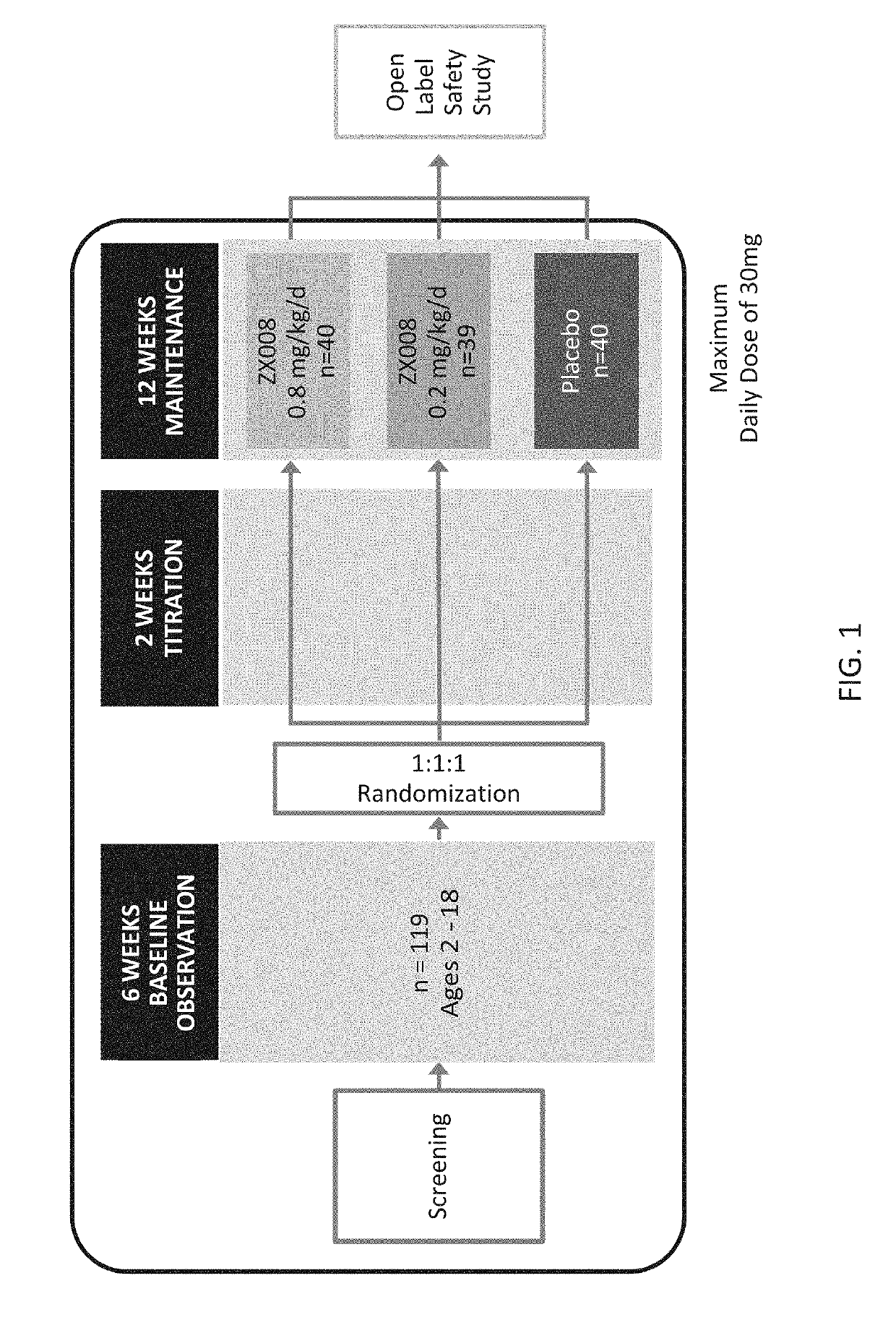
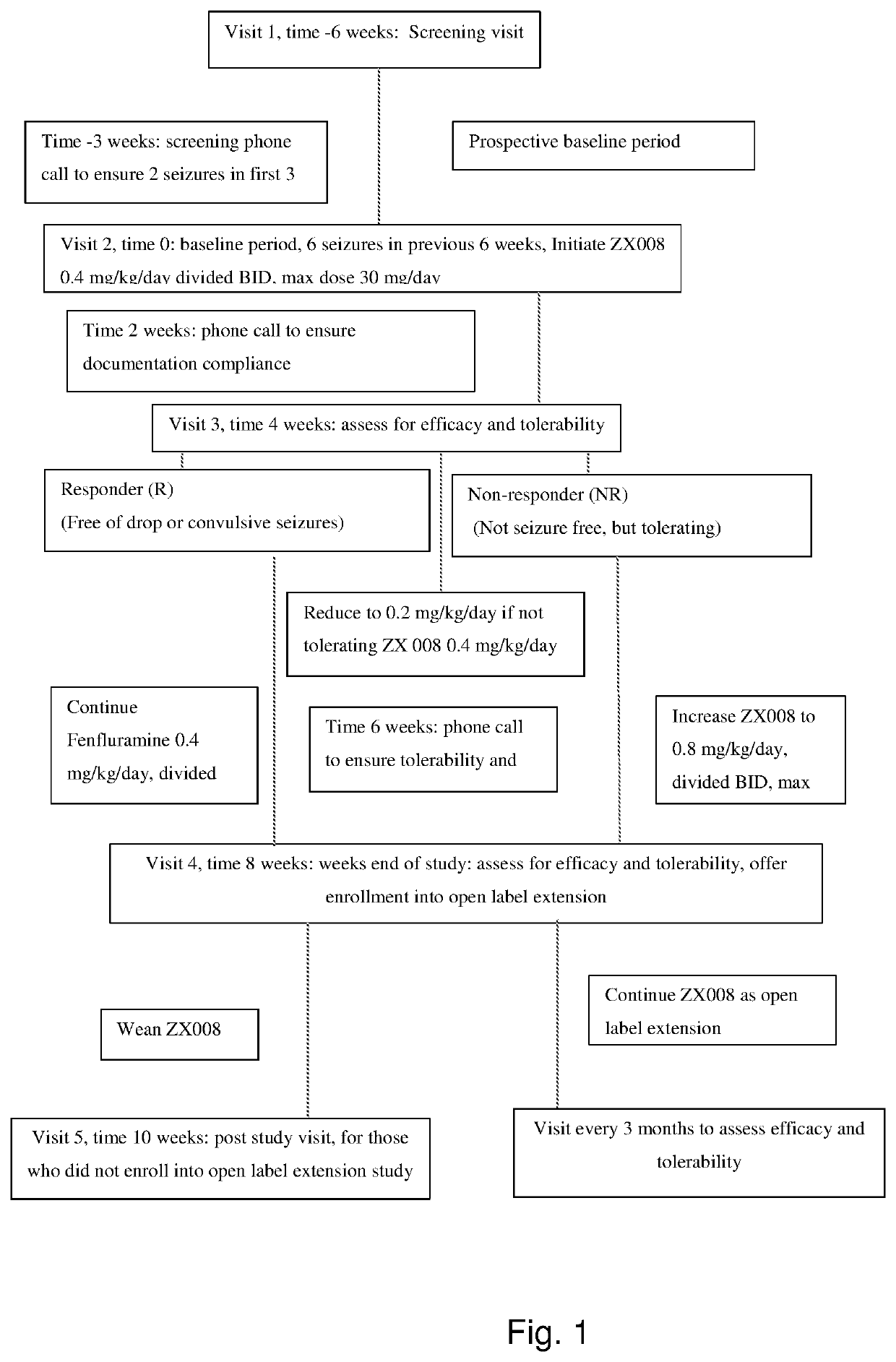
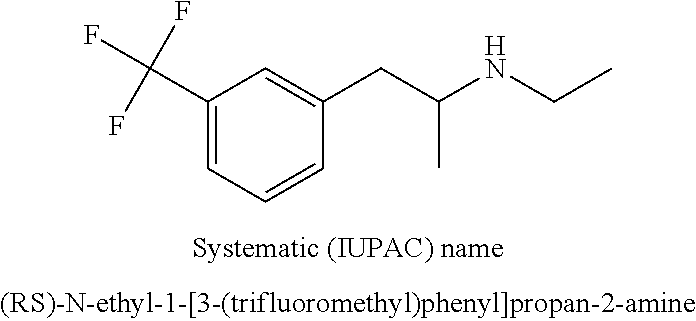
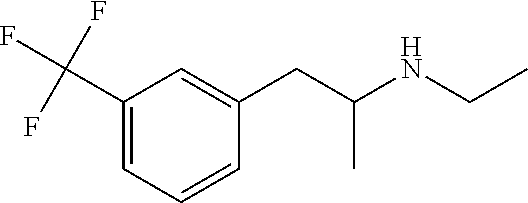
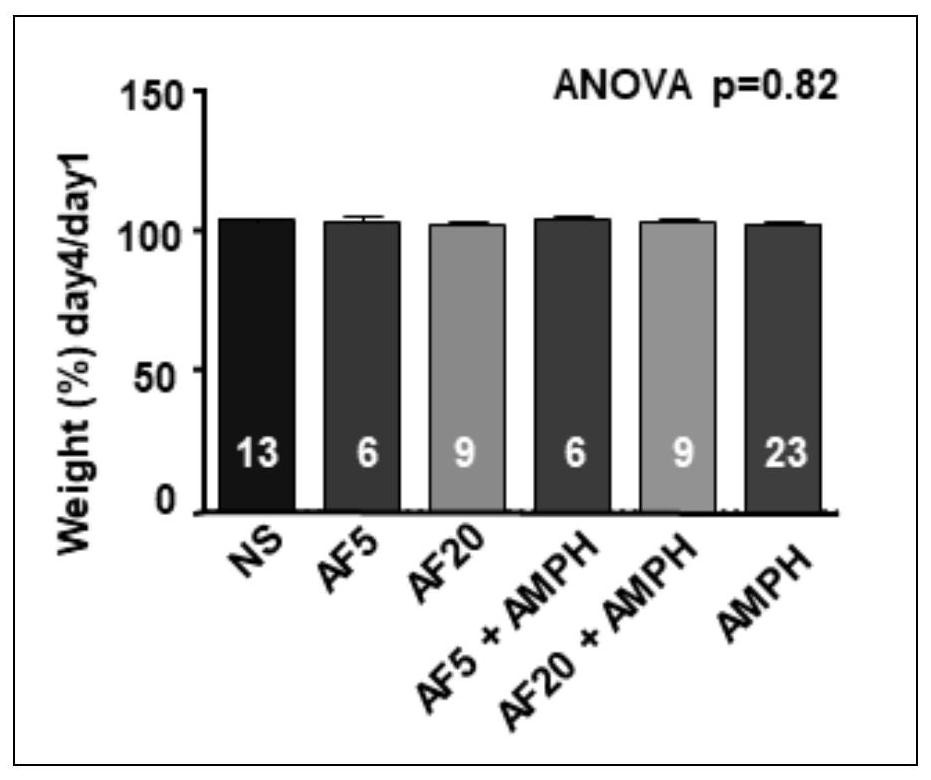
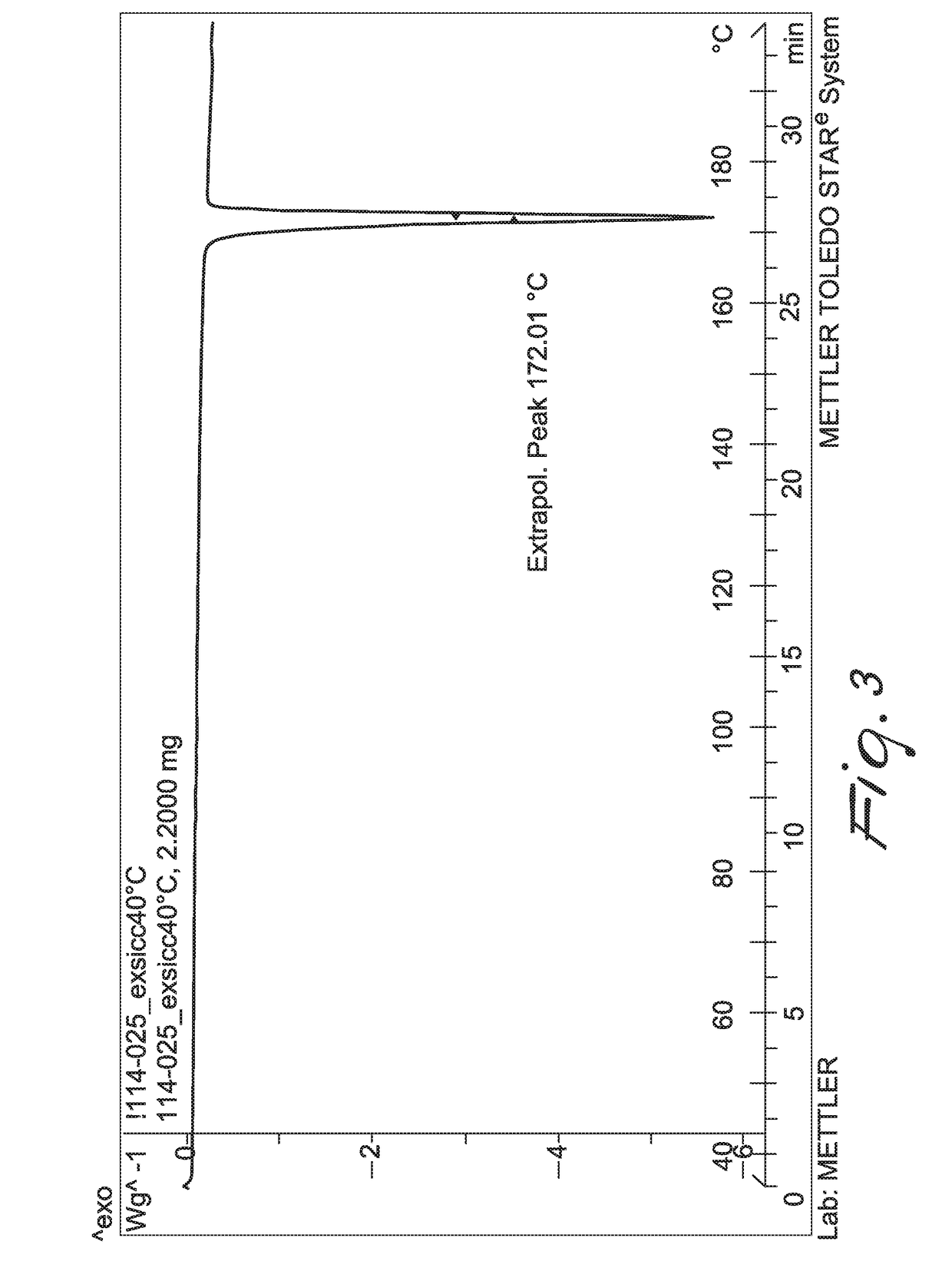
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
41 results about "Fenfluramine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
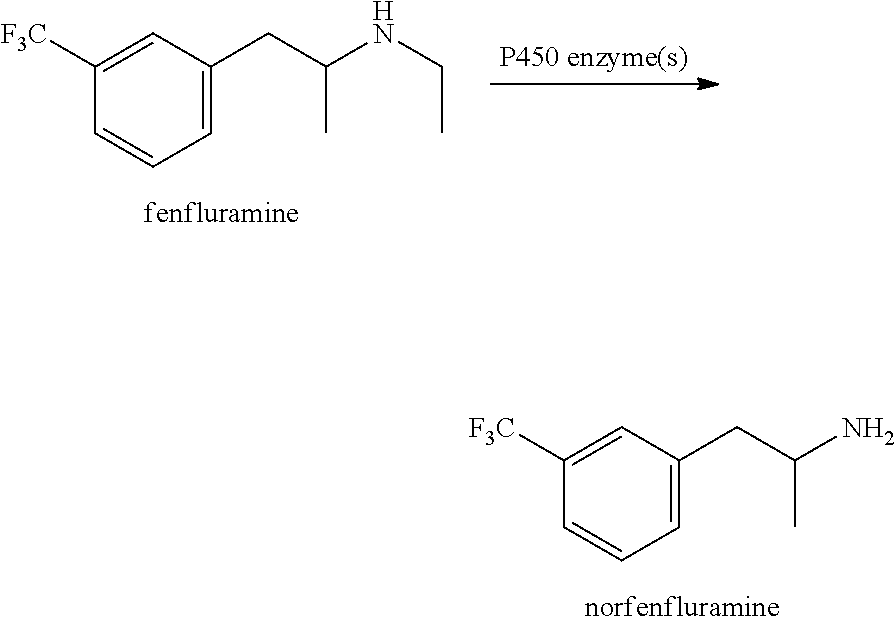
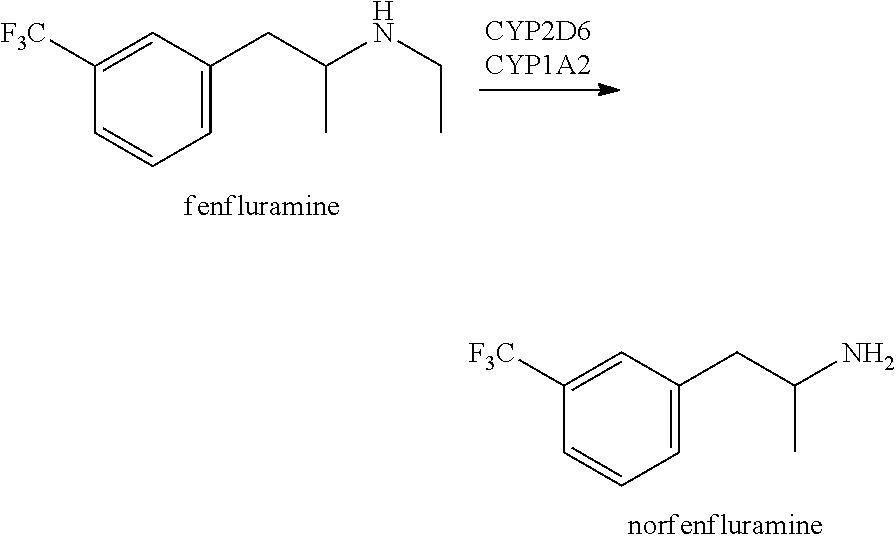
Fenfluramine, formerly sold under the brand name Pondimin among others, is an appetite suppressant which was used to treat obesity and is now no longer marketed. It was used both on its own and, in combination with phentermine, as part of the anti-obesity medication Fen-Phen.
Method for simultaneously detecting seven slimming chemical components which are illegally added to traditional Chinese medicine, health food or cosmetics
InactiveCN102901780AEasy to separateQualitatively accurateComponent separationPhenolphthaleinEphedrine
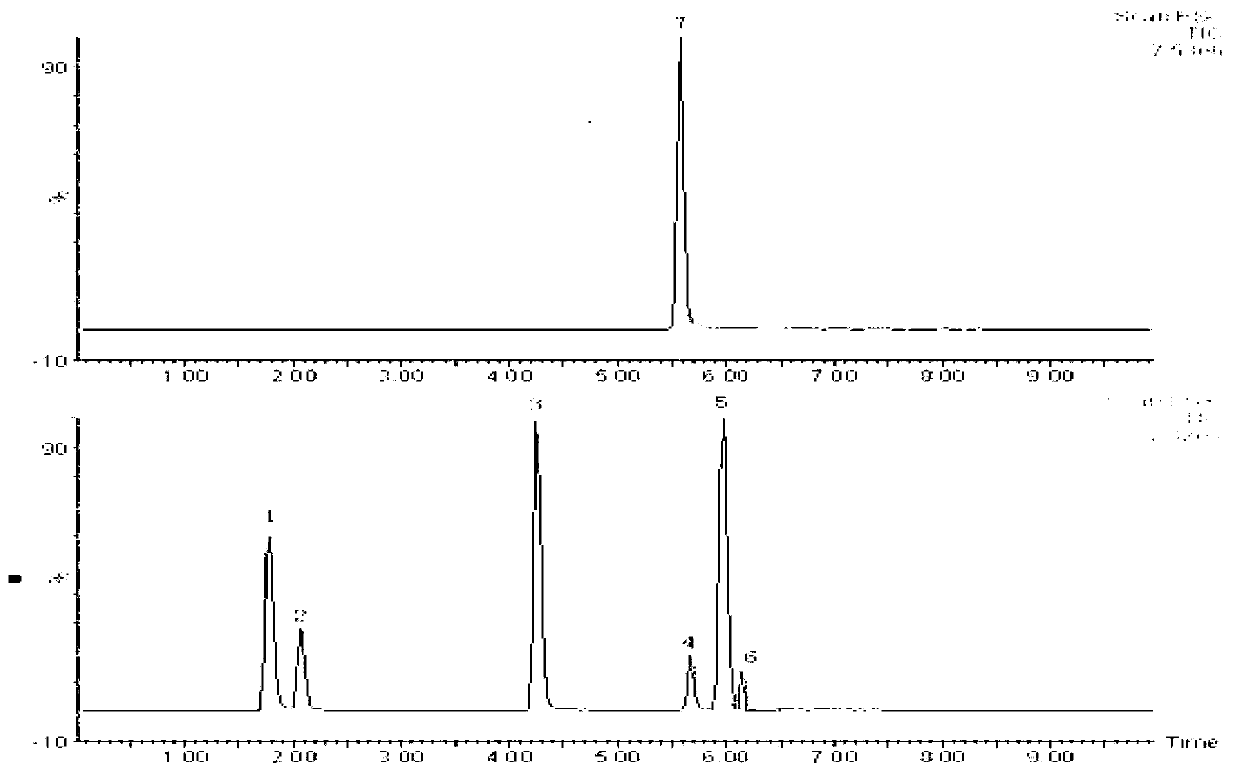
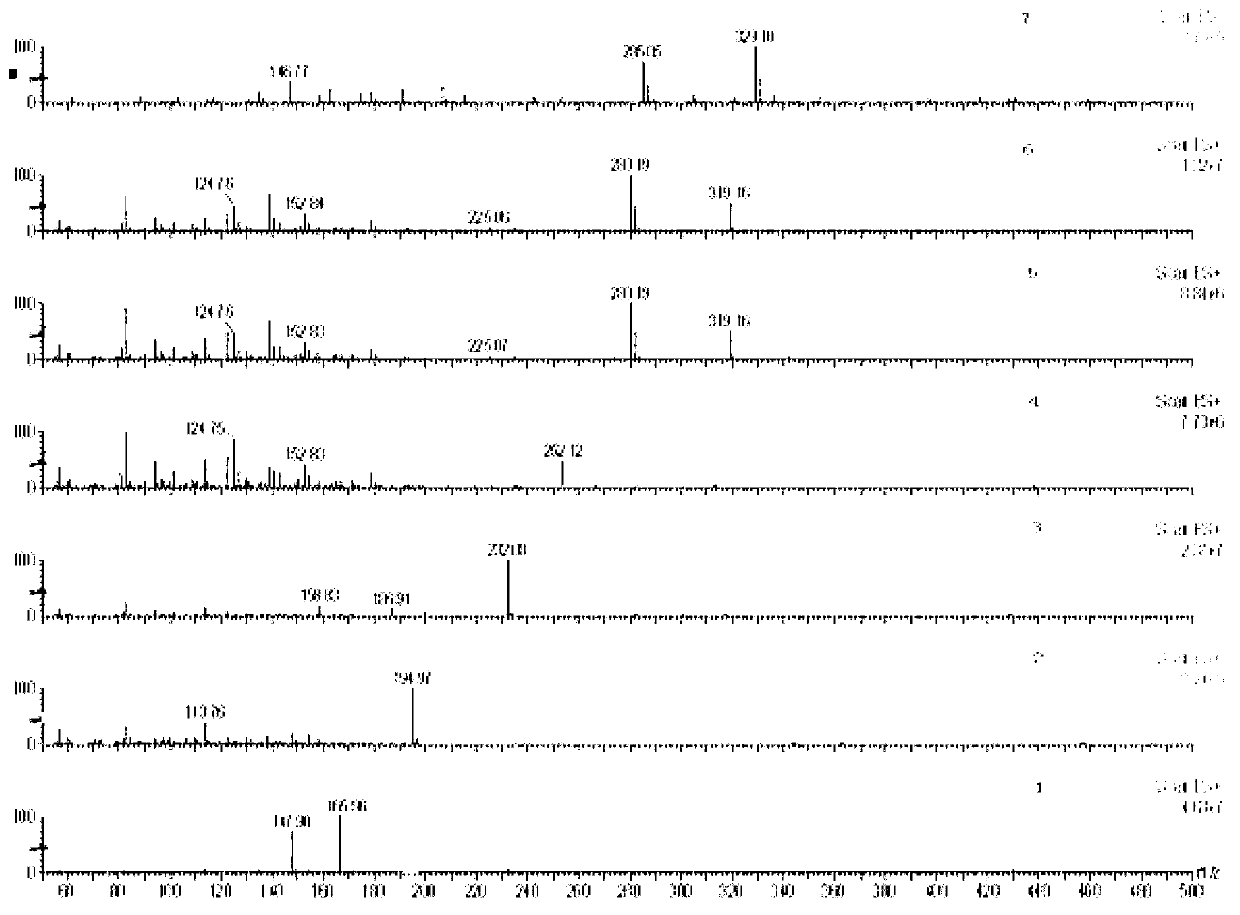
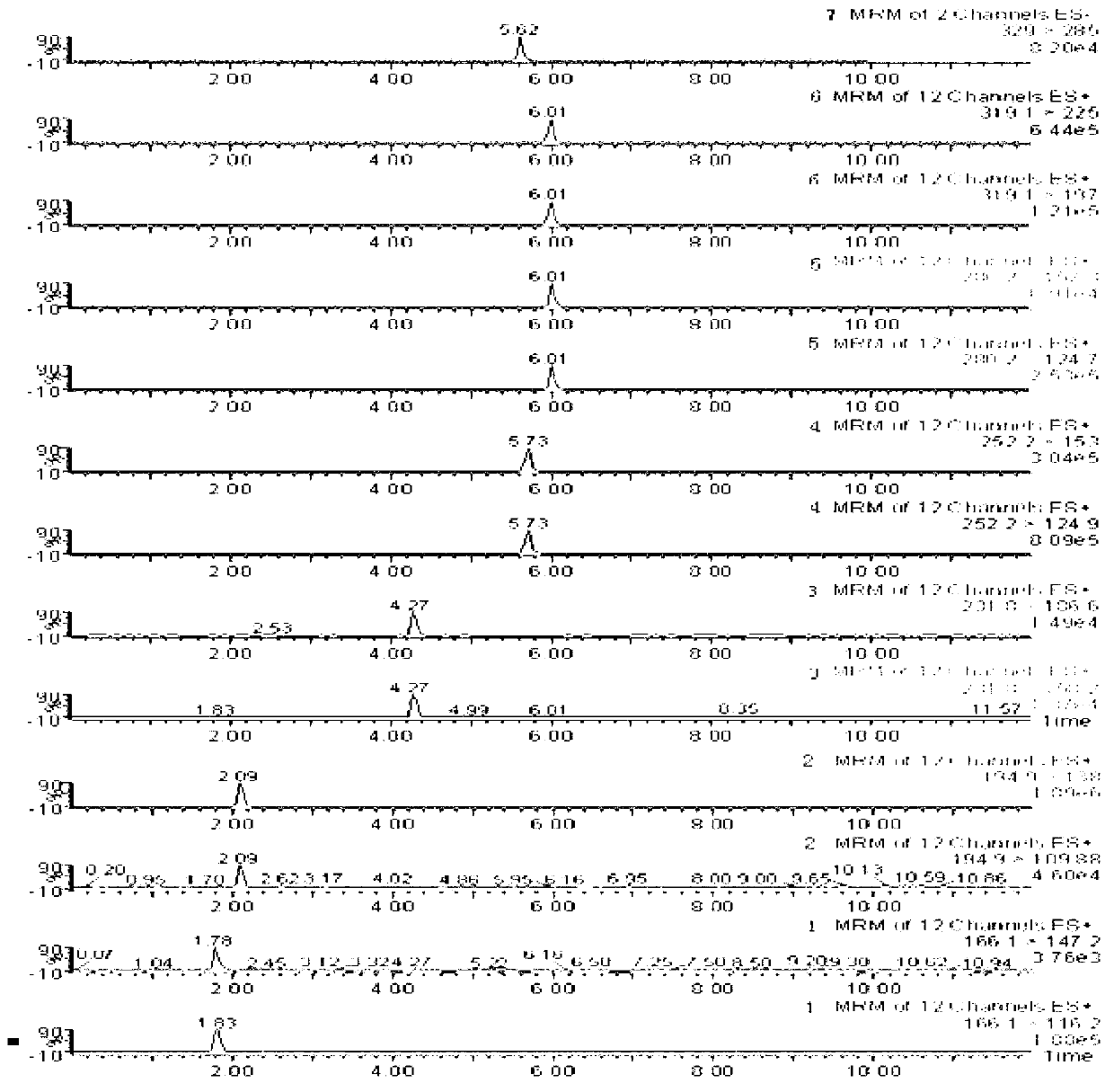
The invention relates to a method for simultaneously detecting seven slimming chemical components which are illegally added to traditional Chinese medicine, health food or cosmetics. According to the method, an appropriate mobile phase and a reasonable elution gradient are adopted during liquid-phase separation, so that the slimming components needing to be detected are effectively separated within 12 min, the analysis time of a sample instrument is shortened by 88%, and the work efficiency is greatly increased. Additionally, the advantages of high separation ability of the HPLC (High Performance Liquid Chromatography) technology, high sensitivity and stronger qualitative ability of the mass spectrum and the like are combined, so screening and confirmation can be simultaneously completed during one operation. By using the method disclosed by the invention, the seven chemical components including sibutramine, fenfluramine, phenolphthalein, ephedrine, caffeine, N,N-bi-demethylation sibutramine and furosemide can be effectively separated, the inspection time can be shortened, and the slimming chemical components which are illegally added to the traditional Chinese medicine, the health food or the cosmetics can be accurately and effectively distinguished.
Owner:HUNAN INST FOR FOOD & DRUG CONTROL
Methods of treating lennox-gastaut syndrome using fenfluramine
A method of treating and / or preventing symptoms of Lennox-Gastaut Syndrome (LGS) also known as Lennox Syndrome in a patient such as a patient previously diagnosed with Lennox Syndrome, by administering an effective dose of fenfluramine or its pharmaceutically acceptable salt to that patient. Lennox Syndrome patients are treated at a preferred dose of less than about 2.0 to about 0.01 mg / kg / day.
Owner:ZOGENIX INT
Method of reduction in convulsive seizure frequency
InactiveUS20190247333A1Reduce convulsive seizure frequencyEliminate seizureOrganic active ingredientsNervous disorderFenfluramineHuman patient
A method of reducing convulsive seizure frequency in a human patient diagnosed with Dravet syndrome, comprising administering to the patient a therapeutically effective dose of fenfluramine or a pharmaceutically acceptable salt, base, acid or amine thereof, and repeating the administering over a period of days until the patient exhibits a significant reduction (e.g., 40% or greater) from baseline in convulsive seizure frequency. In some embodiments of the method, convulsive seizures are completely eliminated for 10 days or more, 20 days or more, 30 days or more, 50 days or more, 100 days or more.
Owner:ZOGENIX INT
Method of reducing seizure type experienced by a dravet patient
InactiveUS20190091174A1Reduce convulsive seizure frequencyEliminate seizureNervous disorderHydroxy compound active ingredientsFenfluramineHuman patient
A method of reducing a particular type of seizure in a human patient diagnosed with Dravet syndrome, by administering to the patient a therapeutically effective dose of fenfluramine or a pharmaceutically acceptable salt, base, acid or amine thereof, and repeating the administering over a period of days until the patient exhibits a reduction from baseline in seizures of a particular type. The reduction may be of one, two or three specific types of seizures.
Owner:ZOGENIX INT
Synergistic composition for the treatment of diabetes mellitus
The present invention relates to a synergistic composition for the treatment of diabetes in a subject in need thereof, said composition-comprising Trigonelline of concentration ranging between 20 to 30%, amino acids of concentration ranging between 20 to 60%, and soluble fiber of concentration ranging between 10 to 60%, optionally along with pharmaceutically acceptable additives, a process thereof and also, a method of treating diabetes.
Owner:INDUS BIOTECH PVT
Compositions and methods for treating respiratory depression with fenfluramine
ActiveUS10517841B1Reduce occurrenceReduce riskNervous disorderRespiratory disorderDiseaseFenfluramine
5-HT receptor agonists are useful in the treatment of a variety of diseases. Provided herein are methods of treating and / or reducing the occurrence of respiratory depression caused by an opioid in a human patient or patient population using a 5-HT receptor agonist, such as, for example, a 5-HT4 agonist (e.g., fenfluramine). Methods of stimulating one or more 5-HT4 receptors in the brain of a patient undergoing treatment with an opioid, wherein the patient is at risk of respiratory depression, by administering a 5-HT4 agonist (e.g., fenfluramine) to a subject in need thereof are provided. Pharmaceutical compositions for use in practicing the subject methods are also provided.
Owner:ZOGENIX INT
Ketogenic diet compatible fenfluramine formulation
ActiveUS10682317B2Improve complianceLacks a nutritive/digestible/glycemic carbohydrateOrganic active ingredientsNervous disorderFenfluramineKetogenic diet
A method of treating symptoms of a subtype of epilepsy, e.g., Dravet syndrome, in a patient diagnosed with a subtype of epilepsy, by administering to the patient an effective dose of a fenfluramine formulation in combination with a ketogenic diet over a period of time sufficient to reduce or completely eliminate seizures in the patient. Also provided are compositions and kits finding use in practicing embodiments of the methods.
Owner:ZOGENIX INT
Method of treating patients with lennox-gastaut syndrome
A method of treating symptoms of Lennox-Gastaut syndrome in a patient diagnosed with Lennox-Gastaut syndrome, by administering an effective dose of fenfluramine to that patient over a period of time sufficient to reduce or completely eliminate seizures in the patient. The fenfluramine may be administering in an oral liquid formulation on a daily basis of 0.7 mg / kg / day, over a period of weeks until seizures are reduced by 25% or more, 50% or more, 75% or more.
Owner:ZOGENIX INT
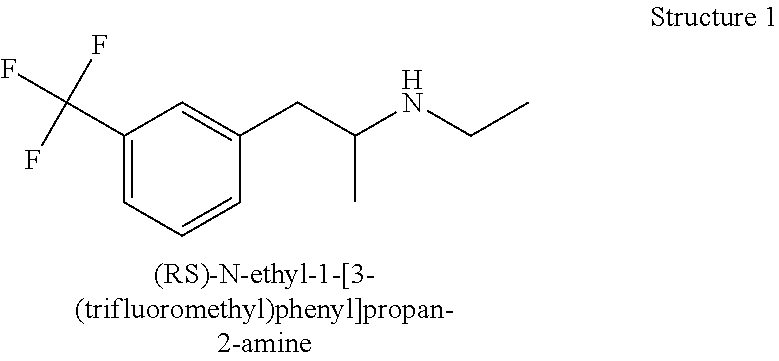
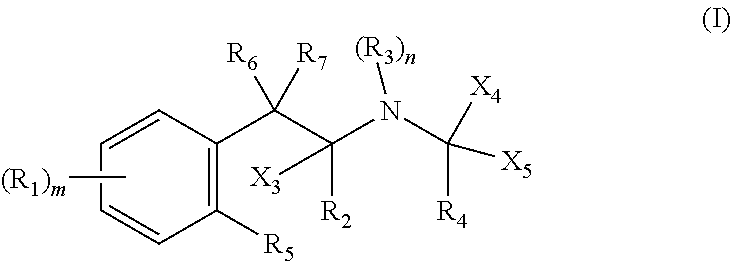
Metabolism resistant fenfluramine analogs and methods of using the same
Metabolism-resistant fenfluramine analogs are provided. The subject fenfluramine analogs find use in the treatment of a variety of diseases. For example, methods of treating epilepsy by administering a fenfluramine analog to a subject in need thereof are provided. Also provided are methods of suppressing appetite in a subject in need thereof. Pharmaceutical compositions for use in practicing the subject methods are also provided.
Owner:ZOGENIX INT
Method of increasing time between convulsive seizures
InactiveUS20190091173A1Eliminate seizureReduce frequencyNervous disorderHydroxy compound active ingredientsFenfluramineHuman patient
A method of increasing an average time between seizures in a human patient diagnosed with Dravet syndrome, comprising administering to the patient a therapeutically effective dose of fenfluramine or a pharmaceutically acceptable salt, base, acid or amine thereof, and repeating the administering over a period of days until the patient exhibits an increase from baseline in average time between convulsive seizures of 6 hours, days weeks or more.
Owner:ZOGENIX INT
Changing cognitive function with fenfluramine
Disclosed herein are methods of improving cognitive function in a patient as measured by, for example, improvement in score on a validated scale that measures cognitive function, such as the Behavior Rating Inventory of Executive Function (BRIEF), by administering the test to a patient and obtaining a pre-treatment test score, treating the patient with fenfluramine or its pharmaceutically acceptable salt, and after treatment, re-administering the test of cognitive function to the patient and obtaining a post-treatment score, to allow observation of an improvement in the test score. In some embodiments, the patient is also being treated for the symptoms of epilepsy.
Owner:ZOGENIX INT
Method of treating selected patient population experiencing dravet syndrome
InactiveUS20190091176A1Eliminate seizureReduce frequencyNervous disorderHydroxy compound active ingredientsTolerabilityCannabidiol
Provided herein is a method of treating a selected patient population, wherein the patient population is selected based on a determination that the patients have previously been non-responsive when treated with cannabidiol. In some embodiments, the method comprises selecting the patient based on a previously failed treatment with cannabidiol, based on lack of efficacy or tolerability. Pharmaceutical compositions and formulations for use in practicing the subject methods are also provided. The method comprises identifying a population of patients diagnosed with Dravet syndrome who were found previously to have been non-responsive when treated with cannabidiol. The selected population of patients is then treated by administering, to each identified patient, a therapeutically effective dose of fenfluramine or a pharmaceutically acceptable salt, base, acid or amine thereof, and repeating the administering over a period of a day or days, or over a period of weeks, months or years, until the patient exhibits a reduction from baseline in convulsive seizure frequency.
Owner:ZOGENIX INT
Method of reduction in convulsive seizure frequency
InactiveUS20190125697A1Eliminate seizureReduce frequencyNervous disorderHydroxy compound active ingredientsFenfluramineHuman patient
A method of reducing convulsive seizure frequency in a human patient diagnosed with Dravet syndrome, comprising administering to the patient a therapeutically effective dose of fenfluramine or a pharmaceutically acceptable salt, base, acid or amine thereof, and repeating the administering over a period of days until the patient exhibits a significant reduction (e.g., 40% or greater) from baseline in convulsive seizure frequency. In some embodiments of the method, convulsive seizures are completely eliminated for 10 days or more, 20 days or more, 30 days or more, 50 days or more, 100 days or more.
Owner:ZOGENIX INT
Compositions and methods for treating seizure disorders
InactiveUS20200297665A1Improve securityOrganic active ingredientsNervous disorderFenfluraminePharmaceutical drug
Functional analogs of fenfluramine are provided. The subject fenfluramine functional analogs find use in the treatment of a variety of diseases. For example, methods of treating epilepsy by administering a fenfluramine analog to a subject in need thereof are provided. Also provided are methods of treating a neurodegenerative disease in a subject in need thereof. Pharmaceutical compositions for use in practicing the subject methods are also provided.
Owner:ZOGENIX INT
Method of treating selected patient population experiencing dravet syndrome
InactiveUS20190091177A1Reduce convulsive seizure frequencyEliminate seizureNervous disorderHydroxy compound active ingredientsFenfluramineTolerability
Provided herein is a method of treating a selected patient population, wherein the patient population is selected based on a determination that the patients have previously been non-responsive when treated with stiripentol. In some embodiments, the method comprises selecting the patient based on a previously failed treatment with stiripentol, based on lack of efficacy or tolerability. Pharmaceutical compositions and formulations for use in practicing the subject methods are also provided. The method comprises identifying a population of patients diagnosed with Dravet syndrome who were found previously to have been non-responsive when treated with stiripentol. The selected population of patients is then treated by administering, to each identified patient, a therapeutically effective dose of fenfluramine or a pharmaceutically acceptable salt, base, acid or amine thereof, and repeating the administering over a period of a day or days, or over a period of weeks, months or years, until the patient exhibits a reduction from baseline in convulsive seizure frequency.
Owner:ZOGENIX INT
Methods of treating doose syndrome using fenfluramine
InactiveUS20200170965A1Preventing and ameliorating seizureNervous disorderDispersion deliveryFenfluraminePharmaceutical medicine
A method of treating and / or preventing symptoms of Doose syndrome in a patient such as a patient previously diagnosed with Doose syndrome, by administering an effective dose of fenfluramine or its pharmaceutically acceptable salt to that patient. Doose syndrome patients are treated at a preferred dose of less than about 10.0 to about 0.01 mg / kg / day.
Owner:ZOGENIX INT
Method of reduction medication in treating dravet syndrome
InactiveUS20190091175A1Reduce convulsive seizure frequencyEliminate seizureNervous disorderHydroxy compound active ingredientsFenfluramineSide effect
A method of reducing dosing of a concomitant medication in a human patient diagnosed with Dravet syndrome, by administering to the patient a therapeutically effective dose of fenfluramine or a pharmaceutically acceptable salt, base, acid or amine thereof, and repeating the administering over a period of days while reducing the dose of one or more concomitant anti-seizure or anti-epileptic drugs (AEDs) from baseline and thereby decreasing the amount of medication given to the patient while reducing adverse side effects. Pharmaceutical compositions and formulations for use in practicing the subject methods are also provided.
Owner:ZOGENIX INT
Methods of treating rett syndrome using fenfluramine
PendingUS20220008389A1Improving cognitive deficitExtended durationNervous disorderHydroxy compound active ingredientsFenfluramineRett syndrome
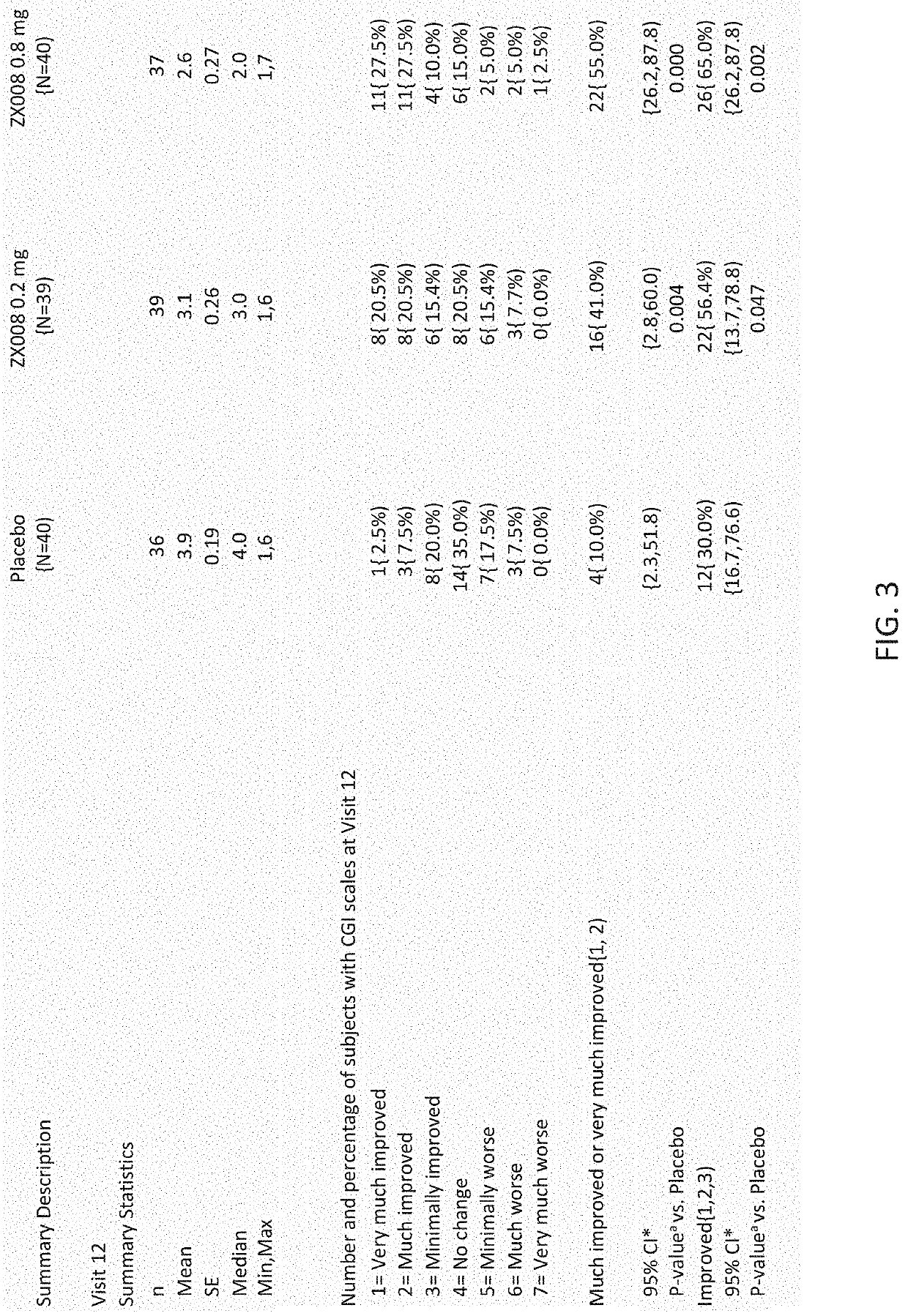
A method of treating and / or preventing symptoms of Rett syndrome (RTT) in a patient such as a patient previously diagnosed with Rett syndrome, by administering an effective dose of a 5-HT1D, 5-HT2A, 5-HT2C or sigma-1 receptor agonist (e.g., fenfluramine or its pharmaceutically acceptable salt) to that patient. RTT patients are treated at a preferred dose of less than about 1.0 mg / kg / day and may be administered as fenfluramine in an amount of between 0.2 to 0.8 mg / kg / day, to a maximum of 30 mg / day in a liquid oral dose.
Owner:ZOGENIX INT
A formulation for improving seizure control
PendingUS20220133652A1Improving seizure controlSeizure control has improvedNervous disorderHydroxy compound active ingredientsGeneral anaesthesiaEpileptic encephalopathy
Described herein is a method of improving seizure control in a patient experiencing uncontrolled seizures persisting 10 minutes or more, comprising administering fenfluramine or a pharmaceutically acceptable salt, base, acid or amine thereof, at a dose of from 0.2 to 1.2 m / kg / day for a period of about 12 hours to about 7 days to a patient having been put into a therapeutic, medically-induced coma via a general anesthetic; and after about 12 hours to about 7 days, weaning the patient from the general anesthetic and assessing whether the seizure control has improved as compared to a pre-treatment time point. The patient experiencing seizures may have epilepsy or epileptic encephalopathy that has led to established status epilepticus (SE), refractory status epilepticus (RSE) or super-refractory status epilepticus (SRSE).
Owner:ZOGENIX INT
Methods of treating doose syndrome using fenfluramine
PendingUS20220226262A1Preventing and ameliorating seizureNervous disorderDispersion deliveryFenfluraminePharmaceutical medicine
A method of treating and / or preventing symptoms of Doose syndrome in a patient such as a patient previously diagnosed with Doose syndrome, by administering an effective dose of fenfluramine or its pharmaceutically acceptable salt to that patient. Doose syndrome patients are treated at a preferred dose of less than about 10.0 to about 0.01 mg / kg / day.
Owner:ZOGENIX INT
Dripping pills of clemastine fumarate and its preparation method
InactiveCN1602860ADisintegration and dissolution fastHigh dissolution ratePill deliveryRespiratory disorderFenfluramineClemastine Fumarate
The invention uses super micro shattering and drop pills producing the technology to produce fenfluramine drop pills, reaching the purposes such as increasing speed, and dissolution of disintegration, increasing the medicine stability, reducing the dose of auxiliary materials and decreasing cost, with rapid effect and convenient use. It can be buccal as well as deglutition, has remarkable conformity, and is especially suitable for children, older man, sicker in bed or with difficulty of swallowing.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
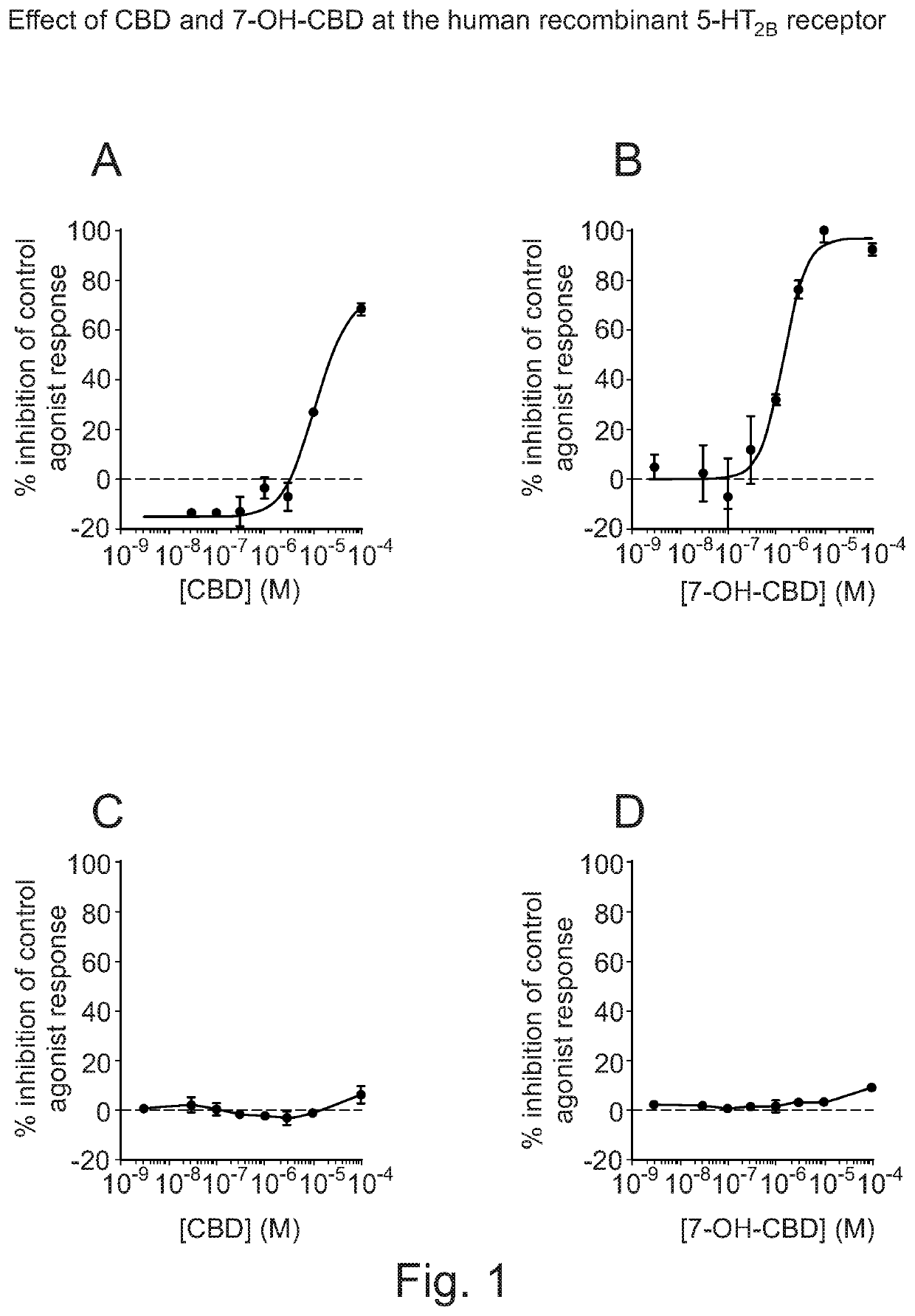
Use of cannabidiol in combination with 5-HT2B receptor agonists or amphetamines in the treatment of epilepsy
The present invention relates to the use of cannabidiol (CBD) in combination with an agonist of 5-HT2B receptors. Such a combination provides protection against the adverse effects caused by agonists of 5-HT2B receptors. The invention further relates to the use of CBD in combination5 with an amphetamine or amphetamine derivative in the treatment of epilepsy. In one embodiment the CBD is used in combination with the amphetamine derivative fenfluramine to produce a significant reduction in seizures. Preferably the CBD used is in the form of a highly purified extract of cannabis such that the CBD is present at greater than 98% of the total extract (w / w) and the other components of the extract are characterised. In particular the cannabinoid10 tetrahydrocannabinol (THC) has been substantially removed, to a level of not more than 0.15% (w / w) and the propyl analogue of CBD, cannabidivarin, (CBDV) is present in amounts of up to 1%. Alternatively, the CBD may be a synthetically produced CBD. In use the CBD in combination with an agonist of 5-HT2B receptors, amphetamine or amphetamine derivative may be formulated for administration separately, sequentially or simultaneously with the15 amphetamine or amphetamine derivative or the combination may be provided in a single dosage form. Where the CBD is formulated for administration separately, sequentially or simultaneously it may be provided as a kit or together with instructions to administer the one or more components in the manner indicated.
Owner:GW RES LTD
Compositions and methods for treating seizure-induced sudden death
ActiveUS20210330610A1Shorten the lengthInhibiting PID periodNervous disorderAmide active ingredientsDiseaseFenfluramine
5-HT receptor agonists are useful in the treatment of a variety of diseases. Provided herein are methods of reducing the incidence and / or severity of seizures in a human patient using a 5-HT receptor agonist, such as, for example, a 5-HT4 agonist (e.g., fenfluramine). Methods of treating epilepsy or epileptic encephalopathy, and / or reducing, ameliorating or eliminating incidence of SUDEP in a subject diagnosed with epilepsy by administering a 5-HT4 agonist (e.g., fenfluramine) to a subject in need thereof are provided. Pharmaceutical compositions for use in practicing the subject methods are also provided.
Owner:ZOGENIX INT
Dripping pills of methoxsalen and its preparation method
InactiveCN1602859ADisintegration and dissolution fastHigh dissolution rateOrganic active ingredientsPill deliveryFenfluramineDissolution
The invention uses super micro shattering and drop pills producing the technology to produce fenfluramine drop pills, reaching the purposes such as increasing speed, and dissolution of disintegration, increasing the medicine stability, reducing the dose of auxiliary materials and decreasing cost, with rapid effect and convenient use. It can be buccal as well as deglutition, has remarkable conformity, and is especially suitable for children, older man, sicker in bed or with difficulty of swallowing.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
Propranolol hydrochloride drop pills and its preparation method
InactiveCN1602849ADisintegration and dissolution fastHigh dissolution rateOrganic active ingredientsPill deliveryFenfluramineDissolution
The invention uses super micro shattering and propranolol drop pills producing the technology to produce fenfluramine drop pills, reaching the purposes such as increasing speed, and dissolution of disintegration, increasing the medicine stability, reducing the dose of auxiliary materials and decreasing cost, with rapid effect and convenient use. It can be buccal as well as deglutition, has remarkable conformity, and is especially suitable for children, older man, sicker in bed or with difficulty of swallowing.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
Synergistic composition for the treatment of diabetes mellitus
A synergistic composition for the treatment of diabetes in a subject in need thereof, said composition-comprising Trigonelline of concentration ranging between 20 to 30%, amino acids of concentration ranging between 20 to 60%, and soluble fiber of concentration ranging between 10 to 60%, optionally along with pharmaceutically acceptable additives, a process thereof and also, a method of treating diabetes.
Owner:INDUS BIOTECH PVT
Application of buagafuran in preparation of medicine for preventing and/or treating related diseases of amphetamine-type drug addiction
ActiveCN111939149ANot addictiveAddiction blockOrganic active ingredientsNervous disorderAmphetamine SulfateDisease
The present invention relates to the use of buagafuran for the prevention and / or treatment of addiction to amphetamine drugs. The amphetamine-type drugs comprise but are not limited to amphetamine, methamphetamine, fenfluramine, phentermine, amphetamine sulfate, methylene dioxymetham-phetamine, Tenamfetamine, dimethyl phenylethylamine or one or more of pharmaceutically acceptable salts of the above-mentioned substances.
Owner:BEIJING UNION PHARMA FACTORY
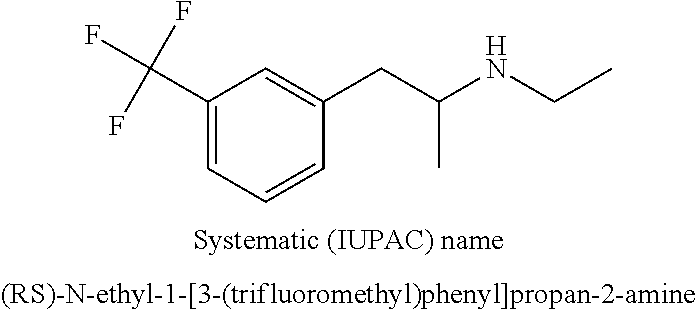
New method for synthesis of fenfluramine, and new compositions comprising it
InactiveUS20180208543A1Organic compound preparationAmino preparation by functional substitutionFenfluramineStereochemistry
A new process for preparing the fenfluramine molecule and new compositions containing fenfluramine obtainable with the claimed process.
Owner:FRAU PHARMA
Rapid detection method for illegally adding illicit drug fenfluramine in health food
ActiveCN109211895AColor is easy to observeClear colorMaterial analysis by observing effect on chemical indicatorPotassium hydroxideIllicit drug
The invention discloses a rapid detection method for illegally adding illicit drug fenfluramine in health food. The method comprises the following steps of: (1) taking a to-be-detected sample, addingwater and a reagent A, shaking, and centrifuging or standing; and (2) taking the supernatant liquid after centrifugation or standing, adding a reagent B and a reagent C, shaking, continuously adding areagent D and a reagent E, shaking, standing, and observing the color of the upper layer solution after standing and layering. The reagent A is one or more of hydrochloric acid solution, sulfuric acid solution, and glacial acetic acid. The reagent B is aqueous solution of p-nitroaniline. The reagent C is one or two of sodium nitrite solution and potassium nitrite solution. The reagent D is one ormore of sodium hydroxide, potassium hydroxide, sodium carbonate, and potassium carbonate. The reagent E is one or more of n-butyl alcohol, chloroform, diethyl ether and, petroleum ether. The detection method is more environmentally friendly, rapid and accurate, obvious in color development, high in sensitivity, simple and convenient to operate, free of any large instruments, and wide in adaptability range.
Owner:广州智汇生物科技有限公司
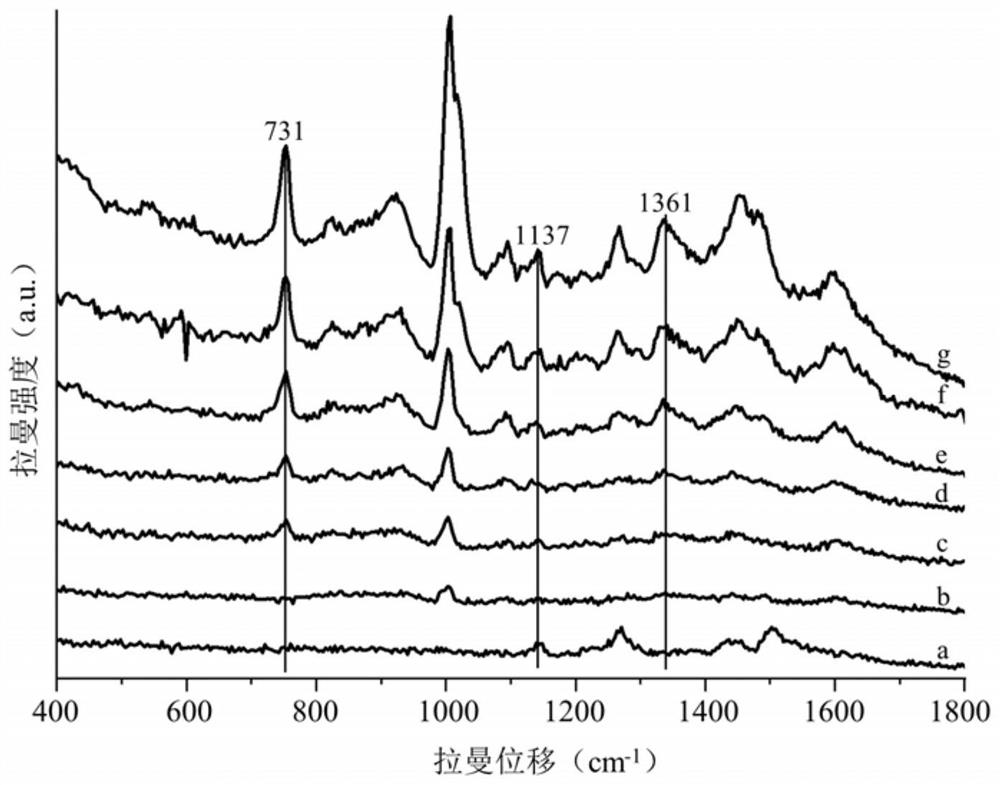
Method for simultaneously detecting sibutramine and fenfluramine in weight-losing health-care products
The invention discloses a method for simultaneously detecting sibutramine and fenfluramine in weight-losing health care products, and belongs to the field of food detection. According to the method disclosed by the invention, the Raman characteristic peaks of sibutramine and fenfluramine which exist at the same time are determined; then pretreating a weight-losing health care product sample, and extracting by adopting an extraction solvent to obtain a solution to be detected; and judging whether sibutramine and fenfluramine exist in the to-be-detected sample or not according to the Raman spectrum of the to-be-detected solution. According to the method disclosed by the invention, the lowest detection concentration of sibutramine and fenfluramine in the weight-losing health-care product is 25mg / kg, qualitative analysis can be carried out on existence of sibutramine and fenfluramine in the weight-losing health-care product, and the detection time of a single sample is controlled within 2min.
Owner:JIANGNAN UNIV +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com