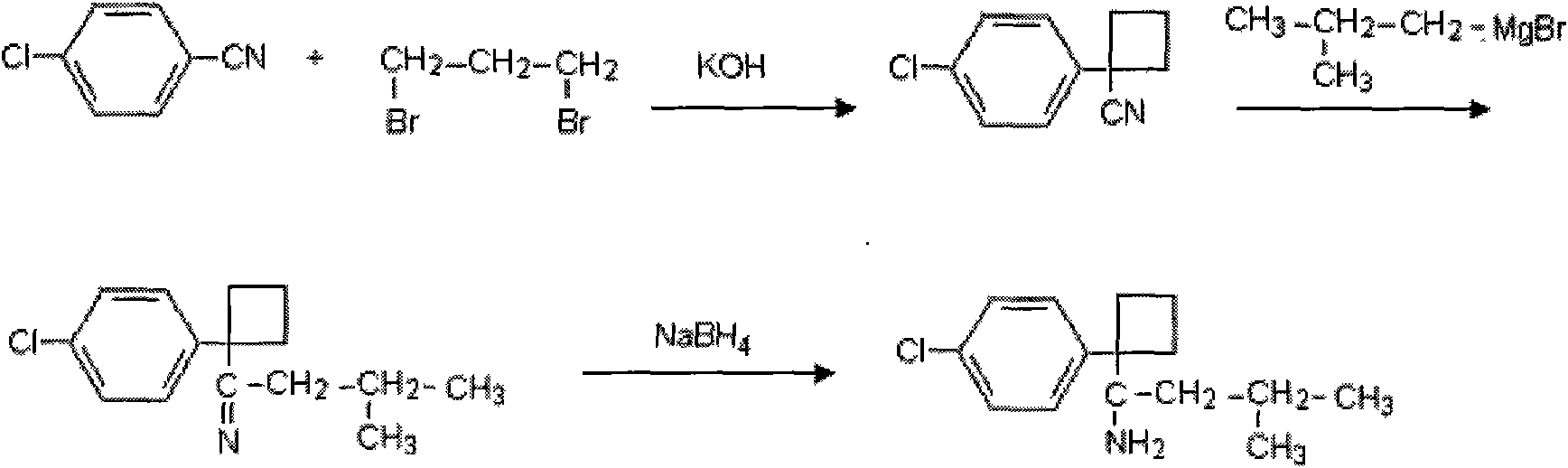
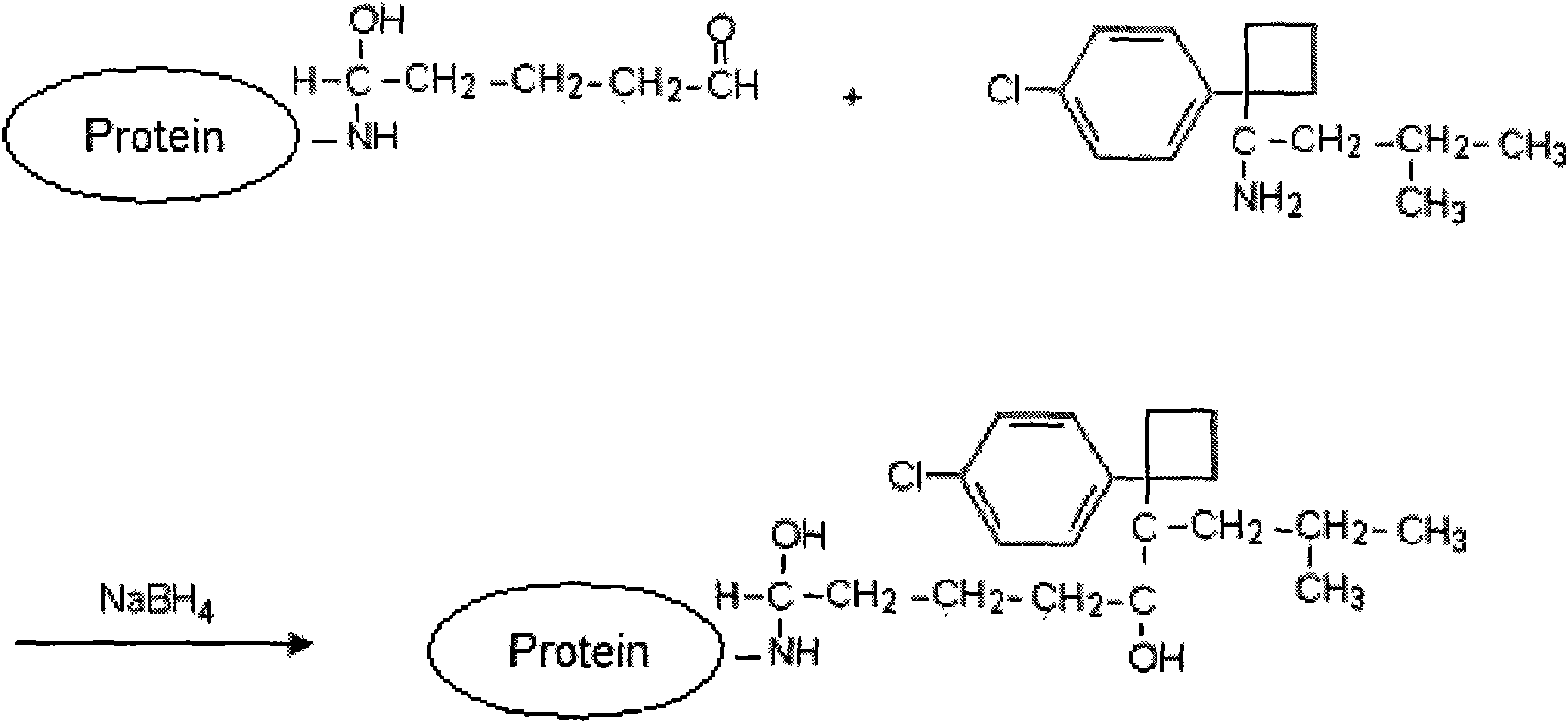
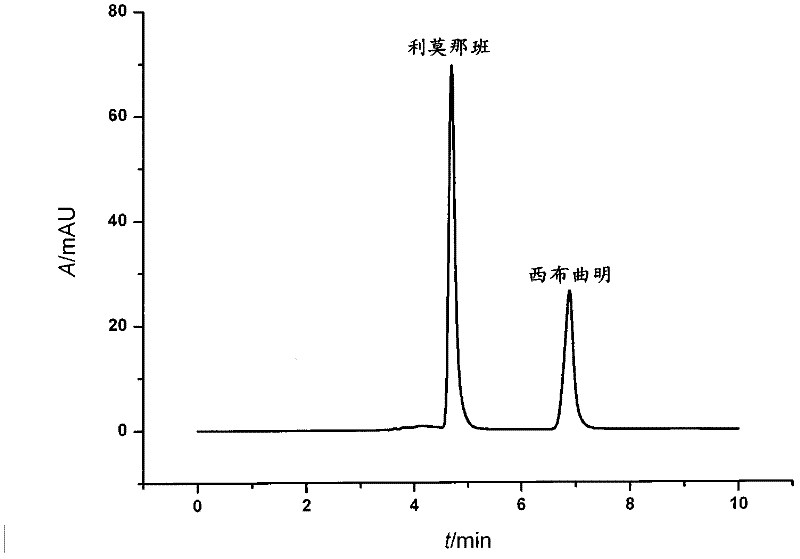
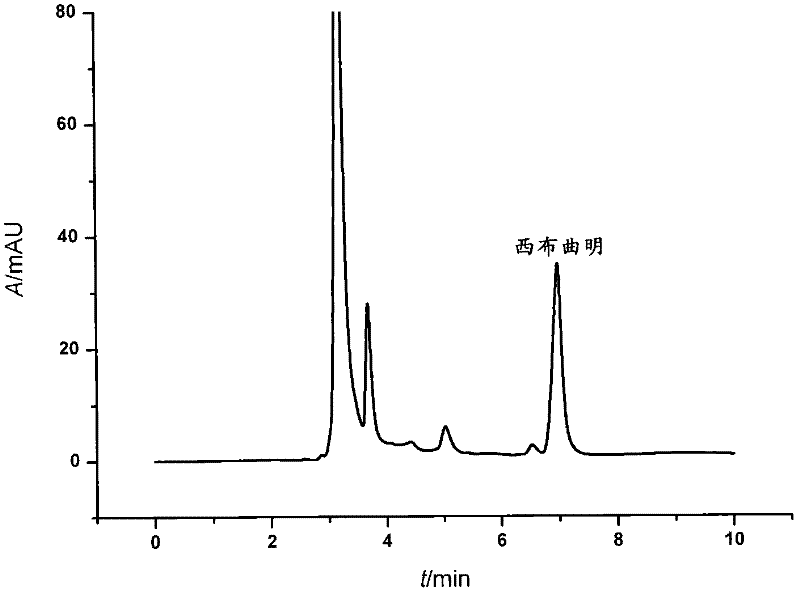
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
47 results about "Sibutramine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Sibutramine, formerly sold under the brand name Meridia among others, is an appetite suppressant which has been discontinued in many countries. Until 2010, it was widely marketed and prescribed as an adjunct in the treatment of obesity along with diet and exercise. It has been associated with increased cardiovascular events and strokes and has been withdrawn from the market in several countries and regions including Australia, Canada, China, the European Union, Hong Kong, India, Mexico, New Zealand, the Philippines, Thailand, the United Kingdom, and the United States. However, the drug remains available in some countries.
Methods of using and compositions comprising (+) sibutramine optionally in combination with other pharmacologically active compounds
This invention encompasses methods for the treatment and prevention of disorders that include, but are not limited to, eating disorders; weight gain; obesity; irritable bowel syndrome; obsessive-compulsive disorders; platelet adhesion; apnea; affective disorders such as attention deficit disorders, depression, and anxiety; male and female sexual function disorders; restless leg syndrome; osteoarthritis; substance abuse including nicotine and cocaine addiction; narcolepsy; pain such as neuropathic pain, diabetic neuropathy, and chronic pain; migraines; cerebral function disorders; chronic disorders such as premenstrual syndrome; and incontinence. The invention further encompasses pharmaceutical compositions and dosage forms which comprise optically pure (+) sibutramine, optionally in combination with a phosphodiesterase inhibitor or a lipase inhibitor.
Owner:SEPACOR INC
Dopamine-Agonist Combination Therapy For Improving Sleep Quality
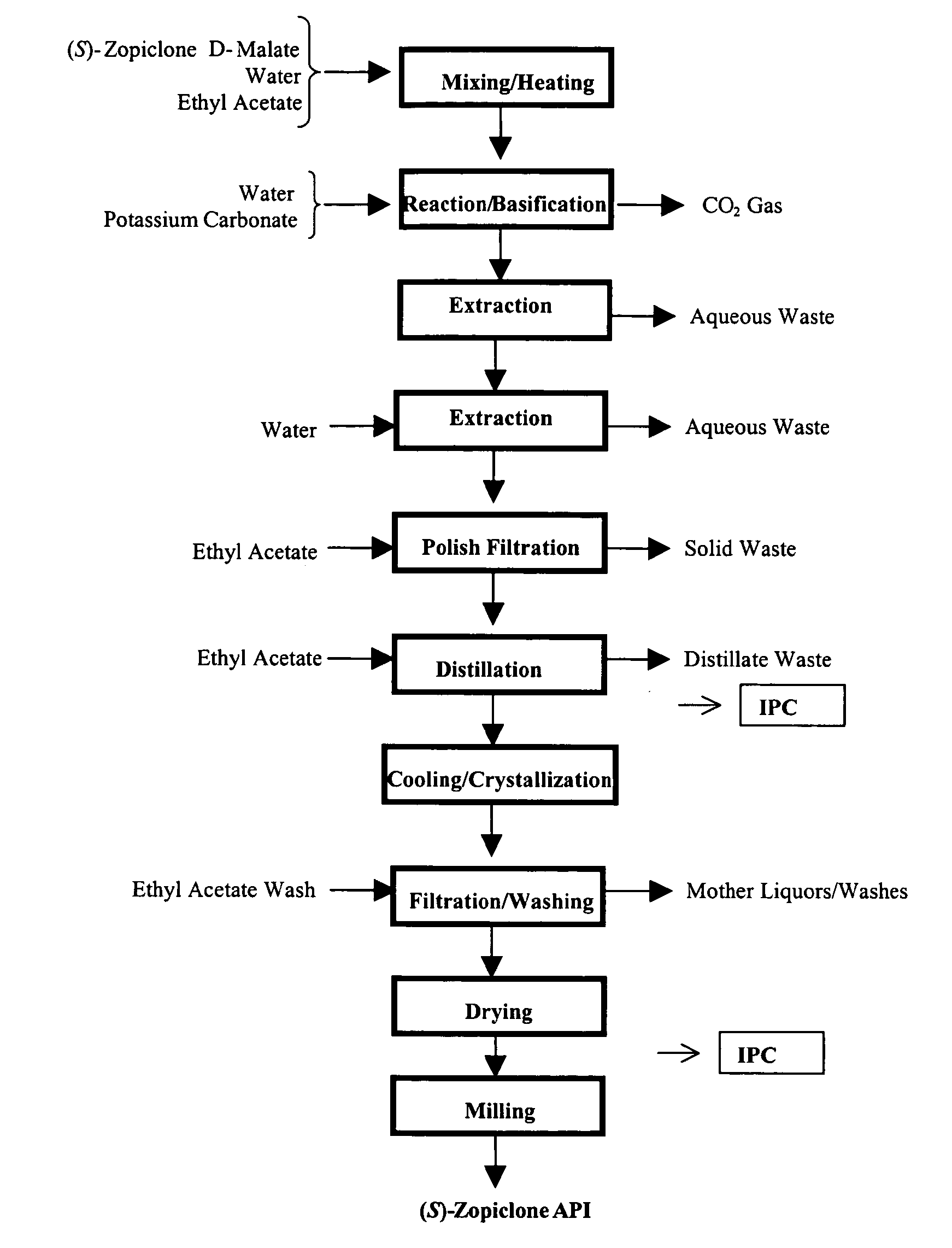
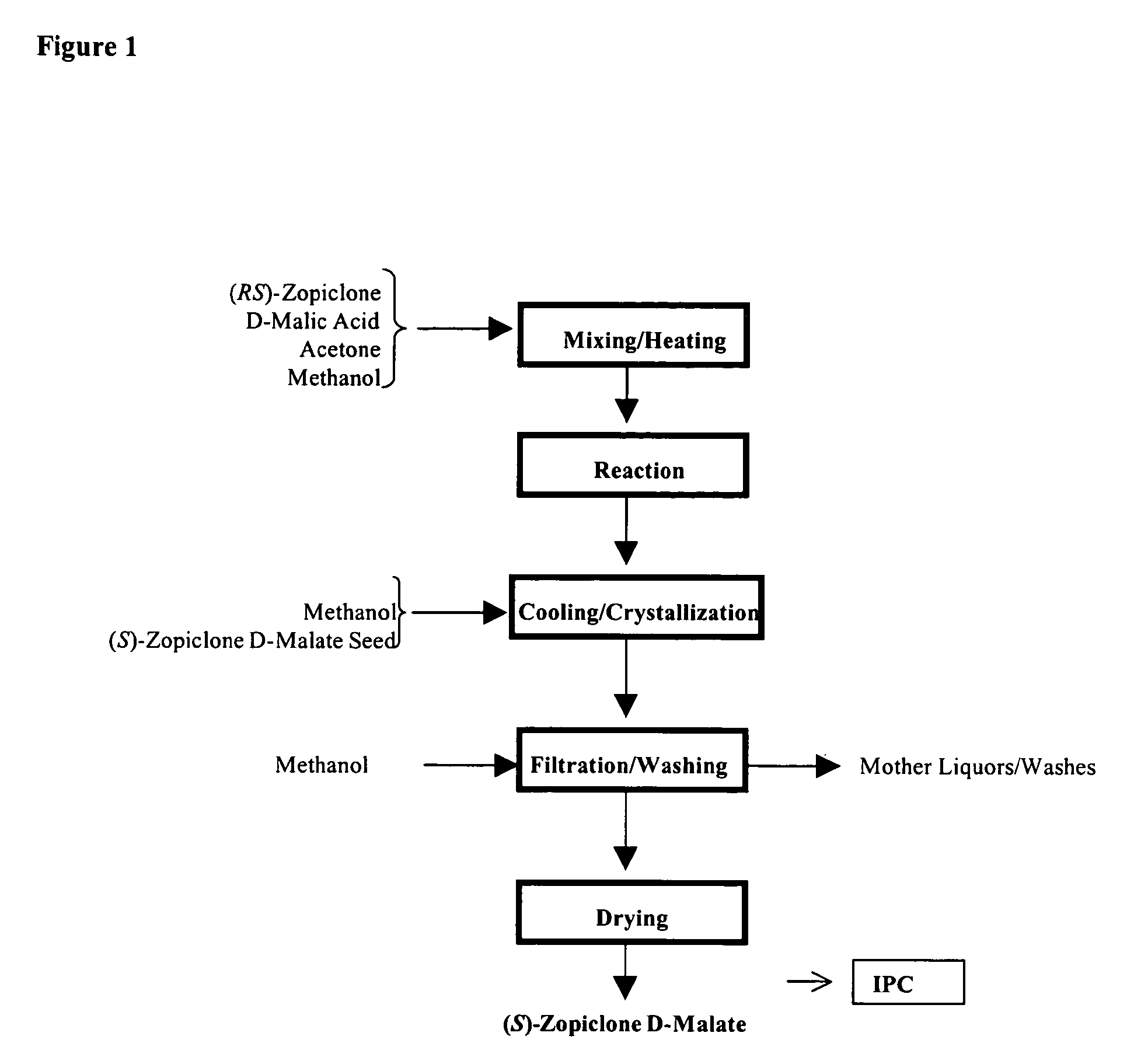
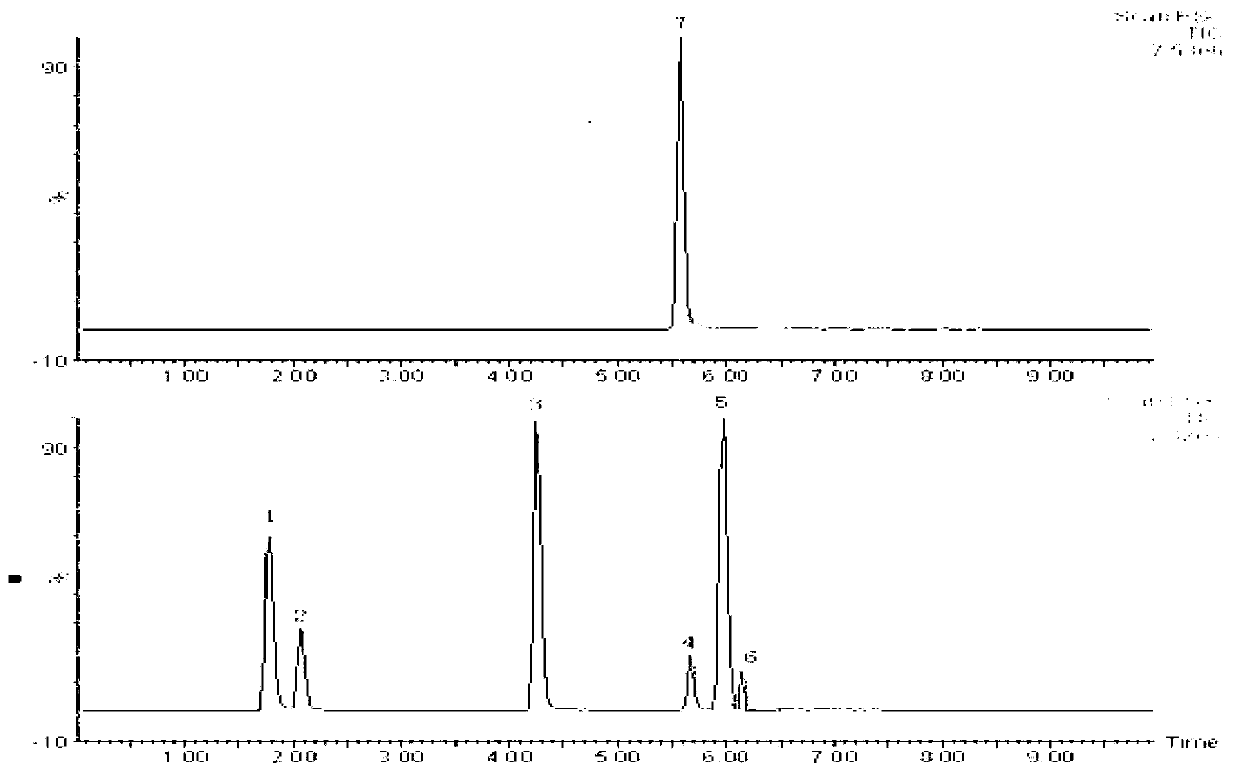
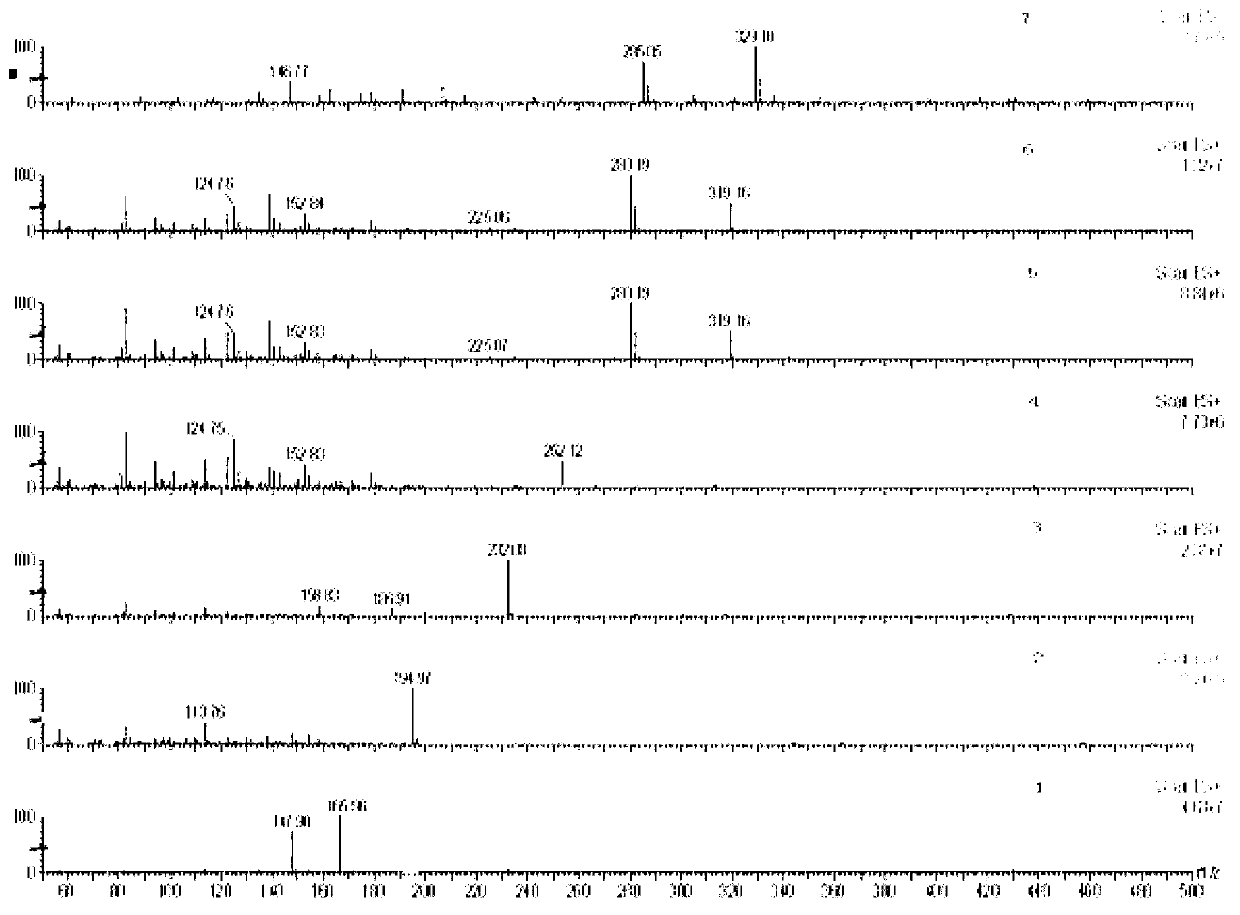
The present invention generally relates to pharmaceutical compositions comprising a dopamine agonist and sedative agent. In a preferred embodiment, the dopamine agonist is optically pure (S)-didesmethylsibutramine. In a preferred embodiment, the sedative agent is optically pure (S)-zopiclone or optically pure (S)—N-desmethylzopiclone. In a preferred embodiment, the dopamine agonist is optically pure (S)-didesmethylsibutramine; and the sedative agent is optically pure (S)-zopiclone or optically pure (S)—N-desmethylzopiclone. The pharmaceutical compositions of the invention are useful in the treatment of restless-leg syndrome and periodic-limb-movement disorder, as well as various sleep disorders. In addition, the present invention relates to a method of treating a patient suffering from restless-leg syndrome, periodic-limb-movement disorder, a sleep abnormality, or insomnia, comprising coadministering a therapeutically effective amount of a dopamine agonist and a therapeutically effective amount of a sedative agent. In a preferred embodiment, the dopamine agonist is optically pure (S)-didesmethylsibutramine. In a preferred embodiment, the sedative agent is optically pure (S)-zopiclone or optically pure (S)—N-desmethylzopiclone. In a preferred embodiment, the dopamine agonist is optically pure (S)-didesmethylsibutramine; and the sedative agent is optically pure (S)-zopiclone or optically pure (S)—N-desmethylzopiclone.
Owner:WOODWARD SPECIALTY LLC
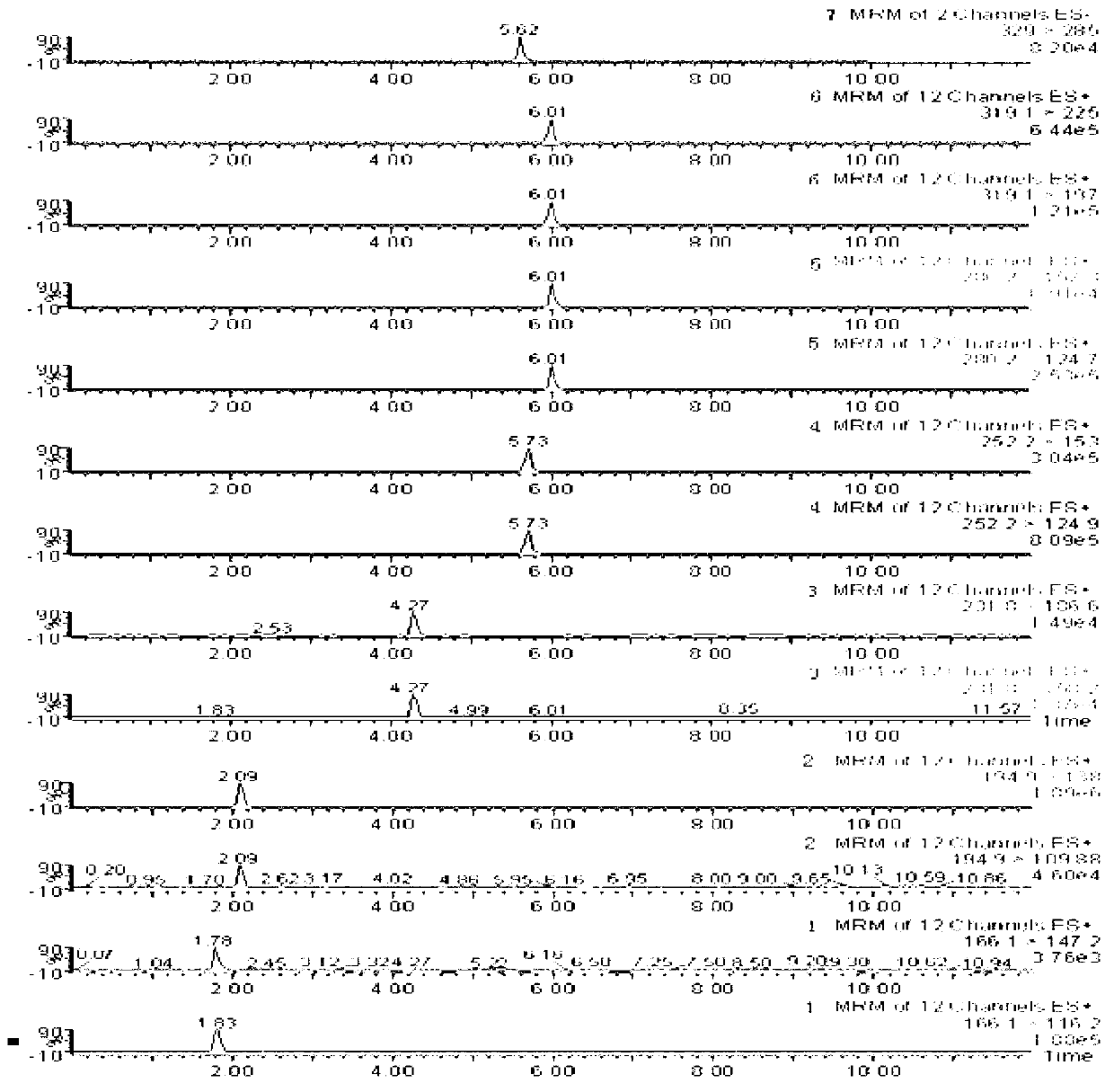
Method for simultaneously detecting seven slimming chemical components which are illegally added to traditional Chinese medicine, health food or cosmetics
InactiveCN102901780AEasy to separateQualitatively accurateComponent separationPhenolphthaleinEphedrine
The invention relates to a method for simultaneously detecting seven slimming chemical components which are illegally added to traditional Chinese medicine, health food or cosmetics. According to the method, an appropriate mobile phase and a reasonable elution gradient are adopted during liquid-phase separation, so that the slimming components needing to be detected are effectively separated within 12 min, the analysis time of a sample instrument is shortened by 88%, and the work efficiency is greatly increased. Additionally, the advantages of high separation ability of the HPLC (High Performance Liquid Chromatography) technology, high sensitivity and stronger qualitative ability of the mass spectrum and the like are combined, so screening and confirmation can be simultaneously completed during one operation. By using the method disclosed by the invention, the seven chemical components including sibutramine, fenfluramine, phenolphthalein, ephedrine, caffeine, N,N-bi-demethylation sibutramine and furosemide can be effectively separated, the inspection time can be shortened, and the slimming chemical components which are illegally added to the traditional Chinese medicine, the health food or the cosmetics can be accurately and effectively distinguished.
Owner:HUNAN INST FOR FOOD & DRUG CONTROL
Combination of an H3 antagonist/inverse agonist and an appetite suppressant
The present invention relates to pharmaceutical compositions comprising therapeutic combinations comprising: one or more H3 antagonists / inverse agonists; one or more appetite suppressants selected from the group consisting of CB1 antagonists / inverse agonists, sibutramine, phentermine and topiramate; and optionally one or more HMG-CoA reductase inhibitors. The invention also relates to medicaments and kits comprising the pharmaceutical compositions of the present invention, and methods of treating obesity, obesity related disorders and diabetes using the pharmaceutical compositions of the present invention.
Owner:SCHERING CORP
Treatment of disorders secondary to organic impairments
A method for treatment of neuropsychiatric symptoms or disorders emanating from primary brain or systemic impairments includes administration of an effective dose of a dopamine, serotonin, and norepinephrine reuptake inhibitor to a human in need of such treatment. The preferred reuptake inhibitor is sibutramine.
Owner:SNOWDEN PHARMA
Combination Therapies for the Treatment of Obesity
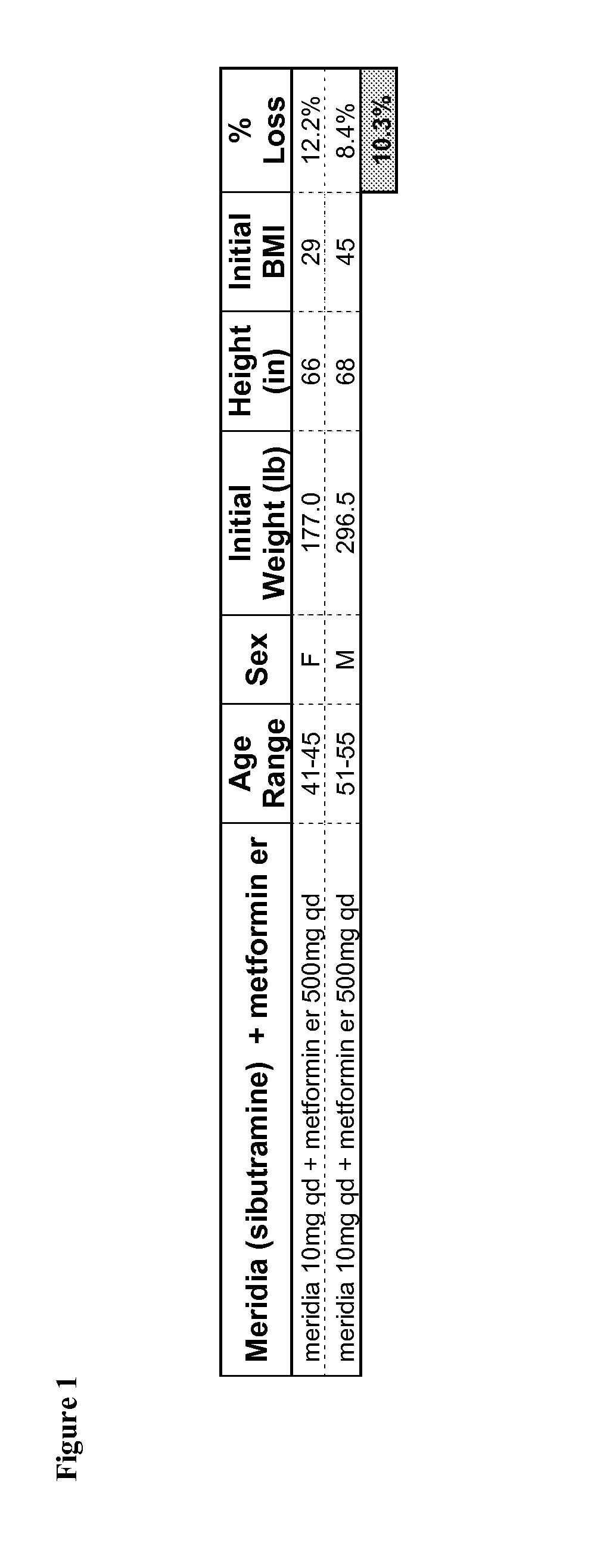
Described are pharmaceutical compositions comprising sibutramine, metformin, and at least one pharmaceutically acceptable carrier or excipient. Another aspect of the present invention relates to a method of treating a patient suffering from obesity or needing to lose weight, comprising the step of co-administering to said patient a therapeutically effective amount of sibutramine and metformin. In certain embodiments, an aforementioned method is practiced in conjunction or tandem with a medical procedure or the use of a medical device or both.
Owner:METABOLOUS PHARMA
Method of using and compositions comprising (-) sibutramine optionally in combination with other pharmacologically active compounds
This invention encompasses methods for the treatment and prevention of disorders that include, but are not limited to, eating disorders; weight gain; obesity; irritable bowel syndrome; obsessive-compulsive disorders; platelet adhesion; apnea; affective disorders such as attention deficit disorders, depression, and anxiety; male and female sexual function disorders; restless leg syndrome; osteoarthritis; substance abuse including nicotine and cocaine addiction; narcolepsy; pain such as neuropathic pain, diabetic neuropathy, and chronic pain; migraines; cerebral function disorders; chronic disorders such as premenstrual syndrome; and incontinence. The invention further encompasses pharmaceutical compositions and dosage forms which comprise optically pure (-) sibutramine, optionally in combination with a phosphodiesterase inhibitor or a lipase inhibitor.
Owner:SEPACOR INC
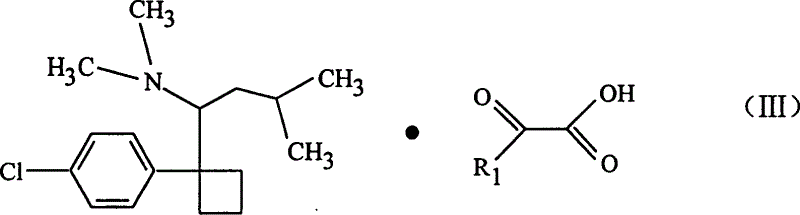
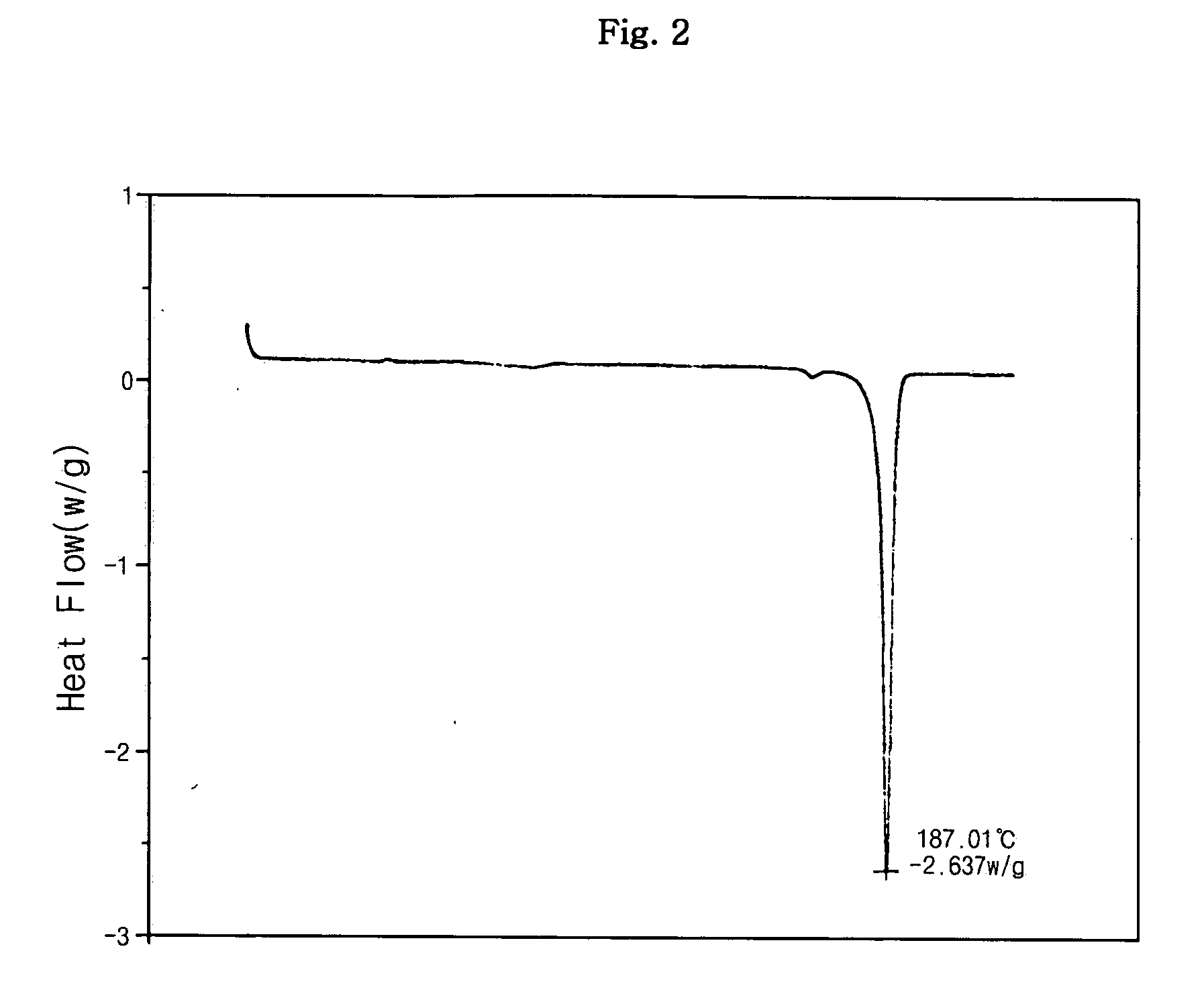
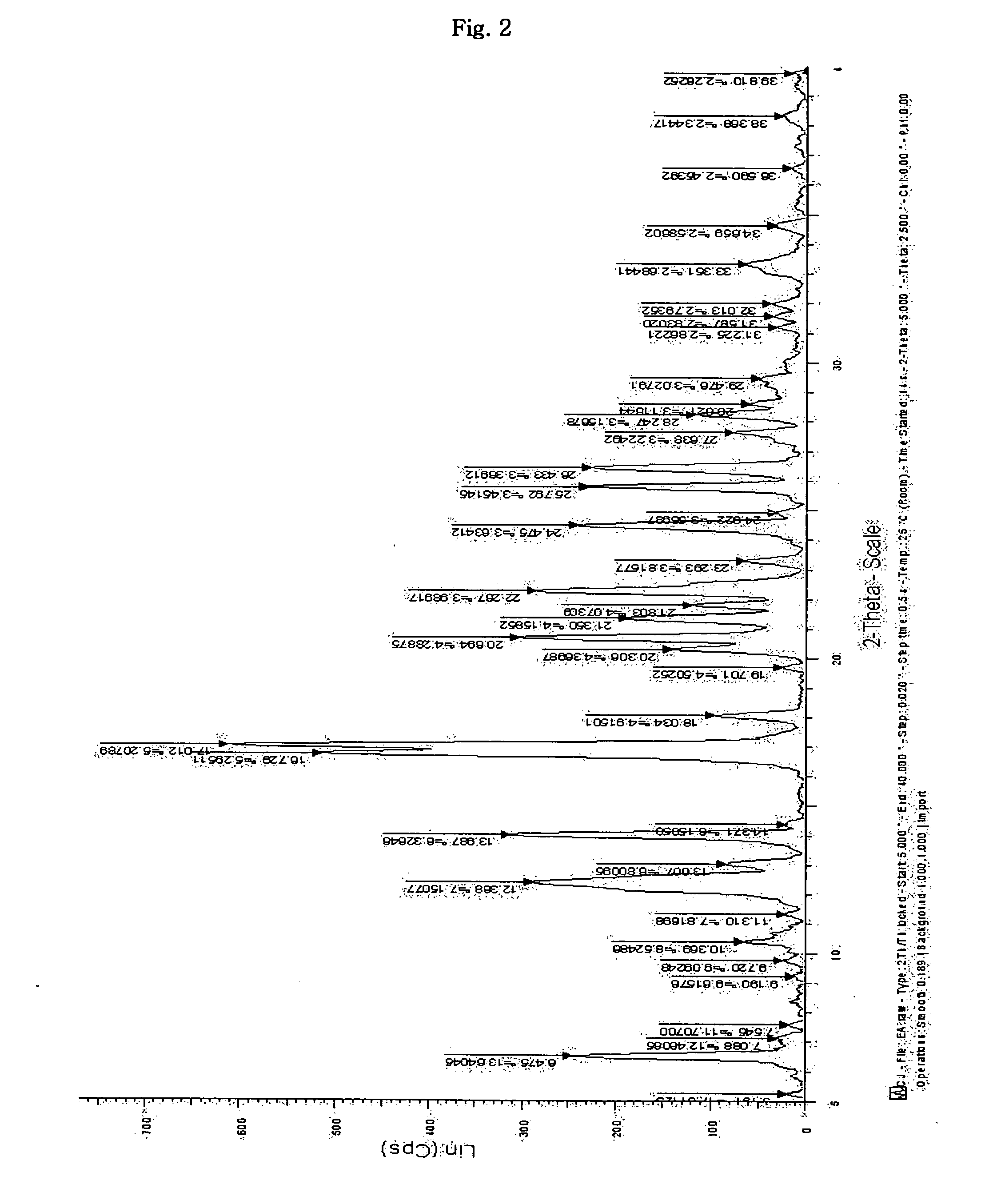
Dicarboxylic acid salt of sibutramine
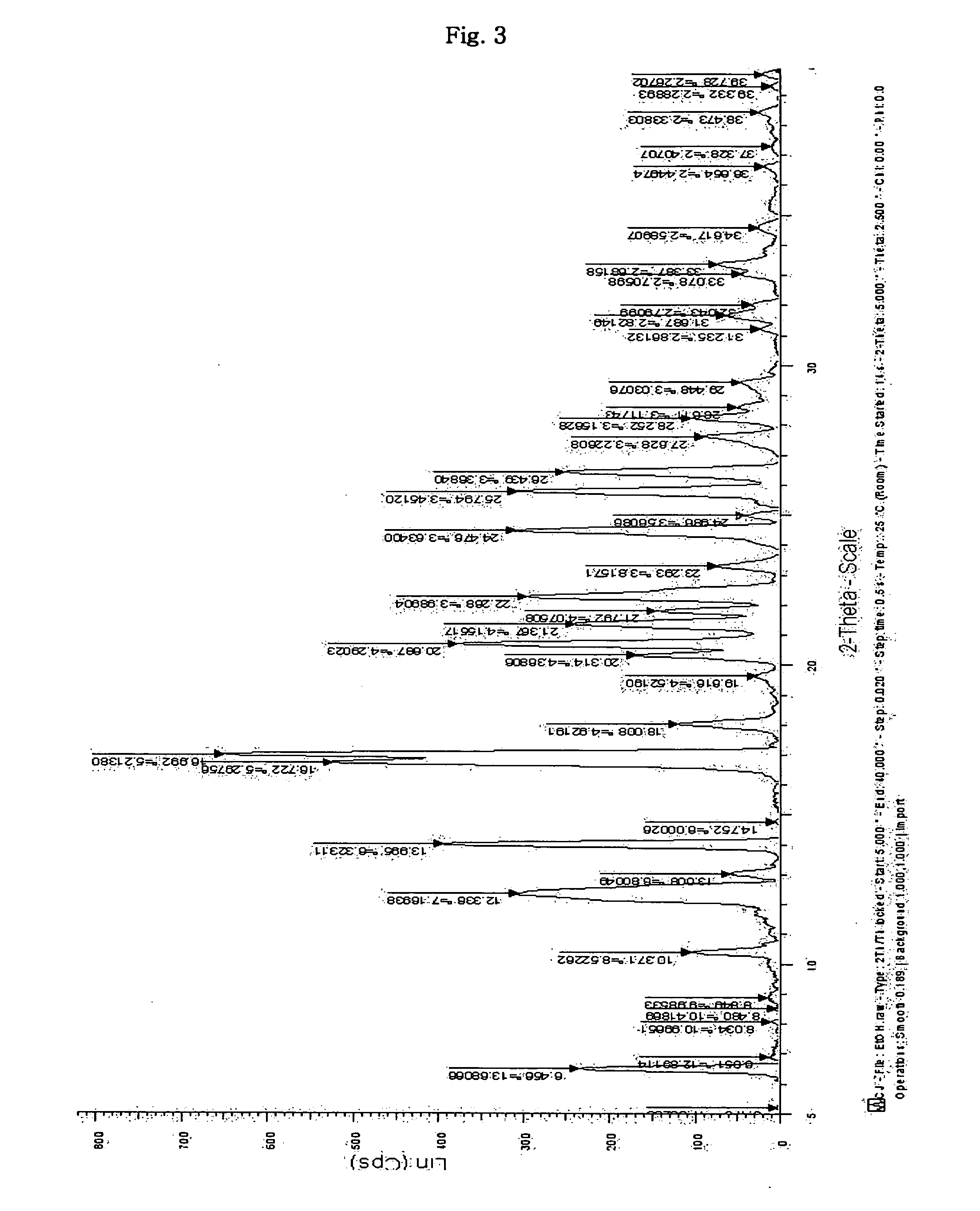
InactiveUS20070191482A1Treating and preventing pathological stateBiocideOrganic active ingredientsDicarboxylic acidCarboxylate
Disclosed is a novel dicarboxylic acid salt of sibutramine, which has good physicochemical properties. Also disclosed are a method of preparing the compound and a pharmaceutical composition comprising the compound.
Owner:CJ CHEILJEDANG CORP
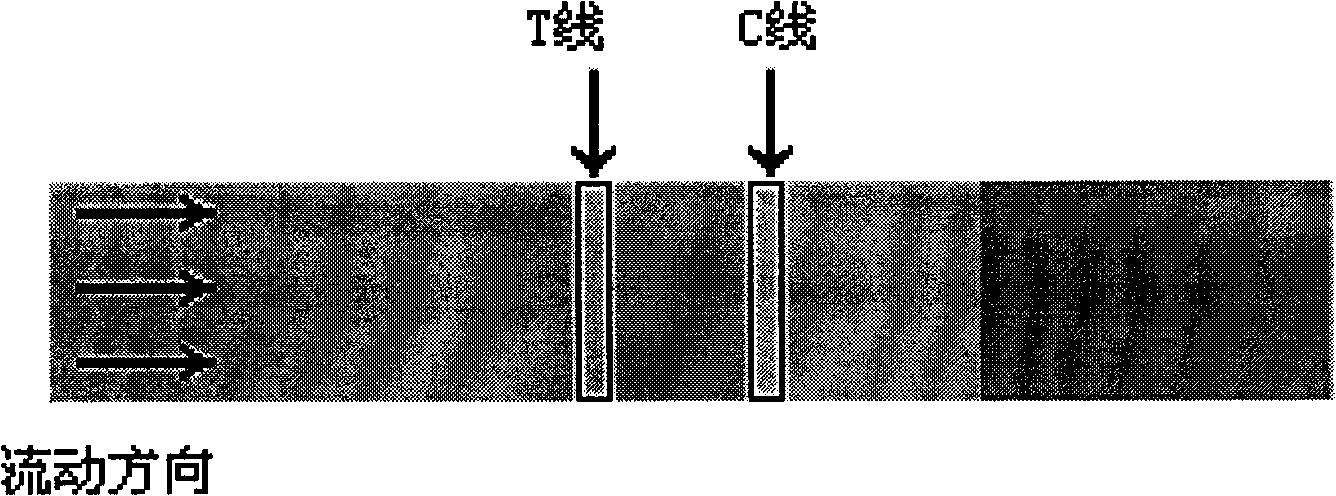
Colloid gold chromatographic test paper strip and preparation method thereof for fast detecting sibutramine
InactiveCN101526533AStrong specificityIncreased sensitivityMaterial analysisGlass fiberCarrier protein
The invention relates to a colloid gold chromatographic test paper strip and a preparation method thereof for fast detecting sibutramine, belonging to the field of detection technique of biological immunoassay methods. The test paper strip comprises a scaleboard, a sample pad, a gold-marking binding pad, a coated film and a water absorbent pad, wherein the sample pad, the gold-marking binding pad, the coated film and the water absorbent pad are sequentially spliced on the scaleboard; the gold-marking binding pad is glass cellucotton for absorbing gold-marking antibodies of sibutramine ramifications; the coated film has a linear adelomorphic detection line T printed by carrier protein solution coupled by the sibutramine ramifications and a linear adelomorphic contrasting line C printed by goat anti-rabbit IgG solution, and the two lines are arranged in parallel. The test paper strip has strong specificity, high sensibility, strong timeliness detected minimum reaching 5ppb, visual, directly-viewing and accurate result display and wide scope of population, is simple, convenient and fast, does not need other reagents and instruments, can be operated in field, can determine detection results within 15 minutes when being added to detected sample solution, and saves cost.
Owner:JIANGNAN UNIV
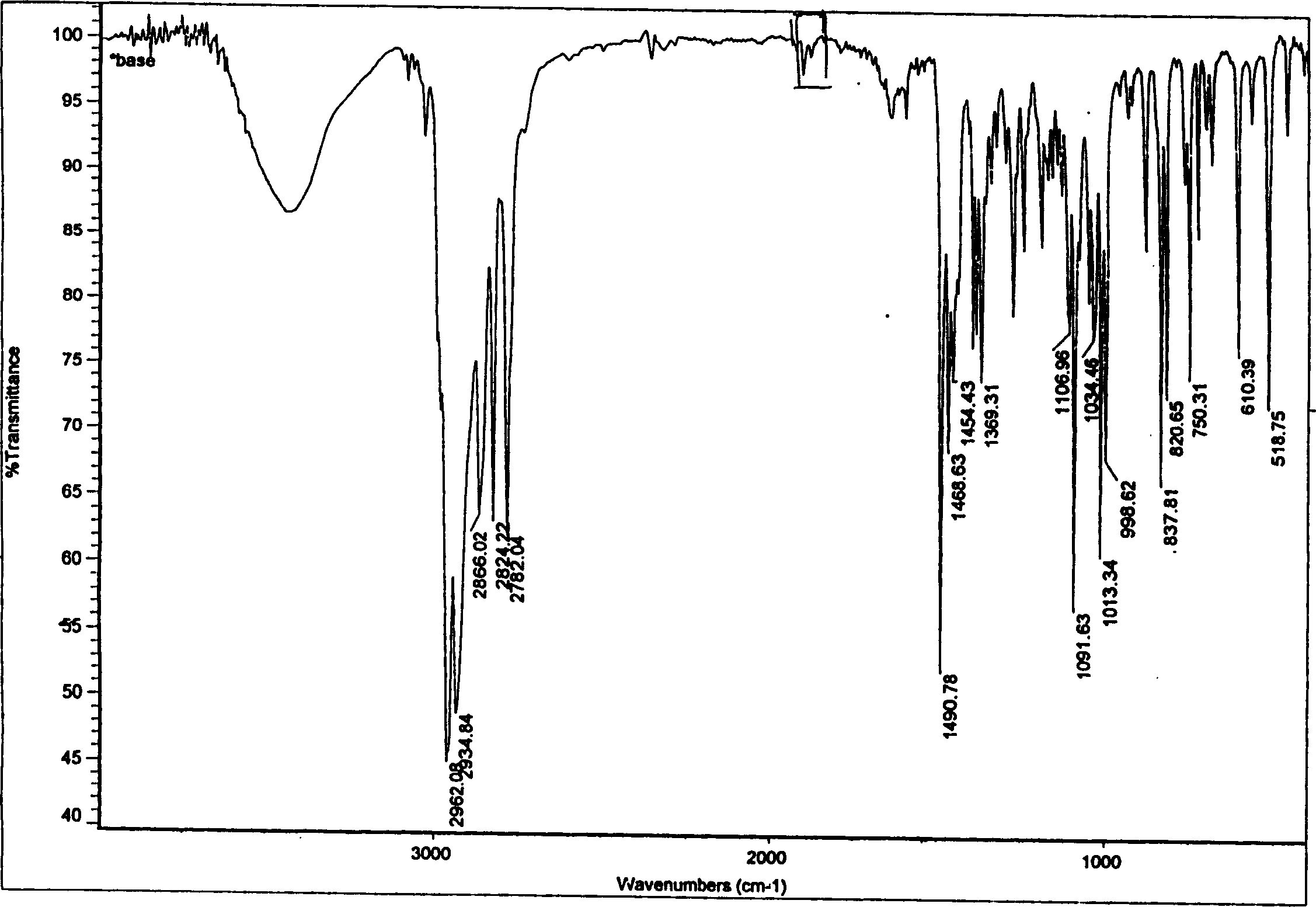
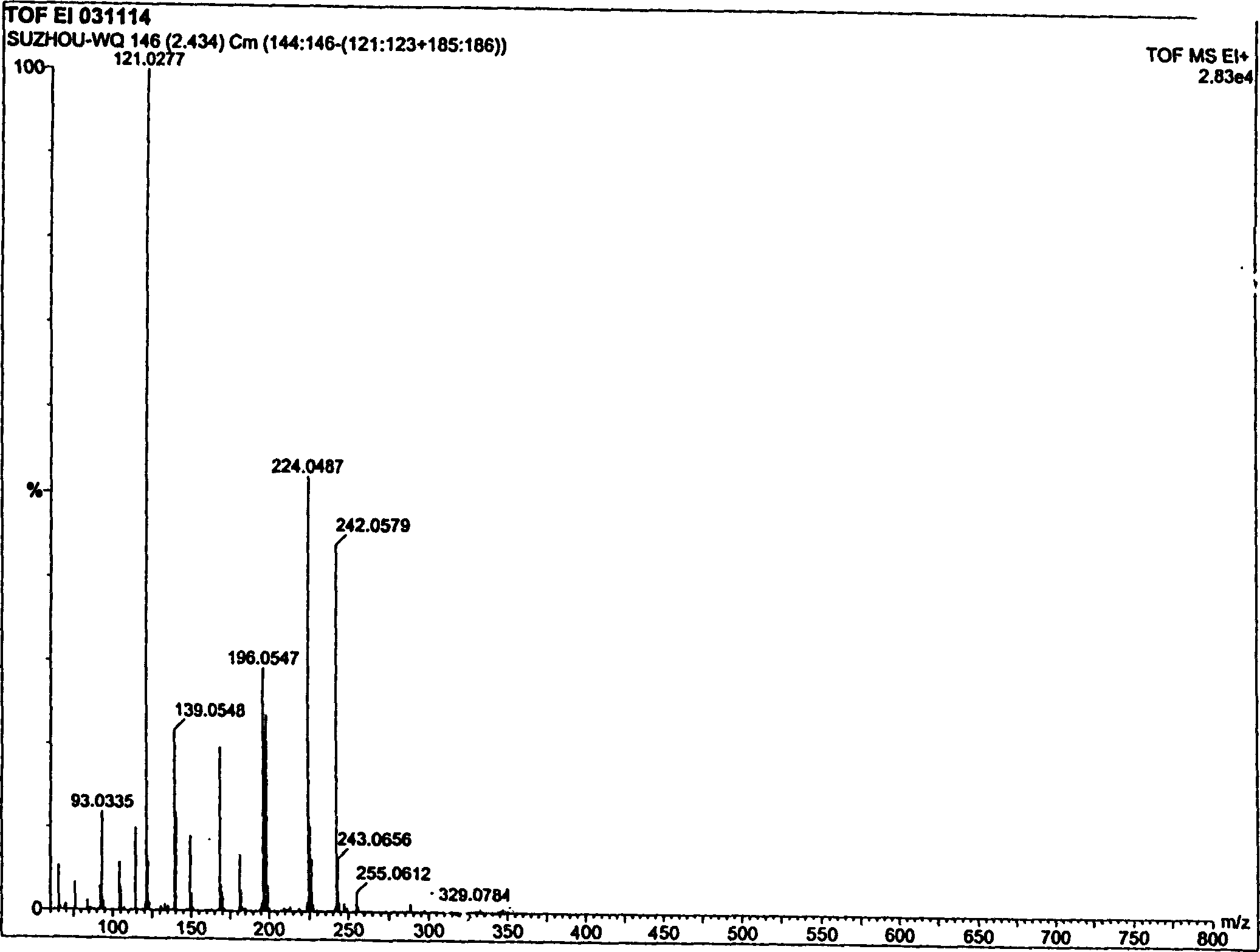
Sibutramine aliphatic salt of organic acid, its preparation process and use
The present invention relates to aromatic organic salt of Sibutramine and its preparation process. The hydrochloride of Sibutramine as initial material is added into water solution of caustic alkali and reacted for some time before ethyl ether extraction and drying to concentrate to obtain white solid free Sibutramine alkali. Organic solution of free Sibutramine alkali and organic solution of ketoacetic acid or similar organic fatty acid are then mixed at room temperature via stirring to react and separate white crystal, which is filtered and dried to obtain the aromatic organic salt of Sibutramine. The salt has excellent diabetes and obesity preventing and treating effect.
Owner:苏州市玮琪生物科技有限公司
Combination Therapies for the Treatment of Obesity
Described are pharmaceutical compositions comprising sibutramine, metformin, topiramate, and at least one pharmaceutically acceptable carrier or excipient. Another aspect of the present invention relates to a method of treating a patient suffering from obesity or needing to lose weight, comprising the step of co-administering to said patient a therapeutically effective amount of sibutramine, metformin, and topiramate. In certain embodiments, an aforementioned method is practiced in conjunction or tandem with a medical procedure or the use of a medical device or both.
Owner:METABOLOUS PHARMA
Colloidal gold chromatography test strip for quickly detecting sibutramine and derivative thereof and preparation method
InactiveCN101980021AStrong specificityIncreased sensitivityMaterial analysisAntigenMonoclonal antibody
The invention discloses a colloidal gold chromatography test strip for quickly detecting sibutramine and derivative thereof and a preparation method thereof. The test strip consists of a lining plate and a sample pad, a gold label bonding pad, a coating film and a water absorption pad engaged on the lining plate in turn, wherein on the coating film, a straight detection line T is coated by using carrier protein solution coupled with the sibutramine and the derivative thereof, and a straight contrast line C is coated by using goat-anti-mouse IgG solution. The method comprises the following steps of: preparing artificial conjugated antigens; preparing monoclonal antibodies of the sibutramine and the derivative thereof; preparing colloidal gold solution; and obtaining a colloidal gold marker and the gold label bonding pad for resisting the monoclonal antibodies of the sibutramine and the derivative thereof. The test strip has the advantages of strong specificity, high sensitivity, strong timeliness and vivid and accurate result display, and is simple, convenient and quick.
Owner:NANTONG EGENS BIOTECH
Weight-lossing fabric and its preparing process
InactiveCN1379149AEliminate side effectsReduce adverse effectsOrganic active ingredientsMetabolism disorderSolventNanometre
A weight-losing fabric is prepared through preparing nanometre microsoftgels from liposoluble sibutramine, VE, essence and oily solvent, and attaching the nanometre microsoftgels to the fabric.
Owner:南京中脉科技发展有限公司
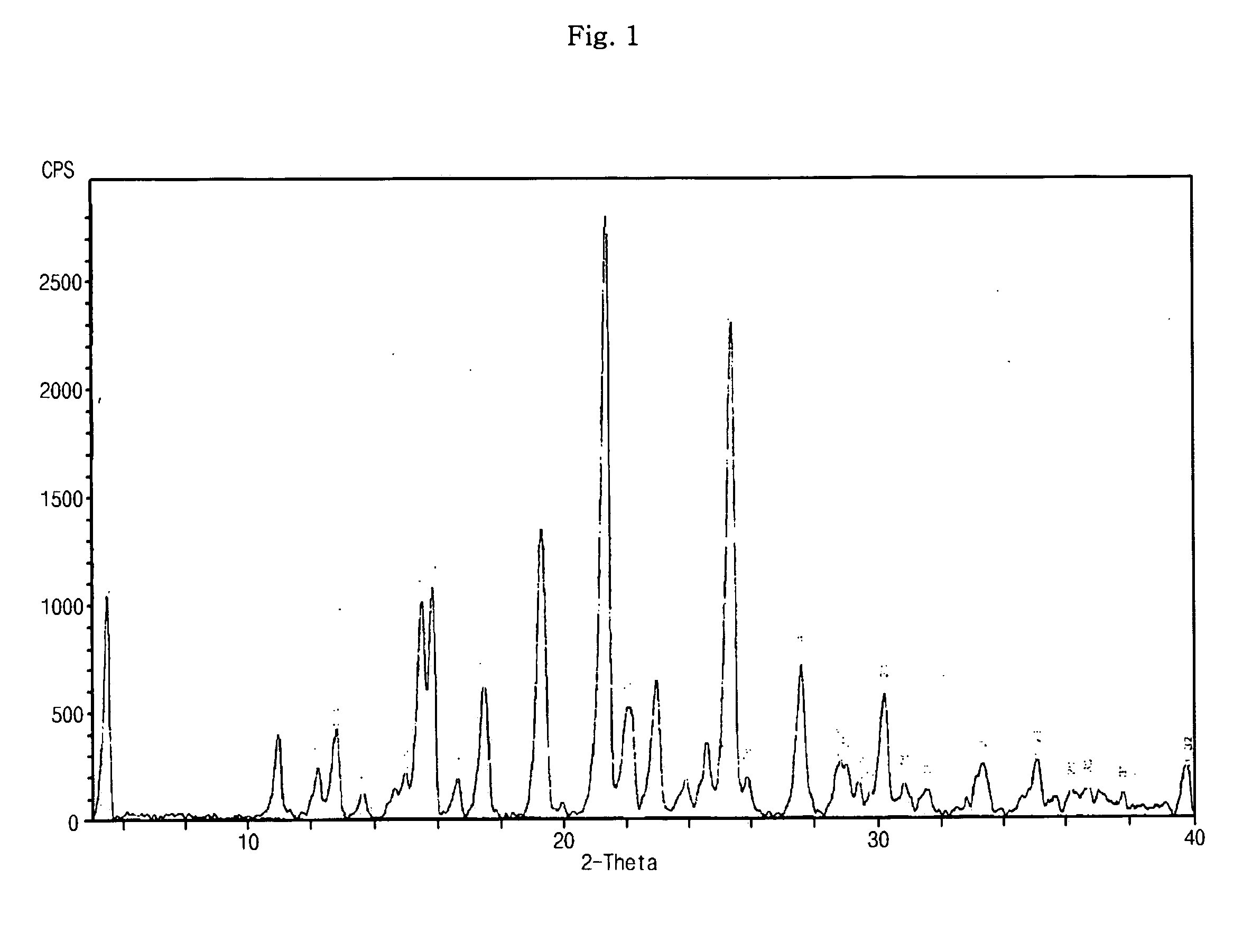
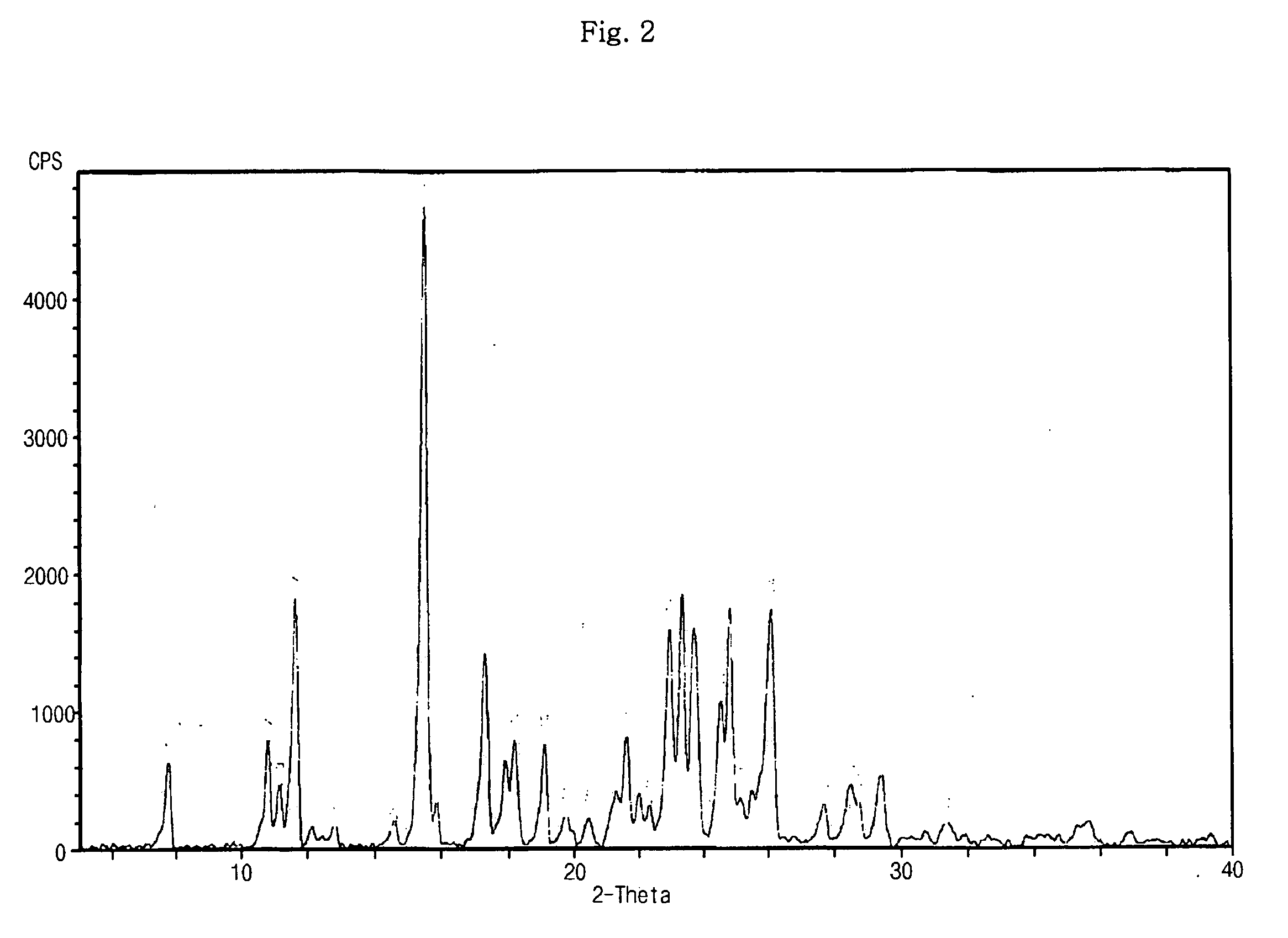
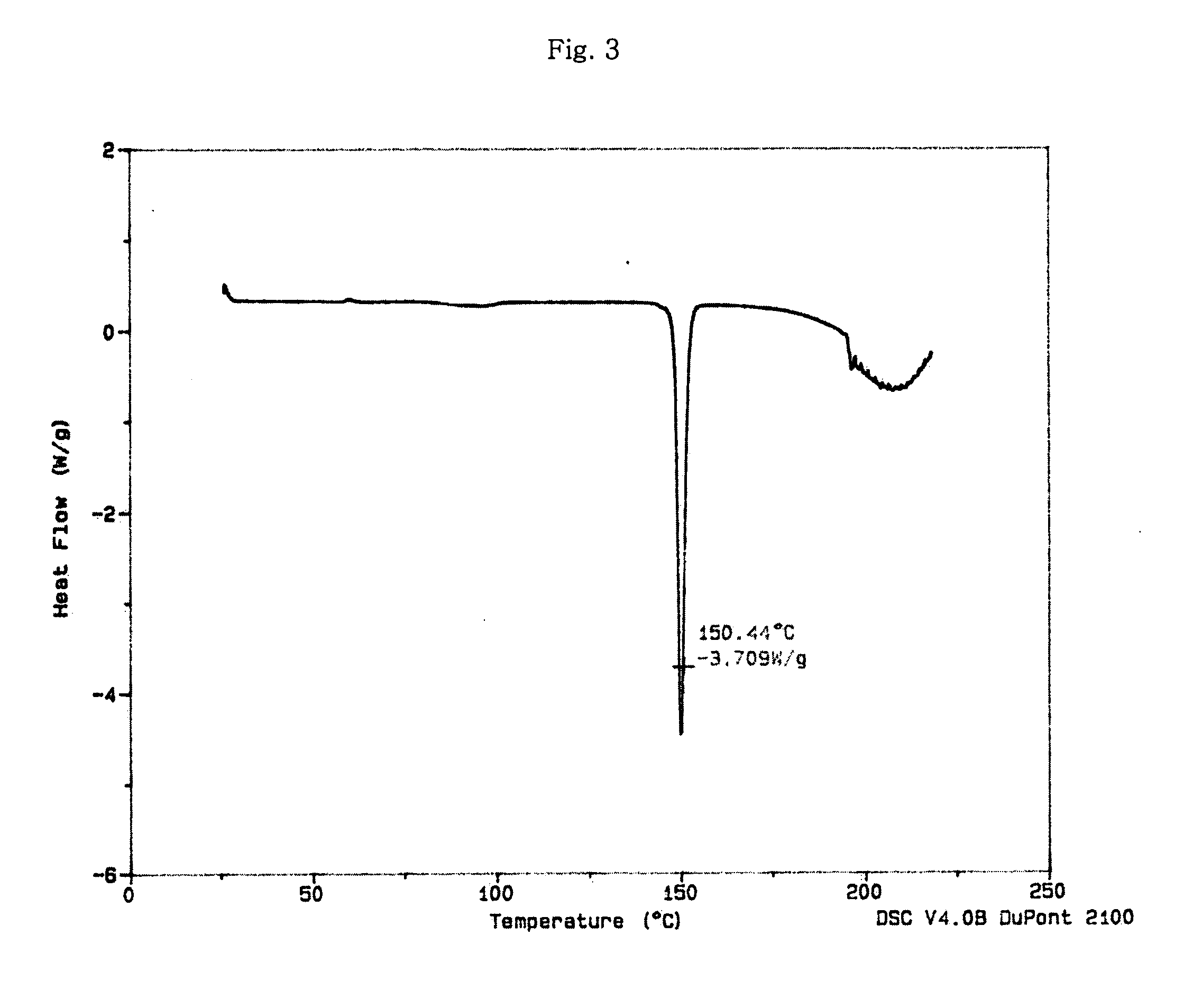
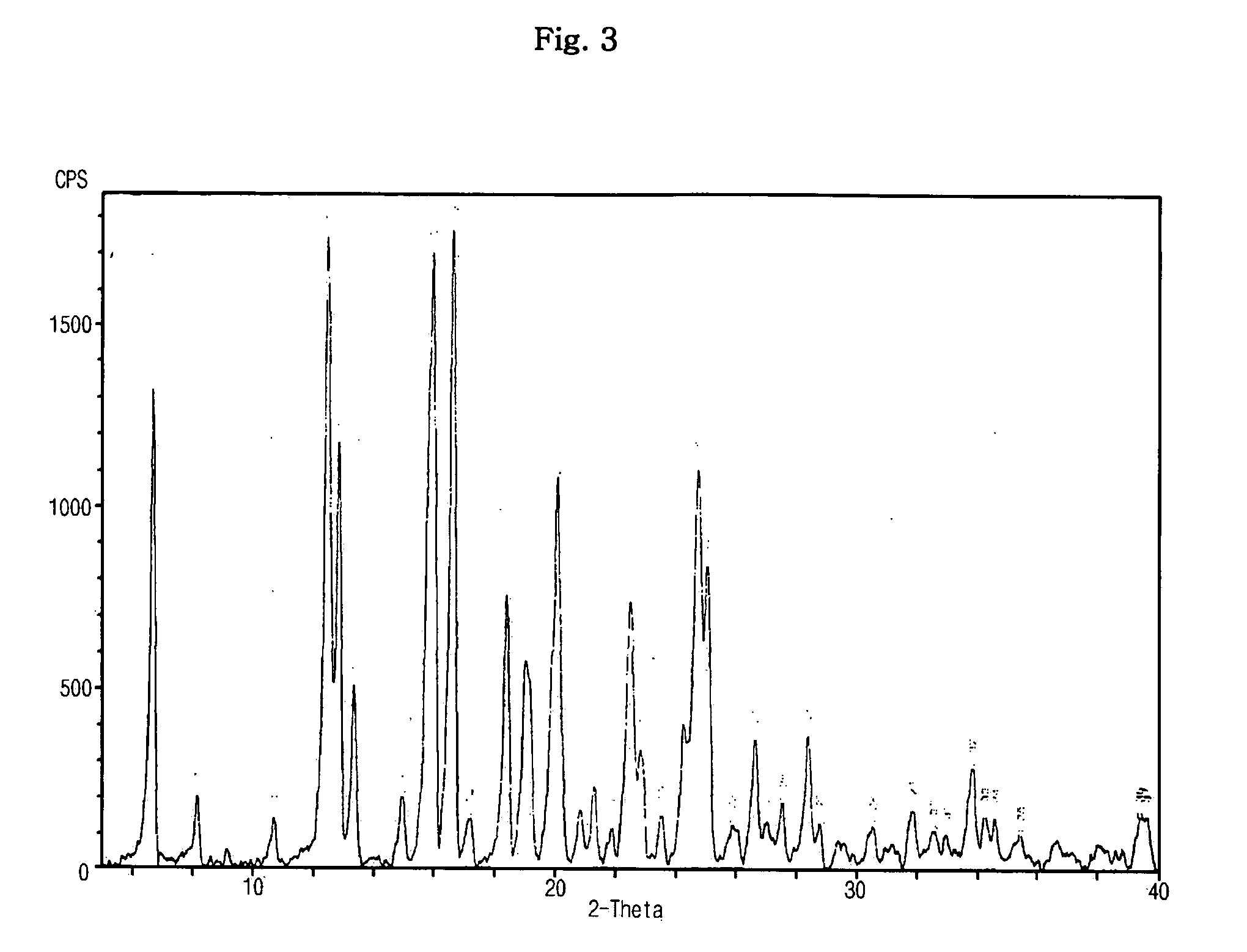
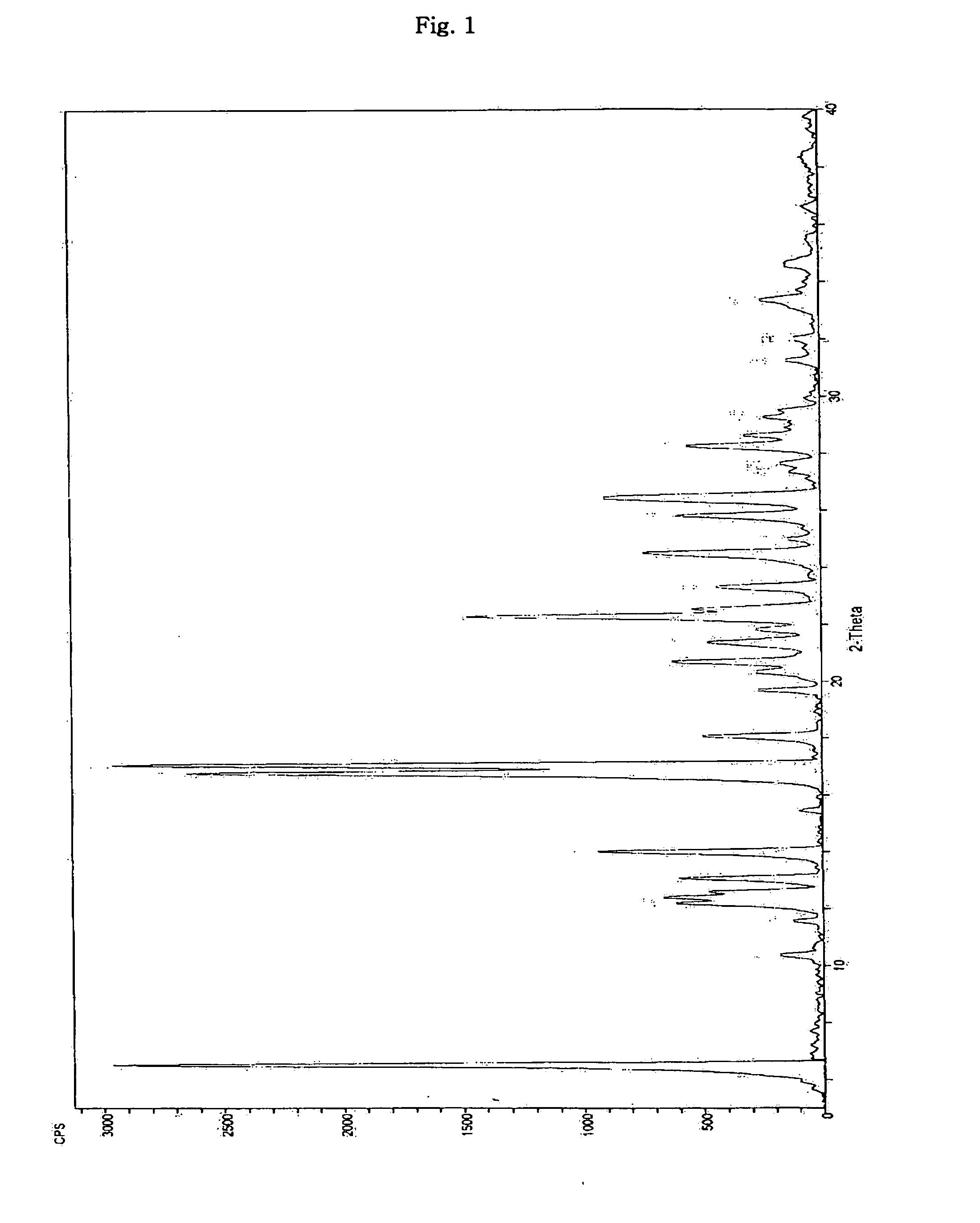
Optically pure Sibutramine and process for preparing salt derivative thereof
InactiveCN101514163AAdvantages of preparation processHigh optical purityAmino compound purification/separationAmino preparation from aminesDextrorotatoryDiastereomer
The invention discloses a preparation process of optically pure Sibutramine. In the process, chiral O,O'-diaryl formacyl tartaric acid is taken as a resolving agent to resolve Sibutramine raceme to obtain diastereomer salt thereof; and the diastereomer salt is separated, alkalized and extracted to obtain optically pure levorotatory Sibutramine or optically pure dextrorotatory Sibutramine. The invention further discloses a preparation process of a salt derivative of the optically pure Sibutramine, and the optically pure Sibutramine obtained by the process is combined with an acid compound to form the salt derivative of the optically pure Sibutramine. The preparation process of the optically pure Sibutramine is characterized by high resolving efficiency, simple and practical process, short resolving cycle and the like; and the used resolving agent can be recovered and recycled. Furthermore, the salt derivative of the optically pure Sibutramine prepared by the invention can expand Sibutramine application scope.
Owner:广州市金匮贸易有限公司
Methods of treating and preventing sexual dysfunction using (+) sibutramine in combination with phosphodiesterase inhibitors
This invention encompasses methods for the treatment and prevention of disorders that include, but are not limited to, eating disorders; weight gain; obesity; irritable bowel syndrome; obsessive-compulsive disorders; platelet adhesion; apnea; affective disorders such as attention deficit disorders, depression, and anxiety; male and female sexual function disorders; restless leg syndrome; osteoarthritis; substance abuse including nicotine and cocaine addiction; narcolepsy; pain such as neuropathic pain, diabetic neuropathy, and chronic pain; migraines; cerebral function disorders; chronic disorders such as premenstrual syndrome; and incontinence. The invention further encompasses pharmaceutical compositions and dosage forms which comprise optically pure (+) sibutramine, optionally in combination with a phosphodiesterase inhibitor or a lipase inhibitor.
Owner:SEPACOR INC
Sibutramine magnetic molecularly imprinted polymer and preparation method thereof
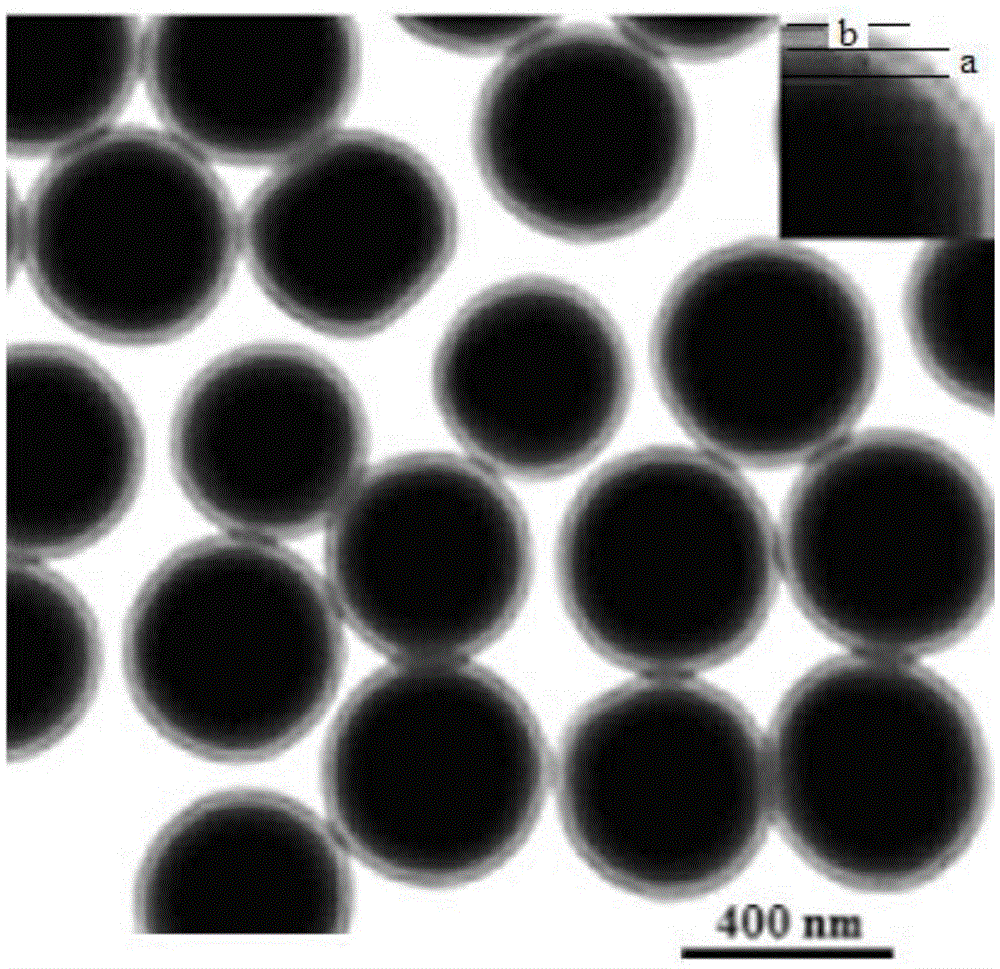
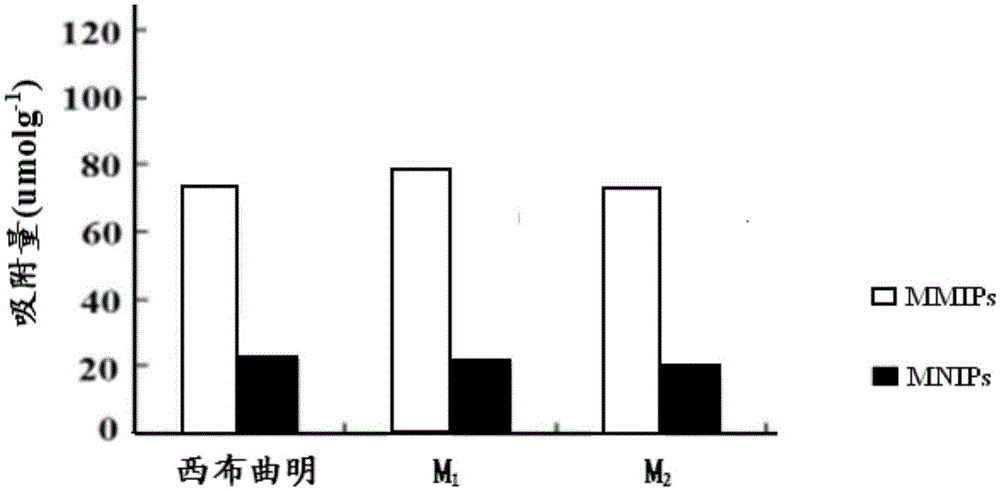
InactiveCN103819632AReduce distractionsImprove adsorption capacityOther chemical processesAlkali metal oxides/hydroxidesMagnetic moleculesMolecularly imprinted polymer
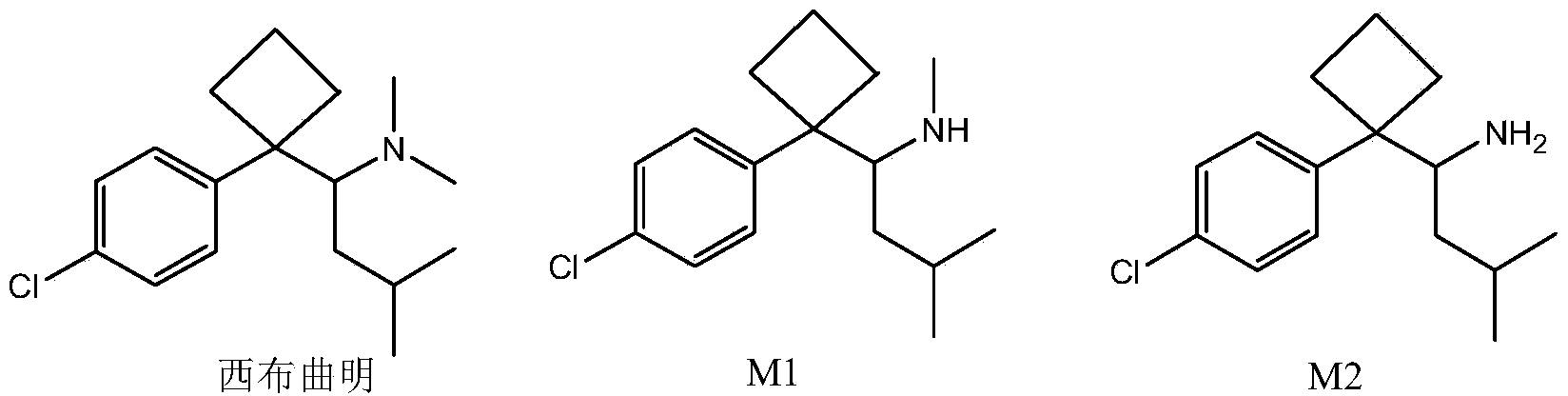
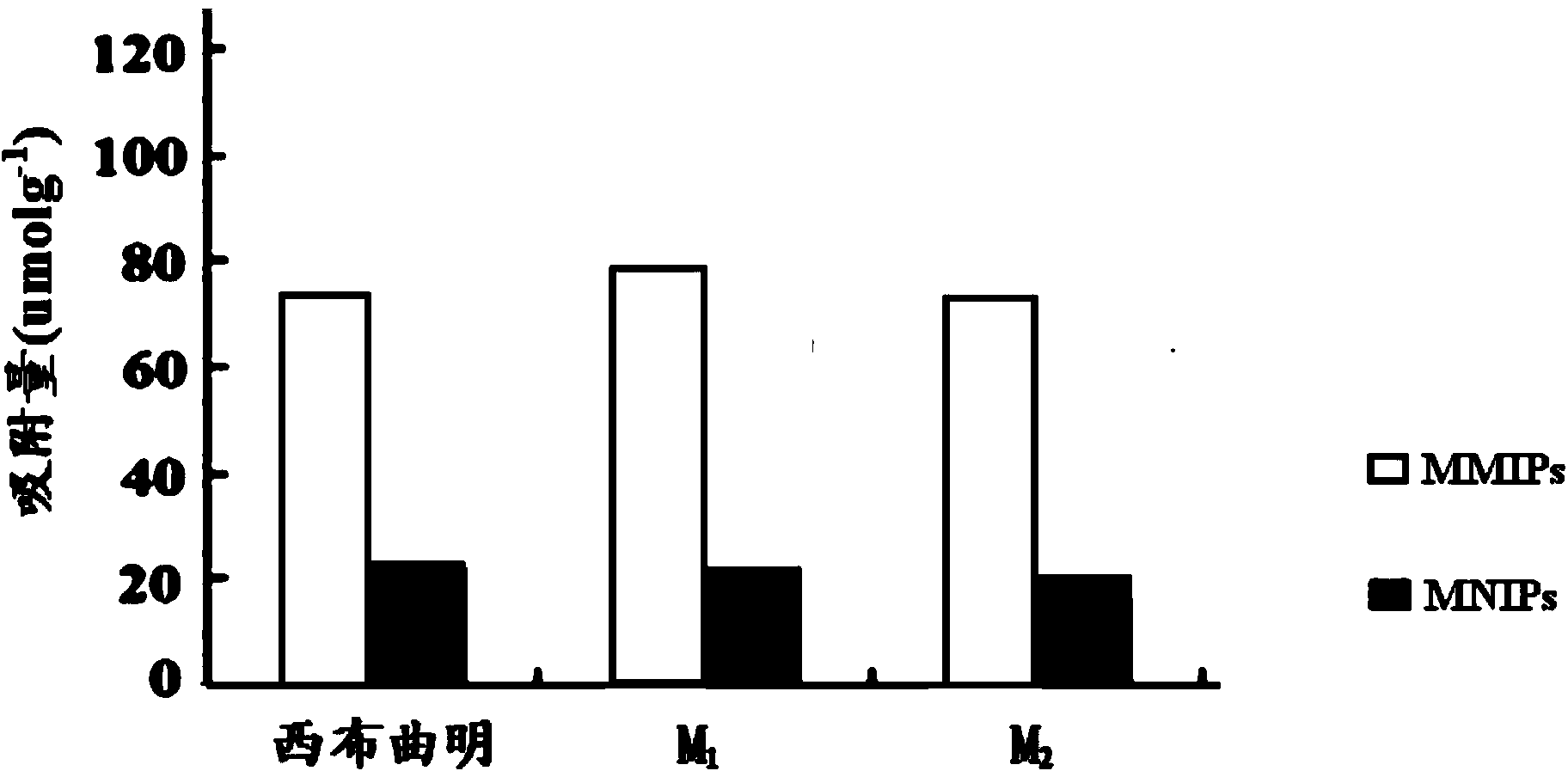
The invention discloses a sibutramine magnetic molecularly imprinted polymer and a preparation method thereof. The molecularly imprinted polymer is a magnetic molecularly imprinted polymer having a core-shell structure composed of a core made of Fe3O4@SiO2 magnetic nano particles and a shell made of sibutramine molecularly imprinted polymers. The magnetic molecularly imprinted polymer has special absorption on sibutramine, thus interference of other components on sibutramine detection can be reduced; moreover, the magnetic molecularly imprinted polymer can be directionally controlled by an external magnetic field, thus the absorption, washing, desorption can be conveniently completed, the operation is convenient, so the magnetic molecularly imprinted polymer is suitable for detection of sibutramine illegally added in food and active metabolite of sibutramine namely M1 and M2.
Owner:NANJING MEDICAL UNIV
Combination and use of drugs
InactiveUS20060276549A1Reduced metabolic activityReduce energy consumptionOrganic active ingredientsBiocideDiseasePharmaceutical drug
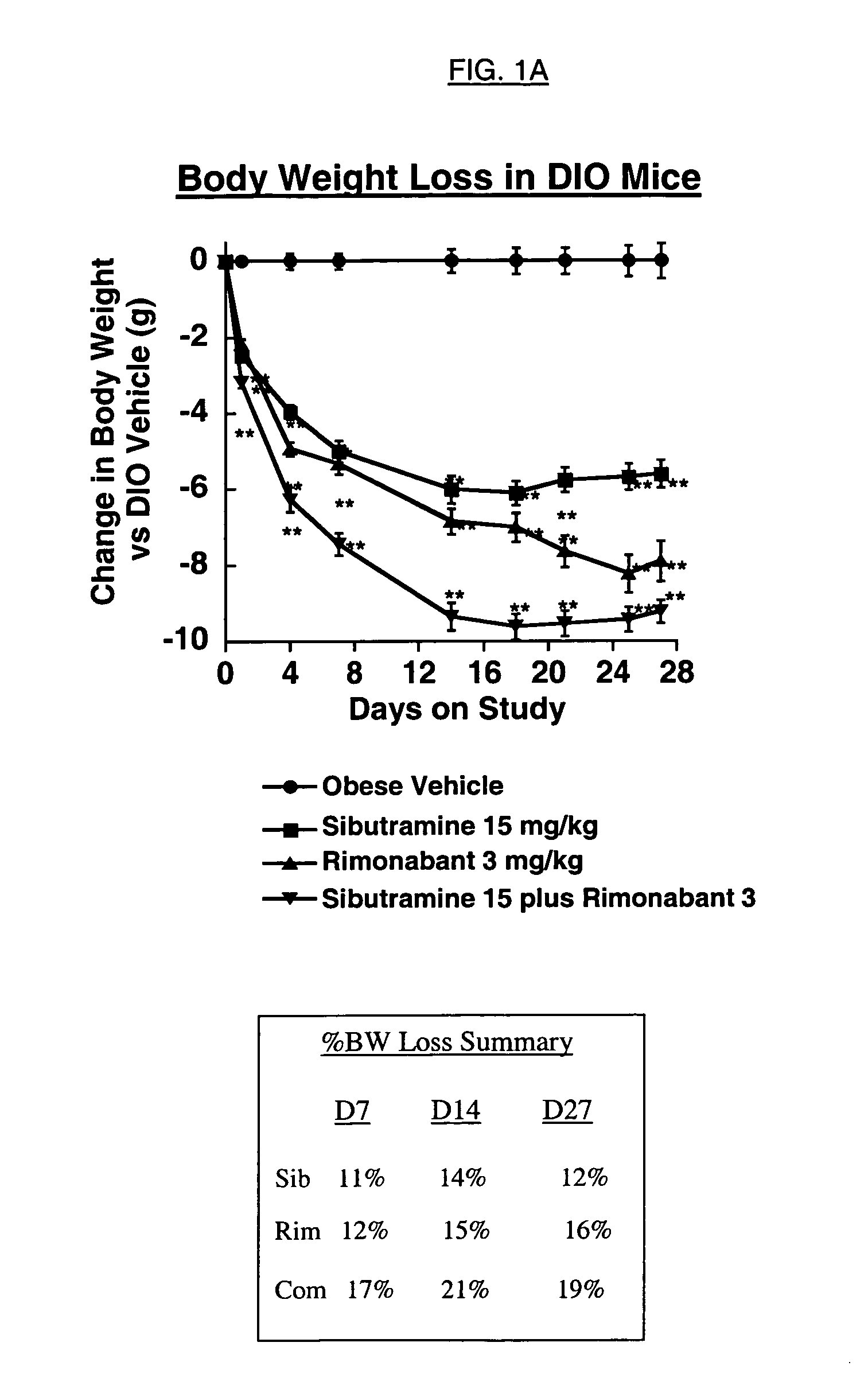
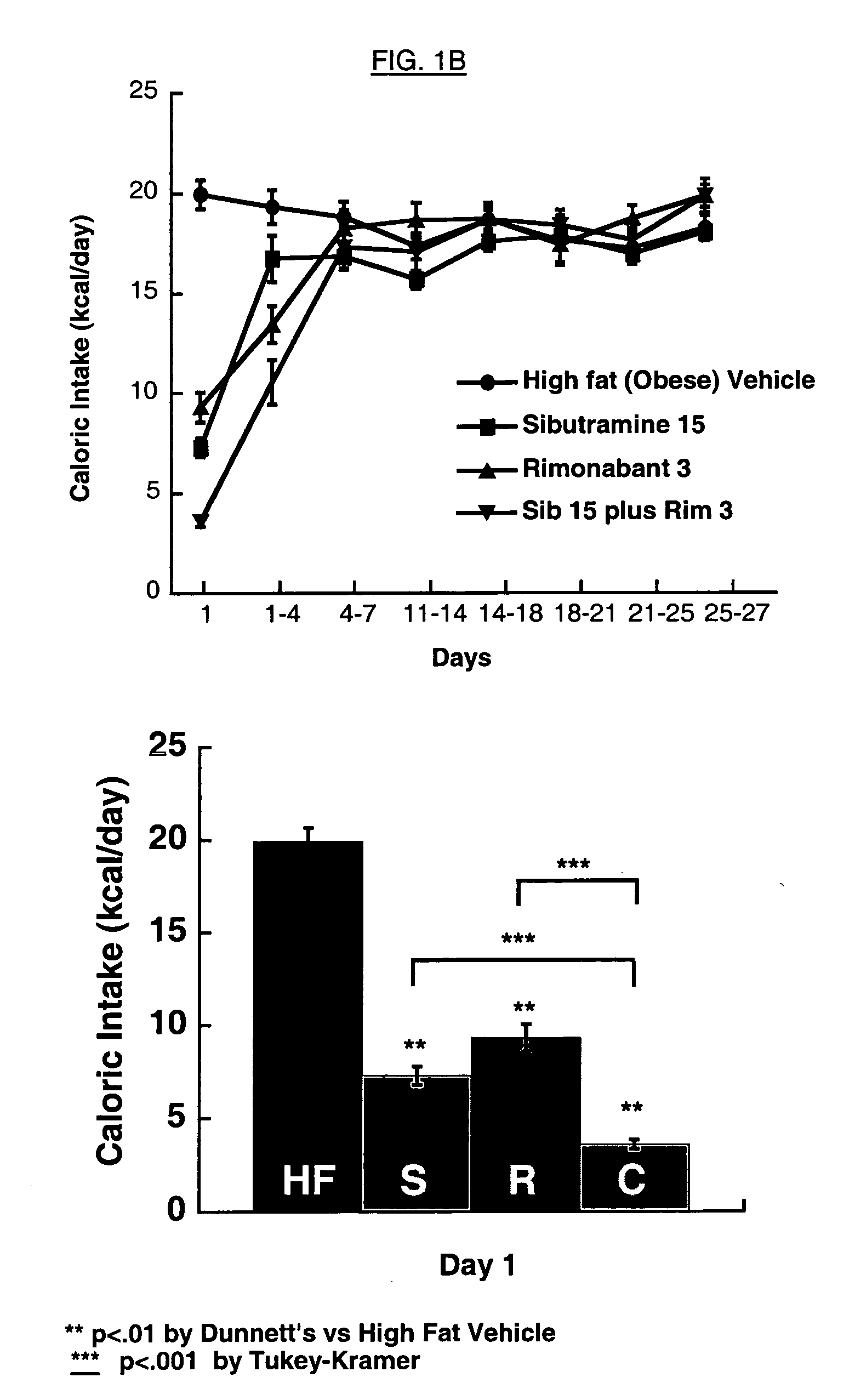
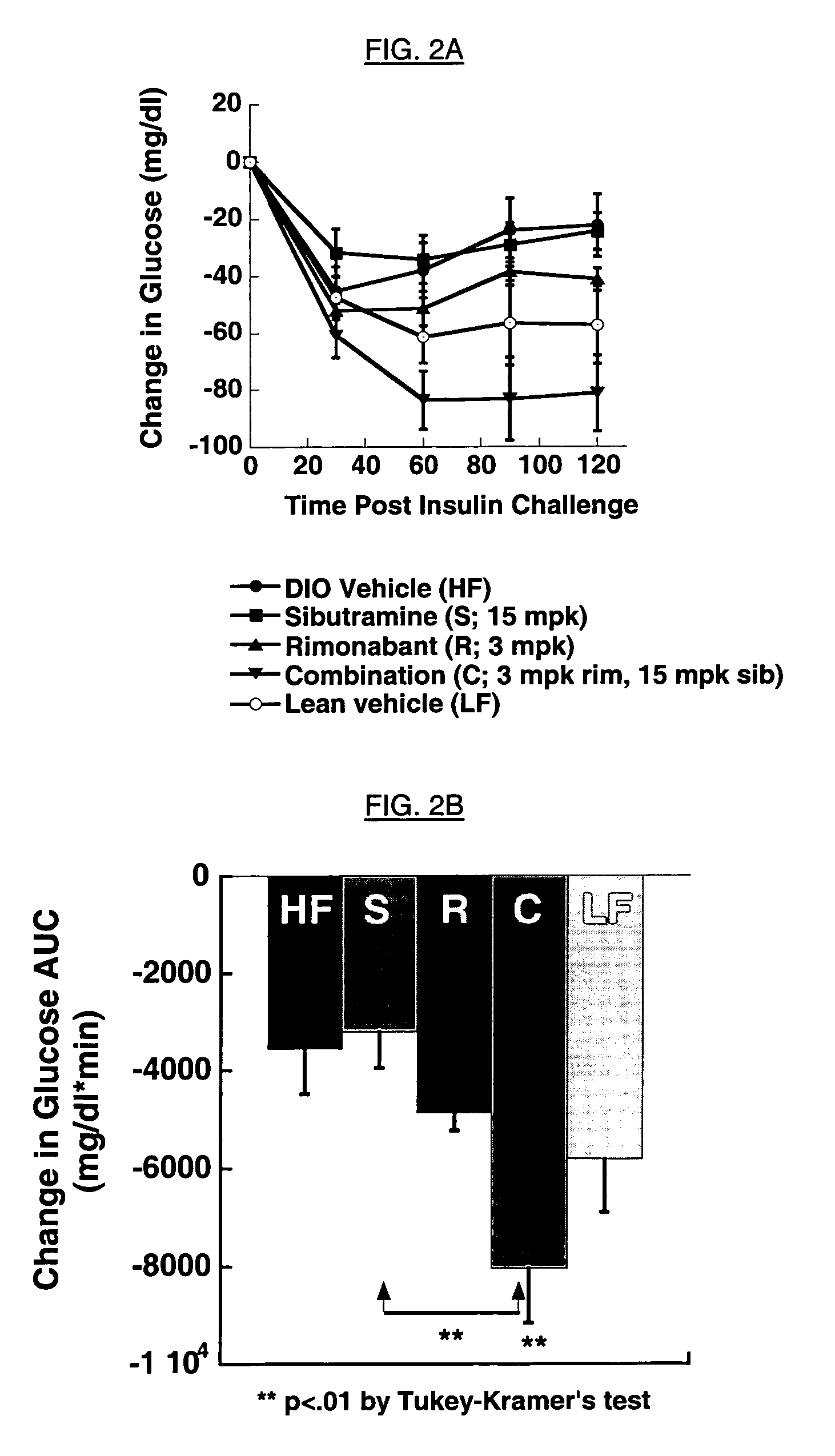
The present invention is directed to preferred pharmaceutical compositions comprising sibutramine and rimonabant and use of sibutramine and rimonabant to treat obesity and obesity related disorders in a patient.
Owner:ABBOTT LAB INC
Sulphonic acid salt of sibutramine
InactiveUS20070191481A1Treating and preventing pathological stateBiocideNervous disorderMedicinal chemistrySibutramine
Disclosed is a novel sulphonic acid salt of sibutramine, which has good physicochemical properties. Also disclosed are a method of preparing the compound and a pharmaceutical composition comprising the compound.
Owner:CJ CHEILJEDANG CORP
Inorganic acid salts of sibutramine
InactiveUS20070191489A1Improve solubilityMaintain good propertiesOrganic active ingredientsBiocideAdditive ingredientCompound (substance)
Disclosed are novel inorganic acid salts of sibutramine, which have good physicochemical properties, and crystalline forms thereof. Also disclosed are pharmaceutical compositions comprising the compounds as effective ingredients, methods of preparing the compounds, and the use of the compounds.
Owner:CJ CHEILJEDANG CORP
Combination of an h3 antagonist/inverse agonist and an appetite suppressant
The present invention relates to pharmaceutical compositions comprising therapeutic combinations comprising: one or more H3 antagonists / inverse agonists; one or more appetite suppressants selected from the group consisting of CBi antagonists / inverse agonists, sibutramine, phentermine and topiramate; and option one or more HMG-CoA reductase inhibitors. The invention also relates to medicaments and kits comprising the pharmaceutical compositions of the present invention, and methods of treating obesity, obesity related disorders and diabetes using the pharmaceutical compositions of the present invention.
Owner:SCHERING AG
Preparation of monoclonal antibody of specific combined sibutramine and demethylated sibutramine
InactiveCN101643506AHigh sensitivityStrong specificitySerum albuminPeptide preparation methodsMonoclonal antibodyBovine serum albumin
The invention discloses preparation of monoclonal antibody of specific combined sibutramine and demethylated sibutramine, comprising the following steps of: synthesizing immune antigen; detecting antigen synthesis; immunizing mouse; screening monoclonal antibody; and identifying the structural specificity of the monoclonal antibody. The synthesis of the immune antigen comprises the following stepsof: (1) mixing glucose, lactose and bovine serum albumin according to mole ratio being 20-25:1, adjusting pH value to 9.0-9.5, and table reacting for 2 hours under the temperature of 40-42 DEG C; and(2) mixing the modified bovine serum albumin, glutaraldehyde and demethylated sibutramine according to mol ratio being 1:(25-30): (80-90), adjusting pH value to be 9.0-9.5, reacting for 6-8 hours under the temperature of 40-42 DEG C, and completely dialyzing in 0.01 M PBS solution with pH value being 7.2-7.4 under the temperature of 4 DEG C. The detection of the antigen synthesis comprises the following steps of: mixing the ovalbumin, the glutaraldehyde and the methyl sibutramine with mol ration being 1: (15-20): (60-75), adjusting pH value to be 9.0-9.5, reacting for 6-8 hours under the temperature of 40-42 DEG C, and completely dialyzing in 0.01 M PBS solution with pH value being 7.2-7.4 under the temperature of 4 DEG C. The preparation has high sensitivity, strong specificity, convenience, simple operation, low cost, and no expensive equipment.
Owner:NANCHANG UNIV
A method for simultaneous detection of rimonabant and sibutramine
InactiveCN102269739AEasy to operateFast analysisComponent separationIsocratic elutionQuality control
The invention discloses a method for simultaneously detecting rimonabant and sibutramine, which comprises the following steps of: preprocessing a sample to be detected, and detecting by using high performance liquid chromatography, wherein the chromatographic conditions of the high performance liquid chromatography are that: a chromatographic column takes octadecylsilane chemically bonded silica as a filler, methanol or acetonitrile is taken as a mobile phase for isocratic elution, and the detection wavelength is 220-225nm. The detection method has the advantages of simple operation, high analysis speed and reliable and sensitive results, and the quality control of a product can be realized by simultaneously detecting and monitoring the rimonabant and the sibutramine.
Owner:SHANGHAI INST OF MEASUREMENT & TESTING TECH
A kind of sibutramine magnetic molecularly imprinted polymer and its preparation method
InactiveCN103819632BReduce distractionsImprove adsorption capacityOther chemical processesAlkali metal oxides/hydroxidesSpecific adsorptionMagnetite Nanoparticles
Owner:NANJING MEDICAL UNIV
Novel omega-3 and omega-6 fatty acid compositions and uses thereof
InactiveUS20130295179A1Preventing functionsReducing secondary adverse eventsHeavy metal active ingredientsBiocideSertralineStimulant
Owner:TERREAUX CHRISTIAN +3
Treatment of disorders secondary to organic impairments
A method for treatment of neuropsychiatric symptoms or disorders emanating from primary brain or systemic impairments includes administration of an effective dose of a dopamine, serotonin, and norepinephrine reuptake inhibitor to a human in need of such treatment. The preferred reuptake inhibitor is sibutramine.
Owner:SNOWDEN PHARMA
Weight-lossing fabric and its preparing process
InactiveCN1190549CEliminate side effectsReduce adverse effectsOrganic active ingredientsMetabolism disorderSolventNanometre
A weight-losing fabric is prepared through preparing nanometre microsoftgels from liposoluble sibutramine, VE, essence and oily solvent, and attaching the nanometre microsoftgels to the fabric.
Owner:南京中脉科技发展有限公司
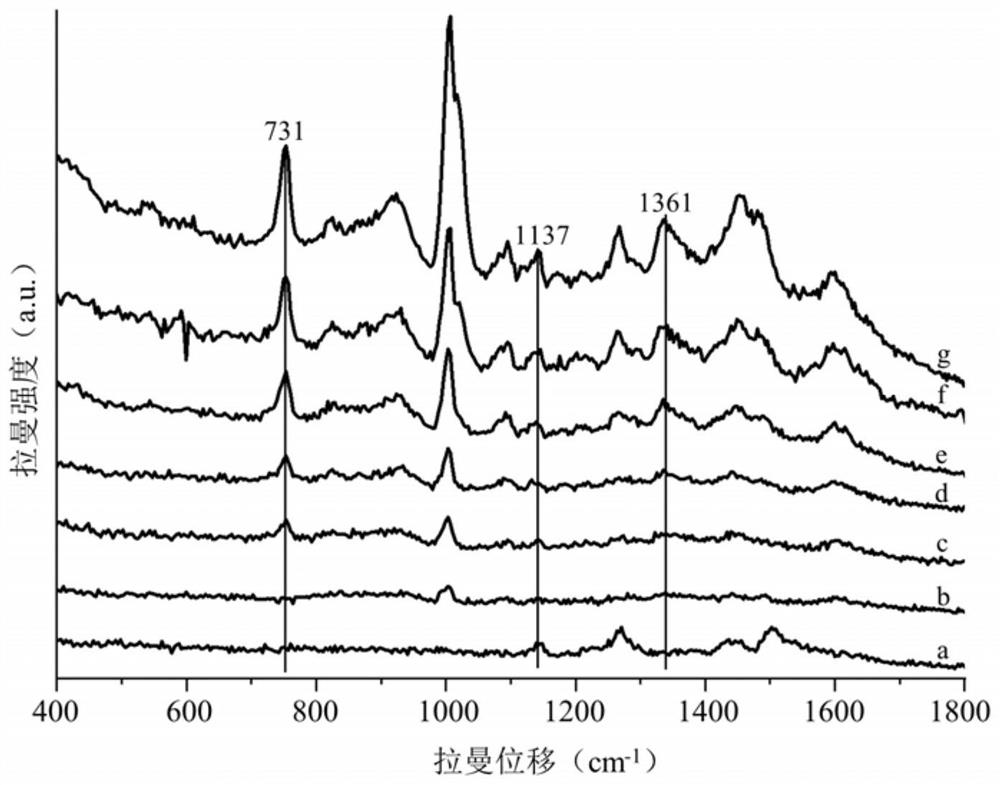
Method for simultaneously detecting sibutramine and fenfluramine in weight-losing health-care products
The invention discloses a method for simultaneously detecting sibutramine and fenfluramine in weight-losing health care products, and belongs to the field of food detection. According to the method disclosed by the invention, the Raman characteristic peaks of sibutramine and fenfluramine which exist at the same time are determined; then pretreating a weight-losing health care product sample, and extracting by adopting an extraction solvent to obtain a solution to be detected; and judging whether sibutramine and fenfluramine exist in the to-be-detected sample or not according to the Raman spectrum of the to-be-detected solution. According to the method disclosed by the invention, the lowest detection concentration of sibutramine and fenfluramine in the weight-losing health-care product is 25mg / kg, qualitative analysis can be carried out on existence of sibutramine and fenfluramine in the weight-losing health-care product, and the detection time of a single sample is controlled within 2min.
Owner:JIANGNAN UNIV +1
Application and preparation method of sibutramine in preparation of information therapeutic drug and novel drug prepared by preparation method
InactiveCN102600118AGood treatment effectEliminate side effectsOrganic active ingredientsMetabolism disorderChemical treatmentSide effect
The invention discloses application and a preparation method of sibutramine in preparation of an information therapeutic drug and a novel drug prepared by the preparation method. The information therapeutic drug is characterized in that the drug does not contact a human body directly during treatment, any substance ingredient of the drug can not be consumed, only drug information is utilized for physical treatment, but the chemical treatment effect can be achieved, a treatment window is enlarged by more than 1000 times, and the side effects of chemical treatment are avoided. After sibutramine is used as the information therapeutic drug, the contents of active ingredients are not changed, and sibutramine can also be used as a conventional preparation in the chemical treatment for secondary effective use.
Owner:北京中卫神农慢性病医学研究院有限公司
Sulphonic acid salt of sibutramine
InactiveUS7429679B2Treating and preventing pathological stateBiocideNervous disorderMedicinal chemistrySibutramine
Owner:CJ CHEILJEDANG CORP
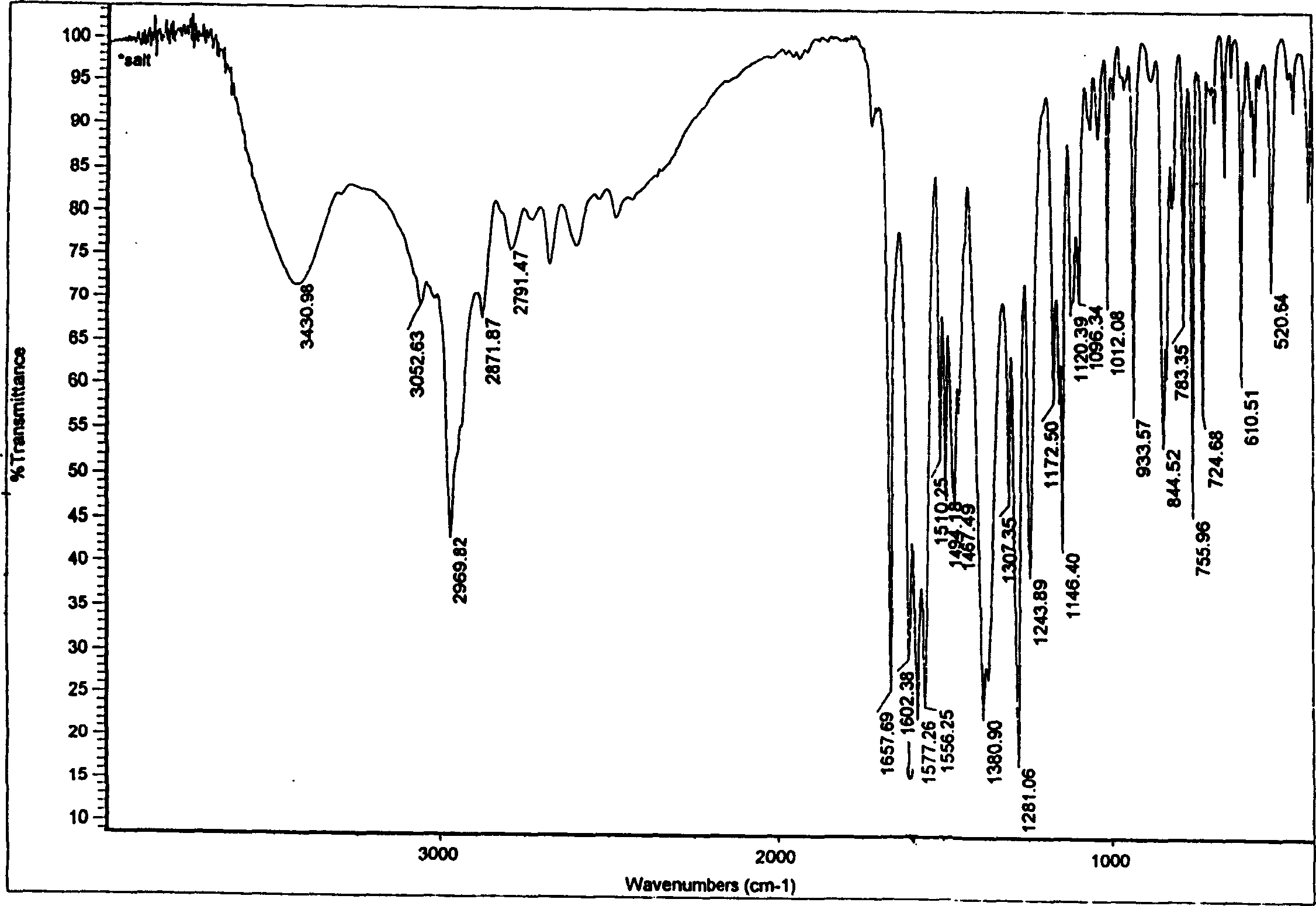
Sibutramine aromatic salt of organic acid and its preparation process
InactiveCN1557802ADevelop medical applicationsGood effectOrganic compound preparationAmino-carboxyl compound preparationOrganic acidRoom temperature
The present invention relates to aromatic organic salt of Sibutramine and its preparation process. The hydrochloride of Sibutramine as initial material is added into water solution of caustic alkali and reacted for some time before ethyl ether extraction and drying to concentrate to obtain white solid free Sibutramine alkali. Organic solution of free Sibutramine alkali and organic solution of Haibensaite or its derivative are then mixed at room temperature via stirring to react and separate white crystal, which is dried to obtain the aromatic organic salt of Sibutramine.
Owner:苏州市玮琪生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com