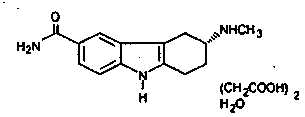
Furotriptan Succinate Extended Release Tablets
A technology of furovatriptan and succinic acid, applied in the field of drug sustained-release tablets, can solve the problems of short half-life, high relapse rate, and low oral bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
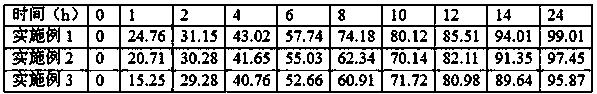
Embodiment 1
[0023] The tablet core is a skeleton tablet, including the following components, and its weight composition is:
[0024] Furotriptan succinate 200 parts
[0025] HPMC 2000 copies
[0026] Pregelatinized starch 1000 parts
[0027] Magnesium stearate 3000 parts
[0028] 125 parts of 95% ethanol solution
[0029] The coating layer comprising the coating solution of the following components is composed of:
[0030] Ethyl cellulose 100 parts
[0031] Cellulose acetate 200 parts
[0032] PEG6000 15 parts
[0033] Furotriptan succinate 8 parts
[0034] The preparation method includes the following steps: (1) Tablet core preparation: Weigh furovatriptan succinate, HPMC, and pregelatinized starch according to the prescribed amount of the tablet core and sieve them, mix them evenly, and prepare them with 95% ethanol solution as a wetting agent. into soft materials, sieve and granulate, dry the wet granules, granulate, add magnesium stearate and talcum powder and mix evenly, put ...
Embodiment 2
[0036] The tablet core is a skeleton tablet, including the following components, and its weight composition is:
[0037] Furotriptan succinate 200 parts
[0038] HPMC 2000 copies
[0039] Pregelatinized starch 1000 parts
[0040] Magnesium stearate 3000 parts
[0041] 125 parts of 95% ethanol solution
[0042] The coating layer comprising the coating solution of the following components is composed of:
[0043] Cellulose acetate 300 parts
[0044] PEG6000 15 copies
[0045] Furotriptan succinate 8 parts
[0046] The preparation method includes the following steps: (1) Tablet core preparation: Weigh furovatriptan succinate, HPMC, and pregelatinized starch according to the prescribed amount of the tablet core and sieve them, mix them evenly, and prepare them with 95% ethanol solution as a wetting agent. into soft materials, sieve and granulate, dry the wet granules, granulate, add magnesium stearate and talcum powder and mix evenly, put the above mixture into a tablet mac...
Embodiment 3
[0048] The tablet core is a skeleton tablet, including the following components, and its weight composition is:
[0049] Furotriptan succinate 200 parts
[0050] HPMC 2000 copies
[0051] Pregelatinized starch 1000 parts
[0052] Magnesium stearate 3000 parts
[0053] 125 parts of 95% ethanol solution
[0054] The coating layer comprising the coating solution of the following components is composed of:
[0055] Ethyl cellulose 300 parts
[0056] PEG6000 15 parts
[0057] Furotriptan succinate 8 parts
[0058] The preparation method includes the following steps: (1) Tablet core preparation: Weigh furovatriptan succinate, HPMC, and pregelatinized starch according to the prescribed amount of the tablet core and sieve them, mix them evenly, and prepare them with 95% ethanol solution as a wetting agent. into soft materials, sieved and granulated, after drying the wet granules, granulate, add magnesium stearate and mix evenly, put the above mixture into a tablet machine and c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com