Polyamine enhanced formulations for triptan compound iontophoresis
A technology of iontophoresis and compounds, applied in electrotherapy, active ingredients of heterocyclic compounds, medical preparations of non-active ingredients, etc., can solve problems such as pollution, expensive programmable controllers, damage, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Example 1: Use of an iontophoretic patch for the delivery of sumatriptan succinate
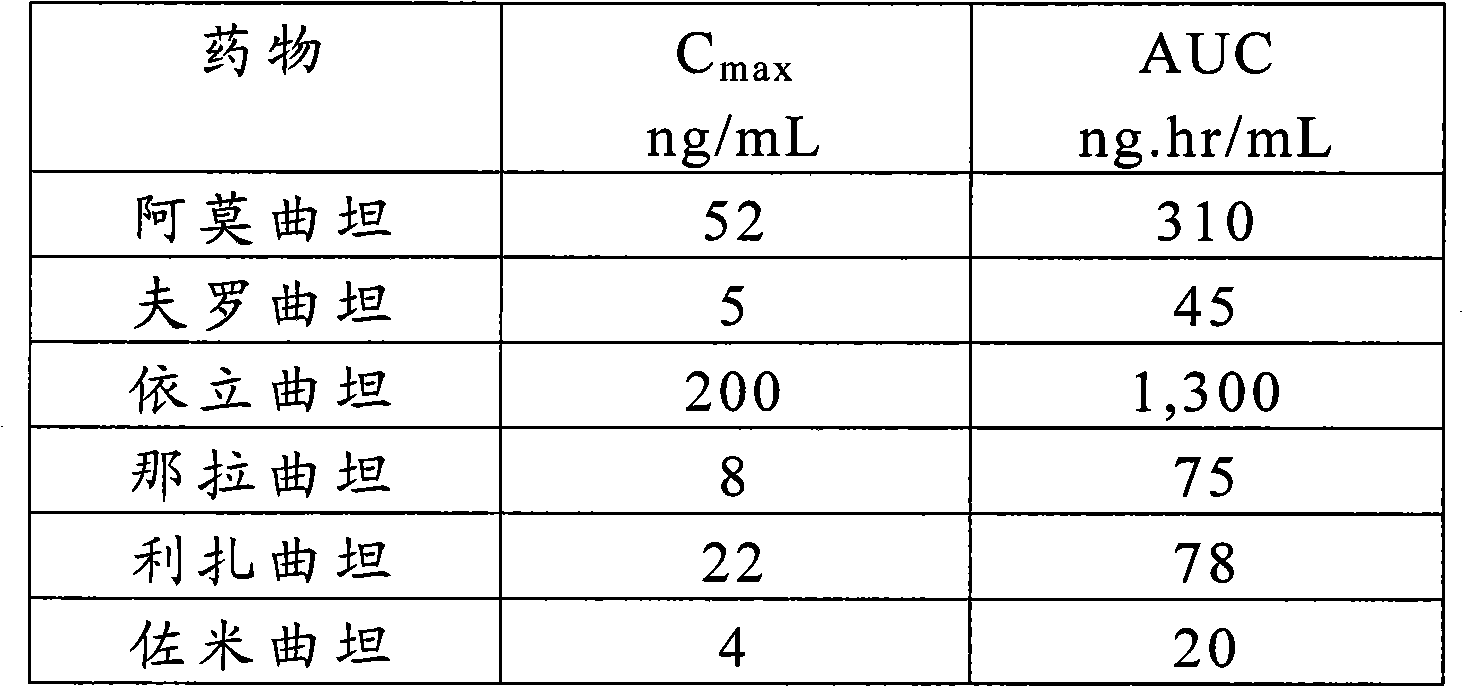
[0096] A single-center, open label, single-dose, five-period study was conducted to compare the pharmacokinetics and Comparison of 100 mg oral sumatriptan succinate. Subjects were required to participate in Treatment A and Treatment B at a minimum.
[0097] The iontophoretic patch used has its own external power source and is designed to be applied to the surface of the skin and deliver the drug systemically.
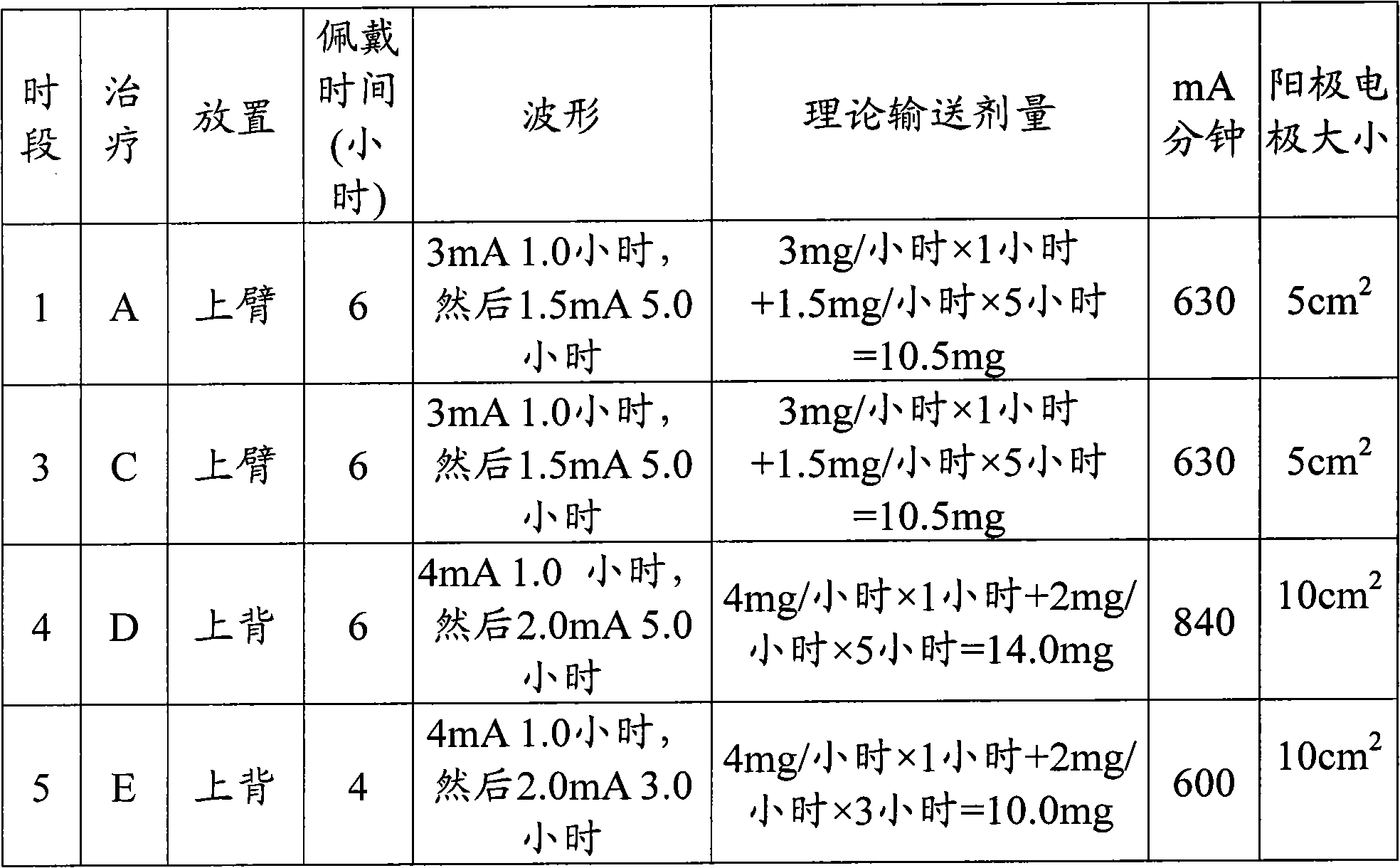
[0098] The patch treatments and the prototype iontophoretic patches prepared for this example are detailed in Table 2 below.
[0099] Table 2 Patch quantitative treatment of iontophoresis
[0100]
[0101] Nine subjects participated in Treatment B, 100 mg sumatriptan succinate oral tablet. The study consisted of a screening visit after treatments A, B, C, D and E. Each treatment period was separated by a 2-day rest period.
[0102] The patches for Treatments A and C we...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com