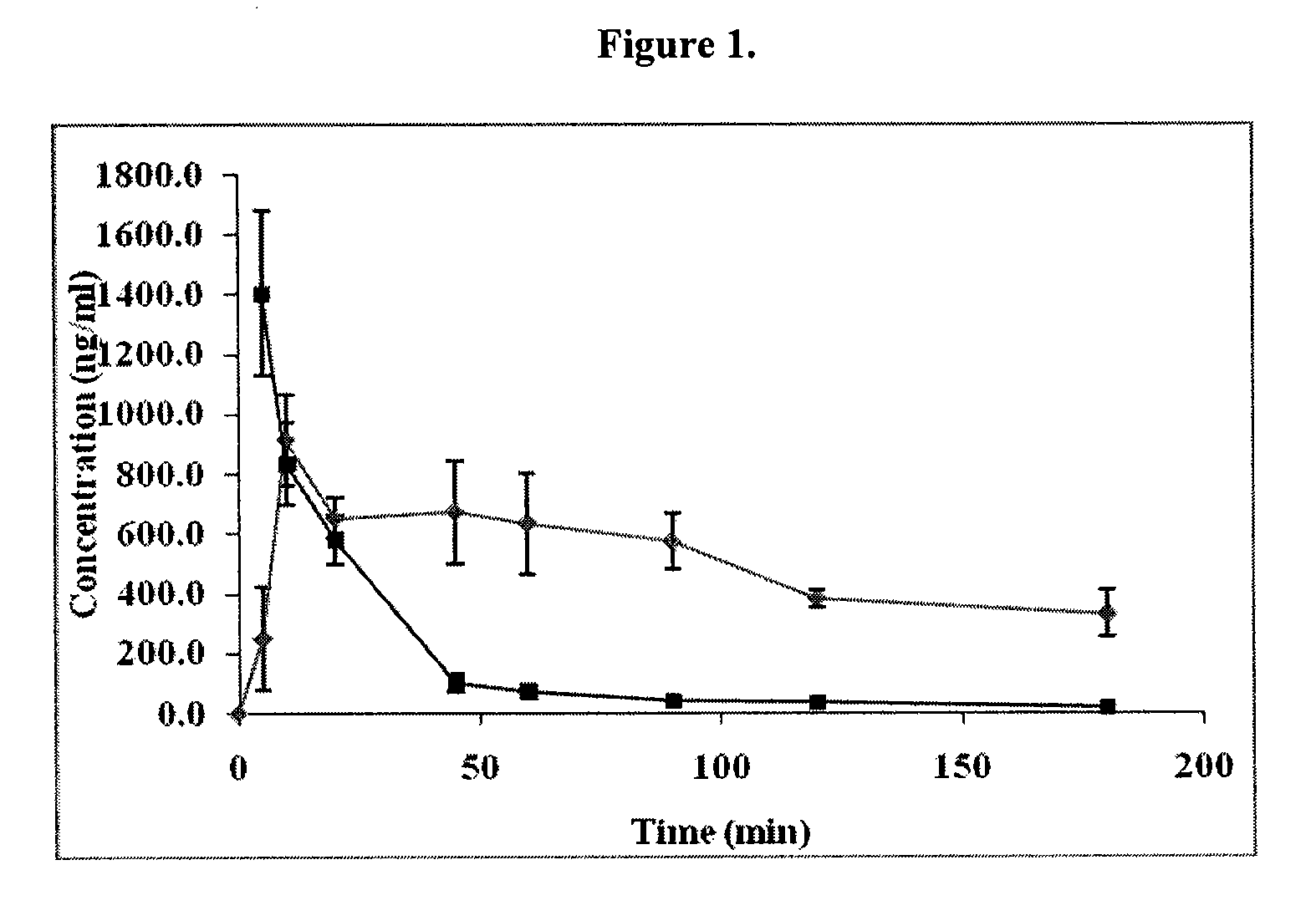
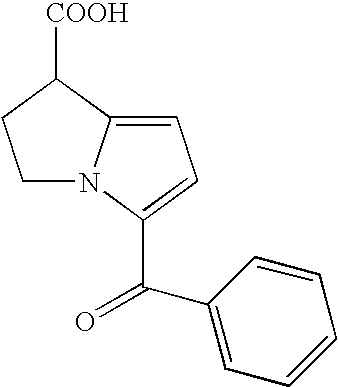
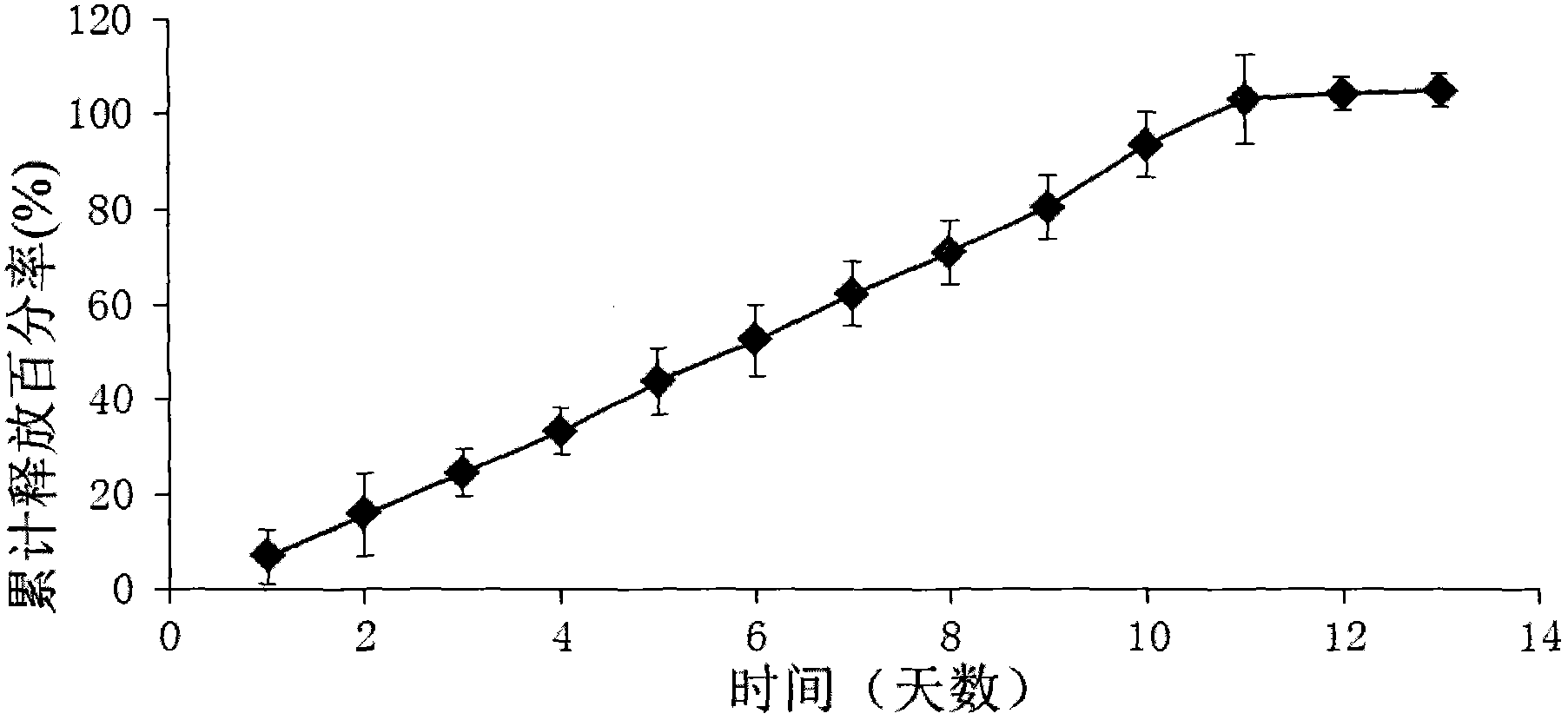
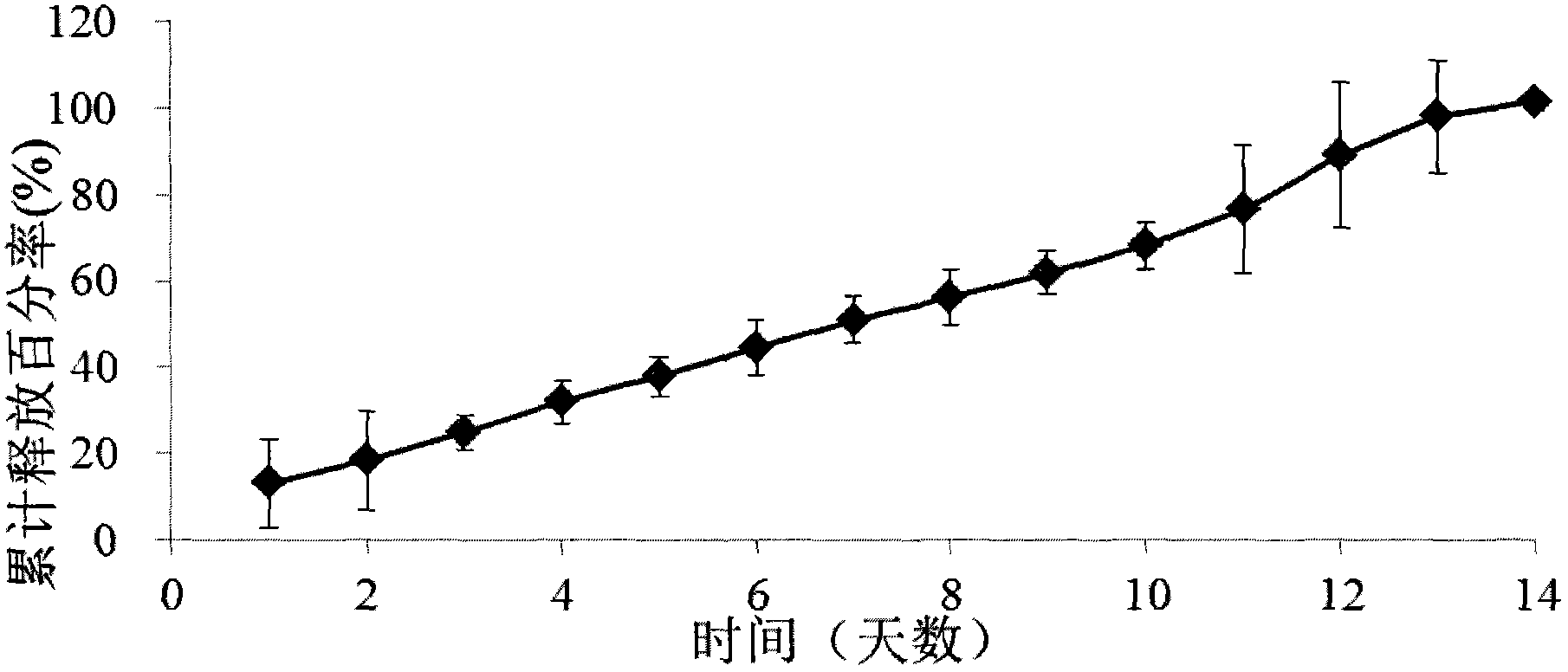
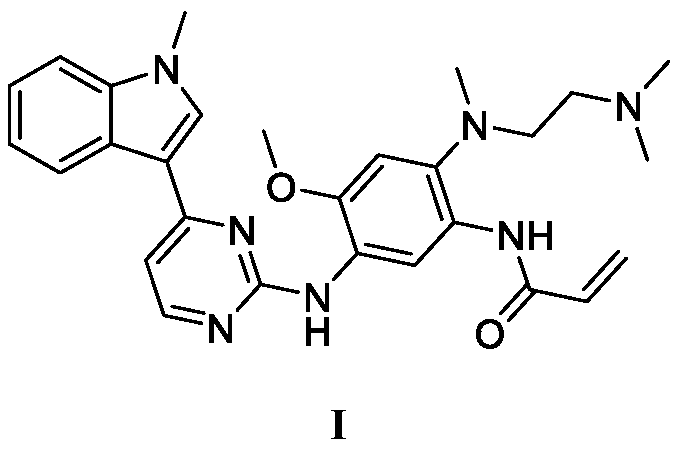
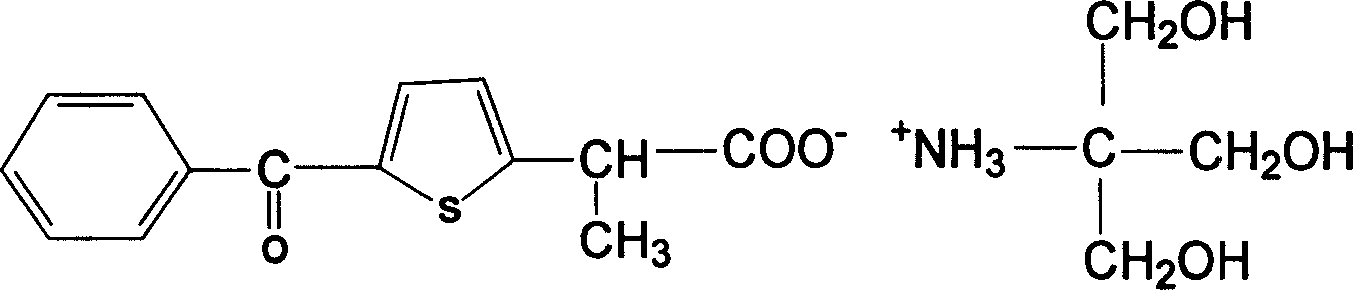
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
70 results about "Ketorolac" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Ketorolac is used for the short-term treatment of moderate to severe pain in adults. It is usually used before or after medical procedures or after surgery.
Injectable long-acting analgesic composition comprising an ester derivative of ketorolac
InactiveUS20060183786A1Good anti-inflammatory effectLong duration of actionBiocideAntipyreticKetorolacAryl radical
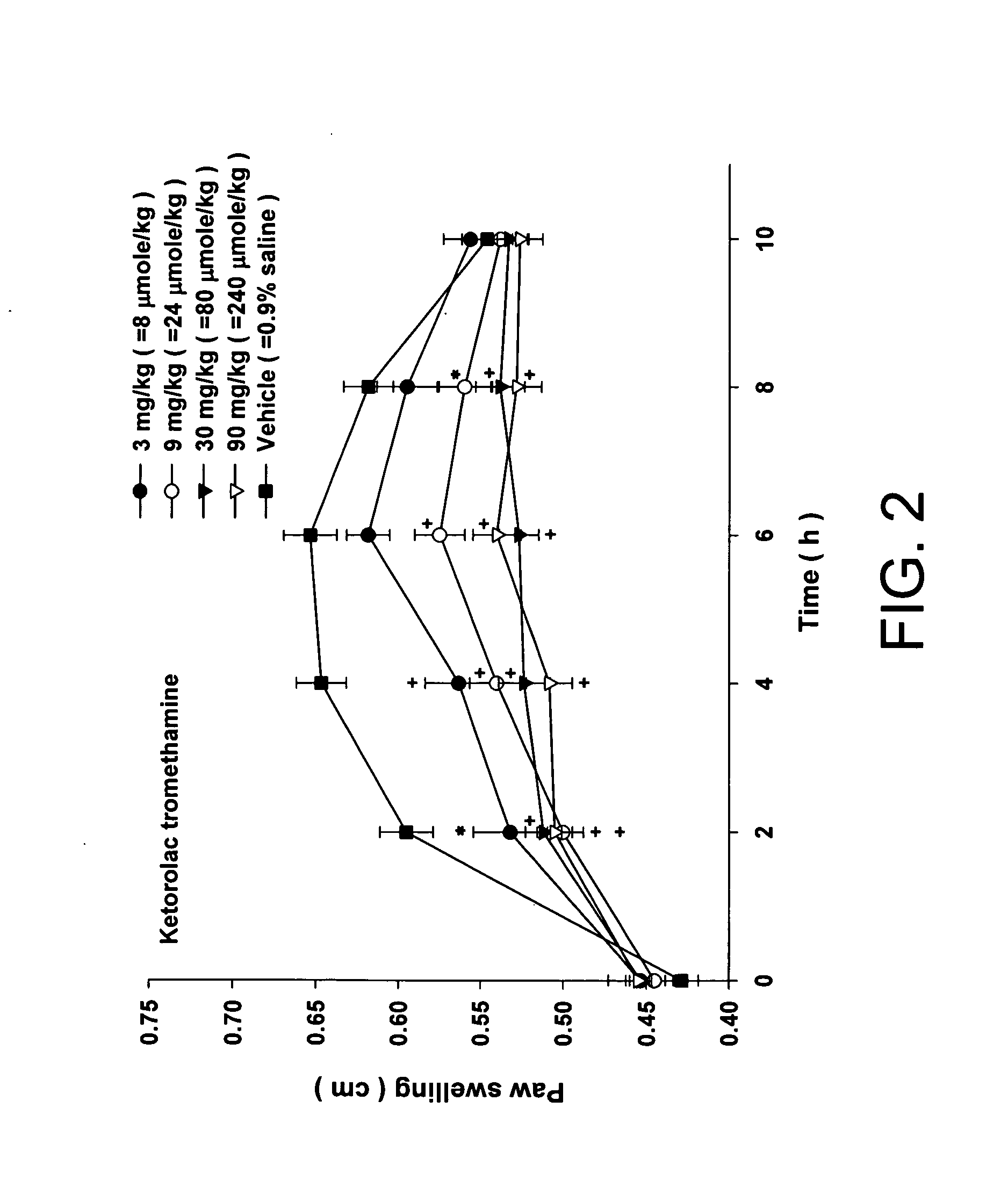
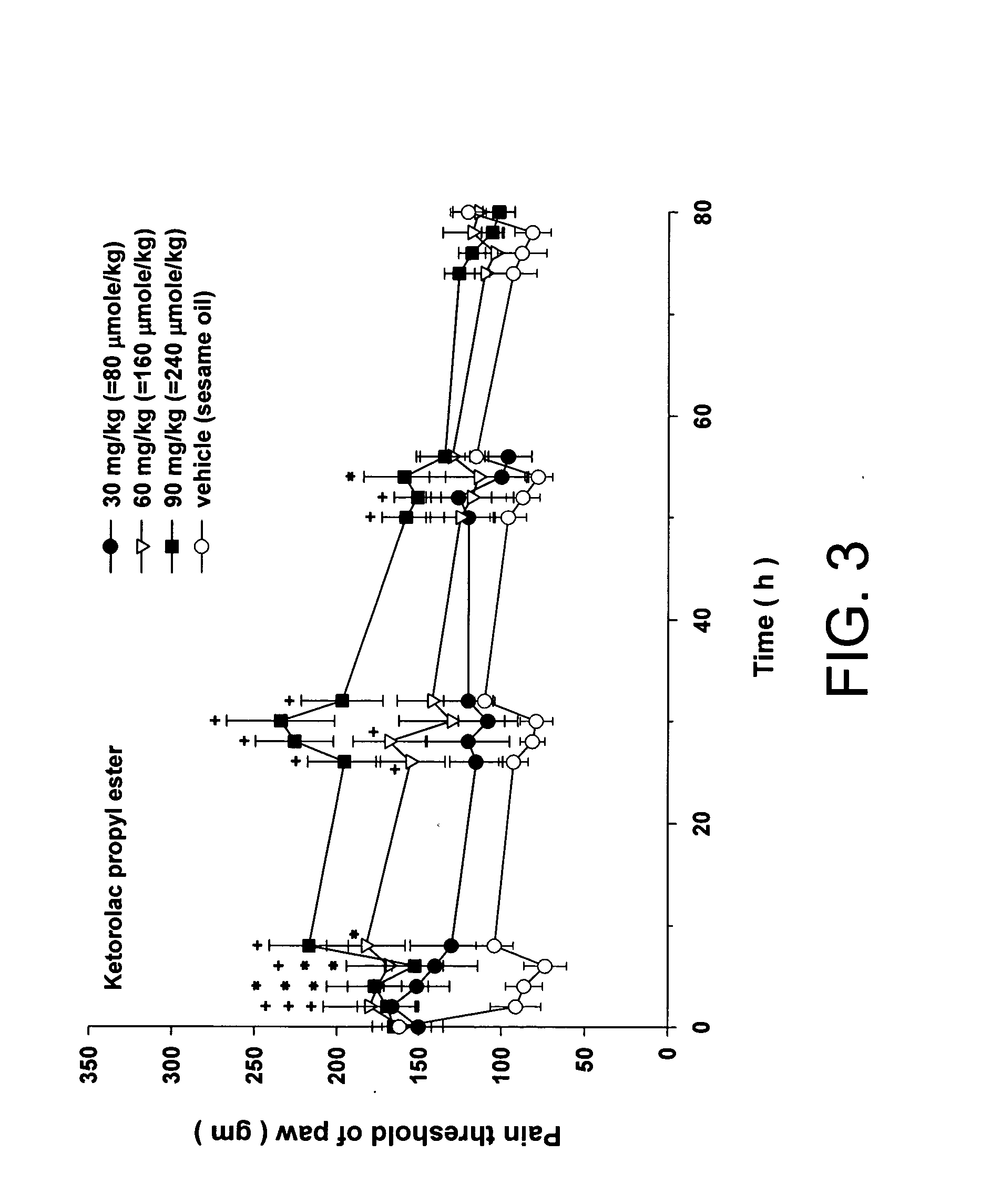
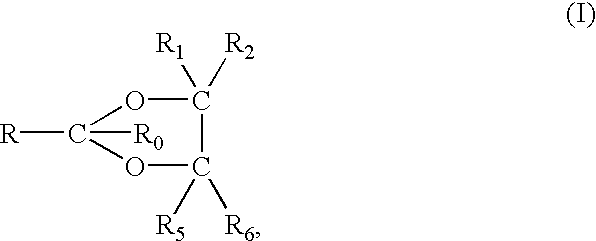
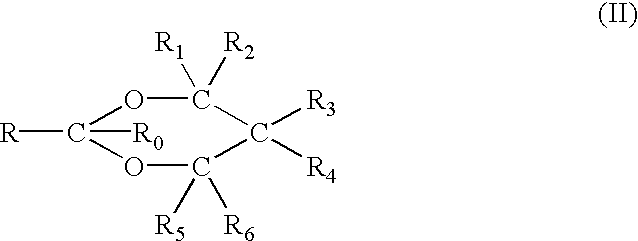
Disclosed herein is an injectable long-acting analgesic composition comprising: (a) a ketorolac ester derivative of formula (I), wherein R is a straight-chain or branched saturated or unsaturated C1-C20 aliphatic group optionally substituted with a C6-C10 aryl group; and (b) a pharmaceutically acceptable oil vehicle. The composition can provide a longer duration of action and, therefore, is suitable for use in the treatment of long-lasting pains and inflammations.
Owner:CHI MEI MEDICAL CENT
Non-steroidal antiinflammatory drug formulations for topical application to the skin
InactiveUS20030082226A1Improve performanceSignificant positive effectOrganic active ingredientsAntipyreticSkin penetrationAntiinflammatory drug
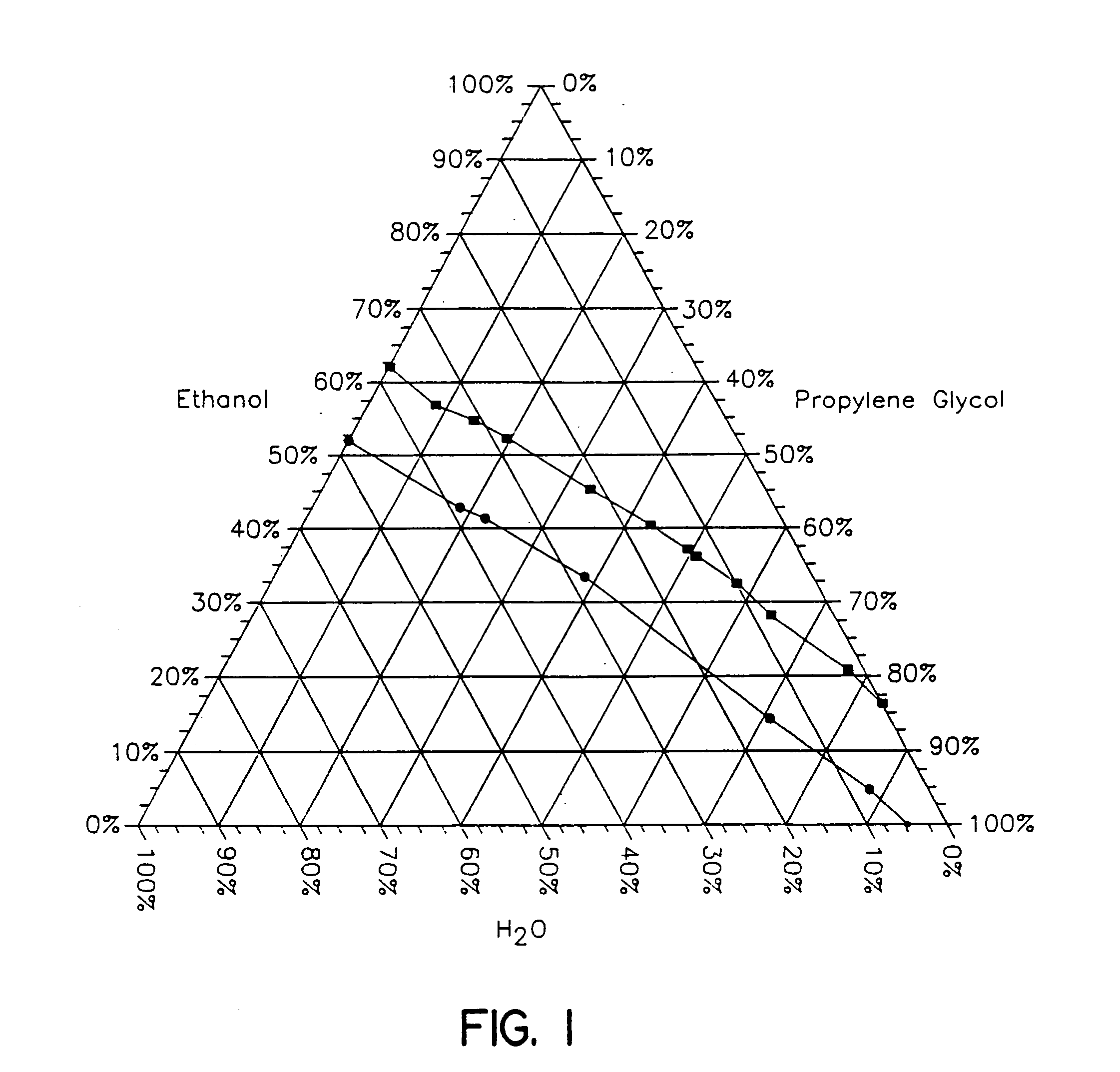
Topical alcoholic or aqueous alcoholic gels containing ibuprofen or other NSAIDs, such as, naproxen, in substantially neutral salt form, have enhanced penetration through skin and may provide rapid pain / inflammation relief by including in the formulation 2-n-nonyl-1,3-dioxolane or other hydrocarbyl derivative of 1,3-dioxolane-or 1,3-dioxane or acetal, as skin penetration enhancing compound. The amount of propylene glycol may be varied to adjust the initial flux of the NSAID through the skin, especially for ibuprofen, naproxen, and ketorolac.
Owner:SAMOUR CARLOS M +2
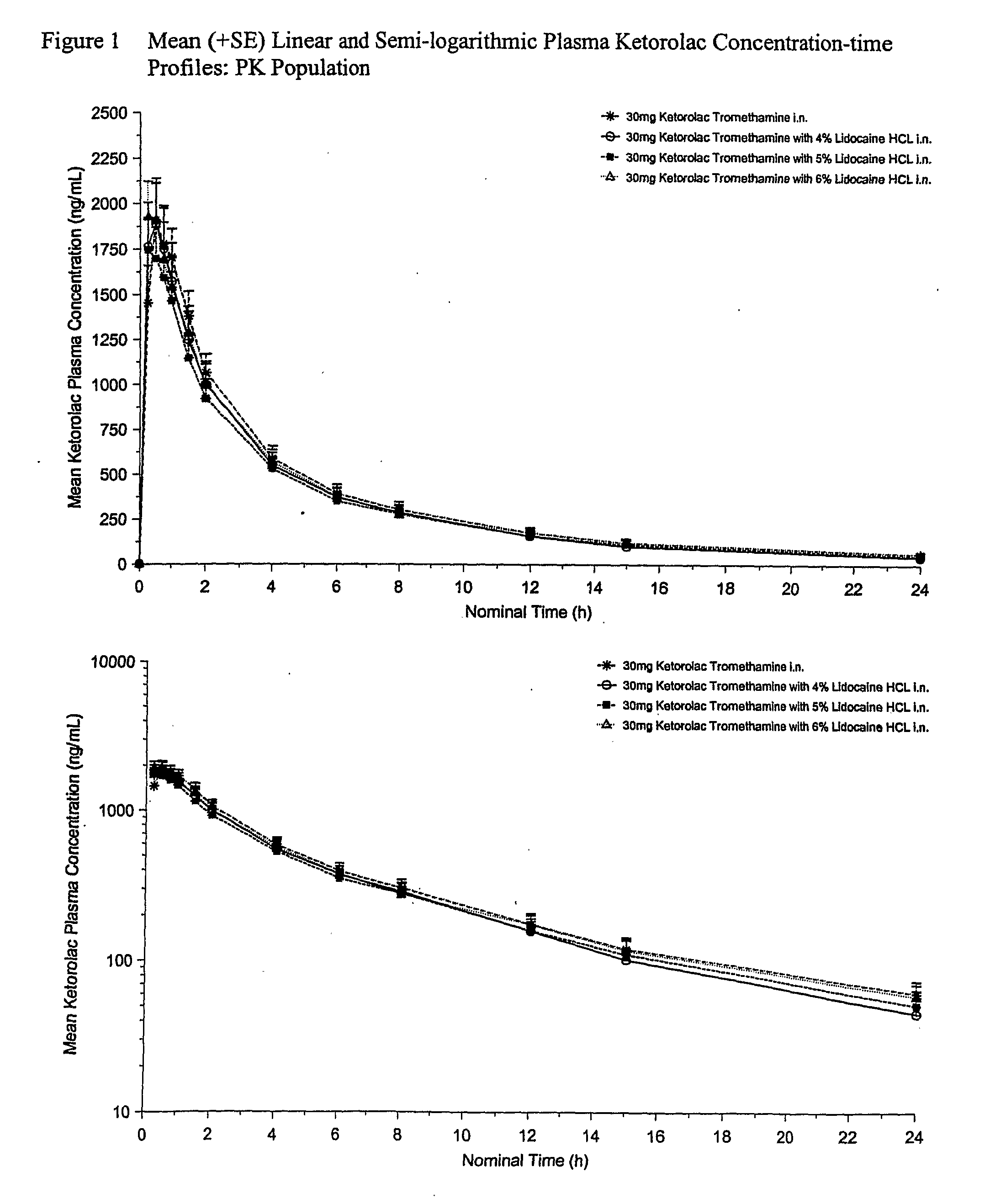
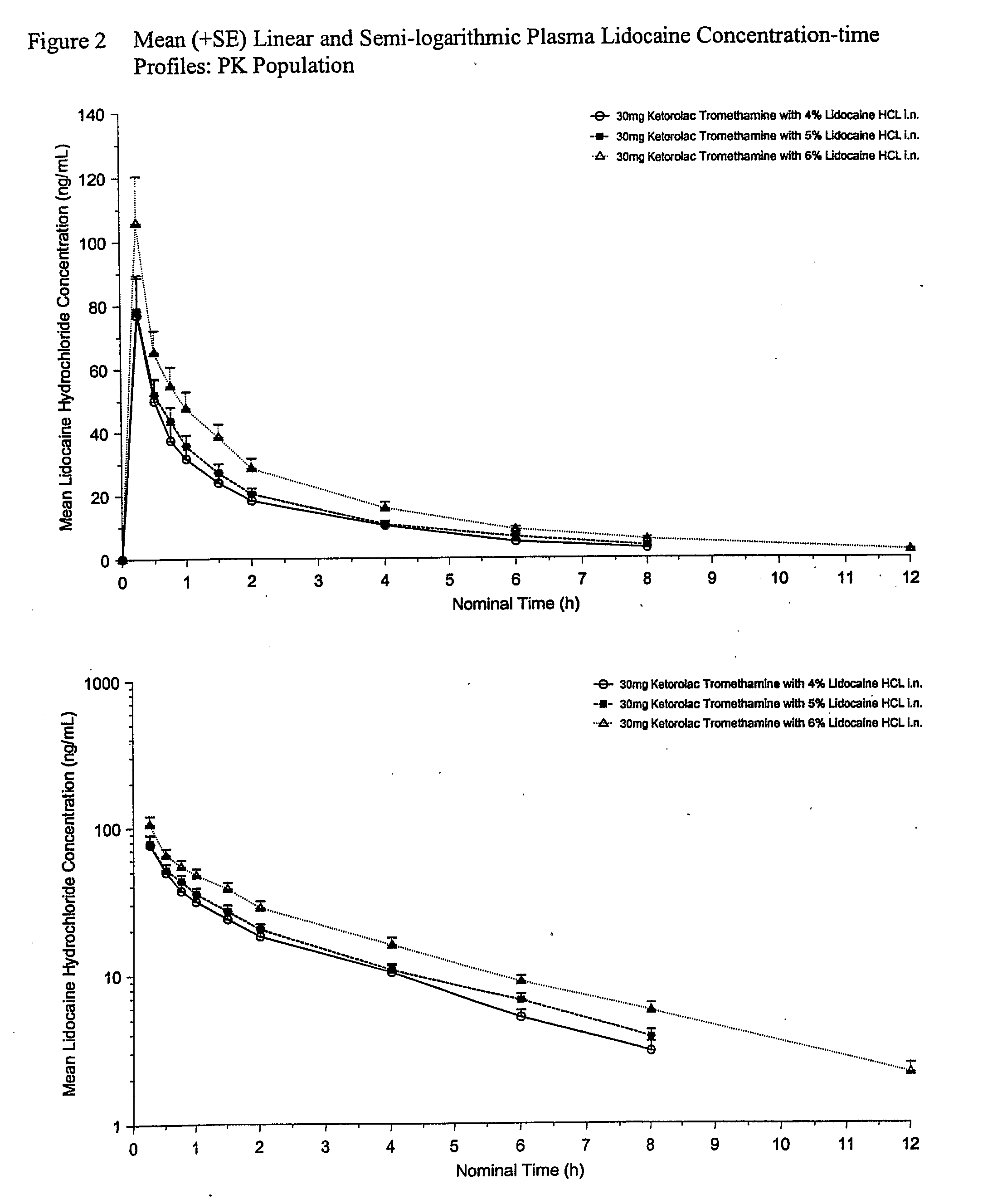
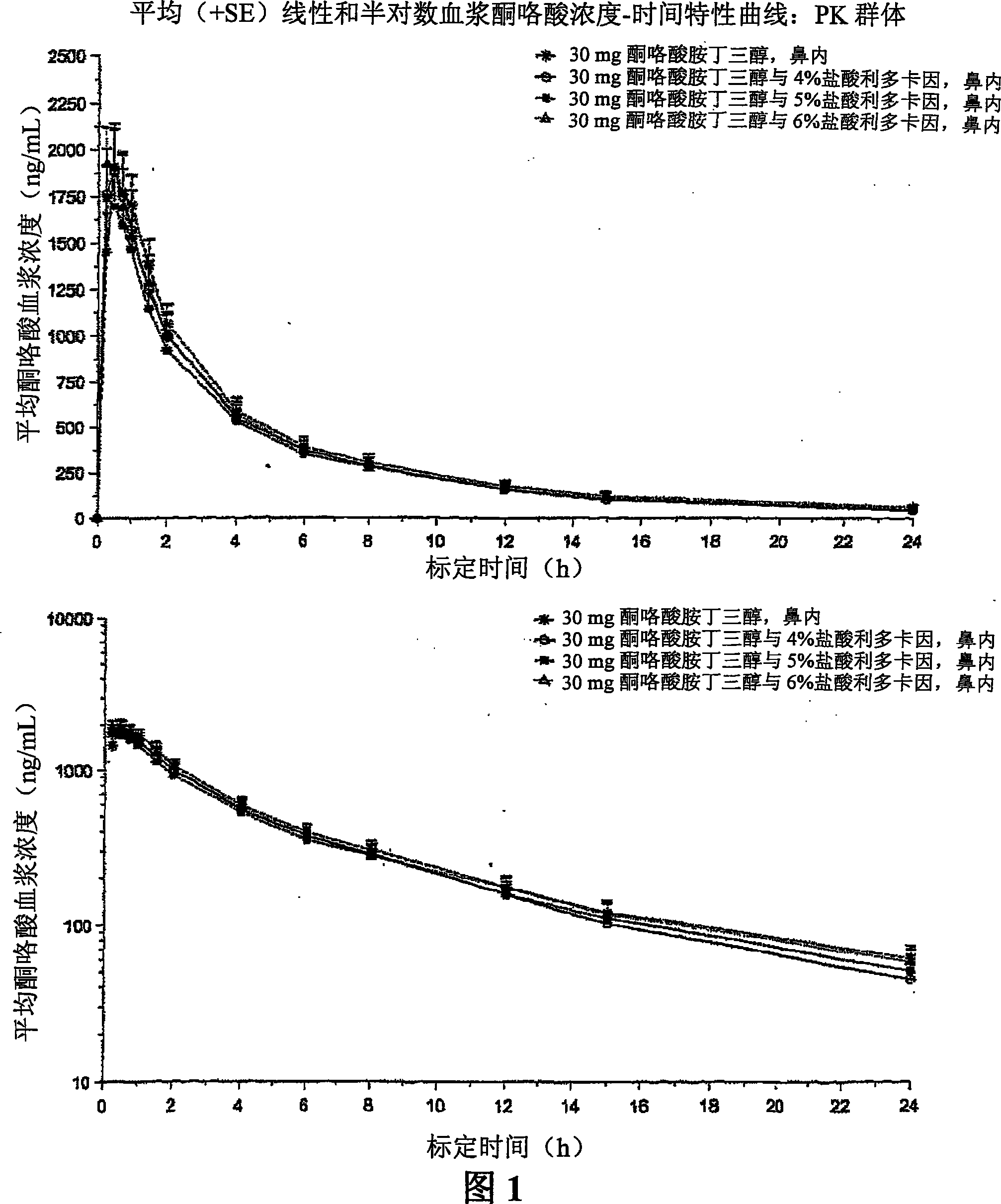
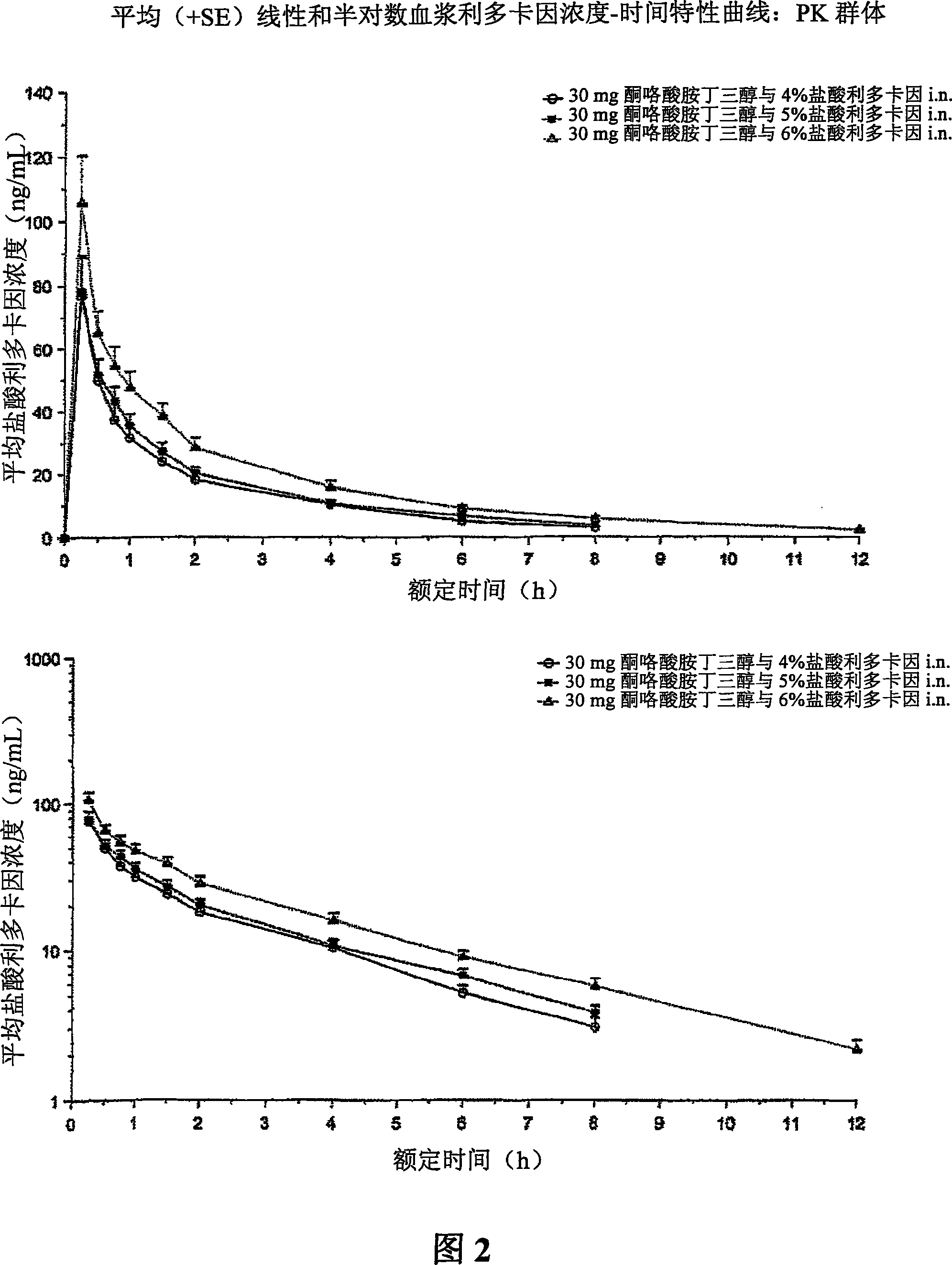
Therapeutic compositions for intranasal administration of ketorolac
Therapeutic compositions, particularly sprayable aqueous compositions, comprise ketorolac or a pharmaceutically acceptable salt, in combination with a local anesthetic, such as lidocaine hydrochloride. The compositions are nasally administered to a subject in need thereof to treat pain or inflammation and have the benefit of reduced stinging and improved efficacy, compared to known nasally administered compositions.
Owner:ROXRO PHARMA INC
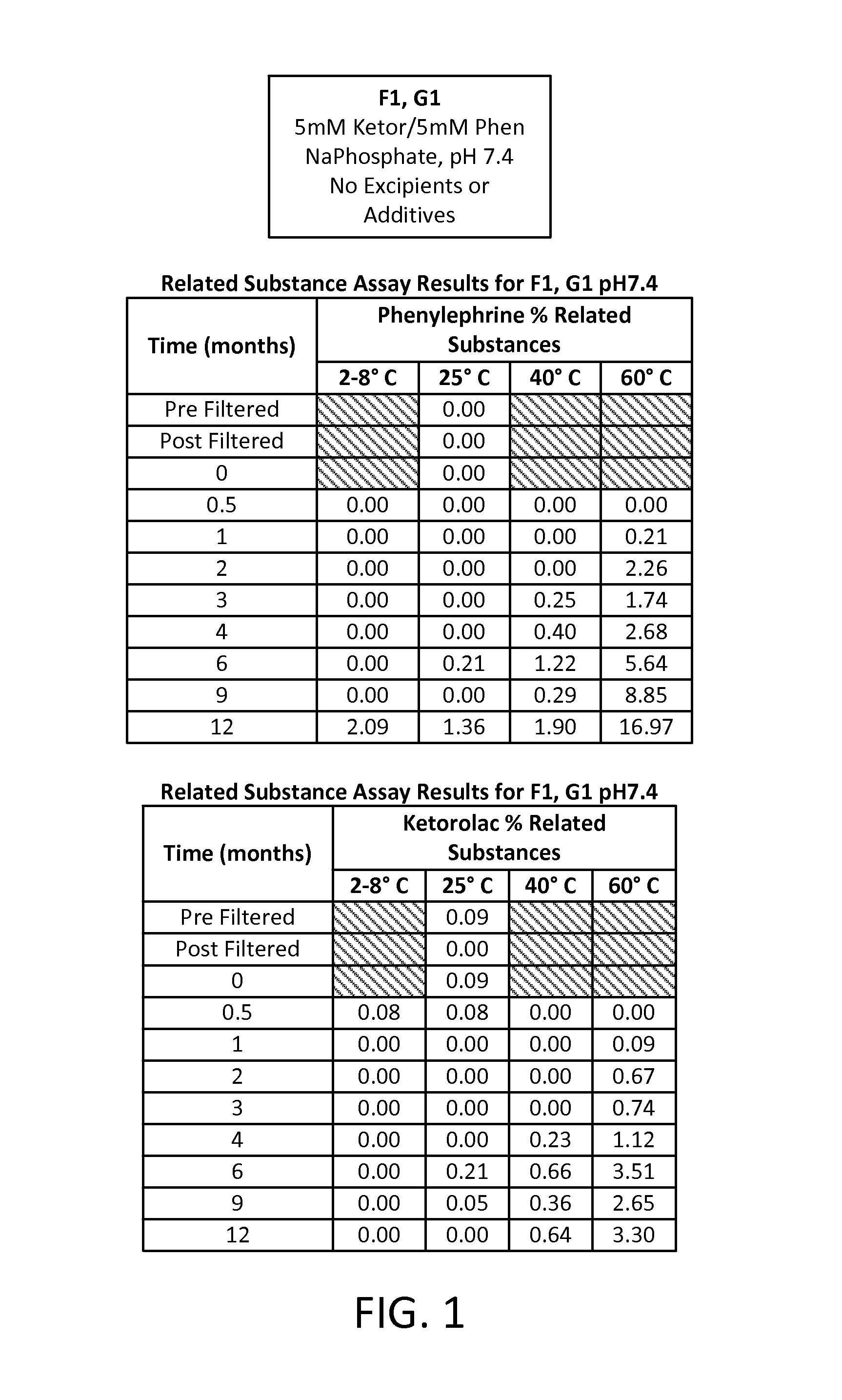
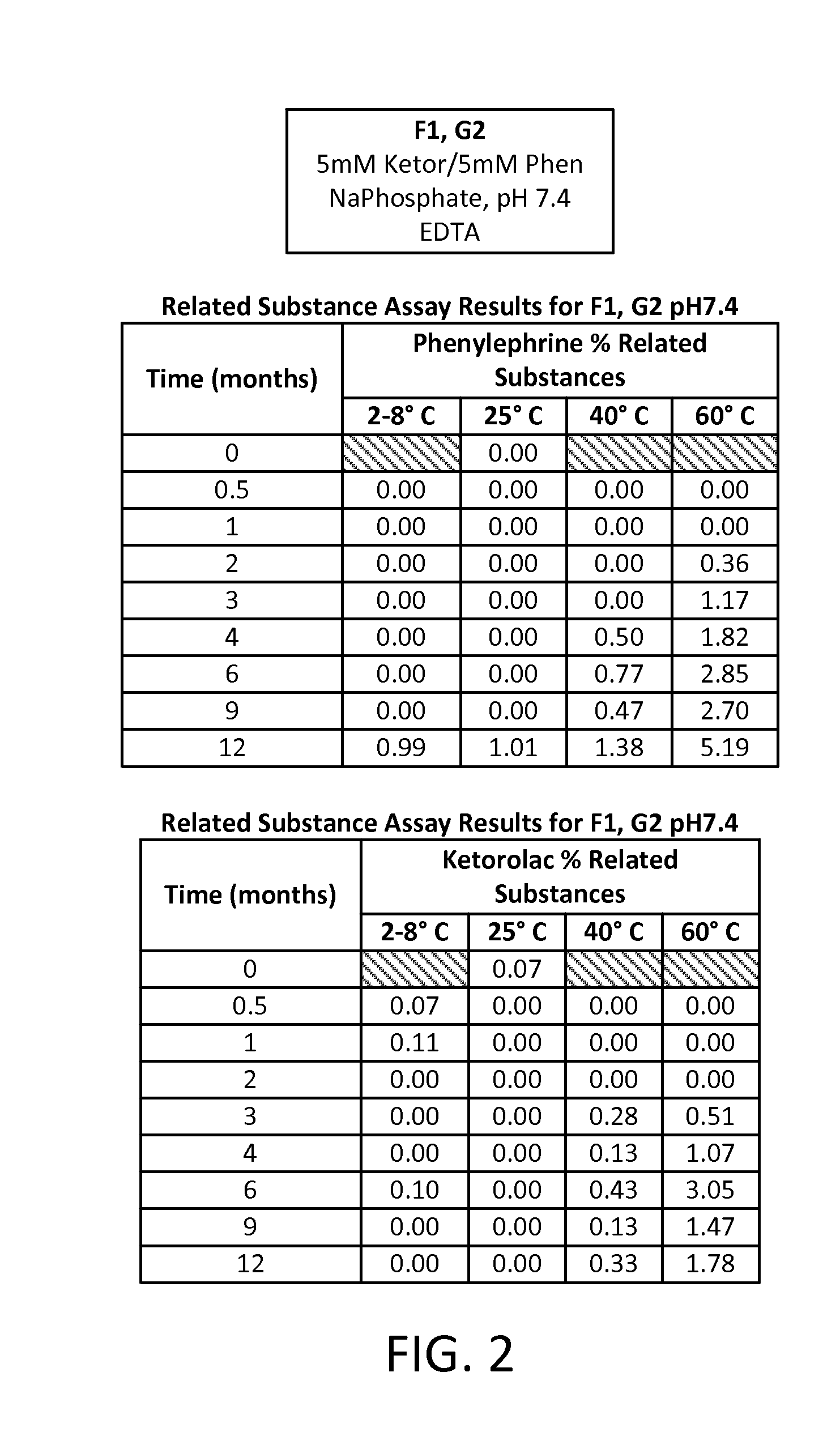
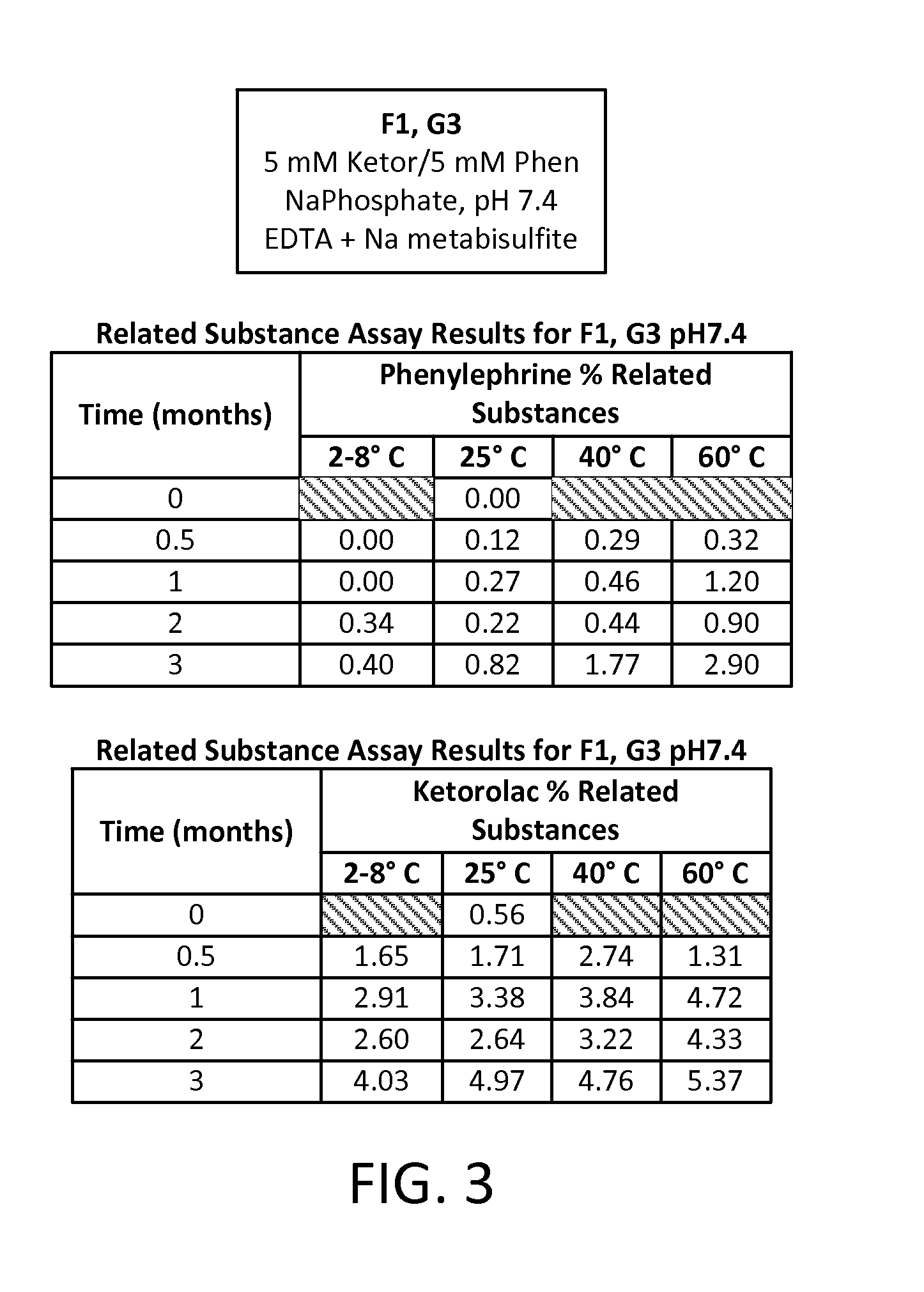
Stable Preservative-free Mydriatic and Anti-inflammatory Solutions for Injection
ActiveUS20140235691A1Avoid Potential ToxicityImprove stabilityBiocideSenses disorderPreservative freeKetorolac
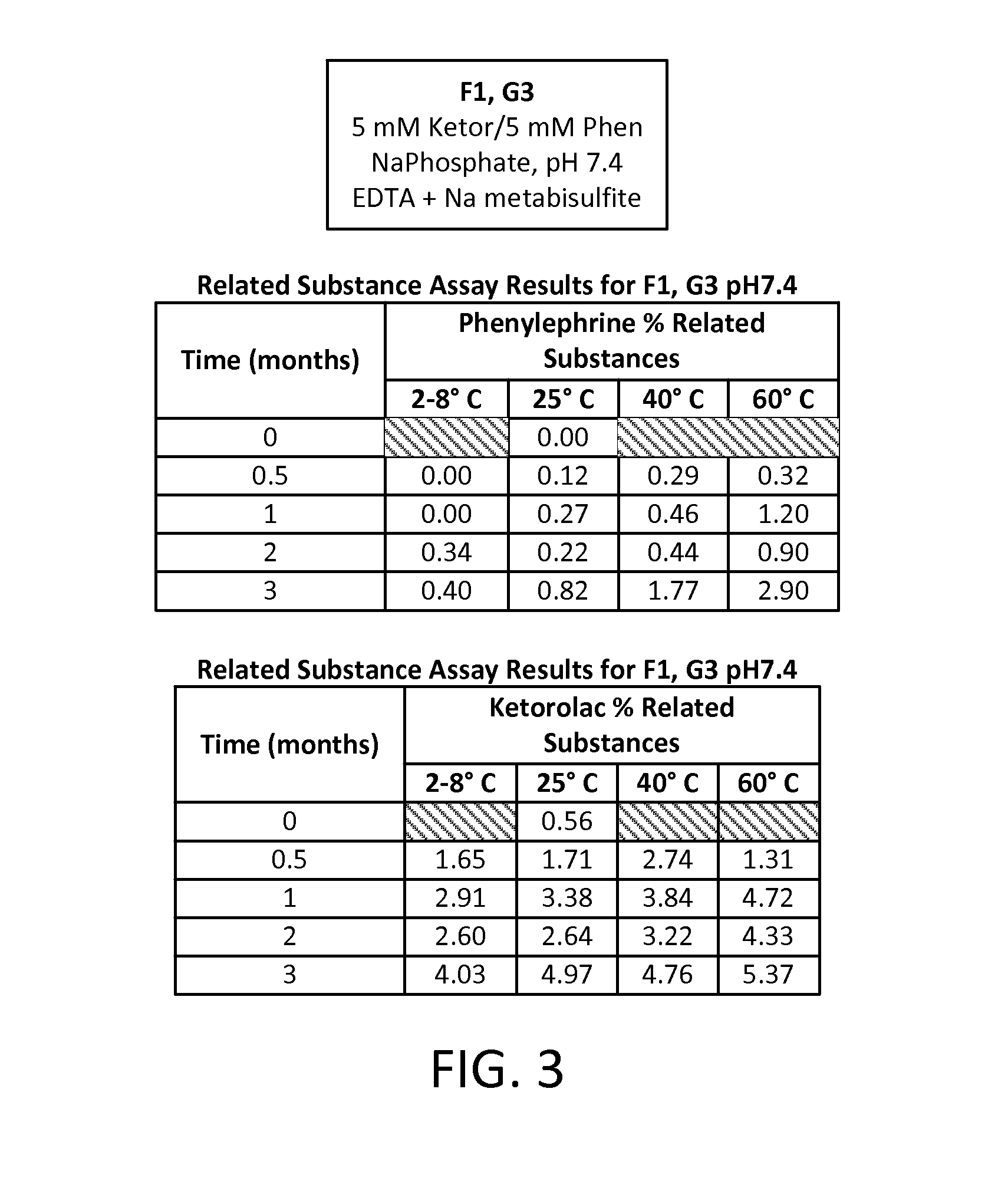
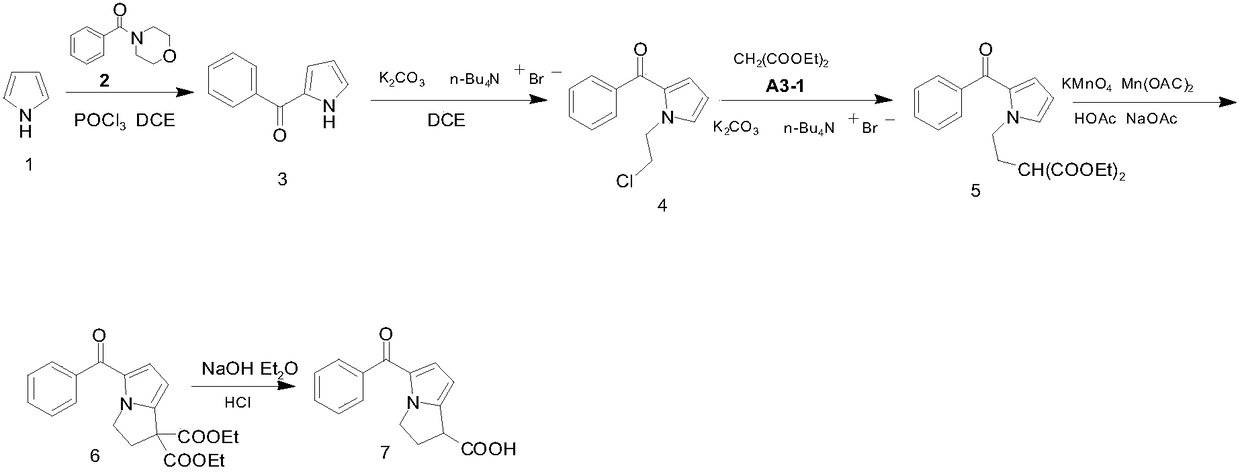
The present invention relates to stable, preservative- and antioxidant-free liquid formulations of phenylephrine and ketorolac for injection.
Owner:RAYNER SURGICAL (IRELAND) LTD
Therapeutic compositions for intranasal administration which include KETOROLAC
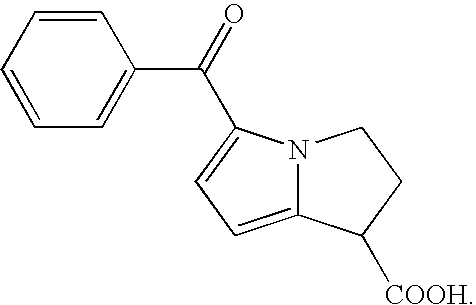
InactiveUS7476689B2Rapidly and thoroughly absorbedFast deliveryPowder deliveryBiocideCarboxylic acidBULK ACTIVE INGREDIENT
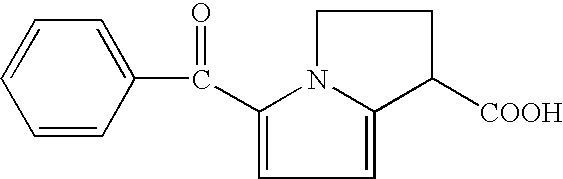
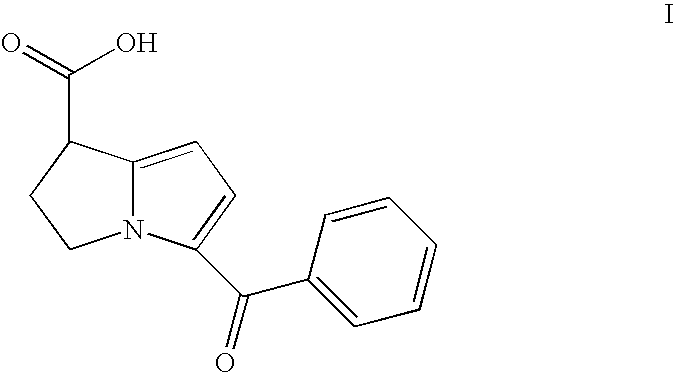
An analgesic / anti-inflammatory pharmaceutical dosage form which comprises an effective amount of an active ingredient selected from the group consisting of racemic 5-benzoyl-2,3-dihydro-1H-pyrrolizine-1-carboxylic acid, optically active forms thereof and pharmaceutically acceptable salts thereof, in combination with a pharmaceutically acceptable excipient or diluent, said dosage form being an intranasally administrable dosage form.
Owner:RECORDATI IRELAND LTD
Topical formulation having effects on alleviating pain/inflammation caused by herpes virus infection
InactiveUS7132452B2Fasten skin recoveryPromote recoveryPowder deliveryBiocideComplete remissionTolmetin
The present invention provides a topical formulation containing NSAID, particularly diclofenac. The topical formulation is particularly useful for alleviating pain / inflammation associated with infection caused by herpes virus, especially herpes simplex virus (HSV) and varicella-zoster virus (VZV). Similar relief can be achieved where diclofenac is replaced with another non-steroidal anti-inflammatory drug (NSAID), which includes, without limitation, etodolac, ketorolac, bromfenac, diflunisal, ibuprofen, fenoprofen, ketoprofen, naproxen, suprofen, meclofenamate, mefenamic acid, piroxicam, meloxicam, indomethacin, sulindac, phenylbutazone, oxyphenbutazone, and tolmetin. The topical formulation is further characterized by its fast relief on pain and / or inflammation associated with infection caused by herpes virus, i.e., a complete relief in no more than seven (7) days after the application of the topical formulation on skins of patients.
Owner:YUNG SHIN PHARMACEUTICALS INDUSTRIAL CO LTD
Non-steroidal anti-inflammatory drug formulations for topical applications to the skin
Topical alcoholic or aqueous alcoholic gels containing ibuprofen or other NSAIDs, such as, naproxen, in substantially neutral salt form, have enhanced penetration through skin and may provide rapid pain / inflammation relief by including in the formulation 2-n-nonyl-1,3-dioxolane or other hydrocarbyl derivative of 1,3-dioxolane or 1,3-dioxane or acetal, as skin penetration enhancing compound. The amount of propylene glycol may be varied to adjust the initial flux of the NSAID through the skin, especially for ibuprofen, naproxen, and ketorolac.
Owner:MACROCHEM CORP
Pharmaceutical Composition Comprising the Combination of a Ketorolac Salt and Vitamins of the-B-Complex for the Treatment of Neuralgia
This invention refers to the pharmaceutical combinations of Ketorolac salts and B-complex; to the methods used to make said combinations; and particularly, to ketorolac and B-complex synergic combinations useful in the treatment of patients that suffer from moderate to severe pain and neuralgias in different body sites.
Owner:LAB SENOSIAIN DE C V
Stable preservative-free mydriatic and anti-inflammatory solutions for injection
ActiveUS9066856B2Avoid Potential ToxicityImprove stabilityBiocideSenses disorderPreservative freeKetorolac
The present invention relates to stable, preservative- and antioxidant-free liquid formulations of phenylephrine and ketorolac for injection.
Owner:RAYNER SURGICAL (IRELAND) LTD
Synthesis process of ketorolac
InactiveCN108191876AEmission reductionThe synthesis process is environmentally friendlyOrganic chemistryKetorolacManganese
The invention discloses a synthesis process of ketorolac, and relates to the technical field of medicine synthesis. The synthesis process solves the technical problems that the existing process can generate a large amount of liquid and solid wastes, and the environment protection is not facilitated. Hydrogen peroxide with side products being water is used as an oxidizing agent; malysite is used asa catalyst; a large number of manganese salts are replaced; when 1kg of ketorolac is reduced, 3.5 to 6.1kg of discharged liquid and solid wastes are reduced; the green and environment-friendly effects are achieved. Benzoyl chloride is directly used as raw materials; the one-step reaction is reduced; the synthesis process is simpler; the methyl tertiary butyl ether is used for replacing the flammable and combustible diethyl ether; the process production safety is improved. The synthesis process has the advantages that the operation is easy; the process conditions can be easily controlled; thefinal product purification and aftertreatment are simple.
Owner:上海仁实医药科技有限公司
Ketorolac Sublingual Spray for the Treatment of Pain
InactiveUS20090246273A1Improve bioavailabilityAmenable to ameliorationBiocidePowder deliveryKetorolacHeadache severe
The present invention provides for compositions and methods for accelerating the rate of delivery of ketorolac to the systemic circulation by sublingual spray administration under the tongue to provide a rapid response in the treatment of pain, especially acute pain associated with postoperative pain and migraine headache. Compositions of ketorolac formulated for sublingual delivery as liquid spray are provided. Also provided are methods of treatment and management of pain.
Owner:US WORLDMEDS
Ketorolac implant and preparation method thereof
InactiveCN103263413AControl releaseControlled and sustained releaseOrganic active ingredientsAntipyreticKetorolacMedicine
The invention provides a ketorolac implant and a preparation method thereof. The implant which contains ketorolac and a degradable carrier is prepared by using a hot melt extrusion method, can release drugs in a zero-level dynamic way within a preset drug release time and can be used for preventing or treating acute or chronic pain and inflammation. The ketorolac implant prepared by the invention is stable in release and free of burst release effect and has great advantages in clinic application.
Owner:广东欧替克生物医学科技有限公司
Therapeutic compositions for intranasal administration of ketorolac
Therapeutic compositions, particularly sprayable aqueous compositions, comprise ketorolac or a pharmaceutically acceptable salt, in combination with a local anesthetic, such as lidocaine hydrochloride. The compositions are nasally administered to a subject in need thereof to treat pain or inflammation and have the benefit of reduced stinging and improved efficacy, compared to known nasally administered compositions.
Owner:ROXRO PHARMA INC
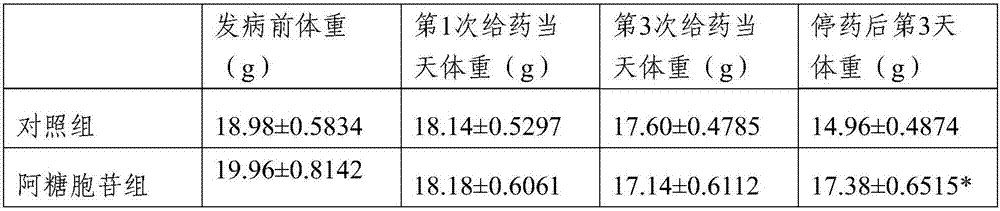
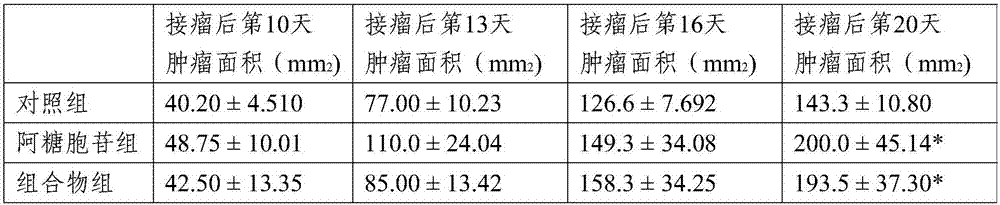
Medicinal composition for treating cachexia and applications of medicinal composition
ActiveCN107137417AReach a state of healingProlong lifeOrganic active ingredientsMetabolism disorderCytarabineKetorolac
The invention relates to applications of cytarabine or an analogue of cytarabine and ketorolac or an analogue of ketorolac in treating cachexia, and in particular provides a medicinal composition for treating cachexia. According to the scheme, for the first time, one or more of cytarabine or the analogue of cytarabine and ketorolac or the analogue of ketorolac are creatively adopted as the active ingredients of the composition for treating cachexia, and remarkable treatment effects are obtained in the animal experiment. Based on the severity degree of cachexia, the medicinal composition has the broad clinical application prospect.
Owner:CHANGCHUN NUOSAI BIOTECH CO LTD
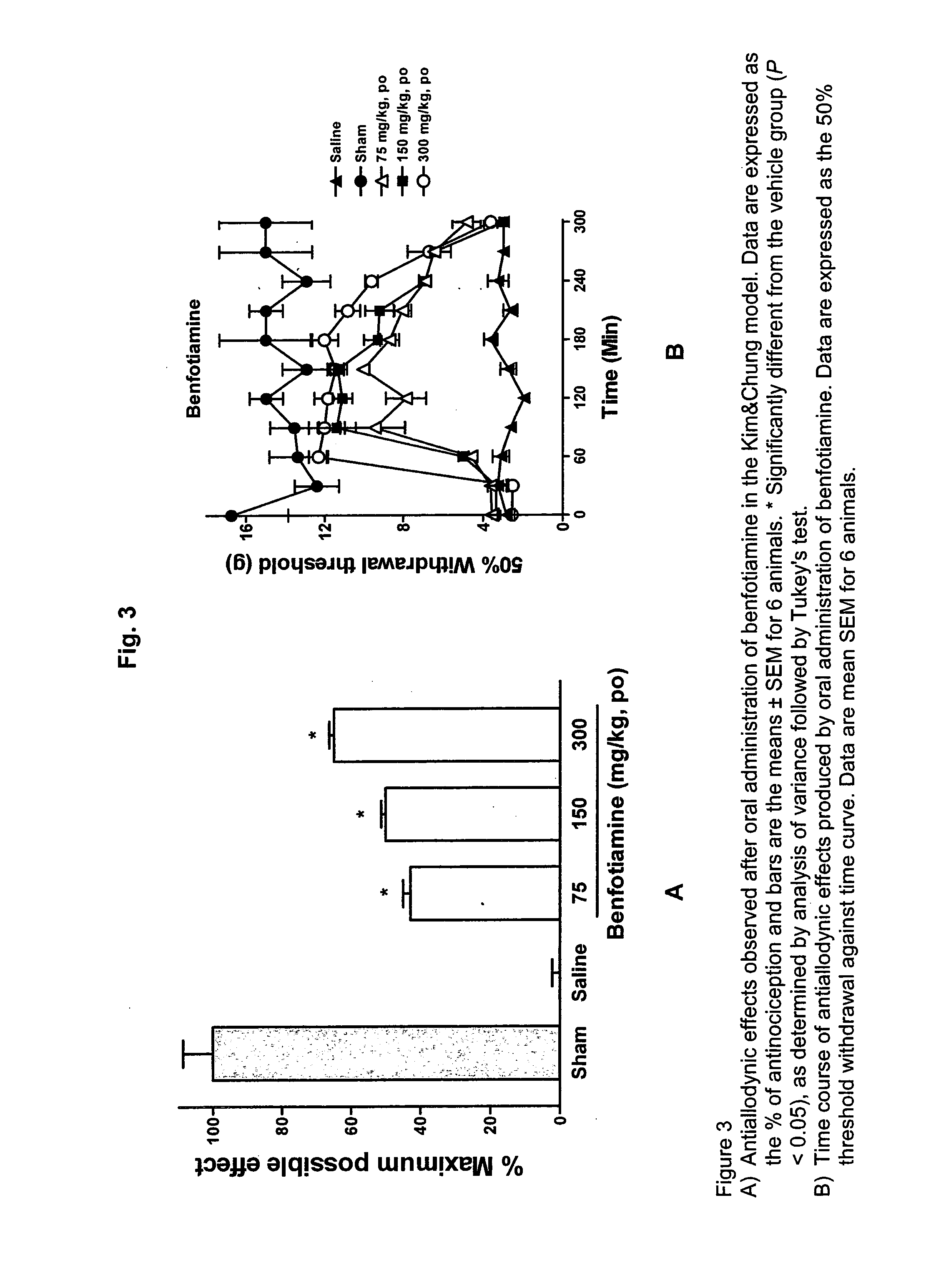
Pharmaceutical compositions containing benfotiamine and one or one more pharmaceutically active agents for the treatment of pain conditions of neuropathic origin
InactiveUS20100279984A1Good synergistic effectEnhanced interactionBiocideNervous disorderDiseaseGabapentin
The present invention relates to pharmaceutical compositions containing benfotiamine and one or more pharmaceutically active agents selected from the group consisting of analgesic acting substances, especially pharmaceutical compositions containing benfotiamine and one or more pharmaceutically active agents selected from the group consisting of gabapentin, pregabalin, XP13512, carbamacepin, amitryptilin, ketorolac, diclofenac, ibuprofen, flurpirtin, paracetamol and dexamethasone, the process for their preparation and their use for the treatment and prevention of conditions and diseases selected from the group consisting of pain conditions of neuropathic origin.
Owner:MERCK PATENT GMBH
Positively charged water-soluble prodrugs of aryl- and heteroarylpropionic acids with very fast skin penetration rate
ActiveCN101506161AGood absorption rateImprove solubilitySenses disorderNervous disorderSolubilityPhosphate
Owner:TECHFIELDS BIOCHEM CO LTD
Phenylephrine ketorolac solution and preparation method
InactiveCN104856990AImprove securityAchieve superimposed effectOrganic active ingredientsSenses disorderPreservative freeSide effect
The invention relates to phenylephrine ketorolac solution and a preparation method, and provides intraocular operation washing concentrated solution without a preservative, an antioxidant and a buffer system. The phenylephrine ketorolac solution is capable of preventing the intraoperative miosis and relieving the postoperation pain, and avoiding the side effects caused by the preservative, the antioxidant and the buffer system.
Owner:WUHAN WUYAO SCI & TECH
Sublingual Formulations of Ketorolac or Salts Thereof
InactiveUS7879901B2Fast absorptionQuick treatmentBiocideHydroxy compound active ingredientsCelluloseKetorolac
The present invention refers to pharmaceutical compositions based on ketorolac or one of its salts pharmaceutically acceptable, as well as the use of ketorolac or one of its salts acceptable from pharmaceutical viewpoint, for preparation of a pharmaceutical composition (tablets) for sublingual administration, with the purpose of accelerating the pharmacological response to ketorolac, without making use of the injectable via. On the other hand, a pharmaceutical composition is described encompassing, as one of its active principles, ketorolac or one of its salts acceptable from pharmaceutical viewpoint, representing from 10 to 15% by weight, in relation to the total weight of the compound and as the essential excipient, a ternary mixture of lactose / sorbitol / cellulose, eventually in a mixture with other excipients acceptable from pharmaceutical viewpoint.
Owner:NATURES PLUS FARM
Oral pharmaceutical compositions containing non-steroidal anti-inflammatory drugs and method for preparing the same
The present invention provides oral pharmaceutical compositions for acetic acid class of non-steroidal anti-inflammatory drug (NSAID), particularly ketorolac. The pharmaceutical composition contains a core, a drug layer (which comprises the drug, a binder, and a disintegrant), a protecting layer, and an enteric coating layer. The oral pharmaceutical compositons are particularly useful for treating patients with moderate to acute pain. The present invention also provides a method for making the pharmaceutical compositions and a method for using the pharmaceutical compositions.
Owner:YUNG SHIN PHARMACEUTICALS INDUSTRIAL CO LTD
Anti-inflammatory and mydriatic intracameral solutions for inhibition of postoperative ocular inflammatory conditions
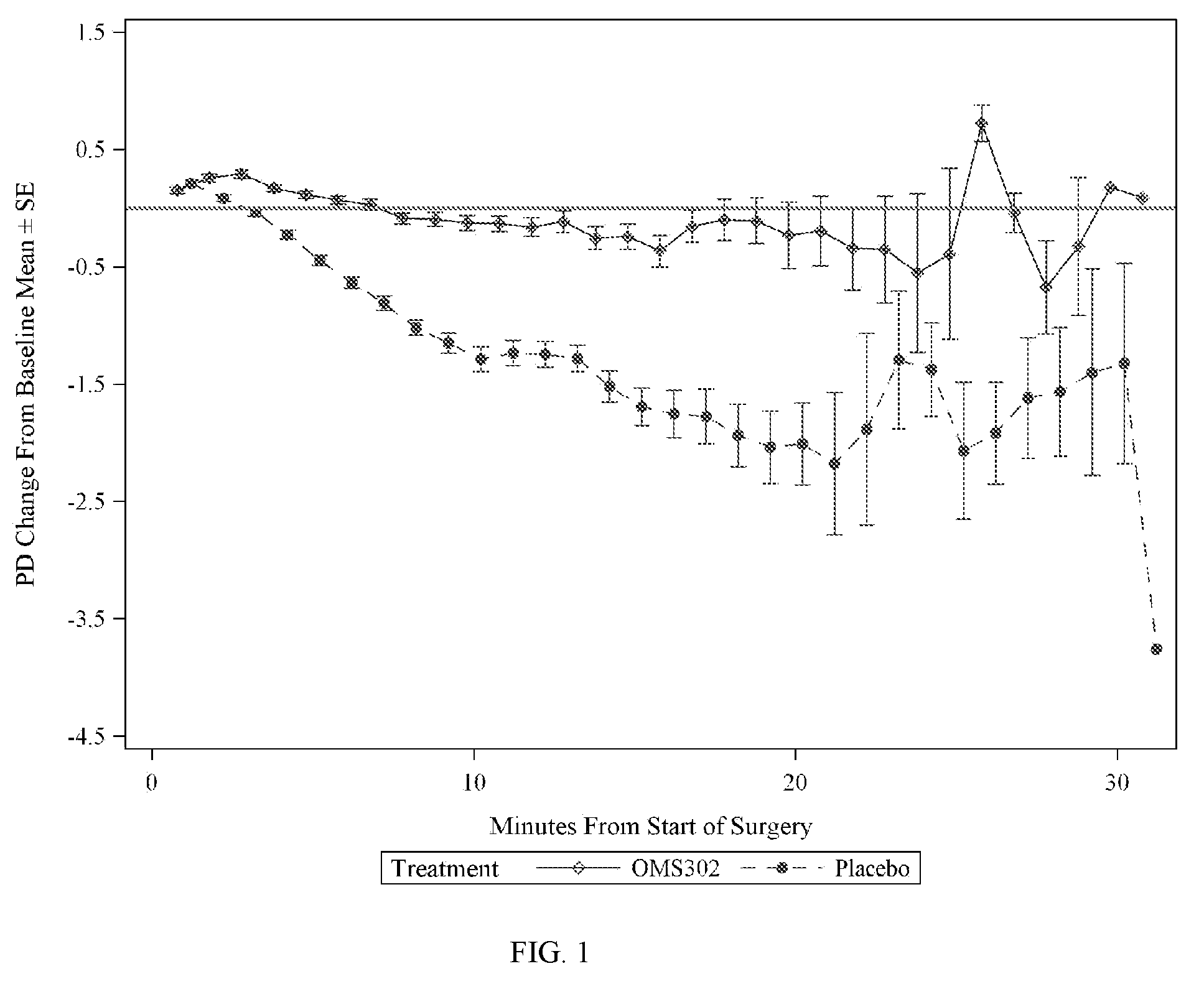
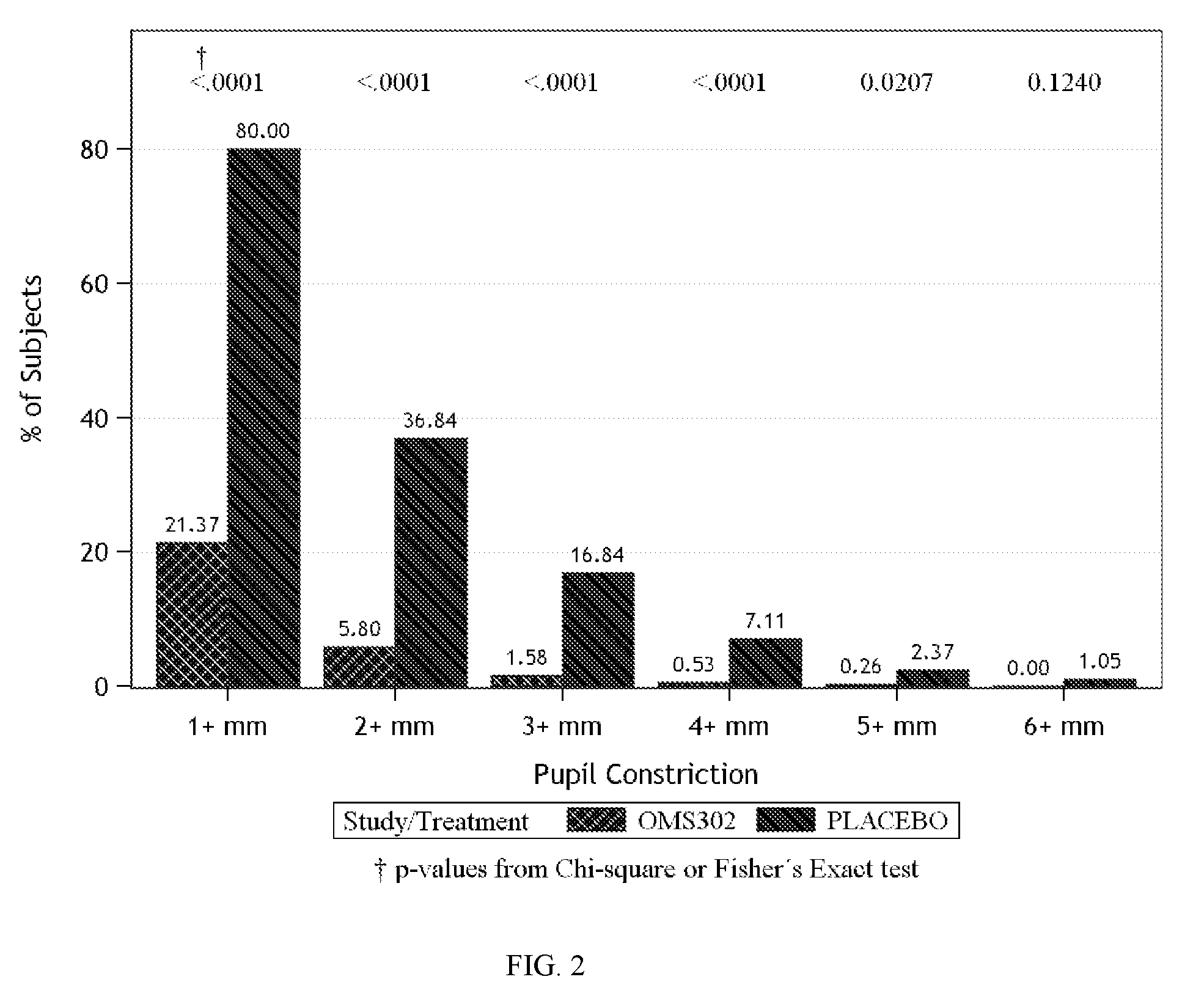
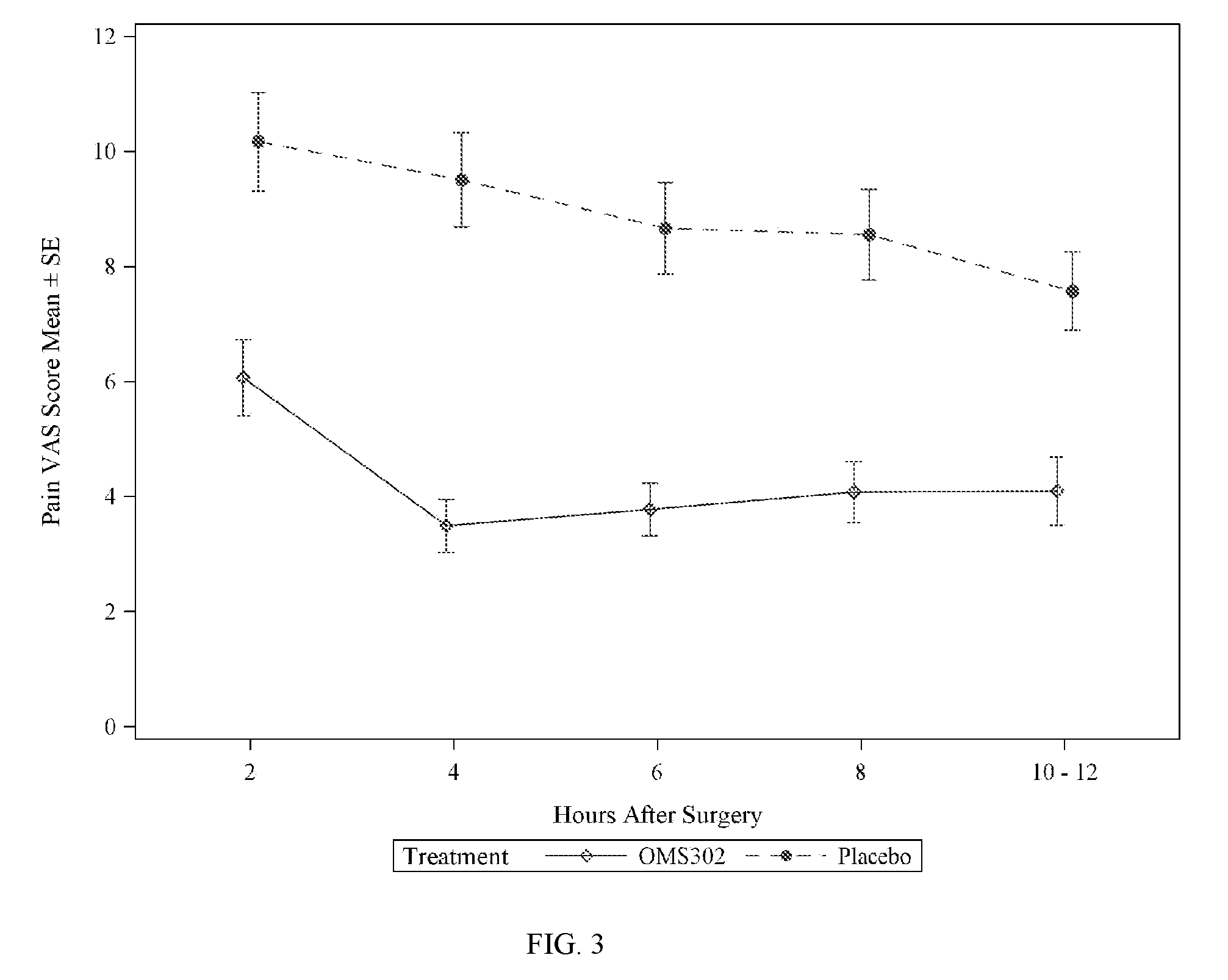
The present invention provides methods for inhibiting postoperative inflammatory conditions following ophthalmologic surgical procedures by administering intraocularly during an ophthalmologic surgical procedure a solution including a nonsteroidal anti-inflammatory agent and an alpha-1 adrenergic mydriatic agent, such as a liquid irrigation solution of ketorolac and phenylephrine.
Owner:OMEROS CORP
Osimertinib ketorolac salt crystal form and preparation method thereof
PendingCN110483486AReduce humidityImprove stabilityOrganic active ingredientsAntipyreticKetorolacX-ray
The invention belongs to the technical field of medicines, and particularly relates to an osimertinib ketorolac salt crystal form and a preparation method thereof. An X-ray diffraction pattern expressed by 2theta and utilizing Cu-Kalpha radiation, of the crystal form, has characteristic peaks at 4.9+ / -0.2 degrees, 6.4+ / -0.2 degrees, 12.2+ / -0.2 degrees, 21.+ / -0.2 degrees and 22.6+ / -0.2 degrees. Theosimertinib ketorolac crystal form is low in hygroscopicity, simple in preparation process, stable in property and suitable for large-scale production.
Owner:LUNAN PHARMA GROUP CORPORATION
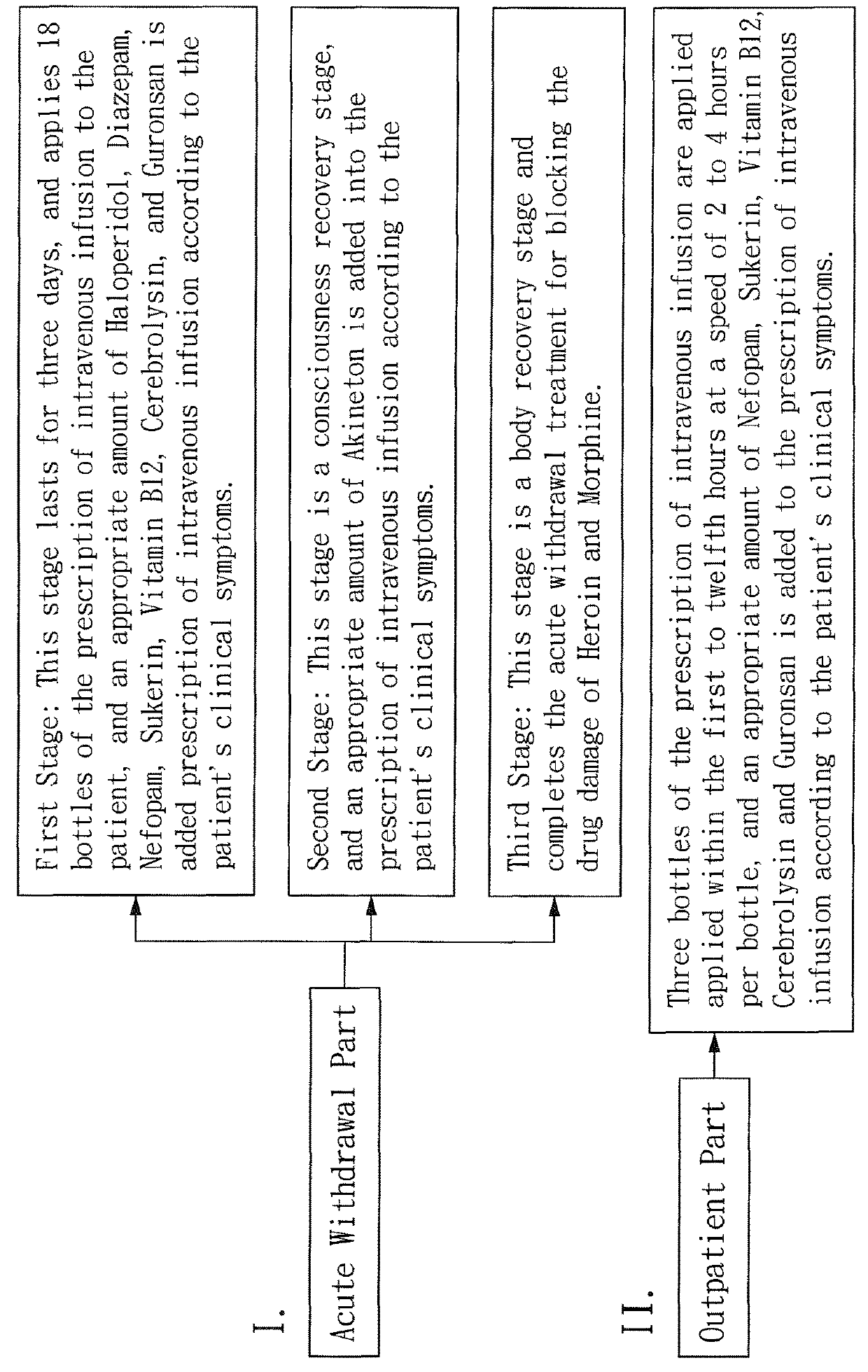
Prescription of intravenous medication for blocking heroin or morphine intoxication path and using thereof
ActiveUS10004781B2Enhancing withdrawalPrevent addictionNervous disorderPeptide/protein ingredientsMorphine poisoningBlock drugs
Owner:CHENG EN CHE
Prescription of Intravenous Medication for Blocking Heroin or Morphine Intoxication Path and Using Thereof
ActiveUS20170157209A1Enhancing withdrawalReduce usageNervous disorderPeptide/protein ingredientsMorphine poisoningBlock drugs
Disclosed are a prescription of intravenous medication for blocking a heroin or morphine intoxication path and its using. The prescription of intravenous infusion includes Cerebrolysin, Piracetam, Cimetidine, Scopolamine Butylbromide, Nefopam, B.C Complex, Vitamin B1, Ascorbic Acid, Ketorolac, Guronsan, Hyoscine Butylbromide and Sukerin. The prescription activates a patient's liver and circulatory system to block drug intoxication. The using for blocking drugs includes an acute withdrawal part, wherein the prescription of intravenous infusion is injected and the timing of drug administration and the safe dosage are controlled according to clinical symptoms, and an appropriate amount of a supplementary medicine such as Haloperidol is added, and the processes of detoxication, relieving symptoms, suppressing restlessness, and sobering are conducted to block acute withdrawal symptoms of an acute withdrawal addict quickly and successfully.
Owner:CHENG EN CHE
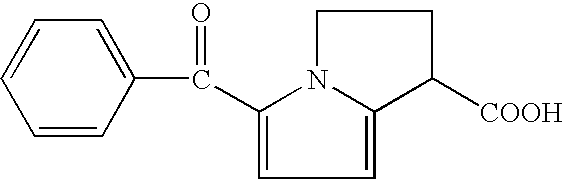
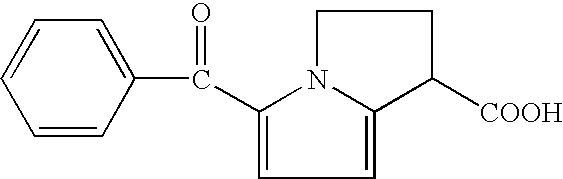
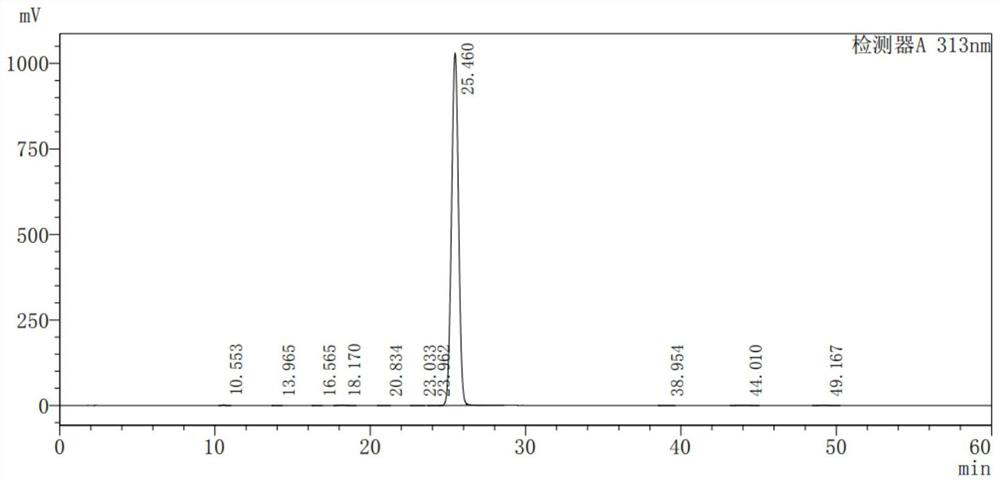
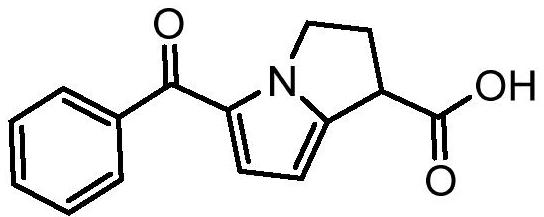
Ketorolac impurity C and preparation method and application thereof
PendingCN112898307AHigh yieldSpecific responseOrganic chemistryTesting medicinal preparationsKetorolacPhysical chemistry
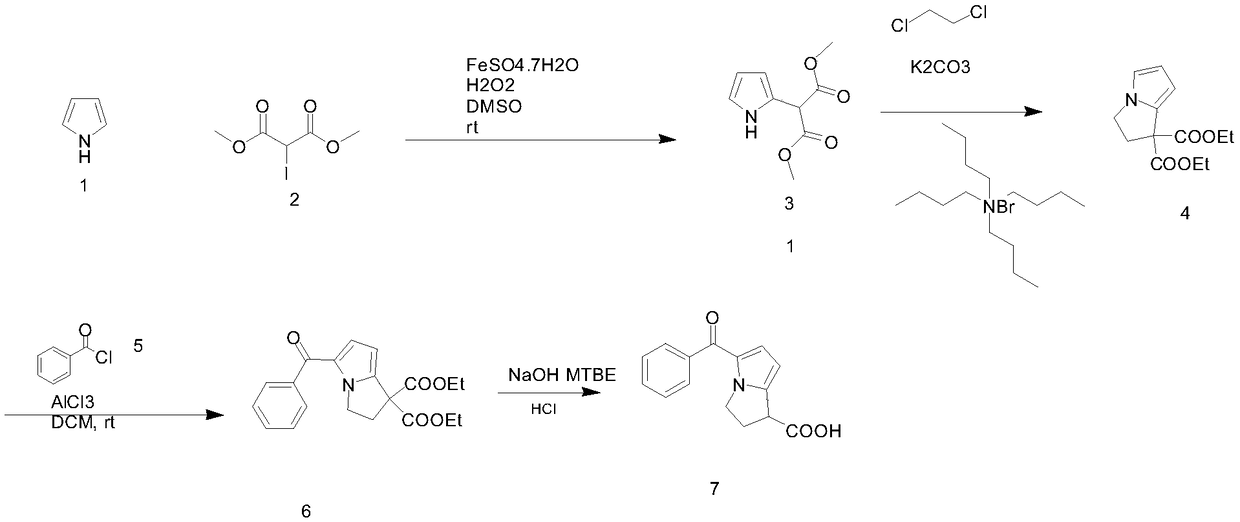
The invention discloses a ketorolac impurity C and a preparation method and application thereof. According to the method, the ketorolac impurity C is prepared by taking pyrrole as an initial raw material through a series of reactions such as substitution, oxidation and alkaline hydrolysis, and the ketorolac impurity C is one of important impurities of ketorolac drugs or preparations thereof and can be used for toxicological and pharmacological researches such as in-vivo absorption and metabolism of ketorolac, and can also be applied to research on stability and quality control of ketorolac preparations. The preparation method of the ketorolac impurity C has the advantages of simplicity in operation, safety in reaction and high purity and yield, and can be widely applied to the fields of impurity analysis, toxicological research, safety detection, stability judgment and the like of ketorolac bulk drugs and preparations thereof.
Owner:THE KEY LAB OF CHEM FOR NATURAL PROD OF GUIZHOU PROVINCE & CHINESE ACADEMY OF SCI
Application of Rac1 activity inhibitor in preparation of drugs for treating Alzheimer disease
InactiveCN109925510AForgetting to improveImprove the symptoms of rapid forgettingOrganic active ingredientsNervous disorderKetorolacWiskostatin
The invention provides an application of a Rac1 activity inhibitor in preparation of drugs for treating an Alzheimer disease. The Rac1 activity inhibitor can be: at least one of EHop-016, CS7171, JKF-034, Secramine, AZD0530, NSC23766, MBQ-167, AZA1, AZA197, Compound 19, ZINC08010136, ZINC07949036, 69391, 1A-116, ITX3, ITX1, CPYPP, GGTase1inhibitor(P61A6, Statins), EHT1864, Compound 1, MLS000532223, R-Ketorolac, OSU-3012, FL172, FRAX597, Phox-11, 187-1 and Wiskostatin. The invention protects the discovery of a mechanism for treating AD by inhibiting the activity of a forgetting regulatory molecule Rac1, and protects the application of a small molecule compound capable of inhibiting Rac1 activity in treating AD.
Owner:BEIJING JOEKAI BIOTECH
Carboxyl contained NSAIDS (nonsteroidal anti-inflammatory drugs) salt
Disclosed are non-steroidal analgesic and analgesic anti-inflammatory agents containing carboxyl including sodium, calcium, zinc, magnesium, N-n-octylgucamine, Arginine, Lycine or Trometamol salts of Loxoprofen, Ketoprofen, Pranoprofen, Tiaprofenic acid, Butibufen, Omolofen, epoxy indene acid, Lobuprofen, Clofenamic acid, Clonixin, Fenoprofen, Benorilate, Flurbiprofen, Alminoprofen, Bucloxic acid, Sulindac, Zidometacin, Acemetacin, Ketorolac, Risedronic acid, Sulindac, Lonaprofen, aspirin, Florfenicol, tiaprofenic acid, overall evaluation shows that trometamol salts are the best choice in terms of physicochemical properties, solvability, stability, local irritation, blood vessel irritation, and bioavailability for oral administration.
Owner:陈文展
Improved preparation method of ketorolac intermediate
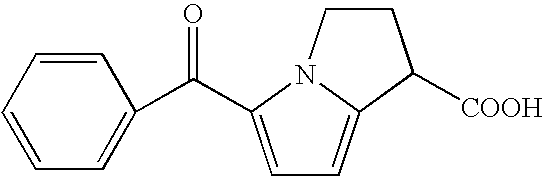
The invention discloses an improved preparation method of a ketorolac intermediate. The intermediate is (5-Benzoyl-1H-pyrrol-2-yl)methanetricarboxylic acid triethyl ester (M-1). The method comprises the following steps: subjecting 2-benzoylpyrrole, methane tricarboxylic acid triethyl ester, manganese acetate dihydrate (trivalent), sodium acetate and acetic anhydride to reacting in an organic solvent; adding an aqueous sodium hydrogen sulfite solution for extraction and liquid separation; and conducting vacuum concentration on an organic phase and pulping the an organic phase with an alcohol solvent. According to the method, post-treatment problems caused by the existence of a large amount of manganese ion compounds and a large amount of solvent acetic acid are effectively solved; meanwhile, the problems of high energy consumption, long period, high cost and the like caused by later concentration are reduced; and the obtained intermediate has the advantages of high purity, high yield and the like and is suitable for industrial production.
Owner:四川尚锐生物医药有限公司
Therapeutic compositions for intranasal administration which include KETOROLAC
InactiveUS7267827B2Rapid systemic deliveryRapidly and thoroughly absorbedBiocideNervous disorderCarboxylic acidBULK ACTIVE INGREDIENT
An analgesic / anti-inflammatory pharmaceutical dosage form which comprises an effective amount of an active ingredient selected from the group consisting of racemic 5-benzoyl-2,3-dihydro-1H-pyrrolizine-1-carboxylic acid, optically active forms thereof and pharmaceutically acceptable salts thereof, in combination with a pharmaceutically acceptable excipient or diluent, said dosage form being an intranasally administrable dosage form.
Owner:EGALET US +1
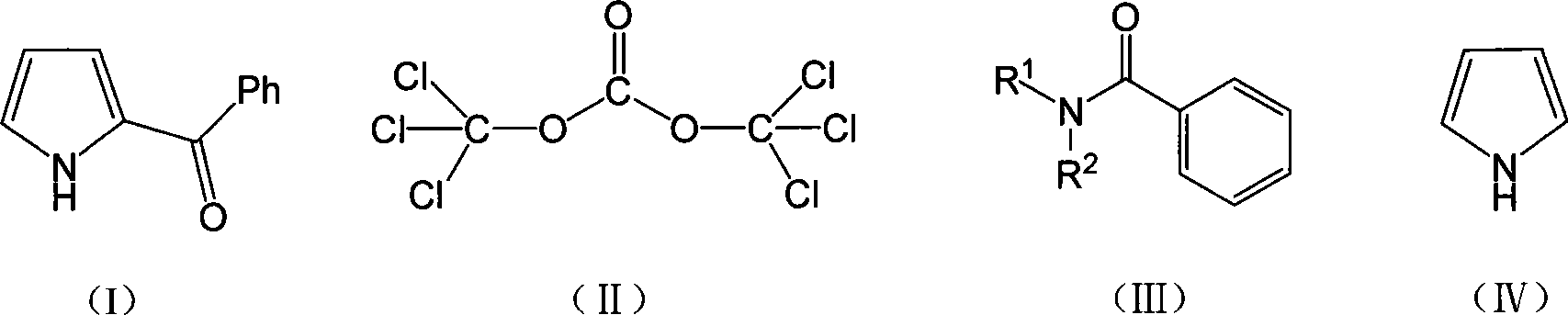
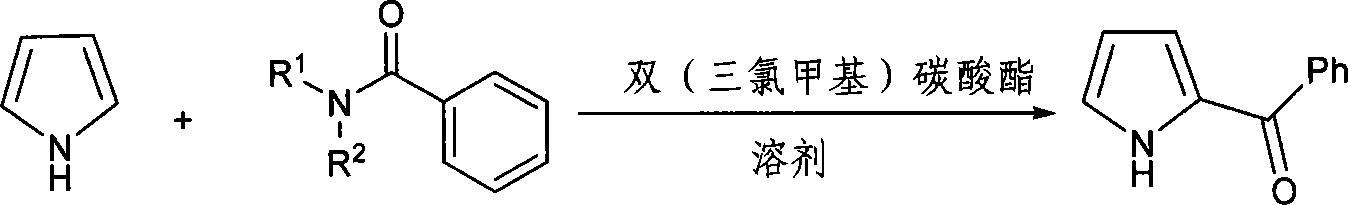
Method for synthesizing ketorolac ammonia butanetriol key intermediate compound benzoyl pyrrole
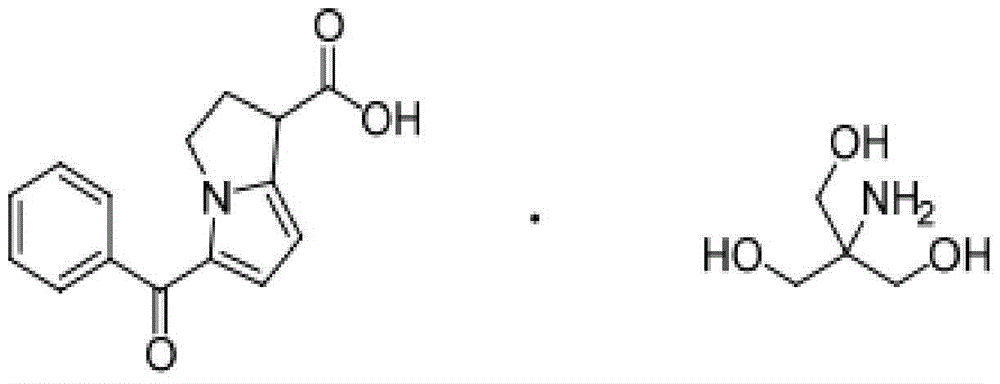
The present invention discloses the synthesis process of benzoyl pyrrole as the key intermediate for ketorolac trometamol. Benzoyl pyrrole is synthesized through the reaction of bis(trichloromethyl) carbonate and N, N-disubstituent amide to form one intermediate, adding pyrrole and reaction in organic solvent at 0-120 deg.c for 2-20 hr, and post-treatment of the resultant to obtain benzoyl pyrrole. The technological scheme of the present invention has reasonable production process, safe and reliable operation, high reaction yield, low cost and other advantages, and possesses broad industrial application foreground.
Owner:ZHEJIANG UNIV OF TECH +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com