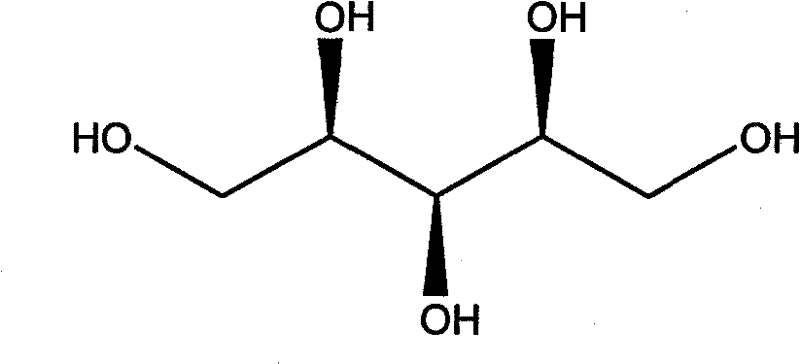
Liquid pharmaceutical composition containing triptan compound and xylitol
A pharmaceutical composition and composition technology, applied in the field of aqueous liquid pharmaceutical composition, can solve problems such as instability of liquid pharmaceutical composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1- 1
[0081] Example 1 - One Week Stress Stability Test
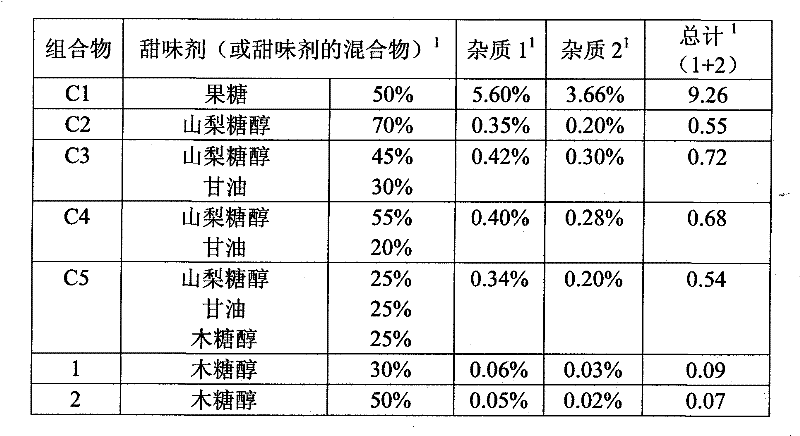
[0082] Aqueous liquid compositions of almotriptan malate in combination with different sweeteners (Table 1 ) were evaluated for physical and chemical stability using a stability screening test at 50°C over a period of one week.
[0083] To prepare each composition, 17.5 mg of almotriptan D, L hydrogen malate was weighed and dissolved in purified water in a 10 ml tubular amber vial. A sweetener or mixture of sweeteners is added to the mixture. After homogenization, the composition was stored at 50°C for 1 week.
[0084] The presence of almotriptan oxidized impurities was followed by High Performance Liquid Chromatography (HPLC) assay. The results are shown in Table 1.
[0085] The total amount of oxidative impurities should be as low as possible, preferably below 0.1% by weight of the amount of almotriptan. In addition, the amount of various impurities (impurity 1 and impurity 2) should also be as low as possible.
[008...
example 2-6
[0094] Example 2-6 months accelerated stability test
[0095] The following composition, Composition 3 (9.5 grams), was prepared and packed into stick packs (polyester 12 microns - Al 9 microns - polyethylene 50 microns):
[0096] -D, L Hydrogen Malate Almotriptan 17.5mg
[0097] - Xylitol 4750mg
[0098] - Fragrance in moderation
[0099] - Pure water added to 100%.
[0100] To prepare the composition, 17.5 mg of almotriptan D,L hydrogen malate were weighed and dissolved in purified water. A fragrance is added to the mixture. After homogenization, xylitol was added. Finally, after homogenization, the composition was stored for 6 months at 40° C. and 75% relative humidity (RH).
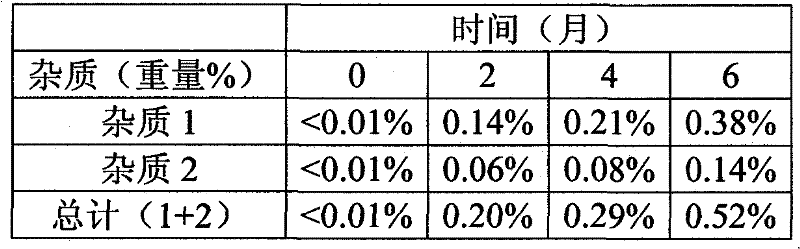
[0101] The presence of almotriptan oxidation impurities was followed by HPLC. The results are shown in Table 2.
[0102] Table 2 - Stability of an aqueous liquid composition of D, L hydrogen malate almotriptan combined with xylitol stored at 40°C and 75% RH for 6 months
[0103]
[0104]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com