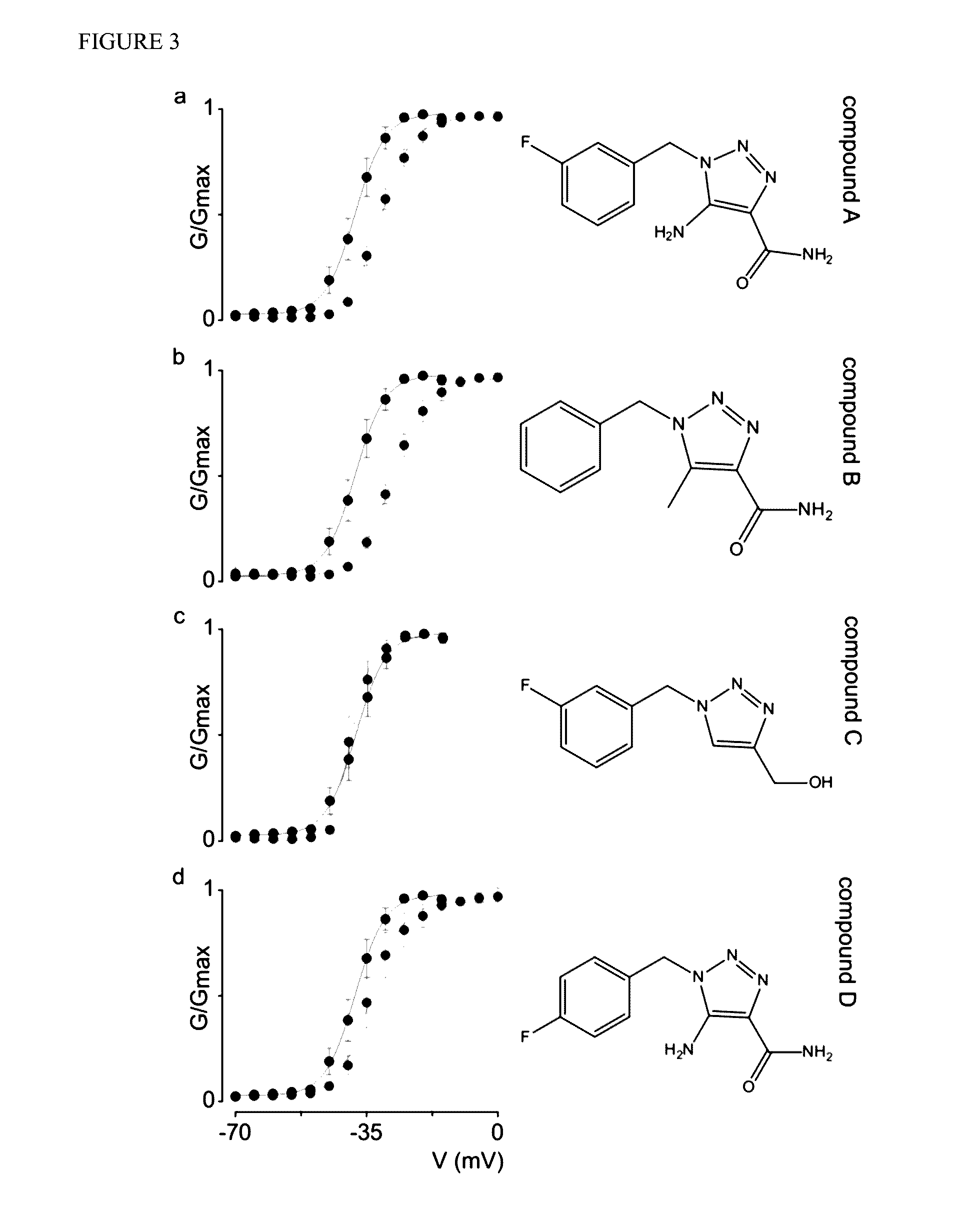
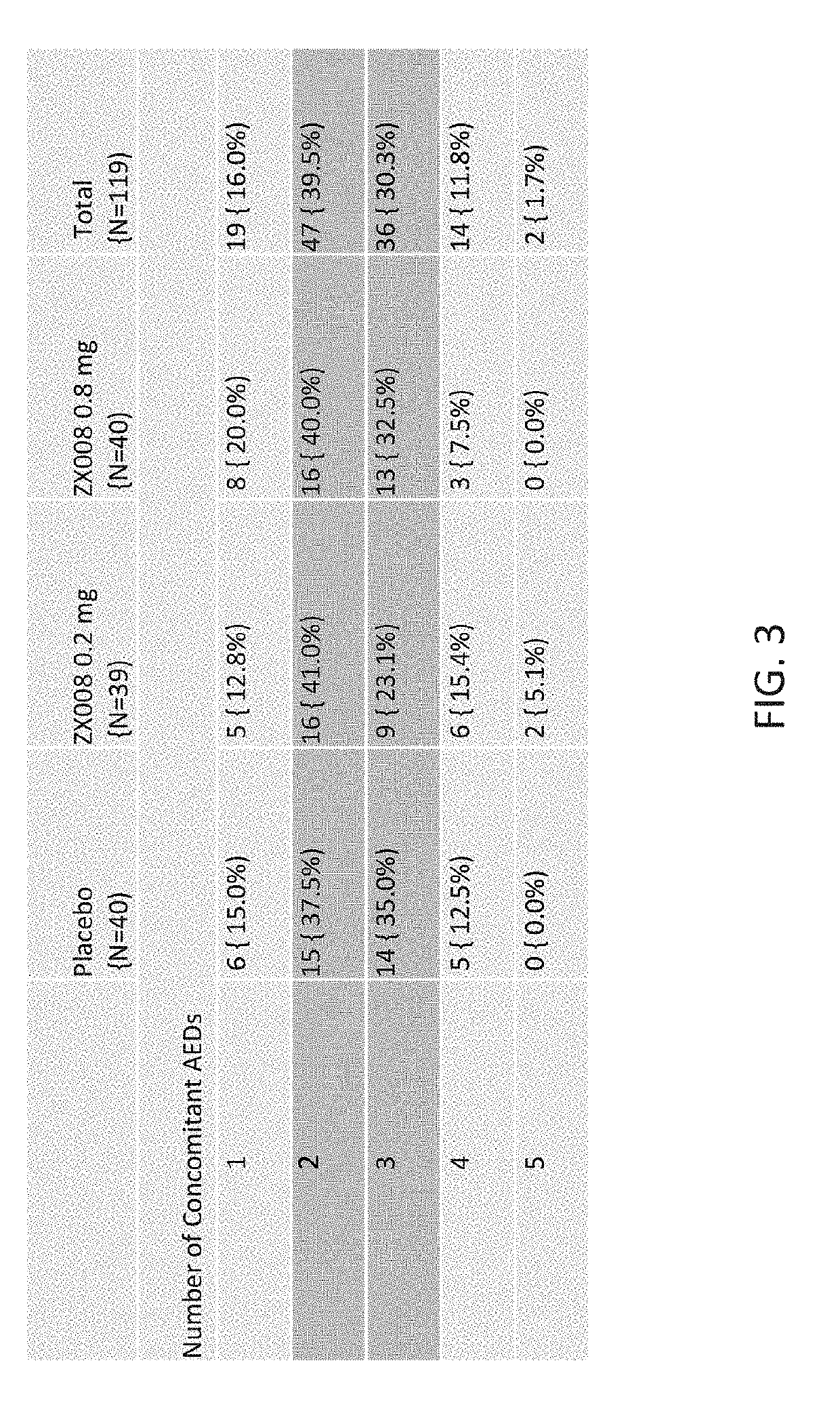
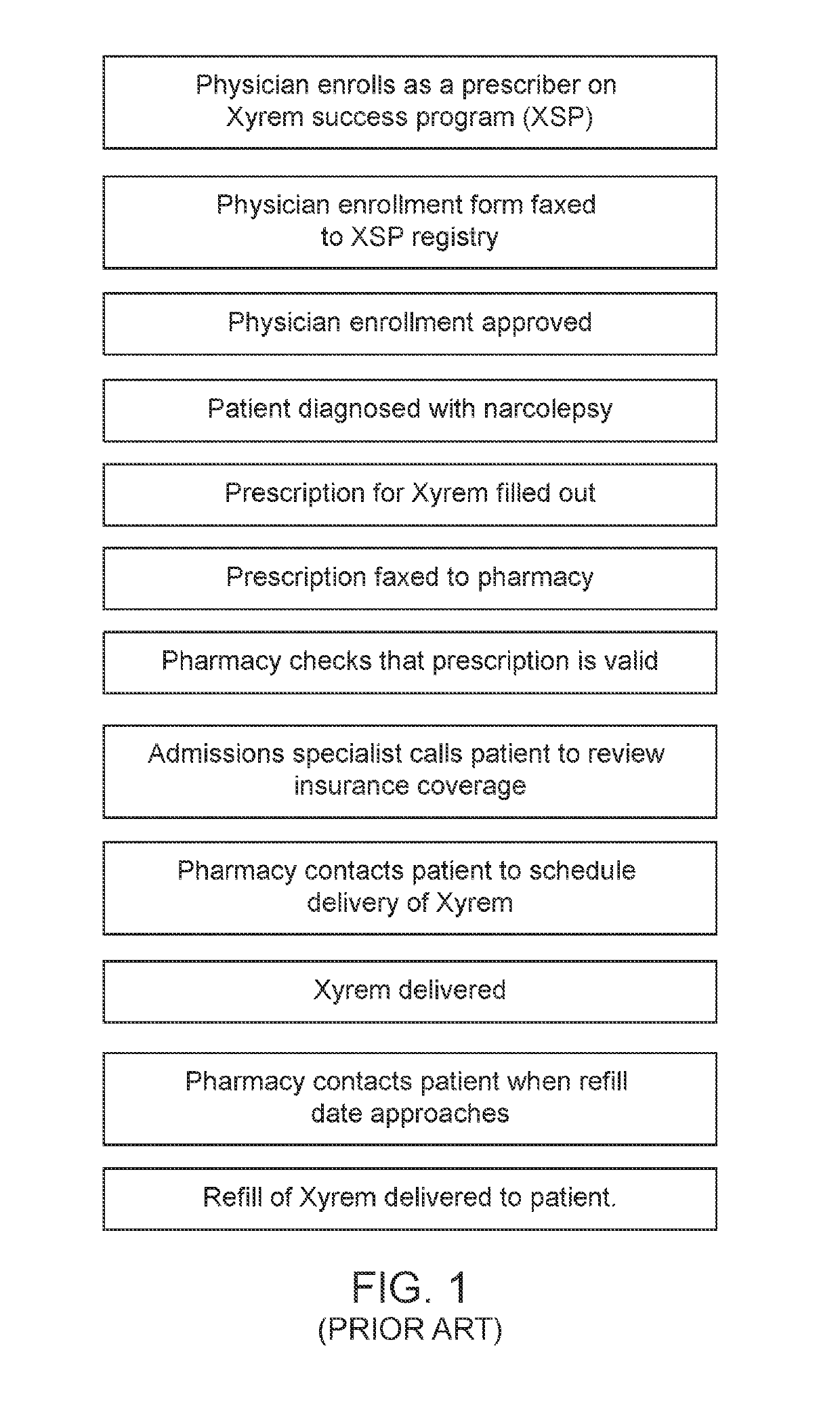
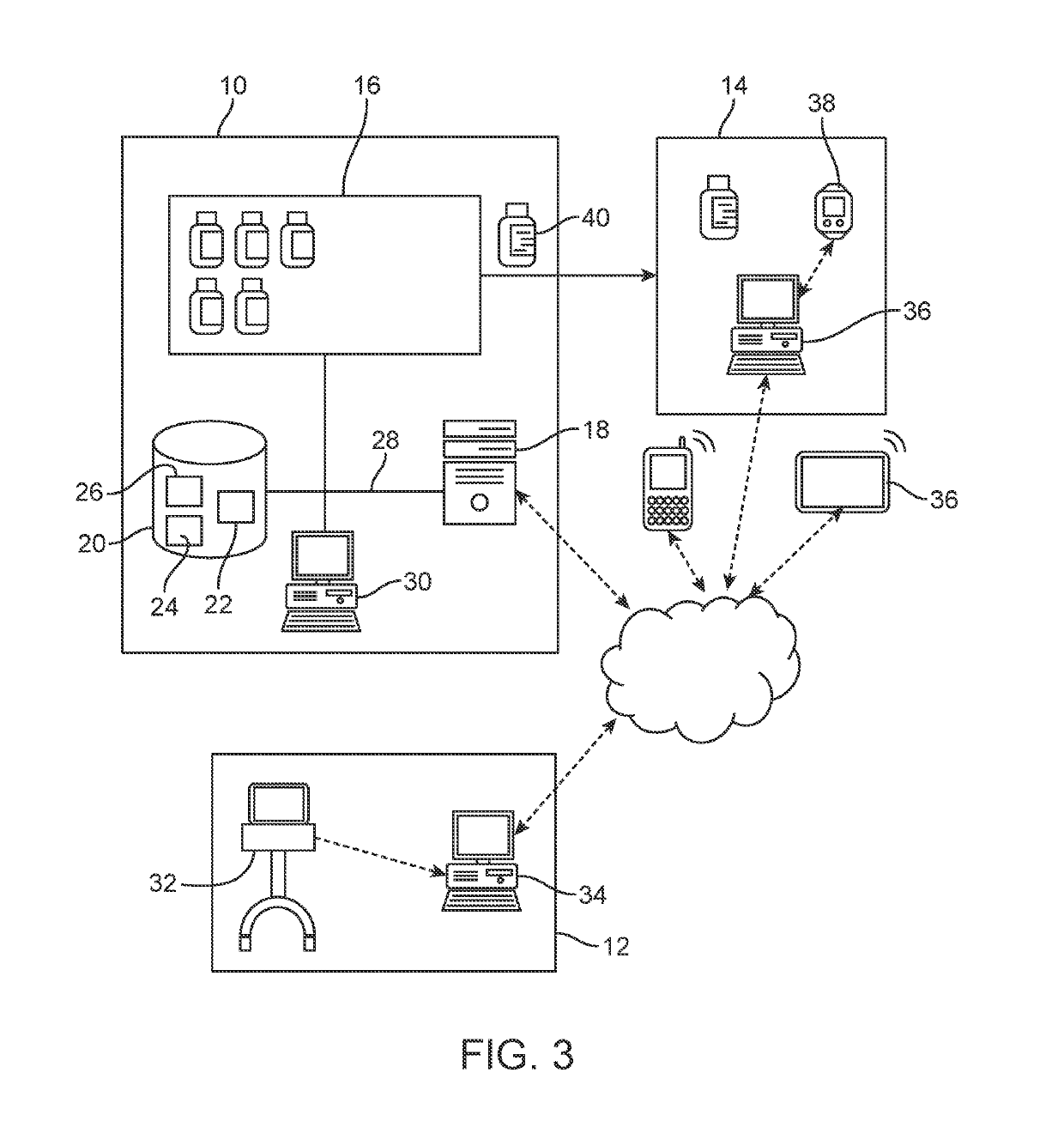
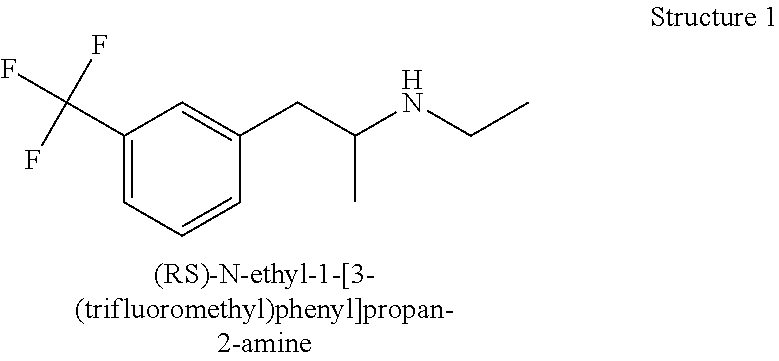
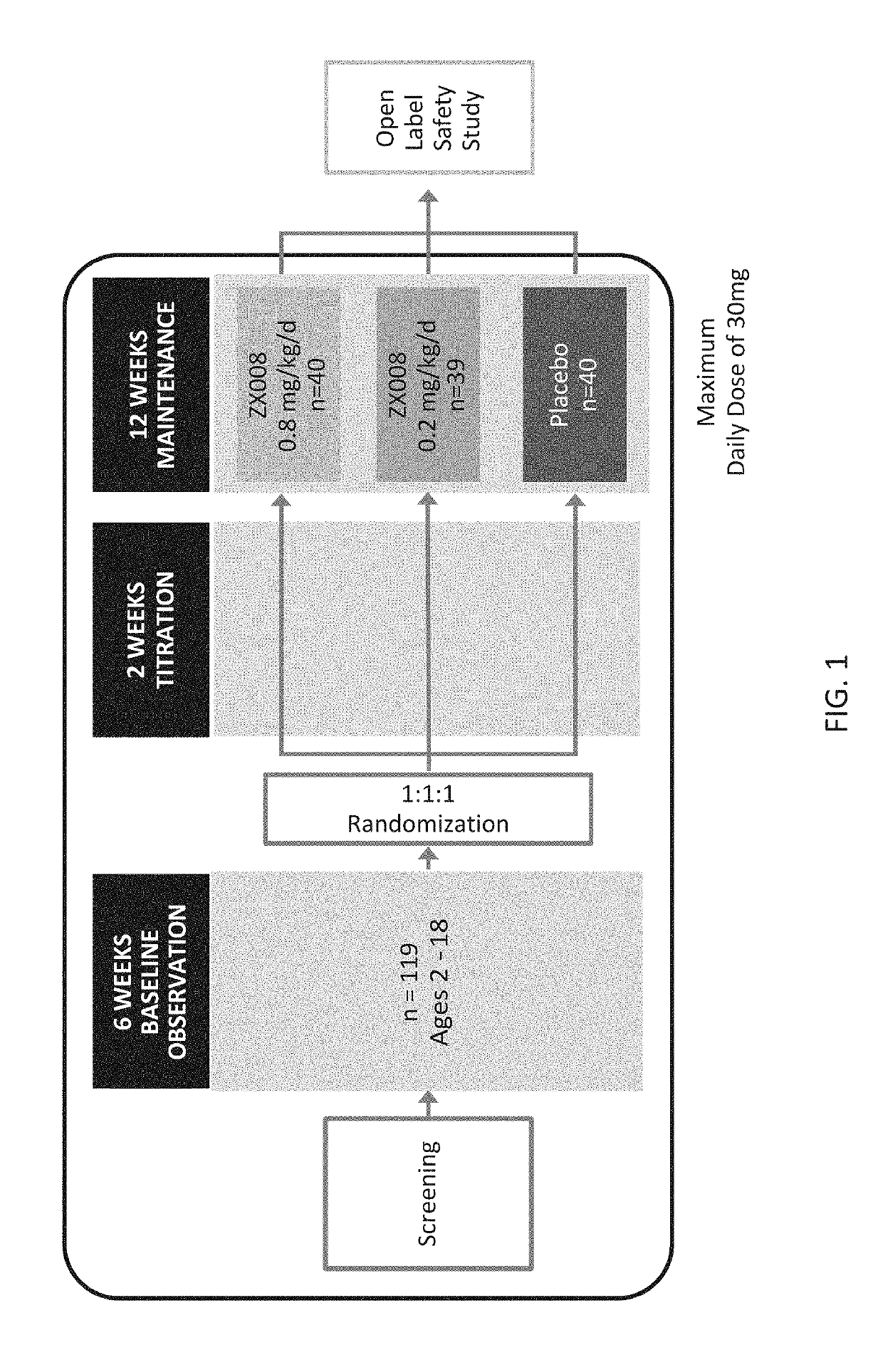
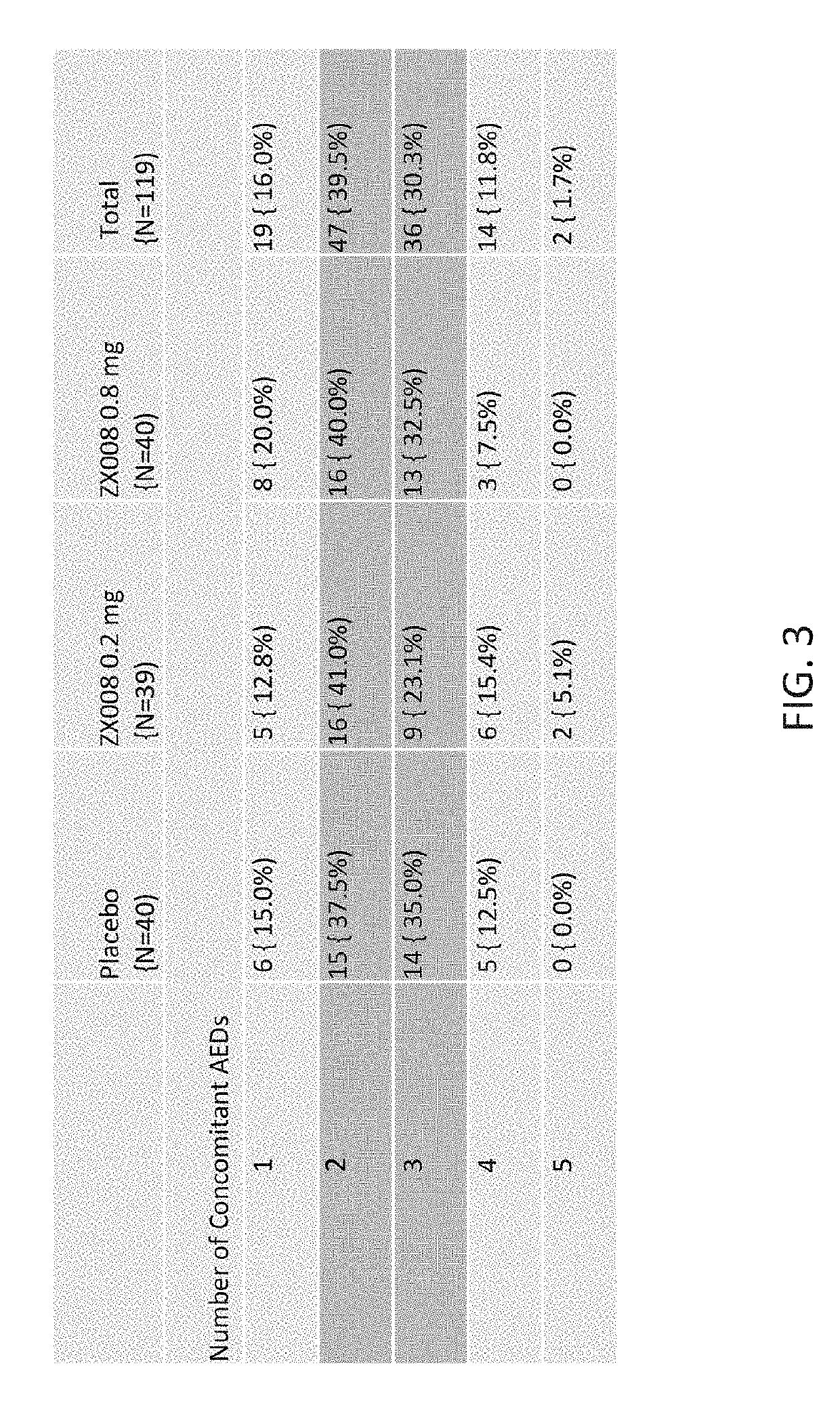
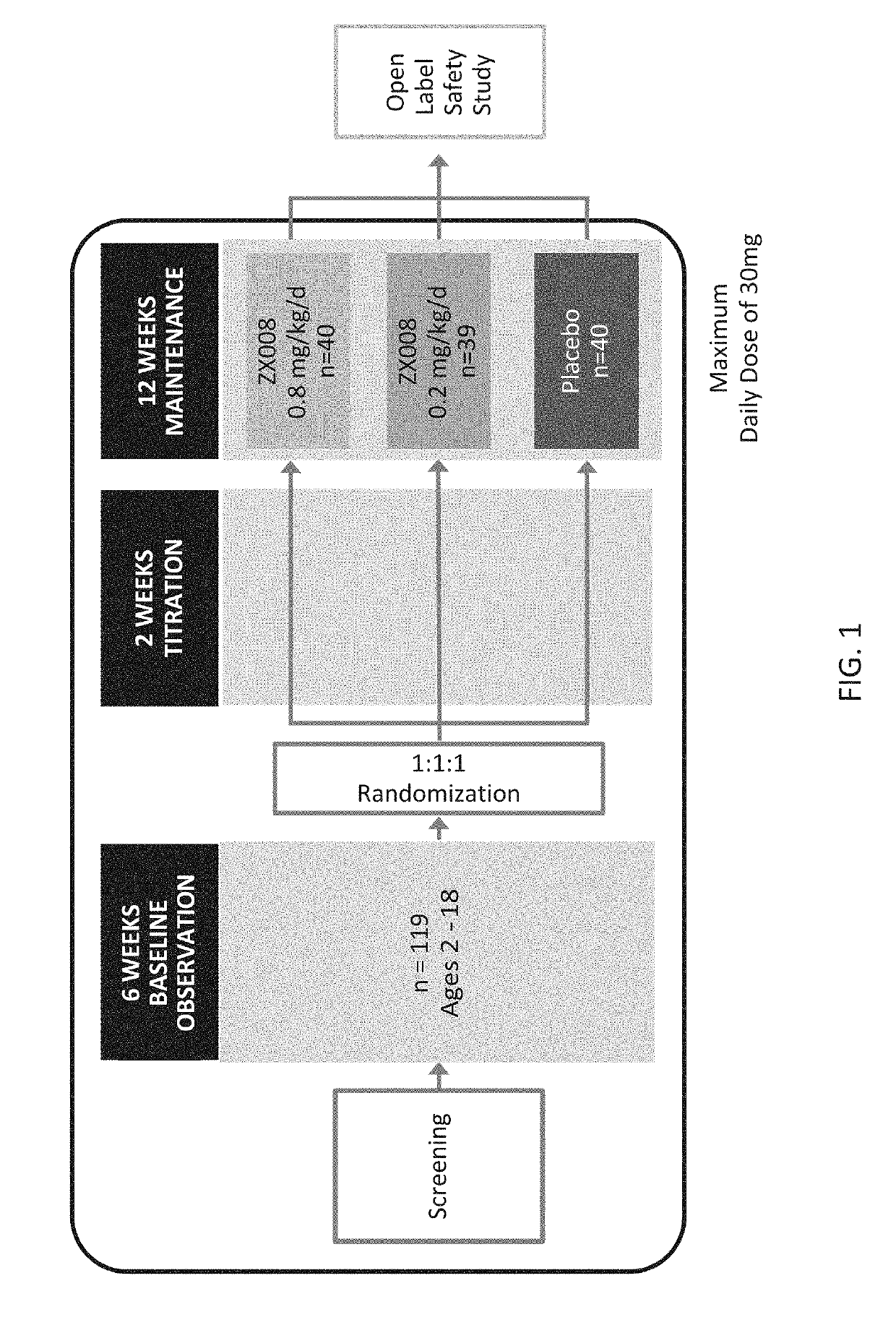
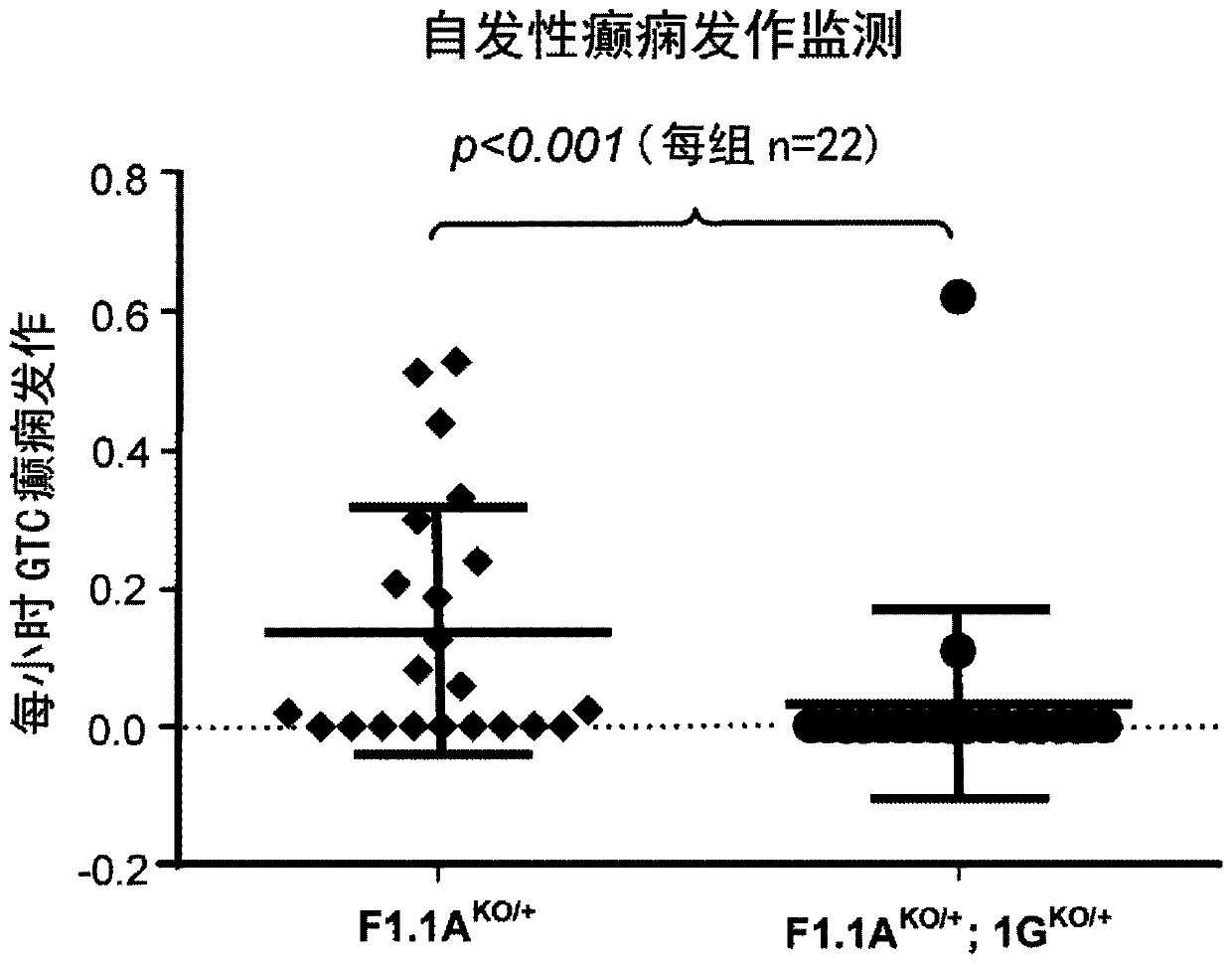
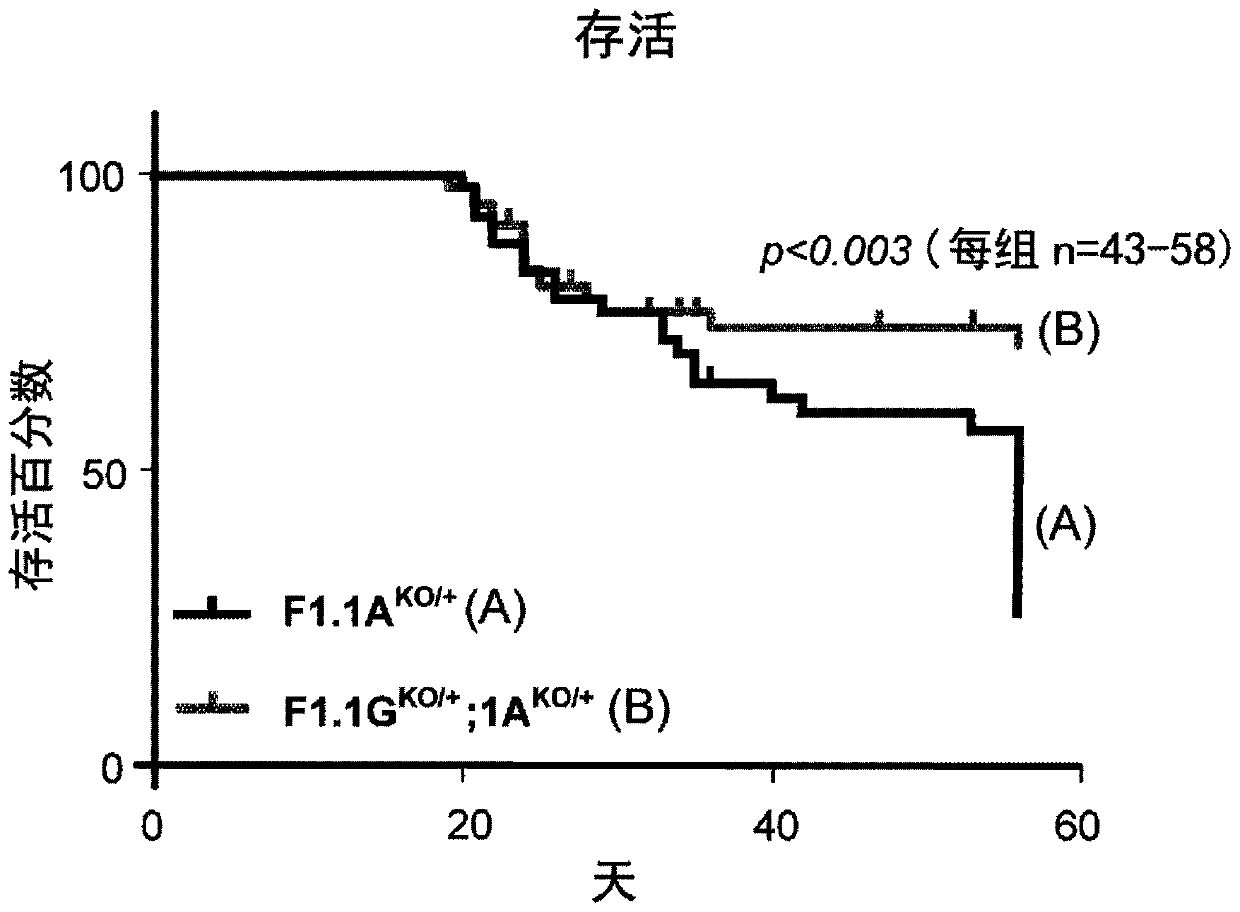
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36 results about "Dravet syndrome" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
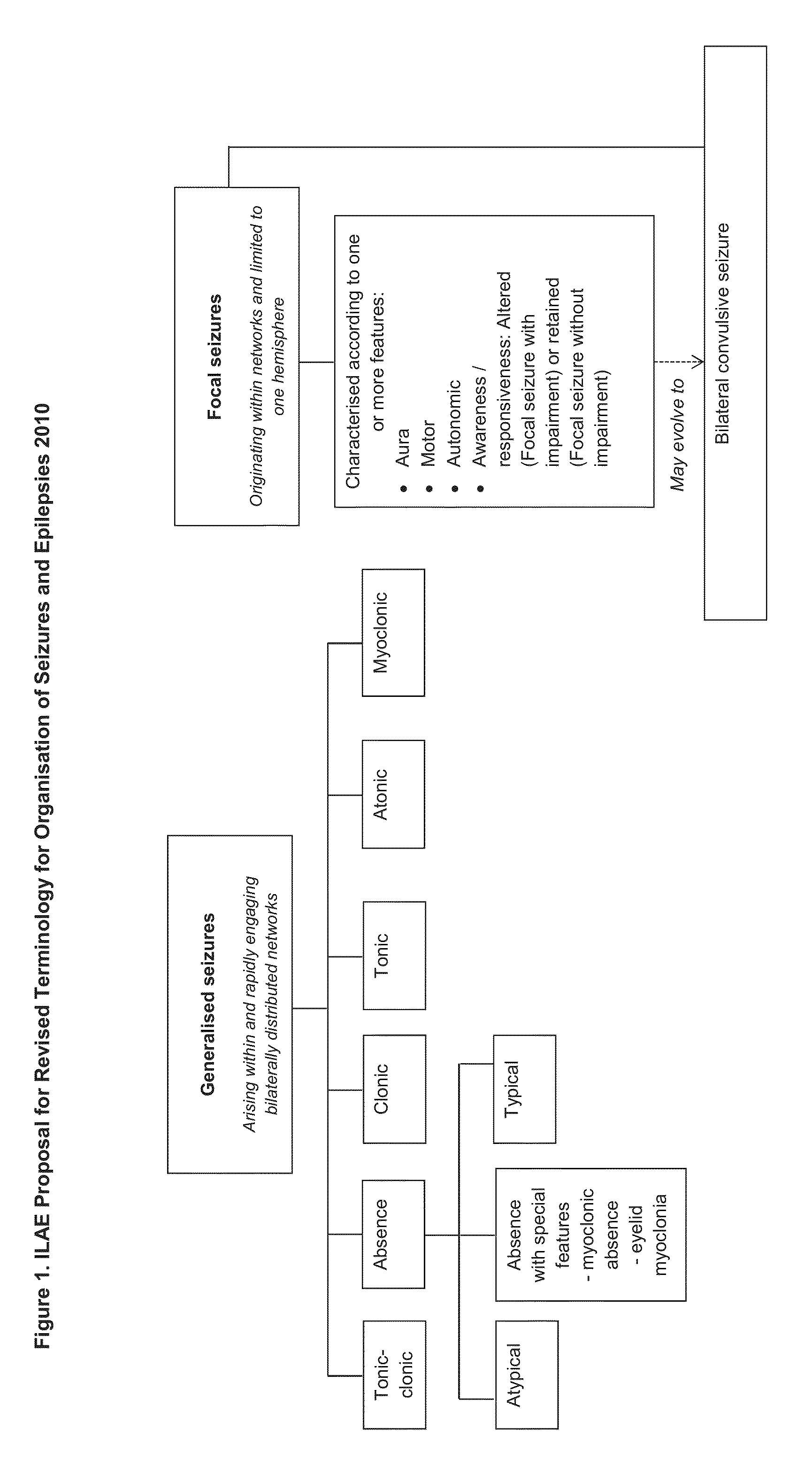
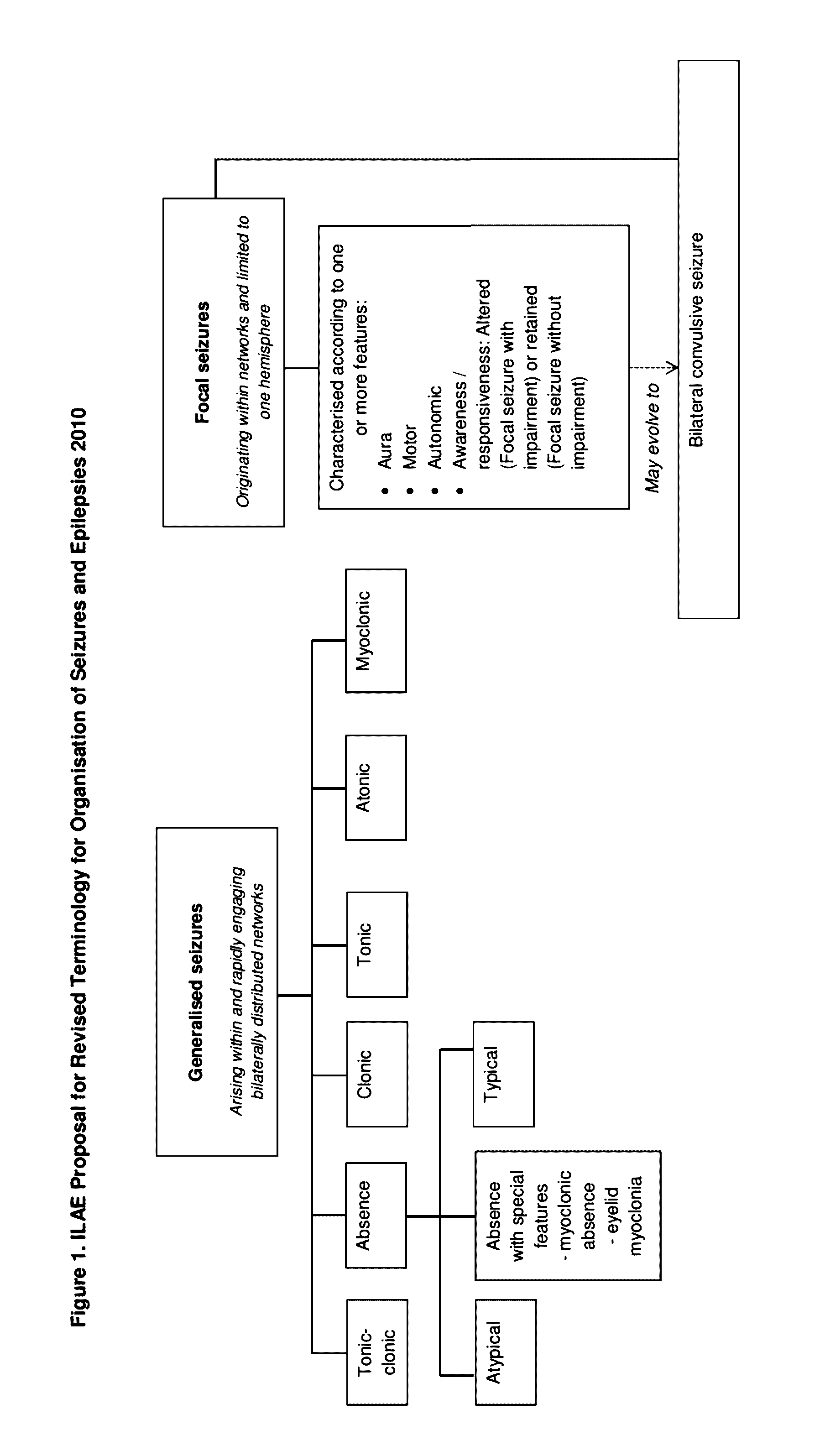
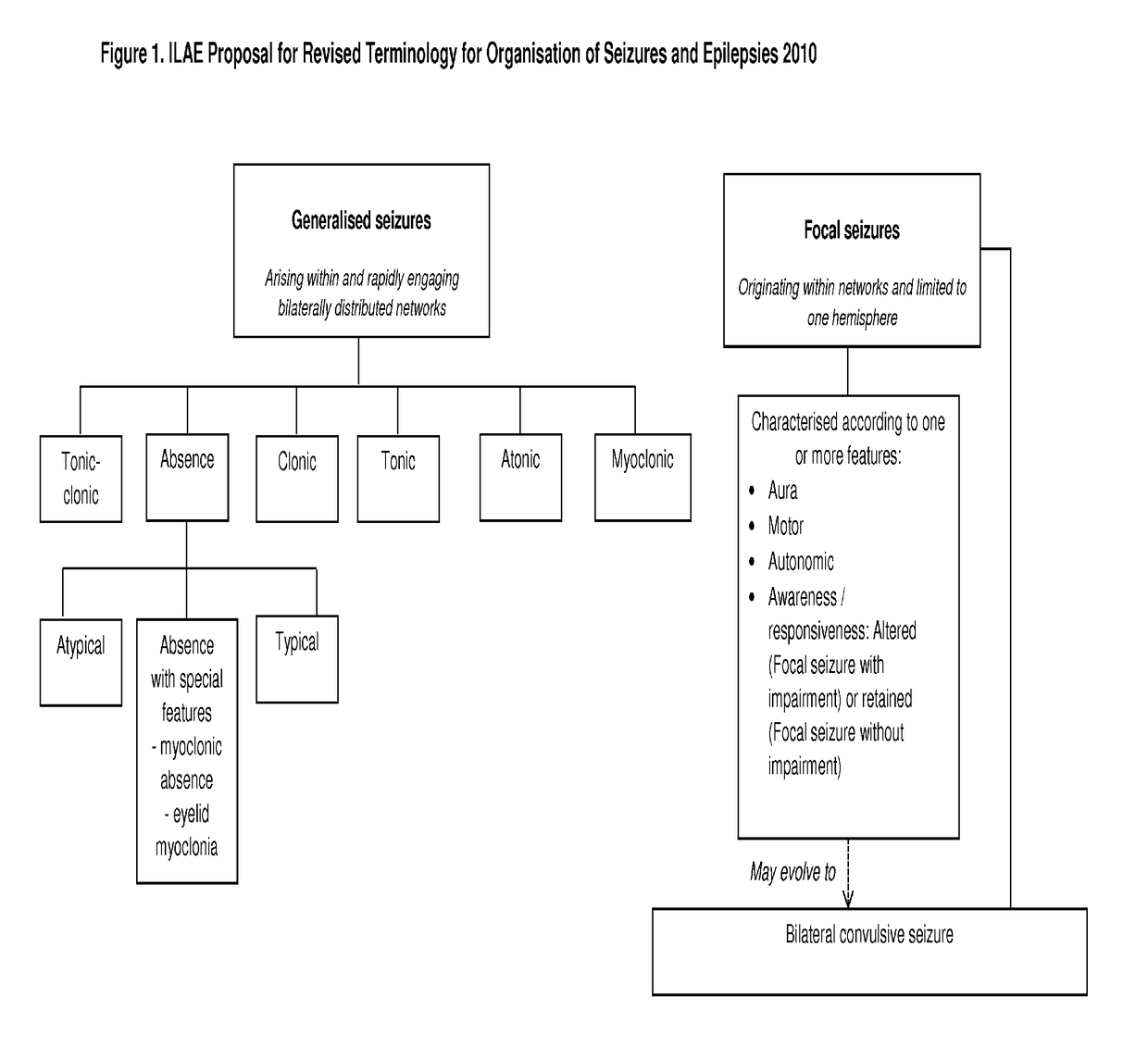
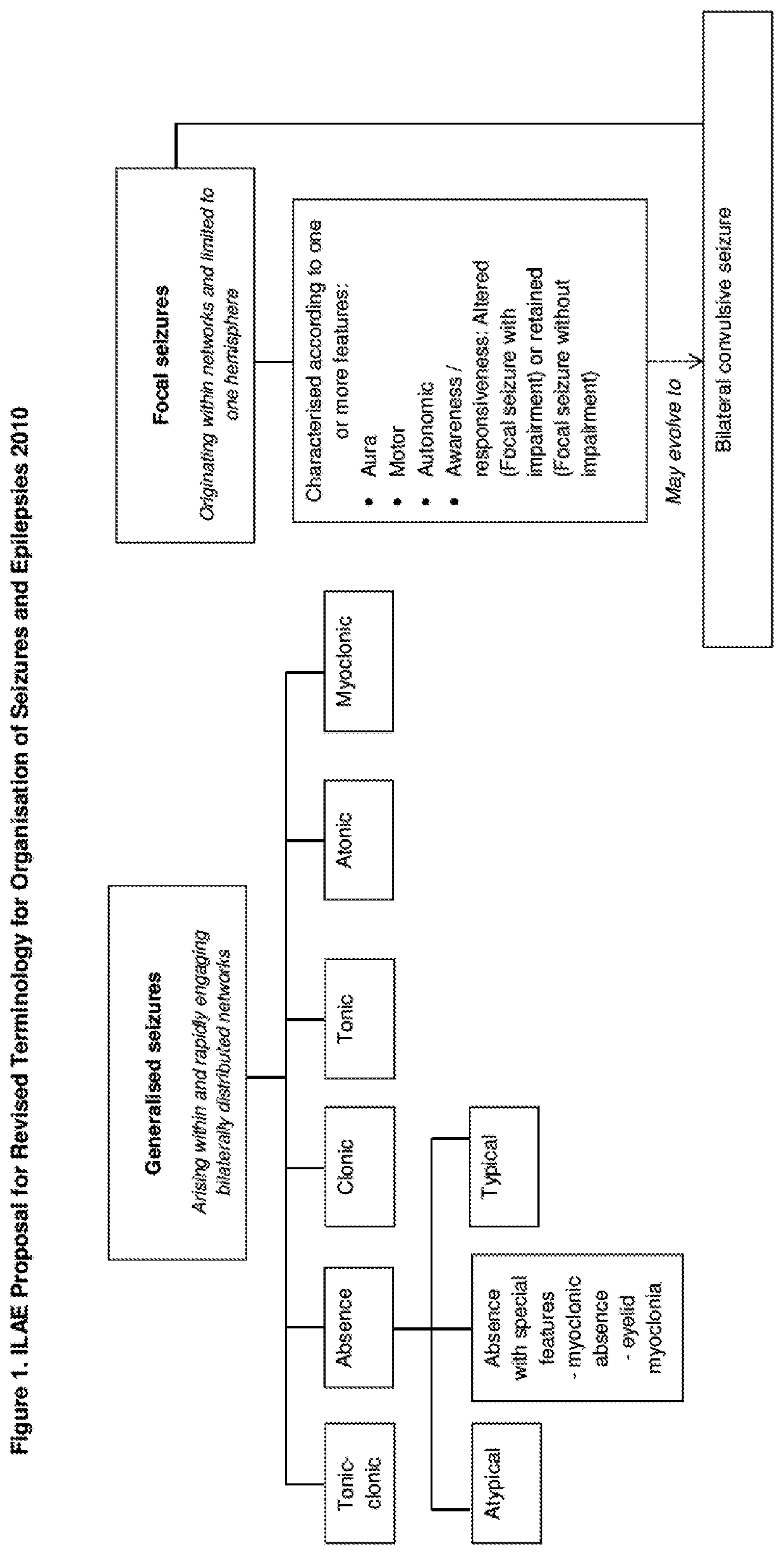
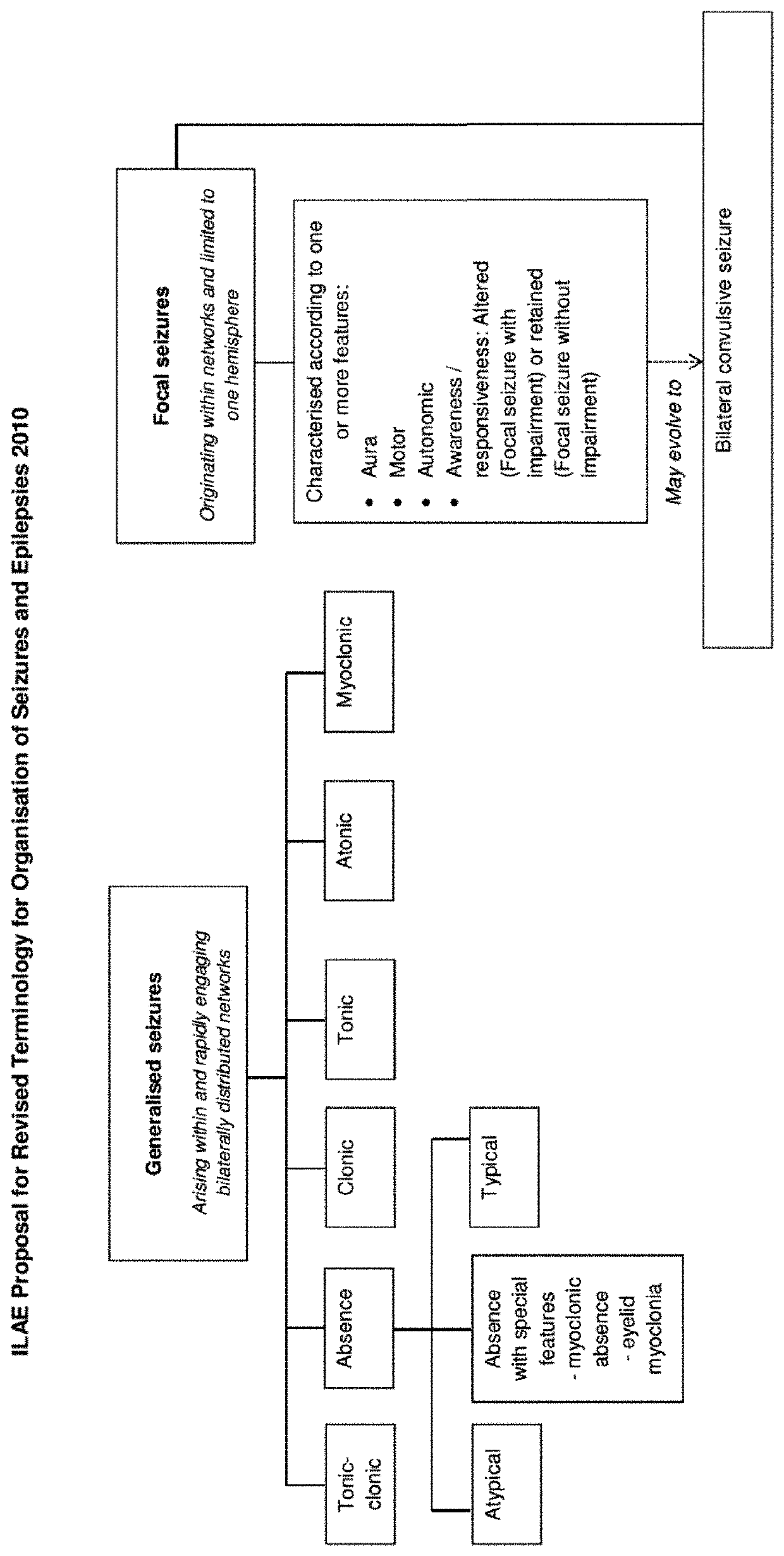
Dravet syndrome, previously known as severe myoclonic epilepsy of infancy (SMEI), is a type of epilepsy with seizures that are often triggered by hot temperatures or fever. It is treated with anticonvulsant medications. It often begins around six months of age.
Use of cannabinoids in the treatment of epilepsy
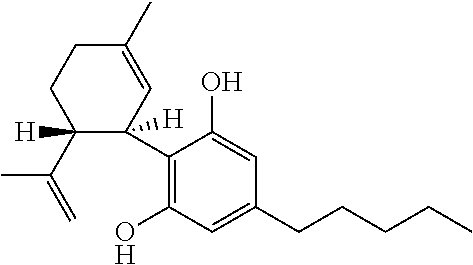
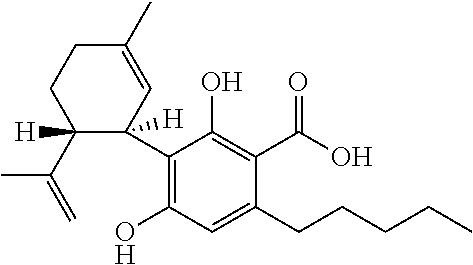
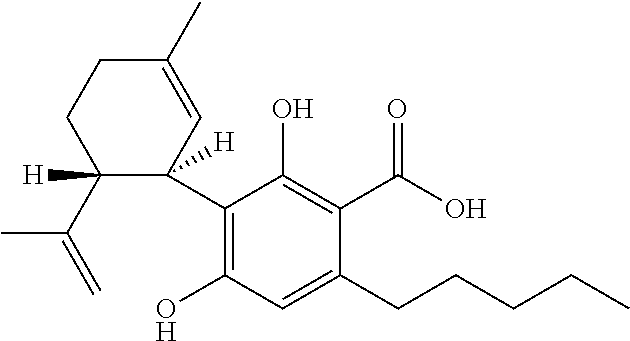
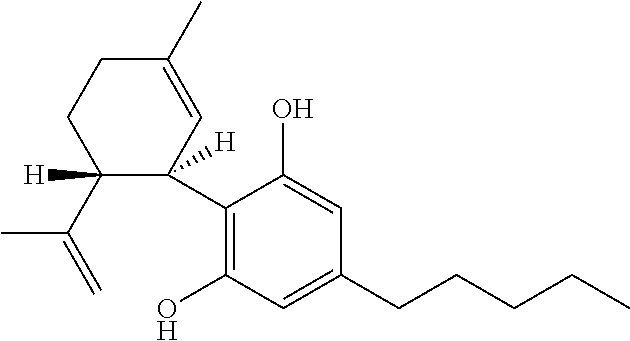
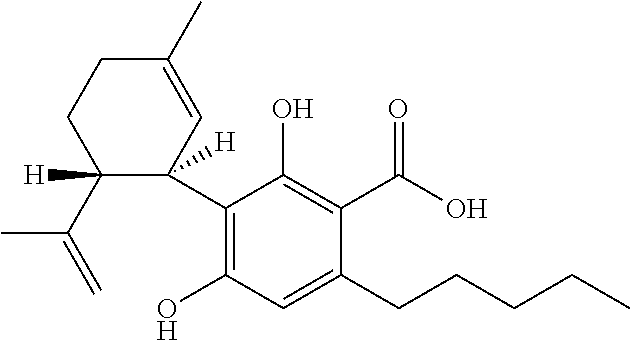
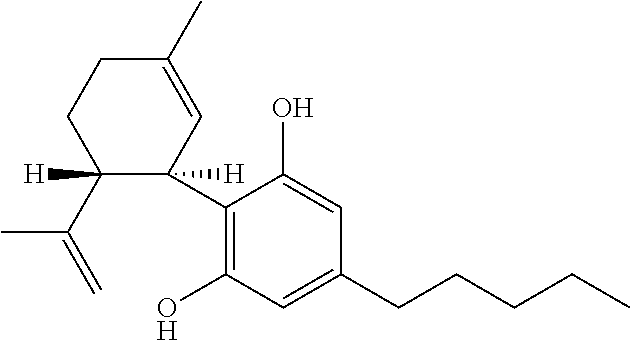
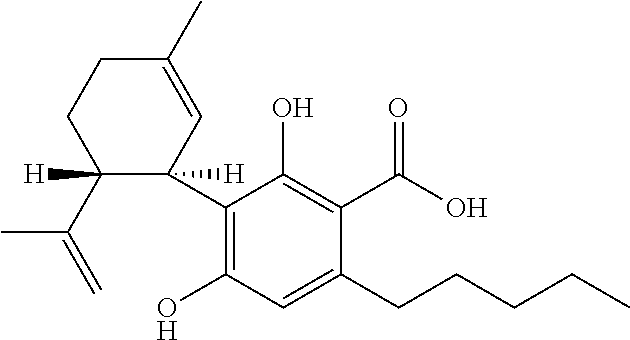
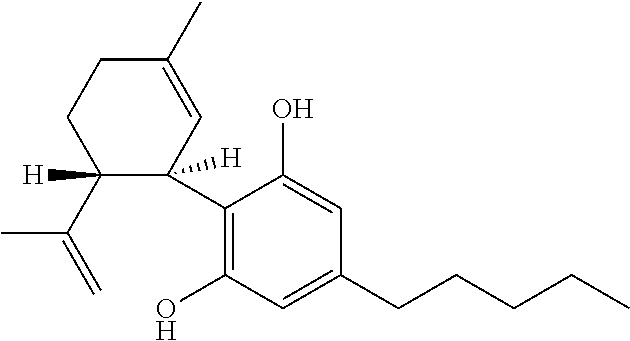
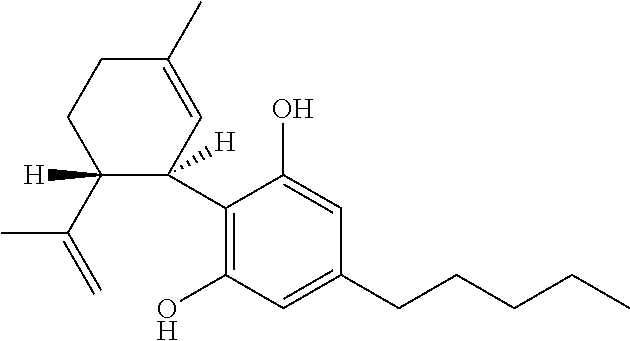
The present disclosure relates to the use of cannabidiol (CBD) in the treatment of absence seizures. In particular, the disclosure relates to the use of CBD for reducing absence seizures in patients suffering with etiologies that include: Lennox-Gastaut Syndrome; Tuberous Sclerosis Complex; Dravet Syndrome; Doose Syndrome; CDKL5; Dup15q; Jeavons syndrome; Myoclonic Absence Epilepsy; Neuronal ceroid lipofuscinoses (NCL) and brain abnormalities. The disclosure further relates to the use of CBD in combination with one or more anti-epileptic drugs (AEDs).
Owner:GW RES LTD
Use of cannabinoids in the treatment of epilepsy
ActiveUS20150359755A1Reduces total convulsive seizure frequencyBiocideNervous disorderCannabinoidDravet syndrome
The present disclosure relates to the use of cannabidiol (CBD) for the reduction of total convulsive seizure frequency in the treatment of “treatment-resistant epilepsy” (TRE). In particular, the disclosure relates to the use of CBD of treating TRE when the TRE is Dravet syndrome; myoclonic absence seizures or febrile infection related epilepsy syndrome (FIRES). The disclosure further relates to the use of CBD in combination with one or more anti-epileptic drugs (AEDs).
Owner:GW RES LTD
Use of cannabinoids in the treatment of epilepsy
The present disclosure relates to the use of cannabidiol (CBD) for the reduction of total convulsive seizure frequency in the treatment of “treatment-resistant epilepsy” (TRE). In particular, the disclosure relates to the use of CBD of treating TRE when the TRE is Dravet syndrome; myoclonic absence seizures or febrile infection related epilepsy syndrome (FIRES). The disclosure further relates to the use of CBD in combination with one or more anti-epileptic drugs (AEDs).
Owner:GW RES LTD
Use of cannabinoids in the treatment of epilepsy
ActiveUS20170007551A1Reduce in quantityReduce doseNervous disorderHydroxy compound active ingredientsAicardi's syndromeFocal Epilepsies
The present invention relates to the use of cannabidiol (CBD) in the treatment of focal seizures. In one embodiment the patients suffering from focal seizures are children and young adults. CBD appears particularly effective in reducing focal seizures in patients suffering with etiologies that include: Lennox-Gastaut Syndrome; Tuberous Sclerosis Complex; Dravet Syndrome; CDKL5; Neuronal ceroid lipofuscinoses (NCL); febrile infection related epilepsy syndrome (FIRES); Aicardi syndrome and brain abnormalities in comparison to other seizure types. Significantly CBD additionally is very effective in the reduction of a sub-type of focal seizures, focal seizures with impairment.
Owner:GW RES LTD
Use of Cannabinoids in the Treatment of Epilepsy
ActiveUS20170172941A1Reduce in quantityReduce doseNervous disorderHydroxy compound active ingredientsEtiologyAicardi's syndrome
The present disclosure relates to the use of cannabidiol (CBD) for the treatment of atonic seizures. In particular the CBD appears particularly effective in reducing atonic seizures in patients suffering with etiologies that include: Lennox-Gastaut Syndrome; Tuberous Sclerosis Complex; Dravet Syndrome; Doose Syndrome; Aicardi syndrome; CDKL5 and Dup15q in comparison to other seizure types. The disclosure further relates to the use of CBD in combination with one or more anti-epileptic drugs (AEDs).
Owner:GW RES LTD
Combination treatment of specific forms of epilepsy
InactiveUS20170071949A1Reduce seizuresEliminate side effectsAnhydride/acid/halide active ingredientsHeterocyclic compound active ingredientsSide effectCo morbidity
Formulations for and methods of treatment of Dravet syndrome that avoid side effects are disclosed. The formulations comprise a 5-HT receptor agonists which does not agonize selected 5-HT receptor subtypes, and in particular does not agonize the receptor subtype 5-HT2B. Also disclosed are combinations of such 5-HT receptor agonists. Also disclosed are combinations of such 5-HT receptor agonists and SSRIs, SNRIs, and triptans for treating co-morbidities associated with Dravet syndrome.
Owner:ZOGENIX INT
Use of cannabinoids in the treatment of epilepsy
ActiveUS20200297656A1Reduce in quantityReduce doseNervous disorderHydroxy compound active ingredientsAicardi's syndromeYoung adult
The present invention relates to the use of cannabidiol (CBD) in the treatment of focal seizures. In one embodiment the patients suffering from focal seizures are children and young adults. CBD appears particularly effective in reducing focal seizures in patients suffering with etiologies that include: Lennox-Gastaut Syndrome; Tuberous Sclerosis Complex; Dravet Syndrome; CDKLS; Neuronal ceroid lipofuscinoses (NCL); febrile infection related epilepsy syndrome (FIRES); Aicardi syndrome and brain abnormalities in comparison to other seizure types. Significantly CBD additionally is very effective in the reduction of a sub-type of focal seizures, focal seizures with impairment.
Owner:JAZZ PHARM RES UK LTD
Use of cannabinoids in the treatment of epilepsy
ActiveUS10709671B2Nervous disorderHydroxy compound active ingredientsAicardi's syndromeFocal Epilepsies
Owner:GW RES LTD
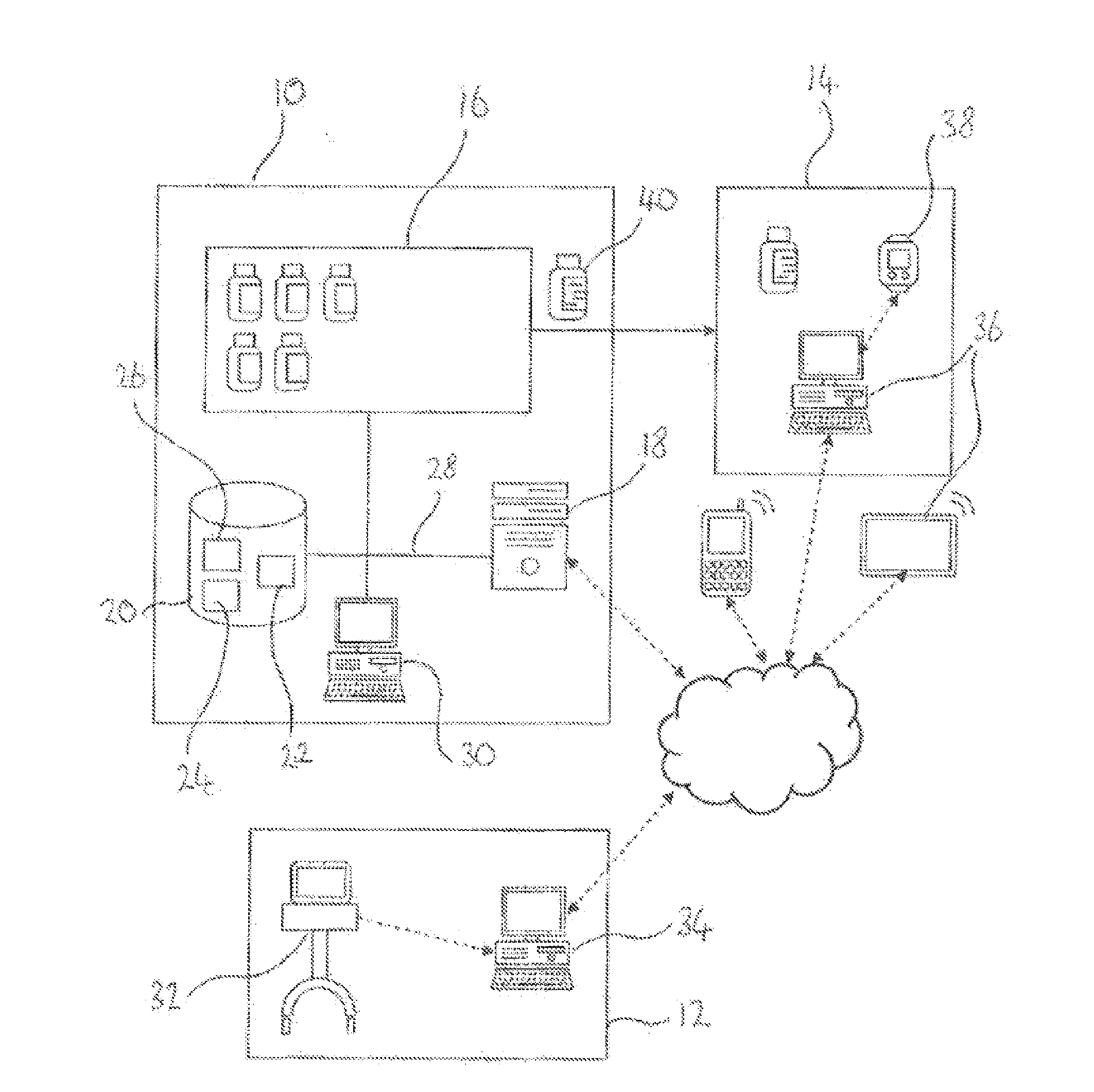
Control system for control of distribution of medication
ActiveUS20160092652A1Improve understandingReduce and prevent instance of misdiagnosisData processing applicationsDigital data information retrievalMedication authorizationMedicine
A system of controlling distribution of a medication in the treatment or prevention of epilepsy is provided. A central controller of the system has a data store and one or more processors for reading and writing data to the data store. The data store comprises a database of patient records, each patient record having a medication authorization field. The central controller can output an authorization of a first prescription of epilepsy medication to a patient in dependence upon genetic test results for the patient and schedules a subsequent test for the patient prior to authorization of a subsequent prescription of epilepsy medication. Also provided are methods in which the subject systems find use. The systems and methods find use in the treatment of severe subtypes of epilepsy or refractory epilepsy, such as Dravet Syndrome.
Owner:ZOGENIX INT
Method of reduction in convulsive seizure frequency
InactiveUS20190247333A1Reduce convulsive seizure frequencyEliminate seizureOrganic active ingredientsNervous disorderFenfluramineHuman patient
A method of reducing convulsive seizure frequency in a human patient diagnosed with Dravet syndrome, comprising administering to the patient a therapeutically effective dose of fenfluramine or a pharmaceutically acceptable salt, base, acid or amine thereof, and repeating the administering over a period of days until the patient exhibits a significant reduction (e.g., 40% or greater) from baseline in convulsive seizure frequency. In some embodiments of the method, convulsive seizures are completely eliminated for 10 days or more, 20 days or more, 30 days or more, 50 days or more, 100 days or more.
Owner:ZOGENIX INT
Derivatives of rufinamide and their use in inhibtion of the activation of human voltage-gated sodium channels
ActiveUS20170029382A1Improve actionGreatly reduces seizuresOrganic active ingredientsNervous disorderDiseaseEpileptic encephalopathy
The present invention provides compounds of formula I which are capable of inhibition of the activation of hNav1.1 or hNav1.6 sodium channels in neurons. Pharmaceutical compositions comprising these compounds are also provided. Methods for prevention and treatment of neurological disorders, including, for example, seizures and seizure disorders, including Lennox-Gastaut Syndrome, Dravet syndrome, epileptic encephalopathies, autism, Familial hemiplegic migraine (FHM), anxiety disorders, including Post-traumatic stress disorder (PTSD), panic disorder and obsessive-compulsive disorder, neuropathic pain, and Rett syndrome by administration of these compounds are also provided.
Owner:AES GLOBAL HLDG PTE LTD +1
Method of reducing seizure type experienced by a dravet patient
InactiveUS20190091174A1Reduce convulsive seizure frequencyEliminate seizureNervous disorderHydroxy compound active ingredientsFenfluramineHuman patient
A method of reducing a particular type of seizure in a human patient diagnosed with Dravet syndrome, by administering to the patient a therapeutically effective dose of fenfluramine or a pharmaceutically acceptable salt, base, acid or amine thereof, and repeating the administering over a period of days until the patient exhibits a reduction from baseline in seizures of a particular type. The reduction may be of one, two or three specific types of seizures.
Owner:ZOGENIX INT
Control system for control of distribution of medication
ActiveUS10452815B2Reduce and prevent instance of misdiagnosisSignificant health implications for the patientDigital data information retrievalDrug and medicationsMedication authorizationMedicine
A system of controlling distribution of a medication in the treatment or prevention of epilepsy is provided. A central controller of the system has a data store and one or more processors for reading and writing data to the data store. The data store comprises a database of patient records, each patient record having a medication authorization field. The central controller can output an authorization of a first prescription of epilepsy medication to a patient in dependence upon genetic test results for the patient and schedules a subsequent test for the patient prior to authorization of a subsequent prescription of epilepsy medication. Also provided are methods in which the subject systems find use. The systems and methods find use in the treatment of severe subtypes of epilepsy or refractory epilepsy, such as Dravet Syndrome.
Owner:ZOGENIX INT
Ketogenic diet compatible fenfluramine formulation
ActiveUS10682317B2Improve complianceLacks a nutritive/digestible/glycemic carbohydrateOrganic active ingredientsNervous disorderFenfluramineKetogenic diet
A method of treating symptoms of a subtype of epilepsy, e.g., Dravet syndrome, in a patient diagnosed with a subtype of epilepsy, by administering to the patient an effective dose of a fenfluramine formulation in combination with a ketogenic diet over a period of time sufficient to reduce or completely eliminate seizures in the patient. Also provided are compositions and kits finding use in practicing embodiments of the methods.
Owner:ZOGENIX INT
Combination treatment of specific forms of epilepsy
InactiveUS20180325909A1Reduce seizuresEliminate side effectsAnhydride/acid/halide active ingredientsHeterocyclic compound active ingredientsSide effectCo morbidity
Formulations for and methods of treatment of Dravet syndrome that avoid side effects are disclosed. The formulations comprise a 5-HT receptor agonists which does not agonize selected 5-HT receptor subtypes, and in particular does not agonize the receptor subtype 5-HT2B. Also disclosed are combinations of such 5-HT receptor agonists. Also disclosed are combinations of such 5-HT receptor agonists and SSRIs, SNRIs, and triptans for treating co-morbidities associated with Dravet syndrome.
Owner:ZOGENIX INT
Method of increasing time between convulsive seizures
InactiveUS20190091173A1Eliminate seizureReduce frequencyNervous disorderHydroxy compound active ingredientsFenfluramineHuman patient
A method of increasing an average time between seizures in a human patient diagnosed with Dravet syndrome, comprising administering to the patient a therapeutically effective dose of fenfluramine or a pharmaceutically acceptable salt, base, acid or amine thereof, and repeating the administering over a period of days until the patient exhibits an increase from baseline in average time between convulsive seizures of 6 hours, days weeks or more.
Owner:ZOGENIX INT
Method of treating selected patient population experiencing dravet syndrome
InactiveUS20190091176A1Eliminate seizureReduce frequencyNervous disorderHydroxy compound active ingredientsTolerabilityCannabidiol
Provided herein is a method of treating a selected patient population, wherein the patient population is selected based on a determination that the patients have previously been non-responsive when treated with cannabidiol. In some embodiments, the method comprises selecting the patient based on a previously failed treatment with cannabidiol, based on lack of efficacy or tolerability. Pharmaceutical compositions and formulations for use in practicing the subject methods are also provided. The method comprises identifying a population of patients diagnosed with Dravet syndrome who were found previously to have been non-responsive when treated with cannabidiol. The selected population of patients is then treated by administering, to each identified patient, a therapeutically effective dose of fenfluramine or a pharmaceutically acceptable salt, base, acid or amine thereof, and repeating the administering over a period of a day or days, or over a period of weeks, months or years, until the patient exhibits a reduction from baseline in convulsive seizure frequency.
Owner:ZOGENIX INT
Method of reduction in convulsive seizure frequency
InactiveUS20190125697A1Eliminate seizureReduce frequencyNervous disorderHydroxy compound active ingredientsFenfluramineHuman patient
A method of reducing convulsive seizure frequency in a human patient diagnosed with Dravet syndrome, comprising administering to the patient a therapeutically effective dose of fenfluramine or a pharmaceutically acceptable salt, base, acid or amine thereof, and repeating the administering over a period of days until the patient exhibits a significant reduction (e.g., 40% or greater) from baseline in convulsive seizure frequency. In some embodiments of the method, convulsive seizures are completely eliminated for 10 days or more, 20 days or more, 30 days or more, 50 days or more, 100 days or more.
Owner:ZOGENIX INT
Method of reduction medication in treating dravet syndrome
InactiveUS20190091175A1Reduce convulsive seizure frequencyEliminate seizureNervous disorderHydroxy compound active ingredientsFenfluramineSide effect
A method of reducing dosing of a concomitant medication in a human patient diagnosed with Dravet syndrome, by administering to the patient a therapeutically effective dose of fenfluramine or a pharmaceutically acceptable salt, base, acid or amine thereof, and repeating the administering over a period of days while reducing the dose of one or more concomitant anti-seizure or anti-epileptic drugs (AEDs) from baseline and thereby decreasing the amount of medication given to the patient while reducing adverse side effects. Pharmaceutical compositions and formulations for use in practicing the subject methods are also provided.
Owner:ZOGENIX INT
Methods for treating epilepsy
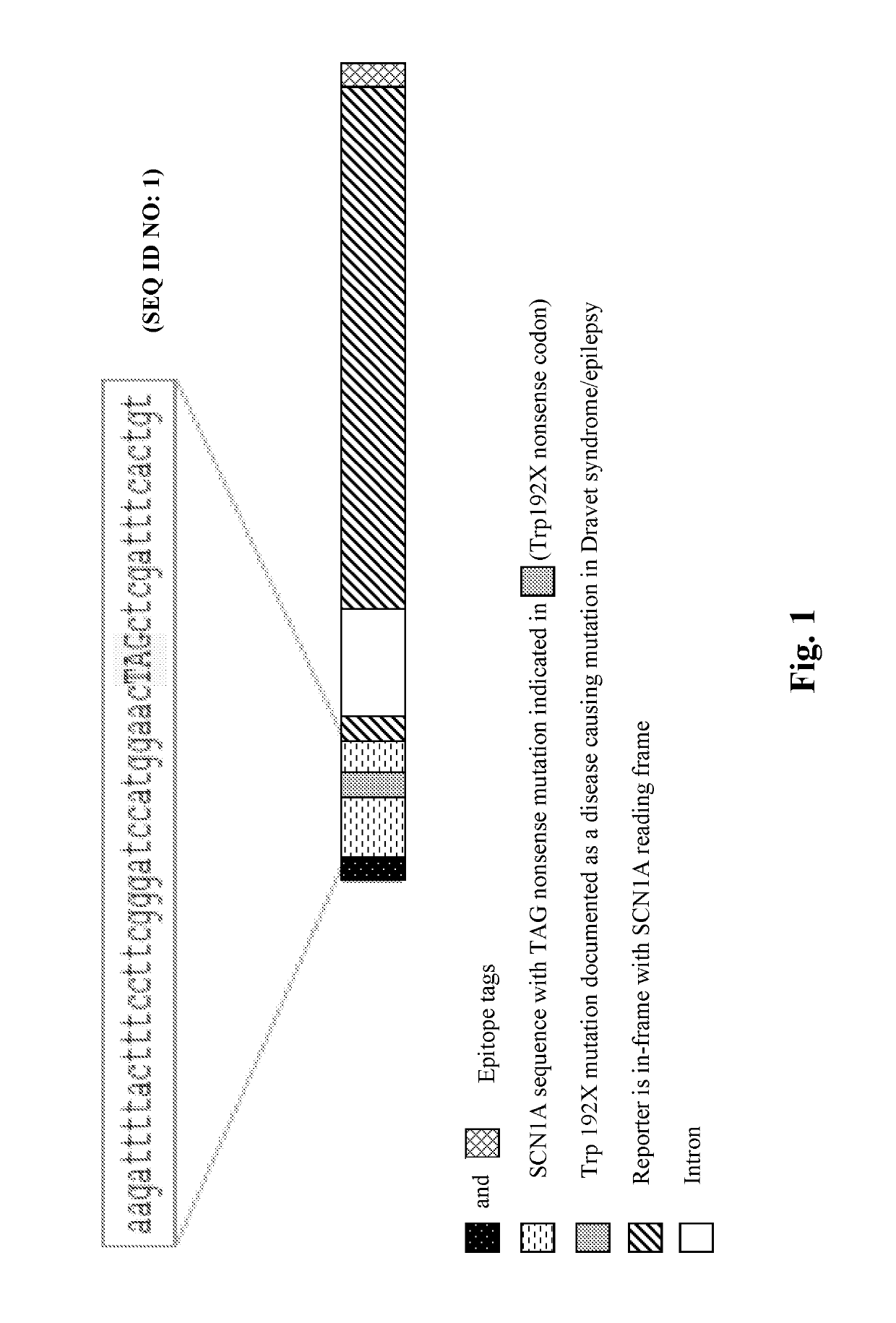
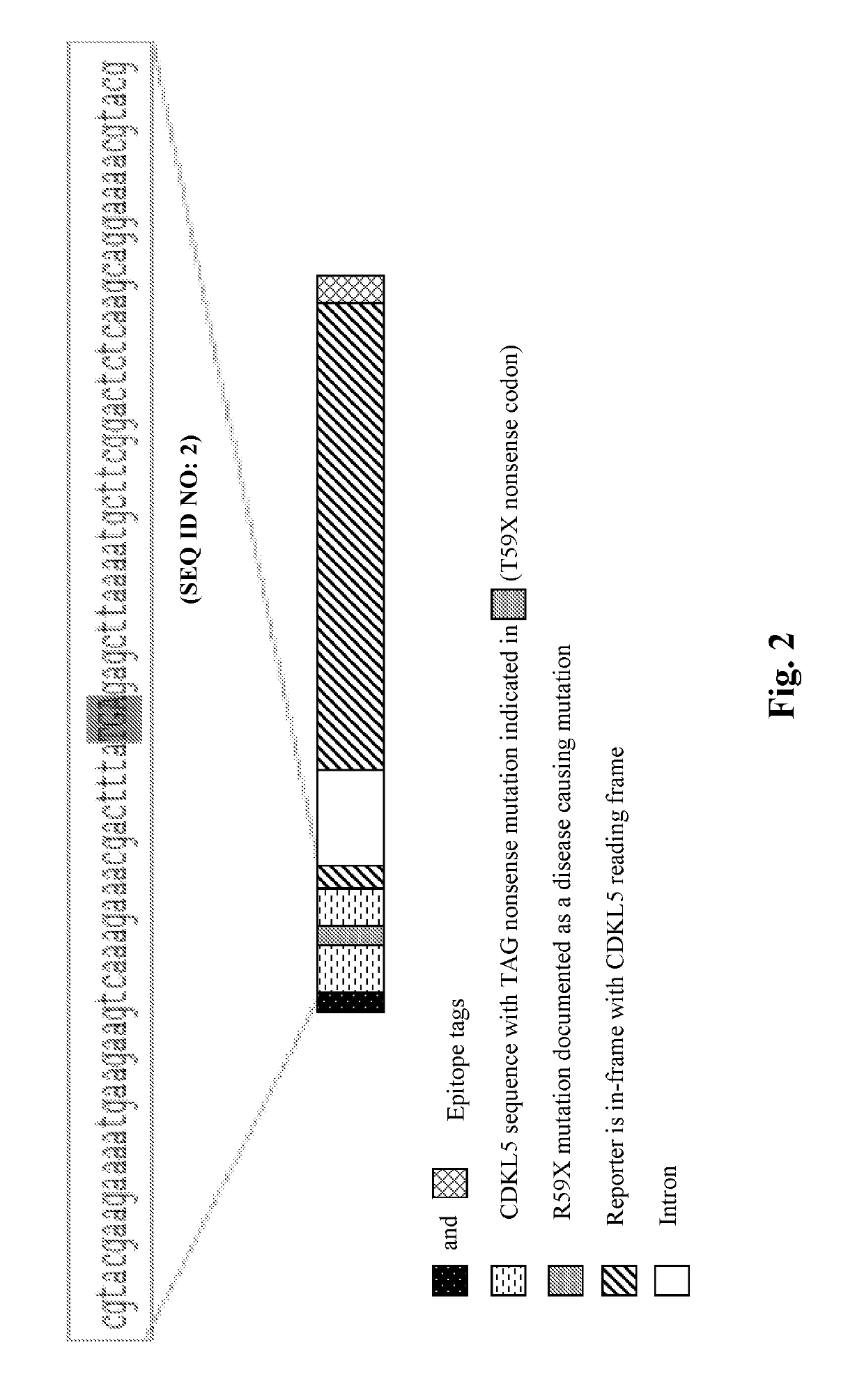
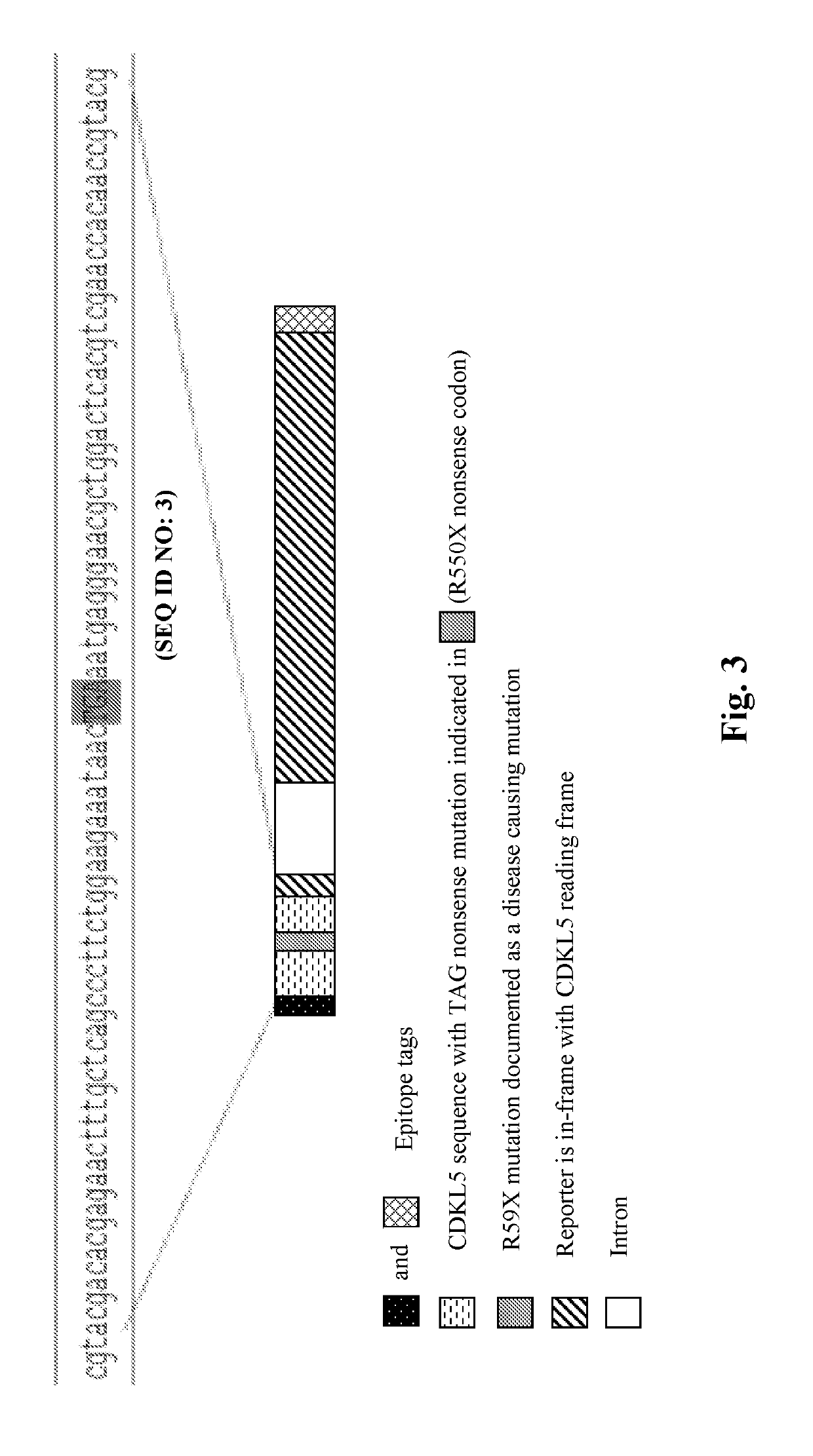
Provided herein are methods of treating, preventing, ameliorating or managing a nonsense mutation mediated epileptic disease, comprising administering a 1,2,4-oxadiazole benzoic acid to a patient having a nonsense mutation mediated epileptic disease. In particular, provided herein are methods of treating, preventing, ameliorating or managing a CDKL5 and / or SCN1A (Dravet syndrome) nonsense mutation mediated epileptic disease.
Owner:PTC THERAPEUTICS INC
Combination treatment of specific forms of epilepsy
PendingUS20220193082A1Reduce seizuresEliminate side effectsAnhydride/acid/halide active ingredientsHeterocyclic compound active ingredientsSide effectReceptor subtype
Formulations for and methods of treatment of Dravet syndrome that avoid side effects are disclosed. The formulations comprise a 5-HT receptor agonists which does not agonize selected 5-HT receptor subtypes, and in particular does not agonize the receptor subtype 5-HT2B. Also disclosed are combinations of such 5-HT receptor agonists. Also disclosed are combinations of such 5-HT receptor agonists and SSRIs, SNRIs, and triptans for treating co-morbidities associated with Dravet syndrome.
Owner:ZOGENIX INT
Cyclopropanamine compound and use thereof
ActiveUS20150291577A1Superior LSD inhibitory actionHigh LSD selectivityBiocideNervous disorderNoonan syndromeNodular sclerosis
The present invention provides a compound having a lysine-specific demethylase-1 inhibitory action, and useful as a medicament such as a prophylactic or therapeutic agent for schizophrenia, developmental disorders, particularly diseases having intellectual disability (e.g., autistic spectrum disorders, Rett syndrome, Down's syndrome, Kabuki syndrome, fragile X syndrome, Kleefstra syndrome, neurofibromatosis type 1, Noonan syndrome, tuberous sclerosis), neurodegenerative diseases (e.g., Alzheimer's disease, Parkinson's disease, spinocerebellar degeneration (e.g., dentatorubural pallidoluysian atrophy) and Huntington's disease), epilepsy (e.g., Dravet syndrome) or drug dependence, and the like. A compound represented by the formulawherein each symbol is as defined in the present specification, or a salt thereof.
Owner:TAKEDA PHARMA CO LTD
Rufinamide and derivatives and their use in modulating the gating process of human voltage-gated sodium channels
ActiveUS20150336904A1Slow recoveryImprove actionBiocideNervous disorderEpileptic encephalopathyNeurological disorder
The present invention provides compounds of formula I which are capable of inhibition of the activation of hNav1.1 or hNav1.6 sodium channels in neurons. Pharmaceutical compositions comprising these compounds are also provided. Methods for prevention and treatment of neurological disorders, including, for example, seizures and seizure disorders, including Lennox-Gastaut Syndrome, Dravet syndrome, epileptic encephalopathies, autism, Familial hemiplegic migraine (FHM), anxiety disorders, including Post-traumatic stress disorder (PTSD), panic disorder and obsessive-compulsive disorder, neuropathic pain, and Rett syndrome by administration of these compounds are also provided.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Method for assessment of potential for development of dravet syndrome and use thereof
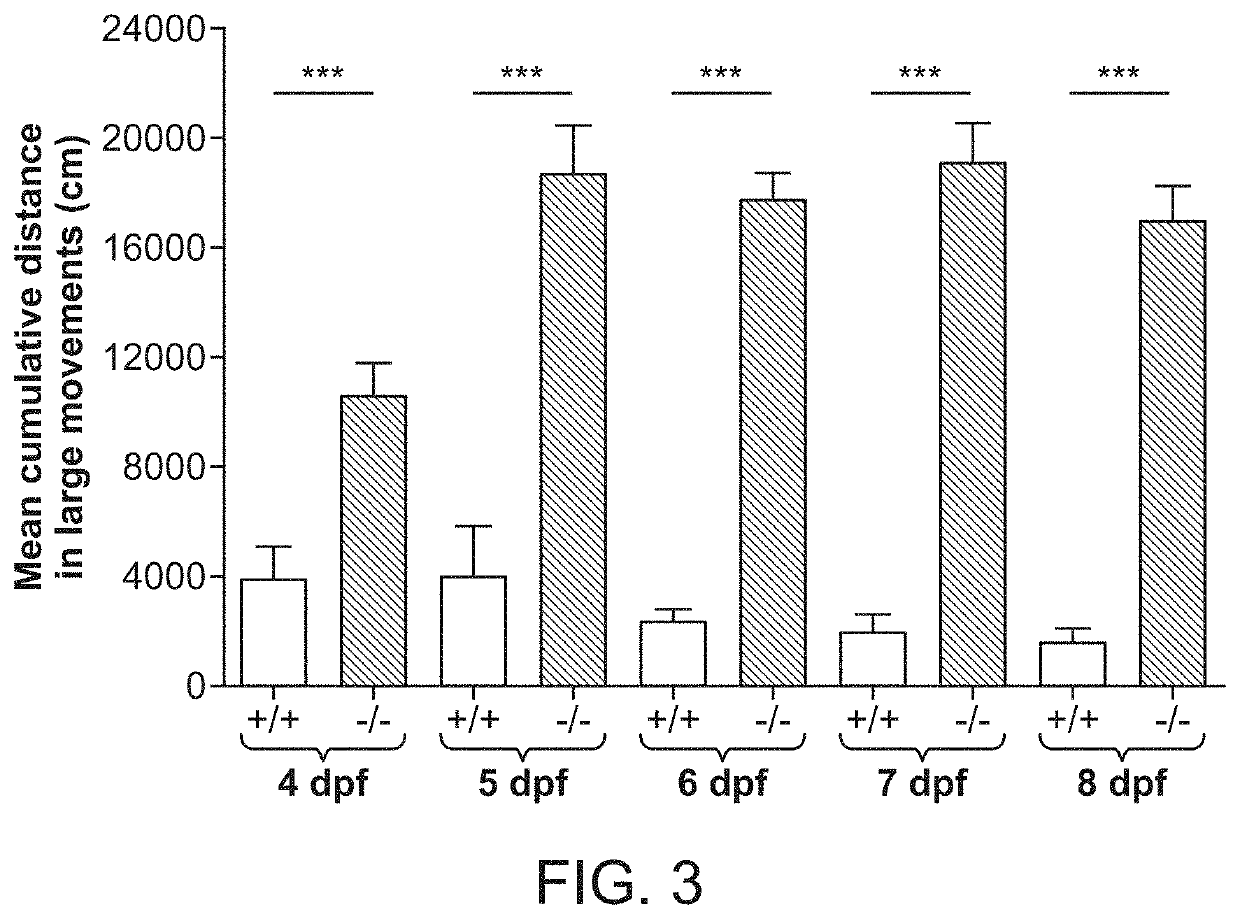
InactiveUS20130036482A1Hinders motivationWorsening and intractableness of Dravet syndromeCompounds screening/testingCompound screeningDravet syndromeBiology
Provided is a method of assessing a potential for development of Dravet syndrome with high accuracy, and use thereof. The method according to the present invention of assessing a potential for development of Dravet syndrome includes, with use of a sample taken from a subject, detecting whether or not a mutation is on α-subunit type 1 of voltage-gated sodium ion channel NaV1.1, and detecting whether or not a mutation is on α-subunit type 1 of voltage-gated calcium ion channel CaV2.1.
Owner:UNIV OKAYAMA
Use of cannabidiol in the treatment of dravet syndrome
PendingUS20220184000A1Improvement of neonatal welfareImprove survivalNervous disorderHydroxy compound active ingredientsCannabidiolDravet syndrome
The present invention relates to the use of cannabidiol (CBD) for use in the treatment of disease modification in Dravet syndrome. In particular the CBD is used to improve neonatal welfare, survival and co-morbidities in patients with Dravet syndrome. Preferably the CBD used is in the form of a botanically derived purified CBD which comprises greater titan or equal to 98% (w / w) CBD and less than or equal to 2% (w / w) of other cannabinoids. The other cannabinoids present are THC at a concentration of less than or equal to 0.1% (w / w); CBD-C1 at a concentration of less titan or equal to 0.15% (w / w); CBDV at a concentration of less titan or equal to 0.8% (w / w); and CBD-C4 at a concentration of less than or equal to 0.4% (w / w). The botanically derived purified CBD preferably also comprises a mixture of both trans-THC and cis-THC. Alternatively, a synthetically produced CBD is used.
Owner:GW RES LTD
Methods for treating epilepsy
Provided herein are methods of treating, preventing, ameliorating or managing a nonsense mutation mediated epileptic disease, comprising administering a 1,2,4-oxadiazole benzoic acid to a patient having a nonsense mutation mediated epileptic disease. In particular, provided herein are methods of treating, preventing, ameliorating or managing a CDKL5 and / or SCN1A (Dravet syndrome) nonsense mutation mediated epileptic disease.
Owner:PTC THERAPEUTICS INC
Use of cannabinoids in the treatment of epilepsy
InactiveUS20220087951A1Shortens dose titration periodReduce generationNervous disorderHydroxy compound active ingredientsAicardi's syndromeAntiepileptic drug
Owner:GW RES LTD
Derivatives of rufinamide and their use in inhibtion of the activation of human voltage-gated sodium channels
ActiveUS9771335B2Improve actionGreatly reduces seizuresOrganic active ingredientsNervous disorderEpileptic encephalopathyBiological activation
The present invention provides compounds of formula I which are capable of inhibition of the activation of hNav1.1 or hNav1.6 sodium channels in neurons. Pharmaceutical compositions comprising these compounds are also provided. Methods for prevention and treatment of neurological disorders, including, for example, seizures and seizure disorders, including Lennox-Gastaut Syndrome, Dravet syndrome, epileptic encephalopathies, autism, Familial hemiplegic migraine (FHM), anxiety disorders, including Post-traumatic stress disorder (PTSD), panic disorder and obsessive-compulsive disorder, neuropathic pain, and Rett syndrome by administration of these compounds are also provided.
Owner:AES GLOBAL HLDG PTE LTD +1
Methods for treating dravet syndrome
Provided herein are methods of treating Dravet syndrome that include administering an effective amount of a T-type calcium channel antagonist to a subject in need of the treatment.
Owner:CAVION INC
Ketogenic diet compatible fenfluramine formulation
ActiveUS20190091178A1Improve complianceLacks a nutritive/digestible/glycemic carbohydrateOrganic active ingredientsNervous disorderFenfluramineKetogenic diet
A method of treating symptoms of a subtype of epilepsy, e.g., Dravet syndrome, in a patient diagnosed with a subtype of epilepsy, by administering to the patient an effective dose of a fenfluramine formulation in combination with a ketogenic diet over a period of time sufficient to reduce or completely eliminate seizures in the patient. Also provided are compositions and kits finding use in practicing embodiments of the methods.
Owner:ZOGENIX INT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com