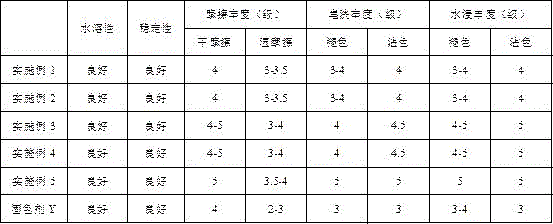
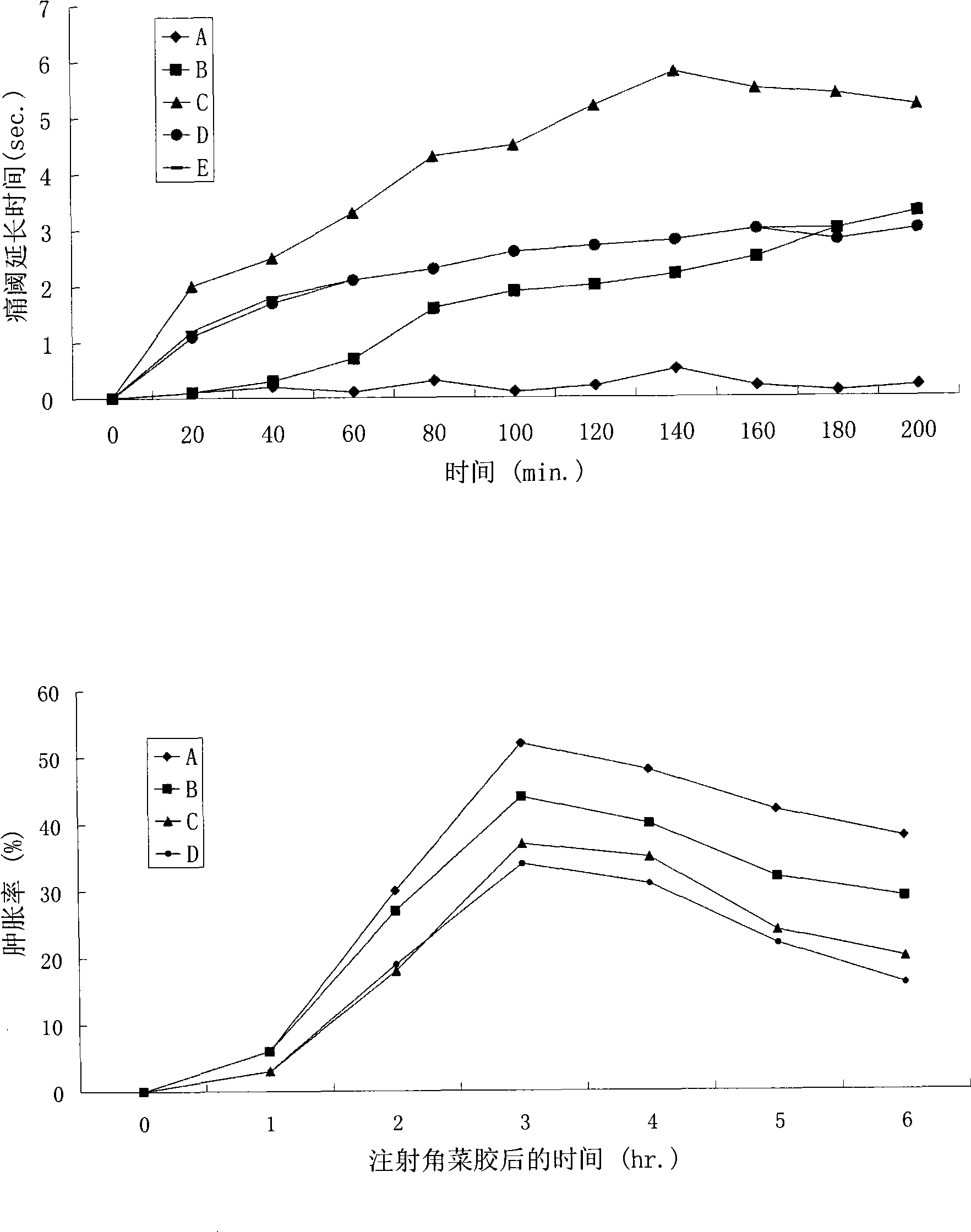
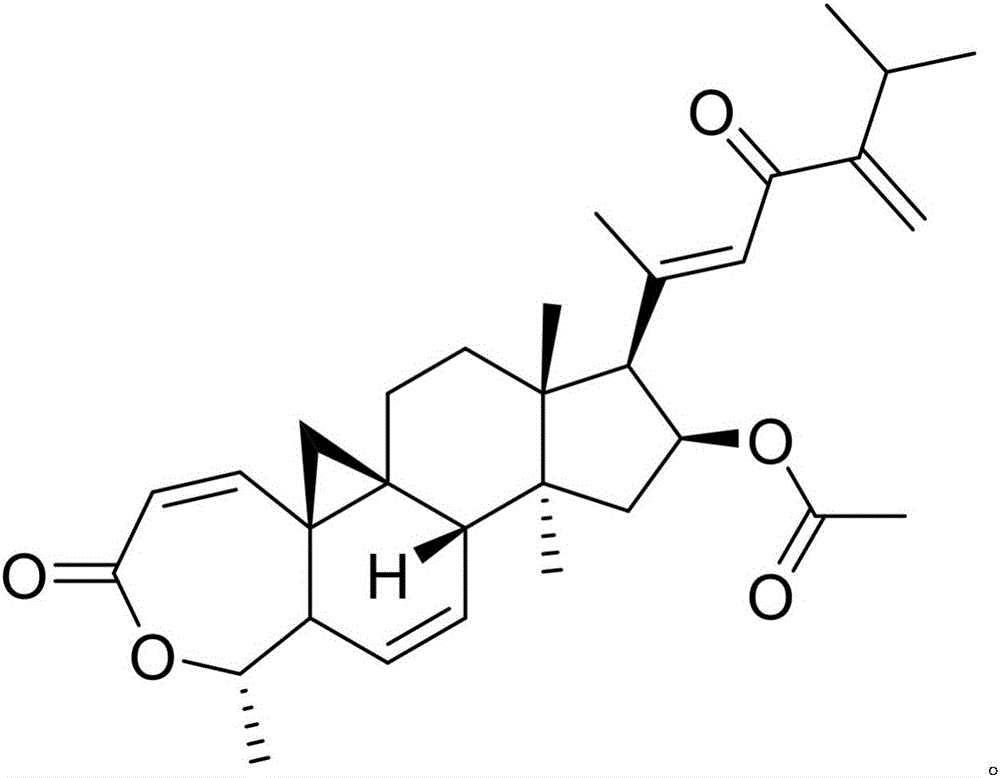
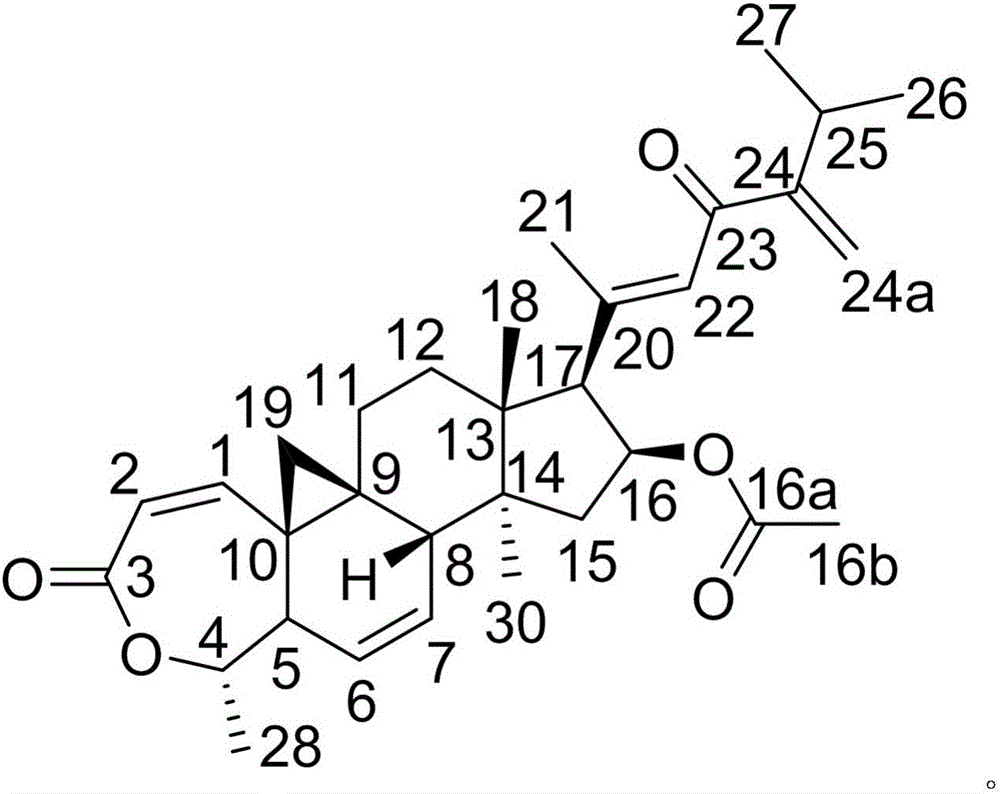
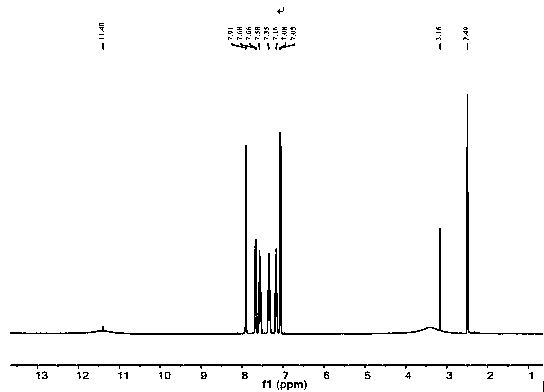
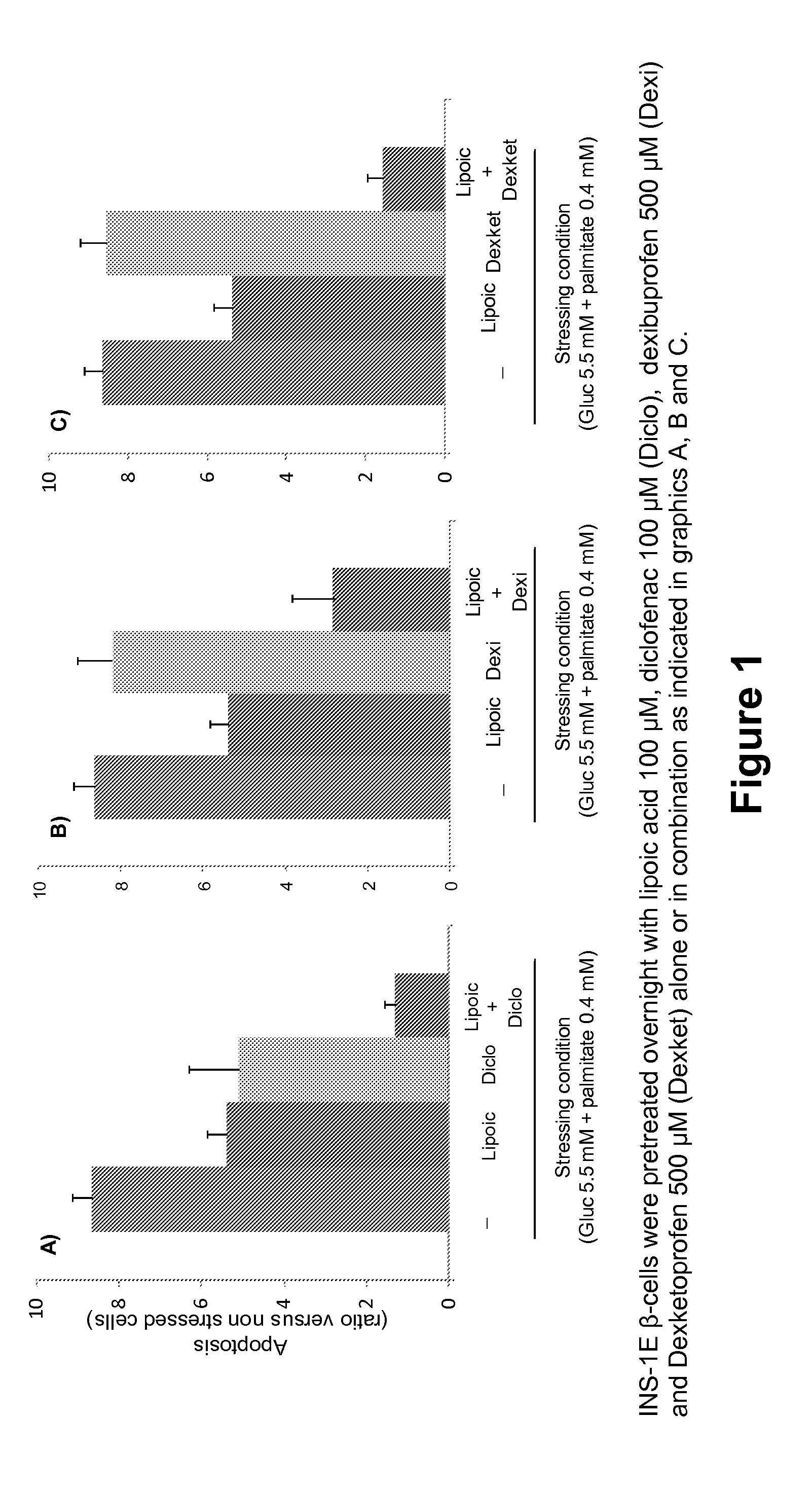
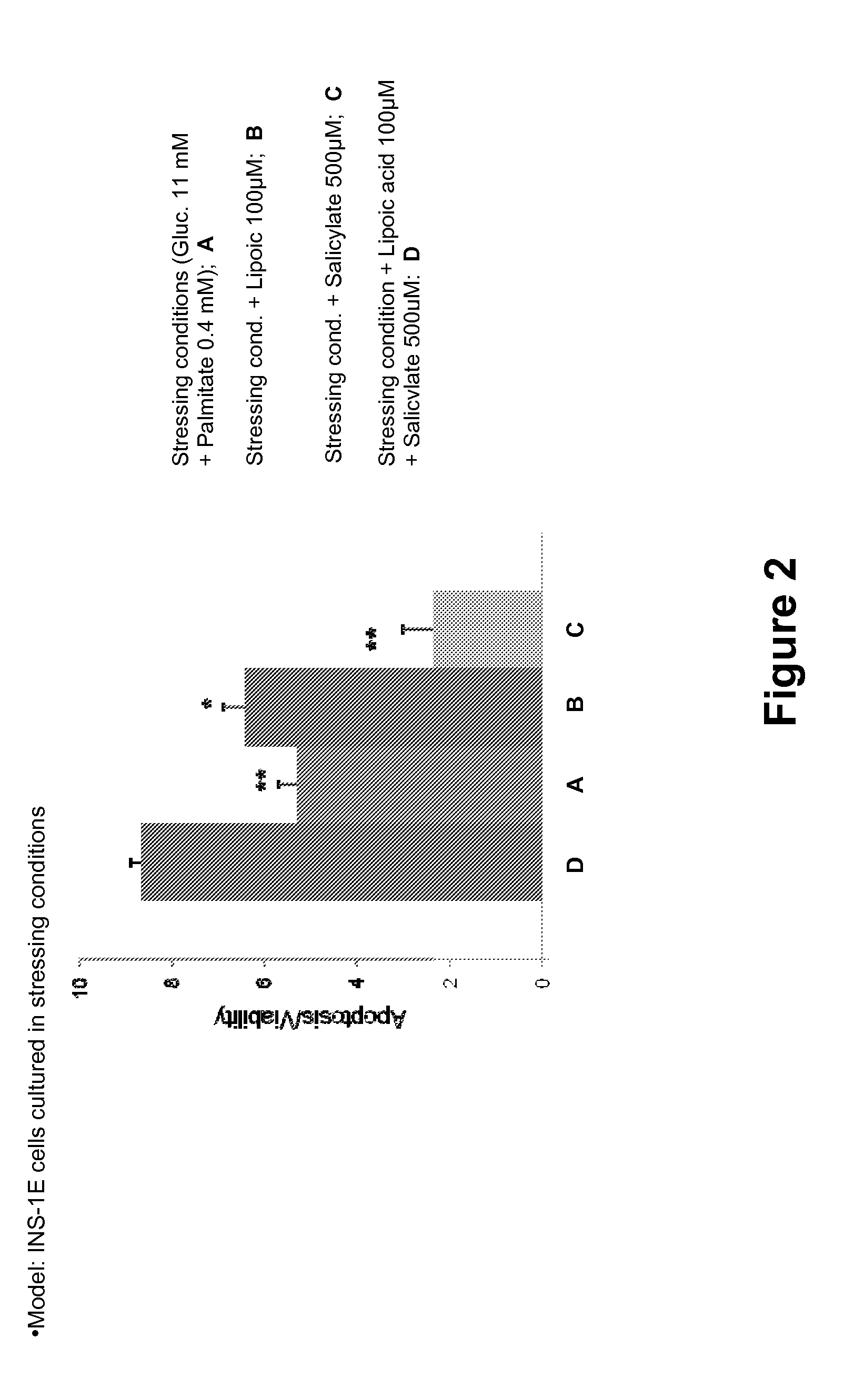
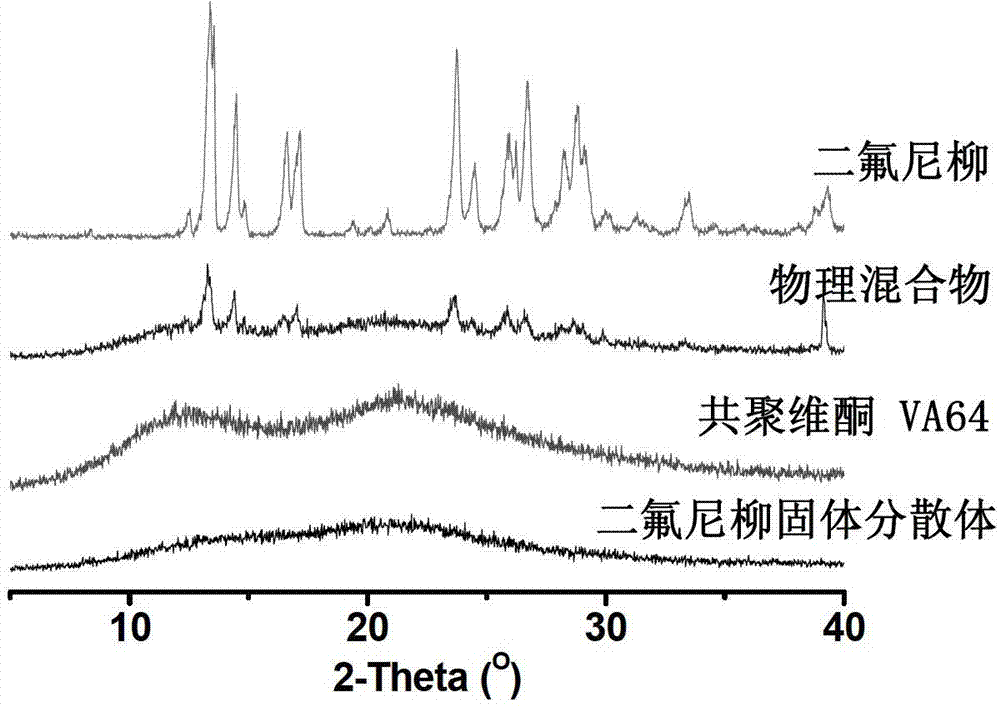
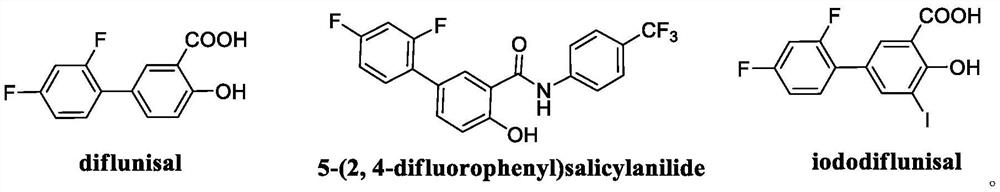
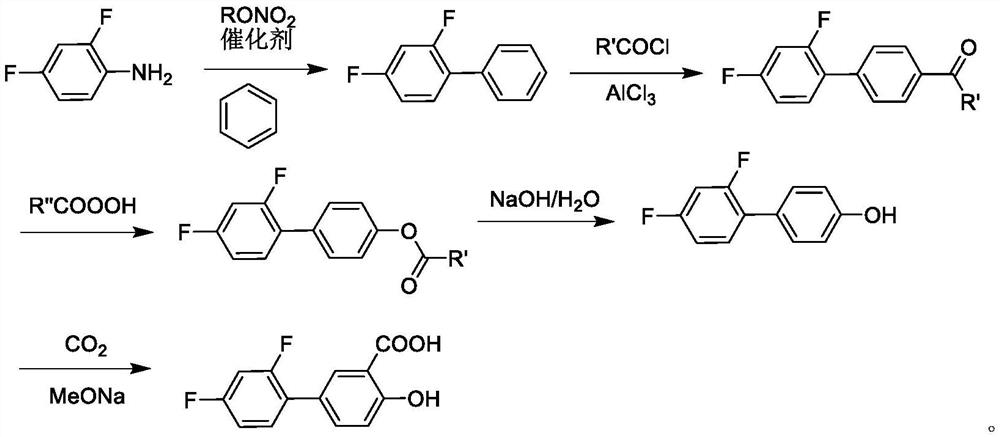
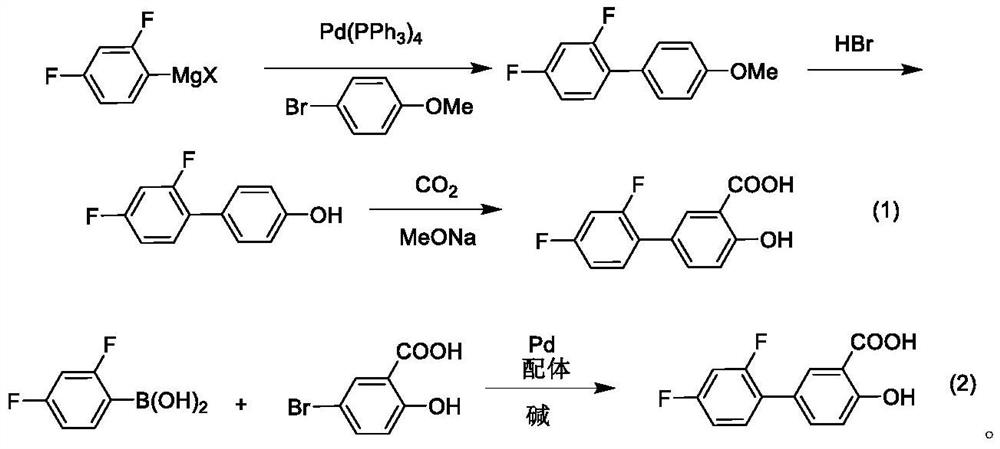
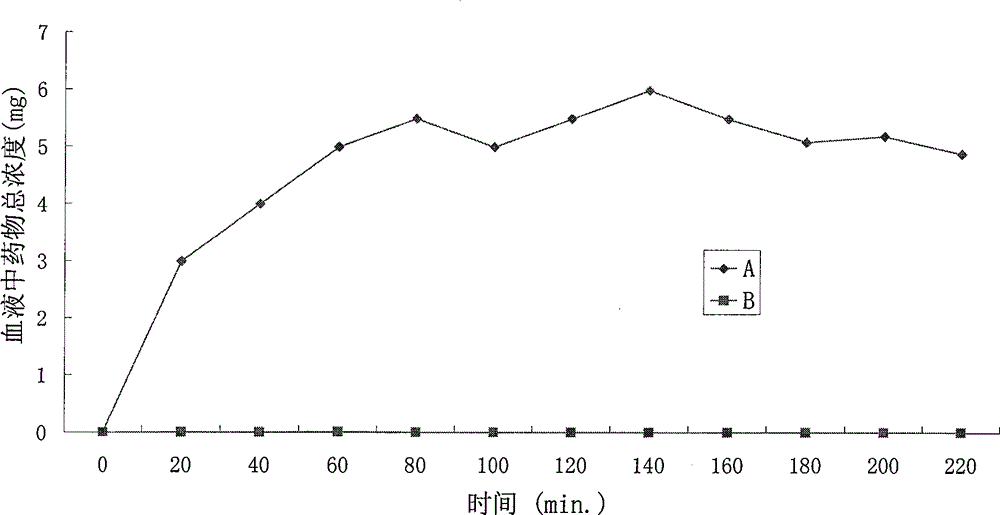
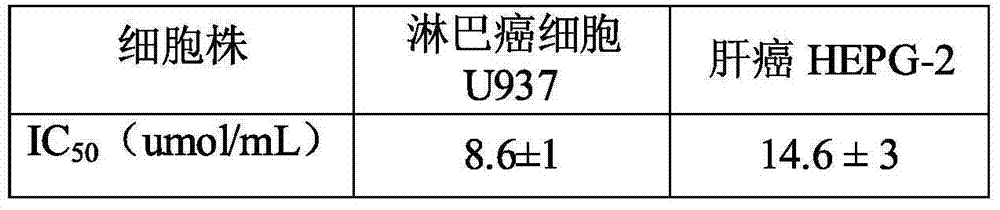
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30 results about "Diflunisal" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
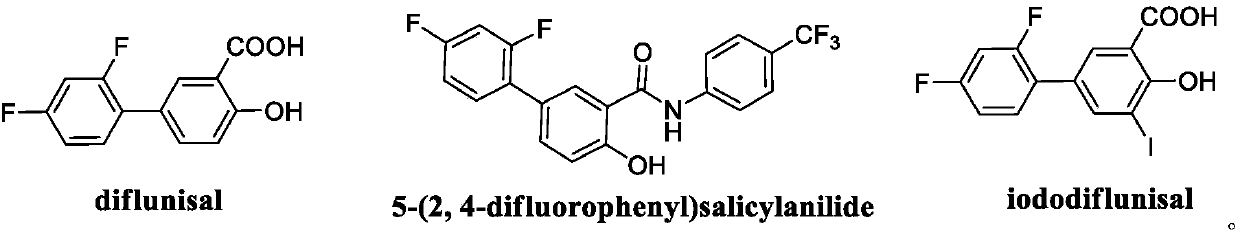
Diflunisal is used to relieve mild to moderate pain from various conditions. It also reduces pain, swelling, and joint stiffness caused by arthritis.
Topical formulation having effects on alleviating pain/inflammation caused by herpes virus infection
InactiveUS7132452B2Fasten skin recoveryPromote recoveryPowder deliveryBiocideComplete remissionTolmetin
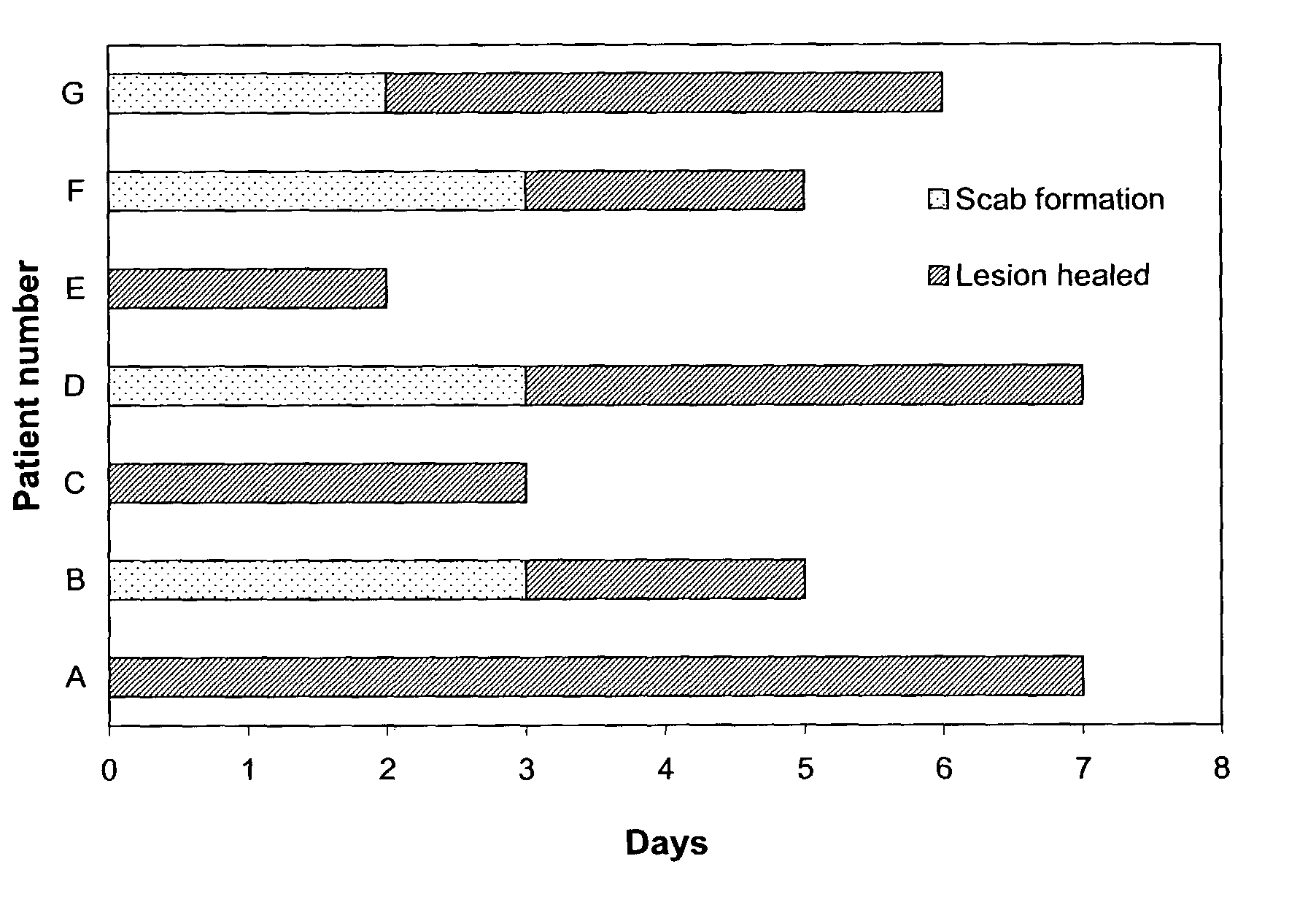
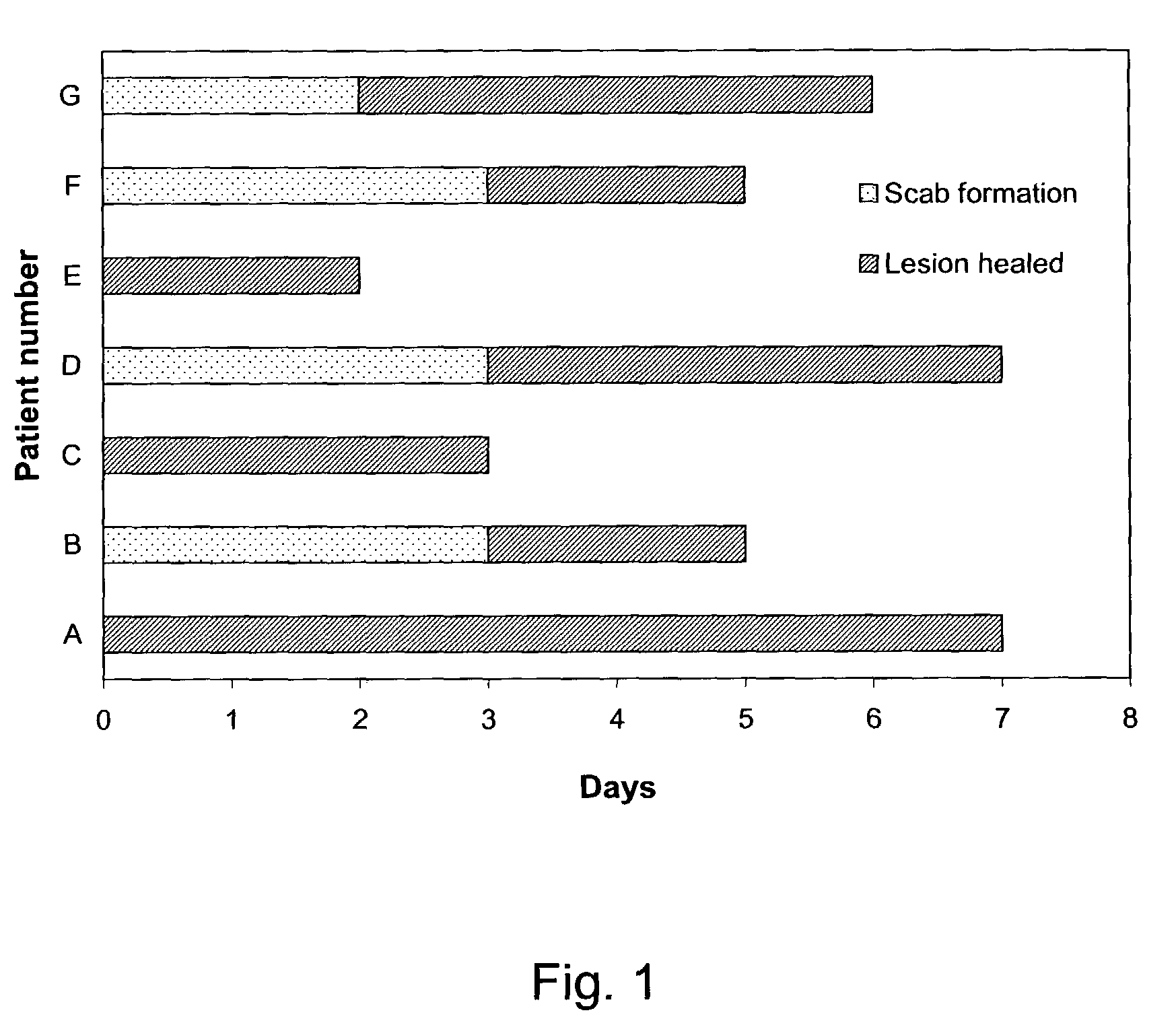
The present invention provides a topical formulation containing NSAID, particularly diclofenac. The topical formulation is particularly useful for alleviating pain / inflammation associated with infection caused by herpes virus, especially herpes simplex virus (HSV) and varicella-zoster virus (VZV). Similar relief can be achieved where diclofenac is replaced with another non-steroidal anti-inflammatory drug (NSAID), which includes, without limitation, etodolac, ketorolac, bromfenac, diflunisal, ibuprofen, fenoprofen, ketoprofen, naproxen, suprofen, meclofenamate, mefenamic acid, piroxicam, meloxicam, indomethacin, sulindac, phenylbutazone, oxyphenbutazone, and tolmetin. The topical formulation is further characterized by its fast relief on pain and / or inflammation associated with infection caused by herpes virus, i.e., a complete relief in no more than seven (7) days after the application of the topical formulation on skins of patients.
Owner:YUNG SHIN PHARMACEUTICALS INDUSTRIAL CO LTD
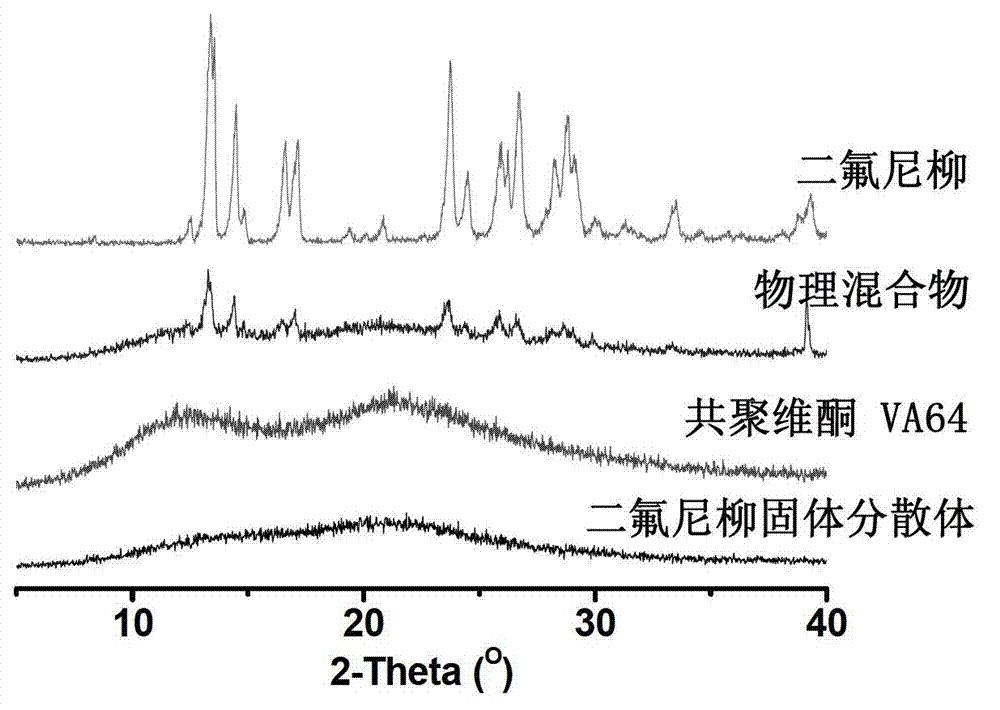
Diflunisal solid dispersion and preparation method thereof
ActiveCN103202811AReduce adverse reactionsNo solvent residueOrganic active ingredientsPowder deliverySolubilityPolyethylene glycol
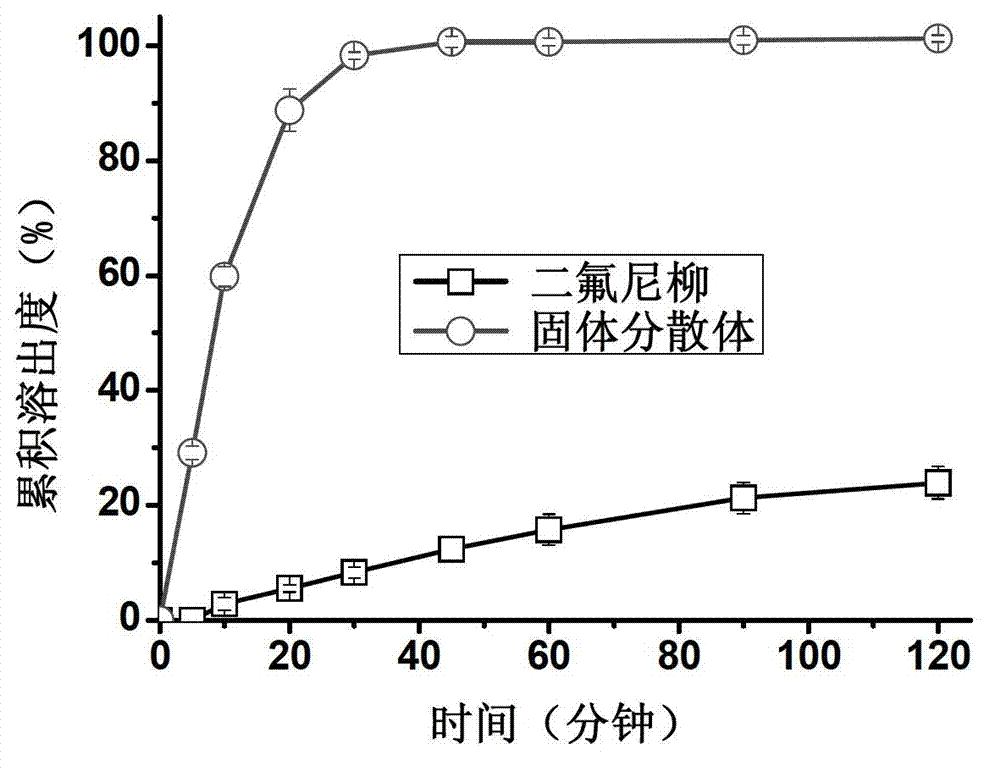
The invention discloses a diflunisal solid dispersion and a preparation method thereof. The solid dispersion is composed of diflunisal as active components and high-polymer carrier materials. The high-polymer carrier materials are 40-90% of the diflunisal in total and selected from povidones, copovidones, and polyethylene glycol / vinyl caprolactam / vinyl acetate copolymer or hydroxypropyl methylcellulose. The preparation method adopts a hot-melting extrusion method. The diflunisal solid dispersion obtained is higher in solubility and quick and high in dissolving, bioavailability of indissolvable drugs is improved, drug dosage is reduced, and adverse drug reaction is reduced.
Owner:SUN YAT SEN UNIV
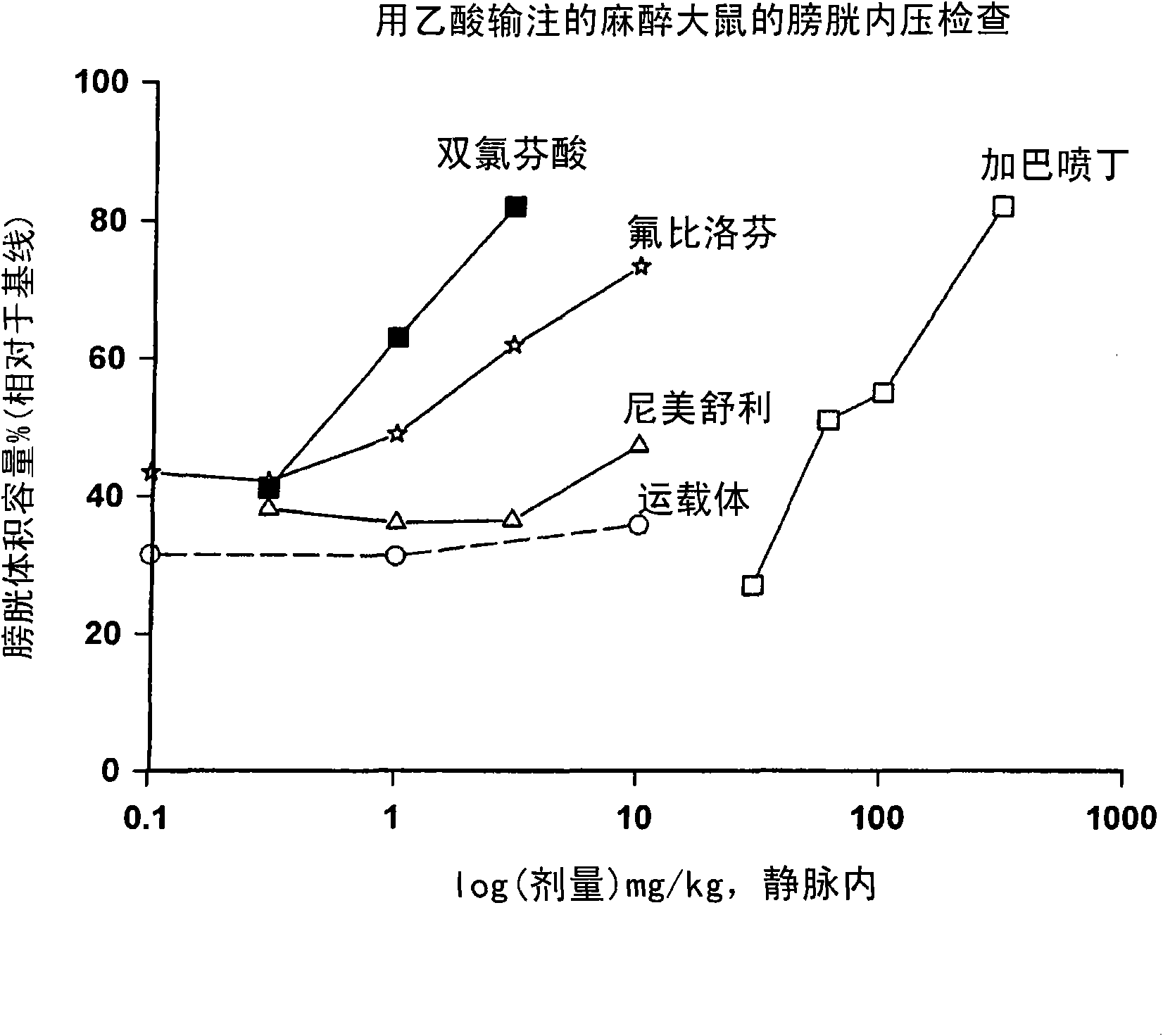
Combination therapy of lower urinary tract disorders with a2d ligands and nsaids
The administration to a mammal of a combination of compounds, at least one of which is an a2d calcium channel subunit (A2d) ligand and at least one of which is a non-steroidal anti-inflammatory drug (NSAID), provides a surprising and potent inhibition of the micturition reflex, superior to that obtained by treatment with an A2d ligand or NSAID alone. Combinations of A2d ligand and NSAIDs are thus useful for treatment of lower urinary tract disorders and symptoms thereof. Preferred A2d ligands are gabapentin and pregabalin. Preferred NSAIDs are celecoxib, diclofenac, diflunisal, flurbiprofen, naproxen, nimesulide or sulindac.
Owner:RECORDATI IRELAND LTD
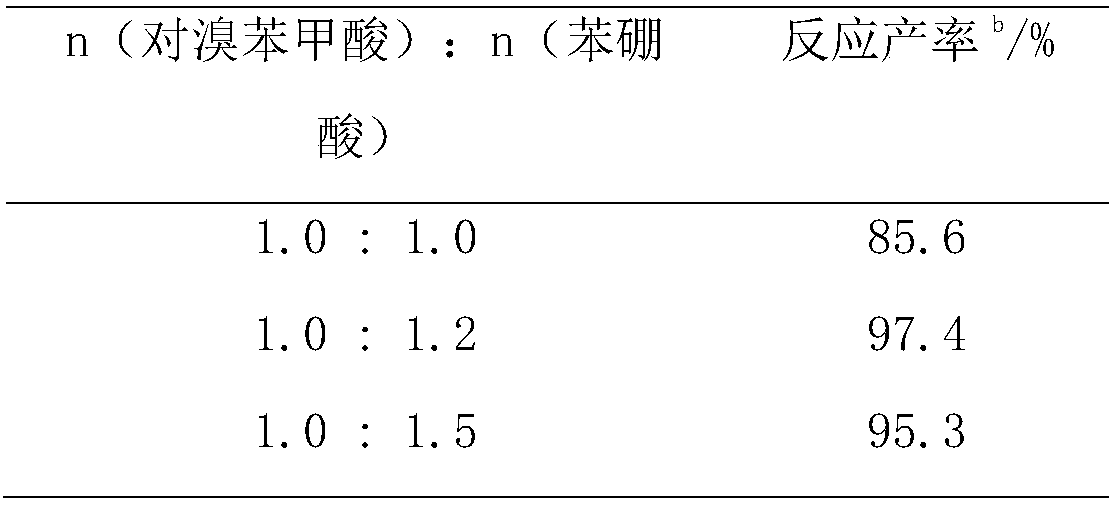
Method for synthesizing biphenylcarboxylic acid compound by using Suzuki coupling reaction
InactiveCN108484385AStable in natureImprove natureOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsTemperature treatmentChemistry
The invention provides a method for synthesizing a biphenylcarboxylic acid compound by using the Suzuki coupling reaction. According to the method, brominated aromatic hydrocarbon and arylboronic acidare used as raw materials, and water-soluble fullerene nanopalladium is used as a catalyst; and the equation of the Suzuki coupling reaction is as described in the specification. In the equation, R1and R2 represent substituents at different positions, may be acceptor or donor substituents, and may be monosubstitutents or polysubstitutent; and R1 and R2 may be identical or different groups. The water-soluble fullerene nanopalladium catalyst is cheap, easily available and environmentally friendly, and has high catalytic activity and stable properties. When the catalyst is used for catalysis ofthe Suzuki coupling reaction, conditions are mild, anhydrous anaerobic treatment and high-temperature treatment are not needed, and cost is low. The method can be applied to the industrial synthesisof non-steroidal anti-inflammatory drugs such as diphenylacetic acid and diflunisal.
Owner:YICHUN UNIVERSITY
Novel diflunisal esters and related compounds
InactiveUS20030220497A1Inhibit synthesisInhibits platelet aggregationOrganic chemistryOrganic compound preparationDiflunisalAnti platelet
O-medium alkyl esters of diflunisal and related compounds are disclosed having anti-platelet activity, hydroxy radical scavenging properties, enhanced hepatic clearance and low ulcerogenic potential. These compounds have general formula (I) wherein n equals 3-13.
Owner:THE UNIV OF QUEENSLAND
Self-assembly system based on hydrophilic polymer and medicine and preparation method thereof
ActiveCN102091332AEasy to realize industrializationEasy to makeMacromolecular non-active ingredientsIndometacinPolymer science
The invention relates to a self-assembly system based on hydrophilic polymer and medicine and a preparation method thereof. The system consists of a hydrophilic polymer and a carboxyl-containing medicine, wherein the hydrophilic polymer is polymine or beta-cyclodextrin modified polymine; and the carboxyl-containing medicine is selected from ibuprofen, ketoprofen, fenoprofen, flurbiprofene, oxaprozin, naproxen, indometacin, sulindac, etodolac, diclofenac, pontal, meclofenamic acid, flufenamic acid, tolfenamic acid, lumiracoxib, licofelone, diflunisal and aspirin. The system is prepared by dissolving the hydrophilic polymer in a certain amount of water, dissolving the medicine in a water soluble solvent, slowly adding the organic solution of the medicine into the aqueous solution of the polymer under the action of ultrasound, placing the mixed solution in a dialysis bag, dialyzing in deionized water with magnetic stirring, replacing the deionized water at certain time interval, filtering dislysate after 5 to 48 hours, cooling and drying.
Owner:ARMY MEDICAL UNIV
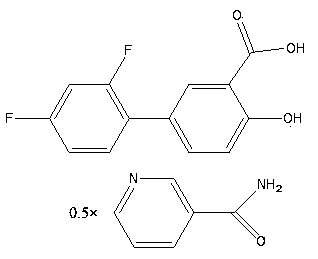
Eutectic of diflunisal and pyridine formamide compounds and preparation method thereof
The invention belongs to the chemical pharmaceutical technical field and in particular relates to an eutectic medicine of 2 ',4 '- difluoro-4-hydroxyl-3-biphenyl carboxylic acid and pyridine carboxamide compounds and a preparation method thereof. The eutectic medicine prepared in the invention is obviously improved in solubility.
Owner:SUZHOU INST OF NANO TECH & NANO BIONICS CHINESE ACEDEMY OF SCI
Diflunisal slow-releasing preparation and its making method
InactiveCN1895258AComposition controlControlled release rateOrganic active ingredientsAntipyreticDiflunisalHydrotalcite
A slow-releasing diflunisal with high anti-inflammatory and antalgic effect and its preparing process are disclosed. It uses the anionic laminar hydrotalcite as its main body and has the intercalated hydrotalcite crystal structure.
Owner:ZHEJIANG UNIV OF TECH
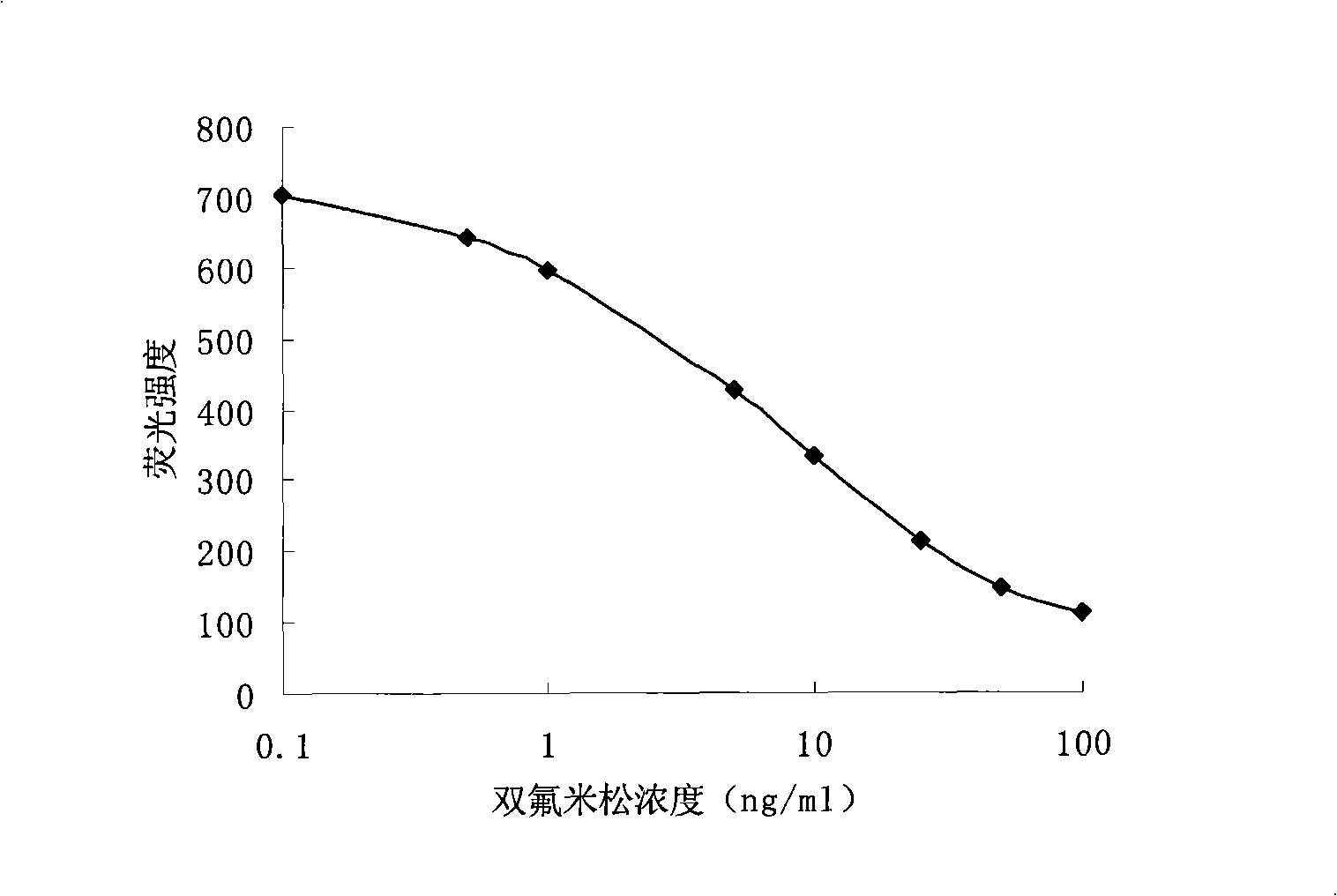
Method for quantum dot mark indirect competition fluoroimmunoassay detection for diflunisal
InactiveCN101308147AEasy to operateHigh fluorescence intensityBiological testingFluorescence/phosphorescenceAntigenImmuno detection
Disclosed is a method of quantum dot-labeled indirect competitive fluorescence immunoassay of diflucortolone, which belongs to the immunoassay method technique field. Quantum dots for labeling antibodies of the invention have the emission spectra of QD650, and the method comprises: directly covering coating antigens in micro-holes of an enzyme label plate, adding diflucortolone standard solution or a sample under test to form an antigen-antibody fluorescence immunity compound body, stimulating and detecting the fluorescence intensity of the formed antigen-antibody fluorescence immunity compound body with a fluorescence enzyme-labeling instrument, and obtaining the concentration of diflucortolone in the sample under test through comparing with the standard solution. The invention can detect the content of diflucortolone in the sample under test without adding chromogenic substance, namely, the concentration of diflucortolone in the sample under test can be detected indirectly through the fluorescence intensity of the antigen-antibody immunity compound body, and both the operation and reaction need only one step; and the quantum dots for labeling antibodies of the invention have advantages of stronger emitted fluorescence intensity and long stabilization time of fluorescence compared with the traditional fluorescence.
Owner:JIANGNAN UNIV
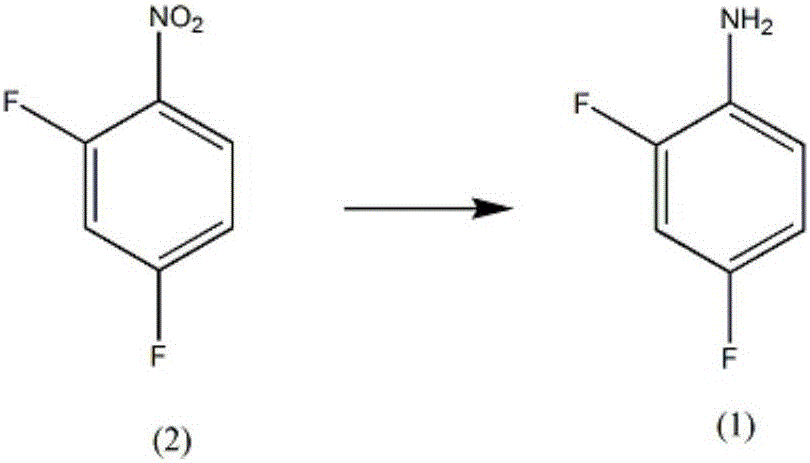
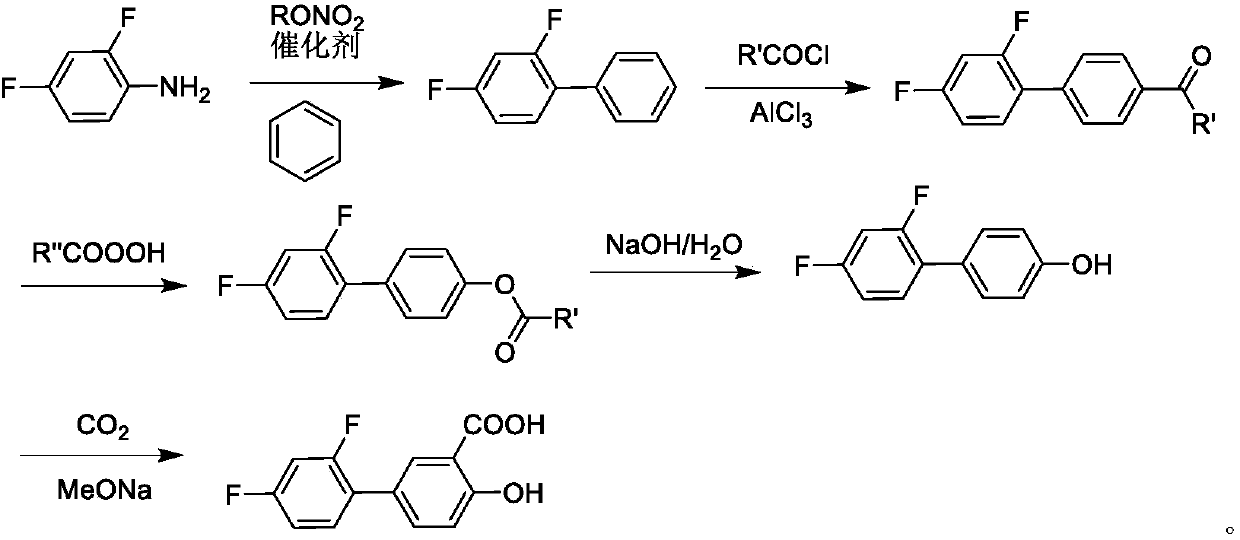
Synthesis method of diflunisal drug intermediate 2,4-difluoroaniline
InactiveCN106008226AHigh reaction yieldOrganic compound preparationAmino compound preparationAcetic acidTrifluoroacetic acid
The invention discloses a synthesis method of a diflunisal drug intermediate 2,4-difluoroaniline. The method comprises the following steps: adding 2,4-difluoronitrobenzene and molybdenum powder to a 2,6-dimethoxyphenol solution, heating the obtained solution to 50-56DEG C, refluxing the solution for 3-5h, carrying out water vapor distillation, carrying out trifluoroacetic acid solution extraction, washing the obtained extract with a salt solution, dewatering the washed extract with a dewatering agent, carrying out reduced pressure distillation, and collecting a 50-55DEG C fraction to obtain 2,4-difluoroaniline. The reaction yield of the above reaction can reach 94% or above, and is obviously higher than that in the background technologies. The invention also provides a new synthesis route. The new synthesis route lays a good foundation for further increasing the reaction yield.
Owner:CHENGDU QIANYE LONGHUA PETROLEUM ENG TECH CONSULTING
Preparation method of active chlorine-resistant and formaldehyde-free color fixing agent, and color fixing agent prepared by method
ActiveCN106758413AImprove wet fastnessImprove color fixationDyeing processPolymer scienceCellulose fiber
The invention discloses a preparation method of an active chlorine-resistant and formaldehyde-free color fixing agent, and the color fixing agent prepared by the method. The preparation method comprises the steps of mixing acrylamide with diflunisal, grinding an obtained mixture, adding a polyethylene glycol monomethyl ether solution, heating up to 68 DEG C, and carrying out stirring treatment for 75min at the temperature of 68 DEG C; after that, adding water glass and a hydrochloric acid solution into an obtained mixed solution, stirring for 33min at the temperature of 62 DEG C, then heating up to 88 DEG C, and dropwise adding n-butyl titanate; then, stirring for 80min at the temperature of 88 DEG C to obtain the color fixing agent. The formaldehyde-free color fixing agent prepared by the method is mainly used for improving the wet-treatment fastness of cellulose fibers / reactive dye-dyed materials, and is excellent in color fixation effect and very high in alkali resistance and high temperature resistance; the dyed materials treated by using the color fixing agent seldom change color, and the color fixing agent does not have a bad influence on the bright colors of reactive dyes; the fabric treated by the color fixing agent does not change color, and the color fixing agent does not affect the chlorine fastness, light fastness and colorfastness to perspiration-light of the dyed materials. The raw materials of the color fixing agent are simple and wide in source, so that the color fixing agent is low in production cost; the color fixing agent does not contain formaldehyde, thus being green and environment-friendly; the color fixing agent improves the quality of the fabric.
Owner:江西德盛精细化学品有限公司
Positively charged water-soluble prodrugs of diflunisal and related compounds with very fast skin penetration rate
The novel positively charged pro-drugs of diflunisal, salicylsalicylic acid, and salicylic acid in the general formula(1) 'Structure 1' and general formula(2) 'Structure 2' were designed and synthesized. The compounds of the general formula(1) 'Structure 1' or general formula(2) 'Structure 2' indicated above can be prepared from functional derivatives of diflunisal, salicylsalicylic acid, or salicylic acid, (for example acid halides or mixed anhydrides), by reaction with suitable alcohols, thiols, or amines. The positively charged amino groups of these pro-drugs not only largely increases the solubility of the drugs, but also bonds to the negative charge on the phosphate head group of membranes and pushes the pro-drug into the cytosol. The results suggest that the pro-drug, diethylaminoethyl 5-(2,4-difluorophenyl) salicylate. AcOH diffuses through human skin ~150 times faster than does diflunisal itself. In plasma, more than 90% of these pro-drugs can change back to the drug in a few minutes. The prodrugs can be used medicinally in treating any diflunisal, salicylsalicylic acid, or salicylic acid-treatable conditions in humans or animals and be administered not only orally, but also transdermally for any kind of medical treatments and avoid most of the side effects of diflunisal, salicylsalicylic acid, or salicylic acid, most notably GI disturbances such as dyspepsia, gastroduodenal bleeding, gastric ulcerations, and gastritis. Controlled transdermal administration systems of the prodrug enables diflunisal, salicylsalicylic acid, or salicylic acid to reach constantly optimal therapeutic blood levels to increase effectiveness and reduce the side effects of diflunisal, salicylsalicylic acid, or salicylic acid.
Owner:TECHFIELDS BIOCHEM CO LTD
Diflunisal pharmaceutical composition and medical application thereof
InactiveCN106046112AIncrease the number of cellsHas therapeutic effectOrganic active ingredientsAntiviralsNatural productMedicine
The invention discloses a diflunisal pharmaceutical composition and medical application thereof. The diflunisal pharmaceutical composition provided by the invention contains diflunisal and a natural product compound (I) novel in structure, when diflunisal and the compound (I) independently affect, CD4<+> cell number and immunologic function can be improved, immunologic balance can be adjusted, and therapeutical effect on Aids is realized; and when diflunisal and the compound (I) jointly act, the therapeutical effect is further improved, a medicine for treating the Aids can be developed, and prominent substantiality characteristic and obvious progress are realized compared with the prior art.
Owner:胡逸穹
Diflunisal enteric-coated tablet and preparation method thereof
InactiveCN109758432ADissolution rate is fastImprove bioavailabilityOrganic active ingredientsAntipyreticSide effectClinical efficacy
The invention relates to a diflunisal enteric-coated tablet and a preparation method thereof. The tablet is prepared from the following components by the weight percentage: diflunisal, a filling agent, a disintegrating agent, a proper amount of an adhesive, a proper amount of a lubricant, an isolation layer of a coating solution and a casing. The clinical curative effect of diflunisal is improved,the irritation of drugs in stomach is avoided, and the irritation of drugs in intestinal tracts is reduced, so the side effects are reduced, quick and efficient effects are achieved, and the bioavailability is significantly improved. Compared with the prior art, the diflunisal enteric-coated tablet has the advantages of novel dosage form, convenient taking, quick action, convenience in carrying,transportation and storage, convenience in use and the like.
Owner:ZHENGZHOU TAIFENG PHARMA CO LTD
Method for one-step synthesis of non-steroidal anti-inflammatory drug diflunisal
ActiveCN108610253AImprove responseEasy to operateOrganic compound preparationCarboxylic compound preparationOrganic solventOrganic synthesis
Relating to the field of organic synthesis, the invention discloses a method for one-step synthesis of non-steroidal anti-inflammatory drug diflunisal. The method includes: controlling the charging sequence, taking 2, 4-difluorophenylboronic acid and 5-bromosalicylic acid as the raw materials, taking a mixed solution of an aqueous phase and an organic solvent as the solvent, conducting ligand-freepalladium catalysis in the presence of an alkali metal salt, carrying out catalytic reaction under the ultrasonic condition of 70-80DEG C for 90-110min for one-step synthesis of a diflunisal crude product, and further conducting refining to obtain the diflunisal product with yield of 98.37% and purity of 99.89%. The method has the characteristics of green and high efficiency, mild reaction conditions, simple and safe process, and the obtained product has high yield and good purity, and has important industrial prospects.
Owner:XIANGTAN UNIV
Combination Therapies For Treating Metabolic Disorders
InactiveUS20140357602A1Improve the level ofAvoid complicationsBiocideSalicyclic acid active ingredientsCombined Modality TherapyInsulin resistance
Owner:GENMEDICA THERAPEUTICS SL
Diflunisal solid dispersion and preparation method thereof
ActiveCN103202811BReduce adverse reactionsNo solvent residuePowder deliveryOrganic active ingredientsSolubilityPolyethylene glycol
The invention discloses a diflunisal solid dispersion and a preparation method thereof. The solid dispersion is composed of diflunisal as active components and high-polymer carrier materials. The high-polymer carrier materials are 40-90% of the diflunisal in total and selected from povidones, copovidones, and polyethylene glycol / vinyl caprolactam / vinyl acetate copolymer or hydroxypropyl methylcellulose. The preparation method adopts a hot-melting extrusion method. The diflunisal solid dispersion obtained is higher in solubility and quick and high in dissolving, bioavailability of indissolvable drugs is improved, drug dosage is reduced, and adverse drug reaction is reduced.
Owner:SUN YAT SEN UNIV
Application of diflunisal in preparation of drug for preventing and treating diabetes
InactiveCN108478583AGood conditionPromote absorptionOrganic active ingredientsMetabolism disorderPharmaceutical drugDiabrezide
The invention aims to provide application of diflunisal in preparation of a drug for preventing and treating diabetes. The diflunisal is used as an effective component in the drug for preventing and treating the diabetes. The concentration of the diflunisal in the drug for preventing and treating the diabetes is 40 to 80 [mu]M. The diflunisal can be well applied to the drug for preventing and treating the diabetes, particularly type II diabetes; the diflunisal can obviously improve insulin resistance and improve the absorption of hepatic cells under insulin resistance for glucose; the effect is outstanding; the aim of preventing and improving the diabetic condition is achieved.
Owner:ZHEJIANG UNIV
Prodrugs of positively charged water-soluble diflunisal and related compounds
ActiveCN105439877BGood absorption rateGood curative effectOrganic active ingredientsSenses disorderSide effectOral medication
The present invention relates to novel positively charged diflunisal, salicyl salicylic acid and prodrugs of salicylic acid shown in general formula (1) "structural formula 1" and general formula (2) "structural formula 2" design and synthesis. The compounds in the above general formula (1) "structural formula 1" and general formula (2) "structural formula 2" can be prepared by reacting diflunisal, salicyl salicylic acid or salicylic acid with appropriate alcohols, mercaptans, or amines have to. These prodrugs can be used medicinally in the treatment of any diflunisal, salicylsalicylic acid or salicylic acid treatable state in humans or animals, not only by oral administration but also by transdermal administration in the treatment, thereby avoiding the Most side effects of diflunisal, salicylsalicylic acid, or salicylic acid were eliminated. The controlled-release transdermal drug delivery system of the prodrug can stabilize the blood drug concentration of diflunisal, salicylsalicylic acid or salicylic acid at the optimal therapeutic level, thereby improving the curative effect and reducing diflunisal, salicylsalicylic acid or salicylic acid. Salicylic acid side effects.
Owner:TECHFIELDS BIOCHEM CO LTD
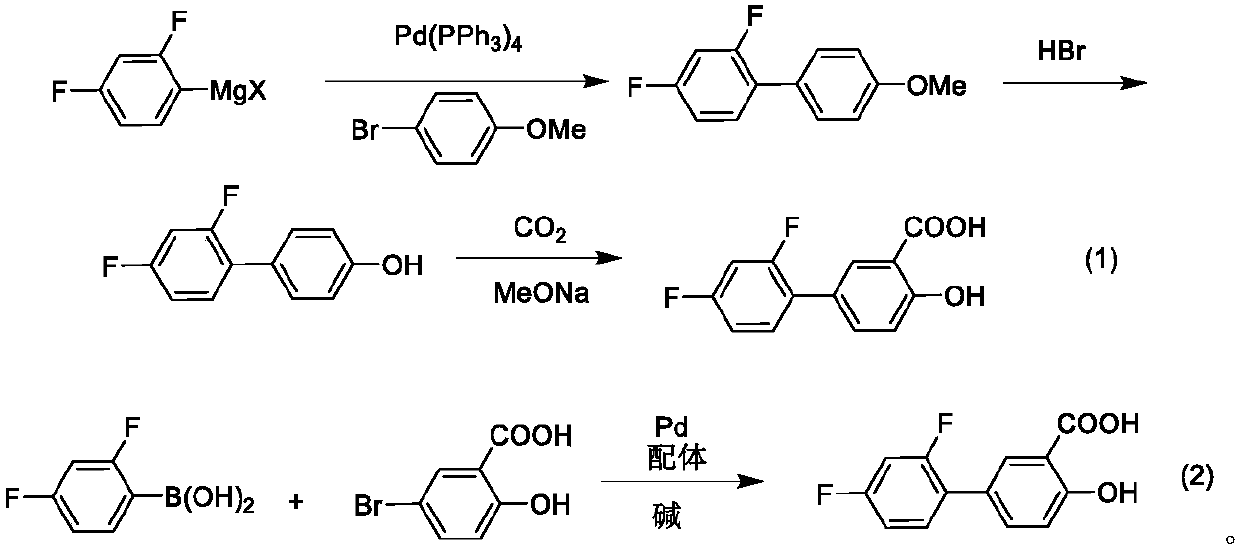
A kind of method for synthesizing diflunisal and its derivatives by one-step method
ActiveCN110372493BLow costEfficiently obtainedPreparation from carboxylic acid saltsOrganic compound preparationIron saltsGrignard reagent
The invention discloses a method for synthetizing diflunisal and a derivative thereof through a one-step method. The method comprises the steps: under joint catalysis of an iron salt, a ligand and titanate, 2,4-difluorophenylmagnesium halide and 5-halogenated salicylic acid or a 5-halogenated salicylic acid derivative are mixed, heated and coupled in a solvent, and the diflunisal and the derivative thereof are obtained. The method has the advantages that (1) high-priced palladium or high-toxicity nickel does not need to be adopted as catalytic metal, only low-toxicity, high-yield and inexpensive iron salts and titanate need to be used, thus the cost is low, and environmental friendliness is achieved; (2) a zinc salt does not need to be used, or step preparation of a boron reagent is not needed, a Grignard reagent is directly used, the preparation steps are few, and raw material and energy consumption is low; and (3) operation is easy and convenient, conditions are mild, amplification is easy, and the method is suitable for industrial production.
Owner:BEIJING NORMAL UNIVERSITY
Positively charged water-soluble prodrugs of diflunisal and related compounds with fast skin penetration rates
The novel positively charged pro-drugs of diflunisal, salicylsalicylic acid, and salicylic acid in the general formula(1) 'Structure 1' and general formula(2) 'Structure 2' were designed and synthesized. The compounds of the general formula(1) 'Structure 1' or general formula(2) 'Structure 2' indicated above can be prepared from functional derivatives of diflunisal, salicylsalicylic acid, or salicylic acid, (for example acid halides or mixed anhydrides), by reaction with suitable alcohols, thiols, or amines. The positively charged amino groups of these pro-drugs not only largely increases the solubility of the drugs, but also bonds to the negative charge on the phosphate head group of membranes and pushes the pro-drug into the cytosol. The results suggest that the pro-drug, diethylaminoethyl 5-(2,4-difluorophenyl) salicylate. AcOH diffuses through human skin ~150 times faster than does diflunisal itself. In plasma, more than 90% of these pro-drugs can change back to the drug in a few minutes. The prodrugs can be used medicinally in treating any diflunisal, salicylsalicylic acid, or salicylic acid-treatable conditions in humans or animals and be administered not only orally, but also transdermally for any kind of medical treatments and avoid most of the side effects of diflunisal, salicylsalicylic acid, or salicylic acid, most notably GI disturbances such as dyspepsia, gastroduodenal bleeding, gastric ulcerations, and gastritis. Controlled transdermal administration systems of the prodrug enables diflunisal, salicylsalicylic acid, or salicylic acid to reach constantly optimal therapeutic blood levels to increase effectiveness and reduce the side effects of diflunisal, salicylsalicylic acid, or salicylic acid.
Owner:TECHFIELDS BIOCHEM CO LTD
Diflunisal film coating agent and preparation method thereof
InactiveCN106361729AIncrease elasticityStrong transdermal absorptionOrganic active ingredientsAntipyreticGlycerolHepatic first pass effect
The invention discloses a diflunisal film coating agent. The diflunisal film coating agent is prepared from, by weight, 1-5 g of diflunisal, 5-15 g of chitosan, 80-150 g of glycerin, 2-7 g of azone, 200-800 ml of purified water and a full dosage of an ethyl alcohol solution. A preparation method of the diflunisal film coating agent comprises the following steps that 1, the chitosan is uniformly dispersed into the surface of purified water, natural soluble inflation is performed, polyvinyl alcohol is added, and stirring is performed to obtain a film formation matrix; 2, diflunisal is added in a glycerin ethyl alcohol solution to be dissolved, and the mixture is added into the film formation matrix in the step 1; 3, ethyl alcohol is added to reach the volume of 1,000 ml, sufficient stirring is performed, and the diflunisal film coating agent is obtained. According to the diflunisal film coating agent, on the basis of partial skin dosage, the liver first-pass effect and the gastrointestinal tract effect are overcome, the bioavailability is improved, and the curative effect is enhanced; the preparation method is reasonable, the content measuring method is high in specificity, the result is accurate, and it is indicated that the established standard can be used for quality control over preparations.
Owner:CHENGDU XIANXIANXIAN BIOTECH CO LTD
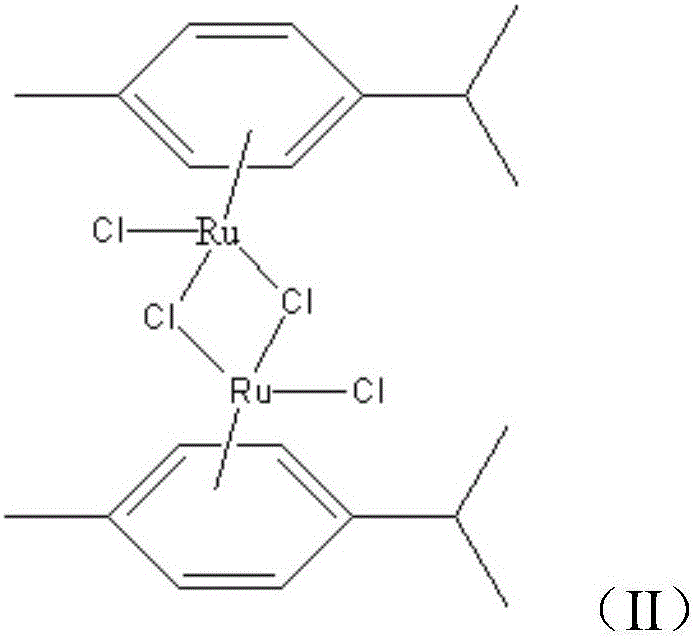
Fluorine benzene ruthenium compound and preparation method and application thereof
InactiveCN104844660AEasy to prepareRaw materials are easy to getRuthenium organic compoundsAntineoplastic agentsSolubilityBenzene
The present invention provides a fluorine benzene ruthenium compound and a preparation method and application thereof. The structure of the fluorine benzene ruthenium compound is shown in formula (I). The fluorine benzene ruthenium compound is synthesized and generated by dichloridized dichloro-2-methyl-isopropyl-benzo dirhodium and phenylacetyl diflunisal and shows a relatively strong inhibition activity on a human lymph cancer cell strain and a liver cancer strain of a human body. The fluorine benzene ruthenium compound can be used for preparing medicines for treating liver cancer and lymph cancer, and can be prepared to injections, tablets, pellets, capsules, suspensions or emulsions. The fluorine benzene ruthenium compound provided by the invention can improve the lipid solubility and the permeability of medicines, enhances the dissolubility of a biological film and prompts the speed of absorption and transmission in vivo, so that the physiological action changes.
Owner:THE SIXTH CONSTR CO LTD OF CHINA NAT CHEM ENG
Method for simultaneously determining contents of coexisting impurities in 2, 4-difluoroaniline
The invention discloses a method for simultaneously determining contents of coexisting impurities in 2, 4-difluoroaniline. According to the method, the contents of coexisting impurities in 2, 4-difluoroaniline are detected by gas chromatography. With the method, the chromatographic peaks are high in separation degree and no mutual interference exists; the peak pattern is good and impurities such as 2, 6-difluoroaniline, 1, 3-dichlorobenzene, 2, 6-difluoronitrobenzene, 2, 4-difluoronitrobenzene, 2, 6-dichloronitrobenzene and 2, 4-dichloronitrobenzene can be accurately detected at the same time.The detection method is operated simply and is easy to control with the low detection cost; the method has a good linear relation, high specificity, precision, stability, sensitivity and repeatability, and high sample adding recovery rate and the detection result is accurate. The quality stability and clinical medication safety of new drugs such as diflunisal and tosufloxacin prepared by taking 2, 4-difluoroaniline as a starting material can be guaranteed.
Owner:HINYE PHARM CO LTD
A kind of fluorophenyl ruthenium compound and its preparation method and application
InactiveCN104844660BEasy to prepareRaw materials are easy to getRuthenium organic compoundsAntineoplastic agentsSolubilityCancer cell
Owner:THE SIXTH CONSTR CO LTD OF CHINA NAT CHEM ENG
Medicine for treating lupus nephritis and preparation method and application thereof
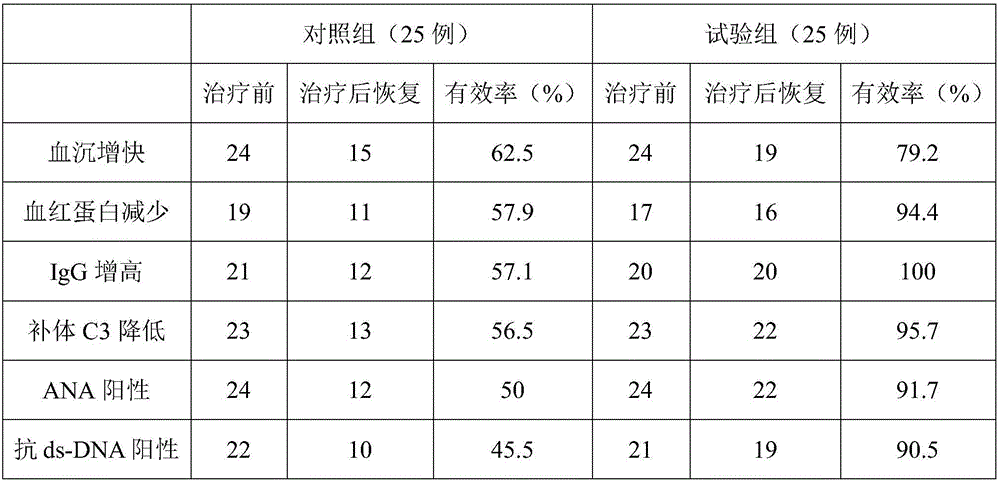
InactiveCN106511358ASignificant effectCurative Effect ConsolidationSalicyclic acid active ingredientsHydroxy compound active ingredientsSide effectMedicine
The invention discloses a medicine for treating lupus nephritis and a preparation method and application thereof. The medicine is prepared from 1-5 parts of hexamethyl disilazane, 11-19 parts of diflunisal, 13-21 parts of monosodium fumarate and 8-16 parts of perillyl alcohol. Diflunisal and monosodium fumarate are mixed and ground, then deionized water is added, the mixture is heated to 75 DEG C and stirred at the temperature, and a mixture A is prepared; hexamethyl disilazane is placed into the mixture A, then the product is sealed and stirred at 88 DEG C, then perillyl alcohol is added, ultrasonic treatment is conducted for 15 min at 63 DEG C, then the product is stirred at 75 DEG C till the product is dry, granulation is conducted, and the medicine is obtained. The medicine is more remarkable in lupus nephritis treating effect, hormone is reduced easily, complications in the treatment process are reduced, the curative effect is enhanced, remarkable, obvious and stable, use is safe and convenient, no toxic and side effects are caused, lupus nephritis does not relapse after healing, the price is low, and the medicine is worthy of clinical popularization.
Owner:郑州莉迪亚医药科技有限公司
Method for synthetizing diflunisal and derivative thereof through one-step method
ActiveCN110372493ALow costEfficiently obtainedPreparation from carboxylic acid saltsOrganic compound preparationIron saltsGrignard reagent
The invention discloses a method for synthetizing diflunisal and a derivative thereof through a one-step method. The method comprises the steps: under joint catalysis of an iron salt, a ligand and titanate, 2,4-difluorophenylmagnesium halide and 5-halogenated salicylic acid or a 5-halogenated salicylic acid derivative are mixed, heated and coupled in a solvent, and the diflunisal and the derivative thereof are obtained. The method has the advantages that (1) high-priced palladium or high-toxicity nickel does not need to be adopted as catalytic metal, only low-toxicity, high-yield and inexpensive iron salts and titanate need to be used, thus the cost is low, and environmental friendliness is achieved; (2) a zinc salt does not need to be used, or step preparation of a boron reagent is not needed, a Grignard reagent is directly used, the preparation steps are few, and raw material and energy consumption is low; and (3) operation is easy and convenient, conditions are mild, amplification is easy, and the method is suitable for industrial production.
Owner:BEIJING NORMAL UNIVERSITY
Drug for treating lupus nephropathy and preparation method and application of drug
InactiveCN106511446ASignificant effectCurative Effect ConsolidationSalicyclic acid active ingredientsSilicon compound active ingredientsSide effectMedicine
The invention discloses a drug for treating lupus nephropathy and a preparation method and application of the drug. The drug consists of the following raw materials: 1-5 parts of hexamethyl disilazane, 11-19 parts of diflunisal, 13-21 parts of Radix Platycodonis and 8-16 parts of Radix Astragali. The preparation method comprises the following steps: mixing and grinding the diflunisal and the Radix Platycodonis, then adding deionized water, heating to the temperature of 75 DEG C, and stirring at the temperature to obtain a mixture A; and placing the hexamethyl disilazane in the mixture A, then sealing and stirring at the temperature of 88 DEG C, adding the Radix Astragali, carrying out ultrasonic treatment for 15 minutes at the temperature of 63 DEG C, stirring at the temperature of 75 DEG C, and granulating to obtain the final product. The drug for treating the lupus nephropathy is remarkable in curative effect, meanwhile, reduction of hormone is facilitated, complications in a treatment process are reduced, the curative effect is consolidated, remarkable, definite and stable, the drug is safe and convenient to use, and does not have any toxic and side effects, the lupus nephropathy does not relapse after being healed, and the drug is low in price and is worthy of being popularized clinically.
Owner:ZHENGZHOU ZHANGMENG NETWORK TECH CO LTD
Positively charged water-soluble prodrug of diflunisal and related compound
ActiveCN105439877AGood absorption rateGood curative effectOrganic active ingredientsSenses disorderSide effectSalicylic acid
The present invention relates to design and synthesis of a novel prodrug of diflunisal, salsalate, and salicylic acid, wherein the prodrug is positively charged, and has a structural formula 1 as shown in the general formula (1), and a structural formula 2 as shown in the general formula (2). The compound with the structural formula 1 as shown in the general formula (1), and the structural formula 2 as shown in the general formula (2) can be prepared by a reaction of diflunisal, salsalate and salicylic acid with appropriate alcohol, mercaptan, or amine. The prodrugs can be used in medicine for the therapy of humans or animals in a state that can be treated with diflunisal, salsalate, or salicylic acid, and in the therapy process, the prodrugs can be orally used, and can be transdermally administrated, so that the most side effects of the diflunisal, salsalate, or salicylic acid are avoided. According to a controlled release transdermal administration system of the prodrug, the concentration of the diflunisal, salsalate, or salicylic acid in blood can be kept stable in the optimal therapy proficiency, so that the efficacy is improved, and the side effects of the diflunisal, salsalate, or salicylic acid are reduced.
Owner:TECHFIELDS BIOCHEM CO LTD
Self-assembly system based on hydrophilic polymer and medicine and preparation method thereof
ActiveCN102091332BEasy to realize industrializationEasy to makeMacromolecular non-active ingredientsIndometacinFlufenamic acid
The invention relates to a self-assembly system based on hydrophilic polymer and medicine and a preparation method thereof. The system consists of a hydrophilic polymer and a carboxyl-containing medicine, wherein the hydrophilic polymer is polymine or beta-cyclodextrin modified polymine; and the carboxyl-containing medicine is selected from ibuprofen, ketoprofen, fenoprofen, flurbiprofene, oxaprozin, naproxen, indometacin, sulindac, etodolac, diclofenac, pontal, meclofenamic acid, flufenamic acid, tolfenamic acid, lumiracoxib, licofelone, diflunisal and aspirin. The system is prepared by dissolving the hydrophilic polymer in a certain amount of water, dissolving the medicine in a water soluble solvent, slowly adding the organic solution of the medicine into the aqueous solution of the polymer under the action of ultrasound, placing the mixed solution in a dialysis bag, dialyzing in deionized water with magnetic stirring, replacing the deionized water at certain time interval, filtering dislysate after 5 to 48 hours, cooling and drying.
Owner:ARMY MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com