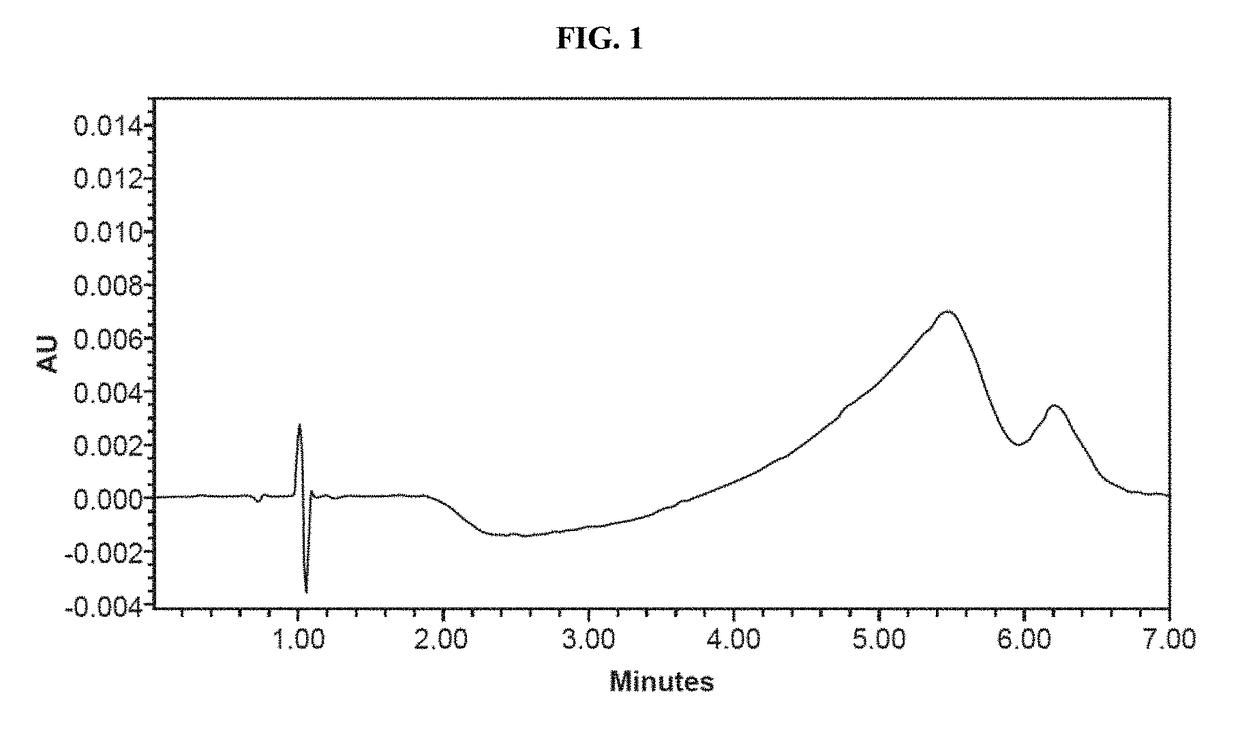
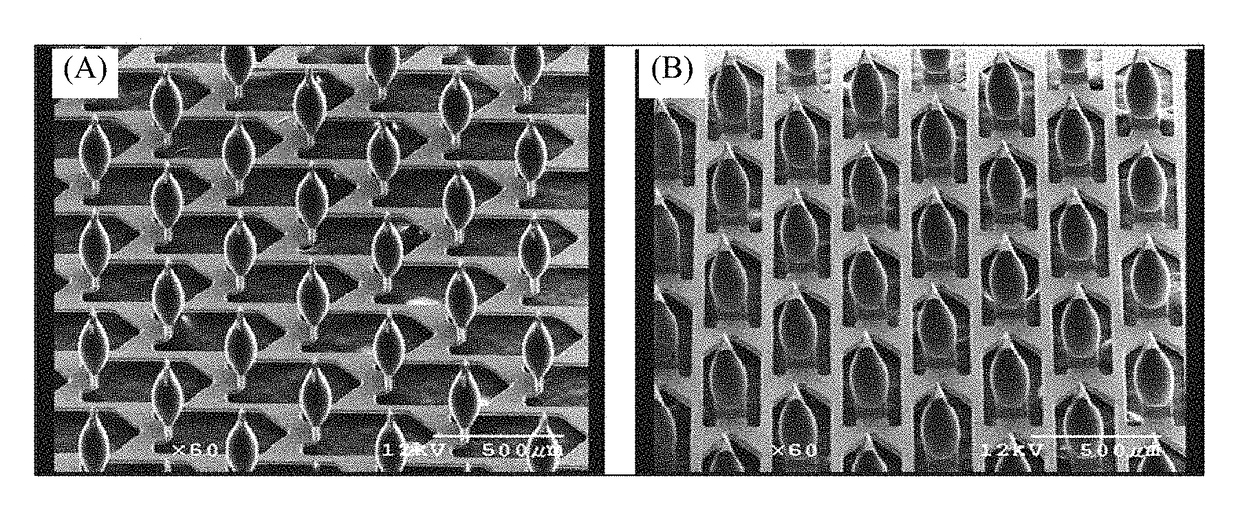
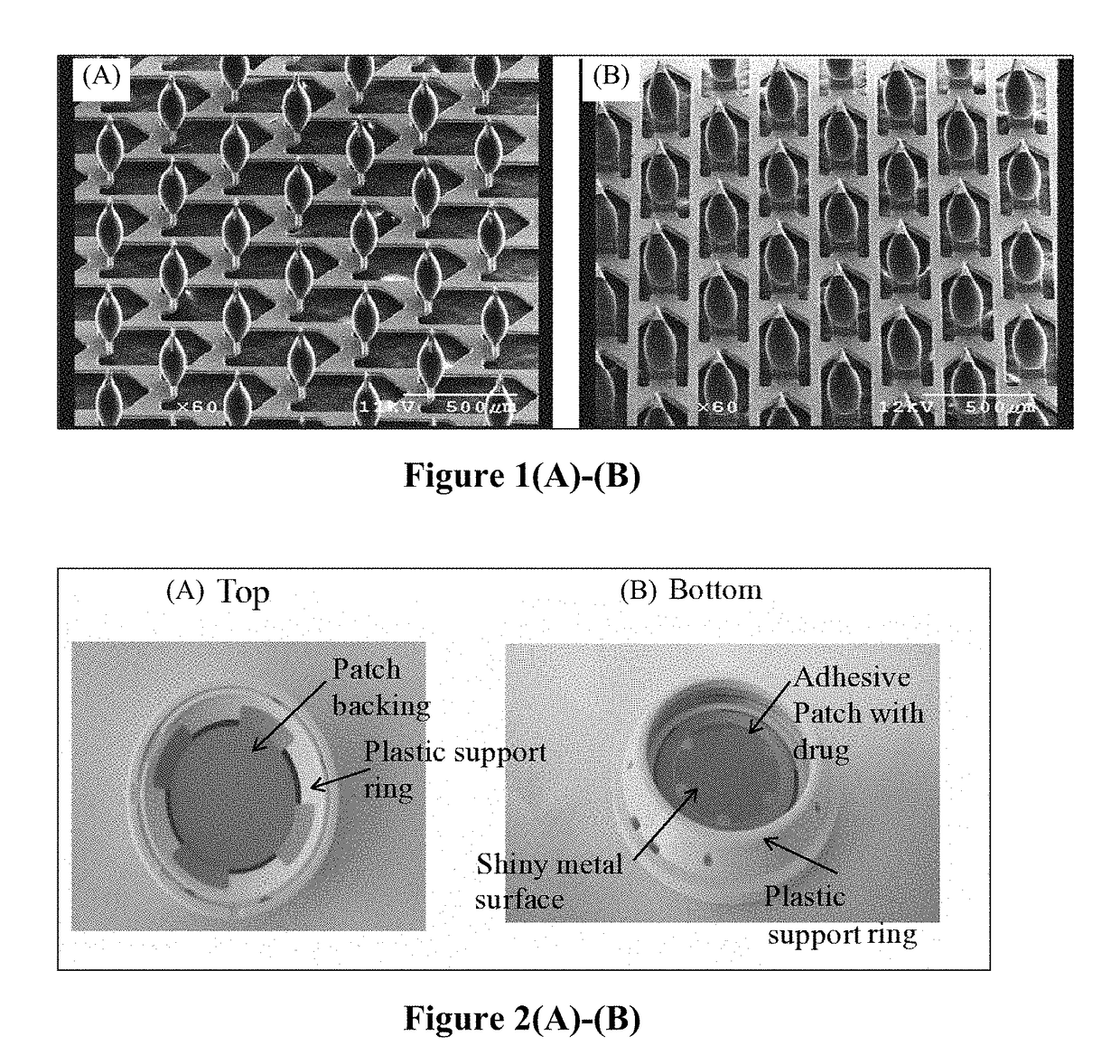
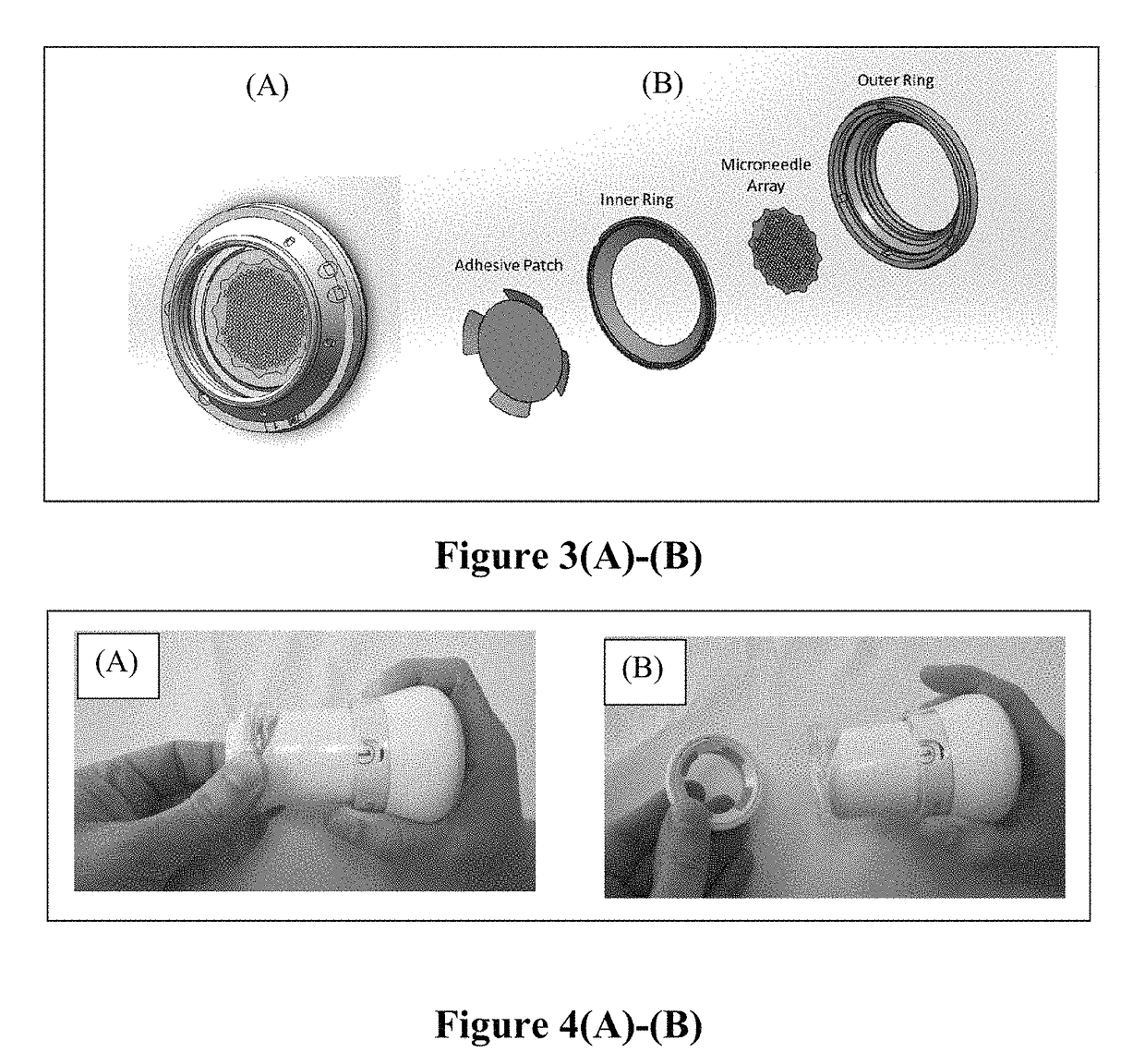
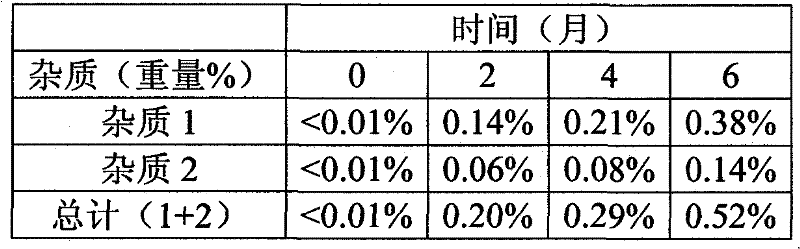
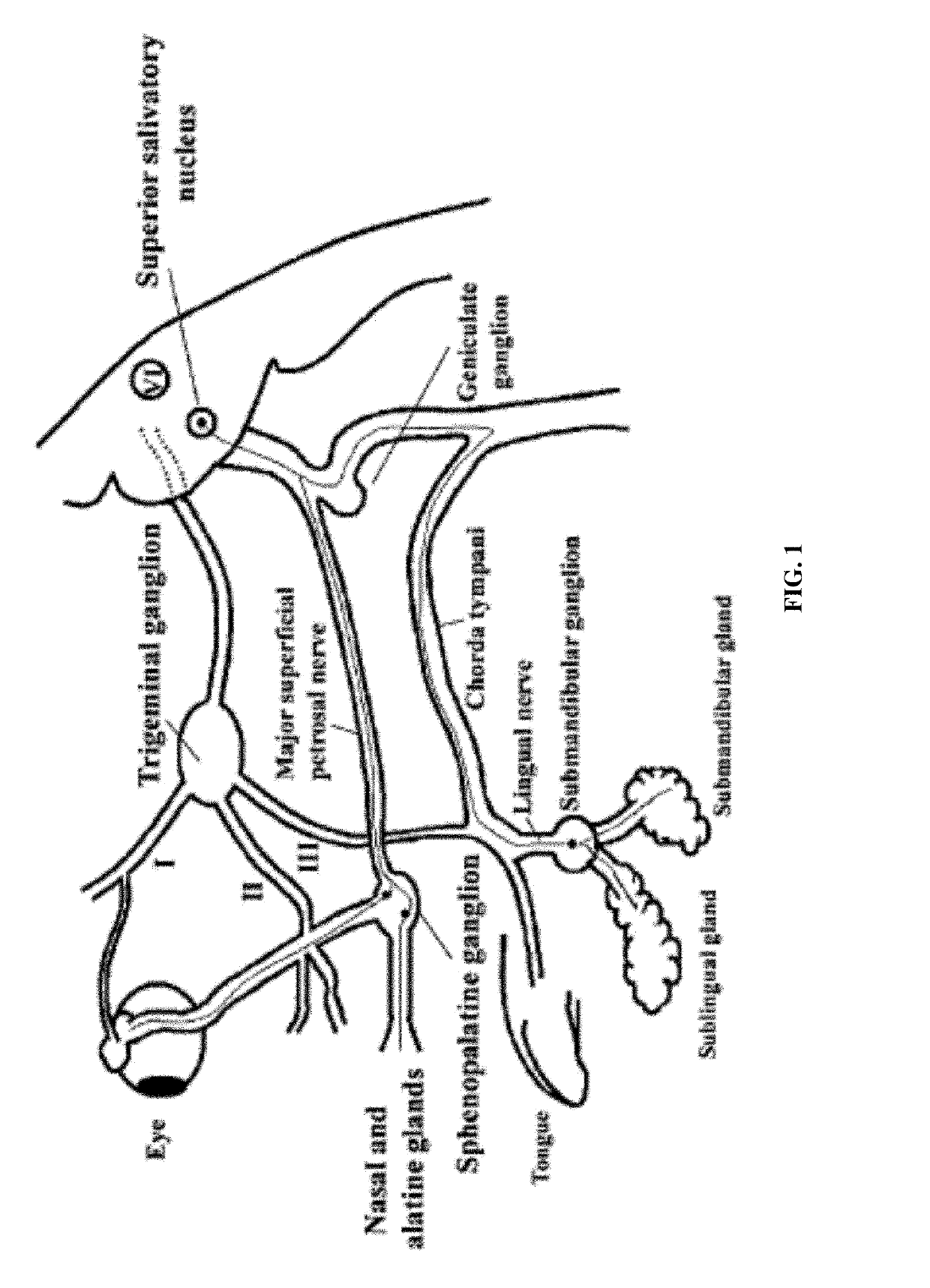
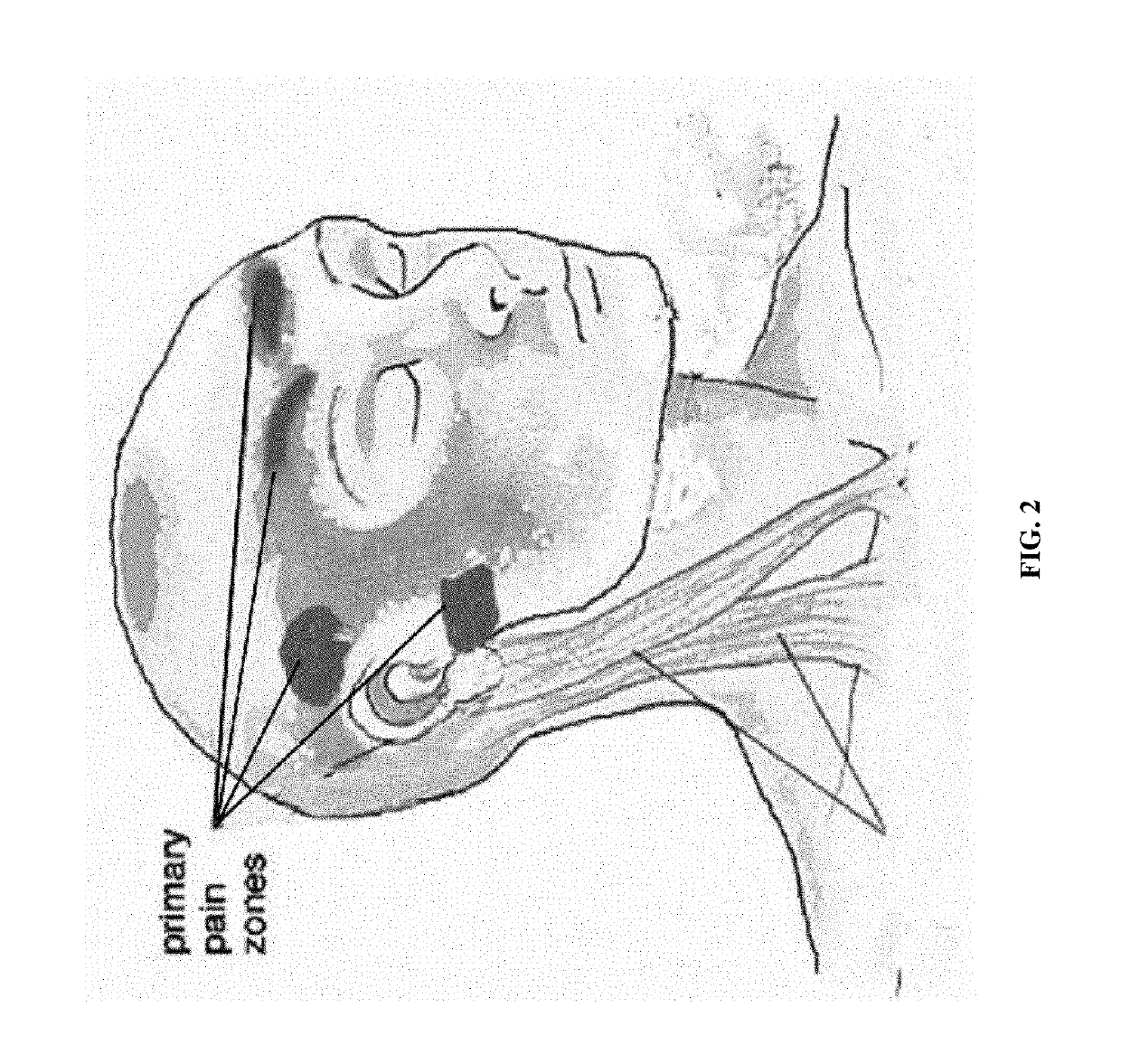
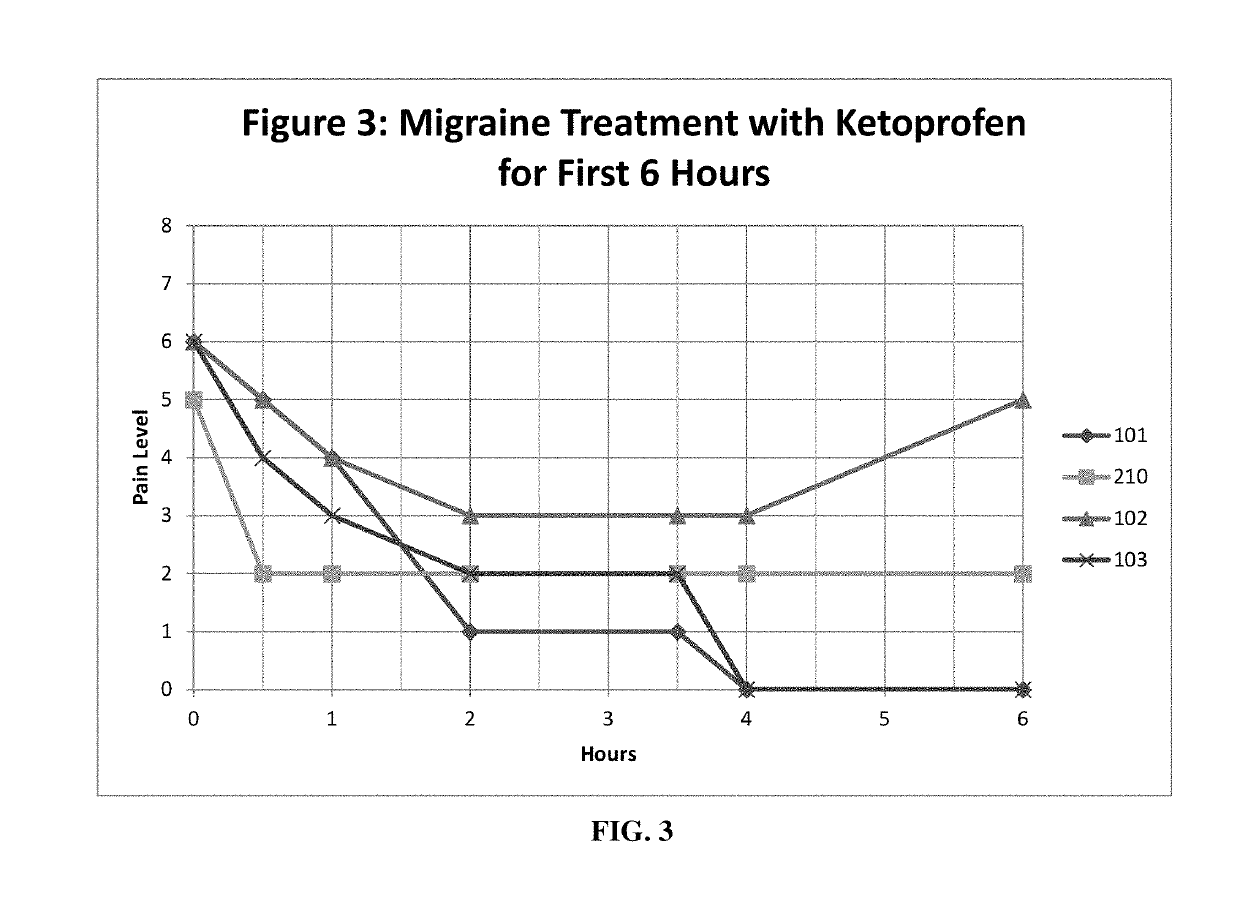
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
35 results about "Triptans" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
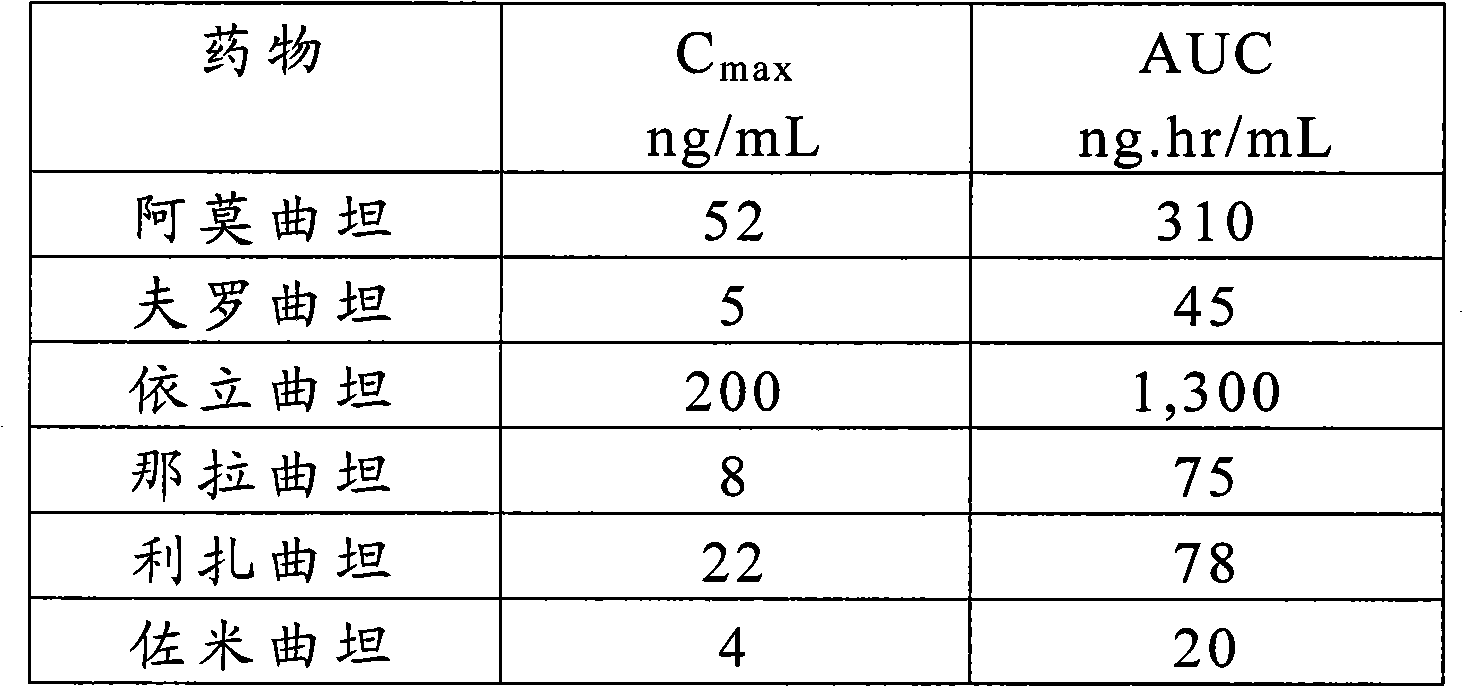
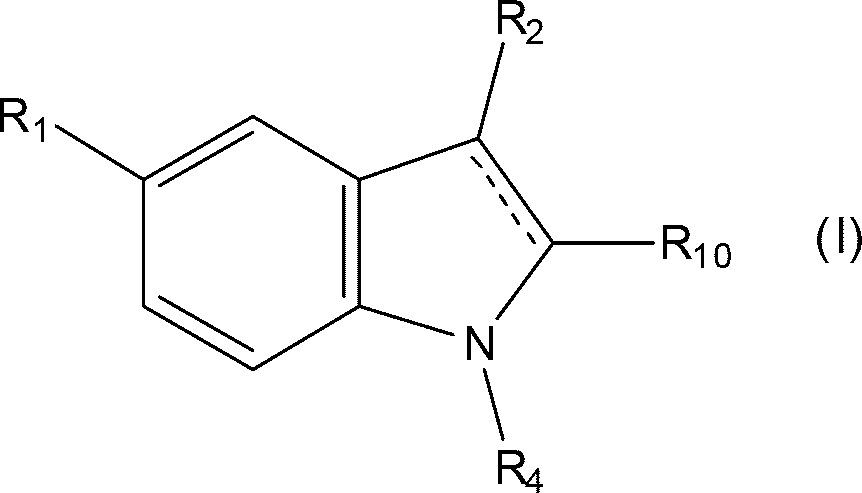
Triptans are a family of tryptamine-based drugs used as abortive medication in the treatment of migraines and cluster headaches. This drug class was first introduced in the 1990s. While effective at treating individual headaches, they do not provide preventive treatment and are not considered a cure. They are not effective for the treatment of tension–type headache, except in persons who also experience migraines. They may be effective in disabling tension–type headaches, which exist on a spectrum of migraine. Triptans do not relieve other kinds of pain.
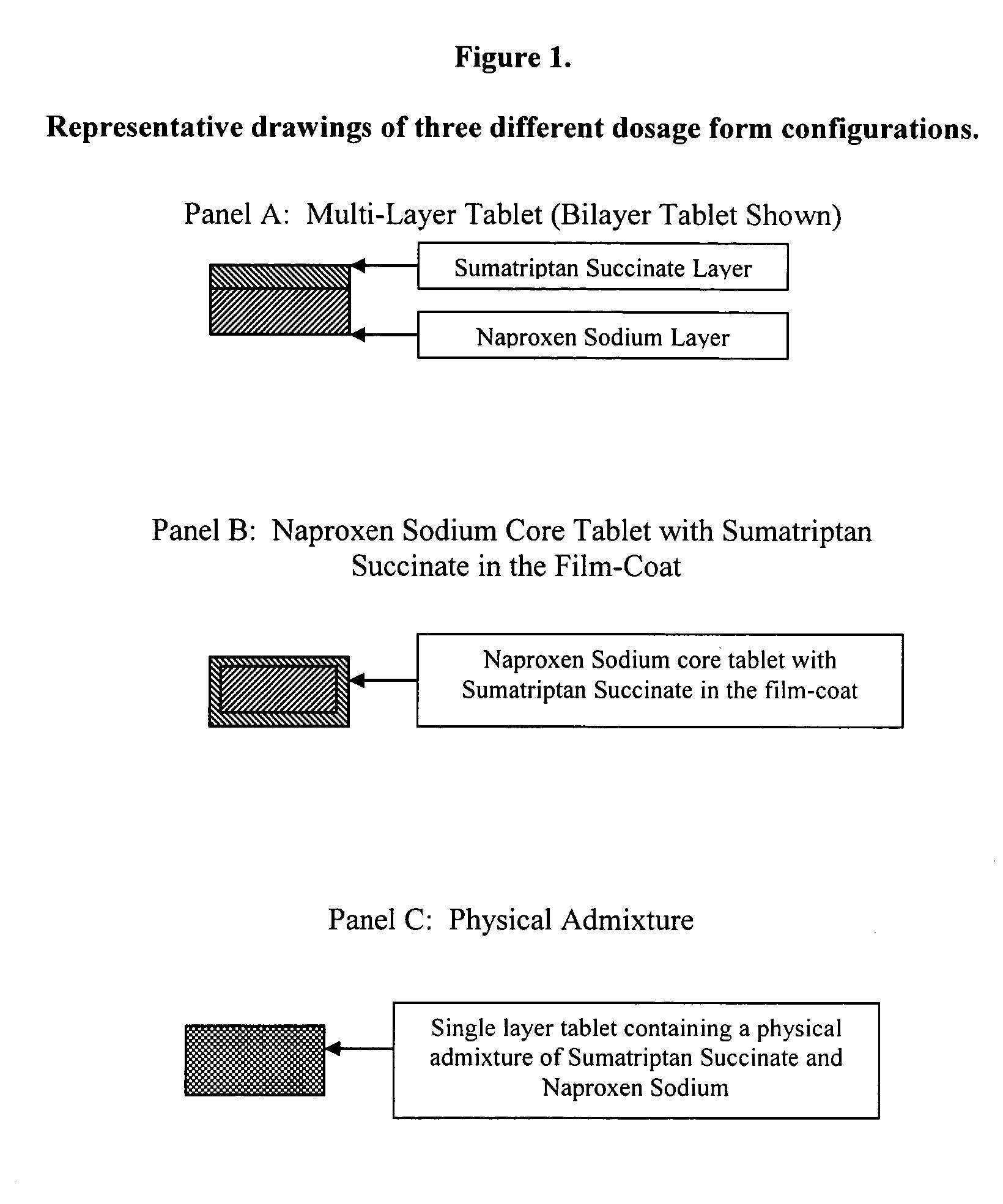
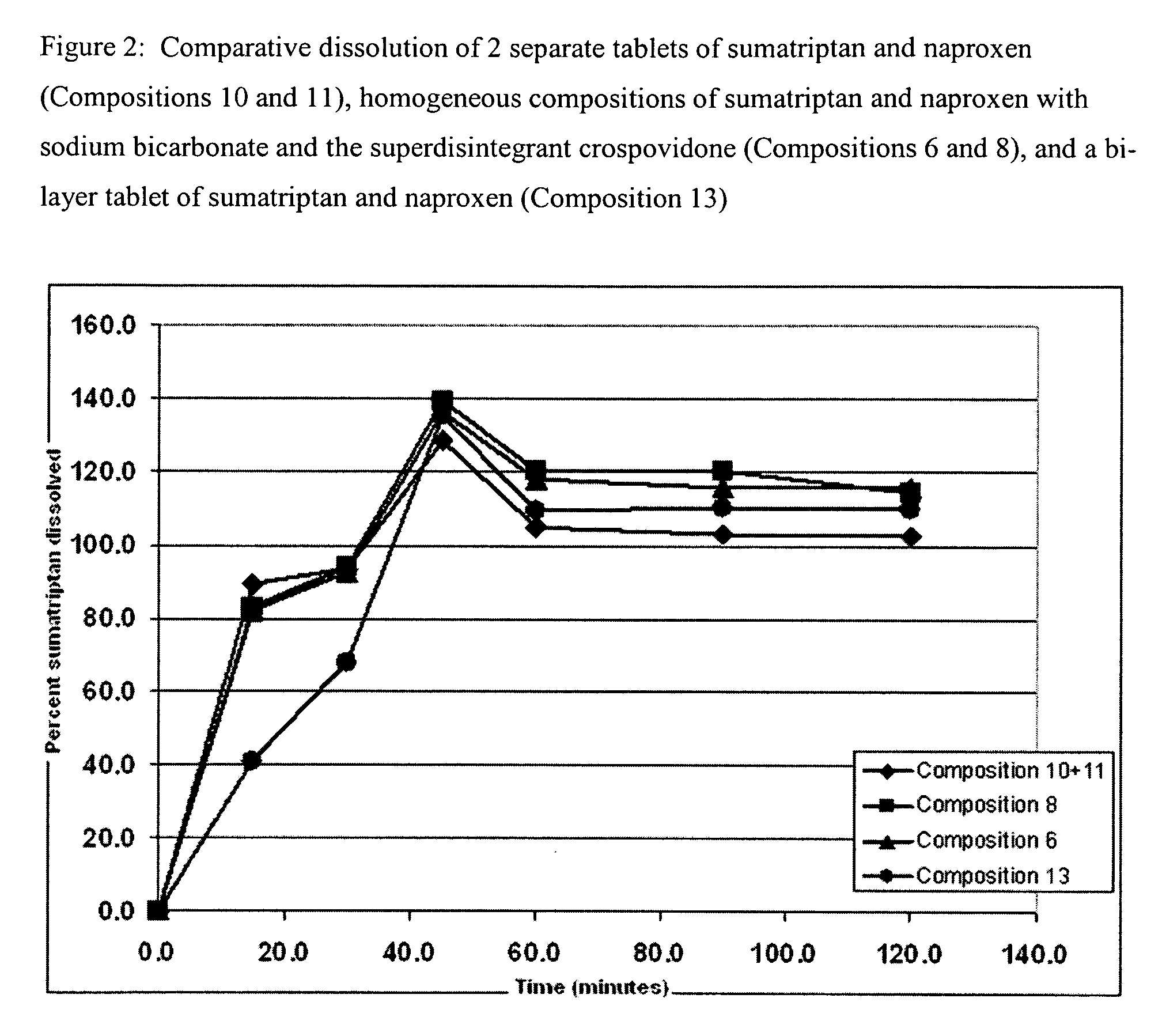
Multilayer dosage forms containing NSAIDs and triptans
The present invention is directed to multilayer pharmaceutical tablets in which an NSAID and a triptan are present in separate and distinct layers. The layers are in a side-by-side configuration, which allows the dissolution of triptan and NSAID to occur independently and immediately.
Owner:NUVO PHARMA IRELAND DESIGNATED ACTIVITY CO
Dosage forms for administering combinations of drugs
The present invention is directed to dosage forms that can be used in therapeutic methods involving the oral co-administration of a combination of at least two drugs, one of which impairs gastrointestinal absorption and one of which does not. The dosage forms are designed so that the drug impairing absorption is not released into the gastrointestinal tract of a patient until after the drugs that do not impair absorption have been released and substantially absorbed. The invention may be used in treatment of migraine using a combination of triptans and NSAIDs or in the treatment of pain using a combination of NSAIDs and opioid analgesics.
Owner:POZEN INC
Rapid dissolution of combination products
Provided are rapidly dissolving pharmaceutical oral dosage forms of triptans and NSAIDs, processes for the preparation thereof, and methods of treatment therewith.
Owner:TEVA PHARM USA INC
Combination of a triptan and an nsaid
InactiveUS20090311335A1Short time to TmaxOrganic active ingredientsPowder deliveryParticulatesPharmacology
Owner:ALKERMES PHARMA IRELAND LTD
Dosage forms for administering combinations of drugs
InactiveUS20070207200A1Efficient and rapid deliveryImpairs absorptionOrganic active ingredientsNervous disorderCo administrationGastrointestinal absorption
The present invention is directed to dosage forms that can be used in therapeutic methods involving the oral co-administration of a combination of at least two drugs, one of which impairs gastrointestinal absorption and one of which does not. The dosage forms are designed so that the drug impairing absorption is not released into the gastrointestinal tract of a patient until after the drugs that do not impair absorption have been released and substantially absorbed. The invention may be used in treatment of migraine using a combination of triptans and NSAIDs or in the treatment of pain using a combination of NSAIDs and opioid analgesics.
Owner:POZEN INC
Methods for treating visceral pain
InactiveUS20090163451A1Minimal effectBiocideSalicyclic acid active ingredientsDiseaseIntestinal structure
The invention features methods of treating visceral pain in humans by administering an effective amount of a 5HT1B or 5HT1D receptor agonist, (e.g., a triptan). These methods can be used, for example, to treat a human suffering from visceral pain secondary to an underlying disease of a visceral organ, such as pancreatitis. Visceral pain treatable by the methods of the invention may also be secondary to a disease of the liver, kidney, ovary, uterus, bladder, bowel, stomach, esophagus, duodenum, intestine, colon, spleen, pancreas, appendix, heart, or peritoneum.
Owner:THE ARIZONA BOARD OF REGENTS ON BEHALF OF THE UNIV OF ARIZONA
Combination treatment of specific forms of epilepsy
InactiveUS20170071949A1Reduce seizuresEliminate side effectsAnhydride/acid/halide active ingredientsHeterocyclic compound active ingredientsSide effectCo morbidity
Formulations for and methods of treatment of Dravet syndrome that avoid side effects are disclosed. The formulations comprise a 5-HT receptor agonists which does not agonize selected 5-HT receptor subtypes, and in particular does not agonize the receptor subtype 5-HT2B. Also disclosed are combinations of such 5-HT receptor agonists. Also disclosed are combinations of such 5-HT receptor agonists and SSRIs, SNRIs, and triptans for treating co-morbidities associated with Dravet syndrome.
Owner:ZOGENIX INT
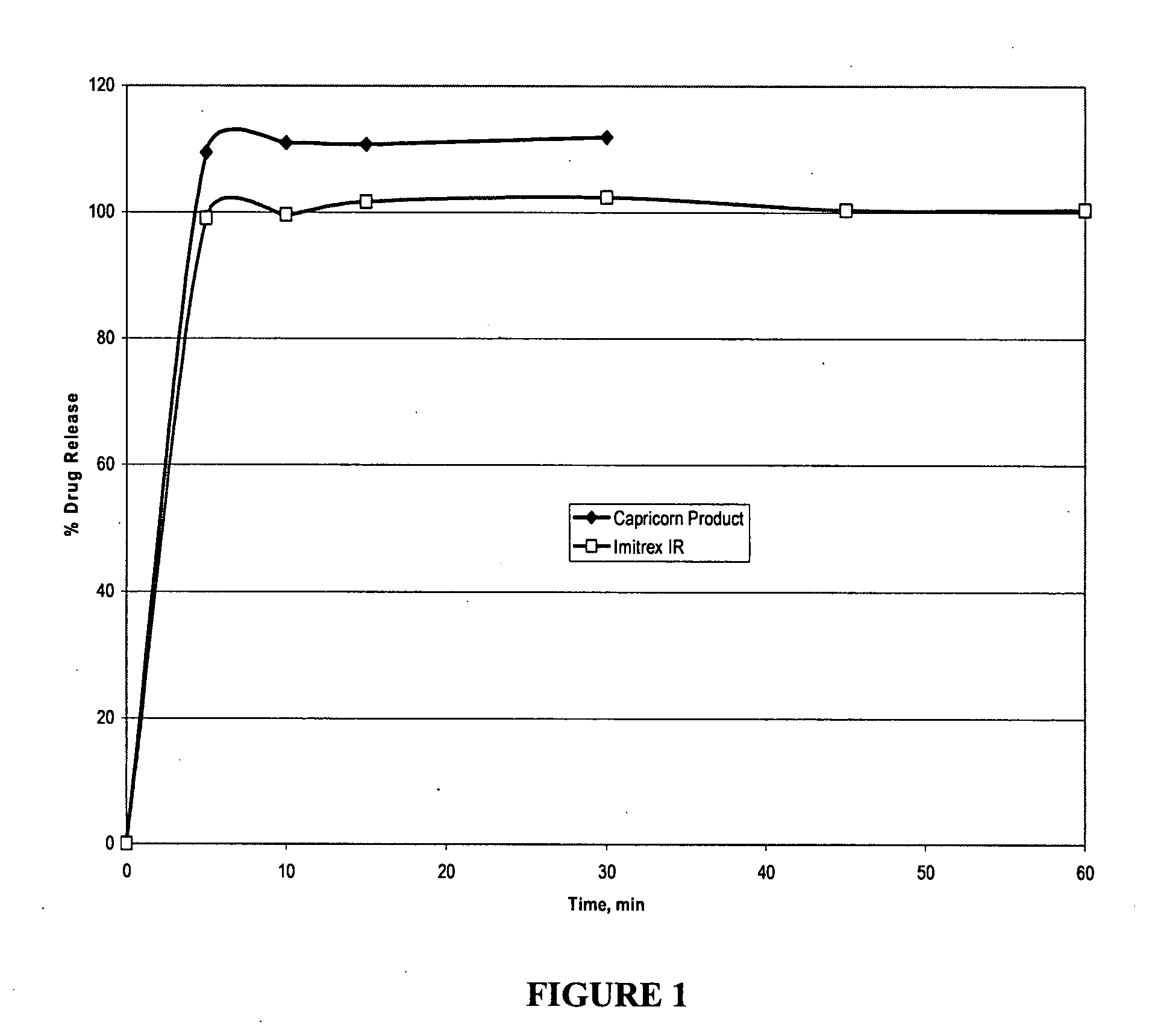
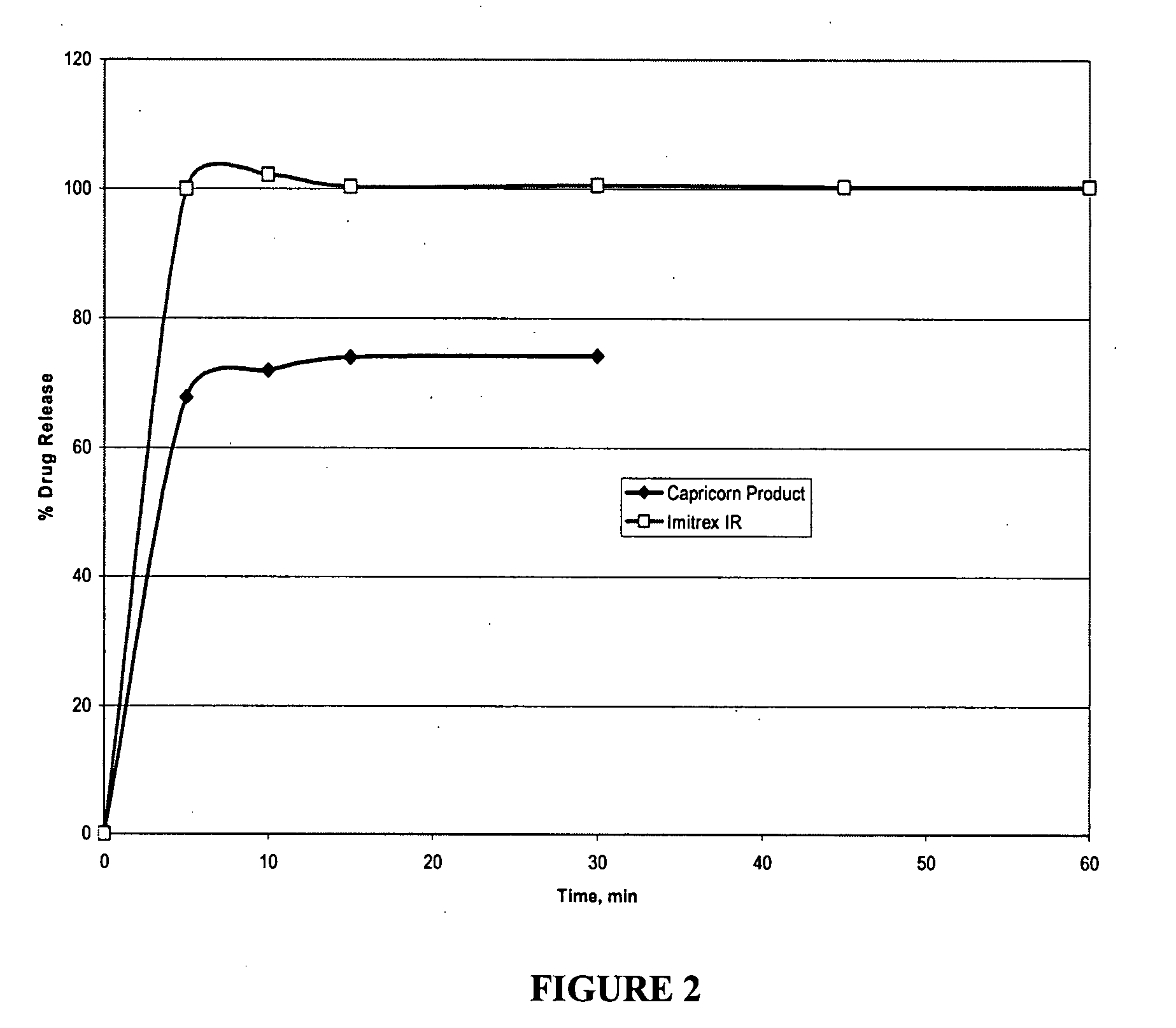
Novel triptan formulations and methods for making them
InactiveUS20070259040A1Improve palatabilityRapid onsetOrganic active ingredientsBiocideCompressible materialMedicine
Rapidly disintegrating oral triptan formulations having superior palatability and methods of making such are provided herein. A rapidly disintegrating oral triptan composition can comprise a triptan compound, a resin, a lubricant, a disintegrant, and a compressible material, where the triptan is admixed with the resin forming a taste-masked triptan composition, which is further admixed with the lubricant, the disintegrant, and the compressible material to form the rapidly disintegrating oral triptan composition.
Owner:CAPRICORN PHARMA INC
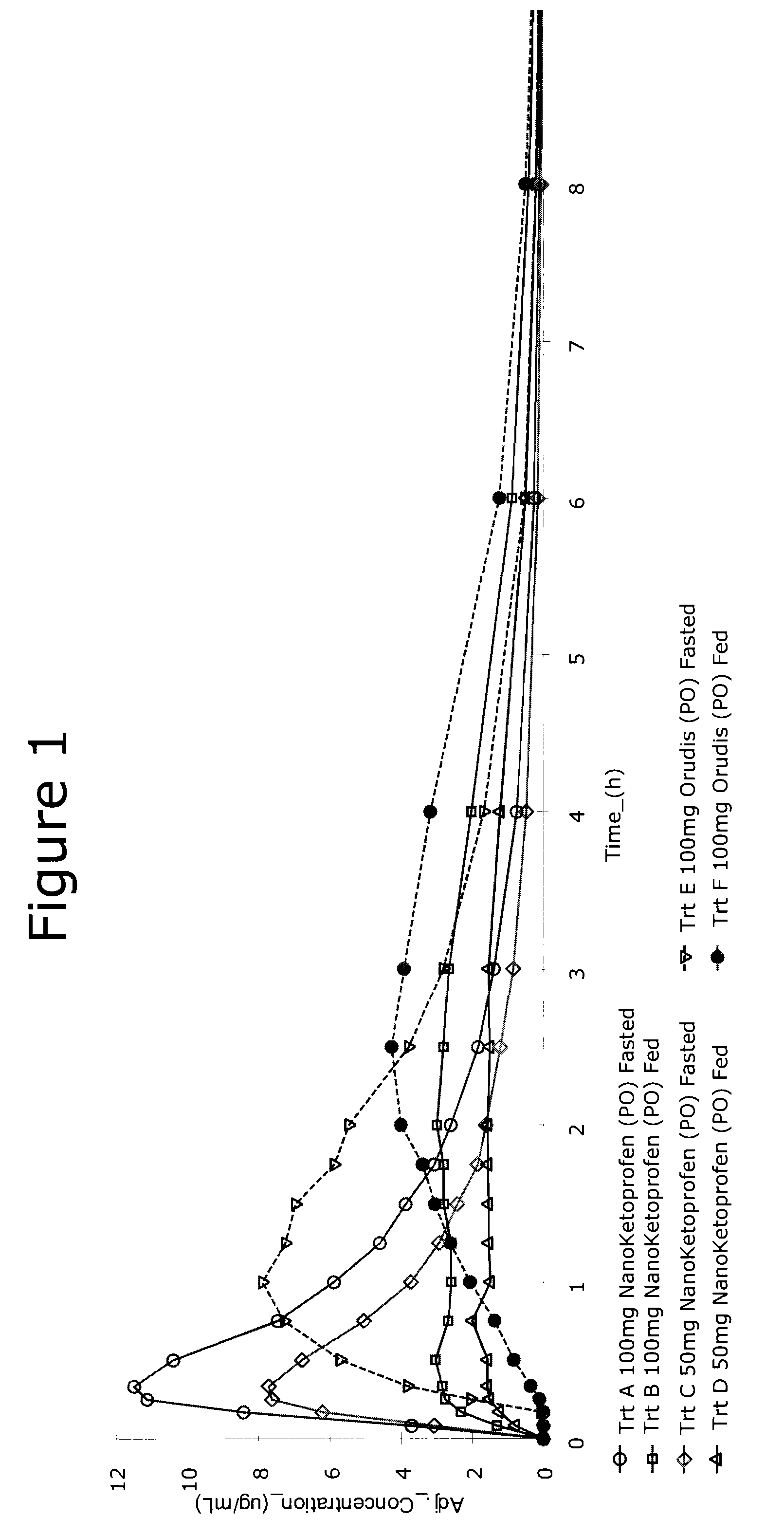
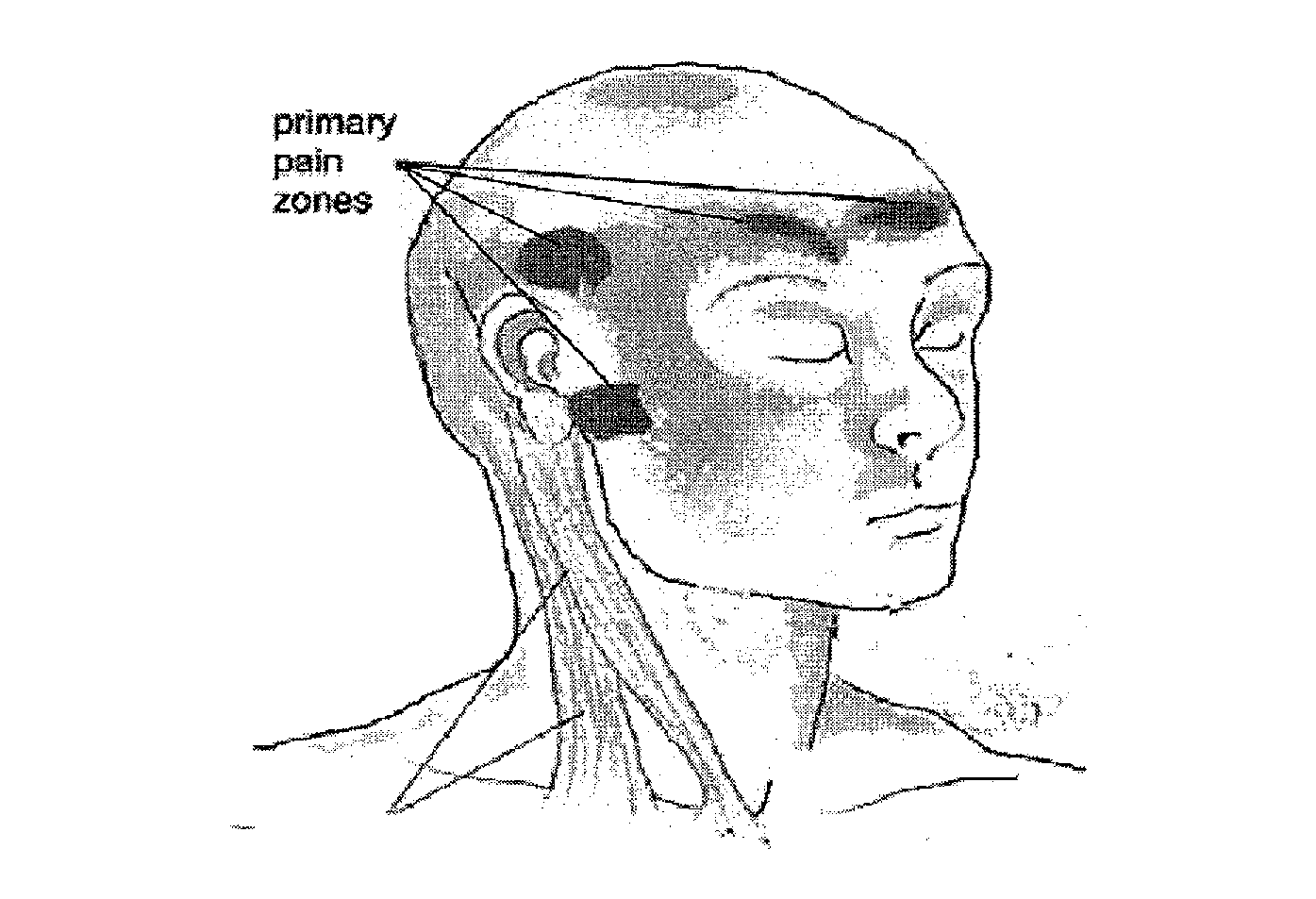
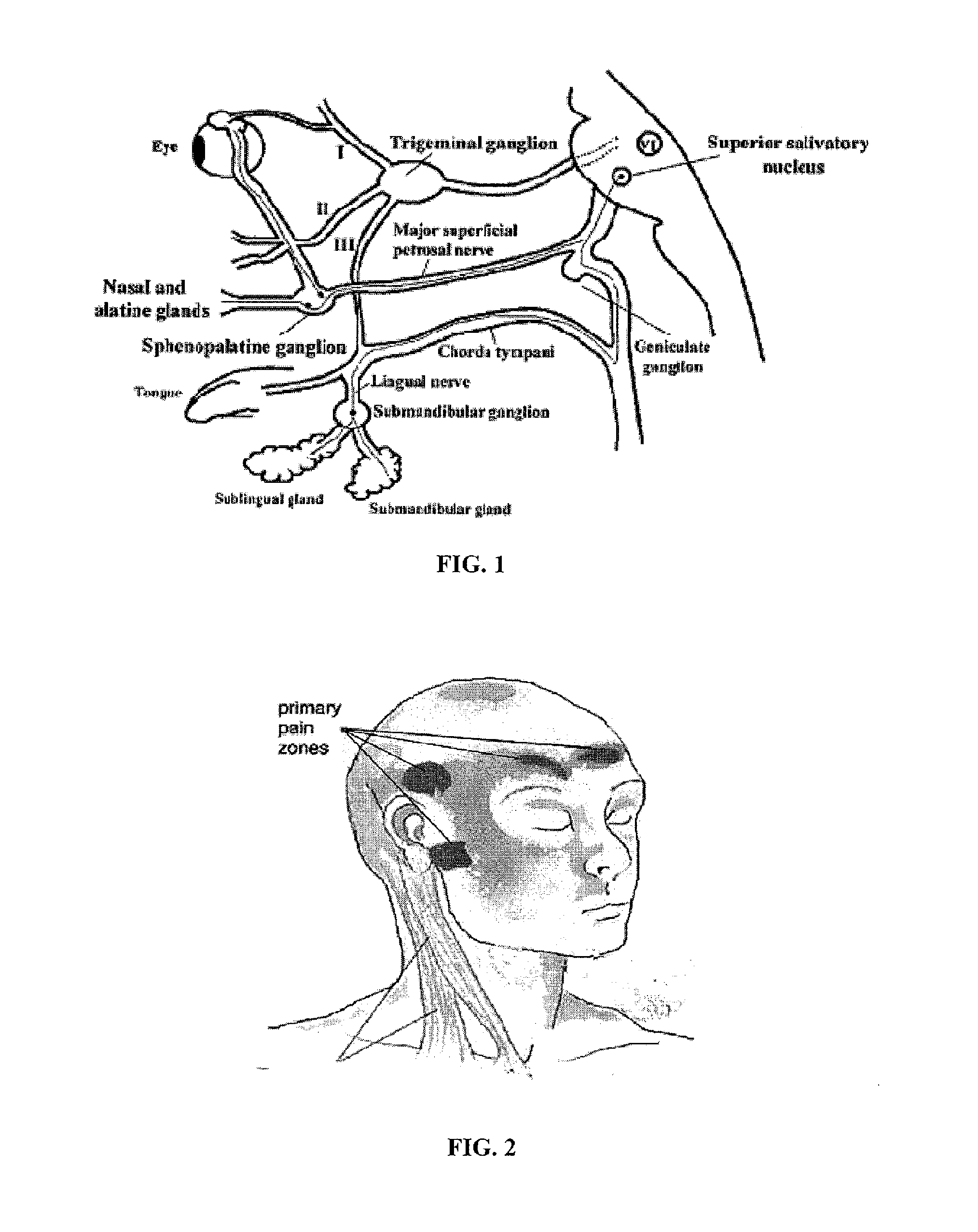
Methods and compositions for treating and preventing trigeminal autonomic cephalgias, migraine, and vascular conditions
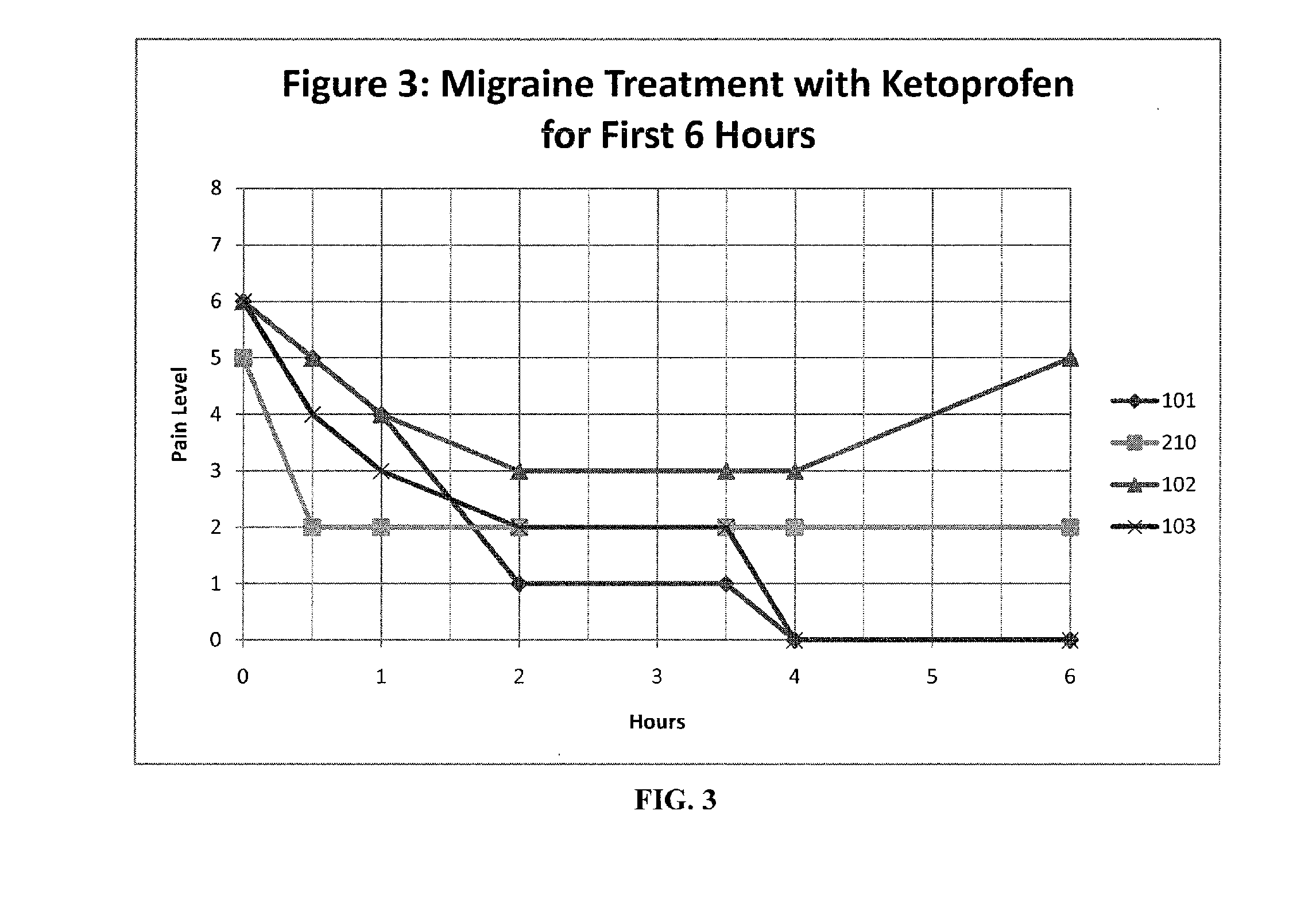
The present invention relates to, among other things, methods for treating trigeminal cephalgias such as migraine and migraine like headaches and other cerebrovascular conditions associated with pain and or inflammation. When non-steroidal anti inflammatory drugs (NSAIDs), such as ketoprofen, are applied locally using specific topical formulations immediate relief of pain is obtained. Intense pain is typically reduced to mild pain or no pain within 30 minutes of application of the topical formulation. The NSAID may be given in combination with other pharmacological agents, such as vasoconstrictors, opioids, decongestants and / or non-opioid migraine drugs, such as triptans and ergots and agents that affect serotonin receptors as agonists, antagonists or partial agonists.
Owner:ACHELIOS THERAPEUTICS
Dosage forms for administering combinations of drugs
Owner:POZEN INC
Dosage forms for administering combinations of drugs
InactiveUS20080175897A1Impairs absorptionGood treatment effectBiocideAnimal repellantsCo administrationTreatment pain
The present invention is directed to dosage forms that can be used in therapeutic methods involving the oral co-administration of a combination of at least two drugs, one of which impairs gastrointestinal absorption and one of which does not. The dosage forms are designed so that the drug impairing absorption is not released into the gastrointestinal tract of a patient until after the drugs that do not impair absorption have been released and substantially absorbed. The invention may be used in treatment of migraine using a combination of triptans and NSAIDs or in the treatment of pain using a combination of NSAIDs and opioid analgesics.
Owner:POZEN INC
Combination treatment of specific forms of epilepsy
InactiveUS20180325909A1Reduce seizuresEliminate side effectsAnhydride/acid/halide active ingredientsHeterocyclic compound active ingredientsSide effectCo morbidity
Formulations for and methods of treatment of Dravet syndrome that avoid side effects are disclosed. The formulations comprise a 5-HT receptor agonists which does not agonize selected 5-HT receptor subtypes, and in particular does not agonize the receptor subtype 5-HT2B. Also disclosed are combinations of such 5-HT receptor agonists. Also disclosed are combinations of such 5-HT receptor agonists and SSRIs, SNRIs, and triptans for treating co-morbidities associated with Dravet syndrome.
Owner:ZOGENIX INT
Novel triptan formulations and methods for making them
Owner:CAPRICORN PHARMA INC
Pharmaceutical compositions
Pharmaceutical compositions and methods are provided to treat headache, headache-associated symptoms, photophobia, or adverse effects associated with triptan administration.
Owner:CHARLESTON LAB
Triptans for the treatment of psoriasis
ActiveUS20120252863A1Reduce the impactThe process is convenient and fastBiocideOrganic active ingredientsOral medicationTryptamine
The present invention provides for a composition comprising a tryptamine based drug that acts as a 5-hydroxytryptamine-1 inhibitor in an amount sufficient to reduce the effects of psoriasis and wherein the composition is formulated for topical or oral administration.
Owner:DASCALU AVI
Method of rapidly achieving therapeutic concentrations of triptans for treatment of migraines
ActiveUS9918932B2Fast concentrationOrganic active ingredientsPharmaceutical delivery mechanismHeadache severeSurgery
Compositions, devices and methods employing therapeutic concentrations of a triptan for treatment of migraine are described. Also described are methods and apparatuses for delivery of zolmitriptan for achieving a Tmax as quick as 2 minutes and not later than 30 minutes in the majority of subjects.
Owner:EMERGEX USA CORP
Intrathecal administration of triptan compositions to treat non-migraine pain
InactiveUS20080064725A1BiocideNervous disorderMetachromatic leukodystrophyMetachromatic leucodystrophy
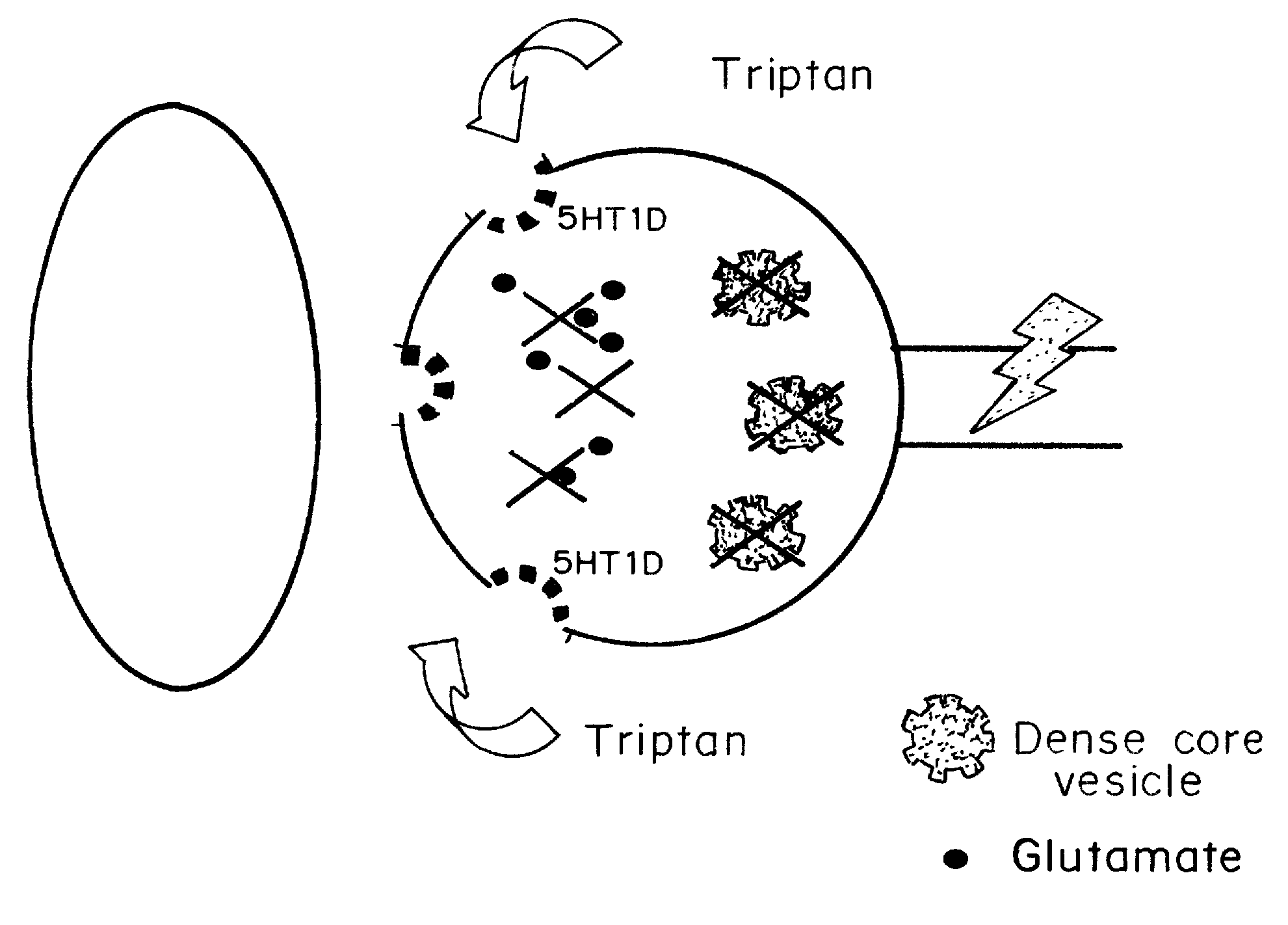
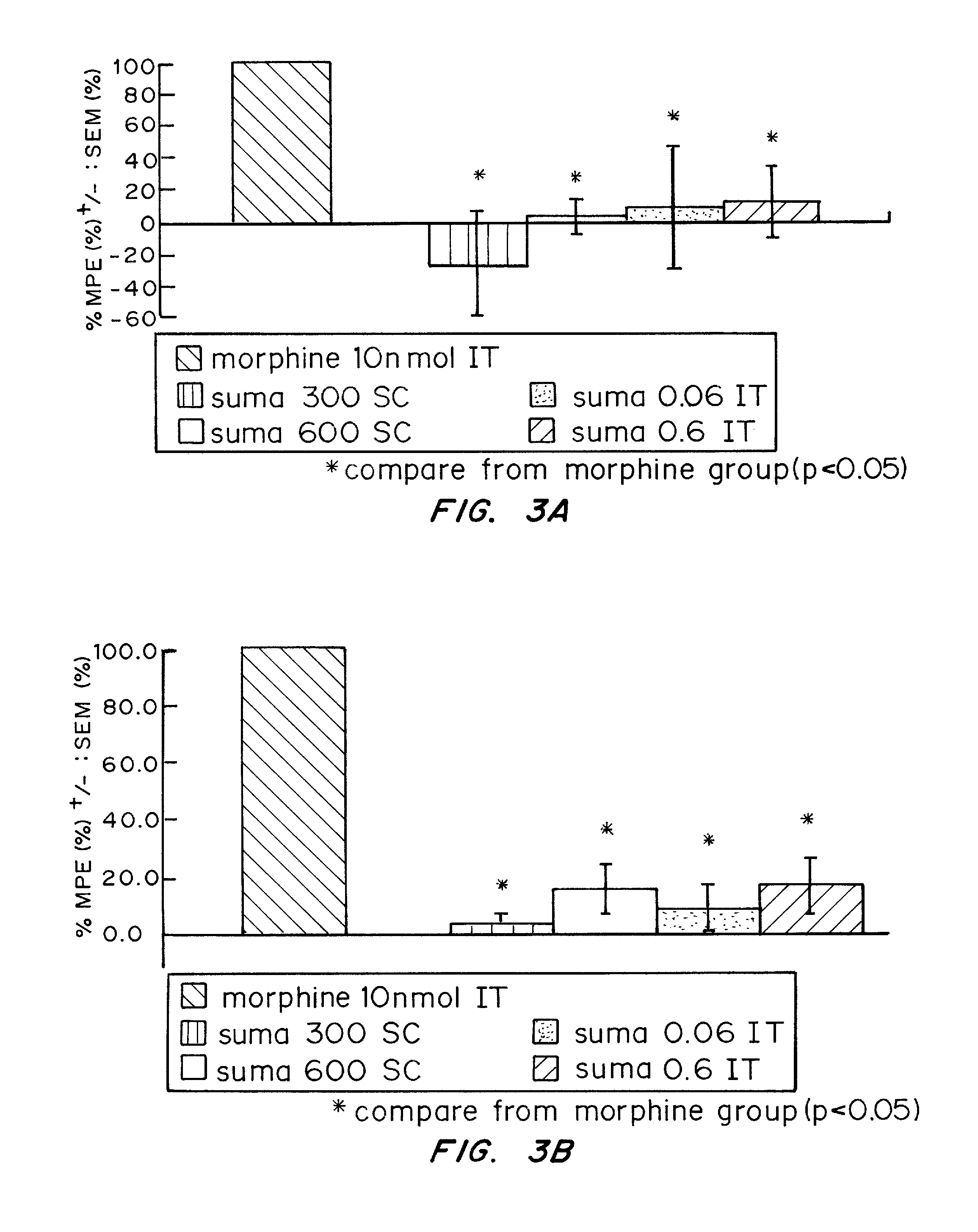
Intrathecal delivery of a pharmaceutically acceptable formulation for intrathecal administration of any drug selectively binding to this receptor to provide pain can be used in any situation in which intrathecal (“IT”) drugs are presently used for pain management. In the preferred embodiment, the drug is a triptan. In another embodiment, a combination of drugs with triptans can be used instead of just the triptan. Exemplary conditions to be treated include cancer pain, chronic back pain, post-herpetic neuralgia, and complex regional pain syndrome types I or II, as well as post-traumatic pain, diabetic vasculopathy, inflammatory radiculopathy, inflammatory plexopathies such as brachial plexopathy (Parsonage Turner syndrome), or lumbar plexopathy, HIV neuropathy, chemotherapy-induced neuropathy (such as vincristine toxicity), erythromelalgia, and inherited painful disorders such as metachromatic leukodystrophy, Friedreich's ataxia, and Fabry's disease. The triptans can also be used in acute pain management, such as in labor management or spinal blockade for surgery, where a spinal formulation of sumatriptan could be combined with traditional opiates for synergistic or additive effects.
Owner:BASBAUM ALLAN
Orally available pharmaceutical formulation suitable for improved management of movement disorders
ActiveUS20150104506A1Good synergyGood curative effectOrganic active ingredientsNervous disorderImmediate releaseBULK ACTIVE INGREDIENT
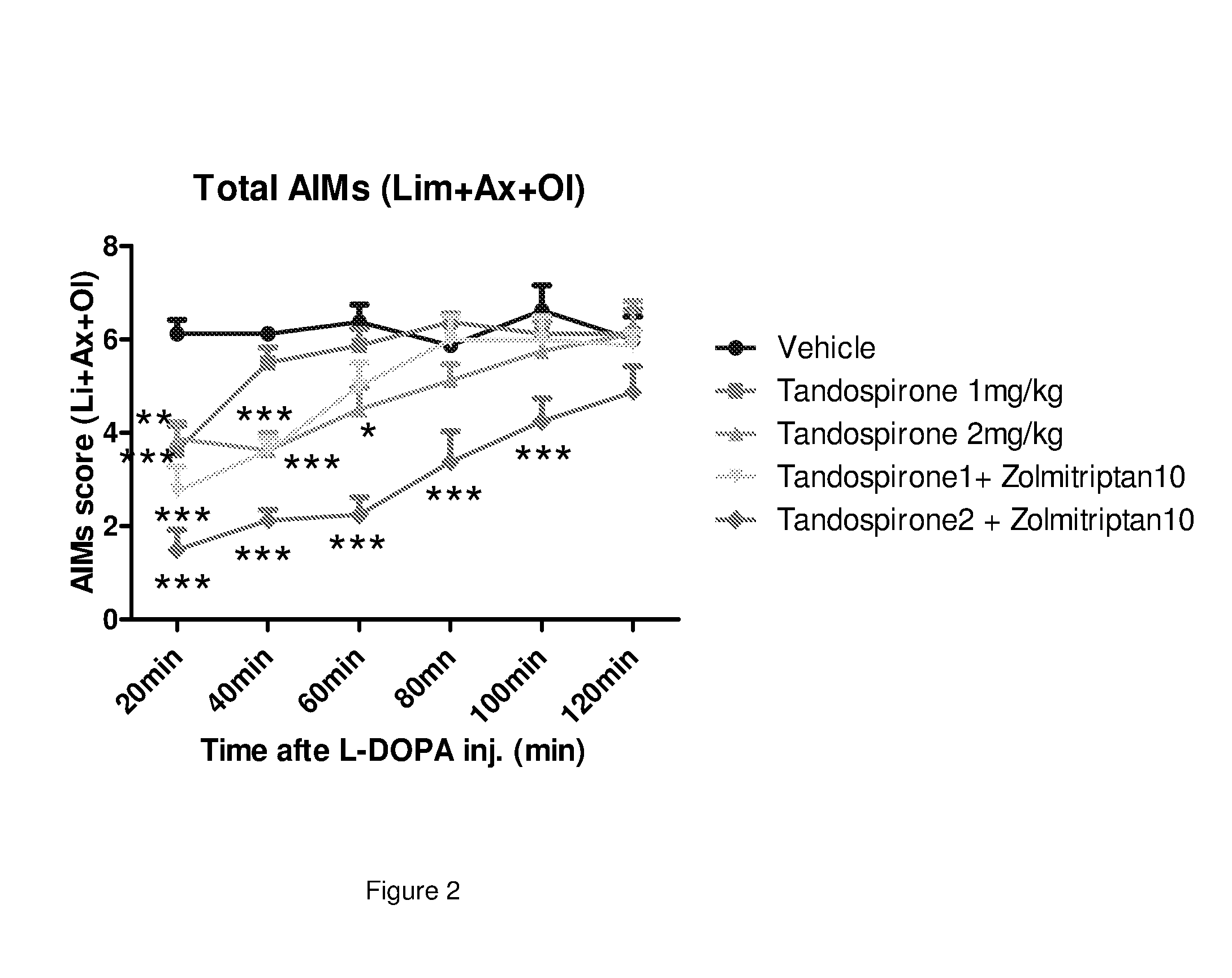
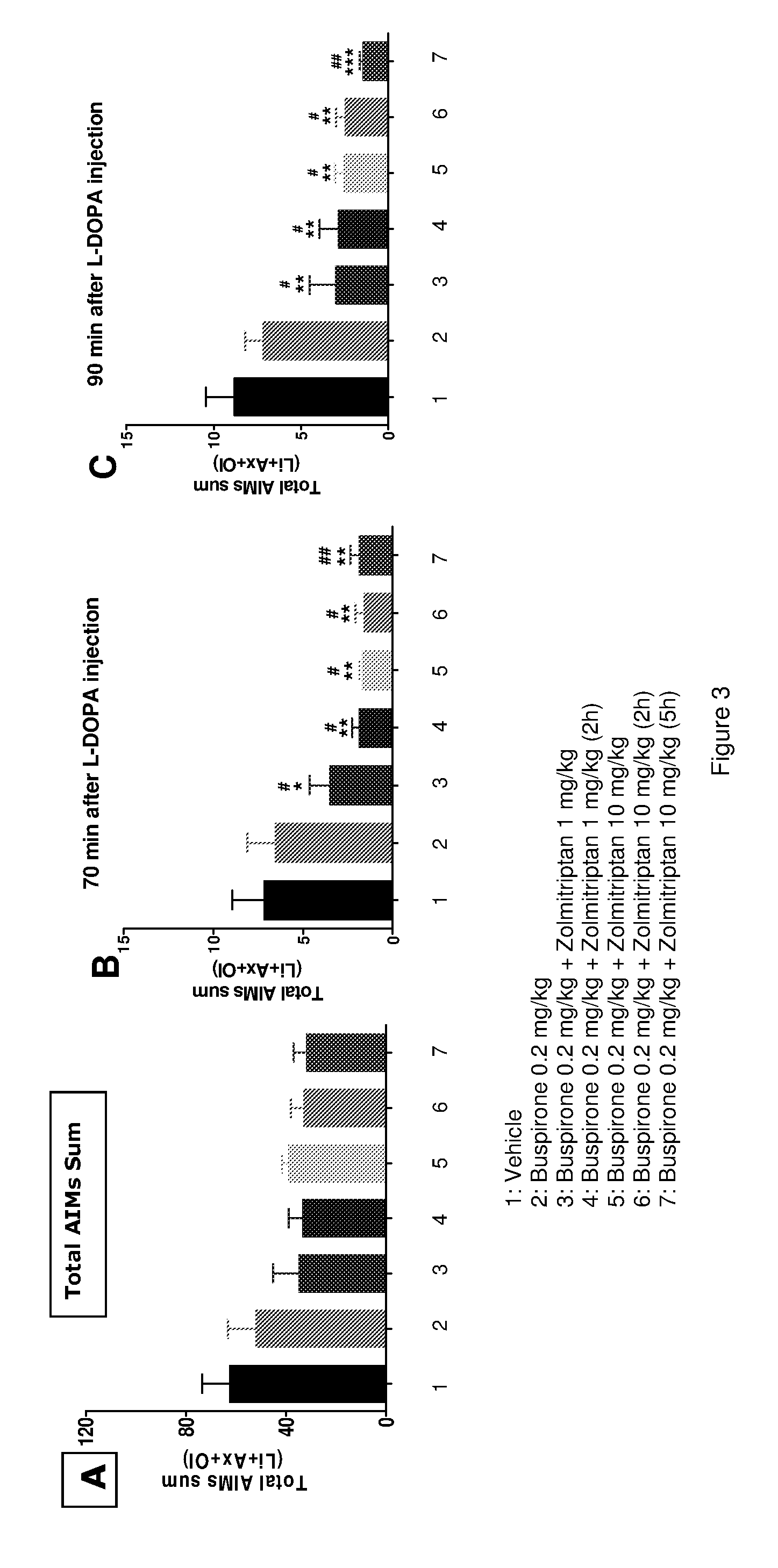
The present invention provides a pharmaceutical formulation for oral administration comprising an agonist of two or more of the 5-HT1B, 5-HT1D and 5-HT1F receptors, such as a triptan, e.g. zolmitriptan, in a matrix constituent with extended release characteristics, and further comprising a 5-HT1A-R agonist, such as buspirone, in a constituent with immediate-release characteristics. The special formulation is particularly well-suited for use in the treatment of movement disorders by combining the two active ingredients in a manner that achieves synergy from both the combination per se and the special release parameters of the pharmaceutical formulation, allowing for ease of administration and reducing the risk of adverse effects of each of the two active ingredients.
Owner:CONTERA PHARMA APS
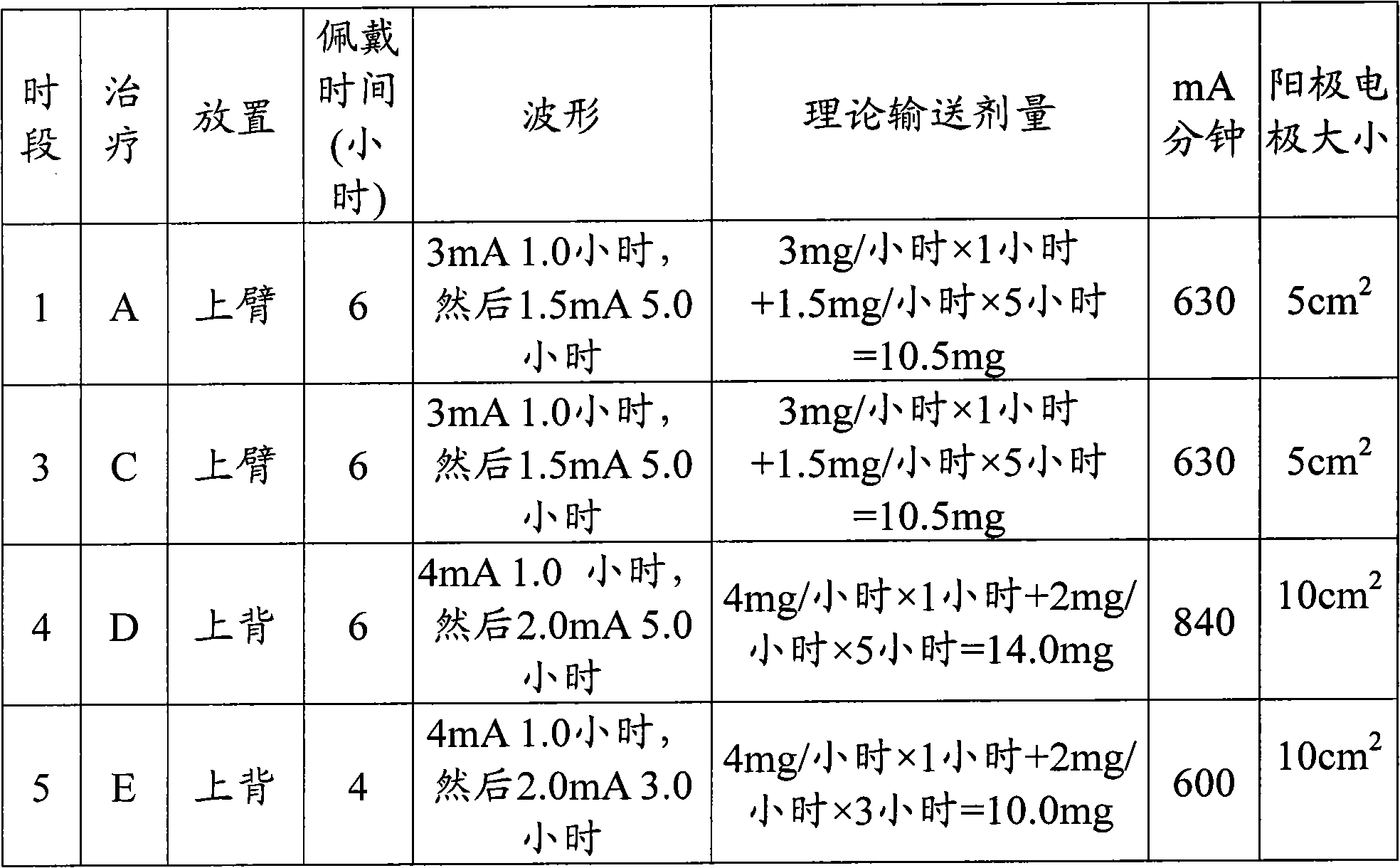
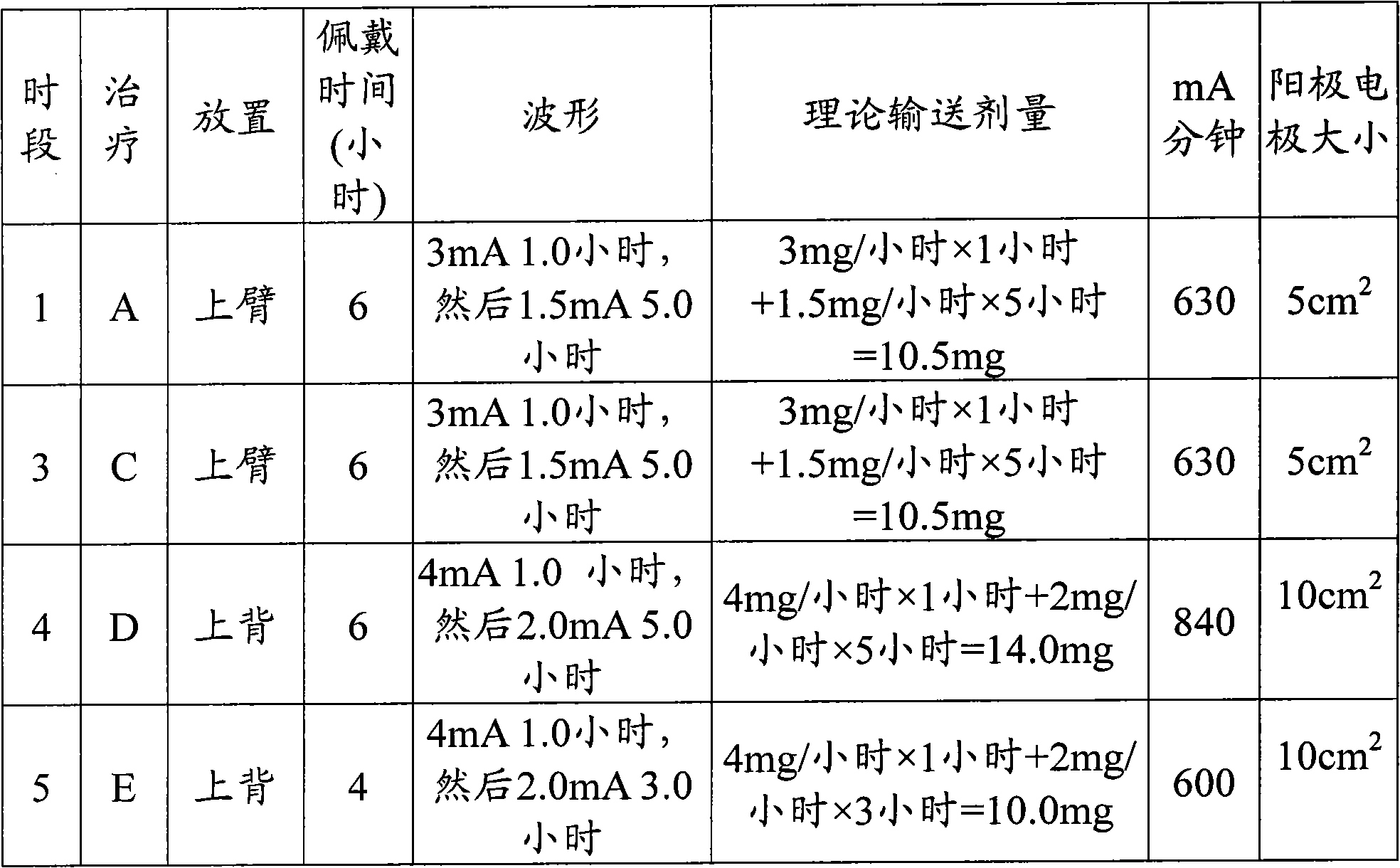
Polyamine enhanced formulations for triptan compound iontophoresis
Owner:TEVA PHARMACEUTICALS INTERNATIONAL GMBH
Pharmaceutical combinations for the treatment of head-aches and migraine attacks as well as blisters and packs that contain them
A pharmaceutical combination comprising at least a triptan and the trimebutine compound [hydrogenated maleate of 2-dimethyl-amino-2-phenylbutyl-3,4,5-trimetoxybenzoate] and an anti-inflammatory or simple analgesic for the treatment of attacks of migraine and other headaches is provided.More particularly, the present invention discloses a pharmaceutical combination comprising triptan and trimebutine for the treatment of migraine attacks.The present invention further discloses blisters and packs containing said pharmaceutical combinations.
Owner:KRYMCHANTOWSKI ABOUCH VALENTY
Methods and compositions for treating and preventing trigeminal autonomic cephalgias, migraine, and vascular conditions
InactiveUS20190105290A1Reducing and preventing symptomOrganic active ingredientsNervous disorderCranial nervesDecongestant
Owner:ACHELIOS THERAPEUTICS
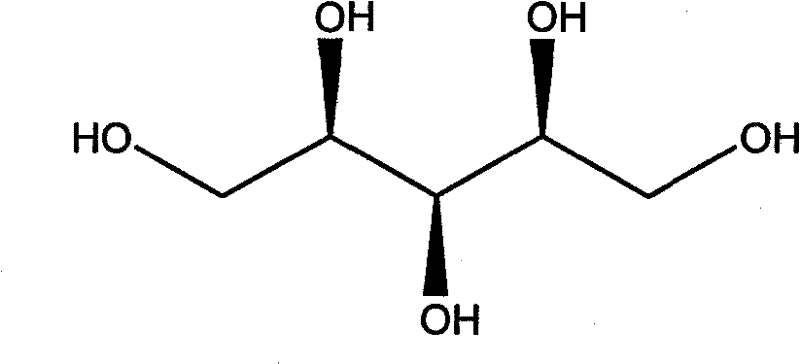
Formulations containing triptan compounds
ActiveCN102933198BPharmaceutical delivery mechanismCarbohydrate active ingredientsGlycoside formationSumatriptan
The invention provides a pharmaceutical composition for intranasal administration comprising a salt of sumatriptan or a physiologically acceptable solvate thereof, an alkyl glycoside or saccharide alkyl ester and optionally at least one pharmaceutically acceptable excipient, wherein the said composition provides Tmax value of less than 30 minutes upon said administration. Other aspects and embodiments are contemplated and described. The invention also provides a pharmaceutical composition for intranasal administration comprising a triptan, a pharmaceutically acceptable vehicle and a mucosal permeation enhancer, wherein upon said administration said composition provides a Tmax substantially equivalent to subcutaneous administration of said triptan. Other aspects and embodiments are contemplated and described.
Owner:DR REDDYS LAB LTD
Galenic form for the oral transmucosal delivery of triptans
ActiveCN101983052AGuaranteed SolubilizationEliminate useOrganic active ingredientsNervous disorderBULK ACTIVE INGREDIENTPharmaceutical formulation
The invention relates to a galenic form for the oral transmucosal delivery of at least one active ingredient of the family of triptans, comprising at least: said active ingredient in base form and in salt form, and an aqueous-alcoholic solution with a titer of at least 15 degrees alcohol, said active ingredient being present in a stable and complete dissolution state in the aqueous-alcoholic solution, for rapid absorption of said active ingredient through the mucosa of the oral cavity and / or of the oropharynx. The invention also relates to a method for obtaining this galenic formulation and to the use thereof.
Owner:菲利普·佩罗维特什 +1
Compound preparation of diclofenac sodium and triptan medicines for treating migraine
InactiveCN102008723AEffective treatmentQuick resultsOrganic active ingredientsNervous disorderSide effectRegimen
The invention discloses a compound preparation of diclofenac sodium and triptan medicines for treating migraine, prepared from diclofenac sodium or pharmaceutical salts thereof and any one triptan medicine or pharmaceutical salts thereof used as main ingredients and matched pharmaceutical vectors. The preparation contains 25-4150mg of diclofenac sodium and 1.0-150mg of triptan medicaments. The invention has the advantages of fully exerting the pharmaceutical complementary synergism according to medicament combination, lessening the adverse reaction related to a certain increased dosage and effectively treating migraine. The compound preparation of the invention has the advantages of short course of treatment, rapid effect, less side effect and low cost.
Owner:邬林祥 +1
Oral film formulation
Pharmaceutical composition comprising at least one triptan and at least one thiol-group containing reducing agent.
Owner:LABTEC
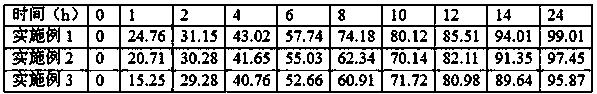
Furotriptan Succinate Extended Release Tablets
ActiveCN104800184BMaintain effective plasma concentrationLow frequency of useOrganic active ingredientsNervous disorderBlood concentrationPolyethylene glycol
The invention relates to a slow-release tablet comprising succinate frovatriptan. The slow-release tablet comprises a tablet core and a coating, wherein the tablet core adopts a matrix tablet, and comprises succinate frovatriptan, hydroxypropyl methylcellulose, pregelatinized starch, magnesium stearate, talcum powder, and a 95% ethanol solution; the coating comprises a slow-release coating material, polyethylene glycol and succinate frovatriptan. The slow-release tablet has the advantages that the administration number of times is reduced, the compliance of a patient is improved, and the effective blood concentration is kept for a relatively long time.
Owner:CP PHARMA QINGDAO CO LTD
Polyamine enhanced formulations for triptan compound iontophoresis
Owner:TEVA PHARMACEUTICALS INTERNATIONAL GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com