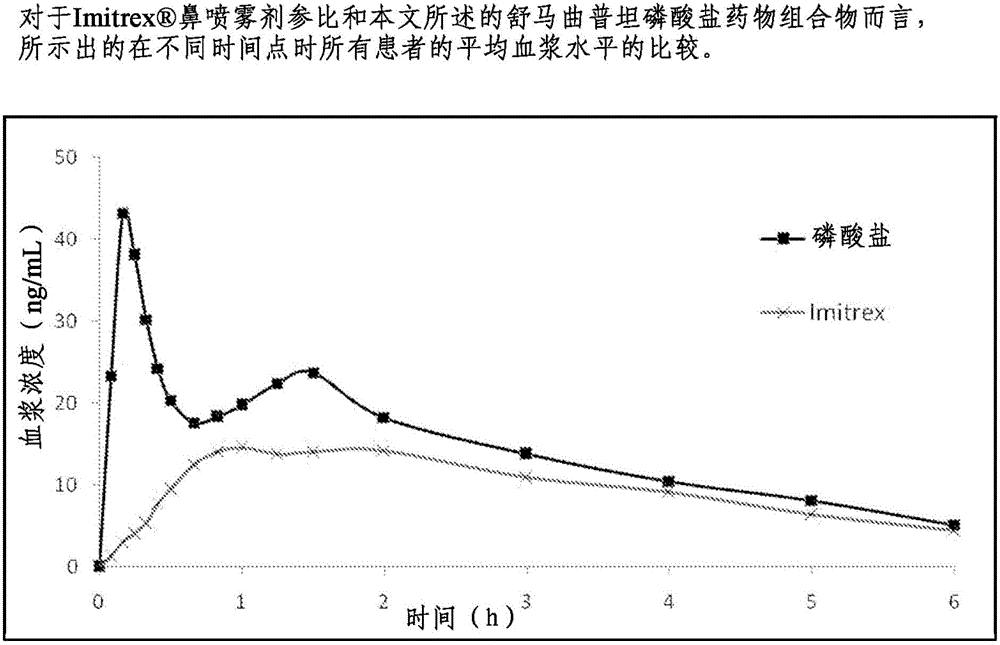
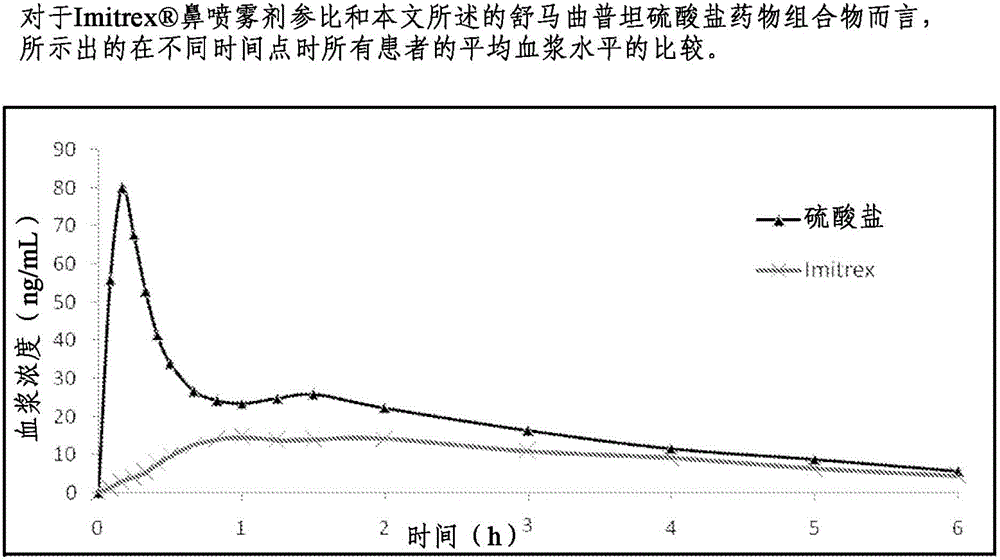
Formulations containing triptan compounds
A kind of composition, the technology of medicine, be used in the preparation field that comprises triptan compound
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0192] Embodiment 1: Preparation of "sumatriptan citrate" composition
[0193] process:
[0194] Step i: Preparation of Phosphate Buffer:
[0195] Dissolve 0.2 g disodium hydrogen phosphate and 10.0 g potassium dihydrogen phosphate in enough water to produce 1000 mL of phosphate buffer.
[0196] Step ii: Preparation of 1N NaOH:
[0197] Dissolve 1.0 g of sodium hydroxide in sufficient water to yield 25 mL of 1 N sodium hydroxide.
[0198] Step iii: Preparation of 5N citric acid monohydrate:
[0199] Dissolve 70.04 g of citric acid monohydrate in a sufficient amount of purified water, and make up its volume to 200 mL with purified water.
[0200] Step iv: Add required amount of citric acid monohydrate (5N) solution to required amount of water and mix well to obtain a homogeneous mixture. To this solution is added the required amount of sumatriptan and the mixture is stirred to dissolve the drug.
[0201] Step v: To check the pH of the solution obtained in step iv, add the...
Embodiment 2
[0210] Embodiment 2: Preparation of "sumatriptan phosphate" composition
[0211] By a similar procedure as described in Example 1, a pharmaceutical composition comprising sumatriptan phosphate was prepared using the following amounts of reaction materials.
[0212] Ingredients / Conditions
Embodiment 3-5
[0213] Embodiment 3-5: Preparation of "sumatriptan formate, acetate and maleate" composition
[0214] Compositions of sumatriptan formate, acetate and maleate can be prepared by following the procedure described in Example 1, using appropriate amounts of the reactants and maintaining appropriate pH conditions.
[0215]
[0216] * Used as a solution of (10N) formic acid in water.
[0217] ** Used as a solution of (10N) acetic acid in water.
[0218] *** Used as a solution of (5N) maleic acid in water.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com