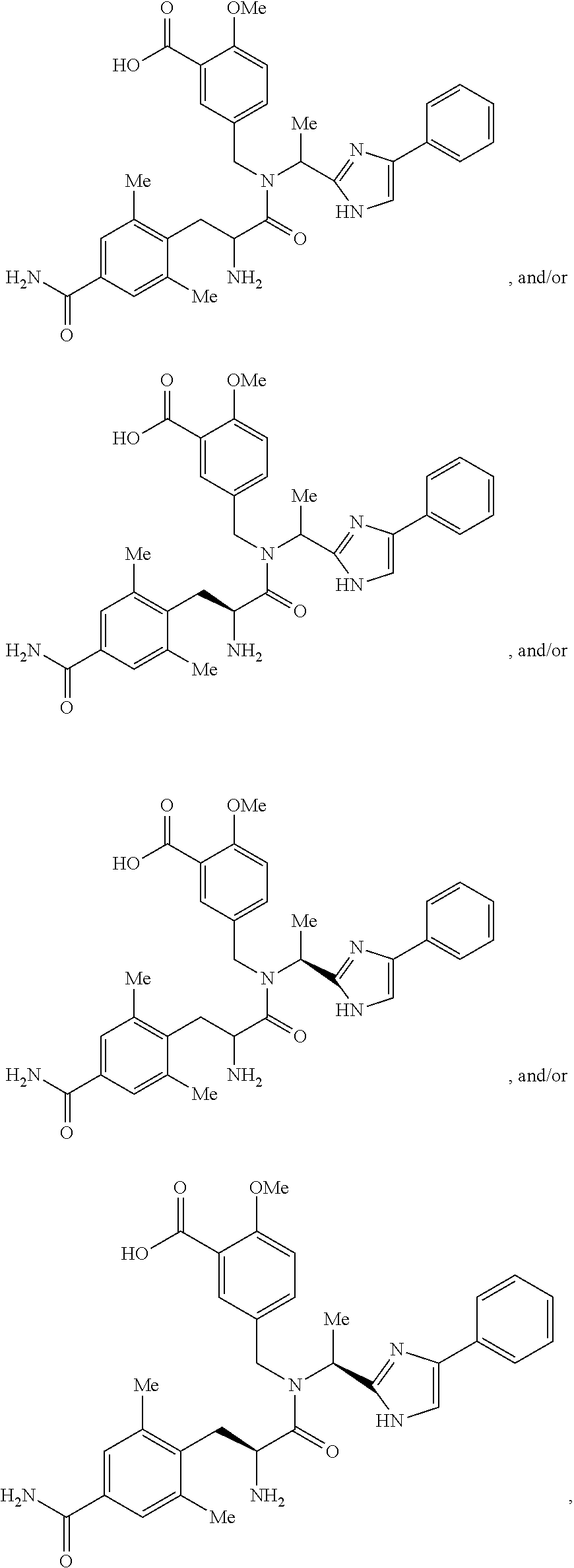
Abuse deterrent solid dosage formulations containing 5-({[2-Amino-3-(4-carbamoyl-2,6-dimethyl-phenyl)-propionyl]-[1-(4-phenyl-1H-imidazol-2-yl)-ethyl]-amino}-methyl)-2-methoxy-benzoic acid, and processes for the preparation and administration of these formulations.
Owner:ALLERGAN HLDG UNLTD
Abuse-deterrent pharmaceutical compositions for controlled release
The present disclosure relates to pharmaceutical compositions that are abuse resistant and may also provide controlled release. The present disclosure also relates to the use of pharmaceutical compositions in the treatment of pain.
Owner:EGALET LTD
Opioid and attention deficit hyperactivity disorder medications possessing abuse deterrent and anti-dose dumping safety features
ActiveUS10183001B1Pharmaceutical delivery mechanismHeterocyclic compound active ingredientsDrug productAbuse deterrent
An abuse deterrent drug product is provided wherein the drug product comprises a matrix and a drug substance in the matrix wherein the drug substance is defined as a 1:1 salt of a pharmaceutically active compound and BNDO.
Owner:PISGAH LAB
Abuse deterrent immediate release biphasic matrix solid dosage form
InactiveUS20150272902A1Deter the abuse of drugsLow release ratePowder deliveryOrganic active ingredientsImmediate releasePsychiatry
An abuse deterrent immediate release biphasic matrix solid dosage form that releases the drug at a desired rate for quick onset of action when a single unit or prescribed units of the dosage form are orally administered but exhibits a reduced rate of release when more than the prescribed number of units, are administered.
Owner:SUN PHARMA INDS
Abuse deterrent solid dosage form for immediate release with functional score
The present disclosure provides an immediate release, abuse deterrent pharmaceutical solid dosage form comprising at least one functional score. In particular, the immediate release, abuse deterrent solid dosage form comprises at least one low molecular weight hydrophilic polymer, at least one high molecular weight hydrophilic polymer, and an effervescent system.
Owner:SPECGX LLC
Abuse-resistant drug formulations
ActiveUS20160317530A1Decrease potential for and inhibit abusePrevent or at least significantly reduce the potential for abuseOrganic active ingredientsInorganic non-active ingredientsMedicineBULK ACTIVE INGREDIENT
Disclosed are abuse resistant oral pharmaceutical compositions that reduce the likelihood of improper administration of drugs that are susceptible to abuse. The oral pharmaceutical formulations contain abuse deterrent agents that cause discomfort to the user when administered in an improper manner and make the extraction of an active ingredient more difficult. Methods of making and using the compositions are also disclosed.
Owner:AMNEAL COMPLEX PROD RES LLC
Abuse Deterrent Solid Dosage Form for Immediate Release with Functional Score
The present disclosure provides an immediate release, abuse deterrent pharmaceutical solid dosage form comprising at least one functional score. In particular, the immediate release, abuse deterrent solid dosage form comprises at least one low molecular weight hydrophilic polymer, at least one high molecular weight hydrophilic polymer, and an effervescent system.
Owner:SPECGX LLC
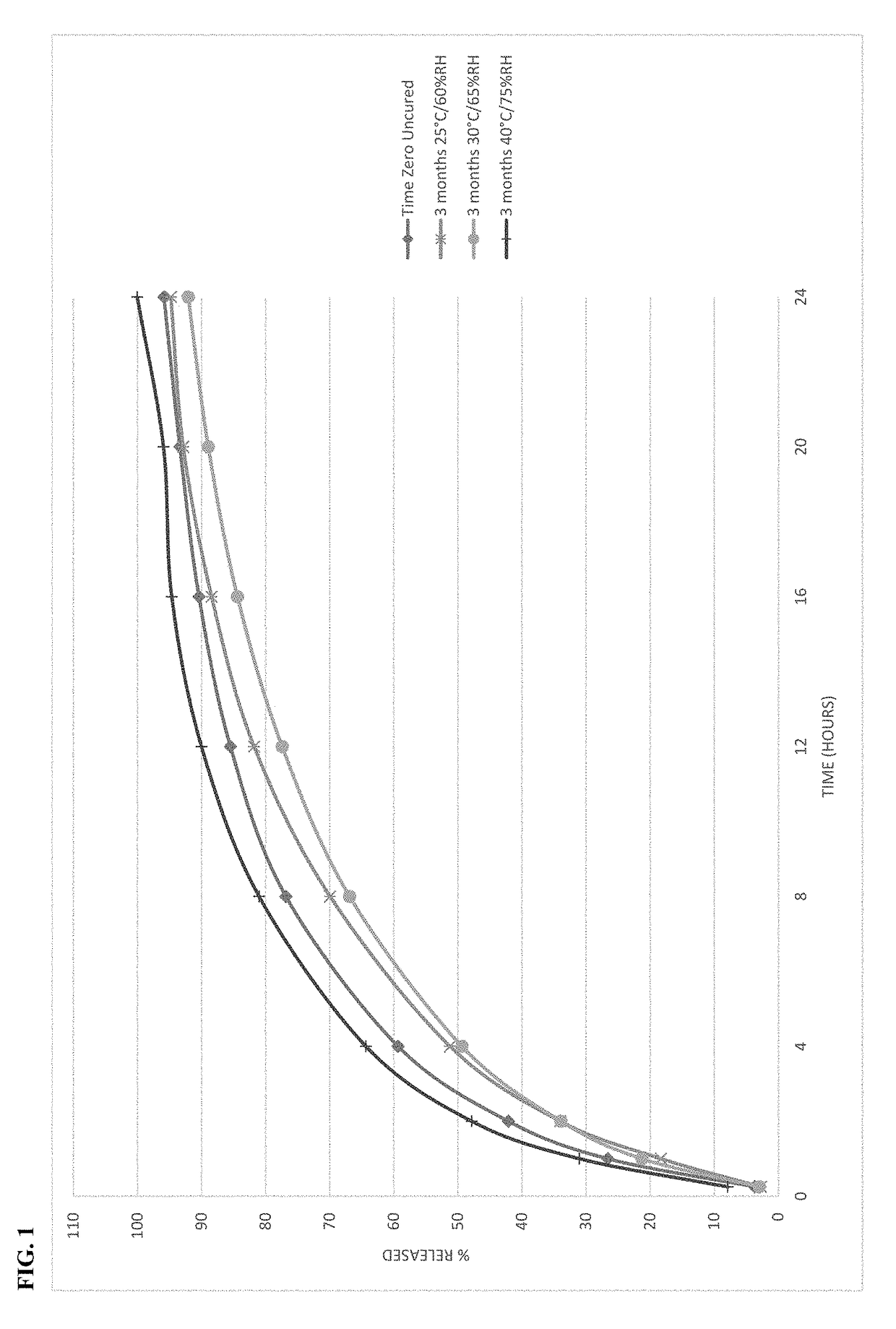
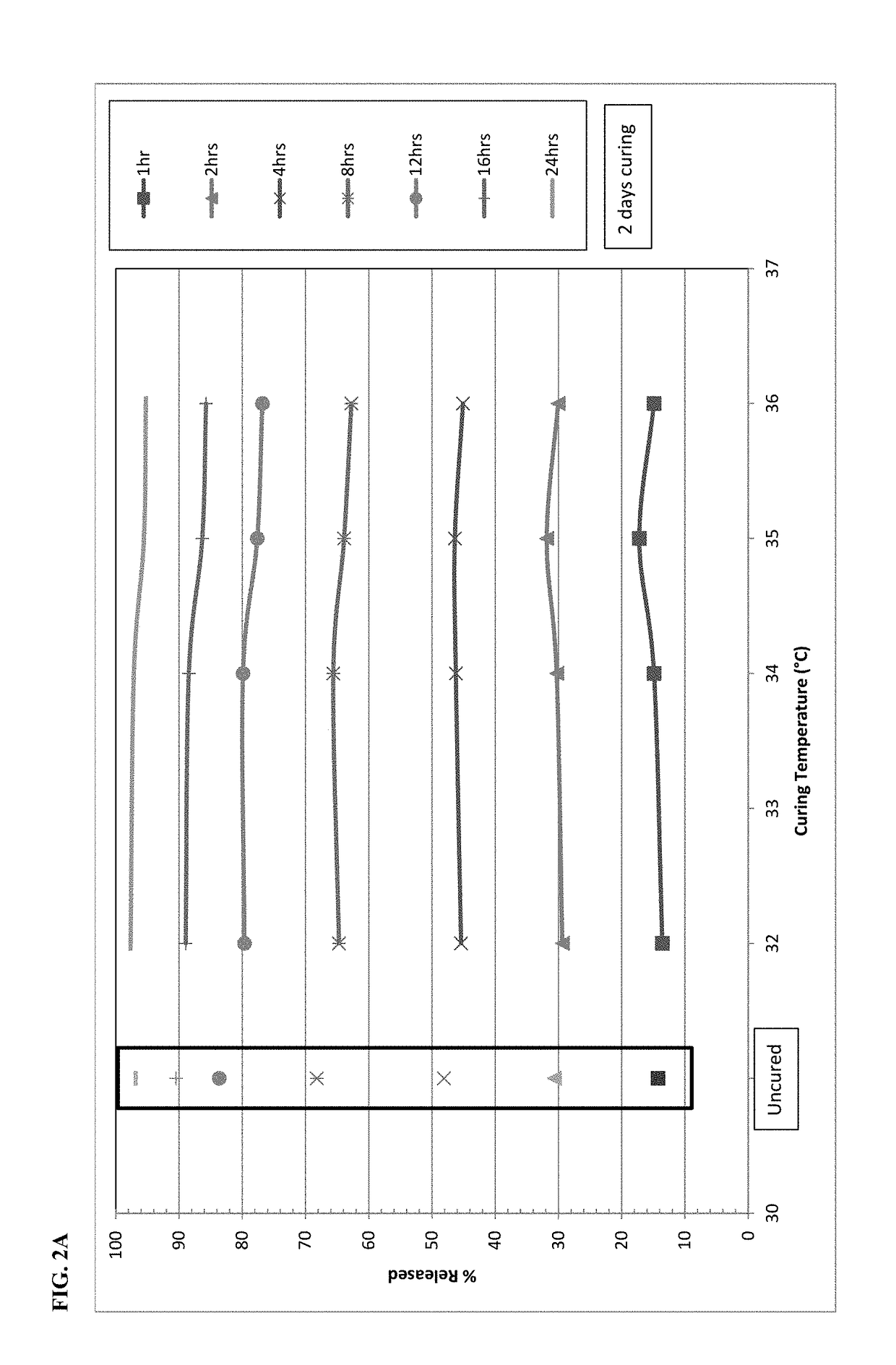
Process of making stable abuse-deterrent oral formulations
The present disclosure relates to cured pharmaceutical compositions designed to reduce the potential for improper administration of drugs that are subject to abuse, the process of curing such composition in order to improve the dissolution stability, method of using the same for treatment of pain.
Owner:COLLEGIUM PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com