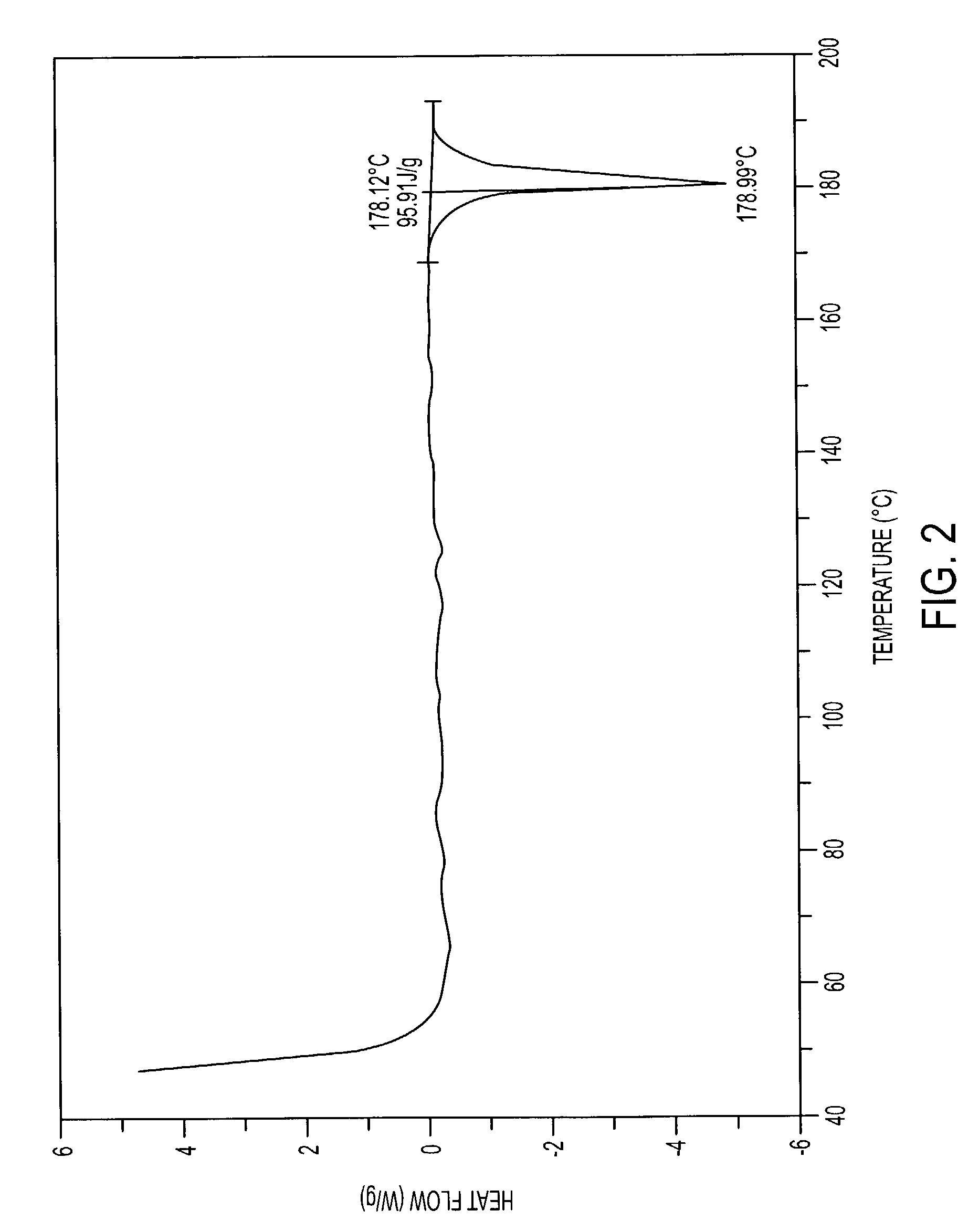
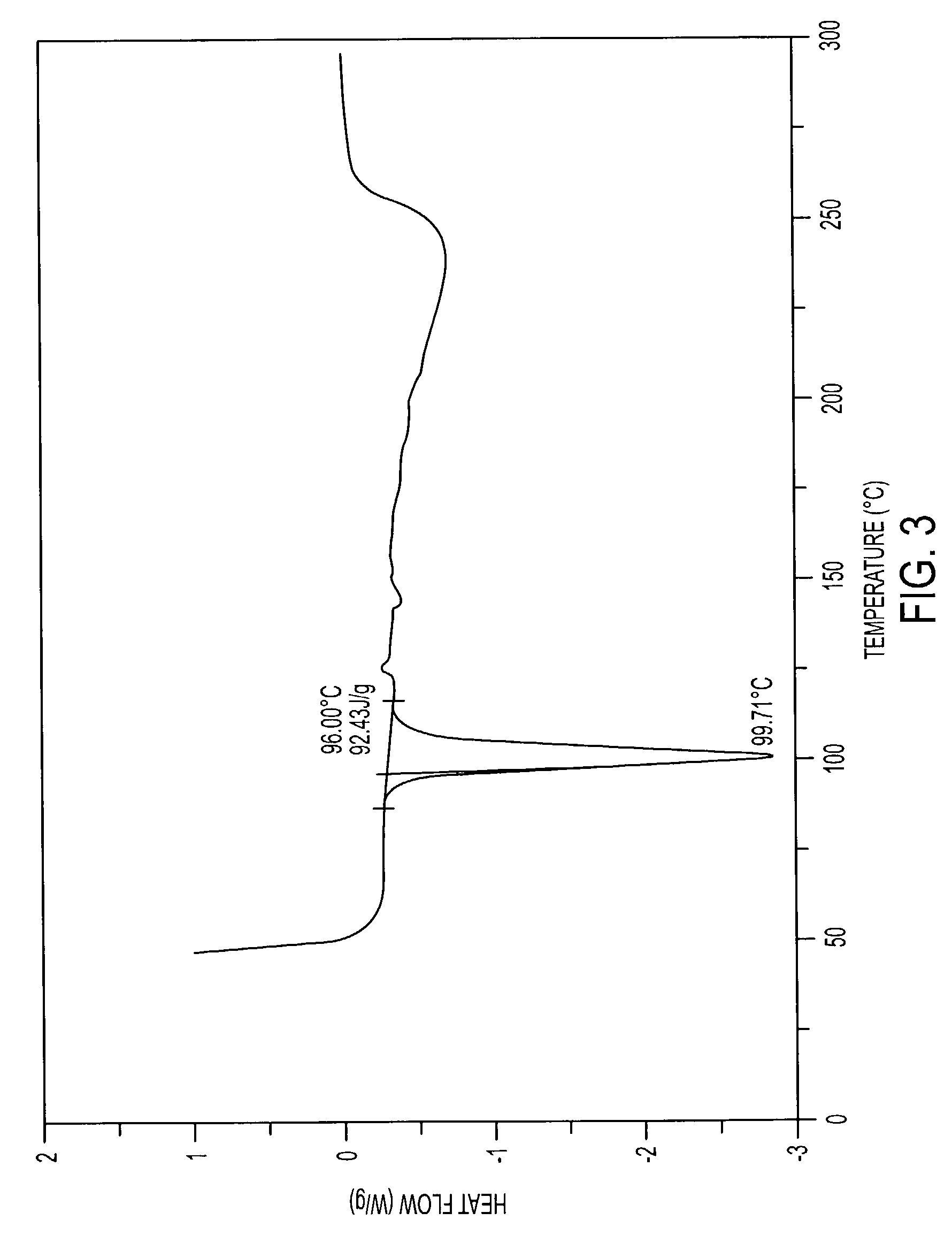
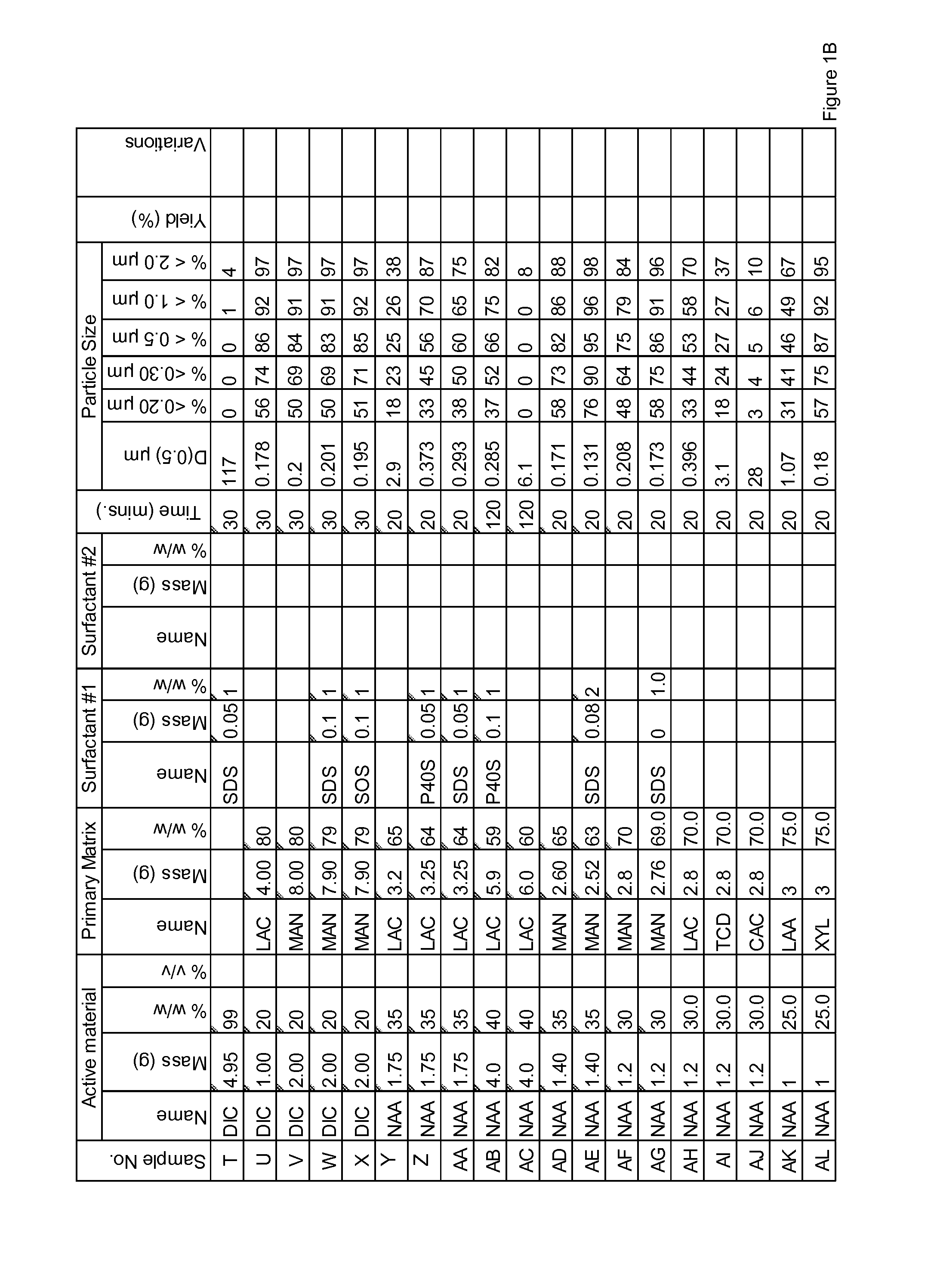
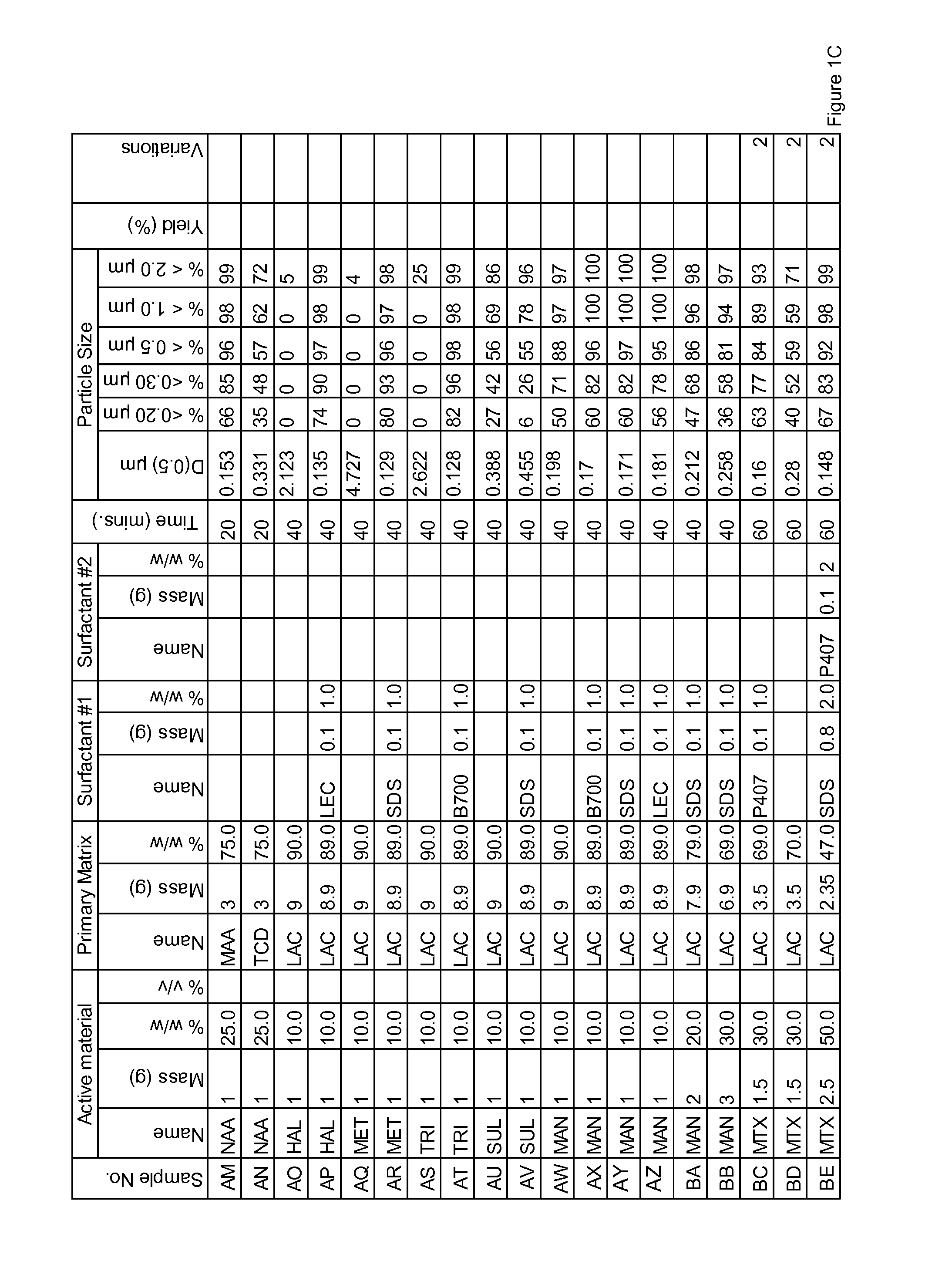
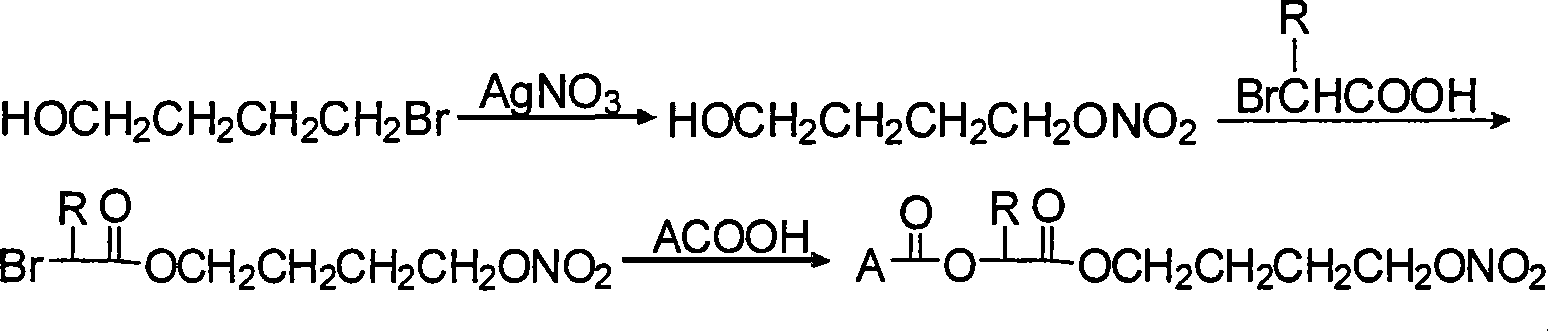Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
309 results about "Diclofenac" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Diclofenac is used to relieve pain and swelling (inflammation) from various mild to moderate painful conditions. It is used to treat muscle aches, backaches, dental pain, menstrual cramps, and sports injuries. It also reduces pain, swelling, and joint stiffness caused by arthritis.
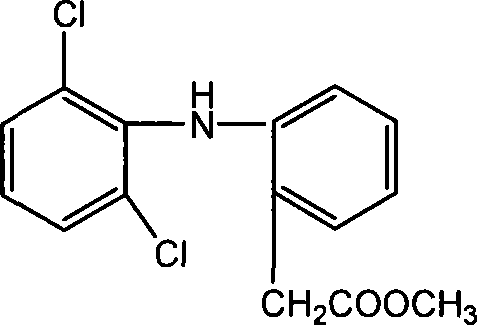
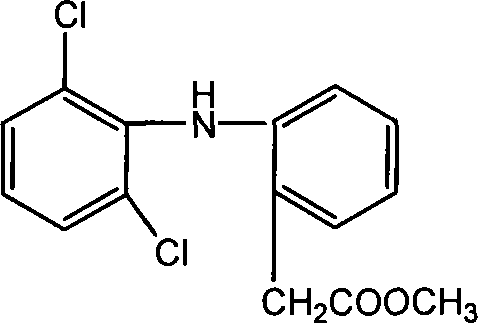
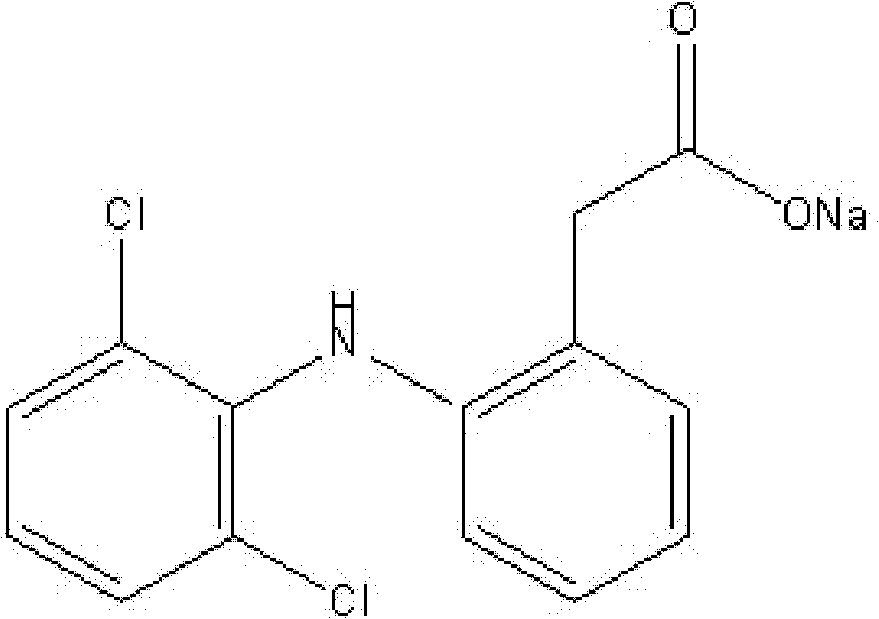
Methyl 2-(2-(2,6-dichlorophenylamino)phenyl)acetate and its synthesizing method and application
InactiveCN101186583AAnti-inflammatory in low dose groupLow analgesic effectOrganic active ingredientsAntipyreticSynthesis methodsChloride
The invention relates to a 2-(2-(2, 6-dichlorophenyl amido) phenyl) methyl acetate, relative synthesis method and application, belonging to the technical field of chemical pharmacy, which comprises that adds acid into diclofenac salt to acidify the salt into diclofenac, reacts diclofenac with methanol, or adds acid into diclofenac salt to acidify the salt into diclofenac, to be reacted with chloracetyl chloride to obtain 2-(2-(2, 6-dichlorophenyl amido) phenyl) methyl acetate. The inventive drug has significant anti-inflammatory and analgesic effects.
Owner:JILIN UNIV
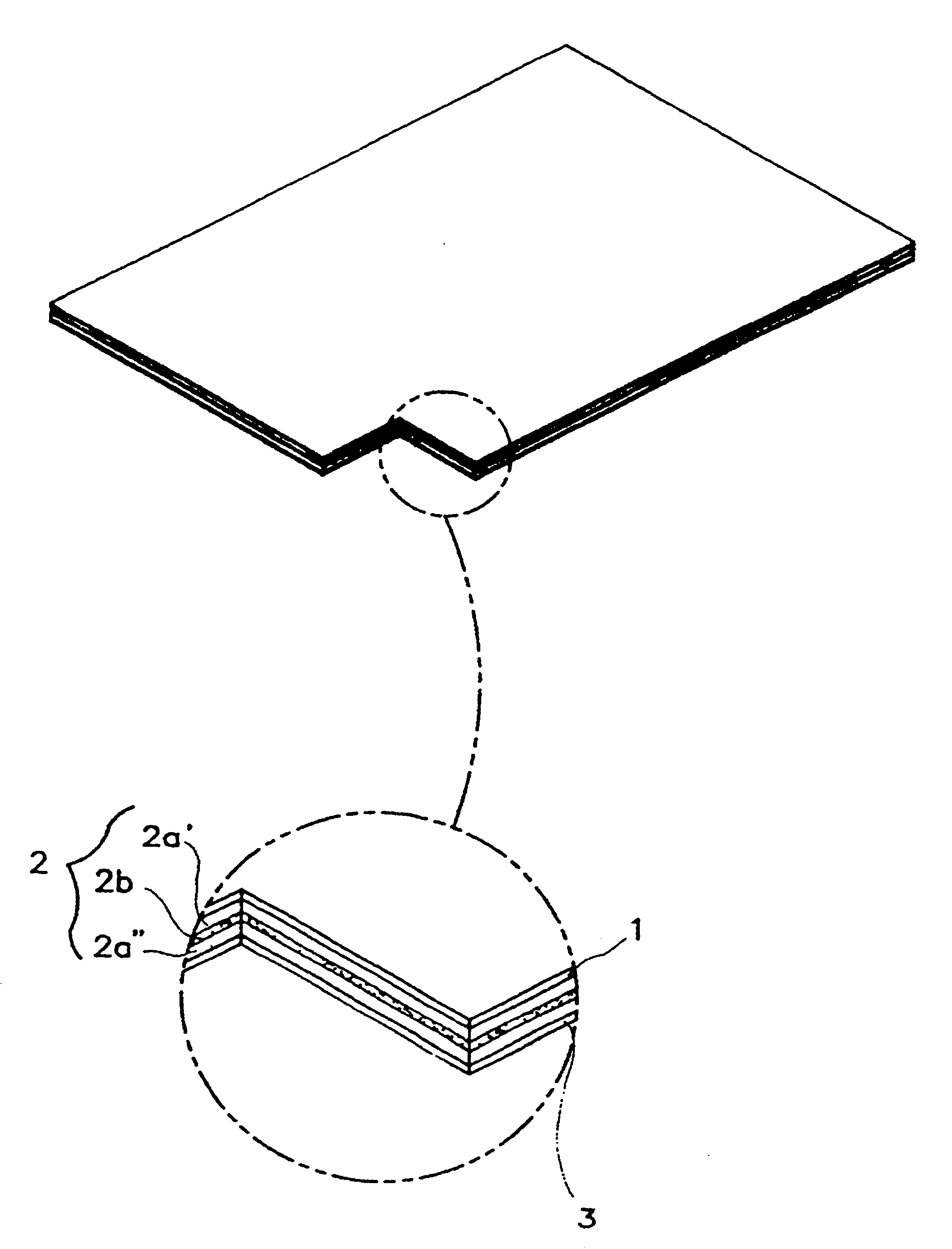
Transdermal drug delivery system for anti-inflammatory analgesic agent comprising diclofenac diethylammonium salt, and the manufacturing method thereof
InactiveUS6723337B1Improve solubilityPromote absorptionPowder deliveryPeptide/protein ingredientsAdditive ingredientDissolution
The invention herein relates to a transdermal drug delivery systern for anti-Inflammatory analgesic. agent comprising diclofenac diethylammonium salt, wherein a backing film (1), a matrix layer (2) containing active ingredients, a release liner (3) which is removed before application onto the skin are laminated therein. Mome particularly, the invention herein relates to a transdermal drug delivery system for anti-inflammatory analgesic agent comprising diclofenac diethylammonum salt, wherein the transdermal penetration and adhesion of the patch to the body are enhanced by means of a matrix layer which comprises a diclofenac diethylammonum salt as active ingredient in addition to acrylic polymer as adhesive constituent, non-ionic surfacant as absorption enhancer, terpene and dissolution assistant, and the volatile and non-volatile constituents of the composition arc separately applied therein for significantly reducing the manufacturing time thereof.
Owner:SAMYANG BIOPHARMLS CORP
Diclofenac gel
ActiveUS20080300311A1Extended drying timeHigh viscosityOrganic active ingredientsBiocideDiclofenac SodiumTopical treatment
The present invention provides a gel formulation comprising diclofenac sodium which has superior transdermal flux properties, which may be used for the topical treatment of pain, such as in osteoarthritis.
Owner:HORIZON THERAPEUTICS IRELAND DAC
Diclofenac compositions for the treatment of skin disorders
InactiveUS20050137164A1Readily availableSimilar and good performanceBiocideOrganic active ingredientsDiseaseActinic keratosis
Novel NSAID pharmaceutical compositions and methods for the treatment of skin disease and disorders such as actinic keratosis using same are disclosed.
Owner:AGIS INDUSTRIES (1983) LTD
Plaster for topical use containing heparin and diclofenac
Plaster for topical use having an analgesic activity and at the same time being able to re-absorb haematomas, comprising:a substrate layer;an adhesive layer in the form of a hydrogel matrix containing a pharmaceutically acceptable diclofenac salt, heparin or a heparinoid;a protective film which can be removed at the moment of use.
Owner:ALTERGON
Anti-Inflammatory and Antioxidant Conjugates Useful for Treating Metabolic Disorders
InactiveUS20100234452A1Improves anti-diabetic effectEliminate side effectsBiocideOrganic chemistryAntioxidantTriflusal
The present invention is directed to methods for treating metabolic disorders with compounds that are conjugates. The conjugates of the present invention are comprised of salicylic acid, triflusal, diflusinal, salsalate, IMD-0354, ibuprofen, diclofenac, licofelone, or HTB, and one or more antioxidants.
Owner:GENMEDICA THERAPEUTICS SL
Nutrigenomics methods and compositions
The present invention provides a proprietary compositions and systems to modulate genetic and metabolomic contributing factors affecting disease diagnosis, stratification, and prognosis, as well as the metabolism, efficacy and / or toxicity associated with specific vitamins, minerals, herbal supplements, homeopathic ingredients, and other ingredients for the purposes of customizing a subject's nutritional supplement formulation to optimize specific health outcomes. Specific to this invention the utilization of certain known polymorphic genes associated with Substance Use Disorder (SUD) are analyzed to target certain genetic anomalies that lead to a high risk and predisposition to SUD. The genotypic patterns are then utilized to provide certain nutritional customized solutions especially related to the attenuation of aberrant abuse of physician prescribed narcotic pain medication across all pain conditions. A priority GENOPROFILE is measured and directs the customization of a subsequent nutraceutical to act as a therapeutic modality. Specifically the treatment includes slow attenuation of the pain medication by incorporating orals (shakes, liquid beverages, pills, tablets, troche, ointments etc.), Intramuscular, Intravenous, intra-rectal and any form necessary to deliver a sufficient amount of an anti-craving and anti-stress nutraceutical. Moreover, the invention includes examples of novel analgesic ointments coupling Synaptamine and such analgesic and other anesthetic compounds including but not limited to Gabapentin, Ketamine, Baclofen, Ketoprofen, Amitriptyline, Lidocaine, Cyclobenzapine, Diclofenac, Menthol, Camphor and Capsaicin. The GENOPROFILE will be used to determine pain sensitivity Intolerance.
Owner:BLUM KENNETH +3
Stable injectable compositions
This invention relates to a stable parenteral aqueous solutions comprising either (a) diclofenac or a pharmaceutically acceptable diclofenac salt and a cyclodextrin, or (b) an inclusion complex of diclofenac or a pharmaceutically acceptable diclofenac salt and a cyclodextrin, or a mixture of (a) and (b), which are suitable for intramuscular and intravenous administration. The solutions contain diclofenac or diclofenac salt, cyclodextrin, and an antioxidant selected from monothioglycerol, or a combination of ethylene diamine tetra-acetic acid and N-acetyl-cysteine.
Owner:SHIMODA BIOTECH PTY LTD
Pain relief and soothing cream and method for making same
emollient and methyl salicylate, where said methyl salicylate comprises between 10 and 30 percent by weight of said cream; a skin stimulant comprising menthol crystals and two or more reactants selected from a group of camphor, cinnamomum cassia, diclofenac, eucalyptus, and ginger to cause a hot and cool stimulus on an epidermal surface; a pain reliever comprising two or more agents selected from a group of benzocaine, emu oil, eucalyptus oil, methylsulfonylmethane and glucosamine; and an inflammatory comprising two or more agents selected from a group of artemisia vulgaris, amica Montana flower extract, licorice root extract, oat extract, and thyme oil.
Owner:TRAN HUYNH D
Pharmaceutically acceptable salts containing local anesthetic and anti-inflammatory activities and methods for preparing the same
The present invention provides pharmaceutically acceptable salts having local anesthetic and anti-inflammatory activities. The preferred pharmaceutically acceptable salt is a diclofenac salt of lidocaine. Diclofenac is a non-steroidal anti-inflammatory drug (“NSAID”). Lidocaine is a local anesthetic. Other NSAID (except the salicylic acid derivatives of NSAID) can be used to replace diclofenac and / or other local anesthetics can be used to replace lidocaine. The pharmaceutically acceptable salts are crystalline compounds, which are distinctively different from either the NSAID alone or the local anesthetic alone, as indicated by differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), High Performance Liquid Chromatography (HPLC) and Fourier-Transformed Infrared Spectroscopy (FTIR) analyses. These pharmaceutically acceptable salts are suitable for use in topical treatment or parenteral injection to treat patients with localized pain, including muscle pain, joint pain, pain associated with herpes infection, and wound pain (such as surgical wound, burn wound etc.).
Owner:YUNG SHIN PHARMACEUTICALS INDUSTRIAL CO LTD
Novel formulation of diclofenac
ActiveUS20130209569A1Appropriate useEasy to separateBiocideOrganic active ingredientsParticulatesDiclofenac
Owner:ICEUTICA PTY LTD
Formulations Of Low Dose Diclofenac And Beta-Cyclodextrin
InactiveUS20070232566A1Good curative effectParticular value in treating pain in a horsePowder deliveryBiocideBeta-CyclodextrinsLow dose
The present invention is directed to a pharmaceutical composition containing a unit dose of a diclofenac compound effective to induce analgesia; and a beta-cyclodextrin compound; wherein the dose of the diclofenac compound is less than 10 mg. The present invention is also directed to methods of treating a subject in need of analgesia with the pharmaceutical compositions of the invention.
Owner:JAVELIN PHARMA INC +1
Novel formulation of diclofenac
ActiveUS20120135047A1Appropriate useEasy to separateOrganic active ingredientsPowder deliveryParticulatesTreated animal
The present invention relates to methods for producing particles of diclofenac using dry milling processes as well as compositions comprising diclofenac, medicaments produced using diclofenac in particulate form and / or compositions, and to methods of treatment of an animal, including man, using a therapeutically effective amount of diclofenac administered by way of said medicaments.
Owner:ICEUTICA PTY LTD
Diclofenac topical formulation
ActiveUS8252838B2Reduce deliveryConvenient timeOrganic active ingredientsBiocideDiclofenac SodiumTopical treatment
The present invention provides a gel formulation comprising diclofenac sodium which has superior transdermal flux properties, which may be used for the topical treatment of pain, such as in osteoarthritis.
Owner:HORIZON THERAPEUTICS IRELAND DAC
Compound transdermal patch used for curing acute and chronic inflammatory pain
InactiveCN101530401AImprove stabilityPromote absorptionOrganic active ingredientsAntipyreticTransdermal patchTreatment effect
The invention discloses a compound transdermal patch used for curing acute and chronic inflammatory pain. The patch comprises a compound penetrating agent and a pressure sensitive adhesive and is characterized by also comprising Xylocaine or hydrochloride thereof and sodium salt or sylvite of diclofenac. The transdermal patch contains the following components by weight percentages: 1-20 percent of Xylocaine or the hydrochloride thereof, 1-20 percent of the sodium salt or sylvite of diclofenac, 1-30 percent of compound penetrating agent and 40-49 percent of pressure sensitive adhesive. Aiming at overcoming the defects of the existing medicinal preparations in treatment and use, the invention provides a compound transdermal patch that is used for curing acute and chronic inflammatory pain, can act on local human body and quickly permeate, and is good in treatment effect, safe and convenient in use.
Owner:中山健康基地孵化器管理有限公司
Method, composition and kit for treating frequent headaches
InactiveUS20140163072A1Avoid nasal cavity ' damageEffective compositionBiocidePeptide/protein ingredientsAnesthetic AgentSphenopalatine Ganglion Block
Methods, compositions and kits for treating a headache in a human patient are provided. The methods comprise intranasally administering to the patient a composition comprising a short-acting local anesthetic, a long-acting local anesthetic and a non-steroidal anti-inflammatory drug. A composition useful for practicing the methods of the disclosure is described which comprises a short-acting local anesthetic, a long-acting local anesthetic and a non-steroidal anti-inflammatory drug, wherein the composition is formulated for intranasal delivery. A kit comprising the composition and an intranasal applicator and a method of systemically delivering the composition to a human patient is also included in the disclosure. The headache-treating effectiveness of a local anesthetic in a Sphenopalatine Ganglion Block (SFGB) is significantly enhanced by coadministering diclofenac.
Owner:RAMON DE JESUS JOSE C
Methods of treating acute pain using diclofenac
New pharmaceutical compositions for oral use containing diclofenac preferably together with alkali metal bicarbonates in amounts of from about 20 to about 80 by weight with respect to diclofenac are described. These compositions are entirely palatable and free from any unpleasant taste or other, side effects; in particular, these formulations permit to obtain in human patients higher Cmax of the active principle and shorter Tmax together with a lower coefficient of variation.
Owner:KOWA PHARMA AMERICA
Pharmaceutical compositions and methods of treatment based on diclofenac
New pharmaceutical compositions for oral use containing diclofenac together with alkali metal bicarbonates in amounts of from 20 to 80 by weight with respect to diclofenac are described. These compositions are entirely palatable and free from any unpleasant taste or other, side effects; in particular, these formulations permit to obtain in human patients higher Cmax of the active principle and shorter Tmax together with a lower coefficient of variation.
Owner:KOWA PHARMA AMERICA
Diclofenac sodium sustained-release tablet and its preparation process
ActiveCN102274200AAvoid condensationReduce usageOrganic active ingredientsAntipyreticDiclofenacAdhesive
The invention relates to the technical field of medicine, in particular to diclofenac sodium sustained release tablets and a preparation process thereof. The diclofenac sodium sustained release tablets provided by the invention comprise the following components in percentage by mass: 16.5 to 39.0 percent of diclofenac sodium, 10.0 to 35.5 percent of sustained release agent, 33.5 to 65.0 percent of filling agent, 2.01 to 8.0 percent of glidant, 0 to 2.5 percent of lubricating agent, and 0 to 8.0 percent of adhesive. According to the diclofenac sodium sustained release tablets and a whole-powder direct tabletting method thereof provided by the invention, by changing the components and the preparation method, the production process is simplified, the production cost is reduced, the production efficiency is improved, the yield is improved to 98.0 to 100 percent, granule condensation is avoided, the surface of the tablets is smooth, meanwhile, consumption of high-concentration alcohol is avoided, and the potential safety hazard in the production process is reduced.
Owner:SINOPHARM GRP ZHIJUN SHENZHEN PINGSHAN PHARMA CO LTD
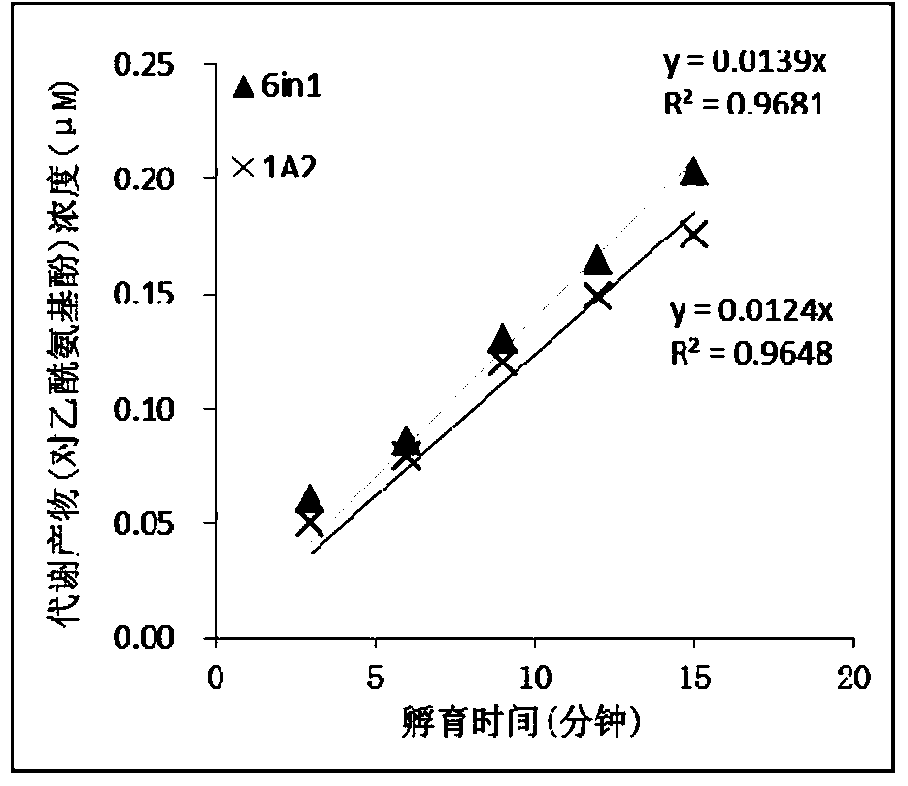
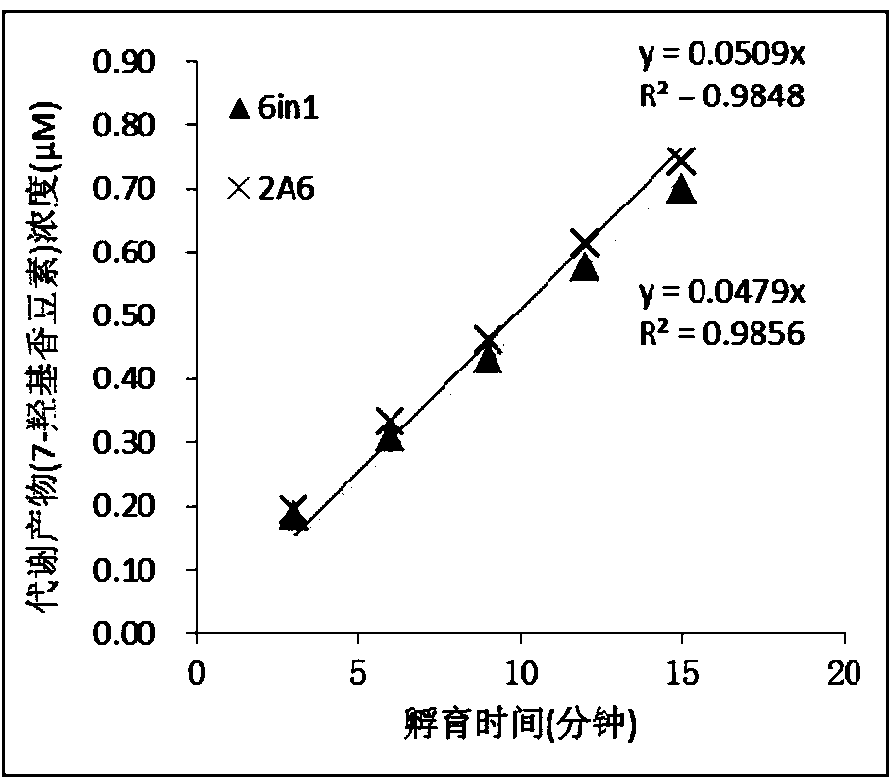
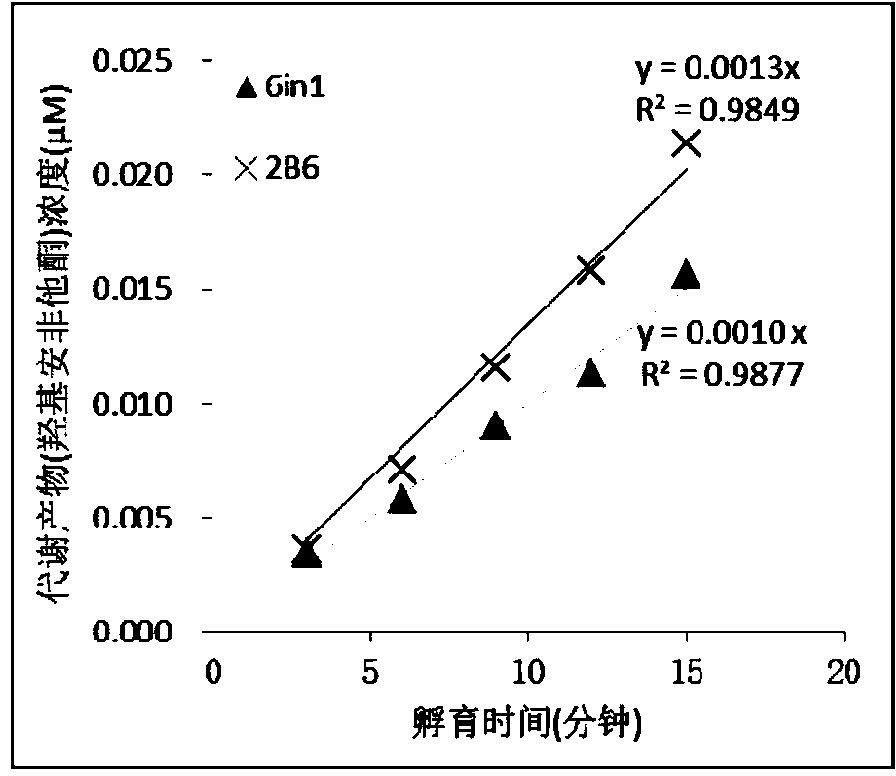
Specific probe substrate composition of cytochrome P450 enzyme and application of specific probe substrate composition
InactiveCN104195218AStrong specificityReduce distractionsComponent separationMicrobiological testing/measurementLiquid chromatography mass spectroscopyIonic Channels
The invention relates to a specific probe substrate composition of cytochrome P450 (CYP450) enzyme and an application of the specific probe substrate composition in subtype enzyme activity detection of the cytochrome P450 enzyme. The composition comprises at least two of phenacetin, coumarin, amfebutamone, dextromethorphan, diclofenac and testosterone. The characteristics of multi-ion channels can be simultaneously detected by the specific probe substrate composition and a liquid chromatography-mass spectrometry technology, and the inhibition conditions of six CYP450 enzymes are determined simultaneously, so that the experiment flux efficiency is greatly improved, and the experiment cost is reduced.
Owner:广东中西达一新药开发有限公司
Carbon dot modified oxygen-doped carbon nitride photocatalyst as well as preparation method and application thereof
InactiveCN108993561AThe synthesis method is simpleLow costPhysical/chemical process catalystsWater/sewage treatment by irradiationWater bathsCatalytic effect
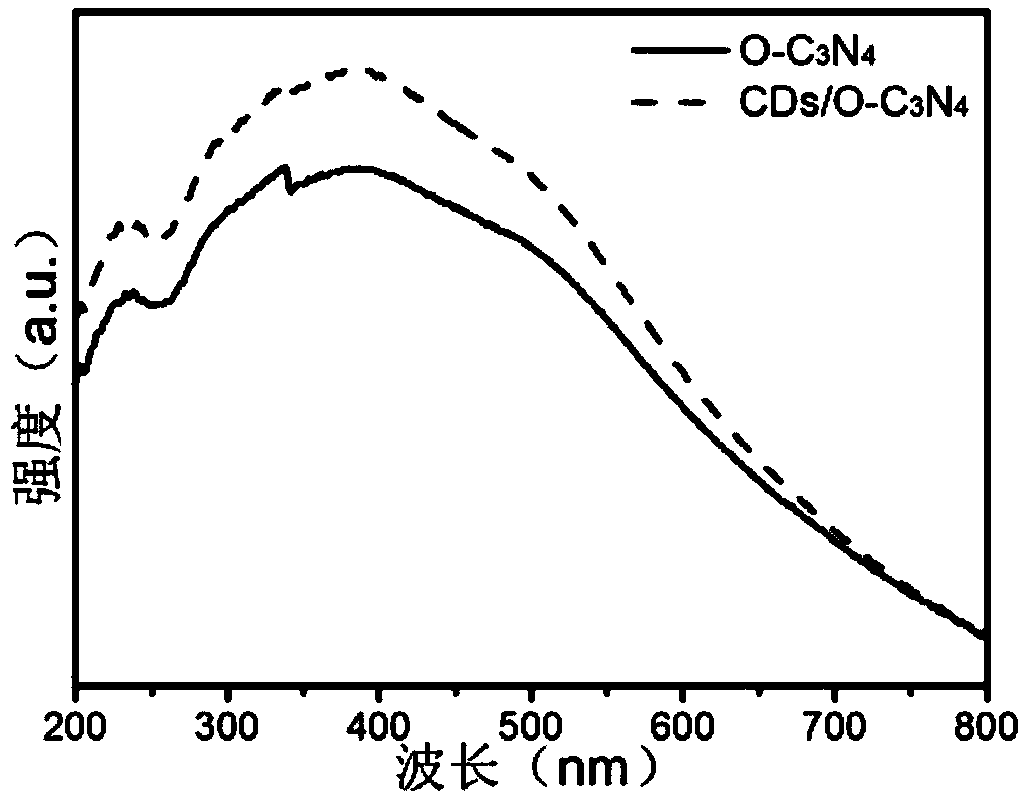
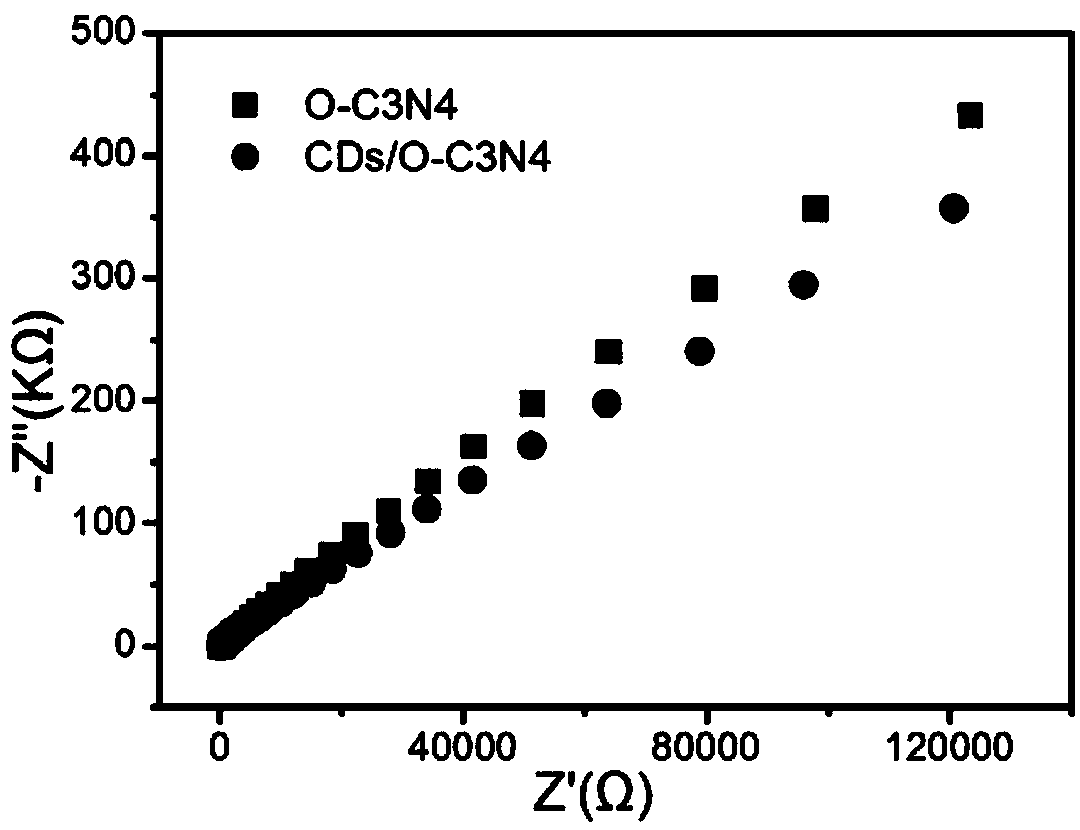
The invention belongs to the technical field of photocatalysis and discloses a carbon dot modified oxygen-doped carbon nitride photocatalyst as well as a preparation method and an application thereof.The method comprises steps as follows: citric acid and urea are added to ultrapure water and subjected to reaction at 170-190 DEG C after being ultrasonically dissolved, a mixed solution is cooled toroom temperature, centrifuged, dried and subjected to constant-volume treatment with the ultrapure water, and a CDs solution is obtained; semicarbazide hydrochloride is subjected to calcining reaction at 540-560 DEG C, cooled, ground and sieved, and O-C3N4 is obtained; O-C3N4 is added to a crucible, the ultrapure water and ethanol are added, then the CDs solution is added, after ultrasonic dissolving, water-bath heating and stirring are performed until the mixture is dry, the calcining reaction is performed at 280-320 DEG C, a product is cooled, ground and sieved, and the photocatalyst is obtained. With the adoption of a thermo polymerization method, the synthetic process is simple, repeatability is good, and basic conditions for large-scale production are provided; the CDs in the photocatalyst can convert long-wavelength light into short-wavelength light, the catalytic effect is improved, and diclofenac can be degraded under different light sources.
Owner:GUANGDONG UNIV OF TECH
Topical formulation having effects on alleviating pain/inflammation caused by herpes virus infection
InactiveUS7132452B2Fasten skin recoveryPromote recoveryPowder deliveryBiocideComplete remissionTolmetin
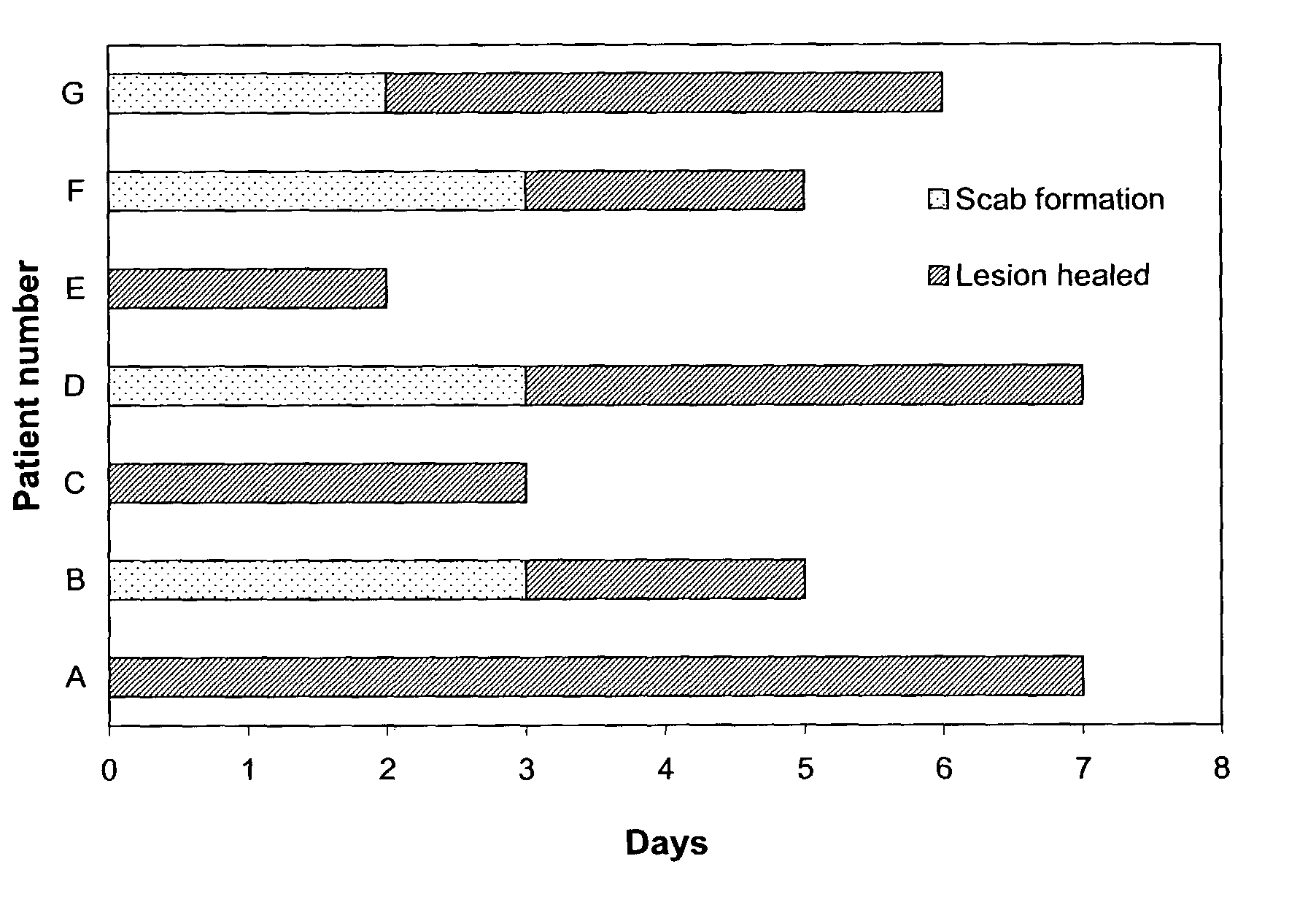
The present invention provides a topical formulation containing NSAID, particularly diclofenac. The topical formulation is particularly useful for alleviating pain / inflammation associated with infection caused by herpes virus, especially herpes simplex virus (HSV) and varicella-zoster virus (VZV). Similar relief can be achieved where diclofenac is replaced with another non-steroidal anti-inflammatory drug (NSAID), which includes, without limitation, etodolac, ketorolac, bromfenac, diflunisal, ibuprofen, fenoprofen, ketoprofen, naproxen, suprofen, meclofenamate, mefenamic acid, piroxicam, meloxicam, indomethacin, sulindac, phenylbutazone, oxyphenbutazone, and tolmetin. The topical formulation is further characterized by its fast relief on pain and / or inflammation associated with infection caused by herpes virus, i.e., a complete relief in no more than seven (7) days after the application of the topical formulation on skins of patients.
Owner:YUNG SHIN PHARMACEUTICALS INDUSTRIAL CO LTD
Micro-nano heterojunction visible light composite photocatalyst, preparation method and applications thereof
InactiveCN106944043AHigh absorption of visible lightImprove photocatalytic performanceWater/sewage treatment by irradiationWater treatment compoundsMicro nanoHeterojunction
The invention relates to a micro-nano heterojunction visible light composite photocatalyst, a preparation method and applications thereof, wherein the micro-nano heterojunction visible light composite photocatalyst is CuBi2O4 / Ag3PO4, the CuBi2O4 in the micro-nano heterojunction visible light composite photocatalyst is a micrometer structure, the Ag3PO4 is a nanometer structure, and the CuBi2O4 closely contacts the Ag3PO4 to form the heterojunction. Compared to the pure Ag3PO4, the prepared micro-nano heterojunction visible light composite photocatalyst has advantages of high visible light absorption intensity, good photocatalytic performance, strong light corrosion resistance, and the like. According to the present invention, after organic wastewater containing difficultly degraded diclofenac is treated with 0.5 g of the CuBi2O4 / Ag3PO4 (1:1, wt%) prepared by using sodium stearate as the additive, the removal rate of 1 L of a 10 mg / L diclofenac solution is 88.73% after 240 min, and the removal rate of the diclofenac solution is 73.69% with the CuBi2O4 / Ag3PO4 being repeatedly used 5 times.
Owner:FOSHAN UNIVERSITY
Compound paracetamol injection for livestock and process for preparing same
InactiveCN101015542AImprove survival rateIncrease weightOrganic active ingredientsAntipyreticDiclofenac SodiumPolyethylene glycol
The invention discloses a compound acetaminophen injection for livestock and its preparation method. the injection comprises acetaminophen 50-200, diclofenac sodium 10-50, polyethylene glycol-400 100-500, propylene glycol 100-500, and sodium bisulfite 10-30. the preparation method comprises (1) mixing polyethylene glycol-400 and propylene glycol, heating to 70-80deg.C, adding diclofenac sodium, stirring, adding acetaminophen, stirring; (2) heating water for injection to 30-40deg.C, adding sodium bisulfite, stirring; and (3) merging the solutions of steps 1 and 2, adjusting pH to 4.5-6.5 with hydrochloric acid or sodium hydroxide, and adding water for injection to 1000 to obtain the final product. The invention strengthen antipyretic and analgesic effects, is effective for treating pain and fever due to different kinds of inflammation, and can improve animal survival rate and increase animal body weight.
Owner:TIANJIN SHENGJI GRP CO LTD
Topical formulation including diclofenac, or a pharmaceutically acceptable salt thereof
InactiveUS7795309B2Improve throughputOrganic active ingredientsBiocideSODIUM LAURYL SULFOACETATEMethyl laurate
There is described a topical formulation. The topical formulation comprises: (i) diclofenac or a pharmaceutically acceptable salt thereof, (ii) a first compound, and (iii) a second compound. The first compound and second compound are different, and each is selected from the group consisting essentially of N-lauroyl sarcosine, sodium octyl sulfate, methyl laurate, isopropyl myristate, oleic acid, glyceryl oleate and sodium lauryl sulfoacetate. It has been discovered that certain combination of compounds are excellent penetration enhancers and, as such, can be incorporated in a topical formulation to facilitate administration of diclofenac or a pharmaceutically acceptable salt thereof. The increased penetration enhancement can also lead to a reduction in the total concentration of skin irritants in the formulation.
Owner:CRESCITA THERAPEUTICS INC
Freeze dry preparation containing diclofenac salt and lidocaine and its preparation method
InactiveCN1689561AReduce volumeSolve solubilityOrganic active ingredientsPowder deliveryOrganic solventAlcohol
The freeze dried powder for injection containing diclofenac salt and lidocaine is prepared with Tween-80, pharmaceutically acceptable solution pH regulator, solution of diclofenac salt and lidocaine of pH 7.0 and in effective treating amount, and through freeze drying. The solution may contain other pharmaceutically acceptable supplementary material. The preparation of the present invention has stable performance, easy transportation, long storage period, and no bad reaction caused by alcohol and other organic solvent.
Owner:BEIJING SIHUAN KEBAO PHARM CO LTD
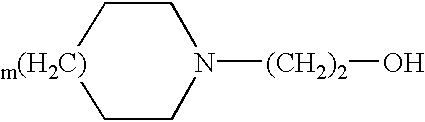
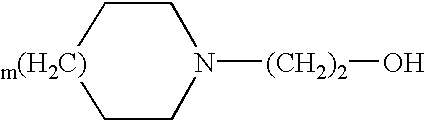
Transdermal delivery system of diclofenac with improved water absorbability and adhesion properties
InactiveUS7691404B2Improve suitabilityExcellent water absorbabilityHydroxy compound active ingredientsInorganic non-active ingredientsActive agentAdhesive
Owner:SAMYANG BIOPHARMLS CORP
Method for removing diclofenac contained in sewage by utilizing copper-iron heterogeneous fenton technology
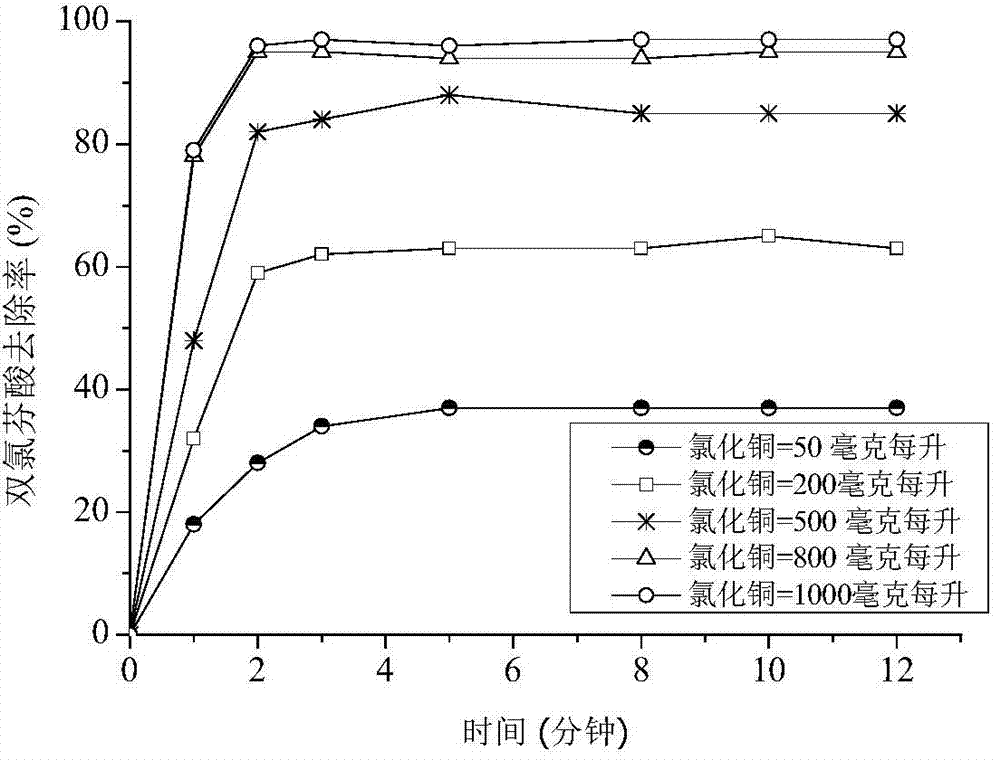
InactiveCN103787484ASimple reaction conditionsGood removal effectWater contaminantsWater/sewage treatment by oxidationReaction temperatureRates reactions
The invention relates to a method for removing diclofenac contained in sewage by utilizing a copper-iron heterogeneous fenton technology. The method comprises the following steps: adding bivalent copper salt, nanometer zero-valent iron and hydrogen peroxide to the sewage which contains the diclofenac under the condition that a pH value is 3-5, reacting for 3-12 minutes, oxygenizing to remove the diclofenac contained in the sewage, wherein the mass concentration of the diclofenac contained in the sewage is 1-50 milligrams / litre, the addition amount of the bivalent copper salt added to every litre of the sewage is 50-1000 milligrams, the addition amount of the nanometer zero-valent iron added to every litre of the sewage is 0.5-3.0 grams, and the addition amount of the hydrogen peroxide added to every litre of the sewage is 400-1000 milligrams. Compared with the prior art, the method disclosed by the invention has the advantages that the bivalent copper salt, the nanometer zero-valent iron and the hydrogen peroxide are added to waste water which contains the diclofenac and a bimetal system can promote the velocity of Fenton reaction because the electron affinity of Cu is relatively higher, thereby accelerating the reaction velocity; the method disclosed by the invention can achieve very good removal effect at normal temperature and pressure because the reaction temperature is unlimited and achieve high removal rate on the diclofenac without secondary pollution and is favorable to the recycling of the sewage.
Owner:TONGJI UNIV
Nonsteroidal antiinflammatories with nitric oxide donors and its preparation method
InactiveCN101053662ALittle side effectsMild reaction conditionsAntipyreticAnalgesicsIndometacinSide effect
The invention relates to a non-steroidal anti-inflammatory drug with nitrogen oxide donor and a method for preparing same, which can be used to eliminate inflammation, relieve fever and stop pain, and can decrease the frequently seen side effect thereof on the gastrointestinal tract. The structure of which is A-O(X)-CO-O(y)-B-ONO2,wherein, A is the non-steroidal anti-inflammatory group; B is the connection group, when x=0, y=1; when x=1 then y=0. The the non-steroidal anti-inflammatory groups includes aspirin, diclofenac, indometacin, lumiracoxib, brufen, ketoprofen, naproxen, piroxicam, and meloxicam. The preparation process are that the bromhydrin (or hydroxybenzene) reacts with silver nitrate into hydroxy nitrate, then reacts with bromo acid into the connection group of nitrogen oxide donor, then connects with the non-steroidal anti-inflammatory drug; or the non-steroidal anti-inflammatory drug condensates with bromo acid into a bromide intermediate, then reacts with silver nitrate into the non-steroidal anti-inflammatory drug with nitrogen oxide donor.
Owner:江苏吴中苏药医药开发有限责任公司
Injectable Preparations Of Diclofenac And Its Pharmaceutically Acceptable Salts
ActiveUS20080153914A1Relieve painHigh viscosityOrganic active ingredientsBiocideInjection siteIntravenous route
The present invention provides injectable formulations of water-soluble salts of diclofenac in single doses of less than 2 ml, which cause significantly less pain at the site of injection and can be administered by intradeltoid route, in addition to intragluteal and slow intravenous route. More specifically the injectable preparations contain 75 mg to 100 mg of water-soluble salts of diclofenac, in about 1 ml injection solution without significantly raising the viscosity of the injection solution without the use surfactants. The formulations are adjusted to pH 6 to 10 containing up to 100 mg of diclofenac salt in a medium comprising of water, along with one or more co-solvent(s) / solubiliser(s), antioxidants, preservatives, buffers, alkali and stabilizers.
Owner:TROIKAA PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com