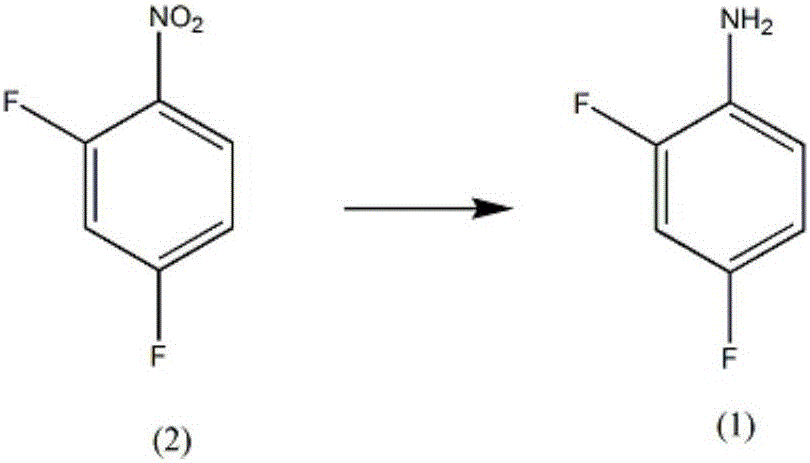
Synthesis method of diflunisal drug intermediate 2,4-difluoroaniline
A technology of difluorophenyl salicylic acid and difluoroaniline, applied in chemical instruments and methods, preparation of organic compounds, preparation of amino compounds, etc., to achieve the effect of increasing the reaction yield and improving the reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] A kind of synthetic method of difluorophenyl salicylic acid medicine intermediate 2,4-difluoroaniline, comprises the steps:
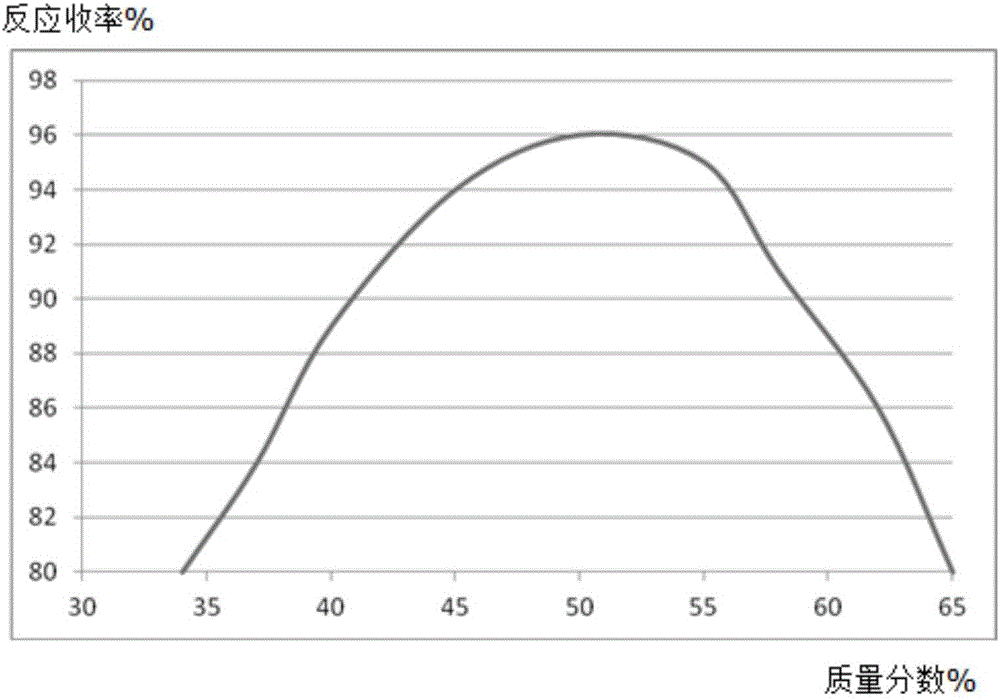
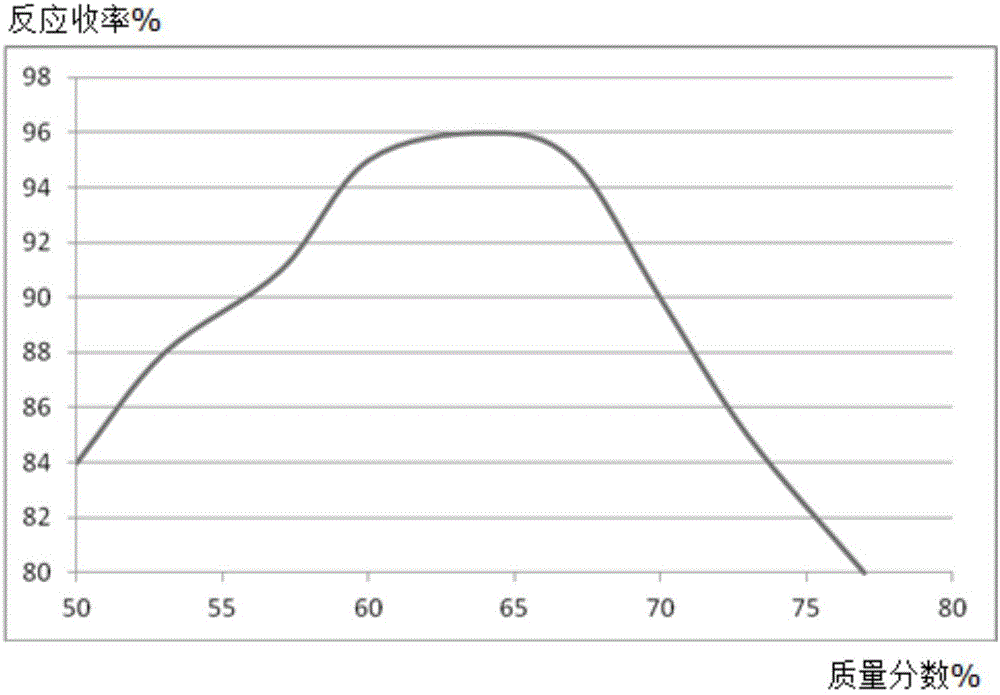
[0020] A, in the reaction vessel that agitator, thermometer, reflux condenser, dropping funnel are installed, add molybdenum powder 0.53mol, mass fraction is 50% 2,6-dimethoxyphenol solution 300ml, control stirring speed at 270rpm, add 0.32mol of 2,4-difluoronitrobenzene, increase the temperature of the solution to 56°C, and reflux for 5h;
[0021] B, steam distillation for 120min, the distillate is extracted with a trifluoroacetic acid solution with a mass fraction of 63%, the extract is washed with diammonium hydrogen citrate solution, dehydrated with anhydrous potassium carbonate dehydrating agent, distilled under reduced pressure at 1.9kPa, and collected The fraction at 55°C yielded 39.58 g of 2,4-difluoroaniline, with a yield of 96%.
Embodiment 2
[0023] A kind of synthetic method of difluorophenyl salicylic acid medicine intermediate 2,4-difluoroaniline, comprises the steps:
[0024] A, in the reaction vessel that agitator, thermometer, reflux condenser, dropping funnel are installed, add molybdenum powder 0.53mol, mass fraction is 50% 2,6-dimethoxyphenol solution 300ml, control stirring speed at 250rpm, add 0.32mol of 2,4-difluoronitrobenzene, increase the temperature of the solution to 53°C, and reflux for 4h;
[0025] B, steam distillation 110min, the distillate is extracted with the trifluoroacetic acid solution of 60% by mass fraction, the extract is washed with potassium sulfate solution, dehydrated with anhydrous magnesium sulfate dehydrating agent, 2.1kPa vacuum distillation, collect 53 ℃ The distillate obtained 38.89 g of 2,4-difluoroaniline, with a yield of 94%.
Embodiment 3
[0027] A kind of synthetic method of difluorophenyl salicylic acid medicine intermediate 2,4-difluoroaniline, comprises the steps:
[0028] A, in the reaction vessel that agitator, thermometer, reflux condenser, dropping funnel are installed, add molybdenum powder 0.53mol, mass fraction is 45% 2,6-dimethoxyphenol solution 300ml, control stirring speed at 230rpm, add 0.32mol of 2,4-difluoronitrobenzene, increase the temperature of the solution to 50°C, and reflux for 3h;
[0029] B, steam distillation for 90min, the distillate is extracted with a trifluoroacetic acid solution with a mass fraction of 60%, the extract is washed with diammonium hydrogen citrate solution, dehydrated with anhydrous potassium carbonate dehydrating agent, distilled under reduced pressure at 1.8kPa, and collected The fraction at 50°C yielded 38.92 g of 2,4-difluoroaniline, with a yield of 94%.
[0030]
Reaction yield%
Example 1
5.5
96
Example 2
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com